Attached files
| file | filename |
|---|---|
| 8-K - PDL BIOPHARMA 8-K 5-24-2011 - PDL BIOPHARMA, INC. | form8k.htm |
| EX-99.2 - EXHIBIT 99.2 - PDL BIOPHARMA, INC. | ex99_2.htm |
Exhibit 99.1

1
Non-Deal Roadshow
May 24-25, 2011

Forward Looking Statements
This presentation contains forward-looking statements, including PDL’s expectations with respect to its future royalty
revenues, expenses, net income, and cash provided by operating activities.
revenues, expenses, net income, and cash provided by operating activities.
Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from those,
express or implied, in these forward-looking statements. Factors that may cause differences between current expectations
and actual results include, but are not limited to, the following:
express or implied, in these forward-looking statements. Factors that may cause differences between current expectations
and actual results include, but are not limited to, the following:
▪The expected rate of growth in royalty-bearing product sales by PDL’s existing licensees;
•The relative mix of royalty-bearing Genentech products manufactured and sold outside the U.S. versus manufactured or
sold in the U.S.;
sold in the U.S.;
•The ability of PDL’s licensees to receive regulatory approvals to market and launch new royalty-bearing products and
whether such products, if launched, will be commercially successful;
whether such products, if launched, will be commercially successful;
•Changes in any of the other assumptions on which PDL’s projected royalty revenues are based;
•Changes in foreign currency rates;
•Positive or negative results in PDL’s attempt to acquire royalty-related assets;
•The outcome of pending litigation or disputes, including PDL’s current dispute with Genentech related to ex-U.S. sales of
Genentech licensed products; and
Genentech licensed products; and
•The failure of licensees to comply with existing license agreements, including any failure to pay royalties due.
Other factors that may cause PDL’s actual results to differ materially from those expressed or implied in the forward-
looking statements in this presentation are discussed in PDL’s filings with the SEC, including the "Risk Factors" sections of
its annual and quarterly reports filed with the SEC. Copies of PDL’s filings with the SEC may be obtained at the "Investors"
section of PDL’s website at www.pdl.com. PDL expressly disclaims any obligation or undertaking to release publicly any
updates or revisions to any forward-looking statements contained herein to reflect any change in PDL’s expectations with
regard thereto or any change in events, conditions or circumstances on which any such statements are based for any
reason, except as required by law, even as new information becomes available or other events occur in the future. All
forward-looking statements in this presentation are qualified in their entirety by this cautionary statement.
looking statements in this presentation are discussed in PDL’s filings with the SEC, including the "Risk Factors" sections of
its annual and quarterly reports filed with the SEC. Copies of PDL’s filings with the SEC may be obtained at the "Investors"
section of PDL’s website at www.pdl.com. PDL expressly disclaims any obligation or undertaking to release publicly any
updates or revisions to any forward-looking statements contained herein to reflect any change in PDL’s expectations with
regard thereto or any change in events, conditions or circumstances on which any such statements are based for any
reason, except as required by law, even as new information becomes available or other events occur in the future. All
forward-looking statements in this presentation are qualified in their entirety by this cautionary statement.
2

|
Company:
|
PDL BioPharma, Inc.
|
|
Ticker:
|
PDLI (NASDAQ)
|
|
Location:
|
Incline Village, Nevada
|
|
Employees:
|
Less than 10
|
|
2010 Revenues:
|
$345 million
|
|
2011- Q1 Revenue:
|
$83 million
|
|
2011 Regular Dividends:
|
$0.15 /share paid on March 15, June 15,
September 15 & December 15 |
|
Q1-2011 Cash Position1:
|
$193 million
|
|
Shares O/S2:
|
~ 140 million
|
|
Average Daily Volume:
|
~ 3 million shares
|
Key Information
3
1. As of March 31, 2011; 2. Not fully diluted

Overview of PDL BioPharma
4

Company Overview
•PDL pioneered the humanization of monoclonal antibodies
which enabled the discovery of a new generation of targeted
treatments for cancer and immunologic diseases
which enabled the discovery of a new generation of targeted
treatments for cancer and immunologic diseases
•PDL’s primary assets are its antibody humanization patents
and royalty assets which consist of its Queen et al. patents
and license agreements
and royalty assets which consist of its Queen et al. patents
and license agreements
•Licensees consist of large biotechnology and pharmaceutical
companies including Roche/Genentech/ Novartis,
Elan/BiogenIdec, Pfizer/Wyeth/J&J and Chugai
companies including Roche/Genentech/ Novartis,
Elan/BiogenIdec, Pfizer/Wyeth/J&J and Chugai
5

Antibody Humanization Technology
• Antibodies are naturally produced by humans to fight
foreign substances, such as bacteria and viruses
foreign substances, such as bacteria and viruses
• In the 1980’s, scientists began creating antibodies in
non-human immune systems, such as those of mice,
that could target specific sites on cells to fight various
human diseases
non-human immune systems, such as those of mice,
that could target specific sites on cells to fight various
human diseases
• However, mouse derived antibodies are recognized by
the human body as foreign substances and may be
rejected by the human immune system
the human body as foreign substances and may be
rejected by the human immune system
6
• PDL’s technology allows for the “humanization” of mouse derived antibodies by moving the
important binding regions from the mouse antibody onto a human framework
important binding regions from the mouse antibody onto a human framework
• PDL’s humanization technology is important because the humanized antibodies retain the binding
and activity levels from the original mouse antibody
and activity levels from the original mouse antibody
• PDL’s technology has been incorporated into antibodies to treat cancer, eye diseases, arthritis,
multiple sclerosis and other health conditions with aggregate annual sales of over $17 billion
multiple sclerosis and other health conditions with aggregate annual sales of over $17 billion

Mission Statement
• Queen et al. Patents
▪ Manage patent portfolio
▪ Manage license agreements
• Purchase new royalty generating assets
▪ Assets that improve shareholder return
▪ Commercial stage assets
▪ Prefer biologics with strong patent protection
• Optimize return for shareholders
7

Corporate Governance
Management
• John McLaughlin
President & CEO
President & CEO
• Christine Larson
VP & CFO
VP & CFO
• Christopher Stone
VP, General Counsel &
Secretary
VP, General Counsel &
Secretary
• Caroline Krumel
VP of Finance
VP of Finance
• Danny Hart
Associate General Counsel
Associate General Counsel
Board of Directors
• Fred Frank
Lead Director
Lead Director
• Jody Lindell
• John McLaughlin
• Paul Sandman
• Harold Selick
8

Licensed Products and Royalty Revenue
9

Licensed Products and Royalty Revenue
10
1. As reported to PDL by its licensee 2. As reported by Roche; assume 1.155 CHF/USD
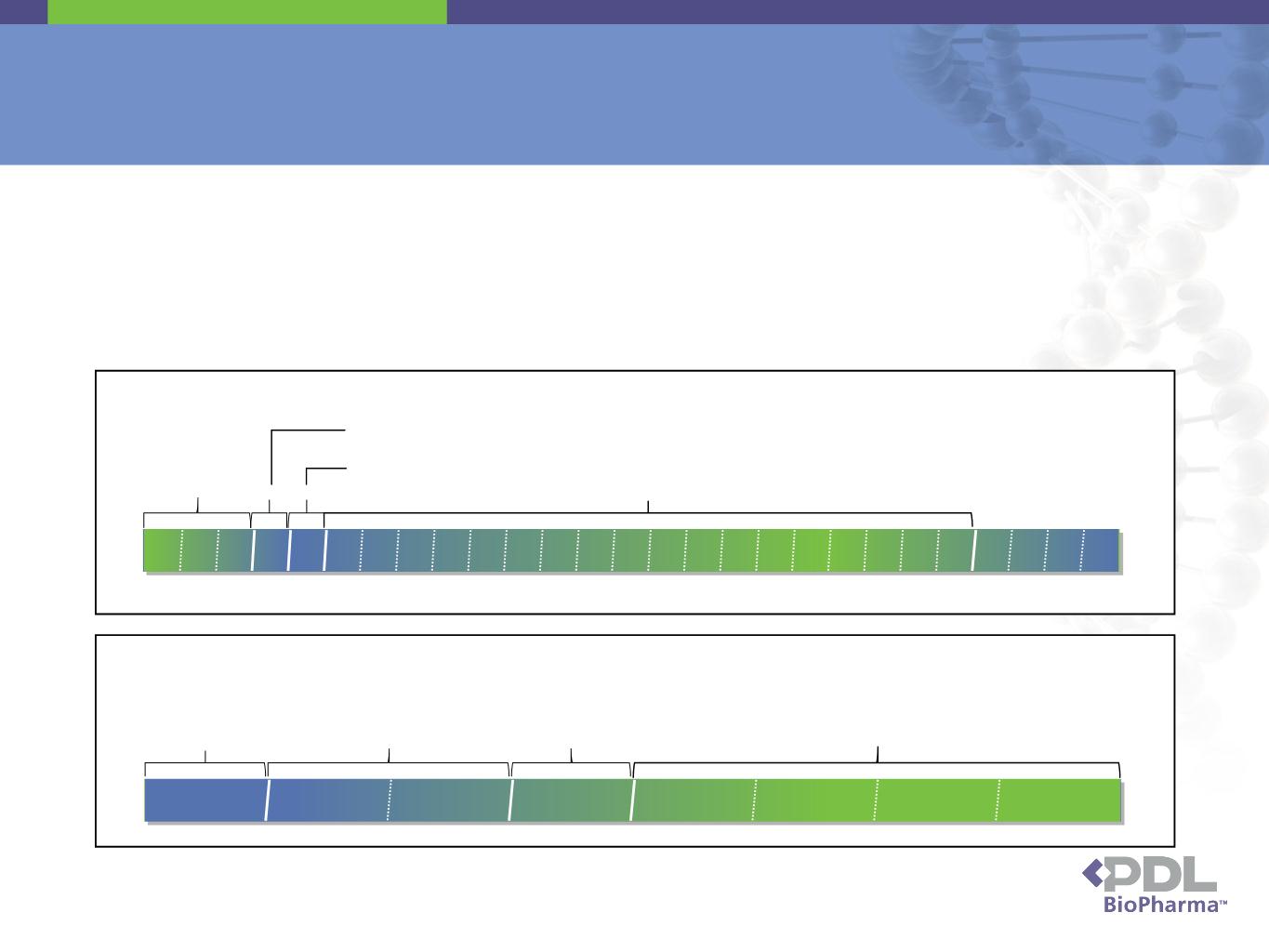
How Long will PDL Receive Royalties from
Queen et al. Patents?
Queen et al. Patents?
• PDL’s revenues consist of royalties generated on sales of licensed products
▪Sold before the expiration of the Queen et al. patents in mid-2013 through end of 2014
or
▪Made prior to the expiration of the Queen et al. patents and sold anytime thereafter
11
Example of Antibody Formulation, Fill and Finish Schedule
½ month
1 month
½ month
2-3 months
Thaw, Formulation &
Vial Filling
Vial Filling
Quality
Release
Release
Packaging
& Quality
& Quality
Inventory
Example of Antibody Bulk Manufacturing Schedule
Cell
Culture
Quality Release
Testing
Testing
Bulk Frozen Storage
1 mo
3 mos
5 mos
10 mos
15 mos
20 mos
27 mos
3 mos
2-18 months
1mo
1mo
Purification to Concentrated Bulk/Frozen

|
Genentech Product Made or Sold in U.S.
|
|
|
Net Sales up to $1.5 Billion
|
3.0%
|
|
Net Sales Between $1.5 Billion and $2.5 Billion
|
2.5%
|
|
Net Sales Between $2.5 Billion and $4.0 Billion
|
2.0%
|
|
Net Sales Over $4.0 Billion
|
1.0%
|
|
Genentech Product Made and Sold Ex-U.S.
|
|
|
All Sales
|
3.0%
|
Queen et al Patents - Royalty Rates
12
• Tysabri and Actemra
• Flat, low single-digit royalty
• Genentech Products (Avastin, Herceptin, Lucentis1 and Xolair)
• Tiered royalties on product made or sold in US
• Flat, 3% royalty on product made and sold outside US
• Blended global royalty rate on Genentech Products in 2010 was 1.9%
• Blended royalty rate on Genentech Products in 2010 made or sold in US was 1.5%
1. As part of a settlement with Novartis, which commercializes Lucentis outside US, PDL agreed to pay to Novartis certain amounts
based on net sales of Lucentis made by Novartis during calendar year 2011 and beyond. The amounts to be paid are less than we
receive in royalties on such sales and we do not currently expect such amount to materially impact our total annual revenues.
based on net sales of Lucentis made by Novartis during calendar year 2011 and beyond. The amounts to be paid are less than we
receive in royalties on such sales and we do not currently expect such amount to materially impact our total annual revenues.

Shift of Manufacturing Sites = Higher Royalties
• Roche is moving some manufacturing ex-US which may result in higher royalties to PDL
due to the flat 3% royalty for Genentech Products made and sold ex-US
due to the flat 3% royalty for Genentech Products made and sold ex-US
▪ Current production at Penzburg (Herceptin) and Basel (Avastin) plants
▪ Two new plants in Singapore (CHO = antibody and e. coli = antibody fragment)
- E. coli (Lucentis) and CHO (Avastin) plants are approved for commercial supply to the US
- E. coli and CHO plants are expected to be approved for commercial supply to the EU in 2011
- Currently, all Lucentis is made in the US
13
Percent of Total Worldwide Sales1
1. As reported to PDL by its licensee
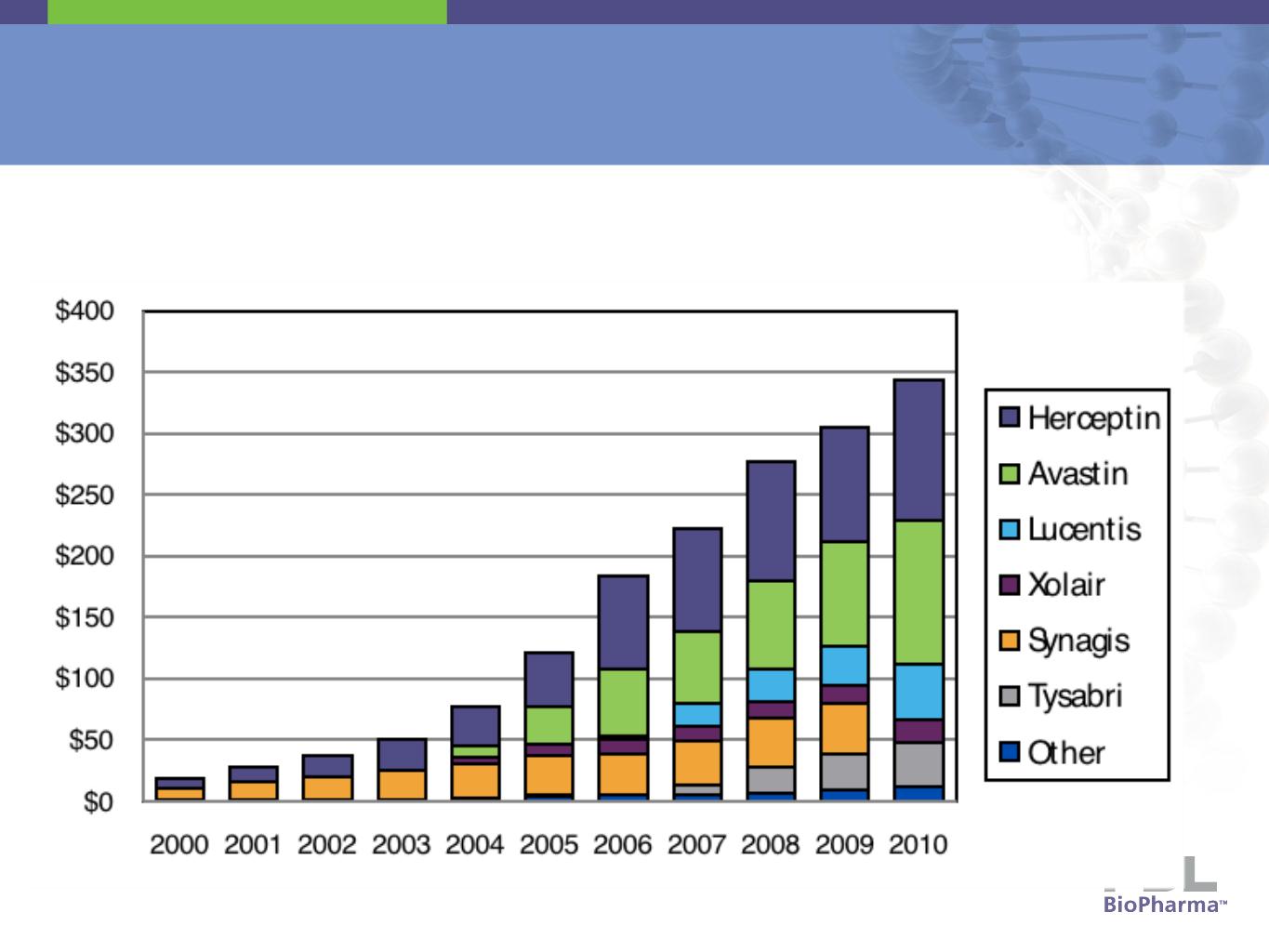
Royalty Revenue & Licensed Products
14
Royalties by Product
($ in millions)

Royalty Products - Approved
15
Royalty Products - Avastin
16
|
Licensee
|
Product
|
Status
|
Indications
|
|
Roche (Genentech)
|
Avastin
|
Approved
sBLA
Phase 3
|
Colorectal Cancer
NSCLC
Metastatic Renal Cell
Glioblastoma
Metastatic Breast HER2- 1st Line
Metastatic Breast HER2- 2nd Line
Ovarian Cancer
Gastric
|
|
|
|||
|
|
|||
|
|
|||
|
Elan
|
|||
•On December 16, 2010, FDA notified Roche/Genentech of its intention to withdraw Avastin’s
approval as first line treatment for HER2- breast cancer in combination with paclitaxel.
approval as first line treatment for HER2- breast cancer in combination with paclitaxel.
•In response to request from Genentech, FDA scheduled a hearing on June 28-29, 2011 to
allow Genentech to present why Avastin should remain FDA-approved for HER2- breast cancer.
allow Genentech to present why Avastin should remain FDA-approved for HER2- breast cancer.
•On December 16, 2010, EMEA narrowed, but did not withdraw Avastin’s approval for first line
treatment of HER2- breast cancer in combination with paclitaxel.
treatment of HER2- breast cancer in combination with paclitaxel.
•Roche lowered its estimate of peak annual sales from of Avastin from CHF8 - CHF9 billion to
CHF7 billion.
CHF7 billion.
•On April 15, 2011, Roche announced that CHMP issued a positive opinion for the use of
Avastin in combination with Xeloda for 1st-line HER 2- breast cancer.
Avastin in combination with Xeloda for 1st-line HER 2- breast cancer.
•Based on our internal model, we project Avastin for treatment of metastatic HER2- breast
cancer represents slightly more than 2% of total PDL royalty revenue.
cancer represents slightly more than 2% of total PDL royalty revenue.
Royalty Products - Avastin
17
|
Licensee
|
Product
|
Status
|
Indications
|
|
Roche (Genentech)
|
Avastin
|
Approved
sBLA
Phase 3
|
Colorectal Cancer
NSCLC
Metastatic Renal Cell
Glioblastoma
Metastatic Breast HER2- 1st Line
Metastatic Breast HER2- 2nd Line
Ovarian Cancer
Gastric
|
|
|
|||
|
|
|||
|
|
|||
|
Elan
|
|||
•On February 7, 2011, Genentech reported that Phase 3 trial in women with previously treated
(recurrent), platinum-sensitive ovarian cancer showed an improvement in progression free
survival in those patients treated with Avastin in combination with chemotherapy (carboplatin
and gemcitabine) followed by continued use of Avastin alone compared to those treated with
chemotherapy alone.
(recurrent), platinum-sensitive ovarian cancer showed an improvement in progression free
survival in those patients treated with Avastin in combination with chemotherapy (carboplatin
and gemcitabine) followed by continued use of Avastin alone compared to those treated with
chemotherapy alone.
•Two previous Phase 3 studies in women with newly diagnosed ovarian cancer demonstrated
that front-line Avastin in combination with standard chemotherapy (carboplatin and paclitaxel),
followed by the continued use of Avastin alone, significantly increased progression free survival
compared to treatment with chemotherapy alone.
that front-line Avastin in combination with standard chemotherapy (carboplatin and paclitaxel),
followed by the continued use of Avastin alone, significantly increased progression free survival
compared to treatment with chemotherapy alone.
•Roche has submitted an application for approval for first line treatment in EU and expects a
decision later in 2011.
decision later in 2011.
•Genentech expects to file an application for approval in US in 2011.

18
|
Phase 2 ISP
|
Retinopathy of Prematurity
|
||
|
|
Herceptin
|
Approved
|
Breast HER2+ Cancer
HER2+ Stomach and Gastro-Esophageal cancers
|
|
|
Lucentis
|
Approved
Approved
Phase 3
|
AMD
RVO
DME
|
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
•On February 16, Research to Prevent Blindness Foundation and the U.S. National Eye Institute
announced results from a trial showing that just 4% of the infants who developed retinopathy of
prematurity and were treated with Avastin suffered a recurrence of the disease compared to
22% of those babies with the disease who received laser treatment.
announced results from a trial showing that just 4% of the infants who developed retinopathy of
prematurity and were treated with Avastin suffered a recurrence of the disease compared to
22% of those babies with the disease who received laser treatment.
•Retinopathy of prematurity is a disease that harms the retina and is the most common cause of
blindness in infants.
blindness in infants.
•Because the trial was not sponsored by Genentech/Roche, it is not clear whether they will seek
approval for this indication.
approval for this indication.
•The publication of this data in the February 17 issue of the New England Journal of Medicine
should result in significant off-label use in this disease.
should result in significant off-label use in this disease.
Royalty Products - Lucentis
|
|
|||
|
|
Lucentis
|
Approved
Approved
Phase 3 (US)
|
AMD
RVO
DME
|
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
•On January 7, Novartis announced that Lucentis has been approved in the EU for the treatment
of visual impairment due to diabetic macular edema (DME).
of visual impairment due to diabetic macular edema (DME).
•DME is a leading cause of blindness in the working-age population in most developed
countries.
countries.
•On February 11, 2011, Genentech announced that one of two Phase 3 studies evaluating in
patients with DME showed that a significantly higher percentage of patients receiving monthly
dosing of Lucentis achieved an improvement in vision of at least 15 letters on the eye chart at
24 months compared to those in a control group, who received a placebo injection.
patients with DME showed that a significantly higher percentage of patients receiving monthly
dosing of Lucentis achieved an improvement in vision of at least 15 letters on the eye chart at
24 months compared to those in a control group, who received a placebo injection.
19
|
|
|||
|
|
Lucentis
|
Approved
Approved
Phase 3
|
AMD
RVO
DME
|
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
•On November 22, 2010, Regeneron and its partner, Bayer, reported top line data from two
Phase 3 trials investigating its VEGF Trap in age-related macular degeneration (AMD) patients
which suggest that it may be injected into the eye every other month with safety and efficacy
comparable to that of monthly dosing of Lucentis.
Phase 3 trials investigating its VEGF Trap in age-related macular degeneration (AMD) patients
which suggest that it may be injected into the eye every other month with safety and efficacy
comparable to that of monthly dosing of Lucentis.
•On December 20, 2010, Regeneron has also reported positive Phase 3 data in the treatment of
retinal vein occlusion (RVO) for which Lucentis is approved.
retinal vein occlusion (RVO) for which Lucentis is approved.
•Unlike the AMD trial, monthly administration was used in the RVO trial, which does not
afford a dosing advantage with respect to Lucentis.
afford a dosing advantage with respect to Lucentis.
•On February 22, 2011, Regeneron and its partner, Bayer, filed an application for approval of its
VEGF Trap for treatment of AMD with a PDUFA date of August 20, 2011 based on priority
review.
VEGF Trap for treatment of AMD with a PDUFA date of August 20, 2011 based on priority
review.
•Regeneron filed suit in February 2011 seeking a summary judgment that it does not infringe
Genentech’s patents.
Genentech’s patents.
•Genentech filed a countersuit in April 2011 asserting that Regeneron is willfully infringing
Genentech’s patents, seeking treble damages and asking for injunctive relief.
Genentech’s patents, seeking treble damages and asking for injunctive relief.
20

Royalty Products - Lucentis
|
Licensee
|
Product
|
Status
|
Indications
|
|
|
HER2+ Stomach and Gastro-Esophageal cancers
|
||
|
|
Lucentis
|
Approved
Approved
Phase 3
|
AMD
RVO
DME
|
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
•On April 4, 2011, Genentech and Johns Hopkins University reported results of a review of files
of 77,886 patients with AMD who received either Avastin off-label or Lucentis.
of 77,886 patients with AMD who received either Avastin off-label or Lucentis.
•Patients receiving Avastin off-label had an 11% increased risk of overall mortality, 57%
increased risk of hemorrhagic cerebrovascular accident, 80% more likely to have ocular
inflammation and 11% more likely to have cataract surgery following treatment than Lucentis
treated patients.
increased risk of hemorrhagic cerebrovascular accident, 80% more likely to have ocular
inflammation and 11% more likely to have cataract surgery following treatment than Lucentis
treated patients.
•Authors of the study note that it is limited due to incomplete information on confounding factors
such as smoking, lipid and blood pressure levels, etc.
such as smoking, lipid and blood pressure levels, etc.
21

Royalty Products - Lucentis
|
|
|||
|
|
Lucentis
|
Approved
Approved
Phase 3
|
AMD
RVO
DME
|
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
•On April 28, 2011, New England Journal of Medicine reported the results from the NEI’s CATT
study comparing Lucentis and Avastin on fixed and variable schedules in the treatment of AMD.
study comparing Lucentis and Avastin on fixed and variable schedules in the treatment of AMD.
•Efficacy results from the first year of the two year study showed that, with respect to the primary
endpoint of mean change in visual acuity (number of lines of letters on an eye chart) at 12
months, less expensive Avastin was not inferior to Lucentis.
endpoint of mean change in visual acuity (number of lines of letters on an eye chart) at 12
months, less expensive Avastin was not inferior to Lucentis.
•It is estimated that off label use of Avastin in the U.S. was 60% prior to the results of the
CATT trial.
CATT trial.
•At 12 months, serious adverse events (primarily hospitalizations) occurred at a 24 percent rate
for patients receiving Avastin and a 19 percent rate for patients receiving Lucentis. However,
preliminary 24 month safety data showed no difference between Lucentis and Avastin treated
patients in terms of death, stroke and all arteriothrombotic events.
for patients receiving Avastin and a 19 percent rate for patients receiving Lucentis. However,
preliminary 24 month safety data showed no difference between Lucentis and Avastin treated
patients in terms of death, stroke and all arteriothrombotic events.
22

Royalty Products - Tysabri
23
|
Licensee
|
Product
|
Status
|
Indications
|
|
|
|||
|
|
|||
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
•Biogen Idec and Elan made regulatory filings with FDA and EMA to update the label of Tysabri
to reflect that anti-JC virus antibody status could be used to stratify the risk of progressive
multifocal leukoencephalopathy (PML).
to reflect that anti-JC virus antibody status could be used to stratify the risk of progressive
multifocal leukoencephalopathy (PML).
•On April 18, 2011, CHMP recommended inclusion of JC virus (JCV) status as a risk factor
the product label for Tysabri in the EU.
the product label for Tysabri in the EU.
•The CHMP also recommended a five-year renewal of the Tysabri’s Marketing Authorization
in the EU.
in the EU.
•On April 22, 2011, FDA disclosed the estimated risk of PML infection in Tysabri treated patients
is 0.3 per 1,000 patients during the first two years of treatment, 1.5 per 1,000 patients during
months 25 to 36, and 0.9 per 1,000 after three years. Limited data is available beyond four
years.
is 0.3 per 1,000 patients during the first two years of treatment, 1.5 per 1,000 patients during
months 25 to 36, and 0.9 per 1,000 after three years. Limited data is available beyond four
years.
|
Licensee
|
Product
|
Status
|
Indications
|
|
Roche (Genentech)
|
Avastin
|
Approved
|
Colorectal Cancer
NSCLC
|
|
|
|||
|
|
Phase 3
|
DME
|
|
|
|
Xolair
|
Approved
sBLA
|
Moderate-Severe Asthma
Pediatric Asthma
|
|
Elan
|
Tysabri
|
Approved
|
Multiple Sclerosis
|
|
Roche (Chugai)
|
Actemra
|
Approved
|
Rheumatoid Arthritis
|
Royalty Products - Actemra
24
•On January 5, 2011, Roche announced that FDA expanded the Actemra label to include
inhibition and slowing of structural joint damage, improvement of physical function, and
achievement of major clinical response in adult patients with moderately to severely active
rheumatoid arthritis.
inhibition and slowing of structural joint damage, improvement of physical function, and
achievement of major clinical response in adult patients with moderately to severely active
rheumatoid arthritis.
•On April 18, 2011, FDA approved Actemra to treat patients age 2 and older with active
systemic juvenile idiopathic arthritis (SJIA).
systemic juvenile idiopathic arthritis (SJIA).
•It is the first and only approved treatment for SJIA, a rare and severe form of arthritis
affecting children.
affecting children.

Potential Royalty Products
- Development Stage
- Development Stage
25

|
Licensee
|
Product
|
Status
|
Indications
|
|
Roche (Genentech)
|
T-DM1
|
Phase 2 & 3
|
Breast HER2+ Cancer
|
|
|
Ocrelizumab
|
Phase 2b
|
Relapsing Remitting Multiple Sclerosis
|
|
|
Pertuzumab
|
Phase 3
|
Metastatic HER2+ Breast Cancer
|
|
Roche
|
Afutuzumab
|
Phase 3
|
Chronic Lymphocytic Leukemia
|
|
Elan/J&J/Pfizer
|
Bapineuzumab
|
Phase 3
|
Alzheimer’s Disease
|
|
Lilly
|
|||
|
Eisai
|
Potential Royalty Products - T-DM1
•On October 13, 2010, Roche/Genentech announced preliminary, six month results from a
Phase 3 trial in second line HER2+ breast cancer patients which showed that 48% of women
treated with T-DM1 had their tumors shrink compared with 41% of those taking the combination
of Herceptin and Taxotere.
Phase 3 trial in second line HER2+ breast cancer patients which showed that 48% of women
treated with T-DM1 had their tumors shrink compared with 41% of those taking the combination
of Herceptin and Taxotere.
•Among the women taking the standard therapy, 75% had side effects of grade 3 or higher
on a 5-point scale, compared with 37% of those getting T-DM1.
on a 5-point scale, compared with 37% of those getting T-DM1.

Potential Royalty Products - Pertuzumab
|
Licensee
|
Product
|
Status
|
Indications
|
|
Roche (Genentech)
|
T-DM1
|
Phase 2 & 3
|
Breast HER2+ Cancer
|
|
|
Ocrelizumab
|
Phase 2b
|
Relapsing Remitting Multiple Sclerosis
|
|
|
Pertuzumab
|
Phase 3
|
Metastatic HER2+ Breast Cancer
|
|
Roche
|
Afutuzumab
|
Phase 3
|
Chronic Lymphocytic Leukemia
|
|
Elan/J&J/Pfizer
|
Bapineuzumab
|
Phase 3
|
Alzheimer’s Disease
|
|
Lilly
|
Solanezumab
|
Phase 3
|
Alzheimer’s Disease
|
•On December 10, 2010, Roche/Genentech reported the results from a Phase 2 trial
investigating the neoadjuvant (prior to surgery) use of pertuzumab and Herceptin plus
chemotherapy for the treatment of early-stage, HER2+ breast cancer.
investigating the neoadjuvant (prior to surgery) use of pertuzumab and Herceptin plus
chemotherapy for the treatment of early-stage, HER2+ breast cancer.
•Treatment significantly improved the rate of complete tumor disappearance in the breast by
more than half compared to Herceptin plus docetaxel, p=0.014.
more than half compared to Herceptin plus docetaxel, p=0.014.
•Roche expects a global regulatory filing of pertuzumab at the end of 2011.
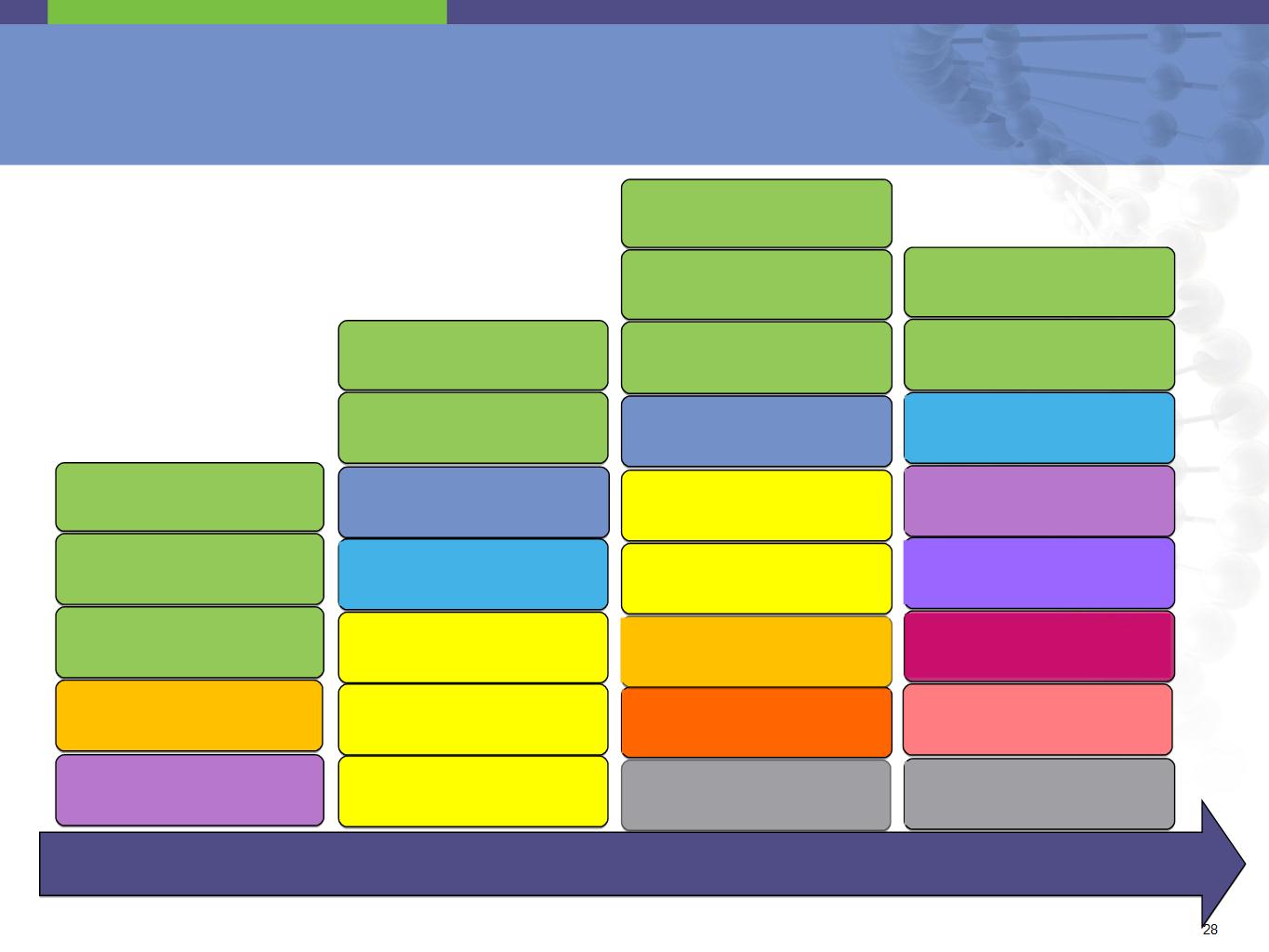
Genentech / Roche - Product Pipeline
2011
2012
2013
Post 2013
Avastin
Ovarian Cancer 1st Line US
Lucentis
Diabetic Macular Edema (US)
Pertuzumab1
mBC HER2+ 1st Line
Avastin + Herceptin
mBC HER+ 2nd Line
Avastin
Relapsed Ovarian Cancer
T-DM1
HER 2+ Advanced mBC
Actemra
RA DMARD H2H (EU)
Actemra
Ankylosing Spondylitis
Herceptin
Subcutaneous Formulation
Avastin & Herceptin
HER2+ mBC 1st Line
Avastin
mCRC TML
Actemra
SC Formulation (EU)
Afutuzumab (GA101)
Chronic Lymphocytic Leukemia
Actemra
Early Rheumatoid Arthritis
Avastin
BC Adjuvant HER2+
Avastin
BC Adj Triple Negative
Herceptin
BC HER 2+ Adj 2 Year
Xolair
Chronic Idiopathic Urticaria
Avastin
Glioblastoma 1st Line
Actemra
SC Formulation (US)
Lucentis
AMD High Dose (US)
Avastin
HER 2- BC adj
Avastin
NSCLC adj
Afutuzumab (GA101)
Non-Hodgkin’s Lymphoma
T-DM1
HER 2+ mBC 1st Line
Pertuzumab1
HER 2+ EBC
Ocrelizumab1
PPMS & RRMS
Lebrikizumab1
Asthma
Rontalizumab1
Systemic Lupus Erythematosus
1. Not a licensed product; source Roche investor update, April 14, 2011
US & EU Filings Calendar

Financial Update
29
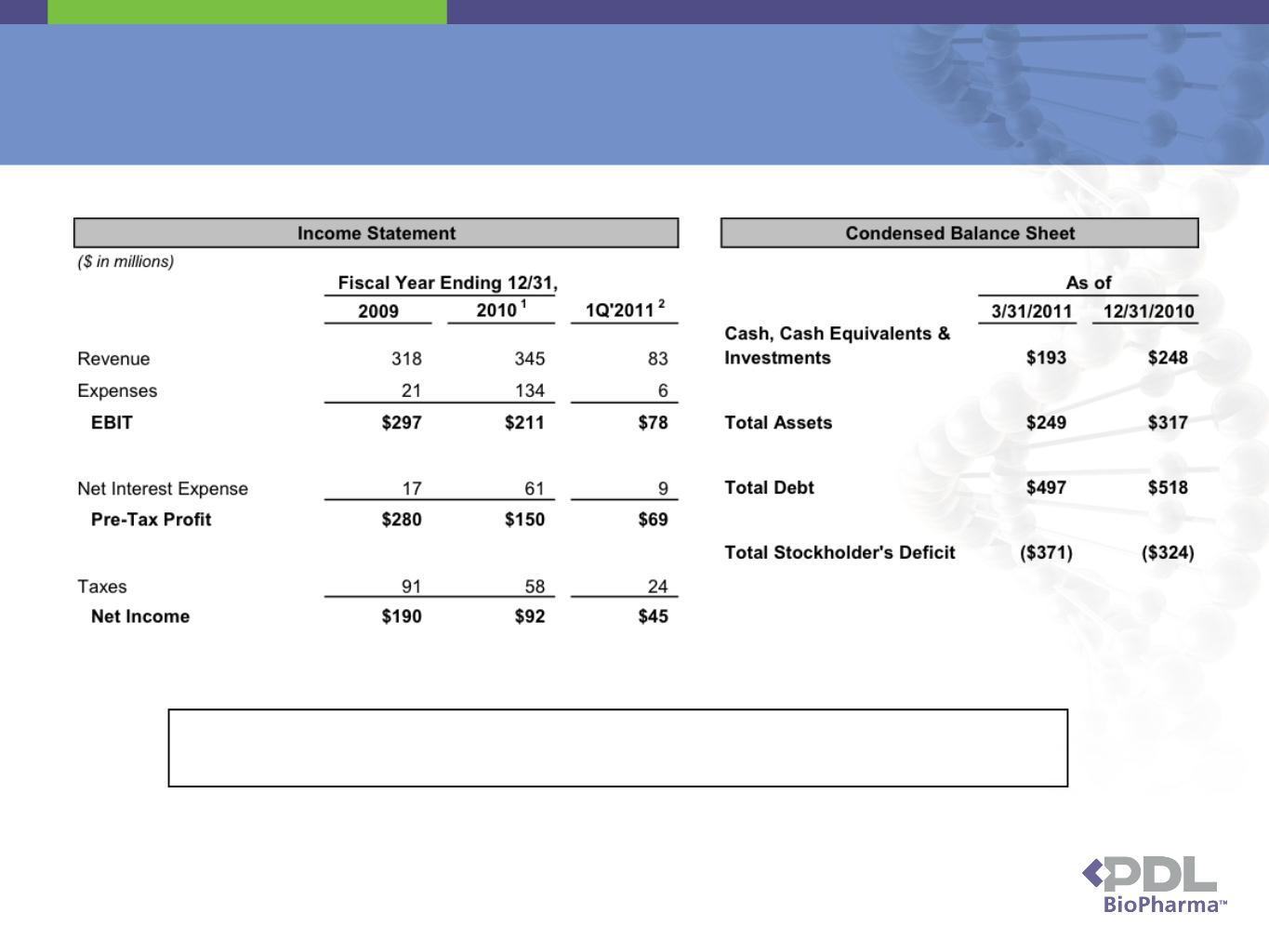
Financial Overview
30
1.Includes $92.5 million one time legal settlement to MedImmune. Net interest expense includes
$17.6 million loss on convertible note retirement.
$17.6 million loss on convertible note retirement.
2.Includes $10.0 million one time legal settlement from UCB.

New 2015 Convertible Notes
• Terms
▪ $135 million convertible, non-callable senior notes due May 2015
- Bankers exercised option to purchase an additional ~$20 million increasing total to ~$155
million
million
▪ Coupon rate is 3.75%
▪ Conversion price is $7.92/share
▪ Bond hedge effectively increases conversion price to $9.31½ /share
• Timing
▪ Transaction was priced on May 10th and closed on May 16th
• Use of Proceeds
▪ Redeem PDL’s convertible notes due in February 2012 with a current
principal balance of $133 million
principal balance of $133 million
▪ Pay cost of convertible note hedge transactions
▪ General corporate purposes
31

$497 Million Debt
• $250 million 2.00% convertible senior notes due February 2012; current principal
balance of $133 million
balance of $133 million
▪Conversion rate is 144.474 shares / $1,000 face amount ($6.92/share)
▪Issued a notice of redemption for cash on May 23 effective as of June 30 for all outstanding Notes at
redemption price of 100.29% (approximately $1,010.3444 per $1,000 principal amount of Notes)
redemption price of 100.29% (approximately $1,010.3444 per $1,000 principal amount of Notes)
▪Using cash from new 2015 Notes
• $180 million 2.875% convertible senior notes due February 2015 placed November 1,
2010
2010
▪Conversion rate is 144.474 shares / $1,000 face amount ($6.92/share)
• $300 million 10.25% secured non-recourse notes; current principal balance of $184
million
million
▪Distributed $200 million of proceeds as special dividend of $1.67/share in December 2009
▪Approximately 40% of Genentech royalties dedicated to quarterly principal and interest payments; principal
repayment fluctuates in relation to royalties received
repayment fluctuates in relation to royalties received
▪Anticipated final maturity is September 2012; legal maturity is March 2015
▪After final maturity, securitized Genentech royalties will be retained by PDL
• In order to pay a dividend, PDL must first satisfy a net assets test at the time of dividend
declaration by Board of Directors
declaration by Board of Directors
32

Legal Matters
33

Recent Resolution of Legal Disputes
• PDL has resolved all challenges to the Queen et al. Patents in the
U.S. Patent and Trademark Office (USPTO) and the European Patent
Office (EPO) as well as its dispute with MedImmune
U.S. Patent and Trademark Office (USPTO) and the European Patent
Office (EPO) as well as its dispute with MedImmune
▪ UCB Pharma
- PDL received $10 million from UCB and PDL agreed not to sue UCB for any royalties related
to Cimzia
to Cimzia
- UCB terminated patent interference proceedings before the USPTO and withdrew its
opposition appeal in the EPO
opposition appeal in the EPO
▪ MedImmune
- PDL paid MedImmune $65 million on February 15, 2011 and will pay them an additional $27.5
million by February 2012
million by February 2012
- MedImmune ceased support of any party in the EPO opposition appeal
▪ Novartis
- PDL dismissed its claims against Novartis in its Nevada lawsuit
- Novartis withdrew its opposition appeal to PDL’s European patent in EPO
- PDL will pay Novartis an amount based on Novartis’ net ex-U.S. sales of Lucentis during
calendar year 2011 and beyond
calendar year 2011 and beyond
▪ BioTransplant
- PDL acquired BioTransplant, a bankrupt company and instructed BioTransplant to withdraw
its opposition appeal in the EPO
its opposition appeal in the EPO
34

Pending Dispute with Genentech and Roche
• In August 2010, Genentech sent a fax on behalf of Roche and Novartis
asserting its products do not infringe PDL’s supplementary protection
certificates (SPCs)
asserting its products do not infringe PDL’s supplementary protection
certificates (SPCs)
▪ Products include Avastin, Herceptin, Lucentis and Xolair
▪ SPCs are extensions of patent term in Europe that are issued on a country-by-country and
product-by-product basis
product-by-product basis
• PDL Response
▪ Genentech’s assertions are without merit
▪ PDL disagrees with Genentech’s assertions of non-infringement
▪ Genentech had waived its rights to challenge our patents, including SPCs in its 2003
Settlement Agreement with PDL
Settlement Agreement with PDL
• 2003 Settlement Agreement
▪ Resolved intellectual property disputes between the two companies at that time
▪ Limits Genentech’s ability to challenge infringement of PDL’s patent rights, including SPCs,
and waives Genentech’s right to challenge or assist other in challenging the validity of our
patent rights
and waives Genentech’s right to challenge or assist other in challenging the validity of our
patent rights
35

Nevada Lawsuit Against Genentech/Roche
•PDL filed a lawsuit against Genentech and Roche in Nevada
state court
state court
▪ Lawsuit states that fax constitutes a breach of 2003 Settlement Agreement
because Genentech assisted Roche in challenging PDL’s patents and SPCs
because Genentech assisted Roche in challenging PDL’s patents and SPCs
▪ Complaint seeks compensatory damages, including liquidated damages and
other monetary remedies set forth in the 2003 Settlement Agreement, punitive
damages and attorney’s fees
other monetary remedies set forth in the 2003 Settlement Agreement, punitive
damages and attorney’s fees
•In November 2010, Genentech and Roche filed two motions to
dismiss
dismiss
▪ They contend that 2003 Settlement Agreement applies only to PDL’s U.S.
patent rights
patent rights
▪ They asserted that the Nevada court lacks personal jurisdiction over Roche
▪ On April 21, 2011, Nevada court heard arguments on two Genentech and
Roche motions
Roche motions
▪ If case proceeds, trial is not yet scheduled and not expected until 2012
36

Optimizing Stockholder Return
37

Business Strategy
• Purchase new royalty assets and
ladder like a bond portfolio
ladder like a bond portfolio
▪ Continue to reinvest in new royalty assets
and pay dividends
and pay dividends
▪ Debt repaid by end of 2015
▪ Company continues as long as it can
generate satisfactory return
generate satisfactory return
• If unable to acquire royalty assets on
attractive terms, build cash reserves to
attractive terms, build cash reserves to
▪ Repay debt
▪ Use all excess cash to pay dividends to
enhance shareholder return
enhance shareholder return
▪ Wind-up company in 2016 timeframe
38
• Queen et al. patents expire end of 2014; we
anticipate royalties will likely continue to ~
2016
anticipate royalties will likely continue to ~
2016
• There are two possible pathways forward
for PDL
for PDL

Optimizing Stockholder Return
• Continuously evaluating
alternatives
alternatives
▪ Dividends
▪ Capital restructure
▪ Share repurchase
▪ Company sale
▪ Purchase of commercial stage, royalty
generating assets
generating assets
39

Investment Highlights
• Strong historic revenue growth from approved products
• Potential for additional indications from existing
products, new product approvals and purchase of new
royalty assets
products, new product approvals and purchase of new
royalty assets
• Potential to grow and diversify revenues with the
addition of new royalty assets
addition of new royalty assets
• Significantly reduced expenses with no R&D burn
• Liquidity - volume averages 3 million shares/day
• Return to stockholders
▪ In 2011, $0.60/share to be paid in quarterly regular dividends of
$0.15/share in March 15, June 15, September 15 and December
15
$0.15/share in March 15, June 15, September 15 and December
15
40

Appendix
41

Avastin
• Licensor
▪Genentech (US) and Roche (ex-US)
• Mechanism
▪As a tumor grows, it exceeds the ability of the local blood supply
to nourish it
to nourish it
▪Tumor causes up regulation of vascular endothelial growth factor
(VEGF) stimulating angiogenesis or the growth of leaky blood
vessels to nourish the tumor
(VEGF) stimulating angiogenesis or the growth of leaky blood
vessels to nourish the tumor
▪Avastin targets and inhibits VEGF reduction in blood vessels
“starving” the tumor
“starving” the tumor
• Approvals
▪Metastatic colorectal cancer, advanced non-small cell lung
cancer, renal cancer, metastatic HER2- breast cancer and
glioblastoma
cancer, renal cancer, metastatic HER2- breast cancer and
glioblastoma
• Sales
▪2010 worldwide net sales of $6.4 billion1
- US is reviewing approval for metastatic HER2- breast cancer and EU has
narrowed this label, resulting in drop in sales for this indication
narrowed this label, resulting in drop in sales for this indication
42
Treatment with Avastin
reduces vascularization
or blood supply of tumor
reduces vascularization
or blood supply of tumor
1. As reported to PDL by its licensee

Herceptin
• Licensor
▪ Genentech (US) and Roche (ex-US)
• Mechanism
▪ Some breast cancer cells make too many (over-express)
copies of a particular gene known as HER2 that causes
rapid growth of the breast cancer cell
copies of a particular gene known as HER2 that causes
rapid growth of the breast cancer cell
▪ Herceptin works by attaching itself to the HER2 receptors
on the surface of breast cancer cells, blocking them from
receiving growth signals and slowing or stopping the
growth of the breast cancer cell
on the surface of breast cancer cells, blocking them from
receiving growth signals and slowing or stopping the
growth of the breast cancer cell
▪ Herceptin also fights breast cancer by alerting the immune
system to destroy cancer cells onto which it is attached
system to destroy cancer cells onto which it is attached
• Approvals
▪ Metastatic HER2+ breast cancer, metastatic HER2+
stomach cancer
stomach cancer
• Sales
▪ 2010 worldwide net sales of $5.4 billion1
43
Without Herceptin treatment,
cell surface receptors signal
into the HER2+ breast cancer
cell to proliferate
cell surface receptors signal
into the HER2+ breast cancer
cell to proliferate
Herceptin binds to cell surface
receptors inhibiting intracellular
signals thus preventing cancer
cell proliferation and signaling
the immune system to “kill” the
cancer cell
receptors inhibiting intracellular
signals thus preventing cancer
cell proliferation and signaling
the immune system to “kill” the
cancer cell
1. As reported to PDL by its licensee

Lucentis
44
• Licensor
▪ Genentech (US) and Novartis (ex-US)
• Mechanism
▪ A form of VEGF known as VEGF-A causes the
formation of leaky blood vessels result in the
swelling in the macula and vision loss
formation of leaky blood vessels result in the
swelling in the macula and vision loss
▪ Lucentis binds to and inhibits VEGF-A before it can
cause the formation of the leaky blood vessels
preserving and sometimes improving vision
cause the formation of the leaky blood vessels
preserving and sometimes improving vision
• Approvals
▪ Wet age-related macular degeneration (AMD),
macular edema or swelling following retinal vein
occlusion, diabetic macular edema
macular edema or swelling following retinal vein
occlusion, diabetic macular edema
• Sales
▪ 2010 worldwide net sales of $3.0 billion1
- Recent NIH study comparing safety and effectiveness of
Lucentis finds less expensive Avastin equally efficacious -
will adversely affect future Lucentis sales for AMD
Lucentis finds less expensive Avastin equally efficacious -
will adversely affect future Lucentis sales for AMD
- It’s estimated that in the U.S. 60% of AMD patients are
already treated with off-label Avastin
already treated with off-label Avastin
Cross
section of
normal
macula at
back of eye
section of
normal
macula at
back of eye
Cross
section of
macula with
AMD causing
loss of vision
section of
macula with
AMD causing
loss of vision
Amsler Grid as
seen through
normal eyes
seen through
normal eyes
Amsler Grid as
seen through
eyes with AMD
seen through
eyes with AMD
1. As reported to PDL by its licensee
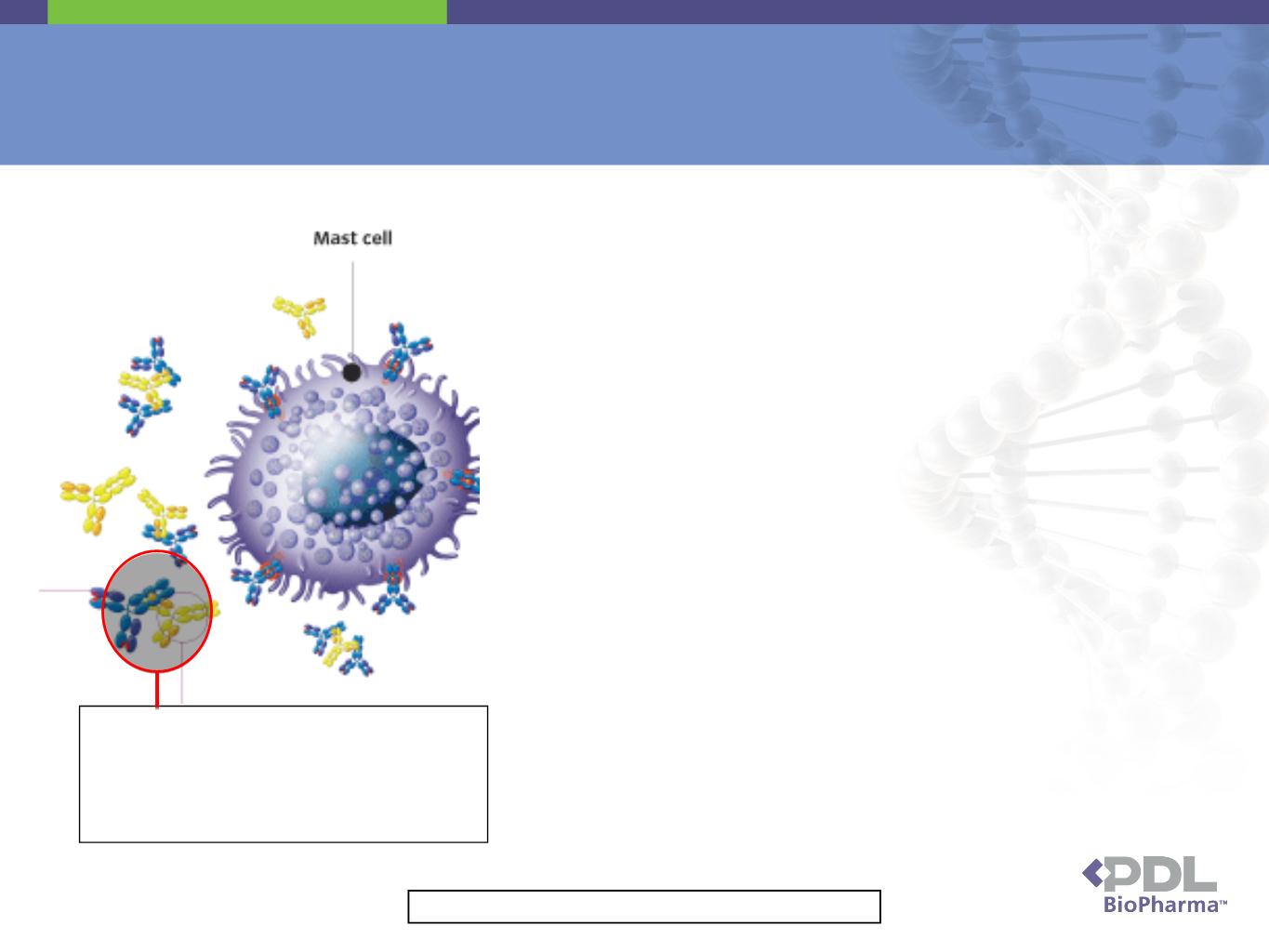
Xolair
45
• Licensor
▪ Genentech (US) and Novartis (ex-US)
• Mechanism
▪ IgE plays a role in allergic disease by
causing the release of inflammatory
mediators from mast cells that result in
sneezing, wheezing and asthma
causing the release of inflammatory
mediators from mast cells that result in
sneezing, wheezing and asthma
▪ Xolair binds to and neutralizes circulating
IgE by preventing IgE from binding to its
mast-cell receptor
IgE by preventing IgE from binding to its
mast-cell receptor
• Approvals
▪ Moderate-to-severe persistent asthma
• Sales
▪ 2010 worldwide net sales of $1.0 billion1
Xolair antibody (yellow) binds to IgE
(blue) preventing IgE from binding to
mast cell. Otherwise, IgE binding to
mast cell would result in wheezing,
sneezing and asthma.
(blue) preventing IgE from binding to
mast cell. Otherwise, IgE binding to
mast cell would result in wheezing,
sneezing and asthma.
1. As reported to PDL by its licensee

Tysabri
46
In MS, the body’s
autoimmune system is
inappropriately activated,
resulting in it attacking the
body. Here, defense cells,
known as T cells, are
activated.
autoimmune system is
inappropriately activated,
resulting in it attacking the
body. Here, defense cells,
known as T cells, are
activated.
Activated T cells are able
to cross the blood brain
barrier affording them
access to nerve cells.
to cross the blood brain
barrier affording them
access to nerve cells.
Activated T cells attack,
and recruit other defense
cells known as
macrophages, to attack
and consume the myelin
sheath or insulation
surrounding nerve fibers.
The resulting holes in the
myelin slow the
transmission of impulses
along the nerve and cause
the symptoms of MS.
and recruit other defense
cells known as
macrophages, to attack
and consume the myelin
sheath or insulation
surrounding nerve fibers.
The resulting holes in the
myelin slow the
transmission of impulses
along the nerve and cause
the symptoms of MS.
1. As reported to PDL by its licensee

Actemra
47
• Licensor
▪ Roche and Chugai
• Mechanism
▪ Rheumatoid arthritis (RA) is an autoimmune disease in
which the body's immune system attacks itself
which the body's immune system attacks itself
▪ One of the defense mechanisms inappropriately activated
in RA is IL-6, which can result in destruction of the cartilage
between joints causing the symptoms of RA
in RA is IL-6, which can result in destruction of the cartilage
between joints causing the symptoms of RA
▪ Actemra binds to and neutralizes IL-6 preventing it from
destroying cartilage thereby blocking one of the causes of
RA
destroying cartilage thereby blocking one of the causes of
RA
• Approvals
▪ Treatment of signs and symptoms in moderate-to-severe
adult RA patients, slowing of structural damage to joints
caused by RA and preservation physical function of joints
afflicted by RA
adult RA patients, slowing of structural damage to joints
caused by RA and preservation physical function of joints
afflicted by RA
• Sales
▪ 2010 worldwide net sales of $459 million1
It is the degradation and
eventual destruction of this
cartilage that causes the
symptoms of RA.
eventual destruction of this
cartilage that causes the
symptoms of RA.
1. As reported by Roche; assume 1.155 CHF/USD
