Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 000-19756

PDL BioPharma, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 94-3023969 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
932 Southwood Boulevard
Incline Village, Nevada 89451
(Address of principal executive offices)
Registrant’s telephone number, including area code
(775) 832-8500
Securities registered pursuant to Section 12(b) of the Act:
| Title of Class |
Name of Exchange on which Registered | |
| Common Stock, par value $0.01 per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File to be submitted required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
| Large accelerated filer x |
Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company ¨ | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of shares of common stock held by non-affiliates of the registrant, based upon the closing sale price of a share of common stock on June 30, 2009 (the last business day of the registrant’s most recently completed second fiscal quarter), as reported on the NASDAQ Global Select Market, was $702,185,330.
As of February 25, 2010, the registrant had outstanding 119,674,377 shares of common stock.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s proxy statement to be delivered to stockholders with respect to the registrant’s 2010 Annual Meeting of Stockholders to be filed by the registrant with the U.S. Securities and Exchange Commission (hereinafter referred to as the “Proxy Statement”) are incorporated by reference into Part III of this Annual Report on Form 10-K. The registrant intends to file its proxy statement within 120 days after its fiscal year end.
Table of Contents
PDL BIOPHARMA, INC.
2009 Form 10-K Annual Report
| PART I |
||||
| Item 1 |
1 | |||
| Item 1A |
8 | |||
| Item 1B |
15 | |||
| Item 2 |
15 | |||
| Item 3 |
15 | |||
| Item 4 |
18 | |||
| PART II |
||||
| Item 5 |
19 | |||
| Item 6 |
20 | |||
| Item 7 |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
22 | ||
| Item 7A |
33 | |||
| Item 8 |
35 | |||
| Item 9 |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
70 | ||
| Item 9A |
70 | |||
| Item 9B |
72 | |||
| PART III |
||||
| Item 10 |
72 | |||
| Item 11 |
72 | |||
| Item 12 |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
72 | ||
| Item 13 |
Certain Relationships and Related Transactions, and Director Independence |
72 | ||
| Item 14 |
72 | |||
| PART IV |
||||
| Item 15 |
72 | |||
| 78 | ||||
Table of Contents
PART I
Forward-looking Statements
This Annual Report contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including any projections of earnings, revenues or other financial items, any statements of the plans and objectives of management for future operations, including any statements concerning new licensing, any statements regarding future economic conditions or performance, and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “may,” “will,” “intends,” “plans,” “believes,” “anticipates,” “expects,” “estimates,” “predicts,” “potential,” “continue” or “opportunity,” or the negative thereof or other comparable terminology. Although we believe that the expectations presented in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our future financial condition and results of operations, as well as any forward-looking statements, are subject to inherent risks and uncertainties, including but not limited to the risk factors set forth below, and for the reasons described elsewhere in this Annual Report. All forward-looking statements and reasons why results may differ included in this Annual Report are made as of the date hereof, and we assume no obligation to update these forward-looking statements or reasons why actual results might differ.
As used in this Annual Report, the terms “we,” “us,” “our,” the “Company” and “PDL” mean PDL BioPharma, Inc. after giving effect to the spin-off described below (unless the context indicates a different meaning). Unless otherwise indicated, our consolidated financial information included in this Annual Report gives effect to the presentation of our biotechnology operations, which we spun-off in December 2008, as discontinued operations and to the presentation of our commercial and manufacturing operations, for which we completed the divestiture in March 2008, also as discontinued operations.
We own or have rights to certain trademarks, trade names, copyrights and other intellectual property used in our business, including PDL BioPharma and the PDL logo, each of which is considered a trademark. All other company names, product names, trade names and trademarks included in this Annual Report are trademarks, registered trademarks or trade names of their respective owners.
| ITEM 1. | BUSINESS |
Overview
We were organized as a Delaware corporation in 1986 under the name Protein Design Labs, Inc. In 2006, we changed our name to PDL BioPharma, Inc. Our business is the management of our antibody humanization patents and royalty assets which consist of our Queen et al. patents and license agreements with numerous biotechnology and pharmaceutical companies pursuant to which we have licensed certain rights under our Queen et al. patents. We receive royalties based on these license agreements on sales of a number of humanized antibody products marketed today and also may receive royalty payments on additional humanized antibody products launched before final patent expiry in 2014. Under most of our licensing agreements, we are entitled to receive a flat-rate or tiered royalty based upon our licensees’ net sales of covered antibodies.
Until December 2008, our business included a biotechnology operation which was focused on the discovery and development of novel antibodies which we spun-off (the Spin-Off) to Facet Biotech Corporation (Facet). From March 2005 until March 2008, we also had commercial and manufacturing operations which we partially divested in 2006 and fully divested in 2008. The financial results of our former biotechnology and manufacturing operations as well as our former commercial operation are presented as discontinued operations in the Consolidated Statements of Operations.
1
Table of Contents
We intend to distribute our income, net of operating expenses, debt service, income taxes and other corporate activities to our stockholders. In 2009, we paid two cash dividends of $59.7 million or $0.50 per share of common stock to our stockholders on April 1, 2009 and October 1, 2009. In December 2009, we paid an additional cash dividend of $199.6 million or $1.67 per share of common stock to our stockholders using a portion of the proceeds from the securitization transaction which is described below. On January 27, 2010, our board of directors declared two cash dividends of $0.50 per share of common stock payable on April 1, 2010 and October 1, 2010 to stockholders of record on March 15, 2010 and September 15, 2010, respectively.
Securitization Transaction
In November 2009, we completed a $300 million securitization transaction in which we monetized 60% of the net present value of the estimated five year royalties from sales of Genentech products (the Genentech Royalties) including Avastin®, Herceptin®, Lucentis®, Xolair®, and future products, if any, under which Genentech may take a license under our related agreements with Genentech. The $300 million QHP PhaRMA Senior Secured Notes due 2015 (the QHP Notes) bear interest at 10.25% per annum and were issued in a non-registered offering by QHP Royalty Sub LLC (QHP), a Delaware limited liability company, and a newly formed, wholly-owned subsidiary of PDL. Concurrent with the securitization transaction and pursuant to the terms of a purchase and sale agreement, we sold, transferred, conveyed, assigned, contributed and granted to QHP, certain rights under our non-exclusive license agreements with Genentech including the right to receive the Genentech Royalties in exchange for QHP’s proceeds from the QHP Notes issuance. The Genentech Royalties and other payments, if any, that QHP will be entitled to receive under the agreements with Genentech, together with any funds made available from certain accounts of QHP, will be the sole source of payment of principal and interest on the QHP Notes. Once all obligations on the QHP Notes have been paid in full, including all other sums payable under the indenture, then the indenture shall cease to be of further effect and all of the security interests in the collateral shall terminate, including the pledge by PDL to the trustee of its equity interest in QHP. At such point, there will be no further restrictions on the Genentech Royalties and PDL shall be free to either keep them in QHP, transfer them back to PDL, the parent company, or to further dispose or monetize them. The anticipated final repayment date of the QHP Notes is December 2012.
Patents and Technology Out-License Agreements
Patents
We have been issued patents in the United States and elsewhere, covering the humanization of antibodies, which we refer to as our Queen et al. patents. Our Queen et al. patents, for which final patent expiry is in December 2014, cover, among other things, humanized antibodies, methods for humanizing antibodies, polynucleotide encoding in humanized antibodies and methods of producing humanized antibodies.
The following is a list of our U.S. and European patents within our Queen et al. patent portfolio:
| Application Number |
Filing Date |
Patent Number |
Issue Date |
Jurisdiction | ||||
| 08/477,728 |
06/07/95 | 5,585,089 | 12/17/96 | United States | ||||
| 08/474,040 |
06/07/95 | 5,693,761 | 12/02/97 | United States | ||||
| 08/487,200 |
06/07/95 | 5,693,762 | 12/02/97 | United States | ||||
| 08/484,537 |
06/07/95 | 6,180,370 | 01/30/01 | United States | ||||
| 09/718,998 |
11/22/00 | 7,022,500 | 04/04/06 | United States | ||||
| 90903576.8 |
12/28/89 | 0 451 216B | 01/24/96 | Europe |
Our European Patent No. 0 451 216B (the ‘216 Patent) expired in December 2009. We have applied for and been granted Supplemental Protection Certificates (SPCs) with respect to the Herceptin®, Synagis®, Xolair®, Raptiva®, Avastin®, Tysabri® and Lucentis® products in many of the jurisdictions in the European Union in connection with the ‘216 Patent. We have also filed SPC applications for Cimzia® in countries of the European Union based on the
2
Table of Contents
‘216 Patent. These SPCs, upon grant thereof, effectively extend our patent protection with respect to these products generally until December 2014, except that the SPCs for Raptiva, Herceptin, and Synagis will generally expire in March 2013, July 2014 and August 2014, respectively. Because SPCs are granted on a jurisdiction-by-jurisdiction basis, the duration of the extension varies slightly in certain jurisdictions. We are not able to file applications for any SPCs after December 2009. Therefore, if a product is first approved for marketing after December 2009 in a jurisdiction that issues SPCs, we will not have patent protection or SPC protection in this jurisdiction with respect to this product. We may still be eligible for royalties notwithstanding the unavailability of SPC protection if the relevant royalty-bearing humanized antibody product is also made, used, sold or offered for sale in or imported from a jurisdiction in which we have an unexpired Queen et al. patent such as the United States.
We are currently in an opposition proceeding with respect to the ‘216 Patent at the European Patent Office. MedImmune filed a declaratory judgment against us related to the Queen et al. patents in December 2008. In February 2009, the U.S. Patent and Trademark Office (PTO) declared an interference proceeding between our U.S. Patent No. 5,585,089 (the ‘089 Patent) and a patent application pending to Adair et al. and, on November 23, 2009, the PTO declared a second interference proceeding between certain claims of the U.S. Patent No. 6,180,370 (the ‘370 Patent) and certain pending claims of Adair et al. UCB Pharma S.A. is the assignee of the Adair et al. applications. See “Item 3—Legal Proceedings.”
Licensing Agreements
We have entered into licensing agreements with numerous entities that are independently developing or have developed humanized antibodies pursuant to which we have licensed certain rights under our Queen et al. patents to make, use, sell, offer for sale and import humanized antibodies. We receive royalties on net sales of products that are made, used or sold prior to patent expiry. In general, these agreements cover antibodies targeting antigens specified in the license agreements. Under most of our licensing agreements, we are entitled to receive a flat-rate or tiered royalty based upon our licensees’ net sales of covered antibodies. Our licensing agreements generally entitle us to royalties following the expiration of our patents with respect to products manufactured prior to patent expiry. We also expect to receive minimal annual maintenance fees from licensees of our Queen et al. patents.
Licensing Agreements for Marketed Products
In each of the years ended December 31, 2009, 2008 and 2007, we received royalties on sales of the nine humanized antibody products listed below, all of which are currently approved for use by the U.S. Food and Drug Administration (FDA) and eight are approved by other regulatory agencies outside the United States. Approval for one of the products, Raptiva, which was marketed by Genentech in the United States and Merck Serono S.A. outside of the United States, was suspended in the European Union and Canada in February 2009 and the product was withdrawn from the United States market in April 2009 due to safety concerns. Thus, we do not expect to receive material amounts of royalties on future sales of Raptiva. For the year ended December 31, 2009, we received $1.2 million in royalties for sales of Raptiva as compared with $3.9 million and $3.7 million for the same periods in 2008 and 2007, respectively. Also, in December 2009, we declared MedImmune in breach of its license agreement with us and canceled the license agreement. For the year ended December 31, 2009, we received $40.7 million in royalties for sales of MedImmune’s Synagis product compared with $40.2 million and $36.7 million for the same periods in 2008 and 2007, respectively. See “Item 3—Legal Proceedings.”
3
Table of Contents
In 2009, 2008 and 2007, we received approximately $305.0 million, $278.7 million and $224.7 million, respectively, of royalty revenues under the license agreements. The licensees with commercial products are identified below:
| Licensees |
Product Names | |
| Genentech, Inc. (Genentech) |
Avastin® | |
| Herceptin® | ||
| Xolair® | ||
| Raptiva® | ||
| Lucentis® | ||
| MedImmune, LLC (MedImmune)(1) |
Synagis® | |
| Elan Corporation, Plc (Elan) |
Tysabri® | |
| Wyeth Pharmaceuticals, Inc. (Wyeth) |
Mylotarg® | |
| Chugai Pharmaceutical Co., Ltd. (Chugai) |
Actemra® /RoActemra® | |
| (1) | In December 2009, we canceled the MedImmune license agreement. See “Item 3-Legal Proceedings.” |
Genentech
We entered into a master patent license agreement, effective September 25, 1998 pursuant to which we granted Genentech a license under our Queen et al. patents to make, use and sell certain antibody products. Our master patent license agreement with Genentech provides for a tiered royalty structure under which the royalty rate Genentech must pay on royalty-bearing products sold in the United States or manufactured in the United States and used or sold anywhere in the world (U.S.-based Sales) in a given calendar year decreases on incremental U.S.-based Sales above certain net sales thresholds. The net sales thresholds and the applicable royalty rates are outlined below:
| Aggregate Net Sales |
Royalty Rate | ||
| Net sales up to $1.5 billion |
3.0 | % | |
| Net sales between $1.5 billion and $2.5 billion |
2.5 | % | |
| Net sales between $2.5 billion and $4.0 billion |
2.0 | % | |
| Net sales exceeding $4.0 billion |
1.0 | % |
As a result of the tiered royalty structure, Genentech’s average annual royalty rate for a given year will decline as Genentech’s U.S.-based Sales increase during that year. Because we receive royalties one quarter in arrears, the average royalty rates for the payments we receive from Genentech in the second calendar quarter for Genentech’s sales from the first calendar quarter have been and are expected to continue to be higher than the average royalty rates for following quarters. The average royalty rates for payments we receive from Genentech are generally lowest in the fourth and first calendar quarters for Genentech’s sales from the third and fourth calendar quarters when Genentech’s U.S.-based Sales bear royalties at the lowest royalty rates.
With respect to royalty-bearing products that are both manufactured and sold outside of the United States (ex-U.S.-based Manufacturing and Sales), the royalty rate that we receive from Genentech is a fixed rate of 3.0% based on a percentage of the underlying ex-U.S.-based Manufacturing and Sales. The mix of U.S.-based Sales and ex-U.S.-based Manufacturing and Sales has fluctuated in the past and may continue to fluctuate in future periods, particularly in light of the 2009 acquisition of Genentech by the Roche Group (Roche). For example, in July 2009 Roche announced its decision to partially close its manufacturing site in Vacaville, California.
4
Table of Contents
The mix of U.S.-based sales and ex-U.S. based Manufacturing and Sales is outlined in the following table:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| U.S. Based Sales |
88 | % | 85 | % | 86 | % | |||
| Ex-U.S. Based Manufacturing and Sales |
12 | % | 15 | % | 14 | % | |||
The information in the table above is based on information provided to us by Genentech. We were not provided the reasons for the shift in the manufacturing split between U.S.-based Sales and ex-U.S.-based Manufacturing and Sales.
In 2009, two of Genentech’s licensed products, Herceptin and Xolair, generated royalties from ex-U.S.-based Manufacturing and Sales. In the first quarter of 2010, for sales occurring in the fourth quarter of 2009, we received royalties for the first time on sales of Avastin that were ex-U.S. based Manufactured and Sold. Roche has also announced that there are new plants in Singapore for the production of Avastin and Lucentis. The Genentech agreement continues until the expiration of the last to expire of our Queen et al. patents but may be terminated by Genentech prior to such expiration upon 60 days written notice or by us upon a material breach by Genentech. Either party may terminate upon the occurrence of certain bankruptcy-related events.
MedImmune
We entered into a patent license agreement, effective July 17, 1997, with MedImmune pursuant to which we granted to MedImmune a license under our Queen et al. patents to make, use and sell antibodies that bind to respiratory syncytial virus. Pursuant to the agreement, we are entitled to receive a flat royalty rate in the low single digits based on MedImmune’s net sales of its Synagis product. Under the terms of the agreement, the agreement continues until the expiration of the last to expire of our Queen et al. patents but may be terminated by MedImmune prior to such expiration upon thirty days written notice. Either party may terminate the agreement upon a material breach by the other party or upon the occurrence of certain bankruptcy-related events. As discussed below, in December 2009, we declared MedImmune in breach of its obligations under the license agreement, canceled the agreement and revoked any licenses and rights granted therein.
MedImmune filed for approval of its motavizumab product, a second generation of Synagis, in the United States in January 2008 and received a Complete Response Letter from the FDA on December 1, 2008 asking for additional information on motavizumab. Astra Zeneca, which owns MedImmune, said it plans to continue discussions with the FDA and, subject to the outcome of those discussions, resubmitted the application for approval in December 2009. Motavizumab is a next-generation follow-on to Synagis for the treatment of respiratory syncytial virus.
In December 2008, MedImmune filed a lawsuit against us in the United States District Court for the Northern District of California seeking a declaratory judgment that the U.S. Queen et al. patents are invalid and/or not infringed by its Synagis and motavizumab products, and that therefore MedImmune owes no royalties under its license agreement with us. In April 2009, MedImmune amended its complaint to allege that, even if our patents are valid and infringed by Synagis and/or motavizumab, MedImmune is now or may have been retroactively entitled to a lower royalty rate on its sales of infringing products under the most favored licensee clause in our agreement. In May 2009, we filed our answer to MedImmune’s lawsuit asserting certain counterclaims and affirmative defenses and requested that the court find (i) that Synagis and motavizumab fall under the scope of the Queen et al. patents and that the sale thereof requires that MedImmune pay us royalties as specified in our license agreement with them; (ii) that the claims we are asserting against MedImmune are valid; (iii) that MedImmune is not entitled to different terms, including a lower royalty rate, as a result of our settlement with Alexion; and (iv) that MedImmune is liable for attorney’s fees and costs related to the action. On August 18, 2009, we attended a mandatory settlement conference with MedImmune held before the Federal District Court for Northern California. No settlement resulted from the meeting. A Markman claim construction hearing took
5
Table of Contents
place on November 5, 2009. A decision was issued from the court on February 22, 2010. The court generally construed the claim language at issue as proposed by PDL.
In December 2009, we sent a letter to MedImmune stating that it is in breach of its obligations under the license agreement, canceling the license agreement and revoking any licenses and rights granted therein. Also in December 2009, we filed a First Amended Answer, Defenses and Counterclaims (the Amended Pleadings) alleging that MedImmune breached the license agreement by (i) failing to pay all royalties due to us from the sale of Synagis, including sales by and through Abbott Laboratories, whom we believe is MedImmune’s exclusive sales representative for such sales outside the United States, and (ii) by demanding that we consent to conditions that are commercially unreasonable and contractually insupportable in order to permit an audit of sales and revenue associated with Synagis by an independent accountant, as required under the license agreement and allege that, as a result of MedImmune’s breach of the license agreement and the Company’s cancelation thereof, MedImmune is infringing the Company’s U.S. Patent No. 6,180,370 by making, using, selling, offering for sale and/or importing Synagis into the United States and by having Synagis made, used, sold, offered for sale and/or imported into the United States. We have requested that the court award to the Company damages resulting from MedImmune’s breach of the license agreement, treble damages resulting from MedImmune’s infringement of the Company’s patent rights, attorney’s fees, and an injunction to prevent MedImmune from further acts of infringement and further request a jury trial on all issues triable by jury. In December 2009, MedImmune filed a Motion for a preliminary injunction against our cancelation of the license agreement and a motion to strike our Amended Pleadings. In January 2010, MedImmune filed a motion for summary judgment seeking a declaratory judgment from the court that MedImmune is entitled under the most favored licensee clause in our agreement to a fully paid-up license as of December 2008 as a result of our agreement with Alexion Pharmaceuticals, Inc. (Alexion), see “Item 3—Legal Proceedings,” and, retroactively to 1998, to a reduced royalty rate on sales of Synagis. A hearing was held on February 26, 2010, related to the preliminary injunction motion, motion to strike and motion for summary judgment. Trial is scheduled to commence on June 14, 2010 at which issues of infringement, validity and contractual rights are expected to be decided. MedImmune has paid us more than $280 million in royalties under the MedImmune agreement with respect to sales of Synagis since the fourth quarter of 1998 through the fourth quarter of 2009. An escrow account has been created for receipt of MedImmune’s February 2010 payment, in which account the funds will be held pendente lite (while the litigation is pending). See “Item 3—Legal Proceedings.”
Elan
We entered into a patent license agreement, effective April 24, 1998, with Elan pursuant to which we granted to Elan a license under our Queen et al. patents to make, use and sell antibodies that bind to the cellular adhesion molecule a4 in patients with multiple sclerosis. Pursuant to the agreement, we are entitled to receive a flat royalty rate in the low single digits based on Elan’s net sales of the Tysabri product. The agreement continues until the expiration of the last to expire of our Queen et al. patents but may be terminated (i) by Elan prior to such expiration upon sixty days written notice, (ii) by either party upon a material breach by the other party or (iii) upon the occurrence of certain bankruptcy-related events.
Wyeth
We entered into a patent license agreement, effective September 1, 1999, with Wyeth pursuant to which we granted to them a license under our Queen et al. patents to make, use and sell antibodies that bind to CD33, an antigen that is found in about 80% of patients with acute myeloid leukemia, and conjugated to a cytotoxic agent. Pursuant to the agreement, we are entitled to receive a flat royalty rate in the low single digits based on Wyeth’s net sales of the Mylotarg product. The agreement continues until the expiration of the last to expire of our Queen et al. patents but may be terminated (i) by Wyeth prior to such expiration upon sixty days written notice, (ii) by either party upon a material breach by the other party or (iii) upon the occurrence of certain bankruptcy-related events.
6
Table of Contents
Chugai
We entered into a patent license agreement, effective May 18, 2000, with Chugai, a majority owned subsidiary of Roche, pursuant to which we granted to Chugai a license under our Queen et al. patents to make, use and sell antibodies that bind to interleukin-6 receptor to prevent inflammatory cascades involving multiple cell types for the treatment of rheumatoid arthritis. Pursuant to the agreement, we are entitled to receive a flat royalty rate in the low single digits based on net sales of the Actemra product (RoActemra in Europe). The agreement continues until the expiration of the last to expire of our Queen et al. patents but may be terminated (i) by Chugai prior to such expiration upon sixty days written notice, (ii) by either party upon a material breach by the other party or (iii) upon the occurrence of certain bankruptcy-related events.
Other
Pursuant to the terms of our Cross License Agreement with Facet, Facet is obligated to pay us a portion of royalties it receives from Roche on sales of the Zenapax® product under an agreement with Roche which was assigned to Facet in connection with the Spin-Off. Roche is obligated to pay Facet royalties only once product sales have reached a certain threshold. We have not received royalties on sales of Zenapax since the first quarter of 2006, and we do not expect to receive royalty revenue from Roche’s sales of Zenapax in the future.
Licensing Agreements for Non-Marketed Products
We have also entered into licensing agreements pursuant to which we have licensed certain rights under our Queen et al. patents to make, use and sell certain products in development that have not yet reached commercialization. Certain of these development-stage products are currently in Phase 3 clinical trials. With respect to these agreements, we may receive milestone payments based on certain development milestones. We may also receive royalty payments if the licensed products receive marketing approval and are manufactured or generate sales before the expiration of our Queen et al. patents. For example, both Eli Lilly and Company (Lilly) and Wyeth have licensed antibodies for the treatment of Alzheimer’s disease that are currently in Phase 3 clinical trials. Another example is teplizumab which is being studied for the treatment of newly-diagnosed type 1 diabetes mellitus and which is the subject of a new license agreement with Lilly that we announced in December 2009.
Major Customers
Our revenues consist almost entirely of royalties, although we also receive periodic milestone payments from licensees of our Queen et al. patents and, in future periods, we may continue to receive milestone payments if the licensed products in development achieve certain development milestones as well as royalty payments if the licensed products receive marketing approval and are manufactured or generate sales before the expiration of our Queen et al. patents. In 2009, 2008 and 2007, Genentech accounted for 71%, 73% and 79% of our revenues, respectively; MedImmune accounted for 13%, 14% and 16% of our revenues, respectively; and Elan accounted for 9%, 7% and 3% of our revenues, respectively.
Employees
As of March 1, 2010, we had seven full-time employees and two part-time employees managing our intellectual property, our licensing operations and other corporate activities as well as providing for certain essential reporting and management functions of a public company. None of our employees are covered by a collective bargaining agreement.
Available Information
We file electronically with the Securities and Exchange Commission (SEC) our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. The public may read and copy any materials we file with the SEC
7
Table of Contents
at the SEC’s Public Reference Room at 450 Fifth Street, NW, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that website is www.sec.gov.
We make available free of charge on or through our website at www.pdl.com our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and proxy statements, as well as amendments to these reports and statements, as soon as practicable after we have electronically filed such material with, or furnished them to, the SEC. You may also obtain copies of these filings free of charge by calling us at (775) 832-8500. Also, our Audit Committee Charter, Compensation Committee Charter, Nominating and Governance Committee Charter, Litigation Committee Charter, Corporate Governance Guidelines and Code of Business Conduct are also available free of charge on our website or by calling the number listed above.
| ITEM 1A. | RISK FACTORS |
You should carefully consider and evaluate all of the information included and incorporated by reference in this Annual Report, including the risk factors listed below. Any of these risks, as well as other risks and uncertainties, could materially and adversely affect our business, results of operations and financial condition, which in turn could materially and adversely affect the trading price of shares of our common stock. Additional risks not currently known or currently material to us may also harm our business.
Keep these risk factors in mind when you read forward-looking statements contained in this Annual Report and the documents incorporated by reference in this Annual Report. These statements relate to our expectations about future events and time periods. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “intends,” “plans,” “believes,” “anticipates,” “expects,” “estimates,” “predicts,” “potential,” “continue” or “opportunity,” the negative of these words or words of similar import. Similarly, statements that describe our reserves and our future plans, strategies, intentions, expectations, objectives, goals or prospects are also forward-looking statements. Forward-looking statements involve risks and uncertainties, and future events and circumstances could differ significantly from those anticipated in the forward-looking statements.
Our antibody humanization patents, which are of significant value to us, are being challenged in various administrative proceedings and a successful challenge could limit our future revenues.
Two of the Queen et al. patents were issued to us by the European Patent Office, the ‘216 Patent and the European Patent No. 0 682 040 Patent (the ‘040 Patent). Eighteen notices of opposition to our ‘216 Patent and eight notices of opposition to our ‘040 Patent were filed by major pharmaceutical and biotechnology companies, among others. On October 14, 2009, the European Patent Office Technical Board of Appeal upheld the Opposition Division’s revocation of our ‘040 Patent on formal issues. The Technical Board of Appeal did not consider substantive issues of patentability. Each of our granted and applied for SPCs are based on the ‘216 Patent. As a result, the European Patent Office Technical Board of Appeal’s decision regarding the ‘040 Patent will not have an effect on our right to receive royalties beyond December 27, 2009. However, an adverse decision in the pending European opposition to our ‘216 Patent will have a material negative impact on our ability to collect royalties on European sales of our licensees’ products manufactured outside the United States and could encourage challenges to our related Queen et al. patents in other jurisdictions including the United States.
In addition, the PTO has declared interference proceedings between certain claims of our patents and certain pending claims of Adair et al under 35 U.S.C. Section 135(a). On February 25, 2009, Interference No. 105,688 was declared between certain claims of the ‘089 Patent and certain pending claims of Adair et al., U.S. Application No. 08/846,658 (the ‘658 Application), and on November 23, 2009, Interference No. 105,705 was declared between certain claims of the ‘370 Patent and certain pending claims of Adair et al., U.S. Application 10/938,117 (the ‘117 Application). Any final decision in an interference proceeding, if adverse to the claim of an applicant, is a final refusal by the PTO of the claims involved. A final judgment adverse to us from which no
8
Table of Contents
appeal or other review has been or can be taken or had constitutes cancellation of the claims involved in the patent and could have a material negative impact on our ability to collect royalties on the sale or manufacture of licensees products in the United States. The ‘216 Patent is also discussed in the following risk factor. See “Item 3—Legal Proceedings.”
Our right to continue to collect royalties based on European sales and manufacture may depend on our ability to enforce our ‘216 Patent claims under our supplementary protection certificates.
We have applied for SPCs under our ‘216 Patent in various European national patent offices to cover Avastin, Herceptin, Xolair, Lucentis, Synagis, Tysabri and Cimzia (collectively, the SPC Products). Because our ‘216 Patent expired on December 28, 2009, our ability to collect revenues for manufacture and sale of the SPC Products in Europe will depend on the enforceability of the patent claims underlying our SPCs which generally expire in 2014. These SPCs extend the enforceability of our ‘216 Patent against the SPC Products, but are subject to the varying, complex and evolving national requirements and standards for enforcement of patent claims pursuant to SPCs. As a result of these factors, we are unable to predict the extent of protection afforded by our SPCs.
We do not anticipate continuing to receive royalties on MedImmune’s sales of Synagis until resolution of our lawsuit with them and, depending on the outcome of that lawsuit, may have to repay previously received royalties.
MedImmune has filed a lawsuit seeking a declaratory judgment that the U.S. Queen et al. patents are invalid, that Synagis and motavizumab do not infringe such patents and therefore no royalties are owed on the Synagis product or motavizumab development product. MedImmune has also asserted that it is entitled to pay a lower royalty rate or no royalty under the most favored licensee clause in our agreement because of other license agreements between the Company and third parties. We have canceled the license agreement because we believe that MedImmune breached the license agreement by failing to pay all royalties due and effectively blocking our contractual right to audit MedImmune’s sales. As a result, we have amended our pleadings in the lawsuit to allege that MedImmune has breached the contract and to request contract damages. Our Amended Pleadings also allege that, as a result of cancelation of the license agreement, sales of Synagis constitute patent infringement and we have requested an injunction against further infringement and treble damages. In the event that MedImmune prevails on the claims in its complaint, we expect that MedImmune will request the court to order a recoupment of payments made to PDL which represent obligations under its license to the Queen et al. patents that have accrued since the date of their claim in December 2008. MedImmune requested that the court order that its February 2010 payment be made to an escrow account pendente lite (while the litigation is pending) and an account was created for this purpose. We may not receive additional payments from MedImmune unless and until the lawsuit is resolved in our favor. In addition, if MedImmune is successful in showing that it has made payments to PDL in excess of its license obligations, we expect that MedImmune will request the court to order recoupment of such excess payments. In the event that we prevail on our claims of patent infringement and breach of the license agreement, we will request that MedImmune pay damages for breach of the license, pay treble damages for patent infringement and either desist further infringement or pay royalties at a rate to be determined. See “Item 3—Legal Proceedings.”
We must protect our patent and other intellectual property rights to succeed.
Our success is dependent in significant part on our ability to protect the scope, validity and enforceability of our patents. The scope, validity, enforceability and effective term of patents can be highly uncertain and often involve complex legal and factual questions and proceedings. When our patents are challenged, they may be invalidated, circumvented or rendered unenforceable by the adjudicating tribunal. A finding that some or all of our patent rights are invalid or unenforceable in such a proceeding may have a material impact on our ability to continue to collect royalty payments from our licensees or conclude new royalty generating license agreements. Similarly, a finding narrowing the scope of some or all of our patent rights could likewise have a material impact on our ability to continue to collect royalty payments from our licensees or conclude new license agreements.
9
Table of Contents
U.S. patents and patent applications may also be subject to interference proceedings and to reexamination or reissue proceedings in the PTO and foreign patents may be subject to opposition or nullification or similar proceedings in the various national and regional patent offices in which we have applied for and received patent rights and/or SPCs. Any of these proceedings could result in either loss of the patent or loss or reduction in the scope of one or more of the claims of the patent. These proceedings could be expensive, last several years and result in a significant reduction in the scope or invalidation of our patents. Any limitation in claim scope could reduce our ability to collect royalties or commence enforcement proceedings based on these patents. Moreover, the scope of a patent in one country does not assure similar scope of a patent with similar claims in another country. Also, claim interpretation and infringement laws vary among countries. As a result of these factors, we are unable to predict the extent of patent protection in any country. See “Item 3—Legal Proceedings.”
We derive a significant portion of our royalty revenues from Genentech and our future success depends on continued market acceptance of their products and approval of their licensed products that are in development.
Our revenues consist almost entirely of royalties from licensees of our Queen et al. patents and, in future periods, we may receive milestone payments if the licensed products in development achieve certain development milestones and royalty payments if the licensed products receive marketing approval before the expiration of our Queen et al. patents. Genentech accounted for 71%, 73% and 79% of our revenues from continuing operations for the years ended December 31, 2009, 2008 and 2007, respectively. Our future success depends primarily upon the continued market acceptance of Genentech and other licensee’s commercialized products and the performance by our licensees of their obligations under the applicable license agreements. In addition, our ability to generate royalty revenue depends upon the ability of Genentech and our other licensees to develop, introduce and deliver products that achieve and sustain market acceptance. For example, 60% of the royalties we currently receive from Genentech are dedicated to service the debt related to the QHP Notes that we, through our wholly-owned subsidiary, QHP, issued in November 2009. We have no control over the sales efforts of Genentech and our other licensees, and our licensees might not be successful. Reductions in the sales volume or average selling price of licensed products could have a material adverse effect on our business.
Our licensees may be unable to maintain regulatory approvals for currently licensed products or obtain regulatory approvals for new products. Safety issues could also result in the failure to maintain regulatory approvals or decrease revenues.
Our licensees are subject to stringent regulation with respect to product safety and efficacy by various international, federal, state and local authorities. Of particular significance are the FDA’s requirements covering research and development, testing, manufacturing, quality control, labeling and promotion of drugs for human use in the United States. As a result of these requirements, the length of time, the level of expenditures and the laboratory and clinical information required for approval of a biologic license application or new drug application are substantial and can require a number of years. In addition, even if our licensees’ products receive regulatory approval, they remain subject to ongoing FDA and other international regulations including, but not limited to, obligations to conduct additional clinical trials or other testing, changes to the product label, new or revised regulatory requirements for manufacturing practices, written advisements to physicians and/or a product recall or withdrawal. Our licensees may not maintain necessary regulatory approvals for their existing licensed products or our licensees may not obtain necessary regulatory approvals on a timely basis, if at all, for any of the licensed products our licensees are developing or manufacturing. The occurrence of adverse events reported by any licensee may result in the revocation of regulatory approvals or decreased sales of the applicable product due to a change in physicians’ willingness to prescribe, or patients’ willingness to use the applicable product. In either case, our revenues could be materially and adversely affected.
10
Table of Contents
For example, in February 2005, Elan and Biogen Idec Inc. (Biogen Idec) announced that they had voluntarily suspended the marketing and commercial distribution of Tysabri, a drug approved for the treatment of multiple sclerosis that is licensed under Queen et al. patents, because of the occurrence of progressive multifocal leukoencephalopathy (PML), a rare and frequently fatal, demyelinating disease of the central nervous system, in certain patients treated with Tysabri. In July 2006, Elan and Biogen Idec reintroduced Tysabri; however, Tysabri’s label now includes prominent warnings regarding the Tysabri’s risks and Elan and Biogen Idec have implemented a risk management program to inform physicians and patients of the benefits and risks of Tysabri and to minimize the risk of PML potentially associated with Tysabri. Regulatory authorities worldwide continue to monitor the safety and efficacy of Tysabri. If physicians prescribe Tysabri less frequently due to the PML risk, or if Elan and Biogen Idec or various regulatory authorities suspend the marketing of Tysabri, the amount of royalties we receive will be adversely affected.
Another example is Raptiva, Genentech’s product for the treatment for psoriasis for which marketing approval was suspended in Europe and in Canada in February 2009 and was then withdrawn from the worldwide market due to safety concerns. We no longer receive royalties on sales of Raptiva.
In addition, the current regulatory framework could change or additional regulations could arise at any stage during our licensees’ product development or marketing which may affect our licensees’ ability to obtain or maintain approval of their licensed products. Delays in our licensees receiving regulatory approval for licensed products or their failure to maintain existing regulatory approvals could have a material adverse effect on our business.
Our licensees face competition.
Our licensees face competition from other pharmaceutical and biotechnology companies. The introduction of new competitive products or follow-on biologics may result in lost market share for our licensees, reduced utilization of licensed products, lower prices and/or reduced licensed product sales, any of which could reduce our royalty revenue and have a material adverse effect on our results of operations.
Our revenues and operating results will likely fluctuate in future periods.
Our royalty revenues may be unpredictable and fluctuate because they depend upon, among other things, the seasonality and rate of growth of sales of licensed products as well as the mix of U.S.-based Sales and ex-U.S.-based Manufacturing and Sales in connection with our master patent license agreement with Genentech.
The Genentech agreement provides for a tiered royalty structure under which the royalty rate Genentech must pay on the U.S.-based Sales in a given calendar year decreases on incremental U.S.-based Sales above certain net sales thresholds. As a result of the tiered royalty structure, Genentech’s average annual royalty rate for a given year declines as Genentech’s U.S.-based Sales increase during that year. Because we receive royalties one quarter in arrears, the average royalty rate for the payments we receive from Genentech in the second calendar quarter—which would be for Genentech’s sales from the first calendar quarter—has been and is expected to continue to be higher than the average royalty rate for following quarters. The average royalty rate for payments we receive from Genentech is generally lowest in the fourth quarter and first calendar quarter of the following year, which would be for Genentech’s sales from the third and fourth calendar quarter, when Genentech’s U.S.-based Sales bear royalties at the lowest royalty rate. With respect to the ex-U.S.-based Manufacturing and Sales, the royalty rate that we receive from Genentech is a fixed rate of 3.0% based on a percentage of the underlying ex-U.S.-based Manufacturing and Sales. The mix of U.S.-based Sales and ex-U.S.-based Manufacturing and Sales has fluctuated in the past and may continue to fluctuate in future periods, particularly in light of the 2009 acquisition of Genentech by Roche. For example, in July 2009 Roche announced its decision to partially close its manufacturing site in Vacaville, California.
Approximately 13% of our royalty revenues for the year ended December 31, 2009 are from sales of Synagis, which is marketed by MedImmune. This product has significantly higher sales in the fall and winter, which to date have resulted in much higher royalties paid to us in our first and second quarters than in other quarters. It is
11
Table of Contents
not known if we will continue to receive royalties on sales of Synagis in 2010 and, if we do so, the seasonality of Synagis sales may continue to contribute to fluctuation in our revenues from quarter to quarter. See “Item 3—Legal Proceedings.”
We intend to reserve from time to time a certain amount of cash in order to satisfy the obligations relating to our convertible notes, which could adversely affect the amount or timing of distributions to our stockholders.
As of December 31, 2009, we had $228 million in principal that remains outstanding under our 2.00% Convertible Senior Notes due February 15, 2012 (the 2012 Notes) and $200 million in principal that remains outstanding under our unsecured 2.75% Convertible Subordinated Notes due 2023 (the 2023 Notes). The 2012 Notes are our senior unsecured debt and have been redeemable by us in whole or in part since February 19, 2010 at 100.57% of principal amount if redeemed between February 19, 2010 and February 14, 2011 and at 100.29% of principal amount if redeemed between February 15, 2011 and the maturity date. The 2023 Notes may be redeemed at our option, in whole or in part, at par value. Holders of the 2023 Notes may require us to repurchase all or a portion of their 2023 Notes at 100% of their principal amount plus any unpaid interest for cash on August 16, 2010 and for cash, or, at our option, shares of our common stock at the then-current conversion price on August 16, 2013 and August 16, 2018. Holders of the 2023 Notes may also require us to repurchase all or a portion of the notes cash upon the occurrence of a repurchase event in which a change in control has occurred or our common stock is neither listed on a U.S. national securities exchange nor approved for trading over-the-counter. Similarly, holders of the 2012 Notes may require us to purchase all or any portion of their 2012 Notes at 100% of their principal amount, plus any unpaid interest, upon a fundamental change resulting in the reclassification, conversion, exchange or cancellation of common stock. Such repurchase event or fundamental change is generally defined to include a merger involving PDL, an acquisition of a majority of PDL’s outstanding common stock, and the change of a majority of PDL’s board of directors without the approval of the board of directors.
We intend to reserve from time to time a certain amount of cash in order to satisfy these repurchase or other obligations relating to the 2023 Notes and 2012 Notes which could adversely affect the amount or timing of any distribution to our stockholders. We may continue to redeem, repurchase or otherwise acquire one or both series of convertible notes in the open market in the future either which could adversely affect the amount or timing of any cash distribution to our stockholders.
If any or all of the 2023 Notes or 2012 Notes are not converted into shares of our common stock before their respective maturity dates, we will have to pay the holders of such notes the full aggregate principal amount of the 2023 Notes or 2012 Notes, respectively, then outstanding. Any of the above payments could have a material adverse effect on our cash position. If we fail to satisfy these repurchase or other obligations, it may result in a default under the indenture which could result in a default under certain of our other debt instruments, if any.
The conversion of any of the 2023 Notes or 2012 Notes into shares of our common stock would have a dilutive effect which could cause our stock price to go down.
The 2023 Notes and 2012 Notes are currently convertible at any time, at the option of the holder, into shares of our common stock. We have reserved shares of our authorized common stock for issuance upon conversion of the 2023 Notes and 2012 Notes. If any or all of the 2023 Notes or 2012 Notes are converted into shares of our common stock, our existing stockholders will experience immediate dilution and our common stock price may be subject to downward pressure.
In connection with the cash dividend paid on December 15, 2009 to stockholders of record on December 1, 2009, the conversion rates of the 2023 Notes and 2012 Notes were adjusted upward. The conversion rate for the 2023 Notes, as adjusted, is 164.7254 shares of common stock per $1,000 principal amount or $6.07 per share of common stock. The conversion rate for the 2023 Notes was previously 131.0339 shares of common stock per
12
Table of Contents
$1,000 principal amount of the 2023 Notes. The conversion rate for the 2012 Notes, as adjusted, is 119.294 shares of common stock per $1,000 principal amount or $8.38 per share of common stock. The conversion rate for the 2012 Notes was previously 94.447 shares of common stock per $1,000 principal amount of the 2012 Notes. Because the conversion rates of the 2023 Notes and 2012 Notes adjust upward upon the occurrence of certain events, such as a dividend payment, our existing stockholders will experience more dilution if any or all of the 2023 Notes or 2012 Notes are converted into shares of our common stock after the adjusted conversion rates became effective.
Our common stock may lose value due to several factors, including the expiration of our Queen et al. patents, the payment of dividends or distributions to our stockholders and failure to meet analyst expectations, and our common stock could be delisted from NASDAQ.
Our revenues consist almost entirely of royalties from licensees of our Queen et al. patents which expire in 2013 and 2014. Unless we develop other revenue streams, we will no longer receive patent-related royalties once our licensees have sold all their inventory of licensed product that was manufactured before the expiration of the Queen et al. patents. As a result, our common stock will likely lose value.
If we fail to meet the expectations of securities analysts or investors, or if adverse conditions prevail or are perceived to prevail with respect to our business, the price of the common stock would likely drop significantly.
In addition to all of the risk factors listed herein, the payment of dividends or distributions to our stockholders may reduce the price of our common stock. If the price of our common stock were to fall below NASDAQ listing standards as we approach the date of patent expiration, our common stock may be delisted. If our common stock were delisted, market liquidity for our common stock could be severely affected, and our stockholders’ ability to sell securities in the secondary market could be limited. Delisting from NASDAQ would negatively affect the value of our common stock. Delisting could also have other negative results, including, but not limited to, the potential loss of confidence by employees, the loss of institutional investor interest and fewer business development opportunities.
Changes in the third-party reimbursement environment may affect product sales from which we generate royalty revenues.
Sales of products from which we generate royalties will depend significantly on the extent to which reimbursement for the cost of such products and related treatments will be available to physicians and patients from various levels of U.S. and international government health administration authorities, private health insurers and other organizations. Third-party payers and government health administration authorities increasingly attempt to limit and/or regulate the reimbursement of medical products and services, including branded prescription drugs. Changes in government legislation or regulation, such as the Medicare Prescription Drug Improvement and Modernization Act of 2003; the Deficit Reduction Act of 2005; the Medicare, Medicaid and State Children’s Health Insurance Program Extension Act of 2007; the Medicare Improvements for Patients and Providers Act of 2009; changes in formulary or compendia listing; or changes in private third-party payers’ policies toward reimbursement for such products may reduce reimbursement of the cost of such products to physicians, pharmacies and distributors. Decreases in third-party reimbursement could reduce usage of such products, sales to collaborators and may have a material adverse effect on our royalties which depend on such product sales. In addition, macroeconomic factors may affect the ability of patients to pay or co-pay for costs or otherwise pay for products from which we generate royalties by, for example, decreasing the number of patients covered by insurance policies or increasing costs associated with such policies.
We must attract, retain and integrate key employees in order to succeed. It may be difficult to recruit, retain and integrate key employees.
To be successful, we must attract, retain and integrate qualified personnel. Our business is managing our antibody humanization patents and royalties assets which requires only a small number of employees. It may be
13
Table of Contents
difficult for us to recruit and retain qualified personnel. If we are unsuccessful in attracting, retaining and integrating qualified personnel, our business could be impaired.
Our agreements with Facet may not reflect terms that would have resulted from arm’s-length negotiations between unaffiliated third parties.
The agreements associated with the spin-off of Facet in December 2008, including the Separation and Distribution Agreement, Tax Sharing and Indemnification Agreement, Transition Services Agreement and Cross License Agreement, were negotiated in the context of the Spin-Off while Facet was still part of PDL and, accordingly, may not reflect more favorable terms that may have resulted from arm’s-length negotiations between unaffiliated third parties.
We may have obligations for which we may not be able to collect under our indemnification rights from Facet.
Under the terms of the separation and distribution agreement with Facet, we and Facet agreed to indemnify the other from and after the Spin-Off with respect to certain indebtedness, liabilities and obligations that were retained by our respective companies. These indemnification obligations could be significant. The ability to satisfy these indemnities, if called upon to do so, will depend upon the future financial strength of each of our companies. We cannot assure you that, if Facet has to indemnify us for any substantial obligations, Facet will have the ability to satisfy those obligations. If Facet does not have the ability to satisfy those obligations, we may be required to satisfy those obligations instead. For example, in connection with the Spin-Off, we entered into amendments to the leases for the facilities in Redwood City, California, which formerly served as our corporate headquarters and which are now occupied by Facet under which Facet was added as a co-tenant under the leases and a Co-Tenancy Agreement under which Facet agreed to indemnify us for all matters related to the leases attributable to the period after the Spin-Off date. Should Facet default under its lease obligations, we would be held liable by the landlord as a co-tenant and, thus, we have in substance guaranteed the payments under the lease agreements for the Redwood City facilities, the disposition of which could have a material adverse effect on the amount or timing of any distribution to our stockholders. As of December 31, 2009, the total lease payments for the duration of the guarantee, which runs through December 2021, are approximately $130.8 million. We would also be responsible for lease related payments including utilities, property taxes and common area maintenance which may be as much as the actual lease payments. See “Item 2—Properties.”
We must evaluate the effectiveness of our disclosure controls and internal control over financial reporting on a periodic basis and publicly disclose the results of these evaluations and related matters.
Our management is required to periodically evaluate the effectiveness of our disclosure controls and procedures and our internal control over financial reporting and our independent registered public accounting firm must attest to the effectiveness of our internal control over financial reporting as of the end of each fiscal year. We are also required to disclose in our periodic reports with the SEC any changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. The rules governing the standards that must be met for management to assess the effectiveness of our internal control over financial reporting are complex and require significant documentation, testing and possible remediation. Compliance with these rules has resulted in increased expenses and the devotion of management resources.
Our evaluation of our disclosure controls and procedures may reveal material weaknesses in our internal control over financial reporting. If we identify a material weakness, we would be required to conclude that our internal control over financial reporting is ineffective and disclose this conclusion which could adversely affect the market price of our common stock. For example, we disclosed we had material weaknesses in our Quarterly Reports on Form 10-Q for the periods ended June 30, 2007, September 30, 2007, March 31, 2008 and June 30, 2008, and our Annual Report on Form 10-K for the year ended December 31, 2007 which we believe have been remediated.
14
Table of Contents
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
In November 2008, we entered into a lease for 3,775 square feet of office space in Incline Village, Nevada which now serves as our corporate headquarters. In February 2010, we entered into a lease amendment to extend our building lease term to May 2011 and obtained an option to further extend the lease until May 2012. Except as set forth above, we do not own or lease other properties.
In July 2006, we entered into two leases and a sublease for the facilities in Redwood City, California, which formerly served as our corporate headquarters and cover approximately 450,000 square feet of office space. Pursuant to amendments to the leases entered into in connection with the Spin-Off, Facet was added as a co-tenant under the leases. As a co-tenant, Facet is bound by all of the terms and conditions of the leases. PDL and Facet are jointly and severally liable for all obligations under the leases, including the payment of rental obligations. However, we also entered into a Co-Tenancy Agreement with Facet in connection with the Spin-Off and the lease amendments pursuant to which we assigned to Facet all rights under the leases, including, but not limited to, the right to amend the leases, extend the lease term or terminate the leases, and Facet assumed all of our obligations under the leases. Pursuant to the Co-Tenancy Agreement, we also relinquished any right or option to regain possession, use or occupancy of these facilities. Facet agreed to indemnify us for all matters associated with the leases attributable to the period after the Spin-Off date and we agreed to indemnify Facet for all matters associated with the leases attributable to the period before the Spin-Off date. In addition, in connection with the Spin-Off, the sublease was assigned by PDL to Facet.
| ITEM 3. | LEGAL PROCEEDINGS |
European Patent Oppositions
Two Queen et al. patents were issued to us by the European Patent Office, the ‘216 Patent and the ‘040 Patent both of which were opposed after grant. A description of those proceedings is below.
Opposition to ‘216 Patent
In November 2003, in an appeal proceeding of a prior action of the Opposition Division of the European Patent Office, the Technical Board of Appeal of the European Patent Office ordered that certain claims in our ‘216 Patent be remitted to the Opposition Division for further prosecution and consideration of issues of patentability, that is, entitlement to priority, novelty, enablement and inventive step. These claims cover the production of humanized antibody light chains that contain amino acid substitutions made under our antibody humanization technology. In April 2007, at an oral proceeding, the Opposition Division upheld claims that are virtually identical to the claims remitted by the Technical Board of Appeal to the Opposition Division. The deadline for filing a notice of appeal has expired. Five opponents filed such notices in a timely manner and, of those, three have filed Grounds of Appeal. The ‘216 Patent remains enforceable during the appeal process. The Technical Board of Appeal has not scheduled a date for the appeal hearing with respect to the ‘216 Patent. We intend to vigorously defend the ‘216 Patent in this proceeding.
Opposition to ‘040 Patent
At an oral hearing in February 2005, the Opposition Division revoked the claims in our ‘040 Patent. The Opposition Division based its decision on formal issues and did not consider substantive issues of patentability. On October 14, 2009, the European Patent Office Technical Board of Appeal upheld the
15
Table of Contents
Opposition Division’s revocation of our ‘040 Patent on formal issues. The Technical Board of Appeal did not consider substantive issues of patentability. Each of our granted and applied for SPCs are based on the ‘216 Patent. As a result, the European Patent Office Technical Board of Appeal’s decision regarding the ‘040 Patent will not affect our right to receive royalties beyond December 28, 2009.
Settlement with Alexion
In March 2007, after the FDA’s market approval of Alexion’s Soliris® humanized antibody product, we filed a lawsuit against Alexion in the United States District Court for the District of Delaware for infringement of certain claims of United States Patent Number 5,693,761, United States Patent Number 5,693,762 and the ‘370 Patent which are three of our Queen et al. patents.
On December 31, 2008, we and Alexion entered into a definitive license agreement and settlement agreement. Under the terms of the agreements, we granted Alexion a license under certain claims in our Queen et al. patents and provided Alexion a covenant not to sue in respect of other claims in our Queen et al. patents, thus permitting Alexion to commercialize Soliris for all indications under our Queen et al. patents. In consideration of this license, Alexion agreed to pay us $25 million, of which Alexion paid $12.5 million in January 2009 and another $12.5 million was paid in May 2009. Following receipt of this second payment, in May 2009, the parties filed with the United States District Court of Delaware a Stipulation and Order of Dismissal dismissing the lawsuit in its entirety with prejudice effective December 31, 2008, subject to the terms and conditions of the license agreement and settlement agreement.
No additional payments will be owed by Alexion to us under our Queen et al. patents in respect of Soliris sales for any indication. As part of the settlement, Alexion has confirmed that our Queen et al. patents claims are valid and that Soliris employs technology covered under our Queen et al. patents. Further, Alexion has agreed not to challenge or assist other parties in challenging the validity of our Queen et al. patents in the future. Under the license agreement, we separately granted Alexion the right to take a royalty-bearing license under our Queen et al. patents to commercialize additional Alexion humanized antibodies that may be covered by our Queen et al. patents in the future. In the event that Alexion takes such a license, Alexion will pay us a royalty of 4.0% of net sales of such non-Soliris products.
Action for Declaratory Judgment by MedImmune
In December 2008, MedImmune, a subsidiary of AstraZeneca plc, filed a lawsuit against us in the United States District Court for the Northern District of California seeking a declaratory judgment that the U.S. Queen et al. patents are invalid and/or not infringed by its Synagis and motavizumab products and, that therefore, MedImmune owes no royalties under its license agreement with us. In April 2009, MedImmune amended its complaint to allege that, even if our patents are valid and infringed by Synagis and/or motavizumab, MedImmune is now or may have been retroactively entitled to a lower royalty rate on its sales of infringing products under the most favored licensee clause in our agreement. In May 2009, we filed our answer to MedImmune’s lawsuit asserting certain counterclaims and affirmative defenses and requested that the court find (i) that Synagis and motavizumab fall under the scope of the Queen et al. patents and that the sale thereof requires that MedImmune pay us royalties as specified in our license agreement with them; (ii) that the claims we are asserting against MedImmune are valid; (iii) that MedImmune is not entitled to different terms, including a lower royalty rate; and (iv) that MedImmune is liable for attorney’s fees and costs related to the action. On August 18, 2009, we attended a mandatory settlement conference with MedImmune held before the Federal District Court for Northern California. No settlement resulted from the meeting. A Markman claim construction hearing took place on November 5, 2009. A decision was issued from the court on February 22, 2010. The court generally construed the claim language at issue as proposed by PDL.
In December 2009, we sent a letter to MedImmune stating that it is in breach of its obligations under the license agreement, canceling the license agreement and revoking any licenses and rights granted therein. Also in
16
Table of Contents
December 2009 we filed the Amended Pleadings alleging that MedImmune breached the license agreement by (i) failing to pay all royalties due to us from the sale of Synagis, including sales by and through Abbott Laboratories, whom we believe is MedImmune’s exclusive sales representative for such sales outside the United States and (ii) by demanding that we consent to conditions that are commercially unreasonable and contractually insupportable in order to permit an audit of sales and revenue associated with Synagis by an independent accountant, as required under the license agreement and allege that, as a result of MedImmune’s breach of the license agreement and the Company’s cancelation thereof, MedImmune is infringing the ‘370 Patent by making, using, selling, offering for sale and/or importing Synagis into the United States and by having Synagis made, used, sold, offered for sale and/or imported into the United States. We have requested that the court award to the Company damages resulting from MedImmune’s breach of the license agreement, treble damages resulting from MedImmune’s infringement of the Company’s patent rights, attorney’s fees, and an injunction to prevent MedImmune from further acts of infringement and request a jury trial on all issues triable by jury. Also in December 2009, MedImmune filed a Motion for a preliminary injunction against our cancelation of the license agreement and filed a motion to strike our Amended Pleadings. In January of 2010, MedImmune filed a motion for summary judgment seeking a declaratory judgment from the court that MedImmune is entitled under the most favored licensee clause in our agreement to a fully paid-up license as of December 2008 as a result of our agreement with Alexion and, retroactively to 1998, a reduced royalty rate on sales of Synagis. A hearing was held on February 26, 2010, related to the preliminary injunction motion, motion to strike and motion for summary judgment.
On November 23, 2009, the PTO declared an interference proceeding between certain claims of the ‘370 Patent, which is involved in the current litigation against MedImmune, and certain pending claims of Adair et al., the 117 Application under 35 U.S.C. 135(a). UCB Pharma S.A. is the assignee of the ‘117 Application. We are unable to predict whether either of these proceedings will impact on the issues or timing of the other proceeding.
Trial is scheduled to start June 14, 2010 at which issues of infringement, validity and contractual rights are expected to be decided. MedImmune has paid us royalties under the MedImmune agreement with respect to sales of Synagis on a quarterly basis since the fourth quarter of 1998 through the fourth quarter of 2009. An escrow account has been created for receipt of MedImmune’s February 2010 payment, in which account the funds will be held pendente lite (while the litigation is pending).
In the event that MedImmune prevails on the claims in its complaint, in either its summary judgment request or at trial, we expect that MedImmune will request the court to order a recoupment of payments made to us which represent obligations under its license to the Queen et al. patents that have accrued since the date of their claim. Alternatively, if MedImmune is successful in showing that it has made payments to us at a higher royalty rate than required pursuant to its license obligations, we expect that MedImmune will request the court to order recoupment of such excess payments.
Interference Proceedings in the U.S. Patent and Trademark Office
On February 25, 2009, the PTO declared an interference proceeding between certain claims of the ‘089 Patent and certain pending claims of Adair et al., the ‘658 Application under 35 U.S.C. 135(a). UCB Pharma S.A. is the assignee of the ‘658 Application. A hearing was held on January 29, 2010 regarding the first phase of the interference, which relates to substantive motions except those for priority of invention. A decision is expected within several months. The PTO has scheduled proceedings for the determination of priority of invention, if necessary.
On November 23, 2009, the PTO declared an interference proceeding between certain claims of the ‘370 Patent and certain pending claims of Adair et al., the ‘117 Application under 35 U.S.C. 135(a). UCB Pharma S.A. is the assignee of the ‘117 Application.
17
Table of Contents
In an interference proceeding, the Board of Patent Appeals and Interferences typically determines questions of priority of the claimed inventions and may also determine questions of patentability. Any final decision, if adverse to the claim of an applicant, is a final refusal by the PTO of the claims involved. The Office may issue a patent to the applicant if the applicant is adjudged the prior inventor. A final judgment adverse to the patentee from which no appeal or other review has been or can be taken or had constitutes cancellation of the claims involved in the patent.
| ITEM 4. | RESERVED |
18
Table of Contents
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock trades on the NASDAQ Global Select Market under the symbol “PDLI.” Prices indicated below are the high and low intra-day sales prices per share of our common stock as reported by the NASDAQ Global Select Market for the periods indicated.
| High | Low | ||||||
| 2009 | |||||||
| First Quarter |
$ | 7.35 | $ | 5.20 | |||
| Second Quarter |
$ | 8.04 | $ | 6.57 | |||
| Third Quarter |
$ | 9.32 | $ | 7.61 | |||
| Fourth Quarter |
$ | 9.13 | $ | 6.32 | |||
| 2008 | |||||||
| First Quarter |
$ | 17.66 | $ | 9.07 | |||
| Second Quarter |
$ | 14.11 | $ | 9.00 | |||
| Third Quarter |
$ | 12.70 | $ | 8.82 | |||
| Fourth Quarter |
$ | 10.24 | $ | 5.64 | (1) | ||
| (1) | Reflects the distribution of Facet common stock in connection with the Spin-Off, where the Facet common stock was valued at $2.60 per PDL share of common stock based on the closing price of Facet common stock on the Spin-Off date. |
As of February 23, 2010, we had approximately 172 common stockholders of record. Most of our outstanding shares of common stock are held of record by one stockholder, Cede & Co., a nominee for the Depository Trust Company. Many brokers, banks and other institutions hold shares of common stock as nominees for beneficial owners which deposit these shares of common stock in participant accounts at the Depository Trust Company. The actual number of beneficial owners of our stock is likely significantly greater than the number of stockholders of record; however, we are unable to reasonably estimate the total number of beneficial owners.
In May 2008, we paid a cash dividend of approximately $506.6 million, or $4.25 per share of common stock, to our stockholders using proceeds from the sales of our commercial operations and an antibody manufacturing plant. In December 2008, following the Spin-Off, the price of our common stock dropped to reflect the separation of Facet from us.
In April 2009 and October 2009, we paid cash dividends of $59.7 million, or $0.50 per share of common stock, and $59.7 million, or $0.50 per share of common stock, respectively, to our stockholders using proceeds from our 2009 earnings. In December 2009, we paid an additional cash dividend of $199.6 million, or $1.67 per share of common stock, to our stockholders using a portion of the proceeds from the issuance of the QHP Notes. In connection with the payment of these dividends, the conversion rates for the 2012 Notes and the 2023 Notes were adjusted upward based on the amount of the dividends and the trading price of our stock in certain periods pursuant to the terms of the applicable indentures.
On January 28, 2010, our board of directors declared two cash dividends of $0.50 per share of common stock payable on April 1, 2010 and October 1, 2010 to stockholders of record on March 15, 2010 and September 15, 2010, respectively. Based on the number of shares of common stock issued and outstanding as of March 1, 2010, we currently expect the aggregate dividends to be approximately $60 million each, which we expect to pay using proceeds from our 2010 earnings and from cash on hand at December 31, 2009.
Our board of directors will evaluate a dividend policy for subsequent years based on net income, debt service, cash requirements for future debt service, income taxes and other corporate activities.
19
Table of Contents
Comparison of Stockholder Returns
The line graph below compares the cumulative total stockholder return on our common stock between December 31, 2004 and December 31, 2009 with the cumulative total return of (i) the NASDAQ Biotechnology Index and (ii) the NASDAQ Composite Index over the same period. This graph assumes that $100.00 was invested on December 31, 2004, in our common stock at the closing sales price for our common stock on that date and at the closing sales price for each index on that date and that all dividends were reinvested. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns and are not intended to be a forecast.
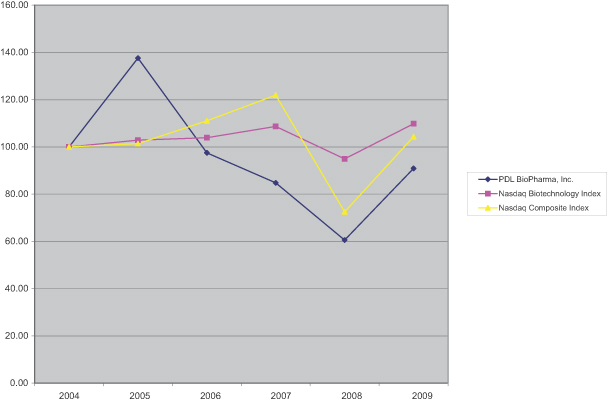
| 12/31/2004 | 12/31/2005 | 12/31/2006 | 12/31/2007 | 12/31/2008 | 12/31/2009 | |||||||
| PDL BioPharma, Inc. |
100.00 | 137.62 | 97.48 | 84.77 | 60.53 | 90.86 | ||||||
| Nasdaq Biotechnology Index |
100.00 | 102.84 | 103.89 | 108.65 | 94.93 | 109.77 | ||||||
| Nasdaq Composite Index |
100.00 | 101.37 | 111.03 | 121.92 | 72.49 | 104.31 |
The information in this section shall not be deemed to be “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference in such filing.
| ITEM 6. | SELECTED FINANCIAL DATA |
The following selected consolidated financial information has been derived from our consolidated financial statements. The information below is not necessarily indicative of the results of future operations and should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of
20
Table of Contents
Operations,” and Item 1A, “Risk Factors,” of this Form 10-K and the consolidated financial statements and related notes thereto included in Item 8 of this Form 10-K in order to fully understand factors that may affect the comparability of the information presented below.
The financial results relating to both our former biotechnology, manufacturing and commercial operations have been presented as discontinued operations for all periods presented in the table below. See Note 18 to the Consolidated Financial Statements for further information.
Consolidated Statements of Operations Data
| Year Ended December 31, | |||||||||||||||
| (In thousands, except per share data) |
2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||
| Revenues: |
|||||||||||||||
| Royalties |
$ | 305,049 | $ | 278,713 | $ | 224,735 | $ | 185,775 | $ | 123,052 | |||||
| License and other |
13,135 | 15,483 | 350 | 850 | 3,597 | ||||||||||
| Total revenues |
318,184 | 294,196 | 225,085 | 186,625 | 126,649 | ||||||||||
| General and administrative expenses |
21,064 | 51,544 | 41,176 | 31,881 | 17,993 | ||||||||||
| Operating income |
297,120 | 242,652 | 183,909 | 154,744 | 108,656 | ||||||||||
| Other non-operating income (expense) |
(16,835) | 682 | 7,164 | 4,448 | (1,948) | ||||||||||
| Income from continuing operations before income taxes |
280,285 | 243,334 | 191,073 | 159,192 | 106,708 | ||||||||||
| Income tax expense |
90,625 | 5,014 | 10,624 | 3,199 | 2,162 | ||||||||||
| Income from continuing operations |
189,660 | 238,320 | 180,449 | 155,993 | 104,546 | ||||||||||
| Loss on discontinued operations, net of income taxes(1) |
- | (169,933) | (201,510) | (286,013) | (271,123) | ||||||||||
| Net income (loss) |
$ | 189,660 | $ | 68,387 | $ | (21,061) | $ | (130,020) | $ | (166,577) | |||||
| Income per basic share from continuing operations |
$ | 1.59 | $ | 2.01 | $ | 1.55 | $ | 1.37 | $ | 1.00 | |||||
| Net income (loss) per basic share |
$ | 1.59 | $ | 0.58 | $ | (0.18) | $ | (1.14) | $ | (1.60) | |||||
| Income per diluted share from continuing operations |
$ | 1.07 | $ | 1.48 | $ | 1.34 | $ | 1.19 | $ | 0.88 | |||||
| Net income (loss) per diluted share |
$ | 1.07 | $ | 0.47 | $ | (0.08) | $ | (0.84) | $ | (1.17) | |||||
| Dividends per share: |
|||||||||||||||
| Cash dividends declared and paid (Note 4) |
$ | 2.67 | $ | 4.25 | $ | - | $ | - | $ | - | |||||
| Stock distribution in connection with the spin off of Facet (Notes 1 and 5) |
$ | - | $ | 2.60 | $ | - | $ | - | $ | - | |||||
Consolidated Balance Sheet Data
| December 31, | |||||||||||||||
| (In thousands) |
2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||
| Cash, cash equivalents, investments and restricted cash |
$ | 303,227 | $ | 147,527 | $ | 440,788 | $ | 426,285 | $ | 333,922 | |||||
| Working capital |
$ | 22,320 | $ | 149,168 | $ | 598,346 | $ | 273,433 | $ | 307,302 | |||||
| Assets held for sale(2) |
$ | - | $ | - | $ | 269,390 | $ | - | $ | - | |||||
| Total assets |
$ | 338,411 | $ | 191,142 | $ | 1,192,192 | $ | 1,141,893 | $ | 1,163,154 | |||||
| Long-term obligations, less current portion |
$ | 460,848 | $ | 510,698 | $ | 534,847 | $ | 536,923 | $ | 507,294 | |||||
| Accumulated deficit |
$ | (333,298) | $ | (522,958) | $ | (591,345) | $ | (570,129) | $ | (440,109) | |||||
| Total stockholders’ equity (deficit) |
$ | (415,953) | $ | (352,569) | $ | 507,610 | $ | 467,541 | $ | 526,065 | |||||
| (1) | The financial results associated with our former biotechnology, manufacturing and commercial operations have been presented as discontinued operations in our Consolidated Statements of Operations. See Note 18 to the Consolidated Financial Statements for further details. |
21
Table of Contents
| (2) | The assets associated with our former commercial operations were presented as “held for sale” on our Consolidated Balance Sheet as of December 31, 2007, and such assets were fully divested in March 2008. See Note 18 to the Consolidated Financial Statements for further details. |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
Our business is the management of our antibody humanization patents and royalty assets which consist of our Queen et al. patents and license agreements with numerous biotechnology and pharmaceutical companies pursuant to which we have licensed certain rights under our patents. We receive royalties based on these license agreements on sales of a number of humanized antibody products marketed by our licensees and also may receive royalty payments on additional humanized antibody products launched before final patent expiry in 2014. Under most of our licensing agreements, we are entitled to receive a flat-rate or tiered royalty based upon our licensees’ net sales of covered antibodies. We receive royalties on net sales of products made, used or sold prior to patent expiry. We have also entered into licensing agreements pursuant to which we have licensed certain rights for development stage products that have not yet reached commercialization including products that are currently in Phase 3 clinical trials.
Until December 2008, our business included a biotechnology operation which was focused on the discovery and development of novel antibodies which we spun-off to Facet. From March 2005 until March 2008, we also had commercial and manufacturing operations which we partially divested in 2006 and fully divested in 2008. The financial results of our former biotechnology and manufacturing operations as well as our former commercial operation are presented as discontinued operations in the Consolidated Statement of Operations for all periods presented in this Annual Report. See Note 18 to the Consolidated Financial Statements for further details.
Recent Developments
In 2009, we paid two cash dividends of $59.7 million, or $0.50 per share of common stock, to our stockholders on April 1, 2009 and October 1, 2009. In December 2009, we paid an additional cash dividend of $199.6 million, or $1.67 per share of common stock, to our stockholders using a portion of the proceeds from the securitization transaction which is described below. As of December 31, 2009, we accrued $0.4 million in other accrued liabilities for estimated dividends payable on unvested restricted stock.
On January 27, 2010, our board of directors declared two cash dividends of $0.50 per share of common stock payable on April 1, 2010 and October 1, 2010 to stockholders of record on March 15, 2010 and September 15, 2010, respectively. Our board of directors will evaluate our dividend policy for subsequent years based on net income, debt service, income taxes and other corporate activities.
In connection with each of the dividend payments, the conversion rates for our outstanding 2012 Notes and 2023 Notes were adjusted upwards. As of December 31, 2009, the conversion rates for the 2012 Notes and the 2023 Notes were 119.294 and 164.7254 shares of common stock per $1,000 principal amount, respectively, or $8.38 and $6.07 per share of common stock for each of the notes, respectively. The adjustment was based on the amount of the dividend and the average trading price of our stock for certain periods before the record date and adjusted for ex-dividend trading pursuant to the terms of the applicable indenture.
In 2009, we repurchased $50 million of the 2023 Notes and $22 million of the 2012 Notes. We will continue to evaluate opportunities to repurchase the convertible notes as market conditions warrant.
Approval for Raptiva, which was marketed by Genentech in United States and by Merck Serono S.A. outside of the United States, was suspended in the European Union and Canada in February 2009 and the product was withdrawn from the United States market in April 2009 due to safety concerns; we do not expect to receive
22
Table of Contents
royalties on future sales of Raptiva. For the year ended December 31, 2009, we received $1.2 million in royalties for sales of Raptiva as compared with $3.9 million and $3.7 million for the same periods in 2008 and 2007, respectively.
In February 2009, the PTO declared an interference proceeding between our ‘089 Patent and a patent application pending to Adair et al. and, on November 23, 2009, the PTO declared a second interference proceeding between certain claims of the ‘370 Patent and certain pending claims of Adair et al. UCB Pharma S.A. is the assignee of the Adair et al. applications. Also, on October 14, 2009, the European Patent Office Technical Board of Appeal upheld the Opposition Division’s revocation of our ‘040 Patent on formal issues. The Technical Board of Appeal did not consider substantive issues of patentability. See “Item 3—Legal Proceedings.”
In November 2009, we completed a $300 million securitization transaction in which we monetized the Genentech Royalties including Avastin, Herceptin, Lucentis, Xolair, and future products, if any, under which Genentech may take a license under our related agreements with Genentech. The QHP Notes bear interest at 10.25% per annum and were issued in a non-registered offering by QHP, a Delaware limited liability company, and a newly formed, wholly-owned subsidiary of PDL. Concurrent with the securitization transaction and pursuant to the terms of a purchase and sale agreement, we sold, transferred, conveyed, assigned, contributed and granted to QHP, certain rights under our non-exclusive license agreements with Genentech including the right to receive the Genentech Royalties in exchange for QHP’s proceeds from the QHP Notes issuance. The Genentech Royalties and other payments, if any, that QHP will be entitled to receive under the agreements with Genentech, together with any funds made available from certain accounts of QHP, will be the sole source of payment of principal and interest on the QHP Notes. Once all obligations on the QHP Notes have been paid in full, including all other sums payable under the indenture, then the indenture shall cease to be of further effect and all of the security interests in the collateral shall terminate, including the pledge by PDL to the trustee of its equity interest in QHP. At such point, there will be no further restrictions on the Genentech Royalties and PDL shall be free to either keep them in QHP, transfer them back to PDL, the parent company, or to further dispose or monetize them. The anticipated final repayment date of the QHP Notes is December 2012.
A Markman claim construction hearing in our lawsuit with MedImmune took place on November 5, 2009. A decision was issued from the court on February 22, 2010. The court generally construed the claim language at issue as proposed by PDL. A trial is scheduled to commence on June 14, 2010 at which issues of infringement, validity and contractual rights are expected to be decided. In December 2009, we sent a letter to MedImmune stating that it is in breach of its obligations under the license agreement, canceling the license agreement and revoking any licenses and rights granted therein. Also in December 2009, we filed the Amended Pleadings alleging that MedImmune breached the license agreement by (i) failing to pay all royalties due to us from the sale of Synagis, including sales by and through Abbott Laboratories, whom we believe is MedImmune’s exclusive sales representative for such sales outside the United States, and (ii) by demanding that we consent to conditions that are commercially unreasonable and contractually insupportable in order to permit an audit of sales and revenue associated with Synagis by an independent accountant, as required under the license agreement and allege that, as a result of MedImmune’s breach of the license agreement and the Company’s cancelation thereof, MedImmune is infringing the ‘370 Patent by making, using, selling, offering for sale and/or importing Synagis into the United States and by having Synagis made, used, sold, offered for sale and/or imported into the United States. We have requested that the court award to the Company damages resulting from MedImmune’s breach of the license agreement, treble damages resulting from MedImmune’s infringement of the Company’s patent rights, attorney’s fees and an injunction to prevent MedImmune from further acts of infringement and request a jury trial on all issues triable by jury. Also in December 2009, MedImmune filed a Motion for a preliminary injunction against our cancelation of the license agreement and a motion to strike our Amended Pleadings. In January of 2010, MedImmune filed a motion for summary judgment seeking a declaratory judgment from the court that MedImmune is entitled under the most favored licensee clause in our agreement to a fully paid-up license as of December 2008 as a result of our agreement with Alexion and, retroactively to 1998, a reduced royalty rate on sales of Synagis. A hearing was held on February 26, 2010, related to the preliminary injunction motion, motion to strike and motion for summary judgment. See “Item 3—Legal Proceedings.”
23
Table of Contents
Critical Accounting Policies and Uses of Estimates
The preparation of our financial statements in conformity with accounting principles generally accepted in the United States of America (GAAP) requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. Actual results could differ materially from those estimates. The items in our financial statements requiring significant estimates and judgments comprise:
Royalty Revenues
Under most of our patent license agreements, we receive royalty payments based upon our licensees’ net sales of covered products. Generally, under these agreements we receive royalty reports from our licensees approximately one quarter in arrears, that is, generally in the second month of the quarter after the licensee has sold the royalty bearing product. We recognize royalty revenues when we can reliably estimate such amounts and collectibility is reasonably assured. As such, we generally recognize royalty revenues in the quarter reported to us by our licensees, that is, royalty revenues are generally recognized one quarter following the quarter in which sales by our licensees occurred. Under this accounting policy, the royalty revenues we report are not based upon our estimates and are typically reported in the same period in which we receive payment from our licensees.
We may also receive annual license maintenance fees, payable at the election of the licensee, to maintain the license in effect. We have no performance obligations with respect to such fees. Maintenance fees are recognized as they are due and when payment is reasonably assured.
Income Taxes
Our income tax provision is based on income before taxes and is computed using the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using tax rates projected to be in effect for the year in which the differences are expected to reverse. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations, or the expected results from any future tax examinations. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes. These factors include, but are not limited to, changes in tax laws, regulations and/or rates, the results of any future tax examinations, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, past levels of research and development spending, acquisitions, changes in our corporate structure and state of domicile and changes in overall levels of income before taxes all of which may result in periodic revisions to our provision for income taxes. We accrue tax related interest and penalties associated with uncertain tax positions and include these in income tax expense in the Consolidated Statements of Operations.
Due to our lack of earnings history prior to the Spin-Off, our gross deferred tax assets had been fully offset by a valuation allowance on our Consolidated Balance Sheets. However, as a result of the Spin-Off, we believe that our history of royalty revenues and the significantly lowered cost structure to support our intellectual property, manage our licensing operations and provide for certain essential reporting and management functions of a public company provided a basis to reverse the valuation allowance on our deferred tax assets. As a result, we expect that our effective income tax rate going forward will be approximately 35%.
Lease Guarantee
In connection with the Spin-Off, we entered into amendments to the leases for our former facilities in Redwood City, California, under which Facet was added as a co-tenant under the leases, and a Co-Tenancy Agreement, under which Facet agreed to indemnify us for all matters related to the leases attributable to the period after the Spin-Off date. Should Facet default under its lease obligations, we could be held liable by the landlord as a co-tenant, and thus, we have in substance guaranteed the payments under the lease agreements for the Redwood City facilities. As
24
Table of Contents
of December 31, 2009, the total lease payments for the duration of the guarantee, which runs through December 2021, are approximately $130.8 million. If Facet were to default, we could also be responsible for lease related costs including utilities, property taxes, and common area maintenance which may be as much as the actual lease payments.
We recorded a liability of $10.7 million on our Consolidated Balance Sheets as of December 31, 2009 and 2008 which was the estimated fair value of this guarantee. We prepared a discounted, probability-weighted cash flow analysis to calculate the estimated fair value of the lease guarantee as of the Spin-Off. We were required to make assumptions regarding the probability of Facet’s default on the lease payment, the likelihood of a sublease being executed, and the times at which these events could occur. These assumptions are based on information that we received from real estate brokers and the then-current economic conditions, as well as expectations of future economic conditions. The fair value of this lease guarantee was charged to additional paid-in capital upon the Spin-Off and any future adjustments to the carrying value of the obligation will also be recorded in additional paid-in capital. On a quarterly basis, we review the underlying cash flow analysis assumptions and update them if necessary. In future periods, we may increase the recorded liability for this obligation if we conclude that a loss, which is larger than the amount recorded, is both probable and estimable.
Summary of 2009, 2008 and 2007 Financial Results
We recognized income from continuing operations of $189.7 million, $238.3 million and $180.4 million for the years ended December 31, 2009, 2008 and 2007, respectively. Our net income for the years ended December 31, 2009 and 2008 was $189.7 million and $68.4 million, respectively, compared to a net loss of $21.1 million for the year ended December 31, 2007. At December 31, 2009, we had cash, cash equivalents, investments and restricted cash of $303.2 million as compared with $147.5 million as of December 31, 2008. At December 31, 2009, we had $754.4 million in total liabilities as compared with $543.7 million as of December 31, 2008.
Revenues
Revenues from continuing operations were $318.2 million, $294.2 million and $225.1 million for the years ended December 31, 2009, 2008 and 2007, respectively, and consist of royalty revenues as well as other license related revenues. During the years ended December 31, 2009, 2008 and 2007, our royalty revenues consisted of royalties and maintenance fees earned on sales of products under license agreements associated with our Queen et al. patents. Over this same time period, our other license related revenues primarily consisted of milestone payments from licensees under our patent license agreements as well as two $12.5 million payments in 2009 and 2008 from our legal settlement with Alexion. Our revenues from continuing operations are comprised almost entirely of royalties, which represent more than 90% of total revenues from continuing operations for each of the past three years.
A summary of our revenues for the years ended December 31, 2009, 2008 and 2007 is presented below:
| (Dollars in thousands) |
2009 | Change from Prior Year % |
2008 | Change from Prior Year % |
2007 | ||||||||
| Revenues |
|||||||||||||
| Royalties |
$ | 305,049 | 9% | $ | 278,713 | 24% | $ | 224,735 | |||||
| License and other |
13,135 | -15% | 15,483 | 4324% | 350 | ||||||||
| Total revenues |
$ | 318,184 | 8% | $ | 294,196 | 31% | $ | 225,085 | |||||
In each of the years ended December 31, 2009, 2008 and 2007, we received royalties on sales of nine humanized antibody products, all of which are currently approved for use by the FDA and eight are approved by other regulatory agencies outside the United States. Approval for one of the products, Raptiva, was suspended in the European Union and Canada in February 2009 and the product was withdrawn from the United States market in
25
Table of Contents
April 2009; we do not expect to receive royalties on future sales of Raptiva. For the year ended December 31, 2009, we received $1.2 million in royalties for sales of Raptiva as compared with $3.9 million and $3.7 million for the same periods in 2008 and 2007, respectively. In December 2009, we declared MedImmune in breach of its license agreement with us and canceled the license agreement. For the year ended December 31, 2009, we received $40.7 million in royalties for sales of MedImmune’s Synagis product compared with $40.2 million and $36.7 million for the same periods in 2008 and 2007, respectively. See “Item 3—Legal Proceedings.” In 2009, 2008 and 2007, we received approximately $305.0 million, $278.7 million and $224.7 million in royalty revenues, respectively, from our licensees.
Under most of our license agreements, we receive a flat-rate or tiered royalty based upon our licensees’ net sales of covered antibody products. Royalty payments are generally due one quarter in arrears, that is, generally in the second month of the quarter after the licensee has sold the royalty-bearing product. Our master patent license agreement with Genentech provides for a tiered royalty structure under which the royalty rate Genentech must pay on U.S.-based Sales in a given calendar year decreases on incremental U.S.-based Sales above certain net sales thresholds.
The net sales thresholds and the applicable royalty rates for Genentech’s U.S.-based Sales are outlined below:
| Aggregate Net Sales |
Royalty Rate | ||
| Net sales up to $1.5 billion |
3.0 | % | |
| Net sales between $1.5 billion and $2.5 billion |
2.5 | % | |
| Net sales between $2.5 billion and $4.0 billion |
2.0 | % | |
| Net sales exceeding $4.0 billion |
1.0 | % |
As a result of the tiered royalty structure, Genentech’s average annual royalty rate for a given year will decline as Genentech’s U.S.-based Sales increase during that year. Because we receive royalties one quarter in arrears, the average royalty rates for the payments we receive from Genentech in the second calendar quarter for Genentech’s sales from the first calendar quarter have been and are expected to continue to be higher than the average royalty rates for following quarters. The average royalty rates for payments we receive from Genentech are generally lowest in the fourth and first calendar quarters for Genentech’s sales from the third and fourth calendar quarters when Genentech’s U.S.-based Sales bear royalties at the lowest royalty rates. With respect to ex-U.S.-based Manufacturing and Sales, the royalty rate that we receive from Genentech is a fixed rate of 3.0% based on a percentage of the underlying ex-U.S.-based Manufacturing and Sales. The mix of U.S.-based Sales and ex-U.S.-based Manufacturing and Sales has fluctuated in the past and may continue to fluctuate in future periods, particularly in light of the 2009 acquisition of Genentech by Roche. For example, in July 2009 Roche announced its decision to partially close its manufacturing site in Vacaville, California.
In addition to the tiered royalty structure for the Genentech products, also contributing to seasonality in our historical revenues are sales of Synagis. This product has significantly higher sales in the fall and winter which to date have resulted in much higher royalties paid to us in our first and second quarters than in other quarters.
Royalties from licensed product sales exceeding more than 10% of our total revenues by licensee and product as a percentage of total revenue are set forth below:
| Licensee |
Product Name |
Year Ended December 31, | ||||||
| 2009 | 2008 | 2007 | ||||||
| Genentech |
Avastin | 27% | 25% | 26% | ||||
| Herceptin |
29% | 33% | 38% | |||||
| Lucentis |
10% | 9% | 9% | |||||
| MedImmune(1) |
Synagis | 13% | 14% | 16% | ||||
| (1) | In December 2009, we canceled the MedImmune license agreement. See “Item 3 – Legal Proceedings.” |
26
Table of Contents
Total revenues from continuing operations for the year ended December 31, 2009 were $318.2 million, an 8% increase from $294.2 million for the same period in 2008, driven primarily by higher underlying product sales of Avastin which is marketed by Genentech and Roche, underlying product sales of Lucentis which is marketed by Genentech and Novartis, and underlying product sales of Tysabri which is marketed by Elan and Biogen Idec. When compared to the prior year, the overall effective royalty rate for sales of the Genentech products, Avastin, Herceptin, Lucentis, Xolair and Raptiva, declined from 1.81% in 2008 to 1.69% in 2009, because of the tiered royalty structure that we have under our master patent license agreement with Genentech. As a result of the tiered royalty structure, Genentech’s average annual royalty rate for a given year will decline as Genentech’s U.S.-based Sales increase during that year. Accordingly, as U.S.-based year over year sales increase, the average royalty rate earned in that year declines when compared to the prior year. Also impacting the overall effective rate earned on sales of Genentech products is the amount of royalties received from Genentech for ex-U.S.-based Manufacturing and Sales for which we receive a flat 3.0% for these sales. The mix of U.S.-based Sales and ex-U.S.-based Manufacturing and Sales fluctuates and may continue to fluctuate in future periods, particularly in light of the aforementioned acquisition of Genentech by Roche.
The mix of U.S.-based sales and ex-U.S. based Manufacturing and Sales of Genentech products for each of the years ended December 31, 2009, 2008 and 2007 is outlined in the following table:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| U.S. Based Sales |
88 | % | 85 | % | 86 | % | |||
| Ex-U.S. Based Manufacturing and Sales |
12 | % | 15 | % | 14 | % | |||
The information in the table above is based on information provided to us by Genentech. We were not provided the reasons for the shift in the manufacturing split between U.S.-based Sales and ex-U.S.-based Manufacturing and Sales.
When compared to the prior year, also impacting revenue results are changes in foreign currency exchange rates. Approximately 50 percent of underlying product sales is in currencies other than U.S. dollars. While foreign currency conversion terms vary by license agreement, generally most agreements require that royalties first be calculated in the currency of sale and then converted into U.S. dollars using the average daily exchange rates for that currency for a specified period at the end of the calendar quarter. Accordingly, when the U.S. dollar weakens against other currencies, the converted amount is greater than it would have been had the U.S. dollar not weakened. For example, in a quarter in which we generate $60 million in revenue, approximately $30 million is based on sales in currencies other than U.S. dollar. If the U.S. dollar weakens across all currencies by ten percent during the conversion period for that quarter, when compared to the same amount of local currency royalties for the prior year, U.S. dollar converted royalties will be approximately $3 million more in the current quarter than in the prior year. In comparison to the year ended December 31, 2008, royalties earned for the year ended December 31, 2009 were negatively impacted by changes in foreign currency exchange rates. The impact on full year revenue is greatest in the second quarter when we receive the largest amount of royalties because of the Genentech tiered royalties are at their highest rate for first quarter sales and because sales of Synagis are highly seasonal.
The following table presents the quarterly, five-day average U.S. dollar per Euro exchange rate for quarterly royalty payments received in each of the years ended December 31, 2009, 2008 and 2007:
| 5 Day Average USD/EUR Rate |
2009 | 2008 | 2007 | |||
| Royalties received in Q1 |
1.41 | 1.45 | 1.31 | |||
| Royalties received in Q2 |
1.34 | 1.56 | 1.33 | |||
| Royalties received in Q3 |
1.40 | 1.56 | 1.35 | |||
| Royalties received in Q4 |
1.47 | 1.46 | 1.41 |
27
Table of Contents
Total revenues from continuing operations for the year ended December 31, 2008 were $294.2 million, a 31% increase from $225.1 million for the same period in 2007, driven primarily by higher underlying product sales of Avastin and Herceptin which are marketed by Genentech and Roche, higher underlying product sales of Lucentis which is marketed by Genentech and Novartis, and higher underlying product sales of Tysabri which is marketed by Elan and Biogen Idec. Also contributing to higher royalty revenues was a higher volume of total ex-U.S.-based Manufacturing and Sales of Herceptin in 2008, for which the royalty rate is a fixed 3% as compared to the tiered-rate fee structure that applies to U.S.-based Sales. The average royalty rate received for sales of the Genentech products declined from 2.02% in 2007 to 1.81% in 2008. Also, when compared to 2007, royalty revenues for the year ended December 31, 2008 were favorably impacted by changes in foreign currency translation rates because the U.S. dollar was significantly weaker in 2008 than it was in 2007. In addition, included in 2008 revenue is a $12.5 million settlement payment from Alexion.
General and Administrative Expenses
A summary of our general and administrative expenses for the years ended December 31, 2009, 2008 and 2007 is presented below:
| (Dollars in thousands) |
2009 | Change from Prior Year % |
2008 | Change from Prior Year % |
2007 | ||||||||
| General and administrative expenses |
$ | 21,064 | -59% | $ | 51,544 | 25% | $ | 41,176 | |||||
The decrease in general and administrative expenses for the year ended December 31, 2009 when compared to the year ended December 31, 2008 was primarily driven by our significantly reduced, post Spin-Off cost structure. After the Spin-Off, we significantly downsized our operations and we currently have fewer than ten employees managing our intellectual property, our licensing operations and other corporate activities, as well as providing for certain essential reporting and management functions of a public company.
Individual components of general and administrative expenses for the year ended December 31, 2009 comprise:
| (In thousands) |
Year Ended December 31, 2009 | ||
| Compensation and benefits |
$ | 3,359 | |
| Legal fees |
10,891 | ||
| Professional fees |
2,374 | ||
| Insurance |
992 | ||
| Depreciation |
991 | ||
| Stock-based compensation |
821 | ||
| Other |
1,636 | ||
| Total general and administrative expenses |
$ | 21,064 | |
In the first quarter of 2009, we recorded a depreciation charge of $0.9 million on certain software assets which were fully depreciated as of March 31, 2009 and are no longer in use. We expect depreciation expense to be significantly less in the future.
In 2008, our total costs and expenses from continuing operations were $51.5 million, an increase of $10.4 million from 2007. This increase was primarily driven by higher legal and consulting expenses associated with the Spin-Off, divestiture of the commercial and manufacturing operations as well as royalty monetization efforts.
28
Table of Contents
Interest and Other Income, Net, and Interest Expense
A summary of our interest and other income, net, and interest expense for the years ended December 31, 2009, 2008 and 2007 is presented below:
| (Dollars in thousands) |
2009 | Change from Prior Year % |
2008 | Change from Prior Year % |
2007 | |||||||||||
| Gain on repurchase of convertible notes |
$ | 1,518 | - | $ | - | - | $ | - | ||||||||
| Interest and other income, net |
1,004 | -93% | 14,901 | -26% | 20,233 | |||||||||||
| Interest expense |
(19,357 | ) | 36% | (14,219 | ) | 9% | (13,069 | ) | ||||||||
| Total non-operating income (expense) |
$ | (16,835 | ) | -2568% | $ | 682 | -90% | $ | 7,164 | |||||||
The gain on repurchase of convertible notes for the year ended December 31, 2009 resulted from the repurchase of $50.0 million in principal value of our 2023 Notes and $22.0 million in principal value of our 2012 Notes.
Interest and other income, net, for the year ended December 31, 2009 decreased from the same period in 2008 due to lower average investment balances as well as lower interest rates earned on our investments. Interest and other income, net, from continuing operations for the year ended December 31, 2008 decreased from 2007 due to lower average investment balances as well as lower interest rates earned on our investments.
Interest expense increased in 2009 when compared to 2008 because of interest expense and amortization of debt issuance costs associated with the QHP Notes. This is partially offset by reduced interest expense associated with the 2012 Notes and the 2023 Notes due to the partial repurchase in 2009 of each of our convertible notes. Interest expense from continuing operations increased during the year ended December 31, 2008 in comparison to 2007 because a portion of interest expense incurred in 2007 was capitalized as part of the construction costs of the Redwood City facility in that period.
Income Taxes
Income tax expense attributable to our continuing operations for the year ended December 31, 2009 was $90.6 million, which resulted primarily from applying the federal statutory income tax rate to income from continuing operations less a net adjustment to re-establish net operating loss carryforwards and certain other adjustments. We no longer pay state taxes because we moved our operations from California to Nevada in December 2008 and Nevada does not impose a corporate income tax. Income tax expense attributable to our continuing operations for the years ended December 31, 2008 and 2007 was $5.0 million and $10.6 million, respectively, which primarily related to federal and state taxes which were reduced by the release of the valuation allowance on our gross deferred tax assets. See Note 17 to the Consolidated Financial Statements for further discussion.
We recognized income tax expenses from our discontinued operations of zero and $7.2 million in 2009 and 2008, respectively, and an income tax benefit of $10.2 million in 2007. See Note 18 to the Consolidated Financial Statements for further discussion.
During the year ended December 31, 2009, we recorded a $0.8 million net decrease in our liabilities related to uncertain tax positions. The future impact of the unrecognized tax benefit of $23.1 million, if recognized, is as follows: $12.3 million would affect the effective tax rate and $10.8 million would result in adjustments to deferred tax assets and corresponding adjustments to the valuation allowance.
Estimated interest and penalties associated with unrecognized tax benefits decreased the provision for income taxes in the Consolidated Statements of Operations by $0.4 million and $0.1 million during the years ended December 31, 2009 and 2008, respectively, and increased the provision for income taxes by $0.1 million during the year ended December 31, 2007. Accrued interest and penalties associated with the underpayment of income taxes were $26,000 and $0.4 million as of December 31, 2009 and 2008, respectively. In general, our income tax
29
Table of Contents
returns are subject to examination by U.S. federal, state and various local tax authorities for tax years 1992 forward. We do not anticipate any additional unrecognized tax benefits in the next twelve months that would result in a material change to our financial position.
As of December 31, 2009, we had deferred tax assets in excess of our deferred tax liabilities of approximately $22.3 million. We recorded a valuation allowance to reduce our deferred tax assets to amounts that are more likely than not to be realized. As of December 31, 2009, we had a valuation allowance of $10.6 million, primarily related to net operating loss carryforwards and research and development tax credits.
Discontinued Operations
Biotechnology and Manufacturing Operations
On December 18, 2008, we spun off our former biotechnology operations to Facet and, in March 2008, we sold our manufacturing operations to Genmab A/S. We did not have discontinued operations for the year ended December 31, 2009. Significant components of our former biotechnology and manufacturing operations, presented as discontinued operations, were as follows:
| Year Ended December 31, | ||||||||
| (In thousands) |
2008 | 2007 | ||||||
| Net revenues |
$ | 27,770 | $ | 33,840 | ||||
| Total costs and expenses |
(150,234 | ) | (244,058 | ) | ||||
| Income tax benefit |
12,964 | 10,377 | ||||||
| Loss from operations |
$ | (109,500 | ) | $ | (199,841 | ) | ||
Commercial Operation
In March 2008, we completed the sale of our former commercial operation. We did not have discontinued operations for the year ended December 31, 2009. Significant components of our former commercial operation, presented as discontinued operation, were as follows:
| Year Ended December 31, | ||||||||
| (In thousands) |
2008 | 2007 | ||||||
| Net revenues |
$ | 66,467 | $ | 204,166 | ||||
| Total costs and expenses |
(106,687 | ) | (205,615 | ) | ||||
| Income tax expense |
(20,213 | ) | (220 | ) | ||||
| Loss from operations |
$ | (60,433 | ) | $ | (1,669 | ) | ||
See Note 18 to the Consolidated Financial Statements for further information associated with our discontinued operations.
Liquidity and Capital Resources
Historically, we financed our operations primarily through public and private placements of debt and equity securities, royalty and other license related revenues, product sales revenues, collaboration and other revenues under agreements with third parties and interest income on invested capital. In 2008, we divested assets associated with our former biotechnology and manufacturing operations as well as our former commercial operation. Since the divestiture of these operations, we have significantly downsized our operations and currently have fewer than ten employees managing our intellectual property, our licensing operations and other corporate activities as well as providing for certain essential reporting and management functions of a public company.
30
Table of Contents
We had cash, cash equivalents, short-term investments and restricted cash in the aggregate of $303.2 million and $147.5 million at December 31, 2009 and 2008, respectively. The $155.7 million increase was primarily attributable to net cash provided by operating activities of $187.0 million, net proceeds from the issuance of the QHP Notes of $285.7 million, and net excess tax benefits from stock-based compensation of $70.6 million, partially offset by our payment of $319.0 million in dividends during 2009 and the repurchase of $72.0 million face value of convertible notes. As a result of our downsized operations, we believe that cash from future royalty revenues, net of operating expenses, debt service and income taxes, plus cash on hand, will be sufficient to fund our operations over the next several years.
We intend to distribute a substantial portion of our income to our stockholders. In 2009, we paid two cash dividends of $0.50 per share of common stock totaling $59.7 million per distribution to our stockholders on April 1, 2009 and October 1, 2009 using proceeds from our cash on hand at December 31, 2008 as well as cash generated from operating activities. In December 2009, we paid an additional cash dividend of $1.67 per share of common stock totaling $199.6 million using a portion of the proceeds from the $300 million securitization transaction.
Effective December 2, 2009, in connection with the payment of the dividend in December 2009, the conversion rates for our outstanding 2012 Notes and 2023 Notes were adjusted upward to 119.294 and 164.7254 shares of common stock per $1,000 principal amount of the Notes or $8.38 and $6.07 per share of common stock, for each of the convertible notes, respectively. The adjustment was based on the amount of the dividend as well as the average trading price of our stock for certain periods before the record date and adjusted for ex-dividend trading pursuant to the terms of the applicable indenture.
Convertible Notes
2012 Notes
The 2012 Notes are convertible at any time, at the holders’ option, into our common stock at a conversion price of 119.294 shares of common stock per $1,000 principal amount of the 2012 Notes or $8.38 per share of common stock, as adjusted from the cash dividend declared in November 2009 and paid on December 15, 2009, and subject to further adjustment on the occurrence of certain events such as a dividend payment. Interest on the 2012 Notes is payable semiannually in arrears on February 15 and August 15 of each year. The 2012 Notes are senior unsecured debt and are redeemable by us in whole or in part on or after February 19, 2010 at 100.57% of principal amount if redeemed between February 19, 2010 and February 14, 2011 and at 100.29% of principal amount if redeemed between February 15, 2011 and the maturity date. The 2012 Notes are not puttable other than in the context of a fundamental change resulting in the reclassification, conversion, exchange or cancellation of our common stock. Such repurchase event or fundamental change is generally defined to include a merger involving PDL, an acquisition of a majority of PDL’s outstanding common stock, and a change of a majority of PDL’s board of directors without the approval of the board of directors.
In 2009, we repurchased $5.0 million face value of our 2012 Notes, at a discount of 10.75% from face value in a privately negotiated transaction with an institutional holder, for aggregate consideration of $4.5 million in cash, plus accrued but unpaid interest. Also in 2009, we repurchased an aggregate of $17.0 million face value of our 2012 Notes, at a discount of 3.0% from face value in privately negotiated transactions with institutional holders, for aggregate consideration of $16.5 million in cash, plus accrued but unpaid interest. The Company recorded a net gain of $0.8 million from the repurchase of these notes.
2023 Notes
The 2023 Notes are convertible at any time, at the holders’ option, into our common stock at a conversion rate of 164.7254 shares of common stock per $1,000 principal amount of the 2023 Notes or $6.07 per share of common stock, as adjusted from the cash dividend declared in November 2009 and paid on December 15, 2009 and subject to further adjustment on the occurrence of certain events such as a dividend payment. Interest on the 2023
31
Table of Contents
Notes is payable semiannually in arrears on February 16 and August 16 of each year. The 2023 Notes are unsecured and are subordinated to all our existing and future senior indebtedness. The 2023 Notes are redeemable at our option, in whole or in part, at par value. Holders of the 2023 Notes may require us to repurchase all or a portion of their 2023 Notes at 100% of their principal amount, plus any unpaid interest, on August 16, 2010, August 16, 2013 and August 16, 2018, and upon the occurrence of a repurchase event in which a change in control has occurred or our common stock is neither listed on a U.S. national securities exchange nor approved for trading over-the-counter. For any 2023 Notes put to us in August 2010, we must pay for the repurchase in cash. For any of the 2023 Notes put to us in August 2013 and August 2018, at our option, we may pay for the repurchase in cash, shares of our common stock or a combination of cash and shares of our common stock at the conversion rate then in effect.
In 2009, we repurchased an aggregate of $50.0 million face value of our 2023 Notes, at a discount of 2.0% from face value in privately negotiated transactions with institutional holders, for aggregate consideration of $49.0 million in cash, plus accrued but unpaid interest. The Company recorded a net gain of $0.7 million from the repurchase of these notes.
Non-Recourse Notes
In November 2009, we completed a $300 million securitization transaction in which we monetized the Genentech Royalties including Avastin, Herceptin, Lucentis, Xolair, and future products, if any, under which Genentech may take a license under our related agreements with Genentech. The QHP Notes bear interest at 10.25% per annum and were issued in a non-registered offering by QHP, our-wholly owned subsidiary. The Genentech Royalties and other payments, if any, that QHP will be entitled to receive under the agreements with Genentech, together with any funds made available from certain accounts of QHP, will be the sole source of payment of principal, interest and premium on the QHP Notes, which will be secured by a continuing security interest granted by QHP in its rights to receive the Genentech Royalties. The amount of quarterly repayment of the principal of the non-recourse notes will vary based upon the amount of future quarterly Genentech Royalties received. The anticipated final repayment date of the QHP Notes is December 2012. The QHP Notes may be redeemed at any time prior to maturity, in whole or in part, at the option of QHP at a make-whole redemption price.
Operating Lease
In February 2010, we entered into a lease amendment to extend our building lease term to May 2011 and obtained an option to further extend the lease until May 2012 for our office in Incline Village, Nevada.
Contractual Obligations
At December 31, 2009, our principal obligations are our convertible notes and our non-recourse notes, which in the aggregate total $728.0 million in principal. As discussed above, the 2012 Notes are not puttable other than in the context of a fundamental change and our 2023 Notes have a put right in August 2010, August 2013 and August 2018. The current conversion price of the 2023 Notes is $6.07 per share of our common stock and, accordingly, we expect that our debt service obligations over the next few years will consist solely of interest payments. To the extent holders of our 2023 Notes require us to repurchase all or a portion of their notes in August 2010 in cash should the then-current trading price of our common stock fall below the conversion price then in effect, we believe we will have sufficient funds for such repurchase from operating income together with our cash on hand at December 31, 2009, although we will evaluate our liquidity situation at such time and determine whether we should also undertake additional financings. We may also redeem, repurchase or otherwise acquire one or both series of convertible notes in the open market in the future which could adversely affect the amount or timing of any distributions to our stockholders. We would make such redemptions or repurchases only if we deemed it to be in our stockholders’ best interest. We may finance such redemptions or repurchases with cash on hand and/or with public or private equity or debt financings if we deem such financings are available on favorable terms.
32
Table of Contents
Our material contractual obligations under lease and debt agreements for the next five years and thereafter are as follows:
| Payments Due by Period | |||||||||||||||
| (In thousands) |
Less Than 1 Year |
1-3 Years | 4-5 Years | More than 5 Years |
Total | ||||||||||
| Operating leases |
$ | 230 | $ | 85 | $ | 5 | $ | - | $ | 320 | |||||
| Convertible notes (including interest payments)(1) |
210,058 | 230,280 | - | - | 440,338 | ||||||||||
| Non-recourse notes (including interest payments)(2) |
109,297 | 248,701 | - | - | 357,998 | ||||||||||
| Total contractual obligations |
$ | 319,585 | $ | 479,066 | $ | 5 | $ | - | $ | 798,656 | |||||
| (1) | The 2023 Notes are shown as being due in “Less Than 1 Year” column because they are puttable to the Company by note holders in August 2010. |
| (2) | Repayment of the non-recourse notes is based on anticipated future royalties to be received from Genentech and the anticipated final payment date is December 2012. |
Off-Balance Sheet Arrangements
In connection with the Spin-Off, we entered into amendments to the leases for our former facilities in Redwood City, California adding Facet as a co-tenant. In addition, we signed a Co-Tenancy Agreement with Facet under which we are obligated to make lease payments for the Redwood City facility in the event that Facet defaults under the lease. Such guarantee is in place for the original term of the leases, or through December 2021. We recorded the estimated fair value of the guarantee of $10.7 million as a long-term liability on our Consolidated Balance Sheets as of December 31, 2009 and 2008. However, our maximum exposure exceeds the amount recorded as a liability on our balance sheet. As of December 31, 2009, the lease payments subject to our guarantee aggregated approximately $130.8 million through December 31, 2021. In addition, should Facet default, we would also be responsible for lease related costs including utilities, property taxes and common area maintenance which may be as much as the actual lease payments.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Foreign Currency Risk
The underlying sales of our licensees’ products are conducted in multiple countries and in multiple currencies throughout the world. While foreign currency conversion terms vary by license agreement, generally most agreements require that royalties first be calculated in the currency of sale and then converted into U.S. dollars using the average daily exchange rates for that currency for a specified period at the end of the calendar quarter. Accordingly, when the U.S. dollar weakens in relation to other currencies, the converted amount is greater than it would have been had the U.S. dollar not weakened. Approximately 50 percent of underlying product sales is in currencies other than U.S. dollars; as such, our revenue may fluctuate due to changes in foreign currency exchange rates and is subject to foreign currency risk. For example, in a quarter in which we generate $60 million in revenue, approximately $30 million is based on sales in currencies other than U.S. dollar. If the U.S. dollar weakens across all currencies by ten percent during the conversion period for that quarter, when compared to the same amount of local currency royalties for the prior year, U.S. dollar converted royalties will be approximately $3 million more in the current quarter than in the prior year.
Interest Rate Risk
As of December 31, 2009, our investment portfolio was approximately $297.0 million and consisted of investments in Rule 2a-7 money market funds. If market interest rates were to have increased by 1% as of December 31, 2009, there would have been no material impact on the fair value of our portfolio. However, credit and liquidity risks could adversely affect the value of our investments in money market funds. If the difference between amortized cost
33
Table of Contents
and outside market valuations becomes significant, the fund’s valuation may change causing the fund to “break the buck” or move from the $1.00 net asset value. Our money market funds maintained a $1.00 net asset value and were not subject to withdrawal restrictions as of December 31, 2009. However, if credit market conditions worsen, the value of our money market funds could be adversely affected.
As of December 31, 2009, the aggregate fair value of our convertible notes was estimated to be $452.3 million, based on available pricing information. The 2023 Notes bear interest at a fixed rate of 2.75% and the 2012 Notes bear interest at a fixed rate of 2.00%. These obligations are subject to interest rate risk because the fixed interest rates under these obligations may exceed current interest rates.
As of December 31, 2009, the aggregate fair value of our non-recourse notes was estimated to be $300.0 million, which approximates the carrying value of the notes because negotiated terms and conditions in November 2009 are consistent with current market rates. The QHP Notes bear interest at a fixed rate of 10.25% per annum. This obligation is subject to interest rate risk because the fixed interest rates under this obligation may exceed current interest rates.
The following table presents information about our material debt obligations that are sensitive to changes in interest rates. The table presents principal amounts and related weighted-average interest rates by year of expected maturity for our debt obligations or the earliest in which the noteholders may put the debt to us. Our convertible notes may be converted to common stock prior to the maturity date.
| (In thousands) |
2010 | 2011 | 2012 | 2013 | 2014 | Thereafter | Total | Fair Value |
|||||||||||||||||
| Convertible notes |
|||||||||||||||||||||||||
| Fixed Rate |
$ | 199,998 | $ | - | $ | 228,000 | $ | - | $ | - | $ | - | $ | 427,998 | $ | 452,332 | (1) | ||||||||
| Avg. Interest Rate |
2.22% | 2.00% | 2.00% | -% | -% | -% | |||||||||||||||||||
| Non-recourse notes |
|||||||||||||||||||||||||
| Fixed Rate(3) |
$ | 77,852 | $ | 100,283 | $ | 121,865 | $ | - | $ | - | $ | - | $ | 300,000 | $ | 300,000 | (2) | ||||||||
| Avg. Interest Rate |
10.25% | 10.25% | 10.25% | -% | -% | -% | |||||||||||||||||||
| (1) | The fair value of the remaining payments under our convertible notes was estimated based on the trading value of these notes at December 31, 2009. |
| (2) | The fair value of the QHP Notes at December 31, 2009 approximates the carrying value of the notes because the terms and conditions at issuance on November 2, 2009 are consistent with current market rates. |
| (3) | Repayment of the Non-recourse Notes is based on anticipated future royalties to be received from Genentech and the anticipated final payment date is December 2012. |
34
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share data)
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 303,227 | $ | 129,058 | ||||
| Restricted cash |
- | 3,469 | ||||||
| Short-term investments |
- | 15,000 | ||||||
| Receivables from licensees |
1,050 | 13,500 | ||||||
| Deferred tax assets |
1,271 | 17,996 | ||||||
| Prepaid and other current assets |
10,288 | 1,658 | ||||||
| Total current assets |
315,836 | 180,681 | ||||||
| Property and equipment, net |
171 | 1,123 | ||||||
| Long-term deferred tax assets |
10,396 | 3,913 | ||||||
| Other assets |
12,008 | 5,425 | ||||||
| Total assets |
$ | 338,411 | $ | 191,142 | ||||
| Liabilities and Stockholders’ Deficit |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 370 | $ | 1,717 | ||||
| Accrued compensation |
2,206 | 7,856 | ||||||
| Accrued interest |
8,812 | 4,434 | ||||||
| Other accrued liabilities |
2,678 | 17,406 | ||||||
| Deferred revenue |
1,600 | 100 | ||||||
| Current portion of convertible notes payable |
199,998 | - | ||||||
| Current portion of non-recourse notes payable |
77,852 | - | ||||||
| Total current liabilities |
293,516 | 31,513 | ||||||
| Convertible notes payable |
228,000 | 499,998 | ||||||
| Non-recourse notes payable |
222,148 | - | ||||||
| Long-term deferred revenue |
- | 1,500 | ||||||
| Other long-term liabilities |
10,700 | 10,700 | ||||||
| Total liabilities |
754,364 | 543,711 | ||||||
| Commitments and contingencies (Note 13) |
||||||||
| Stockholders’ deficit: |
||||||||
| Preferred stock, par value $0.01 per share, 10,000 shares authorized; no shares issued and outstanding |
- | - | ||||||
| Common stock, par value $0.01 per share, 250,000 shares authorized; 119,523 and 119,305 shares issued and outstanding at December 31, 2009 and 2008, respectively |
1,195 | 1,193 | ||||||
| Additional paid-in capital |
(83,850 | ) | 169,196 | |||||
| Accumulated deficit |
(333,298 | ) | (522,958 | ) | ||||
| Total stockholders’ deficit |
(415,953 | ) | (352,569 | ) | ||||
| Total liabilities and stockholders’ deficit |
$ | 338,411 | $ | 191,142 | ||||
See accompanying notes.
35
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Revenues: |
||||||||||||
| Royalties |
$ | 305,049 | $ | 278,713 | $ | 224,735 | ||||||
| License and other |
13,135 | 15,483 | 350 | |||||||||
| Total revenues |
318,184 | 294,196 | 225,085 | |||||||||
| General and administrative expenses |
21,064 | 51,544 | 41,176 | |||||||||
| Operating income |
297,120 | 242,652 | 183,909 | |||||||||
| Gain on repurchase of convertible notes |
1,518 | — | — | |||||||||
| Interest and other income, net |
1,004 | 14,901 | 20,233 | |||||||||
| Interest expense |
(19,357 | ) | (14,219 | ) | (13,069 | ) | ||||||
| Income from continuing operations before income taxes |
280,285 | 243,334 | 191,073 | |||||||||
| Income tax expense |
90,625 | 5,014 | 10,624 | |||||||||
| Income from continuing operations |
189,660 | 238,320 | 180,449 | |||||||||
| Discontinued operations (Note 18): |
||||||||||||
| Loss from operations before income taxes |
— | (162,684 | ) | (211,667 | ) | |||||||
| Income tax expense (benefit) |
— | 7,249 | (10,157 | ) | ||||||||
| Loss on discontinued operations |
— | (169,933 | ) | (201,510 | ) | |||||||
| Net income (loss) |
$ | 189,660 | $ | 68,387 | $ | (21,061 | ) | |||||
| Income (loss) per basic share: |
||||||||||||
| Continuing operations |
$ | 1.59 | $ | 2.01 | $ | 1.55 | ||||||
| Discontinued operations |
— | (1.43 | ) | (1.73 | ) | |||||||
| Net income (loss) per basic share |
$ | 1.59 | $ | 0.58 | $ | (0.18 | ) | |||||
| Income (loss) per diluted share: |
||||||||||||
| Continuing operations |
$ | 1.07 | $ | 1.48 | $ | 1.34 | ||||||
| Discontinued operations |
— | (1.01 | ) | (1.42 | ) | |||||||
| Net income (loss) per diluted share |
$ | 1.07 | $ | 0.47 | $ | (0.08 | ) | |||||
| Shares used to compute income (loss) per basic and diluted share: |
||||||||||||
| Shares used to compute income (loss) per basic share |
119,402 | 118,728 | 116,365 | |||||||||
| Shares used to compute income (loss) per diluted share |
184,400 | 167,869 | 141,480 | |||||||||
See accompanying notes.
36
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net income (loss) |
$ | 189,660 | $ | 68,387 | $ | (21,061 | ) | |||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
||||||||||||
| Asset impairment charges |
- | 3,777 | 5,513 | |||||||||
| Depreciation expense |
991 | 20,909 | 32,150 | |||||||||
| Amortization of convertible notes offering costs |
2,159 | 2,345 | 2,344 | |||||||||
| Amortization of non-recourse notes offering costs |
1,256 | - | - | |||||||||
| Amortization of intangible assets |
- | 1,585 | 32,341 | |||||||||
| Gain on repurchase of convertible notes |
(1,518 | ) | - | - | ||||||||
| Stock-based compensation expense |
821 | 8,783 | 20,578 | |||||||||
| Loss on sale of assets, net |
- | 14,897 | - | |||||||||
| Loss on disposal of equipment |
- | 220 | 763 | |||||||||
| Tax benefit from stock-based compensation arrangements |
64,140 | 19,720 | - | |||||||||
| Net excess tax benefit from stock-based compensation |
(70,610 | ) | (19,317 | ) | - | |||||||
| Changes in assets and liabilities: |
||||||||||||
| Accounts receivable, net |
- | 17,201 | 9,652 | |||||||||
| Interest receivable |
- | 967 | 1,169 | |||||||||
| Inventories |
- | - | (4,218 | ) | ||||||||
| Receivables from licensees |
12,450 | (12,490 | ) | (560 | ) | |||||||
| Other current assets |
(4,903 | ) | (12,497 | ) | 4,091 | |||||||
| Deferred tax asset |
10,242 | (21,909 | ) | - | ||||||||
| Other assets |
- | 568 | (23 | ) | ||||||||
| Accounts payable |
(1,347 | ) | (7,176 | ) | (4,585 | ) | ||||||
| Accrued liabilities |
(16,387 | ) | (32,350 | ) | (4,146 | ) | ||||||
| Other long-term liabilities |
- | 2,859 | 2,956 | |||||||||
| Deferred revenue |
- | 23,670 | (9,991 | ) | ||||||||
| Total adjustments |
(2,706 | ) | 11,762 | 88,034 | ||||||||
| Net cash provided by operating activities |
186,954 | 80,149 | 66,973 | |||||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of investments |
- | (15,000 | ) | (134,588 | ) | |||||||
| Maturities of investments |
15,000 | 70,778 | 291,083 | |||||||||
| Sale of commercial assets |
- | 272,945 | - | |||||||||
| Sale of manufacturing assets |
- | 236,560 | - | |||||||||
| Purchase of property and equipment |
(39 | ) | (3,273 | ) | (94,738 | ) | ||||||
| Proceeds from the sale of property and equipment |
- | - | 20,903 | |||||||||
| Release of (transfer to) restricted cash |
3,469 | 24,805 | (10,005 | ) | ||||||||
| Net cash provided by investing activities |
18,430 | 586,815 | 72,655 | |||||||||
| Cash flows from financing activities |
||||||||||||
| Cash distribution to Facet Biotech Corporation |
- | (405,968 | ) | - | ||||||||
| Proceeds from issuance of common stock, net of cancellations |
1,402 | 15,390 | 27,273 | |||||||||
| Cash dividends paid |
(319,020 | ) | (506,612 | ) | - | |||||||
| Repurchase of convertible notes |
(69,953 | ) | - | - | ||||||||
| Net proceeds from the issuance of non-recourse notes |
285,746 | - | - | |||||||||
| Net excess tax benefit from stock-based compensation |
70,610 | 19,317 | - | |||||||||
| Proceeds from financing of tenant improvements |
- | - | 2,118 | |||||||||
| Payments on other long-term debt |
- | (667 | ) | (7,394 | ) | |||||||
| Net cash provided by (used in) financing activities |
(31,215 | ) | (878,540 | ) | 21,997 | |||||||
| Net increase (decrease) in cash and cash equivalents |
174,169 | (211,576 | ) | 161,625 | ||||||||
| Cash and cash equivalents at beginning of the year |
129,058 | 340,634 | 179,009 | |||||||||
| Cash and cash equivalents at end the year |
$ | 303,227 | $ | 129,058 | $ | 340,634 | ||||||
37
Table of Contents
PDL BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS, continued
(In thousands)
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Supplemental Disclosure of Cash Flow Information |
|||||||||
| Cash paid during the year for interest |
$ | 11,552 | $ | 11,874 | $ | 12,449 | |||
| Cash paid during the year for income taxes |
$ | 29,258 | $ | 8,525 | $ | 162 | |||
| Non-Cash Investing and Financing Activities |
|||||||||
| Transfer of assets, net of liabilities, to Facet Biotech Corporation |
$ | - | $ | 49,651 | $ | - | |||
| Guarantee issued in connection with the Spin-Off (Note 15) |
$ | - | $ | 10,700 | $ | - | |||
| Capitalization of facilities under financing lease transactions, including accrued interest, and corresponding long-term financing |
$ | - | $ | - | $ | 1,549 | |||
| Issuance of escrow shares to former ESP stockholders |
$ | - | $ | - | $ | 12,580 | |||
See accompanying notes.
38
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands, except share data)
| Common Stock | Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Income (loss) |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||
| Shares | Amount | ||||||||||||||||||||
| Balance at December 31, 2006 |
115,006,260 | $ | 1,150 | $ | 1,037,846 | $ | (570,129 | ) | $ | (1,326 | ) | $ | 467,541 | ||||||||
| Issuance of common stock under employee benefit plans, net |
2,065,352 | 21 | 27,252 | - | - | 27,273 | |||||||||||||||
| Stock-based compensation expense for employees |
- | - | 20,513 | - | - | 20,513 | |||||||||||||||
| Stock-based compensation expense for consultants |
- | - | 65 | - | - | 65 | |||||||||||||||
| Issuance of common stock in connection with release of escrow shares from ESP Pharma acquisition |
505,650 | 5 | 12,575 | - | - | 12,580 | |||||||||||||||
| Adoption of accounting for uncertain tax positions |
- | - | - | (155 | ) | - | (155 | ) | |||||||||||||
| Comprehensive loss: |
|||||||||||||||||||||
| Net loss |
- | - | - | (21,061 | ) | - | (21,061 | ) | |||||||||||||
| Change in unrealized gains and losses on investments in available-for-sale securities, net of tax |
- | - | - | - | 536 | 536 | |||||||||||||||
| Change in postretirement liability not yet recognized as net period expense, net of tax |
- | - | - | - | 318 | 318 | |||||||||||||||
| Total comprehensive loss |
(20,207 | ) | |||||||||||||||||||
| Balance at December 31, 2007 |
117,577,262 | 1,176 | 1,098,251 | (591,345 | ) | (472 | ) | 507,610 | |||||||||||||
| Issuance of common stock under employee benefit plans, net |
1,727,304 | 17 | 15,373 | - | - | 15,390 | |||||||||||||||
| Stock-based compensation expense for employees |
- | - | 8,783 | - | - | 8,783 | |||||||||||||||
| Tax benefit from employee stock options |
- | - | 19,720 | - | - | 19,720 | |||||||||||||||
| Guarantee issued in connection with the spin-off of biotechnology operations |
- | - | (10,700 | ) | - | - | (10,700 | ) | |||||||||||||
| Dividends paid |
- | - | (506,612 | ) | - | - | (506,612 | ) | |||||||||||||
| Spin-off of biotechnology operations |
- | - | (455,619 | ) | - | - | (455,619 | ) | |||||||||||||
| Comprehensive income: |
|||||||||||||||||||||
| Net income |
- | - | - | 68,387 | - | 68,387 | |||||||||||||||
| Change in unrealized gains and losses on investments in available-for-sale securities, net of tax |
- | - | - | - | (67 | ) | (67 | ) | |||||||||||||
| Change in postretirement liability not yet recognized as net period expense, net of tax |
- | - | - | - | 539 | 539 | |||||||||||||||
| Total comprehensive income |
68,859 | ||||||||||||||||||||
| Balance at December 31, 2008 |
119,304,566 | 1,193 | 169,196 | (522,958 | ) | - | (352,569 | ) | |||||||||||||
| Issuance of common stock under employee benefit plans, net |
218,319 | 2 | 1,400 | - | - | 1,402 | |||||||||||||||
| Stock-based compensation expense for employees |
- | - | 773 | - | - | 773 | |||||||||||||||
| Stock-based compensation expense for consultants |
- | - | 48 | - | - | 48 | |||||||||||||||
| Tax benefit from employee stock options |
- | - | 64,140 | - | - | 64,140 | |||||||||||||||
| Dividends declared |
- | - | (319,407 | ) | - | - | (319,407 | ) | |||||||||||||
| Net income and comprehensive income |
- | - | - | 189,660 | - | 189,660 | |||||||||||||||
| Balance at December 31, 2009 |
119,522,885 | $ | 1,195 | $ | (83,850 | ) | $ | (333,298 | ) | $ | - | $ | (415,953 | ) | |||||||
See accompanying notes.
39
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2009
1. Organization and Business
PDL BioPharma, Inc. (we, us, our, PDL and the Company) was incorporated in Delaware in 1986. Our business is the management of our antibody humanization patents and royalty assets which consist of our Queen et al. patents and license agreements with numerous biotechnology and pharmaceutical companies. We receive royalties based on sales of humanized antibody products pursuant to certain rights we have licensed under our patents and may also receive royalty payments on new humanized antibody products launched before final patent expiry in December 2014. Generally, our license agreements cover humanized antibodies targeting antigens specified in the license agreements.
Under most of our licensing agreements, we are entitled to receive a flat-rate or tiered royalty based upon our licensees’ net sales of covered antibodies. In each of the years ended December 31, 2009, 2008 and 2007, we received royalties on sales of the nine humanized antibody products, all of which are currently approved for use by the U.S. Food and Drug Administration (FDA) and eight are approved by other regulatory agencies outside the United States. We have also entered into licensing agreements pursuant to which we have licensed certain rights under our patents for development-stage products that have not yet reached commercialization including products that are currently in Phase 3 clinical trials.
Until December 2008, our business included biotechnology operations which were focused on the discovery and development of novel antibodies which we spun off (the Spin-Off) to Facet Biotech Corporation (Facet). From March 2005 until March 2008, we also had commercial operations as well as manufacturing operations which we partially divested in 2006 and fully divested in 2008. The financial results of our former biotechnology, manufacturing and commercial operations are presented as discontinued operations in the Consolidated Statement of Operations. For further information, see Note 18.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP) and pursuant to the rules and regulations of the Securities and Exchange Commission (SEC). Certain amounts in prior periods have been reclassified to conform to the current period presentation.
Principles of Consolidation
Beginning in November 2009, the consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary, QHP Royalty Sub LLC (QHP). Prior to the Spin-Off, the consolidated financial statements included the accounts of PDL BioPharma, Inc. and its wholly-owned subsidiaries which were transferred to Facet and are now presented as discontinued operations, see Note 18. All material intercompany balances and transactions have been eliminated.
Management Estimates
The preparation of financial statements in conformity with GAAP requires the use of management’s estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
40
Table of Contents
Segment Disclosures
We are required to report operating segments and make related disclosures about our products, services, geographic areas and major customers. Our chief operating decision-maker consisted of our executive management. Our chief operating decision-maker reviews our operating results and operating plans and makes resource allocation decisions on a company-wide or aggregate basis. As of December 31, 2009, we operated as one segment. Our operations and facilities are located in Incline Village, Nevada.
Cash Equivalents, Restricted Cash, Investments and Concentration of Credit Risk
We consider all highly liquid investments with initial maturities of three months or less at the date of purchase to be cash equivalents. We place our cash, cash equivalents, investments and restricted cash with high credit quality financial institutions and in securities of the U.S. government, U.S. government agencies and U.S. corporations and, by policy, limit the amount of credit exposure in any one financial instrument.
Fair Value Measurements
The fair value of our financial instruments are estimates of the amounts that would be received if we were to sell an asset or we paid to transfer a liability in an orderly transaction between market participants at the measurement date (exit price). We apply a three-level valuation hierarchy for fair value measurements. The categorization of assets and liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the measurement of fair value. Level 1 inputs to the valuation methodology utilize unadjusted quoted market prices in active markets for identical assets and liabilities. Level 2 inputs to the valuation methodology are other observable inputs, including quoted market prices for similar assets and liabilities, quoted prices for identical and similar assets and liabilities in the markets that are not active, or other inputs that are observable or can be corroborated by observable market data. Level 3 inputs to the valuation methodology are unobservable inputs based upon management’s best estimate of the inputs that market participants would use in pricing the asset or liability at the measurement date, including assumptions about risk. We do not estimate the fair value of our royalty assets.
Revenue Recognition
We recognize royalty, licensing and other revenues from our proprietary patent portfolio covering the humanization of antibodies for use as drugs, in drug development and production. In connection with the divestiture of our former biotechnology, manufacturing and commercial operations, all revenues resulting from product sales and certain license and other revenues, including all revenues that we have recognized in the past from our collaboration partners under collaboration agreements, have been reflected as discontinued operations in the Consolidated Statement of Operations, see Note 18.
Revenues, and their respective accounting treatment for financial reporting purposes, are as follows:
Royalty Revenues
Under most of our patent license agreements, we receive royalty payments based upon our licensees’ net sales of covered products. Generally, under these agreements we receive royalty reports from our licensees approximately one quarter in arrears, that is, generally in the second month of the quarter after the licensee has sold the royalty-bearing product. We recognize royalty revenues when we can reliably estimate such amounts and collectibility is reasonably assured. Accordingly, we recognize royalty revenues in the quarter reported to us by our licensees, i.e., generally royalty revenues are recognized one quarter following the quarter in which sales by our licensees occurred. Under this accounting policy, the royalty revenues we report are not based upon our estimates and such royalty revenues are typically reported in the same period in which cash is received from our licensees.
41
Table of Contents
We may also receive annual license maintenance fees, payable at the election of the licensee to maintain the license in effect. We have no performance obligations with respect to such fees. Maintenance fees are recognized as they are due and when payment is reasonably assured.
License and Other Revenues
Generally there are three types of arrangements we enter into under which we provide access to our proprietary patent portfolio covering the humanization of antibodies.
| • | Under patent license agreements, the licensee typically obtains a non-exclusive license to one or more of our patents. In this arrangement, the licensee is responsible for all of the development work on its product. The licensee has the technical ability to perform the humanization of the antibody it is developing using our patented technology, but needs to obtain a license from us to avoid infringing our patents. We have no future performance obligations under these agreements. Consideration that we receive for patent license agreements is recognized upon execution and delivery of the patent license agreement and when payment is reasonably assured. |
| • | Under patent rights agreements, the licensee purchases a research patent license in exchange for an upfront fee. In addition, the licensee has the right to obtain, in exchange for consideration separate from the upfront fee, patent licenses for commercial purposes for a specified number of drug targets to be designated by the licensee subsequent to execution of the agreement. The licensee performs all of the research, and we have no further performance obligations with respect to the research patent license and the grant of the right to obtain commercial patent licenses; therefore, upon delivery of the patent rights agreement, the earnings process is complete. When a licensee exercises its right to obtain patent licenses to certain designated drug targets for commercial purposes, we recognize the related consideration as revenues upon the licensee’s exercise of such right, execution and delivery of the associated patent license agreement and when payment is reasonably assured. |
| • | Prior to the Spin-Off, under antibody humanization agreements, the licensee would typically pay an upfront fee for us to humanize an antibody. These upfront fees were recognized as the humanization work was performed, which was typically over three to six months, or upon acceptance of the humanized antibody by our licensee if such acceptance clause existed in the agreement. Such amounts are presented as discontinued operations in the Consolidated Statements of Operations. |
We enter into patent license and humanization agreements that may contain milestones associated with reaching particular stages in product development. We recognize “at risk” milestone payments upon achievement of the underlying milestone event and when they are due and payable under the arrangement. Milestones are deemed to be “at risk” when, at the onset of an arrangement, management believes that they will require a reasonable amount of effort to be achieved and are not simply reached by the lapse of time or through a perfunctory effort. Milestones which are not deemed to be “at risk” are recognized as revenue in the same manner as up-front payments. Generally, there are three types of agreements under which a customer would owe us a milestone payment:
| • | Patent license agreements and humanization agreements sometimes require our licensees to make milestone payments to us when they achieve certain progress, such as FDA approval, with respect to the licensee’s product. |
| • | We may also receive certain milestone payments in connection with licensing technology to or from our licensees, such as product licenses. Under these agreements, our licensees may make milestone payments to us when certain levels of development are achieved with respect to the licensed technology. |
| • | Prior to the divestiture of our commercial operations and the Spin-Off, we entered into humanization agreements which provided for the payment of certain milestones to us after the completion of services to perform the humanization process. These milestones generally include delivery of a humanized antibody |
42
Table of Contents
| meeting a certain binding affinity and, at the customer’s election, delivery of a cell line meeting certain criteria described in the original agreement. |
Amounts recognized with respect to our former biotechnology, manufacturing and commercial operations are presented as discontinued operations in the Consolidated Statements of Operations.
Collaboration Revenues
Prior to the divestiture of our commercial operation and the Spin-Off, amounts received from our collaboration partners were recognized as revenue as the related services were performed. In certain instances, our collaboration agreements involved a combination of upfront fees, milestones and development costs where we were not able to establish fair value of all of the undelivered elements. In those cases, we recognized these upfront fees, milestones and reimbursements of development costs as the services were performed. Such amounts are presented as discontinued operations in the Consolidated Statements of Operations.
Product Sales Revenues
Prior to the divestiture of our commercial operation, we recognized revenues from product sales when there was persuasive evidence that an arrangement existed, title passed, the price was fixed and determinable, and collectibility was reasonably assured. Product sales were recognized net of estimated allowances, discounts, sales returns, chargebacks and rebates. Such amounts are presented as discontinued operations in the Consolidated Statements of Operations.
Advertising and Promotional Expenses
Prior to the divestiture of our commercial operation and the Spin-Off, we engaged in promotional activities, which typically took the form of industry publications, journal ads, exhibits, speaker programs and other forms of media. Advertising and promotion expenditures were expensed as incurred. These expenses for the years ended December 31, 2009, 2008 and 2007 were zero, $3.4 million and $19.6 million, respectively, and are presented as discontinued operations in the Consolidated Statements of Operations.
Shipping and Handling Expenses
Prior to the divestiture of our commercial operation and the Spin-Off, we recorded costs associated with shipping and handling of revenue-generating products in cost of product sales, which costs are presented as discontinued operations in the Consolidated Statements of Operations.
Research and Development Expenses
Prior to the divestiture of our commercial operation and the Spin-Off, major components of research and development expenses consisted of personnel costs, including salaries and benefits, clinical development, preclinical work, pharmaceutical development, materials and supplies, payments associated with work completed for us by third-party research organizations and overhead allocations consisting of various administrative and facilities related costs. All research and development costs were charged to expense as incurred and, since they related entirely to our former commercial and biotechnology operations, are reflected as discontinued operations in the Consolidated Statements of Operations. Research and development expenses were zero, $166.9 million and $237.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Comprehensive Income (Loss)
Comprehensive income (loss) comprises net income (loss) adjusted for other comprehensive income (loss) which includes the changes in unrealized gains and losses on our investments in marketable securities, if any, which are excluded from our net income (loss). In addition, other comprehensive income (loss) includes the liability, if any,
43
Table of Contents
that has not yet been recognized as net periodic benefit cost for our postretirement benefit. Our former post-retirement benefit plan was assigned to Facet in connection with the Spin-Off in December 2008. Our comprehensive income (loss) for the years ended December 31, 2009, 2008 and 2007 is presented in the Consolidated Statements of Stockholders’ Equity. As of December 31, 2009 and 2008, we had no unrealized gains or losses on investments and we had assigned the rights and obligations under our former post-employment benefit plan to Facet in connection with the Spin-Off; therefore, our accumulated other comprehensive income (loss) as of December 31, 2009 and 2008 was zero.
Capitalized Software
Prior to the divestiture of our commercial operation and the Spin-Off, we recognized costs incurred in the preliminary planning phase of software development as expense as the costs were incurred. Software development costs incurred in the application development phase were capitalized and were included in property and equipment. For the years ended December 31, 2009 and 2008, we did not capitalize software development costs. For the year ended December 31, 2007, we capitalized software development costs of $4.1 million. Once the developed software was placed into service, these costs were amortized over the estimated useful life of the software.
Foreign Currency Translation
Prior to the divestiture of our commercial operation and the Spin-Off, the U.S. dollar was the functional currency for our former French subsidiary, which was assigned to Facet in connection with the Spin-Off in December 2008. All foreign currency gains and losses are presented as discontinued operations in the Consolidated Statements of Operations and have not been material.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization were computed using the straight-line method over the following estimated useful lives:
| Leasehold improvements |
Shorter of asset life or term of lease | |
| Computer and office equipment |
3 years | |
| Furniture and fixtures |
7 years |
Prior to the Spin-Off, we also had the following:
| Buildings and improvements |
20 years | |
| Laboratory and manufacturing equipment |
7 years |
Depreciation and amortization related to buildings and improvements, laboratory and manufacturing equipment as well as other property and equipment, used by our former biotechnology, manufacturing and commercial operations are presented as discontinued operations in the Consolidated Statements of Operations.
Capitalization of Interest Cost
Prior to the divestiture of our commercial operation and the Spin-Off, we capitalized a portion of our interest on borrowings in connection with significant capital expenditures. Of total interest cost incurred of $16.8 million during the year ended December 31, 2007, we capitalized interest of $3.1 million. We did not capitalize interest in 2009 and 2008. Of the total interest expense, zero, $1.6 million and $0.6 million during the years ended December 31, 2009, 2008 and 2007, respectively, are presented as discontinued operations in the Consolidated Statements of Operations.
44
Table of Contents
Long-Lived Assets
We identify and record impairment losses, as circumstances dictate, on long-lived assets used in operations when events and circumstances indicate that the assets might be impaired and the discounted cash flows estimated to be generated by those assets are less than the carrying amounts of those assets.
Recent Accounting Pronouncements
Management reviewed the most recently issued accounting pronouncements and determined that none were applicable to the Company.
3. Stock-Based Compensation
We recognize compensation expense, using a fair-value based method, for costs associated with all share-based awards including stock options and stock issued to our employees and directors under our stock plans. The value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service periods in our Consolidated Statements of Operations.
We have adopted the simplified method to calculate the beginning balance of the additional paid-in capital (APIC) pool of the excess tax benefit and to determine the subsequent effect on the APIC pool and Consolidated Statements of Cash Flows of the tax effects of employee stock-based compensation awards that were outstanding upon our adoption.
We calculate stock-based compensation expense based on the number of awards ultimately expected to vest, net of estimated forfeitures. We estimate forfeiture rates at the time of grant and revise such rates, if necessary, in subsequent periods if actual forfeitures differ from those estimates. In connection with the Spin-Off of Facet in December 2008 and the termination of our former employees, we adjusted the forfeiture rate assumption to 100% for all option pools except for our current members of the board of directors. As a result, during the fourth quarter of 2008, we recognized a change in estimate for stock-based compensation expense of $2.7 million, which reduced our net loss by such amount, reflecting the amount of stock-based expense recognized in prior periods that was not earned by employees as of their termination on the Spin-Off date. As this amount relates to unvested stock options held by our former employees who were associated with the biotechnology, manufacturing and commercial operations, this adjustment is reflected as discontinued operations in the Consolidated Statements of Operations.
Stock-based compensation expense for employees and directors for the years ended December 31, 2009, 2008 and 2007 was as follows:
| Year Ended December 31, | |||||||||||
| (In thousands) |
2009 | 2008 | 2007 | ||||||||
| General and administrative |
$ | 773 | $ | 879 | $ | 1,406 | |||||
| Discontinued operations |
- | 7,904 | 19,107 | ||||||||
| Total stock-based compensation expense |
773 | 8,783 | 20,513 | ||||||||
| Tax benefit related to current year stock-based compensation |
(271 | ) | (2,861 | ) | - | ||||||
| Stock-based compensation expense included in net income (loss) |
$ | 502 | $ | 5,922 | $ | 20,513 | |||||
We also account for stock options granted to persons other than employees or directors at fair value. Stock options granted to non-employees are subject to periodic re-measurement over their vesting terms. We recognize the resulting stock-based compensation expense during the service period over which the non-employee provides services to us. The stock-based compensation expense related to non-employees for the years ended December 31, 2009, 2008 and 2007 was $48,000, zero and $65,000, respectively.
45
Table of Contents
Valuation Assumptions
The stock-based compensation expense recognized for the years ended December 31, 2009, 2008 and 2007 was determined using the Black-Scholes option valuation model. Option valuation models require the input of subjective assumptions and these assumptions can vary over time. We did not grant any stock options under our stock-based incentive plans or issue shares of common stock under our employee stock purchase plan during 2009, therefore weighted-average assumptions for 2009 are not presented below.
The weighted-average assumptions used for the years ended December 31, 2008 and 2007 were as follows:
| Year ended December 31, | ||||||
| 2008 | 2007 | |||||
| Stock Option Plans |
||||||
| Expected life, in years |
4.0 | 4.0 | ||||
| Risk free interest rate |
2.4 | % | 4.5 | % | ||
| Volatility |
41 | % | 38 | % | ||
| Dividend yield |
- | - | ||||
| Employee Stock Purchase Plans |
||||||
| Expected life, in years |
0.5 | 0.5 | ||||
| Risk free interest rate |
2.8 | % | 5.1 | % | ||
| Volatility |
32 | % | 38 | % | ||
| Dividend yield |
- | - | ||||
Our expected term represents the period that we expect our stock-based awards to be outstanding, which we determined based on historical experience of similar awards, the contractual terms of the stock-based awards, vesting schedules and expectations of future optionee behavior as influenced by changes to the terms of stock-based awards. We base expected volatility on both the historical volatility of our common stock and implied volatility derived from the market prices of traded options of our common stock. We base the risk-free interest rate on the implied yield available on U.S. Treasury zero-coupon issues with a remaining term equal to the expected term of our options at the time of grant. Even though we issued a cash dividend in May 2008 relating to the sales of our former commercial operations and our former antibody manufacturing plant in March 2008, the dividend yield was determined to be zero since we did not have a plan in place to pay any additional cash dividends in the foreseeable future.
Stock-Based Incentive Plans
We currently have one active stock-based incentive plan under which we may grant stock-based awards to our employees, directors and consultants. Prior to 2009, we had five stock-based incentive plans which we could grant stock based awards.
46
Table of Contents
The total number of shares of common stock authorized for issuance, shares of common stock issued upon exercise of options or grant of restricted stock, shares of common stock subject to outstanding awards and available for grant under each of these plans as of December 31, 2009, is set forth in the table below:
| Title of Plan |
Total Shares of Common Stock Authorized |
Total Shares of Common Stock Issued |
Total Shares of Common Stock Subject to Outstanding Awards |
Total Shares of Common Stock Available for Grant | ||||
| 2005 Equity Incentive Plan(1) |
5,200,000 | 314,036 | - | 4,885,964 | ||||
| 2002 Outside Directors Stock Option Plan(2) |
208,250 | 140,750 | 67,500 | - | ||||
| 1999 Non-statutory Stock Option Plan(2) |
5,075,707 | 4,966,183 | 109,524 | - | ||||
| 1999 Stock Option Plan(2) |
5,039,719 | 3,653,150 | 1,386,569 | - | ||||
| 1991 Nonstatutory Stock Option Plan(3) |
13,994,479 | 13,994,479 | - | - | ||||
| 29,518,155 | 23,068,598 | 1,563,593 | 4,885,964 | |||||
| (1) | As of December 31, 2009, there were 148,198 shares of unvested restricted stock awards outstanding. |
| (2) | This plan was terminated in 2009 subject to options outstanding under this plan. |
| (3) | This plan expired in 2001 and we may no longer grant awards under this plan. |
Under our 2005 Equity Incentive Plan, we are authorized to issue a variety of incentive awards, including stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance share and performance unit awards, deferred compensation awards and other stock-based or cash-based awards.
In September 2009, our Compensation Committee terminated the 1991 Nonstatutory Stock Option Plan. Also in September 2009, our Compensation Committee terminated the 1999 Outside Director Stock Option Plan and the 1999 Nonstatutory Stock Option Plan subject to any outstanding options. In June 2009, our stockholders approved amendments to the Company’s 2005 Equity Incentive Plan to expand persons eligible to participate in the plan to include our outside directors. In February 2009, our Compensation Committee terminated the 2002 Outside Directors Stock Option Plan, subject to any outstanding options.
Stock options granted to employees under our stock-based incentive plans in connection with the start of employment customarily vested over four years with 25% of the shares subject to such an option vesting on the first anniversary of the grant date and the remainder of the stock option vesting monthly after the first anniversary at a rate of one thirty-sixth of the remaining non-vested shares subject to the stock option. Stock options granted to employees as additional incentive and for performance reasons after the start of employment customarily vested monthly after the grant date or such other vesting start date set by the company on the grant date at a rate of one forty-eighth of the shares subject to the option. Each outstanding stock option granted prior to mid-July 2005 has a term of 10 years and each outstanding stock option granted after mid-July 2005 has a term of seven years.
Employee Stock Purchase Plan
In addition to the stock-based incentive plans described above, we adopted the 1993 Employee Stock Purchase Plan (ESPP), which was intended to qualify as an “employee stock purchase plan” under Section 423 of the Internal Revenue Code of 1986, as amended. However, after the Spin-Off, the Company’s Compensation Committee terminated the Company’s ESPP in June 2009. Under the ESPP prior to its termination, full-time employees who owned less than 5% of our outstanding shares of common stock were eligible to contribute a percentage of their base salary, subject to certain limitations, over the course of six-month offering periods for
47
Table of Contents
the purchase of shares of common stock. The purchase price for shares of common stock purchased under our ESPP equaled 85% of the fair market value of a share of common stock at the beginning or end of the relevant six-month offering period, whichever was lower. The stock-based compensation expense recognized in connection with our ESPP for the years ended December 31, 2008 and 2007 was $0.3 million and $1.6 million, respectively. No shares of common stock were purchased during the year ended December 31, 2009.
Stock Option Activity
A summary of our stock option activity for the years ended December 31, 2009, 2008 and 2007 is presented below:
| 2009 | 2008 | 2007 | ||||||||||||||||
| (In thousands) |
Shares | Weighted- Average Exercise Price |
Shares | Weighted- Average Exercise Price |
Shares | Weighted- Average Exercise Price | ||||||||||||
| Outstanding at beginning of year |
5,776 | $ | 18.04 | 14,956 | $ | 19.85 | 14,313 | $ | 18.79 | |||||||||
| Granted |
- | - | 1,055 | 9.34 | 3,980 | 21.92 | ||||||||||||
| Exercised |
(213 | ) | 6.57 | (1,775 | ) | 8.23 | (1,664 | ) | 13.69 | |||||||||
| Forfeited |
(3,999 | ) | 17.96 | (8,460 | ) | 22.21 | (1,673 | ) | 21.58 | |||||||||
| Outstanding at end of year |
1,564 | 19.82 | 5,776 | 18.04 | 14,956 | 19.85 | ||||||||||||
| Exercisable at end of year |
1,543 | 20.01 | 5,665 | 18.24 | 9,076 | 19.11 | ||||||||||||
| Weighted-average grant-date fair value of options granted during the year |
N/A | $ | 3.60 | $ | 7.79 | |||||||||||||
The following is a summary of stock options outstanding, stock options exercisable and their aggregate intrinsic value as of December 31, 2009:
| Outstanding | Exercisable | |||||||||||||||||
| Range of Exercise Prices |
Number Outstanding (in thousands) |
Weighted- Average Remaining Contractual Life (years) |
Weighted- Average Exercise Price |
Aggregate Intrinsic Value (in thousands) |
Number Exercisable (in thousands) |
Weighted- Average Exercise Price |
Aggregate Intrinsic Value (in thousands) | |||||||||||
| $5.41 - $14.39 |
92 | 4.70 | $ | 8.57 | 71 | $ | 9.51 | |||||||||||
| $16.00 |
240 | 0.09 | $ | 16.00 | 240 | $ | 16.00 | |||||||||||
| $17.70 |
2 | 4.70 | $ | 17.70 | 2 | $ | 17.70 | |||||||||||
| $18.12 |
500 | 0.09 | $ | 18.12 | 500 | $ | 18.12 | |||||||||||
| $18.71 - $20.83 |
75 | 3.06 | $ | 19.62 | 75 | $ | 19.62 | |||||||||||
| $20.91 |
15 | 4.57 | $ | 20.91 | 15 | $ | 20.91 | |||||||||||
| $20.99 |
20 | 0.09 | $ | 20.99 | 20 | $ | 20.99 | |||||||||||
| $22.60 |
105 | 4.28 | $ | 22.60 | 105 | $ | 22.60 | |||||||||||
| $24.60 |
500 | 0.09 | $ | 24.60 | 500 | $ | 24.60 | |||||||||||
| $25.54 |
15 | 0.09 | $ | 25.54 | 15 | $ | 25.54 | |||||||||||
| Totals |
1,564 | 0.83 | $ | 19.82 | $ | 83 | 1,543 | $ | 20.01 | $ | 53 | |||||||
Aggregate intrinsic value in the table above represents the total pre-tax intrinsic value, based on the closing prices of our common stock of $6.86 on December 31, 2009, which would have been received by the option holders had all option holders exercised their options as of that date. In connection with the Spin-Off of Facet in December 2008, we terminated substantially all employees. As a result, approximately 4 million options with an average exercise price of $17.96 were forfeited during the year ended December 31, 2009. Total unrecognized compensation cost associated with non-vested stock options outstanding as of December 31, 2009 was $50,000, excluding forfeitures, which we expect to recognize over a weighted-average period of one year.
48
Table of Contents
Additional information regarding our options exercised is set forth below:
| Year Ended December 31, | |||||||||
| (In thousands) |
2009 | 2008 | 2007 | ||||||
| Cash received |
$ | 1,402 | $ | 14,661 | $ | 22,778 | |||
| Aggregate intrinsic value |
$ | 326 | $ | 8,495 | $ | 15,856 | |||
Prior to the fourth quarter of 2007, all outstanding stock options contained provisions whereby 25% of the original option grant amount would have accelerated and become immediately vested under certain circumstances in the event of a change in control of the Company. During the fourth quarter of 2007, the Compensation Committee of the board of directors approved a modification to the existing terms of all outstanding stock options held by non-officers of the Company to increase the level of acceleration to 50% of the original grant amount with all other terms and provisions of the options remaining unchanged. In addition, during the fourth quarter of 2007, the Compensation Committee approved a modification to the existing terms of outstanding stock options held by our commercial employees to accelerate the vesting equal to 25% of the original grant amount if and when the sale of the commercial operations occurred prior to a change in control of the Company. Stock-based compensation expense for 2008 included stock option modification charges totaling $4.6 million. The stock option modification charges related to accelerated vesting and extended exercise periods for certain stock options provided in connection with the termination of certain employees and members of the board of directors. The majority of the stock option modification charges related to the termination of certain employees as a result of the sale of the commercial assets and is reflected within discontinued operations.
Restricted Stock
A summary of our restricted stock activity for the years ended December 31, 2009, 2008 and 2007 is presented below:
| 2009 | 2008 | 2007 | ||||||||||||||||
| Number of shares (in thousands) |
Weighted- average grant-date fair value per share |
Number of shares (in thousands) |
Weighted- average grant-date fair value per share |
Number of shares (in thousands) |
Weighted- average grant-date fair value per share | |||||||||||||
| Nonvested at beginning of year |
- | $ | - | 208 | $ | 20.33 | 137 | $ | 20.67 | |||||||||
| Awards granted |
159 | $ | 6.54 | 148 | $ | 9.67 | 143 | $ | 20.00 | |||||||||
| Awards vested |
(5 | ) | $ | 6.43 | (78 | ) | $ | 18.07 | (41 | ) | $ | 20.86 | ||||||
| Forfeited |
(6 | ) | $ | 6.66 | (278 | ) | $ | 15.30 | (31 | ) | $ | 19.65 | ||||||
| Nonvested at end of year |
148 | $ | 6.54 | - | $ | - | 208 | $ | 20.33 | |||||||||
Stock-based compensation expense associated with our restricted stock for the years ended December 31, 2009, 2008 and 2007 was $0.5 million, $0.8 million and $1.2 million, respectively. As of December 31, 2009, the aggregate pre-tax intrinsic value of non-vested restricted stock was $1.0 million. Total unrecognized compensation costs associated with non-vested restricted stock as of December 31, 2009 was $0.5 million, excluding forfeitures, which we expect to recognize over a weighted-average period of one year.
During the fourth quarter of 2007, the Compensation Committee of the board of directors approved a modification to the existing terms of certain restricted stock grants made during the third quarter of 2007 to certain employees of the Company to provide for 100% acceleration of any unvested portion of these grants in the event of a change in control of the Company. All other terms and provisions of the restricted stock grants remain unchanged.
49
Table of Contents
4. Cash Dividends
In April 2008, our board of directors declared a cash dividend equivalent to $4.25 per share of common stock using the proceeds of the sales of our former commercial operations and our former antibody manufacturing plant in March 2008. We paid $506.6 million to our stockholders on May 5, 2008.
In February 2009, our board of directors declared two cash dividends of $0.50 per share of common stock payable on April 1, 2009 and October 1, 2009. We paid $59.7 million to our stockholders on April 1, 2009 and $59.7 million to our stockholders on October 1, 2009. In November 2009, our board of directors declared an additional cash dividend equivalent to $1.67 per share of common stock payable on December 15, 2009. We paid $199.6 million to our stockholders on December 15, 2009. As of December 31, 2009, we had $0.4 million accrued in other accrued liabilities for estimated dividends payable on unvested restricted stock.
In January 2010, our board of directors declared two cash dividends of $0.50 per share of common stock payable on April 1, 2010 and October 1, 2010. For further information, see Note 20.
5. Spin-Off of Facet
On December 17, 2008, we transferred our biotechnology operations to Facet and on December 18, 2008, made a pro rata distribution to our stockholders of record on December 5, 2008 of one share of Facet common stock for every five shares of PDL common stock valued at $2.60 per share of common stock.
In connection with the Spin-Off, on December 17, 2008, PDL and Facet entered into a Separation and Distribution Agreement (the Separation Agreement). The Separation Agreement identifies the assets transferred, liabilities assumed and contracts assigned to Facet as part of the Spin-Off, and describes when and how these transfers, assumptions and assignments occurred. In particular, all of the assets and liabilities associated or primarily used in connection with the biotechnology operations were transferred to Facet, including our intellectual property assets other than our Queen et al. patents. As a result, the primary assets and liabilities retained by us after the Spin-Off are our Queen et al. patents, our convertible notes and our leased office space in Nevada.
On December 18, 2008, we also entered into with Facet (i) a Transition Services Agreement pursuant to which Facet and we will provide each other with a variety of administrative services, including financial, tax, accounting, information technology, legal and human resources services, for a period of time of up to 36 months following the Spin-Off, (ii) a Tax Sharing and Indemnification Agreement that will govern Facet’s and our respective rights, responsibilities and obligations after the Spin-Off with respect to taxes, (iii) a Cross License Agreement relating to our Queen et al. patents and certain other patents and know-how under which we granted to Facet a royalty-free, development license to our Queen et al. patents and a royalty-bearing, commercialization license to our Queen et al. patents, and Facet granted to us a royalty-free license under certain intellectual property Facet owns solely for the purposes of allowing us to perform and fulfill existing obligations that we have under certain agreements with third parties, and (iv) an Employee Matters Agreement which governs the employee benefit obligations of Facet and us as they relate to current and former employees, allocates liabilities and responsibilities relating to employee benefit matters, other than severance plans, that are subject to ERISA in connection with the Spin-Off, including the assignment and transfer of employees, and the establishment of a savings plan and a welfare plan.
In connection with the Spin-Off, we entered into amendments to the leases for the facilities in Redwood City, California, which formerly served as our headquarters, under which Facet was added as a co-tenant under the leases, and a Co-Tenancy Agreement, under which Facet agreed to indemnify us for all matters related to the leases attributable to the period after the Spin-Off date. For further information, see Notes 13 and 15.
50
Table of Contents
The total value of the Facet stock dividend of $455.6 million was based on the value of the net assets that were transferred to Facet in connection with the Spin-Off. The following net assets were transferred to Facet:
| (In thousands) |
||||
| Net assets transferred: |
||||
| Cash and cash equivalents |
$ | 405,968 | ||
| Prepaid and other current assets |
15,768 | |||
| Land, property and equipment, net |
122,373 | |||
| Other intangible assets, net |
7,471 | |||
| Other assets |
2,141 | |||
| Accrued compensation |
(3,365 | ) | ||
| Other accrued liabilities |
(2,333 | ) | ||
| Deferred revenue |
(58,723 | ) | ||
| Debt and other long-term liabilities |
(34,149 | ) | ||
| Accumulated other comprehensive loss |
468 | |||
| Net assets transferred |
$ | 455,619 | ||
Facet’s historical results of operations have been presented as discontinued operations in the Consolidated Statements of Operations. See Note 18 for further details of the discontinued operations results.
6. Net Income (Loss) Per Share
We compute income (loss) per basic share using the weighted-average number of shares of common stock outstanding during the periods presented, less the weighted-average number of shares of restricted stock that are subject to repurchase. We compute income (loss) per diluted share for our continuing operations using the sum of the weighted-average number of common and common equivalent shares outstanding. Common equivalent shares used in the computation of income per diluted share result from the assumed exercise of stock options, the issuance of restricted stock, the assumed issuance of common shares under our ESPP using the treasury stock method, and the assumed conversion of our 2.00% Convertible Senior Notes due 2012 (the 2012 Notes) and our 2.75% Convertible Subordinated Notes due 2023 (the 2023 Notes), including both the effect on interest expense and the inclusion of the underlying shares, using the if-converted method. The adjusted conversion rate for the 2012 Notes is 119.294 shares per $1,000 principal amount of 2012 Notes, or a conversion price of approximately $8.38 per share, effective December 2, 2009. The adjusted conversion rate for the 2023 Notes is 164.7254 shares per $1,000 principal amount of 2023 Notes, or a conversion price of approximately $6.07 per share, effective December 2, 2009. For the year ended December 31, 2007, we also included the release of the contingent shares remaining in escrow from the ESP Pharma acquisition, prior to their release from escrow in April 2007.
51
Table of Contents
The following is a reconciliation of the numerators and denominators of the income (loss) per basic and diluted share computations for the years ended December 31, 2009, 2008 and 2007:
| Year Ended December 31, | ||||||||||
| (In thousands) |
2009 | 2008 | 2007 | |||||||
| Numerator |
||||||||||
| Income from continuing operations used to compute income per basic share from continuing operations |
$ | 189,660 | $ | 238,320 | $ | 180,449 | ||||
| Add back interest expense for convertible notes, net of estimated tax of $3.8 million, $1.4 million, and $2.4 million for the years ended December 31, 2009, 2008 and 2007, respectively (see Note 14) |
7,079 | 10,450 | 9,500 | |||||||
| Income used to compute income per diluted share from continuing operations |
$ | 196,739 | $ | 248,770 | $ | 189,949 | ||||
| Net income (loss) |
$ | 189,660 | $ | 68,387 | $ | (21,061 | ) | |||
| Add back interest expense for convertible notes, net of estimated tax of $3.8 million, $1.4 million, and $2.4 million for the years ended December 31, 2009, 2008 and 2007, respectively (see Note 14) |
7,079 | 10,450 | 9,500 | |||||||
| Income (loss) used to compute net income (loss) per diluted share |
$ | 196,739 | $ | 78,837 | $ | (11,561 | ) | |||
| Denominator |
||||||||||
| Total weighted-average shares used to compute income (loss) per basic share |
119,402 | 118,728 | 116,365 | |||||||
| Effect of dilutive stock options |
18 | 50 | 1,950 | |||||||
| Assumed release of common stock in escrow |
- | - | 153 | |||||||
| Restricted stock outstanding |
42 | 10 | 42 | |||||||
| Assumed conversion of 2012 Notes |
28,809 | 20,542 | 10,555 | |||||||
| Assumed conversion of 2023 Notes |
36,129 | 28,539 | 12,415 | |||||||
| Shares used to compute income per diluted share from continuing operations and net income (loss) per diluted share |
184,400 | 167,869 | 141,480 | |||||||
We excluded 2.5 million, 10.3 million and 8.2 million of outstanding stock options from our diluted earnings per share calculations for the years ended December 31, 2009, 2008 and 2007, respectively, because the average price of the common stock obtainable upon exercise of the options is above the exercise price.
7. Restructuring Charges
During the years ended December 31, 2008 and 2007, we put into place certain restructuring plans under which we recognized involuntary termination benefits and idle facilities charges. As the majority of restructuring charges has been allocated to our former commercial operations and our former biotechnology operations, they are classified as discontinued operations, see Note 18. During 2008 and 2007, we recognized $12.0 million and $6.7 million, respectively, of restructuring expense attributable to discontinued operations. In addition, we recognized approximately $0.2 million of restructuring charges in 2008 attributable to continuing operations, which is classified as general and administrative expenses. The details of the restructuring plans are described below.
52
Table of Contents
The following table summarizes the restructuring activity discussed above:
| (In thousands) |
Personnel Costs |
Facilities Related |
Total | |||||||||
| Balance at December 31, 2006 |
$ | - | $ | - | $ | - | ||||||
| Restructuring charges |
3,616 | 3,052 | 6,668 | |||||||||
| Payments and adjustments |
(3,205 | ) | (1,195 | ) | (4,400 | ) | ||||||
| Interest expense |
- | 55 | 55 | |||||||||
| Balance at December 31, 2007 |
411 | 1,912 | 2,323 | |||||||||
| Restructuring charges |
11,928 | 227 | 12,155 | |||||||||
| Payments and adjustments |
(10,305 | ) | (2,075 | ) | (12,380 | ) | ||||||
| Transfer of liability to Facet |
(1,994 | ) | - | (1,994 | ) | |||||||
| Balance at December 31, 2008 |
40 | 64 | 104 | |||||||||
| Payments and adjustments |
(40 | ) | (64 | ) | (104 | ) | ||||||
| Balance at December 31, 2009 |
$ | - | $ | - | $ | - | ||||||
Company-Wide Restructuring Plan
In March 2008, we commenced a restructuring plan in which we eliminated approximately 120 employment positions in the first quarter of 2008 and approximately 130 additional employment positions over the subsequent 12 months (the Transition Employees). All impacted employees were notified in March 2008. Subsequent to the completion of the restructuring, we had approximately 300 employees. Employees terminated in connection with the restructuring were eligible for a package consisting of severance payments of generally 12 weeks of salary and medical benefits along with up to three months of outplacement services. We recognized severance charges for Transition Employees over their respective estimated service periods. During 2008, we recognized restructuring charges of $9.4 million, which primarily related to post-termination severance costs as well as salary accruals relating to the portion of the 60-day notice period over which the terminated employees would not be providing services to the Company. As the restructuring efforts related primarily to our biotechnology operations, $9.2 million of the total $9.4 million of restructuring charges are presented as discontinued operations. These restructuring charges included expenses associated with employees who were terminated immediately as well as expenses associated with the Transition Employees. The remaining liability associated with these restructuring charges as of December 18, 2008 was transferred to Facet in connection with the Spin-Off.
In addition, in the fourth quarter of 2008, we commenced a restructuring plan pursuant to which we closed our France office and eliminated all related employment positions. In connection with this restructuring effort, we recognized charges of approximately $0.9 million. The liability associated with this restructuring plan was transferred to Facet in December 2008 in connection with the Spin-Off.
Manufacturing Restructuring Plan
In August 2007, we announced a strategic change to focus the Company on the discovery and development of novel antibodies in oncology and select immunologic diseases. As a result, we communicated our intent to sell certain assets that were not aligned with this new strategic direction. In addition, we announced our plans to conduct a thorough review of our organization to ensure that our structure and scope of operations were appropriately aligned with our new strategy and we anticipated a sizeable reduction in our workforce. Restructuring expenses associated with our change in strategic focus of $3.6 million were recognized in 2007 and fully paid in 2008 and are presented as discontinued operations since all related employees were associated with our former biotechnology operations.
53
Table of Contents
Commercial Restructuring
In connection with the divestiture of the commercial operation, we committed in the first quarter of 2008 to provide certain severance benefits to those employees whose employment positions we would likely eliminate in connection with the transactions. We recognized expenses for these severance benefits of $1.8 million during 2008, which are presented as discontinued operations. Substantially all related severance obligations were settled by the end of 2008.
Facilities Related Restructuring Plan
During the third quarter of 2007, we initiated our move from our prior corporate headquarters in Fremont, California to Redwood City, California. In connection with this move, we ceased use of a portion of the leased property in Fremont, California and, as a result, we recognized a restructuring charge of approximately $1.3 million, all of which are presented as discontinued operations. We paid all obligations relating to these leases by the end of the first quarter of 2008, when the leases on these facilities terminated.
In addition, during the second and fourth quarters of 2007, we ceased use of two of our leased facilities in Plymouth, Minnesota. In connection with the sale of our manufacturing assets in March 2008, Genmab assumed our obligations for one of these two facilities. During 2007, we recognized restructuring costs of approximately $1.8 million associated with these leased facilities, all of which are presented as discontinued operations. We have paid all lease and severance obligations, which were not transferred to Facet in connection with the Spin-Off by the end of 2009.
8. Cash Equivalents, Investments and Restricted Cash
As of December 31, 2009, we had invested our excess cash balances primarily in money market funds, and as of December 31, 2008, we had invested our excess cash balances primarily in money market funds and certificates of deposit. Our securities are classified as available-for-sale and are carried at estimated fair value, with unrealized gains and losses reported in accumulated other comprehensive loss in stockholders’ deficit. The estimated fair value is based upon quoted market prices for these or similar instruments. The cost of securities sold is based on the specific identification method. To date, we have not experienced credit losses on investments in these instruments and we do not require collateral for our investment activities.
A summary of our available-for-sale securities at December 31, 2009 and 2008 is presented below:
| (In thousands) |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value | ||||||||
| December 31, 2009 |
||||||||||||
| Money market funds |
$ | 296,969 | $ | - | $ | - | $ | 296,969 | ||||
| Classification on Consolidated Balance Sheets: |
||||||||||||
| Cash equivalents |
$ | 296,969 | ||||||||||
| December 31, 2008 |
||||||||||||
| Money market funds |
$ | 107,041 | $ | - | $ | - | $ | 107,041 | ||||
| Certificate of deposit |
15,000 | - | - | 15,000 | ||||||||
| Total |
$ | 122,041 | $ | - | $ | - | $ | 122,041 | ||||
| Classification on Consolidated Balance Sheets: |
||||||||||||
| Cash equivalents |
$ | 107,041 | ||||||||||
| Short-term investments |
15,000 | |||||||||||
| Total |
$ | 122,041 | ||||||||||
54
Table of Contents
During 2009, 2008 and 2007, we did not recognize any gains or losses on sales of available-for-sale securities.
As of December 31, 2008, we had $3.3 million of restricted cash relating to letters of credit supporting the lease deposit on the Redwood City facilities. The letters of credit were released in 2009 and, therefore, the restricted cash balance as of December 31, 2009 was zero.
As of December 31, 2009 and 2008, our financial assets consisted primarily of money market funds which are considered to be Level 1 assets under the valuation hierarchy and are classified as cash and cash equivalents in our Consolidated Balance Sheets. As of December 31, 2008, we also had $15.0 million of certificates of deposit which are considered to be Level 2 assets.
9. Prepaid and Other Current Assets
Prepaid and other current assets as of December 31, 2009 and 2008 consisted of the following:
| December 31, | ||||||
| (In thousands) |
2009 | 2008 | ||||
| Non-recourse Notes issuance costs |
$ | 3,373 | $ | - | ||
| 2023 Notes issuance costs |
524 | - | ||||
| Prepaid taxes |
5,847 | - | ||||
| Other |
544 | 1,658 | ||||
| Total |
$ | 10,288 | $ | 1,658 | ||
See Note 14 for information about the Non-Recourse Notes and 2023 Notes.
10. Property and Equipment
Property and equipment as of December 31, 2009 and 2008 consisted of the following:
| December 31, | ||||||||
| (In thousands) |
2009 | 2008 | ||||||
| Leasehold improvements |
$ | 112 | $ | 92 | ||||
| Computer and office equipment |
8,989 | 9,127 | ||||||
| Furniture and fixtures |
38 | 37 | ||||||
| Gross property and equipment |
9,139 | 9,256 | ||||||
| Less accumulated depreciation and amortization |
(8,968 | ) | (8,133 | ) | ||||
| Property and equipment, net |
$ | 171 | $ | 1,123 | ||||
11. Other Assets
Other assets as of December 31, 2009 and 2008 consisted of the following:
| December 31, | ||||||
| (In thousands) |
2009 | 2008 | ||||
| 2012 Notes issuance costs |
$ | 2,202 | $ | 3,550 | ||
| 2023 Notes issuance costs |
- | 1,863 | ||||
| Non-recourse Notes issuance costs |
9,624 | - | ||||
| Other |
182 | 12 | ||||
| Total |
$ | 12,008 | $ | 5,425 | ||
55
Table of Contents
See Note 14 for information about the 2012 Notes, the 2023 Notes, and the Non-Recourse Notes.
12. Other Accrued Liabilities
Other accrued liabilities as of December 31, 2009 and 2008 consisted of the following:
| December 31, | ||||||
| (In thousands) |
2009 | 2008 | ||||
| Consulting and services |
$ | 2,154 | $ | 5,357 | ||
| Dividend payable |
386 | - | ||||
| Payable to Facet Biotech Corporation |
1 | 1,100 | ||||
| Restructuring accruals |
- | 104 | ||||
| Accrued income taxes |
81 | 7,340 | ||||
| Other |
56 | 3,505 | ||||
| Total |
$ | 2,678 | $ | 17,406 | ||
13. Commitments and Contingencies
Operating Leases
Current Facilities
We are party to leased facilities under agreements that have expiration dates between 2011 and 2021. We also have leased certain office equipment under operating leases. Rental expense under these arrangements totaled $0.2 million, $7.0 million and $10.7 million for the years ended December 31, 2009, 2008 and 2007, respectively, of which approximately zero, $6.8 million and $10.5 million is classified as discontinued operations, respectively. We currently occupy a leased facility in Incline Village, Nevada, with a lease term through May 2011 and an option to extend the lease until May 2012.
As of December 31, 2009, the future minimum operating lease payments are as follows:
| (In thousands) |
|||
| 2010 |
$ | 230 | |
| 2011 |
80 | ||
| 2012 |
5 | ||
| 2013 |
5 | ||
| Total |
$ | 320 | |
Former Facilities
In July 2006, we entered into two leases (the Leases) and a sublease (the Sublease) for the facilities in Redwood City, California, which formerly served as our headquarters. Pursuant to amendments to the Leases entered into in connection with the Spin-Off (the Lease Amendments), Facet was added as a co-tenant under the Leases. As a co-tenant, Facet is bound by all of the terms and conditions of the Leases. PDL and Facet are jointly and severally liable for all obligations under the Leases, including the payment of rental obligations. However, we also entered into a Co-Tenancy Agreement with Facet in connection with the Spin-Off and the Lease Amendments pursuant to which we assigned to Facet all rights under the Leases, including, but not limited to, the right to amend the leases, extend the lease term or terminate the leases, and Facet assumed all of our obligations under the Leases. In addition, we assigned the Sublease to Facet. In the event that Facet amends the Leases to extend beyond the original expiration date, PDL shall have no liability for any obligations that accrue under the Leases with respect to the period after the original expiration date. Pursuant to the Co-Tenancy Agreement, we also relinquished any right or option to regain possession, use or occupancy of these facilities.
56
Table of Contents
Facet agreed to indemnify us for all matters associated with the Leases attributable to the period after the Spin-Off. Should Facet default under its lease obligations, we would be held liable by the landlord as a co-tenant, and thus, we have in substance guaranteed the payments under the lease agreements for the Redwood City facilities. As of December 31, 2009, the total lease payments for the duration of the guarantee, which runs through December 2021, are approximately $130.8 million. We would also be responsible for lease-related payments including utilities, property taxes, and common area maintenance which may be as much as the actual lease payments. As of December 31, 2009 and December 31 2008, we had a liability of $10.7 million on our Consolidated Balance Sheets related to the estimated fair value of this guarantee. For further information, see Note 15.
Contingencies
As permitted under Delaware law, pursuant to the terms of our bylaws, we have agreed to indemnify our directors and officers and, pursuant to the terms of indemnification agreements we have entered into, we have agreed to indemnify our executive officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving as an officer or director of the Company. While the maximum amount of potential future indemnification is unlimited, we have a director and officer insurance policy that limits our exposure and may enable us to recover a portion of any future amounts paid. We believe the fair value of these indemnification agreements and bylaw provisions is minimal, and accordingly, we have not recorded the fair value liability associated with these agreements as of December 31, 2009 and 2008.
Pierre Fabre - Sales Rebate
Until PDL’s assignment and sale of Busulfex® to Otsuka Pharmaceuticals (Otsuka) in March 2008, Pierre Fabre Medicament (Pierre Fabre) was PDL’s exclusive distributor for Busulfex in Italy. In 2005, Pierre Fabre negotiated a pricing and sales volume agreement with the Agenzia Italiana del Farmaco (AIFA) related to its distribution of Busulfex in which Pierre Fabre agreed to a maximum amount of ex-factory sales of Busulfex in Italy. During 2006 and 2007, Pierre Fabre exceeded those sales limits and, in October 2008, Pierre Fabre received notification to repay EUR 2.13 million to the local Italian authorities for such excess sales. In April 2009, Pierre Fabre sent a letter to Otsuka requesting that it pay 40% of the total amount paid by Pierre Fabre. This letter was, in turn, forwarded to PDL’s attention. In July 2009, the Company responded to Pierre Fabre declining to make payment and stating that there is no basis for reimbursement under PDL’s contractual arrangement with Pierre Fabre. On November 9, 2009, Pierre Fabre sent Otsuka and PDL a second letter demanding reimbursement or a note of credit. On December 9, 2009, the Company replied by letter stating that there is no basis for reimbursement under the contract. As of December 31, 2009, no amounts for this contingency have been accrued and there has been no further communication with Pierre Fabre regarding the reimbursement request. PDL does not believe that any reimbursement is required under the contract or that it is probable that PDL will need to reimburse Pierre Fabre.
14. Convertible Notes and Non-Recourse Notes
2012 Notes
In February 2005, we issued 2.00% Convertible Senior Notes due February 15, 2012 with a principal amount of $250.0 million (2012 Notes). The 2012 Notes are convertible at any time, at the holders’ option, into our common stock at a conversion rate of 119.294 shares of common stock per $1,000 principal amount of the 2012 Notes or $8.38 per share of common stock, as adjusted for the cash dividend paid on December 15, 2009 and subject to further adjustment in certain events. Interest on the 2012 Notes is payable semiannually in arrears on February 15 and August 15 of each year. The 2012 Notes are our senior unsecured debt and are redeemable by us in whole or in part on or after February 19, 2010 at 100.57% of principal amount if redeemed between February 19, 2010 and February 14, 2011 and at 100.29% of principal amount if redeemed between February 15, 2011 and the maturity date. The 2012 Notes are not puttable other than in the context of a fundamental change resulting in the reclassification, conversion, exchange or cancellation of our common stock. Such repurchase
57
Table of Contents
event or fundamental change is generally defined to include a merger involving PDL, an acquisition of a majority of PDL’s outstanding common stock, and a change of a majority of PDL’s board of directors without the approval of the board of directors.
In 2009, the Company repurchased $5.0 million face value of our 2012 Notes, at a discount of 10.75% from face value in a privately negotiated transaction with an institutional holder, for aggregate consideration of $4.5 million in cash, plus accrued but unpaid interest. Also in 2009, the Company repurchased an aggregate of $17.0 million face value of our 2012 Notes, at a discount of 3.0% from face value in privately negotiated transactions with institutional holders, for aggregate consideration of $16.5 million in cash, plus accrued but unpaid interest. The Company recorded a net gain of $0.8 million from the purchase of the debt.
As of December 31, 2009, the remaining gross issuance costs associated with the 2012 Notes totaled $7.3 million. These costs are included in other assets and are being amortized to interest expense over the term of the debt, or approximately seven years.
2023 Notes
In July 2003, we issued 2.75% Convertible Subordinated Notes due August 16, 2023 with a principal amount of $250.0 million (2023 Notes). The 2023 Notes are convertible at any time, at the holders’ option, into our common stock at a conversion rate of 164.7254 shares of common stock per $1,000 principal amount of the 2023 Notes or $6.07 per share of common stock, as adjusted for the cash dividend paid on December 15, 2009, and subject to further adjustment in certain events. Interest on the 2023 Notes is payable semiannually in arrears on February 16 and August 16 of each year. The 2023 Notes are unsecured and are subordinated to all our existing and future senior indebtedness. The 2023 Notes may be redeemed at our option, in whole or in part, at par value. Holders of the 2023 Notes may require us to repurchase all or a portion of their notes at 100% of their principal amount, plus any accrued and unpaid interest, on August 16, 2010, August 16, 2013 and August 16, 2018, and upon the occurrence of a repurchase event in which a change in control has occurred or our common stock is neither listed on a U.S. national securities exchange nor approved for trading over-the-counter. For any 2023 Notes to be repurchased in August 2010, we must pay for the repurchase in cash, and we may pay for the repurchase of any 2023 Notes to be repurchased in August 2013 and August 2018, at our option, in cash, shares of our common stock or a combination of cash and shares of our common stock at the conversion rate then in effect.
In 2009, the Company repurchased an aggregate of $50.0 million face value of our 2023 Notes, at a discount of 2.0% from face value in privately negotiated transactions with institutional holders, for aggregate consideration of $49.0 million in cash, plus accrued but unpaid interest. The Company recorded a net gain of $0.7 million from the purchase of the debt.
In August 2009, we reclassified the 2023 Notes to current liabilities on the Consolidated Balance Sheet as the holders of the 2023 Notes may require us to repurchase all or a portion of the 2023 Notes on August 16, 2010.
As of December 31, 2009, the remaining gross issuance costs associated with the 2023 Notes totaled $6.8 million. These costs are included in other current assets and are being amortized to interest expense over the term of the earliest redemption of the debt, or approximately seven years.
Non-Recourse Notes
On November 2, 2009, we completed a $300 million securitization transaction in which we monetized 60% of the net present value of the estimated five year royalties from sales of Genentech products (the Genentech Royalties) including Avastin®, Herceptin®, Lucentis®, Xolair® and future products, if any, under which Genentech may take a license under our related agreements with Genentech. The QHP PhaRMA Senior Secured Notes due 2015 (the QHP Notes or the Non-recourse Notes) bear interest at 10.25% per annum and were issued
58
Table of Contents
in a non-registered offering by QHP, a Delaware limited liability company, and a newly formed, wholly-owned subsidiary of PDL. Concurrent with the securitization transaction and pursuant to the terms of a purchase and sale agreement, we sold, transferred, conveyed, assigned, contributed and granted to QHP, certain rights under our non-exclusive license agreements with Genentech including the right to receive the Genentech Royalties in exchange for QHP’s proceeds from the QHP Notes issuance. Once all obligations on the QHP Notes have been paid in full, including all other sums payable under the indenture, the indenture shall cease to be of further effect and all of the security interests in the collateral shall terminate, including the pledge by PDL to the trustee of its equity interest in QHP. At such point, there will be no further restrictions on the Genentech Royalties and PDL shall be free to either keep them in QHP, transfer them back to PDL or to further dispose or monetize them.
The Genentech Royalties and other payments, if any, that QHP will be entitled to receive under the agreements with Genentech, together with any funds made available from certain accounts of QHP, will be the sole source of payment of principal, interest and premium on the QHP Notes, which will be secured by a continuing security interest granted by QHP in its rights to receive payments under such agreements and all of its other assets and a pledge by the equity holder (initially PDL) of its equity ownership interest in QHP. The QHP Notes may be redeemed at any time prior to maturity, in whole or in part, at the option of QHP at a make-whole redemption price.
Issuance costs associated with the QHP Notes were $14.3 million. These costs are included in other current assets and other assets and are being amortized to interest expense using the effective interest method over the estimated repayment period, or approximately three years.
The following table summarizes the activity of the 2012 Notes, the 2023 Notes and the Non-Recourse Notes discussed above, as well as the balance and fair value at December 31, 2009:
| (In thousands) |
2012 Notes |
2023 Notes |
Non-recourse Notes |
Total | |||||||||||
| Balance at December 31, 2008 |
$ | 250,000 | $ | 249,998 | $ | - | $ | 499,998 | |||||||
| Issuance |
- | - | 300,000 | 300,000 | |||||||||||
| Repurchases |
(22,000 | ) | (50,000 | ) | - | (72,000 | ) | ||||||||
| Balance at December 31, 2009 |
$ | 228,000 | $ | 199,998 | $ | 300,000 | $ | 727,998 | |||||||
| Fair value(1) |
$ | 219,484 | $ | 232,848 | $ | 300,000 | $ | 752,332 | |||||||
| (1) | As of December 31, 2009, the fair value of the remaining payments under our convertible notes was estimated based on the trading value of our notes. As of December 31, 2009, the fair value of our QHP Notes was estimated to be approximately the carrying value of the notes because terms and conditions at the issuance date of November 2, 2009 are consistent with current market rates. |
As of December 31, 2009, the future minimum principal payments under the 2012 Notes, the 2023 Notes and the Non-Recourse Notes were as follows:
| (In thousands) |
2012 Notes |
2023 Notes(1) |
Non-recourse Notes(2) |
Total | ||||||||
| 2010 |
$ | - | $ | 199,998 | $ | 77,852 | $ | 277,850 | ||||
| 2011 |
- | - | 100,283 | 100,283 | ||||||||
| 2012 |
228,000 | - | 121,865 | 349,865 | ||||||||
| Total |
$ | 228,000 | $ | 199,998 | $ | 300,000 | $ | 727,998 | ||||
| (1) | The 2023 Notes are shown as being due in 2010 because they are puttable to the Company by note holders in August 2010. |
| (2) | Repayment of the Non-recourse Notes is based on anticipated future royalties to be received from Genentech and the anticipated final payment date is December 2012. |
59
Table of Contents
15. Other Long-Term Liabilities
In connection with the Spin-Off, we entered into amendments to the leases for our former facilities in Redwood City, California, under which Facet was added as a co-tenant under the leases, and a Co-Tenancy Agreement, under which Facet agreed to indemnify us for all matters related to the leases attributable to the period after the Spin-Off date. Should Facet default under its lease obligations, we would be held liable by the landlord as a co-tenant, and thus, we have in substance guaranteed the payments under the lease agreements for the Redwood City facilities. As of December 31, 2009, the total lease payments for the duration of the guarantee, which runs through December 2021, are approximately $130.8 million. We would also be responsible for lease-related costs including utilities, property taxes, and common area maintenance which may be as much as the actual lease payments if Facet were to default.
As of December 31, 2009 and 2008, we had a liability of $10.7 million on our Consolidated Balance Sheets for the estimated fair value of this guarantee. We prepared a discounted, probability-weighted cash flow analysis to calculate the estimated fair value of the lease guarantee as of the Spin-Off. We were required to make assumptions regarding the probability of Facet’s default on the lease payment, the likelihood of a sublease being executed, and the times at which these events could occur. These assumptions are based on information that we received from real estate brokers and the current economic conditions, as well as expectations of future economic conditions. The fair value of this lease guarantee was charged to additional paid-in capital upon the Spin-Off and any future adjustments to the carrying value of the obligation will be recorded to additional paid-in capital. On a quarterly basis, we evaluate the underlying cash flow analysis assumptions and update them if necessary.
16. Revenues by Geographic Area and Significant Customers
The following table summarizes revenues from licensees who individually accounted for 10% or more of our total revenues from continuing operations:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Licensees |
|||||||||
| Genentech, Inc. (Genentech) |
71 | % | 73 | % | 79 | % | |||
| MedImmune, LLC (MedImmune) |
13 | % | 14 | % | 16 | % | |||
Royalty revenues and license and other revenues by geographic area are based on the country of domicile of the counterparty to the agreement. The following table summarizes revenues from continuing operations by geographic area:
| Year Ended December 31, | |||||||||
| (In thousands) |
2009 | 2008 | 2007 | ||||||
| United States |
$ | 243,047 | $ | 229,774 | $ | 179,492 | |||
| Europe |
71,684 | 63,339 | 45,018 | ||||||
| Other |
3,453 | 1,083 | 575 | ||||||
| Total revenues |
$ | 318,184 | $ | 294,196 | $ | 225,085 | |||
60
Table of Contents
17. Income Taxes
The provision for income taxes for the years ended December 31, 2009, 2008 and 2007 consisted of the following:
| Year Ended December 31. | |||||||||
| (In thousands) |
2009 | 2008 | 2007 | ||||||
| Current income tax expense (benefit) for continuing operations |
|||||||||
| Federal |
$ | 87,402 | $ | 17,105 | $ | 10,624 | |||
| State |
(573) | 10,086 | - | ||||||
| Foreign |
- | - | - | ||||||
| 86,829 | 27,191 | 10,624 | |||||||
| Deferred income tax (benefit) for continuing operations |
|||||||||
| Federal |
3,796 | (22,177) | - | ||||||
| State |
- | - | - | ||||||
| Foreign |
- | - | - | ||||||
| 3,796 | (22,177) | - | |||||||
| Income tax expense for continuing operations |
90,625 | 5,014 | 10,624 | ||||||
| Income tax expense (benefit) for discontinued operations |
- | 7,249 | (10,157) | ||||||
| Total provision |
$ | 90,625 | $ | 12,263 | $ | 467 | |||
A reconciliation of the income tax provision computed using the U.S. statutory federal income tax rate compared to the income tax provision for continuing operations included in the Consolidated Statements of Operations is as follows:
| Year Ended December 31, | |||||||||
| (In thousands) |
2009 | 2008 | 2007 | ||||||
| Tax at U.S. statutory rate on income before income taxes and discontinued operations |
$ | 98,100 | $ | 85,193 | $ | 66,815 | |||
| Change in valuation allowance |
4,891 | (103,844) | (56,214) | ||||||
| Federal alternative minimum tax |
- | 17,105 | - | ||||||
| State taxes |
(573) | 6,556 | - | ||||||
| Foreign taxes |
- | 4 | 23 | ||||||
| Net operating loss re-establishment |
(9,174) | - | - | ||||||
| Other |
(2,619) | - | - | ||||||
| Total |
$ | 90,625 | $ | 5,014 | $ | 10,624 | |||
As of December 31, 2009 and December 31, 2008, we had federal net operating loss carryforwards of $46.5 million and $220.2 million, respectively. As of December 31, 2009, we had federal and California state research and other tax credit carryforwards of $22.6 million and $20.2 million, respectively. The federal net operating loss and tax credit carryforwards will expire at various dates beginning in the year 2011 through 2028, if not utilized. In addition, as we moved our entire operation outside of California in 2008, it is unlikely that we will realize any future benefit from the California state tax credit carryforwards. The net operating loss carryforwards which resulted from exercises of stock options were not recorded on the Consolidated Balance Sheets. Instead, such unrecognized deferred tax benefits were accounted for as a credit to additional paid-in capital and were realized through a reduction in taxes payable in 2009. In addition, most of the tax credit carryforwards resulted from exercises of stock options and are not recorded on the Consolidated Balance Sheets. Such unrecognized deferred tax benefits will be accounted for as a credit to additional paid-in capital if and when they are realized through a reduction in taxes payable.
61
Table of Contents
Utilization of the federal and state net operating loss and tax credit carryforwards may be subject to a substantial annual limitation due to the “change in ownership” provisions of the Internal Revenue Code of 1986. The annual limitation may result in the expiration of net operating losses and credits before utilization. We have an annual limitation on the utilization of our federal net operating losses of $1.8 million for each of the years ended December 31, 2010 to 2022, and $1.3 million for the year ended December 31, 2023. As of December 31, 2009, we estimate that at least $22.0 million of the $46.5 million of federal net operating loss carryforwards and at least $2.8 million of the $22.6 million of federal tax credit carryforwards will expire prior to their use due to change of ownership provisions.
Deferred income tax assets and liabilities are determined based on the differences between financial reporting and income tax bases of assets and liabilities, as well as net operating loss carryforwards and are measured using the enacted tax rates and laws in effect when the differences are expected to reverse. The significant components of our net deferred tax assets and liabilities are as follows:
| December 31, | ||||||||
| (In thousands) |
2009 | 2008 | ||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 8,552 | $ | - | ||||
| Research and other tax credits |
5,743 | 11,118 | ||||||
| Stock-based compensation |
675 | 6,949 | ||||||
| Reserves and accruals |
608 | 608 | ||||||
| Deferred revenue |
525 | 525 | ||||||
| Intangible assets |
6,290 | 3,974 | ||||||
| Other |
439 | 4,157 | ||||||
| Total deferred tax assets |
22,832 | 27,331 | ||||||
| Valuation allowance |
(10,634 | ) | (5,422 | ) | ||||
| Total deferred tax assets |
12,198 | 21,909 | ||||||
| Deferred tax liabilities: |
||||||||
| Other |
(531 | ) | - | |||||
| Total deferred tax liabilities |
(531 | ) | - | |||||
| Net deferred tax assets |
$ | 11,667 | $ | 21,909 | ||||
During the year ended December 31, 2009, we recorded a $0.8 million net decrease in our liability associated with uncertain tax positions. A reconciliation of our unrecognized tax benefits, excluding accrued interest and penalties, for 2009 and 2008 are as follows:
| December 31, | ||||||||
| (In thousands) |
2009 | 2008 | ||||||
| Balance at the beginning of the year |
$ | 23,922 | $ | 11,576 | ||||
| Increases related to current year tax positions |
- | 7,125 | ||||||
| Increases related to prior year tax positions |
- | 5,576 | ||||||
| Decreases related to prior year tax positions |
(324 | ) | - | |||||
| Expiration of statute of limitations for the assessment of taxes |
(482 | ) | (355 | ) | ||||
| Balance at the end of the year |
$ | 23,116 | $ | 23,922 | ||||
The future impact of the unrecognized tax benefit of $23.1 million, if recognized, is as follows: $12.3 million would affect the effective tax rate and $10.8 million would result in adjustments to deferred tax assets and corresponding adjustments to the valuation allowance.
62
Table of Contents
Estimated interest and penalties associated with unrecognized tax benefits decreased income tax expense in the Consolidated Statements of Operations by $0.4 million and $0.1 million during the years ended December 31, 2009 and 2008, respectively, and increased income tax expense by $0.1 million during the year ended December 31, 2007. Accrued interest and penalties associated with the underpayment of income taxes were $26,000 and $0.4 million as of December 31, 2009 and 2008, respectively. In general, our income tax returns are subject to examination by U.S. federal, state and various local tax authorities for tax years 1992 forward. We do not anticipate any additional unrecognized tax benefits in the next 12 months that would result in a material change to our financial position.
18. Discontinued Operations
Biotechnology and Manufacturing Operations
In December 2008, we spun-off our biotechnology operations to Facet and, in March 2008, we sold our manufacturing operations to Genmab. We did not have discontinued operations for the year ended December 31, 2009. For further information on the Spin-Off, see Notes 1 and 5.
The significant components of our former biotechnology and manufacturing operations for 2008 and 2007, presented as discontinued operations, were as follows:
| Year Ended December 31, | ||||||
| (In thousands) |
2008 | 2007 | ||||
| Net revenues (1) |
$ | 27,770 | $ | 33,840 | ||
| Total costs and expenses |
(150,234) | (244,058) | ||||
| Income tax benefit |
12,964 | 10,377 | ||||
| Loss from operations(2) |
$ | (109,500) | $ | (199,841) | ||
| (1) | Net revenues include revenues recognized under collaboration agreements with Biogen Idec, Inc. (Biogen Idec), which was effective starting in September 2005, and Bristol-Myers Squibb Company (BMS), which was effective starting in September 2008. In addition, we had a collaboration agreement with Roche from 2004 through mid-2007. Under each of the collaboration agreements, we determined that all elements should be accounted for as a single unit of accounting. As we had continuing obligations under the collaboration agreements, we recorded the upfront license fees as deferred revenue, and we were recognizing the amounts over the respective estimated development periods. The upfront license fees from Biogen Idec and BMS were $40 million and $30 million, respectively. Under the agreement with Biogen Idec, we recognized $18.7 million and $24.8 million during the years ended December 31, 2008 and 2007. Under the agreement with BMS, we recognized $5.8 million in 2008. Under the agreement with the Roche Group, we recognized $7.2 million in 2007. |
| (2) | Included within the loss from operations for 2008 is a pre-tax gain of $49.7 million upon the close of the sale of our former Manufacturing Assets to Genmab in March 2008. In addition, loss from operations included $3.8 million and $5.5 million of asset impairment charges for the years ended December 31, 2008 and 2007, respectively. In 2008, such charges associated with the cost of certain research equipment and technologies that were expected to have no future useful life and certain information technology projects that were terminated and have no future benefit to us. In 2007, we recorded a loss of $5.0 million associated with the sale of our former corporate headquarters in Fremont, California. Also included in loss from operations for the years ended December 31, 2008 and 2007 are restructuring charges of approximately $10.1 million and $3.6 million, respectively, see Note 7. |
63
Table of Contents
Commercial Operation
In 2006, we divested four off-branded products that we had acquired in connection with the ESP Pharma Inc. (ESP) business combination in March 2005. In March 2008, we closed the sales of the Commercial Assets, which assets constituted the remaining commercial assets from the ESP acquisition. We sold the rights to IV Busulfex, including trademarks, patents, intellectual property and related assets, to Otsuka for $200 million in cash and an additional $1.4 million for the IV Busulfex inventories. We recognized a pre-tax loss of $64.6 million in connection with the sale of the Commercial Assets during the first quarter of 2008. This loss consisted of the total upfront consideration from the sales of the Commercial Assets of $280.4 million plus the write-off of $10.6 million in net liabilities, less the book values of intangible assets and inventories of $268.2 million, the write-off of goodwill of $81.7 million and transaction fees of $5.7 million.
Also in March 2008, we also sold the rights to Cardene®, Retavase® and ularitide (collectively, the Cardiovascular Assets), including all trademarks, patents, intellectual property, inventories and related assets, to EKR Therapeutics, Inc. (EKR). In consideration for the Cardiovascular Assets, we received upfront proceeds of $85.0 million, $6.0 million of which was placed in an escrow account for a period of approximately one year to cover certain product return related costs under the purchase agreement. In addition, the purchase agreement included contingent consideration of up to $85.0 million in potential future milestone payments as well as potential future royalties on certain Cardene and ularitide product sales. In the third quarter of 2008, we earned and received one of these milestone payments, a $25.0 million milestone payment related to approval by the FDA for a pre-mixed bag formulation of Cardene.
In connection with the sales of the Commercial Assets and the Cardiovascular Assets, we entered into agreements with both Otsuka and EKR to provide certain transition services. We provided these transition services to Otsuka and EKR through 2008 and have substantially completed such obligations under the agreements. Any fees or cost reimbursements received for transition services have been presented as discontinued operations.
In connection with the Spin-Off, we assigned all rights and obligations under the EKR sale agreement to Facet. Therefore, we will not receive any potential future milestone payments or royalties under the agreement with EKR.
The significant components of our commercial operation for 2008 and 2007, presented as discontinued operations, were as follows:
| Year Ended December 31, | ||||||
| (In thousands) |
2008 | 2007 | ||||
| Net revenues(1) |
$ | 66,467 | $ | 204,166 | ||
| Total costs and expenses |
(106,687) | (205,615) | ||||
| Income tax expense |
(20,213) | (220) | ||||
| Loss from operations(2) |
$ | (60,433) | $ | (1,669) | ||
| (1) | In August 2008, EKR received approval from the FDA for a pre-mixed bag formulation of Cardene. Under the terms of the purchase agreement with EKR, we received a $25.0 million milestone payment as a result of this approval; such amount is included in net revenues for 2008. In addition, we recorded favorable changes in estimates to revenue and accounts receivable reserves during 2008, which resulted in an increase to net revenues totaling approximately $2.1 million. |
| (2) | Included within loss from operations for 2008 is $2.5 million that we recognized in connection with certain contingent Retavase manufacturing costs obligations for which we are required to reimburse EKR. |
64
Table of Contents
Also included in total costs and expenses for the year ended December 31, 2008 are restructuring charges of approximately $1.8 million, see Note 7.
19. Legal Proceedings
European Patent Oppositions
Two Queen et al. patents were issued to us by the European Patent Office, European Patent No. 0 451 216B (the ‘216 Patent) and European Patent No. 0 682 040 (the ‘040 Patent) both of which were opposed after grant. A description of those proceedings is below.
Opposition to ‘216 Patent
In November 2003, in an appeal proceeding of a prior action of the Opposition Division of the European Patent Office, the Technical Board of Appeal of the European Patent Office ordered that certain claims in our ‘216 Patent be remitted to the Opposition Division for further prosecution and consideration of issues of patentability, that is, entitlement to priority, novelty, enablement and inventive step. These claims cover the production of humanized antibody light chains that contain amino acid substitutions made under our antibody humanization technology. In April 2007, at an oral proceeding, the Opposition Division upheld claims that are virtually identical to the claims remitted by the Technical Board of Appeal to the Opposition Division. The deadline for filing a notice of appeal has expired. Five opponents filed such notices in a timely manner and, of those, three have filed Grounds of Appeal. The ‘216 Patent remains enforceable during the appeal process. The Technical Board of Appeal has not scheduled a date for the appeal hearing with respect to the ‘216 Patent. We intend to vigorously defend the ‘216 Patent in this proceeding.
Opposition to ‘040 Patent
At an oral hearing in February 2005, the Opposition Division revoked the claims in our ‘040 Patent. The Opposition Division based its decision on formal issues and did not consider substantive issues of patentability. On October 14, 2009, the European Patent Office Technical Board of Appeal upheld the Opposition Division’s revocation of our ‘040 Patent on formal issues. The Technical Board of Appeal did not consider substantive issues of patentability. Each of our granted and applied for SPCs are based on the ‘216 Patent. As a result, the European Patent Office Technical Board of Appeal’s decision regarding the ‘040 Patent will not affect our right to receive royalties beyond December 28, 2009.
Settlement with Alexion Pharmaceuticals, Inc.
In March 2007, after the FDA’s market approval of Alexion Pharmaceuticals, Inc.’s (Alexion) Soliris® humanized antibody product, we filed a lawsuit against Alexion in the United States District Court for the District of Delaware for infringement of certain claims of United States Patent Number 5,693,761, United States Patent Number 5,693,762 and United States Patent Number 6,180,370 (the ‘370 Patent) (collectively, the Patents-in-Suit), which are three of our Queen et al. patents. We sought monetary damages and other relief. In June 2007, Alexion filed an answer denying that its Soliris product infringes the Patents-in-Suit, asserting certain defenses and counterclaiming for non-infringement and invalidity, and thereafter amended its answer to include a defense of unenforceability. In July 2008, the District Court issued a claim construction opinion.
On December 31, 2008, we and Alexion entered into a definitive license agreement and settlement agreement. Under the terms of the agreements, we granted Alexion a license under certain claims in our Queen et al. patents, and provided Alexion a covenant not to sue in respect of other claims in our Queen et al. patents, thus permitting Alexion to commercialize Soliris for all indications under our Queen et al. patents. In consideration of this license, Alexion agreed to pay us $25 million, of which Alexion paid $12.5 million in January 2009 and another $12.5 million was paid in May 2009. In 2008, we recognized $12.5 million in license revenue associated with the
65
Table of Contents
definitive license agreement and settlement agreement that we entered into with Alexion in December 2008. As of December 31, 2008, receivable from licensees on the Consolidated Balance Sheet included the $12.5 million receivable from Alexion.
No additional payments will be owed by Alexion to us under our Queen et al. patents in respect of Soliris sales for any indication. As part of the settlement, Alexion has confirmed that our Queen et al. patents claims are valid and that Soliris employs technology covered under our Queen et al. patents. Further, Alexion has agreed not to challenge or assist other parties in challenging the validity of our Queen et al. patents in the future. Under the license agreement, we separately granted Alexion the right to take a royalty-bearing license under our Queen et al. patents to commercialize additional Alexion humanized antibodies that may be covered by our Queen et al. patents in the future. In the event that Alexion takes such a license, Alexion will pay us a royalty of 4% of net sales of such non-Soliris products.
Action for Declaratory Judgment by MedImmune
In December 2008, MedImmune, a subsidiary of AstraZeneca plc, filed a lawsuit against us in the United States District Court for the Northern District of California seeking a declaratory judgment that the U.S. Queen et al. patents are invalid and/or not infringed by its Synagis® and motavizumab products and, that therefore, MedImmune owes no royalties under its license agreement with us. In April 2009, MedImmune amended its complaint to allege that, even if our patents are valid and infringed by Synagis and/or motavizumab, MedImmune is now or may have been retroactively entitled to a lower royalty rate on its sales of infringing products under the most favored licensee clause in our agreement. In May 2009, we filed our answer to MedImmune’s lawsuit asserting certain counterclaims and affirmative defenses and requested that the court find (i) that Synagis and motavizumab fall under the scope of the Queen et al. patents and that the sale thereof requires that MedImmune pay us royalties as specified in our license agreement with them; (ii) that the claims we are asserting against MedImmune are valid; (iii) that MedImmune is not entitled to different terms, including a lower royalty rate; and (iv) that MedImmune is liable for attorney’s fees and costs related to the action. On August 18, 2009, we attended a mandatory settlement conference with MedImmune held before the Federal District Court for Northern California. No settlement resulted from the meeting. A Markman claim construction hearing took place on November 5, 2009. A decision was issued from the court on February 22, 2010. The court generally construed the claim language at issue as proposed by PDL.
In December 2009, we sent a letter to MedImmune stating that it is in breach of its obligations under the license agreement, canceling the license agreement and revoking any licenses and rights granted therein. Also in December 2009, we filed a First Amended Answer, Defenses and Counterclaims (the Amended Pleadings) alleging that MedImmune breached the license agreement by (i) failing to pay all royalties due to us from the sale of Synagis, including sales by and through Abbott Laboratories, whom we believe is MedImmune’s exclusive sales representative for such sales outside the United States and (ii) by demanding that we consent to conditions that are commercially unreasonable and contractually insupportable in order to permit an audit of sales and revenue associated with Synagis by an independent accountant, as required under the license agreement and allege that, as a result of MedImmune’s breach of the license agreement and the Company’s cancelation thereof, MedImmune is infringing the ‘370 Patent by making, using, selling, offering for sale and/or importing Synagis into the United States and by having Synagis made, used, sold, offered for sale and/or imported into the United States. We have requested that the court award to the Company damages resulting from MedImmune’s breach of the license agreement, treble damages resulting from MedImmune’s infringement of the Company’s patent rights, attorney’s fees, and an injunction to prevent MedImmune from further acts of infringement and request a jury trial on all issues triable by jury. Also in December 2009, MedImmune filed a Motion for a preliminary injunction against our cancelation of the license agreement and filed a motion to strike our Amended Pleadings. In January 2010, MedImmune filed a motion for summary judgment seeking a declaratory judgment from the court that MedImmune is entitled under the most favored licensee clause in our agreement to a fully paid-up license as of December 2008 as a result of our agreement with Alexion and, retroactively to 1998, a reduced royalty rate on sales of Synagis. A hearing was held on February 26, 2010, related to the preliminary injunction motion, motion to strike and motion for summary judgment.
66
Table of Contents
On November 23, 2009, the U.S. Patent and Trademark Office (PTO) declared an interference proceeding between certain claims of Queen et al., the ‘370 Patent, which is involved in the current litigation against MedImmune, and certain pending claims of Adair et al., (the ‘117 Application) under 35 U.S.C. 135(a). UCB Pharma S.A. is the assignee of the ‘117 Application. We are unable to predict whether either of these proceedings will impact on the issues or timing of the other proceeding.
Trial is scheduled to start June 14, 2010 at which issues of infringement, validity and contractual rights are expected to be decided. MedImmune has paid us royalties under the MedImmune agreement with respect to sales of Synagis on a quarterly basis since the fourth quarter of 1998 through the fourth quarter of 2009. An escrow account has been created for receipt of MedImmune’s February 2010 payment, in which account the funds will be held pendente lite (while the litigation is pending). In the event that MedImmune prevails on the claims in its complaint, in either its summary judgment request or at trial, we expect that MedImmune will request the court to order a recoupment of payments made to us which represent obligations under its license to the Queen et al. patents that have accrued since the date of their claim. Alternatively, if MedImmune is successful in showing that it has made payments to us at a higher royalty rate than required pursuant to its license obligations, we expect that MedImmune will request the court to order recoupment of such excess payments.
Interference Proceedings in the U.S. Patent and Trademark Office
On February 25, 2009, the PTO declared an interference proceeding between certain claims of Queen et al., U.S. Patent No. 5,585,089 and certain pending claims of Adair et al., U.S. Application No. 08/846,658 (the ‘658 Application) under 35 U.S.C. 135(a). UCB Pharma S.A. is the assignee of the ‘658 Application. A hearing was held on January 29, 2010 regarding the first phase of the interference, which relates to substantive motions except those for priority of invention. A decision is expected within several months. The PTO has scheduled proceedings for the determination of priority of invention, if necessary.
On November 23, 2009, the PTO declared an interference proceeding between certain claims of the ‘370 patent and certain pending claims of Adair et al., U.S. Application No. 10/938,117 (the ‘117 Application) under 35 U.S.C. 135(a). UCB Pharma S.A. is the assignee of the ‘117 Application.
In an interference proceeding, the Board of Patent Appeals and Interferences typically determines questions of priority of the claimed inventions and may also determine questions of patentability. Any final decision, if adverse to the claim of an applicant, is a final refusal by the PTO of the claims involved. The Office may issue a patent to the applicant if he is adjudged the prior inventor. A final judgment adverse to a patentee from which no appeal or other review has been or can be taken or had constitutes cancellation of the claims involved in the patent.
20. Subsequent Event
On January 28, 2010, our board of directors declared two cash dividends of $0.50 per share of common stock to be paid on April 1, 2010 and October 1, 2010. Based on the number of shares of common stock issued and outstanding as of March 1, 2010, we currently expect the dividends to be approximately $60 million each, which we expect to pay using proceeds from our 2010 earnings and the QHP Notes. In connection with the payment of these dividends, the conversion rates for our outstanding 2012 Notes and 2023 Notes will be adjusted based on the amount of the dividend and the trading price of our stock in certain periods pursuant to the terms of the applicable indentures.
67
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of PDL BioPharma, Inc.
We have audited the accompanying consolidated balance sheets of PDL BioPharma, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations, cash flows, and stockholders’ equity (deficit) for each of the three years in the period ended December 31, 2009. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of PDL BioPharma, Inc. at December 31, 2009 and 2008, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2009, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), PDL BioPharma, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2010 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
Palo Alto, California
March 1, 2010
68
Table of Contents
Quarterly Financial Data (Unaudited)
| 2009 Quarter Ended | ||||||||||||||
| (In thousands, except per share data) |
December 31 | September 30 | June 30 | March 31 | ||||||||||
| Revenues |
$ | 58,252 | $ | 71,446 | $ | 125,864 | $ | 62,622 | ||||||
| Net income |
$ | 28,560 | $ | 46,406 | $ | 77,237 | $ | 37,457 | ||||||
| Net income per basic share |
$ | 0.24 | $ | 0.39 | $ | 0.65 | $ | 0.31 | ||||||
| Net income per diluted share |
$ | 0.17 | $ | 0.29 | $ | 0.47 | $ | 0.23 | ||||||
| 2008 Quarter Ended | ||||||||||||||
| (In thousands, except per share data) |
December 31 | September 30 | June 30 | March 31 | ||||||||||
| Revenues(1) |
$ | 68,658 | $ | 68,817 | $ | 106,532 | $ | 50,189 | ||||||
| Net income (loss) |
$ | 40,639 | (3) | $ | 55,691 | $ | 33,932 | $ | (61,875 | )(2) | ||||
| Net income (loss) per basic share |
$ | 0.34 | $ | 0.47 | $ | 0.29 | $ | (0.53 | ) | |||||
| Net income (loss) per diluted share |
$ | 0.26 | $ | 0.38 | $ | 0.24 | $ | (0.42 | ) | |||||
| (1) | Revenues presented above are those associated with our continuing operations; revenues from product sales and certain license, collaboration and other revenues associated with our former commercial operations and biotechnology operations are presented as discontinued operations, see Note 18. |
| (2) | In March 2008, we recorded a pre-tax gain on the sale of assets of $49.7 million associated with the sale of our manufacturing and related administrative facilities in Brooklyn Park, Minnesota, and related assets therein, to Genmab A/S and the assumption of certain of our lease obligations related to our facilities in Plymouth, Minnesota. |
In March 2008, we also recorded a pre-tax loss of $64.6 million in connection with the sale of the Commercial and Cardiovascular Assets to Otsuka Pharmaceutical Co., Ltd. and EKR Therapeutics, Inc.
See Note 18 to the Consolidated Financial Statements for further information.
| (3) | In December 2008, as a result of the Spin-off and our history of royalty revenue and significantly lower cost structure post-spin, we reversed the valuation allowance on our deferred tax assets as of December 31, 2008 which was approximately $21.9 million. |
69
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2009, our disclosure controls and procedures were effective to ensure the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
Management’s Annual Report on Internal Control over Financial Reporting
PDL, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, is responsible for the preparation and integrity of our Consolidated Financial Statements, establishing and maintaining adequate internal control over financial reporting and all related information appearing in this Annual Report. We evaluated the effectiveness of our internal controls over financial reporting under the Internal Control-Integrated Framework founded by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control-Integrated Framework, our management has assessed our internal control over financial reporting to be effective as of December 31, 2009.
Changes in Internal Controls
There were no changes in our internal controls over financial reporting during the quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting, except we added new internal controls related to PDL’s new wholly-owned subsidiary, QHP Royalty Sub LLC.
Limitations on the Effectiveness of Controls
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within an organization have been detected. We continue to improve and refine our internal controls and our compliance with existing controls is an ongoing process.
Our independent registered public accountants, Ernst & Young LLP, audited the Consolidated Financial Statements included in this Annual Report and have issued an audit report on the effectiveness of our internal control over financial reporting. The report on the audit of internal control over financial reporting appears below, and the report on the audit of the Consolidated Financial Statements appears in Part II, Item 8 of this Annual Report.
70
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of PDL BioPharma, Inc.
We have audited PDL BioPharma, Inc.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). PDL BioPharma, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, PDL BioPharma, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of PDL BioPharma, Inc. as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2009 of PDL BioPharma, Inc. and our report dated March 1, 2010 expressed an unqualified opinion thereon.
/S/ ERNST & YOUNG LLP
Palo Alto, California
March 1, 2010
71
Table of Contents
| ITEM 9B. | OTHER INFORMATION |
Not applicable.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item 10 will be contained in the Proxy Statement for our 2010 Annual Meeting of Stockholders and is incorporated herein by reference.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item 11 will be contained in the Proxy Statement for our 2010 Annual Meeting of Stockholders and is incorporated herein by reference.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item 12 will be contained in the Proxy Statement for our 2010 Annual Meeting of Stockholders and is incorporated herein by reference.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item 13 will be contained in the Proxy Statement for our 2010 Annual Meeting of Stockholders and is incorporated herein by reference.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this Item 14 will be contained in the Proxy Statement for our 2010 Annual Meeting of Stockholders and is incorporated herein by reference.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (a) | The following documents are filed as part of this report: |
| (1) | Index to financial statements |
Our financial statements and the Report of the Independent Registered Public Accounting Firm are included in Part II, Item 8.
| Item |
Page | |
| 35 | ||
| 36 | ||
| 37 | ||
| 39 | ||
| 40 | ||
| 68 |
72
Table of Contents
| (2) | The following schedule is filed as part of this Annual Report and should be read in conjunction with the financial statements: |
Schedule II—Valuation and Qualifying Accounts and Reserves for the years ended December 31, 2009, 2008 and 2007
All other financial statement schedules are omitted because the information is inapplicable or presented in our Consolidated Financial Statements or notes.
| (3) | Index to Exhibits |
| Exhibit |
Exhibit Title | |
| 2.1 | Separation and Distribution Agreement, dated December 17, 2008, between the Company and Facet Biotech Corporation (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed December 23, 2008) | |
| 2.2 | Amendment No. 1 to Separation and Distribution Agreement, dated January 20, 2009, between the Company and Facet Biotech Corporation (incorporated by reference to Exhibit 2.2 to Annual Report on Form 10-K filed March 2, 2009) | |
| 3.1 | Restated Certificate of Incorporation effective March 23, 1993 (incorporated by reference to Exhibit 3.1 to Annual Report on Form 10-K filed March 31, 1993) | |
| 3.2 | Certificate of Amendment of Certificate of Incorporation effective August 21, 2001 (incorporated by reference to Exhibit 3.3 to Annual Report on Form 10-K filed March 14, 2002) | |
| 3.3 | Certificate of Amendment of Certificate of Incorporation effective January 9, 2006 (incorporated by reference to Exhibit 99.1 to Current Report on Form 8-K filed January 10, 2006) | |
| 3.4 | Certificate of Designation, Preferences and Rights of the Terms effective August 25, 2006 (incorporated by reference to Exhibit 3.4 to Registration Statement on Form 8-A filed September 6, 2006) | |
| 3.5 | Amended and Restated Bylaws effective June 4, 2009 (incorporated by reference to Exhibit 99.1 to Current Report on Form 8-K filed June 10, 2009) | |
| 4.1 | Indenture between the Company and J.P. Morgan Trust Company, National Association, dated July 14, 2003 (incorporated by reference to Exhibit 4.1 to Registration Statement on Form S-3 filed September 11, 2003) | |
| 4.2 | Indenture between the Company and J.P. Morgan Trust Company, National Association, dated February 14, 2005 (incorporated by reference to Exhibit 4.1 to Current Report on Form 8-K filed February 16, 2005) | |
| *10.1 | 1991 Stock Option Plan, as amended October 20, 1992 and June 15, 1995, together with forms of Incentive Stock Option Agreement and Nonqualified Stock Option Agreement (incorporated by reference to Exhibit 10.1 to Annual Report on Form 10-K filed March 31, 1996) | |
| *10.2 | 1991 Stock Option Plan, as amended October 17, 1996 (incorporated by reference to Exhibit 10.2 to Annual Report on Form 10-K filed March 14, 2002) | |
| *10.3 | 1999 Stock Option Plan (incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.4 | 1999 Nonstatutory Stock Option Plan, as amended through February 20, 2003 (incorporated by reference to Exhibit 10.3 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.5 | Form of Notice of Grant of Stock Option under the 1999 Stock Option Plan (incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q filed August 14, 2002) | |
73
Table of Contents
| Exhibit |
Exhibit Title | |
| *10.6 | Form of Stock Option Agreement (incentive stock options) under the 1999 Stock Option Plan (incorporated by reference to Exhibit 10.4 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.7 | Form of Stock Option Agreement (nonstatutory stock options) under the 1999 Stock Option Plan (incorporated by reference to Exhibit 10.5 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.8 | Form of Notice of Grant of Stock Option under the 1999 Nonstatutory Stock Option Plan (incorporated by reference to Exhibit 10.3 to Quarterly Report on Form 10-Q/A filed November 14, 2007) | |
| *10.9 | Form of Stock Option Agreement under the 1999 Nonstatutory Stock Option Plan (incorporated by reference to Exhibit 10.6 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.10 | 2002 Outside Directors Stock Option Plan, as amended June 8, 2005 (incorporated by reference to Exhibit 99.2 to Current Report on Form 8-K filed June 14, 2005) | |
| *10.11 | Form of Nonqualified Stock Option Agreement under the 2002 Outside Directors Plan (incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q/A filed November 14, 2007) | |
| *10.12 | 2005 Equity Incentive Plan (incorporated by reference to Exhibit 99.1 to Current Report on Form 8-K filed June 14, 2005) | |
| *10.13 | Form of Notice of Grant of Stock Option under the 2005 Equity Incentive Plan (incorporated by reference to Exhibit 10.7 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.14 | Form of Stock Option Agreement under the 2005 Equity Incentive Plan (incorporated by reference to Exhibit 10.8 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.15 | Form of Notice of Grant of Restricted Stock Award under the 2005 Equity Incentive Plan (incorporated by reference to Exhibit 10.9 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.16 | Form of Restricted Stock Agreement under the 2005 Equity Incentive Plan (for the officers of the Company) (incorporated by reference to Exhibit 10.10 to Quarterly Report on Form 10-Q filed August 9, 2006) | |
| *10.17 | Form of Director and Officer Indemnification Agreement (incorporated by reference to Exhibit 10.1 to Registration Statement on Form S-1 filed December 16, 1991) | |
| *10.18 | Offer Letter between the Company and Mr. John McLaughlin dated November 4, 2008 (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed November 10, 2008) | |
| *10.19 | Offer Letter between the Company and Ms. Christine Larson dated December 15, 2008 (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed December 19, 2008) | |
| 10.20 | Transition Services Agreement, dated December 18, 2008, between the Company and Facet Biotech Corporation (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed December 23, 2008) | |
| 10.21 | Tax Sharing and Indemnification Agreement, dated December 18, 2008, between the Company and Facet Biotech Corporation (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed December 23, 2008) | |
| 10.22 | Patent Licensing Master Agreement between the Company and Genentech, Inc., dated September 25, 1998 (incorporated by reference to Exhibit 10.10 to Quarterly Report on Form 10-Q filed November 16, 1998)† | |
| 10.23 | Amendment No. 1 to Patent Licensing Master Agreement between the Company and Genentech, Inc., dated September 18, 2003 (incorporated by reference to Exhibit 10.45 to Annual Report on Form 10-K filed March 8, 2004)† | |
74
Table of Contents
| Exhibit |
Exhibit Title | |
| 10.24 | Amendment No. 2 to Patent Licensing Master Agreement between the Company and Genentech, Inc., dated December 18, 2003 (incorporated by reference to Exhibit 10.45 to Annual Report on Form 10-K filed March 2, 2009) | |
| 10.25 | Amendment No. 1 to the Herceptin® License Agreement between the Company and Genentech, Inc., dated December 18, 2003 (incorporated by reference to Exhibit 10.47 to Annual Report on Form 10-K filed March 8, 2004) | |
| 10.26 | Patent License Agreement, dated July 17, 1997, between the Company and MedImmune Inc. (incorporated by reference to Exhibit 10.45 to Annual Report on Form 10-K filed March 2, 2009)† | |
| 10.27 | Patent License Agreement, dated April 24, 1998, between the Company and Elan International Services Ltd. (incorporated by reference to Exhibit 10.45 to Annual Report on Form 10-K filed March 2, 2009) † | |
| 10.28 | Amendment to Rights Agreement, dated August 25, 2006 between PDL and Mellon Investor Services, LLC as Rights Agent (incorporated by reference to Exhibit 4.1 to Form 8-K filed February 5, 2009) | |
| *10.29 | Offer Letter between the Company and Christopher Stone, dated December 30, 2008 | |
| *10.30 | Offer Letter between the Company and Karen Wilson, dated April 17, 2009 (incorporated by reference to Exhibit 10.1 to Form 8-K filed April 28, 2009) | |
| 10.31 | Asset Purchase Agreement between the Company and EKR Therapeutics, Inc. dated February 4, 2008 and Amendment No. 1 to Asset Purchase Agreement dated as of March 7, 2008 (incorporated by reference to Exhibit 10.5 to Form 10-Q/A filed May 5, 2009) | |
| 10.32 | Asset Purchase Agreement between the Company and GMN, Inc. dated February 21, 2008 (incorporated by reference to Exhibit 10.5 to Form 10-Q/A filed May 5, 2009) | |
| 10.33 | Amended and Restated 2005 Equity Incentive Plan effective June 4, 2009 (incorporated by reference to Exhibit 10.1 to Form 10-Q filed July 31, 2009) | |
| 10.34 | Indenture, dated November 2, 2009 between PDL and wholly-owned subsidiary QHP Royalty Sub LLC (incorporated by reference to Exhibit 99.1 to Form 8-K filed November 6, 2009) | |
| 10.35 | Purchase and Sale Agreement, dated November 2, 2009 between PDL and wholly-owned subsidiary QHP Royalty Sub LLC (incorporated by reference to Exhibit 99.2 to Form 8-K filed November 6, 2009) | |
| 10.36 | Pledge and Security Agreement, dated November 2, 2009 between PDL and wholly-owned subsidiary QHP Royalty Sub LLC (incorporated by reference to Exhibit 99.3 to Form 8-K filed November 6, 2009) | |
| 10.37 | Bill of Sale, dated November 2, 2009 between PDL and wholly-owned subsidiary QHP Royalty Sub LLC (incorporated by reference to Exhibit 99.4 to Form 8-K filed November 6, 2009) | |
| 14.1 | Code of Business Conduct (incorporated by reference to Exhibit 14.1 to Current Report on Form 8-K filed February 5, 2009) | |
| 21.1 | Subsidiaries of the Registrant | |
| 23.1 | Consent of Independent Registered Public Accounting Firm | |
| 31.1 | Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended | |
| 31.2 | Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended | |
75
Table of Contents
| Exhibit |
Exhibit Title | |
| 32.1 | Certification by the Principal Executive Officer and the Principal Financial Officer, as required by Rule 13a-14(b) or Rule 15d-14(b) of the Securities Exchange Act of 1934, as amended, and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. 1350) |
| * | Management contract or compensatory plan or arrangement. |
| † | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under 17 C.F.R. Sections 200.80(b)(4) and 24b-2. |
76
Table of Contents
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
| (In thousands) |
Balance at Beginning of Year |
Charged to Costs and Expenses |
Deductions(1) | Charged to Other Accounts |
Balance at End of Year | ||||||||||
| Year ended December 31, 2009: |
|||||||||||||||
| Allowances for accounts receivable |
$ | - | $ | - | $ | - | $ | - | $ | - | |||||
| Year ended December 31, 2008: |
|||||||||||||||
| Allowances for accounts receivable |
$ | 17,722 | $ | 4,120 | $ | (13,387) | $ | (8,455) | $ | - | |||||
| Year ended December 31, 2007: |
|||||||||||||||
| Allowances for accounts receivable |
$ | 13,709 | $ | 46,760 | $ | (44,035) | $ | 1,288 | $ | 17,722 | |||||
| (1) | Deductions represent amounts written off against the allowances or reserve. |
77
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| PDL BIOPHARMA, INC. (REGISTRANT) | ||
| By: | /S/ JOHN P. MCLAUGHLIN | |
| John P. McLaughlin | ||
| President and Chief Executive Officer | ||
| Date: March 1, 2010 | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ JOHN P. MCLAUGHLIN (John P. McLaughlin) |
President and Chief Executive Officer and Director |
March 1, 2010 | ||
| /S/ CHRISTINE R. LARSON (Christine R. Larson) |
Vice President and Chief Financial Officer (Principal Financial Officer) |
March 1, 2010 | ||
| /S/ KAREN J. WILSON (Karen J. Wilson) |
Vice President Finance (Principal Accounting Officer) |
March 1, 2010 | ||
| /S/ FREDERICK FRANK (Frederick Frank) |
Director |
March 1, 2010 | ||
| /S/ JOSEPH KLEIN III (Joseph Klein III) |
Director |
March 1, 2010 | ||
| /S/ JODY S. LINDELL (Jody S. Lindell) |
Director |
March 1, 2010 | ||
| /S/ PAUL W. SANDMAN (Paul W. Sandman) |
Director |
March 1, 2010 | ||
| /S/ HAROLD E. SELICK (Harold E. Selick) |
Director |
March 1, 2010 | ||
78
