Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-9034_18k.htm |
| EX-99.2 - EX-99.2 - VIVUS INC | a11-9034_1ex99d2.htm |
Exhibit 99.1
Below is a graphical representation of the poster entitled “Weight Loss and Cardiovascular Risk Reduction Over 2 Years With Controlled-Release Phentermine-Topiramate”:
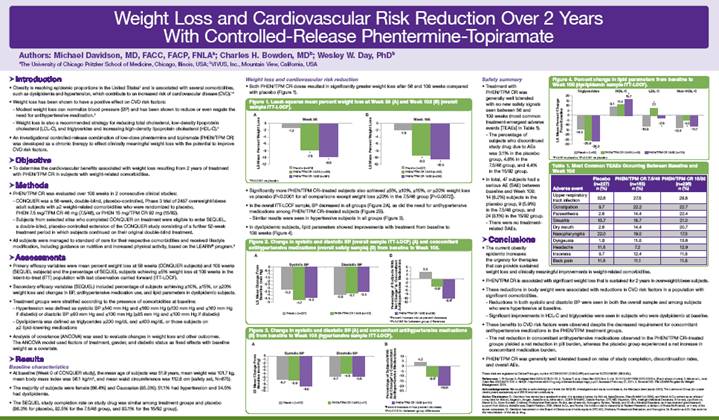
Below is a reproduction of the contents of the poster entitled “Weight Loss and Cardiovascular Risk Reduction Over 2 Years With Controlled-Release Phentermine-Topiramate”:
Authors: Michael Davidson, MD, FACC, FACP, FNLA(a); Charles H. Bowden, MD(b); Wesley W. Day, PhD(b)
(a) The University of Chicago Pritzker School of Medicine, Chicago, Illinois, USA; (b) VIVUS, Inc., Mountain View, California, USA
· Introduction
· Obesity is reaching epidemic proportions in the United States(1) and is associated with several comorbidities, such as dyslipidemia and hypertension, which contribute to an increased risk of cardiovascular disease (CVD).(1)-(4)
· Weight loss has been shown to have a positive effect on CVD risk factors:
· Modest weight loss can normalize blood pressure (BP) and has been shown to reduce or even negate the need for antihypertensive medication.(3)
· Weight loss is also a recommended strategy for reducing total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides and increasing high-density lipoprotein cholesterol (HDL-C).(4)
· An investigational controlled-release combination of low-dose phentermine and topiramate (PHEN/TPM CR) was developed as a chronic therapy to effect clinically meaningful weight loss with the potential to improve CVD risk factors.
· Objective
· To determine the cardiovascular benefits associated with weight loss resulting from 2 years of treatment with PHEN/TPM CR in subjects with weight-related comorbidities.
· Methods
· PHEN/TPM CR was evaluated over 108 weeks in 2 consecutive clinical studies:
· CONQUER was a 56-week, double-blind, placebo-controlled, Phase 3 trial of 2487 overweight/obese adult subjects with >2 weight-related comorbidities who were randomized to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92).
· Subjects from selected sites who completed CONQUER on treatment were eligible to enter SEQUEL, a double-blind, placebo-controlled extension of the CONQUER study consisting of a further 52-week treatment period in which subjects continued on their original double-blind treatment.
· All subjects were managed to standard of care for their respective comorbidities and received lifestyle modification, including guidance on nutrition and increased physical activity, based on the LEARN® program.(5)
· Assessments
· Primary efficacy variables were mean percent weight loss at 56 weeks (CONQUER subjects) and 108 weeks (SEQUEL subjects) and the percentage of SEQUEL subjects achieving >5% weight loss at 108 weeks in the intent-to-treat (ITT) population with last observation carried forward (ITT-LOCF).
· Secondary efficacy variables (SEQUEL) included percentage of subjects achieving >10%, >15%, or >20% weight loss and changes in BP, antihypertensive medication use, and lipid parameters in dyslipidemic subjects.
· Treatment groups were stratified according to the presence of comorbidities at baseline:
· Hypertension was defined as systolic BP >140 mm Hg and <160 mm Hg (>130 mm Hg and <160 mm Hg if diabetic) or diastolic BP >90 mm Hg and <100 mm Hg (>85 mm Hg and <100 mm Hg if diabetic)
· Dyslipidemia was defined as triglycerides >200 mg/dL and <400 mg/dL or those subjects on >2 lipid-lowering medications
· Analysis of covariance (ANCOVA) was used to evaluate changes in weight loss and other outcomes. The ANCOVA model used factors of treatment, gender, and diabetic status as fixed effects with baseline weight as a covariate.
· Results
Baseline characteristics
· At baseline (Week 0 of CONQUER study), the mean age of subjects was 51.9 years, mean weight was 101.7 kg, mean body mass index was 36.1 kg/m2, and mean waist circumference was 112.6 cm (safety set, N=675).
· The majority of subjects were female (66.4%) and Caucasian (85.3%); 51.1% had hypertension and 34.5% had dyslipidemia.
· The SEQUEL study completion rate on study drug was similar among treatment groups and placebo (86.3% for placebo, 82.5% for the 7.5/46 group, and 83.1% for the 15/92 group).
Weight loss and cardiovascular risk reduction
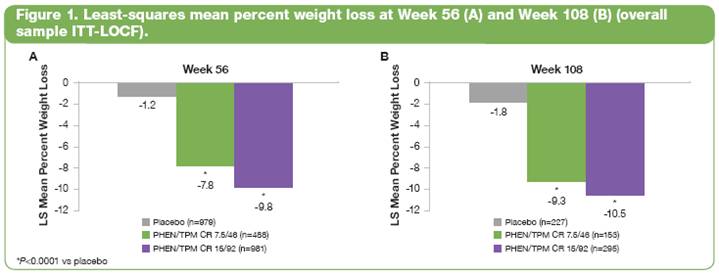
· Both PHEN/TPM CR doses resulted in significantly greater weight loss after 56 and 108 weeks compared with placebo (Figure 1).
· Significantly more PHEN/TPM CR-treated subjects also achieved >5%, >10%, >15%, or >20% weight loss vs placebo (P<0.0001 for all comparisons except weight loss >20% in the 7.5/46 group [P=0.0072]).
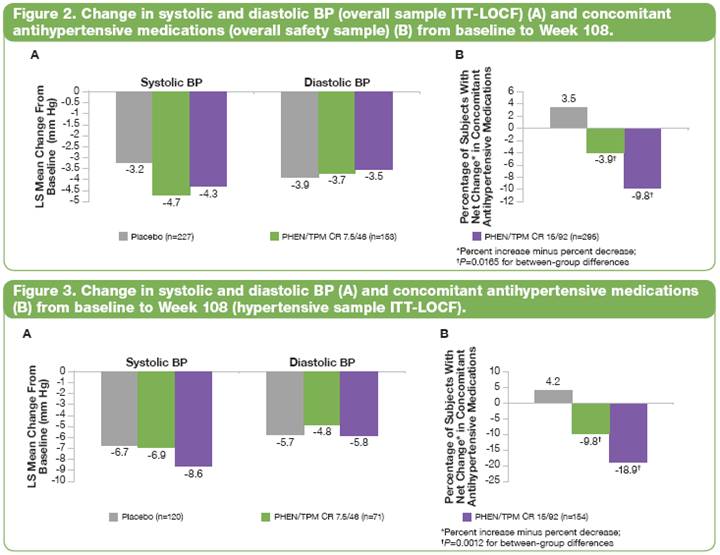
· In the overall ITT-LOCF sample, BP decreased in all groups (Figure 2A), as did the need for antihypertensive medications among PHEN/TPM CR–treated subjects (Figure 2B).
· Similar results were seen in hypertensive subjects in all groups (Figure 3).
· In dyslipidemic subjects, lipid parameters showed improvements with treatment from baseline to 108 weeks (Figure 4).
Safety summary
· Treatment with PHEN/TPM CR was generally well tolerated with no new safety signals seen between 56 and 108 weeks (most common treatment-emergent adverse events [TEAEs] in Table 1).
· The percentage of subjects who discontinued study drug due to AEs was 3.1% in the placebo group, 4.6% in the 7.5/46 group, and 4.4% in the 15/92 group.
· In total, 47 subjects had a serious AE (SAE) between baseline and Week 108: 14 (6.2%) subjects in the placebo group, 9 (5.9%) in the 7.5/46 group, and 24 (8.1%) in the 15/92 group.
· There were no treatment-related SAEs.
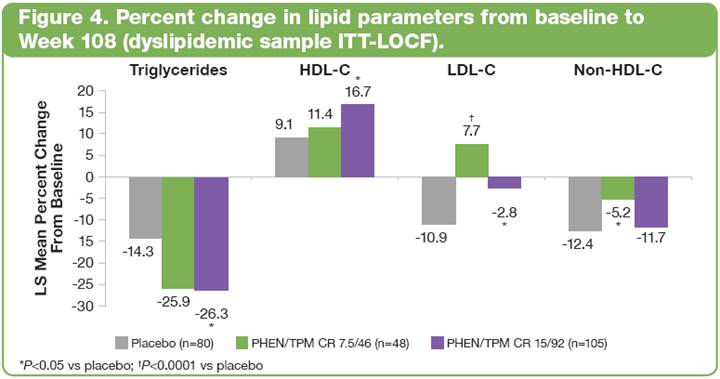
Table 1. Most Common TEAEs Occurring Between Baseline and Week 108
|
Adverse event |
|
Placebo |
|
PHEN/TPM CR 7.5/46 |
|
PHEN/TPM CR 15/92 |
|
|
Upper respiratory tract infection |
|
32.6 |
|
27.5 |
|
28.5 |
|
|
Constipation |
|
9.7 |
|
22.2 |
|
22.7 |
|
|
Paraesthesia |
|
2.6 |
|
14.4 |
|
22.4 |
|
|
Sinusitis |
|
13.7 |
|
15.7 |
|
21.0 |
|
|
Dry mouth |
|
2.6 |
|
14.4 |
|
20.7 |
|
|
Nasopharyngitis |
|
22.0 |
|
19.0 |
|
17.3 |
|
|
Dysgeusia |
|
1.8 |
|
11.8 |
|
13.6 |
|
|
Headache |
|
11.5 |
|
7.2 |
|
12.9 |
|
|
Insomnia |
|
9.7 |
|
12.4 |
|
11.5 |
|
|
Back pain |
|
11.5 |
|
11.1 |
|
11.5 |
|
· Conclusions
· The current obesity epidemic increases the urgency for therapies that can provide sustained weight loss and clinically meaningful improvements in weight-related comorbidities.
· PHEN/TPM CR is associated with significant weight loss that is sustained for 2 years in overweight/obese subjects.
· These reductions in body weight were associated with reductions in CVD risk factors in a population with significant comorbidities.
· Reductions in both systolic and diastolic BP were seen in both the overall sample and among subjects who were hypertensive at baseline.
· Significant improvements in HDL-C and triglycerides were seen in subjects who were dyslipidemic at baseline.
· These benefits to CVD risk factors were observed despite the decreased requirement for concomitant antihypertensive medications in the PHEN/TPM treatment groups.
· The net reduction in concomitant antihypertensive medications observed in the PHEN/TPM CR-treated groups yielded a net reduction in pill burden, whereas the placebo group experienced a net increase in concomitant medication burden.
· PHEN/TPM CR was generally well tolerated based on rates of study completion, discontinuation rates, and overall AEs.
These trials are registered at ClinicalTrials.gov, number NCT00553787 (CONQUER) and number NCT00796367 (SEQUEL).
References: 1. Pi-Sunyer X. Postgrad Med 2009; 121(6);21-33. 2. Tzotzas T. et al. Obes Rev 2010 Nov 3. doi: 10.1111/j.1467-789X.2010.00807.x. [Epub ahead of print]. 3. Mertens IL, et al. Obes Res 2000;8;270-278. 4. NHLBI. http//www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed February 21, 2011. 5. Brownell KD. The LEARN Program for Weight Management. 2004.
Acknowledgements: We would like to acknowledge and thank the SEQUEL investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
Author Disclosures: Dr. Davidson has served as a speaker/member of a speakers bureau for Abbott, AstraZeneca, GlaxoSmitKline (GSK), and Merck & CO; served as an advisor/consultant for Abbott, Aegerion, Amgen, AstraZeneca, Atherotech, CCBR-SYNARC, Daiichi-Sankyo, DTC MD, Esperion, GSK, Intelligent Medical Decisions, Kinemed, LipoScience, Merck & Co, Novo Nordisk, Omthera, Professional Evaluation, Roche, sanofi-aventis, Sonogene, Synarc, Takeda, and Vindico Medical Education; and has received grant/research support from Abbott, AstraZeneca, Daiichi-Sankyo, GSK, Merck & Co, and Roche. He holds no stock ownership in Radiant Research, a division of Swiss BioScience, or in any of the above companies. Dr. Davidson has served on the Board of Directors and holds equity in DTC MD, Omthera, Professional Evaluation, and Sonogene. Dr. Bowden and Dr. Day work for the manufacturer of the study drug.
