Attached files
| file | filename |
|---|---|
| EX-31.1 - ONE Holdings, Corp. | csev_ex311.htm |
| EX-32.1 - ONE Holdings, Corp. | csev_ex321.htm |
| EX-31.2 - ONE Holdings, Corp. | csev_ex312.htm |
| EX-32.2 - ONE Holdings, Corp. | csev_ex322.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2010
Commission file number 333-136643
|
ONE BIO, CORP.
|
||
|
(Exact Name of Registrant as Specified In Its Charter)
|
||
|
Florida
|
59-3656663
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
| 19950 West Country Club Drive, Suite 100, Aventura, Florida | 33180 | |
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
(305)3288662
|
||
|
(Registrant's Telephone Number, Including Area Code)
|
20900 NE 30th Avenue Suite 842 Aventura Florida 33180
Former Name and Address
Securities registered under Section 12(b) of the Act: NONE
Securities registered pursuant to Section 12(g) of the Act: NONE
(Title of Class)
_____________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d0 of the act. Yes o No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in rule 12-b-2 of the Exchange Act. (Check One):
| Large Accelerated Filer | o | Accelerated Filer | o | Non-accelerated Filer | o | Smaller Reporting Company | þ |
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2). Yes o No þ
The aggregate market value of the registrants voting and non-voting common equity held by non-affiliates as of December 31, 2010, was approximately $12,602,431.
The number of shares of common stock outstanding as of March 15, 2011 was 6,725,862.
TABLE OF CONTENTS
| PART I | |||||
| Item 1. | Description of Business | 2 | |||
| Item 1A | Risk Factors | 22 | |||
| Item 1B | Unresolved Staff Comments | 44 | |||
| Item 2. | Description of Property | 44 | |||
| Item 3. | Legal Proceedings | 46 | |||
| Item 4. | [Reserved] | 46 | |||
| PART II | |||||
| Item 5. | Market for Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 47 | |||
| Item 6. | Selected Financial Data | 47 | |||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results | 48 | |||
| Item 8. | Financial Statements | 57 | |||
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 57 | |||
| Item 9A. | Controls and Procedures | 57 | |||
| PART III | |||||
| Item 10. | Directors, Executive Officers and Corporate Governance | 58 | |||
| Item 11. | Executive Compensation | 62 | |||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 62 | |||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 63 | |||
| Item 14 | Principal Accountant Fees and Services | 64 | |||
| Item 15. | Exhibits and Financial Statement Schedules | 65 | |||
| SIGNATURES | 67 | ||||
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934. These statements involve risks and uncertainties, including, among other things, statements regarding our business strategy, future revenues and anticipated costs and expenses. Such forward-looking statements include, among others, those statements including the words “expects,” “anticipates,” “intends,” “believes,” “may,” “will,” “should,” “could,” “plans,” “estimates,” and similar language or negative of such terms. Our actual results may differ significantly from those projected in the forward-looking statements. Factors that might cause or contribute to such differences include, but are not limited to, those discussed in Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this report. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we do not know whether we can achieve positive future results, levels of activity, performance, or goals. Actual events or results may differ materially. We undertake no obligation to publicly release any revisions to the forward-looking statements or reflect events or circumstances taking place after the date of this document.
1
PART I
|
ITEM 1.
|
DESCRIPTION OF BUSINESS
|
1.1 Business
ONE Bio, Corp. (the “Company” and/or "ONE" and/or “Registrant”) (www.onehcorp.com) headquartered in Aventura, FL, is an award winning, innovative company utilizing green process manufacturing to produce raw chemicals and herbal extracts, natural supplements and organic products. ONE is focused on the Asia Pacific region and the United States of America. Key products include widely recognized Solanesol, Ganoderma Tea, CoQ10, Resveratrol, 5-HTP, organic fertilizers, and organic bamboo health food and beverages. ONE's growth plan targets an aggressive organic growth strategy supported by strategic acquisition. We are committed to becoming a leader in agritech utilizing green processes, combining our experience in producing our chemical and herbal extract products, natural supplements and organic products with seasoned North American managerial expertise. Our key market is Asia-Pacific which currently generates approximately 79% of our revenue followed by North and South America that generates 21% of our revenue. (Unless otherwise specified all dollar amounts in this prospectus are in U.S. dollars.) We are headquartered in Aventura, Florida; however, our primary contractually controlled operating enterprises, Sanming Huajian Bio-Engineering Co., Ltd. (“Sanming”) and Jianou Lujian Foodstuff Co., are based in Sanming City and Jianou City, respectively, in the Fujian province of the People’s Republic of China (“PRC”).
1.2 Our History
The Company was incorporated under the laws of the State of Florida on June 30, 2000 under the name of Contracted Services, Inc.
On January 8, 2010, the Company entered into a Securities Purchase and Registration Rights Agreement with certain institutional and accredited investors pursuant to which the investors purchased $3,000,000 in aggregate principal amount of our 8% Convertible Promissory Notes. The Convertible Notes are due on October 11, 2010, and are convertible at the elections of the Investors into shares of our Common Stock at an initial Conversion Price equal to the lesser of (a) $6.077 per share, or (b) 65% of the price per share offered by the Company in any publicly offered Common Stock or other equity-linked financing by the Company that is closed within nine months of the Initial Closing (each, a “New Financing”). Pursuant to the SPA, we also issued to the Investors five (5) year warrants (“Warrants”) to purchase an aggregate of 49,366 shares of our Common Stock at an initial exercise equal to the lesser of (a) $6.077 per share, or (b) 65% of the price per share offered by us in any New Financing. Effective June 30, 2010, the Company modified the terms of its existing bridge loan agreement which was entered into on February 11, 2010. The modifications include the cancellation of both the conversion feature embedded in the note and the detachable warrant, along with a two month extension of the note’s maturity date to December 10, 2010, with an option to further extend the notes maturity to January 10, 2011 (the “Amended Maturity Date”). On December 20, 2010, ONE entered into a loan extension agreement with an effective date of December 10, 2010, with UTA Capital and other investors (the “Investors”) to extend and modify the securities purchase agreement to January 31, 2011. In exchange for the Investors’ agreement to extend the maturity date, the Company agreed to issue to the Investors on January 1, 2011 warrants that shall be immediately exercisable for a period of five (5) years which shall entitle the purchasers to purchase up to an aggregate of 60,000 shares of common stock at an initial exercise price of $3.75 per share. Effective January 31, 2011, the Company entered into a Third Loan Extension Agreement with the Investors to extend the securities purchase agreement to April 1, 2011 with options for further extensions to May, 1, 2011 and June 1, 2011. In exchange for the Investors agreement to extend the maturity dates, we agreed to pay an extension fee of $29,000 and to issue to the Investors five (5) year second extension warrants to purchase 90,000 shares of Common stock at $3.00 per share. If the loan is extended to May 1, the Company agrees to pay a fee of $72,500 and to issue to the Investors 58,000 shares of Common stock for no additional consideration and to cause one or more of our principal shareholders to deposit 300,000 shares of our Common Stock as additional pledged stock under the stockholder pledge and security agreement. The Investors also agreed to convert $150,000 in principal amount of the amended notes into shares of Common stock under the terms of the Third Loan Agreement.
2
In January 2010, the Company issued 10,000 shares of Series A Preferred Stock to the Chairman and the CEO in the amount of 3,733 and 6,267 respectively. This action, approved by the Board, was in consideration and recognition of the agreement by the executives to pledge 6 million shares of common stock in satisfaction of the share pledge condition of the Bridge Loan Financing Agreement. Holders of the Series A Preferred Stock shall be entitled to cast two thousand (2,000) votes for each share held of the Series A Preferred Stock on all matters presented to the shareholders of the Company for a shareholder vote which shall vote along with holders of the Company’s Common Stock on such matters.
On April 14, 2010, the Company entered into a Share Purchase Agreement (the “Selling Shareholder SPA”), with Min Zhao (our director and president of our CHE business unit), (the “Selling Shareholder”), pursuant to which, among other things we acquired from the Selling Shareholder 1,632,150 shares of common stock of Green Planet owned by the Selling Shareholder in consideration for 260,000 shares (adjusted for the 1 for 5 stock splits of August 2010) of our restricted common stock. The number of shares of our common stock issuable to the Selling Shareholder is subject to adjustment as set forth in the Selling Shareholder SPA. As part of this transaction, the Selling Shareholder agreed to a lock-up and leak-out period as further defined in this agreement. At the same time, we received from a certain Green Planet shareholder 1,216,184 shares of common stock of Green Planet. As a consequence of these transactions, we now own 92.5% of the outstanding common stock of Green Planet.
Also on April 14, 2010, the Company entered into an agreement with Green Planet pursuant to which, among other things,
|
(i)
|
that certain Amended and Restated Green Planet Preferred Stock Purchase Agreement made effective as of June 17, 2009, between us and Green Planet (“Amended and Restated GP Preferred Stock Agreement”) was cancelled,
|
|
(ii)
|
we returned to Green Planet the 5,101shares of Green Planet preferred stock that were issued to us pursuant to the Amended and Restated GP Preferred Stock Agreement, and
|
|
(iii)
|
Green Planet returned to us the 200,962 shares (adjusted for the 1 for 5 stock splits of November 2009 and August 2010) of our common stock that we issued to Green Planet pursuant to the Amended and Restated GP Preferred Stock Agreement.
|
Additionally, on April 14, 2010, Green Planet granted to ONE an option to acquire 100% of the stock of Elevated Throne Overseas Ltd. (“Elevated Throne”), Green Planet’s 100% owned BVI subsidiary. In the event we exercise this option, the closing of the transaction will be subject to the approval of Green Planet’s stockholders. As consideration for our exercise of this option, we will be required to
|
(i)
|
convert the $1,700,000 loan we made to Elevated Throne on or about January 19, 2010, into an equity investment in Elevated Throne,
|
|
(ii)
|
convert the $300,000 loan we made to Green Planet on or about September 1, 2009, into a $300,000 equity investment in Elevated Throne,
|
|
(iii)
|
cancel that certain Convertible Note Purchase Agreement between us and Green Planet dated on or about September 1, 2009, and
|
|
(iv)
|
cancel that certain 10% Convertible Bridge Loan Note Due September 1, 2010, in the principal amount of $300,000 from Green Planet to us.
|
On September 14, 2010 FINRA advised the Company that they would process the Company’s 1 for 5 reverse stock split previously disclosed in the Company’s July 27, 2010, Definitive Information Statement on Schedule 14 (c), relating to an amendment to the Company’s Articles of Incorporation to: (i) reduce the number of authorized shares of common stock from 150,000,000 shares to 30,000,000 shares: and (ii) effecting a 1 for 5 reverse split of the Company’s common stock.
3
Subsequent to the filing and effectiveness of above referenced amendment to the Company’s Articles of Incorporation, the Company then filed on August 30, 2010 another amendment to its Articles of Incorporation also previously disclosed in the Company’s July 27, 2010, Definitive Information Statement on Schedule 14 (c) pursuant to which the number of authorized shares of common stock was increased from 30,000,000 shares to 100,000,000.
On December 20, 2010, we delivered written notice to Green Planet Bioengineering, Co., Ltd. (“Green Planet”) that we elected to exercise the option we were granted pursuant to that certain Option Agreement dated April 14, 2010 (the “Option Agreement”), to acquire 100% of the stock of Elevated Throne Overseas Ltd. (“Elevated Throne”), Green Planet’s 100% owned BVI subsidiary and in consideration therefore we agreed to (i) convert the $1,700,000 loan we made to Elevated Throne on or about January 19, 2010, into an equity investment in Elevated Throne, (ii) convert the $300,000 loan we made to Green Planet on or about September 1, 2009, into a $300,000 equity investment in Elevated Throne, (iii) cancel that certain Convertible Note Purchase Agreement between us and Green Planet dated on or about September 1, 2009, and (iv) cancel that certain 10% Convertible Bridge Loan Note Due September 1, 2010, in the principal amount of $300,000 from us to Green Planet, (collectively referred to as the “Transaction”).
On December 20, 2010, we, as the owner of 92.5% of the outstanding common stock of Green Planet by Majority Shareholder Written Consent in Lieu of a Special Meeting of Stockholders approved, authorized, and ratified the Transaction contemplated by the Option Agreement and the exercise by us of the Option as described above retroactive as of April 14, 2010.
On June 4, 2009, Abacus Global Investments, Corp. (“Abacus”) entered into a material definitive agreement pursuant to which Abacus acquired eighteen million seven hundred fifty thousand (18,750,000) shares of the Contracted Services, Inc.’s common stock representing 92.25% of the Company’s issued and outstanding stock. The transaction closed on June 9, 2009. Following the transaction, Abacus Global Investments, Corp. controlled 92.25% of the Registrant’s outstanding capital stock. Additionally, effective June 9, 2009, Marius Silvasan was appointed as a director and as the interim President of the Company.
On June 9, 2009, the Company filed an amendment to its Articles of Incorporation pursuant to which the Company’s name was changed to ONE HOLDINGS, CORP. (“ONE”). The Company also changed its registered address on the same day.
Upon Abacus’ acquisition of majority control of the Company and following the name change to ONE, Abacus changed the Company’s business focus to its current strategy.
On July 22, 2009, the Company acquired from certain shareholders (“GP Shareholders”) of Green Planet Bioengineering Co., Ltd., a Delaware corporation (“Green Planet”) 80% of the issued and outstanding shares of common stock of Green Planet. The Company also acquired 5,101 shares of Green Planet preferred stock which preferred stock provides the Company the right to vote 1,000 votes on all matters submitted to a vote of the shareholders of Green Planet and is convertible into 1,000 shares of Green Planet common stock. The Company acquired the Green Planet preferred stock in consideration for the issuance by the Company to Green Planet of 200,962 shares (adjusted for the 1 for 5 stock splits of November 2009 and August 2010) of the Company’s common stock. As part of this Green Planet transaction, Green Planet and the GP Shareholders deposited into an Escrow thirty-five percent (35%) of the Company’s shares issued to Green Planet and the GP Shareholders and in the event Green Planet’s EBITDA for fiscal year 2009 is less than GP’s EBITDA for fiscal 2008, the number of shares of Registrant’s stock issued to Green Planet and the GP Shareholders shall be proportionately reduced as provided for in the Green Planet Preferred Stock Purchase Agreement (the “Preferred Stock Purchase Agreements”) and the Stock Purchase Agreement (“GP SPA”) with the GP Shareholders. Green Planet and the GP Shareholders are also subject to a lockup and leak out period and has one piggy-back registration right as further defined in the Green Planet Preferred Stock Purchase Agreement and GP SPA. As a result of the Green Planet transactions, the Company has become the majority shareholder of Green Planet and based upon the number of shares outstanding and assuming conversion or the Green Planet preferred stock into common stock, the Company would own approximately 83% of Green Planet’s shares. Green Planet owns 100% of Fujian Green Planet Bioengineering Co., Ltd., (“Fujian Green Planet”), a WFOE, that through a series of contractual arrangements has effective control of the business and operations of and has an irrevocable option to purchase the equity and/or assets of Sanming (a PRC company), a green process manufacturer of high quality health supplements. Consequently, the Company effectively controls the business and operations of Green Planet, Fujian Green Planet (a GP WFOE) and Sanming.
4
On September 3, 2009, ONE acquired from the shareholders of Trade Finance Solutions ("collectively referred to as "TFS Shareholders") 3,990 shares representing 99.75% of the Shareholders' common shares owned in Trade Finance Solutions Inc. ("TFS"). For the TFS shares, each TFS Shareholder is to receive shares of the Registrant's common stock and cash payments as per the Share Purchase Agreement with the TFS Shareholders (“TFS SPA”). The cash component of the purchase price will be calculated on an earn-out basis based on TFS' monthly EBIT (earnings before interest and taxes) beginning with the measuring period as defined in the TFS SPA not to exceed the purchase price of $6,000,000. In addition to the cash portion of the purchase price, the TFS Shareholders shall receive 1 share of ONE common stock (adjusted for forward or reverse splits following the closing) for every $5.00 in EBIT achieved during the measuring period ("TFS Stock Compensation") subject to a maximum TFS Stock Compensation of 1.2 million shares of Registrant's common stock. The TFS Shareholders are subject to a lockup and leak out period as further defined in the TFS SPA. Upon the purchase of the TFS Common Shares from the TFS Shareholders, Registrant has become the majority shareholder of TFS. TFS was acquired to support Registrant’s international expansion by handling the logistics, fulfillment and financing for the group’s sales outside PRC. TFS provides balance sheet financing solutions and mitigates the group’s international credit exposure by insuring all international receivables through A-rated third party insurance providers. The TFS acquisition is strategic to the Company beyond the direct and immediate financial contribution TFS will bring to sales and net income because it will provide the Company the ability to use TFS as an internal financing arm ready, able and dedicated to fund and facilitate the growth of the Company’s core agritech business.
On September 27, 2009, the Company acquired through a variety of transactions, 83% control of Jianou Lujian Foodstuff Co., Ltd. (“JLF”), a company based in PRC. The Registrant executed and consummated a Share Exchange Agreement (the “Supreme Agreement”) by and among: (i) the Company’s 100% owned subsidiary United Green Technology Inc., a Nevada corporation, (“UGTI”), (ii) Supreme Discovery Group Limited, a British Virgin Islands Company (“Supreme”), and (iii) the stockholders who owned 100% of Supreme’s common stock (the “Supreme Shareholders”). Supreme is the parent company of Fujian United Bamboo Technology Company Ltd., a wholly foreign-owned enterprise (“JLF WFOE”) organized under the laws of the PRC. The stockholders of Supreme are Tang Jinrong, Li Lifang and Tang Shuiyou, who are also the owners of JLF. JLF WFOE through a series of contractual arrangements has effective control of the business and operations of and has an irrevocable option to purchase the equity and/or assets of JLF. Consequently, the Company effectively controls the business and operations of Supreme, JLF WFOE and JLF.
Pursuant to the Supreme Agreement, at the closing, the Supreme Shareholders sold, transferred and assigned 100% of the outstanding common stock of Supreme to the Company’s wholly-owned subsidiary UGTI in exchange for (i) cash, (ii) shares of the Company’s common stock, and (iii) 20% of the issued and outstanding shares of common stock of UGTI. The Company thereby acquired through UGTI ownership of all of the outstanding stock of Supreme which owns 100% ownership of JLF WFOE. As part of this transaction the Supreme Shareholders deposited into an Escrow thirty-five percent (35%) of the Company’s shares of common stock issued to them and in the event UGTI’s EBITDA for fiscal year 2010 is less than UGTI’s EBITDA for fiscal 2009, the number of shares of the Company’s stock issued to Supreme Shareholders shall be proportionately reduced as provided for in the Supreme Agreement. Supreme Shareholders are also subject to a lockup and leak out period and have one piggy-back registration right as further defined in the Agreement. JLF is an award winning green-technology agritech company that specializes in the production of organic products and fertilizers based on bamboo. JLF holds bamboo land contracts in Fujian Province, one of PRC’s largest bamboo growing areas. JLF is the third largest bamboo producer in PRC and is the first bamboo company in PRC to gain food safety certification from China (HACCP), Japan (JAS) and Europe (ESFA). JLF was also the first company in PRC to formulate a “zero-to-zero” process starting from cultivation to distribution including the development of organic fertilizers from bamboo skins to eliminate waste.
Effective October 26, 2009, the Company amended its Articles of Incorporation to: (i) authorize a class of preferred stock consisting of 10,000,000 shares, $0.001 par value per share; (ii) designating 10,000 shares of the preferred stock as Series A Preferred Stock; (iii) reducing the number of authorized shares of common stock from 750,000,000 shares to 150,000,000 shares and changing the par value to $0.001 per share; (iv) changing the name of the Registrant to ONE Bio, Corp.; and (v) effecting a five (5) for one (1) reverse split of the Registrant’s Common Stock.
On November 3, 2009, the Company and UGTI cancelled and replaced the UGTI Preferred Stock Purchase Agreement and entered into a Share Purchase Agreement (the “UGTI Share Purchase Agreement”). Pursuant to the UGTI Share Purchase Agreement, we agreed to purchase from UGTI, and UGTI agreed to sell to us 10,000 shares of UGTI Common Stock in consideration for a cash payment of $1,200,000 in which $180,000 was paid in May 2010 and $1,020,000 originally due in November 2010, but deferred until 2011 per Bridge Loan Agreement.. As a result of the foregoing UGTI transactions, the Company is now 98% shareholder of UGTI.
On November 11, 2009, the Company filed an application to list its common stock on the NASDAQ Capital Market. The Company believes it currently fulfills or will shortly meet all applicable NASDAQ Capital Market listing requirements. The Company's listing application is subject to review and approval by NASDAQ's Listing Qualifications Department for compliance with all NASDAQ Capital Market standards.
5
1.2.1 Corporate Structure
The Corporate Structure of ONE consists of the companies under various business units which can be summarized as follows:
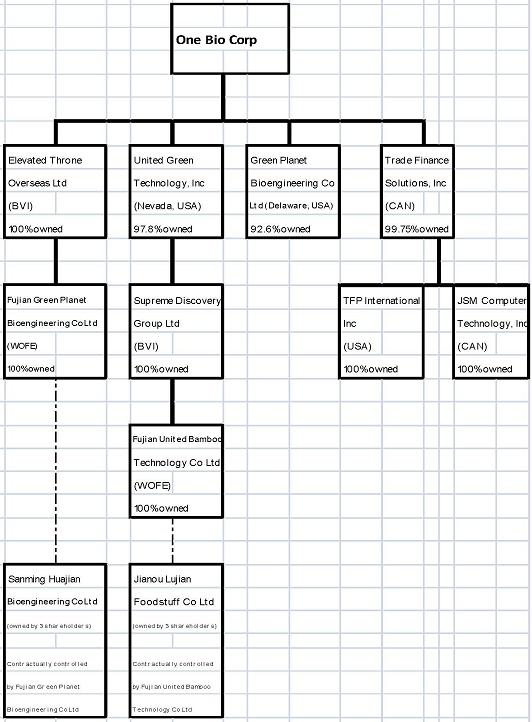
6
1.3 Business Overview
ONE Bio, Corp. headquartered in Aventura, FL, is an award winning, innovative agritech company that utilizes green process manufacturing to produce raw chemicals and herbal extracts, natural supplements and organic products. ONE is focused on the Asia-Pacific region and the United States of America. Key products include widely recognized Solanesol, Ganoderma Tea, CoQ10, Resveratrol and 5-HTP, organic fertilizers, and organic bamboo health food and beverages. ONE's growth plan targets an aggressive organic growth strategy supported by strategic acquisition. We are committed to becoming a leader in agritech utilizing green processes, combining our experience in producing our award winning chemical and herbal extract products with proven North American managerial expertise.
We are headquartered in Aventura, FL, USA; however, our primary operating enterprises, Sanming and JLF are based in Sanming City and Jianou City, respectively, in the Fujian province of PRC. Our key market is Asia-Pacific which currently generates approximately 79% of our revenue followed by North and South America that generates 21% of our revenue.
1.3.1 Operational Summary
Our operations are divided into two principal complementary business units that focus on producing chemical and herbal extracts (our “CHE” business unit) and organic products (our “OP” business unit) utilizing green processes. We also have an internal financing business unit that was acquired to support our international expansion by handling the logistics, fulfillment and financing for the group’s sales outside PRC. TFS provides balance sheet financing solutions and mitigates the group’s international credit exposure by insuring all international receivables through A-rated third party insurance providers.
In order to harvest the benefits of integrating growing and profitable Chinese operating enterprises with the management and financial techniques available to North American enterprises, we adhere to a “Yin-Yang” management strategy based on the Chinese Philosophy of “Correlative Thinking”. The core components of this approach are: constructing a strong, balanced team; addressing the needs of investors; and realizing the importance of diversity.
In practical terms, our strategy is to combine the manufacturing expertise and work ethic of the Eastern world with the investment experience and management skills of seasoned North American companies. Our team in PRC works together with our North American management to grow our core business, and provide transparency with an emphasis on risk management and internal controls.
1.3.1 (a) Elevated Throne and our Chemical and Herbal Extract (“CHE”) operating division
Our CHE business unit operates under the umbrella of our subsidiary Elevated Throne which contractually controls and operates Sanming. Sanming is the principle operating enterprise of this business unit and is located in and organized under the laws of the PRC.
Sanming is a research and development company with a focus on improving human health through the development, manufacture and commercialization of bio-ecological products and over-the-counter products utilizing extractions from tobacco leaves. Sanming's position in the agritech industry comes from its research and development which utilizes patented methods to create downstream products ranging from plant indigenous medicine and pharmaceutical intermediates to eco-friendly products. Since 2007, Sanming has developed a variety of natural organic products using tobacco leaves.
Our CHE unit produces chemical and herbal extracts for use in a wide range of health and wellness products. Utilizing green technology and proprietary processes, this unit extracts health supplements, fertilizers, and pesticides from waste tobacco. Our chemical extraction processes from tobacco leaves and delivers high purity Solanesol (98%) which can be further extracted into Coenzyme Q10 also known as CoQ10. The discarded tobacco leaves are further extracted to produce organic fertilizers both powdered and particulate and thereby substantially eliminates waste in the process. Our CHE unit also extracts from a variety of plants Resveratrol, Sarcandra and 5-HTP, which are key components in many consumer health and wellness products. Additionally, the Company has introduced its first over the counter (“OTC”) product a Ganoderma Tea extract which has been favorably received in the PRC market.
7
We distribute our CHE products through established independent third party distributors who enter into renewable one year distribution agreements with us. Our distributors are focused on the bio-health industry and raw chemical intermediates industry. This permits use to more accurately forecast sales and required production. In addition, feedback from our distributors provides us with good visibility on changes in consumer demand. In addition, we have established distribution channels, such as universities and hospital research centers, through referrals obtained from the support of local government in some provinces.
Our CHE business unit is a research & development company with a focus on improving human health through the development, manufacture and commercialization of bio-ecological products and over-the-counter products utilizing the extractions of tobacco leaves and a variety of other plant materials.
This business unit produces chemical and herbal extracts for use in a wide range of health and wellness products, including:
|
|
●
|
Chemical Extracts
|
|
|
°
|
Solanesol which is extracted from discarded tobacco leaves is the mother chemical intermediate for many high-value bio-chemicals such as Coenzyme Q10 and vitamin-K analogues.
|
|
|
°
|
Coenzyme Q10 (which is a derivative from Solanesol) is a non-specific immune intensifier, which takes part in cell metabolism and respiration.
|
|
|
●
|
Herbal Extracts
|
|
|
°
|
Resveratrol is an active component and a powerful antioxidant extracted from Huzhang (Polygonum cuspidatum).
|
|
|
°
|
5-HTP (5-Hydroxytryptophan) which is extracted from Griffonia seed has been studied and clinically shown to be of benefit in the treatment of primary fibromyalgia, insomnia, depression, and weight control.1
|
|
|
°
|
Ganoderma Tea which is extracted from Rechee Mushrooms and has a long tradition in PRC to promote health and wellness.
|
|
|
°
|
Powdered & Particulate Fertilizers are extracted from discarded tobacco leaves.
|
While various research studies have shown beneficial effects on humans from the consumption of these chemical and herbal extracts, to date we know of no government body like the U.S. Food and Drug Administration or China’s State Food and Drug Administration that has approved these supplements for the treatment of any disease.
The vertically integrated process of the CHE business unit can be summarized as follows:
Access to Raw Materials
- more than 4,000 leased acres of land for herb cultivation in Sanming province
- contractual relationships to purchase discarded tobacco leaves
8
Extraction Process / Value Creation
- green technology and proprietary processes utilized in the extraction processes
- cost-effective process and access to raw materials contribute to gross margins in excess of 50%
Products
- chemical and herbal extracts that are sold as ingredients for medical, health and wellness products
- over-the-counter health and natural supplements
- organic fertilizers
_______________
1 -Birdsall, Timothy C. “5-Hydroxytryptophan: A Clinically-Effective Serotonin Precursor”, Alternative Medicine Review, Vol. 3, Number 4, 1998, p.271.
1.3.1 (b) UGTI and our Organic Products (“OP”) operating division
Our OP business unit operates under the umbrella of our subsidiary United Green Technology Inc. (“UGTI”) which contractually controls and operates Jianou Lujian Foodstuff Co. (“JLF”). JLF is the principle operating enterprise of this business unit and is located in and organized under the laws of the PRC.
JLF is an award-winning green-technology enterprise that specializes in the production of organic products and fertilizers based on bamboo. JLF was also one of the first companies in PRC to formulate a "zero-to-zero" process starting from cultivation to distribution, and taking it further by developing organic fertilizers from bamboo skins and thereby substantially eliminates waste in the process. JLF concentrates on processing bamboo shoots and bamboos which it sells domestically in PRC and exports to other countries. JLF is the third largest bamboo producer in PRC and is the first bamboo company in PRC to gain food safety certification from PRC (HACCP), Japan (JAS) and Europe (ESFA).
Our OP unit manufactures a variety of consumer and commercial-use health and energy drinks, organic food products and fertilizers primarily based on bamboo. Organic food products based on bamboo are low in saturated fat, cholesterol and sodium yet high in dietary fiber, vitamin C, potassium, zinc, and numerous other nutrients, making bamboo shoots popular for weight loss and maintaining a healthy lifestyle. Also, the Moso bamboo leaf extract, which contains soluble and insoluble fiber and antioxidants, is used to make a caffeine-free energy drink or is infused into white rice creating green bamboo rice with health benefits. Our OP unit also uses bamboo skins to produce organic fertilizers, thereby substantially eliminating waste in the process.
Presently, JLF operates production lines to produce both 18L boiled bamboo shoot cans and boiled vegetable cans. This includes the production of 50 kinds of products, such as 18L boiled bamboo shoot cans, boiled bamboo shoot cans with vacuum packing, boiled mixed vegetables, boiled seasoned vegetables and Kamameshi (a Japanese rice dish), with a quality rating that meets national and international standards. The boiled bamboo shoots and mixed vegetables account for the majority of the products sold with approximately 83% of all products sold domestically and approximately 17% exported to other countries such as Japan, Southeast Asia, Europe and North America.
We distribute our OP products directly to large supermarket chains (such as Kobe Bussan in Japan), hotels, hospitals and restaurants. We also distribute our products through a network of independent third party distributors who enter into renewable one year distribution agreements with us.
9
Our OP business unit focuses on improving human health through the development, manufacture and commercialization of a variety of consumer and commercial use organic health and energy drinks, organic food products, organic agricultural products and fertilizers based on bamboo, including:
|
|
●
|
Vacuum packed for freshness:
|
|
|
o
|
Fresh Moso Bamboo shoots
|
|
|
o
|
Seasoned organic bamboo shoots
|
|
|
o
|
Seasoned organic vegetables
|
|
|
o
|
Seasoned organic vegetables with bamboo shoots
|
|
|
●
|
Convenient supermarket packages: cooked
|
|
|
o
|
Seasoned bamboo shoots
|
|
|
o
|
Seasoned vegetables
|
|
|
o
|
Seasoned vegetable and rice packages
|
|
|
●
|
Canned industrial use or commercial use (i.e. for hotels, restaurants):
|
|
|
o
|
Water bamboo shoots
|
|
|
o
|
Water chestnuts
|
|
|
o
|
Water baby corn
|
|
|
o
|
Water mixed vegetables
|
|
|
o
|
Organic fruits
|
|
|
●
|
Extracts: used for organic energy beverages
|
|
|
o
|
Bamboo extracts
|
|
|
●
|
Fertilizers:
|
|
|
o
|
Organic fertilizers using bamboo skin
|
10
The vertically integrated process of the CHE business unit can be summarized as follows:
Access to Raw Materials
- more than 17,000 leased acres of certified Moso bamboo and vegetable land growing water chestnuts, baby corn and others in Fujian province,
- Fujian province is one of the largest bamboo lands in the PRC.
Manufacturing Process / Value Creation
- “zero-to-zero” process from bamboo cultivation to the manufacturing of end products and production of organic fertilizers from waste,
- virtually everything grown is used, reducing the need for fertilizers and cutting energy consumption and costs.
Products
- health and energy drinks and organic food products, including private label products for Kobe Bussan Supermarkets in Japan,
- organic drinks,
- fresh vegetables,
- organic fertilizers
1.3.1 (c) TFS and our financing unit
Our internal financing business unit operates under the umbrella of our 99.75% owned subsidiary Trade Finance Solutions (“TFS”), an Ontario, Canada, company. TFS was established in 2006 to provide creative financing solutions, including purchase order financing, fulfillment services and factoring or invoice discounting for credit worthy customers of eligible goods and services. TFS has a branch office in Miami, Florida. TFS has 2 wholly owned subsidiaries, TFP International Inc., a Florida corporation, which operates the Miami office, and JSM Computer Technology, Inc, an Ontario, Canada, company. The remaining 0.25% of TFS is owned by an individual, who is not an affiliate of ONE Bio.
TFS was acquired to support our international expansion by handling the logistics, fulfillment and financing for the group’s sales outside PRC. TFS provides balance sheet financing solutions and mitigates the group’s international credit exposure by insuring all international receivables through A-rated third party insurance providers. TFS conducts a thorough review and due diligence examination of potential borrowers and the respective borrower’s customers before the particular transaction is approved. An analysis of each individual transaction also takes place through TFS’ credit approval process, in order to ensure that all parties in the transaction receive value, and that TFS will be re-paid according to the terms agreed to in the particular transaction. Security for the financing includes a first lien position on the receivables and assets of the client (UCC, PPSA), personal guaranty and credit insurance. In addition, 100% of TFS’ financing transactions are credit insured with large, multi-national insurance companies. In a typical financing transaction, a letter of credit will be utilized to provide all parties with the protections on agreed to delivery, quality and timelines. TFS’ clients are primarily small and medium size businesses registered and/or located in the United States and Canada, which export their products internationally. Through TFS we also offer purchase order financing to third-party clients that purchase products from our CHE business unit and our OP business unit to assist in the collection process, and expedite cash flow and debt repayment.
Our internal financing business unit offers factoring or invoice discounting financing, where TFS, as factor, purchases the client’s credit insured receivables (i.e., invoices) for products or services satisfactorily rendered to creditworthy customers. By selling receivables to TFS, the client can generate cash almost immediately, instead of waiting the usual 30, 60, 90 days. TFS will verify, insure and control the transaction with the ultimate payer. TFS also offers purchase order financing (or “PO Funding”) which is a mechanism put in place to provide a short-term finance option to clients who have pre-sold finished goods and a requirement to pre-pay suppliers. All these PO Funding transactions are on a “per project” basis. Typically the client has a purchase order from a credit insured customer but does not have the cash resources to pay their supplier upfront. TFS typically requires that the client must have “pre-sold” the goods with strong profit margins. TFS utilizes letters of credit to purchase the goods from the supplier and protect all parties. Once the goods are accepted by the end customer, TFS then factors the invoice and pays off the purchase order facility; and fulfillment services, most of which are provided through TFP and which involves the goods being acquired by TFP on terms negotiated with both supplier and end-client. Here TFP has established credit insurance and marine cargo insurance policies. The customer provides the purchase order and sales contract to TFP, which completes the transaction and disburses the proceeds.
11
Historically TFS has funded its financing transactions through the sale of debentures to institutional investors and high net worth investors, often on an individual transaction basis. The debentures pay quarterly interest to the investors solely from the interest and fees generated from the financing business unit’s activities. The debenture is secured by the assets of TFS with no recourse to other assets or business units of ONE Bio. Through ONE Bio, TFS has greater access to capital to finance its business and through its association with our other business units, access to new international markets.
1.4 Sales and Marketing
The Company’s marketing and sales strategy is characterized by:
|
|
|
|
|
|
|
●
|
High Growth, agri-tech business units that utilize green technology and proprietary processes to grow both organic food products and plants that through the use of proprietary processes, produce chemical and herbal extracts for use in a wide range of health and wellness products;
|
|||
|
|
|
|||
|
|
●
|
Proprietary processes and access to raw materials to generate desirable high gross margins;
|
||
|
|
|
|||
|
●
|
Continuously developing organic products and health products to expand its product offering including items with retail potential which will add demand for existing products;
|
|||
|
|
||||
|
|
●
|
Markets its products throughout PRC, Japan, Europe and the United States;
|
||
|
|
|
|||
|
|
●
|
Direct sales model supported with over 35 sales associates who work with our established direct channel and distribution organizations;
|
||
|
|
●
|
Establish referral programs with major universities where distributors look for new products and technologies;
|
||
|
|
●
|
Multi-channel approach to reach potential distributors and customers (including major retailers such as but not limited to mass merchandisers, large retailers, major hotel chains, hospitals and senior homes);
|
||
|
|
●
|
Use the following channels for visibility with potential distributors:
|
||
|
|
|
|
||
|
|
|
●
|
Web advertising
|
|
|
|
|
|
|
|
|
|
|
|
–
|
Internal web optimization
|
|
|
|
|
|
|
|
|
|
|
–
|
Search engine optimization
|
|
|
|
|
|
|
|
|
|
|
–
|
Sponsored links
|
|
|
|
|
|
|
|
|
●
|
Trade shows
|
||
|
|
|
|
|
|
|
|
|
●
|
Exhibitions
|
|
|
|
|
|
|
|
|
|
|
●
|
Conferences
|
|
|
|
||||
|
|
●
|
Use contacts within local provincial governments to refer us to established distributors.
|
||
12
As for the retail over-the-counter products such as the company’s organic Ganoderma Tea, we run aggressive advertising and marketing campaigns such as:
|
●
|
TV advertising campaign (including static advertisements and interviews)
|
|
|
●
|
Local and national newspapers
|
|
|
●
|
Radio advertising campaign (including static advertisements and interviews)
|
|
|
●
|
Web advertising campaign
|
1.5 Market Analysis
1.5.1 Focus on Asia-Pacific Region
“Asia remains firmly in the lead of the global economic recovery and strong growth in the region is set to continue”, the International Monetary Fund (IMF) said in its latest Regional Economic Outlook for Asia and the Pacific which was released in Jakarta, Indonesia. The expansion in Asia exceeded expectations in the first half of the year, the IMF said, prompting the Fund to revise up its 2010 growth forecast for the region to 8 percent, nearly 1 percentage point higher than its April forecast. Economies across the region are expanding strongly. PRC and India are leading the way with projected 2010 growth rates of 10.5 percent and 9.7 percent, respectively, while Indonesia is expected to grow by 6 percent. In Japan, growth is expected to moderate to a more sustainable pace of 6.8 percent. This continued strength in PRC is evidenced by, among other things, significant growth in retail sales as well as urban fixed-asset investment. Despite the global economic downturn, PRC’s economic growth continues to outpace the rest of the world.
Our product lines and strategic focus are designed to capitalize on this growth. At the same time, many small businesses in Asia-Pacific continue to be undervalued, providing significant opportunities for us to accretively acquire companies that complement our business strategy. Smaller and mid-size Asian companies, partially due to numerous listing requirements in Asia, recognize the value of combining with us and as such, these target companies can be acquired at more reasonable prices. On the cost side, traditionally lower labor costs in the Asia-Pacific Region contribute to higher profit margins, while still maintaining very high product quality standards.
1.5.2 PRC’s Expanding Organic Products Industry
The global organic food market has developed rapidly during the past six years, according to the Gain Report dated October 26, 2010, with organic food and beverage sales reaching $7.2 billion in 2008, an increase of more than 140 percent from the former $3 billion record in 2003. PRC’s participation is on the rise. Some analysts expect domestic sales of organic products in PRC to be as high as $3.6 to $8.7 billion by 2015. PRC’s organic food market is still in the early stages of development but profit margins potentially can be high and market prospects are arguably large.
With the rapid development of living standards in PRC and the Asia-Pacific Region in recent years, organic agriculture and the market for organic foods in PRC and the Asia-Pacific Region are developing at a rate of 30% per annum. In 2005, PRC introduced the China National Organic Product Standard and The Rule on Implementation of Organic Products Certification which covers production certification and imports of organic food products. By the end of 2007, PRC became the second largest area of certified organic cultivation land (4.10 million hectares), producing about 30 categories and more than 500 species of organic products. Due to the advantages of abundant resources, market demand, government support and promotion of health benefits, we believe that PRC's organic food industry will continue to experience strong growth in the future.
13
1.6 Complimentary Strategies
We have assembled a combination of complementary strengths that we believe will enable us to successfully implement our organic growth strategies to capitalize on the large and growing markets for our agritech and organic products, and accretive acquisitions.
|
·
|
Rapid and Sustainable Growth Strategy
|
We believe that our growth strategy allows us to focus our resources to capitalize on opportunities. By first focusing on accretive and synergistic acquisitions, we believe that we will accelerate our expansion beyond what would be achieved through a pure organic growth strategy. Once substantial mass is achieved, we then intend to integrate the acquisitions to improve profitability, achieve production and technology synergies, capitalize on efficiencies of scale and expand our product portfolio, distribution channels and customer base. We believe that this strategy will increase our product diversification, reduce our business risks and increase our accretive opportunities.
|
·
|
Established Platform for Organic Growth
|
We are a vertically integrated company with our own agricultural lands, research and development, proprietary manufacturing processes and distribution channels. Our operating companies are profitable and have established branded products and experienced management teams. We believe that these capabilities position us for organic growth in our product offerings and will serve as a template for successfully integrating additional acquisitions.
|
·
|
Research and Development Team with Ability to Identify Commercially Viable Products
|
Led by our Chief scientist, Dr. Jian Ming Chan, we have assembled what we believe to be a skilled and experienced research and development team that includes engineers, chemists, doctorates and adjunct professors. In addition to possessing a multi-discipline background, our research and development team has affiliations with several prominent research universities in PRC, including the Fudan University. Our team has leveraged these relationships and their own skills to produce, among other things, our proprietary extraction process, which affords us a production cost advantage, and our “zero-to-zero” green production process, which is favored by government authorities. An essential component of our growth strategy is to expand our product offerings through both acquisition and organic growth. We look to our research and development team to continue to provide the new products and innovative production techniques that will fuel our continued organic growth and provide us with a competitive advantage.
|
·
|
Experienced Managers with the Ability to Identify and Integrate Acquisitions
|
We have assembled a team with significant successful mergers and acquisition experience. Our CEO, Marius Silvasan, is a senior executive with over 17 years of experience in business development, M&A, marketing, sales, finance and distribution. Our Chairman, Michael Weingarten, is a seasoned executive and entrepreneur with over 30 years of experience in distribution, manufacturing and managing companies from early stage of development to multimillion dollar corporations worldwide. Mr. Weingarten also has extensive experience in mergers and acquisitions, having participated in over 40 such transactions throughout his career. We believe that that our North American management team positions us properly to participate in the consolidation of our industries.
14
|
·
|
Seasoned Managers with the Ability to Create and Sustain Operating Success
|
Our PRC operations team is led by Min Zhao and Jin Rong Tang, seasoned and highly-qualified managers with extensive industry-specific sales, marketing and production experience. Mr. Zhao has over 20 years of business experience at a number of successful companies where he served in senior positions ranging from general manager to chairman. Mr. Tang was selected as one of the Top Ten Most Outstanding Young Entrepreneurs in 2009 and serves as the Executive Vice President of Fujian Bamboo Association as well as the President of Nanping Association of Young Entrepreneurs. Under their direction, our operating business units have achieved and sustained profitability. We believe that these individuals and their respective teams are well-positioned to execute our growth plans.
|
·
|
Create Win-Win Relationships for Participants
|
We believe that our strategy provides us with an excellent opportunity to participate in the growth of PRC and the entire Asia-Pacific Region. To our acquisition targets, we believe that we offer owners of smaller private companies the opportunity to diversify their investment by being part of a larger multi-faceted public company and at the same time provide access to capital that would not be available to them otherwise. To the Chinese government, we offer a “zero-to-zero” green process, which minimizes waste. To our customers, we offer safe, high-quality, organic products. To our research partners, we offer practical implementation of innovative technology, and to our suppliers, we offer a stable well-managed growth partner.
1.7 Challenges to our Growth
While we believe we will be able to capitalize on our strengths, we are mindful of the significant challenges we face in implementing our growth plan, including:
● Potential Changes in Government Regulation
● Increasing Capital Requirements
● Market Development
● Changes in the Economic Environment
● Changes in Currency Exchange Rates
We believe that our strategy provides us with an excellent opportunity to participate in the growth of PRC and the entire Asia-Pacific region. To our acquisition targets, we believe that we offer owners of smaller private companies the opportunity to diversify their investment by being part of a larger multi-faceted public company and at the same time provide access to capital that would not be available to them otherwise. To the Chinese government, we offer green production processes which reduce waste. To our customers, we offer safe, high-quality, organic products. To our research partners, we offer practical implementation of innovative technology, and to our suppliers, we offer a stable well-managed growth partner.
1.7.1 CHE Organic Growth Strategy
Our CHE business unit’s principal revenue-producing activity involves the extraction of chemical and herbal extracts, including Solanesol, from discarded tobacco leaves and a variety of plants and materials. Our CHE business unit has the contractual relationships necessary to receive tobacco leaves from tobacco companies on an ongoing basis. This business unit also has established relationships to negotiate additional tobacco leaf contracts necessary to support the organic growth rate of our CHE business unit.
15
Our CHE business unit has a patented cost-effective process that extracts Solanesol from discarded tobacco leaves and the resulting residue is further processed to produce organic fertilizer. Because we are able to extract two products from our extraction process, we believe we are able to generate higher margins than we would if we used a traditional single product fermentation extraction process. Additionally, we can further process the Solanesol to produce Coenzyme Q10 (“CoQ10”). Our CHE business unit has the expertise, ability and market demand to expand its manufacturing capacity and to vertically integrate the chemical extract subsidiary. Hence, we intend to invest in increasing our manufacturing capacity to a level adequate to support the CHE business unit’s planned organic growth. As part of our vertical growth strategy, we have added our first over the counter product Ganoderma Tea, which has contributed to our increase in revenue during the fiscal year.
We intend to expand our CHE distribution sales channels horizontally to include other Asia-Pacific countries, Europe and the United States of America. Our entry into these markets will follow the launch of our finished or “end user” over-the-counter products and the establishment of distribution relationships.
1.7.2 OP Organic Growth Strategy
Our OP business unit’s principal revenue-producing activity involves the production of organic food products primarily from bamboo. This business unit continues to lease access to land for its bamboo cultivation as part of its vertical integration and organic growth strategy. We intend to continue to grow our capacity to cultivate bamboo and organic products. During the fiscal year, we have developed our own products for distribution rather than distribute raw materials through the distribution channel which resulted in a significant increase in our revenues.
Our management has also identified several key strategic areas as targeted opportunities for horizontal expansion both in PRC and abroad. This business unit already distributes its products in Japan and PRC. We intend to increase the distribution of our OP products in those markets and to expand into other Asia-Pacific countries, Europe and the United States of America, once we have identified and established distribution relationships in those markets. In conjunction with the expanded sales and distribution initiative, our OP business unit is also in frequent contact and discussions with both the local and federal PRC government to obtain additional land lease rights for rich bamboo. Our OP business unit has been successful in procuring these rights and expects this to continue, resulting in increased production capacity and allowing for expansion of the product lines.
Recruitment and qualification of distributors within the targeted regions has been implemented and the process is ongoing. Distributors catering to organic food retailers and health stores are our primary targets. The existing distribution in each region is assessed and our OP business unit makes the decision to either engage distributors or to sign direct distribution agreements with the retailers to reach its target market. We believe that mix of both strategies can be implemented within a targeted region.
1.7.3 Accretive Growth Strategy
We have a focused growth strategy through acquisition for the next twenty-four to thirty-six months. We believe that we are in a good position to complete one or more strategic acquisitions during said period of time. Furthermore, we believe this strategy will allow us to grow at an accelerated, controlled, and accretive pace. From time to time we are engaged in preliminary discussions with potential acquisition candidates that would be a fit based upon our business model. We are currently engaged in preliminary discussions with a few potential acquisition candidates that we believe fit our business model. It is possible that those preliminary discussions could culminate in one or more strategic acquisitions. However, no assurances can be given that we will pursue or that that we will be able to close any acquisition within our desired time frame. Following our initial twenty-four to thirty-six months growth strategy focused on strategic acquisitions we intend to put a greater focus on organic growth, consolidation and integration of redundant labor, plant and equipment.
16
We believe that there are compelling opportunities to acquire target companies in the region at attractive multiples. The acquisition targets we seek are profitable and well-run companies whose potential is constrained due to lack of access to capital from financial markets. We provide that access along with the added benefit of our management expertise and strategic direction. We intend to achieve scale by acquiring companies or assets that have one or more of the following characteristics:
● An established customer base
● Existing plant and equipment
● Excess production capacity
● Attractive and complementary product portfolio
● Vertical or horizontal integration synergy
● Innovative patents and/or technology
● Processes or products with scalability
● Established complementary distribution channels
We also believe that management expertise is an essential ingredient to achieving scale, both organically and by acquisition. To execute this strategy, we have brought together both the financial and transactional expertise to identify and acquire accretive and synergistic targets and the operational expertise to effectively integrate operations and profitably expand business or product lines. We believe our management and operations teams combine the operational expertise and work ethics of the Asia-Pacific region with the managerial, financial, and transactional skills of North America. Our CHE and OP business units will principally execute and manage our organic growth strategies.
1.8 Manufacturing and distribution of our products in PRC and other countries
Our CHE and OP business units produce different products. Our CHE business unit produces raw materials such as intermediate chemicals and herbal extracts that are primarily focused on the nutraceutical, health supplement and vitamins market segments (see “Chemical and Herbal Extracts” herein for a list of our CHE products). Our OP business unit produces primarily organic food products (see “Organic Products” herein for a list of our OP products). Our CHE and OP business units also produce fertilizers.
We currently distribute our products in PRC and export and distribute our products to Japan and other Asia-Pacific countries. We intend to establish distribution relationships in order to expand our distribution into other Asia-Pacific countries, the U.S and Europe. The manufacture, exportation and distribution of our products in PRC, and Japan and other Asia-Pacific countries are not subject to any health or governmental standards or regulations. However, the exportation of our products will be subject to U.S. standards and regulations once we expand to the U.S. In anticipation of expanding to the U.S., we have registered our facilities with the U.S. FDA. In order to facilitate food, pharmaceutical and product safety, many countries have embraced various manufacturing and quality control standards. Generally compliance with these standards is voluntary and failure to satisfy these standards does not necessarily disqualify manufacturers from exporting their products to a particular country. However, compliance with FDA regulations is required in order to export and distribute products to the U.S. The principal voluntary standards for our current and intended regions of distribution are: current Good Manufacturing Practices (“cGMP”); HACCP (Hazard Analysis Critical Control Points); and JAS (Japanese Agricultural Standard). Under these standards, the burden is on the manufacturer to establish safety and efficacy. Generally these standards focus on how products are produced, maintained and held, the facility where the products are produced, and the documentation that is to be maintained by the producer to demonstrate the producer’s compliance with these standards, and the labeling of the products. The respective governing bodies generally do not certify the producer’s compliance with the applicable standards. If a consumer files a complaint regarding a producer’s product and the product is found to cause a health hazard, the producer’s facilities will generally be inspected by the applicable governing body and if such inspection reveals that the producer’s processes, documentation or products do not meet the applicable standards, the export of the products to the applicable country may be blocked until the producer can demonstrate that the problem has been rectified. Certain of the governing bodies (such as the FDA) may require a recall of the adulterated product. Producers often obtain a certification from an independent certification company (such as Underwriters’ Laboratories) regarding the producer’s satisfaction of the generally accepted standards of the particular country; but such independent certification is voluntary and is not binding on the applicable governing body. However, unlike many of the other governing bodies, JAS issues certifications covering food labeling and products that have been reviewed and inspected by JAS regarding compliance with JAS Standards.
17
The following is a description of the above mentioned standards. All of the following standards, except FDA registration, are voluntary and undertaken by us to enhance the quality and marketability of our products and our failure to satisfy these standards will not preclude us from distributing and/or exporting our products a particular country or region.
|
|
●
|
cGMP (current Good Manufacturing Practices): cGMP are the current accepted standards of design, operation, practice, and sanitization for facilities that manufacture, package or hold foods and dietary supplements. Compliance with the cGMP standards, requires an inspection of the facilities to determine whether these standards are satisfied.
|
|
|
●
|
HACCP (Hazard Analysis Critical Control Points): HACCP is a system used to manage food safety through the analysis and control of biological, chemical, and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product. We believe our CHE and OP facilities utilize HACCP in order to reduce the risk of hazards getting into our products. We believe our OP facility complies with HACCP standards and we have obtained an independent certification in PRC from China Quality Certification Center, an independent certification company. Our CHE business unit facilities have not been certified, and no certification is required.
|
|
|
●
|
FDA Registration: In contemplation and preparation for exporting our products for distribution in the United States, we have registered our CHE and OP facilities with the FDA pursuant to the Public Health Security and Bioterrorism Preparedness and Response Act of 2002. This Act allows the FDA and other authorities to determine the source and cause of any deliberate or accidental contamination of food. For this, the FDA uses information provided by registered food facilities prior to entry into the U.S. of food and beverages for human and animal consumption. Pursuant to this Act, the owner, the operator or agent in charge of a domestic or foreign food facility must register that facility with the FDA and provide necessary information as requested in order to import the food into the U.S. Foreign facilities are required to report emergency contact information. Except for specific exemptions, the registration requirements apply to all facilities that manufacture, process, pack, transport, distribute, receive, or hold food regulated by the FDA, including animal feed, dietary supplements, infant formula, beverages (including alcoholic beverages) and food additives. Failure of a domestic or foreign facility to register, update required elements, or cancel its registration in accordance with this Act is prohibited under the Federal Food, Drug, and Cosmetic Act and a facility that fails to register with the FDA may have its goods seized by the U.S. Customs and Border Protection agency and/or fined. Prior notice of imported foods must be provided to the FDA within prescribed time periods. The notice must include a description of the article, the manufacturer and shipper, the grower (if known), the country of origin, the country from which the article is shipped, and the anticipated port of entry. After such notice has been submitted, the FDA will provide a prior notice confirmation number that the information has been successfully received by the FDA. Once the FDA issues the confirmation number, the FDA reviews the information submitted and determines whether the cargo must be examined. If the review concludes that no further action is necessary, a customs entry may be processed for that importation. If the review concludes that further action is necessary, the importation of the food may be detained at the port of entry or prevented until any problems or errors are corrected.
|
|
|
●
|
JAS (Japanese Agricultural Standard): The JAS standards apply to organic plants, organic processed foods of plant origin, organic livestock products, organic processed foods of animal origin and organic feeds. These standards set forth requirements that producers of these products must comply with, if they wish to label their products “Organic”. Our OP facilities comply with the JAS standards. In addition, our JAS facilities have been certified by a registered certifying body. The JAS certification allows us to attach the organic JAS logo to products produced or manufactured in our OP facilities to indicate that they comply with relevant organic JAS standards. The fact that we maintain one or more JAS Certifications is not a guarantee that each and every OP product we produce will always be entitled to claim organic status. By submitting to these standards, we are able to put the JAS labels on our OP division products, which we believe enhances the marketability of these products. If we were to lose one or both of our JAS Certifications, we would still be able to distribute our products in Japan; however, we would not be permitted to affix the JAS labels to our products until we demonstrate that we are JAS compliant. The JAS standards are voluntary, but necessary, in order to distribute our products with the JAS labels.
|
The fact that we comply with a particular standard, system or regulations (i.e., cGMP, HACCP, FDA, JAS) is not a guarantee that each and every product we produce will be absolutely safe for consumption. Many factors can conspire to cause products such as the ones we manufacture and distribute to be adulterated or impure. If a product we produce is discovered to be adulterated, causes illness or injury, or fails to meet standards for purity or identity, such a problem can lead to interruptions of production, product recalls and claims against us. The applicable regulatory agencies, including the FDA and PRC’s State Food and Drug Administration (“SFDA”) may conduct on-sight inspections of our facilities. If we fail an inspection, the regulatory agency may order us to close one or more facilities. Furthermore, our products may be inspected during the import process into one of our targeted countries, and failure to pass inspection would result in our products being refused entry by that country.
18
We believe we are eligible to distribute in PRC all of our products produced at our facilities. Based on our JAS certification, we believe we are eligible to export and distribute in Japan all of our OP products with JAS Organic labels certified by JAS. Based upon the registration of our CHE and OP facilities with the U.S. FDA, we believe we are eligible to export and distribute all of the products to the U.S.
The following table sets forth the standards/ rules applicable to our CHE and OP business units in the indicated countries:
|
Country
|
Standards applicable to CHE
products in the country/region
|
Standards applicable to OP
products in the country/region
|
||||
|
Japan
|
No compliance required to distribute in Japan and JAS Certification not applicable
|
Voluntary JAS certification necessary for Organic labeling, but not necessary for importation and distribution (certification obtained)
|
||||
|
United States
|
U.S. FDA – facility registration is required
(facilities have been registered)
|
U.S. FDA – facility registration is required
(facilities have been registered)
|
||||
|
China
|
No compliance required
|
No compliance required (Voluntary HACCP compliance has been instituted and independent non-binding certification has been obtained)
|
In the past, we have focused our sales and distribution efforts to PRC and Japan and with only limited distribution of only certain of our OP products and no distribution of our CHE products in Europe. We intend to establish distribution relationships in other Asia-Pacific countries, Europe and the U.S. so that we can expand distribution of our products to these markets. The foregoing notwithstanding, we may from time to time, on a case by case basis, distribute our products in Europe.
1.9 Intellectual Property
Patents approved and issued by the PRC is as follows:
| Patent Name |
Certificate No
|
Date | Expiry | Designer | Owner | |||||
| Synchronization high efficiency process of Solanesol and Nicotine Sulphate |
200610069846.6
|
2006.8.10 | 2026.8.10 |
Zhao Min, ChenYanmei, Liu Caiqing
|
Sanming Huajian Bio- engineering Co Ltd
|
19
The following table is a list of our Patents applications pending approval by the PRC:
|
Patent Name
|
Application No.
|
Date
|
Expiry
|
Designer
|
Owner
|
|||||
|
A Method of Eliminating Plum Bum Products with basic liquid of zymogene mung bean
|
200710009735.0
|
2007.11.1
|
20 years after the application
|
Lin Xuanxian, Chen Jianmin, Chen Yanmei
|
Sanming Huajian Bioengineering Co., Ltd. & Lin Xuan Xian
|
|||||
|
Purified craftsmanship of coenzyme Q10
|
200810072112.2
|
2008.11.13
|
20 years after the application
|
Chen Yanmei, Zhao Min
|
Sanming Huajian Bioengineering Co., Ltd.
|
|||||
|
New craftsmanship of extracting high content resveratrol from polygonum cuspidatum
|
200910306409.5
|
2009.8.31
|
20 years after the application
|
Zhao Min, Chen Yanmei, Yu Feng
|
Sanming Huajian Bioengineering Co., Ltd.
|
|||||
|
Craftsmanship of extracting high content 5-HTP from griffonia seeds
|
200910306408.0
|
2009.8.31
|
20 years after the application
|
Zhao Min, Yu Feng, Chen Yanmei
|
Sanming Huajian Bioengineering Co., Ltd.
|
|||||
|
Craftsmanship and application of extracting isofraxidin and flavonoid from sarcandra glabra at the same time
|
200910307418.6
|
2009.9.22
|
20 years after the application
|
Zhao Min, Yu Feng, Chen Yanmei
|
Sanming Huajian Bioengineering Co., Ltd.
|
|||||
|
One effective method for extracting tanshinone IIA and salvianolic acid B from salvia miltiorrhiza at the same time
|
201010227720.3
|
2010.7.15
|
20 years after the application
|
Zhao Min, Yu Feng, Chen Yanmei
|
Sanming Huajian Bioengineering Co., Ltd.
|
|||||
|
The producing method for a weight-losing milk tea
|
201010227720.3
|
2010.7.15
|
20 years after the application
|
Zhao Min, Yu Feng, Chen Yanmei
|
Green Planet Bioengineering Co., Ltd.
|
|||||
|
A cultivating method of interplanting stevia among tobaccos
|
201010233987.3
|
2010.7.22
|
20 years after the application
|
Zhao Min, Yu Feng, Chen Yanmei
|
Sanming Huajian Bioengineering Co., Ltd.
|
Note- The patent of “Solanesol-clean extraction method” is exclusively owned by Fudan University. However, we have obtained the right to use this technology patent.
Since August 11, 2006, we have been designing the “synchronization and high efficiency process of Solanesol and Nicotine Sulphate” and applied for the patent ownership and have used it in the production process.
20
1.10 Trademarks
The following table is a list of our current trademarks approved and issued by the PRC:
|
Trademark
|
Certificate No.
|
Date
|
Expiry
|
Category
|
Owner
|
Authorized user
|
||||||
|
Paiqianshu
|
4322405
|
2007.4.20
|
2017.4.20
|
No. 30 Refined food from plants, etc.
|
Zhao Min
|
Sanming Huajian Bioengineering Co., Ltd.
|
||||||
|
Jinliang
|
4538612
|
2008.6.28
|
2018.6.27
|
No.3 Cosmetic, household and personal care chemicals, etc.
|
Zhao Min
|
NA
|
||||||
|
GREENPLANET
|
6871472
|
2010.7.14
|
2020.7.13
|
No.5 Medical products, etc.
|
Fujian Green Planet Bio- Engineering, Co., Ltd
|
NA
|
The following table is a list of our trademarks applications pending approval by the PRC:
|
Trademark
|
Application No
|
Category
|
Owner
|
Authorized user
|
||||
|
Jimai QQ
|
4322404
|
No. 30 Refined food from plants, etc.
|
Zhao Min
|
NA (no product)
|
||||
|
Jimai QQ
|
5425649
|
No.1 Fertilizer, chemical products
|
Zhao Min
|
NA (product no longer sold)
|
||||
|
PURESOLAN
|
6869795
|
No.5 Medical products, etc.
|
Fujian Green Planet Bio- Engineering, Co., Ltd
|
|||||
|
Jinglao
|
7897892
|
No. 30 Refined food from plants, etc.
|
Green Star Bioengineering Co., Ltd.
|
Fujian Green Planet Bio- Engineering, Co., Ltd
|
A registered trademark is protected for a term of ten years, renewable for another term of ten years under the PRC trademark law, so long as an application for renewal is submitted to the PRC Trademark Offices within six months prior to the expiration of the initial term. In addition to trademark and patent protection law in PRC, we also rely on contractual provisions to protect our intellectual property rights and brand.
21
1.11 Need For Government Approval
None
1.12 Employees
The Company had approximately 358 full time employees as of December 31, 2010. Of these employees, approximately 18 worked in top management positions, approximately 49 worked in sales, approximately 19 worked in research and development and approximately 49 worked in administration, 14 worked in finance, 47 in procurement/logistics/warehousing and 162 worked in manufacturing. We also hire between 20 and 40 seasonal /part time employees each year.
The Company entered into employment agreements with our management employees containing customary confidentiality and non-competition covenants which prohibit the employee from disclosing confidential information and from engaging in activities that compete with our business during his or her employment with us and, for an upon period after the termination of the employee’s employment with us.
|
ITEM 1A
|
RISK FACTORS
|
Investingin our securities involves a great deal of risk. Careful consideration should be made of the following factors as well as other information included in this prospectus before deciding to purchase our securities. You should pay particular attention to the fact that we conduct substantially all of our operations in PRC and are governed by a legal and regulatory environment that in some respects differs significantly from the legal and regulatory environment that may prevail in the U.S. and other countries. Our business, financial condition and results of operations could be affected materially and adversely by any or all of these risks.
THE FOLLOWING MATTERS MAY HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS, FINANCIAL CONDITION, LIQUIDITY, RESULTS OF OPERATIONS AND PROSPECTS, FINANCIAL OR OTHERWISE. REFERENCE TO THIS CAUTIONARY STATEMENT IN THE CONTEXT OF A FORWARD-LOOKING STATEMENT OR STATEMENTS SHALL BE DEEMED TO BE A STATEMENT THAT ANY ONE OR MORE OF THE FOLLOWING FACTORS MAY CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE IN SUCH FORWARD-LOOKING STATEMENT OR STATEMENTS.
Risks Related to Our Business
We have a limited operating history.
We have a relatively limited operating history. Sanming and JLF, our contractually controlled PRC operating entities, and TFS through which we currently operate our business, commenced operations in April 2004, September 2002, and March 2006, respectively. All three entities were profitable since 2007. The foregoing, notwithstanding, you should consider our future prospects in light of the risks and uncertainties typically experienced by companies such as ours in evolving industries such as the agritech industry in PRC and the Asia-Pacific region. Some of these risks and uncertainties relate to our ability to:
22
|
●
|
offer new and innovative products to attract and retain a larger customer base;
|
|
●
|
attract additional customers and increase spending per customer;
|
|
●
|
increase awareness of our products and brands and continue to develop user and customer loyalty;
|
|
●
|
raise sufficient capital to sustain and expand our business;
|
|
●
|
maintain effective control of our costs and expenses;
|
|
●
|
respond to changes in our regulatory environment;
|
|
●
|
respond to competitive market conditions;
|
|
●
|
manage risks associated with intellectual property rights;
|
|
●
|
integrate any business acquisition properly;
|
|
●
|
attract, retain and motivate qualified personnel; and
|
|
●
|
upgrade our technology to support additional research & development of new products.
|
If we are unsuccessful in addressing any of these risks and uncertainties, our business may be materially and adversely affected.
Although our revenues have grown rapidly, we cannot assure you that we will maintain our profitability or that we will not incur net losses in the future. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in operating losses.
We will continue to encounter risks and difficulties in implementing our business model.
We believe that our business model will allow us to become a leader in developing and producing chemical and herbal extracts and natural supplements and organic products for the health and wellness and organic products industries in which we operate. However, we cannot assure you that our business model will be effective. We are susceptible to risks, including the failure to increase awareness of our products, protect our reputation and develop customer loyalty, the inability to manage our expanding operations, the failure to maintain adequate control of our expenses, and the inability to anticipate and adapt to changing conditions in the markets in which we operate as well as the impact of any changes in government regulation, mergers and acquisitions involving our competitors, technological developments, and other significant competitive and market dynamics. If we are not successful in addressing any or all of these risks, our business may be materially and adversely affected.
Quarterly operating results may fluctuate.
Our quarterly results of operations may fluctuate as a result of a number of factors, including fluctuation in the demand for and shipments of our products and changes in the price of raw material which directly affect the price of our products and may influence the demand for our products. Therefore, quarter-to-quarter comparisons of results of operations have been and will be impacted by the volume of such orders and shipments. In addition, our operating results could be adversely affected by the following factors, among others, such as variations in the mix of product sales, price changes in response to competitive factors, increases in raw material costs and other significant costs, increases in utility costs (particularly electricity) and interruptions in plant operations resulting from the interruption of raw material supplies and other factors.
23
We may incur material product liability claims and/or product recalls, which could increase our costs and adversely affect our reputation, revenues and operating income.
As a manufacturer of products designed for human consumption, we are subject to product liability claims that the use of our products has resulted in injury. Also, in the event the use of our products results in injury, we may be forced to recall any of our products that caused the injury. We do not maintain any product liability insurance. A product liability claim against us could result in costly litigation and could adversely affect our reputation with our customers, which in turn could adversely affect our revenues and operating income.
Certain of the countries where we distribute or intend to distribute our CHE and OP products have embraced various manufacturing and quality control standards for the distribution and/or exportation of products such as the products we produce and the labeling of such products. Even though compliance with these standards is generally voluntary, and we are typically able to distribute and/or export our products into those countries without such compliance, if we are unable to demonstrate our compliance with those standards, the marketability of our products may be adversely effected, which may in turn adversely and materially affect our revenues and profits and the market price for our stock.
The manufacture and distribution of our products are subject to domestic (PRC) manufacturing and quality control standards, and the standards announced by the countries to which we export and intend to export our products, including Japan, Europe and the U.S. Generally these standards focus on how products are produced, maintained and held, the facility where the products are produced, and the documentation maintained by the producer supporting the producer’s compliance with these provisions and the labeling of the products. Additionally certain countries, including the U.S. require registration of facilities and compliance with regulations in order to export and distribute these products in the U.S. If a consumer files a complaint regarding a producer’s product and the product is found to cause a health hazard, the producer’s facilities will generally be inspected by the applicable governing body and if such inspection reveals that the producer’s processes, documentation or products do not meet the applicable standards, the export of the products to the applicable country may be blocked until the producer can demonstrate that the problem has been rectified. Certain of the governing bodies, including the U.S. FDA, may require a recall of the adulterated products and/or levy fines. If a producer’s facilities, processes, documentation or products do not meet the applicable agency’s rules and standards, the export of the products to the applicable country may be denied until the producer can demonstrate that it has satisfied the required standards.
We believe that our CHE and OP facilities, systems, processes, procedures and documentation comply with the standards in the countries where we distribute and export our products. However, the fact that we comply with a particular standard is not a guarantee that each and every product we produce will be absolutely safe for consumption. Many factors can cause products such as the ones we manufacture and distribute to be adulterated or impure. If a product we produce is discovered to be adulterated, causes illness or injury, or fails to meet standards for purity or identity, this can lead to interruptions of production and, recalls or claims against us. Further no assurances can be given that we will be able to achieve or maintain compliance with announced standards, or that a governing body may not prohibit us from distributing or exporting our products to a particular country, which events could have a material adverse on our revenue and profits and the market price of our stock.
Certain of the countries where we distribute and intend to distribute our CHE and OP products regulate the labeling and advertising of products of the type we produce and distribute.
The products produced by our CHE and OP facilities may be subject to standards and regulations of the countries to which we export or intend to export our products which regulations govern the labeling of bulk or individual finished products. In addition, the advertising and marketing of our products may be subject to other rules that govern claims which may be made about products and their contents. These standards and regulations vary from country to country. Often when our products are imported into particular countries, inspectors review the label affixed to the product to determine whether the product should be permitted entry. When our products are purchased in bulk for use as an ingredient in other products, we may make claims regarding our products that the purchaser may rely upon and repeat on a label or in advertising. When our products are sold in finished form we may make claims ourselves about our product on it label or in advertising. The claims we make to our customers, and the statements on our labels and in advertising, are regulated in many countries. If the advertising or labeling of a product we produce fails to comply with the specific laws, rules or regulations that apply in the country where it is sought to be imported and sold, we may be unable to sell it there, we may experience delays in delivery, we may be required to re-label or revise advertising, or we may be precluded from making a claim with respect to the product (i.e., a claim that the product is “Organic”) and any of these events this can lead to loss of sales or claims against us and our revenues and profits and market price for our stock could be materially and adversely affected
24
We may encounter substantial competition in our business and our failure to compete effectively may adversely affect our ability to generate revenue.
The nutritional over-the-counter products and organic products industries are becoming increasingly competitive. Our competitors may have greater market recognition and substantially greater financial, technical, marketing, distribution, purchasing, manufacturing, personnel and other resources than we do. Furthermore, some of our competitors have manufacturing and sales forces that are geographically diversified, allowing them to reduce transportation expenses, tariff costs and currency fluctuations for certain customers in markets where their facilities are located. The principal elements of competition in the nutritional over-the-counter products and organic products industry are, in our opinion, pricing, product availability and quality. In order to succeed in the nutritional over-the-counter products and organic products industry, we must be competitive in our pricing, product availability and quality. If we fail to do so, we will not be able to compete effectively and will lose market share. In such case we may be forced to reduce our margins to retain or acquire that business, which could decrease our revenues or slow our future revenue growth and lead to a decline in profitability. Further, to the extent that, whether as a result of the increased cost of raw materials, the relative strength of the Chinese currency, shipping costs or other factors, we are not able to price our products competitively, our ability to sell our products in both the Chinese domestic and the international markets will suffer.
We may not be able to effectively control and manage our growth.
If our business and markets grow and develop, it may be necessary for us to finance and manage expansion in an orderly fashion. In addition, we may face challenges in managing expanding product offerings. Such circumstances could increase demands on our existing management and facilities. Failure to manage this growth and expansion could interrupt or adversely affect our operations and cause production backlogs, longer product development time frames and administrative inefficiencies.
We may not be able to successfully implement our strategy of acquiring desired target companies or to obtain financing needed to complete the acquisition of target companies.
One of our strategies is to grow through the acquisition of profitable, well-run companies whose businesses are synergistic with our core business. We may encounter substantial competition in connection with our efforts to complete such acquisitions which in turn could increase the cost of such acquisitions. If we are unable to negotiate and close such acquisitions on terms acceptable to us, our anticipated growth may not occur as contemplated or may require us to pay more for a target company which could adversely affect the profitability of such acquired company and negatively affect our results of operations. Additionally, our ability to successfully acquire a desired target company may depend upon our ability to obtain funding for any such acquisition and no assurances can be given that we will be able to obtain such financing, if needed, on terms acceptable to us.
We may not be able to effectively integrate acquired target companies or attain economies of scale.
If we are successful in acquiring desired target companies, we will need to integrate such acquisitions with our existing core business. Failure to effectively integrate the acquired target companies could adversely affect our core business, prevent us from realizing the benefits of economies of scale, or adversely affect our ability to successfully acquire other target companies and thereby adversely impact our growth strategy.
Shortages or disruptions in the availability of raw materials could have a material adverse effect on our business.
We expect that raw materials used to produce and manufacture our products will continue to account for a significant portion of our cost of goods sold in the future. The prices of raw materials fluctuate because of general economic conditions, global supply and demand and other factors causing monthly variations in the costs of our raw materials purchases. The macro-economic factors, together with labor and other business interruptions experienced by certain suppliers, have contributed to periodic shortages in the supply of raw materials, and such shortages may increase in the future. If we are unable to procure adequate supplies of raw material to meet our future production needs and customer demand, shortages could result in a material loss of customers and revenues and adversely impact our results of operations. In addition, supply shortages or disruptions or the loss of suppliers may cause us to procure our raw materials from less cost-effective sources and may have a material adverse affect on our business, revenues and results of operations.
25
Interruptions in growing or otherwise obtaining the raw materials used by our CHE and OP business units, whether due to problems with our crops or problems with our raw material suppliers, may affect our results of operations and our financial performance.
We grow a significant amount of the raw materials we use for our CHE business unit and we also purchase additional raw materials for our CHE business unit from various suppliers. We are not substantially dependent on any of our suppliers for the raw materials used by our CHE business unit. Also, we grow substantially all of the raw materials for our OP business unit and accordingly, to the extent we purchase raw materials from suppliers for our OP business unit, we are not dependent on any of our suppliers for the raw materials used by our OP business unit. Any problems we may experience with our CHE or OP business unit crops could disrupt production or impact our ability to increase production and sales of our CHE or OP products respectively or require us to purchase a greater amount of raw materials from suppliers. With respect to our suppliers, we typically enter into renewable, fixed price agreements. If we experience disruptions in our obtaining supplies for either our CHE business unit or our OP business unit, whether due to problems with our crops or other circumstances, we will need to identify and access alternative sources for such supplies which may in turn increase our costs and our dependence. Also in such event, interruptions in our obtaining the necessary raw materials could disrupt production or impact our ability to increase production and sales of our CHE or OP products and negatively impact our results of operations, financial performance and the price of our common stock.
Due to increased volatility of raw material prices, the timing lag between the raw material purchase and product pricing can negatively impact our profitability.
To the extent we purchase raw materials for our CHE and OP business units, volatility in the prices of raw materials, among other factors, may adversely impact our ability to accurately forecast demand and may have a material adverse impact on our results of operations. We seek to mitigate the impact of changing raw material prices by passing changes in prices to our customers by adjusting prices daily to reflect changes in raw material prices, as is customary in the industry. We may not be able to adjust our product prices rapidly enough in the short-term to recover the costs of increases in raw materials. Our future profitability may be adversely affected to the extent we are unable to pass on higher raw material costs to our customers.
Increases in raw materials prices will increase our need for working capital.
If the prices of raw materials that we may purchase for our CHE business unit and our OP business unit were to increase, our working capital requirements may increase as well. Increases in our working capital requirements can materially adversely impact our results of operations, our cash flow and our available liquidity to fund other business needs. Furthermore, there is no assurance we would be able to finance additional working capital requirements or finance such working capital requirements on favorable terms. If we were unable to obtain financing on favorable terms, our business and results of operations may be adversely affected. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources.”
Increases in raw materials prices may increase credit and default risk with respect to our customers.
Increases in the price of our products, if raw material prices were to rise and be passed on to our customers, may place additional demands on the working capital and liquidity needs of our customers. Accordingly, our customers’ cash flow may be negatively impacted which may have an adverse affect on the timing and amount of payment on our accounts receivable, which would in turn, negatively affect our results of operations.
26
If the nutritional, over-the-counter products and organic products industries do not grow or grow at a slower speed than we project, our sales and profitability may be materially adversely affected.
We currently derive most of our profits from sales of our products in PRC. The continued development of our business depends, in large part, on continued growth in the nutritional over-the-counter products and organic products industries in PRC and the Asia-Pacific region. Although PRC’s nutritional over-the-counter products and organic products industries have grown rapidly in the past, they may not continue to grow at the same growth rate or at all in the future. Any reduced demand for our products, any downturn or other adverse changes in PRC’s nutritional, over-the-counter products and organic products or related industries could severely impact the profitability of our business.
Potential environmental liability could have a material adverse effect on our operations and financial condition.
As a manufacturer, we are subject to various Chinese environmental laws and regulations on air emission, waste water discharge, solid wastes and noise. Although we believe that our operations are in substantial compliance with current environmental laws and regulations, we may not be able to comply with these regulations at all times as the Chinese environmental legal regime is evolving and becoming more stringent. Therefore, if the Chinese government imposes more stringent regulations in the future, we may have to incur additional and potentially substantial costs and expenses in order to comply with new regulations, which may negatively affect our results of operations. Further, no assurance can be given that all potential environmental liabilities have been identified or properly quantified or that any prior owner, operator, or tenant has not created an environmental condition unknown to us. If we fail to comply with any of the present or future environmental regulations in any material aspects, we may suffer from negative publicity and be subject to claims for damages that may require us to pay substantial fines or have our operations suspended or even be forced to cease operations.
Adverse publicity associated with us or our products or similar products manufactured by our competitors could have a material adverse effect on our results of operations.
Over the past several years, there have been various reported and publicized incidents regarding the safety and efficacy of products produced in PRC and the production in PRC of counterfeit products. Because our products include health supplements, energy drinks, organic food products, and products that are used in the manufacture of health supplements, energy drinks, organic food products and fertilizers, we are highly dependent upon market perceptions of the safety and quality of our products. Concerns over the safety of nutraceutical and organic products produced or manufactured in PRC could have an adverse effect on the sale of such products, including products produced or manufactured by us.
We could be adversely affected if any of our products or any similar products produced or manufactured by other companies prove to be, or are alleged to be, unsafe or harmful to humans or to animals, or ineffective or harmful to the environment. Any negative publicity associated with the safety, adverse effects or efficacy resulting from the use or misuse of our products or any similar products manufactured by other companies could also have a material adverse impact on our results of operations. We have not, to date, experienced any significant quality control or safety problems. If in the future we become involved in incidents of the type described above, such problems could have a material adverse effect on our results of operations.
Key employees are essential to growing our business.
We are dependent on the services of Mr. Silvasan our CEO, Mr. Min Zhao, our CHE business unit president, Mr. Jinrong Tang, our OP business unit president, and Ms. Jeanne Chan our Senior VP China to grow our business. Messrs. Silvasan, Zhao and Tang, and Ms. Chan are essential to our ability to continue to grow our business. Each of Messrs Zhao and Tang has established relationships within the industries in which we operate. Each of our key executives has agreed to non-solicitation and non-compete restrictions during the course of their employment with us, however, these restrictions only extend for a one-year period from termination. While none of our key executives have indicated to us that they intend to leave or resign from our Company in the near future, the loss of the services of any of our key executives could materially harm our business because of the cost and time necessary to recruit and train a replacement and to re-establish relationships within the industries in which we operate. Such a loss would also divert management’s attention away from operational matters. Further, we do not maintain, or intend to maintain, key person life insurance for any of our key executives or key employees. If any of them were to leave us, our growth strategy might be hindered, which could limit our ability to increase revenue. In addition, we face competition for attracting skilled personnel. If we fail to attract and retain qualified personnel to meet current and future needs, this could slow our ability to grow our business, which could result in a decrease in market share.
27
We may need additional financing, which may not be available on satisfactory terms or at all.
Our capital requirements may be accelerated as a result of many factors, including timing of acquisition and development activities, underestimates of budget items, unanticipated expenses or capital expenditures, overestimates of revenues, future product opportunities with collaborators, future licensing opportunities and future business combinations. Consequently, we may need to seek additional debt or equity financing, which may not be available on favorable terms, if at all, and which may be dilutive to our shareholders.
We may seek to raise additional capital through public or private equity offerings, debt financings or additional corporate collaboration and licensing arrangements. To the extent we raise additional capital by issuing equity securities, our shareholders may experience dilution. To the extent that we raise additional capital by issuing debt securities, we may incur substantial interest obligations, may be required to pledge assets as security for the debt and may be constrained by restrictive financial and/or operational covenants. Debt financing would also be superior to our shareholders’ interest in bankruptcy or liquidation. To the extent we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or product candidates, or grant licenses on unfavorable terms.
If we fail to adequately protect or enforce our intellectual property rights, or to secure rights to patents of others, the value of our intellectual property rights could diminish.
Our success, competitive position and future revenues will depend in part on our ability to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties.
To date, we have, through our contractually controlled PRC operating entities, applied for or been issued two patents in the PRC. The patents cover the proprietary production process used to produce Solanesol, Co-enzyme Q10, Resveratrol, 5-HTP, organic fertilizer, and potential future products. We cannot predict the degree and range of protection the patents will afford us against competitors. Third parties may find ways to invalidate or otherwise circumvent any patented technology that is a part of our business units. Third parties may attempt to obtain patents claiming aspects similar to our patents. We also have, through our contractually controlled PRC operating entities, applied for or have been issued six trademarks in the PRC. If we need to initiate litigation or administrative proceedings to protect or enforce our intellectual property rights, such actions may be costly whether we win or lose.
Our success will also depend on the skills, knowledge and experience of our scientific and technical personnel, consultants, advisors, licensors and contractors. To help protect our proprietary know-how and inventions for which patents may be unobtainable or difficult to obtain, we rely on trade secret protection and confidentiality agreements. If any of our confidential intellectual property is disclosed, our value could be significantly impaired, and our business and competitive position could suffer.
If we infringe the rights of third parties, we could be prevented from selling products, forced to pay damages and compelled to defend against litigation.
If our products, methods, processes and other technologies infringe on the proprietary rights of other parties, we could incur substantial costs, and may have to obtain licenses (which may not be available on commercially reasonable terms, if at all), redesign our products or processes, stop using the subject matter claimed in the asserted patents, pay damages, or defend litigation or administrative proceedings, which may be costly whether we win or lose. All of the above could result in a substantial diversion of valuable management resources.
We believe we have taken reasonable steps, including comprehensive internal and external prior patent searches, to ensure we have freedom to operate and that our development and commercialization efforts can be carried out as planned without infringing on others’ proprietary rights. However, we cannot guarantee that no third party patent has been filed or will be filed that may contain subject matter of relevance to our development, causing a third party patent holder to claim infringement. Resolving such issues has traditionally resulted, and could in our case result, in lengthy and costly legal proceedings, the outcome of which cannot be predicted accurately.
28
We do not have business insurance coverage in PRC.
Insurance companies in PRC offer limited business insurance products. As a result, we do not have any business liability, loss of data or disruption insurance coverage for our operations. Any business disruption, litigation or natural disaster might cause us to incur substantial costs or divert our resources. In addition, we do not carry insurance with respect to certain risks, including product liability insurance. As a result, any product liability or other claims may materially adversely affect our business, financial condition and results of operations. Furthermore, we may need to stop selling products that result in product liability claims, which could negatively affect the range of products that we offer and the size of our customer base.
Our corporate actions are substantially controlled by our principal shareholders and affiliated entities.
As of December 31, 2010, our principal shareholders and their affiliated entities own approximately 67% of our outstanding common stock, representing a majority of our voting power. These shareholders, acting individually or as a group, could exert substantial influence over matters such as electing directors and approving mergers or other business combination transactions. In addition, because of the percentage of ownership and voting concentration in these principal shareholders and their affiliated entities, elections of our board of directors will generally be within the control of these shareholders and their affiliated entities. While all of our shareholders are entitled to vote on matters submitted to our shareholders for approval, the concentration of shares and voting control presently lies with these principal shareholders and their affiliated entities. As such, it would be difficult for shareholders to propose and have approved proposals not supported by management. There can be no assurances that matters voted upon by our officers and directors in their capacity as shareholders will be viewed favorably by all shareholders of the company.
If we are unable to maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, and cause investors to lose confidence in our reported financial information.
Effective internal controls are necessary for us to provide reliable financial reports and effectively prevent fraud. As a public company, we have significant additional requirements for enhanced financial reporting and internal controls. We are required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, which requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent registered public accounting firm addressing these assessments. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company.
Our management, with the participation of our Chief Executive Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2010. Based upon that evaluation, our CEO concluded that our disclosure controls and procedures were effective and designed to ensure that material information required to be disclosed by us in the reports that we file or submit is recorded, processed, summarized and reported, within the time period specified in the SEC’s rules and regulations and accumulated and communicated to them as appropriate to allow timely decision regarding required disclosure.
Our management is also responsible for establishing and maintaining adequate internal control over financial reporting, which refers to the process designed by, or under the supervision of, our CFO and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles. As of December 31, 2010, our management evaluated the effectiveness of our internal control over financial reporting and concluded that at December 31, 2010, there was no material weakness in internal control over financial reporting, which related to the monitoring and review of work performed in the preparation of audit and financial statements, footnotes and financial data provided by us to our independent accounting firm. Management concluded that our financial disclosure controls and procedures were effective. At any time, if it appears that any control can be implemented to continue to mitigate such weakness, it is immediately implemented.
29
We have implemented internal financial controls and external reporting systems that we believe meet the requirements of the Sarbanes-Oxley Act of 2002. However, we cannot assure you that we will not, in the future, identify areas requiring improvement in our internal control over financial reporting. We cannot assure you that the measures we will take to remediate any areas in need of improvement will be successful or that we will implement and maintain adequate controls over our financial processes and reporting in the future as we continue our growth. If we are unable to establish appropriate internal financial reporting controls and procedures, it could cause us to fail to comply with Sarbanes-Oxley and meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, and cause investors to lose confidence in our reported financial information.
We may not be able to extend our Research and Development Contract with Fudan University
In 2005, we entered into a Research and Development Contract with Fudan University regarding, among other things, our joint ownership with Fudan University of the patented “solanesol-clean extraction method”, research and development of downstream products and assistance and support to be provided by Fudan University. On May 8, 2010, we entered into an Addendum Agreement to extend the expiration date under the Research and Development Contract to July 24, 2013. Because of our relationship with Fudan University, and in particular with Dr. Jian Ming Chan, who has served as Sanming’s Chief Scientist since April 2006, and has continued in that position since we acquired Elevated Throne on June 17, 2009, we believe that this Research and Development Contract will be further extended; however no assurances can be given that this contract will be extended beyond July 24, 2013. If we are unable to further extend the Research and Development Contract, if desirable, we may not have the assistance and support of Fudan University and we may be limited in the improvement of current products or the development of new downstream products.
Risks Associated With Doing Business In PRC
There are substantial risks associated with doing business in PRC, as set forth in the following risk factors.
Substantially all of our assets and operations are located in the PRC and are subject to changes resulting from the political and economic policies of the Chinese government and it is possible that the PRC government could adopt material changes to their policies regarding foreign participation in businesses operating in the PRC, which could have a material and adverse effect on our business and could even cause us to lose all of our assets and operations in PRC.
We conduct our business primarily through our contractually controlled, affiliated PRC operating entities. Our business operations could be restricted by the political environment in the PRC. The PRC has operated as a socialist state since 1949 and is controlled by the Communist Party of China. In recent years, however, the government has introduced reforms aimed at creating a socialist market economy and policies have been implemented to allow business enterprises greater autonomy in their operations. We are generally subject to laws and regulations applicable to foreign investments in PRC and, in particular, laws applicable to wholly foreign-owned enterprises (“WFOE”s). Changes in the political leadership of the PRC may have a significant effect on laws and policies related to the current economic reform programs, other policies affecting business and the general political, economic and social environment in the PRC, including the introduction of measures to control inflation, changes in the rate or method of taxation, import and export tariffs, the imposition of additional restrictions on currency conversion and remittances abroad, foreign investment and regulations relating to raw materials, environmental regulations, land use rights, property and other matters. Moreover, economic reforms and growth in the PRC have been more successful in certain provinces than in others, and the continuation or increases of such disparities could affect the political or social stability of the PRC. Although we believe that the economic reforms and the macroeconomic measures adopted by the Chinese government have had a positive effect on the economic development of PRC, there is no assurance that the Chinese government will continue to pursue the current economic reform policies, or that it will not significantly alter these policies from time to time without notice and the future direction of these economic reforms is uncertain and the uncertainty may decrease the attractiveness of our Company as an investment, which may in turn result in a decline in the trading price of our common stock. Further, we cannot assure you that the PRC government will not alter its policies to further restrict foreign participation in businesses operating in the PRC or even cause us to lose all of our assets and operations in the PRC.
30
Additionally, Chinese legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in PRC. However, the PRC has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in PRC. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their non-binding nature, the limited precedential value or prior court decisions, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the Chinese legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation (if any) of these policies and rules until sometime after the violation. In addition, any litigation in PRC may be protracted and result in substantial costs and diversion of resources and management attention.
We cannot assure you that the current Chinese policies of economic reform will continue. Because of this uncertainty, there are significant economic risks associated with doing business in PRC.
Although the majority of productive assets in PRC are owned by the Chinese government, in recent years the government has implemented economic reform measures that emphasize decentralization and encourage private economic activity. In keeping with these economic reform policies, the PRC has been openly promoting business development in order to bring more business into the PRC. Because these economic reform measures may be inconsistent or ineffective, there are no assurances that:
|
●
|
the Chinese government will continue its pursuit of economic reform policies;
|
|
●
|
the economic policies, even if pursued, will be successful;
|
|
●
|
the economic policies will not be significantly altered from time to time; or
|
|
●
|
business operations in China will not become subject to the risk of nationalization.
|
We cannot assure you that we will be able to capitalize on these economic reforms, assuming the reforms continue. Because our business model is dependent upon the continued economic reform and growth in PRC (as well as other Asia-Pacific countries), any change in Chinese government policy could materially adversely affect our ability to continue to implement our business model. PRC’s economy has experienced significant growth in the past decade, but such growth has been uneven across geographic and economic sectors and has recently been slowing. Even if the Chinese government continues its policies of economic reform, there are no assurances that economic growth in that country will continue or that we will be able to take advantage of these opportunities in a fashion that will provide financial benefit to us.
The Chinese government exerts substantial influence over the manner in which our Chinese subsidiaries and contractually controlled operating entities must conduct our business activities.
The PRC only recently has permitted provincial and local economic autonomy and private economic activities. The government of the PRC has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in the PRC or particular regions of the PRC, and could require us to divest ourselves of any interest we then hold in our PRC subsidiaries, or cause us to lose all of our assets and operations in the PRC.
We may be unable to enforce our rights due to policies regarding the regulation of foreign investments in PRC.
The PRC’s legal system is a civil law system based on written statutes in which decided legal cases have uncertain value as precedent, unlike the common law system prevalent in the United States. The PRC does not have a well-developed, consolidated body of laws governing foreign investment enterprises and its regulations and policies with respect to foreign investments are evolving. As a result, the administration of laws and regulations by government agencies may be subject to considerable discretion and variation, and may be subject to influence by external forces unrelated to the legal merits of a particular matter.
31
Definitive regulations and policies with respect to many of such matters have not yet been published. Statements regarding these evolving policies have been conflicting and any such policies, as administered, are likely to be subject to broad interpretation and discretion and to be modified, perhaps on a case-by-case basis. The uncertainties regarding such regulations and policies present risks which may affect our ability to achieve our stated business objectives. If we are unable to enforce any legal rights we may have under our contracts or otherwise, our ability to compete with other companies in our industry could be limited which could result in a loss of revenue in future periods which could have a material adverse effect on our business, financial condition, results of operations and stock price.
Certain agreements to which we are a party and which are material to our operations lack various legal protections which are customarily contained in similar contracts prepared in the United States.
Our subsidiaries and affiliated entities include companies organized under the laws of the PRC and all of their business and operations are conducted in PRC. We are a party to certain contracts related to our operations in PRC and, in particular, the contracts by which we contractually control Sanming and JLF. While these contracts contain the basic business terms of the agreements between the parties, these contracts do not contain certain clauses which are customarily contained in similar contracts prepared in the U.S., such as representations and warranties of the parties, confidentiality and non-compete clauses, provisions outlining events of defaults, and termination and jurisdictional clauses. Because our contracts in PRC omit these customary clauses, notwithstanding the differences in Chinese and U.S. laws, we may not have the same legal protections as we would if the contracts contained these additional clauses. We anticipate that our Chinese subsidiaries and our contractually controlled PRC operating entities will likely enter into contracts in the future which will likewise omit these customary legal protections. While we have not been subject to any adverse consequences as a result of the omission of these customary clauses, and we consider the contracts to which we are a party to contain all the material terms of our business arrangements with the other party, future events may occur which lead to a dispute which could have been avoided if the contracts included customary clauses in conformity with U.S. standards. Contractual disputes which may arise from this lack of legal protection could divert management’s time from the operation of our business, require us to expend funds attempting to settle a possible dispute, limit the time our management would otherwise devote to the operation of our business, and have a material adverse effect on our business, financial condition and results of operations. Additionally the lack of a well developed body of laws and the uncertain value of decided legal cases a precedent could, if a dispute were to arise, impact the enforceability of such contracts.
PRC regulations relating to acquisitions of PRC companies by foreign entities may create regulatory uncertainties that could restrict or limit our ability to operate. Our failure to obtain the prior approval of the China Securities Regulatory Commission (the “CSRC”), for this offering and the listing and trading of our common stock could have a material adverse effect on our business, operating results, reputation and trading price of our common stock, and may also create uncertainties for this offering.
The PRC State Administration of Foreign Exchange, or “SAFE,” issued a public notice effective November 1, 2005, known as Circular 75, concerning the use of offshore holding companies in mergers and acquisitions in PRC. The public notice provides that if an offshore company controlled by PRC residents intends to acquire a PRC company, such acquisition will be subject to registration with the relevant foreign exchange authorities. The public notice also suggests that registration with the relevant foreign exchange authorities is required for any sale or transfer by the PRC residents of shares in an offshore holding company that owns an onshore company. The PRC residents must each submit a registration form to the local SAFE branch with respect to their ownership interests in the offshore company, and must also file an amendment to such registration if the offshore company experiences material events, such as changes in the share capital, share transfer, mergers and acquisitions, spin-off transactions or use of assets in China to guarantee offshore obligations.
We are committed to complying with and to ensuring that our shareholders who are subject to the regulations will comply with the relevant rules. However, we cannot assure you that all of our shareholders who are PRC residents will comply with our request to make or obtain any applicable registrations or comply with other requirements required by Circular 75 or other related rules. Any future failure by any of our shareholders who is a PRC resident, or controlled by a PRC resident, to comply with relevant requirements under this regulation could subject us to fines or sanctions imposed by the PRC government, including restrictions on our PRC subsidiaries’ abilities to pay dividends or make distributions to us and our ability to increase our investment in our PRC subsidiaries.
32
On August 8, 2006, the PRC Ministry of Commerce (“MOFCOM”), joined by the State-owned Assets Supervision and Administration Commission of the State Council, the State Administration of Taxation, the State Administration for Industry and Commerce, the China Securities Regulatory Commission and SAFE, released a substantially amended version of the Provisions for Foreign Investors to Merge with or Acquire Domestic Enterprises (the “Revised M&A Regulations”), which took effect September 8, 2006. These new rules significantly revised China’s regulatory framework governing onshore-to-offshore restructurings and foreign acquisitions of domestic enterprises. These new rules signify greater PRC government attention to cross-border merger, acquisition and other investment activities, by confirming MOFCOM as a key regulator for issues related to mergers and acquisitions in PRC and requiring MOFCOM approval of a broad range of merger, acquisition and investment transactions. Further, the new rules establish reporting requirements for acquisition of control by foreigners of companies in key industries, and reinforce the ability of the Chinese government to monitor and prohibit foreign control transactions in key industries.
Among other things, the Revised M&A Regulations include new provisions that purport to require that an offshore special purpose vehicle, or “SPV”, formed for listing purposes and controlled directly or indirectly by PRC companies or individuals must obtain the approval of the CSRC prior to the listing and trading of such SPV’s securities on an overseas stock exchange. On September 21, 2006, the CSRC published on its official website procedures specifying documents and materials required to be submitted to it by SPVs seeking CSRC approval of their overseas listings. Our PRC counsel has advised us that, (i) Fujian GP and Fujian United were incorporated by a foreign owned enterprise, and there was no acquisition of the equity or assets of a “PRC domestic company” as such term is defined under the Revised M&A Regulations and (ii) no provision in the Revised M&A Regulations clearly classifies the contractual arrangements between Fujian GP and Sanming, Fujian United and JLF as a type of transaction falling within such rules. Therefore, we were and are not required to obtain the approval of CSRC under the Revised M&A Regulations if we are to pursue a public offering. However, the application of this PRC regulation remains unclear with no consensus currently existing among the leading PRC law firms regarding the scope and applicability of the CSRC approval requirement.
If the CSRC or another PRC regulatory agency subsequently determines that CSRC approval was required for a public offering, we may face regulatory actions or other sanctions from the CSRC or other PRC regulatory agencies. These regulatory agencies may impose fines and penalties on our operations in the PRC, limit our operating privileges in the PRC, delay or restrict the repatriation of the proceeds from the public offering in the PRC, or take other actions that could have a material adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our common stock. The CSRC or other PRC regulatory agencies also may take actions requiring us, or making it advisable for us, to halt the public offering before settlement and delivery of the common stock offered hereby. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that settlement and delivery may not occur.
Also, if later the CSRC requires that we obtain its approval, we may be unable to obtain a waiver of the CSRC approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties and/or negative publicity regarding this CSRC approval requirement could have a material adverse effect on the trading price of our common stock. Furthermore, published news reports in PRC recently indicated that the CSRC may have curtailed or suspended overseas listings for Chinese private companies. These news reports have created further uncertainty regarding the approach that the CSRC and other PRC regulators may take with respect to transactions for such public offering.
It is uncertain how our business operations or future strategy will be affected by the interpretations and implementation of Circular 75 and the Revised M&A Regulations. It is anticipated that application of the new rules will be subject to significant administrative interpretation, and we will need to closely monitor how MOFCOM and other ministries apply the rules to ensure that our domestic and offshore activities continue to comply with PRC law. Given the uncertainties regarding interpretation and application of the new rules, we may need to expend significant time and resources to maintain compliance.
33
We conduct of our business primarily through contractually controlled PRC operating entities and our control of the day-to-day operations of such PRC entities pursuant to contracts in order to comply with Chinese law may not be as effective as conducting business through direct equity ownership of such PRC entities due to uncertainties with respect to the PRC legal system which could materially and adversely affect our results of operations.
We conduct our business primarily through our contractually controlled PRC operating entities. PRC laws and regulations govern our operations in PRC. Our contractually controlled PRC operating entities are generally subject to laws and regulations applicable to foreign investments in PRC and, in particular, laws applicable to wholly foreign-owned enterprises (“WFOEs”). Although members of our executive management team and our shareholders include the executive officers and owners of our contractually controlled PRC operating entities, because we do not directly own our contractually controlled PRC operating entities, we may encounter problems enforcing our rights to control the business affairs and day-to-day operations of such entities. If we find it necessary to take legal action in PRC to enforce our rights under our contracts with the PRC operating entities, we will be subject to the uncertainties of the PRC legal system, where prior court decisions have limited precedential value. Since 1979, PRC legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in PRC. However, PRC has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in PRC. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their non-binding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation, if any, of these policies and rules until sometime after the violation. In addition, any litigation in PRC, regardless of outcome, may be protracted and result in substantial costs and diversion of resources and management attention. Accordingly, notwithstanding our contractual control over our PRC operating entities, such control may not be as effective as if we conducted our business through direct equity owned PRC entities which could materially and adversely affect our results of operations.
Our contractual arrangements with Sanming, JLF and their shareholders may not be as effective in providing control over these entities as direct ownership.
We have no equity ownership interest in Sanming and JLF, we rely on the contractual arrangements of the VIE agreements to control and operate Sanming and JLF. These contractual arrangements may not be as effective in providing control over Sanming and JLF as direct ownership. For example, Sanming and JLF could fail to take actions required for our business or fail to pay dividends to Fujian GP and Fujian United despite its contractual obligation to do so. If Sanming and JLF fail to perform their obligation under the VIE agreements, we may have to rely on legal remedies under PRC law, which may not be effective.
Uncertainty as to the enforceability of our agreements through which we control our contractually controlled PRC operating entities and their day-to-day operations.
We entered into agreements with our contractually controlled PRC operating entities and their equity holders which provide us with the contractual control over the business affairs and the day-to-day operations of such businesses as a means to comply with PRC restrictions relating to foreign investment in PRC entities. Our PRC counsel, DeHeng Law Offices (Bejing), has advised us that such agreements are enforceable and in compliance with PRC laws. Based on such advice, we did not seek PRC government approval regarding our relationships or agreements with our contractually controlled PRC operating entities. Notwithstanding the foregoing, due to the uncertainties and inconsistencies of the applications and interpretations of the PRC laws and regulations and the limited precedential value of prior court decisions in the PRC legal system, we cannot assure you that we will be able to enforce our rights under our agreements with said third party PRC operating entities or otherwise control the business affairs and day-to-day operations of such businesses.
If we make equity compensation grants to persons who are PRC citizens, they may be required to register with the State Administration of Foreign Exchange of the PRC, or “SAFE”. We may also face regulatory uncertainties that could restrict our ability to adopt additional equity compensation plans for our directors and employees and other parties under PRC law.
On April 6, 2007, SAFE issued the “Operating Procedures for Administration of Domestic Individuals Participating in the Employee Stock Ownership Plan or Stock Option Plan of An Overseas Listed Company,” also known as “Circular 78.” It is not clear whether Circular 78 covers all forms of equity compensation plans or only those which provide for the granting of stock options. For any plans which are so covered and are adopted by a non-PRC listed company, such as our company, after April 6, 2007, Circular 78 requires all participants who are PRC citizens to register with and obtain approvals from SAFE prior to their participation in the plan. In addition, Circular 78 also requires PRC citizens to register with SAFE and make the necessary applications and filings if they participated in an overseas listed company’s covered equity compensation plan prior to April 6, 2007. We believe that the registration and approval requirements contemplated in Circular 78 will be burdensome and time consuming.
34
Effective January 5, 2010, we adopted our 2010 Incentive Stock Plan and made stock option grants under the 2010 Incentive Stock Plan to certain of our officers, directors and employees, some of whom are PRC citizens and may be required to register with SAFE. In addition to our officers and directors that receive option grants at the close of this offering, future participants of our 2010 Incentive Stock Plan or any other equity compensation plan we may adopt who are PRC citizens may be required to register with SAFE. If it is determined that any of our equity compensation plans are subject to Circular 78, failure to comply with such provisions may subject us and participants of our equity incentive plan who are PRC citizens to fines and legal sanctions and prevent us from being able to grant equity compensation to our PRC employees. In that case, our business operations may be adversely affected.
Because our business is located in the PRC, we may have difficulty establishing adequate management, legal and financial controls, which we are required to do in order to comply with U.S. securities laws.
PRC companies have historically not adopted a Western style of management and financial reporting concepts and practices, which includes strong corporate governance, internal controls and, computer, financial and other control systems. Although our senior executive officers are seasoned executives with significant experience in operating public companies and corporate governance compliance, most of our middle and top management staff in the PRC are not educated and trained in the Western system. While historically we have not experienced problems in attracting executives or management personnel from PRC’s large pool of professionals, should we need to replace one of our executives or key employees or bring on additional executives and key employees, we may have difficulty hiring new employees in the PRC with such Western system training. As a result of these factors, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices for our PRC subsidiaries and controlled operating entities that meet Western standards even though we have significant contractual controls over our PRC operating entities. Therefore, we may, in turn, experience difficulties in implementing and maintaining adequate internal controls as required under Section 404 of the Sarbanes-Oxley Act of 2002. This may result in significant deficiencies or material weaknesses in our internal controls, which could impact the reliability of our financial statements and prevent us from complying with SEC rules and regulations and the requirements of the Sarbanes-Oxley Act of 2002. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our business.
Restrictions on the convertibility of RMB into foreign currency may limit our ability to make dividends or other payments in U.S. dollars or fund possible business activities outside PRC.
Substantially all of our net revenues are currently generated in RMB. Any future restrictions on currency exchanges may limit our ability to use net revenues generated in RMB to make dividends or other payments in U.S. dollars or fund possible business activities outside PRC. Although the PRC government introduced regulations in 1996 to allow greater convertibility of RMB for current account transactions, significant restrictions still remain, including primarily the restriction that foreign-invested enterprises may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business after providing valid commercial documents. In addition, remittance of foreign currencies abroad and conversion of RMB for capital account items, including direct investment and loans, is subject to government approval in PRC, and companies are required to open and maintain separate foreign exchange accounts for capital account items. Subject to certain procedures, including the payment of certain taxes in the PRC, our PRC business units are currently able to pay dividends to us which we can then use to fund our other business activities. We cannot assure you the Chinese regulatory authorities will not impose more stringent restrictions on the convertibility of RMB, especially with respect to foreign exchange transactions.
Restrictions on making payments to us.
We rely almost entirely on payments from the revenues generated by our contractually controlled PRC operating entities under our contractual arrangements with them. The Chinese government imposes controls on the conversion of the Chinese currency, RMB, into foreign currencies and the remittance of currencies out of PRC. Subject to certain procedures, including the payment of certain taxes in the PRC, our PRC business units are currently able to pay dividends to us which we can then use to fund our other business activities. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency. Furthermore, if our contractually controlled PRC operating entities incur debt on their own in the future, the instruments governing the debt may restrict their ability to make payments. If we are unable to receive all of the revenues from our operations in the PRC through these contractual or dividend arrangements, we may be unable to pay our operating expenses as they come due.
35
We do not have a financial service business license and may be restricted in extending loans directly to other companies, including affiliates.
We do not currently have a financial service business license and PRC laws generally do not permit companies that do not possess a financial service business license to extend loans directly to other companies, including affiliates, without proceeding through a financial agency. The enforcement of these restrictions remains unpredictable, and government authorities may declare these loans void, require the forfeiture of any interest paid and levy fines or other penalties upon the parties involved, among other remedies. In the past, when our subsidiaries or affiliates have required capital, we have funded such needs by providing equity investments and not loans to such entities. In the future, we intend to continue to fund the capital needs of our subsidiaries in the form of additional equity investments. Additionally, because we do not have a financial services business license, our internal financing business unit may be limited in providing funding to our other business units and their customers in PRC that purchase products from us. However, in the future we may seek to obtain the necessary PRC licenses or government approvals in order to provide intercompany loans to our subsidiaries and funding to their customers in PRC who purchase products from us.
Bringing original actions in PRC based on United States or other foreign laws against us, our management or the experts named in the prospectus.
We currently conduct substantially all of our operations in PRC through contractually controlled PRC operating entities, substantially all of our revenues are generated by our operations in PRC, and substantially all of our operating assets are located in PRC. The owners and executive officers who control and operate our contractually controlled PRC operating entities include individuals who are also our executive officers and shareholders who reside within PRC. If the owners and executive officers of our contractually controlled PRC operating entities were to decide to operate such businesses for their own benefit and thereby breach our agreements with them, we may experience difficulties enforcing our agreements with such entities. Additionally, it may not be possible to effect service of process within the United States or elsewhere outside PRC upon our executive officers and other individuals our executive officers who reside in PRC, including with respect to matters arising under U.S. federal securities laws or applicable state securities laws. Moreover, our Chinese counsel has advised us that PRC does not have treaties with the United States or many other countries providing for the reciprocal recognition and enforcement of judgment of courts.
Governmental control of currency conversion may affect the value of your investment.
The Chinese government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of PRC. We receive substantially all of our revenues in RMB. Under our current structure, our income is primarily derived from payments from our PRC business units. Shortages in the availability of foreign currency may restrict the ability of our Chinese subsidiaries and our affiliated entities to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency denominated obligations. Under existing Chinese foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of PRC to pay capital expenses such as the repayment of bank loans denominated in foreign currencies. The Chinese government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay our operating expenses as they come due.
We derive a substantial portion of our sales from PRC and a slowdown or other adverse developments in the PRC economy may materially and adversely affect our customers, demand for our services and our business.
Substantially all of our sales are generated from PRC. We anticipate that sales of our products in PRC will continue to represent a substantial proportion of our total sales in the near future. Although the PRC economy has grown significantly in recent years, we cannot assure you that such growth will continue. The industries in which we are involved in the PRC are relatively new and growing, but we do not know how sensitive these industries are to a slowdown in economic growth or other adverse changes in the PRC economy which may affect demand for our products. A slowdown in overall economic growth, an economic downturn or recession or other adverse economic developments in the PRC may materially reduce the demand for our products and materially and adversely affect our business.
36
Currency fluctuations and restrictions on currency exchange may adversely affect our business, including limiting our ability to convert Chinese RMB into foreign currencies and, if Chinese RMB were to decline in value, reducing our revenue in U.S. dollar terms.
Although our reporting currency is the U.S. dollar, substantially all of our operations are in PRC and the RMB is used as their functional currency and substantially all of our Chinese revenue and expenses are in RMB. We are subject to the effects of exchange rate fluctuations with respect to any of these currencies. For example, the value of the RMB depends to a large extent on Chinese government policies and PRC’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of RMB to the U.S. dollar had generally been stable and the RMB had appreciated slightly against the U.S. dollar. However, on July 21, 2005, the Chinese government changed its policy of pegging the value of Chinese RMB to the U.S. dollar. Under the new policy, Chinese RMB may fluctuate within a narrow and managed band against a basket of certain foreign currencies. It is possible that the Chinese government could adopt a more flexible currency policy, which could result in more significant fluctuation of Chinese RMB against the U.S. dollar. We can offer no assurance that Chinese RMB will be stable against the U.S. dollar or any other foreign currency.
The income statements of our foreign operations are translated into U.S. dollars at the average exchange rates in each applicable period. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign operations into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign operations’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income. In addition, we have certain assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss. We have not entered into agreements or purchased instruments to hedge our exchange rate risks, although we may do so in the future. The availability and effectiveness of any hedging transaction may be limited and we may not be able to successfully hedge our exchange rate risks.
We may have limited legal recourse under PRC law if disputes arise under our contracts with third parties.
The Chinese government has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. If our new business ventures are unsuccessful, or other adverse circumstances arise from these transactions, we face the risk that the parties to these ventures may seek ways to terminate the transactions, or, may hinder or prevent us from accessing important information regarding the financial and business operations of these contractually controlled companies. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the Chinese government, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance, or to seek an injunction under PRC law, in either of these cases, are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. The occurrence of any such events could have a material adverse effect on our business, financial condition and results of operations, including the loss of all of our assets and operations located in PRC. Although legislation in PRC over the past 30 years has significantly improved the protection afforded to various forms of foreign investment and contractual arrangements in PRC, these laws, regulations and legal requirements are relatively new and their interpretation and enforcement involve uncertainties, which could limit the legal protection available to us, and foreign investors, including you. The inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital and could have a material adverse impact on our operations.
37
It may be difficult to protect and enforce our intellectual property rights under PRC law.
Intellectual property rights in PRC are still developing, and there are uncertainties involved in the protection and the enforcement of such rights. We will need to pay special attention to protecting our intellectual property and trade secrets. Failure to do so could lead to the loss of a competitive advantage that could not be compensated by a damages award.
If our land use rights are revoked, we would have no operational capabilities.
Under Chinese law, land is owned by the state or rural collective economic organizations. The state issues to tenants the rights to use property. Use rights can be revoked and the tenants forced to vacate at any time when redevelopment of the land is in the public interest. The public interest rationale is interpreted quite broadly and the process of land appropriation may be less than transparent. Sanming and JLF, our contractually controlled PRC operating entities, rely on these land use rights in their respective operations, and the loss of such rights would have a material adverse effect on these operating divisions and our company and our source of raw materials needed by our CHE and OP business units.
Because we may not be able to obtain business insurance in the PRC, we may not be protected from risks that are customarily covered by insurance in the United States.
Business insurance is not readily available in the PRC. To the extent that we suffer a loss of a type which would normally be covered by insurance in the United States, such as product liability and general liability insurance, we would incur significant expenses in both defending any action and in paying any claims that result from a settlement or judgment.
Future inflation in PRC may inhibit our ability to conduct business in PRC.
In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. During the past ten years, the rate of inflation in PRC has been as high as 20.7% and as low as 2.2%. These factors have led to the adoption by the PRC government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. While inflation has been more moderate since 1995, high inflation may in the future cause the PRC government to impose controls on credit or prices, or to take other action, which could inhibit economic activity in PRC, and thereby harm the market for our products.
We must comply with the Foreign Corrupt Practices Act.
We are required to comply with the United States Foreign Corrupt Practices Act, which prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some of our competitors, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in mainland PRC. If our competitors engage in these practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business or from government officials who might give them priority in obtaining new licenses, which would put us at a disadvantage. Although we inform our personnel that such practices are illegal and therefore not acceptable to us, we cannot assure you that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties.
38
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
We are dependent on our relationship with the local government in the province in which we operate our business. Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in PRC may be harmed by changes in its laws and regulations, including those relating to taxation, environmental regulations, land use rights, property and other matters. We believe that our operations in PRC are in material compliance with all applicable legal and regulatory requirements. However, the central or local governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in PRC or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
Because the M&A Rules permit the government agencies to have scrutiny over the economics of an acquisition transaction and require consideration in a transaction to be paid within stated time limits, we may not be able to negotiate a transaction that is acceptable to our shareholders or sufficiently protect their interests in a transaction.
The M&A Rules have introduced aspects of economic and substantive analysis of the target business and the acquirer and the terms of the transaction by MOFCOM and the other governing agencies through submissions of an appraisal report and the acquisition agreement, all of which form part of the application for approval, depending on the structure of the transaction. The M&A Rules also prohibit a transaction at an acquisition price obviously lower than the appraised value of the Chinese business or assets. The M&A Rules require that in certain transaction structures, the consideration must be paid within strict time periods. Because the Chinese authorities have been unhappy with offshore flips which converted domestic companies into foreign investment enterprises (“FIEs”) in order to take advantage of certain benefits, including reduced taxation, in the PRC, the M&A Rules require foreign sourced capital of not less than 25% of the domestic company’s post-acquisition capital in order to obtain FIE treatment. In asset transactions there must be no harm of third parties and the public interest in the allocation of assets and liabilities being assumed or acquired. These aspects of the M&A Rules will limit our ability to negotiate various terms of the acquisition, including aspects of the initial consideration, contingent consideration, holdback provisions, indemnification provisions and provisions relating to the assumption and allocation of assets and liabilities. Transaction structures involving trusts, nominees and similar entities are prohibited with respect to the acquisitions among related parties. Therefore, we may not be able to negotiate a transaction with terms that will satisfy our investors and protect our shareholders’ interests in an acquisition of a Chinese business or assets.
Compliance with the PRC Antitrust Law may limit our ability to effect an acquisition.
The PRC Antitrust Law was promulgated on August 30, 2007, and became effective on August 1, 2008. The government authorities in charge of antitrust matters in PRC are the Antitrust Commission and other antitrust authorities under the State Council. The PRC Antitrust Law regulates (i) monopoly agreements, including decisions or actions in concert that preclude or impede competition, entered into by business operators; (ii) abuse of dominant market position by business operators; and (iii) concentration of business operators that may have the effect of precluding or impeding competition. The concentration of business operators refers to (i) merger with other business operators; (ii) gaining control over other business operators through acquisition of equity interest or assets of other business operators; and (iii) gaining control over other business operators through exerting influence on other business operators through contracts or other means. In the event of occurrence of any concentration of business operators and to the extent required by the Antitrust Law, the relevant business operators must file with the antitrust authority under the State Council prior to conducting the contemplated business concentration. If the antitrust authority decides not to further investigate whether the contemplated business concentration has the effect of precluding or impeding competition or fails to make a decision within 30 days from receipt of relevant materials, the relevant business operators may proceed to consummate the contemplated business concentration. When we evaluate a potential transaction, we will consider the need to comply with the Antitrust Law and other relevant regulations which may limit our ability to effect an acquisition.
39
Our contractual arrangements with Sanming and JLF may result in adverse tax consequences to us.
As a result of our corporate structure and contractual arrangements, Fujian GP and Fujian United are effectively subject to the 5% PRC business tax on revenues derived from Sanming and JLF pursuant to the VIE agreements. Fujian GP and Fujian United are subject to the business tax, while Sanming and JLF, as manufacturers, instead of a service provider, are not subject to the business tax. Moreover, we would be subject to adverse tax consequences if the PRC tax authorities were to determine that the VIE agreements were not on an arm’s length basis and therefore constitute a favorable transfer pricing. As a result, the PRC tax authorities could request that we adjust the taxable income upward for PRC tax purposes. Such a pricing adjustment could adversely affect us by:
- increasing Sanming and JLF’s tax expenses without reducing Fujian GP and Fujian United’s tax expenses, which could subject Sanming and JLF to late payment fees and other penalties for under-payment of taxes; and/or
- resulting in Fujian GP and Fujian United’s loss of its preferential tax treatment.
Under PRC law, we are required to obtain permits and business licenses, and our failure to do so would adversely impact our ability to conduct business in PRC.
We hold various permits, business licenses, and approvals authorizing our operations and activities, which are subject to periodic review and reassessment by the Chinese authorities. Standards of compliance necessary to pass such reviews change from time to time and differ from jurisdiction to jurisdiction, leading to a degree of uncertainty. If renewals, or new permits, business licenses or approvals required in connection with existing or new facilities or activities, are not granted or are delayed, or if existing permits, business licenses or approvals are revoked or substantially modified, we will suffer a material adverse effect. If new standards are applied to renewals or new applications, it could prove costly to us to meet any new level of compliance.
Requirement of new labor laws in the PRC.
On June 29, 2007, the PRC government promulgated a new labor law, namely, the Labor Contract Law of the PRC, or the New Labor Contract Law, which became effective on January 1, 2008. The New Labor Contract Law imposes stricter obligations on employers. Under the New Labor Contract Law, an employer is obligated to execute written labor contracts with all of its employees, otherwise an employee without a written labor contract will be entitled to claim double monthly salary from the employer. We believe we are in substantial compliance with this law, although at certain times of the year (harvest season) we hire between 20 and 40 seasonal/part time employees each year without execution of a written labor contract. If any of our employees with whom we do not have a written labor contract were to file a valid claim under this law, and we are required to make additional payments to such employee, we do not believe it would have a material impact on our financial results.
The New Labor Contract Law also imposes tougher procedural requirements related to a reduction in workforce and requires certain terminations to be based upon seniority and not merit. In the event we decide to significantly change or decrease our workforce, the New Labor Contract Law could adversely affect our ability to enact such changes in a timely and cost-effective manner that is most advantageous to our business, which may materially and adversely affect our financial condition and results of operations.
40
Our failure to fully comply with PRC labor laws exposes us to potential liability.
Companies operating in PRC must comply with a variety of labor laws, including certain social insurance, housing fund and other staff welfare-oriented payment obligations. There exist uncertainties as to the interpretation, implementation and enforcement of such obligations. If relevant governmental authorities determine that we have not complied fully with such obligations, we may be in violation of applicable PRC labor laws and we cannot assure you that PRC governmental authorities will not impose penalties on us for any failure to comply. In addition, in the event that any current or former employee files a complaint with relevant governmental authorities, we may be subject to making up such staff-welfare oriented obligations as well as paying administrative fines.
Our global income and the dividends that we may receive from our PRC subsidiary may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would have a material adverse effect on our results of operations.
Under the PRC Enterprise Income Tax Law and its implementing rules, both effective from January 1, 2008, an enterprise established outside of the PRC with “de facto management bodies” within the PRC is considered a resident enterprise and will be subject to the enterprise income tax at the rate of 25% on its global income. The implementation rules define the term “de facto management bodies” as “establishments that carry out substantial and overall management and control over the manufacturing and business operations, personnel, accounting, properties, etc. of an enterprise.” The State Administration of Taxation issued the Notice Regarding the Determination of Chinese-Controlled Offshore Incorporated Enterprises as PRC Tax Resident Enterprises on the Basis of De Facto Management Bodies, or SAT Circular 82, on April 22, 2009. SAT Circular 82 provides certain specific criteria for determining whether the “de facto management body” of a Chinese-controlled offshore-incorporated enterprise is located in China. Although SAT Circular 82 only applies to offshore enterprises controlled by PRC enterprises, not those controlled by PRC individuals, like our company, the determining criteria set forth in SAT Circular 82 may reflect the State Administration of Taxation’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC enterprises or individuals. Accordingly, we may be considered a resident enterprise and may therefore be subject to the enterprise income tax at 25% on our global income. If we are considered a resident enterprise and earn income other than dividends from our PRC subsidiary, a 25% enterprise income tax on our global income could significantly increase our tax burden and materially and adversely affect our cash flow and profitability.
We face risks related to natural disasters and health epidemics in PRC, which could have a material adverse effect on our business and results of operations.
Our business could be materially and adversely affected by natural disasters or the outbreak of health epidemics in PRC. For example, in May 2008, Sichuan Province suffered a strong earthquake measuring approximately 8.0 on the Richter scale that caused widespread damage and casualties. In addition, in the last decade, the PRC has suffered health epidemics related to the outbreak of avian influenza and severe acute respiratory syndrome, or SARS. In April 2009, an outbreak of the H1N1 virus, also commonly referred to as “swine flu”, occurred in Mexico and has spread to other countries. Cases of swine flu have been reported in Hong Kong and mainland PRC. If the outbreak of swine flu were to become widespread in PRC or increase in severity, it could have an adverse effect on economic activity in PRC, and our business and operations could be adversely affected. Any future natural disasters or health epidemics in the PRC could also have a material adverse effect on our business and results of operations.
41
Risks Related to our Securities
Our common stock is thinly traded and you may be unable to sell at or near ask prices or at all if you desire to liquidate your shares. There is only a limited and sporadic trading market for our common stock on the OTB Bulletin Board.
Our common stock has historically been sporadically or “thinly-traded” on the OTC Bulletin Board, meaning that the number of persons interested in purchasing our common stocks at or near bid prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a relatively small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow a relatively unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there had been periods of several days or more when trading activity in our shares was minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained.
The market price for our common stock may be volatile given our status as a relatively small company with a small and thinly traded “float” that could lead to wide fluctuations in our share price. The price at which you purchase our common stock may not be indicative of the price that will prevail in the trading market. You may be unable to sell your common stock at or above your purchase price if at all, which may result in substantial losses to you.
The market for our common stock is characterized by price fluctuations when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The fluctuations in our share price are attributable to a number of factors. First, as noted above, our common stock is sporadically and/or thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our shareholders may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of shares of our common stocks are sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Secondly, we are a speculative or “risky” investment due to our fluctuating level of revenues or profits to date and uncertainty of future market acceptance for our current and potential products. As a consequence of this enhanced risk, more risk-averse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. The following factors may add to the volatility in the price of our common stock: actual or anticipated variations in our quarterly or annual operating results; adverse outcomes; and additions or departures of our key personnel, as well as other items discussed under this “Risk Factors” section, as well as elsewhere in this prospectus. Many of these factors are beyond our control and may decrease the market price of our common stock regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common stock will be at any time, including as to whether our common stock will sustain their current market prices, or as to what effect that the sale of shares or the availability of common stock for sale at any time will have on the prevailing market price.
Shareholders should be aware that the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities. The occurrence of these patterns or practices could increase the volatility of our share price. Although we believe that our shares would not currently be considered a penny stock, if our shares were to trade at a price below $5.00 per share and/or not be listed on one of the major exchanges (NSYE, NYSEAmex or NASDAQ) we may be considered a penny stock under the SEC’s rules which would impose restrictions of broker-dealers who recommend our stock to customers.
42
The market price for our securities may be subject to wide fluctuations and our securities may trade below any public offering price that we may pursue.
The initial public offering price of our securities will be determined by negotiations between us and the representative of the underwriters, based on numerous factors. This price may not be indicative of the market price of our securities after any public offering that we may pursue. We cannot assure you that you will be able to resell your securities at or above the public offering price or our net asset value. The securities of a number of Chinese companies and companies with substantial operations in PRC have also experienced wide fluctuations subsequent to their public offerings, including trading at prices substantially below the public offering prices. Among the factors that could affect the price of our securities are risk factors described in this section and other factors, including:
|
●
|
conditions in health and wellness and organic products markets;
|
|
●
|
changes in the economic performance or market valuations of other agritech companies;
|
|
●
|
announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
●
|
general economic or political conditions in PRC;
|
|
●
|
regulatory developments of our industry affecting us, our customers or our competitors;
|
|
●
|
actual or anticipated fluctuations in our quarterly operating results;
|
|
●
|
failure of our quarterly financial and operating results to meet market expectations or failure to meet our previously announced guidance, if any;
|
|
●
|
changes in financial estimates by securities research analysts;
|
|
●
|
changes in the economic performance or market valuations of our competitors;
|
|
●
|
additions or departures of our executive officers and other key personnel;
|
|
●
|
announcements regarding intellectual property litigation (or potential litigation) involving us or any of our directors and officers;
|
|
●
|
fluctuations in the exchange rates between the U.S. dollar and the RMB; and
|
|
●
|
release or expiration of the underwriters’ post-offering lock-up or other transfer restrictions on our outstanding common stock.
|
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular industries or companies. For example, the capital and credit markets have been experiencing volatility and disruption for more than 12 months. Starting in September 2008, the volatility and disruption have reached extreme levels, developing into a global crisis. As a result, stock prices of a broad range of companies worldwide, whether or not they are related to financial services, have declined significantly. These market fluctuations may also have a material adverse effect on the market price of our securities.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate.
We may need additional capital and may sell additional securities or other equity securities or incur indebtedness, which could result in additional dilution to our shareholders or increase our debt service obligations.
We may require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our cash resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities or equity-linked debt securities could result in additional dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
43
If our shares of common stock become subject to the SEC’s penny stock rules, broker-dealers may experience difficulty in completing customer transactions and trading activity in our securities may be adversely affected.
If at any time we have net tangible assets of $5,000,000 or less and our shares of common stock have a market price per share of less than $5.00, transactions in our common stock may be subject to the “penny stock” rules promulgated under the Securities Exchange Act of 1934, as amended. Under these rules, broker-dealers who recommend such securities to persons other than institutional accredited investors must:
|
●
|
make a special written suitability determination for the purchaser;
|
|
●
|
receive the purchaser’s written agreement to the transaction prior to sale;
|
|
●
|
provide the purchaser with risk disclosure documents which identify certain risks associated with investing in “penny stocks” and which describe the market for these “penny stocks” as well as a purchaser’s legal remedies; and
|
|
●
|
obtain a signed and dated acknowledgment from the purchaser demonstrating that the purchaser has actually received the required risk disclosure document before a transaction in a “penny stock” can be completed.
|
If our securities become subject to these rules, broker-dealers may find it difficult to effectuate customer transactions and trading activity in our securities may be adversely affected. As a result, the market price of our securities may be depressed, and you may find it more difficult to sell our securities.
Foreign exchange risk
We carry out the majority of our transactions in RMB. Therefore, we have an exposure to foreign exchange fluctuations. A substantial portion of our cash is held in PRC in interest bearing bank deposits and denominated in RMB. The RMB is not a freely convertible currency. The PRC government may take actions that could cause future exchange rates to vary significantly from current or historical exchange rates. Fluctuations in exchange rates may adversely affect the value of any dividends we declare. Restrictions on the convertibility of RMB into foreign currency may limit our ability to make dividends or other payments in U.S. dollars or fund possible business activities outside of PRC.
|
ITEM 1B
|
UNRESOLVED STAFF COMMENTS
|
NotApplicable
|
ITEM 2
|
DESCRIPTION OF PROPERTY
|
TheCompany’s corporate headquarters is located at 19950 W. Country Club Drive, Suite 100 in Aventura, Florida. Additionally, the Company also has manufacturing and production sites located in the PRC. All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants or allocates landholders a “land use right.”
The Chemical and Herbal Extracts group has its production base located at Jianou Industrial Development Zone, Sanyuan District, Sanming City, Fujian which comprises approximately 691,258 square feet of manufacturing and production site for all its products.
44
|
Land use right
|
Certificate
|
Square feet
|
||
|
Sanming Sanyuan District Jikou Farm
|
Guoyong (2005) 6238
|
584,694
|
||
|
Sanming Sanyuan District Jikou Farm
|
To be issued
|
106,564
|
||
|
Property
|
||||
|
Main factory
|
708976
|
37,502
|
||
|
Synthetic Building
|
708977
|
33,767
|
||
|
Extracting factory
|
708975
|
19,666
|
||
|
Transformer Room / Bolier Room
|
To be issued
|
3,132
|
||
|
Fertilizer factory
|
To be issued
|
9,957
|
||
|
Land lease right
|
Lessor
|
Acres
|
||
|
Jikou, Sanyuan District, Sanming - Polygonum Cuspidatum
|
Forestry Bureau of Sanming
|
494
|
||
|
Jikou, Sanyuan District, Sanming - Sarcandra Glabra I
|
Forestry Bureau of Sanming
|
1,318
|
||
|
Louyuan Mountain, Sanyuan District, Saming - Sarcandra Glabra II
|
Forestry Bureau of Sanming
|
1,648
|
||
|
Changping Village, Masha County, Jianyang - Tea Tree
|
Jianyang Jiuru Liuyun Tea Co., Ltd.
|
1,318
|
The Organic Product Division has its administrative and manufacturing facility located at Shui Xi Bei Jin Keng, Jianou City, Fujian includes 199,364 square feet. and is the production site for its products.
45
| Jian'ou Lujian Foodstuff Co., Ltd. | ||||
|
Land use right
|
Certificate
|
Square feet
|
||
|
Shui Xi Bei Jin Keng, Jianou, Fujian
|
Ou Guoyong (2004) No.089
|
146,035
|
||
|
Shui Xi Bei Jin Keng, Jianou, Fujian
|
Ou Guoyong (2004) No.090
|
53,329
|
||
|
Property
|
||||
|
Main factory
|
Ou Cheng Zi No. 0171337
|
74,598
|
||
|
Domitory
|
Ou Cheng Zi No. 0171338
|
12,104
|
||
|
Land lease right
|
Lessor
|
Acres
|
||
|
Linkou Village, Yushan Town, Jian'ou
|
Linkou Village
|
10,261
|
||
|
Jixi Village, Yushan Town, Jian'ou
|
Jixi Village
|
6,841
|
||
|
ITEM 3
|
LEGAL PROCEEDINGS
|
None
|
ITEM 4
|
[RESERVED]
|
None
46
PART II
|
ITEM 5
|
MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
“Bid”and “ask” offers for the common stock are listed on the NASDAQ OTC-Bulletin Board published by the National Quotation Bureau, Inc. The Company’s common stock began trading in the second quarter of 2009, under the trading symbol, ‘ONEZ”. The symbol was changed to “ONBI” in connection with the Company’s name change in November 2009.
The following table sets forth the high and low bid prices for the Company’s common stock for the periods indicated as reported by the NASDAQ OTC-Bulletin Board. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions (amounts have been restated to reflect the 1 for 5 reverse split of November 16, 2009 and 1 for 5 reverse split of September 14, 2010).
| Bid Prices | ||||||||
| Quarter Ended | High | Low | ||||||
| Fourth Quarter 2010 | $ | 30.75 | $ | 5.99 | ||||
| Third Quarter 2010 | $ | 30.75 | $ | 30.00 | ||||
We have never declared or paid any cash dividends on our common stock. We currently intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate.
|
ITEM 6
|
SELECTED FINANCIAL DATA
|
Thefollowing selected financial data are derived from our consolidated financial statements and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results” and our consolidated financial statements.
|
Year ended December, 31
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
(Dollars in thousands)
|
||||||||||||
|
Consolidated statement of income data:
|
||||||||||||
|
Revenue
|
$ | 52,296 | $ | 22,073 | $ | 10,402 | ||||||
|
Gross Profits
|
20,125 | 9,984 | 6,462 | |||||||||
|
Net income attributable to Company
|
8,677 | 4,773 | 3,350 | |||||||||
|
Consolidated balance sheet data:
|
||||||||||||
|
Current assets
|
$ | 39,760 | $ | 27,518 | $ | 5,527 | ||||||
|
Long term assets
|
30,325 | 27,116 | 11,315 | |||||||||
|
Total assets
|
70,086 | 54,634 | 16,842 | |||||||||
|
Total Liabilities
|
27,496 | 21,552 | 2,503 | |||||||||
47
ITEM 7 MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS
Results of Operations and Financial Condition
As a result of the reverse acquisition transaction with Green Planet Bioengineering, Ltd. (“GPB”), discussed elsewhere in this report, although legally GPB was acquired by ONE, GPB was considered the accounting acquirer for accounting and financial reporting purposes and therefore the financial reporting of ONE is considered to be a continuation of GPB. During the second quarter fiscal 2010, Elevated Throne and its subsidiaries (“ET” or “CHE”) were absorbed into ONE Bio as a direct subsidiary for consolidation as compared to fiscal year 2009, whereby ET was consolidated via GPB. Our two other acquisitions that occurred during 2009, Trade Finance Solutions (“TFS” or “FIN”) and UGTI group. (“OP”) were accounted for as purchases. Accordingly, the information reported in our consolidated financial statements and in the following discussion and analysis of our operating results for the fiscal year ending December 31, 2010 compared to that of 2009, include the operating results of Elevated Throne consolidated with the operating results of TFS and UGTI from their acquisition dates which are September 2, 2009 and September 27, 2009 respectively. Our CHE business unit consists of ET and its subsidiaries. Our OP business unit consists of UGTI, its acquired subsidiary Supreme and its other subsidiaries. Our FIN business unit consists of TFS and its subsidiaries. Please refer to Note 23 in the notes to our consolidated financial statements included in this 10-K filing for segment information.
Year Ended December 31, 2010 versus December 31, 2009
Operating Revenues
The Company generated $52.3 million of revenues from operations for the year ended December 31, 2010 as compared to $22.1 million from operations for the year ended December 31, 2009, an increase of $30.2 million or 137%. The increase in revenue is primarily the result of organic growth strategies and the accounting for a full year’s operation of the OP and FIN businesses in fiscal 2010 as compared to only the fourth quarter in fiscal 2009.
Approximately $24.3 million of the increase in revenue is due to the full year’s inclusion of revenues from the Company’s completed acquisition of FIN and OP in 2009, which contributed revenues of $21.8 million and $11.2 million respectively for the year ended December 31, 2010. For fiscal 2009, the revenues from these acquisitions are only included from the date of their respective acquisitions both of which occurred in September 2009.
Approximately $5.9 million of the increase in revenues (a 27% increase) is due to the increase in sales in the CHE business unit resulting from the continued focus on driving customer value through all product lines. The major contributor to the increase in sales of our CHE business unit were from new products, Sarcandra ($3.0 million) and Ganoderma Tea ($4.6 million); existing products Resveratrol ($1.9 million) and 5-HTP ($1.1 million). Part of the increase in revenue was offset by a decrease in revenue for the Solanesol and organic fertilizer of approximately $4.7 million from the prior year. In addition, the Company took advantage of a shift in the product and customer mix with a broader product portfolio which contributed to the increase in sales. The Company continues to experience an increase in demand for its broad product portfolio which caters to a higher number of customers. The following table reflects the sales volume by product for the CHE business unit and the acquisitions of the OP and Financing business units since their acquisition date.
48
| 2010 Revenues | 2009 Revenues | |||||||
|
CHE Business Unit
|
||||||||
|
Sarcandra
|
$ | 3,049,000 | $ | - | ||||
|
Ganoderma Tea
|
4,585,000 | - | ||||||
|
Solanesol
|
844,000 | 5,591,000 | ||||||
|
Organic fertilizer
|
361,000 | 2,655,000 | ||||||
|
Resveratrol
|
4,325,000 | 2,369,000 | ||||||
|
5-HTP
|
3,752,000 | 2,614,000 | ||||||
|
Others
|
2,308,000 | 69,000 | ||||||
|
Total
|
$ | 19,224,000 | $ | 13,298,000 | ||||
|
OP Business Unit
|
$ | 21,857,000 | $ | 6,105,000 | ||||
|
FIN Business Unit
|
$ | 11,215,000 | $ | 2,670,000 | ||||
|
Total
|
$ | 52,296,000 | $ | 22,073,000 | ||||
Cost of Sales
Cost of sales from operations for the year ended December 31, 2010 was $32.2 million compared to $12.1 million for the same period in 2009, resulting in an increase of $20.1 million or 166%. The increase in cost of sales is directly attributable to the increase in revenue.
Approximately $16.0 million of the increase in cost of sales is due to the inclusion of costs from the Company’s completed acquisition of Financing and OP business units for a full year in 2010 as compared to 2009 which contributed costs of $9.3 million and $6.6 million respectively since the dates of their respective acquisitions which occurred in September 2009.
Approximately $4.1 million of the increase in cost (a 34% increase) in the CHE business unit is primarily related to the higher net sales both organically and a shift in product mix towards broader product lines. Increases in raw material costs drove an overall decrease in gross profit as a percentage of revenues.
It is significant to note that the recent acquisitions of the Financing and OP business units have lower costs of sale which resulted in higher margins as full implementation in operations were effected.
Gross Profits
Gross profits from operations for the year ended December 31, 2010 were $20.1 million compared to $10.0 million for the same period in 2009, resulting in an increase of $10.1 million or 101%. The increase in gross profit is primarily the result of the acquisition and organic growth strategies.
49
Approximately $8.3 million of the increase in gross profit is due to the contribution of gross profit from the Company’s completed acquisitions of the Financing and OP business units for the year ended December 31, 2010. The acquisitions were overall contributing to the increased consolidated gross profit as a percentage of revenues. The individual gross profit percentages of the CHE, OP and Finance business unit for the year 2010 were 50%, 39% and 18% respectively.
Approximately $1.8 million of the increase in gross profit is primarily a result of the CHE unit’s operating performance. Gross profit from the CHE operations for the year ended December 31, 2010 was $9.6 million compared to $7.7 million for the same period in 2009. Correspondingly, gross profit as a percentage of revenues for the year ended December 31, 2010 was 50% compared to 58% for the same period in 2009. As previously discussed, the decrease is a result of increased raw material costs in its core business and the introduction of new products such as Resveratrol and 5-HTP that contribute lower margins until economies of scales are achieved in the manufacturing process than the traditional core products. Additionally, we have not yet been able to pass along all of our production cost increases to our customers in the form of price increases. The following table reflects the gross margin by business unit and product.
|
Gross margin
|
Gross margin
|
Gross margin
|
||||||
|
2010
|
2009
|
|||||||
|
CHE Business Unit
|
||||||||
|
Sarcandra
|
$ | 1,308,000 | $ | - | ||||
|
Ganoderma Tea
|
2,674,000 | - | ||||||
|
Solanesol from tobacco
|
407,000 | 3,059,050 | ||||||
|
Organic fertilizer
|
268,000 | 2,097,030 | ||||||
|
Resveratrol
|
1,716,000 | 1,052,413 | ||||||
|
5-HTP
|
2,132,000 | 1,541,449 | ||||||
|
Others
|
1,065,000 | 16,448 | ||||||
|
Total
|
$ | 9,570,000 | $ | 7,766,390 | ||||
|
OP Business Unit
|
$ | 8,585,000 | $ | 1,846,000 | ||||
|
FIN Business Unit
|
$ | 1,970,000 | $ | 371,800 | ||||
|
Total
|
$ | 20,125,000 | $ | 9,984,190 | ||||
Operating Expenses from Continuing Operations
Operating expenses for the year ended December 31, 2010 at the business units level were $5.0 million as compared to $1.8 million for the year ended December 31, 2009. This represented an increase of $3.1 million or 172%. Operating expenses comprised general and administrative expenditures, research and development and sales and marketing. The increase in the operating expenses as compared to last year is due to the accounting for the full year in 2010 for the acquisitions completed (FIN and OP business units) at the beginning of the fourth quarter 2009 as against only one quarter expense in 2009. The CHE business recorded a $693,000 increase or 38% over last year as a result of additional expenses in adding new products which contributed to increased revenues of 45% in the CHE business over the corresponding period.
50
Operating Profit
Operating profit for the year ended December 31, 2010 at the business units level was $15.2 million versus $8.2 million for the year ended December 31, 2009, an increase of $7.0 million or 85%. The CHE business unit contributed $1.1 million of the increase for a 14% organic growth over the prior year. The OP and Financing units contributed $5.5 million and $357,000 of the increase for the full year of 2010. The Company continues to focus on the execution of its business plan and with the completed acquisitions of the Financing and OP business units, is positioning for continued organic growth among all product lines and subsidiaries.
Financing and Other Income/Expense
Financing expenses includes the interest expense on the Company’s various financial instruments. For the year ended December 31, 2010 the Company recognized $33,336 in interest income from its various instruments. Other expense incurred during fiscal 2010 of $742,000 includes loan discount costs and a reserve for doubtful debt made during the year. The financing expense of $736,000 for 2010 comprises interest cost for various loans and credit lines provided by financial institutions.
Earnings Before Income Taxes and Non-controlling Interests
For the year ended December 31, 2010, earnings before income taxes and non-controlling interest was $12.2 million versus $7.4 million for an increase of $4.8 million or 65%. The increase is the result of organic growth in the CHE business of $1.1 million (14%) and the contribution of the OP and Financing business units of $5.5 million and $357,000 reduced by the increase in corporate expenses and financing cost of $2.2 million.
Provisions for Income Taxes
For the year ended December 31, 2010, provisions for income taxes were $3.3 million versus $2.0 million for the year ended December 31, 2009 for an increase of $1.3 million or 65%. The increase is due to increased profits in certain jurisdiction that was not offset by the increase in costs that was in different tax jurisdictions. Additionally, the increase is associated with the completed acquisitions being accounted for a full year as compared to a three month period in 2009.
Non-controlling Interest
For the year ended December 31, 2010, non-controlling interest was $279,000 versus $612,000 for the prior year period. The non-controlling was created by ONE acquiring or having less than 100% interest in ET, TFS and UGTI. In addition, ONE acquired more equity interest in ET during the second quarter of 2010 resulting in ET being a 100% subsidiary thereby reducing the non-controlling interest share in the net income of ET.
Net Income
Net income attributable to ONE for the year ended December 31, 2010 was $8.7 million as compared to $4.8 million, an increase of $3.9 million or 81% over the prior year. The increase was attributable to the organic growth in the CHE business unit and the acquisitions of the OP and FIN business units which were accounted for a full year as compared to a three month period in 2009.
51
Liquidity and Capital Resources
As discussed elsewhere in this document, the Company has implemented a two pronged strategy, which is:
|
·
|
to encourage and support organic expansion in and synergies between the enterprises it acquires, and
|
|
·
|
to identify and acquire accretive acquisition targets.
|
To be successful, each strategy requires adequate funding from available liquidity resources. Accordingly, ONE Bio has determined that its organic expansion strategy is first supported through organically generated cash flow and supplemented by available borrowing capacity depending on the requirements of the anticipated expansion project. Any liquidity organically generated in excess of reinvestment needs will be channeled to the accretive acquisition strategy or retained for future expansion projects.
We had working capital of $12.8 million at December 31, 2010. Our operating and capital requirements in connection with supporting our expanding operations and introducing new products have been and will continue to be significant to us. Although we are profitable for the years ended December 31, 2008 to 2010, our growth strategy, which is initially focused on accretive acquisitions and organically expanding our product lines will require substantial capital which we may not be able to satisfy solely through our operations.
Based on our current plans for the next 12 months, we anticipate that additional revenues earned from our expanded product line and broadened distribution channels will be the primary organic source of funds for future operating activities in 2011. However, to fund continued expansion of our product line and extend our reach to broader markets, including international markets, and to acquire additional subsidiaries, we may rely on bank borrowings, if available as well as capital raises. The comments discussed below in “Cash Flows for the Year Ended December 31, 2010 compared to December 31, 2009” section address our organic cash resources and the relevant trends and drivers associated with those cash flows.
Financial Position
Total Assets - Our total assets increased $15.5 million or 28% to $70.1 million as of December 31, 2010 from $54.6 million as of December 31, 2009 primarily as a result of post acquisition earnings after tax and non-controlling interest and assets acquired through our acquisitions.
Property, plant and equipment, net – These assets comprise substantially of buildings and machinery and land improvement relating to the production facilities for agricultural basic raw materials. During the year these assets increased $3.3 million (net of depreciation) to $8.9 million at December 31, 2010. The increase was primarily due to land improvement cost as required every 10 years by the relevant authorities in PRC to enhance the plantation bases for agriculture. A concrete road was also constructed to facilitate the transportation of raw materials in the plantation base. In addition, the Company initiated a plant expansion project which is currently under construction and is expected to complete in mid 2012.
52
Cash and cash equivalents - Include all cash, deposits in banks and other highly liquid investments with initial maturities of three months or less to be cash equivalents. Our cash and cash equivalents as of December 31, 2010 and 2009, by geography are:
|
Location of Cash Deposits
|
2010
|
2009
|
||||||
|
PRC
|
$ | 10,201,249 | $ | 3,741,449 | ||||
|
North America
|
473,737 | 1,187,519 | ||||||
|
Total
|
$ | 10,674,986 | $ | 4,928,968 | ||||
Cash and cash equivalents located in the PRC were denominated in Renminbi (“RMB”) and were placed with financial institutions in the PRC. Typically, they are not freely convertible into foreign currencies and the remittance of these funds out of the PRC is subject to exchange control restrictions imposed by the PRC government. In order to improve the accessibility of our cash on a global scale, we have begun a process of transferring certain assets and processes previously centered in our PRC based VIE entities to our WFOE entities. PRC regulations permit WFOE’s to remit funds out of the PRC.
Cash and cash equivalents located in the North America were primarily denominated in Canadian Dollars and were placed in financial institutions in Canada. These deposits are freely convertible into foreign currencies and there are few if any practical exchange control restrictions imposed by the Canadian government.
The increase in cash and cash equivalents is explained more fully by the following discussion of cash flows.
Cash Flows for the Fiscal Year Ended December 31, 2010 compared to December 31, 2009
Cash Flows from Operating Activities
Operating activities provided net cash of $8.8 million and $4.3 million during the years ended December 31, 2010 and 2009, respectively. A major component of net cash provided by operations was net income of $9.0 million and $5.4 million, respectively for the years ended December 31, 2010 and 2009. In 2010, reported net income included the earnings of its acquired subsidiaries for the full year of $5.8 million as compared to 2009 which was from the point of acquisition of $950,139. This increase in net earnings represents the impact of our various organic growth strategies on net earnings.
53
Other than net income, the following are the significant changes in operating activity components:
|
•
|
a $3.0 million increase resulting from depreciation and amortization expenses recorded;
|
|
•
|
a $(5.5) million increase in accounts receivable as a result of new products, expanded lines, increased sales and broadened customer base along with the normal timing of shipping activities, $5.3 million of the increase in accounts receivable resulted from companies acquired during 2009;
|
|
•
|
a $(1.4) million increase in inventory arising from production in various stages of completion in anticipation of revenue growth in the coming year;
|
|
•
|
a $2.8 million increase in accounts payable as a result of better management of supplier credit arising from expanded lines, increased sales and broadened customer base.
|
Cash Flows used in Investing Activities
Our investing activities used $8.7 million in net cash during the year ended December 31, 2010. Net cash used comprised the following significant items:
|
•
|
a $3.7 million increase in property, plant and equipment primarily due to land improvement cost as required every 10 years by the relevant authorities in PRC to enhance the plantation bases for agriculture. A concrete road was also constructed to facilitate the transportation of raw materials in the plantation base. In addition, the Company initiated a plant expansion project which is currently under construction and is expected to complete in mid 2012;
|
|
•
|
a $6.4 million increase in deposit payments for plantation based leases to secure additional plantation bases. These deposits are prepaid and set off against the lease rentals for the various parcels of land varying from 20 to 25 years;
|
|
•
|
a $(2.2) million decrease in loans receivable from increased collection upon completion of the financing activity. TFS is designed to provide accounts receivable and purchase order financing to third parties. Its loan receivables vary directly with the amount of financing activities under contract.
|
Cash Flows from Financing Activities
Our financing activities provided net cash of $6.2 million during the year ended December 31, 2010. The net cash provided comprised the following significant items:
|
•
|
proceeds from the issue of bridge loan of $3.0 million;
|
|
•
|
proceeds from bank loans of $3.9 million;
|
|
•
|
proceeds from loans payable of financing subsidiary of $1.6 million;
|
|
•
|
proceeds from the issue of shares of $390,000
|
|
•
|
advances from stockholders and related parties of $831,000; and
|
|
•
|
repayment of bank loans of $3.3 million.
|
54
Foreign Currency Translation
The Company’s operating entities under the CHE and OP business units maintain their financial records in the functional currency of the PRC, which is the “Renminbi” (RMB), the currency of the primary economic environment in which the entities operate. The Company’s other operating entities under the FIN business unit maintains its financial records in the functional currency of Canada, the Canadian Dollar (“CAD”). For financial reporting purposes, the financial statements are prepared using the functional currency RMB or CAD, which have been translated into United States Dollars. Assets and liabilities are translated at the exchange rates at the balance sheet dates, revenue and expenses are translated at the average exchange rates and stockholders’ equity is translated at historical exchange rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustment to other comprehensive income, a component of stockholders’ equity.
| Exchange Rates | 12/31/2010 | 12/31/2009 | ||||||
| Fiscal period/year end RMB: US$ exchange rate | 6.61 | 6.83 | ||||||
| Average period/yearly RMB: US$ exchange rate | 6.77 | 6.83 | ||||||
Significant Estimates
Critical accounting polices include the areas where we have made what we consider to be particularly subjective or complex judgments in making estimates and where these estimates can significantly impact our financial results under different assumptions and conditions.
We prepare our financial statements in conformity with accounting principles generally accepted in the United States of America. As such, we are required to make certain estimates, judgments and assumptions that we believe are reasonable based upon the information available. These estimates, judgments and assumptions affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the periods presented. Actual results could be different than those estimates
Off-Balance Sheet Arrangements
Our financial statements include consolidated majority owned subsidiaries and consolidated VIE’s of which we are the primary beneficiary.
We do not have any other off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Critical Accounting Policies
The Company’s consolidated financial statements were prepared in accordance with generally accepted accounting principles, which require management to make subjective decisions, assessments and estimates about the effect of matters that are inherently uncertain. As the number of variables and assumptions affecting the judgment increases, such judgments become even more subjective. While management believes its assumptions are reasonable and appropriate, actual results may be materially different from estimated. Management has identified certain critical accounting policies, described below, that require significant judgment to be exercised by management.
55
Revenue recognition
Revenue is recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured.
Revenue is recognized when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable.
Accounting for warrants
The fair value of warrants granted is determined using the Black-Scholes model. Under this model, certain assumptions, including the risk-free interest rate, the expected life of the warrants and the estimated fair value of the Company’s common stock and the expected volatility, are required to determine the fair value of the warrants. If different assumptions had been used, the fair value of the warrants would have been different from the amount the Company computed and recorded, which would have resulted in either an increase or decrease in the compensation expense
Income Taxes
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC Topic 740 formerly SFAS No. 109, “Accounting for Income Taxes”. Under the asset and liability method of SFAS 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carry-forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Impairment of long-lived assets
Long-lived assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets. During the reporting periods, the Company has not identified any indicators that would require testing for impairment.
Accounts receivable
Accounts receivable is stated at cost, net of allowance for doubtful accounts. The Company maintains allowances for doubtful accounts for estimated losses resulting from the failure of customers to make required payments. The Company establishes an allowance for doubtful accounts based on management’s assessment of the collectability of trade and other receivables. A considerable amount of judgment is required in assessing the amount of the allowance. The Company considers the historical level of credit losses and applies percentages to aged receivable categories. The Company makes judgments about the creditworthiness of each customer based on ongoing credit evaluations, and monitors current economic trends that might impact the level of credit losses in the future. If the financial condition of the customers were to deteriorate, resulting in their inability to make payments, a larger allowance may be required.
Market Risks
The Company operates in the United States and other countries that have their own currency. This may cause the Company to experience and be exposed to different market risks such as changes in interest rates and currency deviations.
56
|
ITEM 8
|
FINANCIAL STATEMENTS
|
The financial statements and report of an independent registered certified public accounting firm are included herein immediately following the signature page of this report.
|
ITEM 9
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
None
|
ITEM 9A
|
CONTROLS AND PROCEDURES
|
Disclosure Control and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in company reports filed or submitted under the Securities Exchange Act of 1934, or the “Exchange Act,” is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include without limitation, controls and procedures designed to ensure that information required to be disclosed in company reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our chief executive officer as appropriate to allow timely decisions regarding disclosure.
The Company’s management with the participation of the Company’s Chief Executive Officer and Chief Financial Officer evaluated the effectiveness of the Company’s disclosure controls and procedures as of December 31, 2010. Based upon this evaluation, the Company’s Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures were effective and did not note any material weakness.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate “internal control over financial reporting” as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting refers to the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
|
i.
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
|
|
ii.
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
iii.
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
|
57
As of December 31, 2010 and as reported in the Registrant’s Form 10-K filing, management used the framework set forth in the report entitled “Internal Control – Integrated Framework” published by the Committee of Sponsoring Organizations of the Treadway Commission to evaluate the effectiveness of our internal control over financial reporting. Based on its evaluation, management concluded that at December 31, 2010 there was no material weakness and concluded that the internal control over financial reporting was effective. A material weakness is a deficiency, or a combination of control deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
During the most recently completed fiscal quarter, there has been no change in our internal control over financial reporting that has materially affected or is reasonably likely to materially affect, our internal control over financial reporting.
PART III
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
Generally, each of our directors is elected to a term of one year and serves until his or her successor is elected and qualified. The Directors and Officers of the Company are as follows:
|
Name
|
Age
|
Position
|
Term
|
|||
|
Michael Wiengarten
|
56
|
Chairman of the Board and Director
|
August 2009 – Present
|
|||
|
Marius Silvasan
|
38
|
CEO/Vice Chairman and Director
|
September 2009 – Present
|
|||
|
Cris Neely
|
54
|
CFO and Director
|
August 2009 – Present
|
|||
|
Min Zhao
|
46
|
President China Operations and Director
|
July 2009 – Present
|
|||
|
Qingsheng Fen
|
48
|
Independent Director
|
January 2010 – Present
|
|||
|
James Fernandes
|
40
|
Independent Director
|
January 2010 – Present
|
|||
|
Frank Klees
|
60
|
Independent Director
|
January 2010 – Present
|
|||
|
Jan E. Koe
|
61
|
Independent Director
|
January 2010 – Present
|
|||
|
John Perkins
|
46
|
Independent Director
|
January 2010 – Present
|
Michael S. Weingarten has served as Chairman of the Board of Directors since August 26, 2009. He is a seasoned executive and entrepreneur with over 30 years of experience in distribution, manufacturing and managing companies from early stage to multimillion dollar corporations worldwide. Mr. Weingarten has extensive experience in mergers and acquisitions, having participated in more than 40 such transactions throughout his career. Mr. Weingarten has served as Chairman, CEO and President of both publicly traded and privately owned companies. Mr. Weingarten devotes substantially his full attention to the affairs and day-to-day operations of the Company, although he also currently serves as Chairman of our Trade Finance Solutions Inc. subsidiary, and as Chairman of AMW Latin America Inc. a computer and technology distribution firm located in Doral, Florida, and Keda Consulting Services, Inc., a consulting firm based in Toronto, Ontario, Canada. From 1999 to 2002, he served as the Chairman and Chief Executive Officer for Commercial Consolidators, Corp., a Toronto, Ontario, Canada, based financing firm. From 1997 to 1999, Mr. Weingarten was the Chairman and Chief Executive Officer for Complete Tele-Management, Corp. a telecom firm located in Toronto, from 1994 to 1996, he served as the Chairman and Chief Executive Officer of the Preferred Management, Corp. and Consumer Telephone, Corp. from 1991 to 1993. Mr. Weingarten was the CEO of Network Business Supply, Inc. from 1979 to 1991 and Majestic Paper from 1982 to 1991.
58
Marius Silvasan, has served as our Vice-Chairman, Chief Executive Officer since September 1, 2009, and as, President and Director since June 9, 2009. Mr. Silvasan is a senior executive with over 17 years of experience in business development, M&A, marketing, sales, finance and distribution. Mr. Silvasan has raised capital to fund various acquisitions and assist with organic growth. In addition, Mr. Silvasan has over 5 years experience in investing in China in developing and fast growing Agritech and technology companies. Mr. Silvasan devotes substantially his full attention to the affairs and day-to-day operations of the Company, although he also is currently the Chairman of Abacus World, Corp. and Abacus Global Investments, Corp. located in Miami, Florida, which provides business and financial consulting services to business in the Asian Pacific region. Mr. Silvasan also founded and served as Chairman and Chief Executive officer and director of TelePlus World, Corp (NASDAQ: TLPE) from 1998 to 2009. Earlier in his career, Mr. Silvasan also held the positions of President and CEO for Visioneer Calling Card Inc. and Alliance TeleCard Corp. and National Sales Manager for The Home Phone Club. Mr. Silvasan is a graduate of HEC University in Montreal, and holds an undergraduate degree in business (1995) and an MBA (2003).
Cris Neely, has served as our Chief Financial Officer and Director, since August 11, 2009. Mr. Neely has an over 14 year extensive financial experience as a CFO of public and private corporations with domestic as well as foreign subsidiaries. Previously, from 2006 to 2009, he was the Chief Financial Officer of TelePlus World, Corp. (NASDAQ: TLPE). From 1999 to 2005, Mr. Neely was the Chief Financial Officer of Siemens Enterprise Networks located in Boca Raton, Florida. He also held various other executive positions with Siemens Enterprise Networks including Senior Vice President Business Transformation, Director Internal Audit, Director of Finance for Wireless Terminals and Area Financial Manager. Mr. Neely has also held management positions with ROLM, IBM and Cisco during his career. After leaving Siemens in 2005, Mr. Neely worked as a consultant for small/medium organizations focusing on Sarbanes-Oxley compliance, revenue recognition and financial/operational business assessments. Mr. Neely holds a Bachelor of Business Administration Finance degree from the University of Texas at Arlington, Texas and an MBA from Amberton University, Dallas, Texas.
Min Zhao, has served as a Director and as President of our China operations and as a director since June 30, 2009. Min Zhao is the founder and currently the President of Sanming, our CHE business unit’s contractually controlled PRC operating entity. As President of our China Operations, Mr. Zhao is in charge of and devotes substantially his full attention to the affairs and day-to-day operations of Sanming. He is a pioneer in the agritech and nutraceutical industry in China with more than 10 years of experience. Mr. Zhao has served in senior positions in companies ranging from general manager to chairman. Mr. Zhao has over 20 years of business experience leading various types of companies since graduating from the Chinese People’s Liberation Army University.
Mr. Fan is a researcher specialized in traditional Chinese Medicine and organic and health food research. Mr. Fan’s research encompassed research & development on health food particularly in the preparation of healthy products from different natural plants. Mr. Fan has published 8 books and more than 60 papers in periodicals in China and abroad. Mr. Fan, as chief editor, edited one of the first systematic practical series books in China about health food according to new health food registration management measure. For the last 10 years, Mr. Fan has been a Professor at the Education Ministry food science key laboratory at Nanchang University. Mr. Fan has led 8 national and provincial appraised research projects, and presided over more than 200 health food project appraisals by the National Food and Drug Supervising Administration of China. These projects have been put into production all over China and have yielded significant benefits to over 40 companies. Prior to that Mr. Fan worked as a senior visiting researcher at the Pennsylvania State University, studying the anticancer effects of food. Before that Mr. Fan worked in the Central Laboratory at Jiangxi College of Traditional Chinese Medicine where he pursued his master’s degree in immunity pharmacology at the Shanxi College of Traditional Chinese Medicine. Prior to earning his Master’s degree, Mr. Fan graduated from the Jiangxi College of Traditional Chinese Medicine. Mr. Fan’s research and experience in traditional Chinese Medicine and organic and health food are valuable assets to ONE Bio. Mr. Fan has served as an independent member of our Board of Directors since January 12, 2010.
Mr. Fernandes is an experienced banker, fund manager and accounting professional. Mr. Fernandes is an active private investor in a diversified portfolio of private and publicly traded companies. Among other assignments, Mr. Fernandes is currently the Chairman and President of Solana Capital Solutions, a provider of growth financing for privately held companies in the retail, industrial, and consumer services sectors. Previously, Mr. Fernandes was a senior equity analyst and investment manager with Lazard Asset Management from 2007 to 2009 and Allianz Global Investors from 2005-2007 and Independence Investments LLC from 2002-2005. Prior to Independence Investments, Mr. Fernandes provided financial advisory services for privately held companies at Devland Financial. Mr. Fernandes started his investment management career with TD Asset Management, the investment management division of one of Canada’s largest banks, TD Bank, in 1996. Mr. Fernandes holds a bachelor’s degree in accounting from York University and an MBA from Cornell University’s Johnson Graduate School of Management (2002). He is also a Certified Public Accountant and Chartered Financial Analyst. Mr. Fernandes’ investment banking experience combined with his strong business and finance acumen make him a valuable board member for ONE Bio. Mr. Fernandes has served as an independent member of our Board of Directors since January 12, 2010.
59
Mr. Klees is an accomplished business man, highly regarded and influential politician and active member of the board of several publicly traded corporations. Mr. Klees over the years has developed strong contacts in the Asia-Pacific region. Among several assignments, Mr. Klees currently sits on the boards of Roxul Inc., Northern Ethanol Inc., Universal Energy, Tribute Resources, and National Medical Imaging. In 1990, Mr. Klees co-founded the Municipal Gas Corporation where he served as the company’s Executive Vice President with responsibilities for business development and government relations. Mr. Klees began his business career in financial services in 1974 with the Canada Life Assurance Company, headquartered in Toronto, Canada. Mr. Klees further developed a financial services practice which further expanded into contract negotiations and investment advisory services. In June of 1995, Mr. Klees was elected to the Ontario Legislature, where he has served in numerous senior government positions, including Chief Government Whip, Deputy House Leader, Minister of Tourism and Minister of Transportation. He is currently the Official Opposition Critic for the Ministry of Transportation and the Ministry of Public Infrastructure Renewal. In his over 15 year tenure in the government, Mr. Klees developed strong contacts in the Asia-Pacific region that cover multiple fronts. Mr. Klees continues to provide advisory services to public and private companies, drawing on his extensive business, administration and government experience. Mr. Klees’ extensive and diverse business and public experience combined to his large network of contacts makes Mr. Klees a valuable asset to ONE Bio. Mr. Klees has served as an independent member of our Board of Directors since January 12, 2010.
Mr. Koe is a seasoned investor and principal in several US based private and publicly traded companies. Mr. Koe’s investments cover firms invested in real-estate, agritech and construction. Mr. Koe is a principal of Method K Partners, Inc., a commercial real estate firm located in Arlington Heights, Illinois which he founded in 1988 and GoStar, Inc. a real estate consulting firm which he founded in 2005. Mr. Koe has 30 years of experience in consulting, asset management, leasing and development working with many national real estate firms including Golub & Co., Draper & Kramer Co., Rauch & Co. and Manufacturers Real Estate, a wholly owned affiliate of Manufactures Life Insurance Company. Among other assignments Mr. Koe’s currently provides a variety of real estate services to the medical industry including serving as a real estate consultant for prominent Chicago area medical institutions. Mr. Koe is also a principal in Chicago based, Paving Solutions LLC. Mr. Koe holds a Bachelors degree in Business Administration and Psychology from Luther College in Decorah, Iowa. Mr. Koe’s business acumen and experience in real-estate are valuable assets to ONE Bio as it grows its manufacturing capacity and real-estate foot print to service the worldwide demand for its products. Mr. Koe has served as an independent member of our Board of Directors since January 12, 2010.
Mr. Perkins is currently Senior director, Northern and Southern Europe for Apple Inc. Mr. Perkins held other senior management positions including, VP or Business development, Worldwide Television/Video Division, Thomson Consumer Electronics, co-CEO and co-founder of Egencia, an internet start-up that was acquired by Expedia Inc. and director of Marketing and sales, Southern Europe for Dell Inc. Mr. Perkins was also a consultant for Bain & Company. Mr. Perkins has a MBA from the Wharton school of business as well as an undergraduate business degree (BBA) from Wilfrid Laurier University. Mr. Perkins has served as an independent member of our Board of Directors since January 16, 2010.
Director Compensation
All Directors of the Company will hold office until the next annual meeting of the shareholders, and until their successors have been elected and qualified. Officers of the Company are elected by the Board of Directors and hold office at the pleasure of the Board.
We enter into an Independent Director Agreement with each of our independent directors. Effective January 12, 2010 we entered into independent Directors Agreements with each of Messrs. Fan, Fernandes, Klees and Koe and on January 15, 2010 with Mr. Perkins. Pursuant to the Independent Director Agreements, we have agreed to compensate each of our independent director as follows: (a) on the date of the execution of each Independent Director Agreement, as a bonus for agreeing to serve as an independent director, we agreed to issue to each independent director (i) five hundred (500) shares of our common stock and (ii) a five (5) year option to purchase two thousand (2,000) shares of our common stock at an exercise price equal to the fair market value of a share of the common stock on the date of grant, which vests quarterly at the end of each calendar quarter in equal installments over the 12 month period during the term of the respective Independent Director Agreement; and (b) for services rendered by the respective independent director we granted to each such director (i) two hundred fifty (250) shares of our common stock for each quarter for with the independent Director has served as an independent director, which shares will be issued at the end of each such quarter and (ii) a five (5) year option to purchase one thousand (1,000) shares of our common stock at an exercise price equal to the fair market value of a share of the common stock on the date of grant, which vests quarterly at the end of each calendar quarter in equal installments over the 12 month period during the term of the respective Independent Director Agreement. We also agreed to reimburse our independent directors for certain expenses they incur in performing their duties as independent directors and to indemnify our independent directors except for matters in which the independent director (i) failed to act in good faith and in a manner he/she reasonably believed to be in or not opposed to the best interests of the Company, (ii) had reasonable cause to believe that his/her conduct was unlawful or (iii) where his/her conduct constituted willful misconduct, fraud or knowing violation of law.
60
Involvement in Legal Proceedings
None of our executive officers or directors have been the subject of any order, judgment, or decree of any court of competent jurisdiction, or any regulatory agency permanently or temporarily enjoining, barring suspending or otherwise limiting him from acting as an investment advisor, underwriter, broker or dealer in the securities industry, or as an affiliated person, director or employee of an investment company, bank, savings and loan association, or insurance company, or from engaging in or continuing any conduct or practice in connection with any such activity or in connection with the purchase or sale of any securities.
None of our executive officers or directors has been convicted in any criminal proceeding (excluding traffic violations) or is the subject of a criminal proceeding that is currently pending.
None of our executive officers or directors is the subject of any pending legal proceeding.
Audit Committee
Mr. Fernandes serves as the Chairman and along with Mr. Perkins on the Company’s Audit Committee. The Audit Committee reports to the Board of Directors regarding the appointment of our independent registered public accounting firm, the scope and results of our annual audit, compliance with our accounting and financial policies and management’s procedures and policies relative to the adequacy of our internal accounting controls.
Compensation Committee
Mr. Koe serves as the Chair of the Company’s Compensation Committee with oversight of the employee stock option plan. The Compensation Committee reports to the Board of Directors regarding officer and director compensation arrangements, employee stock option plans, policies and programs of the Company and administer the Company’s equity-based compensation plans for all employees.
Corporate Governance and Nominating Committee
Mr. Klees serves as the Chair of the Company’s Corporate Governance and Nominating Committee. The Corporate Governance and Nominating Committee reports to the Board of Directors regarding the compliance with governance initiatives and nomination of Board members and key officer positions.
Section 16 (A) Beneficial Ownership Reporting Compliance
Section 16 (A) of the Securities Exchange Act of 1934, as amended, requires the Company’s directors, executive officers and persons who own more than 10% of a class of the Company’s equity securities which are registered under the Exchange Act to file with the Securities and Exchange Commission initial reports of ownership and reports of changes of ownership of such registered securities. Such executive officers, directors and greater than 10% beneficial owners are required by Commission regulation to furnish the Company with copies of all Section 16 (A) forms filed by such reporting persons.
To the Company’s knowledge, based solely on a review of the copies of such reports furnished to the Company and on representations that no other reports were required, no person required to file such a report, failed to file during fiscal 2010 and 2009.
Code of Ethics
The Board of Directors adopted a Code of Ethics in September 2009, meeting the requirements of Section 406 of the Sarbanes-Oxley Act of 2002. The Company will provide to any person without charge, upon request, a copy of such Code of Ethics.
61
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
The following is a summary of the compensation paid by ONE Bio, Corp to its Chief Executive Officer’s for the years ended December 31, 2010 and December 31, 2009. There was no compensation paid for these positions for 2008.
[Missing Graphic Reference]
(1) John Corn resigned effective June 2009 with the acquisition of the company by Abacus Global Investments.
Stock Option Awards
The Company’s 2010 Stock Option Plan (“the “Plan”) provides for the issuance of a maximum of 4,500,000 of the issued and outstanding common shares at an exercise price equal to or greater than the market price of the Company’s common shares on the date of the grant to directors, officers, employees and consultants to the Company. The option period for options granted under the Plan is for a maximum period of 5 years. Options granted may be vested over certain time periods within the option period, which will limit the number of options that may be exercised. Each stock option is exercisable into one common share of the Corporation at the price specified in the terms of the option.
In January 2010 the Company issued 15,000 and 500 options at an exercise price of $30.45 and $30.00 per share respectively with a vesting period of 5 years to its non-employee directors. In September 2009, the Company issued 534,000 options to its management staff at exercise prices ranging from $15.00 to $18.75 per share with varied vesting schedules. No options were issued during 2008.
As of September 30, 2010, the 534,000 options issued to its management staff were cancelled.
The following table sets forth information with respect to stock awards and grants of options to purchase our common stock outstanding to the named executive officers which were granted at the time of employment.
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
|
Name of Beneficial Owner
|
Number of Shares
of Common Stock
Beneficially
Owned(1)
|
Beneficially
Owned
%
|
||||||
|
ONE-V Group LLC(3)
|
1,859,000 | 28 | % | |||||
|
Michael Weingarten
|
1,116,200 | 17 | % | |||||
|
Jeanne Chan(4)
|
390,108 | 6 | % | |||||
|
Min Zhao(5)
|
544,806 | 8 | % | |||||
|
Jin Rong Tang
|
408,769 | 6 | % | |||||
|
Marius Silvasan(3)(4)
|
47,529 | 1 | % | |||||
|
Abacus Global Investment (3)
|
96,015 | 1 | % | |||||
|
Cris Neely
|
22,384 | * | ||||||
|
Sally Ou(5)
|
13,933 | * | ||||||
|
Jan Koe(6)
|
60,450 | 1 | % | |||||
|
Quingsheng Fan(6)
|
1,250 | * | ||||||
|
Frank Klees(6)
|
1,250 | * | ||||||
|
James Fernandes(6)
|
46,050 | * | ||||||
|
John Perkins(6)
|
146,850 | 4 | % | |||||
|
All Directors and Executive
|
4,754,594 | 71 | % | |||||
|
Officers as a group
|
||||||||
_______________
* Less than one percent
|
(1)
|
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock subject to securities anticipated to be exercisable or convertible at or within 60 days of the date hereof, are deemed outstanding for computing the percentage of the person holding such option or warrant but are not deemed outstanding for computing the percentage of any other person. The indication herein that shares are anticipated to be beneficially owned is not an admission on the part of the listed stockholder that he, she or it is or will be a direct or indirect beneficial owner of those shares.
|
62
|
(2)
|
Based upon 6,696,417 shares of common stock issued and outstanding as of December 31, 2010.
|
|
(3)
|
Beneficially owns 1,611,000 shares indirectly through ONE-V Group, LLC which is wholly owned by Mr. Silvasan and 96,015 shares owned indirectly through Abacus Global Investments, Corp. of which Mr. Silvasan is the sole director and officer and controlling stockholder
|
|
(4)
|
Mr. Silvasan and Ms. Chan are husband and wife.
|
|
(5)
|
Mr. Min Zhao, our President, China operations and Director, and Ms. Sally Ou, our Vice President of Business Development, China, are husband and wife.
|
|
(6)
|
Messrs. Koe, Fan, Klees and Fernandes were appointed to the Company’s Board of Directors on January 12, 2010, and Mr. Perkins was appointed to the Company’s Board of Directors on January 15 2010, and we entered into agreements with each of them pursuant to which we agreed to issue to each of them 1,500 shares of our common stock and options to purchase shares of our common stock of the Company. As of the date of this filing all of the options were vested. See “Compensation of Directors”.
|
Change in Control
The Company does not anticipate any changes in control of the Company
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
Certain Relationships and Related Transactions, and Director Independence
On April 14, 2010, we acquired from Min Zhao, the President and a director in Green Planet , 1,632,150 shares of common stock of Green Planet through the issuance of 260,000 shares (post split) of common stock in ONE.
Up to and until April 14, 2010, Green Planet is the umbrella entity for our chemical and herbal extracts division, which owns 100% of Elevated Throne and Fujian Green Planet Bioengineering Co., Ltd. (“Fujian G.P.”), the WFOE organized under the laws of the PRC. Fujian G.P., through a series of contractual arrangements has effective control of the business and operations of and has an irrevocable option to purchase the equity and/or assets of Sanming Huajian Bio-Engineering Co., Ltd., a PRC company, which is a green process manufacturer of high quality health supplements, organic fertilizers and pesticides.
As described in this document, ONE exercised an option to acquire Elevated Throne from Green Planet, thereby Elevated Throne became a 100% direct subsidiary of ONE effective April 14, 2010.
Review, Approval or Ratification of Transactions with Related Parties
The terms of the transactions described above were negotiated on an arm’s length basis. Pursuant to the terms of each respective transaction, the above named principal of the acquired target company became a related party. Accordingly, at the time of the approval of each respective transaction by our Board of Directors, that person was not a member of our Board of Directors. We have adopted a policy that requires the review and approval of related party transactions by our independent directors.
63
Director Independence
On January 12, 2010, our Board of Directors of the Company appointed Messrs. Koe, Fan, Klees and Fernandes and on January 15, 2010, appointed Mr. Perkins (the “Independent Directors”) to serve as independent directors. Each of Messrs. Fan, Fernandes, Klees, Koe and Perkins are independent as defined by Rule 5605(a)(2) of the Marketplace Rules of The Nasdaq Stock Market, Inc., as well as under Section 303A.02 of the NYSE, Amex Listed Company Manual (the “Independent Directors”).
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
AuditFees
The aggregate fees billed (or expected to be billed) for each of the fiscal years ended December 31, 2010 and 2009 for professional services rendered by the principal accountant for the audit of the Company’s annual financial statements was $169,500 and $110,000 respectively.
The aggregate fees billed for the fiscal year ended December 31, 2010 for professional services rendered by the principal accountant for the reviews of the Company’s quarterly financial statements included in the Company’s Form 10-QSB’s was approximately $15,000.
Audit Related Fees
None
Tax Fees
The aggregate fees billed (or expected to be billed) for each of the fiscal years ended December 31, 2010 and 2009 for professional services rendered by the principal accountant for tax services of the Company’s annual tax filing was $20,000 and $20,000 respectively
All Other Fees
None
64
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
| (a) | Exhibits | |
| 31.1 Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||
| 31.2 Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||
| 32.1 Certification pursuant to 18 U.S.C. Section 1350 | ||
| 32.2 Certification pursuant to 18 U.S.C. Section 1350 |
| (b) | Reports on form 8-K |
| The Company filed the following report on Form 8-K during the year for which the report is filed.
1. Form 8K filed on January 12, 2010 announcing the appointment of Qingsheng Fan, James Fernandes, Jan Koe and Frank Klees to the Company’s Board of Directors.
2. Form 8K filed on January 19, 2010 announcing initial closing of a financing transaction with UTA Capital and Udi Toledino. Additionally, the Company announced the addition of John Perkins to the Board of Directors.
3. Form 8K filed on January 25, 2010 announcing American Stock Transfer as the new transfer agent for the Company. Additionally, the Company announced its new address in Aventura Florida.
4. Form 8K filed on February 17, 2010 announcing its issuance of $3,000,000 of the Company’s secured 8% convertible notes.
5. Form 8K filed on April 19, 2010 announcing entering into a share Purchase Agreement with Min Zhao for the purchase of 1,632,150 shares of common stock in Green Planet. Additionally, the Company announced the cancellation of the certain Amended and Restated Green Planet Preferred Stock Purchase Agreement made effective as of June 17, 2009; and, announced Green Planet granted the Company an option to acquire 100% of the stock of Elevated Throne Overseas Ltd.
6. Form 8K/A filed on July 29, 2010, incorporating exhibits (a) transfer of 1,632,150 shares in Green Planet to the Company by seller, Min Zhao; (b) amended and restated Articles of Incorporation of Contracted Services, Inc; (c) articles of amendment to Articles of Incorporation of Contracted Services, Inc; (d) articles of amendment to Articles of Incorporation of ONE Holdings, Corp; (e) officer’s certificate certifying sale of 1,631,150 shares by Min Zhao in green Planet to the Company; (f) unanimous written consent by the Company’s directors approving, authorizing and ratifying the purchase of 1,632,150 shares in Green Planet from Min Zhao, cancellation of the certain Amended and Restated Green Planet Preferred Stock Purchase Agreement made effective as of June 17, 2009, and an option to acquire 100% of the stock of Elevated Throne Overseas Ltd.; (g) certification of the Articles of Incorporation and Bye-Laws of Green Planet by the CEO of the Company; and (i) unanimous written consent by the Company’s directors approving, authorizing and ratifying the cancellation of the certain Amended and Restated Green Planet Preferred Stock Purchase Agreement made effective as of June 17, 2009.
|
65
|
7. Form 8K filed on August 18, 2010, announcing the entering of a Loan Extension and Modification Agreement with an effective date of June 30, 2010, with UTA Capital and several other investors.
8. Form 8K filed on August 27, 2010, requesting withdrawal of its registration statement for a proposed secondary offering with the Securities and Exchange Commission;
9. Form 10K filed on September 14, 2010, announcing that FINRA would process the Company’s 1 for 5 reverse stock split as disclosed in the Company’s Definitive Information Statement of July 27, 2010.
10. Form 8K filed on November 23, 2010, announcing the entering of an agreement with Michael Weingarten, the Company’s Chairman and ONE-V Group, LLC for the cancellation of the 10,000 shares of the Company’s Series A Preferred Stock previously issued to them upon the closing of the Company’s pending public stock offering.
11. Form 8K filed on January 7, 2011 announcing that on December 20, 2010, ONE entered into a loan extension agreement with an effective date of December 10, 2010, with UTA Capital and other investors to extend and modify the securities purchase agreement to January 31, 2011.
12. Form 8K filed on January 25, 2011 announcing that the Company had filed with the Securities and Exchange Commission a request to withdraw its registration statement regarding a proposed offering of shares of its common stock.
13. Form 8K filed on February 4, 2011 announcing that the Company had delivered written notice on December 20, 2010 to Green Planet Bioengineering Co., Ltd. of the exercise of the option to acquire 100% of the stock of Elevated Throne Overseas Ltd. Additionally, the Company as the 92.4% owner of Green Planet outstanding common stock, by majority shareholder written consent in lieu of a special meeting of the stockholders, approved, authorized and ratified the transaction contemplated by the option agreement and the exercise by the Company of the option retroactive to April 14, 2010.
14. Form 8K filed on March 9, 2011, announcing that effective January 31, 2011, the Company has entered into a third loan extension agreement with UTA Capital and other investors to extend the Securities Purchase Agreement to April 1, 2011 with an option to further extend to May 1, 2010 and June 1, 2011. Additionally, as of February 28, 2011 the Company entered into a consulting agreement with the investors to provide certain consulting services to us until November 10, 2011
|
66
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized this 31st day of March, 2011.
| ONE Bio, CORP. | |||
|
|
By:
|
/s/ Marius Silvasan | |
| Marius Silvasan | |||
| Chief Executive Officer | |||
67
: Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Date: March 31, 2011 |
By:
|
/s/ Michael Wieingarten | ||
|
Michael Weingarten
|
||||
|
Chairman of the Board and Director
|
||||
| Date: March 31, 2011 | By: | /s/ Marius Silvasan | ||
|
Marius Silvasan
|
||||
|
Chief Executive Officer and Director
|
||||
| Date: March 31, 2011 | By: | /s/ Cris Neely | ||
|
Cris Neely
|
||||
|
Chief Financial Officer
|
||||
| Date: March 31, 2011 | By: | s/ Min Zhao | ||
|
Min Zhao
|
||||
| Director | ||||
| Date: March 31, 2011 | By: | /s/ Qingsheng Fan | ||
|
Qingsheng Fan
|
||||
|
Director
|
||||
|
Date: March 31, 2011
|
By: | /s/ James Fernandes | ||
| James Fernandes | ||||
|
Director
|
||||
| Date: March 31, 2011 | By: | /s/ Frank Klees | ||
| Frank Klees | ||||
| Director | ||||
| Date: March 31, 2011 | By: | /s/ Jan Koe | ||
|
Jan Koe
|
||||
|
Director
|
||||
| Date: March 31, 2011 | By: | /s/ John Perkins | ||
| John Perkins | ||||
| Director |
68
ONE BIO, CORP.
(PREVIOUSLY CONTRACTED SERVICES, INC.)
CONSOLIDATED
FINANCIAL STATEMENTS
FOR THE YEAR ENDED DECEMBER 31, 2010
69
ONE BIO, CORP.
(PREVIOUSLY CONTRACTED SERVICES, INC.)
(Expressed in United States Dollars)
DECEMBER 31, 2010
CONTENTS
| Consolidated Financial Statements: | Page | |||
| Consolidated Balance Sheets | 71 | |||
| Consolidated Statements of Income and Comprehensive Income | 72 | |||
| Consolidated Statements of Cash Flows | 73 | |||
| Consolidated Statements of Changes in Shareholders’ Equity | 74 | |||
| Notes to the Consolidated Financial Statements | 75 | |||
70

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
One Bio, Corp.
We have audited the accompanying consolidated balance sheets of One Bio, Corp. as of December 31, 2010 and 2009 and the related consolidated statements of income and comprehensive income, changes in shareholders' equity, and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of One Bio, Corp. as of December 31, 2010 and 2009 and the results of its consolidated operations and its consolidated cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ JEWETT, SCHWARTZ, WOLFE & ASSOCIATES
Hollywood, Florida
March 31, 2011
|
One Bio, Corp.
|
||||||||
|
Consolidated Balance Sheets
|
||||||||
|
(Stated in US dollars)
|
||||||||
|
As of December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 10,674,986 | $ | 4,928,968 | ||||
|
Receivables, net
|
18,009,266 | 12,006,585 | ||||||
|
Inventory
|
4,505,207 | 2,976,031 | ||||||
|
Loans Receivable
|
2,044,038 | 4,223,863 | ||||||
|
Prepaid expenses
|
4,526,953 | 3,382,882 | ||||||
|
Total current assets
|
39,760,450 | 27,518,329 | ||||||
|
Property, plant and equipment, net
|
8,873,632 | 5,557,911 | ||||||
|
Land use rights
|
1,116,216 | 1,106,056 | ||||||
|
Goodwill
|
1,596,569 | 3,974,908 | ||||||
|
Intangible assets
|
823,668 | 682,058 | ||||||
|
Deposits for acquisition of intangible assets
|
355,425 | 161,151 | ||||||
|
Other Assets
|
17,559,545 | 15,633,438 | ||||||
|
TOTAL ASSETS
|
$ | 70,085,505 | $ | 54,633,851 | ||||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY
|
||||||||
|
LIABILITIES
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$ | 4,455,552 | $ | 1,699,783 | ||||
|
Other payables and accrued liabilities
|
3,064,653 | 2,632,190 | ||||||
|
Loans payable- current portion
|
19,122,823 | 12,868,796 | ||||||
|
Deferred revenues
|
65,035 | 62,995 | ||||||
|
Deferred taxes
|
215,589 | (29,192 | ) | |||||
|
Total current liabilities
|
26,923,652 | 17,234,572 | ||||||
|
Loans payable
|
592,198 | 4,215,855 | ||||||
|
Deferred taxes
|
(20,040 | ) | 101,100 | |||||
|
TOTAL LIABILITIES
|
27,495,810 | 21,551,527 | ||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||
|
SHAREHOLDERS’ EQUITY
|
||||||||
|
Preferred stock : par value $0.001 per share
|
||||||||
|
Authorized : 10,000,000 shares
|
||||||||
|
Issued and outstanding : 10,000 shares in 2010
|
||||||||
|
and 0 shares in 2009
|
10 | - | ||||||
|
Common stock : par value $0.001 per share
|
||||||||
|
Authorized : 100,000,000 shares
|
||||||||
|
Issued and outstanding : 6,696,417 shares in 2010
|
6,696 | 5,834 | ||||||
|
and 5,834,398 shares in 2009
|
||||||||
|
Additional paid-in capital
|
17,003,622 | 16,221,004 | ||||||
|
Statutory reserve
|
3,484,793 | 1,740,016 | ||||||
|
Accumulated other comprehensive income
|
4,863,975 | 1,495,835 | ||||||
|
Retained earnings
|
17,059,191 | 9,965,874 | ||||||
| 42,418,287 | 29,428,563 | |||||||
| Subscriptions receivable | (324,440 | ) | - | |||||
|
Total shareholders' equity of the company
|
42,093,847 | 29,428,563 | ||||||
|
Non-Controlling Interest
|
495,848 | 3,653,761 | ||||||
|
TOTAL EQUITY
|
42,589,695 | 33,082,324 | ||||||
|
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY
|
$ | 70,085,505 | $ | 54,633,851 | ||||
See Notes to Consolidated Financial Statements
71
|
One Bio, Corp.
|
||||||||
|
Consolidated Statements of Income and Comprehensive Income
|
||||||||
|
(Stated in US dollars)
|
||||||||
|
Year ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Revenues
|
$ | 52,296,203 | $ | 22,073,219 | ||||
|
Cost of sales
|
32,170,762 | 12,089,029 | ||||||
|
Gross profits
|
20,125,441 | 9,984,190 | ||||||
|
Operating expenses
|
||||||||
|
General and administrative expenses
|
5,291,908 | 1,110,991 | ||||||
|
Research and development expenses
|
259,878 | 378,497 | ||||||
|
Selling and marketing expenses
|
892,106 | 1,035,949 | ||||||
| 6,443,892 | 2,525,437 | |||||||
|
Income from operations
|
13,681,549 | 7,458,753 | ||||||
|
Interest and financing expense
|
(736,411 | ) | (230,340 | ) | ||||
|
Interest income
|
33,336 | 36,633 | ||||||
|
Other (expense) income
|
(742,135 | ) | 147,236 | |||||
|
Income before income taxes
|
12,236,339 | 7,412,282 | ||||||
|
Provision for income taxes
|
(3,281,163 | ) | (2,027,309 | ) | ||||
|
Net income
|
8,955,176 | 5,384,973 | ||||||
|
Net income attributable to
|
||||||||
|
non-controlling interest
|
(278,564 | ) | (612,159 | ) | ||||
|
Net income attributable to
|
||||||||
|
Company
|
$ | 8,676,612 | $ | 4,772,814 | ||||
|
Earnings per share
|
||||||||
|
- Basic
|
$ | 1.43 | $ | 0.95 | ||||
|
- Diluted
|
$ | 1.35 | $ | 0.93 | ||||
|
Weighted average number of shares outstanding :
|
||||||||
|
- Basic
|
6,056,057 | 5,011,925 | ||||||
|
- Diluted
|
6,423,994 | 5,117,274 | ||||||
|
STATEMENT OF COMPREHENSIVE INCOME
|
||||||||
|
Net Income
|
$ | 8,955,176 | $ | 5,384,973 | ||||
|
Other comprehensive income
|
||||||||
|
Unrealized foreign currency gain (loss)
|
992,346 | (37,286 | ) | |||||
|
Comprehensive income
|
9,947,522 | 5,347,687 | ||||||
|
Comprehensive income attributable to
|
||||||||
|
noncontrolling interest
|
(291,484 | ) | (612,159 | ) | ||||
|
Comprehensive income attributable to the Company
|
$ | 9,656,038 | $ | 4,735,528 | ||||
See Notes to Consolidated Financial Statements
72
|
One Bio, Corp.
|
||||||||
|
Consolidated Statements of Cash Flows
|
||||||||
|
(Stated in US dollars)
|
||||||||
|
Year ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash flows from operating activities
|
||||||||
|
Net Income
|
$ | 8,955,176 | $ | 5,384,973 | ||||
|
Adjustments to reconcile net income to net cash provided by
|
||||||||
|
operating activities :
|
||||||||
|
Depreciation
|
507,297 | 255,555 | ||||||
|
Amortization of intangible assets
|
107,345 | 64,056 | ||||||
|
Amortization of land use rights
|
25,657 | 62,525 | ||||||
|
Amortization of lease prepayments
|
2,311,070 | 254,911 | ||||||
|
Deferred taxes
|
123,641 | 112,528 | ||||||
|
Share-based compensation
|
159,438 | 50,470 | ||||||
|
Loss on disposal of investment
|
14,142 | - | ||||||
|
Changes in operating assets and liabilities :
|
||||||||
|
Receivables, net
|
(5,546,499 | ) | (1,402,183 | ) | ||||
|
Prepaid and other current assets
|
561,526 | (1,831,567 | ) | |||||
|
Inventory
|
(1,401,271 | ) | (693,236 | ) | ||||
|
Accounts payables
|
2,755,769 | 2,011,964 | ||||||
|
Other payables and accrued liabiilities
|
269,525 | - | ||||||
|
Income tax payable
|
(79,909 | ) | - | |||||
|
Deferred revenue
|
- | (86 | ) | |||||
|
Net cash flows provided by operating activities
|
8,762,907 | 4,269,910 | ||||||
|
Cash flows from investing activities
|
||||||||
|
Payments to acquire property, plant and equipment
|
(3,734,130 | ) | (844,615 | ) | ||||
|
Proceeds on sale of equipment
|
80,842 | - | ||||||
|
Deposits paid for plantation based leases
|
(6,352,279 | ) | (3,932,199 | ) | ||||
|
Investment in intangible assets
|
(415,924 | ) | (479,426 | ) | ||||
|
Loans receivable
|
2,179,825 | (1,370,836 | ) | |||||
|
Restricted cash
|
(457,231 | ) | - | |||||
|
Cash acquired from acquisition
|
- | 1,908,338 | ||||||
|
Net cash flows used in investing activities
|
(8,698,897 | ) | (4,718,738 | ) | ||||
|
Cash flows from financing activities
|
||||||||
|
Proceeds from bridge loan
|
3,000,000 | - | ||||||
|
Proceeds of bank loans
|
3,932,363 | 1,860,561 | ||||||
|
Issue of preferred stock
|
10 | - | ||||||
|
Issue of common stock
|
64,720 | 1,754,000 | ||||||
|
Repurchase and cancellation of common shares
|
(80,000 | ) | (740,000 | ) | ||||
|
Repayments of bank loans
|
(3,325,569 | ) | - | |||||
|
Repayments of government loans
|
- | (146,700 | ) | |||||
|
Advances on loans payable
|
1,433,599 | 1,873,567 | ||||||
|
Advances on notes payable
|
- | 4,177 | ||||||
|
Advances from stockholders and related parties
|
831,365 | 143,145 | ||||||
|
Exercise of warrants
|
- | 764 | ||||||
|
Net cash flows provided by financing activities
|
5,856,488 | 4,749,514 | ||||||
|
Effect of foreign currency translation
|
(174,480 | ) | (37,286 | ) | ||||
|
Net increase in cash and cash equivalents
|
5,746,018 | 4,263,400 | ||||||
|
Cash and cash equivalents - beginning of year
|
4,928,968 | 665,568 | ||||||
|
Cash and cash equivalents - end of year
|
$ | 10,674,986 | $ | 4,928,968 | ||||
|
Supplemental disclosures for cash flow information:
|
||||||||
|
Cash paid for interest
|
$ | 1,434,641 | $ | 80,474 | ||||
|
Cash paid for Income taxes
|
$ | 3,334,839 | $ | 1,904,477 | ||||
|
Supplemental disclosure of noncash transactions:
|
||||||||
|
Issue of stock for satisfaction of deposits received in prior year
|
$ | 60,000 | $ | - | ||||
|
Adjustment of goodwill arising from the reduction of TFS
|
||||||||
|
contingent purchase price
|
$ | 2,378,339 | $ | - | ||||
|
TFS net assets acquired
|
$ | - | $ | 3,593,339 | ||||
|
UGTI net assets acquired
|
$ | - | $ | 11,443,822 | ||||
|
Transfer of land use rights
|
$ | - | $ | 5,834,519 | ||||
See Notes to Consolidated Financial Statements
73
|
One Bio, Corp.
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Consolidated Statements of Changes in Stockholders’ Equity
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
(Stated in US dollars)
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Preferred stock
|
Common stock
|
Additional
|
other
|
Total
|
Non
|
|||||||||||||||||||||||||||||||||||||||||||
|
Number
|
Number
|
paid-in
|
Statutory
|
comprehensive
|
Retained
|
Subscriptions
|
Shareholders'
|
Controlling
|
||||||||||||||||||||||||||||||||||||||||
|
of shares
|
Amount
|
of shares
|
Amount
|
capital
|
reserve
|
income
|
earnings
|
receivable
|
Equity
|
Interest
|
Total
|
|||||||||||||||||||||||||||||||||||||
|
Balance, January 1, 2008
|
4,700,292 | $ | 4,701 | $ | 2,999,165 | $ | 334,823 | $ | 691,977 | $ | 2,475,768 | $ | - | $ | 6,506,434 | $ | 2,737,267 | $ | 9,243,701 | |||||||||||||||||||||||||||||
|
Recapitalization
|
3,960 | 4 | 675,216 | - | - | - | 675,220 | 675,220 | ||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for cash
|
6,160 | 6 | 139,885 | - | - | - | 139,891 | 139,891 | ||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for services
|
2,200 | 2 | 12,459 | - | - | - | 12,461 | 12,461 | ||||||||||||||||||||||||||||||||||||||||
|
Issue of warrants for services
|
- | - | 169,739 | - | - | - | 169,739 | 169,739 | ||||||||||||||||||||||||||||||||||||||||
|
Appropriation of statutory reserve
|
- | - | - | 366,638 | - | (366,638 | ) | - | - | |||||||||||||||||||||||||||||||||||||||
|
Unrealized foreign currency gain
|
- | - | - | - | 747,343 | - | 747,343 | 747,343 | ||||||||||||||||||||||||||||||||||||||||
|
Net income
|
- | - | - | - | - | 3,350,299 | 3,350,299 | - | 3,350,299 | |||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2008
|
- | - | 4,712,612 | $ | 4,713 | $ | 3,996,464 | $ | 701,461 | $ | 1,439,320 | $ | 5,459,429 | $ | - | $ | 11,601,387 | $ | 2,737,267 | $ | 14,338,654 | |||||||||||||||||||||||||||
|
Issuance of common shares for cash upon the
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
exercise of warrants
|
30,548 | 30 | 122 | 152 | 152 | |||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares upon the
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
acquisition of assets
|
728,000 | 728 | 10,546,464 | 91,589 | 93,801 | 1,294,796 | 12,027,378 | 304,335 | 12,331,713 | |||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for cash
|
375,200 | 375 | 1,753,625 | 1,754,000 | 1,754,000 | |||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for services
|
17,638 | 18 | 50,099 | 50,117 | 50,117 | |||||||||||||||||||||||||||||||||||||||||||
|
Purchase of common shares
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
for cancellation
|
(29,600 | ) | (30 | ) | (125,770 | ) | (614,200 | ) | (740,000 | ) | (740,000 | ) | ||||||||||||||||||||||||||||||||||||
|
Appropriation of statutory reserve
|
946,966 | (946,966 | ) | - | - | |||||||||||||||||||||||||||||||||||||||||||
|
Unrealized foreign currency gain
|
(37,286 | ) | (37,286 | ) | (37,286 | ) | ||||||||||||||||||||||||||||||||||||||||||
|
Net income
|
4,772,815 | 4,772,815 | 612,159 | 5,384,974 | ||||||||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2009
|
- | - | 5,834,398 | $ | 5,834 | $ | 16,221,004 | $ | 1,740,016 | $ | 1,495,835 | $ | 9,965,874 | $ | - | $ | 29,428,563 | $ | 3,653,761 | $ | 33,082,324 | |||||||||||||||||||||||||||
|
Issuance of preferred shares for cash
|
10,000 | 10 | 10 | 10 | ||||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for cash
|
594,444 | 595 | 383,843 | 384,438 | 384,438 | |||||||||||||||||||||||||||||||||||||||||||
|
Subscriptions receivable
|
(324,440 | ) | (324,440 | ) | ||||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for services
|
63,575 | 63 | 159,375 | 159,438 | 159,438 | |||||||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares upon the
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
acquisition of assets
|
260,000 | 260 | 260 | 260 | ||||||||||||||||||||||||||||||||||||||||||||
|
Repurchase of common shares from executives
|
(16,000 | ) | (16 | ) | (13,584 | ) | (66,400 | ) | (80,000 | ) | (80,000 | ) | ||||||||||||||||||||||||||||||||||||
|
Transfer of reserves upon acquisition
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
of Elevated Throne
|
858,857 | 147,088 | 2,391,413 | 3,397,358 | (3,397,358 | ) | - | |||||||||||||||||||||||||||||||||||||||||
|
Transfer of Green Planet investment in
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
Elevated Throne upon its divestment
|
14,142 | 14,142 | 14,142 | |||||||||||||||||||||||||||||||||||||||||||||
|
Revision of TFS contingent price
|
(40,000 | ) | (40 | ) | (593,339 | ) | (593,379 | ) | (593,379 | ) | ||||||||||||||||||||||||||||||||||||||
|
Correction of Non controlling interest in UGTI
|
(26,676 | ) | (11,237 | ) | (1,105 | ) | 91,059 | 52,041 | (52,041 | ) | - | |||||||||||||||||||||||||||||||||||||
|
Appropriation of statutory reserve
|
1,608,926 | (1,608,926 | ) | - | - | |||||||||||||||||||||||||||||||||||||||||||
|
Unrealized foreign currency gain
|
977,832 | 972 | 978,804 | 12,922 | 991,726 | |||||||||||||||||||||||||||||||||||||||||||
|
Net income
|
8,676,612 | 8,676,612 | 278,564 | 8,955,176 | ||||||||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2010
|
10,000 | $ | 10 | 6,696,417 | $ | 6,696 | $ | 17,003,622 | $ | 3,484,793 | $ | 4,863,975 | $ | 17,059,191 | $ | (324,440 | ) | $ | 42,093,847 | $ | 495,848 | $ | 42,914,135 | |||||||||||||||||||||||||
See Notes to Consolidated Financial Statements
74
1. Organization and Basis of Presentation
Description of the Business
One Bio, Corp. (formerly ONE Holdings, Corp), (formerly Contracted Services, Inc.) (“ONE” and/or the “Company”) was incorporated June 30, 2000 in the State of Florida as Contracted Services, Inc. and changed its name to ONE Holdings, Corp. on June 9, 2009 and subsequently on November 16, 2009 changed its name to ONE Bio, Corp. ONE and its subsidiaries (collectively the “Corporation” or “Company”) are utilizing green processes to produce raw chemicals and herbal extracts, natural supplements and organic products. The Corporation is focused on the Asia Pacific region and the United States of America. The Corporation’s key products include widely recognized Solanesol, CoQ10, Resveratrol, Ganoderma Tea, 5-HTP, organic fertilizers, and organic bamboo health food and beverages.
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”).
Basis of Consolidation
On June 4, 2009 Abacus Global Investments Corp. (“Abacus”) entered into a material definitive agreement pursuant to which Abacus acquired 18,750,000 shares of Contracted Services, Inc.’s common stock representing 92.25% of the Company’s issued and outstanding stock. The transaction closed on June 9, 2009. On June 9, 2009, concurrent with the Abacus acquisition, ONE changed its name from Contracted Services Inc to ONE Holdings Corp. On October 26, 2009 ONE changed its name again from ONE Holdings Corp to ONE Bio Corp. and authorized a 5 for 1 reverse split in its common shares.
At the time of this acquisition, both Abacus and ONE had nominal operations although ONE was a publicly reporting entity. Abacus is in the business of acquiring and selling companies and in providing strategic management consulting services. To our knowledge, Abacus engaged in only the above described transaction in 2009. ONE (at the time known as Contracted Services Inc.) was a small lawn maintenance company. Accordingly, neither ONE nor Abacus had any significant operations or assets.
Abacus entered into the transaction to establish a public vehicle which it could utilize as the core of a business strategy to establish a global bio-engineering group to promote and develop organic products, intermediate chemical extracts and green processes.
Since both companies, ONE and Abacus, were small closely held companies both prior to and, immediately after the Abacus transaction, and because neither Company had substantial operations or assets, ONE viewed the Abacus transaction as a straight forward private transaction between its shareholder Belmont Partners and Abacus. We believe that Abacus accounted for the transaction as a purchase and we concur with that determination. Further, in accordance with the guidance offered in ASC 805-20-55 we concluded that because, at the time, there was no distinct difference in relative size of assets or operations, and because the legal acquiree did not issue any additional shares that were not already in circulation to effect this transaction, this transaction did not meet the characteristics of a reverse acquisition. In addition, neither of the companies were merged.
The Abacus transaction had little or no impact on liquidity or operations other than the minor lawn service operations which were discontinued in anticipation of engaging in the new business strategy discussed above.
The Abacus transaction had no impact on our capital structure
75
On July 22, 2009 (closing date of the reverse acquisition) ONE Bio Corp. (“ONE”) (formerly ONE Holdings Corp), (formerly Contracted Services Inc.) acquired 11,625,333 outstanding common shares and 4,155,066 of outstanding warrants together representing 78.28% of Green Planet Bio-Engineering Co., Ltd (“GPB” or “Green Planet”) from GPB’s majority shareholders. The transaction was accomplished through a share exchange agreement wherein in exchange for GPB common stock and warrants, ONE issued 3,442,043 of its common shares along with a note for $980,349. The total transaction was valued at $12,336,902.
At the time of this acquisition, ONE had nominal operations whereas GPB was a fully operational enterprise whose assets and operating results far exceeded ONE’s. ONE entered into the transaction as its first acquisition in implementing its business strategy to establish a global agritech group to promote and develop organic products, intermediate chemical extracts and green processes.
Both were publicly traded companies. However, ONE’s operations and assets were nominal whereas GPB operations and assets were substantial. Because GPB’s operations and assets were distinctly different in relative size, ONE viewed the GPB transaction as a reverse acquisition in accordance with the guidance offered in ASC 805-10-55-13. Accordingly, although ONE was the legal acquirer, because the transaction met the characteristics of a reverse acquisition, GPB was treated as the acquirer for accounting purposes, and its reported equity was recapitalized. The companies were not merged.
ONE’s capital structure was changed to reflect the shares it issued to acquire GPB under the share exchange agreement. GPB’s capital structure was recapitalized to reflect the number of shares outstanding as ONE’s shares. Par value of common stock was adjusted to reflect ONE’s par with the offset adjusting Additional Paid-in Capital. The following chart reflects the calculation of the exchange ratio of the conversion of Green Planet stock into ONE Bio, Corp common stock as a result of the reverse acquisition.
| Exchange Ratio Calculation | |||||||||||||||
|
Legal
|
Acctg
|
Pre
|
RTO
|
Post
|
|||||||||||
|
ONE Bio, Corp
|
Parent
|
Acquiree
|
107,243,159 | 17,200,000 | 124,443,159 | ||||||||||
|
Green Planet
|
Subsidiary
|
Acquirer
|
15,589,367 | - | 15,589,367 | ||||||||||
| Number of common shares outstanding of Green Planet | 15,589,367 | ||||||||||||||
|
Exchange ratio
|
110 | % | |||||||||||||
| Number of shares deemed outstanding before RTO | 17,200,000 | ||||||||||||||
| Number of shares outstanding from RTO | 124,443,159 | ||||||||||||||
During the year ended December 31, 2009, ONE completed two acquisitions: (i) on September 3, 2009, ONE completed the purchase of 99.75% of Trade Finance Solutions Inc; and (ii) on September 27, 2009, ONE completed the acquisition of 100% of Supreme Discovery Group Limited (“Supreme”) through its subsidiary United Green Technology, Inc. (“UGTI”). Pursuant to this transaction, 20% of UGTI common stock was issued to the Supreme shareholders in consideration for 100% of Supreme. Also ONE acquired 10,000 shares of UGTI preferred stock which have super voting rights resulting in ONE having a 83.3% interest in UGTI. On November 3, 2009, ONE increased its ownership of UGTI to approximately 98%. ONE’s consolidated financial statements incorporate the results of these acquisitions, as of the acquisition date.
During the year ended December 31, 2010, ONE further acquired 1,632,150 shares in GPB through a Share Purchase Agreement dated April 14, 2010 with Min Zhao, our Director and President of GPB for a total consideration of $975,000. At the same time, we received from a certain GPB shareholder, 1,216,184 shares of GPB common stock. As a consequence, of these transactions, ONE now own 92.5% of the outstanding common stock of Green Planet.
76
Additionally, on April 14, 2010, Green Planet granted to ONE an option to acquire 100% of the stock of Elevated Throne Overseas Ltd. (“Elevated Throne”), Green Planet’s 100% owned BVI subsidiary. In the event ONE exercises this option, the closing of the transaction will be subject to the approval of Green Planet’s stockholders. As consideration for ONE’s exercise of this option, ONE will be required to
|
(i)
|
convert the $1,700,000 loan ONE made to Elevated Throne on or about January 19, 2010, into an equity investment in Elevated Throne,
|
|
(ii)
|
convert the $300,000 loan ONE made to Green Planet on or about September 1, 2009, into a $300,000 equity investment in Elevated Throne,
|
|
(iii)
|
cancel that certain Convertible Note Purchase Agreement between ONE and Green Planet dated on or about September 1, 2009, and
|
|
(iv)
|
cancel that certain 10% Convertible Bridge Loan Note Due September 1, 2010, in the principal amount of $300,000 from Green Planet to ONE.
|
On December 20, 2010, ONE delivered a written notice to Green Planet on ONE’s election to exercise the option granted pursuant to that certain Option Agreement dated April 14, 2010 (the “Option Agreement”), to acquire 100% of the stock of Elevated Throne, Green Planet’s 100% owned BVI subsidiary and in consideration ONE agreed to perform all the obligations numbered (i) to (iv) as described in the immediate paragraph above. On the same day, ONE, as the owner of 92.5% of the outstanding common stock of Green Planet by Majority Shareholder Written Consent in Lieu of a Special Meeting of Stockholders approved, authorized, and ratified the Transaction contemplated by the Option Agreement and the exercise by ONE of the Option as described above retroactive as of April 14, 2010.
Included in the Company’s consolidated financial statements are the operational results and financial position of Sanming and JLF. These companies are deemed to be variable interest entities (“VIE”) and were effectively acquired on July 22, 2009 and September 27, 2009 respectively. As variable interest entities the Company includes their operating results in its consolidated financial results based on the execution of certain contractual relationships which grants to the Company operational control. The Company has operational control which obligates the Company to absorb the risk or loss and enables the Company to receive the majority of the VIE residual returns.
To comply with PRC laws and regulations that restrict foreign ownership of companies, the Company operates its China businesses (Sanming and JLF) through Fujian Green Planet and Fujian United Bamboo wholly foreign owned enterprises (“WFOE”), which are wholly owned subsidiaries of Green Planet and UGTI. The WFOE’s have entered into certain irrevocable agreements with Sanming and JLF through which operational and financial control of these businesses reside in the WFOE. The following are the transactional agreements:
Entrusted Management Agreement
Shareholders Voting Proxy Agreement
Exclusive Purchase Option Agreement
Share Pledge Agreement
As a result of these agreements, the Company is considered the primary beneficiary of Sanming and JLF both financially and operationally and accordingly their results are consolidated in the Company’s financial statements.
ONE’s consolidated financial statements include the accounts of all of its majority owned subsidiaries and the accounts of its variable interest entities, of which ONE is the primary beneficiary. All intercompany balances and transactions have been eliminated on consolidation. The functional currency of ONE’s foreign subsidiaries is in the United States Dollar, Canadian Dollar and Chinese Renminbi. Certain prior year balances have been reclassified to conform to current year presentation.
77
2. Summary of Significant Accounting Policies
Cash and Cash Equivalents
Cash and cash equivalents include all cash, deposits in banks and other highly liquid investments with initial maturities of three months or less to be cash equivalents. Our cash and cash equivalents as of December 31, 2010 and 2009, by geography are:
| Location of Cash Deposits | 2010 | 2009 | ||||||
| PRC | $ | 10,201,249 | $ | 3,741,449 | ||||
| North America | 473,737 | 1,187,519 | ||||||
| Total | $ | 10,674,986 | $ | 4,928,968 | ||||
Cash and cash equivalents located in the PRC were denominated in Renminbi (“RMB”) and were placed with financial institutions in the PRC. Typically, they are not freely convertible into foreign currencies and the remittance of these funds out of the PRC is subject to exchange control restrictions imposed by the PRC government. . In order to improve the accessibility of our cash on a global scale, we have begun a process of transferring certain assets and processes previously centered in our PRC based VIE entities to our WFOE entities. PRC regulations permit WFOE’s to remit funds out of the PRC.
Cash and cash equivalents located in North America were primarily denominated in Canadian Dollars and were placed in financial institutions in Canada. These deposits are freely convertible into foreign currencies and there are few if any practical exchange control restrictions imposed by the Canadian government.
Restricted Cash
In accordance with ASC Topic 305 formerly Accounting Review Board (“ARB”) No. 43, Chapter 3A “Current Assets and Current Liabilities”, cash which is restricted as to withdrawal is considered a non-current asset.
Receivables
Accounts receivable are recognized and carried at original invoiced amount less an allowance for uncollectible accounts, as needed.
When evaluating the adequacy of its allowance for doubtful accounts, the Company reviews the collectability of accounts receivable, historical write-offs, and changes in sales policies, customer credibility and general economic tendency.
78
2. Summary of Significant Accounting Policies
Loans Receivables
These assets are non-derivatives financial assets resulting in cash advances by the lender to a borrower under financing facility agreements that mature on a specific date or dates, usually less than one year or on demand. Each cash advance is typically secured by the assignment of proceeds of the borrower’s accounts receivables which usually collect within 90 day cycles from the date of the advance. These assets are initially recognized at their acquired cost adjusted for any write-offs, the allowance for loan losses, any deferred fees or costs on originated loans, less any provision for impairment. Due to the short term nature of the individual advances, their carrying value approximates fair value. Management believes there is no impairment nor were there any write-offs or allowances for loan losses recorded as of December 31, 2010.
Concentration of Credit
The Company extends credit to its customers for which no credit insurance is available. To date the Corporation has not incurred any significant loss due to this activity; however, if such occurrence was to occur, the loss may have an adverse effect on the financial position of the Company.
Capital Leases
The Company’s policy is to record leases, which transfer substantially all benefits and risks incidental to ownership of property, as acquisitions of property and equipment and to record the incurrence of corresponding obligations as liabilities. Obligations under capital leases are reduced by rental payments net of imputed interest.
Inventory
Inventory is stated at the lower of cost and current market value. Costs include the cost of purchase and processing, and other costs. Inventory is stated at cost upon acquisition. The cost of inventory is calculated using the weighted average method. Any excess of the cost over the net realizable value of each item in inventory is recognized as a provision for diminution in the value of inventory.
Net realizable value is the estimated selling price in the normal course of business less the estimated costs to completion and the estimated expenses and related taxes to make the sale.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, less accumulated depreciation. Expenditures for maintenance and repairs, which are not considered improvements and do not extend the useful life of the asset, are expensed as incurred; additions, renewals and betterments are capitalized. When assets are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in the statement of operations in cost of blended products.
Depreciation is provided to recognize the cost of the asset in the results of operations. The Company calculates depreciation using the straight-line method with estimated useful life as follows:
|
|
Building and structural components
|
20 years
|
|
|
Computer equipment and software
|
5 years
|
|
|
Leasehold improvements
|
7 years
|
|
|
Machinery and equipment
|
10 years
|
|
|
Office equipment and furniture
|
5 years
|
|
|
Technology
|
5 - 10 years
|
|
|
Vehicles
|
5 years
|
79
2. Summary of Significant Accounting Policies - continued
Land Use Rights
Land use rights represent the purchased rights to use land granted PRC land authorities. Depending on the PRC land authority, the land use rights can be conveyed in the form of a prepaid lease or a use agreement. Land use rights are stated at cost less accumulated amortization. Amortization is provided using the straight line method over the terms of the agreement which range over a term of 30 to 50 years obtained from the relevant PRC land authorities.
Intangible Assets
Intangible assets consist mainly of proprietary technology and software. The intangible assets are amortized using straight-line method over the life of the assets.
Impairment of Long-lived Assets
In accordance with ASC Topic 360 Formerly Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, certain assets such as property, plant, and equipment, and purchased intangibles, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Intangible assets are tested for impairment annually. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. There were no events or changes in circumstances that necessitated a review of impairment of long lived assets as of December 31, 2010 and December 31, 2009.
Revenue Recognition
The Company recognizes revenues in accordance with the guidance in the Securities and Exchange Commission (“SEC”) ASC Topic 605 Formerly Staff Accounting Bulletin (“SAB”) No. 104. Revenue is recognized when persuasive evidence of an arrangement exists, when the selling price is fixed or determinable, when delivery occurs and when collection is probable. The Company recognizes sales revenue when goods are shipped or ownership has transferred.
Employee Future Benefits
Pursuant to the relevant laws and regulations in the PRC, the Company participates in various defined contribution retirement plans organized by the respective divisions in municipal and provincial governments for its employees. The Company is required to make contributions to the retirement plans in accordance with the contribution rates and basis as defined by the municipal and provincial governments. The contributions are charged to the respective assets or the income statement on an accrual basis. When employees retire, the respective divisions are responsible for paying their basic retirement benefits. The Company does not have any other obligations in this respect.
80
2. Summary of Significant Accounting Policies - continued
Income Taxes
The Company accounts for income taxes in accordance with ASC topic 740 formerly Statement of Financial Accounting Standards (“SFAS”) No. 109, “Accounting for Income Taxes”. Under SFAS No. 109, deferred tax assets and liabilities are determined based on temporary differences between accounting and tax bases of assets and liabilities and net operating loss and credit carry forwards, using enacted tax rates in effect for the year in which the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. A provision for income tax expense is recognized for income taxes payable for the current period, plus the net changes in deferred tax amounts. Any interest and penalties are expensed in the year that the Notice of Assessment is received.
The Company’s practice is to recognize interest and/or penalties to income tax matters in income tax expense.
Use of Estimates
The preparation of consolidated financial statements in accordance with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses for the periods reported. Actual results could differ from those estimates. Significant items that require estimates are goodwill, deferred tax assets, stock-based compensation, deferred tax liabilities and the depreciation, and amortization of the Corporation’s assets.
Costs of Raising Equity Capital
Costs of raising capital include legal, professional fees and agent fees associated with the raising of equity and debt capital.
Incremental costs incurred in respect of raising capital are charged against equity or debt proceeds raised. Costs associated with the issuance of share capital are charged to capital stock upon the raising of share capital. Costs associated with the issuance of debt are part of the carrying value of the debt and charged to operations as non-cash financing expense using the effective interest rate method.
Foreign Currency Translation
In accordance with ASC Topic 830 formerly SFAS No.52, “Foreign Currency Translation”, the financial statements of subsidiaries of the Company are measured using local currency (Canadian Dollar and Chinese Yuan) as the functional currency. Assets and liabilities have been translated at period-end exchange rates and related revenue and expenses have been translated at average exchange rates. Gains and losses resulting from the translation of subsidiaries’ financial statements are included as a separate component of shareholders’ equity accumulated in other comprehensive income.
Fair Value of Financial Instruments
The Company estimates the fair value of its financial instruments based on current interest rates, quoted market values or the current price of financial instruments with similar terms. Unless otherwise disclosed herein, the carrying value of financial instruments, especially those with current maturities such as cash and cash equivalents, accounts receivable, and accounts payable and accrued liabilities are considered to approximate their fair values.
81
2. Summary of Significant Accounting Policies - continued
Fair Value Measurements
In April 2009, the Financial Accounting Standard Board (“FASB”) released ASC 820, Fair Value Measurements and Disclosures, (formerly SFAS No. 157 “Fair Value Measurements”) that defines fair value, establishes a framework for measuring fair value in accordance with U.S. GAAP, and expands disclosures about fair value measurements.
According to ASC 820, investment measured and reported at fair value are classified and disclosed in one of the following hierarchy:
|
Level1 -
|
Quoted prices are available in active markets for identical investments as of the reporting date. The type of investments included in Level 1 included listed equities and listed derivatives.
|
|
Level2 -
|
Pricing inputs are other than quoted prices in active markets, which are either directly or indirectly observable as of the reporting date, and fair value is determined through the use of models or other valuation methodologies. Investments that are generally included in this category include corporate bonds and loans, less liquid and restricted equity securities and certain over-the-counter derivatives.
|
|
Level3 -
|
Pricing inputs are unobservable for the investment and included situations where there is little, if any, market activity for the investment. The inputs into the determination of fair value require significant management judgment or estimation.
|
Stock-Based Compensation Plan
The Corporation uses the fair value-based method for measurement and cost recognition of employee share-based compensation arrangements under the provisions of ASC Topic 718 formerly FASB, SFAS 123 (Revised 2004) and Share-Based Payment (SFAS 123R), using the modified prospective transitional method.
Under the modified prospective transitional method, share-based compensation is recognized for awards granted, modified, repurchased or cancelled subsequent to the adoption of SFAS 123R. In addition, share-based compensation is recognized, subsequent to the adoption of SFAS 123R, for the remaining portion of the vesting period (if any) for outstanding awards granted prior to the date of adoption.
We measure share-based compensation costs on the grant date, based on the calculated fair value of the award. We have elected to treat awards with graded vesting as a single award when estimating fair value. Compensation cost is recognized on a straight-line basis over the employee requisite service period, which in our circumstances is the stated vesting period of the award, provided that total compensation cost recognized at least equals the pro rata value of the award that has vested. Compensation cost is initially based on the estimated number of options for which the requisite service is expected to be rendered. This estimate is adjusted in the period once actual forfeitures are known.
82
2. Summary of Significant Accounting Policies - continued
Earnings Per Share
Earnings (loss) per common share are reported in accordance with ASC Topic 260 formerly SFAS No. 128, “Earnings Per Share”. SFAS No. 128 requires dual presentation of basic earnings per share (“EPS”) and diluted EPS on the face of all statements of earnings, for all entities with complex capital structures. Diluted EPS reflects the potential dilution that could occur from common shares issuable through the exercise or conversion of stock options, restricted stock awards, warrants and convertible securities. In certain circumstances, the conversion of these options, warrants and convertible securities are excluded from diluted EPS if the effect of such inclusion would be anti-dilutive. Fully diluted loss per common share is not provided, when the effect is anti-dilutive.
When the effect of dilution on loss per share is anti-dilutive, diluted loss per share equals the loss per share.
Comprehensive Income
The Company follows ASC Topic 220 formerly Statement of Financial Accounting Standards No. 130, “Reporting Comprehensive Income”. This statement establishes standards for reporting and display of comprehensive income and its components. Comprehensive income is net income plus certain items that are recorded directly to shareholders’ equity bypassing net income. Other than foreign exchange gains and losses, the Company recorded a transfer of preacquistion retained earnings of $2.1 million previously owned by non-controlling interest at the date of ONE acquiring Elevated Throne from Green Planet.
Recent Changes in Accounting Standards
Recent accounting pronouncements that the Company has adopted or that will be required to adopt in the future are summarized below.
In January 2010, the FASB has published ASU 2010-01 “Equity (Topic 505)- Accounting for Distributions to Shareholders with Components of Stock and Cash—a consensus of the FASB Emerging Issues Task Force,” as codified in ASC 505,. ASU No. 2010-01 clarifies the treatment of certain distributions to shareholders that have both stock and cash components. The stock portion of such distributions is considered a share issuance that is reflected in earnings per share prospectively and is not a stock dividend. The amendments in this Update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. Early adoption is permitted. The adoption of this standard did not have an impact on the Company’s (consolidated) financial position and results of operations.
In January 2010, the FASB has published ASU 2010-02 “Consolidation (Topic 810)- Accounting and Reporting for Decreases in Ownership of a Subsidiary—a Scope Clarification,” as codified in ASC 810, “Consolidation.” ASU No. 2010-02 applies retrospectively to April 1, 2009, our adoption date for ASC 810-10-65-1 as previously discussed in this financial note. This ASU clarifies the applicable scope of ASC 810 for a decrease in ownership in a subsidiary or an exchange of a group of assets that is a business or nonprofit activity. The ASU also requires expanded disclosures. The amendments in this Update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. . The adoption of this standard is not expected to have any impact on the Company’s consolidated financial position and results of operations.
83
2. Summary of Significant Accounting Policies - continued
In January 2010, the FASB has published ASU 2010-06 “Fair Value Measurements and Disclosures (Topic 820): - Improving Disclosures about Fair Value Measurements. ASU No. 2010-06 clarifies improved disclosure requirements related to fair value measurements and disclosures – Overall Subtopic (Subtopic 820-10) of the FASB Accounting Standards Codification. The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosure about purchase, sales, issuances, and settlement in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The amendments in this Update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. The adoption of this standard is not expected to have a material impact on the Company’s consolidated financial position and results of operations.
In March 2010, the FASB issued Accounting Standard Update No. 2010-11 “Derivatives and Hedging” (Topic 815). ASU No. 2010-11 update provides amendments to subtopic 815-15, Derivatives and hedging. The amendments clarify about the scope exception in paragraph 815-10-15-11 and section 815-15-25 as applicable to the embedded credit derivatives. The ASU is effective on the first day of the first fiscal quarter beginning after June 15, 2010. Therefore, for a calendar-year-end entity, the ASU becomes effective on July 1, 2010. Early application is permitted at the beginning of the first fiscal quarter beginning after March 5, 2010. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
In March 2010, the FASB issued Accounting Standard Update No. 2010-11 “Derivatives and Hedging” (Topic 815). ASU No. 2010-11 update provides amendments to subtopic 815-15, Derivatives and hedging. The amendments clarify about the scope exception in paragraph 815-10-15-11 and section 815-15-25 as applicable to the embedded credit derivatives. The ASU is effective on the first day of the first fiscal quarter beginning after June 15, 2010. Therefore, for a calendar-year-end entity, the ASU becomes effective on July 1, 2010. Early application is permitted at the beginning of the first fiscal quarter beginning after March 5, 2010. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued Accounting Standard Update No. 2010-12. “Income Taxes” (Topic 740). ASU No.2010-12 amends FASB Accounting Standard Codification subtopic 740-10 Income Taxes to include paragraph 740-10-S99-4. On March 30, 2010 The President signed the Health Care & Education Affordable Care Act reconciliation bill that amends its previous Act signed on March 23, 2010. FASB Codification topic 740, Income Taxes, requires the measurement of current and deferred tax liabilities and assets to be based on provisions of enacted tax law. The effects of future changes in tax laws are not anticipated.” Therefore, the different enactment dates of the Act and reconciliation measure may affect registrants with a period-end that falls between March 23, 2010 (enactment date of the Act), and March 30, 2010 (enactment date of the reconciliation measure). However, the announcement states that the SEC would not object if such registrants were to account for the enactment of both the Act and the reconciliation measure in a period ending on or after March 23, 2010, but notes that the SEC staff “does not believe that it would be appropriate for registrants to analogize to this view in any other fact patterns.” The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
84
2. Summary of Significant Accounting Policies - continued
In April 2010, the FASB issued Accounting Standard Update No. 2010-13 “Stock Compensation” (Topic 718). ASU No.2010-13 provides amendments to Topic 718 to clarify that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity's equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. The amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. The amendments in this Update should be applied by recording a cumulative-effect adjustment to the opening balance of retained earnings. The cumulative-effect adjustment should be calculated for all awards outstanding as of the beginning of the fiscal year in which the amendments are initially applied, as if the amendments had been applied consistently since the inception of the award. The cumulative-effect adjustment should be presented separately. Earlier application is permitted. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued Accounting Standard Update No. 2010-17. “Revenue Recognition-Milestone Method” (Topic 605) ASU No.2010-17 provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. An entity often recognizes these milestone payments as revenue in their entirety upon achieving a specific result from the research or development efforts. A vendor can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved only if the milestone meets all criteria to be considered substantive. Determining whether a milestone is substantive is a matter of judgment made at the inception of the arrangement. The ASU is effective for fiscal years and interim periods within those fiscal years beginning on or after June 15, 2010. Early application is permitted. Entities can apply this guidance prospectively to milestones achieved after adoption. However, retrospective application to all prior periods is also permitted. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued Accounting Standard Update No. 2010-18. “Receivables” (Topic 310). ASU No.2010-18 provides guidance on accounting for acquired loans that have evidence of credit deterioration upon acquisition. Paragraph 310-30-15-6 allows acquired assets with common risk characteristics to be accounted for in the aggregated as a pool. Upon establishment of the pool, the pool becomes the unit of accounting. When loans are accounted for as a pool, the purchase discount is not allocated to individual loans; thus all of the loans in the pool accrete at a single pool rate (based on cash flow projections for the pool). Under subtopic 310-30, the impairment analysis also is performed on the pool as a whole as opposed to each individual loan. Paragraphs 310-40-15-4 through 15-12 establish the criteria for evaluating whether a loan modification should be classified as a troubled debt restructuring. Specifically paragraph 310-40-15-5 states that “a restructuring of a debt constitutes a troubled debt restructuring for purposes of this subtopic if the creditor for economic or legal reasons related to the debtor’s financial difficulties grants a concession to the debtor that it would not otherwise consider.” The ASU is effective for modification of loans accounted for within pools under subtopic 310-30 occurring in the first interim or annual period ending on or after July 15, 2010. The amendments are to be applied prospectively. Early application is permitted. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
85
2. Summary of Significant Accounting Policies - continued
In July 2010, the FASB issued Accounting Standard Update No. 2010-20 (ASU No. 2010-20) “Receivables” (Topic 310). ASU No. 2010-20 provides financial statement users with greater transparency about an entity’s allowance for credit losses and the credit quality of its financing receivables. This update is intended to provide additional information to assist financial statement users in assessing an entity’s credit risk exposures and evaluating the adequacy of its allowance for credit losses. The amendments in this update apply to both public and nonpublic entities with financing receivables, excluding short-term trade accounts receivable or receivables measured at fair value or lower of cost or fair value. The objective of the amendments in ASU No. 2010-20 is for an entity to provide disclosures that facilitate financial statement users’ evaluation of (1) the nature of credit risk inherent in the entity’s portfolio of financing receivables, (2) How that risk is analyzed and assessed in arriving at the allowance for credit losses and (3) The changes and reasons for those changes in the allowance for credit losses. The entity must provide disclosures about its financing receivables on a disaggregated basis. For public entities ASU No. 2010-20 is effective for interim and annual reporting periods ending on or after December 15, 2010. For nonpublic entities ASU No. 2010-20 will become effective for annual reporting periods ending on or after December 15, 2011. The Company is evaluating the impact ASU No. 2010-20 will have on the consolidated financial statements.
In August 2010, the FASB issued Accounting Standard Updates No. 2010-21 (ASU No. 2010-21) “Accounting for Technical Amendments to Various SEC Rules and Schedules” and No. 2010-22 (ASU No. 2010-22) “Accounting for Various Topics – Technical Corrections to SEC Paragraphs”. ASU No 2010-21 amends various SEC paragraphs pursuant to the issuance of Release no. 33-9026: Technical Amendments to Rules, Forms, Schedules and Codification of Financial Reporting Policies. ASU No. 2010-22 amends various SEC paragraphs based on external comments received and the issuance of SAB 112, which amends or rescinds portions of certain SAB topics. Both ASU No. 2010-21 and ASU No. 2010-22 are effective upon issuance. The amendments in ASU No. 2010-21 and No. 2010-22 will not have a material impact on the Company’s consolidated financial statements.
In September 2010, the FASB issued Accounting Standard Update No. 2010-25 (ASU No. 2010-25) “Defined Contribution Pension Plans” (Topic 962). ASU No. 2010-25 clarifies how loans to participants should be classified and measured by defined contribution pension benefits. The amendments in ASU No. 2010-25 affect any defined contribution pension plan that allows participant loans. The amendments in ASU No. 2010-25 require that participant loans be classified as notes receivable from participants, which are segregated from plan investments and measured at their unpaid principal balance plus any accrued but unpaid interest. ASU No. 2010-25 is effective for fiscal years ending after December 15, 2010 and should be applied retrospectively to all prior periods presented. Early adoption is permitted. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
86
2. Summary of Significant Accounting Policies - continued
In October 2010, the FASB issued Accounting Standard Update No. 2010-26 (ASU No. 2010-26) “Accounting for Costs Associated with Acquiring or Renewing Insurance Contracts.” ASU No. 2010-26 addresses diversity in practice regarding the interpretation of which costs relating to the acquisition of new or renewal insurance contracts qualify for deferral. Costs that meet the definition of acquisition costs, as stated in the Master Glossary of the FASB Accounting Standards Codification, are typically recognized as assets and are commonly referred to as deferred acquisition costs. The amendments in this update affect insurance entities that are within the scope of Topic 944 Financial Services – Insurance (which includes but is not limited to stock life insurance entities, mutual life insurance entities, and property and liability insurance entities), that incur costs in the acquisition of new and renewal insurance contracts. The amendments in this update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2011. The amendments in this update should be applied prospectively upon adoption. Retrospective application to all prior periods presented upon the date of adoption also is permitted, but not required. Early adoption is permitted, but only at the beginning of an entity’s annual reporting period. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
In December 2010, the FASB issued Accounting Standard Update No. 2010-28 (ASU No. 2010-28) “Intangibles – Goodwill and Other (Topic 350).” ASU No. 2010-28 addresses questions about entities with reporting units with zero or negative carrying amounts because some entities concluded that Step 1 of the goodwill impairment test, as stated in Topic 350, is passed in those circumstances because the fair value of their reporting unit will generally be greater than zero. The amendments in this update do not provide guidance on how to determine the carrying amount or measure the fair value of the reporting unit. The amendments in this update modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For public entities, the amendments in this update are effective for fiscal years, and interim periods within those years, beginning after December 15, 2010. Early adoption is permitted. For nonpublic entities, the amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Nonpublic entities may early adopt the amendments using effective date for public entities. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
In December 2010, the FASB issued Accounting Standard Update No. 2010-29 (ASU No. 2010-29) “Business Combinations (Topic 805).” ASU No. 2010-29 addresses the diversity in practice about the interpretation of the pro forma revenue and earnings disclosure requirements for business combinations. The amendments in this update specify that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. The amendments in this update also expand the supplemental pro forma disclosures under Topic 805 to include a description of the nature and amount of material, nonrecurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. The amendments in this update are effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. The adoption of this guidance has not had and is not expected to have a material impact on the Company’s consolidated financial statements.
Other ASUs not effective until after December 31, 2010, are not expected to have a significant effect on the Company’s consolidated financial position or results of operations.
87
3. Supplemental Cash Flow Information
|
For the Year Ended December 31,
|
||||||||
| 2010 | 2009 | |||||||
|
Supplemental disclosure
|
||||||||
|
Interest paid
|
$ | 1,434,641 | $ | 80,474 | ||||
|
Income taxes paid
|
$ | 3,334,839 | $ | 1,904,477 | ||||
|
Non-cash transactions
|
||||||||
|
Issue of stock for satisfaction of deposits
|
||||||||
|
In prior period
|
$ | 60,000 | $ | - | ||||
|
Adjustment of goodwill arising from the
|
||||||||
|
reduction of TFS contingent purchase price
|
$ | 2,378,339 | $ | - | ||||
|
TFS Net assets acquired
|
$ | - | $ | 3,593,339 | ||||
|
UGTI net assets acquired
|
$ | - | $ | 11,443,822 | ||||
|
Transfer of land use rights
|
$ | - | $ | 5,834,519 | ||||
4. Accounts Receivables
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Accounts receivables
|
$ | 18,079,696 | $ | 12,017,848 | ||||
|
Allowances for doubtful accounts
|
(70,430 | ) | (11,263 | ) | ||||
|
Accounts receivable, net
|
$ | 18,009,266 | $ | 12,006,585 | ||||
5. Inventory
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Raw material
|
$ | 232,928 | $ | 140,759 | ||||
|
Packaging material
|
90,616 | 59,954 | ||||||
|
Work in progress
|
3,815,837 | 2,610,245 | ||||||
|
Finished goods
|
365,826 | 165,073 | ||||||
| $ | 4,505,207 | $ | 2,976,031 | |||||
88
6. Property, Plant and Equipment
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Buildings and structural components
|
$ | 3,676,087 | $ | 3,580,686 | ||||
|
Machinery
|
1,941,389 | 1,806,118 | ||||||
|
Office equipment and furniture
|
439,484 | 306,685 | ||||||
|
Vehicles
|
218,654 | 109,590 | ||||||
|
Leasehold improvement
|
1,960,756 | - | ||||||
| 8,236,370 | 5,803,079 | |||||||
|
Less: Accumulated depreciation
|
(1,561,851 | ) | (1,130,912 | ) | ||||
| 6,674,519 | 4,672,167 | |||||||
|
Construction in progress
|
2,199.113 | 885,744 | ||||||
| $ | 8,873,632 | $ | 5,557,911 | |||||
As of December 31, 2010, the Company has pledged buildings with a carrying value of $1.87 million as collateral for the revolving loan facility with Industrial & Commercial Bank and $1.16 million as collateral for the short term loan (secured B) facility with China Construction Bank.
The Company has a capital commitment of $4.86 million to complete the building and machinery recorded under construction in progress as of December 31, 2010.
|
During the periods, depreciation is included in:
|
||||||||
|
For the Year Ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cost of sales
|
$ | 273,636 | $ | 158,517 | ||||
|
Administrative expenditures
|
196,920 | 96,942 | ||||||
89
7. Intangible Assets
|
The following is a summary of the Company’s intangible assets:
|
||||||||
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Technology
|
$ | 1,128,966 | $ | 871,680 | ||||
|
Software
|
3,282 | 5,304 | ||||||
| 1,132,248 | 876,984 | |||||||
|
Less: Accumulated amortization
|
(308,580 | ) | (194,926 | ) | ||||
| $ | 823,668 | $ | 682,058 | |||||
As of December 31, 2010, the Company has committed $347,862 to spend on additional technology to improve production techniques for various products.
The estimated aggregate amortization expense for intangible assets for the five succeeding years is $94,857 for 2011 and $94,528 for years 2012 to 2015.
8. Acquisition of Non-controlling interest
On April 14, 2010, Green Planet granted to ONE an option to acquire 100% of the stock of Elevated Throne, a 100% owned BVI subsidiary of Green Planet.
On December 20, 2010, ONE delivered a written notice to Green Planet on ONE’s election to exercise the option granted pursuant to that certain Option Agreement dated April 14, 2010 (the “Option Agreement”), to acquire 100% of the stock of Elevated Throne, Green Planet’s 100% owned BVI subsidiary and in consideration ONE agreed to perform all the obligations as follows:
|
(i)
|
convert the $1,700,000 loan ONE made to Elevated Throne on or about January 19, 2010, into an equity investment in Elevated Throne,
|
|
(ii)
|
convert the $300,000 loan ONE made to Green Planet on or about September 1, 2009, into a $300,000 equity investment in Elevated Throne,
|
|
(iii)
|
cancel that certain Convertible Note Purchase Agreement between ONE and Green Planet dated on or about September 1, 2009, and
|
|
(iv)
|
cancel that certain 10% Convertible Bridge Loan Note Due September 1, 2010, in the principal amount of $300,000 from Green Planet to ONE.
|
On the same day, ONE, as the owner of 92.5% of the outstanding common stock of Green Planet by Majority Shareholder Written Consent in Lieu of a Special Meeting of Stockholders approved, authorized, and ratified the Transaction contemplated by the Option Agreement and the exercise by ONE of the Option as described above retroactive as of April 14, 2010.
90
As a result of the completion of the above transaction, ONE effectively owned 100% of Elevated Throne and thereby acquiring all of the non-controlling interest from Green Planet minority shareholders interest in Elevated Throne.
The following is a condensed statement showing the effects of the transaction on the Income Statement and Balance Sheet for fiscal year 2010:
|
ONE's Proforma
|
ONE's Actual
|
|||||||||||
|
Effects on Income
|
before
|
Effects of
|
Balance after
|
|||||||||
|
Statement
|
Transaction
|
Transaction
|
Transaction
|
|||||||||
|
Non-controlling interest
|
503,261 | (338,742 | ) (a) | 164,519 | ||||||||
|
Net Income of Elevated
|
||||||||||||
|
Throne group
|
$ | 5,508,311 | $ | 5,508,311 | ||||||||
|
Non-controlling interest
|
503,261 | 164,519 | ||||||||||
|
Net Income attributable to
|
||||||||||||
| $ | 5,005,050 | $ | 5,343,792 | |||||||||
|
ONE's Proforma
|
||||||||||||
|
Balance Sheet
|
ONE's Actual
|
|||||||||||
|
before
|
Effects of
|
Balance Sheet
|
||||||||||
|
Effects on Balance Sheet
|
Transaction
|
Transaction
|
after Transaction
|
|||||||||
|
Additional paid-in capital
|
16,144,765 | 858,857 | (b) | 17,003,622 | ||||||||
|
Statutory reserve
|
3,337,705 | 147,088 | (b) | 3,484,793 | ||||||||
|
Accumulated other
|
||||||||||||
|
comprehensive income
|
2,518,185 | 2,391,413 | (b) | 4,909,598 | ||||||||
|
Non-controlling interest
|
3,893,320 | (3,397,358 | )(b) | 495,962 | ||||||||
Notes to effects of transaction on Income Statement and Balance Sheet:
|
(a)
|
This represents the net income attributable to non-controlling interest previously when they owned the minority interest share in Elevated Throne via Green Planet. This is no longer attributable to them upon completion of the transaction during the second quarter of fiscal year 2010.
|
|
(b)
|
Upon completion of the transaction, the equity reserves representing additional paid-in capital, statutory reserve and accumulated other comprehensive income formerly attributable to non-controlling interest are now acquired by ONE.
|
91
9. Goodwill
Goodwill is recorded when the consideration paid for an acquisition of a business exceeds the fair value of identifiable net tangible and intangible assets. Goodwill was recorded with the purchase of TFS.
| Balance at December 31, 2009 | $ | 3,974,908 | ||
| Adjustment of contingent purchase price | (2,378,339 | ) | ||
| Balance at December 31, 2010 | $ | 1,596,569 |
Future adjustments to prior acquisitions may be required primarily due to adjustments to plans formulated in accordance with the ASC Topic 805.
10. Restricted Cash
One of the Company’s operating subsidiaries in the PRC has established a credit facility with a local lender denominated in RMB. The facility was partially guaranteed by the Rural Credit Cooperatives Cooperation of Jianou City. The credit facility provides that the Company deposit 50% of the total credit applied into an Escrow Account as further guaranty against default. As of December 31, 2010, the cash on deposit but restricted as to access was $845,458.
11. Loans Payable
The Company has entered into certain financial agreements and loans payable as follows:
|
Description of Borrowings
|
December 31, 2010
|
December 31, 2009
|
||||||
|
China Construction Bank, Secured A
|
$ | - | $ | 1,465,008 | ||||
|
China Construction Bank. Secured B
|
1,512,447 | - | ||||||
|
Rural Credit Cooperative of Jianou
|
1,361,203 | - | ||||||
|
Rural Credit Cooperative, Secured A
|
- | 1,860,561 | ||||||
|
Rural Credit Cooperative, Secured B
|
- | 732,504 | ||||||
|
Rural Credit Cooperative, Secured C
|
604,979 | - | ||||||
|
Industrial & Commercial Bank
|
1,814,937 | - | ||||||
|
Reverse acquisition of GPB
|
980,349 | 980,349 | ||||||
|
Acquisition of TFS
|
974,750 | 3,000,000 | ||||||
|
Acquisition of Supreme
|
1,238,400 | 2,438,400 | ||||||
|
Financial Services
|
7,254,780 | 6,449,880 | ||||||
|
Bridge Loan
|
3,000,000 | - | ||||||
|
Other
|
973,176 | 157,949 | ||||||
|
Total
|
19,715,021 | 17,084,651 | ||||||
|
Loans payable, current portion
|
19,122,823 | 12,868,796 | ||||||
| Loans payable, less current portion | $ | 592,198 | $ | 4,215,855 | ||||
92
The Company has long term loans payable in 2012 and 2013 of $510,068 and $82,130 respectively.
China Construction Bank, Secured B - The amount represents a short term loan and accrues interest at 115% of the rate quoted by the People’s Bank of China. The loan is secured by land use rights with a carrying value of $106,570 and buildings with a carrying value of $1.16 million. The loan matures on April 30, 2011.
Rural Credit Cooperative of Jianou – The amount represents two short term loans of 6 months each expiring on February 2011 and April 2011. The first term loan is provided at a discount rate of 8% per annum and the second term loan is at 10.4% per annum. The loans are secured through a guarantee deposit of $378,112 and $302,489 respectively with the financial institution.
Rural Credit Cooperative, Secured C – On June 21, 2010, the Company entered into a one year short term loan agreement with Rural Credit Cooperative, which carries interest at the annual rate of 7.965%. The loan is secured by a guarantee company. In return, the Company provided a counter guarantee of $119,437 to the guarantee company. The guarantee company charged a fee of $9,292 per annum.
Industrial & Commercial Bank – On July 7, 2010, the Company entered into a 2 year revolving loan facility agreement with Industrial and Commercial Bank of China (“ICBC”), which carries an interest rate of 85% of the rate stipulated by the People’s Bank of China. Under the terms of the loan facility, usage of the funds is limited to the purchase of certain inventory items. ICBC charged a commission fee of $33,293. The Company pledged land use rights with a carrying value of $916,126, and buildings with a carrying value of $1.87 million as collateral for the revolving loan facility.
Reverse Acquisition of GPB - The amounts represent notes issued in connection with the acquisition of Green Planet Bioengineering Co Ltd. There were two notes issued, one for $445,613 which matured on July 22, 2010 and was extended to June 1, 2011, and one for $534,736 which matures on July 22, 2011.
Acquisition of TFS - The amount represents the contingent cash consideration due in connection with the acquisition of Trade Finance Services, Inc. Payment is due based on the achievement of certain milestones.
Acquisition of Supreme - The amounts represent two notes issued in connection with the acquisition of Supreme Discovery Group Limited. One for $557,280 which matured on September 22, 2010 and was extended to June 1, 2011, and another for $681,120 which matures on September 22, 2011.
Financial Services - Our Financial services business units borrows money from various individual private lenders at prevailing rates, which was 12% for instruments issued in fiscal 2010. These borrowings are collateralized by a first lien on the receivables of Trade Finance and mature in one year. Interest and principal is due at maturity.
Bridge Loan – The amount represents the borrowings from various lenders with an interest rate of 8% per annum which matured on October 11, 2010. The loan was further extended to January 10, 2011. Effective January 31, 2011, the Company entered into an additional loan extension agreement with the lenders to April 1, 2011 with an option to further extend to May 1, 2010 and June 1, 2011.
Other - The amounts are payable to shareholders and related parties. The amounts are interest-free, unsecured and payable on demand.
93
12. Loans Receivable
These assets are non-derivative financial assets resulting in cash advances by the lender to a borrower under financing facility agreements that mature on a specific date or dates, usually less than one year or on demand. We make cash advances under these financing facility agreements which are usually secured by the assignment of proceeds of accounts receivable which usually collect within 90 day cycles from the date of the advance. Management generally, holds the loans until maturity or payoff and values them at their outstanding principal adjusted for any write-offs, the allowance for loan losses, any deferred fees or costs on originated loans. Our loans are collateralized as follows: (a) accounts receivable financing agreements are collateralized by the assignment of the proceeds of trade receivables; (b) purchase order financing agreements are collateralized by the assignment of the assets being purchased or the proceeds of the sale once such assets are sold; further all of our financing agreements are secured by credit insurance.
Because we purchase credit insurance on all our financing transactions our credit insurance underwriter performs extensive credit evaluations, which we rely on. Accordingly, because our advances are fully secured by the proceeds of short term accounts receivables or products being delivered to our borrower’s customer’s and all of our advances are further secured by credit insurance, management does not believe that any allowance for doubtful accounts or loan impairment is required.
13. Land Use Rights
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Cost
|
$ | 1,302,024 | $ | 1,261,185 | ||||
|
Less: Accumulated amortization
|
(185,808 | ) | (155,129 | ) | ||||
| $ | 1,116,216 | $ | 1,106,056 | |||||
General
Land Use Rights are generally associated with the long-term use of land underlying a building or production facility in the PRC. These rights have characteristics similar to a real estate property deed under western law in that they are:
|
1.
|
paid for at initial acquisition
|
|
2.
|
have an extended life, up to 50 years
|
|
3.
|
generally relate to a building or production facility
|
|
4.
|
may be pledged as collateral for debt
|
|
5.
|
require no further payments by the owner unless the land usage or building configuration is modified
|
They differ from a real estate property deed in that:
|
1.
|
the transfer of use rights is not permanent
|
|
2.
|
and therefore they are amortized over their life.
|
Sanming Hujian Bio-Engineering, Co., Ltd.
As of December 31, 2010, the Company acquired land use rights to construct its main operating and production facilities for $1.16 million. The land use rights expire in 2054. The Company recognizes $23,178 in expense annually as amortization of its land use rights. The Company has pledged its land use rights with a carrying value of $916,126 as collateral according to a short term borrowing agreement with the Rural Credit Cooperative.
94
Jianou Lujian Foodstuff, Co.
As of December 31, 2010, the Company acquired land use rights to construct its main operating and production facilities for $143,134. The land use rights expire in 2054. The Company recognizes $2,863 of expense annually as amortization of its land use rights. The Company has pledged all its land use rights as collateral according to a loan agreement with the Jianou Sub-Branch of China Construction Bank.
14. Other Assets
Other assets consist of the following:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Land Lease Rights
|
||||||||
|
Forest Bureau of Sanming City
|
$ | 7,952,448 | $ | 9,502,044 | ||||
|
Sanming Sanyuan Forest Bureau
|
1,966,182 | - | ||||||
|
Jianyang Jiuru Liuyun Tea Co , Ltd
|
604,979 | - | ||||||
|
Yushan Town (Harvest Rights)
|
9,074,685 | 5,567,031 | ||||||
|
Restricted Cash (Note 10)
|
845,458 | 388,227 | ||||||
|
Other
|
37,995 | 176,136 | ||||||
| 20,481,747 | 15,633,438 | |||||||
|
Less: Land Lease Rights, current portion
|
(2,922,202 | ) | - | |||||
| $ | 17,559,545 | $ | 15,633,438 | |||||
The estimated aggregate land lease expense and commitment for the five succeeding years and thereafter is as follows:
|
Year
|
Lease Expense
|
Commitment
|
||||||
|
2011
|
$ | 4,819,363 | $ | 2,132,551 | ||||
|
2012
|
4,819,363 | 2,200,611 | ||||||
|
2013
|
4,819,363 | 4,015,547 | ||||||
|
2014
|
4,819,363 | 11,577,785 | ||||||
|
2015
|
4,698,367 | 1.595.632 | ||||||
|
Thereafter
|
77,325,072 | 60,301,465 | ||||||
|
Total
|
$ | 101,300,891 | $ | 81,823,591 | ||||
The estimated aggregate headquarters sub-lease expense and commitment for the three succeeding years is as follows:
|
Year
|
Lease Expense
|
Commitment
|
||||||
|
2011
|
$ | 146,332 | $ | 142,361 | ||||
|
2012
|
146,332 | 146,632 | ||||||
|
2013
|
121,942 | 125,613 | ||||||
| $ | 414,606 | $ | 414,606 | |||||
95
General
Other assets include items which also represent the right to use land but typically these rights have characteristics similar to real estate leases under western law in that they are:
|
1.
|
paid for through a series of interim payments that permit continued use
|
|
2.
|
may have long lives, up to 30 years, but are less than Land Use rights
|
|
3.
|
generally relate to agricultural uses
|
Transfer of Land Use Rights to Land Lease Rights
In July 2009, the Company filed an application with the PRC requesting the exchange of the land use right agreement dated December 31, 2006 for the development of the 15.33 hectare of land in the Jindong Bio-industry Development Zone located in Sanming City, for a land lease agreement containing 667 hectare of agricultural land located in Sanyuan District of Sanming City. The application included a request to transfer the deposit paid in December 2006 for the land use rights be applied to the land lease agreement to be used to net-off against the rent of the land lease agreement.
The PRC government approved the application for the exchange of land use rights for the new land lease rights in July 2009. This entitled the Company a land lease right of 667 hectares of state owned cultivation bases from the Forestry Bureau of Sanming City. Additionally, the deposit paid on December 31, 2006 for the land use rights was approved to transfer to the land lease to be used to off-set against the rent of the land lease agreements. As a result of the PRC’s approval, we transferred $7,656,134 previous recorded land use rights to land lease rights with the Forestry Bureau of Sanming City. The effect of this transfer had no impact on the statement of operations.
Forestry Bureau of Sanming City
In July 2009, the Company entered into 2 land use agreements with the Forestry Bureau of Sanming City to use agricultural land on 2 plantations to grow essential botanical raw materials to support the Company’s operations. The agreement provides for payments every five years and will expire in 2039. As part of this agreement, the Company was required to prepay the first five year leasing period in 2009 which includes an initial nonrefundable deposit which can be offset against the rental of the last year of the last leasing period.
Sanming Sanyuan Forestry Bureau
In April 2010, the Company entered into a land leasing agreement with Sanming Sanyuan Forestry Bureau. According to the agreement, the Company acquired the rights to a 30 year lease to the sarcandra cultivation base located in Sanming Sanyuan Louyuan Hills. In return, every three years the Company is required to pay the Forestry Bureau prepaid rent. The Company was also required to pay a deposit of which will be netted off against rent for the last year of the leasing period.
Jianyang Jiuru Liuyun Tea Co, Ltd
In November 2010, the Company entered into a 5 year lease agreement with Jianyang Jiuru Liuyun Tea Co, Ltd effective December 1, 2010 in the Changping Village, Masha County, Jianyang City. According to the agreement, the Company will pay the lease rental payments yearly and a non-refundable deposit upon execution of the agreement. The deposit will be netted off against the rental for the last 6 months of the leasing period.
96
Yushan Town (harvest rights)
In 2007, UGTI entered into 2 land leasing agreements with Jixi Village and Linkou Village, Yushan Town for exclusive harvest rights to the bamboo shoots and bamboo grown in the plantations based in the two villages, which are an essential botanical raw materials used to support the UGTI operations. The agreement provides for annual payments on an accelerated schedule which will expire in 2037. As part of this agreement, UGTI was required to make an initial nonrefundable deposit which will be offset against the rental of the last 10 years of the leasing period
15. Defined Contribution Plan
Pursuant to the relevant PRC regulations, the Company is required to make contributions at a rate of 18% of the average salaries for the latest fiscal year-end of Fujian Province to a defined contribution retirement plan organized by a state-sponsored social insurance plan in respect of the retirement benefits for the Company's employees in the PRC. The only obligation of the Company with respect to retirement plan is to make the required contributions under the plan. No forfeited contribution is available to reduce the contribution payable in the future years. The defined contribution plan contributions were charged to the statement of income and comprehensive income.
The Company contributed $85,581 and $52,679 for the years ended December 31, 2010 and 2009, respectively.
16. Related Party Transactions
Amounts due to the related parties are payable to entities controlled by shareholders, officers or directors of the Company as well as transactions with these related parties. These amounts are non-interest bearing, unsecured and not subject to specific terms of repayment unless stated otherwise.
|
For the Year Ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Proceeds paid to an entity whose shareholder
|
||||||||
|
is a director
|
$ | 20,641 | $ | 4,709 | ||||
|
Repurchase of 148,000 common shares
|
- | 740,000 | ||||||
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Demand loans with no interest and
|
||||||||
|
recorded within loans payable
|
$ | 932,500 | $ | - | ||||
| Debentures issued by a subsidiary | 875,000 | 875,000 | ||||||
|
Amount due to shareholders
|
40,676 | 157,950 | ||||||
The above demand loans are associated with a $3.0 million line of credit established February 2, 2010, by the Chairman of the Company and a company owned by the CEO of the Company, to provide working capital to the Company. The Chairman and CEO, respectively, partly funded the loans under the facility from third party borrowings and pledged 486,500 of their individually owned shares of the Company’s common stock as a guarantee to the third parties providing the financing.
The above debentures were issued by a subsidiary to the Chairman of the Company and to an officer of the subsidiary for loans provided to the subsidiary. The loans are collateralized by a first lien on the receivables of the subsidiary and matures in 1 year. Interest is payable at 12% per annum and due at maturity.
The above transactions are in the normal course of operations and have been measured at the exchange amount which is the amount of consideration established and agreed to by the related parties.
97
17. Capital Stock
The Company is authorized to issue 10 million shares of Preferred Stock with a par value of $0.001.
In January 2010, the Company issued 10,000 shares of Series A Preferred Stock to the Chairman of the Board and CEO in the amount of 3,733 and 6,267 respectively. This action, approved by the Board, was in consideration and recognition of the agreement by the executives to pledge 6 million shares of Common Stock in satisfaction of the share pledge condition of the Bridge Loan Financing Agreement. Holders of the Series A Preferred Stock shall be entitled to cast two thousand (2,000) votes for each share held of the Series A Preferred Stock on all matters presented to the shareholders of the Company for a shareholder vote which shall vote along with holders of the Company’s Common Stock on such matters
The Company is authorized to issue 100 million shares of Common Stock with a par value of $0.001. Each common share entitles the holder to one vote.
During the year ended December 31, 2010, the Company had the following Common Stock transactions:
|
1.
|
Issued 260,000 shares to purchase from non-controlling interest in a subsidiary.
|
|
2.
|
Issued 63,575 shares for services rendered.
|
|
3.
|
Repurchased 16,000 shares from executives
|
|
4.
|
Issued 594,444 shares to investors
|
|
5.
|
Reduced 40,000 shares as adjustment of contingent purchase price for a subsidiary
|
The Company executed a 1 for 5 stock split effective August 30, 2010. All shares reported in this filing are reflective of the 1 for 5 split and the following table reflects the beginning and ending balances for the fiscal years presented and the effects of the 1 for 5 split of the common stock.
|
Financial Statement
|
||||||||
|
Date
|
Pre-Split Shares
|
Post-Split Shares
|
||||||
|
1/1/2008
|
23,501,060 | 4,700,212 | ||||||
|
12/31/2008
|
23,563,061 | 4,712,612 | ||||||
|
12/31/2009
|
29,171,990 | 5,834,398 | ||||||
18. Warrants
The Company does not have a formal plan in place for the issuance of stock warrants. However, at times, the Company will issue warrants to non-employees or in connection with financing transactions. The exercise price, vesting period, and term of these warrants is determined by the Company’s Board of Directors at the time of issuance. A summary of warrants at December 31, 2010 and activity during the year then ended is presented below:
98
|
Weighted-
|
||||||||||||||||
|
Average
|
||||||||||||||||
|
Weighted-
|
Remaining
|
|||||||||||||||
|
Average
|
Contractual
|
Aggregate
|
||||||||||||||
|
Exercise
|
Term (in
|
Intrinsic
|
||||||||||||||
|
Shares
|
Price
|
years)
|
Value
|
|||||||||||||
|
|
||||||||||||||||
|
Outstanding at January 1, 2010
|
339,200 | $ | 25.71 | 3.7 | ||||||||||||
|
Issued
|
114,733 | 25.00 | 5.0 | |||||||||||||
|
Exercised
|
- | - | ||||||||||||||
|
Forfeited
|
(98,733 | ) | 25.00 | 5.0 | ||||||||||||
|
Outstanding at December 31, 2010
|
355,200 | $ | 25.68 | 3.7 | $ | 427,412 | ||||||||||
|
Exercisable at December 31, 2010
|
355,200 | $ | 25.68 | 3.7 | $ | 427,412 | ||||||||||
The following information applies to warrants outstanding and exercisable at December 31, 2010:
| Warrants Outstanding |
Warrants Exercisable
|
||||
|
Weighted-
|
|||||
|
Average
|
Weighted-
|
Weighted-
|
|||
|
Remaining
|
Average
|
Average
|
|||
|
Contractual
|
Exercise
|
Exercise
|
|||
|
Shares
|
Term
|
Price
|
Shares
|
Price
|
|
|
|
|||||
|
$20.05-25.00
|
331,200
|
3.7
|
$25.00
|
331,200
|
$25.00
|
|
$25.05-35.00
|
24,000
|
3.9
|
$35.00
|
24,000
|
$35.00
|
|
355,200
|
335,200
|
||||
During the year ended December 31, 2010, the Company issued the following warrants:
99
Bridge Loan –
For the year ended December, 2010, the Company issued warrants to purchase 98,733 shares in connection with a bridge loan financing transaction. Effective June 30, 2010 the bridge loan was modified to reflect an extension of the loan maturity date, cancellation of the loan convertible option and cancellation of warrants associated with the loan.
The Company issued warrants to purchase 16,000 common shares to an individual investor. The fair value of the warrants was determined by the Black-Scholes valuation model. The warrants were subject to certain “lock-up and leak out” provisions and expire on the fifth anniversary of the issuance date.
The Company uses the Black-Scholes valuation model to determine the fair value of warrants on the date of grant. This model derives the fair value of options based on certain assumptions related to the expected stock price volatility, expected life, risk-free interest rates and dividend yield. For the year through December 31, 2010, the Company’s expected volatility is based on actual fluctuations in its share prices. The risk-free interest rate assumption is based upon the U.S. Treasury yield curve appropriate for the term of the expected life of the options.
For the year ended December 31, 2009, the fair value of each warrant grant was estimated on the date of grant using the following weighted-average assumptions.
|
For the Year Ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Expected dividend yield
|
0.0 | % | 0.0 | % | ||||
|
Expected price volatility
|
30.0 | % | 30.0 | % | ||||
|
Risk free interest rate
|
1.35 | % | 1.35 | % | ||||
|
Expected life of warrants in years
|
3.7 | 3.9 | ||||||
19. Stock Based Compensation
During the year ended December 31, 2010, we recognized total non-cash stock-based compensation of $145,842. All stock option expenses and service fees are booked to general and administrative expenses.
We had issued 500,000 options on September 1, 2009 to three management personnel of the Company. Of these options, 20% vest six months from issue date (with $16.25 exercise price) with 40% each vesting on the first and second anniversary of the issue date ($17.50 and $18.75 exercise price, respectively). The options expire on September 1, 2014. The aggregate fair value of the options granted was $23,035 at the date of grant, which was determined using the Black-Scholes option valuation model with the following assumptions: fair value stock price of $3.36 at grant date, risk-free interest rate of 2.33%, volatility of 40%, nil expected dividends and expected life of 5 years. Additionally, we issued 34,000 options to two management personnel which vest on the completion of future milestones. The options have an exercise price of $15.00 and expire three years after achieving milestones.
The above options issued to various management personnel were cancelled as of September 30, 2010.
100
We also issued 15,000 options to five non-employee directors and 500 options to a consultant on January 12, 2010 and recorded an expense of $36,244 for the year ended December 31, 2010. The options vest 25% per quarter over the first year and expire on January 12, 2015, with an exercise price of $30.45. The aggregate fair value of the options granted was $43,973 at the date of grant, which was determined using the Black-Scholes option valuation model with the following assumptions: fair value stock price of $30.45 at grant date, risk-free interest rate of 2.5%, average volatility of 12%, nil expected dividends and expected life of 2 years.
The Options activity during the year ended December 31, 2010 is as follows:
| Number of Options | ||||||||||||||||||||
|
Outstanding
|
Outstanding
|
|||||||||||||||||||
|
as of
|
Granted/
|
as of
|
||||||||||||||||||
|
Exercise
|
January 1,
|
forfeited/
|
December 31,
|
|||||||||||||||||
|
Month of grant
|
price
|
2010
|
Exercised
|
cancelled
|
2010
|
|||||||||||||||
|
|
||||||||||||||||||||
|
September 2009
|
$ | 15.00 | 34,000 | - | (34,000 | ) | - | |||||||||||||
|
September 2009
|
$ | 16.25-18.75 | 500,000 | - | (500,000 | ) | - | |||||||||||||
|
January 2010
|
$ | 30.00 | - | - | 500 | 500 | ||||||||||||||
|
January 2010
|
$ | 30.45 | - | - | 15,000 | 15,000 | ||||||||||||||
| 534,000 | - | (518,500 | ) | 15,500 | ||||||||||||||||
20. Statutory Reserve
The Company’s income is distributable to its shareholders after transfer to statutory reserves as required under relevant PRC laws and regulations and the Company’s articles of association. As stipulated by the relevant laws and regulations in the PRC, the Company is required to maintain a statutory surplus reserve fund which is non-distributable. Appropriation to such reserves is set aside from the net profit after taxation of the statutory financial statements of the respective PRC entity yearly of 10%.
The statutory surplus reserve fund can be used to make up prior year losses, if any, and can be applied for conversion into capital by means of capitalization issue. The appropriation may cease to apply if the balance of the fund has reached 50% of the relevant PRC entity’s registered capital.
21. Taxation
United States
The Company is subject to the United States of America Tax law at tax rate of approximately 40%. No provision for the US federal income taxes has been made as the Company had no taxable income in this jurisdiction for the reporting years. The Company has not provided deferred taxes on undistributed earnings of its non-U.S. subsidiaries or VIE as of December 31, 2010 as it was the Company’s current policy to reinvest these earnings in non-U.S. operations.
BVI
Elevated Throne and Supreme Discovery Group were incorporated in the BVI and, under the current laws of the BVI, are not subject to income taxes.
PRC
The PRC’s legislative body, the National People’s Congress, adopted the unified Corporate Income Tax Law on March 16, 2007. This new tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rate is set at 25% for both domestic enterprises and foreign-invested enterprises.
Accordingly, Fujian Green Planet, Sanming Huajian, Jianou Lujian Foodstuff and Fujian United Bamboo, which are established in the PRC, are subject to PRC enterprise income tax at the rate of 25% on their assessable profits during the years ended December 31, 2010 and 2009.
101
The components of the provision for income taxes are:
|
The components of the provision for income taxes are:
|
||||||||
|
For the Year Ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Current taxes – PRC
|
$ | 3,165,322 | $ | 1,980,394 | ||||
|
Deferred taxes
|
115,831 | 46,915 | ||||||
| $ | 3,281,163 | $ | 2,027,309 | |||||
Deferred tax assets as of December 31, 2010 and 2009 comprise the following:
|
As of December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
The PRC
|
||||||||
|
Current deferred tax liabilities/(assets):
|
||||||||
|
Decelerated amortization of intangible assets
|
$ | (4,537 | ) | $ | (4,395 | ) | ||
|
Provision of expenses
|
(49,224 | ) | (134,673 | ) | ||||
|
Changes in capitalized rental expenses
|
115,958 | 109,876 | ||||||
|
Expenses adjusted due to tax exemption
|
153,392 | - | ||||||
| $ | 215,589 | $ | (29,192 | ) | ||||
|
Non-current deferred tax (assets)/liabilities:
|
||||||||
|
Accelerated amortization of intangible assets
|
$ | (20,040 | ) | $ | 18,679 | |||
|
Provision of expenses
|
- | 28,802 | ||||||
|
Rental expenses capitalized in inventory
|
- | (148,581 | ) | |||||
| $ | (20,040 | ) | $ | 101,100 | ||||
|
The following table reconciles the Group’s effective tax rates:
|
||||||||
|
For the Year Ended December 31,
|
||||||||
| 2010 | 2009 | |||||||
|
PRC income taxes
|
25 | % | 25 | % | ||||
|
Local income tax adjustment
|
1.5 | % | (7.5 | )% | ||||
|
Effective income tax rates
|
26.5 | % | 17.5 | % | ||||
102
22. Contingencies
From time to time, the Company may be exposed to claims and legal actions in the normal course of business, some of which may be initiated by the Corporation. As of December 31, 2010, no material claims were outstanding.
23. Segmented Information
The Company uses the ―management approach in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s executive management for making operational decisions and assessing performance as the source for determining the Company’s reportable segments. Accordingly, in accordance with ASC Topic 280-10 ―Segment Reporting , the Company’s operations comprises three reporting segments engaged in herbal and chemical extracts, organic products and financing within Canada, PRC and the United States.
The following represents the segmented information based on geographical distribution as at December 31:
|
For the Year Ended
|
North
|
|||||||||||
|
December 31, 2010
|
Asia
|
America (1)
|
Total
|
|||||||||
|
Revenues
|
$ | 41,080,761 | $ | 11,215,442 | $ | 52,296,203 | ||||||
|
Cost of sales
|
22,925,137 | 9,245,625 | 32,170,762 | |||||||||
|
Gross profit
|
18,155,624 | 1,969,817 | 20,125,441 | |||||||||
|
Operating expenses
|
3,504,276 | 1,459,748 | 4,964,024 | |||||||||
|
Operating profit
|
$ | 14,651,348 | $ | 510,069 | 15,161,417 | |||||||
|
Corporate expenses,
|
||||||||||||
|
including amortization
|
1,479,869 | |||||||||||
|
Interest/Other expenses
|
1,445,212 | |||||||||||
|
Income before income taxes
|
||||||||||||
|
and non-controlling interest
|
$ | 12,236,336 | ||||||||||
|
Assets at December 31, 2010
|
$ | 58,024,122 | $ | 12,061,383 | $ | 70,085,505 | ||||||
(1) North America segment includes $907,836 of interest income classified as Sales and $867,415 of interest expense classified as Cost of sales.
103
|
For the Year Ended
|
North
|
|||||||||||
|
December 31, 2009
|
Asia
|
America (1)
|
Total
|
|||||||||
|
Revenues
|
$ | 19,402,733 | $ | 2,670,485 | $ | 22,073,218 | ||||||
|
Cost of sales
|
9,479,144 | 2,609,885 | 12,089,029 | |||||||||
|
Gross profit
|
9,923,589 | 60,600 | 9,984,189 | |||||||||
|
Operating expenses
|
1,914,771 | (92,067 | ) | 1,822,704 | ||||||||
|
Operating profit
|
$ | 8,008,818 | $ | 152,667 | 8,161,485 | |||||||
|
Corporate expenses
|
||||||||||||
|
including amortization
|
702,732 | |||||||||||
|
Interest/Other expenses
|
46,471 | |||||||||||
|
Income before income taxes
|
||||||||||||
|
and non-controlling interest
|
$ | 7,412,282 | ||||||||||
|
Assets at December 31, 2009
|
$ | 41,650,473 | $ | 12,983,378 | $ | 54,633,851 | ||||||
(1) Financing segment includes $1,285,316 of interest income classified as Sales; and $648,865 of interest expense classified as Cost of sales.
We currently operate 3 business units as follows:
CHE – Our CHE business unit produces chemical and herbal extracts for use in a wide range of health and wellness products. This business unit is organized under Elevated Throne (previously under Green Planet).
OP – Our OP business unit manufactures a variety of consumer and commercial use health and energy products, organic food products and fertilizers primarily based on bamboo. This business unit is organized under UGTI and its acquired subsidiary Supreme.
FIN – Our Fin business unit was acquired to support our international expansion by handling the logistics, fulfillment and financing for the group’s sales out PRC. FIN provides balance sheet financing solutions and mitigates the group’s international credit exposure by insuring all international receivables through A rated third party insurance providers. This business unit is organized under TFS.
104
The following represents the segmented information based on operating segments as of December 31, 2010:
|
Chemical
|
||||||||||||||||||||
|
and Herbal
|
Organic
|
|||||||||||||||||||
|
For the Year Ended
|
Extracts
|
Products
|
Financing (1)
|
|||||||||||||||||
|
December 31, 2010
|
(CHE)
|
(OP)
|
(FIN)
|
Corporate
|
Total
|
|||||||||||||||
|
Revenues
|
$ | 19,223,711 | $ | 21,857,050 | $ | 11,215,442 | $ | - | $ | 52,296,203 | ||||||||||
|
Cost of sales
|
9,653,267 | 13,271,870 | 9,245,625 | - | 32,170,762 | |||||||||||||||
|
Gross profit
|
9,570,444 | 8,585,180 | 1,969,817 | - | 20,125,441 | |||||||||||||||
|
Operating expenses
|
2,496,527 | 1,007,749 | 1,459,748 | - | 4,964,024 | |||||||||||||||
|
Operating profit
|
$ | 7,073,917 | $ | 7,577,431 | $ | 510,069 | $ | - | 15,161,417 | |||||||||||
|
Corporate expenses,
|
||||||||||||||||||||
|
including amortization
|
1,479,869 | |||||||||||||||||||
|
Interest/Other expenses
|
1,445,209 | |||||||||||||||||||
|
Income before income
|
||||||||||||||||||||
|
taxes and non-
|
||||||||||||||||||||
|
controlling interest
|
$ | 12,236,339 | ||||||||||||||||||
|
Assets at
|
||||||||||||||||||||
|
December 31, 2010
|
$ | 31,442,711 | $ | 26,571,022 | $ | 9,308,011 | $ | 2,763,761 | $ | 70,085,505 | ||||||||||
(1) Financing segment includes $907,836 of interest income classified as Sales; and $867,415 of interest expense classified as Cost of sales.
|
Chemical
|
||||||||||||||||||||
|
and Herbal
|
Organic
|
|||||||||||||||||||
|
For the Year Ended
|
Extracts
|
Products
|
Financing (1)
|
|||||||||||||||||
|
December 31, 2009
|
(CHE)
|
(OP)
|
(FIN)
|
Corporate
|
Total
|
|||||||||||||||
|
Revenues
|
$ | 13,297,616 | $ | 6,105,117 | $ | 2,670,485 | $ | - | $ | 22,073,218 | ||||||||||
|
Cost of sales
|
5,553,342 | 3,925,802 | 2,609,885 | - | 12,089,029 | |||||||||||||||
|
Gross profit
|
7,744,274 | 2,179,315 | 60,600 | - | 9,984,189 | |||||||||||||||
|
Operating expenses
|
1,803,093 | 111,678 | (92,067 | ) | - | 1,822,704 | ||||||||||||||
|
Operating profit
|
$ | 5,941,181 | $ | 2,067,637 | $ | 152,667 | $ | - | 8,161,485 | |||||||||||
|
Corporate expenses,
|
||||||||||||||||||||
|
including amortization
|
702,732 | |||||||||||||||||||
|
Interest/Other expenses
|
46,471 | |||||||||||||||||||
|
Income before income
|
||||||||||||||||||||
|
taxes and non-
|
||||||||||||||||||||
|
controlling interest
|
$ | 7,412,282 | ||||||||||||||||||
|
Assets at
|
||||||||||||||||||||
|
December 31,2009
|
$ | 34,103,266 | $ | 7,547,207 | $ | 7,748,681 | $ | 5,234,697 | $ | 54,633,851 | ||||||||||
During the years ended December 31, 2010 and December 31, 2009, the Company had no sales to any customer that exceeded 10% of total revenues.
105
23. Subsequent Events
Form 8K filed on January 7, 2011 announcing that on December 20, 2010, ONE entered into a loan extension agreement with an effective date of December 10, 2010, with UTA Capital and other investors to extend and modify the securities purchase agreement to January 31, 2011.
Form 8K filed on January 25, 2011 announcing that the Company had filed with the Securities and Exchange Commission a request to withdraw its registration statement regarding a proposed offering of shares of its common stock.
Form 8K filed on March 9, 2011, announcing that effective January 31, 2011, the Company has entered into a third loan extension agreement with UTA Capital and other investors to extend the Securities Purchase Agreement to April 1, 2011 with an option to further extend to May 1, 2010 and June 1, 2011.
106
