Attached files
| file | filename |
|---|---|
| EX-12.1 - EXHIBIT 12.1 - Integer Holdings Corp | c13388exv12w1.htm |
| EX-31.2 - EXHIBIT 31.2 - Integer Holdings Corp | c13388exv31w2.htm |
| EX-21.1 - EXHIBIT 21.1 - Integer Holdings Corp | c13388exv21w1.htm |
| EX-23.1 - EXHIBIT 23.1 - Integer Holdings Corp | c13388exv23w1.htm |
| EX-31.1 - EXHIBIT 31.1 - Integer Holdings Corp | c13388exv31w1.htm |
| EX-32.1 - EXHIBIT 32.1 - Integer Holdings Corp | c13388exv32w1.htm |
Table of Contents
SECURITIES AND EXCHANGE COMMISSION
OF THE SECURITIES EXCHANGE ACT OF 1934
| Delaware | 16-1531026 | |
| (State of Incorporation) | (I.R.S. Employer Identification No.) |
Clarence, New York 14031
(Address of principal executive offices)
(Registrant’s telephone number, including area code)
| Title of Each Class: | Name of Each Exchange on Which Registered: | |
| Common Stock, Par Value $0.001 Per Share | New York Stock Exchange | |
| Preferred Stock Purchase Rights | New York Stock Exchange |
| Large accelerated filer o | Accelerated filer þ | Non- accelerated filer o | Smaller reporting company o |
| Document | Part | |
Proxy Statement for
the 2011 Annual
Meeting of
Stockholders
|
Part III, Item 10 “Directors, Executive Officers and Corporate Governance” |
|
| Part III, Item 11 “Executive Compensation” |
||
| Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” |
||
| Part III, Item 13 “Certain Relationships and Related Transactions, and Director Independence” |
||
| Part III, Item 14 “Principal Accounting Fees and Services” |
| ITEM | PAGE | |||||||
| NUMBER | NUMBER | |||||||
| PART I |
||||||||
| 3 | ||||||||
| 18 | ||||||||
| 27 | ||||||||
| 27 | ||||||||
| 27 | ||||||||
| 27 | ||||||||
| PART II |
||||||||
| 28 | ||||||||
| 29 | ||||||||
| 30 | ||||||||
| 52 | ||||||||
| 54 | ||||||||
| 100 | ||||||||
| 100 | ||||||||
| 100 | ||||||||
| PART III |
||||||||
| 101 | ||||||||
| 101 | ||||||||
| 101 | ||||||||
| 101 | ||||||||
| 101 | ||||||||
| PART IV |
||||||||
| 101 | ||||||||
| 102 | ||||||||
| Exhibit 12.1 | ||||||||
| Exhibit 21.1 | ||||||||
| Exhibit 23.1 | ||||||||
| Exhibit 31.1 | ||||||||
| Exhibit 31.2 | ||||||||
| Exhibit 32.1 | ||||||||
2
Table of Contents
| Acquisition Date | Acquired Company | Business at Time of Acquisition | ||
July 1997
|
Wilson Greatbatch Ltd. | Founded in 1970, designed and manufactured batteries for IMDs and commercial applications. | ||
August 1998
|
Hittman Materials and Medical Components, Inc. | Founded in 1962, designed and manufactured ceramic and glass feedthroughs and specialized porous coatings for electrodes used in IMDs. | ||
August 2000
|
Battery Engineering, Inc. | Founded in 1983, designed and manufactured high-energy density batteries for industrial, commercial, military and medical applications. |
3
Table of Contents
| Acquisition Date | Acquired Company | Business at Time of Acquisition | ||
June 2001
|
Sierra-KD Components division of Maxwell Technologies, Inc. | Founded in 1986, designed and manufactured ceramic electromagnetic filtering capacitors and integrated them with wire feedthroughs for use in IMDs as well as military, aerospace and commercial applications. | ||
July 2002
|
Globe Tool and Manufacturing Company, Inc. | Founded in 1954, designed and manufactured precision enclosures used in IMDs and commercial products used in the aerospace, electronics and automotive sectors. | ||
March 2004
|
NanoGram Devices Corporation | Founded in 1996, developed nanoscale materials for battery and medical device applications. | ||
April 2007
|
BIOMEC, Inc. | Established in 1998, provided medical device design and component integration to early-stage and established customers. | ||
June 2007
|
Enpath Medical, Inc. | Founded in 1981, designed, developed, and manufactured venous introducers and dilators, implantable leadwires, steerable sheaths and steerable catheters. | ||
October 2007
|
IntelliSensing LLC | Founded in 2005, designed and manufactured battery-powered wireless sensing solutions for commercial applications. | ||
November 2007
|
Quan Emerteq LLC | Founded in 1998, designed, developed, and manufactured catheters, stimulation leadwires, microcomponents and assemblies. | ||
November 2007
|
Engineered Assemblies Corporation | Founded in 1984, designed and integrated custom battery solutions and electronics focused on rechargeable systems. | ||
January 2008
|
P Medical Holding SA | Founded in 1994, designed, manufactured and supplied delivery systems, instruments and implants for the orthopaedic industry. | ||
February 2008
|
DePuy Orthopaedics’ Chaumont, France manufacturing facility | Manufactured hip and shoulder implants for DePuy Orthopaedics. |
4
Table of Contents
| Device | Principal Illness or Symptom | |
Pacemakers
|
Abnormally slow heartbeat (Bradycardia) | |
ICDs
|
Rapid and irregular heartbeat (Tachycardia) | |
CRT/CRT-Ds
|
Congestive heart failure | |
Neurostimulators
|
Chronic pain, movement disorders, epilepsy, obesity or depression | |
Drug pumps
|
Diabetes or chronic pain |
| • | Advances in medical technology — New therapies will allow physicians to use IMDs to treat a wider range of patients with various heart diseases. |
| • | Increasing device complexity — Device manufacturers are developing new devices with additional features (such as RF telemetry and MRI conditional capabilities) that will require increased energy, power and filtering capabilities. These new features make Greatbatch Medical technologies and innovations more relevant than ever. |
5
Table of Contents
| • | Expanding patient population — The patient groups that are eligible for CRM devices are increasing and the number of people in the U.S. that are over age 50 is expected to double in the next 10 years. Additionally, recent studies may lead to updated guidelines on clinical indications for CRT—increasing the number of patients who may qualify for these devices. Fast growing emerging markets, especially in Asia and Latin America, are getting significant attention from IMD manufacturers given their large population size, under-penetration, expanding purchasing power and increasing expenditure in medical infrastructure and training. These markets represent another growth driver for the Company. |
| • | Growth within neuromodulation — Neuromodulation applications are growing at a faster pace than traditional markets, and are expected to expand as new therapeutic applications are identified. Many CRM OEM companies, which are strategic partners of Greatbatch Medical, are also OEMs in the neuromodulation market, which positions us to capitalize on this market growth. |
6
Table of Contents
| Product | Description | Principal Product Attributes | ||
Batteries
|
Power sources include: | |||
•
Lithium iodine (“Li Iodine”)
|
High reliability and predictability | |||
•
Lithium silver vanadium oxide (“Li SVO”)
|
Long service life | |||
• Lithium carbon monoflouride (“Li CFx”)
|
Customized configuration | |||
• Lithium ion rechargeable (“Li Ion”)
|
Light weight | |||
• Lithium SVO/CFx
(“QHR” & “QMR”)
|
Compact and less intrusive | |||
Capacitors
|
Storage for energy generated by a battery before delivery to the heart. Used in ICDs and CRT-Ds. | Stores more energy per unit volume (energy density) than other existing technologies Customized configuration |
||
EMI filters
|
Filters electromagnetic interference to limit undesirable response, malfunctioning or degradation in the performance of electronic equipment | High reliability attenuation of EMI RF over wide frequency ranges Customized design | ||
Feedthroughs
|
Allow electrical signals to be brought from inside hermetically sealed IMD to an electrode | Ceramic to metal seal is substantially more durable than traditional seals Multifunctional | ||
Coated electrodes
|
Deliver electric signal from the feedthrough to a body part undergoing stimulation | High quality coated surface Flexible in utilizing any combination of biocompatible coating surfaces Customized offering of surfaces and tips | ||
Precision components
|
• Machined
|
High level of manufacturing precision | ||
• Molded and over molded products
|
Broad manufacturing flexibility | |||
Enclosures and
|
• Titanium
|
Precision manufacturing, flexibility in | ||
related components
|
• Stainless steel
|
configurations and materials | ||
Value-added assemblies
|
Combination of multiple components in a single package/unit | Leveraging products and capabilities to provide
subassemblies and assemblies Provides synergies in component technology and procurement systems |
||
Leads
|
Cardiac, neuro and hearing restoration stimulation leads | Custom and unique configurations that increase therapy effectiveness, provide finished device design and manufacturing | ||
Introducers
|
Creates a conduit to insert infusion catheters, guidewires, implantable ports, pacemaker leads and other therapeutic devices into a blood vessel | Variety of sizes and materials that facilitate problem-free access in a variety of clinical applications | ||
Catheters
|
Delivers therapeutic devices to specific sites in the body | Enable safe, simple delivery of therapeutic and
diagnostic devices, soft tip and steerability Provide regulatory clearance and finished device |
||
Cases and trays
|
Delivery systems for cleaning and sterilizing orthopaedic instruments and implants | Deliver turn-key full service kits | ||
Implants
|
Orthopaedic implants for reconstructive hip, knee, shoulder, trauma and spine procedures | Precision manufacturing, leveraging
capabilities and products Complete processes including sterile packaging and coatings |
||
Instruments
|
Orthopaedic instruments for reconstructive and trauma procedures | Designed to improve surgical techniques, reduce surgery time, increase surgical precision and decrease risk of contamination |
7
Table of Contents
| Product | Description | Principal Product Attributes | ||
Cells
|
• Low-rate
|
Optimized rate capability, shock and vibration resistant, high and low temperature tolerant, high energy density | ||
• Moderate-rate
|
||||
• Spiral (high rate) |
||||
Primary and
rechargeable
battery packs
|
Highly-customized pack solutions | Diverse portfolio of cells in various sizes, temperature ranges, and rate capabilities, custom-designed into battery packs for critical applications | ||
Wireless sensors
|
Complete, customized end-to-end wireless sensing solutions measuring temperature, pressure and flow remotely | Wireless sensors and interactive gateway which can withstand the most extreme internal and external industrial environments, provide critical, real-time data delivered directly to a desktop or laptop |
8
Table of Contents
9
Table of Contents
10
Table of Contents
11
Table of Contents
12
Table of Contents
| Product Line | Competitors | |
Greatbatch Medical |
||
Medical batteries
|
Eagle-Picher | |
Capacitors
|
Critical Medical Components | |
Feedthroughs
|
Alberox (subsidiary of The Morgan Crucible Co. PLC) | |
EMI filtering
|
AVX (subsidiary of Kyocera) | |
| Eurofarad | ||
Enclosures
|
Heraeus | |
| Hudson | ||
Machined and molded components
|
Numerous | |
Value added assembly
|
Numerous | |
Catheters
|
Teleflex | |
Leads
|
Oscor | |
Orthopaedic trays, instruments and implants
|
Accelent | |
| Avalign Technologies | ||
| IMDS | ||
| Micropulse, Inc. | ||
| Norwood Medical | ||
| Orchid | ||
| Sandvik | ||
| Symmetry | ||
| Paragon | ||
| Teleflex | ||
Electrochem |
||
Primary Power Solutions
|
Tracer Technologies, Engineered Power, Saft, Ultralife | |
Secondary Power Solutions
|
Micro Power, Nexergy, Ultralife, Saft | |
Wireless Sensing Solutions
|
Vektek |
13
Table of Contents
14
Table of Contents
15
Table of Contents
Manufacturing |
1,486 | |||
General and administrative |
118 | |||
Sales and marketing |
48 | |||
Research, development and engineering |
204 | |||
Chaumont, France facility |
217 | |||
Switzerland facilities |
188 | |||
Tijuana, Mexico facility |
715 | |||
Total |
2,976 | |||
16
Table of Contents
| • | future sales, expenses and profitability; |
| • | future development and expected growth of our business and industry; |
| • | our ability to execute our business model and our business strategy; |
| • | our ability to identify trends within our industries and to offer products and services that meet the changing needs of those markets; and |
| • | projected capital expenditures. |
17
Table of Contents
18
Table of Contents
19
Table of Contents
20
Table of Contents
| • | a substantial percentage of our costs are fixed in nature, which results in our operations being particularly sensitive to fluctuations in production volumes; |
| • | changes in the relative portion of our revenue represented by our various products and customers, which could result in reductions in our profits if the relative portion of our revenue represented by lower margin products increases; |
| • | timing of orders placed by our principal customers who account for a significant portion of our revenues; and |
| • | increased costs of raw materials or supplies. |
21
Table of Contents
22
Table of Contents
| • | inaccurate assessments of potential liabilities associated with the acquired businesses; |
| • | the existence of unknown or undisclosed liabilities associated with the acquired businesses; |
| • | diversion of our management’s attention from our core businesses; |
| • | potential loss of key employees or customers of the acquired businesses; |
| • | difficulties in integrating the operations and products of an acquired business or in realizing projected revenue growth, efficiencies and cost savings; and |
| • | increases in indebtedness and limitation in our ability to access capital if needed. |
23
Table of Contents
| • | changes in foreign regulatory requirements; |
| • | local product preferences and product requirements; |
| • | longer-term receivables than are typical in the U.S.; |
| • | difficulties in enforcing agreements through certain foreign legal systems; |
| • | less protection of intellectual property in some countries outside of the U.S.; |
| • | trade protection measures and import and export licensing requirements; |
| • | work force instability; |
| • | political and economic instability; and |
| • | complex tax and cash management issues. |
24
Table of Contents
25
Table of Contents
26
Table of Contents
| Location | Sq. Ft. | Own/Lease | Principal Use | |||||
Alden, NY |
125,000 | Own | Medical battery and capacitor manufacturing | |||||
Blaine, MN |
32,400 | Own | Medical device engineering | |||||
Chaumont, France |
59,200 | Own | Manufacturing of orthopaedic and surgical goods | |||||
Clarence, NY |
117,800 | Own | Corporate offices and RD&E | |||||
Clarence, NY |
20,800 | Own | Machining and assembly of components | |||||
Clarence, NY |
18,600 | Lease | Machining and assembly of components | |||||
Cleveland, OH |
16,900 | Lease | Office and lab space for design engineering team | |||||
Columbia City, IN |
40,000 | Lease | Manufacturing of orthopaedic and surgical goods | |||||
Corgemont, Switzerland |
34,400 | Lease | Manufacturing of orthopaedic and surgical goods | |||||
Indianapolis, IN |
82,600 | Own | Manufacturing of orthopaedic and surgical goods | |||||
Minneapolis, MN |
72,000 | Own | Enclosure manufacturing and engineering | |||||
Orvin, Switzerland |
40,400 | Own | Manufacturing of orthopaedic and surgical goods | |||||
Plymouth, MN |
95,700 | Lease | Introducers, catheters and leads manufacturing | |||||
Raynham, MA |
81,000 | Own | Commercial battery manufacturing and RD&E | |||||
Tijuana, Mexico |
144,000 | Lease | Value-added assembly, and feedthrough, electrode and EMI filtering manufacturing | |||||
Warsaw, IN |
3,000 | Lease | Orthopaedic rapid prototyping design center | |||||
27
Table of Contents
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
| High | Low | Close | ||||||||||
2009 |
||||||||||||
First Quarter |
$ | 27.45 | $ | 17.27 | $ | 19.71 | ||||||
Second Quarter |
23.48 | 18.50 | 22.00 | |||||||||
Third Quarter |
23.20 | 20.06 | 21.63 | |||||||||
Fourth Quarter |
22.21 | 17.99 | 19.23 | |||||||||
2010 |
||||||||||||
First Quarter |
$ | 21.69 | $ | 18.99 | $ | 20.90 | ||||||
Second Quarter |
24.43 | 19.94 | 22.00 | |||||||||
Third Quarter |
24.00 | 21.35 | 22.84 | |||||||||
Fourth Quarter |
25.11 | 21.61 | 24.15 | |||||||||
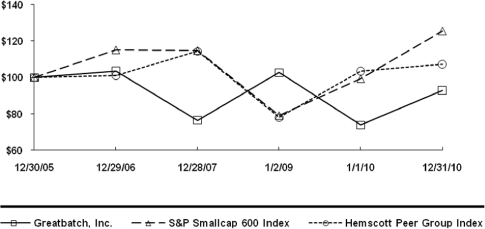
28
Table of Contents
| Years ended | ||||||||||||||||||||
| Dec. 31, | Jan. 1, | Jan. 2, | Dec. 28, | Dec. 29, | ||||||||||||||||
| 2010(1)(3) | 2010(1)(3) | 2009(1)(2) | 2007(1)(2) | 2006(1) | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
Consolidated Statement of Operations Data: |
||||||||||||||||||||
Sales |
$ | 533,425 | $ | 521,821 | $ | 546,644 | $ | 318,746 | $ | 271,142 | ||||||||||
Income (loss) before income taxes |
49,325 | (18,177 | ) | 20,517 | 23,919 | 23,534 | ||||||||||||||
Income (loss) per share |
||||||||||||||||||||
Basic |
$ | 1.44 | $ | (0.39 | ) | $ | 0.63 | $ | 0.54 | $ | 0.74 | |||||||||
Diluted |
1.40 | (0.39 | ) | 0.62 | 0.53 | 0.73 | ||||||||||||||
Consolidated Balance Sheet Data: |
||||||||||||||||||||
Working capital |
$ | 150,922 | $ | 119,926 | $ | 142,219 | $ | 116,816 | $ | 199,051 | ||||||||||
Total assets |
776,976 | 830,543 | 848,033 | 662,769 | 547,827 | |||||||||||||||
Long-term obligations |
289,560 | 317,575 | 379,890 | 247,239 | 205,859 | |||||||||||||||
| (1) | From 2006 to 2010, we recorded material charges in other operating expenses, net, primarily related to our cost savings and consolidation initiatives. Additional information is set forth at Note 9 “Other Operating Expenses, Net” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. | |
| (2) | During 2008, we acquired P Medical Holding, SA (January 2008) and DePuy Orthopaedics’ Chaumont, France facility (February 2008). During 2007, we acquired BIOMEC, Inc. (April 2007), Enpath Medical, Inc. (June 2007), IntelliSensing, LLC (October 2007), Quan Emerteq, LLC (November 2007), and Engineered Assemblies Corporation (November 2007). This data includes the results of operations of these companies subsequent to their acquisition. In connection with these acquisitions, we recorded charges in 2008 and 2007 of $8.7 million and $18.4 million, respectively, related to inventory step-up amortization and the write-off of in process research and development. | |
| (3) | In 2009, we recorded a $34.5 million charge related to litigation involving Electrochem and a $15.9 million write-down of trademarks and tradenames. In 2010, we settled the Electrochem litigation which resulted in a $9.5 million gain. Additional information is set forth at Note 11 “Commitments and Contingencies” and Note 4 “Intangible Assets” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. |
29
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
| • | Our business |
| • | Our acquisitions |
| • | Our customers |
| • | Strategic and financial overview |
| • | Government regulation |
| • | Product development |
| • | Valuation of goodwill and other identifiable intangible assets including IPR&D |
| • | Stock-based compensation |
| • | Inventories |
| • | Tangible long-lived assets |
| • | Provision for income taxes |
| • | Results of operations table |
| • | Fiscal 2010 compared with fiscal 2009 |
| • | Fiscal 2009 compared with fiscal 2008 |
| • | Liquidity and capital resources |
| • | Off-balance sheet arrangements |
| • | Litigation |
| • | Contractual obligations |
| • | Inflation |
| • | Impact of recently issued accounting standards |
30
Table of Contents
31
Table of Contents
32
Table of Contents
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Operating income as reported: |
$ | 68,994 | $ | 1,048 | $ | 34,894 | ||||||
Executive death benefits (SG&A) |
885 | — | — | |||||||||
Consolidation costs |
1,348 | 7,069 | 9,010 | |||||||||
Integration costs |
42 | 3,077 | 5,369 | |||||||||
Asset dispositions, severance and other |
3,168 | 948 | 199 | |||||||||
Electrochem Litigation charge (gain) |
(9,500 | ) | 34,500 | — | ||||||||
Intangible asset write-down |
— | 15,921 | — | |||||||||
Acquired IPR&D |
— | — | 2,240 | |||||||||
Acquisition charges (inventory step-up) |
— | — | 6,422 | |||||||||
Operating income — adjusted |
$ | 64,937 | $ | 62,563 | $ | 58,134 | ||||||
Operating margin — adjusted |
12.2 | % | 12.0 | % | 10.6 | % | ||||||
33
Table of Contents
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Income (loss) before taxes as reported: |
$ | 49,325 | $ | (18,177 | ) | $ | 20,517 | |||||
Executive death benefits (SG&A) |
885 | — | — | |||||||||
Consolidation costs |
1,348 | 7,069 | 9,010 | |||||||||
Integration costs |
42 | 3,077 | 5,369 | |||||||||
Asset dispositions, severance and other |
3,168 | 948 | 199 | |||||||||
Electrochem Litigation charge (gain) |
(9,500 | ) | 34,500 | — | ||||||||
Intangible asset write-down |
— | 15,921 | — | |||||||||
Acquired IPR&D |
— | — | 2,240 | |||||||||
Acquisition charges (inventory step-up) |
— | — | 6,422 | |||||||||
Sub-total |
45,268 | 43,338 | 43,757 | |||||||||
CSN II conversion option discount amortization |
7,876 | 7,311 | 6,786 | |||||||||
Gain on extinguishment of debt & sale of
investment security |
— | — | (3,242 | ) | ||||||||
Adjusted income before taxes |
53,144 | 50,649 | 47,301 | |||||||||
Adjusted provision for income taxes |
17,524 | 14,688 | 14,427 | |||||||||
Adjusted net income |
$ | 35,620 | $ | 35,961 | $ | 32,874 | ||||||
Adjusted diluted EPS |
$ | 1.51 | $ | 1.52 | $ | 1.40 | ||||||
Number of shares(a) |
23,802 | 23,983 | 24,128 | |||||||||
| (a) | Adjusted shares outstanding used for calculating adjusted diluted EPS for 2009 and 2008 include the dilutive impact of outstanding equity awards and our convertible subordinated notes of 1,057,000 and 1,267,000, respectively, that were not dilutive for GAAP purposes. |
34
Table of Contents
| 1. | Develop systems and device solutions for our customers in the markets we operate in; | ||
| 2. | Continued development of MRI compatible leadwires and other Neuromodulation products; | ||
| 3. | Continued development of higher energy/higher density capacitors; | ||
| 4. | The design of next generation steerable catheters, sheaths and introducers; | ||
| 5. | Further develop minimally invasive surgical techniques for the Orthopaedic industry; | ||
| 6. | Develop disposable instrumentation for the Orthopaedic industry; | ||
| 7. | Provide wireless sensing solutions to Electrochem customers; and | ||
| 8. | Develop a charging platform for Electrochem’s secondary offering. |
35
Table of Contents
| Balance Sheet Caption / | ||||
| Nature of Critical | ||||
| Estimate Item | Assumptions / Approach Used | Effect of Variations of Key Assumptions Used | ||
Valuation of goodwill and
other identifiable
intangible assets
including IPR&D When we acquire a company, we allocate the purchase price to the tangible and intangible assets we acquire and liabilities we assume based on their fair value at the date of acquisition, including IPR&D. Intangible assets, such as trademarks and tradenames, are considered non-amortizing intangible assets as they are expected to generate cash flows indefinitely. Goodwill is recorded when the purchase price paid for an acquisition exceeds the estimated fair value of the net identified tangible and intangible assets acquired. Indefinite lived intangibles and goodwill are not amortized but are required to be assessed for impairment on an annual basis or more frequent if certain indicators are present. Definite-lived intangible assets are amortized over their estimated useful lives and are assessed for impairment if certain indicators are present. |
We base the fair value of
identifiable tangible and
intangible assets on
detailed valuations that
use information and
assumptions provided by
management. The fair
values of the assets
acquired and liabilities
assumed are determined
using one of three
valuation approaches:
market, income or cost.
The selection of a
particular method for a
given asset depends on the
reliability of available
data and the nature of the
asset. The market
approach values the asset
based on available market
pricing for comparable
assets. The income
approach values the asset
based on the present value
of risk adjusted cash
flows projected to be
generated by that asset.
The projected cash flows
for each asset considers
multiple factors,
including current revenue
from existing customers,
attrition trends,
reasonable contract
renewal assumptions from
the perspective of a
marketplace participant,
and expected profit
margins giving
consideration to
historical and expected
margins. The cost
approach values the asset
by determining the current
cost of replacing that
asset with another of
equivalent economic
utility. The cost to
replace the asset reflects
the estimated reproduction
or replacement cost, less
an allowance for loss in
value due to depreciation
or obsolescence, with
specific consideration
given to economic
obsolescence if indicated. We perform an annual review on the last day of each fiscal year, or more frequently if indicators of potential impairment exist, to determine if the recorded goodwill and other indefinite lived intangible assets are impaired. We assess goodwill for impairment by comparing the fair value of our reporting units to their carrying value to determine if there is potential impairment. If the fair value of a reporting unit is less than its carrying value, an impairment loss is recorded to the extent that the implied fair value of the goodwill within the reporting unit is less than its carrying value. Fair values for reporting units are determined based on the income and market approaches. Indefinite lived intangible assets such as trademarks and tradenames are evaluated for impairment by using the income approach. Definite-lived intangible assets such as purchased technology, patents and customer lists are reviewed at least quarterly to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment or a change in their remaining useful life. We do not believe that the Greatbatch tradename or goodwill allocated to our Greatbatch Medical or Electrochem segments are at risk of failing step one of future annual impairment tests unless operating conditions significantly deteriorate. |
The use of alternative valuation
assumptions, including estimated cash flows
and discount rates, and alternative
estimated useful life assumptions could
result in different purchase price
allocations. In arriving at the value of
the IPR&D, we additionally consider among
other factors: the in-process projects
stage of completion; commercial feasibility
of the project; the complexity of the work
completed; the projected costs to complete
and commercialize the project; and the
estimated useful life of the technology.
Significant changes in these estimates and
assumptions could impact the value of the
assets and liabilities recorded, which
would change the amount and timing of
future intangible asset amortization
expense. We make certain estimates and assumptions that affect the expected future cash flows of our reporting units for our goodwill impairment testing. These include discount rates, terminal values and projections of future revenues and expenses. Significant changes in these estimates and assumptions could create future impairment losses to our goodwill. The assumptions used in our 2010 impairment test incorporate growth rates disclosed in “2011 Sales Outlook” of this section. For trademarks and tradenames, we make estimates of royalty rates, future revenues and discount rates. Significant changes in these estimates could create future impairments of these indefinite lived intangible assets. Estimation of the useful lives of indefinite- and definite-lived intangible assets is based upon the estimated cash flows of the respective intangible asset and requires significant management judgment. Events could occur that would materially affect our estimates of the useful lives. Significant changes in these estimates and assumptions could change the amount of future amortization expense or could create future impairments of these intangible assets. As of December 31, 2010, we have $402.9 million of intangible assets recorded on our balance sheet representing 52% of total assets. This includes $75.1 million of amortizing intangible assets, $20.3 million of indefinite lived intangible assets and $307.5 million of goodwill. A 1% change in the amortization of our intangible assets would change 2010 net income by approximately $0.06 million, or approximately $0.003 per diluted share. |
36
Table of Contents
| Balance Sheet Caption / | ||||
| Nature of Critical | ||||
| Estimate Item | Assumptions / Approach Used | Effect of Variations of Key Assumptions Used | ||
Stock-based compensation We record compensation costs related to our stock-based awards which include stock options, restricted stock and restricted stock units. We measure stock-based compensation cost at the grant date based on the fair value of the award. Compensation cost for service-based awards is recognized ratably over the applicable vesting period. Compensation cost for performance awards based on Company metrics is reassessed each period and recognized based upon the probability that the performance targets will be achieved. Compensation cost for performance awards based on market metrics (such as total shareholder return) is expensed each period whether the performance metrics are achieved or not. The amount of stock-based compensation expense recognized during a period is based on the portion of the awards that are ultimately expected to vest, excluding market and nonmarket performance award considerations. The total expense recognized over the vesting period will only be for those awards that ultimately vest, excluding market and nonmarket performance award considerations. |
We utilize the
Black-Scholes Options
Pricing Model to determine
the fair value of stock
options. We are required
to make certain
assumptions with respect
to selected Black Scholes
model inputs, including
expected volatility,
expected life, expected
dividend yield and the
risk-free interest rate.
Expected volatility is
based on the historical
volatility of our stock
over the most recent
period commensurate with
the estimated expected
life of the stock options.
The expected life of
stock options granted,
which represents the
period of time that the
stock options are expected
to be outstanding, is
based, primarily, on
historical data. The
expected dividend yield is
based on our history and
expectation of dividend
payouts. The risk-free
interest rate is based on
the U.S. Treasury yield
curve in effect at the
time of grant for a period
commensurate with the
estimated expected life.
The fair value of time-based as well as nonmarket-based performance restricted stock and restricted stock unit awards is equal to the fair value of the Company’s stock on the date of grant. The fair value of market-based performance restricted stock unit awards is determined by utilizing a Monte Carlo simulation model, which projects the value of Greatbatch stock versus our peer group under numerous scenarios and determines the value of the award based upon the present value of these projected outcomes. Compensation cost for nonmarket-based performance awards is reassessed each period and recognized based upon the probability that the performance targets will be achieved. That assessment is based upon actual and expected future performance. Stock-based compensation expense is only recorded for those awards that are expected to vest, excluding market and nonmarket performance award considerations. Forfeiture estimates for determining appropriate stock-based compensation expense are estimated at the time of grant based on historical experience and demographic characteristics. Revisions are made to those estimates in subsequent periods if actual forfeitures differ from estimated forfeitures. |
Option pricing models were developed for
use in estimating the value of traded
options that have no vesting restrictions
and are fully transferable. Because our
share-based payments have characteristics
significantly different from those of
freely traded options, and because changes
in the subjective input assumptions can
materially affect our estimates of fair
values, existing valuation models may not
provide reliable measures of the fair
values of our share-based compensation.
Consequently, there is a risk that our
estimates of the fair values of our
share-based compensation awards may bear
little resemblance to the actual values
realized upon the exercise,
expiration or
forfeiture of those share-based payments in
the future. Stock options may expire
worthless or otherwise result in zero
intrinsic value as compared to the fair
values originally estimated on the grant
date and reported in our consolidated
financial statements. Alternatively, value
may be realized from these instruments that
are significantly in excess of the fair
values originally estimated on the grant
date and reported in our consolidated
financial statements. There are
significant differences among valuation
models. This may result in a lack of
comparability with other companies that use
different models, methods and assumptions. There is a high degree of subjectivity involved in selecting assumptions to be utilized to determine fair value and forfeiture assumptions. If factors change and result in different assumptions in future periods, the expense that we record for future grants may differ significantly from what we have recorded in the current period. Additionally, changes in performance of the Company and its stock price will affect the likelihood that performance-based targets are achieved and could materially impact the amount of stock-based compensation expense recognized. A 1% change in our stock based compensation expense would increase/decrease 2010 net income by approximately $0.04 million, or approximately $0.002 per diluted share. |
37
Table of Contents
| Balance Sheet Caption / | ||||
| Nature of Critical | ||||
| Estimate Item | Assumptions / Approach Used | Effect of Variations of Key Assumptions Used | ||
Inventories Inventories are stated at the lower of cost, determined using the first-in, first-out method, or market. |
Inventory costing requires complex calculations that include assumptions for overhead absorption, scrap, sample calculations, manufacturing yield estimates and the determination of which costs may be capitalized. The valuation of inventory requires us to estimate obsolete or excess inventory as well as inventory that is not of saleable quality. | Variations in methods or assumptions could have a material impact on our results. If our demand forecast for specific products is greater than actual demand and we fail to reduce manufacturing output accordingly, we could be required to record additional inventory write-downs or expense a greater amount of overhead costs, which would have a negative impact on our net income. As of December 31, 2010 we have $101.4 million of inventory recorded on our balance sheet representing 13% of total assets. A 1% write-down of our inventory would decrease 2010 net income by approximately $0.7 million, or approximately $0.03 per diluted share. | ||
Tangible long-lived assets
Property, plant and equipment and other tangible long-lived assets are carried at cost. The cost of property, plant and equipment is charged to depreciation expense over the estimated life of the operating assets primarily using straight-line rates. Tangible long-lived assets are subject to impairment assessment if certain indicators are present. |
We assess the impairment of tangible long-lived assets when events or changes in circumstances indicate that the carrying value of the asset (asset group) may not be recoverable. Factors that we consider in deciding when to perform an impairment review include, but are not limited to: a significant decrease in the market price of the asset (asset group); a significant adverse change in the extent or manner in which a long-lived asset (asset group) is being used or in its physical condition; an accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction; a current-period operating or cash flow loss combined with a history of operating or cash flow losses or a projection or forecast that demonstrates continuing losses associated with the use of a long-lived asset (asset group); or a current expectation that, more likely than not, a long-lived asset (asset group) will be sold or otherwise disposed of significantly before the end of its previously estimated useful life. Recoverability potential is measured by comparing the carrying amount of the asset (asset group) to the related total future undiscounted cash flows. The projected cash flows for each asset (asset group) considers multiple factors, including current revenue from existing customers, proceeds from the sale of the asset (asset group), reasonable contract renewal assumptions, and expected profit margins giving consideration to historical and expected margins. If an asset’s (assets group’s) carrying value is not recoverable through related cash flows, the asset (asset group) is considered to be impaired. Impairment is measured by comparing the asset’s (asset group’s) carrying amount to its fair value. When it is determined that useful lives of assets are shorter than originally estimated, and there are sufficient cash flows to support the carrying value of the assets, we accelerate the rate of depreciation in order to fully depreciate the assets over their shorter useful lives. | Estimation of the useful lives of tangible
assets that are long-lived requires
significant management judgment. Events
could occur, including changes in cash flow
that would materially affect our estimates
and assumptions related to depreciation.
Unforeseen changes in operations or
technology could substantially alter the
assumptions regarding the ability to
realize the return of our investment in
long-lived assets. Also, as we make
manufacturing process conversions and other
facility consolidation decisions, we must
make subjective judgments regarding the
remaining useful lives of our assets,
primarily manufacturing equipment and
buildings. Significant changes in these
estimates and assumptions could change the
amount of future depreciation expense or
could create future impairments of these
long-lived assets (asset groups). As of December 31, 2010 we have $162.6 million of tangible long-lived assets recorded on our balance sheet representing 21% of total assets. A 1% write-down in our tangible long-lived assets would decrease 2010 net income by approximately $1.1 million, or approximately $0.04 per diluted share. |
38
Table of Contents
| Balance Sheet Caption / | ||||
| Nature of Critical | ||||
| Estimate Item | Assumptions / Approach Used | Effect of Variations of Key Assumptions Used | ||
Provision for income taxes In accordance with the liability method of accounting for income taxes, the provision for income taxes is the sum of income taxes both currently payable and deferred. The changes in deferred tax assets and liabilities are determined based upon the changes in differences between the bases of assets and liabilities for financial reporting purposes and the tax bases of assets and liabilities as measured by the enacted tax rates that management estimates will be in effect when the differences reverse. |
In relation to recording the provision for income taxes, management must estimate the future tax rates applicable to the reversal of temporary differences based upon the timing of expected reversal. Also, estimates are made as to whether taxable operating income in future periods will be sufficient to fully recognize any gross deferred tax assets. If recovery is not likely, we must increase our provision for income taxes by recording a valuation allowance against the deferred tax assets that we estimate will not ultimately be recoverable. Alternatively, we may make estimates about the potential usage of deferred tax assets that decrease our valuation allowances. | Changes could occur that would materially
affect our estimates and assumptions
regarding deferred taxes. Changes in
current tax laws and tax rates could affect
the valuation of deferred tax assets and
liabilities, thereby changing the income
tax provision. Also, significant declines
in taxable income could materially impact
the realizable value of deferred tax
assets. At December 31, 2010, we had $23.5
million of gross deferred tax assets on our
balance sheet and a valuation allowance of
$6.5 million has been established for
certain deferred tax assets as it is more
likely than not that they will not be
realized. A 1 percentage point change in the effective tax rate would impact the current year provision for income taxes by $0.5 million, and 2010 diluted earnings per share by $0.02 per diluted share. |
||
| The calculation of our tax liabilities involves dealing with uncertainties in the application of complex tax regulations. Significant judgment is required in evaluating our tax positions and determining our provision for income taxes. During the ordinary course of business, there are many transactions and calculations for which the ultimate tax determination is uncertain. We establish reserves for uncertain tax positions when we believe that certain tax positions do not meet the more likely than not threshold. We adjust these reserves in light of changing facts and circumstances, such as the outcome of a tax audit or the lapse of statutes of limitations. The provision for income taxes includes the impact of reserve provisions and changes to the reserves that are considered appropriate. |
39
Table of Contents
| Year Ended | 2010 vs. 2009 | 2009 vs. 2008 | ||||||||||||||||||||||||||
| Dec. 31, | Jan. 1, | Jan. 2, | $ | % | $ | % | ||||||||||||||||||||||
| Dollars in thousands, except per share data | 2010 | 2010 | 2009 | Change | Change | Change | Change | |||||||||||||||||||||
Greatbatch Medical |
||||||||||||||||||||||||||||
CRM/Neuromodulation |
$ | 303,521 | $ | 305,354 | $ | 286,251 | $ | (1,833 | ) | -1 | % | $ | 19,103 | 7 | % | |||||||||||||
Vascular Access |
38,000 | 35,816 | 39,443 | 2,184 | 6 | % | (3,627 | ) | -9 | % | ||||||||||||||||||
Orthopaedic |
118,748 | 113,897 | 142,446 | 4,851 | 4 | % | (28,549 | ) | -20 | % | ||||||||||||||||||
Total Greatbatch Medical |
460,269 | 455,067 | 468,140 | 5,202 | 1 | % | (13,073 | ) | -3 | % | ||||||||||||||||||
Electrochem |
73,156 | 66,754 | 78,504 | 6,402 | 10 | % | (11,750 | ) | -15 | % | ||||||||||||||||||
Total sales |
533,425 | 521,821 | 546,644 | 11,604 | 2 | % | (24,823 | ) | -5 | % | ||||||||||||||||||
Cost of sales |
359,844 | 355,402 | 390,855 | 4,442 | 1 | % | (35,453 | ) | -9 | % | ||||||||||||||||||
Gross profit |
173,581 | 166,419 | 155,789 | 7,162 | 4 | % | 10,630 | 7 | % | |||||||||||||||||||
Gross profit as a % of sales |
32.5 | % | 31.9 | % | 28.5 | % | ||||||||||||||||||||||
SG&A expenses |
64,510 | 70,294 | 72,633 | (5,784 | ) | -8 | % | (2,339 | ) | -3 | % | |||||||||||||||||
SG&A as a % of sales |
12.1 | % | 13.5 | % | 13.3 | % | ||||||||||||||||||||||
RD&E expenses |
45,019 | 33,562 | 31,444 | 11,457 | 34 | % | 2,118 | 7 | % | |||||||||||||||||||
RD&E as a % of sales |
8.4 | % | 6.4 | % | 5.8 | % | ||||||||||||||||||||||
Electrochem Litigation charge (gain) |
(9,500 | ) | 34,500 | — | (44,000 | ) | -128 | % | 34,500 | 100 | % | |||||||||||||||||
Intangible asset write-down |
— | 15,921 | — | (15,921 | ) | -100 | % | 15,921 | 100 | % | ||||||||||||||||||
Acquired IPR&D |
— | — | 2,240 | — | — | (2,240 | ) | -100 | % | |||||||||||||||||||
Other operating expenses, net |
4,558 | 11,094 | 14,578 | (6,536 | ) | -59 | % | (3,484 | ) | -24 | % | |||||||||||||||||
Operating income |
68,994 | 1,048 | 34,894 | 67,946 | NA | (33,846 | ) | -97 | % | |||||||||||||||||||
Operating margin |
12.9 | % | 0.2 | % | 6.4 | % | ||||||||||||||||||||||
Interest expense |
18,519 | 20,071 | 19,954 | (1,552 | ) | -8 | % | 117 | 1 | % | ||||||||||||||||||
Interest income |
(10 | ) | (324 | ) | (711 | ) | 314 | -97 | % | 387 | -54 | % | ||||||||||||||||
Gain on extinguishment of debt |
— | — | (3,242 | ) | — | — | 3,242 | -100 | % | |||||||||||||||||||
Other (income) expense, net |
1,160 | (522 | ) | (1,624 | ) | 1,682 | NA | 1,102 | -68 | % | ||||||||||||||||||
Provision (benefit) for income taxes |
16,187 | (9,176 | ) | 6,369 | 25,363 | NA | (15,545 | ) | NA | |||||||||||||||||||
Effective tax rate |
32.8 | % | 50.5 | % | 31.0 | % | ||||||||||||||||||||||
Net income (loss) |
$ | 33,138 | $ | (9,001 | ) | $ | 14,148 | $ | 42,139 | NA | $ | (23,149 | ) | NA | ||||||||||||||
Net margin |
6.2 | % | -1.7 | % | 2.6 | % | ||||||||||||||||||||||
Diluted earnings (loss) per share |
$ | 1.40 | $ | (0.39 | ) | $ | 0.62 | $ | 1.79 | NA | $ | (1.01 | ) | NA | ||||||||||||||
40
Table of Contents
| Year Ended | ||||||||||||||||
| December 31, | January 1, | $ | % | |||||||||||||
| Product Lines | 2010 | 2010 | Change | Change | ||||||||||||
Greatbatch Medical |
||||||||||||||||
CRM/Neuromodulation |
$ | 303,521 | $ | 305,354 | $ | (1,833 | ) | -1 | % | |||||||
Vascular Access |
38,000 | 35,816 | 2,184 | 6 | % | |||||||||||
Orthopaedic |
118,748 | 113,897 | 4,851 | 4 | % | |||||||||||
Total Greatbatch Medical |
460,269 | 455,067 | 5,202 | 1 | % | |||||||||||
Electrochem |
73,156 | 66,754 | 6,402 | 10 | % | |||||||||||
Total Sales |
$ | 533,425 | $ | 521,821 | $ | 11,604 | 2 | % | ||||||||
| • | CRM & Neuromodulation: Flat |
| • | Vascular Access: 10% to 20% |
| • | Orthopaedic: 2% to 10% |
| • | Electrochem: 2% to 5% |
41
Table of Contents
| 2010-2009 | ||||
| % Increase | ||||
Capacity & productivity (a) |
-0.9 | % | ||
Selling price (b) |
-0.5 | % | ||
Mix change (c) |
2.8 | % | ||
Other |
-0.8 | % | ||
Total percentage point change to gross profit
as a percentage of sales |
0.6 | % | ||
| (a) | Our gross profit percentage was negatively impacted by excess capacity costs due to our increased infrastructure investment in our Orthopaedic business in comparison to 2009. Modest productivity improvement initiatives partially offset these excess capacity costs. In accordance with our inventory accounting policy, excess capacity costs are expensed. | |
| (b) | Our gross profit percentage was negatively impacted in 2010 by contractual volume price reductions and price concessions made to our larger OEM customers on certain product lines. We expect this pricing pressure to continue in the future. | |
| (c) | Our gross profit percentage was positively impacted by an increase in sales of higher margin products as a percentage of total sales, primarily within our CRM, Vascular Access and Electrochem product lines. |
| 2010-2009 | ||||
| $ Decrease | ||||
Personnel costs (a) |
$ | (2,688 | ) | |
Information technology and consulting (b) |
(1,555 | ) | ||
Allowance for doubtful accounts (c) |
(1,095 | ) | ||
Other |
(446 | ) | ||
Decrease in SG&A |
$ | (5,784 | ) | |
| (a) | Amounts reflect our consolidation and cost reduction initiatives. A portion of these cost savings were reinvested in RD&E. SG&A expenses for 2010 include $0.9 million of death benefits provided to the family of the Company’s former Senior Vice President — Orthopaedics. | |
| (b) | Amounts represent the change in information technology and consulting costs from 2009 and reflect our cost reduction initiatives. | |
| (c) | Amounts primarily relate to lower losses incurred on uncollectible receivables compared to 2009, which included higher Electrochem and Orthopaedic write-offs due to the economic slowdown. |
42
Table of Contents
| Year Ended | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Research and development costs |
$ | 17,378 | $ | 17,707 | ||||
Engineering costs |
34,208 | 26,438 | ||||||
Less cost reimbursements |
(6,567 | ) | (10,583 | ) | ||||
Engineering costs, net |
27,641 | 15,855 | ||||||
Total RD&E |
$ | 45,019 | $ | 33,562 | ||||
| Year Ended | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
(a) 2007 & 2008 facility shutdowns and consolidations |
$ | 1,348 | $ | 7,069 | ||||
(b) Integration costs |
42 | 3,077 | ||||||
(c) Asset dispositions, severance and other |
3,168 | 948 | ||||||
| $ | 4,558 | $ | 11,094 | |||||
| (a) | See Note 9 “Other Operating Expenses, Net” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. |
43
Table of Contents
| (b) | For 2010 and 2009, we incurred costs related to the integration of the companies acquired in 2007 and 2008. The integration initiatives include the implementation of the Oracle ERP system, training and compliance programs as well as the implementation of lean manufacturing and six sigma initiatives. The expenses are primarily for consultants, relocation and travel costs that will not be required after the integrations are completed. | |
| (c) | During the fourth quarter of 2010, we consolidated our Greatbatch Medical business under the leadership of Mauricio Arellano. As part of this consolidation, there was a realignment of resources in which certain positions globally were eliminated and restructured. The severance charges associated with this realignment were $2.3 million of which $0.7 million were paid in the fourth quarter of 2010, with remaining amounts expected to be paid over the next twelve months. A significant portion of the annual savings as a result of this initiative will be reinvested into research and development activities with higher growth opportunities, including further investment in our systems and device projects. During 2009, we incurred approximately $0.6 million in severance charges in connection with various workforce reductions. | |
| During 2010, 2009 and 2008, we recorded write-downs in connection with various asset disposals, which were partially offset by insurance proceeds received. |
44
Table of Contents
| Year Ended | ||||||||||||||||
| January 1, | January 2, | $ | % | |||||||||||||
| Product Lines | 2010 | 2009 | Change | Change | ||||||||||||
Greatbatch Medical |
||||||||||||||||
CRM/Neuromodulation |
$ | 305,354 | $ | 286,251 | $ | 19,103 | 7 | % | ||||||||
Vascular Access |
35,816 | 39,443 | (3,627 | ) | -9 | % | ||||||||||
Orthopaedic |
113,897 | 142,446 | (28,549 | ) | -20 | % | ||||||||||
Total Greatbatch Medical |
455,067 | 468,140 | (13,073 | ) | -3 | % | ||||||||||
Electrochem |
66,754 | 78,504 | (11,750 | ) | -15 | % | ||||||||||
Total Sales |
$ | 521,821 | $ | 546,644 | $ | (24,823 | ) | -5 | % | |||||||
45
Table of Contents
| 2009-2008 | ||||
| % Increase | ||||
Inventory step-up amortization (a) |
1.2 | % | ||
Manufacturing efficiencies (b) |
1.8 | % | ||
Selling price (c) |
-1.1 | % | ||
Mix change (d) |
1.1 | % | ||
Foreign currency (e) |
0.5 | % | ||
Performance-based compensation (f) |
0.5 | % | ||
Other |
-0.6 | % | ||
Total percentage point change to gross profit
as a percentage of sales |
3.4 | % | ||
| (a) | In connection with our acquisitions in 2008 and 2007, the value of inventory on hand was stepped-up to reflect the fair value at the time of acquisition. The amortization of inventory step-up, which is recorded in Cost of Sales, was $6.4 million for 2008. There was no inventory step-up amortization recorded in 2009. | |
| (b) | Our gross profit percentage benefited from manufacturing efficiencies realized due to an increase in CRM and Neuromodulation revenue, as well as the consolidation of our Columbia, MD facility into our Tijuana, Mexico facility in June 2008 and our Blaine, MN facility into our Plymouth, MN facility in April 2009. The additional output absorbs a higher amount of lower fixed costs such as plant overhead and depreciation. | |
| (c) | Our gross profit percentage was negatively impacted in 2009 due to contractual volume price reductions and price concessions made to our larger OEM customers on certain product lines. | |
| (d) | Our gross profit percentage benefited from an increase in sales of CRM and Neuromodulation products as a percentage of total sales during 2009, which typically are higher margin products. | |
| (e) | During 2009, the value of the U.S. dollar strengthened significantly in comparison to the Mexican peso. This foreign currency exchange rate fluctuation resulted in a higher gross profit percentage at our Tijuana, Mexico facility, which has peso denominated expenses but sales denominated in U.S. dollars. | |
| (f) | During 2009, we took cost-cutting measures to help mitigate the impact of the lower revenue levels on operating income. This included adjusting 2009 related discretionary performance based compensation, which benefited Cost of Sales, by approximately $2.5 million versus 2008. |
| 2009-2008 | ||||
| $ Decrease | ||||
Legal costs (a) |
$ | (3,027 | ) | |
Performance-based compensation (b) |
(2,907 | ) | ||
IT and Consulting (c) |
1,658 | |||
Rebranding initiative (d) |
722 | |||
Bad debt expense (e) |
371 | |||
Other |
844 | |||
Net decrease in SG&A |
$ | (2,339 | ) | |
46
Table of Contents
| (a) | Amounts primarily represent lower fees incurred in connection with a patent infringement action which went to trial in 2008, partially offset by higher legal costs incurred in connection with the development and patenting of new technologies in 2009. See Note 11 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. | |
| (b) | During 2009, we took cost-cutting measures to help mitigate the impact of the lower revenue levels on operating income. This included adjusting 2009 related discretionary performance based compensation, which benefited SG&A, by approximately $2.9 million in comparison to 2008. | |
| (c) | Amounts relate to various corporate development initiatives as well as increased IT spending due to our investment in IT infrastructure to support future growth including moving all of our acquired facilities to one common ERP platform. | |
| (d) | During 2009, we launched a new branding initiative to unify our existing businesses under a common vision and consolidated our medical entities under a single brand — Greatbatch Medical. These increased costs primarily relate to consulting costs and the replacement of collateral material in connection with this new brand. | |
| (e) | Amounts primarily relate to increased losses incurred on uncollectible receivables from Electrochem and Orthopaedic customers given the economic slowdown in their related markets. |
| Year Ended | ||||||||
| January 1, | January 2, | |||||||
| 2010 | 2009 | |||||||
Research and development costs |
$ | 17,707 | $ | 18,750 | ||||
Engineering costs |
26,438 | 22,447 | ||||||
Less cost reimbursements |
(10,583 | ) | (9,753 | ) | ||||
Engineering costs, net |
15,855 | 12,694 | ||||||
Total RD&E |
$ | 33,562 | $ | 31,444 | ||||
47
Table of Contents
| Year ended | ||||||||
| January 1, | January 2, | |||||||
| 2010 | 2009 | |||||||
(a) 2005 & 2006 facility shutdowns and consolidations |
$ | — | $ | 663 | ||||
(a) 2007 & 2008 facility shutdowns and consolidations |
7,069 | 8,347 | ||||||
(b) Integration costs |
3,077 | 5,369 | ||||||
(c) Asset dispositions, severance and other |
948 | 199 | ||||||
| $ | 11,094 | $ | 14,578 | |||||
| (a) | See Note 9 “Other Operating Expenses, Net” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. | |
| (b) | For 2009 and 2008, we incurred costs related to the integration of the companies acquired in 2007 and 2008. The integration initiatives include the implementation of the Oracle ERP system, training and compliance programs as well as the implementation of lean manufacturing and six sigma initiatives. The expenses are primarily for consultants, relocation and travel costs that will not be required after the integrations are completed. | |
| (c) | During 2009 and 2008, we recorded write-downs in connection with various asset disposals, partially offset by insurance proceeds received. During 2009, we incurred approximately $0.6 million in severance charges in connection with various workforce reductions due to the lower revenue levels. |
48
Table of Contents
| As of | ||||||||
| December 31, | January 1, | |||||||
| (Dollars in millions) | 2010 | 2010 | ||||||
Cash and cash equivalents(a) |
$ | 22.9 | $ | 37.9 | ||||
Working capital (b) |
$ | 150.9 | $ | 119.9 | ||||
Current ratio (b) |
3.5:1.0 | 1.9:1.0 | ||||||
| (a) | The decrease in cash and cash equivalents from the prior year was primarily due to the repayment of long-term debt of $78.5 million during 2010 which was partially funded by free cash flow of $63.2 million. Note that cash flow from operating activities for 2010 was unfavorably impacted by the $25 million ($16.3 million net of tax) Electrochem Litigation settlement. See Note 11 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. | |
| (b) | Our working capital and current ratio increased in comparison to 2009 due to cash flow from operating activities of $76.9 million, which was primarily used to repay the current portion of long-term debt and the Electrochem Litigation settlement. Note that in 2009 we accrued $34.5 million in connection with the Electrochem Litigation and recognized a $9.5 million gain in the fourth quarter of 2010 after settling the lawsuit for $25 million. See Note 11 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report. |
49
Table of Contents
50
Table of Contents
| Payments due by period | ||||||||||||||||||||
| Less than 1 | More than | |||||||||||||||||||
| CONTRACTUAL OBLIGATIONS | Total | year | 1-3 years | 3-5 years | 5 years | |||||||||||||||
Debt obligations (a) |
$ | 262,726 | $ | 6,030 | $ | 256,696 | $ | — | $ | — | ||||||||||
Operating lease obligations (b) |
15,323 | 2,361 | 4,295 | 4,007 | 4,660 | |||||||||||||||
Purchase obligations (b) |
18,645 | 18,380 | 265 | — | — | |||||||||||||||
Foreign currency contracts (b) |
6,000 | 6,000 | — | — | — | |||||||||||||||
Pension obligations (c) |
11,845 | 957 | 2,146 | 2,284 | 6,458 | |||||||||||||||
Total |
$ | 314,539 | $ | 33,728 | $ | 263,402 | $ | 6,291 | $ | 11,118 | ||||||||||
| (a) | Includes the annual interest expense on our convertible subordinated notes of 2.25%, which is paid semi-annually. Amounts also include the expected interest expense on the $50.0 million outstanding on our line of credit based upon the period end interest rate of 3.16%, which includes the impact of our interest rate swaps outstanding. Amounts do not include $22.8 million of potential deferred tax payments that may be required in connection with our convertible subordinated notes. See Note 6 “Debt” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our debt obligations. | |
| (b) | See Note 11 “Commitments and Contingencies” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our operating lease obligations, purchase obligations and foreign currency contracts. | |
| (c) | See Note 7 “Employee Benefit Plans” of the Notes to Consolidated Financial Statements contained in Item 8 of this report for additional information about our pension plan obligations. These amounts do not include any potential future contributions to our pension plans that may be necessary if the rate of return earned on pension plan assets is not sufficient to fund the rate of increase of our pension liability. Future cash contributions may be required. As of December 31, 2010, our actuarially determined pension benefit obligation exceeded plans assets by $4.6 million. |
51
Table of Contents
52
Table of Contents
53
Table of Contents
| 55 | ||||
| 56 | ||||
| 59 | ||||
| 60 | ||||
| 61 | ||||
| 62 | ||||
| 63 |
54
Table of Contents
/s/ Thomas J. Hook
|
/s/ Thomas J. Mazza | |||||
President & Chief Executive Officer |
Senior Vice President & Chief Financial Officer |
55
Table of Contents
Greatbatch, Inc.
Clarence, New York
56
Table of Contents
57
Table of Contents
Greatbatch, Inc.
Clarence, New York
March 1, 2011
58
Table of Contents
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
ASSETS |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 22,883 | $ | 37,864 | ||||
Accounts receivable, net |
70,947 | 81,488 | ||||||
Inventories |
101,440 | 106,609 | ||||||
Refundable income taxes |
2,763 | — | ||||||
Deferred income taxes |
7,398 | 13,896 | ||||||
Prepaid expenses and other current assets |
6,078 | 13,313 | ||||||
Total current assets |
211,509 | 253,170 | ||||||
Property, plant and equipment, net |
146,380 | 153,601 | ||||||
Amortizing intangible assets, net |
75,114 | 82,076 | ||||||
Trademarks and tradenames |
20,288 | 20,288 | ||||||
Goodwill |
307,451 | 303,926 | ||||||
Deferred income taxes |
2,427 | 2,458 | ||||||
Other assets |
13,807 | 15,024 | ||||||
Total assets |
$ | 776,976 | $ | 830,543 | ||||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 27,989 | $ | 34,395 | ||||
Current portion of long-term debt |
— | 30,450 | ||||||
Income taxes payable |
— | 403 | ||||||
Deferred income taxes |
514 | — | ||||||
Accrued expenses and other current liabilities |
32,084 | 67,996 | ||||||
Total current liabilities |
60,587 | 133,244 | ||||||
Long-term debt |
220,629 | 258,972 | ||||||
Deferred income taxes |
64,290 | 54,043 | ||||||
Other long-term liabilities |
4,641 | 4,560 | ||||||
Total liabilities |
350,147 | 450,819 | ||||||
Commitments and contingencies (Note 11) |
||||||||
Stockholders’ equity: |
||||||||
Preferred stock, $0.001 par value, authorized 100,000,000 shares;
no shares issued or outstanding in 2010 or 2009 |
— | — | ||||||
Common stock, $0.001 par value, authorized 100,000,000
shares; 23,319,492 shares issued and 23,256,897 shares outstanding in 2010
and 23,190,105 shares issued and 23,157,097 shares outstanding in 2009 |
23 | 23 | ||||||
Additional paid-in capital |
298,405 | 291,926 | ||||||
Treasury stock, at cost, 62,595 shares in 2010 and 33,008 shares in 2009 |
(1,469 | ) | (635 | ) | ||||
Retained earnings |
119,400 | 86,262 | ||||||
Accumulated other comprehensive income |
10,470 | 2,148 | ||||||
Total stockholders’ equity |
426,829 | 379,724 | ||||||
Total liabilities and stockholders’ equity |
$ | 776,976 | $ | 830,543 | ||||
59
Table of Contents
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Sales |
$ | 533,425 | $ | 521,821 | $ | 546,644 | ||||||
Cost of sales |
359,844 | 355,402 | 390,855 | |||||||||
Gross profit |
173,581 | 166,419 | 155,789 | |||||||||
Operating expenses: |
||||||||||||
Selling, general and administrative expenses |
64,510 | 70,294 | 72,633 | |||||||||
Research, development and engineering costs, net |
45,019 | 33,562 | 31,444 | |||||||||
Electrochem Litigation charge (gain) (Note 11) |
(9,500 | ) | 34,500 | — | ||||||||
Intangible asset write-down |
— | 15,921 | — | |||||||||
Acquired in-process research and development |
— | — | 2,240 | |||||||||
Other operating expenses, net |
4,558 | 11,094 | 14,578 | |||||||||
Operating income |
68,994 | 1,048 | 34,894 | |||||||||
Interest expense |
18,519 | 20,071 | 19,954 | |||||||||
Interest income |
(10 | ) | (324 | ) | (711 | ) | ||||||
Gain on extinguishment of debt |
— | — | (3,242 | ) | ||||||||
Other (income) expense, net |
1,160 | (522 | ) | (1,624 | ) | |||||||
Income (loss) before provision (benefit) for
income taxes |
49,325 | (18,177 | ) | 20,517 | ||||||||
Provision (benefit) for income taxes |
16,187 | (9,176 | ) | 6,369 | ||||||||
Net income (loss) |
$ | 33,138 | $ | (9,001 | ) | $ | 14,148 | |||||
Earnings (loss) per share: |
||||||||||||
Basic |
$ | 1.44 | $ | (0.39 | ) | $ | 0.63 | |||||
Diluted |
$ | 1.40 | $ | (0.39 | ) | $ | 0.62 | |||||
Weighted average shares outstanding: |
||||||||||||
Basic |
23,070 | 22,926 | 22,525 | |||||||||
Diluted |
23,802 | 22,926 | 22,861 | |||||||||
Comprehensive income (loss): |
||||||||||||
Net income (loss) |
$ | 33,138 | $ | (9,001 | ) | $ | 14,148 | |||||
Foreign currency translation adjustment |
7,896 | 4,562 | (228 | ) | ||||||||
Net change in cash flow hedges, net of tax |
1,027 | (200 | ) | (906 | ) | |||||||
Defined benefit pension plan liability adjustment,
net of tax |
(601 | ) | 862 | (1,942 | ) | |||||||
Comprehensive income (loss) |
$ | 41,460 | $ | (3,777 | ) | $ | 11,072 | |||||
60
Table of Contents
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Cash flows from operating activities: |
||||||||||||
Net income (loss) |
$ | 33,138 | $ | (9,001 | ) | $ | 14,148 | |||||
Adjustments to reconcile net income (loss) to net cash
from operating activities: |
||||||||||||
Depreciation and amortization |
46,447 | 47,229 | 52,168 | |||||||||
Stock-based compensation |
6,884 | 5,204 | 11,211 | |||||||||
Electrochem Litigation charge (gain) |
(9,500 | ) | 34,500 | — | ||||||||
Electrochem Litigation settlement |
(25,000 | ) | — | — | ||||||||
Intangible asset write-down |
— | 15,921 | — | |||||||||
Gain on extinguishment of debt |
— | — | (3,242 | ) | ||||||||
Acquired in-process research and development |
— | — | 2,240 | |||||||||
Other non-cash (gains) losses |
893 | (559 | ) | 2,994 | ||||||||
Deferred income taxes |
15,419 | (10,120 | ) | (704 | ) | |||||||
Changes in operating assets and liabilities: |
||||||||||||
Accounts receivable |
10,922 | 5,876 | (18,640 | ) | ||||||||
Inventories |
7,406 | 6,898 | (21,077 | ) | ||||||||
Prepaid expenses and other assets |
2,111 | (2,364 | ) | (35 | ) | |||||||
Accounts payable |
(7,568 | ) | (12,668 | ) | 14,285 | |||||||
Accrued expenses and other liabilities |
(1,472 | ) | (5,050 | ) | 1,589 | |||||||
Income taxes |
(2,795 | ) | (4,100 | ) | 2,164 | |||||||
Net cash provided by operating activities |
76,885 | 71,766 | 57,101 | |||||||||
Cash flows from investing activities: |
||||||||||||
Purchases of short-term investments |
— | — | (2,010 | ) | ||||||||
Proceeds from maturity/disposition of short-term investments |
— | — | 9,027 | |||||||||
Acquisition of property, plant and equipment |
(16,140 | ) | (19,674 | ) | (44,172 | ) | ||||||
Proceeds from sale of property, plant and equipment |
2,537 | 114 | 170 | |||||||||
Purchase of cost method investment, net of distributions |
— | (1,050 | ) | (4,300 | ) | |||||||
Acquisitions, net of cash acquired |
— | — | (107,577 | ) | ||||||||
Other investing activities |
(321 | ) | (531 | ) | 136 | |||||||
Net cash used in investing activities |
(13,924 | ) | (21,141 | ) | (148,726 | ) | ||||||
Cash flows from financing activities: |
||||||||||||
Principal payments of long-term debt |
(78,450 | ) | (46,000 | ) | (62,058 | ) | ||||||
Proceeds from issuance of long-term debt |
— | 12,000 | 142,000 | |||||||||
Issuance of common stock |
659 | 212 | 2,210 | |||||||||
Other financing activities |
(1,030 | ) | (718 | ) | (495 | ) | ||||||
Net cash provided by (used in) financing activities |
(78,821 | ) | (34,506 | ) | 81,657 | |||||||
Effect of foreign currency exchange on cash and cash equivalents |
879 | (318 | ) | (1,442 | ) | |||||||
Net increase (decrease) in cash and cash equivalents |
(14,981 | ) | 15,801 | (11,410 | ) | |||||||
Cash and cash equivalents, beginning of year |
37,864 | 22,063 | 33,473 | |||||||||
Cash and cash equivalents, end of year |
$ | 22,883 | $ | 37,864 | $ | 22,063 | ||||||
61
Table of Contents
(in thousands)
| Accumulated | ||||||||||||||||||||||||||||||||
| Additional | Treasury | other | Total | |||||||||||||||||||||||||||||
| Common stock | paid-in | stock | Retained | comprehensive | stockholders’ | |||||||||||||||||||||||||||
| Shares | Amount | capital | Shares | Amount | earnings | income (loss) | equity | |||||||||||||||||||||||||
Balance, December 29, 2007 |
22,477 | $ | 22 | $ | 270,124 | (7 | ) | $ | (140 | ) | $ | 81,115 | $ | — | $ | 351,121 | ||||||||||||||||
Stock-based compensation |
— | — | 6,822 | — | — | — | — | 6,822 | ||||||||||||||||||||||||
Net shares issued under stock incentive plans |
266 | 1 | 1,417 | (21 | ) | (601 | ) | — | — | 817 | ||||||||||||||||||||||
Income tax benefit from stock options and
restricted stock |
— | — | 14 | — | — | — | — | 14 | ||||||||||||||||||||||||
Shares issued in connection with the
Quan Emerteq acquisition |
60 | — | 1,473 | — | — | — | — | 1,473 | ||||||||||||||||||||||||
Shares contributed to 401(k) |
168 | — | 3,472 | — | — | — | — | 3,472 | ||||||||||||||||||||||||
Net income |
— | — | — | — | — | 14,148 | — | 14,148 | ||||||||||||||||||||||||
Total other comprehensive loss, net |
— | — | — | — | — | — | (3,076 | ) | (3,076 | ) | ||||||||||||||||||||||
Balance, January 2, 2009 |
22,971 | 23 | 283,322 | (28 | ) | (741 | ) | 95,263 | (3,076 | ) | 374,791 | |||||||||||||||||||||
Stock-based compensation |
— | — | 5,204 | — | — | — | — | 5,204 | ||||||||||||||||||||||||
Net shares issued under stock incentive plans |
24 | — | 214 | (33 | ) | (635 | ) | — | — | (421 | ) | |||||||||||||||||||||
Income tax liability from stock options and
restricted stock |
— | — | (88 | ) | — | — | — | — | (88 | ) | ||||||||||||||||||||||
Shares contributed to 401(k) |
195 | — | 3,274 | 28 | 741 | — | — | 4,015 | ||||||||||||||||||||||||
Net loss |
— | — | — | — | — | (9,001 | ) | — | (9,001 | ) | ||||||||||||||||||||||
Total other comprehensive income, net |
— | — | — | — | — | — | 5,224 | 5,224 | ||||||||||||||||||||||||
Balance, January 1, 2010 |
23,190 | 23 | 291,926 | (33 | ) | (635 | ) | 86,262 | 2,148 | 379,724 | ||||||||||||||||||||||
Stock-based compensation |
— | — | 6,884 | — | — | — | — | 6,884 | ||||||||||||||||||||||||
Net shares issued under stock incentive plans |
129 | — | 179 | (30 | ) | (834 | ) | — | — | (655 | ) | |||||||||||||||||||||
Income tax liability from stock options,
restricted stock and restricted stock units |
— | — | (584 | ) | — | — | — | — | (584 | ) | ||||||||||||||||||||||
Net income |
— | — | — | — | — | 33,138 | — | 33,138 | ||||||||||||||||||||||||
Total other comprehensive income, net |
— | — | — | — | — | — | 8,322 | 8,322 | ||||||||||||||||||||||||
Balance, December 31, 2010 |
23,319 | $ | 23 | $ | 298,405 | (63 | ) | $ | (1,469 | ) | $ | 119,400 | $ | 10,470 | $ | 426,829 | ||||||||||||||||
62
Table of Contents
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
| Principles of Consolidation — The consolidated financial statements include the accounts of Greatbatch, Inc. and its wholly owned subsidiary Greatbatch Ltd. (collectively, the “Company” or “Greatbatch”). All intercompany balances and transactions have been eliminated in consolidation. |
| Nature of Operations — The Company operates its business in two reportable segments — Greatbatch Medical and Electrochem Solutions (“Electrochem”). The Greatbatch Medical segment designs and manufactures systems, components and devices for the Cardiac Rhythm Management (“CRM”), Neuromodulation, Vascular Access and Orthopaedic markets. Our Greatbatch Medical customers include large multi-national original equipment manufacturers (“OEMs”). Greatbatch Medical products include: 1) batteries, capacitors, filtered and unfiltered feedthroughs, engineered components and enclosures used in Implantable Medical Devices (“IMDs”); 2) instruments and delivery systems used in hip and knee replacement, trauma and spine surgeries as well as hip, knee and shoulder implants; and 3) introducers, catheters, steerable sheaths and implantable stimulation leads. Additionally, Greatbatch Medical offers value-added assembly and design engineering services for medical systems and devices within the markets in which it operates. |
| Electrochem is a leader in technology solutions for critical industrial applications, including customized battery power and wireless sensing systems. Originating from the lithium cell invented for the implantable pacemaker by Wilson Greatbatch, our technology and superior quality and reliability is utilized in markets world-wide. |
| Fiscal Year End — The Company utilizes a fifty-two, fifty-three week fiscal year ending on the Friday nearest December 31st. Fiscal years 2010, 2009 and 2008 ended on December 31, 2010, January 1, 2010 and January 2, 2009, respectively. Fiscal years 2010 and 2009 contained fifty-two weeks while fiscal year 2008 contained fifty-three weeks. |
| Fair Value Measurements — Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e. the “exit price”) in an orderly transaction between market participants at the measurement date. Accounting Standards Codification (“ASC”) establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The hierarchy is broken down into three levels based on the reliability of inputs as follows: |
| Level 1— Valuation is based on quoted prices in active markets for identical assets or liabilities that the Company has the ability to access. Level 1 valuations do not entail a significant degree of judgment. |
| Level 2— Valuation is determined from quoted prices for similar assets or liabilities in active markets, quoted prices for identical instruments in markets that are not active or by model-based techniques in which all significant inputs are observable in the market. |
63
Table of Contents
| Level 3— Valuation is based on inputs that are unobservable and significant to the overall fair value measurement. The degree of judgment exercised in determining fair value is greatest for Level 3 valuations. The availability of observable inputs can vary and is affected by a wide variety of factors, including, the type of asset/liability, whether the asset/liability is established in the marketplace, and other characteristics particular to the valuation. To the extent that a valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes the level in the fair value hierarchy within which the fair value measurement in its entirety falls is determined based on the lowest level input that is significant to the fair value measurement in its entirety. |
| Fair value is a market-based measure considered from the perspective of a market participant rather than an entity-specific measure. Therefore, even when market assumptions are not readily available, assumptions are required to reflect those that market participants would use in pricing the asset or liability at the measurement date. |
| The carrying amount of cash and cash equivalents, trade receivables and accounts payable, approximated their fair value as of December 31, 2010 because of the short-term nature of these instruments. Note 12 “Fair Value Measurements” contains additional information on assets and liabilities recorded at fair value in the consolidated financial statements. |
| Cash and Cash Equivalents — Cash and cash equivalents consist of cash and highly liquid, short-term investments with maturities at the time of purchase of three months or less. |
| Concentration of Credit Risk — Financial instruments that potentially subject the Company to concentration of credit risk consist principally of accounts receivable. A significant portion of the Company’s sales are to four customers, all in the medical device industry, and, as such, the Company is directly affected by the condition of those customers and that industry. However, the credit risk associated with trade receivables is partially mitigated due to the stability of those customers. The Company performs on-going credit evaluations of its customers. Note 14 “Business Segment Information” contains information on sales and accounts receivable for these customers. The Company maintains cash deposits with major banks, which from time to time may exceed insured limits. The Company performs on-going credit evaluations of its banks. |
| Allowance for Doubtful Accounts — The Company provides credit, in the normal course of business, to its customers in the form of trade receivables. The Company maintains an allowance for doubtful customer accounts for those receivables that it does not expect to collect. The Company accrues its estimated losses from uncollectable accounts receivable to the allowance based upon recent historical experience, the length of time the receivable has been outstanding and other specific information as it becomes available. Provisions to the allowance for doubtful accounts are charged to current operating expenses. Actual losses are charged against this allowance when incurred. The allowance for doubtful accounts was $1.8 million at December 31, 2010 and $2.5 million at January 1, 2010. |
| Inventories — Inventories are stated at the lower of cost, determined using the first-in first-out method, or market. Write-downs for excess, obsolete or expired inventory are based primarily on how long the inventory has been held as well as our estimates of forecasted net sales of that product. A significant change in the timing or level of demand for our products may result in recording additional write-downs for excess, obsolete or expired inventory in the future. |
64
Table of Contents
| Property, Plant and Equipment — Property, plant and equipment is carried at cost. Depreciation is computed by the straight-line method over the estimated useful lives of the assets, as follows: buildings and building improvements 7-40 years; machinery and equipment 3-8 years; office equipment 3-10 years; and leasehold improvements over the remaining lives of the improvements or the lease term, if less. The cost of repairs and maintenance are expensed as incurred; renewals and betterments are capitalized. Upon retirement or sale of an asset, its cost and related accumulated depreciation or amortization is removed from the accounts and any gain or loss is recorded in operating income or expense. |
| Business Combinations — The Company records its business combinations under the acquisition method of accounting. Under the acquisition method of accounting, the Company allocates the purchase price of each acquisition to the tangible and identifiable intangible assets acquired and liabilities assumed based on their respective fair values at the date of acquisition. The fair value of identifiable intangible assets is based upon detailed valuations that use various assumptions made by management. Any excess of the purchase price over the fair value of the net tangible and intangible assets acquired is allocated to goodwill. Prior to 2009 the Company included all direct acquisition-related costs as part of the purchase price. Effective in 2009, the accounting standard applicable to business combinations changed such that any direct acquisition-related costs are expensed as incurred. |
| On January 7, 2008, the Company acquired P Medical Holding SA (“Precimed”) which had administrative offices in Orvin, Switzerland and Exton, PA, manufacturing operations in Switzerland and Indiana and sales offices in Japan, China and the United Kingdom. Precimed was a leading technology-driven supplier to the Orthopaedic industry. The results of Precimed’s operations were included in the Greatbatch Medical segment from the date of acquisition. The purchase price and other direct costs of Precimed totaled $85.0 million, which was paid in cash. Total assets acquired from Precimed were $143.0 million, of which $82.3 million were intangible assets, including $2.2 million of in process research and development (“IPR&D”) which was immediately expensed, and $47.2 million of goodwill. |
| On February 11, 2008, Precimed completed its previously announced acquisition of DePuy Orthopaedics’ (“DePuy”) Chaumont, France manufacturing facility (the “Chaumont Facility”). The Chaumont Facility produces hip and shoulder implants for DePuy who distributes them worldwide through various DePuy selling entities. This transaction included a new four year supply agreement with DePuy. The results of the Chaumont Facility’s operations were included in the Greatbatch Medical segment from the date of acquisition. The purchase price and other direct costs of the Chaumont Facility totaled $28.7 million, which was paid in cash. Total assets acquired from the Chaumont Facility were $29.3 million, of which $6.6 million was goodwill. |
| The following unaudited pro forma information presents the consolidated results of operations of the Company, Precimed, and the Chaumont Facility as if those acquisitions had occurred as of the beginning of the earliest fiscal year presented. The unaudited pro forma results are presented for illustrative purposes only and do not reflect the realization of potential cost savings, and any related integration costs. Certain cost savings may result from the acquisition; however, there can be no assurance that these cost savings will be achieved. These pro forma results do not purport to be indicative of the results that would have been obtained, or to be a projection of results that may be obtained in the future. Amounts in thousands, except per share amounts: |
| (Unaudited) | Fiscal Year 2008 | |||
Sales |
$ | 555,139 | ||
Net income |
20,128 | |||
Earnings per share: |
||||
Basic |
$ | 0.90 | ||
Diluted |
$ | 0.86 | ||
65
Table of Contents
| The unaudited pro forma information presents the combined operating results of Greatbatch, Precimed, and the Chaumont Facility with the results prior to the acquisition date adjusted to include the pro forma impact of the amortization of acquired intangible assets and depreciation of fixed assets based on the purchase price allocation, the elimination of non-recurring IPR&D charges ($2.2 million) and inventory step-up amortization recorded by Greatbatch ($6.4 million), the adjustment to interest income/expense reflecting the cash paid in connection with the acquisition, including acquisition-related expenses, at Greatbatch’s weighted average interest income/expense rate, and the impact of income taxes on the pro forma adjustments utilizing the applicable statutory tax rate, except for IPR&D which is not deductible for tax purposes. The unaudited pro forma consolidated basic and diluted earnings per share are based on the consolidated basic and diluted weighted average shares of Greatbatch. |
| Amortizing Intangible Assets — Acquired intangible assets other than goodwill and trademarks and tradenames consist primarily of purchased technology, patents and customer lists. The Company amortizes its definite-lived intangible assets on a straight-line basis over their estimated useful lives as follows: purchased technology and patents 5-15 years; customer lists 7-20 years and other intangible assets 1-10 years. |
| Impairment of Long-Lived Assets — The Company assesses the impairment of definite-lived long-lived assets or asset groups when events or changes in circumstances indicate that the carrying value may not be recoverable. Factors that are considered in deciding when to perform an impairment review include: A significant decrease in the market price of the asset or asset group; A significant adverse change in the extent or manner in which a long-lived asset or asset group is being used or in its physical condition; An accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction; A current-period operating or cash flow loss combined with a history of operating or cash flow losses or a projection or forecast that demonstrates continuing losses associated with the use of a long-lived asset or asset group; or a current expectation that, more likely than not, a long-lived asset or asset group will be sold or otherwise disposed of significantly before the end of its previously estimated useful life. The term more likely than not refers to a level of likelihood that is more than 50 percent. |
| Recoverability potential is measured by comparing the carrying amount of the asset or asset group to its related total future undiscounted cash flows. If the carrying value is not recoverable through related cash flows, the asset or asset group is considered to be impaired. Impairment is measured by comparing the asset or asset group’s carrying amount to its fair value. When it is determined that useful lives of assets are shorter than originally estimated, and no impairment is present, the rate of depreciation is accelerated in order to fully depreciate the assets over their new shorter useful lives. |
| Goodwill and trademarks and tradenames recorded are not amortized but are periodically tested for impairment. The Company assesses goodwill for impairment by comparing the fair value of its reporting units to their carrying amounts on the last day of each fiscal year, or more frequently if certain events occur as described above. If the fair value of a reporting unit is less than its carrying value, an impairment loss is recorded to the extent that the implied fair value of the goodwill within the reporting unit is less than its carrying value. Fair values for reporting units are determined based on discounted cash flows and market multiples. Indefinite lived intangible assets such as trademarks and tradenames are assessed for impairment on the last day of each fiscal year, or more frequently if certain events occur as described above, by comparing the fair value of the asset to its carrying value. The fair value is determined by using a relief-from-royalty approach. |
66
Table of Contents
| The Company has determined that, based on the impairment tests performed, no impairment of goodwill has occurred during 2010, 2009 or 2008. During 2009, the Company recognized a $15.9 million impairment charge related to its trademarks and tradenames — See Note 4 “Intangible Assets.” No impairment of the Company’s trademarks and tradenames occurred during 2010 or 2008. |
| Other Long-Term Assets — Other long-term assets includes deferred costs incurred in connection with the Company’s issuance of its convertible subordinated notes and revolving line of credit. These costs are amortized to Interest Expense using the effective interest method over the period from the date of issuance to the put option date (if applicable) or the contractual maturity date, whichever is earlier. Total deferred financing fees amounted to $2.0 million at December 31, 2010 and $3.0 million at January 1, 2010. The amortization of deferred fees is included in Depreciation and Amortization in the Consolidated Statements of Cash Flows. |
| Other long-term assets also include investments in equity securities of entities which the Company does not have the ability to exercise significant influence over and are accounted for using the cost method. Each reporting period, management evaluates these investments to determine if there are any events or circumstances that are likely to have a significant adverse effect on the fair value of the investment. Examples of such impairment indicators include, but are not limited to: a recent sale or offering of similar shares of the investment at a price below the Company’s cost basis; a significant deterioration in earnings performance; a significant adverse change in the regulatory, economic or technological environment of the investee; or a significant doubt about an investee’s ability to continue as a going concern. If an impairment indicator is identified, management will estimate the fair value of the investment and compare it to its carrying value. The estimation of fair value considers all available financial information related to the investee, including, but not limited to, valuations based on recent third-party equity investments in the investee. If the fair value of the investment is less than its carrying value, the investment is impaired and a determination as to whether the impairment is other-than-temporary is made. Impairment is deemed to be other-than-temporary unless the Company has the ability and intent to hold the investment for a period sufficient for a market recovery up to the carrying value of the investment. Further, evidence must indicate that the carrying value of the investment is recoverable within a reasonable period. For other-than-temporary impairments, an impairment loss is recognized equal to the difference between the investment’s carrying value and its fair value. During 2010, the Company recognized a $0.2 million impairment charge related to one of its cost method investments, which had a book value of $0.3 million at December 31, 2010. No impairment of the Company’s cost method investments occurred during 2009 or 2008. |
| The aggregate recorded amount of cost method investments at December 31, 2010 and January 1, 2010 was $11.8 million and $11.9 million, respectively. The Company has determined that these investments are not considered variable interest entities. The Company’s exposure related to these entities is limited to its recorded investment. These investments are in start-up research and development companies whose fair value is highly subjective in nature and subject to future fluctuations, which could be significant. |
| Subsequent event (Unaudited) — Effective January 5, 2011, the Company sold its cost method investment in IntElect Medical, Inc. (“IntElect”) in conjunction with Boston Scientific’s acquisition of IntElect. The Company obtained its ownership interest in IntElect through its acquisition of BIOMEC, Inc. in 2007 and two subsequent additional investments. The Company received $10.5 million in cash proceeds and recognized a pre-tax gain of $4.5 million in the first quarter of 2011 in connection with this transaction. |
67
Table of Contents
| Income Taxes — The consolidated financial statements of the Company have been prepared using the asset and liability approach in accounting for income taxes, which requires the recognition of deferred income taxes for the expected future tax consequences of net operating losses, credits, and temporary differences between the financial statement carrying amounts and the tax bases of assets and liabilities. A valuation allowance is provided on deferred tax assets if it is determined that it is more likely than not that the asset will not be realized. |
| The Company accounts for uncertain tax positions using a more likely than not recognition threshold. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. These tax positions are evaluated on a quarterly basis. The Company recognizes interest expense related to uncertain tax positions as Interest Expense. Penalties, if incurred, are recognized as a component of Selling, General and Administrative Expenses. |
| The Company and its subsidiary file a consolidated U.S. federal income tax return. State tax returns are filed on a combined or separate basis depending on the applicable laws in the jurisdictions where tax returns are filed. The Company also files foreign tax returns on a separate company basis in the countries in which it operates. |
| Convertible Subordinated Notes — For convertible debt instruments that may be settled in cash upon conversion, such as the CSN II notes described in Note 6 “Debt,” the Company accounts for the liability and equity components of those instruments in a manner that will reflect the entity’s nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. |
| Upon issuance, the Company determined the carrying amount of the liability component of CSN II by measuring the fair value of a similar liability that does not have the associated conversion option. The carrying amount of the conversion option was then determined by deducting the fair value of the liability component from the initial proceeds received from the issuance of CSN II. |
| The carrying amount of the conversion option was recorded in Additional Paid-In Capital with an offset to Long-Term Debt and is being amortized using the effective interest method over the period from the date of issuance to the contractual maturity date. Deferred financing fees incurred in connection with the issuance of CSN II, were allocated proportionally to the proceeds of the liability and equity components. The deferred financing fees allocated to the debt component are being amortized using the effective interest method over the period from the date of issuance to contractual maturity date. The deferred financing fees allocated to the equity component were recorded as an offset to Additional Paid-In Capital. The amortization of discount and deferred fees related to the Company’s convertible debt instruments is included in Depreciation and Amortization in the Consolidated Statements of Cash Flows. |
| Derivative Financial Instruments — The Company recognizes all derivative financial instruments in its consolidated financial statements at fair value. Changes in the fair value of derivative instruments are recorded in earnings unless hedge accounting criteria are met. The Company’s interest rate swap (Note 6) and foreign currency contracts (Note 11) outstanding as of December 31, 2010 are designated as cash flow hedges. The effective portion of the changes in fair value of these cash flow hedges is recorded each period, net of tax, in Accumulated Other Comprehensive Income until the related hedged transaction occurs. Any ineffective portion of the changes in fair value of these cash flow hedges is recorded in earnings. In the event the hedged cash flow for forecasted transactions does not occur, or it becomes probable that they will not occur, the Company would reclassify the amount of any gain or loss on the related cash flow hedge to income (expense) at that time. Cash flows related to these derivative financial instruments are included in cash flows from operating activities. |
68
Table of Contents
| Revenue Recognition — The Company recognizes revenue when it is realized or realizable and earned. This occurs when persuasive evidence of an arrangement exists, delivery has occurred, the price is fixed or determinable, the buyer is obligated to pay us (i.e., not contingent on a future event), the risk of loss is transferred, there is no obligation of future performance, collectability is reasonably assured and the amount of future returns can reasonably be estimated. With regards to the Company’s customers (including distributors), those criteria are met at the time of shipment when title passes. The Company includes shipping and handling fees billed to customers in sales. Shipping and handling costs associated with inbound and outbound freight are recorded in Cost of Sales. In certain instances the Company obtains component parts for sub-assemblies from its customers that are included in the final product. These amounts were excluded from Sales and Cost of Sales recognized by the Company. The cost of these customer supplied component parts amounted to $29.9 million, $27.8 million and $35.1 million in 2010, 2009 and 2008, respectively. |
| Product Warranties — The Company allows customers to return defective or damaged products for credit, replacement, or exchange. The Company warrants that its products will meet customer specifications and will be free from defects in materials and workmanship. The Company accrues its estimated exposure to warranty claims based upon recent historical experience and other specific information as it becomes available. |
| Research and Development and Engineering Costs — Research and development costs are expensed as incurred. The primary costs are salary and benefits for personnel, material costs used in development projects and subcontracting costs. Engineering costs are expensed as incurred. Cost reimbursements for engineering services from customers for whom the Company designs products are recorded as an offset to engineering costs upon achieving development milestones specified in the contracts. |
| Net research, development and engineering costs are comprised of the following (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Research and development costs |
$ | 17,378 | $ | 17,707 | $ | 18,750 | ||||||
Engineering costs |
34,208 | 26,438 | 22,447 | |||||||||
Less: cost reimbursements |
(6,567 | ) | (10,583 | ) | (9,753 | ) | ||||||
Engineering costs, net |
27,641 | 15,855 | 12,694 | |||||||||
Total research, development
and engineering costs, net |
$ | 45,019 | $ | 33,562 | $ | 31,444 | ||||||
| Acquired In-Process Research and Development — The Company defines IPR&D as the value assigned to research and development projects acquired that have not yet reached technological feasibility and have no alternative future use. The Company believes a research and development project is not technically feasible until the related products have received regulatory approval. Prior to 2009, when the Company acquired another entity, the portion of the purchase price allocated to IPR&D was immediately expensed on the acquisition date. Effective in 2009, the accounting standard applicable to business combinations changed such that IPR&D projects acquired are now required to be recognized on the balance sheet at fair value as an indefinite-lived intangible asset regardless of whether there is an alternative future use for the IPR&D. In future periods, the IPR&D intangible asset will be amortized or written-down depending on the outcome of the project similar to other indefinite-lived assets — See “Amortizing Intangible Assets” and “Impairment of Long-Lived Assets.” As of December 31, 2010, the Company does not have any IPR&D intangible assets recorded on its balance sheet. |
69
Table of Contents
| Determining the portion of the purchase price to allocate to IPR&D requires the Company to make significant estimates. The amount of the purchase price allocated to IPR&D is determined by estimating the future cash flows of each project and discounting the net cash flows back to their present values. The discount rate used is determined at the time of acquisition in accordance with accepted valuation methods. These estimates also include consideration of the risk of the project not achieving commercial feasibility. |
| Stock-Based Compensation — The Company records compensation costs related to stock-based awards granted to employees based on the estimated fair value of the award on the grant date. Compensation cost for service-based awards is recognized ratably over the applicable vesting period. Compensation cost for nonmarket-based performance awards is reassessed each period and recognized based upon the probability that the performance targets will be achieved. Compensation cost for market-based performance awards is expensed each period whether the performance metrics are achieved or not. |
| The Company utilizes the Black-Scholes option pricing model to estimate the fair value of stock options granted. For service-based and nonmarket-based performance restricted stock and restricted stock unit awards, the fair market value of the award is determined based upon the closing value of the Company’s stock price on the grant date. For market-based performance restricted stock unit awards, the fair market value of the award is determined by utilizing a Monte Carlo simulation model, which projects the value of the Company’s stock under numerous scenarios and determines the value of the award based upon the present value of those projected outcomes. |
| The amount of stock-based compensation expense recognized is based on the portion of the awards that are ultimately expected to vest, excluding market and nonmarket performance award considerations discussed above. The Company estimates pre-vesting forfeitures at the time of grant by analyzing historical data and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. The total expense recognized over the vesting period will only be for those awards that ultimately vest, excluding market and nonmarket performance award considerations. |
| Foreign Currency Translation — The Company translates all assets and liabilities of its foreign subsidiaries, where the U.S. dollar is not the functional currency, at the period-end exchange rate and translates income and expenses at the average exchange rates in effect during the period. The net effect of this translation is recorded in the consolidated financial statements as Accumulated Other Comprehensive Income. Translation adjustments are not adjusted for income taxes as they relate to permanent investments in the Company’s foreign subsidiaries. Net foreign currency transaction gains and losses included in Other (Income) Expense, Net amounted to a loss of $0.9 million for 2010 and a gain of $0.7 million and $0.1 million for 2009 and 2008, respectively. |
| Defined Benefit Pension Plans — The Company recognizes in its balance sheet as an asset or liability the overfunded or underfunded status of its defined benefit pension plans provided to its employees located in Switzerland and France. This asset or liability is measured as the difference between the fair value of plan assets and the benefit obligation of those plans. For a pension plan, the benefit obligation is the projected benefit obligation, which is calculated based on actuarial computations of current and future benefits for employees. Actuarial gains or losses and prior service costs or credits that arise during the period, but are not included as components of net periodic benefit expense, are recognized as a component of Accumulated Other Comprehensive Income. Pension expense is charged to operating expenses. |
70
Table of Contents
| Earnings (Loss) Per Share (“EPS”) — Basic EPS is calculated by dividing Net Income (Loss) by the weighted average number of shares outstanding during the period. Diluted EPS is calculated by adjusting the weighted average number of shares outstanding for potential common shares, which consist of stock options, unvested restricted stock and restricted stock units and contingently convertible instruments. |
| Holders of the Company’s convertible subordinated notes may convert them into shares of the Company’s common stock under certain circumstances — See Note 6 “Debt.” The Company includes the effect of the conversion of these convertible notes in the calculation of diluted EPS using the if-converted method or the treasury method for instruments that may be settled in cash at the Company’s election and which the Company has the ability and intent to settle them in cash, as long as the effect is dilutive. For computation of EPS under conversion conditions, the number of diluted shares outstanding increases by the amount of shares that are potentially convertible during that period. Also, Net Income (Loss) is adjusted for the calculation to add back interest expense on the convertible notes as well as unamortized discount and deferred financing fees amortization recorded during the period. |
| The following table reflects the calculation of basic and diluted EPS (in thousands, except per share amounts): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Numerator for basic EPS: |
||||||||||||
Net income (loss) |
$ | 33,138 | $ | (9,001 | ) | $ | 14,148 | |||||
Effect of dilutive securities: |
||||||||||||
Interest expense on convertible notes and
related deferred financing fees, net of
tax |
241 | — | — | |||||||||
Numerator for diluted EPS |
$ | 33,379 | $ | (9,001 | ) | $ | 14,148 | |||||
Denominator for basic EPS: |
||||||||||||
Weighted average shares outstanding |
23,070 | 22,926 | 22,525 | |||||||||
Effect of dilutive securities: |
||||||||||||
Convertible subordinated notes |
347 | — | — | |||||||||
Stock options and unvested restricted stock |
385 | — | 336 | |||||||||
Denominator for diluted EPS |
23,802 | 22,926 | 22,861 | |||||||||
Basic EPS |
$ | 1.44 | $ | (0.39 | ) | $ | 0.63 | |||||
Diluted EPS |
$ | 1.40 | $ | (0.39 | ) | $ | 0.62 | |||||
| The diluted weighted average share calculations do not include the following as they are not dilutive to the EPS calculations or the performance criteria have not been met as of the reporting date: |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Time-vested stock options, restricted stock
and restricted stock units |
1,061,000 | 1,523,000 | 1,500,000 | |||||||||
Performance-vested stock options and
restricted stock units |
609,000 | 1,026,000 | 515,000 | |||||||||
Convertible subordinated notes |
— | 756,000 | 1,267,000 | |||||||||
71
Table of Contents
| Comprehensive Income (Loss) — The Company’s comprehensive income (loss) as reported in the Consolidated Statements of Operations and Comprehensive Income (Loss) includes net income (loss), foreign currency translation adjustments, the net change in cash flow hedges, and defined benefit pension plan liability adjustments. Accumulated other comprehensive income (loss) is comprised of the following (in thousands): |
| Defined | Foreign | |||||||||||||||||||||||
| benefit | currency | Total | ||||||||||||||||||||||
| pension plan | Cash flow | translation | pre-tax | Tax | Net-of tax- | |||||||||||||||||||
| liability | hedges | adjustment | amount | amount | amount | |||||||||||||||||||
Balance at January 1, 2010 |
$ | (1,455 | ) | $ | (1,701 | ) | $ | 4,334 | $ | 1,178 | $ | 970 | $ | 2,148 | ||||||||||
Net foreign currency translation gain |
— | — | 7,896 | 7,896 | — | 7,896 | ||||||||||||||||||
Unrealized gain on cash flow hedges |
— | 371 | — | 371 | (130 | ) | 241 | |||||||||||||||||
Realized loss on cash flow hedges |
— | 1,209 | — | 1,209 | (423 | ) | 786 | |||||||||||||||||
Net pension liability adjustments |
(559 | ) | — | — | (559 | ) | (42 | ) | (601 | ) | ||||||||||||||
Balance at December 31, 2010 |
$ | (2,014 | ) | $ | (121 | ) | $ | 12,230 | $ | 10,095 | $ | 375 | $ | 10,470 | ||||||||||
| Supplemental Cash Flow Information (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Cash paid during the year for: |
||||||||||||
Interest |
$ | 8,498 | $ | 9,234 | $ | 10,021 | ||||||
Income taxes |
3,826 | 4,473 | 3,811 | |||||||||
Noncash investing and financing activities: |
||||||||||||
Net change in cash flow hedges, net of tax |
$ | 1,027 | $ | (200 | ) | $ | (906 | ) | ||||
Common stock contributed to 401(k) Plan |
— | 4,015 | 3,472 | |||||||||
Property, plant and equipment purchases
included in accounts payable |
2,614 | 1,259 | 2,762 | |||||||||
Unsettled purchase of treasury stock |
— | 632 | 741 | |||||||||
Shares issued in connection with business
acquisition |
— | — | 1,473 | |||||||||
Acquisition of non-cash assets and
liabilities: |
||||||||||||
Assets acquired |
$ | 350 | $ | — | $ | 169,508 | ||||||
Liabilities assumed |
— | — | 58,693 | |||||||||
| Use of Estimates — The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of sales and expenses during the reporting period. Actual results could differ materially from those estimates. |
| Recently Issued Accounting Pronouncements — In the normal course of business, Management evaluates all new accounting pronouncements issued by the Financial Accounting Standards Board (“FASB”), Securities and Exchange Commission (“SEC”), Emerging Issues Task Force (“EITF”), American Institute of Certified Public Accountants (“AICPA”) or other authoritative accounting bodies to determine the potential impact they may have on the Company’s Consolidated Financial Statements. Based upon this review, Management does not expect any of the recently issued accounting pronouncements, which have not already been adopted, to have a material impact on the Company’s Consolidated Financial Statements. |
72
Table of Contents
| 2. | INVENTORIES |
| Inventories are comprised of the following (in thousands): |
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Raw material |
$ | 45,974 | $ | 54,002 | ||||
Work-in-process |
34,659 | 28,329 | ||||||
Finished goods |
20,807 | 24,278 | ||||||
Total |
$ | 101,440 | $ | 106,609 | ||||
| 3. | PROPERTY, PLANT AND EQUIPMENT |
| Property, plant and equipment are comprised of the following (in thousands): |
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Manufacturing machinery and equipment |
$ | 135,108 | $ | 125,524 | ||||
Buildings and building improvements |
71,160 | 68,489 | ||||||
Information technology hardware and software |
32,700 | 32,472 | ||||||
Leasehold improvements |
17,282 | 17,277 | ||||||
Furniture and fixtures |
10,475 | 10,259 | ||||||
Land and land improvements |
10,332 | 10,175 | ||||||
Construction work in process |
11,639 | 7,696 | ||||||
Other |
808 | 790 | ||||||
| 289,504 | 272,682 | |||||||
Accumulated depreciation |
(143,124 | ) | (119,081 | ) | ||||
Total |
$ | 146,380 | $ | 153,601 | ||||
| Depreciation expense for property, plant and equipment during 2010, 2009 and 2008 was $26.1 million, $27.1 million and $25.5 million, respectively. |
| 4. | INTANGIBLE ASSETS |
| Amortizing intangible assets are comprised of the following (in thousands): |
| Gross | Foreign | |||||||||||||||
| carrying | Accumulated | currency | Net carrying | |||||||||||||
| amount | amortization | translation | amount | |||||||||||||
December 31, 2010 |
||||||||||||||||
Purchased
technology and
patents |
$ | 83,023 | $ | (48,187 | ) | $ | 1,212 | $ | 36,048 | |||||||
Customer lists |
46,818 | (10,577 | ) | 2,119 | 38,360 | |||||||||||
Other |
3,519 | (2,862 | ) | 49 | 706 | |||||||||||
Total amortizing
intangible assets |
$ | 133,360 | $ | (61,626 | ) | $ | 3,380 | $ | 75,114 | |||||||
January 1, 2010 |
||||||||||||||||
Purchased
technology and
patents |
$ | 82,673 | $ | (42,289 | ) | $ | 399 | $ | 40,783 | |||||||
Customer lists |
46,818 | (7,264 | ) | 612 | 40,166 | |||||||||||
Other |
3,519 | (2,410 | ) | 18 | 1,127 | |||||||||||
Total amortizing
intangible assets |
$ | 133,010 | $ | (51,963 | ) | $ | 1,029 | $ | 82,076 | |||||||
73
Table of Contents
| Intangible amortization expense was $9.7 million, $10.1 million and $10.7 million for 2010, 2009 and 2008, respectively. All intangible amortization expense is included in Cost of Sales except for amortization primarily related to the Company’s customer lists, which totaled $3.8 million, $3.7 million and $3.9 million for 2010, 2009 and 2008 respectively, and is included in Selling, General and Administrative Expenses. Annual intangible amortization expense is estimated to be $9.8 million for 2011, $9.7 million for 2012, $8.9 million for 2013, $8.2 million for 2014 and $7.1 million for 2015. |
| As a result of the successful rebranding of the Company, during the fourth quarter of 2009, the Company wrote-down its non-Greatbatch trademarks and tradenames by $15.9 million. This charge was recorded based upon the Company’s decision to discontinue use of the associated tradenames and the Company’s determination that there would be no market participants willing to purchase the previously acquired tradenames. In addition to the above, the Company incurred expense of $0.7 million in 2009 related to its rebranding initiative, which includes additional advertising costs, and is included in Selling, General and Administrative Expenses. |
| The change in goodwill during 2010 is as follows (in thousands): |
| Greatbatch | ||||||||||||
| Medical | Electrochem | Total | ||||||||||
Balance at January 1, 2010 |
$ | 293,983 | $ | 9,943 | $ | 303,926 | ||||||
Foreign currency translation |
3,525 | — | 3,525 | |||||||||
Balance at December 31, 2010 |
$ | 297,508 | $ | 9,943 | $ | 307,451 | ||||||
| As of December 31, 2010, no accumulated impairment loss has been recognized for the goodwill allocated to the Company’s Greatbatch Medical or Electrochem segments. |
| 5. | ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES |
| Accrued expenses and other current liabilities are comprised of the following (in thousands): |
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Litigation accrual |
$ | — | $ | 36,000 | ||||
Salaries and benefits |
13,104 | 12,605 | ||||||
Profit sharing and bonuses |
8,443 | 9,544 | ||||||
Warranty |
2,313 | 1,330 | ||||||
Other |
8,224 | 8,517 | ||||||
Total |
$ | 32,084 | $ | 67,996 | ||||
| 6. | DEBT |
| Long-term debt is comprised of the following (in thousands): |
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Revolving line of credit, due 2012 |
$ | 50,000 | $ | 98,000 | ||||
2.25% convertible subordinated notes I, due 2013 |
— | 30,450 | ||||||
2.25% convertible subordinated notes II, due
2013 |
197,782 | 197,782 | ||||||
Unamortized discount |
(27,153 | ) | (36,810 | ) | ||||
Total debt |
220,629 | 289,422 | ||||||
Less: current portion |
— | (30,450 | ) | |||||
Total long-term debt |
$ | 220,629 | $ | 258,972 | ||||
74
Table of Contents
| Revolving Line of Credit — The Company has a senior credit facility (the “Credit Facility”) consisting of a $235 million revolving credit facility, which can be increased to $335 million upon the Company’s request and approval by a majority of the lenders. The Credit Facility also contains a $15 million letter of credit subfacility and a $15 million swingline subfacility. |
| The Credit Facility is secured by the Company’s non-realty assets including cash, accounts receivable and inventories, and has an expiration date of May 22, 2012 with a one-time option to extend to April 1, 2013 at the Company’s option if no default has occurred. Interest rates under the Credit Facility are, at the Company’s option, based upon the current Base Rate, as defined in the credit agreement, or LIBOR rate plus a margin that varies with the Company’s leverage ratio, as defined in the credit agreement for the Credit Facility. If interest is paid based upon the Base Rate, the applicable margin is between minus 1.25% and 0.00%. If interest is paid based upon the LIBOR rate, the applicable margin is between 1.00% and 2.00%. The Company is required to pay a fee on any outstanding letter of credit balance equal to a margin between 1.125% and 2.125%, depending on the Company’s leverage ratio, as defined in the credit agreement. The Company is also required to pay a commitment fee between 0.125% and 0.250% per annum on the unused portion of the Credit Facility based on the Company’s leverage ratio, as defined in the credit agreement. |
| The Credit Facility contains limitations on the incurrence of indebtedness, liens and licensing of intellectual property, investments and certain payments. Additionally, the Credit Facility restricts payments, among other things, including limiting the repurchase of Greatbatch stock to $60 million and the ability of the Company to make cash payments upon conversion of its convertible subordinated notes. These limitations can be waived upon the Company’s request and approval of a simple majority of the lenders. |
| The Credit Facility requires the Company to maintain a ratio of adjusted EBITDA, as defined in the credit agreement, to interest expense of at least 3.00 to 1.00, and a total leverage ratio, as defined in the credit agreement, of not greater than 4.50 to 1.00. The calculation of adjusted EBITDA and leverage ratio excludes non-cash charges. As of December 31, 2010, the Company was in compliance with all covenants. |
| The Credit Facility contains customary events of default. Upon the occurrence and during the continuance of an event of default, a majority of the lenders may declare the outstanding advances and all other obligations under the Credit Facility immediately due and payable. |
| The weighted average interest rate on borrowings under the Company’s revolving line of credit as of December 31, 2010, which does not include the impact of the interest rate swaps described below, was 1.75%. As of December 31, 2010, the Company had $185 million of borrowing capacity available under the Credit Facility. This amount may vary from period to period based upon the debt levels of the Company as well as the level of EBITDA, which impacts the covenant calculations described above. |
| Interest Rate Swaps —In 2008, the Company entered into three receive floating-pay fixed interest rate swaps indexed to the six-month LIBOR rate. As of December 31, 2010, only one of these swaps remains outstanding. The objective of these swaps was to hedge against potential changes in cash flows on the Company’s outstanding revolving line of credit, which is indexed to the six-month LIBOR rate. No credit risk was hedged. The receive variable leg of the swaps and the variable rate paid on the revolving line of credit bear the same rate of interest, excluding the credit spread, and reset and pay interest on the same dates. The Company intends to continue electing the six-month LIBOR as the benchmark interest rate on the debt being hedged. If the Company repays the debt, it intends to replace the hedged item with similarly indexed forecasted cash flows. |
75
Table of Contents
| Information regarding the outstanding interest rate swap as of December 31, 2010 is as follows (dollars in thousands): |
| Current | Fair | |||||||||||||||||||||||||||||||
| Pay | receive | value | Balance | |||||||||||||||||||||||||||||
| Type of | Notional | Start | End | fixed | floating | December 31, | sheet | |||||||||||||||||||||||||
| Instrument | hedge | amount | date | date | rate | rate | 2010 | location | ||||||||||||||||||||||||
Int. rate swap |
Cash flow | $ | 50,000 | 7/7/2010 | 7/7/2011 | 2.16 | % | 0.75 | % | $ | (436 | ) | Other Current Liabilities | |||||||||||||||||||
| The estimated fair value of the interest rate swap represents the amount the Company would have to pay to terminate the contract. No portion of the change in fair value of the interest rate swaps during 2010 was considered ineffective. The amount recorded in Interest Expense related to the interest rate swaps was expense of $1.7 million and $1.4 million during 2010 and 2009, respectively, and income of $0.4 million in 2008. |
| Convertible Subordinated Notes — In May 2003, the Company completed a private placement of $170 million of 2.25% convertible subordinated notes, due June 15, 2013 (“CSN I”). In March 2007, the Company entered into separate, privately negotiated agreements to exchange $117.8 million of CSN I for an equivalent principal amount of a new series of 2.25% convertible subordinated notes due 2013 (“CSN II”) (collectively the “Exchange”) at a 5% discount. The primary purpose of the Exchange was to eliminate the June 15, 2010 call and put option that was included in the terms of CSN I. In connection with the Exchange, the Company issued an additional $80 million aggregate principal amount of CSN II at a price of $950 per $1,000 of principal. In December 2008, the Company entered into privately negotiated agreements under which it repurchased $21.8 million in aggregate principal amount of its outstanding CSN I at $845.38 per $1,000 of principal. The primary purpose of this transaction was to retire the notes, which contained a put option exercisable on June 15, 2010, at a discount, and resulted in a $3.2 million gain. During 2010, the holders of the remaining $30.5 million of CSN I exercised their put option and the notes were repaid with cash on hand. |
| CSN II bear interest at 2.25% per annum, payable semi-annually, and are due on June 15, 2013. The holders may convert the notes into shares of the Company’s common stock at a conversion price of $34.70 per share, which is equivalent to a conversion ratio of 28.8219 shares per $1,000 of principal. The conversion price and the conversion ratio will adjust automatically upon certain changes to the Company’s capitalization. CSN II were issued at a price of $950 per $1,000 of principal. The fair value of CSN II as of December 31, 2010 was approximately $192 million and is based on recent sales prices. |
| The effective interest rate of CSN II, which takes into consideration the amortization of the discount and deferred fees related to the issuance of these notes is 8.5%. The discount on CSN II is being amortized to the maturity date of the convertible notes utilizing the effective interest method. As of December 31, 2010, the carrying amount of the discount related to the CSN II conversion option value was $23.0 million. As of December 31, 2010, the if-converted value of the CSN II notes does not exceed their principal amount as the closing stock price of the Company’s stock of $24.15 per share did not exceed the conversion price of $34.70 per share. |
| The contractual interest and discount amortization for CSN II were as follows (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Contractual interest |
$ | 4,450 | $ | 4,450 | $ | 4,450 | ||||||
Discount amortization |
9,657 | 9,038 | 8,461 | |||||||||
76
Table of Contents
| The notes are convertible at the option of the holders at such time as: (i) the closing price of the Company’s common stock exceeds 150% of the conversion price of the notes for 20 out of 30 consecutive trading days; (ii) the trading price per $1,000 of principal is less than 98% of the product of the closing sale price of common stock for each day during any five consecutive trading day period and the conversion rate per $1,000 of principal; (iii) the notes have been called for redemption; (iv) the Company distributes to all holders of common stock rights or warrants entitling them to purchase additional shares of common stock at less than the average closing price of common stock for the ten trading days immediately preceding the announcement of the distribution; (v) the Company distributes to all holders of common stock any form of dividend which has a per share value exceeding 5% of the price of the common stock on the day prior to such date of distribution; (vi) the Company affects a consolidation, merger, share exchange or sale of assets pursuant to which its common stock is converted to cash or other property; (vii) the period beginning 60 days prior to but excluding June 15, 2013; and (viii) certain fundamental changes, as defined in the indenture agreement, occur or are approved by the Board of Directors. |
| Conversions in connection with corporate transactions that constitute a fundamental change require the Company to pay a premium make-whole amount, based upon a predetermined table as set forth in the indenture agreement, whereby the conversion ratio on the notes may be increased by up to 7.4 shares per $1,000 of principal. The premium make-whole amount will be paid in shares of common stock upon any such conversion, subject to the net share settlement feature of the notes described below. |
| CSN II contains a net share settlement feature that requires the Company to pay cash for each $1,000 of principal to be converted. Any amounts in excess of $1,000 will be settled in shares of the Company’s common stock, or at the Company’s option, cash. The Company has a one-time irrevocable election to pay the holders in shares of its common stock, which it currently does not plan to exercise. |
| The notes are redeemable by the Company at any time on or after June 20, 2012, or at the option of a holder upon the occurrence of certain fundamental changes, as defined in the agreement, affecting the Company. The notes are subordinated in right of payment to all of our senior indebtedness and effectively subordinated to all debts and other liabilities of the Company’s subsidiaries. |
| Deferred Financing Fees — The following is a reconciliation of deferred financing fees for 2010 and 2009 (in thousands): |
Balance at January 2, 2009 |
$ | 4,096 | ||
Amortization during the year |
(1,068 | ) | ||
Balance at January 1, 2010 |
3,028 | |||
Amortization during the year |
(1,023 | ) | ||
Balance at December 31, 2010 |
$ | 2,005 | ||
| 7. | EMPLOYEE BENEFIT PLANS |
| Savings Plan — The Company sponsors a defined contribution 401(k) plan, which covers substantially all of its U.S. based employees. The plan provides for the deferral of employee compensation under Section 401(k) and a discretionary Company match. In 2010, 2009 and 2008, this match was $0.35 per dollar of participant deferral, up to 6% of the total compensation for each participant. Net costs related to this defined contribution plan were $1.5 million in 2010, 2009, and 2008. |
77
Table of Contents
| In addition to the above, under the terms of the 401(k) plan document there is an annual discretionary defined contribution equal to five percent of each employee’s eligible compensation. This amount is contributed to the 401(k) plan in the form of Company stock. Compensation cost recognized related to the defined contribution was $4.4 million in 2008. No discretionary contribution was made for fiscal years 2010 and 2009 as the Company did not meet its EPS targets for those years. As of December 31, 2010, the 401(k) Plan held 607,476 shares of Company stock. |
| Pension Plans — The Company is required to provide its employees located in Switzerland and France certain defined pension benefits. Under these plans, benefits accrue to employees based upon years of service, position, age and compensation. The defined benefit pension plan that provides benefits to the Company’s employees located in Switzerland is a funded contributory plan while the pension plan that provides benefits to the Company’s employees located in France is unfunded and noncontributory. |
| Information relating to the funding position of the Company’s defined benefit pension plans as of the plans measurement date of December 31, 2010 and January 1, 2010 were as follows (in thousands): |
| Year Ended | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Change in projected benefit obligation: |
||||||||
Projected benefit obligation at beginning of year |
$ | 14,294 | $ | 13,439 | ||||
Service cost |
724 | 891 | ||||||
Interest cost |
383 | 407 | ||||||
Prior service cost |
143 | — | ||||||
Plan participants’ contributions |
863 | 839 | ||||||
Actuarial (gain) loss |
257 | (467 | ) | |||||
Benefits paid |
(590 | ) | (1,434 | ) | ||||
Settlements/curtailments |
(1,524 | ) | — | |||||
Foreign currency translation |
1,411 | 619 | ||||||
Projected benefit obligation at end of year |
15,961 | 14,294 | ||||||
Change in fair value of plan assets: |
||||||||
Fair value of plan assets at beginning of year |
10,320 | 7,454 | ||||||
Employer contributions |
913 | 2,283 | ||||||
Plan participants’ contributions |
863 | 839 | ||||||
Actual gain (loss) on plan assets |
(278 | ) | 701 | |||||
Benefits paid |
(559 | ) | (1,415 | ) | ||||
Settlements |
(1,050 | ) | — | |||||
Foreign currency translation |
1,105 | 458 | ||||||
Fair value of plan assets at end of year |
11,314 | 10,320 | ||||||
Projected benefit obligation in excess of plan
assets at end of year |
$ | 4,647 | $ | 3,974 | ||||
Pension liability classified as other current liabilities |
$ | 6 | $ | 15 | ||||
Pension liability classified as long-term liabilities |
$ | 4,641 | $ | 3,959 | ||||
Accumulated benefit obligation at end of year |
$ | 14,218 | $ | 12,877 | ||||
78
Table of Contents
| Amounts recognized in accumulated other comprehensive (gain) loss: |
| Year Ended | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Net (gain) loss occurring during the year |
$ | 916 | $ | (850 | ) | |||
Amortization of losses |
(586 | ) | (129 | ) | ||||
Prior service cost |
143 | — | ||||||
Amortization of prior service cost |
(4 | ) | — | |||||
Foreign currency translation |
90 | (79 | ) | |||||
Pre-tax adjustment |
559 | (1,058 | ) | |||||
Taxes |
42 | 196 | ||||||
Net (gain) loss |
$ | 601 | $ | (862 | ) | |||
| Net pension cost is comprised of the following (in thousands): |
| Year Ended | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Service cost |
$ | 724 | $ | 891 | ||||
Interest cost |
383 | 407 | ||||||
Expected return on plan assets |
(381 | ) | (318 | ) | ||||
Settlements |
87 | — | ||||||
Recognized net actuarial loss |
30 | 129 | ||||||
Net pension cost |
$ | 843 | $ | 1,109 | ||||
| The weighted-average rates used to determine the projected benefit obligations and net pension costs were as follows: |
| Projected benefit obligation | Net pension cost | |||||||||||||||||||
| December 31, | January 1, | |||||||||||||||||||
| 2010 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||
Discount rate |
2.9 | % | 3.0 | % | 3.0 | % | 3.0 | % | 3.6 | % | ||||||||||
Salary growth |
2.5 | % | 2.5 | % | 2.5 | % | 2.5 | % | 2.5 | % | ||||||||||
Expected rate of return
on plan assets |
3.8 | % | 4.0 | % | 4.0 | % | 4.0 | % | 3.8 | % | ||||||||||
Long-term inflation rate |
1.5 | % | 1.5 | % | 1.5 | % | 1.5 | % | 1.1 | % | ||||||||||
| The discount rate used is based on the yields of foreign government bonds with a duration matching the duration of the liabilities plus approximately 50 basis points to reflect the risk of investing in corporate bonds. The expected rate of return on plan assets reflects long-term earnings expectations on existing plan assets and those contributions expected to be received during the current plan year. In estimating that rate, appropriate consideration was given to historical returns earned by plan assets in the fund and the rates of return expected to be available for reinvestment. Rates of return were adjusted to reflect current capital market assumptions and changes in investment allocations. Equity securities and fixed income securities were assumed to earn a return in the range of 7% to 8% and 2.5% to 4.5%, respectively. When these overall return expectations are applied to the pension plan’s target allocation, the expected rate of return is determined to be 3.8%. |
79
Table of Contents
| Plan assets were comprised of the following (in thousands): |
| Fair value measurements using | ||||||||||||||||
| Significant | ||||||||||||||||
| Quoted prices in | other | Significant | ||||||||||||||
| At | active markets for | observable | unobservable | |||||||||||||
| December 31, | identical assets | inputs | inputs | |||||||||||||
| Description | 2010 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Cash |
$ | 84 | $ | 84 | $ | — | $ | — | ||||||||
Equity securities: |
||||||||||||||||
U.S. companies |
969 | 969 | — | — | ||||||||||||
International companies |
2,310 | 2,310 | — | — | ||||||||||||
Emerging markets |
464 | 464 | — | — | ||||||||||||
Fixed income: |
||||||||||||||||
Government & government
agencies |
4,336 | 4,336 | — | — | ||||||||||||
Corporate |
1,034 | 1,034 | — | — | ||||||||||||
Real-estate |
1,081 | — | 1,081 | — | ||||||||||||
Other |
1,036 | 1,036 | — | — | ||||||||||||
Total |
$ | 11,314 | $ | 10,233 | $ | 1,081 | $ | — | ||||||||
| Fair value measurements using | ||||||||||||||||
| Significant | ||||||||||||||||
| Quoted prices in | other | Significant | ||||||||||||||
| At | active markets for | observable | unobservable | |||||||||||||
| January 1, | identical assets | inputs | inputs | |||||||||||||
| Description | 2010 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Cash |
$ | 830 | $ | 830 | $ | — | $ | — | ||||||||
Equity securities: |
||||||||||||||||
U.S. companies |
430 | 430 | — | — | ||||||||||||
International companies |
2,444 | 2,444 | — | — | ||||||||||||
Emerging markets |
496 | 496 | — | — | ||||||||||||
Fixed income: |
||||||||||||||||
Government & government
agencies |
2,521 | 2,521 | — | — | ||||||||||||
Corporate |
2,064 | 1,847 | 217 | — | ||||||||||||
Convertible |
217 | — | 217 | — | ||||||||||||
Insurance contracts |
424 | — | 424 | — | ||||||||||||
Real-estate |
642 | — | 642 | — | ||||||||||||
Other |
252 | — | 252 | — | ||||||||||||
Total |
$ | 10,320 | $ | 8,568 | $ | 1,752 | $ | — | ||||||||
| The fair value of Level 1 pension assets are obtained by reference to the last quoted price of the respective security on the market which it trades. The fair value of Level 2 pension assets are obtained from quoted market prices in inactive markets or valuation models with observable market data inputs to estimate fair value. These observable market data inputs include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, benchmark securities, bids, offers and reference data. |
80
Table of Contents
| The weighted average target and actual pension fund asset allocation as of the valuation date was as follows: |
| 2010 | ||||||||
| Target | Actual | |||||||
Asset Category: |
||||||||
Fixed income |
60 | % | 47 | % | ||||
Equity |
25 | % | 33 | % | ||||
Real-estate |
5 | % | 10 | % | ||||
Cash |
5 | % | 1 | % | ||||
Other |
5 | % | 9 | % | ||||
| 100 | % | 100 | % | |||||
| The target allocation is consistent with the Company’s goal of diversifying the pension plans assets in order to preserve capital while achieving investment results that will contribute to the proper funding of pension obligations and cash flow requirements. |
| Estimated pension benefit payments over the next ten years are as follows (in thousands): |
2011 |
$ | 957 | ||
2012 |
941 | |||
2013 |
1,205 | |||
2014 |
1,121 | |||
2015 |
1,163 | |||
2016-2020 |
6,458 |
| Education Assistance Program — The Company reimburses tuition, textbooks and laboratory fees for college or other job related programs for all of its U.S. based employees. The Company also reimburses college tuition for the dependent children of its full-time U.S. based employees, which vests on a straight-line basis over ten years, up to the applicable local state university tuition rate. For certain employees and executives, the dependent children benefit is not limited. Minimum academic achievement is required in order to receive reimbursement under both programs. Aggregate expenses under the programs were approximately $1.3 million, $1.5 million and $1.3 million in 2010, 2009 and 2008, respectively. |
| 8. | STOCK-BASED COMPENSATION |
| Compensation costs related to stock-based payments totaled $6.9 million, $5.2 million and $6.8 million for 2010, 2009 and 2008, respectively. Of these amounts, $6.0 million, $4.4 million and $5.7 million were included in Selling, General and Administrative Expenses, respectively. The remaining stock-based compensation expense is primarily included in Cost of Sales. During 2010 and 2009, the Company reversed approximately $0.3 million and $2.6 million, respectively, of performance stock-based compensation expense as it was no longer probable that the performance metrics would be achieved on those awards. During 2010, the Company recorded $0.7 million of stock-based compensation expense related to the accelerated vesting of equity awards issued to the Company’s former Senior Vice President — Orthopaedics, who passed away during the year. Stock-based compensation expense included in the 2008 Consolidated Statements of Cash Flows includes costs recognized for stock-based awards and the annual discretionary stock contribution to the Company’s 401(k) Plan. See Note 7 — “Employee Benefit Plans.” |
81
Table of Contents
| Summary of Plans |
| The Company’s 1997 Stock Option Plan, 1998 Stock Option Plan, 2002 Restricted Stock Plan and Non-Employee Directors Plan have been frozen to any new award issuances. Stock option and restricted stock awards remain outstanding under these plans. |
| The Company’s 2005 Stock Incentive Plan (“2005 Plan”), as amended, authorizes the issuance of up to 2,450,000 shares of equity incentive awards including nonqualified and incentive stock options, restricted stock, restricted stock units, stock bonuses and stock appreciation rights subject to the terms of the 2005 Plan. The 2005 Plan limits the amount of restricted stock, restricted stock units and stock bonuses that may be awarded in the aggregate to 850,000 shares of the 2,450,000 shares authorized by the 2005 Plan. |
| The Company’s 2009 Stock Incentive Plan (“2009 Plan”) authorizes the issuance of up to 1,350,000 shares of equity incentive awards including nonqualified and incentive stock options, restricted stock, restricted stock units, stock bonuses and stock appreciation rights subject to the terms of the 2009 Plan. The 2009 Plan limits the amount of restricted stock, restricted stock units and stock bonuses that may be awarded in the aggregate to 200,000 shares of the 1,350,000 shares authorized. |
| As of December 31, 2010, there were 848,595 and 256,361 shares available for future grants under the 2009 Plan and 2005 Plan, respectively. Due to the plan sub-limits, of the shares available for grant only 180,981 and 26,361 shares may be issued under the 2009 Plan and the 2005 Plan, respectively, in the form of restricted stock, restricted stock units or stock bonuses. Currently, there are not enough shares available under the Company’s stock incentive plans to fund its stock-based compensation program grants for 2011. Accordingly, the Company intends to submit a proposal to shareholders at its 2011 Annual Meeting of Stockholders requesting additional shares. |
| Stock Options |
| Stock options granted generally vest over a three or four year period. Stock options expire 10 years from the date of grant. Stock options are granted at exercise prices equal to or greater than the fair value of the Company’s common stock on the date of grant. Performance-based stock options only vest if certain performance metrics are achieved. The performance metrics generally cover a three-year performance period beginning in the year of grant and include the achievement of revenue, adjusted operating earnings and adjusted operating cash flow targets. In 2010, the Company began issuing all performance stock-based awards in the form of restricted stock units. |
| The Company utilizes the Black-Scholes option pricing model to determine the fair value of stock options. Management is required to make certain assumptions with respect to selected model inputs. Expected volatility is based on the historical volatility of the Company’s stock over the most recent period commensurate with the estimated expected life of the stock options. The expected life of stock options, which represents the period of time that the stock options are expected to be outstanding, is based on historical data. The expected dividend yield is based on the Company’s history and expectation of future dividend payouts. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for a period commensurate with the estimated expected life. If factors change and result in different assumptions, the stock option expense that the Company records for future grants may differ significantly from what the Company recorded in the current period. Stock-based compensation expense is only recorded for those awards that are expected to vest. Pre-vesting forfeiture estimates for determining appropriate stock-based compensation expense are estimated at the time of grant based on historical experience. Revisions are made to those estimates in subsequent periods if actual forfeitures differ from estimated forfeitures. For retirement eligible employees, whose awards immediately vest, a 0% forfeiture rate is used. |
82
Table of Contents
| The weighted-average fair value and assumptions used are as follows: |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Weighted-average fair value |
$ | 8.24 | $ | 8.63 | $ | 8.38 | ||||||
Risk-free interest rate |
2.62 | % | 2.03 | % | 2.91 | % | ||||||
Expected volatility |
40 | % | 39 | % | 39 | % | ||||||
Expected life (in years) |
5.4 | 5.6 | 5.2 | |||||||||
Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
Pre-vesting forfeiture rate |
9 | % | 9 | % | 9 | % | ||||||
| The following tables summarize stock option activity: |
| Weighted | ||||||||||||||||
| average | ||||||||||||||||
| Weighted | remaining | Aggregate | ||||||||||||||
| Number of | average | contractual | intrinsic | |||||||||||||
| time-vested | exercise | life | value | |||||||||||||
| stock options | price | (in years) | (in millions) | |||||||||||||
Outstanding at December 28, 2007 |
1,301,167 | $ | 25.04 | |||||||||||||
Granted |
452,964 | 20.21 | ||||||||||||||
Exercised |
(131,100 | ) | 16.85 | |||||||||||||
Forfeited or Expired |
(124,737 | ) | 25.21 | |||||||||||||
Outstanding at January 2, 2009 |
1,498,294 | 24.28 | ||||||||||||||
Granted |
243,920 | 26.53 | ||||||||||||||
Exercised |
(13,736 | ) | 15.45 | |||||||||||||
Forfeited or Expired |
(366,355 | ) | 27.27 | |||||||||||||
Outstanding at January 1, 2010 |
1,362,123 | 23.94 | ||||||||||||||
Granted |
243,155 | 20.57 | ||||||||||||||
Exercised |
(34,196 | ) | 19.26 | |||||||||||||
Forfeited or Expired |
(107,526 | ) | 24.43 | |||||||||||||
Outstanding at December 31, 2010 |
1,463,556 | $ | 23.46 | 6.3 | $ | 3.2 | ||||||||||
Expected to Vest at December
31, 2010 |
1,421,839 | $ | 23.49 | 6.2 | $ | 3.1 | ||||||||||
Exercisable at December 31, 2010 |
1,144,997 | $ | 23.77 | 5.8 | $ | 2.4 | ||||||||||
83
Table of Contents
| Weighted | ||||||||||||||||
| average | ||||||||||||||||
| Number of | Weighted | remaining | ||||||||||||||
| performance- | average | contractual | Aggregate | |||||||||||||
| vested stock | exercise | life | intrinsic value | |||||||||||||
| options | price | (in years) | (in millions) | |||||||||||||
Outstanding at December 28, 2007 |
442,855 | $ | 25.08 | |||||||||||||
Granted |
417,888 | 21.88 | ||||||||||||||
Forfeited or Expired |
(62,179 | ) | 22.24 | |||||||||||||
Outstanding at January 2, 2009 |
798,564 | 23.62 | ||||||||||||||
Granted |
310,407 | 26.53 | ||||||||||||||
Forfeited or Expired |
(106,987 | ) | 24.00 | |||||||||||||
Outstanding at January 1, 2010 |
1,001,984 | 24.48 | ||||||||||||||
Forfeited or Expired |
(257,461 | ) | 26.81 | |||||||||||||
Outstanding at December 31, 2010 |
744,523 | $ | 23.68 | 7.1 | $ | 0.9 | ||||||||||
Expected to Vest at December
31, 2010 |
363,261 | $ | 23.27 | 6.3 | $ | 0.5 | ||||||||||
Exercisable at December 31, 2010 |
216,986 | $ | 22.92 | 5.3 | $ | 0.3 | ||||||||||
| Intrinsic value is calculated for in-the-money options (exercise price less than market price) outstanding and/or exercisable as the difference between the market price of our common shares as of December 31, 2010 ($24.15) and the weighted average exercise price of the underlying stock options, multiplied by the number of options outstanding and/or exercisable. As of December 31, 2010, $3.3 million of unrecognized compensation cost related to non-vested stock options is expected to be recognized over a weighted-average period of approximately 2 years. Shares are distributed from the Company’s authorized but unissued reserve upon the exercise of stock options or treasury stock if available. The Company does not intend to purchase treasury shares to fund the future exercises of stock options. |
| Proceeds from the exercise of stock options are credited to common stock at par value and the excess is credited to additional paid-in capital. A portion of the options outstanding qualify as incentive stock options (“ISO”) for income tax purposes. As such, a tax benefit is not recorded at the time the compensation cost related to the stock options is recorded for book purposes due to the fact that an ISO does not ordinarily result in a tax benefit unless there is a disqualifying disposition. Stock option grants of non-qualified stock options result in the creation of a deferred tax asset, which is a temporary difference, until the time that the option is exercised. The following table provides certain information relating to the exercise of stock options (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Intrinsic value |
$ | 112 | $ | 80 | $ | 974 | ||||||
Cash received |
659 | 212 | 2,210 | |||||||||
Tax (expense) benefit realized |
(41 | ) | 24 | 313 | ||||||||
84
Table of Contents
| Restricted Stock and Restricted Stock Units |
| Time-vested restricted stock and restricted stock unit awards granted typically vest 50% on the second fiscal year-end from the date of the award and 25% on the third and fourth fiscal year-ends from the date of the award. The following table summarizes time-vested restricted stock and stock unit activity: |
| Weighted average | ||||||||
| Activity | fair value | |||||||
Nonvested at December 28, 2007 |
258,134 | $ | 25.14 | |||||
Shares granted |
142,441 | 20.08 | ||||||
Shares vested |
(194,269 | ) | 24.04 | |||||
Shares forfeited |
(22,541 | ) | 21.39 | |||||
Nonvested at January 2, 2009 |
183,765 | 22.84 | ||||||
Shares granted |
100,358 | 26.17 | ||||||
Shares vested |
(104,412 | ) | 23.79 | |||||
Shares forfeited |
(18,713 | ) | 23.49 | |||||
Nonvested at January 1, 2010 |
160,998 | 24.22 | ||||||
Shares granted |
124,747 | 21.11 | ||||||
Shares vested |
(147,434 | ) | 23.05 | |||||
Shares forfeited |
(14,925 | ) | 23.45 | |||||
Nonvested at December 31, 2010 |
123,386 | $ | 22.57 | |||||
| Performance-vested restricted stock granted prior to 2010 vests upon the achievement of certain annual diluted EPS targets by the Company, or the seventh anniversary date of the award. |
| The performance-based restricted stock units granted in 2010 only vest if certain market-based performance metrics are achieved. The amount of shares that ultimately vest range from 0 shares to 266,478 shares based upon the total shareholder return of the Company relative to the Company’s compensation peer group over a three year performance period beginning in the year of grant. |
| The following table summarizes performance-vested restricted stock and stock unit activity related to the Company’s plans: |
| Weighted average | ||||||||
| Activity | fair value | |||||||
Nonvested at December 28, 2007 |
24,000 | $ | 23.07 | |||||
Nonvested at January 2, 2009 |
24,000 | 23.07 | ||||||
Nonvested at January 1, 2010 |
24,000 | 23.07 | ||||||
Shares granted |
289,654 | 14.43 | ||||||
Shares vested |
(21,558 | ) | 15.12 | |||||
Shares forfeited |
(8,299 | ) | 14.56 | |||||
Nonvested at December 31, 2010 |
283,797 | $ | 15.10 | |||||
| The fair value of time-based as well as nonmarket-based performance restricted stock and restricted stock unit awards is equal to the fair value of the Company’s stock on the date of grant. The fair value of market-based performance restricted stock unit awards is determined by utilizing a Monte Carlo simulation model, which projects the value of Greatbatch stock versus the Company’s peer group under numerous scenarios and determines the value of the award based upon the present value of these projected outcomes. |
| The realized tax benefit (expense) from the vesting of restricted stock and restricted stock units was $0.01 million, ($0.1 million) and $0.04 million for 2010, 2009 and 2008, respectively. As of December 31, 2010, there was $5.2 million of total unrecognized compensation cost related to the restricted stock and restricted stock unit awards. That cost is expected to be recognized over a weighted-average period of approximately 2 years. |
85
Table of Contents
| 9. | OTHER OPERATING EXPENSES, NET |
| Other operating expenses, net are comprised of the following (in thousands): |
| Year ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
(a) 2005 & 2006 facility shutdowns and
consolidations |
$ | — | $ | — | $ | 663 | ||||||
(b) 2007 & 2008 facility shutdowns and
consolidations |
1,348 | 7,069 | 8,347 | |||||||||
(c) Integration costs |
42 | 3,077 | 5,369 | |||||||||
(d) Asset dispositions, severance and other |
3,168 | 948 | 199 | |||||||||
| $ | 4,558 | $ | 11,094 | $ | 14,578 | |||||||
| • | Consolidated its medical capacitor manufacturing operations in Cheektowaga, NY and its implantable medical battery manufacturing operations in Clarence, NY into its advanced power source manufacturing facility in Alden, NY; | ||
| • | Consolidated its capacitor research, development and engineering center in Cheektowaga, NY and its Fremont, CA Advanced Research Laboratory into its technology center in Clarence, NY; | ||
| • | Consolidated the EMI filtering manufacturing in Carson City, NV and the feedthrough and electrode manufacturing in Columbia, MD facility into its Tijuana, Mexico facility; and | ||
| • | Completed a plan for consolidating its corporate and business unit organization structure. A significant portion of the annual savings from this initiative was reinvested into research and development activities and business growth opportunities. |
| The total cost of these projects was $24.7 million, which was incurred from 2005 to 2008, and consisted of the following: |
| • | Severance and retention — $7.4 million; | ||
| • | Production inefficiencies, moving and revalidation — $4.6 million; | ||
| • | Accelerated depreciation and asset write-offs — $1.1 million; | ||
| • | Personnel — $8.4 million; and | ||
| • | Other — $3.2 million. |
| All categories of costs were considered to be cash expenditures, except accelerated depreciation and asset write-offs. For 2008, costs relating to these initiatives were included in the Greatbatch Medical business segment. |
| • | Consolidated its Electrochem manufacturing facilities in Canton, MA, Teterboro, NJ and Suzhou, China, into a newly constructed facility in Raynham, MA; | ||
| • | Consolidated its corporate offices in Clarence, NY into its technology center also in Clarence, NY; | ||
| • | Reorganized and consolidated various general and administrative and research and development functions throughout the organization in order to optimize those resources with the businesses it acquired in 2007 and 2008; |
86
Table of Contents
| • | Consolidated its Orchard Park, NY (Electrochem manufacturing), Exton, PA (Orthopaedic corporate office) and Saignelegier, Switzerland (Orthopaedic manufacturing) facilities into existing facilities that had excess capacity; and | ||
| • | Consolidated its manufacturing operations in Blaine, MN into its Plymouth, MN facility. |
| The total cost incurred for these facility shutdowns and consolidations was $17.3 million and included the following: |
| • | Severance and retention — $4.4 million; | ||
| • | Production inefficiencies, moving and revalidation — $5.2 million; | ||
| • | Accelerated depreciation and asset write-offs — $5.3 million; | ||
| • | Personnel — $0.7 million; and | ||
| • | Other — $1.7 million. |
| All categories of costs are considered to be cash expenditures, except accelerated depreciation and asset write-offs. For 2010, costs relating to these initiatives of $0.3 million and $1.0 million were included in the Greatbatch Medical and Electrochem business segments, respectively. For 2009, costs relating to these initiatives of $1.6 million and $5.5 million were included in the Greatbatch Medical and Electrochem business segments, respectively. Costs incurred during 2008 of $0.3 million, $4.7 million and $3.3 million were included in unallocated corporate expenses, Greatbatch Medical and Electrochem business segments, respectively. |
| As a result of these consolidation initiatives, two Greatbatch Medical facilities and one Electrochem facility were classified as assets held for sale. These facilities were recorded at the lower of their carrying amount or estimated fair value less cost to sell. The fair value of these facilities was primarily determined by reference to recent sales data for comparable facilities taking into consideration recent offers, if any, received from prospective buyers of the facilities, which is categorized as Level 2 in the fair value hierarchy. One Greatbatch Medical and one Electrochem facility were sold in 2010, which resulted in net cash proceeds of $2.4 million. The remaining Greatbatch Medical facility, which had a fair value of $1.9 million, was reclassified to Property, Plant and Equipment, Net in 2010 as management decided to utilize this facility for future operations. For 2010, 2009 and 2008, write-downs of $1.0 million, $0.3 million and $1.7 million, respectively, were recorded relating to these facilities and were included in Other Operating Expense, Net. |
| Accrued liabilities related to the 2007 & 2008 facility shutdowns and consolidations are comprised of the following (in thousands): |
| Production | Accelerated | |||||||||||||||||||||||
| Severance | inefficiencies, | depreciation/ | ||||||||||||||||||||||
| and | moving and | asset write- | ||||||||||||||||||||||
| retention | revalidation | offs | Personnel | Other | Total | |||||||||||||||||||
Balance, January 2, 2009 |
$ | 594 | $ | — | $ | — | $ | — | $ | — | $ | 594 | ||||||||||||
Restructuring charges |
1,796 | 2,948 | 671 | 534 | 1,120 | 7,069 | ||||||||||||||||||
Write-offs |
— | — | (671 | ) | — | — | (671 | ) | ||||||||||||||||
Cash payments |
(1,466 | ) | (2,948 | ) | — | (534 | ) | (1,120 | ) | (6,068 | ) | |||||||||||||
Balance, January 1, 2010 |
$ | 924 | $ | — | $ | — | $ | — | $ | — | $ | 924 | ||||||||||||
Restructuring charges |
(137 | ) | 153 | 956 | 76 | 300 | 1,348 | |||||||||||||||||
Write-offs |
— | — | (956 | ) | — | — | (956 | ) | ||||||||||||||||
Cash payments |
(787 | ) | (153 | ) | — | (76 | ) | (300 | ) | (1,316 | ) | |||||||||||||
Balance, December 31, 2010 |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
87
Table of Contents
| During 2010, 2009 and 2008, the Company recorded write-downs in connection with various asset disposals, which were partially offset by insurance proceeds received. |
| 10. | INCOME TAXES |
| The U.S. and international components of income (loss) before provision (benefit) for income taxes were as follows (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
U.S. |
$ | 46,217 | $ | (15,285 | ) | $ | 25,946 | |||||
International |
3,108 | (2,892 | ) | (5,429 | ) | |||||||
| $ | 49,325 | $ | (18,177 | ) | $ | 20,517 | ||||||
88
Table of Contents
| The provision (benefit) for income taxes was comprised of the following (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Current: |
||||||||||||
Federal |
$ | (671 | ) | $ | 827 | $ | 5,860 | |||||
State |
179 | (177 | ) | 693 | ||||||||
International |
1,260 | 294 | 520 | |||||||||
| 768 | 944 | 7,073 | ||||||||||
Deferred: |
||||||||||||
Federal |
15,409 | (9,256 | ) | 3,024 | ||||||||
State |
300 | (153 | ) | (692 | ) | |||||||
International |
(290 | ) | (711 | ) | (3,036 | ) | ||||||
| 15,419 | (10,120 | ) | (704 | ) | ||||||||
| $ | 16,187 | $ | (9,176 | ) | $ | 6,369 | ||||||
| The provision (benefit) for income taxes differs from the U.S. statutory rate due to the following: |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Statutory rate |
35.0 | % | (35.0 | )% | 35.0 | % | ||||||
Swiss tax holiday |
— | — | (7.5 | ) | ||||||||
Federal tax credits |
(2.6 | ) | (5.5 | ) | (4.4 | ) | ||||||
Foreign rate differential |
(0.8 | ) | 1.9 | 4.0 | ||||||||
Uncertain tax positions |
(1.3 | ) | (7.8 | ) | 0.8 | |||||||
In-process research and development |
— | — | 3.0 | |||||||||
State taxes, net of federal benefit |
(0.3 | ) | (1.2 | ) | (0.9 | ) | ||||||
Valuation allowance |
1.7 | (0.1 | ) | 0.9 | ||||||||
Other |
1.1 | (2.8 | ) | 0.1 | ||||||||
Effective tax rate |
32.8 | % | (50.5 | )% | 31.0 | % | ||||||
| In its budget submission to Congress in February 2011, the Obama administration proposed changes to the manner in which the U.S. would tax the international income of U.S. based companies. While it is uncertain how the U.S. Congress may address U.S. tax policy in the future, reform of U.S. taxation, including taxation of international income, continues to be a topic of discussion for Congress. A significant change to the U.S. tax system, including changes to the taxation of international income, could have a material effect on the Company’s effective tax rate. |
| During 2008, the Company received a nine year tax holiday (i.e. reduction in tax rate) from the Canton of Bern, Switzerland, beginning in 2009. This resulted in a one-time reduction of the Swiss deferred tax liabilities of approximately $1.5 million, which is reflected in the 2008 effective tax rate. The tax holiday was granted based upon projections of future capital investment and employment levels in the Canton of Bern. These projections are subject to periodic review by the governmental and tax authorities. If these projections are not met, part or all of the tax holiday may be revoked. If part or all of the tax holiday were revoked, a portion (or all) of the tax benefit recognized in 2008 would be reversed. The Company also negotiated a tax holiday with the Swiss federal authority’s contingent on certain conditions that have not yet been met. As such, this tax holiday will not be recorded until the conditions have been satisfied. |
89
Table of Contents
| Deferred tax assets (liabilities) consist of the following (in thousands): |
| As of | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Tax credits |
$ | 5,896 | $ | 5,318 | ||||
Net operating loss carryforwards |
4,617 | 4,189 | ||||||
Inventories |
4,575 | 4,139 | ||||||
Accrued expenses |
2,563 | 15,871 | ||||||
Stock-based compensation |
5,358 | 5,711 | ||||||
Other |
507 | 807 | ||||||
Gross deferred tax assets |
23,516 | 36,035 | ||||||
Less valuation allowance |
(6,482 | ) | (5,656 | ) | ||||
Net deferred tax assets |
17,034 | 30,379 | ||||||
Property, plant and equipment |
(713 | ) | (3,471 | ) | ||||
Intangible assets |
(40,082 | ) | (35,808 | ) | ||||
Convertible subordinated notes |
(31,218 | ) | (28,789 | ) | ||||
Gross deferred tax liabilities |
(72,013 | ) | (68,068 | ) | ||||
Net deferred tax liability |
$ | (54,979 | ) | $ | (37,689 | ) | ||
Presented as follows: |
||||||||
Current deferred tax asset |
$ | 7,398 | $ | 13,896 | ||||
Current deferred tax liability |
(514 | ) | — | |||||
Noncurrent deferred tax asset |
2,427 | 2,458 | ||||||
Noncurrent deferred tax liability |
(64,290 | ) | (54,043 | ) | ||||
Total net deferred tax liability |
$ | (54,979 | ) | $ | (37,689 | ) | ||
| As of December 31, 2010, the Company has the following carryforwards available: |
| Tax | Begin to | |||||||||||
| Jurisdiction | attribute | Amount | expire | |||||||||
U.S. |
Net Operating Loss | $ | 2.9 million | (1) | 2022 | |||||||
Switzerland |
Net Operating Loss | 11.9 million | (1) | 2011 | ||||||||
State |
Net Operating Loss | 20.2 million | (1) | Various | ||||||||
U.S. and State |
R&D Credit | 1.0 million | Various | |||||||||
State |
Investment Tax Credit | 4.9 million | Various | |||||||||
| (1) | These tax attributes were acquired primarily as part of the Precimed acquisition in 2008. The utilization of certain net operating losses and credits is subject to an annual limitation under Internal Revenue Code Section 382. |
90
Table of Contents
| Certain federal and state net operating loss carryforwards and tax credits per the income tax returns filed included uncertain tax positions taken in prior years. Due to the application of the accounting for uncertain tax positions, the actual tax attributes are larger than the net operating losses and tax credits for which a deferred tax asset is recognized for financial statement purposes. |
| In assessing the realizability of deferred tax assets, management considers, within each taxing jurisdiction, whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. Based on the consideration of the weight of both positive and negative evidence, management has determined that a portion of the deferred tax assets as of December 31, 2010 and January 1, 2010 related to certain state investment tax credits and net operating losses will not be realized. |
| The Company files annual income tax returns in the U.S., various state and local jurisdictions, and in various foreign jurisdictions. A number of years may elapse before an uncertain tax position, for which the Company has unrecognized tax benefits, is examined and finally settled. While it is often difficult to predict the final outcome or the timing of resolution of any particular uncertain tax position, the Company believes that its unrecognized tax benefits reflect the most probable outcome. The Company adjusts these unrecognized tax benefits, as well as the related interest, in light of changing facts and circumstances. The resolution of a matter could be recognized as an adjustment to the provision for income taxes and the effective tax rate in the period of resolution. |
| Below is a summary of changes to the unrecognized tax benefit (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Balance, beginning of year |
$ | 3,418 | $ | 5,686 | $ | 1,678 | ||||||
Additions based upon tax positions related to the
current year |
300 | 396 | 699 | |||||||||
Additions recorded as part of business combinations |
— | — | 3,979 | |||||||||
Reductions related to prior period tax positions |
222 | (1,185 | ) | (373 | ) | |||||||
Reductions relating to settlements with tax authorities |
— | (700 | ) | (233 | ) | |||||||
Reductions as a result of a lapse of the applicable
statute of limitations |
(1,184 | ) | (779 | ) | (64 | ) | ||||||
Balance, end of year |
$ | 2,756 | $ | 3,418 | $ | 5,686 | ||||||
| The tax years that remain open and subject to tax audits varies depending on the tax jurisdiction. The consolidated federal 2008 and 2009 tax years remain open for examination. |
| It is reasonably possible that a reduction in the range of $0.0 million to $1.0 million of the balance of unrecognized tax benefits may occur within the next 12 months as a result of the lapse of the statute of limitations. As of the end of 2010, approximately $1.8 million of unrecognized tax benefits would favorably impact the effective tax rate (net of federal benefit on state issues), if recognized. |
| 11. | COMMITMENTS AND CONTINGENCIES |
| Litigation — The Company is a party to various legal actions arising in the normal course of business. While the Company does not believe, except as indicated below, that the ultimate resolution of any such pending actions will have a material adverse effect on its results of operations, financial position or cash flows, litigation is subject to inherent uncertainties. If an unfavorable ruling were to occur, there exists the possibility of a material adverse impact in the period in which the ruling occurs. |
91
Table of Contents
| As previously reported, in 2002, a former Electrochem customer, Input/Output, Inc., now known as ION Geophysical Corporation (“Input/Output”), commenced an action against the Company. After trial in September 2009, a jury found in favor of Input/Output on fraud, unfair trade practices and breach of contract claims and awarded damages in the amount of $21.7 million. The final judgment in the matter included an award of prejudgment interest bringing the total judgment to approximately $33 million. During 2009, the Company accrued $34.5 million in connection with the Electrochem Litigation. The Company’s post-trial motion for a new trial was denied, and the Company appealed the judgment to the Louisiana Court of Appeal. In December 2010, the Company entered into a settlement agreement with Input/Output. Under terms of this agreement, Input/Output released the Company of any liability in connection with the jury verdict and in return for that release, the Company paid Input/Output $25 million. In the fourth quarter of 2010, the Company recognized a gain for the remaining $9.5 million of the previous accrual. |
| As previously reported, on June 12, 2006, Enpath Medical, Inc. (“Enpath”), a subsidiary of the Company that has since been merged into Greatbatch Ltd., was named as defendant in a patent infringement action filed by Pressure Products Medical Supplies, Inc. (“Pressure Products”). After trial, a jury found that Enpath infringed the Pressure Products patents, but not willfully, and awarded damages in the amount of $1.1 million. The Company appealed the judgment to the U.S. Court of Appeals for the Federal Circuit and countersued Pressure Products for violation of federal and state unfair competition laws. In August 2010, Pressure Products and the Company settled the patent infringement action. In exchange for a cash payment by the Company of $1.5 million, Pressure Products agreed not to sue on the products at issue in that case. The settlement agreement eliminated any restrictions on the Company’s ability to sell its FlowGuard and OptiSeal Introducer products. As part of the settlement, the litigation commenced by the Company against Pressure Products was also dismissed. |
| License agreements — The Company is a party to various license agreements for technology that is utilized in certain of its products. The most significant of these agreements are the licenses for basic technology used in the production of wet tantalum capacitors, filtered feedthroughs and MRI compatible lead systems. Expenses related to license agreements were $2.5 million, $3.3 million and $3.0 million, for 2010, 2009 and 2008, respectively, and are included in Cost of Sales. |
| Product Warranties — The change in product warranty liability was comprised of the following (in thousands): |
| Year Ended | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Beginning balance |
$ | 1,330 | $ | 1,395 | ||||
Additions to warranty reserve |
2,237 | 668 | ||||||
Warranty claims paid |
(1,285 | ) | (733 | ) | ||||
Foreign currency effect |
31 | — | ||||||
Ending balance |
$ | 2,313 | $ | 1,330 | ||||
92
Table of Contents
| Operating Leases — The Company is a party to various operating lease agreements for buildings, equipment and software. The Company incurred operating lease expense of $3.1 million, $3.4 million, and $3.8 million, in 2010, 2009 and 2008, respectively. Minimum future annual operating lease payments are $2.4 million in 2011; $2.2 million in 2012; $2.1 million in 2013; $2.2 million in 2014; $1.8 million in 2015 and $4.7 million thereafter. The Company primarily leases buildings, which accounts for the majority of the future lease payments. Lease expense includes the effect of escalation clauses and leasehold improvement incentives which are accounted for ratably over the lease term. |
| Workers’ Compensation Trust — With respect to its operations in Western New York, the Company is a member of a group self-insurance trust that provides workers’ compensation benefits to eligible employees of the Company and other group member employers. For locations outside of Western New York, the Company utilizes traditional insurance relationships to provide workers’ compensation benefits. Under the terms of the Trust, the Company makes annual contributions to the Trust based on reported salaries paid to the employees using a rate based formula. Based on actual experience, the Company could receive a refund or be assessed additional contributions. For financial statement purposes, no amounts have been recorded for any refund or additional assessment since the Trust has not informed the Company of any such adjustments. Under the trust agreement, each participating organization has joint and several liability for trust obligations if the assets of the trust are not sufficient to cover those obligations. |
| Purchase Commitments — Contractual obligations for the purchase of goods or services are defined as agreements that are enforceable and legally binding on the Company and that specify all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Our purchase orders are normally based on our current capital and manufacturing needs and are fulfilled by our vendors within short time horizons. We enter into blanket orders with vendors that have preferred pricing and terms, however these orders are normally cancelable by us without penalty. As of December 31, 2010, the total contractual obligation related to such expenditures is $18.6 million, the majority of which is expected to be paid in 2011 and will be financed by cash and cash equivalents or financing under the Credit Facility. We also enter into contracts for outsourced services; however, the obligations under these contracts were not significant and the contracts generally contain clauses allowing for cancellation without significant penalty. |
| Foreign Currency Contracts — In December 2007 and January 2008, the Company entered into forward contracts to purchase Swiss francs in order to fund the purchase of Precimed. In January 2008, the Company entered into an additional Euro Dollar forward contract in order to fund the purchase of the Chaumont Facility. The net result of the above contracts, which were settled upon the funding of the respective acquisitions, was a gain of $2.4 million, $1.6 million of which was recorded in 2008 as Other (Income) Loss, Net. |
| In February 2009, the Company entered into forward contracts to purchase 10 million Mexican pesos per month from March 2009 to December 2009 at an exchange rate of 14.85 pesos per one U.S. dollar. These contracts were entered into in order to hedge the risk of peso-denominated payments associated with the operations at the Company’s Tijuana, Mexico facility and were accounted for as cash flow hedges. |
| In December 2009 and February 2010, the Company entered into forward contracts to purchase 6.6 million and 3.3 million, respectively, Mexican pesos per month through December 2010 at an exchange rate of 13.159 pesos and 13.1595 pesos per one U.S. dollar, respectively. These contracts were entered into in order to hedge the risk of peso-denominated payments associated with the operations at the Company’s Tijuana, Mexico facility for 2010 and were accounted for as cash flow hedges. |
| In July 2010, the Company entered into forward contracts to purchase 6.6 million Mexican pesos per month from January 2011 to December 2011 at an exchange rate of 13.2231 pesos per one U.S. dollar. This contract was entered into in order to hedge the risk of peso-denominated payments associated with a portion of the operations at the Company’s Tijuana, Mexico facility for 2011 and are being accounted for as a cash flow hedge. |
93
Table of Contents
| The amount recorded as a reduction of Cost of Sales during 2010 and 2009 related to these forward contracts was $0.5 million and $0.6 million, respectively. As of December 31, 2010, the Company’s outstanding forward contracts had a positive fair value of $0.3 million, which is recorded within Prepaid Expenses and Other Current Assets. No portion of the change in fair value of the Company’s foreign currency contracts during 2010 or 2009 was considered ineffective. |
| Subsequent event (Unaudited) — In February 2011, the Company entered into forward contracts to purchase 3.7 million Mexican pesos per month from March 2011 to December 2011 at an exchange rate of 12.2761 pesos per one U.S. dollar. This contract was entered into in order to hedge the risk of peso-denominated payments associated with a portion of the operations at the Company’s Tijuana, Mexico facility for 2011 and are being accounted for as a cash flow hedge. |
| Self-Insured Medical Plan — In 2010, the Company began self-funding the medical insurance coverage for all of its U.S. based employees. The risk to the Company is being limited through the use of stop loss insurance, which for 2010 had an annual deductible of $0.2 million per covered participant with losses covered up to $4.8 million per covered person. The maximum aggregate loss (i.e. sum of all claims under the $0.2 million deductible) was limited to $9.9 million with a maximum benefit of $1.0 million. For 2011, the annual maximum aggregate loss was raised to $14.2 million and there is no longer a maximum benefit for specific losses per covered person. As of December 31, 2010, the Company has $2.1 million accrued related to the self-insured medical plan, which is recorded within Accrued Expenses. |
| 12. | FAIR VALUE MEASUREMENTS |
| Fair value measurement standards apply to certain financial assets and liabilities that are measured at fair value on a recurring basis (each reporting period). For the Company, these financial assets and liabilities include its derivative instruments. The Company does not have any nonfinancial assets or liabilities that are measured at fair value on a recurring basis. A summary of the valuation methodologies for assets and liabilities measured on a recurring basis is as follows: |
| Foreign currency contracts — The fair value of foreign currency contracts are determined through the use of cash flow models that utilize observable market data inputs to estimate fair value. These observable market data inputs include spot and forward foreign currency exchange rates, interest rates and credit spread curves. In addition to the above, the Company receives fair value estimates from the foreign currency contract counterparty to verify the reasonableness of the Company’s estimates. This fair value calculation was categorized in Level 2 of the fair value hierarchy. |
| Interest rate swap — The fair value of the Company’s interest rate swap is determined through the use of a cash flow model that utilizes observable market data inputs. These observable market data inputs include LIBOR, swap rates, and credit spread curves. In addition to the above, the Company receives a fair value estimate from the interest rate swap counterparty to verify the reasonableness of the Company’s estimate. This fair value calculation was categorized in Level 2 of the fair value hierarchy. |
94
Table of Contents
| The following tables provide information regarding assets and liabilities recorded at fair value on a recurring basis (in thousands): |
| Quoted | Significant | |||||||||||||||
| prices in | other | Significant | ||||||||||||||
| At | active | observable | unobservable | |||||||||||||
| December 31, | markets | inputs | inputs | |||||||||||||
| Description | 2010 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Assets |
||||||||||||||||
Foreign currency contracts (Note 11) |
$ | 315 | $ | — | $ | 315 | $ | — | ||||||||
Liabilities |
||||||||||||||||
Interest rate swap (Note 6) |
436 | — | 436 | — | ||||||||||||
| Quoted | Significant | |||||||||||||||
| prices in | other | Significant | ||||||||||||||
| At | active | observable | unobservable | |||||||||||||
| January 1, | markets | inputs | inputs | |||||||||||||
| Description | 2010 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Liabilities |
||||||||||||||||
Foreign currency contracts |
$ | 89 | $ | — | $ | 89 | $ | — | ||||||||
Interest rate swaps |
1,612 | — | 1,612 | — | ||||||||||||
| Fair value standards also apply to certain nonfinancial assets and liabilities that are measured at fair value on a nonrecurring basis. For example, certain long-lived assets such as goodwill, intangible assets and property, plant and equipment are measured at fair value in connection with business combinations or when an impairment is recognized and the related assets are written down to fair value. A summary of the valuation methodologies for assets and liabilities measured on a nonrecurring basis is as follows: |
| Property, plant and equipment, net — During 2010, one Greatbatch Medical facility, which was previously classified as an asset held for sale, was reclassified to Property, Plant and Equipment, Net as management decided to utilize this facility for future operations. This building was recorded at fair value at the date of reclassification and is now being amortized on a straight-line basis over its remaining estimated useful life. The fair value was determined by reference to recent sales data for comparable facilities. This fair value calculation was categorized in Level 2 of the fair value hierarchy. |
| Cost method investment — The Company holds investments in equity securities that are accounted for as cost method investments, which are classified as Other Long-Term Assets, and are measured at fair value only if certain events or circumstances occur that have a significant adverse effect on the fair value of the investment. During 2010, one cost method investment was written down by $0.2 million as it was determined that its book value exceeded its fair value. The fair value of this investment was determined by reference to recent sales data of similar shares to independent parties in an inactive market. This fair value calculation was categorized in Level 2 of the fair value hierarchy. |
| Asset held for sale — Assets held for sale are recorded at the lower of their carrying amount or estimated fair value less cost to sell. For the properties written-down in 2009, the fair value was determined by reference to recent sales data for comparable facilities taking into consideration recent offers, if any, received from prospective buyers of the facility. This fair value calculation was categorized in Level 2 of the fair value hierarchy. |
95
Table of Contents
| The following tables provide information regarding assets and liabilities recorded at fair value on a nonrecurring basis (in thousands): |
| Quoted | Significant | |||||||||||||||
| prices in | other | Significant | ||||||||||||||
| At | active | observable | unobservable | |||||||||||||
| December 31, | markets | inputs | inputs | |||||||||||||
| Description | 2010 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Assets |
||||||||||||||||
Property, plant and equipment, net (Note 9) |
$ | 1,908 | $ | — | $ | 1,908 | $ | — | ||||||||
Cost method investment |
317 | — | 317 | — | ||||||||||||
| Quoted | Significant | |||||||||||||||
| prices in | other | Significant | ||||||||||||||
| At | active | observable | unobservable | |||||||||||||
| January 1, | markets | inputs | inputs | |||||||||||||
| Description | 2010 | (Level 1) | (Level 2) | (Level 3) | ||||||||||||
Assets |
||||||||||||||||
Assets held for sale (Note 9) |
$ | 3,207 | $ | — | $ | 3,207 | $ | — | ||||||||
| Convertible subordinated notes — The fair value of the Company’s convertible subordinated notes disclosed in Note 6 “Debt” were determined by reference to recent third-party transactions for the Company’s notes in an inactive market. The Company’s convertible subordinated notes are categorized in Level 2 of the fair value hierarchy. |
| Pension plan assets — The fair value of the Company’s pension plan assets disclosed in Note 7 “Employee Benefit Plans” are determined based upon quoted market prices in active markets, quoted market prices in inactive markets or multidimensional relational models with observable market data inputs to estimate fair value. These observable market data inputs include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, benchmark securities, bids, offers and reference data. The Company’s pension plan assets are categorized in Level 1 or Level 2 of the fair value hierarchy. |
| 13. | STOCKHOLDER RIGHTS PLAN |
| On March 1, 2002, the Company’s Board of Directors adopted a stockholder rights plan and declared a dividend distribution of one preferred stock purchase right for each outstanding share of common stock. The dividend was paid to stockholders as of April 30, 2002. Each right, once exercisable, entitles the registered holder to purchase from the Company one one-hundredth of a share of preferred stock of the Company. |
| Under the rights plan, the rights initially trade together with the common stock and are not exercisable. In the absence of further action by the Board of Directors, the rights will become exercisable if a person or group acquires 15 percent or more of the outstanding shares of common stock or a person or group announces its intent to commence a tender or exchange offer without the prior approval of the Board of Directors. |
| The rights plan includes an exchange option. In general, after the rights become exercisable, the Board of Directors may, at its option, affect an exchange of part or all of the rights at a ratio of one share of Common Stock for each right, subject to adjustment in certain circumstances. The rights are also redeemable at any time prior to the time they become exercisable for $0.001 per right, subject to adjustment in certain circumstances. |
96
Table of Contents
| Unless earlier amended, redeemed or exchanged, the rights will expire on March 18, 2012. The issuance of the rights was not a taxable event, does not affect our reported financial condition or results of operations, including our EPS, and does not change the manner in which our common stock is traded. |
| 14. | BUSINESS SEGMENT INFORMATION |
| The Company operates its business in two reportable segments — Greatbatch Medical and Electrochem. The Greatbatch Medical segment designs and manufactures systems, components and devices for the CRM, Neuromodulation, Vascular Access and Orthopaedic markets. The Company’s products include: 1) batteries, capacitors, filtered and unfiltered feedthroughs, engineered components and enclosures used in Implantable Medical Devices; 2) instruments and delivery systems used in hip and knee replacement, trauma and spine surgeries as well as hip, knee and shoulder implants; and 3) introducers, catheters, steerable sheaths and implantable stimulation leads. Additionally, Greatbatch Medical offers value-added assembly and design engineering services for medical systems and devices within the markets in which it operates. |
| Electrochem is a leader in technology solutions for critical industrial applications, including customized battery power and wireless sensing systems for demanding applications in markets such as energy, security, portable medical, environmental monitoring and more. |
| The Company defines segment income (loss) from operations as sales less cost of sales and expenses attributable to segment-specific selling, general and administrative, research, development and engineering expenses, and other operating expenses. Segment income (loss) also includes a portion of non-segment specific selling, general and administrative expenses based on allocations appropriate to the expense categories. The remaining unallocated operating expenses are primarily corporate headquarters and administrative function expenses. Transactions between the two segments are not significant. Segment assets include accounts receivable, inventories, net property, plant and equipment, amortizing intangible assets and goodwill. Corporate assets consist primarily of cash and cash equivalents, non-segment specific deferred income taxes, and net property, plant and equipment. The accounting policies of the segments are the same as those described and referenced in Note 1 “Summary of Significant Accounting Policies.” Sales by geographic area are presented by attributing sales from external customers based on where the products are shipped. |
| The Greatbatch Medical segment results for 2009 include a $15.9 million intangible asset write-down (See Note 4). The Electrochem operating results include a $9.5 million gain and a $34.5 million charge related to the Electrochem Litigation (See Note 11) in 2010 and 2009, respectively. The 2008 Greatbatch Medical segment results include $6.2 million and $2.2 million of inventory step-up amortization and IPR&D expense, respectively, related to the acquisitions in 2007 and 2008. Electrochem segment results for 2008 include $0.2 million of inventory step-up amortization related to an acquisition in 2007. |
97
Table of Contents
| An analysis and reconciliation of the Company’s business segment and product line information to the respective information in the consolidated financial statements is presented below (in thousands): |
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Sales: |
||||||||||||
Greatbatch Medical |
||||||||||||
CRM/Neuromodulation |
$ | 303,521 | $ | 305,354 | $ | 286,251 | ||||||
Vascular Access |
38,000 | 35,816 | 39,443 | |||||||||
Orthopaedic |
118,748 | 113,897 | 142,446 | |||||||||
Total Greatbatch
Medical |
460,269 | 455,067 | 468,140 | |||||||||
Electrochem |
73,156 | 66,754 | 78,504 | |||||||||
Total sales |
$ | 533,425 | $ | 521,821 | $ | 546,644 | ||||||
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Segment income (loss) from operations: |
||||||||||||
Greatbatch Medical |
$ | 62,477 | $ | 46,270 | $ | 49,760 | ||||||
Electrochem |
22,195 | (32,734 | ) | 9,499 | ||||||||
Total segment income from operations |
84,672 | 13,536 | 59,259 | |||||||||
Unallocated operating expenses |
(15,678 | ) | (12,488 | ) | (24,365 | ) | ||||||
Operating income as reported |
68,994 | 1,048 | 34,894 | |||||||||
Unallocated other expense |
(19,669 | ) | (19,225 | ) | (14,377 | ) | ||||||
Income (loss) before provision
(benefit) for
income taxes as reported |
$ | 49,325 | $ | (18,177 | ) | $ | 20,517 | |||||
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Depreciation and amortization: |
||||||||||||
Greatbatch Medical |
$ | 28,117 | $ | 29,869 | $ | 36,987 | ||||||
Electrochem |
2,660 | 2,860 | 2,748 | |||||||||
Total depreciation and amortization
included
in segment income from operations |
30,777 | 32,729 | 39,735 | |||||||||
Unallocated depreciation and amortization |
15,670 | 14,500 | 12,433 | |||||||||
Total depreciation and amortization |
$ | 46,447 | $ | 47,229 | $ | 52,168 | ||||||
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Expenditures for tangible long-lived
assets, excluding acquisitions: |
||||||||||||
Greatbatch Medical |
$ | 15,088 | $ | 11,261 | $ | 11,414 | ||||||
Electrochem |
763 | 910 | 19,602 | |||||||||
Total reportable segments |
15,851 | 12,171 | 31,016 | |||||||||
Unallocated long-lived tangible assets |
1,120 | 7,040 | 16,562 | |||||||||
Total expenditures |
$ | 16,971 | $ | 19,211 | $ | 47,578 | ||||||
98
Table of Contents
| As of | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Identifiable assets, net: |
||||||||
Greatbatch Medical |
$ | 641,591 | $ | 663,539 | ||||
Electrochem |
71,480 | 79,157 | ||||||
Total reportable segments |
713,071 | 742,696 | ||||||
Unallocated assets |
63,905 | 87,847 | ||||||
Total assets |
$ | 776,976 | $ | 830,543 | ||||
| Year Ended | ||||||||||||
| December 31, | January 1, | January 2, | ||||||||||
| 2010 | 2010 | 2009 | ||||||||||
Sales by geographic area: |
||||||||||||
United States |
$ | 243,827 | $ | 245,974 | $ | 266,985 | ||||||
Non-domestic countries: |
||||||||||||
Puerto Rico |
88,369 | 76,823 | 56,941 | |||||||||
Belgium |
58,014 | 29,431 | — | |||||||||
United Kingdom &
Ireland |
56,903 | 66,255 | 75,917 | |||||||||
France |
6,318 | 37,373 | 74,670 | |||||||||
All other |
79,994 | 65,965 | 72,131 | |||||||||
Consolidated sales |
$ | 533,425 | $ | 521,821 | $ | 546,644 | ||||||
| As of | ||||||||
| December 31, | January 1, | |||||||
| 2010 | 2010 | |||||||
Long-lived tangible assets: |
||||||||
United States |
$ | 126,519 | $ | 132,605 | ||||
Foreign countries |
36,095 | 38,478 | ||||||
Consolidated long-lived assets |
$ | 162,614 | $ | 171,083 | ||||
| A significant portion of the Company’s sales and accounts receivable were to four customers as follows: |
| Sales | Accounts Receivable | |||||||||||||||||||
| Year Ended | As of | |||||||||||||||||||
| December 31, | January 1, | January 2, | December 31, | January 1, | ||||||||||||||||
| 2010 | 2010 | 2009 | 2010 | 2010 | ||||||||||||||||
Customer A |
21 | % | 22 | % | 17 | % | 15 | % | 13 | % | ||||||||||
Customer B |
19 | % | 17 | % | 14 | % | 13 | % | 19 | % | ||||||||||
Customer C |
12 | % | 12 | % | 12 | % | 8 | % | 5 | % | ||||||||||
Customer D |
10 | % | 12 | % | 13 | % | 14 | % | 11 | % | ||||||||||
Total |
62 | % | 63 | % | 56 | % | 50 | % | 48 | % | ||||||||||
99
Table of Contents
| 15. | QUARTERLY SALES AND EARNINGS DATA — UNAUDITED |
| 4th Qtr. | 3rd Qtr. | 2nd Qtr. | 1st Qtr. | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
2010 |
||||||||||||||||
Sales |
$ | 133,111 | $ | 127,490 | $ | 140,795 | $ | 132,029 | ||||||||
Gross profit |
44,464 | 41,994 | 45,459 | 41,664 | ||||||||||||
Net income (1) |
13,839 | 5,964 | 7,788 | 5,547 | ||||||||||||
EPS — basic |
0.60 | 0.26 | 0.34 | 0.24 | ||||||||||||
EPS — diluted |
0.59 | 0.25 | 0.33 | 0.24 | ||||||||||||
2009 |
||||||||||||||||
Sales |
$ | 125,808 | $ | 121,470 | $ | 134,725 | $ | 139,818 | ||||||||
Gross profit |
41,646 | 39,137 | 41,472 | 44,164 | ||||||||||||
Net income (loss)
(1)(2) |
(1,534 | ) | (20,693 | ) | 6,562 | 6,664 | ||||||||||
EPS — basic |
(0.07 | ) | (0.90 | ) | 0.29 | 0.29 | ||||||||||
EPS — diluted |
(0.07 | ) | (0.90 | ) | 0.28 | 0.28 | ||||||||||
| (1) | Net gain (loss) in the 2010 fourth quarter and 2009 third quarter, respectively, includes the impact of the Electrochem Litigation. See Note 11 “Commitments and Contingencies.” | |
| (2) | Net loss in the 2009 fourth quarter includes the write-down of intangible assets. See Note 4 “Intangible Assets.” |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
| ITEM 9A. | CONTROLS AND PROCEDURES |
| Management’s Report on Internal Control Over Financial Reporting is incorporated by reference into this Item 9A from the report appearing at Part II, Item 8, “Financial Statements and Supplementary Data.” |
| a. | Evaluation of Disclosure Controls and Procedures — Our management, including the principal executive officer and principal financial officer, evaluated our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) related to the recording, processing, summarization and reporting of information in our reports that we file with the SEC. |
| Our disclosure controls and procedures have been designed to provide reasonable assurance that material information relating to us, including our subsidiaries, is made known to our management, including these officers, by our employees, and that this information is recorded, processed, summarized, evaluated and reported, as applicable, within the time periods specified in the SEC’s rules and forms. Based on their evaluation, as of December 31, 2010, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures are effective. |
| b. | Changes in Internal Control Over Financial Reporting — There have been no changes in our internal control over financial reporting that occurred during our last fiscal quarter to which this Annual Report on Form 10-K relates that have materially affected, or are reasonably likely to materially affect, internal control over financial reporting. |
| ITEM 9B. | OTHER INFORMATION |
100
Table of Contents
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
| The identification of each of the Registrant’s directors is incorporated by reference to the caption “Election of Directors” contained in the Company’s definitive Proxy Statement for its 2011 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission on or about April 14, 2011. |
| The identification of the Company’s executive officers is presented under the caption “Executive Officers of the Company” contained in Part I of this Annual Report on Form 10-K. |
| The other information required by Item 10 is incorporated by reference to the Company’s definitive Proxy Statement for its 2011 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission on or about April 14, 2011. |
| ITEM 11. | EXECUTIVE COMPENSATION |
| Information regarding executive compensation in the Proxy Statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference. |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
| Information regarding security ownership of certain beneficial owners in the Proxy Statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference. |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
| Information regarding certain relationships and related transactions, and director independence in the Proxy Statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference. |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
| Information regarding the fees paid to and services provided by Deloitte & Touche LLP, the Company’s independent registered public accounting firm, in the Proxy Statement for the 2011 Annual Meeting of Stockholders is incorporated herein by reference. |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (a) | LIST OF DOCUMENTS FILED AS PART OF THIS REPORT |
| (1) | Financial statements and financial statement schedules filed as part of this Annual Report on Form 10-K. See Part II, Item 8. “Financial Statements and Supplementary Data.” |
| (2) | The following financial statement schedule is included in this report on Form 10-K (in thousands): |
101
Table of Contents
| Col. B | Additions | Col. E | ||||||||||||||||||
| Balance at | Charged to | Col. D | Balance at | |||||||||||||||||
| Col. A | Beginning | Charged to | Other Accounts- | Deductions - | End of | |||||||||||||||
| Description | of Period | Costs & Expenses | Describe | Describe | Period | |||||||||||||||
December 31, 2010 |
||||||||||||||||||||
Allowance for
doubtful accounts |
$ | 2,452 | $ | (64 | ) | $ | 35 | (4) | $ | (593 | ) (2) | $ | 1,830 | |||||||
Valuation allowance
for deferred income
tax assets |
$ | 5,656 | $ | 761 | (1) | $ | 65 | (4) | $ | — | $ | 6,482 | ||||||||
January 1, 2010 |
||||||||||||||||||||
Allowance for
doubtful accounts |
$ | 1,603 | $ | 961 | $ | — | $ | (112 | )(2) | $ | 2,452 | |||||||||
Valuation allowance
for deferred income
tax assets |
$ | 4,485 | $ | 1,171 | (1) | $ | — | $ | — | $ | 5,656 | |||||||||
January 2, 2009 |
||||||||||||||||||||
Allowance for
doubtful accounts |
$ | 758 | $ | 590 | $ | 374 | (3) | $ | (119 | ) (2) | $ | 1,603 | ||||||||
Valuation allowance
for deferred income
tax assets |
$ | 3,969 | $ | — | $ | 580 | (3) | $ | (64 | ) (1) | $ | 4,485 | ||||||||
| (1) | Valuation allowance recorded in the provision for income taxes for certain net operating losses and tax credits. | |
| (2) | Accounts written off, net of collections on accounts receivable previously written off. | |
| (3) | Balances recorded as a part of our 2008 acquisition of P Medical Holding SA. | |
| (4) | Foreign currency translation effect. |
| Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto. |
| (3) | Exhibits required by Item 601 of Regulation S-K. The exhibits listed on the Exhibit Index of this Annual Report on Form 10-K have been previously filed, are filed herewith or are incorporated herein by reference to other filings. |
| Dated: March 1, 2011 | By | /s/ Thomas J. Hook | ||
| Thomas J. Hook (Principal Executive Officer) | ||||
| President & Chief Executive Officer | ||||
102
Table of Contents
| Signature | Title | Date | ||
/s/ Thomas J. Hook
|
President & Chief Executive Officer & Director |
March 1, 2011 | ||
/s/ Thomas J. Mazza
|
Senior Vice President & Chief Financial Officer (Principal Financial Officer) |
March 1, 2011 | ||
/s/ Marco F. Benedetti
|
Corporate Controller (Principal Accounting Officer) |
March 1, 2011 | ||
/s/ Bill R. Sanford
|
Chairman | March 1, 2011 | ||
/s/ Pamela G. Bailey
|
Director | March 1, 2011 | ||
/s/ Michael Dinkins
|
Director | March 1, 2011 | ||
/s/ Kevin C. Melia
|
Director | March 1, 2011 | ||
/s/ Dr. Joseph A. Miller, Jr.
|
Director | March 1, 2011 | ||
/s/ Peter H. Soderberg
|
Director | March 1, 2011 | ||
/s/ William B. Summers, Jr.
|
Director | March 1, 2011 | ||
/s/ Dr. Helena S. Wisniewski
|
Director | March 1, 2011 |
103
Table of Contents
| EXHIBIT | ||||
| NUMBER | DESCRIPTION | |||
| 3.1 | Amended and Restated Certificate of Incorporation, as amended
(incorporated by reference to Exhibit 3.1 to our quarterly
report on Form 10-Q for the period ended June 27, 2008). |
|||
| 3.2 | Amended and Restated Bylaws (incorporated by reference to
Exhibit 3.2 to our annual report on Form 10-K for the period
ended January 1, 2010). |
|||
| 4.1 | Indenture for 21/4% Convertible Subordinated Debentures Due
2013 dated as of March 28, 2007 (incorporated by reference to
Exhibit 10.3 to our Current Report on Form 8-K filed on March
29, 2007). |
|||
| 4.2 | First Supplemental Indenture dated April 2, 2007
(incorporated by reference to Exhibit 10.3 to our Current
Report on Form 8-K filed on April 4, 2007). |
|||
| 4.3 | Registration Rights Agreement dated as of March 28, 2007 by
and among us and the initial purchasers of the Debentures
described above (incorporated by reference to Exhibit 10.2 to
our Current Report on Form 8-K filed on March 29, 2007). |
|||
| 10.1 | # | 1997 Stock Option Plan (including form of “standard” option
agreement and form of “special” option agreement)
(incorporated by reference to Exhibit 10.1 to our
Registration Statement on Form S-1 (File No. 333-37554)). |
||
| 10.2 | # | 1998 Stock Option Plan (including form of “standard” option
agreement, form of “special” option agreement and form of
“non-standard” option agreement) (incorporated by reference
to Exhibit 10.2 to our Registration Statement on Form S-1
(File No. 333-37554)). |
||
| 10.3 | # | Greatbatch Ltd. Equity Plus Plan Money Purchase Plan
(incorporated by reference to Exhibit 10.3 to our
Registration Statement on Form S-1 (File No. 333-37554)). |
||
| 10.4 | # | Greatbatch Ltd. Equity Plus Plan Stock Bonus Plan
(incorporated by reference to Exhibit 10.4 to our
Registration Statement on Form S-1 (File No. 333-37554)). |
||
| 10.5 | # | Non-Employee Director Stock Incentive Plan (incorporated by
reference to Exhibit A to our Definitive Proxy Statement on
Schedule 14-A filed on April 22, 2002). |
||
| 10.6 | # | Greatbatch, Inc. Executive Short Term Incentive Compensation
Plan (incorporated by reference to Exhibit B to our
Definitive Proxy Statement on Schedule 14A filed on April 20,
2007). |
||
| 10.7 | Credit Agreement dated as of May 22, 2007 by and among
Greatbatch Ltd., the lenders party thereto and Manufacturers
and Traders Trust Company, as administrative agent
(incorporated by reference to Exhibit 10.1 of our Current
Report on Form 8-K filed on May 25, 2007). |
|||
| 10.8 | Amendment No. 1 to Credit Agreement dated as of December 20,
2007 by and among Greatbatch Ltd., the lenders party thereto
and Manufacturers and Traders Trust Company, as
administrative agent. (incorporated by reference to Exhibit
10.8 to our Annual Report on Form 10-K for the year ended
January 1, 2010) |
|||
| 10.9 | Amendment No. 2 to Credit Agreement dated as of November 4,
2008 by and among Greatbatch Ltd., the lenders party thereto
and Manufacturers and Traders Trust Company, as
administrative agent. (incorporated by reference to Exhibit
10.9 to our Annual Report on Form 10-K for the year ended
January 1, 2010) |
|||
| 10.10 | Amendment No. 3 to Credit Agreement dated as of March 31,
2009 by and among Greatbatch Ltd., the lenders party thereto
and Manufacturers and Traders Trust Company, as
administrative agent (incorporated by reference to Exhibit
10.10 to our Annual Report on Form 10-K for the year ended
January 1, 2010). |
|||
104
Table of Contents
| EXHIBIT | ||||
| NUMBER | ||||
| 10.11 | Amendment No. 4 to Credit Agreement dated as of October 30,
2009 by and among Greatbatch Ltd., the lenders party thereto
and Manufacturers and Traders Trust Company, as
administrative agent (incorporated by reference to Exhibit
10.11 to our Annual Report on Form 10-K for the year ended
January 1, 2010). |
|||
| 10.12 | # | 2002 Restricted Stock Plan (incorporated by reference to
Appendix B to our Definitive Proxy Statement on Schedule 14A
filed on April 9, 2003). |
||
| 10.13 | License Agreement dated August 8, 1996, between Greatbatch
Ltd. and Evans Capacitor Company (incorporated by reference
to Exhibit 10.23 to our Registration Statement on Form S-1
(File No. 333-37554)). |
|||
| 10.14 | + | Amendment No. 2 dated December 6, 2002, between Greatbatch
Technologies, Ltd. and Evans Capacitor Company (incorporated
by reference to Exhibit 10.18 to our Annual Report on Form
10-K for the year ended January 3, 2003). |
||
| 10.15 | # | Form of Change of Control Agreement, dated August 14, 2006,
between Greatbatch, Inc. and our executive officers (Thomas
J. Hook, Thomas J. Mazza, Mauricio Arellano, Susan M.
Bratton, Michelle Graham and Timothy G. McEvoy). |
||
| 10.16 | # | Employment Agreement dated April 10, 2010 between Greatbatch,
Inc. and Thomas J. Hook (incorporated by reference to Exhibit
10.1 to our Current Report on Form 8-K filed on April 13,
2010). |
||
| 10.17 | # | 2005 Stock Incentive Plan (incorporated by reference to
Exhibit B to our Definitive Proxy Statement on Schedule 14A
filed on April 20, 2007). |
||
| 10.18 | # | 2009 Stock Incentive Plan (incorporated by reference to
Exhibit A to our Definitive Proxy Statement on Schedule 14A
filed on April 13, 2009). |
||
| 10.19 | # | Form of Restricted Stock Award Letter (incorporated by
reference to Exhibit 10.22 to our Annual Report on Form 10-K
for the year ended December 30, 2005). |
||
| 10.20 | # | Form of Incentive Stock Option Award Letter (incorporated by
reference to Exhibit 10.23 to our Annual Report on Form 10-K
for the year ended December 30, 2005). |
||
| 10.21 | # | Form of Nonqualified Option Award Letter (incorporated by
reference to Exhibit 10.24 to our Annual Report on Form 10-K
for the year ended December 30, 2005). |
||
| 10.22 | # | Form of Stock Option Award Letter (incorporated by reference
to Exhibit 10.25 to our Annual Report on Form 10-K for the year ended December 30, 2005). |
||
| 10.23 | + | Supply Agreement for medical device components dated March
31, 2006, between Greatbatch, Inc. and SORIN/ELA BIOMEDICA
CRM and ELA MEDICAL SAS (incorporated by reference to Exhibit
10.1 to our Quarterly Report on Form 10-Q for the quarterly
period ended March 31, 2006). |
||
| 10.24 | Form of Exchange and Purchase Agreement dated March 22, 2007,
by and between Greatbatch, Inc. and certain other parties
thereto related to its outstanding 2 1/4% Convertible
Subordinated Debentures due 2013. (Incorporated by reference
to Exhibit 10.1 to our Current Report on Form 8-K filed on
March 29, 2007). |
|||
105
Table of Contents
| EXHIBIT | ||||
| NUMBER | DESCRIPTION | |||
| 12.1 | * | Ratio of Earnings to Fixed Charges (Unaudited) |
||
| 21.1 | * | Subsidiaries of Greatbatch, Inc. |
||
| 23.1 | * | Consent of Independent Registered Public Accounting Firm |
||
| 31.1 | * | Certification of Chief Executive Officer pursuant to Rule
13a-14(a) of the Securities Exchange Act. |
||
| 31.2 | * | Certification of Chief Financial Officer pursuant to Rule
13a-14(a) of the Securities Exchange Act. |
||
| 32.1 | ** | Certification of Chief Executive Officer and Chief Financial
Officer pursuant to 18 U.S.C. Section 1350 as adopted
pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||
| * | - Filed herewith. | |
| ** | - Furnished herewith. | |
| # | - Indicates exhibits that are management contracts or compensation plans or arrangements required to be filed pursuant to Item 14(c) of Form 10-K. |
106
