Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pernix Sleep, Inc. | c12414e8vk.htm |
| EX-99.2 - EXHIBIT 99.2 - Pernix Sleep, Inc. | c12414exv99w2.htm |
| EX-10.1 - EXHIBIT 10.1 - Pernix Sleep, Inc. | c12414exv10w1.htm |
Exhibit 99.1

| 13th Annual BIO CEO & Investor Conference Richard W. PascoePresident and CEOFebruary 14, 2011 (r) |

| Forward-Looking Statements This presentation contains forward-looking statements about our business, including our commercialization plans and outlook, future financial results and events that have not yet occurred. Operating in the pharmaceutical industry inherently involves significant risks and uncertainties, including the risks outlined under "Risk Factors" and elsewhere in our Annual Report on Form 10-K and our other filings made with the SEC from time to time. Our actual results may differ materially from our expectations due to these risks and uncertainties, including our near-term dependence on the success of our lead product candidate, Silenor(r), and factors relating to ability to raise sufficient capital, competition, intellectual property protection, industry environment, and other matters. Somaxon undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. 2 |

| Building Shareholder Value by Executing our Corporate Objectives 3 Our goal is to become a leading specialty pharmaceutical company dedicated to creating value for our shareholders through maximizing the potential of our marketed product Silenor(r) and commercializing promising products that treat medical conditions where there is an unmet medical need or high level of patient dissatisfaction. NOTE: Silenor was approved by the FDA for the treatment of insomnia characterized by difficulty with sleep maintenance in March 2010. (r) |

| Somaxon Pharmaceuticals Today Focused specialty pharmaceutical companyBranded prescription productsEstablished commercial organizationFlexible and scalableSilenor: Highly differentiated product with meaningful revenue potentialFirst and only non-scheduled Rx sleep maintenance insomnia treatmentDemonstrated Rx growth since launch in September 2010Favorable initial managed care coverageMultiple Orange Book listed patentsOTC life-cycle management opportunityLaunched Silenor with experienced partner P&GFocused on primary care physicians and pharmaciesDeal structure preserves asset value and company autonomyWell capitalized to fund revenue growthAbility to further invest in Silenor and potential commercial pipeline 4 |

| Recent Somaxon Developments Expansion to 145 sales reps35 additional sales reps deployed in high-value territoriesCombined with P&G = 250 sales repsObtained access to capital at low-rates with $15mm Comerica A/R line of credit3.3% cost of capital (LIBOR + 3.0%)Enhanced senior management teamHired Rob Cutler, VP of Business Development (previously at Biogen-Idec)Focused on leveraging Somaxon's commercial organization 5 |

| Silenor: First-Line Therapy for Sleep Maintenance Insomnia First and only FDA-approved product for sleep maintenance insomnia that is not associated with risk of abuse or physical dependenceMarketed in U.S. since September 20, 2010Differentiated insomnia productPotent H1 antagonist1,2Promotes sleep into hours 7 and 83No next-day residual effectsNot a controlled substance 6 1. Silenor (r) (doxepin) tablets Prescribing Information. San Diego, CA: Somaxon Pharmaceuticals, Inc.; revised March 2010. 2. Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. 3. Data on file. San Diego, CA: Somaxon Pharmaceuticals, Inc. |

| Insomnia is a Large and Accessible Market* Large and growing market$2bn in U.S. sales (2010)70mm U.S. TRx's (2010)Concentrated market<50,000 doctors write half the TRx's30% of highest prescribing doctors are specialistsTop-decile prescribers average >1,000 Rx's/yearSolid "window of opportunity" for SilenorLack of new and differentiated insomnia treatmentsNo near-term "novel" compounds on the horizonDecreased competitive promotional expendituresPrimary details have decreased DTC spending down dramatically 7 (CHART) * Source: IMS Health data. Insomnia Remains Under-diagnosed and Under-treated (70mm Insomniacs) |

| Primary Issue: Sleep Maintenance* * Source: National Sleep Foundation. Summary of findings: 2008 Sleep in America poll. n = 1760 respondents. Maintenance Onset Difficulty Falling Asleep Frequent Nocturnal Awakenings Early Morning Awakenings Difficulty Falling Asleep 29% 42% 26% 8 Sleep in America poll: >60% of respondents reported sleep problemsSilenor addresses the two most common complaints |

| Silenor: Making a Difference - A Patient's Perspective 9 Peg N. (age 50) - New Hampshire |

| Silenor: Demonstrated Immediate and Sustained Effect on Sleep Maintenance 10 (CHART) Wake After Sleep Onset* (WASO) Placebo * * * * * Data on file. San Diego, CA: Somaxon Pharmaceuticals, Inc. * p-value <0.05 Silenor 3mg Silenor 6mg |

| Silenor: Improved Sleep Efficiency in Adults and the Elderly* Adults: Sleep Efficiency - Night 1(35 Night Study) * Krystal A et al. Sleep. 2010;33(11):1533-1561. Data on file. San Diego, CA: Somaxon Pharmaceuticals, Inc. Elderly: Sleep Efficiency - Night 1 (85 Night Study) 11 11 * ** ** ** ** ** * ** * * * * * * * p-value <0.05 * p-value <0.05 Placebo Silenor 3mg Silenor 6mg |

| Silenor: Adverse Events in Controlled Studies 12 Adverse Event* Placebo (N=278) Silenor 3mg (N=157) Silenor 6mg (N=203) Nervous System Disorders Somnolence/Sedation 4% 6% 9% Infections and Infestations Upper Respiratory Tract Infection/Nasopharyngitis 2 4 2 Gastroenteritis 0 2 0 Gastrointestinal Disorders Nausea 1 2 2 Vascular Disorders Hypertension 0 3 <1 * Includes reactions that occurred at a rate of ? 2% in any Silenor-treated group and at a higher rate than placebo. Source: Silenor Prescribing Information. Low-rate of discontinuation seen in clinical trials for both 3mg (1%) and 6mg (0.7%) as compared to placebo (0.6%) |

| Silenor Commercial Strategy Target high-value physicians through experienced sales forceEstablish strong market share within targeted physiciansNon-personal promotion to physiciansIncrease frequency with targeted physiciansBroaden reach to non-targeted physiciansPartnered with leading professional resources (e.g. Medscape and Epocrates)Direct to insomniac campaignComprehensive digital promotion (e.g. websites and WebMD)Ensure broad patient accessTarget plans that drive insomnia Rx'sSeeking preferred status over other branded therapiesTarget Medicare Part D plans given Silenor's favorable clinical profile in elderly populationMinimize patient out-of-pocket costs 13 |

| Highly Leverageable Commercial Organization* Combined 250 sales reps Target 40,000 high-valued physicians>60,000 details to physicians and >20,000 calls to pharmacies*Somaxon's 145 sales reps to call on ~23,000 high-value physiciansSpecialty sales force targeting psychiatrists, neurologists, and sleep, pain and addiction specialists**P&G's 105 sales reps call on 17,000 PCPs and 25,000 pharmacies 14 * During the 4Q 2010. Source: IMS Health data.** PCPs that are high-prescribers of insomnia therapies are considered specialists and high-value physicians. 23,000 HVPs 17,000 PCP's Responsible for 42% of U.S. Insomnia TRx's |

| Productive Non-Personal Marketing to Physicians Cost efficient non-personal marketing has generated 1.8mm HCP impressions* 15 Targeted online advertising 1.4mm HCP impressions WebMD (Medscape) editorial sponsorship and online advertising 200,000 Psych and PCP impressions Epocrates "DocAlert" program 140,000 HCP reviewed "DocAlert" email Tele-detail and sample fulfillment 9,000 high-decile targets E-detail and E-sampling 2,000 high-decile targets * Since September 2010 launch. |

| Direct to Insomniac Campaign Multi-faceted outreach strategy has created nearly 35mm consumer impressions*Silenor.com website - heavy unique visitors trafficWebMD consumer sponsorship and online advertising Targeted consumer advertisingPay-per-click advertisingBanner advertisementsFacebook advertisementsCampaign outperforming industry standards in click-through and conversion rates 16 * Since September 2010 launch. |

| Current Silenor Patient Coverage Distribution* Currently ~200mm commercial lives have access to SilenorActive contract negotiations with national PBMs and Health Plans designed to improve formulary position of SilenorCMS 2011 - eligible for coverageNegotiating with Medicare Part D plansDepartment of Defense - Tier 2 status on TRICARELow monthly out-of-pocket expensesSleep-SaverTM reduces patient out-of-pocket costs to less than $1 per day1Extended through 2011 17 (CHART) Silenor Payor Coverage (% of TRx's) * Source: IMS Health data.1. Based on co-pays of less than $55 per Rx. |

| Current Silenor Prescriber Landscape Specialists drive 35% of Silenor TRx's1Consistently growing the number of Silenor prescribing physicians; doubled month-over-month2Favorable initial uptake in Silenor prescribers2Initial 4% market share2x Rozerem's market share 18 1. Source: IMS Health data. Since September 2010 launch.2. September through November 2010. Source: IMS Health data. (CHART) Silenor Prescriber Mix (% of TRx's) Other 30% PCPs35% Specialists35% |

| Silenor: Patent Coverage 19 Orange Book List Patents Orange Book List Patents Orange Book List Patents Orange Book List Patents Patent Number Issue Date Expiration Date 1. Method of use (transient) 6,211,229 04/03/01 02/17/20 2. Method of use (chronic) 5,502,047 03/26/96 03/22/13 3. Formulation 5,585,115 12/17/96 01/09/15 4. Formulation 5,725,884 03/10/98 01/09/15 5. Formulation 5,866,166 02/02/99 01/09/15 6. Formulation 5,948,438 09/07/99 01/09/15 7. Formulation 6,217,909 04/17/00 01/09/15 8. Formulation 6,103,219 08/15/00 01/09/15 Patent Applications* Patent Applications* Patent Applications* Patent Applications* Patent Number Issue Date Expiration Date 1. Method of use (food effect) 11,781,165 pending 7/20/27 2. Method of use (8th hour) 11,804,720 pending 5/18/27 3. Method of use (elderly) 11,804,722 pending 5/18/27 4. Method of use (weight gain, tolerance, rebound insomnia) 11,867,595 pending 10/4/27 5. Formulation/PK 12,101,917 pending 4/11/28 * If issued, would be listed in the Orange Book. |
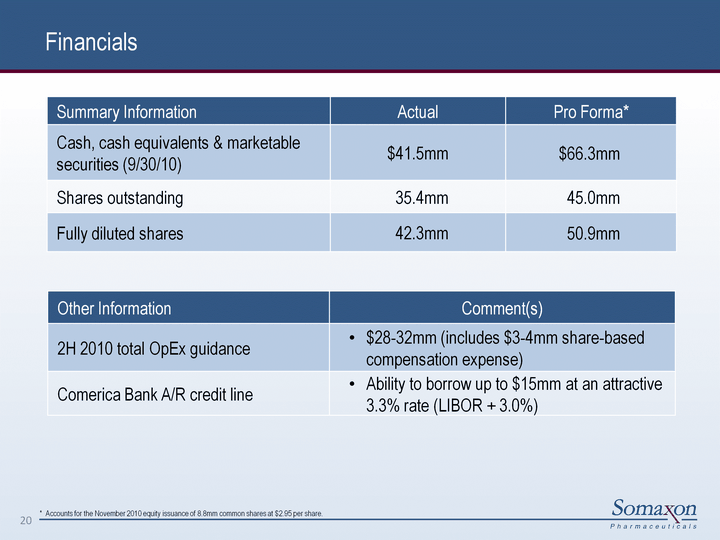
| Financials 20 Summary Information Actual Pro Forma* Cash, cash equivalents & marketable securities (9/30/10) $41.5mm $66.3mm Shares outstanding 35.4mm 45.0mm Fully diluted shares 42.3mm 50.9mm Other Information Comment(s) 2H 2010 total OpEx guidance $28-32mm (includes $3-4mm share-based compensation expense) Comerica Bank A/R credit line Ability to borrow up to $15mm at an attractive 3.3% rate (LIBOR + 3.0%) * Accounts for the November 2010 equity issuance of 8.8mm common shares at $2.95 per share. |

| Strategic Priorities in 2011 Maximize value of SilenorEstablish as first-line therapy for sleep maintenance insomniaPursue life-cycle management opportunitiesLeverage commercial infrastructureCo-promotion deal(s)"Tuck-in" product acquisition/in-licensing(s)Consider broader strategic M&A for multiple productsEffective management of corporate resourcesMaintain total OpEx within stated rangeContinuously assess performance and re-allocate resources appropriately 21 |
| 13th Annual BIO CEO & Investor Conference Richard W. PascoePresident and CEOFebruary 14, 2011 (r) |
