Attached files
| file | filename |
|---|---|
| 8-K - FORM 8K UBS GLOBAL HEALTHCARE CONFERENCE - WEST PHARMACEUTICAL SERVICES INC | file8kform.htm |

WEST PHARMACEUTICAL SERVICES OVERVIEW
Solutions for Injectable Drug Delivery NYSE:WST www.westpharma.com
UBS
21st Global Healthcare Services Conference
New York, NY February 8, 2011

This presentation contains “forward-looking statements”, as that term is defined in the Private
Securities Litigation Reform Act of 1995, that are based on management’s beliefs and
assumptions, current expectations, estimates and forecasts. Statements that are not
historical facts, including statements that are preceded by, followed by, or that include, words
such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate” and other words and terms
of similar meaning are forward-looking statements. West’s estimated or anticipated future
results, product performance or other non-historical facts are forward-looking and reflect our
current perspective on existing trends and information.
Securities Litigation Reform Act of 1995, that are based on management’s beliefs and
assumptions, current expectations, estimates and forecasts. Statements that are not
historical facts, including statements that are preceded by, followed by, or that include, words
such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate” and other words and terms
of similar meaning are forward-looking statements. West’s estimated or anticipated future
results, product performance or other non-historical facts are forward-looking and reflect our
current perspective on existing trends and information.
Many of the factors that will determine the Company’s future results are beyond the ability of
the Company to control or predict. These statements are subject to known or unknown risks
or uncertainties, and therefore, actual results could differ materially from past results and
those expressed or implied in any forward-looking statement. You should bear this in mind as
you consider forward-looking statements. A non-exclusive list of important factors that may
affect future results may be found in West’s filings with the Securities and Exchange
Commission, including our annual report on Form 10-K and our periodic reports on Form 10-
Q and Form 8-K. You should evaluate any statement in light of these important factors.
the Company to control or predict. These statements are subject to known or unknown risks
or uncertainties, and therefore, actual results could differ materially from past results and
those expressed or implied in any forward-looking statement. You should bear this in mind as
you consider forward-looking statements. A non-exclusive list of important factors that may
affect future results may be found in West’s filings with the Securities and Exchange
Commission, including our annual report on Form 10-K and our periodic reports on Form 10-
Q and Form 8-K. You should evaluate any statement in light of these important factors.
Safe Harbor Statement
2

West is a globally diversified manufacturer of products consumed in the healthcare and
consumer markets.
consumer markets.
Every day over 80 million components manufactured by West, and our partner Daikyo, are
used to enhance the quality of healthcare worldwide.
used to enhance the quality of healthcare worldwide.
3

A Diverse, Stable Customer Base
Pharmaceutical/Biotech
Generic
Medical Device
4
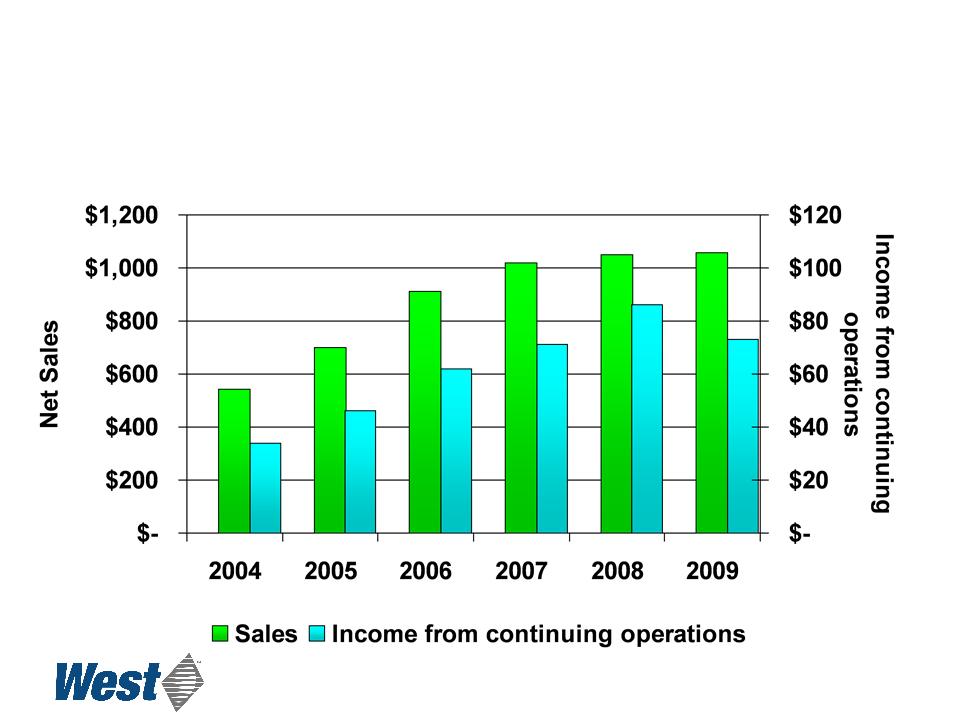
Sales and Income from
Continuing Operations
($ in millions)
Continuing Operations
($ in millions)
5

2010 Segment Reorganization
Packaging Systems
• Established leadership
• High market share
• Stable growth rate
Delivery Systems
• Proprietary devices
• Contract manufacturing
• High projected growth rate
Development
Primary Package
Administration
6

Pharmaceutical Packaging Systems
West MixJect®
and Vial2Bag®
and Vial2Bag®
Prefilled Glass
Cartridge and
Pen
Cartridge and
Pen
Glass
Vials
and
Syringes
Vials
and
Syringes
7

Delivery Systems
West
ConfiDose®
Auto-injector
System
ConfiDose®
Auto-injector
System
West
Electronic
Patch-injector
Electronic
Patch-injector
8
Daikyo Crystal Zenith® is a registered trademark of Daikyo, Seiko, Ltd.

2010 Issues
• General Operating Challenges:
– Pharma M&A continued
– Impact of healthcare reform uncertainty
– Currency volatility; commodity price fluctuations
– Changes in customer order patterns and inventory policies
– Operations restructuring announced in Q4
– Non-recurring 2009 H1N1 Sales
• No Update on 2010 Fourth Quarter or Full-Year Results
• 2010 Year-End Call: February 17, 2011, 9:00 A.M.
9

2011 Market Trends
• Asymmetric global economic recovery
– Europe, US, Japan remain sluggish
– China, India, Brazil stronger
– Continuing Fx, commodity price volatility
• More demanding regulatory environment
– Bydureon
– Benlysta
– Changes to approved indications (Avastin)
• Broader trends in Pharma/Device markets continue:
– Thin near term pipelines
– Pharma M&A - shift to large molecules
– Increased manufacturing outsourcing (India generic growth)
10

• Pharmaceutical Packaging Segment
– Demographics and increasing prevalence of chronic disease
– Biologics
– Growth in Emerging Markets
– Increased access to Healthcare
– Escalating quality expectations: “Zero Defects”
• Delivery Systems Segment
– Demand for combination products that promote safety, dosing accuracy,
ease of use, and deliver cost savings
ease of use, and deliver cost savings
– Drug product life cycle management
– Glass compatibility/breakage issues
What will Drive Future Growth?
11

|
Category
|
Key Customers
|
Projected
Growth |
|
Diabetes
|
|
> 10 %
|
|
Oncology
|
|
> 10 %
|
|
Vaccines
|
|
> 10 %
|
|
Autoimmune
|
|
> 8%
|
IMS April 2010 Report; Business Insights 2009; GBI Research 2009
12
Therapeutic Category Growth Drivers

• Packaging Systems Segment
– Grow incremental value per unit
– Geographic expansion - capacity investments in Asia
– Improved operating efficiency: lean operations
– Strategic acquisitions and partnerships
• Delivery Systems Segment
– Commercialize CZ opportunities
– Develop new platform opportunities - combination products
Growth Strategy
13
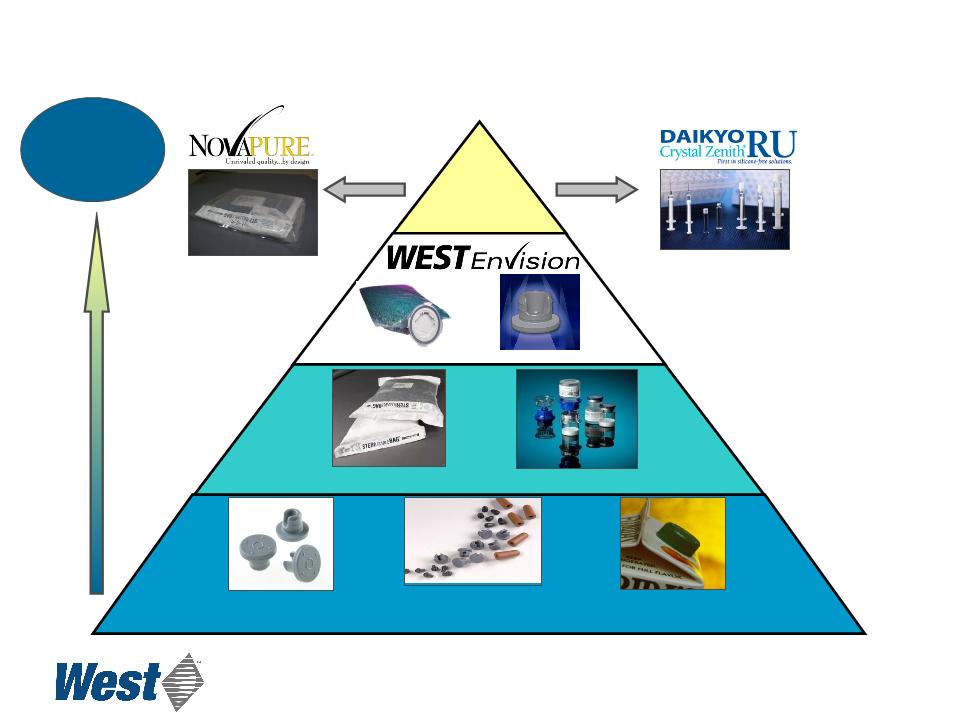
Value Proposition
Proprietary
Products
Products
Revenue and
Margin
Opportunity
Margin
Opportunity
Disposable Device
Components
Components
Westar® RS
Mix2Vial®
Westar® RU
Standard
Components
Components
Consumer
Products
Products
Packaging
Delivery
14

Faster Growth of High-Value Products
Pharmaceutical Packaging Systems
Pharmaceutical Packaging Systems
15

Drivers for Pharma Industry Life Cycle
Management
Management
• To maximize the value of the drug development program
– Extend the value of the molecule as an asset
• Delivery systems as drug formulation life cycle management
– Generate more revenue per dose
• To expand or retain market share in a competitive
environment
environment
– Reduce preparation and administration time
– Improve patient compliance
– Ease of use for self administration
– Accurate delivery of dosage
16
Pharma Industry Drug Life Cycle Management
Phase I
Phase II
Phase III
Post-Market Life Cycle Management
8 - 10 years
2 - 3 years
2 - 3 years
Regulatory
Approval
Discovery
17

Ready Pack™ System
Utilizes West’s proprietary
FluroTec® technology
FluroTec® technology is licensed from Daikyo Seiko, Ltd.
18

RU Vials
RU Seals
Glass
NovaPure™
Utilizes West’s
proprietary
proprietary
FluroTec® technology
CZ®
Daikyo Resin CZ®
Utilizes Daikyo’s
proprietary
proprietary
Crystal Zenith
technology
technology
FluroTec® technology is licensed from Daikyo Seiko, Ltd.
Daikyo Crystal Zenith® is a registered trademark of Daikyo, Seiko, Ltd.
19
Mix2Vial®
Utilizes Medimop’s
proprietary
technologies
proprietary
technologies
Vial Adapters
Utilizes Medimop’s
proprietary
technologies
proprietary
technologies
Ready Pack™ System
Utilizes West’s proprietary
FluroTec® technology
FluroTec® technology is licensed from Daikyo Seiko, Ltd.
20
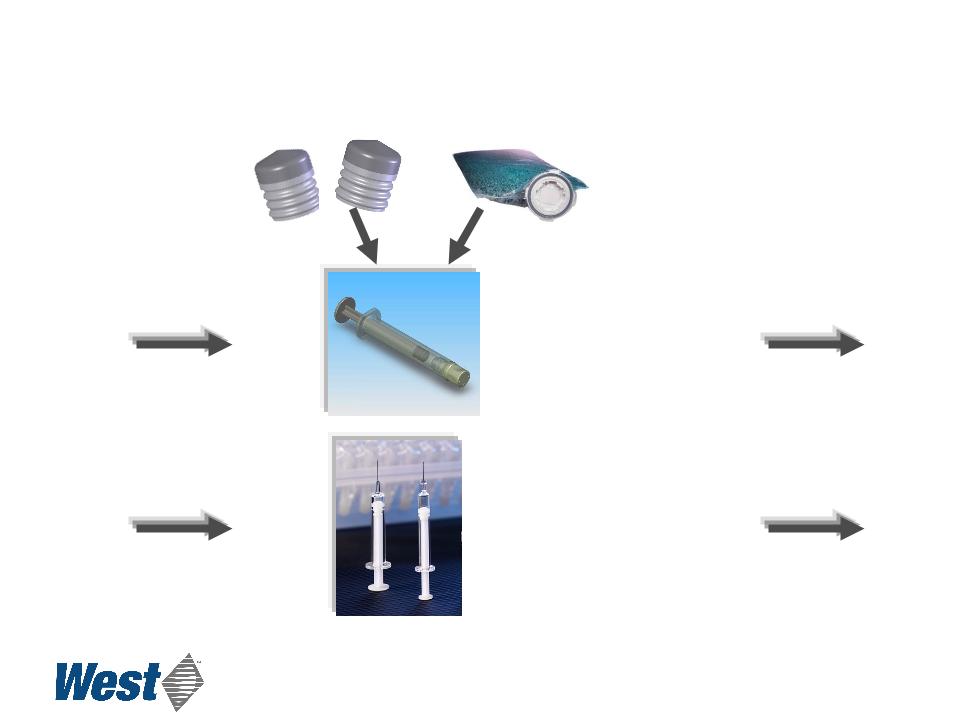
Luer Lock Glass Syringe
PFS Plungers
Utilizes West’s
proprietary
proprietary
FluroTec®
technology
technology
Westar® RU
Plungers in Port Bag
Plungers in Port Bag
Utilizes West’s proprietary
FluroTec® technology
FluroTec® technology
RU CZ® Insert
Needle Syringe
Needle Syringe
Utilizes Daikyo’s
proprietary Crystal
Zenith® technology
proprietary Crystal
Zenith® technology
FluroTec® technology is licensed from Daikyo Seiko, Ltd.
Daikyo Crystal Zenith® is a registered trademark of Daikyo, Seiko, Ltd.
21

Syringe with NovaGuard™
Passive Safety Needle
Passive Safety Needle
Utilizes West’s proprietary
technology
technology
ConfiDose™ Auto Injector
Utilizes West’s proprietary
technology
technology
22

Concerns With Glass Syringes
• Interaction with sensitive biologics
• Protein aggregation (silicone oil)
• Residual chemicals (tungsten, glue)
• Glass flakes
• Breakage
• In process
• Within auto-injector systems
• Recent FDA recalls
• Dimensional variation
• Breakage in auto-injector systems
• Designed for manual injection
• Variable silicone distribution
• Amount of silicone coverage
• Age of barrel
• Inconsistent break force and
sustaining-force
sustaining-force
• Quality
• Cosmetic defects
• High levels of inspection necessary
• High “cost of quality”
Siliconized Glass Syringe
Crystal Zenith Syringe
23
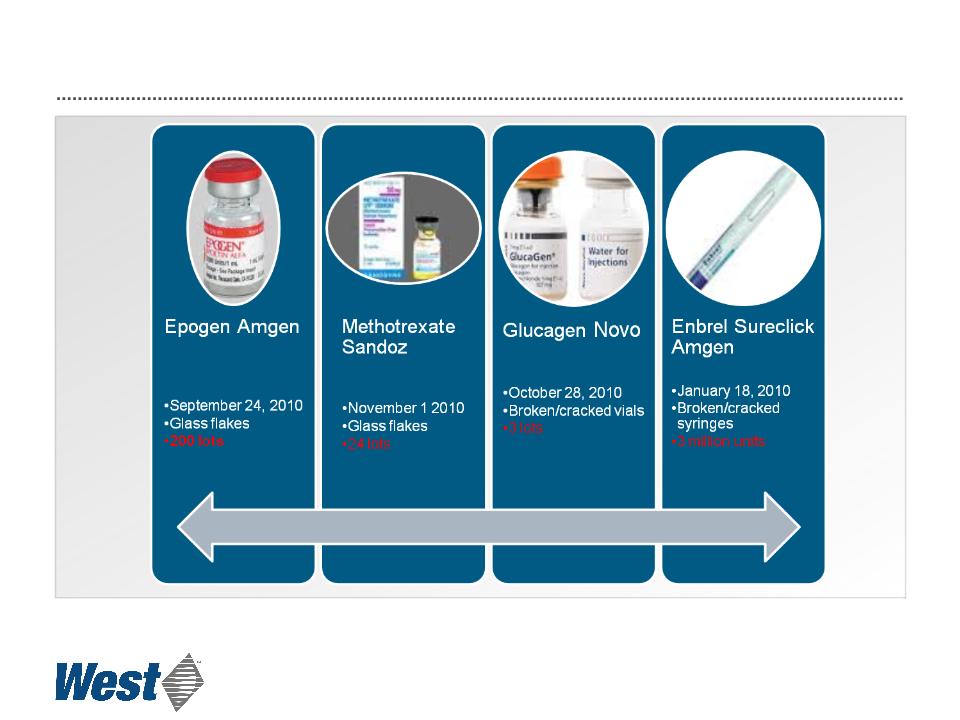
2010 Glass-related Recalls
24

Electronic Patch Injector System
• Controlled, subcutaneous, micro-
infusion delivery of high volumes
and high viscosity drugs
infusion delivery of high volumes
and high viscosity drugs
• Prefilled cartridge, no need for
user filling
user filling
• Based on Daikyo CZ cartridge
• Compact
• Hidden needle for safety
• Single push-button operation
• Fully programmable
25

Prototype Electronic Patch Injector Operation
Programmed by PDA or PC
Dose may be customized
Attached and activated by patient
26
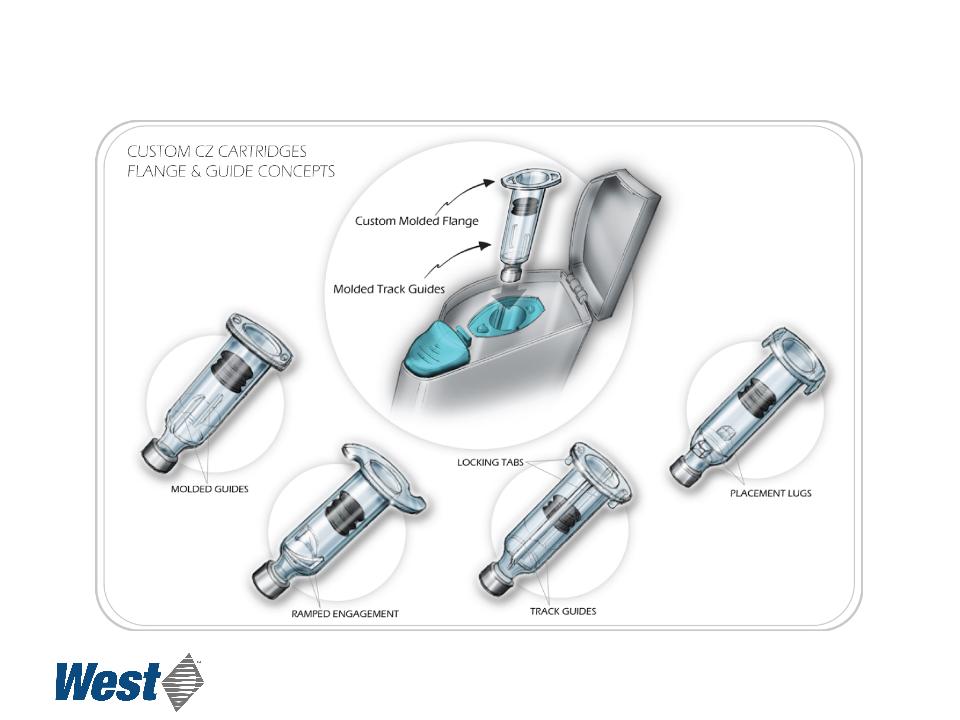
Custom Delivery Device Utilizing Daikyo CZ® Resin
Utilizes Daikyo’s proprietary Crystal Zenith technology
Daikyo Crystal Zenith® is a registered trademark of Daikyo, Seiko, Ltd.
27

West’s Strategy Drives Revenue
Growth in Excess of Unit Growth
Growth in Excess of Unit Growth
Lyo Mixing Station
Launched 1998
Lyo Vial Adapter
Launched in 2003
Prefilled Syringe
Launched in 2004
General trend for level of device
features and functionality
US Sales by Device for 2007*
(One year after Autoinjector launch)
* Data source IMS Health
Taken from presentation at Management Forum Annual Conference in London (March 22 & 23, 2010), Don Rogers, Genentech
Prefilled Auto Injector
Launched in 2006
28
Long-Term Growth Opportunity
Strategic Planning Goals:
• Projected 2014 sales of $0.6 billion
• Projected 2014 Operating margin: > 20%
$1.5 billion market for components with unit
growth 0% to 8% per year, depending on
product and therapeutic segment
growth 0% to 8% per year, depending on
product and therapeutic segment
Strategic Planning Goals:
• Projected 2014 sales of $1.0 billion
• Projected 2014 Operating margin: > 20%
|
Pharmaceutical
Packaging Systems
|
|
Primary Container Solutions
|
|
Pharmaceutical Delivery
Systems |
|
Administration Systems
|
Consolidated 2014 Planning Objectives
• 2014 Sales: $1.6 billion
• 2014 Operating Margin: 19%
29

Our Long-Term Focus
• Pharmaceutical Packaging Systems
– Organic growth (on average) of 3-5% per year
– Margin expansion through operating efficiency, product mix
– Capital investments targeted at enhanced quality and value
• Pharmaceutical Delivery Systems
– Deliver the potential of Daikyo CZ products
– Increase healthcare-consumable contract manufacturing revenue
– Grow proprietary safety and delivery system businesses
• Financial discipline
– Operating cash flow: Discretionary SG&A, R&D and capital spending that
are supported by revenue growth
are supported by revenue growth
– Deliver returns on invested capital (“ROIC”) that regularly exceed weighted
average cost of capital (“WACC”)
average cost of capital (“WACC”)
– Align incentives with financial performance and value creation
30

Summary
• Well positioned
– Substantial market share
– Proprietary technology
– Diversified customer base
– Global footprint
• Stability with growth potential
– Strength in new product pipeline
– Preferred products for biologics
• The financial strength to invest
– Reliable operating cash flow
– Balance sheet strength
• 2010 Year-end call
– February 17, 2011 - 9:00AM
Injectable Container Solutions
Advanced
Injection
Systems
Injection
Systems
Prefillable Syringe Systems
Safety and Administration
Systems
Systems
31

WEST PHARMACEUTICAL SERVICES
Solutions for Injectable Drug Delivery NYSE:WST www.westpharma.com
32
