Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Impax Laboratories, LLC | c10749e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - Impax Laboratories, LLC | c10749exv99w1.htm |
Exhibit
99.2

| J.P. Morgan Healthcare Conference January 10, 2011 Larry Hsu - President & CEOArt Koch - Chief Financial Officer ...Improving Health Through Technology |
| 2 Safe Harbor Statement "Safe Harbor" Statement under the Private Securities Litigation Reform Act of 1995: To the extent any statements made in this presentation contain information that is not historical, these statements are forward-looking in nature and express the beliefs and expectations of management. Such statements are based on current expectations and involve a number of known and unknown risks and uncertainties that could cause the Company's future results, performance or achievements to differ significantly from the results, performance or achievements expressed or implied by such forward-looking statements. Such risks and uncertainties include, but are not limited to, the effect of current economic conditions on the Company's industry, business, financial position, results of operations and market value of its common stock, the ability to maintain an effective system of internal control over financial reporting, fluctuations in revenues and operating income, reductions or loss of business with any significant customer, the impact of competitive pricing and products and regulatory actions on the Company's products, the ability to sustain profitability and positive cash flows, the ability to maintain sufficient capital to fund operations, any delays or unanticipated expenses in connection with the operation of the Taiwan facility, the ability to successfully develop and commercialize pharmaceutical products, the uncertainty of patent litigation, consumer acceptance and demand for new pharmaceutical products, the difficulty of predicting Food and Drug Administration filings and approvals, the inexperience of the Company in conducting clinical trials and submitting new drug applications, reliance on key alliance, collaboration, license and distribution agreements, the availability of raw materials, the ability to comply with legal and regulatory requirements governing the healthcare industry, the regulatory environment, exposure to product liability claims and other risks described in the Company's periodic reports filed with the Securities and Exchange Commission. Forward-looking statements speak only as to the date on which they are made, and Impax undertakes no obligation to update publicly or revise any forward-looking statement, regardless of whether new information becomes available, future developments occur or otherwise. Note: All product sales data included herein are derived from data published by Wolters Kluwer Health for the 12 months ended November 2010. Trademarks referenced herein are the property of their respective owners. (c)2011 Impax Laboratories, Inc. |
| 3 3 Strong Platform for Long Term Growth Unique Targeted High Value ANDAs Offering Long-Term Growth Drivers Generic Brand Drug Delivery Expertise Product Development Formulation Technology Core Competency |

| 4 Dual Strategic Focus Unique targeted ANDAsFirst-to-file, First-to-marketLimited competitionDifficult to formulate and manufacture36 apps pending at FDA62 under development Improve on marketed CNS products505(b)(2) NDA candidatesDevelop IP positions2 products in Phase III1 product in Phase II |

| ...A Leader in Generic Medicines |
| 6 Generic Growth Initiatives |
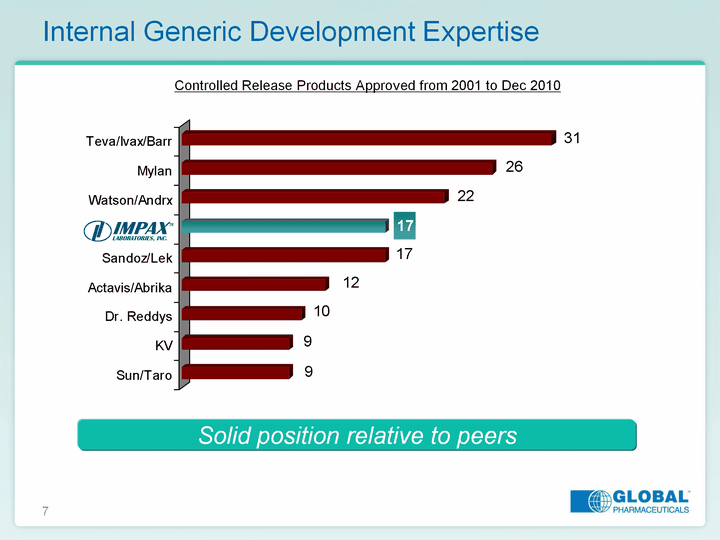
| 7 Internal Generic Development Expertise Sun/Taro KV Dr. Reddys Actavis/Abrika Sandoz/Lek Watson/Andrx Mylan Teva/Ivax/Barr 9 9 10 12 17 17 22 26 31 Solid position relative to peers Controlled Release Products Approved from 2001 to Dec 2010 |
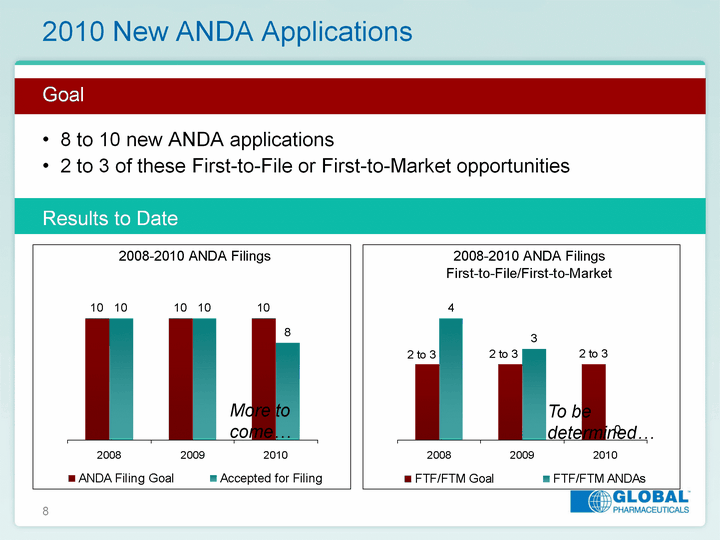
| 8 2010 New ANDA Applications Goal8 to 10 new ANDA applications2 to 3 of these First-to-File or First-to-Market opportunitiesResults to Date 2008 2009 2010 ANDA Filing Goal 10 10 10 Accepted for Filing 10 10 8 More to come... 2008 2009 2010 FTF/FTM Goal 2.5 2.5 2.5 FTF/FTM ANDAs 4 3 0 To be determined... |
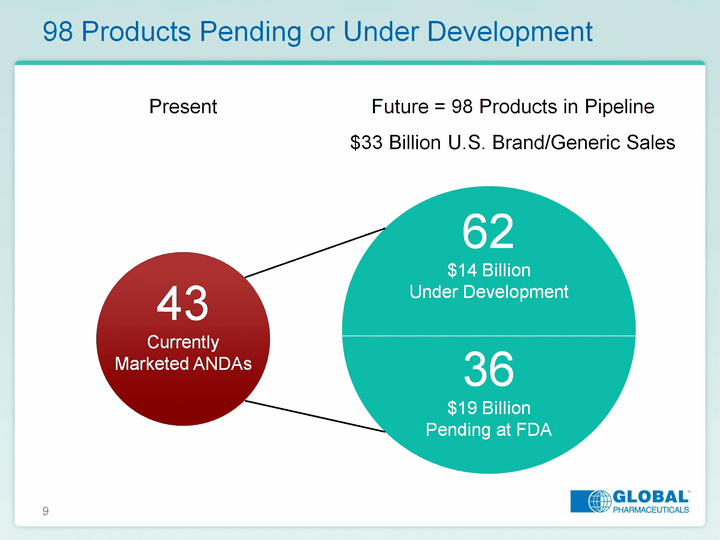
| 9 98 Products Pending or Under Development 43 Currently Marketed ANDAs 62 $14 BillionUnder Development 36 $19 BillionPending at FDA Present Future = 98 Products in Pipeline$33 Billion U.S. Brand/Generic Sales |
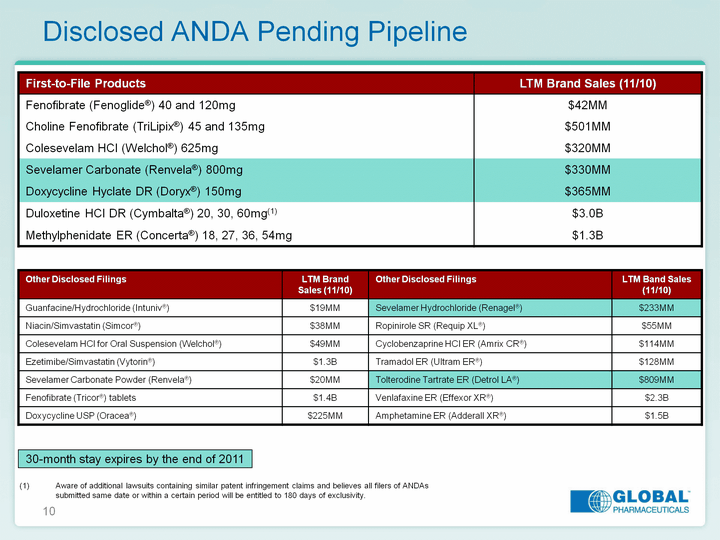
| 10 Disclosed ANDA Pending Pipeline Disclosed ANDA Pending Pipeline Disclosed ANDA Pending Pipeline Aware of additional lawsuits containing similar patent infringement claims and believes all filers of ANDAs submitted same date or within a certain period will be entitled to 180 days of exclusivity. 30-month stay expires by the end of 2011 |
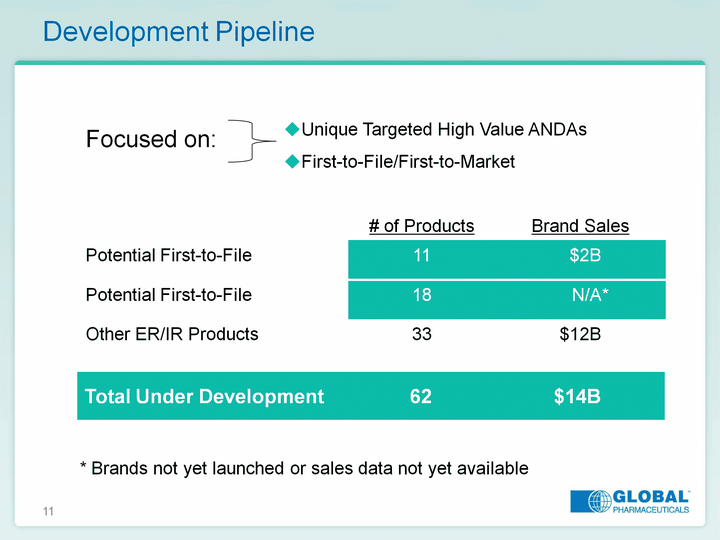
| 11 Development Pipeline Total Under Development 62 $14B * Brands not yet launched or sales data not yet available Unique Targeted High Value ANDAs First-to-File/First-to-Market First-to-File/First-to-Market First-to-File/First-to-Market Focused on: |

| ...Advancing CNS Treatment |
| 13 Impax Pharmaceuticals Background Therapeutic area focus: CNS - NeurologyDevelop products providing significant advancements in clinical outcomes Product differentiationLeveraging proven compounds to improved resultsFormulation expertise lays the foundationControlled-release formulation technologiesBroad drug delivery technologiesFocus on unmet therapeutic needsDevelop IP positions around selected products |
| 14 Brand Growth Initiatives |
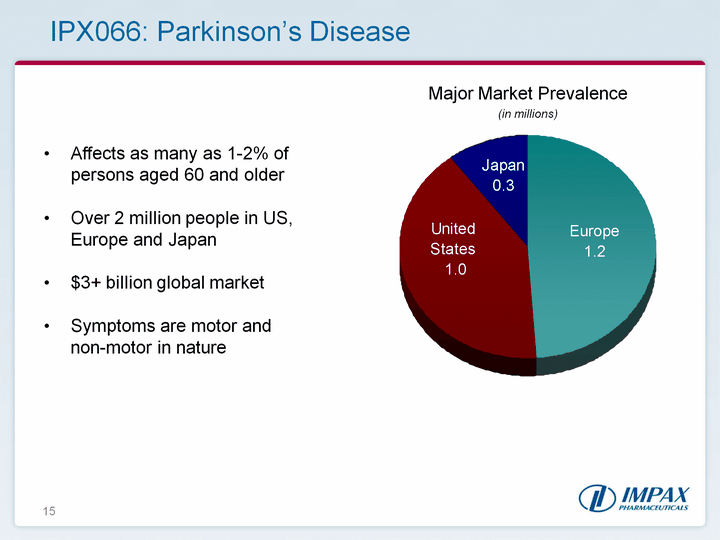
| 15 IPX066: Parkinson's Disease Major Market Prevalence (in millions) Affects as many as 1-2% of persons aged 60 and olderOver 2 million people in US, Europe and Japan$3+ billion global market Symptoms are motor and non-motor in nature |
| 16 IPX066 Potential Benefits... Reduction in "Off" time Reduced dosing frequency Continuous "On" time without troublesome dyskinesia Predictability of "On' time Targeted to address several limitations of current Levodopa therapy IPX066: Controlled-release Carbidopa/Levodopa ...Leading to Improvement in quality of life Improved Motor Symptoms |
| 17 IPX066: Collaboration with GlaxoSmithKline Joint Development and Commercialization of IPX066Agreement reached in December 2010Overview of agreementGSK acquires commercial rights outside of the US & Taiwan Impax received $11.5 million upfront paymentImpax can receive additional milestones of up to $175 millionTiered royalty on GSK net product sales GSK an ideal partner for IPX066Extensive Neuroscience experienceDemonstrated development and commercial experience |
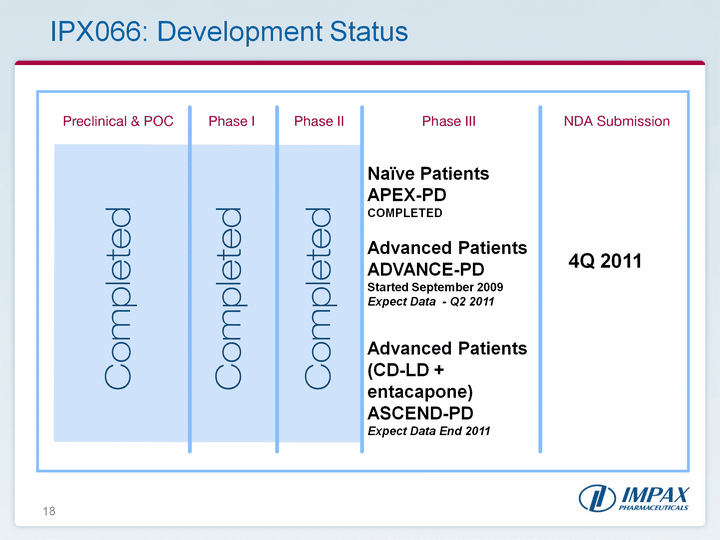
| 18 Naive PatientsAPEX-PD COMPLETED Advanced PatientsADVANCE-PD Started September 2009Expect Data - Q2 2011Advanced Patients (CD-LD + entacapone)ASCEND-PD Expect Data End 2011 4Q 2011 IPX066: Development Status |
| 19 19 IPX066 - Naive PD Patient Phase III Study Phase III randomized, double-blind, placebo-controlled, fixed-dose, parallel arm study of 3 doses of IPX066 vs. placeboEnrolled subjects with early PDConducted under Special Protocol Assessment with FDANorth America and Europe30-week treatment durationPrimary endpoint: change from baseline in the sum of UPDRS Parts II and III scores at week 30 |
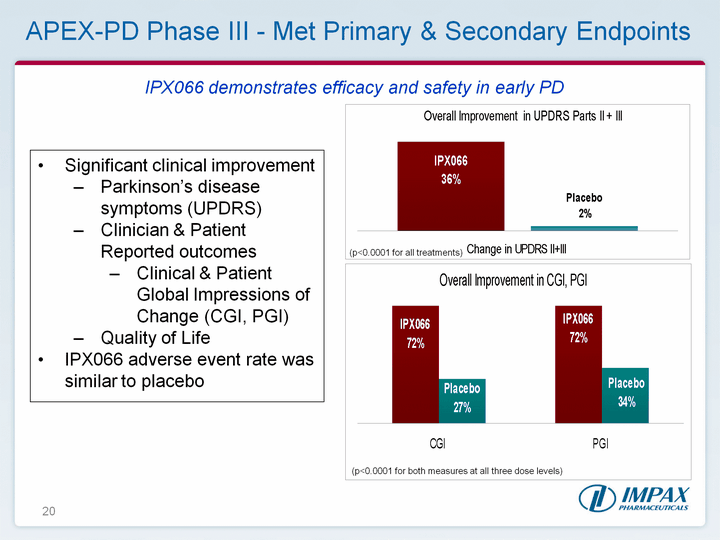
| 20 (p<0.0001 for all treatments) (p<0.0001 for both measures at all three dose levels) APEX-PD Phase III - Met Primary & Secondary Endpoints Significant clinical improvementParkinson's disease symptoms (UPDRS)Clinician & Patient Reported outcomes Clinical & Patient Global Impressions of Change (CGI, PGI)Quality of Life IPX066 adverse event rate was similar to placebo IPX066 demonstrates efficacy and safety in early PD |
| 21 21 IPX066 - Advanced PD Patient Phase III Study Phase III randomized, double-blind, active-control, parallel-group comparison of IPX066 vs. IR carbidopa-levodopaNorth America and Europe22-week treatment durationPrimary endpoint: percentage of "Off" time during waking hours Completed enrollment in Q3 2010Results expected Q2 2011 |
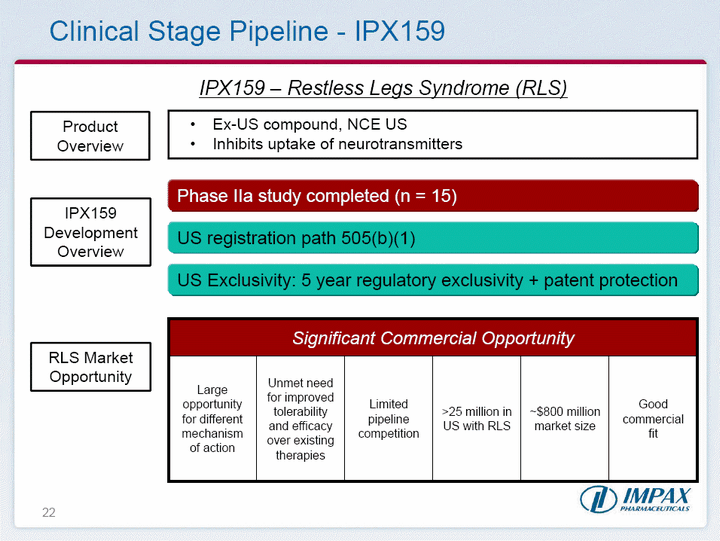
| 22 Clinical Stage Pipeline - IPX159 IPX159 - Restless Legs Syndrome (RLS) IPX159 Development Overview Product Overview Ex-US compound, NCE USInhibits uptake of neurotransmitters RLS Market Opportunity Phase IIa study completed (n = 15) US registration path 505(b)(1) US Exclusivity: 5 year regulatory exclusivity + patent protection Significant Commercial Opportunity Significant Commercial Opportunity Significant Commercial Opportunity Significant Commercial Opportunity Significant Commercial Opportunity Significant Commercial Opportunity Large opportunity for different mechanism of action Unmet need for improved tolerability and efficacy over existing therapies Limited pipeline competition >25 million in US with RLS ~$800 million market size Good commercial fit |
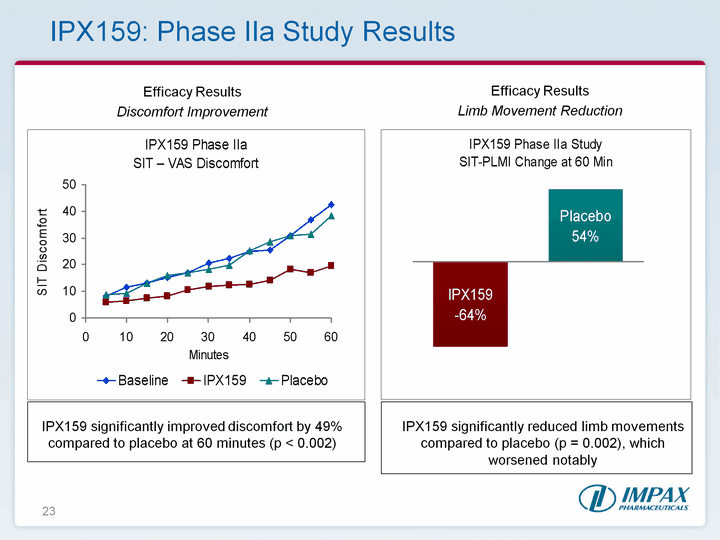
| 23 IPX159: Phase IIa Study Results Efficacy Results Discomfort Improvement Efficacy Results Limb Movement Reduction IPX159 significantly improved discomfort by 49% compared to placebo at 60 minutes (p < 0.002) IPX159 significantly reduced limb movements compared to placebo (p = 0.002), which worsened notably |
| 24 IPX159: Safety Results & Development Strategy Safety Results Safety & tolerability appropriate for moderate to severe RLSMajority of adverse events were mild or moderate in severityDevelopment ActivitiesDevelop for moderate to severe RLS indicationIND filing planned for 1H 2011 Planning Phase IIb study in moderate to severe RLSStart in Q4 2011 / Q1 2012Target results by 2H 2013 |

| 2011 Financial Outlook ...Improving Health Through Technology |
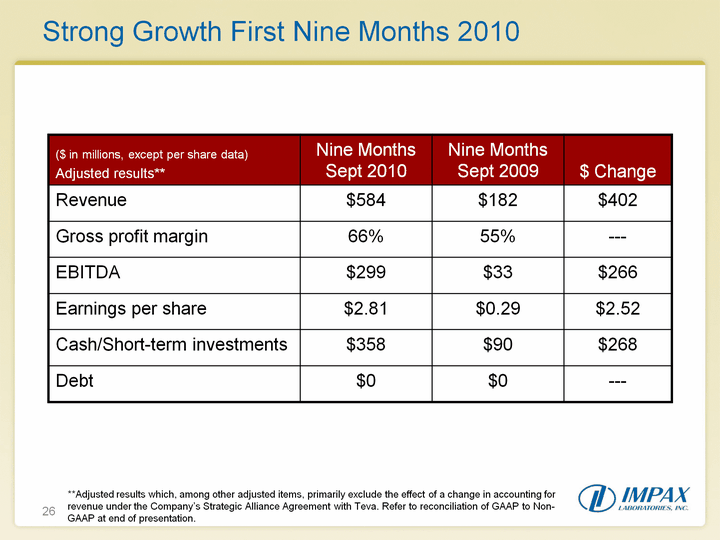
| 26 Strong Growth First Nine Months 2010 **Adjusted results which, among other adjusted items, primarily exclude the effect of a change in accounting for revenue under the Company's Strategic Alliance Agreement with Teva. Refer to reconciliation of GAAP to Non- GAAP at end of presentation. |
| 27 2011 Financial Outlook Top-line performance driven by generic division products:Variability depending on Generic Adderall XR(r)Supply issues and Shire litigationCitizen Petition status Gross margins expected in ~ 50% rangeExpense control is critical management objectiveFree cash flow positive |
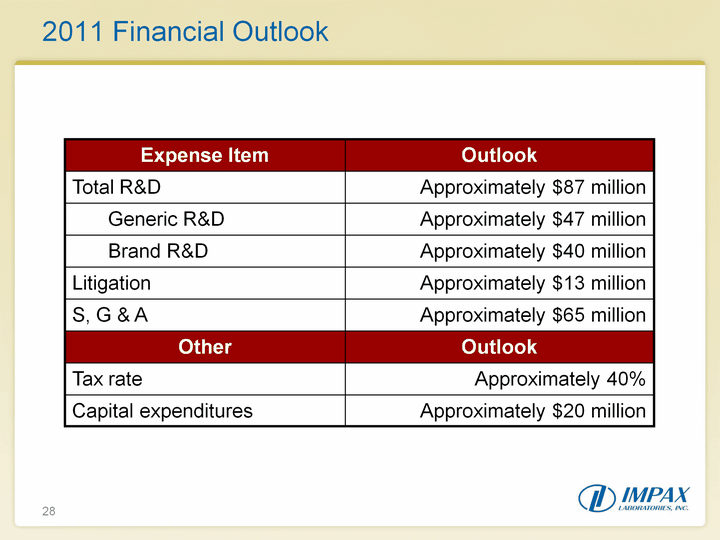
| 28 2011 Financial Outlook 2011 Financial Outlook |
| 29 29 Strong Platform for Long Term Growth Unique Targeted High Value ANDAs Offering Long-Term Growth Drivers Generic Brand Drug Delivery Expertise Product Development Formulation Technology Core Competency |
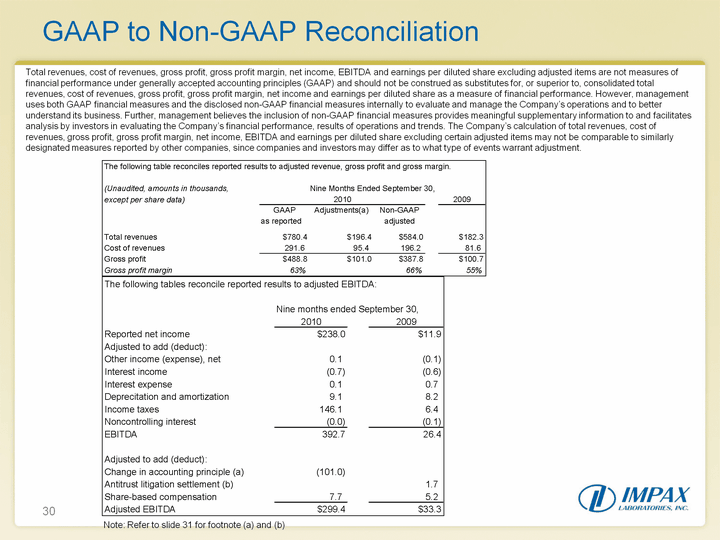
| 30 GAAP to Non-GAAP Reconciliation Total revenues, cost of revenues, gross profit, gross profit margin, net income, EBITDA and earnings per diluted share excluding adjusted items are not measures of financial performance under generally accepted accounting principles (GAAP) and should not be construed as substitutes for, or superior to, consolidated total revenues, cost of revenues, gross profit, gross profit margin, net income and earnings per diluted share as a measure of financial performance. However, management uses both GAAP financial measures and the disclosed non-GAAP financial measures internally to evaluate and manage the Company's operations and to better understand its business. Further, management believes the inclusion of non-GAAP financial measures provides meaningful supplementary information to and facilitates analysis by investors in evaluating the Company's financial performance, results of operations and trends. The Company's calculation of total revenues, cost of revenues, gross profit, gross profit margin, net income, EBITDA and earnings per diluted share excluding certain adjusted items may not be comparable to similarly designated measures reported by other companies, since companies and investors may differ as to what type of events warrant adjustment. Note: Refer to slide 31 for footnote (a) and (b) |
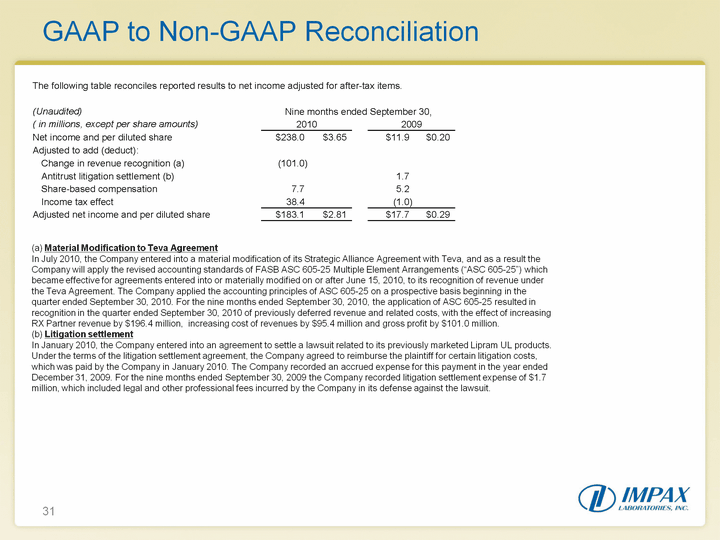
| 31 GAAP to Non-GAAP Reconciliation (a) Material Modification to Teva AgreementIn July 2010, the Company entered into a material modification of its Strategic Alliance Agreement with Teva, and as a result the Company will apply the revised accounting standards of FASB ASC 605-25 Multiple Element Arrangements ("ASC 605-25") which became effective for agreements entered into or materially modified on or after June 15, 2010, to its recognition of revenue under the Teva Agreement. The Company applied the accounting principles of ASC 605-25 on a prospective basis beginning in the quarter ended September 30, 2010. For the nine months ended September 30, 2010, the application of ASC 605-25 resulted in recognition in the quarter ended September 30, 2010 of previously deferred revenue and related costs, with the effect of increasing RX Partner revenue by $196.4 million, increasing cost of revenues by $95.4 million and gross profit by $101.0 million. (b) Litigation settlementIn January 2010, the Company entered into an agreement to settle a lawsuit related to its previously marketed Lipram UL products. Under the terms of the litigation settlement agreement, the Company agreed to reimburse the plaintiff for certain litigation costs, which was paid by the Company in January 2010. The Company recorded an accrued expense for this payment in the year ended December 31, 2009. For the nine months ended September 30, 2009 the Company recorded litigation settlement expense of $1.7 million, which included legal and other professional fees incurred by the Company in its defense against the lawsuit. |
