Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-3050_18k.htm |
Exhibit 99.1
|
|
VIVUS, Inc. (NASDAQ: VVUS) Innovative Products, Novel Therapies |
|
|
Forward-Looking Statements and Information Selected for Presentation During the course of this presentation Vivus will make certain statements in this call that are considered forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as "anticipate," "believe," "planned," "estimated," and "intend," among others. These forward-looking statements are based on Vivus' current expectations, and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the timing and substance of Vivus' written response to the FDA's Complete Response Letter (CRL); the FDA's interpretation of the information Vivus submits relating to the teratogenicity and cardiovascular safety; the FDA's interpretation of the data from our SEQUEL study, or OB-305; their request, if any, to conduct additional clinical trials; substantial competition; uncertainties of patent protection and litigation; reliance on sole source suppliers; limited sales and marketing resources and dependence upon third parties; risks related to the development of innovative products, and risks related to the failure to obtain FDA clearances or approvals and non-compliance with FDA regulations. As with any pharmaceutical in development, there are significant risks in the development, regulatory approval and commercialization of new products. There are no guarantees that our response to the FDA's CRL will be sufficient to satisfy the FDA's safety concerns, and that the FDA will not require us to conduct any additional studies or that any product will receive regulatory approval for any indication or prove to be commercially successful. Vivus does not undertake an obligation to update or revise any forward-looking statements. Investors should read the risk factors set forth in the Vivus Form 10-K for the year ending December 31, 2009, and periodic reports filed with the Securities and Exchange Commission. All investors are encouraged to review each presentation in its entirety at www.vivus.com or as filed with the Securities and Exchange Commission at www.sec.gov. |
|
|
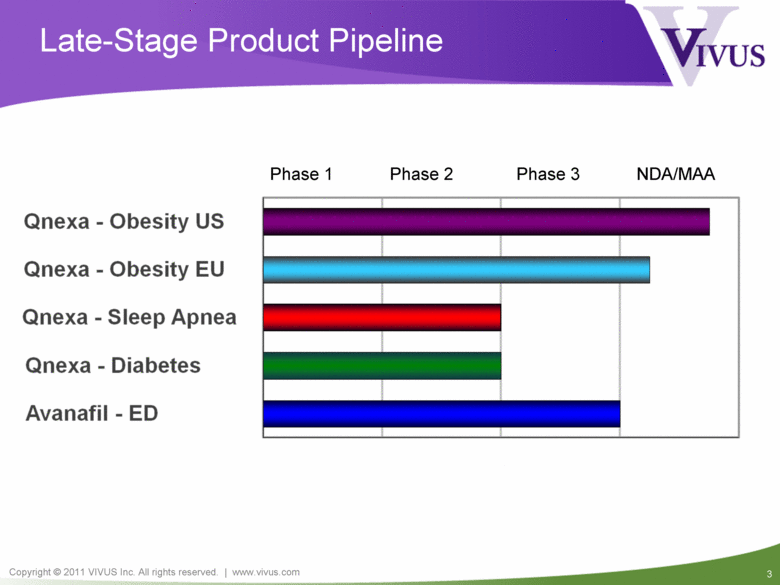
Late-Stage Product Pipeline Phase 1 Phase 2 Phase 3 NDA/MAA Qnexa - Obesity US Qnexa - Obesity EU Qnexa - Sleep Apnea Qnexa - Diabetes Avanafil - ED |
|
|
Prevalence and Consequence of Obesity “Overweight and obesity are associated with increased all-cause mortality.” – NEJM December 20101 Obesity is associated with significant increases in overall mortality from CVD, cancer, diabetes, and kidney disease – JAMA 20072 Epidemiology ~68% (140 million) of US adults are overweight/obese3 ~50% (150 million) of EU adults are overweight/obese4 Obesity related disease costs $147 billion in the US5 $158 billion in the EU6 1 Gonzalez, et al. NEJM 2010; 363:2211-9. 2 Flegal KM, et al. JAMA 2007;298:2028-2037 3 DHHS CDC June 2010, NHANES 2008, BMI > 25 US adult population 4 OECD Health at a Glance, December 2010, WHO The Challenge of Obesity in the WHO European Region and the Strategies for Response 2007 5 Finklestein, et al. Health Affairs 28 no. 5 (2009);w822-w831 6 Rettman A, Focus: Obesity epidemic costs EU a year. euobserver.com, 6/06/06; US$ conversion at €1EU:$1.34USD, xe.com Jan, 2011 |
|
|
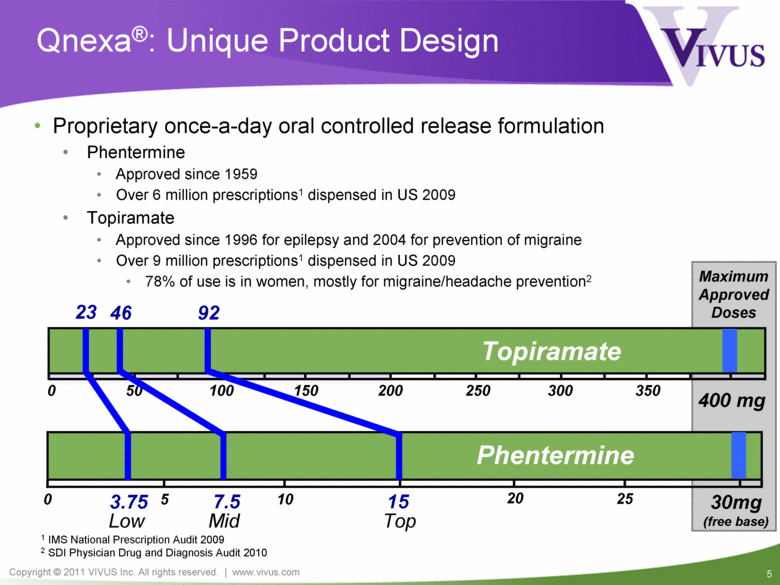
Qnexa®: Unique Product Design Proprietary once-a-day oral controlled release formulation Phentermine Approved since 1959 Over 6 million prescriptions1 dispensed in US 2009 Topiramate Approved since 1996 for epilepsy and 2004 for prevention of migraine Over 9 million prescriptions1 dispensed in US 2009 78% of use is in women, mostly for migraine/headache prevention2 0 400 mg 200 100 300 50 150 250 350 Topiramate 0 30mg (free base) 15 5 10 25 3.75 7.5 Phentermine Maximum Approved Doses 20 23 46 92 Low Mid Top 1 IMS National Prescription Audit 2009 2 SDI Physician Drug and Diagnosis Audit 2010 |
|
|
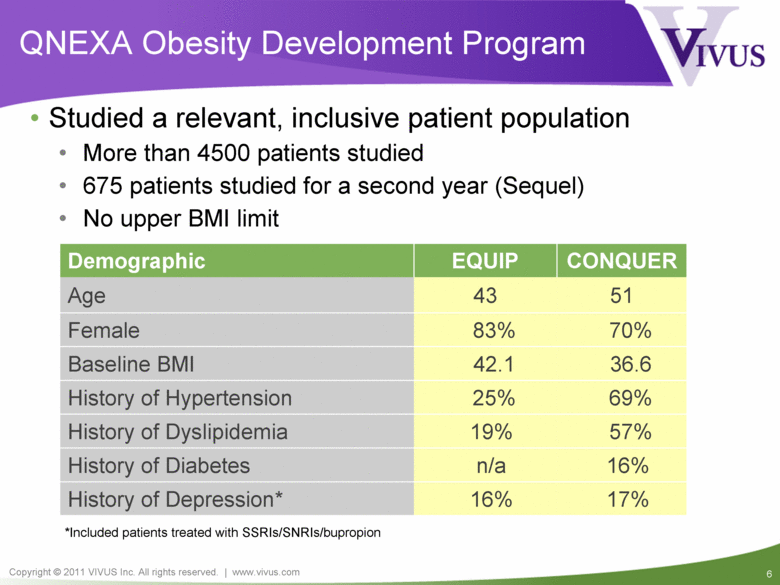
QNEXA Obesity Development Program Demographic EQUIP CONQUER Age 43 51 Female 83% 70% Baseline BMI 42.1 36.6 History of Hypertension 25% 69% History of Dyslipidemia 19% 57% History of Diabetes n/a 16% History of Depression* 16% 17% Studied a relevant, inclusive patient population More than 4500 patients studied 675 patients studied for a second year (Sequel) No upper BMI limit *Included patients treated with SSRIs/SNRIs/bupropion |
|
|
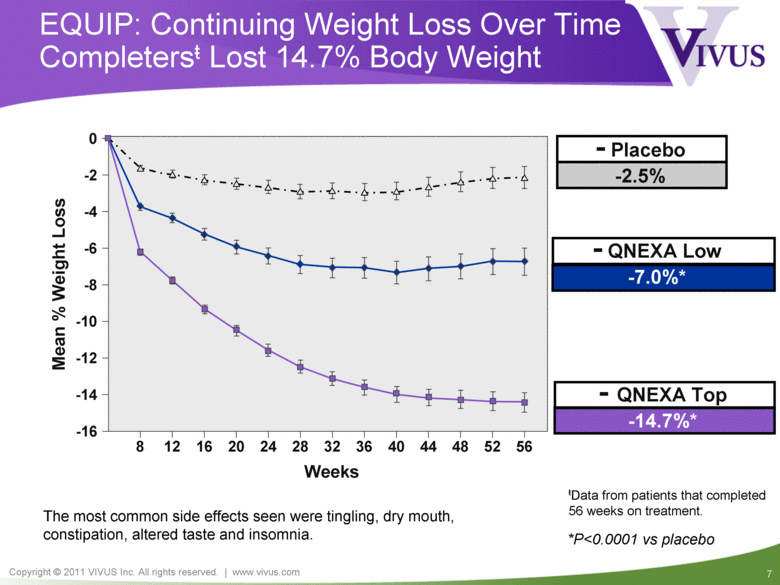
Mean % Weight Loss Weeks 8 12 16 20 24 28 32 36 40 44 48 52 56 -16 -14 -12 -10 -8 -6 -4 -2 0 - QNEXA Low -7.0%* EQUIP: Continuing Weight Loss Over Time Completersŧ Lost 14.7% Body Weight - Placebo -2.5% - QNEXA Top -14.7%* ŧData from patients that completed 56 weeks on treatment. *P<0.0001 vs placebo The most common side effects seen were tingling, dry mouth, constipation, altered taste and insomnia. |
|
|
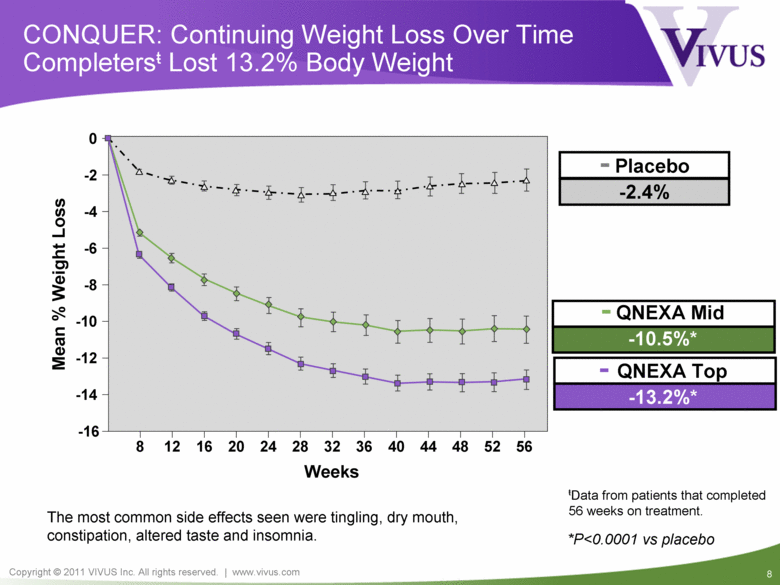
Mean % Weight Loss Weeks 8 12 16 20 24 28 32 36 40 44 48 52 56 -16 -14 -12 -10 -8 -6 -4 -2 0 - QNEXA Mid -10.5%* CONQUER: Continuing Weight Loss Over Time Completersŧ Lost 13.2% Body Weight - QNEXA Top -13.2%* - Placebo -2.4% ŧData from patients that completed 56 weeks on treatment. *P<0.0001 vs placebo The most common side effects seen were tingling, dry mouth, constipation, altered taste and insomnia. |
|
|
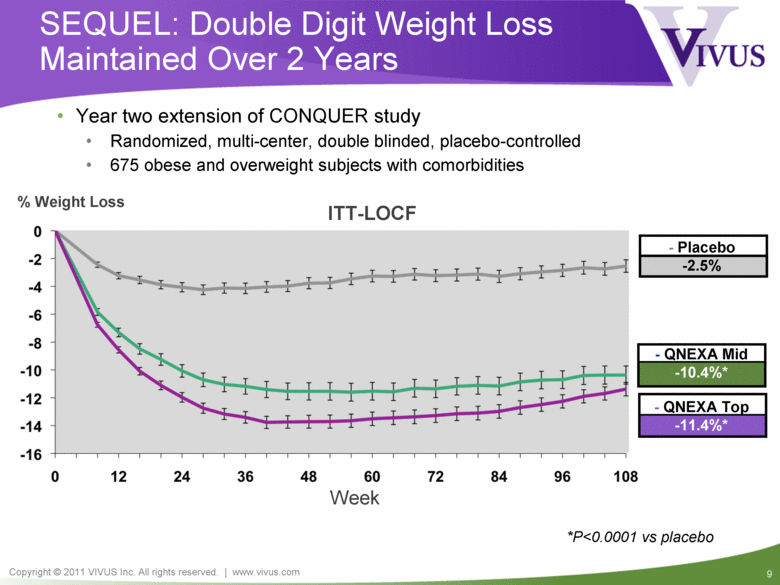
- QNEXA Mid -10.4%* - QNEXA Top -11.4%* SEQUEL: Double Digit Weight Loss Maintained Over 2 Years - Placebo -2.5% ITT-LOCF *P<0.0001 vs placebo % Weight Loss Week Year two extension of CONQUER study Randomized, multi-center, double blinded, placebo-controlled 675 obese and overweight subjects with comorbidities |
|
|
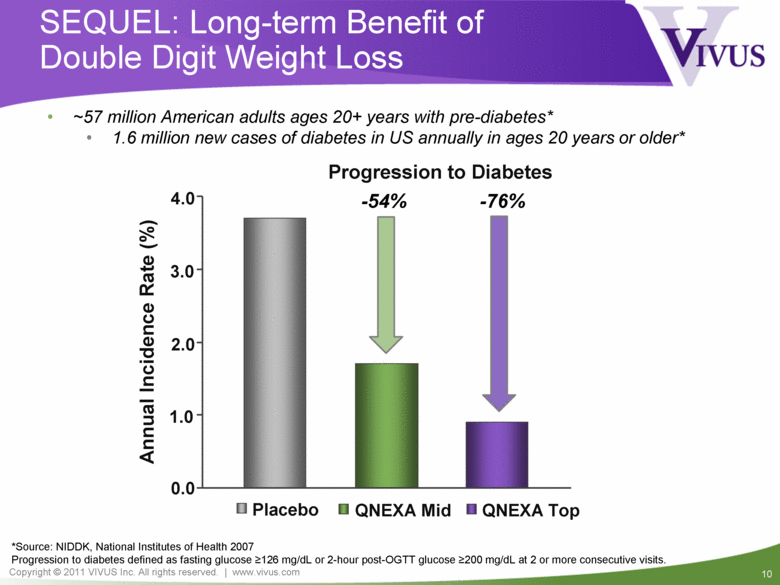
SEQUEL: Long-term Benefit of Double Digit Weight Loss *Source: NIDDK, National Institutes of Health 2007 Progression to diabetes defined as fasting glucose >126 mg/dL or 2-hour post-OGTT glucose >200 mg/dL at 2 or more consecutive visits. Annual Incidence Rate (%) Placebo QNEXA Mid QNEXA Top Progression to Diabetes 0.0 1.0 2.0 3.0 4.0 ~57 million American adults ages 20+ years with pre-diabetes* 1.6 million new cases of diabetes in US annually in ages 20 years or older* -54% -76% |
|
|
SEQUEL: Summary Year two extension of CONQUER study 675 obese and overweight subjects with multiple co-morbidities Sustained double-digit weight loss at end of two years (ITT-LOCF): Top-dose 11.4% Mid-dose 10.4% Placebo 2.5% Up to 76% reduction in progression to diabetes in QNEXA treated patients Significant improvement in blood pressure Safety and tolerability Well tolerated with no new or unexpected adverse events SAE rate similar to placebo with no drug related SAEs (4.0, 2.6, and 4.1%; placebo, mid- and top-dose, respectively) |
|
|
QNEXA US Regulatory Update Briefing documents to address CRL submitted to FDA 12/13/10 Addressed FDA comments in CRL Detailed plan and strategy to evaluate and mitigate the potential teratogenic risks in women of childbearing potential using QNEXA Evidence that the elevation in heart rate associated with phentermine/topiramate does not increase the risk of major adverse cardiovascular events Two-year data (SEQUEL) FDA meeting scheduled second half January 2011 NDA resubmission planned subsequent to meeting |
|
|
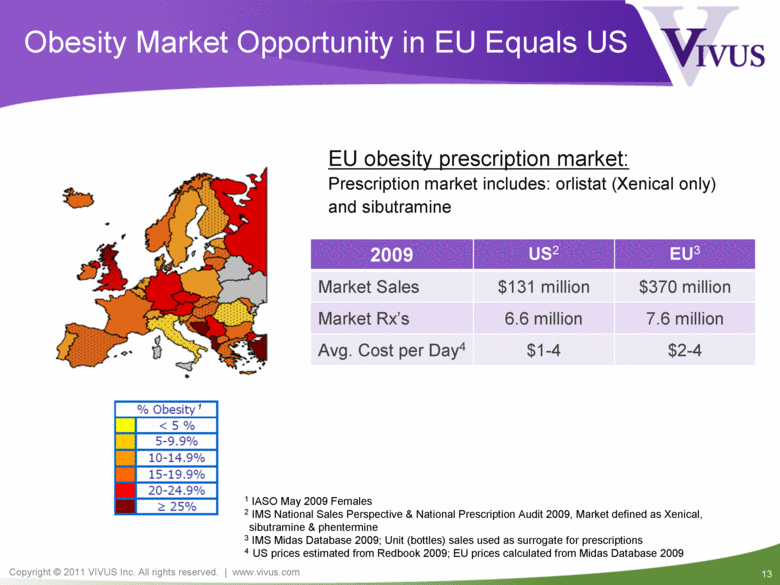
Obesity Market Opportunity in EU Equals US EU obesity prescription market: Prescription market includes: orlistat (Xenical only) and sibutramine 2009 US2 EU3 Market Sales $131 million $370 million Market Rx’s 6.6 million 7.6 million Avg. Cost per Day4 $1-4 $2-4 1 1 IASO May 2009 Females 2 IMS National Sales Perspective & National Prescription Audit 2009, Market defined as Xenical, sibutramine & phentermine 3 IMS Midas Database 2009; Unit (bottles) sales used as surrogate for prescriptions 4 US prices estimated from Redbook 2009; EU prices calculated from Midas Database 2009 % Obesity 1 < 5 % 5-9.9% 10-14.9% 15-19.9% 20-24.9% > 25% |
|
|
QNEXA EU Opportunity MAA Submitted in December 2010 Anticipate review by EMEA to be ~12 months QNEXA met 10% weight loss EMA guideline Market opportunity equal to US Prescription obesity treatments are covered by National Health Plans Seeking partner to launch in the EU |
|
|
Erectile dysfunction afflicts up to 30M men in the US1, Phosphodiesterase-5 inhibitors (PDE5 inhibitors) are the most common drug treatment2 In 2009, PDE5 inhibitors generated 19.5M TRxs and Sales of $1.9B2 Sales CAGR of 13% from 2007 to 2009 50% of patients using PDE5 inhibitors are unsatisfied1, creating opportunities for new entries Physicians switch many patients to another PDE5 inhibitor due to lack of efficacy and/or side effects3 Erectile Dysfunction (ED) Market Opportunity 1 Global Industry Analysts, Inc. – Erectile Dysfunction Drugs: A Global Market Report, April 2010 2 IMS National Sales Perspective & National Prescription Audit 2009 3 Primary market research May 2010 – Life Sciences Strategies Group |
|
|
Significant efficacy seen in all phase 3 studies 80% success rate in SEP2 Fast onset (≤ 15 min) and sustained activity through 6 hrs Highly selective PDE5 inhibitor Low rate of side effects Drug exposure not inhibited by food or alcohol Dosing flexibility 3 dose strengths Avanafil – Erectile Dysfunction |
|
|
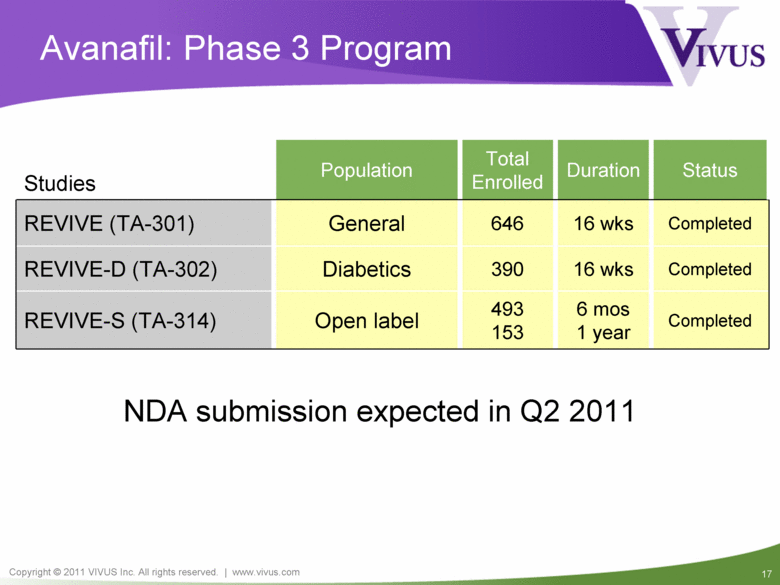
Studies Population Total Enrolled Duration Status REVIVE (TA-301) General 646 16 wks Completed REVIVE-D (TA-302) Diabetics 390 16 wks Completed REVIVE-S (TA-314) Open label 493 153 6 mos 1 year Completed Avanafil: Phase 3 Program NDA submission expected in Q2 2011 |
|
|
Avanafil Long-Term P3 Study (TA-314) Design Open-label safety study in 712 patients at 40 US centers Efficacy 80% of sexual attempts had erections sufficient for intercourse (SEP2) 67% of patients experiences successful intercourse (SEP3) Successful intercourse was achieved as early as 15 minutes Safety 2.8% discontinuation due to AEs Most common side effects reported were headache (5.6%), flushing (3.5%), nasopharyngitis (3.4%) and nasal congestion (2.1%) No drug-related SAEs reported |
|
|
QNEXA Double-digit weight loss sustained at 2 Yrs Significant reduction in co-morbidities Well tolerated, no new or unexpected adverse events Meeting with FDA second half of January 2011 EU Application Filed Avanafil Long-term data confirms safety and efficacy NDA filing planned for Q2 2011 September 30, 2010 cash balance: $158 million VIVUS Investment Opportunity |