Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pernix Sleep, Inc. | c06122e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - Pernix Sleep, Inc. | c06122exv99w1.htm |
Exhibit 99.2

| UBS Global Life Science Conference September 21, 2010 NASDAQ: SOMX |

| Forward Looking Statements This presentation contains forward-looking statements about our business, including our commercialization timeline, plans and outlook, future financial results and events that have not yet occurred. Operating in the pharmaceutical industry inherently involves significant risks and uncertainties, including the risks outlined under "Risk Factors" and elsewhere in our Annual Report on Form 10-K and our other filings made with the SEC from time to time. Our actual results may differ materially from our expectations due to these risks and uncertainties, including our near-term dependence on the success of our lead product candidate, Silenor(r), and factors relating to ability to raise sufficient capital, competition, intellectual property protection, industry environment, and other matters. Somaxon undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. 2 |

| Executing Our Strategy 3 In-license Clinic Drug development 2 Phase II, 4 Phase III Studies NDA Filing, FDA Approval Established co-promote partnership Launched Silenor for insomnia Specialty Pharma Company 3Q 2010 3Q 2010 1Q 2010 2006 2003 |
| Investment Attributes Streamlined specialty pharmaceutical companyCentral nervous system therapeuticsFocused on high value branded pharmaceuticalsEstablished commercial organizationFocused on specialty marketsManagement with deep commercial expertiseSilenor: Highly differentiated product, meaningful revenue potentialAttractive product profileStrong IP and life-cycle opportunities Launching Silenor with experienced partner P&GFocused on primary care and pharmaciesDeal structure preserves asset value and company autonomy 4 |
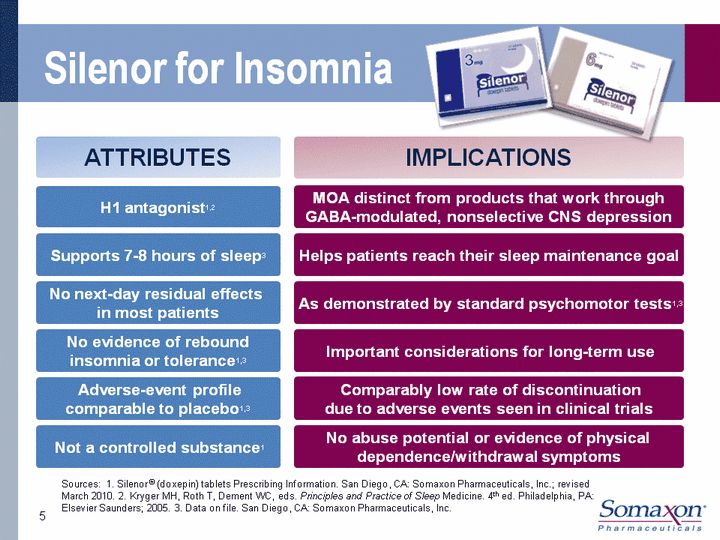
| Silenor for Insomnia 5 H1 antagonist1,2 Supports 7-8 hours of sleep3 No next-day residual effects in most patients No evidence of rebound insomnia or tolerance1,3 Adverse-event profilecomparable to placebo1,3 Helps patients reach their sleep maintenance goal As demonstrated by standard psychomotor tests1,3 Important considerations for long-term use Comparably low rate of discontinuationdue to adverse events seen in clinical trials Not a controlled substance1 No abuse potential or evidence of physical dependence/withdrawal symptoms MOA distinct from products that work through GABA-modulated, nonselective CNS depression Sources: 1. Silenor (r) (doxepin) tablets Prescribing Information. San Diego, CA: Somaxon Pharmaceuticals, Inc.; revised March 2010. 2. Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. 3. Data on file. San Diego, CA: Somaxon Pharmaceuticals, Inc. ATTRIBUTES IMPLICATIONS |

| Insomnia Market Dynamics U.S. market under-diagnosed and under-treated70 million estimated to suffer from insomnia 14 million (20%) are currently on Rx treatmentAssociated with high costs and multiple adverse events1,2,3Annual direct and indirect costs: May be as high as $92.5 - $107.5 billionEstimated total annual cost to employers for sick days: $44.5 billionImpaired memory, cognitive and social functioningIncreases risk for comorbidities2,3Psychiatric disorders; Cardiovascular diseaseImpaired glucose tolerance and diabetes; ObesityMusculoskeletal problems; Chronic pain 6 Sources: IMS Health data. 1. Hossain JL, Shapiro CM. Sleep Breath. 2002;6(2):85-102. 2. Roth T, Culpepper L. Clin Symp. 2008;58(1):3-32. 3. NIH Consensus and State-of- the-Science Statements. 2005;22(2):1-30. |

| Market Insights Physician InsightsInsomnia is a complicated, widespread and ever-increasing problem Agree that final 1/3 of night's sleep is importantClinical experience dictates product perceptions, more than data"My patients will tell me if it is effective or not"Patient InsightsInsomnia described as life marked by a sense of chaos and disorganizationGenerally suffered with moderate-to-severe insomnia for yearsSelf-medicate with OTCs before seeking Physician RxKey switch driver is lack of OTC efficacy and disease severity 7 Source: Somaxon market research. |

| Primary Issue: Sleep Maintenance 8 Silenor addresses the two most common complaints Source: National Sleep Foundation. Summary of findings: 2008 Sleep in America poll. n = 1760 respondents. Maintenance Onset Difficulty Falling Asleep 64% of patients polled had sleep problems... Frequent Nocturnal Awakenings Early Morning Awakenings Difficulty Falling Asleep 29% 42% 26% |

| Large and Accessible Market $2 billion in U.S. sales in 2009Over 67 million U.S. TRx's written in 2009Concentrated market~44,000 doctors write half the TRx's30% of highest prescribing doctors are specialistsTop decile prescribers average >1,000 Rx's /yearSolid "window of opportunity" for new treatmentsA void of new and differentiated insomnia treatments since 2006No "novel" compounds expected to launch in near term Competitive promotional spending is decreasingNumber of primary details is decreasing DTC spending down dramatically year-over-yearConsistent trend across brands 9 Source: IMS Health data. |

| Silenor Commercial Strategy 10 Target high-prescribing physiciansSomaxon sales force targets 18K specialistsP&G sales force targets 17K PCPsCombined effort reaches 40% of prescription market Deploy comprehensive promotional/marketing campaignTargeting physicians and consumers Drive patient access initiativesManaged care; Co-pay assistanceUse sampling advantage Build brand awarenessPublication strategy; medical education; public relations |

| Strategic Partnership Leverages P&G's highly regarded and tenured professional health care sales forcePrimary details for Silenor to physicians and pharmacistsEnables Somaxon to retain significant economic and long-term control of SilenorLife-cycle management: P&G has right of first negotiation to develop and market Silenor for OTC in U.S.Runs through Dec. 31, 2012; mutually renewable thereafter 11 |
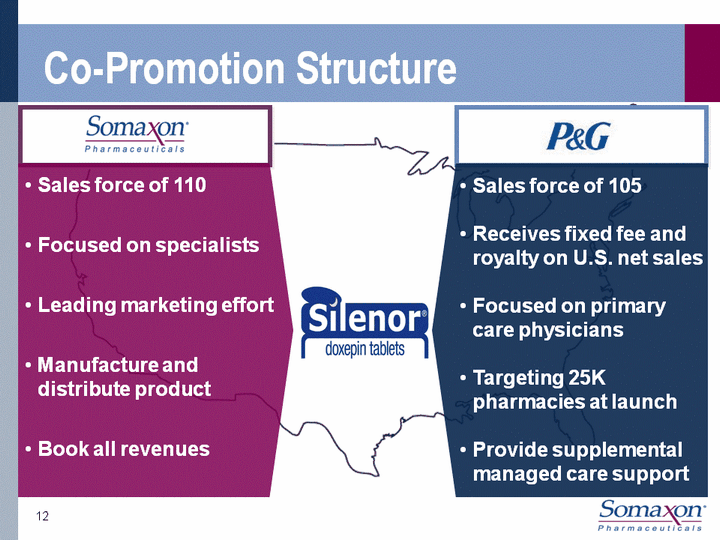
| Co-Promotion Structure 12 Sales force of 110Focused on specialistsLeading marketing effortManufacture and distribute productBook all revenues Sales force of 105Receives fixed fee and royalty on U.S. net salesFocused on primary care physiciansTargeting 25K pharmacies at launchProvide supplemental managed care support |
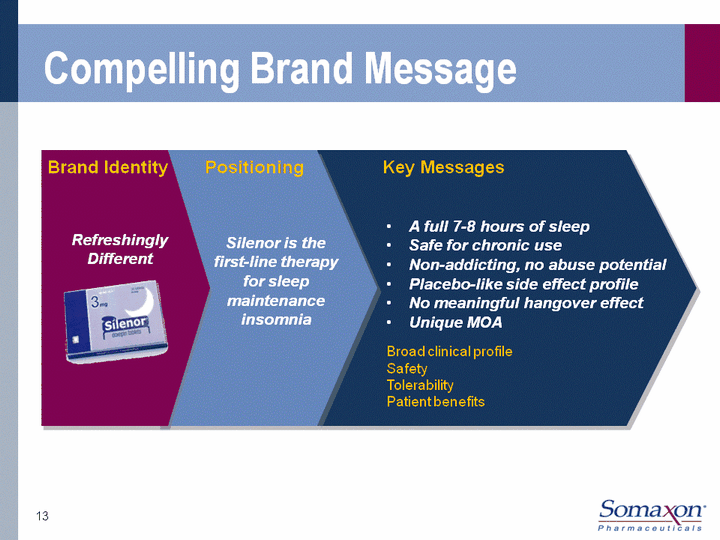
| Compelling Brand Message Brand Identity Positioning Key Messages Silenor is the first-line therapy for sleep maintenance insomnia Refreshingly Different A full 7-8 hours of sleepSafe for chronic useNon-addicting, no abuse potentialPlacebo-like side effect profileNo meaningful hangover effectUnique MOA Broad clinical profileSafetyTolerabilityPatient benefits 13 |

| Professional Campaign Integrated marketing campaign to 35K high value physiciansHigh impact brand awareness programs Supportive sales aids and branded marketing materialsNon-personal promotion to reach non-called-on prescribersWeb-based and media initiatives to reach prescribers25K targeted pharmacy callsUnique dose pack to establish Silenor brand at the pharmacySamples - a competitive advantageDrives physician access7-count Patient Starter Kit to top decile prescribers at launch4-count sample packs for sales rep delivery and direct distribution 14 |

| Comprehensive Branding 15 |

| Direct to Insomniac Campaign Internet rivals traditional DTC ads for Rx info by consumers before seeing MD1Consumers seek insomnia info in general before searching for specific medications1Physicians remain the most important source of Rx educationInternet a close second2 Physician internet use replacing traditional sources of medical info3 16 "Insomnia patients more likely than those with most other health conditions to use the internet for medical information"Trend report 2008 Sources: 1. Somaxon market research, 2008. 2. Trend Report 2008. 3. Manhattan Research |

| Direct to Insomniac Campaign Multi-faceted Outreach Strategy Web property deploymentwww.silenor.com Outreach through Web MDSearch engine optimization ( , , etc.)Public Relations Build awareness of disease and Silenor brandPoint of Care - MD OfficeIn-office videoPatient brochurePoint of Care - PharmacyDirect product messaging to OTC sleep aid and anti-depressant usersComplements P&G efforts 17 |

| Managed Care Targeting Tier 3 at launch for majority of plansMinimize step edits and quantity limits3mg /6mg priced competitively at $151.50/packNormalize the cost to the patient through co-pay assistanceFirst Rx and two refillsConfigured to be delivered as physical part of the sampleOut of pocket expense expected to be less than $1 per day to patientTarget largest/influential commercial plans for potential Tier 2 coverageDossier and formulary kit finalizedPrioritize government programs Product profile supports use in Medicare populationActively pursuing opportunity in the VA/DOD system 18 |

| Financials Financials 19 |

| Building the Enterprise Successfully launch SilenorPotential for meaningful revenueEstablish commercial presenceExtend Silenor franchiseSecure additional IPLife cycle management for Rx productPotential for OTCLeverage existing commercial operationsConsider co-promote opportunitiesFocus on growthIn-license or acquire proprietary marketed or development-stage products 20 |
| Investment Attributes Streamlined specialty pharmaceutical companyCentral nervous system therapeuticsFocused on high value branded pharmaceuticalsEstablished commercial organizationFocused on specialty marketsManagement with deep commercial expertiseSilenor: Highly differentiated product, meaningful revenue potentialAttractive product profileStrong IP and life-cycle opportunities Launching Silenor with experienced partner P&GFocused on primary care and pharmaciesDeal structure preserves asset value and company autonomy 21 |

