Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - LIGAND PHARMACEUTICALS INC | d8k.htm |
 1
1
Investor and Analyst Day
June 24, 2010
Nasdaq: LGND
Exhibit 99.1 |
 Ligand
Pharmaceuticals,
Inc.
2
2
Investor and Analyst Day
John Higgins
President and Chief Executive Officer
June 24, 2010 -
New York |
 3
3
Safe Harbor Statement
The following presentation contains forward-looking statements regarding Ligand’s
prospects, plans and strategies, drug development programs and collaborations.
Forward- looking statements include financial projections, expectations regarding
research and development programs, and other statements including words such as
“will,“ “should,” “could,” “plan,” etc.
Actual events or results may differ from Ligand’s expectations. For example, the
reverse stock split may not be completed, and the intended benefits of the reverse
stock split, expense reductions and drug development programs may not be
realized. In addition there can be no assurance that Ligand will achieve its guidance
in 2010 and 2011.
The forward-looking statements made in the presentation are subject to several risk
factors, including, but not limited to, Ligand’s reliance on collaborative
partners for milestone and royalty payments, regulatory hurdles facing Ligand’s
product candidates, uncertainty regarding Ligand’s product development costs,
the possibility that Ligand’s drug candidates might not be proved to be safe and
efficacious, the commercial performance of Ligand’s approved products, and a
possible failure to successfully combine the businesses of Neurogen and/or Metabasis
with Ligand’s business. Additional risks may apply to forward- looking
statements made in this presentation. The risk factors facing Ligand are explained in greater detail in Ligand’s, filings
with the SEC, including the most recently filed annual reports on Form 10-K and
quarterly reports on Form 10-Q, as well as other public filings.
While forward-looking statements reflect our good faith beliefs (or those of the
indicated third parties), they are not guarantees of future performance. We disclaim
any obligation to update or revise any forward-looking statements, whether as a
result of new information, future events or otherwise.
|
 4
4
Investor and Analyst Day Agenda
Welcome and Company Overview
John Higgins
Financial Highlights
John Sharp
Ligand
Partnered Asset Portfolio
Rob McKay
Promacta
Nezam
H. Afdhal, MD
Expanding the Partnership Pipeline
Syed
Kazmi, PhD
SARM Program
Shalender
Bhasin, MD
Questions & Answers / Reception |
 5
5
Investor Day
•
Ligand’s
Directors own ~9% of the company
•
Other top holders
–
Wellington
–
Biotech Value Fund
–
BlackRock
–
UBS
–
Vanguard
–
Pennant
–
Chesapeake Partners
–
DWS Investment GmbH
–
RA Capital |
 6
6
We Have Delivered
•
We have fully restructured and rebuilt Ligand
•
We have cut costs while building our asset base
•
We have met our financial guidance
•
We have advanced our pipeline
•
We have completed three valuable acquisitions |
 7
7
Our Business Philosophy
•
Run a business that is oriented toward being a financial growth story
–
Our
belief
is
we
should
not
build
a
business
around
“biotech
promises,”
instead
we
should build a business that generates cash flow and makes investors
(shareholders) money
•
Buy or develop a large portfolio of assets that can yield positive cash flow
•
Fiercely manage expenses
–
Unfortunately, this industry has managed to squander vast amounts of money on
programs, infrastructure, and elaborate public enterprises that did not warrant the
investment
–
No
guarantee
what
we
choose
to
fund
will
succeed,
but
we
will
be
very
disciplined
in the process
•
Avoid the biotech financing loop
–
Avoid costly dilution
–
Make better long-term decisions |
 8
8
Perception
Reality
•
The rewards from discovering, developing,
and commercializing a biotech drug can be
enormous
•
True, but the vast majority of programs
fail to ever reach that payoff
•
A biotech company should retain late-stage
and/or commercial rights as the economic
reward will be greater
•
The return is not necessarily greater, in
fact it might be smaller. But for certain
the risks will be greater as costs,
timelines, competitiveness and pricing
pressures in this industry have all gone
up dramatically over the last 20 years.
•
Investors and the pharma
industry as a
whole can make money by investing in a
wide array of assets and de-risking
strategies
•
BUT if an individual
biotech company is
going to increase its chances to succeed,
it has to do things differently than how
companies were run in the early 1990s
Ligand: A Business Model Built on Today’s Reality |
 9
9
Why the Ligand
Royalty Model is So Compelling
•
Integrate most
functional expertise into
the company
•
Short remaining patent life
•
Own products through
late-stage development
or commercialization
Vast portfolio of royalty
partnerships
•
1 or 2 royalty streams
Research and partnering
engine
Ligand
Today
Result
•
Unexciting
•
No growth
•
Fixed value company
•
No engine to drive new
deals
“Yesterday’s”
Royalty Model
“Yesterday’s”
FIPCO Model
Result
•
Large revenue potential
•
Substantial growth
potential
•
Substantially lower
costs and risk
Result
•
High costs and risks,
beholden to wall street for
financing
•
Questionable if the ROI
justifies the model |
 10
10
Why the Ligand
Royalty Model is So Compelling
•
Integrate most
functional expertise into
the company
•
Short remaining patent life
•
Own products through
late-stage development
or commercialization
Vast portfolio of royalty
partnerships
•
1 or 2 royalty streams
Research and partnering
engine
Ligand
Today
Result
•
Unexciting
•
No growth
•
Fixed value company
•
No engine to drive new
deals
“Yesterday’s”
Royalty Model
“Yesterday’s”
FIPCO Model
Result
•
Large revenue potential
•
Substantial growth
potential
•
Substantially lower
costs and risk
Result
•
High costs and risks,
beholden to wall street for
financing
•
Questionable if the ROI
justifies the model |
 11
11
Why the Ligand
Royalty Model is So Compelling
Ligand
Today
Vast portfolio of royalty
partnerships
Research and partnering
engine
Result
•
Large revenue & ER
potential
•
Substantially lower
costs and risks
•
Over 30 programs
•
Long patent lives
•
Some high royalties that
approach a profit split
•
Efficient research
focused on licensing
at early inflection
points |
 12
12
Ligand’s
Potential Downside
Individual project set-backs
Slower growth
Partners drop programs
Ligand’s
Potential Upside
Blockbuster product approved/in-development
Near-term profitability
Well funded/financially disciplined
More royalty partnerships than any peer co
Attractive fully-owned
pipeline
Substantial calendar of news flow
Large NOLs
Risk
Reward
The Investment Proposition: Risk vs. Reward
We believe the upside reward is substantially greater than the downside risk
|
 13
13
Illustrative Sum of the Parts
•
Cash $30 million
•
Over $500 million in NOLs
with estimated ~$100 million net present value
•
Avinza
–
up to $25 million in net present value -
royalty annuity through 2017
•
Promacta
Currently marketed and in development for five other indications
12 years remaining patent life
Tiered royalties averaging to about 9%
How big will this drug be?
•
SERMS –
approved, could launch soon
•
CXCR2,
P-38,
Dinaciclib,
IL-9
–
High-quality,
mid-stage
programs
•
Over 30 partnered programs –
news flow and milestone potential
•
10 fully owned internal programs |
 14
14
Sum of the Parts Illustration
Avinza
•
Promacta
•
SERMs
•
30 other funded programs
•
Fully owned pipeline
Nols
Cash
$180
million Current
Market Capitalization
“Biotech”
Value
How much more
will this grow
over the next few
years?
~$25
~$100
~$30
$155 million |
 15
15
Ligand…Why Now?
•
Given reduced expenses and revenue outlook, we project turning
profitable on an operating basis next year
•
Promacta
is a “now”
story
–
New trials have initiated recently
–
New territories are launching for ITP
–
HepC
P-III trials are winding down with data expected in about a year
•
Ligand
just closed a few great acquisitions which have more than
tripled our pipeline and partnered assets
•
Partners have announced several positive developments in past few
months
•
Data is coming out of our internal pipeline over the next six months
|
 16
16
A View of the Future
•
2 marketed
products
:
Avinza
and
Promacta
•
2 approved
products
pending
launch:
Conbriza
and
Fablyn
•
2 products
announced
to
have
NDA
filings
by
end
of
2012:
Aprela
and
Acadesine
•
9 drug candidates could launch between 2014 and 2018
CXCR2
BACE
P-38
JNK2
CDK
LXR
IL-9
FXR
Hepatitis |
 17
17
Potential Significant Revenue Expansion Over Next Several Years
Illustrative Growth
2016
Promacta
2012
Avinza
Promacta
Conbriza
Avinza
Aprela
Conbriza
Fablyn
Aprela
Acadesine
Fablyn
CDK
>$20 million royalties
CXCR2
Plus license fees
P-38
IL-
9
>$200 million royalties
Plus license fees |
 18
18
2012
2016
>$20 Mil.
>$200 Mil.
Potential Significant Revenue Expansion Over Next Several Years
•
10 fold increase in royalties. Growth due to:
–
New product launches
–
New territories
–
New indications
–
Increasing royalty rates upon sales growth
•
Potential for nearly $100 million in milestones over the same period
•
Annual expenses over this projection period estimated to be $15 to $20
million Illustrative Royalty Revenue Growth |
 Ligand
Pharmaceuticals, Inc.
19
19
Financial Update
Delivering Good Performance
John Sharp
Vice President, Finance
and Chief Financial Officer |
 20
20
Highlights
•
Operating Expenses
•
Overview of Net Operating Losses
•
Financial Guidance
•
Reverse Stock Split |
 21
21
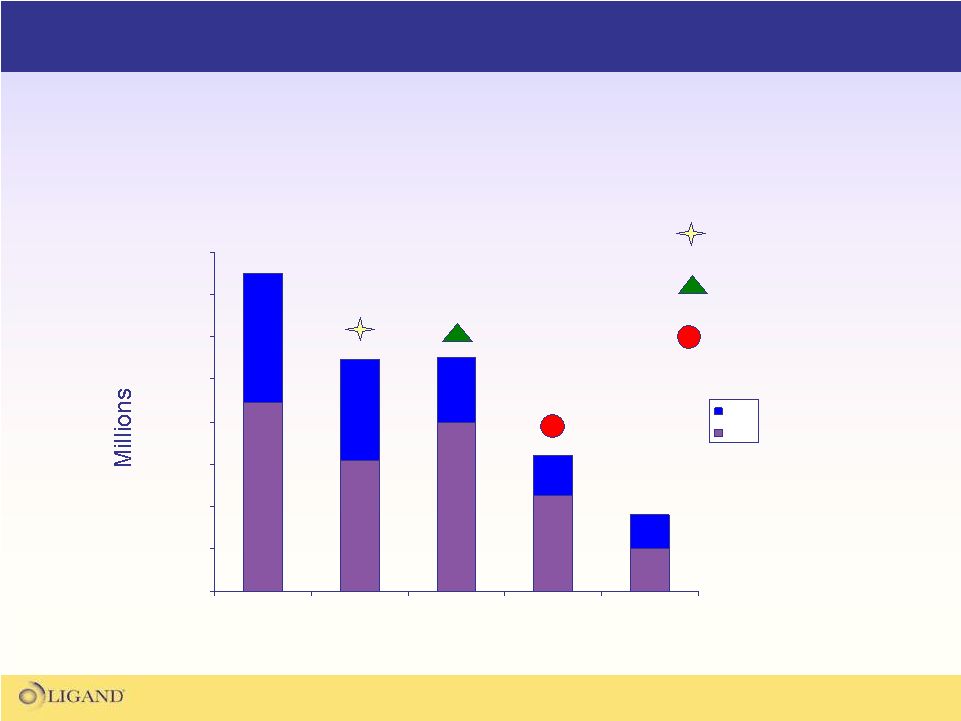
Operating Expense Trend
$0.0
$10.0
$20.0
$30.0
$40.0
$50.0
$60.0
$70.0
$80.0
2007
2008
2009
2010
2011*
G&A
R&D
* High end of expense guidance range
•
Ligand
has significantly reduced expenses over the last several years
•
During the same period, the company closed 3 acquisitions while
significantly expanding its asset base
Pharmacopeia Acquisition
Neurogen Acquisition
Metabasis Acquisition |
 22
22
Value of Net Operating Loss Carryforwards
(NOL)
•
Ligand
has
accumulated
substantial
NOL’s
through
our
operating
history and acquisitions
•
NOL’s
should provide significant relief on taxable income if the
company turns profitable
•
NOL’s
as of December 31, 2009 = $514 million
•
Due
to
tax
code,
NOL’s
are
limited
in
the
quantity
and
the
timing
in
which
they
can
be
used,
so
we
do
not
expect
to
get
a
full
offset
on
taxable income immediately
•
Estimated
net
present
value
of
NOL’s
=
~$100M
•
In near-term, the NOL tax should reduce federal tax rate from 34%
down to 2% (AMT) |
 23
23
Financial Guidance
2010 Guidance:
•
Revenue approximately $25 million
Combination of royalty, contract payments and milestones
•
Operating expenses approximately $30 million
One-third G&A, two-thirds R&D
•
Cash at year end projected to be greater than $30 million
2011 Guidance:
•
Operating
expenses
projected
to
be
in
range
of
$15
-
$18
million
•
Projecting turning profitable on an operating basis and having
positive cash flow from operations |
 24
24
Reverse Split
•
Over Ligand's
history, company raised money through a series of
highly dilutive financings
•
Company initiated restructuring about three years ago but never
addressed its bloated share base
•
Now
is
an
appropriate
time
given
the
progress
of
the
company
and
our
outlook for the business
•
A lower share count and higher stock price provides numerous benefits
including improving trading dynamics, lower costs and increased per
share reported amounts
•
The middle of the reverse split range would yield a share count of
approximately 16 million shares versus 118 million shared currently
outstanding |
 25
25
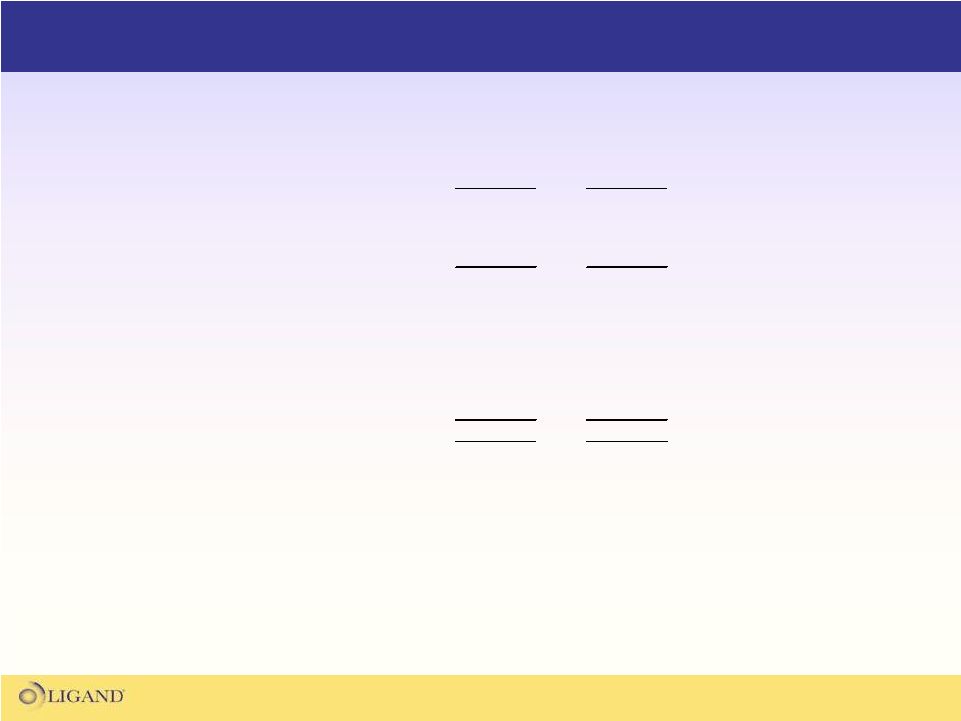
Illustrative Income Statement Assuming Reverse Split
($ in millions)
Pre-split
Post-split
Revenue
40.0
$
40.0
$
Expenses
20.0
20.0
EBIT
20.0
20.0
Interest & other
1.0
1.0
Taxes
(0.4)
(0.4)
Net income
20.6
$
20.6
$
EPS
0.18
$
1.31
$
Shares outstanding
117.6
15.7
*
* assumes midpoint of split range;1-for-7.5 |
 Ligand
Pharmaceuticals, Inc.
26
Ligand
Partnered Asset Portfolio
Value Through Shots on Goal
Rob McKay
Associate Director,
Business Development |
 27
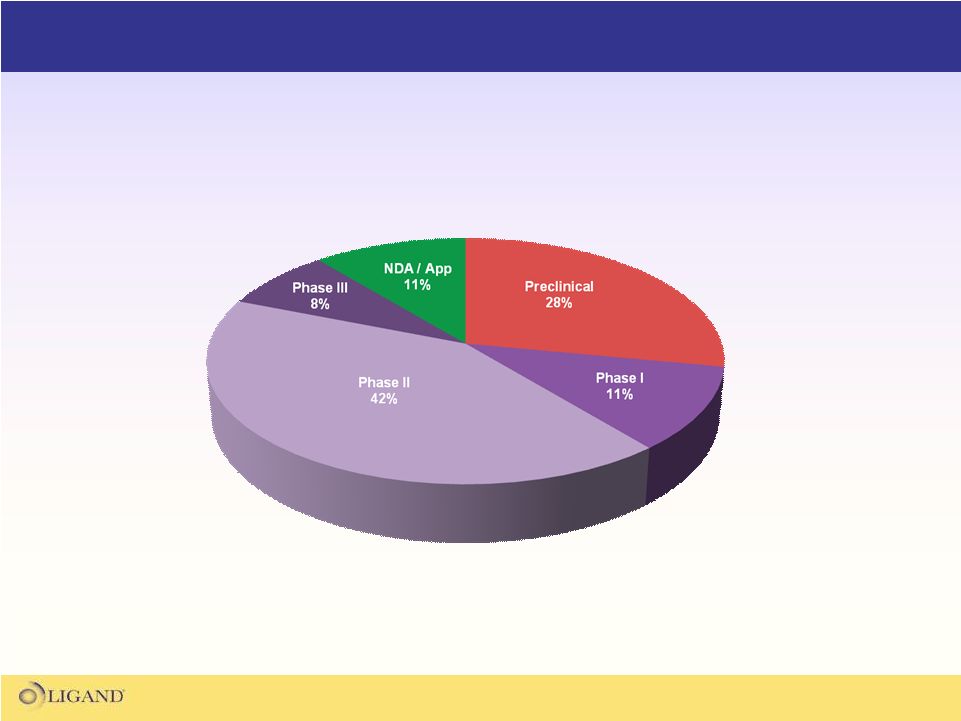
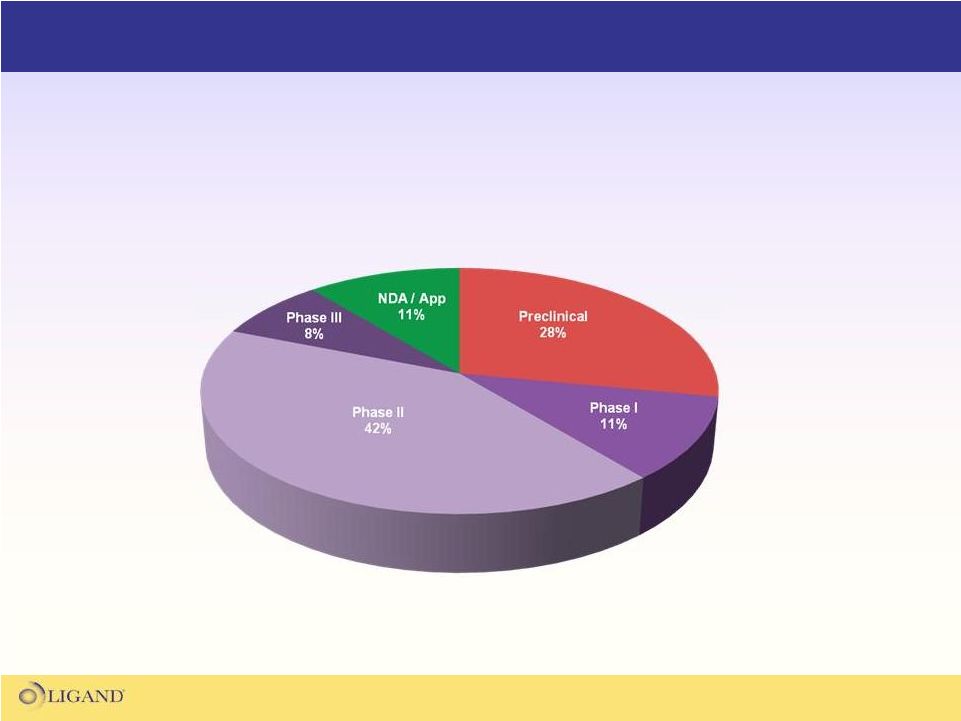
Ligand’s Partnered Asset Portfolio
Ligand has a very large portfolio of partnered programs
which are the core of Ligand's financial growth model |
 28
Ligand’s Partnered Asset Portfolio
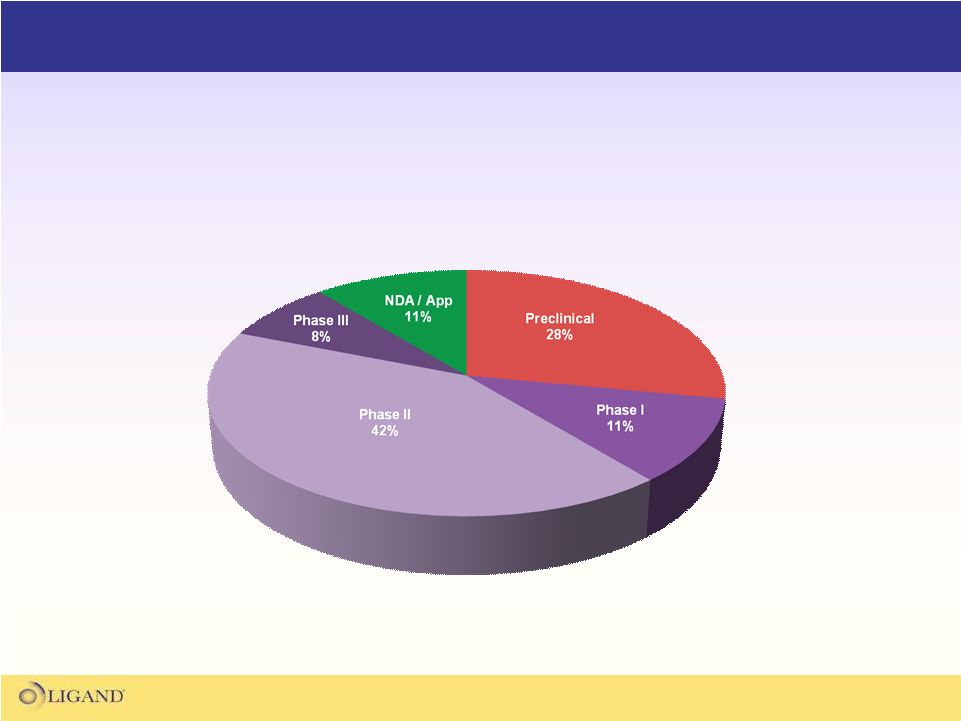
The Ligand partnered portfolio consists of 33 fully funded
programs that have the potential to generate over $500M
in milestones and royalties on future sales |
 29
Ligand’s
Partnered Asset Portfolio
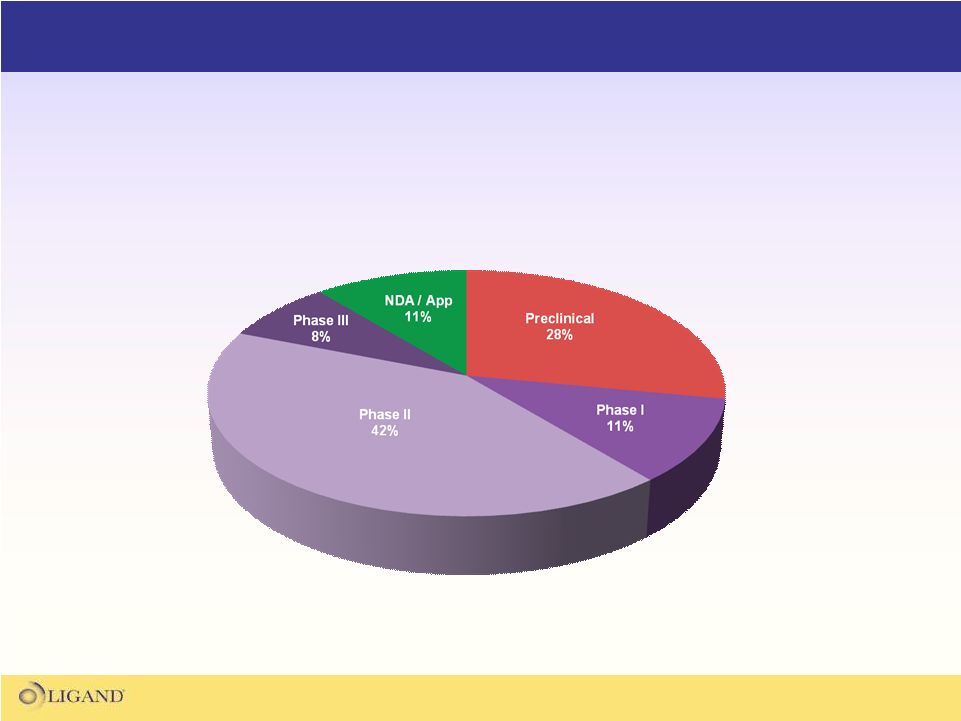
Significant potential for new product launches
and expanded indications the next several years
*
* |
 30
Ligand’s
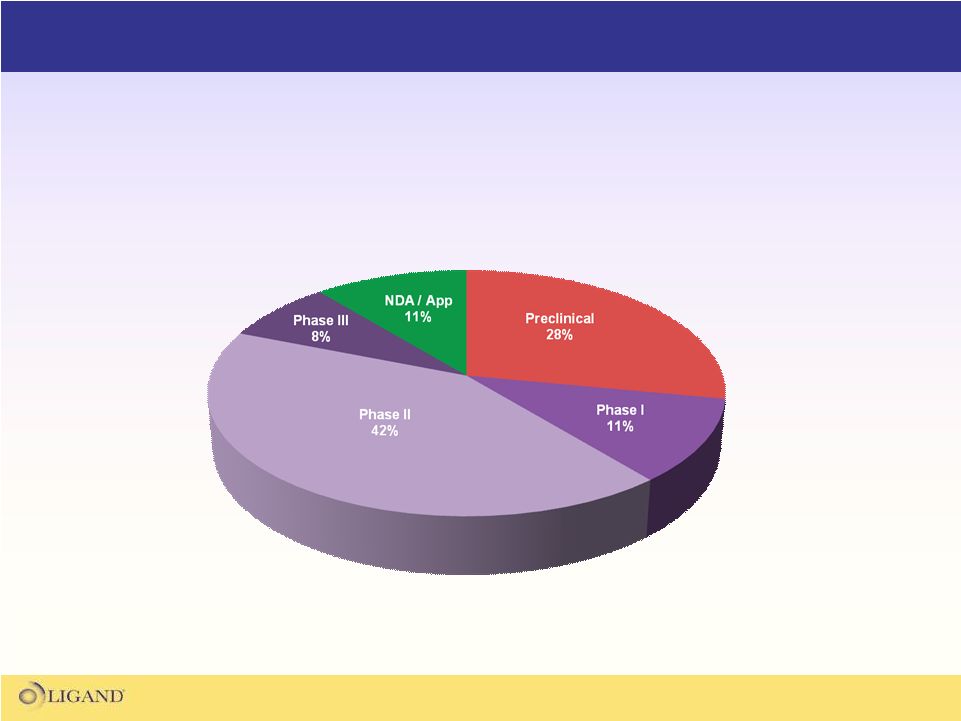
Partnered Asset Portfolio
Over half of the partnered portfolio
is in mid-late stage clinical trials |
 31
Ligand’s
Partnered Asset Portfolio
Significant news flow over the next 18 months
•
Aprela
NDA Filing
•
Promacta-Japan ITP Approval
•
Promacta
HepC
PIII Data
•
SARM PI Data
•BACE PII Initiation
•
IL-9 PII Completion
•
Conbriza
EU Launch
•
Oral EPO Clinical Candidate
•
Glucagon Clinical Candidate
•
TR-beta Clinical Candidate
•
Dinaciclib
PII Completions
•
Acadesine
PIII Data
•
GCGR PI Initiation
•
Promacta
PII Oncology Data
•
Roche HepC
PII Initiation |
 32
Quality Partnerships and Portfolio Value
•
Ligand's
promising long-term growth is firmly based on finding partners who can
deliver our assets into the hands of patients
•
Ligand’s
ideal partners have the following qualities:
–
The expertise to bring each unique asset through development into the
marketplace
–
A need for our product in their portfolio
–
The resources to make it all happen
Long-term Ligand shareholder value is highly based
on the quality of our partnerships |
 33
Examples of Value Through Quality Partnerships
Assessing the value of Ligand’s partnered portfolio
by examining two premier partnerships |
 34
The Merck-Ligand
Collaboration
•
A pipeline of innovative products in established or emerging Merck franchises
•
The potential for milestone and royalty revenue in the near and
long-term •
Regular news flow from a variety of programs
Phase III
CABG
Phase II
COPD
Phase II
Oncology
Phase I
Alzheimer’s
Pre-clinical
Multiple assets under the control of one of the
world’s leading pharmaceutical companies has the potential to
produce enormous value for Ligand
shareholders
*Acadesine
was licensed to Merck by PeriCor
5 Programs
BACE
Dinaciclib
SCH 527123
Acadesine* |
 35
•
What does SCH 527123 do?
Antagonist of chemokine
receptor 2, blocking the activation
of neutrophils.
Reduces airway damage and mucous build up seen in
diseases like COPD
•
What stage of development is SCH 527123 at?
Phase II study for COPD. Expected to be completed in 3Q12
•
Why is SCH 527123 interesting?
12M COPD patients in US with no effective long-term
treatment
Part of the respiratory franchise for Merck
Novel NME with strong patent protection
SCH 527123 (CXCR2 Antagonist)
SCH 527123 is a potential first-in-class, blockbuster medication
for
pulmonary
obstruction
diseases
like
COPD
Airway Obstruction in COPD
(thinkcopddifferently.com-2010) |
 36
•
What does Dinaciclib
do?
Inhibitor of cyclin
dependent kinase
(CDK)
Inhibiting CDK should block cell-cycle progression and
promote apoptosis
•
What
stage
of
development
is
Dinaciclib
at?
Multiple Phase II studies for cancer. The studies will
complete in late 2010 through 2012
•
Why is Dinaciclib
interesting?
Merck is making a large commitment to becoming a player in
the oncology field
Novel kinase
inhibitors are the next wave of therapeutics in
the oncology space
Novel NME with strong patent protection
Dinaciclib
(CDK Inhibitor)
Dinaciclib
is a pro-apoptotic cyclin
dependent kinase
inhibitor with potential anti-neoplastic
activity
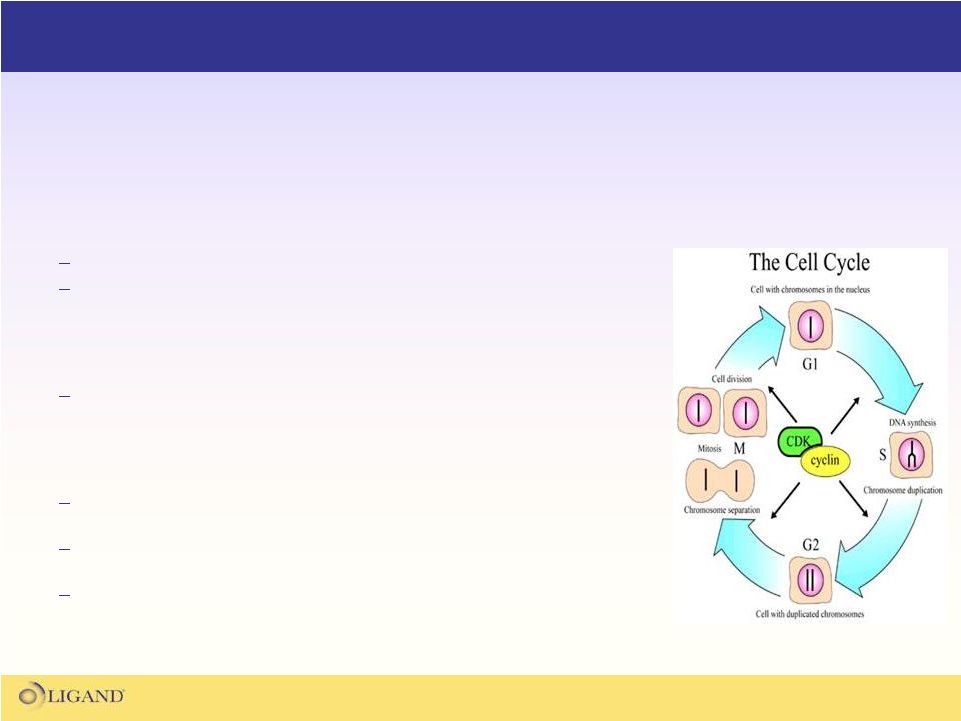
CDK Control of the Cell Cycle
(sandwalk.blogspot.com-2009) |
 37
•
What does a BACE Inhibitor do?
BACE
inhibitors
block
the
activity
beta-secretase
(BACE)
Inhibiting
beta-secretase
should
lower
the
amount
of
beta-
amyloid
protein
produced
in
the
brain,
limiting
the
plaque
formation
that
is
thought
to
be
a
cause
of
Alzheimer’s
disease
•
What stage of development is BACE Program at?
Merck
has
a
BACE
molecule
in
Phase
I
•
58% reduction in beta-amyloid
protein seen in single dose PI
study
Ligand
anticipates a late 2010 Phase II initiation
•
Why are BACE inhibitors interesting?
5-6M patients in the US with little therapeutic options
•
Estimated at more than 15M world-wide
Completely novel mechanism of action
Novel NME with strong patent protection
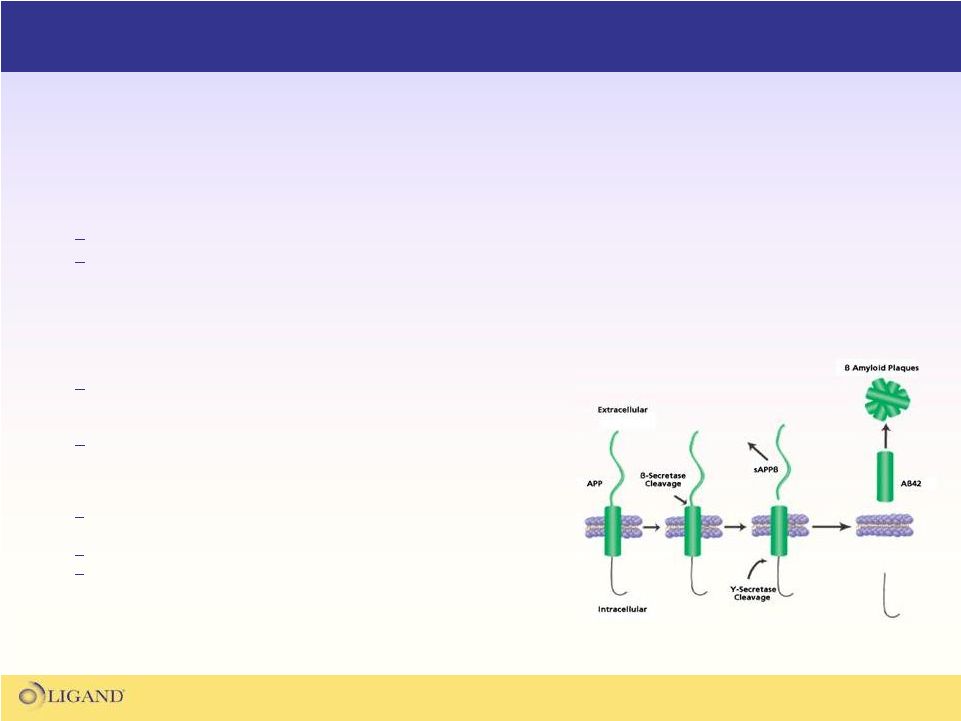
BACE Inhibitor Program
The Merck BACE inhibitor program is developing a novel, first-in-class
therapeutic
for
Alzheimer’s
disease
by
targeting
beta-secretase
BACE Cleavage of beta-amyloid
Protein
(sigmaaldrich.com-2008) |
 38
Merck Collaboration Summary
The Merck collection of assets has the potential to produce one of the next
blockbuster drugs in the Ligand
asset portfolio
Phase III
CABG
Phase II
COPD
Phase II
Oncology
Phase I
Alzheimer’s
Pre-clinical
The opportunity is for any one (or several) of these assets
to become the next Promacta
for Ligand
5 Programs
BACE
Dinaciclib
SCH 527123
Acadesine* |
 39
The GSK-Ligand
Collaboration
•
Increasing royalty revenue over time from expanded indications
•
Regular news flow of filings and approvals
•
Regular news flow from a variety of programs
•
2nd Generation TPO Receptor Agonist licensed to GSK as a follow-on
molecule to Promacta
Approved
ITP
Phase III
HepC
Phase II
Oncology
Phase II
Thrombo
Phase I
Other Indications
GSK and Ligand
have a long-standing collaboration to develop
thrombopoietin
receptor agonists for treatment of thrombocytopenia
Promacta
GSK 2285921*
Promacta
Promacta
Promacta |
 40
•
What does Promacta
do?
Promacta
is a once daily oral medicine that activates
the thrombopoietin
(TPO) receptor
Activation of the TPO receptor causes an increase in
the production of platelets
•
What is Promacta
approved for?
Promacta
is approved for ITP in multiple countries
•
What are the next indications for Promacta?
Promacta
is in Phase III for HepC-related
thrombocytopenia and we project it to launch in 2012
Promacta
is in Phase II for thrombocytopenia related
to various cancer types
Promacta
Facts
Promacta
is a first-in-class therapy for raising platelet levels
in patients experiencing thrombocytopenia |
 41
•
GSK
is
currently
running
two
potentially
large
Phase
III
studies
in
HepC
patients
Study 1 (PEGASYS/Ribavirin)
Study 2 (PEGINTRON/Ribavirin)
•
Both
studies
are
randomized,
double
blind,
placebo
controlled
in
750
patients
currently
on
peginterferon/ribavirin
•
Primary
outcome
is
Sustained
Virologic
Response
(SVR)
6
months
after
treatment
•
Data is expected from both in 3Q11
•
Phase II data showed
65% of patients on 75mg daily of Promacta
finished viral therapy
course, compared to 6% on placebo.
•
10% of all HepC
patients cannot finish viral therapy due to thrombocytopenia, resulting
in a sub-optimal therapeutic response
Promacta
and HepC-Related Thrombocytopenia
The ongoing Phase III studies for HepC-related thrombocytopenia
are the basis for a large expansion of the Promacta
value |
 42
•
The platelet segment is the next frontier in the multi-billion dollar
hematology market
•
Promacta and its backup compound will build a long-term platelet franchise
for GSK/Ligand
•
GSK is expending large amounts of resources and money to expand
Promacta markets and indications
Two 750 patient studies for HepC-related thrombocytopenia
•
Data expected in 2H11
Currently in multiple PI and PII studies for cancer-related thrombocytopenia
Filings and approvals in new countries
•
ITP approval in Japan expected in 2H10
Promacta: Reasons for Excitement
Ligand shareholders have many
reasons to be very excited about Promacta |
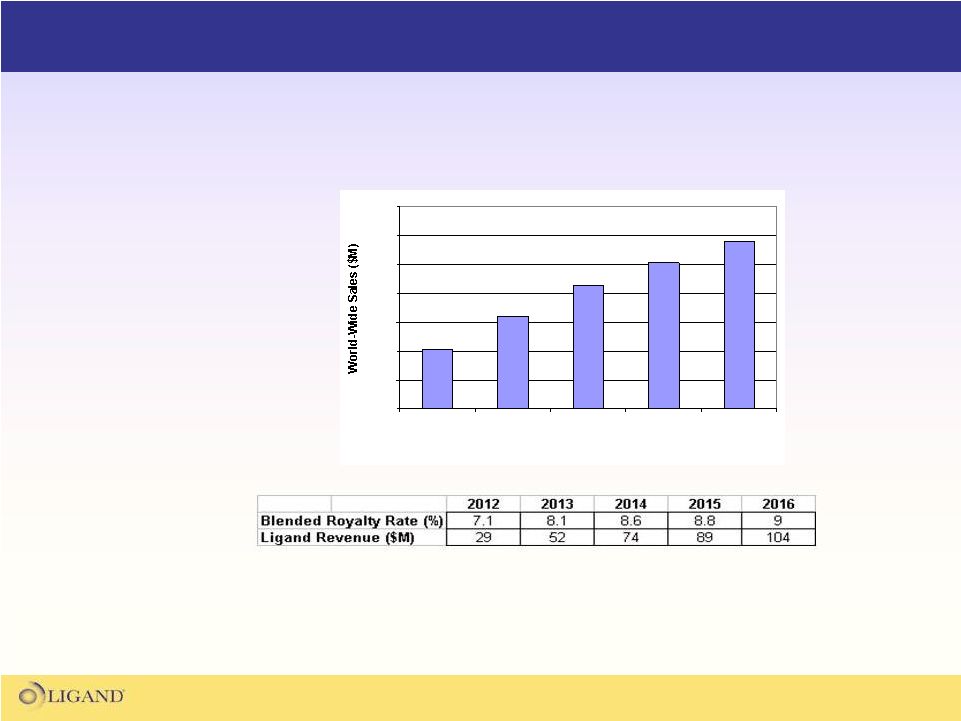 43
What Promacta
Means to Ligand
Independent Analyst
Projected Sales
for Promacta*
Potential royalty revenues from Promacta
are the
basis for transformative growth at Ligand
0
200
400
600
800
1000
1200
1400
2012
2013
2014
2015
2016
Year
Yearly Revenue
Projections for Ligand
from Promacta
Sales**
*Projected sales figures are the average projections from 6 independent analyst
groups, including
Bank
of
America-Merrill
Lynch,
Credit
Suisse,
JP
Morgan,
Morgan
Stanley,
Nomura,
and
UBS
**There can be no assurance these results will be achieved. Projections derived
from analyst revenue estimates. |
 Ligand
Pharmaceuticals, Inc.
44
Promacta
and its Role in Treating
HepC-Related Thrombocytopenia
Nezam
Afdhal, M.D.
Gastroenterology Chief of Hepatology,
Beth Israel Deaconess Medical Center |
 The
Role for Eltrombopag in the Treatment of HepC-Related
Thrombocytopenia N. Afdhal M.D
Beth Israel Deaconess Liver Center
Harvard Medical School |
 Agenda
Eltrombopag Background
Conclusions from the ELEVATE Study
Eltrombopag PII HepC Study Results
The ENABLE 1 and ENABLE 2 PIII Studies |
 The
Unmet Medical Need for Thrombocytopenia Thrombocytopenia is a major factor in
patients being unable to achieve a desired clinical outcome in dozens of
diseases Currently estimated to be nearly two million patients annually in
the US who need to be treated for thrombocytopenia
Current non-drug techniques used to increase platelets (i.e. platelet
transfusion, splenectomy) are costly, risky, and inconvenient.
The need for a more convenient and effective method for combating
thrombocytopenia is clear |
 Liver
Disease–Associated Thrombocytopenia
Platelet counts may be as low as
20,000–40,000/µL
Prevalence of thrombocytopenia
increases with severity of liver disease
Degree of thrombocytopenia correlated
with severity of liver disease
Thrombocytopenia predictive of
reduced 5-year survival
Thrombocytopenia may develop or
worsen with interferon-based therapy
Degree of Liver
Damage
Thrombocytopenia
Prevalence
Normal liver
2.3%
Fatty liver
5.1%
Chronic hepatitis
20.3%
Advanced liver
disease
31.8% |
 Eltrombopag
Thrombopoietin
receptor agonist
Oral, once-daily tablet
Induces megakaryocyte
proliferation and differentiation
Increases platelet counts in
patients with HCV
1
1. McHutchison
J, et al.N
Engl
J Med.
2007;357:2227–2236. |
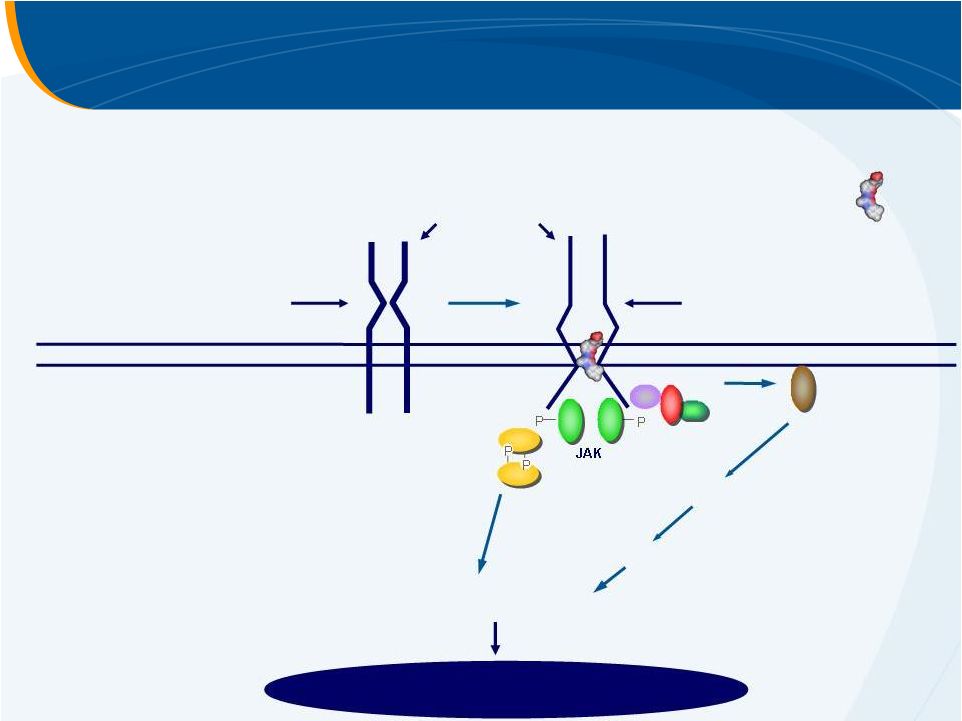 Cytoplasm
STAT
RAS/RAF
MAPKK
p42/44
SOS
SOS
GRB2
SHC
Cell membrane
Eltrombopag
–
Mechanism of Action
thrombopoietin
receptor
inactive receptor
active receptor
Signal Transduction
eltrombopag
Increased platelet production |
 Eltrombopag Clinical Studies
Conclusions from the ELEVATE Study |
 Eltrombopag
in Chronic Liver Disease Patients
with Thrombocytopenia Undergoing an Elective
Invasive Procedure: Results from ELEVATE,
a Randomised Clinical Trial
N.
Afdhal,
E.
Giannini,
G.N.
Tayyab,
A.
Mohsin,
4
J-W.
Lee,
5
A. Andriulli,
6
L. Jeffers,
7
J. McHutchison,
8
F. Campbell,
9
N. Blackman,
10
D. Hyde,
9
A. Brainsky,
11
D. Theodore
12
1. Division of Gastroenterology/Liver Center, Beth Israel Deaconess Medical Center,
Boston, MA, USA; 2. Gastroenterology Unit, Department of Internal
Medicine, University of Genoa, Genoa, Italy; 3. Department of Medicine,
Gastroenterology and Hepatology, Post Graduate Medical Institute, and Lahore
General Hospital, Lahore, Pakistan; 4. Department of Gastroenterology, Services
Hospital Lahore, Services Institute of Medical Sciences, Lahore,
Pakistan; 5. Department of Internal Medicine, Inha
University Hospital and
School of Medicine, Incheon, Korea; 6. Department of Internal Medicine,
Division of Gastroenterology, Casa Sollievo Sofferenza Hospital,
San Giovanni Rotondo, Italy; 7. Center for Liver Diseases, University of
Miami, Miller School of Medicine, Miami, FL, USA; 8. Division of
Gastroenterology, Duke University Medical Center, Durham, NC, USA; 9. Clinical Development,
GlaxoSmithKline, Stockley
Park, Uxbridge, UK; 10. Biometrics and Epidemiology, GlaxoSmithKline,
Collegeville, PA, USA; 11. Clinical Development, GlaxoSmithKline,
Collegeville, PA, USA; 12. Clinical Development, GlaxoSmithKline, Research Triangle Park, NC, USA
1
2
3 |
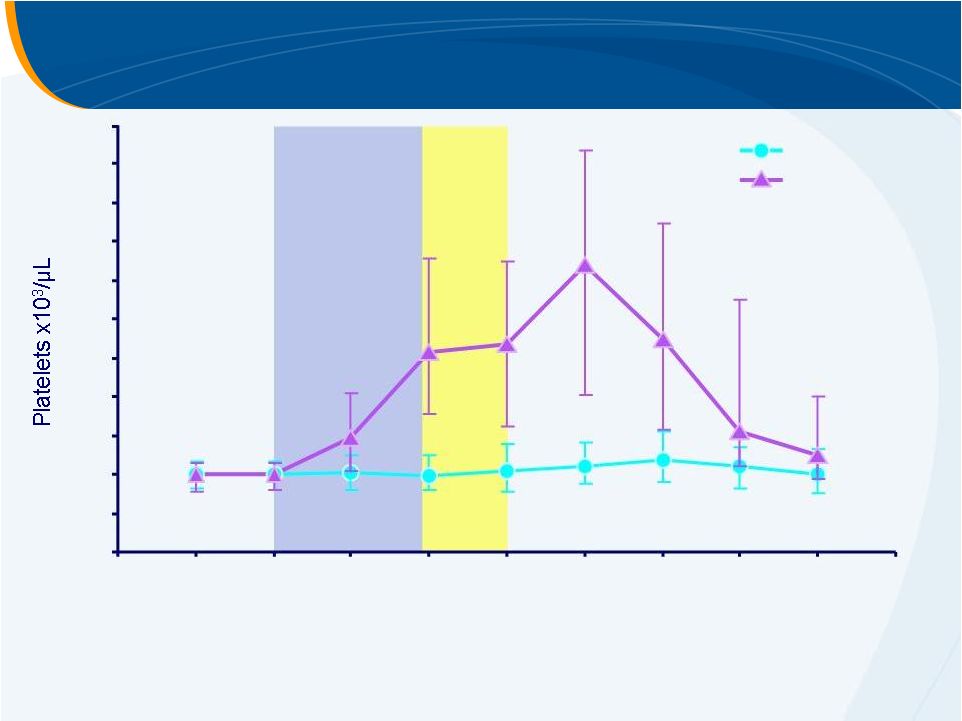 Median Platelet Count from ELEVATE
147
144
145
141
139
134
132
131
50
49
128
125
116
125
120
117
125
127
Placebo
Eltrombopag
n =
n =
Number with available data:
Eltrombopag
Placebo
0
20
40
60
80
100
120
140
160
180
200
220
Screening
Day
1
Day
8
Day
15
Day
16–19
7
DFU
14
DFU
21
DFU
30
DFU
Median (interquartile
range)
Treatment
Period
Procedure |
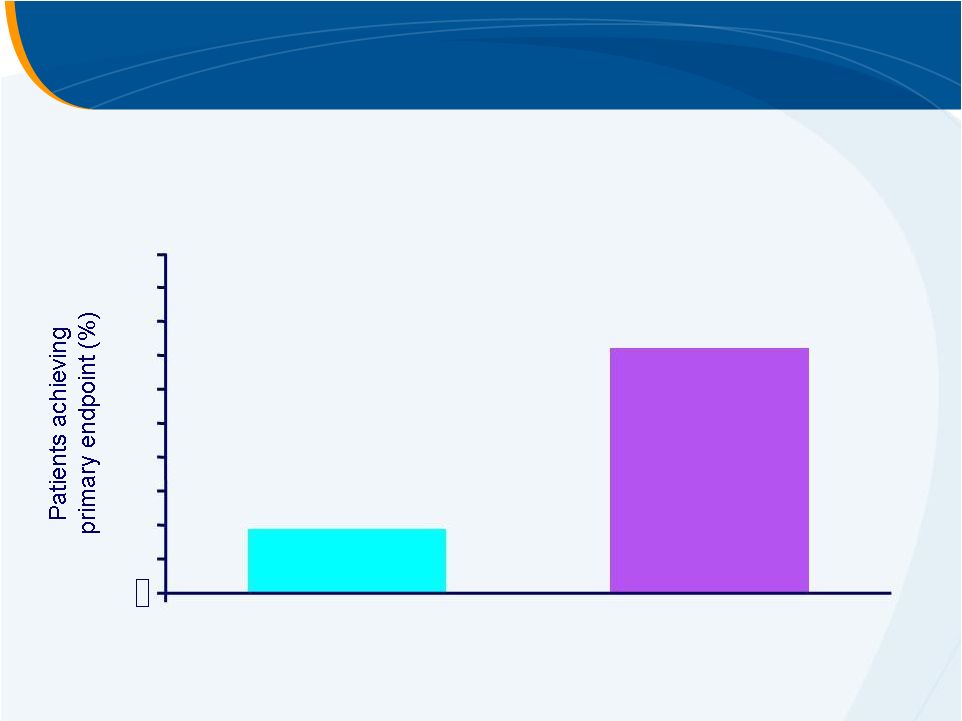 Primary Endpoint: Avoiding Platelet
Transfusions with Elective Invasive Procedure
Difference 53% (95% CI: 43, 62)
p
< 0.0001
0
10
20
30
40
50
60
80
100
Placebo
(N = 147)
Eltrombopag
(N = 145)
70
90
72%
19%
n = 28
n = 104 |
 Selected Adverse Events
Placebo
(N = 145)
Eltrombopag
(N = 143)
n (%)
n (%)
Bleeding
25 (17)
19 (13)
Thrombotic event
2 (1)
6 (4)
Ocular (focus on cataracts / visual
acuity decrease)
6 (4)
6 (4)
Malignancies*
1 (<1)
1 (<1)
* Basal cell carcinoma (Grade 2) reported for one patient receiving placebo and B cell lymphoma (Grade
4) reported for one patient receiving eltrombopag; neither was considered to be related to
treatment by the investigator. |
 Summary of Thrombotic Events
Thrombotic event
Temporal
relationship to last
dose
Temporal
relationship to
procedure
Platelet count at
event (Gi/L)
Procedure
PV / SMV thrombosis
+1
–6 days
417
Brain tumour
resection
PV thrombosis
+5
+4 days
288
Oesophageal
variceal ligation
SMV thrombosis
+8
+7 days
235
Dental extraction
SMV / mesenteric
thrombosis
+9
+7 days
289
HCC ablation
SPV thrombosis
+14
+13 days
241
TACE
PV thrombosis
+38
+34 days
33
Oesophageal
variceal ligation
Acute MI
+20
+19 days
83
Colon resection
Non-occlusive PV and
mesenteric thrombosis
+128
+128 days
Unknown
Oesophagoduo-
denoscopy
PV, portal vein; SMV, superior mesenteric vein; SPV, spleno-portal vein; MI,
myocardial infarction Eltrombopag
Placebo |
 Thrombotic Events and Platelet Count
Platelet count >200,000/µ
L YES
NO
47 patients
(16%)
241 patients
(84%)
YES = 5 (10.6%)
NO = 42 (89.4%)
YES = 3 (1.2%)
NO = 238 (98.8%)
TEs |
 Conclusions
Eltrombopag 75 mg for 14 days
–
Reduced the need for platelet transfusions in CLD patients
with thrombocytopenia undergoing elective invasive
procedures
–
Increased platelets during treatment period and up to 2
weeks following treatment
–
Similar incidence of adverse events and serious adverse
events
–
More thrombotic events in the eltrombopag group |
 Eltrombopag Clinical Studies
HepC Studies |
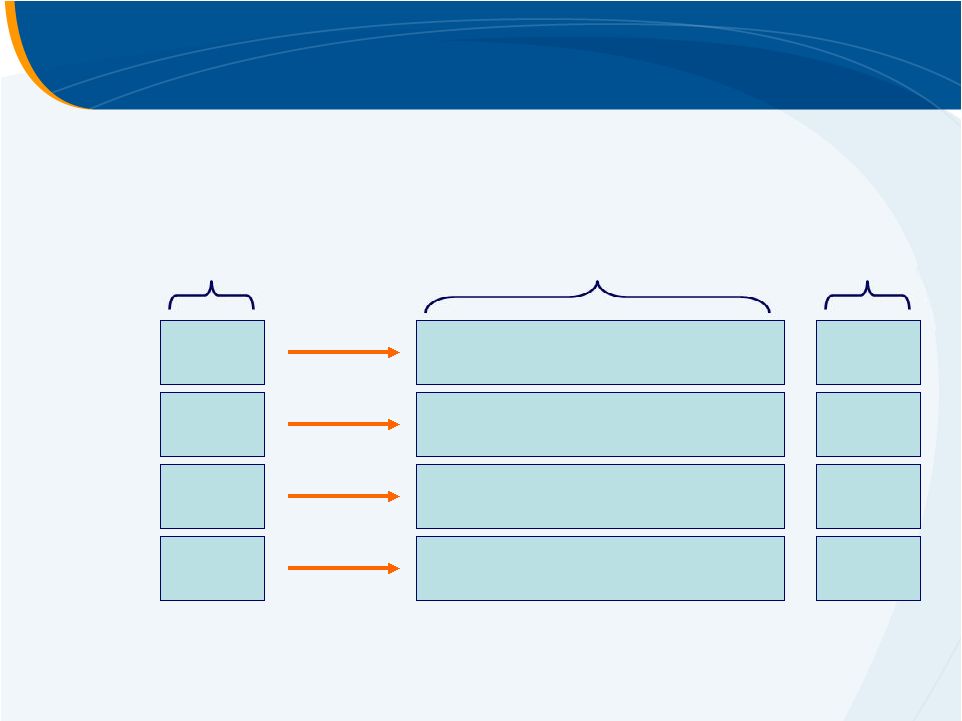 Eltrombopag
PII HepC
Study Design
4 wk
12 wk
PEG-IFN + ribavirin
+ eltrombopag
Pre-antiviral
Eltrombopag
4 wk
4 wk
4 wk
12 wk +
Eltrombopag
12 wk
same here
12 wk
same here
INITIATE
antiviral Rx if platelets
>70–100
K/µL 30 mg
50 mg
75 mg
PART
1 PART
2 4 wk
4 wk
4 wk
4 wk
Follow-up |
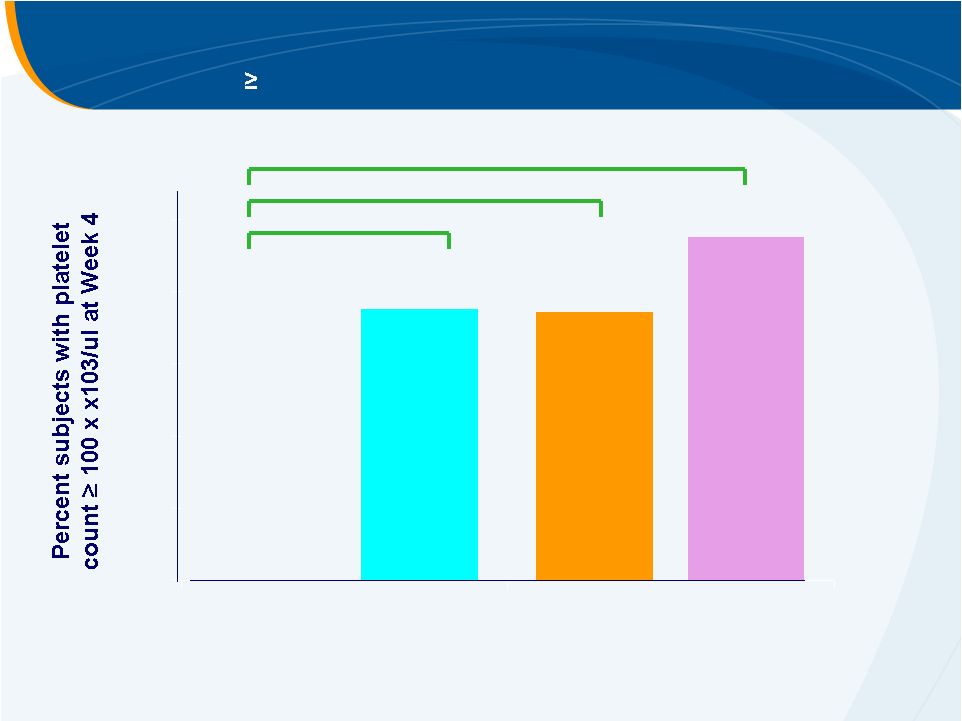 Primary Efficacy Endpoint
Platelet count
100,000/uL at Week 4
PLACEBO
30 mg
50 mg
75 mg
0.0007
0.0003
< 0.0001
0%
74%
75%
95%
0%
20%
40%
60%
80%
100%
McHutchison, NEJM 2007 |
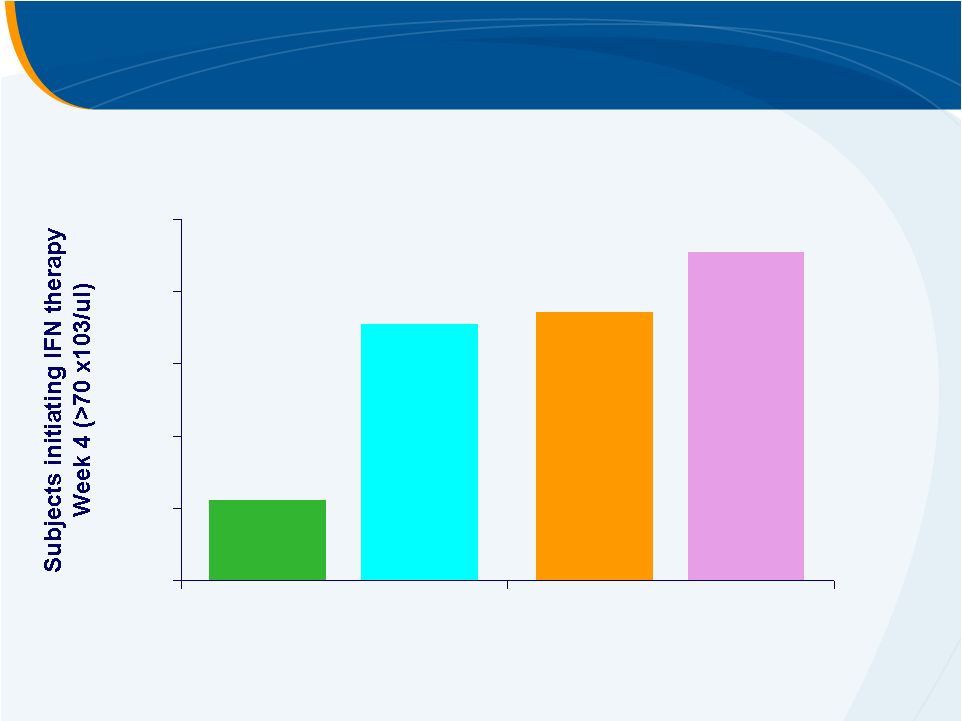 Subjects Initiating PEG-IFN Therapy at Week 4
PLACEBO
30 mg
50 mg
75 mg
22%
74%
71%
91%
0%
20%
40%
60%
80%
100% |
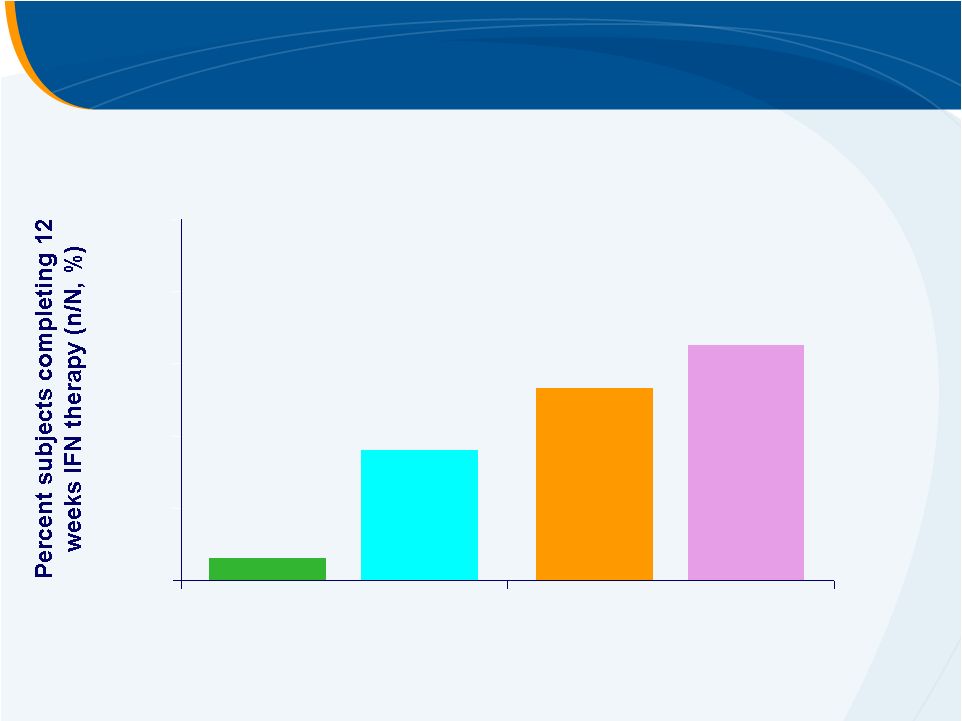 Subjects Completing 12 Weeks of PEG-IFN Therapy
PLACEBO
30 mg
50 mg
75 mg
6%
53%
36%
65%
0%
20%
40%
60%
80%
100% |
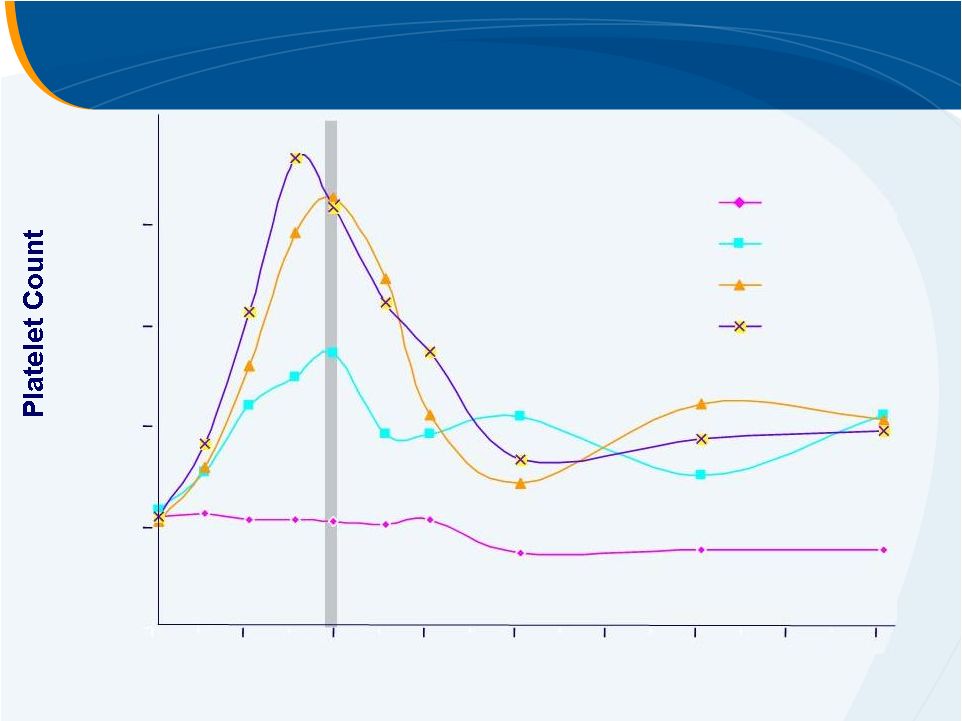 HCV
Phase II Study Median Platelet Count
0
50
100
150
200
250
0
14
28
42
56
70
84
98
112
Study Day
Placebo
30 mg
50 mg
75 mg
INITIATION
MAINTENANCE
McHutchison, AASLD 2006 |
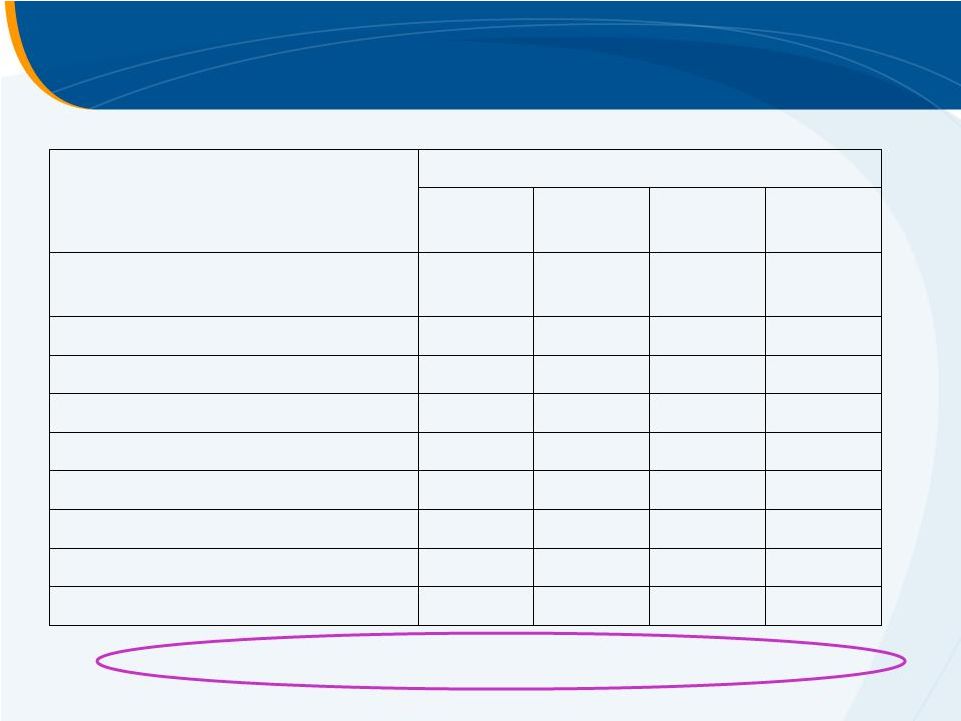 Adverse Events –
Pre-Antiviral Phase
Treatment Group, n (%)
PBO
N=18
30mg
N=14
50mg
N=19
75mg
N=23
Any AE
10 (56)
11 (79)
10 (53)
13 (57)
Headache
3 (17)
5 (36)
3 (16)
4 (17)
Dry mouth
1 (6)
2 (14)
2 (11)
2 (9)
Pruritus
0
0
0
2 (9)
Nausea
0
1 (7)
2 (11)
1 (4)
Fatigue
0
0
2 (11)
1 (4)
Upper abdominal pain
0
2 (14)
2 (11)
0
Insomnia
0
0
2 (11)
0
Arthralgia
0
2 (14)
1 (5)
0
No thromboembolic
or elevated LFT events of concern |
 Conclusions
Eltrombopag
increased platelet counts in subjects in all dose groups
A
significant
number
of
subjects
achieved
the
primary
endpoint
(Week
4)
in all dose groups compared to placebo
Eltrombopag
enabled 45/56 subjects to initiate IFN therapy
–
31 subjects completed 12 weeks of IFN therapy
Preliminary PK findings in general indicate exposure increases with
dose with wide variability
No safety signals of concern in this initial short term study
Safety and efficacy data supports further investigation of eltrombopag
in this patient population |
 2
parallel global Phase III studies
Eltrombopag
to
INitiate
and Maintain Interferon
Antiviral Treatment
to
Benefit Subjects with Hepatitis C related Liver
DiseasE
peginterferon
alfa-2a
(PEGASYS)
plus
ribavirin
–
ENABLE
1
peginterferon
alfa-2b
(PEG-Intron)
plus
ribavirin
–
ENABLE
2
ENABLE 1 & 2 |
 open label
eltrombopag
Eltrombopag
+ PEG-IFN/Rib
Pre-Antiviral Phase
Open-label eltrombopag
(2 –
9 weeks)
Antiviral Phase
Randomised
(up to 48 weeks)
Placebo
+ PEG-IFN/Rib
platelets
>90,000/uL (E1)
or
100,000/uL (E2)
25mg>50mg>75mg>100mg
•
2:1 randomisation eltrombopag:placebo
•
Dose titration of eltrombopag allowed throughout
•
Primary endpoint = proportion of patients achieving SVR (6M off –Tx)
•
N=750 dosed/675 randomised study
•
3 regions, 26 countries, >250 centres
SVR
SVR
6 months
off-Tx
Randomised Withdrawal Design |
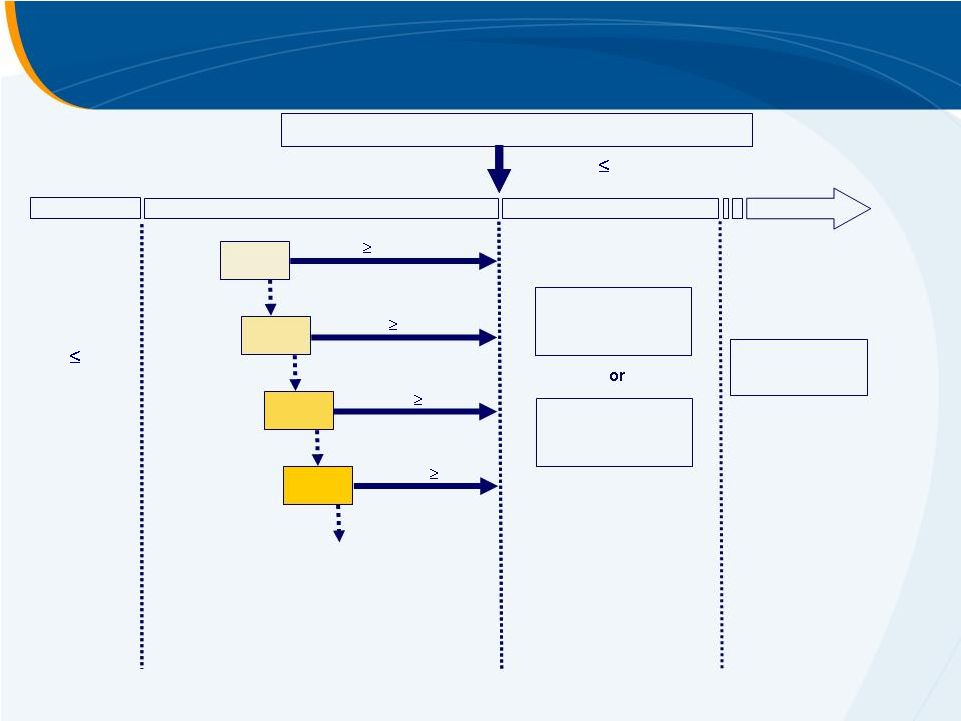 ENABLE 1 & 2 Study Design
Screening
45
Days
Platelets
<75K/µL
25mg
Pre-Antiviral Treatment Phase
Open-label eltrombopag
dosed for
up to 9wks until platelet count
increases to enable initiation of
antiviral therapy.
Part 1 (2-9 Wks)
Open-Label
2 Weeks
Platelets
90K/µL*
Platelets
90K/µL*
Platelets
90K/µL*
*Platelets
<90K/µL
*Platelets
<90K/µL
*Platelets
<90K/µL
WD
START
50mg
1-2 Weeks
75mg
1-2 Weeks
100mg
1-3 Weeks
*Platelets
<90K/µL
Platelets
90K/µL*
*90K/µL (ENABLE 1)
100K/µL (ENABLE 2)
2:1 Randomisation (Eltrombopag:Placebo)
Eltrombopag
plus
Antiviral Therapy
Antiviral Treatment Phase
Randomised either to
maintain dose of
eltrombopag
from Part
1 or to matched
placebo.
Part
2
(
48
Wks)
Double-Blind
Placebo
plus
Antiviral Therapy
or
a
24 Week FU
Ocular/SVR
a
Post-last dose of
investigational
product. |
 SVR
rate defined as percentage of subjects with non-detectable HCV-RNA at
24 weeks post-completion of the planned treatment period
Platelet count
90-100,000/
L in Part 1
Dose modifications
Safety and tolerability
Platelet counts
PK
RVR,
EVR and ETR
Health-related quality of life
Endpoints |
 Eltrombopag is an effective platelet stimulator in CLD
Eltrombopag can reduce need for platelet transfusion in CLD patients
but final dose and safety concerns must be addressed
ENABLE studies will confirm role of eltrombopag in HCV therapy in
2011
Eltrombopag Summary |
 Ligand
Pharmaceuticals,
Inc.
72
Ligand
Business Development
Expanding the Partnership Pipeline
Syed
Kazmi, Ph.D.
Vice President,
Business Development and Strategic Planning |
 73
•
Conduct only early-stage research
Do what we are good at
•
Very successful track record of discovery and IND generation
–
Over 40 clinical candidates, 22 INDs, 5 drugs approved and 3 on the market
Proven and productive technology platform
•
Strong research capabilities for discovering more selective drugs
•
Select high quality discovery programs
Unmet medical needs
Large, non-primary care markets
Likely branded premium pricing
Tissue selective approach to reduce side effects
•
Partner at appropriate value inflection point from pre-IND to clinical proof of
concept
Likely partners -
big pharma
Generate revenue streams from upfronts, milestones and royalties
The Business Model at Ligand |
 74
Ligand Unpartnered Assets
SARM
TRß
Agonist
Glucagon H3
EPO
Androgen
Receptor
Modulator
Thyroid
Receptor-beta
Agonist
Glucagon
Receptor
Antagonist
Histamine H3
Receptor
Antagonist
Oral EPO
Receptor Agonist
Muscle
Wasting
Dyslipidemia
Diabetes
Cognitive
Disorders
Anemia
Phase Ib
Pre-Clinical
Pre-Clinical
Pre-Clinical
Pre-Clinical
Program
Disease
Status |
 75
Ligand Unpartnered Assets
SARM
TRß
Agonist
Glucagon H3
EPO
Androgen
Receptor
Modulator
Thyroid
Receptor-beta
Agonist
Glucagon
Receptor
Antagonist
Histamine H3
Receptor
Antagonist
Oral EPO
Receptor Agonist
Muscle
Wasting
Dyslipidemia
Diabetes
Cognitive
Disorders
Anemia
Phase Ib
Pre-Clinical
Pre-Clinical
Pre-Clinical
Pre-Clinical
Program
Disease
Status |
 76
•
Metabasis
acquisition brings a robust IP portfolio
and industry-leading experience in liver-targeted
drug delivery using proprietary HepDirect
Technology
•
Ligand
brings deep experience in nuclear hormone
receptor chemistry and pharmacology for
discovering
novel
liver-targeted
TR
agonists
for
hyperlipidemia
The Ligand
TR-beta Program
The combination of Ligand
and Metabasis
creates
a synergistic drug discovery platform for
discovering liver-specific TR
agonists |
 77
•
Target validation:
TR activation in liver affects the expression of several genes, leading to
reductions in LDL, triglycerides,Lp(a), and ApoB
TRß
is the major isoform
expressed in liver for tissue-specific effects
•
TR activation in extra-hepatic tissues result in dose-limiting side effects,
including arrhythmia, muscle, bone and thyroid hormones
•
Product Profile:
Oral drug lowering lipids without suppressing thyroid axis or invoking
cardiovascular liabilities
Combination with statins
in patients not reaching LDL goal on current
maximal therapies
•
Avoid mechanism-based safety concerns:
Alterations in thyroid axis –
decrease in T4 and increased TSH levels
Potential for adverse cardiovascular events
Thyroid Receptor
Overview |
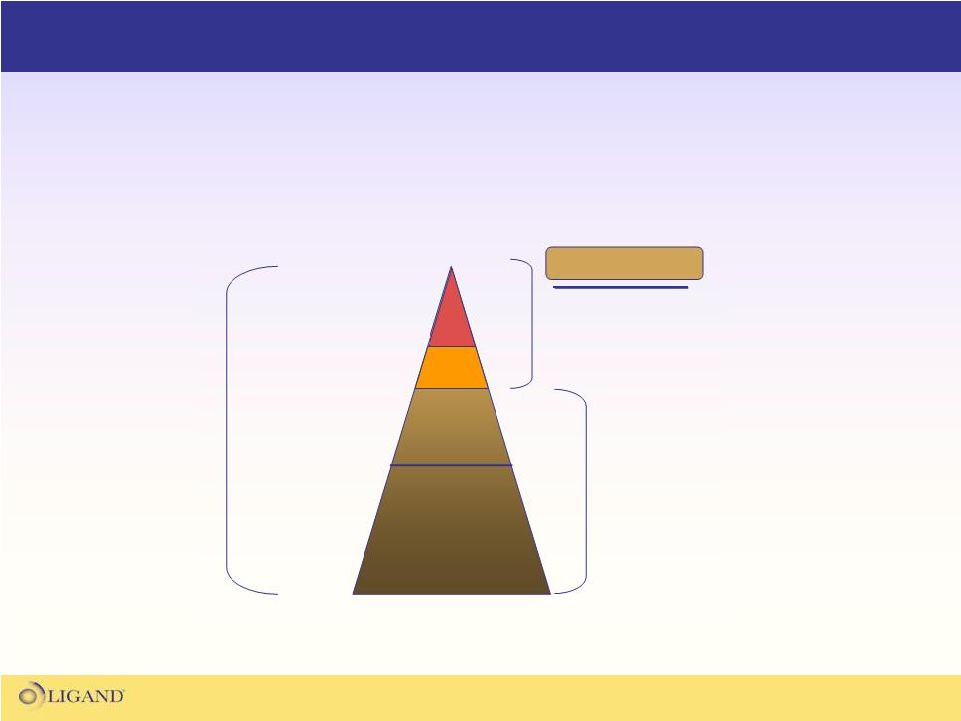 78
Dyslipidemia
–
Unmet
Medical
Need
Low Cardio Risk
Med Cardio Risk
High Cardio Risk
Statin
Intolerant
26M US
Dyslipidemic
Patients
Largely Meeting
LDL Goals on
Existing Therapies
20%
15%
Approximately 9M
Patients in need of
Add-On Therapies
Add-on treatments for patients still not reaching LDL goals
on current maximal therapy is a multi-billion dollar market
TR-b Target Pop. |
 79
MB07811 -
First generation liver-targeted prodrug
•
Phase Ib
study completed in 56 subjects
Clinical proof of concept with dose-dependent decrease in LDL (15-
41%), Apo B, TGs, and Lp(a)
Modest dose-related side effects including thyroid axis suppression in a
small cohort of subjects
2
nd
generation Liver-selective compounds (e.g. MB10866)
•
Improved preclinical safety and efficacy profiles in validated animal
models
•
Lead
optimization
studies
ongoing
to
further
improve
liver
selectivity
Thyroid Receptor
Program Status |
 80
•
Robust Partnering Package
Extensive clinical package with first generation drug candidate to help drive
SARs
2
generation lead candidates representing novel chemistry platforms
Strong IP for a well validated target
Potential for first-in-class NME in a growing area of unmet medical need
•
Partnering Landscape
Established clinical proof of concept with MB07811 (Metabasis) and
Eprotirome
(Karo
Bio)
Well-defined regulatory path to approval
High unmet medical need beyond generic statins
•
Differentiation from Competition
HepDirect
Technology
Nuclear receptor expertise
TR
Partnering Opportunity
nd |
 81
•
LGD-4033, a Selective Androgen Receptor
Modulator
(SARM), for the treatment of muscle
wasting disorders
•
LGD-4033 has desirable tissue-selective
properties
A potent, full agonist on bone with anabolic and anti-
resorptive
effects
A potent, full agonist on skeletal muscle with
anabolic effects
A low potency, weak agonist on prostate and
sebaceous glands with high selectivity resulting in
minimal drive on these tissues
SARM Overview
LGD-4033 retains the beneficial properties of natural
androgens, while mitigating the toxic actions and side
effects of steroidal androgens |
 82
•
Phase I Single Ascending Dose study completed late 2009
LGD-4033 is safe and well tolerated at single doses up to 22mg
Suitable for once-daily dosing
No SAEs
or dose-related clinical significant adverse events reported
•
Phase I Multiple Ascending Dose study ongoing
LGD-4033
doses
of
0.1
-
3mg
once
daily
for
2-3
weeks
Objectives include safety, tolerability, and measurement of muscle protein
biomarkers, lean body mass, and strength (stair climb)
•
90-day
toxicology
studies
ongoing,
allowing
for
seamless
transition
into
Phase II patient populations
SARM Program Status |
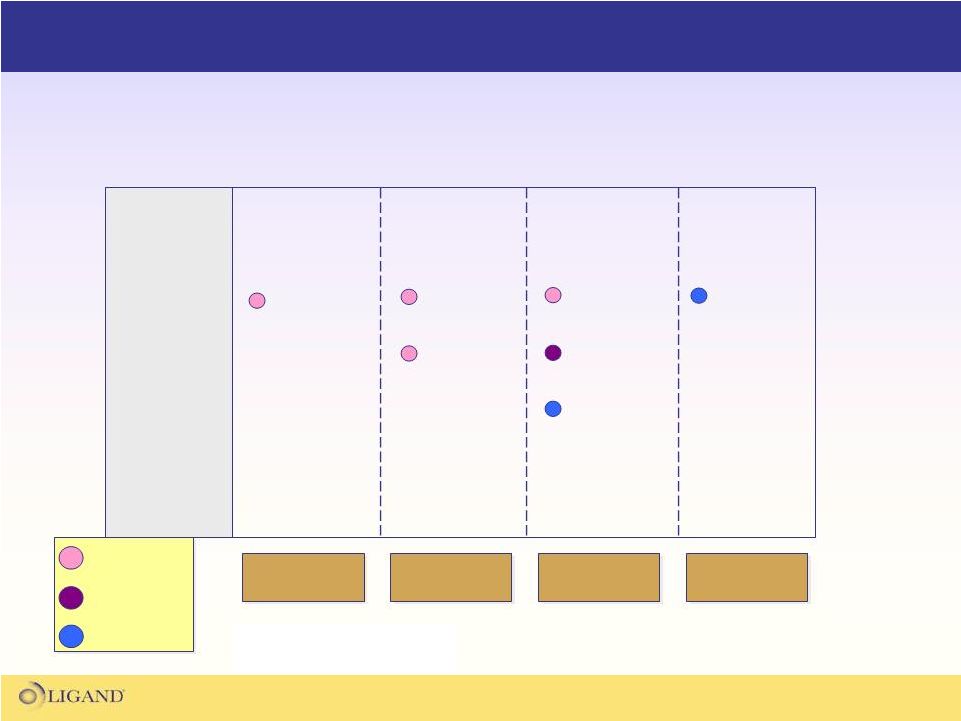 83
The pipeline snapshot below is based upon the developmental agents
listed for treatment of cachexia
(various disease states) and sarcopenia.
Competitive
Pipeline –
Relevant to LGD-4033 Development
Muscle
Wasting
Disease
Development
in the US
Preclinical
Preclinical
Phase II
Phase I
Phase III
Ostarine
(GTx-024)
LGD-3303
SARM
Ghrelin
receptor
Others
Data Source: Thomson-Pharma.com,
LGD-4033
GLPG-0492
Anamorelin
(RC-1291)
VT-122
imidapril |
 84
•
Robust Partnering Package
•
New NME with clinical POC data for a well validated target
•
A large library of novel SARM back-up candidates
•
Blockbuster drug potential in a high unmet medical need area
•
Partnership Landscape
Multiple commercial markets allow for both large and orphan indications
•
Muscle Wasting Disorders (cachexia, sarcopenia, frailty)
•
Rehabilitation
•
Muscular dystrophy
•
Osteoporosis (in men and women)
•
Differentiation from Competition
Higher potency
Oral dosing
Enhanced tissue Selectivity
LGD-4033 Partnering Opportunity |
 Ligand
Pharmaceuticals,
Inc.
85
Selective Androgen Receptor Modulator
LGD-4033
Shalendar
Bhasin, M.D.
Professor of Medicine, Boston University School of Medicine
Chief, Section of Endocrinology, Diabetes, and Nutrition
Director, Boston Claude D. Pepper Older Americans Independence
Center for Function Promoting Anabolic Therapies
Principal Investigator, LGD-4033 Phase Ib
Clinical Trial |
 86
Shalender Bhasin, MD
Professor of Medicine,
Boston University School of Medicine
Chief, Section of Endocrinology, Diabetes, and Nutrition
Director, Boston Claude D. Pepper Older Americans Independence
Center for Function Promoting Therapies
Boston Medical Center
The Unment Medical Need for a SARM Therapeutic
The Unment Medical Need for a SARM Therapeutic |
 Aged Population as a Share of Total U.S.
Population Continues to Grow |
 88
Age-Related Decline in Lean Mass
and Muscle Strength (BLSA; Roy et al 2002)
Age (years)
Leg and Arm Lean Mass (kg)
Quadriceps and Biceps Strength (N)
Age (years) |
 89
Aging baby boomers: 65+ will grow from 35 million in
2000 to over 70 million in 2030.
Recent rate of disability —40% (ACS, 2004).
The oldest old: the fastest growing segment
Grow from 3.0 to 6.2 million in the next decade
Social Security Disability Program and the Supplemental
Security Program pay $62.5 billion yearly to 7.5 million
disabled persons; this amount will grow several-fold.
Growth in Disability Among Older Adults:
The Staggering Economic Cost |
 90
The Unmet Medical Need –
Frailty & Sarcopenia
•
Age-related loss of muscle
mass, strength and
functionality
•
Estimated 10 million
Americans over the age of 65
suffer from sarcopenia
•
Currently no FDA-approved
treatment options available |
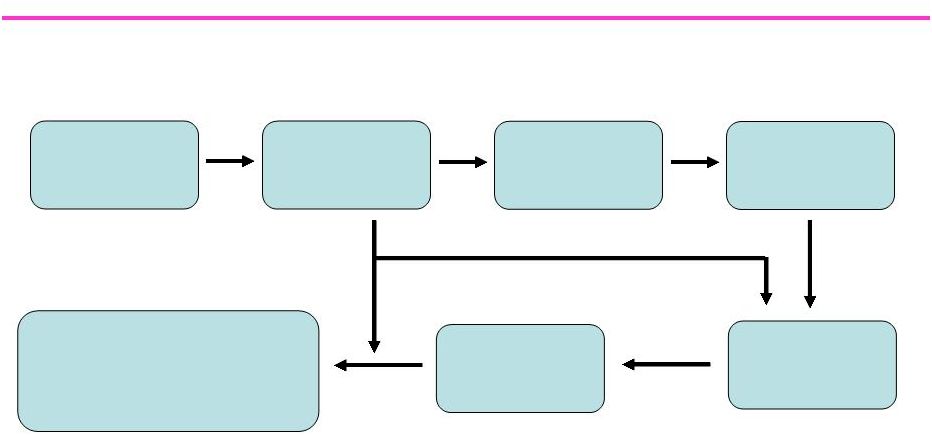 91
Rationale for Function Promoting Anabolic Therapies
Androgen
Therapy
Alterations in
Gene Expression
Stem Cell
Commitment
Increased
Muscle Mass
Increased
Muscle Strength
and Performance
Improved
Physical Function
Decreased Physical Dependence,
Improved Health Perceptions
and Health-Related Outcomes |
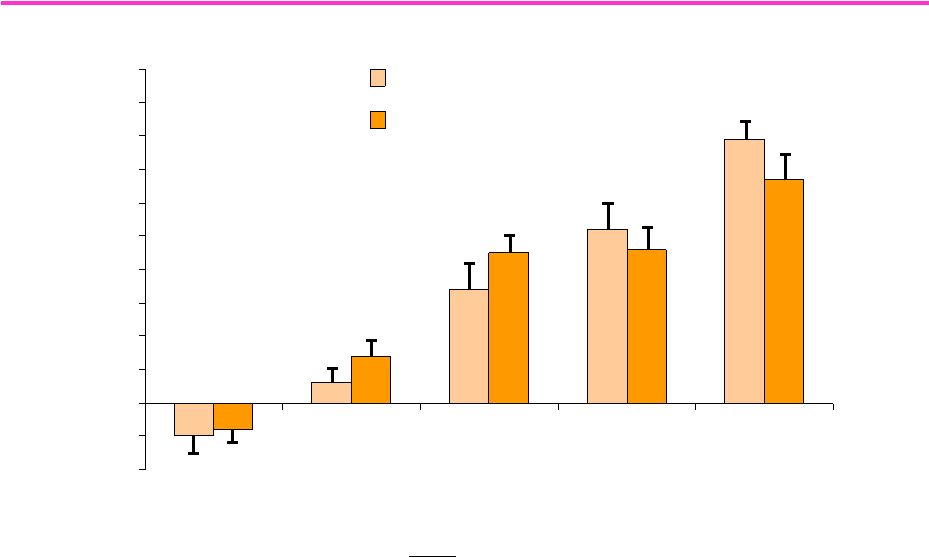 92
-2
-1
0
1
2
3
4
5
6
7
8
9
10
Young Men (18-34)
Older Men (60-75)
T Dose (mg)
25
50
125
300
600
Change in
FFM (kg)
T Dose Effect
P <0.0001
Age Effect
P = 0.22
Change in T X age
P = 0.46
Bhasin et al, JCEM
2005
Testosterone Dose Response in Young and Older Men:
Testosterone Dose Response in Young and Older Men:
Change in Fat Free Mass
Change in Fat Free Mass |
 93
The Need for a SARM Therapeutic
80 years of empiric, clinical, epidemiological, and clinical trials data provide
80 years of empiric, clinical, epidemiological, and clinical trials data
provide unequivocal evidence that androgens increase skeletal muscle mass
and unequivocal evidence that androgens increase skeletal muscle mass and
strength.
strength.
Testosterone is not the answer
Testosterone is not the answer
–
Concern about potential adverse effects on prostate
–
Many older men have microscopic foci of prostate cancer. Testosterone
might make these subclinical foci grow.
–
Inherent bias towards detection of greater number of prostate events in
Testosterone-treated men
We need a SARM
SARMs
are non-steroidal and rogens
that act preferentially on skeletal
muscle while sparing tissues associated with side effects, like the
prostate |
| 94
Potential Indications for SARMS are Broader than Frailty Elderly
•
Osteoporosis
•
Anemia
•
Sexual dysfunction
•
Men with prostate cancer who are deemed cured after
radical prostatectomy
•
Muscle wasting
•
Cancer cachexia
•
COPD
•
Chronic Kidney Disease
Potential Indications for SARMS are Broader than Frailty
|
| 95
•
Selective Androgen Receptor Modulator (SARM) that
has the following properties
–
Anabolic in muscle and bone
–
Restores libido/sexual function (CNS-active)
–
Minimal or no drive on prostate in men
–
Minimal or no virilization in women
–
No adverse cardiovascular or hepatic effects at therapeutic
doses
–
Non-steroidal –
cannot be converted to estrogen
SARM Therapeutic Target Profile |
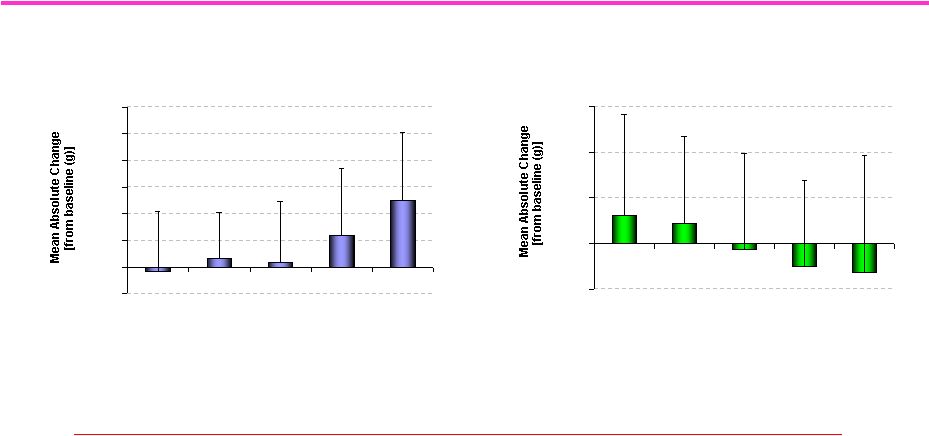 96
Clinical Trial of Ostarine: Increased Total Lean
Mass and Decreased Total Fat Mass
J Dalton. Data presented at 2007 Endo Meeting, Toronto.
We Need More Potent SARMs
with Greater Tissue Selectivity
Total Lean Mass (all subjects)
-500
0
500
1000
1500
2000
2500
3000
Placebo
0.1 mg
0.3 mg
1 mg
3 mg
Dose
# p<0.001 compared to placebo, p<0.0001 compared to baseline
* p=0.055 compared to placebo, p=0.020 compared to baseline
#
*
Total Fat Mass (all subjects)
-500
0
500
1000
1500
Placebo
0.1 mg
0.3 mg
1 mg
3 mg
Dose
# p=0.049 compared to placebo
# |
 97
•
Ligand
is
developing
LGD-4033,
a
Selective
Androgen
Receptor
Modulator
(SARM),
for the treatment of muscle wasting disorders
•
LGD-4033 has desirable tissue-selective properties
–
A
potent,
full
agonist
on
bone
with
anabolic
and
anti-resorptive
effects
–
A potent, full agonist on skeletal muscle with anabolic effects
–
A low potency agonist on prostate with high selectivity resulting in minimal drive
on the prostate
–
A weak, low potency agonist on sebaceous glands resulting in minimal drive on
these tissues LGD-4033 Overview
LGD-4033 retains the beneficial properties of natural androgens, while
mitigating the toxic actions and side effects of steroidal androgens
|
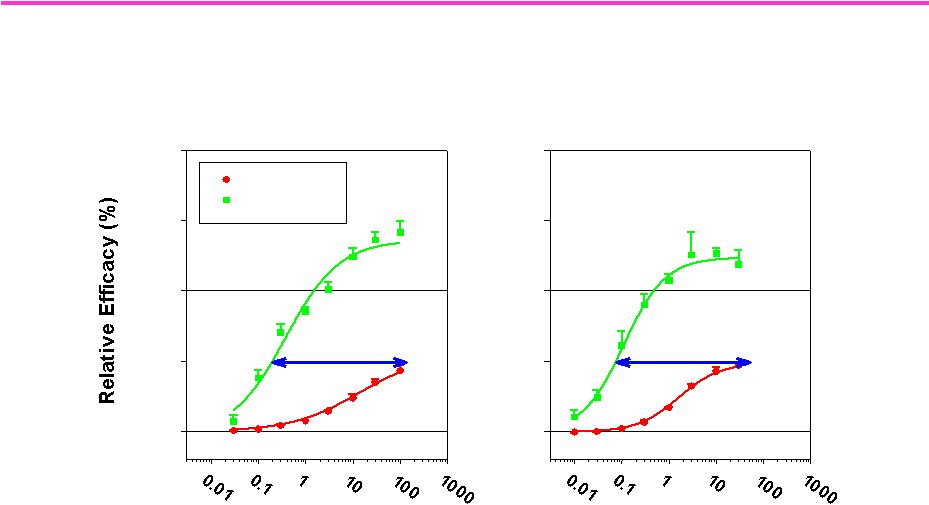 98
Tissue selectivity of LGD-4033
for skeletal muscle vs. prostate
LGD-4033
0
50
100
150
200
Prostate
Muscle
>500x
Dose (mg/kg/day, po)
Ostarine
0
50
100
150
200
>390x
Comparison of LGD-4033 and Ostarine in the rat model for male
hypogonadism |
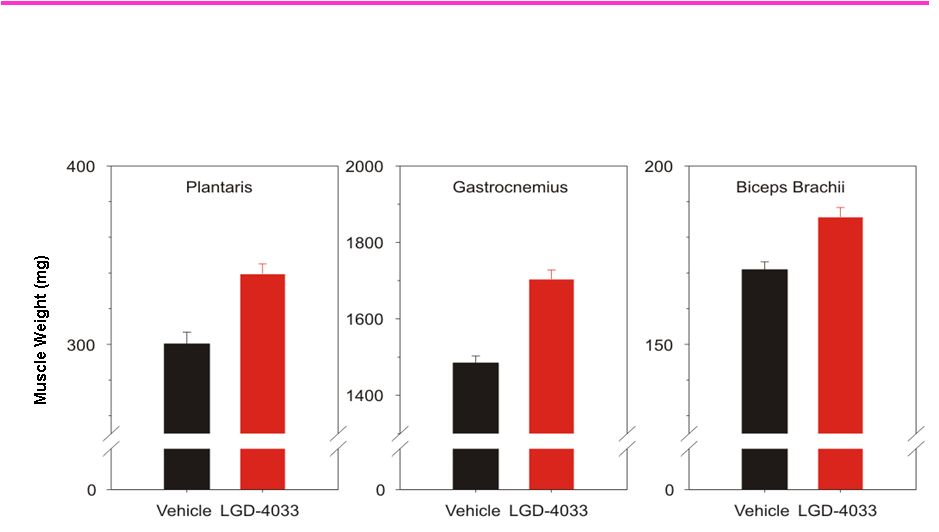 99
LGD-4033 Increases Muscle Mass Throughout the Body
*
*
*
Effect of LGD-4033 on the weight of skeletal muscle in
female rats treated once daily with 3 mg/kg LGD-4033 for 4 weeks
|
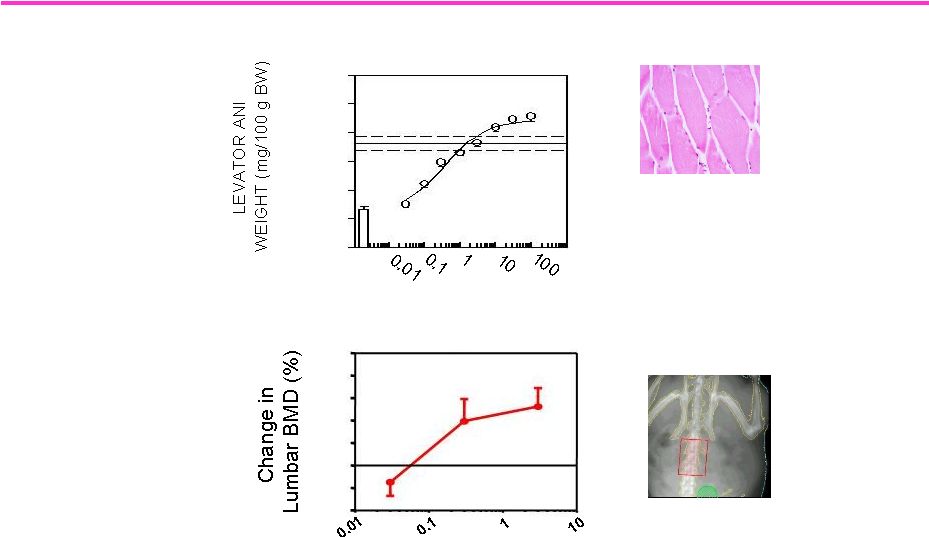 100
LGD-4033: Similar Potency on Muscle and Bone
Indicates Potential for Treating Osteoporosis
Dose (mg/kg/day, po)
-4
-2
0
2
4
6
8
10
Lumbar Spine DEXA Analysis in Female Rats
Skeletal Muscle Weight in Male Rats
Dose (mg/kg p.o.)
0
20
40
60
80
100
120 |
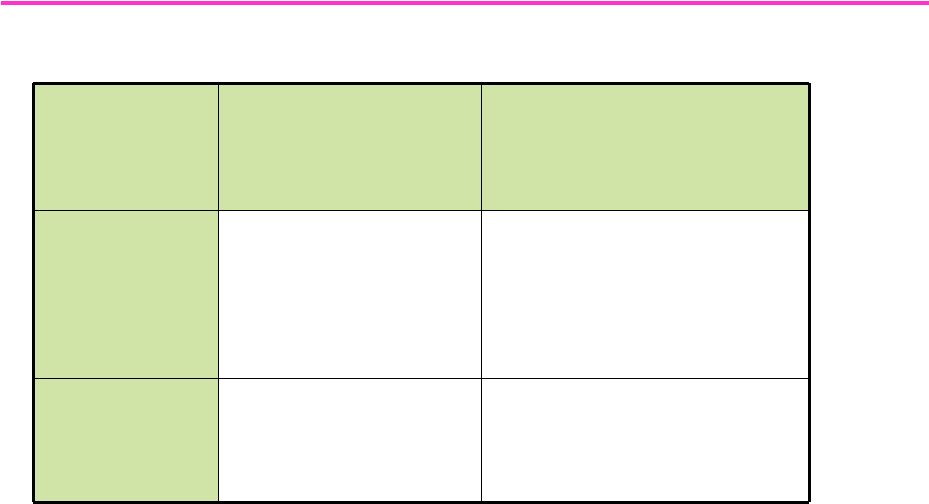 101
Study
Objective
Key Observations
SAD
—
001
Single dose safety and
tolerability /
Pharmacokinetics
0.1-22 mg
Well tolerated at all doses;
Dose proportional AUC; T
1/2
:
~26-38 hrs
No SAE’s
reported
MAD
—
002
Multiple dose safety
and tolerability /
Pharmacokinetics
Study ongoing
LGD-4033
Phase
I
Development
-
Overview |
 102
•
Drug exposure was high and dose-proportional after a
single oral dose suggesting that a low dose will give
sufficient systemic exposure to be therapeutically
effective
•
Elimination half-life (t
1/2
31 hours) consistent with once-
a-day oral dosing
•
No serious adverse events occurred
•
A total of 5 mild-to-moderate AEs
were considered drug-
related by the investigator
–
No AE was dose-related
–
No more than one drug-related AE was observed in any MedRA
category
Summary of Single Ascending Dose Study
(L4033-01) |
 103
L4033-02 Multiple Ascending Dose Study
3 mg Dose
Cohort 4
(N=8)
*
1 mg Dose
Cohort 3
(N=20)
0.3 mg
Dose
Cohort 2
(N=20)
0.1 mg
Dose
Cohort 1
(N=8)
Biomarkers:
DEXA for Lean Body Mass, 1-RM for strength, FSR for muscle
protein synthesis
Healthy Males: 21-50 years
Doses: 0.1, 0.3, 1 & 3 mg
Treatment Duration: 21 days
Objective:
Evaluation of Safety, Tolerability,
Pharmacokinetics (PK) and Pharmacodynamics (PD)
Design:
Single-center, randomized, double-blind, placebo-
controlled, sequential escalation, once-daily for 3 weeks
|
 104
Regulatory Pathway for SARM Drugs
•
Demonstrating improvements in:
–
Physical function
–
Health outcomes, such as quality of life, reduction in falls
•
FDA feedback on required primary end points for Phase IIb as
disclosed by GTx
–
Total lean body mass
–
Performance measure (e.g.Stair climb)
–
Weight is not an acceptable end point
NIA-Academic Task Force is establishing
a regulatory pathway -
Held in US and
Europe |
| 105
Conclusion
•
Growth in the aged population presents an enormous
unmet need for function promoting therapies
•
Testosterone demonstrates the benefits of androgens in
the frail elderly, but adverse effects limit its use
•
SARMs may produce equal or better functional
improvements with more a favorable safety profile
•
LGD-4033 demonstrates a promising profile of high
potency and selectivity in a SARM |
 106
106 |
