Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Impax Laboratories, LLC | c94464e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - Impax Laboratories, LLC | c94464exv99w1.htm |
Exhibit
99.2

| J.P. Morgan Healthcare Conference January 11, 2010 |

| Safe Harbor Statement "Safe Harbor" Statement under the Private Securities Litigation Reform Act of 1995: To the extent any statements made in this presentation contain information that is not historical, these statements are forward-looking in nature and express the beliefs and expectations of management. Such statements are based on current expectations and involve a number of known and unknown risks and uncertainties that could cause Impax's future results, performance or achievements to differ materially from the results, performance or achievements expressed or implied by such forward-looking statements. Such risks and uncertainties include, but are not limited to: the effect of current economic conditions on our industry, business, financial position, results of operations and market value of our common stock; ability to timely file periodic reports required by the Securities Exchange Act; ability to maintain an effective system of internal control over financial reporting; ability to sustain profitability and positive cash flows; ability to maintain sufficient capital to fund operations; any delays or unanticipated expenses in connection with the construction of our Taiwan facility; ability to successfully develop and commercialize pharmaceutical products; the uncertainty of patent litigation; consumer acceptance and demand for new pharmaceutical products; the impact of competitive products and pricing; the difficulty of predicting Food and Drug Administration filings and approvals; inexperience in conducting clinical trials and submitting new drug applications; reliance on key alliance agreements; the availability of raw materials; the regulatory environment; exposure to product liability claims; fluctuations in operating results and other risks described in our periodic reports filed with the Securities and Exchange Commission. You should not place undue reliance on forward looking statements. Forward-looking statements speak only as to the date on which they are made, and Impax undertakes no obligation to update publicly or revise any forward-looking statement, regardless of whether new information becomes available, future developments occur or otherwise. Note: All product sales data included herein are derived from data published by Wolters Kluwer Health for the 12 months ended November 2009. Trademarks referenced herein are the property of their respective owners. (c)2010 Impax Laboratories, Inc. |
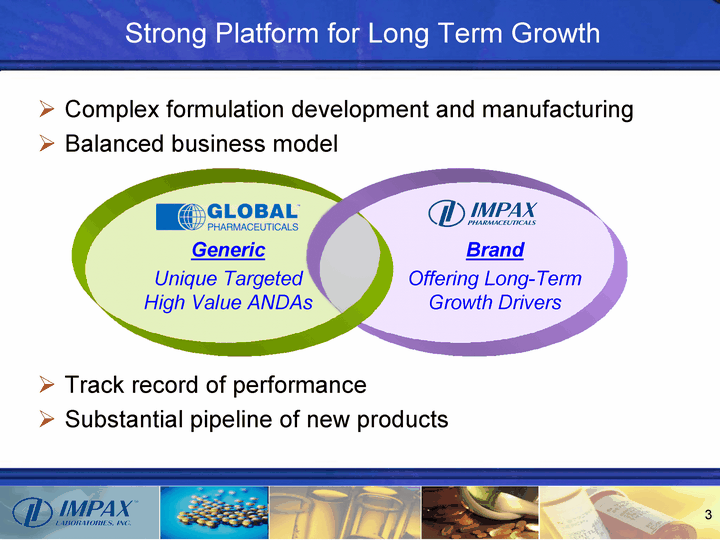
| Complex formulation development and manufacturing Balanced business model Track record of performance Substantial pipeline of new products Strong Platform for Long Term Growth Generic Unique Targeted High Value ANDAs Brand Offering Long-Term Growth Drivers |
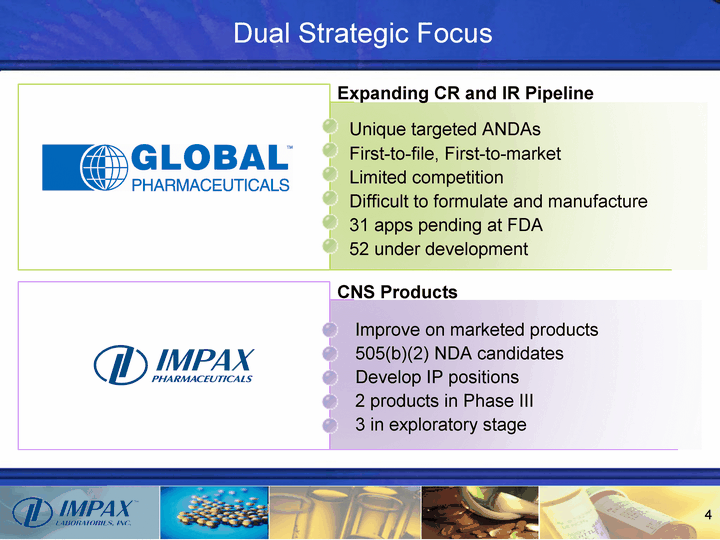
| Dual Strategic Focus Unique targeted ANDAs First-to-file, First-to-market Limited competition Difficult to formulate and manufacture 31 apps pending at FDA 52 under development CNS Products Improve on marketed products 505(b)(2) NDA candidates Develop IP positions 2 products in Phase III 3 in exploratory stage Expanding CR and IR Pipeline |

| Generic Products Division |
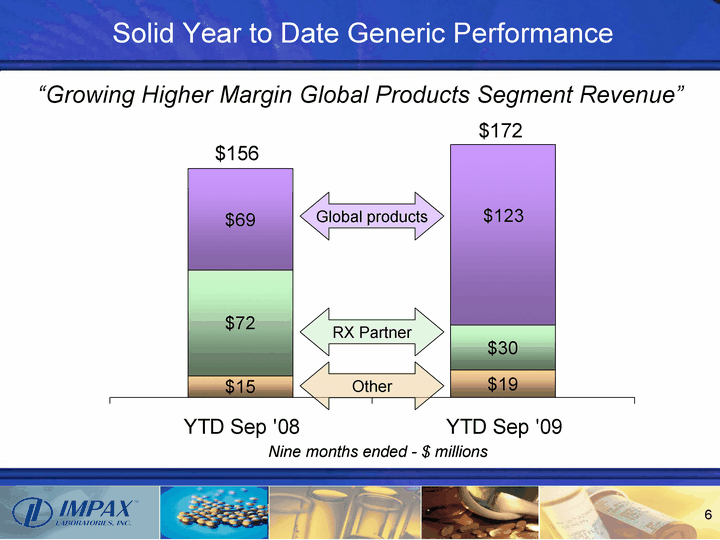
| YTD Sep '08 YTD Sep '09 Other 14.5 18.9 RX Partner 72.1 30.2 Global 69 123.1 Solid Year to Date Generic Performance $156 $172 Nine months ended - $ millions Global products RX Partner Other "Growing Higher Margin Global Products Segment Revenue" |
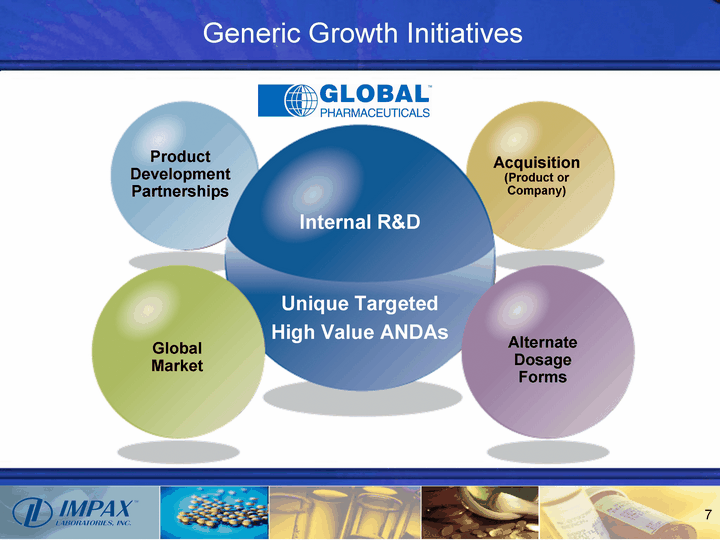
| Generic Growth Initiatives Product Development Partnerships Acquisition (Product or Company) Global Market Alternate Dosage Forms Internal R&D Unique Targeted High Value ANDAs |
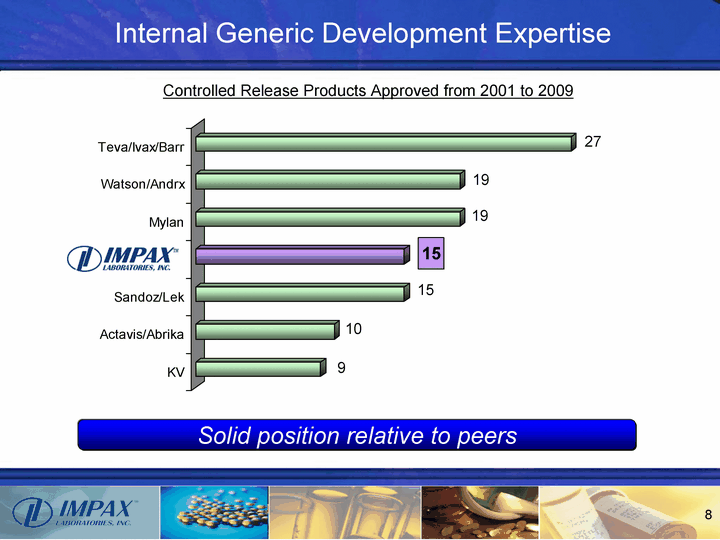
| Internal Generic Development Expertise KV Actavis/Abrika Sandoz/Lek Mylan Watson/Andrx Teva/Ivax/Barr 9 10 15 15 19 19 27 Solid position relative to peers Controlled Release Products Approved from 2001 to 2009 |
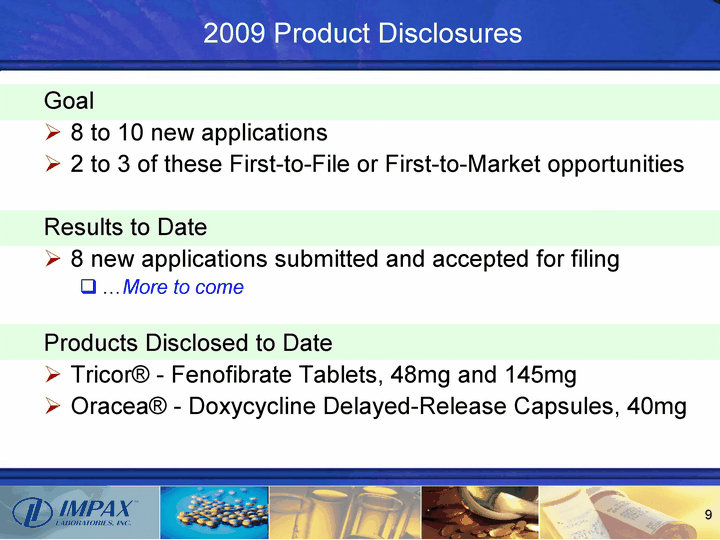
| 2009 Product Disclosures Goal 8 to 10 new applications 2 to 3 of these First-to-File or First-to-Market opportunities Results to Date 8 new applications submitted and accepted for filing ...More to come Products Disclosed to Date Tricor(r) - Fenofibrate Tablets, 48mg and 145mg Oracea(r) - Doxycycline Delayed-Release Capsules, 40mg |
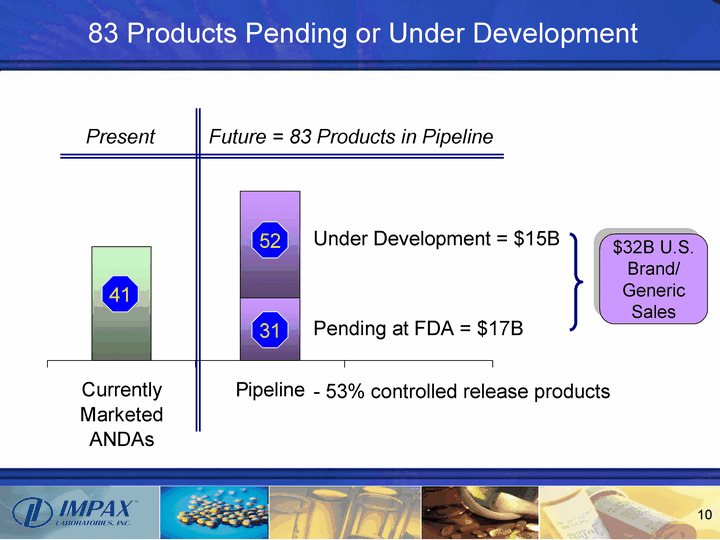
| 83 Products Pending or Under Development Currently Marketed ANDAs Pipeline East 47 26 44 41 31 52 Present Future = 83 Products in Pipeline Pending at FDA = $17B Under Development = $15B - 53% controlled release products $32B U.S. Brand/ Generic Sales |
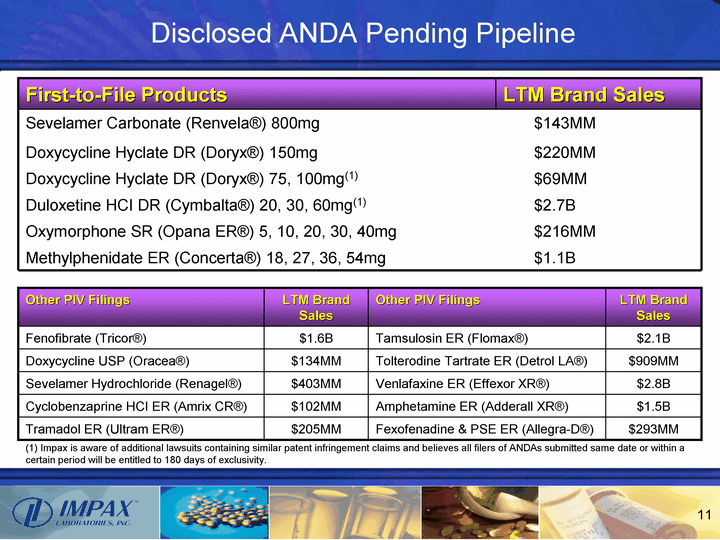
| Disclosed ANDA Pending Pipeline First-to-File Products LTM Brand Sales LTM Brand Sales Sevelamer Carbonate (Renvela(r)) 800mg Sevelamer Carbonate (Renvela(r)) 800mg $143MM Doxycycline Hyclate DR (Doryx(r)) 150mg Doxycycline Hyclate DR (Doryx(r)) 150mg $220MM Doxycycline Hyclate DR (Doryx(r)) 75, 100mg(1) Doxycycline Hyclate DR (Doryx(r)) 75, 100mg(1) $69MM Duloxetine HCI DR (Cymbalta(r)) 20, 30, 60mg(1) Duloxetine HCI DR (Cymbalta(r)) 20, 30, 60mg(1) $2.7B Oxymorphone SR (Opana ER(r)) 5, 10, 20, 30, 40mg Oxymorphone SR (Opana ER(r)) 5, 10, 20, 30, 40mg $216MM Methylphenidate ER (Concerta(r)) 18, 27, 36, 54mg Methylphenidate ER (Concerta(r)) 18, 27, 36, 54mg $1.1B Other PIV Filings LTM Brand Sales Other PIV Filings LTM Brand Sales Fenofibrate (Tricor(r)) $1.6B Tamsulosin ER (Flomax(r)) $2.1B Doxycycline USP (Oracea(r)) $134MM Tolterodine Tartrate ER (Detrol LA(r)) $909MM Sevelamer Hydrochloride (Renagel(r)) $403MM Venlafaxine ER (Effexor XR(r)) $2.8B Cyclobenzaprine HCI ER (Amrix CR(r)) $102MM Amphetamine ER (Adderall XR(r)) $1.5B Tramadol ER (Ultram ER(r)) $205MM Fexofenadine & PSE ER (Allegra-D(r)) $293MM (1) Impax is aware of additional lawsuits containing similar patent infringement claims and believes all filers of ANDAs submitted same date or within a certain period will be entitled to 180 days of exclusivity. |
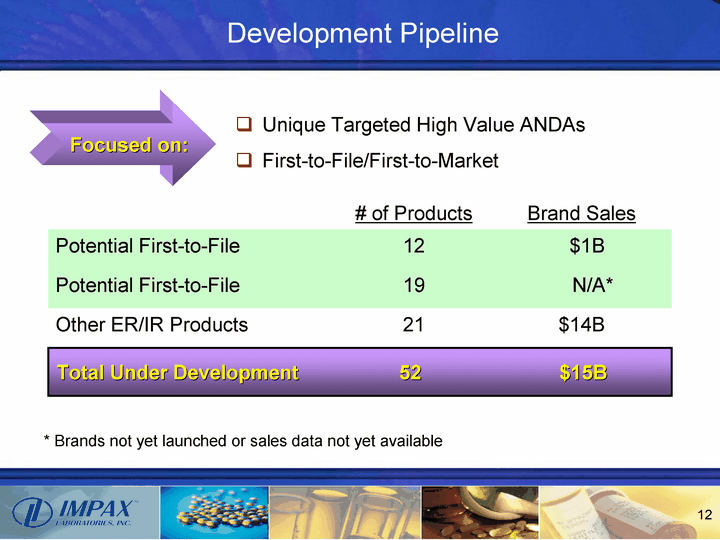
| Development Pipeline Total Under Development 52 $15B * Brands not yet launched or sales data not yet available Unique Targeted High Value ANDAs First-to-File/First-to-Market # of Products Brand Sales Potential First-to-File 12 $1B Potential First-to-File 19 N/A* Other ER/IR Products 21 $14B Focused on: |
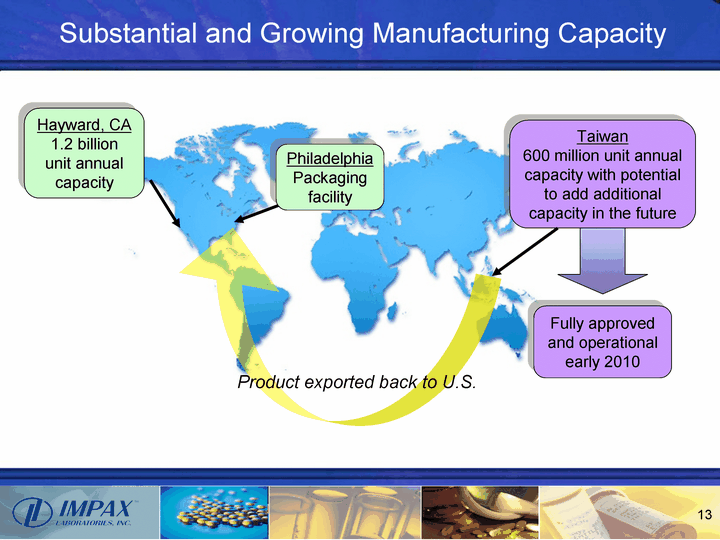
| Substantial and Growing Manufacturing Capacity Taiwan 600 million unit annual capacity with potential to add additional capacity in the future Fully approved and operational early 2010 Hayward, CA 1.2 billion unit annual capacity Product exported back to U.S. Philadelphia Packaging facility |

| Brand Products Division |
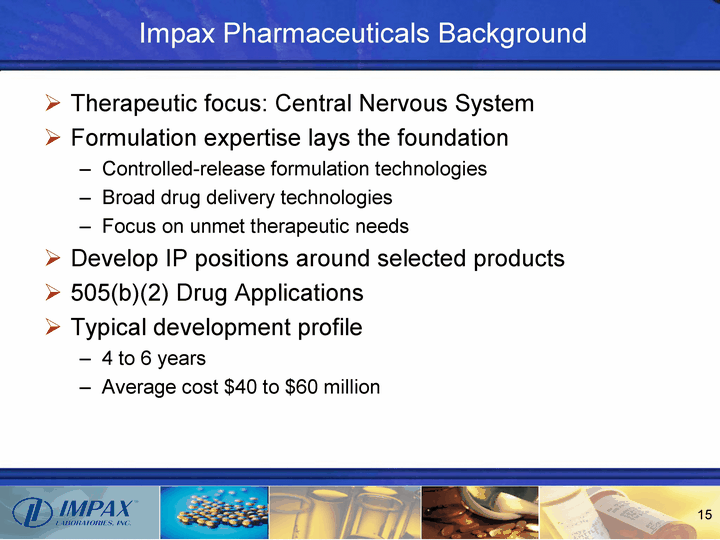
| Impax Pharmaceuticals Background Therapeutic focus: Central Nervous System Formulation expertise lays the foundation Controlled-release formulation technologies Broad drug delivery technologies Focus on unmet therapeutic needs Develop IP positions around selected products 505(b)(2) Drug Applications Typical development profile 4 to 6 years Average cost $40 to $60 million |
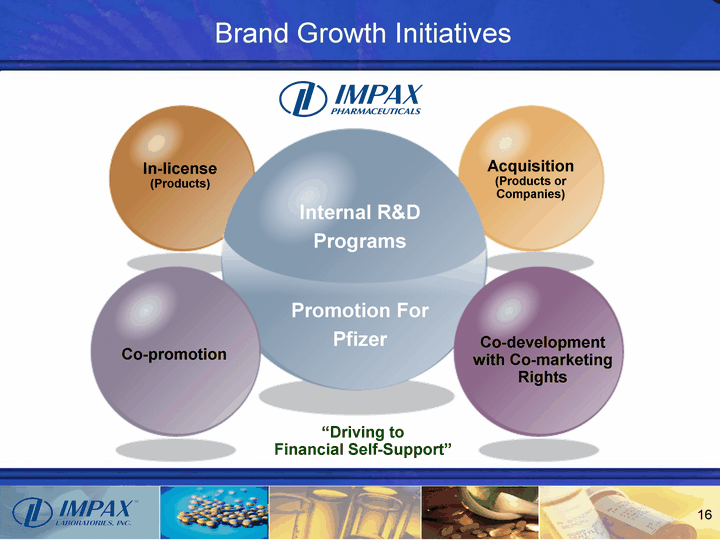
| Brand Growth Initiatives In-license (Products) Acquisition (Products or Companies) Co-promotion Co-development with Co-marketing Rights Internal R&D Programs Promotion For Pfizer "Driving to Financial Self-Support" |
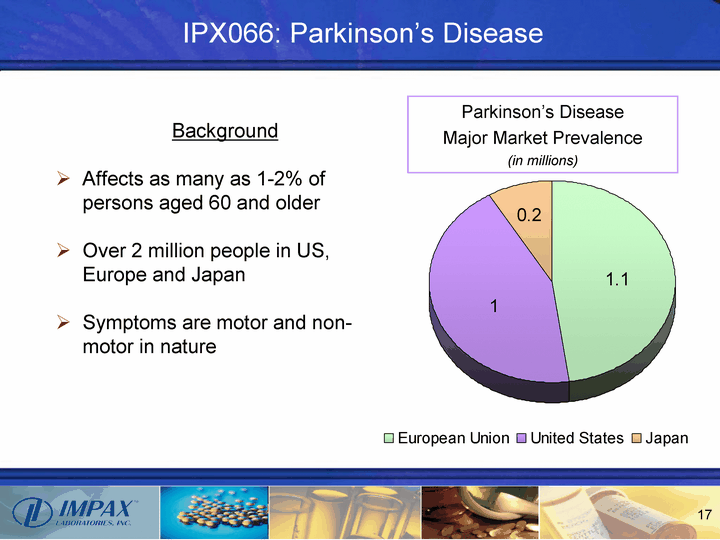
| IPX066: Parkinson's Disease European Union United States Japan 1.1 1 0.2 Parkinson's Disease Major Market Prevalence (in millions) Background Affects as many as 1-2% of persons aged 60 and older Over 2 million people in US, Europe and Japan Symptoms are motor and non- motor in nature |
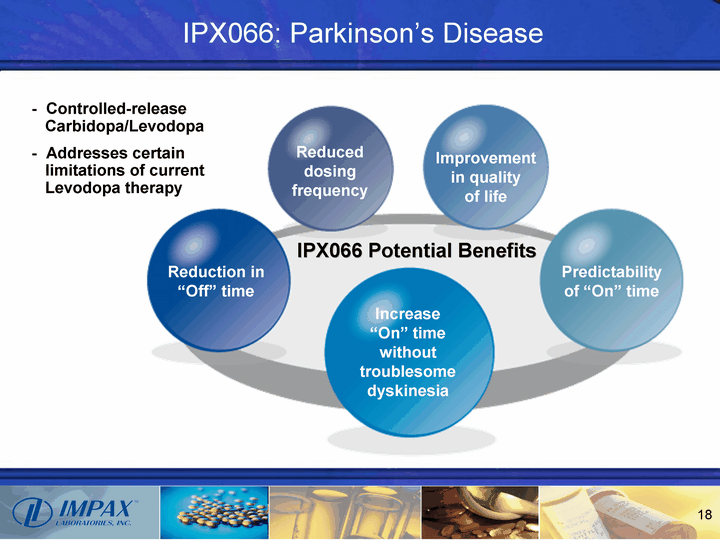
| IPX066 Potential Benefits Reduction in "Off" time Reduced dosing frequency Increase "On" time without troublesome dyskinesia Improvement in quality of life Predictability of "On" time Controlled-release Carbidopa/Levodopa Addresses certain limitations of current Levodopa therapy IPX066: Parkinson's Disease |
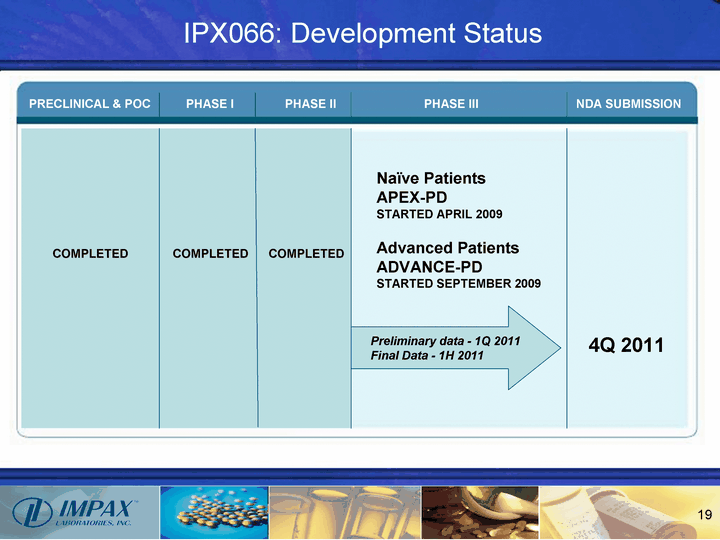
| Naive Patients APEX-PD STARTED APRIL 2009 Advanced Patients ADVANCE-PD STARTED SEPTEMBER 2009 PRECLINICAL & POC PHASE I PHASE II PHASE III NDA SUBMISSION COMPLETED COMPLETED COMPLETED 4Q 2011 Preliminary data - 1Q 2011 Final Data - 1H 2011 IPX066: Development Status |
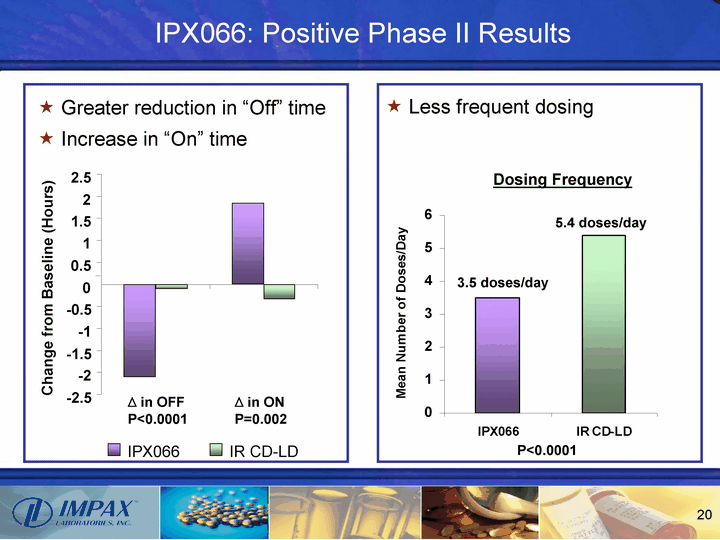
| IPX066: Positive Phase II Results -2.5 -2 -1.5 -1 -0.5 0 0.5 1 1.5 2 2.5 Change from Baseline (Hours) ^ in OFF P<0.0001 ^ in ON P=0.002 Dosing Frequency P<0.0001 3.5 doses/day 5.4 doses/day IPX066 IR CD-LD Greater reduction in "Off" time Increase in "On" time Less frequent dosing |
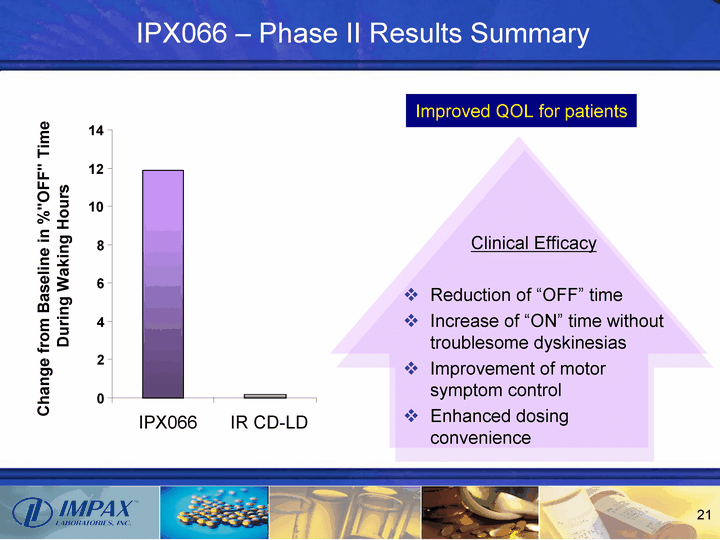
| IPX066 - Phase II Results Summary Clinical Efficacy Reduction of "OFF" time Increase of "ON" time without troublesome dyskinesias Improvement of motor symptom control Enhanced dosing convenience 0 2 4 6 8 10 12 14 IPX066 IR CD-LD Change from Baseline in %"OFF" Time During Waking Hours Improved QOL for patients |
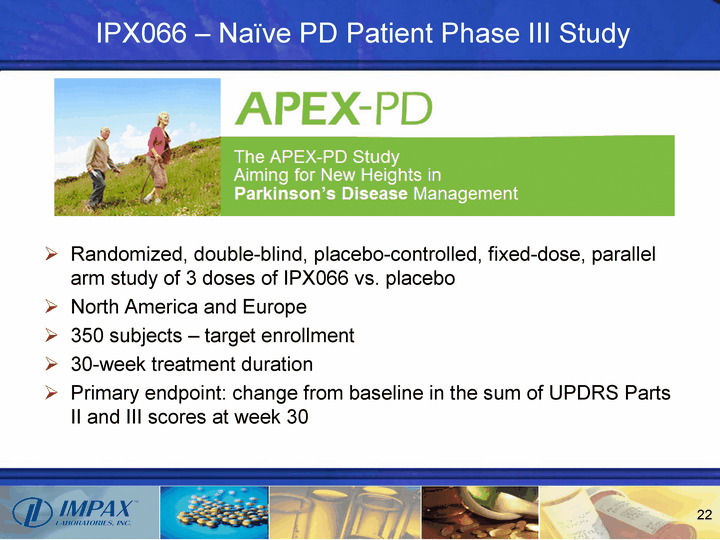
| IPX066 - Naive PD Patient Phase III Study Randomized, double-blind, placebo-controlled, fixed-dose, parallel arm study of 3 doses of IPX066 vs. placebo North America and Europe 350 subjects - target enrollment 30-week treatment duration Primary endpoint: change from baseline in the sum of UPDRS Parts II and III scores at week 30 |

| IPX066 - Advanced PD Patient Phase III Study Randomized, double-blind, active-control, parallel-group comparison of IPX066 vs. IR carbidopa-levodopa North America and Europe 420 subjects - target enrollment 22-week treatment duration Primary endpoint: percentage of "Off" time during waking hours |

| IPX056 Status Spasticity in Multiple Sclerosis Product type: Controlled-release Baclofen Baclofen is drug of choice in treating spasticity Significant unmet need for product offering reduced nocturnal spasticity, less morning stiffness, improved control, and dosing convenience Completed first successful Phase III Clinical successes with IPX066 support acceleration of required studies which will consume the majority of the 2010 spending target Further clinical research on IPX056 is necessarily a second priority |

| Financial Update |
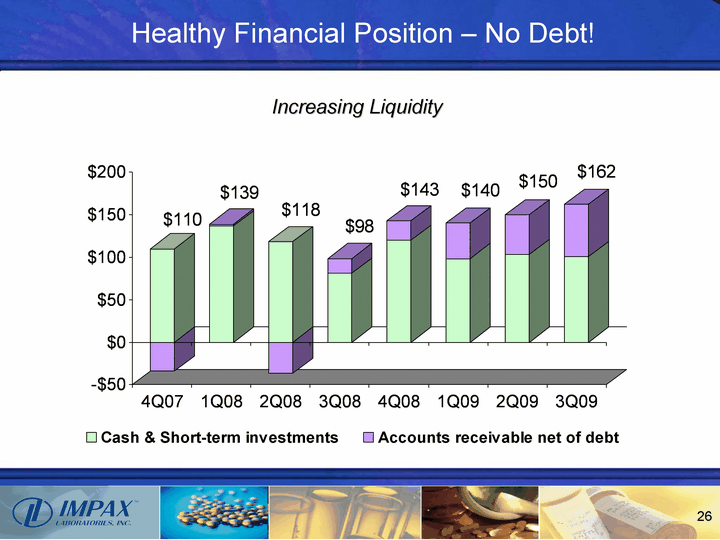
| Healthy Financial Position - No Debt! 4Q07 1Q08 2Q08 3Q08 4Q08 1Q09 2Q09 3Q09 Cash & Short-term investments 109.704 136.571 118.101 81.152 119.985 98.355 103.27 100.527 Accounts receivable net of debt -33.792 2.153 -36.702 17.249 22.9 41.746 46.578 61.543 Increasing Liquidity |

| 2010 Financial Outlook Improved top-line performance driven by generic division products: Significant variability depending on: Generic Adderall XR(r) Citizen Petition status Generic Flomax(r) competitive landscape Gross margins expected in ~ 50% range Expense control is critical management objective 2010 increases more than offset by revenue gains Free cash flow positive Before changes in current assets and liabilities Increase over positive operating cash (before CA/CL) flow for 2009 |
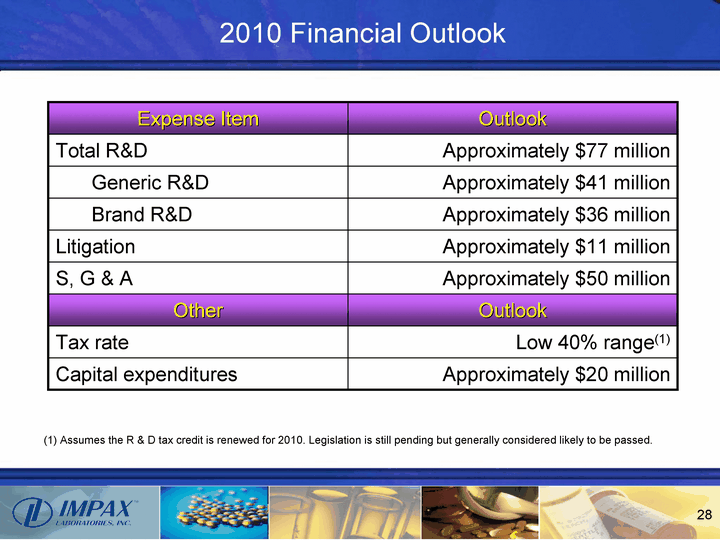
| 2010 Financial Outlook Expense Item Outlook Total R&D Approximately $77 million Generic R&D Approximately $41 million Brand R&D Approximately $36 million Litigation Approximately $11 million S, G & A Approximately $50 million Other Outlook Tax rate Low 40% range(1) Capital expenditures Approximately $20 million (1) Assumes the R & D tax credit is renewed for 2010. Legislation is still pending but generally considered likely to be passed. |
| Complex formulation development and manufacturing Balanced business model Track record of performance Substantial pipeline of new products Strong Platform for Long Term Growth Generic Unique Targeted High Value ANDAs Brand Offering Long-Term Growth Drivers |
