Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a10-1370_18k.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a10-1370_1ex99d1.htm |
Exhibit 99.2
|
|
Qnexa Obstructive Sleep Apnea Phase 2 Results (OB-204) Qnexa |
|
|
VIVUS cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests,“ "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on VIVUS’ current beliefs and expectations. These forward-looking statements include statements regarding the efficacy and safety of Qnexa, the potential for, and timing of, any NDA submission for Qnexa, the commercial and therapeutic potential of Qnexa, and the potential to obtain regulatory approval for, and effectively treat obstructive sleep apnea (OSA) with, Qnexa. The inclusion of forward looking statements should not be regarded as a representation by VIVUS that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to the risk and uncertainties inherent in VIVUS’ business, including, without limitation: additional analyses of data from the Qnexa Phase 3 obesity trials, the results of this single center study in OSA and any other clinical trials of Qnexa may produce negative or inconclusive results, or may be inconsistent with previously announced results or previously conducted clinical trials; the FDA may not agree with VIVUS’ interpretation of efficacy and safety results; earlier clinical trials may not be predictive of future results; Qnexa may not receive regulatory approval on a timely basis or at all, and the FDA may require VIVUS to complete additional clinical, non-clinical or other requirements prior to the submission or the approval of NDAs for a product candidate; the potential for adverse safety findings relating to Qnexa to delay or prevent regulatory approval or commercialization, or result in product liability claims, including serious adverse events that are not characterized by clinical investigators as possibly related to Qnexa; the third parties on whom VIVUS relies to assist with the development programs for Qnexa, including clinical investigators, contract laboratories, clinical research organizations and manufacturing organizations, may not successfully carry out their contractual duties or obligations or meet expected deadlines, and the quality or accuracy of the data or materials generated by such third parties may be of insufficient quality to include in VIVUS’ regulatory submissions; the ability of VIVUS and its licensors to obtain, maintain and successfully enforce adequate patent and other intellectual property protection of its product candidates; VIVUS may be unable to enter a collaboration or partnership relating to Qnexa for promotion to broader markets on attractive terms or at all; and other risks described in VIVUS’ filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and VIVUS undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. Information included herein is based on VIVUS’ review and evaluation of the clinical data. All conclusions and determinations contained herein are subject to VIVUS’ Qnexa analysis of the clinical data. The ultimate determination of the safety and efficacy of Qnexa will be made by the FDA and other relevant regulatory authorities. Forward-Looking Statements |
|
|
Obstructive Sleep Apnea (OSA) is a Serious Unmet Medical Need Obstructive sleep apnea (OSA) is a sleep-related breathing disorder that involves a decrease or complete halt in airflow despite an ongoing effort to breathe Long term effects of OSA include hypertension, stroke and premature death1,2 Obesity is the major risk factor for development of OSA 18 million adults in the US are estimated to have sleep apnea Up to 90% of OSA patients are untreated There is currently no approved pharmacotherapy agent for OSA 1. Marshall, et al., SLEEP 2008 2. Young et al. SLEEP 2008 |
|
|
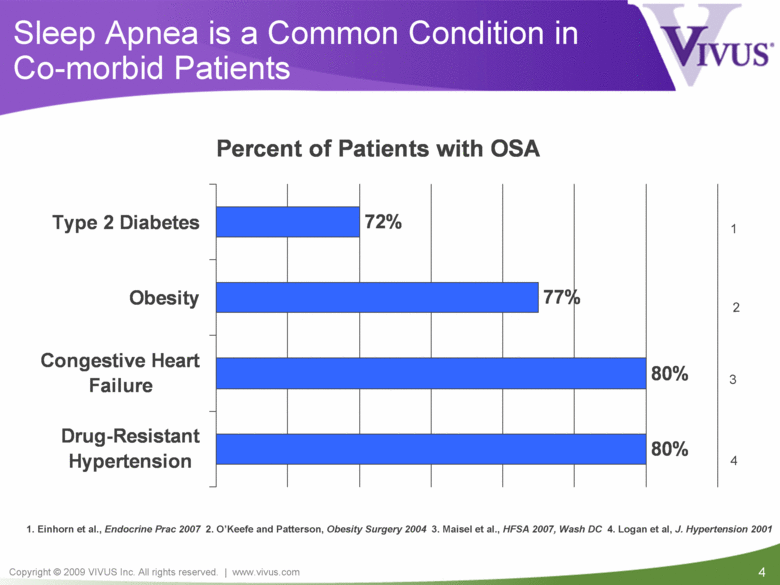
Sleep Apnea is a Common Condition in Co-morbid Patients 1. Einhorn et al., Endocrine Prac 2007 2. O’Keefe and Patterson, Obesity Surgery 2004 3. Maisel et al., HFSA 2007, Wash DC 4. Logan et al, J. Hypertension 2001 1 2 3 4 Percent of Patients with OSA 80% 80% 77% 72% Drug-Resistant Hypertension Congestive Heart Failure Obesity Type 2 Diabetes |
|
|
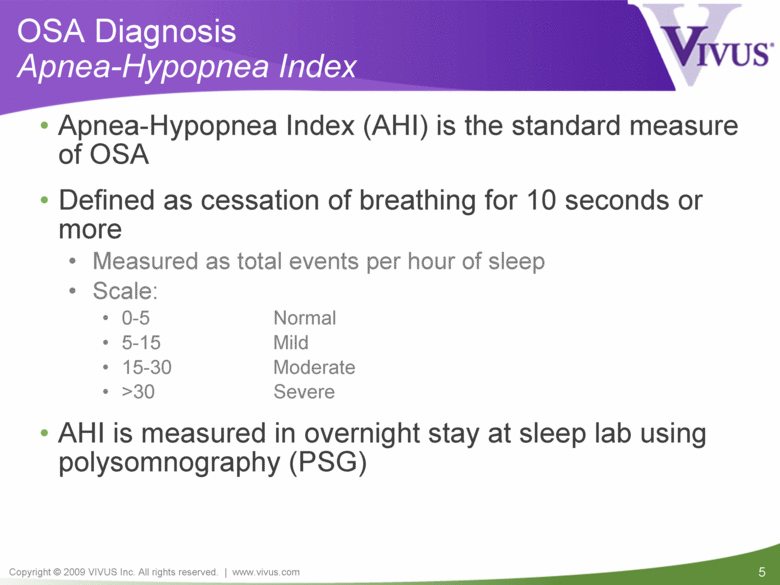
OSA Diagnosis Apnea-Hypopnea Index Apnea-Hypopnea Index (AHI) is the standard measure of OSA Defined as cessation of breathing for 10 seconds or more Measured as total events per hour of sleep Scale: 0-5 Normal 5-15 Mild 15-30 Moderate >30 Severe AHI is measured in overnight stay at sleep lab using polysomnography (PSG) |
|
|
Recognized Treatments for OSA Current treatments for OSA include: Weight loss Oral appliances and devices Surgery Continuous Positive Airway Pressure (CPAP) Device There is currently no approved pharmacotherapy agent for OSA |
|
|
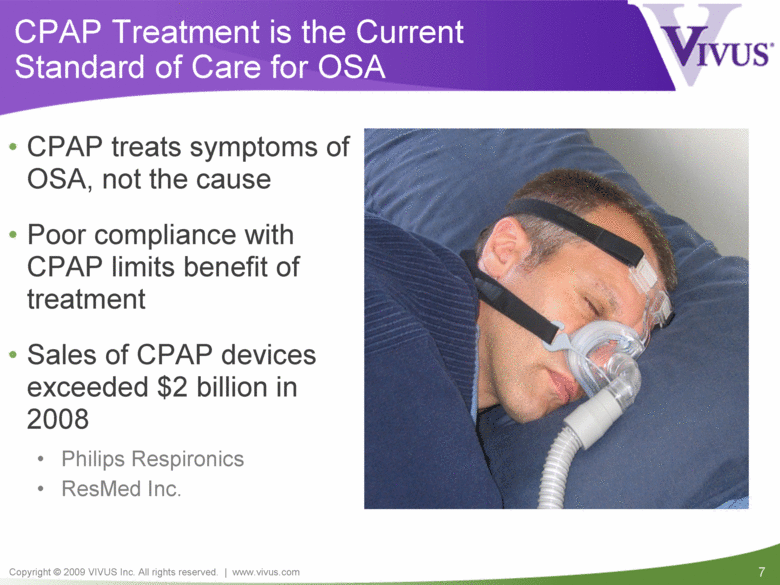
CPAP Treatment is the Current Standard of Care for OSA CPAP treats symptoms of OSA, not the cause Poor compliance with CPAP limits benefit of treatment Sales of CPAP devices exceeded $2 billion in 2008 Philips Respironics ResMed Inc. |
|
|
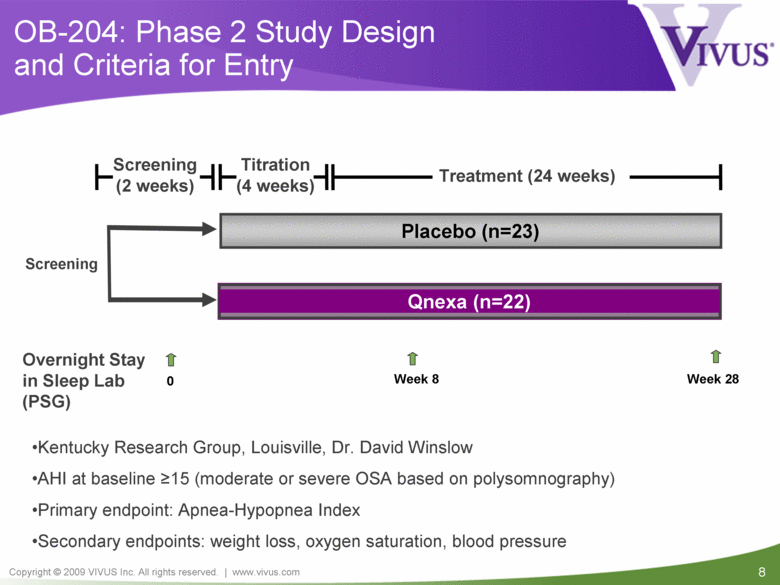
OB-204: Phase 2 Study Design and Criteria for Entry Titration (4 weeks) Treatment (24 weeks) Overnight Stay in Sleep Lab (PSG) Placebo (n=23) 0 Week 8 Week 28 Screening (2 weeks) Screening Kentucky Research Group, Louisville, Dr. David Winslow AHI at baseline >15 (moderate or severe OSA based on polysomnography) Primary endpoint: Apnea-Hypopnea Index Secondary endpoints: weight loss, oxygen saturation, blood pressure Qnexa (n=22) |
|
|
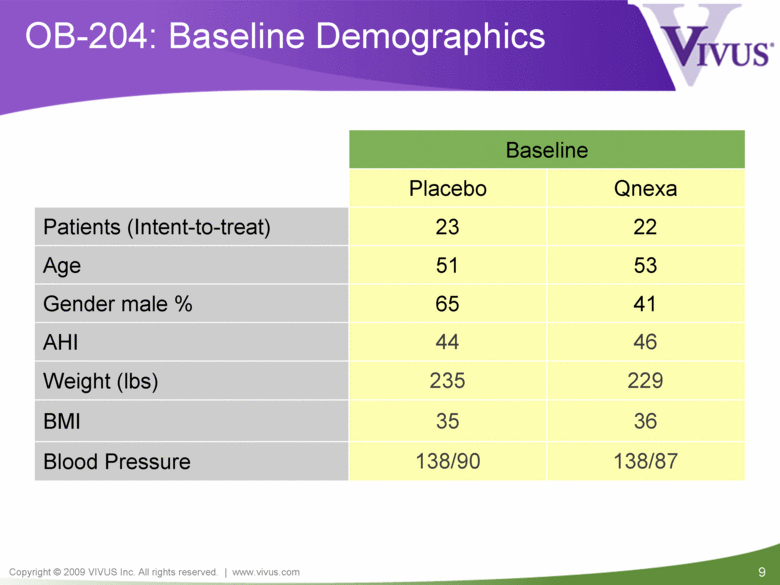
OB-204: Baseline Demographics 36 35 BMI 41 65 Gender male % 138/87 138/90 Blood Pressure Baseline 22 23 Patients (Intent-to-treat) Qnexa Placebo 235 44 51 Age 53 AHI 46 Weight (lbs) 229 |
|
|
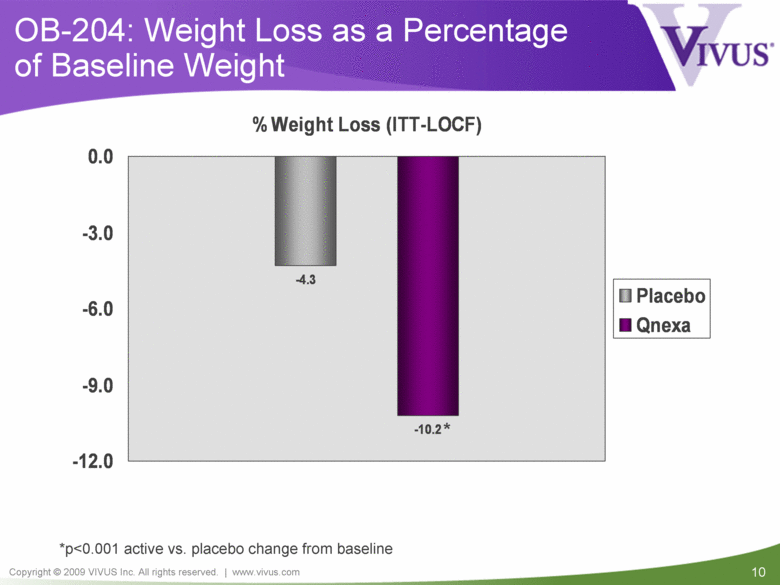
OB-204: Weight Loss as a Percentage of Baseline Weight *p<0.001 active vs. placebo change from baseline * -4.3 -10.2 -12.0 -9.0 -6.0 -3.0 0.0 % Weight Loss (ITT-LOCF) Placebo Qnexa |
|
|
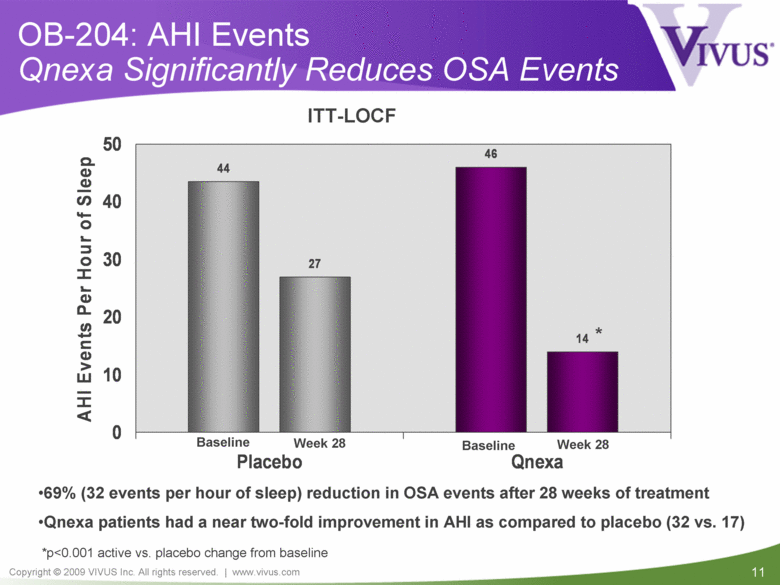
OB-204: AHI Events Qnexa Significantly Reduces OSA Events *p<0.001 active vs. placebo change from baseline 69% (32 events per hour of sleep) reduction in OSA events after 28 weeks of treatment Qnexa patients had a near two-fold improvement in AHI as compared to placebo (32 vs. 17) ITT-LOCF * Baseline Week 28 Week 28 Baseline 46 27 14 44 0 10 20 30 40 50 Placebo Qnexa AHI Events Per Hour of Sleep |
|
|
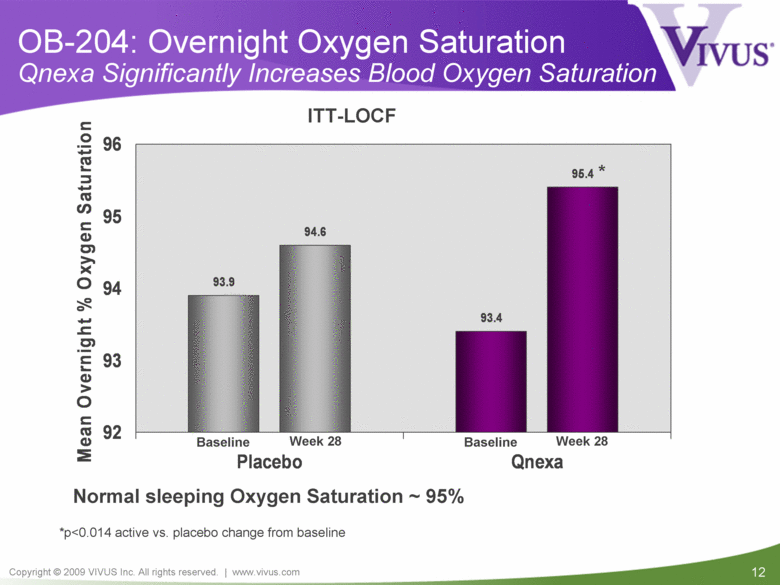
OB-204: Overnight Oxygen Saturation Qnexa Significantly Increases Blood Oxygen Saturation *p<0.014 active vs. placebo change from baseline * ITT-LOCF Normal sleeping Oxygen Saturation ~ 95% Baseline Baseline Week 28 Week 28 93.9 93.4 94.6 95.4 92 93 94 95 96 Placebo Qnexa Mean Overnight % Oxygen Saturation |
|
|
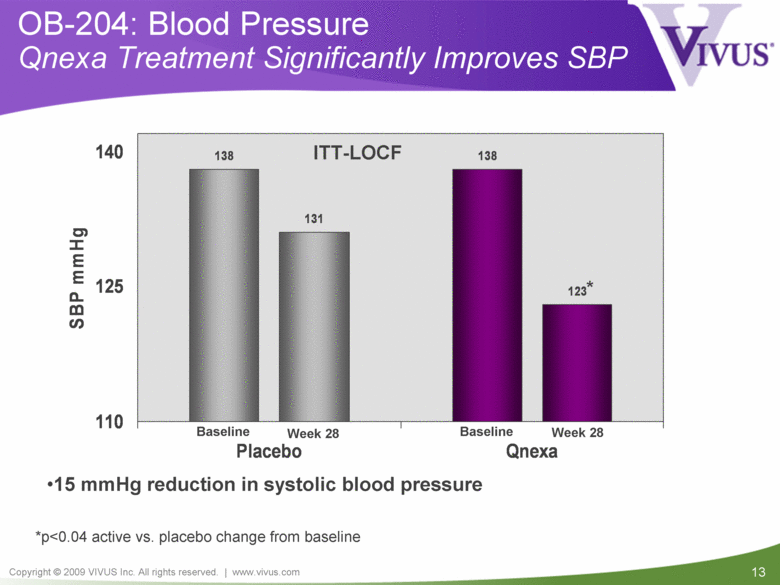
OB-204: Blood Pressure Qnexa Treatment Significantly Improves SBP *p<0.04 active vs. placebo change from baseline ITT-LOCF 15 mmHg reduction in systolic blood pressure * Week 28 Week 28 Baseline Baseline 138 138 131 123 110 125 140 Placebo Qnexa SBP mmHg |
|
|
OB-204: Conclusion Qnexa May be Promising Treatment for OSA Qnexa treatment for 28 weeks produced significant benefits in patients with OSA Number of apnea/hypopnea events decreased by 69% from a mean of 46 events per hour (severe) to 14 events per hour (mild) Lost 10.2% body weight or 23.8 pounds Oxygen saturation improved Systolic blood pressure decreased Most common side effects were dry mouth, altered taste, sinus infection No serious adverse events reported in Qnexa arm Data suggests that OSA is a promising new indication for Qnexa Study results submitted for presentation at scientific meeting Potential to be first pharmacotherapy for OSA |