Attached files
| file | filename |
|---|---|
| 8-K - 8K LAZARD CONFERENCE - WEST PHARMACEUTICAL SERVICES INC | lazard8k.htm |
| EX-99.2 - PRESS RELEASE LAZARD - WEST PHARMACEUTICAL SERVICES INC | pressrelease.htm |

1
Donald E. Morel, Jr., Ph.D.
Chairman and Chief Executive Officer
William J. Federici
Vice President and Chief Financial Officer
Investor Relations Contact:
Michael A. Anderson
Vice President and Treasurer
mike.anderson@westpharma.com
Lazard Capital Markets 6th Annual Healthcare Conference
New York, New York
November 17, 2009
NYSE: WST
westpharma.com
All trademarks and registered trademarks are the property of West Pharmaceutical Services, Inc., unless noted otherwise.

2
This presentation contains forward-looking statements, within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E of the Securities
Exchange Act of 1934, as amended, that are based on management’s beliefs and
assumptions, current expectations, estimates and forecasts. Statements that are not
historical facts, including statements that are preceded by, followed by, or that
include, words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate”
and other words and terms of similar meaning are forward-looking statements. West’s
estimated or anticipated future results, product performance or other non-historical
facts are forward-looking and reflect our current perspective on existing trends and
information.
27A of the Securities Act of 1933, as amended, and Section 21E of the Securities
Exchange Act of 1934, as amended, that are based on management’s beliefs and
assumptions, current expectations, estimates and forecasts. Statements that are not
historical facts, including statements that are preceded by, followed by, or that
include, words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate”
and other words and terms of similar meaning are forward-looking statements. West’s
estimated or anticipated future results, product performance or other non-historical
facts are forward-looking and reflect our current perspective on existing trends and
information.
Many of the factors that will determine the Company’s future results are beyond the
ability of the Company to control or predict. These statements are subject to known or
unknown risks or uncertainties, and therefore, actual results could differ materially
from past results and those expressed or implied in any forward-looking statement.
You should bear this in mind as you consider forward-looking statements. A non-
exclusive list of important factors that may affect future results may be found in
West’s filings with the Securities and Exchange Commission, including our annual
report on Form 10-K and our periodic reports on Form 10-Q and Form 8-K.
ability of the Company to control or predict. These statements are subject to known or
unknown risks or uncertainties, and therefore, actual results could differ materially
from past results and those expressed or implied in any forward-looking statement.
You should bear this in mind as you consider forward-looking statements. A non-
exclusive list of important factors that may affect future results may be found in
West’s filings with the Securities and Exchange Commission, including our annual
report on Form 10-K and our periodic reports on Form 10-Q and Form 8-K.
You should evaluate any statement in light of these important factors.
Forward Looking Statements

3
Who We Are
• Founded in 1923
• Leading global supplier of
components and systems
for injectable drug packaging
and delivery
components and systems
for injectable drug packaging
and delivery
– Vial
closure systems and
prefillable syringe components
prefillable syringe components
– Components
for disposable
delivery systems and diagnostics
delivery systems and diagnostics
– Devices
and device sub-
assemblies
assemblies
– Safety
and administration
systems
systems
• Market capitalization ~$1.3 billion
• Diverse, stable customer base

4
Each and every day,
more than 80 million
West products are used
to enhance the quality of
healthcare around the world.

5
• Global economic conditions remain soft, but show signs of
improvement
improvement
• Ongoing US healthcare reform debate
• Pharma remains under substantial pressure
• Global growth projected at 2.5%
- 5% (down from 5% - 7%)
• North America down 1%
- 2%
• Europe 0% - 1%
• Emerging economies 5% - 7% (India,
China, Brazil)
• Fundamental underlying industry change - consolidation - focus
on large molecule therapies
on large molecule therapies
• Pfizer - Wyeth
• Roche - Genentech
• AstraZeneca - Medimmune
• Lilly - Imclone
• Merck - Schering
Plough
Business Environment

6
Third Quarter Highlights
• Quarterly revenues grew 4.3% vs. 2008, excluding currency
– H1N1-related sales contributed to growth
– Customers’ cyclical inventory adjustments
concluding?
• Adjusted Diluted EPS 21.6% higher than prior year quarter
• Announced Fourth-Quarter Restructuring Plans
• Key Developments:
– Validated CZ insert needle now available
– Plastef acquisition completed
– Official Opening of China Manufacturing Facility
• Updated 2009 Guidance:
– Revenue between $1.03 billion and $1.05 billion
– Adjusted Diluted EPS between $2.08 and $2.13

7
Third Quarter and Year-To-Date Results
($ in millions, except per share data)
($ in millions, except per share data)
|
Quarter 3 |
|
Year-to-date | ||
|
2009 |
2008 |
|
2009 |
2008 |
|
$258.9 |
$256.2 |
Net Sales |
$762.3 |
$806.3 |
|
27.7% |
25.7% |
Gross Margin |
28.8% |
28.9% |
|
$5.1 |
$4.6 |
R&D |
$14.1 |
$14.8 |
|
$44.3 |
$41.5 |
SG&A |
$132.3 |
$122.5 |
|
$22.0 |
$19.7 |
Operating Profit (Non-GAAP) * |
$73.0 |
$94.9 |
|
$15.1 |
$12.2 |
Net Income (Non-GAAP) * |
$49.2 |
$62.6 |
|
$0.45 |
$0.37 |
Diluted EPS (Non-GAAP) * |
$1.45 |
$1.83 |
*2009 Q3 and YTD operating profit, net income and diluted EPS excludes a ($0.02) per share YTD impact of Tech restructuring charges, a $0.01
and $0.06 per share impact, respectively, of discrete tax benefits and a $0.04 Q3/YTD benefit for Brazil tax amnesty.
and $0.06 per share impact, respectively, of discrete tax benefits and a $0.04 Q3/YTD benefit for Brazil tax amnesty.
*2008 Q3 and YTD operating profit, net income and diluted EPS excludes a ($0.03) and $0.11 per share net (loss)/gain, respectively, on the
Nektar contract settlement, a ($0.05) per share YTD impact of Tech restructuring charges and a $0.06 and $0.09 per share impact, respectively,
of discrete tax benefits.
Nektar contract settlement, a ($0.05) per share YTD impact of Tech restructuring charges and a $0.06 and $0.09 per share impact, respectively,
of discrete tax benefits.

8
Capital Management
|
($M) |
9/30/09 |
12/31/08 |
|
Total Debt |
$397.0 |
$386.0 |
|
Total Equity |
$565.7 |
$487.1 |
|
Net Debt to Total Capital |
35.9% |
38.0% |
|
|
|
|
|
Capital Expenditures: |
|
|
|
YTD Spending |
$70.4 |
|
|
Full Year Forecast / Actual |
$110 - $120 |
$131.8 |
|
|
|
|
9
Market Dynamics Support Future Growth
• Increasing number of patients
with chronic illnesses
with chronic illnesses
• Many of these are treated with
biologic drugs
biologic drugs
• Biologic drugs demand ultra-
clean delivery systems
clean delivery systems
• Point of care shift: hospital to
specialty clinic to home
specialty clinic to home
• Need for safe, accurate dosing is
pushing the market toward
integrating the container/
closure system into the delivery
system
pushing the market toward
integrating the container/
closure system into the delivery
system
• Generic growth
Autoimmune Disease
Vaccines
Oncology
Diabetes

10
Why West - Our Competitive Advantage
• Unmatched experience/expertise: drug - material interface
– Global regulatory and technical support
• Protected IP: proprietary materials and technology
• Regulatory barrier to entry: US
NDA and ANDA filings must
include reference to packaging/components in contact
with the drug
include reference to packaging/components in contact
with the drug
1. West Drug Master File (DMF) 1546 is confidential
2. West DMF includes functionality data (multi-year
studies)
3. All primary package changes require new stability/functionality
studies for new filing
studies for new filing
• Global manufacturing footprint
• Market leader in packaging and delivery systems
for biologics

11
Packaging & Delivery Systems for Large Molecules
are a Key Business Driver
are a Key Business Driver
• Currently all large molecule drugs are delivered
by injection
by injection
• West components are preferred and used for the
top selling biotech therapeutics
top selling biotech therapeutics
• Used predominantly to treat chronic diseases
– Diabetes
– Cancer
– Auto-immune
• Fastest growing segment of our business
• Strong profit driver
12
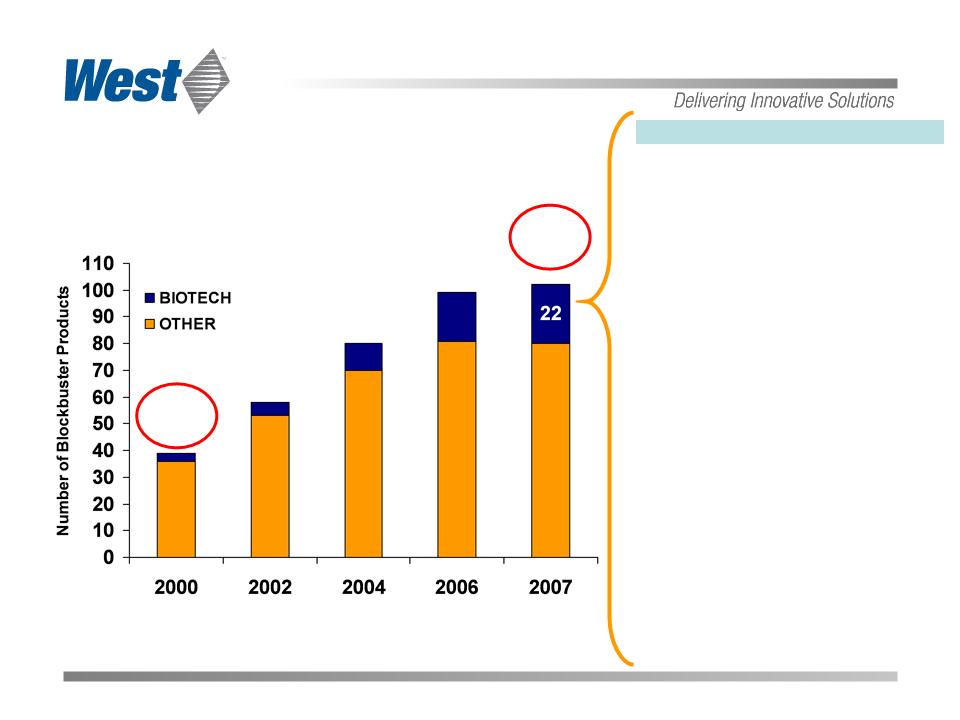
Global Biotech Blockbusters
(Annual Sales >$1Billion)
(Annual Sales >$1Billion)
Total
39
39
Total
106
106
|
2007’s 22 Biotech Blockbusters |
|
Enbrel (Amgen/Wyeth) |
|
Aranesp (Amgen) |
|
Remicade (J&J/SP) |
|
Rituxan (Roche) |
|
Neulasta (Amgen) |
|
Erypo/Procrit (J&J) |
|
Herceptin (Roche) |
|
Epogen (Amgen) |
|
Avastin (Roche) |
|
Humira (Abbott) |
|
Lantus (Sanofi-Aventis) |
|
Avonex (Biogen Idec) |
|
Neorecormon (Roche) |
|
Gardasil (Merck) |
|
Rebif (Serono) |
|
Neupogen (Amgen) |
|
Novorapid (Novo Nordisk) |
|
Erbitux (Imclone/Merck KGaA/BMS) |
|
Lucentis (Roche/Novartis/Genentech) |
|
Synagis (MedImmune) |
|
Humalog (Lilly) |
|
Betaferon (Bayer/Schering AG) |
Sources: IMS 2008 Global Biotech Perspective report and Company
Estimates
Estimates
West supplies components for all
current Biotech Blockbuster products
current Biotech Blockbuster products
13
|
Drug Packaging
(How it is contained) |
|
Primary Container Solutions |
|
Prefillable Syringe Systems |
|
Drug Delivery
(How it gets into the patient) |
|
Administration Systems |
|
Advanced Injection Systems |
Our Growth Strategy
• Build
market share in multi-
component systems for drug
administration
component systems for drug
administration
• Expand
proprietary product portfolio
through innovation and strategic
technology acquisitions
through innovation and strategic
technology acquisitions
• Market
segmentation to generate
incremental value per unit
incremental value per unit
• New
product innovation
• Lean
manufacturing
• Strategic
acquisitions
• Geographic
expansion
• China
• India
14
Core Strategy - Add Incremental Quality and Value
(representative prices per thousand units)
(representative prices per thousand units)
Uncoated 4432/50 V35
$50
$200
4432/50 S2 FluroTec®
Westar RS
Westar RS
$250
4432/50 S2 FluroTec®
Westar RS B2-40 Port Bag
Westar RS B2-40 Port Bag
$300
4432/50 S2 FluroTec®
Westar® RS B2-40
(1000 packs)
Westar® RS B2-40
(1000 packs)
Ready-to-sterilize
components
components
$465
4432/50 Sterile S2 W
(RU) Ready Pack®
(RU) Ready Pack®
(1000 packs)
$2,900
RU Ready Pack® System
(small volume sourcing only)
(small volume sourcing only)
Vials and closure system,
Ready-to-use (sterile)
$3,500
$360

15
China Plastics Facility
16
|
Drug Packaging
(How it is contained) |
|
Primary Container Solutions |
|
Prefillable Syringe Systems |
|
Drug Delivery
(How it gets into the patient) |
|
Administration Systems |
|
Advanced Injection Systems |
Our Growth Strategy
• Build
market share in multi-
component systems for drug
administration
component systems for drug
administration
• Expand
proprietary product portfolio
through innovation and strategic
technology acquisitions
through innovation and strategic
technology acquisitions
• Market
segmentation to generate
organic growth
organic growth
• New
product innovation
• Lean
manufacturing
• Strategic
acquisitions
• Geographic
expansion
• Capacity
build - Europe, Singapore,
North America
North America
• China
plastics
• India
rubber site selection

17
Delivery Systems - Key Programs
Vial2Bag™
Mix2Vial®
MixJect®
NovaGuard™ Safety
Needle Device
Needle Device
(luer-lock syringe)
ConfiDose®
Auto-injector
Auto-injector
Daikyo Crystal Zenith®
éris™
Safety Needle Device
(fixed-needle syringe)

18
Comparison of protein aggregation in glass versus CZ for
a model antibody
a model antibody
Daikyo Crystal Zenith®
Glass

19
Our Long-term Focus
• Superior quality and service
• Margin improvement - lean manufacturing, OEE
• Continued investment for the future
– Innovation: New products that meet the challenge
of biologics
– Geographic expansion
– Technology & product acquisitions
• Financial discipline
– Maintain strong operating cash flow
– Prudent spending: discretionary SG&A,
R&D and Capex
– Maintain a strong balance sheet
– Return on Invested Capital > Weighted
Average Cost of Capital

20
|
Drug Packaging
(How it is contained) |
|
Primary Container Solutions |
|
Prefillable Syringe Systems |
|
Drug Delivery
(How it gets into the patient) |
|
Administration Systems |
|
Advanced Injection Systems |
Five-Year Growth Opportunity
$2 billion combined markets for safety,
prefilled and auto-injectors:
prefilled and auto-injectors:
• Annual
unit growth 6-12%,
depending on segment
depending on segment
• West
2009 sales: $250 million
• 2009
OP margin: 5%
Strategic Planning Goals:
• Projected
2014 sales of $600 million
• Projected
2014 OP margin 20%
$1.5 billion market for packaging
components:
components:
• Annual
unit growth 0-8%, depending
on segment
on segment
• West
2009 sales: $750 million
• 2009
OP margin: 18%
Strategic Planning Goals:
• Projected
2014 sales of $1.0 billion
• Projected
2014 OP margin 20%

21
Summary
• Market leader
• Proprietary technology
• Regulatory expertise
• Customer base
• Global footprint
• Partnerships
– Daikyo
– Mexico
• New product pipeline
• Financial position
– Cash flow
– Balance sheet
• People
Injectable Container Solutions
Advanced
Injection
Systems
Injection
Systems
Prefillable Syringe
Systems
Systems
Safety and Administration
Systems
Systems

22
Donald E. Morel, Jr., Ph.D.
Chairman and Chief Executive Officer
William J. Federici
Vice President and Chief Financial Officer
Investor Relations Contact:
Michael A. Anderson
Vice President and Treasurer
mike.anderson@westpharma.com
Lazard Capital Markets 6th Annual Healthcare Conference
New York, New York
November 17, 2009
NYSE: WST
westpharma.com
All trademarks and registered trademarks are the property of West Pharmaceutical Services, Inc., unless noted otherwise.
