Attached files
| file | filename |
|---|---|
| 8-K - LIGAND PHARMACEUTICALS INCORPORATED 8-K - LIGAND PHARMACEUTICALS INC | a6092591.htm |
| EX-99.1 - EXHIBIT 99.1 - LIGAND PHARMACEUTICALS INC | a6092591ex99_1.htm |
Exhibit 99.2

November 5, 2009 Filed by Ligand Pharmaceuticals Incorporated.Pursuant to Rule 425 under the Securities Act of 1933 and deemed filed pursuant to rule 14a-6 under the Securities and Exchange Act of 1934, as amended.Subject Company: Ligand Pharmaceuticals IncorporatedCommission File No: 001-33093

Safe Harbor Statement The following presentation contains forward-looking statements regarding Ligand’s prospects, plans and strategies, drug development programs and collaborations, and the proposed acquisitions of Neurogen and Metabasis by Ligand. Forward-looking statements include financial projections, expectations regarding research and development programs, and other statements including words such as “will,“ “should,” “could,” “plan,” etc.The forward looking statements made in the presentation are subject to several risk factors, including but not limited to the reliance on collaborative partners for milestone and royalty payments, regulatory hurdles facing product candidates, uncertain product development costs, the possibility that drug candidates might not be proved to be safe and efficacious, the commercial performance of approved products, the failure of Neurogen’s and/or Metabasis’ stockholders to approve the announced mergers, Ligand’s, Neurogen’s and/or Metabasis’ inability to satisfy the conditions of the mergers, or that one or both mergers is otherwise delayed or ultimately not consummated, and a failure of the combined businesses to be integrated successfully. Additional risks may apply to forward-looking statements made in this presentation.The risk factors facing Ligand, Neurogen and Metabasis are explained in greater detail in Ligand’s, Neurogen’s and Metabasis’ filings with the SEC, including the most recently filed annual reports on Form 10-K and quarterly reports on Form 10-Q, as well as other public filings.

Additional Information and Where to Find It Ligand has filed with the SEC and intends to ask the SEC to declare effective a Registration Statement on Form S-4, which includes a proxy statement of Neurogen and other relevant materials in connection with the proposed Neurogen transaction, and intends to file with the SEC a Registration Statement on Form S-4, which will include a proxy statement of Metabasis and other relevant materials in connection with the proposed Metabasis transaction. The proxy statements will be mailed to the respective stockholders of Neurogen and Metabasis. Respective investors and security holders of Neurogen and Metabasis are urged to read the proxy statements and the other relevant materials when they become available, before making any voting or investment decision with respect to the proposed transactions, because they will contain important information about Ligand, Neurogen/Metabasis and the applicable proposed transaction. The proxy statements and other relevant materials (when they become available), and any other documents filed by Ligand, Neurogen or Metabasis with the SEC, may be obtained free of charge at the SEC's website at www.sec.gov. In addition, investors and security holders may obtain free copies of the documents filed with the SEC by Ligand by going to Ligand’s Investor Relations page on its corporate website at www.ligand.com. Investors and security holders may obtain free copies of the documents filed with the SEC by Neurogen by going to Neurogen’s Investors page on its corporate website at www.neurogen.com. Investors and security holders may obtain free copies of the documents filed with the SEC by Metabasis by going to Metabasis’ Investors page on its corporate website at www.mbasis.com.Ligand and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Neurogen and Metabasis in favor of the proposed transactions. Information concerning Ligand’s directors and executive officers is set forth in Ligand’s proxy statement for its 2009 annual meeting of shareholders, which was filed with the SEC on April 29, 2009, and annual report on Form 10-K filed with the SEC on March 16, 2009. Neurogen and Metabasis and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Neurogen and Metabasis, respectively, in favor of the applicable proposed transaction. Information about Neurogen and Metabasis executive officers and directors and their ownership of Neurogen and Metabasis common stock is set forth in Neurogen’s and Metabasis’ amended annual reports on Form 10-K, each of which was filed with the SEC on April 30, 2009. Investors and security holders may obtain more detailed information regarding the direct and indirect interests of Neurogen and Metabasis and their respective executive officers and directors in the respective acquisitions by reading the proxy statements regarding the mergers, which will be filed with the SEC.

Q3 Financial Highlights

Ligand’s Strategy Invest in promising, large market research programsAcquire high-quality, fully funded partnered assetsAssemble broad portfolio of assets - Many partners, many indications, many different molecules in developmentRun efficient business that is not dependent on equity financings Ligand uniquely qualified to drive this strategy Strategy maximizes long-term potential revenueswhile minimizing risks
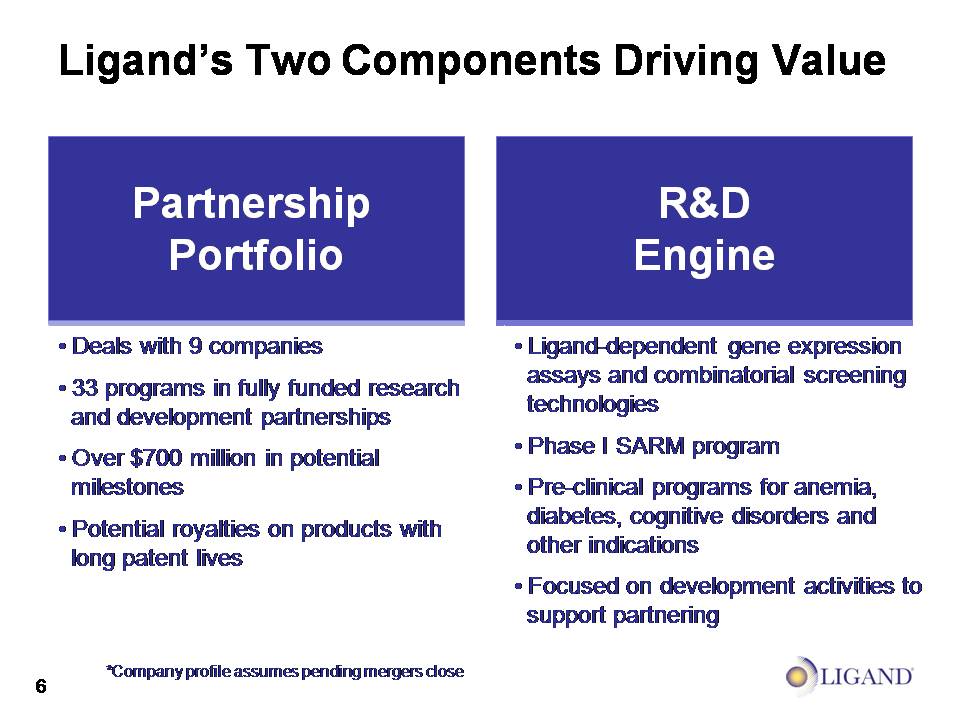
Ligand’s Two Components Driving Value Partnership Portfolio Biotech Business Company profile assumes pending mergers close
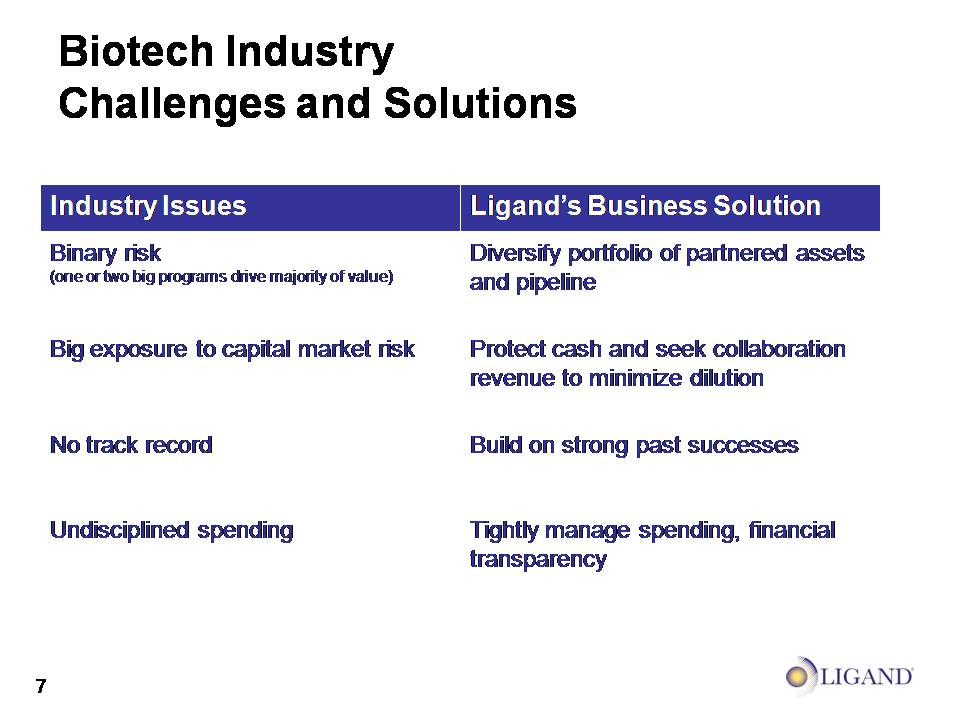
Biotech Industry Challenges and Solutions
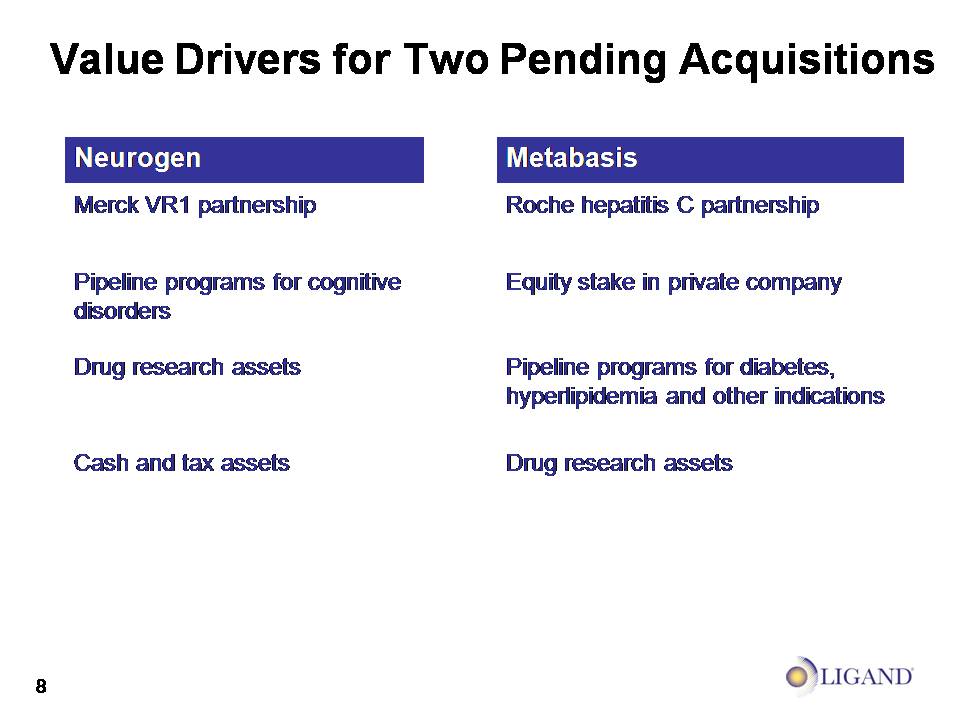
Value Drivers for Two Pending Acquisitions
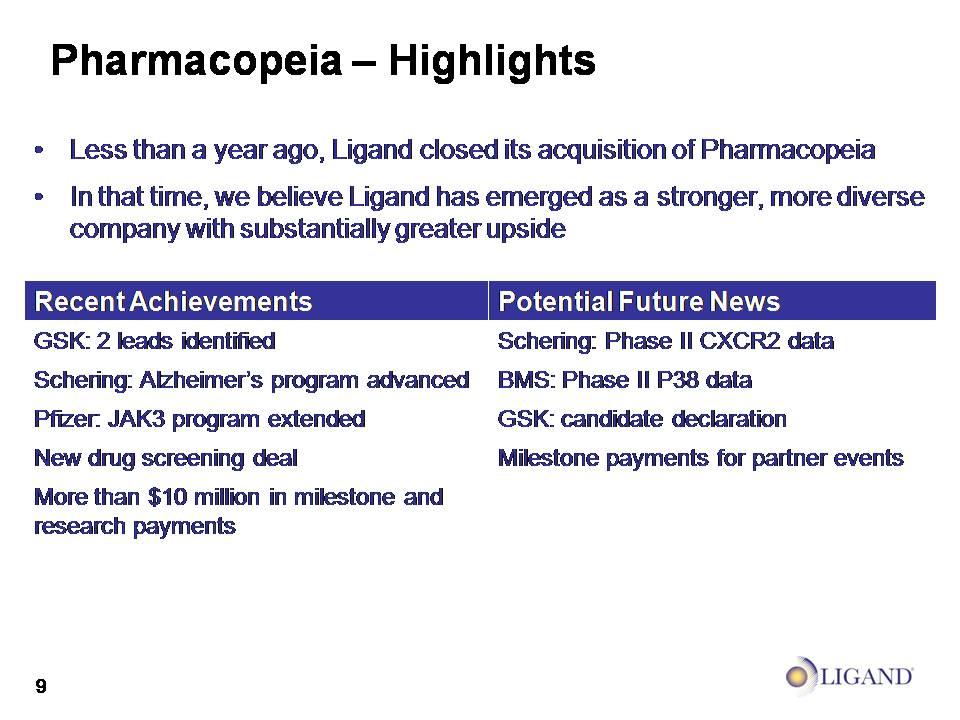
Pharmacopeia – Highlights Less than a year ago, Ligand closed its acquisition of Pharmacopeia In that time, we believe Ligand has emerged as a stronger, more diverse company with substantially greater upside
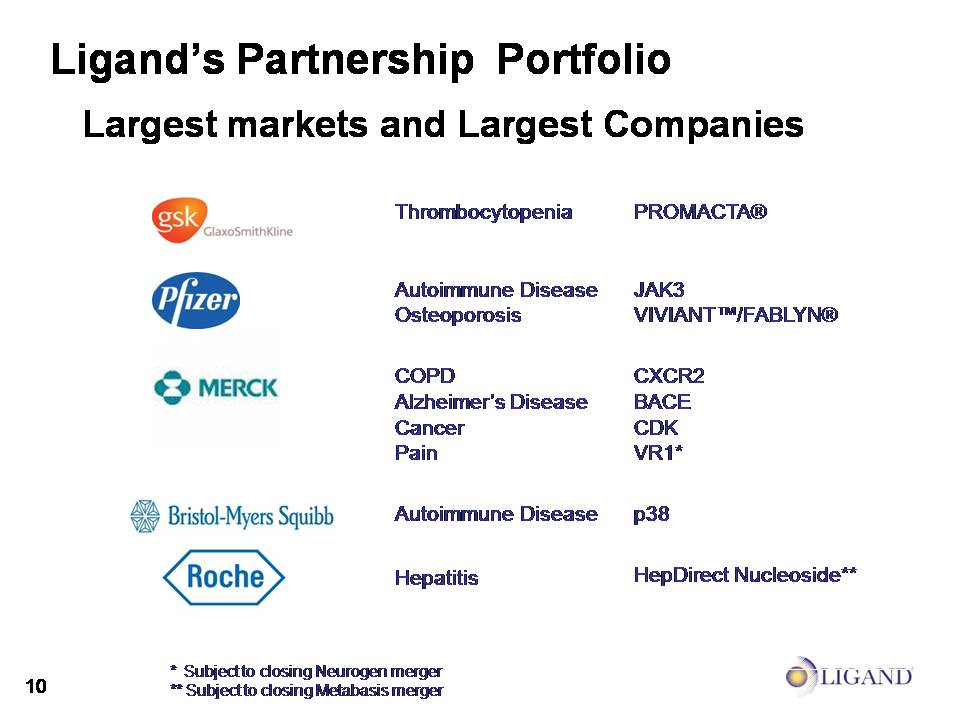
Ligand’s Partnership Portfolio Largest markets and Largest Companies * Subject to closing Neurogen merger** Subject to closing Metabasis merger
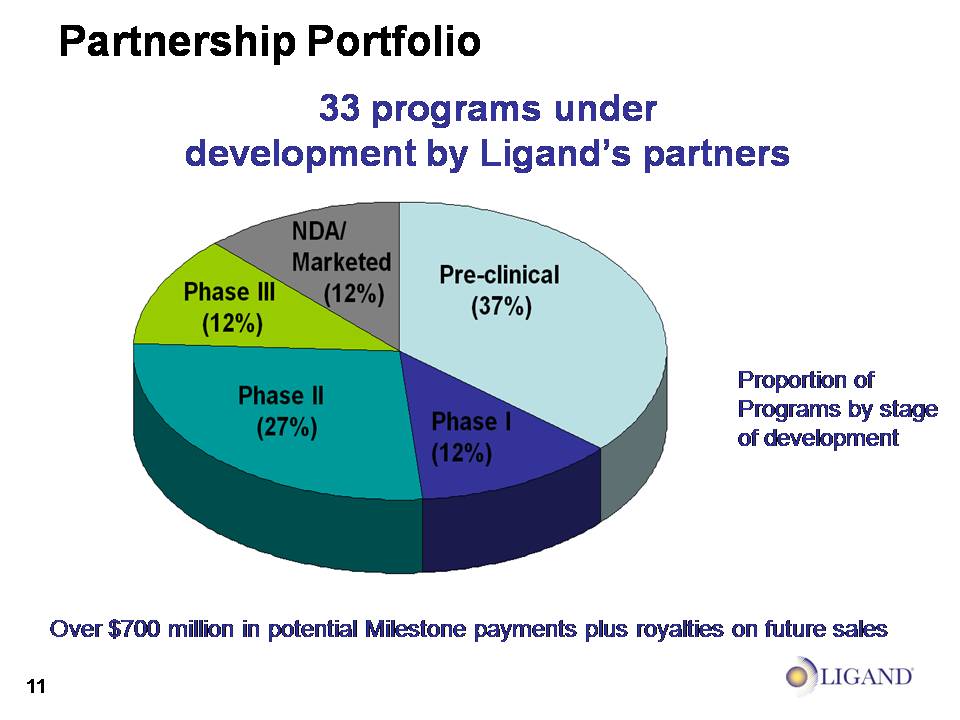
Partnership Portfolio 38 programs under development by Ligand’s partners Pre-clinical (47%) Phase I(13%) Phase II (18%) Phase III (11%) NDA/Marketed (11%) Over $700 million in potential Milestone payments plus royalties on future sales Proportion of Programs by stageof development
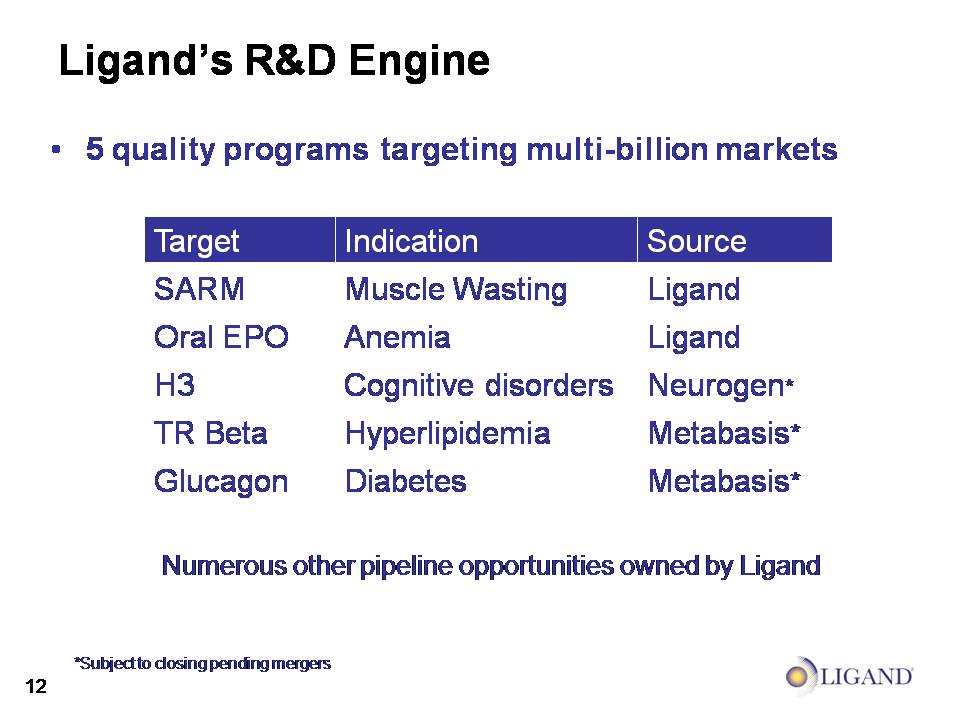
Ligand’s Biotech Business 5 quality programs targeting multi-billion markets *Subject to closing pending mergers Numerous other pipeline opportunities owned by Ligand

Biotech Business Results 5 molecules discovered or co-discovered by Ligand have been approved and currently 3 are on the market Over 15 licensing deals executed by Ligand Most recent deal struck with GSK for thrombocytopenia drug less than one year ago with 16% royalty rate

Research Highlights
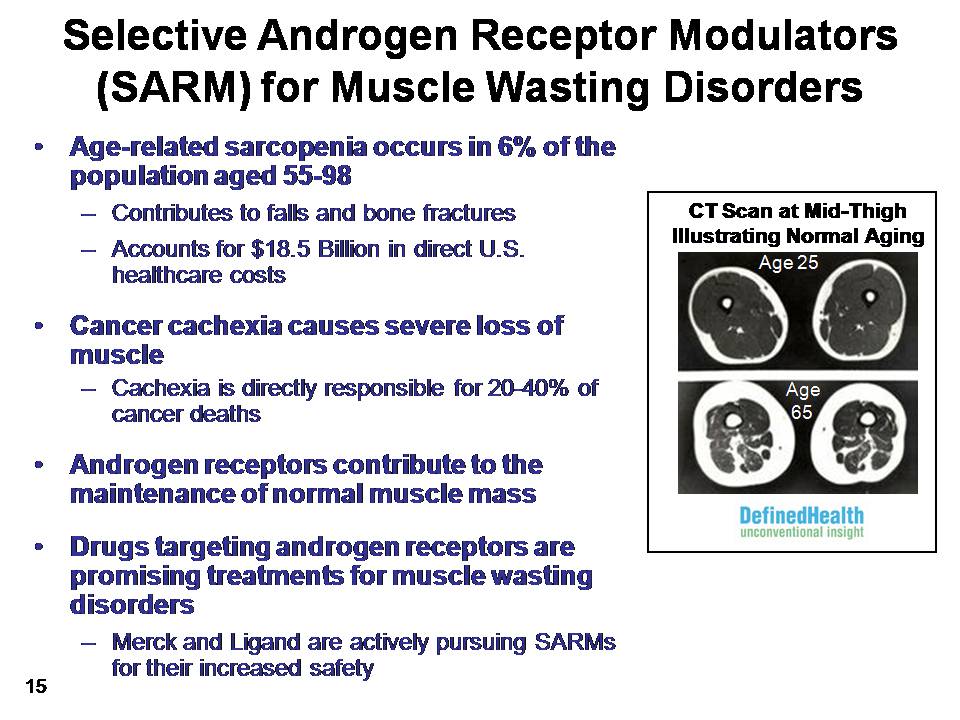
Selective Androgen Receptor Modulators (SARM) for Muscle Wasting Disorders Age-related sarcopenia occurs in 6% of the population aged 55-98Contributes to falls and bone fracturesAccounts for $18.5 Billion in direct U.S. healthcare costsCancer cachexia causes severe loss of muscleCachexia is directly responsible for 20-40% of cancer deathsAndrogen receptors contribute to the maintenance of normal muscle massDrugs targeting androgen receptors are promising treatments for muscle wasting disordersMerck and Ligand are actively pursuing SARMs for their increased safety(Gp:) CT Scan at Mid-ThighIllustrating Normal Aging (Gp:) Age 25 (Gp:) Age65

LGD-4033 SARM PropertiesBest-in-class potency and tissue selectivityFull activity on androgen receptor in skeletal muscle and boneWeak, partial activity in prostate and sebaceous glandsOrally-active, safe and effective in animal modelsLGD-4033 Phase I Single Ascending Dose study recently completedUpcoming EventsA Phase I Multiple Ascending Dose clinical trial will begin shortlyLGD-4033 preclinical data will be presented in the Late Breaking Session of the Gerontology Society of America 62nd Annual Scientific Meeting, November 20, 2009 (Atlanta, GA)
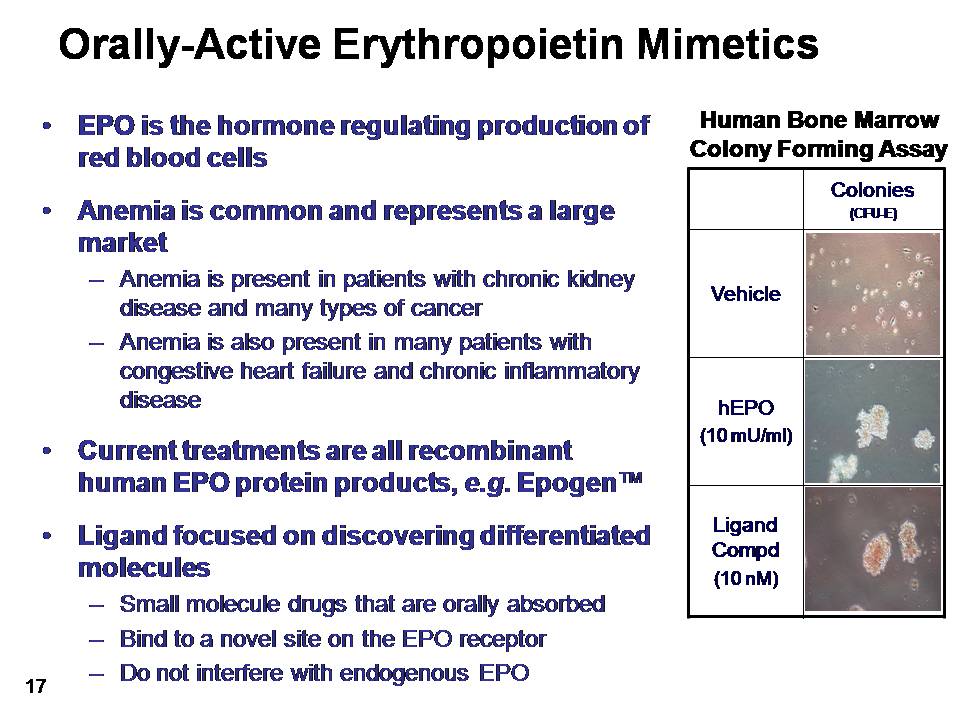
Orally-Active Erythropoietin Mimetics EPO is the hormone regulating production of red blood cellsAnemia is common and represents a large marketAnemia is present in patients with chronic kidney disease and many types of cancerAnemia is also present in many patients with congestive heart failure and chronic inflammatory disease Current treatments are all recombinant human EPO protein products, e.g. Epogen™Ligand focused on discovering differentiated moleculesSmall molecule drugs that are orally absorbedBind to a novel site on the EPO receptorDo not interfere with endogenous EPO Human Bone MarrowColony Forming Assay
Selected Additions to Ligand Pipeline Neurogen and Metabasis Acquisitions Histamine H3 Receptor Antagonist Potential treatment for narcolepsy and mild cognitive impairmentNeurogen’s lead compound is at an advanced preclinical stageGlucagon Receptor AntagonistGlucagon secretion is abnormal in Type 2 Diabetes resulting in excessive glucose production Potential treatment for Type 2 Diabetes by a novel mechanismMetabasis’ lead compound is at an advanced preclinical stageLiver-Directed Thyroid Hormone Receptor-b (TR-b) AgonistOral drug lowering LDL-cholesterol and Lpa without stimulating the heartIntended as add-on therapy in statin-treated patients who continue to have elevated cholesterolMetabasis’ compounds are at Phase I and preclinical stage

Potential Near-Term Milestone and Events
