Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NOVAVAX INC | tm2127238d1_8k.htm |
Exhibit 99.1

Nasdaq: NVAX | September 2021 CORPORATE OVERVIEW AND INVESTOR DECK

2 novavax.com Safe Harbor Statement Certain information, particularly information relating to the future of Novavax, its operating plans and prospects, the ongoing development of NVX - CoV2373 and other Novavax vaccine product candidates, timing of future regulatory filings and actions, anticipated manufacturing capacity, the readiness of our global supply chain and future availability of NVX - CoV2373 at a global scale constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act. Forward - looking statements may generally contain words such as “believe,” “may,” “could,” “will,” “possible,” “can,” “estimate,” “continue,” “ongoing,” “consider,” “intend,” “indicate,” “plan,” “project,” “expect,” “should,” “would,” or “assume” or variations of such words or other words with similar meanings. Novavax cautions that these forward - looking statements are subject to numerous assumptions, risks and uncertainties that change over time and may cause actual results to differ materially from the results discussed in the forward - looking statements. These risks and uncertainties include challenges satisfying, alone or together with partners, various safety, efficacy, and product characterization requirements, including those related to process qualification and assay validation, necessary to satisfy applicable regulatory authorities; difficulty obtaining scarce raw materials and supplies; resource constraints, including human capital and manufacturing capacity, on the ability of Novavax to pursue planned regulatory pathways; challenges meeting contractual requirements under agreements with multiple commercial, governmental, and other entities; and those other risk factors identified in the “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” sections of Novavax' Annual Report on Form 10 - K for the year ended December 31, 2020 and subsequent Quarterly Reports on Form 10 - Q, as filed with the Securities and Exchange Commission, which are available at www.sec.gov and www.novavax.com . Forward - looking statements are based on current expectations and assumptions and currently available data and are neither predictions nor guarantees of future events or performance. Current results may not be predictive of future results. You should not place considerable reliance on forward - looking statements which speak only as of the date hereof. The Company does not undertake to update or revise any forward - looking statements after they are made, whether as a result of new information, future events, or otherwise, except as required by applicable law. Matrix - M and NanoFlu are trademarks of Novavax, Inc. Safe Harbor Statement

3 novavax.com Novavax at - a - Glance 90% Overall Efficacy in PREVENT - 19 Phase 3 Trial 10+ years of Nanoparticle Vaccine Development 150 million Doses per Month Manufacturing Capacity by end of 4Q 2021* 100% Efficacy Against Moderate and Severe Disease $2+ billion in Funding Secured to Date 93% Efficacy Against the Predominantly Circulating VoC and VoI *When all planned capacity is online
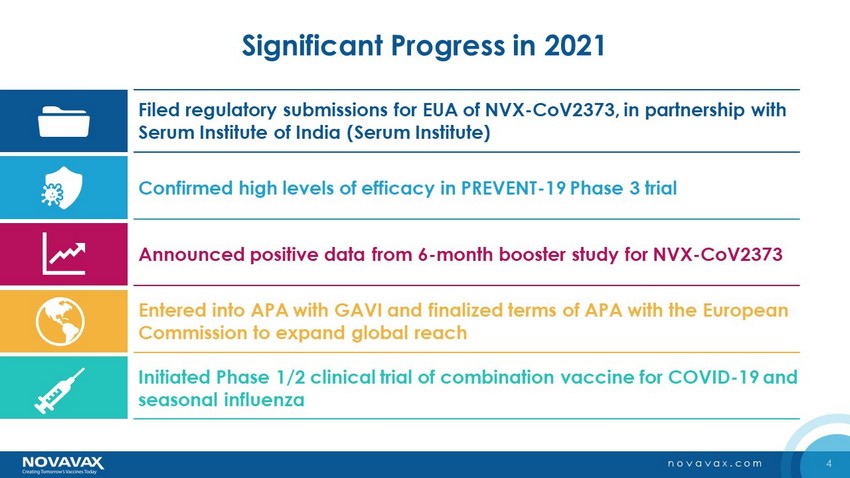
4 novavax.com Significant Progress in 2021 Filed regulatory submissions for EUA of NVX - CoV2373, in partnership with Serum Institute of India (Serum Institute) Confirmed high levels of efficacy in PREVENT - 19 Phase 3 trial Announced positive data from 6 - month booster study for NVX - CoV2373 Entered into APA with GAVI and finalized terms of APA with the European Commission to expand global reach Initiated Phase 1/2 clinical trial of combination vaccine for COVID - 19 and seasonal influenza

5 novavax.com Table of Contents Introduction COVID - 19 Clinical Trial Information Booster Study 6 37 9 51 NVX - CoV2373 Regulatory Pathway 53 NanoFlu Πand Combination Vaccine Programs 56 Strategic Development of COVID - 19 Vaccines NVX - CoV2373 Manufacturing & Distribution 60 Clinical Development Conducted by Partners 64 Upcoming Milestones 67 Page Section

6 novavax.com Introduction

8 novavax.com The picture can't be displayed. NVX - CoV2373 Highlighted in Recent Peer - Reviewed Publications

9 novavax.com COVID - 19 Clinical Trial Information

10 novavax.com NVX - CoV2373 Clinical Development Program ▪ Established dose level in younger and older adults ▪ Confirmed need for adjuvant and 2 dose schedule ▪ Defined immunologic phenotype ▪ Described preliminary safety profile Phase 1/2 US & Australia N=131 Phase 1 N=1,288 Phase 2 Keech et al. NEJM 02 September 2020 ▪ Evaluated preliminary efficacy ▪ Defined safety profile ▪ HIV+ subgroup Phase 2b South Africa N=4,422 Shinde et al. NEJM 20 May 2021 ▪ Licensure - enabling safety data ▪ Licensure - enabling efficacy data ▪ Safety of co - administration with influenza vaccine Phase 3 United Kingdom N=15,203 Heath et al. NEJM 30 June 2021 ▪ Licensure - enabling safety in US population ▪ Licensure - enabling efficacy in US populations Phase 3 US & Mexico N=29,960

11 novavax.com Key Takeaways from NVX - CoV2373 Clinical Trials 3 Publications in The New England Journal of Medicine Immunogenicity Safety Efficacy x Favorable safety and reactogenicity profile x Efficacy confirmed against original COVID - 19 and variant strains x 100% efficacy against moderate and severe disease x Robust immune responses generated (2 doses of 5 µg + Matrix - M Œ adjuvant) 50,000+ Participants E nrolled in Clinical Trials 4 Clinical Trials Conducted Across 4 Continents 3 Crossovers Initiated in Late - stage Trials

12 novavax.com PREVENT - 19 Phase 3 United States and Mexico

13 novavax.com PREVENT - 19 Phase 3 Trial Design 5µg + 50µg Matrix - M adjuvant 2 injections, 21 days apart n = ~20,000 Placebo 2 injections, 21 days apart n = ~10,000 29,960 Adults > 18 years R 2:1 • Primary endpoint : PCR - positive symptomatic mild, moderate or severe COVID - 19 illness diagnosed ≥ 7 days after second dose • 2:1 randomization • Pediatric expansion underway (see slide 23) Randomized, observer - blinded, placebo - controlled trial evaluating efficacy, immunogenicity and safety Placebo 2 injections, 21 days apart 5µg + 50µg Matrix - M adjuvant 2 injections, 21 days apart Protocol version 8.0 posted on Novavax.com

14 novavax.com 90% Overall Vaccine Efficacy Placebo NVX - CoV2373 0.0% 0.2% 0.4% 0.6% 0.8% 1.0% 1.2% 1.4% 1.6% 1.8% 0 7 14 21 28 35 42 49 56 63 70 77 84 91 98 105 112 119 126 133 140 147 154 Event Rate Days from first vaccination Vaccine and placebo rates separate prior to dose 2 (day 21). No evidence of waning efficacy through day 98. = case

15 novavax.com 93% Efficacy Against Predominantly Circulating Variants of Interest and Variants of Concern VoI / VoC represented 82% of cases 6 (13.6%) 1 (2.3%) 1 (2.3%) 1 (2.3%) 0 10 20 30 B.1.526 B.1.526-1 B.1.617 P.2 28 (63.6%) 3 (6.8%) 2 (4.5%) 2 (4.5%) 0 10 20 30 B.1.1.7 B.1.429 B.1.351 P.1 Variant definition source: cdc.gov 9 (17%) Variants of Interest 35 (65%) Variants of Concern 10 (19%) Variants not of Concern/Interest VE = 100% Sequencing performed at University of Washington VE = 93.6% (post - hoc) VoI / VoC VE = 92.6%

16 novavax.com Serious and Severe Events: Infrequent and Balanced Safety summary through crossover (n=25,981) NVX - CoV2373 Placebo 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Any Adverse Event (AE) Any Medically Attended AE Serious AE Any Severe AE AESIs Deaths An AE is any event reported, whether or not related to vaccine. No single adverse event term was reported by more than 1% of participants . *Rate <0.1% * *

17 novavax.com 0% 20% 40% 60% 80% 100% Tenderness Pain Erythema Swelling 0% 20% 40% 60% 80% 100% Tenderness Pain Erythema Swelling Mild Favorable Reactogenicity Profile Local: Pain and Tenderness most common, 3 days duration Systemic: Fatigue, Headache and Muscle Pain, 2 days duration Dose 1 Dose 2 0% 20% 40% 60% 80% 100% Tenderness Pain Erythema Swelling Grade 3+ 0% 20% 40% 60% 80% 100% Tenderness Pain Erythema Swelling Any Grade NVX - CoV2373 Placebo 0% 20% 40% 60% 80% 100% Grade 3+ 0% 20% 40% 60% 80% 100% 0% 20% 40% 60% 80% 100% Headache Muscle pain Fatigue Malaise Nausea/Vomiting Fever Joint Pain Mild 0% 20% 40% 60% 80% 100% Headache Muscle pain Fatigue Malaise Nausea/Vomiting Fever Joint pain Any Grade Local Systemic

18 novavax.com Final Analysis: High Overall Efficacy NVX - CoV2373 (n=17,312)* Placebo (n=8,125)* Total 14 63 Mild 14 49 Moderate 0 10 Severe 0 4 Vaccine Efficacy 90.4% (95% CI: 82.9, 94.6) • Primary efficacy statistical criteria achieved with lower bound of 95% CI >30 • 82% of cases caused by Variants of Interest (“ VoI ”) & Variants of Concern (“ VoC ”) • All breakthrough cases in vaccine group were mild *2:1 randomization
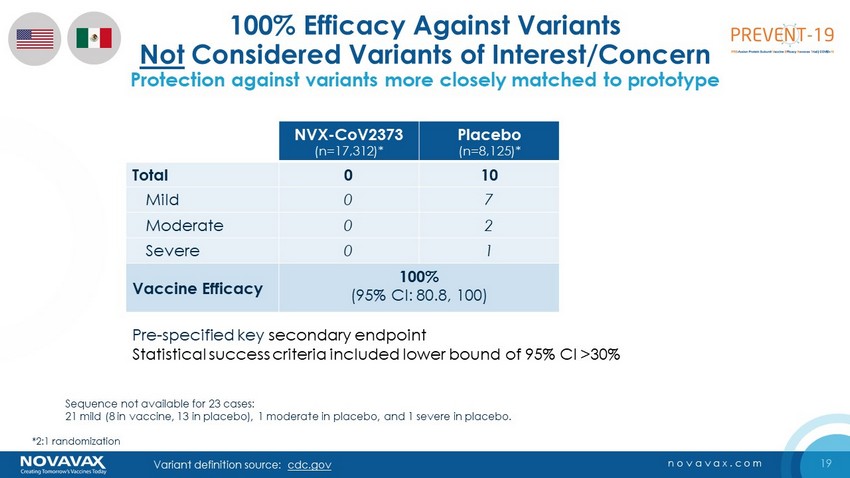
19 novavax.com 100% Efficacy Against Variants Not Considered Variants of Interest/Concern Protection against variants more closely matched to prototype NVX - CoV2373 (n=17,312)* Placebo (n=8,125)* Total 0 10 Mild 0 7 Moderate 0 2 Severe 0 1 Vaccine Efficacy 100% (95% CI: 80.8, 100) Pre - specified key secondary endpoint Statistical success criteria included lower bound of 95% CI >30% Variant definition source: cdc.gov Sequence not available for 23 cases: 21 mild (8 in vaccine, 13 in placebo), 1 moderate in placebo, and 1 severe in placebo. *2:1 randomization

20 novavax.com High Efficacy Against Variants of Interest & Variants of Concern NVX - CoV2373 (n=17,312)* Placebo (n=8,125)* Total 6 38 Mild 6 29 Moderate 0 7 Severe 0 2 Vaccine Efficacy 92.6% (95% CI: 83.6, 96.7) Variant definition source: cdc.gov Efficacy updated in post - hoc analyses. Sequence not available for 23 cases: 21 mild (8 in vaccine, 13 in placebo), 1 moderate in placebo, and 1 severe in placebo. *2:1 randomization

21 novavax.com 100% Efficacy Against Moderate or Severe Disease NVX - CoV2373 (n=17,312)* Placebo (n=8,125)* Total 0 14 Moderate 0 10 Severe 0 4 Vaccine Efficacy 100% (95% CI: 87.0, 100) • Pre - specified secondary endpoint • Post - hoc analysis for Severe disease only: VE = 100% (95% CI: 35, 100) • An additional 6 COVID hospitalizations (including 1 death) occurred in the placebo group but were not included in the efficacy analysis because PCR samples were not evaluated in the central lab *2:1 randomization

22 novavax.com High Efficacy in High - Risk Population High Risk defined as: • ≥65 years of age • <65 years of age with obesity, chronic kidney disease, chronic lung disease, cardiovascular disease, Type 2 diabetes • Life circumstances with frequent COVID exposure (e.g., meat packing plants) or densely populated living conditions NVX - CoV2373 (n=16,493)* Placebo (n=7,723)* Total 13 62 Vaccine Efficacy 91.0% (95% CI: 83.6, 95.0) *2:1 randomization

23 novavax.com PREVENT - 19 Phase 3 Pediatric Expansion 5 µg + 50 µg Matrix - M adjuvant (2 injections: Day 0 and Day 21) n = ~1,500 Placebo (2 injections: Day 0 and Day 21) n = ~750 2,248 Adolescents 12 - 17 years R 2:1 Randomized, observer - blinded, placebo - controlled trial evaluating safety, efficacy and effectiveness Protocol version 8.0 posted on Novavax.com June 2021 Enrollment Complete April 2021 First Dose August 2021 Blinded Crossover Underway
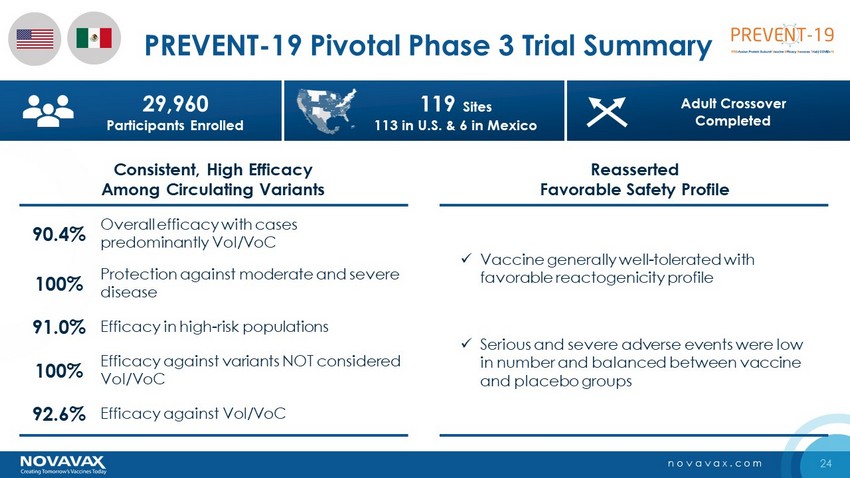
24 novavax.com PREVENT - 19 Pivotal Phase 3 Trial Summary Consistent, High Efficacy Among Circulating Variants Reasserted Favorable Safety Profile x Vaccine generally well - tolerated with favorable reactogenicity profile x Serious and severe adverse events were low in number and balanced between vaccine and placebo groups 100% Protection against moderate and severe disease 90.4% Overall efficacy with cases predominantly VoI / VoC 92.6% Efficacy against VoI / VoC 91.0% Efficacy in high - risk populations 100% Efficacy against variants NOT considered VoI / VoC 29,960 Participants Enrolled 119 Sites 113 in U.S. & 6 in Mexico Adult Crossover Completed

25 novavax.com Phase 3 United Kingdom

26 novavax.com 89% Overall Vaccine Efficacy Placebo NVX - CoV2373 0.0% 0.5% 1.0% 1.5% 2.0% 2.5% 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100 104 108 112 116 120 124 Event Rate Days from first vaccination = case Heath et al. 2021; DOI: 10.1056/NEJMoa2107659 Phase 3 UK

27 novavax.com Safety Events Were Infrequent and Balanced Summary of events 1 through Day 7 after Dose 1 & 2 (n=15,139) 1. Events occurring after receipt of deployed vaccines and reactogenicity events (according to preferred terms) are excluded. 2. Missing information not imputed. 3. According to post hoc analysis based on list of protocol derived preferred terms for PIMMC. 4. According to post hoc analysis based on revised AESI related to COVID - 19 definition. NVX - CoV2373 Placebo 0% 1% 2% 3% 4% 5% MAAEs Any Severe AEs Any Serious AEs AESIs related to COVID PIMMCs 3 Events were infrequent and balanced between vaccine and placebo groups. 2 4 Heath et al. 2021; DOI: 10.1056/NEJMoa2107659 Phase 3 UK
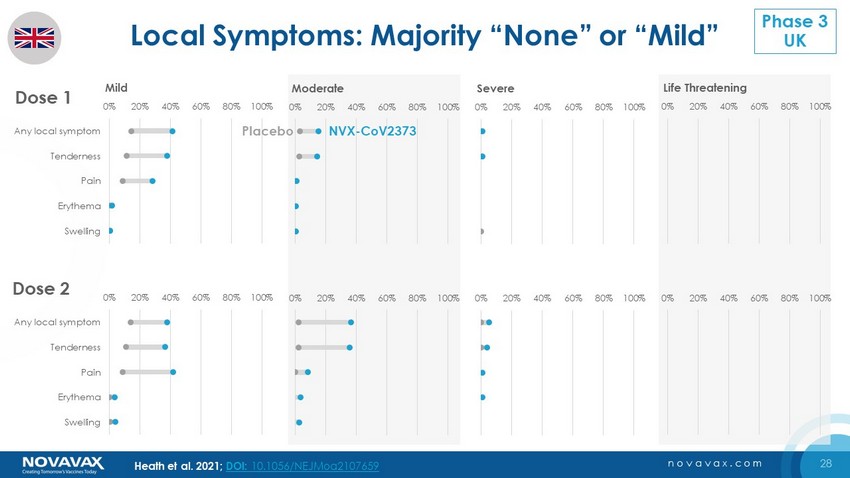
28 novavax.com 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling Life Threatening 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling Severe 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling Mild Local Symptoms: Majority “None” or “Mild” Dose 1 Dose 2 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling Moderate 0% 20% 40% 60% 80% 100% Any local symptom Tenderness Pain Erythema Swelling Mild NVX - CoV2373 Placebo Heath et al. 2021; DOI: 10.1056/NEJMoa2107659 Phase 3 UK

29 novavax.com 0% 20% 40% 60% 80% 100% 0% 20% 40% 60% 80% 100% Life Threatening 0% 20% 40% 60% 80% 100% Any systemic symptom Headache Muscle pain Fatigue Malaise Nausea or vomiting Joint pain Elevated temperature 0% 20% 40% 60% 80% 100% Any systemic symptom Headache Muscle pain Fatigue Malaise Nausea or vomiting Elevated temperature Joint pain Severe 0% 20% 40% 60% 80% 100% Moderate 0% 20% 40% 60% 80% 100% 0% 20% 40% 60% 80% 100% Any systemic symptom Headache Muscle pain Fatigue Malaise Nausea or vomiting Joint pain Elevated temperature Mild Systemic Symptoms: Majority “None” or “Mild” Dose 1 Dose 2 0% 20% 40% 60% 80% 100% Any systemic symptom Headache Muscle pain Fatigue Malaise Nausea or vomiting Elevated temperature Joint pain Mild NVX - CoV2373 Placebo Heath et al. 2021; DOI: 10.1056/NEJMoa2107659 Phase 3 UK

30 novavax.com NVX - CoV2373 Well - Tolerated when Administered with Influenza Vaccine Participants received influenza vaccine or placebo with first dose of NVX - CoV2373 (n=431) Placebo 0% 20% 40% 60% 80% 100% Any local Any systemic Mild 0% 20% 40% 60% 80% 100% Any local Any systemic Moderate 0% 20% 40% 60% 80% 100% Any local Any systemic Severe 0% 20% 40% 60% 80% 100% Any local Any systemic Life Threatening NVX - CoV2373 Placebo + Flu NVX CoV2373 + Flu Toback et al. 2021; DOI : 10.1101/2021.06.09.21258556 Vaccine Efficacy Preserved NVX - CoV2373 NVX - CoV2373 + Flu 90% 88% Influenza HAI and seroconversion responses preserved with co - administration (95%CI: 80.2; 94.6) (95%CI: - 0.2; 98.4) Phase 3 UK

31 novavax.com UK Phase 3 Trial Summary Primary Efficacy Endpoint Achieved Demonstrated Favorable Safety Profile x Safety events were infrequent and balanced between vaccine and placebo groups x When co - administered with influenza: - Generally well - tolerated - Immune responses and vaccine efficacy preserved 96% Efficacy against original COVID - 19 90% Overall efficacy 91% Efficacy in participants with high - risk medical comorbidities 86% Efficacy against Alpha (B.1.1.7) variant (first described in UK) 89% Efficacy in participants ≥ 65 years of age 15,203 Participants Enrolled Adult Crossover Completed Heath et al. 2021; DOI: 10.1056/NEJMoa2107659 Phase 3 UK

32 novavax.com Consistent Efficacy Across Phase 3 Studies UK Phase 3 PREVENT - 19 Overall Efficacy 89.7% 90.4% “Matched” Strain Efficacy 96.4% Prototype 100% (Non - VoI / VoC ) Efficacy Against Variants 86.3% Alpha (B.1.1.7) 93.6% Alpha (B.1.1.7) 92.6% VoI / VoC Efficacy Against Severe Disease NS (all 5 severe cases in placebo group) 100%

33 novavax.com Phase 2b Trial South Africa
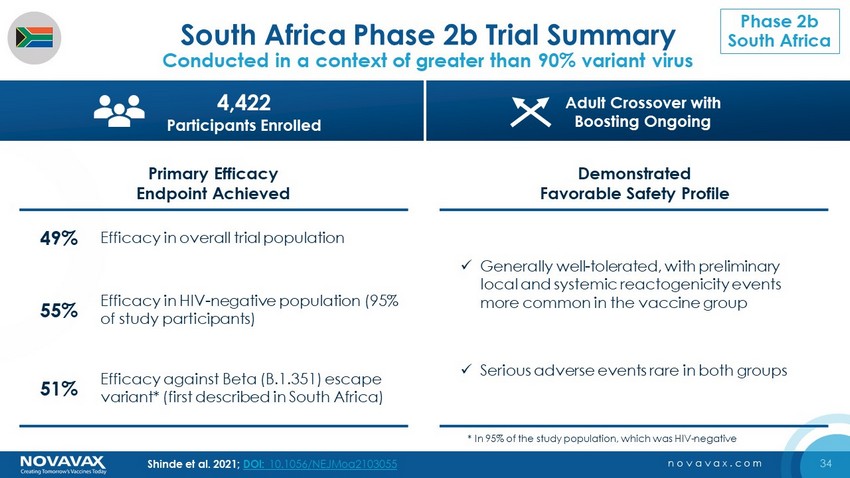
34 novavax.com South Africa Phase 2b Trial Summary Conducted in a context of greater than 90% variant virus Primary Efficacy Endpoint Achieved Demonstrated Favorable Safety Profile x Generally well - tolerated, with preliminary local and systemic reactogenicity events more common in the vaccine group x Serious adverse events rare in both groups 55% Efficacy in HIV - negative population (95% of study participants) 49% Efficacy in overall trial population 51% Efficacy against Beta (B.1.351) escape variant* (first described in South Africa) 4,422 Participants Enrolled Adult Crossover with Boosting Ongoing * In 95% of the study population, which was HIV - negative Shinde et al. 2021; DOI: 10.1056/NEJMoa2103055 Phase 2b South Africa

35 novavax.com Phase 1/2 Trial United States & Australia

36 novavax.com Robust Immune Response 2 doses + Matrix - M adjuvant Convalescent Sera 2 doses: 25µg + Matrix - M adjuvant 2 doses: 5µg + Matrix - M adjuvant 1 dose: 25µg + Matrix - M adjuvant Placebo 100 1,000 10,000 100,000 Day 0 Day 7 Day 21 Day 28 Day 35 Day 49 Day 105 Day 189 Antibody levels at 6 months are within the range of recently recovered patients Anti - Spike IgG (Log 10 EU/mL) Keech et al. 2020; DOI : 10.1056/NEJMoa2026920 Phase 1/2 US & Australia

37 novavax.com Booster Study United States & Australia

38 novavax.com Phase 2 Study Ongoing: Examining Third Dose Day 189 boost complete, immune responses evaluated on Day 217 USA & Australia — N=1,288 | Adults aged 18 - 84 years (n=583; 60 - 84 years) Placebo n=255 5 µg + Matrix - M n=258 25 µg + Matrix - M n=259 5 µg + Matrix - M n=256 25 µg + Matrix - M n=255 Day 21 Day 0 25 µg + Matrix - M Placebo 5 µg + Matrix - M 25 µg + Matrix - M 5 µg + Matrix - M Placebo 5 µg + Matrix - M 25 µg + Matrix - M Placebo Placebo Placebo Placebo Placebo Day 189 Placebo 5ug + Matrix - M Placebo 5ug + Matrix - M Additional boosting planned on Day 357
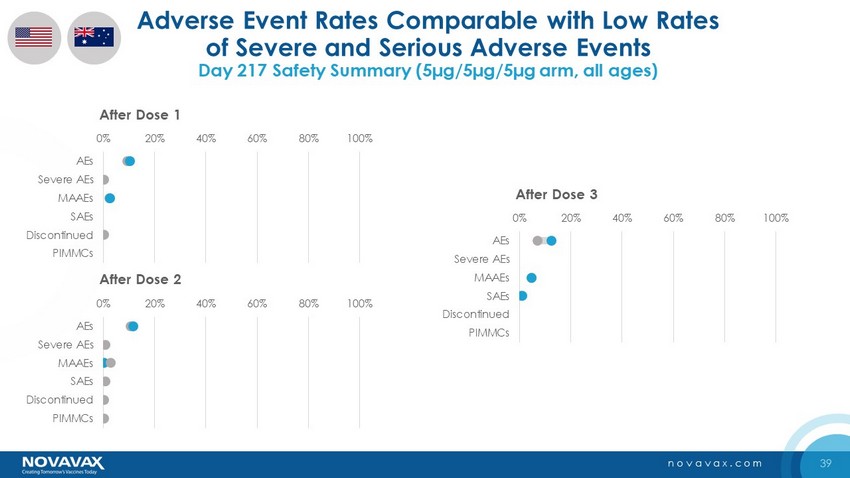
39 novavax.com Adverse Event Rates Comparable with Low Rates of Severe and Serious Adverse Events Day 217 Safety Summary (5µg/5µg/5µg arm, all ages) 0% 20% 40% 60% 80% 100% AEs Severe AEs MAAEs SAEs Discontinued PIMMCs After Dose 1 0% 20% 40% 60% 80% 100% AEs Severe AEs MAAEs SAEs Discontinued PIMMCs After Dose 2 0% 20% 40% 60% 80% 100% AEs Severe AEs MAAEs SAEs Discontinued PIMMCs After Dose 3

40 novavax.com Local Symptoms: Favorable Profile is Consistent; Severe Events are Relatively Infrequent Median duration 2 days, except erythema (2.5 days)
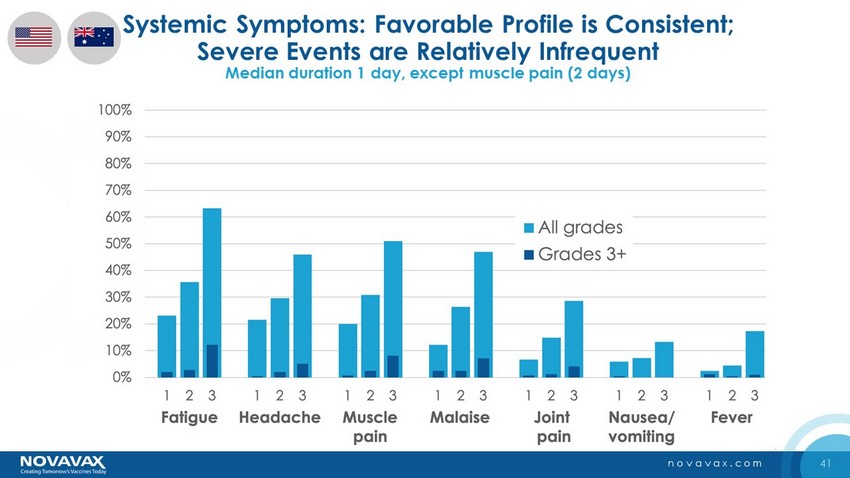
41 novavax.com Systemic Symptoms: Favorable Profile is Consistent; Severe Events are Relatively Infrequent Median duration 1 day, except muscle pain (2 days)

42 novavax.com Robust Anti - Spike IgG Responses Vaccination on Day 0 & 21 with boost on Day 189 106 745 41,621 11,514 5,943 202,406 Placebo 100 1,000 10,000 100,000 1,000,000 Day 0 7 21 28 35 49 105 189 Day 217 Anti - Spike IgG (Log 10 EU/mL) Titers increased ~4.6 - fold compared to peak response seen after primary vaccination series.

43 novavax.com Consistent Anti - Spike IgG Responses Vaccination on Day 0 & 21 with boost on Day 189 Overall Ages 18 - 59 Ages 60 - 84 Placebo 100 1,000 10,000 100,000 1,000,000 Day 0 7 21 28 35 49 105 189 Day 217 Anti - Spike IgG (Log 10 EU/mL) Anti - spike IgG titers increased ~3.9 - fold in adults aged 18 - 59 . Anti - spike IgG titers increased ~5.4 - fold in adults aged 60 - 84 .

44 novavax.com Robust Beta Anti - Spike IgG Responses Vaccination on Day 0 & 21 with boost on Day 189 Placebo 4,413 169,770 100 1,000 10,000 100,000 1,000,000 Day 0 7 21 28 35 49 105 189 Day 217 Anti - Spike IgG (Log 10 EU/mL) Beta IgG

45 novavax.com Increased Wild Type Neutralization R esponses Vaccination on Day 0 & 21 and boost on Day 189 10 33 1,581 65 6,039 10 100 1,000 10,000 Day 0 7 21 28 35 49 105 189 Day 217 IC<50% Wild Type Virus Neutralization (Log 10 ) WT neutralization titers increased ~4.3 - fold compared to peak response seen after primary vaccination series. Neutralization titers increased ~3.7 - fold in adults aged 18 - 59 & ~4.7 - fold in adults aged 60 - 84.

46 novavax.com 1,000 10,000 100,000 1,000,000 UK Ph3 Day 35 PREVENT-19 Day 35 US/AU Ph2 Day 217 Boosted Anti - spike IgG Responses Greater Than Observed in Phase 3 Studies Anti - Spike IgG (EU/mL, Log 10 ) IgG Responses with 95% CI PREVENT - 19 Efficacy Non - VoI / VoC : 100% VoI / VoC : 93% B.1.1.7: 94% UK Phase 3 Efficacy Prototype: 96% B.1.1.7: 86% 3.7 - 4.4x

47 novavax.com 100 1,000 10,000 100,000 UK Ph3 Day 35 PREVENT-19 Day 35 US/AU Ph2 Day 217 Boosted Microneutralization Responses Greater Than Observed in Phase 3 Studies Wild Type Virus Neutralization (Log 10 ) Microneutralization Responses with 95% CI 4.6 - 5.5x PREVENT - 19 Efficacy Non - VoI / VoC : 100% VoI / VoC : 93% B.1.1.7: 94% UK Phase 3 Efficacy Prototype: 96% B.1.1.7: 86%

48 novavax.com After Boosting, All Participants Developed High Levels of Functional hACE2 Responses Against All Variants Post - boost consistency suggests maturation of immune response (n=29) Assays performed by Novavax Discovery 1 10 100 1,000 10,000 Day 35 Day 217 Delta 8.1x increase 50% Receptor Inhibition 1 10 100 1,000 10,000 Day 35 Day 217 Beta 6.6x increase 1 10 100 1,000 10,000 Day 35 Day 217 Alpha 10.8x increase LOD 1 10 100 1,000 10,000 Day 35 Day 217 Ancestral 6x increase average Responses against Delta and Beta suggest comparable efficacy VE = 86.3% VE = 96.4% VE = 93.6%

49 novavax.com Use of NVX - CoV2373 in a Boosting Campaign A single dose of NVX - CoV2373 at 6 months significantly increases immune responses: • Wild - type Neutralization and Anti - Spike IgG levels up >4x over peak primary vaccination response • Increased functional hACE - 2 immune response against variants: • Delta (B.1.617.2): 6.6x increase from peak • Beta (B.1.351): 10.8x increase from peak • Alpha (B.1.1.7): 8.8x increase from peak

50 novavax.com Emerging Shift Toward Booster Doses NVX - CoV2373 Positioned to be Booster of Choice Emerging Booster Policy Recommendations • Waning immunity reinforces need for booster doses • Emerging policy recommendations reflect shift towards booster programs Ongoing and Upcoming Heterologous Boosting Studies Will Further Inform Booster Strategy Com - COV2 Ongoing Cov - Boost Ongoing Heterologous Boosting Study Exp. Fall 2021 Data from Phase 2 Homologous Booster Study in U.S. & Australia Supports NVX - CoV2373’s Ability to Boost • A single dose of NVX - CoV2373 at 6 months significantly increases immune responses Select Countries with Announced Booster Recommendations* *Reflects select countries with booster policy recommendations as of August 2021 OCTAVE - DUO Ongoing

51 novavax.com NVX - CoV2373 Regulatory Pathway

52 novavax.com a The Philippines Indonesi a Drugs Controller General of India European Medicines Agency (EMA) UK Medicines and Healthcare products Regulatory Agency (MHRA) Australian Therapeutic Goods Administration New Zealand Medsafe Health Canada US Food and Drug Administration (FDA) * Regulatory submissions for emergency use authorization filed in partnership with Serum Institute ** List of regulatory filings not in chronological order Filings for Authorization Underway with Additional Filings Expected in 2H 2021 World Health Organization (WHO) • Expect to complete rolling submission filings with WHO, MHRA, EMA and others** • Regulatory submissions f iled for EUA* with India, Indonesia , Philippines • Expect to submit for EUA to FDA TODAY End of 2021

53 novavax.com NanoFlu and Combination Vaccine Programs

54 novavax.com NanoFlu Addresses the Need for Greater and Broader Immune Responses Enhances immune response to generate potent, robust, and long - lasting protective immune responses Eliminates egg - adaptive strain changes that result in mismatch between vaccine and circulating viruses Provides broader protection against antigenic drift and mismatched strains Recombinant nanoparticle technology and Matrix - M adjuvant Next - generation flu vaccine for improved protection

55 novavax.com COVID - NanoFlu Combination Vaccine Development A transformative innovation to fight both illnesses June 2021 Announced data from co - administration sub - study** May 2021 Announced positive preclinical data* September 2021 Initiated phase 1/2 clinical trial of COVID - NanoFlu Combination Vaccine Preclinical Development Clinical Proof of Concept x UK Phase 3 co - administration sub - study completed x Demonstrated viability of simultaneous COVID - 19 and influenza vaccination x Hemagglutination inhibition (HAI) and ACE2 titers were comparable between individual and component vaccines x Maintained clinical and virologic protection against experimental challenge with SARS - CoV - 2 x Induced antibodies against SARS - CoV - 2 neutralizing epitopes common between USA - WA1 (original strain) and Beta (B.1.351) variant * Massare et al. 2021; DOI : 10.1101/2021.05.05.442782 **Toback et al. 2021; DOI : 10.1101/2021.06.09.21258556

56 novavax.com Strategic Development of COVID - 19 Vaccines
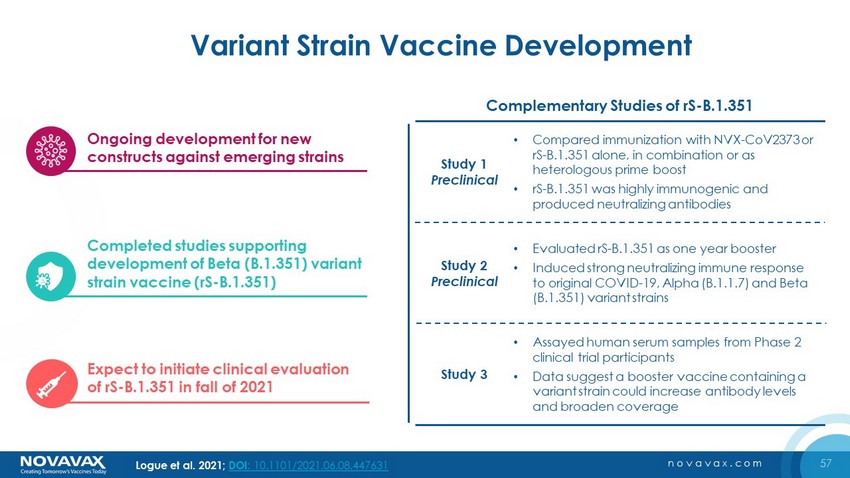
57 novavax.com Variant Strain Vaccine Development Logue et al. 2021; DOI : 10.1101/2021.06.08.447631 Study 1 Preclinical Study 2 Preclinical Study 3 • Compared immunization with NVX - CoV2373 or rS - B.1.351 alone, in combination or as heterologous prime boost • rS - B.1.351 was highly immunogenic and produced neutralizing antibodies • Evaluated rS - B.1.351 as one year booster • Induced strong neutralizing immune response to original COVID - 19, Alpha (B.1.1.7) and Beta (B.1.351) variant strains • Assayed human serum samples from Phase 2 clinical trial participants • Data suggest a booster vaccine containing a variant strain could increase antibody levels and broaden coverage Ongoing development for new constructs against emerging strains Expect to initiate clinical evaluation of rS - B.1.351 in fall of 2021 Completed studies supporting development of Beta (B.1.351) variant strain vaccine (rS - B.1.351) Complementary Studies of rS - B.1.351

58 novavax.com Response in Baboons Immunized 1 Year Ago Boost: 3µg rS - B.1.351 + 50µg Matrix - M adjuvant 10 100 1000 10000 100000 1000000 10000000 0 3 4 5 7 17 26 43 46 48 50 Anti - rS IgG Titer EC 50 (log10) weeks after immunization Geometric mean of 1, 5 or 25µg NVX - CoV2373 + 50µg Matrix - M adjuvant Geometric mean of 25µg NVX - CoV2373 no Matrix - M adjuvant Prime/Boost Original strain Boost Variant strain Dose 1 Dose 2 Dose 1 Dose 2 7 days after dose Logue et al. 2021; DOI : 10.1101/2021.06.08.447631

59 novavax.com 1 10 100 1000 10000 Pre- boost 7 days Post-boost 21 days 35 days 50% Receptor Inhibition Titer Ace2 Receptor Inhibition Increases After Boost at 1 Year Boost: 3µg rS - B.1.351 + 50µg Matrix - M adjuvant LOD 1 10 100 1000 10000 Pre- boost 7 days Post-boost 21 days 35 days 50% Receptor Inhibition Titer Geometric mean of 1, 5 or 25µg NVX - CoV2373 + 50µg Matrix - M adjuvant Geometric mean of 25µg NVX - CoV2373 no Matrix - M adjuvant US - WU1 (Original) Spike Inhibition B.1.351 Spike Inhibition Priming Regimen Logue et al. 2021; DOI : 10.1101/2021.06.08.447631

60 novavax.com NVX - CoV2373 Manufacturing & Distribution

61 novavax.com Practical Benefits Enabling Efficient Distribution Presentation • 10 - dose vials Transportation & Storage • Stable at 2 to 8 ° C Administration • Ready to use Large Global Capacity • Well - characterized technology platform; Dose - sparing

62 novavax.com AGC Biologics Global Supply Chain Established Capacity of approx. 150 million* doses per month starting by end of 4Q 2021 Par Sterile Products PolyPeptide Group FujiFilm TX FujiFilm NC Novavax HQ MD Biofabri FujiFilm UK AGC Biologics PolyPeptide Group Novavax AB Serum Institute SK Bioscience Takeda Novavax facilities Licensed Manufacturer Matrix - M adjuvant production Antigen production Fill/Finish Baxter Siegfried Novavax CZ Jubilant HollisterStier Biologics Manufacturing Centre GSK *When all planned capacity is online

63 novavax.com Agreements Executed for NVX - CoV2373 Ensuring fair and equitable global access • Finalized APA with Gavi • NVAX to provide 350 million doses • Serum Institute to provide balance of the 1.1 billion doses • Ensuring fair and equitable access of NVX - CoV2373 • Doses committed to US government in relation to funding received Up to >400 million doses • SK bioscience granted exclusive license in Republic of Korea • Serum Institute granted exclusive license in India and non - exclusive license in LMICs • Takeda granted exclusive license in Japan ~1.1 billion doses 110 million doses Gavi / COVAX Facility Commitment to US Government Advance Purchase Agreements Licensing Agreements • European Commission • Government of UK • Government of Canada • Commonwealth of Australia • Government of New Zealand • Government of Switzerland

64 novavax.com Clinical Development Conducted by Partners

65 novavax.com NVX - CoV2373 Clinical Development Conducted by Partners Phase 1/2 Japan • Evaluating immunogenicity and safety of NVX - CoV2373 Sponsored by Takeda Enrollment Complete n = 200 ≥ 20 years Phase 2/3 India • Evaluating immunogenicity and safety of NVX - CoV2373 Sponsored by Serum Institute Enrollment Complete in Phase 2 Cohort n = 1,600 18 - 65 years Phase 2 Com - COV2 • Mixed vaccine regimens for primary vaccination • Assessing immune response and safety • NVX - CoV2373 is one of four COVID - 19 vaccines evaluated Conducted by University of Oxford Sponsored By UK Vaccines Taskforce (VTF) Enrollment Complete n = 1,072 ≥ 50 years (n=359 NVX - CoV2373 admin) Phase 2 Cov - Boost • Heterologous boosting in previously vaccinated individuals • Assessing immune response and safety • NVX - CoV2373 is one of seven COVID - 19 vaccines evaluated Conducted by University Hospital Southampton NHS Trust Sponsored by VTF Enrollment Complete n = 2,886 ≥ 30 years (n=446 NVX - CoV2373 admin) Phase 3 OCTAVE - DUO • Evaluating safety and immunogenicity of a third dose in participants with impaired immune systems due to lymphoid malignancies • NVX - CoV2373 is one of three COVID - 19 vaccines evaluated Led by University of Glasgow and University of Birmingham Funded by VTF and UK Research and Innovation Enrollment Ongoing n = 320 (n=107 NVX - CoV2373 admin)

66 novavax.com Malaria Vaccine Candidates / Matrix - M Adjuvant Collaborations R21 with Matrix - M Adjuvant R0.6C with Matrix - M Adjuvant Vaccine created and trial sponsored by University of Oxford in collaboration with Serum Institute, with Matrix - M adjuvant Vaccine created by Statens Serum Institut and trial conducted at Radboud University Medical Center in the Netherlands n = 450 5 - 17 months Phase 2b Africa • Data published in Preprints with The Lancet • 77% efficacy with 50µg of Matrix - M adjuvant • 71% efficacy with 25µg of Matrix - M adjuvant Data Published n = 4,800 5 - 36 months Phase 3 Africa Ongoing Preclinical Study Complete • Demonstrated greater than 80% reduction of transmission of parasite that causes malaria n = 32 18 - 55 years Phase 1 The Netherlands Ongoing

67 novavax.com Upcoming Milestones
68 novavax.com Key Upcoming Milestones By end of 2021 Protection against variants Highly adaptable platform Strong stability profile Favorable safety profile • Expect to c omplete regulatory filings for emergency authorization with the MHRA, WHO, EMA, FDA, New Zealand Medsafe , Health Canada and Australian Therapeutic Goods Administration • Reach anticipated manufacturing capacity of 150 million doses per month • Begin expansive distribution of NVX - CoV2373 • Clinical evaluation of heterologous boosting with NVX - CoV2373 through ongoing and upcoming booster studies

69 novavax.com Pipeline Overview

70 novavax.com Near - Term Vaccine Pipeline Significant Opportunities for Future Development Clinical Development Conducted by Novavax Therapeutic Area Name Preclinical Phase 1 Phase 2 Phase 3 Marketed Coronavirus NVX - CoV2373 (Booster) Variant Strain (Monovalent and / or Bivalent) Seasonal Influenza NanoFlu (Older Adults) (Pre - BLA) Combination Vaccines COVID - NanoFlu NanoFlu / RSV NanoFlu / NVX - CoV2373 / RSV Clinical Development Conducted by Partners Therapeutic Area Name Preclinical Phase 1 Phase 2 Phase 3 Marketed Malaria R21* R0.6C** *Vaccine created and trial sponsored by University of Oxford in collaboration with Serum Institute, with Matrix - M adjuvant **Vaccine created by Statens Serum Institut and trial conducted at Radboud University Medical Center in the Netherlands

71 novavax.com 21 Firstfield Road Gaithersburg, MD 20878 United States novavax.com ir@novavax.com
