Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Brooklyn ImmunoTherapeutics, Inc. | brhc10028789_8k.htm |
Exhibit 99.1

September 2021 mRNA Engineered Cell & Cytokine Medicines A platform company in cell, gene-editing
& cytokine therapies

Disclaimer This presentation is intended to provide summary information about the business of Brooklyn
ImmunoTherapeutics, Inc. (“BTX”). The information in this presentation is in no respects complete, comprehensive or exhaustive, and it should be read in conjunction with BTX’s public filings with the Securities and Exchange Commission,
including information set forth in those filings under “Risk Factors” and similar headings. Forward-Looking Statements. Certain statements presented below on pages 3, 4, 7 and 8 are forward-looking statements for purposes of the safe harbor
provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are any statements that are not statements of historical fact and may be identified by terminology such as “expect,” “plan,” “potential,” “project”
or “will” or other similar words. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results may vary significantly from
BTX’s expectations based on a number of risks and uncertainties, including but not limited to the following: (i) the evolution of BTX’s business model into a platform company focused on cellular, gene editing and cytokine programs; (ii) BTX’s
ability to successfully, cost effectively and efficiently develop its technology and products; (iii) BTX’s ability to successfully commence clinical trials of any products on a timely basis or at all; (iv) BTX’s ability to successfully fund
and manage the growth of its development activities; (v) BTX’s ability to obtain regulatory approvals of its products for commercialization; and (vi) uncertainties related to the impact of the COVID-19 pandemic on the business and financial
condition of BTX, including on the timing and cost of its clinical trials. BTX cannot guarantee any future results, levels of activity, performance or achievements. The industry in which BTX operates is subject to a high degree of uncertainty
and risk due to variety of factors, including those described in BTX’s public filings with the Securities and Exchange Commission, including its Current Report on Form 8-K filed with the Securities and Exchange Commission on May 11, 2021 and
its Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2021 for a more complete discussion of these factors and other risks, particularly under the heading “Risk Factors.” BTX expressly disclaims any obligation to update
forward-looking statements after the date of this presentation. 2

BTX: World’s Only mRNA Cell-Engineering Platform What is mRNA cell-engineering? Messenger RNA (“mRNA”)
is a special class of molecules that contain the instructions that determine how cells function. BTX’s platform is being designed to harness mRNA to engineer cells to treat disease by repairing disease-causing mutations and directing the
formation of stem cells.BTX is developing a platform of gene-editing and cell therapies based on exclusively in-licensed mRNA technologyEngineered mRNA allows gene editing without provoking an immune responsemRNA therapeutics are safe and
highly effective in patientsFast to market – mRNA products have proven accelerated entry into clinical development Low cost of goods sold – mRNA products avoid complex and costly viral-vector manufacturingCan target any geneNo limit to the
number or complexity of cellular modifications that can be madeEnables high-potency mRNA and precision cell therapiesEngineered cells are fully rejuvenated allowing longer-lasting treatments BTX’s in-licensed vehicle safely delivers mRNA to
cells inside and outside the body 3

BTX: Leveraging In-licensed Patent Portfolio to Advance Medicine BTX has an exclusive license from
Factor Bioscience to a portfolio of granted patents around mRNA-based cell engineering that will provide BTX with a competitive advantageBTX’s major platform components:mRNA Cell Reprogramming (25 patents, extensive cellular data)mRNA Gene
Editing (15 patents, extensive cellular data)NoveSlice™ Gene-Editing Protein (15 patents, extensive cellular data)ToRNAdo™ mRNA Delivery Vehicle (4 patents, extensive cell and animal data) 4 NoveSlice and ToRNAdo are trademarks of Factor
Bioscience Limited. 2022 2023 Reverse Merger with NTN Buzztime (NYSE American: BTX) 2021 Established R&D Center in Cambridge, MA In-Licensed mRNA Patents Acquired Novellus Therapeutics IND-Enabling Pre-Clinical Studies IND –
Indication 1 (est.)

Fields of Medicine Addressable with BTX Technology 5
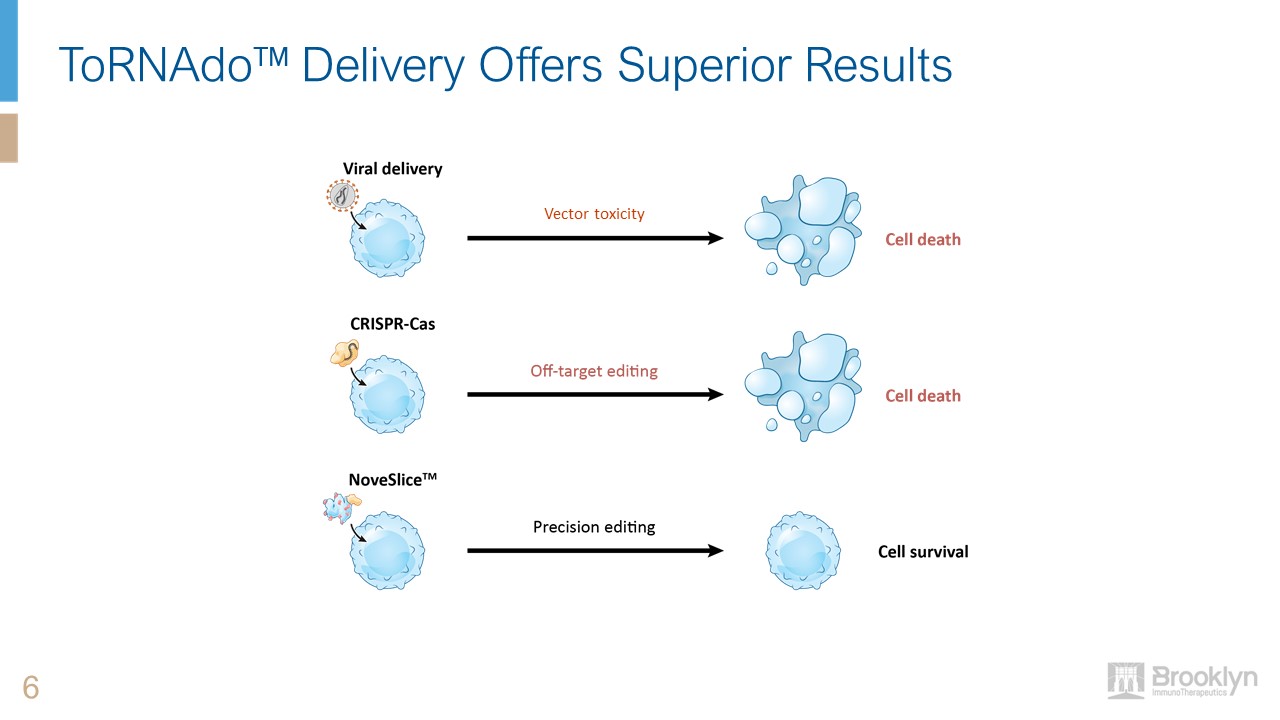
ToRNAdo™ Delivery Offers Superior Results 6 Vector toxicity

An Investment in BTX Today Offers Future Value Breadth and depth of BTX technology offer numerous
opportunities for successful clinical resultsBTX in-licensed patent portfolio offers potential for licensing and royalty revenue in coming yearsBTX technology platform offers predictable path to clinical development, with initial cellular
products expected within 2 yearsBTX patent portfolio offers range of clinical applications at lower cost and lower riskBTX legacy cytokine portfolio and completed Phase 2b trial offer additional valueOpportunity for partnering in the Ph3
registration studyOpportunity for advancing another Ph2 study in a different oncologic indication 7

8 8 BTX CRISPR1 TALENs2 Zinc-Finger Nucleases3 mRNA Vaccines4 CAR-T5 Primary
Technologies mRNA Cell ReprogrammingmRNA Gene EditingNoveSlice™ToRNAdo™ CRISPR(including base-editing) TALENs Zinc-Finger Nucleases None Viral Vectors for CAR-T mRNA Gene Editing Yes No No No No No mRNA Cell
Reprogramming Yes No No No No No mRNA Delivery Yes Electroporation (limited to ex-vivo) Electroporation (limited to ex-vivo) Electroporation (limited to ex-vivo) Vaccines N/A High Off-Target Effects No Yes(short gRNA
recognition sequence) Yes(based on plant-pathogen sequences) Yes(requires extensive screening) N/A N/A Immune response No Yes(bacterial protein) Possible(plant- pathogen sequences) Possible Yes(desired for vaccines) N/A On-Target
Efficiency High Medium Medium Medium N/A N/A Cell Rejuvenation Yes No No No No No BTX Technology: Competitive Landscape Example Companies: 1 Beam Therapeutics, CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics. 2
Allogene Therapeutics, Cellectis, Iovance Biotherapeutics. 3 Sangamo Therapeutics. 4 BioNTech, Moderna. 5 Juno Therapeutics, Kite.

September 2021 A platform company in cell, gene-editing & cytokine therapies mRNA Engineered
Cell & Cytokine Medicines
