Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - SURMODICS INC | srdx-ex991_7.htm |
| EX-10.1 - EX-10.1 - SURMODICS INC | srdx-ex101_6.htm |
| EX-2.2 - EX-2.2 - SURMODICS INC | srdx-ex22_609.htm |
| EX-2.1 - EX-2.1 - SURMODICS INC | srdx-ex21_123.htm |
| 8-K - 8-K - SURMODICS INC | srdx-8k_20210702.htm |

© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 1 Surmodics Expands Thrombectomy Portfolio with Acquisition of Vetex Medical Limited Galway, Ireland Exhibit 99.2
Some of the statements made in this presentation may be considered forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Statements that are not historical or current facts, including statements about the timetable for manufacturing process validation for, and clinical use evaluations of, the ReVene™ thrombectomy system; statements about future product development and product launches; statements about the value creation potential of the acquisition of Vetex Medical Limited (the “Acquisition”) ; statements about the anticipated financial impact of the Acquisition, including the timing of product revenues and earnings accretion from the Acquisition; and statements about the potential for rapid growth in a high-ASP product category, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties, and important factors could cause actual results to differ materially from those anticipated, including (1) our ability to successfully refine and commercialize the ReVene thrombectomy system; (2) our ability to obtain favorable regulatory determinations related to the ReVene thrombectomy system; (3) the final accounting treatment of the Acquisition; (4) possible adverse market conditions; (5) the impacts, duration and severity of the global COVID-19 pandemic and the effects of responses to it on healthcare systems, the general economy, our business partners, and our operations; and (6) the factors identified under “Risk Factors” in Part I, Item 1A of our Annual Report on Form 10-K for the fiscal year ended September 30, 2020, and updated in our subsequent reports filed with the SEC. These reports are available in the Investors section of our website at https://surmodics.gcs-web.com and at the SEC website at www.sec.gov. Forward-looking statements speak only as of the date they are made, and we undertake no obligation to update them in light of new information or future events © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 2 Safe Harbor

© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. Acquisition of Vetex Medical Limited 3 Vetex Medical Limited Founded in 2016 Private Company, based in Galway, Ireland Developed the ReVene™ Thrombectomy Catheter Medtech Innovator 2016 Semi-Finalist Intellectual Property: 4 U.S., 1 EU and 3 Japan granted patents; 4 U.S., 4 EU and 3 Japan filed patents Five full-time employees The Vetex ReVene™ Thrombectomy Catheter has been purposefully designed for the venous vasculature, specifically iliofemoral vessels with mixed morphology and large clot volume

With this acquisition Surmodics, now has two cleared products for both arterial and venous thrombosis which significantly accelerates its strategy of clinical introduction of thrombectomy devices in multiple vascular beds Groundbreaking design with compelling competitive advantages and multi-geography regulatory approvals in a growing and underpenetrated $1.4 billion U.S. market Excellent synergies with Surmodics’ product development, clinical development and manufacturing capabilities create a significantly accelerated timeline for clinical development and introduction of the venous clot removal system Complements our Pounce™ Arterial Thrombectomy portfolio with venous thrombectomy Talented and experienced team to join high-caliber Surmodics R&D team in Ballinasloe, Ireland Strengthens Surmodics’ existing thrombectomy IP portfolio, providing additional opportunities for product development in other vascular beds © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 4 Investment Thesis

Deal Terms $39.9 million upfront payment Up to $7 million, $3.5 million of which is guaranteed, payable upon achievement of certain new product development and regulatory milestones Transaction provides for 100% ownership of Vetex Medical Limited Deal Funding $29.9 million financed with cash on hand $10 million financed with our $25 million credit facility Post-transaction cash & investments total approximately $40 million Fiscal 2021 Guidance Impact We expect to update fiscal 2021 guidance, including the impact from the Vetex acquisition, during our 3rd quarter earnings call Anticipated charge for one-time acquisition costs and intangible asset amortization expense ranging from $0.10 to $0.12 per share Primary balance sheet impact will be the addition of developed technology intangible assets, goodwill and long-term, contingent obligations Catalytic Events We expect product revenues beginning the second half of calendar 2022 We anticipate the acquisition will be accretive beginning the second half of fiscal 2023 © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 5 Deal Terms and Economic Considerations
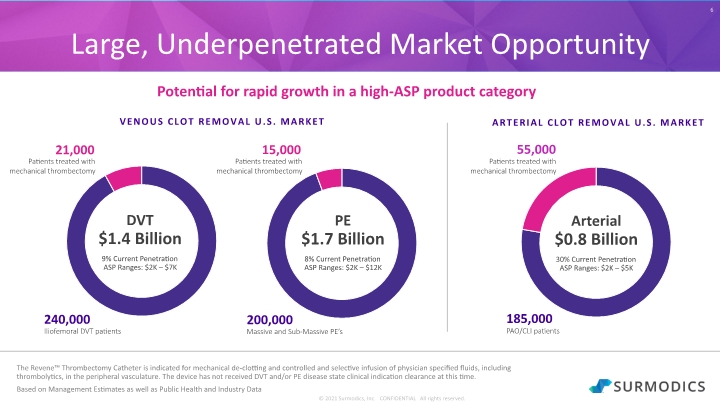
© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 6 Large, Underpenetrated Market Opportunity ARTERIAL CLOT REMOVAL U.S. MARKET Potential for rapid growth in a high-ASP product category DVT $1.4 Billion 9% Current Penetration ASP Ranges: $2K – $7K PE $1.7 Billion 8% Current Penetration ASP Ranges: $2K – $12K Arterial $0.8 Billion 30% Current Penetration ASP Ranges: $2K – $5K VENOUS CLOT REMOVAL U.S. MARKET The Revene™ Thrombectomy Catheter is indicated for mechanical de-clotting and controlled and selective infusion of physician specified fluids, including thrombolytics, in the peripheral vasculature. The device has not received DVT and/or PE disease state clinical indication clearance at this time. Based on Management Estimates as well as Public Health and Industry Data
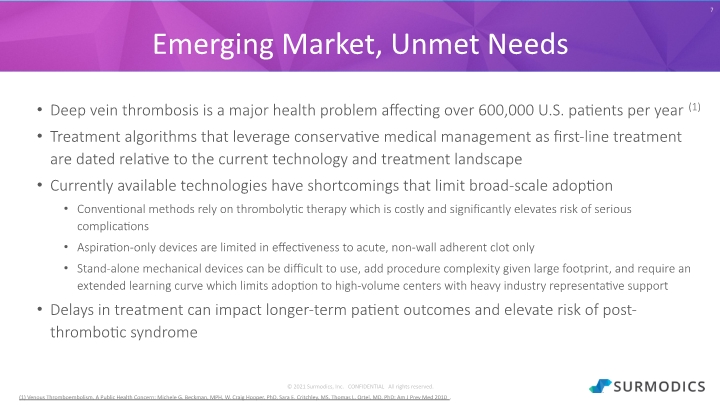
© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. Deep vein thrombosis is a major health problem affecting over 600,000 U.S. patients per year(1) Treatment algorithms that leverage conservative medical management as first-line treatment are dated relative to the current technology and treatment landscape Currently available technologies have shortcomings that limit broad-scale adoption Conventional methods rely on thrombolytic therapy which is costly and significantly elevates risk of serious complications Aspiration-only devices are limited in effectiveness to acute, non-wall adherent clot only Stand-alone mechanical devices can be difficult to use, add procedure complexity given large footprint, and require an extended learning curve which limits adoption to high-volume centers with heavy industry representative support Delays in treatment can impact longer-term patient outcomes and elevate risk of post-thrombotic syndrome 7 Emerging Market, Unmet Needs (1) Venous Thromboembolism, A Public Health Concern; Michele G. Beckman, MPH, W. Craig Hooper, PhD, Sara E. Critchley, MS, Thomas L. Ortel, MD, PhD; Am J Prev Med 2010.
The market needs a stand-alone, easy-to-use, single session device that efficiently removes large clot volume and enables the early treatment of patients in an outpatient setting © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 8 Vetex Venous Thrombectomy Solution Design Goals Aimed at Gaining Widespread Adoption
© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 9 Designed to Improve Upon Current Available Technologies Small footprint device accommodates guidewire positioning and management No IVC filter contraindication Smaller venotomy Dual action of wall-apposing capture basket and Archimedes removal system designed to dislodge, capture and efficiently clear large clots Clot capture basket designed to minimize the need to rotate the device around the vessel to gain 360° clearance via subsequent passes Small and easy to clean basket On-the-table, single session treatment Simple, product interface with intuitive set up and operation Stand-alone, single session therapy – no capital equipment required Designed to be intuitive, approachable and accessible to facilitate widespread adoption Low learning curve intended to establish independent and confident use of device SAFETY PERFORMANCE EASE OF USE ACCESSIBLE TIME EFFICIENT
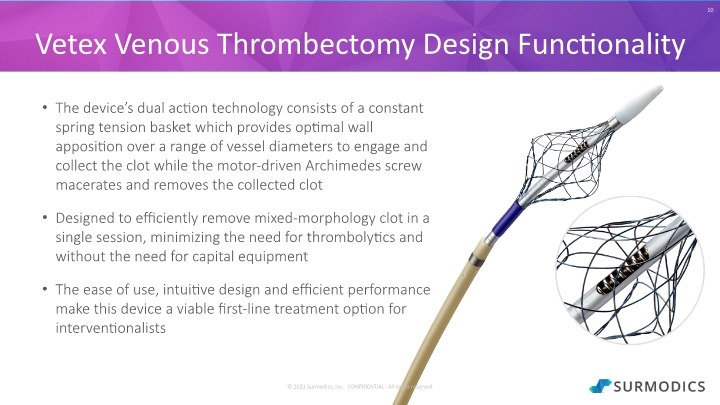
The device’s dual action technology consists of a constant spring tension basket which provides optimal wall apposition over a range of vessel diameters to engage and collect the clot while the motor-driven Archimedes screw macerates and removes the collected clot Designed to efficiently remove mixed-morphology clot in a single session, minimizing the need for thrombolytics and without the need for capital equipment The ease of use, intuitive design and efficient performance make this device a viable first-line treatment option for interventionalists 10 Vetex Venous Thrombectomy Design Functionality © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved.
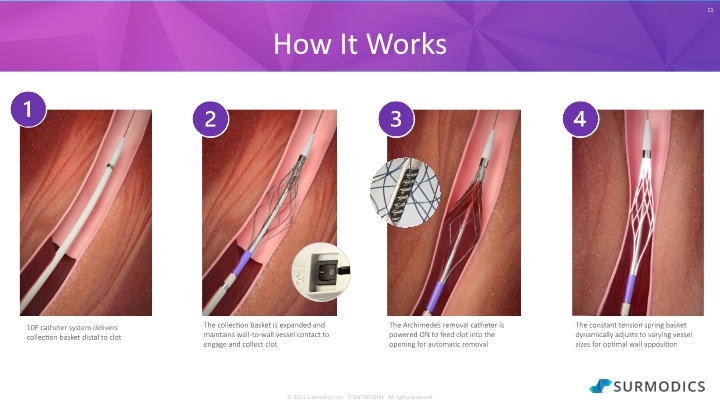
© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 11 How It Works The collection basket is expanded and maintains wall-to-wall vessel contact to engage and collect clot The Archimedes removal catheter is powered ON to feed clot into the opening for automatic removal The constant tension spring basket dynamically adjusts to varying vessel sizes for optimal wall apposition 10F catheter system delivers collection basket distal to clot
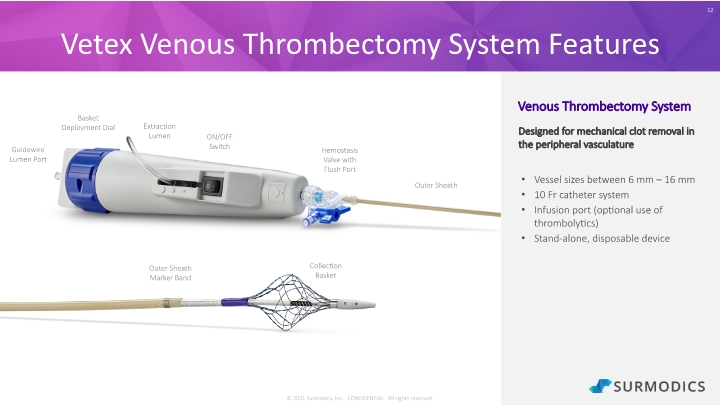
© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. Vetex Venous Thrombectomy System Features 12 Venous Thrombectomy System Designed for mechanical clot removal in the peripheral vasculature Vessel sizes between 6 mm – 16 mm 10 Fr catheter system Infusion port (optional use of thrombolytics) Stand-alone, disposable device Collection Basket Outer Sheath Marker Band Outer Sheath Hemostasis Valve with Flush Port Guidewire Lumen Port Extraction Lumen Basket Deployment Dial ON/OFF Switch
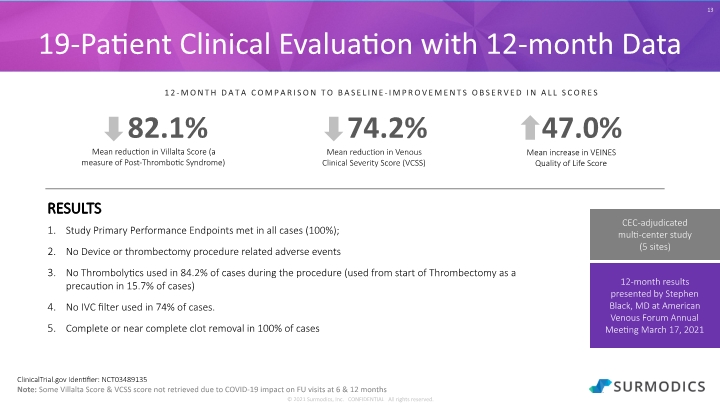
© 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 13 19-Patient Clinical Evaluation with 12-month Data RESULTS Study Primary Performance Endpoints met in all cases (100%); No Device or thrombectomy procedure related adverse events No Thrombolytics used in 84.2% of cases during the procedure (used from start of Thrombectomy as a precaution in 15.7% of cases) No IVC filter used in 74% of cases. Complete or near complete clot removal in 100% of cases ClinicalTrial.gov Identifier: NCT03489135 Note: Some Villalta Score & VCSS score not retrieved due to COVID-19 impact on FU visits at 6 & 12 months 12-MONTH DATA COMPARISON TO BASELINE-IMPROVEMENTS OBSERVED IN ALL SCORES 12-month results presented by Stephen Black, MD at American Venous Forum Annual Meeting March 17, 2021 CEC-adjudicated multi-center study (5 sites)
FDA Clearance received in December of 2020 CE Mark granted May 2021 Approved Indication: For mechanical de-clotting and controlled and selected infusion of physician specified fluids, including thrombolytics, in the peripheral vasculature The Vetex ReVene™ Thrombectomy Catheter currently is not indicated for the treatment of DVT or PE © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 14 Regulatory Status
Surmodics now has two cleared products for both arterial and venous thrombosis Significantly accelerates company strategy of clinical introduction of thrombectomy devices in multiple vascular beds Groundbreaking design with compelling competitive advantages Easy-to-use, stand-alone, single session therapy with intuitive design aimed at widespread adoption User-friendly, low-risk device enables the migration trend of moving more complex procedures to the outpatient setting Multi-geography regulatory approvals in a growing and underpenetrated $1.4 billion U.S. market Excellent synergies with Surmodics’ product development, clinical and manufacturing capabilities create a significantly accelerated timeline Strengthens Surmodics’ existing thrombectomy IP portfolio, providing additional opportunities for product development in other vascular beds © 2021 Surmodics, Inc. CONFIDENTIAL All rights reserved. 15 Accelerates Vision and Enhances Strategic Value
