Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PALISADE BIO, INC. | f8k_042821.htm |
Exhibit 99.1

Restoring Intestinal Barrier Health Corporate Presentation | April 2021 PALI (NASDAQ) | palisadebio.com

2 Forward Looking Statements This communication contains “forward - looking” statements, including, without limitation, statements related to the anticipated benefits of the transactions contemplated by the merger and the private placement financing and the related transactions, the anticipated trading of the combined company’s stock on the Nasdaq Capital Market, and statements related to Palisade’s development programs. Any statements contained in this communication that are not statements of historical fact may be deemed to be forward - looking statements. These forward - looking statements are based upon Palisade’s current expectations. Forward - looking statements involve risks and uncertainties. Palisade’s actual results and the timing of events could differ materially from those anticipated in such forward - looking statements as a result of these risks and uncertainties, which include, without limitation, related to the Company’s ability to advance its preclinical programs and the uncertain and time - consuming regulatory approval process. Additional risks and uncertainties can be found in Seneca’s Registration Statement on Form S - 4 initially filed with the SEC on December 23, 2020, as amended. Palisade expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward - looking statements contained herein to reflect any change in Palisade’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.

3 Merger Transaction Created Palisade Bio, Inc. (Nasdaq: PALI)

4 Leading BioSciences and Seneca Merged to Form Palisade Bio • Nasdaq public listing allows for proper capitalization, liquidity, and strategic visibility – Reverse merger with Seneca provides the public listing under the ticker, PALI – Leading’s management team, expanded board, and top - tier scientific advisory board – Pro forma ownership 25.7% Seneca and 74.3% Leading Bio (including Altium Capital) – In addition to the equity exchange, Seneca stockholders received 1 contingent value right (CVR) for each share of SNCA which entitles holders to receive a payment for future milestone events tied to the proceeds of Seneca asset sales, if any • Concurrent $20 million financing led by Altium Capital – Pro forma cash provides sufficient cash runway that we believe will allow us to achieve key clinical milestones • Palisade Bio focusing exclusively on gastrointestinal (GI) drug development – Scientific foundation focused on therapeutic protection of intestinal mucosal barrier – Two proprietary formulations of oral protease inhibitors for novel indications: LB1148 and PB101 – LB1148: Phase 2 showed 30% improvement in time to normal bowel function following surgery (p<0.001) – LB1148: Phase 3 expected to begin in 2021 to improve GI recovery in neonates undergoing cardiac surgery

5 Accomplished Management Team Leading to Success J.D. Finley Chief Financial Officer, Director Mr. Finley has raised nearly $1 billion in capital for companies during his career. Mr. Finley’s experience includes roles of Tax Specialist, EVP, CFO, CEO, and President at companies including Deloitte Haskins & Sells, MetroGolf Inc., Phillips Capital, Proteus Capital Partners Inc., and PointAcross . Mr. Finley holds a Bachelor of Business Administration degree from Boise State University and a Master of Taxation degree from the University of Denver. Michael J. Dawson, MD Chief Medical Officer Dr. Dawson brings both clinical expertise and a background in research and development to Leading BioSciences. He has served in numerous healthcare leadership positions and hospital committees, including the Chief of Staff of Centinela Hospital Medical Center in Los Angeles. He has carried out hospital based clinical studies for over 25 years, first starting as a medical student with the earliest adenovirus gene transfers studies. Dr. Dawson is a Board Certified Diagnostic Radiologist and CAQ Interventional Radiologist. He received an MD from USC and Bachelors degrees in Biochemistry and Cell Biology in addition to Department Honors in Biology with High Distinction from UCSD. Thomas M. Hallam, PhD Chief Executive Officer, Director Dr. Hallam brings a broad base of pharmaceutical expertise and success, having led virtually every stage of pre - clinical research, clinical development, and product commercialization strategy. Served as an employee and consultant and advisor to some of the world’s leading healthcare companies, including Merck, Roche/Genentech, Pfizer, Mesoblast, Sanofi, Astra - Zeneca, Bausch & Lomb, J&J, Saatchi & Saatchi, and Ogilvy CommonHealth. Dr. Hallam is a former Howard Hughes Medical Institute Research Fellow. He received his PhD in Neuroscience from UC Davis and holds an MBA from USC. John Rodenrys Co - Founder, Chief Technology Officer Mr. Rodenrys has served in several Senior Executive positions at a number of companies including VC funded and Fortune 40 organizations. Positions included CEO, President and COO at Forhealth ( Baxa ), Safetymate , Vistant Corp and Pyxis Corp (Cardinal Health NYSE:CAH) among others. Served on several UCSD review organizations/therapeutic advisory boards CCAT, the von Liebig Center, Advancing Innovations to Market (AIM) and Innovation Advisor. Mr. Rodenrys holds an Engineering degree from RPI and an Executive MBA from Boston University amongst others.

6 Acute and Chronic Disorders Caused by GI Barrier Disruption Focused on pathologies caused by loss of GI epithelial barrier integrity and the consequences to human health GI Epithelial Barrier Biology Expertise in GI - specific protease - targeted therapeutics Integrated discovery and development platform for creating drug pipeline to treat complications from GI barrier disruption Oral serine protease inhibitor with demonstrated clinical benefit in multiple surgical studies; Phase 3 expected to start in H2 2021 Multiple potential clinical and regulatory milestones that may drive value over the next 12 - 18 months R&D Platform Technology Building Broad Pipeline Lead Program Multiple Indications Multiple Upcoming Catalysts

7 LB1148 Overview: Pipeline in a Product – Multiple Indications Drug/Class Indication Research Pre - clinical Phase 1 Phase 2 Phase 3 LB1148* Oral broad - spectrum protease inhibitor* Postoperative return of bowel function Neonatal cardiac surgery GI surgery Prevention of post - surgical abdominal adhesions *Commercial right to LB1148 in Grater China (excluding Taiwan) have been out - licensed to Newsoara

LB1148 Program Overview Pipeline in a Product – Multiple Indications 8 Accelerate Return of Postoperative GI Function Reduce Post - surgical Adhesions Cardiovascular Surgery Gastrointestinal Surgery Abdominal Surgery

9 Unmet Need – Postoperative GI Recovery After Surgery Postoperative return of bowel function is often a limiting determinant of hospital discharge • Patients generally do not leave hospital until they have a bowel movement • Causes major discomfort • Extends hospital stay • Increases medical costs If we save 1 day on length of stay, we would realize $20M more in profits annually. – Mark A. Talamini, MD, MBA Chair of Surgery, SUNY Stony Brook Major Surgeries Cardiovascular Gastrointestinal Abdominal Gynecological LB1148 has demonstrated a 1.3 - day reduction in hospital length of stay National average hospital expenses per inpatient day = $2,400

10 Postoperative Complications Driven by Proteolytic Activity Injury (surgery or hypoperfusion) Compromised mucosal barrier Healthy intestine Protease leak and translocation ↑ Proteolytic activity ↑ Tissue damage/permeability ↑ Autodigestion ↑ Microbiome imbalance/pathology ↑ Pathologic cell signaling activity ↑ Inflammatory cell activation/infiltration ↑ Receptor degradation • Delayed function • Ileus • Adhesions • Inflammation Cascade of disease mechanisms Proteolytic intestinal permeation • Intestinal injury (from surgery or hypoperfusion during surgery) triggers a cascade of disease mechanisms and leads to postoperative complications • Destructive feedback loop where protease leakage leads to additional intestinal damage Postoperative complications
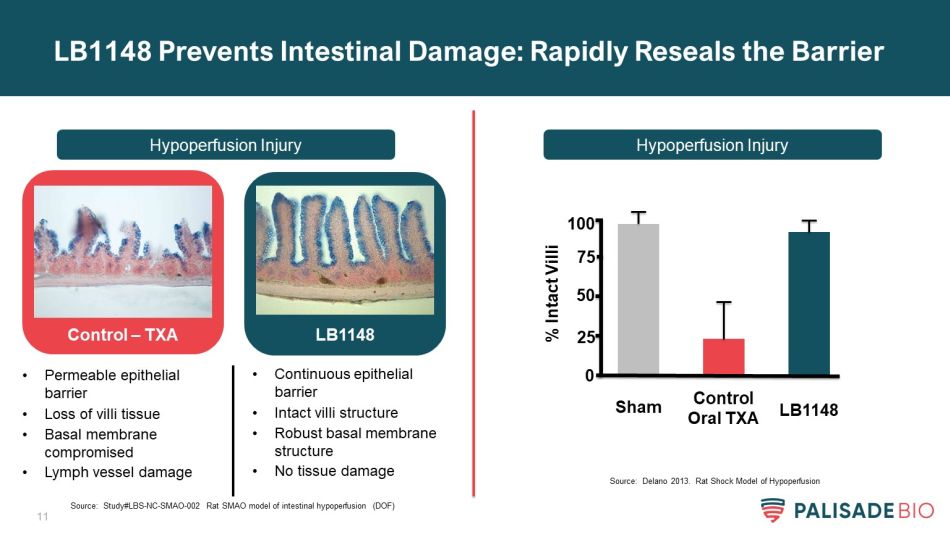
11 LB1148 Prevents Intestinal Damage: Rapidly Reseals the Barrier Hypoperfusion Injury Hypoperfusion Injury Control – TXA LB1148 • Permeable epithelial barrier • Loss of villi tissue • Basal membrane compromised • Lymph vessel damage • Continuous epithelial barrier • Intact villi structure • Robust basal membrane structure • No tissue damage % Intact Villi Sham Control Oral TXA LB1148 0 50 75 100 25 Source: Study#LBS - NC - SMAO - 002 Rat SMAO model of intestinal hypoperfusion (DOF) Source: Delano 2013. Rat Shock Model of Hypoperfusion

12 • Oral small molecule in novel formulation comprised of FDA - approved components – Broad - spectrum serine protease inhibitor (tranexamic acid) – Known safety profile – Plan to utilize 505(b)(2) pathway for approval • Prevents damage to the intestine – Improves time to post - surgical bowel recovery • Issued pharmaceutical composition patents • No new approved drugs for postoperative ileus since 2008 – Entereg has austere black box warning, onerous REMS protocols, and limited utilization LB1148 - Expeditious Regulatory Pathway; Limited Competition

13 Entereg: Only Drug Approved for Accelerating Return of GI Function • Provides a precedent pathway to FDA approval • Developed by GSK/ Adolor /Cubist and now owned by Merck • Indicated to improve post - op recovery of bowel function – Phase 3 demonstrated*: • 17 - hour improvement in return of bowel function • 7 - hour improvement in hospital length of stay • Entereg MOA carries safety risks – LB1148 has completely different MOA • Limitations include*: – In - patient hospital administration only – Short - term use only – Restrictive REMS program – Black Box warning for severe side effects • Utilized in few hospitals * Entereg Prescribing Information Black - box warning: • Increased incidence of myocardial infarction • Available only through restricted program - REMS Entereg’s FDA approval provides regulatory roadmap LB1148 clinical trial design

14 LB1148 Accelerates Return of GI Function – Phase 3 Ready • Oral protease inhibitor addressing gut - barrier pathologies for two indications – Accelerate postoperative return of bowel function – Reduce post - surgical abdominal adhesions • Two completed clinical studies demonstrate statistically significant clinical benefit – 30% improvement in time to return of bowel function after cardiovascular (CV) surgery (p<0.001) • 1.0 - day reduction in ICU length of stay (LOS) (ns) • 1.1 - day reduction in hospital LOS (ns) – 48% improvement in time to return of bowel function after GI surgery (vs. placebo in Entereg study 314*) • 1.3 - day reduction in hospital LOS vs. expected LOS at time of admission (p<0.03) • Zero post - surgical abdominal adhesions documented in 2 nd look surgeries • Fast Track designation granted for neonatal cardiac surgery program, potential for additional expedited pathways with well - defined endpoints • Strong IP portfolio, including pharmaceutical composition patents • Tremendous potential with 6.7 million addressable patients in the US Source: Entereg Prescribing Information

Cardiovascular Surgery (Hypoperfusion Injury) 15

16 CV Surgery Completed Phase 2: Study Design * Study Day 0 is the day of surgery. Screening Period CV Surgery Postoperative / Discharge LB1148 Day - 42 to - 1 Day 0* Day 1 - 14 Follow - up Day 30 Placebo Cardiovascular Surgery • Open - heart CV surgery requiring cardio - pulmonary bypass • Randomized, double - blind, placebo - controlled, in subjects undergoing CABG and/or heart valve replacement surgery • Randomized 1:1 • 7.5 grams QD on Days 0, 1, and 2 Study Design • 120 subjects undergoing elective cardiac surgery • Single center • CRO: IQVIA • Data announced in 2020 Scope & Timelines • ICU length of stay • Hospital length of stay • Organ function • Inflammatory response • Glucose control Secondary Endpoints: • Time to return of bowel function (defined by first bowel movement) Primary Endpoint:

17 CV Surgery Phase 2: Significant Improvement in Bowel Function Top - line data highlights • 30% improvement in return of bowel function – Highly statistically significant; p<0.001 – Median time to bowel recovery: 31.7 hours on LB1148 vs. 45.0 hours on placebo – Same endpoint will be used for Phase 3 • 1.0 - day reduction in ICU LOS (ns) • 1.1 - day reduction in hospital LOS (ns) • No LB1148 - related adverse events LB1148 n=58 Placebo n=52

18 LB1148 Compares Favorably to Entereg: 30% vs 16% Improvement 16% Improvement Proportion achieving endpoint Hours after End of GI Surgery Proportion achieving endpoint Hours after End of CV Surgery 30% Improvement p<0.001 Source: Entereg FDA AdCom; Jan. 23, 2008 Entereg : Approved by FDA based on 16% improvement in return of bowel function LB1148: 30% improvement in return of bowel function; p<0.001 Entereg n=317 Placebo n=312 LB1148 n=58 Placebo n=52 0 24 48 72 96 120 144 188 192 216 240 264

19 Neonatal CV Surgery – Opportunity to Improve Development Status with Faster GI Recovery and Faster Return to Full Feedings • Unmet need for improving GI recovery after neonatal CV surgeries • Impaired GI function delays return to full feedings, which are a key criteria for ICU discharge – $400,000 hospitalization costs as ICU stay can last weeks to months 1 • Delayed feeding linked to developmental complications: – Growth deficiencies – Lower IQ – Neurological disorders – ADHD • Patients at high risk of necrotizing enterocolitis (NEC) – Up to 18% of neonatal CV surgeries develop NEC 2 – 29.6 additional days in hospital with approximately $180,000 in additional costs 3 • 150,000 CV surgeries/year in the US for congenital defects 4 – 13% CV surgeries are in neonates 5 1. J Am Coll Cardiol . 2013 Mar, 61 (10_Supplement) E501 2. Ann Thorac Surg. 2006 Mar;81(3):982 - 7. doi : 10.1016/j.athoracsur.2005.09.001. 3. Arch. Surg. 145, 389 (2010). 4. MMWR. Morb . Mortal. Wkly. 2013. Rep. 66, 41 – 46 (2017) 5. Eur. J. Cardio - thoracic Surg. 51, 301 – 307 (2017) Neonatal Cardiovascular Surgery Opportunity for LB1148 to minimize risk of developmental delays in neonates undergoing CV surgery
20 • Estimated trial costs of <$10M – ~10 sites in the US • Estimated 1 year to enroll study • Homogeneity of patient population LB1148 Planned Phase 3: Lead Indication in Pediatric Heart Surgery • Attractive first indication • Enthusiastic investigators to participate in clinical studies • Strong pricing power • Alignment of incentives for payers and providers • Fast track designation granted • Additional potential for: – Rare pediatric disease exclusivity – Breakthrough therapy designation Neonatal Cardiovascular Surgery

21 Neonatal CV Surgery: Planned Phase 2/3 Study Design Screening Period CV Surgery Postoperative / Discharge LB1148 Day - 30 to - 1 Day 0 Day 1 - 60 Follow - up Day 90 Placebo • Neonatal infants undergoing elective on - pump open - heart surgery to correct congenital heart defects (emphasis on single ventricle patients) Patient Population • 10 patient open - label run - in • ~100 subjects expected to be enrolled over 12 months • 10 U.S. sites • Trial planned to begin mid 2021 • Final data expected in 2022 Scope & Timelines • Time to recovery of GI function • Return to normal feeding • ICU and Hospital LOS • Prevention of NEC • Safety and tolerability Objectives • Randomized, double - blind, placebo - controlled, multi - site Phase 3 Study Study Design Study drug given: 2, 4, and 6 hours prior to surgery twice daily for 3 days after surgery Neonatal Cardiovascular Surgery

Gastrointestinal Surgery (Physical Injury) 22

23 Completed GI Surgery Study: 1 Study, 2 Indications, 2 Data Readouts: Return of Bowel Function and Adhesion Prevention • Open label, investigator - sponsored, single - arm study of LB1148 in subjects undergoing elective GI surgery • Patients take 700 mL of LB1148 as a split dose prior to surgery Study Design • 11 subjects • Single site in Los Angeles, CA Scope & Timelines At time of 2 nd surgery: • Adhesions extent • Adhesions severity Adhesion Indications • Return of bowel function • Hospital length of stay • Bowel obstruction • Hospital re - admittance Functional Indications Screening Period GI Surgery Postoperative / Discharge LB1148 Day - 42 to - 1 Day 0 Day 1 - 14 Follow - up Day 30 Study drug given as a split dose 2 - 6 hours prior to surgery 6 - 12 hours prior to surgery Adhesion Assessment Second Surgery GI Surgery

24 GI Surgery Study Results: 48% Faster than Historical Control Data highlights: • 48% improvement in return of bowel function – Approvable endpoint • No LB1148 - related AEs 2.3 - days earlier first bowel movement 48% better than historical control GI Surgery Source: Ludwig K, et. al.. Arch Surg. 2008 Nov;143(11):1098 - 105. doi: 10.1001/archsurg.143.11.1098. PMID: 19015469. Placebo (Entereg Study 314)

25 GI Surgery Study Results: Significant Improvement in Hospital Length of Stay Versus Expected LOS GI Surgery Time from Surgery to Discharge (Days) Patient’s actual length of stay (LOS) was compared to expected LOS as determined from their billing code at time of admission (GMLOS) 1.3 - day improvement in LOS 20% reduction p = 0.03

26 • Adhesions are scar tissue that forms during the healing process that bind tissues/organs that are not normally connected – HYPOTHESIS: Proteases, released as a result of bowel manipulation during surgeries, lead to adhesion formation • Bands of adhesions can constrict organs and tissues, causing organ strangulation and pain • Surgical re - intervention required after 6 - 10% of GI/GYN procedures to remove adhesions** Post - surgical Adhesions Develop in up to 93% of Abdominal Procedures* * J Chir Viscerale. 2012;149(2):114 - 126. doi:10.1016/j.jviscsurg.2011.11.006 ** Ward, B. C. & Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 165, 91 – 111 (2011). Image used with permission from Clear Passage Physical Therapy, http://www.clearpassage.com/adhesions - and - scar - tissue/abdominal - and - pelvic - adhesions - post - surgical - adhesions/

27 Post - surgical Adhesions Are Costly for Patients and Hospitals Increased healthcare complications: • #1 cause of secondary female infertility • #1 cause of bowel obstruction (up to 75%) • 10 th most frequent cause of emergency surgery • 80% of emergency surgery deaths • 400,000 adhesiolysis surgeries annually • $2.3B annual costs in US Sources: Causes of female infertility, UpToDate 2020 Etter, K., et. Al.. Impact of postcolectomy adhesion - related complications on healthcare utilization. Clin. Outcomes Res. 10, 7 61 – 771 (2018) Non - Adhesion Complications Adhesion Complications Difference Length of Stay 5.2 days 7.2 days 38% Rehospitalization Costs $22,500 $29,800 $7,326/pt.

28 LB1148 dramatically reduces the number of post - surgical adhesions LB1148 Reduces Adhesions by 63% in Animal Studies Animals receiving LB1148 before surgery had 63% fewer adhesions Source: LBS - NC - IL - ADH - 0003 DOF

29 • For the 3 patients with a 2 nd surgery: – No adhesions – No evidence of inflammation GI Surgery Study Results: Zero Adhesions in 3 Patients • 3/11 patients required a 2 nd surgery for complications unrelated to adhesions – Opportunity to measure two FDA approvable endpoints in same patient • bowel function • adhesions First Patient Adhesions Data Bowel Function Data GI Surgery As a surgeon who has performed thousands of abdominal surgeries, the absence of adhesions in three follow - up surgery patients is entirely unexpected Principal investigator’s report:

30 FDA Approved ADEPT with Only a 10% Reduction in Adhesions Source: Adept (4% icodextrin) Package Insert; March 2006 www.accessdata.fda.gov/cdrh_docs/pdf5/P050011B.pdf FDA approved ADEPT based on only 10% of patients achieving “success” (defined as a decrease ≥ 3 sites with adhesions)

31 GI Surgery (PROFILE) Phase 2 – Now Enrolling GI Surgery • Randomized, double - blind, placebo - controlled, multi - site Phase 2 Study • Evaluate LB1148 for return of GI function and adhesions in subjects undergoing elective bowel resection with a laparotomy or minimally invasive approach with planned stoma take - down Study Design • 120 - 200 subjects • 11 U.S. sites • First SIV Oct 2019 • 79 subjects enrolled as of November 2020 • LPE expected in 2021 Scope & Timelines At time of 2 nd surgery: • Presence of adhesions • Impact of adhesions on bowel function and pain Adhesion Indications Primary Endpoint: • Time to return of GI function GI2: (1 st bowel movement) Secondary Endpoints: • Hospital LOS • Hours to resolution of POI Functional Indications Screening Period GI Surgery Postoperative / Discharge LB1148 Day - 42 to - 1 Day 0 Day 1 - 14 Follow - up Day 30 Study drug given as a split dose 2 - 6 hours prior to surgery 6 - 10 hours prior to surgery Adhesion Assessment Up to 7 months after initial surgery Placebo

LB1148 Commercial Opportunity 32
33 LB1148 Can Improve Patient Care While Reducing Costs Oral Drug Taken at Home – No Impact on Surgical Procedure Reduce Healthcare Costs and Improve Margins Well - Characterized Safety Profile Potential to Improve Patient Outcomes Strong Value Proposition Across the Healthcare System with Key Benefits for Widespread Adoption

34 Illustrative Patient Journey: GI Surgery with LB1148 Administration At patient’s local pharmacy, covered by pharmacy benefit Prescription Filled at Pharmacy 2 1 - 2 weeks pre - op Preoperative Medications Taken at Home 3 In - Patient Surgery 5 First bowel movement Leads to discharge orders Return of Bowel Function 6 Recovery at Home Discharge from Hospital 7 8 Admission to Hospital 4 □ Bowel prep □ Anti - nausea □ Antibiotics □ LB1148 1 - 2 weeks pre - op 1 Evening before Surgery Morning of Surgery LB1148 LB1148 Pre - op Physical in Clinic

35 LB1148 Has Large Global Market Opportunity US Market 1.1 million open heart surgeries 5.5 million abdominal surgeries 20 - 40% market penetration could translate to >$2B revenue Partnered China: w/ Newsoara EU and ROW Opportunities Potential to partner with US rights or independently USA Strategy Commercialize by marketing to 5,000 hospitals M&A – Shows Value of Adhesion Market Baxter acquired the medical device Seprafilm from Sanofi for $350M in 2020 • 3.5X sales

36 Upcoming Potential Milestones December 2020 – Announced merger with Seneca Close merger with Seneca Complete financing with Altium Initiate Phase 3 in neonatal cardiac surgeries Data from 1st 10 patients from Phase 3 in neonatal CV surgery Bowel function data from Phase 2 in GI surgery Adhesion data from Phase 2 in GI surgery

5800 Armada Dr, Suite 210 Carlsbad, CA 92008 858 - 704 - 4900 InvestorRelations@palisadebio.com www.palisadebio.com 37
