Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Eloxx Pharmaceuticals, Inc. | tm2111531d1_8k.htm |
| EX-99.2 - EX-99.2 - Eloxx Pharmaceuticals, Inc. | tm2111531d1_ex99-2.htm |
| EX-10.3 - EXHIBIT 10.3 - Eloxx Pharmaceuticals, Inc. | tm2111531d1_ex10-3.htm |
| EX-10.2 - EXHIBIT 10.2 - Eloxx Pharmaceuticals, Inc. | tm2111531d1_ex10-2.htm |
| EX-10.1 - EXHIBIT 10.1 - Eloxx Pharmaceuticals, Inc. | tm2111531d1_ex10-1.htm |
| EX-2.1 - EXHIBIT 2.1 - Eloxx Pharmaceuticals, Inc. | tm2111531d1_ex2-1.htm |
Exhibit 99.1

Creating a Leader in Ribosomal RNA - Targeted Genetic Therapy Eloxx A cquires Zikani Therapeutics April 1, 2021

This presentation contains forward - looking statements, which are generally statements that are not historical facts . Forward - looking statements can be identified by the words "expects," "anticipates," "believes," "intends," "estimates," "plans," "will," "outlook" and similar expressions . Forward - looking statements are based on management's current plans, estimates, assumptions and projections, and speak only as of the date they are made . We undertake no obligation to update any forward - looking statement in light of new information or future events, except as otherwise required by law . Forward - looking statements involve inherent risks and uncertainties, most of which are difficult to predict and are generally beyond our control . Actual results or outcomes may differ materially from those implied by the forward - looking statements as a result of the impact of a number of factors, including : the development of the Company’s readthrough technology ; the approval of the Company’s patent applications ; the Company’s ability to successfully defend its intellectual property or obtain necessary licenses at a cost acceptable to the Company, if at all ; the successful implementation of the Company’s research and development programs and collaborations ; the Company’s ability to obtain applicable regulatory approvals for its current and future product candidates ; the acceptance by the market of the Company’s products should they receive regulatory approval ; the timing and success of the Company’s preliminary studies, preclinical research, clinical trials, and related regulatory filings ; the ability of the Company to consummate additional financings as needed ; the impact of global health concerns, such as the COVID - 19 global pandemic, on our ability to continue our clinical and preclinical programs and otherwise operate our business effectively ; including successfully integrating the combined companies ; as well as those discussed in more detail in our Annual Report on Form 10 - K and our other reports filed with the Securities and Exchange Commission . Forward - looking statements 2
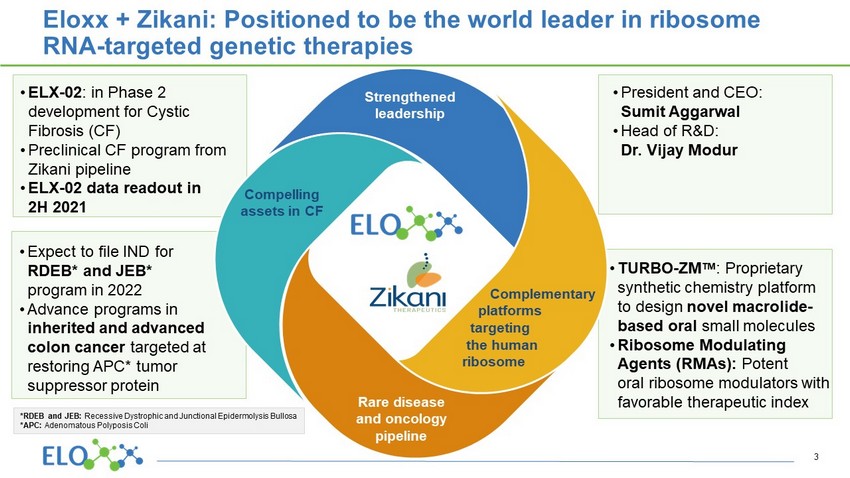
• President and CEO: Sumit Aggarwal • Head of R&D: Dr. Vijay Modur • TURBO - ZM TM : Proprietary synthetic chemistry platform to design novel macrolide - based oral small molecules • Ribosome Modulating Agents (RMAs): Potent oral ribosome modulators with favorable therapeutic index Eloxx + Zikani : Positioned to be the world leader in ribosome RNA - targeted genetic therapies • ELX - 02 : in Phase 2 development for Cystic Fibrosis (CF) • Preclinical CF program from Zikani pipeline • ELX - 02 data readout in 2H 2021 • Expect to file IND for RDEB* and JEB* program in 2022 • Advance programs in inherited and advanced colon cancer targeted at restoring APC* tumor suppressor protein 3 Strengthened leadership Rare disease and oncology pipeline Compelling assets in CF Complementary platforms targeting the human ribosome *RDEB and JEB: Recessive Dystrophic and Junctional Epidermolysis Bullosa *APC: Adenomatous Polyposis Coli

Zikani leadership team to join Eloxx Dr. Vijay Modur Head of Research & Development • 20+ years in translation and drug development • Led Venglustat rare disease program at Sanofi • 30+ years building companies • Closed $2B in equity and debt financings for public and private companies Daniel Geffken Interim Chief Financial Officer Dr. Roger Clark Head of Discovery Sciences • 20+ years medicinal chemistry • Architect of Zikani RMAs Sumit Aggarwal President and CEO • 20+ years investing and transforming healthcare companies • Raised >$150M • Biotech Investor 4 Significantly expands and strengthens leadership team
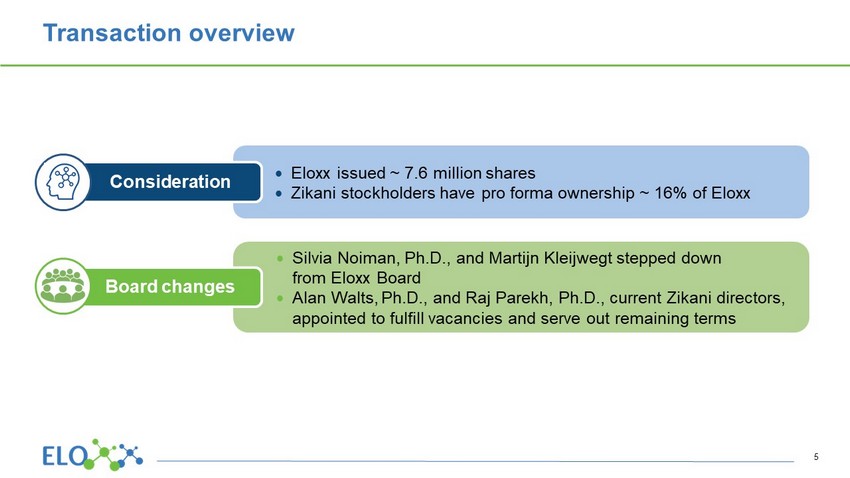
Transaction overview Eloxx issued ~ 7.6 million shares Zikani stockholders have pro forma ownership ~ 16% of Eloxx Consideration Silvia Noiman, Ph.D ., and Martijn Kleijwegt stepped down from Eloxx Board Alan Walts, Ph.D., and Raj Parekh, Ph.D., current Zikani directors, appointed to fulfill vacancies and serve out remaining terms Board changes 5

Acquisition combines complementary platforms in targeting the human ribosome Genetic nonsense mutations • Premature stop codon • Less than full length protein formed = loss of function ERSG RMA Dysregulated Ribosomes • Onco - and mitoribosomes • Selective translation of oncogenic proteins • Aids hyperproliferative phenotype ELX - 02 • ELX - 02 has shown clinically relevant readthrough of CFTR in pre - clinical models Ribosome = “Protein Factory” 6 RMA: Ribosome Modulating Agent ERSG: Eukaryotic Ribosome Selective Glycoside
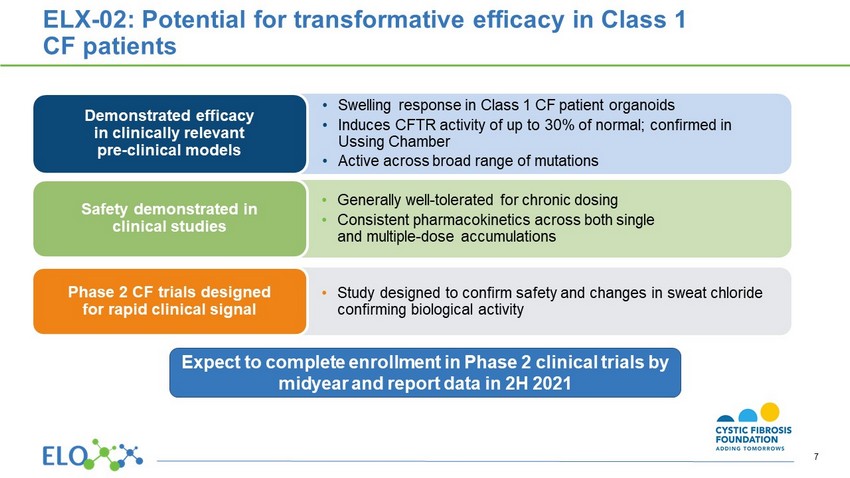
• S welling response in Class 1 CF patient organoids • Induces CFTR activity of up to 30 % of normal; confirmed in Ussing Chamber • Active across broad range of mutations Demonstrated efficacy in clinically relevant pre - clinical models • Generally well - tolerated for chronic dosing • Consistent pharmacokinetics across both single and multiple - dose accumulations Safety demonstrated in clinical studies • Study designed to confirm safety and changes in sweat chloride confirming biological activity Phase 2 CF trials designed for rapid clinical signal ELX - 02: Potential for transformative efficacy in Class 1 CF patients Expect to complete enrollment in Phase 2 clinical trials by midyear and report data in 2H 2021 7
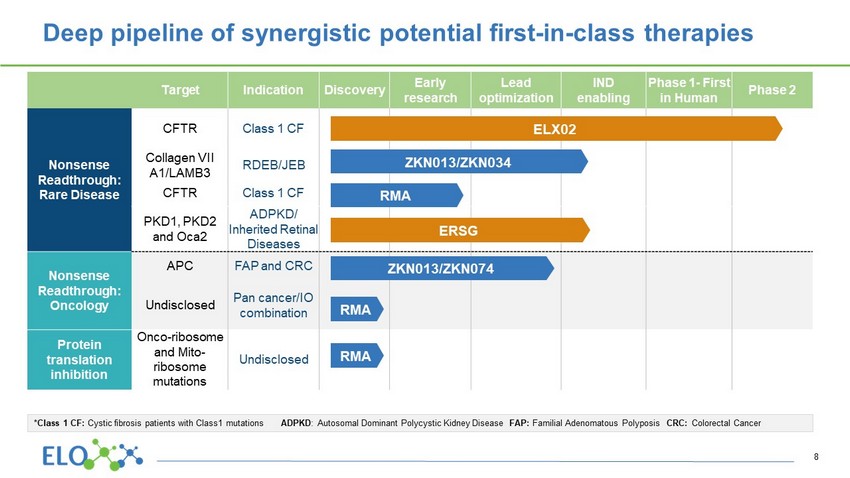
Target Indication Discovery Early research Lead optimization IND enabling Phase 1 - First in Human Phase 2 Nonsense Readthrough : Rare Disease CFTR Class 1 CF Collagen VII A1/LAMB3 RDEB/JEB CFTR Class 1 CF PKD1, PKD2 and Oca2 ADPKD/ Inherited Retinal Diseases Nonsense Readthrough : Oncology APC FAP and CRC Undisclosed Pan cancer/IO combination Protein translation inhibition Onco - ribosome and Mito - ribosome mutations Undisclosed ZKN013/ZKN034 ZKN013/ZKN074 ERSG RMA RMA Deep pipeline of synergistic potential first - in - class therapies ELX02 RMA 8 *Class 1 CF: Cystic fibrosis patients with Class1 mutations ADPKD : Autosomal Dominant Polycystic Kidney Disease FAP: Familial Adenomatous Polyposis CRC: Colorectal Cancer

Zikani Therapeutics Introduction

10 TURBO - ZM TM (Tuning the Ribosome with Zikani Molecules) platform fully unlocks the potential of macrolides Optimize for: • Readthrough • Protein translation inhibition Interact with the peptide sequence Modulate: • PK • Safety: cardiac, liver • Oral bioavailability RMA core required for ribosomal binding A B C D F E X O TURBO - ZM TM : Applying Macrolide SAR to RMA Design TURBO - ZM E ssential for ribosomal binding Modulate cytoplasm and mitochondrial ribosome binding activity
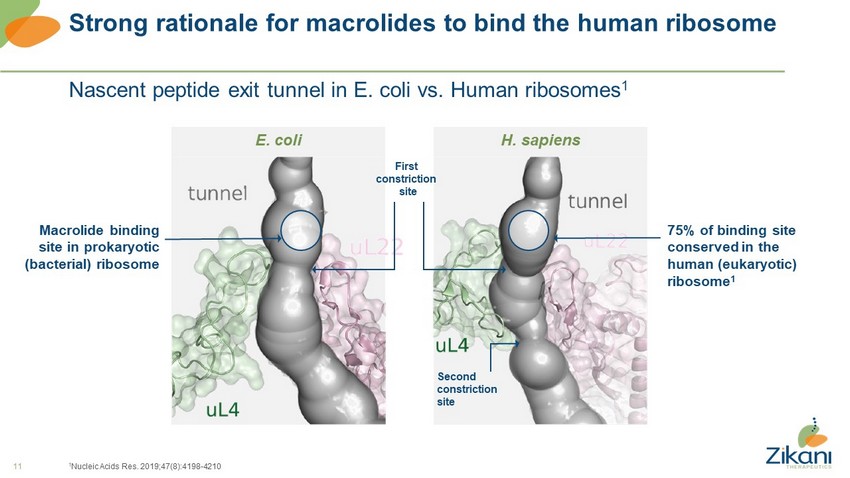
11 Strong rationale for macrolides to bind the human ribosome Nascent peptide exit tunnel in E. coli vs. Human ribosomes 1 1 Nucleic Acids Res. 2019;47(8):4198 - 4210 H. sapiens E. coli Macrolide binding site in prokaryotic (bacterial) ribosome 75% of binding site conserved in the human (eukaryotic) ribosome 1 First constriction site Second constriction site
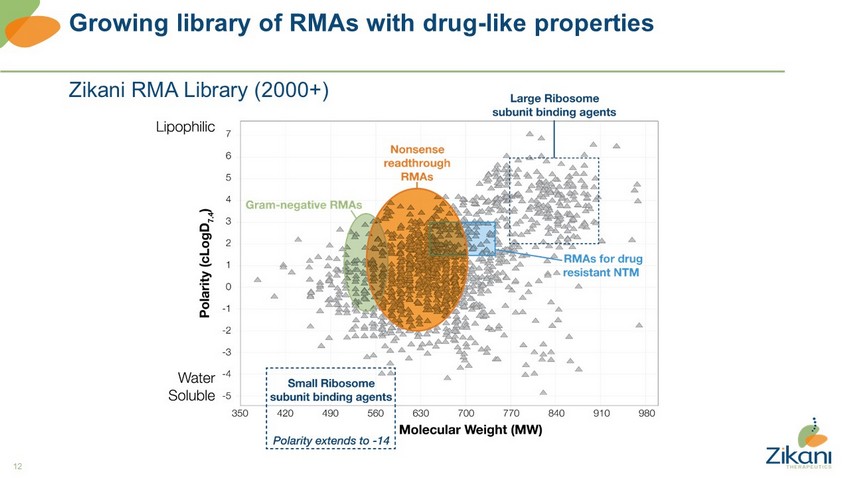
12 Growing library of RMAs with drug - like properties Zikani RMA Library (2000+)
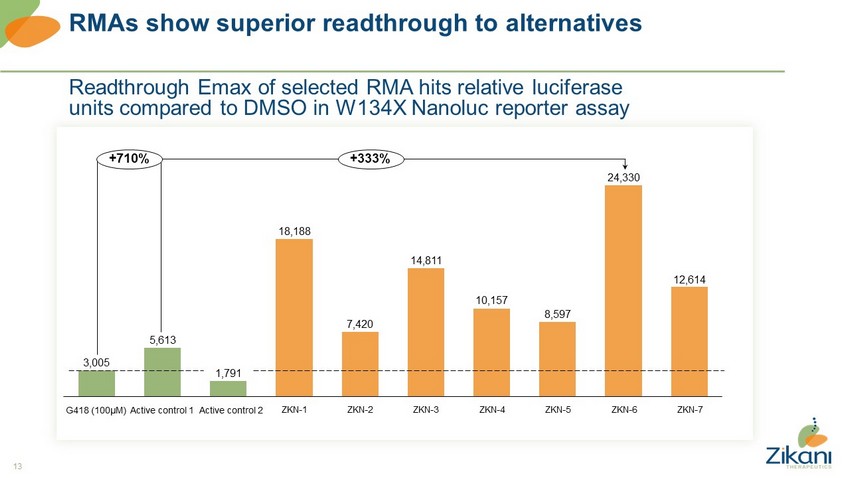
13 RMAs show superior readthrough to alternatives Readthrough Emax of selected RMA hits relative luciferase units compared to DMSO in W134X Nanoluc reporter assay 3,005 5,613 1,791 18,188 7,420 14,811 10,157 8,597 24,330 12,614 ZKN - 3 ZKN - 1 ZKN - 5 ZKN - 2 ZKN - 4 ZKN - 6 +710% +333% ZKN - 7 G418 (100µM) Active control 1 Active control 2
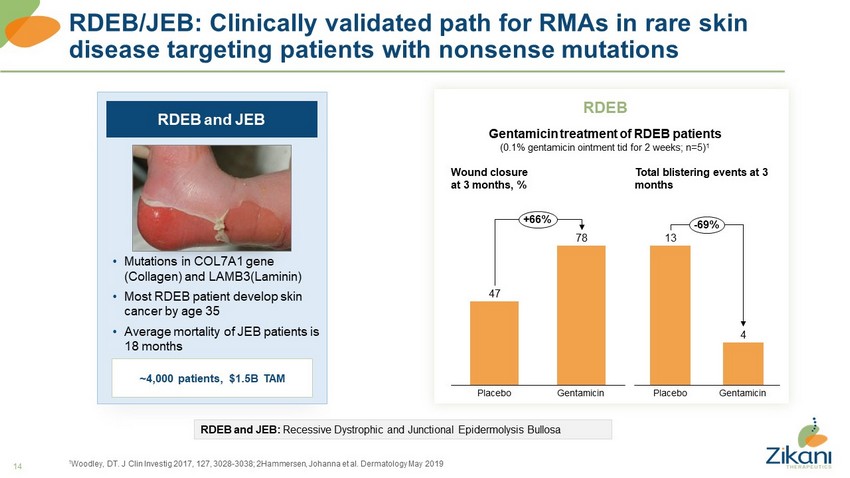
14 RDEB/JEB: Clinically validated path for RMAs in rare skin disease targeting patients with nonsense mutations 1 Woodley, DT. J Clin Investig 2017, 127, 3028 - 3038; 2Hammersen, Johanna et al. Dermatology May 2019 RDEB and JEB • Mutations in COL7A1 gene (Collagen) and LAMB3(Laminin) • Most RDEB patient develop skin cancer by age 35 • Average mortality of JEB patients is 18 months ~4,000 patients, $1.5B TAM RDEB and JEB: Recessive Dystrophic and Junctional Epidermolysis Bullosa RDEB Gentamicin treatment of RDEB patients (0.1% gentamicin ointment tid for 2 weeks; n=5) 1 Wound closure at 3 months, % Total blistering events at 3 months 13 4 Placebo Gentamicin - 69% 47 78 Gentamicin Placebo +66%

15 RDEB: RMAs restore functional collagen protein in primary patient cells comparable to high dose gentamicin COL7 with 48 hr. exposure in RDEB patient derived primary fibroblasts* * Fibroblasts isolated from patients two and five in gentamicin clinical trial. J Clin Invest 2017, 127, 3028 - 3038 ** 48 hours treatment with media and compounds replaced and refreshed at 24 hours. Study repeated twice with equivalent resul ts. Full length protein in Hom R578X COL7A Fibroblasts** Full length protein in R613X/R1683X COL7A Fibroblasts** • Assay with proven translation to clinic • 30 to 60 - day Col7 protein half - life • RMAs compounds exceed clinical efficacy threshold of 10% Gentamicin 845uM Data generated in collaboration with academic partner

16 Class 1 CF: RMA lead showed highest ever readthrough preclinical Ussing chamber assay Summary of Class 1 CF data * Forskolin 10 µM/1µM VX - 770 - both chambers ** VX 809 and RMA data averaged from 2 separate Ussing chamber results • Submitted $2.5M grant to CF Foundation to support through development candidate Data generated at Chantest Het G542X Human Broncho Epithelial (HBE) cells Ussing Chamber Steady state modulator response measurement** RMA (15 uM) DMSO RMA (30 uM) VX - 809 (3uM) G418 (50 uM) G418 (100 uM) +47% “ ” Never seen before impressive single agent activity from non aminoglycoside class – need to advance this program – CF Foundation Encouraged to apply for “Path to Cures” Isc Chloride Transport (µA/cm 2 )

17 APC readthrough: Supported by positive prior clinical success of Erythromycin in FAP 1 Kariv, R. Int J Cancer. 2019 doi: 10.1002/ijc.32557 Clinical trial success in FAP with Erythromycin Erythromycin treatment (250 mg/day po for 4 months) Change in polyp burden at 12 months 1 Change in polyp number - 55% 35% Change in polyp cumulative size 46% - 42% - 1 yr to baseline 8 months post treatment APC mutant Familial Adenomatous Polyposis (FAP) and CRC • Mutations in the Adenomatous Polyposis Coli (APC) gene (tumor suppressor gene) • FAP patients develop CRC by age 40 • 80% of CRC patients have an APC mutation 8,000 - 12,5000 FAP patients in the US/EU; 210,000 CRC patients WW

18 Clear path in treating FAP supported by efficacy in APC mutant cancer patient tumor grafts Efficacy of ZN013 in colorectal cancer patient derived tumor grafts ex - vivo • Ex - vivo sensitivity assessment in tumor grafts • Potent tumor growth inhibition – GI 50 <15uM • Cancer xenograft studies planned in 2021 ZNK013

Strengthened leadership Rare disease and oncology pipeline Compelling assets in CF Complementary platforms targeting the human ribosome 20 Eloxx + Zikani : Positioned to be the world leader in ribosome RNA - targeted genetic therapies

21
