Attached files
| file | filename |
|---|---|
| EX-32.1 - Save Foods Inc. | ex32-1.htm |
| EX-31.1 - Save Foods Inc. | ex31-1.htm |
| EX-21.1 - Save Foods Inc. | ex21-1.htm |
| EX-4.1 - Save Foods Inc. | ex4-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2020
or
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _______________ to _______________
Commission file number 000-56100
SAVE FOODS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-468460 | |
State or other jurisdiction of incorporation or organization |
(I.R.S. Employer Identification No.) |
Kibbutz Alonim, Israel, 3657700
(Address of principal executive offices) (Zip Code)
(347) 468 9583
Registrant’s telephone number, including area code
Securities registered pursuant to Section 12(b) of the Act: None
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| N/A | N/A | N/A |
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.0001 Par Value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [X]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes [X] No [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | [ ] | Accelerated filer | [ ] |
| Non-accelerated filer | [X] | Smaller reporting company | [X] |
| Emerging growth company | [ ] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. [ ]
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ] No [X]
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant as of June 30, 2020, based on the price at which the common equity was last sold on the OTC Market, Pink Tier, on such date, was $11,740,910. For purposes of this computation only, all officers, directors and 10% or greater stockholders of the registrant are deemed to be affiliates.
As of March 29, 2021, there were 1,606,760 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS
| 2 |
This Annual Report on Form 10-K (the “Annual Report”) includes a number of forward-looking statements that reflect management’s current views with respect to future events and financial performance. Forward-looking statements are projections in respect of future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other comparable terminology. Those statements include statements regarding the intent, belief or current expectations of our Company and members of our management team as well as the assumptions on which such statements are based. Prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those contemplated by such forward-looking statements.
These statements are only predictions and involve known and unknown risks, uncertainties and other factors. Readers are urged to carefully review and consider the various disclosures made by us in this Annual Report and in our other reports filed with the Securities and Exchange Commission. We undertake no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes in future operating results over time except as required by law. We believe that our assumptions are based upon reasonable data derived from and known about our business and operations. No assurances are made that actual results of operations or the results of our future activities will not differ materially from our assumptions.
As used in this Annual Report and unless otherwise indicated, the terms “Save Foods,” “we,” “us,” “our,” or “our Company” refer to Save Foods, Inc. Unless otherwise specified, all dollar amounts are expressed in United States dollars.
All information in this Annual Report relating to shares or price per share reflects the 1-for-7 reverse stock split effected by us on February 23, 2021.
| 3 |
Company Overview
We develop eco-friendly “green” solutions for the food industry. Our solutions are developed to improve the food safety and shelf life of fresh produce. We do this by controlling human and plant pathogens, thereby reducing spoilage, and in turn, reducing food loss.
Our products are based on a proprietary blend of food acids which have a synergistic effect when combined with certain types of oxidizing agent-based sanitizers and fungicides at low concentrations. Our “green” products are capable of cleaning, sanitizing and controlling pathogens on fresh produce with the goal of making them safer for human consumption and extending their shelf life by reducing their decay. One of the main advantages of our products is that our active ingredients do not leave any toxicological residues on the fresh produce we treat. In contrary, by forming a temporary protective shield around the fresh produce we treat, our products make it difficult for pathogens to develop and potentially provide protection which also reduces cross-contamination.
The U.S. Food and Drug Administration (the “FDA”) Food Safety Modernization Act (the “FSMA”) is transforming the United States’ food safety system by shifting the focus from responding to foodborne illness to preventing it. According to the recent data from the Centers for Disease Control and Prevention, approximately 48 million people in the United States get sick each year from foodborne diseases. We believe this is a significant public health burden that is largely preventable. Since 2011, the FDA has had a legislative mandate to require comprehensive, science-based preventive controls across the food supply. In the context of fresh produce at packing houses, FDA’s final produce safety rule (with an initial compliance date of January 26, 2018) provides for the use of sanitizers to ensure produce is cleaned from human pathogens.
In addition, most conventional chemical pesticides (fungicides), which are currently used to protect fresh produce from microbial spoilage and reduce food waste, are potentially toxic, they remain on fruit peel and present health concerns, while also polluting the environment. Therefore, the use of these products is strictly regulated and their residue on food and on the environment are carefully monitored. Today’s trends led by both consumers and regulatory bodies are to significantly reduce the use of fungicides and switch to greener solutions In a series of studies conducted in collaboration with a large post-harvest service company during the second quarter of 2020, our products have shown impressive results in extending the shelf life of fresh produce in “organic” (where no fungicides are used at the post-harvest stage) and conventional (where fungicides are being used at the post-harvest stage) settings. On average, our products may reduce the rotten fruits at the retail level by 50%.
We have a unique opportunity to make a positive difference throughout the food value chain from field to fork and address two of the major’s challenges in the food industry today — safety and waste. We target major markets that use conventional chemical pesticides and sanitizers, including the pre- and post-harvest market, the greenhouse market and the fresh-cut market, where our “green” products are used as alternatives for, or mixed with, conventional products in order to reduce (i) health and environmental concerns, and/or (ii) microbial resistance that has reduced the efficacy of conventional chemical pesticides.
Recent Developments
On March 16, 2021, we filed a registration statement on Form S-1 with the U.S. Securities and Exchange Commission in connection with a proposed public offering of our common stock, par value $0.0001 per share (the “Common Stock”, and collectively the “Current Offering”). On January 12, 2021, we applied to list our Common Stock on the Nasdaq Capital Market.
Industry Overview and Market Opportunity
Background
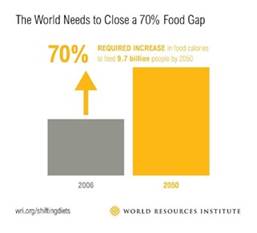
The world’s population is expected to grow to almost 10 billion people by 2050, boosting agricultural demand by some 50%. Providing healthy and safe food to feed the world’s population is one of the biggest challenges of the twenty first century, accentuated with the backdrop of a of a fragile global economy. Globally, around one-third of the food produced (estimated at circa 1.3 billion tons), is lost or wasted along the food chain – from production to consumption.
| 4 |
Fruits and vegetables are considered essential food commodities and demonstrate their best benefits especially when consumed fresh. Consumption as well as production of fresh fruit and vegetables is growing globally; in 2018, the global production of fresh fruit amounted to about 868 million tons, while the production of fresh vegetable amounted to about 1.09 billion tons. According to a report published by technavio in October 2020, the fresh food market size has the potential to grow by 337.76 million tons from 2020 to 2024, growing at a CAGR of almost 3% during the forecast period, and the market’s growth momentum will accelerate during the forecast period due to the steady increase in year-over-year growth. In the United States, according to a report by Grand View Research, increasing health awareness among the U.S population and potential development of secondary diseases due to obesity and unhealthy eating habits are propelling the market of fruit and vegetables to reach an estimated $1.1 billion by 2025.
Food Safety and Food Loss
Food Safety
We believe foodborne diseases are a significant public health concern globally. Hundreds of diseases are caused by eating contaminated food. Many diseases are spread through unwashed or untreated produce. With approximately 48 million people in the United States (one in six) getting sick, 128,000 hospitalized, and 3,000 die each year from foodborne diseases, according to recent data published by the FDA, and 23 million in the European region getting sick due to food borne disease, food safety is another major concern and source of waste, placing a material burden on public health and significant healthcare cost. The economic burden of foodborne illness has been estimated to be as high as $90 billion annually.
When considering the farm-to-fork chain, microbial contamination of fresh produce can occur at multiple steps. Contamination can take place during the cultivation of fresh produce, at harvest, during preparation/washing, within distribution chains and transport to shops, and even at the final step in the consumers’ kitchen. We believe this is a significant public health burden that is largely preventable. The FSMA is transforming the United States food safety system by shifting the focus from responding to foodborne illness to preventing it. The Produce Safety rule of the FSMA establishes, for the first time, science-based minimum standards for the safe growing, harvesting, packing, and holding of fruits and vegetables grown for human consumption. The final rule went into effect on January 26, 2016. Sanitization is a cornerstone of FSMA compliance, which requires preventing or eliminating food safety hazards or reducing such hazards to a minimal level.
Markets require many types of produce to be washed prior to sale in order to remove dirt and other debris. Produce can be contaminated with foodborne pathogens before it enters the packing house, and these pathogens cannot be seen with the naked eye. Inability to visually spot pathogens makes the washing step one of the most important steps in packing because, if washing process is not controlled, it can become a source of cross-contamination (when foodborne pathogens fall off contaminated produce into the water where they can contaminate more produce). These washing steps are defined by the packing house safety managers as critical point because water mixed with organic materials are good conditions for pathogens to develop. Therefore, the use of sanitizers should be introduced during the washing step because they are, most of the time, one of the last treatments applied before the produce meets with the consumer. Sanitizers are designed to inactivate/kill any bacteria in the water, drastically reducing the possibility of cross-contamination. We believe this represents a significant opportunity for us.
| 5 |
Food Loss
The Food and Agriculture Organization of United Nations predicts that about third of the food produced globally are wasted or lost every year. Approximately 644 million tons of fruits and vegetables are thrown away each year (representing 42% of the total food wasted every year). A report published in April 2020, generated by the European Innovation Partnership Agricultural Productivity and Sustainability, estimates that in Europe an estimated 9 million tons of food is lost at the production stage (farm), while up to 16.9 million tons are lost at the processing stage (packing houses, etc.).
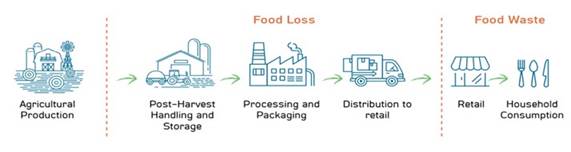
Much of this loss is caused by spoilage, which can be caused by microorganisms – primarily bacteria and mold. In addition, bacteria and fungi represent the highest numbers of incidents of post-harvest microbial diseases in fresh produce worldwide. Taken together, it is estimated that nearly a third of all food grown is lost between the time that it is grown and harvested and the time that it is packaged for retail sale. Such waste equates to roughly $680 billion in industrialized countries and $310 billion in developing countries.
Post-harvest losses due to spoilage represent a significant problem along the supply chain and lead to profit losses in the millions. The main causes of these losses are pest or disease infestation and incorrect storage conditions, which lead to rotting or loss of fresh mass due to respiration and evaporation. Fruits and vegetables are largely damaged after harvest by fungi and bacteria . It is estimated that an average of 45% of harvested fruit and vegetables are lost globally. Post-harvest diseases have been identified as the greatest cause of post-harvest losses in fruits and vegetables, causing significant economic losses. It is estimated that approximately 20 to 25% of the fruit and vegetables harvested are lost due to microbial spoilage during post-harvest handling in developed countries. Furthermore, the demand for fresh fruits and vegetables, especially exotic tropical fruit has contributed to the demand for post-harvest treatments to increase shelf life and maintain quality, resulting in more efficient export trade.
The most common way to protect fresh produce and prevent loss is the use of hazardous chemicals such as fungicides in post-harvest applications. Post-harvest diseases are generally controlled by fungicides. Systemic (non-organic) fungicides, are one of the most commonly used fungicides, for example, citrus fruits in California are completely covered by the fungicides, and the residue is persistent for the life of the fruit providing protection. However, as they tend to affect a single biochemical pathway within the pathogen, fungi may readily develop resistance to systemic fungicides. To avoid potential issues with resistance, maximum concentration of fungicides will be generally used to ensure highly efficient eradication of the targeted pathogen which leaves high residue level on the treated produce.
However, these chemical agents have been applied for many years with few or limited success due to the development of resistance. Further they have severe negative effects on human health, and the environment mainly due to the carcinogenic and/or teratogenic properties of the compounds, and by their cumulative toxic effects.
The effects of exposure to these hazardous chemicals on humans and the environment are a continuing concern as they are intrinsically toxic and pollute the environment through wastewater discharge from the packing house or a discarded fruit. Therefore, the agricultural use of certain pesticides (in the field or in the packing house) has been abandoned in some countries leaving the growers with significant challenges.
| 6 |
To control and monitor the potential negative impacts pesticides might have over time, regulatory agencies that regulate pesticides – for example, the United States Environmental Protection Agency (the “EPA”), the Pest Management Authority Agency in Canada, and the European Food Safety Authority (“EFSA”) in Europe, have defined a maximum residue level (the “MRL”) that can be present on the treated produce. Additionally, more countries require an MRL for the commodity to be imported into their country. As there is increased awareness regarding compliance with MRLs, MRLs have become a much greater concern. These changes have also impacted the market and we believe that consumers spearheaded this change by demanding organic or pesticide-free foods. Consumers have recently increasingly want to understand where and how their food is grown. Retailers and processors have capitalized on what they view as an opportunity to offer more information to consumers. It is more common now for retailers and processors to ask which products have been used on the commodities they are purchasing. There are also retailers and processors banning the use of certain products, requiring any residues to be below the established MRLs. The reduction in MRLs results in lower efficacy of fungicide and increased loss.
We believe that the rising demand for healthy food among the global population will trigger the market’s growth in the forthcoming years. Over the last decade, the organic market in Europe continued to grow and reached €40.7 billion in 2018 with 15.6 million hectares (approximately 38,548,439 acres) (including 2.2 million hectares in Spain, the largest organic area in Europe, followed by 2.0 million hectares in France and 2.0 million hectares in Italy), providing farmers with further added value on their production. The strong growth rates in both production and consumption indicate that the organic market has not yet reached its peak and further growth can still be expected. Organic farming is already responding to further emerging consumer trends such as veganism and demand for locally produced food products, turning these challenges into opportunities.
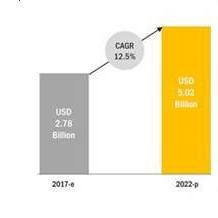
As consumer demand for organic fruits and vegetables is increasing globally and there is an increasing promotion by government organizations for the adoption of environmentally friendly pesticides, the biorational pesticides market estimated at $2.78 billion in 2017 is projected to reach a value of $5.02 billion by 2022, at a compound annual growth rate (the “CAGR”) of 12.5% from 2017. A biorational pesticide is a term used to define any pesticide material that causes relatively no harm to humans or animals and does little or no damage to the environment. We believe that our products could be defined as biorational products.
Analysts have predicted that the organic fresh food market will reach a CAGR of almost 15% by 2023. The market size will increase by $62.23 billion during the forecast period from 2019 to 2023. In addition, strict regulations have been imposed on the usage of pesticides and GMO-produced crops worldwide. This, in turn, has influenced consumer demand for organic fruits and vegetables.
Case Study – Citrus Fruit
Citrus fruit, which represent one of the main fruit produce worldwide with more than 100 million tons produced worldwide, can be infected by many fungal pathogens, and these pathogens can cause considerable losses during storage and transportation. Losses are mainly caused by Penicillium digitatum, P. italicum, Aspergillus flavus and Alternaria alternata for citrus fruit. Post-harvest treatments such as thiabendazole, imazalil, sodium ortho-phenil phenate or other active ingredients have been used for many years. They are currently the most commonly used fungicides effective for controlling post-harvest fungal pathogens in citrus and they are used in citrus packing houses to maintain fresh fruit, control post-harvest decay, and extend fruit shelf life. However, significant problems such as environmental issues and health concerns have risen in the citrus industry due to chemical residues or the occurrence of pathogenic resistant strains which require the use of even higher concentration of these post-harvest treatments. However, currently, the residues of imazalil on citrus fruits is being revised by the European Commission. The EFSA put forward a proposal in 2018 to cut the MRL for imazalil from 5 milligrams per kilogram to 0.01 milligrams per kilogram, causing worry among Europe’s main citrus producing countries and packers exporting their produce to European countries. Due to the significant impact this proposal could have on the citrus industry, the European Council has decided, in the meantime, to start reducing imazalil residues to four milligrams per kilogram for citrus fruit for a limited period of time to allow the citrus industry an extra time to find green and safe alternatives. Our product PeroStar/SaveProtect has already shown its benefits in reducing significantly the residues of imazalil while maintaining the produce shelf life.
| 7 |
Current Market Drivers and Trends
In addition to food safety and food waste concerns, the following market drivers are also shaping the food industry by setting standards and conditions on the main actors in the industry:
| ● | Focus of consumers on health characteristics: consumers are more aware and conscious of the health characteristics of the food they consume. Consumers pay more attention to the qualities of the fresh produce they buy. Particularly in the United States and Europe, products such as berries, avocados, mangoes, pomegranates, papayas and sweet potatoes are gaining popularity and considered “super foods,” and these products are showing a strong annual import growth of 10% to 20%. | |
| ● | Increasing demand for organic produce: the demand for organic products is growing rapidly particularly in Europe and North America, and is closely related to consumer interest in healthy and pure eating. While the increasing demand created potential for oversees supply, it can be challenging and expansive for exporters in tropical climates to comply with the increasingly demanding organic standards. | |
| ● | Success of retailers determined by quality of produce: a recent report by Fruit Logistica published in 2019, based on consumer surveys that involved almost 7,000 consumers in 14 different markets across Europe and North America, demonstrated the increased importance of fresh produce for the profitability of food retailers. According to the report, when choosing the place to buy their groceries, consumers focus on the quality of the stores’ fresh food, with freshness of fruits and vegetable being their top priority. The report also showed that customers who are satisfied with the store’s fresh food quality, would visit the store more frequently than those who are not. In addition, consumers are also willing to pay more for better-quality produce and their basked will be 4% larger. | |
| ● | Promoting sustainability: a large range of sustainability aspects are directly related and affected by the fresh produce industry. Food waste accounts for 8% of global greenhouse gas emissions. Both consumers and businesses, are becoming more aware of the growing importance of sustainability issues. As consumers increasingly embrace social causes, they seek products and brands that align with their values. According to a recent analysis published by Research Insights, nearly six in 10 consumers surveyed are willing to change their shopping habits to reduce environmental impact, nearly eight in 10 respondents indicated sustainability is important for them, and among those respondents that indicated that sustainability is very or extremely important, over 70% indicated that they would pay a premium of 35%, on average, for brands that are sustainable and environmentally responsible. Increasing number of companies in the fresh food sector are investing in sustainability. Survey conducted by Champions 12.3 in 2017 showed that 99% of businesses that invested in reduction of food loss and waste, received a net positive financial return. Primary production companies are investing in aspects of food losses, energy efficiency and carbon footprint, through innovations such as drying produce, on-farm and off-grid cold rooms and post-harvest treatments. | |
| ● | Food retailers seek to reduce their waste and maximize their revenues: more than eight million tons of food are wasted every year in the United States in the retail sector alone, which translates into $18 billion in lost value (cost of waste) every year. Some retailers, including Walmart, have already committed to implement a zero-waste policy by 2025. Prevention solutions across the retail value chain offer the highest returns to retailers and are growing the fastest. | |
| ● | Regulators are promoting the use of safer chemical-based product: for example, the EPA offers a “Safer Choice” label that product manufacturers may use on qualifying products to help consumers and commercial buyers identify products with safer chemical ingredients. The EPA requires that every chemical, regardless of percentage, in a Safer Choice-certified product is evaluated and only the safest ingredients are allowed. |
| 8 |
| ● | Increasing investment in foodtech and agritech companies: according to a recent report published by AgFunder, a venture capital firm active in the foodtech and agritech, startups developing agrifoodtech solutions and products, raised approximately $26.1 billion in 2020, a 15.5% year-over-year increase. Reduction of food waste, extension of the shelf life of fresh produce and reduction of the use of pesticides are main focus of the industry and many companies are addressing these objectives, including: |
| ● | Apeel Sciences, a company developing an edible coating to extend the shelf life of fruit and vegetables, secured in May 2020 a $250 million investment from a leading venture capital, based on a firm valuation of more than $1 billion; | |
| ● | Lineage Logistics, an expert in cold chain management for food, raised $1.6 billion in September 2020. | |
| ● | Zymergen, an expert in biofacturing with applications also for the agricultures with the goal to develop safer crop protection and pest control using their natural products, secured in September 2020 an investment in a total amount of $300 million. | |
| ● | GreenLight Biosciences, an RNA based pest control reducing the use of toxic pesticides, secured in June 2020 an investment of $102 million. | |
| ● | Provivi, a company developing sustainable and safer pest control based on pheromones, closed a $45.5 million financing round in December 2020. | |
| ● | Enko Chem, a company using AI to develop green insecticides, fungicides, and herbicides, announced in June an investment of $45 million. | |
| ● | PeroxyChem, a manufacturer of hydrogen peroxide and peracetic acid and a well-positioned in high-margin specialty applications and applications for environmentally friendly disinfectants, was successfully acquired by Evonik for $640 million. |
The increased consumption of fruits and vegetables in combination with the current regulation and consumers’ demand for healthier food has placed a greater burden on the fresh produce industry to provide food products that are fresher in quality, demonstrate an extended shelf life and are safer to consume.
The aforementioned changes provide a unique opportunity for us to introduce our products. We are aiming to become a significant player in post-harvest green produce treatment, fully responsive to the world’s ongoing change in fruit and vegetables consumption, food safety requirements as well as regulations and consumer demand to eliminate the use of hazardous chemicals.
| 9 |
Our Core Products
Our innovative products address what we believe to be two of the most significant challenges in the food industry: increase food safety and reduce food loss. Our main product lines consist of a proprietary blend of organic food acids applied in post-harvest applications designed to ensure food safety and increase fruit and vegetable’ shelf life by reducing microbial spoilage.
The main steps in post-harvest applications are cleaning, sanitization, and coating (wax). Our products address the cleaning and sanitization application points which are the critical first steps for preserving the quality of fresh produce by controlling microbial contamination related to food safety (e.g., Listeria, Salmonella, E. coli) and food loss due to microbial spoilage (e.g., fungi, mold and yeast). In general, the current process includes an initial washing step to remove soil and other debris, which improves the product appearance and lowers the product temperature. The next step includes sanitation or disinfection methods combined with fungicides that can further reduce the presence and prevent the transfer of spoilage and pathogenic microorganisms on fresh produce surfaces. The last step usually includes application of wax sometimes combined with an additional application of fungicides to prevent or reduce physiological changes and risks of spoilage. Our main products are applied at the cleaning and sanitization steps.
One of the main advantages of our food acid blend is its non-toxic residues that are providing protection to the treated produce. And we believe that all the blend ingredients are recognized by the FDA as GRAS when used as intended in fruit and vegetable wash applications. Moreover, they significantly reduce or eliminate the need for additional post-harvest applications with conventional fungicide by at least 50%, and in some cases entirely, and can reduce food waste due to spoilage by up to 50% (see results below on easy peelers and mango). Our main products are:
| ● | Processing Aids – SavePROTECT or PeroStar: post-harvest treatment added to fruit and vegetable wash water as a processing aid to increase the efficiency of the oxidizing agent present in the water tank against plant pathogens to reduce produce loss; and | |
| ● | Sanitizers - SF3HS and SF3H: post-harvest cleaning and sanitizing solution to control both plant and foodborne pathogens to ensure both food safety as well as increase produce’s shelf life. |
Processing Aids – SavePROTECT or PeroStar
Processing aids are products that are intended to be used with other products to aid the application or enhance the effect of that product. Save Foods processing aids, which are marketed as SavePROTECT in the United States and as PeroStar in Spain Israel and Italy, are based on our proprietary blend of food acids and are added to the wash water at the cleaning and sanitization stages simultaneously with a low concentration of peracetic acid (“PAA”), a sanitizing agent. This food acid blend serves several functions:
| ● | SavePROTECT/PeroStar keeps the process wash waters at a relatively low stable pH level. We have observed that low pH levels strengthen the effectiveness of the PAA and the fungicide used which result in increased sanitation and biocide activity; | |
| ● | PAA-based products are used as disinfectant in wash water. When used with PAA-based products, SavePROTECT/PeroStar may optimize the efficacy of PAA and eliminates the strong odor of PAA, creating a more friendly and safe working environment; | |
| ● | When used with fungicides, including imidazole, imazillil, thiabendazole, etc. – most commonly used fungicides – SavePROTECT/PeroStar may optimize the efficacy of the fungicides used and prevent resistance buildup; | |
| ● | SavePROTECT/PeroStar helps to clean the fruit surface and can improve the performance of the wax applied leading to an improved appearance of treated fruit by leaving a glossy finish on the outer skin of the fruit; and | |
| ● | SavePROTECT/PeroStar helps to extend shelf life. |
During 2020, we ran a series of proof of concept and small trials in collaboration with commercial partners on pears, avocado, easy peelers, lime, mango, bell pepper, lemons, fresh cut vegetables and figs. In February 2021, we initiated a proof of concept in bananas.
| 10 |
Results on Easy Peelers
Easy peelers are citrus fruits that are easier to peel, such as tangerines, mandarins, satsumas, and clementines. As previously described, imazalil is currently one the most commonly used fungicide that is effective in controlling post-harvest fungal pathogens in citrus. Currently, the residues of imazalil on citrus fruit is being revised by the European Commission and have already been reduced, and this reduction poses challenges, especially to packing houses exporting to Europe.
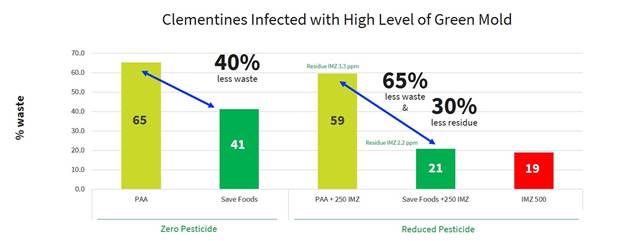
Between February and June 2020, we collaborated with the Israeli branch of one of the largest worldwide post-harvest service companies to demonstrate the safety and ability of PeroStar to meet the new requirement of reduced residue level of imazalil and efficiently control decay against the most common pathogens attacking citrus fruit such as Green mold (Penecillium digitatum) and sour rot (Geotrichum candidum). The experiment simulated the applications in a packing house which tested the use of imazalil with and without our products. The reference used in the trials to compare the results was the maximum amount of imazalil allowed and the current treatment in the packing house which is a combination of PAA and imazalil as well as PAA alone to simulate treatment in organic settings.
To ensure the efficacy of the products, it is customary to deliberately infect the fruit with the target pathogen at a concentration of around 105 and to inoculate it for 16 to 24 hours before treatment. Following the treatments, the fruit was stored in cold storage for between 9 to 21 days and then stored in room temperature for shelf-life evaluation.
During these months, we ran a series of trials from small scale/lab test (between 350 to 500 fruits per trial) to semi-commercial application (more than 1000 fruits per trial). The semi-commercial pilots were run in Ashkelon, Israel on the packing line of Mehadrin in Israel, a well-know and recognized citrus packer.
The results of the trials have shown that PeroStar significantly reduced the need for additional post-harvest applications with imazalil by at least 50%, and in some cases entirely while improving the fruit shelf life, reducing waste). In addition, the use of PeroStar allows the packing house to meet the new limitations of imazalil utilization as well as meet its goal to apply greener and safer products (see graph below).
In addition, over the 2018 to 2019 and 2019 to 2020 citrus fruit seasons in the State of California, the safety of our products was demonstrated on more than 100,000 tons of fresh produce in the aggregate, which, according to the biannual report “Citrus: World Markets and Trade” published in July 2020 by the United States Department of Agriculture, represent more than 12% of the total production in the United States of mandarins/tangerines. The United States is ranked as the number six producer worldwide with around 800,000 tons, while Israel is ranked number ten with around 200,000 tons.
| 11 |
Results on Mangos
We have recently tested our PeroStar on mangos in collaboration with the Israeli-based Volcani Center for Agricultural Research. The goal of the test was to evaluate the effectiveness of PeroStar in preventing decay in harvested mangos in comparison with fludioxonil. Fludioxonil is a fungicide that is commercially available in Israel at a level of 250 to 300 parts per million. Fludioxonil is deemed to be an effective fungicide against fungi that attack the mango post-harvest, yet there is a growing need for “greener” solutions, given Fludioxonil’s level of toxicity.
Mangos was stored for three weeks after treatment at 12°C and an additional week of shelf life at 20°C in what would typically simulate a mango crate shipment to Europe and retailers in similarly distanced markets.
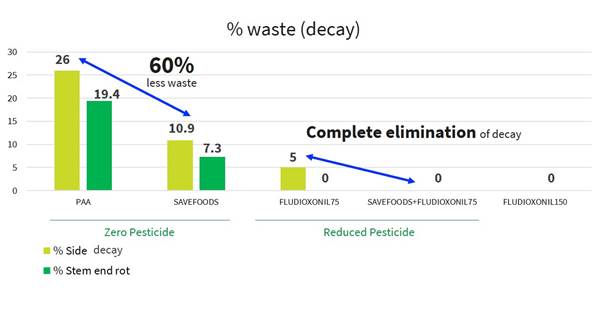
Results after evaluation have shown us that the treatment with PeroStar, improved the biocide activity of the PAA, which resulted in a reduction in both side decay and stem-end rot (common pathogens in mango) leading to an extended shelf life with no use of fungicide (as demonstrated in the picture below). In addition, the results also showed that the combination of PeroStar with a low concentration of fludioxonil reduced the post-harvest decay to zero. The results (as presented in the graph below) demonstrate that applying PeroStar enables mango producers to achieve an improved shelf life of produce compared to the current treatment while reducing the use of conventional chemical pesticides.
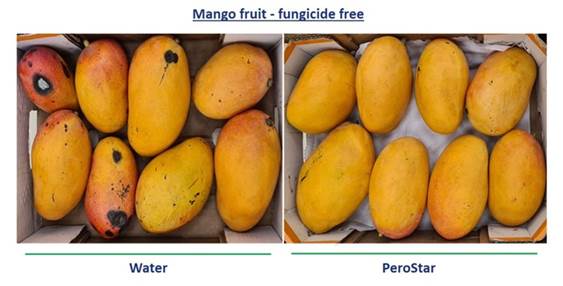
| 12 |
Commercialization Stage
Following a successful pilot in Mexico on Persian lime (where SavePROTECT has reduced to zero the fruit decay after 21 days as shown in the graph below), the packing house has bought a first batch to start the utilization of our product.
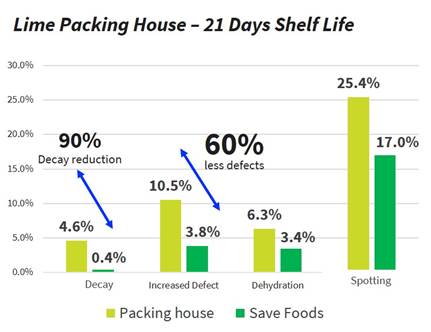
| 13 |
Based on these results, food retailers may benefit from additional income of up to $126 per ton of limes assuming a conservative price of $3,000 per ton lime (based on an average price per pound lime of $1.49 in 2019), as presented in the graph below.
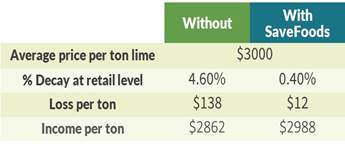
The European Union is a significant target market for our organic food acid blends because of strict regulations that are being imposed on the use of pesticides and GMO-produced crops, as well as health conscious consumers who represent a growing demand for organic fruits and vegetables. In August 2020, we submitted a regulatory dossier for our PeroStar as a processing aid to be used with PAA in Spain and Italy, two of the largest fruit and vegetables producers in Europe. See “Government Regulation and Product Approval” below.
In Israel, with the start of the citrus season, Safe-Pack (Decco Israel) is using our product under a white label name in one of the largest packing house in Israel.
| 14 |
Sanitizer – SF3HS or SF3H
Post-harvest sanitizers are considered a pesticide and regulated by the EPA in the United States. The EPA will review toxicity data and results from tests to show how well the product kills bacteria to determine if the product should be approved. See “Government Regulation and Product Approval” below.
This sub-category of products is based on our proprietary blend of food acids combined with hydrogen peroxide as the oxizider and includes SF3HS and SF3H. We believe that this category of products will be an improved sanitizer as compared to traditional sanitizers. SF3HS and SF3H are public health antimicrobial pesticide products that bear a claim to control by at least a 3 log10 reduction (99.9%) pest microorganisms that pose a threat to human health (foodborne pathogens), and whose presence cannot readily be observed by the consumer.
After we have finalized our toxicological studies, we conducted a series of microbial trials in laboratories in both the United States and Israel in non-Good Laboratory Practice (“non-GLP”) settings in order to evaluate the efficacy of SF3H as an antimicrobial agent to reduce foodborne pathogenic bacteria in “processing water” for fruit and vegetables. We used a modification of the Association of Official Agricultural Chemists Germicidal and Detergent Sanitizing Action of Disinfectants method and test protocol EN1276 (European standard for the evaluation of chemical disinfectant or antiseptic for bactericidal activity). The tested organisms are Listeria monocytogenes, Salmonella enterica and Escherichia coli O157:H7.
The last test was performed by Analytical Lab Group on a mix culture of Listeria monocytogenes with an exposure time of 30 seconds. The results showed more than 99.99999% (>7.51 Log10) reduction. In Israel, the tests were performed by the Institute for Food Microbiology and Consumer Good Health on a single strain for each pathogen (Listeria monocytogenes, Salmonella typhimurium and E. coli) with exposure time of 30 seconds and the results have shown between 99.99% to 99.9999% reduction. Exposure time is a key parameter in sanitization process, therefore allowing a short contact time is a significant advantage over the competition where the current minimum contact time available is 45 seconds.
We plan to conduct GLP efficacy studies during the first half of 2021 to complete our regulatory dossier for the EPA and the FDA to obtain the appropriate regulatory approvals for our products to be marketed and used as “sanitizers” to claim control of foodborne pathogens (food safety) as well as plant pathogens (food loss reduction). We plan to submit our regulatory dossier during the second half of 2021. It is planned that SF3HS/SF3H will be used in post-harvest to control both plant and foodborne pathogens for fruit and vegetables (including microgreens). For additional information regarding the regulatory approval process see “Government Regulation and Product Approval” below.
Results on Avocados
We have tested the efficacy of our SF3H and SF3HS products against Listeria on 40 avocados of which 10 avocados were treated with our SF3H and SF3HS products. The peel of the avocado was punctured and infected with high level of Listeria. The results have shown a 99.99% reduction within fifteen seconds of exposure time. In addition, we have also tested the efficacy of SF3H on avocado’s shelf life compared to current treatments (12 avocados per treatment). The results (demonstrated below) show that after 18 days in room temperature the treated avocados display material reduction in microbial spoilage as compared to avocados treated with water and chlorine, a well-known sanitizer.

| 15 |
Results on Microgreens
An increasing number of studies point to the growing demand for locally sourced, organic vegetables. Various types of “young vegetables,” such as sprouts, microgreens and baby greens, are becoming increasingly popular due to their high nutritional value. Microgreens are deemed premium products and command higher retail value. They also belong to a group of “functional foods” and have high levels of bioactive compounds, while requiring less water and energy to grow, which they do year-round. Currently, microgreens are largely being cultivated in major greenhouses across the United States. According to Agrilyst, an agro-intelligence platform, greenhouse cultivation of microgreens was the highest in South and North East regions, each accounting for 71% and 59% in 2017. While consumers in the United States are more focused on growing leafy greens and microgreens than any other vegetables.
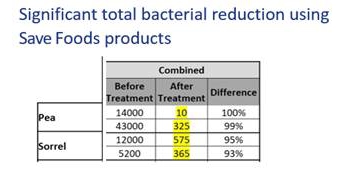
We have tested the efficacy of our SF3H products to control and prevent potential pathogen contamination on microherbs (pea and sorrel) produced by Israeli-based microgreens exporter 2BFresh. Our treatment combined a post-harvest spray application and a fogging treatment to be used in the cooldown storage room. In order to determine the efficacy of the product, 25 swabs were taken across 18 trays (nine of each microgreen species). The results (as presented herein) show more than a 90% reduction of the total bacterial load post-treatment (see table).
We believe that our SF3HS and SF3H provide improved sanitization of bacteria (including E. coli, Salmonella and Listeria) while leaving no toxic residues on fruits and vegetables.
We expect the first commercialization of SF3H and SF3HS at the earliest by the end of the first half of 2022.
Other Products
Our product portfolio also includes the SpuDefender and FreshProtect.
SpuDefender
SpuDefender is one of our EPA-registered products which targets and is designed to control the post-harvest potato sprout. Due to the European Commission’s decision on January 1, 2020 to no longer allow the use of the herbicide chlorpropham (the “CIPC”), the post-harvest potato industry is looking for new solutions. For over 50 years, CIPC was widely used as a sprout suppressing agrochemical agent applied to potatoes that were stored in processing facilities.
| 16 |
Following recent discussions with post-harvest experts and potential customers, we believe our SpuDefender product may offer a successful alternative to CIPC. During 2021, we plan to initiate pilot tests with potential customers to treat potatoes pre-storage (three to six months storage in average).
FreshProtect
FreshProtect is our second EPA registered product, which targets and is designed to control spoilage-creating microorganisms on post-harvest citrus fruit. The registered label of the product only allows us to market and sell our FreshProtect in the United States (excluding California). However, we believe our FreshProtect has a significant potential in reducing the bacterial load entering the packing house in the pre-harvest market. The non-toxicity of our FreshProtect allows its application up to the day of harvest (0-day pre-harvest interval), which is critical to prolong crop protection and reduce microbial spoilage.
We recently ran a proof of concept study under a controlled group environment of different plant fungi responsible for decay which showed promising initial results.
We also conducted a series of smaller studies, consisting of laboratory tests and tests on a limited number of lemon trees whereby we demonstrated significant reduction of decay in treated fruit and reduction in bacterial populations.
The main conclusions of the trials were that FreshProtect with concentration of 1% and 2% applied at 400 gallons per acre materially reduced sour-rot on inoculated fruit. While both rates were also effective against fruit inoculated with P. digitatum, (i.e. fungus found in the soil of citrus-producing areas and major source of post-harvest decay), the 2% concentration of FreshProtect demonstrated significantly more efficacy at reducing sour-rot. Natural incidence of Penicillium spp. (a family of fungi) was also reduced on fruit inoculated with G. candidum, fungus that is a member of the human microbiome.
Furthermore, FreshProtect can be used in combination with several different kinds of pesticides and fertilizers which allows the application of more than one pesticide at once. This in turn reduces cost and facilitates implementation. The graph below summarize these results:
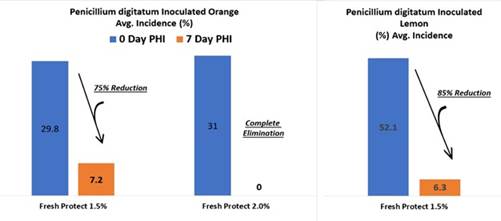
The regulation for pre-harvest (in the field) application especially in California as well as in Israel may take more time than post-harvest application due the potential impact on the environment. Therefore, we expect the product to reach the market during 2022.
| 17 |
Our Strengths
We believe that our main strengths include:
| ● | Strong Management Team with Commitment to Green Products. Led by a team with over 30 years of experience in developing sanitization products and solutions for the agriculture industry, we plan on becoming a significant player in providing consumers with healthy and green fresh produce from farm to fork while endeavoring to ensure food safety and reducing food waste. We believe that our proprietary blend of food acids provides protection to the treated produce and works in synergy with well-known fungicides and sanitizers. This synergy allows us to significantly reduce the concentration of the fungicides that are heavily regulated in several countries and, in certain countries, outright banned and meet the food trends of sustainable and green produce. | |
| ● | Multi-Purpose Products that Simplify Crop Treatment Routine and Save Money. While most chemicals marketed in the industry address either food safety or food waste, our multi-purpose products are intended to provide a solution for both problems, while simplifying crop treatment and achieving cost saving. Our products are capable of cleaning and controlling pathogens that would otherwise render fresh produce as unsafe for human consumption. Our proprietary blend of food acids combined with well-known sanitizers are very efficient against foodborne pathogens like E. coli, Salmonella and Listeria as well as plant pathogens in short contact time (99.999% reduction within 30 seconds of contact). In addition, with multipurpose products, there is no need to order, ship or dispose of bottles of product, resulting in less energy consumed, less CO2, less fuel, and less waste. We believe our focus on natural product chemistries will allow us to continually drive lower costs, higher product gross margins and efficacy through longer shelf life and reduction of food waste. | |
| ● | Strong Intellectual Property Portfolio. We believe that we have built a strong intellectual property position throughout the food chain (from field to fork) as our patents claim compositions and methods that can be used to protect food and agricultural products from decay. We rely on a combination of important intellectual property assets, to protect our innovation. Our employees, consultants, customers, and vendors are subject to confidentiality agreements that protect our proprietary manufacturing processes. Our patent portfolio includes granted patents in the United States, Europe, and Israel, as well as several priority applications, across several patent families, including composition-of-matter claims, methods of use claims, including for treating edible matter, for improving the appearance of edible plant matter, and sterilization methods, as well as for articles for implementing these methods. These patents directly protect a proprietary method for extending life shelf and reducing edible matter from microbial decay. | |
| ● | Commercially Available Products and Seamless Implementation. One of the oxidizers being used with our products is PAA, a well-known and widely used sanitizer. Following the enforcement of the FSMA in connection with the use of sanitizers, more and more packers have been choosing this healthy and eco-friendly sanitizer over chlorine, and this choice facilitates implementation of our products. In addition, the application of our products does not require special equipment as they are used in combination with or replace existing products applied on the packing line or in the mix tank in the field. This allows a relatively cheap, seamless and fast implementation. | |
| ● | Significant Reduction of Hazardous Chemicals Food Residue. All the ingredients of our blend of food acids are recognized by the FDA as GRAS when used as intended in fruit and vegetable wash applications, while oxidizers we use such as hydrogen peroxide rapidly decompose into water and oxygen.. The absence of toxicological residues not only improves food quality but also promotes occupational safety for the employees of packing houses, contributing to a friendlier and safer working environment. |
| 18 |
Our Strategy
In September 2018, the Company changed its organizational structure and management team. After reviewing the Company’s then existing strategy and results of operation, as well as examining the market opportunities, the new management team decided to update the Company’s strategy, reduce the marketing and sales of its existing products, and focus the Company’s efforts and financial resources in developing its next generation products. During the years 2019 and 2020, we developed, validated and tested the efficacy of our next generation product – a blend of food acids – on a variety of crops in small and large scale commercial pilots.
Our strategy is to develop and commercialize our products through strategic partnerships with global post-harvest service companies and with large food distributors and retailers with the intent of: (i) extending the shelf life of fresh produce while reducing (and even eliminating) the use of harmful chemicals (fungicides); (ii) ensuring food safety and shelf life by controlling foodborne pathogens and allow our customers to meet FSMA regulatory requirements; (iii) reducing food loss and the associated carbon “footprint.” Our ultimate goal is to eventually gain presence in a variety of businesses compromising the food industry, including pre-harvest, post-harvest, retail and consumer businesses.
In order to achieve our goals, we intend to:
| ● | Advance our Breakthrough Technologies and Commercialization Efforts. During the first half of 2021, we plan to run a series of additional pilot studies in various commercial collaborations with post-harvest service vendors packing houses and food retailers. | |
| ● | Develop a Strong Marketing Message Around Promoting Safe Food While Avoiding Food Waste. We plan to brand our fresh produce with a “chemical residues free” seal of approval and we believe that like-minded fruit packers around the globe will seek to differentiate themselves from their competitors by obtaining this seal. | |
| ● | Acquire or License Complementary Products and Technologies. We actively search for products and technologies that can enhance our portfolio and grow our business to address all the post-harvest treatments such as fruit coating products or technologies. | |
| ● | Expand to Additional Produce and Geographies. Our plan is to focus first on key countries and regions with the largest markets for our crops, including Mexico, Spain, Italy, Israel and key markets in the United States such as California, Florida and Texas. We are also plan to increase the variety of crops that can be treated with our products, to include produce such as apples, bell peppers, tomatoes and papayas. | |
| ● | Leverage Our Products Through Collaborations. Our focus and expertise in the development of green products for the agritech industry and in post-harvest treatments allow us to be a partner of choice for other businesses looking for development partners and for larger companies wanting to leverage their product such as PAA into new combination products. For example, companies selling or owning fungicides, the MRL of which is being reduced, and that are working in synergy with our products are good partners. This type of collaboration could allow them to continue selling their product. | |
| Our selling and marketing strategy is twofold: | ||
| ● | Establish Collaborations with Food Retailers. Large food retailers play an important role in influencing the decisions of key suppliers down the food chain (i.e. they can dictate to their suppliers which cleaning solutions they will use when treating the fresh produce at their packing house). Food retailers must ensure food safety as well as reducing food loss occurring during distribution, storage and retail. In the United States alone, 4.65 million tons of fresh produce were thrown away at the retail level. This waste cost $8.9 billion and amounted to 12% of the U.S. fresh fruit supply and 10% of the fresh vegetable supply. With an average percentage of 5% of all strawberries, apples, avocadoes, tomatoes and broccoli in the United Kingdom and 12% and 10% of the fresh fruit supply and fresh vegetable supply in the United States, respectively, wasted at the retail and/or distribution level, we believe even a small reduction in fresh produce loss can generate large savings. | |
| 19 |
| ● | Partner with Service Vendors to Fruit and Vegetable Packing Houses. Post-harvest service companies provide packing houses with the necessary machinery and products, such as sanitizers and fungicides to enable the packing houses to treat the produce. Post-harvest service companies face competition due to the current regulations that dictate specific types of treatment products and set tolerable residue levels. In addition, these companies must provide their packing houses with state of the art, cost effective and green product to incentivize the packing houses to build long-term business relationship with them. Moreover, service vendors are important because they have strong influence on the decision of which cleaners, sanitizers, fungicides and waxes that the fruit and vegetable packing houses will use. Additional benefit of partnering with post-harvest service company is their strong global market presence in the relevant geography. In addition, both food retailers and post-harvest service companies have a significant interest in green and efficient post-harvest solutions. |
Selling and Marketing
We concentrate our marketing efforts on high value crops, such as avocado, mango, citrus, apples, pears, bananas, papaya, bell pepper, lettuce and tomatoes and target large producers of these crops as well as large food distributors and retailers. We are currently exploring collaboration opportunities for our SavePROTECT or PeroStar with producers in the United States, Spain, Israel, Italy and Mexico. And we intend during the next 12 months to initiate commercial collaboration with local packers and retailers in our first targeted location previously mentioned.
The table below summarizes the market opportunities for selected produce in our target markets.
| Bananas | Apples and Pears | Avocados | Citrus | Mangos | Papayas | Tomatos | Lettuce and Chicory | Chilli and Green Peppers | Total (in milion tons) | |||||||||||||||||||||||||||||||
| Global Production of the Crop (in million ton)1 | 114.2 | 108.2 | 6.0 | 147.9 | 52.3 | 13.1 | 180.5 | 27.5 | 35.8 | 685.5 | ||||||||||||||||||||||||||||||
| Production of the Crop in the Company’s Target Markets 2 (in million ton) | 2.9 | 10.5 | 2.4 | 25.6 | 2.3 | 1.0 | 28.0 | 6.5 | 5.6 | 84.7 | ||||||||||||||||||||||||||||||
| Production of the Crop in the Company’s Target Markets (in %) | 2.5 | % | 9.7 | % | 39.8 | % | 17.3 | % | 4.3 | % | 7.6 | % | 15.5 | % | 23.6 | % | 15.7 | % | 12.4 | % | ||||||||||||||||||||
| 1. | Average global production for the years 2016, 2017 and 2018. | |
| 2. | Our target markets include Israel, Italy, Mexico, Spain and the United States. |
To support our efforts, we will increase our marketing and sales team as well as services in our target location to support all our efforts.
| 20 |
United States
The first market we target for the sale and distribution of SavePROTECT is the post-harvest citrus industry in the State of California, which alone accounts for approximately 80% of all fruits and vegetables in the United States.
Over the last three years, we have treated more than 180,000 tons of citrus fruit with the different version of our SavePROTECT product. Under the supervision of a world leading packing house to the citrus fruit industry, we evidenced SavePROTECT utility as having a good safety profile, ensuring food safety and in controlling microbial spoilage. We plan to leverage this collaboration in order to further penetrate the citrus based fruit packing industry, both in California and beyond.
The Post-harvest treatment market for fruits and vegetables, which is projected to grow from $1.5 billion in 2019 to $2.3 billion by 2026, growing at a CAGR of 6.5% during the forecast period, is led globally by select companies, including DECCO U.S. Post-Harvest, Inc., (United States), Pace International, (United States), Xeda International (France), John Bean Technologies (United States), and Agrofresh (United States).
Once we have completed our studies and secured the appropriate regulatory approvals, we plan to further penetrate this market. We currently focus on post-harvest treatment for the citrus industry (as well as mangos and avocados) but are conducting pilot studies with leading players in the industry to evaluate and validate our products formulations.
We plan on commencing one commercial pilot study with a global food retailer by the end of the first quarter of 2021.
Israel
On September 11, 2020, we signed a five-year exclusive distribution agreement (the “Distribution Agreement”) with Safe-Pack Products Ltd. (“Safe-Pack”), according to which we granted Safe-Pack an exclusive right to resell, distribute, advertise, and market ours products in the citrus industry in Israel and Palestine. In addition, we agreed to grant Safe-Pack a right of first refusal to be designated as an exclusive distributor of ours in certain agreed upon territory for additional products of ours in the post-harvest market. In consideration for the above rights granted to Safe-Pack, Safe-Pack will submit to us purchase orders of our products at a price specified in the Distribution Agreement. Commencing upon the second calendar year of the agreement, Safe-Pack is required to meet a minimum purchase quota, as shall be mutually agreed upon between the parties. In the event that the parties fail to agree on a quota, the quota shall be equal to last year quota plus 3%.
Spain
We have been collaborating with one of the world’s leading post-harvest treatment service vendors in Spain since June 2020, where we are examining our product on citrus fruit. We believe our product could be an improved alternative to current fungicides that will soon be significantly reduced in this market.
Mexico
We are planning a series of studies in which our SavePROTECT will be applied on tomatoes, bananas, lemons, bell peppers and avocados. The first study to ensure safety of avocados is planned for the first quarter of 2021.
| 21 |
Between August and October of 2020, we have conducted three successful trials in Mexico, on more than 200 kilograms of Persian limes. The results have shown that the addition of SavePROTECT to current treatments extend shelf life. Shelf life was tested for 25 days and results have shown that SavePROTECT substantially reduces decay. Mexico is the largest producer of Persian limes and is deemed to be that country’s second most important citrus fruit.
Intellectual Property
We rely on patents and trade secret protection laws to protect our proprietary products and intellectual property. We entered into confidentiality agreements with our employees, consultants, customers, service providers and vendors that cover, inter alia, our technology and proprietary manufacturing processes.
We currently own five issued patents, one allowed patent, and seven pending patent applications, four of which may be submitted worldwide. Expiration dates of our patents, and any patents which may be granted under our pending patent applications, are from 2031 through 2041. Our patent family includes patents granted in Israel, the United States and Europe.
Compositions and Methods of Treating Edible Matter and Substrates Therefor
This patent family includes granted patents in the United States, Israel, and an allowed application in Europe and is directed to a method for protecting edible matter from decay by applying to the edible matter a disinfecting composition containing, among other things, (1) phosphonic or phosphoric acid, (2) a carboxylic acid, (3) performic acid, (4) a performic acid source (such as formic acid) and an oxidizer (such as hydrogen peroxide).
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||||||||
| SVF-P-001-EP | Europe | Patent | Allowed | 11825901.9 | September 14, 2010 | |||||||||||
| SVF-P-001-IL | Israel | Patent | Issued | 225247 | September 14, 2010 | |||||||||||
| SVF-P-001-IL1 | Israel | Patent | Pending | 254909 | September 14, 2010 | |||||||||||
| SVF-P-001-US1 | United States | Patent | Issued | 10,212,956 | September 14, 2010 | |||||||||||
| SVF-P-001-US2 | United States | Patent | Pending | 16/278,108 | September 14, 2010 | |||||||||||
Methods for Improving the Appearance of Edible Plant Matter
This patent family includes a granted patent in Israel and is directed to a method of improving the appearance of edible plant matter either during the pre-harvest or post-harvest stage. The method includes applying a composition based on phosphonic acid to the edible plant matter.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||||||||||
| SVF-P-002-IL | Israel | Patent | Issued | 229724 | May 30, 2011 | |||||||||||||
Method and Apparatus for Maintaining Fresh Produce in a Transportation Container
This patent family includes granted patents in Israel and in the United States. The patent family is related to a method of using thereof for maintaining fresh produce stored in a transportation container. The apparatus is configured to generate an aerosol of one or more liquid pesticides, thereby reducing pathogenic contamination within the transportation container. This patent family covers any liquid pesticide for use in the above-mentioned apparatus.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||||||||
| SVF-P-003-IL | Israel | Patent | Issued | 227328 | June 23, 2013 | |||||||||||
| SVF-P-003-US | United States | Patent | Issued | 9,487,350 | June 23, 2013 | |||||||||||
| 22 |
Sterilization Compositions and Methods for Use Thereof
This patent family is directed to compositions and methods for reducing pathogen load within a container or on a surface, including inter alia the surface of an edible plant matter. Furthermore, the application is directed to compositions and methods for disinfection of cooling systems.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-004-USP | United States | Patent | Pending | 63/111,197 | November 9, 2020 |
Sterilization Devices and Methods for Use Thereof
This patent family is directed to a device for controlling pathogen load within a container or on a surface by spraying a disinfecting composition in response to a trigger, such as increased pathogenic contamination.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-005-USP | United States | Patent | Pending | 63/111,220 | November 9, 2020 |
Compositions Comprising of Several Organic Acids and Use Thereof
This patent family is directed to kits and methods for controlling pathogen load within or on the surface of an edible plant matter.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-006-PCT | International application | Patent | Pending | PCT/IL2021/050229 | March 1, 2020 |
Combined Fungicidal Preparations and Methods for Use Thereof
This patent family is directed to compositions and to methods for reducing pathogen load on a substrate.
| File Number | Country | Type | Status | Application/Patent Number | Priority Date | |||||
| SVF-P-007-USP | United States | Patent | Pending | 63/042,622 | June 23, 2020 | |||||
| SVF-P-007-USP1-07931-P0004B | United States | Patent | Pending | 63/126,649 | December 17, 2020 |
We cannot be sure that any patent will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future. There is also a significant risk that any issued patents will have substantially narrower claims than those that are currently sought.
Competition
Given that the market for the use of green and “residue free” solutions is evolving, we are continually facing growing competition. The market for post-harvest solutions is fragmented and includes various regional suppliers. The market of post-harvest treatments for fruits and vegetables is dominated by five large players with wide reach across the globe. We believe that a market edge will be given to a company that can solidify its reputation, product quality, customer service and customer intimacy, product innovation, technical service and value creation. Based on these variables, we believe that we compete favorably when compared with the global competition in this market.
Currently, our main competitors are companies providing PAA, chlorine and other sanitization solutions, such as Ozone as well as technology companies developing new biorational fungicides.
| 23 |
We also compete with heavily diversified multi-national chemical conglomerates, who produce various biocide formulations designed to kill or deactivate pathogenic micro-organisms. Of these, two companies are the most significant:
| ● | Peroxychem: Peroxychem is a subsidiary of Evonik Industries AG (Germany). It is a significant worldwide producer of hydrogen peroxide, persulfates and PAA. Peroxychem expected revenue of approximately $300 million in 2018; and | |
| ● | Solvay (Belgium): Similar to Evonik Industries, Solvay is a heavily diversified multinational chemical conglomerate. In fiscal year 2019, the company had approximately €10.2 billion in net sales, spread across the breadth of their product lines. Most relevant to us is their blends of PAA and hydrogen peroxide, sold in two primary formulations – OXYSTRONG for water treatment and PROXITANE for the food industry. |
In addition, we have several indirect competitors, which are companies with whom we seek to make strategic partnerships – large companies specializing in post-harvest solutions for the agricultural industry. Such companies include:
| ● | Decco US Post-Harvest: Decco is a subsidiary of Decco Worldwide, which itself is a division of United Phosphorous Ltd. Decco provides a variety of solutions, both mechanical and chemical, for the post-harvest industry. They produce conventional fungicides (imazalil, thiabendizole, etc.), as well as produce coatings; and | |
| ● | Pace International: Pace International is a subsidiary of the Sumitomo Chemical Company. Similar to Decco, it provides a variety of solutions – primarily in the realm of conventional fungicides and carnauba wax coatings for fruit. |
We also consider Xeda International, JBT and Agrofresh as our indirect competitors (and current or potential collaborators).
The organic market offers a huge trade and income potential for producers, processors and trading companies globally. The rising demand of various organic products has driven the demand of organic post-harvest treatments. Green and organic technologies are increasingly being developed in a global market and several conventional post-harvest product and equipment suppliers, such as Citrosol, Fomesa and Peroxychem, have taken the opportunity and are starting to develop natural products.
Research and Development
In the last two years we spent an aggregate of $1,032,623 on research and development. We focus on developing innovative solutions consisting of new generation, patented products that address immediate and long-term needs. Our research efforts are aimed at optimizing the application protocols of our existing core products such as PeroStar/SavePROTECT for new crops, developing new blend of acids and enhancing our SF3H/SF3HS products’ antimicrobial efficacy while taking into account costs, consumers trends and preferences, which will give the extra value needed to separate our products from those of our competitors in the marketplace.
| 24 |
Post-Harvest
We are currently working on the compatibility and synergistic effect of our processing aids products SavePROTECT/PeroStar with additional post-harvest treatments used in the packing house to provide an efficient, greener and cost-effective solution.
We are also focused on the characterization of our new sanitizers including the identification, improvement and other validations of our formulas. These products are based on a unique stabilization process that blends hydrogen peroxide and food acids to create a broad-spectrum, safe and eco-friendly solution for killing germs. The synergistic effects of combining hydrogen peroxide with food acids produce a stable yet environmentally safe and easy to handle sanitizer. We use a network of experts in related fields, such as microbiology, and food chemistry to obtain all the required regulatory approvals.
In addition, we are in the initial stages of development of natural antimicrobial edible coatings for microbial safety and food quality enhancement comprising our acid blend.
To accurately test the strength of a sanitizing solution, we are working on developing quantitative methods. Similarly, we are developing analytical methods that will enable rapid and effective monitoring of the active ingredients through a novel and improved testing kit that allows for testing at a faster pace and with greater certainty.
Pre-Harvest – FreshProtect
We are also focused on developing new eco-friendly pre-harvest products which will improve post-harvest management practices by reduction of total microbial load, before even entering the packing house. An effective pre-harvest treatment may reduce the need for post-harvest chemical fungicides while increasing profit through reduced spoilage in supply chains. In addition, the potential of addressing pre-harvest treatment might offer new opportunities for treating crops, such as rice and wheat, with large market potential.
In pre-harvest application, one of the main advantages of our products is the non-toxicity of its ingredients, allowing its application up to the day of harvest (0-day pre-harvest interval), which is critical to prolonged crop protection and reduced microbial spoilage while reducing the total bacterial load entering the packing house. Field studies are conducted by Dr. James E. Adaskeveg from University of California, Riverside and the largest grower cooperative in CA in the United States on citrus trees to determine the effectiveness, optimize use protocol and effect on the environment.
Production
In Israel, we work with SasaTech, a reputable chemical production company. Based on our formulation and guidance, SasaTech is producing our PeroStar and any other small-scale formulation that we might need for research and development purposes and trials. SasaTech is particularly regarded for its deep understanding and experience working with oxidizer like hydrogen peroxide and PAA. We also work with Zohar Dalia which we engage on a case-by-case basis.
In the United States we work with Seeler Industries, a national leader in marketing, handling, and in the termination of hydrogen peroxide. Both Seeler and SasaTech, purchase all raw materials necessary for the production of our products.
All ingredients and/or raw materials that are used in the creation of our products are commodities and are readily available for purchase off the shelf.
| 25 |
Government Regulation and Product Approval
Our products are subject to national, state and local government regulations. Based on the product claims and classification, different regulatory and registration requirements may apply at the state, provincial or federal level.
Regulation of our Sanitizers – SF3H and SF3HS
In the United States, the primary federal laws that regulate the sale and distribution of our sanitizer products are the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”) and the Federal Food, Drug and Cosmetic Act (“FFDCA”).
FIFRA is the federal law that regulates the sale and distribution of pesticides and is administered by the EPA. Products that claim or are otherwise intended to control microorganisms on inanimate surfaces, in water and on raw agricultural commodities are regulated, under FIFRA, as pesticides. FIFRA generally requires the pre-market registration of pesticide products. To register a pesticide product, we are required to provide test data and related information to demonstrate that the product is safe and effective under the conditions of use, as specified on the product label. The cost and timeframe to achieve EPA product registration depends on the type of product and the claims made for the product. Registered products are also subject to a number of recordkeeping and reporting obligations which require constant product oversight by companies.
Pursuant to FIFRA and Section 408 of the FFDCA, EPA establishes tolerances for pesticide chemical residues that could remain in or on food, including raw agricultural commodities. A tolerance is the EPA established maximum residue level of a specific pesticide chemical that is permitted in or on a human or animal food in the United States. Generally, any pesticide chemical residue must have either a tolerance or an exemption from the requirement to have a tolerance in order to be permitted in or on human or animal food. FDA enforces the tolerances pursuant to its authority under the FFDCA.
The FFDCA regulates the sale and distribution of drugs, medical devices, cosmetics and foods (including substances added to and found in food such as pesticide residues) and is administered by the FDA. Under the FFDCA, the FDA does not register or approve products that are used on food commodities and certain food-contact surfaces, such as food packaging. However, all substances that are used on food or food-contact surfaces need to be subject to an FDA regulation or permitted through other clearance mechanisms, such as a Food-Contact Notification (FCN), Threshold of Regulation (TOR) opinion, by Prior Sanction or be “Generally Recognized as Safe” or “GRAS”. If all the substances or ingredients in a particular product are cleared for use on food or food-contact surfaces or are GRAS then a company can market a product without obtaining any additional clearances. GRAS substances do not require pre-market approval or clearance by FDA although FDA does have a notification process for GRAS substances.
At the federal level, antimicrobial agents are subject to regulation by FDA and/or EPA, either singly or jointly, depending upon the intended use of the product. Antimicrobial products applied to processed food are solely regulated by the FDA per longstanding FDA and EPA policy outlined in an EPA Notice titled “Legal and Policy Interpretation of the Jurisdiction Under the Federal Food, Drug, and Cosmetic Act of the Food and Drug Administration and the Environmental Protection Agency Over the Use of Certain Antimicrobial Substances” (63 Fed. Reg. 54,532 at 54,536 & 54,541 (Oct. 9, 1998)) and EPA’s Pesticide Registration Manual, Chapter 18. Antimicrobial products applied to raw agricultural commodities (e.g. fruits and vegetables) are jointly regulated by EPA and FDA if their application takes place in a food-processing facility. If the antimicrobial product is applied to a raw agricultural commodity in a treatment facility that solely washes and packs food commodities, and the treatment does not change the status of the food as a raw agricultural commodity, then EPA has sole federal regulatory jurisdiction.
| 26 |
Since our sanitizers will be and are intended to be used solely to treat raw agricultural commodities in post-harvest washing and packing facilities, at the federal level they are regulated solely by EPA (as opposed to FDA): product registration is required under FIFRA and any food residues are regulated under the FFDCA. To complete the registration process, we will be required to submit a number of studies in the form of a registration application or dossier, which has not yet been submitted to EPA. These studies will specifically include: (i) safety studies - six acute toxicity studies (already finalized), (ii) physio-chemical properties testing (already finalized), (iii) one-year storage stability and corrosion (ongoing), and (iv) an efficacy study to demonstrate that the product is an effective sanitizer (studies conducted under non-good laboratory practices already performed and they show the product meets EPA performance standards). We have already identified and engaged with a third-party company in the United States to perform our good laboratory practices efficacy studies.
In addition, every state has its own laws that regulate pesticides and these laws require registration of pesticide products at the state level. Accordingly, products must also be registered in the states in which they are distributed prior to any sale.
Regulation of Our Processing Aid – SavePROTECT or PeroStar
In the United States, our SavePROTECT product does not make any pesticidal claims and is not intended for use as a pesticide and, therefore, is not subject to the registration requirements of FIFRA. However, since the product is used on raw agricultural commodities in food processing facilities, the product is subject to regulation under the FFDCA. We believe that the product is in compliance with the FFDCA since every ingredient in the product can be considered GRAS when used as intended.
Although SavePROTECT is not a pesticide under FIFRA, it is still required to be registered in California because the California statute requires the registration of both pesticide and adjuvant products. Based on the intended use and claims for SavePROTECT, we believe that product falls within California’s definition of a spray adjuvant.
On July 31, 2020 we submitted an “Application for Registration of Adjuvant” for SavePROTECT to the California Department of Pesticide Regulation (CDPR). The dossier submitted included the following studies: (i) acute oral toxicity and acute dermal toxicity studies, (ii) physico-chemical property testing (determination of color, physical state, odor, density, pH, viscosity and oxidation/reduction chemical incompatibility), (iii) validation of the high-performance liquid chromatography method assay, (iv) stability test, and (iv) efficacy data. We anticipate registration of SavePROTECT during the second half of 2021.
In addition, based on the opinion of our U.S. regulatory experts, all SavePROTECT ingredients are GRAS when used as intended.
During the second half of 2021 we plan to submit an application for certification for our SavePROTECT to the Organic Materials Review Institute (the “OMRI”), an international nonprofit organization that determines which input products are allowed for use in organic production and processing. OMRI Listed® products are allowed for use in certified organic operations under the United States Department of Agriculture (the “USDA”) National Organic Program. OMRI reviews input products to verify that they meet the organic standards for use on organic farms or in organic processing. OMRI is recognized by the USDA National Organic Program as a reputable third-party input reviewer in Interim Instruction 3012 of the NOP Handbook. In addition, OMRI is accredited under the International Organization for Standardization (ISO) 17065 by the USDA Quality Assessment Division.
| 27 |
In Europe, processing aids are defined as substances that are added to exert a technological function during food processing and which may end up in the finished product. According to Regulation (EC) No. 1333/2008, processing aids means any substance which (i) is not consumed as food by itself; (ii) is intentionally used in the processing of raw materials, foods or their ingredients, to fulfil a certain technological purpose during treatment or processing; and (iii) may result in the unintentional but technically unavoidable presence in the final product as residues of the substance or its derivatives, provided they do not present any health risk and do not have any technological effect on the final product.
Processing aids are differentiated from food additives, which are substances that are added to food with the intention to exert a technological function within the final food product. Therefore, processing aids must not follow the European Food Safety Authority (the “EFSA”) guideline of “Data Requirements for the Evaluation of Food Additive Applications.”
In Europe, our PeroStar is not considered a processing aids in the enzymatic preparation category and, therefore, PeroStar is only regulated at the national level. While there are no harmonised requirements regarding the registration of a processing aids, some data (such as full composition and some toxicological data) must be disclosed and discussed with the competent authorities before the submission of a registration request.
In Spain, the guidelines for precise documentation for evaluation of technological adjuvants intended to be used in human food, state specific conditions for the assessment, authorisation and use of all other types of processing aids, which are not processing aids in the enzymatic preparation category. We have submitted during the third quarter of 2020 a regulatory dossier as a processing aid for PeroStar in Spain and Italy with very similar information as the regulatory dossier submitted in California.
In Mexico, based on the product composition and the legal status of the substances to be used as food additives, our PeroStar/SavePROTECT can be marketed and used in Mexico as a food additive (processing aid) and no registration is required (pending the final confirmation from Mexican government).
In Israel, the guidelines of the National Food Services, Ministry of Health, define the requirements for cleaning and disinfectant agents used with food. These guidelines state that such cleaning and disinfectant agents applied to the cleaning equipment which comes into direct contact with food, must not contain carcinogens. Specifically, List A and List B published by the Inter-ministerial Committee on Carcinogens, Mutagens and Teratogens of the Ministry of Health identify products and ingredients with carcinogenic, mutagenic and teratogenic properties. Our regulatory consultant in Israel has confirmed that our PeroStar does not contain carcinogens, mutagens and/or teratogens, and, therefore, is considered approved in terms of the relevant regulations of the National Food Services, Ministry of Health, and can be used as an additive to cleaning and disinfectant agents for fresh produce.
Registration of Our SpuDefender and FrehProtect
We currently have registrations for our SpuDefender (EPA Reg. No. 86381-1) and our FreshProtect (EPA Reg. No. 86381-2), at both the federal level and in the individual states where the products are sold for the use in post-harvest settings. To allow the utilization of our FreshProtect in pre-harvest settings, additional studies will need to be submitted to the EPA.
Organizational Structure
We currently have one 98.94% owned subsidiary: Save Foods Ltd., which is incorporated in the State of Israel. Save Foods Ltd. is responsible for all of our research and development and sales activities.
| 28 |
Property and Facilities
Our research and development and manufacturing operations are currently conducted at Kibbutz Alonim (Israel) where we lease approximately 70 square feet of space to run our lab studies. The lease expires on December 31, 2021. Our current monthly rent payment is NIS 4,846 (approximately $1,400) which includes taxes.
We believe that our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business.
Employees
As of March 29, 2021 , we employ two full-time employees and four part-time employees. None of our employees are members of a union or subject to the terms of a collective bargaining agreement.
Legal Proceedings
We are not currently subject to any material legal proceedings.
Company History
We were incorporated under the name Pimi Agro Cleantech, Inc. on April 1, 2009, under the laws of the state of Delaware. On April 11, 2016, we changed our name from Pimi Agro Cleantech, Inc. to Save Foods, Inc. Our subsidiary was incorporated on January 14, 2004 under the name Pimi Marion Holdings Ltd., to exploit the knowledge, intellectual property and business assets of Nir Ecology Ltd., a company founded in September 1989, focused on developing sanitizing solutions for the water and food industry. During the initial years of its activity and until 2009, Pimi Marion Holdings Ltd. focused on the development of new products and applications within the potato-growing industry. On October 5, 2008, Pimi Marion Holdings Ltd. changed its name to Pimi Agro Cleantech Ltd. In September 2018, we changed our organizational structure and leadership team to support our new strategy and objectives. The goal of the organizational change was to drive the Company towards regulatory approvals for our new generation of products. Our revamped strategy was developed following research we conducted on the applicable and potential commercial markets for our products. The results of this research demonstrated a clear and significant market for our new products to be deployed as sanitizers for the agricultural and food tech industries. On May 2, 2019, Pimi Agro Cleantech Ltd. changed its name to Save Foods Ltd.
| 29 |
You should carefully consider the risks described below, as well as the financial or other information in this Annual Report, including our consolidated financial statements and the related notes, before deciding whether to invest in our securities. The risks and uncertainties described below are not the only risks we face. We may face additional risks and uncertainties not currently known to us or that we currently deem to be immaterial. Any of the risks described below, and any such additional risks, could materially adversely affect our business, financial condition or results of operations. In such case, you may lose all or part of your original investment.
Summary Risk Factors
Our business is subject to a number of risks, including risks that may prevent us from achieving our business objectives or may adversely affect our business, financial condition, results of operations, cash flows and prospects that you should consider before making a decision to invest in our Common Stock. These risks are discussed more fully in the section titled “Risk Factors” beginning on page 12 of this Annual Report, and include the following:
● |
we have a history of operating losses and expect to incur additional losses in the future; | |
● |
we have not generated significant revenue from the sale of our products and do not believe that our current cash on hand will be sufficient to fund our growth plans or our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern; | |
● |
even if we complete the Current Offering, we expect that we will need to raise significant additional capital, which we may be unable to obtain; | |
● |
because of our limited operating history, we may not be able to successfully operate our business or execute our business plan; | |
| ● | our products and technology require additional trials; | |
● |
the commercial success of our new generation products, as well as any future products, depends upon the degree of market acceptance by the packing house community as well as by other prospect markets and industries; | |
| ● | the COVID-19 pandemic, or any other pandemic, epidemic or outbreak of an infectious disease, may materially and adversely affect our business and operations; | |
| ● | we may face significant competition from other companies looking to develop or acquire new alternative environmentally friendly solutions for the treatment of fruits and vegetables, and other edible matter; | |
| ● | our success is dependent upon the acceptance of our environmentally friendly solutions for fruits and vegetable; | |
| ● | we may be unable to respond effectively to technological changes in our industry, which could reduce the demand for our products; |
| 30 |
| ● | we currently rely on a limited number of suppliers to produce certain key components of our products. | |
● |
if we are unable to establish sales, marketing and distribution capabilities or enter into successful relationships with third parties to perform these services, we may not be successful in commercializing our products; | |
| ● | we rely on rapidly establishing global distributorship network in order to effectively market our products; | |
● |
the results of our early tests may not be indicative of results in future tests and we cannot assure you that any planned or future tests will lead to results sufficient for the necessary regulatory approvals; | |
● |
our products are highly regulated by governmental agencies in the countries where we conduct business and into which we plan to expand. Our failure to obtain regulatory approvals and registration, to comply with registration and regulatory requirements or to maintain regulatory approvals would have an adverse impact on our ability to market and sell our products; | |
● |
our success is dependent upon our ability to achieve regulatory approvals and registration in the United States and abroad (Mexico, Israel, Spain and Italy), which might take longer periods than expected; | |
● |
the inherent dangers in production and transportation of hydrogen peroxide and highly concentrated organic acids could cause disruptions and could expose us to potentially significant losses, costs or other liabilities; | |
● |
our business and operations may be affected by climate change conditions, which could materially harm our financial results; | |
● |
conditions in the global economy may adversely affect our business, financial condition and results of operation; | |
● |
our relationship with our employees could deteriorate, and certain key employees could leave, which could adversely affect our business and results of operations; | |
| ● | we are subject to risks relating to portfolio concentration; | |
● |
our operating results may fluctuate, which makes our results difficult to predict and could cause our results to fall short of expectations; | |
● |
international expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States, Mexico or Israel; | |
| ● | our business depends to some extent on international transactions; | |
● |
if we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, our ability to compete will be harmed; | |
● |
if we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unprotected know-how, our ability to compete will be harmed; | |
● |
we could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and force us to discontinue selling our products; | |
● |
we may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property; |
● |
we may experience claims that our products infringe the intellectual property rights of others, which may cause us to incur unexpected costs or prevent us from selling our products or services; | |
● |
if we or our contractors or service providers fail to comply with laws and regulations, we or they could be subject to regulatory actions, which could affect our ability to develop, market and sell our products or future products that we may develop and may harm our reputation in our industry; | |
| ● | regulatory reforms may adversely affect our ability to sell our products profitably; | |
| ● | conditions in Israel may limit our ability to manage and market our products, which would lead to a decrease in revenues; | |
● |
we may not be able to enforce covenants not-to-compete under current Israeli law that might result in added competition for our products; | |
● |
it may be difficult to acquire jurisdiction and enforce liabilities against our officers and directors who are based in Israel; and | |
● |
even if we meet the initial listing requirements of the Nasdaq Capital Market, there can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq Capital Market. Our failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a de-listing of our Common Stock. |
| 31 |
Risks Related to Our Financial Condition and Capital Requirements
We have a history of operating losses and expect to incur additional losses in the future.
We have sustained losses in recent years, which as of December 31, 2020, accumulated to $12.3 million, including an operating net loss of $1.3 million and $1.9 million for the years ended December 31, 2020 and 2019, respectively. We are likely to continue to incur significant net losses for at least the next several years as we continue to pursue our strategy, which is currently focused on research and development. Our losses have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. Any failure to achieve and maintain profitability would continue to have an adverse effect on our stockholders’ equity and working capital and could result in a decline in our share price or cause us to cease operations.
We have not generated significant revenue from the sale of our products and do not believe that our current cash on hand will be sufficient to fund our growth plans or our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern.
Our ability to become profitable depends upon our ability to generate revenue. We have not yet generated any material revenues and we do not know when, or if, we will generate any such revenue. We currently have no sources of recurring revenue and are therefore dependent upon external sources for financing our operations. There can be no assurance that we will succeed in obtaining the necessary financing to continue our operations.
This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our audited financial statements do not include any adjustments that might result from the outcome of the uncertainty regarding our ability to continue as a going concern. This going concern opinion could materially limit our ability to raise additional funds through the issuance of equity or debt securities or otherwise. Further reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern. If we cannot continue as a going concern, our investors may lose their entire investment in our Common Stock.
Even if we complete the Current Offering, we expect that we will need to raise significant additional capital, which we may be unable to obtain.
Our capital requirements in connection with our research and development activities and transition to commercial operations have been, and will continue to be significant. We will require additional funds to continue research, development and testing of our technologies and products, to obtain intellectual property protection relating to our technologies when appropriate, and to market our products. There can be no assurance that financing will be available in amounts or on terms acceptable to us, if at all. In either of the aforementioned situations, we may not be able to fully implement its growth plans.
Additional financings that we may require in the future will dilute the percentage ownership interests of our stockholders and may adversely affect our earnings and net book value per share. In addition, we may not be able to secure any such additional financing on terms acceptable to us, if at all. Moreover, if we are unable to obtain such additional capital as discussed above, we will be required to stop our operations, and will resume our activities, only after capital is raised.
| 32 |
Risks Related to Our Business, Industry and Business Operations
Because of our limited operating history, we may not be able to successfully operate our business or execute our business plan.
In September 2018, the Company changed its organizational structure and management team. After reviewing the Company’s then existing strategy and results of operation, as well as examining the market opportunities, the new management team decided to update the Company’s strategy, reduce the marketing and sales of its existing products, and focus the Company’s efforts and financial resources in developing its next generation products. During the years 2019 and 2020, we developed, validated and tested the efficacy of our next generation product – a blend of food acids – on a variety of crops in small and large scale commercial pilots.
Given our limited operating history, it is hard to evaluate our proposed business and prospects. Our proposed business operations will be subject to numerous risks, uncertainties, expenses and difficulties associated with early-stage enterprises. Such risks include, but are not limited to, the following:
| ● | the absence of a lengthy operating history; | |
| ● | insufficient capital to fully realize our operating plan; | |
| ● | expected continual losses for the foreseeable future; | |
| ● | operating in multiple currencies; | |
| ● | our ability to anticipate and adapt to a developing market(s); | |
| ● | acceptance of our products by the pre- and post-harvest industry players and consumers; | |
| ● | limited marketing experience; | |
| ● | a competitive environment characterized by well-established and well-capitalized competitors; | |
| ● | the ability to identify, attract and retain qualified personnel; and | |
| ● | operating in an environment that is highly regulated by a number of agencies. |
Because we are subject to these risks, evaluating our business may be difficult, our business strategy may be unsuccessful and we may be unable to address such risks in a cost-effective manner, if at all. If we are unable to successfully address these risks our business could be harmed.
Our products and technology require additional trials.
The efficacy of our products has only been shown in the limited number of pathogens tested on certain produce and aforementioned climates, and therefore our products have yet to be proven against certain additional and relevant pathogens, produce and market climates to validate the efficacy and benefits of our products. However, due to COVID-19, and the current restrictions on travels, we may delay or postpone certain of our planned trials.
The commercial success of our new generation products, as well as any future products, depends upon the degree of market acceptance by the packing house community as well as by other prospect markets and industries.
In order to achieve high volume sales and attain a leading market share and become the new standard of treatment, our products must not only be approved by the regulators, but also endorsed by the major packing houses and service providers, retailers of fruits and vegetables as well as environmental organizations. Our success depends on our ability to create significant value to the growers, the packing houses and the food retailers. We are aware of this key factor and are focusing on conducting large scale trials with major fruits and vegetables packers and retail suppliers of fresh consumed goods in several countries, in order to show the efficacy of the products and our technology, and to receive the recognition of packers and retailers. However, there can be no assurances that we will succeed in such an endeavor, nor is it clear how long it will take until we receive market recognition.
| 33 |
There can be no assurance that any product that we bring to the market will gain market acceptance by prospective customers. The commercial success of our new generation products and any future product depends in part on the packing house community as well as other industries for various use cases, depending on the acceptance by such industries of our technology as a useful and cost-effective solution compared to current solutions. If our new generation products or any future product does not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of our products will depend on a number of factors, including:
| ● | the results of our large-scale trials; | |
| ● | the cost, safety, efficacy, and convenience of our new generation products; | |
| ● | the acceptance of our products as a superior solution in the fresh produce industry; | |
| ● | the ability of third parties to enter into relationships with us without violating their existing agreements; | |
| ● | the effectiveness of our selling and marketing efforts; | |
| ● | the strength of marketing and distribution support for, and timing of market introduction of, competing products; and | |
| ● | publicity concerning our products or competing products. |
Our efforts to penetrate the packing house industry and educate the marketplace on the benefits of our products may require significant resources and may never be successful.
The COVID-19 pandemic, or any other pandemic, epidemic or outbreak of an infectious disease, may materially and adversely affect our business and operations.
The outbreak of COVID-19, which originated in Wuhan, China, in late 2019, has since spread across the globe, including the United States, Israel and many European countries in which we operate. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. While COVID-19 is still spreading and the final implications of the pandemic are difficult to estimate at this stage, it is clear that it has affected the lives of a large portion of the global population. At this time, the pandemic has caused states of emergency to be declared in various countries, travel restrictions imposed globally, quarantines established in certain jurisdictions and various institutions and companies being closed. We are actively monitoring the pandemic and we are taking all necessary measures to respond to the situation in cooperation with the various stakeholders.
A COVID-19 infection outbreak among our workforce could result in a temporary or long-term disruption in our business activities, including manufacturing and other functions.
Based on guidelines provided by the Israeli Government, employers (including us) are required to prepare and increase as much as possible the capacity and arrangement for employees to work remotely. In that regard, and in compliance with all applicable Israeli rules and guidelines, our offices have remained closed since the middle of March 2020, and all of our employees currently work remotely. In addition, some of our employees, including our Chief Technology Officer, are currently on a temporary leave without pay (furlough) and we have postpend some of our planned field tests due to the current restriction on international travels.
| 34 |
We may face significant competition from other companies looking to develop or acquire new alternative environmentally friendly solutions for the treatment of fruits and vegetables, and other edible matter.
We expect to face significant competition in every aspect of our business, and particularly from other companies that seek to enter our focal market. As regulators continue to move away from current residue chemical solutions, such as chlorpropham or CIPC, existing suppliers of these solutions are continually looking to develop or acquire new alternative environment-friendly solutions that can sustain their market share and revenue streams, or to enable the continuance of CIPC at current levels in new ways of treatment. Additionally, as market opportunity becomes eminent, competitors and new players will most likely attempt to develop similar or comparable solutions. It is possible that superior or more cost-effective alternative technology will emerge that will achieve greater market acceptance and render our products less competitive. Furthermore, existing vendors can cooperate to combat new players by reducing market prices and margins or other competitive initiatives. Our future success will therefore depend, to a large extent, upon our ability to achieve market acceptance of our innovative solutions as well as develop and introduce new products and enhancements to existing products. No assurance can be given that we will be able to compete in such a marketplace.
The market for post-harvest solutions is fragmented with various regional suppliers. The market of post-harvest treatments for fruits and vegetables is dominated by five large players with wide reach across the globe. We believe that the principal factors of competition in our industry include reputation, product quality, customer service and customer intimacy, product innovation, technical service and value creation.
Our success is dependent upon the acceptance of our environmentally friendly solutions for fruits and vegetables.
Our future success is dependent upon the acceptance of our environmentally friendly, non-toxicresidual treatment solutions for fruits and vegetables. While the market is signaling that such a direction is likely, certain trends as well as the future size of this market, and other potential markets for our products, rely upon a number of factors, many of which are beyond our control. For example, both the failure to convince retailers to bear additional costs for “green” fruit and vegetables as well as the failure to persuade consumers to purchase “green” fruits and vegetables for higher prices may adversely affect our business, financial condition, operating results and cash flow going forward.
We may be unable to respond effectively to technological changes in our industry, which could reduce the demand for our products.
Our future business success will depend upon our ability to maintain and enhance our technological capabilities and develop and market products, services and applications that meet changing customer needs and market conditions in a cost-effective and timely manner. Maintaining and enhancing technological capabilities and developing new products may also require significant investments in research and development. We may not be successful in developing new products, services and technology that successfully compete or be able to anticipate changing customer needs and preferences, and our customers may not accept one or more of our new products or services. If we fail to keep pace with evolving technological innovations or fail to modify our products and services in response to customers’ needs or preferences, then our business, financial condition and results of operations could be adversely affected.
We currently rely on a limited number of suppliers to produce certain key components of our products.
We rely on unaffiliated contract manufacturers to produce certain key components of our products. In Israel, we are working with a well-known producer of chemicals, SasaTech, who is responsible for the production of our products. SasaTech is well known for its knowledge and handling of hydrogen peroxide. In the United States, we have worked for the past few years with Seeler Industries, a national leader in the marketing and handling of hydrogen peroxide. There is limited available manufacturing capacity that meets our quality standards and regulatory requirements, especially for the manufacturing of the SF3H and SF3HS with one of their active ingredient – hydrogen peroxide – as well as for FreshProtect with one of its active ingredient – PO3. If we are unable to arrange for sufficient production capacity among our contract manufacturers or if our contract manufacturers encounter production, quality, financial, or other difficulties, including labor or geopolitical disturbances, we may encounter difficulty in meeting customer demands as we seek alternative sources of supply, or we may have to make financial accommodations to such contract manufacturers or otherwise take steps to mitigate supply disruption. We may be unable to locate an additional or alternate contract manufacturer that meets our quality controls and standards and regulatory requirements in a timely manner or on commercially reasonable terms. Any such difficulties could have an adverse effect on our business, financial condition and results of operations, which could be material.
| 35 |
If we are unable to establish sales, marketing and distribution capabilities or enter into successful relationships with third parties to perform these services, we may not be successful in commercializing our products.
We have a limited selling and marketing infrastructure and have limited experience in the sale, marketing or distribution of products. To achieve commercial success for any product for which we have obtained marketing approval, we will need to enter into collaborations with third parties like post-harvest service companies and establish a selling and marketing infrastructure or to out-license our products.
In the future, we may consider building a focused selling and marketing infrastructure to market our products in the United States or elsewhere in the world. There are risks involved with establishing our own sales, marketing and distribution capabilities. For example, recruiting and training a sales force could be expensive and time consuming and could delay any product launch. This may be costly, and our investment would be lost if we cannot retain or reposition our selling and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| ● | our inability to recruit, train and retain adequate numbers of effective selling and marketing personnel; | |
| ● | the inability of sales personnel to obtain access to potential customers; | |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and | |
| ● | unforeseen costs and expenses associated with creating an independent selling and marketing organization. |
If we are unable to establish our own sales, marketing and distribution capabilities or enter into successful arrangements with third parties to perform these services, our revenues and our profitability may be materially adversely affected.
In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our products in our target markets, including first the United States, Mexico, Spain, Italy and Israel, or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We rely on rapidly establishing global distributorship network in order to effectively market our products.
We have developed initial partnerships with local partners. In order to expand selling and marketing globally, and capture leading market share before any potential reaction from the competitors, we will need to rapidly expand geographically and establish a global distribution network. This is likely to put pressure on our management, financial and operational resources. In order to mitigate this factor, once we establish a significant presence in the market, we will proceed to establish strategic partnerships with some of the leading players in the market; however, there are no assurances that we will succeed in establishing such partnerships, which may harm the marketing of our products and the development of our business.
| 36 |
The results of our early tests may not be indicative of results in future tests and we cannot assure you that any planned or future tests will lead to results sufficient for the necessary regulatory approvals.
Our products have been tested in multiple commercial and small-scale pilots on certain types of produce and during specific time of the year. We are currently in the development and optimization phases of these products. Results from our later-stage commercial tests may show lower efficacy than our early-tests conducted previously and we cannot guarantee that when commercialized, our products will be effective and stable and product improvements as well as possible changes in the application and usage protocol may be required. These factors may significantly delay receipts of regulatory approvals, and the introduction of our products into the market. Likewise, we cannot be sure these products will be commercially viable, and have no assurances that we will be able to expand upon our current product offerings or that any such expansion will generate revenue.
Our products are highly regulated by governmental agencies in the countries where we conduct business and into which we plan to expand. Our failure to obtain regulatory approvals and registration, to comply with registration and regulatory requirements or to maintain regulatory approvals would have an adverse impact on our ability to market and sell our products.
Some of our products are subject to technical review and approval by government authorities in each country where we currently conduct our business and where we intend to sell our products.
The regulatory requirements to which we are subject are complex and vary from country to country. To obtain new registrations, it is necessary to have a local registrant, and to understand the country’s regulatory requirements, both at the time an application for registration is submitted and when the registration decision is made, which may be several years later. A significant investment in registration data is required (covering all aspects from manufacturing specifications through storage and transport, use, and, finally, disposal of unwanted product and used containers) to ensure that product performance (e.g. bio efficacy), intrinsic hazards and use patterns are fully characterized. Risk assessments are conducted by government regulatory authorities who make the final decision on whether the documented risk associated with a product and active ingredient is acceptable prior to granting approval for sale. This process may be prolonged due to requirements for additional data or internal administrative processes. There is a risk that registration of a new product may not be obtained or that a product label may be severely reduced, restricting the use of the product. If these circumstances arise, there is a risk that the substantial investments made in product development will generate the projected sales that justified the investment, and our business, financial condition and results of operations may be adversely affected by failure to obtain new registrations.
Products that are already approved may be subject to periodic review by regulatory authorities in many countries. Such reviews frequently require the provision of new data and more complex risk assessments. The outcome of such reviews of existing registrations cannot be guaranteed and registrations may be modified or canceled. Since all government regulatory authorities have the right to review existing registrations at any time, the sustainability of the existing portfolio cannot be guaranteed. Existing registrations may be lost at any time, resulting in an immediate impact on sales. Furthermore, prior to expiration, it is necessary to renew registrations. The renewal period and processes vary by country and may require additional studies to support the renewal process. Failure to comply could result in cancellation of the registration, resulting in an impact on sales.
In addition, new laws and regulations may be introduced, or existing laws and regulations may be changed or may become subject to new interpretations, which could result in additional compliance costs, seizures, confiscations, recalls, monetary fines or delays that could affect us or our customers.
Our success is dependent upon our ability to achieve regulatory approvals and registration in the United States and abroad (Mexico, Israel, Spain and Italy), which might take longer periods than expected.
We are subject to extensive national, state and local government regulation. A critical key to our success and ability to expand our business is our ability to obtain regulatory approvals and registration in the United States and in other countries for the use of our products. The regulatory approvals of some of our products are dependent on trials to show the efficacy and the non-toxicity of our products, and are time and cost consuming. We do not anticipate any significant problems in obtaining future required licenses, permits or approvals that are necessary to expand our business, however such registration filling might take longer period than expected due to various factors including the recent disruptions in regular services as a result of COVID-19, and it might delay obtaining such regulatory approvals, or might cause delays in starting operations on a large scale in these countries and other jurisdictions.
| 37 |
The inherent dangers in production and transportation of hydrogen peroxide and highly concentrated organic acids could cause disruptions and could expose us to potentially significant losses, costs or other liabilities.
Our operations are subject to significant hazards and risks inherent to the transportation of the active ingredient of one of our products – hydrogen peroxide. In high concentrations, our blend of acids has a very low pH which may lead to skin burn and hydrogen peroxide is an aggressive oxidizer and both can corrode many materials. We are working with limited low concentration of the material, however in high concentrations of H2O2 it will react violently. Hydrogen peroxide should be stored in a cool, dry, well-ventilated area and away from any flammable or combustible substances. It should be transported in special tanks and vehicles and should be stored in a container composed of non-reactive materials. These hazards and risks include, but are not limited to fires, explosions, third-party interference (including terrorism) and mechanical failure of equipment at our or third-party facilities. The occurrence of any of these events could result in production and distribution difficulties and disruptions, personal injury or wrongful death claims and other damage to properties.
Our business and operations may be affected by climate change conditions, which could materially harm our financial results.
Our business may be affected from changes in climate conditions as such events would affect the crops and their storability in those cases where there is unusually warm, dry, humid or cold weather before cropping.
In such instances, we may suffer a decrease in revenues as a result of a smaller storage volume of rooms or shorter storage period. We anticipate that once we increase our operations, and enter certain markets which experience or will experience significant climate change, such as above-common rain fall, heat waves, dry air conditions, and unusually cold or prolonged cold weather conditions, such events may materially impact our financial results.
Conditions in the global economy may adversely affect our business, financial condition and results of operation.
Although demand for fresh horticultural products is considered inelastic in developed economies, the fresh produce and citrus industries that we sell to may be affected by material changes in supply, market prices, exchange rates and general economic conditions. Delays or reductions in our customers’ purchasing or shifts to lower-cost alternatives that result from tighter economic market conditions would reduce demand for our products and services and could, consequently, have a material adverse effect on our business, financial condition and results of operations.
Our relationship with our employees could deteriorate, and certain key employees could leave, which could adversely affect our business and results of operations.
Our business involves complex operations and demands a management team to determine and implement our strategy and workforce that is knowledgeable and has expertise in many areas necessary for our operations. As a company focused on research and development in the highly-specialized horticultural post-harvest field, we rely on our ability to attract and retain skilled employees, consultants and contractors, including our specialized research and development. As of March 29, 2021 , we employed two full-time employees and four part-time employees, including the employees employed by our subsidiary, Save Foods Ltd. The departure of highly skilled employees, consultants or contractors or one or more employees who hold key regional management positions could have an adverse impact on our operations, including customers choosing to follow a such regional manager to one of our competitors.
In addition, to execute our growth plan we must attract and retain highly qualified personnel. Competition for these employees exists; new members of management must have significant industry expertise when they join us or engage in significant training which, in many cases, requires significant time before they achieve full productivity. If we fail to attract, train, retain, and motivate our key personnel, our business and growth prospects could be severely harmed.
Furthermore, we are dependent upon the managers to oversee our operations. Thus, there can be no assurance that the managers’ experience will be sufficient to successfully achieve our business objectives. All decisions regarding the management of our affairs will be made exclusively by our officers and directors. In the event these persons are ineffective, our business and results of operation would likely be adversely affected.
| 38 |
We are subject to risks relating to portfolio concentration.
Our business is highly dependent on a small number of products, which are based on our main active ingredients. Our core post-harvest business includes solutions designed to improve the yields of the packing house but mainly ensure food safety and assisting packing houses to meet the new FSMA requirements. Our ability to market and sell products containing our active ingredient to key service providers for treatment in post-harvest food safety industry in order to utilize their market position is important to our future success.
Our operating results may fluctuate, which makes our results difficult to predict and could cause our results to fall short of expectations.
Our operating results may fluctuate as a result of a number of factors, many of which are outside of our control. As a result, comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on our past results as an indication of our future performance. Our quarterly, year-to-date and annual expenses as a percentage of our revenues may differ significantly from our historical or projected rates. Our operating results in future quarters may fall below expectations. Any of these events could cause our stock price to fall. Each of the following factors, among the other risks described herein, may affect our operating results:
| ● | our ability to penetrate the packing house industry with our products; | |
| ● | our ability to generate revenue from our products; | |
| ● | the amount and timing, of operating costs and capital expenditures related to the maintenance and expansion of our businesses, and operations; | |
| ● | our focus on long-term goals over short-term results; | |
| ● | the global economic situation; and | |
| ● | fluctuations in weather conditions. |
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States, Mexico or Israel.
Other than our headquarters and other operations which are located in Israel (as further described below), we currently have limited international operations, but our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval of our product candidates. We also plan to retain sales representatives and third-party distributors, outside of the United States and Israel at a later date. Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits, and licenses; | |
| ● | failure by us to obtain regulatory approvals for the use of our product candidates in various countries; | |
| ● | additional potentially relevant third-party patent or other intellectual property rights; | |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; | |
| ● | limits in our ability to penetrate international markets; | |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; |
| 39 |
| ● | natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; | |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and | |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, as amended (the “FCPA”) its books and records provisions, or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
Our business depends to some extent on international transactions.
As a result of the international nature of our business, we are exposed to risks associated with changes in foreign currency exchange rates. A majority of our revenues and substantially all of our cost of sales are in USD, whilst our management, marketing, sales and R&D costs are in NIS. We are therefore exposed to foreign currency risk due to fluctuations in exchange rates. This may result in gains or losses with respect to movements in exchange rates, which may be significant and may also cause fluctuations in reported financial information that are not necessarily related to our operating results.
Risks Related to Intellectual Property
If we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our products, our ability to compete will be harmed.
Our commercial success depends, in part, on obtaining and maintaining patent and other intellectual property protection for the proprietary blend used in our products and our manufacturing process, as well as continuing to develop and secure trade secrets. We might in the future opt to license intellectual property from other parties. If we, or the other parties from whom we may license intellectual property, fail to obtain and maintain adequate patent or other intellectual property protection for intellectual property used in our products, or if any protection is reduced or eliminated, others could use the intellectual property used in our products, resulting in harm to our competitive business position. In addition, patent and other intellectual property protection may not provide us with a competitive advantage against competitors that devise ways of making competitive products without infringing any patents that we own or have rights to.
U.S. patents and patent applications may be subject to interference proceedings, and U.S. patents may be subject to re-examination proceedings in the U.S. Patent and Trademark Office. Foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings could result in loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope of our protection. Interference, re-examination and opposition proceedings may be costly and time consuming, and we, or the other parties from whom we might potentially license intellectual property, may be unsuccessful in defending against such proceedings. Thus, any patents that we own or might license may provide limited or no protection against competitors. In addition, our pending patent applications and those we may file in the future may have claims narrowed during prosecution or may not result in patents being issued. Even if any of our pending or future applications are issued, they may not provide us with adequate protection or any competitive advantages. Our ability to develop additional patentable technology is also uncertain.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to other parties. In addition, many countries limit the enforceability of patents against other parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States.
| 40 |
If we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unprotected know-how, our ability to compete will be harmed.
Proprietary trade secrets, copyrights, trademarks and unprotected know-how are also very important to our business. We rely on a combination of trade secrets, copyrights, trademarks, confidentiality agreements and other contractual provisions and technical security measures to protect certain aspects of our technology, especially where we do not believe that patent protection is appropriate or obtainable. We require our office holders, employees, consultants and distributers of our products and most third parties to execute confidentiality agreements in connection with their relationships with us. However, these measures may not be adequate to safeguard our proprietary intellectual property and conflicts may, nonetheless, arise regarding ownership of inventions. Such conflicts may lead to the loss or impairment of our intellectual property or to expensive litigation to defend our rights against competitors who may be better funded and have superior resources. Our office holders, employees, consultants and other advisors may unintentionally or willfully disclose our confidential information to competitors. In addition, confidentiality agreements may be unenforceable or may not provide an adequate remedy in the event of unauthorized disclosure. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming, and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our products that we consider proprietary. As a result, other parties may be able to use our proprietary technology or information, and our ability to compete in the market would be harmed.
We could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
Determining whether a product infringes a patent involves complex legal and factual issues, and the outcome of a patent litigation action is often uncertain. No assurance can be given that patents containing claims covering our products, parts of our products, technology or methods do not exist, have not been filed or could not be filed or issued. Furthermore, our competitors or other parties may assert that our products and the methods we employ in the use of our products are covered by U.S. or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware and which may result in issued patents which our current or future products infringe. Also, because the claims of published patent applications can change between publication and patent grant, there may be published patent applications with claims that we infringe. There could also be existing patents that one or more of our products or parts may infringe and of which we are unaware. As the number of competitors in the post-harvest market grows, and as the number of patents issued grows, the possibility of patent infringement claims against us increases.
Infringement actions and other intellectual property claims and proceedings brought against or by us, whether with or without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our business and harm our reputation. Some of our competitors may be able to sustain the costs of complex patent or intellectual property litigation more effectively than we can because they have substantially greater resources.
We cannot be certain that we will successfully defend against allegations of infringement of patents and intellectual property rights of others. In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the other party’s patents or other intellectual property were upheld as valid and enforceable and we were found to infringe the other party’s patents or violate the terms of a license to which we are a party, we could be required to pay damages. We could also be prevented from selling our products unless we could obtain a license to use technology or processes covered by such patents or will be able to redesign the product to avoid infringement. A license may not be available at all or on commercially reasonable terms or we may not be able to redesign our products to avoid infringement. Modification of our products or development of new products could require us to conduct clinical trials and to revise our filings with the applicable regulatory bodies, which would be time consuming and expensive. In these circumstances, we may be unable to sell our products at competitive prices or at all, our business and operating results could be harmed.
| 41 |
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators, or other third parties have an ownership interest in our patents or other intellectual property. Ownership disputes may arise in the future, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may experience claims that our products infringe the intellectual property rights of others, which may cause us to incur unexpected costs or prevent us from selling our products or services.
We continually seek to improve our business processes and develop new products and applications in a crowded patent space that we must continually monitor to avoid infringement. We cannot guarantee that we will not experience claims that our processes and products infringe issued patents (whether present or future) or other intellectual property rights belonging to others.
From time to time, we oppose patent applications that we consider overbroad or otherwise invalid in order to maintain the ability to operate freely in our various business lines without the risk of being sued for patent infringement. If, however, patents are subsequently issued on any such applications by other parties, or if patents belonging to others already exist that cover our products, processes or technologies, we could experience claims for infringement or have to take other remedial or curative actions to continue our manufacturing and sales activities with respect to one or more products. Likewise, our competitors may also already hold or have applied for patents in the United States or abroad that, if enforced or issued, could prevail over our patent rights or otherwise limit our ability to manufacture or sell one or more of our products in the United States or abroad. Any actions asserted against us could include payment of damages for infringement, stopping the use, require that we obtain licenses from these parties or substantially re-engineer our products or processes in order to avoid infringement. We may not be able to obtain the necessary licenses on acceptable terms, or at all, or be able to re-engineer our products successfully. Further, intellectual property litigation is expensive and time-consuming, regardless of the merits of any claim, and could divert our management’s attention from operating our business.
Risks Related to Regulatory Compliance
If we or our contractors or service providers fail to comply with laws and regulations, we or they could be subject to regulatory actions, which could affect our ability to develop, market and sell our products or future products that we may develop and may harm our reputation in our industry.
If we or our manufacturers or other third-party contractors fail to comply with applicable federal, state or foreign laws or regulations, including with respect to food treatment, we could be subject to regulatory actions, which could affect our ability to develop, market and sell our current products or any future products which we may develop in the future and could harm our reputation and lead to reduced demand for or non-acceptance of our proposed products by the market.
Regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in the United States, Mexico, Israel or other countries in which we operate, that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of our products, including in the food health industry. In addition, regulations and guidance may often be revised or reinterpreted by the regulatory authorities in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted, or interpretations changed, and what the impact of such changes, if any, may be.
| 42 |
Risks Related to Our Operations in Israel
Conditions in Israel may limit our ability to manage and market our products, which would lead to a decrease in revenues.
Because most of our operations is conducted in Israel and our management is located in Israel, our operations are directly affected by economic, political, geopolitical and military conditions affecting Israel. Accordingly, political, economic and military conditions in Israel directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have occurred between Israel and its neighboring countries. These conflicts involved missile strikes against civilian targets in various parts of Israel including most recently, central Israel, and negatively affected business conditions in Israel as well as home starts and the building industry in Israel.
Our facilities are in range of rockets that may be fired from Lebanon, Syria or the Gaza Strip into Israel. In the event that our facilities are damaged as a result of hostile action or hostilities otherwise disrupt the ongoing operation of our facilities, our ability to deliver products to customers could be materially and adversely affected. Our commercial insurance in Israel does not cover losses that may occur as a result of acts of war; however, losses as a result of terrorist attacks to our facilities and disruption to the ongoing operations, are covered by our insurance for damages of up to $40 million, if such damages are not covered by the Israeli government. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained and will be adequate in the event we submit a claim. Even if insurance is maintained and adequate, we cannot assure you that it will reduce or prevent any losses that may occur as a result of such actions or will be exercised in a timely manner to meet our contractual obligations with customers and vendors.
In addition, popular uprisings in various countries in the Middle East and North Africa have affected the political stability of those countries. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and these countries, such as Turkey, from which we import a significant amount of our raw materials. Moreover, some countries around the world restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. These restrictions may limit materially our ability to obtain raw materials from these countries or sell our products to companies and customers in these countries. In addition, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods. Such efforts, particularly if they become more widespread, may materially and adversely impact our ability to sell our products out of Israel.
Our employees in Israel, generally males, including executive officers, may be called upon to perform military service on an annual basis until they reach the age of 40 (and in some cases, up to 45 or 49). In emergency circumstances, they could be called to immediate and prolonged active duty. Our operations could be disrupted by the absence of a significant number of our employees related to military service or the absence for extended periods of one or more of our key employees for military service. Such a disruption could materially and adversely affect our business and results of operations. Additionally, the absence of a significant number of the employees of our Israeli suppliers and contract manufacturers related to military service may disrupt their operations, in which event our ability to deliver products to customers may be materially and adversely affected.
Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or a significant downturn in the economic or financial condition of Israel, could materially and adversely affect our operations and product development, cause our revenues to decrease and materially harm the share price of publicly traded companies with operations in Israel, such as us.
In early January 2020, certain events contributed to an increase in hostilities between the United States and Iran, and as a result Iran issued multiple public statements threatening to attack Israel and the United States. These events, coupled with the already mounting tensions between Israel and Iran, may threaten to destabilize the Middle East, the result of which may impact our ability to conduct our business effectively.
| 43 |
The Israeli Parliament (the “Knesset”), for reasons related to political instability, has failed to pass a budget for the year 2020, and certain government ministries, which may be critical to the operation of our business, are without necessary resources and may not receive sufficient funding moving forward. Given the likelihood that the current political stalemate might not be resolved during the next calendar year, our ability to conduct our business effectively may be adversely materially affected.
We may not be able to enforce covenants not-to-compete under current Israeli law that might result in added competition for our products.
We have non-competition agreements with all of our employees, all of which are governed by Israeli law. These agreements prohibit our employees from competing with or working for our competitors, generally during their employment and for up to 12 months after termination of their employment. However, Israeli courts are reluctant to enforce non-compete undertakings of former employees and tend, if at all, to enforce those provisions for relatively brief periods of time in restricted geographical areas, and only when the employee has obtained unique value to the employer specific to that employer’s business and not just regarding the professional development of the employee. If we are not able to enforce non-compete covenants, we may be faced with added competition.
It may be difficult to acquire jurisdiction and enforce liabilities against our officers and directors who are based in Israel.
The majority of our officers and present directors reside outside of the United States and most of our current operations are located outside the United States. As a result, it may not be possible for United States investors to enforce their legal rights, to effect service of process upon our directors or officers or to enforce judgments of United States courts predicated upon civil liabilities and criminal penalties of our directors and officers under federal securities laws. Moreover, we have been advised that Israel does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United States. Further, it is unclear if extradition treaties now in effect between the United States and Israel would permit effective enforcement of criminal penalties of the federal securities laws.
Risks Related to Ownership of Our Common Stock
Even if we meet the initial listing requirements of the Nasdaq Capital Market, there can be no assurance that we will be able to comply with the continued listing standards of the Nasdaq Capital Market. Our failure to meet the continued listing requirements of the Nasdaq Capital Market could result in a de-listing of our Common Stock.
In connection with the Current Offering, we intend to list our Common Stock on the Nasdaq Capital Market. Even if we meet the initial listing requirements of the Nasdaq Capital Market, we cannot assure you that we will be able to comply with the other standards that we are required to meet in order to maintain a listing of our common stock on the Nasdaq Capital Market. If after listing we fail to satisfy the continued listing requirements of the Nasdaq Capital Market, such as the corporate governance requirements or the minimum stockholder’s equity requirement, the Nasdaq Capital Market may take steps to de-list our common stock. Such a de-listing would likely have a negative effect on the price of our Common Stock and would impair our shareholders’ ability to sell or purchase our Common Stock when they wish to do so. In the event of a de-listing, we would take actions to restore our compliance with the Nasdaq Capital Market’s listing requirements, but we can provide no assurance that any action taken by us would result in our common stock becoming listed again, or that any such action would stabilize the market price or improve the liquidity of our Common Stock.
The market price of our Common Stock may be highly volatile.
The market price of our Common Stock is likely to be volatile. Our Common Stock price could be subject to wide fluctuations in response to a variety of factors, including the following:
| ● | reports of adverse events with respect to the commercialization and distribution of our products; | |
| ● | inability to obtain additional funding; |
| 44 |
| ● | any delay in filing a regulatory submission for any of our products and any adverse development or perceived adverse development with respect to the review of that regulatory submission by the EPA, FDA or other regulatory authority; | |
| ● | failure to successfully develop and commercialize our products; | |
| ● | failure to enter into strategic collaborations; | |
| ● | failure by us or strategic collaboration partners to prosecute, maintain or enforce our intellectual property rights; | |
| ● | changes in laws or regulations applicable to future products; | |
| ● | inability to scale up our manufacturing capabilities through third-party manufacturers, inability to obtain adequate product supply for our products or the inability to do so at acceptable prices; | |
| ● | introduction of new products or technologies by our competitors; | |
| ● | failure to meet or exceed financial projections we may provide to the public; | |
| ● | failure to meet or exceed the financial expectations of the investment community; | |
| ● | announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by our competitors; | |
| ● | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our platform technologies, technologies, products or product candidates; | |
| ● | additions or departures of key scientific or management personnel; | |
| ● | significant lawsuits, including patent or stockholder litigation; | |
| ● | changes in the market valuations of similar companies; | |
| ● | sales of our securities by us or our stockholders in the future; and | |
| ● | trading volumes of our securities. |
In addition, companies trading in the stock market have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our Common Stock, regardless of our actual operating performance.
Sales of a substantial number of shares of our Common Stock in the public market by our existing stockholders could cause our share price to fall.
Sales of a substantial number of shares of our Common Stock in the public market, or the perception that these sales might occur, could depress the market price of our Common Stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our Common Stock.
| 45 |
Our principal stockholders, officers and directors beneficially own approximately 39.5% of our outstanding shares of Common Stock. They will therefore be able to exert significant control over matters submitted to our stockholders for approval.
As of March 29, 2021, our principal stockholders, officers and directors beneficially own approximately 39.5% of our outstanding Common Stock. This significant concentration of share ownership may adversely affect the trading price for our Common Stock because investors often perceive disadvantages in owning shares in companies with controlling stockholders. As a result, these stockholders, if they acted together, could significantly influence matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these stockholders may not always coincide with our interests or the interests of other stockholders.
Delaware law and provisions in our amended and restated certificate of incorporation and amended and restated bylaws could make a merger, tender offer or proxy contest difficult, thereby depressing the market price of our Common Stock.
Our status as a Delaware corporation and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, even if a change of control would be beneficial to our existing stockholders. In addition, our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that may make the acquisition of the Company more difficult, including the following:
| ● | our stockholders will only be able to take action at a meeting of stockholders and will not be able to take action by written consent for any matter; | |
| ● | our board of directors is classified into three classes of directors with staggered three-year terms; | |
| ● | a special meeting of our stockholders may only be called by a majority of our board of directors; | |
| ● | advance notice procedures apply for stockholders to nominate candidates for election as directors or to bring matters before an annual meeting of stockholders; and | |
| ● | certain litigation against us can only be brought in Delaware. |
These provisions, alone or together, could discourage, delay or prevent a transaction involving a change in control of the Company. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors of their choosing and to cause us to take other corporate actions they desire, any of which, under certain circumstances, could limit the opportunity for our stockholders to receive a premium for their shares of our Common Stock, and could also affect the price that some investors are willing to pay for our Common Stock.
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or our Common Stock, our stock price and trading volume could decline.
The trading market for our Common Stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding our shares, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analysts who may cover us were to cease coverage of the Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
| 46 |
Shares of our Common Stock are an illiquid investment as there is presently a limited market for our Common Stock. We do not know whether a market for our Common Stock will be sustained or what the trading price of our Common Stock will be and as a result it may be difficult for you to sell your shares of Common Stock.
There is presently a limited market for our Common Stock. Although we intend to list our Common Stock on the Nasdaq Stock Market, an active trading market for our Common Stock may not be sustained. It may be difficult for you to sell your shares of Common Stock without depressing the market price for our Common Stock or at all. Further, an inactive market may also impair our ability to raise capital by selling our Common Stock and may impair our ability to enter into strategic partnerships or acquire companies, products, or services by using our equity securities as consideration.
We do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends, and we do not anticipate paying cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our Common Stock as a source for any future dividend income. Our board of directors has complete discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiary, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors.
We may need additional capital, and the sale of additional shares or equity or debt securities could result in additional dilution to our stockholders.
We believe that our current cash and cash used in operations, together with the net proceeds we anticipate to receive in connection with the closing of our Current Offering, will be sufficient to meet our anticipated cash needs for the next 24 months. We may, however, require additional cash resources due to changed business conditions or other future developments, including further adverse effects to our business from COVID-19 related issues. If these resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain one or more credit facilities. The sale of additional equity securities could result in additional dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. It is uncertain whether financing will be available in amounts or on terms acceptable to us, if at all.
General Risk Factors
Disruptions to our information technology systems due to cyber-attacks or our failure to upgrade and adjust our information technology systems, may materially impair our operations, hinder our growth and materially and adversely affect our business and results of operations.
We believe that an appropriate information technology (“IT”), infrastructure is important in order to support our daily operations and the growth of our business. If we experience difficulties in implementing new or upgraded information systems or experience significant system failures, or if we are unable to successfully modify our management information systems or respond to changes in our business needs, we may not be able to effectively manage our business, and we may fail to meet our reporting obligations. Additionally, if our current back-up storage arrangements and our disaster recovery plan are not operated as planned, we may not be able to effectively recover our information system in the event of a crisis, which may materially and adversely affect our business and results of operations.
| 47 |
In the current environment, there are numerous and evolving risks to cybersecurity and privacy, including criminal hackers, hacktivists, state-sponsored intrusions, industrial espionage, employee malfeasance and human or technological error. High-profile security breaches at other companies and in government agencies have increased in recent years, and security industry experts and government officials have warned about the risks of hackers and cyber-attacks targeting businesses such as ours. Computer hackers and others routinely attempt to breach the security of technology products, services and systems, and to fraudulently induce employees, customers, or others to disclose information or unwittingly provide access to systems or data. We can provide no assurance that our current IT system or any updates or upgrades thereto and the current or future IT systems of our distributors use or may use in the future, are fully protected against third-party intrusions, viruses, hacker attacks, information or data theft or other similar threats. Legislative or regulatory action in these areas is also evolving, and we may be unable to adapt our IT systems or to manage the IT systems of third parties to accommodate these changes. We have experienced and expect to continue to experience actual or attempted cyber-attacks of our IT networks. Although none of these actual or attempted cyber-attacks has had a material adverse impact on our operations or financial condition, we cannot guarantee that any such incidents will not have such an impact in the future.
Failure to comply with anti-bribery, anti-corruption and anti-money laundering laws could subject us to penalties and other adverse consequences.
We are subject to the FCPA and other anticorruption, anti-bribery and anti-money laundering laws in the jurisdictions in which we do business, both domestic and abroad. These laws generally prohibit us and our employees from improperly influencing government officials or commercial parties in order to obtain or retain business, direct business to any person or gain any advantage. The FCPA and other applicable anti-bribery and anti-corruption laws also may hold us liable for acts of corruption and bribery committed by our third-party business partners, representatives and agents. In addition, we leverage third parties to sell our products and conduct our business abroad. We and our third-party business partners, representatives and agents may have direct or indirect interactions with officials and employees of government agencies or state-owned or affiliated entities and we may be held liable for the corrupt or other illegal activities of these third-party business partners and intermediaries, our employees, representatives, contractors, channel partners and agents, even if we do not explicitly authorize such activities. These laws also require that we keep accurate books and records and maintain internal controls and compliance procedures designed to prevent any such actions. While we have policies and procedures to address compliance with such laws, we cannot assure you that our employees and agents will not take actions in violation of our policies or applicable law, for which we may be ultimately held responsible and our exposure for violating these laws increases as our international presence is established and as we increase sales and operations in foreign jurisdictions. Any violation of the FCPA or other applicable anti-bribery, anti-corruption laws and anti-money laundering laws could result in whistleblower complaints, adverse media coverage, investigations, imposition of significant legal fees, loss of export privileges, severe criminal or civil sanctions or suspension or debarment from U.S. government contracts, substantial diversion of management’s attention, a decline in the market price of our Common Stock or overall adverse consequences to our reputation and business, all of which may have an adverse effect on our results of operations and financial condition.
We will incur significant increased costs as a result of the listing of our securities for trading on Nasdaq and our management will be required to devote substantial time to new compliance initiatives as well as compliance with ongoing U.S. requirements.
Upon the listing of securities on Nasdaq, we will become a publicly traded company in the United States. We anticipate that we will incur costs associated with corporate governance requirements of the SEC and Nasdaq, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. We expect these rules and regulations to increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and to make some activities more time consuming and costly. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs and resources, particularly a greater percentage of the time and efforts of our management team will be diverted to these new requirements. Any future changes in the laws and regulations affecting public companies in the United States, including Section 404 and other provisions of the Sarbanes-Oxley Act, and the rules and regulations adopted by the SEC and Nasdaq, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees, or as executive officers.
| 48 |
We face risks related to compliance with corporate governance laws and financial reporting standards.
The Sarbanes-Oxley Act of 2002, as well as related new rules and regulations implemented by the SEC and the Public Company Accounting Oversight Board, require changes in the corporate governance practices and financial reporting standards for public companies. These new laws, rules and regulations, including compliance with Section 404 of the Sarbanes-Oxley Act of 2002 relating to internal control over financial reporting, have materially increased the legal and financial compliance costs of small companies and have made some activities more time-consuming and more burdensome.
We may not have effective internal controls.
In connection with Section 404 of the Sarbanes-Oxley Act of 2002, we need to assess the adequacy of our internal controls, remedy any weaknesses that may be identified, validate that controls are functioning as documented and implement a continuous reporting and improvement process for internal controls. We may discover deficiencies that require us to improve our procedures, processes and systems in order to ensure that our internal controls are adequate and effective and that we are in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act. If the deficiencies are not adequately addressed, or if we are unable to complete all of our testing and any remediation in time for compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the SEC rules under it, we would be unable to conclude that our internal controls over financial reporting are designed and operating effectively, which could adversely affect investor confidence in our internal controls over financial reporting.
Item 1b. unresolved staff comments
None.
We lease offices in various locations in Israel. Our primary locations and their principal terms are as follows:
We previously leased offices located at 20 Habarzel St., Tel Aviv, Israel, which lease began on May 1, 2019 and included approximately 98 square meters for a monthly cost of approximately $1,950 and a monthly management fee of approximately $650 (plus value added tax). We terminated the foregoing lease on August 13, 2020. There are no additional payments required under this lease.
Additionally, on September 1, 2017, we entered into a lease agreement for office space at 156 Fifth Avenue, 10th Floor, New York, NY 10010-7751, and which expired on September 30, 2020. The rent amount payable under the foregoing lease was $7,200, which was paid in the form of an issuance of 720,000 shares of our Common Stock on November 5, 2017. There are no additional payments required under this lease.
Lastly, we entered into a lease agreement with Kibbutz Alonim for a period ending December 31, 2020 with an option to extend the agreement through December 31, 2021, pursuant to which the Company must pay monthly fees in the amount of approximately $1,400. The lease agreement with with Kibbutz Alonim was automatically renewed and it is set to expire on December 31, 2021. Our current monthly rent payment is NIS 4,846 (approximately $1,400) which includes taxes.
None.
item 4. mine safety disclosures.
Not applicable.
| 49 |
item 5. market for registrant’s common equity, related stockholder matters and issuer purchases
Market Information
The shares of our Common Stock are currently traded on the OTC Markets, Pink Tier, under the symbol “SAFO”. The last reported sales price of our Common Stock on the Pink Tier on March 26, 2021, was $15 per share. As of March 14, 2021, there were approximately 177 holders of record of our Common Stock. Action Stock Transfer serves as transfer agent for our Common Stock.
Dividends
We have never paid cash dividends on any of our capital stock and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. We do not intend to pay cash dividends to holders of our Common Stock in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plans
As of the date of this Annual Report, our Company has authorized 289,942 shares of Common Stock for issuance under our 2018 Equity Incentive Plan. The following table presents the information as of December 31, 2020.
| Plan Category | (a)
Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights | (b)
Weighted-average Exercise Price of Outstanding Options, Warrants and Rights | (c)
Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) | |||||||||
| Equity compensation plans approved by security holders | 206,862 | $ | 3.27 | 76,730 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 206,862 | 3.27 | 76,320 | |||||||||
2018 Equity Incentive Plan
We maintain one equity incentive plan – the 2018 Equity Incentive Plan. As of December 31, 2020, the number of shares of Common Stock reserved for the exercise of options granted under Equity Incentive Plan was 289,942.
Our Equity Incentive Plan was adopted by our board of directors in October 2018, and became effective immediately thereafter. The 2018 Equity Incentive Plan permits the grant of incentive stock options to employees of the Company, including officers and directors, and non-statutory stock options, stock appreciation rights, restricted stock, restricted stock units, performance units, performance shares and other stock or cash awards as the administrator of the plan may determine, to the Company’s employees and service providers.
The 2018 Equity Incentive Plan may be administered by the board of directors or by different committees that may be established with respect to different groups of service providers; in that event, the committee established with respect to a group of service providers shall administer the 2018 Equity Incentive Plan with respect to awards granted to members of such group.
Subject to the provisions of the 2018 Equity Incentive Plan, and in the case of a committee, subject to the specific duties delegated by the board to such committee, the administrator will have the authority, in its sole discretion to determine subject to Israeli law and section 16 of the Exchange Act, the grantees of awards and the terms of the grant, including, exercise prices, vesting schedules, acceleration of vesting and conditions and restrictions applicable to an award, as well other matters necessary in the administration of the 2018 Equity Incentive Plan. The 2018 Equity Incentive Plan enables us to issue awards under various tax regimes, including, without limitation, pursuant to Section 102 of the Ordinance, and under Section 3(i) of the Ordinance and Section 422 of the United States Internal Revenue Code of 1986, as amended (the “Code”).
| 50 |
The 2018 Equity Incentive Plan provides that awards granted to our employees, directors and officers who are not controlling shareholders and who are not considered Israeli residents are intended to qualify for special tax treatment under the “capital gain track” provisions of Section 102(b) of the Ordinance as detailed above. Our Israeli non-employee service providers and controlling shareholders may only be granted awards under Section 3(i) of the Ordinance, which does not provide for similar tax benefits.
Awards granted under the 2018 Equity Incentive Plan to U.S. residents may qualify as “incentive stock options” within the meaning of Section 422 of the Code. The exercise price for “incentive stock options” must not be less than the fair market value on the date on which an option is granted, or 110% of the fair market value if the option holder holds more than 10% of our share capital. Notwithstanding the foregoing provisions, options may be granted with a per share exercise price of less than 100% of the fair market value per share on the date of grant pursuant to the issuance or assumption of an option in a transaction to which Section 424(a) of the Code applies in a manner consistent with said Section 424(a).
The vesting schedule of options granted under the 2018 Equity Incentive Plan is set forth in each grantee’s award agreement.
Awards terminate upon the date set out in the grantee’s specific award agreement or at the end of an extended period following the termination of the grantee’s employment or service. In the event of the death of a grantee while employed by or performing service for us or an affiliate, or in the event of termination of a grantee’s employment or services for reasons of disability, the grantee or his or her estate or legal successor (in the case of death), may exercise awards that have vested prior to termination within a period of nine (9) months from the date of disability or death but in any event no later than the expiration date of the awards. If a participant ceases to be a service provider, other than upon the participants termination as the result of the participant’s death or disability, may exercise his or her option within such period of time as is specified in the award agreement to the extent that the option is vested on the date of termination (but in no event later than the expiration of the term of such option as set forth in the award agreement). In the absence of a specified time in the award agreement, the option will remain exercisable for three (3) months following the participant’s termination. Unless otherwise provided by the administrator, if on the date of termination, the participant is not vested as to his or her entire option, the Common Stock covered by the unvested portion of the option will revert to the 2018 Equity Incentive Plan. If after termination, the participant does not exercise his or her option within the time specified by the award agreement or by operation of this paragraph, the option will terminate, and the Common Stock covered by such option will revert to the 2018 Equity Incentive Plan.
Israeli options may not be assigned or transferred other than by will or laws of descent, unless otherwise determined by the committee.
In the event of our proposed dissolution or liquidation, the administrator will notify each participant as soon as practicable prior to the effective date of such proposed transaction. To the extent it has not been previously exercised, an award will terminate immediately prior to the consummation of such proposed action.
In the event of a merger or change in control, each outstanding award will be treated as the administrator determines, including, without limitation, that each award will be assumed or an equivalent option or right substituted by the successor corporation or a parent or subsidiary of the successor corporation. The administrator will not be required to treat all awards similarly in the transaction.
In the event that the successor corporation does not assume or substitute for the award, the participant will fully vest in and have the right to exercise all of his or her outstanding options and stock appreciation rights, including Common Stock as to which such awards would not otherwise be vested or exercisable. All restrictions on restricted stock will lapse, and, with respect to restricted stock units, performance shares and performance units, all performance goals or other vesting criteria will be deemed achieved at target levels and all other terms and conditions met. In addition, if an option or stock appreciation right is not assumed or substituted for in the event of a change in control, the administrator will notify the participant in writing or electronically that the option or stock appreciation right will be fully vested and exercisable for a period of time determined by the administrator in its sole discretion, and the option or stock appreciation right will terminate upon the expiration of such period.
| 51 |
In the year ended December 31, 2020, we granted to our directors and officers options to purchase a maximum aggregated number of 92,574 of the Company’s Common Stock, under our equity incentive plan. 92,574 options were granted at an exercise price of $3.15 to $3.78 per share, and the latest expiration date for such options is July 1, 2030.
Recent Sales of Unregistered Securities
Set forth below is information regarding shares of Common Stock and preferred stock issued, and options granted, by us during the year ended December 31, 2019, that were not registered under the Securities Act. Also included is the consideration, if any, received by us, for such shares and options and information relating to the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
On April 29, 2018, we issued 28,572 shares of Common Stock for total consideration of $30,000.
In August 2018, we issued: (i) 11,128 shares of Common Stock to several existing shareholders of the Company who were owed a sum of $35,000; (ii) 274,986 shares of Common Stock to accredited investors who held shares in Save Foods Ltd. in exchange for 7,218,129 shares of Save Foods Ltd., at the rate of 0.038 shares of Common Stock of the Company for each share of Save Foods Ltd; and (iii) 160,643 shares of Common Stock of the Company to several investors, for total consideration of $506,000 reflecting a price of $3.15 per share of Common Stock.
On September 4, 2018, we issued 25,398 shares of Common Stock to two accredited investors for total consideration of $80,000.
On October 8, 2018, we issued 36,513 shares of Common Stock to an accredited investor for total consideration of $115,000.
During November 2018, we issued an aggregate of 30,689 shares of Common Stock to accredited investors for total consideration of $133,333.
During January 2019, we issued an aggregate of 19,050 shares of Common Stock to accredited investors for total consideration of $120,000.
During February 2019, we issued an aggregate of 35,717 shares of Common Stock to accredited investors for total consideration of $225,000.
During March 2019, we issued (i) 31,748 shares of Common Stock to an accredited investors for total consideration of $216,666, and (iii) warrants to purchase 7,937 shares of Common Stock at an exercise price of $12.60 per share.
During June 2019, we issued: (i) 31,747 shares of Common Stock to accredited investors for total consideration of $200,000; and (ii) 10,004 shares of Common Stock to an accredited investor for total consideration of $84,034 and at the same time issued that accredited investor 10,004 warrants to purchase shares of Common Stock at an exercise price of $12.60.
During August 2019, we issued 11,905 shares of Common Stock to an accredited investor for total consideration of $100,000. In addition, we issued him 11,905 warrants to purchase shares of Common Stock at an exercise price of $12.60.
During July and August 2020, we issued: (i) 67,369 shares of Common Stock in respect of the conversion of convertible loans; (ii) 32,769 units, for an aggregate amount of $250,000, at a price of $7.63 per unit, where each unit consists of one share of Common Stock and one warrant to purchase one share of Common Stock with an exercise price of $8.40 per share.
During September 2020, we issued 13,107 units, for an aggregate amount of $100,000, at a purchase price of $7.63 per unit to Medigus Ltd., where each unit consists of one share of Common Stock and one warrant to purchase one share of Common Stock with an exercise price of $8.40 per share.
| 52 |
During December 2020, we issued 6,350 shares of Common Stock following the exercise of options.
Since December 2017, we issued warrants to purchase an aggregate of 187,128 shares of Common Stock to investors and service providers, with exercise prices ranging from $2.1 to $8.4 per share. As of the date of this Annual Report, 28,572 warrants were exercised and 28,572 warrants were forfeited, such that the total outstanding amount of warrants as of the date of this Annual Report is 129,984.
Since December 2017, we granted to our directors, officers, employees and service providers options to purchase an aggregate of 261,625 shares of Common Stock under our Equity Incentive Plan, with exercise prices ranging from $3.15 to $3.78 per share. As of the date of this Annual Report, 6,350 options were exercised and 43,651 options were forfeited, such that the total outstanding number of options as of the date of this filing is 206,862.
Since December 2017, we issued convertible promissory notes in an aggregate principal amount of $1,013,000 in a series of convertible loan agreements. As of the date of this Annual Report, we issued an aggregate of 67,369 shares of Common Stock as a result of conversion of certain convertible promissory notes, at a conversion price of $7.63 per share.
Issuer Purchases of Equity Securities
During the year ended December 31, 2019, we did not purchase any of our equity securities.
Item 6. selected financial data
As a smaller reporting company, we are not required to provide the information required by this item.
item 7. management’s discussion and analysis of financial condition and results of operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes appearing elsewhere in this Annual Report. In addition to historical information, the following discussion contains forward-looking statements that involve risks, uncertainties and assumptions. See “Forward-looking Statements” for a discussion of the uncertainties and assumptions associated with these statements. Our actual results may differ materially from those discussed below.
Overview
We develop eco-friendly “green” solutions for the food industry. Our solutions are developed to improve the food safety and shelf life of fresh produce. We do this by controlling human and plant pathogens, thereby reducing spoilage, and in turn, reducing food loss.
Our products are based on a proprietary blend of food acids which have a synergistic effect when combined with certain types of oxidizing agent-based sanitizers and fungicides at low concentrations. Our green products are capable of cleaning, sanitizing and controlling pathogens on fresh produce with the goal of making them safer for human consumption and extending their shelf life by reducing their decay. One of the main advantages of our products is that our active ingredients do not leave any toxicological residues on the fresh produce we treat. In contrary, by forming a temporary protective shield around the fresh produce we treat, our products make it difficult for pathogens to develop and potentially provide protection which also reduces cross-contamination.
Components of Results of Operation
Revenues and Cost of Revenues
Our total revenue consists of products and our cost of revenues consists of cost of products.
The following table discloses the breakdown of revenues and costs of revenues:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Revenues from sale of products | $ | 232,274 | $ | 175,823 | ||||
| Cost of sales | (43,405 | ) | (144,548 | ) | ||||
| Gross profit | $ | 188,869 | $ | 31,275 | ||||
Operating Expenses
Our current operating expenses consist of three components — research and development expenses, selling and marketing expenses and general and administrative expenses.
Research and Development Expenses, net
Our research and development expenses consist primarily of salaries and related personnel expenses, share base compensation, professional fees and other related research and development expenses such as field tests.
| 53 |
The following table discloses the breakdown of research and development expenses:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | $ | 39,021 | $ | 177,712 | ||||
| Share based compensation | 91,190 | 75,998 | ||||||
| Professional fees | 130,592 | 178,854 | ||||||
| Laboratory and field tests | 72,593 | 73,968 | ||||||
| Depreciation | 29,319 | 20,544 | ||||||
| Other expenses | 54,285 | 88,547 | ||||||
| Total | $ | 417,000 | $ | 615,623 | ||||
We expect that our research and development expenses will increase as we continue to develop our products and services, field trials and recruit additional research and development employees.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of salaries and related expenses, share based compensation and other expenses.
The following table discloses the breakdown of selling and marketing expenses:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | $ | 30,450 | $ | 148,221 | ||||
| Share based compensation | (20,971 | ) | 64,229 | |||||
| Other expenses | 41,626 | 129,609 | ||||||
| Total | $ | 51,105 | $ | 342,059 | ||||
We expect that our selling and marketing expenses will increase as we continue to increase our selling and marketing efforts including commercial validation pilots and recruit additional employees or contractor to support our selling and marketing efforts in our targeted geographical areas.
General and Administrative Expenses
General and administrative expenses consist primarily of professional services, share based compensation and other non-personnel related expenses.
The following table discloses the breakdown of general and administrative expenses:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Professional services | $ | 443,883 | $ | 461,840 | ||||
| Share based compensation | 416,996 | 283,910 | ||||||
| Legal expenses | 67,492 | 125,753 | ||||||
| Other expenses | 141,738 | 133,396 | ||||||
| Total | $ | 1,070,109 | $ | 1,004,899 | ||||
| 54 |
Comparison of the Year Ended December 31, 2020 to the Year Ended December 31, 2019
Results of Operations
The following table summarizes our results of operations for the years ended December 31, 2020 and 2019, together with the changes in those items in dollars:
| Year Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Revenues from sales of products | $ | 232,274 | $ | 175,823 | ||||
| Cost of sales | (43,405 | ) | (144,548 | ) | ||||
| Gross profit | 188,869 | 31,275 | ||||||
| Research and development expenses | (417,000 | ) | (615,623 | ) | ||||
| Selling and marketing expenses | (51,105 | ) | (342,058 | ) | ||||
| General and administrative expenses | (1,070,109 | ) | (1,004,899 | ) | ||||
| Operating loss | (1,349,345 | ) | (1,931,305 | ) | ||||
| Finance income (expenses), net | (270,393 | ) | (43,408 | ) | ||||
| Other income, net | (2,532 | ) | - | |||||
| Share in losses of affiliated company | - | (15,690 | ) | |||||
| Gain on disposal of affiliated company | 15,690 | - | ||||||
| Net loss | (1,606,580 | ) | (1,990,403 | ) | ||||
| Less: Net loss attributable to non-controlling interests | 13,441 | 18,986 | ||||||
| Net loss attributable to the Company | (1,593,139 | ) | (1,971,417 | ) | ||||
| Loss per share | (1.05 | ) | (1.38 | ) | ||||
| Weighted average number of shares of Common Stock outstanding attributable to shareholders | 1,519,122 | 1,424,045 | ||||||
Revenues
Revenues for the year ended December 31, 2020 were $232,274, an increase of $56,451, or 32%, compared to total revenues of $175,823 for the year ended December 31, 2019. The increase is mainly a result of the Company’s sales of its new products, which the Company commenced in the fourth quarter of 2020.
We do not have backlogs or firm commitments from our customers for our products. Our sales might deteriorate if we fail to achieve commercial success or obtain regulatory approval of any of our products.
Cost of Sales
Cost of sales consists primarily of salaries, materials, transportation and overhead costs of manufacturing our products. Cost of revenues for the year ended December 31, 2020 was $43,405, a decrease of $101,143, or 70%, compared to total cost of revenues of $144,548 for the year ended December 31, 2019. The decrease is mainly a result of the decrease in salaries and related expenses, due to the fact that some of our employee are currently on a temporary leave without pay (furlough), due to the effects of COVID-19 on our business, and a decrease in the overall cost of materials, mainly as a result of inventory elimination made in 2019, due to our efforts to deploy our new solutions.
Gross Profit
Gross loss for the year ended December 31, 2020 was $188,869, an increase of $157,594, or 504%, compared to gross profit of $31,275 for the year ended December 31, 2019. The increase is mainly a result of the increase in revenues and the decrease in cost of revenues, as detailed above.
| 55 |
Research and Development Expenses
Research and development expenses consist of salaries and related expenses, share base compensation, consulting fees, service providers’ costs, related materials and overhead expenses. Research and development expenses for the year ended December 31, 2020 were $417,000, a decrease of $198,623, or 32%, compared to total research and development expenses of $615,623 for the year ended December 31, 2019. The decrease is mainly attributable to: (1) the decrease in professional fees, share based compensation and in payroll; and (2) the decrease in expenses associated with international travel and field trials which have been postponed due to COVID-19.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of salaries and related costs for selling and marketing personnel, travel related expenses and services providers. Selling and marketing expenses for the year ended December 31, 2020 were $51,105, a decrease of $290,954, or 85%, compared to total selling and marketing expenses of $342,058 for the year ended December 31, 2019. The decrease is mainly attributable to the decrease in payroll expenses and service providers used in relation to selling and marketing activities mainly associated with the termination of the employment of our former Vice President of Sales in February 2020.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related expenses including share based compensation and other non-personnel related expenses such as legal expenses and directors and insurance costs. General and administrative expenses for the year ended December 31, 2020 were $1,070,109, an increase of $65,210, or 6%, compared to total general and administrative expenses of $1,004,899 for the year ended December 31, 2019. The increase is mainly a result of the increase in share-based compensation to our service providers and directors offset partially by a decrease in professional fees.
Financing Expenses, Net
Financing expenses, net for the year ended December 31, 2020 were $270,393, an increase of $226,985, or 523%, compared to total financing expenses of $43,408 for the year ended December 31, 2019. The increase is mainly a result of compensation expenses related to the exchange of our convertible loans as well as accrued interest and amortization expenses related to such convertible loans.
Total Comprehensive Loss
As a result of the foregoing, our total comprehensive loss for the year ended December 31, 2020 was $1,593,139, compared to $1,971,417 for the year ended December 31, 2019, a decrease of $378,278, or 19%.
Impact of COVID-19
The global spread of COVID-19 led many countries, including the United States, Europe and Israel (where we maintain material operations), to impose stringent limitations on movement, gatherings, transit of passengers and goods and to close the borders between countries. The responses of governments have notably impacted many economies as well as capital markets worldwide.
Based on guidelines provided by the Israeli Government, employers (including us) are required to prepare and increase as much as possible the capacity and arrangement for employees to work remotely. In that regard, and in compliance with all applicable Israeli rules and guidelines, our offices have remained closed since the middle of March 2020, and all of our employees currently work remotely. In addition, as of the date of this Annual Report, some of our employees are on a temporary leave without pay (furlough), including our Chief Technology Officer, and we have postponed some of our planned field tests due to the current restriction on international travels. However, we did experience any material impact on our financial condition and results of operations due to COVID-19, and we do not expect to experience any material impact on our overall liquidity positions and outlook as a result of the outbreak. Nevertheless, it is not possible at this time to estimate the full impact that the COVID-19 pandemic, the continued spread of COVID-19, and any additional measures taken by governments, health officials or by us in response to such spread, could have on our business results of operations and financial condition.
| 56 |
Critical Accounting Policies and Estimates
We describe our significant accounting policies more fully in Note 2 to our consolidated financial statements included elsewhere in this Annual Report. We believe that the accounting policy described in Note 2 is critical in order to fully understand and evaluate our financial condition and results of operations.
We prepare our financial statements in accordance with U.S. GAAP. At the time of the preparation of the financial statements, our management is required to use estimates, evaluations and assumptions which affect the application of the accounting policy and the amounts reported for assets, obligations, income and expenses. Any estimates and assumptions are continually reviewed. The changes to the accounting estimates are credited during the period in which the change to the estimate is made.
Going Concern Uncertainty
The development and commercialization of our product will require substantial expenditures. We have not yet generated any material revenues and have incurred substantial accumulated deficit and negative operating cash flows. We currently have no sources of recurring revenue and are therefore dependent upon external sources for financing our operations. There can be no assurance that we will succeed in obtaining the necessary financing to continue our operations. As a result, our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Stock-based Compensation
Employees and other service providers of the Company may receive benefits by way of stock-based compensation settled with company options exercised for shares of our Common Stock. The cost of transactions with employees settled with capital instruments is measured based on the fair value of the capital instruments on the granting date. The fair value is determined using an accepted options pricing model. The model is based on share price, grant date and on assumptions regarding expected volatility, expected lifespan, expected dividend, and a no risk interest rate.
The cost of the transactions settled with capital instruments is recognized in profit or loss together with a corresponding increase in the equity over the period in which the performance and/or service takes place, and ending on the date on which the relevant employees are entitled to the benefits (the “Vesting Period”). The aggregate expense recognized for transactions settled with capital instruments at the end of each reporting date and until the Vesting Period reflects the degree to which the Vesting Period has expired and our best estimate regarding the number of warrants that have ultimately vested. The expense or income in profit or loss reflects the change of the aggregate expense recognized as of the end of the reported period.
Convertible Loans
The Company entered into a series of convertible loan agreements with certain lenders to sell convertible promissory notes containing conversion feature. The conversion feature is considered embedded derivative instruments, and is to be recorded at their fair value as its fair value can be separated from the convertible loan and its conversion is independent of the underlying note value. The Company recorded finance expenses in respect of the convertible component in the convertible loan in the excess amount of the convertible component fair value over the face loan amount. The conversion liability is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations.
The Company was using a third-party appraiser to estimate the fair value of the convertible component.
| 57 |
We selected the Black-Scholes-Merton (“Black-Scholes”), as our option pricing model to estimate the fair value of our options awards. The option-pricing model requires a number of assumptions:
Expected dividend yield — The expected dividend yield assumption is based on our historical experience and expectation of no future dividend payouts. We have historically not paid cash dividends and have no foreseeable plans to pay cash dividends in the future.
Volatility — Since the Company is not traded on any stock exchange market, quoted prices of our shares are unavailable. In case of insufficient historical data for a company, the expected volatility is based on similar companies’ stock volatility.
Risk free interest rate — The risk-free interest rate is based on the yield of governmental bonds with equivalent terms.
Contractual term — An option’s contractual term is the amount of time the holder has to exercise the option, per the contract.
Share price — The share price is determined according to the last known closing price of the share of our Common Stock at the grant date.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures. Since our inception through December 31, 2020, we have funded our operations principally with approximately $11,867,746 (net of issuance expenses) from the issuance of shares of shares of our Common Stock, options and loans.
The table below presents our cash flows for the periods indicated:
| Year
Ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Net cash used in operating activities | $ | (798,740 | ) | $ | (1,244,772 | ) | ||
| Net cash provided by (used in) investing activities | 9,065 | (81,655 | ) | |||||
| Net cash provided by financing activities | 741,760 | 1,177,436 | ||||||
| Decrease in cash and cash equivalents | $ | (47,915 | ) | $ | (148,991 | ) | ||
As of December 31, 2020, we had cash of $242,900, as compared to $290,815 as of December 31, 2019. As of December 31, 2020, we had a negative working capital of $290,062, as compared to a negative working capital of $199,212 as of December 31, 2019. The decrease in our cash balance is mainly attributable to our net loss described above and payments to account payables offset by our equity financing and convertible loans.
Operating Activities
Net cash used in operating activities was $798,740 for the year ended December 31, 2020, as compared to $1,244,772 for the year ended December 31, 2019.
| 58 |
Investing Activities
Net cash provided by investing activities was $9,065 for the year ended December 31, 2020, as compared to net cash used in investing activities of $81,655 for the year ended December 31, 2019. The increase is mainly attributable to the decrease in short term bank deposits in banking institutions and proceeds from investment in unconsolidated entity.
Financing Activities
Net cash provided by financing activities was $741,760 for the year ended December 31, 2020, as compared to $1,177,436 for the year ended December 31, 2019. The decrease is mainly the result of a decrease in equity financing and proceeds from convertible loans.
Financial Arrangements
Since our inception, we have financed our operation primarily through proceeds from sales of our shares of Common Stock, convertible loan agreements and grants from the IAA.
During December 2019, January 2020 and March 2020 we entered into a series of convertible loan agreements (the “December 2019 CLAs,” and each a “December 2019 CLA”) with third parties and certain existing shareholders (the “December 2019 Lenders”), pursuant to which the December 2019 Lenders agreed to provide the Company loans in the aggregate amount of $514,000 and in exchange the Company issued to the December 2019 Lenders (i) convertible promissory notes (the “December 2019 Notes”) and (ii) warrants with an exercise price of $8.40.
According to the terms of the December 2019 CLA, the December 2019 Notes bear interest at a rate of 5% per annum and the loan amount represented by the December 2019 Notes will be repaid to the December 2019 Lenders according to the following schedule: (i) the principal amount represented by the December 2019 Notes to be repaid in twenty four equal monthly installments, commencing on the twenty fifth month following the closing of each December 2019 CLA and (ii) the interest accrued on the loan amount to be paid in two bi-annual installments, commencing on the first anniversary of the first payment of the principal amount.
According to the terms of the December 2019 CLA, the outstanding loan amount matures on the earlier of (i) the third anniversary of each December 2019 CLA or (ii) a deemed liquidation event (as defined therein), and the December 2019 Lenders may convert all or any portion of the December 2019 Notes at any time prior to the one-year anniversary of each issuance into shares of Common Stock at a conversion price of $8.40 per share.
On June 24, 2020, we entered into a Securities Purchase Agreement (the “June 2020 SPA”) with the December 2019 Lenders in connection with the sale and issuance of 67,369 units, at a purchase price of $7.63 per unit. Each unit consists of: (i) one share of the Company’s Common Stock; and (ii) one warrant to purchase one share of Common Stock with an exercise price of $8.40. In connection with the June 2020 SPA, the Company issued to the December 2019 Lenders an aggregate of 67,369 shares of Common Stock and warrants to purchase an aggregate of 67,369 shares of Common Stock. The shares of Common Stock were issued on July 2, 2020.
Simultaneous with and conditioned upon the execution of the June 2020 SPA, the Company and each of the December 2019 Lenders, agreed to effectively cancel the December 2019 CLA and the equity securities issued thereunder. In connection therewith, each of the December 2019 Lenders, voluntarily waived any right to receive interest that accrued thereupon pursuant to the December 2019 CLAs.
On September 23, 2020, we entered into a Securities Purchase Agreement (the “September 2020 SPA”) with Medigus Ltd. (“Medigus”) in connection with the sale and issuance of 13,107 units, at a purchase price of $7.63 per unit, and for an aggregate purchase price of $100,000. Each unit consists of: (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of $1.20. In connection with the September 2020 SPA, the Company issued to Medigus an aggregate of 13,107 shares of Common Stock and warrants to purchase an aggregate of 13,107 shares of Common Stock. Furthermore, the September 2020 SPA contemplates an additional investment by Medigus not to exceed $25,000 (the “Additional Investment”), which investment shall be triggered following the parties’ initiation of a proof of concept procedure to test the effectiveness of the Company’s sanitizers and its residual effects against different pathogens. In consideration for the Additional Investment, the Company has agreed to issue an additional 3,277 units at a purchase price of $7.63, which units shall contain the same composition of securities as described in the aforementioned description of the September 2020 SPA.
| 59 |
During September 2020, we entered into a series of convertible loan agreements (each a “September 2020 CLA”) with certain lenders (the “September 2020 Lenders”), to sell convertible promissory notes with an aggregate principal amount of $125,000 (the “September 2020 Notes”). The September 2020 Notes will bear interest at a rate of 5% per annum. The outstanding loan amount will mature on the earlier of (i) the third anniversary of each September 2020 CLA or (ii) a deemed liquidation event (as defined therein). The loan amount represented by the September 2020 Notes will be repaid to the September 2020 Lenders according to the following schedule: (i) the principal amount represented by the September 2020 Notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each September 2020 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. The September 2020 Lenders may convert all or any portion of the September 2020 Notes into shares of Common Stock at any time prior to the closing of an underwritten public offering (the “Mandatory Conversion Event”), at a conversion price of $7.63 per share. In addition, the September 2020 Notes will be automatically converted into shares of Common stock immediately prior to a Mandatory Conversion Event, at a conversion price as shall be determined in connection with the Mandatory Conversion Event.
During October 2020, we entered into a series of convertible loan agreements (each a “October 2020 CLA”) with certain lenders (the “October 2020 Lenders”), to sell convertible promissory notes with an aggregate principal amount of $100,000 (the “October 2020 Notes”). The October 2020 Notes will bear interest at a rate of 5% per annum. The outstanding loan amount will mature on the earlier of (i) the third anniversary of each October 2020 CLA or (ii) a deemed liquidation event (as defined therein). The loan amount represented by the October 2020 Notes will be repaid to the October 2020Lenders according to the following schedule: (i) the principal amount represented by the notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each October 2020 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. The October 2020 Notes will be automatically converted into shares of Common stock immediately prior to a Mandatory Conversion Event, at a conversion price as shall be determined in connection with the Mandatory Conversion Event. In addition, the October 2020 Lenders may convert all or any portion of the notes into shares of Common Stock at any time prior to a Mandatory Conversion Event, at a conversion price of $7.63 per share.
During January 2021, we entered into a series of convertible loan agreements (each a “January 2021 CLA”) with certain lenders (the “January 2021 Lenders”), to sell convertible promissory notes with an aggregate principal amount of $274,000 (the “January 2021 Notes”). The January 2021 Notes bear interest at a rate of 5% per annum. The outstanding loan amount matures on the earlier of (i) the third anniversary of each January 2021 CLA or (ii) a deemed liquidation event (as defined therein). The loan amount represented by the January 2021 Notes will be repaid to the January 2021 Lenders according to the following schedule: (i) the principal amount represented by the notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each January 2021 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. The January 2021 Notes will be automatically converted into shares of Common stock immediately prior to a Mandatory Conversion Event, at a conversion price as shall be determined in connection with the Mandatory Conversion Event. In addition, the January 2021 Lenders may convert all or any portion of the notes into shares of Common Stock at any time prior to a Mandatory Conversion Event, at a conversion price of $7.63 per share.
As part of the September 2020 CLAs, the October 2020 CLAs and the January 2021 CLAs, we entered into a registration rights agreement with each of the September 2020 Lenders, October 2020 Lenders and the January 2021 Lenders, whereby each of such lenders received piggyback registration rights for the shares issuable upon conversion of the September 2020 Notes, October 2020 Notes and the January 2021 Notes to shares of Common Stock.
Current Outlook
We have financed our operations to date primarily through proceeds from sales of our shares of Common Stock. We have incurred losses and generated negative cash flows from operations since inception in 2009. Most of our revenues are currently generated in U.S. dollars from the sale of our products and services.
| 60 |
As of December 31, 2020, our cash and cash equivalents were $242,900. We expect that our existing cash and cash equivalents will be sufficient to fund our current operations until June 2021. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future capital requirements will depend on many factors, including:
| ● | the progress and costs of our studies and other research and development activities; | |
| ● | the costs of manufacturing our products; | |
| ● | the costs and timing of obtaining regulatory approval for our products; | |
| ● | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; | |
| ● | the potential costs of contracting with third parties to provide marketing and distribution services for us or for building such capacities internally; and | |
| ● | the magnitude of our general and administrative expenses. |
Until we can generate significant recurring revenues and profit, we expect to satisfy our future cash needs through debt or equity financings. We cannot be certain that additional funding will be available to us when needed, on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to our products and services. This may raise substantial doubts about our ability to continue as a going concern.
In its report on our financial statements for the year ended December 31, 2020, our independent registered public accounting firm included an explanatory paragraph stating that our recurring losses from operations since inception and our need for additional funding to finance our operations raises substantial doubt about our ability to continue as a going concern.
Off-Balance Sheet Arrangements
We do not currently have any off-balance sheet arrangements.
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2020:
| Total | Less
than 1 year | 1-3 years | 3-5 years | More
than 5 years | ||||||||||||||||
| Operating leases | $ | 15,049 | $ | - | $ | 15,049 | - | - | ||||||||||||
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our current investment policy is to invest available cash in bank deposits with banks that have a credit rating of at least A-minus. Accordingly, some of our cash and cash equivalents is held in deposits that bear interest. Given the current low rates of interest we receive, we will not be adversely affected if such rates are reduced. Our market risk exposure is primarily a result of foreign currency exchange rates, which is discussed in detail in the following paragraph.
Impact of Inflation and Currency Fluctuations
Our functional currency and reporting currency is the U.S. dollar. We incur some of our expenses in other currencies. As a result, we are exposed to the risk that the rate of inflation in countries in which we are active other than the United States will exceed the rate of devaluation of such countries’ currencies in relation to the dollar or that the timing of any such devaluation will lag behind inflation in such countries. To date, we have been affected by changes in the rate of inflation or the exchange rates of other countries’ currencies compared to the dollar, and we cannot assure you that we will not be adversely affected in the future.
| 61 |
The annual rate of inflation in Israel decreased by 0.7% in 2020 and 0.6% in 2019. The New Israeli Shekel (NIS) revaluated against the U.S. dollar and decreased by approximately 7% in 2020 and 8% in 2019.
item 7a. quantitative and qualitative disclosures about market risk
As a smaller reporting company, we are not required to provide the information required by this item.
item 8. financial statements and supplementary data
The information called for by Item 8 is included following the “Index to Financial Statements” on page F-1 of this Annual Report.
item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
item 9a. controls and Procedures
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure.
Our management, including our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures pursuant to Rules 13a-15(e) and 15d-15(e) under the Exchange Act as of December 31, 2020, the end of the period covered by this Annual Report. Based on such evaluation, and due to certain material weaknesses identified by management, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were not effective at a reasonable assurance level as of December 31, 2020 due to (1) the size of the Company and available resources and limited personnel to assist with the accounting and financial reporting function, which results in a lack of segregation of duties (2) lack of a full time Chief Executive Officer and Chief Financial Officer that can oversee day to day operations and the financial reporting function and (3) lack of an Independent Audit Committee that can provide management oversight.
Management’s Report on Internal Control over Financial Reporting
This Annual Report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the company’s registered public accounting firm due to a transition period established by rules of the U.S. Securities and Exchange Commission, or the SEC, for newly public companies.
Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of our registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting or in other factors identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during the period covered by this Annual Report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
| 62 |
Item 10. Directors, Executive Officers and corporate governance
Directors and Executive Officers
The following table sets forth the names and ages of our directors and executive officers, as of March 29, 2021:
| Name | Age | Position | ||
| Prof. Benad Goldwasser (1) (8) | 69 | Chairman of the Board of Directors | ||
| David Palach | 55 | Chief Executive Officer | ||
| Dan Sztybel | 43 | Chief Executive Officer of Save Foods Ltd. | ||
| Shlomo Zakai (2) | 51 | Chief Financial Officer | ||
| Nimrod Ben Yehuda (3) | 67 | Chief Technology Officer | ||
| Dr. Neta Matis | 37 | Vice President of Research and Development | ||
| Dr. Arthur Dawson | 78 | Business Manager of U.S. Operations | ||
| Vered Raz-Avayo (4) (5) (7) | 49 | Director | ||
| Ronen Rosenbloom (5) (6) | 48 | Director | ||
| Israel Berenshtein (5) (6) | 49 | Director | ||
| Amitay Weiss (5) (7) | 58 | Director | ||
| Eliahou Arbib (5) (8) | 54 | Director | ||
| Udi Kalifi (5) | 42 | Director Nominee |
| (1) | Prof. Benad will resign from his position as Chairman of the board of directors of Save Foods, Inc. upon the closing of the Current Offering. We expect Prof. Benad to be nominated as Chairman of the board of directors of Save Foods Ltd. following his resignation. |
| (2) | Mr. Zakai will resign from his position as Chief Financial Officer of the Company upon the closing of the Current Offering. We expect Mr. Zakai to be appointed as Controller of the Company following his resignation. |
| (3) | Mr. Ben Yehuda has been on a temporary leave without pay (furlough) since March 2020. |
| (4) | Ms. Raz-Avayo will resign from her position as a member of the board of directors of the Company upon the closing of the Current Offering. We expect Ms. Raz-Avayo to be appointed as Chief Financial Officer of the Company following the resignation of our current Chief Financial Officer. |
| (5) | Independent Director |
| (6) | Member of Class I with a term ending at the 2021 annual meeting of stockholders. |
| (7) | Member of Class II with a term ending at the 2022 annual meeting of stockholders. |
| (8) | Member of Class III with a term ending at 2023 annual meeting of stockholders. |
Prof. Benad Goldwasser, Chairman of the Board of Directors
Prof. Goldwasser has served as chairman of our board of directors since May 2018. He has also served as chairman of the board of directors of our subsidiary, Save Foods Ltd., since May 2018. Prof. Goldwasser has served as chairman of the board of directors of ScoutCam Inc (OTC: SCTC) since March 2019, and as a member of the board of directors of Innoventric Ltd. since September 2017. Prior to that, Prof. Goldwasser has served as chairman of the board of directors of Medigus Ltd. (Nasdaq and TASE: MDGS) from September 2018 to December 2019, and as a consultant to Shanghai-Israel
Investment Fund from May 2016 to May 2019. In 2016, Prof. Goldwasser launched a venture capital fund partnered with Shanghai Alliance Investment Ltd (SAIL), a Shanghai Government investment company. Prof. Goldwasser has also served on the board of directors of BioCanCell Ltd. (TASE: BICL) from 2013 to 2016. Prof. Goldwasser holds an MD and MBA from Tel-Aviv University. We believe that Prof. Goldwasser is qualified to serve on our board of directors because of his leadership in conceptualizing and developing our brand and business, his entrepreneurship skills and his 27 years of experience building businesses.
| 63 |
David Palach, Chief Executive Officer
Mr. David Palach has served as our chief executive officer since January 2021. Mr. Palach previously served as our co-chief executive officer from November 5, 2020 to January 21, 2021. Mr. Palach has owned and served as chief executive officer of S.T. Sporting LTD and Sun Light Lightning Solutions LTD, companies operating in the environmental industry since 2009 and 2015, respectively. Mr. Palach holds a BBA in Accounting from Baruch College/City University of New York and completed a Directors Course at Bar Ilan University in Israel. Mr. Palach previously maintained a certified public accounting license in the State of Maryland.
Dan Sztybel, Chief Executive Officer of Save Foods Ltd.
Mr. Sztybel has served as the chief executive officer of Save Foods Ltd. since April 2019. Mr. Sztybel previously served as the chief executive officer of the Company from April 2019 to January 2021. Prior to this, Mr. Sztybel has served as our vice president of business development from October 2018 to March 2019. Prior to joining us, Mr. Sztybel has served as principal at Goldmed Ltd., a consulting firm from September 2016 to September 2018. Mr. Sztybel is the founder of Dan Sztybel Consulting Group, a boutique firm advising global leaders and emerging startups in the healthcare field on strategy, partnerships, and investments and has served as its managing director/ since November 2014. Mr. Sztybel is also the co-founder of MyndYou, a digital health-tech company. Prior to that, Mr. Sztybel led the life sciences and healthcare advisory team at Kost, Forer, Gabbay & Kasierer, a member firm of Ernst & Young Global from July 2007 to November 2014. Mr. Sztybel received his B.Sc. and M.Sc. in molecular biology and biotechnology from Bar-Ilan University. Mr. Sztybel also completed a special EY-Kellogg-Recanati business program for employee excellence.
Shlomo Zakai, Chief Financial Officer
Mr. Zakai has served as our chief financial officer since August 2017. Mr. Zakai has served as chief financial officer of UAS Drone Corp. (OTC: USDR) since May 2020, as chief financial officer of Sonovia Ltd. from October 2014 to August 2020, as chief financial officer of Blue Sphere Corporation (OTC: BLSP) from January 2012 to May 2016, and as chief financial officer of Todos Medical Ltd. (OTC: TOMDF) from February 2017 to December 2017. Mr. Zakai has established Shlomo Zakai CPA in in 2004, an accounting firm providing a range of services to publicly traded and private companies. He also previously worked as an accountant for nine years at Kost, Forer, Gabbay & Kasierer, an independent registered public accounting firm and a member firm of Ernst & Young Global, where he last served as a senior manager and worked with technology companies publicly traded on Nasdaq and in Israel. Mr. Zakai holds a BA in Accounting from the College of Management in Rishon Le’Zion, Israel, and is a certified public accountant in Israel.
Nimrod Ben Yehuda, Chief Technology Officer
Mr. Ben Yehuda has served as our chief technology officer since our inception in 2009. Mr. Ben Yehuda has also served as our chief executive officer from March 2014 to May 2017 and from February 2018 to September 2019 and served on our board of directors from August 2005 to October 2019. Mr. Ben Yehuda has been a leading entrepreneur in the area of environmentally friendly solutions using hydrogen peroxide in many applications. Mr. Ben Yehuda is the founder of our subsidiary, Save Foods, Inc. (formerly Pimi Marion Holdings Ltd. and Pimi Agro CleanTech Ltd.), which was established to exploit the knowledge, intellectual property and business assets of Nir Ecology Ltd.
Dr. Neta Matis, Vice President of Research and Development
Dr. Matis has served as our vice president of research and development since January 2019. Prior to joining us, Dr. Matis has served in various roles at Virdia Inc. including head of process development from January 2017 to October 2018 and research and development chemist from January 2012 to December 2017. Dr. Matis has a proven track record of leading innovation and proficiency in developing refinery-scale agricultural processes. Dr. Matis holds a PhD in Chemistry and an MBA from Tel Aviv University.
| 64 |
Dr. Arthur (Art) Dawson, Business Manager of U.S. Operations
Dr. Dawson has served as our business manager of U.S. operations since January 2020. Since March 2001, Dr. Dawson has served as the president of The Dawson Company, a boutique consulting firm with expertise in product commercialization and market development for shelf-life extension products in the post-harvest market, Previously, Dr. Dawson held several key positions in the post-harvest industry, including the general manager of Decco Worldwide from December 1989 to June 1999, one of the largest post-harvest service company worldwide, the vice president postharvest fungicides of Ecogen, Inc. from June 1999 to February 2001, as well as different managerial position at Sunkist Growers, Inc. from October 1973 to November 1983 and Dole Fresh, Inc. from November 1983 to November 1989. Dr. Dawson holds a master’s degree in Plant Science and a Ph.D. in Plant Physiology from the University of California, Riverside. Dr. Dawson also holds a license as a pest control advisor in California.
Vered Raz-Avayo, Director
Ms. Raz-Avayo has served as a member of our board of directors since August 2018. Ms. Raz-Avayo is a business strategy and a financial consultant. She has served on the board of directors of Foresight Autonomous Holdings Ltd. (TASE and Nasdaq: FRSX) since July 2017, Apollo Power Ltd. (TASE: APLP) since December 2017 and Africa Israel Residences Ltd. (TASE: AFRE) since November 2012. Prior to that, Ms. Raz-Avayo served as chief financial officer at one of the companies under the Levayev group from December 1999 to April 2010. In addition, during the last 12 years, Ms. Raz-Avayo served on the board of directors of S.R Accord LTD (TASE: SRAC) since July 2010 until July 2016, Analyst I.M.S mutual funds management (1986) LTD since May 2012 until May 2017, Safe -T group LTD ( Nasdaq: SFET) since March 2016 until March 2019, Naaman group (n.v) LTD since July 2012 until July 2017, TMDA LTD since June 2016 until June 2020. Ms. Raz-Avayo holds a BA in Business Administration – Accounting and Finance, from the College of Management, and an MFA in Film, TV and Screenwriting from the Faculty of Arts of the Tel-Aviv University. Ms. Raz-Avayo is also a certified public accountant in Israel. Ms. Raz-Avayo was selected to serve as a member of our board of directors due to her extensive managerial and consulting experience in finance encompassing a wide range of industries in Israel and overseas.
Ronen Rosenbloom, Director
Mr. Rosenbloom has served as a member of our board of directors since August 2020. Mr. Rosenbloom is an independent lawyer and has been working out of a self-owned law firm specializing in white collar offences since 2004. Mr. Rosenbloom has served on the board of directors of Medigus Ltd. (Nasdaq and TASE: MDGS) since September 2018 and ScoutCam Inc. (OTC: SCTC) since December 2019. Prior to that, Mr. Rosenbloom served as chairman of the Israeli Money Laundering Prohibition committee and the Prohibition of Money Laundering Committee of the Tel Aviv District, both of the Israel Bar Association from November 2015 to December 2019. Mr. Rosenbloom holds an LL.B. from the Ono Academic College, an Israeli branch of University of Manchester. We believe that Mr. Rosenbloom is qualified to serve on our board of directors because of his business experience and expertise and background with regard to legal matters.
Israel Berenshtein, Director
Mr. Berenshtein has served as a member of our board of directors since August 2020. Mr. Berenshtein has also served on the board of directors of Chrion Refineries Ltd. (TASE: CHR) since May 2019 and recently started working as a lawyer in Ben Yakov, Shvimer , Dolv – Law Office. He previously served in the legal department of Sonol Israel Ltd. since April 2010 to December 2020. Before that, Mr. Berenshtein worked as a commercial lawyer and litigator for a leading Israeli law firm from July 2000 to April 2010. Mr. Berenshtein earned an LL.B. in law and an M.A. in political science from Bar Ilan University, Israel. Mr. Berenshtein was admitted to the Israel Bar Association in 2000. We believe that Mr. Berenshtein is qualified to serve on our board of directors due to his extensive legal experience.
Amitay Weiss, Director
Mr. Weiss has served as a member of our board of directors since August 2020. Mr. Weiss has also served as chairman of the board of directors of P.L.T Financial Services Ltd. (TASE: PLTP) since April 2016 and Matomy Media Group Ltd. (LSE: MTMY, TASE: MTMY.TA) since May 2020, as a member of the board of directors of Cofix Group Ltd. (TASE: CFCS) since August 2015, Ziron Ltd. since June 2019, Algomizer Ltd. (TASE: GIX) since March 2019, and Cyntar Ventures Inc. since August 2019, and as a member of the board of directors and chief executive officer of Therapix Biosciences Ltd. (OTC: TRPXY) since August 2020. In April 2016, Mr. Weiss founded Amitay Wiss Management Ltd., an economic consulting company and now serves as its chief executive officer. Mr. Weiss holds a B.A in economics from New England College, M.B.A. in business administration from Ono Academic College in Israel, an Israeli branch of University of Manchester and LL.B from the Ono Academic College. We believe that Mr. Weiss is qualified to serve on our board of directors because of his diverse business, management and leadership experience.
| 65 |
Eliahou Arbib, Director
Mr. Arbib has served as a member of our board of directors since January 2021. Mr. Arbib has also served as chairman of the board of directors of Chiron Refineries Ltd. (TASE: CHR) since September 2016. He has also the current owner and manager of Eliahou Arbib Law Offices, since May 2013. Prior to that, from 1993 until 2000, Mr. Arbib was the managing director of AA Arbib Agriculture Supply Ltd. Mr. Arbib holds an LLB from the Law and Business Academic Center of Ramat Gan, Israel. Mr. Arbib has been an active member of the Israeli Bar Association since 2013, and served as deputy chairman of the Security and Defense Committee of the Israeli Bar Association since 2014. We believe that Mr. Weiss is qualified to serve on our board of directors because of his legal experience as well as experience in the field of agriculture.
Udi Kalifi, Director Nominee
Mr. Klifi has agreed to serve on our board of directors subject to the consummation of the Current Offering. Mr. Kalifi is the owner and manager of Udi Kalifi Law Officer since 2006. He has also served as a member of the board of directors of Matomi Media Group Ltd. (TASE: MTMY) since May 2020. Mr. Kalifi holds an LLB, BSc in Accounting and LLM from the Tel Aviv University, Israel and a master’s degree in law and economics from the University of Bologna, Humbourg and Roterdam. Mr. Kalifi has been an active member of the Israeli Bar Association since 2006. Mr. Kalifi was selected to serve as a member of our board of directors due to his legal and finance experience.
Family Relationships
There are no family relationships among any of our directors or executive officers.
Composition of Board of Directors
Our board of directors will consist of six directors. Our directors are appointed by the board of directors at the annual general meeting. Each director’s term will continue until the annual meeting of the stockholders held following his or her election and the election and qualification of his or her successor, or his or her earlier death, disqualification, resignation or removal.
When considering whether directors have the experience, qualifications, attributes or skills, taken as a whole, to enable our board of directors to satisfy its oversight responsibilities effectively in light of our business and structure, the board of directors focuses primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business.
Arrangements for Election of Directors and Members of Management
There are no arrangements or understandings with major stockholders, customers, suppliers or others pursuant to which any of our executive management or our directors were selected.
Committees of the Board of Directors
Our board of directors directs the management of our business and affairs, as provided by Delaware law, and conducts its business through meetings of the board of directors and standing committees. We intend to establish a standing audit committee, nominating and corporate governance committee and compensation committee following the consummation of the Current Offering. In addition, from time to time, special committees may be established under the direction of the board of directors when necessary to address specific issues.
| 66 |
Risk Oversight
Our board of directors is responsible for overseeing our risk management process. Our board of directors focuses on our general risk management strategy, the most significant risks facing us, and oversees the implementation of risk mitigation strategies by management. Our audit committee is also responsible for discussing our policies with respect to risk assessment and risk management. Our board of directors believes its administration of its risk oversight function has not negatively affected our board of directors’ leadership structure.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is or has been an officer or employee of the Company. None of our executive officers serves as a member of the board of directors or compensation committee (or other committee performing equivalent functions) of any entity that has one or more of its executive officers serving on our board of directors or compensation committee.
Code of Business Conduct and Ethics
Prior to the completion of the Current Offering, we will adopt a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the code will be posted on our website, www.savefoods.co. In addition, we intend to post on our website all disclosures that are required by law or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the code. The information on any of our websites is deemed not to be incorporated in this Annual Report.
item 11. Executive Compensation
Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officers for the years ended December 31, 2020 and 2019.
| Name and principal position | Fiscal Year | Salary ($) | Bonus ($) | Stock awards ($) | Option awards ($) | All other compensation ($) | Total ($) | |||||||||||||||||||||
| David Palach | 2020 | 14,667 | - | - | - | - | 14,667 | |||||||||||||||||||||
| Chief Executive Officer (1) | - | - | - | - | - | - | - | |||||||||||||||||||||
| Dan Sztybel | 2020 | 120,282 | - | - | 135,778 | - | 256,060 | |||||||||||||||||||||
| Former co-Chief Executive Officer, Chief Executive Officer of Save Foods Ltd (2) | 2019 | 137,688 | - | - | 124,081 | - | 261,769 | |||||||||||||||||||||
| Shlomo Zakai | 2020 | 55,721 | - | - | 9,986 | - | 65,707 | |||||||||||||||||||||
| Chief Financial Officer | 2019 | 64,310 | - | - | 32,291 | - | 96,601 | |||||||||||||||||||||
| Nimrod Ben Yehuda | 2020 | 25,304 | - | - | - | - | 25,304 | |||||||||||||||||||||
| Chief Technology Officer | 2019 | 117,014 | - | - | - | - | 117,014 | |||||||||||||||||||||
| Neta Matis | 2020 | 75,202 | - | - | 94,474 | - | 169,676 | |||||||||||||||||||||
| VP Research and Development | 2019 | 44,444 | - | - | 30,235 | - | 74,679 | |||||||||||||||||||||
| (1) | Mr. Palach was appointed as the Company’s co-Chief Executive Officer on November 5, 2020, and as the Company’s Chief Executive Officer of the Company on January 11, 2021, upon the resignation of Mr. Sztybel. |
| (2) | Mr. Sztybel resigned from his position as co-Chief Executive Officer of the Company effective January 11, 2021 and continue serving as the Chief Executive Officer of the subsidiary Save Foods Ltd. |
| 67 |
Outstanding Equity Awards at Fiscal Year-End
The following table provides information about the number of outstanding equity awards held by each of our named executive officers as of December 31, 2020:
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Name | Number
of Securities Underlying Unexercised Options (Exercisable) | Number
of Securities Underlying Unexercised Options (Unexercisable) | Option Exercise Price | Option Expiration Date | Equity Incentive Plan Awards: Number of Unearned Shares That Have Not Vested | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares That Have Not Vested | ||||||||||||||||||
| David Palach | - | - | - | - | - | - | ||||||||||||||||||
| Chief Executive Officer | - | - | - | - | - | - | ||||||||||||||||||
| Dan Sztybel | 10,715 | 3,571 | 3.15 | 1/3/2029 | - | - | ||||||||||||||||||
| Former co- Chief Executive Officer | 16,667 | 11,905 | 3.15 | 4/2/2029 | ||||||||||||||||||||
| 7,143 | 21,429 | 3.78 | 7/1/2030 | |||||||||||||||||||||
| Shlomo Zakai | 6,349 | 3,175 | 3.15 | 1/3/2029 | - | - | ||||||||||||||||||
| Chief Financial Officer | ||||||||||||||||||||||||
| Nimrod Ben Yehuda | - | - | - | - | - | - | ||||||||||||||||||
| Chief Technology Officer | ||||||||||||||||||||||||
| Neta Matis | 4,167 | 2,976 | 3.15 | 1/3/2029 | - | - | ||||||||||||||||||
| VP Research and Development | 2,976 | 4,167 | 3.15 | 10/31/2029 | ||||||||||||||||||||
| 5,286 | 15,857 | 3.15 | 6/23/2030 | |||||||||||||||||||||
| Arthur Dawson | - | - | - | - | - | - | ||||||||||||||||||
| Business Manager of U.S. Operations | - | - | - | - | - | - | ||||||||||||||||||
| 68 |
Employment Agreements with Executive Officers
We, and through our Israeli subsidiary, have entered into written employment agreements with certain of our executive officers. Upon the closing of the Current Offering, we intend to enter into written employment and service agreement with each of our executive officers. Such employment and service agreement will contain customary provisions and representations, including confidentiality, non-competition, non-solicitation and inventions assignment undertakings by the executive officers. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we entered into agreements with each executive officer and director pursuant to which we will indemnify each of them up to a certain amount and to the extent that these liabilities are not covered by directors and officers insurance.
Services Agreement with Dan Sztybel as Chief Executive Officer of Save Foods Ltd.
On October 10, 2018, we entered into a service agreement (as amended on March 28, 2019, the “Save Foods Ltd. CEO Services Agreement”), with Mr. Sztybel and Dan Sztybel Consulting Group Ltd., a consulting services company owned and controlled by Mr. Sztybel, pursuant to which Mr. Sztybel provides us with services as the chief executive officer of Save Foods Ltd. Pursuant to the terms of the Save Foods Ltd. CEO Services Agreement, Mr. Sztybel is currently entitled to a monthly fee in the amount of NIS 47,125 (approximately $14,500) plus value added tax and car allowance in the amount of NIS 3,250 (approximately $1,000) plus value added tax per month. In addition, under the Save Foods Ltd. CEO Services Agreement, Mr. Sztybel was granted options to purchase shares of our Common Stock, including the following:
| (a) | Options to purchase up to 14,286 shares of Common Stock, under our Equity Incentive Plan, in the event that we will receive EPA and FDA approvals by the end of the second quarter of 2020. Such conditions were not met as of June 30, 2020. | |
| (b) | Options to purchase up to 28,572 shares of Common Stock, under our Equity Incentive Plan. |
Both parties may terminate the Save Foods Ltd. CEO Services Agreement at any time for any reason upon a 30-day prior written notice.
Commencing April 2020, the Company and Mr. Sztybel agreed to temporarily reduce the monthly fixed fee to $9,000 per month.
Consulting Agreement with David Palach as Chief Executive Officer
On November 6, 2020, we entered into a consulting agreement with S.T Sporting (1996) Ltd., for the services of David Palach (the “CEO Consulting Agreement”). Pursuant to the terms of the CEO Consulting Agreement, Mr. Palach provides us services as chief executive officer. Pursuant to the terms of the CEO Consulting Agreement, Mr. Palach is currently entitled to a monthly fee in the amount of $8,000 plus value added tax per month and a grant of options to purchase shares of our Common Stock, which amount shall be determined by good faith negotiations by the board of directors on a future date.
| 69 |
Engagement with Shlomo Zakai for Accounting Services
Mr. Zakai is acting as our chief financial officer and our engagement with him is based on a price offer dated September 28, 2017, pursuant to which Mr. Zakai provides us accountant services. Pursuant to the terms of such price offer, Mr. Zakai is entitled to a fixed fee for several services as well as compensation according to an hourly rate. For 2019, the scope of our engagement with Mr. Zakai was in an amount of approximately $64,310.
Commencing January 2020, the Company and Mr. Zakai agreed on a monthly fixed fee of $6,500 per month. Commencing April 2020, the Company and Mr. Zakai Agreed to temporarily reduce the monthly fixed fee to $4,000 per month. For the year ended December 31, 2020, the scope of our engagement with Mr. Zakai was in an amount of approximately $55,721.
Services Agreement with Neta Matis as Vice President of Research and Development
On January 15, 2019, we entered into a service agreement (the “VP Research and Development Services Agreement”), with NSNC Consulting Ltd. (the “Contractor”), pursuant to which Ms. Neta Matis, on behalf of the Contractor, provides us with services as our VP of research and development. Pursuant to the terms of the VP Research and Development Service Agreement, Ms. Matis provides the services in a part time position scope of 50% and is entitled to a monthly fee in the amount of NIS 15,000 (approximately $4,615) plus value added tax. In addition, Ms. Matis will be granted options to purchase up to 7,143 shares of our Common Stock. Such options shall vest over a 36-month period commencing in January 2019 as follows: (i) options to purchase up to 2,381 shares of Common Stock shall vest following the initial 12-month period as of the date of grant, (ii) options to purchase up to 4,762 shares of Common Stock shall vest following the lapse of each additional three-month period thereafter. All other terms and conditions of the options shall be consistent to those applicable under our Equity Incentive Plan. Commencing January 2020, Ms. Matis became a full-time employee and is entitled to a monthly fee in the amount of NIS 30,000 (approximately $9,230) plus value added tax. Commencing April 2020, the Company and Ms. Matis agreed to temporarily reduce the monthly fixed fee to $6,200 per month.
Director Compensation
Summary Compensation Table
The following table sets forth the compensation we paid our non-executive directors during the fiscal year ended December 31, 2020.
| Name | Fees earned or paid in
cash | Stock awards ($) | Option awards ($) | All other compensation ($) | Total ($) | |||||||||||||||
| Prof. Benad Goldwasser | 131,764 | - | 232,530 | - | 364,294 | |||||||||||||||
| Vered Raz-Avayo | - | - | 16,462 | - | 16,462 | |||||||||||||||
| Israel Berenshtein | - | - | - | - | - | |||||||||||||||
| Ronen Rosenbloom | - | - | - | - | - | |||||||||||||||
| 70 |
Service Contracts with Non-Executive Directors
Except for the following agreements, we do not have any written agreements with any of non-officer directors.
Corporate Advisory Consulting Agreement with Goldmed Ltd.
On August 30, 2017, we entered into a corporate advisory consulting agreement (the “Goldmed Agreement”), with Goldmed Ltd., a company wholly owned by Prof. Benad Goldwasser, who currently serves as our active chairman of the board of directors. Pursuant to the Goldmed Agreement, Prof. Goldwasser provides us with services since May 1, 2018. Under the terms of the Goldmed Agreement, Prof. Goldwasser is entitled to a monthly fee in the amount of $5,000, of which $2,000 will acrrue as debt of the Company to Prof. Goldwasser until the Company raises $1,000,000, and to a monthly fee in the amount of $10,000 after the Company will raise more than $2,000,000. The Goldmed Agreement will continue to be in effect unless terminated by either party by providing a 90-day prior written notice. In addition. The Goldmed Agreement provides that for its services as director of the Company’s subsidiary, Save Foods Ltd., the Company will issue to Prof. Goldwasser, under the Equity Incentive Plan, warrants to purchase up to 14,286 shares of our Common Stock at an exercise price of $2.1. Such warrants will vest quarterly over a period of 36 months and will be accelerated in full if we terminate the Goldmed Agreement for no cause prior to the end of the vesting period.
On January 3, 2019, we updated the terms and conditions of the Goldmed Agreement, which now provides that Prof. Goldwasser is entitled to a monthly fee in the amount of $10,000 and a monthly car allowance of $2,000, which will be triggered when the Company raises $1 million, and of which will commence on the date Prof. Goldwasser was elected to serve as a member of the board of directors of the Company. The fee is included in the fees payable to Prof. Goldwasser as a director.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder matters
Security Ownership of Certain Beneficial Owners and Management
The table below provides information regarding the beneficial ownership of our Common Stock as of March 29, 2021 of (i) each of our current directors, (ii) each of the Named Executive Officers, (iii) all of our current directors and officers as a group, and (iv) each person (or group of affiliated persons) known to us who owns more than 5% of our outstanding Common Stock.
The beneficial ownership of our Common Stock is determined in accordance with the rules of the SEC. Under these rules, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. For purposes of the table below, we deem shares of Common Stock issuable pursuant to options that are currently exercisable or exercisable within 60 days of December 31, 2019, if any, to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person. The percentage of shares of Common Stock beneficially owned is based on 10,209,487 shares of Common Stock outstanding as of December 31, 2019.
Unless otherwise indicated below, the address for each beneficial owner listed in the table below is c/o Save Foods, Inc., Kibbutz Alonim, Israel, 3657700.
| Owner | Number of Shares Beneficially Owned(1) | Percentage Beneficially Owned(1) | ||||||
| 5% or more shareholders: | ||||||||
| Amir Uziel / Amir Uziel Economic Consultant Ltd.(2) | 115,512 | 7.1 | % | |||||
| L.I.A Pure Capital Ltd. (3) | 104,938 | 6.5 | % | |||||
| Nimrod Ben Yehuda / Nir Ecology Ltd.(4) | 99,890 | 6.2 | % | |||||
| Nir Reinhold / Buffalo Investments Ltd.(5) | 111,453 | 6.9 | % | |||||
| YAAD Consulting & Management Services (1995) Ltd.(6) | 115,831 | 7.1 | % | |||||
| Directors and named executive officers who are not 5% holders: | ||||||||
| Amitay Weiss(*) | - | - | % | |||||
| Arthur Dawson | - | - | % | |||||
| Prof. Benad Goldwasser(*)(7) | 87,302 | 5.3 | % | |||||
| Dan Sztybel | 44,843 | 2.7 | % | |||||
| David Palach | - | - | % | |||||
| Israel Berenshtein(*) | - | - | % | |||||
| Ronen Rosenbloom(*) | - | - | % | |||||
| Shlomo Zakai | 7,143 | ** | % | |||||
| Vered Raz-Avayo(*) | 5,179 | ** | % | |||||
| Eliahou Arbib(*) | - | - | ||||||
| All directors and named executive officers as a group (11 persons) | 244,357 | 14.5 | % | |||||
| 71 |
| * | Indicates director of the Company. |
| ** | Indicates beneficial ownership of less than 1% of total Common Stock outstanding. |
| (1) | The percentages shown are based on 1,606,760 Common Stock issued and outstanding as of the date of March 29, 2021. |
| (2) | Includes 6,981 shares held by Amir Uziel, and 85,594 shares held by Amir Uziel Economic Consultant Ltd. Amir Uziel is the owner of Amir Uziel Economic Consultant Ltd. and has voting and dispositive power over the shares held by Amir Uziel Economic Consultant Ltd. |
| (3) | Kfir Zilberman is the Owner of L.I.A Pure Capital Ltd. and has the voting and dispositive power over the shares held by L.I.A Pure Capital Ltd. |
| (4) | Includes 14,146 shares held by Nimrod Ben Yehuda, and 85,744 shares held by Nir Ecology Ltd. Nimrod Ben Yehuda is the owner of Nir Ecology Ltd. and has voting and dispositive power over the shares held by Nir Ecology Ltd. |
| (5) | Includes 16,191 shares held by Nir Reinhold, and 82,155 shares held by Buffalo Investments Ltd. Nir Reinhold is the owner of Buffalo Investments Ltd. and has voting and dispositive power over the shares held by Buffalo Investments Ltd. |
| (6) | Includes 3,175 shares held by Itschak Shrem, and 92,995 shares held by YAAD Consulting & Management Services (1995) Ltd. Itschak Shrem is the owner of YAAD Consulting & Management Services (1995) Ltd. and has the voting and dispositive power over the shares held by YAAD Consulting & Management Services (1995) Ltd. |
| (7) | Includes 30,179 shares held by Prof. Benad Goldwasser and 57,143 shares held by Anat Tamir Lotan, a member of Prof. Benad’s immediate family sharing the same household. |
Item 13. Certain relationships and related transactions, and director independence
Related Party Transactions
The following is a description of transactions since January 1, 2018, to which we were a party or will be a party, in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years, and any of our directors, executive officers or holders of more than 5% of our outstanding capital stock, or any immediate family member of, or person sharing the household with, any of these individuals or entities, had or will have a direct or indirect material interest.
Employment Agreements
Certain of our executive officers have employment and service agreements with us. Upon the closing of the Current Offering, we intend to enter into written employment and service agreement with each of our executive officers. Such employment and service agreement will contain customary provisions and representations, including confidentiality, non-competition, non-solicitation and inventions assignment undertakings by the executive officers. However, the enforceability of the noncompetition provisions may be limited under applicable law. See “Management—Employment Agreements with Executive Officers” and “— Service Contracts with Non-Executive Directors.”
| 72 |
Indemnification Agreements
We entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us or will require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer. For further information, see “Executive and Director Compensation—Limitations of Liability and Indemnification.”
Consulting and Service Agreements
Consulting Agreement with Amir Uziel
On March 2, 2021, we entered into a consulting agreement with Amir Uziel (the “Amir Uziel Consulting Agreement”), effective as of January 1, 2021, pursuant to which, Mr. Amir Uziel, a holder of greater than 5% of our Common Stock, provides us with consulting services. Pursuant to the terms of the Amir Uziel Consulting Agreement, Amir Uziel is entitled to a fee in the amount of $100 per hour up to a maximum of 60 hours. The Amir Uziel Consulting Agreement will terminate on December 31, 2021.
Corporate Advisory Consulting Agreement with Nir Ecology Ltd.
On August 30, 2017, we entered into a corporate advisory consulting agreement with Nir Ecology Ltd. (the “Nir Ecology Agreement”), pursuant to which, Mr. Ben Yehuda, our chief technology officer, provided us with services since September 1, 2017. Pursuant to the terms of the Nir Ecology Agreement, Nir Ecology Ltd. was entitled to a monthly fee in the amount of $3,000. The Nir Ecology Agreement remained in effect until August 31, 2019.
Loan Agreements
Loan Agreement with Nimrod Ben Yehuda
On February 26, 2019, we entered into a loan agreement (the “Ben Yehuda Loan Agreement”), with Mr. Nimrod Ben Yehuda, our founder and former director of Nir Ecology Ltd., a company wholly owned by Mr. Ben Yehuda, pursuant to which we extended Nir Ecology Ltd. with a loan in an aggregate amount of NIS 50,000 (approximately $14,021). The loan amount bears interest at an annual compounded rate of four percent from the date the loan was actually provided and until the repayment date.
On March 18, 2019, we entered into a settlement agreement (the “Settlement Agreement”), pursuant to which we agreed to repay Mr. Ben Yehuda NIS 263,929 (the “Ben Yehuda Debt”) that we owed him for his services rendered as chief executive officer of Nir Ecology Ltd. from 2014 through the date of the Settlement Agreement. Pursuant to the Settlement Agreement, we agreed to offset an amount of NIS 50,000 from the Ben Yehuda Loan Agreement and to repay the remaining balance of the Ben Yehuda Debt in 12 monthly payments starting in August 2021. The Ben Yehuda Debt will not bear interest and may be prepaid in whole or in part.
On January 12, 2021, our board of directors approved the prepayment of an additional $5,000 to Mr. Ben Yehuda, ahead of the designated date of August 2021, pursuant to the Company’s right under the Settlement Agreement.
| 73 |
Convertible Loan Agreements
During December 2019, we entered into the December 2019 CLAs, with certain lenders, including three of our holders of greater than 5% of our Common Stock, pursuant to which the December 2019 Lenders agreed to provide us loans in the aggregate amount of $379,000 in exchange for convertible promissory notes and warrants. The following table sets forth the aggregate amount provided by our related parties in the December 2019 CLAs:
| Participant | Loan Amount | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | $ | 100,000 | ||
| L.I.A Pure Capital Ltd. | $ | 35,000 | ||
| YAAD Consulting & Management Services (1995) Ltd | $ | 100,000 | ||
Simultaneous with and conditioned upon the execution of the June 2020 SPA, the Company and each of the December 2019 Lenders, January 2020 Lender and February agreed to effectively cancel the December 2019 CLA and the equity securities issued thereunder. In connection therewith, each of the December 2019 Lenders voluntarily waived any right to receive interest that accrued thereupon pursuant to the December 2019 CLA.
Lease Agreement with YAAD Consulting & Management Services (1995) Ltd.
On September 1, 2019, we entered into a lease agreement, through our Israeli subsidiary, with YAAD Consulting & Management Services (1995) Ltd., a holder of greater than 5% of our Common Stock (the “YAAD Lease Agreement”). Pursuant to the YAAD Lease Agreement, we paid YAAD Consulting & Management Services (1995) Ltd. a monthly payment of NIS 5,000 (approximately $1,430) plus value added tax in consideration for office space located at 20 Raul Wallenberg, Tel Aviv, Israel. The YAAD Lease Agreement remained in effect until September 1, 2019.
Private Placements of our Securities
On June 24, 2020, we entered into the June 2020 SPA with certain investors, including three of our holders of greater than 5% of our Common Stock, pursuant to which we issued an aggregate of 67,369 shares of Common Stock and warrants to purchase an aggregate of 67,369 shares of Common Stock for an aggregate purchase price of $565,868. The following table sets forth the aggregate number of Common Stock issued to our related parties in the June 2020 SPA:
| Participant | Common Stock | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | 13,106 | |||
| L.I.A Pure Capital Ltd. | 4,588 | |||
| YAAD Consulting & Management Services (1995) Ltd | 13,107 | |||
During July 2020, we entered into securities purchase agreements (the “July 2020 SPAs”) with certain investors, including two of our holders of greater than 5% of our Common Stock, pursuant to which we issued an aggregate of 6,554 shares of Common Stock and warrants to purchase an aggregate of 6,554 shares of Common Stock for an aggregate purchase price of $55,046. The following table sets forth the aggregate number of Common Stock issued to our related parties in the July 2020 SPAs:
| Participant | Common Stock | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | 3,277 | |||
| YAAD Consulting & Management Services (1995) Ltd. | 3,277 | |||
| Nir Reinhold / Buffalo Investments Ltd. | 13,107 | |||
| 74 |
During August 2020, we entered into securities purchase agreements (the “August 2020 SPAs”) with certain investors, including three of our holders of greater than 5% of our Common Stock, pursuant to which we issued an aggregate of 13,108 shares of Common Stock and warrants to purchase an aggregate of 13,108 shares of Common Stock for an aggregate purchase price of $110,093. The following table sets forth the aggregate number of Common Stock issued to our related parties in the August 2020 SPAs:
| Participant | Common Stock | |||
| Amir Uziel / Amir Uziel Economic Consultant Ltd. | 6,554 | |||
| L.I.A Pure Capital Ltd. | 3,277 | |||
| YAAD Consulting & Management Services (1995) Ltd | 3,277 | |||
Stock Options
Since our inception, we have granted options to purchase shares of our Common Stock to our officers and certain of our directors. Such option agreements may contain acceleration provisions upon certain merger, acquisition, or change of control transactions. We describe our option plans under “Executive and Director Compensation—Equity Incentive Plan.” If the relationship between us and an executive officer or a director is terminated, except for cause (as defined in the various option plan agreements), options that are vested will generally remain exercisable for three months after such termination.
Policies and Procedures for Related Person Transactions
We intend to adopt a written related person transaction policy, to be effective immediately prior to the Current Offering, setting forth the policies and procedures for the review and approval or ratification of “related person transactions.” For purposes of this policy, a “related person transaction” is any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we and any “related person” are participants involving an amount that exceeds $120,000 including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. Transactions involving compensation for services provided to us as an employee, consultant or director will not be considered related-person transactions under this policy. A “related person” is any executive officer, director or a holder of more than five percent of our Common Stock, including any of their immediate family members and any entity owned or controlled by such persons.
Under this policy, where a transaction has been identified as a related person transaction, management must present information regarding the proposed related-person transaction to our audit committee (or, where review by our audit committee would be inappropriate, to another independent body of our board of directors) for review. In reviewing and approving any such transactions, our audit committee or other independent body of our board of directors, is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval. All of the transactions described in this section occurred prior to the adoption of this policy.
Director Independence
Our board of directors has determined that Vered Raz-Avayo, Ronen Rosenbloom, Israel Berenshtein, Amitay Weiss, Eliahou Arbib and Udi Kalifi do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the rules of The Nasdaq Stock Market LLC.
Item 14. Principal accounting fees and services
Audit and Accounting Fees
The following table sets forth the fees billed to our Company for professional services rendered by Halperin Ilanit CPA, an independent registered public accounting firm, for each of the last two fiscal years ended December 31, 2019 and 2018:
| Services | 2019 | 2018 | ||||||
| Audit fees | $ | 26,000 | $ | 27,655 | ||||
| Audit related fees | - | - | ||||||
| Tax fees | - | - | ||||||
| All other fees | - | - | ||||||
| Total fees | $ | 26,000 | $ | 27,655 | ||||
Audit fees consist of fees for professional services rendered for the audit of our annual financial statements.
Audit Committee Administration of Engagement
We have not established an audit committee and therefore do not have an audit committee pre-approval policy with respect to the engagement of an accountant for audit or non-audit services in place.
| 75 |
Item 15. exhibits, financial statement schedules
* Filed herewith.
+ Management contract or compensatory plan or arrangement.
| 76 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| SAVE FOODS, INC. | ||
| By: | /s/ David Palach | |
| Name: | David Palach | |
| Title: | Chief Executive Officer | |
| Date: | March 29, 2021 | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ David Palach | Chief Executive Officer | March 29, 2021 | ||
| David Palach | (Principal Executive Officer) | |||
| /s/ Shlomo Zakai | Chief Financial Officer | March 29, 2021 | ||
| Shlomo Zakai | (Principal Financial Officer) | |||
| /s/ Benad Goldwasser | Chairman of the Board | March 29, 2021 | ||
| Benad Goldwasser | ||||
| /s/ Ronen Rosenbloom | Director | March 29, 2021 | ||
| Ronen Rosenbloom | ||||
| /s/ Israel Berenshtein | Director | March 29, 2021 | ||
| Israel Berenshtein | ||||
| /s/ Vered Raz-Avayo | Director | March 29, 2021 | ||
| Vered Raz-Avayo | ||||
| /s/ Amitay Weiss | Director | March 29, 2021 | ||
| Amitay Weiss | ||||
| /s/ Eliahou Arbib | Director | March 29, 2021 | ||
| Eliahou Arbib |
| 77 |
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2020
TABLE OF CONTENTS
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
TO THE BOARD OF DIRECTORS AND STOCKHOLDERS OF
SAVE FOODS, INC.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Save Foods, Inc (the “Company”) and its subsidiary, Save Foods Ltd. as of December 31, 2020 and 2019, the related consolidated statements of operations and comprehensive loss, changes in stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1C to the consolidated financial statements, the Company has not yet generated material revenues from its operations to fund its activities and is therefore dependent upon external sources for financing its operations. As of December 31, 2020, the Company has incurred accumulated deficit of $12,277,647 and negative operating cash flows. These factor among others, as discussed in Note 1C to the consolidated financial statements raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 1C to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of’ these uncertainties.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
| /s/ Halperin Ilanit. | |
| Certified Public Accountants (Isr.) |
Tel Aviv, Israel
March 9, 2021
We have served as the Company’s auditor since 2018

| F-2 |
CONSOLIDATED BALANCE SHEETS
(U.S. dollars except share and per share data)
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | 242,900 | 290,815 | ||||||
| Restricted cash (Note 2D) | 22,395 | 38,194 | ||||||
| Accounts receivable, net | 147,941 | 64,003 | ||||||
| Inventories | 16,356 | 16,302 | ||||||
| Other current assets (Note 3) | 65,579 | 15,300 | ||||||
| Total Current assets | 495,171 | 424,614 | ||||||
| Right Of Use asset arising from operating lease | 14,700 | 48,982 | ||||||
| Property and Equipment, Net (Note 4) | 55,194 | 81,119 | ||||||
| Funds in Respect of Employee Rights Upon Retirement | 122,584 | 109,955 | ||||||
| Total assets | 687,649 | 664,670 | ||||||
| Liabilities and Shareholders’ Deficit | ||||||||
| Current Liabilities | ||||||||
| Short-term loan from banking institution (Note 7) | 7,949 | 7,230 | ||||||
| Current maturities of convertible loans | 56,250 | |||||||
| Accounts payable | 203,323 | 235,864 | ||||||
| Other accounts liabilities (Note 5) | 517,711 | 380,732 | ||||||
| Total current liabilities | 785,233 | 623,826 | ||||||
| Fair Value of Convertible Component in Convertible Loans (Note 6) | 54,970 | - | ||||||
| Convertible Loans (Notes 6) | 146,929 | 285,917 | ||||||
| Long term from banking institution (Note 7) | 8,115 | 14,955 | ||||||
| Liability for employee rights upon retirement | 157,855 | 142,091 | ||||||
| Total liabilities | 1,153,102 | 1,066,789 | ||||||
| Stockholders’ Deficit (Note 9) | ||||||||
| Common
stocks of US$ 0.0001 par value each (“Common Stocks”): 495,000,000 shares authorized as of December 31, 2020 and 2019; issued and outstanding 1,606,760 and 1,458,593 shares as of December 31, 2020 and 2019, respectively. | 161 | 146 | ||||||
| Preferred
stocks of US$ 0.0001 par value (“Preferred stocks”): 5,000,000 shares authorized as of December 31, 2020 and 2019; issued and outstanding 0 shares as of December 31, 2020 and 2019. | - | - | ||||||
| Additional paid-in capital | 11,867,585 | 10,329,571 | ||||||
| Foreign currency translation adjustments | (26,275 | ) | (26,275 | ) | ||||
| Accumulated deficit | (12,277,647 | ) | (10,684,508 | ) | ||||
| (436,176 | ) | (381,066 | ) | |||||
| Non-controlling interests | (29,277 | ) | (21,053 | ) | ||||
| Total stockholders’ deficit | (465,453 | ) | (402,119 | ) | ||||
| Total liabilities and stockholders’ deficit | 687,649 | 664,670 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-3 |
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(U.S. dollars except share and per share data)
| Year ended | ||||||||
| December 31 | ||||||||
| 2020 | 2019 | |||||||
| Revenues from sales of products | 232,274 | 175,823 | ||||||
| Cost of sales (Note 11) | (43,405 | ) | (144,548 | ) | ||||
| Gross profit (loss) | 188,869 | 31,275 | ||||||
| Research and development expenses (Note 12) | (417,000 | ) | (615,623 | ) | ||||
| Selling and marketing expenses | (51,105 | ) | (342,058 | ) | ||||
| General and administrative expenses (Note 13) | (1,070,109 | ) | (1,004,899 | ) | ||||
| Operating loss | (1,349,345 | ) | (1,931,305 | ) | ||||
| Financing expenses, net (Note 14) | (270,393 | ) | (43,408 | ) | ||||
| Other expenses, net | (2,532 | ) | - | |||||
| Shares in losses of affiliated company | - | (15,690 | ) | |||||
| Gain on disposal of affiliated company | 15,690 | - | ||||||
| Net loss | (1,606,580 | ) | (1,990,403 | ) | ||||
| Less: Net loss attributable to non-controlling interests | 13,441 | 18,986 | ||||||
| Net loss attributable to the Company | (1,593,139 | ) | (1,971,417 | ) | ||||
| Loss per share (basic and diluted) (Note 16) | (1.05 | ) | (1.38 | ) | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
(U.S. dollars, except share and per share data)
| Number of Shares | Amount | Additional paid-in capital | Accumulated other comprehensive income (loss) | Proceeds
on account of shares | Accumulated deficit | Total
Company’s stockholders’ equity | Non- controlling interest | Total
stockholders’ deficit | ||||||||||||||||||||||||||||
| BALANCE AT JANUARY 1, 2019 | 1,318,422 | 132 | 8,852,461 | (26,275 | ) | 105,000 | (8,713,091 | ) | 218,227 | (6,712 | ) | 211,515 | ||||||||||||||||||||||||
| CHANGES DURING THE YEAR ENDED DECEMBER 31, 2019: | ||||||||||||||||||||||||||||||||||||
| Issuance of shares for cash | 140,171 | 14 | 945,679 | - | (105,000 | ) | - | 840,693 | - | 840,693 | ||||||||||||||||||||||||||
| Value of warrant issued in convertible loans | - | - | 97,406 | - | - | - | 97,406 | - | 97,406 | |||||||||||||||||||||||||||
| Stock based compensation | - | - | 434,025 | - | - | - | 434,025 | 4,645 | 438,670 | |||||||||||||||||||||||||||
| Comprehensive loss for the year | - | - | - | - | - | (1,971,417 | ) | (1,971,417 | ) | (18,986 | ) | (1,990,403 | ) | |||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2019 | 1,458,593 | 146 | 10,329,571 | (26,275 | ) | - | (10,684,508 | ) | (381,066 | ) | (21,053 | ) | (402,119 | ) | ||||||||||||||||||||||
| CHANGES DURING THE YEAR ENDED DECEMBER 31, 2020: | ||||||||||||||||||||||||||||||||||||
| Issuance of shares for cash | 45,876 | 5 | 349,995 | - | - | - | 350,000 | - | 350,000 | |||||||||||||||||||||||||||
| Value of warrant issued in convertible loans | - | - | 34,696 | - | - | - | 34,696 | - | 34,696 | |||||||||||||||||||||||||||
| Conversion of convertible loans | 67,369 | 7 | 585,924 | - | - | - | 585,931 | - | 585,931 | |||||||||||||||||||||||||||
| Exercise of warrants | 28,572 | 2 | 59,998 | - | - | - | 60,000 | - | 60,000 | |||||||||||||||||||||||||||
| Exercise of options | 6,350 | 1 | 19,999 | - | - | - | 20,000 | - | 20,000 | |||||||||||||||||||||||||||
| Stock based compensation | - | - | 487,402 | - | - | - | 487,402 | 5,217 | 492,619 | |||||||||||||||||||||||||||
| Comprehensive loss for the year | - | - | - | - | - | (1,593,139 | ) | (1,593,139 | ) | (13,441 | ) | (1,606,580 | ) | |||||||||||||||||||||||
| BALANCE AT DECEMBER 31, 2020 | 1,606,760 | 161 | 11,867,585 | (26,275 | ) | - | (12,277,647 | ) | (436,176 | ) | (29,277 | ) | (465,453 | ) | ||||||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars)
| Year ended | ||||||||
| December 31 | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Loss for the period | $ | (1,606,580 | ) | $ | (1,990,403 | ) | ||
| Adjustments required to reconcile net loss for the period to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 45,205 | 27,351 | ||||||
| Loss from sales of Property and Equipment | 2,382 | - | ||||||
| Share in losses (gain on disposal) of affiliated company | (15,690 | ) | 15,690 | |||||
| Increase (decrease) in liability for employee rights upon retirement | 15,764 | 16,019 | ||||||
| Stock based compensation | 492,619 | 438,670 | ||||||
| Expenses on convertible loans | 176,216 | 6,147 | ||||||
| Compensation expenses in exchange of instruments | 57,793 | - | ||||||
| Decrease (increase) in accounts receivable | (83,938 | ) | 71,294 | |||||
| Decrease (increase) in inventory | (54 | ) | 21,783 | |||||
| Decrease (increase) in other current assets | (47,575 | ) | 49,739 | |||||
| Increase (decrease) in accounts payable | (32,541 | ) | 39,313 | |||||
| Increase in other accounts payable | 197,659 | 59,625 | ||||||
| Net cash used in operating activities | (798,740 | ) | (1,244,772 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Proceeds from (payments on) investment in unconsolidated entity | 4,864 | (7,567 | ) | |||||
| Decrease (increase) in Short term deposits in banking institutions | 15,799 | (38,194 | ) | |||||
| Increase in funds in respect of employee rights upon retirement | (12,629 | ) | (12,567 | ) | ||||
| Proceeds from sales of Property and Equipment | 1,031 | - | ||||||
| Purchase of Property and Equipment | - | (23,327 | ) | |||||
| Net cash provided by (used in) investing activities | 9,065 | (81,655 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from Secured promissory notes | 135,000 | - | ||||||
| Proceeds from convertible loans | 225,000 | 379,000 | ||||||
| Repayments of long-term loans from banking institutes | (7,272 | ) | (42,257 | ) | ||||
| Repayments of right of use asset arising from operating lease | (40,968 | ) | - | |||||
| Proceeds from stock issued for cash | 350,000 | 840,693 | ||||||
| Exercise of options | 20,000 | - | ||||||
| Exercise of warrants | 60,000 | - | ||||||
| Net cash provided by financing activities | 741,760 | 1,177,436 | ||||||
| INCREASE IN CASH AND CASH EQUIVALENTS | (47,915 | ) | (148,991 | ) | ||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR | 290,815 | 439,806 | ||||||
| CASH AND CASH EQUIVALENTS AT END OF YEAR | $ | 242,900 | $ | 290,815 | ||||
| F-6 |
SAVE FOODS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars)
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid during the year for: | ||||||||
| Interest | $ | 408 | $ | 836 | ||||
| Non cash transactions: | ||||||||
| Disposal of affiliated company | 2,704 | - | ||||||
| Termination of lease agreement | 11,590 | - | ||||||
| Issuance of warrants in convertible loans | 53,388 | 97,406 | ||||||
| Conversion of convertible loans | 528,138 | - | ||||||
| Initial recognition of operating lease right-of-use assets | - | 48,982 | ||||||
| Initial recognition of operating lease liability | - | (48,982 | ) |
The accompanying notes are an integral part of the consolidated financial statements.
| F-7 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL
| A. | Operations |
Save Foods, Inc. (the “Company”) was incorporated on April 1, 2009, under the laws of the State of Delaware. On April 27, 2009, the Company acquired from its stockholders 98.94% of the issued and outstanding shares of Save Foods Ltd., including preferred and common stock.
Save Foods Ltd. was incorporated in 2004 and commenced its operations in 2005. Save Foods Ltd. develops, produces, and focuses on delivering innovative solutions for the food industry aimed at improving food safety and prolonging shelf life of fresh produce.
The Company’s common stock is quoted on the OTC, Pink Tier, under the symbol “SAFO.”
| B. | Reverse stock split |
On April 23, 2019, the Company amended and restated its Certificate of Incorporation to effect a 15 to 1 reverse stock split of the Company’s outstanding Common Stock.
As a result of the reverse stock split, which became effective on June 11, 2019, every 15 shares of the Company’s outstanding Common Stock prior to the effect of that amendment was combined and reclassified into one share of the Company’s Common Stock. No fractional shares were issued in connection with or following the reverse split. The number of outstanding shares of the Company’s Common Stock and par value of the shares remained unchanged.
On February 23, 2021, the Company amended its Certificate of Incorporation to effect a 7 to 1 reverse stock split of the Company’s outstanding Common Stock.
As a result of the reverse stock split, every 7 shares of the Company’s outstanding Common Stock prior to the effect of that amendment was combined and reclassified into one share of the Company’s Common Stock. No fractional shares were issued in connection with or following the reverse split. The number of authorized capital of the Company’s Common Stock and par value of the shares remained unchanged.
All share, stock option and per share information in these condensed consolidated financial statements have been restated to reflect the stock split on a retroactive basis.
| C. | Going concern uncertainty |
Since its incorporation (April 1, 2009), the Company has not had any operations other than those carried out by Save Foods Ltd. The development and commercialization of Save Foods Ltd.’s products will require substantial expenditures. Save Foods Ltd. and the Company have not yet generated sufficient revenues from their operations to fund the Group’s (as defined below) activities and are therefore dependent upon external sources for financing their operations. There can be no assurance that the Company will succeed in obtaining the necessary financing to continue its operations. As of December 31, 2020, the Company had $242,900 in cash, a negative working capital of $290,062 and an accumulated deficit of $12,277,647.
The Company will need to secure additional capital in the future in order to meet its anticipated liquidity needs primarily through the sale of additional Common Stock or other equity securities and/or debt financing. Funds from these sources may not be available to the Company on acceptable terms, if at all, and the Company cannot give assurance that it will be successful in securing such additional capital.
The Company focuses its solutions towards vegetables and fruits which are considered the largest in terms of worldwide consumption. Among other things, the Company tries to cooperate with major fruit packing houses in Israel and abroad.
These factors raise substantial doubt about Save Foods Ltd. and the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| F-8 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL (continue)
| D. | In December 2019, a novel strain of coronavirus, COVID-19, was identified in Wuhan, China. This virus continues to spread globally and, has spread to over 180 countries, including the United States and Israel. The spread of COVID-19 from China to other countries has resulted in the World Health Organization declaring the outbreak of COVID-19 as a “pandemic,” or a worldwide spread of a new disease, on March 11, 2020. Many countries around the world have imposed quarantines and restrictions on travel and mass gatherings to slow the spread of the virus, including in the United States and in Israel. Governments may divert spending from other budgeted resources as they seek to reduce and/or stop the spread of an infectious disease, such as COVID-19. Such events may result in a period of business and manufacturing disruption, and in reduced operations, any of which could materially affect the Company’s business, financial condition and results of operations. The extent to which COVID-19 impacts the Company’s business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others. The Company believes it is taking appropriate actions to mitigate the negative impacts. However, the full impact of COVID-19 is unknown and cannot be reasonably estimated as these events occurred subsequent to year end and are still developing. |
| E. | Risk factors |
The Company and Save Foods Ltd. (collectively, the “Group”) face a number of risks, including uncertainties regarding finalization of the development process, demand and market acceptance of the Group’s products, the effects of technological changes, competition and the development of products by competitors. Additionally, other risk factors also exist, such as the ability to manage growth and the effect of planned expansion of operations on the Group’s future results. In addition, the Group expects to continue incurring significant operating costs and losses in connection with the development of its products and increased marketing efforts. As mentioned above, the Group has not yet generated significant revenues from its operations to fund its activities, and therefore the continuance of its activities as a going concern depends on the receipt of additional funding from its current stockholders and investors or from third parties.
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES
The financial statements were prepared in accordance with accounting principles generally accepted in the United States of America (US GAAP).
| A. | Use of estimates in the preparation of financial statements |
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of expenses during the reporting periods. Actual results could differ from those estimates. As applicable to these financial statements, the most significant estimates and assumptions relate to the going concern assumptions, share based compensation and convertible loans.
| B. | Functional currency |
A majority of the Group’s revenues is generated in U.S. dollars. In addition, most of the Group’s costs are denominated and determined in U.S. dollars and in new Israeli shekels. Management believes that the dollar is the currency in the primary economic environment in which the Group operates. Thus, the functional and reporting currency of the Group is the U.S. dollar.
Accordingly, monetary accounts maintained in currencies other than the dollar are remeasured into dollars in accordance with Accounting Standards Codification (ASC) 830, “Foreign Currency Matters”. All transaction gains and losses of the remeasured monetary balance sheet items are reflected in the statements of operations as financial income or expenses, as appropriate.
| F-9 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| C. | Principles of consolidation |
The consolidated financial statements include the accounts of the Company and its subsidiary, Save Foods Ltd. All significant intercompany balances and transactions have been eliminated on consolidation.
| D. | Cash and cash equivalents, and Restricted cash |
Cash equivalents are short-term highly liquid investments which include short term bank deposits (up to three months from date of deposit), that are not restricted as to withdrawals or use that are readily convertible to cash with maturities of three months or less as of the date acquired.
Restricted cash as of December 31, 2020 and 2019 included a NIS 72,000 ($22,395) and NIS 132,000 ($38,194), respectively collateral account for the Company’s corporate credit cards and a loan and is classified in current assets.
| E. | Accounts receivables |
Accounts receivables are stated at their net realizable value. The allowance against gross accounts receivables reflects the best estimate of losses inherent in the receivables portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other currently available information. As of December 31, 2020, and 2019, an allowance for doubtful debts in the amount of $26,553 and $24,702, respectively, is reflected in net accounts receivables. Accounts receivables are written off after all reasonable means to collect the full amount have been exhausted.
| F. | Property, plant and equipment, net |
| 1. | Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets. When an asset is retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition is reflected in the Statements of Operations and Comprehensive Loss. | |
| 2. | Rates of depreciation: |
| % | ||||
| Furniture and office equipment | 7-15 | |||
| Machines | 10-15 | |||
| Computers | 33 | |||
| Vehicle | 15 |
| G. | Impairment of long-lived assets |
The Group’s long-lived assets are reviewed for impairment in accordance with Accounting Standards Codification (“ASC”) Topic 360, “Property, Plant and Equipment”, whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. No impairment expenses were recorded during the years ended December 31, 2020 or 2019.
| F-10 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| H. | Deferred income taxes |
The Group accounts for income taxes in accordance with ASC Topic 740, “Income Taxes”. Accordingly, deferred income taxes are determined utilizing the asset and liability method based on the estimated future tax effects of differences between the financial accounting and the tax bases of assets and liabilities under the applicable tax law. Deferred tax balances are computed using the enacted tax rates expected to be in effect when these differences reverse. Valuation allowances in respect of deferred tax assets are provided for, if necessary, to reduce deferred tax assets to amounts more likely than not to be realized.
The Group accounts for uncertain tax positions in accordance with ASC Topic 740-10, which prescribes detailed guidance for the financial statement recognition, measurement and disclosure of uncertain tax positions recognized in an enterprise’s financial statements. According to ASC Topic 740-10, tax positions must meet a more-likely-than-not recognition threshold. The Company’s accounting policy is to classify interest and penalties relating to uncertain tax positions under income taxes, however the Company did not recognize such items in its fiscal 2020 and 2019 financial statements and did not recognize any liability with respect to an unrecognized tax position in its balance sheets.
| I. | Liability for employee rights upon retirement |
Save Foods Ltd’s liability for employee rights upon retirement with respect to its Israeli employees is calculated, pursuant to Israeli Severance Pay Law, based on the most recent salary of each employee multiplied by the number of years of employment, as of the balance sheet date. Employees are entitled to one month’s salary for each year of employment, or a portion thereof. Save Foods Ltd. makes monthly deposits to insurance policies and severance pay funds. The liability of the Company is fully provided for.
The deposited funds include profits accumulated up to the balance sheet date. The deposited funds may be withdrawn upon the fulfillment of the obligation pursuant to Israeli severance pay laws or labor agreements. The value of the deposited funds is based on the cash surrender value of these policies, and includes immaterial profits/losses.
Severance expenses for the years ended December 31, 2020 and 2019, amounted to $7,419 and $24,000, respectively.
| J. | Revenue recognition |
Revenues are recognized when delivery has occurred and there is persuasive evidence of an agreement, the fee is fixed or determinable and collection of the related receivables is reasonably assured and no further obligations exist.
Revenues from sales of products are recognized when title and risk and rewards for the products are transferred to the customer.
| K. | Research and development expenses |
Research and development expenses are charged to operations as incurred.
| F-11 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| L. | Royalty-bearing grants |
Royalty-bearing grants from the Israeli Innovation Authority (the “IIA”) for funding approved research and development projects are recognized at the time Save Foods Ltd. is entitled to such grants (i.e. at the time that there is reasonable assurance that the Company will comply with the conditions attached to the grant and that there is reasonable assurance that the grant will be received), on the basis of the costs incurred and reduce research and development costs. The cumulative research and development grants received by the Company from inception through December 2020 amounted to NIS 484,429 (US$150,678).
As of December 31, 2020, and 2019, the Company did not accrue for or pay any royalties to the IIA since no revenues were recognized in respect of the funded projects.
| M. | Inventories |
Inventories are valued at the lower of cost or net realizable value. Cost of raw and packaging materials, purchased products, manufactured finished products and products in process are determined on the average costs basis.
The Company regularly reviews its inventories for impairment and reserves are established when necessary.
| N. | Basic and diluted loss per common stock |
Basic loss per common stock is computed by dividing the loss for the period applicable to shareholders, by the weighted average number of shares of common stock outstanding during the period. Securities that may participate in dividends with the shares of common stock (such as the convertible preferred) are considered in the computation of basic loss per share under the two-class method. However, in periods of net loss, only the convertible preferred shares are considered, since such shares have a contractual obligation to share in the losses of the Company.
In computing diluted loss per share, basic loss per share is adjusted to reflect the potential dilution that could occur upon the exercise of potential shares. Accordingly, in periods of net loss, no potential shares are considered.
| O. | Stock-based compensation |
The Company measures and recognizes the compensation expense for all equity-based payments to employees based on their estimated fair values in accordance with ASC 718, “Compensation-Stock Compensation”. Share-based payments including grants of stock options are recognized in the statement of comprehensive loss as an operating expense based on the fair value of the award at the date of grant. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model. The Company has expensed compensation costs, net of estimated forfeitures, applying the accelerated vesting method, over the requisite service period or over the implicit service period when a performance condition affects the vesting, and it is considered probable that the performance condition will be achieved.
Share-based payments awarded to consultants (non-employees) are accounted for in accordance with ASC Topic 505-50, “Equity-Based Payments to Non-Employees”.
| F-12 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| P. | Fair Value Measurements |
Fair value of certain of the Company’s financial instruments including cash, accounts receivable, account payable, accrued expenses, notes payables, and other accrued liabilities approximate cost because of their short maturities. The Company measures and reports fair value in accordance with ASC 820, “Fair Value Measurements and Disclosure” defines fair value, establishes a framework for measuring fair value in accordance with generally accepted accounting principles and expands disclosures about fair value investments.
Fair value, as defined in ASC 820, is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value of an asset should reflect its highest and best use by market participants, principal (or most advantageous) markets, and an in-use or an in-exchange valuation premise. The fair value of a liability should reflect the risk of non-performance, which includes, among other things, the Company’s credit risk.
Valuation techniques are generally classified into three categories: the market approach; the income approach; and the cost approach. The selection and application of one or more of the techniques may require significant judgment and are primarily dependent upon the characteristics of the asset or liability, and the quality and availability of inputs. Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 also provides fair value hierarchy for inputs and resulting measurement as follows:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for identical assets or liabilities.
Level 2: Quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; inputs other than quoted prices that are observable for the asset or liability; and inputs that are derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level 3: Unobservable inputs for the asset or liability that are supported by little or no market activity, and that are significant to the fair values.
Fair value measurements are required to be disclosed by the Level within the fair value hierarchy in which the fair value measurements in their entirety fall. Fair value measurements using significant unobservable inputs (in Level 3 measurements) are subject to expanded disclosure requirements including a reconciliation of the beginning and ending balances, separately presenting changes during the period attributable to the following: total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings, and a description of where those gains or losses included in earning are reported in the statement of income.
The Company records a debt discount related to the issuance of convertible debts that have conversion features at adjustable rates. The debt discount for the convertible instruments is recognized and measured by allocating a portion of the proceeds as an increase in additional paid-in capital and as a reduction to the carrying amount of the convertible instrument equal to the fair value of the conversion features. The debt discount will be accreted by recording additional non-cash gains and losses related to the change in fair values of derivative liabilities over the life of the convertible notes.
The Company’s financial assets and liabilities that are measured at fair value on a recurring basis by level within the fair value hierarchy are as follows:
| Balance as of December 31, 2020 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Fair Value of convertible component in convertible loan | - | - | 54,970 | 54,970 | ||||||||||||
| Total liabilities | - | - | 54,970 | 54,970 | ||||||||||||
The following table presents the changes in fair value of the level 3 liabilities for the Year ended December 31, 2020:
| Fair value of Convertible component | ||||
| Outstanding at January 1, 2020 | - | |||
| Fair value of issued level 3 liability | 27,762 | |||
| Changes in fair value | 27,208 | |||
| Outstanding at December 31, 2020 | 54,970 | |||
| Q. | Concentrations of credit risk |
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents as well as certain other current assets that do not amount to a significant amount. Cash and cash equivalents, which are primarily held in Dollars and New Israeli Shekels, are deposited with major banks in Israel and United States. Management believes that such financial institutions are financially sound and, accordingly, minimal credit risk exists with respect to these financial instruments. The Company does not have any significant off-balance-sheet concentration of credit risk, such as foreign exchange contracts, option contracts or other foreign hedging arrangements.
| R. | Contingencies |
The Company records accruals for loss contingencies arising from claims, litigation and other sources when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. Legal costs incurred in connection with loss contingencies are expensed as incurred.
| F-13 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2– SIGNIFICANT ACCOUNTING POLICIES (continue)
| S. | New Accounting Pronouncements |
In June 2016, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) No. 2016-13, “Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments.” In November 2018, FASB issued ASU No. 2018-19, “Codification Improvements to Topic 326, Financial Instruments-Credit Losses”, which amends the scope and transition requirements of ASU 2016-13. Topic 326 requires a financial asset (or a group of financial assets) measured at amortized cost basis to be presented at the net amount expected to be collected. The measurement of expected credit losses is based on relevant information about past events, including historical experience, current conditions and reasonable and supportable forecasts that affect the collectability of the reported amount. Topic 326 will originally become effective for the Company beginning January 1, 2020, with early adoption permitted, on a modified retrospective approach. As a smaller reporting company, the effective date for the Company has been delayed until fiscal years beginning after December 15, 2022, in accordance with ASU 2019-10, although early adoption is still permitted. This standard did not have a material impact to the Company’s consolidated financial statements after evaluation.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. The amendments in this ASU simplify the accounting for income taxes, eliminates certain exceptions to the general principles in Topic 740 and clarifies certain aspects of the current guidance to improve consistent application among reporting entities. ASU 2019-12 is effective for fiscal years beginning after December 15, 2021 and interim periods within annual periods beginning after December 15, 2022, though early adoption is permitted, including adoption in any interim period for which financial statements have not yet been issued. This standard is not expected to have a material impact to the Company’s consolidated financial statements.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement. This standard will require entities to disclose the amount of total gains or losses for the period recognized in other comprehensive income that is attributable to fair value changes in assets and liabilities held as of the balance sheet date and categorized within Level 3 of the fair value hierarchy. This ASU will be effective for the Company for annual and interim periods beginning after December 31, 2020. Early adoption of this standard is permitted. This standard did not have a material impact to the Company’s consolidated financial statements.
In August 2020, the FASB issued ASU No. 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging Contracts in Entity s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity s Own Equity. ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models results in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective for public companies for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. Management has not yet evaluated the impact that the adoption of ASU 2020-06 will have on the Company’s consolidated financial statement presentation or disclosures.
Other new pronouncements issued but not effective as of December 31, 2020 are not expected to have a material impact on the Company’s consolidated financial statements.
NOTE 3 – OTHER CURRENT ASSETS
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Prepaid expenses and advances to vendors | 51,020 | 4,811 | ||||||
| Receivables from sale of subsidiary (Note 5) | 2,704 | - | ||||||
| Government Institutions | 11,855 | 10,489 | ||||||
| 65,579 | 15,300 | |||||||
| F-14 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – PROPERTY AND EQUIPMENT, NET
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Computers | 10,328 | 10,328 | ||||||
| Furniture and office equipment | 5,002 | 5,002 | ||||||
| Machines | 130,797 | 134,665 | ||||||
| Vehicles | 85,149 | 85,149 | ||||||
| 231,276 | 235,144 | |||||||
| Less - accumulated depreciation | (139,382 | ) | (117,325 | ) | ||||
| Less – Impairment of long lived assets | (36,700 | ) | (36,700 | ) | ||||
| Total property and equipment, net | 55,194 | 81,119 | ||||||
In the years ended December 31, 2020 and 2019, depreciation expenses were US$ 22,512 and US$ 27,351 respectively, and additional property and equipment were purchased in an amount of US$ 23,327 for the years ended December 31, 2019 (none for the year ended December 31, 2020).
NOTE 5 – OTHER ACCOUNTS LIABILITIES
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Employees and related institutions | 110,220 | 135,901 | ||||||
| Accrued expenses | 392,442 | 184,616 | ||||||
| Right Of Use liability arising from operating lease | 15,049 | 52,093 | ||||||
| Affiliated company (*) | - | 8,122 | ||||||
| 517,711 | 380,732 | |||||||
| (*) | On April 2, 2019, the Company invested 10,000 Canadian Dollars for 20% of the outstanding shares of Savecann Solutions Inc. (“Savescann”) a newly formed company registered in Canada. Savecann intended to market the Company’s solutions to the Cannabis market. | |
| On April 21, 2020, the Company sold its entire holdings in Savecann for total consideration of 10,000 Canadian Dollars ($7,000), of which $2,704 were not paid yet and are presented as part of other current assets. |
| F-15 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – CONVERTIBLE LOANS
| A. | In December 2019, the Company entered into a series of Convertible Loan Agreements (each a “CLA”) with third parties and certain existing shareholders (the “Lenders”), pursuant to which the Lenders agreed to provide the Company loans in the aggregate amount of $379,000 and in exchange the Company issued to the Lenders (i) convertible promissory notes (the “Notes”) and (ii) warrants with an exercise price of $8.40. In January and March 2020, the Company entered into two additional CLA agreements for an aggregate amount of $135,000, consisting of the same terms. | |
| According to the terms of the CLA, the Notes bear interest at a rate of 5% per annum and the loan amount represented by the Notes is to be repaid to the Lenders according to the following schedule: (i) the principal amount represented by the Notes to be repaid in twenty four equal monthly installments, commencing on the twenty fifth month following the closing of each CLA, and (ii) the interest accrued on the loan amount to be paid in two bi-annual installments, commencing on the first anniversary of the first payment of the principal amount. | ||
| In addition, according to the terms of the CLA, the outstanding loan amount matures on the earlier of (i) the third anniversary of each CLA or (ii) a deemed liquidation event (as defined therein), and the Lenders may convert all or any portion of the Notes at any time prior to the one-year anniversary of each issuance into shares of the Company’s Common Stock at a conversion price of $8.40 per share. | ||
| In accordance with ASC 815-15-25, the conversion feature was considered embedded derivative instruments, and is to be recorded at their fair value as its fair value can be separated from the convertible loan and its conversion is independent of the underlying note value. The Company recorded finance expenses in respect of the convertible component in the convertible loan in the excess amount of the convertible component fair value over the face loan amount. The conversion liability is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations. | ||
As a result of the above issuances, the Company recorded in the periods ended March 31, 2020 and December 31, 2019, a total amount of $34,696 and $97,406, respectively, in respect of the detachable warrants, as a credit to stockholders’ equity (additional paid in capital). The fair value of the Warrants was determined using the Black-Scholes pricing model, assuming a risk free rate of 1.6%, a volatility factor of 54.00%, dividend yields of 0% and an expected life of 3 years.
| ||
| On June 24, 2020, the Company entered into a Securities Purchase Agreement (the “SPA”) with the Lenders in connection with the sale and issuance of 69,332 units (“Units”), at a purchase price of $7.63 per Unit. Each Unit consists of: (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of $8.4 (the “Warrant”). In connection with the SPA, the Company issued to the Lenders an aggregate of 67,369 shares of Common Stock and Warrants to purchase an aggregate of 67,369 shares of Common Stock. The shares of Common Stock were issued on July 2, 2020. | ||
| Simultaneous with and conditioned upon the execution of the SPA, the Company and each of the Lenders agreed to effectively cancel the CLA and the equity securities issued thereunder. In connection therewith, each of the Lenders voluntarily waived any right to receive interest that accrued thereupon pursuant to the CLA. | ||
| The Company evaluated the transaction as an exchange of instruments and as a result of the above conversion, recorded a compensation expenses in a total amount of $57,793, as of the exchange date, and as a credit to stockholders’ equity (additional paid in capital). The fair value of the additional shares granted in the conversion was calculated based on the Company’s share price as of the date of the conversion. The fair value of the additional warrants granted in the conversion was determined using the Black-Scholes pricing model, assuming a risk-free rate of 0.21%, a volatility factor of 51.96%, dividend yields of 0% and an expected life of 2.45-2.71 years. | ||
| During the years ended December 31, 2020 and 2019, the Company recorded net interest and amortization expenses in the amount of $199,709 and $4,323, respectively, in respect of the discounts recorded on the CLAs. | ||
| B. | On September 21, 2020, the Company entered into a series of additional convertible loan agreements (each a “2020 CLA”) with certain lenders (the “2020 Lenders”) to sell convertible promissory notes with an aggregate principal amount of $125,000 (each a “2020 Note”). The outstanding loan amount under the 2020 CLA will mature on the earlier of (i) the third anniversary of each 2020 CLA or (ii) a deemed liquidation event (as defined therein), and the 2020 Lenders may convert all or any portion of the 2020 Notes into shares of Common Stock at any time prior to a mandatory conversion event (as defined therein) at a conversion price of $7.63 per share. The 2020 Notes will bear interest at a rate of 5% per annum. The loan amount represented by the 2020 Notes will be repaid to the 2020 Lenders according to the following schedule: (i) the principal amount represented by the 2020 Notes will be repaid in four bi-annual installments, commencing on the first anniversary following the closing of each 2020 CLA, and (ii) the interest accrued on the loan amount will be paid in two bi-annual installments, commencing on the first anniversary of the first payment of that principal amount. |
| F-16 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – CONVERTIBLE LOANS (continue)
| During October 2020, the Company entered into a series of additional 2020 CLAs with additional 2020 Lenders to sell additional 2020 Notes with an aggregate principal amount of $100,000. | ||
| During January 2021, the Company entered into a series of additional 2020 CLAs with additional 2020 Lenders to sell additional 2020 Notes with an aggregate principal amount of $274,000. | ||
| As part of the 2020 CLA, the Company entered into a registration rights agreement with each of the 2020 Lenders, whereby each 2020 Lender received piggyback registration rights for the shares issuable upon conversion of the 2020 Notes to shares of Common Stock. |
The loans are convertible into common Stock upon (i) a completion of underwritten public offering (“Mandatory Conversion”) convert the outstanding loan amount at a share price as shall be determined in the offering, or (ii) at the lender’s discretion (“Optional Conversion”) convert the outstanding loan amount at a share price per share of $7.63.
In accordance with ASC 815-15-25, the conversion feature was considered embedded derivative instruments, and is to be recorded at their fair value as its fair value can be separated from the convertible loan and its conversion is independent of the underlying note value. The Company recorded finance expenses in respect of the convertible component in the convertible loan in the excess amount of the convertible component fair value over the face loan amount. The conversion liability is then marked to market each reporting period with the resulting gains or losses shown in the statements of operations.
The fair value of the convertible component was estimated by third party appraiser as weighted average of the two possible scenarios of the total loan amount conversion: as of September 21, 2020 and October 23, 2020, 70% probability for the Mandatory Conversion and 30% probability for the Optional Conversion and as of December 31, 2020, 75% probability for the Mandatory Conversion and 25% probability for the Optional Conversion.
The Mandatory Conversion (scenario 1) was estimated by the appraiser using the Black-Scholes option pricing model, to compute the fair value of the derivative and to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of issuance dates and as of the balance sheet date:
| September 21, 2020 | October 23, 2020 | December 31, 2020 | ||||||||||
| Dividend yield | 0 | 0 | 0 | |||||||||
| Risk-free interest rate | 0.19 | % | 0.11 | % | 0.09 | % | ||||||
| Expected term (years) | 0.775 | 0.685 | 0.417 | |||||||||
| Volatility | 51.96 | % | 51.96 | % | 48.06 | % | ||||||
| Share price | 6.72 | 5.88 | 8.61 | |||||||||
| Exercise price | 7.63 | 7.63 | 7.63 | |||||||||
| Fair value | 15,208 | 6,457 | 47,499 | |||||||||
The Optional Conversion (scenario 2) was estimated by the appraiser using binomial option pricing model and simulating and waiver of the lender as an exercise price, to compute the fair value of the derivative and to mark to market the fair value of the derivative at each balance sheet date. The following are the data and assumptions used as of the issuance dates and as of balance sheet date:
| September 21, 2020 | October 23, 2020 | December 31, 2020 | ||||||||||
| Dividend yield | 0 | 0 | 0 | |||||||||
| Risk-free interest rate | 0.12-0.16 | % | 0.12-0.2 | % | 0.10-0.14 | % | ||||||
| Volatility | 51.96 | % | 51.96 | % | 48.06 | % | ||||||
| Share price | 6.72 | 5.88 | 8.61 | |||||||||
| Fair value | 26,824 | 15,167 | 77,381 | |||||||||
The fair value of the convertible component was estimated by the third-party appraiser after giving effect to the weighted average of the two possible scenarios as of issuance dates was $27,762 and as of December 31, 2020 was $54,970.
The fair value allocated to the convertible loan was estimated by third party appraiser as the residual value of the proceeds net of the convertible component and was estimated at a value of $203,179 as of December 31, 2020 of which $56,250 is presented under current liabilities and $146,929 is presented under long term liabilities.
| F-17 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars)
NOTE 7 - LONG-TERM LOANS FROM BANKING INSTITUTIONS
| A. | Composition |
Interest
rate at 2020 | December 31, | |||||||||||
| % | 2020 | 2019 | ||||||||||
| Long-term loans | 2.1 | 16,064 | 22,185 | |||||||||
| Less current maturities | (7,949 | ) | (7,230 | ) | ||||||||
| 8,115 | 14,955 | |||||||||||
| B. | Maturity dates: |
| First year | 7,949 | |||
| Second year | 8,115 | |||
| 16,064 |
NOTE 8 – COMMITMENT AND CONTINGENT LIABILITIES
| A. | Save Foods Ltd. is committed to pay royalties to the IIA on the proceeds from sales of products resulting from research and development projects in which the IIA participates by way of grants. In the first 3 years of sales the Company shall pay 3% of the sales of the product which was developed under IIA research and development projects. In the fourth, fifth and sixth years of sales, the Company shall pay 4% of such sales and from the seventh year onwards the Company shall pay 5% of up to 100% of the amount of grants received plus interest at LIBOR. Save Foods Ltd. was entitled to the grants only upon incurring research and development expenditures. There were no future performance obligations related to the grants received from the IIA. As of December 31, 2020, the contingent liabilities with respect to grants received from the IIA, subject to repayment under these royalty agreements on future sales is NIS 484,429 (US$ 150,678), not including interest. | |
| B. | The Company and its subsidiary currently lease office space at Kibbutz Alonim under a short-term operating lease agreement ends at December 31, 2020 with an option to extend the agreement with additional year ended at December 31, 2021. During the years 2020 and 2019, the Company paid an annual rent of $14,967 and $10,605, respectively under the above agreement. The agreement was automatically renewed (option) until December 31, 2021. |
In addition, the Company and Save Foods Ltd. entered into a short term lease agreement for the period ended at May 31, 2019, with a shareholder for the lease of an office and related services for a monthly fee of NIS 5,000 (approximately $1,400).
On May 1, 2019 the Company and Save Foods Ltd. entered into a lease agreement for its offices in Tel Aviv for the period ending December 31, 2020, for the lease of an office and management fees for a monthly fee of NIS 11,214 (approximately $3,450). On August 9, 2020, the Company and the lessor agreed that the Company would pay the lessor a one-time NIS10,000 ($3,100) and the agreement would be terminate as of August 13, 2020
On September 1, 2017, the Company entered into a lease agreement for office space in New York, hereinafter the New York Lease. The New York Lease will expire on September 30, 2020, unless terminated earlier by either party by providing 30 days prior written notice to the other party. The New York Lease rent amount, $7,200, was fully paid for through an issuance of 720,000 shares of our Common Stock on November 5, 2017.
| F-18 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars)
NOTE 8 – COMMITMENT AND CONTINGENT LIABILITIES (continue)
| C. | In July 2011, Save Foods, Ltd. filed with the Commissioner of Patent of the Israeli Patent Office (the “Commissioner”) a claim stating its opposition to a patent application made by Xeda International S.A, (“Xeda”), which would have restricted Save Foods Ltd’s operations. |
In June 2018, the Commissioner accepted Save Foods Ltd’s claims against Xeda’s patent application and, accordingly, rejected Xeda’s application. The Commissioner awarded Save Foods Ltd. with expenses and legal fees in the aggregate amount of approximately NIS 165,000 (approximately $46,000)
In September 2018, Xeda filed an appeal with the District Court in Jerusalem (the “Court”), with respect to the Commissioner’s decision, and in January 2019, the Court dismissed Xeda’s appeal and awarded Save Foods Ltd. expenses and legal fees in the aggregate amount of approximately NIS 50,000 (approximately $13,000).
In February 2019, Xeda filed a request to appeal the Court’s decision with the Israeli Supreme Court. In May 2019, the Israeli Supreme Court rejected Xeda’s request to appeal and awarded Save Foods Ltd. expenses and legal fees in the aggregate amount of NIS 8,000.
| D. | On September 22, 2020, the Company entered into a non-exclusive Commission Agreement with Earthbound Technologies, LLC (“EBT”) for a period of 12 months, according to which EBT shall introduce the Company to potential clients, pre-approved by the Company (“Introduced Parties”) and shall assist the Company in finalizing commercial agreements with the Introduced Parties. In consideration for its services, the Company agreed to pay EBT 12.5% of the net revenues generated from Introduced Parties (during the agreement period and within 18 months following the termination of the agreement) up to a total aggregated amount of $2,000,000, provided that the compensation shall not exceed 25% of the Company’s gross profit under the given commercial agreement signed with the Introduced Party. In addition, in the event that the aggregated net revenues generated from Introduces Parties exceeds $500,000, and subject to the approval of the Board, the Company shall issue to EBT 7,143 options to purchase 7,143 shares of Common Stock at an exercise price of $8.4 per share. In the event that certain additional events detailed in the agreement occur, the Company will also issue to EBT, subject to the approval of the Board, an additional 7,143 options to purchase 7,143 shares of Common Stock at an exercise price of $8.4 per share. |
| E. | On September 22, 2020, the Company entered into a Distribution Agreement (the “Distribution Agreement”), with Safe-Pack Products Ltd (“Safe-Pack”) according to which the Company granted Safe-Pack an exclusive right to resell, distribute, advertise, and market Company’s products related to the citrus industry in Israel and other territories, as well as additional products as shall be mutually agreed upon in the future. In addition, the Company agreed to grant Safe-Pack a right of first refusal to be designated as an exclusive distributor of the Company in certain agreed upon territory for additional products of the Company as they relate to the field of post-harvest. In consideration for the above rights granted to Safe-Pack, Safe-Pack will submit to the Company purchase orders of its products at a price specified in the Distribution Agreement. Commencing upon the second calendar year of the agreement, Safe-Pack is required to meet a minimum purchase quota, as shall be mutually agreed upon between the parties. In the event that the parties fail to agree on a quota, the quota shall be equal to last year quota plus 3%. |
NOTE 9 – SHAREHOLDERS’ EQUITY
Description of the rights attached to the Shares in the Company:
Common stock:
Each share of common stock entitles the holder to one vote, either in person or by proxy, at meetings of stockholders. The holders are not permitted to vote their shares cumulatively. Accordingly, the stockholders of the Company’s common stock who hold, in the aggregate, more than fifty percent of the total voting rights can elect all of the directors and, in such event, the holders of the remaining minority shares will not be able to elect any of such directors. The vote of the holders of a majority of the issued and outstanding shares of common stock entitled to vote thereon is sufficient to authorize, affirm, ratify or consent to such act or action, except as otherwise provided by law.
Transactions:
During January 2019 the Company issued total of 19,050 shares of Common Stock of the Company $0.0001 par value, to accredited investors for total consideration of $120,000.
During February 2019 the Company issued total of 35,717 shares of Common Stock of the Company $0.0001 par value, to accredited investors for total consideration of $225,000.
During March 2019 the Company issued total of 15,874 shares of Common Stock of the Company $0.0001 par value, to accredited investor for total consideration of $100,000.
In addition, during March 2019 the Company issued 7,937 shares of Common Stock of the Company $0.0001 par value, to an accredited investor for total consideration of $50,000 and at the same time issued him 7,937 shares of Common Stock $0.0001 par value for total consideration of $66,666 and 7,937 warrants to purchase the company shares of Common Stock at an exercise price of 84 cents.
During June 2019, the Company issued total of 31,747 shares of Common Stock of the Company $0.0001 par value, to accredited investors for total consideration of $200,000. In addition, During June 2019 the Company issued 10,004 shares of Common Stock of the Company $0.0001 par value, to an accredited investor for total consideration of $84,034 and at the same time issued that accredited investor 10,004 warrants to purchase the company shares of Common Stock at an exercise price of 84 cents.
During August 2019, the Company signed a subscription agreement with an investor according to which the Company will issue total of 11,905 shares of Common Stock of the Company $0.0001 par value, to accredited investor for total consideration of $100,000. In addition, and at the same time issued that accredited investor 11,905 warrants to purchase the company shares of Common Stock at an exercise price of 84 cents.
| F-19 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – SHAREHOLDERS’ EQUITY (continue)
On May 9, 2020, the Company entered into a Securities Purchase Agreement (the “May Agreement”) with an existing shareholder (the “Investor”), pursuant to which the Company sold to the Investor for an aggregated amount of $100,000, 13,107 units at a price per unit of $7.63 (the “2019 Units”), each 2019 Unit consists of (i) one share of Common Stock and (ii) one warrant to purchase one share of Common Stock with an exercise price of $8.40 for a period of 36 months following the issuance date. The shares of Common Stock were issued on July 2, 2020.
On July 2, 2020, the Company issued 67,369 shares of Common Stock in respect of the conversion of convertible loans as detailed in Note 3A above.
During July and August 2020, the Company entered into additional Securities Purchase Agreements with existing shareholders (the “Additional Investors”), pursuant to which the Company sold to the Additional Investors for an aggregate amount of $150,000, 19,662 units, based substantially upon the same terms as in the May Agreement.
On September 23, 2020, the Company entered into a Securities Purchase Agreement (the “Medigus SPA”) with Medigus Ltd. (“Medigus”) in connection with the sale and issuance of 13,107 units for total consideration of $100,000, based substantially upon the same terms as in the May Agreement.
The Medigus SPA contemplates an additional investment by Medigus not to exceed $25,000 (the “Additional Medigus Investment”), which shall be triggered following the parties’ initiation of a proof of concept procedure to test the effectiveness of the Company’s sanitizers and its residual effects on surfaces against different pathogens including COVID-19. In consideration for the Additional Medigus Investment, the Company has agreed to issue an additional 3,277 units at a purchase price of $7.63, which units shall contain the same composition of securities as described in the foregoing description of the Medigus SPA.
On September 22, 2020 and September 24, 2020, the Chairman of the Board of Directors of the Company (the “Board”), exercised a warrant to purchase an aggregate of 28,572 shares of Common Stock, which warrants were granted to him on June 15, 2020 by the Board as a replacement for his recently expired options, which were previously granted to him in April 2018.
During December 2020, two directors on Save Food Ltd exercised 6,350 options under the 2018 Equity Incentive Plan into 6,350 shares of common stock of the Company total consideration of $20,000.
| F-20 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – STOCK OPTIONS
On October 18, 2018, the Company adopted the 2018 Share Incentive Plan (the “2018 Equity Incentive Plan”), pursuant to which the Company’s Board of Directors is authorized to grant up to 190,477 options, exercisable into 190,477 shares of Common Stock of the Company. The purpose of the 2018 Equity Incentive Plan is to offer attract and retain the best available personnel, provide incentive to individuals who perform services for the Company and promote the success of the Company’s business.
On January 3, 2019, the Company granted of 80,954 options under the 2018 Equity Incentive Plan of which 9,524 options are vested quarterly over three years commencing May 15, 2018, 35,715 options are vested 1/3 after a year commencing October 1, 2018 and the remaining 2/3 are vested quarterly over additional two years, 14,286 options are vested quarterly over three years commencing October 1, 2018 and 21,429 options are vested 1/3 after a year commencing January 1, 2019 and the remaining 2/3 are vested quarterly over additional two years
On April, 2019, the Board of Directors of the Company approved the issuance of 28,572 options to purchase 28,572 Company’s Common Stock 0.0001 par value, to Mr. Dan Sztybel, under the Company’s 2018 Equity Incentive Plan. The options shall vest quarterly over three years, commencing April 1, 2019, and shall be exercisable for an exercise price of $3.15 per share. In addition, the Board of Directors of the Company approved the issuance of 14,286 options to purchase 14,286 Company’s Common Stock 0.0001 par value, to Mr. Dan Sztybel, subject to Save Foods Ltd’s obtainment of certain EPA and FDA approvals by the end of the second quarter of 2020. Such conditions did not met as of June 30, 2020.
On November 12, 2019, the Board of Directors of the Company approved the grant of 45,239 options under the 2018 Equity Incentive Plan of which 28,572 options are vested quarterly over three years commencing January 3, 2019, 9,524 options are vested 1/3 after a year commencing January 3, 2019 and the remaining 2/3 are vested quarterly over additional two years, and 7,143 options are vested 1/3 after a year commencing October 1, 2019 and the remaining 2/3 are vested quarterly over additional two years.
In addition, the Board of Directors approved the agreement with a consultant, according to which the consultant would receive 2,858 fully vested options to purchase Company’s shares at exercise price of $6.3 per option for certain “closed” introduction made by the consultant in Chile. No options were granted under this agreement as of December 31, 2020.
On June 23, 2020, the Company granted 21,143 options to purchase its Common Stock under the 2018 Equity Incentive Plan (the “Plan”). The options shall vest quarterly over two years commencing June 23, 2020, whereby 12.50% of the shares covered by the options will vest on the three month anniversary of June 23, 2020, and 12.50% of the shares covered by the options will vest at the end of each subsequent three month period thereafter over the course of the subsequent 21 months.
On July 1, 2020, the Company granted 71,431 options to purchase its Common Stock under the 2018 Equity Incentive Plan. The options shall vest quarterly over two years commencing June 1, 2020, whereby 12.50% of the shares covered by the options will vest on the three month anniversary of June 1, 2020, and 12.50% of the shares covered by the options will vest at the end of each subsequent three month period thereafter over the course of the subsequent 21 months. The fair value of the options was estimated at a value of $344,767 at the date of issuance using the Black-Scholes option pricing model.
In addition, on July 1, 2020, the Board approved an increase to the share option pool under the Plan by 99,466 shares of Common Stock, such that after the increase the total number of shares of Common Stock issuable under the Plan is 289,942 shares of Common Stock.
On September 22, 2020, the Board approved an amendment of the terms of the outstanding options granted to certain employees and directors of the Company. According to the new terms, subject to the consummation of equity financing in excess of $1,000,000 and the completion of listing of the Company’s Common Stock for trade on the Nasdaq, and in the event that the employment or engagement of such grantee is either terminated (not for cause) or otherwise changed thereby resulting in the conclusion of such engagement (including voluntary resignation), all outstanding options of such grantee shall vest immediately and shall be exercisable for a period of three years following the termination date.
| F-21 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 10 – STOCK OPTIONS (continue)
The following table presents the Company’s stock option activity for employees and directors of the Company for the year ended December 31, 2020 and 2019:
Number of Options | Weighted Average Exercise Price | |||||||
| Outstanding at January 1, 2019 | 59,525 | 3.15 | ||||||
| Granted (*) | 109,526 | 3.15 | ||||||
| Exercised | - | - | ||||||
| Forfeited | (4,762 | ) | 3.15 | |||||
| Outstanding at January 1, 2020 | 164,289 | 3.15 | ||||||
| Granted | 92,574 | 3.64 | ||||||
| Exercised | (6,350 | ) | 3.15 | |||||
| Forfeited | (29,365 | ) | 3.15 | |||||
| Expired | (14,286 | ) | 3.15 | |||||
| Outstanding at December 31, 2020 | 206,862 | 3.15 | ||||||
| Number of options exercisable at December 31, 2020 | 97,351 | 3.27 | ||||||
(*) Options that were granted on January 3, 2019 with vesting that commences before December 31, 2018.
The aggregate intrinsic value of the awards outstanding as of December 31, 2020 is US$1,084,465. These amounts represent the total intrinsic value, based on the Company’s stock price of US$ 8.61 as of December 31, 2020, less the weighted exercise price. This represents the potential amount received by the option holders had all option holders exercised their options as of that date.
The fair value of options granted was estimated at the dates of grant using the Black-Scholes option pricing model. The following are the data and assumptions used:
| 2020 | 2019 | |||||||
| Dividend yield | 0 | 0 | ||||||
| Expected volatility (%) (*) | 52 | % | 54 | % | ||||
| Risk-free interest rate (%) (**) | 0.23 | % | 1.56-2.39 | % | ||||
| Expected term of options (years) (***) | 5 | 5 | ||||||
| Exercise price (US dollars) | 3.15-3.78 | 3.15 | ||||||
| Share price (US dollars) | 7.63 | 6.3 | ||||||
| Fair value (US dollars) | 4.83-5.17 | 4.19 | ||||||
| (*) | Due to the low trading volume of the Company’s Common Stock, the expected volatility was based on the historical volatility of the share price of other public companies that operate in the same industry sector as the Company (agricultural chemical industry). | |
| (**) | The risk-free interest rate represented the risk-free rate of US$ zero – coupon US Government Loans. | |
| (***) | Due to the fact that the Company does not have sufficient historical exercise data, the expected term was determined based on the “simplified method” in accordance with SEC Staff Accounting Bulletin No. 110. |
The total fair value estimation of the non-cash compensation of the grant at 2020 and 2019 was approximately $453,976 and $440,848, respectively. Costs incurred in respect of stock-based compensation for employees and directors, for the year ended December 31, 2020 and 2019 were $492,619 and $438,670, respectively.
As of December 31, 2020, there are 76,730 options available for future grants under the 2018 Equity Incentive Plan.
| F-22 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 11 – COST OF SALES
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | 8,074 | 35,142 | ||||||
| Share based compensation | 5,402 | 14,531 | ||||||
| Materials | 16,692 | 69,390 | ||||||
| Vehicle maintenance | 2,063 | 8,455 | ||||||
| Travel expenses | 978 | 3,408 | ||||||
| Transportation and storage | 5,632 | 13,299 | ||||||
| Other expenses | 4,564 | 323 | ||||||
| 43,405 | 144,548 | |||||||
NOTE 12 – RESEARCH AND DEVELOPMENT EXPENSES
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Salaries and related expenses | 39,021 | 177,712 | ||||||
| Share based compensation | 91,190 | 75,998 | ||||||
| Professional fees | 130,592 | 178,854 | ||||||
| Depreciation | 29,319 | 20,544 | ||||||
| Travel expenses | 7,190 | 26,138 | ||||||
| Vehicle maintenance | 13,657 | 26,227 | ||||||
| Rent and asset management | - | 10,582 | ||||||
| Laboratory and Field tests | 72,593 | 73,968 | ||||||
| Other expenses | 33,438 | 25,600 | ||||||
| 417,000 | 615,623 | |||||||
| F-23 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 13 – GENERAL AND ADMINISTRATIVE EXPENSES
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Professional services | 443,883 | 461,840 | ||||||
| Share based compensation | 416,996 | 283,910 | ||||||
| Legal expenses | 67,492 | 125,753 | ||||||
| Insurance | 63,380 | 54,367 | ||||||
| Rent and office maintenance | 11,135 | 38,080 | ||||||
| Levies and tolls | 28,477 | 8,601 | ||||||
| Communications | 1,679 | 1,910 | ||||||
| Depreciation | 13,914 | 2,133 | ||||||
| Travel expenses | 5,305 | 15,310 | ||||||
| Other expenses | 17,848 | 12,995 | ||||||
| 1,070,109 | 1,004,899 | |||||||
NOTE 14 – FINANCING EXPENSES, NET
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Interest and amortization expenses | 202,917 | 4,323 | ||||||
| Currency exchange differences | 34,037 | 28,266 | ||||||
| Changes in fair value of convertible loans | 27,208 | - | ||||||
| Bank charges and other finance expenses, net | 6,231 | 10,819 | ||||||
| 270,393 | 43,408 | |||||||
NOTE 15 – INCOME TAX
| A. | US resident companies are taxed on their worldwide income for corporate income tax purposes at a statutory rate of 21% this reflect certain effects of the Act which includes a reduction in the corporate tax rate from 35% to 21% as well as other changes. No further taxes are payable on this profit unless that profit is distributed. If certain conditions are met, income derived from foreign subsidiaries is tax exempt in the US under applicable tax treaties to avoid double taxation.
| |
Income of the Israeli company is taxable from 2018 and onwards, at corporate tax rate of 23%.
| ||
The Company and Save Foods Ltd. has not received final tax assessments since its inception.
| ||
As of December 31, 2020, the Company and Save Foods Ltd. has estimated carry forward losses for tax purposes of approximately $1,264,000 and $9,412,000, respectively, which can be offset against future taxable income, if any.
|
| F-24 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 15 – INCOME TAX (continue)
| B. | The following is reconciliation between the theoretical tax on pre-tax income, at the tax rate applicable to the Company (federal tax rate) and the tax expense reported in the financial statements: |
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Pretax loss | 1,593,139 | 1,971,417 | ||||||
| Federal tax rate | 21 | % | 21 | % | ||||
| Income tax computed at the ordinary tax rate | 334,559 | 413,998 | ||||||
| Non-deductible expenses | (63,565 | ) | (2,278 | ) | ||||
| Stock-based compensation | (109,772 | ) | (93,970 | ) | ||||
| Tax in respect of differences in corporate tax rates | 13,480 | 26,688 | ||||||
| Losses and timing differences in respect of which no deferred taxes were generated | (174,702 | ) | (344,438 | ) | ||||
| - | - | |||||||
| C. | Deferred taxes result primarily from temporary differences in the recognition of certain revenue and expense items for financial and income tax reporting purposes. Significant components of the Company’s future tax assets are as follows: |
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Composition of deferred tax assets: | ||||||||
| Provision for employee related obligation | 31,627 | 29,353 | ||||||
| Non capital loss carry forwards | 2,350,367 | 2,171,821 | ||||||
| Valuation allowance | (2,381,994 | ) | (2,201,174 | ) | ||||
| - | - | |||||||
| F-25 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 16 – LOSS PER COMMON STOCK
Basic loss per share is computed by dividing net loss by the weighted average number of shares outstanding during the year. The weighted average number of shares of Common Stock used in computing basic and diluted loss per common stock for the years ended December 31, 2020 and 2019, are as follows:
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| Number of shares | ||||||||
| Weighted average number of shares of Common Stock outstanding attributable to shareholders | 1,519,122 | 1,424,045 | ||||||
| Total weighted average number of shares of Common Stock related to outstanding options, excluded from the calculations of diluted loss per share (*) | 206,862 | 164,289 | ||||||
(*) The effect of the inclusion of option and convertible loans in 2020 and 2019 is anti-dilutive.
NOTE 17 – RELATED PARTIES
A. Transactions and balances with related parties
| Year ended December 31 | ||||||||
| 2020 | 2019 | |||||||
| General and administrative expenses: | ||||||||
| Directors compensation | 380,756 | 295,088 | ||||||
| Salaries and fees to officers | 336,433 | 358,370 | ||||||
| Consultants and other fees | 52,331 | - | ||||||
| (*) 769,520 | (*) 653,458 | |||||||
| (*) share based compensation | 394,756 | 272,077 | ||||||
| Research and development expenses: | ||||||||
| Salaries and fees to officers | 25,301 | 116,692 | ||||||
B. Balances with related parties and officers:
| Other accounts payables | 424,515 | 199,983 |
C. Other information:
| A. | On November 5, 2020, the board of directors of the Company appointed Mr. David Palach, to serve as co-Chief Executive Officer of the Company, effective as of the same date. In connection with Mr. Palach’s appointment, the parties entered into a Consulting Agreement pursuant to which the Company and Mr. Palach agreed upon, inter alia, the following engagement terms: (a) a monthly retainer of $8,000, and (b) a grant of options to purchase shares of the Company’s common stock, which amount shall be determined by the Board on a future date. |
| B. | On October 10, 2018 the Board of directors of Save Foods Ltd. approved to engage in consulting agreements with Amir Uziel Economic Consultant Ltd (a company controlled by Amir Uziel) and with L.A Pure Capital Ltd (a company controlled by Kfir Zilberman) at a monthly fee of $1,500. |
| C. | On October 10, 2018 the Board of directors of Save Foods Ltd. approved a monthly directors fee of $1,500 to Itzhak Shrem. |
| F-26 |
SAVE FOODS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars, except share and per share data)
NOTE 17 – SUBSEQUENT EVENTS
| A. | On February 23, 2021, the Company amended its Certificate of Incorporation to effect a 7 to 1 reverse stock split of the Company’s outstanding Common Stock. As a result of the reverse stock split, which became effective on February 23, 2021, every 7 shares of the Company’s outstanding Common Stock prior to the effect of that amendment was combined and reclassified into one share of the Company’s Common Stock. No fractional shares were issued in connection with or following the reverse split. The number of authorized capital of the Company’s Common Stock and par value of the shares remained unchanged. |
All share, stock option and per share information in these condensed consolidated financial statements have been restated to reflect the stock split on a retroactive basis.
| B. | During January 2021, the Company entered into a series of additional 2020 CLAs (See note 6B above) with additional 2020 Lenders to sell additional 2020 Notes with an aggregate principal amount of $274,000. |
| F-27 |
