Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - NOVAVAX INC | tm212728d1_8k.htm |
Exhibit 99.1

Nasdaq: NVAX | January 2021 J.P. MORGAN 39 TH ANNUAL HEALTHCARE CONFERENCE - VIRTUAL

2 novavax.com Safe Harbor Statement Certain information, particularly information relating to future performance and other business matters, including expectations regarding clinical development, market opportunities and anticipated milestones constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act. Forward - looking statements may generally contain words such as “believe,” “may,” “could,” “will,” “possible,” “can,” “estimate,” “continue,” “ongoing,” “consider,” “intend,” “indicate,” “plan,” “project,” “expect,” “should,” “would,” or “assume” or variations of such words or other words with similar meanings. Novavax cautions that these forward - looking statements are subject to numerous assumptions, risks and uncertainties that change over time and may cause actual results to differ materially from the results discussed in the forward - looking statements. Uncertainties include but are not limited to clinical trial results, dependence on third party contractors, competition for clinical resources and patient enrollment and risks that we may lack the financial resources to fund ongoing operations. Additional information on Risk Factors are contained in Novavax’ filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10 - K for the year ended December 31, 2019, our Quarterly Reports on Form 10 - Q, and our Current Reports on Form 8 - K, which are all available at http://www.sec.gov. Forward - looking statements are based on current expectations and assumptions and currently available data and are neither predictions nor guarantees of future events or performance. Current results may not be predictive of future results. You should not place undue reliance on forward - looking statements which speak only as of the date hereof. The Company does not undertake to update or revise any forward - looking statements after they are made, whether as a result of new information, future events, or otherwise, except as required by applicable law. Matrix - M and NanoFlu are trademarks of Novavax, Inc. Safe Harbor Statement

3 novavax.com Agenda • Review of NVX - CoV2373 development • Unique and differentiated vaccine platform • Clinical development program update • Manufacturing and commercial plans • Financial summary

4 novavax.com NVX - CoV2373 Overview

5 novavax.com 2020 NVX - CoV2373 Key Accomplishments DISCOVERY CLINICAL MANUFACTURING FUNDING SARS - CoV - 2 Sequence Published US DoD Funding Praha Vaccines Acquired to Expand Global Supply Chain Large - Scale Manufacturing Initiated Phase 1 - 2 Clinical Trial Initiated Positive Phase 1 Data Announced Phase 2 Trial Initiated Phase 3 UK Trial Initiated Phase 2 Preliminary Data PREVENT - 19 Phase 3 US & Mexico Trial Initiated Additional CEPI Funding Initial CEPI Funding Phase 2b SA Trial Initiated NVX - CoV2373 Identified BMGF Funding OWS Funding NVX - CoV2373 NHP Data JAN FEB MAR APR MAY JUNE JULY AUG SEPT OCT NOV DEC

6 novavax.com 2021: A Pivotal Year for Novavax • Phase 2b South Africa data readout • Phase 3 UK data readout • PREVENT - 19 trial (Phase 3 US/Mexico trial); complete enrollment and data readout • Finalize multiple Advance Purchase Agreements • Gain authorization for use in UK, US, EU and other countries • Commercial scale manufacturing • Commercial revenue expected

7 novavax.com NVX - CoV2373 Progress Built Upon Years of Vaccine Research

8 novavax.com Recombinant Nanoparticle Technology Platform and Matrix - M™ Combined to Create Vaccines That Address Global Public Health Threats Matrix - M adjuvant provides the danger signal to the immune cells, leading to higher quantity and quality immunity Recombinant protein nanoparticles mimic virus surface proteins, stimulate immunity but are not infectious *Coronavirus image: CDC Library Virus infecting cells use surface proteins to attach and infect Recombinant nanoparticle and Matrix - M adjuvant are premixed and stable using standard refrigeration

9 novavax.com SARS - CoV - 2 Full Length Spike Protein • Full - length, natively configured spike protein trimer in a detergent nanoparticle • Formulated with Matrix - M • Highly immunogenic • Spike protein is a validated target NVX - CoV2373: A full - length, prefusion stabilized SARS - CoV - 2 spike (S) glycoprotein + Matrix - M™ Cryo - EM map of trimers showing the spike in prefusion state Bangaru et al., 2020 Transmission Electron Microscopy of CoV2373 Trimers Tian et al., 2020

10 novavax.com Novavax Technology and Programs Validated Through Peer - Reviewed Publications in 2020
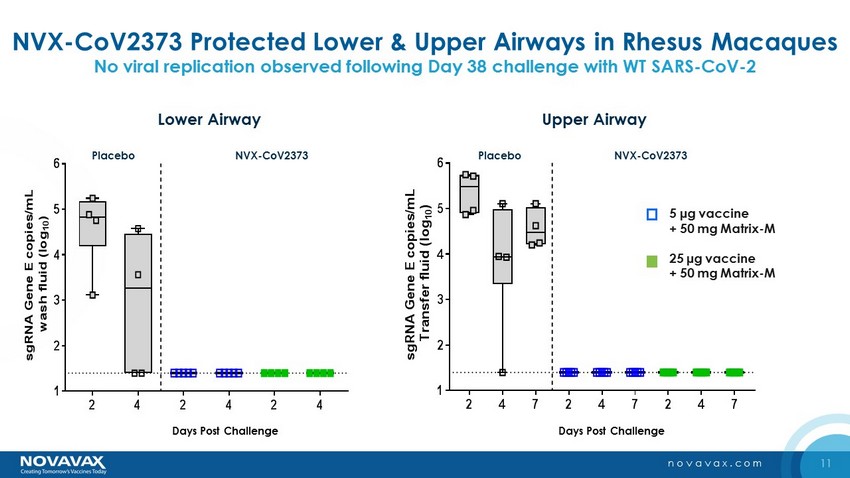
11 novavax.com NVX - CoV2373 Protected Lower & Upper Airways in Rhesus Macaques No viral replication observed following Day 38 challenge with WT SARS - CoV - 2 2 4 2 4 2 4 1 2 3 4 5 6 s g R N A G e n e E c o p i e s / m L w a s h f l u i d ( l o g 1 0 ) Placebo Days Post Challenge 25g vaccine+50g Matrix-M1 5g vaccine+50g Matrix-M1 BAL: Subgenomic RNA NVX-CoV2373 2 4 7 2 4 7 2 4 7 1 2 3 4 5 6 s g R N A G e n e E c o p i e s / m L T r a n s f e r f l u i d ( l o g 1 0 ) Placebo Days Post Challenge 25g vaccine+50g Matrix-M1 5g vaccine+50g Matrix-M1 Nasal Swab: Subgenomic RNA NVX-CoV2373 Lower Airway Upper Airway 5 µg vaccine + 50 mg Matrix - M 25 µg vaccine + 50 mg Matrix - M Placebo Days Post Challenge Days Post Challenge NVX - CoV2373 Placebo NVX - CoV2373

12 novavax.com NVX - CoV2373 Clinical Development Plan Overview

13 novavax.com NVX - CoV2373 Clinical Development Plan US & AUSTRALIA PHASE 1 - 2 PHASE 2b PHASE 3 PHASE 3 SOUTH AFRICA UNITED KINGDOM US & MEXICO (PREVENT - 19) n = 4,404 18 - 65 years (n=245 HIV+) Enrollment Complete Data Expected Q1 ’21 n = 15,203 18 - 84 years (n=400 co - admin with flu vaccine) Enrollment Complete Interim Data Expected Q1 ’21 n ~30,000 ≥18 years Enrolling Interim Data Expected Q2 ’21 n = 1,600 18 - 65 years Enrollment Planned Q1 *Conducted by Serum Institute n = 200 >18 years Enrollment Planned Q1 **Conducted by Takeda 2 - 3 PHASE 1 - 2 PHASE INDIA* JAPAN** 2 PHASE CZECH REPUBLIC n = 1,288 ≥18 years (n=600 >60 years) Enrollment Complete n = 131 18 - 59 years Enrollment Complete Data Published Phase 1 Phase 2 n ~ 120 Age TBC Enrollment Planned Q1

14 novavax.com NVX - CoV2373 Phase 1 - 2 Study (US & Australia)

15 novavax.com US and Australia Phase 1 Study Design 5 µg + 50 µg Matrix - M™ (2 injections: Day 0 and Day 21) n = 131 Adults 18 - 59 years R 25 µg + 50 µg Matrix - M (2 injections: Day 0 and Day 21) Placebo (2 injections: Day 0 and Day 21) N= 258 Placebo (Day 21) 25 µg + 50 µg Matrix - M (Day 0) N= 255 N= 255 Reactogenicity data reviewed by SMC & FDA in advance of Phase 3 study R 1:1:1:1:1 25 µg (2 injections: Day 0 and Day 21)

16 novavax.com Phase 1: Induction of Robust Levels of Anti - S IgG and 100% Neutralization Responses at Day 35 GMEU 63,160 47,521 8,344 95% CI (47,117; 84,666) (33,803; 66,804) (4, 420 ; 15,747) GMT 3,906 3,305 983 95% CI (2,556; 5,970) (2,205; 4,953) (579 ; 1 ,670) *Convalescent Sera donated by Dr Pedro A Piedra Baylor College of Medicine (samples obtained median 19 days after diagnosis, 10% asymptomatic, 77% outpatient ER, 13% hospitalized) Wild - type neutralization assay conducted by the Dr Matthew Frieman Lab University of Maryland School of Medicine

17 novavax.com Phase 1: Robust Immune Response Through Six Months Matrix - M TM required for optimal immune response; 2 adjuvanted doses superior to 1 dose Matrix - M is dose - sparing Phase 1: n = 131; 18 - 59 years of age; Vaccination on Day 0 and D21 (pre - publication) Placebo 2 Doses: 25 µg (no adjuvant) 1 Dose: 25 µg + Matrix - M 2 Doses: 25 µg + Matrix - M 2 Doses: 5 µg + Matrix - M

18 novavax.com US and Australia Phase 2 Study Design Includes ~50% of adults age 60 - 84 years 5 µg + 50 µg Matrix - M™ (2 injections: Day 0 and Day 21) n = 1,288 Adults > 18 years 25 µg + 50 µg Matrix - M (2 injections: Day 0 and Day 21) Placebo (2 injections: Day 0 and Day 21) 5 µg + 50 µg Matrix - M (Day 0) Placebo (Day 21) N= 259 N= 258 25 µg + 50 µg Matrix - M (Day 0) Placebo (Day 21) N= 255 N= 255 R 1:1:1:1:1

19 novavax.com Phase 2: Strong Immune Response in All Age Groups 2 Matrix - M™ - adjuvanted doses of 5 µg and 25 µ g induced comparable, robust immune responses Consistent with Phase 1 data 5 µg Phase 2: n =1288; ≥ 18 years of age (n = 583 >60 years of age) ( pre - publication) 2 Doses: 25 µg + Matrix - M 2 Doses: 5 µg + Matrix - M 1 Dose: 25 µg + Matrix - M 1 Dose: 5 µg + Matrix - M Placebo
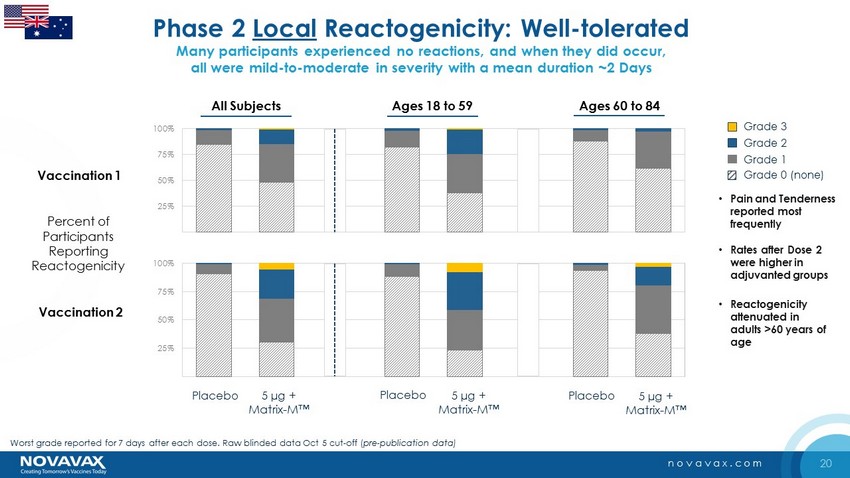
20 novavax.com • Pain and Tenderness reported most frequently • Rates after Dose 2 were higher in adjuvanted groups Percent of Participants Reporting Reactogenicity • Reactogenicity attenuated in adults >60 years of age Phase 2 Local Reactogenicity: Well - tolerated Many participants experienced no reactions, and when they did occur, all were mild - to - moderate in severity with a mean duration ~2 Days 0% 25% 50% 75% 100% 5 µg + Matrix - M™ Placebo Vaccination 2 0% 25% 50% 75% 100% Vaccination 1 Grade 3 Grade 2 Grade 1 Grade 0 (none) 5 µg + Matrix - M™ Placebo 5 µg + Matrix - M™ Placebo Worst grade reported for 7 days after each dose. Raw blinded data Oct 5 cut - off ( pre - publication data) Ages 18 to 59 Ages 60 to 84 All Subjects

21 novavax.com 0% 25% 50% 75% 100% 0% 25% 50% 75% 100% Phase 2 Systemic Reactogenicity: Well - tolerated Many participants experienced no reactions, and when they did occur, all were mild - to - moderate in severity with a mean duration ~2 Days • Fatigue, Headache and Myalgia reported most frequently • Increased rates seen in adjuvanted groups especially after Dose 2 Grade 3 Grade 2 Grade 1 Grade 4 • Reactogenicity attenuated in adults >60 years of age Grade 0 (none) Worst grade reported for 7 days after each dose. Raw blinded data Oct 5 cut - off (pre - publication data) Vaccination 2 Vaccination 1 Ages 18 to 59 Ages 60 to 84 All Subjects Percent of Participants Reporting Reactogenicity 5 µg + Matrix - M™ Placebo 5 µg + Matrix - M™ Placebo 5 µg + Matrix - M™ Placebo

22 novavax.com Phase 1 - 2 Summary: Confirms 5 µg Dose is Well - tolerated, with Strong and Consistent Responses • Consistent results from Phase 1 and Phase 2 • Confirms 5 µg dose for late - stage development • Immunogenicity ▪ High levels of antibody maintained through six months (Phase 1) ▪ Antibody responses compare favorably to convalescent response ▪ Robust antibody responses consistent between Phase 1 and 2 • Safety ▪ No SAEs, no AESIs, AEs balanced and mostly mild/moderate ▪ Vaccine well - tolerated with symptoms of ~2 - day duration

23 novavax.com NVX - CoV2373 Phase 2b Study (South Africa)
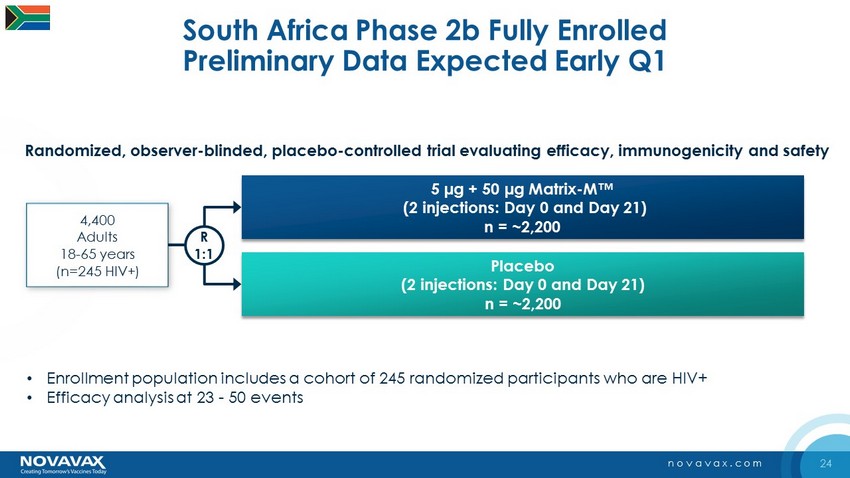
24 novavax.com South Africa Phase 2b Fully Enrolled Preliminary Data Expected Early Q1 5 µg + 50 µg Matrix - M™ (2 injections: Day 0 and Day 21) n = ~2,200 Placebo (2 injections: Day 0 and Day 21) n = ~2,200 4,400 Adults 18 - 65 years (n=245 HIV+) R 1:1 • Enrollment population includes a cohort of 245 randomized participants who are HIV+ • Efficacy analysis at 23 - 50 events Randomized, observer - blinded, placebo - controlled trial evaluating efficacy, immunogenicity and safety

25 novavax.com NVX - CoV2373 UK Phase 3 Study

26 novavax.com UK Phase 3 Fully Enrolled Preliminary Data Expected Early Q1 5 µg + 50 µg Matrix - M™ (2 injections: Day 0 and Day 21) n = ~7,500 Placebo (2 injections: Day 0 and Day 21) n = ~7,500 15,000 Adults > 18 years 25% > age 65 R 1:1 Randomized, observer - blinded, placebo - controlled trial evaluating efficacy, immunogenicity and safety • Primary endpoint: PCR - positive symptomatic mild, moderate or severe COVID - 19 illness diagnosed ≥ 7 days after second dose • Interim analysis at 50 events, final analysis at 100 events

27 novavax.com NVX - CoV2373 PREVENT - 19 US & Mexico Phase 3 Study

28 novavax.com PREVENT - 19 Phase 3 Trial Currently Enrolling Enrollment goals : 25% > age 65 years 15% Black/African American 10 - 20% LatinX 1 - 2% American Indian 5 µg + 50 µg Matrix - M™ (2 injections: Day 0 and Day 21) n = ~20,000 Placebo (2 injections: Day 0 and Day 21) n = ~10,000 n = 30,000 Adults > 18 years R 2:1 • Primary endpoint: PCR - positive symptomatic mild, moderate or severe COVID - 19 illness diagnosed ≥ 7 days after second dose • Interim analysis at 72 events, final analysis at 144 events* Randomized, observer - blinded, placebo - controlled trial evaluating efficacy, immunogenicity and safety *Protocol version 3.0 to be updated on website
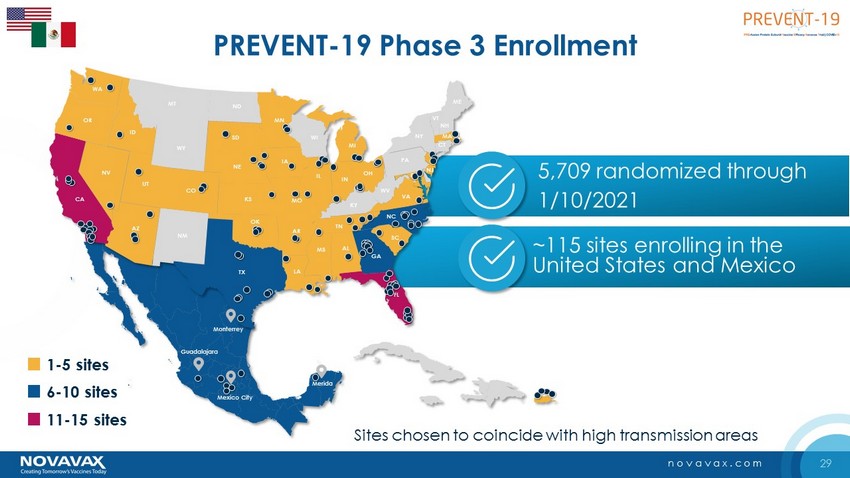
29 novavax.com PREVENT - 19 Phase 3 Enrollment OK GA VA NY FL NM TX KS NE SD ND MT WY CO UT ID AZ NV WA CA OR KY ME PA MI MA CT WV OH IN IL NC TN SC AL MS AR LA MO IA MN WI NJ VT NH DC Mexico City Merida Monterrey Guadalajara 5,709 randomized through 1/10/2021 1 - 5 sites 6 - 10 sites 11 - 15 sites ~115 sites enrolling in the United States and Mexico Sites chosen to coincide with high transmission areas

30 novavax.com NVX - CoV2373 Manufacturing & Distribution
31 novavax.com Practical Benefits Enabling Efficient Distribution Presentation • 10 - dose vials Transportation & Storage • Stable at 2 to 8 ° C Administration • Ready to use Large Global Capacity • Well - characterized technology platform; Dose - sparing

32 novavax.com Global Supply Chain Established Annual capacity of over 2 billion* doses starting in 2021 Par Pharma AGC Biologics PolyPeptide Group FujiFilm TX FujiFilm NC Novavax HQ MD Biofabri FujiFilm UK AGC Biologics PolyPeptide Group Novavax AB Serum Institute SK Bioscience Takeda Novavax facilities Vaccine distribution & license agreement Matrix - M™ production Antigen production Fill/Finish * When all planned capacity is online by mid - 2021 Baxter Siegfried Novavax CZ

33 novavax.com Summary

34 novavax.com Novavax Summary • Strong financial position and revenue realization ▪ >$2 billion non - dilutive financing through CEPI and OWS ▪ Finalize multiple Advance Purchase Agreements • Phase 3 trials on schedule – with efficacy readouts in early Q1 • Commercial scale manufacturing on track with transition to commercial - stage company

35 novavax.com
