Attached files
| file | filename |
|---|---|
| 8-K - HUMANIGEN, INC | f182128k.htm |
Exhibit 99.1

Humanigen January 2021

2 Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this presentation, including information concerning our po ssible or assumed future results of operations and expenses, business strategies and plans, competitive position, business and industry environment and potential gr owth opportunities, are forward - looking statements. Forward - looking statements reflect management's current knowledge, assumptions, judgment and expectations re garding future performance or events. Although management believes that the expectations reflected in such statements are reasonable, they give no assuranc e t hat such expectations will prove to be correct and you should be aware that actual events or results may differ materially from those contained in the forward - looki ng statements. Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forw ard - looking statements, including, without limitation, statements regarding: Our expectations for timing and achievement of the key corporate and COVID - 19 milestones described in the presentation, includin g; The target date for completion of patient enrollment in our Phase 3 trial of lenzilumab in COVID - 19 patients; The anticipated use of lenzilumab in the ACTIV - 5 Trial sponsored by NIAID, and the anticipated scope of that trial and timeline for same; Our potential request for and receipt of an Emergency Use Authorization from FDA for lenzilumab in COVID - 19 and our expectations for filing a BLA; and Our plans to launch and commercialize lenzilumab following receipt of the requisite regulatory authorizations or approvals; a nd Our expectations for timing and achievement of milestones for our pipeline outside of COVID - 19, including in respect of our ZUMA - 19 Phase Ib trial of lenzilumab that is being conducted with Kite, a Gilead company; our ongoing Phase I trial of ifabotuzumab in GBM patients; our plans for a study of lenzilumab in GvHD expected to be conducted with the IMPACT Group in the United Kingdom and a study in the US, and our plans for a study of lenz ilu mab in CMML in Australia; Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack o f p rofitability and need for additional capital to grow our business; our dependence on partners to further the development of our product candidates; the un certainties inherent in the development and launch of any new pharmaceutical product; the outcome of pending or future litigation; and the various risks and uncertainties described in the "Risk Factors" sections of our latest annual and quarterly reports and other filings with the SEC. All forward - looking statements are expressly qualified in their entirety by this cautionary notice. You should not rely upon any forward - looking statements, as predictions of future events. We undertake no obligation to revise or update any forward - looking statements made in this presentation to ref lect events or circumstances after the date hereof, to reflect new information or the occurrence of unanticipated events, to update the reasons why actual results c oul d differ materially from those anticipated in the forward - looking statements, in each case, except as required by law.

3 Company Highlights Leading expertise in Hyper - inflammation and Cytokine Storm across multiple therapeutic applications Strong mid - to - late stage clinical pipeline including potential registration studies in CAR - T (US) and GvHD (UK); collaboration with Kite/Gilead in CAR - T 515+ patient Phase 3 registration study in COVID - 19; ACTIV - 5 200 patient, fully - sponsored COVID - 19 BET study enrolling; positive case control - study published, positive interim analysis Backed by blue - chip institutional shareholders, including Valiant, Citadel, Point 72 and Blackrock as well as management Experienced and execution - oriented management and Board of Directors Potential Emergency Use Authorization, commercial readiness and manufacturing scale - up underway 1 2 6 3 4 5

4 Reduce risk of progression to IMV and/or death Lenzilumab, a dual action monoclonal antibody, which safely replenishes T - Cells and dampens the harmful inflammatory response, can be administered intravenously over a single day, to newly hospitalized and hypoxic COVID - 19 patients, who may or may not have received other COVID - 19 therapies. First - in - Class, Novel MOA x Neutralizes GM - CSF to prevent cytokine storm x Reduces immunogenicity x Higher binding affinity Potential Outcomes (Mayo Case Cohort Study) x 80% relative risk reduction of ventilation (IMV) and/or death x Reduced time to clinical improvement by 6 days 1 x No serious adverse events observed across multiple studies Convenient Care x For all hospitalized patients with SARS - CoV - 2 pneumonia pre - IMV x Administered IV in a single day x Can be combined with current standard of care Lenzilumab is a first - in - class mAb in a Phase 3 study to prevent the cytokine release syndrome in COVID - 19 hospitalized patients. 1 Mayo Clinic Study, GM - CSF Neutralization With Lenzilumab in Severe COVID - 19 Lenzilumab Overview

5 Clinical?Stage PipelineIndication Phase Status Centers Partners Lenzilumab Potential Registration Enabling Prevention / treatment of Hyper-inflammation / Cytokine Storm 3 Recruiting 515+ patients 475+ 24+ US 11 Brazil Company sponsored ACTIV-5/BET Prevention / treatment of Hyper-inflammation / Cytokine Storm 2 Enrolling 200 patients Up to 40 sites ZUMA-19: Break CAR-T Efficacy/Toxicity Linkage Prophylaxis as sequenced therapy with Yescarta in r/r DLBCL 1b/21 Recruiting 10 sites Including MD Anderson and other ZUMA-1 sites Prevention/Treatment of Acute GvHD Allogeneic HSCT 2/3 Advanced planning Up to 23 sites2 CMML Lenz + azacitidine in NRAS, KRAS or CBL mutant-positive newly-diagnosed patients 2 Advanced planning Undisclosed Ifabotuzumab Solid Tumors (Glioblastoma Multiforme) 1 Active, fully Recruited 1 Phase 3 may not be necessary for approval in ZUMA-19; precedent is CAR-Ts to date have been approved on Phase 2 data 2 UK 3 Australia
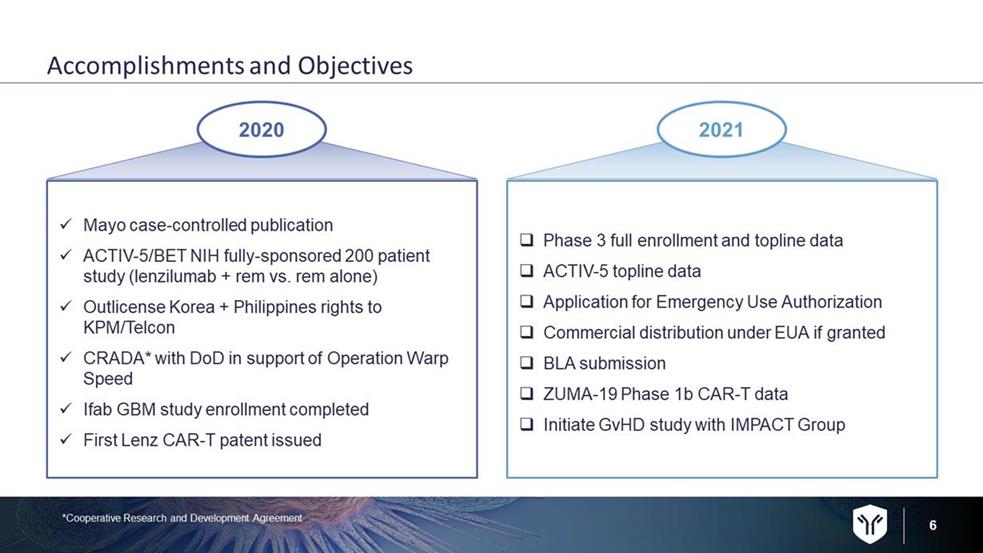
6 Accomplishments and Objectives □ Phase 3 full enrollment and topline data □ ACTIV - 5 topline data □ Application for Emergency Use Authorization □ Commercial distribution under EUA if granted □ BLA submission □ ZUMA - 19 Phase 1b CAR - T data □ Initiate GvHD study with IMPACT Group x Mayo case - controlled publication x ACTIV - 5/BET NIH fully - sponsored 200 patient study (lenzilumab + rem vs. rem alone) x Outlicense Korea + Philippines rights to KPM/Telcon x CRADA* with DoD in support of Operation Warp Speed x Ifab GBM study enrollment completed x First Lenz CAR - T patent issued 2020 2021 *Cooperative Research and Development Agreement

7 3 2 1 Therapeutic for COVID - 19 Patients Prevention / Treatment of Acute GvHD Market Potential/Financial overview

8 Neutralization of GM - CSF to Prevent COVID - 19 Cytokine Storm GM - CSF neutralization prevents CD14+CD16+ inflammatory myeloid cell activation and reduces all downstream monokine production IL - 6 blockade reduces only IL - 6 and does not block inflammatory myeloid cells activation and all downstream monokine production IL - 6 blockade alone has not shown clinical utility as a preventative measure in CAR - T induced Cytokine Storm – IL - 6 and IL - 1 inhibitors have failed in studies in COVID - 19 Three recent independent publications support role of GM - CSF as signature cytokine for hyperinflammation in COVID - 19 1,2,3 Diagram: Temesgen, Zelalem et al. “GM - CSF Neutralization With Lenzilumab in Severe COVID - 19 Pneumonia: A Case - Control Study.” 03 Sep. 2020 1. Blot, Mathieu et al. “ The dysregulated innate immune response in severe COVID - 19 pneumonia that could drive poorer outcome. “J Transl Med. 2020 Dec 3; 18(1):457. doi: 10.1186/s12967 - 020 - 02646 - 9 2. Hue, Sophie et al. “ Uncontrolled Innate and Impaired Adaptive Immune Responses in Patients with COVID - 19 ARDS.” 31 Aug. 2020 3. Gartlan, Kate et al. “Donor T - cell – derived GM - CSF drives alloantigen presentation by dendritic cells in the gastrointestinal tra ct.” Blood Adv. 2019 Oct 8;3(19):2859 - 2865. doi: 10.1182/bloodadvances.2019000053

9 Lenzilumab Phase 3 in Severe or Critical COVID?19 Multicenter, randomized, double-blind, placebo-controlled pivotal trial NCT 04351152 Adults ? 18 yrs Hospitalized, confirmed SARS-CoV-2, COVID-19 pneumonia SpO2 ? 94% and pre IMV (N = 515+, 1:1) Lenzilumab 600mg IV Q8h x 3 on Day 1600mg IV Q8h x 3 on Day 1 Placebo IV Q8h x 3 on Day 1 Daily assessment through Day 28 for 1 and 2 endpoints while hospitalized; If discharged, follow-up assessments on Days 28 and Day 60 Endpoints: ? Time to Recovery ? Ventilator free survival Adults ? 18 yrs Hospitalized, confirmed SARS-CoV-2, COVID-19 pneumonia SpO2 ? 94% and pre IMV (N = 515+, 1:1) All subjects receive institutional standard of care which may include remdesivir or corticosteroids

10 Mayo Clinic Case - Control Trial Design • Case - control study ( n=39) • 12 patients treated with lenzilumab • Lenzilumab treated patients dosed with 600 mg intravenously for three doses • Contemporaneous standard of care control patients selected from electronic medical records from across Mayo clinic enterprise (n=27) • Substantial number of standard of care control patients received other COVID - specific treatments • Outcomes of control patients unknown at time of selection. • Patients matched for age, gender, disease severity Source: Temesgen, Zelalem et al. “GM - CSF Neutralization With Lenzilumab in Severe COVID - 19 Pneumonia: A Case - Control Study.” 03 Sep. 2020

11 Mayo Clinic Case - Control Trial Baseline Characteristics Table 1. Demographics and baseline characteristics Characteristic Lenzilumab group (n=12) Control group (n=27) P-value Age, y 65 (52-70) 68 (61-76) .25 Male 8 (67%) 19 (70%) > .99 Female 4 (33%) 8 (30%) > .99 Race White 9 (75%) 17 (63%) .79 Asian 2 (17%) 5 (19%) > .99 American Indian/Native American 1 (8%) 0 (0%) .36 Comorbidities Diabetes mellitus 7 (58%) 14 (52%) > .99 Hypertension 7 (58%) na na Obesity (BMI > 30) 6 (50%) 9 (33%) .54 Coronary artery disease 2 (17%) 4 (15%) > .99 Kidney transplantation 1 (8%) na na Obstructive lung disease 4 (33%) na na Chronic obstructive pulmonary disease 2 (17%) 11 (41%) .47 Reactive airway disease 1 (8%) na na Temperature (degrees Celcius) 38 (37.25-38.5) 37.5 (37.1-38.4) .76 Inflammatory markers before treatment CRP (<= 8.0 mg/L) 103.2 (52.7-159.9) 74.4 (42.2-131.5) .25 Ferritin (24-336mcg/L) 596.0 (358.3-709.0) 673.0 (406.8-1012.8) .75 IL-6 (<= 1.8 pg/mL) 30.95 (24.18-34.05) 29.20 (13.55-40.70) .87 D-dimer (<=500 ng/mL) 829 (513.5-1298.5) 916.0 (585.0-1299.0) .84 Lymphocyte count before treatment (0.95-3.07x10^9/L) 0.75 (0.55-1.04) 0.76 (0.59-1.01) .91 Oxygen therapy before treatment Nasal cannula (=4 clinical ordinal endpoint scale) 8 (67%) 20 (74%) > .99 High-flow oxygen/NIPPV (=3 clinical ordinal endpoint scale) 4 (33%) 7 (26%) .73 Invasive ventilation (=2 clinical ordinal endpoint scale) 0 (0%) 0 (0%) > .99 SpO2/FiO2 before treatment 280.9 (252.5-317.9) 289.1 (254.9-342.0) .98 • Patients matched for baseline characteristics • Lenzilumab group has slightly higher COVID - 19 relative risk factors • DM2, HTN, BMI • CRP elevated in lenzilumab group but T cell counts similar • Oxygen therapy equally matched

12 Lenzilumab Significantly Improved Clinical Outcomes 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 V e n t i l a t o r - F r e e S u r v i v a l ( % ) Days Post Treatment Lenzilumab Control χ 2 = 3.67, P= .06 Figure 1 A B 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 C u m u l a t i v e I n c i d e n c e o f 2 - P o i n t C l i n i c a l I m p r o v e m e n t ( % ) Days Post Treatment Lenzilumab Control χ 2 = 7.43, P= .006 Time to Clinical Improvement Progression to IMV and/or Death Time to clinical improvement significantly shorter for lenzilumab (5 days vs 11 days) Ventilator - free survival better for lenzilumab

13 Lenzilumab Phase 3 - Interim Analysis * Benefit that is in addition to any benefit from steroids and/or remdesivir in the control arm • Patient characteristics for first 165 patients show good distribution of: • Disease severity • Age • Diversity • Extensive use of concomitant therapies • Estimated HR of 1.37 suggests 37% increase in recoveries with lenzilumab across the 28 - day period Disease Stratification N=165 SpO2<94% or Low - flow 65% High - flow or NIPPV 35% Age Stratification 18 - 64 years 55% >65 years 45% Diversity Hispanic / Latino 28% Black / African American 22% Concomitant Therapies Dexamethasone (or other steroids) or Remdesivir 94% Dexamethasone (or other steroids) 90% Remdesivir 66% Dexamethasone (or other steroids) + Remdesivir 62% Interim Analysis Observed Hazard Ratio 1.37* Events Planned 257 DSMB Recommended Events 402 Power at New Event Rate 90%
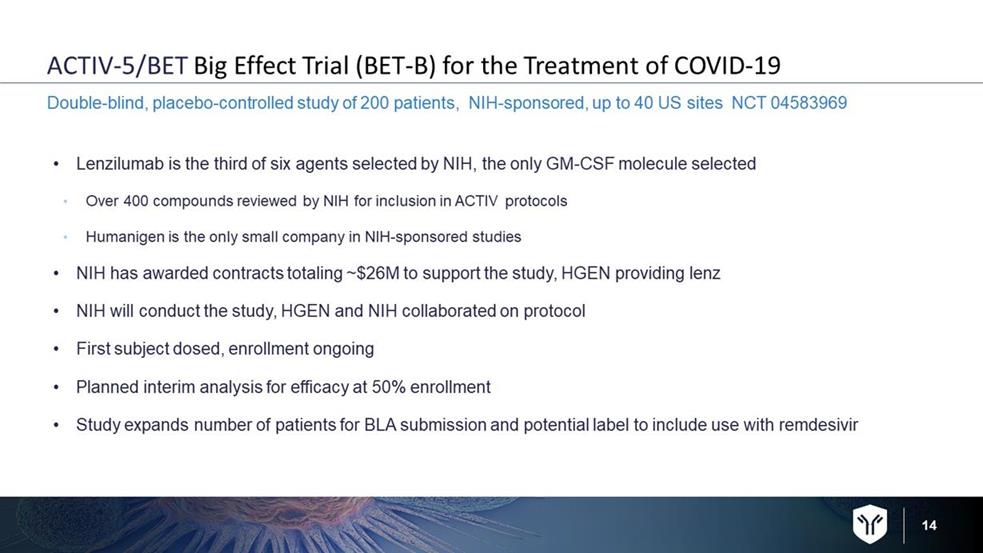
14 ACTIV - 5/BET Big Effect Trial (BET - B) for the Treatment of COVID - 19 • Lenzilumab is the third of six agents selected by NIH, the only GM - CSF molecule selected • Over 400 compounds reviewed by NIH for inclusion in ACTIV protocols • Humanigen is the only small company in NIH - sponsored studies • NIH has awarded contracts totaling ~$26M to support the study, HGEN providing lenz • NIH will conduct the study, HGEN and NIH collaborated on protocol • First subject dosed, enrollment ongoing • Planned interim analysis for efficacy at 50% enrollment • Study expands number of patients for BLA submission and potential label to include use with remdesivir Double - blind, placebo - controlled study of 200 patients, NIH - sponsored, up to 40 US sites NCT 04583969

15 3 2 1 Therapeutic for COVID - 19 Patients Improving efficacy / safety of CAR - T Prevention / Treatment of Acute GvHD 4 Market Potential/Financial overview

16 ZUMA - 19 Potential Registration Study Efficacy Safety Dosing Schedule • 3+3 Design to find recommended phase 2 dose • Cohort 1 with Starting Dose • Cohort 2 with Escalating Dose Clinical Trial Design Phase 1b Phase 2 ▪ At Recommended Dose • Complete Response (CR) • Partial Response (PR) • Objective Response Rate (ORR) • Duration of Response (DOR) • Progression Free Survival (PFS) • Overall Survival (OS) • % Patients with observed T - cell expansion • Kinetics of T - cell expansion • Minimizing Toxicities • NT • CRS • Days in ICU (Exploratory) • Days on Vasopressors (Exploratory) ZUMA - 19 ~ 6 Months Day - 5, - 4, - 3 Day 0 Leukapheresis Lymphodepleting Chemotherapy (Cyclophosphamide/Fludarabine) CAR - T Ongoing follow - up for safety and efficacy Lenzilumab Infusion Top Line Data for Durable Response Evaluation of T - cell kinetics Day 28 Primary endpoint for NT / other key measures Endpoints ClinicalTrials.gov Identifier: NCT04314843

17 3 2 1 Therapeutic for COVID - 19 Patients Improving efficacy / safety of CAR - T Prevention / Treatment of Acute GvHD 4 Market Potential/Financial overview

18 IMPACT Partnership/HGEN GvHD Trial Design Dosing Schedule (every 2 weeks) N= 240 Patients Clinical Trial Design 6 Months Allo Transplant Ongoing follow - up for safety and efficacy Lenzilumab + SOC Day 28 Day 0 GvHD Dx MAGIC biomarker (<48 hours post Dx) Intermediate + High Risk (AA2+AA3) Randomized Low Risk Response rate assessment NRM assessment Phase 2 (case - cohort) • Screened 215/yr • Randomized 110/yr • Total sample size 220 randomized 1:1 Phase 3 • Screened 40 patients • 20 treated with lenzilumab • Go/no - go decision vs. SOC Placebo + SOC

19 4 2 1 Therapeutic for COVID - 19 Patients Improving efficacy / safety of CAR - T Market Potential / Financial overview 3 Prevention / Treatment of Acute GvHD

20 Lenz Commercial Preparation Underway Humanigen is positioned for commercial success in 2021 and beyond. 1 Anticipated lenzilumab EUA in 1H2021 and BLA filing in 2H2021 2 Unique MOA addressing an unmet need in the quest for effective COVID?19 treatments 3 $1B opportunity estimated for lenzilumab in hospitalized and hypoxic COVID?19 patients 4 Anticipate favorable access and reimbursement to support rapid lenzilumab uptake 5 Commercial organization is swiftly scaling and resourced for a successful launch, if EUA granted 6 Experienced leadership team with deep expertise and a proven commercial track record

21 1. Johns Hopkins Coronavirus Resource Center 2. Total Hospitalizations ÷ Total Cases (COVID Tracking Project / Johns Hopkins Coronavirus Resource Center) 3. Fair Health Report as reported in Healthcare Finance News (November 5, 2020) 4. ACTT - 2 355,000+ US COVID Deaths 1 21 Million+ US COVID Cases 1 Of All Cases Require Hospitalization 2 4% ~13.5K Average Daily Cost to Treat Hospitalized COVID Patients 3 89% 4 of Hospitalized Patients are Hypoxic The COVID - 19 pandemic has affected millions and put significant strain on global healthcare systems and institutions. COVID - 19 Disease Landscape

22 Anticipating a Greater Number of Hospitalizations in 2021 Hospitalizations Will Remain Through 2022 and Beyond 2020 2021 2022 and Beyond 600K+ Hospitalizations Despite a vaccine, hospitalizations will increase Despite herd immunity and widespread vaccinations, hospitalizations remain COVID - 19 Hospitalizations ~677,000 COVID - 19 Hospitalizations in 2020 1 The number of hospitalized COVID - 19 patients is expected to rise beyond current levels and remain significant through 2022 and beyond 2 1. Covid Tracking Report 2. Data on file Sizeable and Sustainable Market Opportunity

23 1. Eli Lilly Press Release: December 15, 2020 2. Regeneron Press Release: November 21, 2020 3. H.C. Wainwright, Joseph Pantginis, Ph.D.: December 3, 2020 “2021 revenue is expected to be between $26.5 billion and $28.0 billion, driven by … an estimated $1 billion to $2 billion of revenue from COVID - 19 therapies . ” 1 HHS said it would rapidly distribute Regeneron's 300K doses of COVID - 19 antibody drug to states by end of January “While safe and efficacious vaccines are a large step forward towards ending the COVID - 19 crisis… there will be a continued need for novel anti - inflammatory and immunomodulatory compounds.” - Dr. Matthew Robinson, Austin Infectious Disease Consultants 3 There is consensus across the industry that hospitalizations and the need for therapeutics will continue well into 2021 and b eyo nd. KOL Call Highlights Continued Need for New COVID - 19 Therapeutics Even with Vaccines December 3, 2020 Future Need For COVID - 19 Therapeutics

24 Viral Acute R esponse Cytokine Storm Disease Onset » COVID - 19 can cause uncontrolled inflammatory responses — aka the “Cytokine Storm” — characterized by marked pro - inflammatory cytokine release in patients » These SARS - CoV - 2 - induced immune abnormalities may lead to respiratory failure, cardiac failure, neurological changes, DIC, and multi - organ failure » Patients are often asymptomatic during the first ~5 days following infection, followed by denial, at a point when the viral load is highest » Viral replication can lead to an onset of mild symptoms , such as fever, muscle pain, fatigue, and/or cough COVID - 19 Disease Progression There are 3 key phases of disease progression — early infection, pulmonary / inflammatory, and hyperinflammatory. Hyperinflammatory Phase Pulmonary/Inflammatory Phase Early Infection Phase 1 2 3 Disease Progression

25 Hyperinflammatory Phase Pulmonary/Inflammatory Phase Early Infection Phase 1 2 3 Patient Journey Outpatient Symptom Management COVID - 19 Diagnosis In Hospital Outpatient Invasive Mechanical Ventilation Present to Hospital with Hypoxia (SpO2<94% on Room Air) Low Flow or High Flow Supplemental O2 Treatment Options OTC Treatments Patients quarantine & manage symptoms at home Remdesivir Optimal efficacy when viral load is highest, but often given too late. Efficacy is questionable. 1,2 Corticosteroids Most effective in IMV patients. Concerns remain when administered too early Neutralizing mAbs Patients required to present to outpatient infusion centers Treatment Opportunity Need for an effective therapy that interrupts immune hyper - response and prevents disease progression in hospitalized patients COVID - 19 Disease Progression There is a significant therapeutic need for Hospitalized and Hypoxic Patients 1. “Remdesivir in adults with severe COVID - 19: a randomised, double - blind, placebo - controlled, multicentre trial.” Wang et al. Lanc et. 2020 May 16; 395 (10236): 1569 - 1578 2. WHO Solidarity Trial Bamlanivimab Casirivimab + Imdevimab Patient Journey

26 Understand the needs of hospitalized COVID - 19 patients and support them in their treatment Help physicians understand the value lenzilumab can contribute to better patient outcomes Work with hospital systems and key institutions to ensure patients get timely access to treatment To bring lenzilumab to market, we will focus on addressing the needs of three stakeholders — patients, providers, and payers. Patients Providers Payers / Hospitals Key Stakeholders
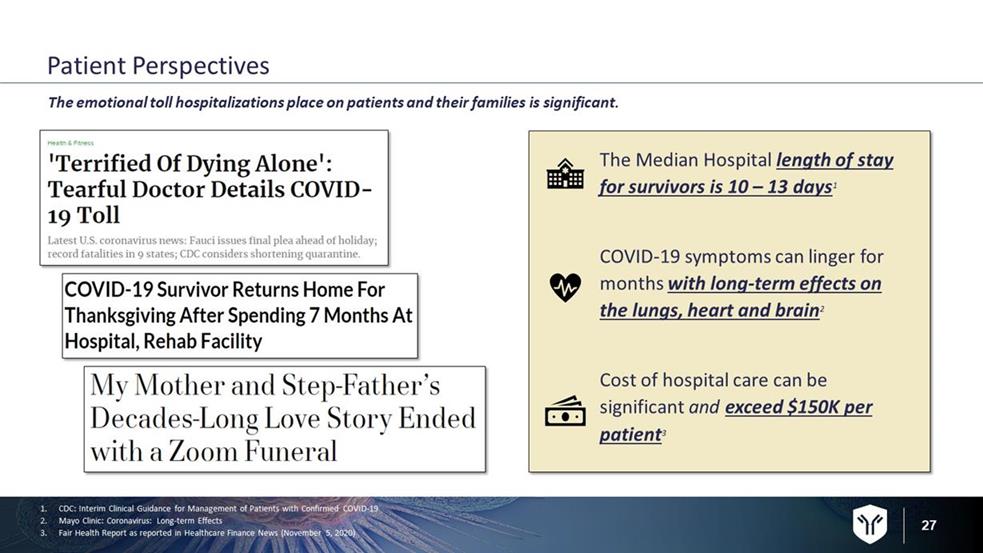
27 The Median Hospital length of stay for survivors is 10 – 13 days 1 COVID - 19 symptoms can linger for months with long - term effects on the lungs, heart and brain 2 Cost of hospital care can be significant and exceed $150K per patient 3 The emotional toll hospitalizations place on patients and their families is significant. 1. CDC: Interim Clinical Guidance for Management of Patients with Confirmed COVID - 19 2. Mayo Clinic: Coronavirus: Long - term Effects 3. Fair Health Report as reported in Healthcare Finance News (November 5, 2020) Patient Perspectives

28 Pulmonologists 12,000 9,000 13,500 Pulmonologists Infectious Disease Physicians Critical Care Physicians Infectious Disease Physicians , Pulmonologists , and Critical Care Physicians Oversee Treatment Decisions for Hospitalized COVID - 19 Patients ~34.5K COVID Specialists 1. PUD Source: NCBI – HHS Author Manuscript (~12k) 2. ID Source: The Lancet: Infectious Disease (~9k) 3. CC Source: Society of Critical Care Medicine (~13.5k) Concentration of COVID - Cases (3% of US Counties Represent 43% of Total Cases) Identify Specialists in Urban Areas with High Number of COVID Cases Target Physicians

29 The Burden of COVID - 19 Will Continue • Hospitals and staff are overwhelmed • Targeted HCPs are eager to reduce COVID - 19 hospitalizations and discharge patients sooner • Despite vaccines, HCPs do not anticipate COVID - 19 hospitalizations disappearing Current Therapies Are Inadequate • Remdesivir is often given too late, and may not be effective in hospitalized patients (reference from WHO Solidarity Study) • Corticosteroids have associated risks and should be reserved from IMV patients, avoided in high - risk patient populations • HCPs recognize the complexity of administration of neutralizing antibodies in an outpatient setting Managing The Cytokine Storm Is Critical • HCPs understand the importance of managing the immune response early to prevent disease progression • There is a lack of effective treatment options for hospitalized and hypoxic patients • Despite vaccines, there will be a continued need for anti - inflammatory and immunomodulatory compounds Lenzilumab Product Profile Is Promising • Majority of prescribers are “Very Likely” to use Lenzilumab in their hospitalized and hypoxic patients • Impressed by lenzilumab efficacy profile and improvement in potential clinical outcomes • The novel MOA targeting GM - CSF and safety profile make lenzilumab an ideal candidate for treatment of COVID - 19 Findings from commissioned market research indicate physicians will prescribe lenzilumab however awareness is low 1 Physician Perspectives 1. Internal market research Q4 2020, n=15

30 • COVID - 19 patients can be hospitalized for a week or more , possibly in the ICU • Cost of care is estimated at ~$13K per day for a hospitalized patient • Inpatient COVID - 19 drug therapies are currently covered by Medicare Part A Given the current market needs and lenzilumab’s unique product profile, we anticipate broad coverage. Hospital Systems and key institutions recognize the need to reduce costly hospitalization days and intubations. Hospital Access and Reimbursement Landscape

31 To help bring lenzilumab to market, we are partnering with an end - to - end commercial partner — Eversana. Go - To - Market Partner An end - to - end commercial partner provides ultimate flexibility, allowing us to quickly scale for a successful launch if EUA is granted Rationale For Selecting End - To - End Partner Enhance flexibility and scalability Reduce commercial risks Minimize financial exposure Maximize reward Maintain control of lenzilumab Leverage significant launch experience x x x x x x » Full launch & commercialization engine » Ready - to - deploy with real - time operational optimization » Dedicated commercial team backed by 3,000+ employees We stand ready to immediately activate our services spanning all disciplines of the product journey to ensure this therapy achieves optimal market growth and measurable patient value,” said Jim Lang, chief executive officer of EVERSANA . “ “

32 Financial Overview Balance at December 31, 2020 (M)* • Cash and cash equivalents $68 • Shares outstanding 55 Uplisting to NASDAQ Achieved on September 18, 2020 *balances are unaudited

33 Upcoming Catalysts and Goals Complete recruitment in Phase 3 COVID - 19 study and ACTIV - 5 EUA submission and subsequent approval Topline data from Phase 3 study Prepare for EUA distribution and scale - up manufacturing, BLA Continue CAR - T recruitment and initiate GvHD study 1 3 2 4 5
