Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ROCKWELL MEDICAL, INC. | rmti-20200924.htm |

NEW INDICATIONS OPPORTUNITIES FOR FERRIC PYROPHOSPHATE CITRATE (FPC) Rockwell Medical, Inc. September 24, 2020 www.RockwellMed.com

OUR COMPANY VISION Transforming Iron Deficiency and Anemia Management Our vision is to establish a new standard of care for patients suffering with iron deficiency and iron deficiency anemia. Iron deficiency anemia afflicts a subset of the two billion people worldwide who are nutritionally iron deficient1. Approximately 10 million people are iron deficient in the United States, including 5 million who have iron deficiency anemia. 2 1. Viteri FE. A new concept in the control of iron deficiency. Biomed Environ Sci. 1998 March;11(1):46-60.

CALL PARTICIPANTS Rockwell Medical Russell Ellison, M.D., M.Sc. President and Chief Executive Officer, Rockwell Medical, Inc. Marc Hoffman, M.D. Chief Medical Officer, Rockwell Medical, Inc. Timothy Chole Vice President of Marketing,Tim Chole Rockwell Medical, Inc. Vice President of Marketing Key Opinion Leaders Connie Sullivan, B.S. Pharm. President and Chief Executive Officer, National Home Infusion Association Inder Anand, M.D., FRCP, D. Phil. Professor Emeritus, Dept. of Cardiovascular Medicine, Univ. of Minnesota 3

FORWARD LOOKING STATEMENTS Certain statements in this presentation may constitute “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, potential new indication opportunities for Ferric Pyrophosphate Citrate (“FPC”), the timing for the commercial launch of Triferic AVNU, the potential for FPC to address the significant unmet need for home infusion patients, market opportunities for new indications, the clinical risk and safety profiles of new indications, the development of a clinical plan and timing of FDA review of FPC for new indications, any future partnerships that Rockwell Medical may enter into, the potential for reimbursement of FPC for new indications, the growth of the home and specialty infusion marketplace, the timing and success of regulatory filings for new indications, the compatibility of FPC with other home infusion drugs, the timing and cost of clinical development plans and clinical study designs. Words such as, “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “could,” “can,” “would,” “develop,” “plan,” “potential,” “predict,” “forecast,” “project,” “intend” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward looking statements. While Rockwell Medical believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Rockwell Medical’s SEC filings), many of which are beyond our control and subject to change. Actual results could be materially different. Risks and uncertainties include, but are not limited to: the impact of the COVID- 19 pandemic (including, applicable federal, state or local orders) on business and clinical development plans; the risk that regulatory authorities delay or fail to approve FPC for new indications; the risk that market opportunities are smaller than estimated; the risk that Rockwell Medical is not able to seek reimbursement for FPC for new indications; the risk that FPC is unsafe for new indications; the risk that clinical study designs, timing and costs are different than estimated; and those risks more fully discussed in the “Risk Factors” section of our Quarterly Report on Form 10-Q for the period ended June 30, 2020, and of our Annual Report on Form 10-K for the year ended December 31, 2019, as such description may be amended or updated in any future reports we file with the SEC. Rockwell Medical expressly disclaims any obligation to update our forward-looking statements, except as may be required by law. 4

ROCKWELL MEDICAL IS REPOSITIONED FOR GROWTH Transforming Iron Deficiency and Anemia Management 1 2 3 4 5 6 Commercial-stage Solid base business Triferic®* - one of the Ferric pyrophosphate Global FPC market Experienced Team company with an • #2 supplier of dialysis most innovative citrate (FPC) platform potential in excess of innovative platform concentrates in the U.S. advancements in iron $1 billion in multiple technology and • >$60M+ revenue management over the Growth expected from indications development pipeline annually last two decades new indications • Broad customer base includes DaVita 2nd generation parenteral iron delivers 100% immediately bioavailable iron with safety similar to placebo 2 FDA approved formulations… - Triferic Dialysate- launched May 2019 - Triferic AVNU (IV)- approved March 2020 5 Triferic® is a registered trademark of Rockwell Medical, Inc. Triferic AVNU is pending with the U.S. Patent and Trademark Office.

FPC NEW INDICATIONS OPPORTUNITY ASSESSMENT Objective and Process to Date OBJECTIVE Expand the use of our ferric pyrophosphate citrate (or FPC) molecule for patients through the development of FPC to treat medical conditions with unmet clinical needs outside of the dialysis setting. PROCESS • Ideation with internal experts • Initiation of a formal indication prioritization project • Engagement of third-party strategic analytics firm • Exhaustive vetting process leveraging qualitative and quantitative market research to rank opportunities • Phase 1: First-pass evaluation of 15+ potential new indications • Phase 2: Deep-dive analysis into top opportunities • Medical Advisory Board Review • Special Cardiology Medical Advisory Board Review • Extensive consultation with home infusion, gastroenterology, cardiology, and iron physiology experts • Completion of market opportunity assessment and clinical development plans 6

AGENDA Introduction & Overview of FPC Russell Ellison, M.D., M.Sc. CEO, Rockwell Medical, Inc. FPC New Indication Opportunity: Iron Deficiency Anemia in Home Infusion Patients • Introduction Russell Ellison, M.D., M.Sc. CEO, Rockwell Medical, Inc. • EXPERT PERSPECTIVE: Home Infusion Therapy Connie Sullivan, B.S. Pharm. President & CEO, National Home Infusion Assoc. • FPC Market Opportunity Timothy Chole VP Marketing, Rockwell Medical, Inc. • Development Plan Marc Hoffman, M.D. CMO, Rockwell Medical, Inc. FPC New Indication Opportunity: Acute Heart Failure • Introduction Russell Ellison, M.D., M.Sc. CEO, Rockwell Medical, Inc. • FPC Market Opportunity Timothy Chole VP Marketing, Rockwell Medical, Inc. • EXPERT PERSPECTIVE: Iron Deficiency in AHF Inder Anand, M.D., FRC, D. Phil. Professor Emeritus, Dept. of Cardiovascular Medicine, Univ. of Minnesota • Development Plan Marc Hoffman, M.D. CMO, Rockwell Medical, Inc. Q&A Session 7

FERRIC PYROPHOSPHATE CITRATE (FPC): OVERVIEW First and only FDA-approved treatment indicated for the replacement of iron as maintenance therapy in adult patients with hemodialysis-dependent chronic kidney disease (HDD-CKD) (Triferic®) • Donates iron directly and completely to transferrin, bypassing liver sequestration • Maintains hemoglobin with no increase in iron stores (ferritin) • Generally well tolerated, with few serious adverse events • Safety profile similar to placebo, with greater than 1.2 million doses • No inflammatory response to FPC • No hepcidin induction 8 Please see Important Safety Information included at the end of this presentation. Full Prescribing Information available at www.Triferic.com
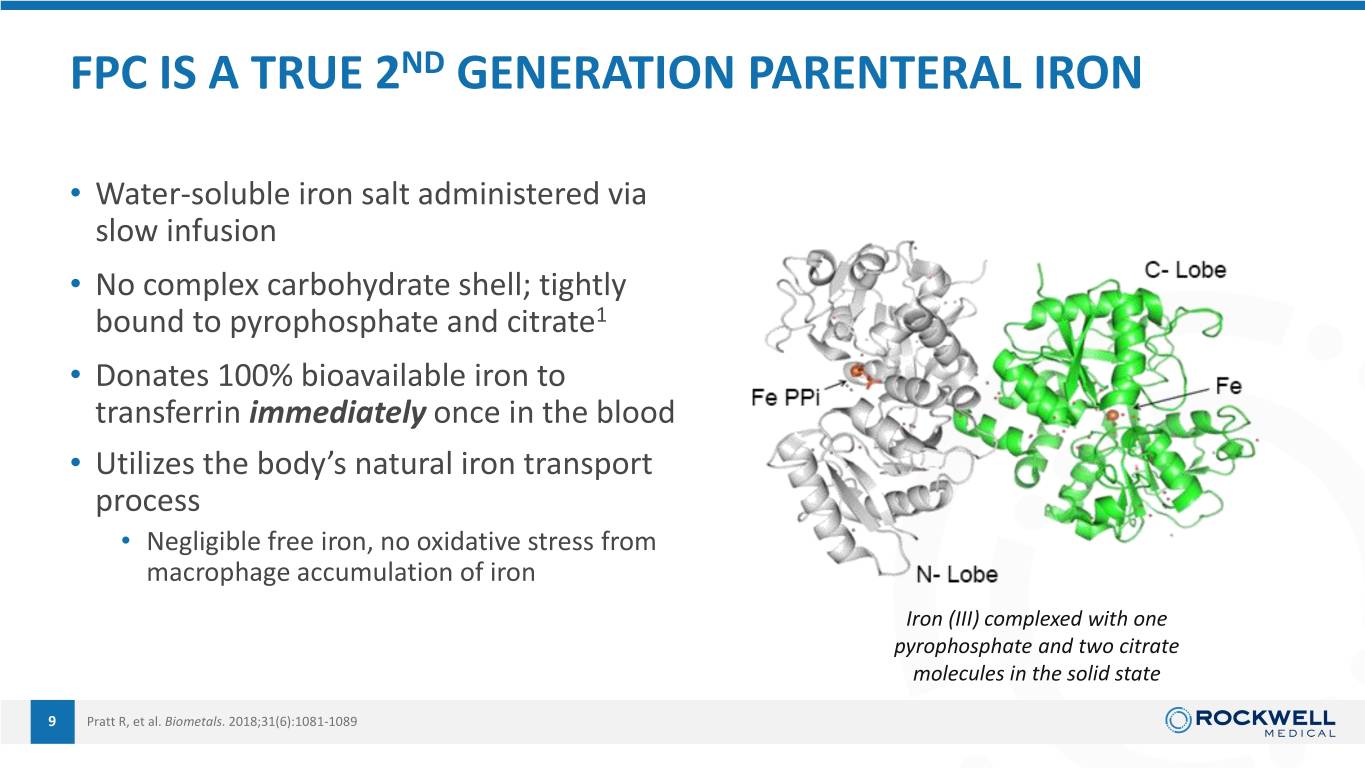
FPC IS A TRUE 2ND GENERATION PARENTERAL IRON • Water-soluble iron salt administered via slow infusion • No complex carbohydrate shell; tightly bound to pyrophosphate and citrate1 • Donates 100% bioavailable iron to transferrin immediately once in the blood • Utilizes the body’s natural iron transport process • Negligible free iron, no oxidative stress from macrophage accumulation of iron Iron (III) complexed with one pyrophosphate and two citrate molecules in the solid state 9 Pratt R, et al. Biometals. 2018;31(6):1081-1089

FPC DONATES BIOAVAILABLE IRON DIRECTLY AND COMPLETELY TO TRANSFERRIN Negligible NTBI Even at the Highest Dose of FPC Administered to Healthy Volunteers 10 mg FPC Non-Transferrin Bound Iron 4 h 260 10 mg FPC Transferrin Bound Iron 4 h 10 mg FPC Total Serum Iron (BL Corr) 4 h 210 TBI nearly equals total serum iron The study also showed that FPC is SD 160 ± cleared from the circulation g/dL 110 without increasing serum hepcidin m levels or biomarkers of oxidative 60 stress or inflammation* –8 –4 0 4 8 12 16 * No increases in cytokines (IL-6) or markers of oxidative stress, e.g. Malondialdhyde (MDA) 10 BL Corr = baseline corrected; NTBI = non–transferrin-bound iron; SD = standard deviation; TBI = transferrin-bound iron. Pratt RD, et al. J Clin Pharmacol. 2017;57(3):312-320.

FEATURES AND BENEFITS OF FPC VS. IV IRON OXIDE NANOPARTICLES (IONP) Macromolecular IV iron is sequestered within the reticuloendothelial system FPC • FPC bypasses the reticuloendothelial system Muscle, and donates iron directly to transferrin RES other In the inflamed patient, increased hepcidin blocks iron release and recycling Circulating red blood Transferrin GI • FPC does not increase inflammation or cells hepcidin levels FPC by-passes the liver and avoids Erythroid sequestration by hepcidin marrow Hepatocytes • Iron from FPC is immediately 100% bioavailable 11

EXCELLENT CLINICAL SAFETY PROFILE OF FPC (TRIFERIC®) DEMONSTRATED IN HEMODIALYSIS PATIENTS Clinical Development Program in the U.S.1,2,3 • No deaths during or attributed to Triferic (>100,000 Doses) • No anaphylaxis • No iron overload (serum ferritin did not increase) • Adverse event rate similar to placebo • No difference in intradialytic hypotension (IDH) • No difference in infections • No difference in vascular access thrombotic events • No clinical laboratory abnormalities • No increase in oxidative stress or inflammation 1. Fishbane SN, Singh AK, Cournoyer SH, et al. Ferric pyrophosphate citrate (Triferic™) Post-Launch Experience in the U.S. administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. 2015;30(12):2019-2026. • Over 1.2 million patient days administered 2. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018. 3. Gupta A, Lin V, Guss C, Pratt R, Ikizler A, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis stimulatingagent use and • No reports of unexpected SAE or changes to U.S. product maintains hemoglobin in hemodialysis patients. Kidney Int. 2015;88(5):1187-1194. information 12

FPC CURRENTLY MARKETED IN RENAL, AT DEVELOPMENT STAGE FOR NEW INDICATIONS Triferic for ESKD Patients… FPC for Additional Indications… Three approved formulations Phase II ready Indicated for iron replacement and maintenance of hemoglobin in adult patients undergoing hemodialysis Priority 1 For treatment of iron deficiency anemia in Triferic Dialysate Triferic Dialysate Triferic AVNU 272 mg Powder Packet 5 mL Ampule 4.5 mL Luer Lock Ampule patients undergoing home infusion therapy Under Consideration For treatment of iron deficiency in hospitalized acute heart failure patients Add one packet to Add one ampule to Administer one every 25 gallons of bicarb every 2.5 gallons of ampule via slow IV solution in the bicarb mixer bicarb solution infusion HCPCS Code: J1444 HCPCS Code: J1443 HCPCS Code: TBD 13

FPC NEW INDICATION OPPORTUNITY: IRON DEFICIENCY ANEMIA IN HOME INFUSION Top Priority 14

CONNIE SULLIVAN, B.S. PHARM. President and CEO, National Home Infusion Association (NHIA) ❖ Home infusion industry leader and patient advocate ❖ Over 25 years experience in home infusion clinical practice, operations, and executive leadership ❖ Expert consultant - United States Pharmacopeia panel developing parenteral nutrition quality standards ❖ Member (for 2020—2025 term) and past Vice Chair (2015-2020) of United States Pharmacopeia Expert Committee for Sterile Compounding ❖ B.S. Pharm. from The Ohio State University 15

HOME INFUSION THERAPY What is home infusion therapy? Specialized services that allow patients to receive intravenous medications at home. Providers are specialized, closed-door pharmacies with expertise in sterile compounding and clinical management of IV therapies. Therapy is supported by multi-disciplinary clinical teams (pharmacists, nurses, dietitians). Home Infusion Provider Patient Care Process… Physician Order Patient Intake Care Plan Service ➢ Receive referral based ➢ Verify insurance coverage ➢ Design the plan of treatment ➢ Purchase, prepare and deliver on physician order for a ➢ Perform a patient medication parenteral medication ➢ assessment for eligibility Make recommendations to the ➢ Ongoing assessments and prescriber for drug regimen monitoring for therapeutic ➢ Coordinate nursing and adjunct therapies services effectiveness, adverse events ➢ Submit for / collect reimbursement Providers are reimbursed for 1 Per diem 2 Drugs 3 Nursing Services three separate components… (covers admin, supplies & equipment) 16
HOME INFUSION THERAPY What are the benefits of home infusion? Allows patients with diseases and disorders requiring multiple on-going infusions of medications to receive them in the comfort of their own home. Benefits include1,2: • Increased quality of life (QOL) • Shorter hospital/skilled nursing facility length of stay • Lower rates of depression, fatigue • Less opioid use • Reduced risk of hospital/facility acquired infections Largest NHIA survey to date of patient satisfaction in home infusion found scores of >97% in 2019.3 1. Healthc (Amst). 2017 Mar;5(1-2):68-80. doi: 10.1016/j.hjdsi.2016.04.004. Epub 2016 Apr 29. 2. https://www.homecaremag.com/march-2019/home-infusion-data 3. Haines D, Sullivan C, Garst R. Assessment of Home Infusion Patient Satisfaction. NHIF 2019. 17

COMMERCIAL PAYERS AND CMS PROVIDE REIMBURSEMENT FOR HOME INFUSION THERAPIES The majority of home infusion therapy patients are covered by commercial insurance. Payers are increasingly motivated to reimburse home infusions to reduce in-office visits, hospitalizations, and save costs. Commercial Payers Payer Sources of Home Infusion Comprehensive coverage. Most often covered under the major medical benefit. Provider Revenue Medicare Part B Reimbursement for drugs, equipment, and services. Current coverage is for drugs that 13.6% Medicare Part D require the use of an item of durable medical equipment (pump for administration). 47.5% 8.9% Medicare Part B Commercial Insurance 4.6% Medicare HMO/Advantage Medicare Part D 5.7% Non-Part D Pharmacy Benefit Reimbursement limited to the drug. Patients may qualify for other coverage (nursing) under Part A, but normally it is out of pocket. 5.3% Medicaid 14.4% Other / government Medicare Advantage Varies with most plans modelling payment after commercial insurers. denotes likely payers / reimbursement mechanisms for FPC as a home infusion therapy 18

THE HOME AND SPECIALTY INFUSION MARKET IS GROWING The home and specialty infusion marketplace is experiencing explosive Unique growth and provides a favorable reimbursement opportunity for Therapy Patients suitable drugs.1 (annually) Anti-infectives 1,441,520 Comparison of Home Infusion Parenteral Nutrition 112,984 Therapy Patients Served 2010 vs. 2019 Traditional Hydration 159,736 2019: Infusion Pain Management 50,648 Therapies Inotropic 85,712 3,224,427 (daily)… 2010: Antineoplastic Chemotherapy 132,464 829,000 Catheter Care 214,280 Other (e.g., steroids, ant-emetics) 720,760 Biologics, Immune Globulin, etc. 306,323 “Opportunities for continued industry growth look promising due to a TOTAL 3,224,427 robust pipeline for specialty drugs…an aging population, and the prospects for a comprehensive Medicare home infusion benefit.” - NHIA Home Infusion Trends Survey 2020 19 1. NHIA Infusion Industry Trends Report 2020.

HOME INFUSION IS EXPECTED TO CONTINUE A GROWTH TREND IN THE FUTURE • Continued industry growth is expected, driven by… • High rates of patient demand, and satisfaction with services • Site of care optimization programs driven by commercial payers • Legislation to expand coverage for Medicare beneficiaries • Robust pipeline of specialty IV treatments • COVID-19 has accelerated existing trends and added… • Use of virtual nursing visits • Oncology shift • Additional information on industry trends available from NHIA (www.nhia.org) 20

IRON DEFICIENCY ANEMIA IS A RECOGNIZED ISSUE FOR TOTAL PARENTERAL NUTRITION PATIENTS1 IDA is a common complication occurring in 40-55% of patients on long-term PN. A majority of patients suffer from conditions/diseases that put them at a high risk for developing IDA. • Patients with IDA can exhibit symptoms of fatigue, shortness of breath, rapid or irregular heartbeats and glossitis – all which affect quality of life. • It is recommended that patients receiving home parenteral nutrition be screened regularly for anemia. • Treatment with parenteral iron for patients with iron deficiency is recommended. • Inadequate response to treatment may be related to continued blood loss, inflammation, ineffective absorption or poor adherence to therapy. “It is imperative that this patient population be monitored regularly and supplied with iron therapy as needed.” – Sullivan, S et al, OSPEN Access 2016 21 1. Sullivan, S, Austhof S. Managing Iron Deficiency Anemia in Patients on Home Parenteral Nutrition. OSPEN Access. 2016. 25:3.

MANAGEMENT OF IRON DEFICIENCY ANEMIA IN HOME INFUSION THERAPY PATIENTS IS A BROKEN PROCESS Many patients on home infusion therapy suffer from diseases with which iron deficiency and anemia are commonly Anemia is untreated until associated, but current treatment patterns are inadequate. symptoms become severe IV iron supplementation is more effective than oral formulations however, concern for adverse events is a deterrent. Urgent Disease related intervention iron deficiency with IV iron or Home infusion of traditional IV iron is limited due to risk of persists transfusion is hypersensitivity and concerns about incompatibility with other required infused drugs.* An office visit for infusion of IV iron is costly, inconvenient, and often does not fit the physician practice care model. Limitations with the current approach can lead to a vicious Follow-up is Intervention cycle of late diagnosis and treatment, inconsistent follow-up, inadequate requires an and increased risk of office visits or hospitalizations. and anemia office visit or recurs hospitalization *E.g., stability of parenteral nutrition lipids when delivered with carbohydrate-based IV iron preparations 22

PROACTIVE MANAGEMENT OF IDA IN HEMODIALYSIS – A MODEL APPROACH FOR HOME INFUSION? Established Guidelines for therapeutic targets Protocols (iron measures, hemoglobin / hematocrit targets, etc.) Iron deficiency is proactively Regular lab testing of iron measures and H&H Monitoring adjustment of iron therapy approach as needed managed in hemodialysis dependent end-stage kidney disease patients Repletion “Loading” doses of IV iron provided to patients that Therapy are absolutely or functionally iron deficient Maintenance Regular doses of IV iron given to support erythropoiesis for Therapy patients with an ongoing negative iron balance 23

IN SUMMARY ❖ Home infusion therapy is a rapidly growing area of medicine with over 3.2 million patients served every year ❖ Growth trend is likely to continue driven by cost savings vs. office-based or hospital care, an improving reimbursement landscape, and emerging standards resulting from the global pandemic ❖ Iron deficiency anemia (IDA) is a persistent co-morbidity and a feasible and effective treatment regimen remains a significant medical unmet need for many patients receiving home infusion ❖ Overall management of IDA is inconsistent for home infusion patients, in part driven by limitations with the currently available iron replacement products 24

A SIGNIFICANT MARKET OPPORTUNITY IDENTIFIED FOR FPC IN THE HOME PARENTERAL NUTRITION PATIENT POPULATION Many patient groups requiring home infusion therapies suffer from diseases that are associated with an incidence of iron deficiency and anemia. For home parenteral nutrition patients specifically, there is a significant market opportunity for FPC and an unmet need for effective proactive iron maintenance therapy. Home parenteral nutrition Total unique patients annually: 113,0001 • # of long-term (all year) HPN patients: 30,000 # of short-term (avg. 45 days) HPN patients: 83,000 • An estimated 40-55% are iron deficient2 , but the majority of patients have a negative iron balance due to low/no dietary iron absorption and inflammation3,4 • Patients receive daily infusions of parenteral nutrition supplements (8-12h per day) • Evidence favors a maintenance iron dose of 1mg per day in adult males4 • Oral iron is almost universally inadequate due to patient inability to absorb • Traditional parenteral iron infrequently used due to risk of hypersensitivity and concerns re: incompatibility with lipids Estimated U.S. FPC market opportunity in HPN pts: $200M per year 1. NHIA Infusion Industry Trends Report 2020. 2. Hwa YL, Rashtak S, Kelly DG, and Murray JA. Iron deficiency in long-term parenteral nutrition therapy. JPEN 2015. 3. NHIA Expert Opinion. Aug 2020. 4. Forbes A. Iron and Parenteral Nutrition. Gastroenterology 2009;137:S47-S54. 25

ADDITIONAL HOME INFUSION THERAPY PATIENTS WITH IRON DEFICIENCY ANEMIA MAY BE CANDIDATES FOR FPC The following home infusion therapies are provided to patients for the treatment of diseases that may be associated with a risk of iron deficiency anemia. Applicability of FPC will depend on length of therapy, rate of iron deficiency and acceptability of alternative therapies such as traditional IV iron loading or oral iron. Antineoplastic chemotherapy Inotropics 1 • Total # of patients: 130,0001 • Total # of patients: 85,700 1 • Length of therapy is medium term (~90 days avg)1 • Length of therapy is long term 3,5 • Estimated 42% of patients are iron deficient2 → 54,600 patients • 50% of ambulatory heart failure patients are iron deficient Hydration therapy Biologics 1 • Total # of patients: 160,000 • Total # of patients: 160,0001 1 • Length of therapy is short term (30-45 days) • Length of therapy lifetime or chronic, intermittent • Common diagnoses include dehydration, cancer, GI disorders • Common diagnoses include Crohn’s Disease, ulcerative colitis, multiple • Can be adjunctive to a primary therapy (HPN, Chemotherapy) sclerosis, and others • Actual incidence of iron deficiency anemia is unknown • Anemia associated with iron deficiency is present in up to 1 in 3 IBD pts.4 Additional total U.S. market opportunity for FPC in home infusion patients estimated to be $400M. 1. NHIA Infusion Industry Trends Report 2020. 4. Kaitha S, et al. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015. 6(3):62-72. 2. Busti E, et al. Anemia and iron deficiency in cancer patients. Role of iron replacement therapy. Pharmaceuticals 2018, 11, 94. 5. von Haehling S, et al. Iron Deficiency in Heart Failure. JACC. 2019. 7(1):36-46. 3. Beale A, et al. Iron Deficiency in Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1569. 26

IRON MANAGEMENT FOR HOME INFUSION WITH FPC VS. TRADITIONAL IV IRON THERAPY APPROACH IRON REPLETION THERAPY IRON MAINTENANCE THERAPY Traditional IV Iron* FPC Traditional IV Iron* FPC (proposed) (proposed) 200 – 500mg per dose 15 – 30 min infusion 2 – 5 doses (office visits) Physician Office Indication not planned Not routinely provided Indication not planned 200 – 500mg per dose 12 - 24mg per dose 7 – 10mg per dose 15 – 30 min infusion 6- 12h infusion 2 - 3hr infusion 2 – 5 days of therapy 6 – 20 days* of therapy Not routinely provided 1 day/week of therapy (infrequently prescribed) Home Infusion Only 2-5 short infusions needed Adoption will depend on Iron maintenance therapy not Could enable on-going iron applicability with patient Use in home infusion limited due routinely provided maintenance dosing safely at treatment regimen to concerns of serious home Safety profile suitable for home use hypersensitivity reactions / Flexible dosing schedule supports Potential to run concurrently with monitoring requirements home infusion services model Office visit commonly required other home infusion drugs No office visit required Inconsistent monitoring / results 27 *calculated 1 g/dl increase in Hgb after 6 days of FPC therapy, 24mg over 12h infusion per day

A MARKET ACCESS PATHWAY IS IN PLACE FOR FPC AS A HOME INFUSION THERAPY • FPC for home infusion will likely be eligible for favorable reimbursement • Drugs reimbursed via buy and bill process for home infusion providers • Coverage available under the Medicare Part B DME POS* benefit (narrow pathway) • Commercial Payers have a universal approach with favorable reimbursement (separate benefit components for drug, per diem equipment & supplies, and nursing services) • Commercial payers see the value and are leading the way with home infusion reimbursement • To ensure optimal (differentiated) pricing and reimbursement, FPC should meet the following criteria… • Approved with NDA • Receive unique J-Code • Differentiated product presentation • Product label describes delivery with an infusion pump • FPC product presentation and dosing regimen can be optimally designed for service model • Ideal product presentation (formulation, fluid volume, packaging) will be designed to fit the desired service model and streamline care • Service providers will recommend drugs to prescribers based on the opportunity to improve patient outcomes – accelerates adoption *Durable Medical Equipment, Prosthetic, Orthotics, and Supplies 28

FERRIC PYROPHOSPHATE CITRATE IS UNIQUELY SUITED FOR USE AS A HOME INFUSION THERAPY Home infusion clinicians are hesitant to recommend IV FPC has been demonstrated to have a safety profile similar iron supplementation due to the potential for severe to placebo in prospective randomized clinical trials.1,2 hypersensitivity risk – however rare. FPC provides 100% immediately bioavailable iron, bypassing Treatment with loading doses of traditional IV iron storage in the liver.3 therapy can temporarily address iron deficiency, but iron deficiency may persist due to inflammation. Iron from FPC is bioavailable even in the presence of inflammation and elevated hepcidin. FPC can be dosed consistently in low doses as a physiologic Managing iron with loading doses of traditional IV iron maintenance dose to address an on-going negative iron is inconsistent for home infusion patients. balance and prevent iron deficiency anemia.3 1. Fishbane SN, Singh AK, Cournoyer SH, et al. Ferric pyrophosphate citrate (Triferic™) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant. 2015;30(12):2019-2026. 2. Gupta A, Lin V, Guss C, Pratt R, Ikizler A, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int. 2015;88(5):1187-1194. 3. TRIFERIC Prescribing Information. Wixom, MI: Rockwell Medical, Inc.; 2018. 29

FPC HOME INFUSION OPPORTUNITY: CLINICAL DEVELOPMENT STRATEGY: UNIQUE NDA • Leverage existing NDA • Preclinical Tox √ • Preclinical Pharmacology √ • Drug Substance √ • Long Term Safety √ • New contents needed for NDA • Drug Product Section New IP • New Package (new dosage not a rate limiting step, e.g. develop new CMC in parallel to clinical program using current dose/strength in clinical trials) • Stability Data • Clinical Data 30

FPC HOME INFUSION OPPORTUNITY: PLANNED REGULATORY APPROACH • Type C meeting with FDA to discuss our clinical development plan (EOY 2020 - early Q1 2021) • Establish efficacy and safety in two well-defined patient populations to minimize variability of clinical study . . . • Uncomplicate clinical assessment • Primary endpoint in clinical development = ∆ Hb • Secondary endpoints; QoL/fatigue scores . . . with the goal of securing a broad label claim of Treatment of IDA in adult patients receiving home infusion therapy regardless of etiology 31

FPC HOME INFUSION OPPORTUNITY: BUILDING ON PRECEDENT Precedents for this strategy are evident in the labels of other iron products, e.g. Feraheme® (ferumoxytol) 1, Injectafer® (ferric carboxymaltose) 2 and Monoferric® (ferric derisomaltose)3 All are indicated for the treatment of iron deficiency anemia (IDA) in adult patients who have intolerance to oral iron or have had unsatisfactory response to oral iron. • Feraheme – There are no data on underlying disease as presented in their label (Two NDC codes) • Injectafer - In clinical studies the primary etiologies of iron deficiency anemia were heavy uterine bleeding and gastrointestinal disorders (Two NDC codes) • Monoferric – does not specify anything further than adult patients with IDA caused by different etiologies (Three NDC codes) 32 1- AMAG Pharmaceuticals, Inc. 2 - AMERICAN REGENT, INC 3 - Pharmacosmos A/S

FPC HOME INFUSION OPPORTUNITY: OUTLINE OF PHASE II AND PHASE III CLINICAL DEVELOPMENT 1) Utilize a Phase II observational study to validate feasibility assumptions and fully characterize treatment setting • Determine iron deficits, by indication • Establish practice treatment patterns and dosage and frequency for repletion and maintenance • 4-6 months • $500k -750k (+ $250k to develop novel product presentation) 2) Dose Scheduling Study • Validate endpoints and confirm dose response (e.g., amount and time required per 1g/dl Hgb increase) • 40-60 patients • 9-12 months • $1- $1.5mm - - - - - - - - - 13-18 months- - - - - - - - - End-Of-Phase 2 Meeting- - - - - - - - - GO/NO-GO Decision- - - - - - - 3) Pivotal Phase III Study (ies) • Strategy will be presented at Type C meeting. Studies would run concurrently. • 200-300 patients, sample size will be determined at feasibility and depend on anticipated treatment effect • $2.5mm - $4.0mm per study, PDUFA Filing fee $2,875,842 • 2 years Program allows for multiple data readouts over the next 3 years. 33

POTENTIAL PHASE III HOME INFUSION STUDY DESIGN* Methods and Cohort Treatment phase** Long Term Follow-up (LTF) 9 month Rx + pts randomized to FPC Maintenance dose 7- 9 month LTF group receiving 10mg FPC/week 2mg/hr. 12°/12° Randomized 2:1 Placebo/SoC placebo-controlled, double-blind CRUISE-like endpoints & Multicenter pts randomized to stopping criteria; clinical trial Hb ∆ -1 or +2 placebo group receiving g/dL SOC HPN n=200-300 HPN SAFETY *support indications for treatment and maintenance of IDA X-Y week run-in, no iron, stable Hb<10-11 ** rolling NDA strategy 34

FPC HOME INFUSION OPPORTUNITY: IN SUMMARY ❖ Home infusion therapy is an area of medicine experiencing explosive growth – a trend that will likely continue in this decade ❖ Iron deficiency anemia (IDA) is a common co-morbidity in many sub-groups of patients receiving home infusion therapy, particularly in those receiving long-term home parenteral nutrition ❖ Management of IDA in home infusion patients is currently a ‘broken’ process due to limitations with currently available parenteral iron products and other factors ❖ FPC can deliver 100% bioavailable iron with a safety profile similar to placebo, and may fill an unmet clinical need as a uniquely suitable home infusion therapy for treatment of IDA ❖ The total U.S. market opportunity for FPC in home infusion is estimated to be near $600M ❖ Clinical development of FPC for the treatment of IDA in home infusion therapy including Ph II and Ph III clinical trials is estimated to be a 27 – 40-month process with a cost of $9.3 – 13.5MM. • Go/No-Go decision at the end of Phase II achieved in 13-18 months at a cost of $1.5- 2.5 MM 35

FPC NEW INDICATION CONCEPT: ACUTE HEART FAILURE Under Consideration 36

HEART FAILURE (OR HF) Inability of the Heart to Meet the Body’s Metabolic Demands Approximately 4.9 million cases in the United States today • Over 400,000 new cases per year • The most common cause of hospitalization in people over 65 years • Increasing numbers of HF patients due to survival from heart attacks and overall aging of the population Location of heart failure • Left ventricular (most common) • Right ventricular • Biventricular < 55% (lab-dependent) 37

HEART FAILURE (OR HF) Inability of the Heart to Meet the Body’s Metabolic Demands Mechanism • HFrEF: HF with reduced ejection fraction (50% of cases) • HFpEF: HF with preserved ejection fraction (50% of cases) Etiology • Ischemic (approx. 2/3 of HFrEF) • Non-ischemic Onset • Acute decompensation (ADHF) • Chronic 38

HEART FAILURE IS INCREASING IN THE U.S. AND IRON DEFICIENCY IS A COMMON CO-MORBIDITY Heart failure in the U.S. is a large and growing patient population. Over a million patients are hospitalized each year with acute decompensated heart failure. Iron deficiency a common co-morbidity in all forms of HF.* Comparison of Hospitalized Acute Systolic Heart Failure Patients # of acute systolic 2010 vs. 2017 heart failure 1,171,4061 patients (HFrEF) 2017: 1.17 million2 # of acute HFrEF 2010: 633,000 patients with iron 650,000 deficiency (54%)2 * “Iron deficiency is a predictor of worse outcomes. If you have iron deficiency with heart failure, your prognosis is poor.” - Academic Cardiologist 39 1. MEDPAR & HCUP databases 2. Beale A, et al. Iron Deficiency in Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1569.

FPC ACUTE HEART FAILURE OPPORTUNITY: MARKET ACCESS & REIMBURSEMENT The majority of heart failure patients admitted to hospital are insured by Medicare / Medicaid through a well- established universal approach usually resulting in a bundled reimbursement structure Payer Source of Inpatient Heart Failure Discharges1 • FPC for heart failure will likely be covered by the Diagnostic Reimbursement 2.0% Other/Missing Group (DRG) bundled payment: 2.8% Uninsured • Bundled payment amount a function of scaled patient severity as determined by diagnoses during hospital stay 11.4% Commercial Insurance • Commercial payers typically proxy to similar bundled payment schema with Medicare reimbursement rates as a benchmark 10.4% Medicaid • To ensure optimal pricing opportunity and market adoption, FPC studies must… • Meet established clinical endpoints which translate to health economic value • Reduced hospital length of stay (LOS) and / or • Reduced 30-day hospital readmission rate 73.5% Medicare 40 1. 2016 HCUP National Inpatient Sample (NIS), Agency for Healthcare Research and Quality (AHRQ).

A SIGNIFICANT MARKET OPPORTUNITY MAY EXIST FOR FPC AS A TREATMENT OF IRON DEFICIENCY IN HOSPITALIZED AHF The AHF opportunity for FPC is associated with a large patient population with a high incidence of iron deficiency.1 FPC has theoretical acute clinical advantages (<30 days) vs. traditional IV iron therapy for iron deficient acute heart failure patients and could have an impact on hospital length of stay and/or 30-day readmissions. • Total # of iron deficient hospitalized AHF patients = 633,000 • Mean length of hospital stay = 5.5 days2 Total market opportunity for • FPC could be safely administered via daily IV infusion FPC as a treatment for iron • 40mg per day via continuous slow infusion for 4 days, total 160mg deficiency in AHF is • FPC can be priced based on health economic value estimated to be • A proven reduction in hospital LOS or 30-day readmissions would $1.01B demonstrate economic value for a hospital • Mean cost per 5.5-day hospital stay: $13,4442 ($2,444 per day) • A 10% reduction in the 30-day re-admission rate or hospital LOS saves the hospital $1,344 per admission 41 1. Enjuanes et al, Okonko et al, Cohen-Solal et al, Nunez et al, Van Aelst et al, multiple peer-reviewed publications on file (2011-2017) 2. www.hcupnet.ahrg.gov – HCUP National Inpatient Sample, 2016 Agency for Healthcare Research and Quality (AHRQ)

INDER ANAND, M.D., FRCP, D. PHIL. ❖ Emeritus Professor of Medicine, University of Minnesota Medical School ❖ Former Director, Heart Failure Program, VA Medical Center Minneapolis MN ❖ Former Senior Advisor, NHLBI, NIH ❖ Founder member of the Heart Failure Society of America (HFSA) ❖ One of the Founder Associate Editors of the Journal of Cardiac Failure ❖ Associate Editor, Journal of Cardiac Failure ❖ Member, Editorial Board, Journal of CV Pharmacology and Therapeutics, High Altitude Medicine & Biology, Italian Journal of Medicine, JACC-Heart Failure ❖ Member, European Board, Journal of Heart Failure ❖ Member, International Advisory Board, Indian Heart Journal ❖ Over 420 Publications ❖ Over 600 Abstracts and book chapters ❖ Over 600 Papers Presented at National and International meetings ❖ Over 80 clinical trials 42

HEART FAILURE, ANEMIA AND IRON DEFICIENCY • Anemia and Iron deficiency (ID) are common comorbidities in heart failure (HF) & often coexist. Together or independently they are associated with worse symptoms, increased hospitalizations and mortality. • Treating anemia in HF with erythropoiesis-stimulating agent (ESA) does not improve outcomes, may be deleterious and is not recommended. • ID is seen in ~50% of chronic and up to 70% of acute decompensated heart failure (ADHF) patients. • Increasing data suggests that IV iron in ambulatory patients with heart failure with reduced ejection fraction (HFrEF) improves symptoms, exercise capacity, but long-term effects on hospitalizations, mortality and safety are unknown. • There are no studies in acute HF or in HF with preserved ejection fraction (HFpEF). • Although studies suggest that iron plays an important role in improving the energetics of HFrEF but such data is not available for HFpEF. 43

Absolute or Relative Iron Deficiency is an Independent Risk Factor in Heart Failure 546 patients with stable HF; LVEF 26 ± 7%. Overall, absolute or relative ID was present in 37% patients; 57% in those with anemia, 32% in those without anemia. ID was associated with death or Heart Tx independent of Absolute or Relative ID was present anemia (HR 1.58, 95% CI 1.14-2.17) in 37% all CHF patients (199/546) 60 40 57% 20 % % of CHF Patients 33% Anemic Non Jankowska et al Eur Heart J (2010) 31, 1872–1880 (Hb 12 g /dL) anemics 44 Jankowska EA, et al. Eur Heart J 2010;31:1872–80

IN HF IRON DEFICIENCY MAY BE A MORE IMPORTANT PROGNOSTIC MARKER THAN ANEMIA Iron deficiency but not anemia remained an independent predictor of mortality (HR] 1.42, 95% CI 1.14-1.77, p = .002) International pooled cohort, 1,506 HF patients with and without ID and anemia 45 Klip et al. Am Heart J 2013;165:575-582.e3.

MECHANISMS OF THE ASSOCIATION OF ID WITH ADVERSE OUTCOMES - MULTIFACTORIAL • Iron is an essential component of all energy producing mitochondrial enzymes. • ID reduces cardiac and skeletal muscle iron content causing both structural and several functional mitochondrial abnormalities, including decrease in high energy stores like adenosine triphosphate (ATP) and Phosphocreatine (PCr). • This explains why ID can cause skeletal and cardiac muscle dysfunction and thereby reduce exercise capacity and worsen HF, independent of anemia. • Fortunately, iron, ATP & PCr can be measured in the skeletal and cardiac muscle non-invasively using Magnetic Resonance Imaging. 46

DOES ORAL IRON REPLACEMENT THERAPY HELP? • Oral iron supplementation is standard therapy for ID; readily available, inexpensive & effectively raises serum iron levels non-HF patients. • Because HF is an inflammatory state, elevated levels of cytokines and the peptide hepcidin prevent iron absorption from the gut and release from body’s iron stores. • Oral iron is also associated with adverse GI effects and not well tolerated by many patients. • Few studies have investigated the effects of oral iron in patients with ID and HF. 47

IRONOUT-HF: ORAL IRON NOT EFFECTIVE • Phase 2 RCT, 225 patients with HFrEF, NYHA class II to IV HF, median LVEF, 25% • Anemic or non-anemic patients Hb; 9-15 g/dL (men) or 9-13.5 g/dL (women) with ID • Randomized: oral iron polysaccharide 150 mg BD/placebo. 48 Lewis, GD et al.JAMA. 2017;317(19):1958-1966. doi:10.1001/jama.2017.5427

STUDIES WITH IV IRON IN HEART FAILURE Efficacy of iron sucrose or FCM investigated in 5 RCT in Outpatients • ↑ Exercise Capacity (Peak VO₂ ; 6MWT) • Improved QoL scores • Improved NYHA class • ↓ NT - proBNP level • Because of small size, outcomes such as death and hospitalization for HF could not be assessed 49 Anand and Gupta Circulation. 2018; 138:80-98

META-ANALYSIS OF 4 RCT COMPARING IV FERRIC CARBOXYMALTOSE AND PLACEBO IN HFREF Individual patient data from 4 RCTs comparing FCM with placebo in 839 Individualpatients patient with HFrEF data from and 4 ID, RCTs 504 comparingrandomized FCM to FCMwith placebo and 335 in to 839 placebo. patients with HFrEF and ID, 504 randomized to FCM and 335 to placebo. CV Deaths and CV Hospitalization Recurrent Event Analysis 50 Anker et al. Eur J Heart Fail. 2017; doi:10.1002/ejhf.823

HOW DOES IV IRON IMPROVE OUTCOMES IN PATIENTS WITH HF AND IRON DEFICIENCY INDEPENDENT OF ANEMIA? • Does IV iron restore iron stores in the body and particularly in the heart and skeletal muscles and how rapid is this effect? • Are these effects associated with improvement in the cardiac and skeletal muscle energetics and outcomes? • Are the effects of IV iron observed in chronic and acute HF? 51 Nunez et al. JAHA. 2020;9: e014254.

IV IRON RESTORES SKELETAL & MYOCARDIAL IRON STORES T2* and T1 Mapping Cardiac Magnetic Resonance Imaging Randomized chronic stable patients with HFrEF and ID to placebo (n=26) or IV FCM (n=27 ) T2* and T1 cardiac magnetic resonance imaging to assess myocardial iron at at baseline, 7 and 30 days after randomization. 52 Myocardial-IRON Trial-Nunez et al. JAHA. 2020;9: e014254 Myocardial-IRON Trial-Nunez et al. JAHA. 2020;9: e014254.

IV IRON RAPIDLY RESTORES SKELETAL AND MYOCARDIAL IRON STORES T2* AND T1 MAPPING CARDIAC MAGNETIC RESONANCE IMAGING THE MYOCARDIAL-IRON TRIAL 53 Nunez et al. JAHA. 2020;9: e014254 ▪Nunez et al. JAHA. 2020;9: e014254.

IRON REPLETION IS ASSOCIATED WITH IMPROVEMENT IN MUSCLE MITOCHONDRIAL ENERGETICS • FERRIC-HF II tested the hypothesis that IV iron in HF enhances skeletal muscle energetics as reflected by shorter PCr recovery half-time (PCr t1/2) on 31P MRS. • 40 patients (50% anemic) HFrEF, NYHF class ≥II and ID were randomized to IV iron isomaltoside or saline, 31P MRS assessed at baseline and 2 weeks post infusion. 54 Edwards…. Okokko et al. Circulation. 2019;139:2386–2398.

HOW DO WE EXPLAIN THESE FINDINGS AND THE NEXT STEPS? • The IV iron studies show that although IV iron rapidly entered the myocardial cells and improved the skeletal muscle mitochondrial function, the functional changes were modest with no appreciable improvement in cardiac function. • Taken together, these findings raise the possibility that the kinetics and bioavailability of FCM and iron isomaltoside, that were used in these studies might be slow and that the unique properties of Ferric Pyrophosphate Citrate (FPC) with its rapid kinetics might more quickly improve cardiac function and symptoms. 55

CLINICAL HYPOTHESIS • Increasing data suggest that IV iron in ambulatory patients with heart failure with reduced ejection fraction (HFrEF) improves symptoms, exercise capacity, but long-term effects on hospitalizations, mortality and safety are unknown • Re-uptake and response of cardiac tissues linked to bioavailability of [macromolecular] IV iron • Replacement with 100% bioavailable iron will get direct to cardiac muscle faster • Effect should be observable during hospital stay • Treat acute condition • Yield an Improvement in Myocardial Energetics, • Improved function, LV Ejection Fraction/Strain • Clinical benefit 56

RATIONALE FOR IV FPC VS. IV IRON OXIDE NANOPARTICLES IN THE HOSPITALIZED AHF PATIENT IONPs are delivered in very large, bolus doses ranging from 100-750mg/dose • These large doses are known to increase inflammation and increase hepcidin which paradoxically prevents the release of iron to the tissue that need it most. • 1020 mg of ferumoxytol given to Iron deficient patients resulted in a 1.35 g/dL increase in hemoglobin – an effective correction of 207 mg iron deficit at 5 weeks. • 1500 mg of ferric carboxymaltose given to Iron deficient patients resulted in a 1.63 g/dL increase in hemoglobin – an effective correction of 245 mg iron deficit at 5 weeks. • IONPs are currently included on both the European Society of Cardiology (ESC) and American Heart Association (AHA) Guidelines for treatment of heart failure. FPC is ideally suited for this population which is already receiving IV drugs for extended periods of time in the hospital (24/7) • FPC is immediately bioavailable getting iron direct to the tissues. • A 2mg/hr infusion would result in 200mg of immediately bioavailable iron within the first four days of hospitalization. • No impact on hepcidin, and no additional iron sequestration effect from hepcidin. 57

FPC FOR ACUTE HEART FAILURE: CLINICAL DEVELOPMENT STRATEGY • Target indication: treatment of Iron Deficiency (with or without anemia) in adult in-patients with acute heart failure (HFrEF) • Over the past 20 years, there has been considerable progress in the treatment of chronic heart failure • Heart failure is still associated with an annual mortality rate of 10% • The search for better treatments is one of the major challenges in cardiology 58

POTENTIAL PROOF OF CONCEPT STUDY DESIGN Baseline MRI: Follow-up MRI: iron stores/energetics iron stores/energetics ventricular function ventricular function Strain Strain Echocardiography Strain Echocardiography Echocardiography Biomarkers Biomarkers Biomarkers BiomarkersDay 1 Day 2 Day 3 Control: Standard of Care Cohort 1: low dose Triferic™ infusion Cohort 2: high dose Triferic™ infusion Improvement in Biomarker Myocardial Improvement: Iron Deficient, Energetics Iron Repletion NT-proBNP, Failing Heart LV Ejection SST2, galectin Fraction/Strain 59

FPC FOR ACUTE HEART FAILURE: What questions would the PoC Study answer? Feel & Function → Clinical Benefit • Improved LV Function demonstrated by ECHO as measured by global longitudinal strain (GLS) • Improvement in energetics measured from Phosphocreatine/Creatine kinase (CK) aka Creatine phosphokinase (CPK) AND phosphocreatine flux from NMR spectroscopy, Compare Timing of changes in Pcr between IV FPC and FCM • Biomarkers: NT-proBNP, troponins 60

FPC ACUTE HEART FAILURE OPPORTUNITY: IN SUMMARY ❖ Over a million patients are hospitalized each year with acute decompensated heart failure. ❖ Iron deficiency a common co-morbidity in all forms of HF (50-70%) representing a significant unmet need. ❖ A significant body of evidence exists to support the use of IV iron as replacement therapy -- therapy is intended for improvement of cardiac energetics (not improvement of Hgb). ❖ Iron uptake and clinical benefit limited by bioavailability for current IV iron products. ❖ FPC uniquely suited for hospitalized acute heart failure – 200mg of bioavailable iron can be delivered during hospital stay (equivalent to over 1 gram of currently available IV iron products). ❖ A mechanistic proof of concept study would determine if 200mg of FPC over 5 days provides a sufficient influx of iron to the cardiac tissue to… • Improve myocardial energetics • Improve heart failure feel and function • Result in improved clinical outcomes (e.g., 6-minute walk test, NYHA class) 61
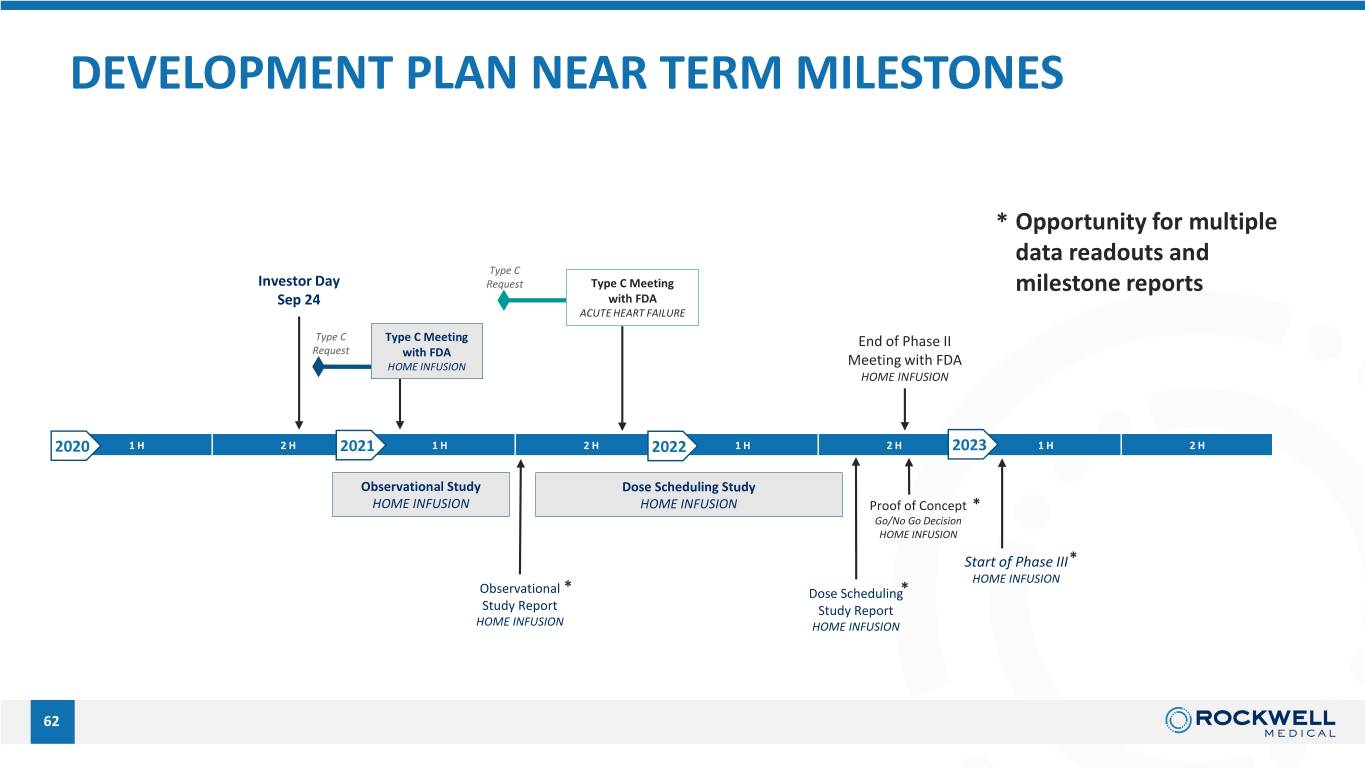
DEVELOPMENT PLAN NEAR TERM MILESTONES * Opportunity for multiple data readouts and Type C Investor Day Request Type C Meeting milestone reports Sep 24 with FDA ACUTE HEART FAILURE Type C Type C Meeting End of Phase II Request with FDA HOME INFUSION Meeting with FDA HOME INFUSION 2020 1 H 2 H 2021 1 H 2 H 2022 1 H 2 H 2023 1 H 2 H Observational Study Dose Scheduling Study HOME INFUSION HOME INFUSION Proof of Concept * 62 Go/No Go Decision HOME INFUSION Start of Phase III* HOME INFUSION Observational * Dose Scheduling* Study Report Study Report HOME INFUSION HOME INFUSION 62

Rockwell Medical Russell Ellison, M.D., M.Sc. President and Chief Executive Officer, Rockwell Medical, Inc. Marc Hoffman, M.D. Chief Medical Officer, Rockwell Medical, Inc. Timothy Chole Q&A Vice President of Marketing,Tim Chole Rockwell Medical, Inc. Vice President of Marketing Key Opinion Leaders Connie Sullivan, B.S. Pharm. President and Chief Executive Officer, National Home Infusion Association Inder Anand, M.D., FRCP, D. Phil. Professor Emeritus, Dept. of Cardiovascular Medicine, Univ. of Minnesota 63

IMPORTANT SAFETY INFORMATION Warnings and Precautions ◦ Serious hypersensitivity reactions, including anaphylactic-type reactions, some of which have been life-threatening and fatal, have been reported in patients receiving parenteral iron products. Patients may present with shock, clinically significant hypotension, loss of consciousness, and/or collapse. Monitor patients for signs and symptoms of hypersensitivity during and after hemodialysis until clinically stable. Personnel and therapies should be immediately available for the treatment of serious hypersensitivity reactions. Hypersensitivity reactions have been reported in 1 (0.3%) of 292 patients receiving TRIFERIC in two randomized clinical trials. ◦ Iron status should be determined on pre-dialysis blood samples. Post dialysis serum iron parameters may overestimate serum iron and transferrin saturation. Adverse Reactions ◦ Most common adverse reactions (incidence ≥3% and at least 1% greater than placebo) in controlled clinical studies include: headache, peripheral edema, asthenia, AV fistula thrombosis, urinary tract infection, AV fistula site hemorrhage, pyrexia, fatigue, procedural hypotension, muscle spasms, pain in extremity, back pain, and dyspnea. Please see full Prescribing Information for more information about TRIFERIC. www.Triferic.com 64 Full prescribing information available at www.Triferic.com
