Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - NOVAVAX INC | tm2030569d1_8k.htm |
Exhibit 99.1

Nasdaq: NVAX | September 10, 2020 Corporate Overview and Investor Deck

2 novavax.com Safe Harbor Statement Certain information, particularly information relating to future performance and other business matters, including expectations regarding clinical development, market opportunities and anticipated milestones constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act. Forward - looking statements may generally contain words such as “believe,” “may,” “could,” “will,” “possible,” “can,” “estimate,” “continue,” “ongoing,” “consider,” “intend,” “indicate,” “plan,” “project,” “expect,” “should,” “would,” or “assume” or variations of such words or other words with similar meanings. Novavax cautions that these forward - looking statements are subject to numerous assumptions, risks and uncertainties that change over time and may cause actual results to differ materially from the results discussed in the forward - looking statements. Uncertainties include but are not limited to clinical trial results, dependence on third party contractors, competition for clinical resources and patient enrollment and risks that we may lack the financial resources to fund ongoing operations. Additional information on Risk Factors are contained in Novavax’ filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10 - K for the year ended December 31, 2019, our Quarterly Reports on Form 10 - Q, and our Current Reports on Form 8 - K, which are all available at http://www.sec.gov. Forward - looking statements are based on current expectations and assumptions and currently available data and are neither predictions nor guarantees of future events or performance. Current results may not be predictive of future results. You should not place undue reliance on forward - looking statements which speak only as of the date hereof. The Company does not undertake to update or revise any forward - looking statements after they are made, whether as a result of new information, future events, or otherwise, except as required by applicable law. Matrix - M and NanoFlu are trademarks of Novavax, Inc. Safe Harbor Statement

3 novavax.com Recent progress leading to significant opportunity Recent positive Phase 1 results for coronavirus vaccine candidate, NVX - CoV2373; Phase 2 clinical trial ongoing in US, Australia and others initiating globally Balance sheet strengthened significantly with ~ $610M in cash at June 30; Recent new hires and promotions have strengthened the leadership team NanoFlu ™ Phase 3 clinical trial achieved all primary endpoints; Preparations for U.S. BLA submission under accelerated approval pathway continuing Over $2 billion in funding for global coronavirus vaccine program; Multiple collaboration and supply agreements completed

4 novavax.com Novavax vaccine pipeline PHASE 1 PHASE 2 PHASE 3 CLINICAL PRECLINICAL PROGRAM DESCRIPTION ResVax™ - RSV F Vaccine - Infants via Maternal Immunization RSV F Vaccine - Older Adults (60+ yrs) RSV F Vaccine - Pediatrics (6 mos – 5 yrs) Combination Influenza/RSV F Vaccine - Older Adults (60+) Ebola GP Vaccine Matrix - M Matrix - M Matrix - M NVX - CoV2373 – Coronavirus Vaccine Candidate Matrix - M NanoFlu™ – Nanoparticle Seasonal Influenza Vaccine - Older Adults (65+ yrs) Matrix - M Completed Phase 3 – March 2020 Successfully achieved all primary endpoints and achieved statistical significance in key secondary endpoints

5 novavax.com NVX - CoV2373 vaccine program
6 novavax.com Recombinant nanoparticle technology platform and Matrix - M™ combined to create NVX - CoV2373 and address global public health threat Matrix - M, a potent and well - tolerated adjuvant broadens immune responses and offers potential dose - sparing Platform combines the power and speed of genetic engineering to produce a new class of highly immunogenic nanoparticles SARS - CoV - 2 virus Enhance immune responses and stimulate high levels of neutralizing antibodies Novavax TECHNOLOGY PLATFORMS *Coronavirus image CDC Library

7 novavax.com Bangaru S. et al, bioRxiv , 2020.08.06.234674; doi : https:// doi.org /10.1101/2020.08.06.234674 Tian et al., bioRxiv , July 2020 SARS - CoV - 2 Protein Spike • Full - length native confirmation trimer nanoparticle formulated with Matrix - M • Liquid formulation in vials, stable at 2 ° C to 8 ° C NVX - CoV2373: A full - length, prefusion stabilized SARS - CoV - 2 spike (S) glycoprotein + Matrix - M™
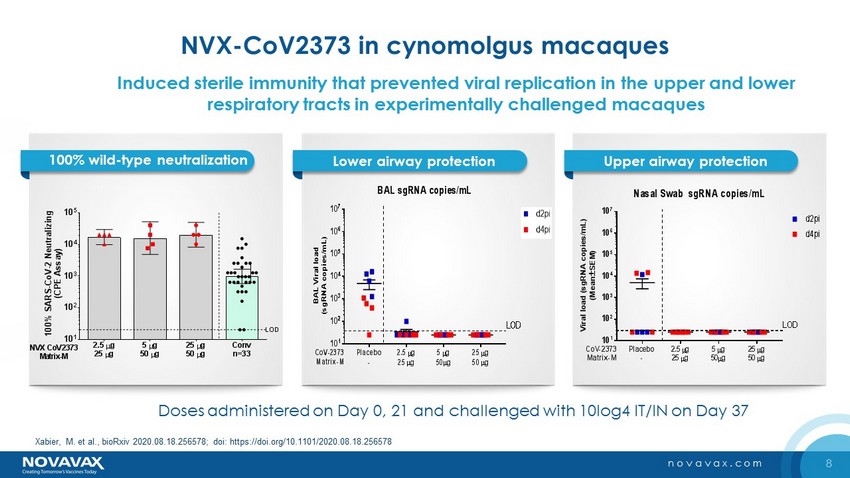
8 novavax.com NVX - CoV2373 in cynomolgus macaques 100% wild - type neutralization Lower airway protection Upper airway protection 2.5 g 25 g 5 g 50 g 25 g 50 g Conv n=33 10 1 10 2 10 3 10 4 10 5 1 0 0 % S A R S - C o V - 2 N e u t r a l i z i n g ( C P E A s s a y ) LOD NVX CoV2373 Matrix-M Doses administered on Day 0, 21 and challenged with 10log4 IT/IN on Day 37 Placebo - 2.5 g 25 g 5 g 50g 25 g 50 g 10 1 10 2 10 3 10 4 10 5 10 6 10 7 BAL sgRNA copies/mL B A L V i r a l l o a d ( s g R N A c o p i e s / m L ) LOD CoV-2373 Matrix-M Placebo - 2.5 g 25 g 5 g 50g 25 g 50 g 10 1 10 2 10 3 10 4 10 5 10 6 10 7 Nasal Swab sgRNA copies/mL V i r a l l o a d ( s g R N A c o p i e s / m L ) ( M e a n S E M ) LOD CoV-2373 Matrix-M d2pi d4pi Induced sterile immunity that prevented viral replication in the upper and lower respiratory tracts in experimentally challenged macaques Xabier , M. et al., bioRxiv 2020.08.18.256578; doi : https://doi.org/10.1101/2020.08.18.256578

9 novavax.com NVX - CoV2373 is stable and will utilize the standard cold chain NVX - CoV2373 binding to hACE2 under stress conditions Tian et al., bioRxiv , July 2020, bioRxiv 2020.06.29.178509; doi : https:// doi.org /10.1101/2020.06.29.178509

10 novavax.com Phase 1/2 published in NEJM “At 35 days, NVX - CoV2373 appeared to be safe, and it elicited immune responses that exceeded levels in Covid - 19 convalescent serum. The Matrix - M1 adjuvant induced CD4+ T - cell responses that were biased toward a Th1 phenotype.” New England Journal of Medicine CONCLUSION:
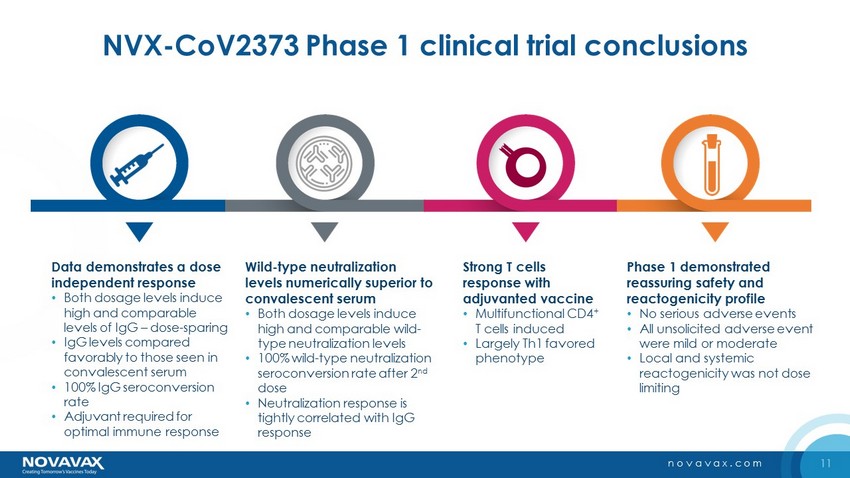
11 novavax.com NVX - CoV2373 Phase 1 clinical trial conclusions Data demonstrates a dose independent response • Both dosage levels induce high and comparable levels of IgG – dose - sparing • IgG levels compared favorably to those seen in convalescent serum • 100% IgG seroconversion rate • Adjuvant required for optimal immune response Strong T cells response with adjuvanted vaccine • Multifunctional CD4 + T cells induced • Largely Th1 favored phenotype Wild - type neutralization levels numerically superior to convalescent serum • Both dosage levels induce high and comparable wild - type neutralization levels • 100% wild - type neutralization seroconversion rate after 2 nd dose • Neutralization response is tightly correlated with IgG response Phase 1 demonstrated reassuring safety and reactogenicity profile • No serious adverse events • All unsolicited adverse event were mild or moderate • Local and systemic reactogenicity was not dose limiting

12 novavax.com 2 doses of vaccine induces high levels of IgG Covid - 19 Convalescent Sera (Baylor) GMEU 8,344 (95% CI 4,420; 15,747) A: Placebo Day 35 GMEU 113 (95% CI: 94; 138) B: 2 dose 25 ug (no adjuvant) Day 35 GMEU 575 (95% CI: 332; 999) C: 2 doses 5 ug + Matrix - M Day 35 GMEU 63,160 (95% CI: 47,117; 84,666) D: 2 doses 25 ug + Matrix - M Day 35 GMEU 47,521 (95% CI: 33,803; 66,804) E: 1 dose 25 ug + Matrix - M Day 35 GMEU 2,932 (95% CI: 1,988; 4,325) Anti - S IgG ELISA units/ml
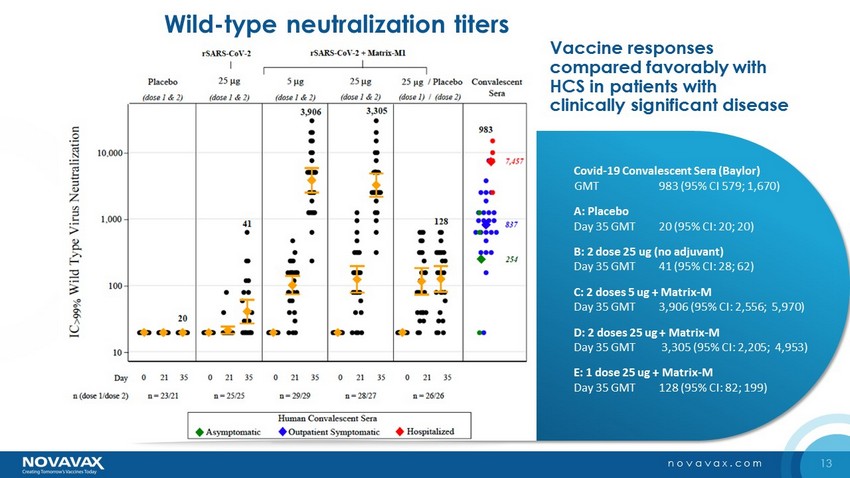
13 novavax.com Covid - 19 Convalescent Sera (Baylor) GMT 983 (95% CI 579; 1,670) A: Placebo Day 35 GMT 20 (95% CI: 20; 20) B: 2 dose 25 ug (no adjuvant) Day 35 GMT 41 (95% CI: 28; 62) C: 2 doses 5 ug + Matrix - M Day 35 GMT 3,906 (95% CI: 2,556; 5,970) D: 2 doses 25 ug + Matrix - M Day 35 GMT 3,305 (95% CI: 2,205; 4,953) E: 1 dose 25 ug + Matrix - M Day 35 GMT 128 (95% CI: 82; 199) Vaccine responses compared favorably with HCS in patients with clinically significant disease 3,906 3,305 254 837 7,457 Wild - type neutralization titers

14 novavax.com Adjuvant and Two Dose Effects: MN at Day 35 Unadjuvanted Two Dose Adjuvanted One Dose Adjuvanted Two Dose The majority vaccinated develop high neutralizing antibodies 25 µg Unadjuvanted Two Doses 5 µg Adjuvanted Two Doses Placebo 25 µg Adjuvanted One Dose 25 µg Adjuvanted Two Doses

15 novavax.com Placebo 25ug x 2 doses (no Matrix - M) 25ug + 50ug Matrix - M x 1 dose 25ug + 50ug Matrix - M x 2 dose 5ug + 50ug Matrix - M x 2 dose 100% wild - type neutralizing titer kinetics to day 49: persistence of immunity
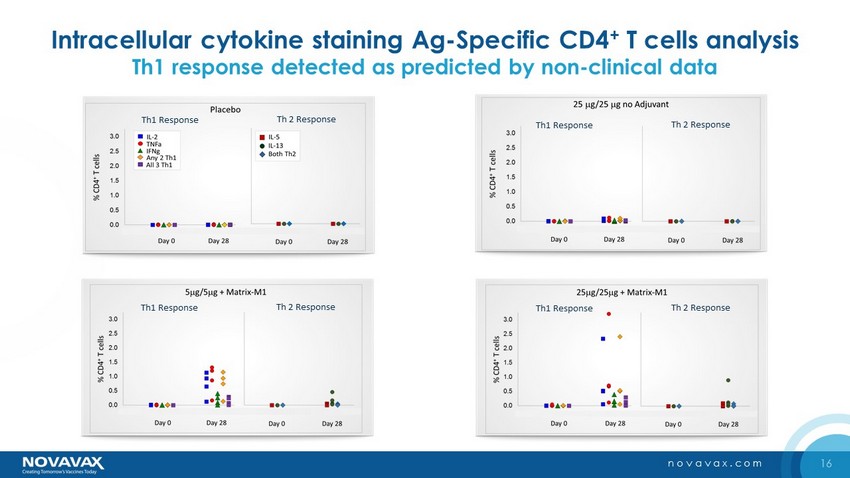
16 novavax.com Intracellular cytokine staining Ag - Specific CD4 + T cells analysis Th1 response detected as predicted by non - clinical data Group D Th1 Response Th 2 Response Group D Th1 Response Th 2 Response Th1 Response Th 2 Response Th1 Response Th 2 Response

17 novavax.com Localized symptoms • The majority of localized reactogenicity symptoms were mild Solicited Localized Symptoms Overall, reactogenicity was mild, and vaccinations were well - tolerated There were no vaccine refusals or dropouts due to systemic reactions

18 novavax.com Systemic symptoms • Reactogenicity increased after Dose 2 • Average duration of reactions <2 days • Majority of reported symptoms remained at ≤ 1 grade (mild or none) Solicited Systemic Symptoms

19 novavax.com Novavax ’ rapid vaccine development demonstrates clinical expertise, endorsed by funding awards to deliver product by the end of 2020 CEPI funding up to $388M received NVX - CoV2373 vaccine candidate identified SARS - CoV - 2 sequence published April May Jan Phase 1 clinical trial initiated Praha Vaccines acquired to expand global supply chain June Contract from U.S. DoD funded up to $60M $1.6B funding from U.S. OWS July August Positive Phase 1 data announced Novavax and FujiFilm Diosynth initiated large scale manufacturing Phase 2 preliminary data Phase 3 initiating ‘Q4 2020

20 novavax.com Network of established supply chain partners to target significant capacity in 2021 Antigen production Matrix M™ production Novavax AB FujiFilm NC FujiFilm TX PolyPeptide Group AGC Biologics Novavax CZ PolyPeptide Group AGC Biologics Vaccine distribution & license agreement Serum Institute Takeda SK biosciences FujiFilm UK
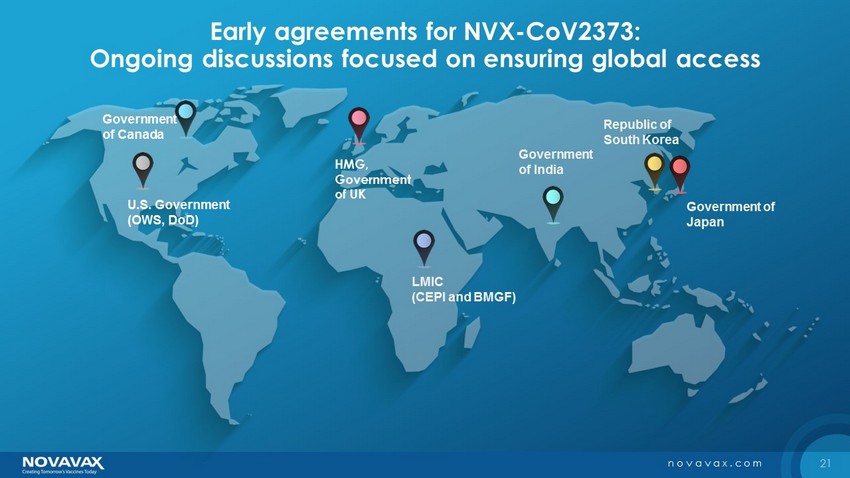
21 novavax.com Early agreements for NVX - CoV2373: Ongoing discussions focused on ensuring global access U.S. Government (OWS, DoD) HMG, Government of UK Government of India Government of Japan Government of Canada Republic of South Korea LMIC (CEPI and BMGF)

22 novavax.com Interim data from Phase 2 clinical trial in Australia and US in 4Q 2020 to advance regulatory strategy Global Pivotal Phase 3 efficacy trial: initiating October to support BLA filing UK Phase 3 efficacy trial: initiating September Expansion of manufacturing capabilities and global supply resources Additional partnerships for collaboration and dose procurement ensuring global access Upcoming milestones to deliver NVX - CoV 2373 to the global market

23 novavax.com www.website.com NanoFlu ™ vaccine program

24 novavax.com NanoFlu ™ addresses the need for greater and broader immune responses Enhances biologic functions to generate potent, robust, and long - lasting protective immune responses Eliminates egg adaptive changes to strains and resulting mismatch between vaccine and circulating viruses Provides broader protection against evolution and antigenic drift Novavax NanoFlu ™ via recombinant nanoparticle technology and Matrix - M adjuvant Next generation flu vaccine for improved protection

25 novavax.com • Demonstrated immunologic HAI antibody responses against all four vaccine strains 2020 U.S. policy objectives encouraging innovative technologies support the need for a new market offering Opportunity for a differentiated flu vaccine brand in a commoditized market with increasing demand for improved effectiveness The combination of these results will form the basis for a future BLA submission using the FDA’s accelerated approval pathway • Well - established and understood direct & indirect distribution / reimbursement systems • Currently exploring pathways to manufacture product for required lot consistency trial Phase 3 immunogenicity data demonstrated the development of robust T cell - mediated responses, differentiating NanoFlu from leading licensed vaccines NanoFlu progress continues

26 novavax.com Financial overview • Strong financial position • Significant funding expected to support activities through Phase 3 clinical trial results for COVID - 19 vaccine development * As of June 30, 2020, ** As of the close on August 31, 2020. Cash and equivalents* > $600 million Market capitalization** $6.8 billion Financial position
