Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - AGENUS INC | d44878dex992.htm |
| EX-99.1 - EX-99.1 - AGENUS INC | d44878dex991.htm |
| 8-K - 8-K - AGENUS INC | d44878d8k.htm |

August 2020 This version of the presentation includes corrections to an error in the original presentation contained on slide 10. Exhibit 99.3

Forward-Looking Statements This presentation contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws, including statements regarding clinical development and regulatory plans and timelines and anticipated corporate milestones. These forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially. These risks and uncertainties include, among others, the factors described under the Risk Factors section of our most recent Quarterly Report on Form 10-Q or Annual Report on Form 10-K filed with the Securities and Exchange Commission. Agenus cautions investors not to place considerable reliance on the forward-looking statements contained in this presentation. These statements speak only as of the date of this presentation, and Agenus undertakes no obligation to update or revise the statements, other than to the extent required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement.
Key Achievements in Advancing Innovative I-O Therapies Delivered 15 INDs (2016 - Present) Produced 11 clinical batches GMP mAbs Positive clinical data released: 26% ORR in 2L cervical cancer (“Bali” + “Zali”) 14.3% ORR in 2L cervical cancer (“Bali” mono) Early responses in AGEN1181 Ph1 (1 CR, 1 CR by PET) “Bali” BLA submission to be initiated 3Q 2020 Generated $575M (2014 - Present) Corporate collaborations Monetization of royalties Delivered on commitments to partners GSK, Merck, Incyte, Gilead
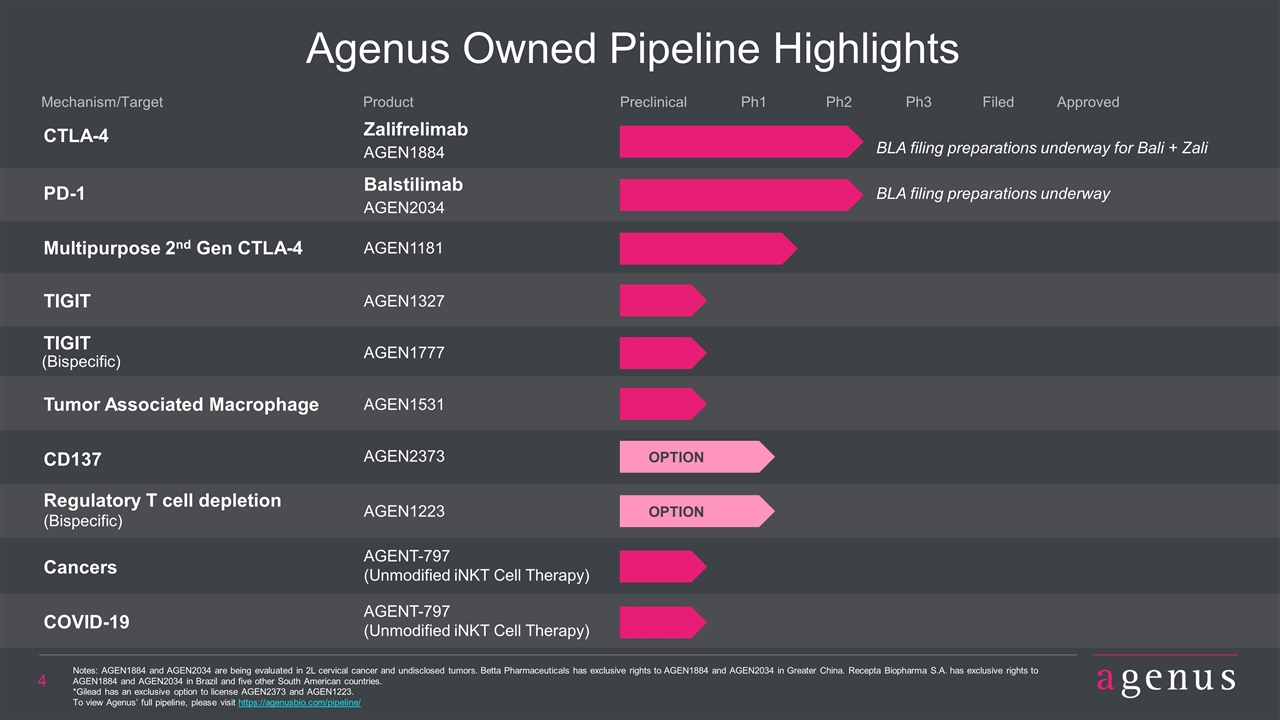
Agenus Owned Pipeline Highlights CTLA-4 Zalifrelimab AGEN1884 PD-1 Balstilimab AGEN2034 Product Mechanism/Target Preclinical Ph1 Ph2 Ph3 Filed Multipurpose 2nd Gen CTLA-4 AGEN1181 TIGIT AGEN1327 TIGIT AGEN1777 (Bispecific) CD137 AGEN2373 Regulatory T cell depletion AGENT-797 (Bispecific) Cancers COVID-19 (Unmodified iNKT Cell Therapy) AGENT-797 (Unmodified iNKT Cell Therapy) AGEN1223 OPTION OPTION Approved Notes: AGEN1884 and AGEN2034 are being evaluated in 2L cervical cancer and undisclosed tumors. Betta Pharmaceuticals has exclusive rights to AGEN1884 and AGEN2034 in Greater China. Recepta Biopharma S.A. has exclusive rights to AGEN1884 and AGEN2034 in Brazil and five other South American countries. *Gilead has an exclusive option to license AGEN2373 and AGEN1223. To view Agenus’ full pipeline, please visit https://agenusbio.com/pipeline/ Tumor Associated Macrophage AGEN1531 BLA filing preparations underway for Bali + Zali BLA filing preparations underway
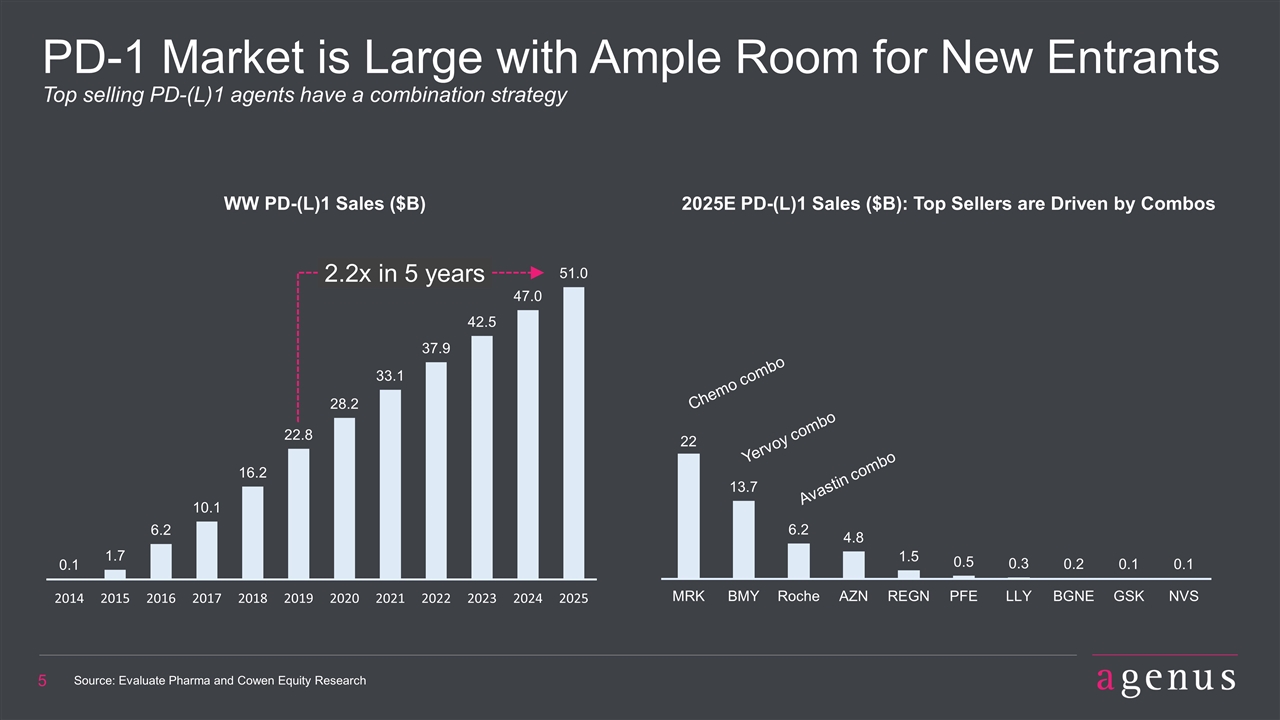
PD-1 Market is Large with Ample Room for New Entrants Top selling PD-(L)1 agents have a combination strategy WW PD-(L)1 Sales ($B) 2025E PD-(L)1 Sales ($B): Top Sellers are Driven by Combos Source: Evaluate Pharma and Cowen Equity Research Chemo combo Yervoy combo Avastin combo 2.2x in 5 years
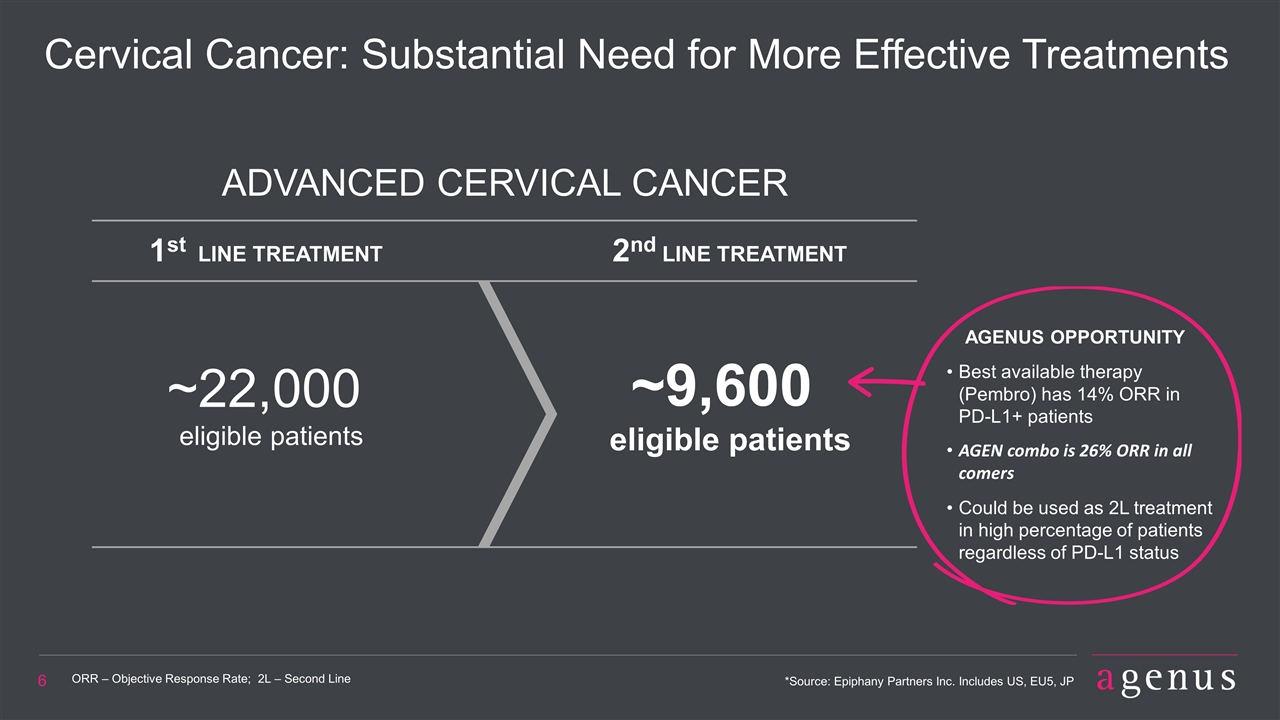
Cervical Cancer: Substantial Need for More Effective Treatments ORR – Objective Response Rate; 2L – Second Line *Source: Epiphany Partners Inc. Includes US, EU5, JP ADVANCED CERVICAL CANCER ~22,000 eligible patients 1st LINE TREATMENT 2nd LINE TREATMENT ~9,600 eligible patients AGENUS OPPORTUNITY Best available therapy (Pembro) has 14% ORR in PD-L1+ patients AGEN combo is 26% ORR in all comers Could be used as 2L treatment in high percentage of patients regardless of PD-L1 status
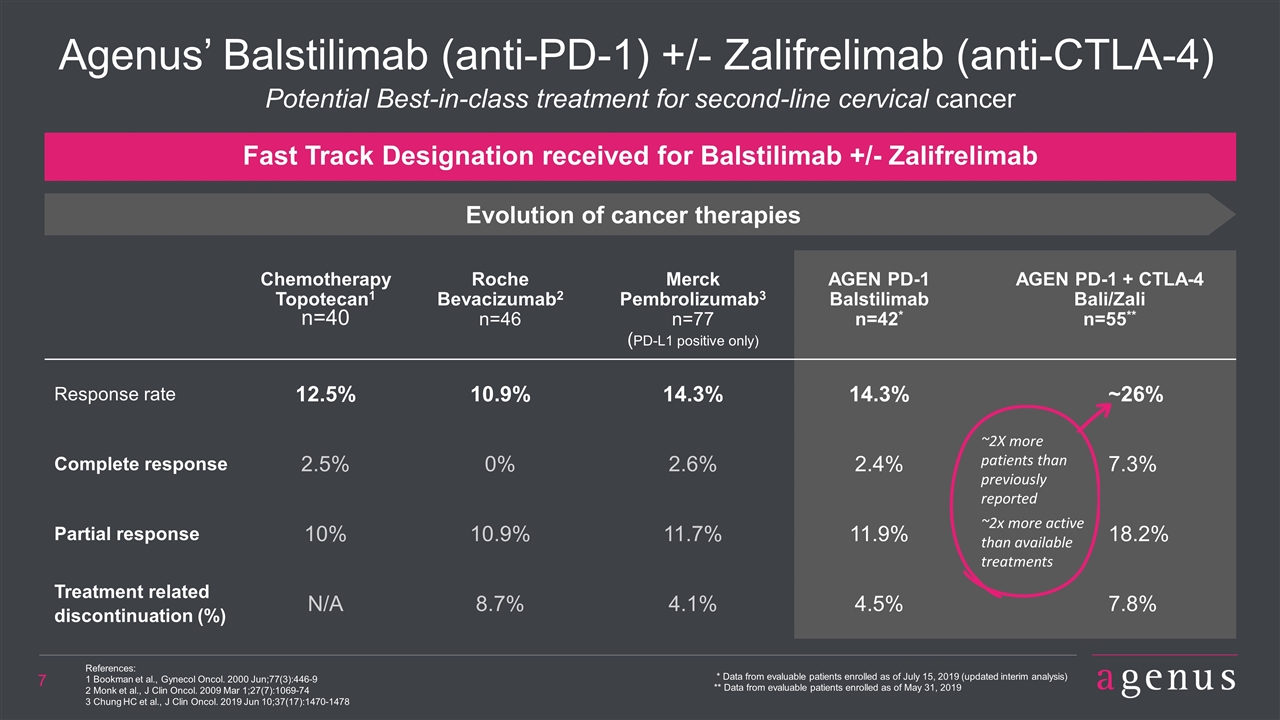
Agenus’ Balstilimab (anti-PD-1) +/- Zalifrelimab (anti-CTLA-4) References: 1 Bookman et al., Gynecol Oncol. 2000 Jun;77(3):446-9 2 Monk et al., J Clin Oncol. 2009 Mar 1;27(7):1069-74 3 Chung HC et al., J Clin Oncol. 2019 Jun 10;37(17):1470-1478 Chemotherapy Topotecan1 n=40 Roche Bevacizumab2 n=46 Merck Pembrolizumab3 n=77 (PD-L1 positive only) AGEN PD-1 Balstilimab n=42* AGEN PD-1 + CTLA-4 Bali/Zali n=55** Response rate 12.5% 10.9% 14.3% 14.3% ~26% Complete response 2.5% 0% 2.6% 2.4% 7.3% Partial response 10% 10.9% 11.7% 11.9% 18.2% Treatment related discontinuation (%) N/A 8.7% 4.1% 4.5% 7.8% Fast Track Designation received for Balstilimab +/- Zalifrelimab Potential Best-in-class treatment for second-line cervical cancer * Data from evaluable patients enrolled as of July 15, 2019 (updated interim analysis) ** Data from evaluable patients enrolled as of May 31, 2019 Evolution of cancer therapies ~2X more patients than previously reported ~2x more active than available treatments
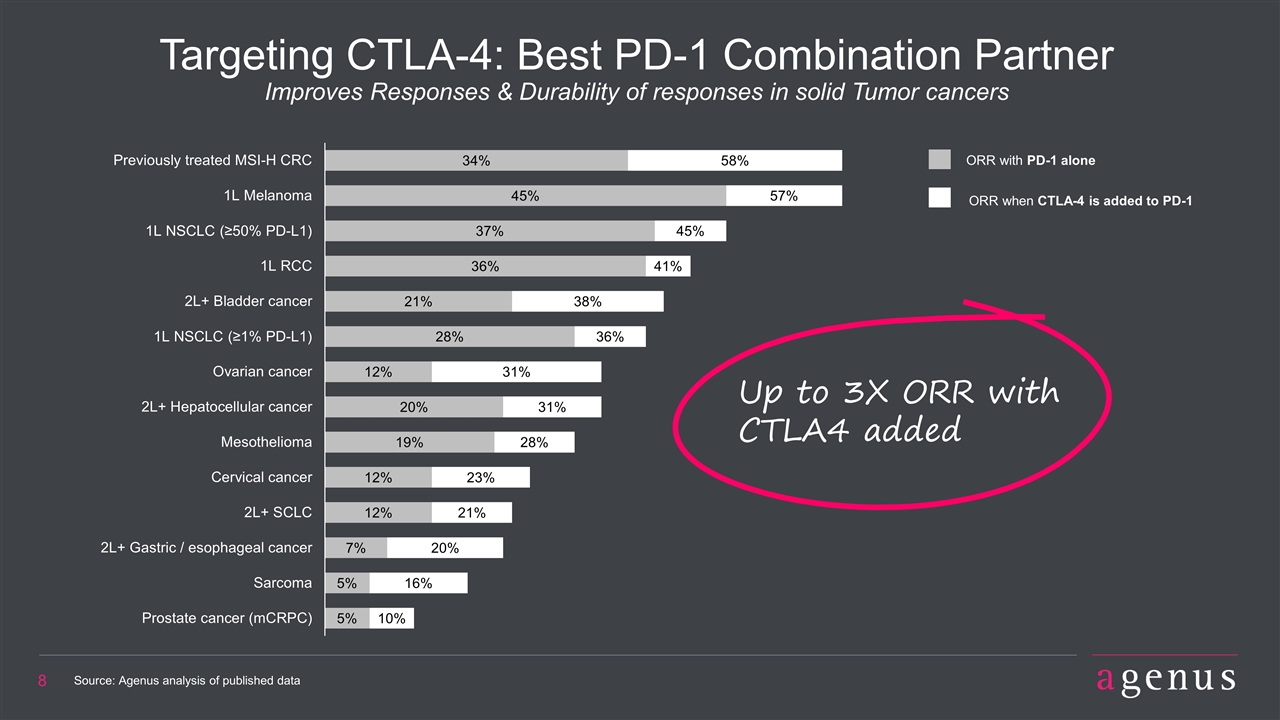
Targeting CTLA-4: Best PD-1 Combination Partner Improves Responses & Durability of responses in solid Tumor cancers Up to 3X ORR with CTLA4 added ORR with PD-1 alone ORR when CTLA-4 is added to PD-1 Source: Agenus analysis of published data
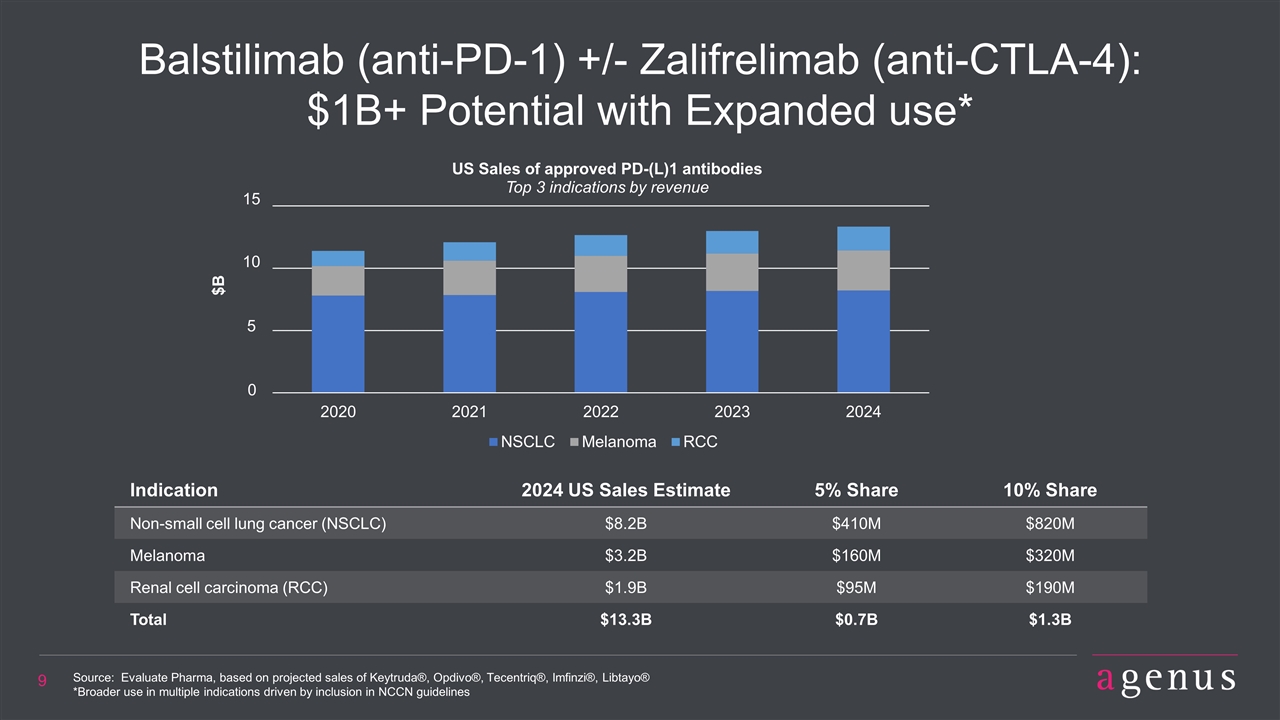
Balstilimab (anti-PD-1) +/- Zalifrelimab (anti-CTLA-4): $1B+ Potential with Expanded use* Indication 2024 US Sales Estimate 5% Share 10% Share Non-small cell lung cancer (NSCLC) $8.2B $410M $820M Melanoma $3.2B $160M $320M Renal cell carcinoma (RCC) $1.9B $95M $190M Total $13.3B $0.7B $1.3B Source: Evaluate Pharma, based on projected sales of Keytruda®, Opdivo®, Tecentriq®, Imfinzi®, Libtayo® *Broader use in multiple indications driven by inclusion in NCCN guidelines 0 5 10 15 $B
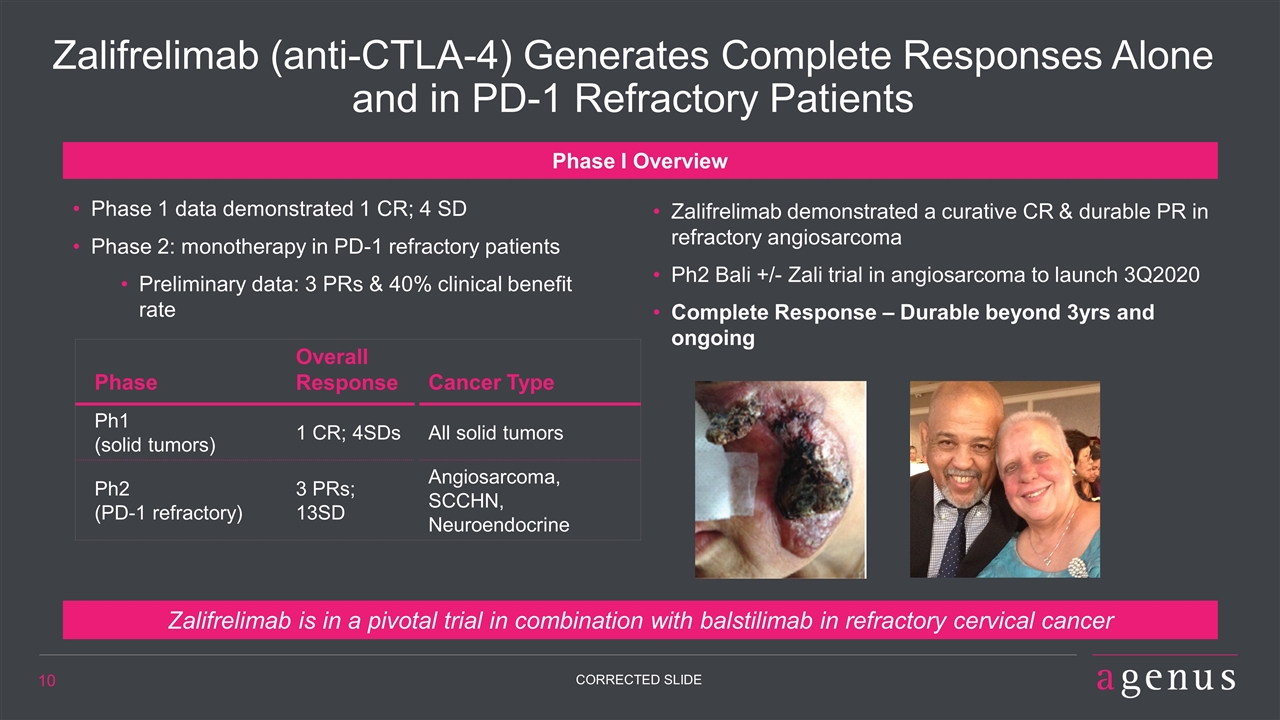
Zalifrelimab (anti-CTLA-4) Generates Complete Responses Alone and in PD-1 Refractory Patients Zalifrelimab demonstrated a curative CR & durable PR in refractory angiosarcoma Ph2 Bali +/- Zali trial in angiosarcoma to launch 3Q2020 Complete Response – Durable beyond 3yrs and ongoing Phase I Overview Phase 1 data demonstrated 1 CR; 4 SD Phase 2: monotherapy in PD-1 refractory patients Preliminary data: 3 PRs & 40% clinical benefit rate Phase Overall Response Cancer Type Ph1 (solid tumors) 1 CR; 4SDs All solid tumors Ph2 (PD-1 refractory) 3 PRs; 13SD Angiosarcoma, SCCHN, Neuroendocrine Zalifrelimab is in a pivotal trial in combination with balstilimab in refractory cervical cancer 10 CORRECTED SLIDE
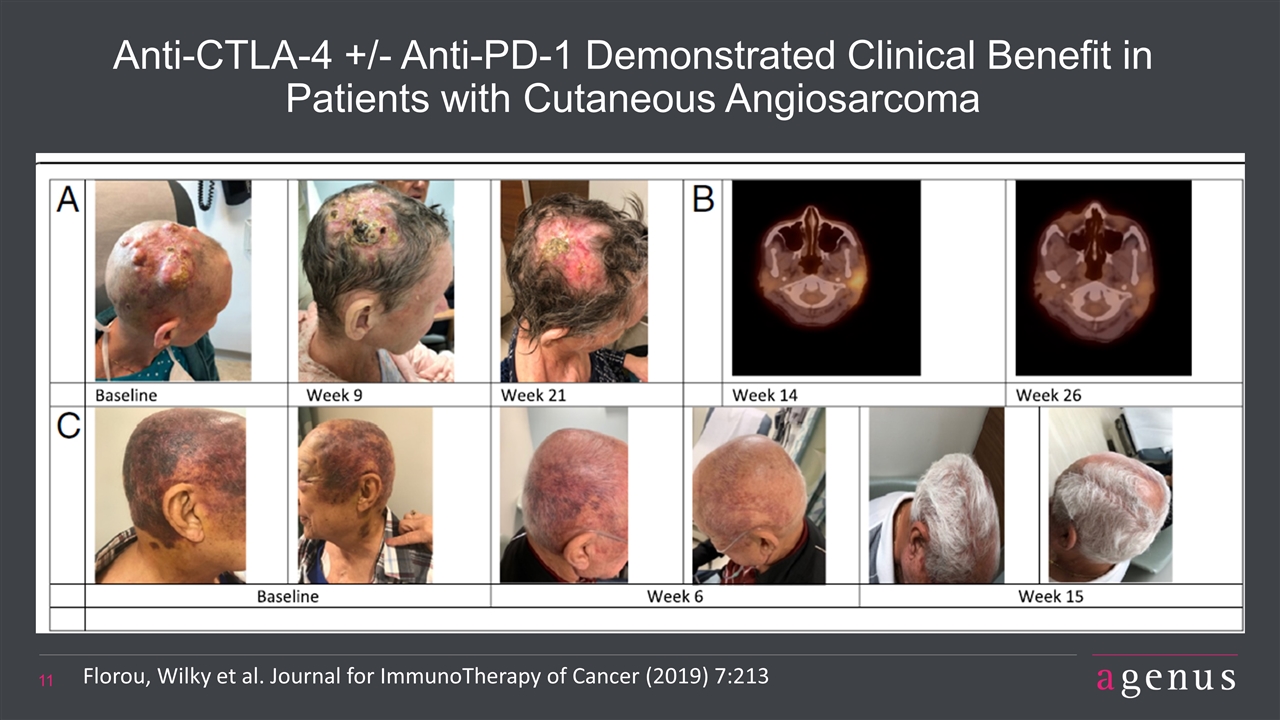
Anti-CTLA-4 +/- Anti-PD-1 Demonstrated Clinical Benefit in Patients with Cutaneous Angiosarcoma Florou, Wilky et al. Journal for ImmunoTherapy of Cancer (2019) 7:213
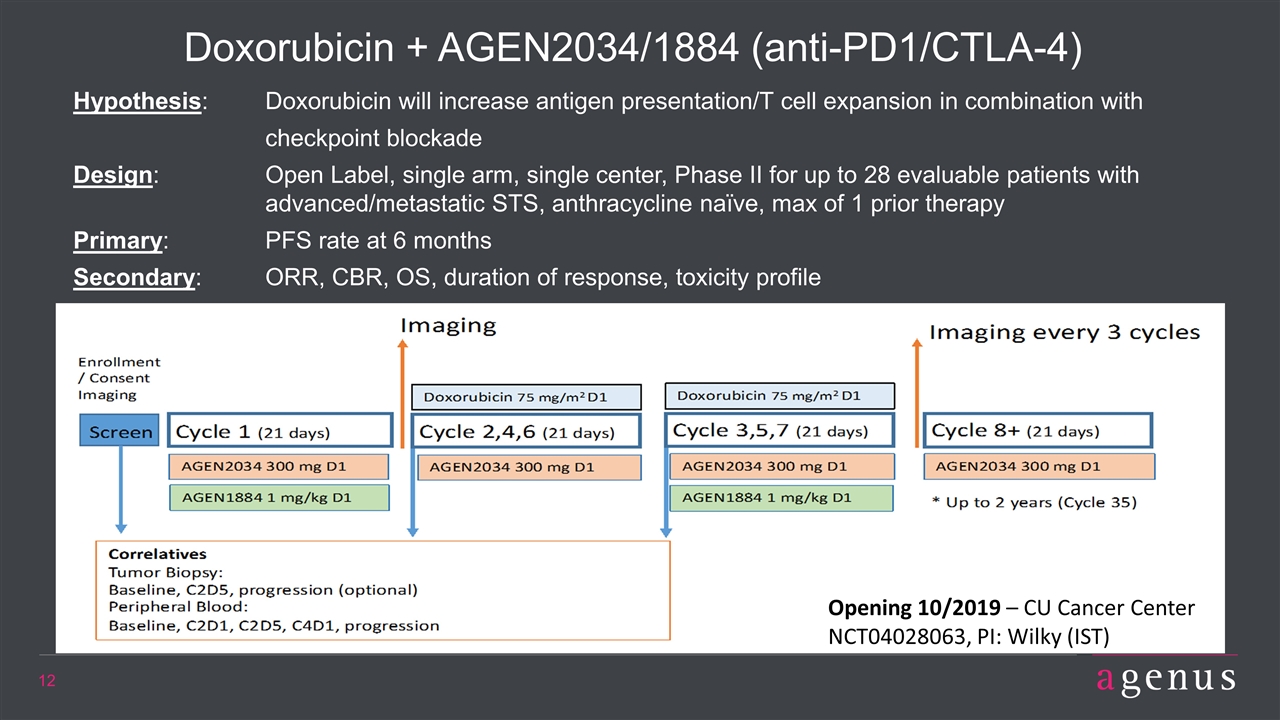
Doxorubicin + AGEN2034/1884 (anti-PD1/CTLA-4) Hypothesis: Doxorubicin will increase antigen presentation/T cell expansion in combination with checkpoint blockade Design: Open Label, single arm, single center, Phase II for up to 28 evaluable patients with advanced/metastatic STS, anthracycline naïve, max of 1 prior therapy Primary: PFS rate at 6 months Secondary: ORR, CBR, OS, duration of response, toxicity profile Opening 10/2019 – CU Cancer Center NCT04028063, PI: Wilky (IST)

Agenus has Generated $575M from Strategic and Financial Transactions (2014 – Present) $172.5M $145M $9M $205M $10M $35M
Agenus’ next Generation I-O Innovations AGEN1181: Multi Functional CTLA-4 AGEN1777: First-in-class TIGIT bispecific AGEN1327: Fc-enhanced TIGIT monospecific AGENT-797: Allogeneic iNKT cell therapy
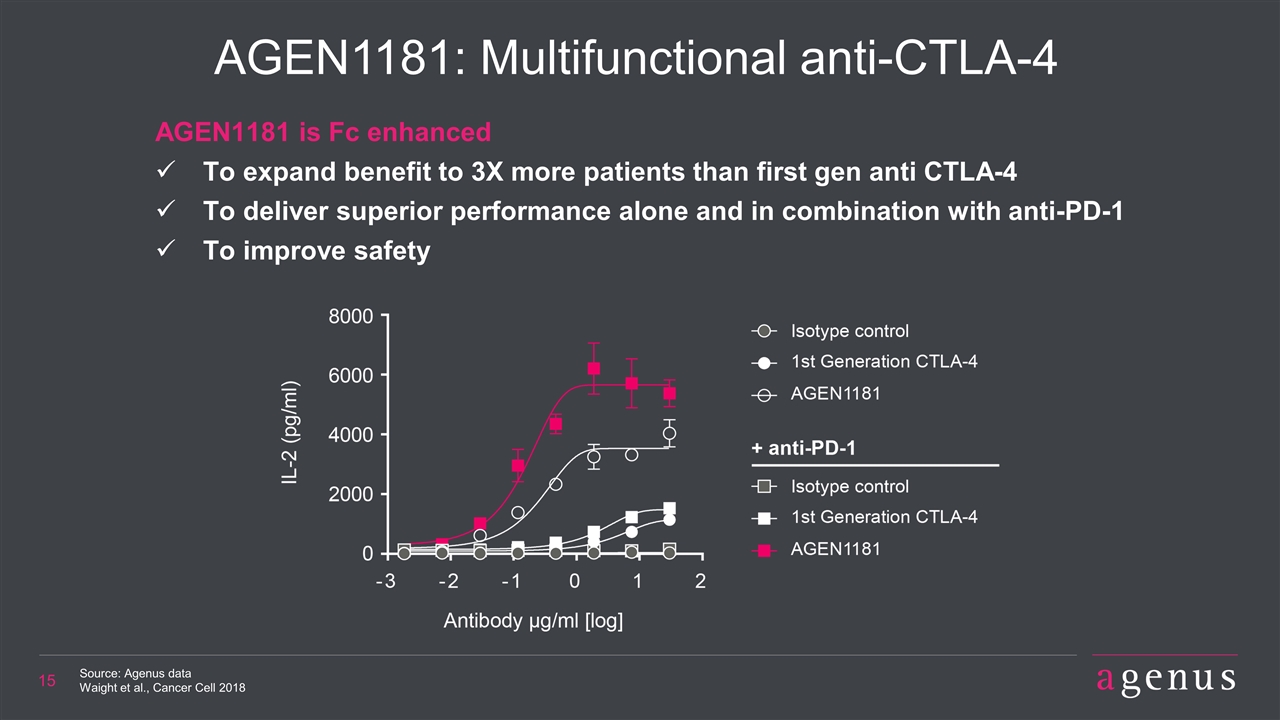
AGEN1181: Multifunctional anti-CTLA-4 Source: Agenus data Waight et al., Cancer Cell 2018 AGEN1181 is Fc enhanced To expand benefit to 3X more patients than first gen anti CTLA-4 To deliver superior performance alone and in combination with anti-PD-1 To improve safety
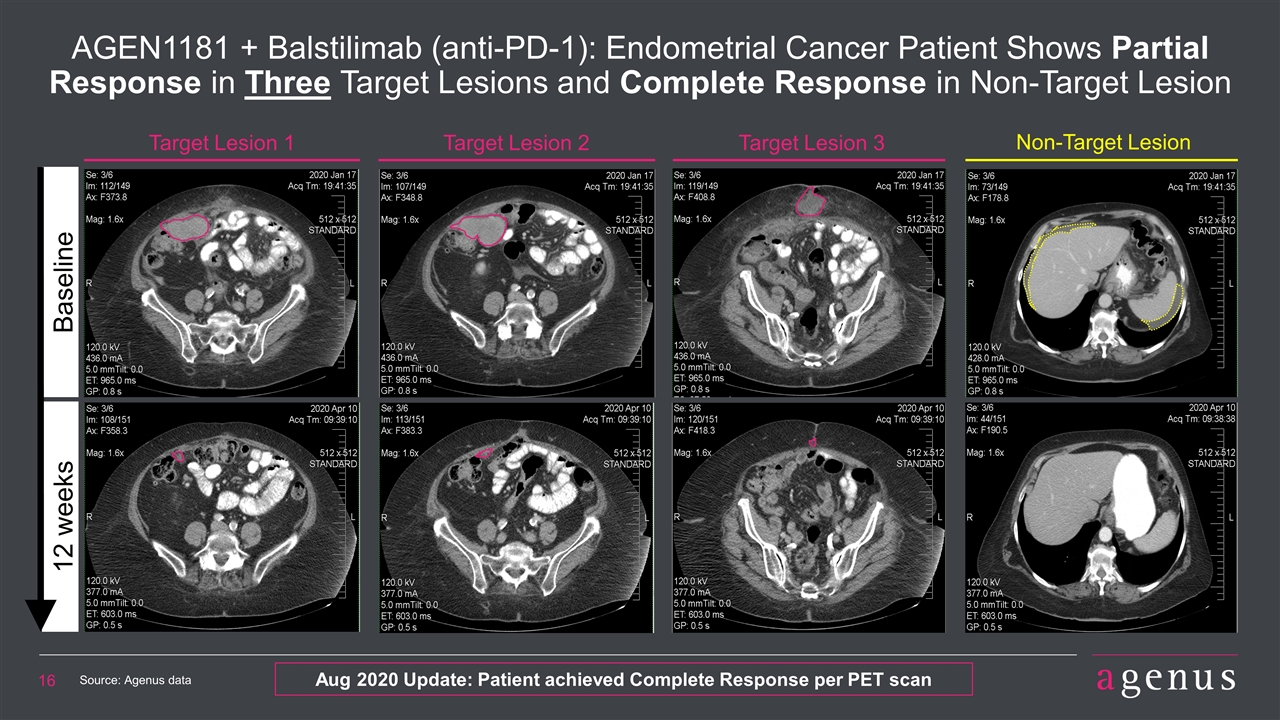
Target Lesion 1 Target Lesion 2 AGEN1181 + Balstilimab (anti-PD-1): Endometrial Cancer Patient Shows Partial Response in Three Target Lesions and Complete Response in Non-Target Lesion Target Lesion 3 Non-Target Lesion Baseline 12 weeks Source: Agenus data Aug 2020 Update: Patient achieved Complete Response per PET scan
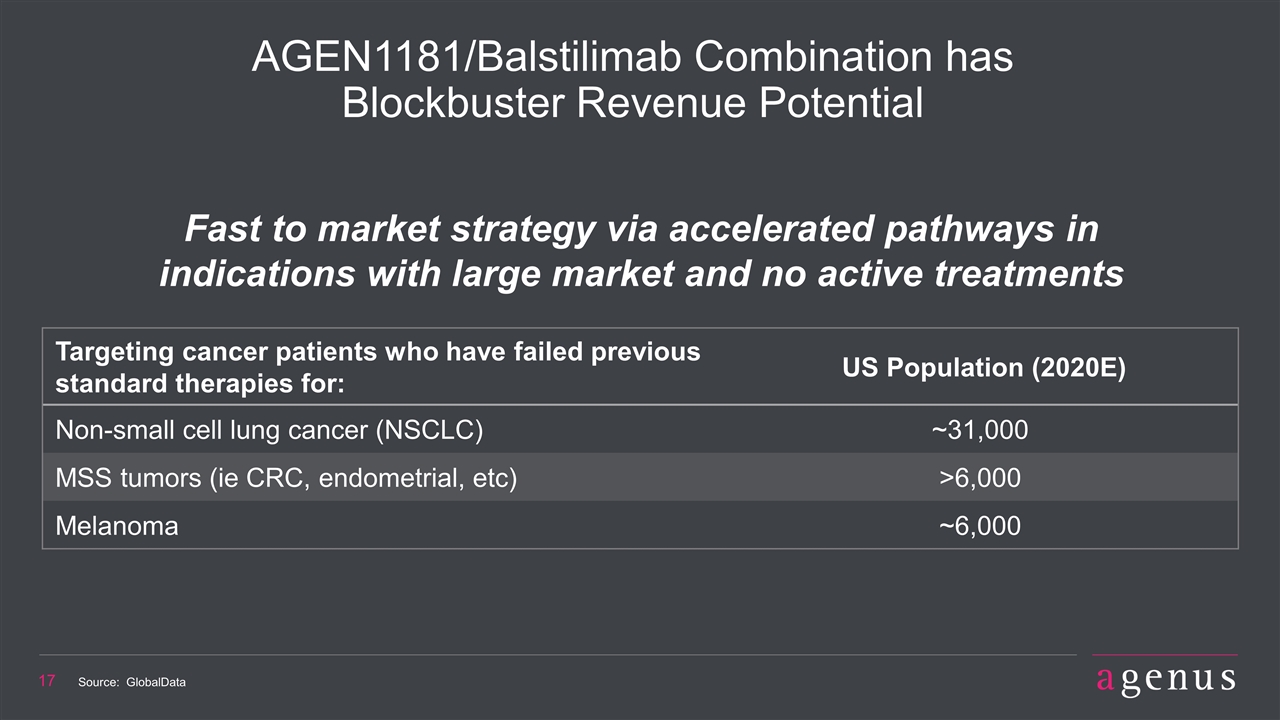
Targeting cancer patients who have failed previous standard therapies for: US Population (2020E) Non-small cell lung cancer (NSCLC) ~31,000 MSS tumors (ie CRC, endometrial, etc) >6,000 Melanoma ~6,000 AGEN1181/Balstilimab Combination has Blockbuster Revenue Potential Fast to market strategy via accelerated pathways in indications with large market and no active treatments Source: GlobalData

TIGIT: Highly Promising Novel Target for Immuno-Oncology What is TIGIT? (aka T cell immunoreceptor with Ig and ITIM domains) A critical regulator of innate and adaptive immune response A key synergistic mechanism to anti PD-1 therapy Hence, a natural partner for PD-1 combination therapy – particularly active in TIGIT-expressing tumors Target of high interest, externally validated by late-stage Roche and Merck TIGIT programs Agenus’ TIGIT programs have unique properties to optimize anti-tumor activity TIGIT Monospecific - AGEN1327: TIGIT antibody differentiated via “Fc enhanced” backbone for superior tumor killing as monotherapy or in combination with PD-1 TIGIT Bispecific - AGEN1777: TIGIT bispecific which also inhibits an undisclosed receptor expressed on T and NK cells
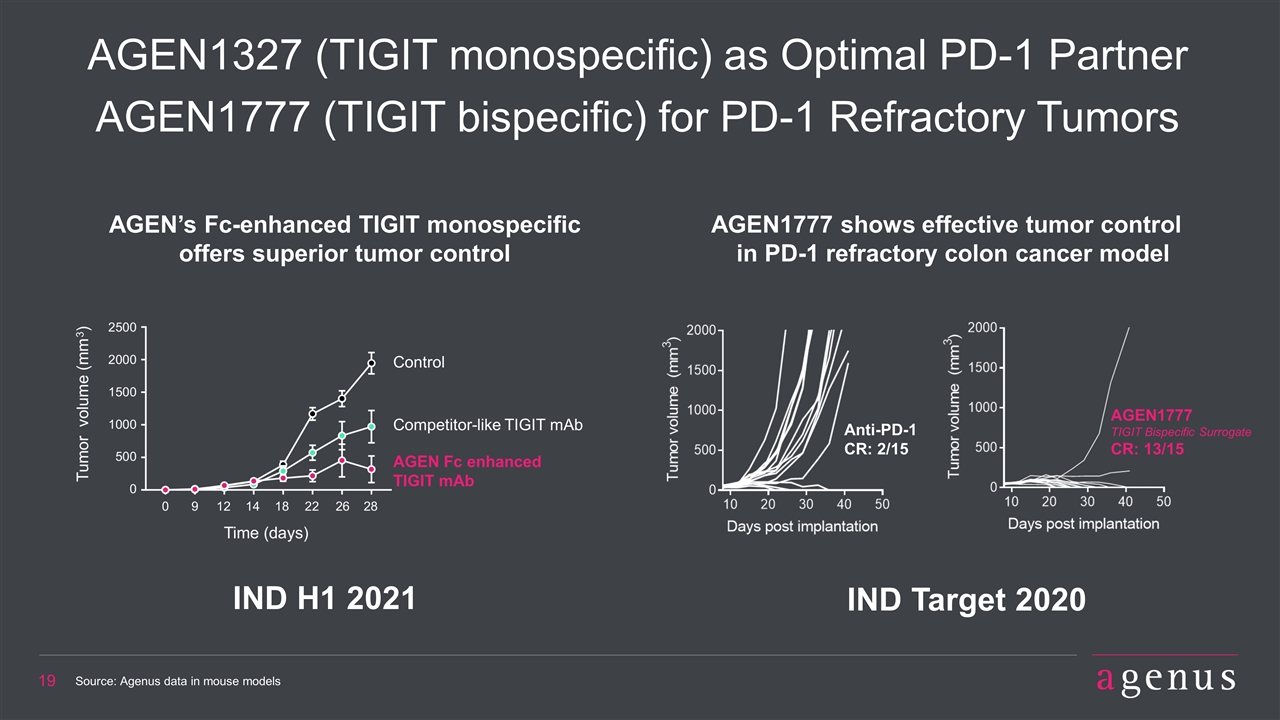
AGEN1327 (TIGIT monospecific) as Optimal PD-1 Partner AGEN1777 (TIGIT bispecific) for PD-1 Refractory Tumors IND H1 2021 Source: Agenus data in mouse models AGEN1777 shows effective tumor control in PD-1 refractory colon cancer model AGEN’s Fc-enhanced TIGIT monospecific offers superior tumor control IND Target 2020 Anti-PD-1 CR: 2/15 AGEN1777 TIGIT Bispecific Surrogate CR: 13/15 Control Competitor-like TIGIT mAb AGEN Fc enhanced TIGIT mAb

Rationale Combination Therapy with AGEN1181 Expands Therapeutic Reach and Enables Curative Responses Agenus at AACR 2020: Fc-enhanced anti-CTLA-4 (AGEN1181) engages multiple mechanisms of action to promote optimal anti-tumor immunity Demonstrates superior tumor control than conventional anti-CTLA-4 (e.g. Yervoy) Promotes curative responses in checkpoint resistant preclinical cancer models in combination with anti-PD-1 and: Vaccine + QS-21 Adoptive T cell therapy iNKT activating Alpha-GalCer adjuvant therapy Radiation therapy Enhances T cell responsiveness in combination with anti-PD-1 or anti-TIGIT mAbs Combination of AGEN1181 with Agenus's balstilimab (anti-PD-1) is advancing in the clinic Expanding the therapeutic potential of anti-PD-1 and anti-CTLA-4 therapy with innovative Fc engineering and rationale combinations for the treatment of solid tumors. Antoine J. Tanne, Sylvia Vincent, Elena Paltrinieri, Sudesh Pawaria, Vidur Patel, Margaret Wilkens, Shalu Kharkwal, Daniel Levey, David Savitsky, Jennifer Buell, Dhan Chand1 1Agenus Inc. or subsidiary thereof (current or former employee), Lexington, MA
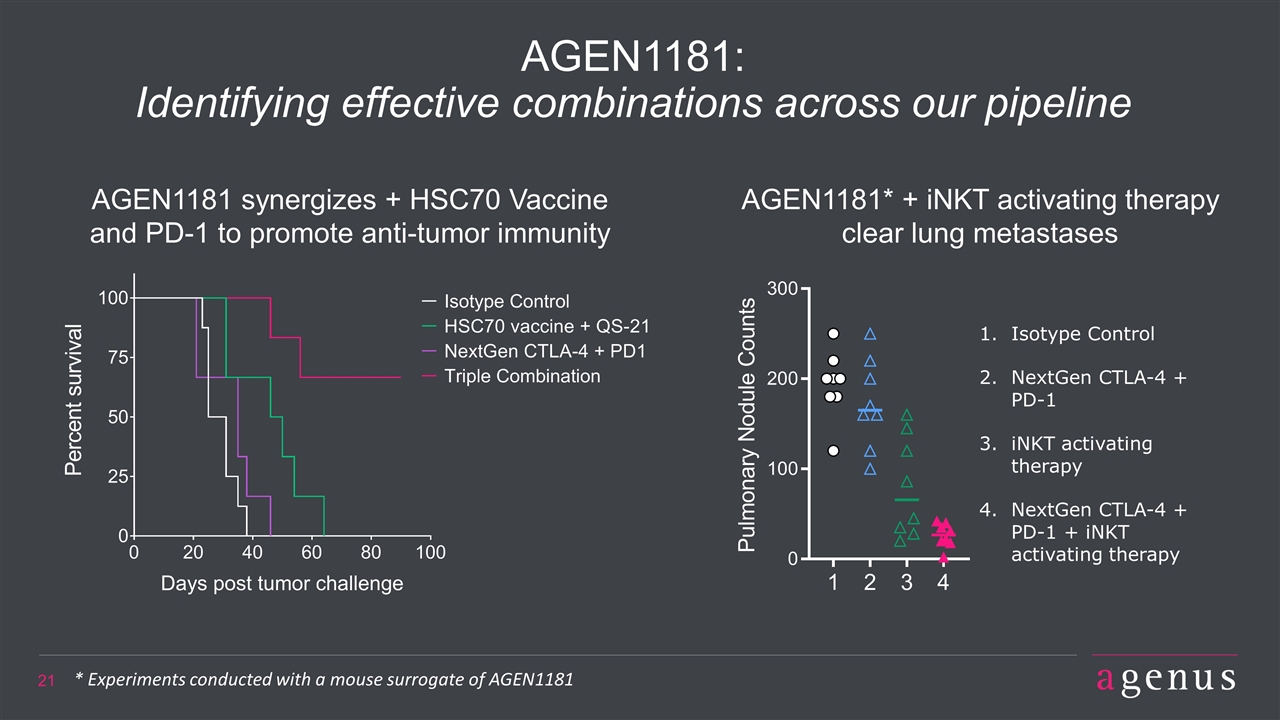
AGEN1181: Identifying effective combinations across our pipeline Isotype Control NextGen CTLA-4 + PD-1 iNKT activating therapy NextGen CTLA-4 + PD-1 + iNKT activating therapy AGEN1181* + iNKT activating therapy clear lung metastases AGEN1181 synergizes + HSC70 Vaccine and PD-1 to promote anti-tumor immunity * Experiments conducted with a mouse surrogate of AGEN1181
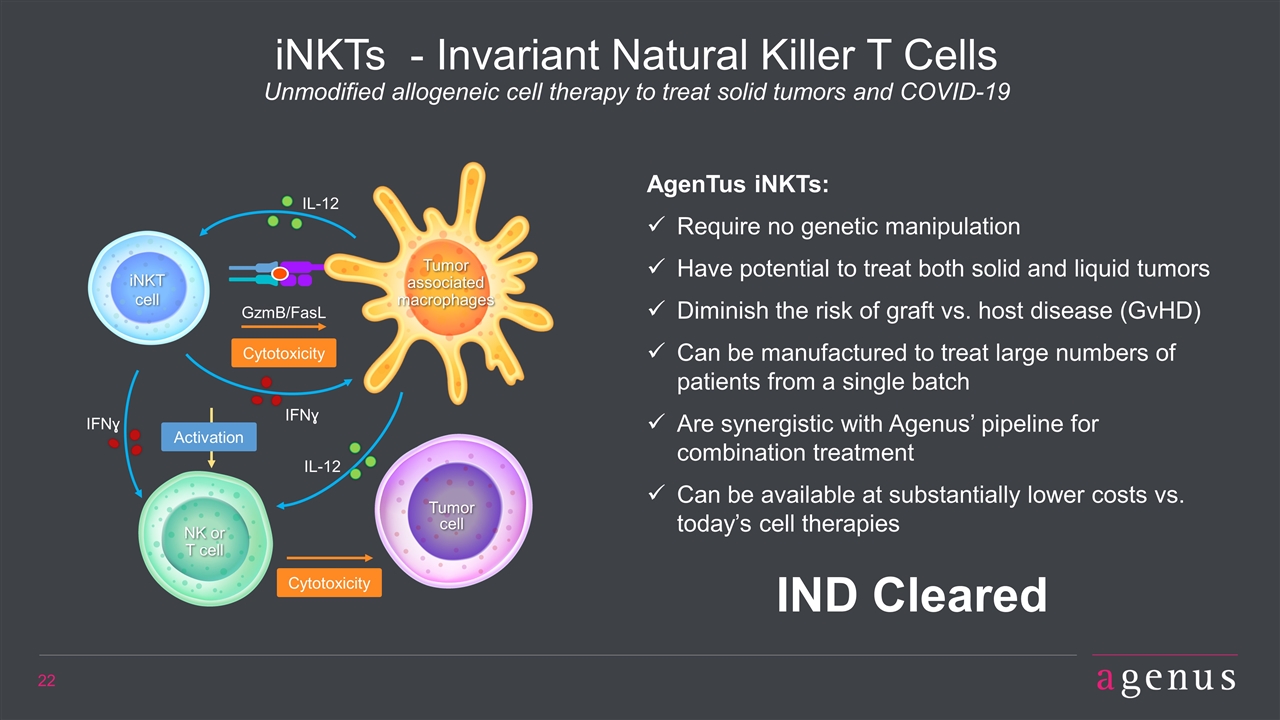
Cytotoxicity GzmB/FasL IFNɣ iNKT cell Tumor cell Cytotoxicity NK or T cell IL-12 IFNɣ Activation iNKTs - Invariant Natural Killer T Cells Unmodified allogeneic cell therapy to treat solid tumors and COVID-19 AgenTus iNKTs: Require no genetic manipulation Have potential to treat both solid and liquid tumors Diminish the risk of graft vs. host disease (GvHD) Can be manufactured to treat large numbers of patients from a single batch Are synergistic with Agenus’ pipeline for combination treatment Can be available at substantially lower costs vs. today’s cell therapies IND Cleared IL-12 Tumor associated macrophages

