Attached files
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☑ ANNUAL REPORT UNDER
SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the fiscal year ended December 31, 2019
------------------------------------------------------
Commission File Number 0-11808
SANARA MEDTECH INC.
(Exact
name of Registrant as specified in its charter)
|
Texas
|
|
59-2219994
|
|
(State or other jurisdiction of incorporation or
organization)
|
|
(I.R.S. Employer Identification No.)
|
|
1200
Summit Ave, Suite 414, Fort Worth, Texas 76102
|
|
(Address
of principal executive offices)
(817)
529-2300
(Registrant’s telephone number, including area
code)
|
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the
Act:
Common
Stock $ .001 par value
Indicate
by check mark if the registrant is a well-known seasoned issuer, as
defined in Rule 405 of the Securities Act. ☐ Yes ☑ No
Indicate
by check mark if the registrant is not required to file reports
pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☑ No
Indicate
by check mark whether the registrant (1) has filed all reports
required to be filed by Section 13 or 15(d) of the Securities
Exchange Act of 1934 during the preceding 12 months (or for such
shorter period that the registrant was required to file such
reports), and (2) has been subject to such filing requirements for
the past 90 days. ☑ Yes ☐ No
Indicate
by check mark whether the registrant has submitted electronically
every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T (§ 232.405 of this
chapter) during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such
files). ☑ Yes ☐ No
Indicate
by check mark whether the registrant is a large accelerated filer,
an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of
“accelerated filer,” “large accelerated
filer” and “smaller reporting company” in
Rule 12b-2 of the Exchange Act.
|
Large
accelerated filer
☐
|
|
Accelerated
filer
☐
|
|
Non-accelerated
filer
☑
|
|
Smaller
reporting company
☑
|
Emerging
growth company
☐
|
If an
emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided
pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act). Yes ☐
No ☑
The
aggregate market value of the voting and non-voting common equity
held by non-affiliates of the registrant as of June 30, 2019 based
on the $5.51 closing price as of such date was approximately
$6,526,022
As of
February 21, 2020, 6,023,732 shares of the Issuer’s $.001 par
value common stock were issued and outstanding.
SANARA MEDTECH INC.
Form 10-K
For the Year Ended December 31, 2019
|
|
|
Page
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LETTER FROM THE
EXECUTIVE CHAIRMAN AND VICE CHAIRMAN
To Our
Shareholders:
2019
was a pivotal year for our Company with continued growth and
substantial progression in the execution of our long-term strategic
plan including our six areas of strategic focus.
2019 Performance
With an
increase in revenues of 34% over 2018, Sanara delivered
double-digit revenue growth for the seventh consecutive year. Total
revenues increased to $11.8 million for 2019 compared to revenues
of $8.8 million for 2018.
To be
poised for future growth, in 2019, we invested in expanding our
sales force and independent distribution network in geographic
areas that hadn’t been covered as well as the related support
infrastructure. In addition, we increased our clinical study to
prove out our product evidence-based healing and value
proposition.
You
will find more detail on all these areas in this
report.
Organization
In
August 2019, we doubled the size of our Board from three directors
to six, including two individuals new to the Company: Kenneth E.
Thorpe, Ph.D. and Ann Beal Salamone, M.S. Both Dr. Thorpe and Ms.
Salamone have extensive health care experience and very
distinguished and successful careers. Dr. Thorpe currently is Chair
of the Department of Health Policy & Management at Emory
University, Atlanta, Georgia and is a frequent national presenter
on issues of health care reform, financing, and insurance. Ms.
Salamone is a member of the National Academy of Engineering and
currently serves as Chairman of the Board of Rochal Industries LLC
based in San Antonio, Texas, and is one of the principal inventors
of Rochal’s many wound care products.
Management Update
In 2019
we implemented one of our strategic plan initiatives to manage our
operations in two distinct divisions: (1) Surgical and (2) Wound
Care. In May, Zachary B. Fleming was appointed to the position of
President, Surgical. Mr. Fleming joined the Company as Vice
President of Sales in November 2017, and successfully led the
surgical team to achieve sales growth of forty percent in 2018 and
thirty-eight percent in 2019. Also, in Mayof 2019, Shawn M. Bowman
was appointed to the position of President, Wound Care. Mr. Bowman
joined the Company as Vice President and General Manager, Wound
Care in September 2018. He has eighteen years of key experience in
the medical device, biologics and pharmaceutical industries. As a
result of their managerial skills and achievements, Mr. Fleming and
Mr. Bowman were appointed Co-Chief Operating Officers in January of
2020 in addition to maintaining their positions as Presidents of
their respective operating divisions.
In
January 2020, the Company engaged Don Stelly to provide operational
expertise in support of the expansion of our wound and skin care
business. Prior to his engagement by Sanara, Mr. Stelly was the
President and Chief Operating Officer of LHC Group, Inc., a
national provider of in-home healthcare and brings a wealth of
experience in the post-acute care setting.
Operations Update
Driving
innovation with new product development and a commercial pipeline
is key to improving patient outcomes. In line with our six focus
areas of wound and skin care which are: debridement, biofilm
management, hydrolyzed collagen, advanced biologics, adjunct
products for Negative Pressure Wound Therapy, and oxygen delivery
systems for the wound bed, the Company introduced the following
products in 2019:
●
PULSAR II™
Advanced Wound Irrigation System (AWI™), a portable, no
touch, painless, hydro-mechanical debridement system that removes
bacteria and necrotic tissue without disrupting healthy
tissue;
●
BIAKŌS™
Antimicrobial Skin & Wound Cleanser, a patented wound
cleansing spray that disrupts extracellular polymeric substances to
eradicate biofilm; and
●
HYCOL™, an
additive free Type I bovine collagen that provides hydrolyzed
collagen fragments to the wound bed that are a fraction of the size
of native collagen.
2020 Events
As we
move forward, the Company will aggressively pursue its mission to
improve patient outcomes through evidence-based healing solutions.
The Company is well positioned to increase its sales of the current
products as a result of its expanded sales force and distribution
capabilities.
In the first quarter of 2020, the Company’s
BIAKŌS™ Antimicrobial Skin & Wound Gel received FDA
clearance. This product, developed by our primary new product
developer, Rochal Industries, is currently going through the
manufacturing and commercialization process and is expected
to launch later this year. We are also in discussions with various
parties to license, acquire, and/or partner in new product
opportunities related to our six focus areas of wound and skin
care.
The
Company continued its record sales in the first two months of 2020,
but, with the unforeseen impact of the COVID-19 virus to the global
economy we expect a downturn in our business primarily due to the
delay in elective surgeries. We are committed to ensuring that our
customers and patients continue to have access to our products
during this difficult time. To manage this situation, we are taking
steps to cut costs, manage cash-flow, and continue to generate
revenue from both our wound care and surgical
businesses.
Sincerely,
Ron
Nixon
Executive
Chairman
J.
Michael Carmena
Vice
Chairman
2
PART I
Item 1. BUSINESS
Background
The
terms “Sanara MedTech,” “we,” “the
Company,” “SMTI,” “our,” and
“us” as used in this report refer to Sanara MedTech
Inc. and its subsidiaries. The Company
was organized on December 14, 2001, as a Texas corporation under
the name eAppliance Innovations, Inc. In June of 2002, MB Software
Corporation, a public corporation formed under the laws of
Colorado, merged with the Company and the Company changed its name
to MB Software Corporation as part of the merger. In May of 2008,
the Company changed its name to Wound Management Technologies,
Inc. In May of 2019, the Company changed its name to Sanara
MedTech Inc.
The
Company’s business is developing, marketing, and distributing
wound and skin care products to physicians, hospitals, clinics and
post-acute care settings. Our products are primarily sold in the
North American advanced wound care and surgical tissue repair
markets. Sanara MedTech products include CellerateRX® Surgical
Activated Collagen® Adjuvant (CellerateRX); HYCOL™
Hydrolyzed Collagen (HYCOL); BIAKŌS™ Antimicrobial Skin
& Wound Cleanser (BIAKŌS AWC); and PULSAR II™
Advanced Wound Irrigation System (AWI™).
The
Company’s exclusive license to sell and distribute
CellerateRX products in the human health care market (excluding
dental and retail) expired on February 27, 2018. The license
permitted the Company to continue to sell and distribute products
through August 27, 2018. Subsequent to the expiration of the
license agreement between the Company and Applied Nutritionals,
LLC, an affiliate of The Catalyst Group, Inc. (Catalyst) acquired
an exclusive license to distribute CellerateRX products in the
United States, Canada and Mexico.
Effective August 28, 2018, the Company consummated definitive
agreements that continued the Company’s operations to market
the its principal products, CellerateRX, through a 50% ownership
interest in a newly formed limited liability company, Cellerate,
LLC. The remaining 50% ownership interest was held by an affiliate
of Catalyst. As part of this transaction, the Company issued a
convertible promissory note to the affiliate of Catalyst which
converted into shares of common stock at an adjusted price of $9.00
per share. Cellerate, LLC conducted operations with an exclusive
sublicense from Catalyst’s affiliate to distribute
CellerateRX products in the United States, Canada and Mexico (the
sublicense was subsequently amended to include worldwide
distribution rights). On March 15, 2019, the Company executed and
closed an agreement with Catalyst to acquire Catalyst’s 50%
equity interest in Cellerate, LLC. After closing the acquisition,
the Company owned 100% of Cellerate, LLC, and as a wholly owned
subsidiary began reporting its financial results on a consolidated
basis beginning March 15, 2019. The Company acquired the remaining
50% equity interest in Cellerate, LLC in exchange for the issuance
of 1,136,815 shares of the Company’s newly created Series F
Convertible Preferred Stock.
The
Cellerate Acquisition was accounted for as a reverse merger and
recapitalization because, immediately following the completion of
the transaction, Catalyst could obtain effective control of the
Company upon exercise of its convertible preferred stock and
promissory note, both of which could occur at Catalyst’s
option. Additionally, Cellerate, LLC’s officers and senior
executive positions continued on as management of the combined
entity after consummation of the Cellerate
Acquisition.
On
February 7, 2020, the affiliate of Catalyst converted its
promissory note into 179,101 shares of common stock and also
converted its Series F Convertible Preferred Stock into 2,273,630
shares of common stock.
The Products
CellerateRX
products are primarily purchased by hospitals and ambulatory
surgical centers for use by surgeons on surgical wounds. HYCOL
products are available through skilled nursing facilities, wound
care clinics and other medical facilities, and are intended for the
management of full and partial thickness wounds including pressure
ulcers, venous and arterial leg ulcers and diabetic foot ulcers.
HYCOL is currently approved for reimbursement under Medicare Part
B. We believe CellerateRX and HYCOL products are unique in
composition, superior to other products in clinical performance,
and demonstrate the ability to reduce costs associated with the
standards of care for their intended uses.
BIAKŌS
AWC is an FDA cleared, patented product that effectively disrupts
extracellular polymeric substances to eradicate biofilm microbes.
BIAKŌS AWC also provides mechanical removal of debris, dirt,
foreign materials, and microorganisms from wounds including stage
I-IV pressure ulcers, diabetic foot ulcers, post-surgical wounds,
first and second-degree burns as well as grafted and donor sites.
BIAKŌS AWC is effective in killing free-floating microbes,
immature, and mature bacterial biofilms and fungal biofilms. In
addition, BIAKŌS AWC safety studies show that it is
non-cytotoxic, non-irritating, and non-sensitizing to healthy skin
and assists in the normal wound healing process. First sales of
BIAKŌS AWC occurred in July 2019
PULSAR
II™ Advanced Wound Irrigation System (AWI™) is a
portable, no touch, painless, selective hydro-mechanical
debridement system that effectively removes bacteria and necrotic
tissue from wounds without disrupting healthy tissue.
3
New Products, Markets and Services
The
Company received notification of FDA clearance for
BIAKŌS™ Antimicrobial Wound Gel in February 2020 and
expects to launch the product in 2020 to complement its
BIAKŌS™ AWC. Both products are effective against
planktonic microbes as well and immature and mature biofilms. When
used together, the cleanser can be used initially to clean a wound
and disrupt biofilms (removing 99% in 10 minutes). The gel can then
be applied and will remain in the wound for up to 72 hours
eliminating biofilms between normal dressing changes.
The
Company also expects to introduce BIAKŌS™ Antimicrobial
Barrier Film (BIAKŌS ABF) in the latter part of 2020.
BIAKŌS ABF is an FDA cleared, first in-class, antimicrobial
spray-on wound dressing that kills microbes while protecting
underlying tissue, helping to remediate damage and prevent further
infection. This product can be used on macerated skin and
wounds.
Marketing, Sales and Distribution
The
Company’s CellerateRX Surgical products are attracting
increased business from hospitals and surgery centers due to their
recognized benefits including efficacy and economic value. The
surgical products are used in specialty areas including total joint
replacement, spine, orthopedic, trauma, vascular, general, plastic
and reconstructive surgeries and podiatry. The surgical products
are sold through a growing network of surgical specialty
distributors and Company representatives who are credentialed to
demonstrate the products in surgical settings.
The
Company’s advanced wound care products are primarily
distributed to the post-acute care settings including long-term
care facilities, home health, wound care centers, and professional
medical offices. Due to the expansion of our distribution network,
the increasing prevalence of diabetic and decubitus (pressure)
ulcers, and the demonstrated efficacy of our products, we believe
demand for our products will grow significantly in 2020. We believe
our products are unique in composition, superior to other products
in clinical performance, and demonstrate the ability to reduce
costs associated with standard wound management. Our wound care
products are sold by Company representatives supplemented by major
medical-surgical distributors, independent distributors, and
durable medical equipment (DME) distributors.
Staffing
As of
March 26, 2020, the Company has a staff of 40, consisting of 38
full-time employees and 2 part time employees.
Competition
The
wound care market is served by a number of large, multi-product
line companies as well as a number of small companies. Our products
compete with primary dressings, advanced wound care products,
collagen matrices and other biopharmaceutical products.
Manufacturers and distributors of competitive products include
Smith & Nephew plc, Acelity L.P. Inc., Medline Industries,
Inc., ACell Inc., and Integra LifeSciences Holdings Corporation.
Many of our competitors are significantly larger than we are and
have greater financial and personnel resources. We believe,
however, that our products outperform our competitors’
currently available products by improving efficacy, reducing the
cost of patient care, and replacing numerous products with a single
primary dressing.
Available Information
The
Company electronically files reports with the Securities and
Exchange Commission (the “SEC”). The SEC maintains an
Internet site (www.sec.gov) that contains reports, proxy and
information statements, and other information regarding issuers
that file electronically with the SEC. Copies of the
Company’s Annual Report on Form 10-K, Quarterly Reports on
Form 10-Q and Current Reports on Form 8-K and amendments to those
reports filed or furnished to the SEC are also available free of
charge through the Company’s website
(http://www.sanaramedtech.com/), as soon as reasonably practicable
after electronically filing with or otherwise furnishing such
information to the SEC, and are available in print to any
shareholder who requests it.
Item 1A. RISK FACTORS
The
following risk factors should be considered with respect to making
any investment in our securities as such an investment involves a
high degree of risk. You should carefully consider the following
risks and the other information set forth elsewhere in this report,
including the financial statements and related notes, before you
decide to purchase shares of our stock. If any of these risks
occur, our business, financial condition and results of operations
could be adversely affected. As a result, the trading price of our
stock could decline, perhaps significantly, and you could lose part
or all of your investment. As used herein, the word
“business” as used in “material adverse effect on
our business”, “adversely affect our business”
and other similar phrases includes any of (or any combination of)
the Company’s present or future operations, financial
performance, margins, revenues, operating margins, stock value,
competitive position, or other indicators of Company
performance.
4
RISKS RELATED TO HOW WE OPERATE OUR BUSINESS:
We had a history of losses in prior years and may not maintain
profitability as we expand our selling efforts.
The
Company has incurred net losses in most years since we began our
current operations in 2004. We plan to continue making significant
investments in our sales and clinical programs which substantially
increase our operating expenses. Consequently, we will need to
continue our revenue growth to become profitable in the future. We
cannot offer any assurance that we will be able to generate future
sales growth. If we fail to achieve profitability, our stock price
may decline and you may lose part or all of your
investment.
Our revenue growth for a particular period is difficult to predict,
and a shortfall in forecast revenues may harm our operating
results.
Because
we are a relatively small company, our revenue growth and
consequently results of operations are difficult to predict. We
plan our operating expense levels based primarily on forecasted
revenue levels. A shortfall in revenue could lead to operating
results being below expectations as we may not be able to quickly
reduce our fixed expenses in response to short-term revenue
shortfalls. We have experienced fluctuations in revenue and
operating results from quarter to quarter and anticipate that these
fluctuations will continue until we achieve a critical mass with
our product sales. These fluctuations can result from a variety of
factors, including:
●
the
uncertainty surrounding our ability to attract new customers and
retain existing customers;
●
the
length and variability of our sales cycle, which makes it difficult
to forecast the quarter in which our sales will occur;
●
issues
in order fulfillment for our products;
●
the
timing of operating expense relating to the expansion of our
business and operations;
●
the
development of new wound care products or product enhancements by
our competitors;
●
actual
events, circumstances, outcomes and amounts differing from
assumptions and estimates used in preparing our operating plan and
how well we execute our strategy and operating plans.
As a
consequence, operating results for a particular future period are
difficult to predict and prior results are not necessarily
indicative of future results. Any of the foregoing factors, or any
other factors discussed elsewhere herein, could have a material
adverse effect on our business.
If our products do not gain market acceptance, we might not be able
to fund future operations.
Several
factors may affect the market acceptance of our products or any
other products we develop or acquire. These include, but are not
limited to:
●
the
price of our products relative to other products for the same
indications;
●
the
perception by physicians and other members of the healthcare
community of the efficacy and safety of our products for their
indicated applications and treatments;
●
changes
in practice guidelines and the standard of care for the targeted
indication
●
the
effectiveness of our sales and marketing efforts or that of our
independent sales distributors.
Our
ability to effectively promote and sell any approved products may
also depend on pricing and cost-effectiveness, including our
ability to produce a product at a competitive price and our ability
to obtain sufficient third-party coverage or reimbursement, if any.
In addition, our efforts to educate the medical community on the
benefits of our products may require significant resources, may be
constrained by FDA rules and policies on product promotion, and may
never be successful. If our products do not gain market acceptance,
we may not be able to fund future operations, including developing,
testing and obtaining regulatory approval for new product
candidates and expanding our sales and marketing efforts for our
approved products, which would cause our business to
suffer.
5
Disruption of, or changes in, our distribution model or customer
base could harm our sales and margins.
If we
fail to manage the distribution of our products properly, or if the
financial condition or operations of our reseller channels weakens,
there may be a material adverse effect on our business.
Furthermore, a change in the mix of our customers between service
provider and enterprise, or a change in the mix of direct and
indirect sales, could adversely affect our business.
Several
factors could also result in disruption of or changes in our
distribution model or customer base, which could harm our sales and
margins, including the following:
●
in some
instances, we compete with some of our resellers through our direct
sales, which may lead these channel partners to use other suppliers
that do not compete; and
●
some of
our resellers may have insufficient financial resources and may not
be able to withstand changes in business conditions.
If we cannot meet our future capital requirements, our business
will suffer.
We have
a history of operating losses and negative cash flow from operating
activities with the exception of 2016 and 2018. As such, we have
utilized funds from offerings of our securities to fund our
operations. Future results of operations involve significant risks
and uncertainties. Factors that could affect our future operating
results and cause actual results to vary materially from
expectations include, but are not limited to, potential demand for
our products, risks from competitors, regulatory approval of our
new products, technological change, and dependence on key
personnel. Although we have taken steps to improve our overall
liquidity, if our cash flow is insufficient, we may be forced
either to secure additional amounts to our bank line of credit or
seek additional equity financing in order to:
●
fund
operating losses;
●
increase
marketing to address the market for wound care and surgical
products;
●
take
advantage of opportunities, including more rapid expansion or
acquisitions of complementary products or businesses;
●
hire,
train and retain employees;
●
develop
and/or distribute new products; and/or
●
respond
to economic and competitive pressures.
If our
capital needs are met through the issuance of equity or convertible
debt securities, the percentage ownership of our current
shareholders may be reduced which may have a negative impact on the
market price of our common stock. Our future success may be
determined in large part by our ability to obtain additional
financing, and the incurrence of indebtedness would result in
increased debt service obligations which could result in operating
and financing covenants that would restrict our operations. There
can be no assurance that such financing would be available or, if
available, that such financing could be obtained upon terms
acceptable to us. If adequate funds are not available, or are not
available on acceptable terms, our operating results and financial
condition may suffer.
Failure to retain and recruit key personnel would harm our ability
to meet key objectives.
Our
success depends, in large part, on our ability to attract and
retain skilled executive, managerial, sales and marketing
personnel. There can be no assurance that we will be able to find
and attract additional qualified employees or retain any such
executive officers and other key personnel. The inability to hire
qualified personnel or the loss of services of our executive
officers or key personnel may have a material adverse effect on our
business.
Failure to manage our planned growth could harm our
business.
Our
ability to successfully market and sell our wound care products and
implement our business plan requires an effective plan for managing
our future growth. We plan to increase the scope of our operations
at a rapid rate. Future expansion efforts will be expensive and may
strain our internal operating resources. To manage future growth
effectively, we must maintain and enhance our financial and
accounting systems and controls, integrate new personnel and manage
expanded operations. If we do not manage growth properly, it could
harm our operating results and financial condition.
6
We operate in a highly competitive industry and face competition
from large, well-established medical device manufacturers as well
as new market entrants.
Competition
from other medical device companies is significant and could be
significantly affected by new product introductions and other
market activities of industry participants. We compete with other
companies in acquiring rights to products or technologies from
third party developers. Although our products have performed well
in customer evaluations, we are a relatively unknown brand in a
market controlled, in large part, by companies with a large
customer base. We may not, even with strong customer accounts, be
able to establish the credibility necessary to secure large
national customers.
Several
factors may limit the market acceptance of our products, including
the timing of regulatory approvals and market entry relative to
competitive products, the availability of alternative products, and
the price of our products relative to alternative products, the
availability of third-party reimbursement and the extent of
marketing efforts by third party distributors or agents that we
retain. There can be no assurance that our products will receive
market acceptance in a commercially viable period of time, if at
all. Furthermore, there can be no assurance that we can develop
products that are more effective or achieve greater market
acceptance than competitive products, or that our competitors will
not succeed in developing or acquiring products and technologies
that are more effective than those being developed by us, that
would render our products and technologies less competitive or
obsolete.
Our
competitors enjoy several competitive advantages over us, including
but not limited to:
●
large
and established distribution networks in the U.S. and/or in
international markets;
●
greater
financial, managerial and other resources for products research and
development, sales and marketing efforts and protecting and
enforcing intellectual property rights;
●
greater
name recognition;
●
larger
consumer base;
●
more
expansive portfolios of intellectual property rights;
●
greater
experience in obtaining and maintaining regulatory approvals and/or
clearances from the FDA and other regulatory agencies.
The
presence of competition in our market may lead to pricing pressure
which would make it more difficult to sell our products at a
profitable price or may prevent us from selling our products at
all. Our failure to compete effectively would have a material
adverse effect on our business.
Security breaches and other disruptions could compromise our
information and expose us to liability, which would cause our
business and reputation to suffer.
In the
ordinary course of our business, we use networks to collect and
store sensitive data, including intellectual property, proprietary
business information and important information of our customers,
suppliers and business partners, as well as personally identifiable
information of our customers and employees. The secure processing,
maintenance and transmission of this information is critical to our
operations. Despite our security measures, our information
technology and infrastructure may be vulnerable to attacks by
hackers or breached due to employee error, malfeasance or other
disruptions. Any such breach could compromise our networks and the
information stored there could be accessed, publicly disclosed,
lost or stolen. Any such access, disclosure or other loss of
information could result in legal claims or proceedings, liability
under laws that protect the privacy of personal information, and
regulatory penalties. Further, such access, disclosure or loss may
cause disruption of our operations and the services we provide to
customers, damage to our reputation, and cause a loss of confidence
in our products and services, which could adversely affect our
business.
We may not be able to maintain sufficient product liability
insurance to cover claims against
us.
Product
liability insurance for the healthcare industry is generally
expensive to the extent it is available at all. We may not be able
to maintain such insurance on acceptable terms or be able to secure
increased coverage as commercialization of our products progresses,
nor can we be sure that existing or future claims against us will
be covered by our product liability insurance. Moreover, the
existing coverage of our insurance policy or any rights of
indemnification and contribution that we may have may not be
sufficient to offset existing or future claims. A successful claim
against us with respect to uninsured liabilities or in excess of
insurance coverage and not subject to any indemnification or
contribution could have a material adverse effect on our
business.
7
RISKS RELATED TO OUR PRODUCTS:
Competitors could invent products superior to ours and cause our
products and technologies to become obsolete.
The
wound care sector of the medical products industry is characterized
by a multitude of technologies and intense competition. Our
competitors currently manufacture and distribute a variety of
products that are, in many respects, comparable to our products.
Many suppliers of competing products are considerably larger and
have much greater resources than we have. In addition, many
specialized products companies have formed collaborations with
large, established companies to support research, development and
commercialization of wound care products which may be competitive
with ours. Academic institutions, government agencies and other
public and private research organizations are also conducting
research activities and may commercialize wound care products on
their own or through joint ventures. It is possible that these
competitors may develop technologies and products that are more
effective than any we currently have. If this occurs, any of our
products and technology affected by these developments could become
obsolete.
We may have exposure to product liability claims.
Although
we have contractual indemnity from the manufacturer of CellerateRX
for certain liability claims related to its production, we could
face a product liability claim outside of the scope of the
contractual indemnity. We do not have, and do not anticipate
obtaining, contractual indemnification from parties supplying raw
materials or parties marketing the products we sell. In any event,
indemnification from the manufacturer of CellerateRX or from any
other party is limited by the terms of the indemnity and by the
creditworthiness of the indemnifying party. A successful product
liability claim or series of claims brought against us could result
in judgments, fines, damages and liabilities that could have a
material adverse effect on our business. We may incur significant
expense investigating and defending these claims, even if they do
not result in liability. Moreover, even if no judgments, fines,
damages or liabilities are imposed on us, our reputation could
suffer, which could have a material adverse effect on our business.
In the event that we do not have adequate insurance or contractual
indemnification, product liability claims relating to defective
products could have a material adverse effect on our
business.
RISKS RELATED TO INTELLECTUAL PROPERTY:
If we are unable to protect our intellectual property rights
adequately, we may not be able to compete effectively.
Part of our success depends on our ability to protect proprietary
rights to technologies used in certain of our products. We rely on
patents, copyrights, trademarks and trade secret laws to establish
and maintain proprietary rights in our technology and products.
However, these legal means afford only limited protection and may
not adequately protect our rights or permit us to gain or keep a
competitive advantage. Patents and patent applications for the
products we have may not be broad enough to prevent competitors
from introducing similar products into the market. Our patents or
our attempts to enforce them may not necessarily be upheld by the
courts. Efforts to enforce any of our proprietary rights could be
time-consuming and expensive, which could adversely affect our
business and prospects and divert management’s
attention. There can be no assurance that our proprietary
rights will not be challenged, invalidated or circumvented or that
the rights will in fact provide competitive advantages to
us.
We may be found to infringe on intellectual property rights of
others.
Third
parties, including customers, may in the future assert claims or
initiate litigation related to exclusive patent, copyright,
trademark and other intellectual property rights to technologies
and related standards that are relevant to us. These assertions may
emerge over time as a result of our growth and the general increase
in the pace of patent claim assertions, particularly in the U.S.
Because of the existence of a large number of patents in the
healthcare field, the secrecy of some pending patents and the rapid
rate of issuance of new patents, it is not economically practical
or even possible to determine in advance whether a product or any
of its components infringes or will infringe the patent rights of
others. The asserted claims or initiated litigation can include
claims against us or our manufacturers, suppliers or customers
alleging infringement of their proprietary rights with respect to
our existing or future products or components of those products.
Regardless of the merit of these claims, they can be
time-consuming, result in costly litigation and diversion of
technical and management personnel, or require us to develop a
non-infringing technology or enter into license agreements. Where
claims are made by customers, resistance even to unmeritorious
claims could damage customer relationships. There can be no
assurance that licenses will be available on acceptable terms and
conditions, if at all, or that our indemnification by our suppliers
will be adequate to cover our costs if a claim were brought
directly against us or our customers. Furthermore, because of the
potential for high court awards that are not necessarily
predictable, it is not unusual to find even arguably unmeritorious
claims settled for significant amounts. If any infringement or
other intellectual property claim made against us by any third
party is successful, or if we fail to develop non-infringing
technology or license the proprietary rights on commercially
reasonable terms and conditions, our business could be materially
and adversely affected.
8
RISKS RELATED TO REGULATIONS:
Our business is affected by numerous regulations.
Government
regulation by the U.S. FDA and similar agencies in other countries
is a significant factor in the development, manufacturing and
marketing of our products and in the acquisition or licensing of
new products. Complying with government regulations is often time
consuming and expensive and may involve delays or actions adversely
impacting the marketing and sale of our current or future
products.
Following
initial regulatory approval of any products that we may develop, we
will be subject to continuing regulatory review, including review
of adverse (drug or device) experiences or reactions and clinical
results that are reported after our products become commercially
available. This would include results from any post-marketing tests
or continued actions required as a condition of approval. The
manufacturing facilities we use (and may use) to make any of our
products may become subject to periodic review and inspection by
the FDA. If a previously unknown problem with a product or a
manufacturing and laboratory facility used or contracted by us is
discovered, the FDA may impose restrictions on that product or on
the manufacturing facility, including requiring us to withdraw the
product from the market. Any changes to an approved product,
including the way it is manufactured or promoted, often requires
FDA approval before the product, as modified, can be marketed. In
addition, for products we develop in the future, we and our
contract manufacturers may be subject to ongoing FDA requirements
for submission of safety and other post-market information. If we
violate regulatory requirements at any stage, whether before or
after marketing approval is obtained, we may be fined, be forced to
remove a product from the market or experience other adverse
consequences, which would materially harm our financial results.
Additionally, we may not be able to obtain the labeling claims
necessary or desirable for product promotion.
Further,
various healthcare reform proposals have emerged at the federal and
state levels. We cannot predict whether foreign, federal, state or
local healthcare reform legislation or regulation affecting our
business may be proposed or enacted in the future, or what effect
any such legislation or regulation would have on our business. The
implementation of new legislation and regulation may lower
reimbursements for our products or reduce medical procedure
volumes, which would likely adversely affect our business. In
addition, the enacted excise tax may materially and adversely
affect our business.
Distribution
of our products outside the U.S. is subject to extensive government
regulation. These regulations, including the requirements for
approvals or clearance to market; the time required for regulatory
review and the sanctions imposed for violations, vary from country
to country. We do not know whether we will obtain regulatory
approvals in such countries or that we will not be required to
incur significant costs in obtaining or maintaining these
regulatory approvals.
If we fail to obtain or experience significant delays in obtaining
regulatory clearances or approvals to market future medical device
products, we will be unable to commercialize these products until
such clearance or approval is obtained.
The
developing, testing, manufacturing, marketing and selling of
medical devices is subject to extensive regulation by governmental
authorities in the U.S. and other countries. The process of
obtaining regulatory clearance and approval of certain medical
technology products is costly and time consuming. Inherent in the
development of new medical products is the potential for delay
because product testing, including clinical evaluation, is required
before many products can be approved for human use. With respect to
medical devices, such as those that we manufacture and market,
before a new medical device, or a new use of, or claim for, an
existing product can be marketed (unless it is a Class I device),
it must first receive either premarket clearance under Section
510(k) of the Federal Food, Drug and Cosmetic Act or approval of a
premarket approval application, or PMA, from the FDA, unless an
exemption applies. In the 510(k)-clearance process, the FDA must
determine that the proposed device is “substantially
equivalent” to a device legally on the market, known as a
“predicate” device, with respect to intended use,
technology and safety and effectiveness to clear the proposed
device for marketing. Clinical data is sometimes required to
support substantial equivalence. The PMA approval pathway requires
an applicant to demonstrate the safety and effectiveness of the
device for its intended use based, in part, on extensive data
including, but not limited to, technical, preclinical, clinical
trial, manufacturing and labeling data. The premarket approval
process is typically required for devices that are deemed to pose
the greatest risk, such as life-sustaining, life-supporting or
implantable devices. Both the 510(k) and premarket approval
processes can be expensive and lengthy and entail significant user
fees.
Failure
to comply with applicable regulatory requirements can result in,
among other things, suspension or withdrawal of approvals or
clearances, seizure or recall of products, injunctions against the
manufacture, holding, distribution, marketing and sale of a product
and civil and criminal sanctions. Furthermore, changes in existing
regulations or the adoption of new regulations could prevent us
from obtaining, or affect the timing of, future regulatory
approvals. Meeting regulatory requirements and evolving government
standards may delay marketing of our new products for a
considerable period of time, impose costly procedures upon our
activities and result in a competitive advantage to larger
companies that compete against us.
9
We
cannot assure you that the FDA or other regulatory agencies will
clear or approve any products developed by us on a timely basis, if
at all, or, if granted, that clearance or approval will not entail
limiting the indicated uses for which we may market the product,
which could limit the potential market for any of these
products.
Changes to the FDA clearance and approval processes or ongoing
regulatory requirements could make it more difficult for us to
obtain FDA clearance or, approval of new products or comply with
ongoing requirements.
New
government regulations may be enacted and changes in FDA policies
and regulations and, their interpretation and enforcement, could
prevent or delay regulatory clearance or approval of new products.
We cannot predict the likelihood, nature or extent of adverse
government regulation that may arise from future legislation or
administrative action, either in the U.S. or abroad. Therefore, we
do not know whether we will be able to continue to comply with such
regulations or whether the costs of such compliance will have a
material adverse effect on our business. Changes could, among other
things, require different labeling, monitoring of patients,
interaction with physicians, education programs for patients or
physicians, curtailment of necessary supplies, or limitations on
product distribution. These changes, or others required by the FDA
could have an adverse effect on our business, and specifically, on
the sales of these products. The evolving and complex nature of
regulatory science and regulatory requirements, the broad authority
and discretion of the FDA and the generally high level of
regulatory oversight results in a continuing possibility that from
time to time, we will be adversely affected by regulatory actions
despite ongoing efforts and commitment to achieve and maintain full
compliance with all regulatory requirements. If we are not able to
maintain regulatory compliance, we will not be permitted to market
our products and our business would suffer.
Modifications to our current products may require new marketing
clearances or approvals or require us to cease marketing or recall
the modified products until such clearances or approvals are
obtained.
Any
modification to an FDA-cleared product that could significantly
affect its safety or efficacy, or that would constitute a major
change or modification in its intended use, requires a new FDA
510(k) clearance or, possibly, a premarket approval (PMA). The FDA
requires every manufacturer to make its own determination as to
whether a modification requires a new 510(k) clearance or PMA, but
the FDA may review and disagree with any decision reached by the
manufacturer. In the future, we may make additional modifications
to our products after they have received FDA clearance or approval
and, in appropriate circumstances, determine that new clearance or
approval is unnecessary. Regulatory authorities may disagree with
our decisions not to seek new clearance or approval and may require
us to obtain clearance or approval for previous modifications to
our products. If that were to occur for a previously cleared or
approved product, we may be required to cease marketing or recall
the modified device until we obtain the necessary clearance or
approval. Under these circumstances, we may also be subject to
significant regulatory fines or other penalties. If any of the
foregoing were to occur, our financial condition and results of
operations could be negatively impacted.
Changes in reimbursement policies and regulations by governmental
or other third-party payers may have an adverse impact on the use
of our products.
A
significant portion of our wound care products are purchased
principally for the Medicare and Medicaid eligible population by
hospital outpatient clinics, wound care clinics, durable medical
equipment (DME) suppliers and skilled nursing facilities (SNFs),
which typically bill various third-party payers, primarily state
and federal healthcare programs (e.g., Medicare and Medicaid), and
managed care plans, for the products and services provided to their
patients. Although our wound care products are currently eligible
for reimbursement under Medicare Part B, adjustments to our
reimbursement amounts or a change in Centers for Medicare &
Medicaid Services’ (CMS) reimbursement policies could have an
adverse effect on our market opportunities in this area. The
ability of our customers to obtain appropriate reimbursement for
products and services from third-party payers is critical to the
success of our business because reimbursement status affects which
products our customers purchase and the prices they are willing to
pay. In addition, our ability to obtain reimbursement approval in
foreign jurisdictions may affect our ability to expand our product
offerings internationally.
Third-party
payers have adopted, and are continuing to adopt, a number of
policies intended to curb rising healthcare costs. These policies
include the imposition of conditions of payment by foreign, state
and federal healthcare programs as well as private insurance plans,
and the reduction in reimbursement amounts applicable to specific
products and services.
Changes
in healthcare systems in the U.S. or internationally in a manner
that significantly reduces reimbursement for procedures using our
products or denies coverage for these procedures would also have an
adverse impact on the acceptance of our products and the prices
which our customers are willing to pay for them.
10
We and our sales personnel, whether employed by us or by others,
must comply with various federal and state anti-kickback,
self-referral, false claims and similar laws, any breach of which
could cause a material adverse effect on our business.
Our
relationships with physicians, hospitals and the marketers of our
products are subject to scrutiny under various federal
anti-kickback, self-referral, false claims and similar laws, often
referred to collectively as healthcare fraud and abuse laws.
Healthcare fraud and abuse laws are complex, and even minor,
inadvertent violations can give rise to liability, or claims of
alleged violations. Possible sanctions for violation of these fraud
and abuse laws include monetary fines, civil and criminal
penalties, exclusion from federal healthcare programs, including
Medicare, Medicaid, the Veterans Administration, Department of
Defense, Public Health Service (PHS), and forfeiture of amounts
collected in violation of such prohibitions could occur. Certain
states have similar fraud and abuse laws that also authorize
substantial civil and criminal penalties for violations. Any
government investigation or a finding of a violation of these laws
may result in an adverse effect on our business. The federal
Anti-Kickback Statute prohibits any knowing and willful offer,
payment, solicitation or receipt of any form of remuneration in
return for the referral of an individual or the ordering or
recommending of the use of a product or service for which payment
may be made by any federal healthcare program, including
Medicare.
The
scope and enforcement of the healthcare fraud and abuse laws is
uncertain and is subject to rapid change. There can be no assurance
that federal or state regulatory or enforcement agencies will not
investigate or challenge our current or future activities under
these laws. Any state or federal investigation, regardless of the
outcome, could be costly and time-consuming. Additionally, we
cannot predict the impact of any changes in these laws, whether
these changes are retroactive or will have effect on a
going-forward basis only.
If we
engage additional physicians on a consulting basis, the agreements
with these physicians will be structured to comply with all
applicable laws, including the federal ban on physician
self-referrals (commonly known as the “Stark Law”) the
federal Anti-Kickback Statute, state anti-self-referral and
anti-kickback laws. Even so, it is possible that regulatory or
enforcement agencies or courts may view these agreements as
prohibited arrangements that must be restructured or for which we
would be subject to other significant civil or criminal penalties.
Because our strategy includes the involvement of physicians who
consult with us on the design of our products, we could be
materially impacted if regulatory or enforcement agencies or courts
interpret our financial relationships with our physician advisors
who refer or order our products to be in violation of one or more
health care fraud and abuse laws. Such government action could harm
our reputation and the reputations of our physician advisors. In
addition, the cost of noncompliance with these laws could be
substantial because we could be subject to monetary fines and civil
or criminal penalties, and we could also be excluded from state and
federal healthcare programs, including Medicare and Medicaid, for
non-compliance.
RISKS RELATED TO OUR GOVERNING DOCUMENTS OR OUR COMMON
STOCK:
The trading price of the shares of our common stock is highly
volatile, and purchasers of our common stock could incur
substantial losses.
The
market price of our common stock has been and is likely to continue
to be highly volatile and could fluctuate widely in response to
various factors, many of which are beyond our control, including
the following:
●
technological
innovations or new products and services by us or by our
competitors;
●
additions
or departures of key personnel;
●
sales
of our common stock;
●
our
ability to execute our business plan;
●
loss of
any strategic relationship;
●
industry
developments;
●
fluctuations
in stock market prices and trading volumes of similar
companies;
●
economic,
political and other external factors;
●
period-to-period
fluctuations in our financial results;
●
regulatory
developments in the U.S. and foreign countries, both generally or
specific to us and our products; and
●
intellectual
property, product liability or other litigation against us;
and
●
Relatively
low trading volume
Although
publicly traded securities are subject to price and volume
fluctuations, it is likely that our common stock will experience
these fluctuations to a greater degree than the securities of more
established and better capitalized organizations.
11
Our common stock does not have a vigorous trading market and you
may not be able to sell your securities when desired.
Although
there is a public market for our common stock, trading volume has
been historically low, which could impact the stock price and the
ability to sell shares of our common stock. We can give no
assurance that a more active and liquid public market for the
shares of our common stock will develop in the future.
The potential sale of large amounts of common stock may have a
negative effect upon the market value of our shares.
Sales
of a significant number of shares of our common stock in the public
market could harm the market price of our common stock and make it
more difficult for us to raise funds through future offerings of
common stock. As additional shares of our common stock become
available for resale in the public market, the supply of our common
stock will increase, which could decrease the price of our common
stock.
In
addition, future sales of large amounts of common stock could
adversely affect or inhibit our ability to raise
capital.
We have not paid, and we are unlikely to pay cash dividends on our
securities in the near future.
We have
not paid and do not currently intend to pay dividends on our common
stock, which may limit the current return available on an
investment in our stock. Future dividends on our stock, if any,
will depend on our future earnings, capital requirements, financial
condition and such other factors as our management personnel may
consider relevant. Currently, we intend to retain earnings, if any,
to increase our net worth and reserves.
A few of our existing shareholders own a large percentage of our
voting stock and have control over matters requiring stockholder
approval and may delay or prevent a change in control.
Our
directors own and through their affiliates control a large
percentage of our common stock (See “Item 12. Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters”). As a result, our directors and their
affiliates could have the ability to exert substantial influence
over all matters requiring approval by our shareholders, including
the election and removal of directors and any proposed merger,
consolidation or sale of all or substantially all of our assets as
well as other corporate transactions. This concentration of control
could be disadvantageous to other shareholders having different
interests. This significant concentration of share ownership may
adversely affect the trading price for our common stock because
investors sometimes perceive disadvantages in owning stock in
companies with controlling stockholders. In addition, our
Certificate of Formation contains a provision which under the Texas
Business Organizations Code could allow the large shareholders who
own a majority of the common stock to approve certain major
transactions without the approval of other shareholders that
otherwise would be required under Texas corporation
law.
Our Board can authorize the issuance of preferred stock, which
could diminish the rights of holders of our common stock and make a
change of control of the Company more difficult even if it might
benefit our shareholders.
The
Board is authorized to issue shares of preferred stock in one or
more series and to fix the voting powers, preferences and other
rights and limitations of the preferred stock. Accordingly, we may
issue shares of preferred stock with a preference over our common
stock with respect to dividends or distributions on liquidation or
dissolution, or that may otherwise adversely affect the voting or
other rights of the holders of common stock. Issuances of preferred
stock, depending upon the rights, preferences and designations of
the preferred stock, may have the effect of delaying, deterring or
preventing a change of control, even if that change of control
might benefit our shareholders.
FORWARD-LOOKING STATEMENTS:
When
used in this Form 10-K or other filings by the Company with the
Securities and Exchange Commission, in the Company’s press
releases or other public or shareholder communications, or in oral
statements made with the approval of an authorized officer of the
Company’s executive officers, the words or phrases
“would be”, “will allow”, “intends
to”, “will likely result”, “are expected
to”, “will continue”, “is
anticipated”, “estimate”, “project”,
“plan”, “believe” or similar expressions
are intended to identify “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act
of 1995.
The
Company cautions readers not to place undue reliance on any
forward-looking statements, which speak only as of the date made,
and advises readers that forward-looking statements involve various
risks and uncertainties. Our management believes its assumptions
are based upon reasonable data derived from and known about our
business and operations. No assurances are made that our actual
results of operations or the results of our future activities will
not differ materially from these assumptions. The Company does not
undertake, and specifically disclaims any obligation, to update any
forward-looking statements to reflect occurrences or unanticipated
events or circumstances after the date of such
statement.
12
ITEM 1B. UNRESOLVED STAFF
COMMENTS
None.
ITEM 2. PROPERTIES
The
Company periodically enters into operating lease contracts for
office space and equipment. Arrangements are evaluated at inception
to determine whether such arrangements constitute a lease. In
accordance with the transition guidance of Accounting Standards
Codification (“ASC”) Topic No. 842, such arrangements
are included in our balance sheet as of January 1,
2019.
Right
of use assets, which we refer to as “ROU assets,”
represent the right to use an underlying asset for the lease term,
and lease
liabilities represent the obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities were
recognized at the transition date based on the present value of
lease payments over the respective lease terms, with the office
space ROU asset adjusted for deferred rent
liability.
The Company has two operating leases: an office space lease with a
remaining lease term of 54 months and a copier lease with a remaining lease
term of 19 months as of
December 31, 2019. In accordance with
the transition guidance of ASC 842, such arrangements are included
in our balance sheet as of January 1, 2019. All other leases are
short-term leases for which practical expediency has been elected
to not recognize lease assets and lease
liabilities.
In
March 2017, and as amended in March 2018, the Company executed a
new office lease effective April 1, 2019 for office space located
at 1200 Summit Ave., Suite 414, Fort Worth, TX 76102. On July 1,
2019, the Company amended its office lease agreement related to its
current office space located at 1200 Summit Ave., Suite 414, Fort
Worth, TX 76102. The amended lease became effective on August 22,
2019 upon completion by landlord of certain leasehold improvements.
Under the terms of the amended lease agreement, the Company leased
an
additional 1,682 rentable square feet of office space which brought
the total square footage leased to 5,877. The amended lease
agreement extends the original term of the lease for a period of 36
months through June 30, 2024. The monthly base rental payments are
as follows:
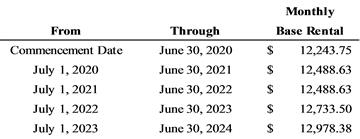
As the implicit rate in the leases is not determinable, the
discount rate applied to determine the present value of lease
payments is the borrowing rate on our line of credit. The office
space lease agreement contains no renewal terms, so no lease
liability is recorded beyond the termination date. The copier lease
can be automatically renewed but no lease liability is recorded
beyond the initial termination date as exercising this option is
not reasonably certain.
In
accordance with ASC 842, the Company has recorded lease assets of
$585,251 and a related lease liability of $598,917 as of December
31, 2019. Cash paid for amounts included in measurement of
operating lease liabilities as of December 31, 2019 was
$95,530 The present value
of our operating lease liabilities is shown below.
Maturity of Operating Lease Liabilities
|
|
December 31,
2019
|
|
2020
|
$150,887
|
|
2021
|
151,317
|
|
2022
|
151,333
|
|
2023
|
154,271
|
|
2024
|
77,870
|
|
Total lease
payments
|
685,678
|
|
Less imputed
interest
|
(86,761)
|
|
Present value of
lease liabilities
|
$598,917
|
As of
December 31, 2019, our operating leases have a weighted average
remaining lease term of 4.5 years and a weighted average discount
rate of 6.25%.
ITEM 3. LEGAL PROCEEDINGS
As of
December 31, 2019, and as of this filing date, the Company has no
outstanding legal proceedings.
ITEM 4. MINE SAFETY
DISCLOSURES
This
item is not applicable.
13
PART II
ITEM 5. MARKET FOR REGISTRANT’S
COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF
EQUITY SECURITIES
The
Company’s common stock is traded on OTCQB market of the OTC
Markets Group under the trading symbol “SMTI.” OTCQB is
one of three tiers established by OTC Markets Group, Inc.
Reverse Stock
Split and Reduction of Authorized Capital Stock
On
May 10, 2019, the Company completed a recapitalization of the
Company which included:
(1) a
1-for-100 reverse stock split of the outstanding Common Stock under
which every shareholder received one share of Common Stock for
every 100 shares of Common Stock held;
(2) the
reduction of the authorized capital stock of the Company to
20,000,000 shares of Common Stock and 2,000,000 shares of preferred
stock; and
(3) the
change of the name of the Company from “Wound Management
Technologies, Inc.” to “Sanara MedTech
Inc.”
In
addition, on June 6, 2019 the Company changed the trading symbol of
its Common Stock to “SMTI”. The post-split Common Stock
is traded under the CUSIP number 79957L100. In connection with the
reverse stock split, the Company adjusted the conversion and voting
provisions of the Series F Convertible Preferred Stock by a factor
of 100 and proportionately adjusted the Company's outstanding
employee stock options.
Record Holders
As of
February 21, 2020, there were 207 shareholders of record and there
were 6,023,732 shares of common stock issued and
outstanding.
The
holders of the common stock are entitled to one vote for each share
held of record on all matters submitted to a vote of shareholders.
Holders of the common stock have no preemptive rights and no right
to convert their common stock into any other securities. There is
no redemption or sinking fund provisions applicable to the common
stock.
Dividends
We have
never declared or paid any cash dividends on our common stock and
we do not intend to pay cash dividends in the foreseeable future.
We currently expect to retain any future earnings to fund our
operations and the expansion of our business.
Recent Sales of Unregistered Securities
Set
forth below is information regarding the issuance and sales of the
Company’s securities without registration for the years ended
December 31, 2018, and 2019:
On
March 6, 2018, the Company issued 226,514 shares of Common Stock
for the conversion of $1,200,000 in convertible debt held by
related parties and $385,594 in accrued interest. In February and
March 2018, the Company issued 1,005,677 shares of Common Stock for
the conversion of 85,561 shares of Series C Convertible Preferred
Stock and $1,050,468 of related Series C Preferred Stock
dividends.
On
October 15, 2019, the “Company closed a private placement
offering of 1,204,820 shares of its common stock at a price of
$8.30 per share. All shares were sold by the Company as newly
issued shares. The purchasers in the offering consist of related
party entities to three members of the Company’s Board of
Directors. The transaction was approved by all of the disinterested
Directors of the Company. The price per share was determined by a
special committee of the Board comprised of disinterested Directors
who considered an independent third-party valuation of the offering
price and other relevant information.
ITEM 6. SELECTED FINANCIAL
DATA
As a
smaller reporting company, we are not required to provide this
information.
14
ITEM 7. MANAGEMENT’S DISCUSSION
AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
The
following discussion and analysis contain forward-looking
statements about future revenues, operating results, plans and
expectations. Forward-looking statements are based on a number of
assumptions and estimates that are inherently subject to
significant risks and uncertainties and our results could differ
materially from the results anticipated by our forward-looking
statements as a result of many known or unknown factors, including,
but not limited to, those factors discussed in Part I, Item 1A.
Risk Factors. In addition, the following discussion should be read
in conjunction with Part 1 of this report on Form 10-K as well as
with our consolidated financial statements and the related Notes
contained in Item 8 of this report.
Overview
The
Company’s business is developing, marketing, and distributing
wound and skin care products to physicians, hospitals, clinics and
post-acute care settings. Our products are primarily sold in the
North American advanced wound care and surgical tissue repair
markets. Sanara MedTech products include CellerateRX® Surgical
Activated Collagen® Adjuvant (“CellerateRX”);
HYCOL™ Hydrolyzed Collagen (“HYCOL”);
BIAKŌS™ Antimicrobial Skin & Wound Cleanser
(“BIAKŌS AWC”); and PULSAR II™ Advanced
Wound Irrigation System.
Liquidity and Capital Resources
Cash on
hand at December 31, 2019 was $6,611,928, compared to $176,421 at
December 31, 2018. Based on our current plan of operations, we
believe this amount, when combined with expected cash flows from
operations and amounts available under our revolving credit
facility will be sufficient to fund our growth strategy and to meet
our anticipated cash needs for at least the next twelve
months.
On
October 15, 2019, the Company closed a private placement offering
of 1,204,820 shares of its Common Stock at a price of $8.30 per
share. The $10 million of cash proceeds of the offering are
expected to be used to fund milestone payments under current and
future product license agreements, repayment of indebtedness under
the Company’s bank line of credit, and operating
expenditures, clinical studies and continued expansion of the
Company’s sales force. On October 16, 2019, the Company paid
down the entire balance of the line of credit of $2,200,000 with
cash proceeds received through the private placement stock
offering.
During
2018 and 2019, our principal sources of liquidity have been our
cash generated from operations, cash provided through a bank line
of credit, and more recently, through a private placement offering
discussed above. Cash consists of cash on deposit with banks.
Historically, we have financed our operations primarily from the
sale of debt and equity securities. No financing activities
occurred in 2018.
We
monitor our cash flow, assess our business plan, and make
expenditure adjustments accordingly. If appropriate, we may pursue
limited financing including issuing additional equity and/or
incurring additional debt. Although we have successfully funded our
past operations through additional bank debt and issuance of
equity, there is no assurance that our capital raising efforts will
be able to attract additional necessary capital at prices
attractive to the Company. If we are unable to obtain additional
funding for operations at any time in the future, we may not be
able to continue operations as proposed. This would require us to
modify our business plan, which could curtail various aspects of
our operations.
In
December 2018, and as amended in June 2019, the Company executed
agreements with Cadence Bank, N.A. which provided Cellerate, LLC
access to a revolving line of credit up to a maximum principal
amount of $2,500,000 which matures in June 2020. The expanded line
of credit is intended to provide the Company with additional
working capital for its product pipeline, sales force expansion and
other corporate purposes. At December 31, 2019, the Company had no
outstanding balance on the line of credit.
For the year ended December 31, 2019, net cash used in
operating activities was $2,167,401 as reported for Successor,
compared to $517,079 provided by operating activities for the year
ended December 31, 2018 as reported for the combined Predecessor
and Successor periods. The higher use of cash in 2019 was due to
investments in our sales force expansion and related sales support
infrastructure, higher inventory purchases, and an increase in
prepaid items related to inventory.
For the
year ended December 31, 2019, net cash used in investing activities
was $1,197,097 as reported for Successor, compared to $27,770 used
in investing activities during the year ended December 31, 2018 as
reported for the combined Predecessor and Successor periods. The
cash used in 2019 investing activities was primarily related to new
product licenses whereby the Company paid $1,500,000 for the
exclusive worldwide rights to market and sell certain FDA cleared
products developed by Rochal Industries, LLC.
For the
year ended December 31, 2019, net cash provided by financing
activities was $9,800,005 as reported for Successor, compared to $0
for the year ended December 31, 2018 as reported for the combined
Predecessor and Successor periods. The cash provided by financing
activities was funded by the October 15, 2019 private placement
offering discussed above.
Results of Operations
To
provide a meaningful presentation and comparison of our results of
operations, this discussion combines the period of January 1, 2018
through August 27, 2018 (Predecessor) with the period of August 28,
2018 through December 31, 2018 (Successor). In the accompanying
consolidated financial statements, a black line separates the
Predecessor and Successor financial statements to highlight the
lack of comparability between these two periods.
15
The
Successor financials for the twelve months ended December 31, 2019
do not include revenues and expenses related to the Predecessor for
the period January 1, 2019 through March 15, 2019. During this
period, the Predecessor’s revenues were approximately $34,000
and expenses were approximately $348,000, which are not reflected
in the Company’s results of operations during the twelve
months ended December 31, 2019.
Revenues. For the year ended December 31, 2019,
the Company generated revenues of $11,766,763 compared to revenues
of $8,779,872 for the year ended December 31, 2018 as reported for
the combined Predecessor and Successor periods, or a 34% increase
from prior year. The higher revenues
in 2019 were primarily due to the continued execution of the
Company’s strategy to expand its sales force and independent
distribution network in both new and existing U.S.
markets.
Cost of goods sold.
Cost of goods sold for the year ended December 31, 2019, was
$1,209,300, compared to costs of goods sold of $852,124 for the
year ended December 31, 2018 as reported for the combined
Predecessor and Successor periods. The increase over prior year was
due to higher sales volume and non-cash obsolescence charges
related to certain raw materials and finished goods
inventory.
Selling, general and administrative expenses
(“SG&A). SG&A
expenses for the year ended December 31, 2019, were $13,297,520
compared to SG&A expenses of $7,715,613 for the year ended
December 31, 2018 as reported for the combined Predecessor and
Successor periods. The higher SG&A expenses in 2019 were
primarily due to increased payroll costs resulting from sales force
expansion and operational support, higher sales commission expense
as a result of higher product sales, and increased marketing costs
related to promotional activities for new and existing product
lines. Direct selling costs represented the vast majority of the
increase in total SG&A costs as we more than doubled the size
of our field sales organization from eight to eighteen in
2019.
The
higher SG&A costs are consistent with the Company's strategy of
building out a larger sales force and independent distribution
network. New sales representatives generally take six to twelve
months on average to begin generating significant revenue. The
Company expects SG&A expenses to decline as a percentage of
revenue in the next two years as the revenue generated by its new
sales force begins to offset the cost of expanding the sales
force.
Interest
expense.
Interest expense was $105,919 for the year ended December 31, 2019,
as compared to $60,608 for the year ended December 31, 2018 as
reported for the combined Predecessor and Successor periods. The
higher interest expense was primarily due to the use of our line of
credit in 2019.
Net income / loss. For the year ended December 31, 2019, the
Company had a net loss of $2,814,088, compared to net income of
$175,464 for the year ended December 31, 2018 as reported for the
combined Predecessor and Successor periods. The net loss in 2019
was due to higher SG&A costs described above, which have been
driven by the Company’s strategy to grow top-line revenue
through significant investments in sales force expansion and
related sales support infrastructure as well as other areas of
administrative support. As mentioned above, the Successor
financials for the year ended December 31, 2019 do not include
revenues and expenses related to the Predecessor for the period
January 1, 2019 through March 15, 2019. During this period,
Predecessor’s revenues were approximately $34,000 and
expenses were approximately $348,000, which are not reflected in
the Company’s results of operations during the year ended
December 31, 2019.
COVID-19 Update
During
the first quarter of 2020, the COVID-19 virus emerged as a threat
to the global economy including the Company. To date, the virus and
the associated slowdown in surgical procedures has had a moderate
impact on the Company's business. With a significant percentage of
the Company's revenue derived from elective surgeries that have
been or will be postponed, management is expecting a decline in
revenue. The Company is proactively taking significant steps to cut
costs, manage cash-flow, and continue to generate revenue from both
its wound care and surgical businesses. The duration and severity
of the impact from the COVID-19 virus is unclear, but management
believes that surgical procedures currently being delayed will
eventually be performed potentially leading to a significant
increase in orders for the Company’s products.
Critical Accounting Policies
Our
discussion and analysis of our financial condition and results of
operations is based on our consolidated financial statements, which
have been prepared in accordance with accounting principles
generally accepted in the U.S. The preparation of these
consolidated financial statements requires us to make estimates and
judgments that affect the reported amounts of assets, liabilities
and expenses. We base our estimates on historical experience and on
various other assumptions that we believe to be reasonable under
the circumstances. The results of these assumptions form the basis
for making judgments about the carrying values of assets and
liabilities that are not readily apparent from other sources. Under
different assumptions or conditions, actual results may differ from
these estimates. We believe the footnotes to the consolidated
financial statements provide the description of the significant
accounting policies necessary in fully understanding and evaluating
our consolidated financial condition and results of
operations.
Off-Balance Sheet Arrangements
None.
ITEM 7A. QUANTITATIVE AND QUALITATIVE
DISCLOSURES ABOUT MARKET RISK
As a
smaller reporting company, we are not required to provide this
information.
16
ITEM 8. FINANCIAL STATEMENTS AND
SUPPLEMENTARY DATA
SANARA MEDTECH INC. AND SUBSIDIARIES
Index to Consolidated Financial Statements
|
Report of Independent Registered Public Accounting
Firm
|
18
|
|
|
|
|
Consolidated Balance Sheets
|
19
|
|
|
|
|
Consolidated Statements of Operations
|
20
|
|
|
|
|
Consolidated Statements of Changes in Stockholders’ Equity
(Deficit)
|
21
|
|
|
|
|
Consolidated Statements of Cash Flows
|
22
|
|
|
|
|
Notes to the Consolidated Financial Statements
|
23
|
17
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the
Shareholders and Board of Directors of
Sanara
MedTech, Inc.
Opinion on the Financial Statements
We have
audited the accompanying consolidated balance sheets of Sanara
MedTech, Inc. and its subsidiaries (collectively, the
“Company”) as of December 31, 2019 and 2018
(Successor), and the related consolidated statements of operations,
stockholders’ equity (deficit), and cash flows for the year
ended December 31, 2019 (Successor), the period from August 28,
2018 to December 31, 2018 (Successor) and for the period from
January 1, 2018 to August 27, 2018 (Predecessor), and the related
notes (collectively referred to as the “financial
statements”). In our opinion, the financial statements
present fairly, in all material respects, the financial position of
the Company as of December 31, 2019 and 2018 (Successor), and the
results of their operations and their cash flows for the year ended
December 31, 2019 (Successor), the period from August 28, 2018 to
December 31, 2018 (Successor) and for the period from January 1,
2018 to August 27, 2018 (Predecessor), in conformity with
accounting principles generally accepted in the United States of
America.
Basis for Opinion
These
financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on the
Company’s financial statements based on our audits. We are a
public accounting firm registered with the Public Company
Accounting Oversight Board (United States) ("PCAOB") and are
required to be independent with respect to the Company in
accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and
the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to
obtain reasonable assurance about whether the financial statements
are free of material misstatement, whether due to error or fraud.
The Company is not required to have, nor were we engaged to
perform, an audit of its internal control over financial reporting.
As part of our audits we are required to obtain an understanding of
internal control over financial reporting but not for the purpose
of expressing an opinion on the effectiveness of the Company's
internal control over financial reporting. Accordingly, we express
no such opinion.
Our
audits included performing procedures to assess the risks of
material misstatement of the financial statements, whether due to
error or fraud, and performing procedures that respond to those
risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting
principles used and significant estimates made by management, as
well as evaluating the overall presentation of the financial
statements. We believe that our audits provide a reasonable basis
for our opinion.
/s/ MaloneBailey, LLP
www.malonebailey.com
We have
served as the Company's auditor since 2014.
Houston,
Texas
March
26, 2020
18
SANARA MEDTECH INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
|
Successor
|
Successor
|
|
|
December 31,
|
December 31,
|
|
Assets
|
2019
|
2018
|
|
Current assets
|
|
|
|
Cash
and cash equivalents
|
$6,611,928
|
$176,421
|
|
Accounts
receivable, net of allowance for bad debt of $60,012 and
$0
|
1,285,165
|
1,022,500
|
|
Royalty
receivable
|
50,250
|
-
|
|
Inventory,
net of allowance for obsolescence of $43,650 and $484
|
746,519
|
465,314
|
|
Prepaid
and other assets
|
161,902
|
26,446
|
|
Total current assets
|
8,855,764
|
1,690,681
|
|
|
|
|
|
Long-term assets
|
|
|
|
Property,
plant and equipment, net of accumulated depreciation of $60,694 and
$511
|
204,953
|
18,777
|
|
Right
of use assets – operating leases
|
585,251
|
-
|
|
Intangible
assets, net of accumulated amortization of $603,580 and
$0
|
1,471,194
|
-
|
|
Total long-term assets
|
2,261,398
|
18,777
|
|
|
|
|
|
Total assets
|
$11,117,162
|
$1,709,458
|
|
|
|
|
|
Liabilities and shareholders' equity
|
|
|
|
Current liabilities
|
|
|
|
Accounts
payable
|
$337,504
|
$156,727
|
|
Accounts
payable – related party
|
68,668
|
36,203
|
|
Accrued
royalties and expenses
|
528,060
|
228,606
|
|
Accrued
bonus and commissions
|
1,588,056
|
701,125
|
|
Operating
lease liability - current
|
117,533
|
-
|
|
Line
of credit
|
-
|
-
|
|
Total current liabilities
|
2,639,821
|
1,122,661
|
|
|
|
|
|
Long-term liabilities
|
|
|
|
Operating
lease liability – long term
|
481,384
|
-
|
|
Convertible
notes payable – related party
|
1,500,000
|
-
|
|
Accrued
interest - related party
|
103,557
|
-
|
|
Total long-term liabilities
|
2,084,941
|
-
|
|
|
|
|
|
Total liabilities
|
4,724,762
|
1,122,661
|
|
|
|
|
|
Shareholders' equity
|
|
|
|
Series
F Convertible Preferred Stock: $10 par value, 1,200,000 shares
authorized; 1,136,815 issued and outstanding as of December 31,
2019 and 1,136,815 issued and outstanding as of December 31,
2018
|
11,368,150
|
11,368,150
|
|
Common
Stock: $0.001 par value, 20,000,000 shares authorized; 3,571,001
issued and outstanding as of December 31, 2019 and none issued and
outstanding as of December 31, 2018
|
3,571
|
-
|
|
Additional
paid-in capital
|
(2,081,829)
|
(10,919,639)
|
|
Retained
earnings (accumulated deficit)
|
(2,675,802)
|
138,286
|
|
Total Sanara MedTech shareholders' equity
|
6,614,090
|
586,797
|
|
Equity attributable
to noncontrolling interest
|
(221,690)
|
-
|
|
Total shareholders' equity
|
6,392,400
|
586,797
|
|
|
|
|
|
Total liabilities and shareholders' equity
|
$11,117,162
|
$1,709,458
|
The accompanying notes are an integral part of these consolidated
financial statements.
19
SANARA MEDTECH INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
Successor
|
Predecessor
|
|
|
|
January 1, 2019 – December 31, 2019
|
August 28, 2018-December 31, 2018
|
January 1, 2018-August 27, 2018
|
|
|
|
|
|
|
Revenues
|
$11,766,763
|
$3,006,320
|
$5,773,552
|
|
|
|
|
|
|
Cost of goods sold
|
1,209,300
|
371,421
|
480,703
|
|
|
|
|
|
|
Gross profit
|
10,557,463
|
2,634,899
|
5,292,849
|
|
|
|
|
|
|
Operating expenses
|
|
|
|
|
Selling,
general and administrative expenses
|
13,067,569
|
2,519,469
|
5,126,650
|
|
Depreciation
and amortization
|
119,951
|
511
|
56,425
|
|
Bad
debt expense
|
110,000
|
-
|
12,558
|
|
Total operating expenses
|
13,297,520
|
2,519,980
|
5,195,633
|
|
|
|
|
|
|
Operating income (loss)
|
(2,740,057)
|
114,919
|
97,216
|
|
|
|
|
|
|
Other income / (expense)
|
|
|
|
|
Other
income
|
10,198
|
23,367
|
570
|
|
Interest
expense
|
(105,919)
|
-
|
(60,608)
|
|
Total other income / (expense)
|
(95,721)
|
23,367
|
(60,038)
|
|
|
|
|
|
|
Net income (loss)
|
(2,835,778)
|
138,286
|
37,178
|
|
|
|
|
|
|
Less:
Net loss attributable to noncontrolling interest
|
(21,690)
|
-
|
-
|
|
|
|
|
|
|
Net income (loss) attributable to Sanara MedTech Inc.
|
(2,814,088)
|
138,286
|
37,178
|
|
|
|
|
|
|
Series
C Preferred Stock dividends
|
-
|
-
|
(28,061)
|
|
Series
C Preferred Stock inducement dividends
|
-
|
-
|
(103,197)
|
|
|
|
|
|
|
Net income (loss) attributable to Sanara MedTech common
shareholders
|
$(2,814,088)
|
$138,286
|
$(94,080)
|
|
|
|
|
|
|
Basic
loss per share of Common stock
|
$(1.32)
|
$-
|
$(0.05)
|
|
|
|
|
|
|
Diluted
loss per share of Common Stock
|
$(1.32)
|
$-
|
$(0.05)
|
|
|
|
|
|
|
Weighted
average number of common shares outstanding basic
|
2,132,745
|
-
|
2,068,941
|
|
|
|
|
|
|
Weighted
average number of common shares outstanding diluted
|
2,132,745
|
-
|
2,068,941
|
The accompanying notes are an integral part of these consolidated
financial statements.
20
SANARA MEDTECH INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(DEFICIT)
|
Predecessor
|
Preferred Stock
Series C
|
Common
Stock
|
Additional
|
|
|
|
Total
|
|||
|
|
$10 par
value
|
$0.001 par
value
|
Paid-In
|
Treasury
Stock
|
Accumulated
|
Noncontrolling
|
Shareholders'
|
|||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Shares
|
Amount
|
Income/(Deficit)
|
Interest
|
Equity
(Deficit)
|
|
Balance at December 31,
2017
|
85,561
|
$855,610
|
1,134,279
|
$1,134
|
$46,114,357
|
(41)
|
$(120)
|
$(46,868,443)
|
$-
|
$102,538
|
|
Conversion of Series C Preferred
Shares
|
(85,561)
|
(855,610)
|
855,605
|
855
|
854,755
|
-
|
-
|
-
|
-
|
-
|
|
Series C
Dividend
|
-
|
-
|
150,067
|
150
|
(150)
|
-
|
-
|
-
|
-
|
-
|
|
Common Stock issued for conversion
of debt
|
-
|
-
|
226,514
|
227
|
1,585,367
|
-
|
-
|
-
|
-
|
1,585,594
|
|
Recognition of stock option
expense
|
-
|
-
|
-
|
-
|
14,867
|
-
|
-
|
-
|
-
|
14,867
|
|
Net income
(loss)
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
37,178
|
-
|
37,178
|
|
Balance at August 27,
2018
|
-
|
$-
|
2,366,465
|
$2,366
|
$48,569,196
|
(41)
|
$(120)
|
$(46,831,265)
|
$-
|
$1,740,177
|
|
Successor
|
Preferred Stock
Series F
|
Common
Stock
|
Additional
|
|
|
|
Total
|
|||
|
|
$10 par
value
|
$0.001 par
value
|
Paid-In
|
Treasury
Stock
|
Accumulated
|
Noncontrolling
|
Shareholders'
|
|||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Shares
|
Amount
|
Income/(Deficit)
|
Interest
|
Equity
(Deficit)
|
|
Issuance of preferred stock upon
formation on August 28, 2018
|
1,136,815
|
$11,368,150
|
-
|
$-
|
$(10,919,639)
|
-
|
$-
|
$-
|
$-
|
$448,511
|
|
Net income
(loss)
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
138,286
|
-
|
138,286
|
|
Balance at December 31,
2018
|
1,136,815
|
$11,368,150
|
-
|
$-
|
$(10,919,639)
|
-
|
$-
|
$138,286
|
$-
|
$586,797
|
|
Reverse
recapitalization
|
-
|
-
|
2,366,465
|
2,366
|
(1,159,929)
|
(41)
|
-
|
-
|
-
|
(1,157,563)
|
|
Treasury stock
retirement
|
-
|
-
|
(41)
|
-
|
-
|
41
|
-
|
-
|
-
|
-
|
|
Repurchase and cancellation of
fractional shares
|
-
|
-
|
(243)
|
-
|
(1,061)
|
-
|
-
|
-
|
-
|
(1,061)
|
|
Private placement stock
issue
|
-
|
-
|
1,204,820
|
1,205
|
9,998,800
|
-
|
-
|
-
|
-
|
10,000,005
|
|
Advance on future noncontrolling
interest distribution
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(200,000)
|
(200,000)
|
|
Net income
(loss)
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(2,814,088)
|
(21,690)
|
(2,835,778)
|
|
Balance at December 31,
2019
|
1,136,815
|
$11,368,150
|
3,571,001
|
$3,571
|
$(2,081,829)
|
-
|
$-
|
$(2,675,802)
|
$(221,690)
|
$6,392,400
|
The accompanying notes are an integral part of these consolidated
financial statements.
21
SANARA
MEDTECH INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
Successor
|
Predecessor
|
|
|
|
January 1, 2019 – December 31, 2019
|
August 28, 2018-December 31, 2018
|
January 1, 2018-August 27, 2018
|
|
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
Net
income (loss)
|
$(2,835,778)
|
$138,286
|
$37,178
|
|
Adjustments
to reconcile net income to net cash used in operating
activities
|
|
|
|
|
Depreciation
and amortization
|
119,951
|
511
|
56,425
|
|
Interest expense on
convertible debt
|
61,934
|
-
|
60,608
|
|
Loss on disposal of
asset
|
15,944
|
-
|
-
|
|
Bad debt
expense
|
110,000
|
-
|
12,558
|
|
Recognition of
vesting stock option expense
|
-
|
-
|
14,867
|
|
Changes
in assets and liabilities:
|
|
|
|
|
(Increase)
decrease in accounts receivable
|
(324,368)
|
(1,022,500)
|
(313,969)
|
|
(Increase)
decrease in inventory
|
(281,205)
|
(16,803)
|
262,886
|
|
(Increase)
decrease in prepaid and other assets
|
(455,960)
|
(26,446)
|
(23,320)
|
|
Increase
(decrease) in accounts payable
|
(65,037)
|
156,727
|
189,388
|
|
Increase
(decrease) in accounts payable related parties
|
(19,599)
|
36,203
|
(36,097)
|
|
Increase
(decrease) in accrued royalties and expenses
|
282,004
|
228,606
|
(170,467)
|
|
Increase
(decrease) in accrued liabilities
|
1,224,713
|
701,125
|
231,313
|
|
Net cash flows provided by (used in) operating
activities
|
(2,167,401)
|
195,709
|
321,370
|
|
|
|
|
|
|
Cash flows from investing activities:
|
|
|
|
|
Purchase
of property and equipment
|
(182,825)
|
(19,288)
|
(8,482)
|
|
Cash
received in reverse acquisition
|
508,973
|
-
|
-
|
|
Repurchase
and cancellation of fractional shares
|
(1,061)
|
-
|
-
|
|
Proceeds
from disposal of assets
|
301
|
|
|
|
Purchase
of intangible assets
|
(1,522,485)
|
-
|
-
|
|
Net cash flows used in investing activities
|
(1,197,097)
|
(19,288)
|
(8,482)
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
Draw
on line of credit
|
2,200,000
|
-
|
-
|
|
Payment
on line of credit
|
(2,200,000)
|
|
|
|
Private
placement stock issue
|
10,000,005
|
-
|
-
|
|
Advance
on future noncontrolling interest distribution
|
(200,000)
|
-
|
-
|
|
Net cash flows from financing activities
|
9,800,005
|
-
|
-
|
|
|
|
|
|
|
Net increase in cash
|
6,435,507
|
176,421
|
312,888
|
|
Cash and cash equivalents, beginning of period
|
176,421
|
-
|
463,189
|
|
Cash and cash equivalents, end of period
|
$6,611,928
|
$176,421
|
$776,077
|
|
|
|
|
|
|
Cash paid during the period for:
|
|
|
|
|
Interest
|
$43,985
|
$-
|
$-
|
|
Income
taxes
|
-
|
-
|
-
|
|
|
|
|
|
|
Supplemental non-cash investing and financing
activities:
|
|
|
|
|
Common
stock issued for dividends on Series C Preferred Stock
|
-
|
-
|
15,007
|
|
Common
stock issued for conversion of Series C Preferred
Stock
|
-
|
-
|
85,561
|
|
Common
stock issued for conversion of Related Party debt and
interest
|
-
|
-
|
1,585,594
|
|
Preferred
Shares issued for inventory contribution
|
-
|
448,511
|
-
|
|
Common
stock issued in reverse capitalization; less cash received of
$508,973
|
1,666,537
|
-
|
-
|
The accompanying notes are an integral part of these consolidated
financial statements.
22
SANARA MEDTECH INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – NATURE OF OPERATIONS
The
Company’s business is developing, marketing, and distributing
wound and skin care products to physicians, hospitals, clinics and
post-acute care settings. Our products are primarily sold in the
North American advanced wound care and surgical tissue repair
markets. Sanara MedTech products include CellerateRX®
Activated Collagen® Adjuvant (CellerateRX); HYCOL™
Hydrolyzed Collagen (HYCOL); BIAKŌS™ Antimicrobial Skin
& Wound Cleanser (BIAKŌS); and PULSAR II™ Advanced
Wound Irrigation System (Pulsar II).
NOTE 2 -- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BACKGROUND AND BASIS OF PRESENTATION
The
terms “Sanara MedTech,” “we,” “the
Company,” “SMTI,” “our,” and
“us” as used in this report refer to Sanara MedTech
Inc. and its subsidiaries. The Company
was organized on December 14, 2001, as a Texas corporation under
the name eAppliance Innovations, Inc. In June of 2002, MB Software
Corporation, a public corporation formed under the laws of
Colorado, merged with the Company and the Company changed its name
to MB Software Corporation as part of the merger. In May of 2008,
the Company changed its name to Wound Management Technologies,
Inc. On May 10, 2019, the
Company changed its name from Wound Management Technologies, Inc.
to Sanara MedTech Inc.
On
August 28, 2018, the Company consummated definitive agreements that
continued operations to market the Company’s principal
products, CellerateRX, through a 50% ownership interest in a newly
formed Texas limited liability company, Cellerate, LLC which began
operations on September 1, 2018. The remaining 50% ownership
interest was held by an affiliate of The Catalyst Group, Inc.
(Catalyst), which acquired the exclusive license to CellerateRX
products. Cellerate, LLC conducts operations with an exclusive
sublicense from the Catalyst affiliate to distribute CellerateRX
products into the wound care and surgical markets in the United
States, Canada and Mexico.
While
the Company had significant influence over the operations of
Cellerate, LLC, the Company did not have a controlling interest.
Catalyst had the controlling vote in the event of a deadlocked vote
by the Board of Managers of Cellerate, LLC. Therefore, the
Company’s investment in Cellerate, LLC was reported using the
equity method of accounting in the
Company’s Quarterly Report on Form 10-Q filed November 14,
2018, and in the Company’s Annual Report on Form 10-K filed
on April 1, 2019.
On
March 15, 2019, the Company acquired Catalyst’s 50% interest
in Cellerate, LLC (the Cellerate Acquisition) in exchange for
1,136,815 shares of the Company’s newly created Series F
Convertible Preferred Stock. Each share of Series F Convertible
Preferred Stock was convertible at the option of the holder, at any
time, into 2 shares of common stock, adjusted for the 1-for-100
reverse stock split of the Company’s common stock which
became effective on May 10, 2019. Additionally, each holder of
Series F Convertible Preferred Stock was entitled to vote on all
matters submitted for a vote of the Company’s shareholders
with votes equal to the number of shares of common stock into which
such holder’s Series F shares could then be converted. Based
on the closing price of the Company’s common stock on March
15, 2019 and the conversion ratio of the Series F Preferred Stock,
the fair value of the preferred shares issued to Catalyst was
approximately $12.5 million. Following the closing of this
transaction, Mr. Ronald T. Nixon, Founder and Managing Partner of
Catalyst, was elected to the Company’s Board of Directors
effective March 15, 2019.
The
Cellerate Acquisition was accounted for as a reverse merger and
recapitalization because, immediately following the completion of
the transaction, Catalyst could obtain effective control of the
Company upon exercise of its convertible preferred stock and
promissory note, both of which could occur at Catalyst’s
option. Additionally, Cellerate, LLC’s officers and senior
executive positions continued on as management of the combined
entity after consummation of the Cellerate Acquisition. For
accounting purposes, Cellerate, LLC was deemed to be the accounting
acquirer in the transaction and, consequently, the transaction was
treated as a recapitalization of Sanara MedTech. As part of the reverse merger and
recapitalization, the net liabilities existing in the Company as of
the date of the merger totaling approximately $1,666,537, which
included $508,973 of cash, were converted to equity as part of this
transaction. No step-up in basis or intangible assets or
goodwill was recorded in this transaction.
As a
result of the reverse merger, Cellerate, LLC’s assets,
liabilities and results of operations are the historical financial
statements of the registrant, and Cellerate, LLC’s assets,
liabilities and results of operations have been combined with
Sanara MedTech effective as of the date of the closing of the
Cellerate Acquisition. The Company’s financial statement
presentation identifies Cellerate, LLC as “Successor”
for the twelve-month period ending December 31, 2019, and on the
balance sheet date of December 31, 2018. Upon its formation on
August 28, 2018, Cellerate LLC succeeded to the business and
operations of Sanara MedTech. As a result, Sanara MedTech is
identified as “Predecessor” for the periods preceding
August 28, 2018.
23
On May
9, 2019, the Company organized Sanara Pulsar, LLC, a Texas limited
liability company, which is owned 60% by the Company’s wholly
owned subsidiary Cellerate, LLC, and 40% owned by Wound Care
Solutions, Limited, an unaffiliated company registered in the
United Kingdom (WCS). Net profits and losses and distributions are
shared by the members in proportion to their respective membership
interests. The Company consolidates the operations and financial
position of Sanara Pulsar.
PRINCIPLES OF CONSOLIDATION
The
financial statements are presented on a comparative basis. The
consolidated balance sheet at December 31, 2018 is identified as
“Successor” and includes the accounts of Cellerate, LLC
only. The consolidated balance sheet at December 31, 2019 is also
identified as “Successor” and includes the accounts of
Cellerate, LLC, Sanara MedTech, and Sanara Pulsar,
LLC.
The
consolidated statement of operations for the year ending December
31, 2019 is identified as “Successor” and includes the
accounts of Cellerate, LLC for the full year, the accounts of
Sanara MedTech for the period March 16, 2019 through December 31,
2019, and the accounts of Sanara Pulsar, LLC since its formation
date of May 9, 2019 through December 31, 2019. The statement of
operations for the period ending August 27, 2018 is identified as
“Predecessor” and includes the accounts of Sanara
MedTech and its wholly owned subsidiaries (excluding Cellerate,
LLC). The statement of operations for the period August 28 through
December 31, 2018 are the accounts of Cellerate, LLC as
“Successor”. A black line separates the Predecessor and
Successor sections to highlight the lack of comparability between
these two periods.
The
consolidated statement of changes in shareholders’ equity
includes two sections. The first section is identified as
“Predecessor” and includes the Sanara MedTech equity
information as previously reported by the Company on its 2017 Form
10-K annual report, and the ending equity balances after the
cancellation of the “Predecessor” equity on August 27,
2018. The second section is identified as “Successor”
which includes a presentation of equity to reflect the
recapitalization of Sanara MedTech. The presentation includes the
issuance of the Series F Preferred Stock, the changes in paid-in
capital, and the restatement of the accumulated deficit. A black
line separates the Predecessor and Successor sections to highlight
the lack of comparability between these two periods
The
consolidated statement of cash flows for the year ending December
31, 2019 is identified as “Successor” and includes the
accounts of Cellerate, LLC for the full year, the accounts of
Sanara MedTech for the period March 16, 2019 through December 31,
2019, and the accounts of Sanara Pulsar, LLC since its formation
date on May 9, 2019 through December 31, 2019. The consolidated
statement of cash flows for the period ending August 27, 2018 is
identified as “Predecessor” and includes the accounts
of Sanara MedTech and its wholly owned subsidiaries (excluding
Cellerate, LLC) for the period ended August 27, 2018. The
consolidated statement of cash flows for the period of August 28,
2018 through December 31, 2018 is identified as
“Successor” and includes only the accounts of
Cellerate, LLC as “Successor”. A black line separates
the Predecessor and Successor sections to highlight the lack of
comparability between these two periods.
USE OF ESTIMATES IN FINANCIAL STATEMENT PREPARATION
The
preparation of the financial statements in conformity with
accounting principles generally accepted in the United States of
America requires management to make estimates and assumptions that
affect the reported amounts of assets and liabilities, the
disclosures of contingent assets and liabilities at the date of the
financial statements, and the amounts of revenues and expenses
during the reporting period. On a regular basis, management
evaluates these estimates and assumptions. Actual results could
differ from those estimates.
CASH, CASH EQUIVALENTS AND MARKETABLE SECURITIES
The
Company considers all highly liquid debt investments purchased with
an original maturity of three months or less to be cash
equivalents. Marketable securities include investments with
maturities greater than three months but less than one year. For
certain of the Company’s financial instruments, including
cash and cash equivalents, accounts receivable, accounts payable
and other accrued liabilities, and amounts due to related parties,
the carrying amounts approximate fair value due to their short
maturities.
INCOME / LOSS PER SHARE
The
Company computes income/loss per share in accordance with
Accounting Standards Codification “ASC” Topic No. 260,
“Earnings per Share,” which requires the Company to
present basic and dilutive income/loss per share when the effect is
dilutive. Basic income/loss per share is computed by dividing
income/loss available to common stockholders by the weighted
average number of common shares available. Diluted income/loss per
share is computed similar to basic income/loss per share except
that the denominator is increased to include the number of
additional common shares that would have been outstanding if the
potential common shares had been issued and if the additional
common shares were dilutive.
24
The calculation of basic
and diluted net loss per share for the years ended December 31,
2019 and 2018 are as follows:
|
|
Successor
|
Predecessor
|
|
|
|
January 1, 2019 – December 31, 2019
|
August 28, 2018-December 31, 2018
|
January 1, 2018-August 27, 2018
|
|
Numerator
for basic and diluted net income (loss) per share:
|
|
|
|
|
Net
income (loss) attributable to Sanara MedTech
|
$(2,814,088)
|
$138,286
|
$37,178
|
|
Series
C Preferred Stock dividends
|
-
|
-
|
(28,061)
|
|
Series
C Preferred Stock inducement dividends
|
-
|
-
|
(103,197)
|
|
Basic
net income (loss) attributable to Sanara MedTech common
shareholders
|
$(2,814,088)
|
$138,286
|
$(94,080)
|
|
Denominator
for basic and diluted net income (loss) per share:
|
|
|
|
|
Weighted
average shares used to compute diluted net income (loss) per
share
|
2,132,745
|
-
|
2,068,941
|
|
|
|
|
|
|
Basic
and diluted net income (loss) per share attributable to common
shareholders
|
$(1.32)
|
$-
|
$(0.05)
|
The following table summarizes the potential shares of common stock
that were excluded from the computation of diluted net loss per
share for the years ended December 31, 2019 and 2018 as such shares
would have had an anti-dilutive effect:
|
|
Successor
|
Predecessor
|
|
|
|
January 1, 2019 – December 31, 2019
|
August 28, 2018-December 31, 2018
|
January 1, 2018-August 27, 2018
|
|
|
|
|
|
|
Options
|
2,059
|
-
|
-
|
|
Convertible
debt
|
178,173
|
-
|
-
|
|
Preferred
Shares
|
2,273,630
|
2,273,630
|
-
|
REVENUE RECOGNITION
The Company recognizes revenue in accordance with ASC Topic
606, Revenue from Contracts with Customers, which was adopted
on January 1, 2018 using the modified retrospective method.
Revenues are recognized when control of the promised goods or
services is transferred to the customer in an amount that reflects
the consideration the Company expects to be entitled to receive in
exchange for transferring those goods or services. Revenue is
recognized based on the following five step model:
|
1. Identification of the contract with a
customer
|
|
2. Identification of the performance obligations in
the contract
|
|
3. Determination of the transaction
price
|
|
4. Allocation of the transaction price to the
performance obligations in the contract
|
|
5. Recognition of revenue when, or as, the Company
satisfies a performance obligation
|
25
The Company recognizes royalty revenue from a licensing agreement
between BioStructures, LLC and the Company. The Company records
revenue each calendar quarter as earned per the terms of the
agreement which stipulates the Company will receive quarterly
royalty payments of at least $50,250.
See Note
3 for more information on
revenue recognition.
ALLOWANCE FOR DOUBTFUL ACCOUNTS
The
Company establishes an allowance for doubtful accounts to ensure
accounts receivable are not overstated due to uncollectible
accounts. Bad debt reserves are maintained based on a variety of
factors, including the length of time receivables are past due and
a detailed review of certain individual customer accounts. If
circumstances related to customers change, estimates of the
recoverability of receivables would be further adjusted. The
Company recorded bad debt expense of $110,000 and $12,588 in 2019
and 2018, respectively. The allowance for doubtful accounts at
December 31, 2019 was $60,012 and the amount at December 31, 2018
was $0. Accounts receivable written-off during 2019 totaled
$90,538. The Successor’s balance sheet at December 31, 2018
did not include an allowance for doubtful accounts of $40,550 which
was carried over from the Predecessor’s December 31, 2018
balance sheet.
INVENTORIES
During
2019 and 2018, inventories were stated at the lower of cost or net
realizable value, with cost computed on a first-in, first-out
basis. Inventories consist of finished goods, powders, gels and the
related packaging supplies. The Company recorded inventory
obsolescence expense of $120,442 in 2019 and $0 in 2018. The
allowance for obsolete and slow-moving inventory had a balance of
$43,650 and $484 at December 31, 2019 and December 31, 2018,
respectively.
PROPERTY AND EQUIPMENT
Property
and equipment are recorded at cost. Depreciation is computed
utilizing the straight-line method over the estimated economic life
of the assets, which ranges from three to ten years. For assets
sold or otherwise disposed of, the cost and related accumulated
depreciation are removed from the accounts, and any related gain or
loss is reflected in income for the period. A summary is as
follows:
|
|
Successor
|
Successor
|
|
|
December 31,
|
December 31,
|
|
|
2019
|
2018
|
|
|
|
|
|
Computers
|
$87,310
|
$5,147
|
|
Office
Equipment
|
22,312
|
-
|
|
Furniture
and fixtures
|
153,995
|
3,328
|
|
Leasehold
Improvements
|
2,030
|
-
|
|
Capital
in progress
|
-
|
10,813
|
|
|
265,647
|
19,288
|
|
|
|
|
|
Less
accumulated depreciation
|
(60,694)
|
(511)
|
|
|
|
|
|
Property
and equipment, net
|
$204,953
|
$18,777
|
As of
December 31, 2019, fixed assets consisted of $265,647 including
furniture and fixtures, computer equipment, phone equipment and the
Company’s tradeshow booth. As of December 31, 2018, fixed
assets consisted of $19,288 including furniture and fixtures,
computer equipment, and phone equipment. Depreciation expense
related to property and equipment was $29,940 for the year ended
December 31, 2019 (Successor), and $511 and $13,076 for the periods
August 28, 2018 through December 31, 2018 (Successor) and January
1, 2018 through August 27, 2018 (Predecessor),
respectively.
26
INTANGIBLE ASSETS
Intangible
Assets are stated at cost of acquisition less accumulated
amortization and impairment loss, if any. Cost of acquisition
includes purchase price and any cost directly attributable to
bringing the asset to its working condition for the intended use.
The Company amortizes its intangible assets on a straight-line
basis over the useful life of the respective assets which is
generally the life of the related patents (if
applicable).
See
Note 6 for more information
on intangible assets.
IMPAIRMENT OF LONG-LIVED ASSETS
Long-lived
assets and certain identifiable intangibles to be held and used by
the Company are reviewed for impairment whenever events or changes
in circumstances indicate that the carrying amount of an asset may
not be recoverable. The Company continuously evaluates the
recoverability of its long-lived assets based on estimated future
cash flows and the estimated liquidation value of such long-lived
assets and provides for impairment if such undiscounted cash flows
are insufficient to recover the carrying amount of the long-lived
assets. If impairment exists, an adjustment is made to write the
asset down to its fair value, and a loss is recorded as the
difference between the carrying value and fair value. Fair values
are determined based on quoted market values, undiscounted cash
flows or internal and external appraisals, as applicable. Assets to
be disposed of are carried at the lower of carrying value or
estimated net realizable value. There was no impairment recorded
during the years ended December 31, 2019 and 2018.
FAIR VALUE MEASUREMENTS
As
defined in Accounting Standards Codification (“ASC”)
Topic No. 820, fair value is the price that would be received to
sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date
(exit price). The Company utilizes market data or assumptions that
market participants would use in pricing the asset or liability,
including assumptions about risk and the risks inherent in the
inputs to the valuation technique. These inputs can be readily
observable, market corroborated, or generally unobservable. ASC 820
establishes a fair value hierarchy that prioritizes the inputs used
to measure fair value. The hierarchy gives the highest priority to
unadjusted quoted prices in active markets for identical assets or
liabilities (level 1 measurement) and the lowest priority to
unobservable inputs (level 3 measurement). This fair value
measurement framework applies at both initial and subsequent
measurement.
The
three levels of the fair value hierarchy defined by ASC Topic No.
820 are as follows:
Level 1
– Quoted prices are available in active markets for identical
assets or liabilities as of the reporting date. Active markets are
those in which transactions for the asset or liability occur in
sufficient frequency and volume to provide pricing information on
an ongoing basis. Level 1 primarily consists of financial
instruments such as exchange-traded derivatives, marketable
securities and listed equities.
Level 2
– Pricing inputs are other than quoted prices in active
markets included in level 1, which are either directly or
indirectly observable as of the reported date. Level 2 includes
those financial instruments that are valued using models or other
valuation methodologies. These models are primarily
industry-standard models that consider various assumptions,
including quoted forward prices for commodities, time value,
volatility factors, and current market and contractual prices for
the underlying instruments, as well as other relevant economic
measures. Substantially all of these assumptions are observable in
the marketplace throughout the full term of the instrument, can be
derived from observable data or are supported by observable levels
at which transactions are executed in the marketplace.
Instruments
in this category generally include non-exchange-traded derivatives
such as commodity swaps, interest rate swaps, options and
collars.
Level 3
– Pricing inputs include significant inputs that are
generally less observable from objective sources. These inputs may
be used with internally developed methodologies that result in
management’s best estimate of fair value.
Our
intangible assets have also been valued using the fair value
accounting treatment. A description of the methodology used,
including the valuation category, is described below in Note 6
“Intangible Assets.”
INCOME TAXES
Income
taxes are accounted for under the asset and liability method,
whereby deferred income taxes are recorded for temporary
differences between financial statement carrying amounts and the
tax basis of assets and liabilities. Deferred tax assets and
liabilities reflect the tax rates expected to be in effect for the
years in which the differences are expected to reverse. A valuation
allowance is provided if it is more likely than not that some or
all, of the deferred tax asset will not be realized.
27
BENEFICIAL CONVERSION FEATURE OF CONVERTIBLE NOTES
PAYABLE
The
convertible feature of certain notes payable may provide for a rate
of conversion that is below the market value of the Company’s
common stock. Such a feature is normally characterized as a
"Beneficial Conversion Feature" ("BCF"). In accordance with ASC
Topic No. 470-20-25-4, the intrinsic value of the embedded
beneficial conversion feature present in a convertible instrument
is recognized separately at issuance by allocating a portion of the
debt equal to the intrinsic value of that feature to additional
paid in capital. When applicable, the Company records the estimated
fair value of the BCF in the consolidated financial statements as a
discount from the face amount of the notes. Such discounts are
accreted to interest expense over the term of the notes using the
effective interest method. There were no beneficial conversion
features recorded in 2019 or 2018.
ADVERTISING EXPENSE
In
accordance with ASC Topic No. 720-35-25-1, the Company recognizes
advertising expenses the first time the advertising takes place.
Such costs are expensed immediately if such advertising is not
expected to occur.
SHARE-BASED COMPENSATION
The
Company accounts for stock-based compensation to employees and
nonemployees in accordance with ASU 2018-07 Topic 718. Stock-based
compensation is measured at the grant date, based on the fair value
of the award, and is recognized as expense over the stipulated
vesting period (if any). The Company estimates the fair value of
stock-based payments using the Black-Scholes option-pricing model
for common stock options and warrants, and the closing price of the
Company’s common stock for common share
issuances.
RECLASSIFICATIONS
Certain
prior period amounts have been reclassified to conform to current
period presentation.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
In May
2014, the Financial Accounting Standards Board (FASB) issued
Accounting Standards Codification (ASC) 606, Revenue from Contracts
with Customers which is to be effective for reporting periods
beginning after December 15, 2017. The Company adopted ASC 606
effective January 1, 2018 and the adoption had no impact on the
Company’s financial position, operations or cash
flows.
In
February 2016, the FASB issued ASC 842 Leases which is to be
effective for reporting periods beginning after December 15, 2018.
The Company adopted the pronouncement effective January 1, 2019.
In accordance with the transition
guidance of ASC 842, such arrangements are included in our balance
sheet as of January 1, 2019. All other leases are short-term leases
for which out of practical expediency the Company has elected to
not recognize lease assets and lease liabilities. As a
result of the adoption of ASC 842, the Company has recorded lease
assets of $585,251 and a related lease liability of $598,917 as of
December 31, 2019.
On June
20, 2018, the FASB issued Accounting Standards Update (ASU)
2018-07, which simplifies the accounting for share-based payments
granted to nonemployees for goods and services. Under the ASU, most
of the guidance on such payments to nonemployees would be aligned
with the requirements for share-based payments granted to
employees. The Company adopted the pronouncement effective January
1, 2019 and the adoption did not have a material impact on the
Company’s financial position, operations or cash
flows.
NOTE 3 – ASC Topic 606, Revenue from Contracts with
Customers
The Company recognizes revenue in accordance with ASC Topic
606, Revenue from Contracts with Customers, which was adopted
on January 1, 2018 using the modified retrospective method.
Revenues are recognized when control of the promised goods or
services is transferred to the customer in an amount that reflects
the consideration the Company expects to be entitled to receive in
exchange for transferring those goods or services. Revenue is
recognized based on the following five step model:
|
1. Identification of the contract with a
customer
|
|
2. Identification of the performance obligations in
the contract
|
|
3. Determination of the transaction
price
|
|
4. Allocation of the transaction price to the
performance obligations in the contract
|
|
5. Recognition of revenue when, or as, the Company
satisfies a performance obligation
|
28
Details of this five-step process are as follows:
Identification of the contract with a customer
Customer purchase orders are generally considered to be contracts
under ASC 606. Purchase orders typically identify specific terms of
products to be delivered, create the enforceable rights and
obligations of both parties, and result in commercial substance. No
other forms of contract revenue recognition, such as the completed
contract or percentage of completion methods, were utilized by the
Company in either 2018 or 2019.
Performance obligations
The Company’s performance obligation is generally limited to
delivery of the requested items to its customers at the agreed upon
quantities and prices.
Determination and allocation of the transaction price
The Company has established prices for its products. These prices
are effectively agreed to when customers place purchase orders with
the Company. Rebates and discounts, if any, are recognized in full
at the time of sale as a reduction of net revenue. Allocation of
transaction prices is not necessary where one performance
obligation exists.
Recognition of revenue as performance obligations are
satisfied
Product revenues are recognized when the products are delivered,
and title passes to the customer.
Disaggregation of Revenue
Revenue
streams from product sales and royalties are summarized below for
the twelve months ended December 31, 2019 and 2018. All revenue was
generated in the United States; therefore, no geographical
disaggregation is necessary.
|
|
Successor
|
Predecessor
|
|
|
|
Twelve Months Ended
December 31,
2019
|
August 28, 2018-December 31, 2018
|
January 1, 2018-August 27, 2018
|
|
Product
sales revenue
|
$11,607,638
|
$3,006,320
|
$5,639,552
|
|
Royalty
revenue
|
159,125
|
-
|
134,000
|
|
Total Revenue
|
$11,766,763
|
$3,006,320
|
$5,773,552
|
The Company recognizes royalty revenue from a licensing agreement
between BioStructures, LLC and the Company. The Company records
revenue each calendar quarter as earned per the terms of the
agreement which stipulates the Company will receive quarterly
royalty payments of at least $50,250. Under the terms of the
development and license agreement the Company executed with
BioStructures, LLC in 2011, royalties of 2.0% are recognized on
sales of products containing the Company’s patented
resorbable bone hemostasis. The minimum annual royalty due to the
Company is $201,000 per year throughout the life of the patent
which expires in 2023. These royalties are payable in quarterly
installments of $50,250. To date, royalties related to this
licensing agreement have not exceeded the annual minimum of
$201,000 ($50,250 per quarter).
NOTE 4 – OTHER SIGNIFICANT TRANSACTIONS
Private Placement Offering
On
October 15, 2019, Sanara MedTech Inc. (the “Company”)
closed a private placement offering of 1,204,820 shares of its
common stock at a price of $8.30 per share. All shares were sold by
the Company as newly issued shares. The purchasers in the offering
consist of related party entities to three members of the
Company’s Board of Directors. The transaction was approved by
all of the disinterested Directors of the Company. The price per
share was determined by a special committee of the Board comprised
of disinterested Directors who considered an independent
third-party valuation of the offering price and other relevant
information.
29
The $10
million of cash proceeds of the offering are expected to be used to
fund milestone payments under current and future product license
agreements, repayment of indebtedness under the Company’s
bank line of credit, and operating expenditures, including clinical
studies and continued expansion of the Company’s sales
force.
The
shares of common stock sold in the transaction were not registered
under the Securities Act of 1933. The Company relied on the
exemption from registration in Section 4(a)(2) of the Securities
Act of 1933, a transaction not involving a public offering. The
Company sold the shares of common stock, without general
solicitation or advertising, to three accredited investors who
represented themselves as being fully informed, with the knowledge
and experience to be capable of evaluating the merits and risks of
the transaction, and with no present intention of selling or
distributing the shares.
Cellerate, LLC
Effective August 28, 2018, the Company consummated definitive
agreements that continued operations to market the Company’s
principal products, CellerateRX® Hydrolyzed Collagen
(CellerateRX), through a 50% ownership interest in a newly formed
Texas limited liability company, Cellerate, LLC. The remaining 50%
ownership interest was held by an affiliate of The Catalyst Group
Inc. (Catalyst), which acquired an exclusive license to distribute
CellerateRX products. Cellerate, LLC conducts operations with an
exclusive sublicense from a Catalyst affiliate to distribute
CellerateRX products into the wound care and surgical markets in
the United States, Canada and Mexico.
While the Company had significant influence over the operations of
Cellerate, LLC, the Company did not have a controlling interest.
Catalyst had the controlling vote in the event of a deadlocked vote
by the Board of Managers of Cellerate, LLC. Therefore, the Company
reported its investment in Cellerate, LLC using the equity method
of accounting in the Company’s Quarterly Report on Form 10-Q
filed November 14, 2018, and in the Company’s Annual Report
on Form 10-K filed on April 1, 2019.
On
March 15, 2019, the Company acquired Catalyst’s 50% interest
in Cellerate, LLC. See Note 2
for more information regarding this acquisition.
The
definitive agreements related to the Cellerate, LLC transaction are
summarized below. The full agreements are attached as exhibits to
the Company’s Quarterly Report on Form 10-Q filed on November
14, 2018.
Contribution Agreement
WCI,
LLC (“WCI”), a wholly owned subsidiary of the Company,
transferred to Cellerate, LLC all of its existing inventories and
certain trademarks and UPC numbers in exchange for its 50%
ownership interest in Cellerate, LLC. The inventories had a net
book value of $448,511 at the time of closing. Additionally, as
part of the transaction, the Company issued a 30-month promissory
note to Catalyst in the principal amount of $1,500,000, bearing
interest at a 5% annual interest rate, compounded quarterly.
Interest is payable quarterly, but may be deferred at the
Company’s election to the maturity of the Note. Outstanding
principal and interest are convertible at Catalyst’s option
into shares of the Company’s common stock at a conversion
price of $9.00 per share.
Catalyst
transferred to Cellerate, LLC in exchange for its 50% ownership
interest an exclusive sublicense to distribute CellerateRX into the
wound care and surgical markets in the United States, Canada and
Mexico. The term of the sublicense extends through August 2028,
with automatic one-year renewals through December 31, 2049, subject
to termination at the end of any renewal term by Catalyst or WCI on
six-months' notice.
Operating Agreement
Cellerate,
LLC’s Operating Agreement provides for the business and
affairs of Cellerate, LLC to be managed by a Board of Managers
consisting of two persons. Catalyst and WCI each has the right to
appoint one person to the Board of Managers who serve indefinite
terms until their resignation, removal or death. The Board of
Managers act by a vote of the Managers, but in the event of a
deadlocked vote, the vote of the Catalyst designated manager will
be controlling, except (i) in the case of a transaction with a
related party or affiliate (other than Cellerate, LLC) of the
Catalyst designee or (ii) Catalyst transfers any portion of its
ownership interest in Cellerate, LLC to a third party or more that
50% of Catalyst’s ownership is transferred to a third party.
The initial Board of Managers is Mr. Ron Nixon as the Catalyst
designee, and Mr. Michael Carmena as the WCI designee. The Board of
Managers manages the general operations of Cellerate, LLC, subject
however to a vote by members of Cellerate, LLC holding two-thirds
of the membership interests in Cellerate, LLC to approve major
actions of Cellerate, LLC.
The
Operating Agreement contains restrictions on transfer of ownership
interests with customary rights of first refusal, co-sale and
buy/sell provisions applicable to each owner.
30
Sublicense Agreement
Cellerate,
LLC has an exclusive sublicense to distribute CellerateRX®
Activated Collagen® products into the wound care and surgical
markets in the United States, Canada and Mexico. The wound care
market comprises the care for external wounds, including the
treatment of external, tunneled or undermined wounds. This would
include pressure ulcers (Stages I-IV), venous stasis ulcers,
diabetic ulcers, ulcers resulting from arterial insufficiency,
surgical wounds, traumatic wounds, first and second-degree burns,
superficial wounds, cuts, scrapes, skin tears, skin flaps and skin
grafts. The term of the sublicense extends through August 2028,
with automatic one-year renewals through December 31, 2049, subject
to termination at the end of any renewal term by either party on
six-months' notice. Cellerate, LLC pays specified royalties based
on Cellerate, LLC’s annual net sales of
CellerateRX.
The
foregoing summary of the Sublicense Agreement does not purport to
be complete and is qualified in its entirety by reference to the
Sublicense Agreement.
Professional Services Agreement
The
Company and Catalyst agreed to provide Cellerate, LLC with certain
professional services needed to conduct the affairs of Cellerate,
LLC through December 31, 2019. The Company and Catalyst were
reimbursed on a monthly basis by Cellerate, LLC for services
performed, consistent with historical costs to provide the
services. The Company also received reimbursement for office lease
and other overhead costs dedicated to supporting Cellerate,
LLC’s activities. These reimbursements from Cellerate, LLC
were recognized by the Company as reductions of selling, general
and administrative expenses.
Promissory Note
As part
of the transaction to form Cellerate, LLC, the Company issued a
30-month convertible promissory note to Catalyst in the principal
amount of $1,500,000, bearing interest at a 5% annual interest
rate, compounded quarterly. Interest is payable quarterly, but may
be deferred at the Company’s election to the maturity of the
Note. Outstanding principal and interest are convertible at
Catalyst’s option into shares of Sanara common stock at a
conversion price of $9.00 per share. On February 7, 2020, Catalyst
converted 100% of the promissory note (including accrued but unpaid
interest) into 179,101 shares of common stock.
NOTE 5 – NOTES PAYABLE
CONVERTIBLE NOTES PAYABLE – RELATED PARTIES
In
August 27, 2018, as part of the partnership transaction with
Catalyst to form Cellerate, LLC, the Company issued a 30-month
unsecured promissory note to Catalyst in the principal amount of
$1,500,000, bearing interest at a 5% annual interest rate,
compounded quarterly. Interest is payable quarterly but may be
deferred at the Company’s election to the maturity of the
Note. Outstanding principal and interest are convertible at
Catalyst’s option into shares of Sanara MedTech common stock
at a conversion price of $9.00 per share.
The
table below summarizes amounts due to related parties, including
accrued interest separately recorded, as of December 31, 2019 and
2018:
|
|
|
Principal Amount
|
Accrued Interest
|
||
|
Note Payable
|
Terms of the agreement
|
2019 Successor
|
2018 Successor
|
2019 Successor
|
2018 Successor
|
|
|
|
|
|
|
|
|
August 27, 2018
Promissory Note
|
A $1,500,000 note
payable (i) interest accrues at 5% per annum and compounds
quarterly (ii) original maturity date of March 1, 2021
|
$1,500,000
|
$-
|
$103,557
|
$-
|
|
|
|
|
|
|
|
|
Total
|
|
$1,500,000
|
$-
|
$103,557
|
$-
|
|
|
|
|
|
|
|
31
NOTE 6 – INTANGIBLE ASSETS
The
carrying values of the Company’s finite-lived intangible
assets are as follows:
|
|
Successor
|
Successor
|
||||
|
|
As of December 31, 2019
|
As of December 31, 2018
|
||||
|
|
|
Accumulated
|
|
|
Accumulated
|
|
|
|
Cost
|
Amortization
|
Net
|
Cost
|
Amortization
|
Net
|
|
|
|
|
|
|
|
|
|
Product
Licenses
|
$1,500,000
|
$(48,876)
|
$1,451,124
|
-
|
-
|
-
|
|
Patent
|
510,310
|
(510,310)
|
-
|
$-
|
$-
|
$-
|
|
Software
& Other
|
64,464
|
(44,394)
|
20,070
|
-
|
-
|
-
|
|
Total
|
$2,074,774
|
$(603,580)
|
$1,471,194
|
$-
|
$-
|
$-
|
Cash
expenditures for intangible assets acquired in 2019 totaled
$1,522,485. This included product licenses of $1,000,000 for
BIAKŌS™ Antimicrobial Wound Gel and Antimicrobial Skin
and Wound Cleanser, and $500,000 for BIAKŌS™
Antimicrobial Barrier Film and Curashield™ No Sting Skin
Protectant. The Company amortizes its intangible assets on a
straight-line basis over the useful life of the respective assets.
Product licenses are amortized over the lives of the related
patents. The BIAKŌS™ patents expire in December 2031 and
October 2033. Other expenditures in 2019 for intangible assets
included $22,485 for website development and computer software.
Intangible assets also include a patent acquired in 2009 with a
historical cost of $510,310. The patent was amortized over its
estimated useful life of 10 years using the straight-line method.
Amortization expense related to intangible assets was $90,011, $0
and $43,349 for the year ended December 31, 2019 (Successor), and
for the periods August 28, 2018 through September 30, 2018
(Successor) and January 1, 2018 through August 27, 2018
(Predecessor), respectively.
The
following table outlines the estimated amortization expense related
to intangible assets held at December 31, 2019:
|
Year Ending December 31,
|
Amount
|
|
|
|
|
2020
|
$122,998
|
|
2021
|
122,998
|
|
2022
|
120,583
|
|
2023
|
115,503
|
|
2024
|
115,503
|
|
Thereafter
|
873,609
|
|
Total
|
$1,471,194
|
NOTE 7 – CUSTOMERS AND SUPPLIERS
The
Company had one customer which accounted for approximately 10%
Company’s sales in 2019, and one customer that accounted for
10% of the outstanding accounts receivable at the end of 2019. The
Company had one customer that accounted for 12% of the outstanding
accounts receivable at the end of 2018. The loss of the sales or
collections generated by these customers could have a significant
effect on the operations of the Company.
The
Company purchases all raw materials inventory for its principal
product from one vendor. If this vendor became unable to provide
materials in a timely manner and the Company was unable to find
alternative vendors, the Company's business, operating results and
financial condition would be materially adversely
affected.
NOTE 8 - COMMITMENTS AND CONTINGENCIES
LICENSE AGREEMENTS AND ROYALTIES
CellerateRX® Activated Collagen®
The
Company has an exclusive sublicense to distribute CellerateRX®
Activated Collagen® products into the wound care and surgical
markets in the United States, Canada and Mexico. The Company pays
specified royalties to Applied Nutritionals, LLC based on annual
net sales of CellerateRX. The term of the sublicense extends
through August 2028, with automatic one-year renewals through
December 31, 2049, subject to termination at the end of any renewal
term by either party on six months' notice. The Company pays
royalties based on its annual net sales of CellerateRX consisting
of 3% of all collected net sales each year up to $12,000,000, 4% of
all collected net sales each year that exceed $12,000,000 up to
$20,000,000, and 5% of all collected net sales each year that
exceed $20,000,000. Minimum royalties of $400,000 per year are
payable for the first five years of the sublicense
agreement.
32
BIAKŌS™ Antimicrobial Barrier Film and
Curashield™ No Sting Skin
Protectant
On
October 1, 2019, the Company executed an additional license
agreement with Rochal Industries, LLC (“Rochal”)
whereby the Company acquired an exclusive world-wide license to
market, sell and further develop certain antimicrobial barrier film
and skin protectant products for use in the human health care
market utilizing certain Rochal patents and pending patent
applications (the “ABF License Agreement”). Currently,
the products covered by the ABF License Agreement are
BIAKŌS™ Antimicrobial Barrier Film and
Curashield™ No Sting Skin
Protectant. The Executive Chairman of the Company is also a
director of Rochal, and indirectly a significant shareholder of
Rochal, and through the potential exercise of warrants a majority
shareholder of Rochal. Another Company director is also a director
and significant shareholder of Rochal.
Key
terms of the ABF License Agreement include:
1.
In consideration
for the license, the Company paid Rochal $500,000 in October
2019.
2.
Subject to the
occurrence of specified Company financing conditions in 2020,
Sanara will also pay Rochal $500,000, which at Rochal’s
option may be in cash or the Company’s Common Stock; or a
combination of cash and Sanara Common Stock.
3.
The
Company will pay Rochal a royalty of:
a.
4% of net sales of
licensed products in countries in which patents are
registered
b.
2% of net sales of
licensed products in countries without patent
protection.
The
minimum annual royalty due to Rochal will be $50,000 beginning with
the first full calendar year following the year in which first
commercial sales of the products occur (the “First Revenue
Year”). The annual minimum royalty will increase by 10% each
subsequent calendar year up to a maximum amount of
$75,000.
4.
Beginning with the
First Revenue Year, the Company will pay an additional royalty
based on specific net profit targets related to the licensed
products. Net profits for the licensed products are defined as net
sales, less cost of goods sold (including royalties) and direct
marketing and selling expenses. The additional royalty will be 25%
of the amount of actual net profits in excess of the established
net profit targets, subject to a maximum of $500,000 for any
calendar year. The established net profit targets for each calendar
year are:
a.
First
Revenue Year - $1,500,000
b.
Second
revenue year - 2021 - $5,000,000
c.
Third
revenue year - $8,000,000
d.
Fourth revenue year -
$10,000,000
e.
Fifth
revenue year - $15,000,000
f.
Beginning with the
sixth revenue year and for each calendar year thereafter, net
profit targets will be equal to the immediately preceding calendar
year’s net profit target incremented by the greater of (1)
50% of the U.S. dollar growth in the amount of net profit in the
current year over net profit in the immediately preceding calendar
year, or (2) the percentage of overall growth of the market for the
category by which the licensed products are generally
described.
Unless
previously terminated or extended by the parties, the ABF License
Agreement will terminate upon expiration of the last U.S. patent in
October 2033.
The
foregoing summary of the ABF License Agreement does not purport to
be complete and is qualified in its entirety by reference to the
ABF License Agreement. The Company filed a copy of the ABF License
Agreement as an exhibit to its Quarterly Report on Form 10-Q filed
on November 14, 2019.
33
BIAKŌS™ Antimicrobial Wound Gel and BIAKŌS™
Antimicrobial Skin and Wound Cleanser
On July
8, 2019, the Company executed a license agreement with Rochal
Industries, LLC (“Rochal”) whereby the Company acquired
an exclusive world-wide license to market, sell and further develop
antimicrobial products for the prevention and treatment of microbes
on the human body utilizing certain Rochal patents and pending
patent applications (the “License Agreement”).
Currently, the products covered by the License Agreement are
BIAKŌS™ Antimicrobial Wound Gel, and FDA cleared
BIAKŌS™ Antimicrobial Skin and Wound Cleanser. The
Executive Chairman of the Company is also a director of Rochal, and
indirectly a significant shareholder of Rochal, and through the
potential exercise of warrants a majority shareholder of Rochal.
Another Company director is also a director and significant
shareholder of Rochal.
Key
terms of the License Agreement include:
1.
In consideration
for the license, the Company paid to Rochal $1,000,000 and agreed
to pay an additional $500,000 upon FDA clearance of the
BIAKŌS™ Antimicrobial Wound Gel product for sale within
the United States.
2.
The Company will
pay Rochal a royalty of:
a.
4% of net sales of
licensed products in countries in which patents are
registered
b.
2% of net sales of
licensed products in countries without patent
protection.
The minimum annual
royalty due to Rochal will be $100,000 beginning with calendar year
2020. The annual minimum royalty will increase by 10% each
subsequent calendar year up to a maximum amount of
$150,000.
3.
Beginning with the
2020 calendar year, the Company will pay an additional royalty
based on specific net profit targets related to the licensed
products. Net profits for the licensed products are defined as net
sales, less cost of goods sold (including royalties) and direct
marketing and selling expenses. The additional royalty will be 25%
of the amount of actual net profits in excess of the established
net profit targets, subject to a maximum of $1,000,000 for any
calendar year. The established net profit targets for each calendar
year are:
a.
2020 -
$1,500,000
b.
2021 -
$5,000,000
c.
2022 -
$8,000,000
d.
2023 -
$10,000,000
e.
2024 -
$15,000,000
f.
Beginning in 2025
and for each calendar year thereafter, net profit targets will be
equal to the immediately preceding calendar year’s net profit
target incremented by the greater of (1) 50% of the U.S. dollar
growth in the amount of net profit in the current year over net
profit in the immediately preceding calendar year, or (2) the
percentage of overall growth of the market for the category by
which the licensed products are generally described.
Unless
previously terminated by the parties, the License Agreement will
expire with the related patents in December 2031.
The
foregoing summary of the License Agreement does not purport to be
complete and is qualified in its entirety by reference to the
License Agreement. The Company filed a copy of the License
Agreement as an exhibit to its Quarterly Report on Form 10-Q filed
on August 14, 2019.
Resorbable Bone Hemostat
The
Company acquired a patent in 2009 for a resorbable bone hemostat
and delivery system for orthopedic bone void fillers. This patent is not part of the Company’s
long-term strategic focus. The Company subsequently licensed
the patent to a third party to market a bone void filler product
for which the Company receives a 3% royalty on product sales over
the life of the patent, which expires in 2023 with annual minimum
royalties of $201,000. The Company pays two unrelated third parties
a combined royalty equal to eight percent (8%) of the
Company’s net revenues or minimum royalties generated from
products that utilize the Company's acquired patented bone hemostat
and delivery system. To date,
royalties received by the Company related to this licensing
agreement have not exceeded the annual minimum of $201,000 ($50,250
per quarter). Therefore, the Company’s annual royalty
obligation under the terms of the license agreement has been
$16,080 ($4,020 per quarter).
OPERATING LEASES
The Company has two operating leases: an office space lease with a
remaining lease term of 54 months and a copier lease with a remaining lease
term of 19 months as of
December 31, 2019. In accordance with
the transition guidance of ASC 842, such arrangements are included
in our balance sheet as of January 1, 2019. All other leases are
short-term leases for which out of practical expediency the Company
has elected to not recognize lease assets and lease
liabilities.
34
In
March 2017, and as amended in March 2018, the Company executed a
new office lease effective April 1, 2019 for office space located
at 1200 Summit Ave., Suite 414, Fort Worth, TX 76102. On July 1,
2019, the Company amended the office lease agreement which became
effective on August 22, 2019 upon completion by landlord of certain
leasehold improvements (the “Commencement Date). Under the
terms of the amended lease agreement, the Company leased an
additional 1,682 rentable square feet of office space which brought
the total square footage leased to 5,877. The amended lease
agreement extends the original term of the lease for a period of 36
months through June 30, 2024. Upon the Commencement Date of the
amended lease, the monthly base rental payments are as
follows:

As the implicit rate in the leases is not determinable, the
discount rate applied to determine the present value of lease
payments is the Company’s incremental borrowing rate of
6.25%. The office space lease agreement contains no renewal terms,
so no lease liability is recorded beyond the termination date. The
copier lease can be automatically renewed but no lease liability is
recorded beyond the initial termination date as exercising this
option is not reasonably certain.
In accordance with the transition guidance of ASC 842, such
arrangements are included in our balance sheet as of January 1,
2019. All other leases are short-term leases for which out of
practical expediency the Company has elected to not recognize lease
assets and lease liabilities. As a result of the adoption of
ASC 842, the Company has recorded lease assets of $585,251 and a
related lease liability of $598,917 as of December 31, 2019. Cash
paid for amounts included in measurement of operating lease
liabilities as of December 31, 2019 was $95,530
OTHER COMMITMENTS
At the
time of the formation of Sanara Pulsar, it and WCS entered into a
supply agreement whereby Sanara Pulsar became the exclusive
distributor in the United States of certain wound care products
that utilize intellectual property developed and owned by WCS. In
2019, the Company advanced to WCS $200,000 and recorded the payment
as a reduction of non-controlling interests. All distributions made
by Sanara Pulsar to its members, not including tax distributions,
will be made exclusively to Cellerate, LLC until such time as
Cellerate, LLC has received an amount of distributions equal to
such advances. In the event WCS’s Form K-l from Sanara Pulsar
for the year 2020 does not allocate to WCS net income of at least
$200,000 ("Target Net Income"), then Cellerate. LLC will, within 30
days after such determination, pay WCS the amount of funds
representing the difference between Target Net Income and the
actual amount of net income shown on WCS’s Form K-1 for the
year 2020. For the years 2021 through 2024 Target Net Income will
increase by 10% each year and in the event WCS’s Form K-1 for
any of those years does not allocate to WCS net income in an amount
at least equal to Target Net Income for such year, then Cellerate,
LLC will, within 30 days after such determination, pay WCS the
amount of funds representing the difference between Target Net
Income and the actual amount of net income shown on WCS’s
Form K-1 for the applicable year.
NOTE 9 – STOCKHOLDERS’ EQUITY
PREFERRED STOCK
On
October 11, 2013, the Company filed a Certificate of Designations,
Number, Voting Power, Preferences and Rights of Series C
Convertible Preferred Stock, under which it designated 100,000
shares of Series C Preferred Stock, par value $10.00. The Series C
Preferred Stock is entitled to accruing dividends (payable, at the
Company’s options, in either cash or stock) of 5% per annum
until October 10, 2016, and 3% per annum until October 10, 2018.
The Series C Preferred Stock was senior to the Company’s
common stock and any other then issued series of the
Company’s preferred stock upon liquidation and entitled to a
liquidation preference per share equal to the original issuance
price of such shares of Series C Preferred Stock together with the
amount of all accrued but unpaid dividends thereon. Each of the
Series C Shares was convertible at the option of the holder into 10
shares of common stock as provided in the Certificate.
Additionally, each holder of Series C Preferred Stock was entitled
to vote on all matters submitted for a vote of the holders of
Common Stock a number of votes equal to the number of full shares
of Common Stock into which such holder’s Series C shares
could then be converted.
35
During
February and March 2018, the Company issued 1,005,677 shares of
Common Stock for the conversion of 85,561 shares of Series C
Convertible Preferred Stock and $1,050,468 of related Series C
dividends. Dividends were converted at $7.00 per share. The Series
C preferred stock earned dividends of $0 and $28,061 for the years
ended December 31, 2019 and December 31, 2018, respectively. As an
inducement to encourage the Series C Preferred Stock shareholders
to convert their Series C Preferred Stock to Common Stock prior to
October 10, 2018, the Company offered to apply the full dividend,
(accelerated to October 10, 2018) upon the shareholders exercise of
their conversion. The fair value of the extra shares of Common
Stock issued to Series C Stock shareholders was $103,197 for
dividends that would have accrued from the date of their conversion
through October 10, 2018. There were no Series C Shares issued or
outstanding as of December 31, 2019 and 2018, and all accrued
dividends were converted to Common Stock.
On
March 13, 2019, the Company established a new series of preferred
stock consisting of 1,200,000 shares of Series F Convertible
Preferred Stock, par value of $10.00 per share. After
proportionally adjusting to reflect a subsequent 1 for 100 reverse
stock split of the Common Stock, each share of Series F Convertible
Preferred Stock is convertible at the option of the holder, at any
time, into 2 shares of Common Stock. Additionally, each holder of
Series F Convertible Preferred Stock is entitled to vote on all
matters submitted for a vote of the Company’s shareholders
with votes equal to the number of shares of Common Stock into which
such holder’s Series F Preferred shares could then be
converted. The Series F Convertible Preferred Stock is senior to
the Company’s Common Stock as to the payment of dividends (if
any) and the distribution of assets. Upon liquidation of the
Company, holders of Series F Convertible Preferred Stock are
entitled to a liquidation preference of $5 per share. As of
December 31, 2019, there were 1,136,815 shares of the Series F
Preferred stock issued and outstanding.
The
Company evaluated the Series F Preferred Stock under FASB ASC 815
and determined that they do not qualify as derivative liabilities.
The Company then evaluated the Series F Preferred Stock for
beneficial conversion features under FASB ASC 470-30 and determined
that none existed.
COMMON STOCK
On
March 6, 2018, the Company issued 226,514 shares of Common Stock
for the conversion of $1,200,000 in convertible debt held by
related parties and $385,594 in accrued interest. In February and
March 2018, the Company issued 1,005,677 shares of Common Stock for
the conversion of 85,561 shares of Series C Convertible Preferred
Stock and $1,050,468 of related Series C Preferred Stock
dividends.
On May
10, 2019 the Company effected a 1-for-100 reverse stock split of
the Company’s issued and outstanding shares of Common Stock.
Concurrent with the reverse stock split, the Company changed its
corporate name from Wound Management Technologies, Inc. to Sanara
MedTech Inc.
The
reverse stock split was previously approved by a majority of
shareholders of the Company’s outstanding voting stock on
March 21, 2019. On May 10, 2019, the Company’s Common Stock
began trading on the OTCQB market under the symbol
“WNDMD” and traded under that symbol until June 6,
2019, at which time the Company changed its trading symbol to
“SMTI”. The post-split Common Stock is traded under a
new CUSIP number 79957L100. In connection with the reverse stock
split, the Company also made a corresponding adjustment to the
Company’s authorized capital stock to reduce the authorized
Common Stock to 20,000,000 shares and the authorized preferred
stock to 2,000,000 shares, effective May 10, 2019.
The
reverse stock split did not change a shareholder’s ownership
percentage of the Company's Common Stock, except for the small
effect where the reverse stock split would result in a shareholder
owning a fractional share. No fractional shares were issued as a
result of the reverse split. Shareholders who were otherwise
entitled to receive a fractional share received a cash payment
based on the market price of a share of the common stock on May 13,
2019.
On
October 15, 2019, Sanara MedTech Inc. (the “Company”)
closed a private placement offering of 1,204,820 shares of its
common stock at a price of $8.30 per share. All shares sold by the
Company were newly issued shares. The purchasers in the offering
were related party entities to three members of the Company’s
Board of Directors. See Note
4 for more information regarding the private placement
transaction.
WARRANTS
There
were no warrants outstanding at December 31, 2019 and December 31,
2018.
36
STOCK OPTIONS
On
December 31, 2017, the Company granted a total of 11,500 options to
five employees. The aggregate fair value of the awards was
determined to be $61,322 and was to be expensed over a three-year
vesting period. On April 13, 2018, the Company granted a total of
2,000 options to one employee and one contractor. The aggregate
fair value of the awards was determined to be $8,943 and was to be
expensed over a three-year vesting period.
The
Company’s stock option agreements include a provision whereby
all outstanding options vest immediately if the Company consummates
a transaction resulting in a change in control of the Company, as
defined in the stock option agreements. The Cellerate Acquisition
on March 15, 2019 (see Note 1 for more information) represented a
change in control of the Company for purposes of the stock option
agreements. Accordingly, all outstanding stock options fully vested
on March 15, 2019. No option expense is reflected in the
consolidated statements of operations in 2019.
The
following tables summarize the outstanding options as of December
31, 2019 and December 31, 2018 for Successor:
|
|
Successor
|
Successor
|
||||
|
|
As of December 31, 2019
|
As of December 31, 2018
|
||||
|
|
|
|
Weighted Average
|
Options
|
Weighted Average
|
Weighted Average
|
|
|
|
Exercise Price
|
Remaining Contract Life
|
Exercise Price
|
Remaining Contract Life
|
|
|
|
||||||
|
Outstanding
at beginning of period
|
13,500
|
$6.00
|
|
-
|
$-
|
|
|
Granted
|
-
|
-
|
|
-
|
-
|
|
|
Exercised
|
-
|
-
|
|
-
|
-
|
|
|
Forfeited
|
(2,000)
|
$6.00
|
|
-
|
$-
|
|
|
Expired
|
-
|
-
|
|
-
|
-
|
|
|
Outstanding
at End of Period
|
11,500
|
$6.00
|
3.14
|
-
|
$-
|
-
|
|
|
|
|
|
|
|
|
|
Exercisable
at End of Period
|
11,500
|
$6.00
|
3.14
|
-
|
$-
|
-
|
NOTE 10 – INCOME TAXES
The
Company accounts for income taxes in accordance with ASC Topic No.
740, “Income Taxes.” This standard requires the Company
to provide a net deferred tax asset or liability equal to the
expected future tax benefit or expense of temporary reporting
differences between book and tax accounting and any available
operating loss or tax credit carry forwards.
After
applying the provisions of Section 382 of the Internal Revenue
Code, the unexpired net operating loss carry forward at December
31, 2019 is approximately $8,934,000, with a portion expiring every
year from 2020 through the 2038 tax year if not used.
As a limited liability company in 2018, the Successor (Cellerate,
LLC) was taxed as a partnership for federal income tax purposes and
therefore had no federal tax asset or liability as of December 31,
2018. The non-current deferred tax asset is summarized
below:
|
|
2019 Successor
|
2018 Successor
|
|
Net
operating loss carry forwards, (21% as of December 31,
2019)
|
$1,876,114
|
$-
|
|
Valuation
allowance
|
(1,876,114)
|
-
|
|
Net
non-current deferred tax asset
|
$-
|
$-
|
A 100%
valuation allowance has been provided for all deferred tax assets,
as the ability of the Company to generate sufficient taxable income
in the future is uncertain.
37
Reconciliations
of the expected federal income tax benefit based on the statutory
income tax rate of 21% to the actual benefit for the years ended
December 31, 2019 and 2018 are listed below. As a limited liability company in 2018, the
Successor (Cellerate, LLC) was taxed as a partnership, therefore,
no reconciliation of federal income tax benefit to actual benefit
is presented for 2018.
|
|
2019 Successor
|
2018 Successor
|
|
|
|
|
|
Expected
federal income tax benefit
|
$605,767
|
$-
|
|
Goodwill
amortization
|
65,957
|
-
|
|
Change
in valuation allowance
|
(294,050)
|
-
|
|
NOL
carryover reduced by expiration
|
(302,134)
|
-
|
|
Pass
through entity income allocation
|
(94,151)
|
-
|
|
Reserve
for bad debt
|
(29,741)
|
-
|
|
Stock-based
compensation
|
48,352
|
-
|
|
Income
tax expense (benefit)
|
$-
|
$-
|
All tax
years starting with 2016 are open for examination.
NOTE 11 – DEBT AND CREDIT FACILITIES
In
December 2018, Cellerate, LLC executed agreements with Cadence
Bank, N.A. (“Cadence”) which provided Cellerate, LLC
access to a revolving line of credit up to a maximum principal
amount of $1,000,000. The line of credit supports short-term
working capital requirements of Cellerate, LLC. The line of credit
is secured by substantially all of the assets of Cellerate, LLC.
The interest rate per annum under this loan is the “Prime
Rate” as it varies from time to time and designated in the
“Money Rates” section of the Wall Street Journal plus
0.75%.
On June
21, 2019, the Company modified the Cadence revolving line of credit
to increase the maximum principal amount from $1,000,000 to
$2,500,000. Most terms of the modification agreement, including
security and interest rate, were unchanged from the original loan
agreement. Significant changes under the terms of the modification
agreement include extending the maturity date from December 16,
2019 to June 19, 2020, and the addition of a financial covenant
requiring the Company to sell additional equity securities in an
amount of at least $5,000,000 no later than December 31,
2019.
The
total outstanding line of credit balance was $2,200,000 at October
15, 2019. On October 16, 2019, the Company paid down the entire
balance of the revolving line of credit with cash proceeds received
through a private placement stock offering. See Note 4 for more
information regarding the private placement offering.
NOTE 12 - PAYABLES TO RELATED PARTIES
As of
December 31, 2019, and 2018, the Company had outstanding payables
to related parties totaling $68,668 and $36,203, respectively. The
payables are unsecured, bear no interest and are due on
demand.
NOTE 13 -- SUBSEQUENT EVENTS
In
accordance with applicable accounting standards for the disclosure
of events that occur after the balance sheet date but before the
financial statements are issued, all significant events or
transactions that occurred after December 31, 2019, are outlined
below:
On
February 7, 2020, The Catalyst Group, Inc., through its affiliates
(collectively, “Catalyst”), converted its entire
holdings of Sanara MedTech Inc.’s 30-month $1,500,000
convertible promissory note and Series F Convertible Preferred
Stock into shares of Sanara Common Stock. The Company issued an
aggregate of 2,452,731 shares of Common Stock in the conversions.
After the conversions, Catalyst controls the voting of a total of
3,416,587 shares of Common Stock, which represents 56.7% of the
6,023,732 shares of Common Stock currently
outstanding.
Catalyst’s
managing partner, Ronald Nixon who is executive chairman of the
Company’s Board of Directors, is the only Catalyst
appointment to the Company’s Board. Catalyst has informed the
Company that it does not presently intend to change the composition
of the Board other than in the course of adding additional
value-added Board members, and notes that the current direction of
the Company was developed by the existing management and Board of
Directors. The Company understands that Catalyst believes that its
objectives for the Company are in line with the plans and future
direction for the Company being put in place by the Company’s
management.
38
On
February 21, 2020, the Company filed a Form S-8 registration
statement which registered an aggregate of 2,000,000 shares of
common stock, par value $0.001 per share (the “Common
Stock”), that may be issued under the Sanara MedTech Inc.
2014 Omnibus Long-Term Incentive Plan. Pursuant to Rule 416 of the
Securities Act of 1933, as amended, this registration statement
also covers such additional and indeterminate number of securities
as may become issuable pursuant to the provisions of the plan
relating to adjustments for changes resulting from a share
dividend, share split or similar change.
ITEM 9. CHANGES IN AND DISAGREEMENTS
WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
None.
ITEM 9A. CONTROLS AND
PROCEDURES
Evaluation of Disclosure Controls and Procedures
In
accordance with Exchange Act Rules 13a-15(e), we carried out
an evaluation, under the supervision and with the participation of
management, including our Principal Executive Officer and Chief
Financial Officer, of the effectiveness of our disclosure controls
and procedures as of the end of the period covered by this report.
Based on the evaluation, our Principal
Executive Officer and Chief Financial Officer concluded that, due
to the small size of the Company and limited segregation of duties,
our disclosure controls and procedures were not effective as of
December 31, 2019.
Management’s Report on Internal Control over Financial
Reporting
Management
is responsible for establishing and maintaining adequate internal
control over financial reporting, as defined in Rules 13a-15(f) and
15d-15(f) under the Exchange Act. Our internal control over
financial reporting is designed to provide reasonable assurance
regarding the reliability of financial reporting and the
preparation of the consolidated financial statements for external
reporting purposes in accordance with generally accepted accounting
principles.
Because
of its inherent limitations, internal control over financial
reporting may not prevent or detect misstatements. Also,
projections of any evaluation of effectiveness of internal control
over financial reporting to future periods are subject to the risk
that controls may become inadequate because of changes in
conditions or that the degree of compliance with the policies or
procedures may deteriorate over time.
Management believes that our policies and procedures provide
reasonable assurance that our operations are conducted with a high
standard of business ethics. In management's opinion, our financial
statements present fairly, in all material respects, our financial
position, results of operations, and cash flows. Management
recognizes that any controls and procedures, no matter how well
designed and operated, can provide only reasonable assurance of
achieving their objectives. Management applies its judgment in
evaluating the cost-benefit relationship of possible controls and
procedures.
The
Company’s management, specifically its Principal Executive
Officer and Chief Financial Officer, has assessed the effectiveness
of the Company’s internal control over financial reporting as
of December 31, 2019 using the criteria set forth by the Committee
of Sponsoring Organizations of the Treadway Commission (COSO) in
Internal Control – Integrated Framework (2013) and SEC
guidance on conducting such assessments. Based on this assessment, management has
concluded that as of December 31, 2019, internal control over
financial reporting was not effective due to the small size of the
Company and limited segregation of duties. Management is currently
evaluating the steps that would be necessary to eliminate this
material weakness.
No Attestation Report of Registered Public Accounting
Firm
This
annual report does not include an attestation report of our
registered public accounting firm regarding internal control over
financial reporting. Management’s report was not subject to
attestation by our registered public accounting firm pursuant to
the rules of the Securities and Exchange Commission that permit us
to provide only management’s report in this annual
report.
ITEM 9B. OTHER
INFORMATION
On
March 10, 2017, the Company and John Siedhoff, the chairman of the
Company’s Board of Directors, entered into an amendment to
the Consulting Agreement, dated April 25, 2016, by and between the
Company and Mr. Siedhoff (the “Amendment”). The
Amendment: (i) changes the name of the consultant under the
Consulting Agreement from John Siedhoff to Twin Oaks Equities, LLC
(an entity controlled by Mr. Siedhoff), and (ii) increases the
monthly compensation payable under the Consulting Agreement from
$15,000 to $20,000, effective as of January 1, 2017. On January 31,
2019 the existing Consulting Agreement was terminated and the
Company re-engaged Mr. Siedhoff to provide certain consulting
services under a new agreement. The new agreement: (i) commences on
February 1, 2019 and continues until December 31, 2020, (ii)
compensation for 2019 is $20,000 per month plus cash reimbursement
of health insurance premium of $1,947 (iii) compensation for 2020
is $10,000 per month.
39
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS
AND CORPORATE GOVERNANCE
Board of Directors
The
following table sets forth the names, ages, and positions of the
current directors of the Company.
|
NAME
|
|
AGE
|
|
POSITION
|
|
YEAR FIRST ELECTED
|
|
Ronald
T. Nixon
|
|
64
|
|
Executive
Chairman
|
|
2019
|
|
James
W. Stuckert
|
|
82
|
|
Director
|
|
2015
|
|
S. Oden
“Denny” Howell Jr.
|
|
80
|
|
Director
|
|
2015
|
|
J.
Michael Carmena
|
|
64
|
|
Vice
Chairman
|
|
2019
|
|
Ann
Beal Salamone
|
|
69
|
|
Director
|
|
2019
|
|
Kenneth
E. Thorpe
|
|
63
|
|
Director
|
|
2019
|
Ronald T.
Nixon, age 64, has been a
director of the Company since March 2019 and has served as
Executive Chairman of the Board since May 2019. As Executive
Chairman, he has been involved in strategy planning, execution and
identifying prospective partnerships and acquisitions opportunities
for the Company. Mr. Nixon is the Founder and has served as
Managing Partner of The Catalyst Group, Inc. since 1990. Mr. Nixon
serves on the board of directors of LHC Group, Inc. as well as a
number of private companies, including Trilliant Surgical, LLC,
Rochal Industries, LLC, and Triad Life Sciences, Inc. Mr. Nixon
holds a Bachelor’s degree in Mechanical Engineering from the
University of Texas at Austin and is a registered professional
engineer in Texas.
James W.
Stuckert, age 82, has been a director of the Company since
September 2015. Mr. Stuckert has been retired since 2016. Mr.
Stuckert served as Chairman and Chief Executive Officer of
J.J.B. Hilliard, W.L. Lyons, LLC from
December 1995 until December 2003, prior to which he served in
executive and broker positions from 1963. J.J.B. Hilliard, W.L.
Lyons, LLC is a full-service financial asset management firm
headquartered in Louisville, Kentucky. Mr. Stuckert was an initial
investor and served 24 years on the board of directors of Royal
Gold, Inc. He previously has served as chairman of SenBanc Fund; a
director of DataBeam, Inc.; a board member of the Securities
Industry Association and chairman of its regional firms committee;
and a past member of the nominating committee of the New York Stock
Exchange. Mr. Stuckert has served as a member of the board of
trustees of the University of Kentucky and as chairman of its
Finance Committee and as chairman of its Presidential Search
Committee. He has also served as chairman of a local
hospital’s investment committee. Mr. Stuckert earned a
Bachelor’s degree in Mechanical Engineering and a Master of
Business Administration degree from the University of
Kentucky.
S. Oden
“Denny” Howell, Jr., age 80, has been a director of the
Company since September 2015. From
1972 to 2020 he served as president of Howell & Howell
Contractors, Inc., a renovation and industrial/commercial painting
contractor. Mr. Howell has been a private investor since 1975 with an emphasis in
pharmaceutical and medical device companies. He has previously
served as a director of a pharmaceutical company and as a member of
board of trustees of Lindsey Wilson College in Columbia,
Kentucky.
Ann Beal
Salamone, M.S., age 69,
has been a director of the Company since August 2019. Ms. Salamone
is a co-founder of Rochal and has
served as its chairman since September 2019, prior to which she
served as its president from 1986 to September 2019. She is one of
the principal inventors of Rochal’s liquid bandages,
antimicrobial compositions and skin regeneration products for burn
and wound treatment, and she has participated in the development of
products for electronics, water purification, personal care and
healthcare. Ms. Salamone has co-founded six companies and invested
in and served on the board of directors of several private
entrepreneurial companies. Ms. Salamone is a member of the National
Academy of Engineering and The Academy of Medicine, Engineering
& Science of Texas.
40
Kenneth E.
Thorpe, Ph.D., age 63,
has been a director of the Company since August 2019. He has been
the Robert W. Woodruff Professor and
Chair of the Department of Health Policy & Management of the
Rollins School of Public Health of Emory University in Atlanta,
Georgia since 1999. From 1983 to 1999 he held faculty positions in
the public health departments at Tulane University, the University
of North Carolina at Chapel Hill, Harvard University and Columbia
University. Since 2007 Dr. Thorpe has served as Chairman of the
Partnership to Fight Chronic Disease. He served on the Board of
Directors of LHC Group, Inc. in 2010; was a consultant in the
Governor’s Office and Legislature of West Virginia in 2011;
and was Co-Chair of the Partnership for the Future of Medicare in
2013. From 1993 to 1995, Dr. Thorpe served as Deputy Assistant
Secretary for Health Policy in the U.S. Department of Health and
Human Services where he coordinated all financial estimates and
program impacts of the Clinton administration’s healthcare
reform proposals. In 1991 he was awarded the Young Investigator
Award as the most promising health services researcher in the
country under age 40 by the Association for Health Services
Research. He has authored multiple articles and books on healthcare
financing, insurance and healthcare reform. Dr. Thorpe received his
Bachelor of Arts degree from the University of Michigan, Master of
Arts degree from Duke University, and Ph.D. from the Rand Graduate
School.
J. Michael
Carmena, age 64, has
served as Vice Chairman of the Board and Principal Executive
Officer of the Company since May 2019, and served as Chief Executive Officer from
February 2018 to May 2019. He served as Chief Financial Officer
from December 2016 to April 2018. Prior to joining the Company, Mr.
Carmena served as Senior Director, Business & Sales Operations
of Smith and Nephew plc (successor to Healthpoint Biotherapeutics)
from 2010 to 2013. He served as Senior Director, Finance &
Administration of Healthpoint Biotherapeutics from 2008 to 2010 and
as Controller from 1998 to 2008, prior to which he held senior
financial positions in a company engaged in oil and gas exploration
and production, ranching and financial asset management. Mr.
Carmena began his professional career in 1978 with Arthur Andersen
& Co. and became a CPA in 1981. Mr. Carmena earned a Bachelor
of Business Administration degree from Texas Christian
University.
Executive Officers
The
following table sets forth the names, ages and positions of the
executive officers of the Company.
|
NAME
|
|
AGE
|
|
POSITION
|
|
Zachary
B. Fleming
|
|
45
|
|
Co-Chief
Operating Officer and President, Surgical
|
|
Shawn
M. Bowman
|
|
44
|
|
Co-Chief
Operating Officer and President, Wound Care
|
|
Michael
D. McNeil
|
|
54
|
|
Chief
Financial Officer
|
|
J.
Michael Carmena
|
|
64
|
|
Principal
Executive Officer
|
Zachary B. Fleming
was appointed to the position of President, Surgical Division on
May 28, 2019, and was named Co-Chief Operating Officer on January
28, 2020. Mr. Fleming joined the Company as Vice President of Sales
in November 2017 and was promoted to Vice President, Surgical in
September 2018. Mr. Fleming will be responsible for the continued
expansion and management of the surgical sales force as well as new
product introductions. Mr. Fleming has spent over fourteen years in
the medical industry with Healthpoint Biotherapeutics, Smith &
Nephew and Sanara MedTech. Mr. Fleming earned a Bachelor of Science
from Indiana University.
Shawn M. Bowman, age
44, has served as President, Wound Care Division since May 2019,
and was named Co-Chief Operating Officer on January 28, 2020. Mr.
Bowman previously served as the Company’s Vice President and
General Manager, Wound Care since September 2018. Mr. Bowman will
be responsible for leading the strategic expansion of the
Company’s wound care division. Mr. Bowman has over eighteen
years of experience in the medical device, biologics and
pharmaceutical industries. Prior to joining Sanara MedTech, Mr.
Bowman built two successful teams as Senior Vice President of
Wellsense, and as a National Sales Director for Smith &
Nephew’s Advanced Wound Management Division. Mr. Bowman
earned a Bachelor of Science in Marketing from the University of
Connecticut.
Michael D. McNeil,
age 54, has served as Chief Financial Officer since April 2018.
Prior to joining the Company, Mr. McNeil served as Controller for
Smith and Nephew’s U.S. Advanced Wound Management Division
from 2012 to 2018. Mr. McNeil previously served as Controller and
Assistant Controller with Healthpoint Biotherapeutics from 1999 to
2012. Prior to his employment at Healthpoint, Mr. McNeil held
several finance and internal audit positions with Burlington
Resources, Snyder Oil Corporation, and Union Pacific Corporation.
Mr. McNeil earned his Bachelor of Science in Business
Administration from the University of Nebraska and is a Texas
certified public accountant.
J. Michael
Carmena has served as an
executive officer of the Company since December 2016 as described
above as a director of the Company.
Indebtedness of Directors and Executive Officers
None of
our directors or officers or their respective associates or
affiliates is indebted to us.
Family Relationships
There
are no family relationships among our directors or executive
officers.
41
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange Act of 1934, as amended
(the “Exchange
Act”) requires our
directors, executive officers, and persons who own more than 10% of
a registered class of our equity securities to file reports with
the SEC of ownership and changes in ownership of our common stock
and other equity securities of the Company. Based solely on a
review of the Section 16(a) forms filed with the SEC and the
representations made by the reporting persons to us, during the
fiscal year ended December 31, 2019, two of our directors, Kenneth
E. Thorpe and Ann Beal Salamone, and two officers, Zachary B.
Fleming and Shawn M. Bowman, each filed late a single form, the
Initial Statement of Beneficial Ownership, due to an oversight by
Company counsel. Family Alignment, LLC and Catalyst Group, Inc.
filed a late amendment to the Form 3 filing of FA Sanara, LLC in
order to become a group for filing purposes with FA Sanara, LLC due
to a delay in receiving EDGAR filing codes from the SEC. All
transactions of our directors and officers were timely
reported.
Meetings and Committees of the Board
Our
business is managed under the direction of the Board of Directors
(the “Board”). The Board meets on a regular basis, at
least quarterly, to review significant developments affecting the
Company and to act on matters requiring the approval of the Board.
In addition to regularly scheduled meetings, the Board also holds
special meetings when the Company faces a matter requiring
attention or action by the Board. The Board does not currently have
a standing audit, compensation, nominating or governance committee.
The entire Board currently performs the functions of each such
committee, participating in all relevant decisions
thereof.
Nominations
The
existing directors work to identify qualified candidates to serve
as nominees for director. When identifying director nominees, the
Board may consider, among other factors, the potential
nominee’s reputation, integrity, independence from the
Company, skills and business, government or other professional
acumen, bearing in mind the composition of the Board and the
current state of the Company and the industry generally. The Board
may also consider the number of other public companies for which
the person serves as director and the availability of the
person’s time and commitment to the Company. In the case of
current directors being considered for re-nomination, the Board
will also consider the director’s tenure as a member of the
Board, the director’s history of attendance at meetings of
the Board and the director’s preparation for and
participation in such meetings.
Shareholders
seeking to nominate director candidates may do so in compliance
with the Company’s Bylaws. Any recommendation must be in
writing to the Corporate Secretary of the Company and provide the
recommended candidate’s name, biographical data and
qualifications.
Following
identification of the need to add or re-elect a director to the
Board, and consideration of the above criteria and any shareholder
recommendations, the Board submits its recommended nominees to the
shareholders for election at a meeting of shareholders. The Board
utilizes this process, rather than a formal nominations committee,
because the directors have found that, for the Company the
functions of a nominations committee are more than adequately
fulfilled by this process.
Board Leadership Structure
The
Board believes its leadership structure is appropriate for the
Company given the size and scope of our business, the experience
and active involvement of our directors, and our corporate
governance practices, which include regular communication with and
interaction between and among the Executive Chairman, the Co-Chief
Operating Officers, the Principal Executive Officer, the Chief
Financial Officer, and the directors.
Risk Management
The
Board is responsible for overseeing the Company’s management
and operations. The Board serves in the role of an audit committee,
fulfilling its responsibilities for general oversight of the
integrity of the Company’s financial statements, the
Company’s compliance with legal and regulatory requirements,
the independent auditor’s qualifications and independence,
and risk assessment and management. We believe that the Board
provides effective oversight of risk management functions. On a
regular basis we perform a risk review wherein the management team
evaluates the risks we expect to face in the upcoming year and over
a longer-term horizon. Plans are then developed to address the
risks identified. In addition, members of our management team
periodically present to the Board the strategies, issues and plans
for the areas of our business for which they are responsible. While
the Board oversees risk management, our management team is
responsible for the Company’s day-to-day risk management
processes. Additionally, the Board requires that management raise
exceptional issues to the Board. We believe this division of
responsibilities is the most effective approach for addressing the
risks we face and that the Board leadership structure supports this
approach.
Meeting Attendance
During
the fiscal year ended December 31, 2019, there were sixteen board
meetings. During 2019, each director attended all board
meetings.
42
Code of Ethics
The
Company adopted a Code of Ethics applicable to all directors,
officers and employees. The Code of Ethics can be found on the
Company’s website at http://sanaramedtech.com under the
Investors Relations tab.
Shareholder Communications with the Board
Any
Company shareholder or other interested party who wishes to
communicate with the non-management directors as a group may direct
such communications by writing to the:
Corporate
Secretary
Sanara
MedTech Inc.
1200
Summit Avenue, Suite 414
Fort
Worth, TX 76102
The
communication must be clearly addressed to the Board of Directors
or to a specific director. If a response is desired, the individual
should also provide contact information such as name, address and
telephone number.
All
such communications will be reviewed initially by the Corporate
Secretary, who will forward to the appropriate director(s) all
correspondence, except for items of the following
nature:
●
advertising;
●
promotions of a
product or service;
●
patently offensive
material; and
●
matters
completely unrelated to the Board’s functions, Company
performance, Company policies or that could not reasonably be
expected to affect the Company’s public
perception.
The
Corporate Secretary will prepare a periodic summary report of all
such communications for the Board. Correspondence not forwarded to
the Board will be retained by the Company and will be made
available to any director upon such director’s
request.
ITEM 11. EXECUTIVE
COMPENSATION
The
following table and the accompanying notes provide summary
information for each of the last two fiscal years concerning cash
and non-cash compensation awarded to, earned by or paid to
executive officers (or those acting in a similar
capacity).
SUMMARY COMPENSATION TABLE
|
Name and
Principal Position
|
|
Year
|
Salary
|
Bonus
|
Stock
Awards
|
Option Awards
(a)
|
Non-equity
incentive compensation
|
Non-qualified
deferred compensation earnings
|
All other
compen-sation
|
Total
|
|
|
|
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Zachary B. Fleming, Co-Chief
Operating Officer and President, Surgical
|
|
2019
|
205,667
|
90,000
|
-
|
-
|
-
|
-
|
-
|
295,667
|
|
|
|
2018
|
161,452
|
68,000
|
-
|
-
|
-
|
-
|
-
|
229,452
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shawn M. Bowman, Co-Chief Operating
Officer and President, Wound Care
|
|
2019
|
205,667
|
80,000
|
-
|
-
|
-
|
-
|
-
|
285,667
|
|
|
|
2018
|
58,833
|
37,000
|
-
|
-
|
-
|
-
|
-
|
95,833
|
|
|
|
|
|
|
|
|
|
|
|
|
|
J. Michael Carmena, Principal
Executive Officer
|
|
2019
|
209,600
|
75,000
|
-
|
-
|
-
|
-
|
-
|
284,600
|
|
|
|
2018
|
207,107
|
60,000
|
-
|
-
|
-
|
-
|
-
|
267,107
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael D. McNeil, Chief Financial
Officer
|
|
2019
|
169,500
|
63,000
|
-
|
-
|
-
|
-
|
-
|
232,500
|
|
|
|
2018
|
123,625
|
45,300
|
-
|
4,472
|
-
|
-
|
-
|
173,197
|
43
Notes to Summary Compensation Table
(a)
The value of option
awards represents the grant date fair value of the stock options,
determined in accordance with FASB ASC Topic 718. The grant date
value was based on the closing price of the stock on the grant date
and determined under the Black Scholes valuation model. The
following assumptions on the date of grant were used in the grant
date fair value: (i) option exercise price equal to the fair market
value of the common stock; (ii) expected option life of 5 years;
(iii) dividend yield of 0%;(iv) risk free rate of return of 2.67%
for options granted in 2018; and (v) volatility of 145.77% for
options granted in 2018.
Employment Agreements
Effective
June 1, 2019, the Company entered into employment agreements with
two of its executive officers, Shawn M. Bowman and Zachary B.
Fleming. Each agreement provides for an initial two-year term, with
automatic one-year renewals unless either party gives prior notice
to the other party of its desire to terminate the agreement. Each
agreement provides for an initial base salary of $225,000, a
one-time bonus payment of $25,000, an annual bonus opportunity
equal to 50% of base salary, and an initial stock grant equal to
$112,500. The initial stock grant vests in one-third increments for
each year completed after the date of issuance. In the event the
executive is terminated by the Company without cause, the executive
shall be entitled to receive a severance package which will include
one year of base salary following the effective date of
termination, paid in twelve equal monthly installments, and
continued participation in any health care benefits provided by the
Company to its employees, provided the executive delivers to the
Company an executed release of claims.
No
executive officer is entitled to payments as a result of a change
in control of the Company.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR END
The
following table provides information concerning outstanding equity
awards as of December 31, 2019, for our named executive officers.
Market values were determined using the last sale price of our
Common Stock on December 31, 2019. No stock awards or performance
awards have been made under the Company’s equity incentive
plan, Those columns have been omitted from the following
table.
|
|
OPTION
AWARDS
|
|||
|
Name
|
Number of
Securities Underlying Unexercised Options
(Exercisable)
|
Number of
Securities Underlying Unexercised Options
(Unexercisable)
|
Option Exercise
Price ($)
|
Option
Expiration Date
|
|
J.
Michael Carmena
|
5,000
|
-
|
6.00
|
12/31/2022
|
|
Michael
D. McNeil
|
1,000
|
-
|
6.00
|
4/13/2023
|
|
Zachary
B. Fleming
|
2,000
|
-
|
6.00
|
12/31/2022
|
|
|
8,000
|
-
|
|
|
2019 DIRECTOR COMPENSATION
We
reimburse each director for reasonable travel expenses related to
such director’s attendance at Board and committee meetings.
During 2019, the Company did not pay cash or equity compensation to
the members of its Board for their service as
directors.
The
Company does not sponsor a pension benefits plan, a non-qualified
deferred compensation plan or a non-equity incentive plan for its
directors.
Retirement Plans
The
Company sponsors a 401(k) tax deferred savings plan, whereby the
Company matches a portion of employees’ contributions in
cash. Participation in the plan is voluntary and all employees of
the Company who are 18 years of age are eligible to participate.
The Company matches employee contributions dollar-for-dollar on the
first 4% of an employee’s pre‑tax earnings, subject to
individual IRS limitations.
The
Company does not sponsor any pension benefit plans and none of the
named executive officers contribute to such a plan.
Non-Qualified Deferred Compensation
The
Company does not sponsor any non-qualified defined compensation
plans or other non-qualified deferred compensation plans and none
of the named executive officers contribute to any such
plans.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN
BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER
MATTERS
44
The
following table provides information as of December 31, 2019, as
adjusted for the 1-for-100 reverse stock split which became
effective May 10, 2019, regarding shares of common stock that may
be issued under the Company's existing equity compensation
plans:
|
Plan
Category
|
Number of shares
to be issued upon the exercise of outstanding options
|
Weighted average
exercise price of outstanding options
|
Number of shares
remaining available for future issuance under equity compensation
plans (excluding shares reflected in the 2nd column)
|
|
Equity compensation
plans approved by shareholders
|
11,500
|
$6.00
|
1,988,500
|
|
Equity compensation
plans not approved by shareholders
|
—
|
—
|
—
|
The
following table sets forth, as of February 21, 2020, the number and
percentage of outstanding shares of our common stock owned by:
(a) each person who is known by us to be the beneficial owner
of more than 5% of our outstanding shares of common stock;
(b) each of our directors; (c) the named executive
officers as defined in Item 402 of Regulation S-K; and
(d) all current directors and executive officers, as a group.
As of February 21, 2020, there were 6,023,732 shares of common
stock issued and outstanding and no shares of Preferred Stock
issued and outstanding.
Beneficial
ownership has been determined in accordance with Rule 13d-3 under
the Exchange Act. Under this rule, certain shares may be deemed to
be beneficially owned by more than one person (if, for example,
persons share the power to vote or the power to dispose of the
shares). In addition, shares are deemed to be beneficially owned by
a person if the person has the right to acquire shares (for
example, upon exercise of an option or warrant) within 60 days of
the date as of which the information is provided. In computing the
percentage ownership of any person, the number of shares is deemed
to include the number of shares beneficially owned by such person
by reason of such acquisition rights. As a result, the percentage
of shares beneficially owned by a person as shown in the following
table does not necessarily reflect the person’s actual voting
power at any particular date.
|
|
Common Stock
|
|
|
OFFICERS AND DIRECTORS:
|
Number of Shares Beneficially Owned
|
Beneficial Ownership Percentage
|
|
Ronald
T. Nixon (1)
|
3,416,587
|
56.7%
|
|
James
W Stuckert (2)
|
941,584
|
15.6%
|
|
S.
Oden “Denny” Howell Jr. (3)
|
481,165
|
8.0%
|
|
J.
Michael Carmena (4)
|
5,000
|
0.1%
|
|
Zachery
Fleming (5)
|
2,000
|
0.0%
|
|
Michael
D. McNeil (6)
|
1,000
|
0.0%
|
|
All directors and executive officers as a group (6
persons)
|
4,847,336
|
80.5%
|
(1)
Ronald
T. Nixon is a director of the Company and a manager of Catalyst
Rochal, LLC, which owns 100% of the equity interest of CGI
Cellerate RX, LLC which owns 2,452,731 shares of the
Company’s common stock. FA Sanara, LLC owns 963,856 shares of
the Company’s common stock. FA Sanara, LLC is managed by
Family Alignment, LLC, which is managed by Catalyst Group, Inc. of
which Mr. Nixon is President. Mr. Nixon, through a relationship of
control of CGI Cellerate RX, LLC and FA Sanara, LLC, may be deemed
to share beneficial ownership of the shares of common stock
beneficially owned by CGI Cellerate RX, LLC and FA Sanara, LLC. Mr.
Nixon has shared power to vote and dispose 3,416,587
shares
(2)
Mr.
James W. Stuckert may be deemed to beneficially own 39,004 shares
held by Diane V. Stuckert and 69,004 shares owned by Ten Grand Ltd.
Mr. Stuckert has the sole power to vote and dispose 941,584
shares.
(3)
Mr.
Denny Howell, Jr is a director of the Company. Mr. Howell has the
sole power to vote and dispose 481,165 shares.
(4)
Mr.
Carmena is an executive officer of the Company and holds stock
options currently exercisable for the purchase of 5,000 shares of
Common Stock. Mr. Carmena has the sole power to vote and dispose
all shares he beneficially owns.
(5)
Mr.
Fleming is an executive officer of the Company and holds stock
options currently exercisable for the purchase of 2,000 shares of
Common Stock. Mr. Fleming has the sole power to vote and dispose
all shares he beneficially owns.
(6)
Mr.
McNeil is an executive officer of the Company and holds stock
options currently exercisable to purchase of 1,000 shares of Common
Stock. Mr. McNeil has the sole power to vote and dispose all shares
he beneficially owns.
45
ITEM 13. CERTAIN RELATIONSHIPS AND
RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The
Company was a participant in the following transactions with
related parties during fiscal years 2019 and 2018.
Mr.
Howell and Mr. Stuckert each held a convertible note from the
Company payable in the principle amount of $600,000 and accrued
interest of $192,797 as of February 18, 2018. On February 19, 2018,
each convertible note and $192,797 of accrued interest were
converted to 113,257 common shares of the Company's
Stock.
The
Company paid Catalyst and a Catalyst affiliate a total of $229,356
in 2019. The payments were related to professional services
provided to Cellerate, LLC by Catalyst and its affiliate under the
terms of the Professional Services agreement between the Catalyst
affiliate and the Company which was executed upon the formation of
Cellerate, LLC. Amounts due to Catalyst and its affiliate totaled
$36,790 at December 31, 2019. The Sanara Executive Chairman is the
Founder and Managing Partner of Catalyst.
The
Company paid Rochal Industries (“Rochal”) a total of
$1,663,073 in 2019, and $12,000 in 2018. In 2019, the Company paid
$1,500,000 to Rochal under two separate new product license
agreements whereby the Company obtained worldwide rights to market
and sell certain FDA cleared products developed by Rochal. Other
amounts were paid to Rochal in 2019 primarily for finished goods
inventory of the licensed products. The Company will pay Rochal
royalties on the licensed products at the rate of 4% of net sales
during the term of the licenses. Amounts due to Rochal totaled
$31,878 at December 31, 2019.
On
October 1, 2019, the Company executed a license agreement with
Rochal whereby the Company acquired an exclusive world-wide license
to market, sell and further develop certain antimicrobial barrier
film and skin protectant products for use in the human health care
market utilizing certain Rochal patents and pending patent
applications (the “ABF License Agreement”). Currently,
the products covered by the ABF License Agreement are
BIAKŌS™ Antimicrobial Barrier Film and Curashield™
No Sting Skin Protectant.
On July
8, 2019, the Company executed a license agreement with Rochal
whereby the Company acquired an exclusive world-wide license to
market, sell and further develop antimicrobial products for the
prevention and treatment of microbes on the human body utilizing
certain Rochal patents and pending patent applications (the
“License Agreement”). Currently, the products covered
by the License Agreement are BIAKŌS™ Antimicrobial Wound
Gel, and FDA cleared BIAKŌS™ Antimicrobial Skin and
Wound Cleanser.
Ronald
T. Nixon, Executive Chairman of the Company, is also a director of
Rochal, and indirectly a significant shareholder of Rochal, and
through the potential exercise of warrants a majority shareholder
of Rochal. Anne Beal Salamone, a director of the Company, is a
shareholder, the former president and current Chairman of the Board
of Rochal.
John C.
Siedhoff was a director of the Company during 2018 and resigned
that position January 31, 2019. During that period Mr. Siedhoff
received compensation in the amount of $260,000 in consulting fees
as a consultant to the Company. On January 31, 2019, Mr. Siedhoff
entered into a consulting agreement with the Company that
terminates December 31, 2020. Under the agreement Mr. Siedhoff
earned consulting fees in the amount of $21,947 per month through
December 31, 2019. For the period January 1, 2020 through December
31, 2020, Mr. Siedhoff will earn $10,000 per month.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND
SERVICES
Audit Fees. We engaged
MaloneBailey, LLP to conduct annual our audits and review of
quarterly financial statements for the years ended December
31, 2019 and December 31, 2018. Audit
fees for services performed were $88,000 and $68,303,
respectively.
Tax Fees. We engaged Haynie
& Company as our accountants for tax-related services
for the years ended December 31, 2019 and December 31, 2018, and
paid $24,951 and $20,903, respectively.
46
All Other Fees. We paid no other fees to independent public
accountants.
Consideration of Non-audit Services Provided by the Independent
Auditors. We paid no other fees to Malone Bailey, LLP for
non-audit services.
Audit Committee Pre-Approval Policy
The
policy of the Board, in its capacity as the Company’s audit
committee, is to pre-approve all audit, audit-related and non-audit
services provided by the independent registered public accounting
firm. These services may include audit services, audit-related
services, and other services. The Board approved all of the fees
described above. The Board may also pre-approve particular services
on a case-by-case basis. The independent public accountants are
required to periodically report to the Board regarding the extent
of services provided by the independent public accountants in
accordance with such pre-approval. The Board may also delegate
pre-approval authority to one or more of its members. Such
member(s) must report any decisions to the Board at the next
scheduled Board meeting.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT
SCHEDULES
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
|
Share
Exchange Agreement between Catalyst CellerateRX, LLC and WNDM
Medical Inc. (Incorporated by reference to Exhibit 2.1 to the
Company’s Current Report on Form 8-K dated March 21,
2019).
|
|
|
|
|
|
|
|
Articles
of Incorporation of Sanara MedTech Inc. (incorporated by reference
to Exhibit 3.1 to the Registrant’s Registration Statement on
Form S-1 filed April 11, 2008).
|
|
|
|
|
|
|
|
Amendment
to Articles of Incorporation of Sanara MedTech Inc. (incorporated
by reference to Exhibit A to the Registrant’s Information
Statement filed with the Commission on May 13, 2008).
|
|
|
|
|
|
|
|
Amendment
to Articles of Incorporation of Sanara MedTech Inc., effective
February 20, 2015 (incorporated by reference to Exhibit 4.4 to the
Registrant’s Registration Statement on Form S-8, filed with
the Commission February 21, 2020).
|
|
|
|
|
|
|
|
Amendment
to Articles of Incorporation of Sanara MedTech Inc. effective May
10, 2019 (incorporated by reference to Exhibit 4.4 to the
Registrant’s Registration Statement on Form S-8, filed with
the Commission February 21, 2020).
|
|
|
|
|
|
|
|
Bylaws
(Incorporated by reference to Exhibit 3.2 to the Company’s
Registration Statement on Form S-1 filed April 11,
2008)
|
|
|
|
|
|
|
|
Certificate
of Designations, of Series F Convertible Preferred Stock
(Incorporated by reference to Exhibit 4.1 to the Company’s
Current Report on Form 8-K filed March 21, 2019)
|
|
|
|
|
|
|
10.1 †
|
|
Wound
Management Technologies, Inc. 2010 Omnibus Long-Term Incentive Plan
dated March 12, 2010 effective subject to shareholder approval on
or before March 11, 2011 (Incorporated by reference to Exhibit 4.1
to the Company’s Quarterly Report on Form 10-Q filed August
16, 2010)
|
|
|
|
|
|
10.2 *†
|
|
Employment
Agreement dated June 1, 2019 between Sanara MedTech Inc. and Shawn
M. Bowman
|
|
|
|
|
|
10.3 *†
|
|
Employment
Agreement dated June 1, 2019 between Sanara MedTech Inc. and
Zachary B. Fleming
|
|
|
|
|
|
|
Contribution
Agreement dated August 27, 2018 between Wound Care Innovations, LLC
and Catalyst Cellerate RX, LLC (Incorporated by reference to
Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q
filed November 14, 2018)
|
|
|
|
|
|
|
|
Operating
Agreement dated August 27, 2018 between Wound Care Innovations, LLC
and Catalyst Cellerate RX, LLC (Incorporated by reference to
Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q
filed November 14, 2018)
|
|
|
|
|
|
|
|
Sublicense
Agreement dated August 27, 2018 between Catalyst Cellerate RX, LLC
and Cellerate, LLC (Incorporated by reference to Exhibit 10.3 to
the Company’s Quarterly Report on Form 10-Q filed November
14, 2018)
|
|
|
|
|
|
47
|
|
Professional
Services Agreement dated August 27, 2018 between Wound Management
Technologies, Inc., Catalyst Cellerate RX, LLC and Cellerate, LLC
(Incorporated by reference to Exhibit
10.4 to the Company’s Quarterly Report on Form 10-Q filed
November 14, 2018)
|
|
|
|
|
|
|
|
Convertible
Promissory Note to Catalyst Cellerate RX, LLC (Incorporated by reference to Exhibit 10.5 to the
Company’s Quarterly Report on Form 10-Q filed November 14,
2018)
|
|
|
|
|
|
|
|
Product
License Agreement dated July 8, 2019 between Sanara MedTech Inc.
and Rochal Industries, LLC (Incorporated by reference to Exhibit
10.1 to the Company’s Quarterly Report on Form 10-Q filed on
August 14, 2019)
|
|
|
|
|
|
|
|
Product
License Agreement dated October 1, 2019 between Sanara MedTech Inc.
and Rochal Industries, LLC (Incorporated by reference to Exhibit
10.1 to the Company’s Quarterly Report on Form 10-Q filed on
November 14, 2019)
|
|
|
|
|
|
|
|
List of
Subsidiaries*
|
|
|
|
|
|
|
|
Certification
of Principal Executive Officer in accordance with 18 U.S.C. Section
1350, as adopted by Section 302 of the Sarbanes-Oxley Act of
2002*
|
|
|
|
|
|
|
|
Certification
of Principal Financial Officer in accordance with 18 U.S.C. Section
1350, as adopted by Section 302 of the Sarbanes-Oxley Act of
2002*
|
|
|
|
|
|
|
|
Certification
of Principal Executive Officer in accordance with 18 U.S.C. Section
1350, as adopted by Section 906 of the Sarbanes-Oxley Act of
2002*
|
|
|
|
|
|
|
|
Certification
of Principal Financial Officer in accordance with 18 U.S.C. Section
1350, as adopted by Section 906 of the Sarbanes-Oxley Act of
2002*
|
|
|
|
|
|
|
101
|
|
Interactive Data Files pursuant to Rule 405 of Regulation
S-T
|
* Filed
herewith
†
Identifies a management contract or compensatory plan
48
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities
Exchange Act of 1934, the registrant has duly caused this report to
be signed on its behalf by the undersigned, thereunto duly
authorized.
|
|
SANARA MEDTECH INC.
|
|
|
|
|
|
|
|
|
March
26, 2020
|
By:
|
/s/ Michael
McNeil
|
|
|
|
|
Michael
McNeil
|
|
|
|
|
Chief
Financial Officer
|
|
Pursuant
to the requirements of the Securities Exchange Act of 1934, this
report has been signed below by the following persons on behalf of
the registrant and in the capacities and on the dates
indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/ J.
Michael Carmena
|
|
PEO
(Principal Executive Officer)
|
|
March
26, 2020
|
|
J. Michael Carmena
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Michael
McNeil
|
|
Chief
Financial Officer (Principal Financial and Accounting
Officer)
|
|
March
26, 2020
|
|
Michael
McNeil
|
|
|
|
|
|
|
|
|
|
|
|
/s/ James
W. Stuckert
|
|
Director
|
|
March
26, 2020
|
|
James
W. Stuckert
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Ronald
T. Nixon
|
|
Chairman
|
|
March
26, 2020
|
|
Mr.
Ronald T. Nixon
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Kenneth E. Thorpe
|
|
Director
|
|
March
26, 2020
|
|
Kenneth
E. Thorpe
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Ann
Beal Salamone
|
|
Director
|
|
March
26, 2020
|
|
Ann
Beal Salamone
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Oden
Howell, Jr.
|
|
Director
|
|
March
26, 2020
|
|
Oden
Howell, Jr.
|
|
|
|
|
49
