Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - NOVAVAX INC | tm2013650d1_ex99-1.htm |
| 8-K - FORM 8-K - NOVAVAX INC | tm2013650d1_8k.htm |
Exhibit 99.2

1 novavax.com NanoFlu Phase 3 clinical trial goals and design Primary objectives • To demonstrate the non - inferior immunogenicity of NanoFlu, relative to Fluzone® Quadrivalent, in terms of hemagglutination inhibition (HAI) antibody responses to all vaccine homologous influenza strains at Day 28. • To describe the safety profile of NanoFlu and Fluzone Secondary objectives • To describe the immunogenicity with both egg - propagated virus and wild - type VLP reagents to all four vaccine - homologous influenza strains and to select drifted strains at Day 28. • To describe the immunogenicity in terms of microneutralization (MN) responses to vaccine - homologous and/or antigenically drifted influenza strains at Day 0 and 28 • To describe the quality and amplitude of cell - mediated immune (CMI) responses in a subset of participants Design Randomized, observer - blinded, active - comparator controlled trial Vaccine strains • WHO - recommended 2019 - 2020 Northern Hemisphere influenza vaccine strains. A/Brisbane (H1N1); A/Kansas (H3N2); B/Maryland (Victoria); B/Phuket (Yamagata) Investigational and comparator vaccines • Hemagglutinin nanoparticle influenza vaccine, quadrivalent with Matrix - M ™ adjuvant (quad - NIV) [NanoFlu] • Quadrivalent inactivated influenza vaccine (IIV4) [Fluzone] Stratification • History of receipt of 2018 - 2019 influenza vaccine Participants • 2,650 clinically stable adults > 65 years of age • Randomized 1:1 (NanoFlu : Fluzone), Single vaccination at Day 0 Study sites • 19 U.S. sites Length of study participation • 1 year (safety assessment through 1 year)

2 novavax.com NanoFlu Phase 3 clinical trial conclusions Primary endpoint met: demonstrated immunologic non - inferiority to Fluzone in terms of hemagglutination inhibition (HAI) antibody responses (assayed with egg - derived virus reagents ) against all four vaccine homologous strains (per CBER criteria). Statistically significant higher HAI antibody responses (assayed with wild - type VLP reagents ) compared to Fluzone: • 24 — 66% improved Day 28 GMTs against homologous strains • 34 — 41% improved Day 28 GMTs against drifted H3N2 strains • 11.4 — 20.4% increased Day 28 seroconversion rate against homologous strains • 14.1 — 16.8% increased Day 28 seroconversion rate against drifted H3N2 strains NanoFlu was well - tolerated

3 novavax.com NanoFlu Fluzone Quad D28 GMT Ratio Assay Strain D28 GMT D28 GMT (NanoFlu / Fluzone) 95% CI HAI: EGG A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 49.3 45.0 1.09 ( 1.03, 1.15) A/Kansas/14/2017 (H3N2) (Homologous) 151.5 126.8 1.19 ( 1.11, 1.27) B/Maryland/15/2016 (Vic) (Homologous) 110.7 106.3 1.03 ( 0.99, 1.07) B/Phuket/3073/2013 (Yam) (Homologous) 168.5 133.9 1.23 ( 1.16, 1.29) Immunogenicity : Egg - or wild - type VLP - based Day 28 HAI GMTs and GMT ratios (NanoFlu / Fluzone) Success: All 95% CI lower bounds are ≥ 0.67 x GMT ratio success criteria met x NanoFlu: 3 — 23% improved responses using egg - based HAI

4 novavax.com NanoFlu Fluzone Quad D28 GMT Ratio Assay Strain D28 GMT D28 GMT (NanoFlu / Fluzone) 95% CI HAI: EGG A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 49.3 45.0 1.09 ( 1.03, 1.15) A/Kansas/14/2017 (H3N2) (Homologous) 151.5 126.8 1.19 ( 1.11, 1.27) B/Maryland/15/2016 (Vic) (Homologous) 110.7 106.3 1.03 ( 0.99, 1.07) B/Phuket/3073/2013 (Yam) (Homologous) 168.5 133.9 1.23 ( 1.16, 1.29) HAI: VLP A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 76.6 62.7 1.24 ( 1.17, 1.32) A/Kansas/14/2017 (H3N2) (Homologous) 153.6 90.7 1.66 ( 1.53, 1.79) B/Maryland/15/2016 (Vic) (Homologous) 62.8 47.2 1.32 ( 1.26, 1.39) B/Phuket/3073/2013 (Yam) (Homologous) 118.3 78.4 1.47 ( 1.40, 1.55) Immunogenicity : Egg - or wild - type VLP - based Day 28 HAI GMTs and GMT ratios (NanoFlu / Fluzone) x NanoFlu: 24 — 66% improved responses using VLP - based HAI x “Superiority” criteria met for homologous H3N2 (66% better)

5 novavax.com NanoFlu Fluzone Quad D28 GMT Ratio Assay Strain D28 GMT D28 GMT (NanoFlu / Fluzone) 95% CI HAI: EGG A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 49.3 45.0 1.09 ( 1.03, 1.15) A/Kansas/14/2017 (H3N2) (Homologous) 151.5 126.8 1.19 ( 1.11, 1.27) B/Maryland/15/2016 (Homologous) 110.7 106.3 1.03 ( 0.99, 1.07) B/Phuket/3073/2013 (Homologous) 168.5 133.9 1.23 ( 1.16, 1.29) HAI: VLP A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 76.6 62.7 1.24 ( 1.17, 1.32) A/Kansas/14/2017 (H3N2) (Homologous) 153.6 90.7 1.66 ( 1.53, 1.79) B/Maryland/15/2016 (Homologous) 62.8 47.2 1.32 ( 1.26, 1.39) B/Phuket/3073/2013 (Homologous) 118.3 78.4 1.47 ( 1.40, 1.55) A/California (“Drifted” H3N2) 115.0 80.6 1.41 ( 1.33, 1.50) A/Cardiff (“Drifted” H3N2) 63.9 45.4 1.34 ( 1.27, 1.43) A/Netherlands (“Drifted” H3N2) 102.3 74.7 1.38 ( 1.30, 1.46) A/South Australia (“Drifted” H3N2) 98.1 70.4 1.36 ( 1.28, 1.44) Immunogenicity : Egg - or wild - type VLP - based Day 28 HAI GMTs and GMT ratios (NanoFlu / Fluzone) x NanoFlu: 34 — 41% improved responses on drifted H3N2s using VLP - based HAI

6 novavax.com NanoFlu Fluzone Quad Absolute SCR Difference Assay Strain SCR SCR NanoFlu - Fluzone Quad 95% CI HAI:EGG A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 22.0% (282/1280) 17.0% (219/1286) 5.0 ( 1.9, 8.1) A/Kansas/14/2017 (H3N2) (Homologous) 41.8% (535/1280) 34.4% (443/1286) 7.3 ( 3.6, 11.1) B/Maryland/15/2016 (Vic) (Homologous) 11.2% (143/1280) 10.7% (137/1286) 0.5 ( - 1.9, 2.9) B/Phuket/3073/2013 (Yam) (Homologous) 31.3% (401/1280) 22.9% (294/1286) 8.5 ( 5.0, 11.9) Immunogenicity: Egg - or wild - type VLP - based Day 28 HAI SCR and SCR difference (NanoFlu - Fluzone) Success: All 95% CI lower bounds are ≥ - 10 x Seroconversion (SCR) difference success criteria met x NanoFlu: 0.5 — 8.5% increased SCR using egg - based HAI

7 novavax.com NanoFlu Fluzone Quad Absolute SCR Difference Assay Strain SCR SCR NanoFlu - Fluzone Quad 95% CI HAI:EGG A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 22.0% (282/1280) 17.0% (219/1286) 5.0 ( 1.9, 8.1) A/Kansas/14/2017 (H3N2) (Homologous) 41.8% (535/1280) 34.4% (443/1286) 7.3 ( 3.6, 11.1) B/Maryland/15/2016 (Vic) (Homologous) 11.2% (143/1280) 10.7% (137/1286) 0.5 ( - 1.9, 2.9) B/Phuket/3073/2013 (Yam) (Homologous) 31.3% (401/1280) 22.9% (294/1286) 8.5 ( 5.0, 11.9) HAI:VLP A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 32.7% (419/1280) 21.4% (275/1286) 11.4 ( 7.9, 14.7) A/Kansas/14/2017 (H3N2) (Homologous) 69.8% (894/1280) 49.5% (636/1286) 20.4 ( 16.6, 24.1) B/Maryland/15/2016 (Vic) (Homologous) 25.1% (321/1280) 13.5% (173/1286) 11.6 ( 8.6, 14.6) B/Phuket/3073/2013 (Yam) (Homologous) 35.4% (453/1280) 17.7% (228/1286) 17.7 ( 14.3, 21.0) Immunogenicity: Egg - or wild - type VLP - based Day 28 HAI SCR and SCR difference (NanoFlu - Fluzone) x NanoFlu: 11.4 — 20.4% increased SCR using VLP - based HAI
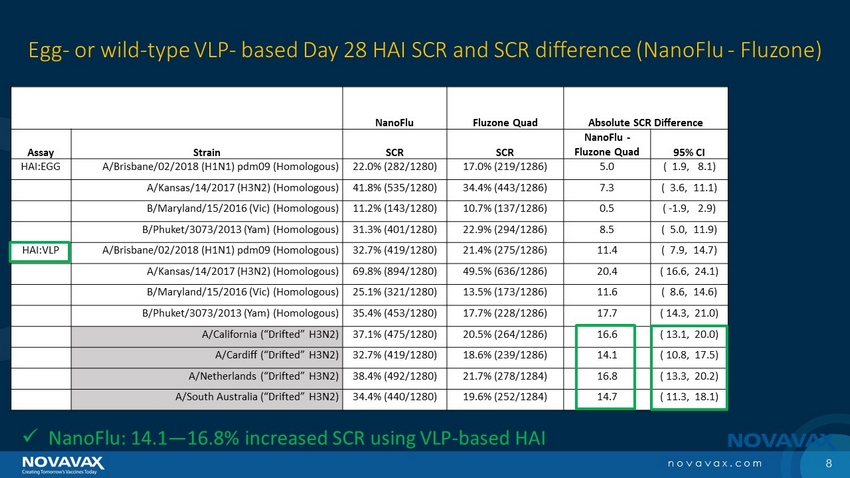
8 novavax.com NanoFlu Fluzone Quad Absolute SCR Difference Assay Strain SCR SCR NanoFlu - Fluzone Quad 95% CI HAI:EGG A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 22.0% (282/1280) 17.0% (219/1286) 5.0 ( 1.9, 8.1) A/Kansas/14/2017 (H3N2) (Homologous) 41.8% (535/1280) 34.4% (443/1286) 7.3 ( 3.6, 11.1) B/Maryland/15/2016 (Vic) (Homologous) 11.2% (143/1280) 10.7% (137/1286) 0.5 ( - 1.9, 2.9) B/Phuket/3073/2013 (Yam) (Homologous) 31.3% (401/1280) 22.9% (294/1286) 8.5 ( 5.0, 11.9) HAI:VLP A/Brisbane/02/2018 (H1N1) pdm09 (Homologous) 32.7% (419/1280) 21.4% (275/1286) 11.4 ( 7.9, 14.7) A/Kansas/14/2017 (H3N2) (Homologous) 69.8% (894/1280) 49.5% (636/1286) 20.4 ( 16.6, 24.1) B/Maryland/15/2016 (Vic) (Homologous) 25.1% (321/1280) 13.5% (173/1286) 11.6 ( 8.6, 14.6) B/Phuket/3073/2013 (Yam) (Homologous) 35.4% (453/1280) 17.7% (228/1286) 17.7 ( 14.3, 21.0) A/California (“Drifted” H3N2) 37.1% (475/1280) 20.5% (264/1286) 16.6 ( 13.1, 20.0) A/Cardiff (“Drifted” H3N2) 32.7% (419/1280) 18.6% (239/1286) 14.1 ( 10.8, 17.5) A/Netherlands (“Drifted” H3N2) 38.4% (492/1280) 21.7% (278/1284) 16.8 ( 13.3, 20.2) A/South Australia (“Drifted” H3N2) 34.4% (440/1280) 19.6% (252/1284) 14.7 ( 11.3, 18.1) Immunogenicity: Egg - or wild - type VLP - based Day 28 HAI SCR and SCR difference (NanoFlu - Fluzone) x NanoFlu: 14.1 — 16.8% increased SCR using VLP - based HAI
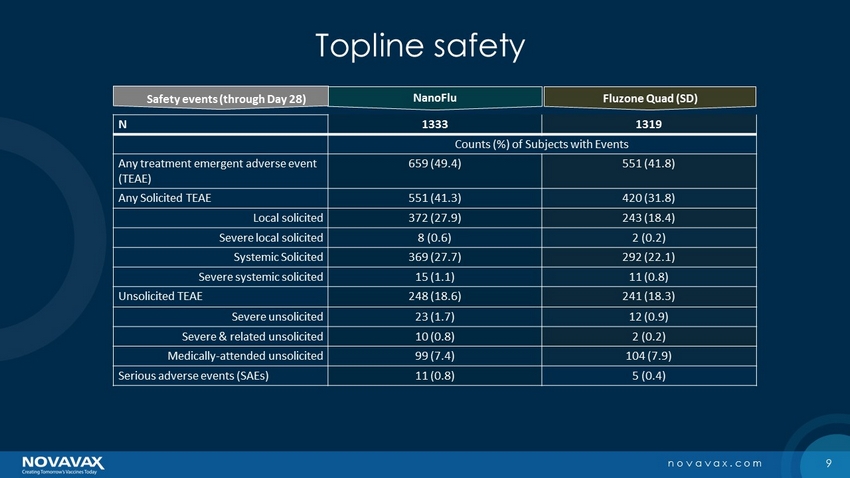
9 novavax.com Topline safety N 1333 1319 Counts (%) of Subjects with Events Any treatment emergent adverse event ( TEAE) 659 (49.4) 551 (41.8) Any Solicited TEAE 551 (41.3) 420 (31.8) Local solicited 372 (27.9) 243 (18.4) Severe local solicited 8 (0.6) 2 (0.2) Systemic Solicited 369 (27.7) 292 (22.1) Severe systemic solicited 15 (1.1) 11 (0.8) Unsolicited TEAE 248 (18.6) 241 (18.3) Severe unsolicited 23 (1.7) 12 (0.9) Severe & related unsolicited 10 (0.8) 2 (0.2) Medically - attended unsolicited 99 (7.4) 104 (7.9) Serious adverse events (SAEs) 11 (0.8) 5 (0.4) NanoFlu Fluzone Quad (SD) Safety events (through Day 28)
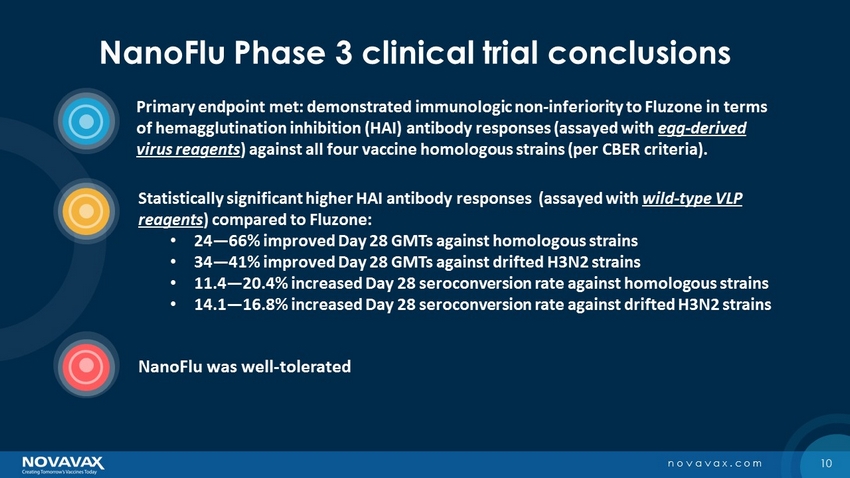
10 novavax.com NanoFlu Phase 3 clinical trial conclusions Primary endpoint met: demonstrated immunologic non - inferiority to Fluzone in terms of hemagglutination inhibition (HAI) antibody responses (assayed with egg - derived virus reagents ) against all four vaccine homologous strains (per CBER criteria). Statistically significant higher HAI antibody responses (assayed with wild - type VLP reagents ) compared to Fluzone: • 24 — 66% improved Day 28 GMTs against homologous strains • 34 — 41% improved Day 28 GMTs against drifted H3N2 strains • 11.4 — 20.4% increased Day 28 seroconversion rate against homologous strains • 14.1 — 16.8% increased Day 28 seroconversion rate against drifted H3N2 strains NanoFlu was well - tolerated
