Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Moleculin Biotech, Inc. | form8-kq12020investorprese.htm |

Developing ground-breaking therapies for highly resistant cancers January 12, 2020 Public Corporate Presentation (Nasdaq: MBRX)

Disclaimer All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission ("SEC") and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. More detailed information about Moleculin is set forth in our filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. 2

Key Points Potential Blockbuster Technologies 3 Noncardiotoxic anthracycline Phase 1 clinical trials 5 STAT3 Inhibitor Drugs showing early activity 2 Inhibitor of glycolysis 2020 Phase 2 trials expected 2 World class collaboration Highly experienced team Exclusive IP/Fast Track 3

4 3 Core Technologies 1 2 3 Next Generation Immune / Transcription Metabolism / Anthracycline Modulators Glycosylation Inhibitor • Avoids multidrug resistance • Enables immune response and • Prodrug of glucose decoy immune memory enables drug-like properties • Little to no cardiotoxicity • Inhibits p-STAT3, • Enables immune checkpoint • Enables “organ-targeted” c-Myc and HIF-1α inhibitors by interfering with therapy glycosylation Annamycin WP1066 WP1122 Potential for Drug Combination Therapies 4

5 Partnerships & Collaborations Our world-leading partnerships & collaborations significantly expand our research capabilities and increase our knowledgebase 5

Delivering Milestones by Year 2016 2017 2018 2019 2020 Projected First Human Data Est. 7 Trials: IND’s filed Begin 4 clinical Annamycin IPO - & 2 Phase 2 & & Approved trials Misfire 5 clinical trials 4+ Phase 1 Trials • Annamycin – Apply for • Annamycin – • Annamycin – • Annamycin – • Annamycin – ODD 1) File FDA IND 1) US trial begins 1) No Cardiotox 1) Poland & US • Prepare for Phase IIB 2) IND approved recruiting 2) 40% efficacy combine into one – 3) IRB approvals at/above 120 IND 2) Poland CTA filed Phase 2 begins begin and approved mg/m2 2) Annamycin • Continue 4) FDA ODD 3) Poland begins 3) Fast Track FDA Sarcomas strengthening pipeline screening patients • WP1066 – • WP1066 – • WP1066 3 Phase 1’s – 1) MDA IND filed • WP1066 – FDA ODD 1) In plasma 1) MDA WP1066 2) MDA IND WP1220 – Poland CTA 2) 5th trial approved 2) Emory WP1066 approved filed & approved • WP1220 – Safe and Pediatric • Benefits of MDA SRA effective in 3 out of 5 3) WP1066 + RAD (WP1732) patients >50% • WP1220 – Phase 2 reduction in CAILS begins 6
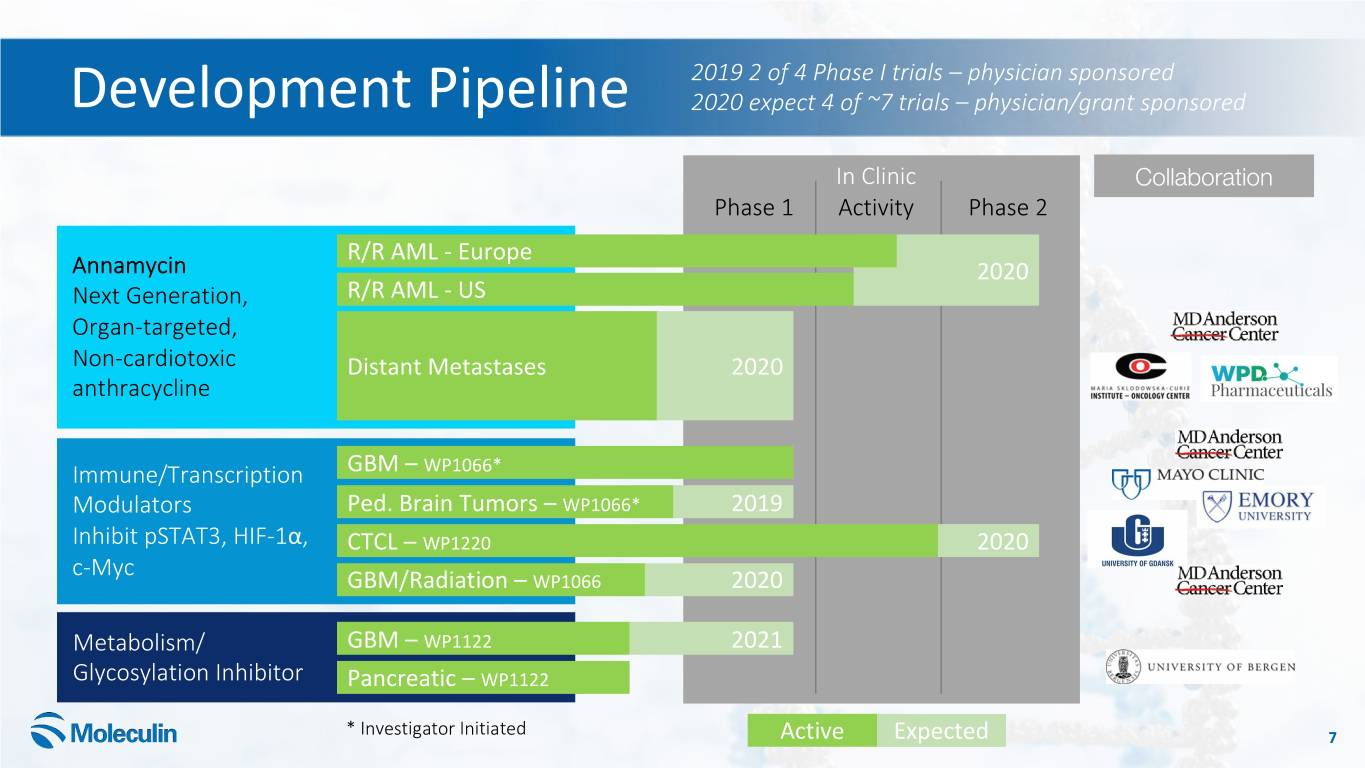
2019 2 of 4 Phase I trials – physician sponsored Development Pipeline 2020 expect 4 of ~7 trials – physician/grant sponsored In Clinic Collaboration Phase 1 Activity Phase 2 R/R AML - Europe Annamycin 2020 Next Generation, R/R AML - US Organ-targeted, Non-cardiotoxic Distant Metastases 2020 anthracycline Immune/Transcription GBM – WP1066* Modulators Ped. Brain Tumors – WP1066* 2019 Inhibit pSTAT3, HIF-1α, CTCL – WP1220 2020 c-Myc GBM/Radiation – WP1066 2020 Metabolism/ GBM – WP1122 2021 Glycosylation Inhibitor Pancreatic – WP1122 * Investigator Initiated Active Expected 7

8 Annamycin Status Annamycin is safe and effective in R/R AML patients1 Annamycin shows activity in untreatable lung models5 Currently approved anthracyclines are ineffective in Annamycin accumulates 5-fold more in lungs that R/R AML patients as a single agent2 standard of care anthracycline Even at subtherapeutic doses, Annamycin as a single More potent than doxorubicin in key tumors that agent has shown efficacy3 in 57% of relapsed patients metastasize to the lungs (sarcoma, colo-rectal) Currently approved anthracyclines are cardiotoxic in Lower toxicity enables longer-term use of Annamycin 65% of patients treated above the LMAE4 Overall survival is significantly increased in triple Annamycin has proven safe well beyond the LMAE negative breast and colon cancer lung mets No cardiotoxicity, even in 7 patients treated above Nation’s leading sarcoma center collaborating to begin LMAE lung mets clinical trial We expect therapeutic dose level to be around 270 to Positioned to replace doxorubicin in a range of 300 mg/m2 (activity reported thus far thru 180 mg/m2) treatments 1. Based on preliminary Phase 1 testing. 2. G.Roboz, et al, International Randomized Phase III Study of Elacytarabine Versus Investigator Choice in Patients With Relapsed/Refractory Acute Myeloid Leukemia, Journal of Clinical Oncology, Volume 32, Number 18, June 20, 2014, pp1919-1929; H.Kantarjian, et al, Acute myeloid leukemia—Major progress over four decades and glimpses into the future, American Journal of Hematology, Vol. 91, No. 1, January 2016, pp131-145. 3. ”Efficacy” defined as complete and partial responses and bridge to transplant. 4. LMAE = Lifetime Maximum Anthracycline Exposure. 5. Based on animal models. 8

Preclinical studies Annamycin – Quick Facts show potential beyond AML Prior leukemia trials 2 Two prior developers in ‘00 & ‘05 2 AML & ALL Prior MTD mg/m 150 - 280 CR’s at or above MTD Prior clearing of bone marrow % 37% - 43% Total patients in these and Prior and current cardiotoxicity 0% breast cancer trials 100+ Latest cohort mg/m2 180 Relapsed patient efficacy Last Reported PR & CR % 30% & 10% 57% & 14% Management believes that, if we replicate prior results, we will have an approvable drug 9

10 Current Trial Versus Two Previous Trials Annamycin Trial (Date) Aronex (2000) Callisto8 (2005) Current (to Date1) Indications AML(17), ALL(3) ALL AML Trial design Phase 1 Phase 1/2 Phase 1/2 • Trials are not Dosage per day for MTD 190 - 230 350 190 100 - 180 TBD 3 days (mg/m2) 5,6 280 150 TBD directly comparable Mucositis/ DLT’s Mucositis TBD4 • 0% cardiotoxicity Hepatotoxicity experienced Patients treated above 20 31(8) 10 100 mg/m2 (expansion) • We believe reaching % Cardiotoxicity 0% 0% 0% prior trial results = Cleared Circulating Blasts 50% 62% NM4 approvable drug PR or BTB NM4 NM4 30%3 CR7 43%2 37.5% 10%3 Source & Notes: For Callisto and Aronex trials see MBRX investigator Brochure summary of prior trials beginning on page 51. For current trial, CRO Theradex. 1) Last reported on 12-4-19; combines US and EU trial data. 2) based on 14 patients, 6 succumbed to disease prior to measurement. 3) Only patients treated at 120 and above are included in the efficacy data. 4) NM = Not measured, TBD = To be determined. 5) Only MB-105 in Poland has exceeded 120 mg/m2. 6) Only in MB-104 in the US treated at 100 mg/m2. 7) Includes all Complete Responses, including CRi’s and bone marrow blasts <5%. 8) Includes only expansion cohort patients in efficacy data. 10

1 1 Key Developments Moleculin has 3 drug candidates in 5 clinical trials Next Generation Anthracycline – Annamycin (2 Clinical Trials) Immune/Transcription Modulators - (3 Clinical Trials) • Positive interim safety and efficacy data from Annamycin • Emory University FDA approved to conduct Phase 1 AML trial in US and Europe pediatric brain tumor trial • 57% efficacy in 9 relapsed AML patients treated at/or above 120 • Continue MD Anderson Phase 1 in adult brain tumors mg/m2 (1 CRi, 2 PRs, 1 BTB) • Efficacy and safety demonstrated in our European Phase 1 • No evidence of cardiotoxicity clinical trial of WP1220 for the topical treatment of • 2 U.S. in second cohort dose level of 120 mg/m ; Europe in cutaneous T-Cell Lymphoma (CTCL) 2 fourth cohort level of 210 mg/m • FDA granted Orphan Drug Designation for WP1066 for the • FDA grants Fast Track Designation of Annamycin for the treatment of glioblastoma, the most aggressive form of treatment of relapsed or refractory acute myeloid (AML) brain tumor • Annamycin active against metastases to the lungs in pre- • WP1066 appears to (a) counteract resistance to checkpoint clinical testing blockade therapy (specifically, immune checkpoint target • Significantly improved survival in an aggressive form for triple PD-L1) and (b) be capable of immune reprogramming negative breast cancer metastasized to the lungs in animal models 11

1 2 Visible Approval Opportunities Based on results to date: If we replicate current results in larger registration trials, we believe we have 2 approvable drugs now, with more to come These would represent 2 “world’s firsts”: 1. Non-cardiotoxic anthracycline 2. STAT3 inhibitor with therapeutic effect in cancer We believe this forms the basis for 2 “blockbuster” drugs (24 mo. horizon) 12

Technology Review 13

Focus on Highly Resistant Tumors Example Common Regulatory Indications Characteristics Advantage • R/R AML • Multidrug resistance • Significant unmet need • Glioblastoma • Immune evasion • Modest gains = new • Pancreatic • Upregulation of oncogenic drug approval transcription factors • Distant metastases • Accelerated approval • Increase in dependence on glycolysis • Other pathway 14

Annamycin (Next Generation Anthracycline) Critical Advantages Leading AML induction therapy drugs are cardiotoxic and lose efficacy due to Annamycin Avoids MDR1 over Current multidrug resistance Anthracyclines Annamycin has little to no cardiotoxicity, is active against multidrug resistant tumors, has been shown to be more potent in AML cell lines and has shown activity in patients who failed standard of care Annamycin accumulates disproportionately in the lungs, liver and pancreas Potential to Annamycin has shown the potential to significantly improve health in a Phase Significantly Improve I/II acute myeloid leukemia (AML) trial Health Orphan Drug status as single agent for relapsed or refractory AML Positioned for Annamycin has Fast Track status in the US and appears to be well suited for an Accelerated Approval accelerated approval pathway in the US, and in Europe Absence of any approved second-line drug for most AML patients represents a significant unmet need Absence of cardiotoxicity and greater efficacy than doxorubicin in a range of solid tumor models suggests significant market potential beyond AML 15

Annamycin Avoids MDR1 Mechanisms Cellular Uptake and Efflux of L-ANN and DOX in HL-60/DOX Cells 10000 L-ANN + VER L-ANN 1000 (L-ANN + VER) + VER (L-ANN + VER) - VER (L-ANN ) + VER DOX + VER (L-ANN ) - VER DOX 100 (DOX + VER) + VER (DOX) + VER (DOX + VER) - VER (DOX) - VER 10 Cells + M Cells + VER Source: Consoli U, Priebe W, Ling YH, Mahadevia R, UPTAKE EFFLUX Griffin M, Zhao S, Perez-Soler R, Andreeff M. Median Channel Fluorescence 1 The novel anthracycline annamycin is not affected by 0 30 60 90 120 150 180 210 240 P-glycoprotein-related multidrug resistance: TIME (minutes) comparison with idarubicin and doxorubicin in HL-60 leukemia cell lines. Blood 88(2):633-44, 7/1996. PMID: 8695811. 16

17 Annamycin is a More Potent Topoisomerase II Poison With a Wider Therapeutic Window than Doxorubicin Induction of DNA-protein cross-links by 4-demethoxy and 4- Topo II lesions and apoptosis induced by annamycin and WP794 in methoxy anthracyclines in CEM leukemic cells with wild-type topo normal cells. A, topo II–mediated DNA-protein cross-links in normal WI-38 II (A) and CEM/VM-1 cells with mutated topo II (B). fibroblasts incubated for 30 minutes with annamycin ( ) and WP794 (u). [14C]thymidine-prelabeled cells were incubated for 30 minutes Dotted and dashed pale lines, tracings of annamycin and WP794 effects, with 4-demethoxy anthracyclines [annamycin (ANN; ), WP769 respectively, in tumor CEM cells from Fig. 2. B, apoptosis induced after a (n), and WP794 (u)] and 4-methoxy compounds [doxorubicin 24-hour incubation of normal WI-38 fibroblasts with annamycin (Ÿ) and (DOX; ☐) and WP744 ( )] followed by DNA-protein cross-link normal prostate PrEC cells with annamycin ( ) and WP794 ( ). Dotted (DPC) determinations. Percentages of total cellular DNA cross- and dashed pale lines, tracings of annamycin and WP794 effects, linked to proteins. Points, mean of two to four experiments carried respectively, in tumor CEM cells from Fig. 5. Points, mean of two out in triplicate; bars, SE. Note the expanded scale in B. experiments; bars, SE. Source: A Trevino, B Woynarowska, T Herman, W Priebe, J Woynarowska; Enhanced topoisomerase II targeting by annamycin and related 4-demethoxy anthracycline analogues; Mol Cancer Ther 2004;3(11): 1403-101 17

L-Annamycin is Dramatically More Potent Than Doxorubicin Against Multidrug Resistant Tumor Cell Lines 400 18 356 16.3 350 16 296 14 300 263 12 250 10.5 10 200 DOX 8 L-ANN 150 117 6 100 4 Resistance Index 63 50 2 1.5 1.4 4 9 5 3 7 0 0 Squamous Lymphocytic Myeloma Myelocytic Breast Breast Small cell lung cancer leukemia leukemia carcinoma carcinoma cancer MDR1 Human Cell Lines MRP1 Human Cell Lines Susceptible KB CEM 8226 HL60 MCF-7 MCF-7 UMCC1 Resistant KB/V1 CEM/Vbl 8226/Dox HL60/Dox MCF-7/Dox MCF-7/VP UMCC1/VP Resistance Index: IC50 of Resistant Cells/IC50 of Susceptible Cells 18

19 L-Annamycin is Non-Cardiotoxic in FDA Required Tests % of Mice with Heart Lesions 100 100 100 100 90 90 80 80 70 70 60 60 60 50 50 43 40 40 30 25 30 20 20 8 10 10 7 0 0 0 0 0 Control Doxorubicin Free L-Annamycin Doxorubicin L-Annamycin Annamycin Dose (%LD10) 50 50 50 20 30 40 20 30 40 Experiment 1 (6 weekly IV injections) Experiment 2 (10 weekly IV injections) 19

In Vivo Antitumor Activity of Annamycin Antitumor Activity Inoculation/ Tumor Treatment Tumor Site End-Point L-ANN DOX L1210 leukemia ip/ip ascitic survival +++ ++ B16 melanoma ip/ip ascitic survival ++ ++ B16 melanoma sc/iv sc TGI ++ + M5076 reticulosarcoma iv/iv liver survival ++ NONE M5076 reticulosarcoma sc/iv sc, liver survival ++ NONE Lewis lung carcinoma iv/iv lungs survival ++ NONE KB human xenograft sc/iv sc TGI ++ ++ KB-V1 human xenograft sc/iv sc TGI ++ +/- 20
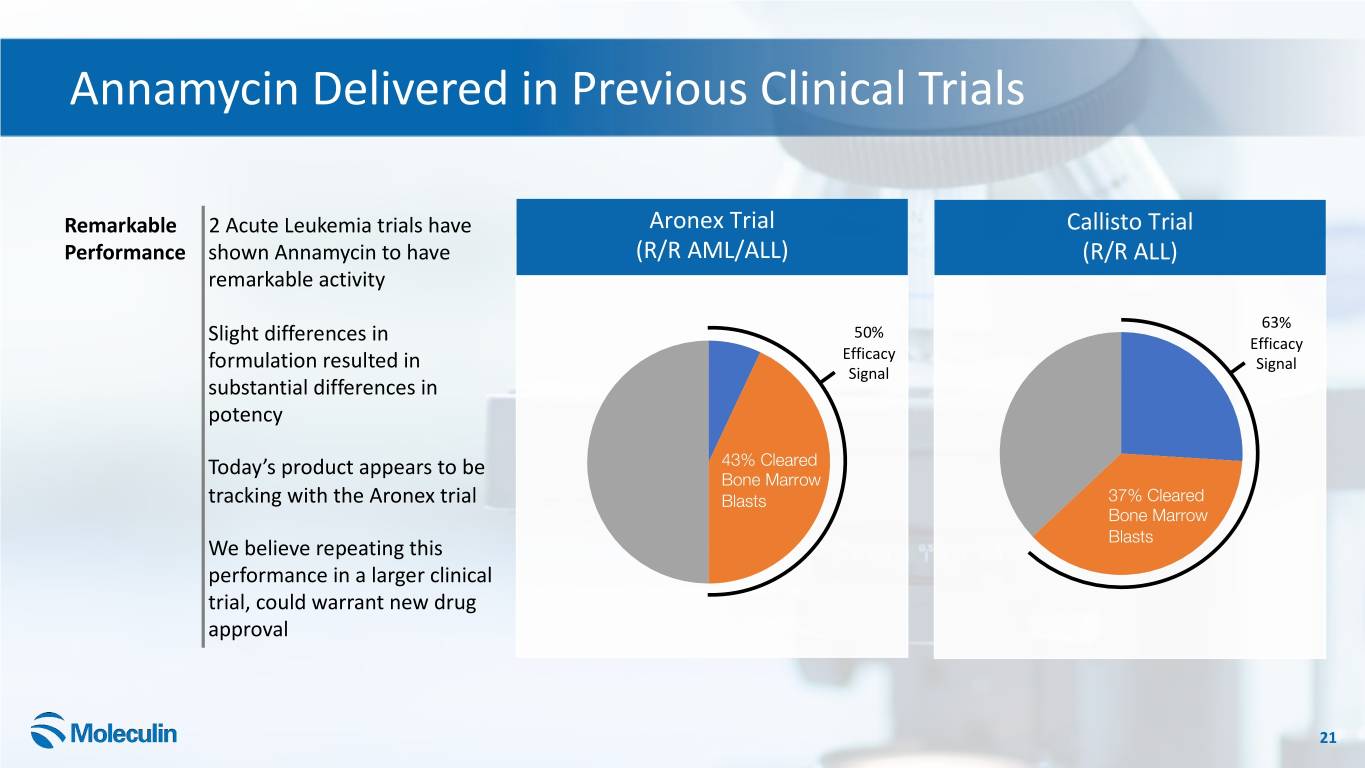
Annamycin Delivered in Previous Clinical Trials Remarkable 2 Acute Leukemia trials have Aronex Trial Callisto Trial Performance shown Annamycin to have (R/R AML/ALL) (R/R ALL) remarkable activity 63% 50% Slight differences in Efficacy Efficacy formulation resulted in Signal Signal substantial differences in potency Today’s product appears to be 43% Cleared Bone Marrow tracking with the Aronex trial 37%Blasts Cleared 37% Cleared Bone Marrow Bone Marrow Blasts We believe repeating this Blasts performance in a larger clinical trial, could warrant new drug approval 21

Annamycin is Delivering Again • Currently in second cohort in US trial 120 mg/m2 and in fourth cohort in Poland 210 mg/m2 • Efficacy to date - 1 Cri, 2 PR and 1 BTB - 40% in patients treated at/or above 120 mg/m2 (3 patients were refractory) • No evidence of cardiotoxicity (together with previous trials, 128 patients have been treated) • A recent review published in Cardiovascular Drugs and Therapy (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5346598/) reported that 65% of patients treated the equivalent of 550 mg/m2of doxorubicin exhibited sub-clinical cardiotoxicity. Of the 5 patients mentioned above who were treated in our European trial above 550 mg/m2, no evidence of cardiotoxicity was detected • Planning to present data to FDA and EMA upon establishment of new RP2D to request acceleration to Phase 2 Registration Trial 22

Annamycin – Organ Targeted Strategy • We now have substantial evidence that, in contrast to doxorubicin, there is elevated accumulation of Annamycin in lung, liver, spleen and pancreas • Distant metastases mouse models show remarkable efficacy • We are moving quickly to begin proof of concept clinical trials targeting distant metastases 23

Annamycin in Lungs is Dramatically Higher than in Plasma Source: Sponsored research at MD Anderson 24

Comparison of Efficacy of L-Annamycin, F-Annamycin and Doxorubicin in Lewis Lung Carcinoma model V e h ic le 1 0 0 D o x S -A n n a 8 0 l F -A n n a a v i 6 0 L -A n n a v r u S 4 0 Source: Ling YH, Priebe W, Yang LY, Burke TG, % Pommier Y, Perez-Soler R. In vitro cytotoxicity, cellular pharmacology, and DNA lesions induced 2 0 by annamycin, an anthracycline derivative with high affinity for lipid membranes. Cancer Res 53(7):1583-9, 4/1993. PMID: 8453627. 0 3 0 5 0 7 0 9 0 1 1 0 D a y s a fte r in o c u la tio n 25

250 250 250 229.7 229.7 229.7 Single Dose PK and Biodistribution 200 200 200 250 229.7 155.7 155.7 155.7 Annamycin has 6 fold 150 150 150 127.7 127.7 127.7 200121.7 121.7 greater concentration121.7 118.6 118.6 118.6 g x h/g or ml) g x h/g g x h/g or ml) g x h/g g x h/g or ml) g x h/g 106.6 100.9 in lungs106.6 vs. 100.9 106.6 100.9 μ μ μ 100.3 100.3 155.7 100.3 100 100 100 84.6 doxorubicin84.6 84.6 150 121.7 127.7 61.2 61.2 61.2 118.6 g x h/g or ml) g x h/g h AUC ( h AUC h AUC ( h AUC h AUC ( h AUC 106.6 100.9 μ - - - 33.2 33.2 33.2 100.3 50 50 50 17.6 17.6 17.684.6 24 24 24 20 29.1 20100 29.1 20 29.1 25.9 25.9 13.225.9 13.2 13.2 15.1 13.7 15.1 13.7 15.1 13.7 61.2 5.4 8 4.7 5.4 8 4.7 5.4 8 4.7 h AUC ( h AUC 0 0 - 0 33.2 50 17.6 24 20 25.9 29.1 Plasma Brain PlasmaHeart KidneyBrain HeartPlasmaLiver15.1 KidneyLungBrain13.7 SpleenLiverHeart LungKidney SpleenLiver Lung 13.2Spleen 5.4 8 4.7 DOX F-Ann 0L-AnnDOX F-Ann L-AnnDOX F-Ann L-Ann Plasma Brain Heart Kidney Liver Lung Spleen 26 DOX F-Ann L-Ann

Efficacy of L-Ann in AML – Organ Distribution Study Experiment description Female B6 albino mice injected with 1 x 10^5 AML1-MLL/ENL-FLT3/ITD-mTurquiose cells at day 1. Treatment was initiated on day 9th with injection of 4 mg/kg of L-Ann. Mouse was sacrificed 3 days after the dose. Animals were perfused with 30 ml of PBS, followed by dissection of bone ( for bone marrow analysis), spleen, lungs, liver and inguinal lymph node. Organs imaged using Leica SP8 confocal system. AML Annamycin Mouse 1 injection 1st dose imaging Days 1 9 12 27

Efficacy of L-Ann in AML – Organ Distribution Study Bone marrow Spleen Lungs Liver Lymph node vehicle Anna - L Green = AML cells Conclusion Blue = Second Harmonic Generation (bone collagen) Significant reduction of AML blasts in bone marrow and spleen. Red = Thy1-RFP (strong in neurons and weak in other cells) Complete elimination of AML cells from lungs, liver and lymph nodes 28

Multidose Toxicity of L-Ann 2 5 3 m g /k g 6 m g /k g 1 0 0 4 m g /k g 8 m g /k g l Source: Poster at the AACR-NCI-EORTC a v Molecular Targets and Cancer Therapeutics i 3 m g /k g Conference, Boston, MA.; entitled “Dose and v r g 2 0 Schedule-Dependent Efficacy of Liposomal , u 4 m g /k g Annamycin in Pre-clinical Models of Acute s h Myeloid Leukemia,” October 2019 g t i n e 5 0 6 m g /k g e w c 1 5 r 8 m g /k g e P 1 0 0 0 1 0 2 0 3 0 4 0 5 0 6 0 0 0 0 0 0 0 0 1 2 3 4 5 6 tim e , d a y s T im e Multidose toxicity studies performed in B6 albino mice shown no deaths or body weigh reduction in mice receiving L-Ann at 6 mg/kg (7 doses) and 4 mg/kg (12 doses). Animals receiving L-Ann at 8 mg/kg showed significant weigh loss and died after third injected dose. Conclusion: Significant lost of body weight was recorded for animals receiving third dose of L-Ann at 8 mg. All mice in this group died or became moribund after fourth dose administration. No evident toxicity was observed for mice in all other experimental groups, including animals receiving 6 mg/kg. No toxic deaths in these group as of today (after 12 doses of L-Ann) and experiment is still in progress. 29

Dose and Schedule Optimization – L-Ann in AML Model V e h ic le V e h ic le 1 0 0 1 0 0 L -A n n a 0 .5 m g /k g 1 -2 -3 L -A n n a 3 m g /k g o n c e a w e e k L -A n n a 4 m g /k g o n c e a w e e k L -A n n a 1 m g /k g 1 -2 -3 l l a a v v i i L -A n n a 2 m g /k g 1 -2 -3 v v r r u u s s t t n n 5 0 5 0 e e c c r r e e P P 0 0 0 5 1 0 1 5 2 0 2 5 3 0 3 5 4 0 0 5 1 0 1 5 2 0 2 5 3 0 3 5 4 0 d a y s d a y s Experiment description Female B6 albino mice injected with 1 x 10^5 AML1-MLL/ENL-FLT3/ITD-mTurquiose cells at day 1. Treatment initiated on day 11. L- Ann injected IV Conclusion Clear dosed – dependent increase of the survival for all tested schedules. The longest survival achieved for group receiving L-Ann at 4 mg/kg on a weekly schedule (38 days). Toxicity was observed for group receiving L-Ann 2 mg/kg on 1-2-3 schedule. Source: Poster at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference, Boston, MA.; entitled “Dose and Schedule-Dependent Efficacy of Liposomal Annamycin in Pre-clinical Models of Acute Myeloid Leukemia,” October 2019 30
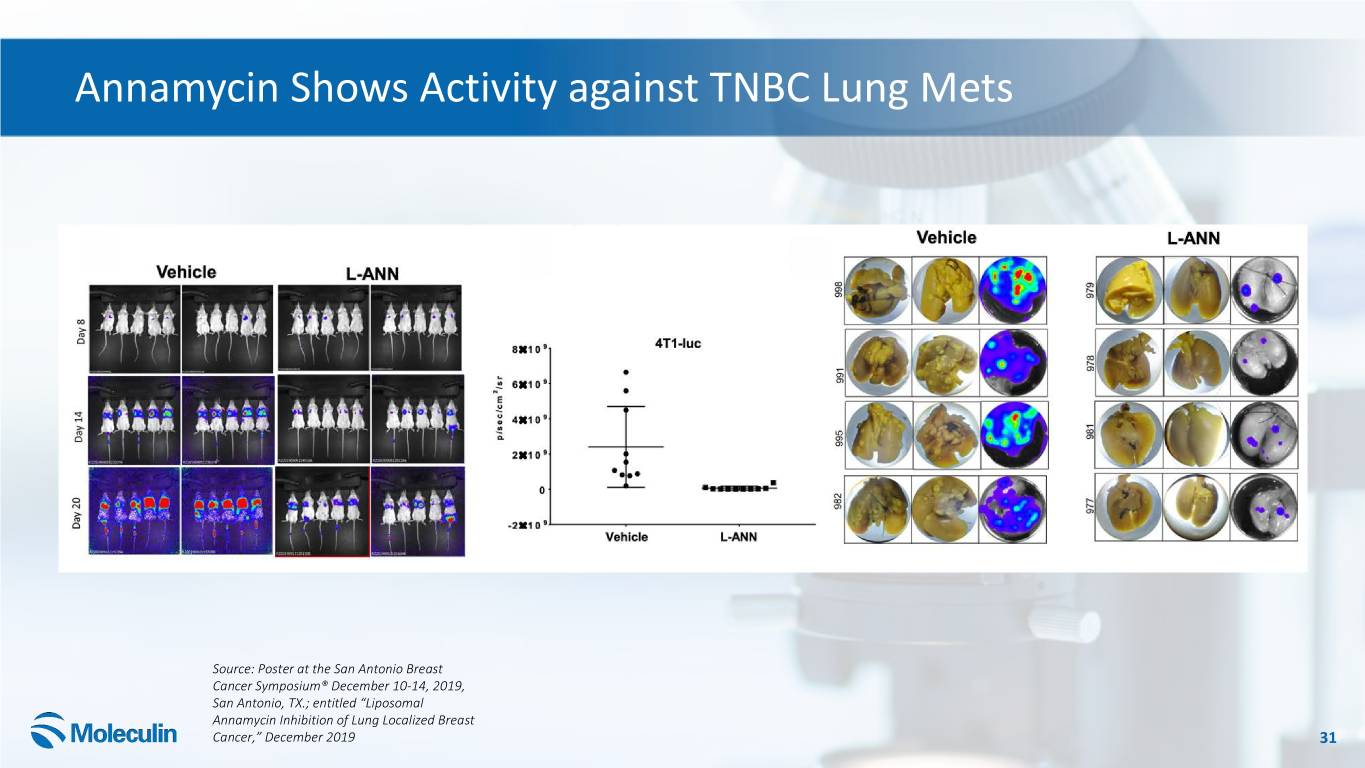
Annamycin Shows Activity against TNBC Lung Mets Source: Poster at the San Antonio Breast Cancer Symposium® December 10-14, 2019, San Antonio, TX.; entitled “Liposomal Annamycin Inhibition of Lung Localized Breast Cancer,” December 2019 31

Annamycin Also Shows Activity against Colon Cancer Lung Mets Source: Poster at the San Antonio Breast Cancer Symposium® December 10-14, 2019, San Antonio, TX.; entitled “Liposomal Annamycin Inhibition of Lung Localized Breast Cancer,” December 2019 32
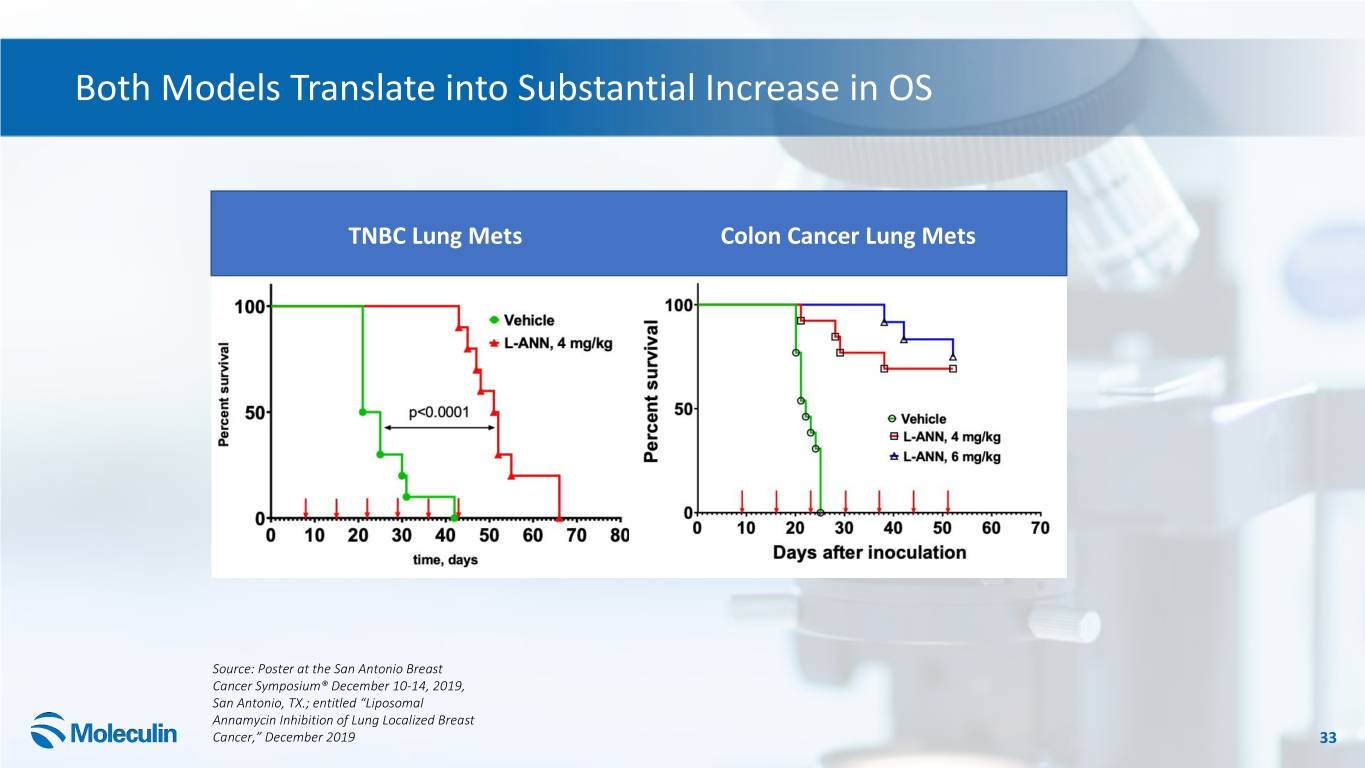
Both Models Translate into Substantial Increase in OS TNBC Lung Mets Colon Cancer Lung Mets Source: Poster at the San Antonio Breast Cancer Symposium® December 10-14, 2019, San Antonio, TX.; entitled “Liposomal Annamycin Inhibition of Lung Localized Breast Cancer,” December 2019 33

Annamycin Recap & Status • Annamycin is a “Next Generation” anthracycline designed to be non-cardiotoxic and avoid multidrug resistance • Although impressive activity was shown in prior acute leukemia trials, developer did not properly close out or establish an appropriate RP2D • Moleculin is repeating Phase 1/2 both in US and EU • US (MB-104) and EU (MB-105) trials have begun treating patients; already showing significant activity in dose escalation phase • Repeat of prior results should afford Annamycin an accelerated approval pathway as a 2nd line induction therapy for R/R AML • Recent animal research shows ability of Annamycin to accumulate preferentially in the lungs, liver and pancreas and substantial activity has been demonstrated in metastases to the lungs 34

WP1066 (Immune / Transcription Modulators) Based On Natural Built from chemical backbone of propolis (caffeic acid STAT3 is Activated by Multiple Upstream Compound benzyl ester) Effectors Unique Dual Action First-in-class drug to both directly inhibit tumor signaling (p-STAT3, HIF-1⍺, c-Myc) while also stimulating patient immune response (Tregs) Activity Against Hardest-to-Treat Pre-clinical testing shows high level of activity against Cancers pancreatic cancer, metastatic melanoma, glioblastoma and others; yet very low potential for toxicity Independently Subject of numerous peer reviewed journals validating Validated findings across multiple institutions around the world 35

STAT3 is a Master Regulator of Tumor Progression Triggering oncogenic transcription activity: • Survival Activated STAT3 • Proliferation Resulting in tumor (p-STAT3) binds to • Inflammation growth and DNA • Invasion metastasis • Metastasis • Angiogenesis • Immunosuppression 36

WP1066 Affects Tumors Directly & Indirectly WP1066 WP1066 modulates transcription factors resulting in direct tumor cytotoxicity while also Immune Transcription stimulating a natural Modulation Modulation immune response by reducing Regulatory T-cells (Tregs) Tumor 37

WP1066 & WP1220 Recap & Status • WP1066 is a small molecule that inhibits p-STAT3 by accelerating proteasomal degradation (not by blocking phosphorylation) without respect to upstream signaling; WP1220 is an analog of WP1066 formulated for topical delivery; WP1066 also inhibits HIF1-α, c-Myc and Tregs • WP1066 is currently in Phase 1 trial at MD Anderson for GBM and melanoma metastasized to the brain (WP1066 crosses BBB); in 3rd cohort of dose ranging; planned surgical expansion • Oral administration (due to lack of solubility) is demonstrating bioavailability in patients; recently began IND-enabling work on WP1732, a fully water-soluble analog of WP1066 that does not cross BBB, and shows impressive accumulation in pancreas • WP1220 is nearing completed a proof of concept trial for the topical treatment of CTCL (next slide) • Recent research suggests WP1066 has the ability to (a) counteract resistance to checkpoint blockade therapy (specifically, immune checkpoint target PD-L1) and (b) induce immune reprogramming in immune-competent mice treated with both radiation and WP1066 (mice developed an immunological memory that prevented regrowth of GBM tumor after tumor cells were reintroduced, resulting in long-term survivors; note: mice with a compromised immune system did not show this effect) 38

WP1220 Proof of Concept Trial - CTCL • Results: 5 patients enrolled between March and July 2019 with 3 patients currently showing efficacy and final patient data to be updated soon. • Three are evaluable for both safety and efficacy after completing 3 months of treatment, with 2 ongoing and evaluable for safety. The only AE reported potentially related to study drug in one of the five patients was a mild contact dermatitis not requiring treatment. • CAILS scores on index lesions were significantly decreased in the first 3 patients. A composite score was obtained for all treated lesions for each patient, and percent changes were calculated from baseline to Day 84. There was a median reduction > 50% (currently 70.8% - range 62.1%-76.2%) for the 3 patients. • Improvement was noted as early as 7 days after initiation of treatment, and maintenance of improvement was also shown at follow up (1 month after discontinuation, as per protocol). The fourth patient has also shown an initial reduction in the composite CAILS score after 56 days (26.7%) and is continuing on treatment. Evaluations of the biopsy samples for histopathology and status of p- STAT3 in treated lesions are in progress. Updated results expected soon. • Conclusions: WP1220, an inhibitor of p-STAT3, has shown demonstrable safety and significant efficacy after at least 3 months of topical treatment in 3 patients with progressive MF, with a continuing trend towards improvement in additional patients currently in treatment. This is the first demonstration that inhibition of the STAT3 activation pathway with topical therapy has impacted the course of this disease. The trial is continuing and updated and more comprehensive data from this study as well as assessment of STAT3 phosphorylation in treated lesions will be reported. 39

WP1122 (Metabolism/Glycosylation Inhibitor) Addicted to Sugar A brain tumor requires as much as 18 to 37 times as much Tumors are hyper-consumers glucose to survive as a healthy brain cell of glucose and can be starved to death without it Starving a Tumor to This eventually led to the theory that, if we feed tumor cells Death a glucose decoy (one that can’t convert into energy), we can kill the tumor This works well in a laboratory setting, but the problem is making these decoys drugable Breakthrough WP1122 is a prodrug of 2-deoxyglucose (2-DG) that increases design half-life, enables transmission across the blood brain barrier and improves other drug-like properties Tumor cell Potential to Change WP1122 (at suboptimal doses) performs as well or better Standard than temozolomide in live human brain tumors; even better of Care performance by combining the two drugs was shown in trials Normal cell 40

WP1122 is Effective In Vivo against Gliomas July 2017 WP1122 used alone has the same or greater activity than temozolomide (Temodar®), a current standard of care in patients diagnosed with glioblastoma Control Temodar WP1122 10 • Human brain tumors 8 injected into mice • WP1122 performed as 6 well or better than 4 temazolomide # of Animals # of • Not shown is that a 2 Orthotopic combination of both 0 Glioblastoma performed even better 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 Model in Mice Days 41

WP1122 Shows In Vivo Activity Against Pancreatic Cancer January 2018 42

WP1122 Recap & Status • WP1122 is a prodrug of 2-DG that increases circulation time and ability to cross BBB and provides other drug-like properties • Utilizing Warburg principle, WP1122 converts to 2-DG in highly glycolytic tumor cells causing autophagy due to energy starvation • Evaluating oral versus IV administration and about to begin IND-enabling work • Recent published literature suggests that 2-DG may also enable deglycosylation of PD-L1 (see abstract in following slide) • WP1234 is an analog of WP1122 with improved organ distribution to the pancreas 43

Financial Review 44

Financial Statement Summary In thousands, except for share and per share data and shares For the 9 Mos For the Year Ended outstanding Ended 09/30/19 12/31/18 (Unaudited) (Unaudited) Statement of Operations Data Revenue - - • ”Capital efficient” Research and development $7,816 $9,728 approach General and administrative and depreciation 4,895 5,297 • Leverage R&D with Loss from operations (12,711) (15,025) Net loss (9,408) (11,876) contractors, sponsored Net loss per common share – basic and diluted $ (0.24) $ (0.46) research, and investigator Basic and diluted weighted average shares outstanding 39,034,303 25,904,170 led trials Balance Sheet Data Cash and cash equivalents $ 15,409 $ 7,134 • Four clinical trials on a Prepaid expenses and other 3,177 840 $10M run-rate R&D spend Total current assets 18,586 7,974 Total assets $30,398 $19,585 Total current liabilities 11,119 3,878 Total liabilities 11,423 5,313 Total stockholders’ equity 18,975 14,272 Total liabilities and stockholders’ equity $30,398 $19,585 The above financial information is summarized from the Company’s most recent 10-K AND 10-Q filed with the SEC (www.sec.gov) 45

Various Sources of Financing Opportunistic Approach • Approx. $20 million raised YTD • Three analysts have reported on MBRX – Roth, Maxim, & Opco • Overnight and intraday deals • ATM – Opco • Equity Line - Lincoln Park Capital • Successful out-licensing of technology in low priority areas The above financial information is summarized from the Company’s most recent 10-K AND 10-Q filed with the SEC (www.sec.gov) 46

4 7 Milestones & Accomplishments 47

Highly Experienced Leadership 48

Executive Management Team Selected Prior Experience Walter Klemp, Chairman, President & CEO Don Picker, PhD Chief Science Officer Jonathan P. Foster, CPA, CGMA EVP & CFO Robert Shepard, MD, FACP Chief Medical Officer Sandra Silberman, MD & PhD Chief Medical Officer – New Products 49

Science Advisory Board Waldemar Elihu Estey, Priebe, PhD MD John Paul Waymack, Marty MD, SCD Tallman, MD Jorge Cortes, James Abbruzzese, MD MD 50

Board of Directors Robert E. George - Chair of Audit and Nominating & Governance Committees Mr. George joined our board of directors upon our IPO. He was a partner with the international accounting firm of PricewaterhouseCoopers (PWC) for 27 years until 2010. Mr. George currently serves as Chairman of the Audit Committee for The University of Texas Health Science Center at Houston and, since June 2011, has been a member of The University of Texas at Austin, McCombs Graduate School of Business accounting faculty. Michael D. Cannon - Chair of Compensation Committee Between 1997 and 2004, Mr. Cannon was the Chief Science Officer, EVP and a Director of SICOR, Inc. until its acquisition by Teva Pharmaceutical Industries, Inc. SICOR focused on generic finished dosage injectable pharmaceuticals, active pharmaceutical ingredients and generic biopharmaceuticals. From July 2005 to December 2009, Mr. Cannon was a member of the scientific advisory board of Trevi Health Ventures LP. Mr. Cannon currently serves on the board of directors of other privately held biotech companies. John M. Climaco, JD – Lead Independent Director Most recently the Executive Vice President of Perma-Fix Medical S.A, a Polish subsidiary of the Perma-Fix Environmental Services, Inc. (NASDAQ: PESI) where he has served as a director since 2013. From 2003 to 2012, Mr. Climaco served as President and Chief Executive Officer, as well as a member of the Board of Directors of Axial Biotech, Inc., which he cofounded in 2003. Mr. Climaco has served as a member of the Board of Directors for Digirad Corporation (NASDAQ: DRAD), PDI, Inc. (NASDAQ: PDII) and InfuSystem Holdings, Inc. (NASDAQ: INFU). From 2001 to 2007, he practiced law for the firm of Fabian and Clendenin. Mr. Climaco holds a J.D. from the University of California Hasting College of the Law. 51

5 2 Partnerships & Collaborations The University of Texas MD Anderson Cancer Center Moleculin actively sponsors ongoing research at The University of Texas MD Anderson Cancer Center. MD Anderson is the largest cancer research center in the world and one of the country’s largest recipients of NIH medical research grants. Dr. Waldemar Priebe, a Professor of Medicinal Chemistry in the Department of Experimental Therapeutics, Division of Cancer Medicine at the University of Texas MD Anderson Cancer Center, discovered the molecules that form the basis for our lead drug candidates. MD Anderson has granted Moleculin royalty-bearing, worldwide, exclusive licenses for the patent and technology rights to all of Moleculin’s drug technologies and continues to collaborate with the Company to translate research discoveries into clinical therapies. Emory University Moleculin entered into an agreement with Emory University to enable expanded cancer research on Moleculin’s WP1066 molecule for the possible treatment of medulloblastoma, a pediatric malignant primary brain tumor. Physician-scientists at Emory University and Children’s Healthcare of Atlanta have requested support to continue research aimed at the development of a novel treatment of medulloblastoma using WP1066 and Moleculin has agreed to supply them with a pure form of WP1066 for preclinical testing for the potential treatment of medulloblastoma. Emory studies so far have indicated that medulloblastoma may be particularly vulnerable to the ability of WP1066 to block the activated form of STAT3, a key signaling protein believed to contribute to the growth and survival of many tumors, including medulloblastoma. Mayo Clinic Research Endeavor Moleculin entered into an agreement with a physician at the Mayo Clinic to enable additional research on Moleculin’s WP1066 molecule for the possible treatment of a rare form of pediatric brain tumor. Mayo Clinic physician-scientists have requested and Moleculin has agreed to supply them with WP1066 for preclinical testing for the potential treatment of pediatric Diffuse Intrinsic Pontine Gliomas (DIPG), a rare and very aggressive form of brain tumor. Mayo Clinic studies have suggested that DIPG may be particularly sensitive to the inhibition of the activated form of a cell-signaling protein called STAT3, a primary target of WP1066, and their preliminary studies have demonstrated significant anti-tumor activity of WP1066 in DIPG in vitro and in vivo tumor models. The University of Iowa Moleculin entered into an agreement with The University of Iowa Pharmaceuticals for the development of a formulation for WP1732, a new molecule for cancer treatment. Based on preclinical testing, WP1732 is believed to be a breakthrough discovery and represents a major expansion of its STAT3 inhibition capability by providing a highly soluble alternative that is ideally suited for IV administration. This agreement marks the beginning of creating a preclinical package to submit to the FDA in order to request Investigational New Drug status. 52

5 3 Partnerships & Collaborations Medical University of Gdansk The Medical University of Gdańsk (MUG) is the largest medical university in northern Poland, located in one of the most beautiful cities in Europe with an old town and beautiful sandy beaches. The MUG educates nearly 6,000 undergraduate and postgraduate students at four Faculties: Faculty of Health Sciences, Faculty of Medicine, Faculty of Pharmacy and the Intercollegiate Faculty of Biotechnology. The MUG offers Premedical Course, Medicine Doctor Programme, Nursing Programme, Nutrition and Dietetics Programme and the Ph.D. Programmes, which are taught fully in English. University of Bergen Moleculin entered into an agreement to collaborate with the University of Bergen to expand research on inhibition of brain metastasis by Moleculin’s pre-clinical drug WP1066 and its unique ability to increase immune system response to cancer and suppression of tumor cell proliferation and survival. Additionally, Moleculin entered into a second collaborative agreement with the University of Bergen to test WP1122 in combination with the drug Avastin(R) (bevacizumab) made by Roche Pharma. Roche Pharma is not a party to the collaborative agreement. DERMIN Sp. z o. o. Dermin Sp. z o. o. (Dermin) is a Polish drug development company that has sublicensed our technologies for use in Poland. We are collaborating with Dermin to conduct a clinical trial in Poland to study WP1220 for the topical treatment of early stage cutaneous T-cell lymphoma or CTCL patients, as well as to coordinate clinical trials of Annamycin in AML patients between clinical sites in Poland and other countries. We are also collaborating with Dermin to prepare WP1122 for clinical development for the possible treatment of glioblastoma. WPD Pharmaceuticals Sp z.o.o. WPD Pharmaceuticals Sp. z.o.o. is a Polish drug development company that has sublicensed our technologies for use in limited territories, including Poland. We are collaborating with WPD to conduct future clinical trials in Poland using one of our licensed drugs utilizing Polish grant monies. Annamycin targeting lung sarcomas is an initial target for WPD. WPD has already received grant funding in excess of $29 million for other drugs. 53

Key Points Potential Blockbuster Technologies 3 Noncardiotoxic anthracycline Phase 1 clinical trials 5 STAT3 Inhibitor Drugs showing early activity 2 Inhibitor of glycolysis 2020 Phase 2 trials expected 2 World class collaboration Highly experienced team Exclusive IP/Fast Track 54

Developing breakthrough therapies for highly resistant cancers Walter Klemp, Chairman & CEO / Jonathan Foster, EVP & CFO info@moleculin.com (713) 300-5160 (Nasdaq: MBRX)
