Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Tempest Therapeutics, Inc. | tm201887-1_8k.htm |
Exhibit 99.1

Exhibit 99.1 Corporate Presentation January 2020

Cautionary Statement Regarding Forward-Looking Statements Certain statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Such forward-looking statements are based on Millendo’s expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, our plans to develop and commercialize our product candidates; the progress and timing of our ongoing and planned clinical trials for our product candidates, including the timing of topline results from the Phase 2b portion of our Phase 2b/3 clinical trial of livoletide in Prader Willi syndrome (“PWS”) patients and the timeline for our Phase 2b clinical study of nevanimibe in congenital adrenal hyperplasia, the potential and timing for a neurokinin 3 receptor antagonist (MLE-301) as a potential treatment of vasomotor symptoms to enter clinical trials; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; and our estimates regarding future revenue, if any, future expenses, the funding of our operations, including whether our cash balance will be sufficient to fund our current operating plans beyond anticipated initial topline results from our livoletide pivotal study in PWS in 1H20, as well as our future capital requirements and needs for additional financing. You should refer to the risk factor disclosure set forth in the periodic reports and other documents we file with the Securities and Exchange Commission available at www.sec.gov, including without limitation, our Annual Report on Form 10-K for our fiscal year ended December 31, 2018 and our Quarterly Report on Form 10-Q for our fiscal quarter ended September 30, 2019. New factors emerge from time to time and it is not possible for Millendo to predict all such factors, nor can Millendo assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements included in this presentation are based on information available to Millendo as of the date of this presentation. Millendo disclaims any obligation to update such forward-looking statements to reflect events or circumstances after the date of this presentation, except as required by applicable law. 2

Millendo Therapeutics (Nasdaq: MLND) A leading endocrine disease company A LEADER IN ENDOCRINE DISEASES Clinical-stage company focused primarily on orphan endocrine significant unmet medical needs diseases with Livoletide: Pivotal stage asset for Prader-Willi syndrome (PWS) Topline results expected 1H20 that could potentially support NDA filing COMPELLING PIPELINE Nevanimibe: Clinical stage asset for classic congenital adrenal hyperplasia (CAH) Ongoing Phase 2b trial with topline data from cohort 1 expected 2H20 MLE-301: Preclinical program for vasomotor symptoms (VMS) associated menopause, with first-in-human trial expected in 2020 with Experienced leadership team to execute on company strategy Financial strength with cash balance of $63.5M* as of December 31, 2019 POSITIONED FOR SUCCESS * unaudited; includes cash, cash equivalents and restricted cash 3
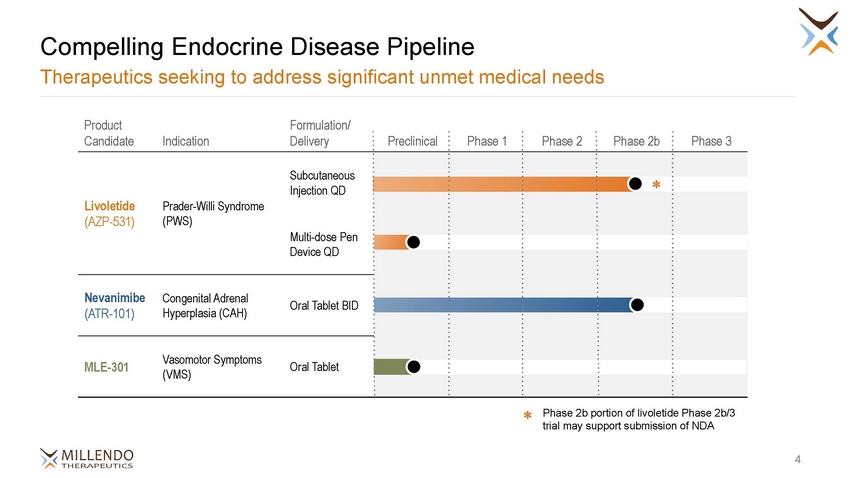
Compelling Endocrine Disease Pipeline Therapeutics seeking to address significant unmet medical needs Product Formulation/ Oral Tablet BID (ATR-101) Hyperplasia (CAH) Oral Tablet (VMS) Phase 2b portion of livoletide Phase 2b/3 trial may support submission of NDA ✽ 4 CandidateIndicationDelivery Preclinical Phase 1 Phase 2 Phase 2b Phase 3 Subcutaneous Injection QD LivoletidePrader-Willi Syndrome (AZP-531)(PWS) Multi-dose Pen Device QD ✽ NevanimibeCongenital Adrenal MLE-301Vasomotor Symptoms

Livoletide for Prader-Willi Syndrome

Prader-Willi Syndrome (PWS) Hyperphagia, a chronic & insatiable hunger, is the top unmet need for PWS patients Livoletide Hyperphagia starts at 5-8 years old and usually lasts until death • • • Leads to overeating and eventual metabolic issues A root cause of morbidity and mortality Patient accidents, including choking, are a significant cause of death Creates significant burden on caregivers and siblings No approved treatment; coping mechanisms include environmental controls such locking refrigerators and cabinets • • Mortality rate is 3x general population • • Average age of death is approximately 30 years* Rate has been relatively unchanged over past two decades * Butler et al. (2017); Manzardo (2018) 6 Urgent need for new therapies as current treatments do not address hyperphagia

Most PWS Patients Are Diagnosed No medicines available to address hyperphagia Livoletide Birth incidence of 1:15K due to a spontaneous genetic defect •Complex disorder resulting from genetic error on chromosome 15 US PWS Market 12,000 10,000 8,000 6,000 4,000 2,000 0 Metabolic comorbidities more common in adolescents and adults • 25% of adult PWS and 9% of adolescent PWS patients have T2D Mean BMI of 41 in adults • Diagnosed hyperphagic PWS population US PWS prevalence Diagnosed PWS population Internal analysis based on McCandless et al. (2019), US Prevalence & Mortality of Prader-Willi syndrome: A Population-based Study of Medical Claims; and Grugni (2008) 7 10,000 8,900 Undiagnosed PWS population 7,000 Pediatric patients; hyperphagia not yet developed

Potential First-In-Class Treatment of Hyperphagia in PWS Distinct pharmacology with objective of replacing functional deficit of UAG Livoletide PWS Patient Journey AG and UAG Levels by Age and Nutritional Phase Hyperphagic aily injectable cyclic rable in vitro and in pharmacological profiles , 2 stable than linear Age: ~0-9 mos. ~9-25 mos. ~2.1-4.5 yrs. ~4.5-8 yrs. ~8 yrs.-adult Healthy Obese Nutritional Phase: 1a Hypotonia w/ difficulty feeding 1b No difficulty feeding, growing 2a Weight increasing w/o increase in 2b Weight increasing w/ increase in 3 Hyperphagic, rarely feels full appropriately appetite appetite Adapted from Kuppens et al. (2015) and Miller et al. (2011) 1Delhanty (2013), 2Granata (2012), 3Julien (2012) 8 Livoletide: an analogue of UAG es in human plasma improved in vivo PK3 • Once-d peptide • Compa vivo to UAG1 • More analogu and has
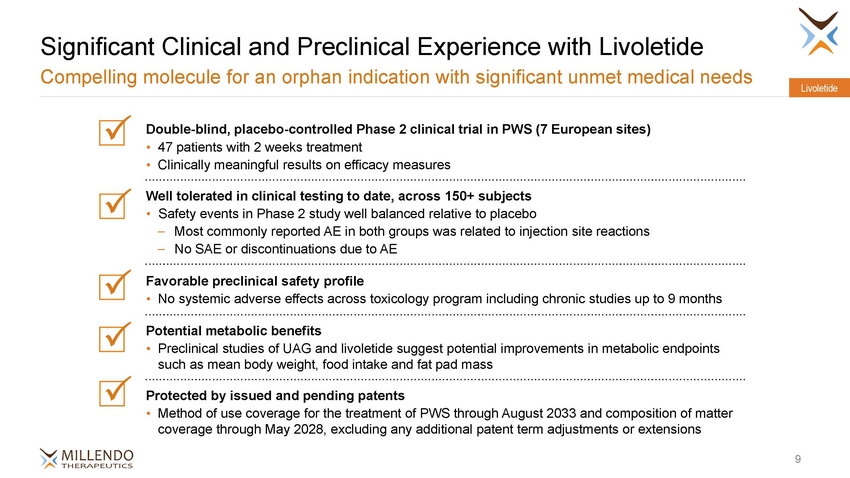
Significant Clinical and Preclinical Experience with Livoletide Compelling molecule for an orphan indication with significant unmet medical needs Livoletide Double-blind, placebo-controlled Phase 2 clinical trial in PWS (7 European sites) • 47 patients with 2 weeks treatment • Clinically meaningful results on efficacy measures Well tolerated in clinical testing to date, across 150+ subjects • Safety events in Phase 2 study well balanced relative to placebo –Most commonly reported AE in both groups was related to injection site reactions –No SAE or discontinuations due to AE Favorable preclinical safety profile • No systemic adverse effects across toxicology program including chronic studies up to 9 months Potential metabolic benefits • Preclinical studies of UAG and livoletide suggest potential improvements in metabolic endpoints such as mean body weight, food intake and fat pad mass Protected by issued and pending patents • Method of use coverage for the treatment of PWS through August 2033 and composition of matter coverage through May 2028, excluding any additional patent term adjustments or extensions 9

2-week Placebo-Controlled Phase 2 PWS Clinical Trial Clinically meaningful decreases in Hyperphagia Questionnaire (HQ) Livoletide Home Residents BL > 10 Home Residents1 All Subjects BL = 12.2 14.1 BL = 12.6 14.8 BL = 17.3 17.9 0 -1 -2 -3 -4 0 -1 -2 -3 -4 -5 -6 -7 0 -1 -2 -3 -4 -5 -6 -7 Change in HQ from baseline Change in HQ from baseline Change in HQ from baseline -5 -6 -7 -4.3 -5.1 -6.2 p = 0.097 p = 0.034 p = 0.019 placebo livoletide 60 μg/kg Allas et al., 2018 HQ is a 9-item, validated behavioral questionnaire to assess food-related behaviors and administered to caregivers. HQ score adjusted for 0 to 36 scale to reect the 9 item HQ-CT. BL= Baseline. 1 Home residents excludes subjects residing in hospital setting; analysis was pre-specified. 10 12 14 -1.0 20 18 -1.6 24 23 -1.6 Similar to the target patient population for pivotal study

Treatment Response Seen Across Broad Set of Livoletide Patients 65% showed ≥ 4-point decrease in Hyperphagia Questionnaire (HQ) Livoletide 40% Home Resident Patients with BL ≥ 10 30% 20% 10% placebo (n=12) livoletide 60 μg/kg (n=14) 0% Worsening No change -1 to -3 -4 to -6 > -6 Improvement Allas et al., 2018 Source: Derived from AZP01-CLI-002 Clinical Study Report 11 % of participants Improvements observed across all 9 items in questionnaire 36% 33% 29% 25%25% 8% 0% 14% 21% 8%

Livoletide PWS Pivotal Study (ZEPHYR) Single, pivotal Phase 2b/3 protocol Livoletide 3-month endpoint Placebo crossover / 9-month extension Phase 2b Placebo crossover / 6-month extension Phase 3 6-month endpoint • Provide flexibility to adjust endpoints and patient population ClinicalTrials.gov Identifier: NCT03790865 12 Successfully recruited 158 patients in eight months for Phase 2b portion of ZEPHYR • Additional cohort of PWS patients ages 4 to 7 will continue to be recruited at active sites Randomized, double-blind, placebo-controlled study • Primary endpoint HQ-CT • PWS patients 4-65 years of age, baseline HQ-CT ≥ 10 • 38 sites in U.S., EU and Australia Phase 2b portion of study may support NDA submission Long-term safety extensions will provide up to 12 months of exposure • EAP program allows for additional long-term data Phase 3 portion will • Assess longer-term durability of effect for hyperphagia • Assess metabolic endpoints that may require longer exposures • Help address data requirements across multiple regulatory agencies Placebo (n~40) livoletide (n~40) Expanded Access Program (EAP) Placebo (n=50) 60 µg/kg livoletide* (n=50) 120 µg/kg livoletide (n=50)
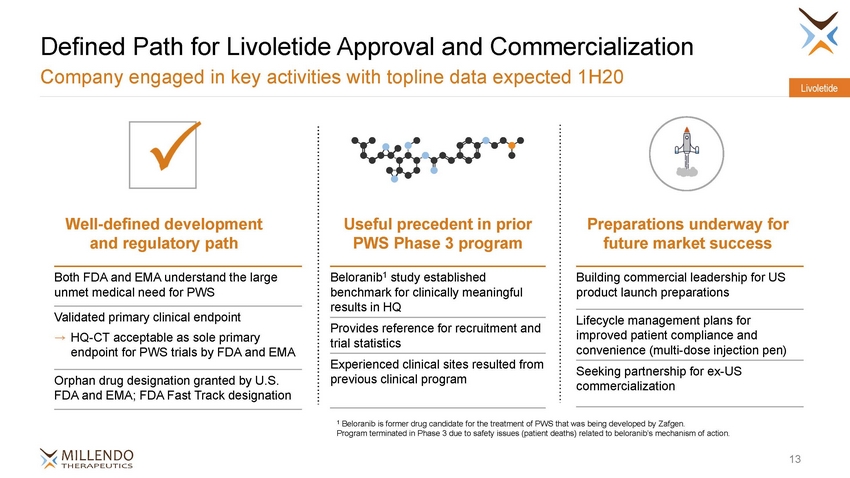
Defined Path for Livoletide Approval and Commercialization Company engaged in key activities with topline data expected 1H20 Livoletide Useful precedent in prior PWS Phase 3 program Preparations underway for Well-defined development and regulatory path future market success Beloranib1 study established benchmark for clinically meaningful results in HQ Both FDA and EMA understand the large unmet medical need for PWS Building commercial leadership for US product launch preparations Validated primary clinical endpoint → HQ-CT acceptable as sole primary endpoint for PWS trials by FDA and EMA Lifecycle management plans for improved patient compliance and convenience (multi-dose injection pen) Provides reference for recruitment and trial statistics Experienced clinical sites resulted from previous clinical program Seeking partnership for ex-US commercialization Orphan drug designation granted by U.S. FDA and EMA; FDA Fast Track designation 1 Beloranib is former drug candidate for the treatment of PWS that was being developed by Zafgen. Program terminated in Phase 3 due to safety issues (patient deaths) related to beloranib’s mechanism of action. 13

Nevanimibe for Congenital Adrenal Hyperplasia

Congenital Adrenal Hyperplasia Goal: Restore Hormonal Balance Achieve physiologic exogenous cortisol doses without excessive androgens Nevanimibe U.S. 18,000 ~40,000 MONOGENIC EUROPE diagnosed at birth Based on Han, Nature Reviews 2013 15 Potentially fatal inability to produce cortisol necessitating chronic treatment CAH PREVALENT CASES 15,000– CAH is a DEFICIENCY through universal screening Doctors’ Current Trade-off (80% of CAH patients out of balance) Excess Cortisol Excess Androgens Psychological effects Hirsutism; amenorrhea Short stature Early puberty Infertility Blood pressure Psychological effects Insulin resistance Short stature Osteoporosis Obesity

Nevanimibe for Congenital Adrenal Hyperplasia in patients (CAH) Potential to achieve better hormonal balance Nevanimibe + Nevanimibe BALANCE Clinical Proof of Concept • Open-label Phase 2 clinical trial in CAH • Favorable safety profile in preclinical and clinical studies Normal levels of ANDROGENS Normal levels of CORTISOL CAH Ph2b Phase 2b clinical trial in CAH ongoing • Orphan drug designation granted by U.S. FDA and EMA Nevanimibe aims to avoid excess hormone symptoms and minimize exogenous cortisol 16 ACAT1 Inhibitor: novel, adrenal-selective MOA inhibits all steroidogenic pathways by targeting starting point of adrenal steroid synthesis

Nevanimibe Proof of Concept Established in CAH Activity demonstrated through reductions in 17-OHP Nevanimibe • Mean change in 17-OHP by Study Visit Phase 2 Study Design placebo nevanimibe Intrasubject dose escalation study (n=10) • 2-week treatment and placebo periods through reduction in key steroids Biological effect demonstrated 17-OHP (ng/dL) • 7 of 10 of subjects demonstrated a biological effect • 2 of 10 subjects met the primary endpoint (17-OHP < 2x ULN) • 70% of subjects experienced a decrease in 17-OHP of ≥ 50% during at least one treatment period well tolerated Nevanimibe was reported to be Visits 1-4 n=10; Visits 5-7 n=9; Visits 8-13 n=8 17

Nevanimibe CAH Phase 2b Study Intrasubject dose escalation study with 16 weeks continuous dosing Nevanimibe Cohort 1: Patients with elevated androgen and 17-OHP levels 17-OHP ≥ 4x ULN baseline period Primary Endpoint N=20-24 (10-12 per cohort) Cohort 2: Patients on high glucocorticoid (GC) doses 17-OHP < 4x ULN GC down-titration/17-OHP stabilization baseline period 17-OHP < 2x ULN 10-12 sites 16 WEEKS OF CONTINUOUS NEVANIMIBE DOSING 500 mg BID, 1000 mg BID, 1500 mg BID, and 2000 mg BID ● Dose titration based on 17-OHP levels Study status: • Topline results from the ongoing Phase 2b study of nevanimibe in patients with anticipated for cohort 1 in 2H20 CAH ClinicalTrials.gov Identifier: NCT03669549 18

MLE-301 for Vasomotor Symptoms

MLE-301, a Selective NK3R Antagonist for Vasomotor Symptoms Over 20 million women in the U.S. suffer from VMS associated with menopause MLE-301 • MLE-301 is a potent, selective antagonist of NK3R KNDy neurons are overactive in menopausal women with VMS KNDy Neuron • NK3R plays a key role in regulating KNDy neurons, which are overactive in menopausal women and play an important role in vasomotor symptoms (VMS) – Robust genetic, pharmacodynamic, and clinical data support its role in VMS GnRH neuron • Early development program will efficiently both safety and efficacy assess ‘Heat dissipation’ neuron Overactive KNDy neurons propagate heat dissipation signals • First clinical trial initiation anticipated in 2020 Vasomotor Symptoms 20 MLE-301 marks a return to a field that Millendo helped advance

Corporate

Significant Potential Value Creation Meaningful corporate and clinical milestones over the next 12 months 1Q19 Initiated ZEPHYR pivotal Phase 2b/3 study of livoletide in PWS 2Q19 Established foundation for commercial organization in Lexington, MA 4Q19 ZEPHYR recruitment completed for Phase 2b portion of study 1H20 Livoletide Phase 2b PWS study topline results 2H20 Nevanimibe Phase 2b CAH study topline results (cohort 1) 2020 MLE-301 Phase 1 study initiation 22 Our mission: to relentlessly pursue therapies that alleviate patient suffering due to orphan endocrine diseases

Millendo is a Leading Company in Endocrine Diseases Topline data readouts in 2020 set the stage for upcoming milestones Livoletide, a potential first-in-class treatment for hyperphagia in PWS patients, with topline data expected 1H20 and significant near-term value creation Nevanimibe for the treatment of CAH, expected to have topline data for cohort 1 in 2H20 MLE-301 for VMS associated with menopause moving to the clinic in 2020 Experienced leadership team to execute on company strategy Financial strength with cash balance of $63.5M* as of December 31, 2019 * unaudited; includes cash, cash equivalents and restricted cash 23

Thank You!
