Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - Lipocine Inc. | tm201752d1_ex99-2.htm |
| 8-K - FORM 8-K - Lipocine Inc. | tm201752d1_8k.htm |

Enabling Oral Drug Delivery to Improve Patient Compliance January 2020 Corporate Presentation Exhibit 99.1

Forward - Looking Statements This presentation contains forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking statement s relate to the Company’s product candidates, the FDA regulatory process related to TLANDO™, the expected timing of Phase 3 trials for TLAND O X R and LPCN 1107 and Phase 2 studies for LPCN 1144 and LPCN 1148, clinical and regulatory processes and objectives, potential benefi ts of the Company’s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties. Actual results may differ materially from the forward - looking statements discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials and studies, the potential advantages of the Company’s product candidates and the Company’s capital needs. The forward - looking statements contained in this presentation are qualified by the detailed discussion of risks and uncertainties set forth in the Company’s annual report on For m 10 - K and other periodic reports filed by the Company with the Securities and Exchange Commission, all of which can be obtained on the Com pany’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations. 2

Clinical Stage Biopharmaceutical Company Innovative Product Candidates for Metabolic and Endocrine Disorders 3 PRODUCT (Indication) PRE - CLINICAL PHASE 1 PHASE 2 PHASE 3 NDA Propriety Drug Delivery Platform LPCN 1144 (Oral Testosterone for Pre - Cirrhotic NASH ) LiFT Phase 2 Paired Biopsy Clinical Study Ongoing TLANDO ™ (Oral Testosterone for Testosterone Replacement Therapy “TRT”) Post Action Meeting Scheduled Jan 2020 TLANDO XR (LPCN 1111, Long Acting Oral Testosterone for Testosterone Replacement Therapy “ TRT” ) Phase 3 Dose Identified LPCN 1148 (Oral Testosterone for Cirrhosis) IND Submission 1Q 2020 LPCN 1107 (Oral HPC for Prevention of PTB) Phase 3 Dose Identified

Enabling Oral Drug Delivery to Improve Patient Compliance LPCN 1144 for Pre - Cirrhotic NASH

LPCN 1144: Oral Testosterone Therapy Differentiated NASH Treatment Candidate Targets Unmet Need Mechanistic Evidence Clinical Experience • Efficacy – NASH resolution and/or fibrosis improvement • Acceptable tolerability for chronic use • Improvement of sarcopenia • Improvement of sexual dysfunction • Improvement of mental health • Anti - steatosis • Anti - inflammatory • Anti - oxidative • Cell regenerative • Meaningfully reduced liver fat • Well tolerated in 700+ subjects with up to 52 - week exposure • Improved sexual and mood dysfunction 5

Association Between T and Liver Disease Pre - clinical Evidence Mouse Model* Method ▪ Testicular - feminized Disease ▪ Hepatic Steatosis ▪ Liver Disease Induction Causes Lower T 6 Induction Method Model T Levels Gene - modified & Diet ob / ob (Obese Mouse) 1 ↓ T db / db (Diabetic Mouse) 2 ↓ T Chemical &/or Diet TAA (Mouse/Rat) 3 ↓ T CCL 4 (Rat/Mouse) 4 ↓ T Diet High Fat Diet (Rabbit) 5 ↓ T 1. Swerdloff et al., Endocrinol 1976 2. Yabiku et al., BMC Endocr Disord 2018 3. Lipocine TAA rat model study 2019 4. Elsawy et al., PeerJ 2019 5. Vignozzi et al., Mol Cell Endocrinol 2014 ▪ Low T Induces Liver Disease * Kelly et al., Life Sci 2014

7 Association Between T and Liver Disease Clinical Evidence 1. Sarkar et al., Gastroenterology 156(6):S - 1258 & Poster Sa1623, Digestive Disease Week 2019 2. Sumida et al., Gastroenterol Hepatol 2015 • Levels of free T decreased significantly with the increased incidence of lobular inflammation, hepatocyte ballooning, NAFLD activity score, and fibrosis 2. Free T (ng/dL) Free T (ng/dL) Fibrosis • Reportedly 75% of Biopsy - Confirmed NASH Male Patients Have Low T (< 372 ng/dL) 1
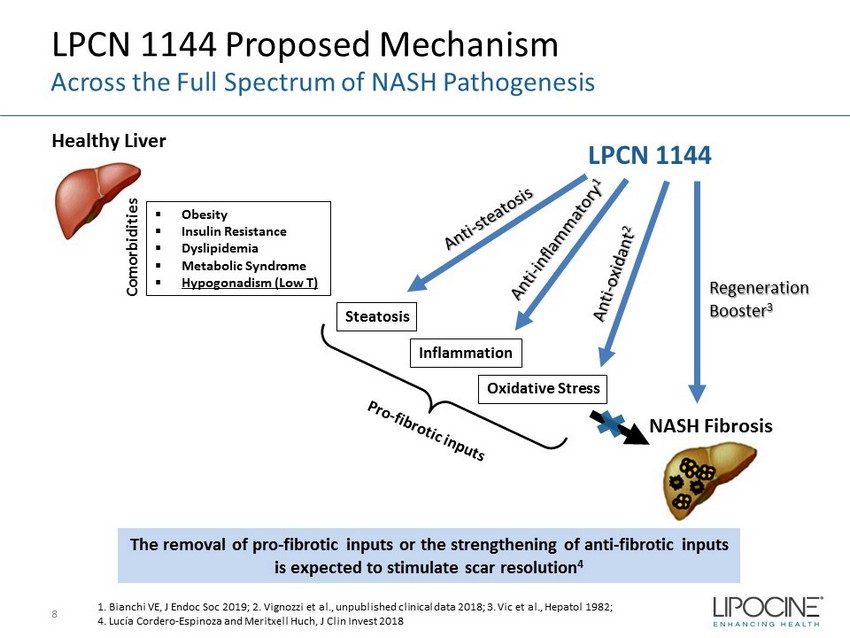
LPCN 1144 Proposed Mechanism 8 Across the Full Spectrum of NASH Pathogenesis Healthy Liver Steatosis Inflammation NASH Fibrosis LPCN 1144 Regeneration Booster 3 ▪ Obesity ▪ Insulin Resistance ▪ Dyslipidemia ▪ Metabolic Syndrome ▪ Hypogonadism (Low T) The removal of pro - fibrotic inputs or the strengthening of anti - fibrotic inputs is expected to stimulate scar resolution 4 Oxidative Stress 1. Bianchi VE, J Endoc Soc 2019; 2. Vignozzi et al., unpublished clinical data 2018; 3. Vic et al., Hepatol 1982; 4. Lucía Cordero - Espinoza and Meritxell Huch , J Clin Invest 2018 Comorbidities

- 42% - 40% -60% -50% -40% -30% -20% -10% 0% Mean BL LF = 18.3% Mean BL LF = 20.5% BL ≥ 8% BL ≥ 10% Relative % Change of Liver Fat % Mean Relative* Liver Fat % Change LPCN 1144: Liver Fat Imaging Study (“LFS”) Results Meaningful Relative Liver Fat % Change and Responder Rate 9 LFS was an open - label, multi - center single - arm 16 - week study (N=36) with LPCN 1144 in hypogonadal males (NCT03868059) LF = liver fat * Mean relative changes of liver fat % were obtained in subjects with BL liver fat ≥ 8% (n=10) and BL ≥ 10% (n=8). ** Responder rate for relative change is % of patients with at least 30% for relative reduction of liver fat % from baseline. 80% 75% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Mean BL LF = 18.3% Mean BL LF = 20.5% BL ≥ 8% BL ≥ 10% % of Responders Responder Rate** for Liver Fat % Change

■ NAFL Free (LF < 5%) ■ NAFL (LF ≥ 5%) 10 NAFLD Free NAFLD Free NAFLD Free NAFLD Free NAFLD Free NAFLD Free 52% 48% Interim (Week 8, N=31) NAFL Free NAFL 63% 38% End of Study (Week 16, N=32) NAFL Free NAFL 34% 66% Baseline (N=32) NAFL Free NAFL LPCN 1144: Longitudinal Treatment Effect Improved NAFL Resolution Over Time NAFL is non - alcoholic fatty liver

LPCN 1144: LiFT Study* Ongoing Liver Fat Intervention with Oral Testosterone Study 11 Phase 2 paired - biopsy clinical study in NASH subjects (NCT04134091) □ Study Design ▪ Three - arm (1:1:1 randomization, two treatments and placebo), multi - center, double - blind ▪ 225mg twice daily (450mg Daily) ▪ 20 - 25 biopsy confirmed NASH male subjects per arm with NAS ≥ 4 ▪ Treatment duration of 36 weeks □ Primary Endpoint ▪ Change in hepatic fat fraction via MRI - PDFF measure □ Secondary Endpoints ▪ Change in NASH activity and fibrosis via liver biopsy scoring ▪ Change in liver enzymes, anthropometric measure, lipids, insulin resistance, inflammatory/fibrosis markers, and labs ▪ Change in quality - of - life degree (SF - 36 and PDQ), weight, BMI, waist circumference, waist to hip ratio, and PAQ activity * Website: www.lift - study.com 24 Weeks 12 Weeks SCREENING TREATMENT: 36 WEEKS Topline MRI - PDFF Results ~Mid 2020 Dose Start Topline Biopsy Results 4Q 2020/1Q 2021 MRI - PDFF Biopsy

Testosterone Replacement Therapy

Confidential □ Close to 6M men with diagnosed hypogonadism 3 □ 2M men being treated 4 1. US Census data. http://www.infoplease.com/us/census/data/demographic.html. 2. Mulligan T, et al. Int J Clin Pract. 2006 Jul;60(7):762 - 9. 3. Araujo, et al. J Clin Endo Metabol 2007. 92(11):4241 - 7. 4. Symphony Healthcare 2014 for FDA Advisory Meeting. 5. IMS Health Sept 2015. Hypogonadism Affects Up to 20M Men 1, 2 Oral TRT has the Potential to Drive Market Expansion reated 1,2 Hypogonadism Under Treatment in US Undiagnosed Hypogonadism 70% Diagnosed Untreated 19% 67% 33% Treated 11% Treatment - naïve 5 Treatment - experienced 13

Testosterone Replacement Therapy (TRT) Market TRT Market is Growing Without an Oral Option 14 Source: Antares Pharma presentation at JEFFERIES 2019 HEALTHCARE CONFERENCE TRx = Total prescriptions 7.0M 7.9M 7% Up 13% Up 6.5M Total Injectables Topicals

Issues with Current Non - oral TRT Options Potential Barrier To Newly Diagnosed and Existing Patients • Black Box Warning – Secondary exposure to testosterone – Pulmonary oil micro embolism (POME) and anaphylaxis shock • Inconvenient application or painful injection • Poor persistence reflects need for oral – Average days on therapy is 100 days • More than 50% of patients need dosage adjustment – Burdensome for patients due to multiple doctor visits 15

16 ~$2B+ opportunity in an established and growing market

TLANDO Regulatory Update Post Action Meeting Scheduled for January 2020 • CRL received November 2019 – One deficiency • Did not meet the three secondary endpoints for maximal testosterone concentrations ( Cmax ) – No CMC deficiencies – FDA confirmed serum data reliable and reflects in vivo T measurements 17

Once Daily Oral Testosterone Replacement Therapy

Future Prescribing Patterns with Entrenchment of Product X BID Future Prescribing Patterns with Entrenchment of Product X QD CE11: Assume Product X is dosed QD vs, BID, adjust percentages to reflect intent - to - prescribe. 1. P<0.01 31% 4% 9% 1% 53% 1% 24% 4% 7% 1% 64% 1% 19 LPCN 1111: Market Research Physician Intent - to - Prescribe Statistically Higher 1 with a QD

Phase 2b Study: Result Summary Met Primary and Secondary Endpoints • Once daily dose for 14 days in an open label, multiple dose PK study in hypogonadal men (n=36 subjects) x Met primary and secondary endpoints x Phase 3 dose identified x No drug related SAEs x Drug related AEs are mild to moderate • Next step: Agree on the Phase 3 design with FDA Confidential 20 20

Upcoming Milestones 21 Near Term Value Drivers Event Expected Timing TLANDO™ Post Action Meeting January 2020 LPCN 1148 File Investigational New Drug Application 1Q 2020 TLANDO XR FDA feedback on Phase 3 protocol 2Q 2020 LPCN 1144 LiFT Study Primary Endpoint Results Mid - 2020

Key Financial Metrics 22 Stock Price, Market Cap, Cash Balance Ticker Symbol LPCN (Nasdaq Capital Market) Closing Stock Price (1/9/20) $0.42/share Market Capitalization (1/9/20) $15.6 million Cash Balance (9/30/19) $16.5 million* † Bank Debt (9/30/19) $7.9 million * $5M restricted and becomes unrestricted upon TLANDO approval † Lipocine raised $6M in gross proceeds from a Registered Direct Offering in November 2019

Lipocine Investment Highlights 23 Robust Pipeline • Unmet need with no approved drug • Most male NASH patients have low testosterone with signs and symptoms of hypogonadism • LPCN 1144 : A differentiated modality with potential for mono/combo pre - cirrhotic NASH therapy • LPCN 1148 : Targeting cirrhosis Oral testosterone targeted for Pre - Cirrhotic NASH/Cirrhosis • ~$2B+ opportunity in an established and growing market • Significant unmet need for an oral TRT • Differentiated product profile with potential for market expansion • Favorable market dynamics • TLANDO™ : BID testosterone • TLANDO XR : Unique long acting once - daily product candidate Potential to be a leader in TRT market in need of an oral option • LPCN 1107 : Superior Cavg to Makena® Orphan designated oral candidate for the prevention of preterm birth

24

Enabling Oral Drug Delivery to Improve Patient Compliance LPCN 1144 for Pre - Cirrhotic NASH

LPCN 1144: Rationale to Target Pre - Cirrhotic NASH 26 1. Estes et al., Hepatol 2018; 2. Williams et al., Gastroenterology. 2011. *near term target Multi - billion $ Opportunity 11 M Male* NASH Patients 17 M NASH Patients in 2015 1 83M NAFLD Patients in US, 2015 1 Underappreciated conditions Unmet Need Estimated Market Currently No Approved Treatment 3 Bhanji et al, Hepatol 2017 4. Hawksworth et al., Sex Med Rev 2019 5. Ali et al., Psychosomatics 2011 6. Assimakopoulos et al., J Psychosom Res 2018 65% 2 Efficacy – NASH Resolution and/or Fibrosis Improvement Acceptable Tolerability for Chronic Use Improvement of Sarcopenia 3 Improvement of Sexual Dysfunction 4 Improvement of Mental Health/QOL 5,6

LPCN 1144: Multi - Dimensional Mechanism of Action Across the Full Spectrum of NASH Pathogenesis 27 Homeostasis Modifier 1, 2 • Alter lipid, cholesterol, and glucose metabolism • Reduce visceral abdominal fat • Modify activity of hepatic lipase, and skeletal muscle/ adipose lipoprotein lipase Anti - inflammatory 2 / Antioxidant/Immuno - modulator 3 • Restore mitochondrial turnover and normalizes oxygen consumption 4 Regeneration Booster 5,6 • Stimulate satellite cells and myocyte precursor resulting in cell differentiation and myocyte proliferation 7 • Increases circulating endothelial progenitor cells (“EPC”) 8 Anabolic/Androgenic Agent 9 • T induces muscle fiber hypertrophy by promoting myogenesis by inhibiting adipogenesis 10 . • Inhibit myostatin 11 • Increase free T (lowering SHBG) • Improve sexual dysfunction 12 1. Shen and Shi, Int J Endocrinol, 2015 2. Kelly and Jones, J Endocrinol, 2013 3. Sinclair et al., J Gastroenterol Hepatol , 2015 4. Linda Vignozzi et al., University of Florence, IT, unpublished, 2018 5. A. Francavilla et al., Digest Dis Sci, 1989 6. Vic et al., Hepatol 1982 7. Sinha - Hikim et al., J Clin Endocrinol Metab , 2004 8. Liao CH et al., Andrology, 2013 9. Gentile MA et al., J Mol Endocrine, 2010 10. Bhasin S., J Gerontol 2003 11. Dasarathy and Merli , J Hepatol . 2016 12. Rizk et al., Curr Opin Urol 2017

Potential of Testosterone Therapy in NAFLD Preclinical Model Results 28 1. Kelly et al., Lif Sci 2014; 2. Nikolaenko et al., BMC Endocrinol 2014; 3. Cai et al., BMC Genomics 2015 Model Mouse Model 1 Rat Model 2 Pig Model 3 Methods ▪ Testicular - feminized + High cholesterol diet ▪ Castrated + High fat diet ▪ Castrated + High fat and cholesterol diet Disease ▪ Hepatic Steatosis ▪ NAFLD ▪ Hepatic steatosis, inflammation, elevated ALT T Therapy Effect ▪ Hepatic lipid deposition ▪ Lipogenesis ▪ Hepatic steatosis ▪ Hepatic apoptosis ▪ Vesicular inflammation ▪ Hepatic lipids ▪ Liver injury ▪ Hepatic steatosis

Potential of Testosterone Therapy Results from high fat diet (HFD) induced rabbit model* Giemsa – PAS Staining Red Oil Staining Masson’s Trichrome Staining TRT ↓ fibrosis TRT ↓ steatosis TRT ↓ inflammation RD HFD HFD +T * Vignozzi et al., Mol Cell Endocrinol 2014 29 T: Testosterone; OCA: Obeticholic Acid Effects on Liver Histology Effects on TNF - α (Inflammatory/Fibrosis Marker)

T Therapy Effects in Liver Regeneration Results from Hepatectomized Rat Model* 30 *90% hepatectomized, Vic et al., Hepatol 1982 40% recovery 60% recovery Total liver mass recovery completed Day 3 Day 4 Day 15 Testosterone Pretreatment Day 0 Day - 30 Testosterone Hepatectomy No Treatment Group 1 (n=50) T Treatment Group 2 (n=50) 100% died within 40 hours 80% survived beyond 40 hours Liver Mass Recovery (50% had a normal life span)

LPCN 1144: Clinical Tolerability Experience Oral Prodrug of Endogenous Testosterone 31 Adverse Reaction N=654 subjects Headache 1.50% Acne 0.90% Hematocrit Increased 1.20% Blood Pressure Increased 0.30% Fatigue 0.20% Hypertension 0.60% □ 654 subjects in multiple completed studies with up to 52 - week exposure ▪ No death, no drug - related SAEs, no major cardiovascular events, no hepato - toxic events were reported.

Sarcopenia is Associated with NAFLD/NASH 1 LPCN 1144 has Potential to Improve Sarcopenia 2,3 32 1. Bhanji et al., Hepatol 2017 2. Bhasin S., J Gerontol 2003 3. Sinclair et al., J Gastroenterol Hepatol 2016 x Sarcopenia endpoint(s) under evaluation in the ongoing LiFT trial 3

Sexual Dysfunction is Associated with NAFLD/NASH 1 LPCN 1144 has Potential to Improve Sexual Dysfunction 2 33 1. Hawksworth et al., Sex Med Rev 2019 2. Rizk et al., Curr Opin Urol 2017 3. SOAR Trial: SOAR Trial: subjects for ALT BL > 40 U/L (N=33) 3 x Sexual dysfunction endpoint(s) under evaluation in the ongoing LiFT trial

Depression is Prevalent in NAFLD Patients 1 LPCN 1144 has Potential to Improve Mental Health 2 34 3 1. Ali et al., Psychosomatics 2011 2. Celec et al., Front Neurosci 2015 3. SOAR Trial: subjects for ALT BL > 40 U/L (N=33) x Mental health endpoint(s) under evaluation in the ongoing LiFT trial

Quality of Life is Poor in NAFLD Patients 1 LPCN 1144 has Potential to Improve Quality of Life 2 35 1. Assimakopoulos et al., J Psychosom Res 2018 2. Almehmadi et al., Arab J Urol 2016 3. SOAR Trial: subjects for ALT BL > 40 U/L (N=33) 3 x Quality of life endpoint(s) under evaluation in the ongoing LiFT trial

LPCN 1144: Resolution of Liver Injury Markers Significant Normalization of Elevated Liver Enzymes* 36 52% 50% 67% 31% 0% 10% 20% 30% 40% 50% 60% 70% ALT AST ALP GGT % of Patients Normalized at EOS N 42 4 6 36 BL 53.6 U/L 59.8 U/L 125.3 U/L 82.2 U/L *1 - Yr SOAR Study (NCT02081300)

- 7.9 - 11.1 - 3.4 - 4.2 - 4.8 - 4.5 - 5.2 - 6.5 -16.0 -14.0 -12.0 -10.0 -8.0 -6.0 -4.0 -2.0 0.0 1 2 Mean Change from Baseline ALT, U/L AST, U/L ALP, U/L GGT, U/L LPCN 1144: Reduction of Liver Injury Markers In Patients with Elevated ALT at Baseline (BL) ▪ Liver enzymes mean change from BL to 1 - Yr EOS ( NCT02081300 ) 37 * Barritt 4 th et al, Contemp Clin Trials, 2017 ** Sanyal et al, Hepatol , 2015 x2 x2 x5 Alanine amino transferase (ALT), Aspartate amino transferase (AST), Alkaline phosphatase (ALP), Gamma - glutamyl transpeptidase (GGT) Subjects (n=81) BL ALT > 30 U/L* Subjects (n=42) BL ALT > 40 U/L** ALT AST ALP GGT ALT AST ALP GGT BL (U/L) 44.5 29.1 68.5 47.1 BL (U/L) 53.6 32.0 74.0 53.6

LPCN 1144: Effects on Serum Lipid Markers In Patients with Elevated Lipids at Baseline (BL) 38 • TG(triglyceride), LDL, Cholesterol, HDL upper normal limit (UNL) is 200 mg/dL, 160 mg/dL, 200 mg/dL, and 60 mg/dL, respective ly. • 1 - Yr SOAR Study (NCT02081300) - 17 - 64 -100 -80 -60 -40 -20 0 BL = 192 mg/dL BL = 320 mg/dL n = 207 n = 73 All subjects BL > ULN Mean TG CBL (mg/dL) TG Change from Baseline - 10 - 19 -25 -20 -15 -10 -5 0 BL = 198 mg/dL BL = 231 mg/dL n = 207 n = 92 All subjects BL > ULN Mean Cholesterol CBL (mg/dL) Cholesterol Change from Baseline - 3 - 15 -25 -20 -15 -10 -5 0 BL = 114 mg/dL BL = 175 mg/dL n = 194 n = 16 All subjects BL > ULN Mean LDL CBL (mg/dL) LDL Change from Baseline - 5.3 - 14.5 -20 -15 -10 -5 0 BL = 48.6 mg/dL BL = 70.8 mg/dL n = 207 n = 34 All subjects BL > ULN Mean HDL CBL (mg/dL) HDL Change from Baseline

39 ~$2B+ opportunity in an established and growing market

TLANDO ™ Attributes Patient and Physician Preferred Option Convenient Oral Route: • No risk of accidental T transference • Non - invasive; easy to use • Less cumbersome/burdensome • Potential for higher persistence/adherence Fixed Dosing Regimen • Easy to use for patients and physicians to prescribe • Unlike most TRT products, fixed “right” dose from the start of therapy with TLANDO ™ for all patients • No additional dose adjustment visits • Not prone to titration decision errors; No risk of patients stuck on wrong dose • Fixed/predictable cost for payers with no titration Differentiated Hypertension (“HTN”) Profile • Marginal (~ 1% )new anti - HTN starts or increase in anti - HTN dose in a year long exposure • Lower incidence of hematocrit increase as compared to injectables Consistent Inter - Day Restoration of T Levels Demonstrated Paradigm Shifting Liver Benefits 40

Enabling Oral Drug Delivery to Improve Patient Compliance

LPCN 1148: Oral T for Cirrhosis No FDA Approved Drug 42 Alcoholic liver disease Nonalcoholic Fatty Liver Disease (NAFLD) Chronic hepatitis B Chronic hepatitis C Cryptogenic 44,478 deaths in 2017 2 Estimated 1.3 M patients with liver cirrhosis 1 Liver Cirrhosis in US 1: Estes C. et al., Hepatology, 2018; 2.Yoon and Chen, National Institute on Alcohol Abuse and Alcoholism; Surveillance Rep ort #114, 2019 3. https://www.niddk.nih.gov/health - information/liver - disease/cirrhosis/symptoms - causes Common Causes 3 https://www.niddk.nih.gov/

High Economic Burden of a Liver Transplant Transplant Only Cure for Liver Cirrhosis 43 30 Days Pre - Transplant: $41,400 Procurement: $94,000 Hospital Transplant Admission: $463,200 Physician during Transplant Admission: $56,000 180 Days Post - Transplant Discharge: $126,900 Op Immunosuppressants & Other Rx: $30,800 Bentley & Phillips, Milliman Research Report 2017 Estimated Total of $812,500/ Transplant in U.S .

Impact of Low Testosterone* on Cirrhosis Progressive drop in T level with increasing disease severity 1 44 Increased risk of major infections, death and/or transplantation rates 1 Worsening of sarcopenia 3 Increased risk of for hepatic decompensation 3 Higher Child - Pugh score 3/ Higher MELD score 4 Severity of portal hypertension and ascites 3 1. Sinclair et al., Liver Transplantation, 2016; 2. Sinclair et al., J Gastroenterol Hepatol . 2016; 3.. Paternostro et al, Hepatol Res 2019; 4.. Sinclair et.al, Liver international, 2016 MELD Score: Model For End - Stage Liver Disease Score; Child - Pugh Score for Cirrhosis Mortality *most cirrhotic male patients have low T 2 KP70

Low Testosterone (T) An independent predictor of mortality in late stage cirrhosis 1 45 □ Low T (<8.3 nmol/L) associated with increased risk of major infections, death and/or transplantation rates 1 1. Sinclair M. et al., J. of Gastro and Hepatology 2015, 2.Moctezuma - Velazquez et al., Ann Hepatol . 2018 □ A significant association between testosterone levels and sarcopenia in male patients 2

LPCN 1107 Prevention of Preterm Birth 46 Enabling Oral Drug Delivery to Improve Patient Compliance

47 • A leading cause of neonatal mortality • ≥ $26 billion economic impact 2 • 10% of all US pregnancies 3 – PTB in singleton pregnancies: 8.1% • Medical costs for PTB infants are ~10x higher than for full term infants 4 1. Pediatric Research (2006) 60, 775 – 776 2. Institute of Medicine of the National Academies. Jul.200 3. National Vital Statistics Reports, Vol 67, No. 8, Nov 7, 2018: Centers for Disease Control and Prevention (2017) 4. J. Maternal - Fetal and Neonatal Medicine, Dec. 2006, 19(12), 773 – 782 Unmet Medical Need One Preterm birth (PTB) every minute 1

LPCN 1107 Opportunity Market Potential: ~1 billion Potential to be the First Oral HPC for Prevention of Recurrent PTB • Preferred route - of - administration is oral • ~140,000 annually pregnancy with history of at least one singleton spontaneous PTB* Potential for Clinical Success • Superior Cavg to Makena® • Targeting high - risk population (similar to MEIS trial) Strong Pharmaco - Economic Justification • Fewer PTB babies with significant healthcare cost savings • Minimize travel related cost/time and healthcare provider cost/time • Premium pricing potential to generic IM injections Strong Exclusivity Position • Orphan Drug Designation • Technology/IP protection 48 *Deutsche Bank Markets Research, 11 June 2015

LPCN 1107 – First Oral HPC for Prevention of PTB Injectable IM 17 - HPC, Makena® and SubQ HPC, Makena® (Standard of Care) 49 LPCN 1107 Oral 17 - HPC □ Twice daily dose • No injection site reactions • Steady state achieved in 7 days □ Higher HPC levels (potentially better efficacy than Makena®) □ Orphan drug designated • Major contribution to patient care □ Therapy duration • Up to 23 weeks □ Total of 18 - 22 injections • Weekly Injections • Viscous oily injection takes up to a minute • Patients experienced injection site pain • Weekly visit to/by health care provider □ Therapy duration • Up to 21 weeks 17 - α Hydroxy Progesterone Caproate (17 - HPC)

50 • Lower % PTB rate can be expected with daily Cavg 2 HPC levels ≥ 8.2 ng/mL 1. Caritis et al., Am J Obstet Gynecol. 2014 (N=315 subjects) 2. Ctrough for IM HPC Cavg , Makena PK - PD Correlation HPC Concentration and PTB Rate 1 46.3% 27.0% 29.6% 31.3% N=315 KP72

LPCN 1107: Dose Finding Clinical Study PK Study: Oral LPCN 1107 vs IM HPC, Makena • Open - label, four - period, four - treatment study • 12 healthy pregnant women - Ages 18 - 35 years; 16 - 18 weeks gestation • All subjects received all four treatments 51 Treatment A 400 mg BID Treatment B 600 mg BID Treatment C 800 mg BID Treatment D 250 mg Weekly Oral HPC, LPCN 1107 IM HPC, Makena® Multiple doses for 8 days Multiple dose: 5 weeks

Dose Finding Study: PK Results * LPCN 1107 vs. Injectable Makena® • Average HPC levels at target LPCN 1107 greater than the comparator, Makena® • HPC levels below 8.2 ng/ml: 0% subjects on LPCN 1107 vs. 20% subjects on IM Injection 52 * PK results obtained from post 5 weeks for weekly IM Injection & post 8 days of BID dosing for LPCN 1107 from the dose finding s tudy Target Dose
