Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Jaguar Health, Inc. | a19-3545_18k.htm |
Forward-Looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding the Company’s plan to file an IND in 2H 2019 for lechlemer for the possible indication of diarrhea caused by cholera, the Company’s belief that lechlemer may offer a possible Priority Review Voucher opportunity, the Company’s statements regarding planned next steps for clinical trials (including the Company’s plan to submit documentation in 1H 2019 to the FDA for the planned formulation of crofelemer for feeding tube administration to support investigation of a pediatric liquid formulation of crofelemer for the possible indication of Congenital Diarrheal Disease (CDD), and the Company’s plan to file an IND and initiate the CDD IIT in mid-2019), the Company’s expectation that it will meet with FDA in Q1 2019 to discuss the pivotal protocol for cancer therapy-related diarrhea (CTD), the Company’s expectation that it will file an IND for the CTD supplemental indication in H1 2019, the Company’s expectations regarding the timing of filings with the SEC, the Company’s expectation that filing of the Canalevia NADA for chemotherapy-induced diarrhea (CID) in dogs will be completed in Q2 2019, the Company’s plans to pursue additional business development deals in H1 & H2 2019, and the timing of data results from planned proof of concept, field and other studies are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this presentation are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control. Please see the risk factors identified in our Annual Report on Form 10-K and our other filings with the SEC. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Readers are also advised that our projected sales do not take into account the royalties and other payments we will need to make to our Licensors and strategic partners. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Crofelemer was discovered through the science of ethnobotany Our Story: From Tree to Bottle
Mytesi (crofelemer 125mg delayed-release tablets) is FDA-approved for symptomatic relief of noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy. Jaguar Health: The Product Portfolio
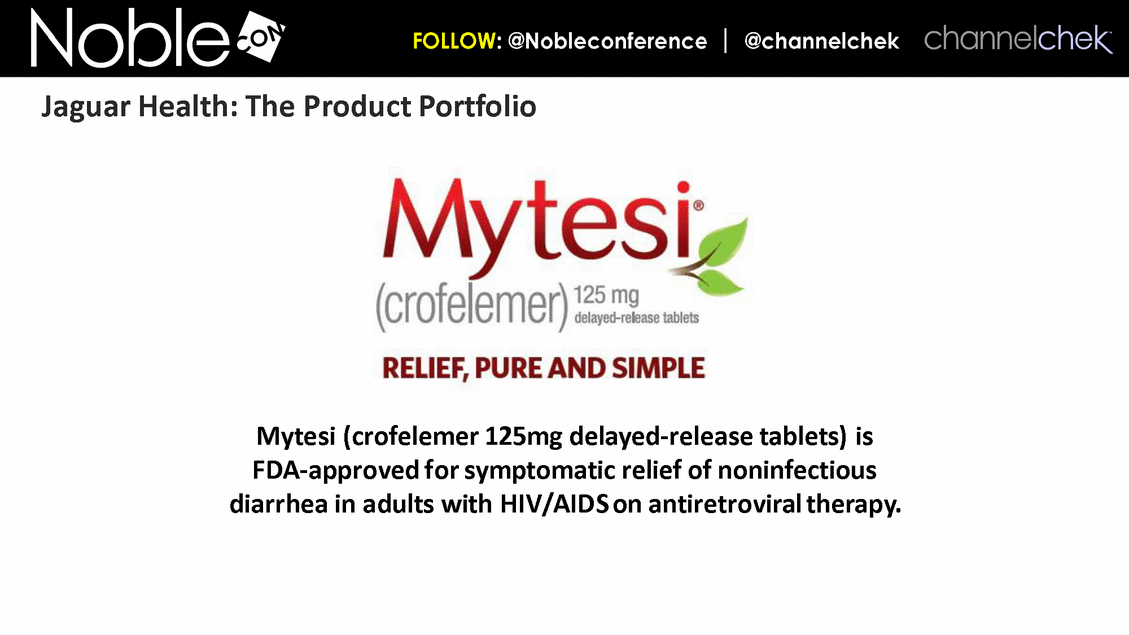
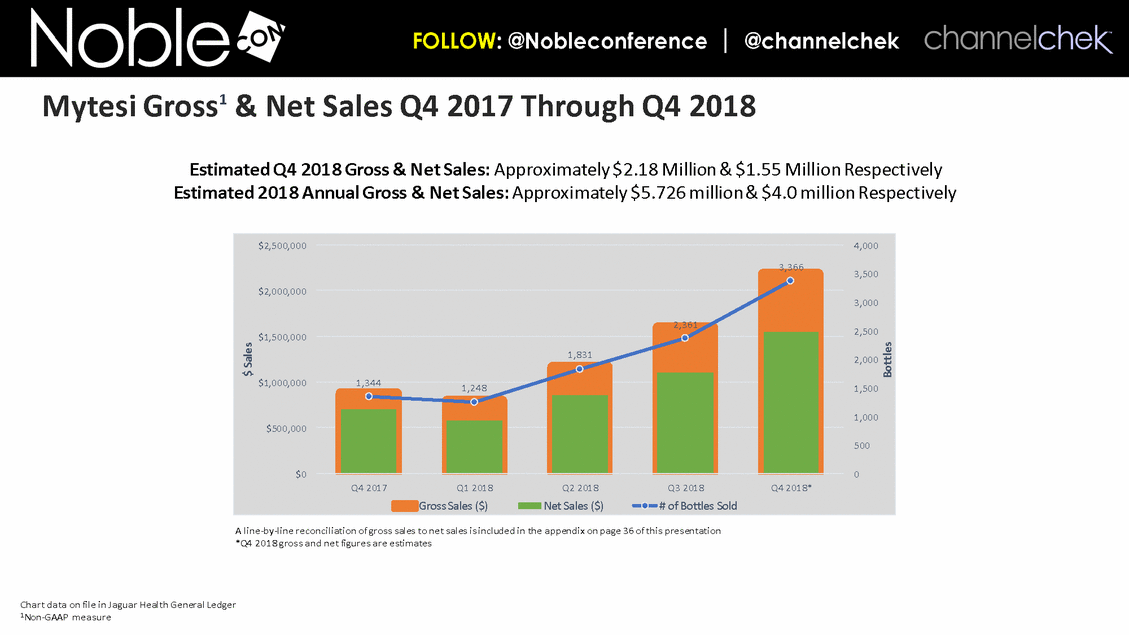
Chart data on file in Jaguar Health General Ledger 1Non-GAAP measure A line-by-line reconciliation of gross sales to net sales is included in the appendix on page 36 of this presentation *Q4 2018 gross and net figures are estimates Estimated Q4 2018 Gross & Net Sales: Approximately $2.18 Million & $1.55 Million Respectively Estimated 2018 Annual Gross & Net Sales: Approximately $5.726 million & $4.0 million Respectively Mytesi Gross1 & Net Sales Q4 2017 Through Q4 2018 1,344 1,248 1,831 2,361 3,366 0 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 $0 $500,000 $1,000,000 $1,500,000 $2,000,000 $2,500,000 Q4 2017 Q1 2018 Q2 2018 Q3 2018 Q4 2018* Bottles $ Sales Gross Sales ($) Net Sales ($) # of Bottles Sold
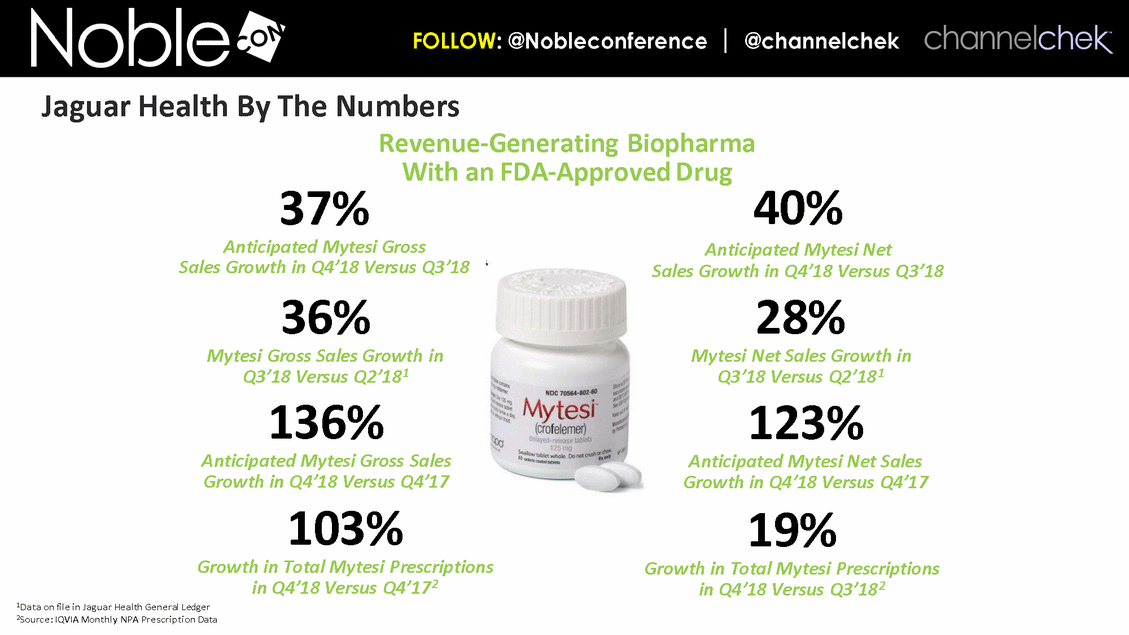
Revenue-Generating Biopharma With an FDA-Approved Drug 19% Growth in Total Mytesi Prescriptions in Q4’18 Versus Q3’182 37% Anticipated Mytesi Gross Sales Growth in Q4’18 Versus Q3’18 36% Mytesi Gross Sales Growth in Q3’18 Versus Q2’181 1Data on file in Jaguar Health General Ledger 2Source: IQVIA Monthly NPA Prescription Data 103% Growth in Total Mytesi Prescriptions in Q4’18 Versus Q4’172 136% Anticipated Mytesi Gross Sales Growth in Q4’18 Versus Q4’17 40% Anticipated Mytesi Net Sales Growth in Q4’18 Versus Q3’18 28% Mytesi Net Sales Growth in Q3’18 Versus Q2’181 123% Anticipated Mytesi Net Sales Growth in Q4’18 Versus Q4’17 Jaguar Health By The Numbers
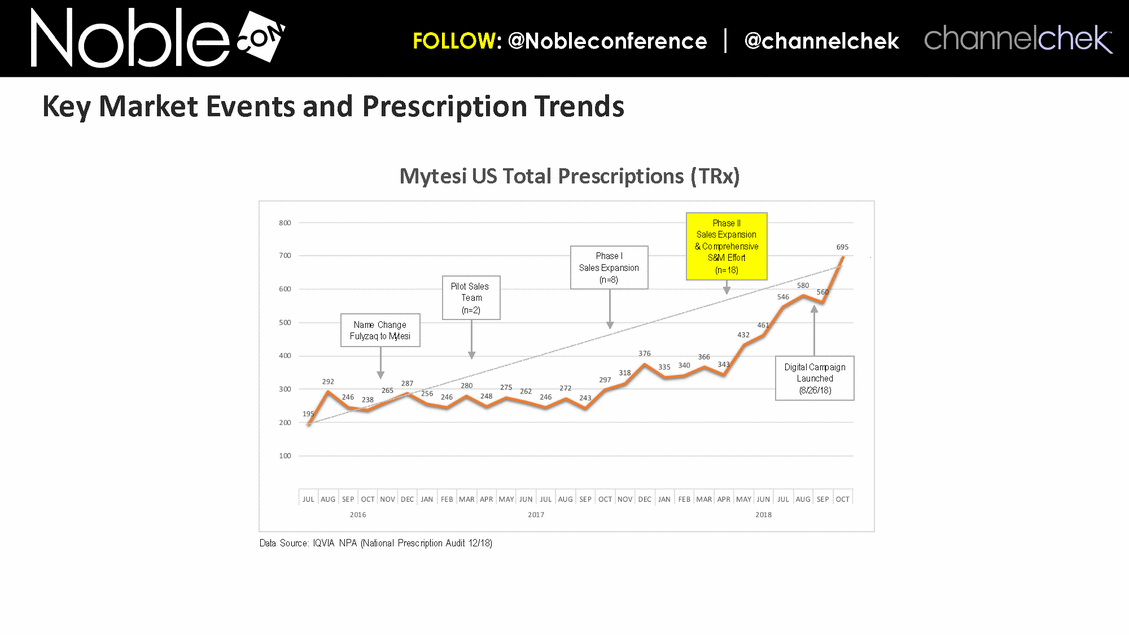
Data Source: IQVIA NPA (National Prescription Audit 12/18) Name Change Fulyzaq to Mytesi Pilot Sales Team (n=2) Phase I Sales Expansion (n=8) Digital Campaign Launched (8/26/18) Phase II Sales Expansion & Comprehensive S&M Effort (n=18) Mytesi US Total Prescriptions (TRx) Key Market Events and Prescription Trends
Our Dual Mission Develop and commercialize proprietary novel GI products for the global marketplace Build value recognition in the company by all stakeholders Break even from Mytesi HIV sales Non-dilutive funding to progress pipeline Napo, a Wholly-owned Subsidiary of Jaguar Health (JAGX)
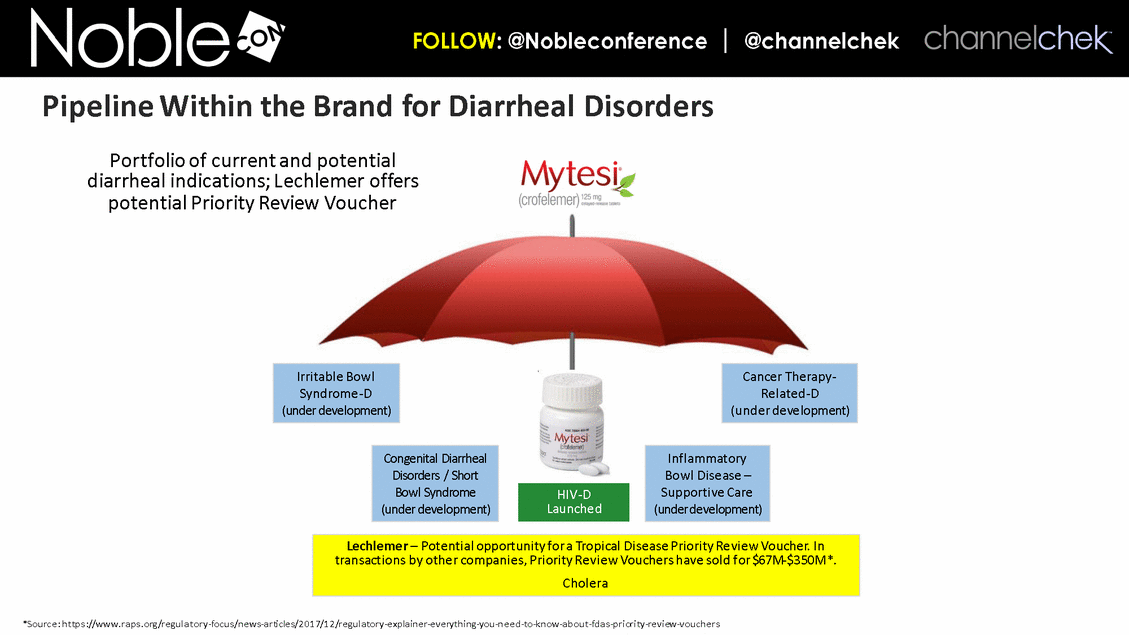
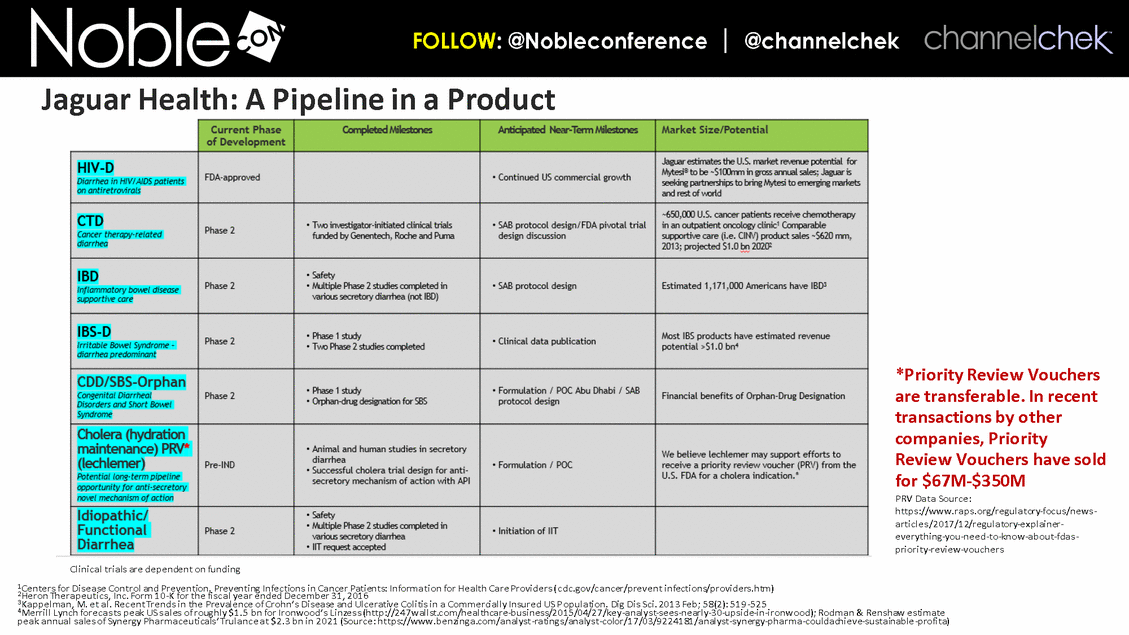
Irritable Bowl Syndrome-D (under development) Cancer Therapy-Related-D (under development) Inflammatory Bowl Disease – Supportive Care (under development) Congenital Diarrheal Disorders / Short Bowl Syndrome (under development) Portfolio of current and potential diarrheal indications; Lechlemer offers potential Priority Review Voucher HIV-D Launched Lechlemer – Potential opportunity for a Tropical Disease Priority Review Voucher. In transactions by other companies, Priority Review Vouchers have sold for $67M-$350M*. Cholera *Source: https://www.raps.org/regulatory-focus/news-articles/2017/12/regulatory-explainer-everything-you-need-to-know-about-fdas-priority-review-vouchers Pipeline Within the Brand for Diarrheal Disorders
On September 24, 2018, Jaguar signed a Distribution, License and Supply Agreement with Knight Therapeutics Inc. Agreement provides Knight Therapeutics with an exclusive right to commercialize current and future Jaguar human health products in Canada and Israel. In accordance with the agreement, the covered territory may be expanded at a later time to include specified countries in Latin America. The Canadian and Israeli markets accounted for an estimated combined total of 2.18% of global pharmaceutical sales in 20141 The markets in the specified countries in Latin America accounted for an estimated combined total of 6.60% of global pharmaceutical sales in 20141 Upon achievement of certain regulatory and sales milestones, Jaguar may receive payments from Knight Therapeutics in an aggregate amount of up to USD $18,019,743 (based on September 23, 2018 USD-CAD exchange rates) payable throughout the initial 15-year term of the agreement. Knight invested US $900k in Jaguar equity. 1The International Federation of Pharmaceutical Manufacturers & Associations “Pharmaceutical Industry and Global Health - Facts and Figures 2017” report Montreal, Canada-based Knight Therapeutics Inc. is a specialty pharmaceutical company focused on acquiring or in-licensing and commercializing innovative pharmaceutical products for the Canadian and select international markets. Knight Therapeutics: License for Canadian and Israeli Markets

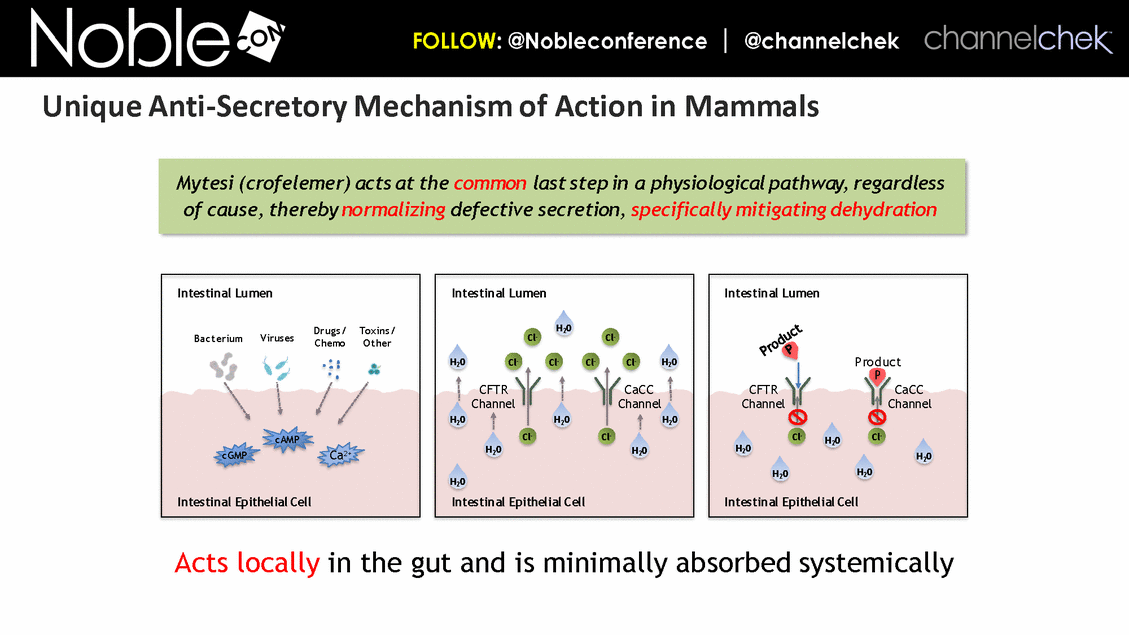
P P Intestinal Lumen Intestinal Epithelial Cell Cl- CFTR Channel CaCC Channel Cl- H20 H20 H20 H20 H20 Cl- Cl- Cl- Cl- Cl- Cl- Intestinal Lumen Intestinal Epithelial Cell Cl- CFTR Channel CaCC Channel Cl- H20 H20 H20 H20 H20 H20 H20 H20 H20 Bacterium Viruses Drugs/ Chemo Toxins/ Other cAMP cGMP Ca2+ Intestinal Epithelial Cell Intestinal Lumen Product Product Acts locally in the gut and is minimally absorbed systemically Mytesi (crofelemer) acts at the common last step in a physiological pathway, regardless of cause, thereby normalizing defective secretion, specifically mitigating dehydration Unique Anti-Secretory Mechanism of Action in Mammals
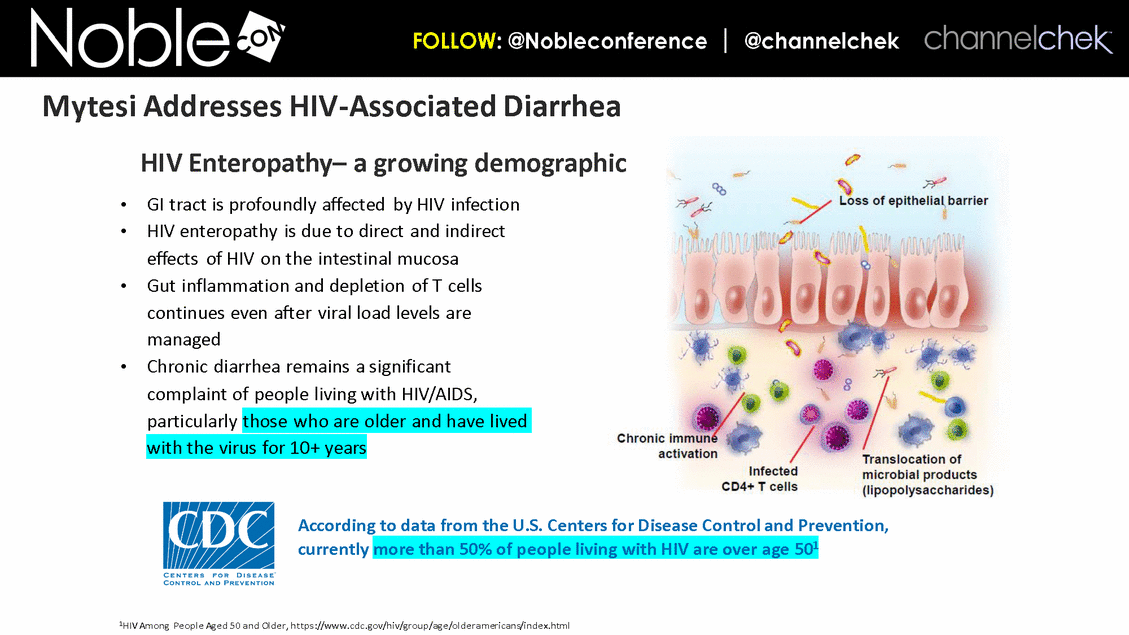
GI tract is profoundly affected by HIV infection HIV enteropathy is due to direct and indirect effects of HIV on the intestinal mucosa Gut inflammation and depletion of T cells continues even after viral load levels are managed Chronic diarrhea remains a significant complaint of people living with HIV/AIDS, particularly those who are older and have lived with the virus for 10+ years According to data from the U.S. Centers for Disease Control and Prevention, currently more than 50% of people living with HIV are over age 501 1HIV Among People Aged 50 and Older, https://www.cdc.gov/hiv/group/age/olderamericans/index.html HIV Enteropathy– a growing demographic Mytesi Addresses HIV-Associated Diarrhea
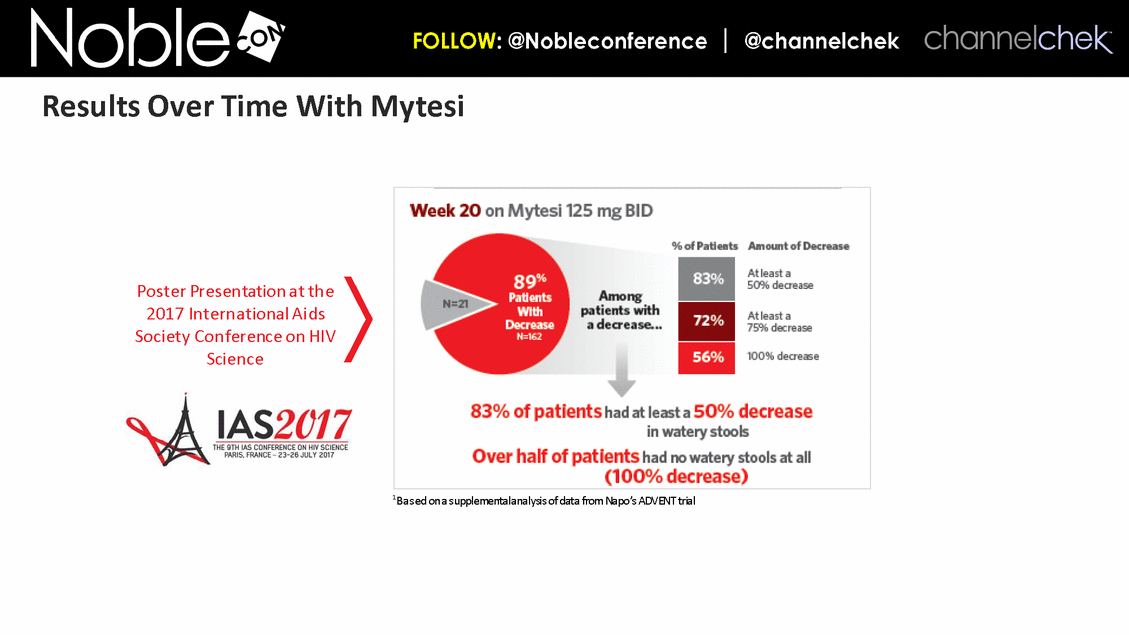
1Based on a supplemental analysis of data from Napo’s ADVENT trial Poster Presentation at the 2017 International Aids Society Conference on HIV Science Results Over Time With Mytesi
Bob Griffing, Chief Commercial Officer, started June 2018 Targeting high-potential HIV prescribers and GIs that actively treat HIV patients 17 strategically positioned reps target major urban areas and the highest-volume prescribers of antiretrovirals and antidiarrheals, such as internal medicine physicians, infectious disease doctors and mid-level practitioners such as nurse practitioners Urban Areas Where We Have or Plan to Place Reps and Conduct Engagement & Education Activities Territory to add (for a total of 17 reps) TBD Mytesi Growth Strategy

The Three ‘E’s” of Patient Focus: Engage Empower Educate

Recent Mytesi Media Coverage

Signed agreement with AIDS Drug Assistance Program (ADAP) in April 2018 Mytesi access available to greater than 90% of ADAP lives nationally Covered by all top 10 commercial insurers (>245 million lives) Covered by all top 10 Managed Medicare plans (>2.4 million lives) Covered by Medicaid in all 50 states Copay coupon program to remove cost as a barrier to filling their prescription Patient Assistance Program to assist patients with benefit verification, prior authorization, and claims appeals ADAP is state administered for persons living with HIV/AIDS authorized under the Ryan White Act of 2016 and often considered insurance of last resort. These persons are not currently receiving or eligible for Medicaid or other Third-Party insurance. Reserved for lower income patients with annual federal income equal to or below 400% of current federal poverty level (2018 poverty level for single household $12,140). Mytesi Coverage and Reimbursement
“If approved for all indications in all countries, crofelemer could be the most utilized product in the world.” - Former Head of Global Health at Gates Foundation Hold global rights to FDA-approved product with: Chronic safety profile Commercial manufacturing in place Multiple potential follow-on indications addressing large patient populations in need Phase 2 and/or proof-of-concept data for most target indications Global Growth Potential

*Priority Review Vouchers are transferable. In recent transactions by other companies, Priority Review Vouchers have sold for $67M-$350M PRV Data Source: https://www.raps.org/regulatory-focus/news-articles/2017/12/regulatory-explainer-everything-you-need-to-know-about-fdas-priority-review-vouchers 1Centers for Disease Control and Prevention. Preventing Infections in Cancer Patients: Information for Health Care Providers (cdc.gov/cancer/prevent infections/providers.htm) 2Heron Therapeutics, Inc. Form 10-K for the fiscal year ended December 31, 2016 3Kappelman, M. et al. Recent Trends in the Prevalence of Crohn’s Disease and Ulcerative Colitis in a Commercially Insured US Population. Dig Dis Sci. 2013 Feb; 58(2): 519-525 4Merrill Lynch forecasts peak US sales of roughly $1.5 bn for Ironwood’s Linzess (http://247wallst.com/healthcare-business/2015/04/27/key-analyst-sees-nearly-30-upside-in-ironwood); Rodman & Renshaw estimate peak annual sales of Synergy Pharmaceuticals’ Trulance at $2.3 bn in 2021 (Source: https://www.benzinga.com/analyst-ratings/analyst-color/17/03/9224181/analyst-synergy-pharma-couldachieve-sustainable-profita) Clinical trials are dependent on funding Jaguar Health: A Pipeline in a Product
A product that is safe and effective, and a trial design that supports this to the statistical satisfaction of FDA Our “Heroes” of Clinical Success

Cancer Therapy-Related Diarrhea SAB Lee Schwartzberg, MD, FACP: Executive Director of the West Cancer Center, a multispecialty oncology practice affiliated with the University of Tennessee; Chief, Division of Hematology/Oncology, the University of Tennessee Health Science Center Eric Roeland, MD: Attending Physician, Center for Palliative Care, Harvard Medical School Hope Rugo, MD: Clinical Professor of Medicine, Director Breast Oncology and Clinical Trials Education, Division of Hematology and Oncology, University of California San Francisco IBD SAB Corey Siegel, MD, MS: Associate Professor of Medicine; Associate Professor of The Dartmouth Institute; Director of the Inflammatory Bowel Disease Center at the Dartmouth-Hitchcock Medical Center Pediatric Indications (SBS and CDD) SAB Mohammed Miqdady, MD: Chief of Pediatric Gastroenterology, Hepatology & Nutrition at Sheikh Khalifa Medical City in Abu Dhabi Martin Martin, MD: Professor, Department of Pediatrics, David Geffen School of Medicine at UCLA Sue Rhee, MD: Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition Pediatric gastroenterologist and liver specialist, UCSF Benioff Children’s Hospital KOLs: Diarrhea Related to HIV and Other Infectious Diseases Patrick Clay, PharmD: Consultant Herbert DuPont, MD: Professor and Director, Center for Infectious Diseases, University of Texas Houston School of Public Health Pradip Bardhan, MBBS, MD: Chief Physician at ICDDR,B, Bangladesh Paulo Pacheco, MD: Clinical Assistant Professor, Department of Medicine, New York University Langone Health Elie Schochet, MD, FACS: Colorectal surgeon, Holy Cross Medical Group KOLs: Cancer Therapy-Related Diarrhea Herbert DuPont, MD: Professor and Director, Center for Infectious Diseases, University of Texas Houston School of Public Health Pablo C. Okhuysen, M.D: Department of Infectious Diseases, Infection Control, and Employee Health, Division of Internal Medicine, MD Anderson Napo Scientific Advisory Board (SAB) Members & Key Opinion Leader (KOL) Advisors to Napo Pravin Chaturvedi, PhD: Chair of Napo’s SABs. Pravin brings 25+ years drug development experience in pharmaceutical/biotech field; Successfully developed crofelemer (Mytesi) (first pivotal adaptive design) KOLs: Diarrhea Related to IBS Anthony Lembo, MD: Director of the GI Motility and Functional Bowel Disorders Program at Beth Israel Deaconess Medical Center and Associate Professor of Medicine at Harvard Medical School Doug Drossman, MD: Co-Director Emeritus, UNC Center for Functional GI and Motility Disorders Adjunct Professor of Medicine and Psychiatry, University of North Carolina School of Medicine William Chey, MD: Professor of Internal Medicine and Professor of Nutritional Sciences, University of Michigan School of Public Health KOLs: Diarrhea Related to IBD Brooks D. Cash, MD, AGAF, FACG, FACP, FASGE: Division Director, Gastroenterology, Hepatology, and Nutrition Visiting Professor of Medicine, The University of Texas McGovern Medical School David Rubin, MD: Joseph B. Kirsner Professor of Medicine Section Chief, Gastroenterology, Hepatology and Nutrition Co-Director, Digestive Diseases Center, University of Chicago Medicine Charles Bernstein, MD: Distinguished Professor of Medicine and Bingham Chair in Gastroenterology Research, University of Manitoba William Sandborn, MD: Director, Inflammatory Bowel Disease Center Chief, Division of Gastroenterology Professor of Medicine, US San Diego Health Scott Lee, MD: Associate Professor of Medicine, Digestive Health Center, University of Washington Medical Center Edward Loftus, Jr., MD: Consultant, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Mayo Clinic Douglas Wolf, MD: Medical Director of IBD Research at Atlanta Gastroenterology Associates. Clinical Assistant Professor of Medicine, Emory University School of Medicine KOLs: Pediatric Indications (SBS and CDD) Jay Thiagarajah, MD, PhD: Attending Physician, Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital. Instructor of Pediatrics, Harvard Medical School James Goldenring, M.D., PhD: Professor of Surgery, Vanderbilt University School of Medicine. Paul W. Sanger Chair in Experimental Surgery. Professor of Cell and Developmental Biology
Cancer Therapy-related Diarrhea (CTD) Diarrhea is a common adverse event reported with cancer treatments “All-grade” diarrhea rates are often 50-80% Dose-limiting toxicity for tyrosine kinase inhibitors (e.g., Neratinib) and EGFR mAbs (e.g., Herceptin) 1https://www.prnewswire.com/news-releases/chemotherapy-induced-nausea-and-vomiting-cinv-market-expected-to-reach-2659-million-by-2022-611755395.html Puma Biotechnology (NASDAQ: PBYI) Neratinib (NerlynxTM): Diarrhea has been reported as the most common side effect of the recently approved CDK 4/6 inhibitor abemaciclib and the pan-HER TKI neratinib, with occurrence ranging from 86% to >95% and grade 3 in over 40% of patients. Supportive care precedent in the Cancer-Therapy Market Approved drugs for chemotherapy-induced nausea and vomiting (CINV) include Sustol, Aloxi, Akynzeo and Sancuso. Allied Market Research estimates that sales of CINV drugs may reach $2.7 billion by 2022 growing ~7.1% per annum.1 Jaguar Health: A Pipeline in a Product
Two Ongoing Investigator Initiated Studies in CTD Crofelemer as salvage anti-diarrheal therapy with investigational breast cancer agent, neratinib Title: Open label study to characterize the incidence and severity of diarrhea in patients with early stage HER2+ breast cancer treated with adjuvant trastuzumab and neratinib followed by neratinib monotherapy, and intensive anti-diarrhea prophylaxis. Primary Objective: Characterize the incidence and severity of diarrhea in patients with early stage breast cancer receiving adjuvant trastuzumab and neratinib followed by 1 year of neratinib monotherapy in the setting of prophylactic anti-diarrheal management. Funded By: Puma Biotechnology Primary objective: Characterize incidence and severity of diarrhea in patients receiving investigational therapy in prophylactic anti-diarrheal management. JAGX completed CID pilot safety study in dogs: 25% of dogs entering study with unformed feces were resolved. Funded By: Genentech/Roche Next Steps: Interim data IIT Georgetown– Q1, 2019 Pivotal protocol discussion with FDA Q1, 2019 Jaguar Health: A Pipeline in a Product

Congenital Diarrheal Disease (CDD) Rare, congenital chronic intestinal channel diseases, occurring exclusively in early infancy Characterized by severe, lifelong diarrhea and a lifelong need for nutritional intake either parenterally or with a feeding tube Incidence much more prevalent in regions where consanguineous marriage is part of the culture, such as in the Gulf Cooperation Council (GCC) and MENA regions Short Bowel Syndrome (SBS) Complex condition characterized by malabsorption of fluids and nutrients due to congenital deficiencies or surgical resection of small bowel segments Patients suffer from symptoms such as debilitating diarrhea, malnutrition, dehydration and imbalances of fluids and salts Can stem from genetic disorder or premature birth Incidence also much more prevalent in GCC and MENA regions Mytesi Pediatric Orphan-Drug Indications
Filed for orphan-drug status in US Received orphan status for SBS Principal investigator: Dr. Mohamad Miqdady, Chief of Pediatric Gastroenterology, Hepatology & Nutrition at Sheikh Khalifa Medical City in Abu Dhabi Jaguar has agreed to support investigator-initiated trial Napo intends to submit documentation in 1H 2019 to the FDA for the planned formulation of crofelemer for feeding tube administration to support this investigation NEXT STEP: Initiation of IIT “With the early and extreme morbidity and mortality suffered by CDD patients, we welcome the opportunity to participate in the investigation of a novel drug to address the devastating diarrhea and dehydration caused by this lifelong disease for which there is currently no available treatment except parenteral nutrition, and help limit the suffering of patients and their family members.” -Dr. Mohamad Miqdady Planned Investigator-Initiated Clinical Trial on CDD at Sheikh Khalifa Medical City, Abu Dhabi, UAE
Study evaluating crofelemer versus placebo 1 hour after Azithromycin in cholera1 Reducing amount of watery stool, 25-30%, 0-6 hour time periods (p=0.025) Indian patient study in adults with severe watery diarrhea2 Statistically significant benefits seen in seven prospectively defined clinical endpoints. Crofelemer superior for overall clinical success, 79% vs. 28% NEXT STEPS: Develop dispersible tablet of lechlemer (an equally effective formulation that is more economically feasible for marketing in resource-constrained regions) File IND in 2H 2019 1Bardhan, et.al., ‘08 US-Japan Cholera Conf. 2Bardhan PK EID,’09 Crofelemer for Diarrhea Caused by Cholera
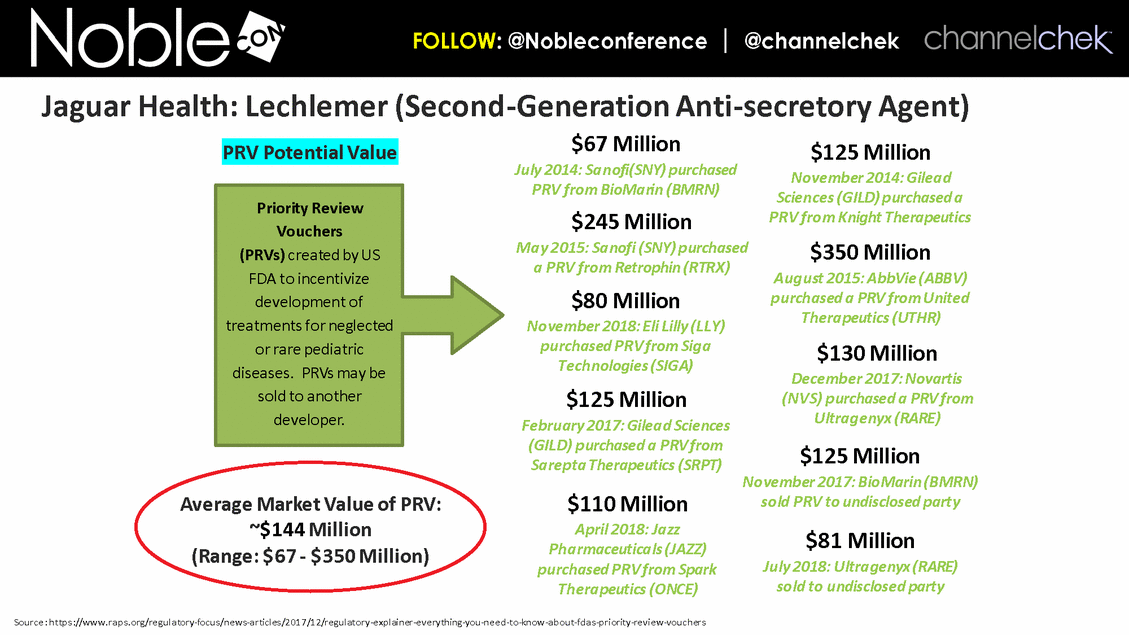
$350 Million August 2015: AbbVie (ABBV) purchased a PRV from United Therapeutics (UTHR) $125 Million February 2017: Gilead Sciences (GILD) purchased a PRV from Sarepta Therapeutics (SRPT) $67 Million July 2014: Sanofi(SNY) purchased PRV from BioMarin (BMRN) $81 Million July 2018: Ultragenyx (RARE) sold to undisclosed party Source: https://www.raps.org/regulatory-focus/news-articles/2017/12/regulatory-explainer-everything-you-need-to-know-about-fdas-priority-review-vouchers Priority Review Vouchers (PRVs) created by US FDA to incentivize development of treatments for neglected or rare pediatric diseases. PRVs may be sold to another developer. $125 Million November 2014: Gilead Sciences (GILD) purchased a PRV from Knight Therapeutics $245 Million May 2015: Sanofi (SNY) purchased a PRV from Retrophin (RTRX) $125 Million November 2017: BioMarin (BMRN) sold PRV to undisclosed party $110 Million April 2018: Jazz Pharmaceuticals (JAZZ) purchased PRV from Spark Therapeutics (ONCE) Average Market Value of PRV: ~$144 Million (Range: $67 - $350 Million) $130 Million December 2017: Novartis (NVS) purchased a PRV from Ultragenyx (RARE) $80 Million November 2018: Eli Lilly (LLY) purchased PRV from Siga Technologies (SIGA) PRV Potential Value Jaguar Health: Lechlemer (Second-Generation Anti-secretory Agent)
Multiple indications Multiple geographies Second-generation anti-secretory (lechlemer) Strategically sequence indication development priorities, second-generation product pipeline development, and partnering goals on a global basis Global partnering for an expanding pipeline provides opportunity for non-dilutive funding and global access to Mytesi and novel anti-secretory agents Global Partnering Driving Pipeline
Q1 2019: Interim IIT results for CTD Q1 2019: First shipment of Equilevia Q1 2019: Meet FDA for CTD pivotal protocol agreement April 2019: File Q4 2018 earnings report as part of 2018 10-K on or before April 1 deadline Q2 2019: Complete filing of Canalevia NADA for CID in dogs Mid-May 2019: File Q1 2019 earnings report Mid-2019: File IND and initiate CDD IIT in Abu Dhabi H1 2019: File IND for CTD supplemental indication Mid-August 2019: File Q2 2019 earnings report H1 & H2 2019: Additional business development deals (non dilutive financing) H2 2019: File IND for lechlemer/cholera (subject to funding) Mid-November 2019: File Q3 2019 earnings report Upcoming Milestones

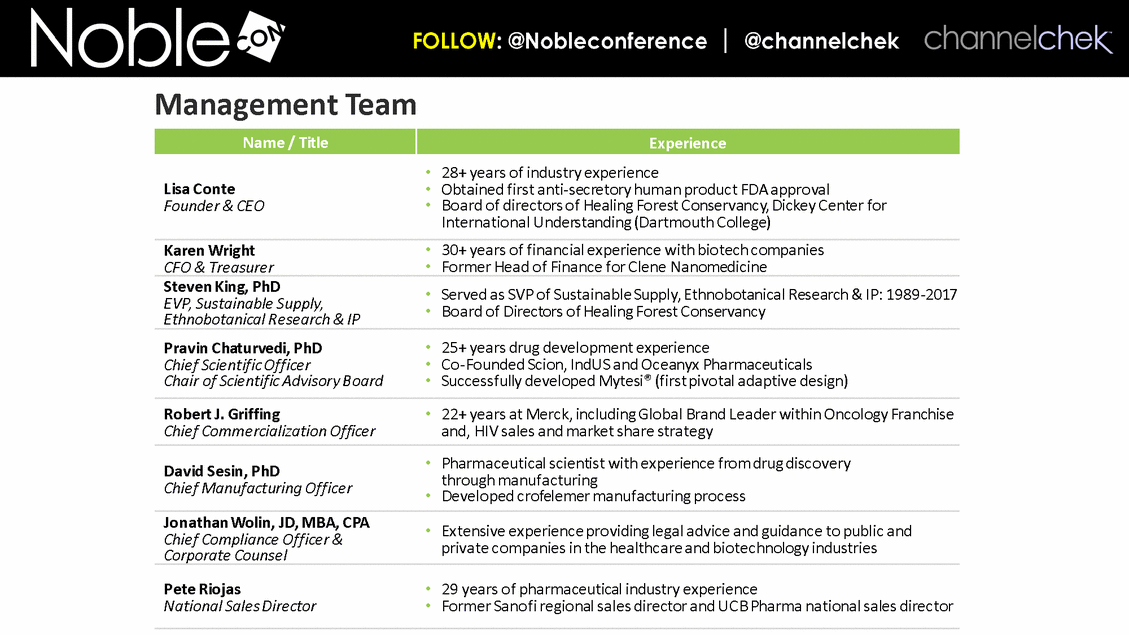
Name / Title Experience Lisa Conte Founder & CEO 28+ years of industry experience Obtained first anti-secretory human product FDA approval Board of directors of Healing Forest Conservancy, Dickey Center for International Understanding (Dartmouth College) Karen Wright CFO & Treasurer 30+ years of financial experience with biotech companies Former Head of Finance for Clene Nanomedicine Steven King, PhD EVP, Sustainable Supply, Ethnobotanical Research & IP Served as SVP of Sustainable Supply, Ethnobotanical Research & IP: 1989-2017 Board of Directors of Healing Forest Conservancy Pravin Chaturvedi, PhD Chief Scientific Officer Chair of Scientific Advisory Board 25+ years drug development experience Co-Founded Scion, IndUS and Oceanyx Pharmaceuticals Successfully developed Mytesi® (first pivotal adaptive design) Robert J. Griffing Chief Commercialization Officer 22+ years at Merck, including Global Brand Leader within Oncology Franchise and, HIV sales and market share strategy David Sesin, PhD Chief Manufacturing Officer Pharmaceutical scientist with experience from drug discovery through manufacturing Developed crofelemer manufacturing process Jonathan Wolin, JD, MBA, CPA Chief Compliance Officer & Corporate Counsel Extensive experience providing legal advice and guidance to public and private companies in the healthcare and biotechnology industries Pete Riojas National Sales Director 29 years of pharmaceutical industry experience Former Sanofi regional sales director and UCB Pharma national sales director Management Team
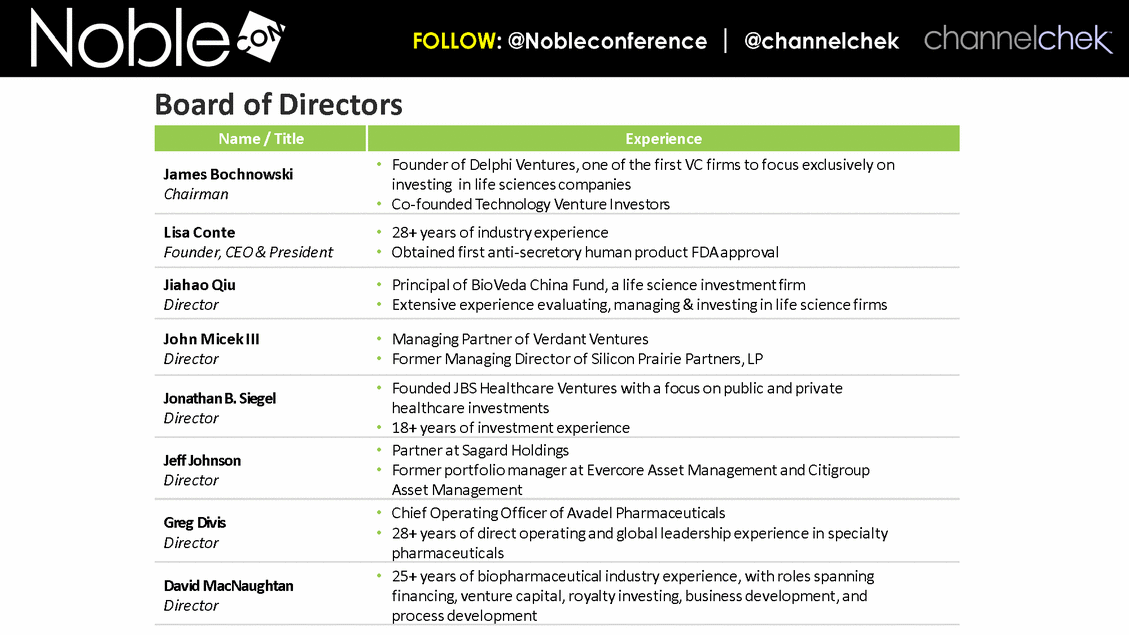
Name / Title Experience James Bochnowski Chairman Founder of Delphi Ventures, one of the first VC firms to focus exclusively on investing in life sciences companies Co-founded Technology Venture Investors Lisa Conte Founder, CEO & President 28+ years of industry experience Obtained first anti-secretory human product FDA approval Jiahao Qiu Director Principal of BioVeda China Fund, a life science investment firm Extensive experience evaluating, managing & investing in life science firms John Micek III Director Managing Partner of Verdant Ventures Former Managing Director of Silicon Prairie Partners, LP Jonathan B. Siegel Director Founded JBS Healthcare Ventures with a focus on public and private healthcare investments 18+ years of investment experience Jeff Johnson Director Partner at Sagard Holdings Former portfolio manager at Evercore Asset Management and Citigroup Asset Management Greg Divis Director Chief Operating Officer of Avadel Pharmaceuticals 28+ years of direct operating and global leadership experience in specialty pharmaceuticals David MacNaughtan Director 25+ years of biopharmaceutical industry experience, with roles spanning financing, venture capital, royalty investing, business development, and process development Board of Directors
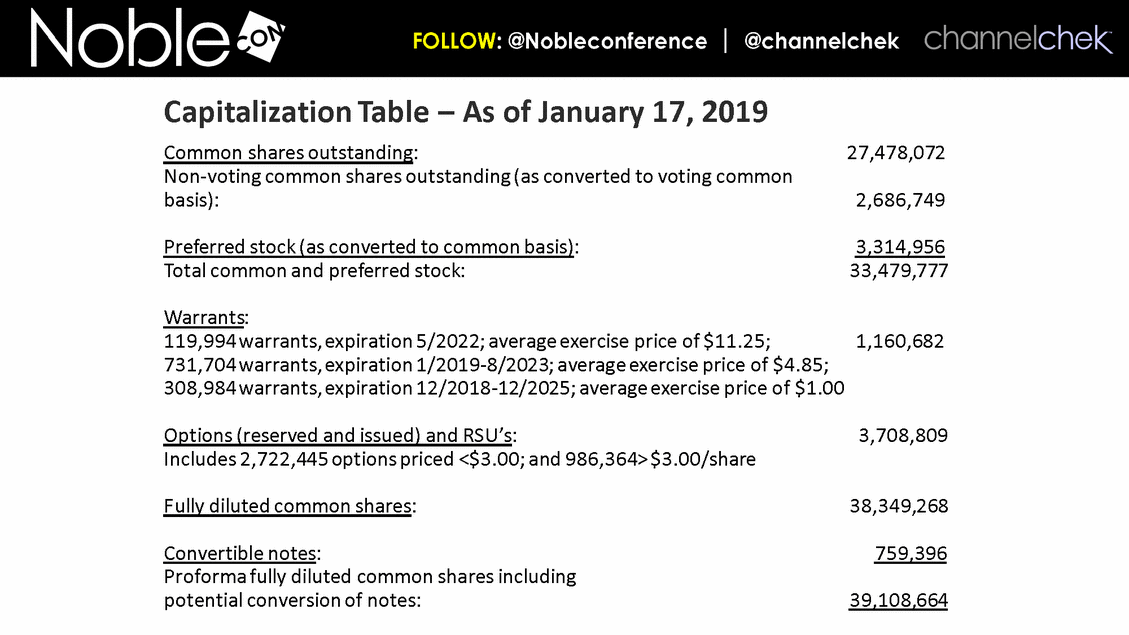
Common shares outstanding: 27,478,072 Non-voting common shares outstanding (as converted to voting common basis): 2,686,749 Preferred stock (as converted to common basis): 3,314,956 Total common and preferred stock: 33,479,777 Warrants: 119,994 warrants, expiration 5/2022; average exercise price of $11.25; 1,160,682 731,704 warrants, expiration 1/2019-8/2023; average exercise price of $4.85; 308,984 warrants, expiration 12/2018-12/2025; average exercise price of $1.00 Options (reserved and issued) and RSU’s: 3,708,809 Includes 2,722,445 options priced <$3.00; and 986,364> $3.00/share Fully diluted common shares: 38,349,268 Convertible notes: 759,396 Proforma fully diluted common shares including potential conversion of notes: 39,108,664 Capitalization Table – As of January 17, 2019
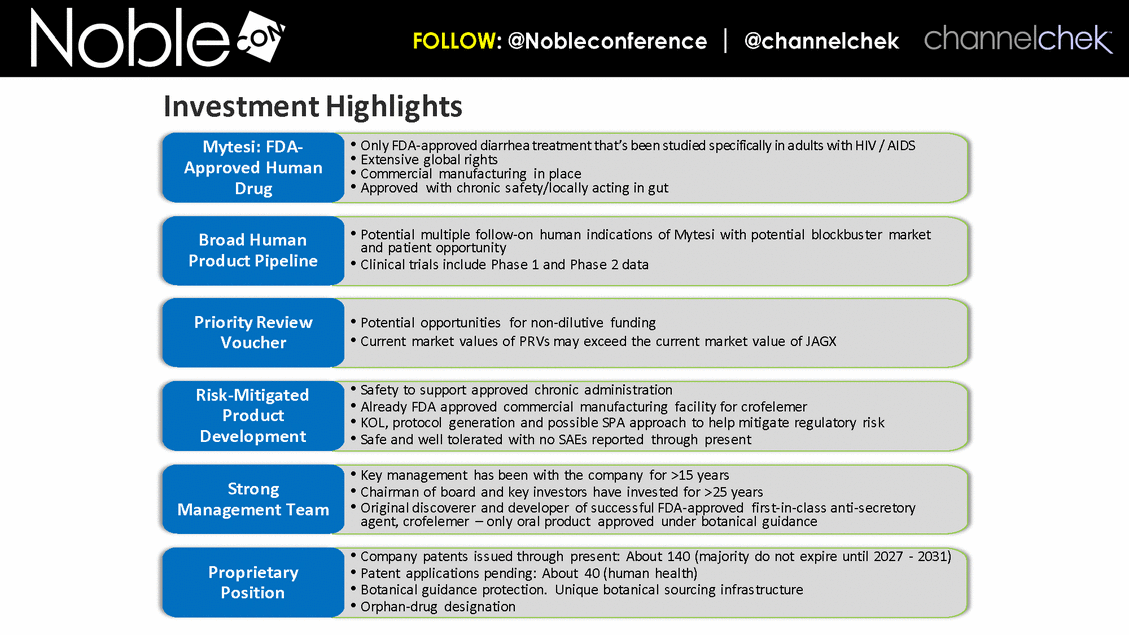
Investment Highlights Only FDA-approved diarrhea treatment that’s been studied specifically in adults with HIV / AIDS Extensive global rights Commercial manufacturing in place Approved with chronic safety/locally acting in gut Mytesi: FDA-Approved Human Drug Potential multiple follow-on human indications of Mytesi with potential blockbuster market and patient opportunity Clinical trials include Phase 1 and Phase 2 data Broad Human Product Pipeline Potential opportunities for non-dilutive funding Current market values of PRVs may exceed the current market value of JAGX Priority Review Voucher Safety to support approved chronic administration Already FDA approved commercial manufacturing facility for crofelemer KOL, protocol generation and possible SPA approach to help mitigate regulatory risk Safe and well tolerated with no SAEs reported through present Risk-Mitigated Product Development Key management has been with the company for >15 years Chairman of board and key investors have invested for >25 years Original discoverer and developer of successful FDA-approved first-in-class anti-secretory agent, crofelemer – only oral product approved under botanical guidance Strong Management Team Company patents issued through present: About 140 (majority do not expire until 2027 - 2031) Patent applications pending: About 40 (human health) Botanical guidance protection. Unique botanical sourcing infrastructure Orphan-drug designation Proprietary Position
It’s a marathon, not a sprint Break the big problem up into little pieces to solve Walk before you run Plan solve recalculate refocus Don’t look at the rocks, find the way through Don’t just do your best, just do it Setting goals is the first step in turning the invisible into the visible

Jaguar Health, Inc. Investor Relations Contact Peter Hodge phodge@jaguar.health

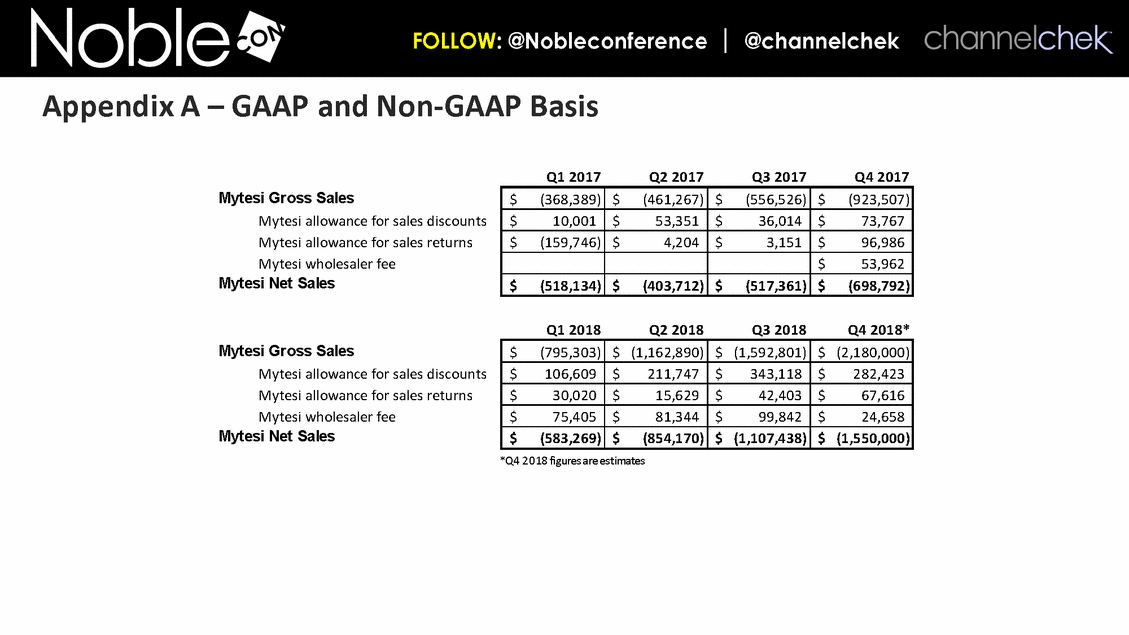
*Q4 2018 figures are estimates Appendix A – GAAP and Non-GAAP Basis Q1 2017 Q2 2017 Q3 2017 Q4 2017 Mytesi Gross Sales $ (368,389) $ (461,267) $ (556,526) $ (923,507) Mytesi allowance for sales discounts $ 10,001 $ 53,351 $ 36,014 $ 73,767 Mytesi allowance for sales returns $ (159,746) $ 4,204 $ 3,151 $ 96,986 Mytesi wholesaler fee $ 53,962 Mytesi Net Sales $ (518,134) $ (403,712) $ (517,361) $ (698,792) Q1 2018 Q2 2018 Q3 2018 Q4 2018* Mytesi Gross Sales $ (795,303) $ (1,162,890) $ (1,592,801) $ (2,180,000) Mytesi allowance for sales discounts $ 106,609 $ 211,747 $ 343,118 $ 282,423 Mytesi allowance for sales returns $ 30,020 $ 15,629 $ 42,403 $ 67,616 Mytesi wholesaler fee $ 75,405 $ 81,344 $ 99,842 $ 24,658 Mytesi Net Sales $ (583,269) $ (854,170) $ (1,107,438) $ (1,550,000)