Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ROCKWELL MEDICAL, INC. | a19-1344_18k.htm |
Certain statements in this presentation may constitute "forward-looking statements" within the meaning of the federal securities laws. Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," "could," "plan," "potential," "predict," "forecast," "project," "plan", "intend" or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Rockwell believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this communication and which are subject to inherent uncertainty. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Rockwell's SEC filings), many of which are beyond our control and subject to change. Actual results could be materially different. Risks and uncertainties include: statements about the timing and success of our planned NDA submission for I.V. Triferic®; the potential market opportunity for I.V. Triferic® and other Rockwell products, as well as the timing for planned commercial activities, including product launch; pricing and reimbursement status for I.V. Triferic®, Dialysate Triferic® and other Rockwell products, including eligibility for add-on reimbursement under TDAPA; timing and success of clinical trials for Triferic®; plans and timing relating to the planned commercialization of Triferic®; the timing and success of our efforts to find commercial partners for Triferic®; and the timing and success of our efforts to renegotiate economic terms of our concentrates business. Rockwell expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law. NASDAQ: RMTI 2 Forward Looking Language

Fully integrated, public (Nasdaq: RMTI), biopharmaceutical company targeting end-stage renal disease (ESRD) and chronic kidney disease (CKD), with a focus on anemia management Newly-formed executive management team, Board of Directors and Scientific Advisory Board with deep renal and pharmaceutical experience Triferic®, an innovative therapeutic for anemia management in hemodialysis patients FIRST and ONLY FDA-approved therapy indicated to maintain hemoglobin - replaces iron lost at every hemodialysis treatment Works like dietary iron in the body and avoids iron toxicity associated with current standard of care therapies Dialysate Triferic® formulation - planned U.S. commercial launch in 1H 2019 I.V. Triferic® formulation - plan NDA filing in 1H 2019 with potential FDA approval in 2020 ; global market potential Orange Book listed patent with expiry in 2029 #2 supplier of “base” dialysis concentrates in the U.S. $50+ million in revenues; relationships with leading companies providing dialysis treatment Strategic asset with potential to expand and generate cash flow to support innovative opportunities Repositioned for growth NASDAQ: RMTI 3 Investment Highlights
Proven track record and expertise in renal sector NASDAQ: RMTI Experienced Management Team 4 Management Experience Years Stuart Paul CEO 25+ Angus Smith CFO 13 Ray Pratt, M.D. Chief Medical Officer 25+ Ajay Gupta, M.D. Chief Scientific Officer 25+ Mike DeYoung VP Operations 25+ Anne Boardman VP Strategic Accounts 25+ Charlie Shiner VP Marketing 15 Jim McCarthy, CLP SVP Business & Corp. Development 25+

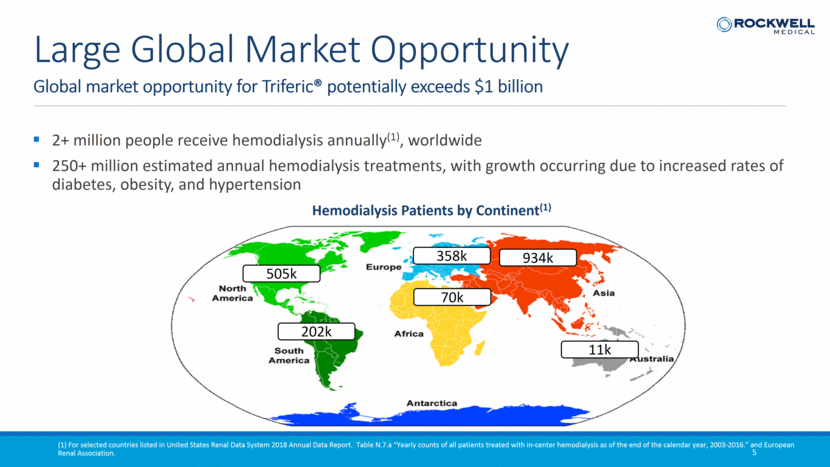
2+ million people receive hemodialysis annually(1), worldwide 250+ million estimated annual hemodialysis treatments, with growth occurring due to increased rates of diabetes, obesity, and hypertension Global market opportunity for Triferic® potentially exceeds $1 billion 5 Large Global Market Opportunity 505k 934k 202k 70k 358k 11k Hemodialysis Patients by Continent(1) (1) For selected countries listed in United States Renal Data System 2018 Annual Data Report. Table N.7.a “Yearly counts of all patients treated with in-center hemodialysis as of the end of the calendar year, 2003-2016.” and European Renal Association.
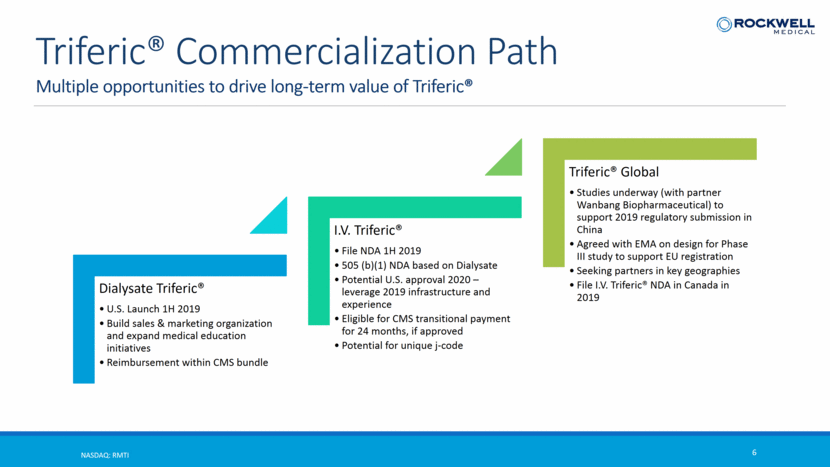
Multiple opportunities to drive long-term value of Triferic® NASDAQ: RMTI 6 Triferic® Commercialization Path Dialysate Triferic® U.S. Launch 1H 2019 Build sales & marketing organization and expand medical education initiatives Reimbursement within CMS bundle I.V. Triferic® File NDA 1H 2019 505 (b)(1) NDA based on Dialysate Potential U.S. approval 2020 – leverage 2019 infrastructure and experience Eligible for CMS transitional payment for 24 months, if approved Potential for unique j-code Triferic® Global Studies underway (with partner Wanbang Biopharmaceutical) to support 2019 regulatory submission in China Agreed with EMA on design for Phase III study to support EU registration Seeking partners in key geographies File I.V. Triferic® NDA in Canada in 2019
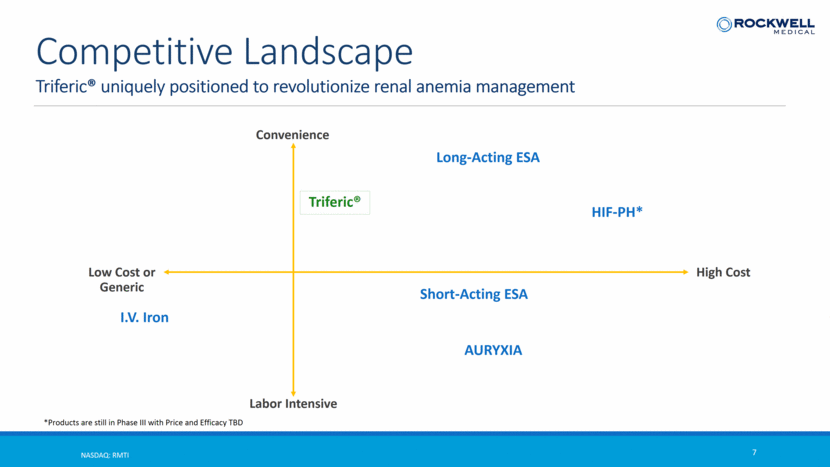
Triferic® uniquely positioned to revolutionize renal anemia management NASDAQ: RMTI 7 Competitive Landscape Low Cost or Generic High Cost Convenience Labor Intensive Long-Acting ESA HIF-PH* Triferic® Short-Acting ESA AURYXIA I.V. Iron *Products are still in Phase III with Price and Efficacy TBD
Blood loss and iron sequestration are leading causes of anemia in hemodialysis (HD) patients 5-7 mg iron loss during every HD treatment (3x/week, 156x/year) Iron losses are best replaced at every HD treatment, similar to concurrent replacement of Ca, Mg, K and bicarbonate losses by dialysis Current standard of care for anemia in HD patients has been focused on acute iron replacement and has significant limitations and risks Risk of iron toxicity; cardiovascular risks of ESAs High cost of ESAs I.V. iron therapy with polymeric iron complexes is associated with: Anaphylaxis/hypersensitivity reactions, infections, cardiovascular disease, calciphylaxis and functional iron deficiency Tissue iron overload with regular, ‘Maintenance’ I.V. iron therapy Hemoglobin and ESA cycling with ‘Load and Hold’ / ‘Intermittent’ I.V. iron therapy Iron loss is unavoidable during hemodialysis and occurs in each and every hemodialysis patient NASDAQ: RMTI 8 Unmet Need in Iron Maintenance Therapy
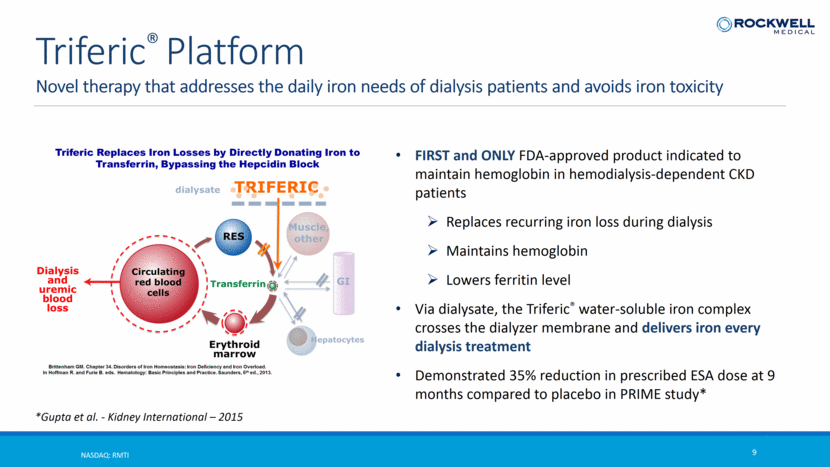
Novel therapy that addresses the daily iron needs of dialysis patients and avoids iron toxicity NASDAQ: RMTI 9 Triferic® Platform FIRST and ONLY FDA-approved product indicated to maintain hemoglobin in hemodialysis-dependent CKD patients Replaces recurring iron loss during dialysis Maintains hemoglobin Lowers ferritin level Via dialysate, the Triferic® water-soluble iron complex crosses the dialyzer membrane and delivers iron every dialysis treatment Demonstrated 35% reduction in prescribed ESA dose at 9 months compared to placebo in PRIME study* *Gupta et al. - Kidney International – 2015
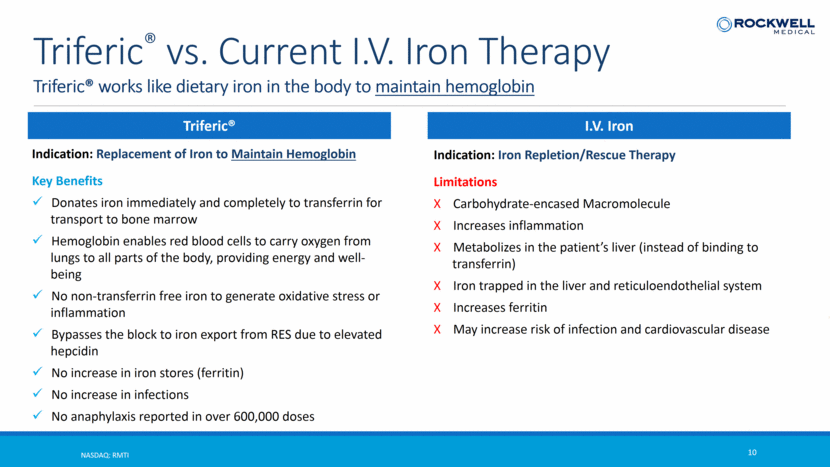
NASDAQ: RMTI 10 Triferic® vs. Current I.V. Iron Therapy Triferic® works like dietary iron in the body to maintain hemoglobin Triferic® Indication: Replacement of Iron to Maintain Hemoglobin Key Benefits Donates iron immediately and completely to transferrin for transport to bone marrow Hemoglobin enables red blood cells to carry oxygen from lungs to all parts of the body, providing energy and well-being No non-transferrin free iron to generate oxidative stress or inflammation Bypasses the block to iron export from RES due to elevated hepcidin No increase in iron stores (ferritin) No increase in infections No anaphylaxis reported in over 600,000 doses Triferic® IV Iron Indication: Iron Repletion/Rescue Therapy Limitations Carbohydrate-encased Macromolecule Increases inflammation Metabolizes in the patient’s liver (instead of binding to transferrin) Iron trapped in the liver and reticuloendothelial system Increases ferritin May increase risk of infection and cardiovascular disease I.V. Iron
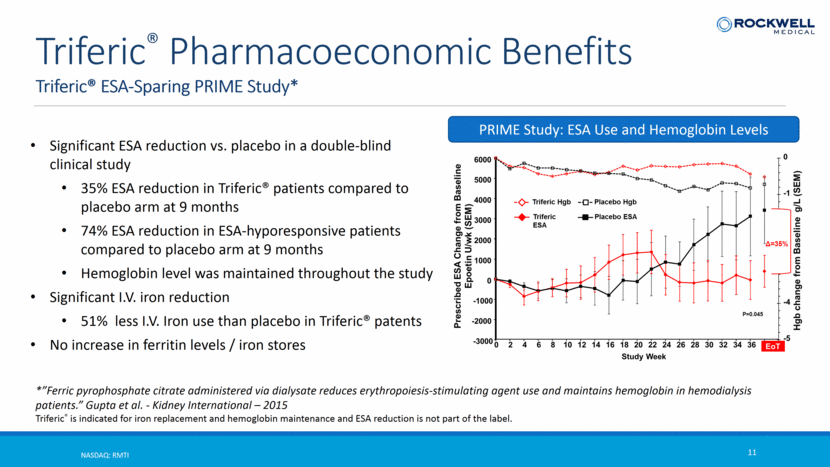
Triferic® ESA-Sparing PRIME Study* NASDAQ: RMTI 11 Triferic® Pharmacoeconomic Benefits *”Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients.” Gupta et al. - Kidney International – 2015 Triferic® is indicated for iron replacement and hemoglobin maintenance and ESA reduction is not part of the label. Significant ESA reduction vs. placebo in a double-blind clinical study 35% ESA reduction in Triferic® patients compared to placebo arm at 9 months 74% ESA reduction in ESA-hyporesponsive patients compared to placebo arm at 9 months Hemoglobin level was maintained throughout the study Significant I.V. iron reduction 51% less I.V. Iron use than placebo in Triferic® patents No increase in ferritin levels / iron stores PRIME Study: ESA Use and Hemoglobin Levels
Demonstrates consistent, well-defined safety profile suitable for long-term iron maintenance therapy NASDAQ: RMTI 12 Triferic® Has a Proven Safety Record Largest safety database submitted for registration of any parenteral iron product 1,440 patients received Triferic® every HD treatment for up to 18 months 870 patient years of exposure in NDA database No reported anaphylaxis in over 600,000 doses administered No increase in intradialytic hypotension No iron toxicity or overload No increase in infections, oxidative stress or inflammation Decrease in blood transfusions of greater than 50%1 Overall adverse event profile similar to placebo Please visit www.triferic.com for full prescribing and safety information 1. Published in Kidney International, Gupta et al, 2015.
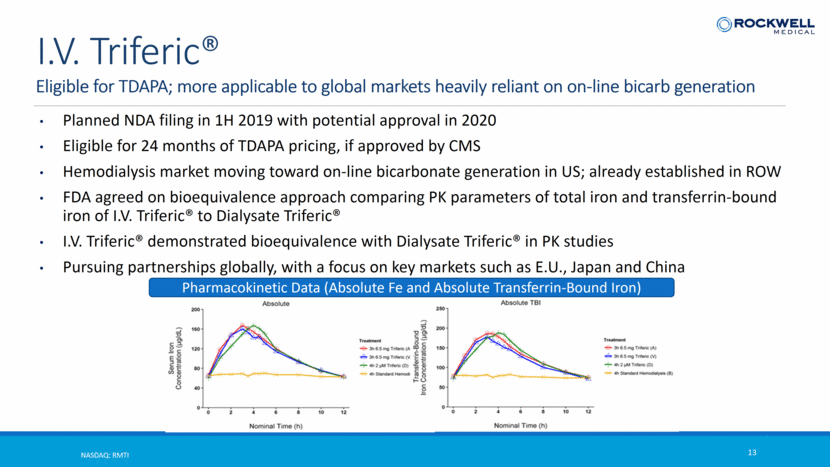
Eligible for TDAPA; more applicable to global markets heavily reliant on on-line bicarb generation NASDAQ: RMTI 13 I.V. Triferic® Planned NDA filing in 1H 2019 with potential approval in 2020 Eligible for 24 months of TDAPA pricing, if approved by CMS Hemodialysis market moving toward on-line bicarbonate generation in US; already established in ROW FDA agreed on bioequivalence approach comparing PK parameters of total iron and transferrin-bound iron of I.V. Triferic® to Dialysate Triferic® I.V. Triferic® demonstrated bioequivalence with Dialysate Triferic® in PK studies Pursuing partnerships globally, with a focus on key markets such as E.U., Japan and China Pharmacokinetic Data (Absolute Fe and Absolute Transferrin-Bound Iron)
Dialysate Triferic® FDA-Approved & Ready to Launch in 2019 NASDAQ: RMTI 14 Potential to Transform Renal Anemia Management Triferic.com; accessed 12/3/2018 Triferic.com Gives Iron When and Where Patients Need it Triferic® enters the blood via dialysate and donates its iron immediately to transferrin, making red blood cells and maintaining hemoglobin. Increases Health Red Blood Cells Hemoglobin enables red blood cells to carry oxygen to all parts of the body, providing energy. Proven Safety Profile No iron trapped in the liver and no increase in ferritin, inflammation, toxicity or infections. No anaphylaxis. Decrease in blood transfusions.
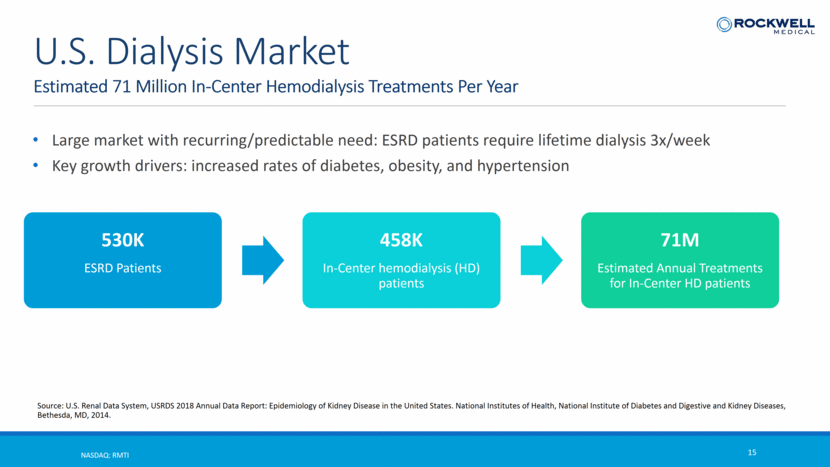
Large market with recurring/predictable need: ESRD patients require lifetime dialysis 3x/week Key growth drivers: increased rates of diabetes, obesity, and hypertension Estimated 71 Million In-Center Hemodialysis Treatments Per Year NASDAQ: RMTI 15 U.S. Dialysis Market Source: U.S. Renal Data System, USRDS 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2014. 530K ESRD Patients 458K In-Center hemodialysis (HD) patients 71M Estimated Annual Treatments for In-Center HD patients
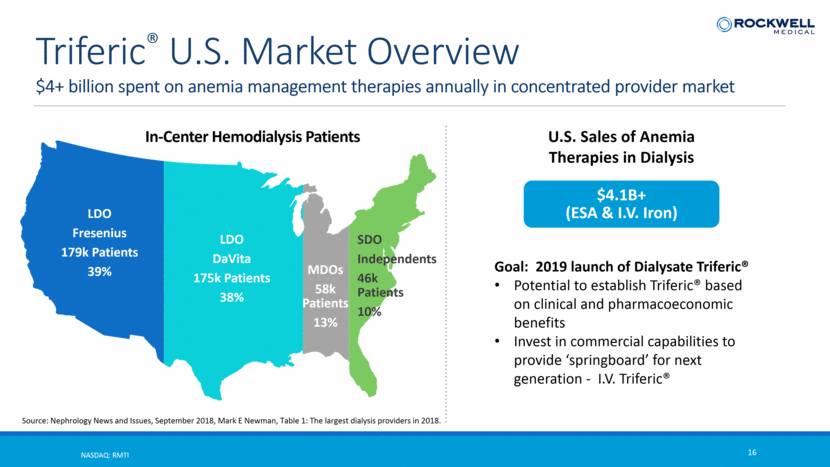
$4+ billion spent on anemia management therapies annually in concentrated provider market NASDAQ: RMTI 16 Triferic® U.S. Market Overview LDO Fresenius 179k Patients 39% LDO DaVita 175k Patients 38% SDO Independents 46k Patients 10% MDOs 58k Patients 13% In-Center Hemodialysis Patients Goal: 2019 launch of Dialysate Triferic® Potential to establish Triferic® based on clinical and pharmacoeconomic benefits Invest in commercial capabilities to provide ‘springboard’ for next generation - I.V. Triferic® Source: Nephrology News and Issues, September 2018, Mark E Newman, Table 1: The largest dialysis providers in 2018. $4.1B+ (ESA & I.V. Iron) U.S. Sales of Anemia Therapies in Dialysis
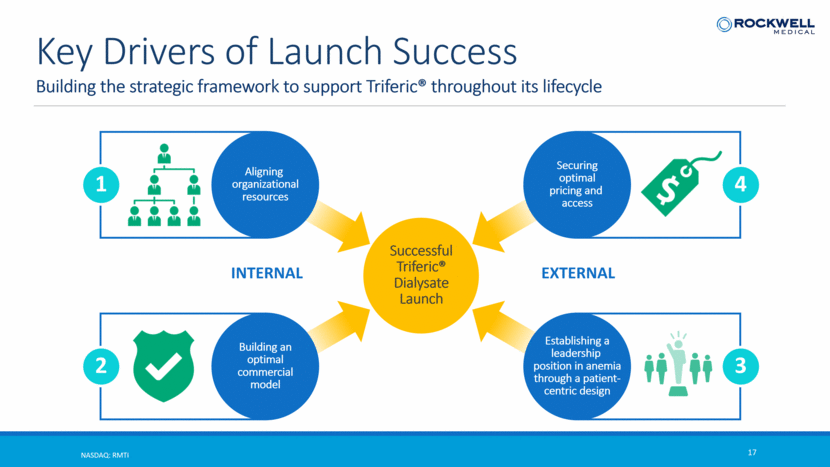
Building the strategic framework to support Triferic® throughout its lifecycle NASDAQ: RMTI 17 Key Drivers of Launch Success INTERNAL Aligning organizational resources 1 Building an optimal commercial model 2 Establishing a leadership position in anemia through a patient- centric design 3 Securing optimal pricing and access 4 EXTERNAL Successful Triferic® Dialysate Launch
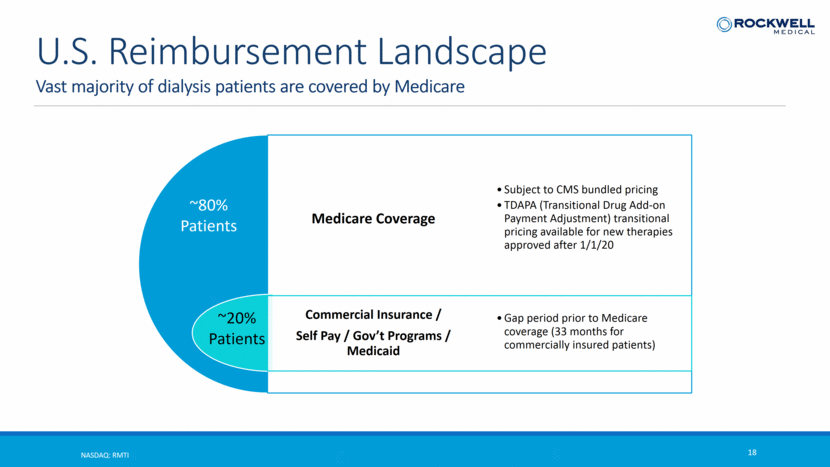
Vast majority of dialysis patients are covered by Medicare NASDAQ: RMTI 18 U.S. Reimbursement Landscape ~80% Patients ~20% Patients Medicare Coverage Commercial Insurance / Self Pay / Gov’t Programs / Medicaid Subject to CMS bundled pricing TDAPA (Transitional Drug Add-on Payment Adjustment) transitional pricing available for new therapies approved after 1/1/20 Gap period prior to Medicare coverage (33 months for commercially insured patients)
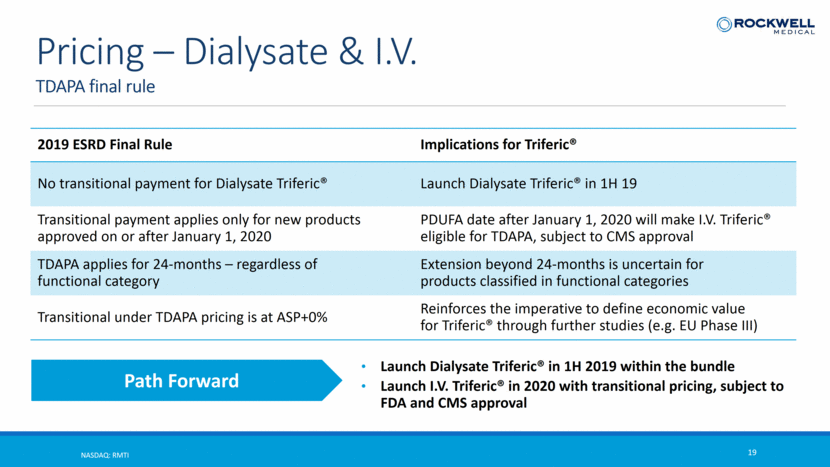
TDAPA final rule NASDAQ: RMTI 19 Pricing – Dialysate & I.V. 2019 ESRD Final Rule Implications for Triferic® No transitional payment for Dialysate Triferic® Launch Dialysate Triferic® in 1H 19 Transitional payment applies only for new products approved on or after January 1, 2020 PDUFA date after January 1, 2020 will make I.V. Triferic® eligible for TDAPA, subject to CMS approval TDAPA applies for 24-months – regardless of functional category Extension beyond 24-months is uncertain for products classified in functional categories Transitional under TDAPA pricing is at ASP+0% Reinforces the imperative to define economic value for Triferic® through further studies (e.g. EU Phase III) Launch Dialysate Triferic® in 1H 2019 within the bundle Launch I.V. Triferic® in 2020 with transitional pricing, subject to FDA and CMS approval Path Forward
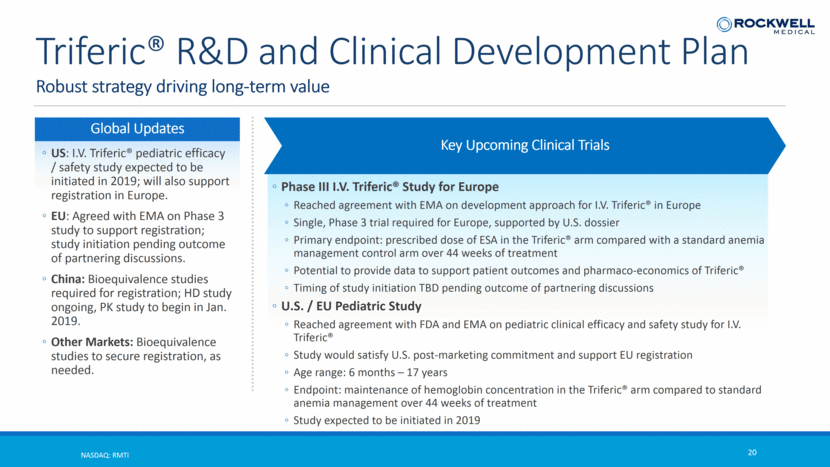
Robust strategy driving long-term value NASDAQ: RMTI 20 Triferic® R&D and Clinical Development Plan Key Upcoming Clinical Trials Phase III I.V. Triferic® Study for Europe Reached agreement with EMA on development approach for I.V. Triferic® in Europe Single, Phase 3 trial required for Europe, supported by U.S. dossier Primary endpoint: prescribed dose of ESA in the Triferic® arm compared with a standard anemia management control arm over 44 weeks of treatment Potential to provide data to support patient outcomes and pharmaco-economics of Triferic® Timing of study initiation TBD pending outcome of partnering discussions U.S. / EU Pediatric Study Reached agreement with FDA and EMA on pediatric clinical efficacy and safety study for I.V. Triferic® Study would satisfy U.S. post-marketing commitment and support EU registration Age range: 6 months – 17 years Endpoint: maintenance of hemoglobin concentration in the Triferic® arm compared to standard anemia management over 44 weeks of treatment Study expected to be initiated in 2019 Global Updates US: I.V. Triferic® pediatric efficacy / safety study expected to be initiated in 2019; will also support registration in Europe. EU: Agreed with EMA on Phase 3 study to support registration; study initiation pending outcome of partnering discussions. China: Bioequivalence studies required for registration; HD study ongoing, PK study to begin in Jan. 2019. Other Markets: Bioequivalence studies to secure registration, as needed.
Advance and establish partnerships in key value-driving territories around the globe NASDAQ: RMTI 21 Triferic® International Priorities Europe Identify partner for I.V. Triferic® Initiate Phase III program in collaboration with partner China Partnered to commercialize Triferic® with Wanbang Biopharmaceutical Initiate 2 studies for Dialysate Triferic® by Jan. 2019 (one initiated 12/18) for completion in 1H 2019 $35 million of potential regulatory and revenue milestone payments Japan Identify partner for I.V. Triferic® Clarify Japan I.V. Triferic® regulatory requirements LATAM Peru and Chile Agreements in place and regulatory submissions completed Expect Peru and Chile regulatory approval in 2019 Exploring additional opportunities Canada Regulatory dossier ready 1H 2019

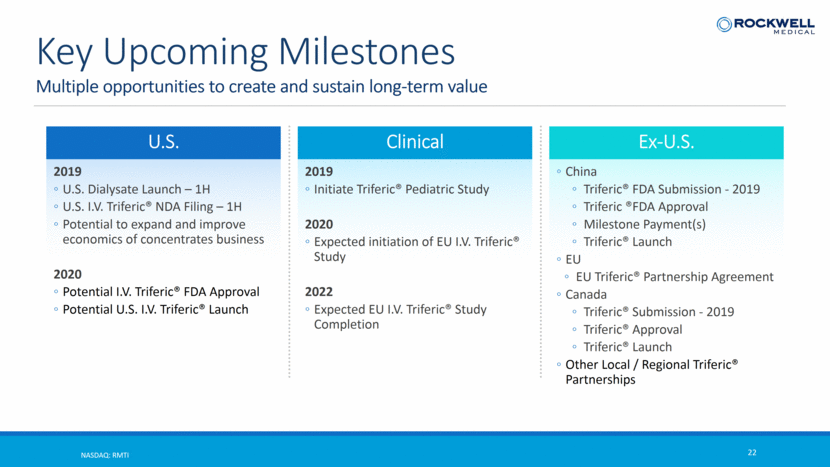
Multiple opportunities to create and sustain long-term value NASDAQ: RMTI 22 Key Upcoming Milestones U.S. 2019 U.S. Dialysate Launch – 1H U.S. I.V. Triferic® NDA Filing – 1H Potential to expand and improve economics of concentrates business 2020 Potential I.V. Triferic® FDA Approval Potential U.S. I.V. Triferic® Launch Clinical 2019 Initiate Triferic® Pediatric Study 2020 Expected initiation of EU I.V. Triferic® Study 2022 Expected EU I.V. Triferic® Study Completion Ex-U.S. China Triferic® FDA Submission - 2019 Triferic ®FDA Approval Milestone Payment(s) Triferic® Launch EU EU Triferic® Partnership Agreement Canada Triferic® Submission - 2019 Triferic® Approval Triferic® Launch Other Local / Regional Triferic® Partnerships
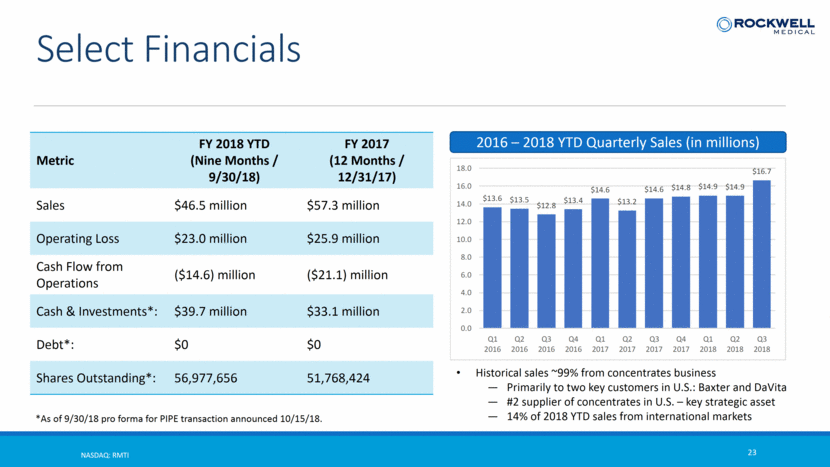
NASDAQ: RMTI 23 Select Financials Metric FY 2018 YTD (Nine Months / 9/30/18) FY 2017 (12 Months / 12/31/17) Sales $46.5 million $57.3 million Operating Loss $23.0 million $25.9 million Cash Flow from Operations ($14.6) million ($21.1) million Cash & Investments*: $39.7 million $33.1 million Debt*: $0 $0 Shares Outstanding*: 56,977,656 51,768,424 *As of 9/30/18 pro forma for PIPE transaction announced 10/15/18. 2016 – 2018 YTD Quarterly Sales (in millions) Historical sales ~99% from concentrates business Primarily to two key customers in U.S.: Baxter and DaVita #2 supplier of concentrates in U.S. – key strategic asset 14% of 2018 YTD sales from international markets $13.6 $13.5 $12.8 $13.4 $14.6 $13.2 $14.6 $14.8 $14.9 $14.9 $16.7 0.0 2.0 4.0 6.0 8.0 10.0 12.0 14.0 16.0 18.0 Q1 2016 Q2 2016 Q3 2016 Q4 2016 Q1 2017 Q2 2017 Q3 2017 Q4 2017 Q1 2018 Q2 2018 Q3 2018
Repositioned for growth NASDAQ: RMTI 24 Why Rockwell? Why Now? Fully integrated, public (Nasdaq: RMTI), biopharmaceutical company targeting end-stage renal disease (ESRD) and chronic kidney disease (CKD), with a focus on anemia management Newly-formed executive management team, Board of Directors and Scientific Advisory Board with deep renal and pharmaceutical experience Triferic®, an innovative therapeutic for anemia management in hemodialysis patients FIRST and ONLY FDA-approved therapy indicated to maintain hemoglobin - replaces iron lost at every hemodialysis treatment Works like dietary iron in the body and avoids iron toxicity associated with current standard of care therapies Dialysate Triferic® formulation - planned U.S. commercial launch in 1H 2019 I.V. Triferic® formulation - plan NDA filing in 1H 2019 with potential FDA approval in 2020 ; global market potential Orange Book listed patent with expiry in 2029 #2 supplier of “base” dialysis concentrates in the U.S. $50+ million in revenues; relationships with leading companies providing dialysis treatment Strategic asset with potential to expand and generate cash flow to support innovative opportunities