Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 PRESS RELEASE - MYRIAD GENETICS INC | mygn-ex992_7.htm |
| 8-K - 8-K GUIDED STUDY CALL - MYRIAD GENETICS INC | mygn-8k_20190104.htm |

Review of GeneSight® Supporting Clinical Data January 4, 2019 Exhibit 99.1

Forward Looking Statements Some of the information presented here today may contain projections or other forward-looking statements regarding future events or the future financial performance of the Company. These statements are based on management’s current expectations and the actual events or results may differ materially and adversely from these expectations. We refer you to the documents the Company files from time to time with the Securities and Exchange Commission, specifically, the Company’s annual reports on Form 10-K, its quarterly reports on Form 10-Q, and its current reports on Form 8-K. These documents identify important risk factors that could cause the actual results to differ materially from those contained in the Company’s projections or forward-looking statements. 2

Overview of Clinical Studies in Depression 3

Overview of Clinical Studies in Depression Studies typically use subjective patient assessment by questionnaire (HAM D-17 score) as the clinical measure of depressive symptoms Three different endpoints are calculated from changes in these scores: Remission, Response, and Symptom Improvement APA guidelines state that Remission is the only acceptable goal of treatment Payers assessed on Remission and Response as part of HEDIS scores 40 consecutive antidepressant studies submitted to FDA in past 20 years No FDA approval was based upon an active drug comparator arm and most enrolled treatment-naïve patients (not more difficult treatment-resistant patients) Only 13% of trials showed statistically significant improvement in remission over placebo Only 30% of trials showed statistically significant improvement in response over placebo Only 70% of trials showed statistically significant improvement in symptoms over placebo 4
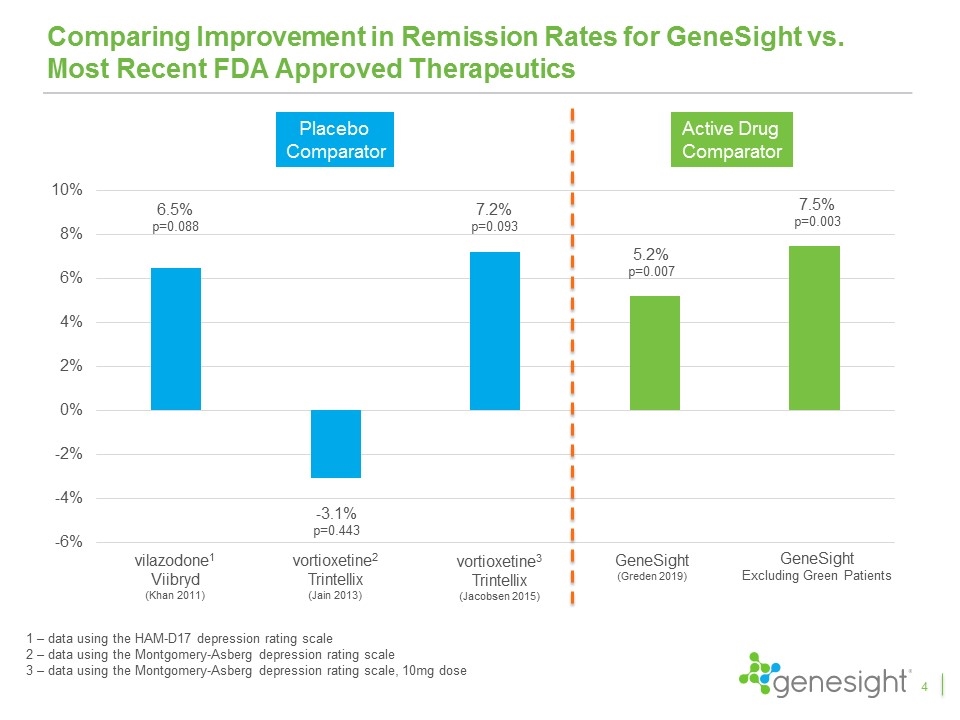
Comparing Improvement in Remission Rates for GeneSight vs. Most Recent FDA Approved Therapeutics 4 vilazodone1 Viibryd (Khan 2011) vortioxetine2 Trintellix (Jain 2013) vortioxetine3 Trintellix (Jacobsen 2015) GeneSight (Greden 2019) 6.5% p=0.088 -3.1% p=0.443 7.2% p=0.093 5.2% p=0.007 1 – data using the HAM-D17 depression rating scale 2 – data using the Montgomery-Asberg depression rating scale 3 – data using the Montgomery-Asberg depression rating scale, 10mg dose Placebo Comparator Active Drug Comparator GeneSight Excluding Green Patients 7.5% p=0.003

GeneSight Clinical Utility Studies Prior to GUIDED 5
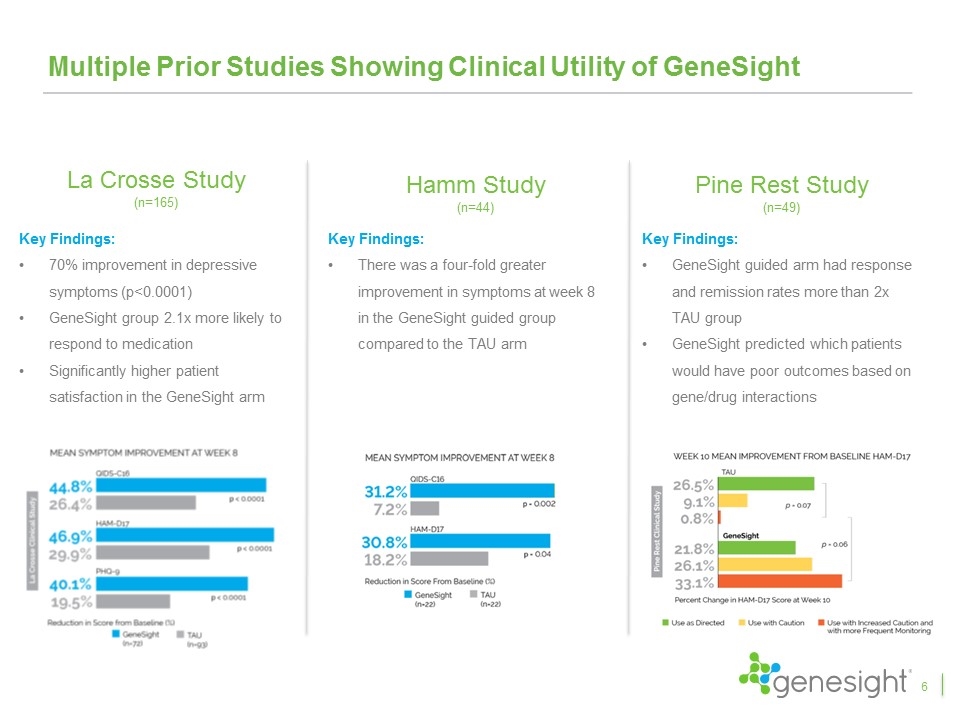
Multiple Prior Studies Showing Clinical Utility of GeneSight La Crosse Study (n=165) Key Findings: 70% improvement in depressive symptoms (p<0.0001) GeneSight group 2.1x more likely to respond to medication Significantly higher patient satisfaction in the GeneSight arm Pine Rest Study (n=49) Key Findings: GeneSight guided arm had response and remission rates more than 2x TAU group GeneSight predicted which patients would have poor outcomes based on gene/drug interactions Hamm Study (n=44) Key Findings: There was a four-fold greater improvement in symptoms at week 8 in the GeneSight guided group compared to the TAU arm 6

Publication Overview GUIDED Study 7
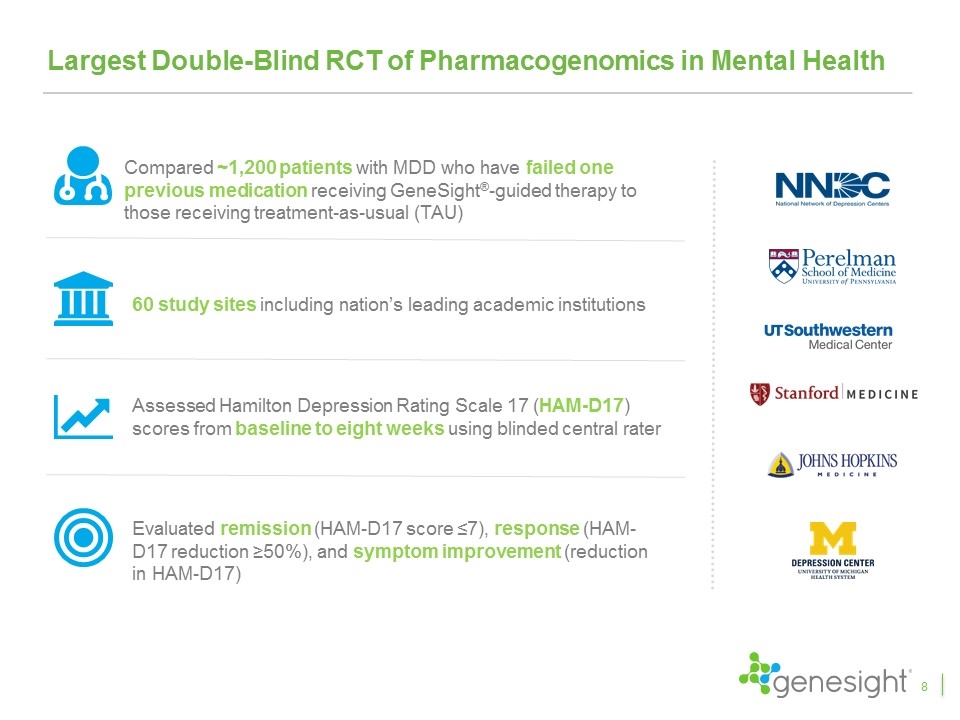
Largest Double-Blind RCT of Pharmacogenomics in Mental Health Compared ~1,200 patients with MDD who have failed one previous medication receiving GeneSight®-guided therapy to those receiving treatment-as-usual (TAU) 60 study sites including nation’s leading academic institutions Assessed Hamilton Depression Rating Scale 17 (HAM-D17) scores from baseline to eight weeks using blinded central rater Evaluated remission (HAM-D17 score ≤7), response (HAM-D17 reduction ≥50%), and symptom improvement (reduction in HAM-D17) 8
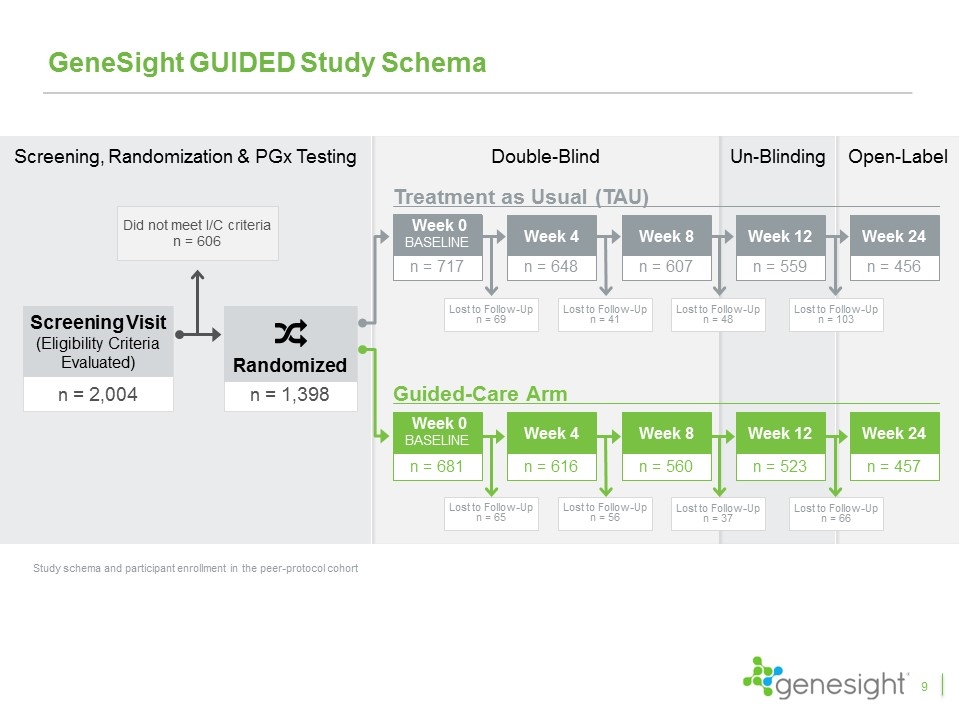
n = 2,004 Screening Visit (Eligibility Criteria Evaluated) Did not meet I/C criteria n = 606 n = 1,398 Randomized Screening, Randomization & PGx Testing GeneSight GUIDED Study Schema Study schema and participant enrollment in the peer-protocol cohort Treatment as Usual (TAU) Guided-Care Arm n = 456 Week 24 n = 457 Week 24 Double-Blind Un-Blinding Open-Label n = 607 Week 8 Lost to Follow-Up n = 48 n = 648 Week 4 Lost to Follow-Up n = 41 n = 717 Week 0 baseline Lost to Follow-Up n = 69 n = 559 Week 12 Lost to Follow-Up n = 103 Lost to Follow-Up n = 65 n = 681 Week 0 baseline n = 616 Week 4 Lost to Follow-Up n = 56 n = 560 Week 8 Lost to Follow-Up n = 37 n = 523 Week 12 Lost to Follow-Up n = 66 9
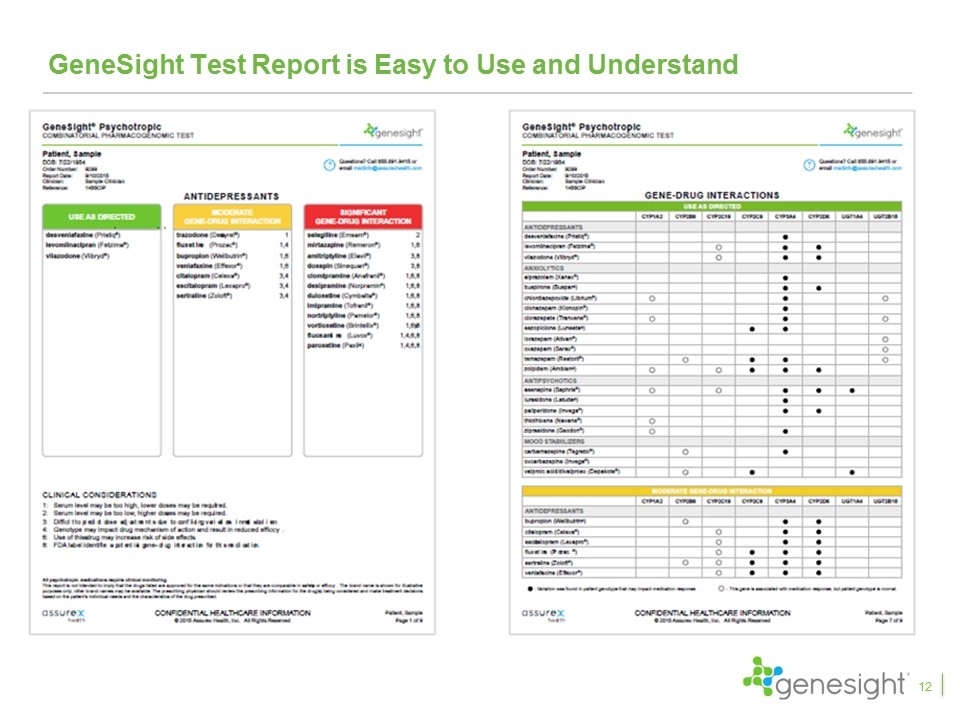
GeneSight Test Report is Easy to Use and Understand 12
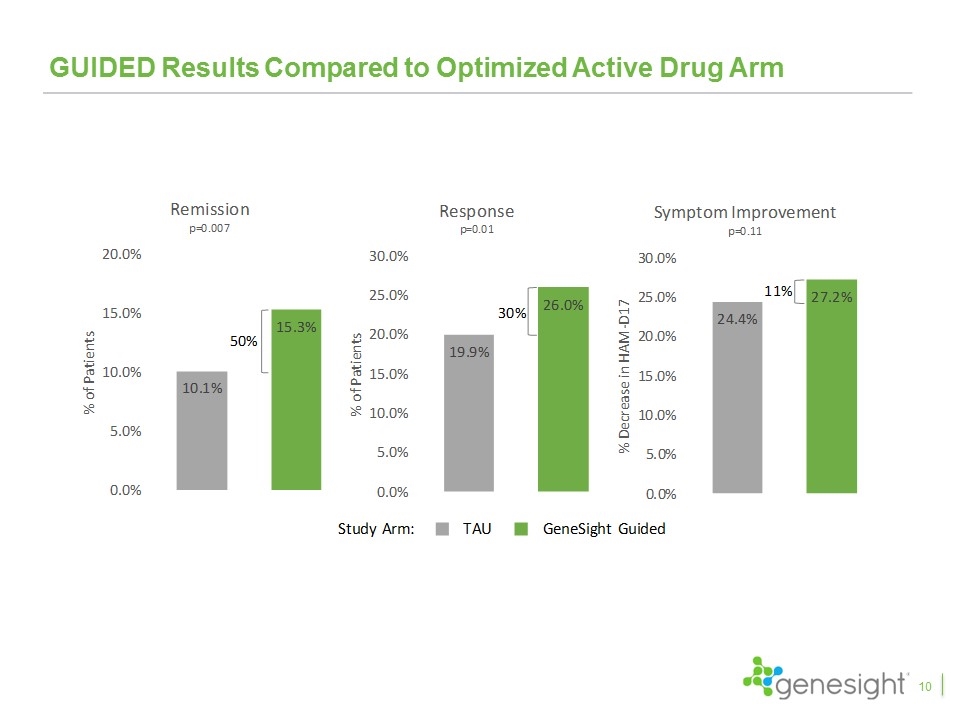
GUIDED Results Compared to Optimized Active Drug Arm 10
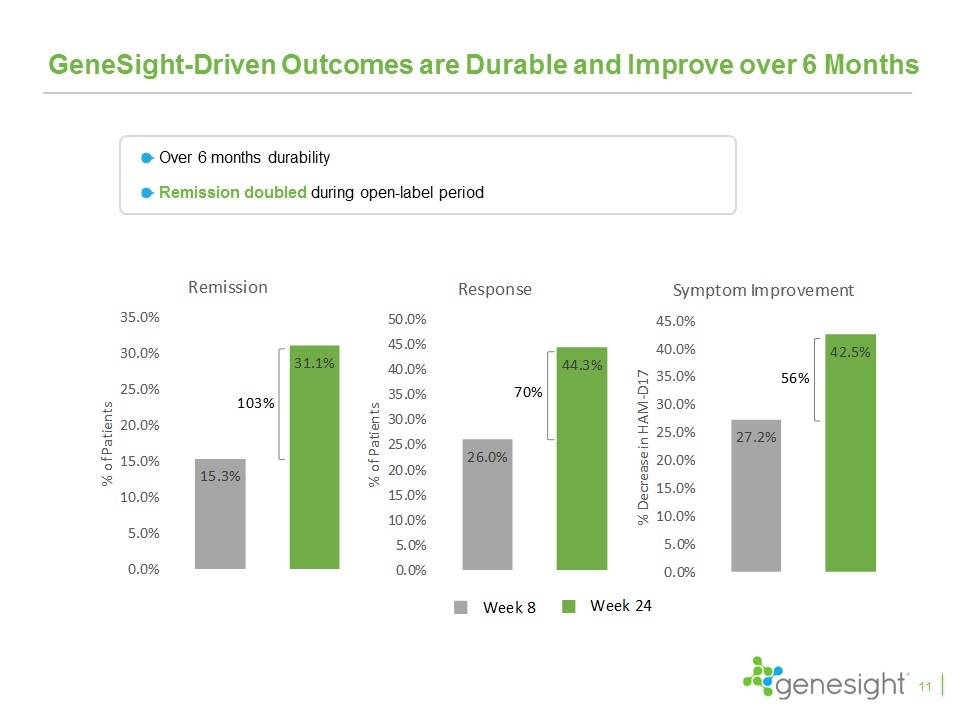
GeneSight-Driven Outcomes are Durable and Improve over 6 Months Over 6 months durability Remission doubled during open-label period 11
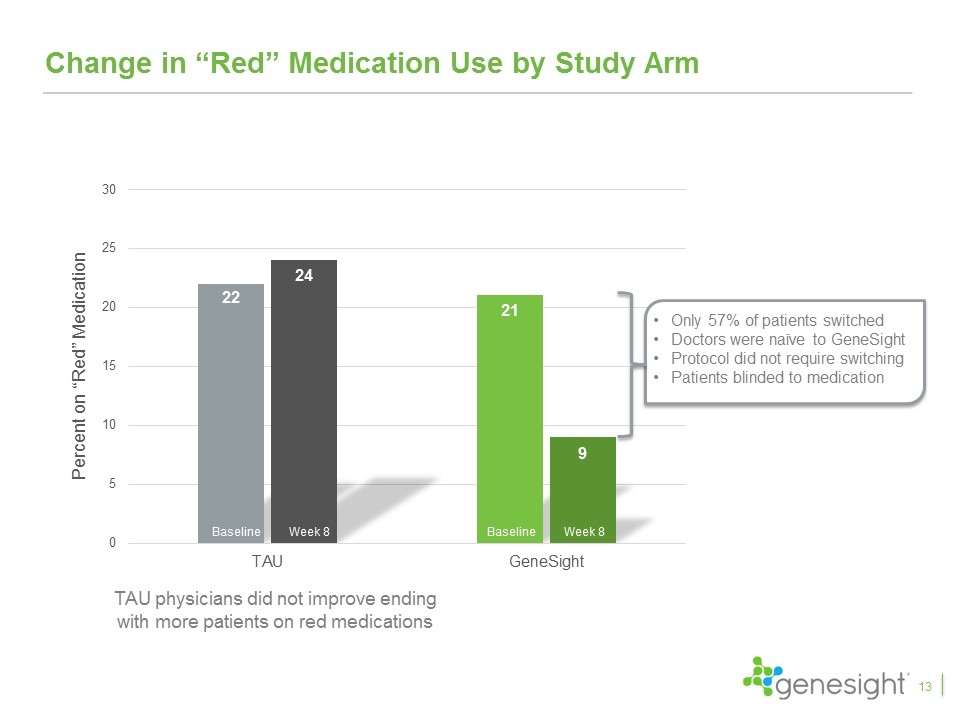
Change in “Red” Medication Use by Study Arm Baseline Week 8 Baseline Week 8 13 Only 57% of patients switched Doctors were naïve to GeneSight Protocol did not require switching Patients blinded to medication TAU physicians did not improve ending with more patients on red medications
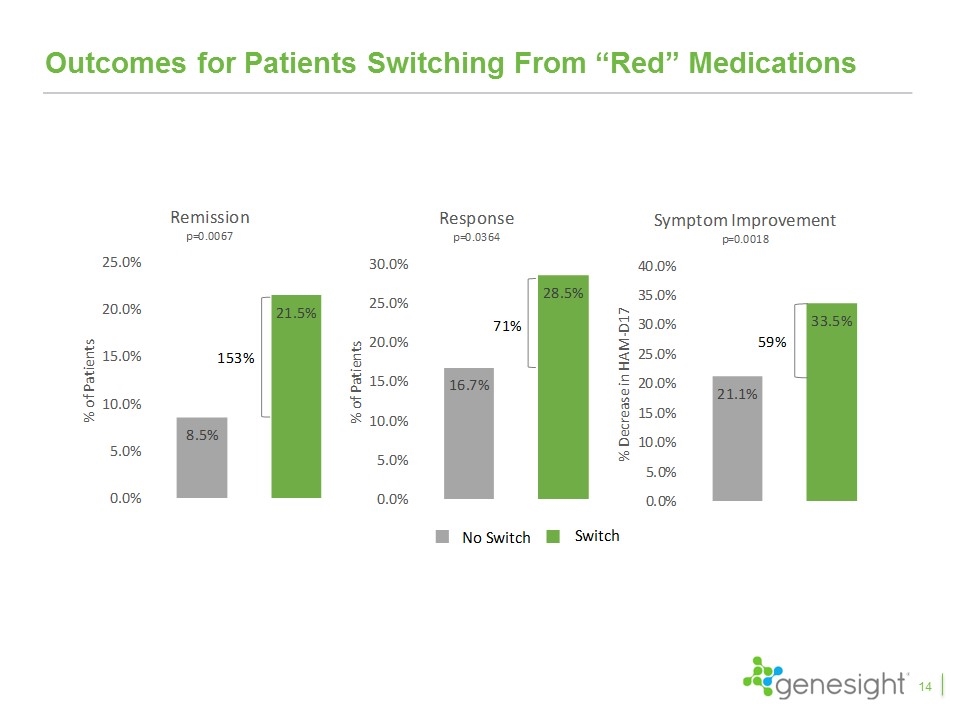
Outcomes for Patients Switching From “Red” Medications 14
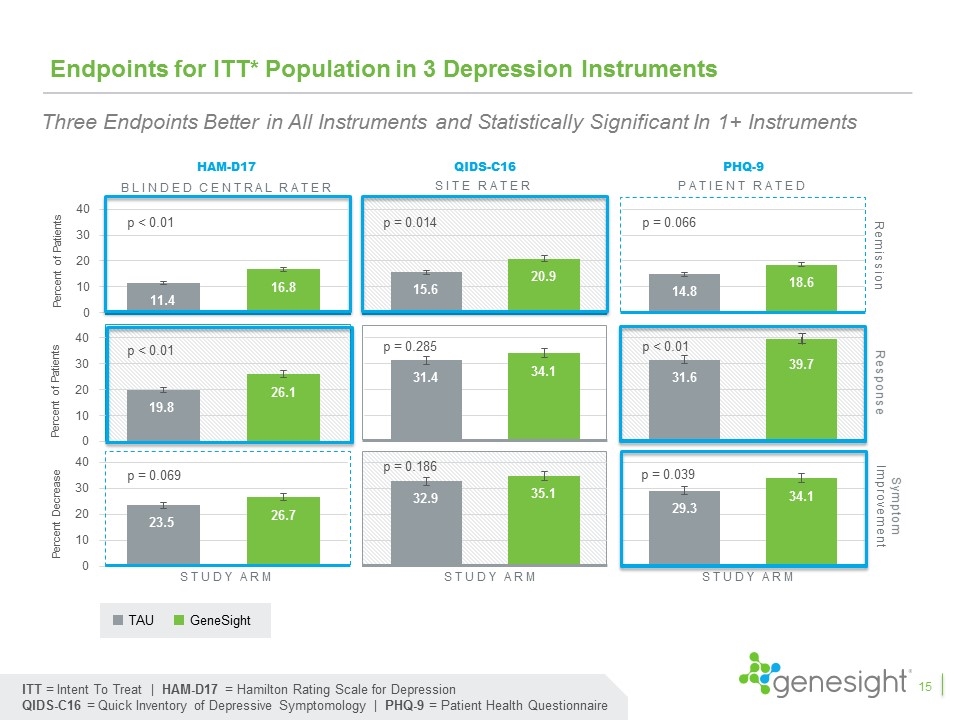
Endpoints for ITT* Population in 3 Depression Instruments HAM-D17 QIDS-C16 PHQ-9 Response Remission Study arm Study arm Study arm Symptom Improvement p < 0.01 p < 0.01 p = 0.069 p = 0.014 p = 0.285 p = 0.186 p = 0.066 p < 0.01 p = 0.039 ITT = Intent To Treat | HAM-D17 = Hamilton Rating Scale for Depression QIDS-C16 = Quick Inventory of Depressive Symptomology | PHQ-9 = Patient Health Questionnaire Blinded central rater Site rater Patient rated TAU GeneSight 15 Three Endpoints Better in All Instruments and Statistically Significant In 1+ Instruments
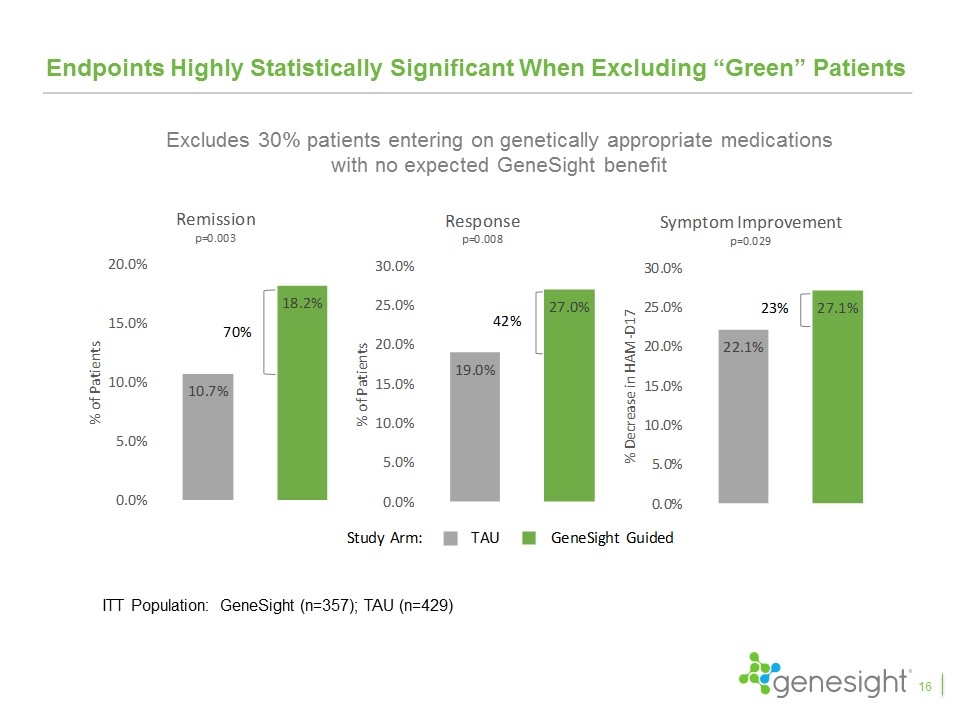
Endpoints Highly Statistically Significant When Excluding “Green” Patients 16 ITT Population: GeneSight (n=357); TAU (n=429) Excludes 30% patients entering on genetically appropriate medications with no expected GeneSight benefit

Publication Overview IMPACT Study 17

IMPACT Study Design Goal was to compare outcomes of patients with major depressive disorder treated by either psychiatrists or primary care physicians using GeneSight to guide therapy selection Performed in cooperation with the Canadian Centre for Mental Health and Addiction (CAMH) Open label study All patients received GeneSight Primary endpoint was the Beck’s Depression Inventory performed at 8 weeks Enrolled 1,871 total patients – 810 treated by primary care providers and 1,061 treated by psychiatrists Patients in the primary care and psychiatrist cohorts were deemed to have no clinically meaningful differences Data important for Medicare to expand LCD to primary care physicians 18
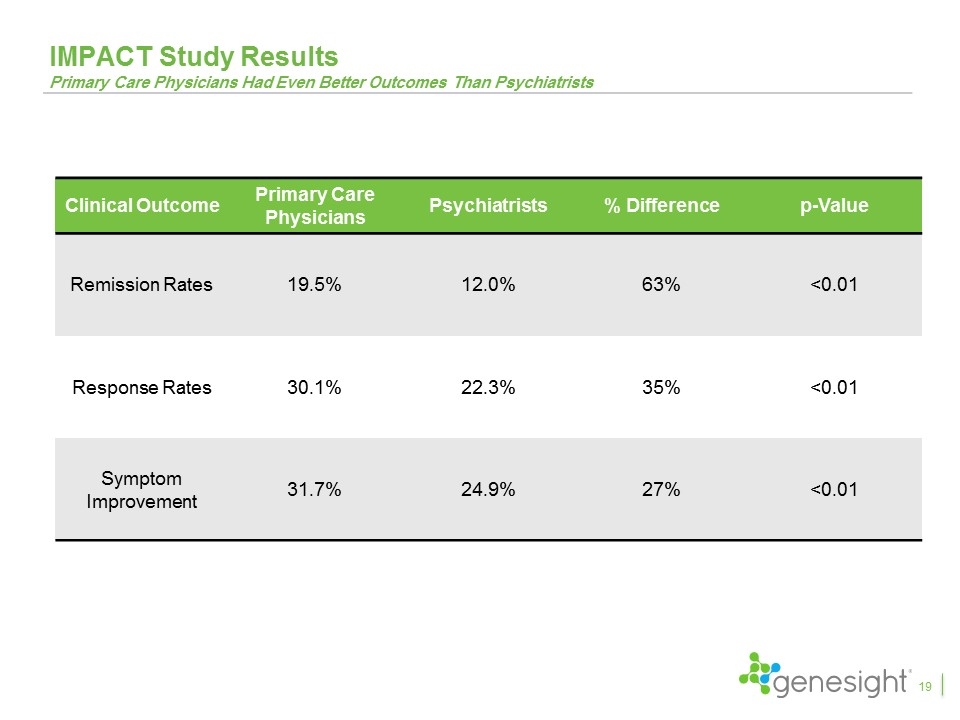
IMPACT Study Results Primary Care Physicians Had Even Better Outcomes Than Psychiatrists Clinical Outcome Primary Care Physicians Psychiatrists % Difference p-Value Remission Rates 19.5% 12.0% 63% <0.01 Response Rates 30.1% 22.3% 35% <0.01 Symptom Improvement 31.7% 24.9% 27% <0.01 19

Publication Overview Health Economic Data 20
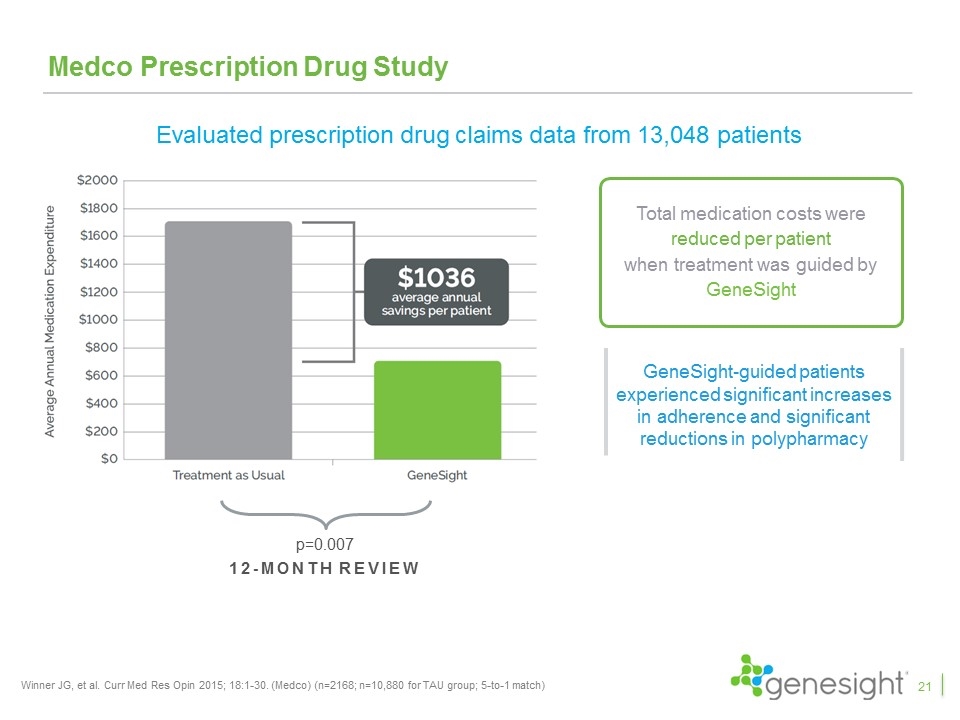
Medco Prescription Drug Study Winner JG, et al. Curr Med Res Opin 2015; 18:1-30. (Medco) (n=2168; n=10,880 for TAU group; 5-to-1 match) 12-month review p=0.007 Total medication costs were reduced per patient when treatment was guided by GeneSight GeneSight-guided patients experienced significant increases in adherence and significant reductions in polypharmacy Evaluated prescription drug claims data from 13,048 patients 21
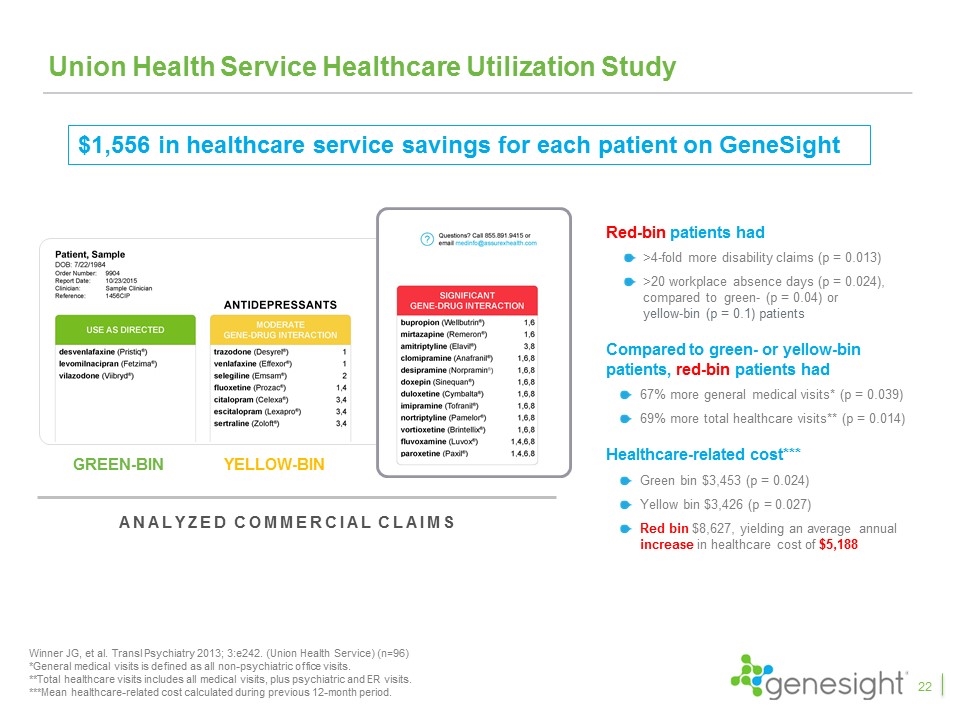
Union Health Service Healthcare Utilization Study Red-bin patients had >4-fold more disability claims (p = 0.013) >20 workplace absence days (p = 0.024), compared to green- (p = 0.04) or yellow-bin (p = 0.1) patients Compared to green- or yellow-bin patients, red-bin patients had 67% more general medical visits* (p = 0.039) 69% more total healthcare visits** (p = 0.014) Healthcare-related cost*** Green bin $3,453 (p = 0.024) Yellow bin $3,426 (p = 0.027) Red bin $8,627, yielding an average annual increase in healthcare cost of $5,188 Winner JG, et al. Transl Psychiatry 2013; 3:e242. (Union Health Service) (n=96) *General medical visits is defined as all non-psychiatric office visits. **Total healthcare visits includes all medical visits, plus psychiatric and ER visits. ***Mean healthcare-related cost calculated during previous 12-month period. Analyzed commercial claims YELLOW-BIN GREEN-BIN RED-BIN $1,556 in healthcare service savings for each patient on GeneSight 22
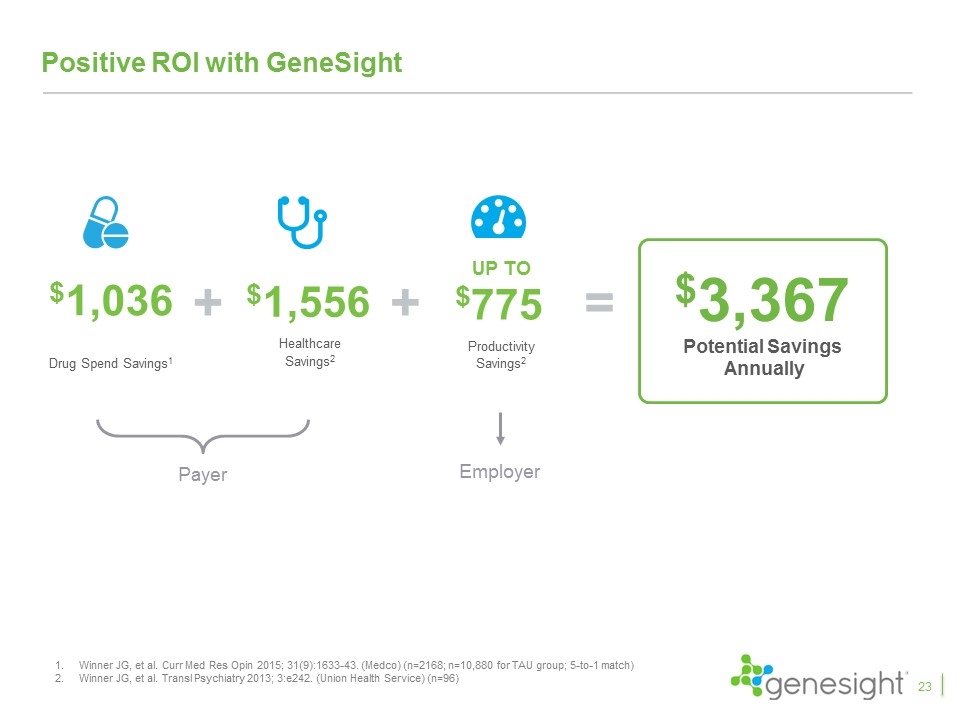
Positive ROI with GeneSight $1,036 Drug Spend Savings1 $1,556 Healthcare Savings2 $3,367 Potential Savings Annually + = + UP TO $775 Productivity Savings2 Payer Employer Winner JG, et al. Curr Med Res Opin 2015; 31(9):1633-43. (Medco) (n=2168; n=10,880 for TAU group; 5-to-1 match) Winner JG, et al. Transl Psychiatry 2013; 3:e242. (Union Health Service) (n=96) 23
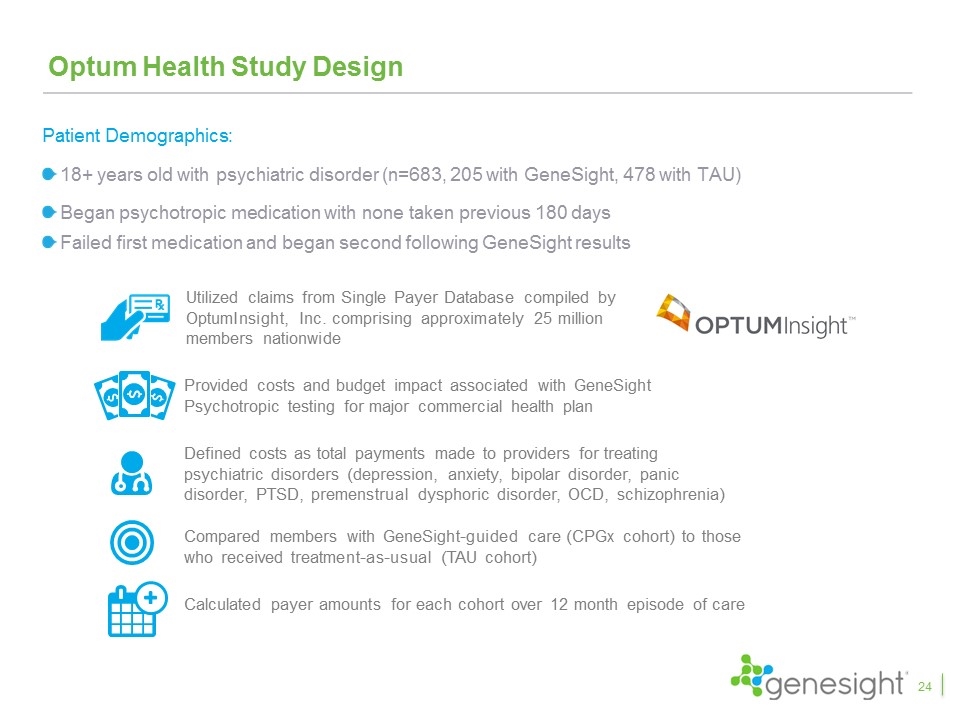
Optum Health Study Design Provided costs and budget impact associated with GeneSight Psychotropic testing for major commercial health plan Utilized claims from Single Payer Database compiled by OptumInsight, Inc. comprising approximately 25 million members nationwide Compared members with GeneSight-guided care (CPGx cohort) to those who received treatment-as-usual (TAU cohort) Defined costs as total payments made to providers for treating psychiatric disorders (depression, anxiety, bipolar disorder, panic disorder, PTSD, premenstrual dysphoric disorder, OCD, schizophrenia) Calculated payer amounts for each cohort over 12 month episode of care Patient Demographics: 18+ years old with psychiatric disorder (n=683, 205 with GeneSight, 478 with TAU) Began psychotropic medication with none taken previous 180 days Failed first medication and began second following GeneSight results 24
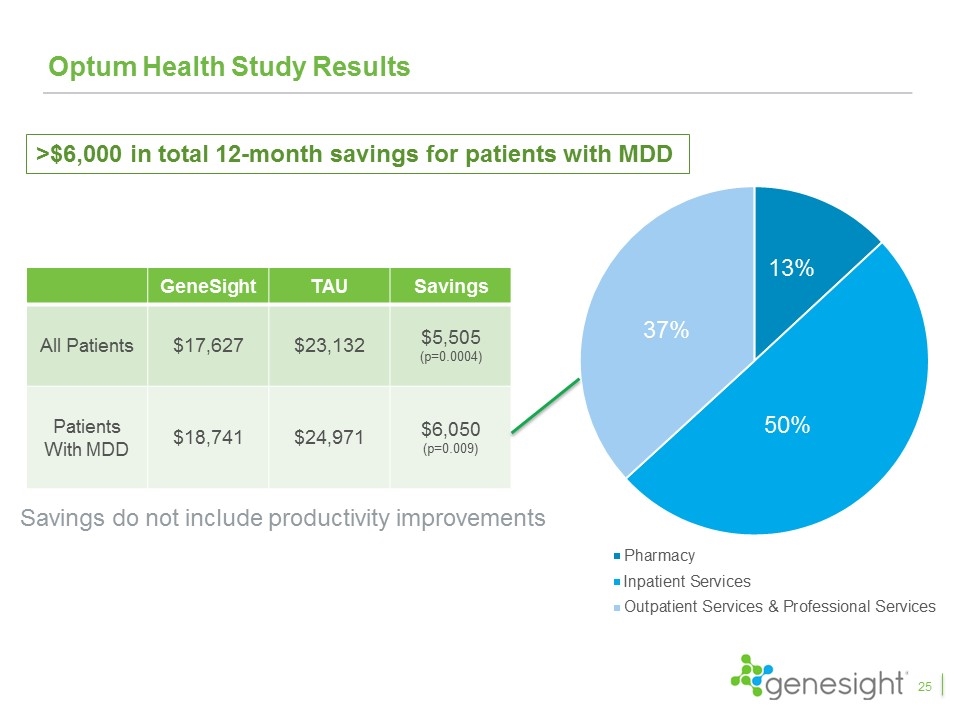
Optum Health Study Results GeneSight TAU Savings All Patients $17,627 $23,132 $5,505 (p=0.0004) Patients With MDD $18,741 $24,971 $6,050 (p=0.009) >$6,000 in total 12-month savings for patients with MDD Savings do not include productivity improvements 37% 13% 50% 25
HEDIS Scores Another Motivation For Payers With GeneSight Healthcare Effectiveness Data and Information Set (HEDIS) is a comprehensive set of standardized performance measures designed to provide purchasers and consumers with the information they need for reliable comparison of health plan performance Behavioral health is an important component of HEDIS scores and for depression the key metrics utilized are remission and response measures These metrics could be used in the future to determine Star Ratings for health insurance plans Medicare Advantage plans can receive additional reimbursement if they have high Star Ratings. Plans with consistent low ratings are discontinued from Medicare Advantage 26

Conclusion & Next Steps 27

Key Takeaways From Clinical Studies The GUIDED study is the fifth favorable clinical study and the first blinded, prospective study GeneSight led to a 50% increase in remission rates, 30% increase in response rates, and 11% improvement in symptoms with remission and response achieving statistical significance Excluding patients entering on “green medications”, GeneSight led to a 70% increase in remission, a 42% increase in response, and a 23% improvement in symptoms, all of which were statistically significant The results continued to improve over the 24 week study period with remission rates increasing to 31%, response rates increasing to 44%, and symptom improvement reaching 43% Patients switching from red medications compared to those that did not saw 153% higher remission rates, 71% higher response rates, and 59% improvement in symptoms and all were highly statistically significant The IMPACT study showed primary care physicians had results even better than psychiatrists when using GeneSight Multiple health economic studies demonstrated significant health care savings 28

Next Steps 29 Begin the tech assessment process with major national payers and request out-of-cycle reviews where appropriate File formal reconsideration request with Medicare to expand LCD to primary care physicians Continue to publish numerous additional GUIDED studies with key opinion leaders Pursue professional guidelines and position papers supporting GeneSight Develop primary care launch plan and direct to consumer initiative to be implemented after expanded reimbursement
