Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PRECIGEN, INC. | d682685d8k.htm |

Precigen Business Update Helen Sabzevari, PhD President, Precigen 26 December 2018 Exhibit 99.1

Forward-looking statements

Today’s business updates Introduction to Precigen’s near-term R&D strategies Precigen’s transformation to an agile R&D engine Agreement overviews for Ziopharm and Merck KGaA Introduction to Precigen’s transformative platform technologies, including UltraCAR-TTM An overview of the opportunity in immunotherapies Overview of PRGN-3006 UltraCAR-T and AML/MDS
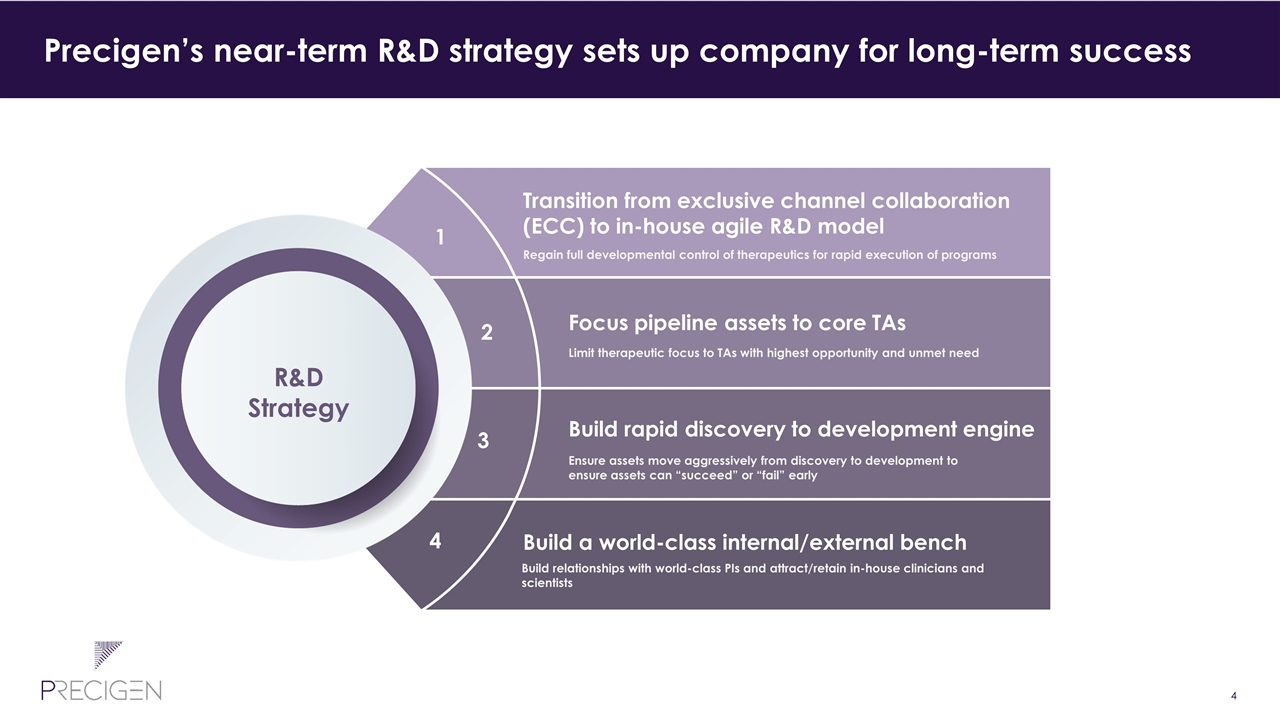
Transition from exclusive channel collaboration (ECC) to in-house agile R&D model Regain full developmental control of therapeutics for rapid execution of programs Focus pipeline assets to core TAs Limit therapeutic focus to TAs with highest opportunity and unmet need Build rapid discovery to development engine Ensure assets move aggressively from discovery to development to ensure assets can “succeed” or “fail” early Build a world-class internal/external bench Build relationships with world-class PIs and attract/retain in-house clinicians and scientists 1 2 3 4 Precigen’s near-term R&D strategy sets up company for long-term success R&D Strategy

Ziopharm license agreement returns rights to pursue CAR targets Agreement Overview Executed October 9, 2018 Replaced existing agreements with Ziopharm Outcome: Precigen has exclusive rights to pursue all CAR targets and Sleeping Beauty system in all tumor types (hematological and solid) subject to: Ziopharm rights are limited to CD19 CAR-T and the right to negotiate for a second undisclosed CAR-T target. Sleeping Beauty licensing to Ziopharm is limited to these two CAR-T cells and use in T-cell Receptor (TCR) therapy Precigen retains rights for the Sleeping Beauty system and membrane bound interleukin-15 (mbIL15) Precigen will receive milestone payments and commercial royalties for Ziopharm’s use of Precigen’s technology Please refer to the 8-K filing on October 12, 2018 for additional details regarding the agreement with Ziopharm

Merck KGaA 2018 agreement assigns exclusive CAR-T development rights to Intrexon, providing Precigen with complete autonomy of its UltraCAR-TTM platform Agreement Overview Expected to close this year Assigns and transfers all Merck KGaA CAR-T rights to Intrexon Outcome: Assigns Merck KGaA’s exclusive CAR-T development rights to Intrexon Establishes Precigen's complete autonomy for its UltraCAR-T platform Merck KGaA will receive $150 million in Intrexon stock and a $25 million convertible note Intrexon to receive $25 million cash investment Merck may receive a 10 percent royalty restricted to the two previous Merck KGaA targets, but will not receive a royalty on other CAR targets Please refer to the 8-K filing on December 20, 2018 for additional details regarding the agreement with Merck KGaA, Darmstadt, Germany
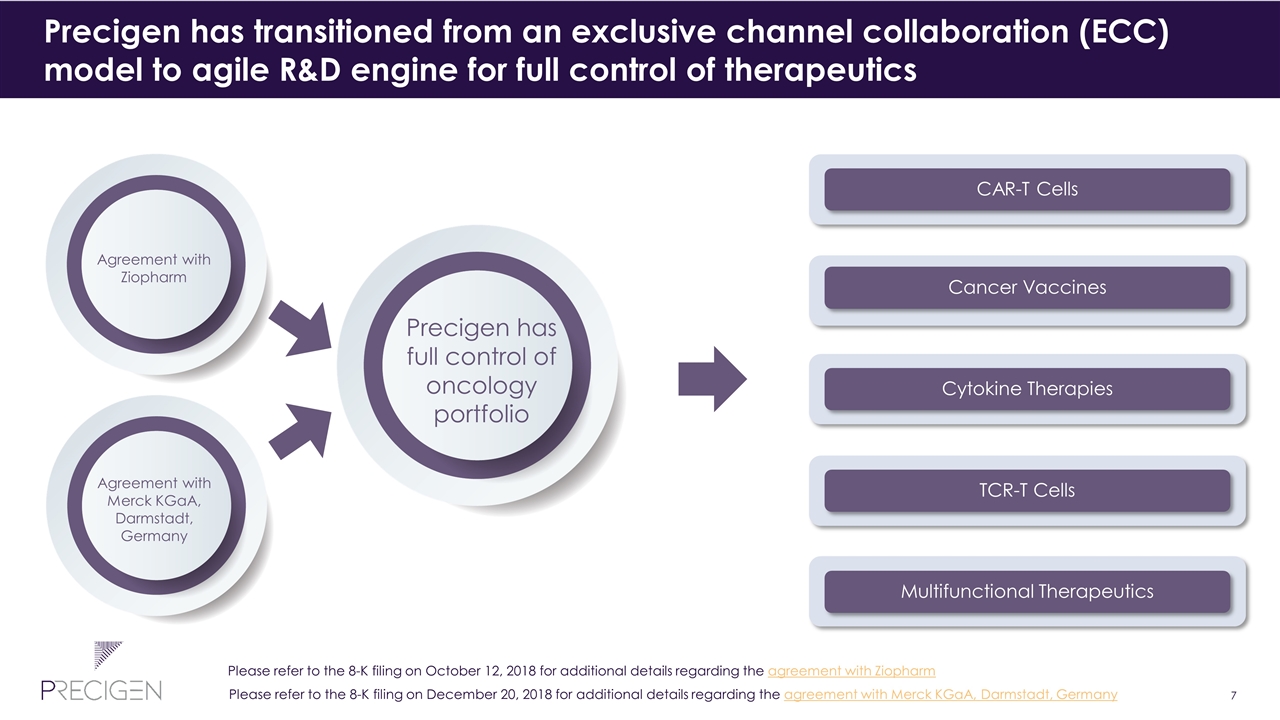
Precigen has transitioned from an exclusive channel collaboration (ECC) model to agile R&D engine for full control of therapeutics Precigen has full control of oncology portfolio Agreement with Ziopharm CAR-T Cells Cancer Vaccines Cytokine Therapies TCR-T Cells Multifunctional Therapeutics Agreement with Merck KGaA, Darmstadt, Germany Please refer to the 8-K filing on December 20, 2018 for additional details regarding the agreement with Merck KGaA, Darmstadt, Germany Please refer to the 8-K filing on October 12, 2018 for additional details regarding the agreement with Ziopharm
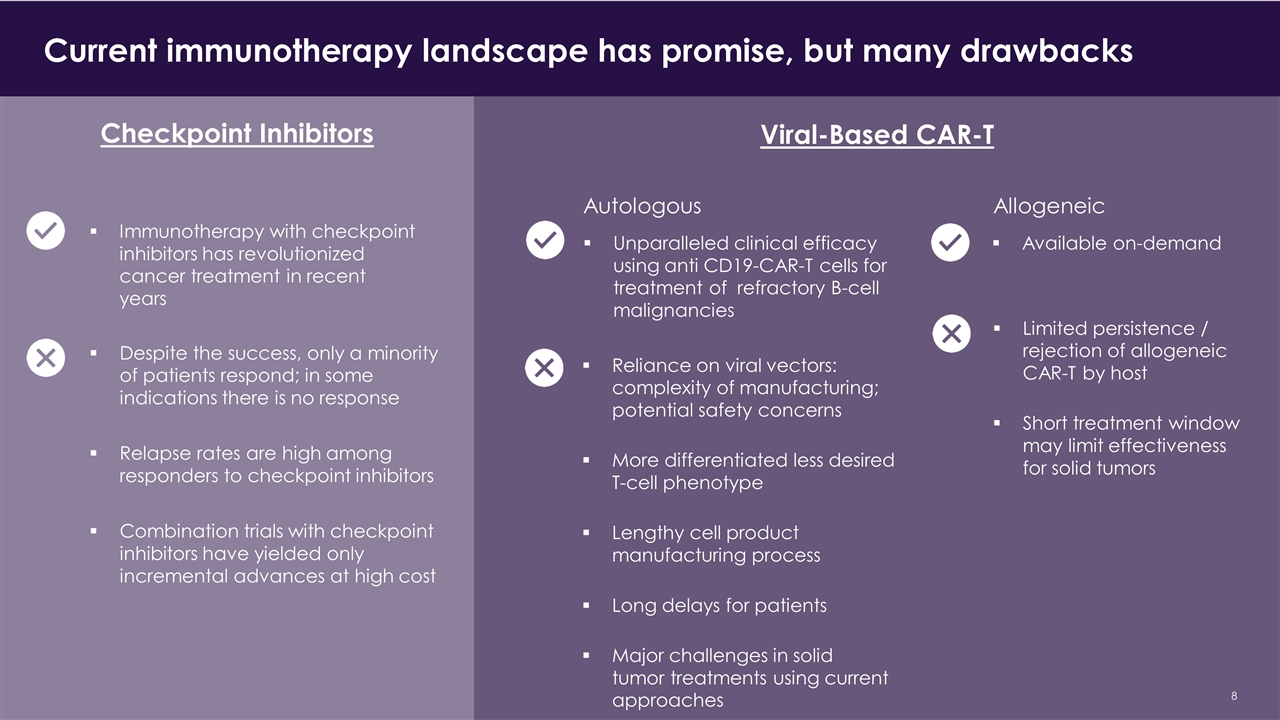
Current immunotherapy landscape has promise, but many drawbacks CONFIDENTIAL Despite the success, only a minority of patients respond; in some indications there is no response Relapse rates are high among responders to checkpoint inhibitors Combination trials with checkpoint inhibitors have yielded only incremental advances at high cost Checkpoint Inhibitors Immunotherapy with checkpoint inhibitors has revolutionized cancer treatment in recent years Viral-Based CAR-T Autologous Unparalleled clinical efficacy using anti CD19-CAR-T cells for treatment of refractory B-cell malignancies Reliance on viral vectors: complexity of manufacturing; potential safety concerns More differentiated less desired T-cell phenotype Lengthy cell product manufacturing process Long delays for patients Major challenges in solid tumor treatments using current approaches Allogeneic Available on-demand Limited persistence / rejection of allogeneic CAR-T by host Short treatment window may limit effectiveness for solid tumors
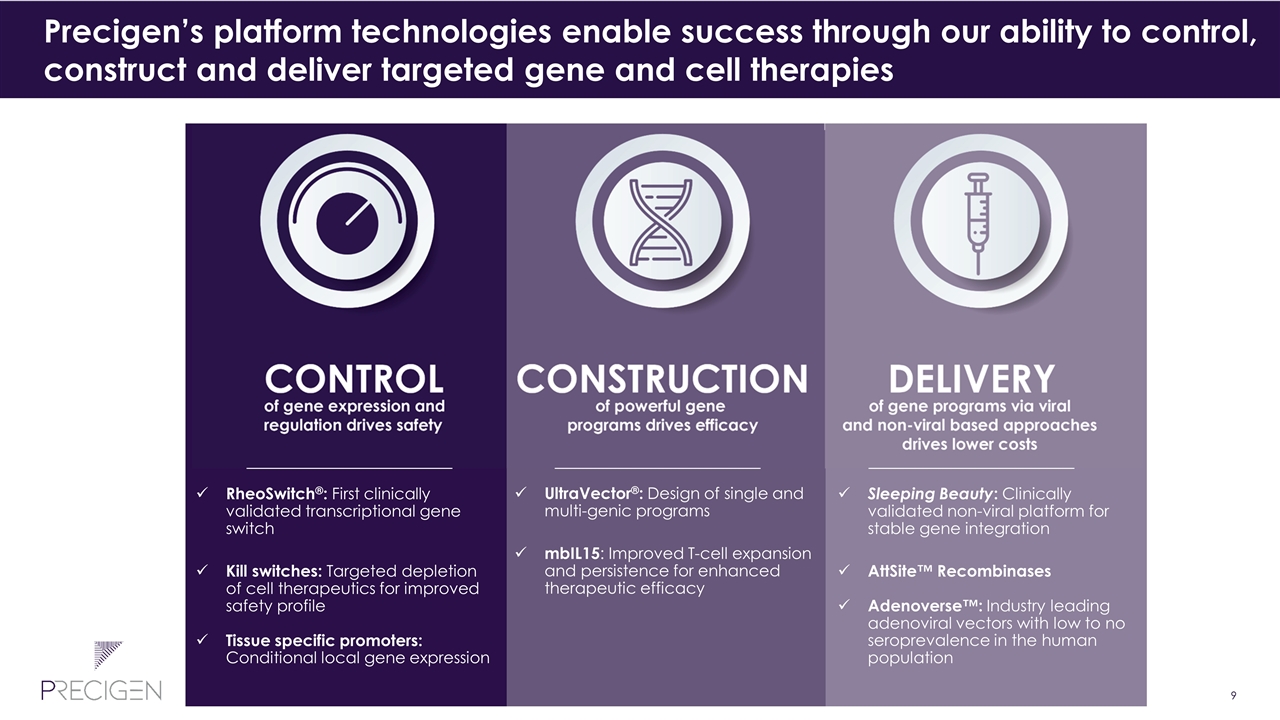
Precigen’s platform technologies enable success through our ability to control, construct and deliver targeted gene and cell therapies RheoSwitch®: First clinically validated transcriptional gene switch Kill switches: Targeted depletion of cell therapeutics for improved safety profile Tissue specific promoters: Conditional local gene expression UltraVector®: Design of single and multi-genic programs mbIL15: Improved T-cell expansion and persistence for enhanced therapeutic efficacy Sleeping Beauty: Clinically validated non-viral platform for stable gene integration AttSite™ Recombinases Adenoverse™: Industry leading adenoviral vectors with low to no seroprevalence in the human population
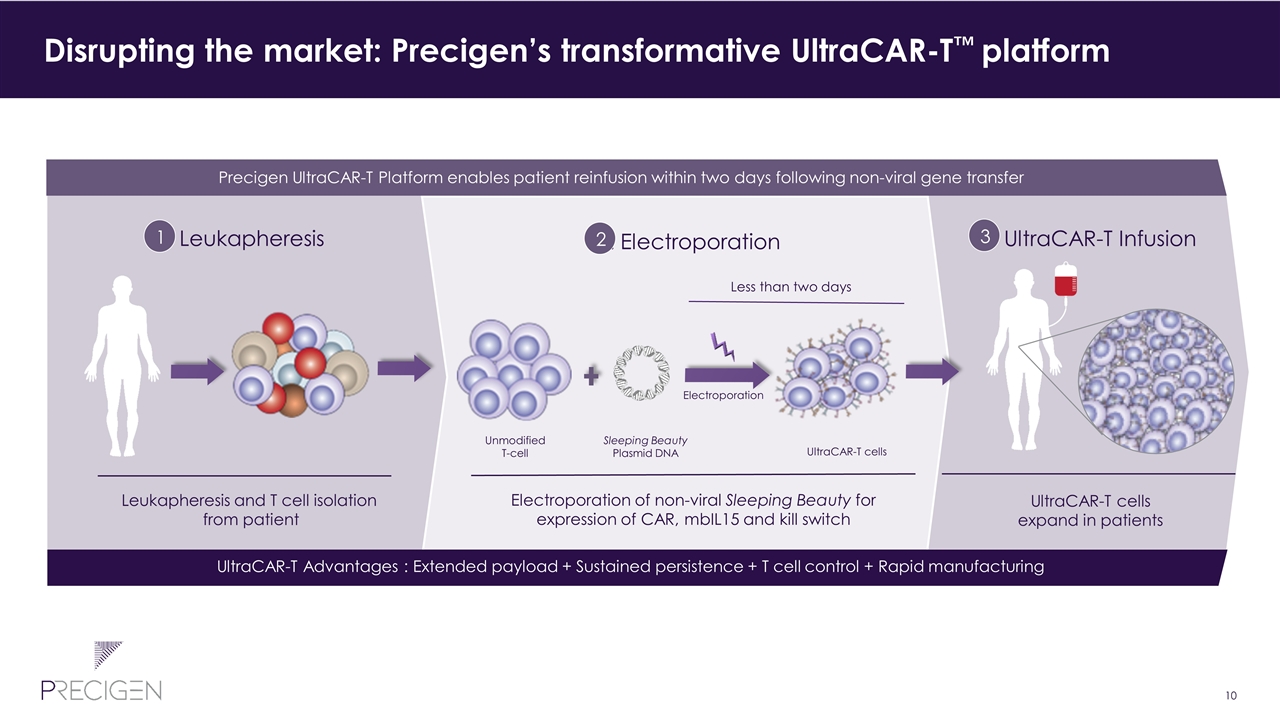
2. Electroporation 1. Leukapheresis 3. UltraCAR-T Infusion Disrupting the market: Precigen’s transformative UltraCAR-T™ platform Less than two days UltraCAR-T advancements Precigen UltraCAR-T Platform enables patient reinfusion within two days following non-viral gene transfer Leukapheresis and T cell isolation from patient UltraCAR-T cells expand in patients UltraCAR-T cells Electroporation of non-viral Sleeping Beauty for expression of CAR, mbIL15 and kill switch Electroporation Unmodified T-cell Sleeping Beauty Plasmid DNA 1 2 3 UltraCAR-T advancements UltraCAR-T Advantages : Extended payload + Sustained persistence + T cell control + Rapid manufacturing
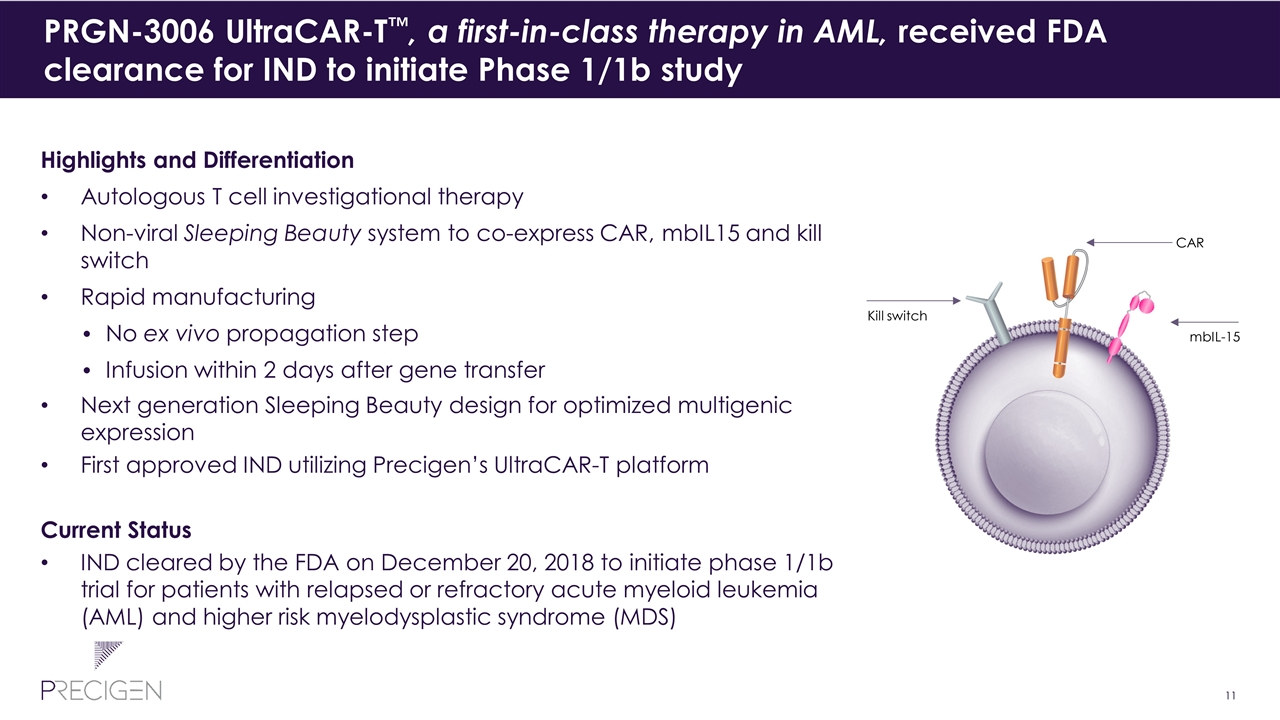
PRGN-3006 UltraCAR-T™, a first-in-class therapy in AML, received FDA clearance for IND to initiate Phase 1/1b study Highlights and Differentiation Autologous T cell investigational therapy Non-viral Sleeping Beauty system to co-express CAR, mbIL15 and kill switch Rapid manufacturing No ex vivo propagation step Infusion within 2 days after gene transfer Next generation Sleeping Beauty design for optimized multigenic expression First approved IND utilizing Precigen’s UltraCAR-T platform Current Status IND cleared by the FDA on December 20, 2018 to initiate phase 1/1b trial for patients with relapsed or refractory acute myeloid leukemia (AML) and higher risk myelodysplastic syndrome (MDS) mbIL-15 CAR Kill switch
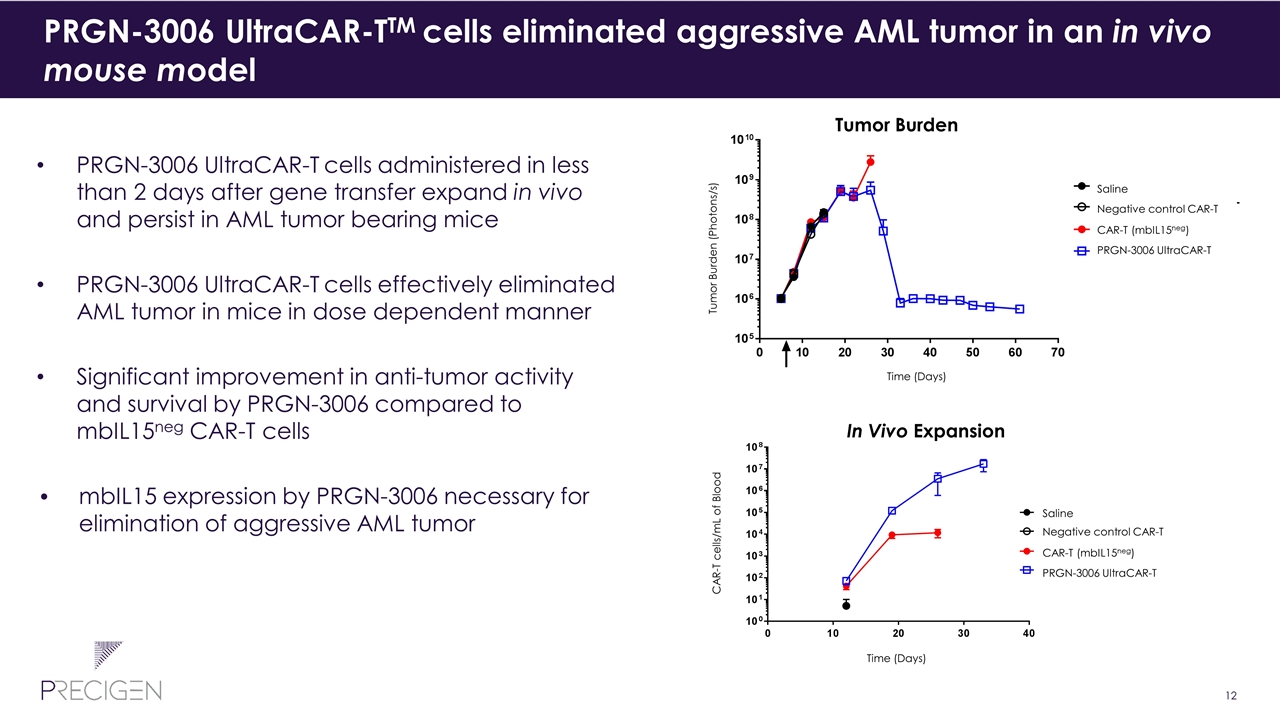
PRGN-3006 UltraCAR-TTM cells eliminated aggressive AML tumor in an in vivo mouse model PRGN-3006 UltraCAR-T cells administered in less than 2 days after gene transfer expand in vivo and persist in AML tumor bearing mice PRGN-3006 UltraCAR-T cells effectively eliminated AML tumor in mice in dose dependent manner Significant improvement in anti-tumor activity and survival by PRGN-3006 compared to mbIL15neg CAR-T cells mbIL15 expression by PRGN-3006 necessary for elimination of aggressive AML tumor Tumor Burden In Vivo Expansion Time (Days) Time (Days) CAR-T cells/mL of Blood Saline Negative control CAR-T CAR-T (mbIL15neg) PRGN-3006 UltraCAR-T Saline Negative control CAR-T CAR-T (mbIL15neg) PRGN-3006 UltraCAR-T Tumor Burden (Photons/s)
PRGN-3006 UltraCAR-TTM targets AML and MDS; patient populations with significant unmet need Acute Myeloid Leukemia (AML) Patient Characteristics and Incidence ~20,000 new cases in US in 2018, mostly in adults1 Among most common types of leukemia in adults1 Uncommon before age 451 Average age of diagnosis is about 681 Survival Poor prognosis with an average 5‐year survival rate of ~25 percent overall2 Less than 5 percent 5‐year survival rate for patients older than 652 Elderly patients median survival ranges from: 3.5 months for patients 65 to 74 years2 1.4 months for patients ≥ 85 years2 Myelodysplastic Syndrome (MDS) Patient Characteristics and Incidence Diseases of bone marrow generally found in adults in their 70s3 Incidence in the US is not known for sure; estimates range from 10,000 each year and higher3 Survival IPSS-R median survival varies: < one year for the "very high" IPSS-R risk group3 > eight years for the "very low" IPSS-R group3 1American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML). Accessed December 2018 via ACS website. 2Thein, M., et al., Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer, 2013. 119(15): p.2720-7. 3American Cancer Society. Key Statistics for Myelodysplastic Syndromes. Accessed December 2018 via ACS website.
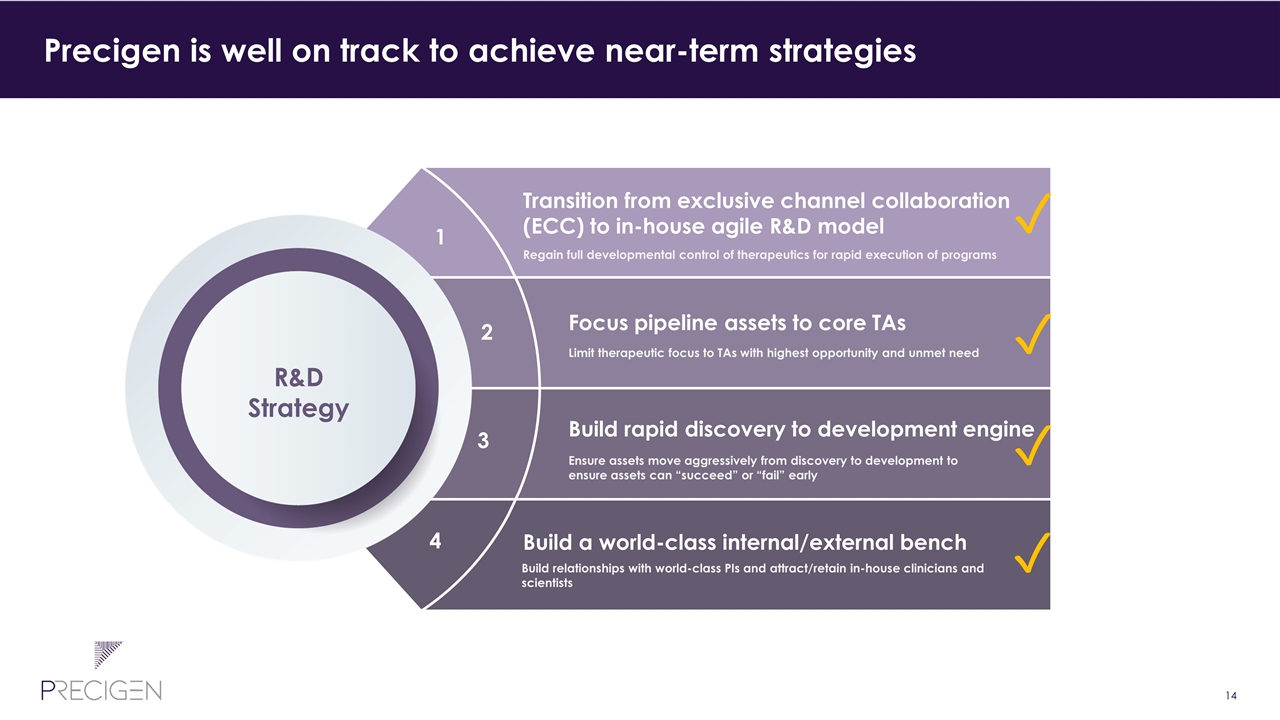
Transition from exclusive channel collaboration (ECC) to in-house agile R&D model Regain full developmental control of therapeutics for rapid execution of programs Focus pipeline assets to core TAs Limit therapeutic focus to TAs with highest opportunity and unmet need Build rapid discovery to development engine Ensure assets move aggressively from discovery to development to ensure assets can “succeed” or “fail” early Build a world-class internal/external bench Build relationships with world-class PIs and attract/retain in-house clinicians and scientists 1 2 3 4 Precigen is well on track to achieve near-term strategies R&D Strategy ✓ ✓ ✓ ✓

