Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Biohaven Pharmaceutical Holding Co Ltd. | a18-41019_1ex99d1.htm |
| 8-K - 8-K - Biohaven Pharmaceutical Holding Co Ltd. | a18-41019_18k.htm |
|
|
December 03, 2018 Advancing Innovative Therapies for Neurological Diseases Phase 3 Topline Rimegepant 75 mg Zydis ODT |
|
|
Disclaimer This presentation contains forward-looking statements within the meaning of “safe harbor” provisions of The Private Securities Litigation Reform Act of 1995, including: statements about our plans to develop and commercialize our product candidates, the timing of our planned regulatory filings, the timing of and our ability to obtain and maintain regulatory approvals for our product candidates and the clinical potential utility of our product candidates, alone and as compared to other existing or potential treatment options. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements and from the Company's current expectations. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. The forward-looking statements in this presentation represent our views as of the date of this presentation. Subsequent events and developments may cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no obligation to do so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. For further information regarding these risks, uncertainties and other factors, you should read the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the ”SEC”) on November 14, 2018 and the Company’s other periodic reports filed with the SEC. References to www.biohavenpharma.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on such website into this presentation by reference. DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES 2 |
|
|
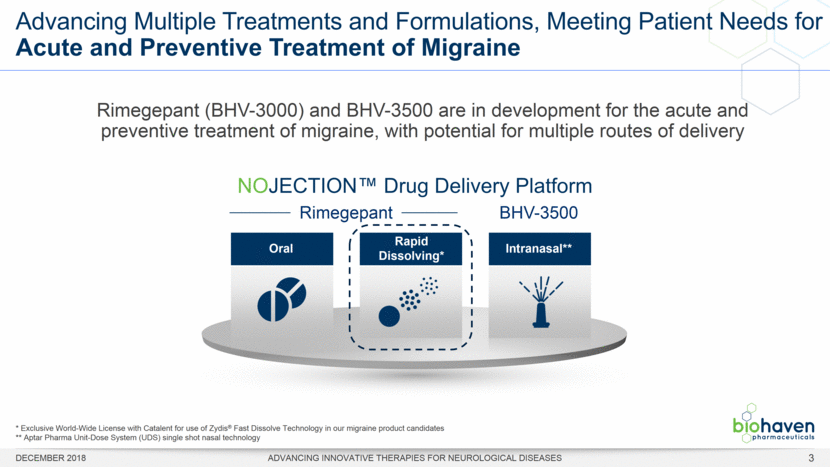
Advancing Multiple Treatments and Formulations, Meeting Patient Needs for Acute and Preventive Treatment of Migraine DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES * Exclusive World-Wide License with Catalent for use of Zydis® Fast Dissolve Technology in our migraine product candidates ** Aptar Pharma Unit-Dose System (UDS) single shot nasal technology Rimegepant (BHV-3000) and BHV-3500 are in development for the acute and preventive treatment of migraine, with potential for multiple routes of delivery NOJECTION™ Drug Delivery Platform BHV-3500 Rimegepant Oral Rapid Dissolving* Intranasal** 3 |
|
|
Rimegepant 75 mg Zydis ODT Phase 3 Topline Summary from Study 303 in the Acute Treatment of Migraine |
|
|
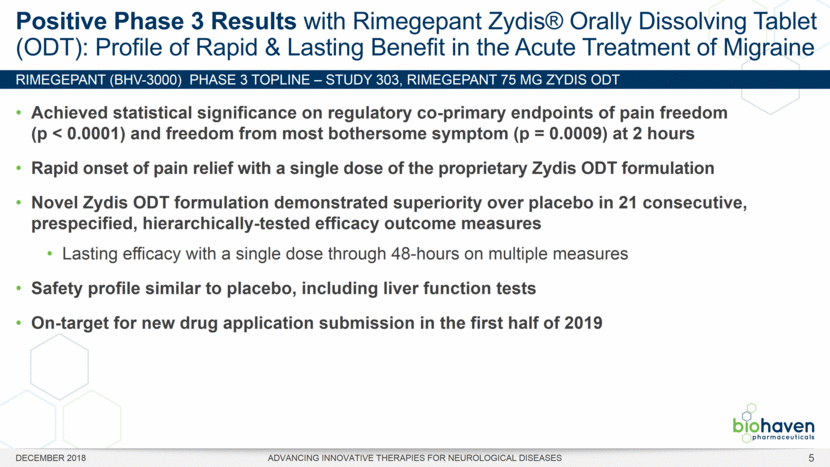
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT Positive Phase 3 Results with Rimegepant Zydis® Orally Dissolving Tablet (ODT): Profile of Rapid & Lasting Benefit in the Acute Treatment of Migraine DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES Achieved statistical significance on regulatory co-primary endpoints of pain freedom (p < 0.0001) and freedom from most bothersome symptom (p = 0.0009) at 2 hours Rapid onset of pain relief with a single dose of the proprietary Zydis ODT formulation Novel Zydis ODT formulation demonstrated superiority over placebo in 21 consecutive, prespecified, hierarchically-tested efficacy outcome measures Lasting efficacy with a single dose through 48-hours on multiple measures Safety profile similar to placebo, including liver function tests On-target for new drug application submission in the first half of 2019 5 |
|
|
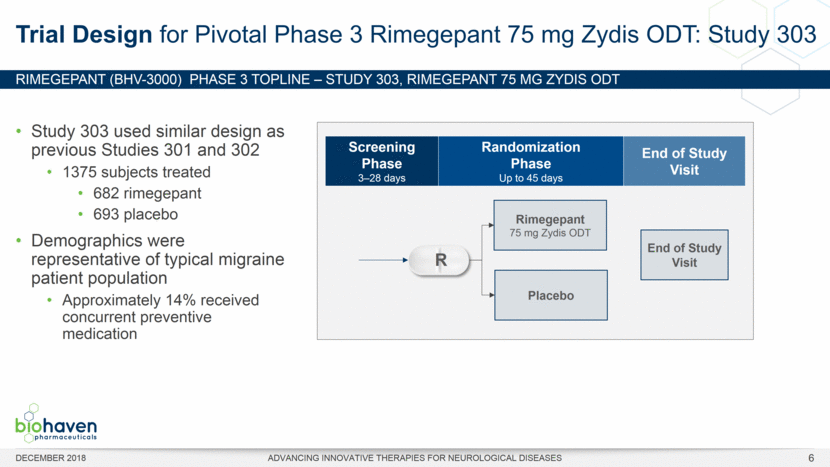
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES Study 303 used similar design as previous Studies 301 and 302 1375 subjects treated 682 rimegepant 693 placebo Demographics were representative of typical migraine patient population Approximately 14% received concurrent preventive medication Trial Design for Pivotal Phase 3 Rimegepant 75 mg Zydis ODT: Study 303 Rimegepant 75 mg Zydis ODT Placebo Screening Phase 3–28 days Randomization Phase Up to 45 days R End of Study Visit End of Study Visit 6 |
|
|
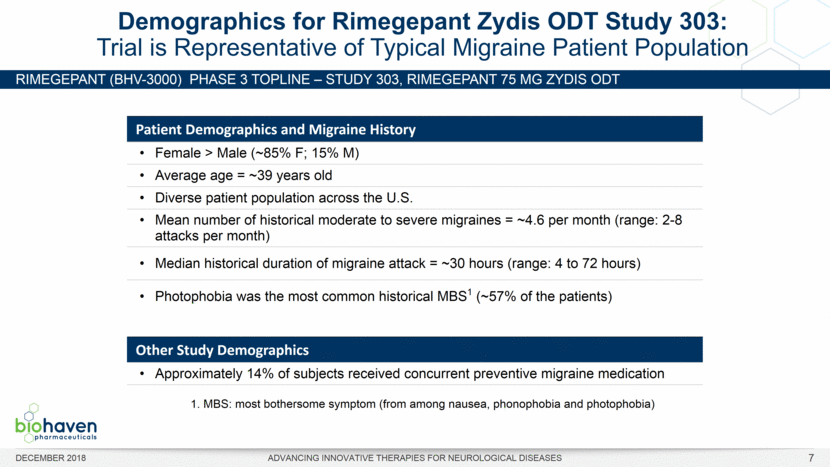
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT Demographics for Rimegepant Zydis ODT Study 303: Trial is Representative of Typical Migraine Patient Population Patient Demographics and Migraine History Female > Male (~85% F; 15% M) Average age = ~39 years old Diverse patient population across the U.S. Mean number of historical moderate to severe migraines = ~4.6 per month (range: 2-8 attacks per month) Median historical duration of migraine attack = ~30 hours (range: 4 to 72 hours) Photophobia was the most common historical MBS1 (~57% of the patients) Other Study Demographics Approximately 14% of subjects received concurrent preventive migraine medication 1. MBS: most bothersome symptom (from among nausea, phonophobia and photophobia) DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES 7 |
|
|
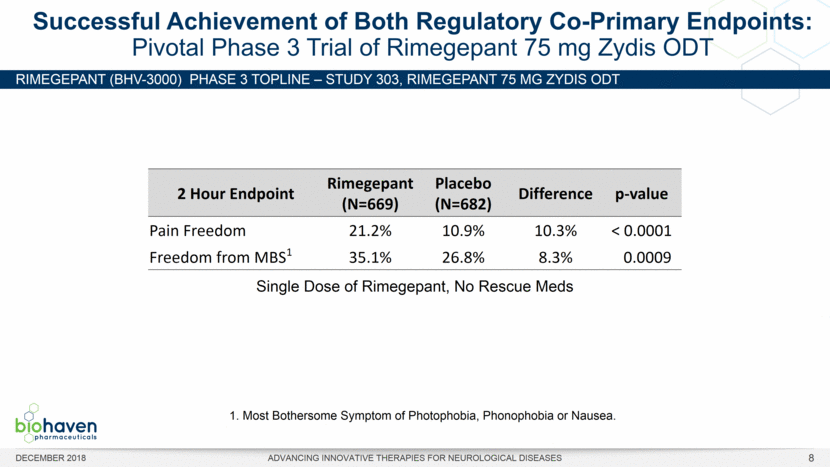
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT Successful Achievement of Both Regulatory Co-Primary Endpoints: Pivotal Phase 3 Trial of Rimegepant 75 mg Zydis ODT 1. Most Bothersome Symptom of Photophobia, Phonophobia or Nausea. DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES 8 2 Hour Endpoint Rimegepant (N=669) Placebo (N=682) Difference p-value Pain Freedom 21.2% 10.9% 10.3% < 0.0001 Freedom from MBS1 35.1% 26.8% 8.3% 0.0009 Single Dose of Rimegepant, No Rescue Meds |
|
|
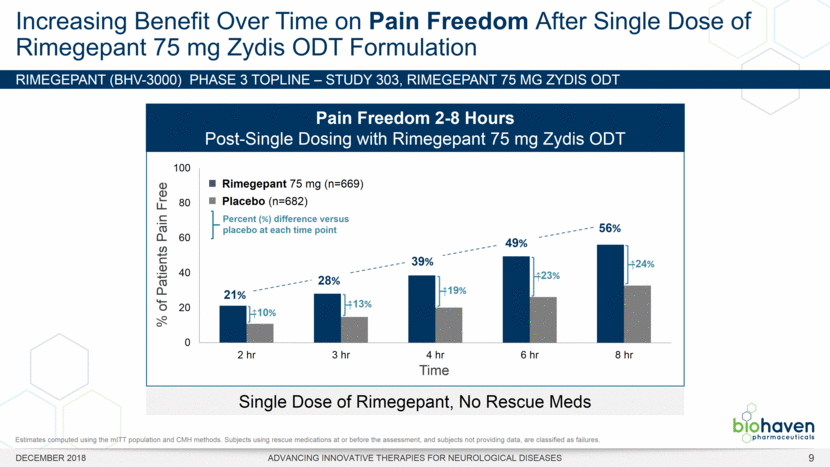
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT Increasing Benefit Over Time on Pain Freedom After Single Dose of Rimegepant 75 mg Zydis ODT Formulation DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES Single Dose of Rimegepant, No Rescue Meds % of Patients Pain Free 10% 13% 19% 23% 24% Pain Freedom 2-8 Hours Post-Single Dosing with Rimegepant 75 mg Zydis ODT Time Rimegepant 75 mg (n=669) Placebo (n=682) Percent (%) difference versus placebo at each time point 21% 56% 28% 39% 49% Estimates computed using the mITT population and CMH methods. Subjects using rescue medications at or before the assessment, and subjects not providing data, are classified as failures. 9 |
|
|
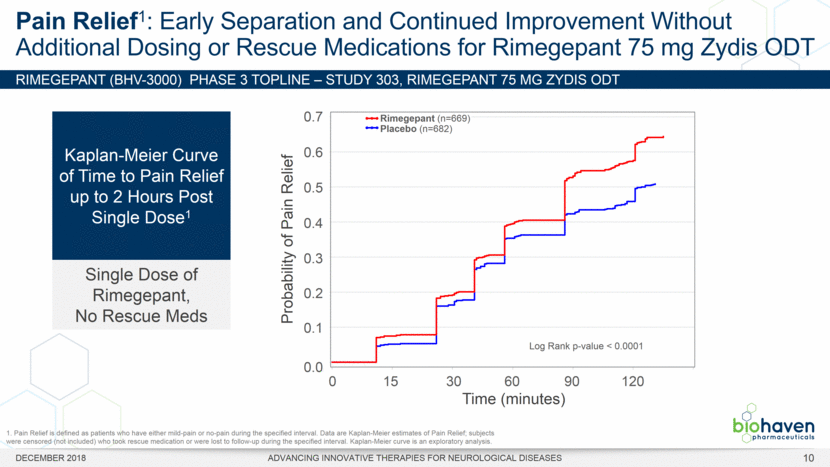
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT Pain Relief1: Early Separation and Continued Improvement Without Additional Dosing or Rescue Medications for Rimegepant 75 mg Zydis ODT Kaplan-Meier Curve of Time to Pain Relief up to 2 Hours Post Single Dose1 Single Dose of Rimegepant, No Rescue Meds DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES Probability of Pain Relief 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 0 30 60 90 120 Time (minutes) 15 Rimegepant (n=669) Placebo (n=682) Log Rank p-value < 0.0001 1. Pain Relief is defined as patients who have either mild-pain or no-pain during the specified interval. Data are Kaplan-Meier estimates of Pain Relief; subjects were censored (not included) who took rescue medication or were lost to follow-up during the specified interval. Kaplan-Meier curve is an exploratory analysis. 10 |
|
|
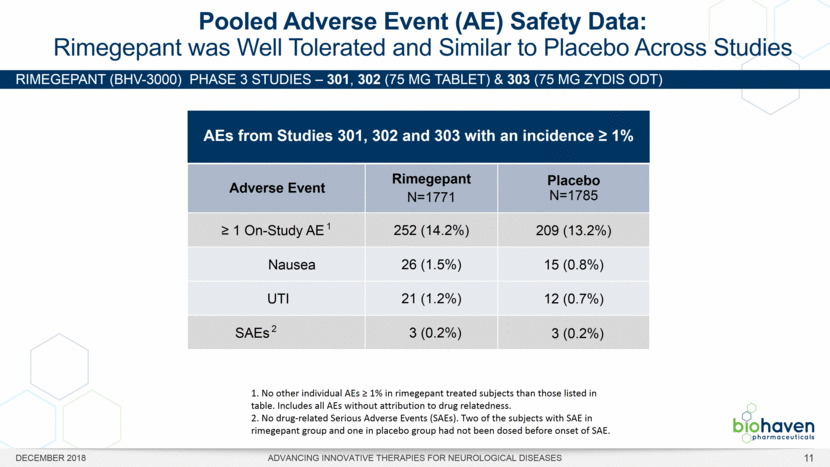
RIMEGEPANT (BHV-3000) Phase 3 studies – 301, 302 (75 mg Tablet) & 303 (75 mg Zydis ODT) Pooled Adverse Event (AE) Safety Data: Rimegepant was Well Tolerated and Similar to Placebo Across Studies AEs from Studies 301, 302 and 303 with an incidence > 1% Adverse Event Rimegepant N=1771 Placebo N=1785 > 1 On-Study AE 1 252 (14.2%) 209 (13.2%) Nausea 26 (1.5%) 15 (0.8%) UTI 21 (1.2%) 12 (0.7%) SAEs 2 3 (0.2%) 3 (0.2%) 1. No other individual AEs > 1% in rimegepant treated subjects than those listed in table. Includes all AEs without attribution to drug relatedness. 2. No drug-related Serious Adverse Events (SAEs). Two of the subjects with SAE in rimegepant group and one in placebo group had not been dosed before onset of SAE. DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES 11 |
|
|
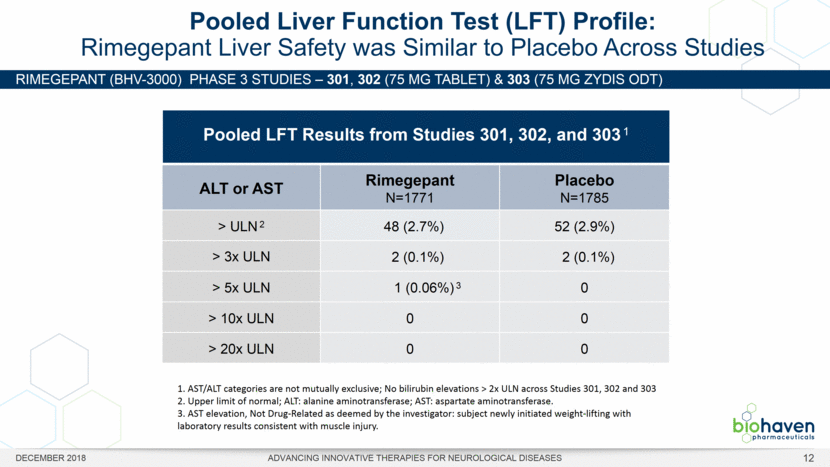
RIMEGEPANT (BHV-3000) Phase 3 studies – 301, 302 (75 mg Tablet) & 303 (75 mg Zydis ODT) Pooled Liver Function Test (LFT) Profile: Rimegepant Liver Safety was Similar to Placebo Across Studies ALT or AST Rimegepant N=1771 Placebo N=1785 > ULN 2 48 (2.7%) 52 (2.9%) > 3x ULN 2 (0.1%) 2 (0.1%) > 5x ULN 1 (0.06%) 3 0 > 10x ULN 0 0 > 20x ULN 0 0 Pooled LFT Results from Studies 301, 302, and 303 1 1. AST/ALT categories are not mutually exclusive; No bilirubin elevations > 2x ULN across Studies 301, 302 and 303 2. Upper limit of normal; ALT: alanine aminotransferase; AST: aspartate aminotransferase. 3. AST elevation, Not Drug-Related as deemed by the investigator: subject newly initiated weight-lifting with laboratory results consistent with muscle injury. DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES 12 |
|
|
RIMEGEPANT (BHV-3000) Phase 3 TOPLINE – Study 303, rimegepant 75 mg ZYDIS ODT Summary: Positive Phase 3 Results with Rimegepant Zydis® ODT – Profile of Rapid and Lasting Benefit in the Acute Treatment of Migraine DECEMBER 2018 ADVANCING INNOVATIVE THERAPIES FOR NEUROLOGICAL DISEASES Rapid onset of pain relief with single dose Numerical separation from placebo as early as 15 minutes and statistically significant by 60 minutes Significantly greater percentage of patients returned to normal functioning by 60 minutes (p = 0.0025) Achieved statistical significance on regulatory co-primary endpoints of pain freedom (p < 0.0001) and freedom from most bothersome symptom (p = 0.0009) at 2 hours Superiority over placebo in 21 consecutive, prespecified, hierarchically-tested efficacy outcome measures, including: Clinical benefit observed through 48 hours on freedom from pain (p < 0.0001), pain relief (p < 0.0001), freedom from most bothersome symptom (p = 0.0018), and freedom from functional disability (p < 0.0001) Safety profile similar to placebo, including liver function tests On-target for new drug application submission in the first half of 2019 13 |
|
|
Rimegepant (BHV-3000) 75 mg Zydis ODT Phase 3 Topline Small Molecule CGRP Receptor Antagonist Q&A |
|
|
Thank You! www.biohavenpharma.com |