Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Biohaven Pharmaceutical Holding Co Ltd. | a18-41019_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Biohaven Pharmaceutical Holding Co Ltd. | a18-41019_1ex99d1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): December 3, 2018
Biohaven Pharmaceutical Holding Company Ltd.
(Exact name of registrant as specified in its charter)
|
British Virgin Islands |
|
001-38080 |
|
Not applicable |
|
(State or other jurisdiction of |
|
(Commission File Number) |
|
(IRS Employer |
c/o Biohaven Pharmaceuticals, Inc.
215 Church Street
New Haven, Connecticut 06510
(Address of principal executive offices, including zip code)
(203) 404-0410
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01 Regulation FD Disclosure.
On December 3, 2018, Biohaven Pharmaceutical Holding Company Ltd. (the “Company”) issued a press release announcing data from the Company’s randomized, controlled Phase 3 clinical trial (BHV3000-303 or Study 303) evaluating the efficacy and safety of its Zydis® orally dissolving tablet (ODT) formulation of rimegepant, an oral calcitonin gene-related peptide (CGRP) receptor antagonist, for the acute treatment of migraine. A copy of the press release is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K.
In addition, on December 3, 2018, members of management of the Company held a conference call to discuss the results of the trial. A copy of the presentation that accompanied the conference call is available on the Company’s website at www.biohavenpharma.com, and is furnished herewith as Exhibit 99.2 to this Current Report on Form 8-K.
The information contained in this Item 7.01, including Exhibits 99.1 and 99.2, are being “furnished” and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liability of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”). The information in this Item 7.01, including Exhibits 99.1 and 99.2, shall not be incorporated by reference into any registration statement or other document pursuant to the Securities Act or into any filing or other document pursuant to the Exchange Act, except as otherwise expressly stated in any such filing.
Item 8.01 Other Events.
On December 3, 2018, the Company announced positive topline data from a randomized, controlled Phase 3 clinical trial (BHV3000-303 or Study 303) evaluating the efficacy and safety of its Zydis® orally dissolving tablet (ODT) formulation of rimegepant, an oral calcitonin gene-related peptide (CGRP) receptor antagonist, for the acute treatment of migraine.
In Study 303, rimegepant Zydis ODT statistically differentiated from placebo on the two co-primary endpoints as well as the first 21 consecutive primary and secondary outcome measures that were pre-specified in hierarchical testing. Consistent with the two previous Phase 3 clinical trials, Study 303 met its co-primary registrational endpoints of pain freedom and freedom from most bothersome symptom (MBS) at 2 hours using a single dose (Table 1). Importantly, patients treated with the rimegepant Zydis ODT formulation began to numerically separate from placebo on pain relief as early as 15 minutes, and this difference was statistically significant at 60 minutes (p < 0.0001) (Figure 1). Additionally, a significantly greater percentage of patients treated with rimegepant Zydis ODT returned to normal functioning by 60 minutes as compared to placebo (p < 0.002). Lasting clinical benefit was observed through 48 hours after a single dose of rimegepant on freedom from pain (p < 0.001), pain relief (p < 0.001), freedom from the most bothersome symptom (p < 0.001), and freedom from functional disability (p < 0.003). Superiority over placebo was also demonstrated in multiple other secondary endpoints. The vast majority of rimegepant Zydis ODT treated patients (85%) did not use any rescue medications.
Table 1: Met Co-Primary Endpoints of Pain Freedom & Freedom from Most Bothersome Symptom
|
2 Hour Endpoint |
|
Rimegepant |
|
Placebo |
|
Difference |
|
p-value |
|
|
Pain Freedom |
|
21.2 |
% |
10.9 |
% |
10.3 |
% |
< 0.0001 |
|
|
Freedom from MBS(1) |
|
35.1 |
% |
26.8 |
% |
8.3 |
% |
0.0009 |
|
|
(1) Most Bothersome Symptom of Photophobia, Phonophobia or Nausea |
Figure 1: Kaplan-Meier Pain Relief Curve Through 2 Hours after Single Dose of Rimegepant 75 Mg Zydis ODT
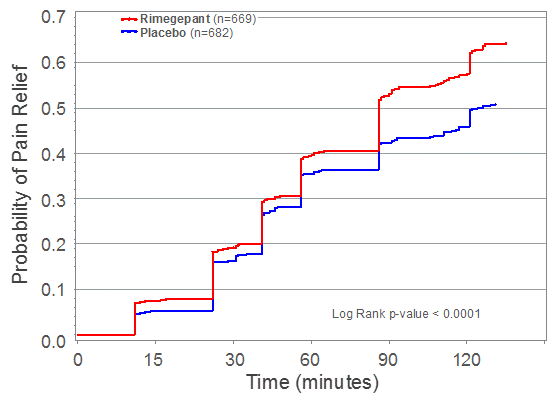
Figure 1 shows the percentage of patients reporting pain relief between 0 and 2 hours after dosing for patients who took a single dose of rimegepant Zydis ODT 75 mg or placebo. Data are Kaplan-Meier estimates of the time to first report of pain relief (report of no pain, or mild pain). Subjects were censored who took rescue medication or were lost to follow-up.
The safety and tolerability observations of rimegepant in Study 303 were consistent with the profile previously observed in Studies 301 and 302. Table 2 shows the pooled safety data across all three trials. In study 303, no single adverse event (AE) occurred in the rimegepant group with an incidence higher than 1.6% and overall rates of AEs were similar to placebo. With regard to liver function tests, one patient treated with placebo and one patient treated with rimegepant showed LFTs > 3x ULN in Study 303. Pooled liver function test results across the three pivotal trials (n=3,556) performed to date showed that rimegepant was similar to placebo with regard to aminotransferase (ALT or AST) levels above the upper limit of normal (ULN) and no patients experienced elevations in bilirubin > 2x ULN (Table 3).
Table 2: Pooled Adverse Event (AE) Safety Data: Rimegepant was Similar to Placebo Across Studies
AEs from Studies 301, 302 and 303 with an incidence > 1%
|
Adverse Event |
|
Rimegepant |
|
Placebo |
|
|
> 1 On-Study AE(1) |
|
252 (14.2%) |
|
209 (13.2%) |
|
|
Nausea |
|
26 (1.5%) |
|
15 (0.8%) |
|
|
UTI |
|
21 (1.2%) |
|
12 (0.7%) |
|
|
SAEs(2) |
|
3 (0.2%) |
|
3 (0.2%) |
|
|
(1) No other individual AEs > 1% in rimegepant treated subjects than those listed in table. Includes all AEs without attribution to drug relatedness. |
|
(2) No drug-related Serious Adverse Events (SAEs). Two of the subjects with SAE in rimegepant group and one in placebo group had not been dosed before onset of SAE. |
Table 3: Pooled Liver Function Test (LFT) Profile: Rimegepant was Similar to Placebo Across Studies
Pooled LFT Results from Studies 301, 302, and 303*
|
ALT or AST |
|
Rimegepant |
|
Placebo |
|
|
> ULN(1) |
|
48 (2.7%) |
|
52 (2.9%) |
|
|
> 3x ULN |
|
2 (0.1%) |
|
2 (0.1%) |
|
|
> 5x ULN |
|
1 (0.06%) (2) |
|
0 |
|
|
> 10x ULN |
|
0 |
|
0 |
|
|
> 20x ULN |
|
0 |
|
0 |
|
|
(1)Upper limit of normal; ALT alanine aminotransferase; AST aspartate aminotransferase |
|
(2)AST elevation, Not Drug-Related as deemed by the investigator: subject newly initiated weight-lifting with laboratory results consistent with muscle injury |
|
*AST/ALT Categories are not mutually exclusive; No bilirubin elevations > 2x ULN across Studies 301, 302 and 303 |
Additional secondary and exploratory outcome measures from this study are anticipated to be presented at upcoming scientific meetings in 2019.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit |
|
Exhibit Description |
|
99.1 |
|
|
|
99.2 |
|
Biohaven Presentation titled “Phase 3 Topline Rimegepant 75 mg Zydis ODT” |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
Biohaven Pharmaceutical Holding Company Ltd. | |
|
|
| |
|
|
|
|
|
|
By: |
/s/ Vlad Coric, M.D. |
|
Date: December 3, 2018 |
|
Vlad Coric, M.D. |
|
|
|
Chief Executive Officer |
