Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - NOVELION THERAPEUTICS INC. | a18-39805_1ex99d1.htm |
| EX-10.3 - EX-10.3 - NOVELION THERAPEUTICS INC. | a18-39805_1ex10d3.htm |
| EX-10.2 - EX-10.2 - NOVELION THERAPEUTICS INC. | a18-39805_1ex10d2.htm |
| EX-10.1 - EX-10.1 - NOVELION THERAPEUTICS INC. | a18-39805_1ex10d1.htm |
| 8-K - 8-K - NOVELION THERAPEUTICS INC. | a18-39805_18k.htm |
Business Update November 2018 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved.

Special Cautionary Statements about this Presentation This presentation includes statements about Novelion’s and Aegerion’s future plans and opportunities. Readers are cautioned that Novelion’s and Aegerion’s ability to pursue and execute on such plans and opportunities is subject to, among other things (including the risks discussed below), Aegerion’s ability to successfully refinance its approximately $36.8 million secured loan from Novelion, its approximately $302.5 million principal amount of 2.0% convertible senior notes due August 15, 2019, and the new $72.5 million facility described herein, which is scheduled to mature on February 15, 2019 but which may be extended, at Aegerion’s option, to June 30, 2019 if certain conditions are satisfied. Effecting such a refinancing, or other wholesale recapitalization or other strategic alternative (which is highly speculative) will be critical for Novelion and Aegerion to continue to execute on the commercial strategy, goals and objectives described in this presentation. Novelion is the parent company of Aegerion, our operating subsidiary. References to “we,” “our” and the “Company” refer to the entire enterprise, whose assets and operations reside at Aegerion, and whose interests may not always be aligned with those of Novelion or its shareholders. The non-GAAP results in this presentation, including, without limitation, blended cash gross margins, are provided as a complement to results provided in accordance with GAAP because management believes, when considered together with the GAAP information, these non-GAAP financial measures help indicate underlying trends in the Company's business, are important in comparing current results with prior period results and provide additional information regarding the Company’s financial performance. Management also uses these non-GAAP financial measures to establish budgets and operational goals that are communicated internally and externally, and to manage the Company's business and evaluate its performance. The non-GAAP financial measures have no standardized meaning under GAAP and therefore may not be comparable to similar measures presented by other companies. The non-GAAP financial measures are not intended to be considered in isolation or as a substitute for, or superior to, the financial measures prepared and presented in accordance with GAAP and should be reviewed in conjunction with the relevant GAAP financial measures. Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. 1

Forward Looking Statements Certain statements in this presentation constitute “forward-looking statements” of Novelion and Aegerion and “forward-looking information” within the meaning of U.S. federal securities laws and applicable Canadian securities laws, including statements regarding the terms of the new financing and expectations regarding use of such funds; expectations regarding Novelion’s and Aegerion’s review of financial and strategic alternatives (which could include a potential debt for equity swap); beliefs about our operational improvement and cost-reduction initiatives, and that Novelion or Aegerion will be able to execute on our commercial strategy and goals; forecasted sales; beliefs about product positioning, including for sales growth and potential pipeline expansion; beliefs about progress of Juxtapid in Japan and growth potential, including significant and rapid international growth potential; beliefs about payor coverage and reimbursement strength for Juxtapid; beliefs that Myalept sales are growing and the potential for a large opportunity in Latin America; expectations for potential new indications for Myalept, such as HMD, and indications of a founder effect and higher prevalence of GL in the Middle East; beliefs about the reimbursement landscape for Myalept, including impact on sales, plans to launch in Germany in November 2018 and expectations for EU published prices; expectations for the growth opportunity for Myalept in Europe; plans to pursue the PL indication in the United States and expectations as to the growth opportunity; expectations, including timing, regarding approval of broader label in Latin America and Canada; beliefs about the efficacy, and level of tolerance, of Myalept and Juxtapid; beliefs about pipeline opportunities for metreleptin; beliefs about product sales, blended cash gross margins, cash flow and EBITDA, including the timing for achieving positive cash flow and EBITDA; commercial plans for Myalept and Juxtapid, including market expansion, U.S. and ex-U.S. regulatory filings and milestones, commercial launch preparations overseas, pricing and reimbursement negotiations and patient conversion initiatives; plans for research and development initiatives for lomitapide and metreleptin, including development plans and beliefs for FCS and HeFH for lomitapide, as well as plans and beliefs for HMD and the clinical utility of metreleptin across a wide range of indications associated with HMD; beliefs about data, including that Aegerion has compelling data to support a potential U.S. label expansion for Myalept, and development plans based lomitapide FCS data; beliefs about the market and market opportunities for Myalept and Juxtapid; beliefs that Juxtapid sales have stabilized; and beliefs about the FCS market are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others, Novelion’s and Aegerion’s ability to meet immediate operational needs and obligations, as well as long-term obligations; the possibility that the restrictions in and other terms of the new loan facility and the related documents could have a negative impact on Novelion’s business and its shareholders (whose interests may not be aligned with those of Aegerion’s holders of Convertible Notes and other lenders); whether Novelion and/or Aegerion will be able to undertake a wholesale recapitalization, which is likely to include a debt for equity swap, and Novelion and/ or Aegerion may be forced to use the protections of the bankruptcy code to effectuate such recapitalization or other alternative; Novelion’s and Aegerion’s ability to identify, pursue and consummate any financial or strategic alternatives; Novelion’s ability to maintain its listing status on Nasdaq; Novelion’s and Aegerion’s ability to continue as a going concern; the risks inherent in the development and commercialization of pharmaceutical products, as well as those identified in Novelion’s filings with the U.S. Securities and Exchange Commission (the “Commission”), including under the heading “Risk Factors” in Novelion’s Annual Report on Form 10-K filed on March 16, 2018 and subsequent filings with the Commission (including Novelion’s upcoming Quarterly Report on Form 10-Q and Novelion’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018), available on the Commission’s website at www.sec.gov. Any such risks and uncertainties could materially and adversely affect our results of operations, cash flows, and our ability to maintain our operations, any of which would have a significant and adverse impact on our stock price. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. Except as required by law, we undertake no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. This presentation also contains “forward-looking information” that constitutes “financial outlooks” within the meaning of applicable Canadian securities laws. This information is provided to give investors general guidance on management’s current expectations of certain factors affecting our business, including our financial results. Given the uncertainties, assumptions and risk factors associated with this type of information, including those described above, investors are cautioned that the information may not be an appropriate subject of reliance for other purposes. Investors and others should note that we communicate with our investors and the public using our company website, www.novelion.com, including, but not limited to, company disclosures, investor presentations and FAQs, SEC filings, press releases, public conference call transcripts and webcast transcripts. The information that we post on this website could be deemed to be material information. As a result, we encourage investors, the media and others interested to review the information that we post there on a regular basis. The contents of our website shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104)

Novelion Therapeutics Inc. (Novelion) is the publicly listed (NASDAQ: NVLN) parent of Aegerion Pharmaceuticals, Inc. (Aegerion). Aegerion is a rare disease pharmaceutical company which develops and commercializes prescription drugs to treat rare metabolic diseases with significant unmet need Aegerion’s main operations are in Cambridge, Massachusetts Approximately 125 employees as of October 2018 Recently implemented significant operational improvements, including a 36% reduction in global workforce and other significant cost reduction efforts, intended to result in sustainability and facilitate the achievement of the Company’s cash flow positive goal Two clinically differentiated on-market products commercialized by Aegerion Juxtapid MTP inhibitor for the treatment for patients with homozygous familial hypercholesterolemia (HoFH) Myalept Leptin hormone replacement therapy, approved in the U.S. for Generalized Lipodystrophy (GL), and in Europe recently for GL & Partial Lipodystrophy (PL) on July 31st, 2018 with EU data and label to be referenced for potential GL and PL approvals in LatAm and Canada Plan to pursue an indication for PL in the U.S.; submission to be made to FDA in near-term Operations in the U.S., Canada, Latin America, Europe and Japan Company at a Glance Company Overview Commercial Products Geographical Coverage 3 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved.
Two on-market, high-margin rare disease products positioned for sales growth and potential pipeline expansion - Approved in U.S., Canada, Colombia, Argentina, Japan, EU Stabilized sales, despite continued competitive pressure from PCSK9s Majority of HoFH patients lack functional LDL receptor, and thus are unlikely to adequately respond to LDL receptor-targeted PCSK9s and could benefit from Juxtapid treatment Launch in Japan progressing well and is expected to provide medium to long-term growth Filed for approval in Brazil in August 2018 Plan to revisit the possibility of developing lomitapide, which carries orphan drug designation in U.S. and EU, for the potential treatment of Familial Chylomicronemia Syndrome (FCS) given recent developments in FCS market FCS prevalence estimated to be 1:1,000,000 to 2:1,000,000 Discussion with FDA in 2012 supports a potential filing based on single Phase 3 placebo-controlled study; plan to re-engage with FDA to align on path forward Also revisiting plans to evaluate the development of lomitapide for severe heterozygous FH Growth Potential DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 4 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved.
- FDA and EMA Approved Only agent that treats the consequences of low leptin, the underlying cause of lipodystrophies GL and PL received European Commission (EC) approval on July 31, 2018; approved for GL in U.S., plan to pursue an indication for PL in the U.S.; submission to be made to FDA in near-term Plan to submit EU dossier for approval in Brazil in H1 2019 Pipeline / Other Leptin Analogs Leptin has the potential to mediate multiple disease states A number of additional potential indications have been identified as possible development candidates Growth Potential DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 5 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved.
Pipeline Overview 6 Discovery Preclinical Phase I Phase II Phase III U.S. PL CLD HMD* NASH* HA* Metreleptin Commentary Lomitapide: Plan to evaluate the development of lomitapide for the treatment of Familial Chylomicronemia Syndrome (FCS) and severe Heterozygous Familial Hypercholesterolemia (HeFH) Metreleptin: In addition to the U.S. PL opportunity, metreleptin has the potential to address multiple disease states based on the treatment of leptin deficiency; we may be required to conduct a placebo-controlled trial prior to filing for approval of PL in the U.S. Next Generation Leptin Opportunities
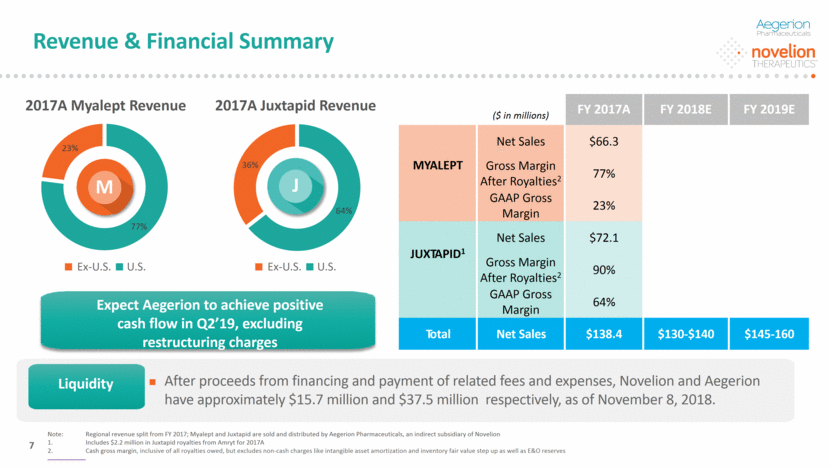
($ in millions) FY 2017A FY 2018E FY 2019E MYALEPT Net Sales $66.3 Gross Margin After Royalties2 77% GAAP Gross Margin 23% JUXTAPID1 Net Sales $72.1 Gross Margin After Royalties2 90% GAAP Gross Margin 64% Total Net Sales $138.4 $130-$140 $145-160 After proceeds from financing and payment of related fees and expenses, Novelion and Aegerion have approximately $15.7 million and $37.5 million respectively, as of November 8, 2018. 2017A Myalept Revenue 2017A Juxtapid Revenue M J Liquidity Revenue & Financial Summary Note: Regional revenue split from FY 2017; Myalept and Juxtapid are sold and distributed by Aegerion Pharmaceuticals, an indirect subsidiary of Novelion Includes $2.2 million in Juxtapid royalties from Amryt for 2017A Cash gross margin, inclusive of all royalties owed, but excludes non-cash charges like intangible asset amortization and inventory fair value step up as well as E&O reserves 7 Ex-U.S. U.S. Ex-U.S. U.S. Expect Aegerion to achieve positive cash flow in Q2’19, excluding restructuring charges Liquidity 64% 36% 77% 23%
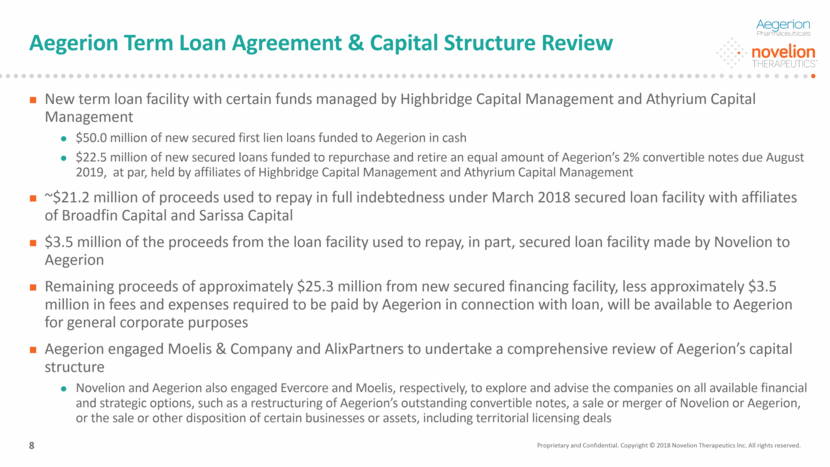
Aegerion Term Loan Agreement & Capital Structure Review New term loan facility with certain funds managed by Highbridge Capital Management and Athyrium Capital Management $50.0 million of new secured first lien loans funded to Aegerion in cash $22.5 million of new secured loans funded to repurchase and retire an equal amount of Aegerion’s 2% convertible notes due August 2019, at par, held by affiliates of Highbridge Capital Management and Athyrium Capital Management ~$21.2 million of proceeds used to repay in full indebtedness under March 2018 secured loan facility with affiliates of Broadfin Capital and Sarissa Capital $3.5 million of the proceeds from the loan facility used to repay, in part, secured loan facility made by Novelion to Aegerion Remaining proceeds of approximately $25.3 million from new secured financing facility, less approximately $3.5 million in fees and expenses required to be paid by Aegerion in connection with loan, will be available to Aegerion for general corporate purposes Aegerion engaged Moelis & Company and AlixPartners to undertake a comprehensive review of Aegerion’s capital structure Novelion and Aegerion also engaged Evercore and Moelis, respectively, to explore and advise the companies on all available financial and strategic options, such as a restructuring of Aegerion’s outstanding convertible notes, a sale or merger of Novelion or Aegerion, or the sale or other disposition of certain businesses or assets, including territorial licensing deals Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. 8
Pro Forma Aegerion Debt Outstanding $50.0 million first lien term loan owed to affiliates of Highbridge Capital Management and Athyrium Capital Management, maturing February 15, 2019 subject to extension to June 30, 2019 if certain conditions are satisfied $36.8 million loan owed to Novelion, maturing July 1, 2019 $22.5 million loan owed to affiliates of Highbridge Capital Management and Athyrium Capital Management, maturing February 15, 2019 subject to extension to June 30, 2019 if certain conditions are satisfied $302.5 million convertible notes issued by Aegerion, maturing August 15, 2019 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. 9 Does not include $32.8 million outstanding DoJ/SEC settlement payment on Balance Sheet as of September 30, 2018

Portfolio Overview: Juxtapid II Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 10

Homozygous Familial Hypercholesterolemia Homozygous Familial Hypercholesterolemia (HoFH) HoFH is a serious, rare genetic disease that impairs the function of the receptor responsible for removing LDL-C (“bad” cholesterol) from the blood. An impairment of low density lipoprotein receptor (“LDL-R”) function results in significant elevation of blood cholesterol levels1 Based on epidemiological data, there are expected to be anywhere from 300-2,000 patients in the U.S. It is typically inherited from both parents1 The significantly elevated LDL-C levels in patients with HoFH may lead to premature and progressive atherosclerosis2 Cuchel M et al. Eur Heart J. 2014;35(32):2146-2157. Goldberg AC et al. J Clin Lipidol. 2011;5(3 Suppl):S1-S8. Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 11
Cash generating product with stabilizing sales and ~90% gross margins(1) (63.9% GAAP gross margins) Due to lomitapide’s MOA, adult HoFH patients are being prescribed lomitapide in greater frequency after using PCSK9s due to the failure of such patients to respond adequately to PCSK9s LOWER patient registry provides 5+ years of long-term efficacy and safety data Potential for significant international growth opportunities with already identified patients and opportunity to launch in several countries DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 12 Stabilizing sales as the third-line agent of choice HoFH null receptor patients do not adequately respond to PCSK9s Opportunity to pick up mipomersen patients following withdrawal from market U.S. Source: Company filings; Management Projections (1) Before non-cash amortization/costs Juxtapid Highlights Partnership with Amryt Reimbursed in Italy, the Netherlands Recently approved for reimbursement by the NHS in the UK Making progress on reimbursement in other EU countries E.U. Rapid growth in Japan with 202 HoFH patients identified Favorable placement in JAS guidelines Approved and preparing to launch in Argentina (Q4 2018) Filed in Brazil in August 2018, expect approval in 1H:2019 Japan / Other Int’l Patent life expected through 2027 Potential new uses (FCS and severe HeFH) Other
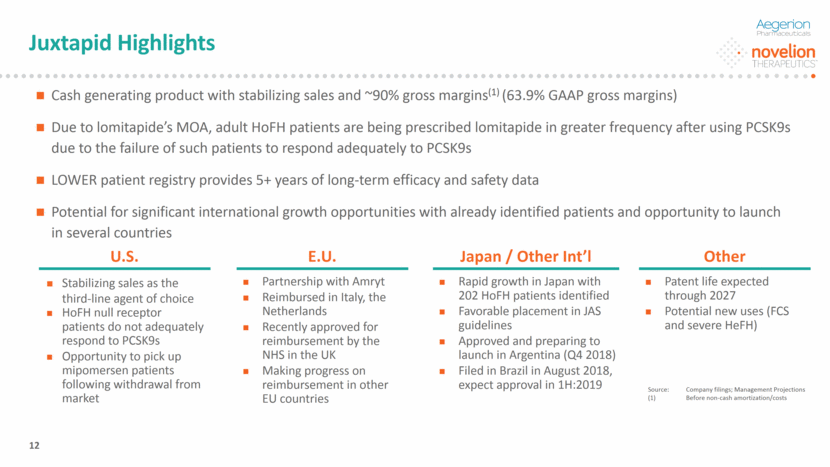
JUXTAPID®: Important Adjunct Treatment Option for Adult HoFH The effect of JUXTAPID on cardiovascular morbidity and mortality has not been established. Current company expectation for therapeutic applicability; subject to FDA approval. Newly Diagnosed 1st Line Treatment New therapies will be introduced 2nd Line Treatment 1st line failures Tolerability Issues 3rd Line Treatment Low Dose Statins, Ezetimibe, Bile Acid Sequestrants High-Dose Statins or PCSK9s JUXTAPID® (Daily Capsule) Mipomersen (Withdrawn from Market) Apheresis JUXTAPID is approved as an adjunct treatment with diet and other lipid-lowering treatments, including LDL apheresis where available, to reduce LDL-C in adult HoFH patients. Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 13
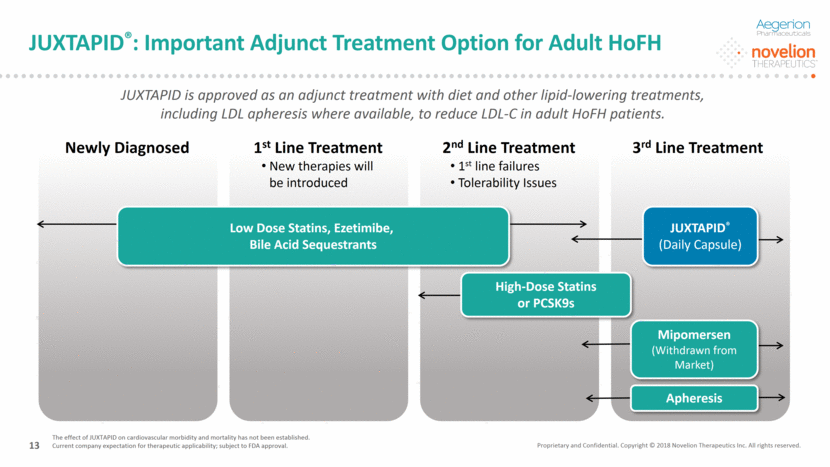
Important Safety Information CONTRAINDICATIONS Pregnancy Concomitant administration of moderate or strong CYP3A4 inhibitors Moderate or severe hepatic impairment or active liver disease including unexplained persistent elevations of serum transaminases WARNINGS AND PRECAUTIONS JUXTAPID can cause elevations in transaminases and hepatic steatosis. Although cases of hepatic failure have not been reported, there is concern that JUXTAPID could induce steatohepatitis, which can progress to cirrhosis over several years. Modify the dose of JUXTAPID if elevations of transaminases are observed and discontinue JUXTAPID for persistent or clinically significant elevations. If transaminase elevations are accompanied by clinical symptoms of liver injury, such as nausea, vomiting, abdominal pain, fever, jaundice, lethargy, flu-like-symptoms, increases in bilirubin 2x ULN, or active liver disease, discontinue treatment with JUXTAPID and identify the probable cause. Use JUXTAPID with caution when co-administered with agents known to be hepatotoxic. Alcohol may increase levels of hepatic fat and induce or exacerbate liver injury. Measure ALT, AST, alkaline phosphatase, and total bilirubin before initiating treatment. During the first year, measure liver-related tests (ALT and AST, at a minimum) prior to each increase in dose or monthly, whichever occurs first. After the first year, do these tests at least every 3 months and before any increase in dose. Females of reproductive potential should have a negative pregnancy test before starting JUXTAPID and should use effective contraception during therapy with JUXTAPID. Given its mechanism of action in the small intestine, JUXTAPID may reduce the absorption of fat-soluble nutrients. Patients treated with JUXTAPID should take daily supplements that contain 400 international units vitamin E and at least 200 mg linoleic acid, 210 mg alpha-linolenic acid (ALA), 110 mg eicosapentaenoic acid (EPA), and 80 mg docosahexaenoic acid (DHA). Gastrointestinal adverse reactions are common and may lead to treatment discontinuation. To reduce the risk of gastrointestinal adverse reactions, patients should adhere to a low-fat diet supplying less than 20% of energy from fat and the dosage of JUXTAPID should be increased gradually. Combination with CYP3A4 inhibitors increases exposure to lomitapide. Strong and moderate CYP3A4 inhibitors should not be used with JUXTAPID. JUXTAPID dosage should not exceed 30 mg daily when used concomitantly with weak CYP3A4 inhibitors. Due to risk of myopathy associated with simvastatin or lovastatin, doses of these agents should be limited when co-administered with JUXTAPID. JUXTAPID increases the plasma concentrations of warfarin. Increases or decreases in the dose of JUXTAPID may lead to supra- or subtherapeutic anticoagulation, respectively. Patients taking warfarin should undergo regular monitoring of the INR, especially after any changes in JUXTAPID dosage. Avoid use of JUXTAPID in patients with rare hereditary disorders of galactose intolerance. Juxtapid: Important Safety Information and Boxed Warning WARNING: RISK OF HEPATOTOXICITY JUXTAPID can cause elevations in transaminases. In the JUXTAPID clinical trial, 10 (34%) of the 29 patients treated with JUXTAPID had at least one elevation in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) 3x upper limit of normal (ULN). There were no concomitant clinically meaningful elevations of total bilirubin, international normalized ratio (INR), or alkaline phosphatase. JUXTAPID also increases hepatic fat, with or without concomitant increases in transaminases. The median absolute increase in hepatic fat was 6% after both 26 and 78 weeks of treatment, from 1% at baseline, measured by magnetic resonance spectroscopy. Hepatic steatosis associated with JUXTAPID treatment may be a risk factor for progressive liver disease, including steatohepatitis and cirrhosis. Measure ALT, AST, alkaline phosphatase, and total bilirubin before initiating treatment and then ALT and AST regularly as recommended. During treatment, adjust the dose of JUXTAPID if the ALT or AST are 3x ULN. Boxed Warning Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 14
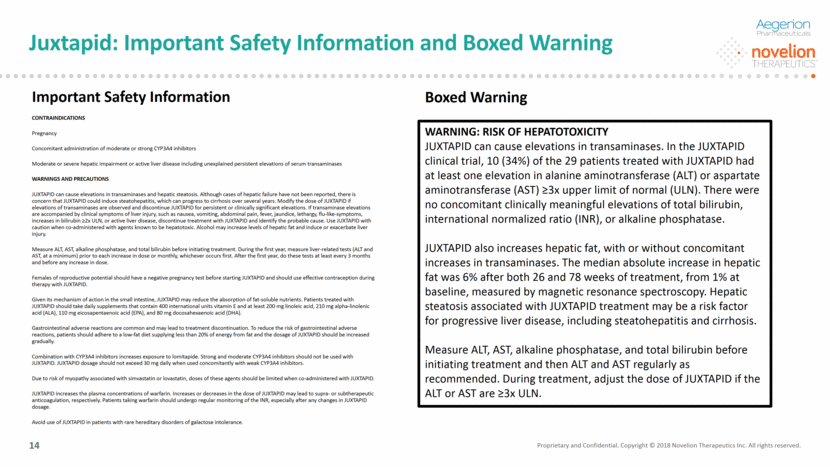
Juxtapid: Reimbursement Landscape Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 15 Strong payor coverage in the U.S. and ex-U.S. ~58% of current US paid patients covered by commercial insurers, 42% covered by government payors Largely overcome third-line step-edit reimbursement issues in the U.S. Continued progress with payors in the EU Lojuxta® recently approved for funding by NHS England (NICE) Mandated reimbursement in Japan, where the product is approved Japanese HoFH Patients registered in the Intractable Diseases List (Nanbyo) receive 100% reimbursement Strong reimbursement in LATAM, where the product is approved or in registration in the markets where we operate Payors in Colombia provide reimbursement for Lomitapide In Brazil and Argentina, the product is purchased by the local payors on a named-patient basis; plan to launch in Argentina in Q4 2018
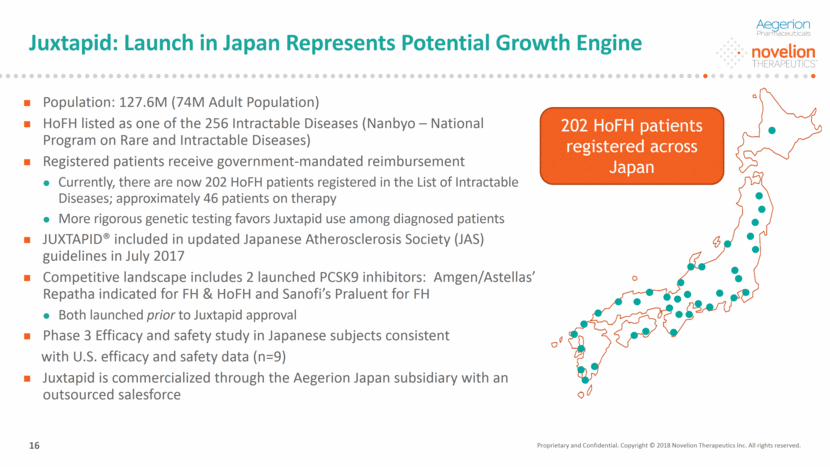
Juxtapid: Launch in Japan Represents Potential Growth Engine 202 HoFH patients registered across Japan Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 16 Population: 127.6M (74M Adult Population) HoFH listed as one of the 256 Intractable Diseases (Nanbyo – National Program on Rare and Intractable Diseases) Registered patients receive government-mandated reimbursement Currently, there are now 202 HoFH patients registered in the List of Intractable Diseases; approximately 46 patients on therapy More rigorous genetic testing favors Juxtapid use among diagnosed patients JUXTAPID® included in updated Japanese Atherosclerosis Society (JAS) guidelines in July 2017 Competitive landscape includes 2 launched PCSK9 inhibitors: Amgen/Astellas’ Repatha indicated for FH & HoFH and Sanofi’s Praluent for FH Both launched prior to Juxtapid approval Phase 3 Efficacy and safety study in Japanese subjects consistent with U.S. efficacy and safety data (n=9) Juxtapid is commercialized through the Aegerion Japan subsidiary with an outsourced salesforce
I Portfolio Overview: Myalept III Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 17
Generalized and Partial Lipodystrophy GL & PL result in abnormal or degenerative conditions of the body's adipose tissue; can be inherited or acquired (resulting in the following sub-categories: AGL, CGL, APL & CPL) Serious progressive diseases: Loss of subcutaneous fat Ectopic deposition of lipid (liver, muscle) Hyperphagia and significant fatigue Metabolic abnormalities often refractory to conventional therapies: Severe insulin resistance Diabetes mellitus Severe hypertriglyceridemia Hepatic steatosis or fatty liver Associated with life altering organ damage if uncontrolled Reduced life expectancy in severe forms GL estimated prevalence = ~ 1:1,000,000 PL estimated prevalence = ~ 3:1,000,000 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 18
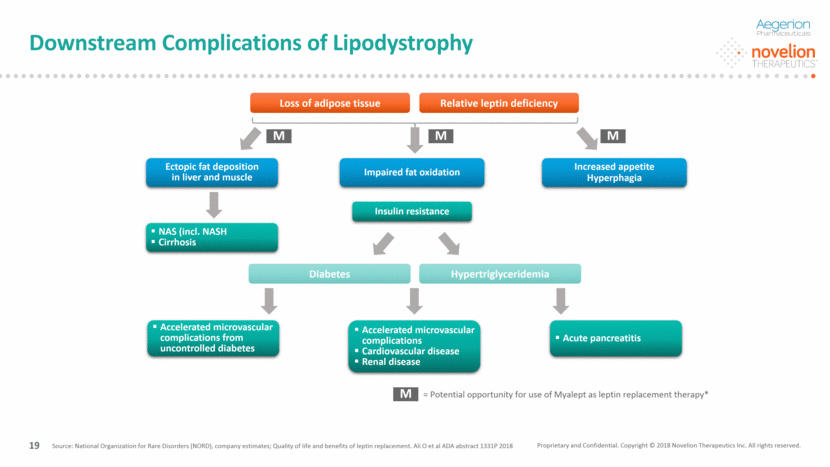
Source: National Organization for Rare Disorders (NORD), company estimates; Quality of life and benefits of leptin replacement. Ali O et al ADA abstract 1331P 2018 Loss of adipose tissue Relative leptin deficiency Ectopic fat deposition in liver and muscle Impaired fat oxidation Increased appetite Hyperphagia NAS (incl. NASH Cirrhosis Insulin resistance Accelerated microvascular complications from uncontrolled diabetes Accelerated microvascular complications Cardiovascular disease Renal disease Acute pancreatitis Diabetes Hypertriglyceridemia M M M M = Potential opportunity for use of Myalept as leptin replacement therapy* Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. Downstream Complications of Lipodystrophy DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 19
Metreleptin is the standard-of-care in the treatment of GL Only agent that treats the underlying cause of lipodystrophies that is low leptin Growing sales with ~70% gross margins(1) (~23% on a GAAP basis) Support NPS in pre-approval countries where permitted Immunogenicity plans agreed with FDA (PMR4) and EU (post approval study) – antibody response has not impacted clinical effect Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 20 Approved for GL; opportunity to greatly expand addressable population with a PL approval; plan to request meeting with FDA on PL Market exclusivity through 2026 Method of use patent expires in July 2027 U.S. Source: Company filings; Management Projections (1) Before non-cash amortization/costs; The time-based royalty rate ranges from mid-single digits to low double digits, increasing annually in years 2016 to 2019 from rates in the low single digits to low double digits, peaking in years 2019 to 2020 at a rate in the low double digits before decreasing in years 2022 through 2025 to rates in the high single digits to mid-single digits. Myalept Highlights GL & PL approval received in July 2018 ~100 patients currently treated on Expanded Access Programs, a majority of which have PL and GL Label includes treatment of patients with GL and PL; estimated ~1,000 addressable patients Market exclusivity in EU to August 2028 E.U. Large potential LATAM opportunity (200+ patients identified for GL with additional for PL) 120+ GL patients identified in Brazil alone Possible founder effect evidenced by large GL patient base EU Label will be basis for filings LATAM / Other Areas Potential new indications, such as HMD A relatively large number of GL patients in the Middle East may indicate a founder effect and support higher prevalence Other
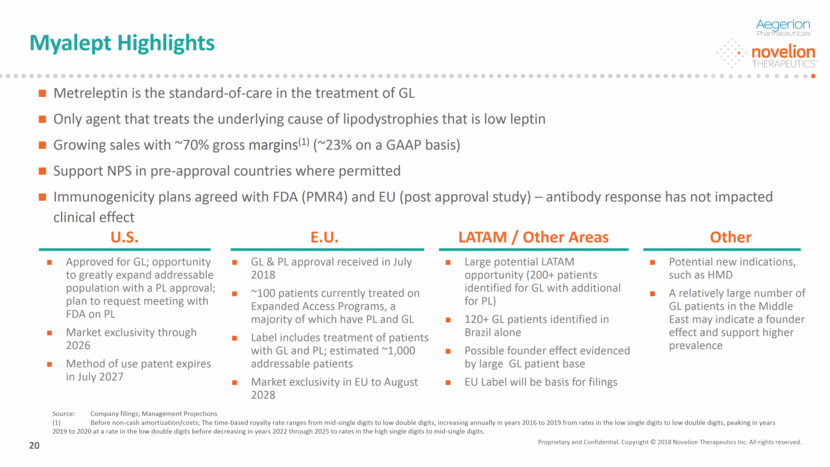
Improve: Glycemic control TG levels Liver function Diabetes mellitus Hypertriglyceridemia Fatty liver Euglycemia Normal TG level Normal liver MYALEPT (metreleptin for injection) is a recombinant analogue of human leptin Regulates energy homeostasis and metabolic function Metreleptin is indicated as an adjunct to diet as replacement therapy to treat complications of leptin deficiency in patients with GL Studies have shown that metreleptin has the potential to dramatically improve conditions such as hyperglycemia, hyperphagia, hypertriglyceridemia and hepatomegaly Clinical observations suggest a potential to positively impact on the quality of life of affected patients MYALEPT is the only agent to treat the underlying cause of lipodystrophies (low leptin) and therefore halt the progression of the disease which effects organ damage and outcome compared to other agents which only tackle the symptoms of hyperglycemia and hypertriglyceridemia Overview of MYALEPT Effect of MYALEPT(1) (1) Source: Kyoto University Faculty of Medicine website Healthy Lipodystrophy Myalept Injection Adipose Tissue Blood Leptin Leptin Leptin Appetite suppression Energy expenditure Insulin sensitizing Improve: Appetite suppression Energy expenditure Insulin sensitizing Hyperphagia Low energy exp. Insulin resistance Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. Metreleptin: The Only Product Approved in the U.S. for the Treatment of GL DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 21
Metreleptin Safety Profile and Limitations of Use < 10% discontinuation due to ADRs, and < 1% due to drug-related ADRs Common adverse drug reactions (ADRs) in >10% of all 113 patients: Weight decreased (15%) Hypoglycemia (13%) Less common ADRs (in < 4% of patients): Injection site reaction, neutralizing antibodies, Decreased appetite, nausea, alopecia Presence of neutralizing antibodies in a small minority of patients did not result in clearly identified clinical sequelae Anaplastic large-cell lymphoma, n = 1; Peripheral T-cell lymphoma, n = 2 Evidence of pre-existing lymphoma and/or bone marrow/hematologic abnormalities in both lymphomas Lymphoma also reported in patients with acquired LD not treated with metreleptin, likely related to underlying predisposition to autoimmune conditions WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA MYALEPT® is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the MYALEPT® REMS PROGRAM See www.Myalept.com for full prescribing information U.S. Prescribing Information Boxed Warning for MYALEPT® Limitations of Use The safety and effectiveness of MYALEPT for the treatment of complications of partial lipodystrophy have not been established. The safety and effectiveness of MYALEPT for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established. MYALEPT is not indicated for use in patients with HIV-related lipodystrophy. MYALEPT is not indicated for use in patients with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without concurrent evidence of congenital or acquired generalized lipodystrophy. Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 22
Myalept: Reimbursement Landscape Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 23 Broad payor coverage in the U.S. for GL ~55% of current US paid patients covered by commercial insurers, ~45% covered by Medicaid/Medicare Significant pre-approval/pre-launch unsolicited sales in LATAM, Turkey and certain EU countries (i.e. France and Italy) on a named-patient basis where permitted by applicable laws EU Pricing and Reimbursement (P&R) payor discussions are ongoing Plan to launch in Germany in November 2018 Expect EU published prices to be consistent with orphan drug analogs with comparable market size
Recent European Approval a Growth Opportunity for Myalept MYALEPTA is indicated as an adjunct to diet as a replacement therapy to treat the complications of leptin deficiency in lipodystrophy (LD) patients with: Confirmed congenital generalised LD (Berardinelli-Seip syndrome) or acquired generalised LD (Lawrence syndrome) in adults and children 2 years of age and above Confirmed familial partial LD or acquired partial LD (Barraquer-Simons syndrome), in adults and children 12 years of age and above for whom standard treatments have failed to achieve adequate metabolic control 24 EU label includes both GL (CGL & AGL) and PL indications (FPL & APL) ~100 patients currently on EAP, with a majority of EAP patients having GL or PL EMEA opportunity of ~1000 treatable patients based on estimated prevalence rates for GL & PL Estimated prevalence rate for GL & PL assumed to be 4 patients/million population (1/M GL & 3/M PL) Concentration of likely prescribers in Centers of Excellence enables Aegerion to commercialize MYALEPTA with relatively small EU team EU Opportunity EU Indication Source: Management Projections
BLA for Metreleptin was filed on April 3rd, 2012, by BMS & Amylin, which was later acquired by BMS in August 2012 Myalept® was approved by the FDA on February 25th, 2014. The FDA concluded that the BLA was approvable in Generalized Lipodystrophy (GL) patients but that its efficacy and safety was not established in PL patients BLA was transferred from BMS to AstraZeneca as part of the acquisition of the diabetes alliance assets, including MYALEPT and Amylin Pharmaceuticals, which was completed in February 2014 Myalept® was approved only for GL when Aegerion acquired the asset in 2014 While clinical data from the same studies were submitted to the EMA, the EMA had more safety data from AE reports and overall determined that Myalept was an effective treatment for patients with both GL and PL As part of the approval in Europe, the following are required: Pediatric GL study Immunogenicity study PL post-approval study Maintain a registry called MEASURE Anticipated applications for approval in LATAM (and possibly Canada) are expected to be based on the broader EMA submission After the approval of Myalepta in PL in Europe, plan to pursue PL indication in the U.S.; briefing documents to be submitted in near-term Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. Partial Lipodystrophy (PL) Growth Opportunity Regulatory History in the U.S. Europe LATAM & Canada Future U.S. Opportunity 25
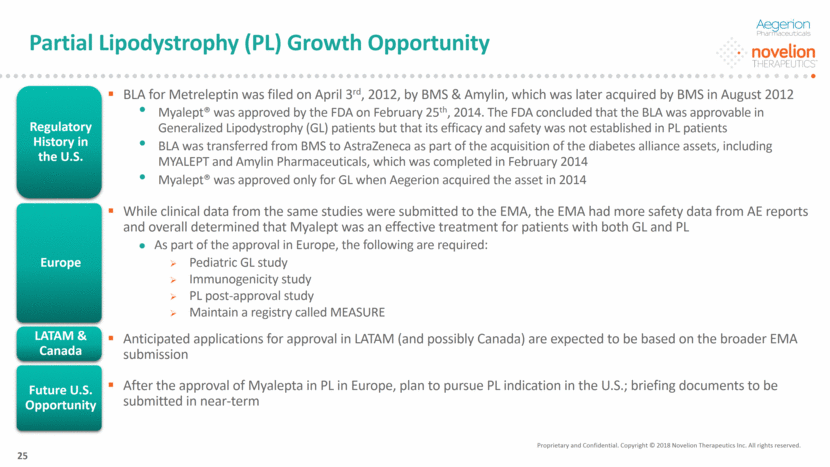
Metreleptin Efficacy in PL Patients Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 26 PL patients experienced a clinically and statistically significant response to Metreleptin after a year of therapy Source: CHMP Oral Explanation, 2/21/2018 Note: PL Subgroup are patients from NIH having TG >5.65 mmol/l and Hba1c >6.5% and leptin <12ng/ml
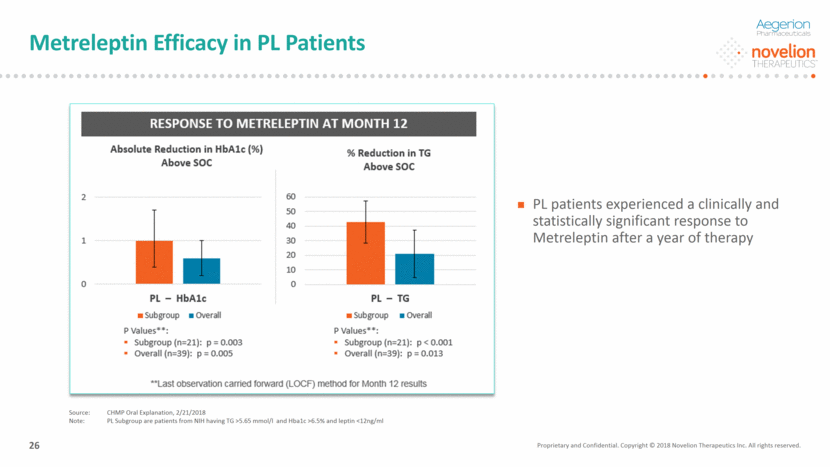
Metreleptin is Generally Well Tolerated in PL Patients PL subgroup n=25(%) PL overall N=73 (%) GL overall N=75 (%) Adverse Drug Reactions Hypoglycemia 4 (16%) 3 (15%) 8 (13%) Fatigue 3 (12%) 4 (5%) 4 (5%) Nausea 1 (4%) 8 (11%) 1 (1%) Headache 0 (0%) 3 (4%) 1( 1%) Weight decrease 1 (4%) 2 (3%) 16 (21%) Blocking activity NAb n=38 5 (26%) 13 (27%) 25 (47%) AE leading to discontinuation N=148 1 (4%) 4 (5%) 6 (8%) Immunogenicity in NAb positive PL Subgroup patients (n=5): Positive clinical outcomes in all except one patient (901-051) death due to anoxic encephalopathy (multiple other pre-existing comorbidities) No related SAE or related severe AE reported in this group No indication of negative impact on NAbs on endogenous leptin in PL patients Metreleptin was generally well tolerated There was no difference in safety profile in <4ng/ml vs >4 ng/ml groups (data not shown) In conclusion, the safety and immunogenicity of metreleptin was the same in PL Subgroup patients across all leptin levels 27 Source: Phase 3 study Note: PL Subgroup are patients from NIH having TG >5.65 mmol/l and Hba1c >6.5% and leptin <12ng/ml Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved.
I Metreleptin Pipeline Opportunities IV Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 28

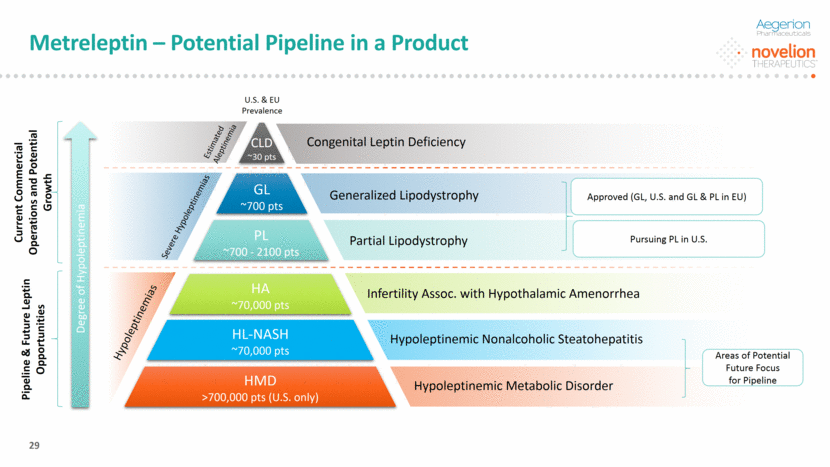
Metreleptin – Potential Pipeline in a Product DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 29 GL ~700 pts PL ~700 - 2100 pts HA ~70,000 pts HL-NASH ~70,000 pts HMD >700,000 pts (U.S. only) Generalized Lipodystrophy Partial Lipodystrophy Infertility Assoc. with Hypothalamic Amenorrhea Hypoleptinemic Nonalcoholic Steatohepatitis Hypoleptinemic Metabolic Disorder Severe Hypoleptinemias Hypoleptinemias U.S. & EU Prevalence Estimated Aleptinemia Congenital Leptin Deficiency Degree of Hypoleptinemia Approved (GL, U.S. and GL & PL in EU) CLD ~30 pts Areas of Potential Future Focus for Pipeline Current Commercial Operations and Potential Growth Pipeline & Future Leptin Opportunities Pursuing PL in U.S.
I Summary V Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) 30

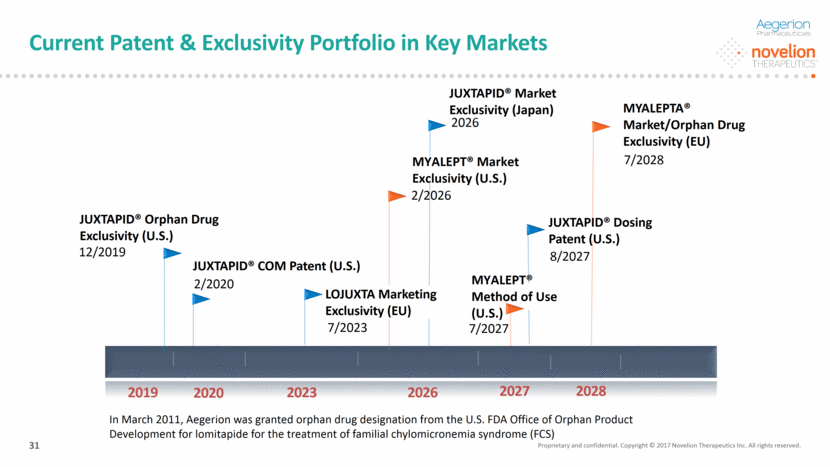
Current Patent & Exclusivity Portfolio in Key Markets Proprietary and confidential. Copyright © 2017 Novelion Therapeutics Inc. All rights reserved. 31 2019 JUXTAPID® Orphan Drug Exclusivity (U.S.) 12/2019 JUXTAPID® COM Patent (U.S.) 2/2020 7/2023 2/2026 8/2027 2020 2023 2026 2027 MYALEPTA® Market/Orphan Drug Exclusivity (EU) 7/2028 MYALEPT® Market Exclusivity (U.S.) JUXTAPID® Market Exclusivity (Japan) 2026 2028 MYALEPT® Method of Use (U.S.) JUXTAPID® Dosing Patent (U.S.) LOJUXTA Marketing Exclusivity (EU) 7/2027 In March 2011, Aegerion was granted orphan drug designation from the U.S. FDA Office of Orphan Product Development for lomitapide for the treatment of familial chylomicronemia syndrome (FCS)
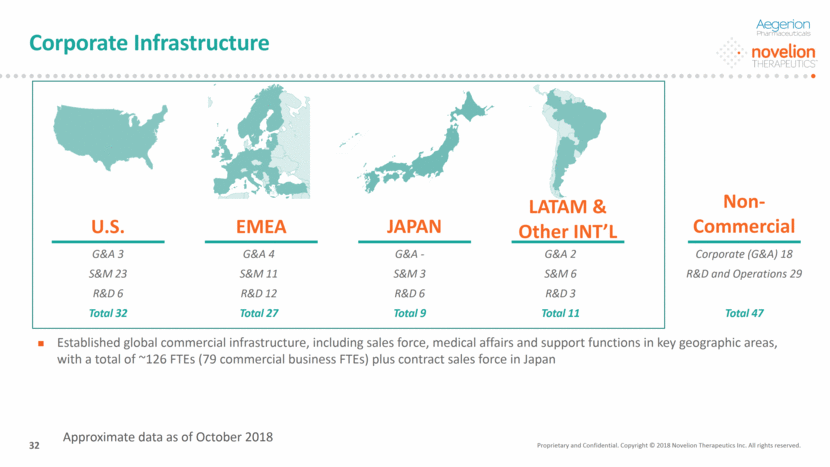
Corporate Infrastructure U.S. G&A 3 S&M 23 R&D 6 Total 32 LATAM & Other INT’L G&A 2 S&M 6 R&D 3 Total 11 EMEA G&A 4 S&M 11 R&D 12 Total 27 JAPAN G&A - S&M 3 R&D 6 Total 9 Established global commercial infrastructure, including sales force, medical affairs and support functions in key geographic areas, with a total of ~126 FTEs (79 commercial business FTEs) plus contract sales force in Japan Non-Commercial Corporate (G&A) 18 R&D and Operations 29 Total 47 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. 32 Approximate data as of October 2018
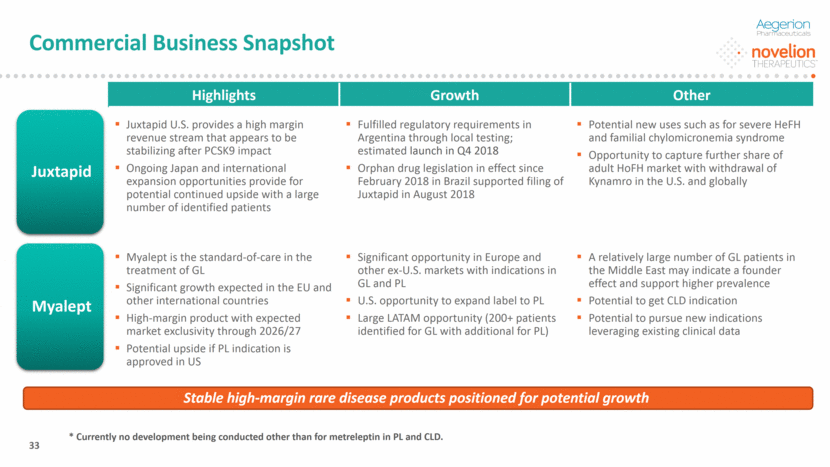
Commercial Business Snapshot DO NOT DELETE Color Palette (246/105/38) (29/167/157) (211/222/229) (0/126/178) Font (104/104/104) Highlights Growth Other Juxtapid U.S. provides a high margin revenue stream that appears to be stabilizing after PCSK9 impact Ongoing Japan and international expansion opportunities provide for potential continued upside with a large number of identified patients Fulfilled regulatory requirements in Argentina through local testing; estimated launch in Q4 2018 Orphan drug legislation in effect since February 2018 in Brazil supported filing of Juxtapid in August 2018 Potential new uses such as for severe HeFH and familial chylomicronemia syndrome Opportunity to capture further share of adult HoFH market with withdrawal of Kynamro in the U.S. and globally Myalept is the standard-of-care in the treatment of GL Significant growth expected in the EU and other international countries High-margin product with expected market exclusivity through 2026/27 Potential upside if PL indication is approved in US Significant opportunity in Europe and other ex-U.S. markets with indications in GL and PL U.S. opportunity to expand label to PL Large LATAM opportunity (200+ patients identified for GL with additional for PL) A relatively large number of GL patients in the Middle East may indicate a founder effect and support higher prevalence Potential to get CLD indication Potential to pursue new indications leveraging existing clinical data 33 Juxtapid Myalept Stable high-margin rare disease products positioned for potential growth * Currently no development being conducted other than for metreleptin in PL and CLD.
Michael Price Executive Vice President & Chief Financial Officer Served since November 2017 following a role as CFO for Noven Pharmaceuticals, Inc where he executed commercial scaling strategies. Mr. Price is a Certified Public Accountant and holds a Masters of Business Administration from Florida State University Geographical Coverage Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. Leadership Team Jeffrey Hackman Chief Operating Officer / Acting CEO Served since November 2017 following a role as head of U.S. Internal Medicine & Oncology for Shire. Prior to joining Shire, Mr. Hackman was region head for North America at Baxalta. He holds a Bachelor of Arts in communications from Lycoming College Ben Harshbarger Global General Counsel Served since November 2016 following a similar role at Aegerion. Also served in legal roles at Cubist Pharmaceuticals, ViaCell and Biogen/Biogen Idec. Mr. Harshbarger served at the law firm of Mintz Levin and holds a J.D. from Boston College and a B.A. from the University of Richmond (VA) Linda Buono Senior Vice President, Human Resources Served since November 2016 following a similar role at Aegerion and more than 25 years at Immunogen where she held a variety of leadership positions in Human Resources Roger Louis Global Chief Compliance Officer Served since November 2016 following a similar role at Aegerion. Also served in compliance and risk management roles at Cubist Pharmaceuticals and Genzyme. Mr. Louis began his legal career at Hale & Dorr and received his J.D. and B.A. from the University of Chicago and Tufts University respectively 34

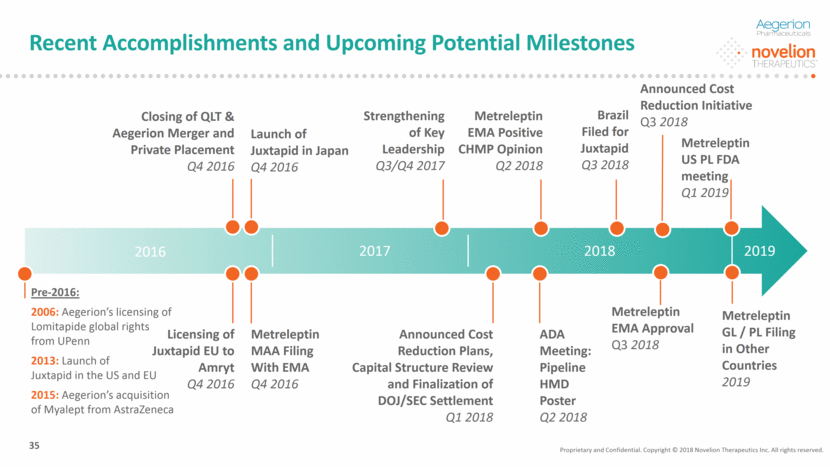
ADA Meeting: Pipeline HMD Poster Q2 2018 Recent Accomplishments and Upcoming Potential Milestones 2016 2017 2018 Closing of QLT & Aegerion Merger and Private Placement Q4 2016 Launch of Juxtapid in Japan Q4 2016 Metreleptin MAA Filing With EMA Q4 2016 35 Metreleptin GL / PL Filing in Other Countries 2019 Pre-2016: 2006: Aegerion’s licensing of Lomitapide global rights from UPenn 2013: Launch of Juxtapid in the US and EU 2015: Aegerion’s acquisition of Myalept from AstraZeneca Licensing of Juxtapid EU to Amryt Q4 2016 Announced Cost Reduction Plans, Capital Structure Review and Finalization of DOJ/SEC Settlement Q1 2018 Strengthening of Key Leadership Q3/Q4 2017 Metreleptin EMA Positive CHMP Opinion Q2 2018 Brazil Filed for Juxtapid Q3 2018 2019 Metreleptin EMA Approval Q3 2018 Metreleptin US PL FDA meeting Q1 2019 Proprietary and Confidential. Copyright © 2018 Novelion Therapeutics Inc. All rights reserved. Announced Cost Reduction Initiative Q3 2018