Attached files
U.S. SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2016
◻ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file Number: 000-17082
Novelion Therapeutics Inc. (Formerly QLT Inc.)
(Exact Name of Registrant as Specified in Its Charter)
British Columbia, Canada | N/A |
(State or Other Jurisdiction of Incorporation or Organization) | (IRS Employer Identification Number) |
887 Great Northern Way, Suite 250, Vancouver, B.C., Canada | V5T 4T5 |
(Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (604) 707-7000
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
Title of class | Name of each exchange on which registered |
Common Shares, without par value | The NASDAQ Global Select Market |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ◻ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ◻ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ◻
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ◻
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ◻ | Accelerated filer | ☒ |
Non-accelerated filer | ◻ (Do not check if a smaller reporting company) | Smaller reporting company | ◻ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ◻ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant as of June 30, 2016 was approximately $46,106,522, based upon the closing price on the NASDAQ Global Select Market reported for such date. As of March 16, 2017, 18,558,072 shares of the registrant’s common shares were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for its 2017 Annual General Meeting of Shareholders are incorporated by reference into Part III of this Annual Report on Form 10-K (Annual Report). The information to be included in Part III of this Annual Report will be provided in accordance with instruction G(3) to Form 10-K no later than May 1, 2017.
FORM 10-K
TABLE OF CONTENTS
PART I | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
PART II | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
PART III | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
PART IV | ||
Item 15. | ||
Item 16. | Summary | |
Explanatory Note
On November 29, 2016, Novelion Therapeutics Inc. (Novelion) (formerly known as QLT Inc.) completed its acquisition of Aegerion Pharmaceuticals, Inc. (Aegerion), through the merger (the Merger) of its indirect, wholly-owned subsidiary Isotope Acquisition Corp. (MergerCo) with and into Aegerion, pursuant to an Agreement and Plan of Merger (as amended, the Merger Agreement), dated as of June 14, 2016, among Novelion, Aegerion and MergerCo. As a result of the Merger, Aegerion became an indirect wholly-owned subsidiary of Novelion. The former stockholders of Aegerion received shares of Novelion as consideration in connection with the Merger.
1
The Merger has been accounted for as a business combination in which Novelion was considered the acquirer of Aegerion. As such, the Consolidated Financial Statements of Novelion are treated as the historical financial statements of the combined companies, with the results of Aegerion being included from November 29, 2016.
All references in this Annual Report to “we,” “us,” “our” and the “Company” refer to Novelion and its consolidated subsidiaries. For periods following the closing of the Merger, such references include Aegerion.
As described more fully in this Annual Report, following the Merger, Novelion continues to conduct research and development related to zuretinol and Aegerion continues to develop and commercialize lomitapide and metreleptin, and each maintains its respective ownership of or licenses covering intellectual property related to such products and remains as party to the regulatory filings and approvals for such products.
Certain portions of this Annual Report may contain information that may no longer be material to our business related to Aegerion’s historical operations. Any comparison of pre-Merger Aegerion revenues and operations with ours may not be helpful to an understanding of our results for the fiscal year ended December 31, 2016 or future periods.
On December 16, 2016, we completed a one-for-five (1:5) consolidation of all of our issued and outstanding common shares, without par value, for shareholders of record as of December 16, 2016 (the Consolidation). All share and per-share data presented in the Company's Consolidated Financial Statements and notes have been retrospectively restated to reflect the Consolidation unless otherwise noted.
Forward-Looking Statements
All statements included or incorporated by reference into this Annual Report, other than statements or characterizations of historical fact, are “forward-looking statements” under applicable laws, regulations and other legal principles and constitute “forward-looking information” within the meaning of applicable Canadian securities laws. Forward-looking statements and information are often identified by words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “forecasts,” “may,” “will,” “should,” “would,” “could,” “potential,” “guidance,” “continue,” “ongoing” and similar expressions, and variations or negatives of these words. Examples of forward-looking statements and information contained in this Annual Report include our statements regarding: the commercial potential for, and market acceptance of, our products; our estimates as to the potential number of patients with the diseases for which our products are approved or for which our product candidates are being developed; our expectations with respect to reimbursement of our products in the United States (U.S.) and elsewhere; our expectations with respect to named patient sales of our products in Brazil and in other countries where such sales are permitted; the potential for and possible timing of approval of our products in countries where we have not yet obtained approval; our plans for further clinical development of our products; the potential for zuretinol to obtain a rare pediatric disease designation and/or priority review voucher, if approved; our expectations regarding future regulatory filings for our products, including planned marketing approval applications with respect to metreleptin to expand the indication for metreleptin in the U.S., subject to discussions with the FDA; our plans for commercial marketing, sales, manufacturing and distribution of our products; our expectations with respect to the impact of competition on our future operations and results; our beliefs with respect to our intellectual property portfolio for our products and the extent to which it allows us to exclusively develop and commercialize our products and product candidates; our expectations regarding the availability of data and marketing exclusivity for our products in the U.S., the European Union (EU), Japan and other countries; our view of potential outcomes of Aegerion’s ongoing Department of Justice (DOJ) and Securities and Exchange Commission (SEC) investigations and shareholder litigation, including the terms of the agreements in principle with respect to the investigations and the memorandum of understanding with respect to the settlement of Aegerion’s shareholder litigation, and investigations in Brazil, and the possible impact and additional consequences of each on our business; our expectations regarding the impact on U.S. sales and patient attrition of JUXTAPID® as a result of the implementation of the modified JUXTAPID Risk Evaluation and Mitigation Strategy program; our expectations regarding our global consolidated tax structure and planning, our ability to achieve tax savings or utilize net operating loss carryforwards and other tax and tax planning activities, including whether we are characterized as a U.S. domestic corporation or passive foreign investment company for U.S. federal income tax purposes; our forecasts regarding sales of our products, our future expenses, our cash position and the timing of any future need for additional capital to fund operations; our ability to successfully integrate the businesses of Aegerion and Novelion; and our ability to manufacture and supply sufficient amounts of our products to meet demand.
The forward-looking statements contained in this Annual Report and in the documents incorporated into this Annual Report by reference are based on our current beliefs and assumptions with respect to future events, all of which are subject to change. Forward-looking statements are based on estimates and assumptions regarding, for example, our financial position and execution of our business strategy, post-merger integration and synergies, resolution of litigation and investigations, future competitive conditions and market acceptance of products, the possibility and timing of future regulatory approvals, expectations regarding our core capabilities, and the availability of sufficient liquidity, each made in light of current conditions and expected future developments,
2
as well as other factors that we believe are appropriate in the circumstances. Forward-looking statements are not guarantees of future performance, and are subject to risks, uncertainties and assumptions that are difficult to predict, including those discussed in the “Risk Factors” section of this Annual Report. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors may impact our operations or results. New risks may emerge from time to time. Past financial or operating performance is not necessarily a reliable indicator of future performance. Given these risks and uncertainties, we can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them does occur, what impact such event will have on our results of operations and financial condition. Our actual results could differ materially and adversely from those expressed in any forward-looking statement in this Annual Report or in our other filings with the SEC.
This Annual Report also contains “forward-looking information” that constitutes “financial outlooks” within the meaning of applicable Canadian securities laws. This information is provided to give investors general guidance on management’s current expectations of certain factors affecting our business, including our financial results. Given the uncertainties, assumptions and risk factors associated with this type of information, including those described above, investors are cautioned that the information may not be appropriate for other purposes. See the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Annual Report.
Except as required by law, we undertake no obligation to revise our forward-looking statements to reflect events or circumstances that arise after the date of this Annual Report or the respective dates of documents incorporated into this Annual Report by reference that include forward-looking statements. Therefore, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in these forward-looking statements.
Trademarks
Novelion™, Aegerion®, JUXTAPID, LOJUXTA®, MYALEPT® and MYALEPTA® are registered trademarks of Novelion or its subsidiary, Aegerion. All other trademarks referenced in this Annual Report are the property of their respective owners.
3
PART I
Item 1. | Business. |
Overview
On November 29, 2016, QLT Inc. (QLT) acquired Aegerion Pharmaceuticals, Inc. (Aegerion) (the Merger) and, changed its name to Novelion Therapeutics Inc. (Novelion). Novelion is a biopharmaceutical company dedicated to developing new standards of care for individuals living with rare diseases.
We have a diversified commercial portfolio through Aegerion, our indirect wholly-owned subsidiary, and we are developing a late-stage pipeline asset, for which we have received orphan drug designation. We have commercial capabilities in North America, Europe, Japan and Latin America, and seek to maximize the potential of our current marketed compounds while creating a strong foundation for other future rare disease therapies by investing in science and clinical development.
We, through Aegerion, now have two commercial products:
◦ | Metreleptin, a recombinant analog of human leptin, is marketed in the U.S. under the brand name MYALEPT (metreleptin) for injection (MYALEPT). MYALEPT is approved in the U.S. as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy (GL). In December 2016, we submitted a marketing authorization application (MAA) to the European Medicines Agency (EMA) to seek approval for metreleptin, under the brand name MYALEPTA, as replacement therapy to treat complications of leptin deficiency in patients with GL and in a subset of patients with partial lipodystrophy (PL). We also expect to submit a supplemental biologics licensing application (sBLA) to the U.S. Food and Drug Administration (FDA) in the first half of 2017, seeking to expand MYALEPT’s indication in the U.S. to the PL subset and plan to file for formal regulatory approvals for metreleptin throughout 2017 and early 2018 in other key markets, including Brazil and Colombia. We offer metreleptin through expanded access programs in countries where permitted by applicable regulatory authorities and under applicable laws, and generate revenue in certain markets where named patient sales are permitted based on the approval of metreleptin in the U.S. In addition to the PL subset, we plan to use our knowledge of the diverse effects of leptin on various physiologic functions to explore new opportunities for metreleptin as a platform drug to potentially treat patients suffering from a range of low leptin-mediated rare and metabolic diseases. We are evaluating and prioritizing these potential opportunities and plan to provide an update in mid-2017. |
◦ | Lomitapide is marketed in the U.S. under the brand name JUXTAPID (lomitapide) capsules (JUXTAPID). JUXTAPID is approved in the U.S. as an adjunct to a low-fat diet and other lipid-lowering treatments, including low-density lipoprotein (LDL) apheresis where available, to reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein B (apo B) and non-high-density lipoprotein cholesterol (non-HDL-C) in adult patients with homozygous familial hypercholesterolemia (HoFH). Lomitapide is approved in the EU, under the brand name LOJUXTA (lomitapide) hard capsules (LOJUXTA) for the treatment of adult patients with HoFH, as well as in Japan, Canada, and a small number of other countries. In December 2016, Aegerion out-licensed the rights to commercialize LOJUXTA in the EU and certain other jurisdictions to Amryt Pharma plc (Amryt) and will receive sales milestones and royalties in the low double-digits on net sales in those jurisdictions. In December 2016, following receipt of reimbursement approval, Aegerion launched JUXTAPID as a treatment for HoFH in Japan. Lomitapide is also sold, on a named patient basis, in Brazil and in a limited number of other countries outside the U.S. where such sales are permitted as a result of the approval of lomitapide in the U.S. or the EU. |
We have one orphan drug-designated product candidate, zuretinol acetate (zuretinol), an oral synthetic retinoid, in late stage development for the treatment of Inherited Retinal Disease caused by underlying mutations in retinal pigment epithelium protein 65 (RPE65) and lecithin: retinol acyltransferase (LRAT) genes (IRD), comprising Leber Congenital Amaurosis (LCA) and Retinitis Pigmentosa (RP). Our clinical and regulatory pathway for the zuretinol program is currently under review, and we expect to provide an update in mid-2017. We are also exploring the potential of submitting to the FDA a request for Rare Pediatric Disease Designation for zuretinol for the treatment of IRD. If zuretinol is approved by the FDA after being designated a Rare Pediatric Disease and we meet certain additional criteria, we may qualify for a Rare Pediatric Disease Priority Review Voucher. Zuretinol was granted orphan drug designations for the treatment of LCA (due to inherited mutations in LRAT or RPE65 genes) and RP (all mutations) by the FDA, and for the treatment of LCA and RP (all mutations) by the EMA. Both the FDA and EMA have acknowledged that the therapeutic indication of zuretinol for the treatment of IRD (patients phenotypically diagnosed as LCA or RP caused by mutations in RPE65 or LRAT genes) falls within these orphan drug designations. The zuretinol program has also been granted two Fast Track designations by the FDA for the treatment of LCA and RP due to inherited mutations in the LRAT and RPE65 genes.
4
We have a new management team and a reconstituted Board of Directors, consisting of four legacy QLT directors, four directors who were serving on the Board of Directors of Aegerion at the time of the Merger and two directors appointed by significant shareholders pursuant to contractual arrangements. Our new management team is comprised of executives who were serving as officers of Aegerion at the time of the Merger and includes individuals with significant experience in the biopharmaceutical industry and with a successful track record of developing and commercializing rare disease and other pharmaceutical products.
During the year ended December 31, 2016, net product sales of lomitapide and metreleptin were $13.6 million, of which $10.8 million was derived from prescriptions for lomitapide and metreleptin written in the U.S. and $2.8 million was derived from prescriptions for lomitapide and metreleptin written outside the U.S. As of December 31, 2016, we had approximately $108.9 million in cash and cash equivalents. Aegerion has approximately $325.0 million principal amount of 2.0% convertible senior notes due August 15, 2019 (the Convertible Notes) outstanding. As further described below in “Investigations and Legal Proceedings”, Aegerion reached, in May 2016, preliminary agreements in principle with the DOJ and the SEC that provide for, among other things, a consolidated monetary package that covers payments due to both the DOJ and the SEC by Aegerion totaling approximately $40 million in the aggregate; to be payable over three years, which is updated from the originally proposed five-year payment schedule contemplated when the preliminary agreement in principle was reached in May 2016. See the “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources” section of this Annual Report for further information.
In the near-term, we expect that the majority of our revenues will continue to be derived from sales of MYALEPT and JUXTAPID in the U.S. We also expect to generate revenues from (i) sales of lomitapide in those countries outside the U.S. in which we have or expect to receive marketing approval, are able to obtain pricing and reimbursement approval at acceptable levels, and elect to commercialize lomitapide, and (ii) sales of both products in a limited number of other countries where they are, or may in the future be, available on a named patient sales basis as a result of existing approvals in the U.S. or EU. We expect that in the near-term, named patient sales of lomitapide and metreleptin in Brazil will continue to be our second largest source of revenues for each product, on a country-by-country basis. We have received named patient sales orders for metreleptin in Argentina in 2016, and have had or expect to continue to have named patient sales of metreleptin in Brazil, Colombia and a select number of countries in the EU, including France and Turkey. We expect net product sales from named patient sales to fluctuate significantly quarter-over-quarter given that named patient sales are derived from unsolicited requests from prescribers. In some countries, including Brazil, orders for named patient sales are for multiple months of therapy, which can lead to some fluctuations in sales depending on the ordering pattern. We believe the investigations into Aegerion’s activities in Brazil have adversely affected named patient sales of lomitapide and metreleptin in that country. See the “Legal Proceedings” section of this Annual Report for further information regarding these investigations. In addition, a proceeding is currently pending with the Brazil Supreme Federal Court to decide whether the government has an obligation to continue to provide, on a named patient sales basis, drugs that have not received regulatory and/or pricing and reimbursement approval in Brazil, like JUXTAPID and MYALEPT. We intend to file for marketing approval in Brazil for both JUXTAPID and MYALEPT, and are currently assessing the timing of these submissions. The result of this above trial and other issues could significantly negatively affect product revenues from named patient sales of JUXTAPID and MYALEPT in Brazil.
Key Operational Objectives
We expect that our near-term efforts will be focused on the following:
• | building and maintaining market acceptance for MYALEPT in the U.S. for the treatment of complications of leptin deficiency in GL patients, and supporting named patient sales of metreleptin in GL in Brazil, particularly in light of local economic challenges and ongoing governmental investigations, and other key countries, including France and Turkey, where such sales are permitted as a result of the U.S. approval or under local law; |
• | preparing for the launch of metreleptin in Europe as a treatment for complications of leptin deficiency in GL patients and a subset of PL, in the event we obtain regulatory, pricing and reimbursement approvals in the EU for metreleptin; |
• | evaluating the potential for future clinical development of metreleptin in additional indications, including a subset of PL, if we are unable to secure approval of such indication with the current metreleptin clinical data package, as well as potentially other low leptin-mediated rare and metabolic diseases; |
• | stabilizing sales of JUXTAPID as a treatment for adult HoFH patients in the U.S. despite competition from PCSK9 inhibitor products, among other factors, which have had a significant adverse impact on sales of JUXTAPID, and gaining market acceptance in the other countries where lomitapide is approved and being commercialized, or may in the future receive approval and be commercialized; |
5
• | managing our costs and expenses to better align with our revenues, and strengthening our capital structure, while supporting approved products in a compliant manner; |
• | continuing to support patient access to and reimbursement for our products in the U.S. without significant restrictions, particularly given the availability of PCSK9 inhibitor products in the U.S., which has adversely impacted reimbursement of JUXTAPID, and given the considerable number of JUXTAPID patients in the U.S. who are on Medicare Part D and the significant percentage of such patients who may not be able to afford their out-of-pocket co-payments for our products, given that the only source of financial support for some such patients may be through patient assistance programs operated by independent charitable 501(c)(3) organizations that may not provide adequate financial assistance; |
• | implementing the modified JUXTAPID Risk Evaluation Management Strategy (REMS) program in the U.S., which includes requirements to recertify all prescribers and pharmacies and a new patient counseling and acknowledgment requirement for existing and new patients, by the July 2, 2017 implementation deadline, while working to limit adult HoFH patient attrition from JUXTAPID as a result of such new requirements; |
• | supporting the recent launch of JUXTAPID in Japan; |
• | continuing to support sales of lomitapide as a treatment for HoFH in Brazil on a named patient basis, particularly in light of the economic challenges, ongoing government investigations, and ongoing court proceedings reviewing the regulatory framework for named patient sales in Brazil, and in other key countries where named patient sales are permitted, despite the availability of PCSK9 inhibitors on a named patient sales basis in such countries; |
• | gaining regulatory, pricing and reimbursement approvals to market our products in countries in which the products are not currently approved and/or reimbursed or for new indications, including obtaining approval of the MAA seeking marketing approval of metreleptin in the EU as a treatment for complications of leptin deficiency in GL patients and a subset of PL, and seeking approval of metreleptin in the U.S. for a subset of PL based on the existing clinical data package for metreleptin; |
• | reviewing the clinical and regulatory pathway for zuretinol to determine the optimal development and business strategy for this product candidate; |
• | engaging in possible further development efforts related to our existing products, and assessing, and possibly acquiring, potential new product candidates targeted at rare diseases where we believe we can leverage our infrastructure and expertise; |
• | minimizing the number of patients who are eligible to receive but decide not to commence treatment with our products, or who discontinue treatment, by supporting activities such as patient support programs, to the extent permitted in a particular country; |
• | continuing to embed a culture of compliance, ethics and integrity throughout Novelion and its subsidiaries; |
• | Aegerion reaching a definitive agreement with the DOJ and the SEC with respect to its ongoing investigations in accordance with the terms of the agreements in principle it entered into in May 2016 and managing other ongoing government investigations pertaining to its products; |
• | Aegerion reaching a definitive agreement with respect to its ongoing securities class action in accordance with the terms of the memorandum of understanding entered into in December 2016 (the MOU); and |
• | defending challenges to the patents or our claims of exclusivity for lomitapide in the U.S., including against potential generic submission with the FDA with respect to lomitapide; and expanding the intellectual property portfolio for our products. |
Investigations and Legal Proceedings
As noted above, Aegerion has been the subject of certain ongoing investigations and other legal proceedings, including investigations by the DOJ and the SEC of Aegerion’s marketing and sales activities related to JUXTAPID, an investigation by federal and state authorities in Brazil to determine whether there have been violations of Brazilian laws related to the promotion of JUXTAPID, and a putative class action lawsuit alleging certain misstatements and omissions related to the marketing of
6
JUXTAPID and the Company’s financial performance in violation of the federal securities laws (the Class Action Litigation). Aegerion reached agreements in principle with the DOJ and the SEC in May 2016 that provides for Aegerion to pay a fine of $40 million, to plead guilty to two misdemeanor misbranding violations of the Food, Drug and Cosmetics Act and to enter into a five-year deferred prosecution agreement with regard to charges that it violated the Health Insurance Portability and Accountability Act (HIPAA) and engaged in obstruction of justice relating to the JUXTAPID REMS program. Aegerion also entered into the MOU with respect to the Class Action Litigation, which provides for a settlement payment by or on behalf of Aegerion of $22.25 million, of which we expect $22.0 million to be funded by insurance carriers and $0.25 million to be funded by Aegerion. See the “Legal Proceedings” section of this Annual Report for further information regarding these and other legal proceedings.
Recent Corporate and Securities Transactions
Merger Transaction with Aegerion. On June 14, 2016, we entered into an Agreement and Plan of Merger (as amended, the Merger Agreement) with Aegerion, pursuant to which on November 29, 2016, our indirect wholly-owned subsidiary, Isotope Acquisition Corp, merged with and into Aegerion, with Aegerion surviving as our wholly-owned subsidiary. Upon completion of the Merger, we changed our name from QLT Inc. to Novelion Therapeutics Inc. and each outstanding share of Aegerion common stock was converted into a right to receive 1.0256 Novelion common shares and Aegerion’s common stock was cancelled and delisted from the NASDAQ Global Select Market (NASDAQ).
Under the Merger Agreement, we also issued certain warrants to the pre-closing shareholders of Novelion. These warrants (the Merger Agreement Warrants) may be exercised for up to an aggregate of 11,301,791 Novelion common shares at an exercise price of $0.05 per share if (i) the previously disclosed DOJ and SEC investigations are settled for amounts in excess of $40 million and/or (ii) the Class Action Litigation is settled for an amount that exceeds the amounts, if any, available under Aegerion’s director and officer insurance coverage in respect of that matter (together, the negotiated thresholds). The number of Novelion common shares for which the Merger Agreement Warrants may be exercised, if any, will vary based on the extent to which the settlements of the matters described above exceed the negotiated thresholds. The Merger Agreement Warrants will not be exercisable for any shares to the extent any excess in respect of such matters is equal to or less than $1.0 million in the aggregate.
Pursuant to the Merger Agreement, effective upon the closing of the Merger, the Board of Directors is composed of four individuals designated by Aegerion, four individuals designated by Novelion, one individual designated by Broadfin Capital, LLC (Broadfin) and one individual designated by Sarissa Capital Management LP (Sarissa). For a specified period of time following the Merger, Sarissa will also have the right to designate one additional member of the Board of Directors.
The aggregate consideration delivered to the former holders of Aegerion common stock in connection with the Merger was approximately 6,060,288 Novelion common shares. Shareholders of Novelion immediately prior to the Merger, including the private placement pursuant to the Unit Subscription Agreement (described below), owned approximately 68% of the outstanding Novelion common shares upon completion of the Merger and stockholders of Aegerion as of immediately prior to the Merger owned approximately 32% of the outstanding Novelion common shares upon completion of the Merger.
Private Placement. Also on June 14, 2016, we entered into a unit subscription agreement (the Unit Subscription Agreement) with the investors’ party thereto (the Investors). Pursuant to the Unit Subscription Agreement, immediately prior to the Merger, the Investors acquired units, for $8.80 per unit, on a post-Consolidation (as defined below) basis, consisting of (i) 2,472,727 Novelion common shares, which includes up to 568,181 Novelion common shares issuable upon exercise of fully paid-up warrants, and (ii) warrants (the Unit Subscription Warrants) exercisable for up to an aggregate of 2,644,952 Novelion common shares at an exercise price of $0.05 per share. The Unit Subscription Agreement Warrants were issued on the same terms and conditions as the Merger Agreement Warrants and are referred to collectively with the Merger Agreement Warrants as the "Warrants" in this Annual Report. The aggregate consideration paid under the Unit Subscription Agreement was approximately $21.8 million, which we intend to continue to use to support future operations and business development initiatives.
Share Consolidation. On December 16, 2016, we completed a one-for-five (1:5) consolidation of all of our issued and outstanding common shares without par value for shareholders of record as of December 16, 2016 (the Consolidation), resulting in a reduction in the issued and outstanding common shares from approximately 92,653,562 to approximately 18,530,323 as of that date. Each shareholder’s percentage ownership in Novelion and proportional voting power remained unchanged after the Consolidation, except for minor changes resulting from the treatment of fractional shares. In connection with the Consolidation, the conversion rate of the Convertible Notes was automatically adjusted from 24.9083 common shares per $1,000 principal amount of such Convertible Notes to 4.9817 common shares per $1,000 principal amount of such Convertible Notes. All share and per-share data presented in the Company's Consolidated Financial Statements and notes have been retrospectively restated to reflect the Consolidation unless otherwise noted.
7
Aralez Investment and Distribution. On December 7, 2015, we entered into an Amended and Restated Share Subscription Agreement (the Amended and Restated Subscription Agreement) with Tribute Pharmaceuticals Canada Inc. (Tribute), POZEN Inc. (POZEN), Aralez Pharmaceuticals plc, (formally known as Aguono Limited) (Aralez Ireland), Aralez Pharmaceuticals Inc. (Aralez Canada), Deerfield Private Design Fund II, L.P., Deerfield International Master Fund, L.P., Deerfield Partners, L.P. (together Deerfield), Broadfin and JW Partners, LP, JW Opportunities Fund, LLC and J.W. Opportunities Master Fund, Ltd. (together the JW Parties) (the Company, Deerfield, Broadfin and the JW Parties are referred to herein collectively as the Co-Investors). The Amended and Restated Subscription Agreement amended and restated a share subscription agreement entered into on June 8, 2015, among QLT, Tribute, POZEN, Aralez Ireland, the Co-Investors and certain other investors. Pursuant to the Amended and Restated Subscription Agreement, immediately prior to and contingent upon the consummation of the merger of Tribute and POZEN (the Aralez Merger), Tribute agreed to sell to us and the other Co-Investors $75.0 million of the common shares of Tribute (the Tribute Shares) in a private placement at a purchase price per share equal to: (a) the lesser of (i) $7.20, and (ii) a five percent discount off of the five-day volume weighted average price per share of POZEN common stock calculated over the five trading days immediately preceding the date of closing of the Aralez Merger, not to be less than $6.25 per share; multiplied by (b) the Aralez Merger exchange ratio of 0.1455. Upon consummation of the Aralez Merger on February 5, 2016, the Tribute Shares were exchanged for common shares of Aralez Canada (the Aralez Shares). We entered into the transaction contemplated by the Amended and Restated Subscription Agreement for the purpose of returning capital to our shareholders pursuant to a special election distribution, payable, at the election of each shareholder of the Company, in either Aralez Shares (approximately 0.13629 of an Aralez Share for each common share of the Company) or cash, subject to pro-ration (the Aralez Distribution), up to a maximum of $15.0 million funded pursuant to the terms of the Backstop Agreement (as described below).
In connection with the Aralez Distribution, on June 8, 2015, we entered into a share purchase agreement (as amended, the Backstop Agreement) with Broadfin and the JW Parties, pursuant to which Broadfin and the JW Parties agreed to purchase up to $15.0 million of the Aralez Shares from us at $6.25 per share. This arrangement provided our shareholders with the opportunity to elect to receive, in lieu of Aralez Shares, up to an aggregate of $15.0 million in cash, subject to proration among the shareholders. As a result, on April 5, 2016 (the Distribution Date), we distributed 4,799,619 Aralez Shares, with a fair value of $19.3 million, and $15.0 million of cash.
Upon consummation of the Aralez Merger on February 5, 2016, we purchased 7,200,000 Aralez Shares (representing 10.1% of the issued and outstanding Aralez Shares), for an aggregate price of $45.0 million. We held the Aralez Shares from February 5, 2016 to the Distribution Date and the Aralez Shares were marked-to-market. As a result, we recognized a $10.7 million loss during the fiscal year ended December 31, 2016, to reflect the change in value from the acquisition date to the Distribution Date.
Marketed Products
As noted above, metreleptin and lomitapide are products that have been and continue to be developed and commercialized by our subsidiary Aegerion. All references to “we”, “us”, “our” and the like in this Annual Report in relation to metreleptin and lomitapide are references to the activities and plans of Aegerion or subsidiaries of Aegerion.
Metreleptin
Metreleptin, a recombinant analog of human leptin, is currently marketed in the U.S. under the brand name MYALEPT. MYALEPT received marketing approval from the FDA in February 2014 as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired GL. In December 2016, we submitted an MAA to the EMA seeking approval for metreleptin as replacement therapy to treat complications of leptin deficiency in patients with GL and in a subset of patients with PL.
We intend to evaluate potential future development of metreleptin in additional indications, including the PL subset, if we are unable to obtain approval of such indication based on the current clinical data package. The role of leptin in human physiology has been further understood during the 20 years since its initial discovery in 1994. In addition to its central role in the regulation of energy homeostasis and glucose and fat metabolism, leptin has diverse effects on various physiologic functions, including the regulation of neuroendocrine function, reproduction, vascular function, bone metabolism, and the immune system. Accordingly, we intend to explore the pleiotropic effects of metreleptin to determine the extent of its potential to treat a wide range of low leptin-mediated rare and metabolic diseases. In furtherance of this, we are evaluating and prioritizing these potential opportunities and plan to provide an update in mid-2017.
Lipodystrophy
Lipodystrophy (LD) is a heterogeneous group of rare syndromes characterized by selective but variable loss of fat tissue. The loss of fat tissue in patients with LD can range from partial to more generalized, and some patients have concomitant accumulation of excess fat tissue centrally. Because of the loss of fat tissue, levels of the fat cell secreted hormone leptin are very low. Leptin is a
8
naturally occurring, hormone derived from fat cells and an important regulator of energy, fat and glucose metabolism, reproductive capacity, and other physiological functions. Circulating levels of leptin closely correlate with the amount of fat mass present.
Due to the lack of fat cells in individuals with LD, energy can no longer be stored as fat in adipose tissues (fat cells) and fat accumulates in the muscles and organs such as heart, liver, and pancreas causing lipotoxicity and end-organ damage. In addition, deposition of fat in these unusual locations leads to extreme insulin resistance and its associated complications, such as diabetes mellitus, hypertriglyceridemia, hepatic steatosis, polycystic ovary syndrome, and high blood pressure. These severe metabolic abnormalities are typically resistant to conventional therapies. As a result of the deficiency of leptin associated with LD, patients experience significant fatigue as well as unregulated appetite. The voracious appetite itself significantly aggravates the metabolic abnormalities that these patients have, and further reduces the ability to successfully treat these metabolic abnormalities with conventional therapies.
Generalized Lipodystrophy
GL is characterized by a near complete lack of adipose tissue and, consequently, leads to early and significant morbidity and mortality. Differentiation of generalized LD (versus PL) is made based on the anatomical distribution of fat loss, which is widespread in GL patients, and the younger age and greater rapidity of onset and severity of the metabolic abnormalities. The severe metabolic abnormalities associated with GL may result in premature diabetic nephropathy, retinopathy, cardiomyopathy, recurrent attacks of acute pancreatitis, hepatomegaly, and organ failure. These complications themselves increase morbidity and mortality due to their known long-term impacts.
Partial Lipodystrophy
PL is characterized by a less uniform loss of fat cells and with a later age of onset. There can be considerable heterogeneity in the extent of fat cell loss, levels of leptin, and degree of metabolic abnormalities. Within the spectrum of PL, there are a subset of patients with more severe disease presentation. In PL patients with relative or near complete leptin deficiency, the metabolic abnormalities and longer impact on disease progression can closely mirror that of patients with GL.
We have defined a subgroup of patients with PL who have clinically similar metabolic disturbances as those patients with GL and who demonstrated clinically significant improvements in metabolic parameters on metreleptin treatment in clinical studies. See Phase 3 Clinical Studies below. Specifically, this subset includes patients with lower leptin levels, and more advanced metabolic abnormalities.
Status in the U.S.
The FDA approved MYALEPT in February 2014, as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired GL. The U.S. prescribing information for MYALEPT specifies that the safety and effectiveness of MYALEPT for the treatment of complications of PL or for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established. MYALEPT is not indicated for use in patients with HIV-related lipodystrophy or in patients with metabolic disease without concurrent evidence of congenital or acquired GL.
MYALEPT has a boxed warning, citing the risk of anti-metreleptin antibodies with neutralizing activity and risk of lymphoma. The consequences of neutralizing antibodies are not well characterized but could reduce how well the leptin found naturally in the body works or how well MYALEPT works. Lymphoma has been observed in acquired forms of LD with or without metreleptin therapy. Since patients with acquired LD typically have underlying autoimmune conditions that may predispose them to risk of lymphoma, a causal link to the use of metreleptin has not been established.
Because of the risk of neutralizing antibodies and the risk of lymphoma, MYALEPT is available in the U.S. only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) (referred to as the MYALEPT REMS program). Under the MYALEPT REMS program, we certify all qualified healthcare providers who prescribe MYALEPT and the pharmacies that dispense the medicine. The goals of the MYALEPT REMS program are to:
• | educate prescribers about the risk of neutralizing antibodies and the risk of lymphoma associated with the use of MYALEPT; and |
• | restrict access to therapy with MYALEPT to patients with a clinical diagnosis consistent with GL. |
The FDA has granted seven years of orphan drug exclusivity for MYALEPT in the U.S. in the treatment of metabolic disorders secondary to lipodystrophy.
We plan to seek approval of MYALEPT in the U.S. for the PL subset based on the existing clinical data package for MYALEPT, through the submission of a supplemental biologics licensing application (sBLA) with the FDA in the first half of 2017.
9
Status outside the U.S.
MYALEPT is currently approved only in the U.S. and Japan. Pursuant to an existing distribution agreement assigned to Aegerion as part of its purchase of metreleptin rights, Shionogi & Co., Ltd. (Shionogi) has rights to market metreleptin in Japan, South Korea and Taiwan. Shionogi received marketing and manufacturing approval in Japan for metreleptin for lipodystrophy in March 2013.
There are currently no approved treatments for GL or PL in the EU. In December 2016, we filed an MAA with the EMA seeking approval for metreleptin as replacement therapy to treat complications of leptin deficiency in patients with GL and in the PL subset. If approved, metreleptin would be marketed in the EU under the tradename MYALEPTA. Metreleptin was granted orphan designation by the European Commission (EC) for the treatment of Barraquer-Simons syndrome (acquired PL), Berardinelli-Seip syndrome (congenital GL), Lawrence syndrome (acquired GL) and familial PL.
We also plan to file applications for regulatory approval of MYALEPT to treat GL and the PL subset in Brazil, Colombia, Argentina and certain other markets over the course of 2017 and early 2018.
When Aegerion acquired metreleptin from AstraZeneca in January 2015, a number of patients were receiving metreleptin therapy free of charge in certain countries outside the U.S. that allow use of a drug, under a compassionate use or other type of expanded access program, before marketing approval has been obtained in such country. Where permitted in accordance with applicable requirements, we have continued to make metreleptin available free of charge under such a program, which has resulted in significant costs to us, given that we have more than 100 patients participating in this program; many of these patients are GL and subset PL patients who will be eligible for paid commercial therapy if we obtain regulatory, pricing and reimbursement approvals in the EU for metreleptin. In 2016, we began generating revenues from named patient sales of metreleptin in certain markets where such sales of metreleptin are possible and to the extent permitted by applicable law and local regulatory authorities. In particular, we are in the process of converting all GL and PL patients currently in the expanded access program in France to a paid program of Autorisation Temporaire d’Utilisation (Temporary Authorization for Use). Metreleptin has also been approved for reimbursement by the Turkish Social Security Association (SGK), and we plan to provide metreleptin on a named patient basis for GL patients, including congenital GL (CGL) patients, and other subsets of lipodystrophy patients, subject to individual assessment in response to unsolicited requests from clinicians. Further, we now supply paid product for individual patients in certain other markets and anticipate further unsolicited requests from clinicians may follow in these countries and potentially other selected markets in the EU where there is a formal mechanism for named patient sales in place. Outside of the EU, we have named patients sales in Brazil, Argentina, and certain other markets.
Phase 3 Clinical Study
The safety and efficacy of metreleptin for the treatment of metabolic disorders associated with LD syndromes in pediatric and adult patients were evaluated in a long-term, open-label, single-arm study conducted at the National Institutes of Health (the NIH). The objective of the NIH trial was to evaluate the efficacy of metreleptin for improving metabolic disorders associated with acquired or inherited lipodystrophy. This investigator-sponsored study was initiated in August 2000.
A total of 107 patients (>= 6 months of age) with a clinical diagnosis of GL or PL, low baseline leptin levels (men < 8 ng/mL, women < 12 ng/mL), and at least one metabolic abnormality (diabetes mellitus, hypertriglyceridemia > 200 mg/dL, fasting insulin levels > 30µU/mL) were enrolled in the NIH study. A total of 66 of the 107 patients had GL and 41 had PL, including 31 patients who were included in the PL subgroup, i.e. those PL patients who have similar metabolic disturbances as patients with GL and who were defined as patients with baseline Hemoglobin A1c (HbA1c) ≥6.5% and/or triglycerides ≥5.65 mmol/L. Among the 66 patients with GL, 45 (68%) had CGL and 21 (32%) had acquired GL. Most patients in the PL subgroup had the familial form (27 patients, 87%); 4 patients (13%) had acquired PL.
Metreleptin was administered subcutaneously once or twice daily in a gender-dependent, weight-based protocol, with step-wise specified titration over the first two months of the study and subsequent dose adjustments based on clinical response. The co-primary efficacy endpoints in the NIH study were defined as:
• | Actual change from baseline in HbA1c at Month 12, and |
• | Percent change from baseline in fasting serum triglycerides at Month 12. |
Treatment with metreleptin led to substantial and sustained improvements in glycemic control and hypertriglyceridemia in patients with GL and in the PL subgroup.
The observed primary efficacy results in GL patients are as follows:
• | A mean change from baseline to Month 12 in HbA1c of -2.2%. |
10
A mean percent change from baseline to Month 12 in triglycerides of -32.1%. The observed primary efficacy results in PL subgroup patients are as follows:
• | A mean change from baseline to Month 12 in HbA1c of -0.9%; and |
• | A mean percent change from baseline to Month 12 in triglycerides of -37.4%. |
In general, changes in fasting plasma glucose followed a similar pattern to changes in HbA1c.
Patients who met target decreases in both parameters were also assessed. In the GL group, 55% of patients achieved both an actual decrease in HbA1c of ≥1% and a ≥30% reduction in triglycerides at Month 12; with over one-third of patients achieving the highest target reductions of a ≥2% actual decrease in HbA1c and a ≥40% reduction in triglycerides. These levels of reduction in baseline metabolic abnormalities were also observed in patients in the PL subgroup. In this subgroup, 30% of patients achieved both an actual decrease in HbA1c of ≥1% and a ≥30% reduction in triglycerides at Month 12. Based on the overall mixed-model repeated measures analysis, which evaluates average levels of HbA1c and triglycerides across all visits, statistically significant decreases in HbA1c from baseline over all analysis visits was observed in the GL group and in the PL subgroup.
Median overall duration of metreleptin treatment was 49.9 months and 29.3 months in GL patients and in PL subgroup patients respectively.
The most common adverse drug reactions (ADRs) occurring in GL patients were weight decrease (reported by fifteen patients; 22.7%) and hypoglycemia (reported by eight patients; 12.1%), followed by decreased appetite, fatigue and neutralizing antibodies (each reported by four patients; 6.1%). The most common ADRs occurring in PL subgroup patients were hypoglycemia and fatigue (each reported by three patients; 9.7%), followed by alopecia (reported by two patients; 6.5%). Over the 14-year study duration, treatment-emergent deaths were reported in 4 (4%) of the 107 patients; treatment-emergent adverse events (TEAEs) leading to death were consistent with the underlying morbidity of LD and included renal failure, cardiac arrest (with pancreatitis and septic shock), progressive end-stage liver disease (chronic hepatic failure), and hypoxic-ischemic encephalopathy. None of the deaths were assessed as drug-related.
The presence of neutralizing antibodies in a small minority of patients did not result in clearly identified clinical sequelae.
Two cases of peripheral T-cell lymphoma and one case of a localized anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (a type of T-cell lymphoma) were reported, all in patients with acquired GL. Lymphoma is known to be associated with autoimmune disease. As the boxed warning for MYALEPT states, T-cell lymphoma has been reported in patients with acquired GL, both treated and not treated with MYALEPT. There was evidence of pre-existing lymphoma and/or bone marrow/hematologic abnormalities in the two patients with peripheral T-cell lymphoma prior to metreleptin therapy, and the third case of anaplastic large cell lymphoma occurred in the context of a specific chromosomal translocation.
Post-Marketing Commitments
As part of the post-marketing commitments to the FDA for metreleptin, we have initiated a long-term, prospective, observational study (product exposure registry) in patients to evaluate serious risks related to the use of the product. The registry will attempt to enroll at least 100 new patients treated with metreleptin. Enrollment will close after five years or after 100 new patients have been enrolled, whichever occurs first. The registry will continue for ten years from the date of last patient’s enrollment.
We have also committed to the EMA, as part of our pediatric investigation plan (PIP), to conduct a study in GL patients under 6 years old to further evaluate the pharmacokinetics, efficacy and safety of metreleptin in this pediatric sub-population.
In addition, three programs are expected to expand the understanding of the immunogenicity of metreleptin. These programs consist of:
• | the development, validation, and implementation of a ligand binding assay to supplement the neutralizing bioassay that tests for the presence of neutralizing antibodies in serum samples from patients with GL, which we have completed; |
• | testing all banked clinical samples from the GL clinical program for the presence of neutralizing antibodies against leptin using the ligand binding assay and to correlate neutralizing antibodies with clinical events, which we have initiated and is ongoing; and |
• | a prospective study to assess the immunogenicity of metreleptin in patients receiving metreleptin, which is in the planning phase. |
The presence of neutralizing antibodies will be assessed using both a validated cell-based assay and a validated ligand-binding assay in samples that are confirmed positive for binding antibodies to leptin. In addition, we are required to conduct certain studies related to the manufacture of metreleptin, including in order to validate new test methods, implement a risk-based reference standard program approach, and reassess product acceptance criteria with a larger data set from more manufactured batches. The remaining three post-marketing commitments related to manufacturing metreleptin are on track for completion by approximately mid-2017,
11
mid-2018 and mid-2019, their respective commitment deadlines. Finally, we have an ongoing commitment to assess spontaneous reports of serious risks related to the use of metreleptin, including the risk to exposed pregnancies and pregnancy outcomes, regardless of indication, for ten years from the date of approval of metreleptin in the U.S.
Estimated Prevalence of GL and PL
There is no patient registry or other method of establishing with precision the actual number of patients with GL and PL in any geography. The data to date suggest that the approximate prevalence of GL in the U.S. is slightly under 1 in 1,000,000 persons and for PL overall is 3 in 1,000,000 persons. Although the data are even more limited, the prevalence in the U.S. of a subset of more severe PL is estimated to be between 0.5 and 1 in 1,000,000 persons. We believe that the prevalence rate of GL and PL, and correspondingly the PL subset, in countries outside the U.S. is likely to be consistent with the prevalence rate in the U.S. There is no guarantee, however, that our estimates are correct. The actual prevalence of GL, PL and the PL subset may be significantly lower than we expect. Ultimately, the actual size of the total addressable market in the U.S. and other key markets where metreleptin is sold, if approved, will be determined only after we have substantial commercial history selling metreleptin.
Lomitapide
Lomitapide, a small molecule microsomal triglyceride transfer protein (MTP) inhibitor, or MTP-I, is currently approved and marketed in a number of countries globally, including the U.S., Japan, Israel, Canada and Colombia under the brand name JUXTAPID, and in the EU under the brand name LOJUXTA, as an adjunct to a low-fat diet and other lipid lowering treatments, to reduce low density lipoprotein cholesterol (LDL-C) in adult patients with HoFH.
HoFH
HoFH is a serious, rare genetic disease that impairs the function of the receptor responsible for removing LDL-C (bad cholesterol) from the blood. An impairment of low density lipoprotein receptor (LDL-R) function results in significant elevation of blood cholesterol levels.
Cholesterol is a naturally occurring molecule that is transported in the blood. The liver and the intestines are the two main sites where cholesterol is packaged and released within the body. The liver synthesizes cholesterol, and provides the body’s intrinsic supply. The intestines are the conduit through which cholesterol enters the body for metabolism. The delivery of cholesterol to peripheral cells in the body provides necessary sources of cellular energy and cell structure. However, excess levels of cholesterol in the blood, also known as hypercholesterolemia, can be the source of significant diseases in humans. HoFH is most commonly caused by genetic mutations in both alleles of the LDL-R gene, but can also be caused by mutations in other genes. To date, more than 1,700 mutations have been identified that can impair the function of the LDL-R gene, with some mutations leading to a total lack of LDL-R activity and others leading to significantly reduced activity in the LDL-R gene. As a result of elevated levels of LDL-C, HoFH patients very often develop premature and progressive atherosclerosis, a narrowing or blocking of the arteries (usually in combination with arterial thrombosis), and are at very high risk of experiencing premature cardiovascular events, such as heart attack or stroke.
There are no universally accepted criteria for the diagnosis of HoFH. Diagnosis is typically made clinically, using the following criteria:
• | significantly elevated LDL-C cholesterol levels (treated or untreated); |
• | physical signs, which may include the presence of cutaneous xanthomas, Achilles tendon thickening, xanthelasma and/or corneal arcus; |
• | limited response to statins that is not attributed to statin intolerance or to another identifiable cause (usually dependent on functional LDL receptors), or limited and/or inadequate response to a PCSK9; |
• | evidence of premature cardiovascular disease (often in the second and third decade of life); and |
• | a positive history of high cholesterol and/or premature cardiovascular disease, consistent with having familial hypercholesterolemia (FH) on both sides of the family. |
HoFH is a rare form of FH and not all patients with the above characteristics will be HoFH patients. Genetic testing may be performed to make a diagnosis of HoFH, but is not routinely used in the U.S. because it has not been widely available, and because genetic testing can fail to detect certain defects given the large number of possible mutations and the number of genes that could be involved, as described above. HoFH patients may have the same defect on both copies of the same gene or may have different defects, one inherited from each parent, on the same gene or defects inherited from each parent on two different genes each affecting the function of the LDL-R. A 2013 article in the European Heart Journal (EHJ), as well as a 2015 article from the American Heart Association (AHA) estimate that current genetic tests may fail to positively detect 10% to 40% of patients with FH. As a result, most physicians in the U.S. and in many other countries use clinical findings and family history on both sides to make a clinical diagnosis of HoFH. Although not widely used, HoFH may also be diagnosed through an assessment of LDL-R function in cultured skin fibroblasts.
12
Physicians treating patients with hypercholesterolemia, including HoFH, are highly focused on lowering levels of LDL-C in their patients. In the U.S., for example, organizations such as the National Cholesterol Education Program (NCEP), the American Heart Association, and the American College of Cardiology have emphasized aggressive management of LDL-C. NCEP guidelines currently recommend that patients at high risk of experiencing a heart attack achieve LDL-C levels of 100 mg/dL or lower through lifestyle changes and drug therapy as appropriate based on their starting levels. International guidelines for adult patients at high risk of experiencing a heart attack, such as those published in the International Journal of Cardiology and the Canadian Journal of Cardiology, and guidelines published in the EHJ in 2014 (2014 EHJ HoFH Guidelines) that are specific to HoFH support LDL-C treatment targets for such patients as low as 70 mg/dL or lower. The American College of Cardiology and the American Heart Association released guidelines in 2013 for patients at high risk of cardiovascular disease caused by atherosclerosis that are focused first on lifestyle changes and statin therapy. The 2014 EHJ HoFH Guidelines made similar recommendations regarding lifestyle changes and statin therapy for the treatment of HoFH and also recommended the use of LDL apheresis, in which cholesterol is removed from the body through mechanical filtration, and the use of other adjunctive treatments, such as lomitapide and mipomersen, for HoFH patients who are within the indication for such products (adults for lomitapide). More recent guidance, such as “The Agenda for Familial Hypercholesterolemia: A Scientific Statement from the American Heart Association” in 2015, added PCSK9 inhibitor treatment for HoFH patients as a recommended treatment. In February 2017, the American Association of Clinical Endocrinologists and American College of Endocrinology published guidelines for management of dyslipidemia and prevention of atherosclerosis. Patients in this “extreme risk” category, including men aged 55 years and younger and women aged 65 years and younger who have established cardiovascular disease accompanied by familial hypercholesterolemia, have an LDL-C goal of <55 mg/dL.
The clinical approach taken with HoFH patients has typically involved an aggressive treatment plan to reduce lipid levels as much as possible through dietary modifications and a combination of available lipid lowering drug therapies. Conventional drug therapies include statins, cholesterol absorption inhibitors and bile acid sequestrants. Less frequently, other drugs, such as niacin and fibrates, have been added to provide some incremental reductions in LDL-C levels, although these agents are typically used to modify mostly lipids other than LDL-C. Because many of these therapies, including statins, act by increasing the activity of LDL-R, HoFH patients, given their impaired LDL-R function, or lack of function, often have an inadequate response to standard therapies. For example, high dose statin therapies that typically produce 46% to 55% reductions in LDL-C levels in the broad hypercholesterolemic patient population, on average, produce a 10% to 25% reduction in patients with HoFH. Patients with HoFH who are unable to reach their recommended target LDL-C levels on drug therapy are sometimes treated using LDL apheresis. Although levels of LDL-C are reduced acutely using apheresis, there is a rapid rebound (usually after approximately four days). Because apheresis provides only temporary reductions in LDL-C levels, it must be repeated frequently. However, typically it is performed one or two times per month. In addition, except in many countries in the EU, apheresis is not readily available, particularly in the U.S., due to the limited number of treatment centers that perform this procedure.
Status in the U.S. and European Union
In December 2012, the FDA approved JUXTAPID as an adjunct to a low-fat diet and other lipid-lowering treatments, including LDL apheresis where available, to reduce LDL-C, total cholesterol, apoliporotein B (apo B) and non-high-density lipoprotein cholesterol (non-HDL-C) in adult patients with HoFH. Our subsidiary, Aegerion, launched JUXTAPID in the U.S. in January 2013. The FDA has granted seven years of orphan drug exclusivity from the date of approval for JUXTAPID in the U.S. in the treatment of HoFH, expiring in December 2019. The U.S. prescribing information for JUXTAPID specifies that the safety and effectiveness of lomitapide have not been established in patients with hypercholesterolemia who do not have HoFH, including those with HeFH, or in pediatric patients, and that the effect of lomitapide on cardiovascular morbidity and mortality has not been determined.
In July 2013, Aegerion received marketing authorization for LOJUXTA in the EU as an adjunct to a low-fat diet and other lipid-lowering medicinal products with or without LDL apheresis in adult patients with HoFH. Despite the prevalence rate, lomitapide does not have orphan medicinal product exclusivity in the EU for the treatment of HoFH because the EMA views the relevant condition, for orphan drug purposes, to include both HoFH and HeFH. The Summary of Product Characteristics (SmPC) approved by the EC for LOJUXTA describes that genetic confirmation of HoFH should be obtained whenever possible, and that other forms of primary hyperlipoproteinemia and secondary causes of hypercholesterolemia (e.g., nephrotic syndrome, hypothyroidism) must be excluded. The SmPC also specifies that the effect of lomitapide on cardiovascular morbidity and mortality has not been determined. As a result of difficulty in obtaining pricing and reimbursement approvals from governmental authorities in key markets of the EU and in an effort to minimize operating expenses required to support EMA post-marketing requirements, Aegerion elected to cease commercialization in the EU and, in December 2016, entered into a license agreement with Amryt under which Amryt was granted an exclusive right to develop and commercialize LOJUXTA in the European Economic Area (EEA), Switzerland, Turkey and certain Middle Eastern and North African territories, including Israel. Under the license agreement, Aegerion maintains the marketing authorizations for LOJUXTA; however, Amryt is responsible for ongoing regulatory and post-marketing obligations and commitments for LOJUXTA. Amryt is also required to pay Aegerion certain sales-related milestone payments and royalties on net product sales in the licensed territories.
13
The prescribing information for lomitapide in the U.S. and the EU warns physicians that lomitapide can cause hepatotoxicity as manifested by elevations in transaminases and increases in hepatic fat, and that physicians are recommended to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and total bilirubin before initiating treatment and then to measure ALT and AST regularly during treatment. During the first year of treatment, physicians must conduct a liver-related test prior to each increase in the dose of lomitapide or monthly, whichever occurs first. After the first year, physicians are required to perform these tests every three months and before increases in dose. The prescribing information in the EU provides further recommendations for monitoring for hepatic steatohepatitis/fibrosis and the risk of progressive liver disease, including annual imaging for tissue elasticity, and measuring of biomarkers and/or scoring methods in consultation with a hepatologist.
Because of the risk of hepatotoxicity, JUXTAPID is available in the U.S. only through a REMS, referred to as the JUXTAPID REMS program. Under the JUXTAPID REMS program, patients must receive education on the JUXTAPID REMS program requirements and we must certify all qualified healthcare providers before they can prescribe JUXTAPID and the pharmacies that will dispense the medicine. The FDA assesses on a periodic basis whether a REMS program is meeting its goals and whether the goals or elements of the program should be modified. In June 2015, Aegerion received a letter from the FDA expressing concern that the JUXTAPID REMS program is not meeting its goals of educating healthcare professionals about the risks of hepatotoxicity and the need to periodically conduct liver tests to monitor patients during treatment with JUXTAPID as set forth on the product label. The letter also expressed concern about the difficulty in assessing whether the goal of restricting access to JUXTAPID to patients with a clinical or laboratory diagnosis consistent with HoFH was being met. In response to the FDA’s concerns, we proposed to the FDA modifications to the JUXTAPID REMS program to improve prescriber awareness of the risk of hepatotoxicity associated with JUXTAPID and the need to monitor patients during treatment, and to reinforce the approved indication and the characteristics of HoFH. On March 11, 2016, Aegerion received from the FDA an additional letter describing certain modifications the FDA considered necessary to the labeling for JUXTAPID and to the JUXTAPID REMS program. In response to the FDA’s proposed modifications to the labeling for JUXTAPID, on April 8, 2016, Aegerion submitted a prior approval labeling supplement to the FDA addressing certain of the FDA’s proposed modifications, including an instruction that patients cease therapy upon the occurrence of severe diarrhea. The labeling changes were approved by the FDA on May 23, 2016. Aegerion submitted a response to the FDA’s proposal regarding modifications to the JUXTAPID REMS program in a prior approval supplement on July 7, 2016. The FDA approved the modifications to the JUXTAPID REMS program on January 3, 2017. The goal of the JUXTAPID REMS program, as modified, is to mitigate the risk of hepatotoxicity associated with the use of JUXTAPID by ensuring that: a) prescribers are educated about the approved indication for JUXTAPID, the risk of hepatotoxicity associated with the use of JUXTAPID, and the need to monitor patients during treatment with JUXTAPID as per product labeling; b) JUXTAPID is dispensed only to patients with a clinical or laboratory diagnosis consistent with HoFH; and c) patients are informed about the risk of hepatotoxicity associated with the use of JUXTAPID and the need for baseline and periodic monitoring. The FDA’s approval letter for the modified REMS program also specified that an authorized generic drug under JUXTAPID’s NDA must have an FDA-approved REMS program prior to marketing.
The originally approved JUXTAPID REMS program consisted of Elements To Assure Safe Use (ETASU), an implementation system, a communication plan and a timetable for submission of assessments of the JUXTAPID REMS program. It also required healthcare professionals who prescribe JUXTAPID and pharmacies that dispense JUXTAPID to be certified, and that JUXTAPID must only be dispensed to patients with evidence or other documentation of safe-use conditions. The ETASU in the modified JUXTAPID REMS program approved by the FDA on January 3, 2017 has been significantly enhanced and requires, among other things, that healthcare professionals and pharmacies complete a recertification process, which includes, for healthcare professionals, required online training and learning assessments by July 2, 2017, in order to continue prescribing and dispensing JUXTAPID; healthcare professionals counsel existing and new JUXTAPID patients on the goals of the JUXTAPID REMS program and, in connection therewith, imposes a new requirement that healthcare professionals and their patients sign a form acknowledging that this counseling has taken place and that the patient understands the goals of the JUXTAPID REMS program; and prescriptions written to a JUXTAPID patient before the healthcare professional completes recertification and the counseling requirements with the patient, including the submission to the REMS coordinating center of an acknowledgment form signed by the healthcare professional and the patient, will not be honored after July 2, 2017. The modified REMS program also requires that prescriptions written after July 2, 2017 must be written on an updated prescription authorization form and includes changes to existing REMS documentation, along with additional required documentation, and new training modules for healthcare professionals and certified pharmacies. The FDA required the modifications to the JUXTAPID REMS program to be implemented by March 2, 2017, and, as noted above, that healthcare professionals and pharmacies complete the recertification process, and healthcare professionals and patients complete the counseling and acknowledgment processes, by July 2, 2017. We have completed the implementation of the modifications to the JUXTAPID REMS program, and we are in the process of educating healthcare professionals, pharmacies, and patients about the JUXTAPID REMS program requirements, including the requirements that must be met by July 2, 2017, and tracking achievement with respect to these requirements. However, we may lose JUXTAPID patients temporarily or permanently, or add new adult HoFH patients at a slower than expected pace, as a result of the implementation of, and enhancements to, the modified JUXTAPID REMS program, as described above, for a variety of reasons, including: the inability to recertify healthcare professionals with existing patients or to certify healthcare professionals who may want to put new patients on
14
JUXTAPID, on a timely basis or at all; the failure of the healthcare professionals and patients, existing or new, to meet the patient counseling requirements and sign and submit the patient acknowledgment form, as required, on a timely basis or at all; the failure of prescriptions for JUXTAPID to meet all of the requirements of the modified JUXTAPID REMS program on or after July 2, 2017 and therefore not being honored by the certified pharmacies after such date, as required under the modified JUXTAPID REMS program, and any payer issues or delays that arise out of new prescriptions being written for patients under the modified JUXTAPID REMS program; and that the enhanced education of the goals of the JUXTAPID REMS program, and related documentation, may cause healthcare professionals to stop or delay treatment with JUXTAPID, or try alternative therapies for adult HoFH patients before starting or continuing JUXTAPID treatment. The ongoing investigations of the SEC and the DOJ, including the consent decree that Aegerion will enter into with FDA related to JUXTAPID REMS program as part of the settlement of these investigations, may also have an effect on the FDA’s requirements for the JUXTAPID REMS program.
Similarly, in the EU, we have adopted risk management plans to help educate physicians on the safety information for LOJUXTA and appropriate precautions to be followed by healthcare professionals and patients.
Status outside the U.S. and the EU
In September 2016, JUXTAPID was approved by the Ministry of Health, Labor and Welfare (MHLW) in Japan for patients with HoFH. Approval in Japan was based on a Phase 3 study we conducted to evaluate the safety and efficacy of JUXTAPID to reduce LDL-C levels in nine adult Japanese HoFH patients. The results of the Phase 3 study were consistent with the known safety and efficacy profile of JUXTAPID. On November 17, 2016, the MHLW approved pricing of JUXTAPID and in December 2016 we launched JUXTAPID in Japan. HoFH is listed as an intractable disease in Japan, and as part of that designation, reimbursement is mandated and patients register with the government to receive comprehensive treatment benefits, including apheresis. According to the 2014 MHLW Japanese Intractable Diseases Information Centers Listing, there are approximately 160 patients registered as diagnosed with HoFH in Japan. JUXTAPID has received orphan drug designation in the treatment of HoFH from the MHLW, which provides ten years of exclusivity.
Lomitapide has also been approved as an adjunct treatment for adult patients with HoFH in other countries outside the U.S. and EU, including Colombia, Mexico, Canada, Israel, Norway, Iceland, Liechtenstein, Taiwan and South Korea. In 2016, we withdrew lomitapide from Mexico and Taiwan, and on February 22, 2017, we withdrew the marketing authorization for lomitapide in South Korea. INVIMA, the regulatory agency responsible for reviewing marketing authorization applications in Colombia, has also granted JUXTAPID five years of post-approval data exclusivity. The indications and prescribing information, including risk information, for lomitapide in these countries are generally comparable to those in the U.S.
Lomitapide is subject to risk management plans in each country in which it is approved outside the U.S. and the EU, except Israel, and such plans require the approval of regulatory authorities prior to reimbursement approval and marketing. The goal of the risk management plans is to help educate physicians on the safety information for lomitapide and appropriate precautions to be followed by healthcare professionals and patients.
We have also filed for marketing approval in Argentina and may file for marketing approval in other countries where, in light of the potential size of the market and other relevant commercial and regulatory factors, it makes business sense to do so. To obtain marketing approval and commercialize JUXTAPID where approved, we must establish, and comply with, numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials, pricing, promotion and distribution of the respective product.
We are also making lomitapide available in certain countries that allow use of a drug, on a named patient basis or under a compassionate use or other type of so-called expanded access program, before marketing approval has been obtained in such country. We charge for lomitapide for authorized pre-approval uses in some of the countries where it is available under an expanded access program, to the extent permitted by applicable law and local regulatory authorities. In 2016, the substantial majority of our revenues from named patient sales of lomitapide were derived from orders from Brazil, where patients have the right to bring legal action through the judicial system to seek access to unapproved drugs for which there are no therapeutic alternatives. We are also generating, or expect to generate, revenues from sales of lomitapide in several other countries on a named patient sales basis in the near-term. In some countries, including Brazil, orders for lomitapide on a named patient sales basis are for multiple patients and multiple months of therapy. We expect net product sales from named patient sales of lomitapide to fluctuate quarter-over-quarter significantly more than sales in the U.S., as a result of the types of orders and unpredictable ordering patterns, government actions, including the ongoing investigations in Brazil, media coverage, economic pressures and political unrest. In addition, a proceeding is currently pending with the Brazil Supreme Federal Court to decide whether the government has an obligation to continue to provide, on a named patient sales basis, drugs that have not received regulatory and/or pricing and reimbursement approval in Brazil, like JUXTAPID and MYALEPT. We intend to file for marketing approval in Brazil for both JUXTAPID and MYALEPT, and are currently assessing the timing of these submissions. The result of the trial and other issues could significantly negatively affect product revenues from named patient sales of JUXTAPID and MYALEPT in Brazil. In certain countries where we charge for lomitapide during the pre-approval phase, we are able to establish the price for lomitapide, while in other countries
15
we need to negotiate the price. In other countries or under certain circumstances, we are providing lomitapide free of charge for permitted pre-approval uses and to the extent permitted by applicable law and local regulatory agencies.
Clinical Development and Post-Marketing Commitments
As part of our post-marketing commitments to the FDA for lomitapide, we completed a juvenile toxicology study in rodents to ascertain the impact, if any, of lomitapide on growth and development prior to initiating a clinical study of lomitapide in pediatric HoFH patients, and have submitted the results of this study to the FDA. In the first quarter of 2015, the FDA issued a Written Request for a study to evaluate lomitapide in pediatric HoFH patients, which, if completed as described, would provide for six months of pediatric exclusivity under the Federal Food, Drug, and Cosmetic Act (FDCA). In the second quarter of 2015, Aegerion decided to decline the FDA’s Written Request regarding its planned study in pediatric HoFH patients, because it believed that the size and complexity of the requested trial created a considerable barrier to the feasibility of the study. Given that we have declined to conduct the study requested by the FDA, we will not be entitled to the six months of additional exclusivity available for conducting a study that is the subject of a Written Request issued by the FDA.
As part of Aegerion’s post-marketing commitments to both the FDA and the EMA for lomitapide, we initiated an observational cohort study in 2014 to generate additional data on the long-term safety profile of lomitapide, the patterns of use and compliance and the long-term effectiveness of lomitapide in controlling LDL-C levels. Our commitment to the FDA is to target enrollment of 300 HoFH patients worldwide, and to study enrolled patients for a period of ten years. The EMA has required that all patients taking lomitapide in the EU be encouraged to participate in the study, and that the study period be open-ended. In connection with the license agreement with Amryt in December 2016, Amryt agreed to bear the costs of conducting this study in the EEA and other relevant territories. In the study, investigators will follow each patient to track malignancies, tumors, teratogenicity, hepatic effects, and gastrointestinal (GI) adverse reactions, events associated with coagulopathy, major adverse cardiovascular events and death. The EMA also required that a vascular imaging study be conducted to determine the impact of lomitapide on vascular endpoints, which Aegerion initiated in 2014 and is now the responsibility of Amryt pursuant to our license agreement with Amryt. In addition, we have completed certain drug-drug interactions studies and submitted the results to the EMA.
Phase 3 Clinical Study (HoFH)
Our Phase 3 clinical study of lomitapide evaluated the safety and effectiveness of lomitapide to reduce LDL-C levels in 29 adult patients with HoFH. The study was a multinational, single-arm, open-label, 78-week trial.
In the Phase 3 study, each patient’s background lipid-lowering therapies were stabilized during a six-week run-in phase prior to dosing, and were maintained through at least the end of the 26-week efficacy phase. All patients received dietary counseling and were instructed to consume a diet containing <20% of energy from total dietary fat. Lomitapide was initiated at a dose of 5 mg daily and gradually escalated to doses of 10 mg, 20 mg, 40 mg, up to 60 mg daily, based on tolerability and acceptable liver enzyme levels. When added to the existing lipid-lowering therapy of the HoFH patients in the study, lomitapide reduced LDL-C by an average of 40% at week 26 in the intent-to-treat population with last observation carried forward for the patients who discontinued prematurely, and reduced LDL-C by an average of 50% for the 23 patients who completed the study through week 26.
Also, approximately 65% of all patients completing the study experienced LDL-C reductions of 50% to 93% from their baseline as measured at the end of week 26. After week 26, during the 52-week safety phase of the study, adjustments to concomitant lipid-lowering treatments were allowed. Average reductions in LDL-C were sustained during chronic therapy.
The most common adverse reactions in the Phase 3 study were gastrointestinal, reported by 27 (93%) of 29 patients. Adverse reactions, reported by greater than or equal to 8 patients (28%) in the HoFH clinical trial, included diarrhea, nausea, vomiting, dyspepsia and abdominal pain. Other common adverse reactions, reported by five to seven (17-24%) patients, included weight loss, abdominal discomfort, abdominal distension, constipation, flatulence, increased ALT, chest pain, influenza, nasopharyngitis, and fatigue. Elevations in liver enzymes and hepatic (liver) fat were also observed. Ten of the 29 patients in the study had at least one elevation in liver enzymes greater than or equal to three times the upper limit of normal (ULN), including four patients who experienced liver enzymes greater than or equal to five times the ULN. During the clinical trial, liver enzyme elevations were managed through dose reduction or temporary discontinuation of dose. There were no clinically meaningful elevations of total bilirubin, international normalized ratio (INR) or alkaline phosphatase, which are other markers of potential harmful effects on the liver. Hepatic fat increased from a baseline of 1% to a median absolute increase of 6% at 26 and 78 weeks.
Nineteen of 23 patients who completed the 78-week pivotal study entered a Phase 3 long-term extension study, and continued lomitapide at their individualized maintenance dose, with 17 (89%) completing 126 weeks of treatment. The primary efficacy endpoint of the extension study was mean percent change in LDL-C from the patient’s baseline, measured at the start of the original pivotal trial, to week 126. In the extension study, mean LDL-C levels were reduced by 45.5% from baseline at week 126. Similar mean percent reductions were observed for apo B, non-HDL-C, and total cholesterol.
16
The adverse reaction profile observed in the extension study was consistent with that observed during the pivotal trial. Gastrointestinal symptoms were the most common adverse reaction, reported in 63% (12/19) of patients in the extension study. Transient aminotransferase elevations (ALT or AST) >= 3x ULN occurred in nine of the patients who completed week 126 of the extension study either in the pivotal phase or the extension phase or both, including five patients who had elevations >= 5x ULN. Of these patients, one patient had an ALT elevation of 24x ULN that was reversible with temporary suspension of lomitapide, and a second patient had a reversible ALT elevation of >= 10-20x ULN following co-administration of other drugs that may precipitate liver injury or interact with lomitapide. One patient who used excessive alcohol was withdrawn from the extension study due to persistent ALT elevations >= 5x ULN. No Hy’s law cases were reported. One sudden cardiac death occurred in a 58 year old patient with known coronary artery disease. Median hepatic fat levels (measured by nuclear magnetic resonance spectroscopy) were 0.7% at baseline and 6.5% at entry to the extension phase and remained stable during approximately 2.5 years of further follow-up (median 7.7% (range 0.6 to 35.2)).
Estimated Prevalence of HoFH
There is no patient registry or other method of establishing with precision the actual number of patients with HoFH in any geography. Medical literature has historically reported the prevalence rate of HoFH as one person in a million, based on an estimated prevalence rate for HeFH of one person in 500. Analysis of HoFH prevalence have been evolving in recent years cumulating in published medical literature that suggests that the actual prevalence of both HeFH and HoFH may be significantly higher than the historical estimate of one person in a million. For example, in 2014, the European Atherosclerosis Society (EAS) Consensus Panel on Familial Hypercholesterolaemia (FH) published an article citing research that would result in an estimate of the prevalence of HoFH in the range of between one person in 300,000 and one person in 160,000 or 3.33 persons per million to 6.25 persons per million, which is consistent with estimates that can be derived from other publications from the last few years. The FDA cited this estimate in its review of PCSK9 inhibitor products in June 2015. There is no guarantee that the prevalence of HoFH is higher than the current medical literature suggests or is even higher than reported in the historical literature. The number of patients with HoFH could actually be significantly lower than we expect. Ultimately the actual size of the total addressable HoFH market in the U.S. will be determined only after we and others have substantial commercial history selling products for the treatment of HoFH.
We believe that the prevalence rate of HoFH in countries outside the U.S. is likely to be consistent with the prevalence rate in the U.S.; however, we expect that our net product sales in countries outside the U.S. are likely to be lower than in the U.S. given significant economic pressures to reduce healthcare costs in certain ex-U.S. countries, resulting in pricing controls, reimbursement restrictions and caps on patients treated and/or drug expenditures, the more widespread availability of apheresis, in certain countries, like Japan, and the possibility that genotyping may be required in some countries, reducing the number of patients diagnosed with HoFH.
Commercialization and Patient Support
We market and sell MYALEPT and JUXTAPID through our Aegerion subsidiary. We believe that the key priorities for the successful commercialization of our products in the countries in which we have received marketing approval, and the preparation for commercialization in the countries in which our products may be approved include:
• | commercializing MYALEPT and JUXTAPID in the U.S. with a new commercialization strategy featuring, among other things, the use of a small contract sales force and analysis of claims data and other information to help identify potential GL and adult HoFH patients; and reorganizing and realigning our sales organization in support of key centers of excellence for both MYALEPT and JUXTAPID; |
• | stabilizing sales of JUXTAPID as a treatment for adult HoFH patients in the U.S. despite competition from PCSK9 inhibitor products, among other factors, which have had a significant adverse impact on sales of JUXTAPID; |
• | educating and training healthcare providers about our products and the diseases they are approved to treat; |
• | continuing to support patient access to and reimbursement for our products in the U.S. without significant restrictions, particularly given the availability of PCSK9 inhibitor products in the U.S., which has adversely impacted reimbursement of JUXTAPID, and given the considerable number of JUXTAPID patients in the U.S. who are on Medicare Part D and the significant percentage of such patients who may not be able to afford their out-of-pocket co-payments for our products, given that the only source of financial support for some such patients may be through patient assistance programs operated by independent charitable 501(c)(3) organizations that may not provide adequate financial assistance; |
• | obtaining pricing and reimbursement approval for metreleptin on acceptable terms and price levels if it is approved in the EU and other countries outside the U.S., and for lomitapide in countries outside the U.S. where it is or becomes approved; and |
17
• | minimizing the number of patients who, although eligible to receive treatment with our products, decide not to commence such treatment, or who discontinue treatment, through activities such as patient support programs, to the extent permitted in a particular country. |
Our commercial initiatives are designed to support these priorities. We believe that it is possible to commercialize our products in the U.S. and other select markets with a relatively small specialty sales force. As part of a broad cost-reduction plan to significantly reduce operating expenses and extend our cash position as the availability of competitive therapies continues to impact lomitapide sales in the U.S., approximately 116 positions were eliminated from Aegerion’s workforce in 2016, including significant reductions in the U.S. sales force and related functions, such as marketing and sales operations.
In connection with the cost-reduction plan, the significant decline in JUXTAPID sales, and an acknowledgment that there is a lack of widespread medical awareness of the diseases that our products are intended to treat, we undertook a re-evaluation of our commercial strategy. We have defined and implemented a new global strategy to achieve our commercial objectives for metreleptin and lomitapide in approved markets in a cost-effective manner. Our new commercial strategy involves a reorganization and realignment of our sales organization, which includes the use of small contract sales forces in the U.S. and Japan. The principal goals of our commercial strategy are to grow sales of MYALEPT in the U.S. and to stabilize and support current and future sales of JUXTAPID, as well as prepare for launch in other jurisdictions in which we have submitted for approval of our products, such as MYALEPTA in the EU, if approved for sale.
As noted above, our commercial organization in the U.S. includes a small, recently hired contract sales force, experienced in marketing drugs for the treatment of rare disorders or endocrinology indications. This contract sales force works with our sales management to educate and train healthcare providers who treat GL and adult HoFH patients about the safety and efficacy of MYALEPT or JUXTAPID, as applicable. The most frequent physician call points for our products are endocrinologists, lipidologists and cardiologists. In addition to reorganizing our sales force, we have implemented the use of de-identified claims data to more precisely target physicians who may have treated patients with GL or adult HoFH.
Outside the U.S., we mainly use country managers to market and sell our products where they are approved, and plan to hire similar employees in other key countries as business needs dictate. In Japan, we sell JUXTAPID through a small contract sales force. The rights to commercialize LOJUXTA in the EU and certain other jurisdictions were out-licensed to Amryt in December 2016. In certain other countries outside the U.S., we have engaged, or plan to engage local distributors to conduct permitted commercial and pre-approval activities.
We also have an in-house global marketing team that, along with third-party contractors, supports our commercialization and disease awareness efforts in the countries in which our products are approved, and permitted educational and disease awareness activities in other parts of the world.
Another key aspect of our commercialization efforts is obtaining market access for our products, which primarily represents securing pricing and reimbursement approvals on acceptable levels, without the imposition of significant restrictions, such as caps, significant step edits or other similar measures, from private and government payers where our products are approved. In the U.S., we have a small U.S. market access team, which is primarily responsible for working with insurance plans, health maintenance organizations and other payers on securing reimbursement and formulary status for MYALEPT and JUXTAPID. Outside the U.S., we support this effort through the work of global asset teams and country managers. One of our main market access objectives, which is conducted in conjunction with our medical and health economics teams, is to strengthen the value proposition for MYALEPT and JUXTAPID for payers through the generation of critical market access studies to enhance patient, physician and payer knowledge of GL and HoFH and the real-world burden of these diseases.
We believe the pricing for our products in the U.S. is consistent with the level of pricing for other ultra-orphan drugs that treat diseases with comparable prevalence rates. The majority of payers in the U.S. are providing coverage for our products, and with respect to JUXTAPID, most payers in the U.S. have not required genotyping. Many payers in the U.S. have, however, imposed requirements, conditions or limitations as conditions to coverage and reimbursement for JUXTAPID as a result of the commercial availability of PCSK9 inhibitor products, which often includes a requirement that HoFH patients have not achieved an adequate LDL-C response on PCSK9 inhibitor products before access to lomitapide is approved. For patients currently taking JUXTAPID, several U.S. pharmacy benefit managers (PBMs) are using prior authorization requiring current JUXTAPID patients to “step through” the less expensive PCSK9 inhibitor product, and additional PBMs and payers may follow this practice. We have been engaging with PBMs to discuss and negotiate potential agreements to limit these so-called “step edits”, which may require us to provide discounts and other price protections and would impact the net revenues from JUXTAPID. One of the key goals of our U.S. market access team is to work with key payers to try to reach contractual terms to address these and similar issues. For MYALEPT, some U.S. payers require additional information such as a leptin level test for patients, which may delay or otherwise impact reimbursement. The cost of MYALEPT and JUXTAPID in the U.S. may result in co-pay amounts for some patients that are prohibitive, and prevent these patients from being able to commence therapy on MYALEPT or JUXTAPID, respectively. We provide support to eligible commercial patients for certain drug co-pays and co-insurance obligations for MYALEPT treatment.
18
We also have a direct co-pay assistance program that provides support to eligible commercial patients for certain drug co-pays and co-insurance obligations for JUXTAPID. From time to time, we make donations to support patient assistance programs operated by independent charitable 501(c)(3) organizations in the U.S. that assist eligible GL and adult HoFH patients, as determined solely by the organization, with certain co-payments or co-insurance requirements for their drug therapies, which may include metreleptin or lomitapide. We do not have control or input into the decisions of these organizations. We believe that investigations and enforcement actions by certain government agencies, however, may have caused a reduction in contributions to these third-party patient organizations, which may prevent these organizations from providing adequate financial assistance, including assistance with co-payment obligations, to individuals who would otherwise be unable to afford our products. A considerable number of JUXTAPID patients in the U.S. are Medicare Part D patients and a significant percentage of such patients may not be able to afford their out-of-pocket co-payments for JUXTAPID, which could result in such patients seeking an alternative free drug or ceasing treatment with our products, given that the only source of financial support for such patients may be through patient assistance programs operated by independent charitable 501(c)(3) organizations that may not provide adequate financial assistance, due to reductions in contributions to such organizations.
A comprehensive patient support program for MYALEPT in the U.S. is provided through our specialty pharmacy, which includes educational resources about MYALEPT and GL; insurance verification and reimbursement support; disease education; monitoring and support of adherence; injection training; providing patients with information about potential sources of financial assistance; and a free drug program for certain eligible uninsured and underinsured patients. A similar program for patients who have been prescribed JUXTAPID, providing comparable education, adherence and insurance verification services as those described above, plus nutritional counseling, is provided by a small internal team of customer care managers. These customer care managers are supported in their efforts to provide support to JUXTAPID patients by a small internal team of reimbursement case managers and field-based clinical nurse or dietitian educators.
Medical Affairs
We have a medical affairs function in the U.S., the EU and certain other countries which supports independent medical education programs and investigator-initiated studies by providing financial grants in a number of medical and disease-related areas. The responsibilities of medical affairs personnel also include assisting in organizing scientific and medical advisory boards to obtain input from experts and practitioners on a variety of medical topics relevant to our products and the diseases our products treat; providing training; providing education to physicians through the dissemination of medical information and publications; and providing support in connection with our post-approval clinical commitments. We are in the process of rebuilding the global medical affairs department to help facilitate execution of our strategic plans.
Significant Customers
For the year ended December 31, 2016, one customer accounted for 34.5% of Aegerion’s net product sales, and such customer accounted for 28.5% of our accounts receivable balance.
Products in Development
In addition to the lifecycle management initiatives described above, our research and development efforts are also focused on our product candidate, zuretinol acetate (zuretinol, formerly known as QLT091001). Zuretinol is an orally administered synthetic retinoid replacement for 11-cis-retinal, which is a key biochemical component of the visual retinoid cycle.
Zuretinol for IRD caused by RPE65 and LRAT Gene Mutations
We are currently developing zuretinol for the treatment of IRD caused by RPE65 and LRAT gene mutations, and we intend to develop zuretinol for this indication, which includes LCA and RP, in adult and pediatric subjects.
IRD caused by RPE65 and LRAT Gene Mutations (LCA and RP)
IRDs are clinically and genetically heterogeneous diseases caused by 261 known gene mutations. LCA and RP are inherited, progressive, retinal degenerative diseases that arise from genetic mutations of enzymes or proteins required in the biochemistry of vision. There are no FDA or EMA approved pharmacologic therapeutic treatments for LCA or RP.
LCA is characterized by abnormalities such as roving eye movements and sensitivity to light, and manifests in severe vision loss from birth. Both rod and cone photoreceptors are affected in LCA. Eye examinations of infants with LCA reveal normal appearing retinas; however, low level of retinal activity, measured by electroretinography, indicates very little visual function. RP is a set of hereditary retinal diseases demonstrating clinical features similar to LCA. RP is also characterized by degeneration of rod and cone photoreceptors, but it presents with a more variable loss of vision in late childhood to adulthood. Deficits in dark adaptation and peripheral vision are particular hallmarks of RP. LCA and RP diseases result from genetic mutations, including RPE65 or
19
LRAT, which result in an inadequate production of 11- cis-retinal, an essential component of the visual retinoid cycle. Zuretinol is a replacement therapy for 11- cis-retinal.
The clinical characteristics and progression of disease in LCA and RP overlap as do some of their genetic causes. At least seven of the known LCA disease genes, including LRAT and RPE65, have also been linked to the clinical appearance of RP. Despite disease heterogeneity and terminology, there is an overlap in the genetic mechanisms underlying some forms of LCA and RP such as those caused by LRAT and RPE65 gene mutations where 11-cis-retinal production is either severely or completely compromised. RP is the most common inherited retinal disease, and is generally the diagnosis given to patients who begin to lose vision after the first decade of life, whereas the diagnosis of LCA is given to patients who have central vision loss soon after birth. There is no universally accepted diagnostic term for patients with characteristics in between; clinicians have considered such cases as either LCA or severe RP. As a result of these factors, we have classified both LCA and RP due to inherited deficiency of RPE65 and LRAT genes as IRD.
Status in the U.S. and EU
Zuretinol has received orphan drug designations for the treatment of LCA (due to inherited mutations in the LRAT and RPE65 genes) and RP (all mutations) by the FDA, and for the treatment of LCA and RP (all mutations) by the EMA. These designations provide market exclusivity for the drug for this use in the applicable jurisdiction after a product is approved for 10 years (possibly subject to reduction) in the EU and seven years in the U.S. Orphan drug designation in the EU can also provide an additional two years of market exclusivity for pediatric orphan drug designated drug products. The FDA has also formally acknowledged that the orphan drug designations granted by the FDA on zuretinol for the treatment of LCA (due to inherited mutations in LRAT or RPE65 genes) and RP (all mutations) also cover zuretinol for the treatment of IRD caused by LRAT or RPE65 gene mutations, including severe early childhood onset retinal dystrophy, which disease/condition we believe comprises both LCA due to inherited mutations in LRAT or RPE65 genes and RP. The EMA also formally acknowledged that a therapeutic indication of zuretinol for the treatment of patients with IRD, who have been phenotypically diagnosed as LCA or RP caused by mutations in RPE65 or LRAT gene, would fall under the orphan drug designations of treatment of LCA and treatment of RP.
Zuretinol has also been granted two Fast Track designations by the FDA for the treatment of LCA and autosomal recessive RP due to mutations in LRAT and RPE65 genes. The FDA has also acknowledged that our two Fast Track designations encompass the treatment of IRD caused by LRAT or RPE65 gene mutations. The FDA’s Fast Track is a process designed to facilitate the development and expedite the review of drugs that are intended for the treatment of serious or life-threatening diseases or conditions and demonstrate the potential to address an unmet medical need.
In addition to the Fast Track and orphan drug designations previously granted to us by the FDA for zuretinol, we are currently exploring the potential of submitting to the FDA a request for rare pediatric disease designation of zuretinol for the treatment of IRD caused by LRAT or RPE65 gene mutations, which indication includes LCA and RP. In order to obtain a rare pediatric disease designation for zuretinol, we must demonstrate to the FDA’s satisfaction that this indication is for the treatment or prevention of a rare disease or condition that affects fewer than 200,000 individuals in the U.S., or that affects more than 200,000 individuals in the U.S. and there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for this disease or condition will be recovered from sales in the U.S. for that product, and is a serious or life-threatening disease in which the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years. Under the FDCA, a sponsor who receives approval of an NDA for a rare pediatric disease and meets certain additional criteria, may qualify for a rare pediatric disease priority review voucher (PRV). A PRV can be redeemed to receive priority review under an expedited timeframe for a subsequent marketing application for a different product. A PRV may also be sold or transferred from the initial sponsor to another sponsor. The voucher may be further transferred any number of times before it is used. Pursuant to the 21st Century Cures Act, FDA’s authority to award rare pediatric disease PRVs has been extended until 2020, and until 2022 for products that receive rare pediatric disease designation by 2020.
The molecule in zuretinol is not eligible for composition of matter protection per se, as it was previously known in the scientific community. However, upon FDA approval, we believe that the active pharmaceutical ingredient in zuretinol may qualify as a new chemical entity, or NCE, which provides for five years of exclusivity following approval. This five-year period of market exclusivity would run concurrently with the seven year period of orphan drug exclusivity period that we expect to receive should zuretinol be approved by the FDA. We intend to seek NCE exclusivity; however, there is no assurance that zuretinol will qualify and gain the five-year exclusivity period, even if zuretinol is approved. We also plan to secure regulatory exclusivity for zuretinol in the EU; however, there can be no assurance that we will be successful in securing approval or regulatory exclusivity in the EU.
Clinical Studies and Compassionate Use
Phase 1b studies. We have completed a Phase 1b multi-center, open-label clinical proof-of-concept trial to evaluate the safety profile and effects of a single seven-day course of treatment with zuretinol on various parameters of retinal function and quality of life in patients with LCA and autosomal recessive RP due to inherited mutations in RPE65 or LRAT genes.
20
We have also completed a Phase 1b multi-center, open-label clinical trial of repeated treatments of zuretinol in patients with LCA and autosomal recessive RP due to inherited mutations in RPE65 or LRAT genes. In this study, patients that were treated with a single course of zuretinol in our previously completed Phase 1b clinical trial received up to three 7-day courses of zuretinol to assess visual outcomes and safety following retreatment. Visual field (VF) was assessed using Goldman Visual Field (GVF) and visual acuity (VA) was assessed using best-corrected visual acuity (BCVA) at baseline and days 7, 14, 30 and 60 after each treatment course, then bimonthly until the next treatment course. Patients received treatment with doses of 10, 40 or 60 mg/m2, with the majority of patients receiving 40 mg/m2.
Results of the Phase 1b retreatment study reported in September 2014 showed clinically meaningful improvements in VF and VA. Nineteen of 27 patients (70%) had an increase in VF retinal area from baseline of at least ³ 20% in at least 1 eye at 2 consecutive visits within 6 months from the start of any zuretinol treatment course. In addition, 70% of patients had an increase in VA from baseline of at least ³ 5 letters in at least 1 eye at 2 consecutive visits within 6 months from the start of any zuretinol treatment course. The percentage of VF and VA responders identified by disease and mutation is summarized below. Over the course of the entire study spanning multiple treatment courses, these responses were durable, with the visual field response lasting an average of 235 days (min-max = 7 - 742 days), and the visual acuity response lasting an average of 232 days (min-max = 7 - 616 days). From the cumulative data, mean GVF improvement over baseline throughout the study time period following a single, seven-day dosing period was 117% in LCA patients and 30% for RP patients.
Table: Results for Visual Field and Visual Acuity Responders
N | Visual Field Responders(a) Number (%) of Patients | Visual Acuity Responders (b) Number (%) of Patients | ||
All Patients | 27 | 19 (70%) | 19 (70%) | |
All LCA | 13 | 7 (54%) | 10 (77%) | |
All RP | 14 | 12 (86%) | 9 (64%) | |
All RPE65 | 15 | 11 (73%) | 8 (53%) | |
All LRAT | 12 | 8 (67%) | 11 (92%) | |
a: >20% change in retinal area from baseline at 2 consecutive visits in at least 1 eye within 6 months of any treatment course.
b: >5 letter increase from baseline at 2 consecutive visits in at least 1 eye within 6 months of any treatment course.
Adverse events reported in the trial were consistent with the retinoid class of medications and were transient and/or reversible. One serious adverse drug reaction (intracranial hypertension (ICH), a known class effect of retinoids), was reported in the study and it was resolved.
Natural History Study. In 2016, we completed a retrospective, uncontrolled, multicenter, case history study to determine the natural history of visual function in subjects with IRD phenotypically diagnosed as LCA or RP caused by autosomal recessive mutations in RPE65 or LRAT (IRD). The objective of the natural history study was to gather data on the natural progression of the disease in subjects over time, including from childhood to adulthood. The study included both subjects who had previously received treatment in clinical trials with our synthetic oral retinoid product, zuretinol, as well as subjects who had not received prior treatment with zuretinol. For subjects who had received prior treatment with zuretinol, the study included a retrospective review of subjects’ medical records prior to and after treatment with zuretinol. The intention of the study was to assess and compare visual outcomes in patients treated with zuretinol, relative to the treatment of naïve patients, in order to assess the extent to which zuretinol may improve or prolong visual function relative to the natural disease progression in these subjects. The preliminary analysis of the study data suggests that IRD subjects, without therapeutic intervention, demonstrate a continuing decline in VF and eventually VA over time. Final analysis of the study data is ongoing and expected to be completed in the first half of 2017.
Compassionate Use Program. We provide zuretinol to patients under our compassionate use program on a named-patient basis. Under the compassionate use program, zuretinol may be made available to patients who participated in our completed Phase 1b clinical trial of zuretinol for the treatment of LCA and RP.
Estimated Prevalence of LCA and RP
Given the very low prevalence in these ultra-orphan diseases, there is limited epidemiological data available to determine definitively the potential patient population for treatment with zuretinol, and as such, there is significant uncertainty around the estimated total potential addressable patient population worldwide. Current epidemiological estimates based on medical literature suggest that approximately 2.5% of the autosomal recessive RP subjects and 6-10% of all LCA subjects possess mutations in the RPE65 gene, while approximately 1% of RP and LCA subjects have mutations in the LRAT gene. LCA is estimated to affect
21
approximately one in 81,000 newborns worldwide, while the overall RP prevalence is estimated at one in 4,000 newborns worldwide. Based on our current market research, we estimate the total potential addressable LCA patient population for zuretinol at 1,000 to 2,000 patients worldwide and the total potential addressable RP patient population at 2,000 to 4,000 patients worldwide. Our most recent epidemiological data estimate the prevalence of IRD subjects with RPE65 or LRAT mutations at 4100 patients in the U.S. and the five major European markets, a portion of whom have the late stage of the disease and may not benefit from zuretinol therapy. While geographic differences in the gene pool may cause fluctuations, the prevalence of LCA, RP, and LRAT and RPE65 mutation distribution in other countries are believed to be comparable with the U.S. and five major European markets based on current medical literature. However, there is no guarantee these current estimates are correct, and the analysis of the prevalence in IRD remains ongoing in the medical literature.
Our estimates of the total potential addressable patient population are based on our current market research, including estimated diagnosis rates. The uncertainty around the estimated total potential addressable patient population is exacerbated by the fact that the course and progression of photoreceptor dysfunction and degeneration in the retina over time varies considerably between individuals with IRD, and the rate and progression of decline of vision function in patients is currently not well understood. Over the course of the disease, we believe that IRD patients who do not retain a sufficient number of functionally viable photoreceptors may not benefit from treatment with zuretinol.
Zuretinol Development Status
Following completion of our Phase 1b studies of zuretinol, we have engaged in discussions with the FDA and EMA to determine the regulatory pathway and optimal clinical trial protocol study design for potential registration trials for zuretinol. We continue to undertake clinical and development activities towards future clinical studies of zuretinol. In parallel, we are currently conducting a review of our regulatory and clinical pathway for the zuretinol program, taking into account the current competitive and regulatory landscape. We are scheduled to meet with the FDA in May 2017 to further discuss our development plans, and also intend to request a meeting with the EMA to discuss our program following our meeting with the FDA. We expect to provide an update in mid-2017.
Manufacturing, Supply and Distribution
We and our contract manufacturers are subject to the FDA’s current Good Manufacturing Practices (cGMP) regulations and other rules and regulations prescribed by regulatory authorities outside the U.S.
Metreleptin is a recombinant protein biologic that is produced using conventional fermentation, isolation, and purification processing techniques. The drug product is provided in nominal 10 mg vials that are reconstituted prior to injection. We are considering development of new presentations in nominal 2.5 mg and 5 mg vials. Lomitapide is a small molecule drug that is synthesized with readily available raw materials using conventional chemical processes. Hard gelatin capsules are prepared in 5 mg, 10 mg, 20 mg, 30 mg, 40 mg and 60 mg strengths.
We currently have no manufacturing facilities, and limited personnel with manufacturing experience. We rely on contract manufacturers to produce drug substance for metreleptin and lomitapide and to produce drug product for commercial supplies and for our clinical trials. Aegerion has long-term supply agreements with metreleptin drug substance and drug product manufacturers, which were assigned to Aegerion in connection with the acquisition of metreleptin in 2015. In February 2017, the contract manufacturer for metreleptin drug product received a Warning Letter from the FDA citing significant violations of current good manufacturing practice (CGMP) regulations at the manufacturing facility where metreleptin drug product is manufactured. In response, the manufacturer may make modifications to the line on which metreleptin drug product is filled, and has committed, in the long-term, to transition the filling of certain drug products, including metreleptin drug product, to a newer line at the same facility and to cooperating with customers on a transition timeline to re-validate the filling process on the new line, such that this transition does not impact supply. Assuming a reasonable timeline for the future transition to and validation of the new filling line, we would have sufficient inventory to handle any downtime in the manufacturing process. Aegerion also has long-term supply agreements with lomitapide drug substance and drug product manufacturers. We have sufficient inventory of metreleptin and lomitapide drug substance to maintain a supply for more than one year. We do not have any other agreements in place for redundant supply or a second source for drug substance or drug product for either product.
In the U.S., metreleptin and lomitapide are each distributed through a single specialty pharmacy that distributes the product directly to patients and, under limited circumstances, to other purchasers. The specialty pharmacy that distributes MYALEPT takes title upon delivery of MYALEPT to such pharmacy. The specialty pharmacy that distributes JUXTAPID does not take title to JUXTAPID. Title transfers upon delivery of JUXTAPID to the purchaser. Both of our specialty pharmacies also provide certain patient program support services, accounts receivables and other order-to-cash services on our behalf. For named patient sales and other expanded access distribution, we use third-party providers to distribute our products either directly to the purchaser in the applicable country or to our local third-party distributor or service provider for such country.
22
In connection with our development of zuretinol, we utilize a small number of qualified third-party contractors, currently located in North America and Europe, to manufacture and supply key raw materials, active pharmaceutical ingredient and drug product. Key raw materials in the production of the active pharmaceutical ingredient and drug product can be sourced from multiple vendors.
Financial Information about Segments and Geographic Areas
We operate in one segment. Financial information about this segment required herein is contained in the “Consolidated Financial Statements and Supplementary Data” section of this Annual Report, and the geographic information required herein is contained in Note 19 - Segmentation Information in the Notes to the Consolidated Financial Statements and is incorporated by reference herein.
Competition
Our industry is highly competitive and subject to rapid and significant technological change. Our competitors and potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. All of these competitors currently engage in, have engaged in or may engage in the future in the development, manufacturing, marketing and commercialization of pharmaceuticals that compete with or may in the future compete with lomitapide, metreleptin, zuretinol or other products or product candidates we may develop or acquire. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Academic institutions, government agencies and other public and private research organizations also are conducting research activities, seeking patent protection and may commercialize products on their own or through joint ventures. The existence of these products, or other products or treatments of which we are not aware, or products or treatments that may be developed in the future, may adversely affect the marketability of products developed by us. Key competitive factors affecting the commercial success of lomitapide, metreleptin, zuretinol and any other products or product candidates that we develop or acquire include efficacy, safety and tolerability profile, reliability, physician acceptance, level of generic competition, convenience of dosing, price and reimbursement.
MYALEPT is the first and only product approved in the U.S. for the treatment of complications of leptin deficiency in patients with GL. There are, however, a number of therapies approved to treat these complications independently that are not specific to GL. Certain clinical complications of GL, including diabetes and hypertriglyceridemia, may be treated with insulin and/or oral medications, such as metformin, insulin secretagogues, fibrates, or statins. Patients with GL often have an inadequate response to these therapies.
We are seeking regulatory approval of metreleptin as a treatment for GL and a PL subset in the EU and intend to seek regulatory approval of metreleptin for that PL subset in the U.S. and in other key countries where it makes business sense to do so. We are aware of an investigational drug currently in development by Akcea Therapeutics (Akcea), a subsidiary of Ionis Pharmaceuticals, Inc. (Ionis), being studied in patients with familial PL with diagnosed type 2 diabetes mellitus or hypertriglyceridemia, which, if approved and depending on the labeled indication, could potentially compete with metreleptin, if metreleptin is approved for the treatment of a subset of PL which comprises a portion of familial PL.
The market for cholesterol-lowering therapeutics is large and competitive with many drug classes. Lomitapide is approved in the U.S., Japan, the EU and in certain other countries as an adjunct to a low-fat diet and other lipid-lowering treatments to reduce LDL-C in adult HoFH patients. As a treatment for HoFH, JUXTAPID competes in the U.S. and certain other countries with Kynamro. Developed by Ionis and acquired by Ionis and Kastle Therapeutics in May 2016, Kynamro is an antisense apolipoprotein B-100 inhibitor which is taken as a weekly subcutaneous injection. JUXTAPID also faces significant competition in the treatment of adult HoFH patients with a class of drugs known as PCSK9 inhibitors. In July 2015, Regeneron Pharmaceuticals, Inc. (Regeneron) and Sanofi announced that the FDA had approved the BLA for their PCSK9 inhibitor candidate, alirocumab, for use in addition to diet and maximally tolerated statin therapy in adult HeFH patients and in patients with clinical atherosclerotic cardiovascular disease who require additional lowering of LDL-C. In September 2015, following the positive opinion of the Committee for Medicinal Products for Human Use (CHMP) of the EMA, the EC approved alirocumab for the treatment of adult patients with HeFH or mixed dyslipidemia as an adjunct to diet, either in combination with a statin, or statin with other lipid-lowering therapies in patients unable to reach their LDL-cholesterol goals with the maximally-tolerated statin, or alone or in combination with other lipid-lowering therapies for patients who are statin intolerant, or for whom a statin is contraindicated. The FDA approved Amgen Inc.’s (Amgen) BLA for its anti-PCSK9 antibody, evolocumab, in August 2015, as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with HeFH or clinical atherosclerotic cardiovascular disease, who require additional lowering of LDL-C; and as an adjunct to diet and other LDL-lowering therapies for the treatment of patients with HoFH, who require additional lowering of LDL-C. In July 2015, the EC Commission approved the marketing authorization of evolocumab for the same indication as alirocumab, and for the treatment for certain patients with high cholesterol, including patients aged 12 years and over with HoFH in combination with other lipid-lowering therapies. In January 2016, the MHLW approved evolocumab for the treatment of patients with FH or hypercholesterolemia who have high risk of cardiovascular events and do not adequately
23
respond to statins, and in July 2016 the MHLW approved alirocumab for the same indication. Health Canada and ANVISA, the regulatory agency responsible for reviewing marketing authorization applications in Brazil, have approved evolocumab for the treatment of patients with HoFH. Other companies, including Roche Holding AG and Alnylam Pharmaceuticals, Inc., in collaboration with The Medicines Company, are also developing PCSK9 inhibitor products.
The introduction of PCSK9 inhibitors in the U.S. has negatively impacted sales of JUXTAPID and we expect this negative trend to continue. This impact results from several factors, including: healthcare professionals switching some existing JUXTAPID patients to a PCSK9 inhibitor product; healthcare professionals trying most new adult HoFH patients on a PCSK9 inhibitor product before trying JUXTAPID; the provision of free PCSK9 drug to adult HoFH patients by the companies that are commercializing PCSK9 inhibitor products, which such companies may have ceased, but which historically has had a negative impact on the rate at which new patients start treatment on JUXTAPID and has caused more patients than we expected to discontinue JUXTAPID and switch their treatment to PCSK9 inhibitor products; and actions by insurance companies, managed care organizations and other private payers in the U.S. that have required, or may require in the future, HoFH patients to demonstrate an inability to achieve an adequate LDL-C response on PCSK9 inhibitor products before access to JUXTAPID is approved, or may impose other hurdles to access or other significant restrictions or limitations on reimbursement, or may require switching of JUXTAPID patients to PCSK9 inhibitor products. Many U.S. insurance companies, managed care organizations and other private payers now require that HoFH patients fail to achieve an adequate response in LDL-C reduction on PCSK9 inhibitor products before providing reimbursement for JUXTAPID. For patients currently taking JUXTAPID, we believe that all U.S. payers require prior authorization, which may influence a switch of the current JUXTAPID patients to the less expensive PCSK9 inhibitor product. We believe that many of the PCSK9 inhibitor switches from current JUXTAPID patients have been at the direction of the prescribing physician. Ultimately, the physician may decide to switch the adult HoFH patient back to JUXTAPID, if the patient does not reach a goal of LDL-C response while being treated with a PCSK9 inhibitor product. It is unknown how many adult HoFH patients, if any, may be switched back to JUXTAPID or the period of time in which this would take place. We expect physicians will continue to consider use of JUXTAPID for those adult HoFH patients who do not adequately respond to PCSK9 inhibitor products. Additionally, we expect that the availability of PCSK9 inhibitor products in commercial markets outside of the U.S., will have similarly negative effects on sales, including named patient sales, of lomitapide outside the U.S., particularly in Brazil, Canada and Japan, where PCSK9 inhibitor products have been approved by the regulatory authorities. If the continued negative impact of PCSK9 inhibitors is greater than we expect, it may make it more difficult for us to generate revenues and achieve profitability. Also, although there are no other MTP-I compounds currently approved by the FDA for the treatment of hyperlipidemia in humans, there may be other MTP-I compounds in development.
In addition, in the EU, patients with HoFH who are unable to reach their recommended target LDL-C levels on conventionally used drug therapies are commonly treated using LDL apheresis, in which cholesterol is removed from the body through mechanical filtration. Although levels of LDL-C are reduced acutely using apheresis, there is a rapid rebound. Because apheresis provides only temporary reductions in LDL-C levels, it must be repeated frequently, typically one or two times per month. The widespread use and availability of apheresis as a treatment for HoFH in the EU, combined with the lower cost of apheresis as compared to LOJUXTA, made it more difficult for us to obtain commercially acceptable pricing and reimbursement approvals for LOJUXTA in the key markets of the EU. As a result of this, and in an effort to reduce costs associated with EMA post-marketing requirements, Aegerion elected to cease commercialization in the EU, and in December 2016, entered into a license agreement with Amryt under which Amryt was granted an exclusive right to develop and commercialize LOJUXTA in the European Economic Area, Switzerland, Turkey, and certain Middle Eastern and North African countries, including Israel.
We may also face future competition from companies selling generic alternatives of lomitapide or metreleptin in countries where we do not have patent coverage, orphan drug status or another form of data or marketing exclusivity or where patent coverage or data or marketing exclusivity has expired, is not enforced, or may, in the future, be challenged.
Inherited retinal diseases are clinically and genetically heterogeneous diseases caused by 261 known gene mutations. Zuretinol is currently under investigation for the treatment of IRD caused by RPE65 or LRAT genetic mutations, which includes LCA and RP. Both RPE65 and LRAT genes are essential for the production of 11-cis-retinal in the visual retinoid cycle to enable vision. Zuretinol may potentially serve to replace 11-cis-retinal in patients with either RPE65 or LRAT gene mutations manifested clinically as LCA2, LCA14, RP20, or RP Juvenile. Spark Therapeutics, Inc.’s (Spark) product candidate, SPK-RPE65 (voretigene neparvovec), is a form of RPE65 gene therapy targeting treatment of inherited retinal disease caused by mutations in the RPE65 gene, manifested clinically as either LCA2 or RP20. Spark has completed Phase 3 trials with SPK-RPE65 and has publicly disclosed that it has initiated a rolling BLA submission with the FDA which is expected to be completed in 2017, and expects to file an MAA with the EMA thereafter. Spark recently released four year data from its Phase 1 trial suggesting an extended duration of action and efficacy in bilateral mobility testing (MT) and full field sensitivity threshold testing (FST) correlated to VF improvement. SPK-RPE65 has received breakthrough therapy and orphan drug designations from the FDA and orphan drug designation from the EMA. In addition, we are aware of a number of early stage gene therapy and optogenetic approaches in development for LCA patients. We are also aware of a retinal implant (Argus ® II) developed by Second Sight Medical Products Inc. (Second Sight) to treat late stage RP,
24
which received FDA approval under a Humanitarian Device Exemption in February 2013, as well as two implantable medical devices for RP that are approved in the European market: Argus ® II (Second Sight) and Alpha IMS (Retina Implant AG).
We may also face future competition from companies selling generic alternatives of our products in countries where we do not have patent coverage, orphan drug status or another form of data or marketing exclusivity or where patent coverage or data or marketing exclusivity has expired or may, in the future, be challenged.
Many of our competitors and potential competitors have substantially greater financial, technical and human resources than we do, which is exacerbated by several factors related to our business, including the negative impact of PCSK9 inhibitor products on the JUXTAPID business, recent reductions in force, and the uncertainty about the timing and magnitude of the financial and other aspects of the resolution of Aegerion’s ongoing DOJ and SEC investigations. Many of these companies also have significantly greater experience in the discovery and development of drug candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, competitors may be more successful than we may be in commercialization, obtaining marketing approvals for drugs and achieving and maintaining widespread market acceptance.
Patents, Trademarks and Proprietary Rights
Our business relies on patents covering inventions licensed from third parties, and on other means to protect our technology, inventions and improvements that are commercially important to our business. Our policy is to file patent applications on a worldwide basis in those jurisdictions where we consider it beneficial, depending on the subject matter and our commercialization strategy.
Our success will depend significantly on our ability to:
• | obtain and maintain patent and other proprietary protection for the products, technology, inventions and improvements we consider important to our business; |
• | defend our patents; |
• | preserve the confidentiality of our trade secrets; and |
• | operate without infringing the patents and proprietary rights of third parties. |
Patent Rights
Lomitapide. Our lomitapide patent portfolio consists of seven issued U.S. patents and issued patents in Europe, Australia, South Korea, New Zealand and Japan and pending applications in the U.S., Japan, Canada, and India, all of which have been licensed to us in a specific field. A five-year patent term extension has been granted for our U.S. patent covering the composition of matter of lomitapide, originally scheduled to expire in early 2015, and will now expire in 2020. The non-U.S. patents directed to the composition of matter of lomitapide have expired. Our five method of use patents in the U.S. cover certain dosing regimens for lomitapide, with one such patent expiring in 2027 and the other four patents expiring in 2025. Two separate inter partes review (IPR) petitions were filed with the Patent Trial and Appeal Board (PTAB) of the U.S. Patent and Trademark Office in August 2015 by the Coalition for Affordable Drugs VIII L.L.C. (CFAD) challenging the validity of two of these method of use patents. On March 6, 2017, the PTAB determined that the CFAD failed to show that the claims of these patents were unpatentable. Our non-U.S. patents, including the European Patent Office (EPO) methods of use patent, expire in 2025. The EPO method of use patents may be eligible for up to three years of supplemental protection in certain EPO countries, and we are seeking such protection in the countries in which LOJUXTA is approved, on a country-by-country basis. An opposition was filed with respect to the EPO method of use patent, but has since been revoked.
Since December 21, 2016, an ANDA or 505(b)(2) NDA may be submitted for JUXTAPID if it contains a Paragraph IV certification of patent invalidity or non-infringement. If we instigate a suit against an ANDA or 505(b)(2) applicant for patent infringement within 45 days of receiving a Paragraph IV notice, the FDA is prohibited from approving the ANDA or 505(b)(2) application for a period of 30 months. If the notice is given and suit filed between December 21, 2016 and December 21, 2017, the 30-month stay does not begin until December 21, 2017. The FDA may approve the proposed competitor product before the expiration of the 30-month stay if a court finds our patents invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
Moreover, if one or more ANDA filers were to receive approval to sell a generic or follow-on version of JUXTAPID, those competitor products could potentially be marketed as early as December 21, 2019 (the date on which JUXTAPID’s orphan drug exclusivity ends) and JUXTAPID would become subject to increased competition at that time. The FDA’s approval letter for the modified JUXTAPID REMS program, received on January 3, 2017, specified that an authorized generic drug under the JUXTAPID NDA must have an FDA-approved REMS program prior to marketing.
Metreleptin. Our metreleptin patent portfolio consists of three issued U.S. patents and issued patents in Europe, Canada, Israel, Australia, New Zealand, Mexico, China, South Korea and Japan, all of which have been licensed to us. The U.S. patent covering
25
the composition of matter of metreleptin was scheduled to expire in 2016, but an interim extension has been granted extending the term for one year until a final determination of a request for patent term extension is made. The non-U.S. patents directed to the composition of matter of metreleptin have expired. The patent family covering metreleptin methods of use, directed to treating human lipoatrophy, is co-owned by Amgen, University of Texas and the National Institutes of Health, and is licensed to us from Amgen. We are in discussions with one of the co-owners to obtain the co-owner’s consent to the sublicense granted by Amgen, and to in-license the co-owners’ rights. We do not have a direct license from this co-owner. The two method of use patents in the U.S. expire in 2022 and 2023, and the non-U.S. patents issued in certain European countries, Canada, and Australia, and pending in Japan, expire in 2022. An application for a patent term extension in the U.S. with respect to MYALEPT has been filed which, if granted, will be applied to either the U.S. composition of matter patent or the method of use patent, to extend one of these patents by 1,206 days. Also, metreleptin qualifies for 12-year biologic exclusivity under the Biologics Price Competition and Innovation Act (the BPCI Act), which will expire in 2026. If approved by the EMA, metreleptin would be entitled to 10 years of market exclusivity in the EU.
Zuretinol. Our zuretinol patent portfolio consists of six issued U.S. patents, and issued patents in Europe, Japan, Canada, and other countries, as well as pending patent applications in countries including the U.S. and Europe. These patents and patent applications relate to zuretinol pharmaceutical compositions and uses thereof, including methods of using of zuretinol for the treatment of LCA and RP, and expire between 2025 and 2032. Certain zuretinol patent families are owned by the University of Washington, which has licensed the patents and patent applications to Retinagenix LLC (Retinagenix), and are exclusively sub-licensed to us by Retinagenix.
Other Patents, Trademarks and Proprietary Rights
In addition to patent protection, we also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in our product areas.
We require our potential business collaborators, clinical investigators, sponsored researchers, employees and consultants who might have access to or be provided with proprietary information to sign confidentiality agreements.
We have included information about risks and uncertainties relating to protection of our proprietary information in the “Risk Factors” section of this Annual Report.
We or Aegerion own registered trademarks including NOVELION, MYALEPT, JUXTAPID, MYALEPTA, and LOJUXTA in the U.S., EU and in other jurisdictions.
Licensing
Metreleptin
Amgen Inc.
In connection with Aegerion’s acquisition of MYALEPT in January 2015, Aegerion acquired a license agreement between Amgen and Amylin Pharmaceuticals, Inc., dated February 7, 2006 (the Amgen License) pursuant to which Aegerion obtained an exclusive worldwide license from Amgen to certain know-how and patents and patent applications covering the composition of matter and methods of use of metreleptin to develop, manufacture and commercialize a preparation containing metreleptin (the Amgen Licensed Products).
As part of the Amgen License, Aegerion also obtained an exclusive sublicense of Amgen’s exclusive rights to certain metreleptin-related patents and patent applications owned by the Rockefeller University and exclusively licensed to Amgen under a license agreement dated April 14, 1995, as amended (the Rockefeller License) and an exclusive sublicense of Amgen’s non-exclusive rights to certain metreleptin-related patents and patent applications owned by The Regents of the University of California and non-exclusively licensed to Amgen under a license agreement dated July 13, 2005 (the UCSF License). Amgen retains rights to conduct research, development, manufacturing and commercialization activities with respect to products other than the Amgen Licensed Products.
Aegerion may grant sublicenses under the licenses and sublicenses granted by Amgen, subject to certain limitations, including Amgen’s right of first offer for any out-license, partnership, co-development, commercialization, co-promotion or similar agreement related to metreleptin or the Amgen Licensed Products, which expires in February 2021. Under this license agreement, Amgen must notify Aegerion of any potential third-party partnership regarding any intellectual property rights controlled by Amgen in the neurology field and Aegerion will have a right of first negotiation for any license, partnership, co-development, commercialization, co-promotion or similar agreement, which expires in February 2021.
Aegerion is required to make royalty payments to Amgen, Rockefeller University and BMS on net sales of each Amgen Licensed Product on a country-by-country basis (i) at a royalty rate in the low double digits where the Amgen Licensed Product has patent
26
protection or market exclusivity granted by a regulatory authority at the time of regulatory approval in the applicable country during the applicable royalty term, which runs on a country-by-country basis until the later of (a) the expiration of the last-to-expire valid claim covering an Amgen Licensed Product in the applicable country, (b) expiration of any market exclusivity granted by a regulatory authority, and (c) ten years from the date on which an Amgen Licensed Product is first sold to a third party in a country after regulatory approval for the Amgen Licensed Product has been granted in such country (Amgen Royalty Term) or (ii) at a royalty rate in the mid-single digits to low double digits where the Amgen Licensed Product receives patent protection or market exclusivity following the time of regulatory approval in the applicable country, in either case subject to a variety of customary reductions.
Under the Amgen License, Aegerion is also required to directly meet certain payment obligations under the Rockefeller License and UCSF License. Aegerion is required to make royalty payments to Rockefeller University on net sales of each product with patent rights or know-how in the field of obesity genes, obesity gene products, and molecules that modulate or mediate their action and/or regulation on a country-by-country basis at a range of royalty rates in the low single digits depending on whether the product has an orphan product designation or not until the later to occur of expiration of (i) patent protection, (ii) any market exclusivity period granted in the applicable country, or (iii) any data exclusivity period in the applicable country (with certain limitations related to the number of units sold). Since acquiring this license agreement in January 2015, Aegerion has paid a one-time $5.0 million milestone payment to Rockefeller in February 2015, which was due twelve months following the receipt of marketing approval for MYALEPT in the U.S. Aegerion will also be required to pay to Rockefeller University a percentage in the low double digits of any upfront license fees or one-time fees Aegerion receives in consideration for a sublicense of the licensed rights. There are no material payment obligations outstanding under the UCSF License.
The Amgen License will terminate upon the expiration of the last Amgen Royalty Term for any Amgen Licensed Product. Aegerion has the right to terminate the Amgen License for convenience upon 90 days prior written notice to Amgen or for Amgen’s uncured material breach of the Amgen License, or becoming subject to specified bankruptcy or liquidation events. Amgen may terminate the Amgen License for Aegerion’s uncured failure to make payments to Amgen or if Aegerion is the subject of specified bankruptcy or liquidation events.
Aegerion made royalty payments to Amgen related to the sales of MYALEPT through November 29, 2016. There were no royalty payments made to Amgen from November 30, 2016 to December 31, 2016. We had $1.2 million remaining balance in royalties payable as of December 31, 2016.
Shionogi & Co., Ltd.
In connection with Aegerion’s acquisition of MYALEPT in January 2015, Aegerion acquired a license agreement between Shionogi and Amylin Pharmaceuticals, Inc., dated July 8, 2009 pursuant to which Shionogi was granted an exclusive sublicense to the patent rights licensed under the Amgen License and the Rockefeller License to develop and commercialize the Amgen Licensed Products and know-how for use in the treatment of lipodystrophy in humans in Japan, South Korea and Taiwan (the Shionogi Territory). This license agreement does not provide Shionogi with manufacturing rights. Shionogi may grant further sublicenses under the license, subject to certain limitations.
The license agreement requires that Shionogi use commercially reasonable efforts to develop, obtain regulatory approvals for, and commercialize the Amgen Licensed Products in the Shionogi Territory. Shionogi is required to make royalty payments to Aegerion on net sales of each Amgen Licensed Product at a range of royalty rates in the mid-to high-single digits dependent on the amount of net sales. Shionogi made royalty payments to Aegerion related to sales of MYALEPT in Japan through November 29, 2016. During the period from November 30, 2016 to December 31, 2016, Aegerion did not receive any royalty payments from Shionogi. Shionogi will be required to make milestone payments to Aegerion of up to an aggregate of approximately $25.0 million if and when Shionogi achieves certain commercialization milestones. Such milestone payments are payable only once. Under the license agreement, Shionogi has also agreed to directly comply with the payment obligations under the Rockefeller License and Amgen License, as set forth under those agreements, relating to its activities under this license agreement.
The license agreement with Shionogi will terminate upon the expiration of the last Amgen Royalty Term for any Amgen Licensed Product with respect to which Shionogi has a license under this license agreement. Aegerion has the right to terminate this license agreement for Shionogi’s uncured material breach of the license agreement, failure to make any payment due to Aegerion, a procedural default, or becoming subject to specified bankruptcy or liquidation events. Shionogi may terminate this license agreement for Aegerion’s uncured material breach of this license agreement, failure to make payments due to Shionogi, or if Aegerion is the subject of specified bankruptcy or liquidation events, or if Shionogi determines it is not feasible to develop, launch or sell the Amgen Licensed Products due to scientific, technical, regulatory or commercial reasons. Aegerion may also terminate this license agreement at any time without cause by exercising our buy-back option for a one-time fee to Shionogi equal to (i) a number in the low single digits times the amount of expenses and fees incurred by Shionogi in developing the Amgen Licensed Products plus (ii) an amount no more than a number in the mid-double digits times monthly net sales of the Amgen Licensed Products by Shionogi in the month the option is exercised.
27
Lomitapide
University of Pennsylvania
In May 2006, Aegerion entered into a license agreement with The Trustees of the University of Pennsylvania, (UPenn) pursuant to which Aegerion obtained an exclusive, worldwide license from UPenn to certain know-how and a range of patent rights applicable to lomitapide. In particular, Aegerion obtained a license to certain patents and patent applications owned by UPenn relating to the dosing of MTP-I compounds, including lomitapide, and certain patents and patent applications and know-how covering the composition of matter of lomitapide that were assigned to UPenn by Bristol-Myers Squibb Company (BMS) for use either as a monotherapy or with other dyslipidemic therapies to treat patients with HoFH. Aegerion also has the right to use lomitapide in the field of monotherapy or in combination with other dyslipidemic therapies for treatment of patients with other severe forms of hypercholesterolemia unable to come within 15% of NCEP’s LDL-C goal on maximal tolerated oral therapy, as determined by the patient’s prescribing physician, or with severe combined hyperlipidemia unable to come within 15% of NCEP’s non-HDL-C goal on maximal tolerated oral therapy, as determined by the patient’s prescribing physician, or with severe hypertriglyceridemia unable to reduce TG <1,000 on maximal tolerated therapy. We refer to the patents and patent applications assigned by BMS to UPenn and licensed to Aegerion by UPenn as the BMS-UPenn assigned patents.
To the extent that rights under the BMS-UPenn assigned patents were not licensed to Aegerion under our license agreement with UPenn or were retained by UPenn for non-commercial education and research purposes, those rights, other than with respect to lomitapide, were licensed by UPenn back to BMS on an exclusive basis pursuant to a technology donation agreement between UPenn and BMS. In the technology donation agreement, BMS agreed not to develop or commercialize any compound, including lomitapide, covered by the composition of matter patents included in the BMS-UPenn assigned patents in the field licensed to Aegerion exclusively by UPenn. Through our license with UPenn, as provided in the technology donation agreement, we have the exclusive right with respect to the BMS-UPenn assigned patents regarding their enforcement and prosecution in the field licensed exclusively to Aegerion by UPenn.
The license from UPenn covers, among other things, the development and commercialization of lomitapide alone or in combination with other active ingredients in the licensed field. The license is subject to customary non-commercial rights retained by UPenn for non-commercial educational and research purposes. Aegerion may grant sublicenses under the license, subject to certain limitations. Aegerion is required to make royalty payments to UPenn at a range of royalty rates in the high single digits on net sales of lomitapide in countries where lomitapide has patent protection, and of any other products covered by the license (subject to a variety of customary reductions), and share with UPenn specified percentages of sublicensing royalties and certain other consideration that we receive under any sublicenses that we may grant. Aegerion made royalty payments to UPenn through November 29, 2016. There were no royalty payments made to UPenn during the period from November 30, 2016 to December 31, 2016. We had $1.3 million remaining balance in royalties payable to UPenn as of December 31, 2016. Aegerion will be required to make development milestone payments to UPenn of up to an aggregate of approximately $2.6 million if we decide to develop lomitapide for indications within the licensed field other than HoFH, and we achieve certain milestones in such development efforts. All such development milestone payments for these other indications are payable only once, no matter how many licensed products for these other indications are developed.
The license agreement with UPenn will remain in effect on a country-by-country basis until the expiration of the last-to-expire licensed patent right covering the product in the applicable country. Aegerion has the right to terminate this license agreement for convenience upon 60 days prior written notice to UPenn or for UPenn’s uncured material breach of the license agreement. UPenn may terminate this license agreement for Aegerion’s uncured material breach of the license agreement, uncured failure to make payments to UPenn or if Aegerion is the subject of specified bankruptcy or liquidation events.
Zuretinol
Retinagenix LLC
Under the terms of a co-development agreement (the Retinagenix Agreement) we entered into with Retinagenix in April 2006, we obtained an exclusive, worldwide license and sub-license under certain intellectual property rights owned by Retinagenix or licensed to Retinagenix by the University of Washington, related to zuretinol, the synthetic retinoid compound under development. We have been responsible for using commercially reasonable and diligent efforts to develop and commercialize, in certain major markets and other markets as we reasonably determine, one or more products covered by the licensed rights or developed using such licensed rights for use in diagnosing, treating or preventing certain human diseases and conditions. We are also responsible for committing certain annual funding to support research and development of such products. Under the license agreement between Retinagenix and the University of Washington (the UW Agreement), Retinagenix has similar obligations, and is required to meet specific development milestones within certain timeframes, one of which was required to be achieved by December 31, 2016. However, the UW Agreement contains provisions for extensions of those dates in certain circumstances. Based on the terms of the Retinagenix Agreement and the UW Agreement, and our significant development clinical spend on the zuretinol program, we
28
believe that we are entitled to an extension of that milestone date until December 31, 2017, and that we may be entitled to certain additional extensions to December 31, 2019, along with a potential additional extension of up to 12 months should enrollment in a planned trial be delayed, provided that we continue to comply with the relevant provisions of the license agreements and expend certain minimum amounts on the development of zuretinol. However, it is possible that we may not be able to achieve the specified development milestone by December 31, 2019. As a result, we and Retinagenix have begun discussing a renegotiation of that milestone with the University of Washington. We are currently conducting a review of the zuretinol development program, the results of which will assist us in determining when we believe that the remaining development milestone can be expected to be achieved.
Pursuant to the Retinagenix Agreement, Retinagenix is eligible to receive the following milestone payments: (i) $1.0 million upon initiation of the first pivotal trial for the first target indication which uses such products, (ii) $1.5 million upon completion of a filing seeking EU approval or Japan approval for the use of such products in the first indication and (iii) up to a total of an additional $10.0 million upon the achievement of other specified development or regulatory milestones and, for each of up to two additional indications, up to a total of $9.0 million upon achievement of specified development or regulatory milestones. If we commercialize such products, we will also pay Retinagenix royalties of between 4% and 6% of net sales, subject to reduction under certain specified circumstances. Retinagenix is also eligible to receive up to a total of $15.0 million upon achievement of specified cumulative sales milestones for such products. The term of the Retinagenix Agreement expires on the later of the expiration of 10 years after first commercial sale of licensed products, or the expiration, lapse or abandonment of all licensed patents. Retinagenix can terminate the agreement earlier if we fail in any material respect to meet our diligence requirements, and we may terminate the agreement for convenience. Each party may terminate the agreement for uncured material breach by the other party.
Government Regulation
Government authorities in the U.S., at the federal, state and local level, the EU, EU Member States, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of lomitapide, metreleptin, zuretinol and other products that we may acquire or develop. Our products must be approved by the FDA through the NDA or the BLA process before they may be legally marketed in the U.S., and must be approved by foreign regulatory authorities via various procedures before they can be marketed in the applicable country, including the EMA or the competent authorities of the EU Member States before they can be placed on the market in the EU.
U.S. Drug and Biologic Development Process
In the U.S., the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act (FDCA) and implementing regulations, and regulates biologics under the Public Health Service Act (PHSA). The process of obtaining marketing approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject a company to administrative or judicial sanctions. These sanctions could include, among other things, the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of enforcement-related letters, product recalls, product seizures, changes to the conditions surrounding marketing approval such as labeling changes or changes to a Risk Evaluation and Mitigation Strategies (REMS) program, total or partial suspension of production or distribution, injunctions, civil money penalties, fines, refusals of government contracts, debarment, restitution, disgorgement of profits, or civil or criminal investigations and penalties.
The process required by the FDA before a drug or biologic may be marketed in the U.S. is extensive and generally involves the following:
• | completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices (GLP) and other applicable regulations; |
• | submission to the FDA of an investigational new drug application (IND), which must become effective before human clinical trials may begin; |
• | performance of human clinical trials, including adequate and well-controlled trials, according to Good Clinical Practices (GCP) to establish the safety and efficacy of the proposed drug for its intended use, or the safety, purity, and potency of a biological product; |
• | approval by an independent Institutional Review Board (IRB), representing each clinical site before each clinical trial may be initiated; |
• | submission to the FDA of an NDA or BLA; |
• | completion of registration batches and validation of the manufacturing process to show that we are capable of consistently producing quality batches of product; |
29
• | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current Good Manufacturing Practice (cGMP) to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
• | FDA review and approval of the NDA or BLA. |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all, and for what indications they will be approved, if any.
Once a pharmaceutical candidate is identified for development it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies, to assess the safety and quality of the product. Animal studies must be performed in compliance with FDA’s GLP regulations and the U.S. Department of Agriculture’s Animal Welfare Act. Human clinical trials cannot commence until an IND application is submitted and becomes effective. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor also will include a protocol detailing, among other things, the objectives of the first phase of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated, if the first phase lends itself to an efficacy evaluation. Preclinical testing may continue after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during studies due to safety concerns or non-compliance, or other reasons.
All clinical trials must be conducted under the supervision of one or more qualified investigators. The conduct of clinical trials is subject to extensive regulation, including FDA’s bioresearch monitoring regulations and GCP requirements, which establish standards for conducting, recording data from, and reporting the results of, clinical trials, and are intended to assure that the data and reported results are credible and accurate, and that the rights, safety, and well-being of study participants are protected. These regulations include the requirement that all research subjects provide informed consent. Further, an IRB must review and approve the plan for any clinical trial before it commences at any institution. An IRB considers, among other things, whether the risks to individuals participating in the trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the information regarding the trial and the consent form that must be provided to each trial subject or his or her legal representative, and continues to provide oversight of the study until it is completed. Additionally, companies sponsoring the clinical trials, investigators, and IRBs also must comply with regulations and guidelines for complying with the protocol and investigational plan, adequately monitoring the clinical trial, and timely reporting of adverse events. Foreign studies conducted under an IND must meet the same requirements that apply to studies being conducted in the U.S. Data from a foreign study not conducted under an IND may be submitted in support of an NDA or BLA if the study was conducted in accordance with GCP and the FDA is able to validate the data.
Each new clinical protocol must be submitted for FDA review, and to the IRBs for approval. Protocols detail, among other things, the objectives of the study, the primary and secondary endpoints of the study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
• | Phase 1. The investigational drug is initially introduced into healthy human subjects, and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing may be conducted in patients with the target diseases. |
• | Phase 2. This phase involves trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
• | Phase 3. This phase involves trials undertaken after preliminary evidence of effectiveness has been obtained and are intended to further evaluate dosage and clinical efficacy and safety of the drug, or the safety, purity, and potency of a biological product, in an expanded patient population, often at geographically dispersed clinical trial sites. These trials are intended to establish the overall risk/benefit ratio of the product, and to provide an adequate basis for product approval and product labeling. |
Progress reports detailing developments associated with the clinical testing program must be submitted at least annually to the FDA, and safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or animal test results that suggest a significant risk to human subjects. Phase 1, Phase 2, and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an
30
IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. Further, success in either preclinical studies or early-stage clinical trials does not assure success in later-stage clinical trials. Sponsors of all controlled clinical trials, except for Phase 1 trials, are required to submit certain clinical trial information for inclusion in the public clinical trial registry and results data bank maintained by the National Institutes of Health, which are publicly available at http://clinicaltrials.gov.
Concurrent with clinical trials, companies usually complete additional studies in non-human models, and must also develop additional information about the chemistry and physical characteristics of the product, and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements.
The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the product, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA generally is subject to the payment of a user fee, although NDAs or BLAs for designated orphan drugs are exempt from this fee.
In addition, under the Pediatric Research Equity Act of 2007 (PREA) an application or supplement to an application must contain data to assess the safety and effectiveness of the drug or biologic for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant deferrals or full or partial waivers for submission of this data. Unless otherwise required by regulation, PREA does not apply to any drug or biologic for an indication for which orphan designation has been granted.
The FDA conducts a preliminary review of all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an application for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. FDA is required to refer an NDA for a new chemical entity (NCE) to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions, or explain why such review is not necessary. Other NDAs or BLAs may also be referred to an advisory committee for evaluation and recommendation. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval or licensure process is lengthy and difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information are submitted, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval or licensure. Data obtained from clinical trials are not always conclusive; and the FDA may interpret data differently than we interpret the same data. The FDA may issue a complete response letter, which generally outlines the deficiencies in the submission and may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the application. The FDA reviews an application to determine, among other things, whether a drug is safe and effective for its intended use, or whether a biologic is safe, pure, and potent, and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. Before approving an NDA or BLA, the FDA will typically inspect the facility or facilities where the product is manufactured. In addition, the FDA often will conduct a bioresearch monitoring inspection of the clinical trial sites involved in conducting pivotal studies to ensure data integrity and compliance with applicable GCP requirements.
Applications receive either standard or priority review. A product representing a major advance in treatment or treatment where no adequate therapy exists may receive priority review. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act (PDUFA), FDA has ten months in which to complete its initial review of a standard new molecular entity (NME) NDA or original BLA and six months for a priority review NME NDA, BLA, or efficacy supplement. FDA does not always meet its PDUFA goal dates and in certain circumstances the PDUFA goal date may be extended. In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and which provide meaningful therapeutic benefit over existing treatments, may receive accelerated approval. In that situation, the product may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, a sponsor of a drug or biologic receiving accelerated approval must perform post-marketing studies to validate the surrogate endpoint or otherwise confirm the effect of the product on a clinical endpoint, and the product may be subject to accelerated withdrawal procedures. Priority review and accelerated approval do not change the standards for approval, but may expedite the approval process for certain products.
31
If a product receives marketing approval, the approval may be significantly limited to specific diseases, dosages or patient populations, or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may impose distribution and use restrictions and other limitations on labeling and communication activities with respect to an approved product via a REMS program, to mitigate serious risks, which could include Medication Guides, patient package inserts, physician communication plans, and/or Elements To Assure Safe Use (ETASU). ETASU may include restricted distribution methods, patient registries and other risk minimization tools. MYALEPT is subject to a REMS program, due to the risks of serious adverse sequelae, as a result of the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or MYALEPT, and the risk of lymphoma. The MYALEPT REMS program aims to educate prescribers about these risks and to restrict access to MYALEPT by requiring prescriber certification, pharmacy certification, and prescriber attestation that each patient has a diagnosis consistent with GL. Because of the risk of liver toxicity, JUXTAPID is also available in the U.S. only through a REMS program, which was modified and approved by the FDA on January 3, 2017. The goal of the modified JUXTAPID REMS program, as discussed earlier in the “Business” section of this Annual Report, is to mitigate the risk of hepatotoxicity associated with the use of JUXTAPID by ensuring that: a) prescribers are educated about the approved indication for JUXTAPID, the risk of hepatotoxicity associated with the use of JUXTAPID, and the need to monitor patients during treatment with JUXTAPID as per product labeling; b) JUXTAPID is dispensed only to patients with a clinical or laboratory diagnosis consistent with homozygous familial hypercholesterolemia; and c) patients are informed about the risk of hepatotoxicity associated with the use of JUXTAPID and the need for baseline and periodic monitoring. The ETASU in the modified JUXTAPID REMS program approved by the FDA on January 3, 2017 has been significantly enhanced and imposes requirements on healthcare professionals, pharmacies, and patients. See the “Business-Lomitapide” section of this Annual Report for further information regarding the modified JUXTAPID REMS program. The REMS programs for each product restrict distribution and sales of our products and impose ongoing implementation requirements that could be burdensome or costly.
The Hatch-Waxman Act, Marketing Exclusivity in the U.S. and Patent Term Restoration
The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, established two abbreviated approval pathways for drug products that are in some way follow-on versions of already approved NDA products.
Generic Drugs. A generic version of an approved drug is approved by means of an Abbreviated New Drug Application (ANDA) through which the sponsor demonstrates that the proposed product is identical or bioequivalent to the approved, brand-name drug, referred to as the Reference Listed Drug (RLD). Generally, an ANDA must contain data and information showing that the proposed generic product and RLD have the same active ingredient(s), in the same strength and dosage form, to be delivered via the same route of administration; are intended for the same uses; have the same labeling; and has been showing through bioequivalence testing to be therapeutically equivalent to the RLD. An ANDA need not independently demonstrate the proposed product’s safety and effectiveness; rather the proposed product’s safety and effectiveness are inferred from the fact that the product is demonstrated to be bioequivalent to the RLD, which the FDA previously found to be safe and effective. These drugs are commonly referred to as “generic equivalents” to the RLD, and they can be substituted by pharmacists under prescriptions written for the RLD.
505(b) (2) NDAs. If a product is similar, but not identical, to an already approved product, it may be submitted for approval via an NDA under FDCA section 505(b) (2). Unlike an ANDA, the sponsor is permitted to rely to some degree on the FDA’s finding that the RLD is safe and effective, but the sponsor must submit its own product-specific safety and effectiveness data to support the differences between the proposed and reference products.
RLD Patents. An NDA sponsor must identify to the FDA any patents that claim the drug substance, drug product or method of using the drug. These patents are among the information about the product that is listed in the FDA publication, Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book). The sponsor of an ANDA or 505(b) (2) application seeking to rely on an approved product as the RLD must make one of several certifications regarding each listed patent. For example, a “Paragraph III” certification is the sponsor’s statement that it will wait for the patent to expire before obtaining approval for its product. A “Paragraph IV” certification is an assertion that the patent does not block approval of the later product, either because the patent is invalid or unenforceable or because the patent, even if valid, is not infringed by the new product. If the ANDA applicant does not challenge the listed patents, the ANDA will not be approved until all the listed patents claiming the referenced product have expired.
Marketing Exclusivities in the U.S.
The Hatch-Waxman Act provides periods of regulatory exclusivity for products that would serve as RLDs for an ANDA, or 505(b) (2) application. If a drug is an NCE, generally meaning that the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance, there is a period of five years from the product’s approval during which the FDA may not accept for filing any ANDA or 505(b) (2) application for a drug with the same active moiety. However, an ANDA or 505(b) (2) NDA may be submitted after four years if it contains a Paragraph IV certification of patent invalidity or non-infringement. According to the Orange Book, JUXTAPID has NCE exclusivity that will expire on December 21, 2017, which means that since December 21, 2016 an ANDA or 505(b) (2) NDA may be submitted for
32
JUXTAPID. We believe that the active pharmaceutical ingredient in zuretinol may qualify as a NCE, in which case it would receive five years of market exclusivity following FDA approval. We intend to seek NCE exclusivity; however, there is no assurance that zuretinol will qualify and gain the five-year exclusivity period, even if zuretinol is approved.
A product that is not an NCE may qualify for three years of marketing exclusivity following approval of a drug product that contains an active moiety that has been previously approved, when the application contains new clinical investigations, other than bioavailability studies, were conducted or sponsored by the applicant and are deemed by the FDA to be essential to the approval of the application, for example, for new indications, strengths or dosage forms of an existing drug. This exclusivity period does not preclude filing or review of an ANDA or 505(b) (2) application; rather, FDA is precluded from granting final approval to the ANDA or 505(b) (2) application until three years after approval of the RLD. In addition, this three-year exclusivity covers only the conditions of use associated with the new clinical investigations and, as a general matter, does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for generic versions of the original, unmodified drug product.
Once the FDA accepts for filing an ANDA or 505(b) (2) application containing a Paragraph IV certification, the applicant must within 20 days provide notice to the RLD NDA holder and patent owner that the application with patent challenge has been submitted, and provide the factual and legal basis for the applicant’s assertion that the patent is invalid or not infringed. If the NDA holder or patent owner files suit against the ANDA or 505(b) (2) applicant for patent infringement within 45 days of receiving the Paragraph IV notice, the FDA is prohibited from approving the ANDA or 505(b) (2) application for a period of 30 months from the date of receipt of the notice. If the RLD has NCE exclusivity and the notice is given and suit filed during the fifth year of exclusivity, the 30-month stay does not begin until five years after the RLD approval. The FDA may approve the proposed product before the expiration of the 30-month stay if, within that time period, the patent involved expires, the parties settle the lawsuit or a court finds the patent invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
Pediatric exclusivity is another type of exclusivity available in the U.S. under Section 505A of the FDCA. Pediatric exclusivity, if granted, provides an additional six months to an existing exclusivity period, including orphan drug exclusivity, or delay in approval resulting from certain patent certifications. This six-month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric trial or trials and submission of pediatric data that fairly responds to an FDA-issued “Written Request” for such a trial or trials. The data need not show the product to be safe or effective in the pediatric population studied; rather if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by FDA within statutory time limits, any periods of regulatory exclusivity or Orange Book -listed patent protections that cover the drug are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve an ANDA or 505(b) (2) application owing to regulatory exclusivity or listed patents. In the first quarter of 2015, the FDA issued a Written Request for a study to evaluate lomitapide in pediatric HoFH patients, which, if completed as described, would provide for 6 months of pediatric exclusivity under the FDCA. In the second quarter of 2015, we decided to decline the FDA’s Written Request regarding the study in pediatric HoFH patients, because we believe that the size and complexity of the requested trial created a considerable barrier to the feasibility of the study. Given that we have declined to conduct the study requested by the FDA, we will not be entitled to the six months of additional exclusivity available for conducting a study that is the subject of a Written Request issued by the FDA.
Patent Term Restoration
The Hatch-Waxman Act established a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. The maximum period of restoration is five years and cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension, and the extension must be applied for prior to expiration of the patent and within 60 days of the NDA approval. The PTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. A five year patent term extension has been granted for our U.S. patent covering the composition of matter of lomitapide, extending the patent term to 2020 from the originally scheduled expiration of early 2015. An application for a patent term extension in the U.S. with respect to MYALEPT has been filed which, if granted, we will apply to either the U.S. composition of matter patent or the method of use patent, to extend one of these patents by 1,206 days.
The Biologics Price Competition and Innovation Act
The BPCI Act, enacted in 2010, as part of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (the Health Care Reform Act), authorizes the FDA to license a biological product that is biosimilar to, and possibly interchangeable with, a PHSA-licensed reference biological product through an abbreviated pathway. The BPCI Act establishes criteria for determining that a product is biosimilar to an already-licensed biologic (a reference product), and
33
establishes a process by which an abbreviated BLA for a biosimilar product is submitted, reviewed and approved. The BPCI Act provides periods of exclusivity that protect a reference product from biosimilar competition. Under the BPCI Act, innovator manufacturers of original reference products are granted twelve years of exclusive use before biosimilar versions of such products can be licensed for marketing in the U.S. This means that the FDA may not approve an application for a biosimilar version of a reference product until twelve years after the date of approval of the reference product (with a potential six-month extension of exclusivity if certain pediatric studies are conducted and the results reported to FDA), although a biosimilar application may be submitted four years after the date of licensure of the reference product. Additionally, the BPCI Act establishes procedures by which the biosimilar applicant must provide information about its application and product to the reference product sponsor, and by which information about potentially relevant patents is shared and litigation over patents may proceed in advance of approval. The BPCI Act also provides a period of exclusivity for the first biosimilar to be determined by the FDA to be interchangeable with the reference product. Under the BPCI Act, metreleptin has twelve years of exclusivity in the U.S. from February 24, 2014, the date of the product’s approval by the FDA.
The objectives of the BPCI Act are conceptually similar to those of the Hatch-Waxman Act, which established abbreviated pathways for the approval of small molecule drug products. The FDA has not yet issued proposed regulations setting forth its interpretation of the BPCI Act’s provisions but has issued guidance documents in 2015 related to BPCI Act implementation concerning biosimilarity and interchangeability, BLA submission requirements, and exclusivity. We anticipate that contours of the BPCI Act will be further defined through issuance of additional guidance documents by the FDA, proposed regulations, and decisions in the course of considering specific applications.
Like pediatric exclusivity applicable to drug products approved under the FDCA, pediatric exclusivity applicable to biological reference products is subject to an exception. Pediatric exclusivity will not apply to either the 12-year reference product or the seven-year orphan drug exclusivity periods if the FDA determines later than nine months prior to the expiration of such period that the study reports a BLA sponsor submitted in response to a written request for pediatric studies met the terms of that request.
The BPCI Act sets forth a complex mechanism for resolving patent disputes that involves a step-wise exchange of information prior to the initiation of a patent infringement lawsuit against a biosimilar or interchangeable product sponsor. Unlike the Hatch-Waxman Act, the BPCI Act provides no automatic stay on approval of a biosimilar or interchangeable product application.
Modifications to or repeal of all or certain provisions of the Health Care Reform Act are expected as a result of the outcome of the recent presidential election and Republicans maintaining control of Congress, consistent with statements made by President Donald Trump and members of Congress during the presidential campaign and following the election. As noted above, the BPCI Act was enacted as part of the Healthcare Reform Act. Although there has been no direct discussion, to our knowledge, of repealing the BPCI Act, if there is a repeal of all or parts of the Health Care Reform Act, this could impact the BPCI Act provisions as well. We cannot predict the ultimate content, timing or effect of any changes to the Health Care Reform Act or any resulting impact on the BPCI Act.
U.S. Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug or biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S., or for which there is no reasonable expectation that development and production costs will be recovered from sales of the drug for such disease or condition in the U.S. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. If a product that has orphan drug designation subsequently receives FDA approval and is the first drug approved for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve for seven years any other applications to market the same drug for the same indication, except in limited circumstances such as a demonstration that the subsequent drug is clinically superior or the inability of the existing manufacturer to supply the market. Orphan drug exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval for an orphan product that the FDA finds to be the “same drug” as our product candidate for the same indication or disease. If a drug or biologic designated as an orphan drug receives marketing approval for an indication broader than the scope of its designation, it may be no longer entitled to orphan drug exclusivity. In addition to creating a 12-year period of reference product exclusivity, the BPCI Act clarifies the interaction of that exclusivity with orphan drug exclusivity, such that the licensure of a biosimilar or interchangeable version of a reference biological product that was designated and approved as an orphan drug may only occur after the later of the expiration of any applicable seven-year orphan drug exclusivity or the 12-year reference product exclusivity (or seven and one-half years and 12.5 years with pediatric exclusivity). The FDA has granted seven years of orphan drug exclusivity for JUXTAPID in the treatment of HoFH which is scheduled to expire on December 21, 2019, and seven years of orphan drug exclusivity for MYALEPT in the treatment of GL which is scheduled to expire on February 24, 2021. Zuretinol has received orphan drug designations for the treatment of LCA (due to inherited mutations in the LRAT or RPE65 genes) and RP (all mutations) by the FDA. The FDA has also formally acknowledged that the orphan drug designations granted by the FDA on zuretinol also cover the treatment of IRD caused by LRAT or RPE65 gene mutations, which disease/condition we believe comprises both LCA due to
34
inherited mutations in LRAT or RPE65 genes and RP. Since the extent and scope of our patent protection for zuretinol is limited, orphan drug designation is especially important for this product candidate.
Fast Track Designation
The FDA’s Fast Track program is intended to facilitate the development and review of drugs that are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for such a disease or condition. Under the program, the sponsor of a new drug may request that the FDA designate the drug for a specific indication as a Fast Track product concurrent with or after the filing of the IND for the product candidate. A drug that receives Fast Track designation may be eligible for more frequent meetings with the FDA to discuss the drug’s development; more frequent written correspondence from the FDA about the design of the proposed clinical trials; and rolling review, meaning the sponsor may submit its NDA in sections rather than wait until the entire NDA is complete. Drugs with Fast Track designation may be more likely to become eligible for a Priority Review, which provides for FDA review of an NDA for a NME within a six-month time frame from the time the complete NDA is accepted for filing (60 days after submission), as opposed to the ten-month time frame for a Standard Review. The FDA grants Priority Review for products that demonstrate the potential to be a significant improvement in the safety or effectiveness of the treatment, prevention, or diagnosis of a serious condition. Zuretinol has been granted Fast Track designations by the FDA for the treatment of LCA and autosomal recessive RP due to inherited mutations in the LRAT and RPE65 genes.
Rare Pediatric Disease Priority Review Voucher
In addition to the Fast Track and orphan drug designations previously granted to us by the FDA for zuretinol, we are currently exploring the potential of submitting to the FDA a request for rare pediatric disease designation of zuretinol for the treatment of IRD caused by LRAT or RPE65 gene mutations, which indication includes LCA and RP. In order to obtain a rare pediatric disease designation for zuretinol, we must demonstrate to FDA’s satisfaction that this indication is for the treatment or prevention of a rare disease or condition that affects fewer than 200,000 individuals in the U.S., or that affects more than 200,000 individuals in the U.S. and there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for this type of disease or condition will be recovered from sales in the U.S. for that product, and is a serious or life-threatening disease in which the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years. Under the FDCA, a sponsor who receives approval of an NDA for a product that is for the prevention or treatment of a rare pediatric disease and meets certain additional criteria, may qualify for a rare pediatric disease priority review voucher (PRV). A PRV can be redeemed to receive priority review under an expedited timeframe for a subsequent marketing application for a different product. A PRV may also be sold or transferred from the initial sponsor to another sponsor. The voucher may be further transferred any number of times before it is used. Pursuant to the 21st Century Cures Act, FDA’s authority to award rare pediatric disease PRVs has been extended until 2020, and until 2022 for products that receive rare pediatric disease designation by 2020.
Post-Approval Requirements in the U.S.
Once an approval is granted, products are subject to continuing regulation by the FDA. The FDA may withdraw the approval, following notice and an opportunity for a hearing, if, among other things, compliance with certain regulatory standards is not maintained or if safety or efficacy problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. If new safety issues are identified following approval, the FDA may require the NDA sponsor to take certain measures, such as revising the approved labeling to reflect the new safety information, conducting post-market studies or clinical trials to assess the new safety information, and/or implementing or changing a REMS program to mitigate newly-identified risks. These are often referred to as Phase 4 or post-marketing studies, and may involve additional clinical trials, nonclinical testing and surveillance programs to monitor the safety of approved products which have been commercialized. After approval, most changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to prior FDA review and approval. Manufacturers and other entities involved in the manufacture and distribution of approved drugs or licensed biologics are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements and other laws. In its approval of JUXTAPID, the FDA required three post-marketing requirements; the MYALEPT approval was associated with 15 post-marketing requirements and commitments. See Marketed Products - Lomitapide - Post-Marketing Commitments and Marketed Products - Metreleptin - Post-Marketing Commitments for details of our post-marketing commitments to applicable regulatory authorities.
Companies engaged in manufacturing drug products or their components must comply with applicable cGMP requirements and product-specific regulations enforced by the FDA and other regulatory agencies. Compliance with cGMP includes adhering to requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, and records and reports. The FDA regulates and inspects equipment, facilities, and processes used in manufacturing pharmaceutical products prior to approval. If, after receiving approval, a company makes a material change in manufacturing
35
equipment, location, or process (all of which are, to some degree, incorporated in the NDA or BLA), additional regulatory review and approval may be required. Manufacturers of approved products also are subject to significant annual establishment and product user fees.
The FDA also conducts regular, periodic visits to re-inspect equipment, facilities, and processes following the initial approval of a product. Failure to comply with applicable cGMP requirements and conditions of product approval may lead the FDA to take enforcement actions, which may range from issuing a warning letter to seeking sanctions, including fines, civil penalties, injunctions, suspension of manufacturing operations, operating restrictions, withdrawal of FDA approval, seizure or recall of products, and criminal prosecution. Accordingly, manufacturers must continue to spend time, money and effort to maintain cGMP compliance. We cannot be certain that our present or future third-party manufacturers will consistently comply with cGMP and other applicable FDA regulatory requirements.
In addition, companies manufacturing or distributing drug products pursuant to FDA approvals are subject to record-keeping requirements; requirements on reporting of adverse experiences with the drug, and providing the FDA with updated safety and efficacy or safety, purity, and potency information for drugs and biologics, respectively; compliance within REMS program reporting obligations; drug and biologic sampling and distribution requirements; compliance with certain electronic records and signature requirements, and compliance with FDA promotion and advertising requirements. As discussed more fully below, the FDA strictly regulates labeling, advertising, promotion and other types of information regarding marketed products that are placed on the market. Drugs and biologics may be promoted only for their approved indications and in accordance with the provisions of the approved labeling.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and guidance are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether or when further legislative changes or changes to FDA regulations, guidance or interpretations may occur or what the impact of such changes, if any, may be.
Foreign Regulation
In addition to regulations in the U.S., our business will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. In addition, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. For example, in the EU, the conduct of clinical trials is currently governed by the EU Clinical Trials Directive 2001/20/EC, which imposes obligations and procedures that are similar to those provided in applicable U.S. laws. The EU Good Clinical Practice and EU Good Laboratory Practice standards must also be respected during the conduct of the trials. Prior to commencement of a clinical trial in an EU Member State, an application for authorization of a clinical trial must be submitted to the competent authority and the competent Ethics Committee of the relevant EU Member State in which the clinical trial takes place. The competent authorities of the EU Member States in which the clinical trial is conducted must authorize the conduct of the trial, and the independent Ethics Committee must grant a positive opinion in relation to the conduct of the clinical trial in the relevant EU Member State before the commencement of the trial. Any substantial changes to trial protocol or other information submitted with the clinical trial applications must be notified to or approved by the relevant competent authorities and ethics committees. However, under the new EU Clinical Trials Regulation No. 536/2014, which is expected to take effect in late 2018, a more harmonized procedure will apply, with clinical trial authorization and other applications or notifications being submitted through a centralized EU clinical trials portal.
The approval process for clinical trials in other countries outside the U.S. and the EU varies from country to country, and the time may be longer or shorter than that required for FDA approval. In all cases, clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain marketing authorization for a medicinal product in the EU, companies must submit an application for marketing authorization based on the ICH Common Technical Document to the competent authorities of the EU Member States or to the EMA. Applicants need to demonstrate the quality, safety and efficacy of the medicinal product in the application for marketing authorization. This requires applicants to conduct human clinical trials to generate the necessary clinical data. Moreover, applicants are required to include, as part of the application for marketing authorization, the results of all studies performed and details of all information collected in compliance with an agreed Pediatric Investigation Plan (PIP) approved by the PDCO, or a decision by the EMA granting a product-specific or class waiver for pediatric use or deferral for the conduct of the PIP.
Medicinal products are authorized in the EU through one of several different procedures, either by the competent authorities of the EU Member States through the decentralized procedure, mutual recognition procedure, or national procedure, or through the
36
centralized authorization procedure by which the EC takes a decision to grant a marketing authorization following a positive opinion by the EMA.
The centralized procedure provides for the grant of a single marketing authorization by the EC that is valid for all EU Member States and three of the four EFTA States (Iceland, Liechtenstein and Norway). The centralized procedure is compulsory for certain medicinal products, including medicinal products derived from certain biotechnological processes, orphan medicinal products, advanced therapy medicinal products and products with a new active substance indicated for the treatment of certain diseases. It is optional for those products that are a significant therapeutic, scientific or technical innovation, or the authorization of which would be in the interest of public. Under the centralized procedure in the EU, the timeframe for the evaluation of a marketing authorization application by the EMA Committee for Medicinal Products for Human Use (CHMP) is, in principle, 210 days from receipt of a valid application for marketing authorization. This time period excludes any clock stops when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP and if the applicant requests a re-examination of the CHMP opinion. Accelerated evaluation might be granted, following a substantiated request from the applicant, by the CHMP in exceptional cases, when a medicinal product is of a major public health interest particularly from the point of view of therapeutic innovation. Justification of what constitutes a major public interest is on a case by case basis. The justification should include the major benefits expected and present the arguments to support the claim that the medicinal product introduces new methods of therapy or improves on existing methods, thereby addressing to a significant extent the greater unmet needs for maintaining and improving public health. In this circumstance of an accelerated assessment, the opinion of the CHMP is given, in principle, within 150 days. Regardless of the assessment procedure, the opinion of the CHMP will be provided to the EC who will take the final decision on the application for centralized marketing authorization of a medicinal product. In light of the United Kingdom’s (UK) vote in 2016 to leave the EU, the so-called Brexit vote, there may be changes forthcoming in the scope of the centralized approval procedure as the terms of that exit are negotiated between the UK and the EU.
The decentralized marketing authorization procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU Member State in which the product is to be marketed. One national competent authority, the “reference” Member State, selected by the applicant, assesses the application for marketing authorization. As part of this procedure, an applicant submits an application for marketing authorization, or dossier, and related materials, including a draft summary of product characteristics (SmPC), and draft labeling and package leaflet, to the reference EU Member State and the concerned EU Member States. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment report and drafts of the related SmPC, labeling and package leaflet within 120 days after receipt of a valid application. The competent authorities of the other EU Member States, the “concerned” Member States, are subsequently required to grant marketing authorization for their territory on the basis of this assessment within 90 days of receipt thereof. The only exception to this obligation arises where the competent authorities provide evidence of potential serious risk to public health which would require this authorization to be refused. Similarly, the mutual recognition procedure is based on the acceptance by the competent authorities of the EU Member States of the marketing authorization of a medicinal product by the competent authorities of other EU Member States. The holder of a national marketing authorization may submit an application to the competent authority of an EU Member State requesting that this authority recognize the marketing authorization delivered by the competent authority of another EU Member State. The reference EU Member State prepares a draft assessment report and drafts of the related SmPC, labeling and package leaflet within 90 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States, which within 90 days of receipt must each decide whether to approve the assessment report and the related materials. For both the decentralized and mutual recognition procedures, if a concerned EU Member State does not approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points may eventually be referred to the Coordination Group for Mutual Recognition and Decentralized Procedures (CMDh) whose decision is binding on all EU Member States. If the CMDh does not reach an agreement, the disputed points are forwarded to the CHMP. The CHMP then adopts an opinion in the matter, which is forwarded to the EC, which makes the final decision regarding the application for a decentralized or mutual recognition marketing authorization. LOJUXTA was granted a marketing authorization by the EC under the centralized procedure. Because Aegerion was not able to provide comprehensive clinical data on efficacy and safety under normal conditions of use due to the rarity of the disease, and in light of Aegerion’s commitments to conduct an appropriate risk-mitigation program, LOJUXTA was approved under exceptional circumstances. This type of marketing authorization requires an annual reassessment of the risk/benefit of LOJUXTA by the CHMP, for which Amryt is now responsible. As part of the post-marketing commitments to the FDA, Aegerion is conducting an observational cohort study to generate more data on the long-term safety profile of lomitapide in the treatment of patients with HoFH, the patterns of use and compliance and the long-term effectiveness of controlling LDL-C levels. The EMA has required that all patients taking lomitapide in the EU be encouraged to participate in the study, and that the study period be open-ended. Amryt will bear the costs of conducting this study in the EEA and other relevant territories. In the study, physicians will follow each patient to track malignancies, tumors, teratogenicity, hepatic effects, and GI adverse reactions, events associated with coagulopathy, major adverse cardiovascular events and death. The EMA also required that a vascular imaging study be conducted to determine the impact of lomitapide on vascular endpoints, which is now the responsibility of Amryt.
37
Innovative medicinal products authorized in the EU on the basis of a full marketing authorization application (as opposed to an application for a generic marketing authorization that relies on the results of pre-clinical and clinical trials available in the marketing authorization dossier for another, previously approved, reference medicinal product) are entitled to eight years’ data exclusivity beginning on the date of the grant of the first marketing authorization for the innovative product in the EU. During this period applicants for approval of generics of these innovative products cannot rely on data contained in the marketing authorization dossier submitted for the innovative medicinal product. Innovative medicinal products are also entitled to ten years’ marketing exclusivity, which also begins on the date of the grant of the first marketing authorization for the innovative product in the EU. During this ten-year period no generic medicinal product can be placed on the EU market. The ten-year period of market exclusivity can be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder for the innovative product obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Lomitapide has eight years’ data exclusivity and ten years’ marketing exclusivity in the EU from July 31, 2013, the date of the EC’s approval of lomitapide.
The EMA grants orphan designation to promote the development of products that treat life-threatening or chronically debilitating conditions affecting not more than five in 10,000 people in the EU. In addition, orphan drug designation can be granted only if, for economic reasons, the medicinal product would be unlikely to be developed without incentives and if there is no other satisfactory method approved in the EU of diagnosing, preventing, or treating the condition, or if such a method exists, the proposed medicinal product must potentially be of significant benefit to patients affected by the condition. The application for orphan designation must be granted by the EC before an application for marketing authorization of the medicinal product is submitted. Upon grant of marketing authorization for the medicinal products, orphan designation provides ten years of market exclusivity for the orphan medicinal product in the orphan indication. During this ten-year period, with a limited number of exceptions, the competent authorities of the EU Member States and the EMA may not accept applications for marketing authorization, accept an application to extend an existing marketing authorization or grant marketing authorization for other similar medicinal products for the same orphan indication. Under an exception, marketing authorization could be granted to a similar medicinal product with the same orphan indication before the expiry of the ten years if the holder of the marketing authorization for the original orphan medicinal product has given its consent or if the manufacturer of the original orphan medicinal product is unable to supply sufficient quantities. Marketing authorization may also be granted to a similar medicinal product with the same orphan indication if this product is safer, more effective or otherwise clinically superior to the original orphan medicinal product. Moreover, the exclusivity period for the original orphan medicinal product may be reduced to six years if the designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. Despite the prevalence rate, lomitapide does not have orphan medicinal product exclusivity in the EU for the treatment of HoFH because the EMA views the relevant condition, for orphan drug purposes, to include both HoFH and HeFH. In 2012, metreleptin was granted orphan designation by the EC for the treatment of Barraquer-Simons syndrome (acquired PL), Berardinelli-Seip syndrome (congenital GL), Lawrence syndrome (acquired GL) and familial PL. Zuretinol was granted orphan designation by the EMA in 2011 for the treatment of LCA and RP (all mutations) and in 2014, the EMA also formally acknowledged that a therapeutic indication of zuretinol for the treatment of patients with IRD, who have been phenotypically diagnosed as LCA or RP caused by mutations in RPE65 or LRAT genes, would fall under the orphan drug designations of treatment of LCA and treatment of RP. We also plan to seek regulatory exclusivity for zuretinol in the EU; however, there can be no assurance that we will be successful in securing approval or regulatory exclusivity in the EU.
In the EU, certain patents may qualify for a supplemental protection certificate that would extend patent protection for up to five years after patent expiration upon marketing authorization in the EU. Grant of such supplemental protection certificate is, however, subject to strict conditions and it is not automatic. We believe that our EPO method of use patent covering certain dosing regimens for lomitapide which expires in 2025 may be eligible for up to three years of supplemental protection in certain EPO countries, and we are seeking such protection in the EU Member States, on a country-by-country basis.
Similar to the U.S., marketing authorization holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA and/or the competent authorities of the EU Member States. This oversight applies both before and after grant of manufacturing and marketing authorizations. It includes control of compliance with EU GMP rules, manufacturing authorizations, pharmacovigilance rules and requirements governing advertising, promotion, sale, and distribution, recordkeeping, importing and exporting of medicinal products.
Failure to comply with the EU Member State laws implementing the EU Community Code on medicinal products and other EU Member State laws that apply to the promotion of medicinal products, statutory health insurance, bribery and anti-corruption or with other applicable regulatory requirements can result in enforcement action by the EU Member State authorities, which may include any of the following: fines, imprisonment, orders forfeiting products or prohibiting or suspending their supply to the market, orders to suspend, vary, or withdraw the marketing authorization or requiring the manufacturer to issue public warnings, or to conduct a product recall. The collection and use of personal health data and other personal information in the EU is governed by the provisions of the Data Protection Directive as implemented into national laws by the EU Member States. This Directive
38
imposes a number of strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting. There is, moreover, a growing trend towards imposition of an obligation of public disclosure of clinical trial data in the EU which adds to the complexity of obligations relating to the processing of health data from clinical trials. Such public disclosure obligations are provided in the new EU Clinical Trials Regulation, EMA disclosure initiatives, and voluntary commitments by industry. The Data Protection Directive also includes requirements relating to the consent of the individuals to whom the personal data relates, the information provided to the individuals prior to processing their personal data or personal health data, notification of data processing obligations to the competent national data protection authorities and the security and confidentiality of the personal data. The Data Protection Directive also prohibits the transfer of personal data to countries outside of the EU Member States that are not considered by the EC to provide an adequate level of data protection. These countries include the U.S. Failure to comply with the requirements of the Data Protection Directive and the related national data protection laws of the EU Member States may result in fines and other administrative penalties. Data protection authorities from the different EU Member States may interpret the EU Data Protection Directive and national laws differently, which adds to the complexity of processing personal data in the EU. Guidance developed at both EU level and at national level in individual EU Member States concerning implementation and compliance practices are often updated or otherwise revised. The EU General Data Protection Regulation (EU No. 2016/679), which will apply from May 25, 2018, will introduce new data protection requirements in the EU and substantial fines for breaches of the data protection rules. The EU Data Protection Regulation, which will be applicable two years after the date of its publication in the Official Journal for the European Union, will increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the new EU data protection rules. This may be onerous and increase our cost of doing business.
Expanded Access
In certain countries, drug products approved in the U.S. or EU can be accessed by patients before the drug has obtained marketing approval in such country. There are various forms of this access. They include the actual purchase of product by the purchaser, which is often times the government for patients, on a named patient basis, providing the product free of charge on a named patient basis, and providing the product on a compassionate use basis. Each country has its own laws and regulations that apply to these forms of access and the extent and nature of such laws and regulations vary by country. We have made lomitapide available in Brazil and other countries that allow such use, and we plan to continue to consider access to additional countries in compliance with applicable laws and regulations. When Aegerion acquired metreleptin from AstraZeneca in January 2015, there were a number of patients receiving metreleptin therapy free of charge in certain countries outside the U.S. that allow use of a drug, under a compassionate use or other type of expanded access program, before marketing approval has been obtained in such country. Where permitted in accordance with applicable requirements, we have continued to make metreleptin available free of charge under such a program, which has resulted in significant costs to us. In 2016, we began generating revenues from named patient sales of metreleptin in certain markets where named patient sales of metreleptin are possible and to the extent permitted by applicable law and local regulatory authorities. In particular, we are in the process of converting all GL and PL patients currently in the expanded access program in France to a paid program under the Autorisation Temporaire d’Utilisation (Temporary Authorization for Use). Metreleptin has also been approved for reimbursement by the Turkish Social Security Association (SGK), and we plan to provide metreleptin on a named patient basis for GL patients, including CGL patients, and other subsets of lipodystrophy patients, subject to individual assessment in response to unsolicited requests from clinicians. We also provide zuretinol to patients under our compassionate use program on a named-patient basis to patients who participated in our completed Phase 1b clinical trial of zuretinol for the treatment of LCA and RP.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists regarding the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of pharmaceutical products depend in significant part on the availability and adequacy of third-party reimbursement. Third-party payers include government health administrative authorities, including authorities at the U.S. federal and state level, managed care providers, private health insurers and other organizations. Third-party payers are increasingly challenging the prices charged for, examining the medical necessity of, and assessing the cost-effectiveness of medical products and services.
In the U.S., the Medicare program provides health insurance for people who are 65 or older, as well as certain people with disabilities and other conditions irrespective of their age. The Medicare program is funded by the federal government and administered by the Centers for Medicare & Medicaid Services (CMS). Medicare Part D is a voluntary prescription drug benefit, through which beneficiaries may enroll in prescription drug plans offered by private entities under contract with CMS for coverage of certain outpatient prescription drugs. Medicare Part D generally provides coverage for medically necessary self-administered drugs (i.e. drugs that do not need to be administered by a healthcare practitioner). JUXTAPID and MYALEPT may be covered under Medicare Part D. Coverage and reimbursement for outpatient drugs under Part D is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs the plan will cover and at what tier or level. The availability of coverage under Medicare Part D may affect demand for
39
JUXTAPID and MYALEPT. In order for JUXTAPID and MYALEPT to remain on or be included on the formularies of Part D prescription drug plans, we may have to offer discounts on the price of those products. In addition, manufacturers, including Aegerion, are required to provide a 50% discount on brand name prescription drugs utilized by Medicare Part D beneficiaries when those beneficiaries reach the so-called “donut hole” in their drug benefits in a particular year (i.e. a coverage gap between initial coverage and catastrophic coverage). We believe that investigations and enforcement actions by certain government agencies may have caused a reduction in contributions to third-party patient assistance programs operated by independent charitable 501(c)(3) organizations that assist patients, including Medicare Part D beneficiaries, in accessing treatment for certain diseases and conditions. If a lack of available funds prevents these patient assistance programs from providing adequate financial assistance, including assistance with co-payment obligations, to individuals who would otherwise be unable to afford our products, our revenues may decline below our expectations.
Medicaid is a health insurance program with mandatory coverage for certain low-income children, families, pregnant women, and people with disabilities. States also have the option of expanding Medicaid coverage to low-income individuals generally and many states have done so. Medicaid is jointly funded by the federal and state governments, but administered by the states. In general, state Medicaid programs are required to cover drugs and biologics of manufacturers that have entered into a Medicaid Drug Rebate Agreement, as discussed further below, although such drugs and biologics may be subject to prior authorization or other utilization controls.
Coverage of drugs and biologics by private health insurance varies. Private payers may use a variety of utilization management techniques designed to control costs, including requiring pre-approval of coverage for drug therapies before reimbursing healthcare providers or patients that use such therapies. In addition, a payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be provided. Coverage may not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development.
Government and private third-party payers have a variety of methodologies for paying for drugs and biologics. Payers are increasingly considering new metrics as the basis for reimbursement rates, such as average sales price, average manufacturer price (AMP) or actual acquisition cost. Recent changes to the Medicaid Drug Rebate Program, effective April of 2016, require state Medicaid programs to reimburse certain brand name covered outpatient drugs at actual acquisition cost plus a dispensing fee. The impact of these evolving reimbursement mechanics on the willingness of providers to furnish JUXTAPID or MYALEPT or other products we may market and the prices we can command for these products is difficult to predict.
We participate in various government programs or contracts that require us to calculate and report certain prices for our products to government agencies or provide rebates or discounted pricing on products purchased to certain purchasers or government payers. The requirements for calculating prices and rebates are complex and subject to change. We may also have reimbursement obligations or be subject to penalties if we fail to provide timely and accurate information to the government, pay the correct rebates or offer the correct discounted pricing.
We participate in the Medicaid Drug Rebate Program. Under the Medicaid Drug Rebate Program, we are required to pay a rebate for each unit of our product reimbursed by a state Medicaid program as a condition of having federal funds made available to the states for our drugs under Medicaid and Medicare Part B. Those rebates are based on pricing data that we report on a monthly and quarterly basis to CMS, the federal agency that administers the Medicaid Drug Rebate Program. We may also participate in state Medicaid supplemental rebate programs which require payment of an incremental rebate to state Medicaid programs for covered utilization of our products. Price reductions as well as price increases that exceed the rate of inflation for our products, such as the price increase for MYALEPT in February of 2015, may result in increasing the rebates we are required to pay under the Medicaid Drug Rebate Program or state Medicaid supplemental rebate programs and the discounts we are required to offer under the Public Health Service (PHS) 340B drug pricing discount program (the 340B Program), as discussed below.
To maintain coverage of our products under the Medicaid Drug Rebate Program and Medicare Part B, we are required to extend significant discounts to certain “covered entities” (defined by statute to include certain types of hospitals and other healthcare providers that receive federal grants) that purchase products under the 340B Program. The 340B Program requires participating manufacturers to agree to charge such covered entities no more than the 340B “ceiling price” for the manufacturers’ covered outpatient drugs. The 340B ceiling price is calculated using a statutory formula, which is based on the AMP and rebate amount for the covered outpatient drug as calculated under the Medicaid Drug Rebate Program. “Orphan drugs” -those designated under section 526 of the FDCA, such as JUXTAPID and MYALEPT-are exempt from the ceiling price requirements with respect to drugs purchased by certain covered entities (i.e. rural referral centers, sole community hospitals, critical access hospitals, and free-standing cancer hospitals). The Healthcare Reform Act also obligates the Health Resources and Services Administration (HRSA), the agency which administers the 340B Program, to promulgate various regulations and implement processes to improve the integrity of the 340B Program. The status of new and pending regulations and guidance is uncertain under the new presidential administration.
40
Drug products are subject to discounted pricing when purchased by federal agencies via the Federal Supply Schedule (FSS). FSS participation is required for a drug product to be covered and reimbursed by certain federal agencies and for coverage under Medicaid, Medicare Part B and the 340B Program. FSS pricing is negotiated periodically with the Department of Veterans Affairs. FSS pricing is intended not to exceed the price that a manufacturer charges its most-favored non-federal customer for its product. In addition, prices for drugs purchased by the Veterans Administration, Department of Defense (including drugs purchased by military personnel and dependents through the Tricare Retail Pharmacy program), Coast Guard, and PHS are subject to a cap on pricing (known as the federal ceiling price) and may be subject to an additional discount if pricing increases more than the rate of inflation. Aegerion participates in the FSS. Aegerion also participates in the Tricare Retail Pharmacy program, under which we pay quarterly rebates on utilization of JUXTAPID and MYALEPT when the products are dispensed through the Tricare Retail Pharmacy network to Tricare beneficiaries.
In addition, in the U.S., the cost of pharmaceuticals continues to generate substantial governmental and third-party payer interest. We expect that the pharmaceutical industry will experience pricing pressures due to the increasing influence of managed care (and related implementation of managed care strategies to control utilization), additional federal and state legislative and regulatory proposals to regulate pricing of drugs, limit coverage of drugs or reduce reimbursement for drugs, public scrutiny and the presidential administration’s agenda to control the price of pharmaceuticals through government negotiations of drug prices in Medicare Part D and importation of cheaper products from abroad.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, in the EU, the sole legal instrument at the EU level governing the pricing and reimbursement of medicinal products is Council Directive 89/105/EEC (the Price Transparency Directive). The aim of this Directive is to ensure that pricing and reimbursement mechanisms established in the EU Member States are transparent and objective, do not hinder the free movement of and trade in medicinal products in the EU, and do not hinder, prevent or distort competition on the market. The Price Transparency Directive does not provide any guidance concerning the specific criteria on the basis of which pricing and reimbursement decisions are to be made in individual EU Member States, nor does it have any direct consequence for pricing nor reimbursement levels in individual EU Member States. The EU Member States are free to restrict the range of medicinal products for which their national health insurance systems provide reimbursement, and to control the prices and/or reimbursement levels of medicinal products for human use. An EU Member State may approve a specific price or level of reimbursement for the medicinal product, or alternatively adopt a system of direct or indirect controls on the profitability of the company responsible for placing the medicinal product on the market, including volume-based arrangements, caps and reference pricing mechanisms.
Health Technology Assessment (HTA) of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States, including the United Kingdom, France, Germany, Ireland, Italy and Sweden. The HTA process in the EU Member States is governed by the national laws of these countries. HTA is the procedure according to which the assessment of the public health impact, therapeutic impact, and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. HTA generally focuses on the clinical efficacy and effectiveness, safety, cost, and cost-effectiveness of individual medicinal products as well as their potential implications for the healthcare system. Those elements of medicinal products are compared with other treatment options available on the market. The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product vary between EU Member States. A negative HTA of one of our products by a leading and recognized HTA body, such as the National Institute for Health and Care Excellence (NICE) in the United Kingdom, could not only undermine our ability to obtain reimbursement for such product in the EU Member State in which such negative assessment was issued, but also in other EU Member States. For example, EU Member States that have not yet developed HTA mechanisms could rely to some extent on the HTA performed in countries with a developed HTA framework, such as the United Kingdom, when adopting decisions concerning the pricing and reimbursement of a specific medicinal product.
In 2011, Directive 2011/24/EU was adopted at EU level. This Directive concerns the application of patients’ rights in cross-border healthcare. The Directive is intended to establish rules for facilitating access to safe and high-quality cross-border healthcare in the EU. It also provides for the establishment of a voluntary network of national authorities or bodies responsible for HTA in the individual EU Member States. The purpose of the network is to facilitate and support the exchange of scientific information concerning HTAs. This could lead to harmonization between EU Member States of the criteria taken into account in the conduct of HTA and their impact on pricing and reimbursement decisions.
To obtain reimbursement or pricing approval in some countries, including the EU Member States, we may be required to conduct studies that compare the cost-effectiveness of our product candidates to other therapies that are considered the local standard of care. In the case of metreleptin, in preparation for seeking reimbursement and pricing approval, if metreleptin is approved by the
41
EMA, we are conducting local and regional studies to ascertain the impact of metreleptin on morbidity, mortality and patients' quality of life, in order to maximize the product's value proposition to payers. There can be no assurance that any country will allow favorable pricing, reimbursement and market access conditions for any of our products, or that it will be feasible to conduct additional cost-effectiveness studies, if required.
In certain of the EU Member States, products that are designated as orphan medicinal products may be exempted or waived from having to provide certain clinical, cost-effectiveness and other economic data in connection with their filings for pricing/reimbursement approval. As noted above, LOJUXTA was not granted orphan designation by the EMA for treatment of HoFH. As such, it is not eligible for benefits related to orphan designation. Therefore, Amryt may not be able to provide all of the data required to obtain pricing/reimbursement approvals in certain EU Member States, which has and could, in the future, result in delays of pricing/reimbursement approvals for LOJUXTA, LOJUXTA not obtaining pricing/reimbursement approval at all, or LOJUXTA obtaining approvals at less than acceptable levels or with significant restrictions on use or reimbursement, all of which thereby potentially negatively impacting sales milestone and royalty payments Aegerion receives under its license agreement with Amryt.
U. S. Healthcare Reform
Our revenue and operations could be affected by changes in healthcare spending and policy in the U.S. We operate in a highly regulated industry and new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, the method of delivery or payment for health care products and services could negatively impact our business, operations and financial condition. As noted above, the U.S. Congress and state legislatures from time to time propose and adopt initiatives aimed at cost containment, which could impact our ability to sell our products profitably. For example, the Healthcare Reform Act substantially changed the way healthcare is financed by both governmental and private insurers. The law contains a number of provisions that affect coverage and reimbursement of drug products and/or that could potentially reduce the demand for our products such as:
• | increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care; and |
• | requiring drug manufacturers to provide a 50% discount on Medicare Part D brand name prescription drugs sold to Medicare beneficiaries whose prescription drug costs cause the beneficiaries to be subject to the Medicare Part D coverage cap (i.e. the so-called donut hole). |
In 2012, the Supreme Court of the U.S. heard challenges to the constitutionality of the individual mandate and the viability of certain provisions of the Healthcare Reform Act. The Supreme Court’s decision upheld most of the Healthcare Reform Act and determined that requiring individuals to maintain “minimum essential” health insurance coverage or pay a penalty to the Internal Revenue Service was within Congress’s constitutional taxing authority. However, the Supreme Court struck down a provision in the Healthcare Reform Act that penalized states that choose not to expand their Medicaid programs through an increase in the Medicaid eligibility income limit from a state’s current eligibility levels to 133% of the federal poverty limit. As a result of the Supreme Court’s ruling, some states have decided not to expand Medicaid. For each state that does not choose to expand its Medicaid program, there will be fewer insured patients overall. Any reduction in the number of insured patients could impact our sales, business and financial condition.
Modifications to or repeal of all or certain provisions of the Healthcare Reform Act are expected as a result of the outcome of the recent presidential election and Republicans maintaining control of Congress, consistent with statements made by President Donald Trump and members of Congress during the presidential campaign and following the election. We cannot predict the ultimate content, timing or effect of any changes to the Healthcare Reform Act or other federal and state reform efforts.
In addition, other legislative changes have been proposed and adopted since the Healthcare Reform Act was enacted. The Budget Control Act of 2011 includes provisions to reduce the federal deficit. The Budget Control Act, as amended, resulted in the imposition of 2% reductions in Medicare payments to providers which began in April, 2013 and will remain in effect through 2025 unless additional congressional action is taken.
Promotional Activities and Interactions with Healthcare Providers and Patients
The FDA and other regulatory agencies strictly regulate promotional claims about prescription drug and biological products through, among other things, standards and regulations for direct-to-consumer advertising, communications regarding unapproved uses, industry-sponsored scientific and educational activities, and promotional activities involving the Internet and social media. A product cannot be commercially promoted before it is approved. In general, after approval, a drug product may not be promoted for uses that are not approved by the FDA, the EC, and the competent authorities of the EU Member States or such other regulatory agencies as reflected in the product’s prescribing information. Moreover, the promotion of prescription-only medicinal products to non-healthcare professionals is prohibited in the EU. In the U.S., healthcare professionals are generally permitted to prescribe
42
drugs and biologics for “off-label” uses-that is, uses not described in the drug’s labeling-because the FDA does not regulate the practice of medicine. However, FDA regulations impose stringent restrictions on manufacturers’ communications regarding off-label uses. A manufacturer may not promote a drug or biologic for off-label use, but under very specific conditions, it may be permissible for a manufacturer to engage in non-promotional, truthful, non-misleading communication regarding off-label information. If a company is found to have promoted off-label uses, it may become subject to adverse publicity and enforcement action by the FDA, the DOJ, or the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil and criminal fines and agreements that materially restrict the manner in which a company promotes or distributes drug or biological products. The federal government has levied large civil and criminal fines against companies for alleged improper promotion, and has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
Healthcare providers and other stakeholders will play a primary role in the recommendation and prescription of our products. Our future arrangements with third-party payers and customers will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute the products for which we obtain marketing approval. Restrictions under applicable U.S. federal and state healthcare laws and regulations include the following:
• | The federal healthcare Anti-Kickback Statute prohibits persons from, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any healthcare item or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to, among others, arrangements between pharmaceutical manufacturers, on the one hand, and prescribers, purchasers, formulary managers and organizations that provide financial assistance to patients, on the other. Violations of the federal Anti-Kickback Statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. The Healthcare Reform Act, among other things, clarified that liability may be established under the federal Anti-Kickback Statute without proving actual knowledge of the statute or specific intent to violate it. In addition, the Healthcare Reform Act amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. There are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute that protect certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. |
• | The federal civil False Claims Act imposes civil penalties and provides for civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment of government funds, or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government, or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. Several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may also implicate the federal civil False Claims Act. Federal civil False Claims Act violations may result in treble monetary damages and penalties and exclusion from participation in federal healthcare programs. Civil liability under the False Claims Act or misdemeanor violation of federal health care laws gives the Inspector General of the Department of Health and Human Services (IG) the discretion to exclude a company’s products from reimbursement by federal healthcare programs. This discretion to exclude often leads companies to negotiate corporate integrity agreements with the IG so their products may continue to receive reimbursement. |
• | The federal criminal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact, making any materially false, fictitious, or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services. There are also criminal penalties, including imprisonment and criminal fines, for making or presenting a false, fictitious or fraudulent claim to the federal government. Conviction under any of the aforementioned federal criminal statutes requires mandatory exclusion from participation in federal healthcare programs. |
• | The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires certain pharmaceutical manufacturers to engage in extensive tracking of payments and other transfers of value to physicians and teaching hospitals and to submit such data to CMS, which will then make all of this data publicly available on the CMS website. Pharmaceutical manufacturers, such as our subsidiary, Aegerion, with products for which payment is available |
43
under Medicare, Medicaid, or the State Children’s Health Insurance Program are required to track reportable payments and transfers of value during each calendar year and must submit a report on or before the 90th day of each calendar year disclosing reportable payments made in the previous calendar year. Failure to comply with the reporting obligations may result in civil monetary penalties.
• | Analogous state laws and regulations, such as state anti-kickback and false claims laws, apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by Medicaid or other state programs or, in several states, apply regardless of the payer. Several states now require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to certain healthcare providers in those states. Some of these states also prohibit certain marketing-related activities including the provision of gifts, meals, or other items to certain healthcare providers. In addition, several states require pharmaceutical companies to implement compliance programs or marketing codes. |
We are also subject to laws and regulations covering data privacy and the protection of health-related and other personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues which may affect our business, including recently enacted laws in many jurisdictions where we operate. Numerous U.S. federal and state laws and regulations, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and protection of personal information. Failure to comply with laws and regulations covering data privacy and the protection of health-related and other personal information could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business. In addition, we obtain patient health information from most healthcare providers who prescribe our products and research institutions we collaborate with, and they are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996, as amended by HIPAA. Although we are not directly subject to HIPAA other than with respect to providing certain employee benefits, we could potentially be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
Efforts to ensure that our business activities and business arrangements will comply with applicable healthcare laws and regulations could be costly. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. As noted above, in late 2013, our subsidiary, Aegerion, received a subpoena from the DOJ, represented by the U.S. Attorney’s Office in Boston, requesting documents regarding its marketing and sale of JUXTAPID in the U.S., as well as disclosures related to the same. See the “Legal Proceedings” section of this Annual Report for further information regarding ongoing investigations, including the preliminary agreements in principle Aegerion reached with the SEC and the DOJ, and other legal proceedings.
The number and complexity of both federal and state laws continues to increase, and additional governmental resources are being used to enforce these laws and to prosecute companies and individuals who are believed to be violating them. In particular, the Healthcare Reform Act includes a number of provisions aimed at strengthening the government’s ability to pursue anti-kickback and false claims cases against pharmaceutical manufacturers and other healthcare entities, including substantially increased funding for healthcare fraud enforcement activities, enhanced investigative powers, and amendments to the False Claims Act that make it easier for the government and whistleblowers to pursue cases for alleged kickback and false claim violations. While it is too early to predict what effect these changes will have on our business, we anticipate that government scrutiny of pharmaceutical sales and marketing practices will continue for the foreseeable future and subject us to the risk of government investigations and enforcement actions. For example, federal enforcement agencies recently have shown interest in pharmaceutical companies’ product and patient assistance programs, including manufacturer reimbursement support services and relationships with specialty pharmacies. Some of these investigations have resulted in significant civil and criminal settlements. As noted above, our subsidiary, Aegerion, is the subject of certain ongoing investigations by the DOJ and the SEC and is also the subject of a putative class action lawsuit filed against it and certain of its former executive officers in the U.S. District Court for the District of Massachusetts alleging certain misstatements and omissions related to the marketing of JUXTAPID and Aegerion’s financial performance in violation of the federal securities laws. See the “Legal Proceedings” section of this Annual Report for further information regarding these investigations and other legal proceedings.
In the EU, the advertising and promotion of our products is also subject to EU Member States’ laws concerning promotion of medicinal products, interactions with physicians, misleading and comparative advertising and unfair commercial practices. In addition, other legislation of individual EU Member States may apply to the advertising and promotion of medicinal products. These laws require that promotional materials and advertising in relation to medicinal products comply with the product’s SmPC as approved by the competent authorities. The SmPC is the document that provides information to physicians concerning the safe and effective use of the medicinal product. It forms an intrinsic and integral part of the marketing authorization granted for the medicinal product. Promotion of a medicinal product that does not comply with the SmPC is considered to constitute off-label promotion. The off-label promotion of medicinal products is prohibited in the EU. The applicable laws at EU level and in the individual EU Member States also prohibit the direct-to-consumer advertising of prescription-only medicinal products. Violations
44
of the rules governing the promotion of medicinal products in the EU could be penalized by administrative measures, fines and imprisonment. These laws may further limit or restrict the advertising and promotion of our products to the general public and may also impose limitations on our promotional activities with healthcare professionals.
Interactions between pharmaceutical companies and physicians are also governed by strict laws, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct in the individual EU Member States. The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medicinal products is prohibited in the EU. The provision of benefits or advantages to physicians is also governed by the national anti-bribery laws of the EU Member States. One example is the UK Bribery Act 2010 (the UK Bribery Act). This Act applies to any company incorporated in or “carrying on business” in the UK, irrespective of where in the world the alleged bribery activity occurs. This Act could have implications for our interactions with physicians both in and outside the UK. Violation of these laws could result in substantial fines and imprisonment.
Payments made to physicians in certain EU Member States must be publicly disclosed. Moreover, agreements with physicians must often be the subject of prior notification and approval by the physician’s employer, his/her competent professional organization, and/or the competent authorities of the individual EU Member States. These requirements are provided in the national laws, industry codes, or professional codes of conduct, applicable in the EU Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Other Regulation
Our international operations are subject to compliance with the Foreign Corrupt Practices Act (FCPA) which prohibits corporations and individuals from paying, offering to pay, or authorizing the payment of anything of value to any foreign government official, government staff member, political party, or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. We also may be implicated under the FCPA by the activities of our partners, collaborators, contract research organizations, vendors or other agents. The FCPA also requires us, as a public company, to make and keep books and records that accurately and fairly reflect all of our transactions and to devise and maintain an adequate system of internal accounting controls. An aspect of the SEC’s ongoing investigation into Aegerion’s disclosures and activities relates to alleged FCPA violations in Brazil. These potential violations are excluded from the preliminary agreements in principle with the DOJ and the SEC.
Our international operations could also be subject to compliance with national laws of the individual EU Member States, such as the UK Bribery Act. The UK Bribery Act applies to any company incorporated in or “carrying on business” in the UK, irrespective of where in the world the offending conduct occurs. The UK Bribery Act prohibits the provision of an “advantage” intended to induce or reward “improper performance” of the recipient’s function. Offenses under the UK Bribery Act include the offer, promise or provision of a bribe to any person including non-UK government officials and private persons, as well as the request, acceptance or agreement to receive a bribe. The failure by a company to prevent third parties from providing a bribe on its behalf could also constitute an offense under the UK Bribery Act. This Act applies to bribery activities both in the public and private sector. Liability in relation to breaches of the UK Bribery Act is strict. This means that it is unnecessary to demonstrate elements of a corrupt state of mind. However, a defense of having in place adequate procedures designed to prevent bribery is available.
We are also subject to compliance with the anti-bribery laws of other countries, including Brazil. Our activities outside the U.S. or those of our employees, licensees, distributors, manufacturers, clinical research organizations, or other third parties who act on our behalf or with whom we do business could subject us to investigation or prosecution under foreign or U.S. laws. For example, federal and São Paulo authorities in Brazil are each conducting an investigation to determine whether there have been any violations of Brazilian laws related to the sales of JUXTAPID in Brazil. See the “Legal Proceedings” section of this Annual Report for further information regarding these investigations and other legal proceedings.
We are subject to a variety of financial disclosure and securities trading regulations as a public company in Canada and the U.S., including laws relating to the oversight activities of the Canadian securities administrators and the SEC, and the rules and regulations of NASDAQ and the Toronto Stock Exchange (TSX), on which our shares are traded. In addition, the Financial Accounting Standards Board, the Canadian securities administrators, the SEC, and other bodies that have jurisdiction over the form and content of our Consolidated Financial Statements and other public disclosure are issuing and amending proposed and existing pronouncements designed to ensure that companies display relevant and transparent information relating to their respective businesses.
Our present and future business has been and will continue to be subject to various other laws and regulations. Various laws, regulations and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage, movement, import and export and use and disposal of hazardous or potentially hazardous substances, including radioactive compounds and infectious disease agents, used or that we may use in the future in connection with our development work are or may be applicable to our activities. Certain agreements entered into by us involving exclusive license
45
rights or acquisitions may be subject to national or supranational antitrust regulatory control, the effect of which cannot be predicted. The extent of government regulation, which might result from future legislation or administrative action, cannot accurately be predicted.
Research and Development Costs
A significant portion of our operating expenses are related to research and development. During the years ended December 31, 2016, 2015, and 2014, our total company-sponsored research and development expenses were 14.8 million, 9.8 million, and 13.8 million, respectively. See the Products in Development section above and in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Annual Report.
Employees
As of December 31, 2016, Novelion and its subsidiaries had approximately 163 employees, 113 of whom were engaged in research, development, commercial, clinical and regulatory affairs, quality control and assurance, and 50 of whom were engaged in finance, business development, information technology, human resources, intellectual property and legal.
When required, we also engage independent consultants and contractors to perform various professional services including, but not limited to, financial, advisory, clinical, regulatory, supply chain, sales and other commercial services.
Corporate Information
Novelion, formerly known as QLT, was originally formed in 1981 under the laws of the Province of British Columbia. Our principal headquarters are located at 887 Great Northern Way, Suite 250, Vancouver, British Columbia, Canada, and our telephone number is 604-707-7000.
Where You Can Find More Information
You are advised to read this Annual Report in conjunction with other reports and documents that we file from time to time with the SEC. Copies of our annual reports on Form 10-K will be furnished without charge to any person who submits a written request directed to the attention of our Secretary, at our offices located at 887 Great Northern Way, Suite 250, Vancouver, B.C., Canada V5T 4T5. You may also obtain copies of these reports from the SEC at the SEC’s Public Reference Room at 100 F Street, N.E. Washington, D.C. 20549. In addition, the SEC maintains information for electronic filers (including Novelion) at its website at www.sec.gov. The public may obtain information regarding the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The System for Electronic Document Analysis and Retrieval (SEDAR) also provides access to most public securities documents and information filed by issuers (including Novelion) with the thirteen provincial and territorial securities regulatory authorities (Canadian Securities Administrators or CSA) at its website at www.sedar.com. We make our periodic and current reports available on our internet website, free of charge, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Our internet address is www.novelion.com.
Item 1A. Risk Factors.
Risks Associated with Product Development and Commercialization
Our business depends on the success of metreleptin and lomitapide. We may not be able to meet expectations with respect to sales of these products and revenues from such sales and if we are not able to meet such expectations, we may not be able to attain or maintain positive cash flow and profitability in the time periods we anticipate, or at all.
Our business depends on the success of metreleptin and lomitapide. Through Aegerion, we have a limited history of generating revenues from the sale of such products, and we anticipate that we will continue to incur significant costs and expend significant operating and management resources associated with developing and commercializing them. Even with these investments, which we may not be able to make on a timely basis or at the levels we would desire due to the decline in our total revenues, these products may not be successful.
Our ability to meet expectations with respect to sales of these products and revenues from such sales, and to attain profitability and maintain positive cash flow from operations, in the time periods we anticipate, or at all, will depend on the commercial success of these products in the U.S. and other markets, which will depend on, among other factors, obtaining timely regulatory approvals, without onerous restrictions or limitations in the resulting label, obtaining or maintaining favorable pricing for and reimbursement of these products in the U.S. and in key countries outside the U.S., and the availability of financial assistance for individuals who otherwise cannot afford our products. Commercial success also will depend on continued acceptance by the medical community and market demand and medical need for these products, including, in the case of lomitapide, in light of the availability of PCSK9
46
inhibitor products, which has had a significant adverse impact on sales of lomitapide in the U.S. We expect that named patient sales in Brazil in the near-term will continue to be our largest source of revenues on a country-by-country basis outside the U.S.; however, we expect net product sales from named patient sales in Brazil and other countries to continue to fluctuate quarter-over-quarter significantly more than sales in the U.S. If metreleptin or lomitapide does not achieve or maintain commercial success, our future operating results and financial condition may be materially adversely affected, and we may not achieve profitability.
We may not be able to maintain or expand market acceptance for metreleptin and lomitapide in the U.S. or to gain market acceptance in markets outside the U.S. where we commercialize such products, and, for lomitapide, we may continue to see a significant number of patients who choose not to start or stay on therapy.
The commercial success of metreleptin and lomitapide will depend primarily upon our ability to maintain and expand the acceptance of these products by the medical community, including physicians and healthcare payers, and by the relevant patients in the U.S., and to gain and maintain such acceptance in countries outside the U.S. where such products are commercialized.
Some physicians and congenital or acquired GL patients may determine that the benefits of metreleptin in treating complications of leptin deficiency in GL do not outweigh the risks, including those risks set forth in the boxed warning for MYALEPT in the U.S., which warn of the risk of anti-metreleptin antibodies with neutralizing activity and the risk of lymphoma. Likewise, some physicians and homozygous familial hypercholesterolemia (HoFH) patients may determine that the benefits of lomitapide in reducing low-density lipoprotein cholesterol (LDL-C) levels do not outweigh the risks, including those risks described in the boxed warning for JUXTAPID in the U.S. and in the prescribing information for lomitapide in the other countries in which it is approved, which warn that lomitapide can cause hepatotoxicity.
Because of the risk of hepatotoxicity, JUXTAPID is available in the U.S. only through a Risk Evaluation Management Strategy (REMS) program, referred to as the JUXTAPID REMS program. Under the JUXTAPID REMS program, patients must receive education on the JUXTAPID REMS program requirements and we must certify all qualified healthcare providers before they can prescribe JUXTAPID and the pharmacies that will dispense the medicine. The U.S. Food and Drug Administration (FDA) assesses on a periodic basis whether a REMS program is meeting its goals and whether the goals or elements of the program should be modified. In June 2015, Aegerion Pharmaceuticals, Inc. (Aegerion) received a letter from the FDA expressing concern that the JUXTAPID REMS program is not meeting its goals of educating healthcare professionals about the risks of hepatotoxicity and the need to periodically conduct liver tests to monitor patients during treatment with JUXTAPID as set forth on the product label. The letter also expressed concern about the difficulty in assessing whether the goal of restricting access to JUXTAPID to patients with a clinical or laboratory diagnosis consistent with HoFH was being met. In response to the FDA’s concerns, we proposed to the FDA modifications to the JUXTAPID REMS program to improve prescriber awareness of the risk of hepatotoxicity associated with JUXTAPID and the need to monitor patients during treatment, and to reinforce the approved indication and the characteristics of HoFH. On March 11, 2016, Aegerion received from the FDA an additional letter describing certain modifications the FDA considered necessary to the labeling for JUXTAPID and to the JUXTAPID REMS program. In response to the FDA’s proposed modifications to the labeling for JUXTAPID, on April 8, 2016, Aegerion submitted a prior approval labeling supplement to the FDA addressing certain of the FDA’s proposed modifications, including an instruction that patients cease therapy upon the occurrence of severe diarrhea. The labeling changes were approved by the FDA on May 23, 2016. Aegerion submitted a response to the FDA’s proposal regarding modifications to the JUXTAPID REMS program in a prior approval supplement on July 7, 2016. The FDA approved the modifications to the JUXTAPID REMS program on January 3, 2017. The goal of the JUXTAPID REMS program, as modified, is to mitigate the risk of hepatotoxicity associated with the use of JUXTAPID by ensuring that: a) prescribers are educated about the approved indication for JUXTAPID, the risk of hepatotoxicity associated with the use of JUXTAPID, and the need to monitor patients during treatment with JUXTAPID as per product labeling; b) JUXTAPID is dispensed only to patients with a clinical or laboratory diagnosis consistent with homozygous familial hypercholesterolemia; and c) patients are informed about the risk of hepatotoxicity associated with the use of JUXTAPID and the need for baseline and periodic monitoring. The FDA’s approval letter for the modified REMS Program also specified that an authorized generic drug under JUXTAPID’s New Drug Application (NDA) must have an FDA-approved REMS program prior to marketing.
The originally approved JUXTAPID REMS program consisted of Elements To Assure Safe Use (ETASU), an implementation system, a communication plan and a timetable for submission of assessments of the JUXTAPID REMS program. It also required healthcare professionals who prescribe JUXTAPID and pharmacies that dispense JUXTAPID to be certified, and that JUXTAPID must only be dispensed to patients with evidence or other documentation of safe-use conditions. The ETASU in the modified JUXTAPID REMS program approved by the FDA on January 3, 2017 has been significantly enhanced and requires, among other things, that healthcare professionals and pharmacies complete a recertification process, which includes, for healthcare professionals, required online training and learning assessments, by July 2, 2017 in order to continue prescribing and dispensing JUXTAPID; healthcare professionals counsel existing and new JUXTAPID patients on the goals of the JUXTAPID REMS program and, in connection therewith, imposes a new requirement that healthcare professionals and their patients sign a form acknowledging that this counseling has taken place and that the patient understands the goals of the JUXTAPID REMS program; and prescriptions
47
written to a JUXTAPID patient before the healthcare professional completes recertification and the counseling requirements with the patient, including the submission to the REMS coordinating center of an acknowledgment form signed by the healthcare professional and the patient, will not be honored after July 2, 2017. The modified REMS program also requires that prescriptions written after July 2, 2017 must be written on an updated prescription authorization form and includes changes to existing REMS documentation, along with additional required documentation, and new training modules for healthcare professionals and certified pharmacies. The FDA required the modifications to the JUXTAPID REMS program to be implemented by March 2, 2017 and, as noted above, that healthcare professionals and pharmacies complete the recertification process, and healthcare professionals and patients complete the counseling and acknowledgment processes, by July 2, 2017. We have completed the implementation of the modifications to the JUXTAPID REMS program, and we are in the process of educating healthcare professionals, pharmacies, and patients about the JUXTAPID REMS program requirements, including the requirements that must be met by July 2, 2017, and tracking achievement with respect to these requirements. However, we may lose JUXTAPID patients temporarily or permanently, or add new adult HoFH patients at a slower than expected pace, as a result of the implementation of, and enhancements to, the modified JUXTAPID REMS program, as described above, for a variety of reasons, including: the inability to recertify healthcare professionals with existing patients or to certify healthcare professionals who may want to put new patients on JUXTAPID, on a timely basis or at all; the failure of the healthcare professionals and patients, existing or new, to meet the patient counseling requirements and sign and submit the patient acknowledgment form, as required, on a timely basis or at all; the failure of prescriptions for JUXTAPID to meet all of the requirements of the modified JUXTAPID REMS program on or after July 2, 2017 and therefore not being honored by the certified pharmacies after such date, as required under the modified JUXTAPID REMS program, and any payer issues or delays that arise out of new prescriptions being written for patients under the modified JUXTAPID REMS program; and that the enhanced education of the goals of the JUXTAPID REMS program, and related documentation, may cause healthcare professionals to stop or delay treatment with JUXTAPID, or try alternative therapies for adult HoFH patients before starting or continuing JUXTAPID treatment. The ongoing investigations of the Securities and Exchange Commission (SEC) and the Department of Justice (DOJ), including the consent decree that Aegerion will enter into with FDA related to JUXTAPID REMS program as part of the settlement of these investigations, may also have an effect on the FDA’s requirements for the JUXTAPID REMS program.
In addition, we have adopted risk management plans in other countries where we have obtained approval of lomitapide to help educate physicians on the safety information for lomitapide and appropriate precautions to be followed by healthcare professionals and patients. Other countries that may approve metreleptin or lomitapide may require risk management plans that may be similar to or more onerous than those we have adopted to date. The prescribing information for each product also describes a number of additional contraindications, warnings, and precautions, including those related to pregnancy and potential adverse interactions with other drugs, and other potential adverse reactions, that could limit the market acceptance of metreleptin and lomitapide. These contraindications, warnings, and precautions make it more difficult for some patients to decide to begin therapy or to stay on therapy. GI adverse reactions, which are common with lomitapide and the risk of which can be reduced only by adherence to a low-fat diet, and elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also lead to treatment discontinuation in a significant percentage of lomitapide patients. With respect to metreleptin, concerns related to the route of administration of metreleptin, as a daily injection, may deter some patients from beginning therapy or staying on therapy. As a result, even if a physician prescribes one of our products, the prescription may not result in a patient beginning therapy or staying on therapy. The degree of market acceptance of our products will also depend on a number of other factors, including:
• | physicians’ views as to the scope of the approved indication and limitations on use and warnings and precautions contained in the approved labeling or prescribing information for our products, including the boxed warnings on the MYALEPT and JUXTAPID labels and the modifications to the JUXTAPID label to include language instructing patients to cease therapy upon the occurrence of severe diarrhea; |
• | the willingness of insurance companies, managed care organizations, other private payers, and government entities in the U.S. that provide reimbursement for medical costs to continue to provide reimbursement for MYALEPT and JUXTAPID at the price at which we offer them and without imposing restrictions on the use of the product, such as, for MYALEPT, leptin level tests, which delay or otherwise impact reimbursement; |
• | the ability and willingness of GL and HoFH patients to pay, or to arrange for payment assistance with respect to, any patient cost-sharing amounts for MYALEPT applicable under their insurance coverage, and the availability of co-pay assistance; |
• | the extent to which the changes to the JUXTAPID REMS program, approved by the FDA on January 3, 2017, including the requirements set forth above, may negatively affect the ability or willingness of a physician to prescribe JUXTAPID, a patient to be willing to initiate or continue on JUXTAPID therapy, or insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs to continue to provide reimbursement for JUXTAPID; |
48
• | the extent to which changes to the labeling for JUXTAPID instructing patients to cease therapy upon the occurrence of severe diarrhea may negatively affect the ability or willingness of a physician to prescribe JUXTAPID, a patient to be willing to initiate or continue on JUXTAPID therapy, or insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs to continue to provide reimbursement for JUXTAPID; |
• | the efficacy, safety and tolerability of competitive therapies, including, in the case of lomitapide, PCSK9 inhibitor products; |
• | the extent of the negative impact of the availability of PCSK9 inhibitor products on sales of JUXTAPID in the U.S., which has caused a significant number of JUXTAPID patients to discontinue JUXTAPID and switch to a PCSK9 inhibitor product, and has significantly decreased the rate at which new HoFH patients start treatment with JUXTAPID; |
• | the provision of free PCSK9 inhibitor drug to adult HoFH patients by the companies that are commercializing PCSK9 inhibitor products, which such companies may have ceased, but which historically has had a negative impact on the rate at which new patients start treatment with lomitapide and has caused more patients than we expected to discontinue lomitapide and switch their treatment to PCSK9 inhibitor products; |
• | pricing and the perception of physicians and payers as to cost effectiveness of our products in relation to other therapies that treat GL and HoFH, respectively, including therapies with a price substantially lower than that of our products, which, in the case of lomitapide, includes PCSK9 inhibitor products and apheresis; and |
• | the effectiveness of our sales, marketing and distribution strategies and our ability to achieve these strategies, particularly in light of our conversion from a full-time employee sales force to the use of primarily a contract sales force in the U.S. in early 2017, the use of a contract sales force in Japan, and the continuing challenges to the lomitapide business, including, among other things, the impact of competitive products on JUXTAPID sales. |
If we are not able to achieve a high degree of market acceptance of metreleptin in the treatment of GL and lomitapide in the treatment of adult patients with HoFH, we may not be able to achieve our revenue goals or other financial goals or to achieve profitability or to maintain cash-flow positive operations in the time periods we expect, or at all.
If we fail to obtain or maintain orphan drug exclusivity for our products or product candidate in any country where exclusivity is available, we will have to rely on our data and marketing exclusivity, if any, and on our intellectual property rights, to the extent there is coverage in such country, which may reduce the length of time that we can prevent competitors from selling generic versions of our products or product candidate.
We have obtained orphan drug exclusivity for JUXTAPID in the U.S. for the treatment of HoFH, for MYALEPT in the U.S. for the treatment of GL, and for zuretinol in the U.S. for the treatment of LCA (due to inherited mutations in the LRAT or RPE65 genes) and RP (all mutations), which the FDA has formally acknowledged also cover zuretinol for the treatment of IRD, including SECORD, which disease/condition we believe comprises both LCA (due to inherited mutations in the LRAT or RPE65) genes and RP. Since the extent and scope of our patent protection for zuretinol is limited, orphan drug designation is especially important for this product candidate.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug or biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S., or for which there is no reasonable expectation that development and production costs will be recovered from sales of the drug for such disease or condition in the U.S. Orphan drug designation must be requested before submitting an NDA or Biologics License Application (BLA). After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. If a product that has orphan drug designation subsequently receives FDA approval and is the first drug approved for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve for seven years any other applications to market the same drug for the same indication, except in limited circumstances such as a demonstration that the subsequent drug is clinically superior or the inability of the existing manufacturer to supply the market. Orphan drug exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval for an orphan product that the FDA finds to be the “same drug” as our product candidate for the same indication or disease. If a drug or biologic designated as an orphan drug receives marketing approval for an indication broader than the scope of its designation, it may be no longer entitled to orphan drug exclusivity. In addition to creating a 12-year period of reference product exclusivity, the Biologics Price Competition and Innovation Act (BPCI Act) clarifies the interaction of that exclusivity with orphan drug exclusivity, such that the licensure of a biosimilar or interchangeable version of a reference biological product that was designated and approved as an orphan drug may only occur after the later of the expiration of any applicable seven-year orphan drug exclusivity or the 12-year reference product exclusivity (or seven and one-half years and 12.5 years with pediatric exclusivity).
49
The BPCI Act, enacted in 2010, as part of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (the Health Care Reform Act), authorizes the FDA to license a biological product that is biosimilar to, and possibly interchangeable with, a Public Health Service Act (PHSA)-licensed reference biological product through an abbreviated pathway. The BPCI Act establishes criteria for determining that a product is biosimilar to an already-licensed biologic (a reference product), and establishes a process by which an abbreviated BLA for a biosimilar product is submitted, reviewed and approved. The BPCI Act provides periods of exclusivity that protect a reference product from biosimilar competition. Under the BPCI Act, innovator manufacturers of original reference products are granted twelve years of exclusive use before biosimilar versions of such products can be licensed for marketing in the U.S. This means that the FDA may not approve an application for a biosimilar version of a reference product until twelve years after the date of approval of the reference product (with a potential six-month extension of exclusivity if certain pediatric studies are conducted and the results of such studies are reported to FDA), although a biosimilar application may be submitted four years after the date of licensure of the reference product. Additionally, the BPCI Act establishes procedures by which the biosimilar applicant must provide information about its application and product to the reference product sponsor, and by which information about potentially relevant patents is shared and litigation over patents may proceed in advance of approval. The BPCI Act also provides a period of exclusivity for the first biosimilar to be determined by the FDA to be interchangeable with the reference product. Under the BPCI Act, metreleptin has twelve years of exclusivity in the U.S. from February 24, 2014, the date of the product’s approval by the FDA.
The European Medicines Agency (EMA) grants orphan designation to promote the development of products that treat life-threatening or chronically debilitating conditions affecting not more than five in 10,000 people in the European Union (EU). In addition, orphan drug designation can be granted only if, for economic reasons, the medicinal product would be unlikely to be developed without incentives and if there is no other satisfactory method approved in the EU of diagnosing, preventing, or treating the condition, or if such a method exists, the proposed medicinal product must potentially be of significant benefit to patients affected by the condition. The application for orphan designation must be granted by the European Commission (EC) before an application for marketing authorization of the medicinal product is submitted. Upon grant of marketing authorization for the medicinal products, orphan designation provides ten years of market exclusivity for the orphan medicinal product in the orphan indication. During this ten-year period, with a limited number of exceptions, the competent authorities of the EU Member States and the EMA may not accept applications for marketing authorization, accept an application to extend an existing marketing authorization or grant marketing authorization for other similar medicinal products for the same orphan indication. Under an exception, marketing authorization could be granted to a similar medicinal product with the same orphan indication before the expiry of the ten years if the holder of the marketing authorization for the original orphan medicinal product has given its consent or if the manufacturer of the original orphan medicinal product is unable to supply sufficient quantities. Marketing authorization may also be granted to a similar medicinal product with the same orphan indication if this product is safer, more effective or otherwise clinically superior to the original orphan medicinal product. Moreover, the exclusivity period for the original orphan medicinal product may be reduced to six years if the designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. Despite the prevalence rate, lomitapide does not have orphan medicinal product exclusivity in the EU for the treatment of HoFH because the EMA views the relevant condition, for orphan drug purposes, to include both HoFH and HeFH. In 2012, metreleptin was granted orphan designation by the EC for the treatment of Barraquer-Simons syndrome (acquired partial lipodystrophy (PL)), Berardinelli-Seip syndrome (congenital GL), Lawrence syndrome (acquired GL) and familial PL. Zuretinol was granted orphan designation by the EMA in 2011 for the treatment of LCA and RP (all mutations) and in 2014, the EMA also formally acknowledged that a therapeutic indication of zuretinol for the treatment of patients with IRD, who have been phenotypically diagnosed as LCA or RP caused by mutations in RPE65 or LRAT genes, would fall under the orphan drug designations of treatment of LCA and treatment of RP. We also plan to seek regulatory exclusivity for zuretinol in the EU; however, there can be no assurance that we will be successful in securing approval or regulatory exclusivity in the EU.
Orphan drug exclusive marketing rights in the U.S. may also be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. Our failure to obtain or maintain orphan drug exclusivity would require us to rely on our data and marketing exclusivity, if any, and on our intellectual property rights for our products and product candidate, which may reduce the length of time that we can prevent competitors from selling generic versions of our products or product candidate. For further information, see “Risks Related to Our Intellectual Property.” If we do not obtain data exclusivity for our products or product candidate, our business may be materially harmed.
In Japan, where we launched JUXTAPID in December 2016, we have received orphan drug designation for JUXTAPID in the treatment of HoFH from Japan’s regulatory authority, the MHLW. We also have other forms of regulatory exclusivity for our products and product candidate in certain other markets. However, there are many other countries, including some key markets for lomitapide, like Brazil, in which we do not have intellectual property coverage for our products or product candidate, and where neither orphan drug exclusivity nor data and marketing exclusivity is available.
50
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. In the U.S., even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care, which could materially adversely affect our financial condition.
The number of patients affected by the diseases for which our products are approved, or for which we may seek approval, is small, and has not been established with precision. Our assumptions and estimates regarding prevalence may be wrong. If the actual number of patients is smaller than we estimate or if any approval is based on a narrower definition of these patient populations, our revenue and ability to achieve profitability and to maintain cash-flow positive operations from our product businesses will be adversely affected, possibly materially.
There is no patient registry or other method of establishing with precision the actual number of HoFH or GL and PL patients with the diseases our products treat in any geography. There is significant uncertainty around the estimated total potential addressable patient population for treatment with zuretinol worldwide.
Medical literature has historically reported the prevalence rate of HoFH as one person in a million, based on an estimated prevalence rate for HeFH of one person in 500. Analysis of HoFH prevalence have been evolving in recent years cumulating in published medical literature that suggests that the actual prevalence of both HeFH and HoFH may be significantly higher than the historical estimate of one person in a million. For example, in 2014, the European Atherosclerosis Society (EAS) Consensus Panel on Familial Hypercholesterolemia (FH) published an article citing research that would result in an estimate of the prevalence of HoFH in the range of between one person in 300,000 and one person in 160,000 or 3.33 persons per million to 6.25 persons per million, which is consistent with estimates that can be derived from other publications from the last few years. The FDA cited this estimate in its review of PCSK9 inhibitor products in June 2015. There is no guarantee that the prevalence of HoFH is higher than the current medical literature suggests or is even higher than reported in the historical literature. The number of patients with HoFH could actually be significantly lower than we expect. Ultimately the actual size of the total addressable HoFH market in the U.S. will be determined only after we and others have substantial commercial history selling products for the treatment of HoFH.
We believe that the prevalence rate of HoFH in countries outside the U.S. is likely to be consistent with the prevalence rate in the U.S.; however, we expect that our net product sales in countries outside the U.S. are likely to be lower than in the U.S. given significant economic pressures to reduce healthcare costs in certain ex-U.S. countries, resulting in pricing controls, reimbursement restrictions and caps on patients treated and/or drug expenditures, the more widespread availability of apheresis, in certain countries, like Japan, and the possibility that genotyping may be required in some countries, reducing the number of patients diagnosed with HoFH.
The data to date suggest that the approximate prevalence of GL in the U.S. is slightly under 1 in 1,000,000 persons and for PL overall is 3 in 1,000,000 persons. Although the data are even more limited, the prevalence in the U.S. of a subset of more severe PL is estimated to be between 0.5 and 1 in 1,000,000 persons. We believe that the prevalence rate of GL and PL, and correspondingly the PL subset, in countries outside the U.S. is likely to be consistent with the prevalence rate in the U.S. There is no guarantee, however, that our estimates are correct. The actual prevalence of GL, PL and the PL subset may be significantly lower than we expect. Ultimately, the actual size of the total addressable market in the U.S. and other key markets where metreleptin is sold, if approved, will be determined only after we have substantial commercial history selling metreleptin.
Current epidemiological estimates based on medical literature suggest that approximately 2.5% of the autosomal recessive RP subjects and 6-10% of all LCA subjects possess mutations in the RPE65 gene, while approximately 1% of RP and LCA subjects have mutations in the LRAT gene. LCA is estimated to affect approximately one in 81,000 newborns worldwide, while the overall RP prevalence is estimated at one in 4,000 newborns worldwide. Based on our current market research, we estimate the total potential addressable LCA patient population for zuretinol at 1,000 to 2,000 patients worldwide and the total potential addressable RP patient population at 2,000 to 4,000 patients worldwide. Our most recent epidemiological data estimate the prevalence of IRD subjects with RPE65 or LRAT mutations at 4,100 patients in the U.S. and the five major European markets, a portion of whom have the late stage of the disease and may not benefit from zuretinol therapy. While geographic differences in the gene pool may cause fluctuations, the prevalence of LCA, RP, and LRAT and RPE65 mutation distribution in other countries are believed to be comparable with the U.S. and five major European markets based on current medical literature. However, there is no guarantee these current estimates are correct, and the analysis of the prevalence in IRD remains ongoing in the medical literature.
Estimating the prevalence of a rare disease is difficult and may rely on an amalgam of information from multiple sources, resulting in potential under- or over-reporting. There is no guarantee that our assumptions and beliefs are correct, or that the methodologies used have generated accurate estimates. Medical literature has historically estimated the prevalence of the diseases our products treat to be significantly lower than our estimates. The actual prevalence of these diseases may be significantly lower than we expect. Ultimately, the actual size of the total addressable market will be determined only after we have substantial commercial
51
history selling the relevant product. If the total addressable market for our products and product candidate, if eventually approved and commercialized, in the U.S. and other key markets is smaller than we expect, then it may be more difficult for us to achieve our revenue goals and estimates and to attain profitability and meet our expectations with respect to cash flow operations.
Our market is subject to intense competition. If we are unable to compete effectively, we may not be able to achieve our revenue goals or achieve profitability or maintain cash-flow positive operations in the time periods we expect, or at all, and lomitapide, metreleptin or any other product candidate that we develop or acquire may be rendered noncompetitive or obsolete.
Our industry is highly competitive and subject to rapid and significant technological change. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. All of these competitors currently engage in, have engaged in or may engage in the future in the development, manufacturing, marketing and commercialization of new pharmaceuticals, some of which may compete with lomitapide, metreleptin, zuretinol or other products or product candidates we may acquire, license or develop. Smaller or early stage companies may also be significant competitors, particularly through collaborative arrangements with large, established companies. Key competitive factors affecting the commercial success of lomitapide, metreleptin and any other products that we develop or acquire are likely to be efficacy, safety and tolerability profile, reliability, convenience of dosing, price and reimbursement.
The market for cholesterol-lowering therapeutics is large and competitive with many drug classes. Lomitapide is approved in the U.S., Japan, the EU and in certain other countries as an adjunct to a low-fat diet and other lipid-lowering treatments to reduce LDL-C in adult HoFH patients. As a treatment for HoFH, JUXTAPID competes in the U.S. and certain other countries with Kynamro. Developed by Ionis Pharmaceuticals, Inc. (Ionis) and acquired by Ionis and Kastle Therapeutics in May 2016, Kynamro is an antisense apolipoprotein B-100 inhibitor which is taken as a weekly subcutaneous injection. JUXTAPID also faces significant competition in the treatment of adult HoFH patients with a class of drugs known as PCSK9 inhibitors. In July 2015, Regeneron Pharmaceuticals, Inc. (Regeneron) and Sanofi announced that the FDA had approved the BLA for their PCSK9 inhibitor candidate, alirocumab, for use in addition to diet and maximally tolerated statin therapy in adult HeFH patients and in patients with clinical atherosclerotic cardiovascular disease who require additional lowering of LDL-C. In September 2015, following the positive opinion of the Committee for Medicinal Products for Human Use (CHMP) of the EMA, the EC approved alirocumab for the treatment of adult patients with HeFH or mixed dyslipidemia as an adjunct to diet, either in combination with a statin, or statin with other lipid-lowering therapies in patients unable to reach their LDL-C goals with the maximally-tolerated statin, or alone or in combination with other lipid-lowering therapies for patients who are statin intolerant, or for whom a statin is contraindicated. The FDA approved Amgen Inc.’s (Amgen) BLA for its anti-PCSK9 antibody, evolocumab, in August 2015, as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with HeFH or clinical atherosclerotic cardiovascular disease, who require additional lowering of LDL-C; and as an adjunct to diet and other LDL-lowering therapies for the treatment of patients with HoFH, who require additional lowering of LDL-C. In July 2015, the EC Commission approved the marketing authorization of evolocumab for the same indication as alirocumab, and for the treatment for certain patients with high cholesterol, including patients aged 12 years and over with HoFH in combination with other lipid-lowering therapies. In January 2016, the MHLW approved evolocumab for the treatment of patients with FH or hypercholesterolemia who have high risk of cardiovascular events and do not adequately respond to statins, and in July 2016 the MHLW approved alirocumab for the same indication. Health Canada and Brazil’s National Health Surveillance Agency (ANVISA) have approved evolocumab for the treatment of patients with HoFH. Other companies, including Roche Holding AG and Alnylam Pharmaceuticals, Inc., in collaboration with The Medicines Company, are also developing PCSK9 inhibitor products.
The introduction of PCSK9 inhibitors in the U.S. has negatively impacted sales of JUXTAPID and we expect this negative trend to continue. This impact results from several factors, including: healthcare professionals switching some existing JUXTAPID patients to a PCSK9 inhibitor product; healthcare professionals trying most new adult HoFH patients on a PCSK9 inhibitor product before trying JUXTAPID; the provision of free PCSK9 drug to adult HoFH patients by the companies that are commercializing PCSK9 inhibitor products, which such companies may have ceased, but which historically has had a negative impact on the rate at which new patients start treatment on JUXTAPID and has caused more patients than we expected to discontinue JUXTAPID and switch their treatment to PCSK9 inhibitor products; and actions by insurance companies, managed care organizations and other private payers in the U.S. that have required, or may require in the future, HoFH patients to demonstrate an inability to achieve an adequate LDL-C response on PCSK9 inhibitor products before access to JUXTAPID is approved, or may impose other hurdles to access or other significant restrictions or limitations on reimbursement, or may require switching of JUXTAPID patients to PCSK9 inhibitor products. Many U.S. insurance companies, managed care organizations and other private payers now require that HoFH patients fail to achieve an adequate response in LDL-C reduction on PCSK9 inhibitor products before providing reimbursement for JUXTAPID. For patients currently taking JUXTAPID, we believe that all U.S. payers require prior authorization, which may influence a switch of the current JUXTAPID patients to the less expensive PCSK9 inhibitor product. We believe that many of the PCSK9 inhibitor switches from current JUXTAPID patients have been at the direction of the prescribing physician. Ultimately, the physician may decide to switch the adult HoFH patient back to JUXTAPID, if the patient does not reach a goal of
52
LDL-C response while being treated with a PCSK9 inhibitor product. It is unknown how many adult HoFH patients, if any, may be switched back to JUXTAPID or the period of time in which this would take place. We expect physicians will continue to consider use of JUXTAPID for those adult HoFH patients who do not adequately respond to PCSK9 inhibitor products. Additionally, we expect that the availability of PCSK9 inhibitor products in commercial markets outside of the U.S. will have similarly negative effects on sales, including named patient sales, of lomitapide outside the U.S., particularly in Brazil, Canada and Japan, where PCSK9 inhibitor products have been approved by the regulatory authorities. If the continued negative impact of PCSK9 inhibitors is greater than we expect, it may make it more difficult for us to generate revenues and achieve profitability. Also, although there are no other microsomal triglyceride transfer protein (MTP) inhibitor (MTP-I) compounds currently approved by the FDA for the treatment of hyperlipidemia in humans, there may be other MTP-I compounds in development.
In addition, in the EU, patients with HoFH who are unable to reach their recommended target LDL-C levels on conventionally used drug therapies are commonly treated using LDL apheresis, in which cholesterol is removed from the body through mechanical filtration. Although levels of LDL-C are reduced acutely using apheresis, there is a rapid rebound. Because apheresis provides only temporary reductions in LDL-C levels, it must be repeated frequently, typically one or two times per month. The widespread use and availability of apheresis as a treatment for HoFH in the EU, combined with the lower cost of apheresis as compared to LOJUXTA (lomitapide) hard capsules (LOJUXTA), made it more difficult for us to obtain commercially acceptable pricing and reimbursement approvals for LOJUXTA in the key markets of the EU. As a result of this, and in an effort to reduce costs associated with EMA post-marketing requirements, Aegerion elected to cease commercialization in the EU, and in December 2016, entered into a license agreement with Amryt Pharma plc (Amryt) under which Amryt was granted an exclusive right to develop and commercialize LOJUXTA in the European Economic Area, Switzerland, Turkey, and certain Middle Eastern and North African countries, including Israel.
MYALEPT is the first and only product approved in the U.S. for the treatment of complications of leptin deficiency in patients with GL. There are, however, a number of therapies approved to treat these complications independently that are not specific to GL. Certain clinical complications of GL, including diabetes and hypertriglyceridemia, may be treated with insulin and/or oral medications, such as metformin, insulin secretagogues, fibrates, or statins. Patients with GL often have an inadequate response to these therapies.
We may also face future competition from companies selling generic alternatives of our products in countries where we do not have patent coverage, orphan drug status or another form of data or marketing exclusivity or where patent coverage or data or marketing exclusivity has expired, is not enforced, or may, in the future, be challenged.
Many of our current and potential competitors have substantially greater financial, technical and human resources than we do, and significantly greater experience in the discovery and development of drug candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining marketing approvals for drugs and achieving and maintaining widespread market acceptance.
Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize, and may render lomitapide, metreleptin, zuretinol or any other product or product candidate that we acquire, license or develop obsolete or non-competitive before we can recover the expenses of developing and commercializing the product. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render lomitapide, metreleptin, zuretinol or any other product or product candidate that we acquire, license or develop non-competitive or obsolete.
As a result of the side effects observed in the clinical and preclinical studies for each of lomitapide and metreleptin, the prescribing information for each of lomitapide and metreleptin contains or is expected to contain significant limitations on use and other important warnings and precautions, including boxed warnings in the U.S. labeling. Our products may continue to cause such side effects or have other properties that could impact market acceptance and dropout rates, result in adverse limitations in any approved labeling or other adverse regulatory consequences, including delaying or preventing additional marketing approval in territories outside the U.S., EU and other countries where lomitapide is approved, or in the case of metreleptin, marketing approval outside the U.S.
The MYALEPT label in the U.S. has a boxed warning, citing the risk of anti-metreleptin antibodies with neutralizing activity and risk of lymphoma. The prescribing information for lomitapide in the U.S. and the EU and in the other countries in which lomitapide is approved contains significant limitations on use and other important warnings and precautions, including a boxed warning in the JUXTAPID labeling, and warnings in the LOJUXTA prescribing information citing concerns over liver toxicity associated with use of lomitapide.
53
The most common adverse reactions in a Phase 3 study of lomitapide were gastrointestinal, reported by 27 (93%) of 29 patients. Adverse reactions, reported by greater than or equal to 8 patients (28%) in the HoFH clinical trial, included diarrhea, nausea, vomiting, dyspepsia and abdominal pain. Other common adverse reactions, reported by five to seven (17-24%) patients, included weight loss, abdominal discomfort, abdominal distension, constipation, flatulence, increased ALT, chest pain, influenza, nasopharyngitis, and fatigue. Elevations in liver enzymes and hepatic (liver) fat were also observed. Ten of the 29 patients in the study had at least one elevation in liver enzymes greater than or equal to three times the upper limit of normal (ULN), including four patients who experienced liver enzymes greater than or equal to five times the ULN. During the clinical trial, liver enzyme elevations were managed through dose reduction or temporary discontinuation of dose. There were no clinically meaningful elevations of total bilirubin, international normalized ratio (INR) or alkaline phosphatase, which are other markers of potential harmful effects on the liver. Hepatic fat increased from a baseline of 1% to a median absolute increase of 6% at 26 and 78 weeks.
In a Phase 3 study of metreleptin, the most common adverse drug reactions occurring in GL patients were weight decrease (reported by fifteen patients; 22.7%) and hypoglycemia (reported by eight patients; 12.1%), followed by decreased appetite, fatigue and neutralizing antibodies (each reported by four patients; 6.1%). The most common adverse drug reactions (ADRs) occurring in PL subgroup patients were hypoglycemia and fatigue (each reported by three patients; 9.7%), followed by alopecia (reported by two patients; 6.5%). Over the 14-year study duration, treatment-emergent deaths were reported in 4 (4%) of the 107 patients; treatment-emergent adverse events (TEAEs) leading to death were consistent with the underlying morbidity of lipodystrophy (LD) and included renal failure, cardiac arrest (with pancreatitis and septic shock), progressive end-stage liver disease (chronic hepatic failure), and hypoxic-ischemic encephalopathy. None of the deaths were assessed as drug-related.
The safety and efficacy of metreleptin for the treatment of metabolic disorders associated with LD syndromes in pediatric and adult patients were evaluated in a long-term, open-label, single-arm study conducted at the National Institutes of Health (the NIH). The objective of the NIH trial was to evaluate the efficacy of metreleptin for improving metabolic disorders associated with acquired or inherited lipodystrophy. This investigator-sponsored study was initiated in August 2000. Two cases of peripheral T-cell lymphoma and one case of a localized anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (a type of T-cell lymphoma) were reported, all in patients with acquired GL. Lymphoma is known to be associated with autoimmune disease. As the boxed warning for MYALEPT states, T-cell lymphoma has been reported in patients with acquired GL, both treated and not treated with MYALEPT. There was evidence of pre-existing lymphoma and/or bone marrow/hematologic abnormalities in the two patients with peripheral T-cell lymphoma before metreleptin therapy, and the third case of anaplastic large cell lymphoma occurred in the context of a specific chromosomal translocation.
As part of our post-marketing commitment to the FDA and Health Canada for lomitapide, we have initiated an observational cohort study to generate more data on the long-term safety profile of lomitapide, the patterns of use and compliance and the long-term effectiveness of controlling LDL-C levels. As part of the post-marketing commitments to the FDA for metreleptin, we plan to initiate a long-term, prospective, observational study (product exposure registry) in patients to evaluate serious risks related to the use of the product. We have completed the first, and have begun the second, of three sequential programs to expand the understanding of the immunogenicity of metreleptin, and we have initiated certain studies related to the manufacturing of metreleptin. A failure to meet post-marketing commitments to the FDA or other regulatory authorities could impact our ability to continue to market lomitapide or metreleptin, respectively, in countries where we are unable to meet such commitments.
In addition, as part of our observational cohort studies or in the conduct of additional clinical studies or in post-marketing surveillance of our products, we or others may identify additional safety information on known side effects or new undesirable side effects caused by our products, or the data may raise other issues with respect to our products, and, in that event, a number of potentially significant negative consequences could result, including:
• | we may experience a negative impact on market acceptance and dropout rates; |
• | regulatory authorities may suspend, withdraw or alter their approval of the relevant product; |
• | regulatory authorities may require the addition of labeling statements, such as warnings or contraindications or distribution and use restrictions, such as, for example, the modifications to the JUXTAPID label to include language instructing patients to cease therapy upon the occurrence of severe diarrhea; |
• | regulatory authorities may require us to issue specific communications to healthcare professionals, such as “Dear Doctor” letters; |
• | regulatory authorities may issue negative publicity regarding the relevant product, including safety communications; |
• | we may be required to change the way the relevant product is administered, conduct additional preclinical studies or clinical trials or restrict the distribution or use of the relevant product; |
• | we could be sued and held liable for harm caused to patients; |
54
• | the regulatory authorities may require us to amend the relevant REMS or risk management plan; and |
• | our reputation may suffer. |
As part of the development of the commercial manufacturing process of lomitapide, we tightened specifications for lomitapide drug substance such that the commercial drug substance differs from the material used in our Phase 3 trial in certain physical parameters and specifications that we assessed not to be clinically meaningful. Exposure measurements collected in the Japanese pharmacokinetic and pharmacodynamic (PK/PD) study using material meeting current commercial specifications, however, do not align with certain earlier data generated under different circumstances using pre-commercial materials. Importantly, there was no evidence of a relationship between increases in dose or exposure and elevations in ALT or AST levels in the PK/PD study. While we do not expect the differences between our commercial material and our clinical material to have adverse efficacy or safety consequences, there is a risk that we may see unexpected differences in the type or severity of side effects with the commercial product from those observed in our Phase 3 trial. There is also the risk that regulatory authorities may not agree with our assessment of the differences between the materials or the potential impact of such differences or may require changes in the prescribing information.
Any known safety concerns for our products and product candidate or any unknown safety issues that may develop could prevent us from achieving or maintaining market acceptance of the respective product or product candidate, could affect our ability to obtain or retain marketing approval of the respective product or product candidate in one or more countries, or result in onerous restrictions on such approval, or could affect our ability to achieve our financial goals.
If we are unable to establish and maintain effective sales, marketing and distribution capabilities, or to enter into agreements with third parties to do so, we will be unable to successfully commercialize our products or product candidates.
We are selling JUXTAPID and MYALEPT in the U.S., in part, by using a contract sales force, and are also selling JUXTAPID in Japan using a contract sales force. We are marketing and selling, or plan to market and sell, lomitapide directly, using our own marketing and sales resources, in certain key countries outside the U.S. in which lomitapide is, or may be, approved, or where we can make lomitapide available on a named patient sales basis, in either case where it makes business sense to do so. We also plan to market and sell metreleptin directly in key countries outside of the U.S., if approved in such countries. We use, and plan to use, third parties to provide warehousing, shipping, third-party logistics, invoicing, collections and other distribution services on our behalf in the U.S. and in other countries throughout the world. For example, we currently have a contract with a single specialty pharmacy distributor in the U.S. for the distribution of lomitapide, a single specialty pharmacy distributor in the U.S. for the distribution of metreleptin, a single distributor in Brazil, and single distributors, importers and/or specialty pharmacies in certain other countries. We have entered into, or may selectively seek to establish, distribution and similar forms of arrangements to reach patients in certain geographies that we do not believe we can cost-effectively address with our own sales and marketing capabilities. If we are unable to establish and maintain the capabilities to sell, market and distribute our products, either through our own capabilities or through arrangements with third parties, and to effectively manage those third parties when we choose to use them, or if we are unable to enter into distribution agreements in those countries that we do not believe we can cost-effectively address with our own sales and marketing capabilities, we may not be able to successfully sell our products. We cannot guarantee that we will be able to establish and maintain our own capabilities or to enter into and maintain favorable distribution agreements with third-parties on acceptable terms, if at all.
To the extent that we enter into arrangements with third parties to perform sales, marketing or distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to commercialize our products ourselves. We likely will have limited control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively, and may also, despite our compliance diligence reviews, audits and training, engage in non-compliant activities that, directly or indirectly, impact the use or sales of our products or damage our relationships with relevant stakeholders. Any performance failure, inability or refusal to perform on the part of our specialty pharmacy distributors in the U.S., our third-party sales forces in the U.S. or Japan, our distributor in Brazil, or our third-party service providers in certain other countries, or any failure to renew existing agreements or enter into new agreements when these relationships expire, could, for a period of time, impair our marketing, sales or named patient supply of our products. Furthermore, even following Aegerion’s 2016 reductions in force, which were intended to align overall company expenses with top-line revenues, in light of decreasing lomitapide revenues, our expenses associated with building up and maintaining the sales force and distribution capabilities around the world may be substantial compared to the revenues we may be able to generate on sales of our products. If we are unable to establish and effectively maintain adequate sales, marketing and distribution capabilities, whether independently or with third parties, particularly as we continue to assess our cost structure in light of declining revenues of JUXTAPID in the U.S., we may not be able to generate product revenue consistent with our expectations and may not become profitable or maintain cash flow positive operations.
55
Our success is dependent upon obtaining regulatory approval for our products and product candidates. The regulatory approval process is costly and lengthy, and we may not receive the regulatory approvals we seek for commercialization and reimbursement of our products and product candidates.
We are currently permitted to market lomitapide in only a small number of countries on a commercial basis, and to market metreleptin in the U.S. Shionogi holds a marketing authorization for metreleptin in Japan under a distribution agreement assigned to us as part of the our acquisition of the metreleptin assets. There is no assurance that we will be able to obtain marketing authorizations for either product in additional countries, or any marketing authorization for zuretinol. To obtain marketing approvals, we must establish, and comply with, numerous and varying regulatory requirements regarding safety and efficacy and governing, among other things, clinical trials, pricing, promotion and distribution of the respective product. Approval procedures vary among countries, and can involve additional product testing and additional administrative review periods. Marketing approval in one country does not ensure such approval in another.
Regulatory authorities in countries where we seek approval for our products may not be satisfied with the design, size, endpoints or efficacy and safety results of the pivotal trial of the product, or the risk/benefit profile of the product, and may reject our applications for approval. For example, we filed to register JUXTAPID as a marketed product in Brazil, and, in May 2014, appealed a rejection of the registration by ANVISA. We subsequently withdrew our appeal, and while we intend to resubmit our marketing application with additional data and information, we are currently assessing the timing of this submission. Even if we do resubmit our application, as we intend to do, we may not be successful in receiving regulatory approval to market JUXTAPID in Brazil. It is also possible that regulatory authorities in countries where we are seeking, or may in the future seek, approval may disagree with our assessment that certain changes made to lomitapide’s physical parameters and specifications as compared to the material used in the pivotal trial are not clinically meaningful. If regulatory authorities require additional studies or trials for either of our products or changes to specifications, we would incur increased costs and delays in the marketing approval process and may not be able to obtain approval.
In addition, regulatory authorities in countries outside the U.S. and EU are increasingly requiring risk management plans and post-marketing commitments, which may be more onerous than those required in the U.S. and EU. In certain countries, if the post-marketing commitment is a post-marketing study that would qualify as an interventional or similar form of study, we may be required to provide free product to participants in the study in such country even if our products are reimbursed there. The time required to obtain approval in other countries may differ from that required to obtain FDA approval or marketing authorization in the EU. In several countries outside the U.S. in which we are commercializing lomitapide, and in which we intend to commercialize metreleptin, if approved by the relevant regulatory authority, a product must also receive pricing and reimbursement approval before it can be commercialized broadly. This can result in substantial delays in commercializing products in such countries, and the price that is ultimately approved may be lower than the price for which we expect to offer, or would be willing to offer, lomitapide or metreleptin, in such countries, and may impact pricing in other countries. Pricing and reimbursement approval in one country does not ensure such approvals in another. Failure to obtain the approvals necessary to commercialize lomitapide or metreleptin in other countries at reimbursement levels that are acceptable to us or any delay or setback in obtaining such approvals would impair our ability to develop foreign markets for lomitapide and metreleptin. For example, because commercially acceptable pricing and reimbursement approvals for LOJUXTA were not obtained in several of the key markets of the EU, and in an effort to reduce costs associated with EMA post-marketing requirements, Aegerion elected to cease commercialization of LOJUXTA in the EU and, in December 2016, entered into a license agreement with Amryt under which Amryt was granted an exclusive license to develop and commercialize LOJUXTA in the EEA, Switzerland, Turkey, and certain Middle Eastern and North African countries, including Israel.
We rely on named patient sales in certain territories, but there is no assurance that named patient sales of lomitapide will continue at current levels, or at all, or that significant levels of named patient sales of metreleptin will be achieved in any country, or at all.
In Brazil and in a limited number of other countries where permitted based on U.S. or EU approval, lomitapide and metreleptin are available on a named patient sales or equivalent basis. There is no assurance that named patient sales will continue to be authorized in any particular country. If violations of any laws or governmental regulations are found to have occurred, significant civil lawsuits may be filed by the Public Prosecution office, and administrative penalties imposed by Brazilian regulatory authorities and additional damages and fines. Under certain circumstances, we could be barred from further sales to federal and/or state governments in Brazil, including sales of JUXTAPID and/or MYALEPT, due to penalties imposed by Brazilian regulatory authorities or through civil actions initiated by federal or state public prosecutors. We believe significant media coverage in Brazil in 2016 may increase the likelihood that such authorities will initiate inquiries and/or litigation. In addition, we believe the investigations in Brazil have contributed to a slower turn-around between price quotation and orders, including re-orders, from the federal government, and, in some cases, delays in orders and re-orders from the government of the State of São Paulo after a patient has obtained access to JUXTAPID through the judicial process. These delays may continue, and we may experience other
56
delays or suspension of the ordering process. Similarly, there has been, and may continue to be, some reluctance by physicians to prescribe JUXTAPID, and some patients to take or stay on JUXTAPID, while the investigations are ongoing, particularly given that some of the investigators in Brazil made formal inquiries of certain prescribers of JUXTAPID in 2016 and there has been significant local media coverage of such inquiries and our activities in Brazil. For example, in the second quarter of 2015 and in the second quarter of 2016, we observed a significant increase in patients discontinuing therapy in Brazil, and we believe that the increases during those times were due in part to the investigations. In addition, a proceeding is currently pending with the Brazil Supreme Federal Court to decide whether the government has an obligation to continue to provide, on a named patient sales basis, drugs that have not received regulatory and/or pricing and reimbursement approval in Brazil, like JUXTAPID and MYALEPT. We intend to file for marketing approval in Brazil for both JUXTAPID and MYALEPT, and are currently assessing the timing of these submissions. The result of the trial and other issues could negatively affect product revenues from named patient sales of JUXTAPID and MYALEPT in Brazil.
We do not yet know the full extent of the impact that the approval of PCSK9 inhibitor products in the U.S., or the approval of a PCSK9 inhibitor product in Brazil in April 2016, will have on the named patient sales of lomitapide in Brazil or any other country. We also do not know whether we will be permitted to sell metreleptin on a named patient basis in any countries besides Brazil, Argentina, Colombia, and the few other countries where we are currently permitted to do so. In certain countries, we may decide not to pursue named patient sales even if permitted to do so. Even if named patient sales or their equivalent sales are permitted in a certain country, and we elect to make lomitapide or metreleptin available on such basis in such country, there is no guarantee that physicians in such country will prescribe the product, and that patients will be willing to start therapy, or that the country will agree to pay for the product at all or at a level that is acceptable to us or, after access is granted, will continue to pay for the product at the levels initially approved, without delay or imposing other hurdles on payment, or at all, particularly in Brazil, in light of the recent approval of a PCSK9 inhibitor by the local Brazilian regulatory authority, ongoing state and federal government investigations, a decision by the Brazilian pharmaceutical industry association that we violated its Code of Conduct, and recent coverage by Brazilian media. There is no guarantee that we will generate sales or substantial revenue from such sales.
If named patient sales do not meet our expectations in key named patient sales markets, particularly Brazil, we may not be able to meet our expectations with respect to sales of lomitapide and metreleptin or revenues from such sales, maintain cash flow positive operations, or meet our expectations with respect to profitability in the time periods we anticipate or at all. There are also countries where we choose to make lomitapide and metreleptin available under an expanded access program at no cost prior to approval in such country. This program may result in significant expenses, and could impact our financial results.
We depend on single third-party manufacturers to produce our drug substance and drug product for each of our products and our product candidate. This may increase the risk that we will not have sufficient quantities of our products or product candidate, or will not be able to obtain such quantities at an acceptable cost, which could negatively impact commercialization of our products or delay, prevent or impair our clinical development programs.
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We rely on contract manufacturers to produce drug substance and drug product for commercial supplies and for our clinical trials.
We have long-term supply agreements with our lomitapide drug product and drug substance manufacturers, as well as supply agreements with our metreleptin drug substance and drug product manufacturers. We do not have agreements in place for redundant supply or a second source for drug substance or drug product for either of our products or our product candidate, zuretinol. Any termination or non-renewal of our agreements with our contract manufacturers could impact availability of lomitapide or metreleptin for commercial sale in any country where such product is approved for commercial sale or sold on a named patient basis, or may delay further clinical development or marketing approval of such product in additional countries. If for any reason our contract manufacturers cannot or will not perform as agreed, we may be required to replace such manufacturer. If we are unable to maintain arrangements for third-party manufacturing, or are unable to do so on commercially reasonable terms, or are unable to obtain timely regulatory approvals in connection with our contract manufacturers, we may not be able to successfully commercialize our products or complete development of our products or product candidate. We may incur significant added costs and substantial delays in identifying and qualifying any replacement manufacturers, and in obtaining regulatory approval to use such replacement manufacturer in the manufacture of our products. Any such delays could result in significant delay in the supply of a product candidate for an ongoing trial due to the need to replace a third-party manufacturer could delay completion of the trial. If for any reason we are unable to obtain adequate supplies of lomitapide, metreleptin, zuretinol or any other product candidate that we develop or acquire, or the drug substances used to manufacture them, it will be more difficult for us to compete effectively, generate revenue, meet our expectations for financial performance and further develop our products or product candidate. In addition, if we are unable to assure a sufficient quantity of the drug for patients with rare diseases or conditions, we may lose any orphan drug exclusivity to which the product otherwise would be entitled.
57
We rely on our contract manufacturers to utilize processes that consistently produce drug substance and drug product to their required specifications, including those imposed by the FDA, the EMA and other regulatory authorities, as applicable. There can be no assurance that our contractors will consistently be able to produce commercial supplies of drug substance or drug product meeting the approved specifications. A number of factors could cause production interruptions at the facilities of our contract manufacturers, including equipment malfunctions, facility contamination, labor problems, raw material shortages or contamination, natural disasters, disruption in utility services, terrorist activities, human error or disruptions in the operations of our suppliers. We have experienced failures by our third-party manufacturers to produce product that meets our specifications in the past, and any future failure by our third-party manufacturers to produce product that meets specifications could lead to a shortage of lomitapide or metreleptin.
The manufacture of biologic pharmaceuticals, such as metreleptin, is more difficult and more risky than the manufacture of small molecule pharmaceuticals, such as lomitapide or zuretinol. The process of manufacturing biologics is highly susceptible to product loss due to contamination, oxidation, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in metreleptin or the facilities of our contract manufacturer, we may need to cease manufacturing for an extended period of time to investigate and remediate the contaminant. A contamination, recall, raw material shortage, or other supply disruption could adversely impact or disrupt commercial manufacturing of metreleptin or could result in a withdrawal of metreleptin from the market. This, in turn, could adversely affect our ability to satisfy demand for metreleptin, which could materially and adversely affect our operating results and expectations for financial performance.
The FDA, the EMA and other regulatory authorities require that product candidates and drug products be manufactured according to current Good Manufacturing Practice (cGMP). Any failure by our third-party manufacturers to comply with cGMP could lead to a shortage of our products. In addition, such failure could be the basis for action by the FDA, the EMA or regulatory authorities in other territories or countries to withdraw approvals previously granted to us and for other regulatory action, including seizure, injunction or other civil or criminal penalties. For example, in February 2017, the contract manufacturer for metreleptin drug product received a Warning Letter from the FDA citing significant violations of cGMP regulations at the manufacturing facility where metreleptin drug product is manufactured. In response, the manufacturer may make modifications to the line on which metreleptin drug product is filled, and has committed, in the long-term, to transition the filling of certain drug products, including metreleptin drug product, to a newer line at the same facility and to cooperating with customers on a transition timeline to re-validate the filling process on the new line, such that this transition does not impact supply. Assuming a reasonable timeline for the future transition to and validation of the new filling line, we would have sufficient inventory to handle any downtime in the manufacturing process. The failure by the metreleptin drug product manufacturer to respond adequately to the Warning Letter, a delay in the future transition to, or validation of, a new filling line for metreleptin drug product, or the failure of any of our third-party manufacturers to address any concerns raised by the FDA or foreign regulators, could lead to plant shutdown or the delay or withholding of product approval by the FDA in additional indications, or by foreign regulators in any indication, including, with respect to metreleptin, the MAA in the EMA for GL and the PL subset. Certain countries may impose additional requirements on the manufacturing of drug products or drug substance, and on our third-party manufacturers, as part of the regulatory approval process for our products in such countries. The failure by us or our third-party manufacturers to satisfy such requirements could impact our ability to obtain or maintain approval of our products in such countries.
We may face resistance from certain private, government and other third-party payers and from healthcare professionals and patients given the prices we charge for metreleptin and lomitapide. We may not be able to achieve our revenue goals or achieve profitability or maintain cash-flow positive operations from the metreleptin or lomitapide businesses in the time periods we expect, or at all, if reimbursement for these products is limited or delayed.
Market acceptance and sales of metreleptin and lomitapide will continue to depend on insurance coverage and reimbursement policies, and may be affected by healthcare reform measures. Government authorities and third-party payers, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment and predictability. Government authorities and these third-party payers have attempted to control costs by limiting coverage and limiting the amount of reimbursement for particular medications. If we fail to successfully secure and maintain reimbursement coverage for our products at levels that are acceptable to us or are significantly delayed in doing so or if onerous conditions are imposed or announced by private payers, government authorities or other third-party payers on such reimbursement, we will have difficulty achieving or maintaining market acceptance of our products and our business and ability to achieve our financial expectations will be harmed.
Given that GL and HoFH are rare diseases with extremely small patient populations, we have set prices for MYALEPT and JUXTAPID in the U.S. that are significantly higher than that of most pharmaceuticals, in order to generate enough revenue to fund our operating costs and potentially enable us to become profitable. We also expect to increase the price of metreleptin and lomitapide
58
from time to time in the future. We believe our pricing for our products in the U.S. is consistent with the level of pricing for other ultra-orphan drugs that treat diseases with comparable prevalence rates. The majority of payers in the U.S. are providing coverage for our products and, with respect to JUXTAPID, most payers in the U.S. have not required genotyping to determine a diagnosis of HoFH for reimbursement purposes. Many payers in the U.S. have, however, imposed requirements, conditions or limitations as conditions to coverage and reimbursement for JUXTAPID as a result of commercial availability of PCSK9 inhibitor products, which often includes a requirement that HoFH patients have not achieved an adequate LDL-C response on PCSK9 inhibitor products before access to lomitapide is approved. For patients currently taking JUXTAPID, several U.S. pharmacy benefit managers (PBMs) are using a prior authorization requiring current JUXTAPID patients to “step through” the less expensive PCSK9 inhibitor product, and additional PBMs and payers may follow this practice. We have been engaging with pharmacy benefit managers (PBMs) to discuss and negotiate potential agreements to limit these so-called “step edits”, which may require us to provide discounts and other price protections and would impact our net revenues from JUXTAPID.
During the payer review process for MYALEPT in the U.S., some U.S. payers are requiring additional patient information, such as a leptin level test, which may delay or otherwise impact reimbursement. The cost of JUXTAPID and MYALEPT in the U.S. may result in cost-sharing amounts for some patients that are prohibitive, and prevent these patients from being able to commence therapy on JUXTAPID or MYALEPT, respectively. We provide support to eligible commercial patients for certain drug co-pays and co-insurance obligations for JUXTAPID and MYALEPT treatment. From time to time, we provide financial support to patient assistance programs operated by independent charitable 501(c)(3) organizations in the U.S. that assist eligible HoFH and GL patients, as determined solely by the organization, with certain co-payments or co-insurance requirements for their drug therapies, which may include lomitapide or metreleptin. We do not have control or input into the decisions of these organizations. Our support of any 501(c)(3) organization and our own co-pay assistance programs could result in significant costs to us.
In certain countries outside the U.S. where lomitapide is or may be approved, or where metreleptin may be approved, we are seeking or expect to seek a price that is significantly higher than that of most pharmaceuticals, and which reflects the rare nature of the diseases our products treat. There is no assurance that government agencies in such countries that are responsible for reimbursement of healthcare costs or other third-party payers in such countries will agree to provide coverage for our products at the prices we expect to propose, or at all. In many countries outside the U.S., the proposed pricing for a drug must be approved by governmental authorities before it may be lawfully sold. The requirements governing drug pricing vary widely from country to country. For example, in March 2015, as part of negotiating a price agreement for lomitapide in Italy with the Italian Medicines Agency (AIFA), we, among other things, agreed to an annual payment cap for the first twelve months after the final publication of the agreement. In July 2016, because pharmaceutical expenses in Italy in 2015 exceeded AIFA’s planned budget, AIFA requested that pharmaceutical companies, including Aegerion, refund part of their 2015 revenues in Italy to manage this over-expenditure. As a result, LOJUXTA 2015 revenues in Italy were subject to a 20% rebate, which was paid in the third quarter of 2016. We expect AIFA will request a similar refund in connection with 2016 revenues in Italy. Amryt is responsible for any rebate applied to a period after it had assumed commercialization responsibility for the EEA. We expect that other countries will seek, and in certain cases, have sought and received, price and patient number and other price restrictions for our products and product candidates, if approved, or in some cases, if sold on a named patient basis. In some countries outside the U.S., we have faced, and will continue to face significant delays or impediments to obtaining reimbursement due to lengthy pricing negotiations with governmental authorities or the decisions of pricing authorities or authorities that indirectly impact pricing or reimbursement. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies, which may not be possible for us to do.
Outside the U.S., the macroeconomic climate, or local regulations or practices, may adversely affect our ability to set and charge a sufficiently high price to generate adequate revenue in those markets. The price of lomitapide, or metreleptin if approved, in one country may adversely affect the price in other countries. We may elect not to launch our products in any country where it does not make commercial sense to do so given the approved price or other conditions. In addition, while we do not expect to obtain approval of our products outside of rare disease indications, in the future if we were to obtain such approval for new indications with a higher prevalence rate than our existing indications, it may be more difficult for us to obtain or maintain our current price levels and targets for lomitapide and metreleptin. For example, due to the broader indication for MYALEPT in Japan, MYALEPT is sold by Shionogi in Japan at a price significantly lower than the U.S. price. Even if we are successful in obtaining pricing and reimbursement approval for lomitapide in a country, such countries may impose onerous conditions on reimbursement, which may include genotyping or the use of other therapies, such as apheresis, prior to the use of lomitapide.
In addition, outside the U.S., products that have orphan designation may be exempted or waived from having to provide certain clinical, cost-effectiveness and other economic data in connection with their filings for pricing/reimbursement approval or filings for therapeutic reviews which impact pricing and reimbursement approvals or negotiations. We may not be able to provide all of the data required to obtain pricing/reimbursement approvals in certain countries outside the U.S. where we seek to commercialize our products, if approved, or we may not satisfactorily meet the technical or substantive requirements of such submissions or receive ratings from pricing or other regulatory authorities commensurate with our expectations or that would support the price
59
levels we want for our products, which could result in delays of pricing/reimbursement approvals for our products, our products not obtaining pricing/reimbursement approval at all, or our products obtaining approvals at less than acceptable levels or with significant restrictions on use or reimbursement. We may also face pricing and reimbursement pressure in the U.S. and other countries as a result of prices charged for competitive products or therapies.
We are making lomitapide and metreleptin available, or plan to do so, in countries that allow use of a drug, on a named patient basis or under a compassionate use or other type of so-called expanded access program, before marketing approval has been obtained in such countries. We obtain reimbursement for lomitapide and metreleptin for authorized pre-approval uses in some of these countries to the extent permitted by applicable law and local regulatory authorities. In other countries or under certain circumstances, we are providing our products free of charge for permitted pre-approval uses. We do not yet know the impact that the availability in the U.S., Japan, and Brazil of PCSK9 inhibitor products will have on our named patient sales in Brazil and in other countries where we currently sell lomitapide on a named patient sales basis. There is no assurance that we will be able to obtain reimbursement at all or at acceptable levels or to maintain reimbursement for our products in any country under an expanded access program. In certain countries where we seek reimbursement for the product during the pre-approval phase, we are able to establish the price for the product, while in other countries we need to negotiate the price. Such negotiations may not result in a price acceptable to us, in which case we may elect not to distribute our products in such country prior to approval or we may curtail distribution. In addition, in certain countries, such as Brazil, the price we are able to charge for named patient sales prior to approval may be higher than the price that is approved by governmental authorities after approval.
The amount of reimbursement for JUXTAPID and MYALEPT and the manner in which government and private payers in the U.S. may reimburse for our potential future products are uncertain.
The impact of evolving reimbursement mechanics on the willingness of providers to furnish JUXTAPID or MYALEPT or other products we may market and the prices we can command for these products is difficult to predict. Legislative changes to the Public Health Service Section 340B drug pricing program (the 340B Program), the Medicaid Drug Rebate Program, and the Medicare Part D prescription drug benefit also could impact our revenues. If reimbursement is not available or available only to limited levels or if the mix of patients for our products is more heavily weighted to patients reimbursed under government programs, we may not be able to generate sufficient revenue to meet our operating costs or to achieve our revenue and profitability goals and to achieve and maintain positive cash flow in the timeframe that we expect, or at all. Price reductions and other discounts we offer or may offer for our products, and significant price increases, such as the price increase for MYALEPT in February 2015, typically result in increasing the rebates we are required to pay under the Medicaid Drug Rebate Program or state Medicaid supplemental rebate programs and the discounts we are required to offer under the 340B Program. For example, we currently pay a significant rebate for MYALEPT that could offset the majority of revenues from Medicaid patients and will have a continued significant impact in future quarters. The degree of such impact on our overall financial performance will depend on the percentage of MYALEPT patients that have Medicaid as their primary insurance coverage and the quantity of units ordered per patient. In addition, a considerable number of JUXTAPID patients in the U.S. are Medicare Part D patients and a significant percentage of such patients may not be able to afford their out-of-pocket co-payments for JUXTAPID and MYALEPT, which could result in such patients seeking an alternative free drug or ceasing treatment with our products, given that the only source of financial support for such patients may be through independent 501(c)(3) patient organizations that may not provide adequate financial assistance, due to reductions in contributions to such patient organizations. This could have a material adverse effect on our revenues or financial condition.
In addition, in the U.S., the cost of pharmaceuticals continues to generate substantial governmental and third-party payer interest. We expect that the pharmaceutical industry will experience pricing pressures due to the increasing influence of managed care (and related implementation of managed care strategies to control utilization), additional federal and state legislative and regulatory proposals to regulate pricing of drugs, limit coverage of drugs or reduce reimbursement for drugs, public scrutiny, and the current presidential administration’s agenda to control the price of pharmaceuticals through government negotiations of drug prices in Medicare Part D and importation of cheaper products from abroad. While we cannot predict what executive, legislative and regulatory proposals will be adopted or other actions will occur, such events could have a material adverse effect on our business, financial condition and profitability.
The FDA, the EU Member States and other regulatory agencies outside the U.S. and the EU enforce laws and regulations prohibiting the promotion of off-label uses. Aegerion is currently the subject of a DOJ investigation regarding its marketing and selling of JUXTAPID in the U.S. Enforcement actions by these agencies can result in significant liability.
The FDA, the competent authorities of the EU Member States and other regulatory agencies outside the U.S. and the EU strictly regulate the promotional claims that may be made about prescription drug products. In particular, a drug product may not be promoted in a jurisdiction prior to approval or for uses that are not approved by the FDA, the EC, the competent authorities of the EU Member States or such other regulatory agencies, as applicable, as reflected in the product’s approved prescribing information
60
or summary of product characteristics. In the U.S., promotion of products for unapproved (or off-label) uses may result in enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA. Promotion of products for off-label uses in the U.S. can also result in false claims litigation under federal and state statutes, which can lead to consent decrees, civil money penalties, restitution, criminal fines and imprisonment, and exclusion from participation in Medicare, Medicaid and other federal and state healthcare programs. As noted above, Aegerion has been the subject of certain investigations by the DOJ and the SEC, for which it reached preliminary agreements in principle in 2016. The preliminary agreements in principle reached with the DOJ contemplates that Aegerion will enter into a corporate integrity agreement, FDA consent decree and deferred prosecution agreement, each of which will be costly to negotiate and may require Aegerion to expend significant costs and resources to implement and maintain compliance. In addition, Aegerion may see new governmental investigations of or actions against it citing additional theories of recovery. Aegerion may also be subject to regulatory and/or enforcement action by federal agencies, private insurers and states’ attorneys general. See the “Legal Proceedings” section of this Annual Report for further information regarding ongoing investigations, including the preliminary agreements in principle reached with the SEC and the DOJ, and other legal proceedings.
The ongoing investigations and litigation involving Aegerion and future investigations or litigation in which we are involved could have a material adverse effect on our business, financial condition, results of operations, and share price, and divert the attention of our management from operating our business and may be disruptive to our employees, possibly resulting in further employee attrition. In addition, the existence of the investigations and related activities has impacted, and may continue to impact, the willingness of some physicians prescribe JUXTAPID and/or MYALEPT.
Our relationships with customers and payers in the U.S. are subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, and reputational harm and could diminish future earnings and prevent us from achieving our forecasted financial results.
Healthcare providers and others play an important role in the recommendation and prescription of our products. Our arrangements with third-party payers and customers in the U.S. expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including the federal healthcare Anti-Kickback Statute, the False Claims Act, the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the Physician Payment Sunshine Act, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products. Restrictions under applicable U.S. federal and state healthcare laws and regulations include the following:
• | The federal healthcare Anti-Kickback Statute prohibits persons from, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any healthcare item or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to, among others, arrangements between pharmaceutical manufacturers, on the one hand, and prescribers, purchasers, formulary managers and organizations that provide financial assistance to patients, on the other. Violations of the federal Anti-Kickback Statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. The Healthcare Reform Act, among other things, clarified that liability may be established under the federal Anti-Kickback Statute without proving actual knowledge of the statute or specific intent to violate it. In addition, the Healthcare Reform Act amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. There are a number of statutory exceptions and regulatory safe harbors to the federal Anti-Kickback Statute that protect certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. |
• | The federal civil False Claims Act imposes civil penalties and provides for civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented false or fraudulent claims for payment of government funds, or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government, or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. Several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may also implicate the federal civil False Claims Act. Federal civil False Claims Act violations may result in treble monetary damages and penalties and exclusion from participation in federal healthcare programs. Civil liability under the False Claims Act or misdemeanor violation of federal health care laws gives the Inspector General (IG) of the |
61
Department of Health and Human Services the discretion to exclude a company’s products from reimbursement by federal healthcare programs. This discretion to exclude often leads companies to negotiate corporate integrity agreements with the IG so their products may continue to receive reimbursement.
• | The federal criminal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact, making any materially false, fictitious, or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services. There are also criminal penalties, including imprisonment and criminal fines, for making or presenting a false, fictitious or fraudulent claim to the federal government. Conviction under any of the aforementioned federal criminal statutes requires mandatory exclusion from participation in federal healthcare programs. |
• | The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires certain pharmaceutical manufacturers to engage in extensive tracking of payments and other transfers of value to physicians and teaching hospitals and to submit such data to Centers for Medicare & Medicaid Services (CMS), which will then make all of this data publicly available on the CMS website. Pharmaceutical manufacturers, such as our subsidiary, Aegerion, with products for which payment is available under Medicare, Medicaid, or the State Children’s Health Insurance Program are required to track reportable payments and transfers of value during each calendar year and must submit a report on or before the 90th day of each calendar year disclosing reportable payments made in the previous calendar year. Failure to comply with the reporting obligations may result in civil monetary penalties. |
• | Analogous state laws and regulations, such as state anti-kickback and false claims laws, apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by Medicaid or other state programs or, in several states, apply regardless of the payer. Several states now require pharmaceutical companies to report expenses relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to certain healthcare providers in those states. Some of these states also prohibit certain marketing-related activities including the provision of gifts, meals, or other items to certain healthcare providers. In addition, several states require pharmaceutical companies to implement compliance programs or marketing codes. |
We are also subject to laws and regulations covering data privacy and the protection of health-related and other personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues which may affect our business, including recently enacted laws in many jurisdictions where we operate. Numerous U.S. federal and state laws and regulations, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and protection of personal information. Failure to comply with laws and regulations covering data privacy and the protection of health-related and other personal information could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business. In addition, we obtain patient health information from most healthcare providers who prescribe our products and research institutions we collaborate with, and they are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996, as amended by HIPAA. Although we are not directly subject to HIPAA other than with respect to providing certain employee benefits, we could potentially be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
Failure to comply with these regulatory frameworks could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business.
In addition to the DOJ investigation of Aegerion described in detail in the “Legal Proceedings” section of this Annual Report, we or our subsidiaries could become subject to other government investigations and related subpoenas. Subpoenas are often associated with previously filed qui tam actions, or lawsuits filed under seal under the federal civil False Claims Act. Qui tam actions are brought by private plaintiffs suing on behalf of the federal government for alleged federal civil False Claims Act violations. The time and expense associated with responding to subpoenas, and any related qui tam or other actions, may be extensive, and we cannot predict the results of our review of the responsive documents and underlying facts or the results of such actions. Any investigation, including the investigations described in detail in the “Legal Proceedings” section of this Annual Report, could result in civil and/or criminal sanctions being levied against us or Aegerion, including significant fines, sanctions, and other negative consequences that will have a material adverse effect on our business, financial condition, results of operations and/or cash flows. Aegerion’s preliminary agreements in principle with the DOJ and the SEC, if finalized, will result in material fines, sanctions and other remedies against Aegerion, as described in detail in the “Legal Proceedings” section of this Annual Report. Even if such matters can be resolved without incurring significant additional penalties, responding to subpoenas and investigations in general is costly and time-consuming. Moreover, responding to any additional government investigations, defending any claims raised, and any resulting fines, restitution, damages and penalties, settlement payments and administrative actions, as well as any related
62
actions brought by shareholders or other third parties, could have further material adverse impacts beyond those attributable to the DOJ and the SEC investigations described in detail in the “Legal Proceedings” section of this Annual Report, including on our reputation, our business, financial condition, results of operations, and share price. These investigations have diverted, and may continue to divert, the attention of our management from operating our business, and have been disruptive, and may continue to be disruptive, to our employees, possibly resulting in employee attrition. In addition, the existence of the investigations described in detail in the “Legal Proceedings” section of this Annual Report and related activities have impacted, and may continue to impact, the willingness of some physicians to prescribe JUXTAPID and/or MYALEPT.
The number and complexity of both federal and state laws continues to increase, and additional governmental resources are being used to enforce these laws and to prosecute companies and individuals who are believed to be violating them. In particular, the Healthcare Reform Act includes a number of provisions aimed at strengthening the government’s ability to pursue anti-kickback and false claims cases against pharmaceutical manufacturers and other healthcare entities, including substantially increased funding for healthcare fraud enforcement activities, enhanced investigative powers, and amendments to the False Claims Act that make it easier for the government and whistleblowers to pursue alleged violations of the Anti-Kickback Statute, the Food, Drug and Cosmetics Act (FDCA), the False Claims Act and other relevant laws. While it is too early to predict what effect these changes will have on our business, we anticipate that government scrutiny of pharmaceutical sales and marketing practices will continue for the foreseeable future and subject us to the risk of government investigations and enforcement actions. For example, federal enforcement agencies recently have shown interest in pharmaceutical companies’ product and patient assistance programs, including manufacturer reimbursement support services and relationships with specialty pharmacies, as well as contributions by companies to third-party 501(c)(3) organizations that assist patients in accessing treatment for certain diseases and conditions. Some of these investigations have resulted in significant civil and criminal settlements. Responding to a government investigation or enforcement action would be expensive and time-consuming, and could have a material adverse effect on our business and financial condition and growth prospects. As noted above, Aegerion is the subject of certain ongoing investigations by the DOJ and the SEC and is also the subject of a putative class action lawsuit filed against it and certain of its former executive officers in the U.S. District Court for the District of Massachusetts alleging certain misstatements and omissions related to the marketing of JUXTAPID and Aegerion’s financial performance in violation of the federal securities laws. See the “Legal Proceedings” section of this Annual Report for further information regarding these investigations and other legal proceedings.
Enacted and future legislation and related implementing regulations may increase the difficulty and cost for us to commercialize lomitapide, metreleptin, zuretinol or any other product candidate for which we obtain marketing approval, and may affect the prices we are able to obtain for them, if and where approved.
In the U.S., there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that restrict or regulate post-approval activities, and may affect our ability to profitably sell JUXTAPID, MYALEPT, zuretinol or any other product candidate for which we obtain marketing approval. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes for JUXTAPID or MYALEPT may be. In addition, increased scrutiny by Congress of the FDA’s approval process may subject us to more stringent product labeling and post-marketing testing and other requirements.
In the U.S., most outpatient prescription drugs, including JUXTAPID and MYALEPT, may be covered under Medicare Part D. Medicare Part D prescription drug plans are authorized to use formularies where they can limit the number of drugs that will be covered in any therapeutic class and/or impose differential cost sharing or other utilization management techniques. This places pressure on us to contain and reduce costs. Changes to Medicare Part D that give plans more freedom to limit coverage or manage utilization, and/or other cost reduction initiatives in the program could decrease the coverage and price that we receive for any approved products, and could seriously harm our business.
The Healthcare Reform Act substantially changed the way healthcare is financed by both governmental and private insurers. The law contains a number of provisions that affect coverage and reimbursement of drug products and/or that could potentially reduce the demand for our products such as:
• | increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care; and |
• | requiring drug manufacturers to provide a 50% discount on Medicare Part D brand name prescription drugs sold to Medicare beneficiaries whose prescription drug costs cause the beneficiaries to be subject to the Medicare Part D coverage cap (i.e. the so-called donut hole). |
In 2012, the Supreme Court of the U.S. heard challenges to the constitutionality of the individual mandate and the viability of certain provisions of the Healthcare Reform Act. The Supreme Court’s decision upheld most of the Healthcare Reform Act and
63
determined that requiring individuals to maintain “minimum essential” health insurance coverage or pay a penalty to the Internal Revenue Service was within Congress’s constitutional taxing authority. However, the Supreme Court struck down a provision in the Healthcare Reform Act that penalized states that choose not to expand their Medicaid programs through an increase in the Medicaid eligibility income limit from a state’s current eligibility levels to 133% of the federal poverty limit. As a result of the Supreme Court’s ruling, some states have decided not to expand Medicaid. For each state that does not choose to expand its Medicaid program, there will be fewer insured patients overall. Any reduction in the number of insured patients could impact our sales, business and financial condition.
Modifications to or repeal of all or certain provisions of the Healthcare Reform Act are expected as a result of the outcome of the recent presidential election and Republicans maintaining control of Congress, consistent with statements made by President Donald Trump and members of Congress during the Presidential campaign and following the election. We cannot predict the ultimate content, timing or effect of any changes to the Healthcare Reform Act or other federal and state reform efforts. There is no assurance that federal or state health care reform will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative, judicial or administrative changes relating to healthcare reform will affect our business.
In addition, other legislative changes have been proposed and adopted since the Healthcare Reform Act was enacted. The Budget Control Act of 2011 includes provisions to reduce the federal deficit. The Budget Control Act, as amended (BCA), resulted in the imposition of 2% reductions in Medicare payments to providers which began in April 2013 and will remain in effect through 2025 unless additional congressional action is taken. Any significant spending reductions affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented and/or any significant taxes or fees that may be imposed on us, as part of any broader deficit reduction effort or legislative replacement to the BCA, could have an adverse impact on our results of operations.
Countries outside the U.S. may make changes to their healthcare systems which may in the future affect the revenues we generate from sales of lomitapide and, if approved outside of the U.S., metreleptin, zuretinol and other product candidates for which we obtain approval.
We face extensive regulatory requirements, and may still face future development and regulatory difficulties.
Even after marketing approval, a regulatory authority may still impose significant restrictions on a product’s indications, conditions for use, distribution or marketing or impose ongoing requirements for post-marketing surveillance, post-approval studies or clinical trials. JUXTAPID is available in the U.S. only through the JUXTAPID REMS program. We must certify all healthcare providers who prescribe JUXTAPID and the pharmacies that dispense the medicine, and under the modified REMS program approved by the FDA on January 3, 2017, as described below, existing and new patients must now formally acknowledge that they understand the goals of the JUXTAPID REMS program and have undergone counseling by their prescriber to this effect. The FDA has also required that we periodically assess the effectiveness of the JUXTAPID REMS program. The FDA assesses on a periodic basis whether a REMS program is meeting its goals and whether the goals or elements of the plan should be modified. The FDA approved changes to the JUXTAPID REMS program on January 3, 2017. Such modifications to the JUXTAPID REMS program, which are extensive and need to be implemented by July 2, 2017, and the labeling modifications may negatively affect the ability or willingness of a healthcare professional to prescribe JUXTAPID, a patient to be willing to initiate or continue on therapy, or insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs to continue to provide reimbursement for JUXTAPID, in which case we will have difficulty achieving or maintaining market acceptance of JUXTAPID, and our business and ability to achieve our financial expectations will be harmed. The outcome of the investigations of the SEC and the DOJ may also have an effect on the FDA’s requirements for the JUXTAPID REMS program. For additional information regarding changes to the JUXTAPID REMS program, see the risk factor captioned “We may not be able to maintain or expand market acceptance for metreleptin and lomitapide in the U.S. or to gain market acceptance in markets outside the U.S. where we commercialize such products, and, for lomitapide, we may continue to see a significant number of patients who choose not to start or stay on therapy.”
MYALEPT is also available only through the MYALEPT REMS program, due to potential for development of anti-metreleptin antibodies and the associated risks of serious adverse sequelae (such as severe infections, excessive weight gain, glucose intolerance, diabetes mellitus) and risk of lymphoma. As a part of this program, we must certify all healthcare providers who prescribe MYALEPT, certify the pharmacies that dispense the medicine, and obtain prescriber attestation that each patient has a diagnosis consistent with GL. We are responsible for maintaining, monitoring and evaluating the implementation of the MYALEPT REMS program.
Regulatory authorities have significant post-marketing authority, including, the authority to require labeling changes based on new safety information, and to require post-marketing studies or clinical trials to evaluate serious safety risks related to the use of a drug or biologic. For example, in July 2015, the FDA notified Aegerion that they considered post-marketing reports of
64
anaphylaxis to be new safety information, and requested that we add it to the prescribing information for MYALEPT. Aegerion complied with that request. We are subject to certain post-marketing commitments to the FDA and the EMA with respect to lomitapide and metreleptin. We expect that the regulatory authorities in certain other countries outside the U.S. and EU where our products are, or may be, approved may impose post-approval obligations, including patient registries, and requirements that may in some countries be more onerous than those imposed by the FDA and EMA. Depending on the nature of these post-marketing studies, we may be required to provide our products free of charge to participants in the studies in certain countries even if we have pricing and reimbursement approval in such countries, which would negatively impact our level of revenues.
Where our products are approved outside the U.S. or are in the future approved, we are and will also be subject to other ongoing regulatory requirements governing the labeling, packaging, storage, advertising, distribution, promotion, recordkeeping and submission of safety, REMS, risk management program and other post-marketing information, including adverse reactions, and any changes to the approved product, product labeling, or manufacturing process. As a company with limited internal resources and expertise in these areas, we rely on third parties to facilitate our compliance with many of these extensive regulatory requirements, which often include detailed record keeping and reporting requirements. We and the third parties we work with may not be able to fully comply with these requirements or the reports we file with regulatory authorities may result in changes to our post-marketing compliance requirements. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA, the EMA, the competent authorities of the EU Member States and other regulatory authorities for compliance with cGMP, and other regulations.
If we, or third-party service providers acting on our behalf, or our drug substance or drug product or the manufacturing facilities for our drug substance or drug product, fail to comply with applicable regulatory requirements, including global pharmacovigilance requirements and meeting the requirements of the JUXTAPID REMS program, a regulatory agency may:
• | issue warning letters or untitled letters; |
• | seek an injunction or impose civil or criminal penalties or monetary fines; |
• | suspend, withdraw or alter the conditions of our marketing approval; |
• | require us to provide corrective information to healthcare practitioners; |
• | require us to modify our product labels; |
• | suspend any ongoing clinical trials; |
• | require entrance into a consent decree, which is a component of Aegerion’s preliminary agreement in principle with the DOJ related to the JUXTAPID REMS program, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; |
• | refuse to approve pending applications or supplements to applications submitted by us; |
• | suspend or impose restrictions on operations, including costly new manufacturing requirements; |
• | seize or detain products, refuse to permit the import or export of products or request that we initiate a product recall; |
• | impose further refinements and enhanced obligations under existing risk management and other forms of post-marketing requirements and programs; or |
• | refuse to allow us to enter into supply contracts, including government contracts. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our products and product candidate and to generate revenue.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program or other governmental pricing programs in the U.S., we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We participate in various government programs or contracts that require us to calculate and report certain prices for our products to government agencies or provide rebates or discounted pricing on products purchased to certain purchasers or government payers. The requirements for calculating prices and rebates are complex and subject to change. For example, new regulations that govern our obligations under the Medicaid Drug Rebate Program took effect in April of 2016. Changes to such requirements may affect our business and operations. We may also have reimbursement obligations or be subject to penalties if we fail to provide timely and accurate information to the government, pay the correct rebates or offer the correct discounted pricing.
65
We participate in the Medicaid Drug Rebate Program. Under the Medicaid Drug Rebate Program, we are required to pay a rebate for each unit of our product reimbursed by a state Medicaid program as a condition of having federal funds made available to the states for our drugs under Medicaid and Medicare Part D. Those rebates are based on pricing data that we report on a monthly and quarterly basis to CMS, the federal agency that administers the Medicaid Drug Rebate Program. We may also participate in state Medicaid supplemental rebate programs which require payment of an incremental rebate to state Medicaid programs for covered utilization of our products. Price reductions as well as price increases that exceed the rate of inflation for our products, such as the price increase for MYALEPT in February of 2015, may result in increasing the rebates we are required to pay under the Medicaid Drug Rebate Program or state Medicaid supplemental rebate programs and the discounts we are required to offer under the Public Health Service (PHS) 340B drug pricing discount program (the 340B Program), as discussed below.
To maintain coverage of our products under the Medicaid Drug Rebate Program and Medicare Part D, we are required to extend significant discounts to certain “covered entities” (defined by statute to include certain types of hospitals and other healthcare providers that receive federal grants) that purchase products under the 340B Program. The 340B Program requires participating manufacturers to agree to charge such covered entities no more than the 340B “ceiling price” for the manufacturers’ covered outpatient drugs. The 340B ceiling price is calculated using a statutory formula, which is based on the average manufacturer price (AMP) and rebate amount for the covered outpatient drug as calculated under the Medicaid Drug Rebate Program. “Orphan drugs” - those designated under section 526 of the FDCA, such as JUXTAPID and MYALEPT - are exempt from the ceiling price requirements with respect to drugs purchased by certain covered entities (i.e. rural referral centers, sole community hospitals, critical access hospitals, and free-standing cancer hospitals). The Healthcare Reform Act also obligates the Health Resources and Services Administration (HRSA), the agency which administers the 340B Program, to promulgate various regulations and implement processes to improve the integrity of the 340B Program. The status of new and pending regulations and guidance is uncertain under the new presidential administration.
Pricing and rebate calculations vary among products and programs. The calculations are complex and are often subject to interpretation by governmental or regulatory agencies and the courts. For example, the Medicaid rebate amount is computed each quarter based on our submission to the CMS of our AMP and best price for the quarter. If we become aware that our reporting for prior quarters was incorrect, or has changed as a result of recalculation of the pricing data, we will be obligated to resubmit the corrected data for a period not to exceed twelve quarters from the quarter in which the data originally were due. Such restatements and recalculations would serve to increase our costs for complying with the laws and regulations governing the Medicaid Drug Rebate Program. Any corrections to our rebate calculations could result in an overage or underage in our rebate liability for past quarters, depending on the nature of the correction. Price recalculations also may affect the price that we will be required to charge certain safety net providers under the Public Health Service 340B drug discount program. In February 2015, we significantly increased the U.S. wholesale acquisition cost per 11.3 mg vial of MYALEPT. As a result of this substantial price increase, we continue to expect a significant gross-to-net adjustment for Medicaid rebates which will offset the majority of revenue from Medicaid and negatively impact net product sales in future quarters, since Medicaid rebates directly reduce our net product sales. The degree of such impact on our overall financial performance will depend on the percentage of MYALEPT patients that have Medicaid as their primary insurance coverage and the quantity of units ordered per patient. To date, approximately 34% of patients prescribed MYALEPT have been Medicaid beneficiaries. The number of patients prescribed MYALEPT in the future who are Medicaid beneficiaries could be higher than historical rates.
We are liable for errors associated with our submission of pricing data and for overcharging government payers. For example, in addition to retroactive rebates and the potential for 340B Program refunds, if we are found to have knowingly submitted false AMP or best price information to the government, we may be liable for civil monetary penalties in the amount of $100,000 per item of false information. Our failure to submit monthly/quarterly AMP and best price data on a timely basis could result in a civil monetary penalty of $10,000 per day for each day the submission is late beyond the due date. In the event that CMS were to terminate our rebate agreement, no federal payments would be available under Medicaid or Medicare Part D for our products. In addition, if we overcharge the government in connection with our Federal Supply Schedule (FSS) contract or under any other government program, we will be required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges could result in allegations against us under the federal civil False Claims Act and other laws and regulations.
To maintain coverage of our products under the Medicaid Drug Rebate Program and Medicare Part D and when purchased by four federal agencies, we are required to participate in the FSS pricing program. Under this program, we are obligated to make JUXTAPID and MYALEPT available for procurement on an FSS contract at a negotiated price and also charge a price to four federal agencies-VA, Department of Defense (DoD), Public Health Service, and Coast Guard-that is no higher than the statutory Federal Ceiling Price (FCP). The FCP is based on the non-federal average manufacturer price (Non-FAMP), which we calculate and report to the VA on a quarterly and annual basis. If a company misstates Non-FAMPs or FCPs it must restate these figures. Pursuant to the Veterans Health Care Act of 1992 (VHCA), knowing provision of false information in connection with a Non-FAMP filing can subject a manufacturer to penalties of $100,000 for each item of false information.
66
FSS contracts are federal procurement contracts that include standard government terms and conditions, separate pricing for each product, and extensive disclosure and certification requirements. In addition to the four agencies described above, all other federal agencies and some non-federal entities are authorized to access FSS contracts. FSS contractors are permitted to charge FSS purchasers other than the four federal agencies “negotiated pricing” for covered drugs that is not capped by the FCP; instead, such pricing is negotiated based on a mandatory disclosure of the contractor’s commercial “most favored customer” pricing. Moreover, all items on FSS contracts are subject to a standard FSS contract clause that requires FSS contract price reductions under certain circumstances where pricing to an agreed “tracking” customer is reduced. In July 2016, we concluded negotiations with the Department of Veterans Affairs (VA), and effective August 15, 2016, we have an FSS contract for both JUXTAPID and MYALEPT.
We also participate in the Tricare Retail Pharmacy program, under which we pay quarterly rebates on utilization of JUXTAPID and MYALEPT when the products are dispensed through the Tricare Retail Pharmacy network to Tricare beneficiaries. The rebates are calculated as the difference between annual Non-FAMP and FCP.
If we overcharge the government in connection with VA FSS pricing program or Tricare Retail Pharmacy program, whether due to a misstated FCP or otherwise, we are required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges could result in allegations against us under the False Claims Act and other laws and regulations.
Unexpected refunds to the U.S. government, and responding to a government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Product development is a long, expensive and uncertain process, and we may terminate one or more of our development programs. If we do not achieve our projected development goals in the timeframes we expect and announce, or otherwise terminate one or more of our development programs, marketing approval and commercialization of our product candidates may be delayed or otherwise cease. As a result, our credibility may suffer, our share price may decline and we may incur significant expenses that could adversely affect our prospects, our financial condition, or results of operations.
For strategic and operational planning purposes, we may estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development goals. These milestones may include the commencement or completion of scientific studies and clinical trials and the submission and approval of regulatory filings. From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones are based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in many cases for reasons beyond our control.
We may determine to discontinue certain programs because we determine they do not have potential or we may elect to suspend, terminate or modify one or more of our programs, which could include changing our clinical or business model for further development, including by attempting to extract or monetize value from the program by either selling, out-licensing or potentially partnering part or all of the program. For example, our product candidate, zuretinol, is under evaluation in clinical programs for the treatment of (i) IRD caused by RPE65 and LRAT gene mutations, which includes LCA and RP (autosomal recessive) (IRD 02) and (ii) RP (autosomal dominant) (RP 01). In addition, through Aegerion, we expect to submit a supplemental biologics licensing application (sBLA) to the FDA in the first half of 2017 to expand MYALEPT’s indication in the U.S. to the PL subset, to seek formal regulatory approvals for metreleptin in GL and the PL subset in other key markets, including Brazil and Colombia, and to use our knowledge of the diverse effects of leptin on many physiologic functions to explore new opportunities for metreleptin as a platform drug to potentially treat patients suffering from a range of low leptin-mediated rare and metabolic diseases. We are evaluating and prioritizing these potential opportunities and plan to provide an update in mid-2017. If we terminate and seek to monetize part or all of a program in which we have invested significant resources, or we continue to expend further resources on a program and subsequently fail to achieve our intended goals, our prospects may suffer, as we will have expended resources on a program that may not provide a suitable return, if any, on our investment and we may have missed the opportunity to allocate those resources to potentially more productive uses. In addition, in the event of a termination of a product candidate or program, we may incur significant expenses and costs associated with the termination of the program, which could adversely affect our financial condition or results of operations.
Failures or delays in the completion or commencement of any of our ongoing or planned clinical trials of our products or product candidate could result in increased costs to us and delay, prevent or limit our ability to generate revenue with respect to the relevant product or product candidate in a new territory or indication.
The commencement and completion of clinical trials may be delayed or prevented for a number of reasons, including:
67
• | difficulties obtaining regulatory clearance to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial; |
• | delays in reaching or failing to reach agreement on acceptable terms with prospective clinical research organizations (CROs) and trial sites, and problems with the performance of CROs; |
• | insufficient or inadequate supply or quality of a product candidate or other materials necessary to conduct our clinical trials, or other manufacturing issues; |
• | difficulties obtaining institutional review board (IRB) approval or Ethics Committee’s positive opinion to conduct a clinical trial at a prospective site; |
• | challenges recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including the size and nature of a patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the nature of trial protocol, the availability of approved treatments for the relevant disease and the competition from other clinical trial programs for similar indications; |
• | severe or unexpected drug-related side effects experienced by patients in a clinical trial; and |
• | difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to the rigors of the trials, lack of efficacy, side effects or personal issues, or who are lost to further follow-up. |
For example, in May 2016, in connection with our MAA filing for metreleptin for the treatment of GL patients and a subset of PL patients in the EU, the EMA’s PDCO issued a summary report regarding the metreleptin pediatric investigation plan (PIP). The PDCO expressed concern that the number of young patients with lipodystrophy included in the clinical trials proposed to be included in the PIP is very limited, and that no information on metreleptin as used by European patients was provided. In July 2016, the PDCO approved our proposal that we conduct a study in GL patients below the age of 6, as a deferred commitment. Given the prevalence of GL and other factors, conducting a clinical trial in GL patients in the EU below a defined age will likely make such a trial lengthy in nature and potentially difficult to complete. Even if we conduct a study in pediatric GL patients, we may not be able to show, to the satisfaction of the EMA, that metreleptin is safe and effective in pediatric patients under the age of 6, and we may never receive approval for this indication in the EMA. The lack of approval to market metreleptin for the pediatric GL population outside of the U.S. would limit expansion of our product revenue potential.
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results or the results of other clinical, preclinical or nonclinical studies. In addition, a clinical trial may be suspended or terminated by us, the FDA, the competent authorities of the EU Member States and other countries, the IRBs or the Ethics Committees at the sites, or a data safety monitoring board overseeing the clinical trial at issue, or other regulatory authorities due to a number of factors, including:
• | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
• | failure to respect applicable data privacy obligations; |
• | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities; |
• | unforeseen safety issues or lack of effectiveness; and |
• | lack of adequate funding to continue the clinical trial. |
If we do not meet our timelines or milestones as publicly announced, the market approval and commercialization of the relevant product or product candidate may be delayed, and our credibility may be adversely affected and, as a result, our share price may decline.
We rely on third parties to conduct our clinical trials and registry studies and to perform related services, and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such clinical trials and compliance with post-marketing requirements. We may become involved in commercial disputes with these parties.
We do not have the ability to independently conduct clinical trials or registry studies, or perform pharmacovigilance and REMS monitoring and reporting, and we rely on third parties such as contract research organizations, medical institutions, academic institutions, clinical investigators, specialty pharmacies and other third-party service providers to perform these functions. Our reliance on these third parties for clinical development, pharmacovigilance and REMS activities reduces our control over these activities. However, if we sponsor clinical trials, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Given our ownership of lomitapide, metreleptin, and zuretinol, we are responsible for REMS activities in connection with marketing lomitapide and metreleptin in the U.S. and pharmacovigilance monitoring and reporting for all of our products on a global basis, except that Shionogi is responsible for these
68
activities for metreleptin in Japan, Korea and Taiwan and Amryt is responsible for these activities for lomitapide in the EEA, Switzerland, Turkey and certain Middle Eastern and North African territories, including Israel. Moreover, the FDA and the competent authorities in the EU and Japan require us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties does not relieve us of these responsibilities and requirements. Furthermore, these third parties may have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they provide is compromised or delayed due to the failure to adhere to regulatory requirements or our clinical trial protocols, or for other reasons, our development programs may be extended, delayed or terminated, additional marketing approvals for lomitapide, metreleptin, zuretinol or any other product candidate may be delayed or denied in the targeted indication or jurisdiction, and we may be delayed or precluded in our efforts to successfully commercialize lomitapide, metreleptin, zuretinol or any other product for targeted indications or in the targeted jurisdiction or it may impact existing approvals.
In addition, we may, from time to time, become involved in commercial disputes with these third parties, for example regarding the quality of the services provided by these third parties or our ultimate liability to pay for services they purported to provide, or the value of such services. In some cases, we may be required to pay for work that was not performed to our specifications or not utilized by us, and these obligations may be material.
We do not have in-house drug discovery capabilities, and will need to acquire or license existing drug compounds from third parties to expand our product candidate pipeline.
We currently have no in-house drug discovery capabilities. Accordingly, if we are to expand our product candidate pipeline, we will need to acquire or license existing compounds from third parties. We will face significant competition in seeking to acquire or license promising drug compounds. Many of our competitors for such promising compounds may have significantly greater financial resources and more extensive experience in preclinical testing and clinical trials, obtaining regulatory approvals and manufacturing and marketing pharmaceutical products, and thus, may be a more attractive option to a potential licensor than us. Further, the ongoing SEC and DOJ investigations may negatively impact our ability to complete strategic acquisitions or licensing arrangements. If we are unable to acquire or license additional promising drug compounds, we will not be able to expand our product candidate pipeline, which may adversely impact our future profitability and growth prospects and increase the risk of insolvency.
Positive results in preclinical studies and earlier clinical trials of our products or product candidates may not be replicated in later clinical trials, or changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which could result in development delay or a failure to obtain marketing approval or affect market acceptance.
Positive results in preclinical or clinical studies of lomitapide, metreleptin, zuretinol or any other product candidate that we acquire, license or develop may not be predictive of similar results in humans during further clinical trials. Accordingly, there is, for example, a possibility that any potential future clinical development of metreleptin in pediatric patients or new indications may generate results that are not consistent with the results of the Phase 3 clinical study for the product or other relevant studies. The results of such clinical trials may not be sufficient to gain approval of metreleptin in any pediatric population or new indication or may generate data that negatively impact the existing data and labels for approved indications. Our preclinical studies or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical trials. Moreover, preclinical and clinical data can be susceptible to varying interpretations and analysis, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain FDA or other regulatory approval for their products.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, as a result of which we may need to amend clinical trial protocols. Amendments may require us to resubmit our clinical trial protocols to the FDA, IRBs, Ethics Committees or the competent authorities of the applicable jurisdictions for review and approval, which may impact the cost, timing or successful completion of a clinical trial. If we experience delays in completion of, or if we terminate, any of our clinical trials or generate results that differ from earlier clinical trial results, the commercial prospects for the applicable product may be harmed.
Recent federal legislation and actions by state and local governments may permit re-importation of drugs from foreign countries into the U.S., including foreign countries where the drugs are sold at lower prices than in the U.S. Similarly, purchasers in the EU are permitted to purchase products in one EU Member State and import it into another EU Member State where the price may be higher. These practices could materially adversely affect our operating results and our overall financial condition.
69
The Medicare Prescription Drug, Improvement and Modernization Act contains provisions that may change importation laws and expand pharmacists’ and wholesalers’ ability to import lower priced versions of an approved drug and competing products from Canada, where there are government price controls. These changes to U.S. importation laws, which will not take effect unless and until the Secretary of Health and Human Services certifies that the changes will pose no additional risk to the public’s health and safety, may result in a significant reduction in the cost of products to consumers. While the Secretary of Health and Human Services has not yet announced any plans to make this required certification, we may ultimately face the risk that a distributor or other purchaser of JUXTAPID or MYALEPT in the U.S. will be permitted to import lower priced product from a country outside the U.S. that places price controls on pharmaceutical products. This risk may be particularly applicable to JUXTAPID and MYALEPT as drugs that currently command premium prices, and especially to JUXTAPID, as a drug that is formulated for oral delivery. In addition, some states and local governments have implemented importation schemes for their citizens and, in the absence of federal action to curtail such activities, other states and local governments may launch importation efforts.
In the EU, a purchaser cannot be restricted from purchasing a medicinal procedure in one EU Member State and importing the product into another EU Member State in which it is also subject to marketing authorization. This activity is called parallel importing. As a result, a purchaser in one EU Member State where lomitapide or, if approved, metreleptin, is sold at a high price may seek to import the product from another EU country where the product is sold at a lower price.
The re-importation of lomitapide or metreleptin into the U.S. market from a foreign market and the parallel importation of lomitapide, and, if approved, metreleptin, among countries of the EU or other regions could negatively impact our revenue and anticipated financial results, possibly materially.
We face potential product liability exposure, and, if claims are brought against us, we may incur substantial liability.
The use of any product or product candidate in clinical trials and the sale of any product for which we have or obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers or others selling or otherwise coming into contact with our product and product candidates. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in:
• | decreased demand for our products and any product candidate for which we obtain marketing approval; |
• | impairment of our business reputation and exposure to adverse publicity; |
• | increased warnings on product labels; |
• | withdrawal of clinical trial participants; |
• | costs as a result of related litigation; |
• | distraction of management’s attention from our primary business; |
• | substantial monetary awards to patients or other claimants; |
• | loss of revenue; and |
• | the inability to successfully commercialize our products or any product candidate for which we obtain marketing approval. |
We have obtained product liability insurance coverage for both our clinical trials and our commercial exposures with a $25.0 million annual aggregate coverage limit. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. On occasion, large judgments have been awarded in class action lawsuits relating to drugs that had unanticipated side effects or warnings found to be inadequate. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial. A product liability claim or series of claims brought against us could harm our reputation and cause our share price to decline and, if the claim is successful and judgments exceed our insurance coverage, could have a material adverse impact on our business, financial condition, results of operations and prospects.
A variety of risks associated with our business operations outside the U.S. could materially adversely affect our business.
In each country outside the U.S. in which lomitapide is approved, or where we are making lomitapide or metreleptin available on a named patient or compassionate use basis before it has obtained marketing approval, we are subject to additional risks related to international business operations, directly and as a result of the activities of third parties with whom we do business, including:
• | differing regulatory requirements for drug approvals in foreign countries; |
70
• | pricing, pricing deals and reimbursement approvals that have a negative impact on our global pricing strategy; |
• | potentially reduced protection for intellectual property rights; |
• | the potential for parallel importing; |
• | unexpected changes in tariffs, trade barriers and regulatory requirements; |
• | economic weakness, including inflation, or political instability in particular foreign economies and markets, including, for example, the current political instability in Brazil, our largest source of revenues on a country-by-country basis outside the U.S.; |
• | compliance with foreign and U.S. laws, rules, regulations or industry codes, including data privacy requirements, labor relations laws, anti-competition regulations, import, export and trade restrictions, and required reporting of payments to healthcare professionals and others; |
• | negative consequences from changes in applicable tax laws; |
• | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
• | workforce uncertainty in countries where labor unrest is more common than in the U.S.; |
• | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
• | dependence upon third parties to perform distribution, pharmacovigilance, quality control testing, collections and other aspects of the distribution, supply chain and commercialization of our products that are required to be performed in order to conduct such activities in international markets, and our ability to effectively manage such third parties; and |
• | business interruptions resulting from geopolitical and economic events or actions, including social unrest, economic crises, war, terrorism, or natural disasters. |
In addition to the foregoing, we are subject to anti-corruption laws, including the Foreign Corrupt Practices Act (FCPA) and various other anti-corruption laws. The FCPA generally prohibits companies and their intermediaries from making improper payments to foreign officials for the purpose of obtaining or keeping business and/or other benefits. An aspect of the SEC’s ongoing investigation into Aegerion’s disclosures and activities relates to alleged FCPA violations in Brazil. These potential violations are excluded from the preliminary agreements in principle with the DOJ and the SEC.
Our activities outside the U.S. and those of our employees, licensees, distributors, manufacturers, clinical research organizations and other third parties who act on our behalf or with whom we do business subject us to the risk of investigation and prosecution under foreign and U.S. laws. For example, as described in detail in the “Legal Proceedings” section of this Annual Report, federal and state authorities in Brazil are conducting an investigation to determine whether there have been violations of Brazilian laws related to the promotion of JUXTAPID in Brazil. These issues could negatively affect our ability to generate product revenue for JUXTAPID consistent with our expectations, and may impact our ability to achieve and maintain profitability or maintain cash-flow-positive operations. Prescriptions for and sales of MYALEPT in Brazil may also be negatively affected.
Despite our ongoing efforts to ensure compliance with foreign and domestic laws, our employees, agents, and companies with which we do business may nevertheless take actions in violation of our policies, for which we may be ultimately held responsible. If so, we may be subject to criminal or civil penalties or other punitive measures, including restrictions on our ability to continue selling in certain markets. Any such outcome, or any allegation or investigation regarding such actions involving us, could harm our reputation and have a material adverse impact on our business, financial condition, results of operations and prospects.
We may not be able to obtain a rare pediatric disease priority review voucher for zuretinol, if we decide to request it.
We are exploring the potential of submitting to the FDA a request for rare pediatric disease designation for zuretinol for the treatment of IRD caused by LRAT or RPE65 gene mutations. If zuretinol is approved by the FDA after being designated a rare pediatric disease and we meet certain additional criteria, we may qualify for a rare pediatric disease priority review voucher. There can be no assurance that the FDA will conclude that zuretinol for the treatment of IRD caused by LRAT or RPE65 gene mutations meets the requirements for rare pediatric disease designation. Even if we decide to submit this request to the FDA, we obtain rare pediatric disease designation and zuretinol is approved for this indication, there is no guarantee that the FDA will conclude that the requirements for a rare pediatric disease priority review voucher are met. Additionally, the FDA generally may not grant rare pediatric disease priority review vouchers after September 30, 2020, although a drug that has received rare pediatric disease designation by September 30, 2020, remains eligible to receive a rare disease priority review voucher if it is approved for marketing
71
no later than September 30, 2022. In light of these deadlines, there is no guarantee that if we request and obtain rare pediatric disease designation, the FDA will approve zuretinol in time for us to receive a rare pediatric disease priority review voucher.
The occurrence of cyber incidents, or a deficiency in cybersecurity, could negatively impact our business by causing a disruption to our operations, a compromise or corruption of confidential information, exposure to legal and regulatory action, or damage to our patient, partner or employee relationships, any of which could subject us to loss and harm our reputation.
A cyber incident is considered to be any event that threatens the confidentiality, integrity or availability of information resources. More specifically, a cyber incident is an intentional attack or an unintentional event that can include gaining unauthorized access to systems to disrupt operations, corrupt data or steal confidential information about patients, suppliers, partners or employees. A number of companies have recently experienced serious cyber incidents and breaches of their information technology systems. Cyber incidents pose risks both to our internal systems and to those we have outsourced, including the risk of operational interruption, damage to our reputation and relationships with patients, partners and employees, and private data exposure. We have implemented processes, procedures and controls to help mitigate these risks. However, these measures, as well as our increased awareness of the risk of a cyber incident, do not guarantee that our reputation, operations and financial results would not be adversely affected by such an incident.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, proprietary business information and patient data. This includes, where required or permitted by applicable laws, personally identifiable information. Certain third parties with whom we contract also collect and store such data related to clinical trial subjects and patients. The secure maintenance of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise information stored on our networks or those of our partners. Any access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, recovery costs, disruption our operations, including delays in our regulatory approval efforts, and damage our reputation, which could adversely affect our business.
Risks Related to Our Intellectual Property
If our patent position does not adequately protect our products and product candidate, others could compete against us more directly, which would harm our business, possibly materially.
Our lomitapide patent portfolio consists of seven issued U.S. patents and issued patents in Europe, Australia, New Zealand, South Korea and Japan and pending applications in the U.S., Japan, Canada, and India, all of which have been licensed to us in a specific field. A five-year patent term extension for our U.S. patent covering the composition of matter of lomitapide, which was originally scheduled to expire in early 2015, has been granted and the patent will now expire in 2020. The non-U.S. patents directed to the composition of matter of lomitapide issued in certain jurisdictions of the EU, Canada, Israel and Japan have expired. Our five method-of-use patents in the U.S. cover certain dosing regimens for lomitapide, with one such patent expiring in 2027 and the other four patents expiring in 2025. The non-U.S. patents directed to methods-of-use issued in certain jurisdictions of the EU, Japan, and South Korea are scheduled to expire in 2025. The method-of-use patent may be eligible for up to three years of supplemental protection in certain European countries, and we are seeking such protection in the countries in which LOJUXTA is approved, on a country-by-country basis. An opposition was filed by a third-party with respect to the European method-of-use patent, but such opposition has since been revoked.
On August 28, 2015, the Coalition for Affordable Drugs VIII L.L.C. (CFAD) filed two separate inter partes review (IPR) petitions with the Patent Trial and Appeal Board (PTAB) of the U.S. Patent and Trademark Office (U.S. PTO), challenging the validity of U.S. Patent Nos. 7,932,268, and 8,618,135, which are directed to methods-of-use for lomitapide. On March 6, 2017, the PTAB determined that the CFAD failed to show that the claims of these patents were unpatentable. We cannot predict whether an appeal or a request for a rehearing on this determination will be filed, or if additional IPR challenges will be filed by another entity, or the outcome of any future IPR.
An Abbreviated New Drug Application (ANDA) or 505(b)(2) NDA may be submitted for JUXTAPID on or after December 21, 2016 if it contains a Paragraph IV certification of patent invalidity or non-infringement. If we instigate a suit against an ANDA or 505(b)(2) applicant for patent infringement within 45 days of receiving a Paragraph IV notice, the FDA is prohibited from approving the ANDA or 505(b)(2) application for a period of 30 months. If the notice is given and suit filed between December 21, 2016 and December 21, 2017, the 30-month stay does not begin until December 21, 2017. The FDA may approve the proposed competitor product before the expiration of the 30-month stay if a court finds our patents invalid or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.
72
Moreover, if one or more ANDA filers were to receive approval to sell a generic or follow-on version of JUXTAPID, those competitor products could potentially be marketed, and we would become subject to increased competition, as early as December 21, 2019, the date on which JUXTAPID’s orphan drug exclusivity ends, although we expect that any such launch would be delayed until February 21, 2020, the date on which JUXTAPID's composition of matter patent expires.
Our metreleptin patent portfolio consists of three issued U.S. patents and issued patents in Europe, Canada, Israel, Australia, New Zealand, Mexico, China, South Korea and Japan, all of which have been licensed to us. The U.S. patent covering the composition of matter of metreleptin was scheduled to expire in 2016, but an interim extension has been granted extending the term for one year until a final determination of a request for patent term extension is made. The non-U.S. patents directed to the composition of matter of metreleptin have expired. The patent family covering metreleptin methods of use, directed to treating human lipoatrophy, is co-owned by Amgen, University of Texas and the National Institutes of Health, and is sublicensed to us from Amgen. We have each additional co-owner’s consent to the sublicense granted by Amgen, and we have an exclusive license from one of the co-owners to this patent family in addition to the sublicense from Amgen. If we are unable to maintain license and/or enforcement rights from each of the co-owners, we may be prevented from enforcing these patent rights against a competitor in the U.S. or in foreign jurisdictions. The two method-of-use patents in the U.S. expire in 2022 and 2023, and the non-U.S. patents issued in certain European countries, Canada, and Australia, and pending in Japan, expire in 2022. An application for a patent term extension in the U.S. with respect to MYALEPT has been filed which, if granted, will be applied to either the U.S. composition of matter patent or the method-of-use patent, to extend one of these patents by 1,206 days. Also, as noted above, metreleptin qualifies for 12-year biologic exclusivity under the BPCI Act, which will expire in 2026. If approved by the EMA, metreleptin would be entitled to 10 years of market exclusivity in the EU.
Our zuretinol patent portfolio includes six granted U.S. patents, and issued patents in Europe, Japan, Canada, and other countries, as well as pending patent applications in countries including the U.S. and Europe. These patents and patent applications relate to zuretinol pharmaceutical compositions and uses thereof, including methods of using of zuretinol for the treatment of LCA and RP, and expire between 2025 and 2032. Certain zuretinol patent families are owned by the University of Washington, which has licensed the patents and patent applications to Retinagenix LLC (Retinagenix), and are exclusively sub-licensed to us by Retinagenix. For additional information regarding the license agreement with Retinagenix, see the risk factor captioned “If we fail to comply with our obligations in our license agreements for our product candidates, we could lose license rights that are important to our business.” Zuretinol has been granted orphan drug designations for the treatment of LCA (due to inherited mutations in LRAT or RPE65 genes) and RP (all mutations) by the FDA, and for the treatment of LCA and RP (all mutations) by the EMA, which would entitle it to orphan drug exclusivity, if submitted for approval to and approved by the FDA and EMA. The molecule zuretinol acetate is not, however, eligible for composition of matter protection in the U.S. or elsewhere, because it was previously known in the scientific community. Therefore, we may not be able to prevent competitors from commercializing zuretinol acetate for the treatment of diseases that fall outside of the scope of our patents protecting these methods.
Our commercial success with respect to our products will depend significantly on our ability to protect our existing patent position with respect to our products and product candidate, as well as our ability to obtain and maintain adequate protection of other intellectual property for our technologies, product candidates and any future products in the U.S. and other countries. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve our expected financial results. Our ability to use the patents and patent applications licensed to us to protect our business will also depend on our ability to comply with the terms of the applicable licenses and other agreements and to obtain requisite licenses. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the U.S., and we may encounter significant problems in protecting our proprietary rights in these countries.
There are many countries, including some key markets for lomitapide and metreleptin, like Brazil, in which we do not have intellectual property coverage, and where neither orphan drug exclusivity nor data and marketing exclusivity is available.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated or circumvented. U.S. patents and patent applications may also be subject to interference proceedings, ex parte reexamination, IPR and post-grant review proceedings and supplemental examination and may be challenged in district court. Patents granted in certain other countries may be subjected to opposition or comparable proceedings lodged in various national and regional patent offices. These proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such interference, re-examination, opposition, post-grant review, IPR, supplemental examination or revocation proceedings may be costly. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, product candidates and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
73
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
• | we will be able to successfully commercialize our product before some or all of our relevant patents expire, or in countries where we do not have patent protection; |
• | we or our licensors were the first to make the inventions covered by each of our pending patent applications and patents; |
• | we or our licensors were the first to file patent applications for these inventions; |
• | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
• | any of our pending patent applications or those we have licensed will result in issued patents; |
• | any of our patents or those we have licensed will be valid or enforceable; |
• | we are able to license patents or pending patents that are necessary or desirable to enforce or protect our patent rights on commercially reasonable terms or at all; |
• | any patents issued to us or our licensors and collaborators will provide a basis for any additional commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
• | we will develop additional proprietary technologies or product candidates that are patentable; or |
• | the patents of others will not have an adverse effect on our business. |
If we do not obtain protection under the Hatch-Waxman Act, the BPCI Act and similar foreign legislation by extending the patent terms and obtaining regulatory exclusivity for our products or product candidates, our business may be materially harmed.
The Hatch-Waxman Act established a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. Our application seeking a five-year patent term extension for our U.S. patent covering the composition of matter of lomitapide, has been granted, extending the patent term of this patent to 2020. An application for a patent term extension in the U.S. with respect to MYALEPT has been filed which, if granted, we will apply to either the U.S. composition of matter patent or the method-of-use patent, to extend one of these patents by 1,206 days. We are also seeking three years of supplemental protection for our European Patent Office (EPO) method-of-use patent in certain EPO countries in which LOJUXTA is approved. However, we may not be granted an extension in a particular country if we, for example, fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the applicable time period of the extension or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or restoration of the term of any such extension is less than we request, our competitors, including manufacturers of generic alternatives, may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially.
In addition, the FDA has classified lomitapide as a new chemical entity (NCE) in the U.S. and it is therefore eligible for data exclusivity under the Hatch-Waxman Act. A drug can be classified as a NCE if the FDA has not previously approved any other new drug containing the same active moiety. An NCE that is granted marketing approval may, even in the absence of patent protection, be eligible for five years of data exclusivity in the U.S. following marketing approval. This data exclusivity precludes submission of 505(b)(2) applications or ANDAs that reference the NCE application for four years if certain patents covering the NCE or its method-of-use expire or are challenged by a generic applicant. In addition, the FDA has granted seven years of orphan drug exclusivity from the date of approval for JUXTAPID in the U.S. in the treatment of HoFH, expiring in December 2019.
With the enactment of the BPCI Act, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing reference product. Under the BPCI Act, an application for a biosimilar product cannot be approved by the FDA until 12 years after the original reference product was approved under a BLA. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation, and meaning are subject to uncertainty. The BPCI Act is described in detail in the risk factor captioned “If we fail to obtain or maintain orphan drug exclusivity for our products or product candidate in any country where exclusivity is available, we will have to rely on our data and marketing exclusivity, if any, and on our intellectual property rights, to the extent there is coverage in such country, which may reduce the length of time that we can prevent competitors from selling generic versions of our products or product candidate.” While it is uncertain when such processes intended to implement the BPCI Act may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for metreleptin. In particular, the approval of a biological product biosimilar to one of our products could have a material impact on our business because it may be significantly less costly to bring to market and may be priced significantly lower than our products.
74
While metreleptin, which is approved under a BLA, qualifies for the 12-year period of exclusivity, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider metreleptin to be a reference product for competing products, potentially creating the opportunity for competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for metreleptin in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
Innovative medicinal products authorized in the EU on the basis of a full marketing authorization application (as opposed to an application for marketing authorization that relies on data in the marketing authorization dossier for another, previously approved medicinal product) are entitled to eight years’ data exclusivity. During this period, applicants for approval of generics of these innovative products cannot rely on data contained in the marketing authorization dossier submitted for the innovative medicinal product. Innovative medicinal products are also entitled to ten years’ market exclusivity. During this ten-year period no generic medicinal product can be placed on the EU market. The ten-year period of market exclusivity can be extended to a maximum of 11 years if, during the first eight years of those ten years, the Marketing Authorization Holder for the innovative product obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. If we are not able to gain or exploit the period of data exclusivity, we may face significant competitive threats to our commercialization of these compounds from other manufacturers, including the manufacturers of generic alternatives. Further, even though our compounds are considered to be NCEs and we were able to gain the period of data exclusivity, another company nevertheless could also market another version of the drug if such company submits a full NDA or a full application for marketing authorization in the EU with a complete human clinical trial program and obtains marketing approval of its product.
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology, products and any product candidates could be significantly diminished.
We may rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information, and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA is currently considering whether to make additional information publicly available on a routine basis, and the EMA is planning to amplify its disclosure rules. These changes could mean that information that we may consider to be trade secrets or other proprietary information may be disclosed, and it is not clear at the present time how the FDA’s and EMA’s disclosure policies may change in the future, if at all. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our products and any product candidates.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. There could be issued patents of which we are not aware that our products or product candidates infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to infringe. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products or product candidates infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes.
The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may obtain patents in the future and allege that our products or product candidates or the use of our technologies infringes these patent claims or that we are employing their proprietary technology without authorization. Likewise, third parties may challenge or infringe upon our existing or future patents.
Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
• | the patentability of our inventions relating to our product or any product candidates; and |
• | the enforceability, validity or scope of protection offered by our patents relating to our product or any product candidates. |
75
Even if we are successful in these proceedings, we may incur substantial costs and divert management’s time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion.
In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
• | incur substantial monetary damages; |
• | encounter significant delays in bringing our product candidates to market; and |
• | be precluded from manufacturing or selling our product candidates. |
In such event, our business could be adversely affected, possibly materially.
If we fail to comply with our obligations in our license agreements for our product candidates, we could lose license rights that are important to our business.
Our existing license agreements with respect to our products and product candidate impose, and we expect any future license agreements that we enter into will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with such obligations, we could lose license rights that are important to our business. For example, under the terms of a co-development agreement (the Retinagenix Agreement) we entered into with Retinagenix in April 2006, we obtained an exclusive, worldwide license and sub-license under certain intellectual property rights owned by Retinagenix or licensed to Retinagenix by the University of Washington related to zuretinol, the synthetic retinoid compound under development. We have been responsible for using commercially reasonable and diligent efforts to develop and commercialize in certain major markets, and other markets as we reasonably determine, one or more products covered by the licensed rights or developed using such licensed rights for use in diagnosing, treating or preventing certain human diseases and conditions. We are also responsible for committing certain annual funding to support research and development of such products. Under the license agreement between Retinagenix and the University of Washington (the UW Agreement), Retinagenix has similar obligations, and is required to meet specific development milestones within certain timeframes, one of which was required to be achieved by December 31, 2016. However, the UW Agreement contains provisions for extensions of those dates in certain circumstances. Based on the terms of the Retinagenix Agreement, the UW Agreement, and our significant development clinical spend on the zuretinol program, we believe that we are entitled to an extension of that milestone date until December 31, 2017, and that we may in the future be entitled to certain additional extensions to December 31, 2019, along with a potential additional extension of up to 12 months should enrollment in a planned trial be delayed, provided that we continue to comply with the relevant provisions of the license agreements and expend certain minimum amounts on the development of zuretinol. However, it is possible that we may not be able to achieve the specified development milestone by December 31, 2019, or such later date to which we may be entitled to an extension under the agreements. As a result, we and Retinagenix have begun discussing a renegotiation of that milestone with the University of Washington. We are currently conducting a review of the zuretinol development program, the results of which will assist us in determining when we believe that the remaining development milestone can be expected to be achieved. The failure to successfully renegotiate amendments to one or both of the license agreements related to zuretinol to allow for additional time beyond December 31, 2019 to achieve the remaining development milestone would greatly limit the future potential of the zuretinol program and could have a material adverse effect on our financial condition and business plans.
If we fail to comply with the obligations and restrictions under our license agreements, the applicable licensor may have the right to terminate the license, in which case we might not be able to market any product that is covered by the licensed patents. Any breach or termination of the license agreements applicable to our products would have a significant adverse effect on our business because of our reliance on the commercial success of our products.
Risks Related to Employee Matters and the Recently Completed Merger
Our future success depends on our ability to hire and retain our key executives and to attract, retain, and motivate qualified personnel.
Our success depends upon retaining, recruiting and motivating key employees. Experienced employees in the biopharmaceutical and biotechnology industries are in high demand and competition for their talents can be intense. We have entered into employment agreements with certain members of our executive, commercial, medical, finance, legal, development, and regulatory teams, but any employee may terminate his or her employment with us at any time. Employees may experience uncertainty about their future
76
roles in light of the Merger and ongoing integration of the companies. Uncertainty may also adversely affect our ability to attract, motivate and retain executives and other key employees and keep them focused on applicable strategies and goals. The loss of the services of any of these executives or key employees, or our inability to recruit desirable candidates, could impede the achievement of our development and commercialization objectives.
The size of our organization, and the Aegerion organization, has been significantly reduced, and we may encounter difficulties in managing the Aegerion business as a result of this reduction, or the attrition that has occurred since Aegerion’s reductions in force in February and July 2016, which could disrupt operations. In addition, the anticipated benefits and savings from the reductions may not be achieved.
In February 2016, the Board of Directors of Aegerion approved a cost-reduction plan that eliminated 80 positions from its workforce, representing a reduction in employees of approximately 25% of Aegerion. In July 2016, the Board of Directors of Aegerion approved an additional cost-reduction and restructuring plan that eliminated approximately 28 positions from its workforce, representing a reduction in employees of approximately 13% of Aegerion. Additional reductions in the size of the organization have occurred due to the restructuring of Aegerion’s commercial organization and in connection with the Merger, representing, in the aggregate, the elimination of approximately 27 positions from the workforces of Aegerion and Novelion. The reductions in force, and the attrition thereafter, have resulted in the loss of longer-term employees, the loss of institutional knowledge and expertise and the reallocation and combination of certain roles and responsibilities across the organization, all of which could adversely affect our operations.
Given the complexity and global nature of our business, we must continue to implement and improve our managerial, operational and financial systems, manage our facilities and continue to recruit and retain qualified personnel. This will be made more challenging given the reductions in force described above, the integration efforts in respect of the recently completed Merger and additional measures we may take to reduce costs to better align with projected revenues, particularly lower revenues for JUXTAPID in the U.S. As a result, our management may need to divert a disproportionate amount of its attention away from our day-to-day activities, and devote a substantial amount of time to managing these activities. Further, the restructuring, integration efforts in respect of the recently completed Merger and possible additional cost containment measures may yield unintended consequences, such as attrition beyond our intended reductions in force or as a result of the recently completed Merger and reduced employee morale. This has resulted in employees who were not affected by the reductions in force or recently completed Merger seeking alternate employment. In addition, we may not achieve anticipated benefits from the reductions in force. Due to our limited resources, we may not be able to effectively manage our operations or recruit and retain qualified personnel, which may result in weaknesses in our infrastructure and operations, risks that we may not be able to comply with legal and regulatory requirements, loss of business opportunities, loss of employees and reduced productivity among remaining employees. If our management is unable to effectively manage these transitions, reductions in force and additional cost containment measures, our expenses may be more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. As a result, our future financial performance and our ability to commercialize lomitapide and metreleptin successfully, and to compete effectively, would be negatively affected.
We incurred substantial expenses in connection with the Merger and expect to continue to incur substantial expenses related to integration of the companies, and our failure to successfully integrate the businesses of Aegerion and Novelion in the expected timeframe or manage our expanded operations would adversely affect our future results.
We have incurred and expect to continue to incur substantial expenses related to the Merger and the integration of Aegerion and Novelion. Our ability to realize the anticipated benefits from the Merger will depend, in part, on our ability to successfully integrate the companies’ operations. If the companies are not able to achieve these objectives within the anticipated timeframe, or at all, the anticipated benefits of the Merger may not be realized fully, or at all, or may take longer to realize than expected, and the value of our common shares may be adversely affected. In addition, the integration of Novelion’s and Aegerion’s respective businesses will be a time-consuming and expensive process. For example, there are a large number of processes, policies, procedures, operations, technologies and systems that must be integrated, including purchasing, accounting and finance, sales, billing, payroll, research and development, sales and marketing and benefits. In addition, the ongoing operation of locations in Cambridge, Massachusetts and Vancouver, British Columbia could result in inefficiencies, creating additional expenses for the companies. Proper planning and effective and timely implementation will be critical to avoid any significant disruption to the companies’ operations. It is possible that the integration process could result in the loss of key employees, the disruption of ongoing business or the identification of inconsistencies in standards, controls, procedures and policies that adversely affect the companies’ abilities to maintain relationships with customers, suppliers, manufacturers, creditors, lessors, clinical trial investigators or managers and other business partners or to achieve the anticipated benefits of the Merger. Delays encountered in the integration process could have a material adverse effect on the companies’ revenues, expenses, operating results and financial condition, including the value of our common shares. Specifically, risks include, among other factors, the companies’ inability to effectively:
77
• | coordinate standards, compliance programs, controls, procedures and policies, business cultures and compensation structures; |
• | integrate and harmonize financial reporting and information technology systems of the two companies; |
• | manage operations in a manner that supports and protects the tax benefits related to, and that may be realized from, our Canadian domicile; |
• | coordinate research and drug candidate development efforts to effectuate their product capabilities; |
• | compete against companies serving the market opportunities expected to be available to the companies following the Merger; |
• | manage inefficiencies associated with integrating the operations of the companies; |
• | identify and eliminate redundant or underperforming personnel, operations and assets; |
• | manage the diversion of management’s attention from business matters to integration issues; |
• | control additional costs and expenses in connection with, and as a result of, the Merger; |
• | conduct successful clinical development programs for their respective strategic product candidates and products and achieve regulatory approval for product candidates in major geographic areas; |
• | define and develop successful commercial strategies for our products in markets in which they are approved for sale and obtain reimbursement for such products in these markets; |
• | resolve Aegerion’s ongoing investigations and litigation and manage any future litigation and investigations that may arise from any such resolution of the ongoing investigations and litigation; |
• | service Aegerion’s significant indebtedness; |
• | commercialize Aegerion’s products at commercially attractive margins and generate revenues in line with our expectations, particularly in light of Aegerion’s 2016 reductions in force, the impact of competitive products on JUXTAPID sales and the continuing challenges to the lomitapide business; and |
• | raise capital through equity or debt financing on attractive terms to support the development and commercialization of our products and product candidate. |
While we have assumed that a certain level of expenses will be incurred, there are many factors beyond our control that could affect the total amount or timing of integration expenses. Moreover, many of the expenses that will be incurred are, by their nature, difficult to estimate accurately. These expenses likely will result in our taking significant charges against consolidated earnings, and the amount and timing of such charges are uncertain at present.
In addition, the actual integration may result in additional and unforeseen expenses, and the anticipated benefits of the integration plan may not be realized. Actual cost synergies, if achieved at all, may be lower than we expect and may take longer to achieve than anticipated. If we are not able to adequately address these challenges, we may be unable to successfully integrate the operations of our business, or to realize the anticipated benefits and cost synergy savings of the integration.
As a result of the Merger, our business became significantly more complex. There can be no assurance that we will effectively manage the increased complexity without experiencing operating inefficiencies or control deficiencies. Significant management time and effort is required to effectively manage the increased complexity of the larger organization and our failure to successfully do so could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Risks Related to Our Financial Position and Capital Requirements
We have incurred significant operating losses since our inception, and have not yet achieved profitability for any fiscal year.
We have incurred losses in each year since our inception. As of December 31, 2016, we had an accumulated deficit of approximately $587.2 million. Substantially all of our operating losses resulted from costs incurred in connection with our development programs and from selling, general and administrative costs associated with our operations. The losses we have incurred to date, combined with potential future losses, have had and may continue to have an adverse effect on our shareholders’ (deficit) equity and working capital.
78
We expect to incur expenses related to the commercialization of metreleptin and lomitapide in the U.S. and in the key countries in which lomitapide is currently approved and in which we intend to commercialize lomitapide, including Japan, or in which lomitapide or metreleptin may be approved and commercialized in the future, and expected distribution of our products in Brazil and certain other countries as part of named patient supply or compassionate use; manufacturing costs for both metreleptin and lomitapide; the conduct of our observational cohort studies and other post-marketing commitments to the FDA for lomitapide and metreleptin, including the implementation of the modified JUXTAPID REMS program and change to the coordinating center for such program; the conduct of any post-marketing commitments imposed by regulatory authorities in countries outside the U.S. and EU where our products are, or may be, approved; other possible clinical development activities for our products and product candidate, including an anticipated clinical trial for metreleptin in the pediatric population or a subset thereof, and activities related to metreleptin lifecycle management and the evolution of the zuretinol program; regulatory activities for our products; and business development activities. We expect to incur significant royalties, sales, marketing, and outsourced manufacturing expenses, as well as research and development expenses. In addition, we expect to continue to incur additional costs associated with operating as a public company and in connection with ongoing government investigations, and the potential outcome thereof, including the proposed payments to the DOJ and the SEC totaling approximately $40 million, implementing and complying with the corporate integrity agreement, FDA consent decree and deferred prosecution agreement contemplated by the preliminary agreement in principle with the DOJ, and a securities class action lawsuit, as described in detail in the “Legal Proceedings” section of this Annual Report.
Because of the numerous risks and uncertainties associated with developing and commercializing pharmaceutical products, we are unable to predict with certainty the extent of any future losses or when we will become profitable, if at all.
Servicing Aegerion’s debt requires a significant amount of cash. Aegerion may not have sufficient cash flow from its business to make payments on its debt, and it may not have the ability to raise the funds necessary to settle conversions of, or to repurchase, the Convertible Notes upon a fundamental change, which could adversely affect our business, financial condition and results of operations on a consolidated basis.
In August 2014, Aegerion incurred indebtedness in the amount of $325.0 million in aggregate principal with additional accrued interest under the 2.00% convertible senior notes due August 15, 2019 (the Convertible Notes), for which interest is payable semi-annually in arrears on February 15 and August 15 of each year. Aegerion’s business may not generate cash flow from operations in the future sufficient to service its debt. If Aegerion is unable to generate such cash flow, it may be required to adopt one or more alternatives, such as selling or licensing assets, further reducing the size of its workforce and curtailing operations and planned development activities, restructuring debt or obtaining financing on terms that may be onerous. Aegerion’s ability to refinance this indebtedness will depend on the capital markets and our financial condition on a consolidated basis at such time. Aegerion may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on these debt obligations.
In addition, holders of the Convertible Notes have the right to require the repurchase of their notes for cash upon the occurrence of a fundamental change at a repurchase price equal to 100% of the respective principal amount, plus accrued and unpaid interest, if any. Subject to certain exceptions as provided in the indenture governing the Convertible Notes, a fundamental change includes (a) delisting of Novelion’s common shares, (b) liquidation of Aegerion, (c) the acquisition of 50% or more of the voting interests in Aegerion, (d) an event in which Aegerion merges or consolidates with another entity and (e) an event in which Aegerion conveys, sells, transfers or leases all or substantially all of its assets to another entity. Among the exceptions provided in the indenture are for transactions described in (c), (d) and (e) in which (i) Aegerion’s common stock holders immediately prior to the transaction have the right to exercise, directly or indirectly, 50% or more of the total voting power of the capital stock of the continuing or surviving entity or transferee or parent thereof following the transaction or (ii) 90% of the consideration paid for Aegerion’s common stock in a transaction consists of stock that is or will be quoted on the New York Stock Exchange or NASDAQ.
Further, unless Aegerion elects to deliver solely our common shares to settle a conversion of Convertible Notes, Aegerion would be required to settle a portion or all of the conversion obligation through the payment of cash, which could adversely affect its liquidity. Aegerion may not have enough available cash or be able to obtain financing at the time it is required to make repurchases of Convertible Notes surrendered therefor or Convertible Notes being converted. The failure to repurchase Convertible Notes at a time when the repurchase is required by the indenture or to pay any cash payable on future conversions of the Convertible Notes as required by the indenture would constitute a default under the indenture. A default under the indenture or a fundamental change itself could also lead to a default under agreements governing our or Aegerion’s current and future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, Aegerion may not have sufficient funds to repay the indebtedness and repurchase the Convertible Notes or make cash payments upon conversions thereof. In addition, even if holders of the Convertible Notes do not elect to convert their Convertible Notes, Aegerion could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the Convertible Notes as a current rather than long-term liability, which would result in a material reduction of Aegerion’s net working capital.
79
Aegerion’s indebtedness and other financial obligations and contractual commitments, could adversely affect our financial health and our ability to respond to changes in our business.
Aegerion’s significant indebtedness, and our financial obligations and contractual commitments, could have important consequences. For example, it could:
• | make us more vulnerable to adverse changes in general U.S. and worldwide economic, industry and competitive conditions and adverse changes in government regulation; |
• | limit our flexibility in planning for, or reacting to, changes in our business and our industry; |
• | place us at a disadvantage compared to our competitors who have less debt; and |
• | limit our ability to borrow additional amounts for working capital, capital expenditures, acquisitions, general corporate purposes or other purposes, or raise additional capital through equity or other types of financings. |
Our leverage resulting from our or Aegerion’s debt could materially and adversely affect our ability to finance our operations or capital needs or to engage in other business activities. In addition, we cannot be sure that additional financing will be available when required or, if available, will be on terms satisfactory to us. Further, even if we are able to obtain additional financing, we may be required to use proceeds to repay a portion of Aegerion’s debt. Any of these factors could materially and adversely affect our business, financial condition and results of operations on a consolidated basis. In addition, if we or Aegerion incur additional indebtedness, the risks related to our business and our ability to service or repay our indebtedness would increase.
Our internal controls over financial reporting could fail to prevent or detect misstatements or have material weaknesses.
Our internal controls over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Any failure to maintain effective internal controls or to timely effect any necessary improvement or remediate any lapse in our internal control and disclosure controls could, among other things, result in losses from fraud or error, require significant resources and divert management’s attention, harm our reputation, causing investors to lose confidence in our reported financial and other information, and expose use to legal or regulatory proceedings, all of which could have a material adverse effect on our financial condition, results of operations and cash flows.
During 2016, a material weakness in internal control over financial reporting was identified relating to business combinations. Management is taking steps to remediate this material weakness and performed additional analysis and procedures to conclude that the Consolidated Financial Statements included in this Annual Report fairly present, in all material respects, our financial condition and results of operations as of and for the year ended December 31, 2016. See "Management's Annual Report On Internal Control Over Financial Reporting." However, we may be unable to remediate this weakness effectively, and, even if we do remediate this weakness, we may in the future identify additional material weaknesses.
We may never be profitable.
Our ability to become profitable depends upon our ability to generate significant revenue. We may never generate substantial revenues from the sale of metreleptin or lomitapide. Our ability to generate revenues sufficient to achieve profitability currently depends on a number of factors, including our ability to:
• | build and maintain market acceptance for MYALEPT in the U.S. for the treatment of GL, and supporting named patient sales of metreleptin in GL in Brazil, France, Turkey, and other key countries where such sales are permitted as a result of the U.S. approval or under local law; |
• | prepare for the launch of metreleptin in Europe as a treatment for complications of leptin deficiency in GL patients and a subset of PL, in the event we obtain regulatory, pricing and reimbursement approvals in the EU for metreleptin; |
• | pursue possible lifecycle management opportunities for metreleptin, including potential future clinical development of metreleptin in additional indications; |
• | stabilize sales of JUXTAPID as a treatment for adult HoFH patients in the U.S. despite competition from PCSK9 inhibitor products, among other factors, which have had a significant adverse impact on sales of JUXTAPID, and gain such market acceptance in the other countries where lomitapide is approved and being commercialized, including Japan, or may in the future receive approval and be commercialized; |
• | manage our costs and expenses to better align with our revenues and strengthening our capital structure, while supporting approved products in a compliant manner; |
80
• | continue to have named patient sales of our products in Brazil and other key countries where such sales can occur as a result of the FDA approval, particularly in light of local economic challenges, ongoing governmental investigations, and ongoing court proceedings in Brazil reviewing the regulatory framework for named patient sales; |
• | obtain timely regulatory approval of metreleptin in the EU and other key international markets as a treatment for patients with GL or a subset of PL, and an expansion of the indication in the U.S. to include the PL subset, subject to discussions with the FDA, and obtain timely regulatory approval of lomitapide in other key international markets as a treatment for patients with HoFH where it makes business sense to seek approval, in each case without onerous restrictions or limitations in the resulting label; |
• | gain pricing and reimbursement approvals to market our products in countries in which we elect to seek, and eventually obtain, regulatory approval, at acceptable prices and without significant restrictions, discounts, caps or other cost containment measures, and to effectively launch our products in those countries where it makes business sense to do so, including approval of metreleptin in the EU for GL and the PL subset and in the U.S. for the PL subset, subject to discussions with the FDA; |
• | reviewing the clinical and regulatory pathway for zuretinol to determine the optimal development and business strategy for this product candidate; |
• | minimize the number of patients who are eligible to receive but decide not to commence treatment with our products, or who discontinue treatment, including with lomitapide, due to tolerability issues, and with metreleptin, due to its route of administration as a daily injection, through activities such as patient support programs, to the extent permitted in a particular country; |
• | effectively estimate the size of the total addressable market for our products; |
• | maintain reimbursement policies for JUXTAPID and MYALEPT in the U.S. that do not impose significant restrictions on reimbursement and a payer mix that does not include significantly more Medicaid patients than the current payer mix; |
• | minimize the expected negative impact of the availability of PCSK9 inhibitor products on sales of lomitapide outside the U.S., including in Japan, where we launched JUXTAPID in December 2016 and where a PCSK9 inhibitor product is available, and the degree to which the availability of PCSK9 inhibitor products outside the U.S., and the potential availability of named patient sales of PCSK9 inhibitor products outside the U.S., impacts named patient sales of lomitapide outside the U.S., particularly in Brazil; and |
• | effectively respond to requirements of insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs in the U.S. to require that newly diagnosed adult HoFH patients be treated with PCSK9 inhibitor products prior to JUXTAPID treatment, that current JUXTAPID patients switch to PCSK9 inhibitor products, and that potential JUXTAPID patients fail to adequately respond to PCSK9 inhibitor products before providing reimbursement for JUXTAPID at the prices at which we offer JUXTAPID. |
In addition, as described above, federal enforcement agencies recently have shown interest in pharmaceutical companies’ product and patient assistance programs, including manufacturer reimbursement support services and relationships with specialty pharmacies, and contributions to charitable organizations that assist patients in accessing treatment for certain diseases and conditions. In addition to the risks associated with the costs of responding to government investigation or enforcement actions (such as a False Claims Act action), federal enforcement agencies’ increased attention to such programs and contributions may lead to changes that adversely affect our business. For example, we believe that investigations and enforcement actions by these agencies have resulted in a reduction in contributions to third-party 501(c)(3) organizations that assist patients in accessing treatment for certain diseases and conditions. If a lack of available funds prevents these third-party 501(c)(3) organizations from providing adequate financial assistance, including assistance with co-payment obligations, to individuals who would otherwise be unable to afford our products, our revenues may decline below our expectations.
Our products may not gain or maintain long-term market acceptance or achieve or maintain commercial success. In addition, we anticipate incurring significant costs associated with commercializing our products, and meeting our post-marketing commitments, in connection with our ongoing clinical efforts related to our products and in connection with defense against government investigations and other legal actions. We may not continue to generate substantial revenue from sales of lomitapide, or generate substantial revenue from sales of metreleptin. We may not achieve profitability. If we are unable to continue to generate significant product revenue, we will not become profitable, and may be unable to continue operations without additional funding.
We will likely need to raise substantial additional capital in the future. If additional capital is not available at all or on acceptable terms when we need it, we will have to delay, reduce or cease operations.
Aegerion's acquisition of metreleptin, the significant negative impact of PCSK9 inhibitor products on U.S. JUXTAPID sales, the payments by Aegerion to the government under the preliminary agreements in principle with respect to the ongoing investigations, if finalized, and the costs and expenses Aegerion has incurred, and expects to continue to incur, in connection with ongoing government investigations have significantly diminished the capital we have to fund anticipated and unanticipated expenses.
81
In light of the reduction in capital available for Aegerion’s operations in 2016, Aegerion implemented reductions in force intended to better align our operating expenses with our expected revenues, out-licensed lomitapide in the EU, and withdrew lomitapide from certain other global markets. We may need to implement additional cost containment measures based on our updated forecast of expected revenues, particularly due to the declining revenues from JUXTAPID in the U.S. There can be no assurance, however, that these reductions in force and cost containment measures will result in the cost savings we anticipate or that additional cost containment measures will be capable of being obtained or implemented. Accordingly, we will likely need to seek additional capital through debt or equity financing to service our indebtedness, strengthen our cash position and fund our operations. We may not be able to obtain additional capital when we need it or such capital may not be available on terms that are favorable to us, particularly while Aegerion’s preliminary agreements in principle to resolve the aspects of the ongoing DOJ and SEC government investigations remain subject to final approvals and while the Convertible Notes are outstanding. We may also pursue opportunities to obtain additional external financing in the future through lease arrangements related to facilities and capital equipment, collaborative research and development agreements, and license agreements, in order to, among other things, finance additional potential product acquisitions and maintain sufficient resources for unanticipated events. Any such additional financing may not be available when we need it or may not be available on terms that are favorable to us. Our need to raise additional capital in the future, and the size of any such financings, will depend on many factors, including:
• | the success of our commercialization efforts and the level of revenues generated from sales of metreleptin and lomitapide in the U.S.; |
• | the level of revenue received from named patient sales of metreleptin and lomitapide in Brazil and other key countries where a mechanism exists to sell the product on a pre-approval basis in such country based on U.S. approval of such products or EU approval of lomitapide, particularly in light of the availability of a PCSK9 inhibitor product in Brazil and the ongoing court proceedings in Brazil reviewing the regulatory framework for named patient sales; |
• | the level of physician, patient and payer acceptance of lomitapide and metreleptin; |
• | our ability to continue to manage our costs and expenses to better align with our revenues and strengthen our capital structure, while supporting approved products in a compliant manner; |
• | gaining regulatory and pricing and reimbursement approvals to market our products in countries in which the products are not currently approved and/or reimbursed, where it makes business sense to seek such approval, without significant restrictions, discounts, caps or other cost containment measures, including regulatory and pricing and reimbursement approval of metreleptin in the EU, in connection with which we filed an MAA in the EMA in December 2016, and regulatory approval of metreleptin in the U.S. for a subset of PL based on the existing clinical data package for metreleptin, subject to discussions with the FDA; |
• | the extent of the negative impact of the availability of PCSK9 inhibitor products on sales of JUXTAPID in the U.S., which, among other factors, have caused a significant number of JUXTAPID patients to discontinue JUXTAPID and switch to a PCSK9 inhibitor product, and significantly decreased the rate at which new HoFH patients start treatment with lomitapide; |
• | the provision of free PCSK9 inhibitor drug to adult HoFH patients by the companies that are commercializing PCSK9 inhibitor products, which such companies may have ceased, but which historically has had a negative impact on the rate at which new patients start treatment with lomitapide and has caused more patients than we expected to discontinue lomitapide and switch their treatment to PCSK9 inhibitor products; |
• | requirements of insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs in the U.S. to require that newly diagnosed adult HoFH patients be treated with PCSK9 inhibitor products prior to JUXTAPID, that current JUXTAPID patients switch to PCSK9 inhibitor products, and that patients fail to adequately respond to PCSK9 inhibitor products before providing reimbursement for JUXTAPID at the prices at which we offer JUXTAPID; |
• | the willingness of insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs in the U.S. to continue to provide reimbursement for our products at the prices at which we offer our products without imposing any additional major hurdles to access or other significant restrictions or limitations, and the ability and willingness of HoFH and GL patients to pay, or to arrange for payment assistance with respect to, any patient cost-sharing amounts for our products applicable under their insurance coverage, particularly in light of recent reductions in contributions to 501(c)(3) patient organizations by pharmaceutical companies; |
• | the cost of building and maintaining the sales and marketing capabilities necessary for the commercialization of our products for their targeted indications in the market(s) in which each has received regulatory approval and we elect to commercialize such products, to the extent reimbursement and pricing approvals are obtained, and certain other key international markets, if approved; |
• | the timing and costs of future business development opportunities; |
• | the timing and cost of seeking regulatory approvals and conducting potential future clinical development of metreleptin in additional indications, pursuing possible lifecycle management opportunities for metreleptin, and conducting potential development of the zuretinol program; |
82
• | the cost of filing, prosecuting and enforcing patent claims, including the cost of defending any challenges to the patents or our claims of exclusivity; |
• | the status of ongoing government investigations and lawsuits, including the disclosure of possible or actual outcomes, including regarding the preliminary agreements in principle that have been reached with the DOJ and the SEC; |
• | the costs of our manufacturing-related activities and the other costs of commercializing our products; |
• | the costs associated with ongoing government investigations and lawsuits, including any damages, settlement amounts, fines or other payments, or implementation of compliance related agreements or consent decrees, that may result from settlements or enforcement actions related to government investigations or whether we are successful in our efforts to defend ourselves in, or to settle on acceptable terms, ongoing or future litigation; |
• | the levels, timing and collection of revenue received from sales of our products in the future; |
• | the timing and costs of satisfying our debt obligations, including interest payments and any amounts due upon the maturity of such debt, including under the Convertible Notes; |
• | the cost of our observational cohort studies and other post-marketing commitments, including to the FDA and in any other countries where our products are ultimately approved; and |
• | the timing and cost of other clinical development activities. |
We may be unable to obtain additional financing on favorable terms, or at all. If we are unable to obtain additional financing, including for purposes of settling conversions of the Convertible Notes, we may be required to delay, reduce or cease operations, including our planned development, sales and marketing and business development efforts. Any of these outcomes would harm our business, financial condition and operating results. The source, timing and availability of any future financing will depend principally upon equity and debt market conditions, interest rates and, more specifically, on our commercial success, the status of Aegerion’s ongoing government investigations and the results of our future development efforts. Any additional sources of financing could involve the issuance of our equity securities, which would have a dilutive effect on our shareholders.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights. Shareholders may also experience dilution as a result of the exercise of outstanding warrants or the conversion of Convertible Notes into shares.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders.
For example, our existing shareholders may be diluted if the Convertible Notes are converted by their holders into Novelion common shares. Additionally, in connection with the acquisition of Aegerion and the private placement that immediately preceded such acquisition, we issued the Warrants. Shareholders may be diluted if the Warrants become exercisable following the settlement of the Class Action Litigation and/or the settlement of the DOJ and the SEC investigations, in each case, for an amount in excess of the negotiated thresholds. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
Risks Related to our Common Shares and Jurisdiction of Incorporation
The market price of our common shares has been, and may continue to be, highly volatile.
Our share price is highly volatile and is subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including the following:
• | the short-term or long-term success or failure of our commercialization efforts and the level of revenues generated from sales of our products in the U.S.; |
• | the level of revenue we receive from named patient sales of our products in Brazil and other key countries where a mechanism exists to sell the product on a pre-approval basis in such country based on U.S. approval of such products or EU approval of lomitapide, particularly in light of the regulatory approval of Amgen’s PCSK9 inhibitor product in Brazil in April 2016, the potential availability of that and other PCSK9 inhibitor products on a named patient sales basis in Brazil, and the ongoing court proceedings in Brazil reviewing the regulatory framework for named patient sales; |
• | the short-term or long-term success or failure of the commercialization of our products in key countries outside the U.S. in which we have or obtain approval, and the level of revenues we generate; |
83
• | our ability to accurately forecast net product sales and operating expenses, and to meet such forecasts; |
• | our ability, or lack thereof, to manage our costs and expenses to better align with our revenues, and strengthen our capital structure, while supporting approved products in a compliant manner; |
• | the timing and cost of seeking regulatory approvals and conducting potential future clinical development of metreleptin in additional indications, pursuing possible lifecycle management opportunities for metreleptin, and conducting potential development of the zuretinol program; |
• | any issues that may arise with our supply chain for our products; |
• | any adverse regulatory decisions, or regulatory issues that arise, made with respect to our products; |
• | any issues that may arise with respect to the safety of our products; |
• | the perception of the terms of the preliminary agreements in principle reached with the DOJ and the SEC and in connection with their investigations, and any adverse consequences that may result from such preliminary agreements in principle, such as additional litigation or investigations, and risks related to finalization of the preliminary agreements in principle and outstanding required approvals in respect thereof; |
• | our ability to defend ourselves successfully against claims made in securities class action lawsuits, and, if we are unsuccessful in such defense or decide to settle, the type and amount of any damages, settlement amounts, fines or other payments or adverse consequences that may result; |
• | the extent to which the changes to the JUXTAPID REMS program, approved by the FDA on January 3, 2017, including the requirements set forth elsewhere in these “Risk Factors” and the “Business” section of this Annual Report, may negatively affect the ability or willingness of a physician to prescribe JUXTAPID, a patient to be willing to initiate or continue on JUXTAPID therapy, or insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs to continue to provide reimbursement for JUXTAPID; |
• | the extent to which changes to the labeling for JUXTAPID instructing patients to cease therapy upon the occurrence of severe diarrhea may negatively affect the ability or willingness of a healthcare professional to prescribe JUXTAPID, a patient to be willing to initiate or continue on therapy, or insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs to continue to provide reimbursement for JUXTAPID; |
• | any adverse actions or decisions related to our intellectual property or marketing or data exclusivity, or any action by a third party to gain approval of a generic or biosimilar product, including, for lomitapide, for which a generic challenge could have been filed with the FDA as of December 21, 2016; |
• | fluctuations in stock market prices and trading volumes of similar companies; |
• | general market conditions and overall fluctuations in U.S. and Canadian equity markets; |
• | low trading volume and short interest positions in our common shares; |
• | international financial market conditions, including the ongoing sovereign debt crisis in the EU; |
• | variations in our quarterly operating results; |
• | changes in our financial guidance or securities analysts’ estimates of our financial performance; |
• | announcements of investigations or litigation, and updates to the status of investigations and litigation, or other notifications from enforcement or regulatory authorities related to our business or business practices; |
• | announcements of clinical data, regulatory submissions, product launches, new products, services or technologies, commercial relationships, acquisitions or other events by us or our competitors; |
• | changes in or materially incorrect application of accounting principles; |
• | issuance by us of new securities, or sales of large blocks of our common shares, including sales by our executive officers, directors and significant shareholders; |
• | the dilutive effect of the Convertible Notes or any other equity or equity-linked financings or alternative strategic arrangements; |
• | the acceleration of our or Aegerion’s long-term debt; |
• | additional changes in, or loss of, key personnel; |
• | success or failure of products within our therapeutic areas of focus; |
84
• | discussion of us or our share price by the financial press and in online investor communities; |
• | our relationships with and the conduct of third parties on which we depend; and |
• | other risks and uncertainties described in these risk factors. |
In particular, our revenue guidance relating to 2017 is predicated on many assumptions, most notably that we have correctly forecast our U.S. and non-U.S. revenues for both of our products, including for sales of JUXTAPID in Japan and for named patient sales of both products to the federal Ministry of Health in Brazil, and that sales continue as we have forecasted to those patients who have previously received JUXTAPID or MYALEPT and, in Brazil, to new patients who have obtained federal court orders for JUXTAPID or MYALEPT treatment, particularly in light of the ongoing investigations in Brazil and the trial currently being heard by the Brazil Supreme Federal Court to decide whether the government has an obligation to provide drugs, such as JUXTAPID and MYALEPT, that have not received regulatory and/or pricing and reimbursement approval in Brazil. Such factors may cause additional delays or eventually the suspension of the ordering process in Brazil. We have also assumed that our forecasts concerning named patient sales of metreleptin in other key markets, including France and Turkey, are correct. If any of our assumptions turn out to be incorrect, including our assumptions with respect to our ability to build and maintain market acceptance for our products for GL and HOFH in territories in which they have been approved or are eligible for named patient sales, or the extent of the negative impact of the availability of PCSK9 inhibitor products in the U.S., the EU, Japan, and Brazil on our sales of lomitapide in those countries and in other countries where PCSK9 inhibitors are, in the future, approved or available on a named patient basis, our 2017 financial results could be weaker than expected, and the price of our common shares could decline, perhaps precipitously.
In addition, the stock market in general, and the market for small pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Also, broad market and industry factors may negatively affect the market price of our common shares, regardless of our actual operating performance. In the past, following periods of volatility in the market in a company’s stock, securities class-action litigation has often been instituted against such a company. Aegerion, and certain of its former executive officers, have been named as defendants in a federal securities class action lawsuit filed against it alleging that Aegerion and the officers made certain false and misleading statements in violation of federal securities laws. See “Legal Proceedings” section of this Annual Report for further information. These proceedings have, and similar litigation could, if instituted against us, result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business and financial condition.
Our business could be negatively affected as a result of proxy contests and other actions of activist shareholders.
Proxy contests have been waged against many companies in the biopharmaceutical industry over the last few years. If faced with a proxy contest, we may not be able to successfully defend against the contest, which would be disruptive to our business. Even if we are successful, our business could be adversely affected by a proxy contest because:
• | responding to proxy contests and other actions by activist shareholders may be costly and time-consuming and may disrupt our operations and divert the attention of our management and our employees; |
• | perceived uncertainties as to our future direction may result in our inability to consummate potential acquisitions, collaborations or in-licensing opportunities and may make it more difficult to attract and retain qualified personnel and business partners; and |
• | if individuals are elected to our Board of Directors with a specific agenda different from our strategy for creating long-term shareholder value, it may adversely affect our ability to effectively and timely execute on our strategic plans and create additional value for our shareholders. |
Anti-takeover provisions in our Articles, certain provisions under the BCBCA, the Canadian take-over bid rules and our advance notice policy could prevent or delay transactions that our shareholders may favor and may prevent shareholders from changing the direction of our business or management.
Provisions of our Articles and certain provisions under the British Columbia Business Corporations Act (BCBCA) may discourage, delay or prevent a merger or acquisition that our shareholders may consider favorable, including transactions in which shareholders might otherwise receive a premium for their shares, and may also frustrate or prevent any attempt by shareholders to change our direction or management. For example, these provisions:
• | require a 66 2/3% majority of shareholder votes cast in favor of a resolution to effect various amendments to our Articles; and |
85
• | require shareholder proposals for matters to be acted upon by shareholders at shareholder meetings to be submitted pursuant to, and in accordance with, the applicable provisions of the BCBCA for inclusion in the Company’s proxy materials by a date that is not later than three months prior to the anniversary date of the prior year’s shareholder meeting. |
Canada’s take-over bid rules provide that take-over bids for Canadian issuers will be subject to a minimum 105-day deposit period, subject to certain exceptions. The take-over bid rules, together with the above-noted provisions, among others, whether alone or together, could delay or impede hostile takeovers and changes in control. Any provision of our constating documents that has the effect of delaying or deterring a change in control could limit the opportunity for our shareholders to receive a premium for their common shares and could also affect the price that some investors are willing to pay for our common shares.
We also have a shareholder-approved advance notice policy which establishes advance notice requirements for nominations for election to our Board of Directors. This policy may delay or impede changes to the composition of our Board of Directors or management.
Provisions of Canadian law may delay, prevent or make undesirable an acquisition of all or a significant portion of our common shares or assets.
The Investment Canada Act subjects an acquisition of control of us by a non-Canadian to government review if our enterprise value as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant Minister is satisfied that the investment is likely to be of net benefit to Canada. This could prevent or delay a change of control and may eliminate or limit strategic opportunities for shareholders to sell their common shares.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation, including the right to bring actions or enforce judgments against us and certain of our directors, and these differences may make our common shares less attractive to investors.
We are incorporated under the laws of the Province of British Columbia, Canada, and therefore certain of the rights of holders of its shares are governed by Canadian law, including the provisions of the BCBCA, and by our Notice of Articles and Articles. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations and these differences may make our common shares less attractive to investors. For example, certain of our directors and officers reside principally outside of the U.S. and a portion of our assets and a portion of the assets of these persons are located outside the U.S., and, as a consequence, it may not be possible for an investor to effect service of process within the U.S. on us or those persons. Furthermore, it may not be possible for an investor to enforce judgments obtained in U.S. courts based upon the civil liability provisions of U.S. federal securities laws or other laws of the U.S. against us or those persons.
An investor may be unable to bring actions or enforce judgments against us and certain of our directors.
We are incorporated under the laws of the Province of British Columbia. Certain of our directors and officers reside principally outside of the U.S. and a portion of our assets and a portion of the assets of these persons are located outside the U.S. Consequently, it may not be possible for an investor to effect service of process within the U.S. on us or those persons. Furthermore, it may not be possible for an investor to enforce judgments obtained in U.S. courts based upon the civil liability provisions of U.S. federal securities laws or other laws of the U.S. against us or those persons.
We do not intend to pay dividends on our common shares and, consequently, a shareholder’s ability to achieve a return on investment will depend on appreciation in the price of our common shares.
We have never declared or paid any cash dividend on our common shares, and do not currently intend to do so for the foreseeable future. We currently anticipate that we will retain any future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Therefore, the success of an investment in our common shares will depend upon any future appreciation in the value of such shares. There is no guarantee that our common shares will appreciate in value or even maintain the price at which our shareholders have purchased their common shares.
Future sales of our common shares may cause our share price to decline.
Sales of a substantial number of shares of our common shares in the public market or a sale of securities convertible into common shares or the perception that these sales might occur, could significantly reduce the market price of our common shares and impair our ability to raise adequate capital through the sale of additional equity securities. If our existing shareholders sell, or if the market believes our existing shareholders will sell, substantial amounts of our common shares in the public market, the trading price of our common shares could decline significantly. If additional shares are sold, or if it is perceived that they will be sold, in the public
86
market, the price of our common shares could decline substantially. We have registered approximately 13,000,000 common shares that are subject to outstanding options to purchase common shares and restricted stock unit awards and reserved for issuance under our equity plans, and will need additional shares for equity awards in the near future. These common shares can be freely sold in the public market upon issuance, subject to vesting restrictions.
Changes in our effective income tax rate could adversely affect our results of operations.
We or our subsidiaries are subject to income and other taxes in Canada, the U.S., and many other tax jurisdictions throughout the world. Tax rates in these jurisdictions may be subject to significant change. Our effective income tax rate can vary significantly between periods due to a number of complex factors, including, but not limited to: (i) interpretations of existing tax laws; (ii) the accounting for business combinations, including accounting for contingent consideration; (iii) the tax impact of existing or future healthcare reform legislation; (iv) changes in accounting standards; (v) changes in the mix of earnings in the various tax jurisdictions in which we operate; (vi) the outcome of examinations by the Canada Revenue Agency, the U.S. Internal Revenue Service (IRS) and other foreign tax authorities; (vii) adjustments to income taxes upon finalization of income tax returns; (viii) the accuracy of our estimates for unrecognized tax benefits; and (ix) increases or decreases to valuation allowances recorded against deferred tax assets. If our effective tax rate increases, our operating results and cash flow could be adversely affected.
Any changes to existing accounting pronouncements or taxation rules or practices may cause adverse fluctuations in our reported results of operations or affect how we conduct our business.
A change in accounting pronouncements or taxation rules or practices can have a significant effect on our reported results and may affect our reporting of transactions completed before the change is effective. New accounting pronouncements, taxation rules and varying interpretations of accounting pronouncements or taxation rules have occurred in the past and may occur in the future. The change to existing rules, future changes, if any, or the need for us to modify a current tax or accounting position may adversely affect our reported financial results or the way we conduct our business.
We may be treated as a U.S. domestic corporation for U.S. federal income tax purposes.
Under current U.S. federal tax law, a corporation is generally considered for U.S. federal income tax purposes to be a tax resident in the jurisdiction of its organization or incorporation. Accordingly, under the generally applicable U.S. federal income tax rules, because we are incorporated under the laws of British Columbia, Canada, we would be classified as a non-U.S. corporation (and, therefore, not a U.S. tax resident) for U.S. federal income tax purposes. Section 7874 of the Internal Revenue Code of 1986, as amended (the Code) provides an exception to this general rule, under which a non-U.S. incorporated entity will nevertheless be treated as a U.S. corporation for U.S. federal income tax purposes (and, therefore, as a U.S. tax resident subject to U.S. federal income tax on its worldwide income) if each of the following three conditions are met: (i) the non-U.S. corporation, directly or indirectly, acquires substantially all of the properties held directly or indirectly by a U.S. corporation (including through the acquisition of all of the outstanding shares of the U.S. corporation), (ii) the non-U.S. corporation’s “expanded affiliated group” does not have “substantial business activities” in the non-U.S. corporation’s country of organization or incorporation and tax residence relative to the expanded affiliated group’s worldwide activities and (iii) after the acquisition, the former shareholders of the acquired U.S. corporation hold at least 80% (by either vote or value) of the shares of the non-U.S. acquiring corporation by reason of holding shares in the U.S. acquired corporation (taking into account the receipt of the non-U.S. corporation’s shares in exchange for the U.S. corporation’s shares) as determined for purposes of Section 7874 (this test is referred to as the 80% ownership test).
On April 4, 2016, the U.S. Treasury Department (Treasury) and the IRS issued the Temporary Section 7874 Regulations, which, among other things, require certain adjustments that generally increase, for purposes of the 80% ownership test, the percentage of the shares of the acquiring non-U.S. corporation deemed owned (within the meaning of Section 7874) by the former shareholders of the acquired U.S. corporation by reason of holding shares in such U.S. corporation. It is possible that Aegerion shareholders could be deemed to acquire for purposes of Section 7874 more than 80% of Novelion in the Merger and that as a result we will be treated as a U.S. corporation for U.S. federal income tax purposes. If we were to be treated as a U.S. corporation for U.S. federal tax purposes, we could suffer adverse tax consequences, including potential U.S. income taxes on future profits distributed from non-U.S. subsidiaries and loss of eligibility for benefits under the income tax treaty between Canada and the U.S.
If the Section 7874 percentage is calculated to be at least 60% or more (but less than 80%), Section 7874 can limit the ability of the acquired U.S. corporation and its U.S. affiliates to utilize U.S. tax attributes such as net operating losses to offset U.S. taxable income resulting from certain transactions. Additionally, related rules may impose an excise tax under Section 4985 of the Code on the gain recognized by certain “disqualified individuals” (including officers and directors of a U.S. company) on certain stock-based compensation held by such individuals at a rate equal to 15%. We may, if we determine that it is appropriate, provide disqualified individuals with payments to offset this excise tax, so that, on a net after-tax basis, they would be in the same position as if no such excise tax had been applied.
87
After taking into account the relevant adjustments under the temporary 7874 regulations and based on the facts and circumstances as of the date of the Merger, the Section 7874 percentage following the Merger is expected to be less than 60% and, thus, we do not expect the excise tax to apply to "disqualified individuals." However, if the IRS determines that the exercise tax does apply, the payment of such excise tax will be costly to us.
We may not be able to achieve tax savings as a result of the Merger.
Even if we are not treated as a U.S. corporation for U.S. federal income tax purposes under the inversion rules discussed above, there can be no assurance as to the effective tax rates applicable to our future revenue. For example, the ability of the companies to locate personnel and to integrate and manage operations in a manner that supports and protects the tax benefits that potentially may be realized from Novelion’s Canadian tax domicile is uncertain and complex.
Potential improvements to our effective tax rate that may result from Novelion’s Canadian tax domicile have not been reflected in the pro forma financial information.
We may not be able to use our net operating loss carryforwards (NOLs) to offset future taxable income for U.S. or Canadian federal income tax purposes.
As of December 31, 2016, we had NOLs for U.S. federal income tax purposes of approximately $14.3 million, which expire at various dates through 2036.
Under Section 382 of the Code, if a corporation subject to the Code undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change U.S. net operating loss carryforwards (NOLs), and other pre-change tax attributes (such as research tax credits) to offset its post-change income may be limited. We underwent an “ownership change” within the meaning of Section 382 and 383 of the Code as a result of the Merger, and therefore an annual limit may be imposed on the amount of NOLs that may be used to offset future taxable income. Such annual limit is generally the product of the total value of a company’s outstanding equity immediately prior to an “ownership change” (subject to certain adjustments) and the applicable federal long term tax exempt interest rate. Certain of our U.S. subsidiaries underwent an “ownership change” (as defined above) and triggered the limitation on use of NOLs in 2005, 2012, and 2016. Due to the ownership changes, we have determined that our U.S. subsidiaries, including Aegerion, will only be able to utilize a small percentage of their NOLs and tax attributes. In connection with the Merger, we have determined that Aegerion had a net unrealized built-in-loss (“NUBIL”). The NUBIL was determined based on the difference between the fair market value of Aegerion’s assets and their tax basis as of the ownership change date. Because of the NUBIL, certain deductions recognized during the five-year period beginning on the date of the Section 382 ownership change are subject to the same limitation as the NOL carryforwards. Our U.S. subsidiaries may also experience ownership changes in the future as a result of subsequent shifts in share ownership. As a result, if our U.S. subsidiaries earn net taxable income, their ability to use their pre-change NOL carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liabilities. In addition, at the U.S. state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
As of December 31, 2016, we had NOLs for Canadian federal income tax purposes of approximately $152.6 million, which expire at various dates through 2036. The extent to which we can utilize any or all of our NOLs will depend on many factors, including the jurisdiction applicable to any future taxable revenue of Novelion.
Our ability to use NOLs will also depend on the amount of taxable income generated in future periods. The NOLs may expire before we can generate sufficient taxable income to use the NOLs.
We may be treated as a passive foreign investment corporation (a PFIC) for U.S. federal income tax purposes, which could result in adverse U.S. federal income tax consequences to U.S. holders and may deter certain U.S. investors from purchasing our shares.
We believe that we were a PFIC for the taxable years ended December 31, 2008 through 2015. Based on the price of our common shares and the composition of our assets, we believe that we will not be deemed a PFIC for U.S. federal income tax purposes for the taxable year ended December 31, 2016. The determination of whether we are a PFIC is made annually and depends on the particular facts and circumstances (such as the valuation of its assets, including goodwill and other intangible assets) and also may be affected by the application of the PFIC rules, which are subject to differing interpretations.
The rules governing PFICs can have adverse tax effects on U.S. holders which may be mitigated by making certain elections for U.S. federal income tax purposes, which elections may or may not be available. If we are determined to be a PFIC in any year, a U.S. holder of common shares in such year will be required to file an annual information return on IRS Form 8621 regarding
88
distributions received on such common shares and any gain realized on disposition of such common shares and will generally be required to file an annual information return with the IRS (also on IRS Form 8621, which PFIC shareholders are required to file with their U.S. federal income tax or information return) relating to their ownership of our common shares. Additionally, if we are classified as a PFIC in any taxable year, with respect to which a U.S. holder owns common shares, we generally will continue to be treated as a PFIC with respect to such U.S. holder in all succeeding taxable years, regardless of whether we continue to meet the tests described above, unless the U.S. holder makes a “deemed sale election.” Treatment as a PFIC could deter certain U.S. investors from purchasing our common shares, which could have an adverse impact on our share price. For purposes of this risk factor, a “U.S. holder” is a beneficial owner of our common shares or warrants that is for U.S. federal income tax purposes: (a) an individual who is a citizen or resident of the U.S.; (b) a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the U.S., any state thereof or the District of Columbia; (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source; or (d) a trust (i) if a court within the U.S. can exercise primary supervision over its administration, and one or more U.S. persons have the authority to control all of the substantial decisions of that trust, or (ii) that has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
Item 1B. | Unresolved Staff Comments. |
Not applicable.
Item 2. | Properties. |
During 2016, we exercised a one-year renewal option on our existing lease in Vancouver, British Columbia, where our head office is located. We currently lease approximately 8,475 square feet of space under the terms of this agreement. The lease term applicable to this space expires on August 31, 2017.
Our U.S. operational office, which is located at One Main Street in Cambridge, Massachusetts, consists of approximately 31,571 square feet of office space under a lease that expires in April 2019.
We also lease office spaces in Japan, the UK, Switzerland, Germany, France, Italy, Canada, Brazil, and Turkey, with approximately 8,414 square feet of office space in the aggregate. Our international lease agreements expire at various dates through the year 2021.
In addition to the locations listed above, we hold inventory at various locations, including international locations, managed by third parties.
Item 3. | Legal Proceedings. |
In late 2013, our subsidiary, Aegerion, received a subpoena from the DOJ, represented by the U.S. Attorney’s Office in Boston, requesting documents regarding our marketing and sale of JUXTAPID in the U.S., as well as related disclosures. We believe the DOJ is seeking to determine whether Aegerion, or any of its current or former employees, violated civil and/or criminal laws, including, but not limited to, the securities laws, the Federal False Claims Act, the FDCA, the Anti-Kickback Statute, and the FCPA. The investigation is ongoing.
In late 2014, Aegerion received a subpoena from the SEC requesting certain information related to Aegerion’s sales activities and disclosures related to JUXTAPID. The SEC also has requested documents and information on a number of other topics, including documents related to the investigations by government authorities in Brazil into whether Aegerion’s activities in Brazil violated Brazilian anti-corruption laws, and whether Aegerion’s activities in Brazil violated the FCPA. We believe the SEC is seeking to determine whether Aegerion, or any of its current or former employees, violated securities laws. The investigation is ongoing.
In May 2016, Aegerion reached preliminary agreements in principle with the DOJ and the SEC to resolve their investigations into the marketing and sales activities and disclosures relating to JUXTAPID. Under the terms of the preliminary agreement in principle with the DOJ, Aegerion would plead guilty to two misdemeanor misbranding violations of the FDCA. One count would be based on its alleged marketing of JUXTAPID with inadequate directions for use (21 U.S.C. §§ 352(f)), and the second count would involve an alleged failure to comply with a requirement of the JUXTAPID REMS program (21 U.S.C. §§ 352(y)). Aegerion would separately enter into a five-year deferred prosecution agreement with regard to a charge that Aegerion violated HIPAA. As part of the resolution of the DOJ investigation, we expect Aegerion to enter into a civil settlement agreement with the DOJ to resolve alleged violations of the False Claims Act, and a non-monetary consent decree with the FDA. We also expect to negotiate a corporate integrity agreement with the Department of Health and Human Services.
Under the terms of the preliminary agreement in principle with the SEC staff, the SEC’s Division of Enforcement will recommend that the SEC accept a settlement offer from Aegerion on a neither-admit-nor-deny basis that contains alleged negligent violations
89
of Sections 17(a)(2) and (3) of the Securities Act of 1933, as amended, related to certain statements we made in 2013 regarding the conversion rate of patients receiving JUXTAPID prescriptions, with remedies that include censure, an order prohibiting future violations of the securities laws and payment of a civil penalty.
The preliminary agreements in principle provide for a consolidated monetary package that covers payments due to both the DOJ and the SEC. The consolidated monetary package covers payments due to both the DOJ and the SEC by Aegerion totaling approximately $40 million in the aggregate, to be payable over three years, which is updated from the originally proposed five-year payment schedule contemplated when the preliminary agreement in principle was reached in May 2016. Certain outstanding amounts would accrue interest at a rate of 1.75% per annum. Such payments are subject to acceleration in the event of certain change of control transactions or the sale of JUXTAPID or MYALEPT. As of December 31, 2016, Aegerion had accrued an aggregate of $40.6 million for the payments to be provided to the DOJ and the SEC under the consolidated monetary package, and an aggregate of $1.0 million for any relator attorney fees and settlement. In March 2017, the final relator agreements were signed and the Company paid out the attorney fees and settlement payments.
The terms of the preliminary agreements in principle described above may change following further negotiations and other terms of the final settlement remain subject to further negotiation. The preliminary agreement in principle with the DOJ is subject to approval of supervisory personnel within the DOJ and relevant federal and state agencies, and approval by a U.S. District Court judge of the criminal plea and sentence and the civil settlement agreement. The preliminary agreement in principle with the SEC is subject to review by other groups in the SEC and approval by the Commissioners of the SEC.
The preliminary agreements in principle do not cover the DOJ and the SEC’s inquiries concerning Aegerion’s operations in Brazil, any potential claims by relators for attorneys’ fees, or any employment claims that may been brought by relators.
We continue to cooperate with the DOJ and the SEC with respect to their investigations. As part of this cooperation, the DOJ has requested documents and information related to donations Aegerion made in 2015 and 2016 to 501(c)(3) organizations that provide financial assistance to patients. As part of this inquiry, the DOJ may pursue theories that will not be covered by the preliminary agreement in principle with the DOJ. Other pharmaceutical and biotechnology companies have disclosed similar inquiries regarding donations to patient assistance programs operated by independent charitable 501(c)(3) organizations.
In addition, federal and state authorities in Brazil are conducting an investigation to determine whether there have been violations of Brazilian laws related to the promotion of JUXTAPID in Brazil. In July 2016, the Ethics Council of Interfarma fined Aegerion approximately $0.5 million for violations of the industry association’s Code of Conduct, to which Aegerion is bound due to its affiliation with Interfarma. Also, the Board of Directors of Interfarma imposed an additional penalty of suspension of Aegerion’s membership, without suspension of Aegerion’s membership contribution, for a period of 180 days for Aegerion to demonstrate the implementation of effective measures to cease alleged irregular conduct, or exclusion of our membership in Interfarma if such measures are not implemented. Aegerion paid approximately $0.5 million related to this fine during the third quarter of 2016. On March 27, 2017, after the suspension period ended, Interfarma’s Board of Directors decided to reintegrate Aegerion, enabling it to participate regularly in Interfarma activities, subject to meeting certain obligations. Also in July 2016, Aegerion received an inquiry from a Public Prosecutor Office of the Brazilian State of Paraná asking it to respond to questions related to recent media coverage regarding JUXTAPID and its relationship with a patient association to which Aegerion made donations for patient support. At this time, we do not know whether the Public Prosecutor’s inquiry will result in the commencement of any formal proceeding against Aegerion, but if Aegerion’s activities in Brazil are found to violate any laws or governmental regulations, Aegerion may be subject to significant civil lawsuits to be filed by the Public Prosecution office, and administrative penalties imposed by Brazilian regulatory authorities and additional damages and fines. Under certain circumstances, Aegerion could be barred from further sales to federal and/or state governments in Brazil, including sales of JUXTAPID and/or MYALEPT, due to penalties imposed by Brazilian regulatory authorities or through civil actions initiated by federal or state public prosecutors. As of the filing date of this Annual Report, we cannot determine if a loss is probable as a result of the investigations and inquiry in Brazil and whether the outcome will have a material adverse effect on our business and, as a result, no amounts have been recorded for a loss contingency.
In January 2014, a putative class action lawsuit was filed against Aegerion and certain of its former executive officers in the U.S. District Court for the District of Massachusetts (the Court) alleging certain misstatements and omissions related to the marketing of JUXTAPID and Aegerion’s financial performance in violation of the federal securities laws. The case is captioned KBC Asset Management NV et al. v. Aegerion Pharmaceuticals, Inc. et al., No. 14-cv-10105-MLW. On March 11, 2015, the Court appointed co-lead plaintiffs and lead counsel. Co-lead plaintiffs filed an amended complaint on June 1, 2015. Aegerion filed a motion to dismiss the amended complaint for failure to state a claim on July 31, 2015. On August 21, 2015, co-lead plaintiffs filed a putative second amended complaint. On September 4, 2015, Aegerion moved to strike the second amended complaint for the co-lead plaintiffs’ failure to seek leave of court to file a second amended pleading. Oral argument on the motion to strike was held on March 9, 2016. On March 23, 2016, plaintiffs filed a motion for leave to amend. Aegerion opposed this motion to amend, and following a hearing on April 29, 2016, the Court took defendants’ motion to strike and plaintiffs’ motion for leave to amend under
90
advisement. On May 13, 2016, co-lead plaintiffs and defendants filed a joint motion wherein the parties stipulated that co-lead plaintiffs could file a third amended pleading within 30 days of the motion, which the Court granted on May 18, 2016, thereby mooting defendants’ pending motion to strike the second amended pleading and co-lead plaintiffs’ motion for leave to file a second amended pleading. The Court also entered a briefing schedule for defendants to file responsive pleadings, co-lead plaintiffs to file any opposition, and defendants to file reply briefs. A third amended complaint was filed on June 27, 2016. On July 22, 2016, co-lead plaintiffs and defendants filed a joint motion to stay the briefing schedule while they pursued mediation, which the Court granted on August 10, 2016. Through mediation, the co-lead plaintiffs and defendants reached an agreement in principle to settle the litigation on November 29, 2016. On January 17, 2017, the co-lead plaintiffs filed a stipulation of settlement with the Court that contained the settlement terms as agreed upon by the parties, including that Aegerion and its insurance carriers would contribute $22.25 million to a settlement fund for the putative class. The insurance carriers have agreed to cover $22.0 million of this amount, with Aegerion responsible for the remainder of $0.25 million. The proposed settlement is subject to a number of procedural steps and is subject to approval by the Court. Accordingly, we cannot predict the outcome of this action or when it will be resolved. We have recorded a loss contingency of $22.25 million and insurance proceeds receivable of $22.0 million at December 31, 2016.
On September 22, 2015, we commenced an action in the Supreme Court of British Columbia against Valeant Pharmaceuticals International, Inc. for breach of contract under the terms of the asset purchase agreement with Valeant (the Valeant Agreement), entered into on September 21, 2012, pursuant to which we sold all of our assets related to Visudyne ®, including our Qcellus™ laser and certain other photodynamic therapy intellectual property, with respect to failure to pay a $5.0 million laser earn-out payment and failure to use commercially reasonable efforts to promptly obtain the laser registrations for the Qcellus laser in the U.S. As of December 31, 2016, we have recorded a long-term accounts receivable at its estimated fair value of zero and this zero fair value reflected management’s assessment of collection risk, the impact of the passage of time and the potential collection costs associated with the Valeant litigation. For additional information, refer to Note 16 - Contingencies, Commitments and Guarantees - Related to the Sale of Visudyne.
Item 4. | Mine Safety Disclosures. |
Not applicable.
91
PART II
Item 5. | Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities. |
Our common shares are traded in Canada on TSX and in the U.S. on NASDAQ under the symbol “NVLN”. The following table sets forth the high and low sales prices for our common shares in each of 2016 and 2015, as quoted on TSX and NASDAQ, as adjusted to give effect to the Consolidation discussed in Note 1- Description of Business, included in the “Consolidated Financial Statements and Supplementary Data” section of this Annual Report:
Toronto Stock Exchange | NASDAQ Global Select Market | ||||||||||||||
2016 | High (CAD$) | Low (CAD$) | High (U.S.$) | Low (U.S.$) | |||||||||||
First Quarter | C$ | 19.40 | C$ | 12.55 | $ | 11.85 | $ | 8.19 | |||||||
Second Quarter | C$ | 14.10 | C$ | 8.00 | $ | 9.37 | $ | 6.10 | |||||||
Third Quarter | C$ | 14.10 | C$ | 8.50 | $ | 10.95 | $ | 6.52 | |||||||
Fourth Quarter | C$ | 14.75 | C$ | 10.25 | $ | 13.80 | $ | 7.65 | |||||||
2015 | |||||||||||||||
First Quarter | C$ | 28.95 | C$ | 23.25 | $ | 21.19 | $ | 16.13 | |||||||
Second Quarter | C$ | 27.50 | C$ | 21.50 | $ | 19.18 | $ | 14.03 | |||||||
Third Quarter | C$ | 27.60 | C$ | 17.75 | $ | 18.92 | $ | 11.46 | |||||||
Fourth Quarter (1) | C$ | 21.15 | C$ | 17.00 | $ | 14.30 | $ | 10.81 | |||||||
(1) Prior to completing the Merger with Aegerion on November 29, 2016, our shares traded under the symbol “QLTI” on NASDAQ.
The last reported sale price of the common shares on TSX and on NASDAQ on March 1, 2017 was CAD$14.40 and USD$10.80, respectively.
As of February 28, 2017, there were 1,119 registered holders of our common shares, 1,002 of whom were residents of the U.S. Of the total 18,533,029 common shares outstanding, the portion held by registered holders resident in the U.S. was 15,954,267 or 86.09%.
We have never paid any cash dividends on our common shares and we do not anticipate paying such cash dividends in the foreseeable future. We currently anticipate that we will retain all future earnings, if any, for use in the development of our business.
Equity Compensation Plan Information as of December 31, 2016
Information regarding our equity compensation plans is included in the “Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters” section of this Annual Report and incorporated in this Item 5 by reference.
Performance Graph
The following performance graph compares cumulative total shareholder return on the common shares of NVLN for the last five fiscal years with the total cumulative return of the NASDAQ Composite Index (U.S.), the NASDAQ Biotechnology Index, and the S&P/TSX Composite Index over the same period. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends, however no dividends have been declared on our common shares to date.
92
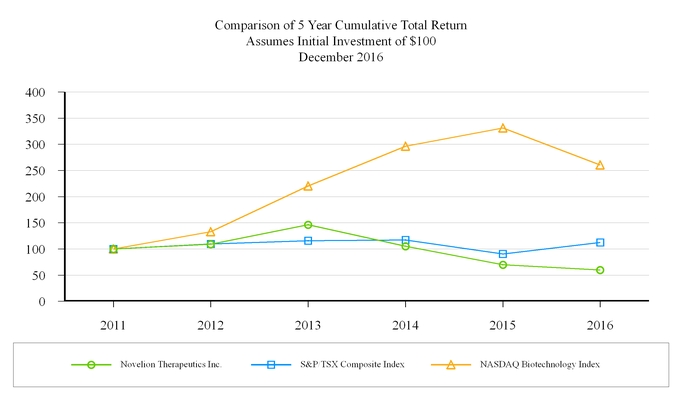
December 31, 2011 | December 31, 2012 | December 31, 2013 | December 31, 2014 | December 31, 2015 | December 31, 2016 | |||||||
Novelion Therapeutics Inc. | 100.00 | 109.17 | 146.40 | 105.40 | 69.92 | 59.79 | ||||||
S&P/TSX Composite Index | 100.00 | 109.52 | 115.83 | 117.17 | 90.22 | 112.31 | ||||||
NASDAQ Biotechnology Index | 100.00 | 132.74 | 220.37 | 296.19 | 331.05 | 260.37 | ||||||
The graph above assumes $100 invested on December 31, 2011 in common shares of Novelion and in each index. The shareholder return shown above are historical and not necessarily indicative of future price performance, and we do not make or endorse any predictions as to future shareholder returns.
Dividend Policy
We have not declared or paid any dividends on our common shares since inception. The declaration of dividend payments is at the sole discretion of our Board of Directors. The Board of Directors may declare dividends in the future depending upon numerous factors that ordinarily affect dividend policy, including the results of our operations, our financial position and general business conditions.
Recent Sales of Unregistered Securities
On June 14, 2016, we entered into the Unit Subscription Agreement with the Investors. Pursuant to the Unit Subscription Agreement, immediately prior to the Merger, the Investors acquired units, for $8.80 per unit, on a post-Consolidation basis, consisting of (i) 2,472,727 Novelion common shares, which includes up to 568,181 Novelion common shares issuable upon exercise of fully paid-up warrants, and (ii) Warrants exercisable for up to an aggregate of 2,644,952 Novelion common shares at an exercise price of $0.05 per common share. The aggregate consideration received under the Unit Subscription Agreement was approximately $21.8 million, which we intend to continue to use to support future operations and business development initiatives.
Use of Proceeds from Registered Securities
Not applicable.
93
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
During the year ended December 31, 2016, there were no common share repurchases.
Exchange Controls and Other Limitations Affecting Holders of Common Shares
There is no limitation imposed by Canadian law or the Notice of Articles or Articles of the Company on the right of non-residents to hold or vote common shares in the Company, other than those imposed by the Investment Canada Act (Canada) (the Investment Act). Generally speaking, the Investment Act establishes the following two principal procedures for certain investments involving Canadian businesses, as defined by the Investment Act, by an individual, government or agency thereof, corporation, partnership, trust or joint venture that is not a “Canadian,” as defined in the Investment Act (a non-Canadian): either the filing of an application for review which, except in certain limited circumstances, must be filed before closing and the non-Canadian cannot complete its investment until the Minister responsible for the Investment Act has determined that the investment is “likely to be of net benefit to Canada,” or the filing of a notice, which must be filed within 30 days after the completion of the investment. A notice is not subject to substantive review and is required for investments by a non-Canadian that involve either the establishment of a new Canadian business or that involve an acquisition of control of a Canadian business but the prescribed thresholds for review are not exceeded. Subject to the possible application of the national security provisions, the Investment Act does not apply to investments in existing Canadian businesses that do not result in an acquisition of control, as defined under the Investment Act.
A direct investment by a non-Canadian to acquire control of a Canadian business is a reviewable investment where the value of the assets of the corporation, based on the corporation’s fiscal year immediately preceding the investment, is CAD$5 million or more. Higher limits apply for direct acquisitions by or from World Trade Organization (WTO) member country investors, as described below.
The acquisition of a majority of the voting interests of an entity or of a majority of the undivided ownership interests in the voting shares of an entity that is a corporation is deemed to be acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of voting shares. The acquisition of less than one-third of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is deemed not to be acquisition of control of that corporation. Certain transactions in relation to common shares in the Company would be exempt from review from the Investment Act, including:
a. | acquisition of common shares by a person in the ordinary course of that person’s business as a trader or dealer in securities; |
b. | acquisition of control of us in connection with the realization of security granted for a loan or other financial assistance and not for any purpose related to the provisions of the Investment Act; and |
c. | acquisition of control of us by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of the Company, through the ownership of voting interests, remains unchanged. |
Under the Investment Act, a direct investment in common shares of the Company by a non-Canadian who is a WTO investor (as defined in the Investment Act) would be reviewable only if it were an investment to acquire control of the Company and the enterprise value of the Company was CAD$600 million or more. Legislation has been introduced to increase this threshold to CAD$1 billion on April 24, 2017. A different threshold applies to an acquisition by a state-owned enterprise (SOE). Currently, where the acquisition is by a SOE, the investment would be reviewable if the value of our assets was CAD$375 million or more. This threshold is expected to increase to CAD$379 million in the near-term future as it is subject to an annual adjustment on the basis of a prescribed formula in the Investment Act to reflect the change in Canada’s gross domestic product.
The Minister responsible under the Investment Act can, within a prescribed period, require the review of an investment by a non-Canadian (even one that does not amount to an acquisition of control, and/or does not meet the review thresholds set out above) on grounds that it is likely to be injurious to national security. Ultimately, the Cabinet can prohibit the completion of an investment, or require divestment of control of a completed investment, or impose terms and conditions on an investment where the investment is injurious to national security.
See also the “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Certain Canadian and U.S. Federal Income Tax Information for U.S. Residents - U.S. Federal Income Tax Information” section of this Annual Report.
94
Item 6. | Selected Financial Data. |
Annual Financial Data
The following selected financial data should be read in conjunction with our Consolidated Financial Statements and notes to our Consolidated Financial Statements and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained elsewhere in this Annual Report on Form 10-K (Annual Report). The selected Consolidated Statements of Operations data for the years ended December 31, 2016, 2015 and 2014 and Consolidated Balance Sheets data as of December 31, 2016 and 2015 have been derived from our Consolidated Financial Statements appearing elsewhere in this Annual Report. The selected Consolidated Statements of Operations data for the years ended December 31, 2013 and 2012 and Consolidated Balance Sheets data as of December 31, 2014, 2013 and 2012 have been derived from our Consolidated Financial Statements prepared in accordance with U.S. GAAP which are not included in this Annual Report. All per-share data has been retrospectively adjusted to give effect to the Consolidation discussed in Note 1. Historical results are not necessarily indicative of future results.
Years Ended December 31, | |||||||||||||||||||
2016 (1) | 2015 (3) | 2014 (4) | 2013 (5) | 2012 (6) | |||||||||||||||
CONSOLIDATED STATEMENTS OF OPERATIONS DATA | (in thousands, except per share amounts) | ||||||||||||||||||
Net product sales | $ | 13,574 | $ | — | $ | — | $ | — | $ | — | |||||||||
Cost of product sales | 5,971 | — | — | — | — | ||||||||||||||
Selling, general and administrative expenses | 29,525 | 16,222 | 17,682 | 6,986 | 15,082 | ||||||||||||||
Research and development expenses | 14,784 | 9,790 | 13,803 | 18,509 | 24,578 | ||||||||||||||
Loss from continuing operations | (52,870 | ) | (23,009 | ) | (4,005 | ) | (25,838 | ) | (42,264 | ) | |||||||||
Net (loss) income | (52,870 | ) | (23,009 | ) | (4,071 | ) | (24,871 | ) | 45,698 | ||||||||||
Basic and diluted net (loss) income per common share (2) | |||||||||||||||||||
Continuing operations | (4.69 | ) | (2.20 | ) | (0.40 | ) | (2.55 | ) | (4.20 | ) | |||||||||
Discontinued operations | — | — | — | 0.10 | 8.75 | ||||||||||||||
Net (loss) income per common share (2) | $ | (4.69 | ) | $ | (2.20 | ) | $ | (0.40 | ) | $ | (2.45 | ) | $ | 4.55 | |||||
As of December 31, | |||||||||||||||||||
2016 (1) | 2015 (3) | 2014 (4) | 2013 (5) | 2012 (6) | |||||||||||||||
CONSOLIDATED BALANCE SHEETS DATA | (in thousands) | ||||||||||||||||||
Cash and cash equivalents (7) | $ | 108,927 | $ | 141,824 | $ | 155,908 | $ | 118,521 | $ | 307,384 | |||||||||
Total assets | 480,782 | 145,166 | 160,371 | 163,867 | 401,218 | ||||||||||||||
Working capital | 47,337 | 139,253 | 153,900 | 157,587 | 352,014 | ||||||||||||||
Debt financing and convertible notes | 225,584 | — | — | — | — | ||||||||||||||
Accumulated deficit | (587,208 | ) | (534,338 | ) | (511,329 | ) | (507,258 | ) | (482,387 | ) | |||||||||
Total shareholders’ equity | $ | 135,787 | $ | 141,341 | $ | 156,512 | $ | 157,784 | $ | 388,318 | |||||||||
1. | On November 29, 2016, we completed the Merger. Our financial position at the end of 2016 included Aegerion’s financial position. Our results of operations for 2016 consisted of Aegerion’s financial performance from November 29, 2016 to December 31, 2016. |
2. | Per share amounts have been retrospectively restated to reflect the one -for- five share consolidation of Novelion’s common stock effective on December 16, 2016. |
3. | On September 15, 2015, the InSite Merger Agreement was terminated after InSite’s board of directors notified QLT that they had reviewed a second unsolicited offer from Sun Pharmaceuticals Industries Ltd. and determined that it was superior to the proposed InSite Merger with QLT. The Sun Proposal was an all-cash offer to acquire InSite for $0.35 per share of InSite common stock. As a result, InSite notified QLT that it was exercising its right to terminate the InSite Merger Agreement in order to enter into an agreement with Sun, and InSite paid QLT a termination fee of $2.7 million. During the year ended December 31, 2015, QLT incurred consulting and transaction fees of $9.4 million in connection with the pursuit of the InSite Merger and the strategic transactions as described below under Note 3 - Terminated Merger Transactions. |
4. | On October 8, 2014, the Auxilium Merger Agreement among QLT, Auxilium, HoldCo, and AcquireCo, terminated after Auxilium delivered a notice of termination to QLT informing QLT that Auxilium’s board of directors had determined that the Endo Proposal was a superior proposal under the terms of the Auxilium Merger Agreement. Due to this change in recommendation by Auxilium’s board of directors and in accordance with the termination |
95
provisions of the Auxilium Merger Agreement, on October 9, 2014 Auxilium paid QLT a termination fee of $28.4 million. On October 22, 2014, pursuant to the terms of our financial advisory services agreement with Credit Suisse, we paid Credit Suisse a fee of $5.7 million in connection the termination of the Auxilium Merger Agreement. During the year ended December 31, 2014, QLT incurred consulting and transaction fees of $10.2 million in connection with our pursuit of the Auxilium Merger.
5. | On June 27, 2013, we completed a $200.0 million special cash distribution, by way of a reduction of the paid-up capital of the Company’s common shares (the Cash Distribution). The Cash Distribution was approved by the Company’s shareholders at QLT’s annual and special shareholders’ meeting on June 14, 2013. All shareholders of record as of June 24, 2013 (the Record Date) were eligible to participate in the Cash Distribution and received a payment of approximately $3.92 per share based upon the 51,081,878 common shares issued and outstanding on the Record Date. |
6. | On April 3, 2013, we completed the sale of our punctal plug drug delivery system technology to Mati pursuant to an asset purchase agreement. During the year ended December 31, 2013, we recognized a $1.1 million gain within discontinued operations, which represented $1.2 million of sale proceeds net of the $0.2 million carrying value of certain equipment sold and a negligible amount of other transaction fees. |
7. | On September 24, 2012, we completed the sale of our Visudyne business to Valeant pursuant to an asset purchase agreement. During the year ended December 31, 2012, we recognized a pre-tax gain of $101.4 million related to this transaction within discontinued operations. |
Quarterly Financial Data (Unaudited)
Set out below is selected consolidated financial information for each of the fiscal quarters of 2016 and 2015.
(In thousands, except per share information) Quarter Ended | December 31 (a) | September 30 | June 30 | March 31 | |||||||||||
2016 | |||||||||||||||
Net product sales | $ | 13,574 | $ | — | $ | — | $ | — | |||||||
Cost of product sales | 5,971 | — | — | — | |||||||||||
Selling, general and administrative expenses | 16,038 | 3,138 | 4,451 | 5,898 | |||||||||||
Research and development expenses | 6,010 | 2,855 | 2,929 | 2,990 | |||||||||||
Loss from continuing operations | (19,920 | ) | (5,936 | ) | (5,120 | ) | (21,894 | ) | |||||||
Net loss | (19,920 | ) | (5,936 | ) | (5,120 | ) | (21,894 | ) | |||||||
Basic and diluted net loss per common share (b) (d) | (1.48 | ) | (0.55 | ) | (0.50 | ) | (2.05 | ) | |||||||
(In thousands, except per share information) Quarter Ended | December 31 | September 30 | June 30 | March 31 | |||||||||||
2015 (c) | |||||||||||||||
Net product sales | $ | — | $ | — | $ | — | $ | — | |||||||
Cost of product sales | — | — | — | — | |||||||||||
Research and development expenses | 2,036 | 2,142 | 3,404 | 2,208 | |||||||||||
Loss from continuing operations | (3,680 | ) | (2,682 | ) | (10,751 | ) | (5,896 | ) | |||||||
Net loss | (3,680 | ) | (2,682 | ) | (10,751 | ) | (5,896 | ) | |||||||
Basic and diluted net loss per common share (b) (d) | (0.30 | ) | (0.25 | ) | (1.05 | ) | (0.60 | ) | |||||||
a. | On November 29, 2016, we completed the Merger. Our financial position at the end of Q4 2016 included Aegerion’s financial position. Our results of operations for Q4 2016 included Aegerion’s financial performance from November 29, 2016 to December 31, 2016. |
b. | Per share amounts have been retrospectively restated to reflect the one -for- five share consolidation of Novelion’s common stock effective on December 16, 2016. |
c. | On September 15, 2015, the InSite Merger Agreement was terminated after InSite’s board of directors notified QLT that they had reviewed a second unsolicited offer from Sun Pharmaceuticals Industries Ltd. and determined that it was superior to the proposed InSite Merger with QLT. The Sun Proposal was an all-cash offer to acquire InSite for $0.35 per share of InSite common stock. Due to this change in recommendation by InSite’s board of directors and in accordance with the termination provisions of the InSite Merger Agreement, InSite paid QLT a termination fee of $2.7 million. During the year ended December 31, 2015, QLT incurred consulting and transaction fees of $9.4 million in connection with the pursuit of the InSite Merger (as described under Note 3 - Terminated Merger Transactions). |
d. | Basic and diluted income (loss) per share are determined separately for each quarter. As a result, the sum of the quarterly amounts may differ from the annual amounts disclosed in the Consolidated Financial Statements due to the use of different weighted average numbers of shares outstanding. |
96
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
All statements included or incorporated by reference into this Annual Report on Form 10-K (Annual Report), other than statements or characterizations of historical fact, are “forward-looking statements” under applicable laws, regulations and other legal principles and constitute “forward-looking information” within the meaning of applicable Canadian securities laws. Forward-looking statements and information are often identified by words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “believes,” “seeks,” “estimates,” “forecasts,” “may,” “will,” “should,” “would,” “could,” “potential,” “guidance,” “continue,” “ongoing” and similar expressions, and variations or negatives of these words. Examples of forward-looking statements and information contained in this Annual Report include our statements regarding: the commercial potential for, and market acceptance of, our products; our estimates as to the potential number of patients with the diseases for which our products are approved or for which our product candidates are being developed; our expectations with respect to reimbursement of our products in the U.S. and elsewhere; our expectations with respect to named patient sales of our products in Brazil and in other countries where such sales are permitted; the potential for and possible timing of approval of our products in countries where we have not yet obtained approval; our plans for further clinical development of our products; the potential for zuretinol to obtain a rare pediatric disease designation and/or priority review voucher, if approved; our expectations regarding future regulatory filings for our products, including planned marketing approval applications with respect to metreleptin to expand the indication for metreleptin in the U.S., subject to discussions with the FDA; our plans for commercial marketing, sales, manufacturing and distribution of our products; our expectations with respect to the impact of competition on our future operations and results; our beliefs with respect to our intellectual property portfolio for our products and the extent to which it allows us to exclusively develop and commercialize our products and product candidates; our expectations regarding the availability of data and marketing exclusivity for our products in the U.S., the EU, Japan and other countries; our view of potential outcomes of Aegerion’s ongoing Department of Justice (DOJ) and Securities and Exchange Commission (SEC) investigations and shareholder litigation, including the terms of the agreements in principle with respect to the investigations and the memorandum of understanding with respect to the settlement of Aegerion’s shareholder litigation, and investigations in Brazil, and the possible impact and additional consequences of each on our business; our expectations regarding the impact on U.S. sales and patient attrition of JUXTAPID® as a result of the implementation of the modified JUXTAPID Risk Evaluation and Mitigation Strategy program; our expectations regarding our global consolidated tax structure and planning, our ability to achieve tax savings or utilize net operating loss carryforwards and other tax and tax planning activities, including whether we are characterized as a U.S. domestic corporation or passive foreign investment company for U.S. federal income tax purposes; our forecasts regarding sales of our products, our future expenses, our cash position and the timing of any future need for additional capital to fund operations; our ability to successfully integrate the businesses of Aegerion and Novelion; and our ability to manufacture and supply sufficient amounts of our products to meet demand.
The forward-looking statements contained in this Annual Report and in the documents incorporated into this Annual Report by reference are based on our current beliefs and assumptions with respect to future events, all of which are subject to change. Forward-looking statements are based on estimates and assumptions regarding, for example, our financial position and execution of our business strategy, post-merger integration and synergies, resolution of litigation and investigations, future competitive conditions and market acceptance of products, the possibility and timing of future regulatory approvals, expectations regarding our core capabilities, and the availability of sufficient liquidity, each made in light of current conditions and expected future developments, as well as other factors that we believe are appropriate in the circumstances. Forward-looking statements are not guarantees of future performance, and are subject to risks, uncertainties and assumptions that are difficult to predict, including those discussed in the “Risk Factors” section of this Annual Report. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors may impact our operations or results. New risks may emerge from time to time. Past financial or operating performance is not necessarily a reliable indicator of future performance. Given these risks and uncertainties, we can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them does occur, what impact such event will have on our results of operations and financial condition. Our actual results could differ materially and adversely from those expressed in any forward-looking statement in this Annual Report or in our other filings with the SEC.
This Annual Report also contains “forward-looking information” that constitutes “financial outlooks” within the meaning of applicable Canadian securities laws. This information is provided to give investors general guidance on management’s current expectations of certain factors affecting our business, including our financial results. Given the uncertainties, assumptions and risk factors associated with this type of information, including those described above, investors are cautioned that the information may not be appropriate for other purposes.
Except as required by law, we undertake no obligation to revise our forward-looking statements to reflect events or circumstances that arise after the date of this Annual Report or the respective dates of documents incorporated into this Annual Report by reference that include forward-looking statements. Therefore, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in these forward-looking statements.
97
Background
As described above, on November 29, 2016, we closed the Merger with Aegerion Pharmaceuticals, Inc. (Aegerion). As of November 29, 2016, after giving effect to the Merger, the pre-Merger shareholders of QLT Inc. (QLT) collectively owned approximately 68% and the pre-Merger stockholders of Aegerion owned approximately 32% of our outstanding common shares.
The Merger has been accounted for as a business combination in which Novelion was considered the acquirer of Aegerion. As such, the Consolidated Financial Statements of Novelion are treated as the historical financial statements of the combined companies, with the results of Aegerion being included from November 29, 2016. We have a new management team and a reconstituted Board of Directors, consisting of four legacy QLT directors, four directors who were serving on the Board of Directors of Aegerion at the time of the Merger and two directors appointed by significant shareholders pursuant to contractual arrangements. Our new management team is comprised of executives who were serving as officers of Aegerion at the time of the Merger and includes individuals with significant experience in the biopharmaceutical industry and a successful track record of developing and commercializing rare disease and other pharmaceutical products.
For periods prior to the closing of the Merger, therefore, our discussion below relates to the historical business and operations of Novelion. Certain portions of this Annual Report may contain information that may no longer be material to our business related to Aegerion’s historical operations. Any comparison of pre-Merger Aegerion revenues and operations with ours may not be helpful to an understanding of our results for the fiscal year ended December 31, 2016 or future periods.
As noted above, all references in this Annual Report to “we,” “us,” “our” and the “Company” refer to Novelion and its consolidated subsidiaries. For periods following the closing of the Merger, such references include Aegerion. As described more fully in this Annual Report, following the Merger, Novelion continues to conduct research and development related to zuretinol and Aegerion continues to develop and commercialize lomitapide and metreleptin, and each maintain its respective ownership of or licenses covering intellectual property related to such products and remain as party to the regulatory filings and approvals for such products.
Business Overview
We are a biopharmaceutical company dedicated to developing new standards of care for individuals living with rare diseases. On November 29, 2016, we completed the Merger with Aegerion. We, through Aegerion, now have two commercial products:
◦ | Metreleptin, a recombinant analog of human leptin, is marketed in the United States (U.S.) under the brand name MYALEPT (metreleptin) for injection (MYALEPT). MYALEPT is approved in the U.S. as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy (GL). In December 2016, we submitted a marketing authorization application (MAA) to the European Medicines Agency (EMA) to seek approval for metreleptin, under the brand name MYALEPTA, as replacement therapy to treat complications of leptin deficiency in patients with GL and in a subset of patients with partial lipodystrophy (PL). We also expect to submit a supplemental biologics licensing application (sBLA) to the U.S. Food and Drug Administration (FDA) in the first half of 2017, seeking to expand MYALEPT’s indication in the U.S. to the PL subset and plan to file for formal regulatory approvals for metreleptin in GL and the PL subset throughout 2017 and early 2018 in other key markets, including Brazil and Colombia. We offer metreleptin through expanded access programs in countries where permitted by applicable regulatory authorities and under applicable laws, and generate revenue in certain markets where named patient sales are permitted based on the approval of metreleptin in the U.S. In addition to the PL subset, we plan to use our knowledge of the diverse effects of leptin on various physiologic functions to explore new opportunities for metreleptin as a platform drug to potentially treat patients suffering from a range of low leptin-mediated rare and metabolic diseases. We are evaluating and prioritizing these potential opportunities and plan to provide an update in mid-2017. |
◦ | Lomitapide is marketed in the U.S. under the brand name JUXTAPID (lomitapide) capsules (JUXTAPID). JUXTAPID is approved in the U.S. as an adjunct to a low-fat diet and other lipid-lowering treatments, including low-density lipoprotein (LDL) apheresis where available, to reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein B (apo B) and non-high-density lipoprotein cholesterol (non-HDL-C) in adult patients with homozygous familial hypercholesterolemia (HoFH). Lomitapide is approved in the European Union (EU), under the brand name LOJUXTA (lomitapide) hard capsules (LOJUXTA) for the treatment of adult patients with HoFH, as well as in Japan, Canada, and a small number of other countries. In December 2016, Aegerion out-licensed the rights to commercialize LOJUXTA in the EU and certain other jurisdictions to Amryt Pharma plc (Amryt) and will receive sales milestones and royalties on net sales in those jurisdictions. In December 2016, Aegerion launched JUXTAPID as a treatment for HoFH in Japan, after receiving reimbursement approval. Lomitapide is also sold, on a named patient basis, in Brazil and in a limited number of other countries outside the U.S. where such sales are permitted as a result of the approval of lomitapide in the U.S. or the EU. |
98
We also have one orphan drug-designated product candidate, zuretinol acetate (zuretinol), an oral synthetic retinoid, in late stage development for the treatment of IRD caused by underlying mutations in retinal pigment epithelium protein 65 (RPE65) and lecithin: retinol acyltransferase (LRAT) genes, comprising Leber Congenital Amaurosis (LCA) and Retinitis Pigmentosa (RP). Our clinical and regulatory pathway for the zuretinol program is currently under review, and we expect to provide an update in mid-2017. We are also exploring the potential of submitting to the FDA a request for Rare Pediatric Disease Designation for zuretinol for the treatment of IRD. If zuretinol is approved by the FDA after being designated a Rare Pediatric Disease and we meet certain additional criteria, we may qualify for a Rare Pediatric Disease Priority Review Voucher. Zuretinol was granted orphan drug designations for the treatment of LCA (due to inherited mutations in LRAT or RPE65 genes) and RP (all mutations) by the FDA, and for the treatment of LCA and RP (all mutations) by the EMA. Both the FDA and EMA have acknowledged that the therapeutic indication of zuretinol for the treatment of IRD (patients phenotypically diagnosed as LCA or RP caused by mutations in RPE65 or LRAT genes) falls within these orphan drug designations. The drug has also been granted two Fast Track designations by the FDA for the treatment of LCA and RP due to inherited mutations in the LRAT and RPE65 genes.
We have a new management team and a reconstituted Board of Directors, consisting of four legacy QLT directors, four directors who were serving on the Board of Directors of Aegerion at the time of the Merger and two directors appointed by significant shareholders pursuant to contractual arrangements. Our new management team is comprised of executives who were serving as officers of Aegerion at the time of the Merger and includes individuals with significant experience in the biopharmaceutical industry and a successful track record of developing and commercializing rare disease and other pharmaceutical products.
During the year ended December 31, 2016, in the period after completion of the Merger, net product sales of lomitapide and metreleptin were $13.6 million, of which $10.8 million was derived from prescriptions for lomitapide and metreleptin written in the U.S., and $2.8 million was derived from prescriptions for lomitapide and metreleptin written outside the U.S. As of December 31, 2016, we had approximately $108.9 million in cash and cash equivalents. Aegerion has approximately $325.0 million principal amount of 2.0% convertible senior notes due August 15, 2019 (the Convertible Notes). See the “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources” section of this Annual Report for further information.
In the near-term, we expect that the majority of revenues will continue to be derived from sales of MYALEPT and JUXTAPID in the U.S. We also expect to generate revenues from (i) sales of lomitapide in those countries outside the U.S. in which we have or expect to receive marketing approval, are able to obtain pricing and reimbursement approval at acceptable levels, and elect to commercialize lomitapide, particularly in Japan and (ii) sales of both products in a limited number of other countries where they are, or may in the future be, available on a named patient sales basis as a result of existing approvals in the U.S. or EU. We expect that in the near-term, named patient sales of lomitapide and metreleptin in Brazil will continue to be our second largest source of revenues for each product, on a country-by-country basis. We received named patient sales orders for metreleptin in Argentina in 2016, and have had and expect to continue to have named patient sales of metreleptin in Brazil, Colombia and a select number of countries in the EU, including France and Turkey. We expect net product sales from named patient sales to fluctuate significantly quarter-over-quarter given that named patient sales are derived from unsolicited requests from prescribers. In some countries, including Brazil, orders for named patient sales are for multiple months of therapy, which can lead to some fluctuations in sales depending on the ordering pattern. We believe the investigations into Aegerion’s activities in Brazil have adversely affected named patient sales of lomitapide and metreleptin in that country. See the “Legal Proceedings” section of this Annual Report for further information regarding these investigations. In addition, a proceeding is currently pending with the Brazil Supreme Federal Court to decide whether the government has an obligation to continue to provide, on a named patient sales basis, drugs that have not received regulatory and/or pricing and reimbursement approval in Brazil, like JUXTAPID and MYALEPT. We intend to file for marketing approval in Brazil for both JUXTAPID and MYALEPT, and are currently assessing the timing of these submissions. The result of the trial and other issues could significantly negatively affect product revenues from named patient sales of JUXTAPID and MYALEPT in Brazil.
We expect that our near-term efforts will be focused on the following:
• | building and maintaining market acceptance for MYALEPT in the U.S. for the treatment of complications of leptin deficiency in GL patients, and supporting named patient sales of metreleptin in GL in Brazil, particularly in light of local economic challenges and ongoing governmental investigations, and other key countries, including France and Turkey, where such sales are permitted as a result of the U.S. approval or under local law; |
• | preparing for the launch of metreleptin in Europe as a treatment for complications of leptin deficiency in GL patients and a subset of PL, in the event we obtain regulatory, pricing and reimbursement approvals in the EU for metreleptin; |
• | evaluating the potential for future clinical development of metreleptin in additional indications, including a subset of PL, if we are unable to secure approval of such indication with the current metreleptin clinical data package, as well as potentially other low leptin-mediated rare and metabolic diseases; |
99
• | stabilizing sales of JUXTAPID as a treatment for adult HoFH patients in the U.S. despite competition from PCSK9 inhibitor products, among other factors, which have had a significant adverse impact on sales of JUXTAPID, and gaining market acceptance in the other countries where lomitapide is approved and being commercialized, or may in the future receive approval and be commercialized; |
• | managing our costs and expenses to better align with our revenues, and strengthening our capital structure, while supporting approved products in a compliant manner; |
• | continuing to support patient access to and reimbursement for our products in the U.S. without significant restrictions, particularly given the availability of PCSK9 inhibitor products in the U.S., which has adversely impacted reimbursement of JUXTAPID, and given the considerable number of JUXTAPID patients in the U.S. who are on Medicare Part D and the significant percentage of such patients who may not be able to afford their out-of-pocket co-payments for our products, given that the only source of financial support for some such patients may be through patient assistance programs operated by independent charitable 501(c)(3) organizations that may not provide adequate financial assistance; |
• | implementing the modified JUXTAPID Risk Evaluation Management Strategy (REMS) program in the U.S., which includes requirements to recertify all prescribers and pharmacies and a new patient counseling and acknowledgment requirement for existing and new patients, by the July 2, 2017 implementation deadline, while working to limit adult HoFH patient attrition from JUXTAPID as a result of such new requirements; |
• | supporting the recent launch of JUXTAPID in Japan; |
• | continuing to support sales of lomitapide as a treatment for HoFH in Brazil on a named patient basis, particularly in light of the economic challenges, ongoing government investigations, and ongoing court proceedings reviewing the regulatory framework for named patient sales in Brazil, and in other key countries where named patient sales are permitted, despite the availability of PCSK9 inhibitors on a named patient sales basis in such countries; |
• | gaining regulatory, pricing and reimbursement approvals to market our products in countries in which the products are not currently approved and/or reimbursed or for new indications, including obtaining approval of the MAA seeking marketing approval of metreleptin in the EU as a treatment for complications of leptin deficiency in GL patients and a subset of PL, and seeking approval of metreleptin in the U.S. for a subset of PL based on the existing clinical data package for metreleptin; |
• | reviewing the clinical and regulatory pathway for zuretinol to determine the optimal development and business strategy for this product candidate; |
• | engaging in possible further development efforts related to our existing products, and assessing, and possibly acquiring, potential new product candidates targeted at rare diseases where we believe we can leverage our infrastructure and expertise; |
• | minimizing the number of patients who are eligible to receive but decide not to commence treatment with our products, or who discontinue treatment, by supporting activities such as patient support programs, to the extent permitted in a particular country; |
• | continuing to embed a culture of compliance, ethics and integrity throughout Novelion and its subsidiaries; |
• | Aegerion reaching a definitive agreement with the DOJ and the SEC with respect to its ongoing investigations in accordance with the terms of the agreements in principle it entered into in May 2016 and managing other ongoing government investigations pertaining to its products; |
• | Aegerion reaching a definitive agreement with respect to its ongoing securities class action in accordance with the terms of the memorandum of understanding entered into in December 2016 (the MOU); and |
• | defending challenges to the patents or our claims of exclusivity for lomitapide in the U.S., including against potential generic submission with the FDA with respect to lomitapide; and expanding the intellectual property portfolio for our products. |
100
Investigations and Legal Proceedings
As noted above, Aegerion has been the subject of certain ongoing investigations and other legal proceedings, including investigations of Aegerion’s marketing and sales activities of JUXTAPID by the DOJ and the SEC, an investigation by federal and state authorities in Brazil to determine whether there have been violations of Brazilian laws related to the promotion of JUXTAPID, and a putative class action lawsuit alleging certain misstatements and omissions related to the marketing of JUXTAPID and the Company’s financial performance in violation of the federal securities laws (the Class Action Litigation). Aegerion reached agreements in principle with the DOJ and the SEC in May 2016 that provide for Aegerion to pay a fine of $40 million, to plead guilty to two misdemeanor misbranding violations of the Food, Drug and Cosmetics Act and to enter into a five-year deferred prosecution agreement with regard to charges that it violated the Health Insurance Portability and Accountability Act (HIPAA) and engaged in obstruction of justice relating to the JUXTAPID REMS program. Aegerion also entered into the MOU with respect to the Class Action Litigation, which provides for a settlement payment by or on behalf of Aegerion of $22.25 million, of which we expect $22.0 million will be funded by insurance carriers and $250,000 will be funded by Aegerion. See the “Legal Proceedings” section of this Annual Report for further information regarding these investigations and legal proceedings.
Recent Corporate and Securities Transactions
Merger Transaction with Aegerion. On June 14, 2016, we entered into an Agreement and Plan of Merger (as amended, the Merger Agreement) with Aegerion, pursuant to which on November 29, 2016 our indirect wholly-owned subsidiary, Isotope Acquisition Corp, merged with and into Aegerion, with Aegerion surviving as our wholly-owned subsidiary (the Merger). Upon completion of the Merger, on November 29, 2016, each outstanding share of Aegerion common stock was converted into a right to receive 1.0256 Novelion (pre-Consolidation) common shares and Aegerion’s common stock was cancelled and delisted from NASDAQ.
Pursuant to the Merger Agreement, we also issued certain warrants to the pre-closing shareholders of Novelion. These warrants (the Merger Agreement Warrants) may be exercised for up to an aggregate of 11,301,791 Novelion common shares at an exercise price of $0.05 per share if (i) the previously disclosed DOJ and SEC investigations are settled for amounts in excess of $40 million and/or (ii) the Class Action Litigation is settled for an amount that exceeds the amounts, if any, available under Aegerion’s director and officer insurance coverage in respect of that matter (together, the negotiated thresholds). The number of Novelion common shares for which the Merger Agreement Warrants may be exercised, if any, will vary based on the extent to which the settlements of the matters described above exceed the negotiated thresholds. The Merger Agreement Warrants will not be exercisable for any shares to the extent any excess in respect of such matters is equal to or less than $1.0 million in the aggregate.
Pursuant to the Merger Agreement, effective upon the closing of the Merger, the Novelion board of directors is composed of four individuals designated by Aegerion, four individuals designated by Novelion, one individual designated by Broadfin Capital, LLC (Broadfin) and one individual designated by Sarissa Capital Management LP (Sarissa). For a specified period of time following the Merger, Sarissa will also have the right to designate one additional member of the board of directors of Novelion.
The aggregate consideration delivered to the former holders of Aegerion common stock in connection with the Merger was approximately 6,060,288 Novelion common shares. Shareholders of Novelion immediately prior to the Merger, including the private placement pursuant to the Unit Subscription Agreement (described below), owned approximately 68% of the outstanding Novelion common shares upon completion of the Merger and stockholders of Aegerion as of immediately prior to the Merger owned approximately 32% of the outstanding Novelion common shares upon completion of the Merger.
Private Placement. Also on June 14, 2016, we entered into a unit subscription agreement (the Unit Subscription Agreement) with the investors party thereto (the Investors). Pursuant to the Unit Subscription Agreement, immediately prior to the Merger, the Investors acquired units, for $8.80 per unit, on a post-Consolidation (as defined below) basis, consisting of (i) 2,472,727 Novelion common shares, which includes up to 568,181 Novelion common shares issuable upon exercise of fully paid-up warrants, and (ii) warrants (the Unit Subscription Agreement Warrants) exercisable for up to an aggregate of 2,644,952 Novelion common shares at an exercise price of $0.05 per share. The Unit Subscription Agreement Warrants were issued on the same terms and conditions as the Merger Agreement Warrants and are referred to collectively with the Merger Agreement Warrants as the Warrants in this Annual Report. The aggregate consideration paid under the Unit Subscription Agreement was approximately $21.8 million, which we intend to continue to use to support future operations and business development initiatives.
Share Consolidation. On December 16, 2016, we completed a one-for-five (1:5) consolidation of all of our issued and outstanding common shares, without par value, for shareholders of record as of December 16, 2016 (the Consolidation), resulting in a reduction in the issued and outstanding common shares from approximately 92,653,562 to approximately 18,530,323 as of that date. Each shareholder’s percentage ownership in Novelion and proportional voting power remained unchanged after the Consolidation, except for minor changes resulting from the treatment of fractional shares. In connection with the Consolidation, the conversion rate of the Convertible Notes was automatically adjusted from 24.9083 common shares per $1,000 principal amount of such Convertible Notes to 4.9817 common shares per $1,000 principal amount of such Convertible Notes.
101
Aralez Investment and Distribution. On December 7, 2015, we entered into an Amended and Restated Share Subscription Agreement (the Amended and Restated Subscription Agreement) with Tribute Pharmaceuticals Canada Inc. (Tribute), POZEN Inc. (POZEN), Aralez Pharmaceuticals plc, (formally known as Aguono Limited) (Aralez Ireland), Aralez Pharmaceuticals Inc. (Aralez Canada), Deerfield Private Design Fund II, L.P., Deerfield International Master Fund, L.P., Deerfield Partners, L.P. (together Deerfield), Broadfin and JW Partners, LP, JW Opportunities Fund, LLC and J.W. Opportunities Master Fund, Ltd. (together the JW Parties) (the Company, Deerfield, Broadfin and the JW Parties are referred to herein collectively as the Co-Investors). The Amended and Restated Subscription Agreement amended and restated a share subscription agreement entered into on June 8, 2015 among the Company, Tribute, POZEN, Aralez Ireland, the Co-Investors and certain other investors. Pursuant to the Amended and Restated Subscription Agreement, immediately prior to and contingent upon the consummation of the merger of Tribute and POZEN (the Aralez Merger), Tribute agreed to sell to us and the other Co-Investors $75.0 million of the common shares of Tribute (the Tribute Shares) in a private placement at a purchase price per share equal to: (a) the lesser of (i) $7.20, and (ii) a five percent discount off of the five-day volume weighted average price per share of POZEN common stock calculated over the five trading days immediately preceding the date of closing of the Aralez Merger, not to be less than $6.25 per share; multiplied by (b) the Aralez Merger exchange ratio of 0.1455. Upon consummation of the Aralez Merger on February 5, 2016, the Tribute Shares were exchanged for common shares of Aralez Canada (the Aralez Shares). We entered into the transaction contemplated by the Amended and Restated Subscription Agreement for the purpose of returning capital to our shareholders pursuant to a special election distribution, payable, at the election of each shareholder of the Company, in either Aralez Shares (approximately 0.13629 of an Aralez Share for each common share of the Company) or cash, subject to pro-ration (the Aralez Distribution), up to a maximum of $15.0 million funded pursuant to the terms of the Backstop Agreement (as described below).
In connection with the Aralez Distribution, on June 8, 2015, we entered into a share purchase agreement (as amended, the Backstop Agreement) with Broadfin and the JW Parties, pursuant to which Broadfin and the JW Parties agreed to purchase up to $15.0 million of the Aralez Shares from us at $6.25 per share. This arrangement provided our shareholders with the opportunity to elect to receive, in lieu of Aralez Shares, up to an aggregate of $15.0 million in cash, subject to proration among the shareholders. As a result, on April 5, 2016 (the Distribution Date), we distributed 4,799,619 Aralez Shares, with a fair value of $19.3 million, and $15.0 million of cash.
Upon consummation of the Aralez Merger on February 5, 2016, we purchased 7,200,000 Aralez Shares (representing 10.1% of the issued and outstanding Aralez Shares), for an aggregate price of $45.0 million. We held the Aralez Shares from February 5, 2016 to the Distribution Date and the Aralez Shares were marked-to-market. As a result, we recognized a $10.7 million loss during the fiscal year ended December 31, 2016, to reflect the change in value from the acquisition date to the Distribution Date.
Terminated Merger Transactions. On June 8, 2015, QLT entered into an agreement and plan of merger (as amended and restated on each of July 16, 2015 and August 26, 2015) (the InSite Merger Agreement) with InSite Vision Incorporated, a Delaware corporation (InSite). On September 15, 2015, the InSite Merger Agreement was terminated by InSite and InSite paid QLT a termination fee of $2.7 million. Refer to Note 3 - Terminated Merger Transactions in the Notes to the Consolidated Financial Statements for further details.
On June 25, 2014, QLT entered into an agreement and plan of merger (the Auxilium Merger Agreement) with Auxilium Pharmaceuticals, Inc., a Delaware corporation (Auxilium). On October 8, 2014, the Auxilium Merger Agreement was terminated by Auxilium and Auxilium paid QLT a termination fee of $28.4 million. Refer to Note 3 - Terminated Merger Transactions in the Notes to the Consolidated Financial Statements for further details.
Acquisition
On November 29, 2016, we completed the Merger. Commencing from the acquisition date, our Consolidated Financial Statements reflect the assets, liabilities, operating results and cash flows of Aegerion, and, in accordance with our domestic and international reporting periods, our Consolidated Financial Statements for the year ended December 31, 2016 reflect legacy Aegerion operations from November 29, 2016 to December 31, 2016. For additional information related to this transaction, see Note 5 - Acquisition to our Consolidated Financial Statements included in this Annual Report.
Financial Highlights
• | Total revenue was $13.6 million for 2016, representing revenue from selling lomitapide and metreleptin through our indirect wholly-owned subsidiary, Aegerion, after the acquisition date in 2016. |
• | Costs of sale were $6.0 million for 2016 representing costs of selling lomitapide and metreleptin through our indirect wholly-owned subsidiary, Aegerion, after the acquisition date in 2016. |
102
• | Selling, general and administrative expenses increased from $16.2 million in 2015 to $29.5 million in 2016, a 82.0% increase. This increase was primarily due to our recognition, starting on November 29, 2016, of 100% of Aegerion’s financial performance due to our acquisition of Aegerion on November 29, 2016 and our recognition of $8.0 million in total in relation to the advisory fees we paid to Greenhill for the completion of QLT’s $45 million investment in Aralez and the completion of the Merger. |
• | Research and development expenses increased from $9.8 million in 2015 to $14.8 million in 2016, a 51.0% increase. This increase was primarily driven by our recognition, starting on November 29, 2016, of 100% of Aegerion’s financial performance due to our acquisition of Aegerion on November 29, 2016. |
• | We used $34.4 million of net cash flows from operations for 2016, which were primarily due to our recognition, starting from November 29, 2016, of 100% of Aegerion’s financial performance including cash activities due to our acquisition of Aegerion on November 29, 2016. Cash and cash equivalents totaled approximately $108.9 million as of December 31, 2016. |
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. (GAAP). The preparation of these Consolidated Financial Statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results and experiences may differ materially from these estimates.
While our significant accounting policies are more fully described in Note 2 - Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements appearing in the “Consolidated Financial Statements and Supplementary Data” section of this Annual Report, we believe that the accounting policy related to Purchase Price Allocation for Business Combinations is the most critical to aid you in fully understanding and evaluating our reported financial results, and affecting the more significant judgments and estimates that we use in the preparation of our Consolidated Financial Statements.
Business Combinations
Acquired businesses are accounted for using the acquisition method of accounting, which requires that the purchase price be allocated to the net assets acquired at their respective fair values. Any excess of the purchase price over the estimated fair values of the net assets acquired is recorded as goodwill. We report provisional amounts when measurements are incomplete as of the end of the reporting period. We complete our purchase price allocation within a measurement period and which does not extend beyond one year after the acquisition date.
The present-value models used to estimate the fair values of acquired inventory and intangible assets incorporate significant assumptions, including, but not limited to: assumptions regarding the probability of obtaining marketing approval; estimated selling price, estimates of the timing and amount of future cash flows from potential product sales and related expenses; and the appropriate discount rate selected to measure the risks inherent in the future cash flows, the assessment of the asset’s life cycle and the competitive trends impacting the assets, including consideration of any technical, legal, regulatory or economic barrier to entry as well as expected changes in standards of practice for indications addressed by the asset and tax rates.
Recently Issued and Recently Adopted Accounting Standards
See Note 2 - Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements for a discussion of recently adopted and new accounting pronouncements.
Results of Operations
Comparison of the Years Ended December 31, 2016 and 2015
The following table summarizes the results of our operations for each of the years ended December 31, 2016 and 2015, together with the changes in those items in dollars and as a percentage:
103
Years Ended December 31, | |||||||||||||||
(in thousands except percentage and per share information) | 2016 (1) | 2015 | Change | % | |||||||||||
Net product sales | $ | 13,574 | $ | — | $ | 13,574 | 100.0 | % | |||||||
Costs of product sales | 5,971 | — | 5,971 | 100.0 | % | ||||||||||
Operating expenses: | |||||||||||||||
Selling, general and administrative expenses | 29,525 | 16,222 | 13,303 | 82.0 | % | ||||||||||
Research and development expenses | 14,784 | 9,790 | 4,994 | 51.0 | % | ||||||||||
Termination fee | — | (2,667 | ) | 2,667 | (100.0 | )% | |||||||||
Total operating expenses | 44,309 | 23,345 | 20,964 | 89.8 | % | ||||||||||
Loss from operations | (36,706 | ) | (23,345 | ) | (13,361 | ) | 57.2 | % | |||||||
Interest (expense) income, net | (2,960 | ) | 277 | (3,237 | ) | (1,168.6 | )% | ||||||||
Fair value loss on investment | (10,740 | ) | — | (10,740 | ) | (100.0 | )% | ||||||||
Other (expense) income, net | (1,999 | ) | 81 | (2,080 | ) | (2,567.9 | )% | ||||||||
Loss before provision for income taxes | (52,405 | ) | (22,987 | ) | (29,418 | ) | 128.0 | % | |||||||
Provision for income tax expense | (465 | ) | (22 | ) | (443 | ) | 2,013.6 | % | |||||||
Net loss | $ | (52,870 | ) | $ | (23,009 | ) | $ | (29,861 | ) | 129.8 | % | ||||
Basic and diluted net loss per common share | $ | (4.69 | ) | $ | (2.20 | ) | $ | (2.49 | ) | 113.2 | % | ||||
(1) On November 29, 2016, we completed the Merger. Our financial position at the end of 2016 included Aegerion’s financial position. Our results of operations for 2016 consisted of Aegerion’s financial performance from November 29, 2016 to December 31, 2016.
For 2016 compared to 2015, the increase in net product sales, costs of product sales, selling, general and administrative (SG&A) expenses and research and development (R&D) expenses, other income, and interest expense was primarily due to our recognition, starting on November 29, 2016, of 100% of Aegerion’s financial performance due to our acquisition of Aegerion on November 29, 2016.
We expect that revenues, cost of product sales, SG&A and R&D expenses and interest expense will increase significantly in 2017 and beyond compared to 2016, relative to prior periods, as we will incorporate a full year of financial performance of Aegerion in 2017 and beyond while 2016 only includes Aegerion's financial performance subsequent to the acquisition date of November 29, 2016.
Revenue
We reported revenue for the first year in 2016, which represented net product sales of MYALEPT and JUXTAPID from November 29, 2016 to December 31, 2016 by our newly acquired wholly-owned subsidiary Aegerion. In 2015, we did not have any commercial product and did not generate any revenue.
Metreleptin
We generated revenues from net product sales of MYALEPT of approximately $5.0 million for the year ended December 31, 2016. Sales generated were comprised primarily of sales to patients within the U.S. Prospectively, outside of sales generated within the U.S., we expect that prescriptions for named patient sales in Brazil will be our largest source of revenues, on a country-by-country basis. The future net product sales of metreleptin are highly dependent on our ability to continue to find GL patients and to build market acceptance for MYALEPT in the U.S. In addition, we will continue to pay significant Medicaid rebates for MYALEPT, which will have a negative impact in future quarters. The degree of such impact on our overall financial performance will depend on the percentage of MYALEPT patients that have Medicaid as their primary insurance coverage and the quantity of units ordered per patient.
Lomitapide
We generated revenues from net product sales of JUXTAPID of $8.6 million in the year ended December 31, 2016. This amount is comprised primarily of sales to patients within the U.S. Prospectively, outside of sales generated within the U.S., we expect that prescriptions for named patient sales in Brazil will be our largest source of revenues, on a country-by-country basis. However, we expect that net product sales from named patient sales in Brazil will fluctuate quarter-over-quarter given that orders for named patient sales are typically for multiple months of therapy which can lead to some fluctuation in sales depending on the ordering pattern. Future revenues may also be negatively affected by the availability of PCSK9 inhibitor products.
104
We expect net product sales from named patient sales of lomitapide and metreleptin in Brazil to fluctuate quarter-over-quarter significantly more than sales in the U.S., as a result of the types of orders and unpredictable ordering patterns, government actions, including the ongoing investigations in Brazil, media coverage, and economic pressure.
Costs of Product Sales
We reported costs of product sales in 2016 to recognize the costs of selling MYALEPT and JUXTAPID by Aegerion from November 29, 2016 to December 31, 2016. In 2015, we did not have any net product sales or revenues, and therefore we did not recognize costs of product sales.
We recorded costs of product sales of $6.0 million for the year ended December 31, 2016. Costs of sales includes the cost of inventory sold, amortization of acquired product rights, which result from the acquisition of JUXTAPID and MYALEPT as of the acquisition date, manufacturing and supply chain costs, product shipping and handling costs, as well as estimated royalties payable related to the sale of MYALEPT and JUXTAPID. We expect cost of product sales for both products to fluctuate consistently with expected changes in net product sales.
Selling, General and Administrative Expenses
During the year ended December 31, 2016, SG&A expenses from continuing operations increased by $13.3 million to $29.5 million, compared to $16.2 million for the same period in 2015. A portion of the increase was attributed to our recognition of $8.0 million in total in relation to the advisory fees we paid to Greenhill for the completion of Novelion's $45.0 million investment in Aralez and the completion of the Merger. The remaining increase was mainly due to our recognition, starting on November 29, 2016, of 100% of the SG&A expenses of our newly acquired wholly-owned subsidiary, Aegerion, which were primarily comprised of employee-related expenses, including stock-based compensation, and litigation expense.
Research and Development Expenses
During the year ended December 31, 2016, R&D expenditures from continuing operations were $14.8 million compared to $9.8 million for the same period in 2015. The $5.0 million increase was primarily due to our recognition, starting on November 29, 2016, of 100% of the R&D expenses of MYALEPT (metreleptin) and JUXTAPID (lomitapide) due to our acquisition of Aegerion on November 29, 2016. These expenses were primarily comprised of employee related expenses, including stock-based compensation, and consulting costs for the period.
Termination Fees
We did not incur termination fees during the year ended December 31, 2016.
In 2015, we recognized a $2.7 million termination fees in connection with the termination of the Insite Merger Agreement on September 15, 2015.
Interest Expense
We recognized $3.0 million interest expense in 2016, which represents the amortization of the debt discount and interest incurred in December 2016 in relation to the issuance by Aegerion in August 2014 of $325.0 million in aggregate principal of 2.00% convertible senior notes due August 15, 2019 (the Convertible Notes) for which interest is payable semi-annually in arrears on February 15 and August 15 of each year. Before 2015, we financed our operations through equity and existing resources and did not have any debt.
Fair Value Loss on Investment
We recognized $10.7 million fair value loss on investment in 2016, which represents realized loss as a result of the mark-to-market of the Aralez shares held by QLT from February 5, 2016 to April 5, 2016 (the Distribution Date). Refer to Note 4 - Strategic Transactions in the Notes to the Consolidated Financial Statements for further details.
Income Taxes
During the year ended December 31, 2016, the provision for income taxes was $0.5 million, an increase of $0.4 million over the same period in 2015. The provision for income taxes consisted of a current tax expense, which relates primarily to our profitable operations in our foreign tax jurisdictions offset by a current tax benefit for the reversal of interest related to our liability on uncertain tax positions.
During the year ended December 31, 2015, the provision for income taxes from continuing operations was insignificant and primarily relates to the accrual of interest on uncertain tax provisions.
105
For the years ended December 31, 2016 and 2015, we considered it more likely than not that some portion or all of the recorded deferred tax assets will not be realized in a future period based on all available evidence. As a result of our evaluations, we concluded that there was insufficient positive evidence to overcome the more objective negative evidence related to our cumulative losses and other factors. Accordingly, for the year ended December 31, 2015, we maintained a full valuation against our domestic and foreign deferred tax assets. For the year ended December 31, 2016, we maintained a full valuation allowance against our U.S., Canadian and Swiss deferred tax assets. The Company did not maintain a valuation allowance against its remaining foreign subsidiaries as these companies are generally profitable under the Company’s transfer pricing model and those earnings are further considered permanently invested in the respective foreign jurisdictions. In future periods, we will continue to evaluate whether there is sufficient positive evidence to overcome the more objective negative evidence in determining whether we will continue to maintain a full valuation allowance.
The Company has not provided for U.S. income taxes on the undistributed earnings of its foreign subsidiaries, as it currently plans to permanently reinvest these amounts and has the intent and ability to do so. As of December 31, 2016, the Company has approximately $1.5 million of undistributed foreign earnings.
As of December 31, 2016, prior to the deferred income tax asset offset described below, our provision for uncertain tax benefits (UTP Provision) was $7.7 million, compared to $7.3 million as of December 31, 2015. Given that we have sufficient deferred tax assets to shelter these potential liabilities, approximately $7.3 million of the UTP Provision has been offset on the Consolidated Balance Sheet as of December 31, 2016, compared to $6.9 million as of December 31, 2015 in accordance with ASU No. 2013-11 - Income Taxes (Topic 740): Presentation of Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists, which was adopted prospectively effective January 1, 2014. The remaining net UTP Provision of $0.4 million at December 31, 2016 and $0.3 million at December 31, 2015 is reflected on the Consolidated Balance Sheet.
Comparison of the Years Ended December 31, 2015 and 2014
The following table summarizes the results of our operations for each of the years ended December 31, 2015 and 2014, together with the changes in those items in dollars and as a percentage:
Years Ended December 31, | |||||||||||||||
(in thousands except percentage and per share information) | 2015 | 2014 | Change | % | |||||||||||
Net product sales | $ | — | $ | — | $ | — | — | % | |||||||
Cost of product sales | — | — | — | — | % | ||||||||||
Operating expenses: | |||||||||||||||
Selling, general and administrative expenses | 15,646 | 16,791 | (1,145 | ) | (6.8 | )% | |||||||||
Research and development expenses | 9,790 | 13,803 | (4,013 | ) | (29.1 | )% | |||||||||
Depreciation | 576 | 891 | (315 | ) | (35.4 | )% | |||||||||
Restructuring charges | — | 744 | (744 | ) | (100.0 | )% | |||||||||
Termination fee | (2,667 | ) | (28,400 | ) | 25,733 | (90.6 | )% | ||||||||
Total operating expenses | 23,345 | 3,829 | 19,516 | 509.7 | % | ||||||||||
Loss from operations | (23,345 | ) | (3,829 | ) | (19,516 | ) | 509.7 | % | |||||||
Interest income, net | 277 | 113 | 164 | 145.1 | % | ||||||||||
Other income (expense), net | 81 | (481 | ) | 562 | (116.8 | )% | |||||||||
Loss from continuing operations before income taxes | (22,987 | ) | (4,197 | ) | (18,790 | ) | 447.7 | % | |||||||
Provision for income tax (expense) recovery | (22 | ) | 192 | (214 | ) | (111.5 | )% | ||||||||
Net loss from continuing operations | (23,009 | ) | (4,005 | ) | (19,004 | ) | 474.5 | % | |||||||
Loss from discontinued operations, net of income taxes | — | (66 | ) | 66 | (100.0 | )% | |||||||||
Net loss | $ | (23,009 | ) | $ | (4,071 | ) | $ | (18,938 | ) | 465.2 | % | ||||
Basic and diluted net loss per common share | $ | (2.20 | ) | $ | (0.40 | ) | $ | (1.80 | ) | 450.0 | % | ||||
Research and Development Expenses
During the year ended December 31, 2015, research and development (R&D) expenditures from continuing operations were $9.8 million compared to $13.8 million for the same period in 2014. The $4.0 million (29%) decrease was primarily due to higher costs
106
incurred in 2014 related to certain toxicity studies, preparatory activities for the zuretinol pivotal trial, our zuretinol study in impaired dark adaptation (IDA) subjects, and trailing costs from the zuretinol Phase 1b retreatment study in LCA and RP subjects (the Retreatment Study), which was substantially completed in 2013. The 2015 decline in R&D expenditures was also attributable to lower salary and overhead costs resulting from R&D headcount attrition, the foreign exchange impact of the weakening Canadian dollar, and downsizing of our lease space as described under the Contractual Obligations section below.
During the year ended December 31, 2014, R&D expenditures from continuing operations were $13.8 million compared to $18.5 million for the same period in 2013. The $4.7 million (25%) decrease was primarily due to higher costs incurred in 2013 related to our Retreatment Study and savings realized in 2014 related to the continuing impact of our 2012 workforce reduction and other restructuring activities. These R&D expenditure decreases were partially offset by higher costs incurred in 2014 related to our preparatory activities for the zuretinol pivotal trial, IDA study, and higher stock-based compensation expense associated with stock option grants.
Trailing R&D expenditures related to our former Visudyne® business and former punctal plug drug delivery technology (the PPDS Technology) are presented as discontinued operations on the Consolidated Statements of Operations. For additional discussion on these expenditures, refer to the Income from Discontinued Operations, Net of Income Taxes section below.
Total cumulative costs incurred through December 31, 2015 related to zuretinol were $118.0 million.
For a more detailed description of our zuretinol development program, refer to the “Business - Our Products in Development” section of this Annual Report.
Selling, General and Administrative Expenses
During the year ended December 31, 2015, selling, general and administration (SG&A) expenses were $15.7 million compared to $16.8 million for the same period in 2014. The $1.1 million (7%) decrease was primarily due to a decrease in transaction and consulting fees related to our exploration and pursuit of certain strategic alternatives. During the year ended December 31, 2015, we incurred $9.4 million of transaction and consulting fees related to our pursuit of the InSite Merger and strategic transactions described above, compared to $10.2 million of similar fees incurred in 2014 related to our pursuit of the Auxilium Merger. In addition, during the year ended December 31, 2015, we incurred $0.2 million of general consulting fees related to our consideration of future strategic options, compared to $0.6 million of similar fees incurred in 2014. Furthermore, 2015 SG&A expense was positively impacted by a decrease in directors fees related to our October 2014 appointment of Dr. Geoffrey Cox as Interim Chief Executive Officer and a decrease in overall operating costs related to the downsizing of our lease space as well as the foreign exchange impact of the weakening Canadian dollar. These cost savings were substantially offset by higher stock-based compensation expense associated with the June 7, 2015 accelerated vesting of all unvested stock options as described above and a decrease in the amount of overhead expenses allocated to our R&D programs due to R&D headcount attrition.
Depreciation
During the years ended December 31, 2015 and 2014, depreciation expense was $0.6 million, and $0.9 million, respectively. The progressive decline in depreciation expense is primarily due to assets reaching the end of their useful lives.
Restructuring charges
During the year ended December 31, 2012, we restructured our operations to focus our resources on our clinical development programs related to our synthetic retinoid, zuretinol, for the treatment of certain inherited retinal diseases. The cumulative cost of the restructuring, which was substantially complete in 2014, was $19.6 million. We did not incur restructuring expenses during the year ended December 31, 2015.
During the year ended December 31, 2014, we recorded a charge of $0.7 million related to certain severance and termination benefits paid to QLT’s former Senior Vice President, Business Development and Commercial Operations, whose employment terminated effective May 31, 2014.
Termination Fees
In connection with the termination of the InSite Merger Agreement on September 15, 2015, InSite paid us a $2.7 million termination fee.
In connection with the termination of the Auxilium Merger Agreement on October 9, 2014, Auxilium paid us a $28.4 million termination fee.
107
Net Foreign Exchange Losses
For the years ended December 31, 2015 and 2014, net foreign exchange gains (losses) represent the impact of foreign exchange fluctuations on our monetary assets and liabilities that are denominated in currencies other than the U.S. dollar (principally the Canadian dollar). See the Liquidity and Capital Resources: Interest and Foreign Exchange Rates section below.
Interest Income
During the years ended December 31, 2015 and 2014, interest income was $0.3 million and $0.1 million, respectively. Interest income in 2015 includes $0.1 million of interest earned on a secured note granted to InSite in connection with the proposed InSite Merger described under Note 4 - Strategic Transactions in the Notes to the Consolidated Financial Statements.
Fair Value Change in Contingent Consideration
Assets recognized in connection with contingent consideration owed to Novelion related to previous divestitures are estimated, measured and recorded at the present value of future expected payments. Fair value changes primarily arise from the following factors: accretion; cash collected during the period, which decreases the balance of future expected cash flows owed to us; and changes in the projected amount and timing of the expected future cash flows.
No fair value changes were recorded in 2015 given that the remaining contingent consideration owing from our previous sale of our subsidiary, QLT USA, Inc., and Eligard® to TOLMAR Holding, Inc. (Eligard Contingent Consideration) was collected in full by the end of 2014.
During the year ended December 31, 2014, we recorded a net fair value loss of $0.5 million, which consisted of a $1.5 million fair value gain related to our Eligard Contingent Consideration offset by a $2.0 million fair value decrease recorded for the Laser Earn-Out Payment to account for the increased uncertainty, collection risk associated with the passage of time and potential collection costs.
Income from Discontinued Operations, Net of Income Taxes
In accordance with the accounting standard for discontinued operations, the results of operations related to our former PPDS Technology and Visudyne business have been excluded from continuing operations and reported as discontinued operations for all periods presented.
During the year ended December 31, 2014, we incurred a loss of $0.1 million from discontinued operations, which primarily consisted of certain residual costs related to the former sale of our Visudyne business.
Income Taxes
During the year ended December 31, 2015, the provision for income taxes from continuing operations was insignificant and primarily relates to the accrual of interest on uncertain tax provisions.
During the year ended December 31, 2014, the $0.2 million income tax recovery from continuing operations primarily relates to a $0.4 million reversal of interest accrued on uncertain tax positions which decreased in 2014 due to the expiration of the statute of limitations. This recovery was partially offset by the tax impact of gains from fair value changes in our previous Eligard related contingent consideration asset balance and interest accrued on remaining uncertain tax positions.
The 2015 and 2014 provisions for income taxes also reflect that we had insufficient evidence to support the current or future realization of the tax benefits associated with our development expenditures.
As of December 31, 2015, prior to the deferred income tax asset offset described below, our provision for uncertain tax positions (UTP Provision) was $7.3 million, compared to $5.6 million as of December 31, 2014 - $5.6 million. Approximately $5.5 million of this UTP Provision as of December 31, 2015 relates to tax filing positions taken on certain transaction costs incurred in 2015 and 2014, $1.4 million relates to uncertain tax positions that are currently under audit examination and the remaining balance relates to other tax positions on uncertain tax matters from prior years. Given that we have sufficient tax deferred tax assets to shelter these potential liabilities, approximately $6.9 million of the UTP Provision has been offset on the Consolidated Balance Sheet as of December 31, 2016, compared to $5.2 million as of December 31, 2014 in accordance with ASU No. 2013-11- Income Taxes (Topic 740): Presentation of Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists, which was adopted prospectively effective January 1, 2014. The remaining net UTP Provision of $0.3 million as of December 31, 2015, compared to $0.4 million as of December 31, 2014 is reflected on Consolidated Balance Sheet and is expected to decrease in 2016 upon the expiration of the statute of limitations applicable to certain tax positions taken on uncertain tax matters in prior years.
108
As of December 31, 2015 and 2014, our respective net deferred tax assets were zero given that we had a full valuation allowance applied against these specific tax assets. The valuation allowance is reviewed periodically and if management’s assessment of the “more likely than not” criterion for accounting purposes changes, the valuation allowance is adjusted accordingly. See Note 13 - Income Taxes in the Notes to the Consolidated Financial Statements.
Liquidity and Capital Resources
General
We have historically financed our operating and capital expenditures through existing cash resources. As a result of the Merger, we now have, through Aegerion, two commercial products, metreleptin and lomitapide, which generate revenues. In connection with the Merger, we entered into the Unit Subscription Agreement with the Investors. The aggregate consideration received pursuant to the Unit Subscription Agreement was approximately $21.8 million, which we intend to use to support future operations and business development initiatives. In August 2014, Aegerion issued $325.0 million in aggregate principal amount of 2.00% convertible senior notes due August 15, 2019 (the Convertible Notes), for which interest is payable semi-annually in arrears on February 15 and August 15 of each year. Aegerion’s ability to refinance this indebtedness will depend on the capital markets and our financial condition on a consolidated basis at such time, if any that it elects to pursue refinancing. In addition, as further described in the “Legal Proceeding” section above, Aegerion reached, in May 2016, preliminary agreements in principle with the DOJ and the SEC that provide for, among other things, a consolidated monetary package that covers payments due to both the DOJ and the SEC by Aegerion totaling approximately $40 million in the aggregate, to be payable over three years, changed from the originally proposed five-year payment schedule contemplated when the preliminary agreement in principle was reached in May 2016.
During the year ended December 31, 2016, we generated $13.6 million of revenues from net product sales. As of December 31, 2016, we had $108.9 million in cash and cash equivalents on hand.
Going forward, we expect to fund our current and planned operating requirements principally through our cash flows from operations, as well as our existing cash resources and proceeds from Aegerion’s potential refinancing of the Convertible Notes, and other potential financing methods, including utilizing equity. We believe that our existing funds, when combined with cash generated from operations, are sufficient to satisfy our operating needs and our working capital, milestone payments, capital expenditure and debt service requirements for at least one year from the date of this Annual Report. We may, from time to time, also seek additional funding through strategic alliances and additional equity and debt financings or from other sources, should we identify a significant new opportunity. For information related to certain risks that could negatively impact our financial position or future results of operations, see the “Risk Factors” and “Quantitative and Qualitative Disclosures About Market Risk” sections of this Annual Report.
Sources and Uses of Cash
The following table sets forth the major sources and uses of cash for the years ended December 31, 2016, 2015 and 2014:
Years Ended December 31, | |||||||||||
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Net cash (used in)/provided by: | |||||||||||
Operating activities | $ | (34,356 | ) | $ | (19,359 | ) | $ | 455 | |||
Investing activities | 25,327 | 34 | 36,672 | ||||||||
Financing activities | (23,519 | ) | 5,508 | 509 | |||||||
Effect of exchange rates on cash | (349 | ) | (267 | ) | (249 | ) | |||||
Net (decrease) increase in cash and cash equivalents | $ | (32,897 | ) | $ | (14,084 | ) | $ | 37,387 | |||
Changes in net cash provided by(used in) operating activities, investing activities and financing activities in 2016 compared to 2015 and 2014 were mainly attributable to our recognition, starting on November 29, 2016, of 100% of the cash flow activities of our newly acquired indirect, wholly-owned subsidiary Aegerion, including, among others, cash generated from the net product sales of MYALEPT and JUXTAPID, cash used to maintain inventory of those products, and cash used to support the SG&A and R&D activities.
We expect operating and financing cash flow activities to increase significantly in 2017 and beyond compared to 2016, relative to prior periods, as we will incorporate a full year of cash flow activities of Aegerion in 2017 and beyond, while in 2016 only cash activities after the acquisition date of November 29, 2016 was included.
109
Cash (Used in) Provided by Operating Activities
During the year ended December 31, 2016, cash used by operating activities was $34.4 million compared to $19.4 million of cash used in operating activities in the same period in 2015. The $15.0 million decrease in operating cash flows was primarily attributable to the following:
• | A significant increase in the net loss recognized by the Company year-over-year. |
• | A negative operating cash flow variance of $8.0 million related to advisory fees paid to Greenhill in connection with the completion of Novelion's $45.0 million investment in Aralez and the completion of Novelion's acquisition of Aegerion. |
• | A positive operating cash flow variance of $10.7 million related to a loss recorded based on the mark-to-market adjustment on the Aralez investment to reflect changes in value from the acquisition date, February 5, 2016, through the distribution date, April 5, 2016. |
• | Negative operating cash flows were noted as a result of the Company's acquisition of Aegerion, which included cash flows from the acquisition date of November 29, 2016 through December 31, 2016. Significant items noted related to payments made for deal-related consulting fees and litigation during the period. These negative operating cash outflows were offset by period amortization of the JUXTAPID and MYALEPT intangible assets recognized in conjunction with the Merger. |
During the year ended December 31, 2015, cash used by operating activities was $19.4 million compared to $0.5 million of cash provided by operating activities in the same period in 2014. The $19.9 million decrease in operating cash flows was primarily attributable to the following:
• | A $25.7 million negative cash flow variance related to a $28.4 million termination fee received in 2014 related to the proposed Auxilium Merger as compared to the $2.7 million termination received in 2015 related to the proposed InSite Merger (refer to Note 3 - Terminated Merger Transactions for more information); |
• | A negative operating cash flow variance of $1.5 million related to the portion of the Eligard Contingent Consideration that was received in 2014 and recognized as part of cash used in operations. |
• | A positive cash flow variance of $5.0 million resulting from: (i) lower salary costs related to changes in the R&D and SG&A head count, lower lease costs and the foreign exchange impact of the weakening Canadian dollar, and (ii) higher cash outlays in the prior year related to the following 2014 research and development activities: toxicity studies, preparatory activities for the zuretinol pivotal trial and trailing costs from our Retreatment Study. These positive cash flow variances were partially offset by increased cash outlays in 2015 related to: (i) the transfer and outsourcing of our analytical and bio-analytical testing functions to certain contract research organizations and (ii) spending related to the commencement of our natural history study. |
• | A positive cash flow variance of $1.5 million due to lower consulting and advisory fees paid during the year ended December 31, 2015 as compared to the same period in 2014. During the year ended December 31, 2015, we paid $8.7 million of transaction and consulting fees related to our exploration and pursuit of the InSite Merger and the Aralez Distribution. In comparison, during the year ended December 31, 2014, we paid $10.2 million of transaction and consulting fees related to our pursuit of the Auxilium Merger. |
• | A $0.9 million positive cash flow variance associated with restructuring charges paid out in 2014 for accrued severance and termination benefits. |
During the year ended December 31, 2014, cash provided by operating activities was $0.5 million compared to $25.8 million of cash used in the same period in 2013. The $26.3 million increase in operating cash flows was primarily attributable to the following factors:
• | A positive cash flow variance related to the $28.4 million termination fee received in connection with the proposed Auxilium Merger. |
• | A positive cash flow variance of $2.9 million from lower spending on restructuring costs; |
• | A positive operating cash flow variance from $8.8 million of lower operational spending associated with our 2012 restructuring initiatives; |
• | A negative cash flow variance of $10.2 million associated with consulting and transaction fees paid in 2014 in connection with the proposed Auxilium Merger; |
• | A negative operating cash flow variance related to the fair value change in contingent consideration of $2.6 million; |
• | A negative operating cash flow variance of $0.6 million related to the reversal of certain liabilities recorded for uncertain tax positions; and |
• | A negative operating cash flow variance from other income items of $0.4 million. |
110
Cash Provided by Investing Activities
During the year ended December 31, 2016, cash flows provided by investing activities was $25.3 million compared to $0.03 million in 2015, a $25.3 million increase, which was mainly attributable to the cash the Company acquired from Aegerion as a result of the Merger.
During the year ended December 31, 2015, cash flows provided by investing activities was insignificant.
During the year ended December 31, 2014, cash flows provided by investing activities primarily consisted of $36.6 million of Eligard Contingent Consideration received and $0.1 million of net proceeds related to the sale of certain property, plant and equipment.
Cash (Used in) Provided By Financing Activities
During the year ended December 31, 2016, $23.5 million cash used in financing activities was mainly attributable to the $45.0 million cash outflows used to purchase 7,200,000 Aralez Shares (representing 10.1% of the issued and outstanding Aralez Shares) at a price of $6.25 per share in connection with the Aralez Merger on February 5, 2016, offset by $21.5 million of proceeds received in connection with the issuance of the Company’s common shares in a private placement to the Investors in connection with the Merger.
During the year ended December 31, 2015, cash flows provided by financing activities consisted of $5.5 million of proceeds received in connection with the issuance of common shares for stock options exercised.
During the year ended December 31, 2014, cash flows provided by financing activities consisted of $0.5 million of proceeds received in connection with the issuance of common shares for stock options exercised.
Interest and Foreign Exchange Rates
We are exposed to market risk related to changes in interest and foreign currency exchange rates, each of which could adversely affect the value of our current assets and liabilities. At December 31, 2016, we had $108.9 million in cash and cash equivalents and our cash equivalents had an average remaining maturity of approximately 27.6 days. If market interest rates were to increase immediately and uniformly by one hundred basis points from levels at December 31, 2016, the fair value of the cash equivalents would decline by an immaterial amount due to the short remaining maturity period.
To the extent that Novelion holds a portion of its monetary assets and liabilities in a currency other than the functional currency of the entity, we are subject to revaluation gains and losses. These revaluation gains and losses are included in operations for the period.
At December 31, 2016 and 2015, we had no outstanding forward foreign currency contracts.
Contractual Obligations
In the normal course of business, we enter into purchase commitments related to daily operations. We have entered into certain operating lease agreements with the following minimum annual commitment:
Payments due by period | |||||||||||||||||||
Contractual Obligations (1) (in thousands) | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||
Long-term debt (including interest)(2) | $ | 342,060 | $ | 6,500 | $ | 335,560 | $ | — | $ | — | |||||||||
Operating Leases | 6,508 | 3,083 | 3,284 | 141 | — | ||||||||||||||
Total contractual obligations (3) | $ | 348,568 | $ | 9,583 | $ | 338,844 | $ | 141 | $ | — | |||||||||
(1) The following contractual obligations have been excluded from the table above due to the reasons stated below:
i. | Uncertain Tax Positions |
As disclosed under Note 13 - Income Taxes in the Notes to the Consolidated Financial Statements for the year ended December 31, 2016, we have identified certain potential long-term liabilities associated with uncertain tax positions. Given that we are unable to reasonably or reliably estimate the timing of these future payments, if any, due to uncertainties about the timing and/or future outcomes of tax audits that may arise, these uncertain tax liabilities have been excluded from the table above.
111
ii. | Purchase Orders |
As of December 31, 2016, we have certain open purchase orders related to potential and/or expected future expenditures. The total $17.7 million value of these purchase orders is not currently reflected on our Consolidated Balance Sheets and has been excluded from the table above given that the amounts are not fixed contractual obligations and would only give rise to liabilities to the extent that goods and services are provided to Novelion. In addition, all of our material research contracts with third-parties have normal course termination and cancellation provisions. These purchase orders reflect estimated future expenditures based on existing arrangements and do not reflect any future modifications to, or terminations of, existing contracts or potential new contracts. Approximately $17.4 million of our open purchase orders consists of expected expenditures related to ongoing research contracts with third-party organizations and $0.3 million relates to expected general and administrative expenses that are in the normal course of business.
iii. | Contract Research Organization (CRO) Agreement |
We have engaged CROs to provide research, safety and project management services (the “Services”) in connection with the execution of our potential clinical trials and existing registries. The estimated amount of Services is excluded from the table above given that Services have not yet been performed as of the December 31, 2016 balance sheet date and they would only give rise to liabilities to the extent that Services are provided to us and pass through expenses are incurred. As of December 31, 2016, the Company had total potential commitments of approximately $42.1 million under these agreements. The amount reflected is based on the existing contracts and does not reflect any inflation, future modification to, or termination of, the existing contracts or anticipated or potential new contracts. The agreements with our selected CROs contain normal course termination and cancellation provisions. In the event of cancellation of these agreements, the Company would be obligated to pay for all direct fees, pass through costs, and services performed or incurred through the termination date. In addition, we would be required to reimburse the CROs for all future non-cancelable obligations to third parties, where such obligations were created in connection with services authorized by Novelion.
iv. | Milestone Obligations |
We have also committed to make potential future milestone payments to certain third parties as part of our licensing, development, and purchase agreements. Payments under these arrangements are generally contingent and payable upon achievement of certain developmental, regulatory or commercial milestones. During the year ended December 31, 2016, none of these payments have been triggered by the specified developmental, regulatory or commercial milestones. For more information refer to Note 16 - Contingencies, Commitments and Guarantees in the Notes to the Consolidated Financial Statements for the year ended December 31, 2016 and Item 1. Business of this Annual Report.
Under Aegerion's license agreement with UPenn, Aegerion will be required to make development milestone payments of up to an aggregate amount of $2.6 million if we decide to develop lomitapide for indications within the licensed field other than HoFH. All such development milestone payments for these other indications are payable only once, no matter how many licensed products for these other indications are developed. We have not initiated plans to develop lomitapide for indications within the licensed field other than HoFH.
As described in the Business Overview section above, under the license agreement between Retinagenix and the University of Washington (the UW Agreement), a specific development milestone was required to be achieved by December 31, 2016. However, the UW Agreement contains provisions for extensions of that date in certain circumstances. Based on the terms of the Retinagenix Agreement and the UW Agreement, and our significant development clinical spend on the zuretinol program, we believe that we are entitled to an extension of that milestone date until December 31, 2017, and that we may be entitled to certain additional extensions to December 31, 2019, along with a potential additional extension of up to 12 months should enrollment in a planned trial be delayed, provided that we continue to comply with the relevant provisions of the license agreements and expend certain minimum amounts on the development of zuretinol. However, it is possible that we may not be able to achieve the specified development milestone by December 31, 2019. As a result, we and Retinagenix have begun discussing a renegotiation of that milestone with the University of Washington. We are currently conducting a review of the zuretinol development program, the results of which will assist us in determining when we believe that the remaining development milestone can be expected to be achieved.
(2) Included in the long-term debt (including interest) line within the contractual obligations table is $325.0 million in convertible debt, which can potentially be settled in our common shares.
(3)This table does not include (i) any milestone payments which may become payable to third parties under license agreements as the timing and likelihood of such payments are not known; (ii) any royalty payments to third parties as the amounts, timing and likelihood of such payments are not known; and (iii) contracts that are entered into in the ordinary course of business which are not material in the aggregate in any period presented above.
In January 2015, Aegerion acquired metreleptin pursuant to the Asset Purchase Agreement with AstraZeneca. Metreleptin, a recombinant analog of human leptin, is currently marketed in the U.S. under the brand name MYALEPT. MYALEPT received marketing approval from the FDA in February 2014 as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with GL. Under the terms of the Asset Purchase Agreement, Aegerion paid AstraZeneca $325.0 million to acquire the global rights to develop, manufacture and commercialize metreleptin, subject to an existing distributor license with Shionogi covering Japan, South Korea and Taiwan. The distribution agreement with Shionogi was assigned to Aegerion as part of the transaction. Aegerion also assumed certain other assets and liabilities of AstraZeneca related to the metreleptin program. In connection with the acquisition, Aegerion assumed an agreement, as amended, with a contract manufacturer of MYALEPT. An amendment, which was disclosed to us after the closing of the MYALEPT acquisition, commits us to spend approximately 0.37 million Euros per week in contract manufacturing costs for a minimum of twelve weeks per year with a maximum of sixteen weeks per year. The amount does not reflect any inflation, future modification to, or termination of, the existing contract or anticipated or potential new contract.
In connection with the acquisition of MYALEPT in January 2015, Aegerion acquired a license agreement between Amgen Inc. (Amgen) and Amylin Pharmaceuticals, Inc., dated February 7, 2006 (the Amgen License) pursuant to which Aegerion obtained an exclusive worldwide license from Amgen to certain know-how and patents and patent applications covering the composition of matter and methods of use of metreleptin to develop, manufacture and commercialize a preparation containing metreleptin (the Amgen Licensed Products).
112
As part of the Amgen License, Aegerion also obtained an exclusive sublicense of Amgen’s exclusive rights to certain metreleptin-related patents and patent applications owned by the Rockefeller University and exclusively licensed to Amgen under a license agreement dated April 14, 1995, as amended (the Rockefeller License) and an exclusive sublicense of Amgen’s non-exclusive rights to certain metreleptin-related patents and patent applications owned by The Regents of the University of California and non-exclusively licensed to Amgen under a license agreement dated July 13, 2005 (the UCSF License). Amgen retains rights to conduct research, development, manufacturing and commercialization activities with respect to products other than the Amgen Licensed Products.
Aegerion may grant sublicenses under the licenses and sublicenses granted by Amgen, subject to certain limitations, including Amgen’s right of first offer for any out-license, partnership, co-development, commercialization, co-promotion or similar agreement related to metreleptin or the Amgen Licensed Products, which expires in February 2021. Under this license agreement, Amgen must notify Aegerion of any potential third-party partnership regarding any intellectual property rights controlled by Amgen in the neurology field and we will have a right of first negotiation for any license, partnership, co-development, commercialization, co-promotion or similar agreement, which expires in February 2021.
Aegerion is required to make royalty payments to Amgen, Rockefeller University and BMS on net sales of each Amgen Licensed Product on a country-by-country basis (i) at a royalty rate in the low double digits where the Amgen Licensed Product has patent protection or market exclusivity granted by a regulatory authority at the time of regulatory approval in the applicable country during the applicable royalty term, which runs on a country-by-country basis until the later of (a) the expiration of the last-to-expire valid claim covering an Amgen Licensed Product in the applicable country, (b) expiration of any market exclusivity granted by a regulatory authority, and (c) ten years from the date on which an Amgen Licensed Product is first sold to a third-party in a country after regulatory approval for the Amgen Licensed Product has been granted in such country (Amgen Royalty Term) or (ii) at a royalty rate in the mid-single digits to low double digits where the Amgen Licensed Product receives patent protection or market exclusivity following the time of regulatory approval in the applicable country, in either case subject to a variety of customary reductions.
Under the Amgen License, Aegerion is also required to directly meet certain payment obligations under the Rockefeller License and UCSF License. Aegerion is required to make royalty payments to Rockefeller University on net sales of each product with patent rights or know-how in the field of obesity genes, obesity gene products, and molecules that modulate or mediate their action and/or regulation on a country-by-country basis at a range of royalty rates in the low single digits depending on whether the product has an orphan product designation or not until the later to occur of expiration of (i) patent protection, (ii) any market exclusivity period granted in the applicable country, or (iii) any data exclusivity period in the applicable country (with certain limitations related to the number of units sold). Since acquiring this license agreement in January 2015, Aegerion has paid a one-time $5.0 million milestone payment to Rockefeller in February 2015, which was due twelve months following the receipt of marketing approval for MYALEPT in the U.S. Aegerion will also be required to pay to Rockefeller University a percentage in the low double digits of any upfront license fees or one-time fees it receives in consideration for a sublicense of the licensed rights. There are no material payment obligations outstanding under the UCSF License.
The Amgen License will terminate upon the expiration of the last Amgen Royalty Term for any Amgen Licensed Product. Aegerion has the right to terminate the Amgen License for convenience upon 90 days prior written notice to Amgen or for Amgen’s uncured material breach of the Amgen License, or becoming subject to specified bankruptcy or liquidation events. Amgen may terminate the Amgen License for our uncured failure to make payments to Amgen or if we are the subject of specified bankruptcy or liquidation events.
Aegerion made royalty payments related to the sales of MYALEPT under the Amgen license through November 29, 2016 and there were no payments made between the period from November 30, 2016 to December 31, 2016. We had $1.2 million remaining balance in royalties payable as of December 31, 2016.
In addition, Aegerion is required to make royalty payments at a range of royalty rates in the high single digits on net sales of lomitapide in countries where lomitapide has patent protection, and in respect of any other products covered by the license (subject to a variety of customary reductions), and share with UPenn specified percentages of sublicensing royalties and other consideration that we receive under any sublicenses that we may grant.
In December 2016, Aegerion entered into a license agreement with Amryt under which Amryt was granted an exclusive right to develop and commercialize LOJUXTA in the European Economic Area (EEA), Switzerland, Turkey and certain Middle Eastern and North African territories, including Israel. Under the license agreement, Aegerion maintains the marketing authorizations for LOJUXTA; however, Amryt is responsible for ongoing regulatory and post-marketing obligations and commitments for LOJUXTA. Amryt is also required to pay us certain sales-related milestone payments and royalties on net product sales in the licensed territories.
113
Aegerion made royalty payments to UPenn through November 29, 2016 and there were no payments made during the period from November 30, 2016 to December 31, 2016. We had $1.3 million remaining balance in royalties payable to UPenn as of December 31, 2016.
Future Funding Requirements
Our need to raise additional capital in the future, and the size of any such financings, will depend on many factors, including:
• | the success of our commercialization efforts and the level of revenues generated from sales of metreleptin and lomitapide in the U.S.; |
• | the level of revenue received from named patient sales of metreleptin and lomitapide in Brazil and other key countries where a mechanism exists to sell the product on a pre-approval basis in such country based on U.S. approval of such products or EU approval of lomitapide, particularly in light of the availability of a PCSK9 inhibitor product in Brazil and the ongoing court proceedings in Brazil reviewing the regulatory framework for named patient sales; |
• | the level of physician, patient and payer acceptance of lomitapide and metreleptin; |
• | our ability to manage our costs and expenses to better align with our revenues and strengthen our capital structure, while supporting approved products in a compliant manner; |
• | gaining regulatory and pricing and reimbursement approvals to market our products in countries in which the products are not currently approved and/or reimbursed, where it makes business sense to seek such approval, without significant restrictions, discounts, caps or other cost containment measures, including regulatory and pricing and reimbursement approval of metreleptin in the EU, in connection with which we filed an MAA in the EMA in December 2016, and regulatory approval of metreleptin in the U.S. for a subset of PL based on the existing clinical data package for metreleptin, subject to discussions with the FDA; |
• | the extent of the negative impact of the availability of PCSK9 inhibitor products on sales of JUXTAPID in the U.S., which, among other factors, have caused a significant number of JUXTAPID patients to discontinue JUXTAPID and switch to a PCSK9 inhibitor product, and significantly decreased the rate at which new HoFH patients start treatment with lomitapide; |
• | the provision of free PCSK9 inhibitor drug to adult HoFH patients by the companies that are commercializing PCSK9 inhibitor products, which such companies may have ceased, but which historically has had a negative impact on the rate at which new patients start treatment with lomitapide and has caused more patients than we expected to discontinue lomitapide and switch their treatment to PCSK9 inhibitor products; |
• | requirements of insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs in the U.S. to require that newly diagnosed adult HoFH patients be treated with PCSK9 inhibitor products prior to JUXTAPID, that current JUXTAPID patients switch to PCSK9 inhibitor products, and that patients fail to adequately respond to PCSK9 inhibitor products before providing reimbursement for JUXTAPID at the prices at which we offer JUXTAPID; |
• | the willingness of insurance companies, managed care organizations, other private payers, and government entities that provide reimbursement for medical costs in the U.S. to continue to provide reimbursement for our products at the prices at which we offer our products without imposing any additional major hurdles to access or other significant restrictions or limitations, and the ability and willingness of HoFH and GL patients to pay, or to arrange for payment assistance with respect to, any patient cost-sharing amounts for our products applicable under their insurance coverage, particularly in light of recent reductions in contributions to 501(c)(3) patient organizations by pharmaceutical companies; |
• | the cost of building and maintaining the sales and marketing capabilities necessary for the commercialization of our products for their targeted indications in the market(s) in which each has received regulatory approval and we elect to commercialize such products, to the extent reimbursement and pricing approvals are obtained, and certain other key international markets, if approved; |
• | the timing and costs of future business development opportunities; |
• | the timing and cost of seeking regulatory approvals and conducting potential future clinical development of metreleptin in additional indications, pursuing possible lifecycle management opportunities for metreleptin, and conducting potential development of the zuretinol program; |
• | the cost of filing, prosecuting and enforcing patent claims, including the cost of defending any challenges to the patents or our claims of exclusivity; |
114
• | the status of ongoing government investigations and lawsuits, including the disclosure of possible or actual outcomes, including regarding the preliminary agreements in principle that have been reached with the DOJ and the SEC; |
• | the costs of our manufacturing-related activities and the other costs of commercializing our products; |
• | the costs associated with ongoing government investigations and lawsuits, including any damages, settlement amounts, fines or other payments, or implementation of compliance related agreements or consent decrees, that may result from settlements or enforcement actions related to government investigations or whether we are successful in our efforts to defend ourselves in, or to settle on acceptable terms, ongoing or future litigation; |
• | the levels, timing and collection of revenue received from sales of our products in the future; |
• | the timing and costs of satisfying our debt obligations, including interest payments and any amounts due upon the maturity of such debt, including under the Convertible Notes; |
• | the cost of our observational cohort studies and other post-marketing commitments, including to the FDA and in any other countries where our products are ultimately approved; and |
• | the timing and cost of other clinical development activities. |
We may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. The source, timing and availability of any future financing will depend principally upon equity and debt market conditions, interest rates and, more specifically, on the extent of our commercial success and our continued progress in our regulatory and development activities. There can be no assurance that external funds will be available on favorable terms, if at all.
Off-Balance Sheet Arrangements
In connection with the sale of assets and businesses, we provide indemnities with respect to certain matters, including product liability, patent infringement, contractual breaches and misrepresentations, and we provide other indemnities to third parties under the clinical trial, license, service, manufacturing, supply, distribution and other agreements that we enter into in the normal course of our business. If the indemnified party were to make a successful claim pursuant to the terms of the indemnity, we would be required to reimburse the loss. These indemnities are generally subject to threshold amounts, specified claims periods and other restrictions and limitations. As of December 31, 2016, no amounts have been accrued in connection with such indemnities.
Except as described above and the contractual arrangements described in the Contractual Obligations section above, we do not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
CERTAIN CANADIAN AND U.S. FEDERAL INCOME TAX INFORMATION FOR U.S. RESIDENTS
The following is a summary of certain Canadian and U.S. federal income tax considerations applicable to holders of common shares of the Company. These tax considerations are stated in brief and general terms and are based on Canadian and U.S. law currently in effect. There are other potentially significant Canadian and U.S. federal income tax considerations and provincial, state and local income tax considerations with respect to ownership and disposition of the common shares which are not discussed herein. The tax considerations relative to ownership and disposition of the common shares may vary from shareholder to shareholder depending on the shareholder’s particular status. Accordingly, shareholders and prospective shareholders are encouraged to consult with their tax advisors regarding tax considerations which may apply to the particular situation.
Canadian Federal Tax Information
The following is a general summary of the principal Canadian federal income tax considerations generally applicable to a holder of common shares of the Company who, at all relevant times, for purposes of the Income Tax Act (Canada) (the Canadian Tax Act) (i) is not, or is not deemed to be, a resident of Canada, (ii) holds the common shares as capital property, (iii) deals at arm’s length with, and is not affiliated with, the Company and (iv) does not and will not use or hold, and is not and will not be deemed to use or hold, common shares of the Company in connection with carrying on a business in Canada (a Non-Resident Holder).Special rules, which are not discussed in this summary, may apply to a Non-Resident Holder that is an insurer carrying on business in Canada and elsewhere. Common shares of the Company will generally be considered to be capital property to a holder thereof, unless the shares are held in the course of carrying on a business or were acquired in a transaction considered to be an adventure in the nature of trade.
Dividends paid, deemed to be paid, or credited on the common shares held by Non-Resident Holders will generally be subject to Canadian withholding tax at the rate of 25% of the gross amount of the dividend unless the rate is reduced by an applicable income
115
tax convention or treaty. The Canada-U.S. Income Tax Convention (1980) (the Convention) provides that the withholding tax rate on dividends paid on the common shares to U.S. residents who qualify for the benefit of the Convention will generally be reduced to 15% of the gross amount of the dividend.
A Non-Resident Holder will generally not be subject to Canadian income tax in respect of any gain realized on the disposition of common shares unless the common shares constitute “taxable Canadian property” to such Non-Resident Holder and such Non-Resident Holder is not entitled to relief under an applicable income tax treaty or convention. Generally, provided the common shares are then listed on a designated stock exchange for purposes of the Canadian Tax Act (which includes the TSX and the NASDAQ), the common shares will not be “taxable Canadian property” to a Non-Resident Holder unless, at any particular time during the 60-month period immediately preceding the disposition (i) 25% or more of the issued shares of any class or series of the capital stock of the Company were owned by such Non-Resident Holder, by persons with whom the Non-Resident Holder did not deal at arm’s length, or any combination thereof and (ii) the shares derived more than 50% of their fair market value directly or indirectly from one or any combination of real or immovable property situated in Canada, Canadian resource properties or timber resource properties (as defined in the Canadian Tax Act), or options in respect of, or interests or rights in any of the foregoing. A gain realized upon the disposition of the common shares by a U.S. resident who qualifies for the benefits of the Convention that is otherwise subject to Canadian tax may be exempt from Canadian tax under the Convention.
Where the common shares are disposed of by way of an acquisition of such common shares by the Company, other than a purchase in the open market in the manner in which common shares normally would be purchased by any member of the public in the open market, the amount paid by the Company in excess of the paid-up capital of such common shares will be treated as a dividend and will be subject to non-resident withholding tax as described above.
U.S. Federal Income Tax Information
Special U.S. federal income tax rules apply to “U.S. Holders” (as defined below) of shares of a “passive foreign investment company” (a PFIC). As previously disclosed, the Company believes, but cannot offer any assurance, that it was classified as a PFIC for one or more taxable years prior to 2000, and that it was not a PFIC during any of the taxable years from the taxable year ended December 31, 2000 through the taxable year ended December 31, 2007. The Company further believes that it was a PFIC for the taxable years ended December 31, 2008 through 2015, which significantly impacts the U.S. federal income tax consequences to U.S. Holders. The Company believes that it will not be deemed a PFIC for the taxable years ending December 31, 2016 and December 31, 2017. The Company’s actual PFIC status for a given taxable year will not be determinable until the close of such year and, accordingly, no assurances can be given regarding the Company’s PFIC status in 2017 or any future year. See further discussion of the PFIC rules below. In addition, the following assumes that the common shares are held as a capital asset within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the Code).
This summary is of a general nature only and is not intended for non-U.S. Holders. Furthermore, it is not intended to constitute, and should not be construed to constitute, legal or tax advice to any particular U.S. Holder, and it does not address U.S. federal income tax considerations that may be relevant to U.S. Holders that are subject to special treatment under U.S. federal income tax law. U.S. Holders are urged to consult their own tax advisors as to the tax consequences in their particular circumstances.
U.S. Holders
A “U.S. Holder” is a holder of the Company’s common shares that is (i) an individual who is a citizen or resident of the U.S. for U.S. federal income tax purposes; (ii) a corporation (or other entity taxed as a corporation for U.S. federal income tax purposes) created or organized under the laws of the U.S., any U.S. state or the District of Columbia; (iii) an estate the income of which is subject to U.S. federal income taxation regardless of the income’s source; or (iv) a trust (a) if a U.S. court is able to exercise primary supervision over the trust’s administration and one or more U.S. persons, as defined under Section 7701(a)(30) of the Code, have authority to control all of the trust’s substantial decisions; or (b) that was in existence on August 20, 1996, was treated as a U.S. person under the Code on the previous day and has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Sale or Other Disposition of Common Shares
Subject to different treatment pursuant to the PFIC rules discussed below, if a U.S. Holder engages in a sale, exchange or other taxable disposition of such U.S. Holder’s common shares, (i) such U.S. Holder will recognize gain or loss equal to the difference between the amount realized by such U.S. Holder and such U.S. Holder’s adjusted tax basis in the common shares, (ii) any such gain or loss will be capital gain or loss, and (iii) such capital gain or loss will be long-term capital gain or loss if the holding period of the common shares exceeds one year as of the date of the sale. Such gain generally is treated as U.S. source gain for U.S. foreign tax credit limitation purposes.
If the Company purchases common shares from a U.S. Holder, such transaction will be treated as a taxable sale or exchange of the common shares by the U.S. Holder if the transaction meets certain conditions under U.S. federal income tax rules, or otherwise will be treated as a distribution by the Company in respect of the U.S. Holder’s common shares, as described below.
116
Distributions on Common Shares
Subject to different treatment pursuant to the PFIC rules discussed below, a distribution with respect to our common shares generally will be treated as a dividend, taxable as ordinary income, to the extent of the Company’s current or accumulated earnings and profits, as determined under U.S. federal income tax principles. In general, to the extent that the amount of the distribution exceeds the Company’s current and accumulated earnings and profits, the excess first will be treated as a tax-free return of capital that will reduce the holder’s tax basis in the holder’s common shares, and to the extent of any remaining portion in excess of such tax basis, the excess will be taxable as capital gain. Any such capital gain will be long-term capital gain if the U.S. Holder has held the common shares for more than one year at the time of the distribution. However, under U.S. Treasury regulations regarding the treatment of PFICs, a purchase of common shares from a U.S. Holder by the Company that does not qualify as a “sale or exchange” under U.S. federal income tax rules, and hence is treated as a distribution, is in fact treated as a distribution in full for PFIC purposes regardless of whether there are any earnings and profits.
A dividend received by a corporate U.S. Holder generally will not be eligible for a dividends-received deduction. In addition, a dividend received by an individual U.S. Holder will not qualify for the 15% reduced maximum rate if the Company is a PFIC in the year in which the dividend is paid or in the preceding year.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by the Company with respect to our common shares will constitute “passive category income” or, in the case of certain U.S. Holders, “general category income.”
Passive Foreign Investment Company
A non-U.S. corporation generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying relevant look-through rules with respect to the income and assets of subsidiaries, either 75% or more of its gross income is “passive income” (the income test) or 50% or more of the average value of its assets consists of assets that produce, or are held for the production of, passive income (the asset test). For this purpose, passive income generally includes, among other things, dividends, interest, certain rents and royalties and gains from the disposition of passive assets.
The Company believes that it was a PFIC for 2008 through 2015 but that it will not be deemed a PFIC for 2016. Please be aware that the Company’s status as a PFIC can have significant adverse tax consequences for U.S. Holders.
A U.S. Holder that holds common shares while the Company is a PFIC may be subject to increased tax liability upon the sale, exchange or other disposition of the common shares or upon the receipt of certain distributions, regardless of whether the Company is a PFIC in the year in which such disposition or distribution occurs. These adverse tax consequences will not apply, however, if (i) a U.S. Holder timely filed and maintained (and in certain cases, continues to maintain), or timely files and maintains, as the case may be, a qualified electing fund (QEF) election to be taxed annually on the U.S. Holder’s pro rata portion of the Company’s earnings and profits, (ii) the U.S. Holder timely made or makes, as the case may be, a mark-to-market election as described below, or (iii) a U.S. Holder is eligible to make a “purging” election and timely does so, as described below.
The adverse tax consequences include:
a. | “Excess distributions” by the Company are subject to the following special rules. An excess distribution generally is the excess of the amount a PFIC distributes to a shareholder during a taxable year over 125% of the average amount it distributed to the shareholder during the three preceding taxable years or, if shorter, the part of the shareholder’s holding period before the taxable year. Distributions with respect to the common shares made by the Company during the taxable year to a U.S. Holder that are excess distributions must be allocated ratably to each day of the U.S. Holder’s holding period. The amounts allocated to the current taxable year and to taxable years prior to the first year in which the Company was classified as a PFIC are included as ordinary income in a U.S. Holder’s gross income for that year. The amount allocated to each other prior taxable year is taxed as ordinary income at the highest tax rate in effect for the U.S. Holder in that prior year (without offset by any net operating loss for such year) and the tax is subject to an interest charge at the rate applicable to deficiencies in income taxes (the special interest charge). |
b. | The entire amount of any gain realized upon the sale or other disposition of the common shares will be treated as an excess distribution made in the year of sale or other disposition and as a consequence will be treated as ordinary income and, to the extent allocated to years prior to the year of sale or disposition, will be subject to the special interest charge described above. |
QEF Election. A U.S. Holder of shares in a PFIC may make a QEF election with respect to such PFIC to elect out of the tax treatment discussed above. Generally, a QEF election, on U.S. Internal Revenue Service (IRS) Form 8621, should be made with
117
the filing of a U.S. Holder’s U.S. federal income tax return for the first taxable year for which both (i) the U.S. Holder holds common shares of the Company, and (ii) the Company was a PFIC. A U.S. Holder that timely makes a valid QEF election with respect to a PFIC will generally include in gross income for a taxable year (i) as ordinary income, such holder’s pro rata share of the corporation’s ordinary earnings for the taxable year, and (ii) as long-term capital gain, such holder’s pro rata share of the corporation’s net capital gain for the taxable year. However, the QEF election is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. The Company will provide, upon request, all information and documentation that a U.S. Holder making a QEF election is required to obtain for U.S. federal income tax purposes (e.g., the U.S. Holder’s pro rata share of ordinary income and net capital gain, and a “PFIC Annual Information Statement” as described in applicable U.S. Treasury regulations, which will be made available on the Company’s website).
Deemed Sale Election. If the Company is a PFIC for any year during which a U.S. Holder holds common shares, but the Company ceases in a subsequent year to be a PFIC (which could occur, for example, if the Company were a PFIC for 2015 but is not a PFIC for 2016), then a U.S. Holder can make a “purging” election, in the form of a deemed sale election, for such subsequent year in order to avoid the adverse PFIC tax treatment described above that would otherwise continue to apply because of the Company having previously been a PFIC. If such election is timely made, the U.S. Holder would be deemed to have sold the common shares held by the holder at their fair market value, and any gain from such deemed sale would be taxed as an excess distribution (as described above). The basis of the common shares would be increased by the gain recognized, and a new holding period would begin for the common shares for purposes of the PFIC rules. The U.S. Holder would not recognize any loss incurred on the deemed sale, and such a loss would not result in a reduction in basis of the common shares. After the deemed sale election, the U.S. Holder’s common shares with respect to which the deemed sale election was made would not be treated as shares in a PFIC, unless the Company subsequently becomes a PFIC. A U.S. Holder may also be able to make a deemed sale election with respect to the Company’s subsidiaries that are PFICs, if any. The rules regarding deemed sale elections are very complex. U.S. Holders are strongly urged to consult their tax advisors about the deemed sale election with regard to the Company and any subsidiaries.
Mark-to-Market Election. Alternatively, a U.S. Holder of “marketable shares” (as defined below) in a PFIC may make a mark-to-market election for such shares to elect out of the adverse PFIC tax treatment discussed above. If a U.S. Holder makes a mark-to-market election for shares of marketable shares, the holder will include in income each year an amount equal to the excess, if any, of the fair market value of the shares as of the close of the holder’s taxable year over the holder’s adjusted basis in such shares. A U.S. Holder is allowed a deduction for the excess, if any, of the adjusted basis of the shares over their fair market value as of the close of the taxable year. However, deductions are allowable only to the extent of any net mark-to-market gains on the shares included in the holder’s income for prior taxable years. Amounts included in a U.S. Holder’s income under a mark-to-market election, as well as gain on the actual sale or other disposition of the shares, are treated as ordinary income. Ordinary loss treatment also applies to the deductible portion of any mark-to-market loss on the shares, as well as to any loss realized on the actual sale or disposition of the shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such shares. A U.S. Holder’s basis in the shares will be adjusted to reflect any such income or loss amounts. However, the special interest charge and related adverse tax consequences described above for non-electing holders may continue to apply on a limited basis if the U.S. Holder makes the mark-to-market election after such holder’s holding period for the shares has begun.
The mark-to-market election is available only for “marketable shares,” which are shares that are traded in other than de minimis quantities on at least 15 days during each calendar quarter on a qualified exchange or other market, as defined in applicable U.S. Treasury regulations. The Company’s common shares are listed on TSX and quoted on NASDAQ, each of which constitutes a “qualified exchange or other market” under applicable U.S. Treasury regulations. U.S. Holders of common shares are urged to consult their tax advisors as to whether the common shares would qualify for the mark-to-market election.
Subsidiary PFICs. To the extent any of the Company’s subsidiaries is also a PFIC, a U.S. Holder will also be deemed to own shares in such lower-tier PFIC and could incur a liability for the deferred tax and special interest charge described above if either (i) the Company receives a distribution from, or disposes of all or part of its interest in, the lower-tier PFIC, or (ii) the U.S. Holder disposes of all or part of such holder’s common shares. In addition, the mark-to-market election cannot be made for a subsidiary of a PFIC if the shares of such subsidiary is not itself marketable shares.
PFIC Reporting Requirement. Unless otherwise provided by the U.S. Treasury, each U.S. person that is a direct or indirect shareholder of a PFIC is required to file an annual report on IRS Form 8621 containing such information as the U.S. Treasury may require. U.S. Holders should consult their tax advisors regarding any reporting requirements that may apply to them and the effect, if any, this reporting may have on their ownership and disposition of our common shares.
THE APPLICABILITY AND CONSEQUENCES OF THE PFIC RULES ARE EXCEEDINGLY COMPLEX. IN ADDITION, THE FOREGOING SUMMARY DOES NOT ADDRESS ALL OF THE POTENTIAL U.S. FEDERAL INCOME TAX CONSEQUENCES WITH RESPECT TO PFIC STATUS THAT MAY BE RELEVANT TO A PARTICULAR INVESTOR IN LIGHT OF SUCH INVESTOR’S PARTICULAR CIRCUMSTANCES OR THAT MAY BE RELEVANT TO INVESTORS THAT ARE SUBJECT TO SPECIAL TREATMENT UNDER U.S. FEDERAL INCOME
118
TAX LAW. ACCORDINGLY, INVESTORS ARE STRONGLY URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE APPLICATION OF THE PFIC RULES TO THEM AND THE ADVISABILITY OF MAKING ANY OF THE ELECTIONS DESCRIBED ABOVE.
Outstanding Share Data
Under the Novelion 2016 Equity Incentive Plan (formerly known as the QLT 2000 Incentive Stock Plan) dated April 25, 2013 and amended and restated effective November 29, 2016 and further amended and restated effective December 1, 2016 (the NVLN Plan), the maximum number of common shares, without par value, that are allotted for stock option and restricted stock unit grants under the NVLN Plan is 3,067,994. As of December 31, 2016, there are 648,432 remaining common shares available for future grants under the NVLN Plan.
As of February 17, 2017, there were 18,533,029 common shares issued and outstanding, which totaled $199.8 million in share capital. As of February 17, 2017, we had 1,729,927 stock options outstanding of which 224,304 were exercisable at a weighted average exercise price of $8.87 per share. Each stock option is exercisable for one common share. As of February 17, 2017, we had 889,220 RSU’s outstanding and none of which were vested, and 28,400 deferred share units outstanding of which 28,400 were vested. The cash value of the deferred share units outstanding as of February 17, 2017 were $236,800.
Under the amended and restated Aegerion 2010 Stock Option and Incentive Plan (the Aegerion 2010 Plan), the maximum number of common shares, without par value, that are allotted for stock option and restricted stock unit grants under the Aegerion 2010 Plan is 143,912. As of December 31, 2016, there are no remaining common shares available for future grants under the Aegerion 2010 Plan. As of February 17, 2017, we had 10,561 stock options outstanding at a weighted average exercise price of $7.70 per share, none of which were exercisable. Each stock option is exercisable for one common share. As of February 17, 2017, we had 129,164 RSU’s outstanding, none of which are vested.
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. |
Market risk is the potential loss arising from adverse changes in the financial markets, including interest rates and foreign currency exchange rates.
Interest Rate Risk
Aegerion has outstanding $325.0 million 2.0% Convertible Notes due August 15, 2019 (the Convertible Notes). The Convertible Notes have a fixed annual interest rate of 2.0% and we, therefore, do not have economic interest rate exposure on the Convertible Notes. However, the fair value of the Convertible Notes is exposed to interest rate risk. Generally, the fair value of the Convertible Notes will increase as interest rates fall and decrease as interest rates rise. These Convertible Notes are also affected by the price and volatility of our common shares and will generally increase or decrease as the market price of our common shares changes. As of December 31, 2016, the fair value of the Convertible Notes was estimated by us to be $240.4 million. For additional discussion on the Convertible Notes, refer to the Note 10 - Convertible Notes, Net in the Notes to the Consolidated Financial Statements for the year ended December 31, 2016. As of December 31, 2016 and 2015, we had no other assets or liabilities with significant interest rate sensitivity.
Foreign Currency Exchange Risk
We are also exposed to risks associated with foreign currency exchange rate fluctuations related to our international subsidiaries in which we continue to help support operations with financial contributions. We do not currently hedge our foreign currency exchange rate risk. We manage this foreign currency risk, in part, through operational means including managing foreign currency revenues in relation to same currency costs as well as managing foreign currency assets in relation to same currency liabilities. We are also exposed to the potential earnings effects from intercompany foreign currency assets and liabilities that arise from normal trade receivables and payables and other intercompany loans. These subsidiaries’ financial statements are re-measured into their respective functional currencies using current or historical exchange rates. Such re-measurement adjustments could have an adverse effect on the Company’s results of operations.
Investment Risk
At December 31, 2016 and 2015, the Company did not have any investments in debt or equity securities and as such was not exposed to risks associated with any other-than-temporary decline in fair value of these investments. At December 31, 2016, the Company had $50.0 million investment in treasury bills and $18.3 million investment in money market funds, which collectively have a weighted average remaining maturity of approximately 27.6 days. Any fluctuation in fair value of these cash equivalents will be an immaterial amount due to the short remaining maturity period.
See the “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources - Interest and Foreign Exchange Rates” section of this Annual Report, which is incorporated by reference herein.
119
120
Item 8. | Consolidated Financial Statements and Supplementary Data. |
Page | |
121
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Novelion Therapeutics Inc. (formerly QLT Inc.)
We have audited the accompanying consolidated balance sheets of Novelion Therapeutics Inc. and subsidiaries (the “Company”) as of December 31, 2016 and 2015, and the consolidated statements of operations, comprehensive loss, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such Consolidated Financial Statements present fairly, in all material respects, the financial position of Novelion Therapeutics Inc. and subsidiaries as of December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2016, based on the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 30, 2017, expressed an adverse opinion on the Company’s internal control over financial reporting because of a material weakness.
/s/Deloitte LLP
Chartered Professional Accountants
March 30, 2017
Vancouver, Canada
122
Novelion Therapeutics Inc. (Formerly QLT Inc.)
Consolidated Balance Sheets
As of December 31, | |||||||
(in thousands, except share information) | 2016 | 2015 | |||||
ASSETS | |||||||
Current assets | |||||||
Cash and cash equivalents (Note 14) | $ | 108,927 | $ | 141,824 | |||
Restricted cash (Note 14) | 390 | — | |||||
Accounts receivable, net (Note 2) | 9,339 | 287 | |||||
Inventories - current (Note 6) | 15,718 | — | |||||
Insurance proceeds receivable (Note 16) | 22,000 | — | |||||
Prepaid expenses and other current assets | 9,762 | 625 | |||||
Total current assets | 166,136 | 142,736 | |||||
Inventories - non-current (Note 6) | 59,003 | — | |||||
Property and equipment, net (Note 7) | 4,159 | 430 | |||||
Accounts receivable - non-current (Note 16) | — | 2,000 | |||||
Intangible assets, net (Note 8) | 250,324 | — | |||||
Other assets | 1,160 | — | |||||
Total assets | $ | 480,782 | $ | 145,166 | |||
LIABILITIES | |||||||
Current liabilities | |||||||
Accounts payable | $ | 17,609 | $ | 1,656 | |||
Accrued liabilities (Note 9) | 37,180 | 1,827 | |||||
Provision for legal settlement (Note 16) | 64,010 | — | |||||
Total current liabilities | 118,799 | 3,483 | |||||
Long-term liabilities: | |||||||
Convertible notes, net (Note 10) | 225,584 | — | |||||
Uncertain tax position liabilities, net (Note 13) | 381 | 342 | |||||
Other liabilities | 231 | — | |||||
Total liabilities | 344,995 | 3,825 | |||||
Contingencies, Commitments and Guarantees (Note 16) | |||||||
SHAREHOLDERS’ EQUITY | |||||||
Share capital (Note 11) | |||||||
Common shares, without par value, 100,000,000(1) shares authorized at December 31, 2016 and 2015; 18,530,323 and 10,565,489(1) shares issued at December 31, 2016 and 2015, respectively. | 551,259 | 475,333 | |||||
Additional paid-in-capital | 69,149 | 97,377 | |||||
Accumulated deficit | (587,208 | ) | (534,338 | ) | |||
Accumulated other comprehensive items | 102,587 | 102,969 | |||||
Total shareholders’ equity | 135,787 | 141,341 | |||||
Total liabilities and shareholders’ equity | $ | 480,782 | $ | 145,166 | |||
(1) Amounts have been retrospectively restated for all prior periods presented to reflect the one-for-five share consolidation of the Company’s common stock effected on December 16, 2016. See Note 1- Description of the Business for details. | |||||||
See the accompanying Notes to the Consolidated Financial Statements. | |||||||
123
Novelion Therapeutics Inc. (Formerly QLT Inc.)
Consolidated Statements of Operations
For the Years Ended December 31, | |||||||||||
(in thousands, except per share information) | 2016 | 2015 | 2014 | ||||||||
Net product sales | $ | 13,574 | $ | — | $ | — | |||||
Cost of product sales | 5,971 | — | — | ||||||||
Operating expenses | |||||||||||
Selling, general and administrative | 29,525 | 16,222 | 17,682 | ||||||||
Research and development | 14,784 | 9,790 | 13,803 | ||||||||
Restructuring charges | — | — | 744 | ||||||||
Termination Fee (Note 3) | — | (2,667 | ) | (28,400 | ) | ||||||
Total operating expenses | 44,309 | 23,345 | 3,829 | ||||||||
Loss from operations | (36,706 | ) | (23,345 | ) | (3,829 | ) | |||||
Interest (expense) income, net | (2,960 | ) | 277 | 113 | |||||||
Fair value loss on investment (Note 4) | (10,740 | ) | — | — | |||||||
Other income (expense), net | (1,999 | ) | 81 | (481 | ) | ||||||
Loss from continuing operations before income taxes | (52,405 | ) | (22,987 | ) | (4,197 | ) | |||||
Provision for Income tax (expense) recovery (Note 13) | (465 | ) | (22 | ) | 192 | ||||||
Loss from continuing operations | $ | (52,870 | ) | $ | (23,009 | ) | $ | (4,005 | ) | ||
Loss from discontinued operations, net of income taxes | — | — | (66 | ) | |||||||
Net loss | $ | (52,870 | ) | $ | (23,009 | ) | $ | (4,071 | ) | ||
Basic and diluted net loss per common share (1) (Note 15) | |||||||||||
Continuing operations (1) | $ | (4.69 | ) | $ | (2.20 | ) | $ | (0.40 | ) | ||
Discontinued operations (1) (2) | — | — | — | ||||||||
Net loss per common share (1) | $ | (4.69 | ) | $ | (2.20 | ) | $ | (0.40 | ) | ||
Weighted-average shares outstanding—basic and diluted (thousands) (1) | |||||||||||
Basic and diluted (1) | 11,284 | 10,434 | 10,225 | ||||||||
(1) Amounts have been retrospectively restated for all prior periods presented to reflect the one-for-five share consolidation of the Company’s common stock effected on December 16, 2016. See Note 1 - Description of Business for details. | |||||||||||
(2) Rounded to zero. | |||||||||||
See the accompanying Notes to the Consolidated Financial Statements. | |||||||||||
124
Novelion Therapeutics Inc. (Formerly QLT Inc.)
Consolidated Statements of Comprehensive Loss
For the Years Ended December 31, | |||||||||||
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Net loss | $ | (52,870 | ) | $ | (23,009 | ) | $ | (4,071 | ) | ||
Other comprehensive loss, net of tax: | |||||||||||
Foreign currency translation | (382 | ) | — | — | |||||||
Other comprehensive loss | (382 | ) | — | — | |||||||
Comprehensive loss | $ | (53,252 | ) | $ | (23,009 | ) | $ | (4,071 | ) | ||
See the accompanying Notes to the Consolidated Financial Statements. | |||||||||||
125
Novelion Therapeutics Inc. (Formerly QLT Inc.)
Consolidated Statements of Shareholders’ Equity
(in thousands, except share and per share information) | Common Shares | Additional Paid-In | Accumulated | Accumulated Other Comprehensive | Total Shareholders’ | |||||||||||||||||
Shares (1) | Amount | Capital | Deficit | Income | Equity | |||||||||||||||||
Balance at December 31, 2013 | 10,215,985 | $ | 466,229 | $ | 95,844 | $ | (507,258 | ) | $ | 102,969 | $ | 157,784 | ||||||||||
Exercise of stock options, for cash, at prices ranging from CAD $22.7 to CAD $26.9 per share (1) | 20,809 | 750 | (241 | ) | — | — | 509 | |||||||||||||||
Shares issued in connection with RSUs vested (Note 12) | 2,800 | 55 | (55 | ) | — | — | — | |||||||||||||||
Uncertain tax position liability recovery (Note 13) | — | — | 837 | — | — | 837 | ||||||||||||||||
Stock-based compensation expense (Note 12) | — | — | 1,453 | — | — | 1,453 | ||||||||||||||||
Net loss and comprehensive loss | — | — | (4,071 | ) | — | (4,071 | ) | |||||||||||||||
Balance at December 31, 2014 | 10,239,594 | 467,034 | 97,838 | (511,329 | ) | 102,969 | 156,512 | |||||||||||||||
Exercise of stock options, for cash, at prices ranging from CAD $20.4 to CAD $22.7 per share (1) | 313,095 | 8,077 | (2,569 | ) | — | — | 5,508 | |||||||||||||||
Shares issued in connection with RSUs vested (Note 12) | 12,800 | 222 | (222 | ) | — | — | — | |||||||||||||||
Stock-based compensation expense (Note 12) | — | — | 2,330 | — | — | 2,330 | ||||||||||||||||
Net loss and comprehensive loss | — | — | (23,009 | ) | — | (23,009 | ) | |||||||||||||||
Balance at December 31, 2015 | 10,565,489 | 475,333 | 97,377 | (534,338 | ) | 102,969 | 141,341 | |||||||||||||||
Shares issued in connection with the Acquisition of Aegerion (Note 5) | 6,060,288 | 59,381 | — | — | — | 59,381 | ||||||||||||||||
Shares issued in a private placement net of share issuance cost (Note 11) | 1,904,546 | 16,545 | 4,936 | — | — | 21,481 | ||||||||||||||||
Stock-based compensation expense (Note 12) | — | 797 | — | — | 797 | |||||||||||||||||
Cash distribution to shareholders (Note 4) | — | — | (15,000 | ) | — | — | (15,000 | ) | ||||||||||||||
Aralez shares distributed to shareholders (Note 4) | — | — | (19,296 | ) | — | — | (19,296 | ) | ||||||||||||||
Uncertain tax position liability recovery (Note 13) | — | — | 335 | — | — | 335 | ||||||||||||||||
Foreign currency translation adjustment | — | — | — | — | (382 | ) | (382 | ) | ||||||||||||||
Net loss | — | — | — | (52,870 | ) | — | (52,870 | ) | ||||||||||||||
Balance at December 31, 2016 | 18,530,323 | $ | 551,259 | $ | 69,149 | $ | (587,208 | ) | $ | 102,587 | $ | 135,787 | ||||||||||
(1) Amounts have been retrospectively restated for all prior periods presented to reflect the one-for-five share consolidation of the Company’s common stock effected on December 16, 2016. Refer to Note 1 - Description of Business for further details. | ||||||||||||||||||||||
See the accompanying Notes to the Consolidated Financial Statements. | ||||||||||||||||||||||
126
Novelion Therapeutics Inc. (Formerly QLT Inc.)
Consolidated Statements of Cash Flows
For the Years Ended December 31, | |||||||||||
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Cash (used in) provided by operating activities | |||||||||||
Net loss | $ | (52,870 | ) | $ | (23,009 | ) | $ | (4,071 | ) | ||
Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | |||||||||||
Depreciation | 264 | 576 | 891 | ||||||||
Amortization of intangible assets | 2,134 | — | — | ||||||||
Stock-based compensation | 797 | 2,330 | 1,453 | ||||||||
Noncash interest expense | 2,676 | — | — | ||||||||
Fair value change in contingent consideration (Notes 14, 16) | 2,042 | — | 2,000 | ||||||||
Unrealized foreign exchange gain (losses) | 118 | (120 | ) | 75 | |||||||
Loss (gain) on sale of long-lived assets | 50 | (36 | ) | — | |||||||
Fair value loss on investment (Note 4) | 10,704 | — | — | ||||||||
Deferred income taxes | (214 | ) | 18 | (177 | ) | ||||||
Impairment of long-lived assets | — | 11 | — | ||||||||
Changes in assets and liabilities, excluding the effect of acquisition: | |||||||||||
Accounts receivable | (893 | ) | 10 | 73 | |||||||
Inventories | 2,079 | — | — | ||||||||
Prepaid and other assets | 705 | 442 | 810 | ||||||||
Accounts payable | 4,441 | (184 | ) | (607 | ) | ||||||
Accrued liabilities | (6,389 | ) | 570 | 108 | |||||||
Accrued restructuring | — | — | (130 | ) | |||||||
Income taxes receivable/payable | — | 33 | 30 | ||||||||
Net cash (used in) provided by operating activities | (34,356 | ) | (19,359 | ) | 455 | ||||||
Cash provided by investing activities | |||||||||||
Cash acquired through acquisition (Note 5) | 28,290 | — | — | ||||||||
Cash consideration for acquisition - loan to Aegerion (Note 5) | (3,000 | ) | — | — | |||||||
Proceeds from sale of long-lived assets | 192 | 43 | 115 | ||||||||
Purchases of property and equipment | (155 | ) | (9 | ) | (25 | ) | |||||
Proceeds from contingent consideration (Notes 16) | — | — | 36,582 | ||||||||
Net cash provided by investing activities | 25,327 | 34 | 36,672 | ||||||||
Cash (used in) provided by financing activities | |||||||||||
Issuance of common shares | 21,481 | 5,508 | 509 | ||||||||
Cash distribution paid to common shareholders | (15,000 | ) | — | — | |||||||
Settlement of Backstop Agreement (Note 4) | 15,000 | — | — | ||||||||
Aralez investment | (45,000 | ) | — | — | |||||||
Net cash (used in) provided by financing activities | (23,519 | ) | 5,508 | 509 | |||||||
Exchange rate effect on cash | (349 | ) | (267 | ) | (249 | ) | |||||
Net (decrease) increase in cash and cash equivalents | (32,897 | ) | (14,084 | ) | 37,387 | ||||||
Cash and cash equivalents, beginning of period | 141,824 | 155,908 | 118,521 | ||||||||
Cash and cash equivalents, end of period | $ | 108,927 | $ | 141,824 | $ | 155,908 | |||||
Supplemental cash flow information | |||||||||||
Cash paid for interest | $ | 33 | $ | — | $ | — | |||||
Cash paid for taxes | $ | 105 | $ | — | $ | — | |||||
Non-cash financing activities | |||||||||||
Shares issued in the acquisition (Note 5) | $ | 59,088 | $ | — | $ | — | |||||
Convertible notes of Aegerion, at fair value (Note 10) | $ | 222,900 | $ | — | $ | — | |||||
Non-cash investment activities | |||||||||||
Purchases of property and equipment included in accounts payable | $ | 61 | $ | — | $ | — | |||||
See the accompanying Notes to the Consolidated Financial Statements. | |||||||||||
127
Novelion Therapeutics Inc. (Formerly QLT Inc.)
Notes to Consolidated Financial Statements
1. Description of Business.
Novelion Therapeutics Inc. (Novelion or the Company) (formerly QLT, Inc.) is a biopharmaceutical company dedicated to developing new standards of care for individuals living with rare diseases. On June 14, 2016, the Company entered into an Agreement and Plan of Merger (as amended, the Merger Agreement) with Aegerion Pharmaceuticals, Inc. (Aegerion), pursuant to which on November 29, 2016, Novelion completed the acquisition (the Merger) of Aegerion. Aegerion is a rare disease biopharmaceutical company with two commercial products and global operations, through which the Company assumed certain assets and liabilities of the acquired entity, including $28.7 million in cash, cash equivalents and restricted cash and two revenue streams which will serve as further funding for Novelion's operations. Upon closing the acquisition, QLT Inc. changed its name to Novelion Therapeutics Inc. As further detailed in Note 5, the acquisition has been accounted for as a business combination in which Novelion was considered the accounting acquirer of Aegerion. As such, the Consolidated Financial Statements of Novelion include the results of Aegerion from November 29, 2016.
Novelion has two commercial products, metreleptin and lomitapide from the acquisition of Aegerion and one orphan drug-designated product candidate, zuretinol acetate (zuretinol). Metreleptin, a recombinant analog of human leptin, is currently marketed in the U.S. under the brand name MYALEPT for injection. MYALEPT is approved in the U.S. as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy (GL). Lomitapide, which is marketed in the U.S. under the brand name JUXTAPID capsules, is approved in the U.S. as an adjunct to a low-fat diet and other lipid-lowering treatments, including low-density lipoprotein (LDL) apheresis where available, to reduce low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), apolipoprotein B (apo B) and non-high-density lipoprotein cholesterol (non-HDL-C) in adult patients with homozygous familial hypercholesterolemia (HoFH). Lomitapide is also approved in the European Union (EU), under the brand name LOJUXTA hard capsules for the treatment of adult patients with HoFH, as well as in Japan, Canada, and a small number of other countries. Zuretinol is an oral synthetic retinoid that is in late stage development for the treatment of inherited retinal disease (IRD) caused by underlying mutations in RPE65 and LRAT genes, comprising LCA and RP.
Under the Merger Agreement, the Company issued certain warrants to the pre-closing shareholders of Novelion. These warrants (the Merger Agreement Warrants) may be exercised for up to an aggregate of 11,301,791 Novelion common shares at an exercise price of $0.05 per share if (i) the previously disclosed DOJ and SEC investigations are settled for amounts in excess of $40 million and/or (ii) the putative class action lawsuit alleging certain misstatements and omissions related to the marketing of JUXTAPID and the Company’s financial performance in violation of the federal securities laws is settled for an amount that exceeds the amounts, if any, available under Aegerion’s director and officer coverage in respect of that matter (together, the negotiated thresholds). The number of common shares for which the Merger Agreement Warrants may be exercised, if any, will vary based on the extent to which the settlements of the matters described above exceed the negotiated thresholds. The Merger Agreement Warrants will not be exercisable for any shares to the extent any excess in respect of such matters is equal to or less than $1.0 million in the aggregate.
Also on June 14, 2016, the Company entered into a unit subscription agreement (the Unit Subscription Agreement) with the investors’ party thereto (the Investors). Pursuant to the Unit Subscription Agreement, immediately prior to the Merger, the Investors acquired units, for $8.80 per unit, consisting of (i) 1,904,546 Novelion common shares and 531,208 fully paid-up warrants (the Paid-Up Warrants), which may be exercised for up to 568,181 Novelion common shares, and (ii) 2,472,727 warrants (the Unit Subscription Agreement Warrants) exercisable for up to an aggregate of 2,644,952 Novelion common shares at an exercise price of $0.05 per share. The Unit Subscription Agreement Warrants were issued on the same terms and conditions as the Merger Agreement Warrants and are referred to collectively with the Merger Agreement Warrants as the “Contingent Warrants” in the Notes to the Consolidated Financial Statements. Refer to Note 11- Share Capital and Note 16 - Contingencies, Commitments and Guarantees for further information.
On December 16, 2016, the Company completed a one-for-five (1:5) consolidation of all of its issued and outstanding common shares (the Consolidation), resulting in a reduction in the issued and outstanding common shares from approximately 92,653,562 to approximately 18,530,323. Shares reserved under the Company’s equity and incentive plans were adjusted to reflect the Consolidation. All share and per-share data presented in the Company's Consolidated Financial Statements and notes have been retrospectively restated to reflect the Consolidation unless otherwise noted. Since the par value of the common shares is zero, neither the recorded value for common shares nor the paid-in capital has been retrospectively restated to reflect the Consolidation.
As noted above, all references in the notes to the Consolidated Financial Statements to the “Company” refer to Novelion and its consolidated subsidiaries. For periods before the closing of the Merger, where the specific entities are referred to within the Consolidated Financial Statements, unless otherwise stated, “QLT” refers to QLT Inc. and its wholly-owned subsidiaries and
128
“Aegerion” refers to Aegerion Pharmaceuticals, Inc. and its wholly-owned subsidiaries. Following the Merger, Novelion continues to conduct research and development related to zuretinol and Aegerion continues to develop and commercialize lomitapide and metreleptin, and each maintains its respective ownership of or licenses covering intellectual property related to such products and remains as party to the regulatory filings and approvals for such products.
2. Significant Accounting Policies.
Basis of Presentation and Principles of Consolidation
The accompanying Consolidated Financial Statements have been prepared in accordance with accounting principles generally accepted in the United States (GAAP). All amounts herein are expressed in U.S. dollars (USD) unless otherwise noted.
The accompanying Consolidated Financial Statements include operations of Novelion Therapeutics Inc. and its wholly-owned subsidiaries. All intercompany transactions and balances have been eliminated.
In management’s opinion, the Consolidated Financial Statements reflect all adjustments (including reclassifications of normal recurring adjustments) necessary to present fairly the financial position of Novelion as of December 31, 2016 and 2015 and the result of operations and cash flows for all periods presented.
Use of Estimates
The preparation of Consolidated Financial Statements in accordance with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements, and the reported amounts of expenses during the reporting periods presented. Significant estimates and assumptions are required when determining the fair value of contingent assets and liabilities, the valuation of the convertible notes, and the valuation of the assets and liabilities acquired in a business combination including inventory and intangible assets. Significant estimates and assumptions are also required in determination of stock-based compensation and income tax. Our estimates often are based on complex judgments, probabilities and assumptions that we believe to be reasonable but that are inherently uncertain and unpredictable. For any given individual estimate or assumption made by us, there may also be other estimates or assumptions that are reasonable. Actual results may differ from estimates made by management. Changes in estimates are reflected in reported results in the period in which they become known.
Reporting and Functional Currency
Novelion’s reporting currency is the USD and the Company's operations utilize the USD or local currency as the functional currency, where applicable.
Transactions in other currencies are recorded in the functional currency at the rate of exchange prevailing when the transactions occur. Monetary assets and liabilities denominated in other currencies are re-measured into the functional currency at rate of exchange in effect at the balance sheet date. Exchange gains and losses arising from re-measurement of foreign currency-denominated monetary assets and liabilities are included in income in the period in which they occur.
For foreign entities where the local currency is the functional currency, assets and liabilities denominated in local currencies are translated into USD at end-of-period exchange rates and the resultant translation adjustments are reported, net of their related tax effects, as a component of accumulated other comprehensive items in equity.
Discontinued Operations
The results of operations, including the gain on disposal for businesses that have been sold or are classified as held for sale, are excluded from continuing operations and reported as discontinued operations for all periods presented. The Company sold its Visudyne business in 2012 and sold its punctal plug drug delivery system technology (PPDS Technology) in 2013. The Company has not had any continued involvement with the Visudyne business or the PPDS Technology following their sale. Amounts billed in connection with the provision of these transition services are included within discontinued operations.
Cash and Cash Equivalents
Cash and cash equivalents consist of highly liquid instruments purchased with an original maturity of three months or less at the date of purchase. As of December 31, 2016 and December 31, 2015, the Company held $108.9 million and $141.8 million in cash and cash equivalents, respectively, consisting of cash and money market funds.
129
Restricted Cash
Restricted cash represents amounts deposited with Silicon Valley Bank (SVB) to collateralize the Company’s corporate credit card program and a letter of credit for the Company’s facility lease in Cambridge, Massachusetts. As of December 31, 2016, $0.4 million was held at SVB as security and hence is presented as restricted cash on the Consolidated Balance Sheet.
Accounts Receivable
The majority of the Company's accounts receivable arise from product sales and primarily represent amounts due from distributors, named patients, and other entities. The Company monitors the financial performance and creditworthiness of large customers to properly assess and respond to changes in their credit profile. The Company provides reserves against account receivables for estimated losses that may result from a customer’s inability to pay. Amounts determined to be uncollectible are charged or written-off against the reserve. To date, the Company's historical reserves and write-offs of accounts receivable have not been significant.
Inventories and Cost of Product Sales
Inventories are stated at the lower of cost or market price with cost determined on a first-in, first-out basis. Inventories acquired in a business combination are required to be fair valued at initial recognition. See "Business Combinations" section below for details.
Inventory is maintained on the Company’s Consolidated Balance Sheets until the inventory is sold, donated as part of the Company’s compassionate use program, or used for clinical development. Inventory that is sold is recognized as cost of product sales in the Consolidated Statements of Operations, inventory that is donated as part of the Company’s compassionate use program is recognized as a selling, general and administrative expense in the Consolidated Statements of Operations, expired inventory is disposed of and the related costs are recognized as cost of product sales in the Consolidated Statements of Operations, and inventory used for clinical development is recognized as research and development expense in the Consolidated Statements of Operations.
Inventories are reviewed periodically to identify slow-moving inventory based on sales activity, both projected and historical, as well as product shelf-life. The portion of the slow-moving inventory not expected to be sold within one year is classified as long-term inventory in the Company's accompanying Consolidated Balance Sheets.
If the asset becomes impaired or is abandoned, the carrying value is written down to its fair value, and an impairment charge is recorded in the period in which the impairment occurs. In evaluating the recoverability of inventories produced, the Company considers the probability that revenue will be obtained from the future sale of the related inventory.
Cost of product sales includes the cost of inventory sold, manufacturing and supply chain costs, product shipping and handling costs, charges for excess and obsolete inventory, amortization of acquired intangibles, as well as royalties payable to The Trustees of the University of Pennsylvania (UPenn) related to the sale of lomitapide and royalties payable to Amgen Inc. (Amgen), Rockefeller University and Bristol-Myers Squibb (BMS) related to the sale of metreleptin.
Contingent Consideration
The contingent consideration is initially recognized and measured at fair value, and are subsequently revalued at the end of each reporting period. Resulting changes in fair value are reported in continuing operations on the Consolidated Statements of Operations and comprehensive loss. See Note 16 - Contingencies, Commitments and Guarantees and Note 14 - Fair Value of Financial Instruments for more information on the Company’s historic contingent consideration asset balance.
Prepaid Manufacturing Costs
Cash advances paid by the Company prior to receipt of the inventory are recorded as prepaid manufacturing costs and included in prepaid expenses and other current assets. The cash advances are subject to forfeiture if the Company terminates the scheduled production. The Company expects the carrying value of the prepaid manufacturing costs to be fully realized. As of December 31, 2016, $1.4 million was recorded as prepaid manufacturing costs and hence was reported under prepaid expenses and other current assets on the Consolidated Balance Sheet. As of December 31, 2015, the Company did not record any prepaid manufacturing costs.
Property and Equipment
Property and equipment are stated at cost and depreciated using the straight-line method based on estimated economic lives of 3 to 5 years for computer software and hardware, and 5 years for office furniture, fixtures, research equipment and other equipment. Leasehold improvements are amortized over the lesser of the estimated useful lives of the improvements or the remaining lease term, which include lease extensions when reasonably assured. Repair and maintenance costs are expensed as incurred.
Intangible Assets
Intangible assets with definite useful lives are amortized to their estimated residual values over their estimated useful lives and reviewed for impairment if certain events occur.
130
Impairment of Long-lived Assets
Impairment testing and assessments of remaining useful lives are performed when a triggering event occurs that could indicate a potential impairment. Such test first entails comparison of the carrying value of the long-lived asset to the undiscounted cash flows expected from that asset. If impairment is indicated by this test, the long-lived assets are written down by the amount, if any, by which the discounted cash flows expected from the long-lived asset exceeds its carrying value.
Business Combinations
The Company evaluates acquisitions of assets and other similar transactions to assess whether or not each such transaction should be accounted for as a business combination by assessing whether or not the Company has acquired inputs and processes that have the ability to create outputs. If the Company determines that an acquisition qualifies as a business, the Company applies the acquisition method of accounting which requires that the purchase price be allocated to the net assets acquired at their respective fair values. Goodwill is calculated as the excess of the consideration transferred over the net assets recognized and represents the future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Goodwill is not amortized and is not deductible for tax purposes. The Company reports provisional amounts when measurements are incomplete as of the end of the reporting period. We complete our purchase price allocation within a measurement period and which does not extend beyond one year after the acquisition date.
Contingent consideration in a business combination is included as part of the acquisition cost and is recognized at fair value as of the acquisition date. Fair value is generally estimated by using a probability-weighted discounted cash flow approach. Any liability resulting from contingent consideration is re-measured to fair value at each reporting date until the contingency is resolved. These changes in fair value are recognized in earnings in other income (expense), net.
The present-value models used to estimate the fair values of acquired inventory and intangibles incorporate significant assumptions, including, but not limited to: assumptions regarding the probability of obtaining marketing approval; estimated selling price, estimates of the timing and amount of future cash flows from potential product sales and related expenses; and the appropriate discount rate selected to measure the risks inherent in the future cash flows, the assessment of the asset’s life cycle and the competitive trends impacting the assets, including consideration of any technical, legal, regulatory or economic barrier.
Transaction costs associated with business combinations are expensed as incurred. The Company's Consolidated Financial Statements include the results from operations of an acquired business after transaction date.
Contingencies
The Company records a liability in the Consolidated Financial Statements for litigation related matters when a loss is considered probable and the amount can be reasonably estimated. If the loss is not probable or a range cannot reasonably be estimated, no liability is recorded in the Consolidated Financial Statements.
Convertible Notes
The accounting guidance for convertible notes requires the Company to separately account for the liability and equity components of the Convertible Notes by allocating the proceeds between the liability component and the embedded conversion option. The carrying amount of the liability component is initially valued at the fair value of a similar liability that does not have an associated convertible feature. The equity component of the Convertible Notes was determined by deducting the fair value of the liability component from the fair value of the Convertible Notes as a whole on the date of acquisition. The excess of the principal amount of the liability component over its carrying amount, or debt discount, is amortized to interest expense using the effective interest method over the life of the Convertible Notes. The equity component is not re-measured as long as it continues to meet the conditions for equity classification.
Contingent Warrants
The Company accounted for the Contingent Warrants in accordance with the guidance regarding the accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock. The Contingent Warrants met the requirements to be accounted for as derivative instruments as the Contingent Warrants are variable and indexed to an event other than the fair value of the Company’s shares. See Note 11 (e) - Share Capital - Cash, Share and Warrant Distributions and Note 11 (d) - Share Capital - Private Placement for more information.
Paid-Up Warrants
The Company accounted for the Paid-Up Warrants issued in the Private Placement in accordance with the guidance regarding the accounting for derivative financial instruments indexed to, and potentially settled in, a company’s own stock. The Paid-Up Warrants met the requirements to be accounted for as equity instruments. The proceeds related to the sale of the Paid-Up Warrants are included in additional paid-in capital in the Consolidated Balance Sheets. See Note 11(d) - Share Capital - Private Placement for more information.
131
Revenue Recognition
The Company applies the revenue recognition guidance in accordance with Financial Accounting Standards Board Accounting Standards Codification (ASC) Subtopic No. 605-15, Revenue Recognition—Products. The Company recognizes revenue from product sales when there is persuasive evidence that an arrangement exists, title to product and associated risk of loss has passed to the customer, the price is fixed or determinable, collectability is reasonably assured and the Company has no further performance obligations.
Lomitapide
In the U.S., JUXTAPID® is only available for distribution through a specialty pharmacy, and is shipped directly to the patient. JUXTAPID is not available in retail pharmacies. Prior authorization and confirmation of coverage level by a patient’s private insurance plan or government payer are currently prerequisites to the shipment of product to the patient in the U.S. Revenue from sales in the U.S. covered by the patient’s private insurance plan or government payer is recognized once the product has been received by the patient. For uninsured amounts billed directly to the patient, revenue is recognized at the time of cash receipt as collectability is not reasonably assured at the time the product is received by the patient. To the extent amounts are billed in advance of delivery to the patient, the Company defers revenue until the product has been received by the patient.
The Company also records revenue on sales in countries where lomitapide is available on a named patient basis, and typically paid for by a government authority or institution. In many cases, these sales are facilitated through a third-party distributor that takes title to the product upon acceptance. Because of factors such as the pricing of lomitapide, the limited number of patients, the short period from product sale to delivery to the end-customer and the limited contractual return rights, these distributors typically only hold inventory to supply specific orders for the product. The Company recognizes revenue for sales under these named patient programs upon product acceptance by either the named patient or the third-party distributor. In the event the payer’s creditworthiness has not been established, the Company recognizes revenue on a cash basis if all other revenue recognition criteria have been met.
The Company records distribution and other fees paid to its distributors as a reduction of revenue, unless the Company receives an identifiable and separate benefit for the consideration and the Company can reasonably estimate the fair value of the benefit received. If both conditions are met, the Company records the consideration paid to the distributor as an operating expense. At this time, neither condition has been met and therefore, the fees paid to the Company’s distributors are recorded as a reduction of revenue. The Company records revenue net of estimated discounts and rebates, including those provided to Medicare, Medicaid, Tricare and other government programs in the U.S. and other countries. Allowances are recorded as a reduction of revenue at the time revenues from product sales are recognized. Allowances for government rebates and discounts are established based on the actual payer information, which is reasonably estimated at the time of delivery. These allowances are adjusted to reflect known changes in the factors that may impact such allowances in the quarter those changes are known.
From time to time, the Company provides financial support to patient assistance programs operated by independent charitable 501(c)(3) organizations which assist patients in the U.S. in accessing treatment for HoFH. These patient assistance programs assist HoFH patients according to eligibility criteria defined independently by the charitable organization. The Company records donations made to these patient assistance programs as selling, general and administrative expense. Any payments received from these patient assistance programs on behalf of a patient who is taking lomitapide for the treatment of HoFH are recorded as a reduction of selling, general and administrative expense rather than as revenue.
Beginning in 2015, the Company also offers a branded co-pay assistance program for eligible patients with commercial insurance in the U.S. who are on JUXTAPID therapy. The branded co-pay assistance program assists commercially insured patients who have coverage for JUXTAPID, and is intended to reduce each participating patient’s portion of the financial responsibility for JUXTAPID’s purchase price up to a specified dollar amount of assistance. The Company records revenue net of amounts paid under the branded specific co-pay assistance program for each patient.
Metreleptin
In the U.S., MYALEPT is only available through an exclusive third-party distributor that takes title to the product upon shipment. MYALEPT is not available in retail pharmacies. The distributor may contractually hold inventory for no more than 21 business days. The Company recognizes revenue for these sales once the product is received by the patient as it is currently unable to reasonably estimate the rebates owed to certain government payers at the time of receipt by the distributor. Prior authorization and confirmation of coverage level by a patient’s private insurance plan or government payer are currently prerequisites to the shipment of product to a patient in the U.S. Revenue from sales in the U.S. covered by the patient’s private insurance plan or government payer is recognized once the product has been received by the patient.
The Company records distribution and other fees paid to its distributor as a reduction of revenue, unless the Company receives an identifiable and separate benefit for the consideration and the Company can reasonably estimate the fair value of the benefit
132
received. If both conditions are met, the Company records the consideration paid to the distributor as an operating expense. At this time, neither condition has been met and therefore, these fees paid to the distributor are recorded as a reduction of revenue. The Company records revenue from sales of MYALEPT net of estimated discounts and rebates, including those provided to Medicare and Medicaid in the U.S. Allowances for government rebates and discounts are established based on the actual payer information, which is reasonably estimable at the time of delivery, and the government-mandated discounts applicable to government-funded programs. These allowances are adjusted to reflect known changes in the factors that may impact such allowances in the quarter those changes are known. To date, such adjustments have not been significant.
From time to time, the Company provides financial support to patient assistance programs operated by independent charitable 501(c)(3) organizations which assist eligible patients in the U.S. in accessing treatment for GL. These patient assistance programs assists GL patients according to eligibility criteria defined independently by the organization. The Company records donations made to these patient assistance programs as selling, general and administrative expense.
Beginning in 2015, the Company also offers a branded co-pay assistance program for eligible patients with commercial insurance in the U.S. who are on MYALEPT therapy. The branded co-pay assistance program assists commercially insured patients who have coverage for MYALEPT, and is intended to reduce each participating patient’s portion of the financial responsibility for MYALEPT’s purchase price up to a specified dollar amount of assistance. The Company records revenue net of amounts paid under the branded specific co-pay assistance program for each patient.
Research and Development Expenses
Research and development expenses are charged to expense as incurred. Research and development expenses comprise costs incurred in performing research and development activities, including personnel-related costs, stock-based compensation, facilities-related overhead, clinical trial costs, costs to support certain medical affairs activities, manufacturing costs for clinical and pre-clinical materials as well as other contracted services, license fees, and other external costs. Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made in accordance with the provisions of ASC No. 730 - Research and Development.
Income Taxes
Income taxes are reported using the asset and liability method, whereby deferred tax assets and liabilities are recognized for the future tax consequences attributable to: (i) differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and (ii) operating loss and tax credit carryforwards using applicable enacted tax rates. An increase or decrease in these tax rates will increase or decrease the carrying value of future net tax assets resulting in an increase or decrease to net income. Income tax credits, such as investment tax credits, are included as part of the provision for income taxes. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities. Significant estimates are required in determining the Company’s provision for income taxes and uncertain tax positions. Some of these estimates are based on interpretations of existing tax laws or regulations. Various internal and external factors may have favorable or unfavorable effects on the Company’s future effective tax rate. These factors include, but are not limited to, changes in tax laws, regulations and/or rates, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, results of tax audits by tax authorities, future levels of research and development spending, changes in estimates related to repatriation of undistributed earnings of foreign subsidiaries, and changes in overall levels of pre-tax earnings. The realization of the Company’s deferred tax assets is primarily dependent on whether the Company is able to generate sufficient capital gains and taxable income prior to expiration of any loss carry forward balance. A valuation allowance is provided when it is more likely than not that a deferred tax asset will not be realized. The assessment of whether or not a valuation allowance is required often requires significant judgment including the long-range forecast of future taxable income and the evaluation of tax planning initiatives. Adjustments to the deferred tax valuation allowances are made to earnings in the period when such assessments are made.
The Company records tax benefits for all years subject to examination based upon management’s evaluation of the facts, circumstances and information available at the reporting date. There is inherent uncertainty in quantifying income tax positions. The Company has recorded tax benefits for those tax positions where it is more likely than not that a tax benefit will result upon ultimate settlement with a tax authority that has all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will result, no tax benefit has been recognized in the Consolidated Financial Statements. See Note 13 - Income Taxes for additional information.
Stock-Based Compensation
The Company accounts for its stock-based compensation to employees in accordance with ASC No. 718 - Compensation - Stock Compensation and to non-employees in accordance with ASC No. 505-50 - Equity-Based Payments to Non-Employees. For service-based awards, compensation expense is recognized using the ratable method over the requisite service period, which is typically the vesting period. For awards that vest or begin vesting upon achievement of a performance condition, the Company recognizes compensation expense when achievement of the performance condition is deemed probable using a straight-line model
133
over the implicit service period. Certain of the Company’s awards that contain performance conditions also require the Company to estimate the number of awards that will vest, which the Company estimates when the performance condition is deemed probable of achievement. For awards that vest upon the achievement of a market condition, the Company recognizes compensation expense over the derived service period. For equity awards that have previously been modified, any incremental increase in the fair value over the original award has been recorded as compensation expense on the date of the modification for vested awards or over the remaining service period for unvested awards. See Note 12 - Stock-Based Payments for further information about the Company’s equity incentive plans.
The Company has a Directors’ Deferred Share Unit Plan ("DDSU Plan") for the Company’s directors. Given that vested Deferred Share Units ("DSUs") are convertible to cash only, the Company recognizes compensation expense for DSUs based on the market price of the Company’s shares. The Company also records an accrued liability to recognize the expected financial obligation related to the future settlement of these DSUs as they vest. Each reporting period, the expected obligation is revalued for changes in the market value of Novelion’s common shares.
The Company issues restricted stock units ("RSUs") to its employees and directors as consideration for their provision of future services. Restricted stock-based compensation expense is measured based on the fair value market price of Novelion’s common shares on the grant date and is recognized over the requisite service period, which coincides with the vesting period. RSUs can only be exchanged and settled for Novelion’s common shares, on a one-to-one basis, upon vesting.
Comprehensive Loss
Comprehensive loss combines net loss and other comprehensive items. Other comprehensive items represent certain amounts that are reported as components of shareholders’ equity in the accompanying Consolidated Balance Sheets, including currency translation adjustments.
Net Loss Per Common Share
Basic net loss per common share is computed using the weighted average number of common shares outstanding during the period. Diluted net income per common share is computed in accordance with the treasury-shares and if-converted methods, which uses the weighted average number of common shares outstanding during the period and also includes the dilutive effect of common shares potentially issuable from outstanding stock-based awards.
Recent Accounting Pronouncements- Not Yet Adopted
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards update (ASU) No. 2014-09, “Revenue from Contracts with Customers” (ASU 2014-09). ASU 2014-09 represents a comprehensive new revenue recognition model that requires a company to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which a company expects to be entitled in exchange for those goods or services. This ASU sets forth a new five-step revenue recognition model which replaces the prior revenue recognition guidance in its entirety and is intended to eliminate numerous industry-specific pieces of revenue recognition guidance that have historically existed. In August 2015, the FASB issued ASU No. 2015-14, “ Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date,” which defers the effective date of ASU 2014-09 by one year, but permits companies to adopt one year earlier if they choose (i.e. the original effective date). As such, ASU 2014-09 will be effective for annual and interim reporting periods beginning after December 15, 2017. In March and April 2016, the FASB issued ASU No. 2016-08 “Revenue from Contracts with Customers (Topic 606): Principal versus Agent Consideration (Reporting Revenue Gross versus Net)” and ASU No. 2016-10 “Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing,” respectively, which clarify the guidance on reporting revenue as a principal versus agent, identifying performance obligations and accounting for intellectual property licenses. In addition, in May 2016, the FASB issued ASU No. 2016-12 “Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients,” which amends certain narrow aspects of Topic 606, and in December 2016, the FASB issued ASU No. 2016-20 “Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers,” which amends certain narrow aspects of Topic 606. The new standard may be adopted using either the full retrospective method, in which case the standard would be applied to each prior reporting period presented, or the modified retrospective method, in which case the cumulative effect of applying the standard would be recognized at the date of initial application. In the fourth quarter of 2016, the Company engaged an external accounting firm to assist with the new standard adoption and has made significant progress in the assessment. Based on the progress, the Company expects to complete its assessment by the second quarter of 2017.
In July 2015, the FASB issued ASU No. 2015-11, “Simplifying the Measurement of Inventory” (ASU 2015-11). ASU 2015-11 states that an entity should measure inventory at the lower of cost or net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. For public entities, ASU 2015-11 is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The amendments in this update should be applied prospectively and early application is permitted. The Company
134
does not expect the adoption of ASU 2015-11 to impact the Company’s consolidated results of operations and financial position.
On February 25, 2016, the FASB issued ASU No. 2016-02 - Leases, its new standard on accounting for leases. The new guidance will require organizations that lease assets (referred to as lessees) for terms of more than 12 months, to recognize on the balance sheet the assets and liabilities associated with the rights and obligations created by those leases. Consistent with current guidance, the recognition, measurement, and presentation of the expenses and cash flows associated with a particular lease will depend on its classification as a capital or operating lease. However, unlike current GAAP, which only requires capital leases to be reflected on the balance sheet, ASU No. 2016- 02 will require both types of leases to be recognized on the balance sheet. ASU No. 2016-02 also aligns many of the underlying principles of the new lessor model with those in ASC No. 606 - Revenue from Contracts with Customers, and will require lessors to increase the transparency of their exposure to changes in value of their residual assets and how they manage the associated exposure. ASU No. 2016-02 will be effective for annual periods beginning after December 15, 2018, and interim periods within those annual reporting periods. Management is currently assessing the impact ASU No. 2016-02 will have on the Company’s Consolidated Financial Statements.
On March 30, 2016, the FASB issued ASU No. 2016-09 - Improvements to Employee Stock-Based Payment Accounting, ASU 2016-09 changes how companies account for certain aspects of share-based payments to employees including: (a) requiring all income tax effects of awards to be recognized in the income statement, rather than in additional paid in capital, when the awards vest or are settled, (b) eliminating the requirement that excess tax benefits be realized before companies can recognize them, (c) requiring companies to present excess tax benefits as an operating activity on the statement of cash flows rather than as a financing activity, (d) increasing the amount an employer can withhold to cover income taxes on awards and still qualify for the exception to liability classification for shares used to satisfy the employer’s statutory income tax withholding obligation, (e) requiring an employer to classify the cash paid to a tax authority when shares are withheld to satisfy its statutory income tax withholding obligation as a financing activity on its statement of cash flows and (f) electing whether to account for forfeitures of share-based payments by (1) recognizing forfeitures of awards as they occur or (2) estimating the number of awards expected to be forfeited and adjusting the estimate when it is likely to change, as is currently required. ASU 2016-09 is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. The Company has adopted the new guidance on January 1, 2017. Upon adoption the Company will account for forfeitures when they occur, instead of estimating the number of awards that are expected to vest under current GAAP. The Company will retrospectively adopt the provision of this guidance related to forfeitures by utilizing the modified retrospective transition method. The adoption of ASU No. 2016-09 will not materially impact the Company’s Consolidated Financial Statements.
On August 26, 2016, the FASB issued ASU No. 2016-15 - Classification of Certain Cash Receipts and Cash Payments, which amends the guidance in ASC No. 230 on the classification of certain items in the statement of cash flows. The primary purpose of ASU No. 2016-15 is to reduce the diversity in practice by making amendments that add or clarify the guidance on eight specific cash flow issues. ASU No. 2016-15 is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. ASU No. 2016-15 must be applied retrospectively to all periods presented, but may be applied prospectively from the earliest date practicable if retrospective application would be impracticable. Management is currently assessing the impact ASU No. 2016-15 will have on the Company’s Consolidated Financial Statements.
On October 24, 2016, the FASB issued ASU No. 2016-16 - Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other than Inventory, which improves the accounting for the income tax consequences of intra-entity transfers of assets other than inventory and eliminates the exception for an intra-entity transfer of an asset other than inventory. ASU No. 2016-16 is effective for annual reporting periods beginning after December 15, 2017, including interim reporting periods within those annual reporting periods. ASU No. 2016-16 should be applied on a modified retrospective basis through a cumulative-effect adjustment directly to retained earnings as of the beginning of the period of adoption. Upon adoption, prior periods will be retrospectively adjusted. Management does not expect the adoption of ASU No. 2016-16 will materially impact the Company’s Consolidated Financial Statements.
On November 17, 2016, the FASB issued ASU No. 2016-18 “Statement of Cash Flows (Topic 230) - Restricted Cash” (ASU 2016-18). ASU 2016-18 states that a statement of cash flows should explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. Therefore, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. ASU 2016-18 is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted, including adoption in an interim period, and all updates should be applied using a retrospective transition method. The Company is currently evaluating the impact ASU 2016-18 will have on the Company’s Consolidated Statement of Cash Flows.
On January 5, 2017, the FASB issued ASU No. 2017-01 Business Combinations (Topic 805): Clarifying the Definition of a Business, which provides a more robust framework to use in determining when a set of assets and activities is a business. It also provides more consistency in applying the guidance, reduces the costs of application and makes the definition of a business more operable. ASU No. 2017-01 is effective for annual periods beginning after December 15, 2017, including interim periods within those
135
periods. Management is currently assessing the impact ASU No. 2017-01 will have on the Company’s Consolidated Financial Statements.
Recently Adopted Accounting Pronouncements
On September 25, 2015, the FASB issued Accounting Standards Update (ASU) No. 2015-16 - Business Combinations (Topic 805) Simplifying the Accounting for Measurement-Period Adjustments. Under the guidance, an acquirer must recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. ASU No. 2015-16 also requires acquirers to present separately on the face of the income statement, or disclose in the notes, the portion of the amount recorded in current-period earnings by line item that would have been recorded in previous reporting periods if the adjustment to the provisional amounts had been recognized as of the acquisition date. ASU No. 2015-16 was effective for annual periods, and interim periods, beginning after December 15, 2015 and did not impact the Company’s financial position or results of operations.
3. Terminated Merger Transactions.
On June 8, 2015, QLT entered into an Agreement and Plan of Merger (as amended and restated on each of July 16, 2015 and August 26, 2015) (the InSite Merger Agreement) with InSite Vision Incorporated, a Delaware corporation (InSite). On September 15, 2015, the InSite Merger Agreement was terminated by InSite's board of directors. As a result, InSite paid QLT a termination fee of $2.7 million. In addition, in conjunction with the entry into the InSite Merger Agreement, on June 8, 2015 QLT granted InSite a secured line of credit (the Secured Note) for up to $9.9 million to fund continuing operations through to the completion of the proposed InSite merger. Upon termination of the InSite Merger Agreement, InSite’s repayment obligations under the Secured Note were accelerated and InSite paid QLT $5.8 million on September 15, 2015, which consisted of $5.7 million of principal drawn from the Secured Note and $0.1 million of accrued interest.
On June 25, 2014, the Company entered into an Agreement and Plan of Merger (the Auxilium Merger Agreement) with Auxilium Pharmaceuticals, Inc., a Delaware corporation (Auxilium). On October 8, 2014, the Auxilium Merger Agreement was terminated by Auxilium's board of directors. In connection with the termination of the Auxilium Merger Agreement, on October 9, 2014, Auxilium paid QLT a termination fee of $28.4 million. On October 22, 2014, pursuant to the terms of QLT's financial advisory services agreement with Credit Suisse Securities (USA) LLC (Credit Suisse), QLT paid Credit Suisse a portion of the breakup fee equal to $5.7 million. QLT’s financial advisory services agreement with Credit Suisse was subsequently terminated.
During the year ended December 31, 2015 and 2014, QLT incurred $10.2 million and $9.4 million of consulting and transaction fees in connection with QLT’s pursuit of the Auxilium and InSite Mergers, respectively. These $19.6 million of consulting and transaction fees, which is net of the $5.7 million portion of the breakup fee paid to Credit Suisse, has been reflected as part of Selling, General and Administrative expenses on the Consolidated Statements of Operations.
4. Strategic Transactions.
Aralez Investment and Distribution
On December 7, 2015, QLT entered into an Amended and Restated Share Subscription Agreement (the Amended and Restated Subscription Agreement) with Tribute Pharmaceuticals Canada Inc. (Tribute), POZEN Inc. (POZEN), Aralez Pharmaceuticals plc, (formally known as Aguono Limited) (Aralez Ireland) and certain other investors for the purpose of returning capital to QLT's shareholders in either Aralez Shares (approximately 0.13629 of an Aralez Share for each common share of the Company) or cash, subject to pro-ration (the Aralez Distribution), up to a maximum of $15.0 million funded pursuant to the terms of the Backstop Agreement (as described below).
In connection with the Aralez Distribution, on June 8, 2015, QLT entered into a share purchase agreement (as amended, the Backstop Agreement) with Broadfin Healthcare Master Fund, Ltd. (Broadfin) and the JW Partners, LP, JW Opportunities Fund, LLC and JW Opportunities Master Fund, Ltd. (together the JW Parties), pursuant to which Broadfin and the JW Parties agreed to purchase up to $15.0 million of the Aralez Shares from the QLT at $6.25 per share. This arrangement provided QLT’s shareholders with the opportunity to elect to receive, in lieu of Aralez Shares, up to an aggregate of $15.0 million in cash, subject to proration among the shareholders.
On February 5, 2016, QLT purchased 7,200,000 Aralez Shares (representing 10.1% of the issued and outstanding Aralez Shares), for an aggregate price of $45.0 million. On April 5, 2016 (the Distribution Date), QLT distributed 4,799,619 Aralez Shares with a fair value of $19.3 million, and $15.0 million of cash to shareholders of record on February 16, 2016.
136
QLT held the Aralez Shares from February 5, 2016 to the Distribution Date and the Aralez Shares were marked-to-market. As a result, QLT recognized a $10.7 million loss during the year ended December 31, 2016, to reflect the change in value from the acquisition date to the Distribution Date.
Pursuant to QLT’s financial advisory services agreement with Greenhill dated December 4, 2014 (as amended, the Greenhill Agreement), QLT paid Greenhill a $4.0 million advisory fee in connection with the completion of QLT’s $45.0 million investment in Aralez and exploration of other strategic initiatives described under Note 3 - Terminated Merger Transactions.
Private Placement related to Aralez
On June 8, 2015, QLT entered into a Share Purchase and Registration Rights Agreement (as amended, the Share Purchase and Registration Rights Agreement) with Broadfin, JW Partners, LP, JW Opportunities Fund, LLC, EcoR1 Capital Fund Qualified, L.P. and EcoR1 Capital Fund, LP (the QLT Investors). The Share Purchase and Registration Rights Agreement provided that QLT would, following the completion of the Aralez Distribution, issue and sell to the QLT Investors a certain number of QLT common shares for an aggregate purchase price of $20.0 million, reflecting a per share purchase price of $1.87. In light of the termination of the InSite Merger Agreement and the board’s determination that QLT’s cash requirements at that time did not justify the dilution that would be caused by this private placement, on April 28, 2016, QLT and the QLT Investors mutually agreed to terminate the Share Purchase and Registration Rights Agreement.
5. Acquisition.
Aegerion Pharmaceuticals, Inc.
On November 29, 2016, Novelion completed its acquisition of Aegerion and each share of Aegerion’s common stock was exchanged for 1.0256 Novelion (pre-Consolidation) common shares (the Exchange Ratio). Immediately after the Merger, the Company had approximately 18,530,323 common shares outstanding; former shareholders of Novelion held approximately 68% of the Company, and former stockholders of Aegerion held approximately 32% of the Company.
The Merger has been accounted for as a business combination under the acquisition method, with Novelion as the accounting acquirer and Aegerion as the “acquired” company. The operating results of Aegerion from November 29, 2016 are included in the accompanying Consolidated Statements of Operations for the year ended December 31, 2016. The Consolidated Balance Sheet as of December 31, 2016 reflect the acquisition of Aegerion, effective November 29, 2016.
The acquisition consideration in connection with the Merger was approximately $62.4 million and consisted of the following (in thousands, except for share and per share information):
Number of Novelion common shares issued in connection with the acquisition of Aegerion | 6,060,288 | ||||||
Novelion share price on November 29, 2016 | $ | 9.75 | |||||
Fair value of Novelion common shares issued to Aegerion stockholders | $ | 59,088 | |||||
Liability assumed (2)(3) | 3,000 | ||||||
Stock compensation assumed (1) | 293 | ||||||
Total acquisition consideration | $ | 62,381 | |||||
(1) The fair value of Aegerion in-the-money options and RSUs attributed to pre-combination services that were outstanding on November 29, 2016 and settled in connection with the Merger.
(2) Represents a term loan facility provided by QLT to Aegerion on June 14, 2016, concurrently with the execution of the Merger Agreement. Aegerion borrowed $3 million against the term loan and the loan remained outstanding as of November 29, 2016.
(3) Includes 10,565,879 Merger Agreement Warrants to purchase up to 11,301,791 common shares issued pursuant to the Merger Agreement, which were recognized as a liability with a fair value of zero as of November 29, 2016. Refer to Note 11 - Share Capital and Note 16 - Contingencies, Commitments and Guarantees for further details.
The estimated fair value of the assets acquired and liabilities assumed are provisional as of December 31, 2016 and are based on information that is currently available to the Company. Additional information is being gathered to finalize these provisional measurements, particularly with respect to intangible assets, inventory, and deferred income taxes. Accordingly, the measurement of the assets acquired and liabilities assumed may change significantly upon finalization of the Company’s valuations and completion of the purchase price allocation, both of which are expected to occur no later than one year from the acquisition date. The following table summarizes the provisional fair values of the assets acquired and liabilities assumed at the Merger date (in thousands):
137
November 29, 2016 | ||||||||
Cash and cash equivalents | $ | 28,290 | ||||||
Restricted cash | 390 | |||||||
Accounts receivable (1) | 8,182 | |||||||
Inventories | 76,800 | |||||||
Prepaid expenses and other current assets | 9,839 | |||||||
Insurance proceeds receivable | 22,000 | |||||||
Property and equipment, net | 4,020 | |||||||
Intangible assets | 252,458 | |||||||
Other Assets | 1,352 | |||||||
Accounts payable | (11,459 | ) | ||||||
Accrued liabilities | (41,883 | ) | ||||||
Provision for legal settlement | (63,968 | ) | ||||||
Long-term debt | (222,908 | ) | ||||||
Other liabilities | (732 | ) | ||||||
Net assets acquired | $ | 62,381 | ||||||
(1) As of the Merger date, the fair value of accounts receivable approximated the book value acquired. The amount not expected to be collected was insignificant.
• | Legal Matters - Aegerion has been the subject of certain ongoing investigations and other legal proceedings. See Note 16 - Contingencies, Commitments and Guarantees for further information regarding these and other legal proceedings. |
• | Tax Matters - Net liabilities for income taxes payable approximated $0.1 million and unrecognized tax benefits approximated $0.9 million as of the acquisition date. A net deferred tax asset related to Aegerion’s foreign subsidiaries approximated $1.1 million as of the acquisition date. |
The valuation of the intangible assets acquired and related amortization periods are as follows:
Valuation (in thousands) | Amortization period (in years) | ||||
Developed Technology: | |||||
JUXTAPID | $ | 42,300 | 10.75 | ||
MYALEPT | 210,158 | 9.25 | |||
Total | $ | 252,458 | |||
The preliminary fair values of the intangibles were estimated using a multi-period excess earnings approach. Under this method, an intangible assets fair value is equal to the present value of the after-tax cash flows attributable solely to the intangible asset over its remaining useful life. To calculate fair value, the Company used cash flows discounted at 11%.
The fair values of the purchased inventories were also estimated using a discounted present value income approach. To calculate fair value, the Company used cash flows discounted at 11%. There was no goodwill recorded as part of the acquisition of Aegerion on the acquisition date.
Novelion recognized acquisition-related transaction costs associated with the Merger during the year ended December 31, 2016 totaling approximately $4.0 million. These costs, which related primarily to bank fees, legal and accounting services, and fees for other professional services, were expensed as incurred, and reported as the selling, general and administrative expenses (SG&A) in the accompanying Consolidated Statements of Operations.
Actual and Pro Forma Impact of Acquisition
The following table presents the amount of Aegerion net product sales and net loss included in the Company's Consolidated Statement of Operations from November 29, 2016 through December 31, 2016:
138
(in millions, except for per share information) | November 29, 2016 - December 31, 2016 | ||||
Net product sales | $ | 13.6 | |||
Net loss | (6.3 | ) | |||
Basic and diluted net loss per share | $ | (0.34 | ) | ||
The following supplemental unaudited pro forma information presents the financial results as if the Merger had occurred on January 1, 2015 for the years ended December 31, 2016 and 2015.
Unaudited Supplemental Pro Forma Consolidated Results | |||||||
Year ended December 31, | |||||||
(in millions, except for per share information) | 2016 | 2015 | |||||
Net product sales | $ | 153.2 | $ | 239.9 | |||
Net loss | (207.8 | ) | (96.3 | ) | |||
Basic and diluted loss per share | $ | (18.41 | ) | $ | (9.23 | ) | |
This supplemental pro forma information has been prepared for comparative purposes and does not purport to reflect what the Company’s results of operations would have been had the acquisition occurred on January 1, 2015, nor does it project the future results of operations of the Company or reflect the expected realization of any cost savings associated with the acquisition. The actual results of operations of the Company may differ significantly from the pro forma adjustments reflected here due to many factors. The unaudited supplemental pro forma financial information includes various assumptions, including those related to the provisional purchase price allocation of the assets acquired and the liabilities assumed from Aegerion.
6. Inventories.
The components of inventory are as follows:
December 31, | |||||||
2016 | 2015 | ||||||
(in thousands) | |||||||
Work-in-process | $ | 20,219 | $ | — | |||
Finished goods | 54,502 | — | |||||
Total | $ | 74,721 | $ | — | |||
As part of the Merger, the Company acquired $76.8 million of inventory. During the years ended December 31, 2016, 2015 and 2014, the Company did not record any expense related to the excess or obsolete inventory in the Consolidated Statements of Operations.
7. Property and Equipment.
Property and equipment consists of the following:
December 31, 2016 | |||||||||||
(in thousands) | Cost | Accumulated Depreciation | Net Book Value | ||||||||
Leasehold improvements | $ | 1,869 | $ | 263 | $ | 1,606 | |||||
Office furniture and equipment | 539 | 20 | 519 | ||||||||
Research equipment | 1,962 | 1,810 | 152 | ||||||||
Computer and office equipment | 10,236 | 8,693 | 1,543 | ||||||||
Construction in progress | 339 | $ | — | 339 | |||||||
$ | 14,945 | $ | 10,786 | $ | 4,159 | ||||||
139
December 31, 2015 | |||||||||||
(in thousands) | Cost | Accumulated Depreciation | Net Book Value | ||||||||
Leasehold improvements | $ | 207 | $ | 207 | $ | — | |||||
Office furniture and equipment | 258 | 246 | 12 | ||||||||
Research equipment | 3,471 | 3,092 | 379 | ||||||||
Computer and office equipment | 9,844 | 9,805 | 39 | ||||||||
$ | 13,780 | $ | 13,350 | $ | 430 | ||||||
Depreciation expense was $0.3 million, $0.6 million and $0.9 million for the years ended December 31, 2016, 2015 and 2014, respectively.
As part of the Merger, the Company acquired $4.0 million of property and equipment.
In connection with the downsizing of the Company’s lease space in 2015 (see Note 16 - Contingencies, Commitments and Guarantees for more information), the Company retired the use of certain property, plant and equipment with zero or minimal net book values.
8. Intangible Assets.
The following is a summary of intangible assets held by the Company at December 31, 2016 and 2015 (in thousands):
Cost basis: | Balance as of December 31, 2015 | 2016 Acquisitions | Balance as of December 31, 2016 | ||||||
Definite-lived intangibles: | |||||||||
Developed Technology - Juxtapid (weighted average life of 10.75 years) | $ | — | $ | 42,300 | $ | 42,300 | |||
Developed Technology - Myalept (weighted average life of 9.25 years) | — | 210,158 | 210,158 | ||||||
Total definite-lived intangibles (weighted average life of 9.50 years) | $ | — | $ | 252,458 | $ | 252,458 | |||
Accumulated amortization: | Balance as of December 31, 2015 | 2016 Amortization | Balance as of December 31, 2016 | ||||||
Definite-lived intangibles: | |||||||||
Developed Technology - Juxtapid | $ | — | $ | (328 | ) | $ | (328 | ) | |
Developed Technology - Myalept | — | (1,806 | ) | (1,806 | ) | ||||
Total definite-lived intangibles | $ | — | $ | (2,134 | ) | $ | (2,134 | ) | |
Net intangible assets | $ | — | $ | 250,324 | |||||
As part of the Merger, the Company acquired $252.5 million of intangible assets. Amortization expense for the year ended December 31, 2016 totaled $2.1 million, and zero for the years ended December 31, 2015 and 2014, respectively. Estimated amortization of intangible assets for the five fiscal years subsequent to December 31, 2016 is as followings (in thousands):
Years Ending December 31, | Estimated Amortization of Intangible Assets | |||
2017 | $ | 25,614 | ||
2018 | 25,614 | |||
2019 | 25,614 | |||
2020 | 25,614 | |||
2021 | $ | 25,614 | ||
140
Novelion will test definite-lived intangible assets for impairment when events and changes in circumstances indicate that the carrying amount of these definite-lived intangible assets may not be recoverable. Impairment loss is recognized if the carrying value of definite-lived intangible is not recoverable and its carrying value exceeds its fair value. Any impairment charges resulting from intangible asset impairment assessments are recorded to asset impairments charges on the Company’s Consolidated Statements of Operations.
9. Accrued Liabilities.
Accrued liabilities consist of the following:
December 31 | |||||||
(in thousands) | 2016 | 2015 | |||||
Accrued employee compensation and related costs | $ | 7,920 | $ | 1,460 | |||
Accrued sales allowances | 7,849 | — | |||||
Other accrued liabilities | 21,411 | 367 | |||||
Total | $ | 37,180 | $ | 1,827 | |||
10. Convertible Notes, Net.
In August 2014, Aegerion issued Convertible Notes with an aggregate principal amount of $325.0 million. The Convertible Notes are governed by the terms of an indenture and a supplemental indenture between Aegerion and The Bank of New York Mellon Trust Company, N.A., as the Trustee. The following are the key terms of the Convertible Notes:
• | The Convertible Notes are senior unsecured obligations of Aegerion and bear interest at a rate of 2.0% per year, payable semi-annually in arrears on February 15 and August 15 of each year, beginning on February 15, 2015. The Convertible Notes will mature on August 15, 2019, unless earlier repurchased or converted. |
• | After the Merger, the Convertible Notes are convertible into the Company’s common shares at a conversion rate of 4.9817 common shares per $1,000 principal amount of the Convertible Notes, as adjusted for the Exchange Ratio and the Consolidation. Aegerion can, at its election, settle the conversion of the Convertible Notes through payment or delivery of cash, common shares, or a combination of cash and common shares. |
• | On or after February 15, 2019 until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or any portion of their Convertible Notes, in multiples of $1,000 principal amount, at the option of the holder. |
• | The indenture does not contain any financial covenants or restrict the Company’s ability to repurchase the Company’s securities, pay dividends or make restricted payments in the event of a transaction that substantially increases the Company’s level of indebtedness. |
• | The indenture contains customary terms and covenants and events of default. If an event of default (other than certain events of bankruptcy, insolvency or reorganization involving Aegerion) occurs and is continuing, the Trustee by notice to Aegerion, or the holders of at least 25% in principal amount of the outstanding Convertible Notes by written notice to Aegerion and the Trustee, may declare 100% of the principal of and accrued and unpaid interest, if any, on all of the Convertible Notes to be due and payable. Upon such a declaration of acceleration, such principal and accrued and unpaid interest, if any, will be due and payable immediately. Upon the occurrence of certain events of bankruptcy, insolvency or reorganization involving Aegerion, 100% of the principal and accrued and unpaid interest, if any, on the Convertible Notes will become due and payable automatically. Notwithstanding the foregoing, the indenture provides that, upon Aegerion’s election, and for up to 180 days, the sole remedy for an event of default relating to certain failures by Aegerion to comply with certain reporting covenants in the indenture consists exclusively of the right to receive additional interest on the Convertible Notes. |
On November 29, 2016, the Company completed its acquisition of Aegerion. The acquisition method of accounting requires an accounting acquirer to measure liabilities assumed at fair value on the acquisition date, as such, both the liability and equity component of the Convertible Notes were re-measured at fair value at the Merger date. The fair value of the Convertible Notes as of the Merger date was approximately $222.9 million, which was determined by taking into consideration valuations obtained from third-party pricing services. The pricing services utilize pricing model incorporating variables such as coupon, maturity, delta, conversion ratio, parity, corporate actions and equity market closing prices to calculate conversion premiums and sensitivity values and to generate the fair value of the Convertible Notes. As of the Merger date, management attributed the fair value entirely
141
to the liability component of the Convertible Notes for the following reasons: 1) as of the Merger date, the conversion price ($200.74) was significantly higher than the price of Novelion common shares ($9.75), and 2) management did not expect the price of Novelion common shares to raise above the conversion price before the Convertible Notes expire in August 2019. Novelion recorded $222.9 million as the opening balance for the liability component of the Convertible Notes and reported zero balance for the equity component of the Convertible Notes post-Merger on its Consolidated Financial Statements.
The Company’s outstanding Convertible Notes balances as of December 31, 2016 consisted of the following (in thousands):
Liability component: | |||
Principal | $ | 324,998 | |
Less: debt discount | (99,414 | ) | |
Net carrying amount | $ | 225,584 | |
Equity component | $ | — | |
(1) Represents interest payable within one year after December 31, 2016.
The Company determined that the expected life of the debt was equal to the five year term on the Convertible Notes. The effective interest rate on the liability component was 16.42% for the period from the acquisition date through December 31, 2016. The following table sets forth total interest expense recognized related to the Convertible Notes during the year ended December 31, 2016 (in thousands):
Years Ending December 31, 2016 | |||
Contractual interest expense | $ | 577 | |
Amortization of debt discount | 2,676 | ||
Total | $ | 3,253 | |
Future minimum payments under the Company’s Convertible Notes are as follows (in thousands):
Years Ending December 31, | |||
2017 | $ | 6,500 | |
2018 | 6,500 | ||
2019 | 329,060 | ||
342,060 | |||
Less amounts representing interest | (17,062 | ) | |
Less amortization of debt discount, net | (99,414 | ) | |
Net carrying amount of convertible notes | $ | 225,584 | |
11. Share Capital.
(a) Authorized Shares
At December 31, 2016 and 2015, the Company was authorized to issue 100 million shares without par value. Dividends on the common shares will be paid when, and if, declared by the Board of Directors. Each holder of common shares is entitled to vote on all matters and is entitled to one vote for each share held.
The Company will, at all times, reserve and keep available, out of its authorized but unissued common share, sufficient shares to affect the conversion of shares for stock options, restricted stock units, convertible notes and warrants.
(b) Share Consolidation
On December 16, 2016, the Company effected a one-for-five consolidation of all of its issued and outstanding common shares without par value. As noted above, all share and per-share data presented in the Company’s Consolidated Financial Statements and notes have been retrospectively restated to reflect the Consolidation unless otherwise noted. Shares reserved and outstanding under the Company’s equity and incentive plans were adjusted to reflect the Consolidation.
142
(c) Merger Consideration
On November 29, 2016, an indirect wholly-owned subsidiary of Novelion acquired Aegerion for total consideration of $62.4 million. The consideration included 6,060,288 Novelion common shares valued at $59.1 million and 10,565,879 Merger Agreement Warrants exercisable for up to an aggregate of 11,301,791 common shares at an exercise price of $0.05 per share that were valued at zero on November 29, 2016 on the Company’s Consolidated Balance Sheet. Refer to Note 5 - Acquisition for additional information.
(d) Private Placement
Also on June 14, 2016, the Company entered into the Unit Subscription Agreement with the Investors. Pursuant to the Unit Subscription Agreement, immediately prior to the Merger, the Investors acquired units, for $8.80 per unit, consisting of (i) 2,472,727 Novelion common shares, which includes up to 568,181 Novelion common shares issuable upon exercise of the 531,208 Paid-Up Warrants, and (ii) 2,472,727 Unit Subscription Agreement Warrants exercisable for a maximum of 2,644,952 Novelion common shares at an exercise price of $0.05 per common share. The net consideration received from the private placement was $21.5 million ($21.8 million gross consideration net of $0.3 million share issuance cost), which was recorded as equity and allocated based on the relative fair values of the common shares and the Paid-Up Warrants at the time of issuance. The Unit Subscription Agreement Warrants were issued on the same terms and conditions as the Merger Agreement Warrants. As of December 31, 2016, the Unit Subscription Agreement Warrants were recognized as a liability on the Company's Consolidated Balance Sheet with a fair value of zero.
(e) Cash, Share and Warrant Distributions
On April 5, 2016, based on the shareholder election, Novelion distributed $15 million of cash and 4,799,619 Aralez Shares, with a fair value of $19.3 million, to its shareholders of record on February 16, 2016 according to the Amended and Restated Subscription Agreement.
On November 23, 2016, the Company issued 10,565,879 Merger Agreement Warrants to its shareholders of record on November 17, 2016. As of December 31, 2016, the Merger Agreement Warrants were recognized as a liability on the Company's Consolidated Balance Sheet with a fair value of zero.
12. Stock-Based Payments.
The Company currently maintains one equity incentive plan, the Novelion 2016 Equity Incentive Plan (the NVLN Plan), formerly known as QLT 2000 Incentive Stock Plan, under which it may grant non-qualified stock options, incentive stock options, and restricted stock units (“RSUs”) to employees, directors and consultants of Novelion and its affiliates. Common shares of Novelion will be issued upon exercise of stock options and the vesting of RSUs.
The Company issues stock options and RSUs with service conditions, which are generally the vesting periods of the awards. The Company has also issued RSUs that vest upon the satisfaction of certain performance conditions or the satisfaction of certain market conditions.
No financial assistance is provided by Novelion to the participants under the NVLN Plan. Under the terms of the NVLN Plan, Novelion is entitled to grant awards in respect of its unissued common shares up to a maximum of 4,760,000 shares.
In connection with the Merger, Novelion assumed the Aegerion 2010 Stock Option and Incentive Plan (Aegerion’s 2010 Option Plan) from Aegerion. On the closing of the Merger, on November 29, 2016, all the outstanding out-of-the-money Aegerion stock options were cancelled. All the outstanding and unexercised in-the-money Aegerion stock options were exchanged for adjusted options of Novelion and all the outstanding Aegerion RSUs held by Aegerion RSU holders were exchanged for adjusted RSUs of Novelion, in each case appropriately adjusted to reflect the Exchange Ratio.
Aegerion’s 2010 Option Plan was approved by Aegerion’s stockholders in October 2010. Aegerion’s 2010 Option Plan allowed Aegerion to make grants of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock awards, restricted stock units, unrestricted stock awards, cash-based awards, performance share awards and dividend equivalent rights. All full-time and part-time officers, employees, non-employee directors and other key persons, including consultants and prospective employees, of Aegerion were eligible to participate in Aegerion’s 2010 Option Plan.
The Company does not plan to issue additional awards under Aegerion’s 2010 Option Plan. There are 10,561 options and 133,351 RSUs outstanding under Aegerion’s 2010 Stock Option Plan as of December 31, 2016.
Determining the Fair Value of Stock Awards
(a) Stock Options
143
The fair value of stock options is measured with service-based and performance-based vesting criteria to employees, consultants and directors on the date of grant using the Black-Scholes option pricing model.
The Black-Scholes option pricing model was developed for use in estimating the value of traded options that have no vesting restrictions and are fully transferable. In addition, option pricing models require the input of highly subjective assumptions, including the expected stock price volatility. The expected volatility and expected life of the Company’s stock options is projected based upon historical and other economic data trended into future years. The risk-free interest rate assumption is based upon observed interest rates appropriate for the expected life of the Company’s stock options. The risk-free interest rate is determined by reference to implied yields available from U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant. Forfeitures are estimated based on the Company’s historical analysis of both options and awards that forfeited prior to vesting.
The weighted-average grant date fair values of stock options granted during the years ended December 31, 2016, 2015 and 2014, were USD $7.12, CAD $10.60, and CAD $9.35, respectively.
The following weighted-average assumptions were used to value stock options granted in each of the following years:
Years Ended December 31, | ||||||||
2016 | 2015 | 2014 | ||||||
Expected share price volatility | 38.23 | % | 41.30 | % | 42.40 | % | ||
Risk-free interest rate | 1.93 | % | 1.40 | % | 1.60 | % | ||
Expected life of options (years) | 6.19 | 6.80 | 6.80 | |||||
Expected dividend yield | — | — | — | |||||
As of December 31, 2016, 205,030 options were outstanding under the NVLN Plan, which are exercisable at prices ranging between $7.15 and $35.51 per common share. The number of options issued and outstanding under the NVLN Plan represents 9.1% (2015 - 0.8%, 2014 - 4.1%) of the Company’s issued and outstanding common shares.
The Company’s stock option activity for the year ended December 31, 2016 is as follows:
Weighted | ||||||||||||
Weighted- | Average | |||||||||||
Average | Remaining | Aggregate | ||||||||||
Number of | Exercise Price | Contractual | Intrinsic Value (2) | |||||||||
stock options (2) | Per Share (2) | Life (years) | (in thousands) | |||||||||
Outstanding at December 31, 2015 (3) | 85,630 | C$ | 25.35 | 8.26 | C$ | — | ||||||
Granted (1) | 1,629,563 | $ | 8.27 | |||||||||
Exercised | — | $ | — | $ | — | |||||||
Forfeited/cancelled | (600) | $ | 25.35 | |||||||||
Outstanding at December 31, 2016 | 1,714,593 | $ | 9.01 | 9.65 | $ | 528 | ||||||
Vested and expected to vest at December 31, 2016 | 1,231,203 | $ | 9.24 | 9.58 | $ | 437 | ||||||
Exercisable at December 31, 2016 | 205,030 | $ | 13.86 | 8.49 | $ | 140 | ||||||
(1) The Aegerion stock options assumed by the Company in connection with the Merger are included in grants made during the year ended December 31, 2016 in the above table. The total number of assumed Aegerion stock options was 10,561 with a weighted-average exercise price of $7.70 per share.
(2) Shares and per share amounts have been retrospectively restated for all prior periods presented to reflect the one-for-five share consolidation.
(3) Intrinsic value and exercise price of the stock options granted before 2016 were based on the underlying Novelion common shares listed on the Toronto Stock Exchange (TSX), and thus in Canadian dollars. Stock options granted in 2016 and after are and will be based on the underlying Novelion common shares listed on NASDAQ and thus in USD.
In connection with the strategic transactions announced on June 8, 2015 as described under Note 3 - Terminated Merger Transactions and Note 4 - Strategic Transactions, on June 7, 2015 the Board of Directors accelerated the vesting provisions applicable to 217,294 options outstanding and unvested at that date. The impact of the accelerated vesting of these stock options on stock-based compensation expense for the year ended December 31, 2015 was $1.5 million.
1,509,563 stock options were outstanding and unvested at December 31, 2016 (2015 - zero , 2014 - 256,796) and the total estimated unrecognized compensation cost related to unvested stock options is $4,922,028 (2015 - zero, 2014 - $2,021,000)and the weighted-
144
average period over which such costs are expected to be recognized is 2.83 years (December 31, 2015 - 0 years, December 31, 2014 - 2.20 years).
The aggregate intrinsic values of options outstanding and exercisable as of December 31, 2016, 2015, and 2014 are as follows:
(In thousands) | 2016 | 2015 | 2014 | ||||||||
Aggregate intrinsic value of options outstanding (1) | $ | 528 | C$ | — | C$ | 2,435 | |||||
Aggregate intrinsic value of options exercisable (1) | 140 | — | 240 | ||||||||
(1) Intrinsic value of the stock options granted before 2016 were based on the underlying Novelion common shares listed on the Toronto Stock Exchange (TSX), and thus in Canadian dollars. Stock options granted in 2016 and after are and will be based on the underlying Novelion common shares listed on NASDAQ and thus in USD.
New common shares are issued upon exercise of stock options. The intrinsic value of stock options exercised and the related cash from exercise of stock options during the years ended December 31, 2016, 2015 and 2014 are as follows:
(In thousands) | 2016 | 2015 | 2014 | ||||||||
Intrinsic value of stock options exercised | $ | — | $ | 4,930 | $ | 230 | |||||
Cash from exercise of stock options | — | 5,508 | 509 | ||||||||
(b) Deferred Share Units
Under the DDSU Plan, at the discretion of the Board of Directors, directors can receive all or a percentage of their equity-based compensation in the form of DSUs. DSUs vest in thirty-six (36) successive and equal monthly installments beginning on the first day of the first month after the grant date. A vested DSU can only be settled by conversion to cash (no share is issued), and is automatically converted after the director ceases to be a member of the Board unless the director is removed from the Board for just cause. Prior to conversion, the value of each DSU, at any point in time, is equivalent to the latest closing price of Novelion’s common shares on TSX on that trading day. When converted to cash, the value of a vested DSU is equivalent to the closing price of a Novelion’s common share on the trading day immediately prior to the conversion date.
DSU activity is presented below:
Number of DSUs (1) | ||||
Outstanding at December 31, 2015 | 30,800 | |||
Granted | 8,960 | |||
Redeemed | (11,360 | ) | ||
Cancelled | — | |||
Outstanding at December 31, 2016 | 28,400 | |||
Vested at December 31, 2016 | 28,400 | |||
(1) Shares and per share amounts have been retrospectively restated for all prior periods presented to reflect the one-for-five share consolidation.
The obligation to settle DSUs in cash is recorded as a liability in the Company’s Consolidated Financial Statements and is marked-to-market at the end of each reporting period. See Note 9 - Accrued Liabilities. Cash payments under the DDSU Plan during the years ended December 31, 2016, 2015, and 2014 were as follows:
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Cash payments under the DDSU plan | $ | 105 | $ | — | $ | — | |||||
145
The cash payments in 2016 related to two former members of the Novelion board of directors who departed from the board after the acquisition closed on November 29, 2016. The Company’s obligation to settle the remaining vested and unsettled 28,400 common shares underlying DSUs held by former directors was $0.2 million as of December 31, 2016.
In connection with the Merger, the vesting provisions applicable to all of the outstanding and unvested DSUs were accelerated on November 29, 2016. The acceleration of the vesting provisions of all the outstanding and unvested DSUs during the year ended December 31, 2016 resulted in an additional $62,251 DSU compensation expense recognized on the Company's accompanying Consolidated Statements of Operations.
(c) Restricted Stock Units
The Company has outstanding time-vested, market-based and performance-based RSUs. Time-vested RSUs are awarded to eligible employees and entitle the grantee to receive common shares at the end of a vesting period, subject solely to the employee’s continuing employment. The majority of time-vested RSUs vest in two equal annual installments beginning a year from the grant date. Aegerion’s market-based RSUs were awarded to employees of Aegerion on July 29, 2014 and were replaced by Novelion’s market-based RSUs at the time of the acquisition. All the market-based RSUs vest when the price the day the stock price closes on the value at or above the value of the original new hire strike price and expire on July 29, 2019. Because the current stock price of the Company is significantly lower than the original new hire strike price, we expect all the market-based RSUs will expire without being vested. The performance-based RSUs are awarded to eligible employees and entitle the grantee to receive shares of common stock if specified performance goals are achieved during the performance period and the grantee remains employed during the subsequent vesting period. The majority of performance-based RSUs vest in three equal annual installments beginning upon goal achievement. Upon vesting, each RSU represents the right to receive one common share of the Company.
RSU transactions for the years ended December 31, 2016 are as following:
Number of RSUs (3) | ||||
Outstanding at December 31, 2015 | — | |||
Granted (1) (2) | 1,036,735 | |||
Redeemed | — | |||
Cancelled | (7,854 | ) | ||
Outstanding at December 31, 2016 | 1,028,881 | |||
(1) The weighted-average grant date fair value of the RSUs granted during the year ended December 31, 2016 was $8.82 (December 31, 2015 - zero, December 31, 2014 - CAD $20.4).
(2) The assumed Aegerion RSUs from the Merger are included in grants made during the fiscal year ended December 31, 2016 in the above table. The total number of assumed Aegerion RSUs was 140,605 with a weighted-average merger date valuation of $9.53 per share.
(3) Shares and per share amounts have been retrospectively restated for all prior periods presented to reflect the one-for-five share consolidation.
RSUs are generally subject to forfeiture if employment terminates prior to the release of vesting restrictions. The Company expenses the cost of the RSUs with service-based vesting conditions, which is determined to be the fair value of the shares of common stock underlying the RSUs at the date of grant, ratably over the period during which the vesting restrictions lapse. The fair value of RSUs with market-based vesting conditions is determined using a Monte Carlo simulation and the Company recognizes compensation expense over the derived service period.
In connection with the strategic transactions announced on June 8, 2015 (see Note 3 - Terminated Merger Transactions and Note 4 - Strategic Transactions), the Board of Directors accelerated the vesting provisions applicable to 12,800 RSUs that were outstanding and unvested on June 7, 2015. The impact of the accelerated vesting of these RSUs during the year ended December 31, 2015 was an additional $0.2 million RSU compensation expenses recognized in the Company's 2015 results of operations.
1,028,881 RSUs were outstanding and unvested as of December 31, 2016 (December 31, 2015 - zero, December 31, 2014 - 12,800). In addition, the total estimated unrecognized compensation cost related to RSUs as of December 31, 2016 is $9.0 million (December 31, 2015 - zero, December 31, 2014 - $0.2 million) and the weighted-average period over which such costs are expected to be recognized is 1.77 years (December 31, 2015 - zero years, December 31, 2014 - 2.34 years).
The impact on the Company’s results of operations of stock-based compensation expense for the years ended December 31, 2016, 2015, and 2014 is as follows:
146
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Research and development | $ | 155 | $ | 1,267 | $ | 911 | |||||
Selling, general and administrative | 683 | 1,108 | 702 | ||||||||
Total stock-based compensation expense | $ | 838 | $ | 2,375 | $ | 1,613 | |||||
13. Income Taxes.
Loss before provision for income taxes, classified by source of (loss)/income, is as follows:
(in thousands) | December 31, 2016 | December 31, 2015 | December 31, 2014 | ||||||||
Canada | $ | (46,733 | ) | $ | (22,987 | ) | $ | (4,197 | ) | ||
U.S. | (9,521 | ) | — | — | |||||||
Other Foreign | 3,849 | — | — | ||||||||
Loss before provision for income taxes | $ | (52,405 | ) | $ | (22,987 | ) | $ | (4,197 | ) | ||
Provision for income taxes for the years ended December 31, 2016, 2015 and 2014 are as follows:
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Current: | |||||||||||
Canada | $ | 105 | $ | — | $ | — | |||||
U.S. state, net of federal income tax benefit | (2 | ) | — | — | |||||||
Other Foreign | (517 | ) | — | — | |||||||
$ | (414 | ) | $ | — | $ | — | |||||
Deferred: | |||||||||||
Canada | $ | — | $ | (22 | ) | $ | 192 | ||||
Other Foreign | (51 | ) | — | — | |||||||
(51 | ) | (22 | ) | 192 | |||||||
(Provision for) recovery of income taxes | $ | (465 | ) | $ | (22 | ) | $ | 192 | |||
Differences between the Company’s statutory income tax rates and its effective income tax rates, as applied to the loss from continuing operations before income taxes, are reconciled as follows:
(in thousands) | 2016 | 2015 | 2014 | ||||||||
Loss from continuing operations before income taxes | $ | (52,405 | ) | $ | (22,987 | ) | $ | (4,197 | ) | ||
Canadian statutory tax rates | 26.00 | % | 26.00 | % | 26.00 | % | |||||
Expected income tax recovery | 13,625 | 5,977 | 1,091 | ||||||||
Net decrease (increase) in valuation allowance | (13,684 | ) | (2,486 | ) | 731 | ||||||
Non-taxable portion of capital gains | — | — | 230 | ||||||||
Investment tax credits | 868 | (222 | ) | 1,628 | |||||||
Stock-based compensation | (133 | ) | (606 | ) | (377 | ) | |||||
Foreign rate differential | 532 | — | — | ||||||||
Non-taxable (deductible) expenditures | — | 76 | 1,752 | ||||||||
Changes in uncertain tax positions | — | (1,784 | ) | (4,793 | ) | ||||||
Adjustments to capital losses for settlement of uncertain tax positions | — | (560 | ) | — | |||||||
Other | (1,673 | ) | (417 | ) | (70 | ) | |||||
(Provision for) recovery of income taxes | $ | (465 | ) | $ | (22 | ) | $ | 192 | |||
147
Deferred taxes are recognized for temporary differences between the basis of assets and liabilities for financial statement and income tax purposes. The primary components of the Company’s deferred tax assets and liabilities comprised of the following:
(in thousands) | 2016 | 2015 | |||||
Deferred tax assets: | |||||||
Net operating loss carryforwards | $ | 51,808 | $ | 45,365 | |||
Research and development credits | 14,028 | 14,164 | |||||
Stock-based compensation | 108 | — | |||||
Capitalized research expenses | 2,124 | — | |||||
Capital loss carryforwards | 37,452 | 36,207 | |||||
Depreciable and amortizable assets | 13,597 | 1,645 | |||||
Other temporary differences | 13,736 | 197 | |||||
Total gross deferred tax assets | 132,853 | 97,578 | |||||
Valuation allowance | (131,858 | ) | (97,578 | ) | |||
Net deferred tax assets | $ | 995 | $ | — | |||
As of December 31, 2016, the Company has a $1.0 million net deferred tax asset attributable to its profitable foreign subsidiaries. Additionally, as of December 31, 2016, the Company has $131.9 million of valuation allowance recorded against its Canadian, U.S. and Switzerland deferred tax assets. The valuation allowance increase of $34.3 million is primarily the result of the acquisition of Aegerion. If the Company is subsequently able to utilize all or a portion of the deferred tax assets for which the remaining valuation allowance has been established, then the Company may be required to recognize these deferred tax assets through the reduction of the valuation allowance which could result in a material benefit to results of operations in the period in which the benefit is determined.
The Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets and concluded that based on the Company’s history of operating losses that it is more likely than not that the benefit of its deferred tax assets will not be realized.
At December 31, 2016, the Company had approximately $166.9 million of foreign and federal net operating loss carryforwards, of which $152.6 million relate to Canada and $14.3 million relate to the Company’s U.S. subsidiaries. The loss carryforwards expire at various dates through 2036. As of December 31, 2016, the Company also had approximately $11.8 million of state net operating loss carryforwards that expire at various dates through 2036. As of December 31, 2016, the Company also had approximately $13.5 million of Canadian national and provincial research and development credits available for carryforward. The research and development credit carryforwards expire at various dates through 2036. As of December 31, 2016, the Company's Canadian Scientific, Research and Experimental Development pool was $13.4 million. Furthermore, as of December 31, 2016, the Company had approximately $289.5 million of Canadian capital loss carryforwards, which carryforward indefinitely. The deferred tax benefit of these loss carryforwards and research and development credits is ultimately subject to final determination by the respective taxation authorities.
Upon acquiring a company that has U.S. federal and state net operating loss carryforwards and federal and state tax credits, the Company prepared an assessment to determine if it has a legal right to use the acquired net operating losses and tax credits. In performing this assessment the Company followed the regulations within the Internal Revenue Code Sections 382 and 383. The Company determined that the U.S. net operating losses and tax credits acquired in the Aegerion acquisition will be subject to limitations under Internal Revenue Code Sections 382 and 383. Due to the ownership changes, the Company has determined that its U.S. subsidiaries, including Aegerion, will only be able to utilize approximately $14.3 million of its NOLs before expiration as a result of the annual Section 382 limitation. For Aegerion, $166.6 million of federal net operating losses and $30.2 million of federal general business credits will expire before becoming available under the Section 382 limitation. Additionally, $96.9 million of state net operating losses and $0.2 million of state credits will expire before becoming available under the Section 382 limitation. The U.S. subsidiaries may also experience ownership changes in the future as a result of subsequent shifts in share ownership that could further limit the use of the available net operating losses and credits. Additionally, Aegerion determined that at the date of the ownership change, it had a net unrealized built-in loss (“NUBIL”). The NUBIL is determined based on the difference between the fair market value of the Company’s assets and their tax basis at the ownership change.
In accordance with ASC 740, the Company recorded a reserve for uncertain tax positions of approximately $7.7 million offset by $7.3 million of the Company's deferred tax assets at December 31, 2016, for a net of $0.4 million for unrecognized tax benefits as of December 31, 2016.
The following table summarizes the activity related to the Company’s unrecognized tax benefits:
148
(in thousands) | 2016 | 2015 | 2014 | |||||||||
Total uncertain tax position liabilities as of January 1, | $ | 7,278 | $ | 5,557 | $ | 1,846 | ||||||
Current year acquisitions | 911 | — | — | |||||||||
Increases related to current year tax positions | — | 347 | 5,169 | |||||||||
Changes in tax positions of a prior period | — | 1,934 | 11 | |||||||||
Lapse due to statute of limitations | (342 | ) | — | (1,469 | ) | |||||||
Settlements with taxing authorities | (187 | ) | (560 | ) | — | |||||||
Total uncertain tax position liabilities as of December 31, | $ | 7,660 | $ | 7,278 | $ | 5,557 | ||||||
Of this amount of unrecognized tax benefits, approximately $7.3 million, $6.9 million and $5.2 million would be fully offset by the Company's deferred tax asset at December 31, 2016, 2015 and 2014. The Company recognizes interest and penalties as a component of income tax expense. At December 31, 2016, 2015 and 2014 the Company had not accrued interest or penalties as a result of the deferred assets available to offset its unrecognized tax benefits. The increase in unrecognized tax benefits during the year was the result of transfer pricing adjustments made at the Company’s Swiss subsidiary as a result of its acquisition of Aegerion. During 2016, the Company settled a dispute with the Canadian Revenue Authority surrounding its capital loss carryover as a result of its divestiture of its Eligard contingent consideration which resulted in a decrease of the unrecognized tax benefits. Additionally during 2016, the period in which the 2008 Canadian income tax return was subject to reassessment expired, which resulted in a reduction of the unrecognized tax benefits surrounding its share by buyback costs that arose during that tax year.
Given that the potential net 2016 liability increase of $0.4 million, the potential 2015 liability increase of $1.7 million and the potential net 2014 liability increase of $3.7 million are fully or partially sheltered by the Company’s existing deferred tax assets, the Company has offset a portion of the total liability on the Company’s Consolidated Balance Sheets in accordance with ASU No. 2013-11 - Income Taxes (Topic 740): Presentation of Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists. ASU No. 2013-11 was adopted on a prospective basis effective January 1, 2014.
The Company and its subsidiaries file income tax returns in Canada, the U.S., and various U.S. states and in foreign jurisdictions. The Canadian income tax returns are generally subject to tax examination for the tax years ended December 31, 2010 through December 31, 2016. The U.S., U.S. state and foreign income tax returns are generally subject to tax examinations for the tax years ended December 31, 2013 through December 31, 2016. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the tax authorities.
The Company monitors the undistributed earnings of its foreign subsidiaries and, as necessary, provides for income taxes on those earnings that are not deemed permanently invested. As of December 31, 2016 the Company has approximately $1.5 million of undistributed earnings at its foreign subsidiaries. These earning are deemed permanently invested by the Company.
14. Fair Value of Financial Instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. ASC No. 820 - Fair Value Measurements and Disclosures established a fair value hierarchy for those instruments measured at fair value that distinguishes between fair value measurements based on market data (observable inputs) and those based on the Company’s own assumptions (unobservable inputs). This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
• | Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. The Company’s Level 1 assets consist of cash, money market investments, and restricted cash. |
• | Level 2—Inputs other than quoted prices in active markets that are observable for the asset or liability, either directly or indirectly. The Company’s Level 2 liabilities consist of convertible notes. |
• | Level 3—Inputs that are unobservable for the asset or liability. The Company does not have any Level 3 assets and liabilities as of December 31, 2016. |
149
The fair value measurements of the Company’s financial instruments at December 31, 2016 and 2015 are summarized in the tables below:
December 31, 2016 | |||||||||||||||
Significant | |||||||||||||||
Quoted Prices in | Other | Significant | |||||||||||||
Active Markets for | Observable | Unobservable | Balance at | ||||||||||||
Identical Assets | Inputs | Inputs | December 31, | ||||||||||||
(in thousands) | (Level 1) | (Level 2) | (Level 3) | 2016 | |||||||||||
Assets: | |||||||||||||||
Cash | $ | 40,693 | $ | — | $ | — | $ | 40,693 | |||||||
Money market funds | 68,234 | — | — | 68,234 | |||||||||||
Restricted cash | 390 | — | — | 390 | |||||||||||
Total assets | $ | 109,317 | $ | — | $ | — | $ | 109,317 | |||||||
Liabilities: | |||||||||||||||
Convertible notes, net - long-term | — | 225,584 | — | 225,584 | |||||||||||
Total liabilities | $ | — | $ | 225,584 | $ | — | $ | 225,584 | |||||||
December 31, 2015 | |||||||||||||||
Significant | |||||||||||||||
Quoted Prices in | Other | Significant | |||||||||||||
Active Markets for | Observable | Unobservable | Balance at | ||||||||||||
Identical Assets | Inputs | Inputs | December 31, | ||||||||||||
(in thousands) | (Level 1) | (Level 2) | (Level 3) | 2015 | |||||||||||
Assets: | |||||||||||||||
Cash | $ | 141,824 | $ | — | $ | — | $ | 141,824 | |||||||
Accounts receivable - Laser Earn-Out Payment (1) | — | — | 2,000 | 2,000 | |||||||||||
Total assets | $ | 141,824 | $ | — | $ | 2,000 | $ | 143,824 | |||||||
(1) Represents the estimated fair value of the Laser Earn-Out Payment as described in Note 16 - Contingencies, Commitments and Guarantees. The fair value of the Laser Earn-Out Payment was estimated using a probability weighted approach to examine various possible outcomes with respect to the timing and amount that may be collected.
The fair value of the Convertible Notes, which differs from their carrying values, is influenced by interest rates, the Company’s share price and share price volatility and is determined by prices for the Convertible Notes observed in market trading which are Level 2 inputs. The estimated fair value of the Convertible Notes at December 31, 2016 was $240.4 million. See Note 10 - Convertible Notes, Net for further information.
The Company’s financial instruments that are exposed to credit risks consist primarily of cash, cash equivalents, restricted cash and accounts receivable. To limit the Company’s credit exposure, cash and cash equivalents are deposited with high-quality financial institutions in accordance with its treasury policy goal to preserve capital and maintain liquidity. The Company’s treasury policy limits investments to certain money market securities issued by governments, financial institutions and corporations with investment-grade credit ratings, and places restrictions on maturities and concentration by issuer. The Company maintains its cash, cash equivalents and restricted cash in bank accounts, which, at times, exceed federally insured limits. The Company has not experienced any credit losses in these accounts and does not believe it is exposed to any significant credit risk on these funds.
The Company is subject to credit risk from its accounts receivable related to its product sales of lomitapide and metreleptin. The majority of the Company’s accounts receivable arise from product sales in the U.S. For accounts receivable that have arisen from named patient sales outside of the U.S., the payment terms are predetermined and the Company evaluates the creditworthiness of each customer or distributor on a regular basis. The Company periodically assesses the financial strength of the holders of its accounts receivable to establish allowances for anticipated losses, if necessary. The Company does not recognize revenue for uninsured amounts billed directly to a patient until the time of cash receipt as collectability is not reasonably assured at the time the product is received. To date, the Company has not incurred any credit losses.
150
15. Basic and Diluted Net Loss per Common Share.
Basic net loss per common share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period.
Diluted net loss per common share is computed by dividing the net loss by the weighted-average number of unrestricted common shares and dilutive common share equivalents outstanding for the period, determined using the treasury-shares and if-converted methods. Since the Company has had net losses for all periods presented, all potentially dilutive securities are anti-dilutive. Accordingly, basic and diluted net loss per common share are equal.
The following table sets out the computation of basic and diluted net loss per common share:
(in thousands, except per share data) | 2016 | 2015 | 2014 | ||||||||||
Numerator: | |||||||||||||
Loss from continuing operations | $ | (52,870 | ) | $ | (23,009 | ) | $ | (4,005 | ) | ||||
Loss from discontinued operations, net of income taxes | — | — | (66 | ) | |||||||||
Net loss | $ | (52,870 | ) | $ | (23,009 | ) | $ | (4,071 | ) | ||||
Denominator: (thousands) | |||||||||||||
weighted-average common shares outstanding | 11,284 | 10,434 | 10,225 | ||||||||||
Basic and diluted net loss per common share | |||||||||||||
Continuing operations | $ | (4.69 | ) | $ | (2.20 | ) | $ | (0.40 | ) | ||||
Discontinued operations (1) | — | — | — | ||||||||||
Net loss per common share | $ | (4.69 | ) | $ | (2.20 | ) | $ | (0.40 | ) | ||||
(1) Rounded to zero.
The following table sets forth potential common shares issuable upon the exercise of outstanding options, warrants, the vesting of RSUs and the conversion of the Convertible Notes (prior to consideration of the treasury shares and if-converted methods) (see Note 12 - Stock-Based Payments for details), which were excluded from the computation of diluted net loss per share because such instruments were anti-dilutive (in thousands):
December 31, 2016 | December 31, 2015 | December 31, 2014 | |||||||
Stock options | 1,715 | 86 | 418 | ||||||
Unvested restricted stock units | 1,029 | — | 13 | ||||||
Warrants | 14,515 | 568 | — | ||||||
Convertible notes | 1,619 | — | — | ||||||
Total | 18,878 | 654 | 431 | ||||||
16. Contingencies, Commitments and Guarantees.
Lease Obligations
The Company leased certain office facilities and office equipment under operating leases. The future estimated operating lease payments for office space and office equipment over the next five years are summarized as follows (in thousands):
151
Year Ending December 31: | Lease Commitments | ||
2017 | $ | 3,083 | |
2018 | 2,400 | ||
2019 | 884 | ||
2020 | 128 | ||
Thereafter | 13 | ||
Total | $ | 6,508 | |
Rent expense under operating leases was approximately $0.5 million, $0.4 million, and $0.5 million for the years ended December 31, 2016, 2015 and 2014, respectively.
During 2015, the Company entered into a sublease agreement (as amended, the Lease Agreement) to downsize its existing space in Vancouver, British Columbia, where its headquarters is located. The Company currently leases approximately 8,475 square feet of space under the terms of the Lease Agreement. The lease term applicable to this space expires on August 31, 2017.
The Company's U.S. operational office, which is located at One Main Street in Cambridge, Massachusetts, consists of approximately 31,571 square feet of office space under a lease that expires in April 2019.
The Company also leases office spaces in Japan, the UK, Switzerland, Germany, France, Italy, Canada, Brazil, and Turkey, with approximately 8,414 square feet of office space in the aggregate. The Company's international lease agreements expire at various dates through the year 2021.
In addition to the locations listed above, the Company holds inventory at various locations, including international locations, managed by third parties.
Other Commitments
Amgen Licensing Agreements
Metreleptin. In connection with Aegerion's acquisition of MYALEPT in January 2015, Aegerion acquired a license agreement between Amgen Inc. (Amgen) and Amylin Pharmaceuticals, Inc., dated February 7, 2006 (the Amgen License) pursuant to which an exclusive worldwide license was obtained from Amgen to certain know-how and patents and patent applications covering the composition of matter and methods of use of metreleptin to develop, manufacture and commercialize a preparation containing metreleptin (the Amgen Licensed Products).
As part of the Amgen License, an exclusive sublicense of Amgen’s exclusive rights to certain metreleptin-related patents and patent applications owned by the Rockefeller University and exclusively licensed to Amgen under a license agreement dated April 14, 1995, as amended (the Rockefeller License) and an exclusive sublicense of Amgen’s non-exclusive rights to certain metreleptin-related patents and patent applications owned by The Regents of the University of California and non-exclusively licensed to Amgen under a license agreement dated July 13, 2005 (the UCSF License) were obtained. Amgen retains rights to conduct research, development, manufacturing and commercialization activities with respect to products other than the Amgen Licensed Products.
Sublicenses under the licenses are permitted and are subject to certain limitations, including Amgen’s right of first offer for any out-license, partnership, co-development, commercialization, co-promotion or similar agreement related to metreleptin or the Amgen Licensed Products, which expires in February 2021. Under this license agreement, Amgen must notify us of any potential third-party partnership regarding any intellectual property rights controlled by Amgen in the neurology field and the Company will have a right of first negotiation for any license, partnership, co-development, commercialization, co-promotion or similar agreement, which expires in February 2021.
Aegerion is required to make royalty payments to Amgen, Rockefeller University and BMS on net sales of each Amgen Licensed Product on a country-by-country basis (i) at a royalty rate in the low double digits where the Amgen Licensed Product has patent protection or market exclusivity granted by a regulatory authority at the time of regulatory approval in the applicable country during the applicable royalty term, which runs on a country-by-country basis until the later of (a) the expiration of the last-to-expire valid claim covering an Amgen Licensed Product in the applicable country, (b) expiration of any market exclusivity granted by a regulatory authority, and (c) 10 years from the date on which an Amgen Licensed Product is first sold to a third-party in a country after regulatory approval for the Amgen Licensed Product has been granted in such country (Amgen Royalty Term) or (ii) at a royalty rate in the mid-single digits to low double digits where the Amgen Licensed Product receives patent protection or
152
market exclusivity following the time of regulatory approval in the applicable country, in either case subject to a variety of customary reductions.
Under the Amgen License, Aegerion is also required to directly meet certain payment obligations under the Rockefeller License and UCSF License. Aegerion is required to make royalty payments to Rockefeller University on net sales of each product with patent rights or know-how in the field of obesity genes, obesity gene products, and molecules that modulate or mediate their action and/or regulation on a country-by-country basis at a range of royalty rates in the low single digits depending on whether the product has an orphan product designation or not until the later to occur of expiration of (i) patent protection, (ii) any market exclusivity period granted in the applicable country, or (iii) any data exclusivity period in the applicable country (with certain limitations related to the number of units sold). Aegerion is required to pay to Rockefeller University a percentage in the low double digits of any upfront license fees or one-time fees it receives in consideration for a sublicense of the licensed rights. There are no material payment obligations outstanding under the UCSF License.
The Amgen License will terminate upon the expiration of the last Amgen Royalty Term for any Amgen Licensed Product. Aegerion has the right to terminate the Amgen License for convenience upon 90 days prior written notice to Amgen or for Amgen’s uncured material breach of the Amgen License, or becoming subject to specified bankruptcy or liquidation events. Amgen may terminate the Amgen License for the Aegerion’s uncured failure to make payments to Amgen or if the Company is the subject of specified bankruptcy or liquidation events.
During the period from November 30, 2016 to December 31, 2016, there were no royalty payments made by the Company related to sales of MYALEPT. As of December 31, 2016, $1.2 million remained as an accrual balance in royalties payable to Amgen.
Shionogi & Co., Ltd.
Metreleptin. In connection with Aegerion's acquisition of MYALEPT in January 2015, Aegerion acquired a license agreement between Shionogi and Amylin Pharmaceuticals, Inc., dated July 8, 2009 pursuant to which Shionogi was granted an exclusive sublicense to the patent rights licensed under the Amgen License and the Rockefeller License to develop and commercialize the Amgen Licensed Products and know-how for use in the treatment of lipodystrophy in humans in Japan, South Korea and Taiwan (the Shionogi Territory). This license agreement does not provide Shionogi with manufacturing rights. Shionogi may grant further sublicenses under the license, subject to certain limitations.
The license agreement requires that Shionogi use commercially reasonable efforts to develop, obtain regulatory approvals for, and commercialize the Amgen Licensed Products in the Shionogi Territory. Shionogi is required to make royalty payments to Aegerion on net sales of each Amgen Licensed Product at a range of royalty rates in the mid-to high-single digits dependent on the amount of net sales. During the period from November 30, 2016 to December 31, 2016, Aegerion did not receive any royalty payments from Shionogi. Shionogi will be required to make milestone payments to Aegerion of up to an aggregate of approximately $25.0 million if and when Shionogi achieves certain commercialization milestones. Such milestone payments are payable only once. Under the license agreement, Shionogi has also agreed to directly comply with the payment obligations under the Rockefeller License and Amgen License, as set forth under those agreements, relating to its activities under this license agreement.
The license agreement with Shionogi will terminate upon the expiration of the last Amgen Royalty Term for any Amgen Licensed Product with respect to which Shionogi has a license under this license agreement. Aegerion has the right to terminate this license agreement for Shionogi’s uncured material breach of the license agreement, failure to make any payment due to Aegerion, a procedural default, or becoming subject to specified bankruptcy or liquidation events. Shionogi may terminate this license agreement for Aegerion’s uncured material breach of this license agreement, failure to make payments due to Shionogi, or if Aegerion is the subject of specified bankruptcy or liquidation events, or if Shionogi determines it is not feasible to develop, launch or sell the Amgen Licensed Products due to scientific, technical, regulatory or commercial reasons. Aegerion may also terminate this license agreement at any time without cause by exercising its buy-back option for a one-time fee to Shionogi equal to (i) a number in the low single digits times the amount of expenses and fees incurred by Shionogi in developing the Amgen Licensed Products plus (ii) an amount no more than a number in the mid-double digits times monthly net sales of the Amgen Licensed Products by Shionogi in the month the option is exercised.
University of Pennsylvania Licensing Agreement
Lomitapide. In May 2006, Aegerion entered into license agreement with The Trustees of the University of Pennsylvania, (UPenn) pursuant to which it obtained an exclusive, worldwide license from UPenn to certain know-how and a range of patent rights applicable to lomitapide. In particular, Aegerion obtained a license to certain patent and patent applications owned by UPenn relating to the dosing of microsomal triglyceride transfer protein inhibitors, including lomitapide, and certain patents and patent applications and know-how covering the composition of matter of lomitapide that were assigned to UPenn by BMS in the field of monotherapy or in combination with other dyslipidemic therapies, which are therapies for the treatment of patients, with abnormally high or low levels of plasma cholesterol or triglycerides.
153
Aegerion is obligated under this license agreement to use commercially reasonable efforts to develop, commercialize, market and sell at least one product covered by the licensed patent rights, such as lomitapide.
Aegerion will be required to make specified royalty payments on net sales of products, at a range of royalty rates in the high single digits on net sales of lomitapide in countries where lomitapide has patent protection, and of any other products covered by the license (subject to a variety of customary reductions), and share with UPenn specified percentages of sublicensing royalties and certain other consideration that the Company receives under any sublicenses that Aegerion may grant. During the period from November 30, 2016 to December 31, 2016, there were no royalty payments made to UPenn. As of December 31, 2016, $1.3 million remained as an accrual balance in royalty payable UPenn.
This license agreement will remain in effect on a country-by-country basis until the expiration of the last-to-expire licensed patent right in the applicable country. Aegerion has the right to terminate this license agreement for UPenn’s uncured material breach of the license agreement or for convenience upon 60 days’ prior written notice to UPenn, subject to certain specific conditions and consequences. UPenn may terminate this license agreement for Aegerion’s uncured material breach of the license agreement, its uncured failure to make payments to UPenn or if Aegerion is the subject of specified bankruptcy or liquidation events.
Retinagenix LLC
Zuretinol. Under the terms of the April 2006 co-development agreement (as amended, the Retinagenix Agreement) QLT entered into with Retinagenix LLC (Retinagenix), it obtained an exclusive, worldwide license and sub-license to certain intellectual property rights owned or controlled by Retinagenix related to the synthetic retinoid compound under development. Under the terms of this agreement, QLT is responsible for using commercially reasonable and diligent efforts to develop and commercialize in certain major markets and other markets as it reasonably determines, one or more products covered by the licensed rights or developed using such licensed rights for use in diagnosing, treating or preventing certain human diseases and conditions. QLT is also responsible for committing certain annual funding to support research and development of such products. Under the license agreement between Retinagenix and the University of Washington (the UW Agreement), Retinagenix has similar obligations, and is required to meet specific development milestones within certain timeframes, one of which was required to be achieved by December 31, 2016. However, the UW Agreement contains provision for extensions of those dates in certain circumstances. Based on the terms of the Retinagenix Agreement and the UW Agreement, and the Company’s significant development clinical spend on the zuretinol program, management believes that the Company is entitled to an extension of that milestone date until December 31, 2017, and that the Company may be entitled to certain additional extensions to December 31, 2019, along with a potential additional extension of up to 12 months should enrollment in a planned trial be delayed, provided that the Company continues to comply with the relevant provisions of the license agreements and expend certain minimum amounts on the development of zuretinol. However, it is possible that the Company may not be able to achieve the specified development milestone by December 31, 2019. As a result, management of the Company and Retinagenix have begun discussing a renegotiation of that milestone with the University of Washington. The Company is currently conducting a review of the zuretinol development program, the results of which will assist the Company in determining when the remaining development milestone can be expected to be achieved.
Pursuant to the Retinagenix Agreement, Retinagenix is eligible to receive the following milestone payments: (i) $1.0 million upon initiation of the first pivotal trial for the first target indication which uses such products, (ii) $1.5 million upon completion of a filing seeking EU approval or Japan approval for the use of such products in the first indication and (iii) up to a total of an additional $10.0 million upon the achievement of other specified development or regulatory milestones and, for each of up to two additional indications, up to a total of $9.0 million upon achievement of specified development or regulatory milestones. If the Company commercializes such products, it will also pay Retinagenix royalties of between 4% and 6% of net sales, subject to reduction under certain specified circumstances. Retinagenix is also eligible to receive up to a total of $15.0 million upon achievement of specified cumulative sales milestones for such products. The term of the Retinagenix Agreement expires on the later of the expiration of 10 years after first commercial sale of licensed products, or the expiration, lapse or abandonment of all licensed patents. Retinagenix can terminate the agreement earlier if the Company fails in any material respect to meet its diligence requirements, and the Company may terminate the agreement for convenience. Each party may terminate the agreement for uncured material breach by the other party.
Financial Advisory Services Milestone Obligation
On February 5, 2016, pursuant to the Greenhill Agreement, QLT paid Greenhill a $4.0 million advisory fee in connection with the completion of QLT’s $45.0 million investment in Aralez and exploration of other strategic initiatives described under Note 3 - Terminated Merger Transactions and Note 4 - Strategic Transactions. The recognition and payment of the advisory fee was both contingent upon the satisfaction of various terms and conditions, which were met on February 5, 2016, and subject to the outcome of certain external factors and uncertainties, which were settled by February 5, 2016 but were beyond the Company’s control.
154
Indemnities
In connection with the sale of assets, the Company provided indemnities with respect to certain matters, including product liability, patent infringement, contractual breaches and misrepresentations, and the Company provides other indemnities to third parties under the clinical trial, license, service, supply and other agreements that it enters into in the normal course of its business. If the indemnified party were to make a successful claim pursuant to the terms of the indemnity, the Company would be required to reimburse the loss. These indemnities are generally subject to threshold amounts, specified claims periods and other restrictions and limitations. As of December 31, 2016, no amounts have been accrued in connection with such indemnities.
Development and Post Marketing Regulatory Commitments
Novelion and Aegerion have engaged Contract Research Organizations (CROs) to provide research, safety and project management services (the Services) in connection with the execution of their potential clinical trials and existing registries. Services would only give rise to liabilities to the extent that services are provided to Novelion or Aegerion, as applicable, and pass through expenses are incurred. As of December 31, 2016, the Services have not yet been performed and the Company has potential commitments of approximately $42.1 million under these agreements. The amount reflected is based on the existing contracts and does not reflect any inflation, future modification to, or termination of, the existing contract or anticipated or potential new contracts.
Contingencies
Upon the acquisition of Aegerion, the Company assumed the assets and liabilities related to the following contingencies (in thousands):
Insurance Proceeds Receivable | ||||
Class action lawsuit insurance proceeds | $ | 22,000 | ||
Provision for Legal Settlement | ||||
Class action lawsuit settlement | $ | (22,250 | ) | |
DOJ and SEC settlement | (40,635 | ) | ||
Relator legal settlement | (620 | ) | ||
Relators legal fees | (405 | ) | ||
Other litigation settlement | (100 | ) | ||
Total provision for legal settlement | $ | (64,010 | ) | |
DOJ/SEC Investigations
In late 2013, Aegerion received a subpoena from the DOJ, represented by the U.S. Attorney’s Office in Boston, requesting documents regarding Aegerion’s marketing and sale of JUXTAPID in the U.S., as well as related disclosures. Aegerion believes the DOJ is seeking to determine whether it, or any of its current or former employees, violated civil and/or criminal laws, including but not limited to, the securities laws, the Federal False Claims Act, the Food and Drug Cosmetic Act, the Anti-Kickback Statute, and the Foreign Corrupt Practices Act of 1977 (FCPA). The investigation is ongoing.
In late 2014, Aegerion received a subpoena from the SEC requesting certain information related to its sales activities and disclosures related to JUXTAPID. The SEC also has requested documents and information on a number of other topics, including documents related to the investigations by government authorities in Brazil into whether Aegerion’s activities in Brazil violated Brazilian anti-corruption laws, and whether Aegerion’s activities in Brazil violated the U.S. Foreign Corrupt Practices Act. Aegerion believes the SEC is seeking to determine whether Aegerion, or any of its current or former employees, violated securities laws. The investigation is ongoing.
In May 2016, Aegerion reached preliminary agreements in principle with the DOJ and the SEC to resolve their investigations into the marketing and sales activities and disclosures relating to JUXTAPID. Under the terms of the preliminary agreement in principle with the DOJ, Aegerion would plead guilty to two misdemeanor misbranding violations of the Food, Drug and Cosmetic Act. One count would be based on its alleged marketing of JUXTAPID with inadequate directions for use (21 U.S.C. §§ 352(f)), and the second count would involve an alleged failure to comply with a requirement of the JUXTAPID Risk Evaluation and Mitigation Strategies (REMS) program (21 U.S.C. §§ 352(y)). Aegerion would separately enter into a five-year deferred prosecution agreement with regard to a charge that Aegerion violated the Health Insurance Portability and Accountability Act. As part of the resolution of the DOJ investigation, Aegerion is expected to enter into a civil settlement agreement with the DOJ to resolve alleged violations of the False Claims Act and, a non-monetary consent decree with the FDA. Aegerion also expects to negotiate a corporate integrity agreement with the Department of Health and Human Services.
155
Under the terms of the preliminary agreement in principle with the SEC staff, the SEC’s Division of Enforcement will recommend that the SEC accept a settlement offer from Aegerion on a neither-admit-nor-deny basis that contains alleged negligent violations of Sections 17(a)(2) and (3) of the Securities Act of 1933, as amended, related to certain statements Aegerion made in 2013 regarding the conversion rate of patients receiving JUXTAPID prescriptions, with remedies that include censure, an order prohibiting future violations of the securities laws and payment of a civil penalty.
The preliminary agreements in principle provide for a consolidated monetary package that covers payments due to both the DOJ and the SEC. The consolidated monetary package includes payments to the DOJ and the SEC by Aegerion totaling approximately $40 million in the aggregate, to be payable over three years, which is updated from the originally proposed five-year payment schedule contemplated when the preliminary agreement in principle was reached in May 2016. Certain outstanding amounts would accrue interest at a rate of 1.75% per annum. Such payments are subject to acceleration in the event of certain change of control transactions or the sale of JUXTAPID or MYALEPT. Upon completion of the Merger, the Company fair valued the contingent liability related to the DOJ and the SEC investigations as $40.6 million, which is consistent with the amounts to be provided to the DOJ and the SEC under the consolidated monetary package, and an aggregate of $1.0 million for any relator attorney fees and settlement. In March 2017, the final relator agreements were signed and we paid out the attorney fees and settlement payments.
The terms of the preliminary agreements in principle described above may change following further negotiations and other terms of the final settlement remain subject to further negotiation. The preliminary agreement in principle with the DOJ is subject to approval of supervisory personnel within the DOJ and relevant federal and state agencies, and approval by a U.S. District Court judge of the criminal plea and sentence and the civil settlement agreement. The preliminary agreement in principle with the SEC is subject to review by other groups in the SEC and approval by the Commissioners of the SEC.
The preliminary agreements in principle do not cover the DOJ and the SEC’s inquiries concerning Aegerion’s operations in Brazil, any potential claims by relators for attorneys’ fees, or any employment claims that may been brought by relators.
DOJ inquiries into patient assistance programs
Aegerion continues to cooperate with the DOJ and the SEC with respect to their investigations. As part of this cooperation, the DOJ has requested documents and information related to donations Aegerion made in the years ended December 31, 2015 and 2016 to patient assistance programs operated by independent charitable 501(c)(3) organizations. As part of this inquiry, the DOJ may pursue theories that will not be covered by the preliminary agreement in principle with the DOJ. Other pharmaceutical and biotechnology companies have disclosed similar inquiries regarding donations to patient assistance programs operated by independent charitable 501(c)(3) organizations.
Investigations in Brazil
In addition, federal and state authorities in Brazil are each conducting investigations to determine whether there have been violations of Brazilian laws related to the promotion of JUXTAPID in Brazil. In July 2016, the Ethics Council of the national pharmaceutical industry association, Interfarma, fined Aegerion approximately$0.5 million for violations of the industry association’s Code of Conduct, to which Aegerion is bound due to its affiliation with Interfarma. Also, the Board of Directors of Interfarma imposed an additional penalty of suspension of Aegerion’s membership, without suspension of Aegerion’s membership contribution, for a period of 180 days for Aegerion to demonstrate the implementation of effective measures to cease alleged irregular conduct, or exclusion of its membership in Interfarma if such measures are not implemented. Aegerion paid approximately $0.5 million related to this fine during the third quarter of 2016. On March 27, 2017, after the suspension period ended, Interfarma’s Board of Directors decided to reintegrate Aegerion, enabling it to participate regularly in Interfarma activities, subject to meeting certain obligations. Also, in July 2016, Aegerion received an inquiry from a Public Prosecutor Office of the Brazilian State of Paraná asking it to respond to questions related to recent media coverage regarding JUXTAPID and its relationship with a patient association to which Aegerion made donations for patient support. At this time, Aegerion does not know whether the Public Prosecutor’s inquiry will result in the commencement of any formal proceeding against Aegerion, but if Aegerion’s activities in Brazil are found to violate any laws or governmental regulations, Aegerion may be subject to significant civil lawsuits to be filed by the Public Prosecution office, and administrative penalties imposed by Brazilian regulatory authorities and additional damages and fines. Under certain circumstances, Aegerion could be barred from further sales to federal and/or state governments in Brazil, including sales of JUXTAPID and/or MYALEPT, due to penalties imposed by Brazilian regulatory authorities or through civil actions initiated by federal or state public prosecutors. As of December 31, 2016, Aegerion cannot determine if a loss is probable as a result of the investigations and inquiry in Brazil and whether the outcome will have a material adverse effect on its business and, as a result, no amounts have been recorded for a loss contingency.
Shareholder Class Action Lawsuit
In January 2014, a putative class action lawsuit was filed against Aegerion and certain of its former executive officers in the U.S. District Court for the District of Massachusetts (the Court) alleging certain misstatements and omissions related to the marketing of JUXTAPID and Aegerion’s financial performance in violation of the federal securities laws. The case is captioned KBC Asset Management NV et al. v. Aegerion Pharmaceuticals, Inc. et al., No. 14-cv-10105-MLW. Through mediation, the co-lead plaintiffs
156
and defendants reached an agreement in principle to settle the litigation on November 29, 2016. On January 17, 2017, the co-lead plaintiffs filed a stipulation of settlement with the Court that contained the settlement terms as agreed upon by the parties, including that Aegerion and its insurance carriers would contribute $22.25 million to a settlement fund for the putative class. The insurance carriers have agreed to cover $22.0 million of this amount, with Aegerion responsible for the remainder of $0.25 million. The proposed settlement is subject to a number of procedural steps and is subject to approval by the Court. Accordingly, management of the Company cannot predict the outcome of this action or when it will be resolved. Upon the completion of the Merger, the Company estimated the fair value of the amounts and recorded a loss contingency of $22.25 million and $22 million to reflect the insurance proceeds it expects to receive.
Contingent Consideration
Related to the Sale of Visudyne®
On September 24, 2012, the Company completed the sale of its Visudyne business to Valeant Pharmaceuticals International, Inc. (Valeant). Subject to the achievement of certain future milestones, the Company is eligible to receive the following additional consideration: (i) a milestone payment of $5.0 million if receipt of the registration required for commercial sale of the Qcellus ™ laser in the U.S. (the Laser Registration) is obtained by December 31, 2013, $2.5 million if the Laser Registration is obtained after December 31, 2013 but before January 1, 2015, and $0 if the Laser Registration is obtained thereafter (the Laser Earn-Out Payment); (ii) up to $5.0 million in each calendar year commencing January 1, 2013 (up to a maximum of $15.0 million in the aggregate) for annual net royalties exceeding $8.5 million pursuant to the Amended and Restated PDT Product Development, Manufacturing and Distribution Agreement with Novartis Pharma AG (the Novartis Agreement) or from other third-party sales of Visudyne outside of the U.S.; and (iii) a royalty on net sales attributable to new indications for Visudyne, if any should be approved by the U.S. Food and Drug Administration (FDA).
On September 26, 2013, the FDA approved the premarket approval application (PMA) supplement for the Qcellus laser and on October 10, 2013, the Company invoiced Valeant for the $5.0 million Laser Earn-Out Payment. Valeant subsequently disputed payment on the basis that it believes the Laser Earn-Out Payment remains contingent upon receipt of additional governmental authorizations with regard to the Qcellus laser. As a result, on September 22, 2015 the Company commenced an action in the Supreme Court of British Columbia against Valeant for breach of contract. See "Legal Proceedings" section of this Annual Report for further details.
While management of the Company believes that the $5.0 million Laser Earn-Out Payment has been triggered and is currently due and payable by Valeant, the outcome of such a dispute and litigation is uncertain and there may be difficulty in recovering damages and collecting the Laser Earn-Out Payment in full. As of December 31, 2015, Laser Earn-Out Payment was recorded as a long-term accounts receivable on the Company’s Consolidated Balance Sheet at its estimated fair value of $2.0 million. As of December 31, 2016, the fair value for the Laser Earn-Out Payment was reduced to zero. The fair value estimate of the Laser Earn-Out Payment was derived using a probability weighted approach to examine various possible outcomes with respect to the timing and amount that may be collected. In addition, it also reflects management’s assessment of collection risk, the impact of the passage of time and potential collection costs associated with the Valeant litigation. The remaining estimated fair value of the contingent consideration, which relates to estimated future net royalties pursuant to the Novartis Agreement, is currently valued at zero. For the years ended December 31, 2016 and 2015, the Company received no proceeds related to the collection of the contingent consideration for the Company’s previous sale of Visudyne.
Related to the Sale of the PPDS Technology
On April 3, 2013, Novelion completed the sale of its punctal plug drug delivery system technology for approximately $1.3 million (the PPDS Technology) to Mati Therapeutics Inc. (Mati) pursuant to the terms of Novelion’s asset purchase agreement with Mati (the Mati Agreement). Under the terms of the Mati Agreement, Novelion is eligible to receive future potential payments upon completion of certain product development and commercialization milestones that could reach $19.5 million (or exceed that amount if more than two products are commercialized), a low single digit royalty on worldwide net sales of all products using or developed from the PPDS Technology and a fee on payments received by Mati in respect of the PPDS Technology other than net sales revenues. For the years ended December 31, 2016 and 2015, the Company received no proceeds related to the collection of this contingent consideration.
17. Employee Benefit Plan.
The Company maintains a defined contribution 401(k) plan (the Plan) in which substantially all of its or its subsidiaries’ permanent U.S. employees are eligible to participate. Employee contributions are voluntary and are determined on an individual basis, limited by the maximum amounts allowable under U.S. federal tax regulations. The Company makes matching contributions of 50% of the first 6% of its U.S. employees’ contributions to the Plan. Additionally, for certain employees outside of the U.S., the Company contributes amounts for retirement benefits required by applicable local laws. The Company recorded employer contribution
157
expense of $27,331 during the year ended December 31, 2016 and recorded employer contribution expense of zero during the years ended 2015, and 2014. The Company assumed the Plan upon the completion of the Merger.
18. Related Party Transactions.
The Company did not enter into any transactions with related parties, other than compensation arrangements, expense allowances and other similar items in the ordinary course of business in the years ended December 31, 2016, 2015 and 2014.
19. Segmentation information.
The Company currently operates in one business segment, pharmaceuticals, which is focusing on the development and commercialization of its lead products. The Company's CEO is the Company's chief operating decision maker (CODM). The Company does not operate any separate lines of business or separate business entities with respect to its products. Accordingly, the Company does not accumulate discrete financial information with respect to separate service lines and does not have separately reportable segments. Enterprise-wide disclosures about product revenues and long-lived assets by geographic area and information relating to major customers are presented below.
Net Product Sales
The following table summarizes total net product sales from external customers by product and by geographic region. Net product sales represent sale of MYALEPT and JUXTAPID through Aegerion subsequent to the acquisition, from November 29, 2016 to December 31, 2016 and are attributed to countries based on the location of the customer.
For the Year Ended December 31, 2016 | ||||||||||||||||
(in thousands) | U.S. | Italy | Other Foreign Countries | Total | ||||||||||||
MYALEPT | $ | 4,685 | $ | — | $ | 268 | $ | 4,953 | ||||||||
JUXTAPID | 6,134 | 1,209 | 1,278 | 8,621 | ||||||||||||
Total | $ | 10,819 | $ | 1,209 | $ | 1,546 | $ | 13,574 | ||||||||
Net product sales generated from customers outside of the U.S. were primarily derived from named patient sales in Argentina, Colombia and Italy.
The total net product sales from customers in Canada subsequent to the Merger, from November 29, 2016 to December 31, 2016 was approximately $0.1 million, which related to the sales of JUXTAPID.
Significant Customers
For the year ended December 31, 2016, one customer accounted for 34.5% of the Company’s net product sales, and such customer accounted for 28.5% of the Company’s accounts receivable balance.
Long-lived Assets
The Company’s long-lived assets primarily comprised intangible assets, inventories, and properties and equipment. As of December 31, 2016, 100% of the Company's intangible assets were held by Aegerion. 65.5% of the intangible assets were attributable to Aegerion's U.S. business and the remaining 34.5% were attributable to Aegerion's Bermuda business. Approximately 92.7% of the Company’s properties and equipment were located in the U.S., 5.1% were located in Canada, and the remaining 2.2% were located in other foreign countries. For the Company's long-term inventory, approximately 52.0% was located in the U.S. and 48.0% was located in countries outside of the U.S.
Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
Item 9A. | Controls and Procedures |
Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, evaluated, as of the end of the period covered by this Annual Report, the effectiveness of our disclosure controls and procedures. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date were not effective due to a material weakness in our internal controls over the financial reporting process related
158
to business combinations. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed under the supervision of our principal executive officer and principal financial officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with accounting principles generally accepted in the United States. Management evaluated the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013). Management’s evaluation did not include an assessment of the effectiveness of internal control over financial reporting for Aegerion, which we acquired effective November 29, 2016. Aegerion's financial statements represent approximately 39% and 82% of the net and total assets, respectively, 100% of revenues and 12% of the net loss of the Company's Consolidated Financial Statements as of and for the year ended December 31, 2016.
Management, under the supervision and with the participation of the principal executive officer and principal financial officer, assessed the effectiveness of our internal control over financial reporting. Based on this evaluation, management determined that there was a material weakness in our controls over the financial reporting process as we did not design and maintain sufficiently precise or effective review and approval controls over business combinations. As a result, management concluded that we did not maintain effective internal control over financial reporting as of December 31, 2016.
The Company is committed to remediating the control deficiencies that constituted the above material weakness by implementing changes to its internal control over financial reporting. Management is responsible for implementing changes and improvements in the internal control over financial reporting and for remediating the control deficiencies that gave rise to the material weaknesses. To remediate the material weakness described above, the Company is currently evaluating the controls and procedures the Company will design and put in place to address this material weakness and plan to implement appropriate measures as part of this effort.
Our independent registered public accounting firm, Deloitte LLP, has audited our Consolidated Financial Statements included in this Annual Report and have issued a report on the effectiveness of our internal control over financial reporting as of December 31, 2016 . Their report on the audit of internal control over financial reporting appears below.
Changes to Internal Controls over Financial Reporting
During our fourth quarter of fiscal 2016 , except as described above, there were no changes made in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. | Other Information |
None.
159
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Novelion Therapeutics Inc. (formerly QLT Inc.)
We have audited the internal control over financial reporting of Novelion Therapeutics Inc. and subsidiaries (the “Company”) as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. As described in Management’s Report on Internal Control over Financial Reporting, management excluded from its assessment the internal control over financial reporting at Aegerion Pharmaceuticals Inc., which was acquired on November 29, 2016 and whose financial statements represents approximately 39% and 82% of the net and total assets, respectively, 100% of revenues, and 12% of the net loss of the Company’s Consolidated Financial Statements as of and for the year ended December 31, 2016. Accordingly, our audit did not include the internal control over financial reporting at Aegerion Pharmaceuticals Inc. The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on that risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed by, or under the supervision of, the company's principal executive and principal financial officers, or persons performing similar functions, and effected by the company's board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness has been identified and included in management’s assessment. Management has identified a material weakness in the Company’s controls over the financial reporting process as the Company did not design and maintain sufficiently precise or effective review and approval controls over business combinations. This material weakness was considered in determining the nature, timing and extent of audit tests applied in our audit of the Consolidated Financial Statements as of and for the year ended December 31, 2016 of the Company, and this report does not affect our report on such financial statements.
In our opinion, because of the effect of the material weakness identified above on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of December 31, 2016, based on the criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Consolidated Financial Statements as of and for the year ended December 31, 2016 of the Company and our report dated March 30, 2017 expressed an unqualified opinion on those financial statements.
/s/Deloitte LLP
Chartered Professional Accountants
March 30, 2017
Vancouver, Canada
160
PART III
Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this item will be contained in our definitive proxy statement, or Proxy Statement, which will be filed with the SEC in connection with our 2017 Annual General Meeting of Shareholders. Such information is incorporated herein by reference.
Code of Ethics
Our Board of Directors has adopted a code of conduct (the Code) that applies to our directors, officers and employees. The Code is available on the corporate governance section of our website (which is a subsection of the “Investors” section of our website) at the following address: www.novelion.com. We intend to disclose on our website any amendments or waivers to the Code that are required to be disclosed by SEC rules. You may also request a printed copy of the code, without charge, by writing to us at Novelion Therapeutics Inc., 887 Great Northern Way, Suite 250, Vancouver, B.C., Canada V5T 4T5 Attn: Investor Relations.
Item 11. | Executive Compensation |
The information required by this item will be contained in our Proxy Statement, which will be filed with the SEC in connection with our 2017 Annual General Meeting of Shareholders. Such information is incorporated herein by reference.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
The information required by this item will be contained in our Proxy Statement, which will be filed with the SEC in connection with our 2017 Annual General Meeting of Shareholders. Such information is incorporated herein by reference.
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item will be contained in our Proxy Statement, which will be filed with the SEC in connection with our 2017 Annual General Meeting of Shareholders. Such information is incorporated herein by reference.
Item 14. | Principal Accountant Fees and Services |
The information required by this item will be contained in our Proxy Statement, which will be filed with the SEC in connection with our 2017 Annual General Meeting of Shareholders. Such information is incorporated herein by reference.
161
PART IV
Item 15. | Exhibits, Financial Statement Schedules |
(a) The following documents are filed as part of this Annual Report:
1. | Consolidated Financial Statements (see Item 8). |
2. | All information is included in the Consolidated Financial Statements or notes thereto. |
3. | Exhibits: |
See Exhibit Index.
162
Item 16. | Summary |
Not applicable.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
NOVELION THERAPEUTICS INC. | ||
Date: March 30, 2017 | By: | /s/ Mary Szela |
Mary Szela | ||
Chief Executive Officer | ||
(principal executive officer) and Director | ||
Date: March 30, 2017 | By: | /s/ Gregory D. Perry |
Gregory D. Perry | ||
Chief Financial and Administrative Officer | ||
(principal financial officer ) | ||
Date: March 30, 2017 | By: | /s/ Barbara Chan |
Barbara Chan | ||
President and Chief Accounting Officer, Aegerion Pharmaceuticals, Inc. | ||
(principal accounting officer ) | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below appoints severally, Mary Szela, Gregory D. Perry and Benjamin Harshbarger, and each one of them, his or her attorneys-in-fact, each with the power of substitution for him or her in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the SEC, hereby ratifying and confirming all that each attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Capacity | Date | ||
/s/ Mary Szela | Chief Executive Officer (principal executive officer) and Director | March 30, 2017 | ||
Mary Szela | ||||
/s/ Gregory D. Perry | Chief Financial and Administrative Officer (principal financial officer) | March 30, 2017 | ||
Gregory D. Perry | ||||
/s/ Barbara Chan | President and Chief Accounting Officer, Aegerion Pharmaceuticals, Inc. (principal accounting officer) | March 30, 2017 | ||
Barbara Chan | ||||
/s/ Jason Aryeh | Chairman of the Board of Directors | March 30, 2017 | ||
Jason Aryeh | ||||
/s/ Geoffrey Cox | Director | March 30, 2017 | ||
Geoffrey Cox | ||||
163
/s/ Kevin Kotler | Director | March 30, 2017 | ||
Kevin Kotler | ||||
/s/ Jorge Plutzky | Director | March 30, 2017 | ||
Jorge Plutzky | ||||
/s/ Stephen Sabba | Director | March 30, 2017 | ||
Stephen Sabba | ||||
/s/ Sandford D. Smith | Vice Chair of the Board of Directors | March 30, 2017 | ||
Sandford D. Smith | ||||
/s/ Donald K. Stern | Director | March 30, 2017 | ||
Donald K. Stern | ||||
/s/ John Thomas, Jr. | Director | March 30, 2017 | ||
John Thomas, Jr. | ||||
/s/ Anne VanLent | Director | March 30, 2017 | ||
Anne VanLent | ||||
164
EXHIBIT INDEX
The exhibits listed below are filed as part of this Annual Report. References under the caption “Location” to exhibits or other filings indicate that the exhibit or other filing has been filed, that the indexed exhibit and the exhibit or other filing referred to are the same and that the exhibit or other filing referred to is incorporated by reference.
Table of Contents
Exhibit | Description of Document | Location | ||
2.1 | # | Asset Purchase Agreement, dated September 21, 2012, by and between the Company and Valeant. | Exhibit 10.65 to the Company’s Current Report on Form 8-K, filed with the SEC on September 27, 2012. | |
2.2 | # | Asset Purchase Agreement, dated April 3, 2013, by and between the Company and Mati Therapeutics Inc. | Exhibit 10.70 to the Company’s Current Report on Form 8-K, filed with the SEC on April 9, 2013. | |
2.3 | # | Asset Purchase Agreement, dated November 5, 2014, by and among Aegerion Pharmaceuticals, Inc., Amylin Pharmaceuticals, LLC and, solely for purposes of Sections 2.1.1, 2.2.1 and 2.3.2, AstraZeneca Pharmaceuticals LP. | Exhibit 10.29 to Aegerion Pharmaceuticals, Inc.’s Amendment No. 1 to the Annual Report on Form 10-K, filed with the SEC on July 7, 2015. | |
2.4 | First Amendment to Asset Purchase Agreement dated January 9, 2015, by and among Aegerion Pharmaceuticals, Inc., Amylin Pharmaceuticals, LLC and, solely for purposes of Sections 2.1.1, 2.2.1 and 2.3.2, AstraZeneca Pharmaceuticals LP. | Exhibit 10.30 to Aegerion Pharmaceuticals, Inc.’s Annual Report on Form 10-K, filed with the SEC on March 2, 2015. | ||
2.5 | Share Purchase Agreement, dated June 8, 2015, by and among the Company, Broadfin Healthcare Master Fund, Ltd., JW Partners, LP, JW Opportunities Fund, LLC, EcoR1 Capital Fund Qualified, L.P. and EcoR1 Capital Fund, L.P. | Exhibit 2.3 to the Company’s Current Report on Form 8-K, filed with the SEC on June 12, 2015. | ||
2.6 | Share Purchase and Registration Rights Agreement, dated June 8, 2015, by and among the Company, Broadfin Healthcare Master Fund, Ltd., JW Partners, LP, JW Opportunities Fund, LLC, EcoR1 Capital Fund Qualified, L.P. and EcoR1 Capital Fund, L.P. | Exhibit 2.4 to the Company’s Current Report on Form 8-K, filed with the SEC on June 12, 2015. | ||
2.7 | Letter agreement, dated June 8, 2015, by and among the Company, Broadfin Healthcare Master Fund, Ltd., JW Partners, LP, JW Opportunities Fund, LLC, EcoR1 Capital Fund Qualified, L.P. and EcoR1 Capital Fund, L.P. | Exhibit 2.5 to the Company’s Current Report on Form 8-K, filed with the SEC on June 12, 2015. | ||
2.8 | Second Amended and Restated Agreement and Plan of Merger, dated August 26, 2015, by and among InSite Vision Incorporated, the Company and Isotope Acquisition Corp. | Exhibit 2.1 to the Company’s Current Report on Form 8-K, filed with the SEC on August 28, 2015. | ||
2.9 | Amended and Restated Share Subscription Agreement, dated December 7, 2015, among the Company, Tribute Pharmaceuticals Canada Inc., POZEN Inc., Aralez Pharmaceuticals, Inc., Aralez Pharmaceuticals Plc, Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., Deerfield Partners, L.P., Broadfin Healthcare Master Fund, Ltd., JW Partners, LP, JW Opportunities Fund, LLC, and J.W. Opportunities Master Fund, Ltd. | Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on December 11, 2015. | ||
2.10 | Unit Subscription Agreement, dated June 14, 2016, by and among the Company, Deerfield International Master Fund, L.P., Deerfield Partners, L.P., Broadfin Healthcare Master Fund, Ltd., JW Partners LP, JW Opportunities Master Fund, Ltd., The K2 Principal Fund L.P., Healthcare Value Partners, L.P., Tiger Legatus Capital Management, LLC, Sarissa Capital Domestic Fund LP, Sarissa Capital Offshore Master Fund LP, Armistice Capital Master Fund, Ltd., Levcap Alternative Fund, L.P., Ulysses Partners, L.P., Ulysses Offshore Fund, Ltd. and Jason Aryeh, as amended as applied to Broadfin Healthcare Master Fund, Ltd. on September 9, 2016. | Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on December 5, 2016. | ||
165
Exhibit | Description of Document | |||
2.11 | Agreement and Plan of Merger, dated June 14, 2016, and Amendment No. 1 thereto, by and among Aegerion Pharmaceuticals, Inc., the Company and Isotope Acquisition Corp. | Annex A to the Company’s Amendment No. 1 to the Registration Statement on Form S-4, filed with the SEC on September 12, 2016. | ||
3.1 | Articles of the Company, dated May 25, 2005. | Exhibit 3.2 to the Company’s Current Report on Form 8-K, filed with the SEC on June 1, 2005. | ||
3.2 | Notice of Articles of the Company, dated November 29, 2016. | Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed with the SEC on December 5, 2016. | ||
4.1 | Specimen Common Share Certificate of the Company. | Exhibit 4.1 to the Company’s Current Report on Form 8-K, filed with the SEC on December 16, 2016. | ||
4.2 | Indenture, dated August 15, 2014, by and between Aegerion Pharmaceuticals, Inc. and The Bank of New York Mellon Trust Company, N.A., as Trustee, relating to the 2.00% Convertible Senior Notes Due 2019. | Exhibit 4.1 to Aegerion Pharmaceuticals, Inc.’s Current Report on Form 8-K, filed with the SEC on August 15, 2014. | ||
4.3 | Supplemental Indenture, dated November 29, 2016, by and between Aegerion Pharmaceuticals, Inc. and The Bank of New York Mellon Trust Company, N.A., as Trustee, relating to the 2.00% Convertible Senior Notes Due 2019. | Exhibit 4.1 to Aegerion Pharmaceuticals, Inc.’s Current Report on Form 8-K, filed with the SEC on November 29, 2016. | ||
10.1 | # | License Agreement, dated February 7, 2006, by and between Amylin Pharmaceuticals, LLC and Amgen Inc. | Exhibit 10.32 to Aegerion Pharmaceuticals, Inc.’s Annual Report on Form 10-K, filed with the SEC on March 2, 2015. | |
10.2 | # | Patent License Agreement, dated May 19, 2006, as amended September 27, 2006, by and between Aegerion Pharmaceuticals, Inc. and University of Pennsylvania. | Exhibit 10.6 to Aegerion Pharmaceuticals, Inc.’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010. | |
10.3 | # | License Agreement, dated July 8, 2009, by and between Amylin Pharmaceuticals, LLC and Shionogi & Co., Ltd. | Exhibit 10.31 to Aegerion Pharmaceuticals, Inc.’s Annual Report on Form 10-K, filed with the SEC on March 2, 2015. | |
10.4 | Amended and Restated PDT Product Development, Manufacturing and Distribution Agreement, dated October 16, 2009, by and between the Company and Novartis Pharma AG. | Exhibit 10.48 to the Company’s Current Report on Form 8-K, filed with the SEC on October 22, 2009. | ||
10.5 | # | Co-Development Agreement, dated April 4, 2006, by and between the Company and Retinagenix, LLC, as amended by letter agreements dated August 10, 2006, September 11, 2008 and October 20, 2010. | Exhibit 10.54 to the Company’s Annual Report on Form 10-K, filed with the SEC on March 1, 2011. | |
10.6 | Sublease, dated June 2, 2015, by and between Discovery Parks Realty Corp. and the Company. | Filed herewith. | ||
10.7 | Sublease Amending and Expansion Agreement, dated January 4, 2016, by and between Discovery Parks Realty Corp. and the Company. | Filed herewith. | ||
10.8 | Sublease Renewal Agreement, dated May 18, 2016, by and between Discovery Parks Realty Corp. and the Company. | Filed herewith. | ||
10.9 | Lease, dated January 1, 2011, by and between Aegerion Pharmaceuticals, Inc. and RREEF America REIT II CORP. PPP. | Exhibit 10.1 to Aegerion Pharmaceuticals, Inc.’s Current Report on Form 8-K, filed with the SEC on January 6, 2011. | ||
10.10 | First Amendment to Lease, dated November 7, 2011, by and between Aegerion Pharmaceuticals, Inc. and RREEF America REIT II CORP. PPP. | Exhibit 10.22 to Aegerion Pharmaceuticals, Inc.’s Annual Report on Form 10-K, filed with the SEC on March 15, 2012. | ||
10.11 | Second Amendment to Lease, dated September 4, 2012, by and between Aegerion Pharmaceuticals, Inc. and RREEF America REIT II Corp. PPP. | Exhibit 10.1 to Aegerion Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q, filed with the SEC on November 9, 2012. | ||
10.12 | Third Amendment to Lease, dated June 19, 2013, by and between Aegerion Pharmaceuticals, Inc. and RREEF America REIT II Corp. PPP. | Exhibit 10.1 to Aegerion Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q, filed with the SEC on August 9, 2013. | ||
166
Exhibit | Description of Document | Location | ||
10.13 | Fourth Amendment to Lease, dated January 1, 2014, by and between Aegerion Pharmaceuticals, Inc. and RREEF America REIT II Corp. PPP. | Exhibit 10.25 to Aegerion Pharmaceuticals, Inc.’s Annual Report on Form 10-K, filed with the SEC on March 3, 2014. | ||
10.14 | Fifth Amendment to Lease, dated July 19, 2016, by and between Aegerion Pharmaceuticals, Inc. and RREEF America REIT II Corp. PPP. | Exhibit 10.3 to Aegerion Pharmaceuticals, Inc.’s Quarterly Report on Form 10-Q, filed with the SEC on November 4, 2016. | ||
10.15 | Loan and Security Agreement, dated June 14, 2016, by and between the Company and Aegerion Pharmaceuticals, Inc. | Exhibit 10.2 to Aegerion Pharmaceuticals, Inc.’s Current Report on Form 8-K, filed with the SEC on June 15, 2016. | ||
10.16 | Letter agreement, dated December 7, 2015, by and among the Company, Broadfin Healthcare Master Fund, Ltd., JW Partners, LP, JW Opportunities Fund, LLC, and J.W. Opportunities Master Fund, Ltd. | Exhibit 10.2 to the Company’s Current Report on Form 8-K, filed with the SEC on December 11, 2015. | ||
10.17 | Form of Warrant Agreement. | Exhibit C of Amendment No. 1 to the Agreement and Plan of Merger from Annex A to the Company’s Amendment No. 1 to the Registration Statement on Form S-4, filed with the SEC on September 12, 2016. | ||
10.18 | * | Deferred Share Unit Plan For Non-Employee Directors of the Company. | Exhibit 10.32 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 9, 2005. | |
10.19 | * | Aegerion Pharmaceuticals, Inc. 2010 Stock Option and Incentive Plan. | Exhibit 10.2 to Aegerion Pharmaceuticals, Inc.’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010. | |
10.20 | * | Aegerion Pharmaceuticals, Inc. Form of Incentive Stock Option Agreement for Executive Officers and forms of Non-Qualified Stock Option Agreement and Restricted Stock Award Agreement for Directors. | Exhibit 10.6 to Aegerion Pharmaceuticals, Inc.’s Annual Report on Form 10-K, filed with the SEC on March 18, 2013. | |
10.21 | * | Novelion 2016 Equity Incentive Plan. | Exhibit 99.4 to the Company’s Registration Statement on Form S-8, filed with the SEC on December 5, 2016. | |
10.22 | * | Form of Stock Option Award Grant Notice and Stock Option Award Agreement (Directors) under the Novelion 2016 Equity Incentive Plan. | Filed herewith. | |
10.23 | * | Form of Stock Option Award Grant Notice and Stock Option Award Agreement (Employees) under the Novelion 2016 Equity Incentive Plan. | Filed herewith. | |
10.24 | * | Form of Stock Option Award Grant Notice and Stock Option Award Agreement (Executives) under the Novelion 2016 Equity Incentive Plan. | Filed herewith. | |
10.25 | * | Form of Performance Restricted Stock Unit Award Grant Notice and Performance Restricted Stock Unit Award Agreement under the Novelion 2016 Equity Incentive Plan. | Filed herewith. | |
10.26 | * | Form of Restricted Stock Unit Award Grant Notice and Restricted Stock Unit Award Agreement under the Novelion 2016 Equity Incentive Plan. | Filed herewith. | |
10.27 | Form of Indemnity Agreement (Directors). | Filed herewith. | ||
10.28 | * | Form of Indemnity Agreement (Officers). | Filed herewith. | |
10.29 | * | Employment Agreement, dated January 7, 2016, by and between Aegerion Pharmaceuticals, Inc. and Mary T. Szela. | Exhibit 10.1 to Aegerion Pharmaceuticals, Inc.’s Form 8-K, filed with the SEC on January 11, 2016. | |
10.30 | * | Employment Agreement, dated October 23, 2014, by and between the Company and Geoffrey F. Cox. | Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on October 28, 2014. | |
10.31 | * | Amendment to Employment Agreement, dated April 22, 2015, by and between the Company and Geoffrey F. Cox. | Exhibit 10.79 to the Company’s Current Report on Form 8-K, filed with the SEC on April 23, 2015. | |
167
Exhibit | Description of Document | Location | ||
10.32 | * | Second Amendment to Employment Agreement, dated October 8, 2015, by and between the Company and Geoffrey F. Cox. | Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on October 9, 2015. | |
10.33 | * | Third Amendment to Employment Agreement, dated April 7, 2016, by and between the Company and Geoffrey F. Cox. | Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on April 11, 2016. | |
10.34 | * | Fourth Amendment to Employment Agreement, dated June 17, 2016, by and between the Company and Geoffrey F. Cox. | Exhibit 10.9 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 9, 2016. | |
10.35 | * | Fifth Amendment to Employment Agreement, dated September 16, 2016, by and between the Company and Geoffrey F. Cox. | Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on September 16, 2016. | |
10.36 | * | Form of employee stock option grant to Geoffrey F. Cox. | Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on October 28, 2014. | |
10.37 | * | Employment Offer Letter, dated November 28, 2016, between Novelion Services USA, Inc. and Gregory D. Perry. | Filed herewith. | |
10.38 | * | Employment Agreement, dated January 5, 2015, by and between the Company and Glen Ibbott. | Exhibit 10.76 to the Company’s Current Report on Form 8-K, filed with the SEC on January 5, 2015. | |
10.39 | * | Amendment to Employment Agreement, dated November 5, 2015, by and between the Company and Glen Ibbott. | Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed with the SEC on November 12, 2015. | |
10.40 | * | Second Amendment to Employment Agreement, dated June 17, 2016, by and between the Company and Glen Ibbott. | Exhibit 10.10 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 9, 2016. | |
10.41 | * | Employment Agreement, by and between the Company and Dori Assaly, dated June 14, 2013, as amended November 5, 2015. | Exhibit 10.29 to the Company’s Annual Report on Form 10-K, filed with the SEC on February 25, 2016. | |
10.42 | * | Amendment to Employment Agreement, dated June 17, 2016, by and between the Company and Dori Assaly. | Exhibit 10.11 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 9, 2016. | |
10.43 | * | Employment Agreement, by and between the Company and Lana Janes, dated January 1, 2010, as amended November 5, 2015. | Exhibit 10.27 to the Company’s Annual Report on Form 10-K, filed with the SEC on February 25, 2016. | |
10.44 | * | Change of Control Agreement between the Company and Lana Janes, dated June 1, 2015. | Exhibit 10.28 to the Company’s Annual Report on Form 10-K, filed with the SEC on February 25, 2016. | |
10.45 | * | Amendment to Employment Agreement, dated June 17, 2016, by and between the Company and Lana Janes. | Exhibit 10.12 to the Company’s Quarterly Report on Form 10-Q, filed with the SEC on August 9, 2016. | |
10.46 | * | Employment Offer Letter, dated November 28, 2016, between Novelion Services USA, Inc. and Benjamin Harshbarger. | Filed herewith. | |
10.47 | * | Employment Offer Letter, dated November 28, 2016, between Novelion Services USA, Inc. and Roger Louis. | Filed herewith. | |
10.48 | * | Employment Offer Letter, dated November 28, 2016, between Novelion Services USA, Inc. and Remi Menes. | Filed herewith. | |
21.1 | Subsidiaries of the Company. | Filed herewith. | ||
23.1 | Consent of Deloitte LLP. | Filed herewith. | ||
24.1 | Power of Attorney. | Contained on signature page hereto. | ||
31.1 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002: Mary Szela, Chief Executive Officer (Principal Executive Officer). | Filed herewith. | ||
168
Exhibit | Description of Document | Location | ||
31.2 | Certification pursuant to 18 U.S.C. Section 1350. as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002: Gregory D. Perry, Chief Financial and Administrative Officer (Principal Financial Officer). | Filed herewith. | ||
32.1 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002: Mary Szela, Chief Executive Officer (Principal Executive Officer). | Filed herewith. | ||
32.2 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002: Gregory D. Perry, Chief Financial and Administrative Officer (Principal Financial Officer). | Filed herewith. | ||
101.1 | The following financial statements from the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, formatted in Extensible Business Reporting Language: Consolidated Balance Sheets; Consolidated Statements of Operations; Consolidated Statements of Comprehensive Income; Consolidated Statements of Cash Flows; Consolidated Statements of Shareholders’ Equity; and Notes to Consolidated Financial Statements. | |||
________________________________
Notes:
* Denotes executive compensation plans or arrangements.
# Confidential treatment has been received for certain provisions of this Exhibit. Confidential portions have been omitted and filed separately with the SEC.
169
