Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Loxo Oncology, Inc. | a18-39556_18k.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a18-39556_1ex99d1.htm |
Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future, "likely," "may," "should," "will" and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our future financial performance, business plans and objectives, timing and success of our clinical trials, our ability to obtain regulatory approval or the timing of regulatory filings, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, the timing or success of commercialization, our partnership with Bayer, financing plans, competitive position and industry environment. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: those related to our future financial performance, our ability to raise additional funding when needed, our ability to develop and maintain partnerships, our ability to identify and develop new products in a timely manner, the outcome, cost and timing of our product development activities and clinical trials, market size and acceptance of our targeted small molecule therapeutics and diagnostics, our ability to maintain, protect and enhance our brand and intellectual property, our ability to continue to stay in compliance with applicable laws and regulations, our ability to scale our business and make key hires and such other factors as discussed under the section titled “Risk Factors” and elsewhere in our annual report on Form 10-K and quarterly reports on Form 10-Q that we filed with the Securities and Exchange Commission (“SEC”) as well as our other filings and the documents incorporated by reference therein, with the SEC. Any forward-looking statement made by us in this presentation and the accompanying oral presentation is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Certain information in this slide deck regarding our product candidates is derived from information provided by industry sources. We believe such information is accurate and that the sources from which is has been obtained are reliable. However, we cannot guarantee the accuracy of, and have not independently verified such information. 2
Our Approach Loxo Oncology builds highly selective medicines designed to target genomically defined cancers Each of our clinical programs meets the following criteria: The genomic alteration in question is an oncogenic driver, not a mere passenger event in the cancer cell We believe we can develop a selective medicine with best-in-class potential Patients with the alteration can be identified through testing 3
Building the Loxo Oncology Pipeline 4 In-house R&D In-licensing & Acquisitions Discovery Collaborations
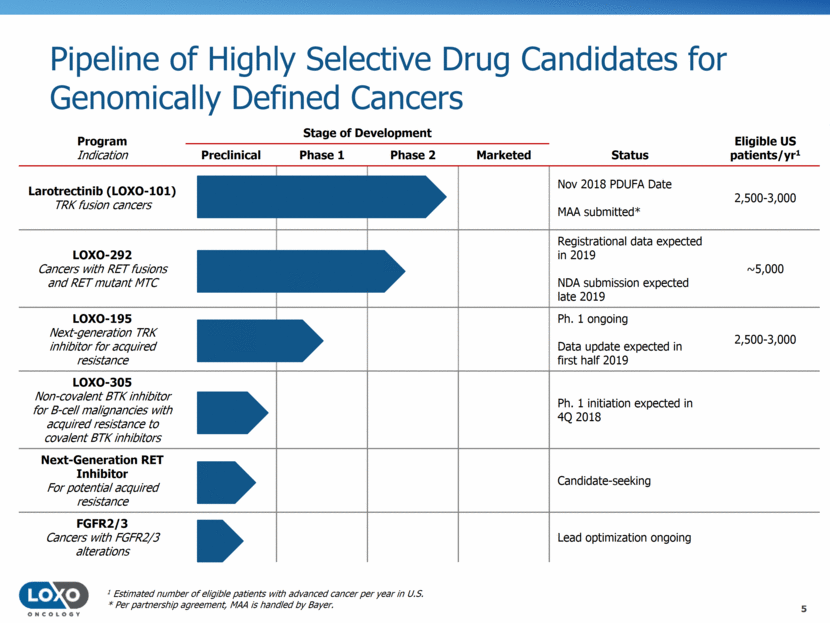
Pipeline of Highly Selective Drug Candidates for Genomically Defined Cancers 5 Program Indication Stage of Development Eligible US patients/yr1 Preclinical Phase 1 Phase 2 Marketed Status Larotrectinib (LOXO-101) TRK fusion cancers Nov 2018 PDUFA Date MAA submitted* 2,500-3,000 LOXO-292 Cancers with RET fusions and RET mutant MTC Registrational data expected in 2019 NDA submission expected late 2019 ~5,000 LOXO-195 Next-generation TRK inhibitor for acquired resistance Ph. 1 ongoing Data update expected in first half 2019 2,500-3,000 LOXO-305 Non-covalent BTK inhibitor for B-cell malignancies with acquired resistance to covalent BTK inhibitors Ph. 1 initiation expected in 4Q 2018 Next-Generation RET Inhibitor For potential acquired resistance Candidate-seeking FGFR2/3 Cancers with FGFR2/3 alterations Lead optimization ongoing 1 Estimated number of eligible patients with advanced cancer per year in U.S. * Per partnership agreement, MAA is handled by Bayer.
6 Larotrectinib (TRK)
Bayer Global Partnership for TRK Franchise 7 Global partnership with Bayer to develop and commercialize larotrectinib and LOXO-195, announced November 14, 2017 Up to $1.55B in upfront, regulatory, and commercial milestones Loxo will lead global development activities and U.S. regulatory activities Development costs shared 50/50 globally In the U.S., Loxo and Bayer will co-promote the products and share commercial costs and profits on a 50/50 basis Bayer will lead ex-U.S. regulatory activities and worldwide commercial activities Bayer will pay tiered, double-digit royalties on ex-U.S. net sales

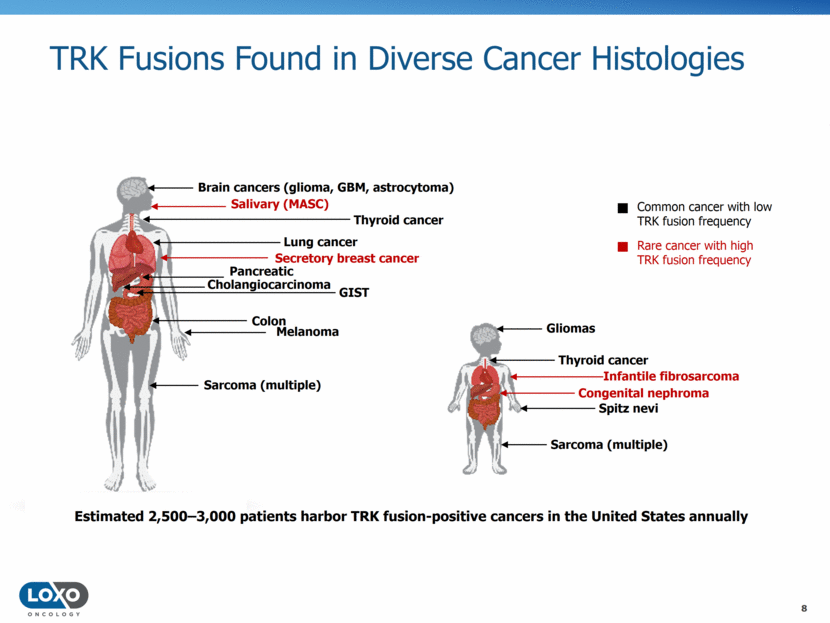
TRK Fusions Found in Diverse Cancer Histologies 8 Brain cancers (glioma, GBM, astrocytoma) Salivary (MASC) Lung cancer Secretory breast cancer Melanoma Colon Sarcoma (multiple) Infantile fibrosarcoma Gliomas Thyroid cancer Congenital nephroma Sarcoma (multiple) Common cancer with low TRK fusion frequency Rare cancer with high TRK fusion frequency Cholangiocarcinoma Pancreatic GIST Thyroid cancer Spitz nevi Estimated 2,500–3,000 patients harbor TRK fusion-positive cancers in the United States annually
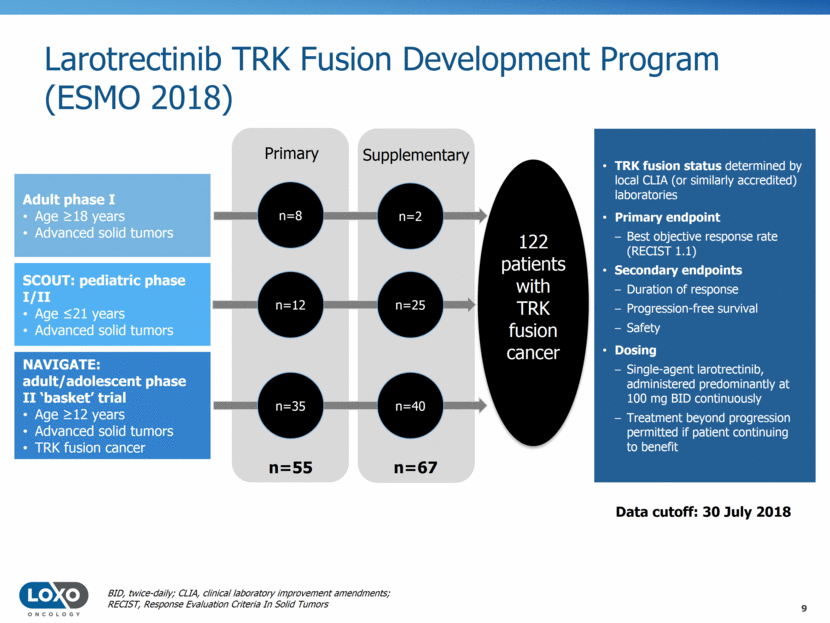
Larotrectinib TRK Fusion Development Program (ESMO 2018) Adult phase I Age 18 years Advanced solid tumors SCOUT: pediatric phase I/II Age 21 years Advanced solid tumors NAVIGATE: adult/adolescent phase II ‘basket’ trial Age 12 years Advanced solid tumors TRK fusion cancer TRK fusion status determined by local CLIA (or similarly accredited) laboratories Primary endpoint Best objective response rate (RECIST 1.1) Secondary endpoints Duration of response Progression-free survival Safety Dosing Single-agent larotrectinib, administered predominantly at 100 mg BID continuously Treatment beyond progression permitted if patient continuing to benefit 122 patients with TRK fusion cancer Primary Supplementary n=2 n=25 n=8 n=12 n=55 n=67 n=40 n=35 Data cutoff: 30 July 2018 BID, twice-daily; CLIA, clinical laboratory improvement amendments; RECIST, Response Evaluation Criteria In Solid Tumors 9
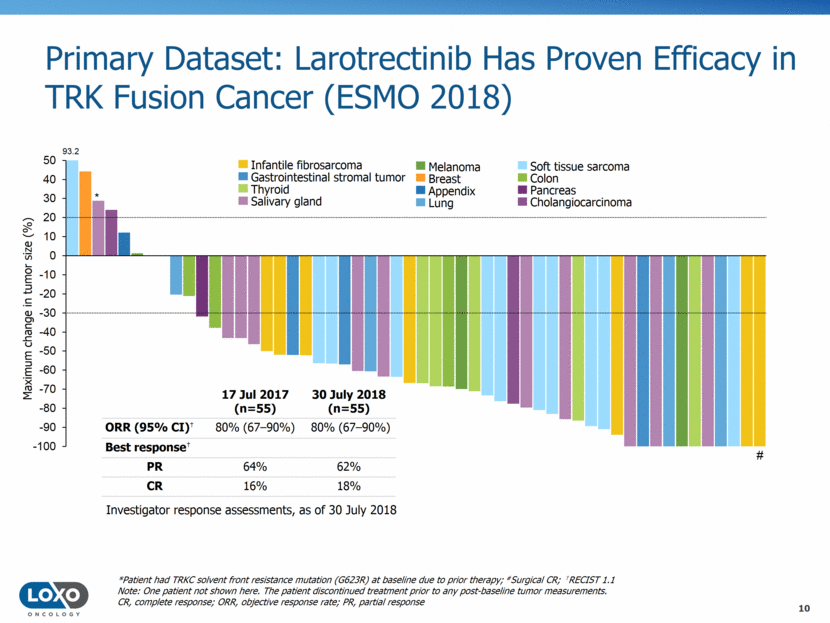
Primary Dataset: Larotrectinib Has Proven Efficacy in TRK Fusion Cancer (ESMO 2018) 17 Jul 2017 (n=55) 30 July 2018 (n=55) ORR (95% CI)† 80% (6790%) 80% (6790%) Best response† PR 64% 62% CR 16% 18% # *Patient had TRKC solvent front resistance mutation (G623R) at baseline due to prior therapy; #Surgical CR; †RECIST 1.1 Note: One patient not shown here. The patient discontinued treatment prior to any post-baseline tumor measurements. CR, complete response; ORR, objective response rate; PR, partial response * Maximum change in tumor size (%) Infantile fibrosarcoma Gastrointestinal stromal tumor Thyroid Salivary gland Melanoma Breast Lung Appendix Soft tissue sarcoma Colon Pancreas Cholangiocarcinoma 93.2 Investigator response assessments, as of 30 July 2018 10 -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 50
Supplementary Dataset: Larotrectinib Efficacy Consistent with Primary Dataset (ESMO 2018) Primary (n=55) Supplementary* (n=54) ORR (95% CI)† 80% (6790%) 81% (6991%) Best response† PR 62% 65% CR 18% 17% Infantile fibrosarcoma Gastrointestinal stromal tumor Thyroid Salivary gland Congenital mesoblastic nephroma Unknown primary Lung Soft tissue sarcoma Colon Bone sarcoma Melanoma # Maximum change in tumor size (%) *Evaluable patients; includes 9 unconfirmed PRs pending confirmation; does not include 13 patients continuing on study and awaiting initial response assessment; #Surgical CR; †RECIST 1.1 Note: One patient not shown here. The patient discontinued treatment prior to any post-baseline tumor measurements. CR, complete response; ORR, objective response rate; PR, partial response Investigator response assessments, as of 30 July 2018 11
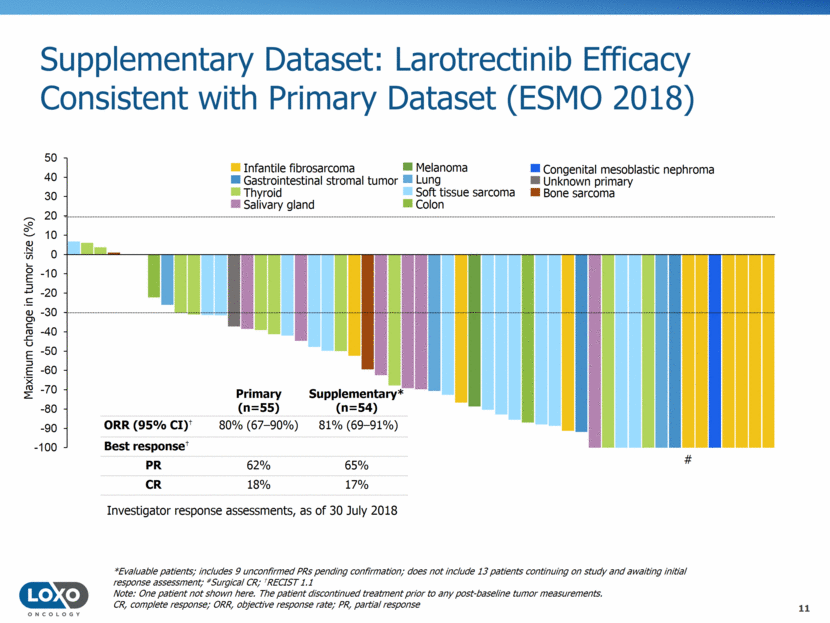
Integrated Dataset: Larotrectinib is Efficacious Regardless of Tumor Type (ESMO 2018) Infantile fibrosarcoma Soft tissue sarcoma Thyroid Salivary gland Melanoma Breast Lung Appendix Gastrointestinal stromal tumor Colon Pancreas Cholangiocarcinoma Congenital mesoblastic nephroma Unknown primary Bone sarcoma Maximum change in tumor size (%) ‡Includes 9 unconfirmed PRs pending confirmation; does not include 13 patients continuing on study and awaiting initial response assessment *Patient had TRKC solvent front resistance mutation (G623R) at baseline due to prior therapy; #Surgical CR; †RECIST 1.1 Note: Two patients not shown here. These patients discontinued treatment prior to any post-baseline tumor measurements. CR, complete response; ORR, objective response rate; PR, partial response Investigator response assessments, as of 30 July 2018 Integrated‡ (n=109) ORR (95% CI)† 81% (7288%) Best response† PR 63% CR 17% # 93.2 # * # 12
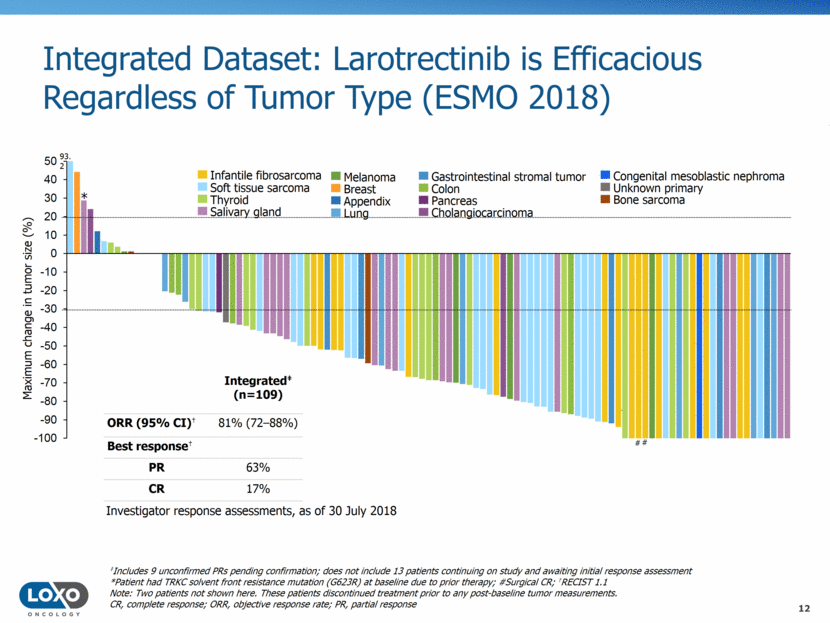
Integrated Dataset: Larotrectinib is Efficacious Regardless of Age (ESMO 2018) Maximum change in tumor size (%) ‡Includes 9 unconfirmed PRs pending confirmation; does not include 13 patients continuing on study and awaiting initial response assessment ͌Age <21 years *Patient had TRKC solvent front resistance mutation (G623R) at baseline due to prior therapy; #Surgical CR; †RECIST 1.1 Note: Two patients not shown here. These patients discontinued treatment prior to any post-baseline tumor measurements. CR, complete response; ORR, objective response rate; PR, partial response Investigator response assessments, as of 30 July 2018 Adult patients Pediatric patients ͌ Integrated‡ (n=109) ORR (95% CI)† 81% (7288%) Best response† PR 63% CR 17% # 93.2 # * # 13
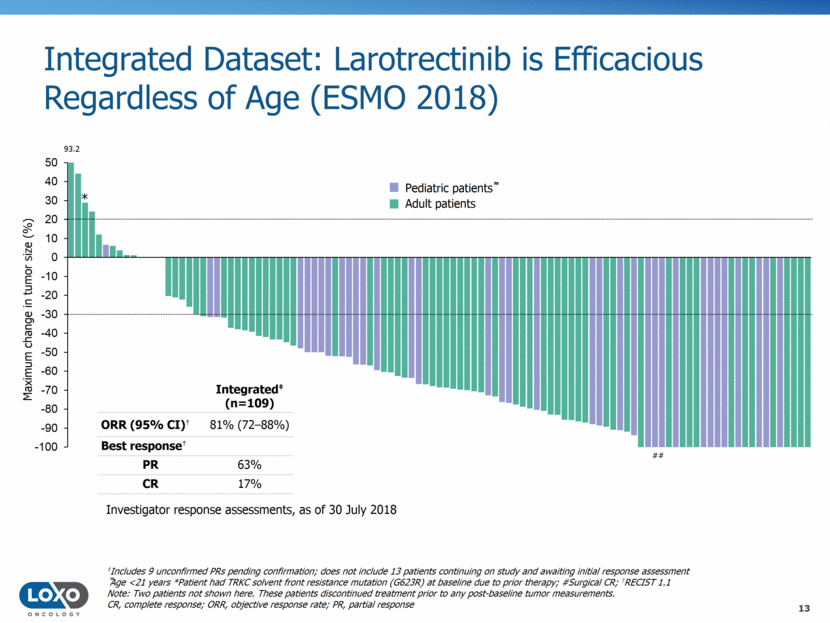
Overall treatment duration (months) Median time to response = 1.8 months Integrated Dataset (n=122): Duration of Larotrectinib Treatment (ESMO 2018) Treatment after progression Treatment after surgery Surgical CR Treatment ongoing n=122 patients 84% of responding patients and 73% of all patients remain on treatment or underwent surgery with curative intent Investigator response assessments, as of 30 July 2018 CR, complete response 14
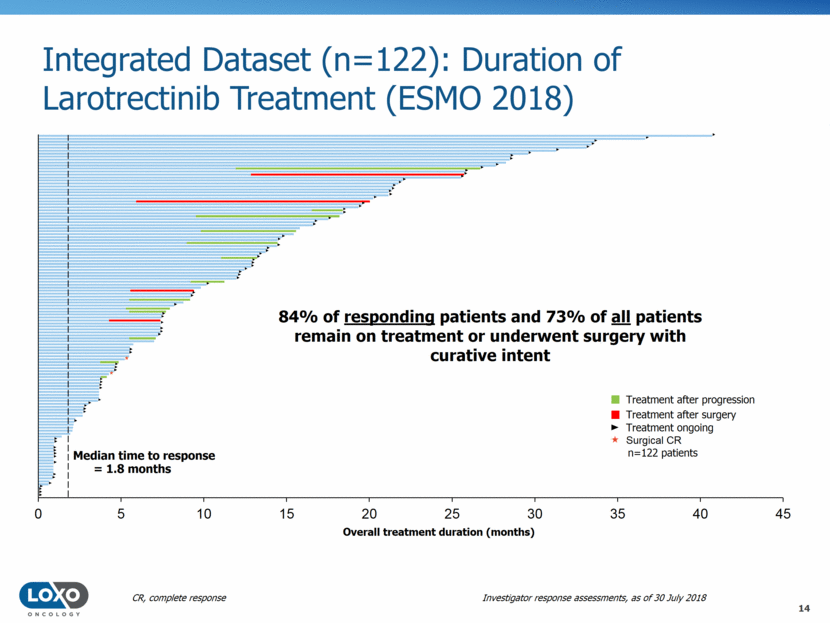
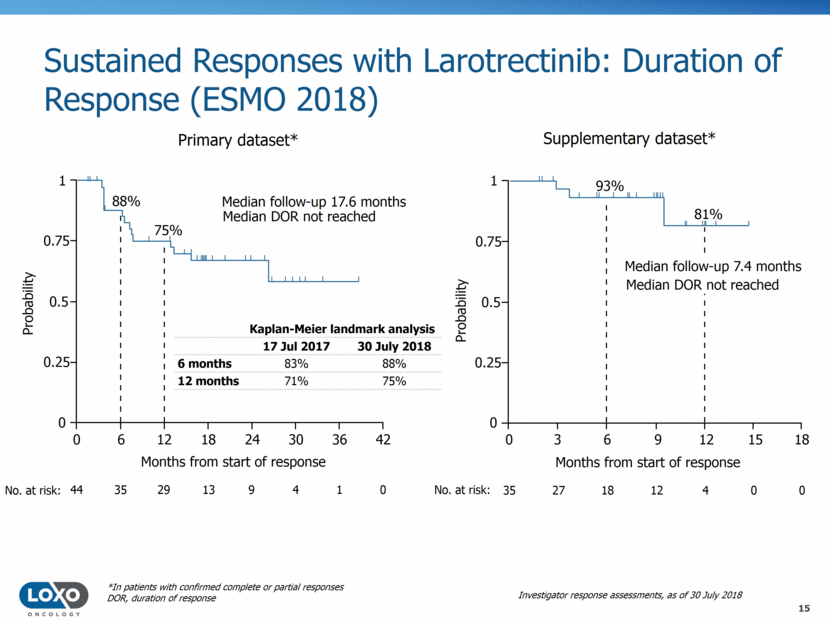
Sustained Responses with Larotrectinib: Duration of Response (ESMO 2018) 0.75 Median follow-up 17.6 months Median DOR not reached 88% 75% Probability Months from start of response 1 0.5 0.25 0 0 6 12 18 24 30 36 42 44 No. at risk: 35 29 13 9 4 1 0 0.75 93% 81% 1 0.5 0.25 0 0 12 6 9 3 15 18 35 4 18 12 27 0 0 Median follow-up 7.4 months Median DOR not reached No. at risk: Primary dataset* Supplementary dataset* Kaplan-Meier landmark analysis 17 Jul 2017 30 July 2018 6 months 83% 88% 12 months 71% 75% Probability Months from start of response *In patients with confirmed complete or partial responses DOR, duration of response Investigator response assessments, as of 30 July 2018 15
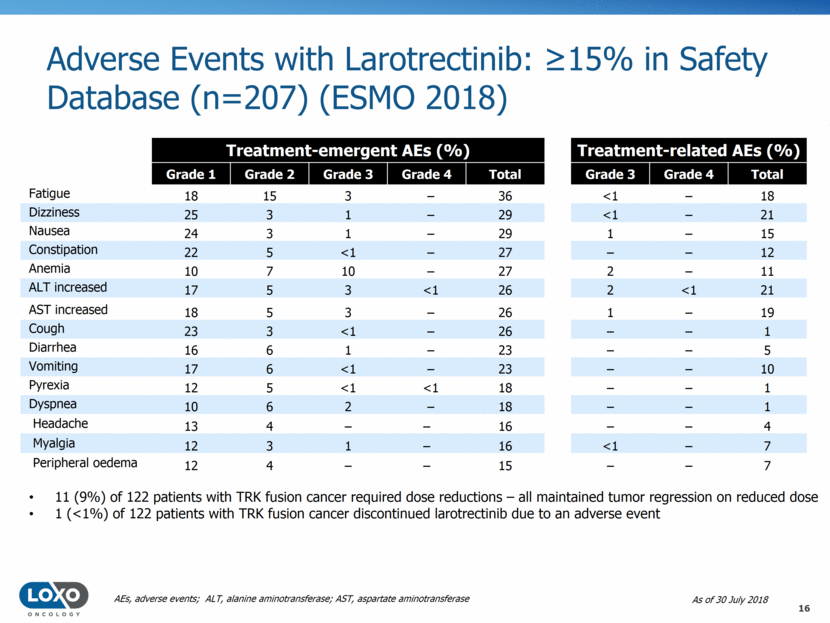
Adverse Events with Larotrectinib: 15% in Safety Database (n=207) (ESMO 2018) 11 (9%) of 122 patients with TRK fusion cancer required dose reductions – all maintained tumor regression on reduced dose 1 (<1%) of 122 patients with TRK fusion cancer discontinued larotrectinib due to an adverse event As of 30 July 2018 AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase Treatment-emergent AEs (%) Treatment-related AEs (%) Grade 1 Grade 2 Grade 3 Grade 4 Total Grade 3 Grade 4 Total Fatigue 18 15 3 – 36 <1 – 18 Dizziness 25 3 1 – 29 <1 – 21 Nausea 24 3 1 – 29 1 – 15 Constipation 22 5 <1 – 27 – – 12 Anemia 10 7 10 – 27 2 – 11 ALT increased 17 5 3 <1 26 2 <1 21 AST increased 18 5 3 – 26 1 – 19 Cough 23 3 <1 – 26 – – 1 Diarrhea 16 6 1 – 23 – – 5 Vomiting 17 6 <1 – 23 – – 10 Pyrexia 12 5 <1 <1 18 – – 1 Dyspnea 10 6 2 – 18 – – 1 Headache 13 4 – – 16 – – 4 Myalgia 12 3 1 – 16 <1 – 7 Peripheral oedema 12 4 – – 15 – – 7 16
Larotrectinib Regulatory Timing Breakthrough therapy designation (BTD) granted in July 2016 BTD indication: “For the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments” Rolling NDA submission completed in March 2018 PDUFA date Nov 26, 2018 MAA submitted on August 27, 2018* Potential for larotrectinib to be the first new cancer drug approved with a genomically-defined, tumor-agnostic indication * Per partnership agreement, MAA is handled by Bayer. 17
18 LOXO-195 (TRK)
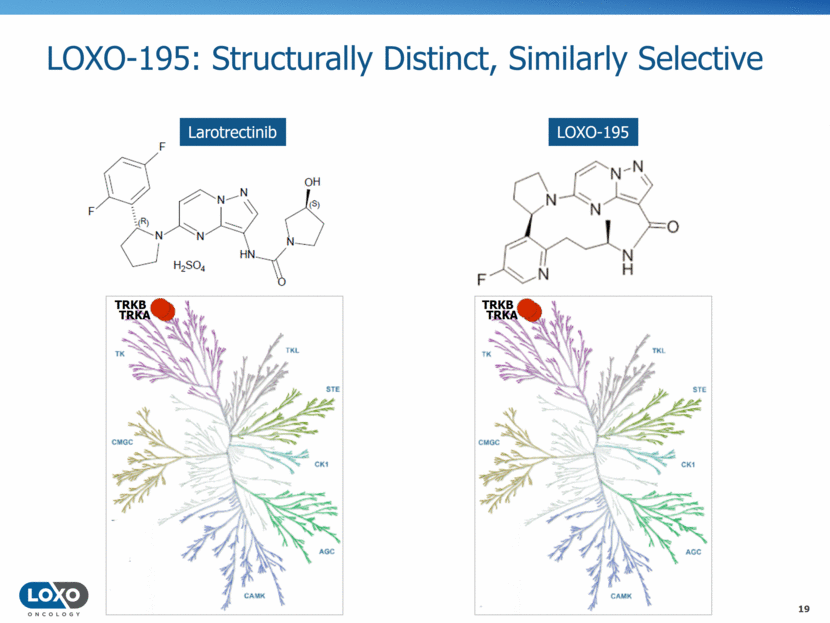
LOXO-195: Structurally Distinct, Similarly Selective 19 LOXO-195 Larotrectinib TRKB TRKB TRKA TRKA
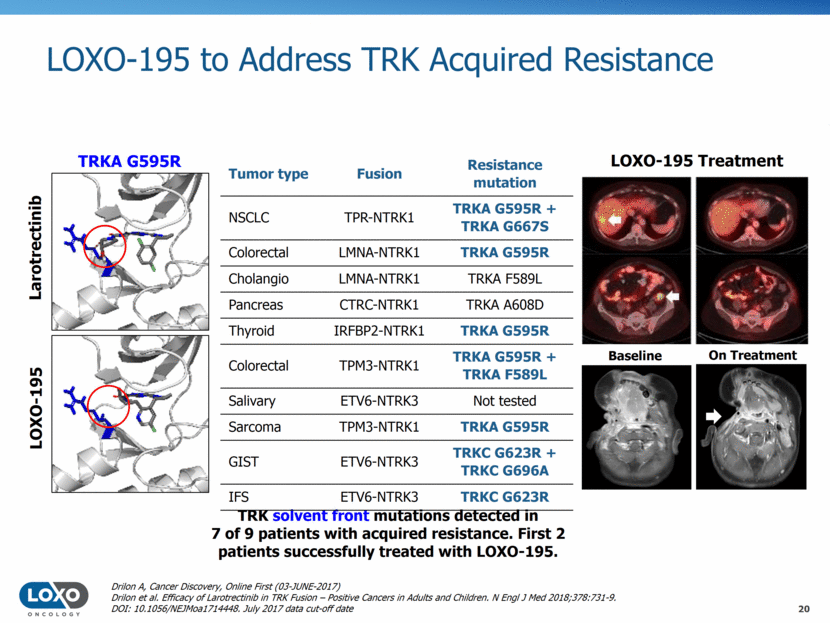
LOXO-195 to Address TRK Acquired Resistance 20 TRKA G595R TRK solvent front mutations detected in 7 of 9 patients with acquired resistance. First 2 patients successfully treated with LOXO-195. LOXO-195 Treatment Larotrectinib LOXO-195 Baseline On Treatment Tumor type Fusion Resistance mutation NSCLC TPR-NTRK1 TRKA G595R + TRKA G667S Colorectal LMNA-NTRK1 TRKA G595R Cholangio LMNA-NTRK1 TRKA F589L Pancreas CTRC-NTRK1 TRKA A608D Thyroid IRFBP2-NTRK1 TRKA G595R Colorectal TPM3-NTRK1 TRKA G595R + TRKA F589L Salivary ETV6-NTRK3 Not tested Sarcoma TPM3-NTRK1 TRKA G595R GIST ETV6-NTRK3 TRKC G623R + TRKC G696A IFS ETV6-NTRK3 TRKC G623R Drilon A, Cancer Discovery, Online First (03-JUNE-2017) Drilon et al. Efficacy of Larotrectinib in TRK Fusion – Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. DOI: 10.1056/NEJMoa1714448. July 2017 data cut-off date
LOXO-195 Clinical Development Phase 1/2 trial enrolling Next clinical data update expected in first half 2019 Plan to address patients with TRK fusion cancers harboring solvent front mutations, xDFG mutations, and gatekeeper mutations Loxo Oncology is well-positioned to be the first oncology company to simultaneously develop a first-generation inhibitor and a second-generation inhibitor to address acquired resistance 21
22 LOXO-292 (RET)

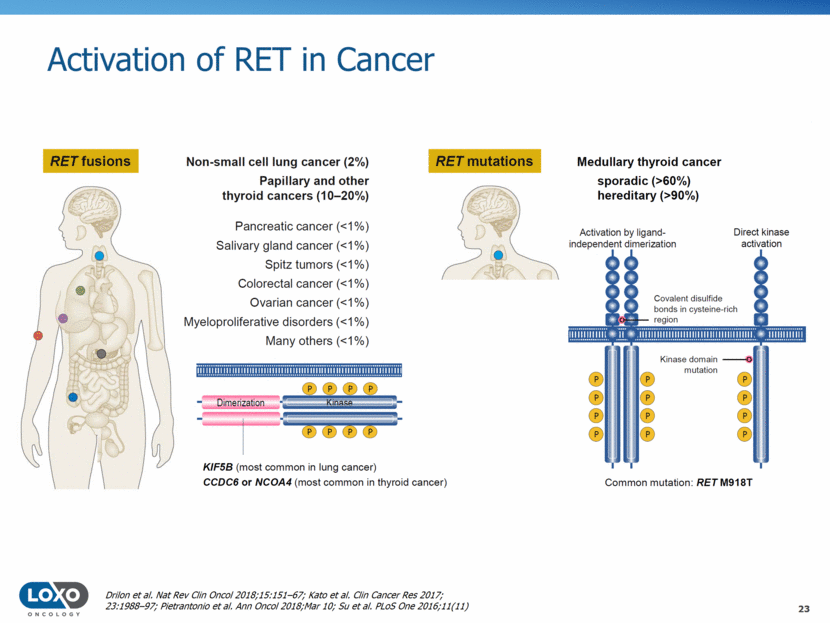
Activation of RET in Cancer 23 Drilon et al. Nat Rev Clin Oncol 2018;15:151–67; Kato et al. Clin Cancer Res 2017; 23:1988–97; Pietrantonio et al. Ann Oncol 2018;Mar 10; Su et al. PLoS One 2016;11(11)
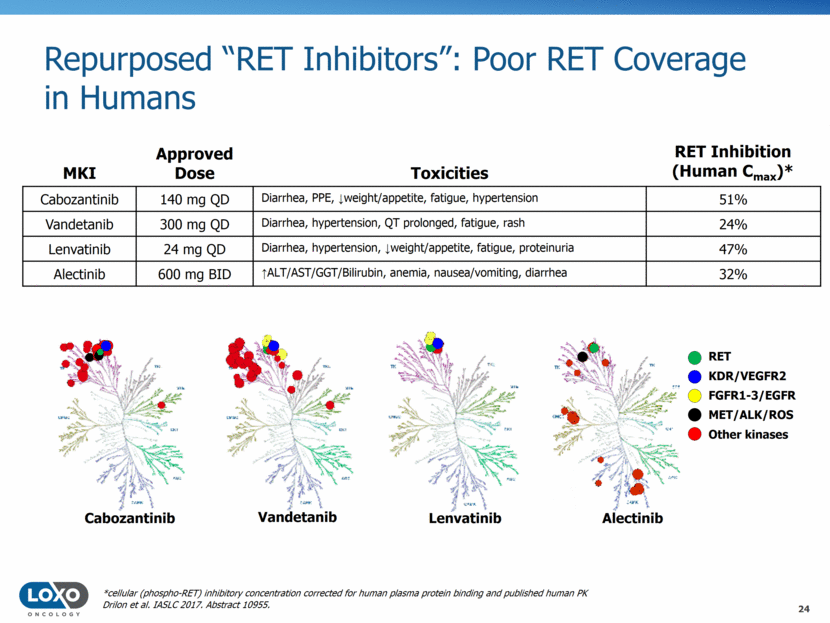
Cabozantinib Vandetanib Lenvatinib Alectinib *cellular (phospho-RET) inhibitory concentration corrected for human plasma protein binding and published human PK RET KDR/VEGFR2 MET/ALK/ROS Other kinases FGFR1-3/EGFR MKI Approved Dose Toxicities Cabozantinib 140 mg QD Diarrhea, PPE, weight/appetite, fatigue, hypertension 51% Vandetanib 300 mg QD Diarrhea, hypertension, QT prolonged, fatigue, rash 24% Lenvatinib 24 mg QD Diarrhea, hypertension, weight/appetite, fatigue, proteinuria 47% Alectinib 600 mg BID ALT/AST/GGT/Bilirubin, anemia, nausea/vomiting, diarrhea 32% RET Inhibition (Human Cmax)* Repurposed “RET Inhibitors”: Poor RET Coverage in Humans 24 Drilon et al. IASLC 2017. Abstract 10955.
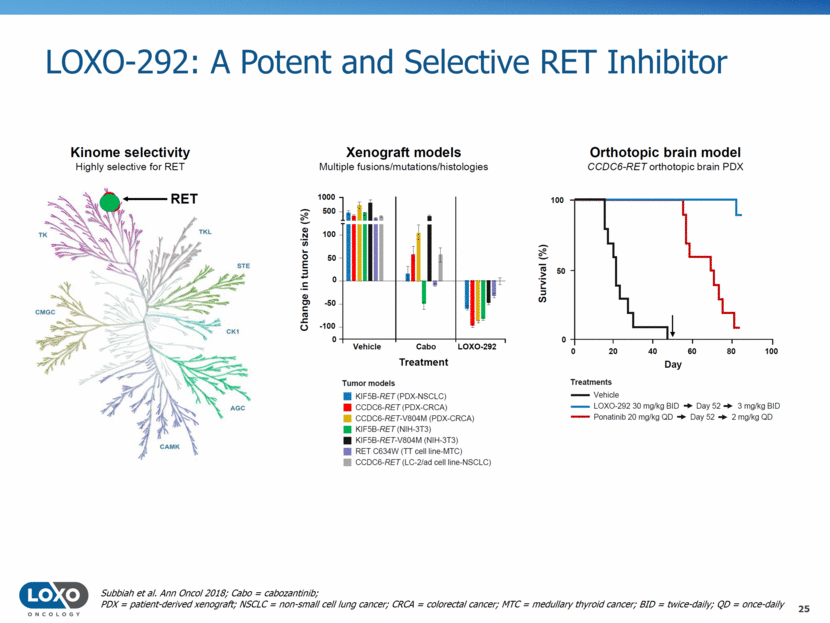
LOXO-292: A Potent and Selective RET Inhibitor 25 Subbiah et al. Ann Oncol 2018; Cabo = cabozantinib; PDX = patient-derived xenograft; NSCLC = non-small cell lung cancer; CRCA = colorectal cancer; MTC = medullary thyroid cancer; BID = twice-daily; QD = once-daily
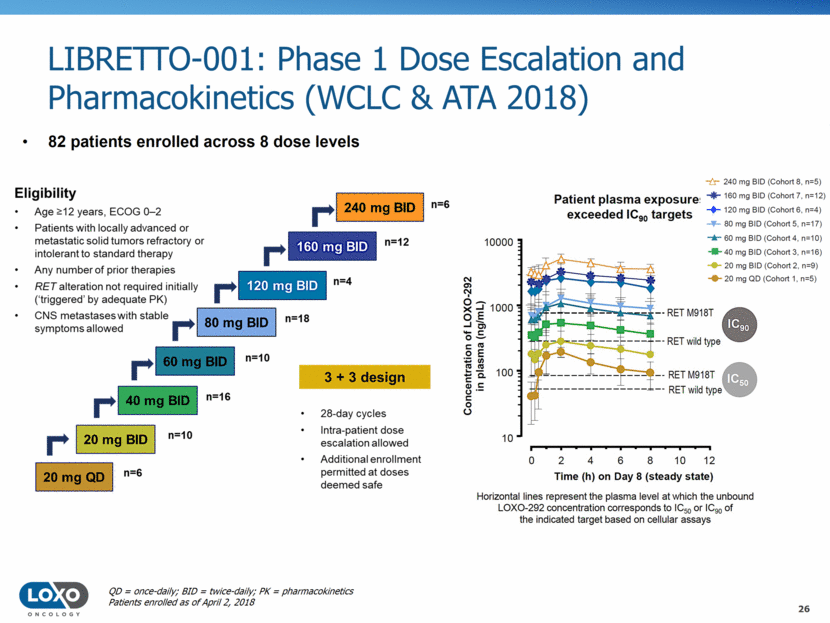
LIBRETTO-001: Phase 1 Dose Escalation and Pharmacokinetics (WCLC & ATA 2018) 26 82 patients enrolled across 8 dose levels QD = once-daily; BID = twice-daily; PK = pharmacokinetics Patients enrolled as of April 2, 2018
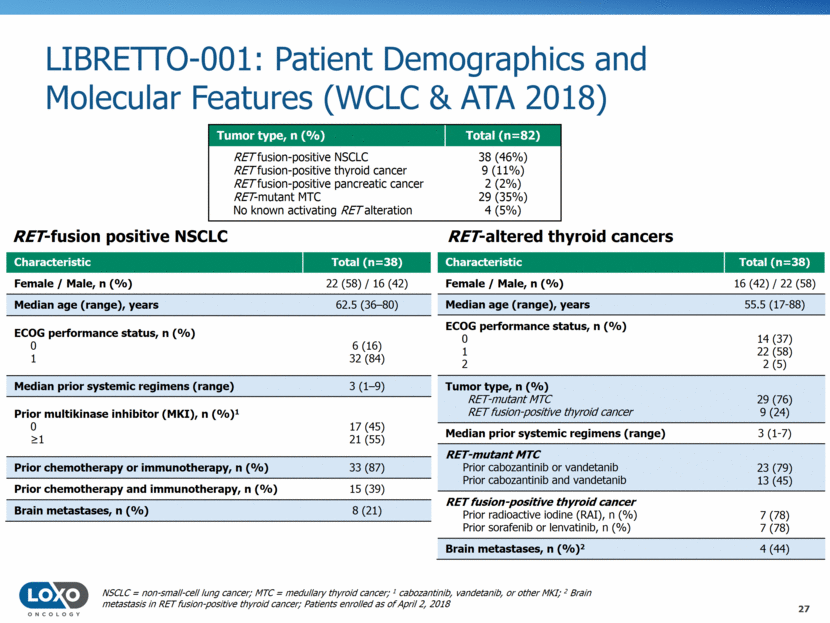
LIBRETTO-001: Patient Demographics and Molecular Features (WCLC & ATA 2018) 27 NSCLC = non-small-cell lung cancer; MTC = medullary thyroid cancer; 1 cabozantinib, vandetanib, or other MKI; 2 Brain metastasis in RET fusion-positive thyroid cancer; Patients enrolled as of April 2, 2018 Tumor type, n (%) Total (n=82) RET fusion-positive NSCLC RET fusion-positive thyroid cancer RET fusion-positive pancreatic cancer RET-mutant MTC No known activating RET alteration 38 (46%) 9 (11%) 2 (2%) 29 (35%) 4 (5%) Characteristic Total (n=38) Female / Male, n (%) 16 (42) / 22 (58) Median age (range), years 55.5 (17-88) ECOG performance status, n (%) 0 1 2 14 (37) 22 (58) 2 (5) Tumor type, n (%) RET-mutant MTC RET fusion-positive thyroid cancer 29 (76) 9 (24) Median prior systemic regimens (range) 3 (1-7) RET-mutant MTC Prior cabozantinib or vandetanib Prior cabozantinib and vandetanib 23 (79) 13 (45) RET fusion-positive thyroid cancer Prior radioactive iodine (RAI), n (%) Prior sorafenib or lenvatinib, n (%) 7 (78) 7 (78) Brain metastases, n (%)2 4 (44) RET-altered thyroid cancers Characteristic Total (n=38) Female / Male, n (%) 22 (58) / 16 (42) Median age (range), years 62.5 (36–80) ECOG performance status, n (%) 0 1 6 (16) 32 (84) Median prior systemic regimens (range) 3 (1–9) Prior multikinase inhibitor (MKI), n (%)1 0 1 17 (45) 21 (55) Prior chemotherapy or immunotherapy, n (%) 33 (87) Prior chemotherapy and immunotherapy, n (%) 15 (39) Brain metastases, n (%) 8 (21) RET-fusion positive NSCLC
LOXO-292 Safety Profile (WCLC & ATA 2018) 28 All doses and patients, n=82 Treatment-emergent AEs (10% overall) Treatment-related AEs Grade 1 Grade 2 Grade 3 Grade 4 Total Grade 3 Grade 4 Total Diarrhea 15% 7% 1% – 23% 1% – 11% Fatigue 9% 13% – – 22% – – 17% Dry Mouth 21% – – – 21% – – 13% Constipation 17% 2% – – 20% – – 4% Hypomagnesemia 12% 1% – – 13% – – 2% Cough 11% 1% – – 12% – – 1% Headache 10% 1% 1% – 12% – – 1% Nausea 9% 4% – – 12% – – 5% 8 treatment-emergent AEs, regardless of attribution, in 10% of patients; most were Grade 1 and judged not related to LOXO-292 Four patients experienced treatment-related AEs grade 3 (all grade 3): diarrhea, increased ALT/AST, thrombocytopenia (DLT @ 240mg BID), tumor lysis syndrome (DLT @ 240mg BID); all were reversible with dose interruption 160mg BID selected as RP2D, with dose exploration ongoing at 200 mg BID to further characterize LOXO-292 safety and efficacy AE = adverse event; DLT = dose limiting toxicity; ALT = alanine aminotransferase; AST = aspartate aminotransferase; RP2D = recommended phase 2 dose; Note: Total %s for any given AE may be different than the sum of the individual grades, due to rounding. Patients enrolled as of April 2, 2018. Follow-up as of July 19, 2018.
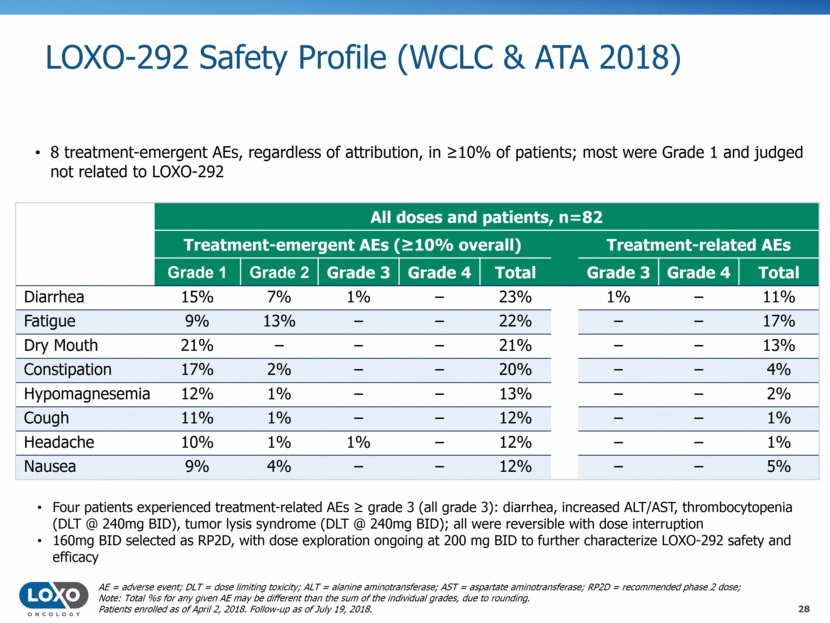
Efficacy of LOXO-292 in RET-Altered Cancers 29 40 Maximum change in tumor size (%) RET fusion-positive NSCLC 20 0 # # # * -20 -40 -60 -80 -100 RET fusion-positive pancreatic cancer RET fusion-positive thyroid cancer RET-mutant medullary thyroid cancer Patients enrolled as of April 2, 2018. Follow-up as of July 19, 2018 Note: 7 patients not displayed; 4 due to treatment discontinuation prior to first post-baseline response assessment, 3 due to nonmeasurable disease at baseline (2 stable disease, and 1 complete response). *Complete response; #Unconfirmed response awaiting confirmatory response assessment. NSCLC, non-small cell lung cancer.
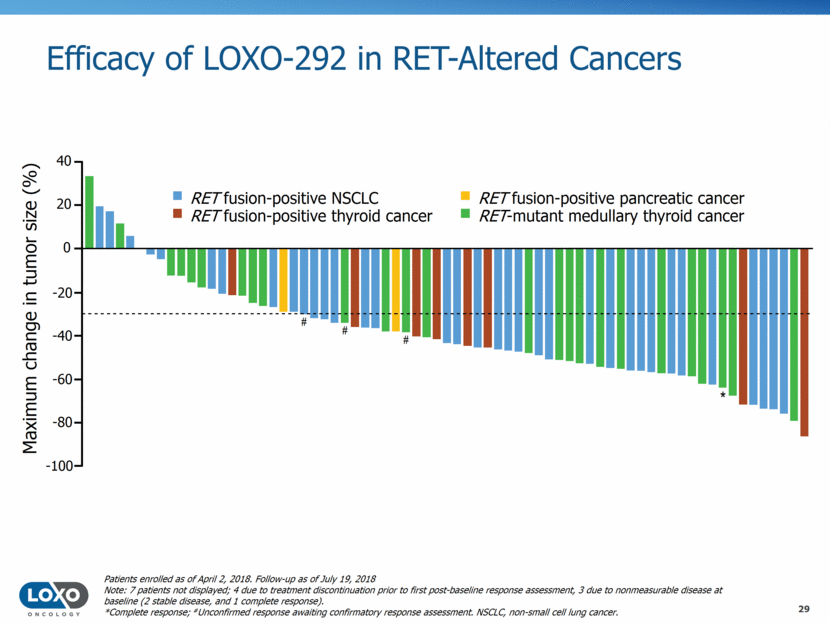
Efficacy of LOXO-292 in RET Fusion-Positive NSCLC (WCLC 2018) 30 ORR (95% CI) 68% (n=26/38) (51–83%) Confirmed ORR* (95% CI) 68% (n=25/37) (50–82%) CR – PR** 26 SD 8 PD 2 NE 2 40 20 0 –20 –40 –60 –80 –100 Best tumor response (%) Prior Chemo Prior IO Prior MKI 20 mg QD 20 mg BID 40 mg BID 60 mg BID 80 mg BID 120 mg BID 160 mg BID 240 mg BID Starting dose All unconfirmed responses reported at ASCO have since been confirmed RECIST 1.1 responses were seen at all starting dose levels, prior to any intrapatient dose escalation, and in 18/26 (69%) responding patients at each patient’s starting dose Activity independent of prior therapy 4/4 confirmed intracranial responses (1 CR, 3 PR) in patients with measurable CNS lesions pending confirmation; * Excludes one patient with unconfirmed PR pending confirmation at time of data cut-off; ** 25 confirmed PR, 1 unconfirmed PR pending confirmation NSCLC patients enrolled as of April 2, 2018. Follow-up as of July 19, 2018.
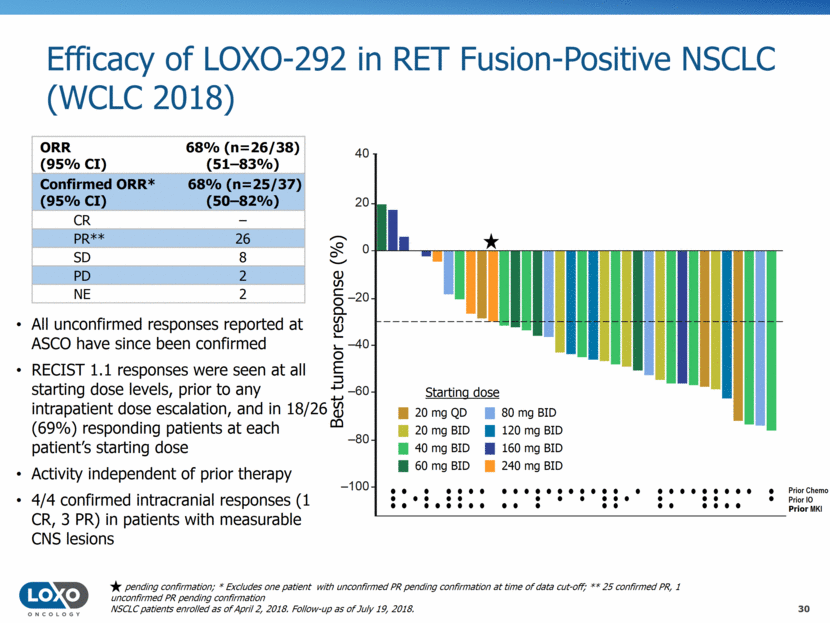
Duration of LOXO-292 Treatment in RET Fusion-Positive NSCLC (WCLC 2018) 31 NSCLC patients enrolled as of April 2, 2018. Follow-up as of July 19, 2018. First response Treatment after progression Still on treatment 24/38 (63%) escalated to/treated with 160 mg BID (RP2D) 25/26 (96%) responding patients remain on therapy 24/26 (92%) responses ongoing, 17 responses 6 months Median follow up: 8.5 (0.3–14.1) months, 9.5 (4.4–14.1) months for responding patients Time on treatment (months) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
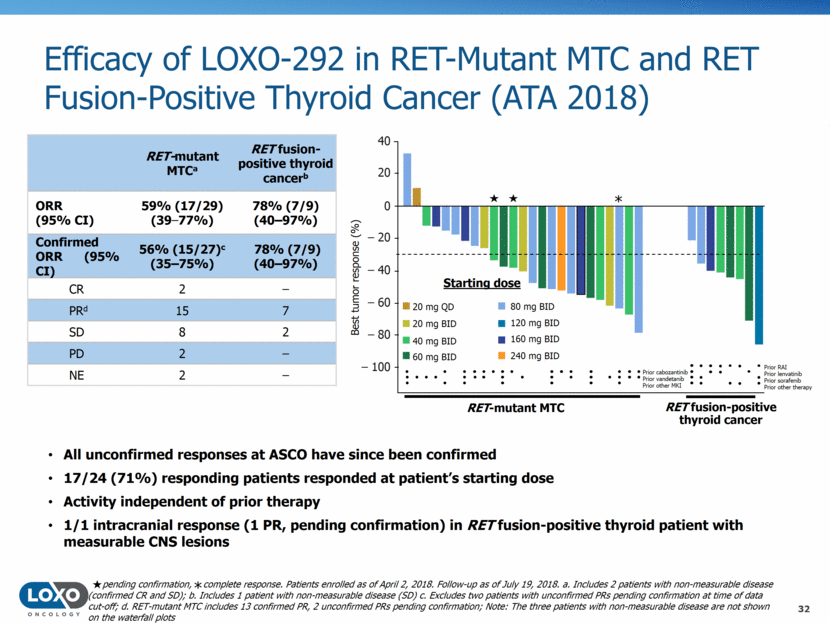
Efficacy of LOXO-292 in RET-Mutant MTC and RET Fusion-Positive Thyroid Cancer (ATA 2018) 32 All unconfirmed responses at ASCO have since been confirmed 17/24 (71%) responding patients responded at patient’s starting dose Activity independent of prior therapy 1/1 intracranial response (1 PR, pending confirmation) in RET fusion-positive thyroid patient with measurable CNS lesions RET-mutant MTCa RET fusion-positive thyroid cancerb ORR (95% CI) 59% (17/29) (39–77%) 78% (7/9) (40–97%) Confirmed ORR (95% CI) 56% (15/27)c (35–75%) 78% (7/9) (40–97%) CR 2 – PRd 15 7 SD 8 2 PD 2 – NE 2 – 20 mg QD 20 mg BID 40 mg BID 60 mg BID 80 mg BID 120 mg BID 160 mg BID 240 mg BID Starting dose Best tumor response (%) Prior cabozantinib Prior vandetanib Prior other MKI – 80 – 60 – 40 – 20 0 20 40 – 100 * Prior RAI Prior lenvatinib Prior sorafenib Prior other therapy RET-mutant MTC RET fusion-positive thyroid cancer pending confirmation, complete response. Patients enrolled as of April 2, 2018. Follow-up as of July 19, 2018. a. Includes 2 patients with non-measurable disease (confirmed CR and SD); b. Includes 1 patient with non-measurable disease (SD) c. Excludes two patients with unconfirmed PRs pending confirmation at time of data cut-off; d. RET-mutant MTC includes 13 confirmed PR, 2 unconfirmed PRs pending confirmation; Note: The three patients with non-measurable disease are not shown on the waterfall plots *
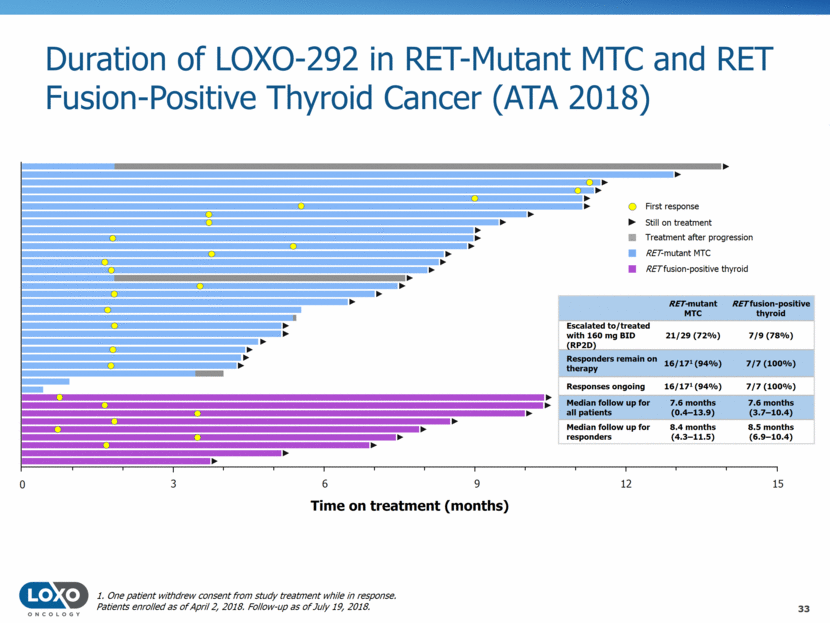
Duration of LOXO-292 in RET-Mutant MTC and RET Fusion-Positive Thyroid Cancer (ATA 2018) 33 1. One patient withdrew consent from study treatment while in response. Patients enrolled as of April 2, 2018. Follow-up as of July 19, 2018. Time on treatment (months) RET-mutant MTC RET fusion-positive thyroid Escalated to/treated with 160 mg BID (RP2D) 21/29 (72%) 7/9 (78%) Responders remain on therapy 16/171 (94%) 7/7 (100%) Responses ongoing 16/171 (94%) 7/7 (100%) Median follow up for all patients 7.6 months (0.4–13.9) 7.6 months (3.7–10.4) Median follow up for responders 8.4 months (4.3–11.5) 8.5 months (6.9–10.4) First response Treatment after progression Still on treatment RET-mutant MTC RET fusion-positive thyroid 0 3 9 12 15 6
LOXO-292 Next Steps LIBRETTO-001 Phase 2 cohorts currently enrolling at 160 mg BID Regulatory – BTDs received September & October 2018 Based on written meeting minutes from FDA, NDA filing expected in late 2019 utilizing data generated from the ongoing LIBRETTO-001 clinical trial Timeline integrates standard NDA activities which are ongoing and planned, including clinical pharmacology studies, non-clinical studies, and manufacturing BTD indication statements RET Fusion NSCLC: “for the treatment of patients with metastatic RET-fusion-positive non-small cell lung cancer who require systemic therapy and have progressed following platinum-based chemotherapy and an anti-PD-1 or anti-PD-L1 therapy” RET Mutant MTC: “for the treatment of patients with RET-mutant medullary thyroid cancer who require systemic therapy, have progressed following prior treatment and have no acceptable alternative treatment options” RET Fusion Thyroid Cancer: “for the treatment of patients with advanced RET fusion-positive thyroid cancer who require systemic therapy, have progressed following prior treatment and have no acceptable alternative treatment options.” Diagnostics Enrolling based on local testing Working with Illumina to clinically validate TST170 as companion diagnostic Next data update is expected to be the registrational dataset, in 2019 34

35 LOXO-305 (BTK)
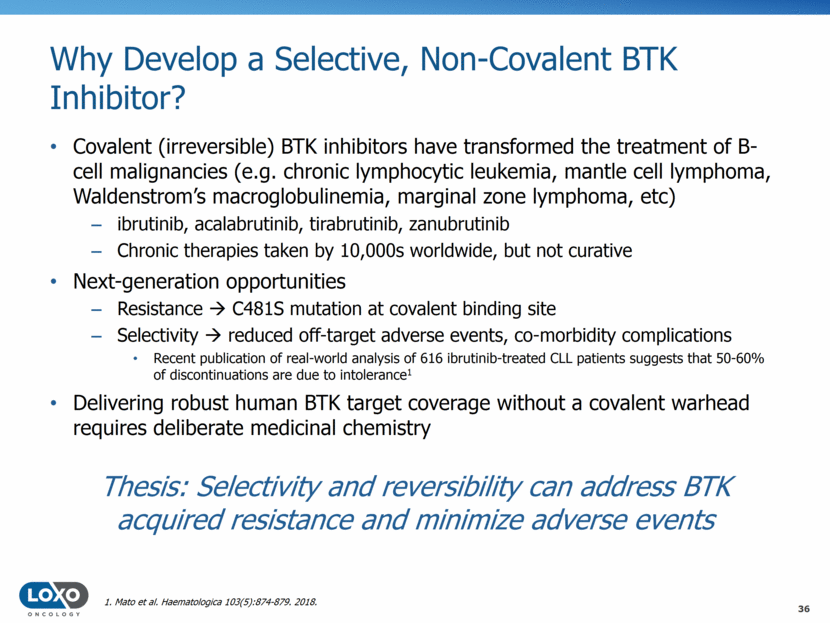
Why Develop a Selective, Non-Covalent BTK Inhibitor? Covalent (irreversible) BTK inhibitors have transformed the treatment of B-cell malignancies (e.g. chronic lymphocytic leukemia, mantle cell lymphoma, Waldenstrom’s macroglobulinemia, marginal zone lymphoma, etc) ibrutinib, acalabrutinib, tirabrutinib, zanubrutinib Chronic therapies taken by 10,000s worldwide, but not curative Next-generation opportunities Resistance C481S mutation at covalent binding site Selectivity reduced off-target adverse events, co-morbidity complications Recent publication of real-world analysis of 616 ibrutinib-treated CLL patients suggests that 50-60% of discontinuations are due to intolerance1 Delivering robust human BTK target coverage without a covalent warhead requires deliberate medicinal chemistry 36 Thesis: Selectivity and reversibility can address BTK acquired resistance and minimize adverse events 1. Mato et al. Haematologica 103(5):874-879. 2018.
C481S: Emerging, Class-wide Acquired Resistance Covalent BTK inhibitors all bind to BTK cysteine at position 481 A mechanism of acquired resistance has emerged called a C481S mutation that may explain 60-70% of progression events (cysteine mutates to serine)1 Likely to occur across all covalent BTK inhibitors (e.g. ibrutinib1, acalabrutinib2, tirabrutinib, zanubrutinib) Emerging in CLL, MCL, and WM In CLL, median overall survival post-relapse <2 years Non-covalent BTK inhibitors may re-induce a new response and restore disease control by potent binding to BTK without reliance on cysteine at position 481 Testing for C481S is not yet routine, but technically straightforward, utilizing blood, bone marrow, or lymph node tissue 37 1. Woyach et al. JCO 35; 13. 2017. 2. Byrd et al. NEJM 374;4. 2016.
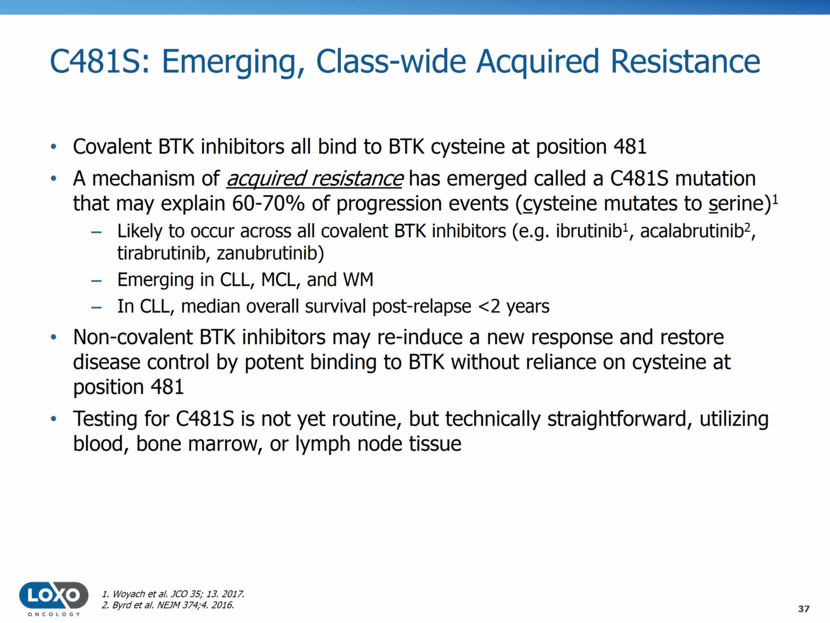
LOXO-305 is Highly Selective in Cellular Assays 180 Kinases Analyzed 38 BTK TEC
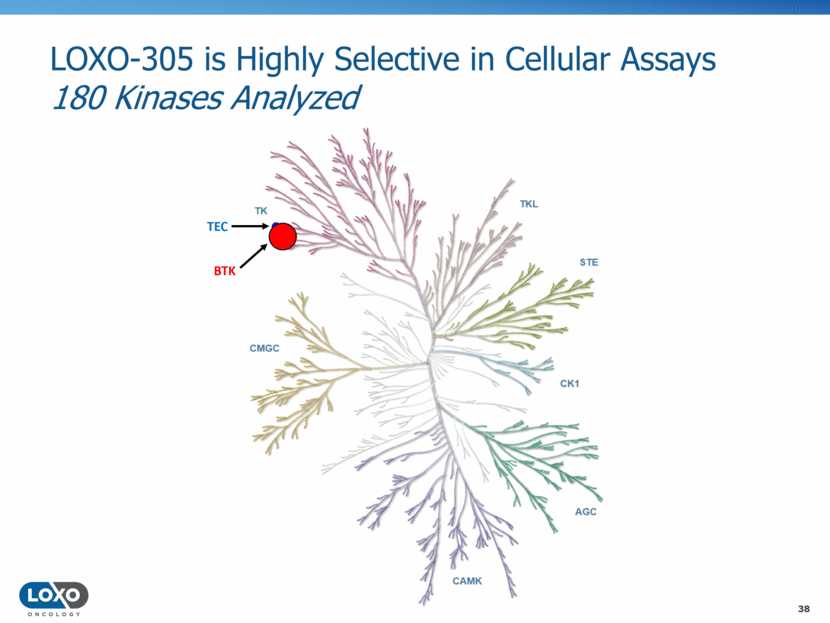
LOXO-305 Inhibits BTK Autophosphorylation and Signaling Activity in Cells (SOHO 2018) 39 RAMOS RA1 (Burkitt’s lymphoma) BTK phospho-Y223 (Area) (mean ± SD) BTK phospho-Y223 (Area) (mean ± SD) BTK phospho-Y223 (Area) (mean ± SD) LOXO-305 IC50 = 8.8 ± 1.8 nM LOXO-305 IC50 = 3.13 ± 0.6 nM Ibrutinib nM LOXO-305 and ibrutinib effects on BTK autophosphorylation of tyrosine 223 (Y223) and phosphorylation of PLC2 tyrosine 1217 (Y1217). HEK293 cells stably expressing BTK (A and B) and BTK C481S (C and D) were treated for 2 h with LOXO-305 or ibrutinib and analyzed by immunoblot (A and C). B and D: BTK Y223 phosphorylation was normalized to total BTK and IC50 values were calculated using a 4-parameter fit in GraphPad Prism 7.04 software. E and F: Ramos RA1 human Burkitt’s lymphoma cells were treated for 2 h with LOXO-305 or ibrutinib followed by 5 min stimulation with anti-IgM. E: Samples were analyzed by immunoblot for phosphorylation of BTK Y223 and PLC2 Y1217. F: LOXO-305 dose response curve of BTK Y223 phosphorylation in Ramos RA1 cells. BTK Y223 phosphorylation was normalized to total BTK and IC50 values were calculated using a 4-parameter fit in GraphPad Prism 7.04 software A B C D E F 0.0001 0.01 1 100 10000 0 200000 400000 600000 800000 1000000 1200000 1400000 Inhibitor concentration (nM) B T K p h o s p h o - Y 2 2 3 ( A r e a ) ( m e a n ± S D ) LOXO-305 + anti-IgM Ibrutinib + anti-IgM - anti-IgM LOXO-305 IC 50 : 3.13 ± 0.6 nM
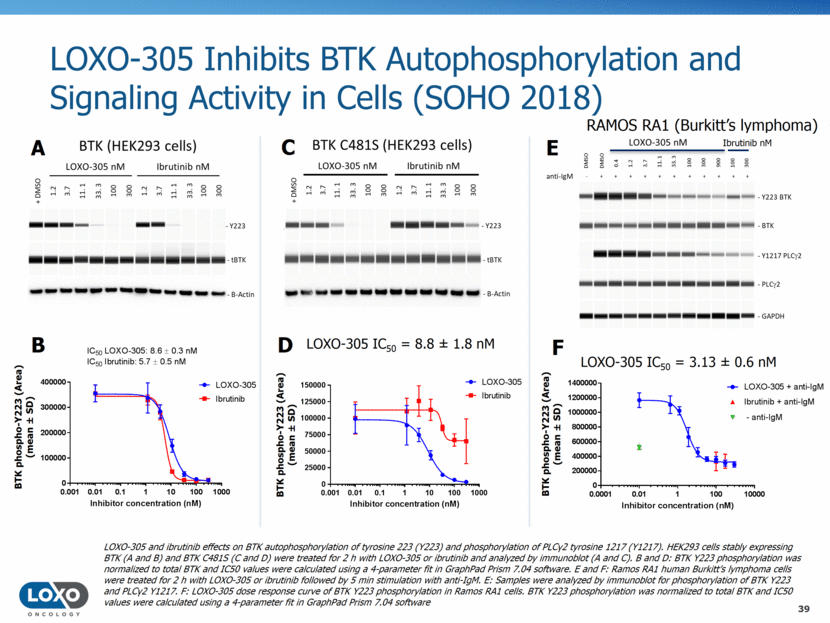
LOXO-305 is Predicted to Achieve High BTK and BTK C481S Target Coverage (SOHO 2018) 40 Time Time Predicted Human Exposure at Steady State (Oral Dosing) Predicted human exposure of LOXO-305 and BTK target coverage. Two planned dose levels of LOXO-305 were modeled using the GastroPlus TM software suite. These illustrate human dose predictions for dose levels planned in the phase 1 dose escalation trial and are not necessarily indicative of the first and second cohorts of the trial. The predicted BTK (wild-type and C481S) target coverage lines were derived from the LOXO-305 inhibition values of autophosphorylation in BTK and BTK C481S HEK293 cells and the plasma protein binding of LOXO-305.
LOXO-305: Next Steps Phase 1 clinical trial initiation planned for 4Q 2018 Opportunity for patient enrichment in Phase 1 As more patients are treated with irreversible BTK inhibitors, more patients will relapse Expect single agent activity at biologically relevant doses 41

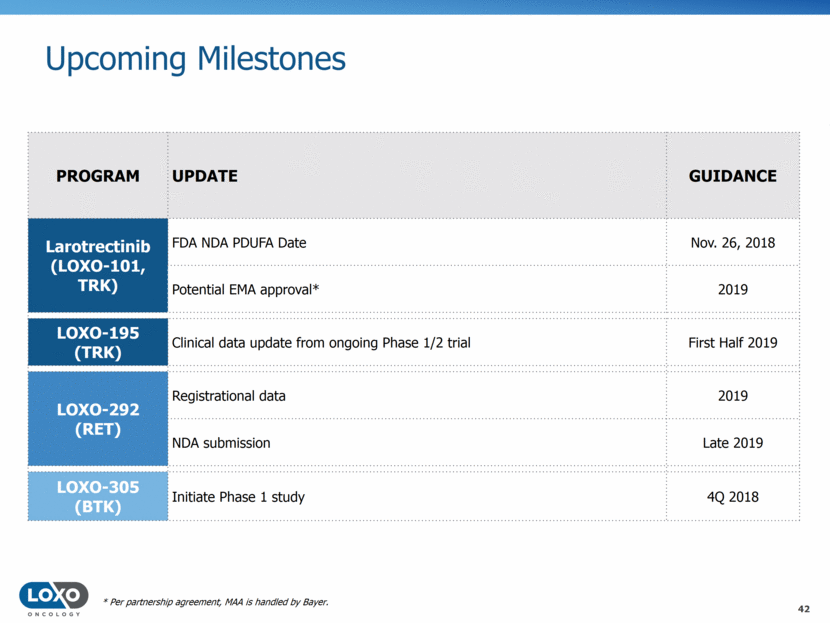
Upcoming Milestones 42 PROGRAM UPDATE GUIDANCE Larotrectinib (LOXO-101, TRK) FDA NDA PDUFA Date Nov. 26, 2018 Potential EMA approval* 2019 LOXO-195 (TRK) Clinical data update from ongoing Phase 1/2 trial First Half 2019 LOXO-292 (RET) Registrational data 2019 NDA submission Late 2019 LOXO-305 (BTK) Initiate Phase 1 study 4Q 2018 * Per partnership agreement, MAA is handled by Bayer.
Financials and Capitalization Nasdaq: LOXO Shares Outstanding*: 30.6M basic, 4.2M options ($53.52 weighted avg strike price) Cash, cash equivalents, and investments*: $647.6M Bayer partnership milestones $450M in milestones associated with larotrectinib regulatory approvals and first commercial sale events in certain major markets $275M expected in 2018; $125M expected in 2019 $200M in milestones associated with LOXO-195 regulatory approvals and first commercial sale events in certain major markets $25M milestone upon reaching a certain U.S. net sales threshold $475M in ex-U.S. sales milestones 43 * As of Q3 2018 10-Q


