Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - ALIMERA SCIENCES INC | pr.htm |
| 8-K - 8-K - ALIMERA SCIENCES INC | alim8k.htm |

Exhibit 99.2 Third Quarter 2018 Earnings Presentation Tuesday, November 6, 2018

Safe Harbor Statement This presentation contains “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, regarding, among other things, Alimera’s anticipated continued momentum through the end of 2018 and into 2019; anticipated addition of an indication for uveitis in Europe; belief that it is closing in on breakeven Adjusted EBITDA; belief that a better balance in sales strategy in Germany will quickly bring ILUVIEN back to the previous level of sales in hospitals and that this improvement, together with the current shortage of Ozurdex in Germany will lead to a rebound in sales in the fourth quarter and a return to growth in Germany; anticipated launches of ILUVIEN by its distribution partners in Spain and France; anticipated registration and pricing confirmation in the UAE and Kuwait prior to the end of 2018, which Alimera believes will lead to increased utilization of ILUVIEN in those countries in 2019; belief that it can maintain or improve its growth in consolidated net revenue in 2019; and its intentions to add additional users and drive deeper penetration into existing accounts and see more patients return for retreatments from three years prior. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual results to differ materially from those projected in its forward-looking statements. Meaningful factors which could cause actual results to differ include, but are not limited to, (a) a slowdown or reduction in Alimera’s sales in late 2018 and early 2019 due to a reduction in end user demand, unanticipated competition, regulatory issues, or other unexpected circumstances, (b) Alimera may not receive approval for non-infectious posterior uveitis for ILUVIEN in Europe, the approval process may be delayed significantly, or Alimera may be unable to meet any post-market requirements, (c) the anticipated launches of ILUVIEN by Alimera’s distribution partners in Spain and France may be delayed or may not occur, or (d) other factors discussed in the “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” sections of Alimera's Annual Report on Form 10-K for the year ended December 31, 2017 and Alimera’s Quarterly Report on Form 10-Q for the three months ended June 30, 2018, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at http://www.sec.gov. Additional factors may also be set forth in those sections of Alimera’s Quarterly Report on Form 10-Q for the three months ended September 30, 2018, to be filed with the SEC soon. In addition to the risks described above and in Alimera's Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, other unknown or unpredictable factors also could affect Alimera's results. There can be no assurance that the actual results or developments anticipated by Alimera will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Alimera. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved. All forward-looking statements contained in this presentation are expressly qualified by the cautionary statements contained or referred to herein. Alimera cautions investors not to rely too heavily on the forward-looking statements Alimera makes or that are made on its behalf. These forward-looking statements speak only as of the date of this presentation (unless another date is indicated). Alimera undertakes no obligation, and specifically declines any obligation, to publicly update or revise any such forward-looking statements, whether as a result of new information, future events or otherwise. 2 © 2018 Alimera Sciences | NASDAQ: ALIM

Third Quarter 2018 Overview +13% $11.1M • Net Revenue of Growth $11.1 Million • Record U.S. end user $9.8M demand of 977 units • Adjusted EBITDA loss improved to $521,000 3Q17 3Q18 Revenue 3 © 2018 Alimera Sciences | NASDAQ: ALIM

U.S. End User Demand 1200 977 1000 955 896 851 850 837 827 800 777 788 705 600 533 536 502 467 400 200 167 0 First Quarter Second Quarter Third Quarter Fourth Quarter 2015 2016 2017 2018 4 © 2018 Alimera Sciences | NASDAQ: ALIM

U.S. Distributor Orders and End User Demand • Historical 1200 inconsistency 1028 1004 1000 980 960977 between distributor 955 896 orders and end user 873 850 864 851 853 827 837 demand* 804 777 788 800 750 705 • 1Q18, 2Q18 and 608 600 3Q18 distributor 536 533 502 501 500 volume was in line 467 460 475 with end user 400 demand 293 * End user demand represents 200 167 units purchased by physicians and pharmacies from Alimera’s distributors 0 1Q15 2Q15 3Q15 4Q15 1Q16 2Q16 3Q16 4Q16 1Q17 2Q17 3Q17 4Q17 1Q18 2Q18 3Q18 Demand Revenue © 2018 Alimera Sciences | NASDAQ: ALIM 5

Income Statement ($000’s) Three Months Ended Three Months Ended Nine Months Ended Nine Months Ended September 30,2018 September 30,2017 September 30,2018 September 30,2017 Revenue $11,137 $9,784 $31,856 $26,770 Operating $12,367 $12,570 $38,244 $35,089 Expenses Net Loss ($3,450) ($5,285) ($15,134) ($14,777) Adj. EBITDA* ($521) ($1,844) ($4,411) ($4,999) *Adjusted EBITDA is earnings before interest taxes, depreciation, amortization, stock-based compensation expenses, net unrealized gains and losses from foreign currency exchange transactions, gains and losses from the change in the fair value of derivative warrant liability and losses on extinguishment of debt. See slides 8 and 9 of this presentation for reconciliation of this Non-GAAP financial measure. 6 © 2018 Alimera Sciences | NASDAQ: ALIM
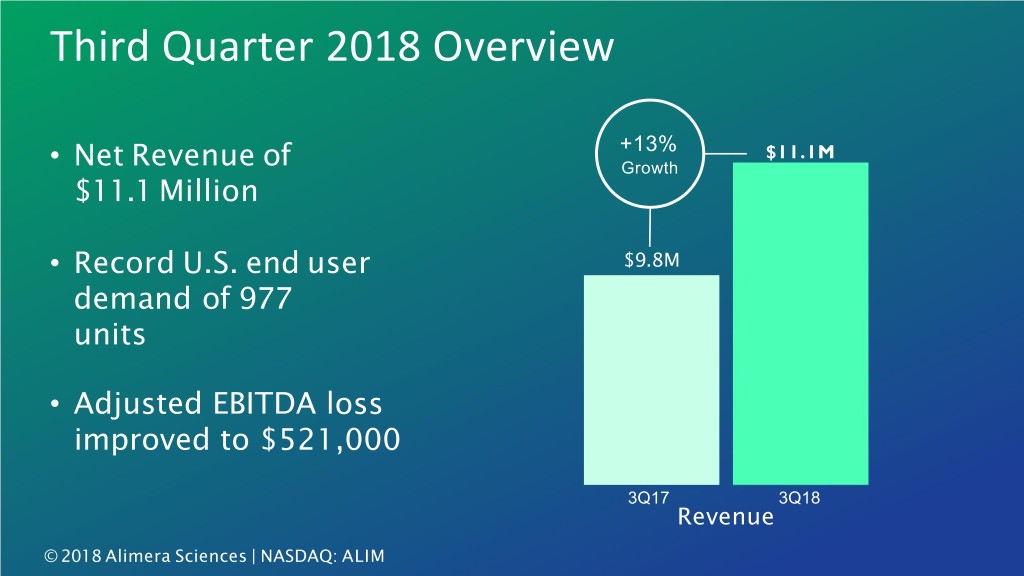
Third Quarter 2018 Overview +13% $11.1M • Net Revenue of Growth $11.1 Million • Record U.S. end user $9.8M demand of 977 units • Adjusted EBITDA loss improved to $521,000 3Q17 3Q18 Revenue 7 © 2018 Alimera Sciences | NASDAQ: ALIM

Reconciliation of GAAP Net Loss to NON-GAAP Adjusted EBITDA Alimera believes that the non-GAAP financial information provided in this presentation can assist investors in the overall understanding of its financial performance when considered together with GAAP figures. This presentation contains a discussion of certain non-GAAP financial measures, as defined in Regulation G of the Securities Exchange Act of 1934, as amended. Alimera reports its financial results in compliance with GAAP, but believes that these non-GAAP measures will be a more relevant measure of Alimera's operating performance. For the purpose of this presentation, “Adjusted EBITDA” is earnings before interest, taxes, depreciation, amortization, stock-based compensation expenses, net unrealized gains and losses from foreign currency exchange transactions, gains and losses from the change in the fair value of derivative warrant liability and losses on extinguishment of debt. Alimera uses Adjusted EBITDA in the management of its business. Accordingly, Adjusted EBITDA for the third quarter of 2018 has been presented in certain instances excluding items identified in the reconciliations provided. “Non-GAAP Net Loss per Common Share” is Net Loss, after the elimination of the gain on the extinguishment of Alimera’s Series B Convertible Preferred Stock resulting from its exchange in September 2018 for new Series C Convertible Preferred Stock, divided by weighted average shares outstanding. This gain was unrelated to Alimera’s operating performance. These non-GAAP financial measures, as presented, may not be comparable to similarly titled measures reported by other companies since not all companies may calculate these measures in an identical manner and, therefore, they are not necessarily an accurate measure of comparison between companies. The presentation of these non-GAAP financial measures is not intended to be considered in isolation or as a substitute for guidance prepared in accordance with GAAP. The principal limitation of these non-GAAP financial measures is that they exclude significant elements that are required by GAAP to be recorded in Alimera's financial statements. In addition, they are subject to inherent limitations as they reflect the exercise of judgments by management in determining these non-GAAP financial measures. To compensate for these limitations, Alimera presents its non-GAAP financial results in connection with its GAAP results. For a reconciliation of Adjusted EBITDA to its most directly comparable GAAP financial measure, see the table towards the end of this presentation. For reconciliations of Non-GAAP Net Loss per Common Share to its most directly comparable GAAP financial measures, see the table located in Alimera’s earnings release from yesterday. © 2018 Alimera Sciences | NASDAQ: ALIM 8
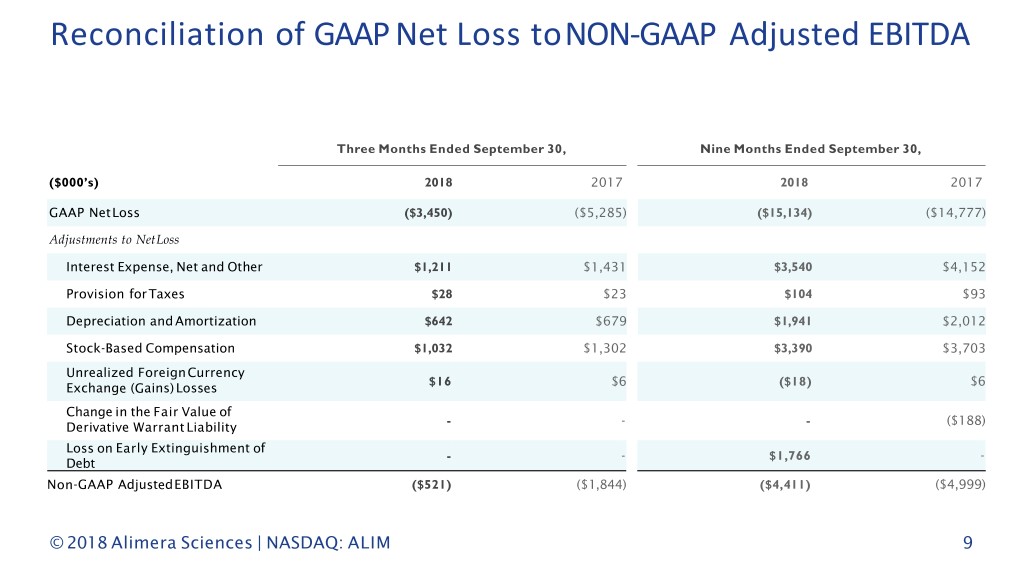
Reconciliation of GAAP Net Loss toNON-GAAP Adjusted EBITDA Three Months Ended September 30, Nine Months Ended September 30, ($000’s) 2018 2017 2018 2017 GAAP NetLoss ($3,450) ($5,285) ($15,134) ($14,777) Adjustments to NetLoss Interest Expense, Net and Other $1,211 $1,431 $3,540 $4,152 Provision for Taxes $28 $23 $104 $93 Depreciation and Amortization $642 $679 $1,941 $2,012 Stock-Based Compensation $1,032 $1,302 $3,390 $3,703 Unrealized ForeignCurrency $16 $6 ($18) $6 Exchange (Gains) Losses Change in the Fair Value of - - - ($188) Derivative Warrant Liability Loss on Early Extinguishment of - - $1,766 - Debt Non-GAAP AdjustedEBITDA ($521) ($1,844) ($4,411) ($4,999) © 2018 Alimera Sciences | NASDAQ: ALIM 9

Third Quarter 2018 Earnings Presentation Tuesday, November 6, 2018
