Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Cardiff Oncology, Inc. | d632248d8k.htm |
| EX-99.2 - EX-99.2 - Cardiff Oncology, Inc. | d632248dex992.htm |

Company Update Wednesday, October 24th, 2018 Exhibit 99.1
Licensed Drug Candidate from NMS Onvansertib – Polo-like Kinase 1 (PLK1) Inhibitor Largest oncology research and development company in Italy Developed anthracycline class of drugs (doxorubicin) Leader in protein kinase drug development (Polo-like Kinase Inhibitors) Identification and validation of molecular targets focused on driver oncogenes Excellent track record licensing innovative drugs to pharma/biotech companies including: Genentech (Roche), Ignyta (Roche), Novartis Oncology Drug Discovery Developing Oncology Drugs That Target Mitosis IND = Investigational New Drug Licensed global development and commercialization rights for Onvansertib Nerviano will continue manufacturing GMP API and finished drug Two active INDs in place with the FDA Financing in place to advance clinical programs
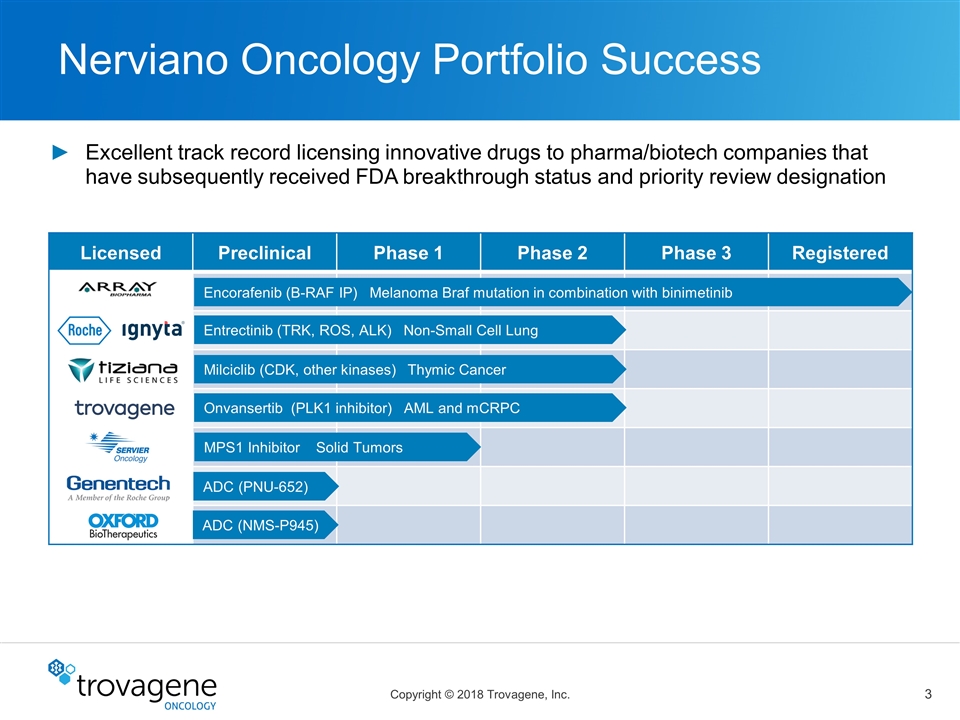
Nerviano Oncology Portfolio Success Licensed Preclinical Phase 1 Phase 2 Phase 3 Registered Encorafenib (B-RAF IP) Melanoma Braf mutation in combination with binimetinib Entrectinib (TRK, ROS, ALK) Non-Small Cell Lung Milciclib (CDK, other kinases) Thymic Cancer MPS1 Inhibitor Solid Tumors Onvansertib (PLK1 inhibitor) AML and mCRPC ADC (PNU-652) ADC (NMS-P945) Excellent track record licensing innovative drugs to pharma/biotech companies that have subsequently received FDA breakthrough status and priority review designation
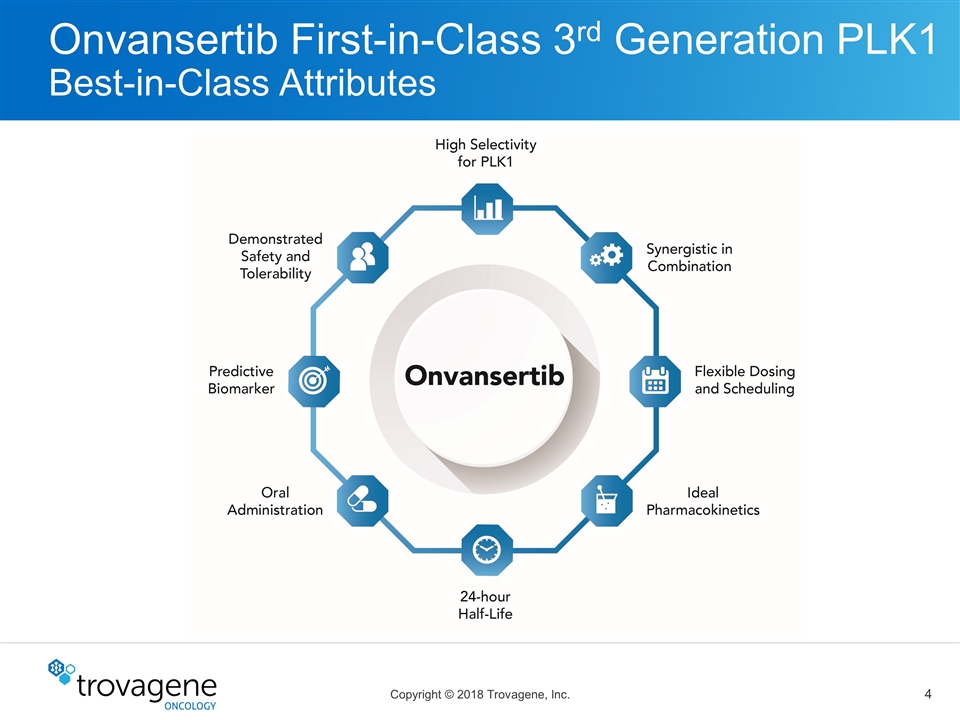
Onvansertib First-in-Class 3rd Generation PLK1 Best-in-Class Attributes

Strategy for Oncology Drug Development Taking a precision cancer medicine approach to develop Onvansertib (PCM-075) Leveraging a proven cancer target, PLK1 Incorporating predictive clinical biomarkers Combining Onvansertib with already approved drugs Phase 1b/2 trial of Onvansertib + cytarabine or decitabine in Acute Myeloid Leukemia (AML) Phase 2 trial of Onvansertib + abiraterone acetate (Zytiga®)/prednisone in metastatic Castration-Resistant Prostate Cancer (mCRPC)
Clinical Development Roadmap Acute Myeloid Leukemia Prostate Cancer Colorectal Cancer
Orphan Drug Designation (ODD) in AML In the U.S. and Europe Regulatory and Financial Incentives Market Exclusivity

Japan Partnering Initiative Complementary therapeutic combination with already approved drug Enhance efficacy and market sustainability
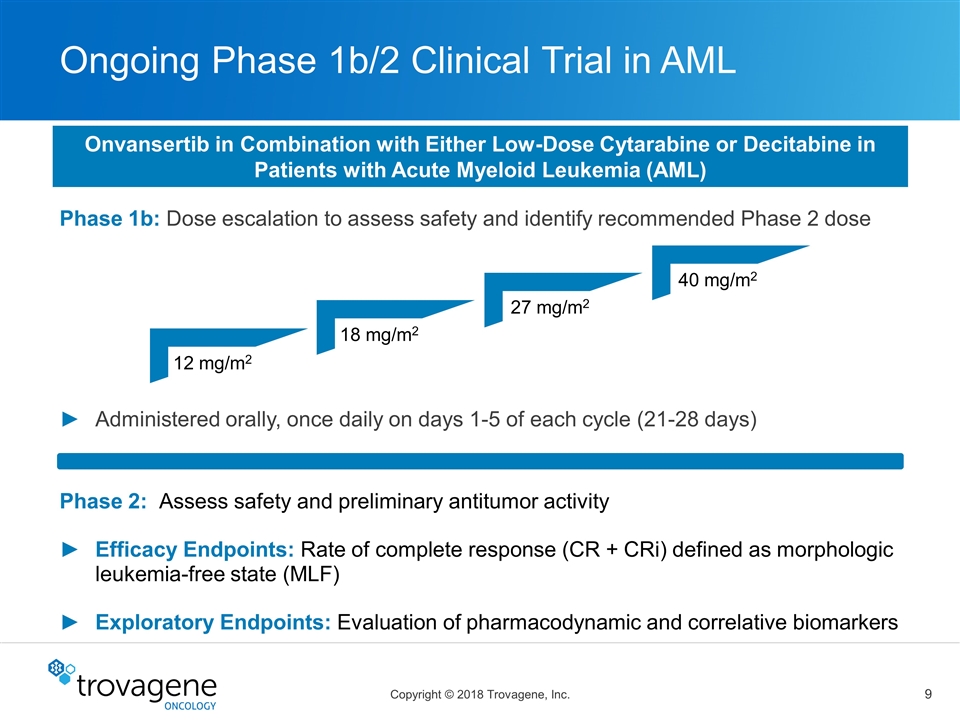
Ongoing Phase 1b/2 Clinical Trial in AML Phase 1b: Dose escalation to assess safety and identify recommended Phase 2 dose Administered orally, once daily on days 1-5 of each cycle (21-28 days) 12 mg/m2 18 mg/m2 27 mg/m2 40 mg/m2 Phase 2: Assess safety and preliminary antitumor activity Efficacy Endpoints: Rate of complete response (CR + CRi) defined as morphologic leukemia-free state (MLF) Exploratory Endpoints: Evaluation of pharmacodynamic and correlative biomarkers Onvansertib in Combination with Either Low-Dose Cytarabine or Decitabine in Patients with Acute Myeloid Leukemia (AML)
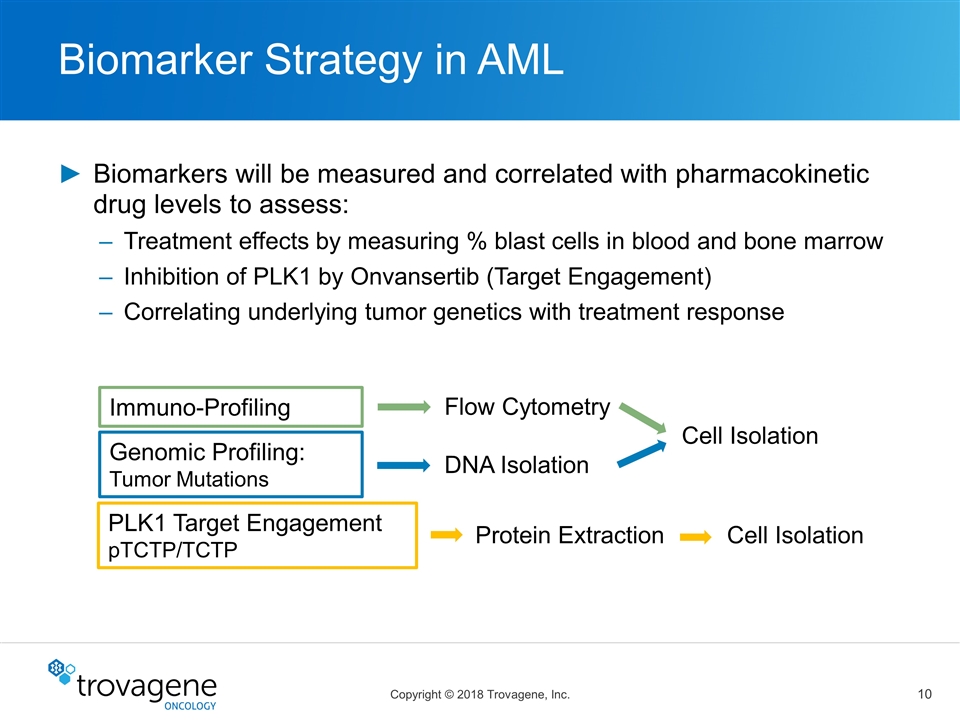
Biomarker Strategy in AML Biomarkers will be measured and correlated with pharmacokinetic drug levels to assess: Treatment effects by measuring % blast cells in blood and bone marrow Inhibition of PLK1 by Onvansertib (Target Engagement) Correlating underlying tumor genetics with treatment response Genomic Profiling: Tumor Mutations Immuno-Profiling Cell Isolation DNA Isolation Flow Cytometry Cell Isolation Protein Extraction PLK1 Target Engagement pTCTP/TCTP
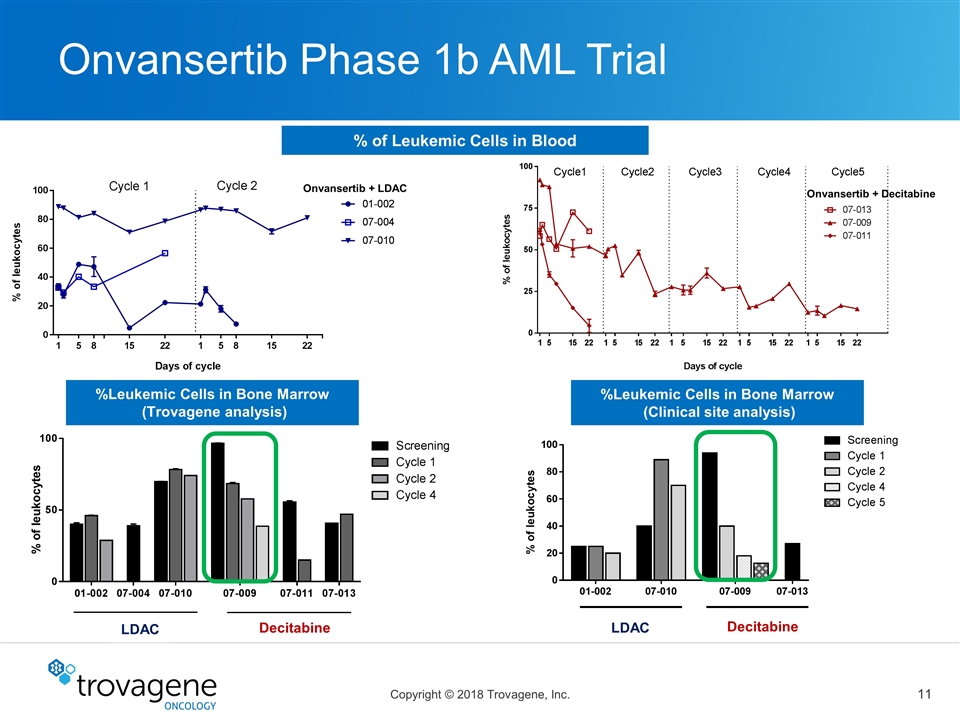
Onvansertib Phase 1b AML Trial % of Leukemic Cells in Blood Onvansertib + LDAC Onvansertib + Decitabine %Leukemic Cells in Bone Marrow (Trovagene analysis) LDAC Decitabine %Leukemic Cells in Bone Marrow (Clinical site analysis) LDAC Decitabine
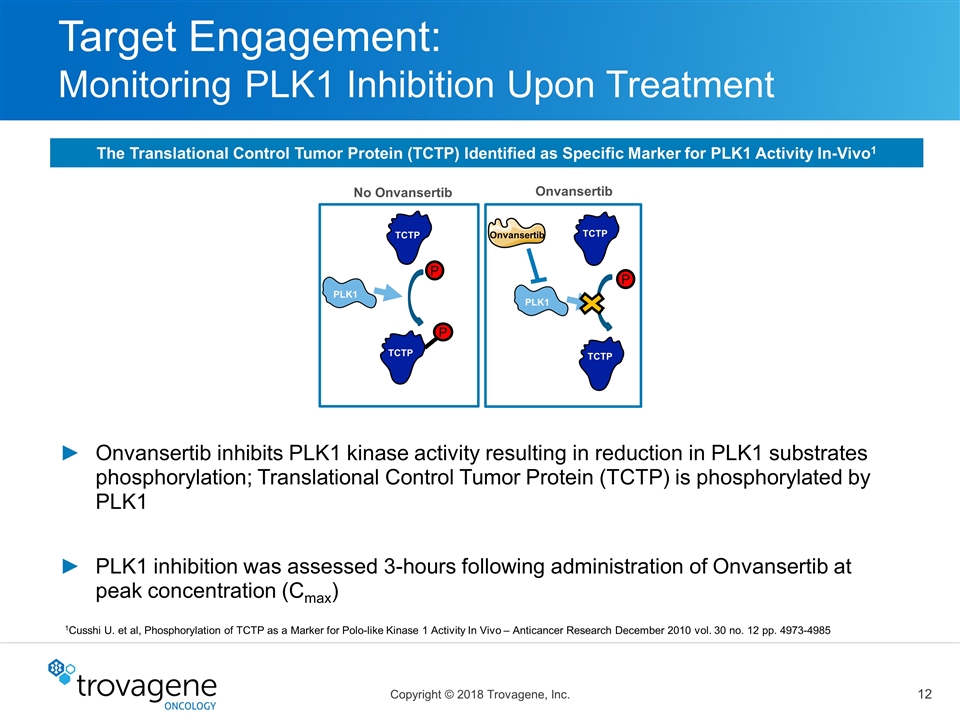
Target Engagement: Monitoring PLK1 Inhibition Upon Treatment TCTP PLK1 TCTP P No Onvansertib P Onvansertib PLK1 TCTP TCTP Onvansertib P Onvansertib inhibits PLK1 kinase activity resulting in reduction in PLK1 substrates phosphorylation; Translational Control Tumor Protein (TCTP) is phosphorylated by PLK1 PLK1 inhibition was assessed 3-hours following administration of Onvansertib at peak concentration (Cmax) 1Cusshi U. et al, Phosphorylation of TCTP as a Marker for Polo-like Kinase 1 Activity In Vivo – Anticancer Research December 2010 vol. 30 no. 12 pp. 4973-4985 The Translational Control Tumor Protein (TCTP) Identified as Specific Marker for PLK1 Activity In-Vivo1

Ongoing Phase 2 Clinical Trial in mCRPC Efficacy Endpoints Effect of Onvansertib in combination with Zytiga®/prednisone on disease control assessed by prostate-specific antigen (PSA) decline or stabilization pre- and post-treatment Safety Endpoint Safety of Onvansertib in combination with Zytiga®/prednisone Exploratory Endpoint Target inhibition of PLK1, evaluation of relevant biomarkers and correlation with patient response and genomic profile Onvansertib in Combination with Zytiga® and Prednisone in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Dosing Regimen Onvansertib – 24 mg/m2 Days 1-5 (21-Day Cycle) + Zytiga®/prednisone daily 4 Cycles = 12 Weeks Disease Control based on PSA level Duration Evaluation
PLK1 and Abiraterone Acetate (Zytiga®) Metastatic Castration-Resistant Prostate Cancer (mCRPC) All metastatic prostate cancer patients become castration-resistant PLK1 dependent microtubule dynamics promotes androgen receptor (AR) signaling1,2 PLK1 inhibition improves abiraterone efficacy3 Inhibition of PLK1 represses androgen signaling pathway4 PLK1 inhibitors may add important therapeutic benefit for the treatment of castration-resistant prostate cancer patients5 1Xianzeng, Hou, Zhiguo, Li – PLK1-Dependent Microtubule Dynamics Promotes Androgen Receptor Signaling in Prostate Cancer; Prostate. 2013 September; 73(12): 1352–1363. doi:10.1002/pros.22683; 2 Arpaporn, Deeraksa, Jing, Pan - Plk1 is upregulated in androgen-insensitive prostate cancer cells and its inhibition leads to necroptosis; Oncogene. 2013 June 13; 32(24): 2973–2983. doi:10.1038/onc.2012.309; 3Clemens, Thoma – Prostate Cancer: PLK-1 Inhibition Improves Abiraterone Efficacy; Nature Reviews Urology volume11, page603 (2014); 4Zhang Z1, Chen L – Inhibition of PLK1 Represses Androgen Signaling Pathway in Castration-Resistant Prostate Cancer; Cell Cycle. 2015;14(13):2142-8. doi: 10.1080/15384101.2015.1041689; 5Klaus, Strebhardt - Drugging Plk1: An attractive approach to inhibit androgen receptor signaling; Cell Cycle. 2015 Jul 18; 14(14): 2193–2194
PSA: NCCN Recommended Biomarker Trial Eligibility and Efficacy for mCRPC1 PSA is a validated biomarker assessing disease stability or progression Prostate Cancer Clinical Trials Working Group (PCWG)1 has set criteria for the use of blood PSA levels: Trial eligibility (defining progression) Initial assessment of efficacy 1PCWG2: Sher et al, JCO, 2008, PCWG3: Sher et al, JCO, 2016
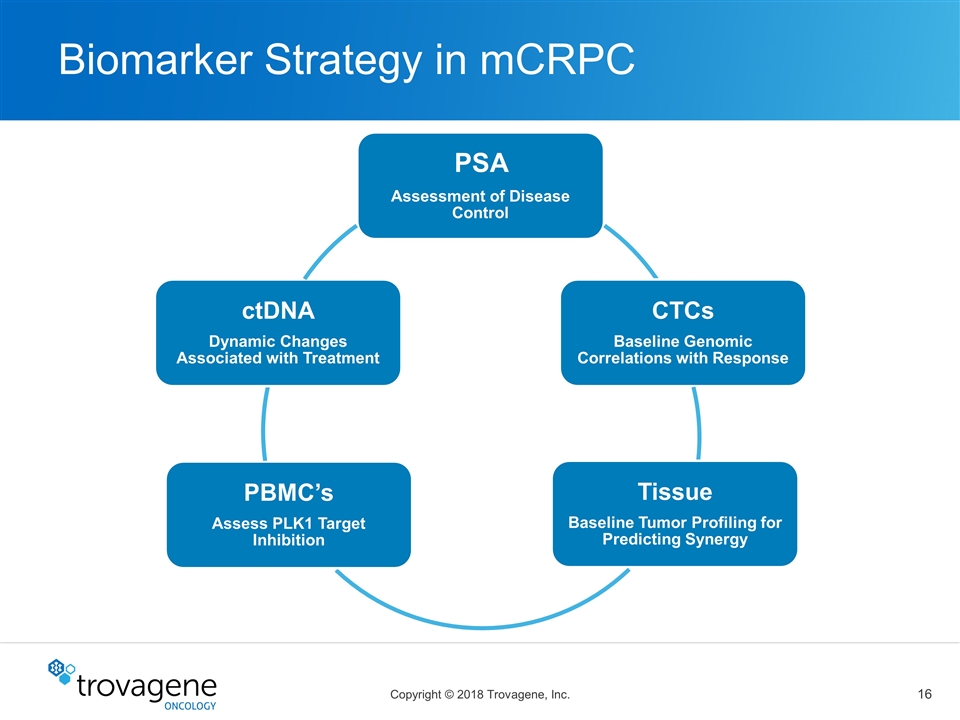
Biomarker Strategy in mCRPC PSA Assessment of Disease Control CTCs Baseline Genomic Correlations with Response Tissue Baseline Tumor Profiling for Predicting Synergy PBMC’s Assess PLK1 Target Inhibition ctDNA Dynamic Changes Associated with Treatment
Colorectal Cancer: Unmet need in mCRC 140K new cases of CRC in 2018 with 64.5% 5 year survival1 ~51K deaths per year from mCRC1 Tumor biomarkers drive therapy decisions for 1st line mCRC therapy2 ~50% mCRC is RAS wild-type: FOLFOX/FOLFIRI + AntiEGFR therapy ~50% mCRC is RAS mutant (KRAS): FOLFOX/FOLFIRI/FOLFOXIRI ~5% mCRC has microsatellite instability (MSI): candidate for immunoRx Large unmet need in RAS mutant CRC2 No targeted therapies are available for RAS mutant CRC 2nd line therapies have ~5% response rate in metastatic CRC (mCRC) 1https://seer.cancer.gov/statfacts/html/colorect.html; 2King et al, Frontline Strategies for Metastatic CRC, 2016, Amer J Hem/Onc; Loree&Kopetz, Recent Developments in treatment of mCRC, 2017, Ther Adv Med Onc;
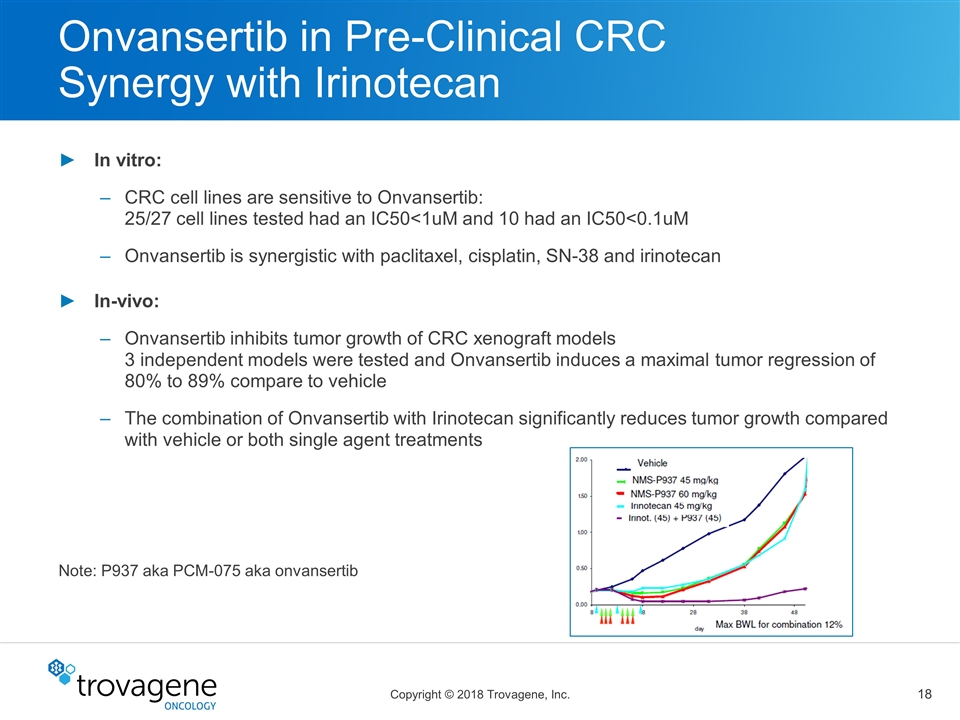
Onvansertib in Pre-Clinical CRC Synergy with Irinotecan In vitro: CRC cell lines are sensitive to Onvansertib: 25/27 cell lines tested had an IC50<1uM and 10 had an IC50<0.1uM Onvansertib is synergistic with paclitaxel, cisplatin, SN-38 and irinotecan In-vivo: Onvansertib inhibits tumor growth of CRC xenograft models 3 independent models were tested and Onvansertib induces a maximal tumor regression of 80% to 89% compare to vehicle The combination of Onvansertib with Irinotecan significantly reduces tumor growth compared with vehicle or both single agent treatments Note: P937 aka PCM-075 aka onvansertib

Value Creation Milestones In our Acute Myeloid Leukemia trial (AML): We anticipate reaching our maximum tolerated dose and recommended Phase 2 dose within the next couple of months; Enrolling patients in Phase 2 in the first half of 2019; Providing efficacy and safety data readouts throughout the year; and Developing our companion diagnostic In our metastatic Castration-Resistant Prostate Cancer (mCRPC) trial: We expect to complete patient enrollment in the first half of 2019; Present efficacy and safety data; and Initiate plans for the follow-on randomized Phase 2b trial In metastatic Colorectal Cancer (mCRC): Filing our IND and protocol for our Phase 2 trial in colorectal cancer by the end of this year; and Initiating this trial in 2019 On the collaboration and partnering front: We are working to formalize a Japanese partnership; and Explore opportunities to expand our clinical development program to sites in Europe

For additional information or questions please contact: ir@trovagene.com
