Attached files
| file | filename |
|---|---|
| EX-99.5 - EXHIBIT 99.5 - SELECTA BIOSCIENCES INC | acr2018presentationoctob.htm |
| EX-99.3 - EXHIBIT 99.3 - SELECTA BIOSCIENCES INC | a993acrposterdect2018fin.htm |
| EX-99.2 - EXHIBIT 99.2 - SELECTA BIOSCIENCES INC | a992acrpostersuaandada20.htm |
| EX-99.1 - EXHIBIT 99.1 - SELECTA BIOSCIENCES INC | exhibit991_pressrelease.htm |
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8k_prese.htm |
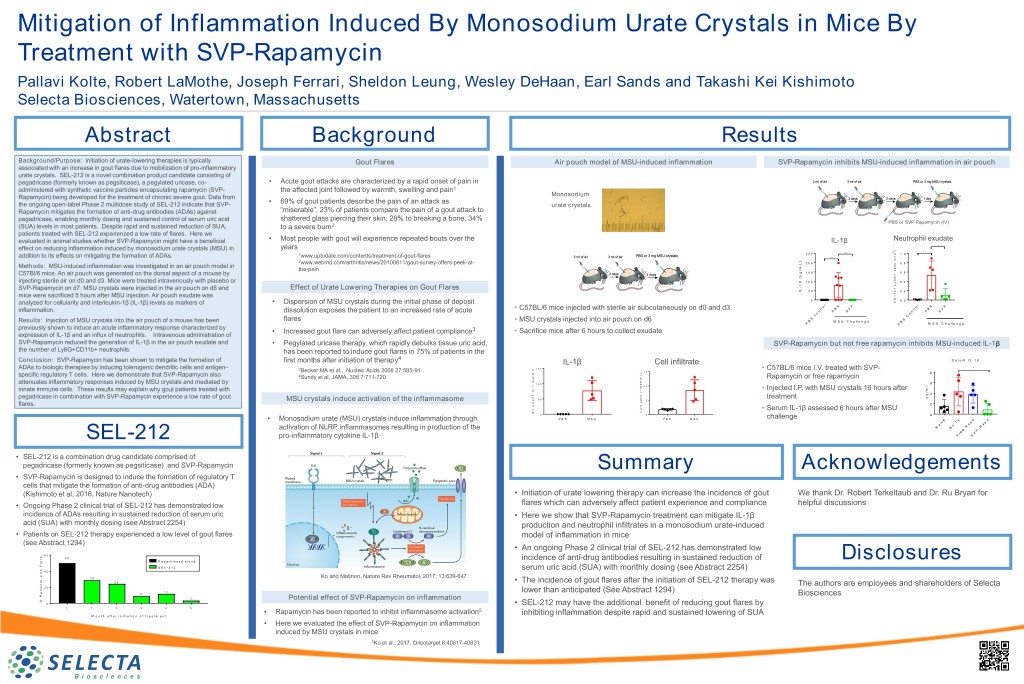
Mitigation of Inflammation Induced By Monosodium Urate Crystals in Mice By Treatment with SVP-Rapamycin Pallavi Kolte, Robert LaMothe, Joseph Ferrari, Sheldon Leung, Wesley DeHaan, Earl Sands and Takashi Kei Kishimoto Selecta Biosciences, Watertown, Massachusetts Abstract Background Results Background/Purpose: Initiation of urate-lowering therapies is typically Gout Flares Air pouch model of MSU-induced inflammation SVP-Rapamycin inhibits MSU-induced inflammation in air pouch associated with an increase in gout flares due to mobilization of pro-inflammatory urate crystals. SEL-212 is a novel combination product candidate consisting of pegadricase (formerly known as pegsiticase), a pegylated uricase, co- • Acute gout attacks are characterized by a rapid onset of pain in administered with synthetic vaccine particles encapsulating rapamycin (SVP- the affected joint followed by warmth, swelling and pain1 Rapamycin) being developed for the treatment of chronic severe gout. Data from Monosodium • 69% of gout patients describe the pain of an attack as the ongoing open-label Phase 2 multidose study of SEL-212 indicate that SVP- urate crystals Rapamycin mitigates the formation of anti-drug antibodies (ADAs) against “miserable”, 23% of patients compare the pain of a gout attack to pegadricase, enabling monthly dosing and sustained control of serum uric acid shattered glass piercing their skin, 28% to breaking a bone, 34% PBS or SVP-Rapamycin (IV) (SUA) levels in most patients. Despite rapid and sustained reduction of SUA, to a severe burn2 patients treated with SEL-212 experienced a low rate of flares. Here we evaluated in animal studies whether SVP-Rapamycin might have a beneficial • Most people with gout will experience repeated bouts over the IL-1β Neutrophil exudate years * effect on reducing inflammation induced by monosodium urate crystals (MSU) in ) 6 * * * * 1 2 5 0 0 1 . 0 addition to its effects on mitigating the formation of ADAs. www.uptodate.com/contents/treatment-of-gout-flares * * 1 x ( ) 2 2 0 0 s 0 . 8 www.webmd.com/arthritis/news/20100611/gout-survey-offers-peek-at- L l l e Methods: MSU-induced inflammation was investigated in an air pouch model in m / the-pain c g 1 5 0 0 . 6 C57Bl/6 mice. An air pouch was generated on the dorsal aspect of a mouse by + p ( G 6 ß injecting sterile air on d0 and d3. Mice were treated intravenously with placebo or 1 0 0 y 0 . 4 L 1 - + SVP-Rapamycin on d7. MSU crystals were injected in the air pouch on d8 and Effect of Urate Lowering Therapies on Gout Flares L b I 5 0 0 . 2 1 mice were sacrificed 5 hours after MSU injection. Air pouch exudate was 1 0 D 0 . 0 analyzed for cellularity and interleukin-1β (IL-1β) levels as markers of • Dispersion of MSU crystals during the initial phase of deposit C l l o S P S P r o • C57BL/6 mice injected with sterile air subcutaneously on d0 and d3. t B V t r B V inflammation. dissolution exposes the patient to an increased rate of acute n P S n P S o o c C S S flares • MSU crystals injected into air pouch on d6 B Results: Injection of MSU crystals into the air pouch of a mouse has been M S U C h a l l e n g e B P M S U C h a l l e n g e previously shown to induce an acute inflammatory response characterized by P • Increased gout flare can adversely affect patient compliance3 • Sacrifice mice after 6 hours to collect exudate expression of IL-1β and an influx of neutrophils. Intravenous administration of SVP-Rapamycin reduced the generation of IL-1β in the air pouch exudate and • Pegylated uricase therapy, which rapidly debulks tissue uric acid, SVP-Rapamycin but not free rapamycin inhibits MSU-induced IL-1β the number of Ly6G+CD11b+ neutrophils. has been reported to induce gout flares in 75% of patients in the 4 Conclusion: SVP-Rapamycin has been shown to mitigate the formation of first months after initiation of therapy IL-1β Cell infiltrate S e r u m I L - 1 ADAs to biologic therapies by inducing tolerogenic dendritic cells and antigen- 1 5 0 * 3 ) • C57BL/6 mice I.V. treated with SVP- Becker MA et al., Nucleic Acids 2008 27:585-91 l 1 5 8 ) m l specific regulatory T cells. Here we demonstrate that SVP-Rapamycin also 4 / g Sundy et al, JAMA, 306 7:711-720 m Rapamycin or free rapamycin / p 6 ( attenuates inflammatory responses induced by MSU crystals and mediated by 6 1 0 0 e l 1 1 0 0 - 1 m L • Injected I.P. with MSU crystals 16 hours after * innate immune cells. These results may explain why gout patients treated with I / ( 4 g h s l p c pegadricase in combination with SVP-Rapamycin experience a low rate of gout 5 0 l treatment e MSU crystals induce activation of the inflammasome u 5 c o 2 p flares. e v r i i • Serum IL-1β assessed 6 hours after MSU L A 0 0 0 P B S M S U P B S M S U challenge • Monosodium urate (MSU) crystals induce inflammation through e x a a i v T p p a a a o R R activation of NLRP inflammasomes resulting in production of the N N - e P e r V SEL-212 pro-inflammatory cytokine IL-1β F S • SEL-212 is a combination drug candidate comprised of pegadricase (formerly known as pegsiticase) and SVP-Rapamycin Summary Acknowledgements • SVP-Rapamycin is designed to induce the formation of regulatory T cells that mitigate the formation of anti-drug antibodies (ADA) (Kishimoto et al, 2016, Nature Nanotech) • Initiation of urate lowering therapy can increase the incidence of gout We thank Dr. Robert Terkeltaub and Dr. Ru Bryan for • Ongoing Phase 2 clinical trial of SEL-212 has demonstrated low flares which can adversely affect patient experience and compliance helpful discussions incidence of ADAs resulting in sustained reduction of serum uric • Here we show that SVP-Rapamycin treatment can mitigate IL-1β acid (SUA) with monthly dosing (see Abstract 2254) production and neutrophil infiltrates in a monosodium urate-induced • Patients on SEL-212 therapy experienced a low level of gout flares model of inflammation in mice (see Abstract 1294) • An ongoing Phase 2 clinical trial of SEL-212 has demonstrated low 6 0 e 5 0 r incidence of anti-drug antibodies resulting in sustained reduction of a P e g a d r i c a s e a l o n e Disclosures l F S E L - 2 1 2 h serum uric acid (SUA) with monthly dosing (see Abstract 2254) t 4 0 i w 2 9 Ko and Matinon, Nature Rev Rheumatol, 2017, 13:639-647 s t 2 4 • The incidence of gout flares after the initiation of SEL-212 therapy was n The authors are employees and shareholders of Selecta e 2 0 i t 1 2 a lower than anticipated (See Abstract 1294) 9 P Biosciences 3 Potential effect of SVP-Rapamycin on inflammation % 0 • SEL-212 may have the additional benefit of reducing gout flares by 1 1 2 3 4 5 • Rapamycin has been reported to inhibit inflammasome activation5 M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t inhibiting inflammation despite rapid and sustained lowering of SUA • Here we evaluated the effect of SVP-Rapamycin on inflammation induced by MSU crystals in mice 5Ko et al., 2017, Oncotarget 8:40817-40831
