Attached files
| file | filename |
|---|---|
| EX-99.5 - EXHIBIT 99.5 - SELECTA BIOSCIENCES INC | acr2018presentationoctob.htm |
| EX-99.4 - EXHIBIT 99.4 - SELECTA BIOSCIENCES INC | a994acrposterpreclinical.htm |
| EX-99.2 - EXHIBIT 99.2 - SELECTA BIOSCIENCES INC | a992acrpostersuaandada20.htm |
| EX-99.1 - EXHIBIT 99.1 - SELECTA BIOSCIENCES INC | exhibit991_pressrelease.htm |
| 8-K - 8-K - SELECTA BIOSCIENCES INC | selectabiosciences8k_prese.htm |
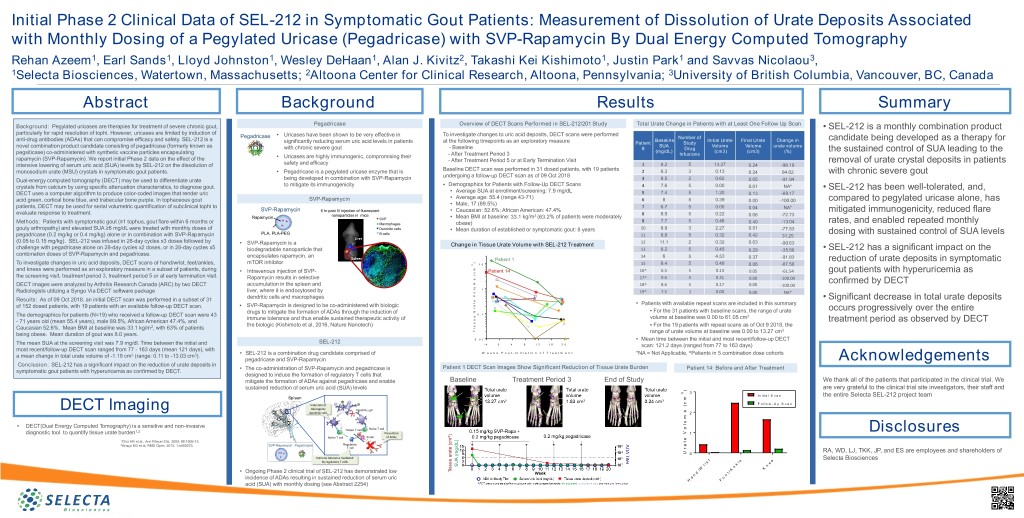
Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Measurement of Dissolution of Urate Deposits Associated with Monthly Dosing of a Pegylated Uricase (Pegadricase) with SVP-Rapamycin By Dual Energy Computed Tomography Rehan Azeem1, Earl Sands1, Lloyd Johnston1, Wesley DeHaan1, Alan J. Kivitz2, Takashi Kei Kishimoto1, Justin Park1 and Savvas Nicolaou3, 1Selecta Biosciences, Watertown, Massachusetts; 2Altoona Center for Clinical Research, Altoona, Pennsylvania; 3University of British Columbia, Vancouver, BC, Canada Abstract Background Results Summary Pegadricase Overview of DECT Scans Performed in SEL-212/201 Study Total Urate Change in Patients with at Least One Follow Up Scan Background: Pegylated uricases are therapies for treatment of severe chronic gout, • SEL-212 is a monthly combination product particularly for rapid resolution of tophi. However, uricases are limited by induction of • Uricases have been shown to be very effective in To investigate changes to uric acid deposits, DECT scans were performed Pegadricase Number of anti-drug antibodies (ADAs) that can compromise efficacy and safety. SEL-212 is a Baseline Initial Urate Final Urate Change in candidate being developed as a therapy for significantly reducing serum uric acid levels in patients at the following timepoints as an exploratory measure Patient Study novel combination product candidate consisting of pegadricase (formerly known as SUA Volume Volume urate volume with chronic severe gout - Baseline # Drug pegsiticase) co-administered with synthetic vaccine particles encapsulating (mg/dL) (cm3) (cm3) (%) the sustained control of SUA leading to the • Uricases are highly immunogenic, compromising their - After Treatment Period 3 Infusions rapamycin (SVP-Rapamycin). We report initial Phase 2 data on the effect of the - After Treatment Period 5 or at Early Termination Visit removal of urate crystal deposits in patients intensive lowering of serum uric acid (SUA) levels by SEL-212 on the dissolution of safety and efficacy 1 8.2 5 13.27 0.24 -98.19 Baseline DECT scan was performed in 31 dosed patients, with 19 patients monosodium urate (MSU) crystals in symptomatic gout patients. • Pegadricase is a pegylated uricase enzyme that is 2 6.3 3 0.13 0.24 84.62 with chronic severe gout undergoing a follow-up DECT scan as of 09 Oct 2018 Dual-energy computed tomography (DECT) may be used to differentiate urate being developed in combination with SVP-Rapamycin 3 8.5 2 0.62 0.05 -91.94 crystals from calcium by using specific attenuation characteristics, to diagnose gout. to mitigate its immunogenicity • Demographics for Patients with Follow-Up DECT Scans 4 7.6 5 0.00 0.01 NA* • Average SUA at enrollment/screening: 7.9 mg/dL • SEL-212 has been well-tolerated, and, DECT uses a computer algorithm to produce color-coded images that render uric 5 7.4 5 1.20 0.13 -89.17 • Average age: 55.4 (range 43-71) acid green, cortical bone blue, and trabecular bone purple. In tophaceous gout SVP-Rapamycin 6 8 5 0.39 0.00 -100.00 compared to pegylated uricase alone, has • Male, 17 (89.5%) patients, DECT may be used for serial volumetric quantification of subclinical tophi to 7 6.7 5 0.00 0.04 NA* SVP-Rapamycin 6 hr post IV injection of fluorescent • Caucasian: 52.6%; African American: 47.4% mitigated immunogenicity, reduced flare evaluate response to treatment. 8 8.9 5 0.22 0.06 -72.73 Rapamycin nanoparticles in mice • Mean BMI at baseline: 33.1 kg/m2 (63.2% of patients were moderately SVP 9 7.7 5 0.46 Methods: Patients with symptomatic gout (≥1 tophus, gout flare within 6 months or Macrophages obese) 0.40 -13.04 rates, and enabled repeated monthly gouty arthropathy) and elevated SUA ≥6 mg/dL were treated with monthly doses of Dendritic cells • Mean duration of established or symptomatic gout: 8 years 10 8.9 3 2.27 0.51 -77.53 PLA, PLA-PEG B cells dosing with sustained control of SUA levels pegadricase (0.2 mg/kg or 0.4 mg/kg) alone or in combination with SVP-Rapamycin 11 8.8 5 0.32 0.42 31.25 (0.05 to 0.15 mg/kg). SEL-212 was infused in 28-day cycles x3 doses followed by • SVP-Rapamycin is a Change in Tissue Urate Volume with SEL-212 Treatment 12 11.1 2 0.32 0.03 -90.63 challenge with pegadricase alone on 28-day cycles x2 doses, or in 28-day cycles x5 biodegradable nanoparticle that 13 8.2 5 0.45 0.29 -35.56 • SEL-212 has a significant impact on the combination doses of SVP-Rapamycin and pegadricase. encapsulates rapamycin, an 14 6 5 4.53 0.37 -91.83 Patient 1 reduction of urate deposits in symptomatic To investigate changes in uric acid deposits, DECT scans of hand/wrist, feet/ankles, mTOR inhibitor ) 3 1 0 15 6.4 5 0.48 0.06 -87.50 and knees were performed as an exploratory measure in a subset of patients, during m Patient 14 16^ 6.5 5 0.13 • Intravenous injection of SVP- c 0.05 -61.54 gout patients with hyperuricemia as ( the screening visit, treatment period 3, treatment period 5 or at early termination visit. Rapamycin results in selective e 17^ 9.6 5 0.31 0.00 -100.00 m confirmed by DECT DECT images were analyzed by Arthritis Research Canada (ARC) by two DECT accumulation in the spleen and 18^ 8.6 5 0.17 0.00 -100.00 u l 1 Radiologists utilizing a Syngo Via DECT software package liver, where it is endocytosed by o 19^ 7.5 1 0.00 0.00 NA* V dendritic cells and macrophages • Significant decrease in total urate deposits Results: As of 09 Oct 2018, an initial DECT scan was performed in a subset of 31 e t • Patients with available repeat scans are included in this summary • SVP-Rapamycin is designed to be co-administered with biologic a of 152 dosed patients, with 19 patients with an available follow-up DECT scan. r occurs progressively over the entire U • For the 31 patients with baseline scans, the range of urate drugs to mitigate the formation of ADAs through the induction of 0 . 1 The demographics for patients (N=19) who received a follow-up DECT scan were 43 e volume at baseline was 0.00 to 61.05 cm3 immune tolerance and thus enable sustained therapeutic activity of u treatment period as observed by DECT - 71 years old (mean 55.4 years), male 89.5%, African American 47.4%, and s the biologic (Kishimoto et al, 2016, Nature Nanotech) s • For the 19 patients with repeat scans as of Oct 9 2018, the Caucasian 52.6%. Mean BMI at baseline was 33.1 kg/m2, with 63% of patients i T range of urate volume at baseline was 0.00 to 13.27 cm3 being obese. Mean duration of gout was 8.0 years. 0 . 0 1 • Mean time between the initial and most recent/follow-up DECT SEL-212 The mean SUA at the screening visit was 7.9 mg/dl. Time between the initial and - 4 0 4 8 1 2 1 6 2 0 scan: 121.2 days (ranged from 77 to 163 days) most recent/follow-up DECT scan ranged from 77 - 163 days (mean 121 days), with • SEL-212 is a combination drug candidate comprised of W e e k s P o s t - I n i t i a t i o n o f T r e a t m e n t *NA = Not Applicable, ^Patients in 5 combination dose cohorts a mean change in total urate volume of -1.18 cm3 (range: 0.11 to -13.03 cm3). pegadricase and SVP-Rapamycin Acknowledgements Conclusion: SEL-212 has a significant impact on the reduction of urate deposits in • The co-administration of SVP-Rapamycin and pegadricase is Patient 1 DECT Scan Images Show Significant Reduction of Tissue Urate Burden Patient 14: Before and After Treatment symptomatic gout patients with hyperuricemia as confirmed by DECT. designed to induce the formation of regulatory T cells that mitigate the formation of ADAs against pegadricase and enable Baseline Treatment Period 3 End of Study We thank all of the patients that participated in the clinical trial. We sustained reduction of serum uric acid (SUA) levels are very grateful to the clinical trial site investigators, their staff and ) 3 3 I n i t ia l S c a n the entire Selecta SEL-212 project team Spleen m c F o l lo w - U p S c a n Induction of ( tolerogenic Dendritic cell DECT Imaging e dendritic cells 2 m u • DECT(Dual Energy Computed Tomography) is a sensitive and non-invasive l Naïve T cell o 1,2 Mitigation of Helper T cell V Disclosures diagnostic tool to quantify tissue urate burden Prevention 1 ImmunogenicityNaïve T cell B cell of ADAs 1 e Choi HK et al., Ann Rheum Dis. 2009, 68:1609-12. with Tolerogenic t 2 Regulatory Araujo EG et al, RMD Open. 2015, 1:e000075. SVP-Rapamycin Pegadricase Nanoparticles a T cell r RA, WD, LJ, TKK, JP, and ES are employees and shareholders of U 0 Immune tolerance mediated Selecta Biosciences by regulatory T cells t e e i s l e r k n n W K / / A • Ongoing Phase 2 clinical trial of SEL-212 has demonstrated low d t n o incidence of ADAs resulting in sustained reduction of serum uric a o H F acid (SUA) with monthly dosing (see Abstract 2254)
