Attached files
| file | filename |
|---|---|
| EX-99.4 - EX-99.4 - Tempest Therapeutics, Inc. | a18-18482_1ex99d4.htm |
| EX-99.2 - EX-99.2 - Tempest Therapeutics, Inc. | a18-18482_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Tempest Therapeutics, Inc. | a18-18482_1ex99d1.htm |
| EX-10.1 - EX-10.1 - Tempest Therapeutics, Inc. | a18-18482_1ex10d1.htm |
| EX-3.1 - EX-3.1 - Tempest Therapeutics, Inc. | a18-18482_1ex3d1.htm |
| EX-2.4 - EX-2.4 - Tempest Therapeutics, Inc. | a18-18482_1ex2d4.htm |
| EX-2.3 - EX-2.3 - Tempest Therapeutics, Inc. | a18-18482_1ex2d3.htm |
| EX-2.2 - EX-2.2 - Tempest Therapeutics, Inc. | a18-18482_1ex2d2.htm |
| EX-2.1 - EX-2.1 - Tempest Therapeutics, Inc. | a18-18482_1ex2d1.htm |
| 8-K - 8-K - Tempest Therapeutics, Inc. | a18-18482_18k.htm |
Disclaimer This presentation includes forward-looking statements. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of Millendo Therapeutics, Inc. (“Millendo,” “we,” “us” or “our”) our business strategy and plans, the clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Additional Information and Where You Can Find It This presentation may be deemed to be solicitation material in respect of the proposed transaction between OvaScience, Inc. (OvaScience) and Millendo Therapeutics, Inc. (Millendo). In connection with the proposed transaction between OvaScience and Millendo, OvaScience intends to file relevant materials with the Securities and Exchange Commission (SEC), including a registration statement that will contain a proxy statement and prospectus. OVASCIENCE URGES INVESTORS AND STOCKHOLDERS TO READ THESE MATERIALS CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT OVASCIENCE, THE PROPOSED TRANSACTION AND RELATED MATTERS. This presentation is not a substitute for the registration statement, proxy statement, prospectus or any other document that OvaScience may file with the SEC or send to OvaScience shareholders in connection with the proposed transaction. Investors and shareholders will be able to obtain free copies of the proxy statement, prospectus and other documents filed by OvaScience with the SEC (when they become available) through the website maintained by the SEC at www.sec.gov. In addition, investors and shareholders will be able to obtain free copies of the proxy statement, prospectus and other documents filed by OvaScience with the SEC by contacting Investor Relations by mail at OvaScience, Inc., Attn: Investor Relations, 9 Fourth Avenue, Waltham, Massachusetts 02451. Investors and stockholders are urged to read the proxy statement, prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the proposed transaction. Participants in the Solicitation – OvaScience and Millendo, and each of their respective directors and executive officers and certain of their other members of management and employees, may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. Information about OvaScience’s directors and executive officers is included in OvaScience’s Annual Report on Form 10-K for the year ended December 31, 2017, filed with the SEC on March 15, 2018, and the proxy statement for OvaScience’s 2018 annual meeting of stockholders, filed with the SEC on April 30, 2018. Additional information regarding these persons and their interests in the transaction will be included in the proxy statement relating to the transaction when it is filed with the SEC. These documents can be obtained free of charge from the sources indicated above. Non-Solicitation – This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No public offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. 2

Millendo Therapeutics Leading orphan endocrine disease company Clinical-stage company focused on orphan endocrine diseases • Clinically-validated molecules with differentiated mechanisms • High unmet medical needs in orphan indications A LEADER IN ORPHAN ENDOCRINE DISEASES Livoletide: Pivotal-stage asset for Prader-Willi syndrome • Placebo-controlled Phase 2 clinical trial in PWS was positive demonstrating a reduction in hyperphagia and negative food related behaviors • Phase 2b/3 study initiating in Q1 2019 Nevanimibe: Multiple Phase 2 trials for serious adrenal diseases • Congenital adrenal hyperplasia: POC in Phase 2; Phase 2b initiating in Q3 2018 • Cushing’s syndrome: Phase 2 study enrolling 3 FIRST-IN-CLASS PROGRAMS Strong leadership in place to execute on company strategy • World-class management team with highly relevant scientific, clinical, regulatory and commercial expertise EXPERIENCED TEAM Backed by highly regarded healthcare investors • New Enterprise Associates, Frazier Healthcare Partners, Roche Venture Fund, and others BLUE CHIP INVESTORS 3
Millendo Executive Leadership Relevant and successful drug development and commercialization expertise JEFF BRINZA Chief Administrative Officer & General Counsel JULIA OWENS, PhD Chief Executive Officer PHARIS MOHIDEEN, MD Chief Medical Officer THOMAS HOOVER SVP Commercial Strategy RYAN ZEIDAN, PhD SVP Development ANDREW SPENCER, PhD SVP Preclinical R&D JENNIFER MINAI VP Finance 4

Livoletide (AZP-531)for Prader-Willi Syndrome

Livoletide Nevanimibe Prader-Willi Syndrome (PWS) Well-characterized genetic orphan endocrine disease Complex disorder attributed primarily to spontaneous genetic error on CHROMOSOME 15 Prader-Willi syndrome is characterized by: Hyperphagia: Chronic feeling of insatiable hunger Starts at 5-8 years old and usually lasts until death Hallmark of condition Intellectual, emotional disabilities and psychiatric disorders PREVALENT CASES 8K–11K Multiple hormonal deficiencies resulting in short stature and incomplete sexual development Sleep disturbances and GI issues ~1:15,000 BIRTH INCIDENCE1,2 30-40 YRS MEDIAN AGE / MORTALITY 1 Lionti, 2015 2 Ehara, 1995 6
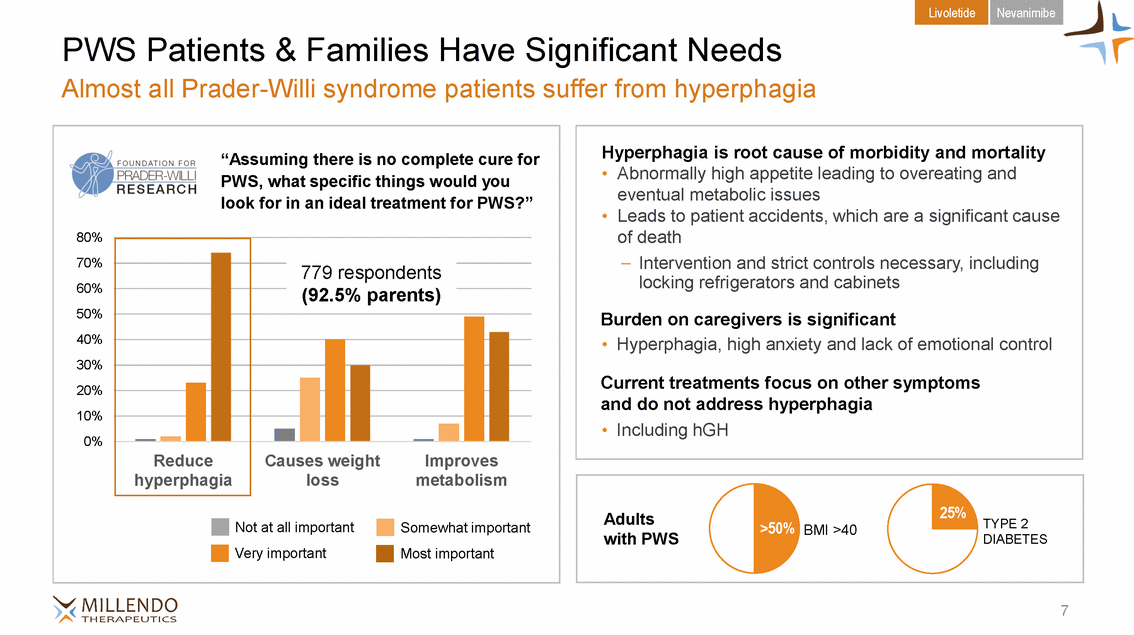
Livoletide Nevanimibe PWS Patients & Families Have Significant Needs Almost all Prader-Willi syndrome patients suffer from hyperphagia PWS, what specific things would you 60% with PWS DIABETES 7 Adults>50% BMI >40TYPE 2 “Assuming there is no complete cure for look for in an ideal treatment for PWS?” 80% 70% 50% 40% 30% 20% 10% 0% Not at all importantSomewhat important Very importantMost important 779 respondents (92.5% parents) Reduce hyperphagia Causes weightImproves lossmetabolism Hyperphagia is root cause of morbidity and mortality • Abnormally high appetite leading to overeating and eventual metabolic issues • Leads to patient accidents, which are a significant cause of death – Intervention and strict controls necessary, including locking refrigerators and cabinets Burden on caregivers is significant • Hyperphagia, high anxiety and lack of emotional control Current treatments focus on other symptoms and do not address hyperphagia • Including hGH
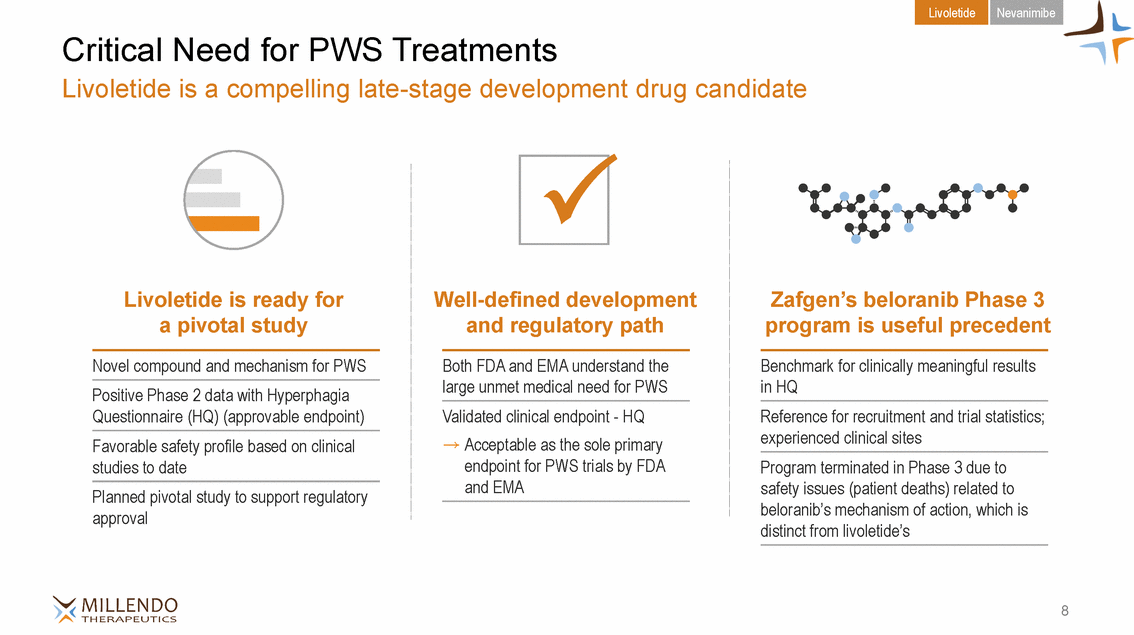
Livoletide Nevanimibe Critical Need for PWS Treatments Livoletide is a compelling late-stage development drug candidate P Livoletide is ready for a pivotal study Zafgen’s beloranib Phase 3 Well-defined development and regulatory path program is useful precedent Novel compound and mechanism for PWS Both FDA and EMA understand the large unmet medical need for PWS Benchmark for clinically meaningful results in HQ Positive Phase 2 data with Hyperphagia Questionnaire (HQ) (approvable endpoint) Validated clinical endpoint - HQ Acceptable as the sole primary endpoint for PWS trials by FDA and EMA Reference for recruitment and trial statistics; experienced clinical sites Favorable safety profile based on clinical studies to date Program terminated in Phase 3 due to safety issues (patient deaths) related to beloranib’s mechanism of action, which is distinct from livoletide’s Planned pivotal study to support regulatory approval 8
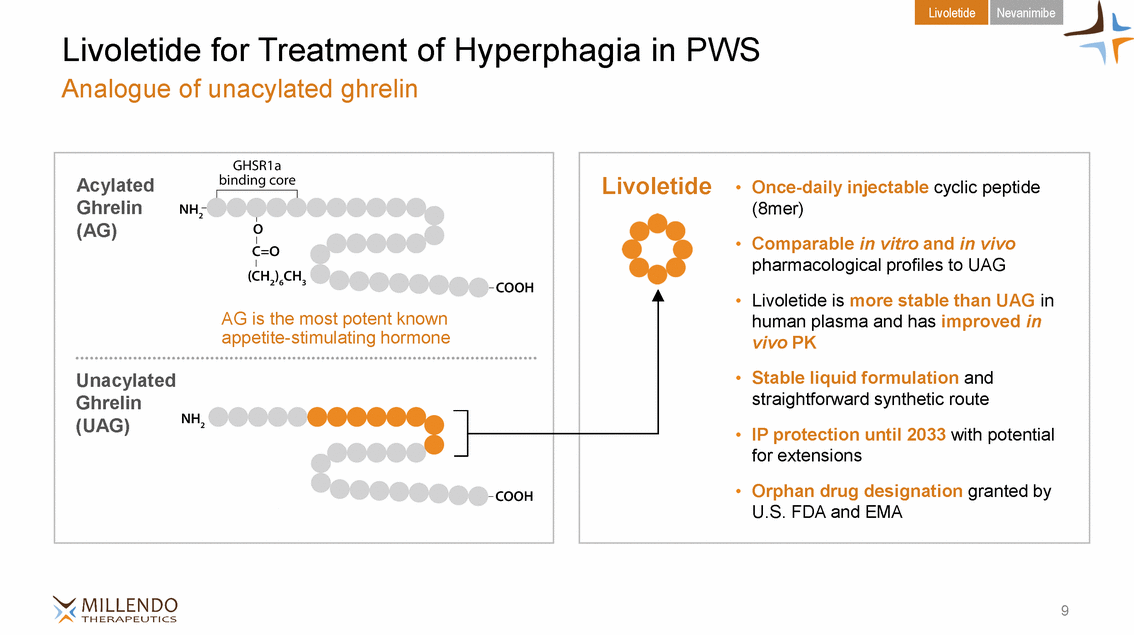
Livoletide Nevanimibe Livoletide for Treatment of Hyperphagia in PWS Analogue of unacylated ghrelin etide • Once-daily injectable cyclic peptide vivo PK 9 Acylated Ghrelin (AG) AG is the most potent known appetite-stimulating hormone Unacylated Ghrelin (UAG) Livol (8mer) • Comparable in vitro and in vivo pharmacological profiles to UAG • Livoletide is more stable than UAG in human plasma and has improved in • Stable liquid formulation and straightforward synthetic route • IP protection until 2033 with potential for extensions • Orphan drug designation granted by U.S. FDA and EMA
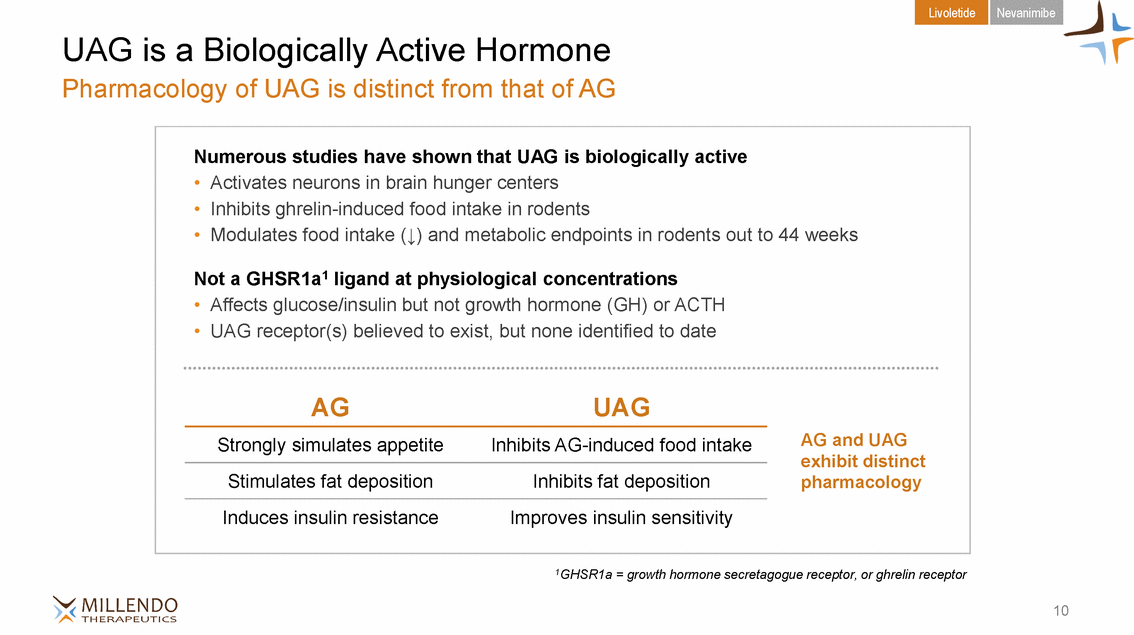
Livoletide Nevanimibe UAG is a Biologically Active Hormone Pharmacology of UAG is distinct from that of AG exhibit distinct 1GHSR1a = growth hormone secretagogue receptor, or ghrelin receptor 10 Numerous studies have shown that UAG is biologically active • Activates neurons in brain hunger centers • Inhibits ghrelin-induced food intake in rodents • Modulates food intake and metabolic endpoints in rodents out to 44 weeks Not a GHSR1a1 ligand at physiological concentrations • Affects glucose/insulin but not growth hormone (GH) or ACTH • UAG receptor(s) believed to exist, but none identified to date AGUAG Strongly simulates appetiteInhibits AG-induced food intake AG and UAG Stimulates fat depositionInhibits fat depositionpharmacology Induces insulin resistanceImproves insulin sensitivity
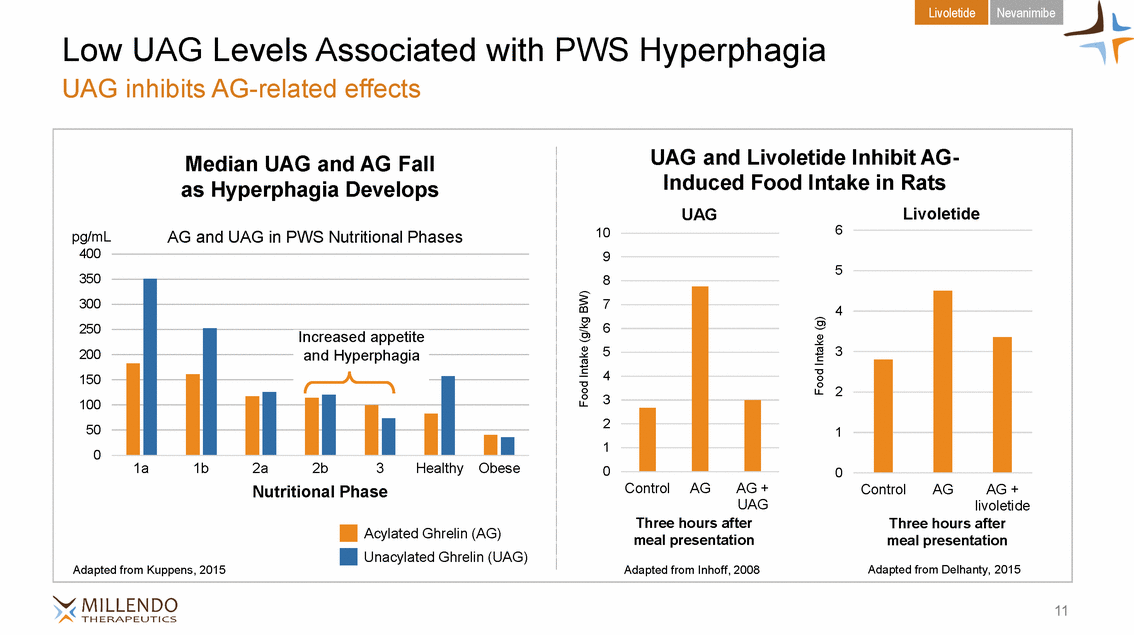
Livoletide Nevanimibe Low UAG Levels Associated with PWS Hyperphagia UAG inhibits AG-related effects Induced Food Intake in Rats as Hyperphagia Develops 350 8 2 Ph1aase Ph1abse Ph2aase Ph2abse Pha3se 3 Healthy Obese 0 0 UAG livoletide Acylated Ghrelin (AG) meal presentation meal presentation Adapted from Kuppens, 2015 Adapted from Inhoff, 2008 Adapted from Delhanty, 2015 11 Food Intake (g/kg BW) Food Intake (g) Median UAG and AG FallUAG and Livoletide Inhibit AG-UAGLivoletide pg/mLAG and UAG in PWS Nutritional Phases106 4009 5 30074 2506 20053 1504 1003 5021 01 1a1bN2autrition2bal PhaseControlAGAG +ControlAGAG + Three hours afterThree hours after Unacylated Ghrelin (UAG) Increased appetite and Hyperphagia
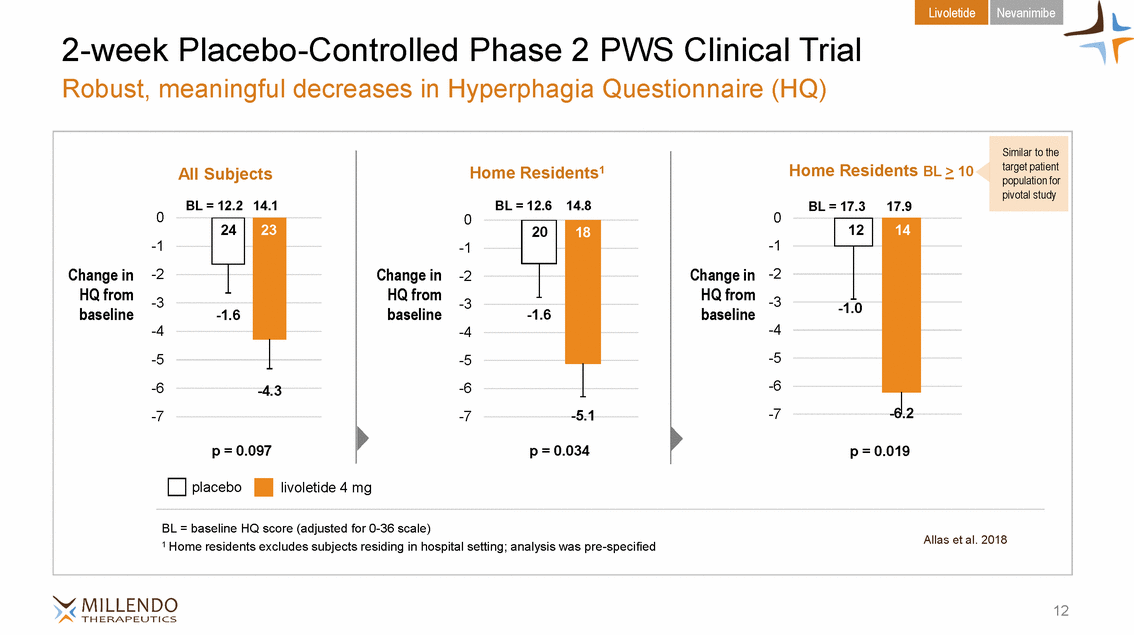
Livoletide Nevanimibe 2-week Placebo-Controlled Phase 2 PWS Clinical Trial Robust, meaningful decreases in Hyperphagia Questionnaire (HQ) 0 0 0 baseline baseline baseline Allas et al. 2018 1 Home residents excludes subjects residing in hospital setting; analysis was pre-specified 12 All SubjectsHome Residents1 Home Residents BL > 10 BL = 12.2 14.1BL = 12.614.8BL = 17.317.9 -1-1-1 Change in-2Change in-2Change in-2 HQ from-3HQ from-3HQ from-3 -4-4-4 -5-5-5 -6-4.3-6-6 -7-7 -5.1 -7-6.2 p = 0.097p = 0.034p = 0.019 placebolivoletide 4 mg BL = baseline HQ score (adjusted for 0-36 scale) 12 14 -1.0 20 18 -1.6 24 23 -1.6 Similar to the target patient population for pivotal study
Livoletide Nevanimibe Livoletide Phase 2 Safety Summary Well tolerated, no safety signals P Safety events well balanced relative to placebo • Subjects reporting any adverse event (AE) – – Livoletide: 60.9% Placebo: 58.3% • Most commonly reported AE in both groups were related injection site with placebo having a higher frequency No serious AE reported or discontinuations due to AE No unusual or unexpected AE to • • P No significant changes in vital signs or safety labs 13

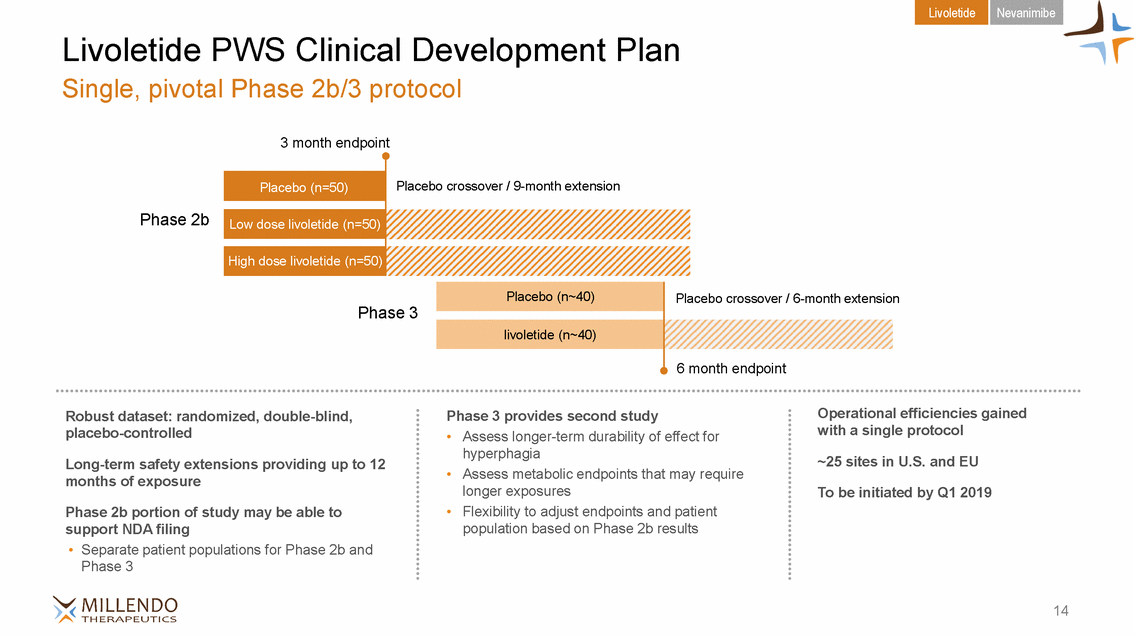
Livoletide Nevanimibe Livoletide PWS Clinical Development Plan Single, pivotal Phase 2b/3 protocol 3 month endpoint Placebo crossover / 9-month extension Phase 2b Placebo crossover / 6-month extension Phase 3 6 month endpoint Operational efficiencies gained with a single protocol Robust dataset: randomized, double-blind, placebo-controlled Phase 3 provides second study • Assess longer-term durability of effect for hyperphagia Assess metabolic endpoints that may require longer exposures Flexibility to adjust endpoints and patient population based on Phase 2b results ~25 sites in U.S. and EU Long-term safety extensions providing up to 12 months of exposure • To be initiated by Q1 2019 • Phase 2b portion of study may be able to support NDA filing • Separate patient populations for Phase 2b and Phase 3 14 livoletide (n~40) Placebo (n~40) High dose livoletide (n=50) Low dose livoletide (n=50) Placebo (n=50)
Nevanimibe (ATR-101) for Orphan Adrenal Diseases

Livoletide Nevanimibe Nevanimibe for Orphan Adrenal Diseases Novel MOA has potential to address serious adrenal diseases P CAH Ph2b CS Ph2 ACAT1 Target Data to Date Clinical Trial Status Orphan Adrenal Diseases Clinical proof-of-concept from open-label Phase 2 clinical trial in CAH Adrenal-selective inhibitor of acetyl-CoA acetyltransferase 1 (ACAT1) Phase 2b clinical trial in CAH to start in Q3 2018 Classic congenital adrenal hyperplasia (CAH) Phase 2 clinical trial in CS ongoing Endogenous Cushing’s syndrome (CS) Favorable safety profile in preclinical and clinical studies Inhibits adrenocortical steroidogenesis at a proximal level Well-defined mechanism of action 16 CAH CS
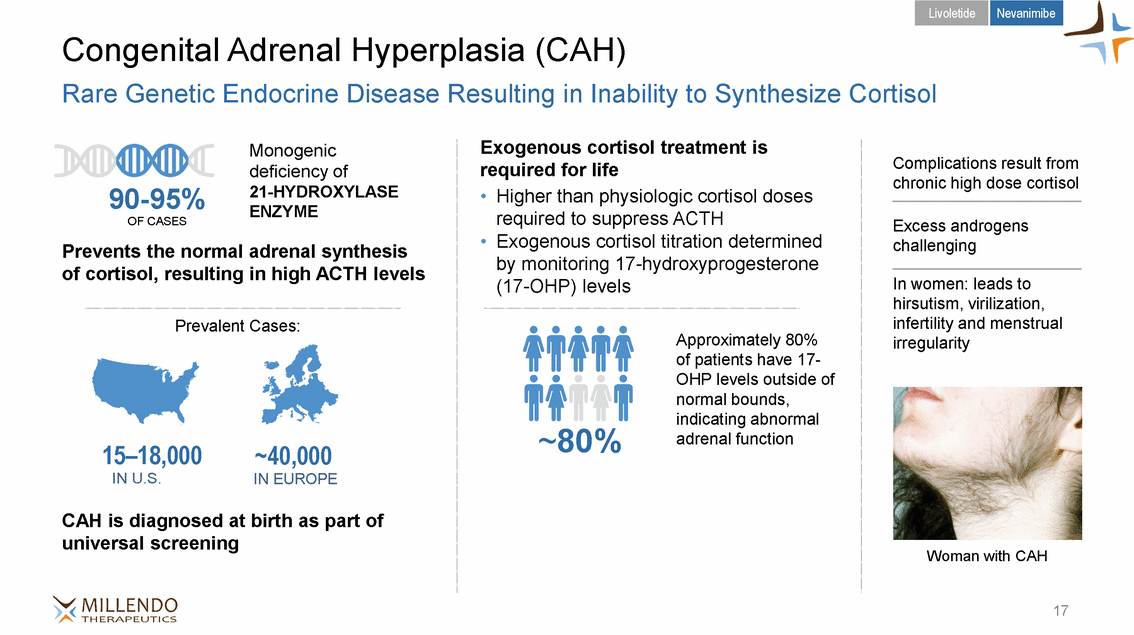
Livoletide Nevanimibe Congenital Adrenal Hyperplasia (CAH) Rare Genetic Endocrine Disease Resulting in Inability to Synthesize Cortisol Exogenous cortisol treatment is required for life Monogenic deficiency of 21-HYDROXYLASE ENZYME Complications result from chronic high dose cortisol 90-95% • Higher than physiologic cortisol doses required to suppress ACTH Exogenous cortisol titration determined by monitoring 17-hydroxyprogesterone (17-OHP) levels OF CASES Excess androgens challenging • Prevents the normal adrenal synthesis of cortisol, resulting in high ACTH levels In women: leads to hirsutism, virilization, infertility and menstrual irregularity Prevalent Cases: Approximately 80% of patients have 17-OHP levels outside of normal bounds, indicating abnormal adrenal function ~80% 15–18,000 IN U.S. ~40,000 IN EUROPE CAH is diagnosed at birth as part of universal screening Woman with CAH 17
Livoletide Nevanimibe Nevanimibe Proof of Concept Established in CAH Activity demonstrated through reductions in 17-OHP P Biological effect demonstrated through reduction in key steroids and steroid precursors • Mean reductions in 17-OHP were observed at all nevanimibe doses while mean increases were observed during all placebo treatments – Aligned with expectations and CAH pathophysiology Significant reductions (up to 72%) in 17-OHP observed even in patients with unusually high 17-OHP levels at baseline 7 of 10 of subjects demonstrated a biological effect 2 of 10 subjects met the primary endpoint (17-OHP < 2x upper limit of normal) Phase 2b study overview • Will include 2 cohorts at 8-10 sites in EU Intra-subject dose escalation with 3 months continuous dosing Study protocol final; expected to start in Q3 2018 • • • • • P Nevanimibe was reported to be well tolerated P Orphan drug designation granted by U.S. FDA and EMA 18
Millendo is a Leading Company in Rare Endocrine Diseases Novel agents in orphan diseases with unmet needs and little innovation Rapidly and efficiently advance development of, obtain approval for, and commercialize livoletide for the treatment of PWS Pursue development of, obtain approval for, and commercialize nevanimibe for the treatment of CAH and CS Continue to expand our pipeline by leveraging our expertise in in-licensing and acquiring product candidates Build a specialized sales and marketing organization in the United States targeting endocrinologists Maximize the value of our portfolio by strategically collaborating in select markets 19
Millendo Therapeutics–OvaScience Merger Definitive agreement for all-stock merger announced on August 9, 2018 Expected to be completed in 4Q 2018; new company will trade on Nasdaq under MLND Requires OvaScience shareholder approval among other customary conditions Concurrent financing from Millendo existing investors of $30 million New company’s cash balance expected to be at least $70 million Leadership: Dr. Julia Owens, CEO of Millendo Board will include 6 nominees from Millendo and one from OvaScience On a pro forma basis, current OvaScience holders will own approximately 20% of the combined company and current Millendo investors will own approximately 80% of the combined company (before accounting for the additional financing transaction) 20
TRANSFORMING FERTILITY


