Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Strongbridge Biopharma plc | a18-18296_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Strongbridge Biopharma plc | a18-18296_1ex99d1.htm |
| 8-K - 8-K - Strongbridge Biopharma plc | a18-18296_18k.htm |
Forward-looking statements This document contains forward-looking statements relating to the Company’s strategy, objectives, business development plans and financial position. All statements other than statements of historical facts included in this document, including, without limitation, statements regarding the Company’s future financial position, strategy, anticipated investments, costs and results, status and results of clinical trials, size of patient population, plans, outcomes of product development efforts, intellectual property portfolio and objectives of management for future operations, may be deemed to be forward-looking statements. You can identify forward-looking statements by words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions that convey uncertainty or future events or outcomes. These forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause the Company’s actual results, performance, or achievements or industry results to be materially different from those contemplated, projected, forecasted, estimated or budgeted, whether expressed or implied, by these forward-looking statements. Given these risks and uncertainties, investors should not place undue reliance on forward-looking statements as a prediction of actual results. A discussion of certain of these risks may be found in the filings the Company makes with the U.S. Securities and Exchange Commission. None of these forward-looking statements constitutes a guarantee of the future occurrence of such events or of actual results. These statements are based on data, assumptions, and estimates that the Company believes are reasonable. The forward-looking statements contained in this document are made only as of the date hereof. Except as otherwise required by law, the Company expressly disclaims any obligation or undertaking to release publicly any updates of any forward-looking statements contained in this document to reflect any change in its actual results, assumptions, expectations or any change in events, factors, conditions, or circumstances on which any forward-looking statement contained in this document is based. 2

Strongbridge Biopharma plc: Building a portfolio of therapeutically-aligned vertical franchises in rare diseases Rare Endocrine Rare Neuromuscular Rare Disease Franchise #3 MACRILEN (macimorelin) The 1st and only FDA-approved oral drug for diagnosing adult growth hormone deficiency (Orphan Drug Exclusivity) The 1st and only FDA-approved drug for Primary Periodic Paralysis, an ultra-rare genetic neuromuscular condition (Orphan Drug Exclusivity) RECORLEV Next-generation cortisol inhibitor for Cushing’s Syndrome PHASE 3 (Orphan Drug Designation) VELDOREOTIDE Extended Release Potential next-generation somatostatin analog for Acromegaly PRECLINICAL (Orphan Drug Designation) Business development opportunities Business development opportunities 3
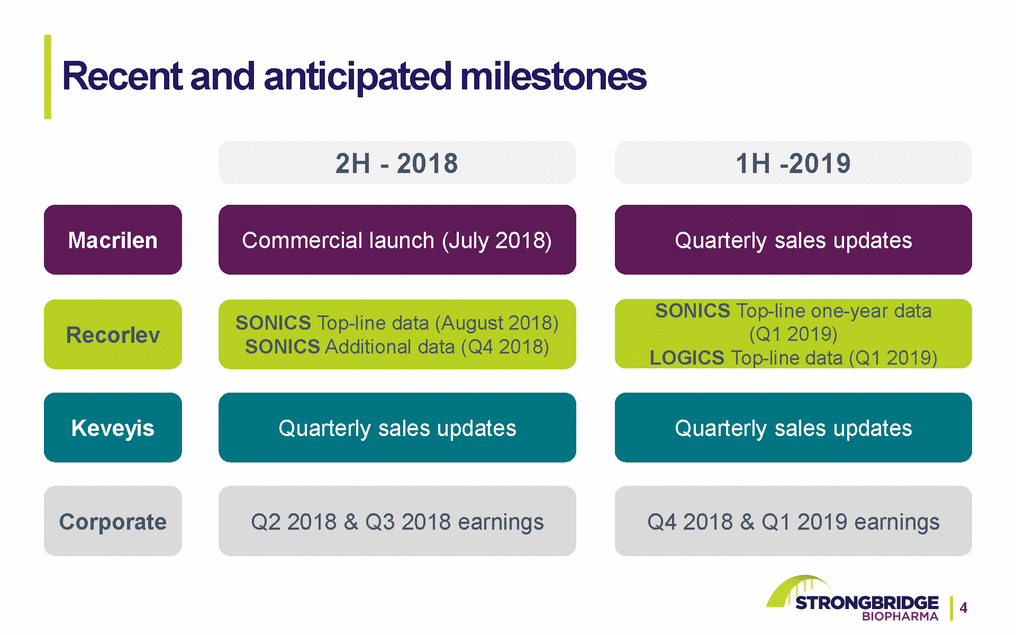
Recent and anticipated milestones 2H - 20181H -2019 Macrilen Commercial launch (July 2018) Quarterly sales updates RecorlevSONICS Top-line data (August 2018) SONICS Additional data (Q4 2018) SONICS Top-line one-year data (Q1 2019) LOGICS Top-line data (Q1 2019) Keveyis Quarterly sales updates Quarterly sales updates Corporate Q2 2018 & Q3 2018 earningsQ4 2018 & Q1 2019 earnings 4
Recorlev (levoketoconazole)

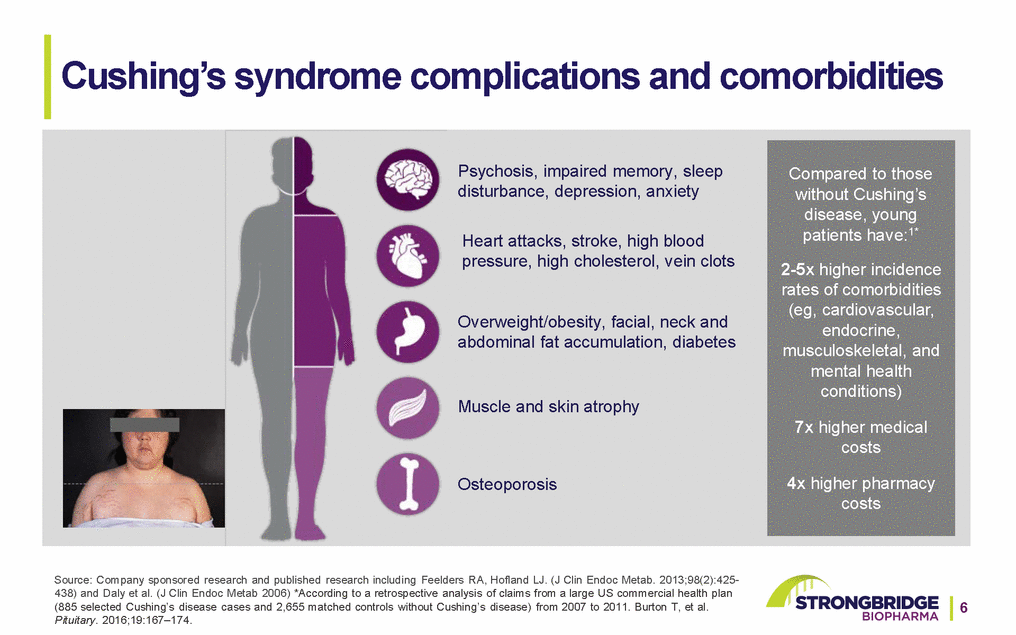
Cushing’s syndrome complications and comorbidities Psychosis, impaired memory, sleep disturbance, depression, anxiety Heart attacks, stroke, high blood pressure, high cholesterol, vein clots Overweight/obesity, facial, neck and abdominal fat accumulation, diabetes Muscle and skin atrophy Osteoporosis Compared to those without Cushing’s disease, young patients have:1* 2-5x higher incidence rates of comorbidities (eg, cardiovascular, endocrine, musculoskeletal, and mental health conditions) 7x higher medical costs 4x higher pharmacy costs Source: Company sponsored research and published research including Feelders RA, Hofland LJ. (J Clin Endoc Metab. 2013;98(2):425-438) and Daly et al. (J Clin Endoc Metab 2006) *According to a retrospective analysis of claims from a large US commercial health plan (885 selected Cushing’s disease cases and 2,655 matched controls without Cushing’s disease) from 2007 to 2011. Burton T, et al.6 Pituitary. 2016;19:167–174.
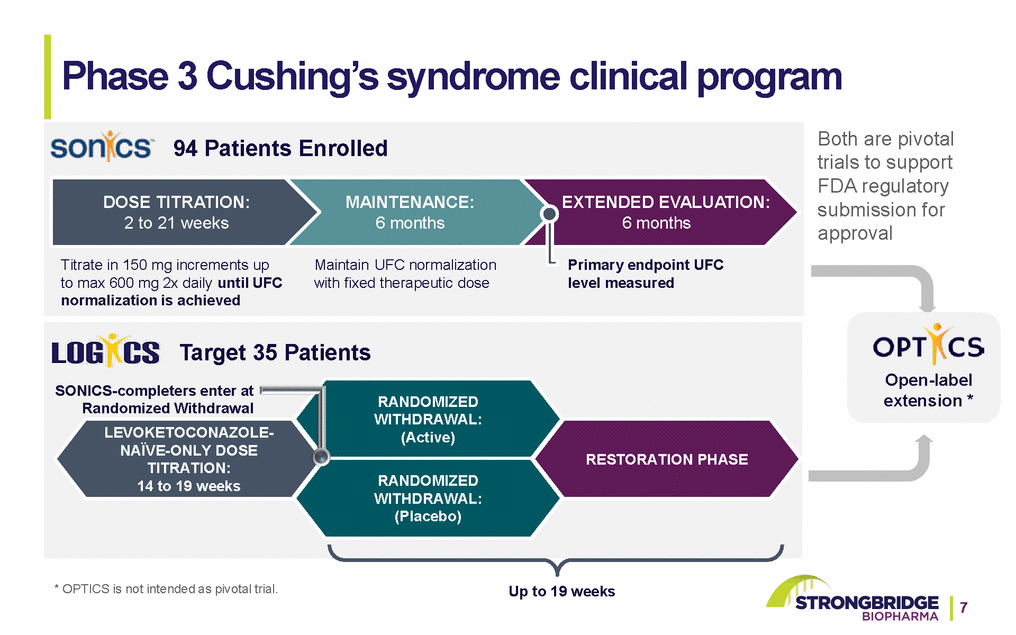
Phase 3 Cushing’s syndrome clinical program 94 Patients EnrolledBoth are pivotal trials to support FDA regulatory DOSE TITRATION: 2 to 21 weeks Titrate in 150 mg increments up to max 600 mg 2x daily until UFC normalization is achieved MAINTENANCE: 6 months Maintain UFC normalization with fixed therapeutic dose EXTENDED EVALUATION: 6 months Primary endpoint UFC level measured submission for approval Target 35 Patients SONICS-completers enter at Randomized Withdrawal LEVOKETOCONAZOLE-NAÏVE-ONLY DOSE TITRATION: 14 to 19 weeks RANDOMIZED WITHDRAWAL: (Active) RANDOMIZED WITHDRAWAL: (Placebo) RESTORATION PHASE Open-label extension * * OPTICS is not intended as pivotal trial. Up to 19 weeks
SONICS Study Design: Single Arm, Open-Label Phase 3 2 – 21 weeks 6 months 6 months Dose Titration Maintenance Extended Evaluation Increase dose in 150mg increments up to max of 600mg 2x daily until UFC normalization Maintain UFC normalization for 6 months with no dose increase Primary endpoint 94 patients enrolled 77 patients entered 61 patients completed Secondary endpoints
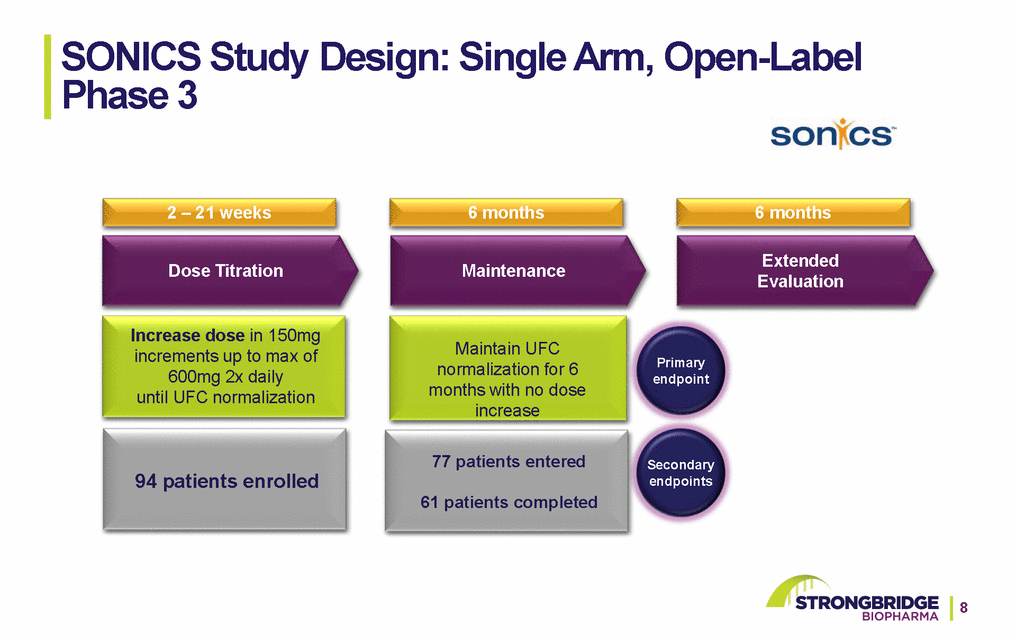
SONICS Patient Demographics
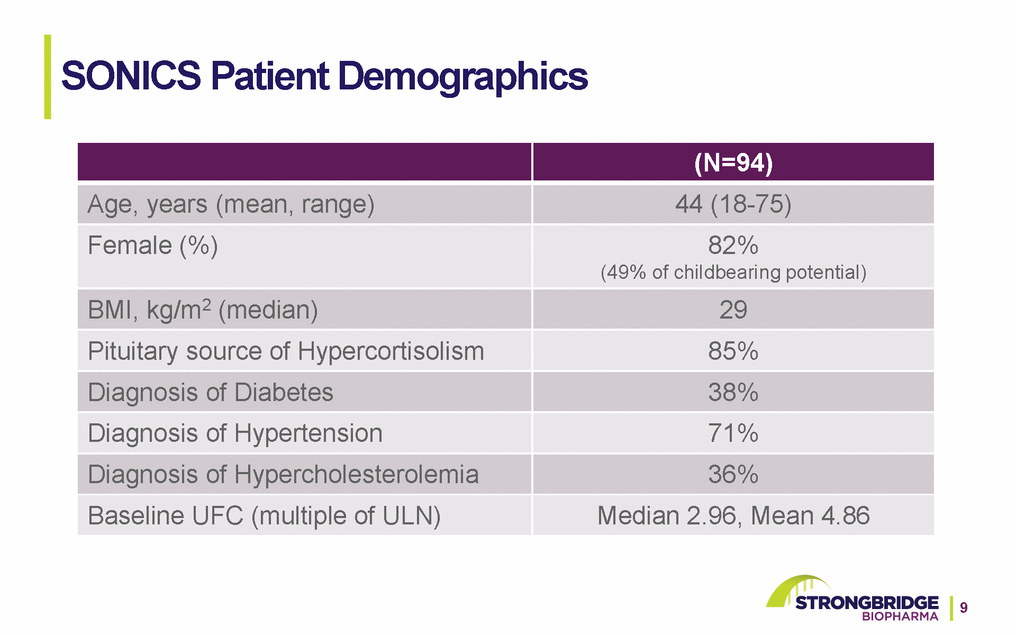
Full and Partial UFC Responder Analysis At End Of Maintenance Phase Analysis based on repeated measures model. Least squares mean estimate and associated 95% confidence interval CI: 95% Confidence interval around the least squares means estimate from repeated measures model *1-sided vs. null hypothesis of 20% or lower rate **Data based on 55 maintenance phase completers with both baseline and month 6 UFC data available
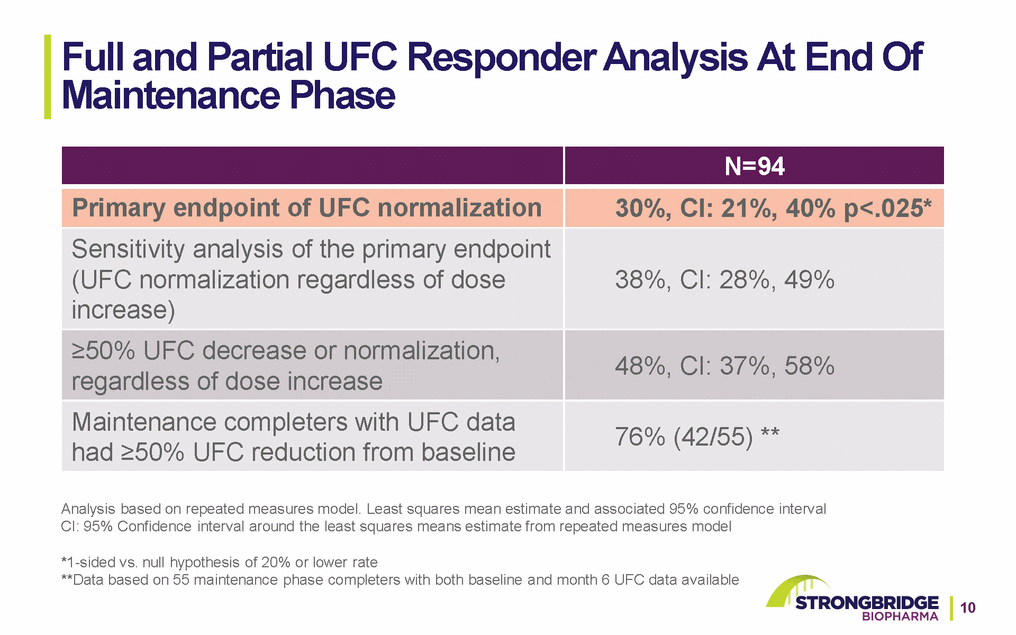
SONICS Key Secondary Endpoints at Month 6 *Hochberg adjustment applied to p-values to control type 1 error (except Body Mass Index); reductions from baseline based on least squares mean changes from repeated measures model. No significant changes observed in blood pressure or c-reactive protein; small but statistically significant decrease observed in HDL-c
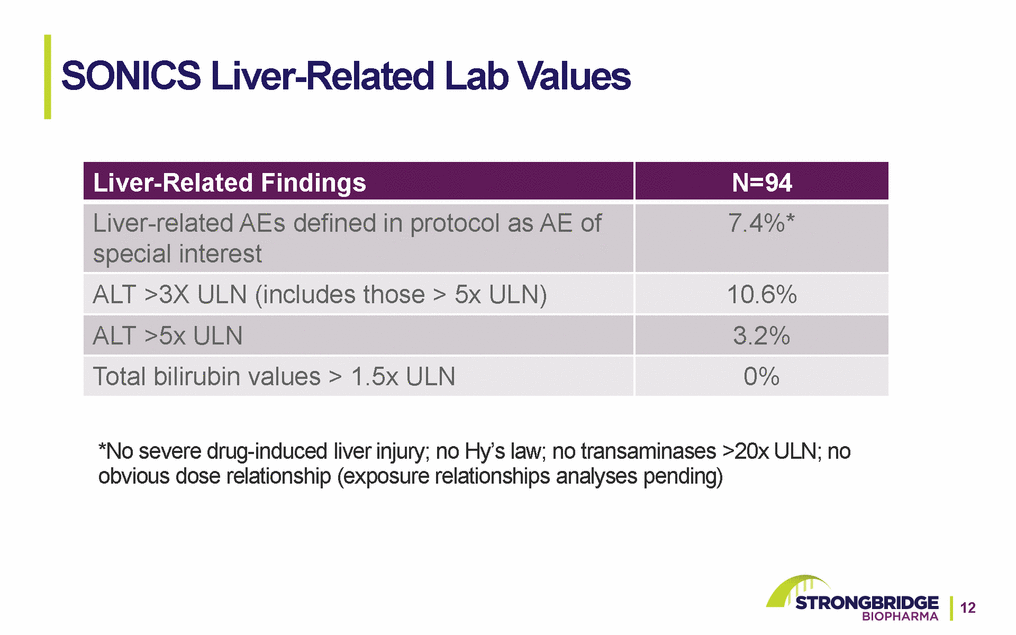
SONICS Liver-Related Lab Values *No severe drug-induced liver injury; no Hy’s law; no transaminases >20x ULN; no obvious dose relationship (exposure relationships analyses pending)
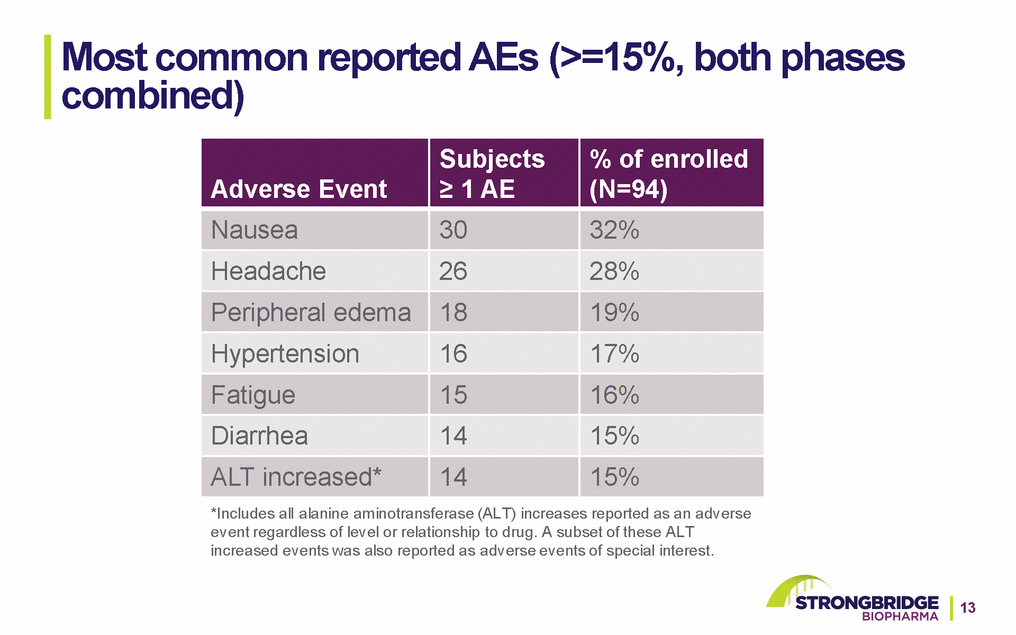
Most common reported AEs (>=15%, both phases combined) *Includes all alanine aminotransferase (ALT) increases reported as an adverse event regardless of level or relationship to drug. A subset of these ALT increased events was also reported as adverse events of special interest.
Recorlev Next Steps Strongbridge intends to discuss accelerated approval and the regulatory pathway with the FDA Report one year safety and efficacy data from SONICS (Q1 2019) Complete enrollment and report results (Q1 2019) from Phase 3 randomized withdrawal LOGICS study
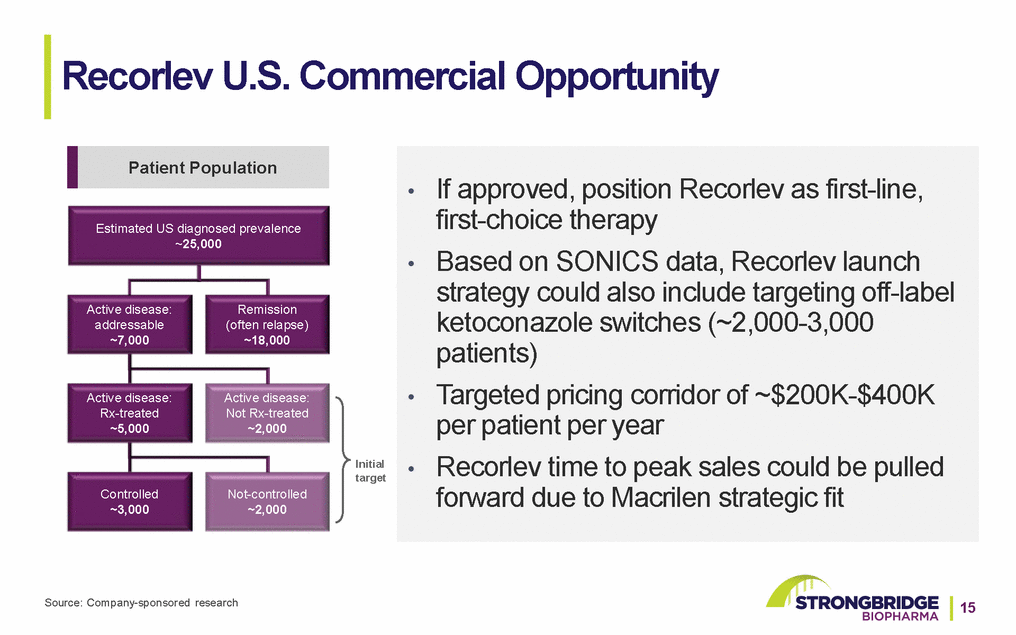
Recorlev U.S. Commercial Opportunity Patient Population Estimated US diagnosed prevalence ~25,000 •If approved, position Recorlev as first-line, first-choice therapy •Based on SONICS data, Recorlev launch strategy could also include targeting off-label Active disease: addressable ~7,000 Active disease: Rx-treated ~5,000 Controlled ~3,000 Remission (often relapse) ~18,000 Active disease: Not Rx-treated ~2,000 Not-controlled ~2,000 Initial target ketoconazole switches (~2,000-3,000 patients) •Targeted pricing corridor of ~$200K-$400K per patient per year •Recorlev time to peak sales could be pulled forward due to Macrilen strategic fit Source: Company-sponsored research15
Macrilen (macimorelin)

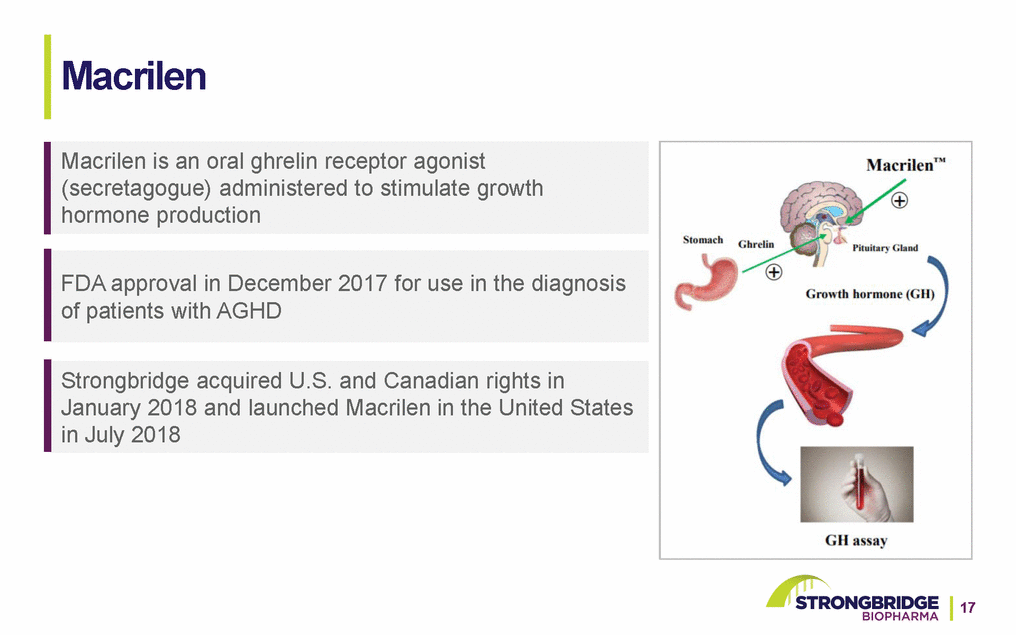
Macrilen Macrilen is an oral ghrelin receptor agonist (secretagogue) administered to stimulate growth hormone production FDA approval in December 2017 for use in the diagnosis of patients with AGHD Strongbridge acquired U.S. and Canadian rights in January 2018 and launched Macrilen in the United States in July 2018 17
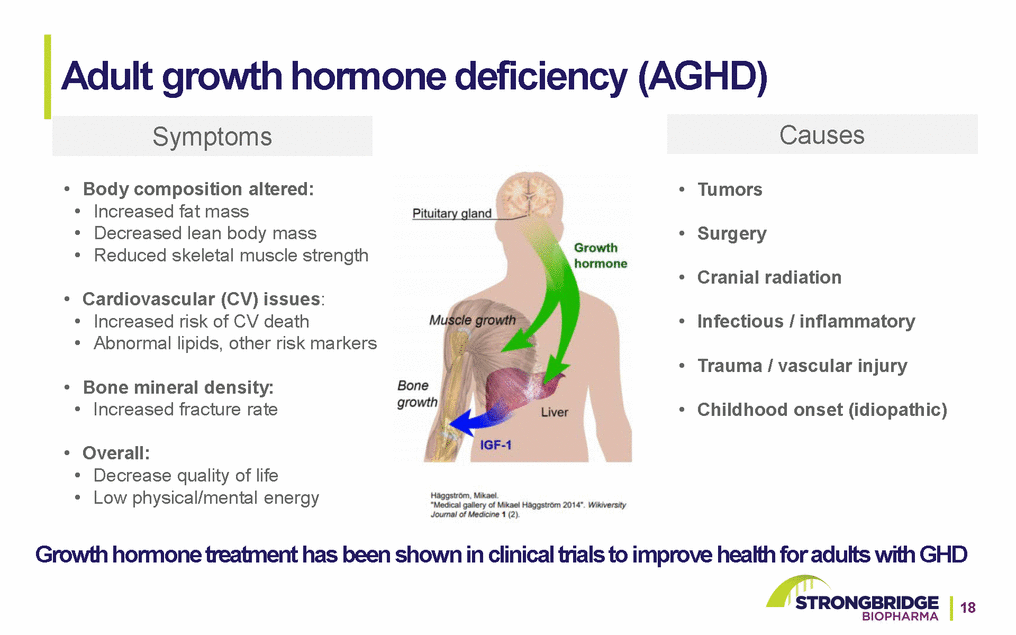
Adult growth hormone deficiency (AGHD) Symptoms Causes • Body composition altered: • Increased fat mass • Decreased lean body mass • Reduced skeletal muscle strength • Cardiovascular (CV) issues: • Increased risk of CV death • Abnormal lipids, other risk markers • Bone mineral density: • Increased fracture rate • Overall: • Decrease quality of life • Low physical/mental energy • Tumors • Surgery • Cranial radiation • Infectious / inflammatory • Trauma / vascular injury • Childhood onset (idiopathic) Growth hormone treatment has been shown in clinical trials to improve health for adults with GHD
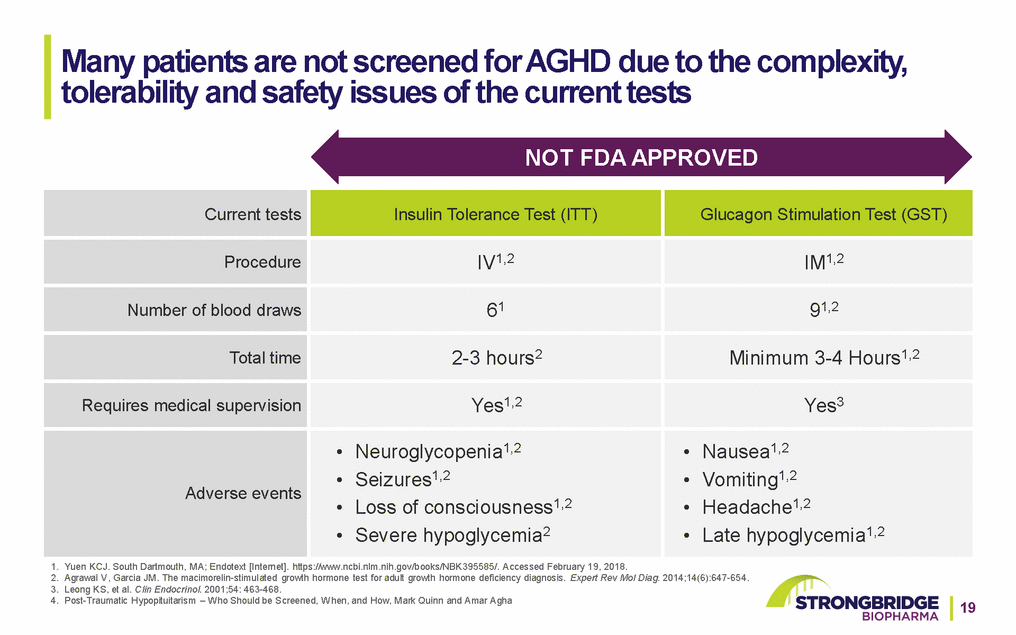
Many patients are not screened for AGHD due to the complexity, tolerability and safety issues of the current tests NOT FDA APPROVED 1. Yuen KCJ. South Dartmouth, MA; Endotext [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK395585/. Accessed February 19, 2018. 2. Agrawal V, Garcia JM. The macimorelin-stimulated growth hormone test for adult growth hormone deficiency diagnosis. Expert Rev Mol Diag. 2014;14(6):647-654. 3. Leong KS, et al. Clin Endocrinol. 2001;54: 463-468. 4. Post-Traumatic Hypopituitarism – Who Should be Screened, When, and How, Mark Quinn and Amar Agha
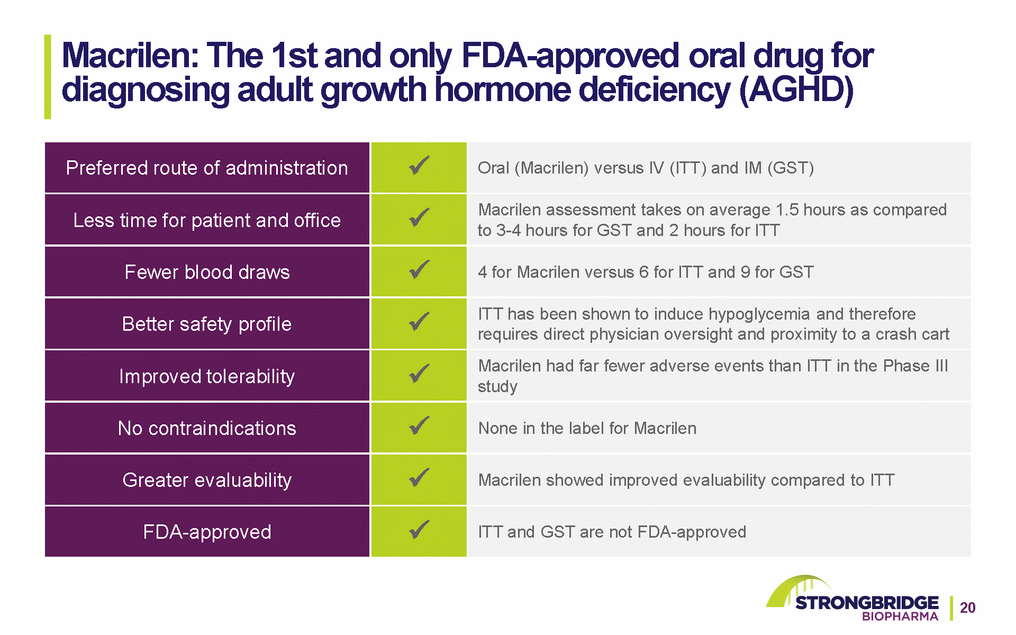
Macrilen: The 1st and only FDA-approved oral drug for diagnosing adult growth hormone deficiency (AGHD)
Macrilen accurately diagnoses AGHD: comparison to ITT Insulin Tolerance Test (ITT) (GH cutoff 5.1 ng/mL) Positive Negative Total Tests Macrilen (GH cutoff 2.8 ng/mL) Positive 55 4 59 Negative 19 62 81 Total 74 66 140 AGRE EMENT 74%* 94%* 84% •Positive agreement higher (89%) for high-risk AGHD category •Negative agreement 86-94% •Labeling reflective of overall and risk-stratified agreements •<1% of Macrilen tests were not evaluable vs. 17% of ITTs •Macrilen was highly reproducible (91%) in the same patient Negative Agreement: 93.94% (CI: 85.20%, 98.32%)-Met pre-defined performance criterion (i.e. excluded negative agreement with ITT of less than 75% with 95% confidence), Positive Agreement: 74.32% (CI: 62.84%, 83.78%)-Did not meet pre-defined performance criterion (i.e. could not exclude positive agreement with ITT of less than 70% with 95% confidence) * Co-Primary efficacy endpoints; a positive test is one that fails to reach the respective test’s GH cutoff 21
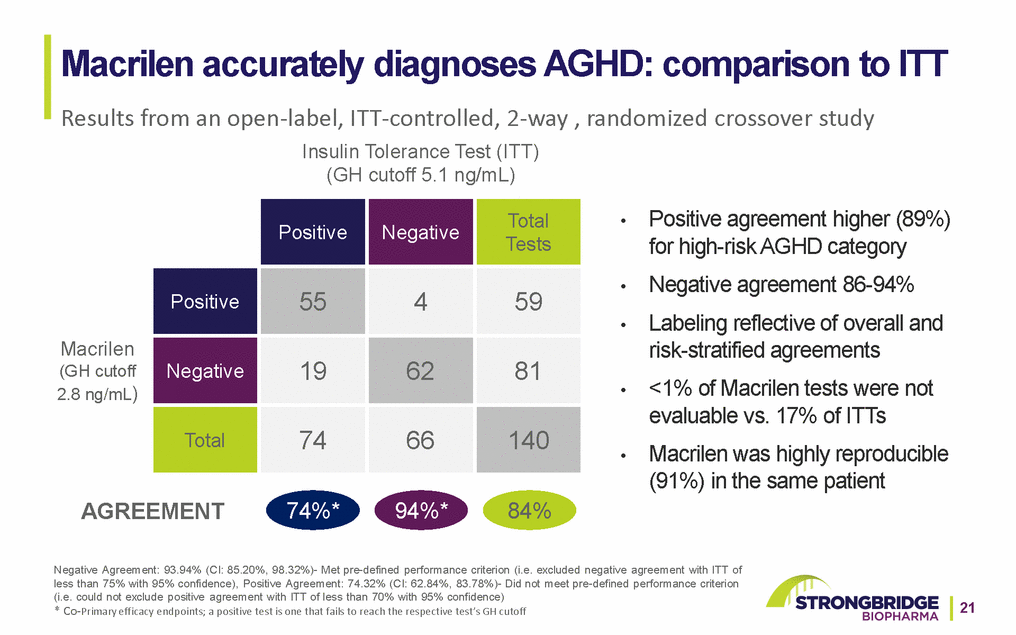
Macrilen Safety Summary •Macrilen well tolerated as compared to ITT (see table) •Most common adverse reactions with Macrilen: •Dysgeusia (4.5%); dizziness, fatigue, and headache (3.9% each); nausea (3.2%) •Most adverse reactions were mild; none severe or led to failure to complete test •QTcF upper 90% confidence limit about 11 ms at doses 2x-4x higher than used for GH stimulation (NO hERG inhibition) •Concomitant QT-prolonging drugs should be avoided •NO need to perform ECG before or after use of Macrilen •DDI potential: Discontinue strong CYP3A4 inducers (avoids false-positive results) 22
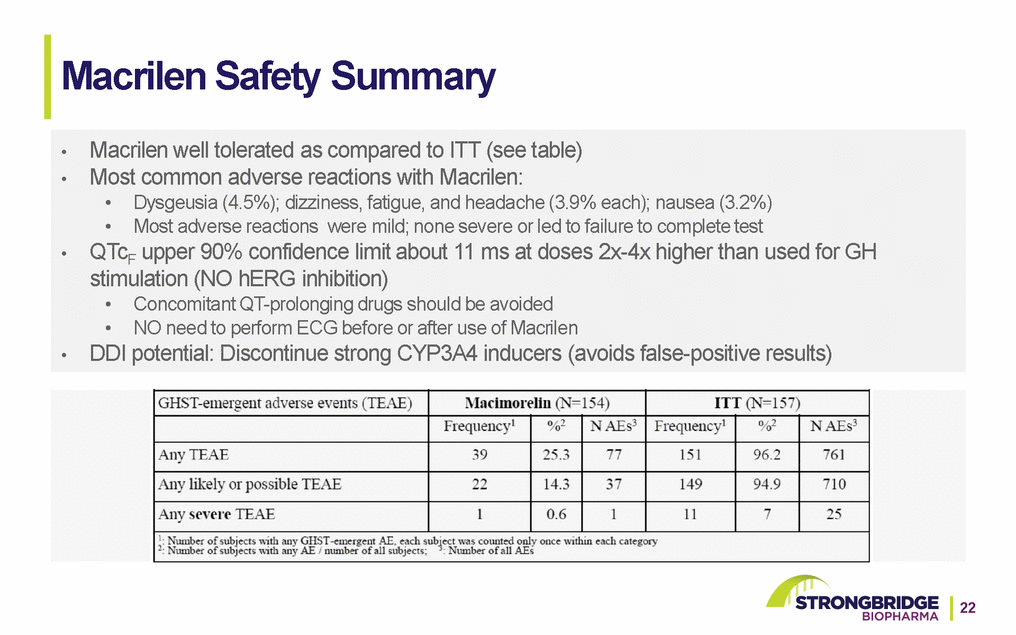
Macrilen Market Opportunity 40-60k Annual Tests* 33% increase in AGHD testing** TBI’s 2.8M per year*** (on-label: no further clinical studies needed) Pre-Macrilen Potential Post-Macrilen Growth Opportunity • Expected increase in testing for AGHD because of Macrilen WAC price per unit of $4,500 • All patients with moderate to severe traumatic brain injury (TBI) require evaluation of pituitary function. *Oppenheimer 2011—manufacturer-sponsored research; Navigant, 2009—manufacturer-sponsored research; Symphony, 2017—manufacturer-sponsored research; TVG Research—manufacturer-sponsored research, 2017; Lumelian, 2017—manufacturer-sponsored research. **Results of quantitative (n=40 endocrinologists) and qualitative (n=5) market research conducted by a 3rd party sponsored by Strongbridge (2017). ***https://www.cdc.gov/traumaticbraininjury/severe.html. 23
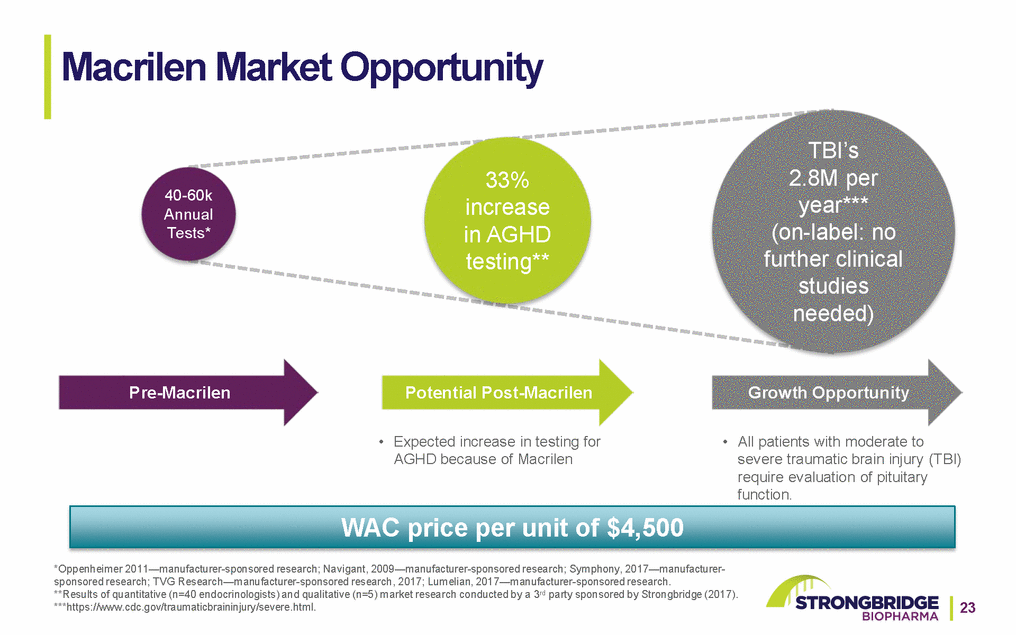
Flexible and Scalable Distribution & Services Model Third-Party Logistics Specialty Distributors Specialty Wholesale Distribution HOPD*, Physician Clinic, VA Specialty Pharmacy Network Macrilen Service Center Reimbursement and Order Triage Specialty Pharmacy Distribution Inbound Service Requests Physician Practice *Hospital Outpatient Department 24
Veldoreotide Extended Release

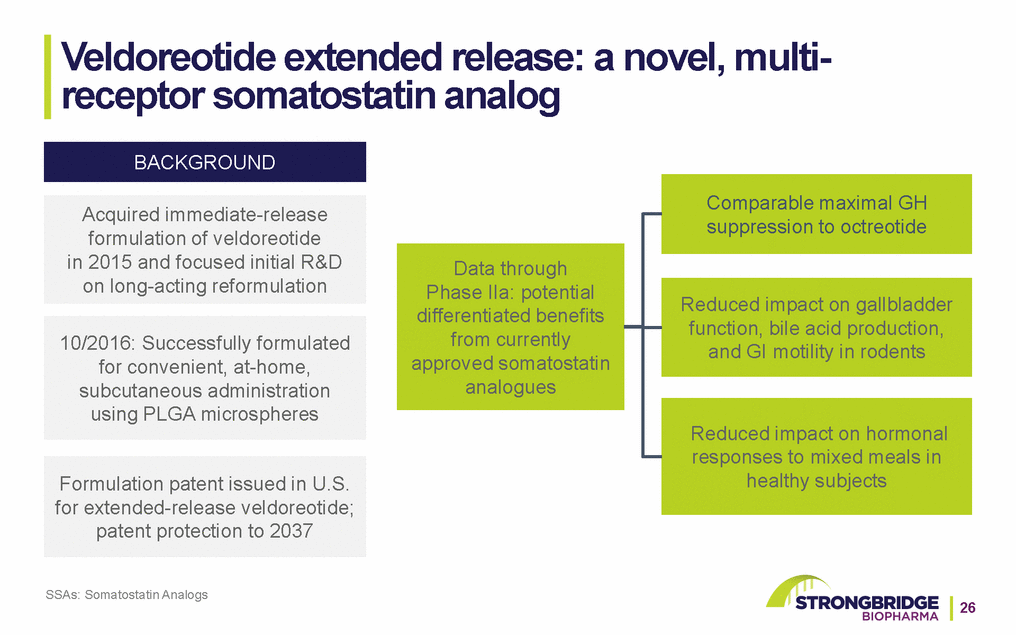
Veldoreotide extended release: a novel, multi-receptor somatostatin analog BACKGROUND Acquired immediate-release formulation of veldoreotide in 2015 and focused initial R&D on long-acting reformulation 10/2016: Successfully formulated for convenient, at-home, subcutaneous administration using PLGA microspheres Formulation patent issued in U.S. for extended-release veldoreotide; patent protection to 2037 SSAs: Somatostatin Analogs Data through Phase IIa: potential differentiated benefits from currently approved somatostatin analogues Comparable maximal GH suppression to octreotide Reduced impact on gallbladder function, bile acid production, and GI motility in rodents Reduced impact on hormonal responses to mixed meals in healthy subjects 26
Keveyis (dichlorphenamide) The first and only FDA-approved therapy for primary periodic paralysis* * FDA-approved treatment for hyperkalemic, hypokalemic, and related variants of primary periodic paralysis

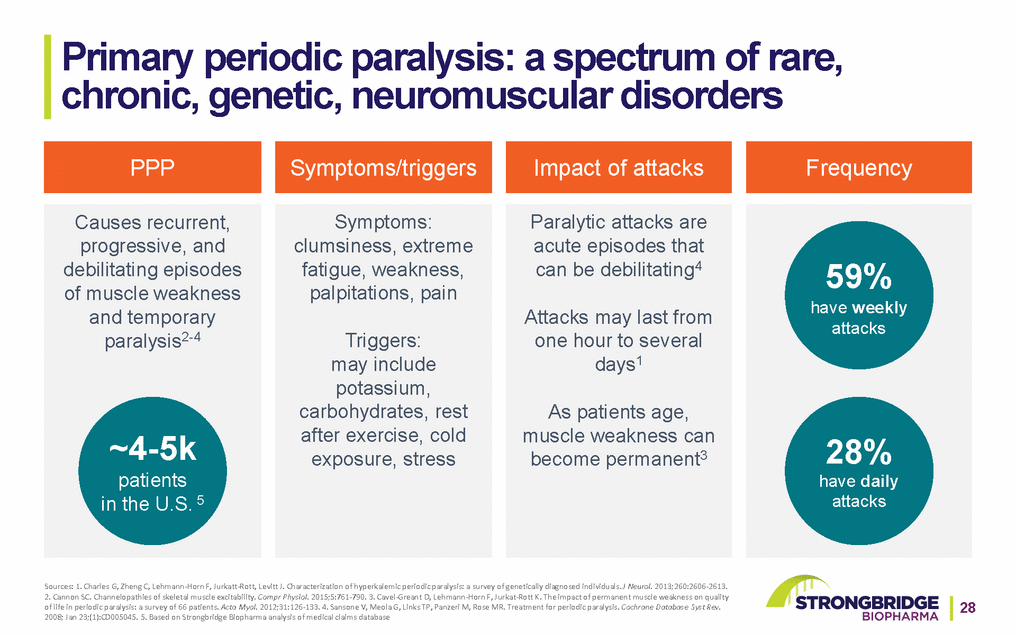
Primary periodic paralysis: a spectrum of rare, chronic, genetic, neuromuscular disorders PPPSymptoms/triggersImpact of attacks Frequency Causes recurrent, progressive, and debilitating episodes of muscle weakness and temporary paralysis2-4 ~4-5k patients in the U.S. 5 Symptoms: clumsiness, extreme fatigue, weakness, palpitations, pain Triggers: may include potassium, carbohydrates, rest after exercise, cold exposure, stress Paralytic attacks are acute episodes that can be debilitating4 Attacks may last from one hour to several days1 As patients age, muscle weakness can become permanent3 59% have weekly attacks 28% have daily attacks Sources: 1. Charles G, Zheng C, Lehmann-Horn F, Jurkatt-Rott, Levitt J. Characterization of hyperkalemic periodic paralysis: a survey of genetically diagnosed individuals. J Neurol. 2013;260:2606-2613. 2. Cannon SC. Channelopathies of skeletal muscle excitability. Compr Physiol. 2015;5:761-790. 3. Cavel-Greant D, Lehmann-Horn F, Jurkat-Rott K. The impact of permanent muscle weakness on quality of life in periodic paralysis: a survey of 66 patients. Acta Myol. 2012;31:126-133. 4. Sansone V, Meola G, Links TP, Panzeri M, Rose MR. Treatment for periodic paralysis. Cochrane Database Syst Rev.28 2008; Jan 23;(1):CD005045. 5. Based on Strongbridge Biopharma analysis of medical claims database
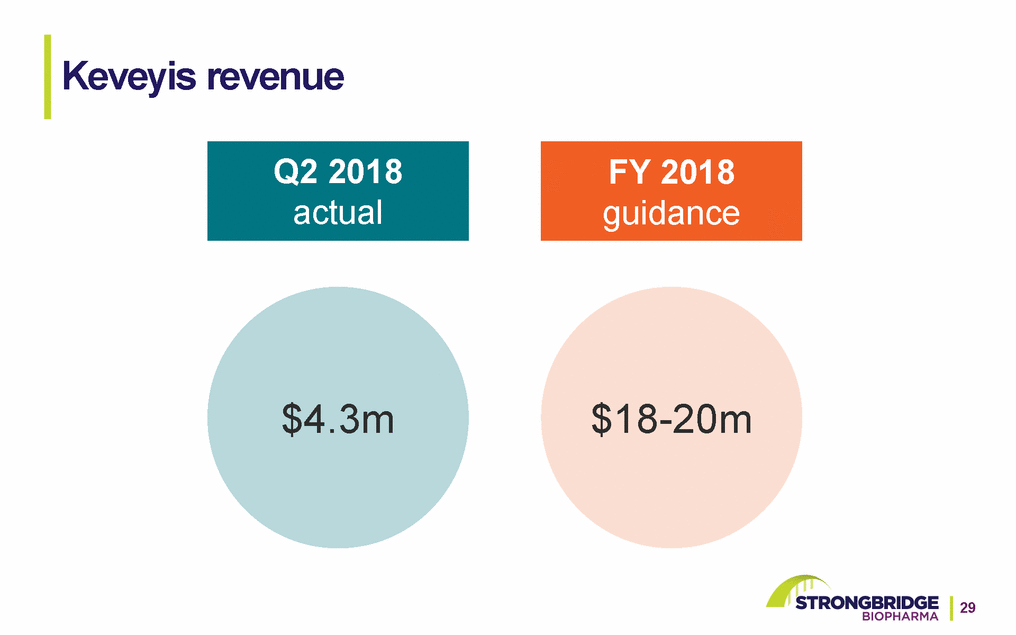
Keveyis revenue Q2 2018 actual FY 2018 guidance $4.3m $18-20m
About Strongbridge

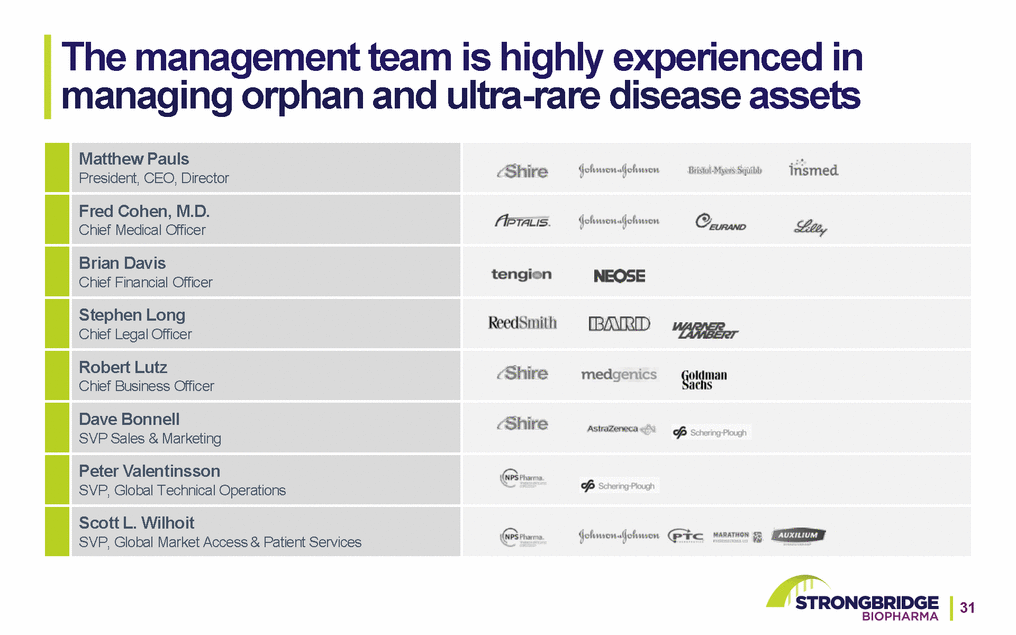
The management team is highly experienced in managing orphan and ultra-rare disease assets
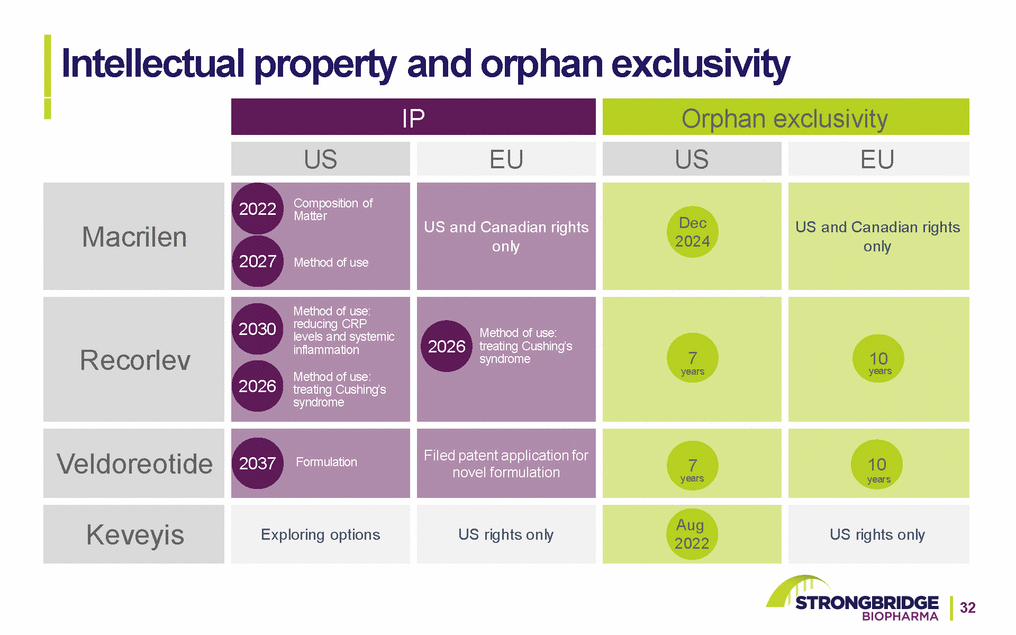
Intellectual property and orphan exclusivity
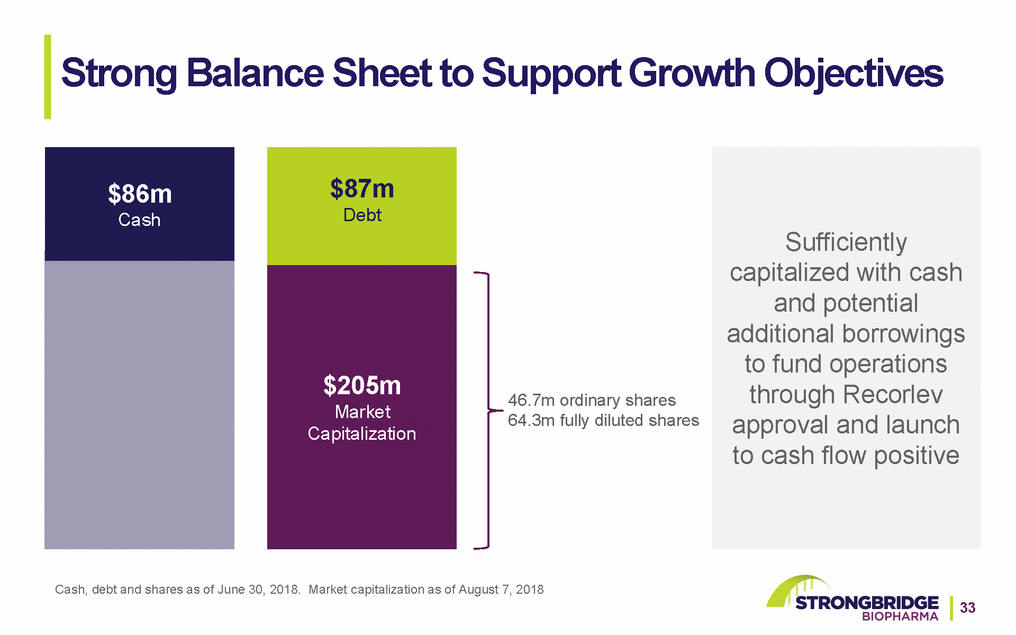
Strong Balance Sheet to Support Growth Objectives $86m Cash $87m Debt $205m Market Capitalization 46.7m ordinary shares 64.3m fully diluted shares Sufficiently capitalized with cash and potential additional borrowings to fund operations through Recorlev approval and launch to cash flow positive Cash, debt and shares as of June 30, 2018. Market capitalization as of August 7, 2018
Strongbridge Biopharma plc


