Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Strongbridge Biopharma plc | sbbp-20191231ex3219a542d.htm |
| EX-31.1 - EX-31.1 - Strongbridge Biopharma plc | sbbp-20191231ex31160edd2.htm |
| EX-23.1 - EX-23.1 - Strongbridge Biopharma plc | sbbp-20191231ex231062bbe.htm |
| EX-21.1 - EX-21.1 - Strongbridge Biopharma plc | sbbp-20191231ex211488331.htm |
| EX-10.26 - EX-10.26 - Strongbridge Biopharma plc | sbbp-20191231ex10264568a.htm |
| EX-10.25 - EX-10.25 - Strongbridge Biopharma plc | sbbp-20191231ex10255df5a.htm |
| EX-10.16 - EX-10.16 - Strongbridge Biopharma plc | sbbp-20191231ex101628adb.htm |
| EX-10.8 - EX-10.8 - Strongbridge Biopharma plc | sbbp-20191231ex10841ffff.htm |
| EX-4.1 - EX-4.1 - Strongbridge Biopharma plc | sbbp-20191231ex4119e9447.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
OR
For the transition period from to
Commission file number: 001-37569
Strongbridge Biopharma plc
(Exact name of Registrant as specified in its charter)
|
Ireland |
98-1275166 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
900 Northbrook Drive
Suite 200
Trevose, PA 19053
+1 610‑254‑9200
(Address of principal executive offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Name of each exchange on which registered |
|
Ordinary shares, par value $0.01 per share |
|
The NASDAQ Global Select Market |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
|
None |
|
(Title of Class) |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer ☐ |
Accelerated Filer ☒ |
||
|
Non-Accelerated Filer ☐ |
Smaller reporting company ☒ |
||
|
|
Emerging growth company ☒ |
||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). ☐ Yes ☒ No
The aggregate market value of the voting and non-voting stock held by non-affiliates of the Registrant, as of June 30, 2019, the last business day of the Registrant’s most recently completed second fiscal quarter, was approximately $159,280,967. Solely for purposes of this disclosure, ordinary shares held by executive officers and directors of the Registrant as of such date have been excluded because such persons may be deemed to be affiliates. This determination of executive officers and directors as affiliates is not necessarily a conclusive determination for any other purposes.
54,247,285 ordinary shares were issued and outstanding as of February 17, 2020.
DOCUMENTS INCORPORATED BY REFERENCE: NONE
Strongbridge Biopharma plc
2
CAUTIONARY STATEMENT REGARDING FORWARD‑LOOKING STATEMENTS AND MARKET DATA
This Annual Report on Form 10-K (“Annual Report”) contains forward‑looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Annual Report, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and results of clinical trials, size of market or patient population, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products, are forward‑looking statements. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward‑looking statements. The words “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “might,” “objective,” “plan,” “potential,” “predict,” “project,” “positioned,” “seek,” “should,” “target,” “will,” “would,” or the negative of these terms or other similar expressions are intended to identify forward‑looking statements, although not all forward‑looking statements contain these identifying words. These forward‑looking statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate and management’s beliefs and assumptions, are not guarantees of future results, performance or developments and involve known and unknown risks, uncertainties and other factors that may cause our actual results or developments to differ materially from the expectations contained in the forward-looking statements. Such risks and uncertainties include those described throughout this Annual Report and particularly in “Risk Factors” in Part I, Item 1A of this Annual Report. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward‑looking statements. Readers are urged to carefully review and consider the various disclosures made in this Annual Report and in other documents we file from time to time with the Securities and Exchange Commission (the “SEC”) that disclose risks and uncertainties that may affect our business. The forward‑looking statements included in this Annual Report do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make. In addition, the forward-looking statements in this Annual Report are made as of the date of this filing, and we do not undertake, and expressly disclaims any duty, to update such statements, whether as a result of new information, new developments or otherwise, except to the extent that disclosure may be required by law.
Solely for convenience, the trademarks and trade names in this Annual Report are referred to without the ® and ™ symbols, but absence of such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. The trademarks, trade names and service marks appearing in this Annual Report are the property of their respective owners.
Unless the context requires otherwise, references in this Annual Report to “Strongbridge,” “we,” “us” and “our” refer to Strongbridge Biopharma plc.
As used in this Annual Report, the term “levoketoconazole” refers to our investigative drug product candidate being developed for the potential treatment of endogenous Cushing’s syndrome. The term “Recorlev” refers to the proposed branded pharmaceutical name used by the Company for levoketoconazole, if approved, and is used interchangeably in this Annual Report with the term levoketoconazole, unless the context requires otherwise. The safety and efficacy of Recorlev (levoketoconazole) for the treatment of endogenous Cushing’s syndrome have not been established.
3
Overview
We are a global, commercial-stage biopharmaceutical company focused on the development and commercialization of therapies for rare diseases with significant unmet needs.
Our first commercial product is Keveyis (dichlorphenamide), the first and only treatment approved by the U.S. Food and Drug Administration (the “FDA”) for hyperkalemic, hypokalemic, and related variants of primary periodic paralysis (“PPP”), a group of rare hereditary disorders that cause episodes of muscle weakness or paralysis.
We have two clinical-stage product candidates for rare endocrine diseases, Recorlev and veldoreotide. Recorlev (levoketoconazole) is a cortisol synthesis inhibitor currently being studied for the treatment of endogenous Cushing's syndrome. Veldoreotide is a next-generation somatostatin analog being investigated for potential applications in conditions amenable to somatostatin receptor activation. Both levoketoconazole and veldoreotide have received orphan designation from the FDA and the European Medicines Agency (“EMA”).
Our Franchises
Rare Endocrine Franchise
|
· |
Recorlev (levoketoconazole), a cortisol synthesis inhibitor, is in Phase 3 clinical development for the treatment of endogenous Cushing’s syndrome. Endogenous Cushing’s syndrome is a rare endocrine disorder characterized by sustained elevated cortisol levels that most commonly result from a benign tumor of the pituitary gland. We believe that levoketoconazole, which is the isolated, “left-handed” enantiomer of ketoconazole, has the potential to become the new standard of care for the drug therapy of endogenous Cushing’s syndrome. In August 2018, we announced top-line results from our multinational, pivotal Phase 3 SONICS trial evaluating Recorlev for treatment of endogenous Cushing’s syndrome. The open-label, single-arm SONICS trial achieved statistical significance of its pre-specified primary endpoint, with 30 percent of patients achieving normalization of mean urinary free cortisol (“UFC”) following six months of maintenance treatment with Recorlev without a dose increase during maintenance therapy (one-sided p=0.0154, 2-sided p = 0.038 vs the null hypothesis of less than or equal to 20%). Sensitivity analyses as well as secondary and exploratory endpoints of UFC response were supportive of the primary endpoint. |
LOGICS is our second Phase 3 clinical trial of Recorlev for the treatment of endogenous Cushing's syndrome. The LOGICS trial is intended to supplement the long-term efficacy and safety data from the SONICS trial. LOGICS includes a double-blind, placebo-controlled, randomized-withdrawal phase of approximately 8 weeks duration targeting enrollment of approximately 46-54 patients in the randomized withdrawal phase of the trial following an open-label titration and maintenance phase of approximately 14 weeks. Top-line data following the randomized-withdrawal phase of LOGICS is expected at the end of second quarter or during the third quarter 2020. The addition of a concurrent control group in LOGICS is an attempt to address the FDA’s request for such a control group that was absent in our SONICS trial.
We intend to file for marketing authorizations in the United States and potentially elsewhere. Following consultations with the FDA, we have determined that the 505(b)(2) approval pathway, which permits a New Drug Application (“NDA”) applicant to rely on data from studies that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference, is the appropriate pathway for a Recorlev NDA. Because NDA approval can rely in part on data already accepted by the FDA or otherwise publicly available, an abbreviated and reduced development program may be possible. We intend to rely on published literature and the FDA’s prior findings concerning the safety and/or effectiveness of ketoconazole in our NDA for Recorlev. A similar marketing authorization pathway is available in most of the rest of the world, and we believe that the studies supporting U.S. approval will likewise support approvals to market Recorlev elsewhere, including in the European Union.
4
|
· |
Veldoreotide modified-release, a novel multi-receptor targeted somatostatin analog (“SSA”) that was previously in Phase 2 development as an immediate release formulation. Based on the differentiated activation pattern of somatostatin receptor subtypes (“SSTs”) and the preclinical and clinical profile of immediate-release veldoreotide, we believe that modified-release veldoreotide is a next-generation somatostatin analog with potential applications in conditions amenable to somatostatin receptor activation. Veldoreotide has been granted orphan drug designation by the FDA and the EMA for treatment of acromegaly. The formulation for veldoreotide modified-release is based upon PLGA microspheres. PLGA is a well-known polymer, which has been widely applied in modified-release formulations due to its biocompatibility, biodegradability, and favorable release kinetics. We have initiated nonclinical studies and are planning clinical studies that seek to determine additional differentiating features of veldoreotide in both endocrine and non-endocrine conditions. |
Rare Neuromuscular Franchise
|
· |
Keveyis (dichlorphenamide), an oral carbonic anhydrase inhibitor and the only therapy approved in the United States to treat hyperkalemic, hypokalemic and related variants of PPP. PPP is a rare genetic, neuromuscular disorder that can cause extreme muscle weakness and/or paralysis; some forms are also commonly associated with myotonia or muscle stiffness. It often interferes with daily activities and, as patients get older, it can lead to permanent muscle weakness. The two most common forms of this disorder are “hyperkalemic” and “hypokalemic” periodic paralysis. Keveyis was approved by the FDA in August 2015 and has orphan drug exclusivity status in the United States through August 7, 2022. |
Product Sales
Our product sales in 2019 resulted from sales of Keveyis. We operate in one operating reporting segment. We recognize net product sales at the time our product is received by the single specialty pharmacy. Keveyis is subsequently sold to patients, who are covered by payors that may provide for government-mandated or privately negotiated rebates with respect to the purchase of Keveyis.
Our Strategy
Our goal is to transform the lives of patients by building a leading franchise‑based, commercially-oriented biopharmaceutical company addressing rare diseases with significant unmet medical needs. We are focused on developing, in‑licensing, acquiring and eventually commercializing products and product candidates that target rare diseases across various therapeutic areas.
To achieve our goal, we are pursuing the following strategies:
|
· |
Focus on rare diseases, including development of Recorlev. We are selling or developing treatments for rare diseases, initially PPP and endogenous Cushing’s syndrome. Rare diseases typically have a high unmet need for innovative treatment options and may have reduced competitive pressures in some cases. Drug development for the treatment of rare diseases often requires smaller clinical trials than for common diseases. Product candidates focused on rare diseases also often qualify for orphan drug designation, which in the United States provides for seven years of market exclusivity and in the European Union provides for 10 years of market exclusivity after regulatory approval to market has been granted. |
|
· |
Independently commercialize Keveyis and other products in the United States and for other geographies pursue either independent commercialization or other arrangements with partners/distributors. We launched Keveyis in the United States in April 2017 and continue to commercialize it. Our other rare disease product candidates, if approved, may be marketed in the United States, the European Union, and, selectively, in other key global markets. Given the relatively small prescriber bases for Keveyis and our two product candidates, we believe we can use a relatively small, focused field-based team to effectively promote and support our products and patients. We have established sales, marketing, market access and patient service capabilities in the United States to market Keveyis. We believe that many of the capabilities |
5
involved in our commercialization activities related to Keveyis will provide synergies to our commercialization of Recorlev, if approved. We believe that our ability to execute on this strategy is enhanced by the significant prior commercial experience of key members of our management team. |
|
· |
Expand our portfolio through a disciplined in‑licensing and acquisition strategy. We plan to source new product candidates by in‑licensing or acquiring them. Our management team seeks to mitigate the potential risks of this strategy by adhering to our disciplined criteria of focusing on in‑licensing or acquisition of products that are already commercially available or that have clinical data that we believe suggest a relatively high probability of success for development and an attractive potential return on investment. As a result of our management team’s experience in sourcing, selecting, in‑licensing, and acquiring products and product candidates, we were successful in acquiring the U.S. rights to Keveyis and the U.S. and Canadian rights to Macrilen, as well as augmenting our rare endocrine franchise by adding veldoreotide to our product pipeline. |
|
· |
Utilize a franchise model built on rare disease therapeutic areas. We will evaluate options to grow our company by in‑licensing and acquiring products and product candidates that target rare diseases in therapeutically aligned franchises with significant commercial opportunity. We believe that complementary products and product candidates will allow us to significantly leverage our expertise as well as our development and commercial infrastructure. |
|
· |
Expand indications of products and product candidates within our franchises. In addition to identifying products and product candidates that can form the basis of new rare disease franchises, we also intend to seek opportunities to develop our potential products and product candidates for additional indications within their respective therapeutic franchises. We believe that this approach will enable us to maximize our commercial potential by further leveraging our existing resources and expertise. |
Our Product Candidate Pipeline
The following table illustrates our product candidates by stage:
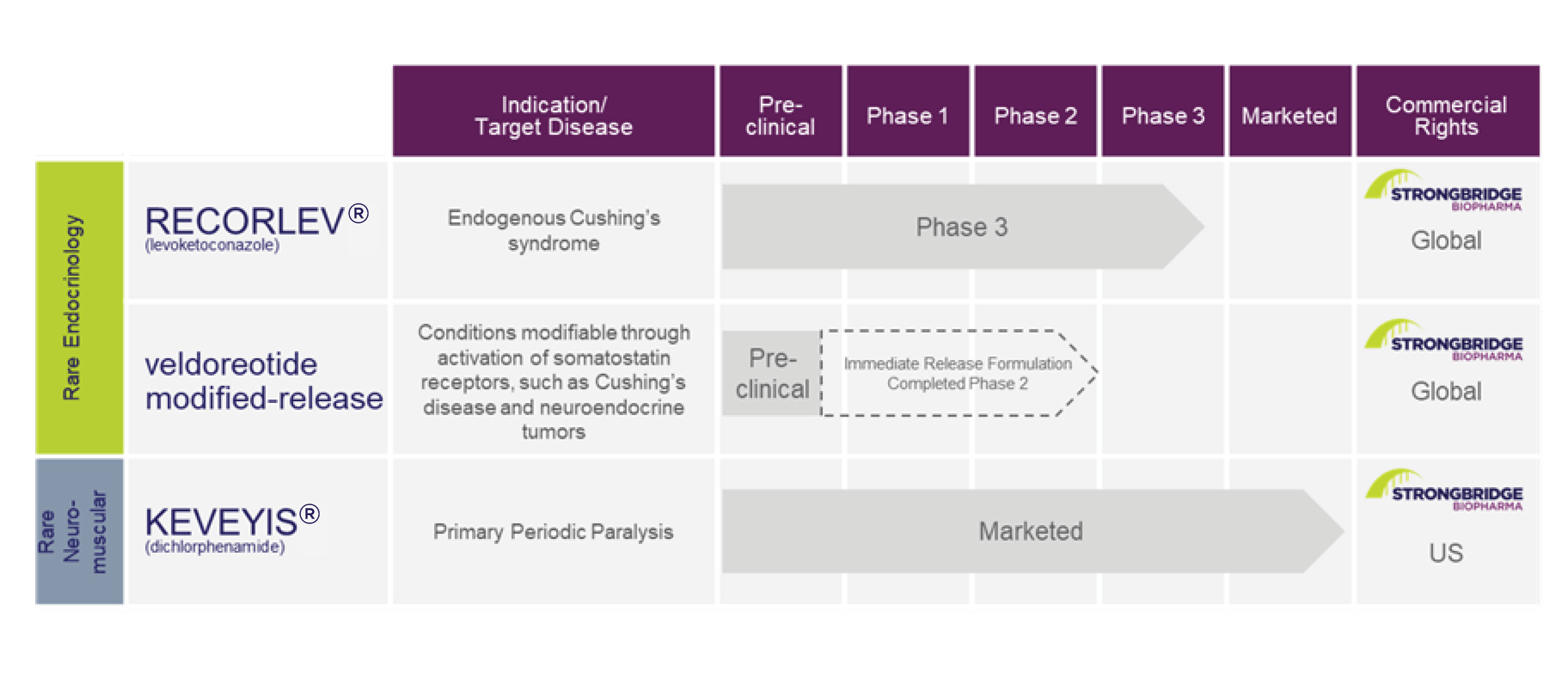
6
Our Rare Endocrine Franchise
Recorlev
Overview
Recorlev (levoketoconazole) is a cortisol synthesis inhibitor that we are developing for the treatment of endogenous Cushing’s syndrome. The active pharmaceutical ingredient in Recorlev, levoketoconazole, exerts its primary therapeutic effect by blocking the synthesis of cortisol in the adrenal glands, leading to the reduction and, ideally, the normalization of blood cortisol. Recorlev has been granted orphan drug designation by the FDA and the EMA and is being developed using a dose regimen of twice daily oral administration.
Ketoconazole, although not approved for such use in the United States, is the most frequently prescribed drug therapy for endogenous Cushing’s syndrome. It is used to reduce blood cortisol and treat comorbidities associated with Cushing’s syndrome. Molecules of ketoconazole form as mirror images, referred to as enantiomers. Manufactured ketoconazole consists of two cis enantiomers, dextroketoconazole and levoketoconazole, that are found in equal amounts, and is therefore referred to as a racemate. Levoketoconazole is a pure form of one of the two enantiomers of ketoconazole. Single‑enantiomer drugs, like Recorlev, may offer safety and efficacy advantages over racemates because one of the enantiomers in a racemate can have safety issues or be less effective in the treatment of the disorder or disease. The more therapeutically favorable enantiomer may be known as the eutomer. We believe that levoketoconazole is the eutomer of ketoconazole with respect to cortisol synthesis inhibition and treatment of endogenous Cushing’s syndrome.
Levoketoconazole inhibits the cortisol synthesis pathway at several points. Based on recent top-line interim results from our SONICS trial, we believe that Recorlev can have a beneficial impact on hypercortisolism, the hallmark of endogenous Cushing’s syndrome, as well as benefits related to several comorbidities of endogenous Cushing’s syndrome, including those associated with cardiovascular disease risk, such as diabetes, weight gain and elevation in LDL-cholesterol. In addition, we believe that Recorlev may offer an advantageous safety profile in a representative population with endogenous Cushing’s syndrome. We believe that Recorlev has the potential to become a new standard of care for the drug therapy of endogenous Cushing’s syndrome because it may provide a favorable efficacy, safety and tolerability profile compared to current drug therapies, including ketoconazole. Based on results of the SONICS trial, Recorlev effectively reduces UFC, in contrast to Korlym, and demonstrates anti‑hyperglycemic effects, in contrast to Signifor and Signifor LAR. In addition, we believe Recorlev may have an improved safety profile compared with that of ketoconazole.
Overview of Endogenous Cushing’s Syndrome
There are two variants of Cushing’s syndrome: exogenous, which is caused by factors outside the body (e.g., corticosteroid or cortisol-like medications) and endogenous, which is caused by factors within the body. The signs and symptoms may be the same in both forms. The much more common form is exogenous Cushing’s syndrome, which is often found in people taking cortisol‑like medications for long periods of time or for shorter periods of time using more potent forms. Cortisol‑like medications are often used to treat inflammatory disorders such as asthma and rheumatoid arthritis. Unlike endogenous Cushing’s syndrome, exogenous Cushing’s syndrome may be alleviated by withdrawing the inciting medication.
Endogenous Cushing’s syndrome is a rare endocrine disorder characterized by sustained elevated blood cortisol. Cortisol is a hormone produced in the adrenal gland and is naturally secreted as an end-product of the activity of the hypothalamic‑pituitary‑adrenal axis. Corticotropin‑releasing‑hormone (“CRH”) is secreted from the hypothalamus and stimulates the secretion and release of adrenocorticotropin (“ACTH”) from the pituitary gland, which in turn stimulates cortisol (and other hormone) secretion from the adrenal gland. Cortisol itself exerts negative feedback control on both CRH in the hypothalamus and ACTH in the pituitary gland, thereby reducing CRH and ACTH secretion, keeping cortisol levels in a normal range.
7
The most common form of endogenous Cushing’s syndrome is called Cushing’s disease, which is typically caused by a benign pituitary tumor that secretes ACTH autonomously. Cushing’s disease represents approximately 70% to 80% of patients with endogenous Cushing’s syndrome. Other causes of endogenous ACTH-dependent Cushing’s syndrome include extrapituitary tumors producing ACTH, known as ectopic ACTH, or less often CRH (ectopic CRH). The source of ectopic ACTH/CRH secretion is most often small‑cell carcinoma of the lung or bronchial carcinoid tumors, but neuroendocrine tumors found in many different organs can also be sources. In a smaller number of cases, approximately 20%, endogenous Cushing’s syndrome is ACTH‑independent, meaning that it does not arise through tumor secretion of ACTH but rather results from excess secretion of cortisol itself in the adrenal gland by adrenocortical tumors, either benign or malignant, or by non‑malignant enlargement of the adrenal glands called hyperplasia.
In patients with endogenous Cushing’s syndrome, the normal feedback mechanisms of the hypothalamic‑pituitary‑adrenal axis are disrupted as a result of a tumor autonomously secreting ACTH, CRH or cortisol. This causes chronic exposure to high circulating cortisol levels that give rise to the clinical state of Cushing’s syndrome. The most common signs and symptoms of the syndrome include: weight gain, especially in the upper body with a rounded face (“moon face”) and extra fat on the upper back and above the collarbones; high blood sugar or diabetes mellitus; high blood pressure or hypertension; thin bones or osteoporosis; muscle loss or sarcopenia; thin, fragile skin that bruises easily; purple‑red stretch marks called striae, usually over the abdomen and under the arms; depression and difficulty thinking clearly; too much facial hair, or hirsutism, usually noticed only in women; irregular or absent menstrual periods and infertility; reduced sex drive or libido; and in children, poor height growth.
An estimated 25,000 patients in the United States and 40,000 patients in Europe are diagnosed with endogenous Cushing’s syndrome. When first diagnosed, patients are most commonly adults aged 20 to 50 and five times more often women than men. However, endogenous Cushing’s syndrome is believed to be underdiagnosed due to lack of disease recognition, resulting in a delay in diagnosis of six years on average. Endogenous Cushing’s syndrome patients are believed to have a mortality risk two to three times that of the age-and-gender-matched general population, with cardiovascular disease, venous thrombosis and infections being the primary causes of death.
Current Treatment Landscape and Limitations of Current Treatment Options
Treatment of endogenous Cushing’s syndrome varies depending on the cause of the disease. For patients with Cushing’s disease, initial treatment is almost always the attempted surgical removal of the pituitary tumor. In anticipation of surgery and when surgery is not effective or not feasible, drug or radiation therapy, or both, is used to suppress excessive cortisol production and the accompanying clinical symptoms.
A typical approach to drug therapy is to inhibit cortisol synthesis through the oral administration of an inhibitor of enzymes that regulate adrenal cortisol synthesis. Ketoconazole acts in this way and is the most widely used drug therapy for endogenous Cushing’s syndrome in the United States. Although approved in the European Union for this indication, ketoconazole is not approved for this indication by the FDA and is therefore prescribed “off-label”. The percentage of endogenous Cushing’s syndrome patients treated with ketoconazole monotherapy who achieve normalized levels of cortisol, assessed by measuring UFC has been reported from retrospective, uncontrolled studies, with varying definitions of normalization, to be between 33% and 100%. Data from a retrospective study of 200 patients in 14 French centers solely treated with ketoconazole for endogenous Cushing’s syndrome between 1995 and 2012 suggested that ketoconazole controlled cortisol in approximately 50% of patients and improved clinical symptoms. Also, beneficial effects of oral ketoconazole on clinical symptoms and signs that drive the morbidity and mortality of endogenous Cushing’s syndrome have been reported including reduction in high blood pressure, improvement of diabetes, and normalization of hypokalemia, or low potassium blood levels. However, some patients treated with ketoconazole experience tolerability issues and, in some cases, liver injury (also known as hepatotoxicity).
As a result of the hepatotoxicity risk, the FDA has issued a boxed warning to prescribers in the labeling describing the use of ketoconazole to treat fungal infections, the only approved indication for ketoconazole in the United States. The FDA has also cautioned that safety and effectiveness have not been established for off-label use of ketoconazole in Cushing’s syndrome. Although elevations in liver enzymes associated with ketoconazole are generally mild to moderate and reversible upon cessation of drug, in rare cases, severe hepatotoxicity may occur (estimated as one in every 10,000 to 15,000 patients). In extremely rare cases, ketoconazole-related liver injury may be irreversible and
8
result in death or require liver transplantation. In July 2013, the Committee for Medicinal Products for Human Use (“CHMP”) recommended that ketoconazole be withdrawn for use as an antifungal agent in the European Union. The EMA adopted the CHMP recommendation in August 2013, and the recommendation was subsequently confirmed by the European Commission. In September 2014, HRA Pharma received a recommendation of approval from the EMA for ketoconazole for the treatment of endogenous Cushing’s syndrome, based on the well‑established use of ketoconazole in medical practice as well as documentation from retrospective studies in the literature.
Metyrapone is another cortisol synthesis inhibitor that blocks cortisol in a different way than ketoconazole or Recorlev. It is not approved for treatment of Cushing’s syndrome in the US but is used off-label. Metyrapone is approved for use in the United Kingdom and certain other countries as a therapeutic drug for CS. Elsewhere, including in the US, it is approved as a diagnostic agent in Cushing’s disease. A drug that works through a similar pathway as metyrapone, called Isturisa (osilodrostat), was granted a marketing authorization in the European Union on January 15, 2020. Recordati, the owner of the global rights to osilodrostat, has filed an NDA for approval of osilodrostat with the FDA in the U.S. Etomidate is an intravenously administered sedative that potently inhibits 11β-hydroxylase, like metyrapone and osilodrostat, and is highly effective to reduce cortisol, but its use is typically limited to the inpatient setting.
An alternative medical approach to treating Cushing’s syndrome targets pituitary tumors that produce ACTH (i.e., in Cushing’s disease). Among Cushing’s disease patients, the dopamine agonist cabergoline, which is not approved for use to treat Cushing’s disease in the United States, has been shown to achieve normalization of UFC levels, gold-standard evidence of disease control, in about 30% of patients. The SSA (somatosatin analog) pasireotide, which is marketed as Signifor and Signifor LAR for the treatment of Cushing’s disease in the United States, has shown normalization of UFC levels with stable dosing of the immediate-release formulation in 15% of patients at a dosage of 600 µg twice-daily and in 26% of patients at a dosage of 900 µg twice-daily over a 6-month period. Certain SSAs, including Signifor, are known to have undesirable side effects on glucose metabolism. Forty percent of patients with Cushing’s disease treated with Signifor in its Phase 3 clinical trial reported the occurrence of hyperglycemia‑related adverse events, and in the cohort receiving Signifor 900 µg twice-daily, glycated hemoglobin (“HbA1c”) increased from 5.8% at baseline to 7.3% at Month 6.
Another alternative drug therapy, Korlym, or mifepristone, works by inhibiting the action of cortisol at the cortisol-receptor level but does not lower blood cortisol levels, which actually tend to increase during therapy. As a result of this mechanism of action, it is not possible to monitor response (i.e., effectiveness and safety) to Korlym by measuring UFC or cortisol levels (from blood or saliva), which are the standard ways clinicians monitor disease progression and response to treatment. As a result, Korlym is usually titrated and monitored through use of clinical signs and symptoms improvements (e.g., blood sugar reductions). Korlym has been approved in the United States to control hyperglycemia secondary to hypercortisolism in patients with endogenous Cushing’s syndrome who also have diabetes mellitus. About one-third of patients with endogenous Cushing syndrome have diabetes. Korlym is contraindicated in pregnant women and in women with a history of unexplained vaginal bleeding, as its side effects include termination of pregnancy, endometrial thickening and vaginal bleeding. It is also frequently associated with hypokalemia.
Mitotane is an adrenolytic agent (i.e. it destroys the adrenal gland at higher doses) that inhibits steroidogenesis non-selectively at low doses, mainly at 20,22-desmolase (cholesterol side-chain cleavage). It seems to be used primarily in adrenocortical cancer, where it had an FDA indication. There are no prospective clinical trials describing the use of mitotane in non-malignant endogenous CS, and it is not approved for that use.
We believe that the efficacy and usage limitations and safety concerns associated with currently available drug therapies for endogenous Cushing’s syndrome are an important reason why a significant unmet medical need exists among endogenous Cushing’s syndrome patients with persistent or recurrent disease post-surgery. In a survey we commissioned in 2014 of 89 U.S. physicians treating patients with Cushing’s syndrome, when asked, “Of your patients on medication to manage cortisol levels, what percentage are well controlled?”, the physicians estimated that only approximately 37% of such patients were well controlled. A recent multicenter study of 230 Cushing’s disease patients followed for up to 27.5 years and treated with any modality (i.e., surgery, radiation or drugs) found that only 49% had documented biochemical control. We believe that our potential addressable market for Recorlev includes diagnosed
9
endogenous Cushing’s syndrome patients that at any time are eligible for drug therapy, including patients anticipating surgery, for whom surgery or radiation is not feasible, is contraindicated or has been unsuccessful.
Levoketoconazole Mechanism of Action and Preclinical Results
Recorlev, like ketoconazole, is a cortisol synthesis inhibitor that inhibits the cortisol synthesis pathway at multiple points. The following graphic illustrates the cortisol synthesis pathway:
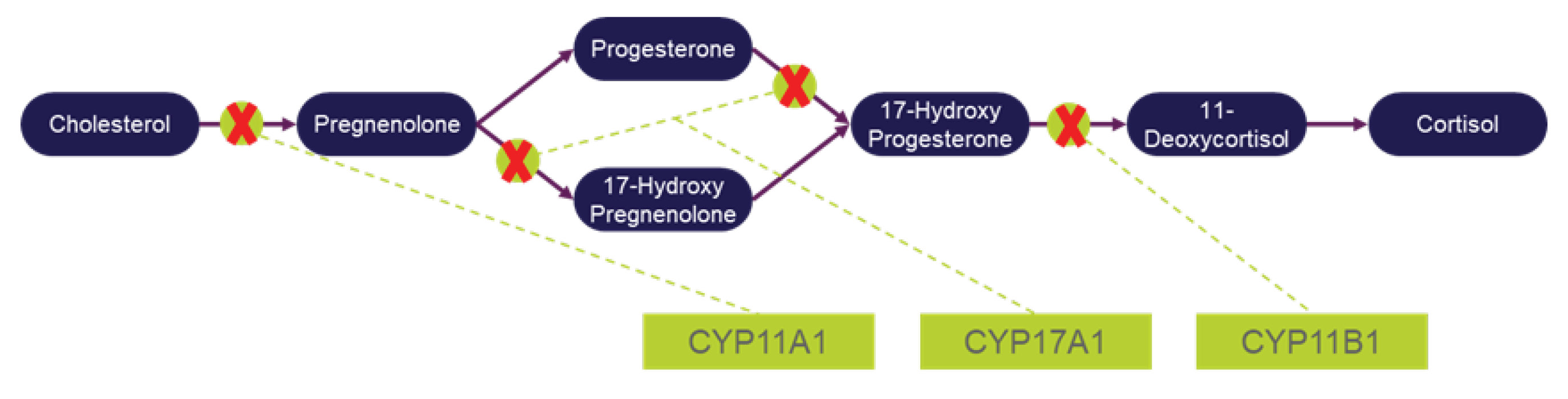
Preclinical and pharmacokinetic data provide evidence that the efficacy of levoketoconazole to treat hypercortisolism is due primarily to the ability of levoketoconazole to inhibit adrenal cortisol synthesis. This conclusion is inferred from evidence that the antipode of levoketoconazole (dextroketoconazole) is a far less potent inhibitor of cortisol synthesis in vitro. Furthermore, the relatively greater potency of levoketoconazole to inhibit cortisol (and androgen) synthesis implies that, all else being equal, a lower dose of levoketoconazole could result in the same or better efficacy as a higher dose of ketoconazole, thus potentially reducing the risk of toxicity, such as liver toxicity, if such toxicity is contributed approximately equally by each enantiomer of ketoconazole. These conclusions are based on the following:
|
· |
In in vitro studies, Recorlev was found to have markedly higher potency than its mirror‑image enantiomer, dextroketoconazole, in inhibiting the key human enzymes that regulate cortisol synthesis (CYP11B1 and CYP17A1). The inhibitory potency in vitro of levoketoconazole on these enzymes is approximately twice that of ketoconazole, precisely the ratio that would be expected if levoketoconazole accounted for essentially all of the in vitro potency of ketoconazole. Combined with the pharmacokinetic profile of the enantiomers (below), these data suggest that essentially all in vivo cortisol inhibition observed following administration of ketoconazole can be ascribed to the single levoketoconazole enantiomer (the active ingredient of Recorlev). |
|
· |
The pharmacokinetics of the enantiomers also suggest a potentially larger therapeutic index of levoketoconazole relative to ketoconazole. The two enantiomers found within ketoconazole are present in equal amounts, but in a Phase 1 clinical trial in healthy subjects, it was observed that administration of ketoconazole resulted in integrated blood concentrations (i.e., exposure) of the single enantiomer, levoketoconazole that exceeded those of the other enantiomer, dextroketoconazole, by approximately three times. This observation suggests either that (i) dextroketoconazole is extracted by the liver to a greater extent than the other single enantiomer, levoketoconazole, and may therefore contribute more than levoketoconazole to the observed liver toxicity of ketoconazole or (ii) levoketoconazole is preferentially absorbed. Even if the liver-clearance of the enantiomers is not different, the higher blood levels of levoketoconazole upon dosing with racemate suggest that a lower amount of drug administration of the single levoketoconazole enantiomer may result in equivalent efficacy to a higher amount of drug administration of ketoconazole, assuming that levoketoconazole accounts for essentially all of the in vivo cortisol inhibition of the racemate (ketoconazole—see above). |
|
· |
Compared with ketoconazole, it was observed in in vitro studies that levoketoconazole is less potent than the dextroketoconazole in inhibiting the activity of CYP7A. CYP7A is the first and rate‑limiting enzyme for production of bile acids in the liver. While a role of CYP7A in liver injury is not established, this |
10
finding suggests a possible differential effect of the ketoconazole enantiomers on metabolic and detoxifying enzymes in the liver contributing to the reduced hepatotoxicity potential of levoketoconazole. |
Clinical Trials of Recorlev in Diabetes
Previously, Recorlev (then named DIO-902) was studied clinically for the treatment of type 2 diabetes. DiObex, our licensee from 2004 to 2008, initiated three clinical trials to investigate the use of levoketoconazole for treatment of type 2 diabetes and two clinical drug-drug interaction studies in healthy volunteers. Results from these studies established the clinical pharmacology profile of levoketoconazole, contributed to an understanding of its potential efficacy in type 2 diabetes and established preliminary clinical safety and tolerability profiles.
Thirty-seven patients with Type 2 Diabetes Mellitus (“T2DM”) were enrolled in a Phase 1/2a double-blind, placebo-controlled, parallel-group study and administered levoketoconazole at 200, 400, or 600 mg once daily (QD), ketoconazole 400 mg QD, or placebo, for 14 days (Study DIO-501). A total of 21 patients with T2DM received levoketoconazole. Levels of LDL-cholesterol were significantly decreased in patients treated with levoketoconazole. Non-significant trends suggestive of improvement in glycemic control and reduction in cortisol secretion relative to placebo were observed.
Administration of levoketoconazole in patients with T2DM was safe and well tolerated. Headache and nausea were the most frequently reported adverse events (“AE”), some of which were considered drug-related. No clinically significant changes in hematology, blood chemistry, and urinalysis were noted in any treatment group. No treatment-related changes in markers of liver injury (LFTs) were reported. Plasma area under the concentration-time curves (AUCs) and maximum concentration (Cmax) increased in a non-proportional manner over the dose range of 200 mg to 400 mg; clearance was decreased at 600 mg QD.
Two Phase 2b studies (one main study plus an open-label extension) designed to evaluate the efficacy of levoketoconazole in combination with atorvastatin in patients with T2DM were voluntarily terminated early due to the perceived high regulatory and commercial hurdles for the approval and use of levoketoconazole in T2DM at the time of development in 2008, given the emerging benefits and risks profile observed. Study DIO-502 was a 4-month, double-blind, randomized, placebo-controlled, eight-arm dose-ranging trial of levoketoconazole (150 mg to 450 mg QD) with concomitant administration of metformin and either atorvastatin 10 mg or matching placebo for atorvastatin 10 mg. Study DIO-503 was an open-label, follow-on extension to Study DIO-502 to evaluate safety, tolerability, and pharmacodynamics after 24 weeks of dosing with levoketoconazole in combination with metformin and atorvastatin or placebo in patients with T2DM. At the time of study terminations, of 133 patients enrolled in the combined studies, 129 were treated, and 97 received at least one dose of levoketoconazole. A total of 47 patients received treatment with levoketoconazole between one and three months duration, while 38 patients exceeded three months of dosing. The frequency of AEs reported was generally similar across treatment arms. Diarrhea was the most frequently reported AE overall with administration of levoketoconazole. No SAEs were reported in the terminated studies.
A safety signal of elevated serum transaminases was identified in the DIO-502 and DIO-503 studies. Three of 129 randomized and treated patients were discontinued prematurely from the studies as required by the safety monitoring plan for elevated LFTs. All three were receiving levoketoconazole and atorvastatin study therapies at the time of their adverse events. Among these three patients, LFTs returned to normal after study drug was discontinued. Three other patients had modest elevations in LFT levels that led to elective premature study withdrawal (i.e. not dictated by the
11
safety monitoring plan). The LFT levels in these three patients also returned to normal after study drug was discontinued. Four additional patients required close monitoring of LFTs following an observed LFT abnormality, per protocol, and had resolution of their LFT abnormalities while receiving study drug. A detailed analysis of the liver transaminase elevations in this study showed that there was no correlation between the dose of levoketoconazole and abnormal liver transaminases. A safety signal consistent with drug-induced QTc prolongation was also observed in one of the drug-drug interaction studies.
Owing to the known risks of liver injury and QTc prolongation with ketoconazole and the observed safety signals in the Phase 2 program in type 2 diabetes, suspected liver injury and QTc prolongation (including related cardiac adverse events) were pre-defined as adverse events of special interest (“AESIs”) in the Phase 3 Cushing’s syndrome studies.
Recorlev Phase 3 Clinical Trials in Cushing’s syndrome
SONICS Phase 3 Clinical Trial
We enrolled 94 patients in our SONICS Phase 3 clinical trial in the United States, Canada, the European Union and the Middle East. This clinical trial was conducted pursuant to a U.S. Investigational New Drug for Recorlev for the treatment of endogenous Cushing’s syndrome that took effect in May 2013. The last patient visit occurred in November 2018.
Following a screening phase, SONICS had three distinct treatment phases. During the dose titration (DT) phase, patients started dosing at 150 mg twice daily (300 mg total daily dose) and titrated in 150 mg increments up to a maximum 600 mg twice daily (1,200 mg total daily dose) as needed and tolerated to control cortisol and improve patient signs and symptoms. Following the dose titration phase, once an individualized therapeutic dose had been reached, patients entered the maintenance phase during which the dose was to be regarded as fixed unless it needed to be changed for safety reasons, including loss of efficacy. At the end of the six-month maintenance phase, the UFC responder rate, which is the primary endpoint of the clinical trial, was determined. Patients who completed the maintenance phase were asked to enter an extended evaluation phase to provide additional safety and efficacy data. Throughout the entire clinical trial, various measurements for safety and efficacy were taken.
The primary endpoint of the clinical trial was the proportion of patients with UFC response to Recorlev, defined as a reduction in mean 24‑hour UFC levels to levels that are equal to or less than the upper level of normal range following six months of treatment in the maintenance phase without a dose increase (during the maintenance phase).
Key secondary endpoints included the number of patients with at least a 50% decrease in UFC levels, changes in blood sugar, blood pressure, cholesterol and weight compared to baseline, effects on clinical signs and symptoms of Cushing’s syndrome, quality of life measures obtained from the Cushing’s syndrome quality of life questionnaire and the severity of depression obtained from the Beck’s Depression Inventory II.
The clinical trial was also designed to investigate the pharmacokinetics of Recorlev in patients with endogenous Cushing’s syndrome.
12
Below is a diagram of the SONICS clinical trial design:

Several elements of the SONICS clinical trial design were informed by the clinical development pathway of currently approved drug therapies in the United States and the European Union. Additionally, we incorporated advice from the CHMP and FDA into the design of the clinical trial. The FDA recommended use of a concurrent control group in SONICS. However, SONICS utilized an open‑label, single‑arm design because use of a placebo control in a long-term, parallel-arm monotherapy design was considered unethical or infeasible to enroll, depending on the specific country or clinical trial site under consideration. Studies lacking a concurrent control group are more likely to be subject to unanticipated variability in study results that can potentially lead to flawed conclusions because they do not allow for discrimination of patient outcomes. As a result, even if we achieved the clinical trial’s endpoints, the FDA or other regulatory authorities could view our study results as potentially biased. We have attempted to control for bias in our SONICS trial via the use of strict evidence of active disease at baseline based on objective measures, an objectively measured primary endpoint with repeated longitudinal assessments, and implementation of a strict data restriction plan that severely limited exposure to efficacy data at the Sponsor.
The primary objective of normalized mean UFC (mUFC) without an increase in the therapeutic dose over the preceding six months was achieved among 30% of all enrolled patients at the final visit of the 6-month maintenance phase. Statistical significance of the primary endpoint analyzed using the intent-to-treat population was achieved, having excluded an mUFC normalization rate at the 6-month time point of 20% or lower (1-sided p = 0.0154; 2‑sided p = 0.031.) Sensitivity analyses and secondary and exploratory analyses of mUFC response were all supportive of and suggested greater efficacy of levoketoconazole than the primary endpoint analysis, indicative of a conservative primary analysis method.
Evidence of clinical benefit from levoketoconazole was further demonstrated by improvements in several pre-defined, key secondary endpoints of cardiovascular risk (i.e., cardiometabolic comorbidities of CS) in the maintenance-completer study population including highly statistically significant (maintaining overall type 1 error at 5%) and clinically meaningful decreases from baseline in mean fasting glucose, hemoglobin A1C, total and low-density lipoprotein‑cholesterol and body weight.
Safety and tolerability findings throughout the DT and maintenance phases indicate that levoketoconazole was generally well tolerated, with a discontinuation rate due to adverse events of 13% and no new safety signals observed relative to the prior experience with the drug in type 2 diabetes. Fourteen of 94 patients (15%) reported one or more serious adverse events (SAE), and in 4 patients an SAE was deemed drug-related by investigators (1 case of elevated liver function tests, 2 cases of prolonged QTc, and 1 case of adrenal insufficiency). One patient death not considered drug-related (colon cancer; preferred terms of adenocarcinoma of colon and metastases to liver), was reported during the maintenance phase.
Liver-related adverse events were considered adverse events of special interest (AESIs) in SONICS and are of particular interest in light of serious hepatotoxicity reported rarely among users of ketoconazole. Seven (7.4%) patients were reported as having an AESI related to the liver, and five of the seven discontinued study drug permanently at the time of the event; the other two resumed study drug after interruption, although one of the two later discontinued.
13
Transaminases were measured routinely at least every other week during dose titration and at least monthly during the maintenance phase. At the baseline visit, 96% of patients enrolled had alanine aminotransferase (ALT) within the normal reference range. During treatment ALT did not exceed 20x ULN in any patient, and no Hy’s Law cases were reported. Three (3.2%) patients were recorded with a post-baseline ALT value greater than 5x ULN and an additional seven (7.4%) of patients had at least 1 ALT value greater than 3x ULN at any time after baseline. Most ALT elevations occurred during dose titration, and all elevations over 3x ULN occurred by Month 2 of the maintenance phase. The aspartate aminotransferase values measured in these cases moved in the same direction as ALT but were less elevated. No patient had a total bilirubin level >1.5x ULN at any time. There was no obvious dose relationship among the cases, and each case was fully reversible upon drug interruption/discontinuation without clinical sequelae. It is not yet known if such events are predictable within an individual, but routine monitoring effectively identified elevated transaminase cases when they were mild and usually asymptomatic.
ECGs with centrally over-read QTc were monitored routinely during the study at the same intervals as LFT monitoring. Five (5.3%) patients were reported as having a QT/cardiac-related AESI; in every case the patient was asymptomatic. There were no discontinuations due to QTc prolongation. No arrhythmias were reported. In each case of prolonged QTc, study drug was resumed after temporary interruption. A total of nine (10%) patients had at least one QTc value representing an increase of more than 60 milliseconds from baseline, and two (2.1%) patients had a QTc interval above the pre-defined mandatory drug-interruption threshold of 500 milliseconds. Routine ECG monitoring effectively identified patients with QTc prolongation without any clinical sequelae. Therefore, we believe QTc prolongation safety issues can be appropriately managed with product labeling.
Complete results from our SONICS trial through the end of the maintenance phase were subsequently published in a peer-reviewed journal. Fleseriu M. et al. Efficacy and safety of levoketoconazole in the treatment of endogenous Cushing’s syndrome (SONICS): a phase 3, multicentre, open-label, single-arm trial. Lancet Diabetes Endocrinology; published online Sep 18, 2019.
The Top-Line results of our Extended Evaluation Phase of our Phase 3 SONICS trial of Recorlev showed that Recorlev was generally well-tolerated during the extended evaluation phase and no new drug-related safety signals were observed:
|
· |
60 out of 61 trial participants who completed the maintenance phase elected to participate in the extended evaluation phase; |
|
· |
Of the 60 patients that entered the extended evaluation phase, 46 patients completed it; |
|
· |
Data were collected twice, at three-month intervals, which is common practice for the long-term follow-up of chronic medical therapy for endogenous Cushing’s syndrome; |
|
· |
Four patients (6.7%) discontinued due to adverse events; |
|
· |
No patients (0%) experienced an increase in either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than three times (3x) the upper limit of normal and there were no (0%) reported adverse events of special interest (AESI) related to liver injury or dysfunction; |
|
· |
The most commonly reported (≥5%) treatment-emergent adverse events (TEAEs) in the extended evaluation phase were arthralgia (7%), QTc prolongation (7%), headache (7%), hypokalemia (7%), and nasopharyngitis (5%); QTc prolongation greater than 460 msec was not observed in the extended evaluation phase; and |
|
· |
Nausea (2%) and headache (7%) were reported at lower rates as compared to the previously reported aggregate rates of 32% (nausea) and 28% (headache) from the dose titration and maintenance phases. |
A summary of Recorlev extended evaluation phase efficacy results:
|
· |
In this exploratory evaluation, an observed-case analysis of completers was used to evaluate mUFC responders; |
14
|
· |
At the end of the extended evaluation phase, normalization of mUFC was observed in 41% of patients, and normalization of, or at least 50% improvement in, mUFC was observed in 68% of patients; |
|
· |
Clinically meaningful improvements in key cardiovascular risk markers (hemoglobin A1c, fasting glucose, total and LDL-cholesterol) were observed throughout the extended evaluation phase; and |
|
· |
Weight loss and reduction in body mass index (BMI) continued throughout the extended evaluation phase. |
LOGICS Phase 3 Clinical Trial
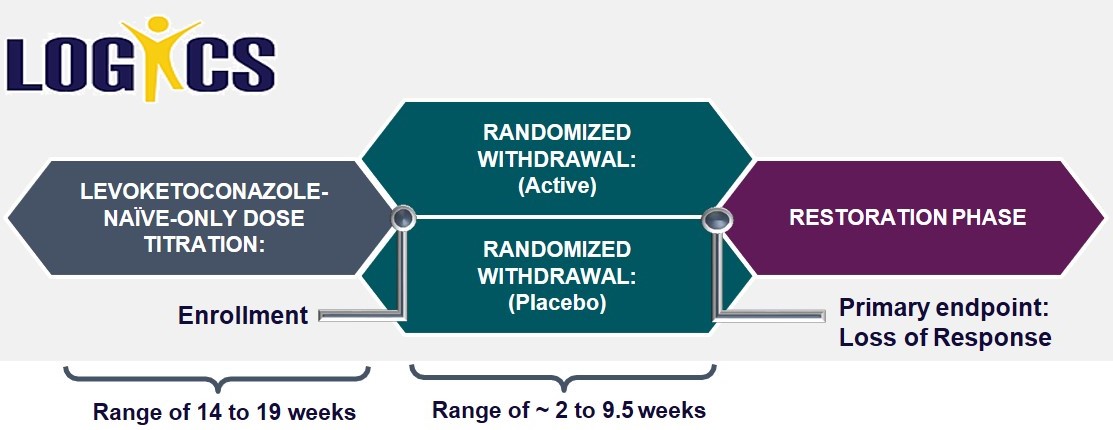
LOGICS is our second Phase 3 clinical trial of Recorlev for the treatment of endogenous Cushing's syndrome. The LOGICS trial is intended to supplement the long-term efficacy and safety data from the SONICS trial. LOGICS includes a double-blind, placebo-controlled, randomized-withdrawal phase of 8 weeks duration, targeting enrollment of approximately 46-54 patients in the randomized withdrawal phase of the trial following an open-label titration and maintenance phase of approximately 14 weeks. Top-line data following the randomized-withdrawal phase of LOGICS is expected at the end of second quarter or during the third quarter 2020. The addition of a concurrent control group in LOGICS is an attempt to address the FDA’s request for such a control group that was absent in our SONICS trial.
Following a screening phase, LOGICS has three distinct treatment phases for patients who did not participate in SONICS and two distinct phases for some of those who did participate in SONICS. The first phase, which is only intended for patients new to levoketoconazole or for those who require re-establishment of a therapeutic dose, is dose titration and maintenance. During the dose titration and maintenance phase, patients start at 150 mg twice daily dosing (300 mg total daily dose) and titrate in 150 mg increments up to a maximum 600 mg twice daily dosing (1,200 mg total daily dose) as needed and as tolerated to achieve and maintain normalization of mUFC for at least 4 weeks at their individualized therapeutic dose. The total duration of this phase is a minimum of approximately 14 weeks and up to approximately 19 weeks. SONICS-completers who were currently receiving a stable therapeutic dose skip dose titration and maintenance and proceed directly to the second phase, where they are joined by those who progressed through the first phase. The second phase is randomized-withdrawal, during which patients are randomly assigned to either continue active treatment with levoketoconazole or be switched to a matching placebo using the same tablet number. The primary efficacy endpoint comes at the end of the randomized-withdrawal period, which lasts approximately 8 weeks for each patient (and may end sooner if a “rescue” is needed). The primary endpoint is the proportion of patients with a loss of established UFC response in the placebo group compared with that proportion in the levoketoconazole group. The final phase of LOGICS is the restoration phase, during which all patients once again receive active therapy. In order to conceal the therapy in the randomized-withdrawal phase, it was necessary to blind the therapy during restoration using twice the number of tablets (one active and one placebo). Throughout the entire clinical trial, various measurements for safety and efficacy are taken.
15
Below is a diagram of the LOGICS clinical trial design:
OPTICS Phase 3 Clinical Trial
In 2018, we initiated a long-term, open-label extension trial with Recorlev (“OPTICS”) to capture longer-term safety, tolerability and efficacy data from patients who complete either SONICS or LOGICS and who choose to continue therapy with Recorlev. OPTICS will continue to accrue data for a minimum of three years, with a plan to allow patients to continue participating in the trial until Recorlev has become available in their own country. We might be required by the FDA and the EMA to collect additional safety data post‑approval.
Clinical Trials Summary
We believe that SONICS has (1) demonstrated consistent and significant clinical benefit by meeting the primary endpoint of the trial, specifically the responder rate measured as normalization of UFC levels at the 6-month time point without need for dose increase during the 6-month maintenance phase and (2) shown consistent improvement of objectively quantifiable biomarkers of endogenous Cushing’s syndrome comorbidities, such as blood glucose, blood lipids, blood pressure or weight, and improvement of other clinical signs and symptoms of endogenous Cushing’s syndrome, and thus it would be regarded by regulators as adequate proof of efficacy in this rare disease with a high unmet medical need. Therefore, we consider LOGICS as a mechanism for providing independent evidence of efficacy of Recorlev in a placebo-controlled and blinded trial rather than serving as sole or primary evidence of efficacy for Recorlev in endogenous Cushing’s syndrome. Furthermore, if approved, LOGICS has the potential to provide adequate evidence of efficacy durability beyond one year of therapy in the subset of patients who were previously enrolled in SONICS. Finally, we believe that the combination of SONICS and LOGICS will provide an adequate demonstration of the long-term safety and tolerability of Recorlev in patients with endogenous Cushing’s syndrome. In total, over 160 unique patients with this condition will have been treated with Recorlev in our SONICS and LOGICS trials, and some patients will have been treated with Recorlev for more than 4 years at the time of first NDA submission.
Recorlev Regulatory Background
In the United States, levoketoconazole is considered a new active substance. Upon completion of the clinical development program for Cushing’s Syndrome, we intend to file for marketing authorizations in the United States and elsewhere. In the United States, an NDA, which is a prerequisite to marketing authorization, can be submitted under one of a number of approval paths defined in the Federal Food, Drug, and Cosmetic Act. Following consultations with the
16
FDA, we determined that the 505(b)(2) approval pathway, which permits an NDA applicant to rely on data from studies that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference, is the appropriate pathway for a Recorlev NDA. Because a 505(b)(2) NDA approval can rely in part on data already accepted by the FDA or otherwise publicly available, an abbreviated and reduced development program may be possible. In the case of Recorlev, we intend to rely in our NDA on published literature and the FDA’s prior findings concerning the safety and/or effectiveness of ketoconazole. A similar marketing authorization path is available in most of the rest of the world, and we anticipate that the studies supporting U.S. approval will likewise support approvals to market Recorlev elsewhere, including in the European Union. The FDA has acknowledged that no additional preclinical investigations will be required for Recorlev prior to an NDA filing. The EMA’s CHMP has requested a study of reproductive toxicity that may be completed prior to filing for marketing authorization in Europe, pending further discussions.
In March 2019, we conducted a Type C meeting with the Division of Metabolic and Endocrine Products (DMEP) of the FDA. The DMEP stated in its meeting minutes that the FDA generally requests that a sponsor conduct two adequate and well-controlled clinical studies for the proposed indication of a drug candidate under 21 CFR 314.126(b)(2). The DMEP also noted that the FDA recognizes situations when a single trial may be sufficient. The DMEP reiterated that the characteristics of an “adequate and well-controlled” investigation under 21 CFR 314.126 include the use of a control group (e.g., placebo concurrent control, dose-comparison concurrent control), randomization and evaluation of primary endpoints that directly measure clinical benefits, or supported by evidence of clinical benefit. For this reason, while the DMEP indicated that it would consider, as a review issue, the adequacy of a New Drug Application (NDA) submission with data from our SONICS trial as the sole Phase 3 evidence supporting the efficacy of RECORLEV, the DMEP nonetheless recommended that we complete a second trial and include the results from that trial in addition to data from our SONICS trial in our NDA submission.
We currently expect, if supported by the data, to submit an NDA for Recorlev approximately six months after reporting top-line results from our LOGICS trial that will include data from each of the SONICS and LOGICS trials. The DMEP stated in its meeting minutes that our clinical pharmacology program for Recorlev, as described to them, appears reasonable to support an NDA filing for Recorlev provided that the data generated are found to be suitable.
Veldoreotide Modified-Release —a Novel Somatostatin Analogue
Overview
In June 2015, we acquired veldoreotide, a novel multi‑receptor targeted somatostatin analog (“SSA”) that has the potential to be a next-generation somatostatin analog to provide a new and differentiated treatment option for patients with conditions amenable to somatostatin receptor activation. We acquired veldoreotide as part of our strategy to build our rare endocrine franchise. At the time of acquisition, veldoreotide was in Phase 2 clinical development as a treatment for acromegaly in its original, immediate-release formulation. Acromegaly is a rare endocrine disorder that most commonly results from a benign tumor of the pituitary gland, leading to excess production of growth hormone and IGF‑1. The treatment goal is the normalization of growth hormone and IGF‑1, which is the main cause of the detrimental clinical signs and symptoms of acromegaly.
SSAs are peptides that are currently administered as deep subcutaneous or intramuscular injections, typically as long-acting formulations for monthly injections. They are the most commonly used drug therapy for the treatment of acromegaly and work by binding to specific subtypes of somatostatin receptors SSTs that are expressed by the tumor. Binding of SSAs to these SSTs leads to the beneficial inhibition of growth hormone secretion but can also result in the unwanted inhibition of secretion of other endocrine hormones such as insulin and glucagon in the pancreas. Like other current SSAs, veldoreotide is a peptide that we are developing for injection. In contrast to approved SSAs, veldoreotide activates a different subset of SSTs. Like the marketed SSAs, it binds and activates signaling via SST2 and SST5. However, in contrast to the approved SSAs, which primarily target one or the other of SST2 or SST5, veldoreotide binds and activates SST2 and SST5 approximately with equal potency. Veldoreotide also has a high affinity for SST4, a receptor believed to be important to modulating pain signals in the peripheral nervous system. Veldoreotide does not bind to SST3 or the mu-opiate receptor at pharmacological concentrations. In vitro data suggest that a higher proportion of human adenomas are a target for growth hormone inhibition by veldoreotide as compared to octreotide, which is referred to as a single receptor targeted SSA that binds and activates predominantly via SST2, potentially resulting in an
17
increased responder rate. Nonclinical data indicate that postprandial insulin secretion and gallbladder motility are both less inhibited by veldoreotide as compared with octreotide.
Based on the differentiated activation pattern of veldoreotide upon binding to SST subtypes, we believe that veldoreotide may offer an improved efficacy and safety profile relative to existing drug therapies for acromegaly and other conditions that are modifiable through activation of somatostatin receptors. In the three clinical studies of immediate-release veldoreotide completed outside the United States in healthy volunteers veldoreotide was able to suppress stimulated growth hormone levels to a similar extent as octreotide, and, when compared with immediate-release subcutaneous octreotide, there was less blunting of insulin in response to a mixed meal or oral glucose load. In two studies of patients with acromegaly, veldoreotide caused a similar degree of suppression of elevated serum growth hormone as octreotide. Veldoreotide has been granted orphan drug designation for the treatment of acromegaly by the FDA and the EMA.
Immediate release veldoreotide would require three injections per day for therapeutic effect and therefore we believe it is not commercially feasible. We have formulated veldoreotide as a modified-release, long-acting product using PLGA microsphere technology. Preliminary nonclinical studies suggest that an injection volume suitable for subcutaneous administration may be feasible for once-weekly dosing in humans. Depending on the results of ongoing exploratory nonclinical studies, we may elect to pursue a development pathway for veldoreotide modified-release that may include acromegaly or may include therapeutic uses outside of acromegaly or endocrinology in general. These ongoing nonclinical studies will provide more information on the development path for veldoreotide in 2020. Regardless of indication(s) to be pursued, further development will require preclinical safety (toxicology) studies as well as manufacturing scale-up before the newly formulated product can enter clinical testing. We anticipate that such preclinical studies, once begun, will take at least 18 months to complete.
Completed Clinical Trials
Five clinical trials of veldoreotide have been performed to date: three in healthy male volunteers and two in patients with acromegaly, all of which employed an immediate-release, short‑acting formulation injected subcutaneously. At the time the clinical trials described below were conducted, veldoreotide was named DG3173. These trials were conducted by Aspireo Pharmaceuticals Ltd., other than DG3173‑I‑001, which was conducted by Develogen AG.
The Phase 1 clinical trials involved 122 healthy subjects and the Phase 2 clinical trial involved 28 patients with acromegaly. No SAEs were observed, and mostly mild adverse events typical for SSAs such as injection site reactions and gastrointestinal side effects were reported. There was no evidence that veldoreotide adversely affects the liver, kidneys or other organ systems, including the cardiovascular system. Data from the multiple ascending dose clinical trial in healthy subjects (Study I 002) showed inhibition of growth hormone comparable to octreotide, but no or less inhibition of insulin secretion and less effect on glucose levels. The single ascending dose trial in patients with acromegaly (Study II 01) and the continuous infusion study in patients with acromegaly (Study II 02) confirmed that veldoreotide also suppresses excessively produced growth hormone to a similar maximal extent as octreotide.
18
We believe the preliminary clinical findings from these trials corroborate the profile of veldoreotide observed in nonclinical studies, which suggested inhibition of growth hormone secretion without detrimental effects on post‑meal insulin or glucose metabolism. These preliminary findings contrast favorably with the well‑described insulin and glucose perturbations caused by octreotide, lanreotide and pasireotide, and we may conduct additional clinical trials to evaluate the clinical profile of veldoreotide and possibly its differentiation from existing SSAs. We believe veldoreotide potentially could confer therapeutic advantages relative to currently approved SSAs as a treatment for somatostatin-responsive conditions. The following table summarizes the completed clinical trials with immediate-release veldoreotide.
|
Clinical Trial |
|
Clinical Trial Descriptions |
|
Patients |
|
Year and Status |
|
Location |
|
Dose |
|
DG3173‑II‑02 |
|
Phase 2 Trial of the Effect of Subcutaneous Infusions of Three Doses of Veldoreotide on Growth Hormone Levels in Untreated Acromegaly Patients |
|
8 |
|
2013/2014 Completed. Bioanalytical report issued. |
|
Ukraine |
|
920‑5520 µg continuous infusion over 23 hours |
|
DG3173‑II‑01 |
|
Phase 2 Trial of the Effect of Single Ascending Doses of Veldoreotide and 300 µg of Octreotide on Human Growth Hormone Levels in Untreated Acromegaly Patients |
|
20 |
|
2012 Completed. Study report issued. |
|
Ukraine |
|
300‑1800 µg QD |
|
DG3173‑I‑003 |
|
Phase 1 Placebo‑Controlled, Trial to Assess the Pharmacodynamic Effect on Glucose Metabolism of Single Doses of Veldoreotide, Octreotide and Placebo in Healthy Male Patients |
|
8 |
|
2013 Completed. Study report issued. |
|
Switzerland |
|
300‑1800 µg QD |
|
DG3173‑I‑002 |
|
Phase 1 Trial to Compare the Safety and Pharmacologic Activity of Repeated Doses of Veldoreotide and Veldoreotide Plus Octreotide with Octreotide and Placebo and Establish Their Pharmacokinetic Interaction in Healthy Male Patients |
|
42 |
|
2012/2013 Completed. Study report issued. |
|
Switzerland |
|
100‑1800 µg TID |
|
DG3173‑I‑001 |
|
Phase 1 Double‑Blind, Placebo-Controlled Trial to Investigate Safety, Tolerability and Pharmacokinetics of Single Escalating Dosing of Veldoreotide in Healthy Male Patients |
|
72 |
|
2008 Completed. Study report issued. |
|
Germany |
|
10‑2000 µg QD |
Macrilen
In January of 2018, Strongbridge Ireland Limited, one of our wholly-owned subsidiaries, acquired the U.S. and Canadian rights to Macrilen (macimorelin), the first and only oral drug approved by the FDA for the diagnosis of patients with adult growth hormone deficiency. We launched Macrilen in the United States in July 2018.
In December 2018, we sold Strongbridge Ireland Ltd. to Novo for $145 million plus tiered royalties on net sales of Macrilen through 2027. Between January 1, 2019 and December 31, 2021, Novo will pay to us 12% of annual net sales of Macrilen in the U.S. Between January 1, 2022 and December 31, 2027, Novo will pay to us (i) 4% of any
19
portion of annual net sales in the U.S. up to $100 million and (ii) 8% of any portion of annual net sales in the U.S. greater than $100 million. The royalty payments are subject to certain conditions and reductions, including if Macrilen is no longer covered by a valid claim of a patent in the United States and Novo or its affiliates no longer hold exclusive marketing rights granted by the FDA. In connection with the sale, we entered into a services agreement with Novo pursuant to which Novo agreed to fund the costs of 23 of our field-based employees to provide full-time ongoing services to Novo, including the promotion of Macrilen in the U.S., for a period of three years. In December 2019, we reached an agreement with Novo to terminate the services agreement. We received a $6 million payment in connection with such termination, and we will no longer provide services to Novo.
Our Rare Neuromuscular Franchise
In December 2016, we acquired the U.S. marketing rights to Keveyis (dichlorphenamide) from Taro Pharmaceuticals North America, Inc., a subsidiary of Taro Pharmaceutical Industries Ltd. Keveyis is the first and only therapy approved in the United States to treat hyperkalemic, hypokalemic and related variants of PPP, a group of rare hereditary disorders that cause episodes of muscle weakness or paralysis.
Overview of PPP and Keveyis
PPP is a rare, genetic, neuromuscular disorder related to a defect in muscle ion channels with multiple variants and subtypes. The disease is characterized by episodes of muscle weakness and paralysis. It often interferes with daily activities and, as patients get older, it can lead to permanent muscle weakness. PPP may be localized (“focal”) or more widespread (“generalized”), and it often goes underdiagnosed and/or undertreated. Types of periodic paralysis are differentiated by criteria including underlying genetic mutations and changes in blood potassium during an episode. The two most common forms of PPP are hypokalemic, when episodes can be induced by low blood levels of potassium, and hyperkalemic, when episodes are associated with elevated levels of blood potassium. We believe, based on our market research, that there are approximately 4,000 to 5,000 patients in the United States diagnosed with PPP.
Keveyis is an oral carbonic anhydrase inhibitor that was approved by the FDA in the United States in August 2015 to treat hyperkalemic, hypokalemic and related variants of PPP. The exact mechanism(s) through which oral carbonic anhydrase inhibitors, and Keveyis in particular, decrease the frequency and severity of periodic paralysis attacks is unknown. However, it is believed that their effects are mediated both locally (i.e., in muscle) and systemically. It is not known whether their effects are disease-modifying. Keveyis has received orphan drug exclusivity status in the United States through August 7, 2022.
Following FDA approval in August 2015, Keveyis was marketed by Taro. In May 2016, Taro announced the cessation of their commercial sales and related promotional activities for Keveyis. Taro supplied Keveyis to patients on a non-commercial basis through a single specialty pharmacy in the United States from May 2016 until our acquisition of the U.S. marketing rights to Keveyis in December 2016. We continued to supply Keveyis to patients on a non-commercial basis until launching Keveyis in April 2017. After acquiring the U.S. marketing rights for Keveyis, we established sales, marketing, market access and patient service capabilities.
Because a large percentage of the people who suffer from PPP remain undiagnosed or inadequately treated, we developed programs to educate the medical community and patients about this illness. In addition, we established a field-based force of medical science liaisons. We use a single, specialty pharmacy to provide reimbursement, clinical and distribution support for Keveyis and to develop cost-sharing and patient assistance programs to support qualified, commercially insured patients, federal- and state-insured patients, and uninsured or under-insured patients. We also donate money to independent charitable foundations dedicated to this cause. Our ultimate goal is to ensure that no PPP patient is denied access to Keveyis for financial reasons.
Clinical Development of Keveyis
The efficacy of KEVEYIS was evaluated in two clinical studies, Study 1 and Study 2.
20
Study 1
Study 1 was a 9-week, double blind, randomized, placebo-controlled, multi-center study. Study 1 consisted of two substudies: a substudy in patients with hypokalemic periodic paralysis (n=44), and a substudy in patients with hyperkalemic periodic paralysis (n=21). The primary efficacy endpoint in both substudies was the average number of self-reported attacks of muscle weakness per week over the final 8 weeks of the trial. Withdrawal from the study for acute severe worsening was also assessed as an endpoint.
In Study 1, the tested dose of Keveyis was 50 mg b.i.d. for treatment-naïve patients. Patients already receiving dichlorphenamide prior to the study continued on the same dose if randomized to Keveyis during the study. In patients taking acetazolamide prior to the study, the daily dose of Keveyis was set at 20% of the daily acetazolamide dose. Dose reduction for tolerability was permitted.
In the hypokalemic periodic paralysis substudy, median age of patients was 45 years and 73% of patients were male. Patients treated with Keveyis (n=24) had 2.2 fewer attacks per week than patients (n=20) treated with placebo (p=0.02). None of the patients randomized to Keveyis reached the endpoint of acute worsening, vs. five patients randomized to placebo. The mean dose of Keveyis at Week 9 was 94 mg/day.
In the hyperkalemic periodic paralysis substudy, median age of patients was 43 years and 43% of patients were male. During the double-blind treatment period, patients treated with Keveyis (n=12) had 3.9 fewer attacks per week than patients treated with placebo (n=9) (p=0.08). None of the patients randomized to Keveyis reached the endpoint of acute worsening, vs. two patients randomized to placebo. The mean dose of Keveyis at Week 9 was 82 mg/day.
Study 2
Study 2 was a 35-week, double blind, placebo-controlled, randomized, multi-center, two-period crossover study. Study 2 also consisted of two substudies: a substudy in patients with hypokalemic periodic paralysis (n=42), and a substudy in patients with hyperkalemic periodic paralysis (n=31), including patients with Paramyotonia Congenita (together termed potassium-sensitive periodic paralysis or PSPP). The primary endpoint in the hypokalemic periodic paralysis substudy was the incidence of acute intolerable worsening (based on attack frequency or severity) necessitating withdrawal. The primary endpoint in the hyperkalemic periodic paralysis substudy was the average number of self-reported attacks of muscle weakness per week. Dosing was determined similarly to Study 1.
In the hypokalemic periodic paralysis substudy, mean age of patients was 38 years and 79% of patients were male. Acute intolerable worsening was observed in 2 patients on Keveyis vs. 11 patients on placebo (p=0.02). The mean dose of Keveyis at the end of the study was 96 mg/day.
In the hyperkalemic periodic paralysis substudy, mean age of patients was 37 years and 58% of patients were male. Patients treated had 2.3 fewer attacks per week on Keveyis than on placebo (p=0.006). The mean dose of Keveyis at the end of the study was 73 mg/day.
Commercialization Strategy
After acquiring the U.S. marketing rights for Keveyis in December 2016, we established sales, marketing, market access and patient services capabilities. We believe, based on our market research, that there are approximately 4,000 to 5,000 patients in the U.S. diagnosed with PPP and we believe that we can address the market by targeting physicians who are managing patients with PPP, including neuromuscular specialists, general neurologists and primary care physicians.
Given the current stage of product development of our product candidates, we have not yet fully established a commercialization infrastructure specifically dedicated to our product candidates still in development, although we do plan to leverage our current commercial infrastructure where possible. As with Keveyis, in the U.S. we intend to independently commercialize our rare disease-focused product candidate, if approved. For product candidates approved outside of the U.S. we will evaluate the best approach to commercialization on a country by country basis—we may
21
choose to independently commercialize but we could choose to partner, use a distributor, or sell the rights among other choices. We believe that we can address the market of our current late-stage product candidate by targeting endocrinologists that are focused on the diagnosis and treatment of rare pituitary disorders primarily stemming from benign tumors.
Our commercial strategy for our late-stage rare endocrine product candidate, if approved, will encompass promoting its unique benefits, as well as a concerted effort to raise awareness about the underlying diseases among the physician/patient community with the goal of increasing the rate of diagnosis when the symptoms may otherwise be overlooked. We believe the combination of our commercial efforts and our late-stage rare endocrine product candidate profile will facilitate our ability to successfully gain usage in our target markets.
Manufacturing
We do not have internal manufacturing capabilities and intend to continue to rely on third parties to produce Keveyis and our product candidates.
We have a supply agreement with Taro to produce Keveyis. We are obligated to purchase certain annual minimum amounts of product totaling approximately $29 million over a six-year period from Taro. As of December 31, 2019, our remaining obligation was $19.0 million. The supply agreement may extend beyond the orphan exclusivity period unless terminated by either party pursuant to the terms of the agreement. If the supply agreement is terminated by Taro at the conclusion of the orphan exclusivity period, we have the right to manufacture the product on our own or have the product manufactured by a third party on our behalf.
The manufacturing, packaging and distribution of Recorlev drug product for clinical trials following Good Manufacturing Practices (“GMPs”), is currently outsourced under contracts to experienced contract manufacturers. We expect to enter into similar arrangements for veldoreotide.
Intellectual Property of our Products and Product Candidates
We actively seek to protect the intellectual property and proprietary technology that we believe is important to our business, including seeking, maintaining, enforcing and defending patent rights for our products, product candidates and methods of treatment, whether developed internally or licensed from third parties. Our success will depend on our ability to obtain and maintain patent and other protection (including exclusivity through orphan drug designation) for our products, product candidates and methods of treatment, preserve the confidentiality of any know‑how and operate without infringing the valid and enforceable patents and proprietary rights of third parties.
Our policy is to seek to protect our proprietary position generally by filing patent applications initially at the USPTO. After this initial phase, patent applications claiming priority to the initial application are filed in various countries, including the United States, Europe and Canada. In each case, we determine the strategy and territories required after discussion with our patent counsel with the goal of obtaining relevant coverage in territories that are or may be commercially important to us and our product candidates. We will additionally rely on orphan drug designation exclusivity and patent term extensions when available. We also rely on trade secrets and know‑how relating to our underlying product technologies. Prior to making any decision on filing any patent application, we consider with our patent counsel whether patent protection is the most sensible strategy for protecting the invention concerned or whether the invention should be maintained as confidential.
We own or license 49 granted patents, of which seven are U.S. issued patents. We also own or license 30 pending patent applications, of which 19 are U.S. patent applications.
We maintain trademark registrations and/or trademark applications for “Strongbridge Biopharma” and “Recorlev” in key geographies that include the United States, Australia, Brazil, China, Europe, Israel, India, Japan, Mexico, and Canada, among others. We also maintain trademark registrations and/or trademark applications for various additional potential trademarks for potential use if we determine not to utilize Recorlev as the branded pharmaceutical name for our levoketoconazole product candidate, once approved.
22
Recorlev
We own 46 issued patents related to our product candidate, Recorlev. Issued claims in these patents are directed to methods of treatment of various diseases or conditions associated with elevated cortisol levels or activity using Recorlev. The patents have been granted in major territories including the U.S., Europe, China and Japan and expire in 2026, 2027 and 2030. We have three pending U.S. patent applications and 1 Patent Cooperation Treaty (PCT) patent application directed to methods of treating a disease or condition associated with elevated cortisol levels or activity, including Cushing’s syndrome, with Recorlev. One of the issued patents in the United States is directed to reducing C-reactive protein levels and systemic inflammation through administration of a once-daily dose of Recorlev that expires in 2030. In addition to any patent exclusivity, we intend to rely on orphan drug designation exclusivity for Recorlev.
Veldoreotide
We own two issued patents and one patent application in the U.S. related to our product candidate, veldoreotide. One of the patents issued in the U.S. contains claims covering a modified-release formulation and a method of manufacturing the formulation. This patent expires in 2037. We have filed eight patent applications that include substantially similar proposed claims in other countries including China, Japan, Canada and various countries in Europe. In addition to any patent exclusivity, we intend to rely on orphan drug designation exclusivity for veldoreotide.
Keveyis
We acquired U.S. marketing rights to Keveyis in late 2016. We are not aware of any issued patents related to Keveyis. We have filed fourteen patent applications in the United States and three PCT patent applications related to Keveyis. Although we intend to rely primarily on orphan drug designation exclusivity for Keveyis, we also expect to continue to prosecute such patent applications and explore additional life cycle management opportunities for Keveyis.
Laws and Regulations Regarding Patent Terms
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non‑provisional application. In the United States, a patent term may be shortened if a patent is terminally disclaimed over another patent or as a result of delays in a patent prosecution by the patentee. A patent’s term may be lengthened by a patent term adjustment, which compensates a patentee for administrative delays by the USPTO in granting a patent. The patent term of a European patent is 20 years from its effective filing date, which, unlike in the U.S., is not subject to patent term adjustments in the same way as U.S. patents.
The term of a patent that covers an FDA‑approved drug or biologic may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984 (the “Hatch‑Waxman Act”) permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug or biologic is under regulatory review. Patent extensions cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other jurisdictions to extend the term of a patent that covers an approved drug, for example Supplementary Protection Certificates. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. We anticipate that some of our issued patents may be eligible for patent term extensions, but such extensions may not be available and, therefore, any commercial monopoly may be restricted.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our scientific knowledge, technology, and development experience provide us with competitive advantages, we face potential competition from many different
23
sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing products and new products that may become available in the future. Many of our competitors, alone or with their strategic partners, have greater experience than we do in conducting preclinical studies and clinical trials, and obtaining FDA, EMA and other regulatory approvals, and have substantially greater financial, technical and other resources than we do, such as larger research and development, clinical, marketing and manufacturing organizations. As a result, these companies may obtain regulatory approval for competing products more rapidly than we are able and may be more effective in selling and marketing their products. Companies that complete clinical trials, obtain required regulatory authority approvals and commence commercial sale of their drugs before their competitors may achieve a significant competitive advantage, and our commercial opportunity could be reduced or eliminated if competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Drugs resulting from our research and development efforts or from our joint efforts with collaboration partners therefore may not be commercially competitive with our competitors’ existing products or products under development.
We are aware of several companies focused on developing or marketing therapies for rare neuromuscular and endocrine disorders. For our product candidates, the main competitors include:
|
· |
Recorlev: A number of therapies are currently approved or in various stages of development for endogenous Cushing’s syndrome. Currently, there are no therapies broadly marketed for the treatment of endogenous Cushing’s syndrome patients in the U.S. Korlym (mifepristone) is a cortisol receptor blocker indicated to control hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing’s syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or are not candidates for surgery. Signifor (pasireotide) and Signifor LAR are marketed by Recordati in the United States and are indicated for the treatment of adult patients with Cushing's disease (a subset of Cushing’s syndrome) for whom pituitary surgery is not an option or has not been curative. A number of products, including ketoconazole, mytripone, cabergoline, mitotane and etomidate are used off-label for the treatment of Cushing’s Syndrome in the U.S. Ketoconazole, metyrapone and mitotane are marketed by HRA Pharma in certain European countries. Products in development include Osilodrostat (LCI699), an 11β-HSD2 inhibitor, is currently in Phase 3 clinical development in Cushing’s disease in the United States by Recordati, and received EMA approval in Cushing’s disease in the European Union in 2019. Corcept Therapeutics is developing relacorilant (CORT125134), a selective glucocorticoid receptor antagonist, currently in Phase 3 for Cushing’s syndrome. AstraZeneca PLC. is developing AZD-4017 inhibitor of 11beta-hydroxysteroid dehydrogenase 1 (11BHSD1), currently in Phase 2. Synchronicity Pharma Inc. is developing SHP-1705, which acts by modulating cryptochrome (Cry) receptor activity, currently in Phase 1. |
|
· |
Keveyis: Acetazolamide, an oral carbonic anhydrase inhibitor, is used frequently off-label for the prophylactic and sometimes acute treatment of PPP. Potassium supplements are indicated for use in |
24
hypokalemic periodic paralysis in the United States and are frequently used either chronically or for emergency treatment of episodes in that form of PPP. Several other types of drugs have been reported to have benefits for chronic or acute use in one or more than one PPP variant, including potassium-sparing diuretics, beta receptor agonists, mexelitine and other sodium channel blockers, and others. We are not aware of drugs currently in development for prophylactic chronic treatment of PPP. |
Government Regulation
Product Approval Process in the United States
The safety, clinical testing, manufacturing, quality, labeling, storage, distribution, record keeping, advertising, promotion, import, export and marketing, among other things, of our product candidates are subject to extensive regulation by governmental authorities in the United States and other countries. The FDA under the Federal Food, Drug, and Cosmetic Act regulates pharmaceutical products in the United States. The steps required before a drug may be approved for marketing in the United States generally include:
|
· |
the completion of preclinical laboratory tests and animal tests conducted under Good Laboratory Practices, (“GLPs”), and other applicable regulations; |
|
· |
the submission to the FDA of an IND application for human clinical testing, which must be reviewed by the FDA and become effective before human clinical trials commence; |
|
· |
the successful performance of adequate and well‑controlled human clinical trials conducted in accordance with current Good Clinical Practices (“cGCP”) to establish the safety and efficacy of the product candidate for each proposed indication; |
|
· |
analysis of clinical trial data and preparation of submission to the FDA of an NDA; |
|
· |
the submission to the FDA of an NDA; |
|
· |
the FDA’s acceptance of the NDA; |
|
· |
satisfactory completion of an FDA inspection of the manufacturing facilities at which the product is made to assess compliance with current Good Manufacturing Practices (“cGMPs”) to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
|
· |
satisfactory completion of FDA inspections of clinical trial sites and GLP toxicology studies; and |
|
· |
the FDA’s review and approval of an NDA prior to any commercial marketing or sale of the drug in the United States. |
The testing and approval process requires substantial time, effort, and financial resources, and the receipt and timing of any approval is uncertain.
Preclinical studies include laboratory evaluations of the product candidate, as well as animal studies to assess the potential safety and efficacy of the product candidate. The results of the preclinical studies, together with manufacturing information, analytical data and a proposed clinical trial protocol, are submitted to the FDA as part of the IND, which must become effective before clinical trials may be commenced. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the clinical trials as outlined in the IND prior to that time and places the IND on clinical hold. In this case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed. A clinical hold may occur at any time during the life of an IND, due to safety concerns or non‑compliance, and may affect one or more specific studies or all studies conducted under the IND.
Clinical trials involve the administration of the product candidates to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator. Clinical trials are conducted under
25
protocols detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Further, each clinical trial must be reviewed and approved by an independent institutional review board (“IRB”), either centrally or individually at each institution at which the clinical trial will be conducted. The IRB will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution. Progress reports detailing the status of the clinical trials must be submitted to the FDA annually. Sponsors must also report to the FDA serious and unexpected adverse reactions, any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigation brochure, or any findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the drug. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
Clinical trials are typically conducted in three sequential phases prior to approval, but the phases may overlap. These phases generally include the following:
|
· |
Phase 1. Phase 1 clinical trials represent the initial introduction of a product candidate into human subjects, frequently healthy volunteers. In Phase 1, the product candidate is usually tested for safety, including adverse effects, dosage tolerance, absorption, distribution, metabolism, excretion and pharmacodynamics. |
|
· |
Phase 2. Phase 2 clinical trials usually involve studies in a limited patient population to (1) evaluate the efficacy of the product candidate for specific indications, (2) determine dosage tolerance and optimal dosage, and (3) identify possible adverse effects and safety risks. |
|
· |
Phase 3. Phase 3 clinical trials are conducted to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population at geographically dispersed clinical trial sites, and to provide sufficient data for the statistically valid evidence of safety and efficacy. |
Phase 4 clinical trials are conducted after approval to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations, or when otherwise requested by the FDA in the form of post‑market requirements or commitments. Failure to promptly conduct any required Phase 4 clinical trials could result in withdrawal of approval.
Clinical trials are inherently uncertain and any phase may not be successfully completed. A clinical trial may be suspended or terminated by the FDA, IRB or sponsor at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides ongoing oversight and safety reviews to determine whether or not a clinical trial may move forward at designated checkpoints based on access to certain data from the clinical trial. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Sponsors have the opportunity to meet with the FDA at certain points during the development of a new drug to share information about the data gathered to date and for the FDA to provide advice on the next phase of development. These meetings may be held prior to the submission of an IND, at the end of Phase 2 and/or before an NDA is submitted. Meetings may be requested at other times as well.
The results of preclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information on the manufacture, composition and quality of the product, proposed labeling and other relevant information are submitted to the FDA in the form of an NDA requesting approval to market the product. The NDA must be accompanied by a significant user fee payment. The FDA has substantial discretion in the approval process and may refuse to accept any application, for example if the NDA is not sufficiently complete, or decide that the data is insufficient for approval and require additional preclinical, clinical or other studies.
In addition, under the Pediatric Research Equity Act (“PREA”), an NDA or supplement to an NDA must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric
26
subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan drug designation has been granted. However, if only one indication for a product has orphan drug designation, a pediatric assessment may still be required for any applications to market that same product for the non‑orphan indication(s).
Once the NDA submission has been submitted, the FDA has 60 days after submission of the NDA to conduct an initial review to determine whether it is sufficient to accept for filing. NDAs receive either a standard or priority review. Under the Prescription Drug User Fee Act, the FDA sets a goal date by which it plans to complete its review. For a standard review, this is typically 10 months from the date of submission of the NDA application. The review process is often extended by FDA requests for additional information or clarification. Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless the manufacturing facility complies with cGMPs and may also inspect clinical trial sites for integrity of data supporting safety and efficacy. The FDA may also convene an advisory committee of external experts to provide input on certain review issues relating to risk, benefit and interpretation of clinical trial data. The FDA is not bound by the recommendations of an advisory committee, but generally follows such recommendations in making its decisions. The FDA may delay approval of an NDA if applicable regulatory criteria are not satisfied and/or the FDA requires additional testing or information. The FDA may require post‑marketing testing and surveillance to monitor safety or efficacy of a product.
After the FDA evaluates the NDA and conducts inspections of manufacturing facilities where the drug product and/or its API will be produced, it may issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A Complete Response Letter generally outlines the deficiencies in the NDA submission and may require substantial additional clinical testing, such as an additional pivotal Phase 3 clinical trial(s), clinical data, and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. Even if such additional information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
The FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy (“REMS”), plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post‑market studies or clinical trials. Such post‑market testing may include Phase 4 clinical trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
Orphan Drug Designation
Under the Orphan Drug Act of 1983, the FDA may grant orphan drug designation to a drug or biological product intended to treat an orphan disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States, or if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making a drug product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan product designation must be requested before submitting an NDA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as demonstrating clinical superiority to the product with orphan exclusivity. The designation of such drug also entitles a party to financial incentives, such as opportunities for grant funding towards clinical trial costs, tax advantages and user‑fee waivers. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan product exclusivity also could block
27
the approval of one of our products for seven years if a competitor obtains approval of the same drug or biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug product designated as an orphan product receives regulatory approval for an indication broader than that for which it is designated, it may not be entitled to orphan product exclusivity. Orphan drug status in the European Union has similar but not identical benefits in that jurisdiction.
Post‑Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product distribution, advertising and promotion, and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval and may require additional clinical trials and NDA submissions. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third‑party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained, or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information, imposition of post‑market studies or clinical trials to assess new safety risks, or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, but are not limited to:
|
· |
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
|
· |
fines, warning letters or holds on post‑approval clinical trials; |
|
· |
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
|
· |
product seizure or detention, or refusal to permit the import or export of products; or |
|
· |
injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off‑label uses, and a company that is found to have improperly promoted off‑label uses may be subject to significant liability.
Moreover, the federal Drug Supply Chain Security Act imposes obligations on manufacturers of pharmaceutical products, among others, related to product tracking and tracing. Among the requirements of this new federal legislation, manufacturers are required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. Further, this legislation requires manufacturers to comply with detailed drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally
28
adulterated products, as well as products that are the subject of fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Foreign Regulation
In order to market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable foreign regulatory authorities before we can commence clinical trials or marketing of the product in foreign countries and jurisdictions. Although many of the issues discussed above with respect to the United States apply similarly in the context of the European Union, the approval process varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Other Healthcare Laws
In addition to FDA restrictions on the marketing of pharmaceutical products, federal and state healthcare laws restrict certain business practices in the biopharmaceutical industry. These laws include, but are not limited to, anti‑kickback, false claims, data privacy and security, and transparency statutes and regulations.
The federal Anti‑Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, purchasing, leasing, arranging for, ordering or recommending any good, facility, item or service for which payment is made, in whole or in part, under Medicare, Medicaid or any other federal healthcare programs. The term “remuneration” has been broadly interpreted to include anything of value, including for example, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash, waivers of payment, ownership interests and providing anything at less than its fair market value. The federal Anti‑Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers and formulary managers on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, the exceptions and safe harbors are drawn narrowly, and our future practices may not in all cases meet all of the criteria for a statutory exception or safe harbor protection. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable regulatory safe harbor does not make the conduct per se illegal under the federal Anti‑Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case‑by‑case basis based on a cumulative review of all of its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare program covered business, the statute has been violated. Additionally, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively, the “PPACA”), amended the intent requirement under the Anti‑Kickback Statute and criminal healthcare fraud statutes (discussed below) such that a person or entity no longer needs to have actual knowledge of the statute or the specific intent to violate it in order to have committed a violation. In addition, PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti‑Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act (discussed below). Due to the breadth of these federal and state anti‑kickback laws, and the potential for additional legal or regulatory change in this area, it is possible that our current and future sales and marketing practices and/or our future relationships with physicians might be challenged under these laws, which could cause harm to us.
The civil monetary penalties statute imposes penalties against any person or entity who, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
29
The federal false claims laws prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, and thus non‑covered, uses.
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, including private third‑party payors, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of, or payment for, healthcare benefits, items or services.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and its implementing regulations, imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates—independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, and newly empowered state attorneys general with the authority to enforce HIPAA. In January 2013, the Office for Civil Rights of the U.S. Department of Health and Human Services issued the Final Omnibus Rule under HIPAA pursuant to HITECH that makes significant changes to the privacy, security, and breach notification requirements and penalties. The Final Omnibus Rule, which generally took effect in September 2013, enhances certain privacy and security protections, and strengthens the government’s ability to enforce HIPAA. The Final Omnibus Rule also enhanced requirements for both covered entities and business associates regarding notification of breaches of unsecured protected health information. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways. These state laws may not have the same effect and often are not preempted by HIPAA, thus complicating compliance efforts.
Additionally, PPACA also included the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Failure to comply with required reporting requirements could subject applicable manufacturers and others to substantial civil money penalties.
Also, many states have similar healthcare statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Certain states require pharmaceutical companies to implement a comprehensive compliance program that includes a limit or outright ban on expenditures for, or payments to, individual medical or health professionals and/or require pharmaceutical companies to track and report gifts and other payments made to physicians and other healthcare providers.
Because we commercialize products that are reimbursed under federal and other governmental healthcare programs, we have developed a comprehensive compliance program that establishes internal controls to facilitate adherence to the rules and healthcare program requirements. Although compliance programs and adherence thereto may mitigate the risk of violation of and subsequent investigation and prosecution for violations of the above laws, the risks cannot be entirely eliminated. If our operations are found to be in violation of any of the health care laws or regulations
30
described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal, civil and/or administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion of products from reimbursement under government programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings and/or the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. To the extent that any of our products are sold in a foreign country, we are subject to similar foreign laws and regulations, which may include, for instance, applicable post‑marketing requirements, including safety surveillance, fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
Pharmaceutical Coverage, Pricing and Reimbursement
In both domestic and foreign markets, our sales depend in part on the availability of coverage and adequate reimbursement from third‑party payors. Third‑party payors include government authorities, managed care providers, private health insurers and other organizations. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third‑party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our commercial products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of the products. Sales of these products therefore depend substantially, both domestically and abroad, on the extent to which the costs of these products are paid by third‑party payors. These third‑party payors are increasingly focused on containing healthcare costs by challenging the price and examining the cost‑effectiveness of medical products and services.
In addition, significant uncertainty exists as to the coverage and reimbursement status of newly approved healthcare product candidates. The market for our product candidates for which we may receive regulatory approval will depend significantly on access to third‑party payors’ drug formularies, or lists of medications for which third‑party payors provide coverage and reimbursement. The industry competition to be included in such formularies often leads to downward pricing pressures on pharmaceutical companies. Also, third‑party payors may refuse to include a particular branded drug in their formularies or otherwise restrict patient access to a branded drug when a less costly generic equivalent or other alternative is available. Furthermore, third‑party payor reimbursement to providers for our product candidates may be subject to a bundled payment that also includes the procedure administering our products. To the extent there is no separate payment for our product candidates, there may be further uncertainty as to the adequacy of reimbursement amounts. Because each third‑party payor individually approves coverage and reimbursement levels, obtaining coverage and adequate reimbursement is a time consuming, costly and sometimes unpredictable process. We may be required to provide scientific and clinical support for the use of any product to each third‑party payor separately with no assurance that approval would be obtained, and we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost‑effectiveness and/or medical necessity of our products. This process could delay the market acceptance of any product and could have a negative effect on our future revenues and operating results. We cannot be certain that our product candidates will be considered cost‑effective or medically necessary. Because coverage and reimbursement determinations are made on a payor‑by‑payor basis, obtaining acceptable coverage and reimbursement from one payor does not guarantee the Company will obtain similar acceptable coverage or reimbursement from another payor. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. If we are unable to obtain coverage of, and adequate reimbursement and payment levels for, our product candidates from third‑party payors, physicians may limit how much or under what circumstances they will prescribe or administer them and patients may decline to purchase them. This in turn could affect our ability to successfully commercialize our products and impact our profitability, results of operations, financial condition and future success.
Furthermore, in many foreign countries, particularly the countries of the European Union, the pricing of prescription drugs is subject to government control. In some non‑U.S. jurisdictions, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. We may face competition for our product candidates from lower‑priced products in foreign countries that have placed price
31
controls on pharmaceutical products. In addition, there may be importation of foreign products that compete with our own products, which could negatively impact our profitability.
Healthcare Reform
In the United States and foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system that could affect our current and future business and operations.
In particular, there have been and continue to be a number of initiatives at the U.S. federal and state level that seek to reduce healthcare costs and pharmaceutical costs. Initiatives to reduce the federal deficit and to reform healthcare delivery are increasing cost‑containment efforts. We anticipate that Congress, state legislatures and the private sector will continue to review and assess alternative controls on healthcare spending through limitations on the growth of private health insurance premiums and Medicare and Medicaid spending, the creation of large insurance purchasing groups, price controls on pharmaceuticals and other fundamental changes to the healthcare delivery system. Any proposed or actual changes could limit or eliminate our spending on development projects and affect our ultimate profitability.
In March 2010, PPACA was signed into law. PPACA has substantially changed the way healthcare is financed by both governmental and private insurers. PPACA, among other things: established an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; revised the methodology by which rebates owed by manufacturers for covered outpatient drugs under the Medicaid Drug Rebate Program are calculated; increased the statutory minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program; addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected; extended the Medicaid Drug Rebate Program to prescriptions of individuals enrolled in Medicaid managed care organizations; required manufacturers to offer 50% point‑of‑sale discounts on negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models. For years the U.S. Congress has been assessing new legislation designed to repeal and replace core sections of the PPACA. The Trump Administration has also taken executive actions to undermine or delay implementation of the PPACA. In January 2017, the President signed an Executive Order directing applicable federal agencies to waive, defer, grant exemptions from, or delay the implementation of any provision of the PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices.
Members of the United States Congress and the Trump administration have expressed an intent to pass legislation or adopt executive orders to fundamentally change or repeal parts of the PPACA.
Since January 2017, President Trump has signed two Executive Orders and other directives designed to delay the implementation of certain provisions of PPACA or otherwise circumvent some of the requirements for health insurance mandated by PPACA. On October 13, 2017, an Executive Order was signed terminating the cost sharing subsidies that reimburse insurers under the PPACA. Several state Attorneys Generals filed suit to stop the administration from terminating the subsidies, but their request for a restraining order was denied by a federal judge in California on October 25, 2017. Further, on June 14, 2018 the United States Court of Appeals for the Federal Circuit ruled that the federal government was not required to pay more than $12.0 billion in PPACA risk corridor payments to third-party payors. The effects of this gap in reimbursement on third-party payors, the viability of the PPACA marketplace, providers, and our business, are not yet known.
Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the PPACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under PPACA have been signed into law. On December 22, 2017, for instance, the President signed into
32
law the Tax Cuts and Jobs Act of 2017 (the “Tax Act”), which repealed the “individual mandate” of the PPACA. The repeal of the individual mandate is expected to cause millions fewer Americans to be insured in 2027 and premiums in insurance markets may rise. Further, on December 14, 2018, a U.S. District Court Judge in the Northern District of Texas ruled that the individual mandate is a critical and inseverable feature of PPACA, and therefore, because it was repealed as part of the Tax Cuts and Jobs Act, the remaining provisions of PPACA are invalid as well. While this judge, as well as the Trump Administration and CMS, have stated that the ruling will have no immediate effect, it is unclear how this judicial decision, subsequent appeals, and other efforts to repeal and replace PPACA will impact PPACA and our business.
In addition, CMS has recently proposed regulations that would give states greater flexibility in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the PPACA for plans sold through such marketplaces. The Bipartisan Budget Act of 2018, or the BBA, among other things, amends the PPACA, effective January 1, 2019, to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.” In July 2018, the CMS published a final rule permitting further collections and payments to and from certain PPACA qualified health plans and health insurance issuers under the PPACA risk adjustment program in response to the outcome of federal district court litigation regarding the method CMS uses to determine this risk adjustment. Moreover, CMS issued a final rule in 2018 that will give states greater flexibility, starting in 2020, in setting benchmarks for insurers in the individual and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the PPACA for plans sold through such marketplaces. Further, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which have resulted in several Congressional inquiries and proposed and enacted federal and state legislation, designed to, among other things, bring more transparency to product pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump Administration’s budget proposal for fiscal year 2019 contains further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients.
At the state level, individual states in the United States are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
In the future, there may continue to be additional proposals relating to the reform of the U.S. healthcare system, some of which could further limit the prices we will be able to charge for our product candidates, or the amounts of reimbursement available for our product candidates. If future legislation were to impose direct governmental price controls and access restrictions, it could have a significant adverse impact on our business. Managed care organizations, as well as Medicaid and other government agencies, continue to seek price discounts. Some states have implemented, and other states are considering, measures to reduce costs of the Medicaid program, and some states are considering implementing measures that would apply to broader segments of their populations that are not Medicaid‑eligible. Due to the volatility in the current economic and market dynamics, we are unable to predict the impact of any unforeseen or unknown legislative, regulatory, payor or policy actions, which may include cost containment and healthcare reform measures. Such policy actions could have a material adverse impact on our profitability.
33
Segment and Geographical Information
Information on our total revenues by product attributed to customers who represented at least 10% of our total revenues in each of 2019 and 2018, is included in Note 15 to our consolidated financial statements.
The following table represents total long-lived assets by location (in thousands):
|
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
United States |
|
$ |
291 |
|
$ |
294 |
|
Total long-lived assets (1) |
|
$ |
291 |
|
$ |
294 |
|
(1) |
Long-lived assets consist of property and equipment. |
Employees
As of December 31, 2019, we had 71 full‑time employees, working in the United States or Ireland. Of these full‑time employees, 27 were engaged in research and development, 30 were engaged in commercial activities including sales, marketing and market access, and 14 were engaged in other general and administrative activities.
Corporate Information
Strongbridge Biopharma plc, an Irish public limited company, was established on May 26, 2015 under the name Cortendo plc.
Our principal office in Ireland is located at Suite 206, Fitzwilliam Hall, Fitzwilliam Place, Dublin 2, D02 T292, Ireland. Our principal executive office is located at 900 Northbrook Drive, Suite 200, Trevose, Pennsylvania, 19053, USA, and our telephone number is +1 610‑254‑9200
Our website is www.strongbridgebio.com. The information on, or that can be accessed through, our website is not part of and should not be incorporated by reference into this Annual Report.
Available Information
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, beneficial ownership reports on Forms 3, 4 and 5 and proxy statements, as well as all amendments to those reports are available free of charge through our investor relations website at www.strongbridgebio.com, as soon as reasonably practicable after such reports are electronically filed with, or furnished to, the Securities and Exchange Commission (“SEC”). The information found on our website is not part of this or any other report that we file with or furnish to the SEC. Our SEC filings are also available to the public over the Internet at the SEC’s website at www.sec.gov.
34
Certain factors may have a material adverse effect on our business, financial condition and results of operations. You should carefully consider the risks and uncertainties described below, in addition to other information contained in this Annual Report, including our consolidated financial statements and related notes. Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occurs and, as a result, the market price of our ordinary shares could decline and you could lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may have similar adverse effects on us.
Risks Related to Our Business, Industry and Operations
We have generated only limited revenues from product sales to date, we have a history of net losses and negative cash flows, and we may never achieve or maintain profitability.
Until we acquired the U.S. marketing rights to Keveyis, in December 2016, we were a development-stage biopharmaceutical company. We have a limited operating history and have not yet demonstrated an ability to obtain regulatory approval for, or manufacture and commercialize, a product candidate.
Since inception, we have incurred significant operating losses. We have devoted substantially all of our financial resources to identifying, in-licensing, acquiring and developing our product candidates, conducting clinical trials, commercializing Keveyis, which we launched in April 2017, and Macrilen, our second commercial product, which we launched in July 2018 and subsequently sold the rights to in December 2018, and providing general and administrative support for these operations.
To date, we have financed our operations primarily through private placements of equity securities, the proceeds from our initial public offering of ordinary shares in the United States in October 2015 and subsequent follow-on public offerings, our at-the-market facility, debt financings and our sale of our subsidiary, Strongbridge Ireland Limited, which owned the rights to Macrilen.
Our ability to achieve and maintain profitability in the future will depend on our ability to obtain regulatory approval for our product candidates and to generate sufficient revenues from product sales and royalties.
Our future revenue will be dependent, in part, upon the size of the markets in the territories for which our products receive regulatory approval, the accepted prices for our products, the ability to obtain coverage and adequate reimbursement, and whether we own the commercial rights for that territory. If the number of our addressable patients is not as significant as we estimate, the indication approved by regulatory authorities is narrower than we expect, or the treatment population is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenue from our products.
Furthermore, we anticipate that our expenses may continue to increase as we:
|
· |
change or add manufacturers or suppliers; |
|
· |
seek regulatory approvals for our product candidates that successfully complete clinical trials; |
|
· |
seek to maintain, protect and expand our intellectual property portfolio; |
|
· |
seek to attract and retain skilled personnel; |
|
· |
create additional infrastructure to support our commercialization efforts; |
|
· |
experience any delays or encounter issues with any of the above, including, but not limited to, failed preclinical studies or clinical trials, complex results, safety issues or other regulatory challenges that may require either longer follow-up of existing preclinical studies or clinical trials or limitation of additional preclinical studies or clinical trials in order to pursue regulatory approval; |
35
|
· |
make up-front, milestone or other payments under any asset acquisition, supply, or license arrangements; and |
|
· |
seek to identify, assess, in-license, acquire and develop additional product candidates. |
The net losses we incur before achieving profitability may fluctuate significantly from quarter-to-quarter and year-to-year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. Moreover, if we incur substantial losses, we could be liquidated, and the value of our shares might be significantly reduced or the shares might be of no value.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to successfully execute on our business strategy would decrease the value of our company and could impair our ability to raise capital, expand our business or continue our operations. A decline in the value of our company could cause you to lose all or part of your investment.
We are highly dependent on our key personnel, including our executive chairman, chief operating officer, chief financial officer and chief medical officer, as well as our ability to recruit, retain and motivate additional qualified personnel.
We are highly dependent on John Johnson, our Executive Chairman, Richard Kollender, our Chief Operating Officer, Robert Lutz, our Chief Financial Officer, and Dr. Fredric Cohen, our Chief Medical Officer, as well as certain other executive officers. Any member of management or employee can terminate his or her relationship with us at any time. Although we have included non-compete provisions in their respective employment agreements such arrangements might not be sufficient for the purpose of preventing such key personnel from entering into agreements with any of our competitors. The inability to recruit and retain qualified personnel, or the loss of Messrs. Johnson, Kollender or Lutz, Dr. Cohen or certain other executive officers, could result in competitive harm as we could experience delays in reaching our in-licensing, acquisition, development and commercialization objectives.
We also depend substantially on highly qualified managerial, sales and technical personnel who are difficult to hire and retain. There is currently a shortage of skilled personnel in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill sets. In addition, failure to succeed in preclinical studies or clinical trials may make it more challenging to recruit and retain qualified personnel. Recruiting and retaining other qualified employees, consultants and advisors for our business, including scientific and technical personnel, will be critical to our success.
If we are unable to effectively build, train and equip our sales force, our ability to successfully commercialize Keveyis, and any other products we acquire or for which we receive regulatory approval will be harmed.
Prior to our launch of Keveyis in April 2017, we had no experience commercializing products on our own. In order to successfully commercialize Keveyis, and any other products we acquire or for which we receive regulatory approval, we must maintain our current sales, marketing, distribution, managerial and other non-technical capabilities. The continued maintenance and/or potential expansion of our commercial organization will be expensive and time-consuming, and our resources may be limited compared to some of our competitors.
We have expended significant time and resources to train our sales force to be effective in their sales efforts for Keveyis. However, we have and are likely to continue to experience turnover of the sales representatives that we have hired or will hire, requiring us to train new sales representatives.
If we are unable to effectively train our sales force and equip them with effective materials, including medical and sales literature to help them inform and educate physicians and other potential customers about the benefits of Keveyis and any other products we acquire or for which we receive regulatory approval, and their proper administration and label indication, as well as any associated patient access programs, our efforts to successfully commercialize
36
products could be put in jeopardy, which could have a material adverse effect on our financial condition, share price and operations.
Future revenues from product sales and/or royalties may be lower than expected.
Efforts to educate the medical community and third-party payors on the benefits of Keveyis and any other product candidates for which we receive regulatory approval may require significant resources and may not be successful. If the products we promote do not achieve an adequate level of market acceptance, we may not generate significant revenues from product sales and/or royalties or any profits from operations. The degree of market acceptance of Keveyis and any product candidates for which we receive regulatory approval will depend on a variety of factors, including, but not limited to:
|
· |
whether clinicians and potential patients perceive the products or product candidates to have better efficacy, safety and tolerability profile, and ease of use compared with alternative therapies; |
|
· |
the timing of market introduction; |
|
· |
the number and quality of current and future competing products; |
|
· |
our ability to provide acceptable evidence of safety and efficacy; |
|
· |
the prevalence and severity of any side effects; |
|
· |
relative convenience and ease of administration; |
|
· |
cost‑effectiveness; |
|
· |
patient diagnostics and screening infrastructure in each market; |
|
· |
marketing and distribution support; and |
|
· |
availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third‑party payors, both public and private. |
In addition, the potential market opportunity for any pharmaceutical product is difficult to estimate precisely. Our estimates of the potential market opportunity for Keveyis and our two product candidates are predicated on several key assumptions derived from industry knowledge and publications, third-party research reports and other surveys. While we believe that our internal assumptions are reasonable, these assumptions may be inaccurate. If any of our assumptions proves to be inaccurate, then the actual market for these products and product candidates, if approved, could be smaller than our estimates of the potential market opportunity. If the actual markets for these products and product candidates, if approved, are smaller than we expect, or if they fail to achieve an adequate level of acceptance by physicians, health care payors and patients, the revenue we recognize from product sales and/or royalties may be limited and we may be unable to achieve or maintain profitability. Further, given the limited number of treating physicians, if we are unable to convince a significant number of such physicians of the value of these products, we may be unable to achieve a sufficient market share to make our products profitable.
We operate in a highly competitive and rapidly changing industry, which may result in competitors discovering, developing or commercializing competing products before or more successfully than we do, or our entering a market in which a competitor has commercialized an established competing product, and we may not be successful in competing with them.
The development and commercialization of pharmaceutical products is highly competitive and subject to significant and rapid technological change. Our success is highly dependent upon our ability to in-license, acquire, develop and obtain regulatory approval for new and innovative drug products on a cost-effective basis and to market them successfully. In doing so, we face and will continue to face intense competition from a variety of businesses, including large, fully integrated, well-established pharmaceutical companies who already possess a large share of the
37
market, specialty pharmaceutical companies and biopharmaceutical companies, academic institutions, government agencies and other private and public research institutions in Europe, the United States and other jurisdictions. In addition, for so long as we are entitled to receive royalties from Novo from their Macrilen sales, our success will depend in part on Novo’s ability to compete in this challenging environment.
We anticipate this competition to increase in the future as new companies enter the neuromuscular, endocrinology and rare diseases markets. In addition, the health care industry is characterized by rapid technological change, and new product introductions or other technological advancements could make some or all of our products obsolete.
The highly competitive nature of and rapid technological changes in the biotechnology and pharmaceutical industries could render our products and product candidates and/or Macrilen, obsolete or non-competitive. Our competitors may, among other things:
|
· |
have similar or better product candidates or technologies; |
|
· |
possess greater financial and human resources as well as supporting clinical data; |
|
· |
develop and commercialize products that are safer, more effective, less expensive, or more convenient or easier to administer; |
|
· |
obtain regulatory approval more quickly; |
|
· |
establish superior proprietary positions; |
|
· |
have access to greater manufacturing capacity; |
|
· |
seek patent protection that competes with our product candidates; |
|
· |
implement more effective approaches to sales and marketing; or |
|
· |
enter into more advantageous collaborative arrangements for research, development, manufacturing and marketing of products. |
Additional competitors could enter the market with generic versions of our products, which may result in a decline in sales of affected products.
Under the Hatch-Waxman Act, a pharmaceutical manufacturer may file an abbreviated new drug application (“ANDA”), seeking approval of a generic copy of an approved innovator product. Under the Hatch-Waxman Act, a manufacturer may also submit an NDA under section 505(b)(2) that references the FDA's prior approval of the innovator product. A 505(b)(2) NDA product may be for a new or improved version of the original innovator product. Hatch-Waxman also provides for certain periods of regulatory exclusivity, which preclude FDA approval, or, in some circumstances, FDA filing and reviewing, of an ANDA or 505(b)(2) NDA. These include, subject to certain exceptions, the period during which an FDA-approved drug is subject to orphan drug exclusivity.
We will rely on orphan drug exclusivity in the marketing and sales of Keveyis and intend to rely on orphan drug exclusivity in the marketing and sale of Recorlev, if approved. In addition to the benefits of regulatory exclusivity, an innovator NDA holder may have patents claiming the active ingredient, method of use, product formulation or an approved use of the drug, which would be listed with the product in the FDA publication, "Approved Drug Products with Therapeutic Equivalence Evaluations," known as the "Orange Book." If there are patents listed in the Orange Book, a 505(j) or 505(b)(2) applicant that seeks to market its product via an ANDA before expiration of the patents must include in the ANDA what is known as a "Paragraph IV certification," challenging the validity or enforceability of, or claiming non-infringement of, the listed patent or patents. Notice of the certification must be given to the innovator, too, and if within 45 days of receiving notice the innovator sues to protect its patents, final approval of the ANDA is stayed for 30 months, or as lengthened or shortened by the court.
38
Accordingly, with respect to Keveyis, and if Recorlev or any of our other product candidates is approved, competitors could file ANDAs for generic versions of our product candidates, or 505(b)(2) NDAs that reference our product candidates, respectively. If there are patents listed for our products or product candidates in the Orange Book, those ANDAs and 505(b)(2) NDAs would be required to include a certification as to each listed patent indicating whether the ANDA applicant does or does not intend to challenge the patent. We cannot predict whether any patents issuing from our pending patent applications will be eligible for listing in the Orange Book, how any generic competitor would address such patents, whether we would sue on any such patents or the outcome of any such suit.
We may not be successful in securing or maintaining proprietary patent protection for products and technologies we develop or license. Moreover, if any patents that are granted and listed in the Orange Book are successfully challenged by way of a Paragraph IV certification and subsequent litigation, the affected product could immediately face generic competition and its sales would likely decline rapidly and materially. Should sales decline, we may have to write off a portion or all of the intangible assets associated with the affected product and our ability to generate revenue could be compromised. In addition, for so long as we are entitled to receive royalties from Novo Nordisk, any challenges faced in maintaining the proprietary patent protection associated with Macrilen that have a negative impact on Novo’s product sales will have a negative effect on us.
We expect that we will need additional funding in order to complete the development of veldoreotide and to commercialize our two product candidates if they are ultimately approved by the FDA, EMA or any comparable foreign regulatory agency.
We are currently advancing two product candidates through clinical development, Recorlev and veldoreotide. While we expect that our costs associated with the clinical development of Recorlev will decrease as we complete the associated clinical trials, we expect that we will require additional capital to complete the clinical development of veldoreotide and will require further funding to commercialize Recorlev and/or veldoreotide if either or both of them are ultimately approved for marketing by the FDA, EMA or any comparable foreign regulatory agency. Our future funding requirements will depend on many factors, including, but not limited to:
|
· |
the amount and timing of revenue that we receive from Keveyis sales and Macrilen royalties and the sales from any product candidates that are approved for marketing; |
|
· |
the costs of expanding our sales, marketing, distribution and administrative capabilities; |
|
· |
the scope, rate of progress, results and cost of our clinical trials, nonclinical testing, and other related activities; |
|
· |
the cost of formulation, process development, manufacturing of clinical supplies, and establishing commercial supplies of our product candidates and any other product candidates that we may develop, in-license or acquire; |
|
· |
the timing of any regulatory approvals; |
|
· |
the number and characteristics of product candidates that we pursue, including any additional product candidates we may in-license or acquire; |
|
· |
the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; |
|
· |
the cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us or our product candidates; |
|
· |
the cost, timing and outcomes of regulatory approvals; |
|
· |
the terms and timing of any collaborative, licensing and other arrangements that we may establish, including any required milestone and royalty payments thereunder; and |
39
|
· |
the emergence of competing technologies and their achieving commercial success before we do or other adverse market developments; and |
|
· |
the price we can charge for current and future products in the United States and globally, and the amount of third party reimbursement we can secure, and any sales deductions required. |
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of product revenue, royalty income, equity offerings, debt financings, grants, and license and development agreements in connection with any collaborations. We do not have any committed external source of funds. In the event we seek additional funds, we may raise additional capital through the sale of equity or convertible debt securities. In such an event, the ownership interests of our current shareholders will be diluted, and the terms of these securities may include liquidation or other preferences that would adversely affect their rights as shareholders. Debt financing, if available, could result in increased fixed payment obligations and may involve agreements that include restrictive covenants, such as limitations on our ability to incur additional debt, make capital expenditures, acquire, sell or license intellectual property rights or declare dividends, and other operating restrictions that could hurt our ability to conduct our business. Further, if we raise additional funds through collaborations, strategic alliances, or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our intellectual property or future revenue streams. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may compromise our ability to develop and commercialize our product candidates, if approved. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares to decline.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product or product candidate that is approved, or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired.
We have no manufacturing capabilities and currently depend on one supplier to manufacture Keveyis. We also depend on a limited number of other suppliers to manufacture our product candidates for use in clinical trials. If these suppliers are unable or unwilling to continue manufacturing for us and we are unable to contract quickly with alternative sources our business will be harmed.
Taro produces all of our requirements for Keveyis. We rely on other third-parties to manufacture our product candidates for use in clinical trials. If any of these vendors is unable or unwilling to meet our future requirements, we may not be able to manufacture our products in a timely manner. Our current arrangements with these manufacturers are terminable by such manufacturers, subject to certain notice provisions.
We may not be successful in executing our research programs or business development efforts.
Research programs and business development efforts to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful. Our research programs, business development efforts or licensing attempts may fail to yield additional complementary or successful product candidates for clinical development and commercialization for a number of reasons, including, but not limited to, the following:
|
· |
our research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates with a high probability of success for development progression; |
40
|
· |
we may not be able or willing to assemble sufficient resources or expertise to in‑license, acquire or discover additional product candidates; |
|
· |
we may not be able to agree to acceptable terms with the licensor or owner of any product candidates we seek to in-license or acquire; |
|
· |
our product candidates may not succeed in preclinical studies or clinical trials; |
|
· |
we may not succeed in formulation or process development; |
|
· |
our product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive regulatory approval; |
|
· |
competitors may develop alternatives that render our product candidates obsolete or less attractive; |
|
· |
product candidates that we develop may be covered by third parties’ patents or other exclusive rights; |
|
· |
product candidates that we develop may not allow us to leverage our expertise and our development and commercial infrastructure as currently expected; |
|
· |
the market for a product candidate may change during our program so that such a product may become unreasonable to continue to develop; |
|
· |
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
|
· |
a product candidate may not be accepted as safe and effective by patients, the medical community or third‑party payors. |
If any of these events occurs, we may not be successful in executing our growth strategy or our growth strategy may not deliver the anticipated results.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
We have limited financial and managerial resources. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
If we acquire other businesses or in-license or acquire other product candidates and are unable to integrate them successfully, our financial performance could suffer.
If we are presented with appropriate opportunities, we may acquire other businesses or product candidates. The integration of new businesses and/or product candidates may produce unforeseen operating difficulties and expenditures, and may absorb significant management attention that would otherwise be directed to the ongoing development of our business. Also, in any future in-licensing or acquisition transactions, we may issue shares of stock that would result in dilution to existing shareholders, incur debt, assume contingent liabilities or create additional expenses related to amortizing intangible assets, any of which might harm our financial results and cause our stock price to decline. Any financing we might need for future transactions may be available to us only on terms that restrict our business or impose costs that reduce our net income.
41
Our business and operations would suffer in the event of system failures.
Our computer systems, as well as those of our clinical research organizations (“CROs”), and other contractors and consultants, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, including hurricanes, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of preclinical study or clinical trial data from completed, ongoing or planned preclinical studies or clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
Risks Related to Our Clinical Trials, Government Regulation and Legal Proceedings
Clinical trials are very expensive, time consuming and difficult to design and implement, and involve uncertain outcomes. Furthermore, results of earlier preclinical studies and clinical trials may not be predictive of results of future preclinical studies or clinical trials.
To obtain the requisite regulatory approvals to market and sell any of our product candidates, we must demonstrate through extensive preclinical studies and clinical trials that our product candidates are safe and effective in humans. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and earlier clinical trials may not be predictive of the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials.
In some instances, there can be significant variability in safety or efficacy results between earlier and later stages of clinical trials of the same product candidate. These discrepancies may be due to a number of factors, including changes in clinical trial procedures set forth in protocols, differences in the size and type of the patient populations, adherence to the administration regimen and other clinical trial protocols, and the rate of dropout among clinical trial participants.
In the case of our two product candidates results from any Phase 3 clinical trials may differ from earlier results due to the larger number of patients, clinical trial sites and additional countries involved in our Phase 3 clinical trials. Furthermore, different countries have different standards of care and different levels of access to care for patients, which in part drives the heterogeneity of the patient populations that enroll in our studies. In August 2018, we announced statistically significant positive top-line results from our SONICS Phase 3 clinical trial for Recorlev. However, there can be no assurances that the final results will be positive or that the results from our LOGICS trial, which is expected at the end of second quarter or during the third quarter 2020, will be positive.
In addition, because we were not involved in and had no control over the preclinical and clinical development of veldoreotide prior to our acquisition of this product candidate in June 2015, we are dependent on the prior research and development of veldoreotide having been conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, the accuracy of reported results of all clinical trials conducted prior to our acquisition, and the correct interpretation of collected data from these clinical trials. If any of these assumptions prove to be incorrect, we could experience increased costs and delays in the development of veldoreotide, which could hurt our ability to generate future revenues from this product candidate.
We may experience delays in our ongoing or future preclinical studies or clinical trials, and we do not know whether future preclinical studies or clinical trials will begin on time, need to be redesigned, enroll an adequate number
42
of subjects or patients on time or be completed on schedule, if at all. Clinical trials may be delayed, suspended or terminated for a variety of reasons, including delay or failure to:
|
· |
obtain authorization from regulators or IRBs to commence a clinical trial at a prospective clinical trial site; |
|
· |
reach agreements on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites; |
|
· |
recruit and enroll a sufficient number of patients in clinical trials to ensure adequate statistical power to detect statistically significant treatment effects; |
|
· |
address any noncompliance with regulatory requirements or safety concerns that arise during the course of a clinical trial; |
|
· |
have patients complete clinical trials or return for post‑treatment follow‑up; |
|
· |
have CROs or other third parties comply with regulatory requirements, adhere to the trial protocol or meet contractual obligations in a timely manner or at all; |
|
· |
identify a sufficient number of clinical trial sites and initiate them within the planned timelines; and |
|
· |
manufacture sufficient quantities of the product candidate to complete clinical trials. |
In addition, we are currently conducting clinical trials in a number of countries outside the United States. Unforeseen global instability, including political instability, or instability from an outbreak of pandemic or contagious disease, such as the novel coronavirus, in or around the countries in which we conduct our clinical trials, could affect our ability to enroll patients in our clinical trials in these countries, prevent patients already enrolled from completing our clinical trials, and/or cause other trial delays or otherwise adversely impact our clinical trials.
We may find it difficult to enroll patients in our clinical trials given the limited number of patients who have the diseases for the treatment of which our product candidates are being studied. Difficulty in enrolling patients in our clinical trials could delay or prevent clinical trials of our product candidates.
Successful and timely completion of clinical trials will require that we enroll a sufficient number of patient candidates. Clinical trials may be subject to delays as a result of patient enrollment taking longer than anticipated or patient withdrawal. Patient enrollment depends on many factors, including the size and nature of the patient population, eligibility criteria for the clinical trial, the proximity of patients to clinical sites, the design of the clinical protocol, the availability of competing clinical trials, the availability of new drugs approved for the indication the clinical trial is investigating, and clinicians' and patients' perceptions as to the safety and potential advantages of the product candidate being studied in relation to other available therapies.
Because we are focused on addressing rare diseases, there are limited patient pools from which to draw in order to complete our clinical trials in a timely and cost-effective manner. Delays in the completion of any clinical trial of our product candidates will increase our costs, slow down our product candidate development and approval process, and delay or potentially jeopardize our ability to commence product sales and generate revenue. In addition, many of the factors that may lead to a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
43
We rely on third parties to conduct our nonclinical and clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or comply with regulatory requirements, we may be exposed to sub-optimal quality and reputational harm, we may not be able to obtain regulatory approval for or commercialize our product candidates, and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third parties, including CROs, collaborative partners, and independent investigators to analyze, collect, monitor, and manage data for our ongoing nonclinical and clinical programs. We rely on third parties for execution of our nonclinical and clinical studies, and for estimates regarding costs and efforts completed, and we control only certain aspects of their activities. We are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory, and scientific standards, and our reliance on the CROs and other third parties does not relieve us of our regulatory responsibilities. We and our CROs and other vendors and partners are required to comply with GMP, GCP, and GLP, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, and comparable foreign regulatory authorities for all of our product candidates in development. Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators, study sites, and other contractors. If we or any of our CROs or other vendors and partners, including the sites at which clinical studies are conducted, fail to comply with applicable regulations, the data generated in our nonclinical and clinical studies may be deemed unreliable and the FDA, EMA, or comparable foreign regulatory authorities may deny approval and/or require us to perform additional nonclinical and clinical studies before approving our marketing applications, which would delay the approval process. We cannot make assurances that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical studies comply with GCP regulations or that nonclinical studies comply with GLP regulations. In addition, our clinical studies must be conducted with products produced under GMP regulations. If the regulatory authorities determine that we have failed to comply with GLP, GMP, or GCP regulations, they may deny approval of our product candidates and/or we may be required to repeat clinical or nonclinical studies, which would delay the regulatory approval process.
Our CROs and other vendors and partners are not our employees, and except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our on-going nonclinical and clinical programs. If our vendors and partners do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements, or for other reasons, our clinical studies may be extended, delayed, or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. CROs and other vendors and partners may also generate higher costs than anticipated as a result of changes in scope of work or otherwise. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If any of our relationships with these third parties terminate, we may not be able to enter into arrangements with alternative vendors or do so on commercially reasonable terms. Switching or adding additional vendors involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new vendor commences work. As a result, delays may occur, which can materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our vendors and partners, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition, and business prospects.
The regulatory approval process of the FDA, EMA or any comparable foreign regulatory agency may be lengthy, time consuming and unpredictable.
We cannot be certain that any of our product candidates will be successful in clinical trials or receive regulatory approval. The FDA, EMA and other comparable foreign regulatory agencies have substantial discretion in the approval process and in determining when or whether regulatory approval will be obtained for any of our product candidates. Even if we believe the data collected from clinical trials of our product candidates are promising, such data may not be sufficient to support approval by the FDA, EMA or any comparable foreign regulatory agency. Many companies that
44
believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain regulatory approval for the product candidates.
Furthermore, while certain of our employees have prior experience with submitting marketing applications to the FDA, EMA and comparable foreign regulatory agencies, we, as a company, have not submitted such applications for our product candidates. Applications for any of our product candidates could fail to receive regulatory approval for many reasons, including, but not limited to, the following:
|
· |
the FDA, EMA or any comparable foreign regulatory agency may disagree with the design or implementation of our clinical trials or our interpretation of data from nonclinical trials or clinical trials; |
|
· |
the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval, including reliance on foreign clinical data; |
|
· |
the data collected from clinical trials of our product candidates may not be sufficient to support a finding that has statistical significance or clinical meaningfulness or support the submission of an NDA or other submission, or to obtain regulatory approval in the United States or elsewhere; |
|
· |
we may be unable to demonstrate to the FDA, EMA or any comparable foreign regulatory agency that a product candidate’s risk‑benefit ratio for its proposed indication is acceptable; |
|
· |
the FDA, EMA or any comparable foreign regulatory agency may fail to approve the manufacturing processes, test procedures and specifications or facilities of third‑party manufacturers with which we contract for clinical and commercial supplies; and |
|
· |
the approval policies or regulations of the FDA, EMA or any comparable foreign regulatory agency may significantly change in a manner rendering our clinical data insufficient for approval. |
In communications we had with the FDA, they recommended use of a concurrent control group in our SONICS Phase 3 clinical trial. However, SONICS utilizes an open-label, single-arm design because use of a placebo control in a parallel-arm monotherapy design was considered unethical or infeasible to enroll, depending on the specific country or clinical trial site under consideration. Studies lacking an active control group are more likely to be subject to unanticipated variability in study results that can potentially lead to flawed conclusions because they do not allow for discrimination of patient outcomes. In August 2018, we announced statistically significant positive top-line results from our SONICS Phase 3 clinical trial. However, even if we achieve the clinical trial’s endpoints for this clinical trial, the FDA or other regulatory authorities could view our study results as potentially biased due to our lack of an active control group.
In March 2019, we conducted a Type C meeting with the Division of Metabolic and Endocrine Products (DMEP) of the FDA. The DMEP stated in its meeting minutes that the FDA generally requests that a sponsor conduct two adequate and well-controlled clinical studies for the proposed indication of a drug candidate under 21 CFR 314.126(b)(2). The DMEP also noted that the FDA recognizes situations when a single trial may be sufficient. The DMEP reiterated that the characteristics of an “adequate and well-controlled” investigation under 21 CFR 314.126 include the use of a control group (e.g., placebo concurrent control, dose-comparison concurrent control), randomization and evaluation of primary endpoints that directly measure clinical benefits, or supported by evidence of clinical benefit. For this reason, while the DMEP indicated that it would consider, as a review issue, the adequacy of a New Drug Application (NDA) submission with data from our SONICS trial as the sole Phase 3 evidence supporting the efficacy of RECORLEV, the DMEP nonetheless recommended that we complete a second trial and include the results from that trial in addition to data from the SONICS trial in our NDA submission.
Our LOGICS study is a second Phase 3 clinical trial of Recorlev for the treatment of endogenous Cushing’s syndrome. The LOGICS trial is intended to supplement the long-term efficacy and safety data from our SONICS trial via a double-blind, placebo-controlled, randomized-withdrawal design that targets approximately 46-54 patients for enrollment into the randomized withdrawal phase of the trial. The final number of patients enrolled into the randomized withdrawal phase will depend on the observed rate of early discontinuation in such phase. We currently expect to
45
receive LOGICS top-line data at the end of second quarter or during the third quarter 2020. The addition of a concurrent control group in LOGICS is an attempt to address FDA’s request for such a control group that was lacking in SONICS.
We currently expect, if supported by the data, to submit an NDA for Recorlev approximately six months after reporting top-line results from the LOGICS trial that will include data from each of the SONICS and LOGICS trials. The DMEP stated in its meeting minutes that our clinical pharmacology program for Recorlev, as described to them, appears reasonable to support an NDA filing for Recorlev provided that the data generated are found to be suitable.
In addition, following FDA consultation, we have determined that the 505(b)(2) approval pathway, which permits an NDA applicant to rely on data from studies that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference, is the appropriate pathway for a Recorlev NDA. We intend to rely on published literature and the FDA’s prior findings concerning the safety and/or effectiveness of ketoconazole in our NDA for Recorlev and on similar processes in other jurisdictions. There can be no assurances, however, that the 505(b)(2) approval pathway in the United States, or similar approval pathways outside of the United States, will be available for Recorlev or that the FDA or other regulatory authorities will approve Recorlev through an application based on such pathways.
We generally plan to seek regulatory approval to commercialize our product candidates in the United States, the European Union and other key global markets. To obtain regulatory approval in other countries, we must comply with regulatory requirements of such other countries regarding safety, efficacy, chemistry, manufacturing and controls, clinical trials, commercial sales, pricing and distribution of our product candidates. Even if we are successful in obtaining approval in one jurisdiction, we cannot ensure that we will obtain approval in any other jurisdictions. Failure to obtain marketing authorization for our product candidates in any jurisdiction will result in our being unable to market and sell such products. Similarly, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
Any of our current or future product candidates could take a significantly longer time to gain regulatory approval than we expect or may never gain regulatory approval. This could delay or eliminate any potential product revenue by delaying or terminating the potential commercialization of our product candidates.
Physicians may accept Keveyis and/or Macrilen slowly or may never accept them, which would adversely affect our financial results.
Physicians will prescribe Keveyis only if they determine, based on experience, clinical data, side effect profiles and other factors, that it is preferable to other treatments, even if those products are not approved for PPP. Because PPP is rare, most physicians are inexperienced in the care of patients with the illness and it may be difficult to persuade them to prescribe Keveyis. Other factors that may affect the commercial success of Keveyis include:
|
· |
the preference of some physicians for more familiar, long-standing, off-label treatments for PPP, such as acetazolamide; |
|
· |
long-term persistency and compliance with therapy; |
|
· |
competition from alternative therapies, such as potassium supplements, diuretics, beta receptor agonists, mexiletine and other sodium channel blockers; |
|
· |
the cost-effectiveness of Keveyis and the availability of third-party insurance coverage and reimbursement; and |
|
· |
the product labeling required by the FDA. |
Physicians will prescribe Macrilen only if they determine, based on experience, clinical data, side effect profiles and other factors, that it is preferable to other diagnostic methods, even if those methods are not approved for diagnosing AGHD. Because AGHD is rare, most physicians are inexperienced in the diagnosis of patients with the illness and it may
46
be difficult to persuade them to prescribe Macrilen. Other factors that may affect the commercial success of Macrilen include:
|
· |
the preference of some physicians to utilize Arginine, an injectable product that is the only other FDA-approved product indicated for use in diagnosing AGHD, or one of several other products that are used off-label to diagnose AGHD, of which the two most frequently prescribed products are the injectables glucagon and insulin; |
|
· |
the cost-effectiveness of Macrilen and the availability of third-party insurance coverage and reimbursement; and |
|
· |
the product labeling required by the FDA. |
The failure of Keveyis and Macrilen to achieve commercial success could prevent us from generating sufficient product sales and royalties to fully fund our commercial and development activities.
If serious adverse, undesirable or unacceptable side effects are identified during the development of our product candidates or following regulatory approval, we may be required to take actions that could significantly harm our business, financial condition, and results of operations.
If any of our product candidates are found to be associated with serious adverse, undesirable or unacceptable side effects, we may need to abandon their development or limit development to certain uses or sub-populations in which such side effects are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many compounds that initially show promise in preclinical or early stage testing have later been found to cause side effects that have restricted their use and prevented further development of the compound for larger indications.
In our clinical trials of Recorlev to date, adverse events have included headache, nausea, back pain, dizziness, diarrhea and liver enzyme elevations among others. For veldoreotide, which is given by subcutaneous injections, adverse events have included injection site reaction such as swelling, itching and pain. Headache and gastrointestinal effects such as nausea and diarrhea have also been observed for veldoreotide. These adverse events can be dose-dependent and may increase in frequency and severity if we increase the dose to increase efficacy. Occurrence of serious treatment-related side effects could impede clinical trial enrollment, require us to halt the clinical trial, and prevent receipt of regulatory approval from the FDA, EMA or any comparable foreign regulatory agency. They could also adversely affect physician or patient acceptance of our product candidates.
Discovery of previously unknown problems, or increased focus on a known problem, with an approved product may result in restrictions on its permissible uses, including withdrawal of the medicine from the market. Currently, ketoconazole is required to include a "black box" warning on its label for use as an antifungal related to liver toxicity in the United States. Manufactured ketoconazole consists of two enantiomers, dextroketoconazole and levoketoconazole, that are found in equal amounts, and is therefore referred to as a racemate mixture. Recorlev is a single-enantiomer drug, a pure form of one of the two enantiomers (levoketoconazole) of ketoconazole. If Recorlev is required to include a similar "black box" warning on its label, it may limit our ability to commercialize the product, if approved.
Additionally, if we or others identify undesirable side effects caused by Keveyis or any other products candidates for which we receive regulatory approval, a number of potentially significant negative consequences could result, including, but not limited to:
|
· |
withdrawal by regulatory authorities of approvals of such product; |
|
· |
seizure of the product by regulatory authorities; |
|
· |
recall of the product; |
|
· |
restrictions on the marketing of the product; |
47
|
· |
requirement by regulatory authorities of additional warnings on the label, such as a black box warning; |
|
· |
requirement that we create a medication guide outlining the risks of such side effects for distribution to patients; |
|
· |
commitment to expensive additional safety studies prior to launch as a prerequisite of approval by regulatory authorities of such product; |
|
· |
commitment to expensive post‑marketing studies as a prerequisite of approval by regulatory authorities of such product; |
|
· |
initiation of legal action against us claiming to hold us liable for harm caused to patients; and |
|
· |
harm to our reputation and resulting harm to physician or patient acceptance of our products. |
Any of these events could negatively impact the commercial prospects of the affected product and could significantly harm our business, financial condition, and results of operations. In addition, for so long as we are entitled to receive royalties from Novo from their Macrilen sales, our operations will be negatively impacted by any adverse side effects found to be associated with Macrilen.
We may become exposed to costly and damaging product liability claims, either in connection with the sale of our approved products or when testing our product candidates in the clinic, and our product liability insurance may not cover all damages from such claims.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing, and use of pharmaceutical products. The current and future use of product candidates by us in clinical trials, and the sale of any approved products, may expose us to liability claims. These claims might be made by patients that use the product, healthcare providers, pharmaceutical companies or others selling such products, and/or other third parties we have agreed to indemnify. Any claims against us, regardless of their merit, could be difficult and costly to defend, and could compromise the market acceptance of our products or any prospects for commercialization of our product candidates, if approved.
Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. Physicians and patients may not comply with product instructions or may ignore warnings regarding potential adverse effects and patients who should not use our products. If any of our products or product candidates were to cause adverse side effects during clinical trials or after approval, we may be exposed to substantial liabilities.
We have limited product liability insurance that offers coverage we believe to be appropriate for a company such as ours. We intend to extend our product liability insurance coverage to any product candidate for which we obtain marketing approval. However, this insurance may be prohibitively expensive or may not fully cover our potential liabilities. Our inability to obtain adequate insurance coverage at an acceptable cost could prevent or inhibit the commercialization of our products or result in meaningful underinsured or uninsured liability. Defending a lawsuit could be costly and significantly divert management's attention from conducting our business. If we were sued successfully, our liability could be substantial.
Our ability to successfully commercialize Keveyis and any other product candidates for which we receive regulatory approval will depend in part on the extent to which governmental authorities and health insurers establish adequate coverage and reimbursement levels and pricing policies.
Our ability to successfully commercialize Keveyis and any other product candidates for which we receive regulatory approval will depend, in part, on the extent to which coverage and reimbursement for these products will be available from government and health administration authorities, private health insurers and other third-party payors. To manage healthcare costs, many governments and third-party payors increasingly scrutinize the pricing of new therapies and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage
48
and adequate reimbursement to such new technologies. In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (the “Medicare Modernization Act”) changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly under a new Part D and introduced a new reimbursement methodology based on average sale prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost-reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement that we receive for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors. In light of such challenges to prices and increasing levels of evidence of the benefits and clinical outcomes of new technologies, we cannot be sure that coverage will be available for these products, and, if available, that the reimbursement rates will be adequate. If adequate levels of coverage and reimbursement for these products is unavailable, our ability to generate revenue from product sales and/or royalties will be compromised.
Because each third-party payor individually approves coverage and reimbursement levels, obtaining coverage and adequate reimbursement is a time consuming, costly and sometimes unpredictable process. We may be required to provide scientific and clinical support, medical necessity or both for the use of a product to each third-party payor separately with no assurance that approval would be obtained, and we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness, medical necessity or both of a product. This process could delay the market acceptance of our products and could have a negative effect on our future revenues and operating results.
Third-party payors may deny coverage and reimbursement status altogether of a given drug product, or cover the product, but may also establish prices at levels that are too low to enable us to realize an appropriate return on our investment in product development. Because the rules and regulations regarding coverage and reimbursement change frequently, in some cases on short notice, even when there is favorable coverage and reimbursement, future changes may occur that adversely impact such favorable coverage and reimbursement status. Further, the net reimbursement for drug products may be subject to additional reductions if there are changes to laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States.
The unavailability or inadequacy of third-party coverage and reimbursement could negatively affect the market acceptance of Keveyis and any product candidates for which we receive regulatory approval and the future revenues we may expect to receive from these products. Similarly, for so long as we are entitled to receive royalties from Novo from their Macrilen sales, any difficulties Novo experiences with respect to third-party coverage and reimbursement could negatively impact our operations.
In addition, we are unable to predict what additional legislation or regulation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect such legislation or regulation would have on our business.
We will be subject to ongoing obligations and continued regulatory requirements, which may result in significant additional expense.
Keveyis and any of our product candidates that receive regulatory approval will remain subject to continued regulatory review. Any regulatory approvals that we receive for our product candidates may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials and surveillance to monitor the safety and efficacy of the product candidate. In addition, if the FDA, the EMA or any comparable foreign regulatory authority approves any of our product candidates, we will be subject to ongoing regulatory obligations and oversight by regulatory authorities, including with respect to the manufacturing processes, labeling, packing, distribution, storage and adverse event reporting, advertising and marketing, and recordkeeping and, potentially, other post-marketing obligations, all of which may result in significant expense and limit our ability to commercialize such products. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued
49
compliance with cGMPs and cGCPs for any clinical trials that we conduct post-regulatory approval. Our three Phase 3 clinical trials of Recorlev are collecting safety data for over approximately 160 patients, and we currently expect that we will be required by the FDA and the EMA to collect additional safety data post-approval.
In addition, approved products, manufacturers and manufacturers' facilities are subject to continual review and periodic inspections. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
· |
restrictions on the marketing or manufacturing of the product; or |
|
· |
withdrawal of the product from the market, or voluntary or mandatory product recalls. |
In addition, approved products, manufacturers and manufacturers' facilities are subject to continual review and periodic inspections. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
· |
restrictions on the marketing or manufacturing of the product; |
|
· |
withdrawal of the product from the market, or voluntary or mandatory product recalls; |
|
· |
fines, disgorgement of profits or revenues, warning letters or holds on clinical trials; |
|
· |
refusal by the FDA to approve pending applications or supplements to approved applications filed by us; |
|
· |
suspension or revocation of product approvals; |
|
· |
product seizure or detention, or refusal to permit the import or export of products; and |
|
· |
injunctions or the imposition of civil or criminal penalties. |
If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements. The policies of the FDA, the EMA or any comparable foreign regulatory agency may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any regulatory approval that we may have obtained, which would compromise our ability to achieve or sustain profitability.
We and our collaborators and contract manufacturers are subject to significant regulation with respect to the manufacturing of pharmaceutical products. The manufacturing facilities on which we rely may not continue to meet regulatory requirements or may not be able to meet supply demands.
All entities involved in the preparation of pharmaceutical products for clinical trials or commercial sale, including our existing contract manufacturers for our products and product candidates, are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with cGMP. These regulations govern manufacturing processes and procedures, including record keeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our product candidates that may not be detectable in final product testing. We, our collaborators or our contract manufacturers must supply all necessary documentation in support of an NDA or foreign equivalent on a timely basis and must adhere to GLP and cGMP regulations enforced by the FDA and other regulatory agencies through their facilities inspection program. Some of our contract manufacturers have never produced a commercially-approved pharmaceutical product and therefore have not obtained the requisite regulatory authority approvals to do so. The facilities and quality systems of some or all of our
50
collaborators and third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates or any of our other potential products.
In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or our other potential products or the associated quality systems for compliance with the regulations applicable to the activities being conducted. Although we oversee the contract manufacturers, we cannot control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the regulatory requirements. If these facilities do not pass a pre-approval plant inspection, regulatory approval of the products may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever.
The regulatory authorities also may, at any time following approval of a product for sale, audit the manufacturing facilities of our collaborators and third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us or a third party to implement, and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility.
If we, our collaborators or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA or another applicable regulatory authority could impose regulatory sanctions including, among other things, refusal to approve a pending application our product candidates, withdrawal of an approval or suspension of production, fines, injunctions, civil penalties, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could harm our business.
Additionally, if the supply from one approved manufacturer is interrupted, an alternative manufacturer would need to be qualified through an NDA supplement or equivalent foreign regulatory filing, which could result in further delay. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could cause us to incur higher costs and could cause the delay or termination of clinical trials, regulatory submissions, required approvals or commercialization of our product candidates. Furthermore, if our suppliers fail to meet contractual requirements and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical trials may be delayed or we could lose potential revenue.
We rely on third parties to conduct our nonclinical and clinical trials and if these third parties perform in an unsatisfactory manner, our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party CROs to conduct and monitor and manage data for our ongoing nonclinical and clinical programs. Once we have established contractual relationships with such third-party CROs, we will have only limited control over their actual performance of these activities. Nevertheless, we are responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol, legal, regulatory, environmental and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our CROs and other vendors are required to comply with current cGMP, cGCP, and GLP, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Union and any comparable foreign regulatory agency for all of our product candidates in nonclinical and clinical development. Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators, trial sites and other contractors. If we or any of our CROs or vendors fail to comply with applicable regulations, the data generated in our nonclinical and clinical trials may be deemed unreliable and the FDA, EMA or any comparable foreign regulatory agency may require us to perform additional nonclinical and clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that all of our clinical trials comply with cGCP regulations. In addition, our clinical trials must be conducted
51
with products produced under cGMP regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
Our business involves the controlled use of hazardous materials, chemicals, biologicals and radioactive compounds. Substantially all such use is outsourced to third-party CRO manufacturers and clinical sites. Although we believe that our third-party CROs’ safety procedures for handling and disposing of such materials comply with industry standards, there will always be a risk of accidental contamination or injury. By law, radioactive materials may only be disposed of at certain approved facilities. If liable for an accident, or if it suffers an extended facility shutdown, we or our CROs could incur significant costs, damages or penalties.
Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing nonclinical and clinical programs. If our CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. Our CROs may also generate higher costs than anticipated. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If any of our relationships with these third-party CROs terminates, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms. If we are able to replace a CRO, switching or adding additional CROs involves additional cost and requires management time and focus and there is a natural transition period when a new CRO commences work. As a result, delays could occur, which could hurt our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future.
Enacted and future legislation may increase the difficulty and cost for us to obtain regulatory approval of, and commercialize our, product candidates and may affect the prices we may set.
In the United States and the European Union, there have been a number of legislative, regulatory and proposed changes regarding the healthcare system. These changes could prevent or delay regulatory approval of our product candidates, restrict or regulate post-approval activities, and affect our ability to sell profitably any products for which we obtain regulatory approval and begin to commercialize.
As a result of legislative proposals and the trend toward managed health care in the United States, third-party payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs. In the United States, the Medicare Modernization Act changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly under a new Part D and introduced a new reimbursement methodology based on average sale prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost-reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement that we receive for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow the Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act (“PPACA”), as amended by the Health Care and Education Reconciliation Act, a sweeping law intended, among other things, to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry, and impose additional health policy reforms. PPACA, among other things: increased the statutory minimum Medicaid rebates a manufacturer must pay under the Medicaid Drug Rebate Program; addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for
52
drugs that are inhaled, infused, instilled, implanted or injected; and established a new Medicare Part D coverage gap discount program in which manufacturers must provide 50% point-of-sale discounts on negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period as a condition for the manufacturer's outpatient drugs to be covered under Part D and implemented payment system reforms, including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models. Further, the PPACA imposed a significant annual nondeductible fee on entities that manufacture or import specified branded prescription drug products and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs. There have been judicial and Congressional challenges to certain aspects of PPACA, as well as recent efforts by the Trump administration to repeal or replace certain aspects of PPACA and we expect such challenges and amendments to continue. The Tax Cuts and Jobs Act of 2017, or the Tax Act, was signed into law, which included a provision which repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On December 14, 2018, a Texas U.S. District Court Judge ruled that PPACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Act. Additionally, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit ruled that that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of PPACA are invalid as well. It is unclear how these decisions, future decisions, subsequent appeals, and other efforts to repeal and replace PPACA will impact PPACA and our business.
Moreover, other legislative changes have also been proposed and adopted in the United States since PPACA was enacted. On August 2, 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021 was unable to reach required goals, thereby triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect on April 1, 2013 and will stay in effect through 2024 unless additional Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other health care funding, which could compromise the ability of patients and third-party payors to purchase our product candidates.
We expect that additional healthcare reform measures will likely be adopted in the future, any of which may increase our regulatory burdens and operating costs and limit the amounts that federal, state and foreign governments will reimburse for healthcare products and services, which could result in reduced demand for our products, if approved, or additional pricing pressures. There is no assurance that the PPACA, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
In the European Union, proposed new clinical trial regulations will centralize clinical trial approval, which eliminates redundancy, but in some cases this may extend timelines for clinical trial approvals due to potentially longer wait times. Proposals to require specific consents for use of data in research, among other measures, may increase the costs and timelines for our product development efforts. Austerity measures in certain European nations may also affect the prices we are able to seek if our products are approved, as discussed below.
Both in the United States and in the European Union, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, whether the regulations, guidance or interpretations will be changed, or what the impact of such changes on the regulatory approvals of our product candidates, if any, may be.
53
Pricing for pharmaceutical products has come under increasing scrutiny by governments, legislative bodies and enforcement agencies. These activities may result in actions that have the effect of reducing our revenue or harming our business or reputation.
Pharmaceutical product pricing is subject to enhanced government and public scrutiny and calls for reform. There has recently been intense publicity regarding the pricing of pharmaceutical products generally, including publicity and pressure resulting from the prices charged for new products as well as price increases for older products that the government and public deem excessive. We may experience downward pricing pressure on the price of our products due to social or political pressure to lower the cost of drugs, which could reduce our revenue and future profitability. Many companies in our industry have received governmental requests for documents and information relating to drug pricing and patient support programs. In December 2017, we received letters from the offices of U. S. Senators Amy Klobuchar, Susan Collins and Tammy Baldwin, and Senator Claire McCaskill, Ranking member of Homeland Security and Governmental Affairs Committee, that requested information relating to the marketing and sales of Keveyis. The letters request information principally relating to the pricing of Keveyis. We have cooperated with these voluntary requests for information, as well as additional follow-up requests in 2018. We could incur significant expense and experience reputational harm as a result of these or other similar future inquiries, as well as reduced market acceptance and demand for our products, which could harm our ability to market our products in the future. These factors could also result in changes in our product pricing and distribution strategies, reduced demand for our products and/or reduced reimbursement of products, including by federal health care programs such as Medicare and Medicaid and state health care programs.
In addition, federal and state governments may adopt policies affecting drug pricing and contracting practices outside of the context of federal programs such as Medicare and Medicaid, which may adversely affect our business. For example, several states have adopted laws that require drug manufacturers to provide advance notice of certain price increase and to report information relating to those price increases.
The Trump Administration and the U.S. Congress have indicated an interest in taking measures pertaining to drug pricing, including potential proposals relating to Medicare price negotiations, and importation of drugs from other countries. On May 11, 2018, the Trump Administration, through the Department of Health and Human Services, requested comments on a "Blueprint to Lower Drug Prices and Reduce Out-of-Pocket Costs," which outlines proposals and policy considerations intended to improve competition; lower patient out-of-pocket costs; enhance negotiation; and provide incentives for lower manufacturer list prices. The U.S. Department of Health and Human Services has solicited feedback on some of these measures and, at the same time, has implemented others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. Notably, some of the Trump Administration’s and other proposals will require authorization through additional legislation to become effective, but others do not, and the U.S. Congress and the Trump Administration have each indicated that they will continue to seek new legislative and/or administrative measures to control drug costs.
Our relationships with customers, consultants and payors are subject to applicable fraud and abuse, privacy and security, transparency and other healthcare laws and regulations, which, if violated, could expose us to criminal sanctions, civil penalties, exclusion from government healthcare programs, contractual damages, reputational harm and/or diminished profits and future earnings.
Healthcare providers, physicians and others play and will play a primary role in the recommendation and prescription of our current products an any products for which we may in the future obtain regulatory approval and commercialize. Our current and future arrangements with third‑party payors, consultants, customers, physicians and others may expose us to broadly applicable fraud and abuse and other healthcare federal and state laws and regulations, including in the United States, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain regulatory approval. Potentially applicable healthcare laws and regulations include, but are not limited to, the following:
|
· |
the U.S. federal Anti‑Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying remuneration, directly or indirectly, |
54
overtly or covertly, in cash or in kind, to induce or in return for, purchasing, leasing, ordering, arranging for, or recommending the purchase, lease, or order of, any good, facility, item or service for which payment may be made under U.S. government healthcare programs such as Medicare and Medicaid; |
|
· |
the federal civil and criminal false claims laws and civil monetary penalties laws, including civil whistleblower or qui tam actions, which prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government, or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay or transmit money or property to the federal government; |
|
· |
the Privacy Rule or the Security Rule of HIPAA, as amended by HITECH, and its implementing regulations, which impose various obligations with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
|
· |
the health care fraud provisions of HIPAA, which impose criminal liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third‑party payors, or to obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of, or payment for, healthcare benefits, items or services; |
|
· |
the federal Physician Payments Sunshine Act under PPACA and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics and medical supplies to annually report to the Centers for Medicare & Medicaid Services information related to payments and other transfers of value made by such manufacturers to physicians and teaching hospitals, and ownership and investment interests held by physicians or their immediate family members; and |
|
· |
analogous laws and regulations, such as state anti‑kickback and false claims laws, which may apply to sales or marketing arrangements, research, distribution and claims involving healthcare items or services reimbursed by state governmental and non‑governmental third‑party payors, including private insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, and state requirements for manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures and other restrictions on drug manufacturer marketing practices. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under the U.S. federal Anti-Kickback Statute and analogous state laws, it is possible that some of our current and future business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, PPACA, among other things, amends the intent requirement of the U.S. federal Anti-Kickback Statute and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them in order to be in violation. Moreover, PPACA provides that the government may assert that a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations, agency guidance or case law involving applicable healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to, without limitation, significant civil, criminal and administrative penalties, damages, fines, exclusion from U.S. government funded healthcare programs, such as Medicare and Medicaid, imprisonment, disgorgement, enhanced government reporting and oversight, contractual damages,
55
reputational harm, diminished profits and future earnings and/or the curtailment or restructuring of our operations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses or divert our management's attention from the operations of our business. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to similar penalties, including, without limitation, criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department's Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors and other partners from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties for clinical trials outside of the United States, to sell our products abroad once we enter a commercialization phase and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors and other partners, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
Risks Related to Our Intellectual Property
If we or our licensors are unable to obtain and maintain effective patent or license rights for our approved products, product candidates or any future product candidates, or if the scope of the patent or license rights obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
In addition to the regulatory exclusivity maintained by Keveyis and our product candidates with regulatory orphan drug status, we rely upon a combination of patents, trade secret protection, license rights and confidentiality agreements to protect the intellectual property related to our products and product candidates. Our success depends in large part on our and our licensors' ability to obtain and maintain patent and other intellectual property protection in the United States and in other countries, as well as license rights, with respect to our proprietary technology, products and product candidates. Furthermore, our right to receive royalty payments from Novo from Macrilen sales, and the amount of any royalty payments, will depend on Novo Nordisk’s ability to protect the Macrilen patent portfolio.
We have sought to protect our proprietary position by filing, where possible, patent applications in the United States and abroad related to our novel technologies and products that are important to our business. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our research and development and manufacturing processes before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
56
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unsolved. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our products or product candidates in the United States or in foreign countries. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions remain confidential for a period of time after filing, and some remain so until issued. Therefore, we cannot be certain that we were the first to file any patent application related to our products or product candidates, or whether we were the first to make the inventions claimed in our owned patents or pending patent applications, nor can we know whether those from whom we license patents were the first to make the inventions claimed or were the first to file. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our products or product candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed, found unenforceable or invalidated, which could allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our products or product candidates, prevent others from designing around our claims or provide us with a competitive advantage. Any of these outcomes could impair our ability to prevent competition from third parties.
We have filed several patent applications covering various aspects of our products and product candidates. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patent, or whether any issued patents will be found invalid and unenforceable or will be challenged by third parties. Any successful opposition to these patents or any other patents owned by or licensed to us after patent issuance, or the loss or other impairment of any license rights relating to our products or product candidates, could deprive us of rights necessary for the successful commercialization of any products or product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product or product candidate under patent protection could be reduced.
We may not have sufficient patent terms to effectively protect our products and business.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is first filed. Although various extensions may be available, the life of a patent, and the protection it affords, is limited. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such product candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours or otherwise provide us with a competitive advantage. Even if patents covering our products or product candidates are obtained, once the patent life has expired for a product, we may be open to competition from generic medications.
Although patent term extensions in the United States and under supplementary protection certificates in the European Union may be available to extend the patent exclusivity term for our products or product candidates, we cannot provide any assurances that any such patent term extension will be obtained and, if so, for how long.
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Assuming the other requirements for patentability are met, in the United States prior to March 15, 2013, the first to invent the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act (the “AIA”), enacted on September 16, 2011, the United States has moved to a first inventor to file system. The AIA also includes a number of significant changes that affect the way patent
57
applications will be prosecuted and may also affect patent litigation. The effects of these changes are currently unclear as the United States Patent and Trademark Office (the “USPTO”) is still implementing various regulations, the courts have yet to address many of these provisions and the applicability of the act and new regulations on specific patents discussed herein have not been determined and would need to be reviewed. In general, the AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. In addition, recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Third-party claims of intellectual property infringement may expose us to substantial liability or prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our ability to develop, manufacture, market and sell our products that have been approved for sale, and to use our proprietary technology without alleged or actual infringement, misappropriation or other violation of the patents and proprietary rights of third parties. There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and reexamination proceedings before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we will market products and are developing product candidates. Some claimants may have substantially greater resources than we do and may be able to sustain the costs of complex intellectual property litigation to a greater degree and for longer periods of time than we could. In addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our products and product candidates may be subject to claims of infringement of the intellectual property rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to compositions, formulations, methods of manufacture or methods of treatment related to the use or manufacture of Keveyis or our product candidates. We cannot be sure that we know of each and every patent and pending application in the United States and abroad that is relevant or necessary to the commercialization of Keveyis or our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents upon which our products or product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our products or product candidates, any compositions formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product or product candidate unless we obtained a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our compositions, formulations, or methods of treatment, prevention or use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product or product candidate unless we obtained a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize Keveyis or one or more of our product candidates, if approved. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys' fees for willful infringement, pay royalties,
58
redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
Furthermore, to the extent any claims of infringement are brought by third parties against Novo related to Macrilen, we may be exposed to indemnification claims from Novo under the terms of the Macrilen Acquisition Agreement.
We may become involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time consuming and unsuccessful.
Competitors may infringe upon our patents or the patents of our licensors. Although we are not currently involved in any litigation, if we or one of our licensing partners were to initiate legal proceedings against a third party to enforce a patent covering one of our products or product candidates, the defendant could counterclaim that the patent covering our product or product candidate is invalid and/or unenforceable, or request declaratory judgment that there is no infringement. In patent litigation in the United States, defendant counterclaims alleging invalidity, noninfringement and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, nonobviousness or non-lack of enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld material relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, or at all. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs, and distract our management and other employees. In addition, the uncertainties associated with litigation could compromise our ability to successfully market Keveyis, raise the funds necessary to continue our clinical trials, continue our research programs, and license necessary technology from third parties or enter into development partnerships that would help us bring our product candidates to market, if approved.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could hurt the market price of our ordinary shares.
Failure to secure or maintain adequate protection for our trademarks could adversely affect our business.
We have filed a U.S., Canadian, Brazilian and International (Madrid Protocol) trademark application designating Australia, China, European Community, India, Israel, Japan, Mexico and Turkey for the mark, "Strongbridge Biopharma." If the U.S. or any foreign trademark offices raise any objections, we may be unable to overcome such objections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Oppositions or cancellation proceedings have been filed and may in the future be filed against our trademarks, and our trademarks may not survive such proceedings.
Furthermore, third parties may allege in the future, that a trademark or trade name that we elect to use for our product candidates may cause confusion in the marketplace. We evaluate such potential allegations in the course of our business, and such evaluations may cause us to change our commercialization or branding strategy for our product candidates, which may require us to incur additional costs. Moreover, any name we propose to use with our product candidate in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA objects to any of our proposed proprietary product names,
59
we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA.
At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, domain names or copyrights may be ineffective and could result in substantial costs and diversion of resources.
In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks alleging that the use of a corporate name or logo, product names or other signs by which we distinguish our products and services are infringing their trademark rights. The outcome of such claims is uncertain and may adversely affect our freedom to use our corporate name or other relevant signs. If litigation arises in this area, it may lead to significant costs and diversion of management and employee attention.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to develop and manufacture our product candidates, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees, and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor's discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may harm our business.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We may employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, and we are not currently subject to any claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties, we may in the future be subject to such claims. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
Although we are not currently experiencing any claims challenging the inventorship of our patents or ownership of our intellectual property, we may in the future be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Even if we are successful in
60
defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our products or product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Further, licensing partners may not prosecute patents in certain jurisdictions in which we may obtain commercial rights, thereby precluding the possibility of later obtaining patent protection in these countries. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful.
Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Our Ordinary Shares
The price of our ordinary shares may be volatile and may fluctuate due to factors beyond our control.
The market price of our ordinary shares may be volatile and subject to wide fluctuations in response to a variety of factors, many of which are beyond our control, including:
|
· |
revenues from product sales and royalties; |
|
· |
positive or negative results of testing and clinical trials by us, strategic partners or competitors; |
|
· |
delays in in‑licensing or acquiring additional complementary product candidates; |
|
· |
any delay in the commencement, enrollment and the ultimate completion of clinical trials; |
|
· |
technological innovations or commercial product introductions by us or competitors; |
|
· |
failure to successfully develop, obtain approval and commercialize any of our product candidates; |
|
· |
changes in government regulations; |
|
· |
developments concerning proprietary rights, including patents and litigation matters; |
|
· |
public concern relating to the commercial value or safety of any of our products or product candidates; |
|
· |
financing or other corporate transactions, or inability to obtain additional funding; |
61
|
· |
failure to meet or exceed expectations of the investment community; |
|
· |
announcements of significant licenses, acquisitions, strategic partnerships or joint ventures by us or our competitors; |
|
· |
publication of research reports or comments by securities or industry analysts; or |
|
· |
general market conditions in the pharmaceutical industry or in the economy as a whole. |
|
· |
positive or negative results from our lifecycle efforts on Keveyis |
|
· |
Any of the risks described herein |
The share price of publicly traded emerging biopharmaceutical and drug discovery and development companies has been highly volatile and is likely to remain highly volatile in the future. In addition, the stock market in general has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of individual companies. Broad market and industry factors may hurt the market price of companies' stock, including ours, regardless of actual operating performance.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, the price of our ordinary shares and our trading volume could decline.
The trading market for our ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If too few securities or industry analysts commence or continue coverage of our company, the trading price for our ordinary shares would likely be negatively affected. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our ordinary shares could decrease, which might cause the price of our ordinary shares and trading volume to decline.
Future sales, or the possibility of future sales, of a substantial number of our ordinary shares could adversely affect the price of our ordinary shares.
Future sales of a substantial number of our ordinary shares, or the perception that such sales will occur, could cause a decline in the market price of our ordinary shares. As of February 17, 2020, we have 54,247,285 ordinary shares outstanding, and 19,048,124 shares issuable upon the exercise of outstanding stock options, restricted stock units and warrants.
We have filed Registration Statements on Form S-8 registering ordinary shares that we may issue under our equity compensation plans. These ordinary shares can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements. If a large number of our ordinary shares or securities convertible into our ordinary shares are sold in the public market after they become eligible for sale, the sales could reduce the trading price of our ordinary shares and impede our ability to raise future capital.
We are also party to an Equity Distribution Agreement with JMP Securities LLC (“JMP Securities”), pursuant to which we may offer and sell ordinary shares having an aggregate offering price of up to $40 million from time to time through JMP Securities, acting as agent. As of December 31, 2019, we have issued an aggregate of $8.9 million in ordinary shares to JMP Securities under the Equity Distribution Agreement, leaving $31.1 million in ordinary shares available for issuance. Whether we choose to affect future sales under this agreement will depend on a number of factors, including, among others, market conditions and the trading price of our ordinary shares relative to other sources of capital. The issuance from time to time of ordinary shares through this “at-the-market” facility program or in any other equity offering, or the perception that such sales may occur, could reduce the trading price of our ordinary shares.
62
An active market in our ordinary shares may not be liquid enough for investors to resell our ordinary shares.
The listing of our ordinary shares on the Nasdaq Global Select Market does not assure that a meaningful, consistent and liquid trading market exists. In general trading volume in our ordinary shares has been limited and an active trading market for our shares may not be sustained. If an active market for our ordinary shares is not sustained, it may be difficult for investors to sell their shares without depressing the market price for the shares or at all.
We have never paid cash dividends, do not expect to pay dividends in the foreseeable future and our ability to pay dividends, or repurchase or redeem our ordinary shares, is limited by law.
We have not paid any dividends since our inception and do not anticipate paying any dividends on our ordinary shares in the foreseeable future. Even if future operations lead to significant levels of distributable profits, we currently intend that any earnings will be reinvested in our business and that dividends will not be paid until we have an established revenue stream to support continuing dividends. The proposal to pay future dividends to shareholders will in addition effectively be at the sole discretion of our board of directors after taking into account various factors our board of directors deems relevant, including our business prospects, capital requirements, financial performance and new product development. In addition, payment of future dividends is subject to certain limitations under the Irish Companies Act 2014 (the “Irish Companies Act”). The Irish Companies Act, among other requirements, requires Irish companies to have distributable reserves available for distribution equal to or greater than the amount of the proposed dividend. Accordingly, investors cannot rely on dividend income from our ordinary shares and any returns on an investment in our ordinary shares will likely depend entirely upon any future appreciation in the price of our ordinary shares.
We believe we were classified as a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes in some of the past years and we may be classified as a PFIC in future years , which could result in adverse U.S. federal income tax consequences to U.S. Holders of our ordinary shares.
A non-U.S. corporation generally will be classified as a PFIC for U.S. federal income tax purposes for any taxable year if either (1) 75% or more of its gross income for such year consists of certain types of "passive" income or (2) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year produce or are held for the production of passive income. For this purpose, "passive income" generally includes, among other items of income, dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income, and a non-U.S. corporation is treated as owning a proportionate share of the assets and earning a proportionate share of the income of any other corporation in which such non-U.S. corporation owns, directly or indirectly, more than 25% of the value of such other corporation's stock. Based on our income, assets and activities in past years, we believe that we were a PFIC in past years, and we may be classified as a PFIC for the current taxable year and for future years depending on the income, assets, and activities in such taxable years.
A U.S. Holder that holds ordinary shares during any taxable year in which we are a PFIC would be subject to substantially increased U.S. federal income tax liability, including upon the receipt of any "excess distributions" from us and upon the sale or other disposition of our ordinary shares. Although certain elections may be available to mitigate the adverse impact of the PFIC rules, such elections may result in a current U.S. federal tax liability prior to any distribution on or disposition of our ordinary shares. Further, there can be no assurances that we will supply U.S. Holders with information that such U.S. Holders are required to report under the rules governing such elections. Accordingly, the acquisition of our ordinary shares may not be an appropriate investment for certain holders that are not tax-exempt organizations. U.S. Holders should consult their tax advisers regarding the application of the PFIC rules to an investment in our ordinary shares.
Our shareholder’s rights are governed by Irish law and differ from the rights of shareholders under U.S. law.
We are a public limited company incorporated under the laws of Ireland. Therefore, the rights of holders of ordinary shares are governed by Irish law and by our memorandum and articles of association. These rights differ from the typical rights of shareholders in U.S. corporations. In certain cases, facts that, under U.S. law, would entitle a shareholder in a U.S. corporation to claim damages may not give rise to a cause of action under Irish law entitling a
63
shareholder in an Irish company to claim damages. For example, the rights of shareholders to bring proceedings against us or against our directors or officers in relation to public statements are more limited under Irish law than under the civil liability provisions of the U.S. securities laws.
Our shareholders may have difficulties enforcing, in actions brought in courts in jurisdictions located outside the United States, judgments obtained in the U.S. courts under the U.S. securities laws. In particular, if a shareholder sought to bring proceedings in Ireland based on U.S. securities laws, the Irish court might consider that:
|
· |
it did not have jurisdiction; |
|
· |
it was not the appropriate forum for such proceedings; |
|
· |
applying Irish conflict of laws rules, U.S. laws (including U.S. securities laws) did not apply to the relationship between you and us or our directors and officers; or |
|
· |
the U.S. securities laws were of a penal nature or violated Irish public policy and should not be enforced by the Irish court. |
Our shareholders should also be aware that Irish law does not allow for any form of legal proceedings directly equivalent to the class action available in the United States.
A future transfer of our ordinary shares, other than one effected by means of the transfer of book-entry interests in DTC, may be subject to Irish stamp duty.
The rate of Irish stamp duty, when applicable, on the transfer of shares in an Irish-incorporated company is 1% of the price paid, or the market value of the shares acquired, whichever is greater. Payment of Irish stamp duty is generally a legal obligation of the transferee. We expect that most of our ordinary shares will be traded through the Depositary Trust Company (“DTC”), or through brokers who hold such shares on behalf of customers through DTC. As such, the transfer of ordinary shares should be exempt from Irish stamp duty based on established practice of the Irish Revenue Commissioners. We received written confirmation from the Irish Revenue Commissioners on June 22, 2015 that a transfer of our ordinary shares held through DTC and transferred by means of a book-entry interest would be exempt from Irish stamp duty. However, if you hold your ordinary shares directly of record, rather than beneficially through DTC, or through a broker that holds your ordinary shares through DTC, any transfer of your ordinary shares may be subject to Irish stamp duty. The potential for Irish stamp duty to arise could adversely affect the price and liquidity of our ordinary shares. In addition, the terms of our eligibility agreement with DTC requires us to provide certain indemnities relating to Irish stamp duty to third parties. If liability were to arise as a result of the indemnities provided under the terms of the eligibility agreement, we may face significant unexpected costs.
Anti-takeover provisions in our Articles and under Irish law could make an acquisition of us more difficult, limit attempts by our shareholders to replace or remove our current directors and management team, and limit the market price of our ordinary shares.
Our Articles contain provisions that may delay or prevent a change of control, discourage bids at a premium over the market price of our ordinary shares and adversely affect the market price of our ordinary shares and the voting and other rights of the holders of our ordinary shares. These provisions include:
|
· |
dividing our board of directors into three classes, with each class serving a staggered three‑year term; |
|
· |
permitting our board of directors to issue preference shares without shareholder approval, with such rights, preferences and privileges as they may designate; |
|
· |
provisions which allow our board of directors to adopt a shareholder rights plan upon such terms and conditions as it deems expedient and in our best interests; |
64
|
· |
establishing an advance notice procedure for shareholder proposals to be brought before an annual meeting, including proposed nominations of persons for election to our board of directors; and |
|
· |
the ability of our board of directors to fill vacancies on our board in certain circumstances. |
These provisions do not make us immune from takeovers. However, these provisions will apply even if the offer may be considered beneficial by some shareholders. In addition, these provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management team by making it more difficult for shareholders to replace members of our board of directors, which is responsible for appointing the members of our management.
Irish law differs from the laws in effect in the United States with respect to defending unwanted takeover proposals and may give our board of directors less ability to control negotiations with hostile offerors.
We are subject to the Irish Takeover Rules. Under the Irish Takeover Rules, our board of directors is not permitted to take any action that might frustrate an offer for our ordinary shares once our board of directors has received an approach that may lead to an offer or has reason to believe that such an offer is or may be imminent, subject to certain exceptions. Potentially frustrating actions such as (1) the issue of shares, options, restricted share units or convertible securities, (2) material acquisitions or disposals, (3) entering into contracts other than in the ordinary course of business or (4) any action, other than seeking alternative offers, which may result in frustration of an offer, are prohibited during the course of an offer or at any earlier time during which our board of directors has reason to believe an offer is or may be imminent. These provisions may give our board of directors less ability to control negotiations with hostile offerors than would be the case for a corporation incorporated in the United States.
Our status as an "emerging growth company" and “smaller reporting company” could make our ordinary shares less attractive to investors.
We are an "emerging growth company," as defined in the JOBS Act and will continue to be an emerging growth company until December 31, 2020. As an "emerging growth company," we are permitted to rely on certain exemptions from various reporting requirements that are applicable to other public companies that are not "emerging growth companies," including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, exemptions from the requirements to provide certain executive compensation disclosures, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation or seeking shareholder approval of any golden parachute payments not previously approved.
We are also a “smaller reporting company”, and we will remain a smaller reporting company until the fiscal year following the determination that our public float is $250 million or more measured on the last business day of our second fiscal quarter, or our annual revenues are $100 million or more during the most recently completed fiscal year and our public float is $700 million or more measured on the last business day of our second fiscal quarter. Similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosure, and have certain other reduced disclosure obligations, including, among other things, being required to provide only two years of audited financial statements and not being required to provide selected financial data or supplemental financial information.
We cannot predict if investors will find our ordinary shares less attractive because we rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and the price of our ordinary shares may be more volatile.
Certain provisions of the warrants issued in our 2016 private placement could impede a sale of the company.
In the event of a sale of the company, the terms of the warrants issued to investors in our December 2016 private placement require us to use our best efforts to ensure the holders of such warrants will have a continuing right to purchase shares of the acquirer and, if our efforts are unsuccessful, to make a payment to such warrant holders based on a Black-Scholes valuation (using variables as specified in the warrant agreements). Such payment must be made in cash
65
in the event that the acquisition results in our shareholders receiving cash from the acquirer at the closing of the transaction, and must be made in shares of the Company (with the value of each ordinary share determined according to the calculation specified in the warrant agreements) in the event that the acquisition results in our shareholders receiving shares in the acquirer or other entity at the closing of the transaction. In the event that our shareholders receive both cash and shares at the closing of the transaction, such payment to the warrant holders shall also be made in both cash and shares in the same proportion as the consideration received by the shareholders.
Notwithstanding the foregoing, in the event that as a result of an acquisition the warrants will be exercisable for anything other than shares or securities that are listed on a regulated market (within the meaning of the Markets in Financial Instruments Directive (2004/39(EC))) or a U.S. national securities exchange, the warrant holders will be entitled to demand to receive a cash payment in an amount equal to the Black-Scholes Value per warrant (calculated in accordance with the warrants) contemporaneously with or promptly after the consummation of such acquisition.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
Our Irish corporate headquarters are located at Suite 206, Fitzwilliam Hall, Fitzwilliam Place, Dublin 2, D02 T292, Ireland. In addition, we lease 22,069 square feet of office space in Trevose, Pennsylvania. We believe that our existing office space is adequate to meet our current needs, and that suitable additional alternative spaces will be available in the future on commercially reasonable terms.
From time to time, we are a party to legal proceedings arising in the ordinary course of business. We are not currently a party to any other legal proceedings that we believe could have a material adverse effect on financial condition or results of operations.
ITEM 4. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our ordinary shares are listed on the Nasdaq Global Select Market under the symbol “SBBP”.
Stockholders
As of December 31, 2019, there were approximately 23 stockholders of record of our ordinary shares. The actual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
66
Securities Authorized for Issuance Under Equity Compensation Plans
See Part III, Item 12. “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” for information relating to our equity compensation plans.
Recent Sale of Unregistered Securities and Use of Proceeds
None.
Purchases of Equity Securities By the Issuer and Affiliated Purchasers
None.
ITEM 6. SELECTED FINANCIAL DATA
The following tables set forth a summary of our consolidated financial data. We have derived the consolidated statement of operations data for the years ended December 31, 2019, 2018, 2017, 2016 and 2015 and the balance sheet data as of December 31, 2019, 2018 and 2017 from our consolidated audited financial statements. You should read this data together with the consolidated financial statements and related notes appearing elsewhere in this Annual Report and the section in this filing titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The historical results are not necessarily indicative of the results to be expected for any future periods. All of our operations are continuing operations and we have not proposed or paid dividends in any of the periods presented.
|
|
|
December 31, |
||||||||||||||
|
|
|
2019 |
|
2018 |
|
2017 |
|
2016 |
|
2015 |
|
|||||
|
|
|
(in thousands) |
||||||||||||||
|
Consolidated Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
21,676 |
|
$ |
18,027 |
|
$ |
7,046 |
|
$ |
— |
|
$ |
— |
|
|
Royalty revenues |
|
|
36 |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
Total revenues |
|
|
21,712 |
|
|
18,027 |
|
|
7,046 |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of intangible assets) |
|
$ |
3,822 |
|
$ |
3,986 |
|
$ |
1,483 |
|
$ |
— |
|
$ |
— |
|
|
Selling, general and administrative |
|
|
49,058 |
|
|
63,336 |
|
|
36,292 |
|
|
14,875 |
|
|
22,719 |
|
|
Research and development |
|
|
30,903 |
|
|
25,441 |
|
|
17,268 |
|
|
20,023 |
|
|
20,135 |
|
|
Amortization of intangible assets |
|
|
5,022 |
|
|
7,187 |
|
|
5,022 |
|
|
— |
|
|
— |
|
|
Impairment of intangible asset |
|
|
— |
|
|
— |
|
|
20,723 |
|
|
15,828 |
|
|
— |
|
|
Total cost and expenses |
|
|
88,805 |
|
|
99,950 |
|
|
80,788 |
|
|
50,726 |
|
|
42,854 |
|
|
Operating loss |
|
|
(67,093) |
|
|
(81,923) |
|
|
(73,742) |
|
|
(50,726) |
|
|
(42,854) |
|
|
Total other income (loss), net |
|
|
19,410 |
|
|
114,310 |
|
|
(37,970) |
|
|
(631) |
|
|
(1,229) |
|
|
(Loss) income before income taxes |
|
|
(47,683) |
|
|
32,387 |
|
|
(111,712) |
|
|
(51,357) |
|
|
(44,083) |
|
|
Income tax (expense) benefit |
|
|
(1,768) |
|
|
(536) |
|
|
(1,771) |
|
|
2,638 |
|
|
450 |
|
|
Net (loss) income |
|
$ |
(49,451) |
|
$ |
31,851 |
|
$ |
(113,483) |
|
$ |
(48,719) |
|
$ |
(43,633) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
67
|
|
|
December 31, |
|||||||
|
|
|
2019 |
|
2018 |
|
2017 |
|||
|
|
|
(in thousands) |
|||||||
|
Consolidated Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
57,032 |
|
$ |
122,490 |
|
$ |
57,510 |
|
Marketable securities |
|
|
21,072 |
|
|
— |
|
|
— |
|
Total assets |
|
|
117,638 |
|
|
170,285 |
|
|
103,925 |
|
Long-term debt |
|
|
— |
|
|
— |
|
|
37,794 |
|
Total liabilities |
|
|
45,448 |
|
|
57,330 |
|
|
115,839 |
|
Total stockholders’ equity (deficit) |
|
|
72,190 |
|
|
112,955 |
|
|
(11,914) |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion summarizes the significant factors affecting the operating results, financial condition, liquidity and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction with the financial statements and the related notes thereto included elsewhere in this Annual Report. The statements in this discussion regarding industry outlook, our expectations regarding our future performance, liquidity and capital resources and all other non‑historical statements in this discussion are forward‑looking statements and are based on the current beliefs of our management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those discussed in or implied by forward‑looking statements as a result of various factors, including those discussed below and elsewhere in this Annual Report, particularly in the section titled “Risk Factors.”
Overview
We are a global, commercial-stage biopharmaceutical company focused on the development and commercialization of therapies for rare diseases with significant unmet needs.
Our first commercial product is Keveyis (dichlorphenamide), the first and only treatment approved by the U.S. Food and Drug Administration (the “FDA”) for hyperkalemic, hypokalemic, and related variants of primary periodic paralysis (“PPP”), a group of rare hereditary disorders that cause episodes of muscle weakness or paralysis.
We have two clinical-stage product candidates for rare endocrine diseases, Recorlev and veldoreotide. Recorlev (levoketoconazole) is a cortisol synthesis inhibitor currently being studied for the treatment of endogenous Cushing's syndrome. Veldoreotide is a next-generation somatostatin analog being investigated for potential applications in conditions amenable to somatostatin receptor activation. Both Recorlev and veldoreotide have received orphan designation from the FDA and the European Medicines Agency (“EMA”).
In January 2018, Strongbridge Ireland Limited., one of our wholly-owned subsidiaries, acquired the U.S. and Canadian rights to Macrilen (macimorelin), the first and only oral drug approved by the FDA for the diagnosis of patients with adult growth hormone deficiency. We launched Macrilen in the United States in July 2018. In December 2018, we sold Strongbridge Ireland Ltd. to Novo Nordisk Healthcare AG (“Novo”) for $145 million plus the right to receive tiered royalties on net sales of Macrilen through 2027. In addition, Strongbridge U.S. Inc, another of our wholly-owned subsidiaries, entered into an agreement with Novo Nordisk Inc., a subsidiary of Novo (“NNI”), pursuant to which NNI was to fund the costs of 23 of our field-based employees to provide full-time ongoing services to NNI, including the promotion of Macrilen in the United States, for a period of three years. Novo also purchased 5.2 million of our ordinary shares at a purchase price of $7.00 per share. In December 2019, we reached an agreement with Novo to terminate the services agreement. We received a $6 million payment in connection with such termination, and we will no longer provide services to Novo.
68
Financial Operations Overview
Product Revenue, net
Revenues from sales of our products are recorded at the net sales price (transaction price), which includes estimates of variable consideration for which reserves are established and that result from rebates, co-pay assistance and other allowances that are offered by us and the patients’ payors. These reserves are based on the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to our customer) or a current liability (if the amount is payable to a party other than our customer). Where appropriate, these estimates may take into consideration a range of possible outcomes that are probability-weighted for relevant factors such as our historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Overall, these reserves reflect our best estimates of the amount of consideration to which we are entitled based on the terms of the contract. For a complete discussion of accounting for net product revenue, see Note 3, "Revenue recognition" to our consolidated financial statements.
Cost of Sales
Cost of sales includes third-party acquisition costs, third-party warehousing and product distribution charges.
Selling, General and Administrative Expenses
Selling, general and administrative expenses include personnel costs, costs for outside professional services and other allocated expenses. Personnel costs consist of salaries, bonuses, benefits, travel and stock‑based compensation. Outside professional services consist of legal, accounting and audit services, commercial evaluation and strategy services, sales, market access, marketing, investor relations, public relations, recruiting and other consulting services.
Research and Development Expenses
We expense all research and development costs as incurred. Our research and development expenses consist primarily of costs incurred in connection with the development of our product candidates, including:
|
· |
personnel‑related costs, such as salaries, bonuses, benefits, travel and other related expenses, including stock‑based compensation; |
|
· |
expenses incurred under our agreements with CROs, clinical sites, contract laboratories, medical institutions and consultants that plan and conduct our preclinical studies and clinical trials. We recognize costs for each grant project, preclinical study or clinical trial that we conduct based on our evaluation of the progress to completion, including the use of information and data provided to us by our external research and development vendors and clinical sites. |
|
· |
costs associated with regulatory filings; and |
|
· |
costs of acquiring preclinical study and clinical trial materials, and costs associated with formulation and process development; and |
We do not allocate personnel‑related research and development costs, including stock‑based compensation or other indirect costs, to specific programs, as they are deployed across multiple projects under development.
Amortization of Intangible Asset
Amortization of intangible assets relates to the amortization of our product rights to Keveyis. This intangible asset is being amortized over an eight-year period using the straight-line method.
69
Other Income, Net
Other income, net, consists of unrealized gain and losses on the remeasurement of the fair value of warrant liability, interest income generated from our cash and cash equivalents, foreign exchange gains and losses, and gains and losses on investments. We also record income and expenses relating to our service agreement with Novo Nordisk Inc (NNI) to fund the costs of 23 of our field-based employees to provide full-time ongoing services to NNI, including the promotion of Macrilen in the United States in Other Income. In December 2019, we reached an agreement with Novo to terminate the services agreement. We received a $6 million payment in connection with such termination; which is reflected in Other Income; we will no longer provide services to Novo.
Critical Accounting Policies and Estimates
This operating and financial review of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our financial statements appearing elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies and estimates are critical.
Revenue Recognition
Revenues from sales of our products are recorded at the net sales price (transaction price), which includes estimates of variable consideration for which reserves are established and that result from rebates, co-pay assistance and other allowances that are offered by us and the patients’ payors. These reserves are based on the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to our customer) or a current liability (if the amount is payable to a party other than our customer). Where appropriate, these estimates may take into consideration a range of possible outcomes that are probability-weighted for relevant factors such as our historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Overall, these reserves reflect our best estimates of the amount of consideration to which we are entitled based on the terms of the contract. For a complete discussion of accounting for net product revenue, see Note 3, "Revenue recognition" to our consolidated financial statements.
Warrant Liability
The fair values of certain outstanding warrants were measured using the Black-Scholes option-pricing model. Inputs used to determine estimated fair value of the warrant liabilities include the fair value of the underlying stock at the valuation date, the term of the warrants, risk-free interest rates, and the expected volatility of the underlying stock. The significant unobservable inputs used in the fair value measurement of the warrant liabilities were the fair value of the underlying stock at the valuation date and the estimated term of the warrants. Generally, increases (decreases) in the fair value of the underlying stock and estimated term result in a directionally similar impact to the periodic fair value measurement of outstanding warrants.
Intangible Assets
Certain intangible assets were acquired as part of an asset purchase and have been capitalized at their acquisition date at fair value. Acquired definite life intangible assets are amortized using the straight-line method over their respective estimated useful lives. We evaluate the potential impairment of intangible assets if events or changes in
70
circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate.
In connection with the Asset Purchase and Supply Agreement we entered into with Taro Pharmaceuticals North America, Inc., we paid Taro an upfront payment in two installments of $1 million in December 2016 and $7.5 million in March 2017. We have concluded that the supply price payable by us exceeds fair value and, therefore, have used a discounted cash flow method with a probability assumption to value the payments in excess of fair value at $29.3 million, for which we have recorded an intangible asset and corresponding liability. This liability is amortized as we purchase inventory over the term of the agreement. In addition, we incurred transaction costs of $2.4 million resulting in the recording of an intangible asset of $40.2 million. This intangible asset is being amortized over an eight-year period using the straight-line method.
As of December 31, 2019, no impairment of intangible assets has been identified.
Goodwill
We test goodwill for impairment on an annual basis or whenever events occur that may indicate possible impairment. This analysis requires us to make a series of critical assumptions to (1) evaluate whether any impairment exists and (2) measure the amount of impairment.
Because we have one operating segment, when testing for a potential impairment of goodwill, we are required to estimate the fair value of our business as a whole and determine the carrying value. If the estimated fair value is less than the carrying value of our business, then we are required to estimate the fair value of all identifiable assets and liabilities in a manner similar to a purchase price allocation for an acquired business. Only after this process is completed can the goodwill impairment be determined, if any.
We did not record a charge for impairment for the years ended December 31, 2019 and 2018. As of December 31, 2019, there were no events or changes in circumstances indicating possible impairment.
Stock‑based compensation
We account for stock‑based compensation awards in accordance with Financial Accounting Standards Board (“FASB”) ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock‑based payments including grants of stock options and restricted stock and modifications to existing stock options, to be recognized in the consolidated statements of operations based on their fair values.
We record compensation expense for service-based awards over the vesting period of the award on a straight-line basis. Compensation expense related to awards with performance-based vesting conditions is recognized over the requisite service period using the accelerated attribution method to the extent achievement of the performance condition is probable.
We estimate the fair value of our awards with service conditions using the Black‑Scholes option pricing model, which requires the input of subjective assumptions, including (i) the expected stock price volatility, (ii) the expected term of the award, (iii) the risk‑free interest rate and (iv) expected dividends.
We have estimated the expected term of employee service-based stock options using the “simplified” method, whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option, due to our lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury Bond rate with a maturity date commensurate with the expected term of the associated award. We have never paid dividends, and do not expect to pay dividends in the foreseeable future.
We account for forfeitures as they occur as opposed to estimating forfeitures. We record stock-based compensation expense only for those awards that are expected to vest.
71
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences of differences between the carrying amounts and tax bases of assets and liabilities and operating loss carryforwards and other attributes using enacted rates expected to be in effect when those differences reverse. Valuation allowances are provided against deferred tax assets that are not more likely than not to be realized.
We assess our ability to realize deferred tax assets. Changes in future earnings projections, among other factors, may cause us to adjust our valuation allowance on deferred tax assets. Any such adjustments would impact our income tax expense in the period in which it is determined that these factors have changed.
We recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2019, and 2018, we had no accrued interest or penalties related to uncertain tax positions and no amounts have been recognized in our statements of operations.
Results of Operations
Comparison of the Years Ended December 31, 2019 and 2018
The following table sets forth our results of operations for the years ended December 31, 2019 and 2018.
|
|
|
Year Ended |
|
|
|
|
||||
|
|
|
December 31, |
|
Change |
|
|||||
|
|
|
2019 |
|
2018 |
|
$ |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
21,676 |
|
$ |
18,027 |
|
$ |
3,649 |
|
|
Royalty revenues |
|
|
36 |
|
|
— |
|
|
36 |
|
|
Total revenues |
|
|
21,712 |
|
|
18,027 |
|
|
3,685 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of intangible assets) |
|
$ |
3,822 |
|
$ |
3,986 |
|
$ |
(164) |
|
|
Selling, general and administrative |
|
|
49,058 |
|
|
63,336 |
|
|
(14,278) |
|
|
Research and development |
|
|
30,903 |
|
|
25,441 |
|
|
5,462 |
|
|
Amortization of intangible assets |
|
|
5,022 |
|
|
7,187 |
|
|
(2,165) |
|
|
Total cost and expenses |
|
|
88,805 |
|
|
99,950 |
|
|
(11,145) |
|
|
Operating loss |
|
|
(67,093) |
|
|
(81,923) |
|
|
14,830 |
|
|
Other income, net |
|
|
19,410 |
|
|
114,310 |
|
|
(94,900) |
|
|
(Loss) income before income taxes |
|
|
(47,683) |
|
|
32,387 |
|
|
(80,070) |
|
|
Income tax expense |
|
|
(1,768) |
|
|
(536) |
|
|
(1,232) |
|
|
Net (loss) income |
|
$ |
(49,451) |
|
$ |
31,851 |
|
$ |
(81,302) |
|
Net Product Sales and Cost of Sales.
Net product sales were $21.7 million for the year ended December 31, 2019, an increase of $3.6 million compared to the year ended December 31, 2018. Product sales from Keveyis increased by $4.7 million due to the continued sales growth of Keveyis. Included in the year ended December 31, 2018 is $1.1 million from Macrilen sales, the rights to which we sold in December 2018.
72
Selling, General and Administrative Expenses
The following table summarizes our selling, general and administrative expenses during the years ended December 31, 2019 and 2018:
|
|
|
Year Ended |
|
|
|
|
||||
|
|
|
December 31, |
|
Change |
|
|||||
|
|
|
2019 |
|
2018 |
|
$ |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Compensation and other personnel costs |
|
$ |
22,481 |
|
$ |
28,157 |
|
$ |
(5,676) |
|
|
Outside professional and consulting services |
|
|
15,929 |
|
|
28,110 |
|
|
(12,181) |
|
|
Stock-based compensation expense |
|
|
6,552 |
|
|
6,012 |
|
|
540 |
|
|
Severance |
|
|
3,248 |
|
|
— |
|
|
3,248 |
|
|
Facility costs |
|
|
848 |
|
|
1,057 |
|
|
(209) |
|
|
Total selling, general and administrative expenses |
|
$ |
49,058 |
|
$ |
63,336 |
|
$ |
(14,278) |
|
Selling, general and administrative expenses were $49.1 million for the year ended December 31, 2019, a decrease of $14.3 million compared to the year ended December 31, 2018. Outside professional and consulting services decreased $12.2 million during the year ended December 31, 2019, with $2.7 million of this decrease due to a reduction in expenses relating to Keveyis and $8.4 million of this decrease due to the prior year period including expenses associated with the launch of Macrilen, the rights to which we sold in December 2018. Compensation and other personnel costs decreased $5.7 million due to employee costs associated with Macrilen being reimbursed by Novo. In 2019, we incurred a one-time charge of $3.2 million for severance costs.
Research and Development Expenses
The following table summarizes our research and development expenses during the years ended December 31, 2019 and 2018:
|
|
|
Year Ended |
|
|
|
|
||||
|
|
|
December 31, |
|
Change |
|
|||||
|
|
|
2019 |
|
2018 |
|
$ |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Product development and supporting activities |
|
$ |
19,940 |
|
$ |
18,228 |
|
$ |
1,712 |
|
|
Compensation and other personnel costs |
|
|
5,911 |
|
|
5,418 |
|
|
493 |
|
|
Preclinical development |
|
|
2,425 |
|
|
— |
|
|
2,425 |
|
|
Stock-based compensation expense |
|
|
2,045 |
|
|
1,795 |
|
|
250 |
|
|
Severance |
|
|
582 |
|
|
— |
|
|
582 |
|
|
Total research and development expenses |
|
$ |
30,903 |
|
$ |
25,441 |
|
$ |
5,462 |
|
Research and development expenses were $30.9 million for the year ended December 31, 2019, an increase of $5.5 million compared to the year ended December 31, 2018. The $1.7 million increase in expenses for product development and supporting activities was primarily due to additional clinical development expenses associated with Recorlev and $2.4 million expenses for preclinical development relates to life cycle work for Keveyis and work relating to veldoreotide.
73
Amortization of Intangible Assets
Amortization of intangible assets was $5.0 million for the year ended December 31, 2019 a decrease of $2.2 million due to the prior year including amortization of the intangible asset related to Macrilen, the rights to which we sold in December 2018.
Other Income, Net
|
|
|
Year Ended |
|
|
|
|
||||
|
|
|
December 31, |
|
Change |
|
|||||
|
|
|
2019 |
|
2018 |
|
$ |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Income from field services agreement |
|
$ |
12,616 |
|
$ |
— |
|
$ |
12,616 |
|
|
Expense from field services agreement |
|
|
(6,652) |
|
|
— |
|
|
(6,652) |
|
|
Unrealized gain on fair value of warrants |
|
|
11,386 |
|
|
16,337 |
|
|
(4,951) |
|
|
Interest expense |
|
|
— |
|
|
(12,515) |
|
|
12,515 |
|
|
Loss on extinguishment of debt |
|
|
— |
|
|
(21,549) |
|
|
21,549 |
|
|
Gain on sale of subsidiary |
|
|
— |
|
|
130,832 |
|
|
(130,832) |
|
|
Other income, net |
|
|
2,060 |
|
|
1,205 |
|
|
855 |
|
|
Total other income, net |
|
$ |
19,410 |
|
$ |
114,310 |
|
$ |
(94,900) |
|
Total other income, net, decreased by $94.9 million for the year ended December 31, 2019 as compared to the year ended December 31, 2018. The decrease was primarily due to our gain on sale of subsidiary of $130.8 million, which occurred in December 2018. The 2018 period included offsets of $12.5 million of interest expense and $21.5 million loss on extinguishment of debt. In 2019, we had a $5.0 million decrease in the unrealized gain on the fair value of our warrant liability in 2019, primarily resulting from a decrease in our stock price. In addition, during the 2019 period, we recorded $12.6 million in income relating to our field based service agreement with NNI, which includes the $6 million payment from Novo to terminate the agreement. We incurred $6.7 million of field services related expenses in 2019.
Income Tax Expense
We recorded income tax expense of $1.8 million for the year ended December 31, 2019 arising from intercompany interest income.
Liquidity and Capital Resources
We believe that our cash, cash equivalent and marketable securities of $78.1 million at December 31, 2019 will be sufficient to allow us to fund planned operations for at least 12 months beyond the issuance date of these financial statements.
Cash used to fund operating expenses is affected by the timing of when we pay expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
Our future funding requirements will depend on many factors, including the following:
|
· |
the amount of revenue that we receive from sales of Keveyis; |
|
· |
the cost and timing of establishing sales, marketing, distribution and administrative capabilities; |
|
· |
the scope, rate of progress, results and cost of our clinical trials testing and other related activities for Recorlev and veldoreotide; |
74
|
· |
the number and characteristics of product candidates that we pursue, including any additional product candidates we may in‑license or acquire; |
|
· |
the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; |
|
· |
the cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us or our product candidates; |
|
· |
the cost, timing and outcomes of regulatory approvals; |
|
· |
the terms and timing of any collaborative, licensing and other arrangements that we may establish, including any required milestone and royalty payments thereunder; and |
|
· |
the emergence of competing technologies and their achieving commercial success before we do or other adverse market developments. |
We may never achieve profitability, and unless and until we do, we will continue to need to raise additional capital. We plan to continue to fund our operations and capital funding needs through equity, debt financing or other financings along with revenues from Keveyis. There can be no assurances, however, that additional funding will be available on terms acceptable to us.
January 2018 Public Offering of Ordinary Shares
On January 25, 2018, we sold 5,000,000 ordinary shares in a public offering at a price to the public of $6.75 per ordinary share for net proceeds of approximately $31.7 million, after deducting underwriting discounts and commissions and offering expenses payable by us.
On February 26, 2018, we sold an additional 255,683 ordinary shares to the underwriters in connection with their partial exercise of the option to purchase additional shares that was granted to them under the underwriting agreement for additional net proceeds of approximately $1.6 million, after deducting underwriting discounts and commissions and offering expenses payable by us.
At-The-Market Facility
We have entered into an Equity Distribution Agreement with JMP Securities LLC (“JMP Securities”), pursuant to which we may offer and sell ordinary shares having an aggregate offering price of up to $40,000,000 from time to time through JMP Securities, acting as agent. During the year ended December 31, 2018, we sold an aggregate of 1,281,903 ordinary shares under the ATM facility for net proceeds of approximately $8.6 million after payment of fees of $0.3 million to JMP Securities. As of December 31, 2019, we have approximately $31.1 million available for sale under our ATM facility.
75
Cash Flows
Comparison for the Years Ended December 31, 2019 and 2018:
|
|
|
Year Ended |
|
||||
|
|
|
December 31 |
|
||||
|
|
|
2019 |
|
2018 |
|
||
|
|
|
(in thousands) |
|
||||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(44,784) |
|
$ |
(84,576) |
|
|
Investing activities |
|
|
(20,761) |
|
|
134,330 |
|
|
Financing activities |
|
|
87 |
|
|
15,226 |
|
|
Net (decrease) increase in cash and cash equivalents |
|
$ |
(65,458) |
|
$ |
64,980 |
|
Operating Activities
Net cash used in operating activities was $44.8 million for the year ended December 31, 2019 compared to $84.6 million for the year ended December 31, 2018. The decrease in net cash used in operating activities resulted from an increase in total revenues of $2.6 million, a decrease in inventory purchases of $2.1 million and reduced expenditures in our commercial activities for Keveyis and Macrilen, which we sold in December 2018. The prior period had expenditures for the launch of Macrilen, the rights to which we sold in December 2018.
Investing Activities
Net cash used in investing activities was $20.8 million for the year ended December 31, 2019 due to purchasing of marketable securities. Net cash provided by investing was $134.3 million for the year ended December 31, 2018, primarily due to the $159.3 million proceeds from our sale of Macrilen product rights and Strongbridge Ireland Limited.
Financing Activities
Net cash provided by financing activities was $87,000 for the year ended December 31, 2019 compared to net cash provided by financing activities of $15.2 million for the year ended December 31, 2018. The decrease in net cash provided by financing activities resulted primarily from our payment to CRG Servicing LLC (“CRG”) in December 2018 of $94.5 million to pay off our outstanding debt, offset by receipt in January 2018 of $44.9 million in proceeds from the amendment to our senior credit facility with CRG, and $65.8 million in net proceeds from issuance of our ordinary shares and exercise of warrants and stock options.
Contractual Obligations and Other Commitments
The following is a summary of our contractual obligations and other commitments as of December 31, 2019:
|
|
|
Payments due by period |
|
|||||||||||||
|
|
|
Less than |
|
|
|
|
|
|
|
More than |
|
|
|
|
||
|
|
|
1 year |
|
1 to 3 years |
|
3 to 5 years |
|
5 years |
|
Total |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minimum contract purchases pursuant to supply agreements |
|
$ |
4,987 |
|
|
14,060 |
|
|
— |
|
$ |
— |
|
$ |
19,047 |
|
|
Operating leases |
|
$ |
470 |
|
|
1,180 |
|
|
— |
|
$ |
— |
|
$ |
1,650 |
|
|
Total contractual obligations |
|
$ |
5,457 |
|
$ |
15,240 |
|
$ |
— |
|
$ |
— |
|
$ |
20,697 |
|
76
We enter into agreements in the normal course of business with vendors for clinical trials, preclinical studies, and other services and products for operating purposes. Future payment obligations under these agreements, which are cancelable at any time by us, generally upon 30 days prior written notice, are not included in this table of contractual obligations.
We are obligated to make future payments to third parties due to payments that become due and payable upon the achievement of certain commercialization milestones. As the amount and timing of these milestones are not probable and estimable, such commitments have not been included on our consolidated balance sheets or in the contractual obligations table above.
Off‑Balance Sheet Arrangements
We do not have variable interests in variable interest entities or any off‑balance sheet arrangements.
Recent Accounting Pronouncements
See Note 2, “Summary of significant accounting policies and basis of presentation - Recently issued accounting pronouncements” to our consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to certain market risks in the ordinary course of our business. These risks primarily include interest rate risks as described below.
Interest Rate Risk
We had cash and cash equivalents and marketable securities of $78.1 million as December 31, 2019, which consisted mostly of funds held in the United States. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments, including cash equivalents and marketable securities, are in the form of U.S. Treasury securities, asset-backed securities, and commercial paper. Such interest‑earning instruments carry a degree of interest rate risk. To date, fluctuations in interest income have not been significant. Due to the short-term nature and low-risk profile of our investment portfolio, we do not expect that an immediate 100 basis point change in market interest rates would have a material effect on the fair market value of our investment portfolio.
The financial statements and supplementary data required by this item are listed in Item 15 – “Exhibits and Financial Statement Schedules” of this Annual Report.
ITEM 9. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
None
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Exchange Act and the rules and regulations thereunder, is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well
77
designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by SEC Rule 13a‑15(b), we carried out an evaluation, under the supervision and with the participation of our management, our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as of the end of the period covered by this report. Based on the foregoing, our Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2019 at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting refers to a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Under the supervision and with the participation of management, the Chief Financial Officer, the Company carried out an evaluation of the effectiveness of its internal control over financial reporting as of December 31, 2019, based on the Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based upon this evaluation, management has concluded that, as of December 31, 2019, the Company’s internal control over financial reporting was effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate due to changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of our registered public accounting firm regarding our internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the rules of the SEC that permit us to provide only management’s report in this Annual Report for so long as we qualify as an emerging growth company.
Changes in Internal Control over Financial Reporting
There were no changes in the Company's internal control over financial reporting that occurred during the period covered by this Annual Report that have materially affected, or are reasonably likely to materially affect, the Company's internal control over financial reporting.
None
78
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table presents information about our officers and directors as of February 17, 2020.
|
NAME |
|
AGE |
|
POSITION |
|
|
Executive Officers |
|
|
|
|
|
|
John H. Johnson |
|
62 |
|
Executive Chairman and Director |
|
|
Fredric Cohen, M.D. |
|
55 |
|
Chief Medical Officer |
|
|
Robert Lutz |
|
51 |
|
Chief Financial Officer |
|
|
Richard S. Kollender |
|
50 |
|
Chief Operating Officer |
|
|
Stephen Long |
|
54 |
|
Chief Legal Officer |
|
|
Scott Wilhoit |
|
57 |
|
Chief Commercial Officer |
|
|
|
|
|
|
|
|
|
Non‑Employee Directors |
|
|
|
|
|
|
David Gill |
|
65 |
|
Director |
|
|
Garheng Kong, M.D., Ph.D. |
|
44 |
|
Director |
|
|
Jeffrey W. Sherman, M.D., FACP |
|
65 |
|
Director |
|
|
Mårten Steen, M.D., Ph.D. |
|
44 |
|
Director |
|
|
Hilde H. Steineger, Ph.D. |
|
53 |
|
Director |
|
Unless otherwise indicated, the current business addresses for our executive officers and directors is 900 Northbrook Drive, Suite 200, Trevose, Pennsylvania 19053, United States.
Executive Officers
John H. Johnson has served as our Executive Chairman since November 2019, and has served as chairman of our board of directors since March 2015. Mr. Johnson recently served as Chief Executive Officer and a board member of Melinta Pharmaceuticals, Inc. through September 2019, having served as interim Chief Executive Officer since October 2018. Mr. Johnson is also a member of the board of directors of Portola Pharmaceuticals, Inc. From January 2012 until August 2014, Mr. Johnson served as the President and Chief Executive Officer of Dendreon Corporation and as its Chairman from January 2012 until June 2014. From January 2011 until January 2012, he served as the Chief Executive Officer and a member of the board of Savient Pharmaceuticals, Inc. From November 2008 until January 2011, Mr. Johnson served as Senior Vice President and President of Eli Lilly and Company’s Oncology unit. He was also Chief Executive Officer of ImClone Systems Incorporated, which develops targeted biologic cancer treatments, from August 2007 until November 2008, and served on ImClone’s board of directors until it was acquired by Eli Lilly in November 2008. From 2005 to 2007, Mr. Johnson served as Company Group Chairman of Johnson & Johnson’s Worldwide Biopharmaceuticals unit, President of its Ortho Biotech Products LP and Ortho Biotech Canada units from 2003 to 2005, and Worldwide Vice President of its CNS, Pharmaceuticals Group Strategic unit from 2001 to 2003. Prior to joining Johnson & Johnson, he also held several executive positions at Parkstone Medical Information Systems, Inc., OrthoMcNeil Pharmaceutical Corporation and Pfizer, Inc. Mr. Johnson is the former Chairman of Tranzyme Pharma, Inc., former lead independent director of Sucampo Pharmaceuticals, Inc and a former director of Histogenics Corporation and AVEO Pharmaceuticals, Inc. He previously served as a member of the board of directors for the Pharmaceutical Research and Manufacturers of America and the Health Section Governing Board of Biotechnology Industry Organization. Mr. Johnson holds a B.S. from the East Stroudsburg University of Pennsylvania.
Fredric Cohen, M.D. has served as our Chief Medical Officer since November 2016. Dr. Cohen joined Strongbridge in August 2015 and held roles of increasing responsibility, including Senior Vice President, Global Research and Development, and Vice President, Clinical Research and Development, prior to his promotion to Chief Medical Officer. Fred is an endocrinologist by training with approximately 25 years of drug and business development experience, most recently focused in development and commercialization of rare disease and specialty products. Prior to joining Strongbridge, Fred provided strategic and operational counsel to life science companies, actively supporting their development and licensing functions. Prior to that, he served as Executive Director, Clinical Pipeline, at Aptalis Pharma,
79
where he was responsible for innovation strategy as well as building and advancing the company’s specialty pharma pipeline. He has also held research and development positions with Johnson & Johnson and Eli Lilly & Company. Fred holds an M.D. from Pennsylvania State University College of Medicine and an A.B. in biology from Franklin and Marshall College.
Robert Lutz has served as our Chief Financial Officer since September 2019. He previously served as our Chief Business Officer from October 2014 to September 2019. Prior to joining the Company, Mr. Lutz worked from December 2004 to April 2014 at Shire Plc, a publicly traded specialty biopharmaceutical company prior to being purchased by Takeda Pharmaceutical Company Ltd., where he most recently served as Vice President and held key leadership positions in the Specialty Pharmaceutical division. Prior to Shire Plc, Mr. Lutz worked in a variety of roles, including Vice President of Finance, for Cinergy Corp., an electric and gas utility company. Mr. Lutz also worked as a Senior Analyst at Alan B. Slifka and Co., a hedge fund, after having started his career at Goldman Sachs Group Inc., where he served as a Financial Analyst in its principal investment area. He holds a B.A. in economics and computer science from Amherst College and an M.B.A. from the Kellogg School of Management
Richard S. Kollender has served as our Chief Operating Officer since September 2019. He previously served as a Class II director of our board of directors from March 2015 until September 2019, and was Chairman of the audit committee and a member of the compensation committee. Since January 2011, he has served as a Partner and Executive Manager of Quaker Partners Management, LP, a healthcare investment firm, which he initially joined in 2003, and was promoted to Partner in 2005. In addition, from August 2016 through September 2018, Mr. Kollender served as Chief Business Officer and Chief Financial Officer of Rapid Micro Biosystems, a Quaker Partners’ portfolio company, where he continues to serve on the board of directors. Mr. Kollender held positions in sales, marketing and worldwide business development at GlaxoSmithKline (“GSK”), and served as investment manager at S.R. One, the corporate venture capital arm of GSK. Mr. Kollender holds a B.A. in accounting from Franklin and Marshall College and an M.B.A. and a certificate degree in the Graduate Program in Health Administration and Policy, both from the University of Chicago, and practiced as a certified public accountant for six years at public accounting firms including KPMG.
Stephen Long has served as our Chief Legal Officer since March 2015 and as Company Secretary since September 2015. Prior to joining Strongbridge, Mr. Long served as Counsel at the law firm of Reed Smith LLP, from April 2013 to February 2015. He previously served at C.R. Bard, Inc., a medical device manufacturing company, from October 2000 to May 2012 in the roles of Vice President, General Counsel, as Vice President, and Secretary, and as Associate General Counsel. Mr. Long also served as Assistant General Counsel, Consumer Healthcare, at Warner‑Lambert Company, and as Counsel for the company’s pharmaceutical division from February 1998 to September 2000. Mr. Long held positions earlier in his career at the law firm of Willkie Farr & Gallagher and Bankers Trust Company. Mr. Long received his B.S. from the School of Industrial and Labor Relations at Cornell University and his J.D. from Albany Law School of Union University.
Scott Wilhoit has served as our Chief Commercial Officer since May 2019. Mr. Wilhoit joined Strongbridge in January 2017 and held roles of increasing responsibility, including Senior Vice President, Global Market Access, Patient Services and Advocacy, prior to his promotion to Chief Commercial Officer. Mr. Wilhoit has over 30 years of industry experience, much of which has been focused on commercializing products in a variety of rare disease categories. Most recently, Mr. Wilhoit served as Vice President, Market Access and Patient Services at Marathon Pharmaceuticals, where he designed and developed the access strategy for the company’s first commercial rare disease product. Previously, Mr. Wilhoit served as Vice President, Market Access and Health Services at PTC Therapeutics leading pre-launch market access strategic planning for the company’s Duchenne Muscular Dystrophy treatment. Prior to that, he served as Vice President, Pricing, Access and Patient Services at NPS Pharmaceuticals (acquired by Shire). Mr. Wilhoit has also served in a variety of positions with increasing responsibility with Clarus Therapeutics, Auxilium Pharmaceuticals, Biovail Corporation and Johnson & Johnson. Mr. Wilhoit served as a Field Artillery Officer in the U.S. Army and holds a BS in Criminology from Missouri Western State University.
Non‑Employee Directors
David N. Gill has served as a member of our board of directors since September 2019. Mr. Gill served as the President and Chief Financial Officer of EndoChoice, Inc., a medical device company focused on gastrointestinal disease
80
from April 2016 through the sale of the company to Boston Scientific in November 2016 and as Chief Financial Officer from August 2014 to April 2016. Since August 2019, Mr. Gill has served as chairman of the board of directors of Melinta Therapeutics, Inc. Mr. Gill also currently serves as a director of Y-mAbs Therapeutics, Inc. Evolus, Inc., and STRATA Skin Sciences, Inc. Previously he served on the board of directors of Histogenics, Inc. from January 2015 to July 2019. Earlier in his career, Mr. Gill served in a variety of senior executive leadership roles for several publicly-traded companies, including NxStage Medical, Inc., CTI Molecular Imaging, Inc., Interland Inc. and Novoste Corporation. Mr. Gill holds a B.S. degree, cum laude, in Accounting from Wake Forest University and an M.B.A. degree, with honors, from Emory University, and was formerly a certified public accountant.
Qualifications: Mr. Gill has extensive experience as an executive in the medical device industry. This experience and his extensive prior and current service as a director of other public life sciences companies make him a valuable contributor to our board of directors.
Garheng Kong, M.D., Ph.D. has served as Lead Independent Director of our board of directors since November 2019 and has served as a member of our board of directors since September 2015. In July 2013, he founded, and has since served as managing partner of, HealthQuest Capital, a healthcare venture growth fund. Dr. Kong was a general partner at Sofinnova Ventures, a venture firm focused on life sciences, from September 2010 to December 2013. From May 2000 to September 2010, he worked at Intersouth Partners, a venture capital firm, serving most recently as a general partner. Dr. Kong currently serves as a director of Venus Concept, Alimera Sciences, Inc. and Laboratory Corporation of America Holdings. Dr. Kong previously served on the board of directors of Histogenics Corporation, Melinta Therapeutics, Inc. and Avedro, Inc. Dr. Kong holds a B.S. from Stanford University and an M.D., Ph.D. and M.B.A. from Duke University.
Qualifications: Dr. Kong brings to the Board extensive knowledge and experience in both the healthcare and finance fields due to his medical background and his work in life science-related venture capital firms and has significant prior board experience with both public and privately held companies.
Jeffrey W. Sherman, M.D., FACP has served as a member of our board of directors since October 2016. Since 2009, he has served as Chief Medical Officer and Executive Vice President of Horizon Therapeutics plc. He has also served as a member of the Xeris Pharmaceuticals board of directors since April 2018. He previously held positions at IDM Pharma, Takeda Global Research and Development, NeoPharm, Searle/Pharmacia, Bristol-Myers Squibb, and is a past president of the Drug Information Association (DIA). He is a member of a number of professional societies, a diplomat of the National Board of Medical Examiners and the American Board of Internal Medicine, and also serves on the Board of Advisors of the Center for Information and Study on Clinical Research Participation (CISCRP). Dr. Sherman earned his MD from the Rosalind Franklin University of Medicine and Science/The Chicago Medical School. He completed internship, residency and chief residency programs in internal medicine at Northwestern University Feinberg School of Medicine, where he currently serves as an adjunct assistant professor and a member of the alumni board, and a fellowship program in infectious diseases at the University of California San Francisco, where he was also a research associate at the Howard Hughes Medical Institute in allergy and immunology. He received a BA in Biology from Lake Forest College.
Qualifications: With over 25 years of research, clinical development, regulatory and commercialization experience within the biopharmaceutical industry, Dr. Sherman brings critical knowledge and expertise to our board of directors relating to the development and commercialization of biopharmaceutical products. In addition, his experience serving in senior leadership positions at multiple public biopharmaceutical companies provides him with keen insight into the issues facing these companies.
Mårten Steen, M.D., Ph.D. has served as a member of our board of directors since December 2014. Since April 2010, he has served as a Partner of HealthCap VI LP, a venture capital firm investing in life science companies. Prior to HealthCap, from February 2008 until March 2010, Dr. Steen served as director at Merck Serono SA, a biopharmaceutical company. He previously served on the boards of Ultragenyx Pharmaceutical Inc., Wilson Therapeutics AB, Altimmune, Inc. and FerroKin Biosciences. Dr. Steen holds a B.Sc. in Business Administration, an M.D., and a Ph.D. in Clinical Chemistry, all from Lund University.
81
Qualifications: Dr. Steen brings extensive venture capital experience in evaluating opportunities and managing healthcare portfolio businesses as well as multiple board experiences at other biopharmaceutical companies, providing him with critical insights on the issues facing our Company. His experience working with global business development, focusing on both product and technology licensing, is also extremely valuable to management.
Hilde H. Steineger, Ph.D. has served as a member of our board of directors since January 2014. She is currently Chief Executive Officer at Staten Biotechnology. She also serves as Chief Operations Officer and Co-founder of NorthSea Therapeutics B.V. Dr. Steineger is a board member of Nordic Nanovector ASA. Dr. Steineger previously served as Head of Strategic Innovation Management in Nutrition & Health Division of BASF, and as Head of Global Omega-3 Innovation Management at Pronova BioPharma ASA, a BASF company, from April 2013 to May 2015. From August 2007 to June 2010, Dr. Steineger was Head of Investor Relations for Pronova BioPharma and Vice President Business Development in Pronova BioPharma from November 2009 to April 2013. She previously served as a member of the board of directors of PCI Biotech AS, Afiew AS, Algeta ASA, Weifa AS, Inven2 AS, Alertis AS, Clavis Pharma ASA and Biotech Pharmacon ASA. Dr. Steineger holds a Ph.D. in medical biochemistry from University of Oslo and an MSc in molecular biology/biotechnology.
Qualifications: Dr. Steineger brings extensive experience in the business/finance and life sciences areas, including as a financial analyst covering life sciences companies, as a venture capitalist at a life science venture fund and as head of business development at a leading pharmaceutical company. This broad experience from a diverse set of industries has provided Dr. Steineger with the opportunity to develop strong analytical and leadership skills which, along with her medical biochemistry background, allows her to provide valuable insight to our board of directors.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires our officers and directors, and persons who own more than 10% of a registered class of our equity securities, to file reports of securities ownership and changes in such ownership with the SEC.
Based solely on our review of the reports filed with the SEC and written representations from the reporting persons, we believe that all Section 16(a) filing requirements applicable to our directors and officers and 10% stockholders were timely met during 2019 other than one late Form 4 filed on June 5, 2019 for Mr. Wilhoit reflecting a stock option award granted to Mr. Wilhoit on May 28, 2019.
Code of Business Conduct and Ethics
Our Code of Business Conduct and Ethics is applicable to all of our directors, officers and employees and is posted on the Investors section of our website, which is located at www.strongbridgebio.com. Our Code of Business Conduct and Ethics provides that our directors, officers and employees are expected to avoid any action, position or interest that conflicts with the interests of our company or gives the appearance of a conflict. We expect that any amendment to this code, or any waivers of its requirements, will be disclosed on our website. Information contained on, or that can be accessed through, our website is not incorporated by reference into this document, and you should not consider information on our website to be part of this document.
Audit Committee
The current members of our audit committee are, David Gill, Hilde H. Steineger and Jeffrey Sherman, with Mr. Gill serving as chairman. Our board of directors has determined that each member of our audit committee is independent under Rule 10A-3 of the Exchange Act and the applicable listing requirements of Nasdaq, and that each member of our audit committee satisfies the other listing requirements of Nasdaq for audit committee membership. Our board of directors has also determined that two of the three members of our audit committee, Mr. Gill and Dr. Steineger, qualify as an “audit committee financial expert,” as such term is defined by the SEC, and that he or she has the requisite level of financial sophistication required by the continued listing standards of Nasdaq.
82
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table (2019 and 2018)
The following table sets forth information concerning cash and non-cash compensation paid for 2019 and 2018 to certain of our executive officers (referred to herein as “our executive officers”).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
Stock |
|
|
Option |
|
All Other |
|
|
|
||||
|
|
|
|
|
Salary |
|
Bonus |
|
|
Awards |
|
|
Awards |
|
Compensation |
|
|
|
||||
|
Name and position |
|
Year |
|
($) |
|
($)(1) |
|
($)(2) |
|
($)(2) |
|
($)(3) |
|
Total |
|
||||||
|
John H. Johnson(4) |
|
2019 |
|
$ |
114,400 |
|
$ |
- |
|
$ |
- |
|
$ |
274,411 |
|
$ |
202,388 |
|
$ |
591,199 |
|
|
Executive Chairman |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Matthew Pauls (5) |
|
2019 |
|
|
524,425 |
|
|
230,345 |
|
|
233,500 |
|
|
1,306,841 |
|
|
156,218 |
|
|
2,451,329 |
|
|
Former Chief Executive Officer |
|
2018 |
|
|
540,000 |
|
|
400,000 |
|
|
- |
|
|
1,692,093 |
|
|
18,837 |
|
|
2,650,929 |
|
|
Fredric Cohen, M.D. |
|
2019 |
|
|
427,859 |
|
|
145,472 |
|
|
114,415 |
|
|
385,518 |
|
|
20,563 |
|
|
1,093,827 |
|
|
Chief Medical Officer |
|
2018 |
|
|
411,403 |
|
|
235,987 |
|
|
- |
|
|
560,808 |
|
|
25,056 |
|
|
1,233,254 |
|
|
Robert Lutz |
|
2019 |
|
|
373,298 |
|
|
144,375 |
|
|
114,415 |
|
|
359,381 |
|
|
25,458 |
|
|
1,016,927 |
|
|
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Brian Davis (6) |
|
2019 |
|
|
261,283 |
|
|
88,785 |
|
|
93,400 |
|
|
354,481 |
|
|
216,062 |
|
|
1,014,010 |
|
|
Former Chief Financial Officer |
|
2018 |
|
|
374,351 |
|
|
201,676 |
|
|
- |
|
|
555,973 |
|
|
23,414 |
|
|
1,155,414 |
|
|
(1) |
The amounts in this column represent the discretionary bonuses paid with respect to 2019 and 2018 performance. |
|
(3) |
All other compensation received that does not properly report in any other column of the table including insurance premiums paid by Strongbridge with respect to term life insurance, company match on employee’s 401(k) contributions and club membership fees. Included in Mr. Pauls’ amount is $139,000 of payments made in 2019 in connection with his termination of employment with the Company. Included in Mr. Johnson’s amount is $201,926 received as director compensation for 2019, which includes $78,326 of fees earned and $123,600 in stock awards granted to Mr. Johnson. See “Director Compensation (2019)” for additional information concerning the compensation paid to Mr. Johnson as a director in 2019. Included Mr. Davis’s amount is $181,000 of payments made in 2019 in connection with his termination of employment with the Company. |
|
(4) |
Mr. Johnson was appointed Executive Chairman (principal executive officer) of the Company effective November 1, 2019. |
|
(5) |
Mr. Pauls resigned as Chief Executive Officer effective November 1, 2019. |
|
(6) |
Mr. Davis resigned as Chief Financial Officer effective September 3, 2019. |
Narrative to Summary Compensation Table
We have entered into an Executive Chairman Agreement with Mr. Johnson and employment agreements with each of Mr. Lutz and Dr. Cohen, as well as with Messrs. Pauls and Davis prior to their resignation. These agreements outline the terms of the employment relationship, including any potential severance benefits. We believe that these agreements provide certainty to our management team and help to retain the leadership necessary for our company to succeed.
Executive Chairman Agreement
We entered into an Executive Chairman Agreement with Mr. Johnson, pursuant to which Mr. Johnson will serve as Executive Chairman until the date our new Chief Executive Officer commences employment with us (unless terminated sooner by reason of Mr. Johnson’s death, disability, resignation or removal). Under the terms of the Executive Chairman Agreement, Mr. Johnson will be paid a monthly salary of not less than $57,200 and will be eligible to participate in and receive benefits under our employee benefit plans that are generally made available to our executive officers. Mr. Johnson was also granted an option to purchase 275,000 shares of common stock (the “Johnson Option”) under the Company’s 2015 Equity Compensation Plan (the “2015 Plan”), at an exercise price equal to the closing price
83
per share of the Company’s common stock as reported on Nasdaq on the grant date. The Johnson Option will vest and become exercisable over a period of four years from the grant date, with 6.25% of the grant vesting on each of the 16 quarterly anniversaries of the grant date.
The Executive Chairman Agreement will be effective for an initial term (the “Johnson Initial Term”) of six months beginning November 1, 2019. Following the Johnson Initial Term, the Executive Chairman Agreement will be automatically extended for additional one-month periods (each a “Johnson Additional Term”) unless, at least 30 days prior to the then-scheduled date of expiration of the Johnson Initial Term or 15 days prior to the then-scheduled expiration of any Johnson Additional Term, as applicable, either (i) the board of directors gives notice to Mr. Johnson that it is electing not to extend the term of the Executive Chairman Agreement, or (ii) Mr. Johnson gives notice to the board of directors that he is electing not to extend the term of the Executive Chairman Agreement.
The Executive Chairman Agreement provides for severance benefits detailed below under “Potential Payments upon Terminations of Employment or Following a Change in Control.”
Employment Agreements
We entered into employment agreements with each of Dr. Cohen and Mr. Lutz for their service as Chief Medical Officer and Chief Financial Officer, respectively. The agreements are effective until terminated by either the Company or the executive officer, in either case in accordance with the terms of the agreement. Under the terms of the employment agreements, Dr. Cohen is entitled to receive an annual base salary of $442,834 and Mr. Lutz is entitled to receive an annual base salary of $404,250. Pursuant to the terms of these agreements, the annual incentive bonus targets for Dr. Cohen and Mr. Lutz are 40% of their annual base salary. Our executive officers are also entitled to participate in benefits offered by us for similarly situated employees, including the Company’s paid time-off policy.
Prior to their resignations, we were also a party to employment agreements with Messrs. Pauls and Davis. Under these agreements, Messrs. Pauls and Davis were entitled to receive an annual basis salary of $572,100 and $387,453, respectively, with an annual incentive bonus target of 55% and 40% of their annual base salaries, respectively. Messrs. Pauls and Davis were also entitled to participate in benefits offered by us for similarly situated employees, including the Company’s paid time-off policy.
The employment agreements with our executive officers provide for severance benefits detailed below under “Potential Payments upon Terminations of Employment or Following a Change in Control.” Each employment agreement also contains a non‑competition provision, which applies during the term of employment and for one year following termination, and a restrictive covenant with respect to non‑disclosure of confidential information, which remains in effect during the term of employment and at all times thereafter.
Other Benefits
Our executive officers (including the named executive officers) are eligible to participate in our employee benefit plans on the same basis as our other employees, including our health and welfare plans and our 401(k) plan. Under our 401(k) plan, participants may elect to make both pre‑ and post‑tax contributions to their accounts in the plan, and we match 100% of those contributions up to 4% of compensation. Our executive officers are not eligible for retirement benefits other than under our 401(k) plan. We are not required to, and have not, set aside any amounts relating to pension or retirements.
Consulting Agreement with Mr. Davis
Effective September 3, 2019, the Company entered into a consulting agreement with Mr. Davis (the “Davis Consulting Agreement”), pursuant to which Mr. Davis agreed to provide certain advisory services to the Company relating to the Company’s business, financial reporting or financial statements, or other business matters. Pursuant to the terms of the consulting agreement, the Company agreed to pay Mr. Davis $5,000 per month (or such pro rata portion in the event of termination) through the close of business on September 2, 2020 the “Davis Initial Term”. In addition, the Davis Consulting Agreement provides that, notwithstanding any contrary provision of the 2015 Plan, any vested stock
84
options held by Mr. Davis as of the effective date of the Davis Consulting Agreement will continue to be exercisable during the Davis Initial Term.
Outstanding Equity Awards as of December 31, 2019
The following table includes certain information with respect to option that were outstanding as of December 31, 2019 for our executive officers.
|
|
|
Option Awards |
|
Stock Awards |
|
|
||||||||||||
|
|
|
Number of |
|
Number of |
|
|
|
|
|
|
|
|
|
|
|
Market |
|
|
|
|
|
Securities |
|
Securities |
|
|
|
|
|
|
|
|
Number of |
|
|
Value of |
|
|
|
|
|
Underlying |
|
Underlying |
|
|
|
|
|
|
|
|
Shares or |
|
|
Shares or |
|
|
|
|
|
Unexercised |
|
Unexercised |
|
Option |
|
|
|
|
|
Units of |
|
|
Units of |
|
|
|
|
|
|
Options |
|
Options |
|
Exercise |
|
|
|
Option |
|
Stock That |
|
|
Stock That |
|
|
|
|
|
|
(#) |
|
(#) |
|
Price |
|
Grant |
|
Expiration |
|
Have Not |
|
|
Have not |
|
|
|
|
Name |
|
Exercisable |
|
Unexercisable |
|
($) |
|
Date |
|
Date |
|
Vested (#) |
|
|
Vested ($) |
(1) |
|
|
|
John Johnson |
|
18,181 |
|
— |
|
$ |
10.74 |
|
3/17/2015 |
|
3/17/2020 |
|
|
|
|
|
|
|
|
|
|
18,181 |
|
— |
|
$ |
13.43 |
|
3/17/2015 |
|
3/17/2020 |
|
|
|
|
|
|
|
|
|
|
18,181 |
|
— |
|
$ |
16.11 |
|
3/17/2015 |
|
3/17/2020 |
|
|
|
|
|
|
|
|
|
|
13,224 |
|
— |
|
$ |
17.55 |
|
10/16/2015 |
|
10/16/2025 |
|
|
|
|
|
|
|
|
|
|
40,000 |
|
— |
|
$ |
5.50 |
|
5/12/2016 |
|
5/12/2026 |
|
|
|
|
|
|
|
|
|
|
40,000 |
|
— |
|
$ |
4.40 |
|
5/11/2017 |
|
5/11/2027 |
|
|
|
|
|
|
|
|
|
|
40,000 |
|
— |
|
$ |
7.75 |
|
5/15/2018 |
|
5/15/2028 |
|
|
|
|
|
|
|
|
|
|
— |
|
275,000 |
(2) |
$ |
1.56 |
|
11/14/2019 |
|
11/14/2029 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40,000 |
|
$ |
83,600 |
(5) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Matthew Pauls |
|
303,181 |
|
— |
(3) |
$ |
15.71 |
|
5/26/2015 |
|
5/26/2025 |
|
|
|
|
|
|
|
|
|
|
150,251 |
|
14,062 |
(3) |
$ |
3.94 |
|
2/26/2016 |
|
2/26/2026 |
|
|
|
|
|
|
|
|
|
|
187,500 |
|
93,751 |
(3) |
$ |
2.90 |
|
2/23/2017 |
|
2/23/2027 |
|
|
|
|
|
|
|
|
|
|
153,125 |
|
87,500 |
(3) |
$ |
6.65 |
|
2/5/2018 |
|
2/5/2028 |
|
|
|
|
|
|
|
|
|
|
75,000 |
|
100,000 |
(3) |
$ |
4.67 |
|
2/20/2019 |
|
2/20/2029 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fredric Cohen, M.D. |
|
81,818 |
|
— |
|
$ |
18.12 |
|
8/5/2015 |
|
8/5/2025 |
|
|
|
|
|
|
|
|
|
|
28,125 |
|
1,875 |
(2) |
$ |
3.94 |
|
2/26/2016 |
|
2/26/2026 |
|
|
|
|
|
|
|
|
|
|
35,000 |
|
5,000 |
(4) |
$ |
4.16 |
|
6/13/2016 |
|
6/13/2026 |
|
|
|
|
|
|
|
|
|
|
7,500 |
|
2,500 |
(4) |
$ |
3.90 |
|
11/23/2016 |
|
11/23/2026 |
|
|
|
|
|
|
|
|
|
|
118,938 |
|
54,062 |
(2) |
$ |
2.90 |
|
2/23/2017 |
|
2/23/2027 |
|
|
|
|
|
|
|
|
|
|
50,750 |
|
65,250 |
(2) |
$ |
6.65 |
|
2/5/2018 |
|
2/5/2028 |
|
|
|
|
|
|
|
|
|
|
22,125 |
|
95,875 |
(2) |
$ |
4.67 |
|
2/20/2019 |
|
2/20/2029 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24,500 |
|
$ |
51,205 |
(6) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Robert Lutz |
|
15,150 |
|
— |
|
$ |
14.37 |
|
6/25/2015 |
|
6/25/2025 |
|
|
|
|
|
|
|
|
|
|
59,063 |
|
3,937 |
(2) |
$ |
3.94 |
|
2/26/2016 |
|
2/26/2026 |
|
|
|
|
|
|
|
|
|
|
120,313 |
|
54,687 |
(2) |
$ |
2.90 |
|
2/23/2017 |
|
2/23/2027 |
|
|
|
|
|
|
|
|
|
|
49,875 |
|
64,125 |
(2) |
$ |
6.65 |
|
2/5/2018 |
|
2/5/2028 |
|
|
|
|
|
|
|
|
|
|
20,625 |
|
89,375 |
(2) |
$ |
4.67 |
|
2/20/2019 |
|
2/20/2029 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24,500 |
|
$ |
51,205 |
(6) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A. Brian Davis |
|
54,545 |
|
— |
|
$ |
15.71 |
|
5/26/2015 |
|
5/26/2025 |
|
|
|
|
|
|
|
|
|
|
133,363 |
|
— |
|
$ |
18.80 |
|
7/21/2015 |
|
7/21/2020 |
|
|
|
|
|
|
|
|
|
|
56,875 |
|
— |
|
$ |
3.94 |
|
2/26/2016 |
|
2/26/2026 |
|
|
|
|
|
|
|
|
|
|
112,500 |
|
— |
|
$ |
2.90 |
|
2/23/2017 |
|
2/23/2027 |
|
|
|
|
|
|
|
|
|
|
43,125 |
|
— |
|
$ |
6.65 |
|
2/5/2018 |
|
2/5/2028 |
|
|
|
|
|
|
|
|
|
|
13,563 |
|
— |
|
$ |
4.67 |
|
2/20/2019 |
|
2/20/2029 |
|
|
|
|
|
|
|
|
(1) |
The market value of shares of stock that have not vested is based on the closing price of our common stock on December 31, 2019, or $2.09 per share. |
85
|
(2) |
These options vest in 16 equal quarterly installments commencing with the first quarter subsequent to the grant date. These options will fully vest and become exercisable upon a change of control provided that the executive is employed on the date of such change of control. |
|
(3) |
These options vest through November 30, 2020, in each case according to their vesting schedule, and all options that were vested as of the date of Mr. Pauls’s resignation (or that will vest through November 30, 2020) will remain exercisable through November 30, 2020; however, the options that vest solely during the month of November 2020 will remain exercisable through February 26, 2021. |
|
(4) |
One-fourth of the shares underlying these options vested on the one-year anniversary of the Date of Grant and the remaining three-fourths of the shares underlying these options vest/vested in quarterly installments after the one-year anniversary of the Date of Grant. These options will fully vest and become exercisable upon a change in control provided that the executive is employed on the date of such change in control. |
|
(5) |
The restricted stock units vest on May 15, 2020, provided Mr. Johnson provides services to the Company through such date. |
|
(6) |
The restricted stock units vest on February 20, 2021. |
Prior to September 3, 2015, we did not have an equity compensation plan. Grants of stock options to the executive officers and other individuals were made through individual grant agreements.
2020 Equity Awards
On January 27, 2020, our board of directors approved grants of stock options for Dr. Cohen and Mr. Lutz in the amounts of 300,000 and 275,000, respectively. These stock options vest in sixteen equal quarterly installments beginning April 27, 2020, provided the executive officer is employed by the Company on each vesting date. All stock options will fully vest upon a change of control of our company.
Potential Payments Upon Terminations of Employment or Following a Change of Control
Executive Chairman Agreement
Pursuant to the terms of the Executive Chairman Agreement, we may terminate Mr. Johnson’s employment at any time; provided, however, that if we terminate Mr. Johnson’s employment, Mr. Johnson will be entitled to receive, subject (in the case of clauses (ii) through (iv)) to his execution and non-revocation of a customary release of claims in favor of the Company and its affiliates, (i) any accrued but unpaid base salary and vested benefits through the date his employment is terminated, (ii) if his employment is terminated on or before the last day of the Johnson Initial Term, a single lump sum cash amount equal to the base salary that he would have otherwise received had his employment not been terminated, from the date his employment is terminated through the expiration of the Johnson Initial Term; (iii) subject to his election, a payment of 100% of the COBRA payments for health and welfare coverage that he held as of the date his employment is terminated (for a period of 18 months); and (iv) if his employment is terminated other than pursuant to a delivery of notice by us not to renew the Executive Chairman Agreement, immediate acceleration of vesting of all of his outstanding equity awards. In addition, upon any involuntary termination of Mr. Johnson’s service on the board of directors, the Executive Chairman Agreement provides for immediate acceleration of vesting of all of his outstanding equity awards.
Employment Agreements
The employment agreements with our other executive officers (including Mr. Lutz and Dr. Cohen, and Messrs. Pauls and Davis prior to their resignations) provide that, upon a termination of employment by our company without “cause,” or by the executive for “good reason,” or due to the executive’s death, subject to the execution of a release of claims, he or she will be entitled to (1) an amount equal to the sum of 12 months of base salary and the target bonus, paid in installments over the 12‑month period following termination, (2) a pro rata portion of the annual bonus that he would have been entitled to receive for the calendar year that includes the termination date, based on the actual achievement of the applicable performance goals, and (3) medical and dental benefits provided by us that are at least equal to the level of benefits provided to other similarly situated active employees until the earlier of (a) 12 months following the termination date and (b) the date the executive becomes covered under a subsequent employer’s medical and dental plans.
86
In the event there is a change of control of our company and, during the 24‑month period following the change of control, any of our executive officers is terminated by us without cause, by the executive for good reason, or due to the executive’s death or, he or she will be entitled to the severance benefits detailed below and all unvested equity or equity‑based awards held by the executive will accelerate and vest. The severance benefits include (1) an amount equal to the sum of 18 months base salary and the target bonus, paid in installments over the 18‑month period following termination; and (2) the medical and dental benefits provided by us until the earlier of (a) one year following the termination date and (b) the date the executive becomes covered under a subsequent employer’s medical and dental plans.
Under the employment agreements, “cause” is defined as (1) the conviction of, or plea of guilty or nolo contendere to, any felony or any crime involving theft, embezzlement, dishonesty or moral turpitude, (2) any act constituting willful misconduct, deliberate malfeasance, dishonesty, or gross negligence in the performance of the individual’s duties, (3) the willful and continued failure to perform any of the individual’s duties, which has not been cured within 30 days following written notice from us, or (4) any material breach by the individual of the employment agreement or any other agreement with us, which has not been cured within 30 days following written notice from us. “Good reason” is defined as any of the following reasons unless cured by us within a specified period: (1) a material reduction of the individual’s base salary, other than a reduction that is applicable to other senior executives in the same manner and proportion, (2) the assignment of duties or responsibilities which are materially inconsistent with the individual’s position, (3) a change in the principal location at which the individual performs his or her duties to a new location that is more than 50 miles from the prior location or (4) a material breach of the employment agreement by us. “Change of control ” is defined as the occurrence of any of the following: (a) any person or group of persons becomes the beneficial owner, directly or indirectly, of securities of the Company representing more than fifty percent (50%) of the combined voting power of the Company’s then outstanding securities; provided that if the person or group of persons is already deemed to own more than 50% of the total fair market value or total voting power, then the acquisition of additional stock by such person or group of persons shall not constitute an additional change of control; (b) the stockholders of the Company approve a plan of complete liquidation of the Company; (c) the sale or disposition of all or substantially all of the Company’s assets; or (d) a merger, consolidation or reorganization of the Company with or involving any other entity, other than a merger, consolidation or reorganization that would result in the voting securities of the Company outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity) at least 50% of the combined voting power of the Company (or such surviving entity) outstanding immediately after such merger, consolidation or reorganization owned in approximately the same proportion of such ownership by each of the prior shareholders as prior to the transaction. The following acquisitions are not considered to be a change of control of the Company: (A) an acquisition by the Company or entity controlled by the Company, or (B) an acquisition by an employee benefit plan (or related trust) sponsored or maintained by the Company.
The employment agreements also provide that, in the event that any of our other executive officers is subject to the excise tax under Section 4999 of the Code, the payments that would be subject to the excise tax will be reduced to the level at which the excise tax will not be applied unless such executive would be in a better net after‑tax position by receiving the full payments and paying the excise tax.
Payments to Messrs. Pauls and Davis in Connection with their Resignations
Pursuant to the terms of his employment agreement, in connection with his resignation effective November 1, 2019, Mr. Pauls will receive severance payments for 18 months, consisting of 18 months of salary and his annual incentive bonus target amount. In addition, pursuant to its authority under the 2015 Plan, the board of directors agreed to extend the vesting and exercisability of the stock options granted to Mr. Pauls on February 26, 2016, February 23, 2017, February 5, 2018 and February 20, 2019 (the “Pauls Options”), such that all Pauls Options that were unvested as of the date of his resignation will continue to vest through November 30, 2020, in each case according to their vesting schedule, and all Pauls Options that were vested as of the date of his resignation (or that will vest through November 30, 2020) will remain exercisable by Mr. Pauls through November 30, 2020; provided, however, that Pauls’ Options that vest solely during the month of November 2020 will remain exercisable by Mr. Pauls through February 26, 2021.
87
Pursuant to the terms of his employment agreement, in connection with his resignation on effective September 3, 2019, Mr. Davis will receive severance payments for 12 months, consisting of 12 months of salary and his annual incentive bonus target amount. In addition, pursuant to its authority under the 2015 Plan, the board of directors agreed to extend the exercisability of Mr. Davis’ vested stock options (as of September 3, 2019) through September 2, 2020.
Director Compensation (2019)
Our board of directors’ compensation program for fiscal year 2019 provided for the following:
|
· |
Annual Cash Retainer—$40,000 |
|
· |
Additional Annual Cash Retainers |
|
· |
Non‑Executive Chairman of the Board Retainer—$35,000 |
|
· |
Audit Committee Chair Retainer—$20,000 |
|
· |
Compensation Committee Chair Retainer—$15,000 |
|
· |
Nomination and Governance Committee Chair Retainer—$10,000 |
|
· |
Audit Committee Member (other than Chairman) Retainer—$10,000 |
|
· |
Compensation Committee Member (other than Chairman) Retainer—$7,000 |
|
· |
Nomination and Governance Committee Member (other than Chairman) Retainer—$4,500 |
|
· |
Transaction Committee Member Retainer—$8,000 |
|
· |
Equity Compensation |
|
· |
Annual Equity Grant—40,000 restricted stock units, vesting in full on the first anniversary of the date of grant, as determined by our board of directors, provided that the director continues to provide services as a member of our board of directors continuously from the date of grant through the vesting date |
Our directors earned compensation in 2019 for their service on the board as summarized below:
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
Fees earned |
|
|
Stock Awards (1) |
|
|
Total |
|
||
|
Name |
|
Year |
|
($) |
|
|
($) |
|
|
($) |
|
||
|
John H. Johnson(2) |
|
2019 |
|
$ |
78,326 |
|
$ |
123,600 |
|
|
$ |
201,926 |
|
|
Richard S. Kollender (3) |
|
2019 |
|
|
50,039 |
|
|
123,600 |
|
|
|
173,639 |
|
|
Garheng Kong, M.D., Ph.D. |
|
2019 |
|
|
51,358 |
|
|
123,600 |
|
|
|
174,958 |
|
|
Jeffrey W. Sherman, M.D., FACP |
|
2019 |
|
|
49,717 |
|
|
123,600 |
|
|
|
173,317 |
|
|
Mårten Steen, M.D., Ph.D. |
|
2019 |
|
|
57,858 |
|
|
123,600 |
|
|
|
181,458 |
|
|
Hilde H. Steineger, Ph.D. |
|
2019 |
|
|
49,717 |
|
|
123,600 |
|
|
|
173,317 |
|
|
David Gill (4) |
|
2019 |
|
|
21,666 |
|
|
128,500 |
|
|
|
150,166 |
|
|
(1) |
Amounts shown represent the aggregate grant date fair value of the restricted stock unit (RSU) awards, computed in accordance with FASB ASC Topic 718. |
88
|
(2) |
Mr. Johnson was appointed Executive Chairman effective November 1, 2019. As of that date, he was no longer entitled to receive any additional compensation in respect of his service as a member of the board of directors. See “Summary Compensation Table (2019 and 2018)” for additional compensation paid to Mr. Johnson as Executive Chairman in 2019. |
|
(3) |
Mr. Kollender was appointed Chief Operating Officer effective September 3, 2019, at which time he resigned from the board of directors. |
|
(4) |
Mr. Gill was appointed to the board of directors effective September 3, 2019. |
The following table includes a summary of outstanding stock options and restricted stock unit grants as of December 31, 2019 for those individuals serving as directors in fiscal year 2019. See “Outstanding Equity Awards as of December 31, 2019” for Mr. Johnson’s outstanding equity awards as of December 31, 2019.
|
|
|
Restricted Stock Units |
|
Options |
|
Name |
|
Outstanding |
|
Outstanding |
|
David Gill |
|
50,000 |
|
— |
|
Richard S. Kollender (1) |
|
100,000 |
|
372,188 |
|
Garheng Kong, M.D., Ph.D. |
|
40,000 |
|
154,385 |
|
Jeffrey W. Sherman, M.D., FACP |
|
40,000 |
|
140,000 |
|
Mårten Steen, M.D., Ph.D. |
|
40,000 |
|
154,918 |
|
Hilde H. Steineger, Ph.D. |
|
40,000 |
|
154,918 |
|
(1) |
The number of outstanding restricted stock units includes a restricted stock unit award of 60,000 ordinary shares of the Company granted to Mr. Kollender on September 3, 2019 in connection with his appointment as Chief Operating Officer and the number of outstanding options includes a nonqualified stock option to purchase 215,000 ordinary shares of the Company granted to Mr. Kollender on September 3, 2019 in connection with his appointment as Chief Operating Officer. |
Non‑Employee Director Equity Compensation Plan
Our board of directors has adopted, and our shareholders have approved, the Non‑Employee Director Equity Compensation Plan (the “Non‑Employee Director Plan”). The Non‑Employee Director Plan provides for the grant of nonstatutory stock options, stock awards, and restricted stock units to our non‑employee directors. The Non‑Employee Director Plan is effective as of September 3, 2015.
Authorized Shares. As of the date of this report, a total of 1,099,514 ordinary shares have been reserved for issuance pursuant to the Non‑Employee Director Plan. The ordinary shares that we have reserved for issuance pursuant to the Non‑Employee Director Plan (the “Share Pool”) will be increased on the first day of each fiscal year, in an amount equal to one‑half percent (0.5%) of the outstanding shares on the last day of the immediately preceding fiscal year. The Share Pool will be reduced on the date of grant, by one ordinary share of our ordinary for each award under the Non‑Employee Director Plan; provided that awards that are valued by reference to shares of our ordinary but are required to be paid in cash pursuant to their terms will not reduce the Share Pool. If and to the extent options terminate, expire, or are canceled, forfeited, exchanged, or surrendered without having been exercised, or if any stock awards or awards of restricted stock units (including restricted stock received upon the exercise of options) are forfeited, the ordinary shares subject to such awards will again be available for awards under the Share Pool. Notwithstanding the foregoing, shares tendered by individual grantees, or withheld by us, as full or partial payment to us upon the exercise of options will not become available for issuance again under the Non‑Employee Director Plan.
Plan Administration. Our board administers the Non‑Employee Director Plan. Subject to the provisions of the Non‑Employee Director Plan, our board has the power to determine the terms of the awards, including the exercise price, the number of shares of our ordinary shares subject to each such award, the exercisability of the awards and the form of consideration, if any, payable upon exercise. To the maximum extent permitted by law, no member of our board will be liable for any action taken or decision made in good faith relating to the Non‑Employee Director Plan or any award granted thereunder.
Stock Options. The exercise price of options granted under the Non‑Employee Director Plan may be equal to or greater than the fair market value of our ordinary shares on the date of grant. The term of an option may not exceed ten
89
years. After the termination of service of a non‑employee director for any reason other than death, disability or cause (as defined in the Non‑Employee Director Plan), he or she may exercise the vested portion of his or her option for 90 days. If termination is due to death (or death occurs within 90 days after the director’s termination date) or disability, the vested portion of the option will remain exercisable for one year. However, in no event may an option be exercised later than the expiration of its term. All options are forfeited upon a termination for Cause. In addition, if a non‑employee director has engaged in conduct that constitutes cause, any shares acquired upon exercise of an option for which we have not yet delivered the share certificates shall be automatically forfeited to us in exchange for payment of the exercise price paid for such shares.
Stock Awards. Stock awards may be granted under the Non‑Employee Director Plan. Stock awards are grants of our ordinary shares that vest in accordance with terms and conditions established by the board. The board will determine the number of shares granted as stock awards to a non‑employee director and the consideration, if any, to be paid for such shares. The board may impose whatever conditions to vesting it determines to be appropriate (for example, the board may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the board, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Ordinary shares subject to stock awards that do not vest are subject to forfeiture.
Restricted Stock Units. Restricted stock units may be granted under the Non‑Employee Director Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one ordinary share. The board determines the terms and conditions of restricted stock units, including the vesting criteria (which may include accomplishing specified performance criteria or continued service to us) and the form and timing of payment. The amount payable as a result of the vesting of a restricted stock unit will be distributed as soon as practicable following the vesting date and in no event later than the fifteenth date of the third calendar month of the year following the vesting date of the restricted stock unit (or as otherwise permitted under Section 409A of the Internal Revenue Code); provided, however, that an individual grantee may, if and to the extent permitted by our board, elect to defer payment of restricted stock units in a manner permitted by Section 409A of the Internal Revenue Code. Notwithstanding the foregoing, the board, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Non‑Transferability of Awards. Unless our board provides otherwise, the Non‑Employee Director Plan generally does not allow for the transfer of awards and only the recipient of an award may exercise an award during his or her lifetime.
Certain Adjustments. In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the Non‑Employee Director Plan, the board will adjust the number and class of shares that may be delivered under the Non‑Employee Director Plan and/or the number, class and price per share of shares covered by each outstanding award.
Change of Control. The Non‑Employee Director Plan provides that in the event of a change of control, as defined in the Non‑Employee Director Plan, where we are not the surviving corporation (or we survive only as a subsidiary of another corporation), unless our board determines otherwise, all outstanding awards will be assumed by, or replaced with comparable awards by, the surviving corporation (or a parent or subsidiary of the surviving corporation). In the event the surviving corporation in such change of control (or a parent or subsidiary of the surviving corporation) does not assume or replace the outstanding awards with comparable awards, (i) we will provide written notice of such change of control to each individual grantee with outstanding awards; (ii) all outstanding options will automatically accelerate and become fully vested and exercisable; (iii) all outstanding stock awards will become vested and deliverable in accordance with the Non‑Employee Director Plan; and (iv) all outstanding restricted stock units will become vested and deliverable in accordance with the Non‑Employee Director Plan.
Notwithstanding the foregoing, if there is a change of control, our board may require that grantees surrender outstanding options in exchange for a payment of cash or stock equal to the amount by which the fair market value of the shares exceeds the exercise price and/or, after giving grantees an opportunity to exercise options, terminate all unexercised options, with such surrender or termination taking place as of the date of the change of control or such other date that our board specifies.
90
Amendment; Termination. Our board has the authority to amend, suspend or terminate the Non‑Employee Director Plan provided such action does not impair the existing rights of any participant. The Non‑Employee Director Plan automatically terminates in 2025, unless we terminate it sooner. We will obtain shareholder approval of any amendment to the Non‑Employee Director Plan as required by applicable law or listing requirements.
Equity Compensation Plan
Our board of directors has adopted, and our shareholders have approved, the 2015 Equity Compensation Plan (the “2015 Plan”). The 2015 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to our employees and any parent or subsidiary corporations’ employees, and for the grant of nonstatutory stock options, stock awards, and restricted stock units to our employees, directors and consultants and our parent or subsidiary corporations’ employees and consultants. The 2015 Plan is effective as of September 3, 2015.
Authorized Shares. As of the date of this report, a total of 7,114,308 ordinary shares have been reserved for issuance pursuant to the 2015 Plan. The ordinary shares that we have reserved for issuance pursuant to the 2015 Plan (the “Share Pool”) will be increased on the first day of each fiscal year in an amount equal to four percent (4.0%) of the outstanding ordinary shares on the last day of the immediately preceding fiscal year. A maximum of 1,000,000 ordinary shares may be subject to awards made under the 2015 Plan to any individual during a calendar year, subject to adjustment as provided in the 2015 Plan. The maximum number of ordinary shares that may be issued under the 2015 Plan as incentive stock options is 7,114,308. The Share Pool will be reduced on the date of grant, by one ordinary share for each award under the 2015 Plan; provided that awards that are valued by reference to ordinary shares but are required to be paid in cash pursuant to their terms will not reduce the Share Pool. If and to the extent options terminate, expire, or are canceled, forfeited, exchanged, or surrendered without having been exercised, or if any stock awards or awards of restricted stock units (including restricted stock received upon the exercise of options) are forfeited, the ordinary shares subject to such awards will again be available for awards under the Share Pool. Notwithstanding the foregoing, the following ordinary shares will not become available for issuance under the 2015 Plan: (i) shares tendered by individual grantees, or withheld by us, as full or partial payment to us upon the exercise of options granted under the 2015 Plan and (ii) shares withheld by, or otherwise remitted to us to satisfy an individual grantee’s tax withholding obligations upon the lapse of restrictions on stock awards, or the exercise of options granted under the 2015 Plan.
Plan Administration. Our compensation committee administers the 2015 Plan. Subject to the provisions of the 2015 Plan, our compensation committee has the power to determine the terms of the awards, including the exercise price, the number of shares of our ordinary shares subject to each such award, the exercisability of the awards and the form of consideration, if any, payable upon exercise. To the maximum extent permitted by law, no member of our board or our compensation committee will be liable for any action taken or decision made in good faith relating to the 2015 Plan or any award granted thereunder.
Stock Options. The exercise price of options granted under the 2015 Plan may be equal to or greater than the fair market value of our ordinary shares on the date of grant. The term of an option may not exceed ten years, except that the term of an incentive stock option granted to any employee who owns more than 10% of the voting power of all classes of our outstanding stock must not exceed five years and the exercise price must equal to at least 110% of the fair market value of our ordinary shares on the grant date. After the termination of service of an employee, director or consultant for any reason other than death, disability or cause (as defined in the 2015 Plan), he or she may exercise the vested portion of his or her option for 90 days. If termination is due to death (or death occurs within 90 days after the individual’s termination date) or disability, the vested portion of the option will remain exercisable for one year. However, in no event may an option be exercised later than the expiration of its term. All options are forfeited upon a termination for Cause. In addition, if an employee, director or consultant has engaged in conduct that constitutes cause, any shares acquired upon exercise of an option for which we have not yet delivered the share certificates shall be automatically forfeited to us in exchange for payment of the exercise price paid for such shares.
Stock Awards. Stock awards may be granted under the 2015 Plan. Stock awards are grants of ordinary shares that vest in accordance with terms and conditions established by the compensation committee. The compensation committee will determine the number of shares granted as stock awards to any employee, director, or consultant and the consideration, if any, to be paid for such shares. The compensation committee may impose whatever conditions to vesting it determines to be appropriate (for example, the compensation committee may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the compensation
91
committee, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Ordinary shares subject to stock awards that do not vest are subject to forfeiture.
Restricted Stock Units. Restricted stock units may be granted under the 2015 Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one ordinary share. The compensation committee determines the terms and conditions of restricted stock units, including the vesting criteria (which may include accomplishing specified performance criteria or continued service to us) and the form and timing of payment. The amount payable as a result of the vesting of a restricted stock unit will be distributed as soon as practicable following the vesting date and in no event later than the fifteenth date of the third calendar month of the year following the vesting date of the restricted stock unit (or as otherwise permitted under Section 409A of the Internal Revenue Code); provided, however, that an individual grantee may, if and to the extent permitted by our compensation committee, elect to defer payment of restricted stock units in a manner permitted by Section 409A of the Internal Revenue Code. Notwithstanding the foregoing, the compensation committee, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Performance‑Based Awards. Certain stock awards or restricted stock units granted under the 2015 Plan may be granted in a manner that should be deductible by us under Section 162(m) of the Internal Revenue Code. These awards, referred to as performance‑based awards, will be determined based on the attainment of written performance goals approved by the compensation committee. The performance‑based awards will be based upon one or more of the following objective criteria: (i) consolidated earnings before or after taxes (including earnings before interest, taxes, depreciation and amortization); (ii) net income; (iii) operating income; (iv) earnings per share; (v) return on shareholders’ equity; (vi) attainment of strategic and operational initiatives; (vii) customer income; (viii) economic value‑added models; (ix) maintenance or improvement of profit margins; (x) stock price (including total shareholder return), including, without limitation, as compared to one or more stock indices; (xi) market share; (xii) revenues, sales or net sales; (xiii) return on assets; (xiv) book value per share; (xv) expense management; (xvi) improvements in capital structure; (xvii) costs; and (xviii) cash flow. The foregoing criteria may relate to the company, one or more of our subsidiaries or one or more of our divisions or units, or any combination of the foregoing, and may be applied on an absolute basis and/or be relative to one or more peer group companies or indices, or any combination thereof, all as determined by the compensation committee. In addition, to the degree consistent with the Internal Revenue Code, the performance criteria may be calculated without regard to extraordinary, unusual and/or non‑recurring items. With respect to performance‑based awards, (i) the compensation committee will establish the objective performance goals applicable to a given period of service while the outcome for that performance period is substantially uncertain and no later than 90 days after the commencement of that period of service (but in no event after 25% of that period of service has elapsed) and (ii) no awards will be granted to any participant for a given period of service until the compensation committee certifies that the objective performance goals (and any other material terms) applicable to that period have been satisfied.
Non‑Transferability of Awards. Unless our compensation committee provides otherwise, the 2015 Plan generally does not allow for the transfer of awards and only the recipient of an award may exercise an award during his or her lifetime.
Certain Adjustments. In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2015 Plan, the compensation committee will adjust the number and class of shares that may be delivered under the 2015 Plan and/or the number, class and price per share of shares covered by each outstanding award, and the numerical share limits set forth in the 2015 Plan.
Change of Control. The 2015 Plan provides that in the event of a change of control, as defined in the 2015 Plan, where we are not the surviving corporation (or we survive only as a subsidiary of another corporation), unless our compensation committee determines otherwise, all outstanding awards will be assumed by, or replaced with comparable awards by, the surviving corporation in such change of control (or a parent or subsidiary of the surviving corporation). In the event the surviving corporation (or a parent or subsidiary of the surviving corporation) in such change of control does not assume or replace the outstanding awards with comparable awards, (i) we will provide written notice of such change of control to each individual grantee with outstanding awards; (ii) all outstanding options will automatically accelerate and become fully vested and exercisable; (iii) all outstanding stock awards will become vested and deliverable in accordance with the 2015 Plan; and (iv) all outstanding restricted stock units will become vested and deliverable in accordance with the 2015 Plan.
92
Notwithstanding the foregoing, if there is a change of control, our board may require that grantees surrender outstanding options in exchange for a payment of cash or stock equal to the amount by which the fair market value of the shares exceeds the exercise price or, after giving grantees an opportunity to exercise options, terminate all unexercised options, with such surrender or termination taking place as of the date of the change of control or such other date that our board specifies.
Amendment; Termination. Our board has the authority to amend, suspend or terminate the 2015 Plan provided such action does not impair the existing rights of any participant. The 2015 Plan automatically terminates in 2025, unless we terminate it sooner. We will obtain shareholder approval of any amendment to the 2015 Plan as required by applicable law or listing requirements.
2017 Inducement Plan
On February 23, 2017, our board of directors adopted the 2017 Inducement Plan (the “Inducement Plan”), pursuant to which we (along with our affiliates and subsidiaries) may grant equity-based awards to new employees. The purpose of the Inducement Plan is to attract valued employees by offering them a greater stake in our success and a closer identity with us, and to encourage ownership of our ordinary shares by such employees.
The Inducement Plan was adopted without shareholder approval pursuant to Rule 5635(c)(4) of the NASDAQ Listing Rules. In accordance with Rule 5635(c)(4) of the NASDAQ Listing Rules, awards under the Inducement Plan may only be made to individuals who were not previously an employee or a non-employee director of the Company or any of our subsidiaries (or who had a bona fide period of non-employment with the Company and our subsidiaries) who is hired by the Company or a subsidiary. Subject to adjustments described in the Inducement Plan, we may issue up to 2,750,000 of our ordinary shares in the form of stock options, stock awards and restricted stock units to eligible recipients.
Administration. Our compensation committee administers the Inducement Plan and is authorized to determine, among other things, the persons to whom inducement awards will be made and the terms of such awards.
Stock Options. The exercise price of options granted under the Inducement Plan will be equal to or greater than the fair market value of our ordinary shares on the date the options are granted, and the term of any option will not exceed ten years from the date of the grant. After a termination of service for any reason other than death, disability or cause (as defined in the Inducement Plan), the grantee of an option award may exercise the vested portion of his or her option for 90 days. If termination is due to death (or death occurs within 90 days after the individual’s termination date) or disability, the vested portion of the option will remain exercisable for one year. However, in no event may an option be exercised later than the expiration of its term. All options are forfeited upon a termination for cause. In addition, if an employee, director or consultant has engaged in conduct that constitutes cause, any shares acquired upon exercise of an option for which we have not yet delivered the share certificates will be automatically forfeited to us in exchange for payment of the exercise price paid for such shares.
Stock Awards and Restricted Stock Units. Ordinary shares issued or transferred pursuant to stock awards may be issued or transferred for consideration or for no consideration, and may be subject to restrictions or no restrictions, as determined by the compensation committee. Each restricted stock unit will be granted with respect to one ordinary share or will have a value equal to the fair market value of one ordinary share. Restricted stock units will be paid in cash, ordinary shares, or other securities, other awards or other property, as determined by the compensation committee, upon the lapse of the restrictions applicable thereto. The amount payable as a result of the vesting of a restricted stock unit will be distributed as soon as practicable following the vesting date and in no event later than the fifteenth date of the third calendar month of the year following the vesting date of the restricted stock unit (or as otherwise permitted under Section 409A of the Internal Revenue Code); provided, however, that an individual grantee may, if and to the extent permitted by our compensation committee, elect to defer payment of restricted stock units in a manner permitted by Section 409A of the Internal Revenue Code. Except as otherwise set forth in an award agreement, if a grantee ceases to be employed by, or provide services to, us, any stock award or restricted stock units held by the grantee that are subject to transfer restrictions will be forfeited.
Non‑Transferability of Awards. Except as otherwise permitted by an award agreement or by our compensation committee, the Inducement Plan generally does not allow for the transfer of awards made under the Inducement Plan, except by will or by the laws of descent and distribution.
93
Certain Adjustments. In the event of certain changes in our capitalization, to prevent dilution or enlargement of the benefits or potential benefits available under the Inducement Plan, the compensation committee will adjust the number and class of shares that may be delivered under the Inducement Plan and/or the number, class and price per share of shares covered by each outstanding award, and the numerical share limits set forth in the Inducement Plan.
Change of Control. The Inducement Plan provides that in the event of a change of control, as defined in the Inducement Plan, where we are not the surviving corporation (or we survive only as a subsidiary of another corporation), unless our compensation committee determines otherwise, all outstanding awards will be assumed by, or replaced with comparable awards by, the surviving corporation in such change of control (or a parent or subsidiary of the surviving corporation). In the event the surviving corporation (or a parent or subsidiary of the surviving corporation) in such change of control does not assume or replace the outstanding awards with comparable awards, (i) we will provide written notice of such change of control to each individual grantee with outstanding awards; (ii) all outstanding options will automatically accelerate and become fully vested and exercisable; (iii) all outstanding stock awards will become vested and deliverable in accordance with the Inducement Plan; and (iv) all outstanding restricted stock units will become vested and deliverable in accordance with the Inducement Plan.
Notwithstanding the foregoing, if there is a change of control, our board may require that grantees surrender outstanding options in exchange for a payment of cash or stock equal to the amount by which the fair market value of the shares exceeds the exercise price or, after giving grantees an opportunity to exercise options, terminate all unexercised options, with such surrender or termination taking place as of the date of the change of control or such other date that our board specifies.
Amendment; Termination. Our board has the authority to amend or terminate the Inducement Plan at any time; provided, however, that the board will not amend the Inducement Plan without shareholder approval if such approval is required in order to comply with applicable laws or stock exchange requirements. The Inducement Plan automatically terminates in 2027, unless we terminate it sooner.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of February 17, 2020 by:
|
· |
each of our directors and director nominees; |
|
· |
each of our “named executive officers”; |
|
· |
all of our directors and executive officers as a group; and |
|
· |
each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our ordinary shares. |
The percentages in the columns entitled “Percentage of Shares Beneficially Owned” are based on a total of 54,247,285 ordinary shares outstanding as of February 17, 2020.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to our ordinary shares. Our ordinary shares subject to options that are currently exercisable or exercisable within 60 days of February 17, 2020 are considered outstanding and beneficially owned by the person holding the options for the purpose of calculating the percentage ownership of that person but not for the purpose of calculating the percentage ownership of any other person. Except as otherwise noted, the persons and entities in this table have sole voting and investing power with respect to all of the ordinary shares beneficially owned by them, subject
94
to community property laws, where applicable. Except as otherwise set forth below, the address of the beneficial owner is c/o Strongbridge Biopharma plc, 900 Northbrook Drive, Suite 200, Trevose, Pennsylvania 19053.
|
|
|
Number of Shares Beneficially Owned |
|
Percentage of Shares Beneficially Owned |
|
5% Shareholders |
|
|
|
|
|
Caxton Alternative Management LP |
(1) |
9,127,987 |
|
16.2% |
|
Novo Nordisk A/S |
(2) |
5,242,000 |
|
9.7% |
|
Longitude Venture Partners III, L.P |
(3) |
4,500,000 |
|
8.3% |
|
Growth Equity Opportunities Fund III, LLC |
(4) |
4,141,308 |
|
7.6% |
|
HealthCap VI, L.P. |
(5) |
3,781,008 |
|
6.9% |
|
Executive Officers and Directors |
|
|
|
|
|
John H. Johnson |
(6) |
204,955 |
|
* |
|
Matthew Pauls |
(7) |
976,039 |
|
1.8% |
|
Fredric Cohen, M.D. |
(8) |
388,127 |
|
* |
|
Robert Lutz |
(9) |
306,775 |
|
* |
|
A. Brian Davis |
|
409,154 |
|
* |
|
David Gill |
|
— |
|
* |
|
Garheng Kong, M.D., Ph.D. |
|
154,384 |
|
* |
|
Jeffrey W. Sherman, M.D., F.A.C.P. |
|
140,000 |
|
* |
|
Mårten Steen, M.D., Ph.D. |
|
154,918 |
|
* |
|
Hilde H. Steineger, Ph.D. |
|
154,918 |
|
* |
|
All current directors and executive officers as a group (13 persons) |
|
3,751,116 |
|
4.4% |
*less than one percent
|
(2) |
Based on the information disclosed in a Schedule 13G filed with the SEC on December 21, 2018 by Novo Nordisk A/S, in which the reporting person reported sole voting and dispositive power with respect to 5,242,000 ordinary shares. The address of the reporting person is Novo All, DK-2880 Bagsværd, Denmark. |
|
(3) |
Based on the information disclosed in a Schedule 13G/A filed with the SEC on February 12, 2019 by Longitude Venture Partners III, L.P. (“LVPIII”), Longitude Capital Partners III, LLC (“LCPIII”), Juliet Tammenoms Bakker and Patrick G. Enright, in which the reporting persons reported shared voting and dispositive power with respect to 4,500,000 ordinary shares. The address of the reporting persons is 2740 Sand Hill Road, Second Floor, Menlo Park, California 94025. |
|
(4) |
Based on the information disclosed in a Schedule 13D/A filed with the SEC on January 6, 2017 by Growth Equity Opportunities Fund III, LLC (“GEO”), New Enterprise Associates 14, L.P. (“NEA 14”), NEA Partners 14, L.P. (“NEA Partners 14”), NEA 14 GP, LTD (“NEA 14 GP”), M. James Barrett, Peter J. Barris, Forest Baskett, Anthony A. Florence, Jr., Patrick J. Kerins, David M. Mott, Scott D. Sandell, Peter W. Sonsini, and Ravi Viswanathan, in which each reporting person reported shared voting and dispositive power with respect to 4,141,308 ordinary shares. The number reported in the table above includes warrants to purchase up to an aggregate of 1,000,000 ordinary shares, which became exercisable subsequent to the Schedule 13D/A filed by the reporting persons. The reporting persons will be prohibited from exercising the warrants, if after giving effect to such exercise, they (together with any of their affiliates) would together beneficially own in excess of 4.99% of our ordinary shares outstanding immediately after giving effect to such exercise or 9.99% if the holder beneficially owns greater than 4.99% of the number of ordinary shares outstanding notwithstanding the ordinary shares issuable upon exercise of this warrant. NEA 14 is the sole member of GEO, NEA Partners 14 is the sole general partner of NEA 14, and NEA 14 GP is the sole general partner of NEA Partners 14. Messrs. Barrett, Barris, Baskett, Florence, Kerins, Mott, Sandell, Sonsini and Viswanathan are the directors of NEA 14 GP. Each reporting person disclaims beneficial |
95
ownership of the ordinary shares reported other than those ordinary shares which such person owns of record. The address of each of GEO, NEA 14, NEA Partners 14, and NEA 14 GP is New Enterprise Associates, 1954 Greenspring Drive, Suite 600, Timonium, MD 21093. The address of the principal business office for each of Messrs. Barris, Florence, Kerins and Mott is New Enterprise Associates, 5425 Wisconsin Avenue, Suite 800, Chevy Chase, MD 20815. The address of the principal business officer for each of Messrs. Baskett, Sandell, Sonsini and Viswanathan is New Enterprise Associates, 2855 Sand Hill Road, Menlo Park, CA 94025. |
|
(5) |
Based on the information disclosed in a Schedule 13G/A filed with the SEC on February 6, 2020 by HealthCap VI, L.P. (“HealthCap”) and HealthCap VI GP S.A. (“HealthCap GP”), in which each reporting person reported shared voting and dispositive power with respect to 3,781,008 ordinary shares, which includes (i) 154,918 ordinary shares issuable upon exercise of options that are exercisable within 60 days and (ii) a warrant to purchase up to an additional 400,000 ordinary shares. The reporting persons will be prohibited from exercising the warrants, if after giving effect to such exercise, they (together with any of their affiliates) would together beneficially own in excess of 4.99% of our ordinary shares outstanding immediately after giving effect to such exercise or 9.99% if the holder beneficially owns greater than 4.99% of the number of ordinary shares outstanding notwithstanding the ordinary shares issuable upon exercise of this warrant. HealthCap GP is the sole general partner of HealthCap. The address of HealthCap and HealthCap GP is 18, Avenue d’Ouchy, 1006 Lausanne, Switzerland. |
|
(6) |
This number includes 17,188 ordinary shares that are subject to options that are or will vest and become exercisable within 60 days as of February 17, 2020. |
|
(7) |
This number includes 62,499 ordinary shares that are subject to options that are or will vest and become exercisable within 60 days as of February 17, 2020. |
|
(8) |
This number includes 23,187 ordinary shares that are subject to options that are or will vest and become exercisable within 60 days as of February 17, 2020. |
|
(9) |
.This number includes 21,749 ordinary shares that are subject to options that are or will vest and become exercisable within 60 days as of February 17, 2020. |
Equity Compensation Plan Information
The table below sets forth information with respect to ordinary shares that may be issued under our equity compensation plans issued as of December 31, 2019:
|
|
|
|
|
|
|
|
|
|
Number of Securities |
|
|
|
|
|
|
|
|
|
|
|
Remaining |
|
|
|
|
Number of Securities to be |
|
|
|
|
|
Available for Future |
||
|
|
|
Issued Upon |
|
|
Weighted-Average |
|
Issuance |
|||
|
|
|
Exercise of Outstanding |
|
|
Exercise Price of Outstanding |
|
Under Equity Compensation |
|||
|
|
|
Options, Warrants |
|
|
|
Options, Warrants |
|
Plans (Excluding Securities |
||
|
Plan Category |
|
and Rights |
|
|
and Rights |
|
Reflected in Column (a)) |
|||
|
|
|
(a) |
|
|
(b) |
|
(c) |
|||
|
Equity compensation plans approved by security holders |
|
|
7,097,609 |
(1) |
|
$ |
4.91 |
|
|
725,531 |
|
Equity compensation plans not approved by security holders |
|
|
1,576,127 |
(2) |
|
|
4.85 |
|
|
1,099,776 |
|
Total |
|
|
8,673,736 |
|
|
|
|
|
|
1,825,307 |
|
(1) |
This number includes the following: (i) 5,998,095 ordinary shares subject to outstanding awards granted under the 2015 Equity Compensation Plan as of December 31, 2019, of which 5,495,896 ordinary shares were subject to outstanding stock options and 502,199 ordinary shares were subject to outstanding restricted stock unit awards; and (ii) 1,099,514 ordinary shares subject to outstanding awards granted under the Non-Employee Director Equity Compensation Plan as of December 31, 2019, of which 827,363 ordinary shares were subject to outstanding stock options and 272,151 ordinary shares were subject to outstanding restricted stock unit awards. |
|
(2) |
This number represents ordinary shares subject to outstanding awards granted under the 2017 Inducement Plan, of which 1,559,127 ordinary shares were subject to outstanding stock options and 17,000 ordinary shares were subject to outstanding restricted stock unit awards as of December 31, 2019. |
96
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Related Party Transactions
On January 25, 2018, we sold 5,000,000 ordinary shares in a public offering. One of our existing shareholders holding in excess of 5% of our outstanding shares prior to the public offering, Broadfin Capital LLC, purchased shares in the public offering for $2.0 million.
Policies and Procedures for Related Party Transactions
We have adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our voting securities and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the prior consent of our audit committee. Any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our voting securities or any member of the immediate family of any of the foregoing persons, in which the amount involved exceeds $120,000 and such person would have a direct or indirect material interest, must first be presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, our audit committee is to consider the material facts of the transaction, including, but not limited to: the benefits to the Company; the impact on a director’s independence in the event the transaction involves a director, an immediate family member of a director or an entity in which a director is a general partner, shareholder or executive officer; the availability of other sources for comparable products or services; the terms of the transaction; and the terms available to unrelated third parties or to employees generally. All of the transactions described above were entered into prior to the adoption of such policy, but after presentation, consideration and approval by our board of directors.
Director Independence
Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that each of Mr. Gill and Drs. Kong, Sherman, Steen and Steineger, representing five of our six directors, is independent under the applicable rules and regulations of the Nasdaq Stock Market (“Nasdaq”). In making such determinations, the board of directors considered the relationships that each such non‑employee director has with the Company and all other facts and circumstances the board of directors deemed relevant in determining their independence. As Executive Chairman, Mr. Johnson no longer qualifies as an independent director under the applicable rules and regulations of Nasdaq.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Principal Accountant Fees and Services
The following table sets forth the aggregate fees billed by Ernst & Young our independent registered public accounting firm as described below:
|
|
|
2019 |
|
2018 |
|
||
|
Fee Category: |
|
(in thousands) |
|
||||
|
Audit Fees(1) |
|
$ |
589 |
|
$ |
730 |
|
|
Audit-Related Fees(2) |
|
|
8 |
|
|
144 |
|
|
Tax Fees(3) |
|
|
— |
|
|
— |
|
|
All Other Fees |
|
|
— |
|
|
— |
|
|
Total Fees |
|
$ |
597 |
|
$ |
874 |
|
|
(1) |
Audit fees consist of fees for the audit of our financial statements, the review of our interim financial statements and statutory audits. |
97
|
(2) |
Audit-related fees included fees for consultations concerning financial and accounting matters not classified as audit services. |
|
(3) |
Tax fees consists of fees incurred for tax compliance, tax advice and tax planning and includes fees for tax return preparation and tax consulting. |
The aggregate fees included in the Audit Fees are billed for the fiscal year. The aggregate fees included in the Audit-related fees and Tax Fees are fees billed in the fiscal year.
All such accountant services and fees were pre-approved by our audit committee in accordance with the “Pre-Approval Policies and Procedures” described below.
Pre-approval policies and procedures
The audit committee of our board of directors has adopted policies and procedures for the pre-approval of audit and non-audit services for the purpose of maintaining the independence of our independent auditor. We may not engage our independent auditor to render any audit or non-audit service unless either the service is approved in advance by the audit committee, or the engagement to render the service is entered into pursuant to the audit committee’s pre-approval policies and procedures.
98
EXHIBIT INDEX
|
|
|
|
|
2.1 |
|
|
|
2.2 |
|
|
|
3.1 |
|
|
|
3.2 |
|
|
|
4.1* |
|
|
|
10.1 |
|
|
|
10.2 |
|
|
|
10.3† |
|
|
|
10.4† |
|
|
|
10.5 |
|
|
|
10.6 |
|
|
|
10.7 |
|
|
|
10.8* |
|
|
|
10.9 |
|
|
|
10.10 |
|
|
|
10.11 |
|
|
|
10.12 |
|
|
|
10.13 |
|
99
|
10.14 |
|
|
|
10.15 |
|
|
|
10.16* |
|
|
|
10.17 |
|
|
|
10.18 |
|
|
|
10.19 |
|
|
|
10.20 |
|
|
|
10.21 |
|
|
|
10.22 |
|
|
|
10.23 |
|
|
|
10.24 |
|
|
|
10.25* |
|
|
|
10.26* |
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
101.INS** |
|
XBRL Instance Document |
|
101.SCH** |
|
XBRL Taxonomy Extension Schema Document |
|
101.CAL** |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
101.LAB** |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
101.PRE** |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
101.DEF** |
|
XBRL Taxonomy Extension Definitions Linkbase Document |
* Filed or furnished herewith.
† Confidential treatment granted as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission.
100
101
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
STRONGBRIDGE BIOPHARMA PLC |
||||
|
|
|
|
|||
|
|
|
|
|||
|
|
By: |
/s/ Robert Lutz |
|||
|
|
|
Name: Robert Lutz |
|||
|
Title: Chief Financial Officer |
|||||
|
Date: February 28, 2020 |
|||||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Robert Lutz, his or her attorney-in-fact, with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto
and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this report has been signed by the following persons on the dates and in the capacities indicated below:
|
|
|
|
|
|
|
/s/ Robert Lutz |
|
Chief Financial Officer (principal executive |
|
February 28, 2020 |
|
Robert Lutz |
|
officer and principal financial officer) |
|
|
|
|
|
|
|
|
|
/s/ Steven McElwaine |
|
Executive Director, Corporate Controller |
|
February 28, 2020 |
|
Steven McElwaine |
|
|
|
|
|
|
|
|
|
|
|
/s/ John H. Johnson |
|
Executive Chairman and Director |
|
February 28, 2020 |
|
John H. Johnson |
|
|
|
|
|
|
|
|
|
|
|
/s/ David Gill |
|
Director |
|
February 28, 2020 |
|
David Gill |
|
|
|
|
|
|
|
|
|
|
|
/s/ Garheng Kong |
|
Director |
|
February 28, 2020 |
|
Garheng Kong |
|
|
|
|
|
|
|
|
|
|
|
/s/ Jeffrey Sherman |
|
Director |
|
February 28, 2020 |
|
Jeffrey Sherman |
|
|
|
|
|
|
|
|
|
|
|
/s/ Marten Steen |
|
Director |
|
February 28, 2020 |
|
Marten Steen |
|
|
|
|
|
|
|
|
|
|
|
/s/ Hilde Steineger |
|
Director |
|
February 28, 2020 |
|
Hilde Steineger |
|
|
|
|
102
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Strongbridge Biopharma plc
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Strongbridge Biopharma Plc. (the Company) as of December 31, 2019 and 2018, the related consolidated statements of operations and comprehensive (loss) income, shareholders' equity and cash flows for each of the three years in the period ended December 31, 2019, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2015.
Philadelphia, Pennsylvania
February 28, 2020
F-1
STRONGBRIDGE BIOPHARMA plc
(In thousands, except share and per share data)
|
|
December 31, |
|
December 31, |
|
||
|
|
2019 |
|
2018 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
57,032 |
|
$ |
122,490 |
|
|
Marketable securities |
|
21,072 |
|
|
— |
|
|
Accounts receivable |
|
2,289 |
|
|
1,626 |
|
|
Inventory |
|
1,993 |
|
|
3,946 |
|
|
Prepaid expenses and other current assets |
|
1,157 |
|
|
4,236 |
|
|
Total current assets |
|
83,543 |
|
|
132,298 |
|
|
Property and equipment, net |
|
291 |
|
|
294 |
|
|
Right of use asset, net |
|
789 |
|
|
— |
|
|
Intangible asset, net |
|
25,110 |
|
|
30,132 |
|
|
Goodwill |
|
7,256 |
|
|
7,256 |
|
|
Other assets |
|
649 |
|
|
305 |
|
|
Total assets |
$ |
117,638 |
|
$ |
170,285 |
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
Accounts payable |
$ |
3,331 |
|
$ |
1,184 |
|
|
Accrued and other current liabilities |
|
20,962 |
|
|
16,065 |
|
|
Total current liabilities |
|
24,293 |
|
|
17,249 |
|
|
Warrant liability |
|
4,127 |
|
|
15,513 |
|
|
Supply agreement liability, noncurrent |
|
15,947 |
|
|
24,568 |
|
|
Other long-term liabilities |
|
1,080 |
|
|
— |
|
|
Total liabilities |
|
45,447 |
|
|
57,330 |
|
|
Commitments and contingencies (Note 10) |
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
Deferred shares, $1.098 par value, 40,000 shares authorized, issued and outstanding at December 31, 2019 and December 31, 2018 |
|
44 |
|
|
44 |
|
|
Ordinary shares, $0.01 par value, 600,000,000 shares authorized at December 31, 2019 and December 31, 2018; 54,205,852 and 54,122,074 shares issued and outstanding at December 31, 2019 and December 31, 2018 |
|
542 |
|
|
541 |
|
|
Additional paid-in capital |
|
332,085 |
|
|
323,402 |
|
|
Accumulated deficit |
|
(260,483) |
|
|
(211,032) |
|
|
Accumulated other comprehensive income |
|
3 |
|
|
— |
|
|
Total stockholders’ equity |
|
72,191 |
|
|
112,955 |
|
|
Total liabilities and stockholders’ equity |
$ |
117,638 |
|
$ |
170,285 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-2
STRONGBRIDGE BIOPHARMA plc
Consolidated Statements of Operations and Comprehensive (Loss) Income
(In thousands, except share and per share data)
|
|
|
Year Ended |
|||||||
|
|
|
December 31, |
|||||||
|
|
|
2019 |
|
2018 |
|
2017 |
|||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
21,676 |
|
$ |
18,027 |
|
$ |
7,046 |
|
Royalty revenues |
|
|
36 |
|
|
— |
|
|
— |
|
Total revenues |
|
|
21,712 |
|
|
18,027 |
|
|
7,046 |
|
|
|
|
|
|
|
|
|
|
|
|
Cost and expenses: |
|
|
|
|
|
||||
|
Cost of sales (excluding amortization of intangible assets) |
|
$ |
3,822 |
|
$ |
3,986 |
|
$ |
1,483 |
|
Selling, general and administrative |
|
|
49,058 |
|
|
63,336 |
|
|
36,292 |
|
Research and development |
|
|
30,903 |
|
|
25,441 |
|
|
17,268 |
|
Amortization of intangible assets |
|
|
5,022 |
|
|
7,187 |
|
|
5,022 |
|
Impairment of intangible asset |
|
|
— |
|
|
— |
|
|
20,723 |
|
Total cost and expenses |
|
|
88,805 |
|
|
99,950 |
|
|
80,788 |
|
Operating loss |
|
|
(67,093) |
|
|
(81,923) |
|
|
(73,742) |
|
Other income (loss), net: |
|
|
|
|
|
|
|
|
|
|
Income from field services agreement |
|
|
12,616 |
|
|
— |
|
|
— |
|
Expense from field services agreement |
|
|
(6,652) |
|
|
— |
|
|
— |
|
Unrealized gain (loss) on fair value of warrants |
|
|
11,386 |
|
|
16,337 |
|
|
(30,218) |
|
Interest expense |
|
|
— |
|
|
(12,515) |
|
|
(4,313) |
|
Loss on extinguishment of debt |
|
|
— |
|
|
(21,549) |
|
|
(3,545) |
|
Gain on sale of subsidiary |
|
|
— |
|
|
130,832 |
|
|
— |
|
Other income, net |
|
|
2,060 |
|
|
1,205 |
|
|
106 |
|
Total other income (loss), net |
|
|
19,410 |
|
|
114,310 |
|
|
(37,970) |
|
(Loss) income before income taxes |
|
|
(47,683) |
|
|
32,387 |
|
|
(111,712) |
|
Income tax expense |
|
|
(1,768) |
|
|
(536) |
|
|
(1,771) |
|
Net (loss) income |
|
|
(49,451) |
|
|
31,851 |
|
|
(113,483) |
|
Other comprehensive income |
|
|
|
|
|
|
|
|
|
|
Unrealized gain on marketable securities |
3 |
— |
— |
||||||
|
Comprehensive (loss) income |
|
$ |
(49,448) |
|
$ |
31,851 |
|
$ |
(113,483) |
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income attributable to ordinary shareholders: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(49,451) |
|
$ |
31,851 |
|
$ |
(113,483) |
|
Diluted |
|
$ |
(60,837) |
|
$ |
15,514 |
|
$ |
(113,483) |
|
Net (loss) income per share attributable to ordinary shareholders: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.91) |
|
$ |
0.69 |
|
$ |
(3.11) |
|
Diluted |
|
$ |
(1.10) |
|
$ |
0.31 |
|
$ |
(3.11) |
|
Weighted-average shares used in computing net (loss) income per share attributable to ordinary shareholders: |
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
54,182,499 |
|
|
46,297,088 |
|
|
36,544,825 |
|
Diluted |
|
|
55,383,030 |
|
|
49,724,503 |
|
|
36,544,825 |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Consolidated Statements of Shareholders’ Equity (Deficit)
(In thousands except share amounts)
|
|
|
Strongbridge Biopharma plc Shareholders |
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
Accumulated Other |
|
Total |
|
||||||
|
|
|
Ordinary Shares |
|
Deferred Shares |
|
Paid-In |
|
Accumulated |
|
Comprehensive |
|
Shareholders’ |
|
||||||||||
|
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
Deficit |
|
Income |
|
Equity (Deficit) |
|
||||||
|
Balance—December 31, 2016 |
|
35,335,026 |
|
$ |
353 |
|
40,000 |
|
$ |
44 |
|
$ |
195,975 |
|
$ |
(129,400) |
|
$ |
— |
|
$ |
66,972 |
|
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(113,483) |
|
|
— |
|
|
(113,483) |
|
|
Stock-based compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
5,167 |
|
|
— |
|
|
— |
|
|
5,167 |
|
|
Issuance of shares, net of offering costs |
|
4,429,799 |
|
|
44 |
|
— |
|
|
— |
|
|
26,340 |
|
|
— |
|
|
— |
|
|
26,384 |
|
|
Issuance of shares in connection with at-the-market facility, net of costs |
|
10,300 |
|
|
* |
|
— |
|
|
— |
|
|
73 |
|
|
— |
|
|
— |
|
|
73 |
|
|
Exercise of stock options |
|
196,081 |
|
|
2 |
|
— |
|
|
— |
|
|
624 |
|
|
— |
|
|
— |
|
|
626 |
|
|
Exercise of warrants |
|
178,606 |
|
|
2 |
|
— |
|
|
— |
|
|
(2) |
|
|
— |
|
|
— |
|
|
— |
|
|
Issuance of warrants related to loan agreements, net |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
2,347 |
|
|
— |
|
|
— |
|
|
2,347 |
|
|
Balance—December 31, 2017 |
|
40,149,812 |
|
$ |
401 |
|
40,000 |
|
$ |
44 |
|
$ |
230,524 |
|
$ |
(242,883) |
|
$ |
— |
|
$ |
(11,914) |
|
|
Net income |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
31,851 |
|
|
— |
|
|
31,851 |
|
|
Stock-based compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
7,807 |
|
|
— |
|
|
— |
|
|
7,807 |
|
|
Issuance of shares, net of offering costs |
|
5,255,683 |
|
|
53 |
|
— |
|
|
— |
|
|
33,455 |
|
|
— |
|
|
— |
|
|
33,508 |
|
|
Issuance of shares to Novo |
|
5,242,000 |
|
|
52 |
|
— |
|
|
— |
|
|
22,331 |
|
|
— |
|
|
— |
|
|
22,383 |
|
|
Issuance of shares to CRG |
|
656,929 |
|
|
7 |
|
— |
|
|
— |
|
|
2,798 |
|
|
— |
|
|
— |
|
|
2,805 |
|
|
Issuance of shares in connection with at-the-market facility, net of costs |
|
1,281,903 |
|
|
13 |
|
— |
|
|
— |
|
|
8,583 |
|
|
— |
|
|
— |
|
|
8,596 |
|
|
Exercise of warrants |
|
1,384,062 |
|
|
14 |
|
— |
|
|
— |
|
|
10,619 |
|
|
— |
|
|
— |
|
|
10,633 |
|
|
Issuance of warrants related to loan agreements |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
7,663 |
|
|
— |
|
|
|
|
|
7,663 |
|
|
Exercise of stock options |
|
50,654 |
|
|
* |
|
— |
|
|
— |
|
|
96 |
|
|
— |
|
|
— |
|
|
96 |
|
|
Ordinary shares issued, net of shares withheld for employee taxes |
|
101,031 |
|
|
1 |
|
— |
|
|
— |
|
|
(474) |
|
|
— |
|
|
— |
|
|
(473) |
|
|
Balance—December 31, 2018 |
|
54,122,074 |
|
$ |
541 |
|
40,000 |
|
$ |
44 |
|
$ |
323,402 |
|
$ |
(211,032) |
|
|
— |
|
$ |
112,955 |
|
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(49,451) |
|
|
— |
|
|
(49,451) |
|
|
Stock-based compensation |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
8,597 |
|
|
— |
|
|
— |
|
|
8,597 |
|
|
Exercise of stock options |
|
43,841 |
|
|
1 |
|
— |
|
|
— |
|
|
178 |
|
|
— |
|
|
— |
|
|
179 |
|
|
Ordinary shares issued, net of shares withheld for employee taxes |
|
39,937 |
|
|
* |
|
— |
|
|
— |
|
|
(92) |
|
|
— |
|
|
— |
|
|
(92) |
|
|
Unrealized gain on marketable securities |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
3 |
|
|
3 |
|
|
Balance—December 31, 2019 |
|
54,205,852 |
|
$ |
542 |
|
40,000 |
|
$ |
44 |
|
$ |
332,085 |
|
$ |
(260,483) |
|
|
3 |
|
$ |
72,191 |
|
* Represents an amount less than $1.
The accompanying notes are an integral part of these consolidated financial statements.
F-4
STRONGBRIDGE BIOPHARMA plc
Consolidated Statements of Cash Flow
(In thousands)
|
|
|
Year Ended |
|
|||||||
|
|
|
December 31, |
|
|||||||
|
|
|
2019 |
|
2018 |
|
2017 |
|
|||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(49,451) |
|
$ |
31,851 |
|
$ |
(113,483) |
|
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
— |
|
|
— |
|
|
— |
|
|
Change in fair value of warrant liability |
|
|
(11,386) |
|
|
(16,337) |
|
|
30,218 |
|
|
Impairment of intangible asset |
|
|
— |
|
|
— |
|
|
20,723 |
|
|
Stock-based compensation |
|
|
8,597 |
|
|
7,807 |
|
|
5,167 |
|
|
Amortization of intangible assets |
|
|
5,022 |
|
|
7,187 |
|
|
5,022 |
|
|
Accretion of discounts on marketable securities |
|
|
(385) |
|
|
— |
|
|
— |
|
|
Interest and related guarantee fees paid in kind |
|
|
— |
|
|
— |
|
|
896 |
|
|
Amortization of debt discounts and debt issuance costs |
|
|
— |
|
|
1,484 |
|
|
482 |
|
|
Loss on extinguishment of debt |
|
|
— |
|
|
21,549 |
|
|
3,545 |
|
|
Gain on sale of subsidiary |
|
|
— |
|
|
(130,832) |
|
|
— |
|
|
Deferred income tax expense |
|
|
— |
|
|
— |
|
|
1,958 |
|
|
Depreciation |
|
|
77 |
|
|
46 |
|
|
10 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(663) |
|
|
(42) |
|
|
(1,584) |
|
|
Inventory |
|
|
1,618 |
|
|
(3,437) |
|
|
(511) |
|
|
Prepaid expenses and other current assets |
|
|
3,079 |
|
|
(3,028) |
|
|
(444) |
|
|
Other assets |
|
|
(798) |
|
|
381 |
|
|
(536) |
|
|
Accounts payable |
|
|
2,153 |
|
|
(63) |
|
|
158 |
|
|
Accrued and other liabilities |
|
|
(2,647) |
|
|
(1,142) |
|
|
3,043 |
|
|
Net cash used in operating activities |
|
|
(44,784) |
|
|
(84,576) |
|
|
(45,336) |
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
Payment for acquisitions |
|
|
— |
|
|
(24,655) |
|
|
(7,500) |
|
|
Purchases of property and equipment |
|
|
(74) |
|
|
(326) |
|
|
— |
|
|
Purchases of marketable securities |
|
|
(56,187) |
|
|
— |
|
|
— |
|
|
Sales and maturities of marketable securities |
|
|
35,500 |
|
|
— |
|
|
— |
|
|
Proceeds from sale of subsidiary |
|
|
— |
|
|
159,311 |
|
|
— |
|
|
Net cash (used in) provided by investing activities |
|
|
(20,761) |
|
|
134,330 |
|
|
(7,500) |
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Proceeds from long-term debt, net |
|
|
— |
|
|
44,930 |
|
|
39,987 |
|
|
Payment for loss on extinguishment of debt |
|
|
— |
|
|
(9,990) |
|
|
(1,300) |
|
|
Repayment of long-term debt |
|
|
— |
|
|
(85,000) |
|
|
(22,261) |
|
|
Proceeds from issuance of ordinary shares, net |
|
|
— |
|
|
33,508 |
|
|
26,384 |
|
|
Proceeds from share subscription to Novo |
|
|
— |
|
|
22,383 |
|
|
— |
|
|
Proceeds from issuance of ordinary shares in connection with at-the-market offering |
|
|
— |
|
|
8,596 |
|
|
73 |
|
|
Proceeds from exercise of warrants |
|
|
— |
|
|
1,176 |
|
|
— |
|
|
Proceeds from exercise of stock options |
|
|
179 |
|
|
96 |
|
|
626 |
|
|
Payments related to tax withholding for net-share settled equity awards |
|
|
(92) |
|
|
(473) |
|
|
— |
|
|
Net cash provided by financing activities |
|
|
87 |
|
|
15,226 |
|
|
43,509 |
|
|
Net (decrease) increase in cash and cash equivalents |
|
|
(65,458) |
|
|
64,980 |
|
|
(9,327) |
|
|
Cash and cash equivalents—beginning of period |
|
|
122,490 |
|
|
57,510 |
|
|
66,837 |
|
|
Cash and cash equivalents—end of period |
|
$ |
57,032 |
|
$ |
122,490 |
|
$ |
57,510 |
|
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for: |
|
|
|
|
|
|
|
|
|
|
|
Interest |
|
$ |
— |
|
$ |
11,122 |
|
$ |
2,935 |
|
|
Income taxes other, net of refunds |
|
$ |
419 |
|
$ |
1 |
|
$ |
127 |
|
|
Supplemental non-cash financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Issuance of shares to settle debt |
|
$ |
— |
|
$ |
2,805 |
|
$ |
— |
|
|
Changes in unrealized gain on marketable securities |
|
$ |
3 |
|
$ |
— |
|
$ |
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-5
STRONGBRIDGE BIOPHARMA plc
Notes to Consolidated Financial Statements
We are a global, commercial-stage biopharmaceutical company focused on the development and commercialization of therapies for rare diseases with significant unmet needs.
Our first commercial product is Keveyis (dichlorphenamide), the first and only treatment approved by the U.S. Food and Drug Administration (the “FDA”) for hyperkalemic, hypokalemic, and related variants of primary periodic paralysis (“PPP”), a group of rare hereditary disorders that cause episodes of muscle weakness or paralysis.
We have two clinical-stage product candidates for rare endocrine diseases, Recorlev and veldoreotide. Recorlev (levoketoconazole) is a cortisol synthesis inhibitor currently being studied for the treatment of endogenous Cushing's syndrome. Veldoreotide is a next-generation somatostatin analog being investigated for potential applications in conditions amenable to somatostatin receptor activation. Both Recorlev and veldoreotide have received orphan designation from the FDA and the European Medicines Agency (“EMA”).
In January 2018, Strongbridge Ireland Limited., one of our wholly-owned subsidiaries, acquired the U.S. and Canadian rights to Macrilen (macimorelin), the first and only oral drug approved by the FDA for the diagnosis of patients with adult growth hormone deficiency. We launched Macrilen in the United States in July 2018. In December 2018, we sold Strongbridge Ireland Limited. to Novo Nordisk Healthcare AG (“Novo”) for $145 million plus the right to receive tiered royalties on net sales of Macrilen through 2027. In addition, Strongbridge U.S. Inc, another of our wholly-owned subsidiaries, entered into an agreement with Novo Nordisk Inc., subsidiary of Novo (“NNI”), pursuant to which NNI funded the costs of 23 of our field-based employees to provide full-time ongoing services to NNI, including the promotion of Macrilen in the United States, for a period of three years. Novo also purchased 5.2 million of our ordinary shares at a purchase price of $7.00 per share. In December, 2019, we reached an agreement with Novo to terminate the services agreement. We received a $6 million payment in connection with such termination and we no longer provide services to Novo.
Liquidity
We believe that our cash, cash equivalents and marketable securities of $78.1 million at December 31, 2019 will be sufficient to allow us to fund planned operations for at least 12 months beyond the issuance date of these consolidated financial statements.
We may never achieve profitability, and unless and until we do, we will continue to need to raise additional capital. We plan to continue to fund our operations and capital funding needs through equity or debt financing along with revenues from Keveyis. There can be no assurances, however, that additional funding will be available on terms acceptable to us.
2. Summary of significant accounting policies and basis of presentation
Basis of presentation and principles of consolidation
The accompanying consolidated financial statements include the accounts of our wholly owned subsidiaries, Strongbridge U.S. Inc. (Trevose, Pennsylvania, United States), Strongbridge Dublin Limited (Dublin, Ireland), Cortendo AB (Gothenburg, Sweden) and Cortendo Cayman (Georgetown, Cayman Islands). All intercompany balances and transactions have been eliminated in consolidation. These audited consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States (“U.S. GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the FASB.
F-6
Revenue recognition
We follow ASC Topic 606, Revenue from Contracts with Customers (“ASC 606”), effective for revenue accounting. Topic 606 applies to all contracts with customers, except for contracts that are within the scope of other standards, such as leases, insurance, collaboration arrangements and financial instruments. Under Topic 606, an entity recognizes revenue when its customer obtains control of promised goods or services, in an amount that reflects the consideration the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of Topic 606, the entity performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. We apply the five-step model to contracts only when it is probable that we will collect the consideration we are entitled to receive in exchange for the goods or services we transfer to the customer. At contract inception, once the contract is determined to be within the scope of Topic 606, we assess the goods or services promised within each contract, determine those that are performance obligations, and assess whether each promised good or service is distinct. We then recognize as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. For a complete discussion of accounting for net product revenue, see Note 3.
Inventory and cost of sales
Inventory is stated at the lower of cost or net realizable value where cost is determined using the first-in, first-out method.
Cost of sales includes the cost of inventory sold, which includes third-party acquisition costs, third-party warehousing and product distribution charges.
Leases
We account for leases in accordance with Accounting Standards Codification Topic 842, Leases, (“ASC 842”). We determine if an arrangement is a lease at contract inception. A lease exists when a contract conveys to us the right to control the use of identified property, plant, or equipment for a period of time in exchange for consideration. The definition of a lease embodies two conditions: (1) there is an identified asset in the contract that is land or a depreciable asset (i.e., property, plant, and equipment), and (2) we have the right to control the use of the identified asset.
Operating leases where we are the lessee are included in Right of use (“ROU”) assets and Other current liabilities and Other long-term liabilities on our Consolidated Balance Sheets. The lease liabilities are initially and subsequently measured at the present value of the unpaid lease payments at the lease commencement date.
Key estimates and judgments include how we determined (1) the discount rate we use to discount the unpaid lease payments to present value, (2) lease term and (3) lease payments.
ASC 842 requires a lessee to discount its unpaid lease payments using the interest rate implicit in the lease or, if that rate cannot be readily determined, its incremental borrowing rate. Because our leases do not provide an implicit rate, we use our incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. Our incremental borrowing rate for a lease is the rate of interest we would have to pay on a collateralized basis to borrow an amount equal to the lease payments under similar terms.
The lease term for all of our leases includes the noncancellable period of the lease. Lease payments included in the measurement of the lease asset or liability are comprised of our fixed payments.
The ROU asset is initially measured at cost, which comprises the initial amount of the lease liability adjusted for lease payments made at or before the lease commencement date less any lease incentives received.
F-7
For operating leases, the ROU asset is subsequently measured throughout the lease term at the carrying amount of the lease liability, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of lease incentives received. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
We monitor for events or changes in circumstances that require a reassessment of a lease. If a reassessment results in the remeasurement of a lease liability, a corresponding adjustment is made to the carrying amount of the corresponding ROU asset unless doing so would reduce the carrying amount of the ROU asset to an amount less than zero. In that case, the amount of the adjustment that would result in a negative ROU asset balance is recorded in profit or loss.
We have elected not to recognize ROU assets and lease liabilities for all short-term leases that have a lease term of 12 months or less. We recognize the lease payments associated with our short-term leases as an expense on a straight-line basis over the lease term. Variable lease payments associated with these leases are recognized and presented in the same manner as for all our other leases.
We adopted ASC 842 using a modified retrospective transition approach as of the effective date, as permitted by the amendments in ASU 2018-11. As a result, we were not required to adjust our comparative period financial information for effects of the standard or make the new required lease disclosures for periods before the date of adoption (i.e., January 1, 2019). We have elected to adopt the package of transition practical expedients and, therefore, have not reassessed (1) whether existing or expired contracts contain a lease, (2) lease classification for existing or expired leases or (3) the accounting for initial direct costs that were previously capitalized. We did not elect the practical expedient to use hindsight for leases existing at the adoption date. Further, we do not expect the amendments in ASU 2018-01: Land Easement Practical Expedient to have an effect on us because we do not enter into land easement arrangements.
Income and Expense from Field Services Agreement
In connection with our sale of our subsidiary, Strongbridge Ireland Limited, which owned the rights to Macrilen to Novo, Strongbridge U.S. Inc, one of our wholly-owned subsidiaries, entered into a field services agreement with Novo Nordisk Inc., a subsidiary of Novo (“NNI”), pursuant to which NNI agreed to fund the costs of 23 of our field-based employees to provide full-time ongoing services to NNI, including the promotion of Macrilen in the United States. This agreement was terminated effective December 1, 2019. Our income and expense under the field services agreement are recorded as non-operating income and expense, respectively.
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. We must apply significant judgment in this process. Actual results could materially differ from those estimates.
Cash, cash equivalents and marketable securities
We consider all short‑term highly liquid investments with an original maturity at the date of purchase of three months or less to be cash equivalents. Cash and cash equivalents consist of account balances at banks and money market accounts, respectively.
We invest our excess cash balances in marketable securities of highly rated financial institutions. We seek to diversify our investments and limit the amount of investment concentrations for individual institutions, maturities and investment types. We classify marketable debt securities as available-for-sale and, accordingly, record such securities at fair value. We classify these securities as current assets as these investments are intended to be available to us for use in funding current operations. There were no marketable securities with a maturity of greater than one year as of December 31, 2019.
Unrealized gains and losses on marketable debt securities are recorded as a separate component of accumulated other comprehensive income (loss) included in stockholders’ equity.
F-8
Concentration of credit risk and other risks and uncertainties
As part of our cash and investment management processes, we perform periodic evaluations of the credit standing of the financial institutions with which we deposit our cash or purchase cash equivalents or marketable securities, and we have not sustained any credit losses from instruments held at these financial institutions.
Fair value of financial instruments
Fair value accounting is applied for all financial assets and liabilities and non‑financial assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually).
We are required to disclose information on all assets and liabilities reported at fair value that enables an assessment of the inputs used in determining the reported fair values. FASB ASC Topic 820, Fair Value Measurements and Disclosures (“ASC 820”), establishes a hierarchy of inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of us. Unobservable inputs are inputs that reflect our assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances. The fair value hierarchy applies only to the valuation inputs used in determining the reported fair value of the investments and is not a measure of the investment credit quality. The three levels of the fair value hierarchy are described as follows:
Level 1—Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that we have the ability to access at the measurement date.
Level 2—Valuations based on quoted prices for similar assets or liabilities, or quoted prices in markets that are not active, and for which all significant inputs are observable, either directly or indirectly.
Level 3—Valuations that require inputs that reflect our own assumptions that are both significant to the fair value measurement and unobservable. To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment we exercise in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
In December 2016, we issued warrants in connection with our private placement of ordinary shares. Pursuant to the terms of the warrant agreement, we could be required to settle the warrants in cash in the event of an acquisition of the Company and, as a result, the warrants are required to be measured at fair value and reported as a liability in the consolidated balance sheet. We recorded the fair value of the warrants upon issuance using the Black-Scholes Model and are required to revalue the warrants at each reporting date with any changes in fair value recorded on our statement of operations. The valuation of the warrants is considered under Level 3 of the fair value hierarchy due to the need to use assumptions in the valuation that that are both significant to the fair value measurement and unobservable. The change in the fair value of the Level 3 warrant liabilities is reflected in the statement of operations for the years ended December 31, 2019, 2018 and 2017.
F-9
Property and equipment, net
Property and equipment, net, consists of office equipment such as furniture, fixtures and computers. Depreciation expense for the years ended December 31, 2019 and 2018, was not significant. The following useful lives were used for the various classifications of property and equipment, net:
|
|
|
Amortization |
|
|||
|
|
|
Periods |
|
|||
|
Computer hardware |
|
3 |
- |
5 |
years |
|
|
Computer software |
|
2 |
- |
5 |
years |
|
|
Furniture and fixtures |
|
2 |
- |
5 |
years |
|
|
Leasehold improvements |
|
Lesser of useful life or remaining lease term |
|
|||
Intangible assets
Certain intangible assets were acquired as part of an asset purchase and have been capitalized at their acquisition date fair value. Acquired definite life intangible assets are amortized using the straight-line method over their respective estimated useful lives. We evaluate the potential impairment of intangible assets if events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate.
Goodwill
We test goodwill for impairment on an annual basis or whenever events occur that may indicate possible impairment. This analysis requires us to make a series of critical assumptions to (1) evaluate whether any impairment exists and (2) measure the amount of impairment.
Because we have one operating segment, when testing for a potential impairment of goodwill, we are required to estimate the fair value of our business and determine the carrying value. If the estimated fair value is less than the carrying value of our business, then we are required to estimate the fair value of all identifiable assets and liabilities in a manner similar to a purchase price allocation for an acquired business. Only after this process is completed can the goodwill impairment be determined, if any.
To estimate the fair value of the business, primarily a market‑based approach is applied, utilizing our public market value. We did not record a charge for impairment for our goodwill for the years ended December 31, 2019, 2018, and 2017.
Stock‑based compensation
We account for stock‑based compensation awards in accordance with Financial Accounting Standards Board (“FASB”) ASC Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock‑based payments including grants of stock options and restricted stock and modifications to existing stock options, to be recognized in the consolidated statements of operations based on their fair values.
We record compensation expense for service-based awards over the vesting period of the award on a straight-line basis. Compensation expense related to awards with performance-based vesting conditions is recognized over the requisite service period using the accelerated attribution method to the extent achievement of the performance condition is probable.
We estimate the fair value of our awards with service conditions using the Black‑Scholes option pricing model, which requires the input of subjective assumptions, including (i) the expected stock price volatility, (ii) the expected term of the award, (iii) the risk‑free interest rate and (iv) expected dividends.
F-10
We have estimated the expected term of employee service-based stock options using the “simplified” method, whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option, due to our lack of sufficient historical data. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury Bond rate with a maturity date commensurate with the expected term of the associated award. We have never paid dividends and do not expect to pay dividends in the foreseeable future.
We account for forfeitures as they occur as opposed to estimating forfeitures. We record stock-based compensation expense only for those awards that are expected to vest.
Income taxes
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”). The Tax Act makes broad and complex changes to the U.S. tax code, including, but not limited to, reducing the top U.S. federal corporate tax rate from 35 percent to 21 percent; requiring companies to pay a one-time transition tax on certain un-repatriated earnings of foreign subsidiaries; generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; eliminating the corporate alternative minimum tax (“AMT”) and changing how existing AMT credits can be realized; creating the base erosion anti-abuse tax (“BEAT”), a new minimum tax; creating a new limitation on deductible interest expense; and changing rules related to uses and limitations of net operating loss carryforwards created in tax years beginning after December 31, 2017.
The Tax Act reduces our U.S. corporate income tax rate from 34% to 21%, effective January 1, 2018. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. As a result of the reduction in the U.S. corporate income tax rate from 34% to 21% under the Tax Act, we revalued our ending net deferred tax assets and liabilities at December 31, 2017.
We recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2019, 2018 and 2017, we had no accrued interest or penalties related to uncertain tax positions and no amounts have been recognized in our statements of operations.
Net (loss) income per share
Basic net (loss) income per share is calculated by dividing the net (loss) income attributable to shareholders by the weighted-average number of ordinary shares outstanding during the period. Diluted net (loss) income per share is calculated by dividing the net (loss) income attributable to shareholders by the weighted‑average number of ordinary shares outstanding for the period, including any dilutive effect from outstanding stock options and other equity-based awards.
F-11
Net (loss) income per share was calculated as follows for the periods indicated below:
|
|
|
Year Ended |
|||||||
|
|
|
December 31, |
|||||||
|
(in thousands, except per share data) |
|
2019 |
|
2018 |
|
2017 |
|||
|
Basic Net (Loss) Income Per Share |
|
|
|
|
|
|
|
|
|
|
Basic net (loss) income |
|
$ |
(49,451) |
|
$ |
31,851 |
|
$ |
(113,483) |
|
Unrealized gain on fair value of warrants |
|
$ |
11,386 |
|
$ |
16,337 |
|
$ |
- |
|
Diluted net (loss) income |
|
$ |
(60,837) |
|
$ |
15,514 |
|
$ |
(113,483) |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average ordinary shares outstanding |
|
|
54,182,499 |
|
|
46,297,088 |
|
|
36,544,825 |
|
|
|
|
|
|
|
|
|
|
|
|
Basic net (loss) income per share |
|
$ |
(0.91) |
|
$ |
0.69 |
|
$ |
(3.11) |
|
|
|
|
|
|
|
|
|
|
|
|
Diluted Net (Loss) Income Per Share |
|
|
|
|
|
|
|
|
|
|
Diluted net (loss) income |
|
$ |
(60,837) |
|
$ |
15,514 |
|
$ |
(113,483) |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average ordinary shares outstanding |
|
|
54,182,499 |
|
|
46,297,088 |
|
|
36,544,825 |
|
Dilutive warrants, stock options and RSUs |
|
|
1,200,531 |
|
|
3,427,415 |
|
|
- |
|
Weighted-average shares used to compute diluted net income (loss) per share |
|
|
55,383,030 |
|
|
49,724,503 |
|
|
36,544,825 |
|
|
|
|
|
|
|
|
|
|
|
|
Diluted net (loss) income per share |
|
$ |
(1.10) |
|
$ |
0.31 |
|
$ |
(3.11) |
Shares used in the diluted net loss per share calculations exclude anti‑dilutive ordinary share equivalents, which consist of outstanding stock options, unvested restricted stock units and warrants, if applicable.
|
|
|
December 31, |
|||||
|
|
|
2019 |
2018 |
2017 |
|||
|
Warrants |
|
|
1,803,253 |
|
1,642,539 |
|
7,555,003 |
|
Stock options issued and outstanding |
|
|
9,192,684 |
|
4,444,830 |
|
6,104,715 |
|
Unvested RSUs |
|
|
791,350 |
|
— |
|
267,250 |
Recently issued accounting pronouncements
In January 2017, the FASB issued ASU 2017-04, Intangibles - Goodwill and Other: Simplifying the Accounting for Goodwill Impairment. ASU 2017-04 removes Step 2 of the goodwill impairment test, which requires a hypothetical purchase price allocation. A goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. This standard, which will be effective for us beginning in the first quarter of fiscal year 2020, is required to be applied prospectively. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We do not expect the adoption of this standard to have a significant impact on our financial statements or internal controls.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments - Credit Losses: Measurement of Credit Losses on Financial Instruments. ASU 2016-13 requires an entity to measure and recognize expected credit losses for certain financial instruments, including trade receivables, as an allowance that reflects the entity's current estimate of credit losses expected to be incurred. For available-for-sale debt securities with unrealized losses, the standard requires allowances to be recorded through net income instead of directly reducing the amortized cost of the investment under the current other-than-temporary impairment model. The standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019, with early adoption permitted. We do not expect the adoption of this standard to have a significant impact on our financial statements or internal controls.
F-12
3. Revenue recognition
Product revenue, net
We sell Keveyis to one specialty pharmacy provider (the “Customer”), who is the exclusive distributor of Keveyis in the United States. The Customer subsequently resells Keveyis to patients, most of whom are covered by payors that may provide for government-mandated or privately negotiated rebates with respect to the purchase of Keveyis.
Revenues from sales of Keveyis are recognized when we satisfy a performance obligation by transferring control of the product to the Customer. Transfer of control occurs upon receipt of the product by the Customer. We expense incremental costs related to the set-up of contracts with the Customer when incurred, as these costs do not meet the criteria for capitalization.
Disaggregation of Revenue
The following table summarizes revenue by product for the twelve months ended December 31, 2019 and 2018 (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
|
December 31, 2017 |
|
|
Products |
|
|
|
|
|
|
|
|
|
|
|
Keveyis |
|
$ |
21,676 |
|
$ |
16,802 |
|
$ |
7,046 |
|
|
Macrilen |
|
|
— |
|
|
1,225 |
|
|
— |
|
|
Total |
|
$ |
21,676 |
|
$ |
18,027 |
|
$ |
7,046 |
|
Reserves for variable consideration
Revenues from sales of Keveyis are recorded at the net sales price (transaction price), which includes estimates of variable consideration for which reserves are established and that result from rebates, co-pay assistance and other allowances that are offered between us and the patients’ payors. There is no variable consideration reserve for returns as we do not accept returns of Keveyis. These reserves are based on the amounts earned or to be claimed on the related sales and are classified as reductions of accounts receivable (if the amount is payable to the Customer) or a current liability (if the amount is payable to a party other than the Customer). Where appropriate, these estimates may take into consideration a range of possible outcomes that are probability-weighted for relevant factors such as our historical experience, current contractual and statutory requirements, specific known market events and trends, industry data and forecasted customer buying and payment patterns. Overall, these reserves reflect our best estimates of the amount of consideration to which we are entitled based on the terms of the contract. The amount of variable consideration that is included in the transaction price may be constrained, and is included in the net sales price only to the extent that it is probable that a significant reversal in the amount of the cumulative revenue recognized will not occur in a future period. Actual amounts of consideration ultimately received may differ from our estimates. We reassess our estimates on an ongoing basis. If actual results in the future vary from our estimates, we will adjust our estimates. Any such adjustments would affect net product revenue and earnings in the period such variances become known.
Trade discount: We provide the Customer with a discount that is explicitly stated in our contract and is recorded as a reduction of revenue in the period the related product revenue is recognized. In addition, we receive sales order management, data and distribution services from the Customer. To the extent the services received are distinct from our sale of Keveyis to the Customer, these payments are classified in selling, general and administrative expenses in our consolidated statement of operations and comprehensive (loss) income.
F-13
Funded Co-pay Assistance Program: We contract with a third-party to manage the co-pay assistance program intended to provide financial assistance to qualified insured patients. The calculation of the accrual for co-pay assistance is based on an estimate of claims and the cost per claim that we expect to receive associated with Keveyis that has been recognized as revenue, but remains in the distribution channel inventories at the end of each reporting period. These payments are consideration payable to the Customer and the related reserve is recorded in the same period the related revenue is recognized, resulting in a reduction of product revenue and the establishment of a current liability which is included in accrued expenses on the consolidated balance sheet.
Government rebates: We are subject to discount obligations under state Medicaid programs and Medicare. We estimate our Medicaid and Medicare rebates for the estimated patient mix. These reserves are recorded in the same period the related revenue is recognized, resulting in a reduction of product revenue and the establishment of a current liability, which is included in accrued expenses on the consolidated balance sheet. For Medicaid, accruals are based on estimates of future Medicaid beneficiary utilization applied to the Medicaid unit rebate formula established by the Center for Medicaid and Medicare Services. Effective January 1, 2019, manufacturers of pharmaceutical products are responsible for 70% of the patient’s cost of branded prescription drugs related to the Medicare Part D Coverage Gap. In order to estimate the cost to us of this Medicare coverage gap responsibility, we estimate the number of patients in the prescription drug coverage gap for whom we will owe an additional liability under the Medicare Part D program. Our liability for these rebates consists of estimates of claims for the current quarter and estimated future claims that will be made for Keveyis that have been recognized as revenue, but remains in the distribution channel inventories at the end of each reporting period.
Temporary Supply and Patient Assistance Programs: We provide free Keveyis to uninsured patients who satisfy pre-established criteria for either the Temporary Supply Program or the Patient Assistance Program. Patients who meet the Temporary Supply Program eligibility criteria may receive a temporary supply of free Keveyis for no more than sixty days while there is a determination of the patient’s third-party insurance, prescription drug benefit or other third-party coverage for Keveyis. The Patient Assistance Program provides free Keveyis for up to twelve months to uninsured patients who satisfy pre-established criteria for financial need. We do not recognize any revenue related to these free products and the associated costs are classified in selling, general and administrative expenses in our consolidated statements of operations and comprehensive (loss) income.
4. Fair value measurement
The following table sets forth the fair value measurements by level within the fair value hierarchy, that are measured on a recurring basis. Our level 3 instrument consist of the ordinary share warrant liability. The fair values of the outstanding warrants were measured using the Black-Scholes option-pricing model. Inputs used to determine estimated fair value of the warrant liabilities include the estimated fair value of the underlying stock at the valuation date, the estimated term of the warrants, risk-free interest rates, expected dividends and the expected volatility of the underlying stock. The significant unobservable inputs used in the fair value measurement of the warrant liabilities were the volatility rate and the estimated term of the warrants. Generally, increases (decreases) in the fair value of the underlying stock and estimated term would result in a directionally similar impact to the fair value measurement.
F-14
The following table presents our assets and liabilities that are measured at fair value on a recurring basis for the periods presented (in thousands):
|
|
|
As of December 31, 2019 |
|
||||||||||
|
|
|
Level I |
|
Level II |
|
Level III |
|
Total |
|
||||
|
Cash equivalents |
|
|
56,544 |
|
|
— |
|
|
— |
|
|
56,544 |
|
|
Marketable securities |
|
|
— |
|
|
21,072 |
|
|
— |
|
|
21,072 |
|
|
Total assets |
|
$ |
56,544 |
|
$ |
21,072 |
|
$ |
— |
|
$ |
77,616 |
|
|
Warrant liability |
|
|
— |
|
|
— |
|
|
4,127 |
|
|
4,127 |
|
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
4,127 |
|
$ |
4,127 |
|
|
|
|
As of December 31, 2018 |
|
||||||||||
|
|
|
Level I |
|
Level II |
|
Level III |
|
Total |
|
||||
|
Cash equivalents |
|
|
122,300 |
|
|
— |
|
|
— |
|
|
122,300 |
|
|
Total assets |
|
$ |
122,300 |
|
$ |
— |
|
$ |
— |
|
$ |
122,300 |
|
|
Warrant liability |
|
|
— |
|
|
— |
|
|
15,513 |
|
|
15,513 |
|
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
15,513 |
|
$ |
15,513 |
|
The following table presents a reconciliation of our level 3 Warrant liability (in thousands):
|
|
|
|
As of December 31, 2019 |
|
Balance as of December 31, 2018 |
|
$ |
15,513 |
|
Unrealized gain on fair value of warrants for the year ended December 31, 2019 |
|
|
(11,386) |
|
Balance as of December 31, 2019 |
|
$ |
4,127 |
5. Intangible assets and goodwill
The gross carrying amount of in‑process research and development, acquired developed product rights and goodwill is as follows (in thousands):
|
|
|
As of December 31, 2019 |
|
|||||||||||||
|
|
|
Beginning of Period |
|
Additions |
|
Sold |
|
Amortization |
|
End of Period |
|
|||||
|
Keveyis |
|
$ |
30,132 |
|
$ |
— |
|
$ |
— |
|
$ |
(5,022) |
|
$ |
25,110 |
|
|
Goodwill |
|
|
7,256 |
|
|
— |
|
|
— |
|
|
— |
|
|
7,256 |
|
|
Total |
|
$ |
37,388 |
|
$ |
— |
|
$ |
— |
|
$ |
(5,022) |
|
$ |
32,366 |
|
|
|
|
As of December 31, 2018 |
|
|||||||||||||
|
|
|
Beginning of Period |
|
Additions |
|
Sold |
|
Amortization |
|
End of Period |
|
|||||
|
Keveyis |
|
$ |
35,155 |
|
$ |
— |
|
$ |
— |
|
$ |
(5,023) |
|
$ |
30,132 |
|
|
Macrilen |
|
|
— |
|
|
24,834 |
|
|
(22,670) |
|
|
(2,164) |
|
|
— |
|
|
Goodwill |
|
|
7,256 |
|
|
— |
|
|
— |
|
|
— |
|
|
7,256 |
|
|
Total |
|
$ |
42,411 |
|
$ |
24,834 |
|
$ |
(22,670) |
|
$ |
(7,187) |
|
$ |
37,388 |
|
F-15
Estimated amortization of our acquired developed product rights intangible asset for the five years subsequent to December 31, 2019 is as follows (in thousands):
|
2020 |
|
$ |
5,022 |
|
2021 |
|
|
5,022 |
|
2022 |
|
|
5,022 |
|
2023 |
|
|
5,022 |
|
2024 |
|
|
5,022 |
Our finite-lived intangible asset consists of acquired developed product rights obtained from our acquisition of U.S. marketing rights to Keveyis (dichlorphenamide) from a subsidiary of Taro Pharmaceutical Industries Ltd. (“Taro”).
Pursuant to the terms of the Asset Purchase Agreement and Supply Agreement that we entered into with Taro in December 2016, we paid Taro an upfront payment in two installments of $1 million in December 2016 and $7.5 million in March 2017. We concluded that the supply price payable by us exceeds fair value and, therefore, used a discounted cash flow method with a probability assumption to value the payments in excess of fair value at $29.3 million, for which we have recorded an intangible asset and corresponding liability. This liability is being reduced as we purchase inventory over the term of the Supply Agreement that we entered into with Taro in December 2016. In addition, we incurred transaction costs of $2.4 million. The transaction resulted in the recording of an intangible asset of $40.2 million. This asset is being amortized over an eight-year period using the straight-line method.
6. Marketable securities
Marketable securities consist of the following:
|
|
|
As of December 31, 2019 |
|||||||
|
|
|
Amortized Cost |
|
Net Unrealized Gain |
|
Fair Value |
|||
|
Commercial Paper |
|
$ |
11,889 |
|
$ |
— |
|
$ |
11,889 |
|
U.S. treasury securities |
|
|
2,982 |
|
|
— |
|
|
2,982 |
|
Asset-backed securities |
|
|
6,198 |
|
|
3 |
|
|
6,201 |
|
Total marketable securities |
|
$ |
21,069 |
|
$ |
3 |
|
$ |
21,072 |
Accrued liabilities consist of the following (in thousands):
|
|
|
December 31, |
|
December 31, |
|
||
|
|
|
|
|
|
|
||
|
|
|
2019 |
|
2018 |
|
||
|
Employee compensation |
|
$ |
4,452 |
|
$ |
5,717 |
|
|
Consulting and professional fees |
|
|
4,335 |
|
|
4,145 |
|
|
Accrued sales allowances |
|
|
2,990 |
|
|
2,233 |
|
|
Severance |
|
|
2,968 |
|
|
— |
|
|
Supply agreement - current portion |
|
|
2,773 |
|
|
1,638 |
|
|
Accrued taxes |
|
|
1,892 |
|
|
535 |
|
|
Accrued royalties |
|
|
806 |
|
|
802 |
|
|
Lease liability - current portion |
|
|
374 |
|
|
— |
|
|
Other |
|
|
372 |
|
|
995 |
|
|
Total accrued liabilities |
|
$ |
20,962 |
|
$ |
16,065 |
|
F-16
8. Leases
We lease office space under operating leases. Our leases have initial lease terms ranging from one to five years. Our lease agreements contain provisions for future rent increases.
As of December 31, 2019, future minimum commitments under facility operating leases were as follows (in thousands):
|
|
|
Operating |
|
|
|
|
|
leases |
|
|
|
|
|
|
|
|
|
2020 |
|
|
470 |
|
|
2021 |
|
|
481 |
|
|
2022 |
|
|
492 |
|
|
2023 |
|
|
207 |
|
|
Total minimum lease payments |
|
$ |
1,650 |
|
The components of lease cost for the quarter ended December 31, 2019 are as follows (in thousands):
|
|
|
|
|
|
|
|
|
Year Ended |
|
|
|
|
December 31, 2019 |
|
Lease costs |
|
|
|
|
Amortization of right of use assets |
|
$ |
335 |
|
Interest on lease liabilities |
|
|
122 |
|
Total lease cost |
|
$ |
457 |
Amounts reported in the Consolidated Balance Sheet for leases where we are the lessee as of December 31, 2019 were as follows (in thousands):
|
|
|
|
December 31, 2019 |
|
Operating Leases |
|
|
|
|
Right of use asset |
|
$ |
789 |
|
Lease liability |
|
$ |
1,080 |
|
|
|
|
|
|
Remaining lease term |
|
|
|
|
Operating leases |
|
|
3 years 5 months |
|
|
|
|
|
|
Discount rate |
|
|
|
|
Operating leases |
|
|
7.69% |
Ordinary share warrants are accounted for in accordance with applicable accounting guidance provided in ASC Topic 815, Derivatives and Hedging — Contracts in Entity’s Own Equity (“ASC Topic 815”), as either derivative liabilities or as equity instruments depending on the specific terms of the warrant agreement.
F-17
Warrants outstanding and warrant activity for the year ended December 31, 2019 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding |
|
|
|
|
|
|
Exercise |
|
Expiration |
|
Warrants |
|
Warrants |
|
December 31, |
|
|
|
|
|
Classification |
|
Price |
|
Date |
|
Issued |
|
Exercised |
|
2019 |
|
|
|
Warrants in connection with private equity placement |
|
Liability |
|
$ |
2.50 |
|
6/28/2022 |
|
7,000,000 |
|
(1,970,000) |
|
5,030,000 |
|
|
Warrants in connection with Horizon and Oxford loan agreement |
|
Equity |
|
$ |
2.45 |
|
12/28/2026 |
|
428,571 |
|
(267,857) |
|
160,714 |
|
|
Warrants in connection with CRG loan agreement |
|
Equity |
|
$ |
7.37 |
|
7/14/2024 |
|
394,289 |
|
— |
|
394,289 |
|
|
Warrants in connection with CRG loan amendment in January 2018 |
|
Equity |
|
$ |
10.00 |
|
1/16/2025 |
|
1,248,250 |
|
— |
|
1,248,250 |
|
|
|
|
|
|
|
|
|
|
|
9,071,110 |
|
|
|
6,833,253 |
|
10. Commitments and contingencies
(a) Commitments to Taro Pharmaceuticals Industries Ltd.
In December 2016, we acquired the U.S. marketing rights to Keveyis (dichlorphenamide) from Taro. Under the terms of an Asset Purchase Agreement, we paid Taro an upfront payment in two installments of $1 million in December 2016 and $7.5 million in March 2017, and will pay an aggregate of $7.5 million in potential milestones upon the achievement of certain product sales targets. Taro has agreed to continue to manufacture Keveyis for us under an exclusive supply agreement through the orphan exclusivity period. We are obligated to purchase certain annual minimum amounts of product totaling approximately $29 million over a six-year period. As of December 31, 2019, our remaining obligation was $19.0 million. Our Supply Agreement with Taro may extend beyond the orphan exclusivity period unless terminated by either party pursuant to the terms of the agreement. If terminated by Taro at the conclusion of the orphan exclusivity period, we have the right to manufacture the product on our own or have the product manufactured by a third party on our behalf. We are also required to reimburse Taro for their royalty obligation resulting from their sale of Keveyis to us.
(b) Indemnifications
In the ordinary course of business and in connection with the sale of assets and businesses and other transactions, we often indemnify our counterparties against certain liabilities that may arise in connection with the transaction or that are related to events and activities prior to or following a transaction, such as breaches of contracts, unfavorable tax consequences and employee liabilities. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we may be required to reimburse the loss and such amount could be material to our financial statements. Where appropriate, the obligation for such indemnifications is recorded as a liability. Because the amount of these types of indemnifications generally is not specifically stated, the overall maximum amount of the obligation under such indemnifications cannot be reasonably estimated. However, we believe that the likelihood of a material liability being triggered under these indemnification obligations is not probable at this time.
Our 401(k) Employee Savings Plan (“401(k) Plan”) is available to all employees. We have elected a Safe-Harbor provision for the 401(k) Plan in which participants are always fully vested in their employer contributions. We match 100% of the first 4% of participating employee contributions. Our contributions were approximately $818,000, $704,000 and $173,000 for the years ended December 31, 2019, 2018 and 2017, respectively. Our contributions are made in cash. Our ordinary shares are not an investment option available to participants in the 401(k) Plan.
F-18
For the years ended December 31, 2019, 2018 and 2017, the components of income (loss) before income taxes were as follows (in thousands):
|
|
|
Year Ended |
|
|||||||
|
|
|
December 31, |
|
|||||||
|
|
|
|
2019 |
|
2018 |
|
2017 |
|
||
|
Sweden |
|
$ |
(1,989) |
|
$ |
4,712 |
|
$ |
(19,249) |
|
|
Ireland |
|
|
(23,708) |
|
|
73,409 |
|
|
(47,211) |
|
|
Cayman Islands |
|
|
(1,587) |
|
|
(701) |
|
|
(21,709) |
|
|
U.S. |
|
|
(20,399) |
|
|
(45,033) |
|
|
(23,543) |
|
|
Total |
|
$ |
(47,683) |
|
$ |
32,387 |
|
$ |
(111,712) |
|
The components of income tax expense (benefit) for the years ended December 31, 2019, 2018 and 2017 were as follows (in thousands):
|
|
|
Year Ended |
|
|||||||
|
|
|
December 31, |
|
|||||||
|
|
|
2019 |
|
2018 |
|
2017 |
|
|||
|
Current tax (benefit) expense: |
|
|
|
|
|
|
|
|
|
|
|
Sweden |
|
$ |
1,767 |
|
$ |
535 |
|
$ |
— |
|
|
Ireland |
|
|
— |
|
|
— |
|
|
(22) |
|
|
U.S. |
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
— |
|
|
— |
|
|
(151) |
|
|
State |
|
|
1 |
|
|
1 |
|
|
(14) |
|
|
Total current tax expense (benefit) |
|
$ |
1,768 |
|
$ |
536 |
|
$ |
(187) |
|
|
Deferred tax expense (benefit): |
|
|
|
|
|
|
|
|
|
|
|
Sweden |
|
$ |
— |
|
$ |
12,395 |
|
$ |
(4,586) |
|
|
Ireland |
|
|
(8,484) |
|
|
(13,337) |
|
|
1,280 |
|
|
U.S. |
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
|
(7,914) |
|
|
(1,785) |
|
|
39 |
|
|
State |
|
|
(784) |
|
|
(354) |
|
|
(2,392) |
|
|
Change in valuation allowance |
|
|
17,182 |
|
|
3,081 |
|
|
7,617 |
|
|
Total deferred tax expense (benefit) |
|
|
— |
|
|
— |
|
|
1,958 |
|
|
Total tax expense (benefit) |
|
$ |
1,768 |
|
$ |
536 |
|
$ |
1,771 |
|
With the exception of Sweden, we have net operating loss carryforwards in all other countries. For the Ireland and United States operations, we have not reflected any benefit of net operating loss carryforwards (“NOLs”) in the accompanying financial statements. In 2017, Strongbridge U.S. Inc. generated book loss as we decided this entity will markets and commercializes certain products. As such, given the high level of expenses incurred, it is not more likely than not we will recognize all deferred tax assets which results in us establishing a full valuation allowance against its deferred tax assets. During 2018, we transferred all our intercompany intellectual property to Ireland, resulting in current taxes due in Sweden after the utilization of all NOLs in Sweden, which previously had a full valuation allowance, and the creation of amortizable deferred tax assets in Ireland, which have a full valuation allowance. We recorded income tax expense of $1.8 million for the year ended December 31, 2019 arising from intercompany interest income.
Deferred taxes are recognized for temporary differences between the bases of assets and liabilities for financial statement and income tax purposes. The tax effect of temporary differences that give rise to significant portions of the deferred tax assets are as follows (in thousands):
F-19
|
|
|
Year Ended |
|
||||
|
|
|
December 31, |
|
||||
|
|
|
2019 |
|
2018 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
$ |
16,283 |
|
$ |
4,932 |
|
|
Stock-based compensation |
|
|
6,811 |
|
|
4,944 |
|
|
Lease Liability |
|
|
404 |
|
|
107 |
|
|
Intangible Amortization |
|
|
628 |
|
|
|
|
|
Other deferred activity |
|
|
250 |
|
|
497 |
|
|
Tax credits |
|
|
14,533 |
|
|
10,826 |
|
|
Interest disallowance |
|
|
9,687 |
|
|
9,889 |
|
|
Intangibles |
|
|
13,240 |
|
|
13,240 |
|
|
Total deferred tax assets |
|
|
61,836 |
|
|
44,435 |
|
|
Valuation allowance |
|
|
(61,617) |
|
|
(44,435) |
|
|
Deferred tax assets recognized |
|
|
219 |
|
|
— |
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Lease Liability - Right of Use |
|
|
(219) |
|
|
— |
|
|
Total deferred tax liabilities |
|
|
(219) |
|
|
— |
|
|
Net deferred tax assets (liabilities) |
|
$ |
— |
|
$ |
— |
|
We have evaluated the positive and negative evidence bearing upon the realizability of our deferred tax assets. Based on our history of operating losses, aside from the gain associated with the sale of our subsidiary, we have concluded that it is more likely than not that the benefit of our deferred tax assets will not be realized. The valuation allowance increased by approximately $17.2 million and $2.7 million during the years ended December 31, 2019 and 2018, respectively, due primarily to net operating losses.
Our effective income tax rate differs from the ultimate parent company, Strongbridge Biopharma plc, Irish domestic statutory rate of 12.5% for the year ended December 31, 2019, 2018 and 2017.
|
|
|
Year Ended |
|
||||
|
|
|
December 31, |
|
||||
|
|
|
2019 |
|
2018 |
|
2017 |
|
|
Ireland statutory income tax rate |
|
12.50 |
% |
12.50 |
% |
12.50 |
% |
|
Foreign tax differential between Sweden, U.S., Cayman Island and Ireland |
|
1.39 |
|
7.55 |
|
3.99 |
|
|
Federal tax credits |
|
7.77 |
|
(4.58) |
|
— |
|
|
Change in valuation allowance |
|
(36.04) |
|
9.51 |
|
(6.82) |
|
|
State income taxes |
|
1.64 |
|
(1.09) |
|
1.43 |
|
|
Permanent differences |
|
2.92 |
|
4.01 |
|
(5.04) |
|
|
Rate change - tax impact |
|
— |
|
— |
|
(7.09) |
|
|
Foreign exchange remeasurement of Swedish deferred tax asset |
|
— |
|
— |
|
0.83 |
|
|
Provision to return |
|
0.53 |
|
3.95 |
|
(1.33) |
|
|
Sale of subsidiary |
|
— |
|
(37.58) |
|
— |
|
|
Net operating loss adjustment |
|
5.57 |
|
7.72 |
|
— |
|
|
Other |
|
— |
|
(0.34) |
|
(0.06) |
|
|
Effective income tax rate |
|
(3.72) |
% |
1.65 |
% |
(1.59) |
% |
At December 31, 2019, we have no Swedish NOLs and approximately $60.2 million of Irish NOLs, which have an indefinite life, and approximately $27.0 million of U.S. federal and $29.1 million of state NOLs, which begin to expire in 2031. Due to recent tax reform, federal U.S. net operating losses generated after January 1, 2018 now have an indefinite life. Through December 31, 2015, we operated through a permanent establishment in both Sweden and the United States. As a result of utilizing the Swedish NOLs, we have written off all attributes associated with the prior U.S. branch. At December 31, 2019, we had $14.3 million of U.S. federal orphan drug tax credit carryforwards, which begin
F-20
to expire in 2032, and $0.2 million of U.S. federal research and development tax credit carryforwards, which begin to expire in 2031.
Utilization of the NOLs may be subject to limitations under U.S. Internal Revenue Code Section 382 if there is a greater than 50% ownership change as determined under applicable regulations.
We file income tax returns in Sweden, Ireland, the United States, and various states within the United States. In the normal course of business, we are subject to examination by federal, state and foreign jurisdictions, where applicable. Our tax years are still open under statute from inception to present. All open years may be examined to the extent that tax credit or net operating loss carryforwards are used in future periods.
13. Ordinary shares
Voting rights and privileges
As of December 31, 2019, and December 31, 2018, there are 600,000,000 authorized ordinary shares and 54,205,852 and 54,122,074 outstanding ordinary shares, respectively.
The holders of our ordinary shares are entitled to one vote for each ordinary share held at all meetings of shareholders without limitation and written actions in lieu of meetings. The holders are entitled to receive dividends if and when declared by our Board of Directors. No dividends have been declared or paid since our inception. The holders are entitled to share ratably in our assets available for distribution to stockholders, in the event of any voluntary or involuntary liquidation.
In addition, on May 26, 2015 we issued 40,000 deferred shares with a €1.00 par value per share (US$1.098). The deferred shares are issued in order to satisfy an Irish legislative requirement to maintain a minimum level of issued share capital denominated in euro. The deferred shares carry no voting rights and are not entitled to any dividend or distribution.
Equity financings
On December 18, 2018, we sold 5,242,000 shares of our ordinary shares to Novo for $7.00 per share and an aggregate purchase price of $36.7 million. We accounted for the $14.3 million premium paid over the market price of our ordinary shares as additional gain on the sale of our subsidiary.
On January 25, 2018, we sold 5,000,000 ordinary shares in a public offering at a price to the public of $6.75 per ordinary share for net proceeds of approximately $31.7 million, after deducting underwriting discounts and commissions and offering expenses paid by us.
On February 26, 2018, we sold an additional 255,683 ordinary shares as part of our January 2018 public offering at a price of $6.75 per ordinary share for net proceeds of approximately $1.6 million, after deducting underwriting discounts and commissions and offering expenses paid by us.
We entered into an equity distribution agreement with JMP Securities LLC (“JMP Securities”) on April 28, 2017, pursuant to which we may sell, at our option, from time to time, up to an aggregate of $40 million in ordinary shares of the Company through JMP Securities, as sales agent. We will pay JMP Securities a commission equal to 3% of the gross proceeds from the sale of ordinary shares under the ATM Facility. Pursuant to the terms of the equity distribution agreement, we reimbursed JMP Securities for certain out-of-pocket expenses, including the fees and disbursements of counsel to JMP Securities, incurred in connection with establishing the ATM Facility and have provided JMP Securities with customary indemnification rights. During the year ended December 31, 2018, we sold an aggregate of 1,281,903 ordinary shares under the ATM Facility for net proceeds of approximately $8.6 million and paid fees of $0.3 million to JMP Securities. As December 31, 2019, we have approximately $31.1 million available for sale under our ATM facility.
F-21
Shares reserved for issuance
There were 9,984,034 and 8,579,511 ordinary shares reserved for future issuance upon exercise of stock options and restricted stock vesting as of December 31, 2019 and 2018, respectively. As of December 31, 2019, we have 6,833,253 ordinary shares reserved for outstanding warrants.
Our board of directors has adopted the 2017 Inducement Plan (the “Inducement Plan”). The Inducement Plan provides for the grant of equity-based awards to new employees. The purpose of the Inducement Plan is to attract valued employees by offering them a greater stake in our success and a closer identity with us, and to encourage ownership of our ordinary shares by such employees. The Inducement Plan became effective on February 23, 2017. As of December 31, 2019, 1,099,776 ordinary shares are available for issuance pursuant to the Inducement Plan.
Our board of directors has adopted, and our shareholders have approved, the 2015 Equity Compensation Plan (the “2015 Plan”). The 2015 Plan provides for the grant of incentive stock options to our employees and any parent or subsidiary corporation’s employees, and for the grant of nonstatutory stock options, stock awards, and RSUs to our employees, directors and consultants and our parent or subsidiary corporations’ employees and consultants. The 2015 Plan became effective on September 3, 2015. As of December 31, 2019, 725,531 ordinary shares are available for issuance pursuant to the 2015 Plan.
Our board of directors has adopted, and our shareholders have approved, the Non‑Employee Director Equity Compensation Plan (the “Non‑Employee Director Plan”). The Non‑Employee Director Plan provides for the grant of nonstatutory stock options, stock awards, and RSUs to our non‑employee directors. The Non‑Employee Director Plan became effective on September 3, 2015. As of December 31, 2019, no ordinary shares are available for issuance pursuant to the Non‑Employee Director Plan.
A summary of the outstanding stock options activity for the year ended December 31, 2019 is as follows:
|
|
|
Options Outstanding |
|
||||||||
|
|
|
|
|
|
|
|
Weighted- |
|
|
|
|
|
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
|
|
Weighted- |
|
Remaining |
|
|
|
|
|
|
|
|
|
|
Average |
|
Contractual |
|
|
|
|
|
|
|
|
Number of |
|
Exercise |
|
Term |
|
Aggregate |
|
||
|
|
|
Shares |
|
Price |
|
(Years) |
|
Intrinsic Value |
|
||
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
Outstanding—January 1, 2019 |
|
8,579,511 |
|
$ |
7.35 |
|
7.57 |
|
$ |
3,281 |
|
|
Granted |
|
2,788,900 |
|
$ |
3.85 |
|
|
|
|
|
|
|
Forfeited and cancelled |
|
(2,131,886) |
|
$ |
5.20 |
|
|
|
|
|
|
|
Exercised |
|
(43,841) |
|
$ |
4.12 |
|
|
|
|
|
|
|
Outstanding—December 31, 2019 |
|
9,192,684 |
|
$ |
6.58 |
|
5.96 |
|
$ |
164 |
|
|
Vested and exercisable—December 31, 2019 |
|
5,466,055 |
|
$ |
7.69 |
|
4.78 |
|
$ |
— |
|
F-22
Stock‑based compensation expense
We recognized stock‑based compensation expense for employees and non‑employees in the accompanying consolidated statements of operations as follows (in thousands):
|
|
|
Year Ended |
|||||||
|
|
|
December 31, |
|||||||
|
|
|
2019 |
|
2018 |
|
2017 |
|||
|
Selling, general and administrative |
|
$ |
6,552 |
|
$ |
6,012 |
|
$ |
4,027 |
|
Research and development |
|
|
2,045 |
|
|
1,795 |
|
|
1,140 |
|
Total stock-based compensation |
|
$ |
8,597 |
|
$ |
7,807 |
|
$ |
5,167 |
As of December 31, 2019, the total unrecognized compensation expense related to unvested options was $9.2 million, which we expect to recognize over an estimated weighted‑average period of 2.55 years. Included in the 2019 stock-based compensation amount is $179,000 of expenses relating to stock option modifications.
In determining the estimated fair value of the stock‑based awards, we use the Black‑Scholes option‑pricing model and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment.
The fair value of stock option awards was estimated with the following assumptions:
|
|
|
Year Ended |
|
||||
|
|
|
December 31, |
|
||||
|
|
|
2019 |
|
2018 |
|
2017 |
|
|
Expected term (in years) |
|
5.57 |
|
6.11 |
|
5.98 |
|
|
Risk-free interest rate |
|
1.38%-2.61% |
|
2.25% - 3.04% |
|
1.78-2.26 |
|
|
Expected volatility |
|
76.5%-80.85% |
|
78.2% - 85% |
|
78.2% - 85% |
|
|
Dividend rate |
|
—% |
|
—% |
|
—% |
|
Restricted stock units
We grant RSUs to employees and to members of our board of directors. RSUs that are granted to employees vest two years from the date of issuance, provided that the employee is employed by us on such vesting date. RSUs that are granted to directors, vest on the one-year anniversary of the grant date, provided that the director continues to serve as a member of the board of directors continuously from the grant date through such one-year anniversary. All RSUs will fully vest upon a change of control of our company. If and when the RSUs vest, we will issue one ordinary share for each whole RSU that has vested, subject to satisfaction of the employee’s or director’s tax withholding obligations. The RSUs will cease to be outstanding upon the issuance of ordinary shares upon vesting. We recorded expense, which is included in the stock-based compensation table above, of $1.4 million and $0.5 million for the year ended December 31, 2019 and 2018, respectively. As of December 31, 2019, the total unrecognized compensation expense related to unvested RSUs is $1.6 million, which we expect to recognize over an estimated weighted‑average period of 1.06 years.
A summary of our unvested RSUs as of December 31, 2019 is as follows:
|
|
|
Number of |
|
|
|
|
Shares |
|
|
Outstanding—January 1, 2019 |
|
143,100 |
|
|
Granted |
|
1,001,000 |
|
|
Forfeited |
|
(283,500) |
|
|
Vested |
|
(69,250) |
|
|
Unvested—December 31, 2019 |
|
791,350 |
|
F-23
15. Segment and other information
Operating segments are identified as components of an enterprise about which separate discreet financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions on how to allocate resources and assess performance. We view our operations and manage our business in one operating segment.
All of our sales were in the United States.
The following table represents total long-lived assets by location (in thousands):
|
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
United States |
|
$ |
291 |
|
$ |
294 |
|
Total long-lived assets (1) |
|
$ |
291 |
|
$ |
294 |
|
(1) |
Long-lived assets consist of property and equipment. |
Customer concentration
The following table presents the gross sales from customers that represented more than 10% of our gross sales included in our single operating segment:
|
|
|
2019 |
|
2018 |
|
Customer A |
|
100% |
|
92% |
16. Sale of subsidiary
In December 2018, we sold Strongbridge Ireland Limited, whose only asset was the rights to Macrilen, to Novo for $145 million. As part of the sale, we are entitled to receive tiered royalties based on net sales of Macrilen through 2027. We have received an immaterial amount of royalties on net sales of Macrilen to date. In addition, Strongbridge U.S. Inc. entered into a services agreement with NNI pursuant to which we agreed to provide 23 field-based employees of Strongbridge U.S. Inc. to NNI to provide commercial services related to Macrilen, including the promotion of Macrilen in the United States, for a period of three years. Novo also purchased 5.2 million of our ordinary shares at a purchase price of $7.00 per share. We accounted for the $14.3 million excess fair value of Novo’s share purchase over the market price of our ordinary shares as additional gain on the sale. We incurred $5.8 million of expenses relating to the sale, which were recorded as part of the gain on the sale. We originally acquired the product rights to Macrilen in January 2018 for $24.8 million and recorded amortization in 2018 of $2.2 million resulting in a net book value of $22.6 million at the time of sale.
In December 2019, we reached an agreement with Novo to terminate the services agreement and we no longer provide these services to Novo. We received a $6 million payment from Novo, which was recorded to income from field service agreement in our Consolidated Statements of Operations and Comprehensive (Loss) Income.
F-24
17. Quarterly consolidated financial information (unaudited)
This table summarizes the unaudited consolidated financial results of operations for the quarters ended:
|
` |
|
March 31, |
|
June 30, |
|
September 30, |
|
December 31, |
||||
|
2019 Quarter Ended |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
4,333 |
|
$ |
6,073 |
|
$ |
5,677 |
|
$ |
5,593 |
|
Royalty revenues |
|
|
10 |
|
|
6 |
|
|
7 |
|
|
13 |
|
Cost of sales (excluding amortization of intangible assets) |
|
|
813 |
|
|
1,022 |
|
|
1,001 |
|
|
986 |
|
Amortization of intangible asset |
|
|
1,256 |
|
|
1,255 |
|
|
1,255 |
|
|
1,256 |
|
Total costs and expenses |
|
|
18,683 |
|
|
20,921 |
|
|
20,358 |
|
|
19,999 |
|
Other (expense) income |
|
|
(1,348) |
|
|
9,272 |
|
|
3,831 |
|
|
7,655 |
|
Income tax expense |
|
|
(677) |
|
|
(400) |
|
|
(691) |
|
|
— |
|
Net loss |
|
|
(18,434) |
|
|
(8,247) |
|
|
(13,790) |
|
|
(8,980) |
|
Net loss per ordinary share, basic (1) |
|
|
(0.34) |
|
|
(0.15) |
|
|
(0.25) |
|
|
(0.17) |
|
Net loss per ordinary share, diluted (1) |
|
|
(0.34) |
|
|
(0.30) |
|
|
(0.31) |
|
|
(0.17) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except share and per share data) |
|
March 31, |
|
June 30, |
|
September 30, |
|
December 31, |
||||
|
2018 Quarter Ended |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net product sales |
|
$ |
3,870 |
|
$ |
4,296 |
|
$ |
5,347 |
|
$ |
4,514 |
|
Cost of sales (excluding amortization of intangible assets) |
|
|
667 |
|
|
753 |
|
|
1,441 |
|
|
1,125 |
|
Amortization of intangible assets |
|
|
1,769 |
|
|
1,872 |
|
|
1,876 |
|
|
1,670 |
|
Total costs and expenses |
|
|
17,243 |
|
|
20,663 |
|
|
26,763 |
|
|
24,108 |
|
Other (expense) income |
|
|
(12,914) |
|
|
16,070 |
|
|
4,174 |
|
|
106,980 |
|
Income tax expense |
|
|
— |
|
|
(1) |
|
|
— |
|
|
(535) |
|
Net (loss) income |
|
|
(28,723) |
|
|
(2,923) |
|
|
(20,559) |
|
|
84,056 |
|
Net (loss) income per ordinary share, basic (1) |
|
|
(0.66) |
|
|
(0.06) |
|
|
(0.44) |
|
|
1.73 |
|
Net (loss) income per ordinary share, diluted (1) |
|
|
(0.66) |
|
|
(0.43) |
|
|
(0.55) |
|
|
1.64 |
|
(1) |
Net loss per share amounts may not agree to the per share for the full year due to the use of weighted-average shares for each period. |
F-25
