Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - KalVista Pharmaceuticals, Inc. | kalv-ex991_6.htm |
| 8-K - 8-K - KalVista Pharmaceuticals, Inc. | kalv-8k_20180727.htm |

Corporate Presentation July 2018 Exhibit 99.2

This presentation and the accompanying oral presentation contain “forward-looking” statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future, "likely," "may," "should," "will" and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our future financial performance, business plans and objectives, timing and success of our clinical trials, our ability to obtain regulatory approval or the timing of regulatory filings, the potential therapeutic benefits and economic value of our lead product candidates, financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: those related to our future financial performance, our ability to raise additional funding when needed, our ability to develop and maintain partnerships, our ability to identify and develop new products in a timely manner, the outcome, cost and timing of our product development activities and clinical trials, market size and acceptance of our products, our ability to maintain, protect and enhance our brand and intellectual property, our ability to continue to stay in compliance with applicable laws and regulations, our ability to scale our business and make key hires and such other factors as discussed under the section titled “Risk Factors” and elsewhere in our definitive proxy statement and quarterly reports on Form 10-Q that we file with the Securities and Exchange Commission (“SEC”) as well as our other filings and the documents incorporated by reference therein, with the SEC. Any forward-looking statement made by us in this presentation and the accompanying oral presentation is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Forward-Looking Statements

Company Highlights Discovery and development of small molecule protease inhibitors, with leading expertise on plasma kallikrein role in disease mechanisms Creating a portfolio of oral plasma kallikrein inhibitors to treat orphan disease hereditary angioedema (HAE) and diabetic macular edema (DME) HAE program KVD900 advancing to Phase 2 as acute therapy, with data mid-2019 Collaboration with Merck in DME; lead program KVD001 Phase 2 data H2 2019 Internal discovery and development capabilities enable high productivity and strong IP positions, supporting best-in-class programs $51.1 million at April 30, 2018 funds beyond key data points in both DME and HAE Additional $14.6 million financing in July 2018
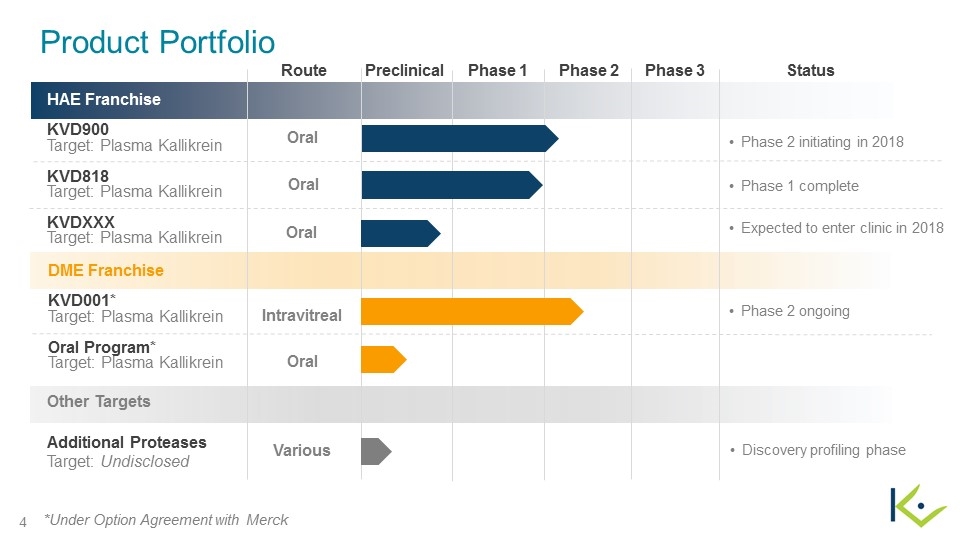
HAE Franchise KVD900 Target: Plasma Kallikrein KVD818 Target: Plasma Kallikrein KVDXXX Target: Plasma Kallikrein DME Franchise KVD001* Target: Plasma Kallikrein Oral Program* Target: Plasma Kallikrein Other Targets Additional Proteases Target: Undisclosed Oral Route Preclinical Phase 1 Phase 2 Status Oral Intravitreal Oral Various Phase 2 initiating in 2018 Phase 2 ongoing Discovery profiling phase Oral Phase 1 complete Expected to enter clinic in 2018 *Under Option Agreement with Merck Product Portfolio Phase 3

Hereditary Angioedema (HAE)

Hereditary Angioedema (HAE) Rare, genetic condition causing painful and dramatic swelling in various parts of the body Orphan disease: incidence 1 in 10,000 to 1 in 50,0001 Driven by uncontrolled plasma kallikrein activity leading to excessive bradykinin release; primarily caused by defect in C1 inhibitor activity Plasma kallikrein inhibition is a validated method for the treatment of HAE – product approved targeting this enzyme All current approved therapies injected/infused - high unmet need for oral HAE market is estimated to be over $2 billion market by 2020 We intend to bring at least three molecules through Phase 1 and target both acute and prophylactic segments 1 www.haei.org
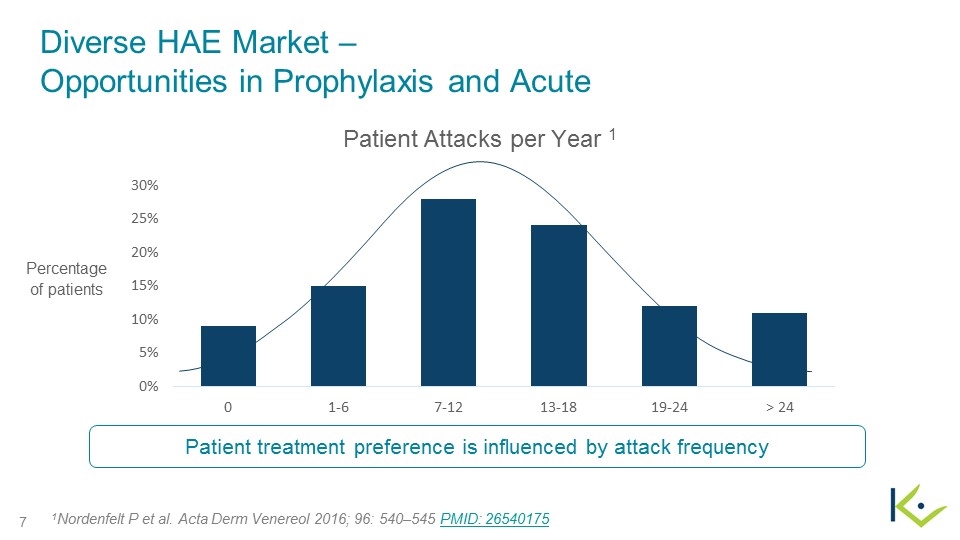
Diverse HAE Market – Opportunities in Prophylaxis and Acute 1Nordenfelt P et al. Acta Derm Venereol 2016; 96: 540–545 PMID: 26540175 Patient Attacks per Year 1 Patient treatment preference is influenced by attack frequency Percentage of patients
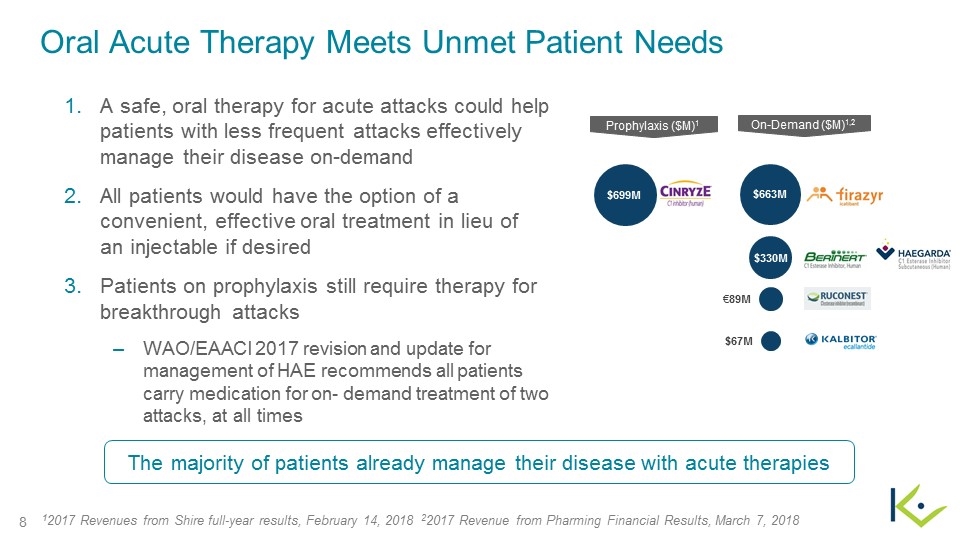
Oral Acute Therapy Meets Unmet Patient Needs On-Demand ($M)1,2 Prophylaxis ($M)1 A safe, oral therapy for acute attacks could help patients with less frequent attacks effectively manage their disease on-demand All patients would have the option of a convenient, effective oral treatment in lieu of an injectable if desired Patients on prophylaxis still require therapy for breakthrough attacks WAO/EAACI 2017 revision and update for management of HAE recommends all patients carry medication for on- demand treatment of two attacks, at all times The majority of patients already manage their disease with acute therapies $663M $699M $330M €89M $67M 12017 Revenues from Shire full-year results, February 14, 2018 22017 Revenue from Pharming Financial Results, March 7, 2018
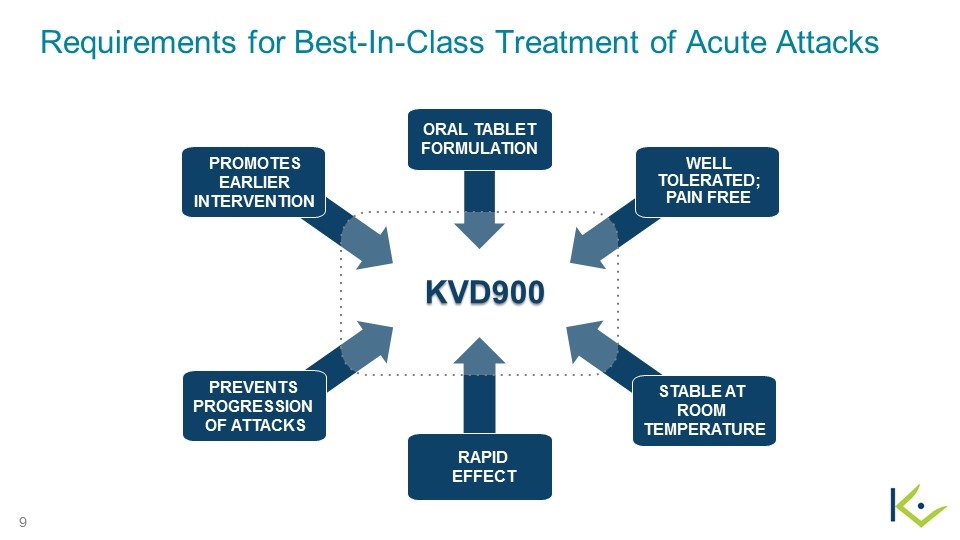
Requirements for Best-In-Class Treatment of Acute Attacks Well Tolerated; Pain Free Oral tablet formulation Promotes Earlier Intervention KVD900 Prevents progression of Attacks Rapid effect Stable AT room temperature

KVD900 Phase 1 Overview Double-blind, randomized investigation of oral KVD900 in healthy, male volunteers Single ascending dose with cross-over to tablet formulation 8 cohorts assessed up to 600 mg Safety and tolerability AEs, laboratory measurements and clinical outcomes Pharmacokinetics PK profile and PK parameters Pharmacodynamics Ex vivo assays assessing enzyme activity and plasma kininogen substrate cleavage
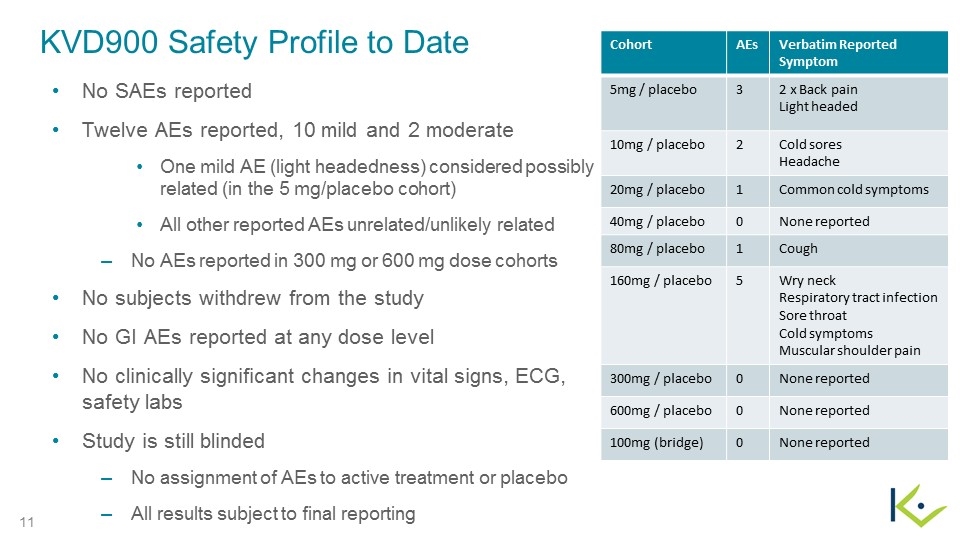
KVD900 Safety Profile to Date No SAEs reported Twelve AEs reported, 10 mild and 2 moderate One mild AE (light headedness) considered possibly related (in the 5 mg/placebo cohort) All other reported AEs unrelated/unlikely related No AEs reported in 300 mg or 600 mg dose cohorts No subjects withdrew from the study No GI AEs reported at any dose level No clinically significant changes in vital signs, ECG, safety labs Study is still blinded No assignment of AEs to active treatment or placebo All results subject to final reporting Cohort AEs Verbatim Reported Symptom 5mg / placebo 3 2 x Back pain Light headed 10mg / placebo 2 Cold sores Headache 20mg / placebo 1 Common cold symptoms 40mg / placebo 0 None reported 80mg / placebo 1 Cough 160mg / placebo 5 Wry neck Respiratory tract infection Sore throat Cold symptoms Muscular shoulder pain 300mg / placebo 0 None reported 600mg / placebo 0 None reported 100mg (bridge) 0 None reported
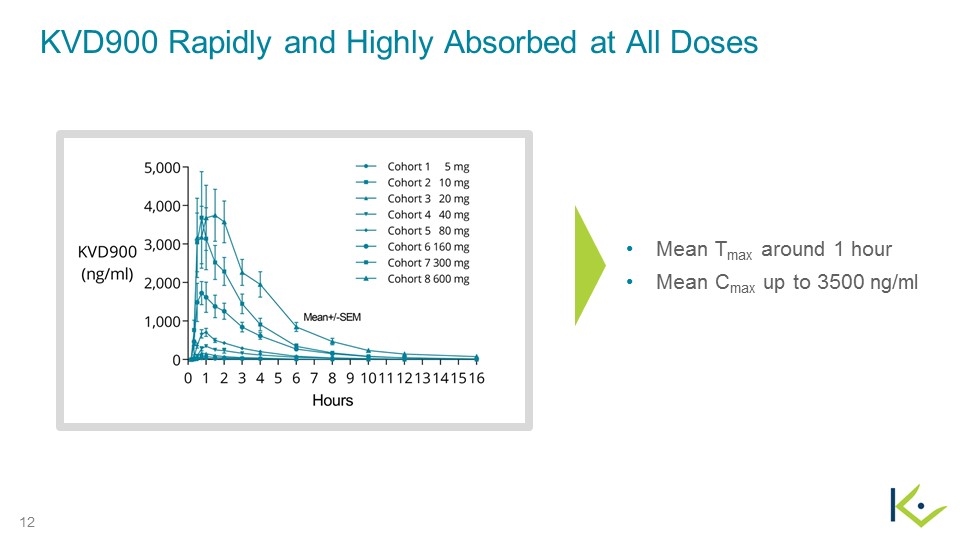
KVD900 Rapidly and Highly Absorbed at All Doses Mean Tmax around 1 hour Mean Cmax up to 3500 ng/ml
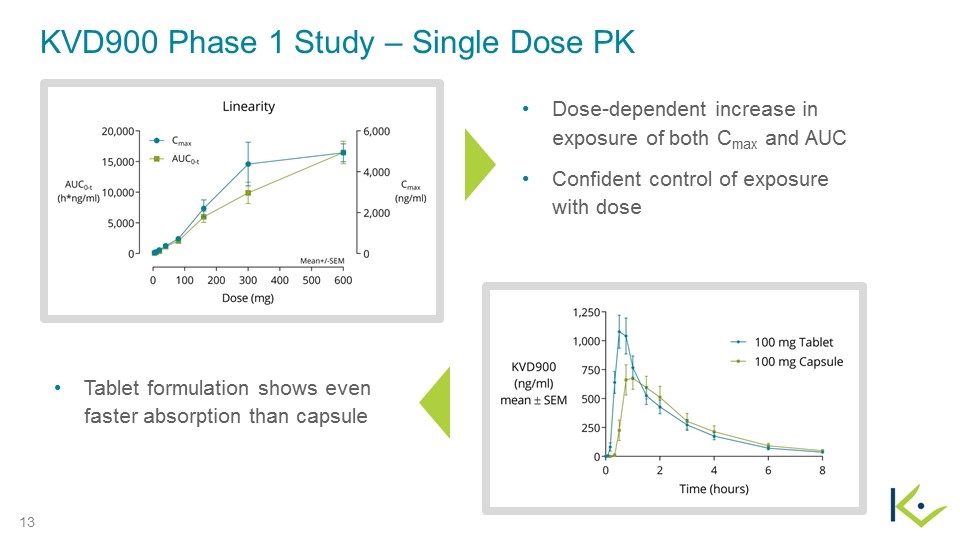
KVD900 Phase 1 Study – Single Dose PK Dose-dependent increase in exposure of both Cmax and AUC Confident control of exposure with dose Tablet formulation shows even faster absorption than capsule
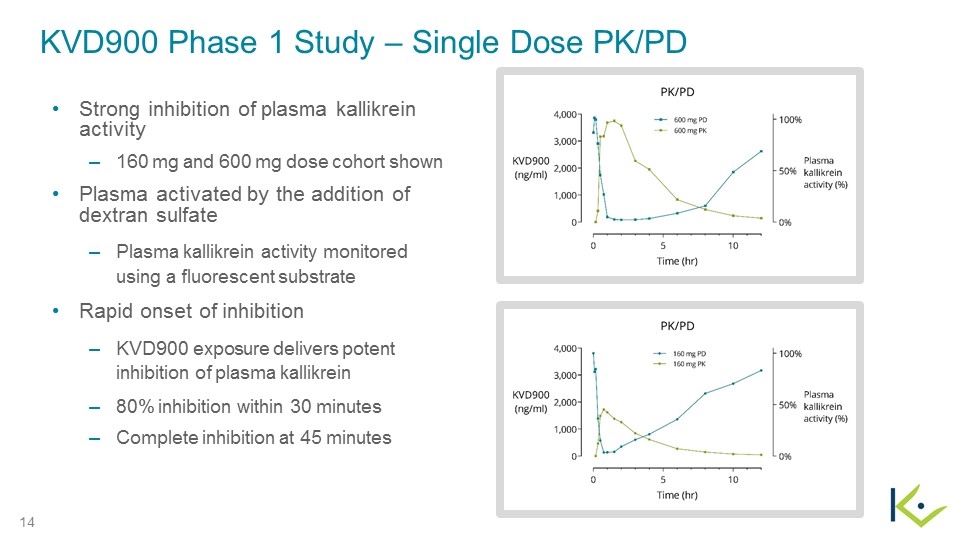
KVD900 Phase 1 Study – Single Dose PK/PD Strong inhibition of plasma kallikrein activity 160 mg and 600 mg dose cohort shown Plasma activated by the addition of dextran sulfate Plasma kallikrein activity monitored using a fluorescent substrate Rapid onset of inhibition KVD900 exposure delivers potent inhibition of plasma kallikrein 80% inhibition within 30 minutes Complete inhibition at 45 minutes
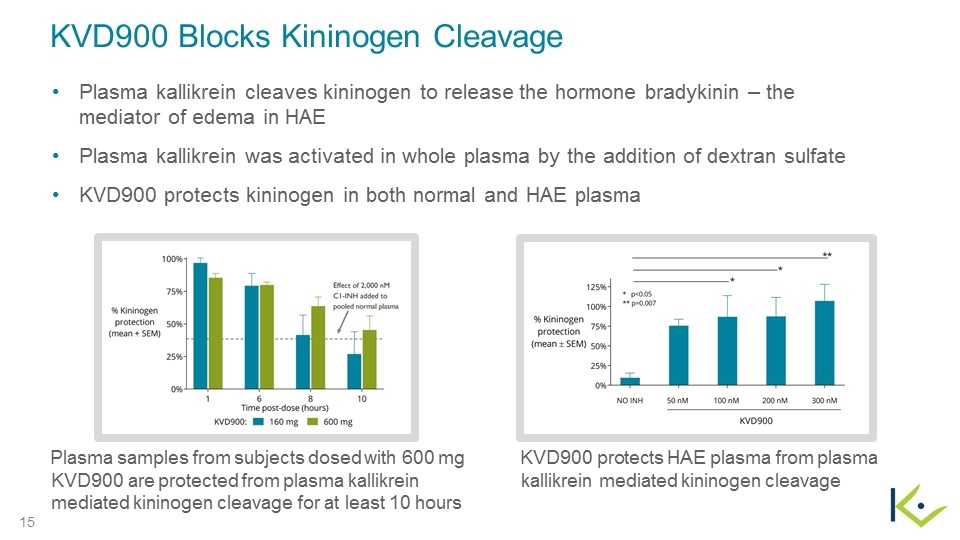
KVD900 Blocks Kininogen Cleavage Plasma kallikrein cleaves kininogen to release the hormone bradykinin – the mediator of edema in HAE Plasma kallikrein was activated in whole plasma by the addition of dextran sulfate KVD900 protects kininogen in both normal and HAE plasma Plasma samples from subjects dosed with 600 mg KVD900 are protected from plasma kallikrein mediated kininogen cleavage for at least 10 hours KVD900 protects HAE plasma from plasma kallikrein mediated kininogen cleavage
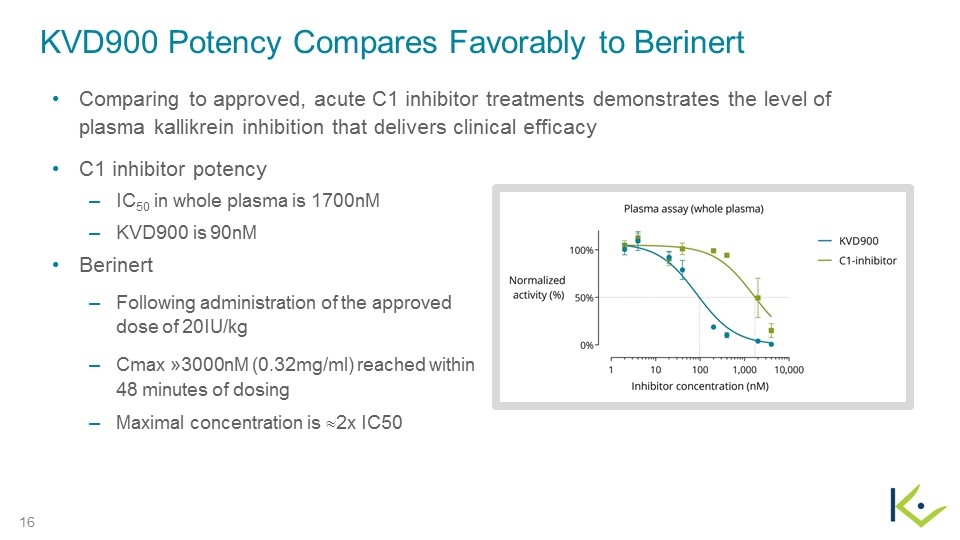
KVD900 Potency Compares Favorably to Berinert C1 inhibitor potency IC50 in whole plasma is 1700nM KVD900 is 90nM Berinert Following administration of the approved dose of 20IU/kg Cmax »3000nM (0.32mg/ml) reached within 48 minutes of dosing Maximal concentration is »2x IC50 Comparing to approved, acute C1 inhibitor treatments demonstrates the level of plasma kallikrein inhibition that delivers clinical efficacy
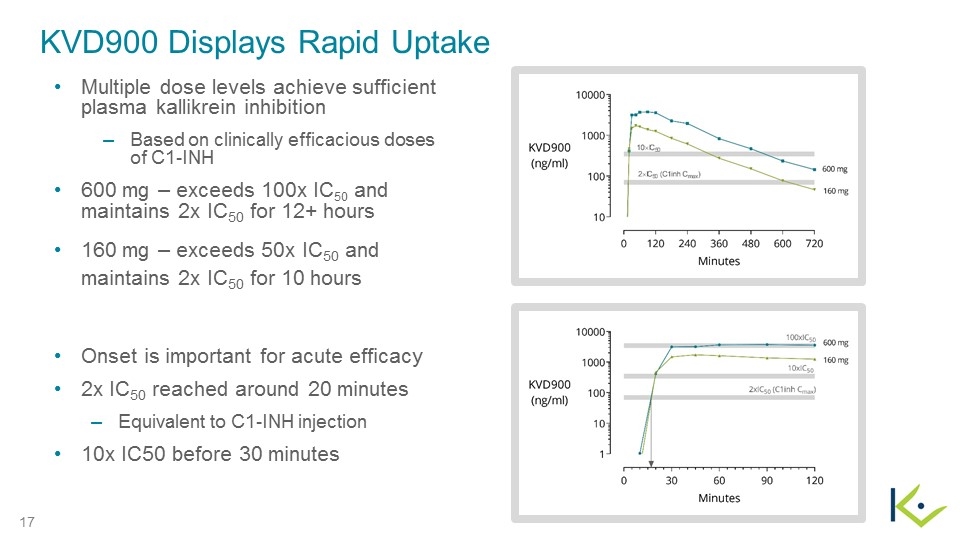
KVD900 Displays Rapid Uptake Multiple dose levels achieve sufficient plasma kallikrein inhibition Based on clinically efficacious doses of C1-INH 600 mg – exceeds 100x IC50 and maintains 2x IC50 for 12+ hours 160 mg – exceeds 50x IC50 and maintains 2x IC50 for 10 hours Onset is important for acute efficacy 2x IC50 reached around 20 minutes Equivalent to C1-INH injection 10x IC50 before 30 minutes

KVD900: On-Demand Treatment of Acute HAE Attacks Data suggests an ideal fit with best-in-class treatment for acute oral therapy Rapid absorption leads to effective concentrations in 30 minutes or less, which is equivalent to injected Systemic levels above effective concentration maintained for up to 10 hours No SAEs or other dose limiting AEs/tolerability issues No GI effects Short residence time to minimize any tolerability findings or impact on other medications KVD900 has the potential to be a patient-friendly treatment which offers the opportunity to intervene early resulting in higher efficacy Efficacy of acute injectable treatments is often undermined by late dosing Early treatment has been shown to be key in maximizing treatment outcomes Potential label extension for treatment on prodromal symptoms à episodic prophylaxis
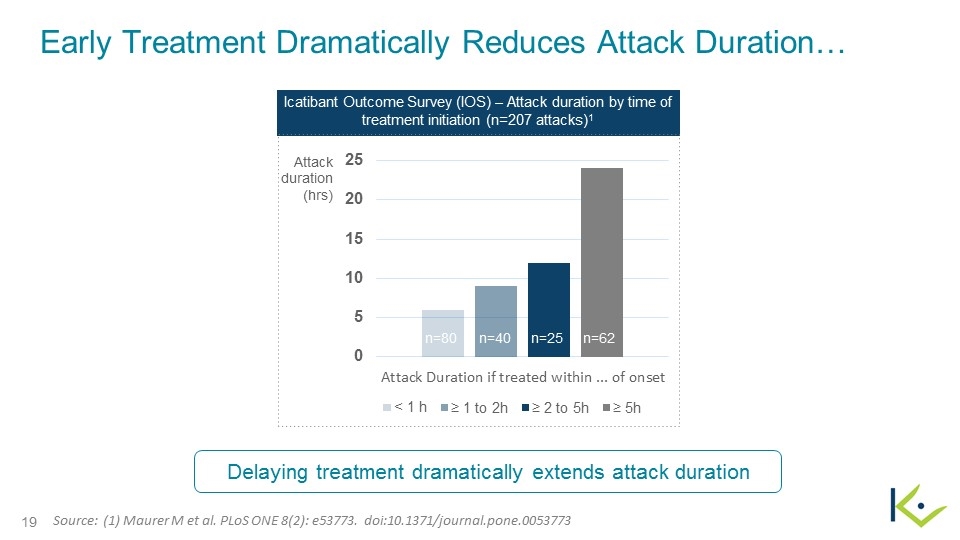
Early Treatment Dramatically Reduces Attack Duration… Attack duration (hrs) n=40 n=25 n=62 n=80 Icatibant Outcome Survey (IOS) – Attack duration by time of treatment initiation (n=207 attacks)1 Source: (1) Maurer M et al. PLoS ONE 8(2): e53773. doi:10.1371/journal.pone.0053773 Delaying treatment dramatically extends attack duration
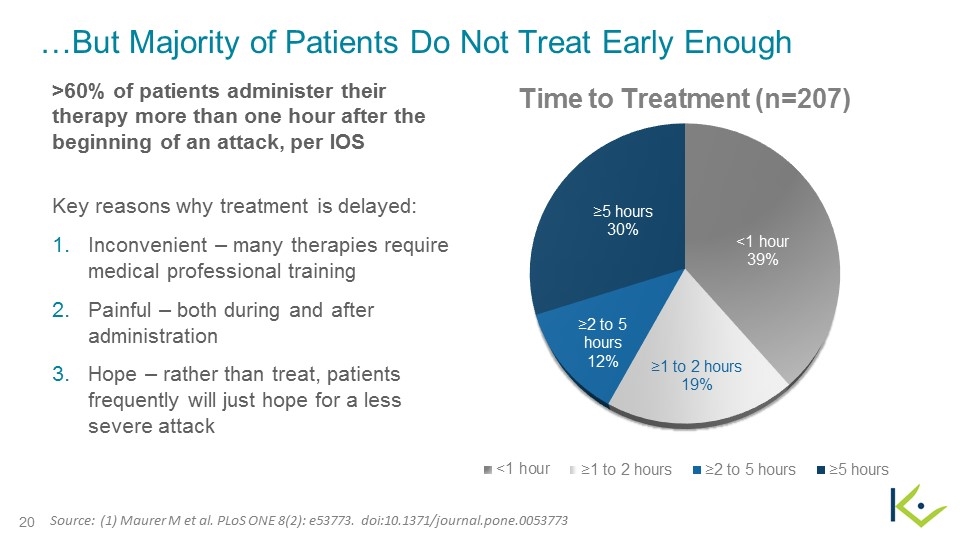
…But Majority of Patients Do Not Treat Early Enough >60% of patients administer their therapy more than one hour after the beginning of an attack, per IOS Key reasons why treatment is delayed: Inconvenient – many therapies require medical professional training Painful – both during and after administration Hope – rather than treat, patients frequently will just hope for a less severe attack Source: (1) Maurer M et al. PLoS ONE 8(2): e53773. doi:10.1371/journal.pone.0053773
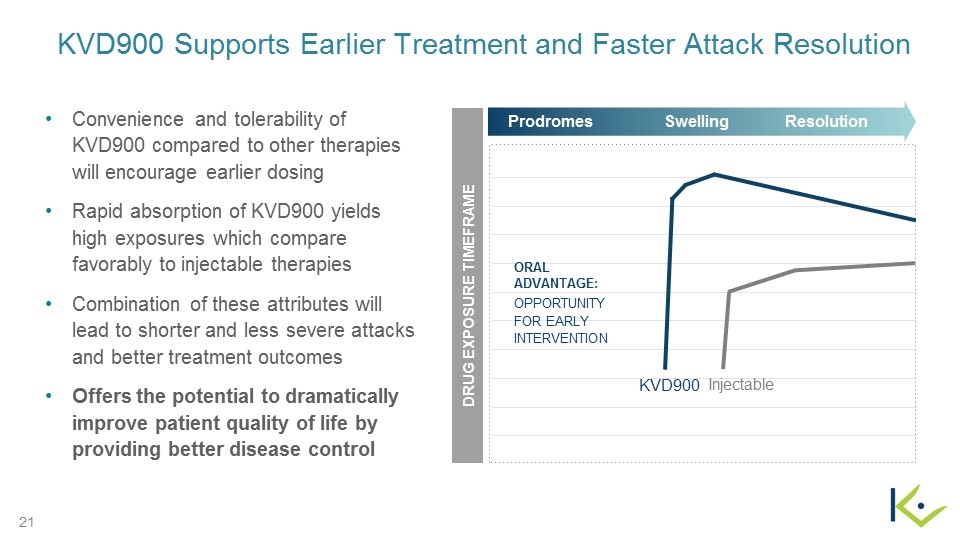
KVD900 Supports Earlier Treatment and Faster Attack Resolution Convenience and tolerability of KVD900 compared to other therapies will encourage earlier dosing Rapid absorption of KVD900 yields high exposures which compare favorably to injectable therapies Combination of these attributes will lead to shorter and less severe attacks and better treatment outcomes Offers the potential to dramatically improve patient quality of life by providing better disease control Resolution Prodromes Swelling DRUG EXPOSURE TIMEFRAME OPPORTUNITY FOR EARLY INTERVENTION KVD900 Injectable ORAL ADVANTAGE:
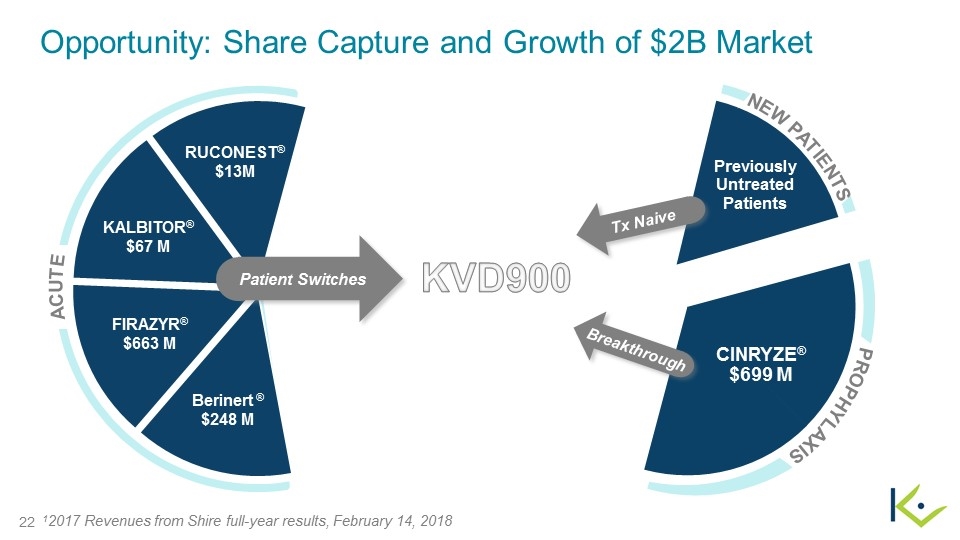
12017 Revenues from Shire full-year results, February 14, 2018 Previously Untreated Patients PROPHYLAXIS FIRAZYR® $663 M KALBITOR® $67 M Berinert ® $248 M ACUTE RUCONEST® $13M Patient Switches CINRYZE® $699 M Tx Naive Breakthrough KVD900 NEW PATIENTS Opportunity: Share Capture and Growth of $2B Market

KVD900 Next Steps Food effect cohort will be completed before we begin the Phase 2 Moving into single dose Phase 2 efficacy study Investigation of PK/PD in HAE patients and efficacy in acute treatment of attacks Anticipated to begin recruitment before YE 2018 Expected completion mid-2019 Enables regulatory interactions to establish phase 3 requirements Potential to move directly to pivotal trials Fast Track and Orphan Drug applications in 2019 Well defined regulatory pathway for treatment of acute attacks
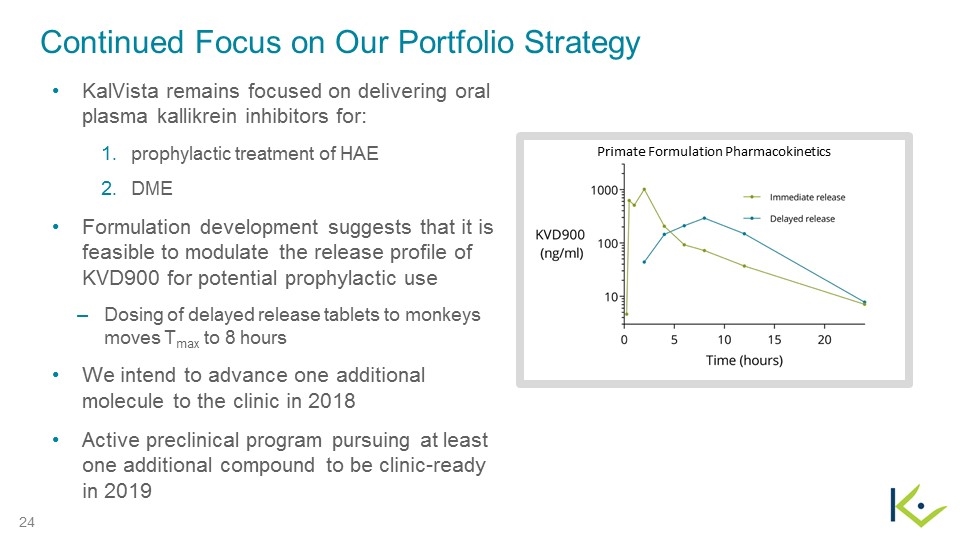
Continued Focus on Our Portfolio Strategy KalVista remains focused on delivering oral plasma kallikrein inhibitors for: prophylactic treatment of HAE DME Formulation development suggests that it is feasible to modulate the release profile of KVD900 for potential prophylactic use Dosing of delayed release tablets to monkeys moves Tmax to 8 hours We intend to advance one additional molecule to the clinic in 2018 Active preclinical program pursuing at least one additional compound to be clinic-ready in 2019 Primate Formulation Pharmacokinetics

Diabetic Macular Edema (DME)

Diabetic Macular Edema: Over $1 Billion Market Retinal swelling due to leaky blood vessels in the macula – a leading cause of blindness Approximately 900,000 patients in the United States have active DME and are at serious risk of vision loss – affects over 16% of diabetes patients1 Standard of care is anti-VEGF injected into the eye – currently there are no oral treatments for DME Up to 40% of patients do not adequately respond and continue to have impaired visual function and macular edema – significant unmet clinical need Plasma kallikrein has been identified as a potential VEGF-independent mediator of DME KalVista developed KVD001 as an IVT therapy, and is also working to develop an orally delivered plasma kallikrein inhibitor therapy for DME The basis of Merck collaboration announced in October 2017 1Diabetes Care, 2012
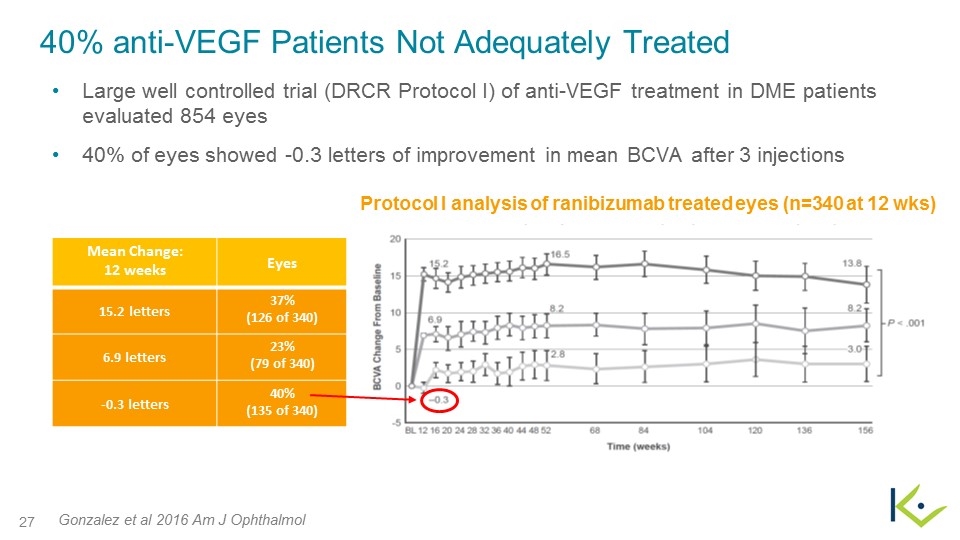
Protocol I analysis of ranibizumab treated eyes (n=340 at 12 wks) 40% anti-VEGF Patients Not Adequately Treated Large well controlled trial (DRCR Protocol I) of anti-VEGF treatment in DME patients evaluated 854 eyes 40% of eyes showed -0.3 letters of improvement in mean BCVA after 3 injections Gonzalez et al 2016 Am J Ophthalmol Mean Change: 12 weeks Eyes 15.2 letters 37% (126 of 340) 6.9 letters 23% (79 of 340) -0.3 letters 40% (135 of 340)
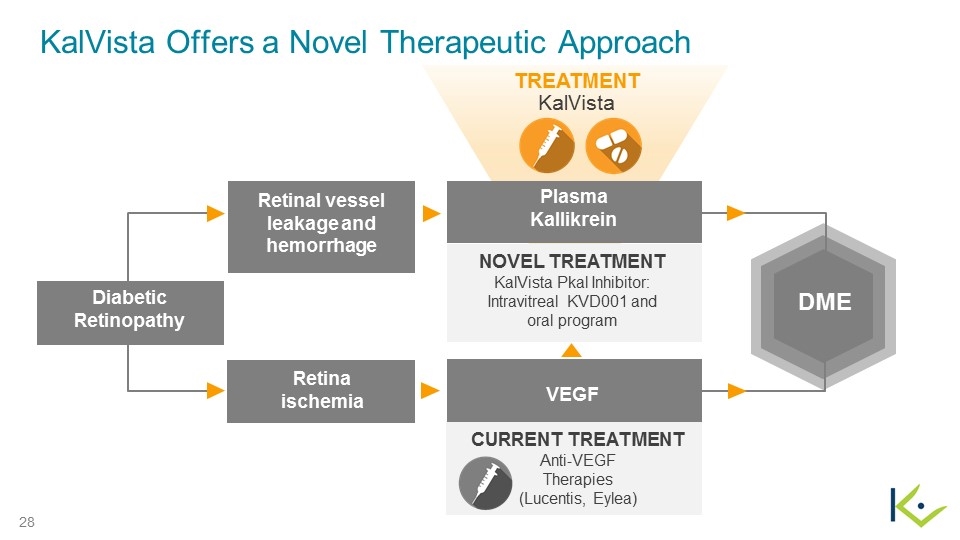
DME Diabetic Retinopathy Retina ischemia CURRENT TREATMENT Anti-VEGF Therapies (Lucentis, Eylea) VEGF NOVEL TREATMENT KalVista Pkal Inhibitor: Intravitreal KVD001 and oral program Retinal vessel leakage and hemorrhage Plasma Kallikrein TREATMENT KalVista KalVista Offers a Novel Therapeutic Approach
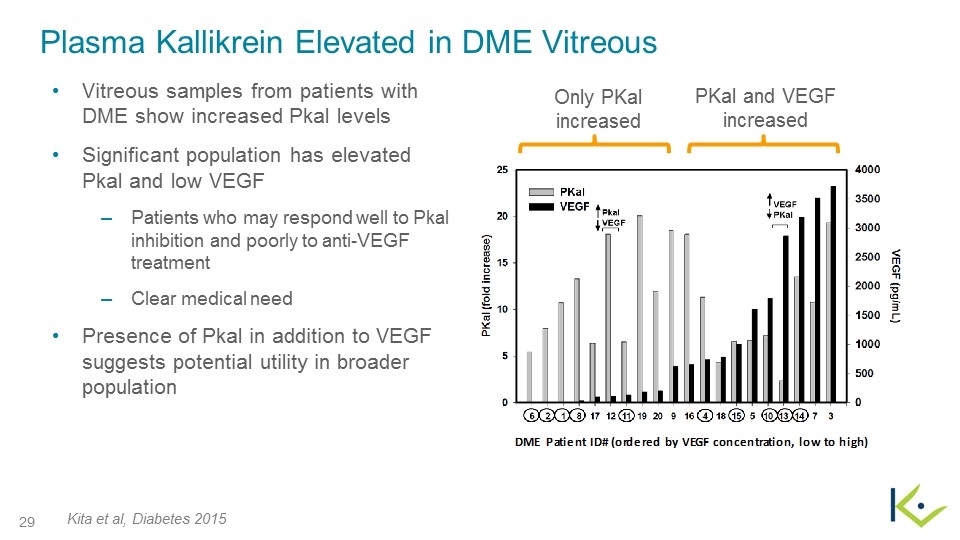
Plasma Kallikrein Elevated in DME Vitreous Vitreous samples from patients with DME show increased Pkal levels Significant population has elevated Pkal and low VEGF Patients who may respond well to Pkal inhibition and poorly to anti-VEGF treatment Clear medical need Presence of Pkal in addition to VEGF suggests potential utility in broader population Kita et al, Diabetes 2015 PKal and VEGF increased Only PKal increased
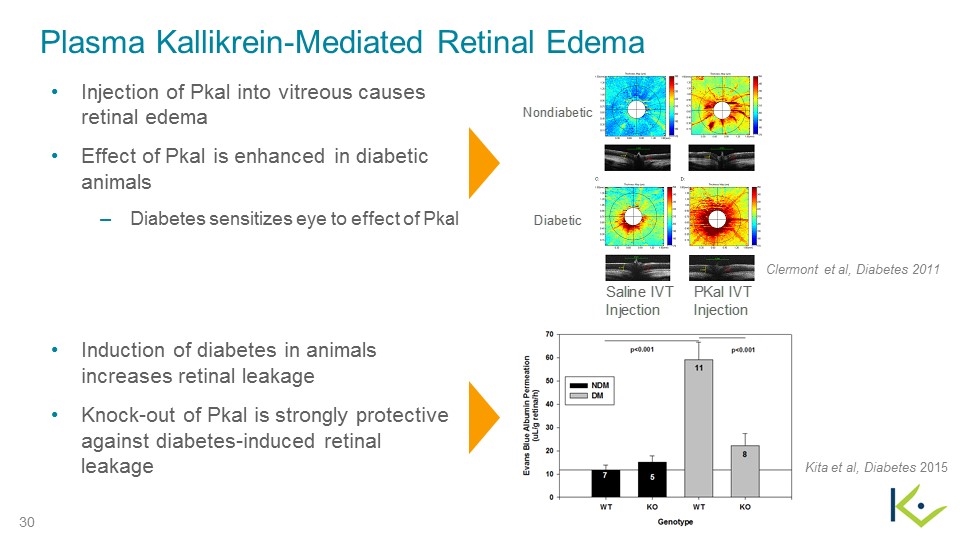
Clermont et al, Diabetes 2011 Kita et al, Diabetes 2015 Injection of Pkal into vitreous causes retinal edema Effect of Pkal is enhanced in diabetic animals Diabetes sensitizes eye to effect of Pkal Induction of diabetes in animals increases retinal leakage Knock-out of Pkal is strongly protective against diabetes-induced retinal leakage Plasma Kallikrein-Mediated Retinal Edema
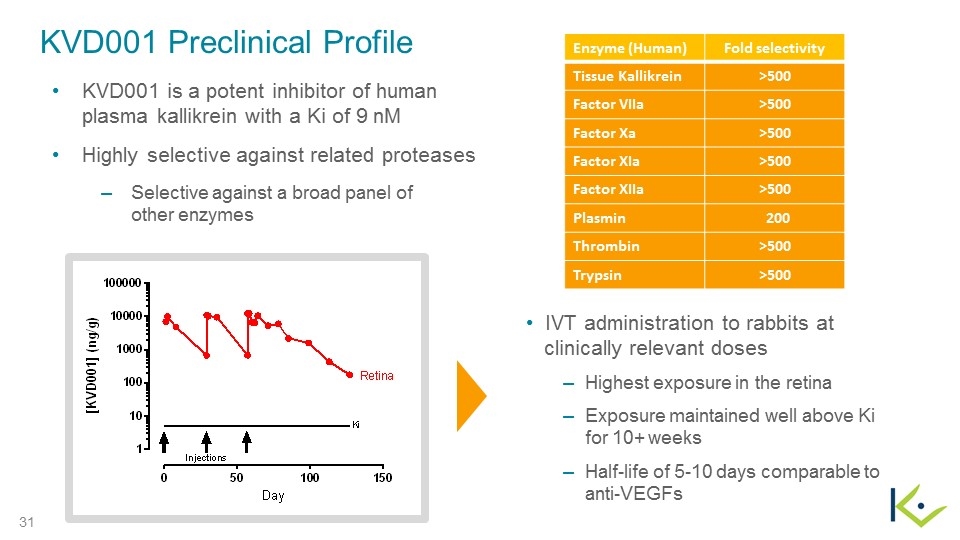
KVD001 Preclinical Profile KVD001 is a potent inhibitor of human plasma kallikrein with a Ki of 9 nM Highly selective against related proteases Selective against a broad panel of other enzymes IVT administration to rabbits at clinically relevant doses Highest exposure in the retina Exposure maintained well above Ki for 10+ weeks Half-life of 5-10 days comparable to anti-VEGFs Enzyme (Human) Fold selectivity Tissue Kallikrein >500 Factor VIIa >500 Factor Xa >500 Factor XIa >500 Factor XIIa >500 Plasmin 200 Thrombin >500 Trypsin >500
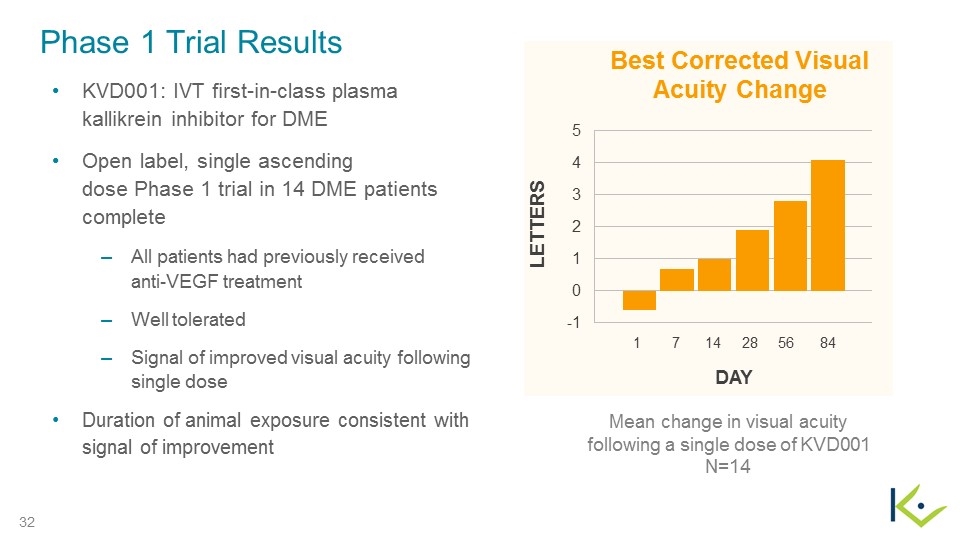
Phase 1 Trial Results KVD001: IVT first-in-class plasma kallikrein inhibitor for DME Open label, single ascending dose Phase 1 trial in 14 DME patients complete All patients had previously received anti-VEGF treatment Well tolerated Signal of improved visual acuity following single dose Duration of animal exposure consistent with signal of improvement Mean change in visual acuity following a single dose of KVD001 N=14 DAY 1 7 14 28 56 84 LETTERS

Option Agreement With Merck on DME Programs In October 2017, KalVista and Merck announced a collaboration for KVD001, KalVista’s intravitreal DME program, as well as future orally-delivered plasma kallikrein inhibitors for DME developed by KalVista Merck has two options, to acquire KVD001 and/or oral DME assets until a specified time following certain data on each Until the options are exercised, KalVista retains full ownership and control of the assets KalVista to execute and fund the Phase 2 KVD001 trial and other activities Merck pays all costs post-exercise $37 million upfront payment to KalVista $715 million in potential additional milestone payments Tiered sales royalties on global net sales Merck acquired a 9.9% stake in KalVista in a concurrent PIPE
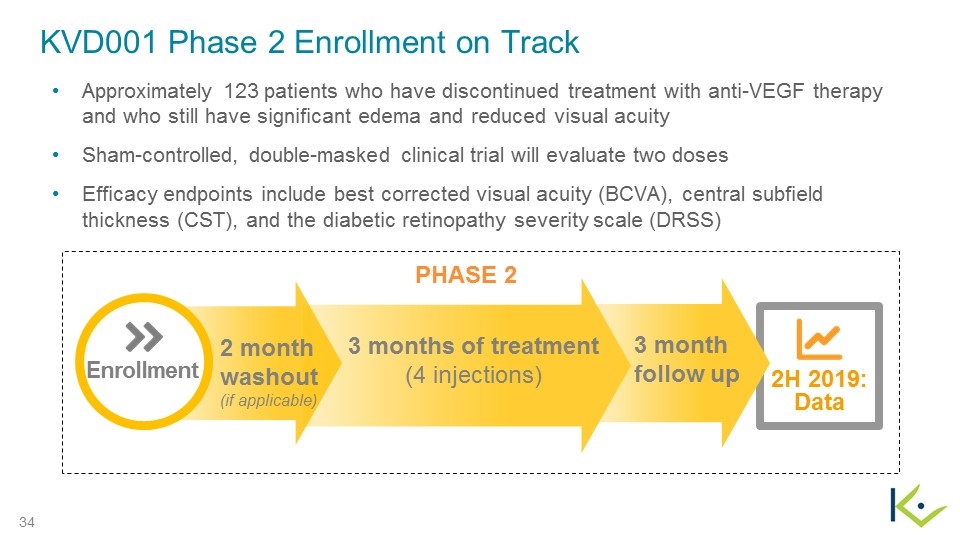
KVD001 Phase 2 Enrollment on Track Approximately 123 patients who have discontinued treatment with anti-VEGF therapy and who still have significant edema and reduced visual acuity Sham-controlled, double-masked clinical trial will evaluate two doses Efficacy endpoints include best corrected visual acuity (BCVA), central subfield thickness (CST), and the diabetic retinopathy severity scale (DRSS) 3 months of treatment (4 injections) Enrollment PHASE 2 3 month follow up Data 2H 2019: 2 month washout (if applicable)

HAE portfolio update KVD001 enrollment update KVD900 Phase 2 clinical trial initiates Late 2018 KVDXXX regulatory filing YE 2018 KVD900 Phase 2 completed Mid-2019 KVD001 Phase 2 data H2 2019 2H 2017 P P Company Achievements and Milestones

NASDAQ: KALV
