Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - VARIAN MEDICAL SYSTEMS INC | tv499256_ex99-1.htm |
| 8-K - FORM 8-K - VARIAN MEDICAL SYSTEMS INC | tv499256_8k.htm |
Exhibit 99.2

J. Michael Bruff Senior Vice President, Investor Relations investors@varian.com July 25, 2018 Third Quarter Fiscal Year 2018

This presentation is intended exclusively for investors. It is not intended for use in Sales or Marketing. 2 Forward - Looking Statements Except for historical information, this presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 . Statements concerning industry outlook, including growth drivers, future trends in cancer incidence and trends in cancer treatment needs, demand, innovation and growth opportunities ; Varian Medical System, Inc . ’s (”Varian” or the “company”) future orders, revenues, operating expenses, tax rate, cash flows, backlog or earnings growth ; future financial results and the expected charges relating to the refinancing of the MPTC loan ; potential impact of tariffs or a global trade war, market acceptance of or transition to new products or technology such as our Edge® radiosurgery system, TrueBeam®, HyperArc TM , 360 Oncology TM , HALCYON TM , image - guided radiation therapy, stereotactic radiosurgery and proton therapy, and any statements using the terms “could”, “believe”, “expect”, “outlook”, “anticipate”, ”vision”, “estimate”, “future”, “horizon”, “aiming”, “driving”, “target” or similar statements are forward - looking statements that involve risks and uncertainties that could cause the company’s actual results to differ materially from those anticipated . Such risks and uncertainties include global economic conditions and changes to trends for cancer treatment regionally ; the impact of changes to the Affordable Health Care for America Act (including excise taxes on medical devices) and any further healthcare reforms (including changes to Medicare and Medicaid), and/or changes to third - party reimbursement levels ; currency exchange rates and tax rates ; the impact of the Tax Cuts and Jobs Act ; demand for the company’s products ; the company’s ability to develop, commercialize, and deploy new products ; the company’s ability to meet Food and Drug Administration (FDA) and other regulatory requirements for product clearances or to comply with FDA and other regulatory regulations or procedures, changes in the regulatory environment, including with respect to FDA requirements ; the company’s assessment of the goodwill associated with its particle therapy business, challenges associated with the successful commercialization of the company’s particle therapy business ; the risks associated with providing financing for the construction and start - up operations of proton therapy centers ; the effect of adverse publicity ; the company’s reliance on sole or limited - source suppliers ; the company’s ability to maintain or increase margins ; the impact of competitive products and pricing ; the potential loss of key distributors or key personnel ; challenges to public tender awards and the loss of such awards or other orders ; and the other risks listed from time to time in the company’s filings with the Securities and Exchange Commission, which by this reference are incorporated herein . The company assumes no obligation to update or revise the forward - looking statements in this presentation because of new information, future events, or otherwise . Reconciliations to GAAP financials can be found at http : //investors . varian . com/financialstatements and the appendix to this presentation . Varian has not filed its Form 10 - Q for the quarter ended June 29 , 2018 . As a result, all financial results described here should be considered preliminary, and are subject to change to reflect any necessary adjustments, or changes in accounting estimates, that are identified prior to the time the company files the Form 10 - Q . Medical Advice Disclaimer Varian as a medical device manufacturer cannot and does not recommend specific treatment approaches. Individual treatment res ult s may vary.

Long - Term Strategy

Eclipse ™ Treatment planning system RapidPlan ™ Knowledge - based planning software Calypso ® Real - time tracking technology Edge ™ System Full - body radiosurgery platform Halcyon ™ Image - guided IMRT treatment platform TrueBeam ® /VitalBeam ™ Trilogy ® /Clinac ® /UNIQUE ™ Treatment procedures with ease, speed and accuracy Varian Brachytherapy Product suite for planning and delivery ProBeam ® Proton therapy systems Radiation oncology OIS Radiosurgery Brachytherapy Radiation oncology treatment planning Proton therapy Radiation therapy Global leader in radiation therapy ARIA ® Oncology information system InSightive ™ Oncology analytics Radiation Therapy Market growing ~4% CAGR to ~$6B in 2022 * * Based on company estimates * and industry reports and data 4 HyperArc ™ High - definition radiotherapy Multi - Criteria Optimization (MCO) Enhanced control of plan optimization Graphics Processing Unit (GPU) Support Faster dose calculation and plan optimization

Long - term growth and value creation strategy 5 Global Leader in Radiation Therapy Radiation oncology OIS Radiosurgery Brachytherapy Radiation oncology treatment planning Proton therapy Radiation therapy Global Leader in Multi - Disciplinary, Integrated Cancer Care Solutions Interventional oncology Surgical oncology Diagnostic imaging Radiation oncology Precision medicine Medical oncology Generate insights Call on all oncologists Aggregate data Disseminated insights Build AI/ML capabilities
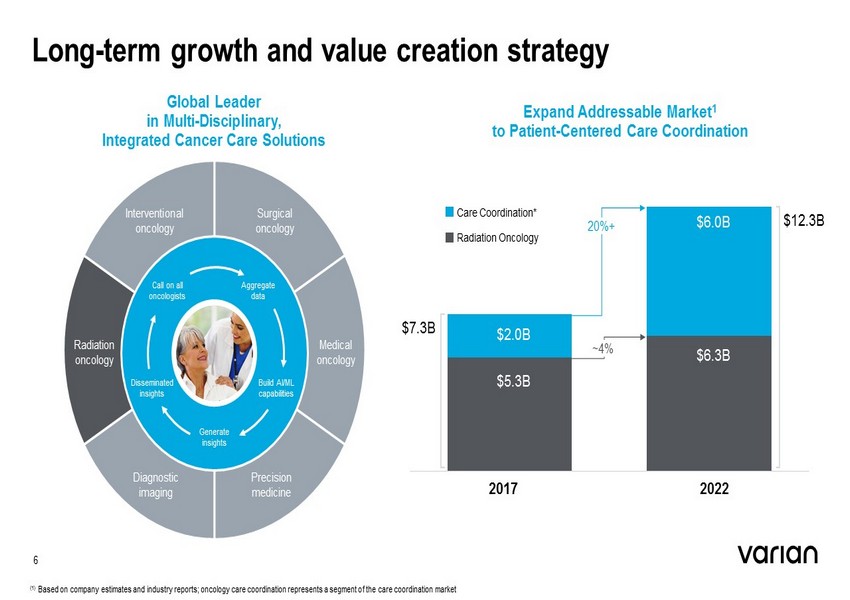
Long - term growth and value creation strategy Global Leader in Multi - Disciplinary, Integrated Cancer Care Solutions 6 Interventional oncology Surgical oncology Diagnostic imaging Radiation oncology Precision medicine Medical oncology Generate insights Call on all oncologists Aggregate data Disseminated insights Build AI/ML capabilities Expand Addressable Market 1 to Patient - Centered Care Coordination $2.0B $5.3B $6.0B $6.3B 20%+ $12.3B $7.3B Care Coordination* Radiation Oncology 2017 2022 ~4% (1) Based on company estimates and industry reports; oncology care coordination represents a segment of the care coordination mar ke t

Growth priorities and strategic enablers 7 We are here Global Leader in Radiation Therapy Global Leader in Multi - Disciplinary, Integrated Cancer Care Solutions Long - Term Growth and Value Creation Strategy Strengthen Leadership in Radiation Therapy Extend Global Footprint Expand Addressable Market Growth Priorities High Quality Care Through Innovation Build Software Services & Big Data Expertise Operational Efficiency Optimize Cash Conversion & Capital Structure Strategic Enablers Where we are headed

Global market leader with >50% share in radiation therapy, maintaining share in a 7% growth market Grew linac installed base by 4%; total installed base of 8,046 units R&D up 8% maintaining commitment to driving high - quality, organic innovation Orders Growth in the quarter ⁃ Oncology orders growth of 11% in the 3 rd quarter, or +9% in constant currency (cc), with orders growth in the Americas and EMEA ⁃ Oncology orders growth of 6% (+5% cc) over the trailing twelve months Since launch last May, Halcyon treatment platform ⁃ 98 orders to date ⁃ Nearly 60% are incremental ⁃ >40% for greenfield sites 8 Growth priority: Strengthen our leadership in radiation therapy (1) Based on company estimates and industry reports and data

~45% of Halcyon orders in emerging markets, since May 2017 launch ⁃ ~90% of emerging market orders to date were for incremental units Expanding access in Taiwan, with the acquisition of Cooperative CL Enterprises (COOP), a leading local distributor of radiotherapy equipment ~55% international orders mix in Oncology in the quarter; ⁃ EMEA orders growth of 27% (+21% cc); trailing twelve months have been the strongest in Varian’s history for EMEA ⁃ Double - digits Latin American orders growth 9 Growth priority: Extend global footprint

10 Growth priority: Expand addressable market Velocity 4.0 launched with RapidSphere, a module for Y90 Selective Internal Radiation Therapy (SIRT) dosimetry analysis, extending Velocity to the interventional oncology market Eclipse MCO orders of nearly 320 licenses to date (4Q FY17 launch) Unique software customers grew 6% during the 3 rd quarter; newer applications fuelling enthusiasm for Varian software solutions ⁃ Software revenues growth of 13% ⁃ Installed base grew >80% for RapidPlan, our knowledge - based treatment planning software; biggest orders quarter since launch

3Q FY 2018 Business Highlights

Varian launches Halcyon 2.0 with kilovoltage cone - beam CT imaging at ESTRO Calypso Anchored Beacon transponder for lung receives FDA 510k clearance First patient treated with Varian HyperArc high - definition radiotherapy Varian publishes 2017 Sustainability Report AUG OCT DEC JAN FEB MARCH 2018 Varian Eclipse users generate highest scoring plans in international treatment plan competition Varian installs Cyclotron at Georgia Proton Treatment Center Varian announces Penn Medicine treats world’s first patient on Halcyon system JUL 2017 NOV Halcyon receives Shonin approval in Japan Varian opens new facility in Brazil Varian partners with Penn Medicine for proton therapy training and education Halcyon receives ANVISA registration in Brazil Peter MacCallum Cancer Centre in Australia standardizes on Varian solution for cancer treatment planning Halcyon receives Taiwan FDA approval Varian ranks first in overall manufacturer, system, and service performance in 2017 Survey of Radiation Oncology Professionals SEP Varian acquires Mobius Medical Systems Halcyon receives AERB Certificate for Import and Supply in India ARIA, Eclipse and TrueBeam named category leaders in 2018 Best in KLAS Report Varian acquires Evinance Innovation Eclipse customers take top Overall spots at World Championships of Treatment Planning Varian signs memorandum of understanding with Ping An to expand access to high - quality cancer care in China Year in review 12 MAY APR JUN 2018 Varian signs training & education cooperation agreement with Brazil Ministry of Health and science and technology institutions Varian wins "Best After - Sales Service Performance Award for Radiotherapy Products" in China Varian acquires leading radiotherapy equipment distributor in Taiwan Varian launches Velocity 4.0, including RapidSphere Varian installs ProBeam cyclotron at UCLH proton beam therapy center

0% 5% 10% 15% 20% 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 +2 pts Y/Y Cash Flow from Operations ($M) $0 $50 $100 $150 $200 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Non - GAAP Operating Earnings (% of Revenues) Financial highlights Operating Trends* Strong performance in key financial measures • Revenues up 12% (10% in cc) due to hardware and services growth in Oncology operating segment • Cash Flow from Operations down 34%, due to a large cash receipt from Georgia Proton Treatment Center in the prior year period Oncology DSO down from 121 to 106 days • GAAP operating earnings down 1 percentage point to 12.9%, due to acquisition - related expenses and impairment from expected refinancing of Maryland Proton Treatment Center • Non - GAAP operating earnings up 2 percentage points to 16.9%, due to strong revenue and gross margin expansion enabling targeted investments in R&D, Sales, and Marketing * All financial metrics, including historical figures, reflect the new revenue recognition standard, ASC 606. 13 Revenues ($M) GAAP Operating Earnings (% of Revenues) $550 $600 $650 $700 $750 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 0% 5% 10% 15% 20% 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 - 34% Y/Y +12% Y/Y - 1 pt Y/Y

Varian linac installed base Installed Base Trends Varian strengthened its global leadership in radiation therapy • Each geography grew respective installed base in the third quarter 14 Q3FY17 8,046 Total Q2FY18 7,766 Q4FY17 Q1FY18 Q3FY18 +4%

Key wins Americas EMEA APAC Spain – Ortego Foundation, Catalunya 9 TrueBeams, 3 Halcyon systems Montefiore 1 TrueBeam, 1 Halcyon Unity Point 5 TrueBeam/Edge systems China – Chang Gung Memorial Hospital 1 Edge Australia – Peter MacCallum 4 TrueBeams Vietnam – VINMEC International Hospital TrueBeam order Japan – Iwate Medical University 1 TrueBeam, 1 TrueBeam STx UPMC 3 TrueBeam orders, 2 TrueBeam upgrades 15 HCA Multi - site order Great Britain – NHS Leeds 20 TrueBeams Nigeria – Lagos LUTH 1 VitalBeam, 1 Halcyon Poland – Gorzow Wielkopolski SZPITAL 2 TrueBeams Kenya – Nairobi West 1 Halcyon, 1 TrueBeam, and 1 VitalBeam

3Q FY 2018 Financial Overview

Varian consolidated (GAAP) Key P&L Financial Metrics 17 • Gross orders of $767 million down 5% Oncology orders +11% (+9% cc), with orders growth in the Americas and EMEA No new ProBeam orders in the quarter; 4 orders in the trailing twelve months; pipeline remains strong • Revenues of $709 million growing at 12% (+10% cc) Oncology revenues +18% (+16% cc) • Gross margin rate improved 227 bps • SG&A increased 33%, driven by: Investments in Sales and Marketing to drive long - term growth; G&A scaling with revenue growth $13 million acquisition - related expenses, primarily driven by acquisition costs and the loss from the Sirtex investment hedge, partially offset by the breakup fee $11 million impairment from expected refinancing of Maryland Proton Treatment Center • R&D investment up 8%, maintaining commitment to innovation • GAAP Diluted EPS growth of 4% driven by revenue growth and gross margin improvements • Grew linac installed base 4% Note: Unless noted otherwise, all ‘Orders’ reflects Gross Orders, all growth rates are in dollars, and year on year, and all num bers reflect continuing operations. VARIAN FISCAL 2018 $M 3Q Y/Y YTD Y/Y Gross Orders $767 -5% $2,099 2% Revenues $709 12% $2,117 12% Product $365 14% $1,124 13% Services $344 10% $993 10% Gross Margin $314 18% $935 16% % of Revenues 44.2% 227 bps 44.2% 157 bps SG&A $163 33% $453 0% % of Revenues 22.9% 367 bps 21.4% -251 bps R&D $60 8% $174 10% % of Revenues 8.4% -32 bps 8.2% -11 bps Operating Earnings $92 3% $307 57% % of Revenues 12.9% -107 bps 14.5% 418 bps Diluted EPS ($) $0.79 4% $0.36 -77% Installed Base (Linac) 8,046 4%

Varian consolidated (non - GAAP) Key P&L Financial Metrics 18 Note: Unless noted otherwise, all ‘Orders’ reflects Gross Orders, all growth rates are in dollars, and year on year, and all num bers reflect continuing operations. • Gross orders of $767 million down 5% Oncology orders +11% (+9% cc), with orders growth in the Americas and EMEA No new ProBeam orders in the quarter; 4 orders in the trailing twelve months; pipeline remains strong • Revenues of $709 million growing at 12% (+10% cc) Oncology revenues +18% (+16% cc) • Gross margin rate improved 235 bps • SG&A increased 17%, driven by investments in Sales and Marketing to drive long - term growth; G&A scaling with revenue growth • R&D investment up 8%, maintaining commitment to innovation • Non - GAAP EPS increased 28% due to strong growth and operational execution Excludes impact from $13 million acquisition - related expenses, and $11 million impairment from expected refinancing of Maryland Proton Treatment Center • Grew linac installed base 4% VARIAN FISCAL 2018 $M 3Q Y/Y YTD Y/Y Gross Orders $767 -5% $2,099 2% Revenues $709 12% $2,117 12% Product $365 14% $1,124 13% Services $344 10% $993 10% Gross Margin $316 18% $940 16% % of Revenues 44.5% 235 bps 44.4% 159 bps SG&A $136 17% $391 2% % of Revenues 19.3% 80 bps 18.5% -178 bps R&D $60 8% $174 10% % of Revenues 8.4% -32 bps 8.2% -11 bps Operating Earnings $119 26% $374 39% % of Revenues 16.9% 187 bps 17.7% 348 bps Diluted EPS ($) $1.04 28% $3.26 47% Installed Base (Linac) 8,046 4%

Varian consolidated Key Balance Sheet and Cash Flow Metrics • Cash Flow from Operations down 34%, due to a large cash receipt from Georgia Proton Treatment Center in prior year period Trailing twelve - month cash flow from operations of $476 million Oncology DSO down from 121 to 106 days • Continuing to strengthen financial flexibility through operational execution and by steadily reducing debt 19 VARIAN FISCAL 2017 FISCAL 2018 $M 3Q 4Q 1Q 2Q 3Q Cash & Cash Equivalents $658 $716 $823 $740 $536 Cash Flow from Operations $155 $130 $179 $66 $102 Y/Y % 63% -15% 118% 104% -34% Total Debt $364 $350 $340 $255 $18

Oncology operating segment Key Financial Metrics • Orders growth of 11% (+9% cc), with orders growth in the Americas and EMEA Oncology orders mix: North America ~45%, International ~55% 17 new orders for Halcyon in the quarter • Product revenues growth of 29% driven by robust linac system sales, services, and software • Services revenues growth of 10% driven by increased installed base and higher contract value from TrueBeam systems • Operating leverage improved due to disciplined scaling of G&A spend relative to revenue growth 20 Note: Unless noted otherwise, all ‘Orders’ reflects Gross Orders, all growth rates are in dollars, and year on year, and all num bers reflect continuing operations. (1) Operating earnings includes an allocation of corporate costs based on relative revenues between the operating segments. The all ocated corporate costs excludes certain transactions or adjustments that are considered non - operational in nature, such as restructurin g and impairment charges, significant litigation matters and acquisition related items. VARIAN FISCAL 2018 $M 3Q Y/Y YTD Y/Y Gross Orders $763 11% $2,047 8% Revenues $667 18% $2,015 14% Product $327 29% $1,029 18% Services $340 10% $986 10% Gross Margin $306 19% $923 16% % of Revenues 45.8% 29 bps 45.8% 81 bps Operating Earnings 1 $126 20% $407 19% % of Revenues 18.8% 36 bps 20.2% 78 bps Installed Base (Linac) 8,046 4%

21 Oncology gross orders by geography AMERICAS 9% 3Q Y/Y (+9% cc) 2% 3Q TTM 1 (+2% cc ) APAC - 7% 3Q Y/Y ( - 9% cc) - 3% 3Q TTM ( - 4% cc ) EMEA 27% 3Q Y/Y (+21% cc) 20% 3Q TTM (+14% cc ) (1) In North America, trailing twelve - month orders growth of 4% in dollars and constant currency

Particle Therapy operating segment Key Financial Metrics • No new orders for ProBeam systems in the quarter; 4 orders in the trailing twelve months; pipeline remains strong • 7 operational sites with 25 rooms in 5 countries; 16 more sites in progress across the world • Revenues, margins and related growth rates may be volatile until we get to operational scale across the business 22 Note: Unless noted otherwise, all ‘Orders’ reflects Gross Orders, all growth rates are in dollars, and year on year, and all num bers reflect continuing operations. (1) Operating earnings includes an allocation of corporate costs based on relative revenues between the operating segments. The all ocated corporate costs excludes certain transactions or adjustments that are considered non - operational in nature, such as restructurin g and impairment charges, significant litigation matters and acquisition related items. VARIAN FISCAL 2018 $M 3Q Y/Y YTD Y/Y Gross Orders $4 -97% $52 -67% Revenues $42 -39% $103 -21% Product $38 -43% $96 -23% Services $4 95% $7 12% Gross Margin $8 -6% $12 -6% % of Revenues 19.5% 677 bps 11.3% 178 bps Operating Earnings 1 ($10) NM ($43) NM % of Revenues -24.1% NM -41.6% NM

ProBeam proton therapy system sites Operational Centers Centers Under Development 23 Treatment rooms ProBeam Sites Operational rooms Operational Sites 70 25 7 23

FY 2018 Guidance

Varian consolidated Updated Fiscal Year 2018 Guidance 25 (1) These values are presented on a non - GAAP basis. We have not provided a reconciliation of non - GAAP guidance measures to the corresponding GAAP measures on a forward - looking basis due to potential significant variability and limited visibility of the ex cluded items. (2) Cash Flow is shown on a Total Company basis, including Varex for FY16 and FY17. VARIAN $M FY16 Actual FY17 Actual FY18 Guidance Revenues $2,594 $2,619 9% to 11% Non-GAAP Operating Earnings as percent of revenues 1 18.0% 15.1% 17.5% to 18.0% Non-GAAP EPS ($) 1 $3.67 $3.26 $4.43 to $4.48 Weighted Average Diluted Shares (M) 96 93 93 Non-GAAP Effective Tax Rate 1 25.5% 23.4% 20% Cash Flow from Operations 2 $356 $399 $475 to $550 Updating full fiscal year 2018 guidance after considering the following: • First, continued projected market growth • Second, our products and solutions are resonating, driving our market leadership • Third, recent fluctuations in the currency markets, and • Fourth, the impact of tax cuts and their implications to our tax rate Additionally, we have reflected in our guidance the projected impact of the tariffs imposed on July 6 th by the United States Trade Representative

3Q FY 2018 Financial Overview

Non - GAAP disclosure 27 Discussion of Non - GAAP Financial Measures This presentation includes the following non - GAAP financial measures derived from our Condensed Consolidated Statements of Earnings : non - GAAP operating earnings, non - GAAP net earnings and non - GAAP net earnings per diluted share . We define non - GAAP operating earnings from continuing operations as operating earnings from continuing operations excluding amortization of intangible assets, restructuring charges, legal costs, impairment charges and acquisition and integration related expenses and benefits . These measures are not presented in accordance with, nor are they a substitute for U . S . generally accepted accounting principles, or GAAP . In addition, these measures may be different from non - GAAP measures used by other companies, limiting their usefulness for comparison purposes . The non - GAAP financial measures should not be considered in isolation from measures of financial performance prepared in accordance with GAAP . Investors are cautioned that there are material limitations associated with the use of non - GAAP financial measures as an analytical tool . We have provided a reconciliation of each non - GAAP financial measure used in this earnings release to the most directly comparable GAAP financial measure . We have not provided a reconciliation of non - GAAP guidance measures to the corresponding GAAP measures on a forward - looking basis due to the potential significant variability and limited visibility of the excluded items discussed below . We utilize a number of different financial measures, both GAAP and non - GAAP, in analyzing and assessing the overall performance of our business, in making operating decisions, forecasting and planning for future periods, and determining payments under compensation programs . We consider the use of the non - GAAP measures to be helpful in assessing the performance of the ongoing operation of our business . We believe that disclosing non - GAAP financial measures provides useful supplemental data that, while not a substitute for financial measures prepared in accordance with GAAP, allows for greater transparency in the review of our financial and operational performance . We also believe that disclosing non - GAAP financial measures provides useful information to investors and others in understanding and evaluating our operating results and future prospects in the same manner as management and in comparing financial results across accounting periods and to those of peer companies . Non - GAAP operating earnings and non - GAAP net earnings exclude the following items, except for significant effects of tax legislation, which are only excluded from non - GAAP net earnings : Amortization of intangible assets : We do not acquire businesses and assets on a predictable cycle . The amount of purchase price allocated to intangible assets and the term of amortization can vary significantly and are unique to each acquisition or purchase . We believe that excluding amortization of intangible assets allows the users of our financial statements to better review and understand the historic and current results of our operations, and also facilitates comparisons to peer companies . Acquisition and integration - related expenses and benefits : We incur expenses or benefits with respect to certain items associated with our acquisitions, such as transaction costs, hedging gains and losses, changes in the fair value of contingent consideration liabilities, gain or expense on settlement of pre - existing relationships, integration costs, breakup fees etc . We exclude such expenses or benefits as they are related to acquisitions and have no direct correlation to the operation of our on - going business . Restructuring and impairment charges : We incur restructuring and impairment charges that result from events, which arise from unforeseen circumstances and/or often occur outside of the ordinary course of our on - going business . Although these events are reflected in our GAAP financials, these unique transactions may limit the comparability of our on - going operations with prior and future periods . Significant effects of tax legislation : We may incur significant effects of tax legislation that are generally unrelated to the level of business activity in the period in which the effects of such legislation are reported . We exclude such expenses because we believe this does not accurately reflect the underlying performance of our continuing business operations . This exclusion is applicable to non - GAAP net earnings only . Significant litigation charges or benefits and legal costs : We may incur charges or benefits as well as legal costs from time to time related to litigation and other contingencies . We exclude these charges or benefits, when significant, as well as legal costs associated with significant legal matters, because we do not believe they are reflective of on - going business and operating results . We apply our GAAP consolidated effective tax rate to our non - GAAP financial measures, other than when the underlying item has a materially different tax treatment . From time to time in the future, there may be other items that we may exclude if we believe that doing so is consistent with the goal of providing useful information to investors and management . Non - GAAP items are generally included in selling, general and administrative expenses, except for significant effects of tax legislation and unless otherwise specified .

GAAP to non - GAAP reconciliation 28 (1) Includes $1.2 million, $1.3 million, $1.2 million, $1.5 million, and $1.9 million, respectively, in cost of revenues for the per iods presented. (2) Includes the hedging loss related to the Australian dollar purchase price for Sirtex Medical Limited amounting to $16.4 million in the second quarter and $13.3 million in the third quarter of 2018, acquisition - re lated expenses of $3.3 million in the second quarter and $8.4 million in the third quarter of 2018, partially offset by the net $9.0 million Sirtex breakup fee in the third quarter of 2018. (3) Represents the tax effect of a change in law related to the U.S. Tax Cuts and Jobs Act. The corporate rate reduction resulted in a remeasurement of our Deferred Tax Assets of $37.8 million in the first quarter, $2.5 million in the second quarter, and $1.6 million in the third quarter of 2018. The mandatory deemed repatriation of unremitted foreign earnings resu lte d in an estimated charge of $169.3 million in the first quarter and $3.7 million in the second quarter of 2018. (4) Excludes immaterial net earnings from continuing operations attributable to noncontrolling interests for the periods presente d. THE FOLLOWING TABLE RECONCILES GAAP AND NON-GAAP FINANCIAL MEASURES FOR VARIAN'S CONTINUING OPERATIONS Dollars and shares in millions, except for share amounts 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Non-GAAP adjustments Amortization of intangible assets (1) $2.9 $3.1 $3.1 $3.5 $4.1 Restructuring charges 2.8 3.8 - - - Legal costs 0.2 - - - - Impairment charges - 13.1 - 11.1 11.0 Acquisition-related expenses (2) 0.5 0.5 1.5 19.7 12.7 Other - 0.8 - 0.1 0.2 Total non-GAAP adjustments to operating earnings 6.4 21.3 4.6 34.4 28.0 Tax effects of non-GAAP adjustments (0.6) (3.4) (1.0) (7.1) (5.6) Significant effects of tax legislation (3) - - 207.1 6.2 1.6 Total net earnings from continuing operations impact from non-GAAP adjustments $5.8 $17.9 $210.7 $33.5 $24.0 Operating earnings reconciliation GAAP operating earnings from continuing operations $88.5 $104.2 $121.4 $94.3 $91.5 Total operating earnings from continuing operations impact from non-GAAP adjustments 6.4 21.3 4.6 34.4 28.0 Non-GAAP operating earnings from continuing operations $94.9 $125.5 $126.0 $128.7 $119.5 Net earnings (loss) and net earnings (loss) per diluted share reconciliation GAAP net earnings (loss) from continuing operations attributable to Varian (4) $69.7 $78.7 ($112.3) $73.2 $72.6 Total net earnings from continuing operations impact from non-GAAP adjustments 5.8 17.9 210.7 33.5 24.0 Non-GAAP net earnings from continuing operations attributable to Varian $75.5 $96.6 $98.4 $106.7 $96.6 GAAP net (loss) earnings per diluted share from continuing operations $0.75 $0.85 ($1.22) $0.79 $0.79 Non-GAAP net earnings per diluted share from continuing operations $0.82 $1.04 $1.06 $1.15 $1.04 Shares used in computing GAAP net earnings per diluted share 92.4 92.6 91.6 92.6 92.5 Shares used in computing non-GAAP net earnings per diluted share 92.4 92.6 92.7 92.6 92.5

Total revenues by sales classification 29 CC – Constant currency $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Product revenues $320.2 $399.7 $365.6 $393.8 $365.0 Y/Y 18% 8% 14% Service revenues $312.6 $322.1 $312.9 $336.1 $344.1 Y/Y 7% 13% 10% Total revenues $632.8 $721.8 $678.5 $729.9 $709.1 Y/Y 13% 10% 12% Y/Y – CC 11% 6% 10% Product revenues as a percentage of total revenues 51% 55% 54% 54% 51% Service revenues as a percentage of total revenues 49% 45% 46% 46% 49%

Total revenues by product type 30 CC – Constant currency $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Y/Y Hardware $281.4 $352.5 $320.4 $341.7 $314.6 12% Oncology $214.9 $303.4 $293.1 $311.6 $276.5 29% Particle Therapy $66.5 $49.1 $27.3 $30.1 $38.1 -43% Software $110.4 $120.1 $115.1 $126.7 $125.1 13% Oncology $110.4 $120.1 $115.1 $126.7 $125.1 13% Particle Therapy - - - - - - Service $241.0 $249.2 $243.0 $261.5 $269.4 12% Oncology $239.1 $245.8 $241.2 $259.7 $265.6 11% Particle Therapy $1.9 $3.4 $1.8 $1.8 $3.8 95% Total revenues $632.8 $721.8 $678.5 $729.9 $709.1 12% CC 10% Hardware as a percentage of total revenues 45% 49% 47% 47% 44% Software as a percentage of total revenues 17% 17% 17% 17% 18% Service as a percentage of total revenues 38% 34% 36% 36% 38%

Total revenues by region 31 CC – Constant currency $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Americas revenues $336.3 $368.6 $356.7 $340.2 $342.6 Y/Y 20% 0% 2% Y/Y – CC 20% -1% 2% EMEA revenues $169.5 $220.4 $193.0 $253.8 $229.7 Y/Y 5% 37% 35% Y/Y – CC -2% 23% 28% APAC revenues $127.0 $132.8 $128.8 $135.9 $136.8 Y/Y 8% 0% 8% Y/Y – CC 10% -3% 6% Total revenues $632.8 $721.8 $678.5 $729.9 $709.1 Y/Y 13% 10% 12% Y/Y – CC 11% 6% 10%

Total Oncology revenues by sales classification 32 CC – Constant currency $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Product revenues $253.7 $350.6 $338.3 $363.7 $326.9 Y/Y 20% 9% 29% Y/Y – CC 18% 7% 26% Service revenues $310.7 $318.7 $311.1 $334.3 $340.3 Y/Y 8% 13% 10% Y/Y – CC 6% 11% 7% Total revenues $564.4 $669.3 $649.4 $698.0 $667.2 Y/Y 14% 10% 18% Y/Y – CC 12% 6% 16% Product as a percentage of total Oncology Systems revenues 45% 52% 52% 52% 49% Service as a percentage of total Oncology Systems revenues 55% 48% 48% 48% 51% Oncology Systems revenues as a percentage of total revenues 89% 93% 96% 96% 94%

Total Oncology revenues by region 33 CC – Constant currency $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Americas revenues $286.9 $347.1 $337.4 $321.2 $323.2 Y/Y 16% -3% 13% Y/Y – CC 16% -3% 13% EMEA revenues $157.6 $196.9 $183.5 $241.2 $208.5 Y/Y 9% 44% 32% Y/Y – CC 2% 29% 24% APAC revenues $119.9 $125.3 $128.5 $135.6 $135.5 Y/Y 15% 2% 13% Y/Y – CC 17% 0% 11% Total revenues $564.4 $669.3 $649.4 $698.0 $667.2 Y/Y 14% 10% 18% Y/Y – CC 12% 6% 16%

Total Particle Therapy revenues by sales classification 34 $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Product revenues $66.5 $49.1 $27.3 $30.1 $38.1 Y/Y 2% 0% -43% Service revenues $1.9 $3.4 $1.8 $1.8 $3.8 Y/Y -49% 61% 95% Total revenues $68.4 $52.5 $29.1 $31.9 $41.9 Y/Y -4% 2% -39% VPT revenues as a percentage of total revenues 11% 7% 4% 4% 6%

Oncology gross orders by region 35 CC – Constant currency $M 3Q FY17 4Q FY17 1Q FY18 2Q FY18 3Q FY18 Americas orders $331.9 $479.0 $299.5 $341.4 $362.9 Y/Y 2% 2% 9% Y/Y – CC 2% 2% 9% EMEA orders $204.5 $315.4 $190.4 $199.3 $259.1 Y/Y 19% 11% 27% Y/Y – CC 13% 0% 21% APAC orders $151.3 $153.0 $130.0 $123.4 $141.3 Y/Y 6% 3% -7% Y/Y – CC 6% 1% -9% Total orders $687.7 $947.4 $619.9 $664.1 $763.3 Y/Y 7% 5% 11% Y/Y – CC 6% 1% 9%

Our promise 36 People powering victories Imagine a world without fear of cancer. We do, every day. We innovate new technologies for treating cancer and for connecting clinical teams to advance patient outcomes. Through ingenuity we inspire new victories and empower people in the fight against cancer. We are Varian.
