Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF PARITZ & COMPANY, P.A. - SPRING PHARMACEUTICAL GROUP, INC. | exh23-1.htm |
As filed with the Securities and Exchange Commission on July 13, 2018
Registration No. 333-
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CHINA YCT INTERNATIONAL GROUP, INC.
CHINA YCT INTERNATIONAL GROUP, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
2834
|
30-0781441
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(Primary Standard Industrial Classification Code Number)
|
(I.R.S. Employer Identification Number)
|
c/o Shandong Spring Pharmaceutical Co., Ltd.
Economic Development Zone, Gucheng Road,
Sishui County, Shandong Province 373200
People's Republic of China
+86 0537-4268271
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Harvard Business Services, Inc.
16192 Coastal Hwy,
Lewes, DE 19958
(302) 645-7400
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Steven W. Schuster, Esq.
Xuan (Shane) Wu, Esq.
McLaughlin & Stern, LLP
260 Madison Avenue, 18 FL
New York, NY, 10016
Tel: (212) 448-1100
|
|
Mitchell Nussbaum, Esq.
Angela Dowd, Esq.Loeb & Loeb LLP
345 Park Avenue
New York, NY 10154
T: 212.407.4000
|
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
(Check one):
☐ Large accelerated filer
☐ Accelerated filer
☐ Non-accelerated filer (Do not check if a smaller reporting company)
☒ Smaller reporting company
☐ Emerging growth company
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered
|
Proposed Maximum Aggregate Offering Price(1)
|
Amount of Registration Fee(1)
|
||||||
|
Shares of common stock, par value $0.001 (2) (3)(4)
|
$
|
8,625,000
|
$
|
1073.81
|
||||
|
Underwriter' warrants(4)
|
—
|
—
|
||||||
|
Shares of common stock underlying underwriter' warrants(3)(5)
|
$
|
603,750
|
$
|
75.17
|
||||
|
Total:
|
$
|
9,228,750
|
$
|
1148.98
|
||||
|
(1)
|
Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the "Securities Act").
|
|
(2)
|
Includes the total offering price of additional shares of common stock the underwriters have the option to purchase to cover over-allotments, if any.
|
|
(3)
|
Pursuant to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of additional shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions.
|
|
(4)
|
We have agreed to issue to Maxim Group LLC (the "Underwriter"), the underwriter for this offering, warrants representing 7% of the total number of securities issued in the offering, including any over-allotment securities (the "Underwriter's Warrants"). The Underwriter's Warrants will be exercisable beginning six months after the effective date of this registration statement at a per share price equal to 110% of the common stock public offering price and will expire three years from the date on which they are initially exercisable. Resales of the Underwriter's Warrants on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, are registered hereby. Resales of shares issuable upon exercise of the Underwriter's Warrants are also being similarly registered on a delayed or continuous basis hereby. See "Underwriting." In accordance with Rule 457(g) under the Securities Act, because the shares of the Registrant's common stock underlying the Underwriter's Warrants are registered hereby, no separate registration fee is required with respect to the warrants registered hereby
|
|
(5)
|
The Registrant amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall hereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until the registration statement shall become effective on such date as the Commission, acting pursuant to Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION, DATED JULY 13 , 2018
|

China YCT International Group, Inc.
We are offering [●] shares of our common stock in a firm commitment underwritten public offering at a public offering price of $[●] per share.
Prior to this offering, our stock has been quoted on the OTCQB under the symbol "CYIG". On July 13, 2018, the last reported sales prices of our common stock on the OTCQB was $0.82 per share. We intend to apply to list our common stock on The Nasdaq Capital Market under the symbol "CTYY". We cannot assure you that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on The Nasdaq Capital Market.
Investing in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 8 of this prospectus to read about factors that you should consider before buying shares of our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
|
|
Per Common Share
|
Total
|
||||||
|
Public offering price
|
$
|
[●]
|
$
|
|||||
|
Underwriting discount (1)
|
$
|
[●]
|
$
|
|||||
|
Proceeds to us, before expenses
|
$
|
[●]
|
$
|
|||||
|
(1) The underwriter will receive compensation in addition to the underwriting discount. See "Underwriting" beginning on page 70 of this prospectus for a description of compensation payable to the underwriters. In addition, we have agreed to reimburse the underwriter for other out-of-pocket expenses relating to this offering up to a maximum of $150,000 for all such expenses.
|
We have granted the underwriter the option for a period of 45 days to purchase up to [●] additional shares of common stock (up to 15% of the number of shares of common sold in the primary offering) solely to cover over-allotments, if any. If the underwriter exercises its right to purchase additional shares of common stock to cover over-allotments in full, we estimate that we will receive gross proceeds of $ [●] from the sale of the common stock being offered and net proceeds of $[●] after deducting $[●] for underwriting discounts and commissions.
The underwriter expects to deliver the shares against payment in New York, New York, on or about [●], 2018.
Sole Book-Running Manager
Maxim Group LLC
The date of this Prospectus is [●]
1
TABLE OF CONTENTS
| Page | |
|
PROSPECTUS SUMMARY
|
3 |
|
RISK FACTORS
|
8 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
23 |
|
USE OF PROCEEDS
|
24 |
|
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
|
24 |
|
DIVIDEND POLICY
|
25 |
|
CAPITALIZATION
|
26 |
|
DILUTION
|
27 |
|
SELECTED CONSOLIDATED FINANCIAL DATA
|
27 |
|
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
29 |
|
DESCRIPTION OF BUSINESS
|
37 |
|
REGULATION
|
52 |
|
LEGAL PROCEEDINGS
|
57 |
|
DESCRIPTION OF PROPERTY
|
57 |
|
MANAGEMENT
|
58 |
|
EXECUTIVE COMPENSATION
|
64 |
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
66 |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
66 |
|
DESCRIPTION OF CAPITAL STOCK
|
67 |
|
UNDERWRITING
|
70 |
|
LEGAL MATTERS
|
74 |
|
EXPERTS
|
74 |
|
WHERE YOU CAN FIND MORE INFORMATION
|
74 |
|
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
F -1 |
2
Neither we nor the underwriter has authorized anyone to provide you with any information or to make any representations other than as contained in this prospectus or in any free writing prospectuses we have prepared. Neither we nor the underwriter takes responsibility for, or provides any assurance about the reliability of, any information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of the common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
No action is being taken in any jurisdiction outside the U.S. to permit a public offering of our common stock or possession or distribution of this prospectus in any such jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the U.S. are required to inform themselves about and to observe any restrictions on this offering and the distribution of this prospectus applicable to those jurisdictions.
Our logo and some of our trademarks and tradenames are used in this prospectus. This prospectus also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks, tradenames and service marks referred to in this prospectus may appear without the ®, TM and SM symbols, but those references are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensor to these trademarks, tradenames and service marks.
Industry and Market Data
This prospectus contains estimates and information concerning our industry, including market size and growth rates of the markets in which we participate, that are based on industry publications and reports. This information involves many assumptions and limitations, and you are cautioned not to give undue weight to these estimates. We have not independently verified the accuracy or completeness of the data contained in these industry publications and reports. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in "Risk Factors," that could cause results to differ materially from those expressed in these publications and reports.
We have also relied on statistics provided by a variety of publicly-available sources regarding China's expectations of growth. We did not, directly or indirectly, sponsor or participate in the publication of such materials, and these materials are not incorporated in this prospectus other than to the extent specifically cited in this prospectus.
Information contained in, and that can be accessed through, our web site www.yctgroup.com shall not be deemed to be part of this prospectus or incorporated herein by reference and should not be relied upon by any prospective investors for the purposes of determining whether to purchase the securities offered hereunder.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before investing in our common stock. You should carefully read the entire prospectus, including "Risk Factors", "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes included elsewhere in this prospectus, before making an investment decision.
In this prospectus, unless otherwise noted or as the context otherwise requires, the "Company" ,"CYIG", "we", "us" and "our" refer to the combined business of China YCT International Group, Inc., a Delaware corporation, Landway Nano Bio-Tech Group, Inc. ("Landway Nano") a Delaware corporation, and Shandong Spring Pharmaceutical Co., Ltd. ("Shandong Spring Pharmaceutical"), which is our 97%-owned subsidiary formed under the laws of the People's Republic of China. "China" or "PRC" refers to the People's Republic of China, excluding Hong Kong Special Administrative Region of China, Macau Special Administrative Region of China and the Taiwan Region.
3
This prospectus contains translations of certain Chinese currency Renminbi or RMB amounts into U.S. dollar amounts at specified rates solely for the convenience of the reader. The relevant exchange rates are listed below:
|
|
For the years ended
March 31,
|
|||||||||||
|
|
2018
|
2017
|
2016
|
|||||||||
|
Period Ended RMB: USD exchange rate
|
6.2881
|
6.8993
|
6.4612
|
|||||||||
|
Period Average RMB: USD exchange rate
|
6.6254
|
6.7287
|
6.3214
|
|||||||||
Our Company
Overview
We are a vertically integrated and innovative health care company focusing on developing, manufacturing, marketing and distributing modernized Traditional Chinese Medicines ("TCM") and health care products in China. Our proprietary product, Huoliyuan capsule, is a China Food and Drug Administration ("CFDA") approved prescription TCM that has a wide range of therapeutic benefits. We believe that it is the only TCM of its kind made in slow-release capsule form for improved absorption rate and therapeutic effects. In addition, in an effort to capitalize on the initiative of developing plant-based oil products promulgated by the State Forestry Administration of China, we are actively engaging in research and development and production of acer truncatum bunge seed oil products. We currently have approximately 5,880 mu (approximately 968.65 acres) of acer truncatum bunge plantation base located in Sishui, Shandong Province coupled with modern production facilities. Production from acer truncatum bunge trees in 2018 will enable us to cease sole reliance on third party producers of our raw material and achieve cost savings and enhance control of raw material supplies. We believe we are the only company in China to have achieved industrial-scale production and vertically integrated capability for acer truncatum bunge seed oil products. We plan to continue increasing the breadth our acer truncatum bunge seed oil product portfolio and improving our capabilities to produce raw materials and finished products as we envision this particular business segment will become a major growth driver for us in the future.
We have three distinctive business segments:
|
·
|
developing, manufacturing and selling Huoliyuan capsules, a medicine made primarily from panax ginseng leaves extract,
|
|
·
|
developing, manufacturing and selling seed oil products from acer truncatum bunge trees, a maple tree native to China, and
|
|
·
|
distributing health care supplement products manufactured by another company in the PRC.
|
We conduct our sales and marketing activities for the acer truncatum bunge seed oil products and health care supplement products produced by Shandong Yongchuntang Group Co., Ltd. ("Shandong Yongchuntang") primarily through seven major distributors who collectively control a 200,000+ person direct sales force, covering 33 provinces throughout China. We also have an internal sales and marketing team responsible for developing key sales strategies and campaigns, and utilize independent distributors for sales of Huoliyuan capsules.
We have realized significant growth in revenue and net income in recent periods. We realized $64,942,737 in revenue, representing an increase of 15.0% or $8,479,573, for the year ended March 31, 2018, as compared to $56,463,164 for the prior year. We also realized net income of $11,738,488, representing a 16.7% or $1,683,834 increase, for the year ended March 31, 2018, compared to $10,054,654 for the prior year.
4
Our Growth Opportunity
We believe that sales of our diversified products are growing and evolving. Drivers of growth include:
|
·
|
Policies from several levels of the Chinese government which support and encourage development of acer truncatum bunge seed oil industry
|
|
·
|
China's increasing expenditures on medicines and healthcare products due to China's increasing per capita disposable income and the growth of China's aging population
|
|
·
|
Use of the cost-effective licensed direct sales program and development of relationships with major distributors to increase the distribution and sales of our acer truncatum bunge seed oil products and health care products purchased from Shandong Yongchuntang as we expand geographically and add new products
|
The upcoming harvest of acer truncatum bunge pods and the establishment of a cooperative relationship with local farmers who plant the acer truncatum bunge trees is expected to increase our supply of acer truncatum bunge pods for processing the seeds into oil, which should lower the per unit cost and eliminate our reliance on third party suppliers for our raw material.
Our Competitive Strengths
We believe the following competitive strengths have contributed to, or will contribute to, our recent and ongoing growth:
Dominant Producer of Acer Truncatum Bunge Seed Oil
We believe that we are the largest producer of acer truncatum bunge seed oil in China, accounting for 47.7% of the market in China in 2017. We have also obtained a food production license for the production of edible vegetable oil from the Food and Drug Administration of Sishui County, and expect to produce blended edible oil products that contain acer truncatum bungee seed oil as one of the major ingredients. We believe that being the dominant producer and having stronger vertically integrated production capabilities will provide us with advantages in pricing, production and distribution of our products and spur further growth.
Diversified Revenue and Profit Stream
We generate revenue through the sales of diversified products, including prescription medicines, acer truncatum bunge seed oil products, and health care products purchased from Shandong Yongchuntang . With a diversified revenue stream, which can reduce financial stress during times of economic decline by relying on more than a single product, we believe alternative revenue sources provide the diversification to create a more financially stable company as well as additional opportunities for growth.
Strong Relationship with Key Distributors
We conduct our sales primarily through our seven distributors, who collectively control a person direct sales force of over 200,000 people and use person-to-person marketing to promote and sell our acer truncatum bunge seed oil products and nine products purchased from Shandong Yongchuntang. Compared to health and nutrition products that are distributed through traditional market means, these personal marketing efforts are supported by various mediums, including our marketing content, websites, events and social business solutions.
Reputable Brands
Our acer truncatum bunge seed oil products are distributed under the recognized brand name "Bao Feng San Yi". In addition, we believe that we are the largest producer of Huoliyuan capsules and tablets in China, that our Huoliyuan capsule is the only herbal medicine of its type made in the form of a capsule, and we believe that we are recognized by customers as a leader in the Huoliyuan market. Other peer products are produced in the form of tablets. The capsule permits the medication to be formulated for slow release. We believe that our reputable brand name will be of benefit to our future expansion and growth efforts.
5
Raw Materials Competitive Pricing
Our goal is to be a vertically integrated acer truncatum bunge seed oil and blended edible oil producer once our own trees begin production, which is expected to occur in the fall of 2018. While to date we have produced acer truncatum bunge seed oil from raw materials purchased from third parties, we began to develop and cultivate our own trees in June 2013. We have leased 5,880 mu (approximately 2,324.77 acres) of farmland for terms of 30 years and 14 years, respectively, and have planted approximately seven million trees at a cost of approximately $49 million through March 31, 2018. Having our own tree base will significantly reduce our cost to produce the seed oils compared to our competitors that purchase the acer truncatum bunge pods from third parties. The intended principal use of the proceeds that we will receive from this offering is to expand and further automate our manufacturing facilities.
Experienced Management
We are led by highly experienced and entrepreneurial executive officers. Mr. Tinghe Yan, our founder, Chairman and Chief Executive Officer, has over twenty years of experience in corporate management within the food and food supplements industries. Mr. Yan founded Shandong Spring Pharmaceutical in 2006. From 1998 to 2006, Mr. Yan was employed as the Chairman and General Manager of Shandong Yongchuntang, which manufactures a wide variety of food supplements and health care products and is currently the exclusive supplier of nine health care products distributed by Shandong Spring Pharmaceutical.
Corporate information
Our principal executive office is located at c/o Shandong Spring Pharmaceutical Co., Ltd., Economic Development Zone, Gucheng Road, Sishui County, Shandong Province 373200, People's Republic of China, and our telephone number is +86 0537-4268171. Our website address is www.yctgroup.com. The information contained therein or connected thereto shall not be deemed to be incorporated into this prospectus or the registration statement of which it forms a part.
6
The Offering
The following summary contains basic information about the offering and the securities we are offering and is not intended to be complete. It does not contain all the information that is important to you. For a more complete understanding of the securities we are offering, please refer to the section of this prospectus titled "Description of Capital Stock".
|
Securities being offered
|
[●] shares of common stock
|
|
Offering Price
|
$[●] per share.
|
|
Over-allotment option
|
We have granted the underwriter the option to purchase up to [●] additional shares of common stock, solely to cover over-allotments, if any, at the price to the public set forth on the cover page to this prospectus less the underwriting discounts and commissions. The over-allotment option is exercisable for 45 days from the date of this prospectus.
|
|
Common stock outstanding before this offering
|
29,839,168 shares of common stock(1)
|
|
Common stock outstanding after this offering
|
[●] shares of common stock (or [●] shares if the underwriter exercises its over-allotment option in full).(2)
|
|
Use of proceeds
|
We estimate that our net proceeds from this offering will be $[●] million (or approximately $[●] million if the underwriters' option to purchase additional shares of our common stock from us is exercised in full), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of the offering for (i) the expansion and automation of our manufacturing facilities for acer truncatum bunge seed oil products, (ii) the purchase of equipment and (iii) general working capital to meet the needs of our continued development. See "Use of Proceeds" for additional information
|
|
Risk factors
|
Investing in our securities involves a high degree of risk. You should read carefully the "Risk Factors" section beginning on page 8 of this prospectus for a discussion of factors that you should carefully consider before deciding to invest in our securities.
|
|
OTCQB
Marketplace ticker symbol
|
"CYIG"
|
|
Proposed Nasdaq Symbol and Listing
|
We intent to apply to list our common stock on The Nasdaq Capital Market under the symbol "CTYY". There can be no assurance that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on The Nasdaq Capital Market.
|
|
Transfer Agent and Registrar:
|
Interwest Transfer Co., Inc.
|
|
(1)
|
The number of shares of our common stock outstanding is based on 29,839,168 shares outstanding as of July 13, 2018, which excludes:
|
|
●
|
3,000,000 shares of our common stock that have been reserved for future issuance under our 2018 Stock Incentive Plan.
|
|
●
|
500,000 restricted shares of common stock issuable to a consultant should our common stock be listed on The Nasdaq Stock Market.
|
|
●
|
400,000 restricted shares of common stock issuable upon the exercise of a warrant at an exercise prices of $2.00 per share, which warrant is exercisable six months after our common stock is listed on The Nasdaq Stock Market. For a discussion on the terms of the outstanding warrant, see "Description of Capital Stock—Warrants".
|
|
●
|
[●] shares of common stock issuable upon the exercise of warrants to be issued to the Underwriter in connection with this offering.
|
|
(2)
|
Except as otherwise stated herein, the information in this prospectus assumes no exercise by the underwriter of its over-allotment option.
|
7
RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this prospectus, before making an investment decision. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, the trading price of our shares of common stock could decline, and you may lose all or part of your investment. You should read the section entitled "Cautionary Note Regarding Forward Looking Statements" for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this prospectus.
Risks Relating to Our Business and Industry
We are dependent on our relationship with Shandong Yongchuntang for a significant portion of our business.
Shandong Yongchuntang is currently the exclusive supplier of nine health care products distributed by Shandong Spring Pharmaceutical. Any problems with our relationship with Shandong Yongchuntang or with the operations of Shandong Yongchuntang could have a material adverse impact on our revenues and growth. The distribution of products manufactured by Shandong Yongchuntang accounted for $26,297,055, or 40.5% and $20,159,631, or 35.7%, of our revenue in the fiscal years ended March 31, 2018 and 2017, respectively. We sublicense Shandong Yongchuntang's license from the Chinese government to conduct direct sales marketing, which accounted for all of our sales of Shandong Yongchuntang's products. We also sell our acer truncatum bunge seed oil products under this sublicense, which accounted for $12,844,950, or 19.8% percent of our revenue in the fiscal year ended March 31, 2018.
We are dependent upon two lines of products for a majority of our revenues,
A substantial portion of our revenue is derived from sales of Huoliyuan capsules and acer truncatum bunge seed oil products. Any material problems in the manufacture, sale or distribution of acer truncatum bunge seed oil products or Huoliyuan capsules, some of which may be beyond our control, could have a material adverse effect on our revenues and growth. For example, in 2014, sales of Huoliyuan capsules were adversely affected by a crisis in China regarding a widespread quality failure in capsules materials, although the market demand for Huoliyuan capsules has since been stabilized. Sales of Huoliyuan capsules and acer truncatum bunge seed oil products, accounted for $38,645,682, or 59.5%, of our revenue in the fiscal year ended March 31, 2018, and for $36,303,533, or 64.3% in the fiscal year ended March 31, 2017, respectively.
We are dependent on the services of our executive officers, including Tinghe Yan, our Chief Executive Officer, Chuamin Li, our Chief Financial Officer, and Maogong Sun, our general manager. Any loss in their services without suitable replacement may adversely affect our operations.
Our success depends to a significant degree on the services rendered to us by our key employees, and our continued success will depend on our ability to retain their services. In particular, we rely on the services of Tinghe Yan, Chuanmin Li, and Maogang Sun, given their experience in the traditional healthcare and edible oils industries, in product development, and in sales and marketing and their relationships with our customers and suppliers. Mr. Yan has served as our Chairman and Chief Executive Officer since 2007. Mr. Yan founded Shandong Spring Pharmaceutical, and he has served as its Chairman since January 2006. Mr. Li has been our Chief Financial Officer since 2005. Mr. Sun has served as our general manager, deputy general manager and director of pharmaceutical production, quality management, marketing, for Shandong Spring Pharmaceutical since 2006. We do not have key man life insurance on any officers.
The loss of the services of any of key employees, including members of our senior management team, without suitable replacement or the inability to attract and retain qualified personnel with sufficient experience in our industry would adversely affect our operations and, hence, our revenue and profits.
8
Unexpected factors may hamper our efforts to implement our business plan.
Our business plan contemplates that we will become a fully integrated grower, manufacturer and marketer of various herbal products. We commenced manufacture of Huoliyuan capsules in 2010; and our business shifted from largely distributing health and beauty aids manufactured by Shandong Yongchuntang, to manufacturing and marketing Huoliyuan capsules and acer truncatum bunge based products. In order to fully implement our business plan, we will have to successfully complete the development of an agricultural facility and an industrial facility. The complexity of this undertaking means that we are likely to face many challenges, some of which are not yet foreseeable. Problems may occur with our raw material production and with the roll-out of efficient manufacturing processes. If we are not able to minimize the costs and delays that result, our business plan may fall short of its goals, and the current profitability of our distribution activities may be offset by losses from the herbal business.
Acer truncatum bunge seed oil is claimed to have nutritional and health benefits, but we cannot assure you that all or any of the claims made by others are accurate.
Studies and users of oil extracted from acer truncatum bunge seeds claim that use of the oil has health benefits. These users generally use acer truncatum products due to word-of-mouth or other advertising by manufacturers of the product. Additionally, there are little to no restrictions or health regulations on acer truncatum bunge seed oil in China at this time. Neither the Chinese Food and Drug Administration nor State Food and Drug Administration (SFDA) of China has opined on acer truncatum bunge seed oil at this time. While there are some research papers that claim that there are health benefits that support brain health by way of high levels of nervonic acid found in acer truncatum bunge seed oil, we cannot make any assurances that these claims are accurate.
The development of products based on acer truncatum bunge based products may not succeed.
We have devoted substantial resources to the development of products based on acer truncatum bunge and estimate that the development of the project which has taken five years with a total investment of approximately $49 million. However, there can be no assurance that such product development will be successful. While we intend to finance the development of our products from internally generated capital and the proceeds of this offering, there is no assurance that our other operations will generate sufficient capital to finance such development or, if insufficient, that alternate financing will be available.
Our business is currently dependent on our relationships with three suppliers.
Shandong Yongchuntang is the sole supplier of nine health care products that we distribute. We also currently depend on one vendor for 44.5% of the raw material we use for the production of Huoliyuan capsules and one vendor for 11.85% of the raw material we use for the production of acer truncatum bunge seed oil. If our relationship with any of these vendors is terminated, interrupted or materially modified or if the businesses of any of these vendors suffer adverse developments, our financial condition would be materially and adversely affected.
We are subject to the risk of natural disasters.
We eventually intend to produce most of the raw materials for our production of acer truncatum bunge seed oils. In particular, we are growing our own acer truncatum bunge trees. Those trees are very sensitive crops, which can be readily damaged by harsh weather, by disease, and by pests. If our crops are destroyed by drought, flood, storm, blight, or the other perils of farming, we will not be able to meet the demands of our manufacturing facility, which will then become inefficient and unprofitable. In addition, if we are unable to produce sufficient products to meet demand, our distribution network is likely to atrophy. This would result in an immediate loss of income and could also have a long term negative effect on our ability to grow our business,
If we lose control of our distribution network, our business will suffer.
We depend on our direct sales marketing distribution network, and particularly upon our seven largest distributors, which, together account for 40.2% of our sales, for the success of our business. Competitors may seek to disrupt or pull our distribution network away from us. In addition, if dominant members of our distribution network become dissatisfied with their relationship with Shandong Spring Pharmaceutical, a concerted effort by the distribution network could force us to accept less favorable financial terms from the distribution network. Any one of these possibilities, if realized, would have an adverse effect on our business.
9
Increased government regulation of our production and/or marketing operations could diminish our profits.
At present, there is no significant government regulation of the health claims that sellers in our industry make regarding their products. In addition, there is only limited government regulation of the conditions under which we manufacture our products, although the production of the Huoliyuan capsules, which is a prescription medicine, is subject to increasing regulations in China. Other developed countries, such as the United States and in particular, members of the European Community, have far more extensive regulation of the operations of nutraceuticals and plant-based cosmetics, including strict limitations on the health-related claims that can be made without scientifically-tested evidence. China may, increase its regulation of our activities. To the extent that new regulations require us to conduct a regimen of scientific tests of the efficacy of our products, the expense of such testing would reduce our profitability. In addition, to the extent that the health benefits of some of our products are not able to be fully supported by scientific evidence, our sales might be reduced.
Our business and growth will suffer if we are unable to hire and retain key personnel, who are in high demand.
Our future success depends on our ability to attract and retain highly skilled agronomists, biologists, chemists, industrial technicians, production supervisors, and marketing personnel. In general, qualified individuals are in high demand in China, and there are insufficient experienced personnel to fill the demand. In a specialized scientific field, such as ours, the demand for qualified individuals is even greater. If we are unable to successfully attract or retain the personnel we need to succeed, we will be unable to implement our business plan.
The production of traditional Chinese medicines depends on the supply of quality medicinal raw materials.
The production of traditional Chinese medicines depends on the supply of Chinese medicinal raw materials of suitable quality. For the fiscal years ended March 31, 2018 and 2017, the cost of raw materials used by us for our production accounted for 30.11% and 14.33%, respectively, of our total cost of revenues. While we expect the maturation of our acer truncatum bunge trees to mitigate our reliance on third parties for the supply of raw material for one of our products, the supply and market prices of these and other raw materials may be adversely affected by various factors such as weather conditions and the occurrence of natural disasters or sudden increases in demand that would impact our costs of production. There is no assurance that we would be able to pass on any resulting increase in costs to our customers, and therefore, any substantial fluctuation in supply or the market prices of raw materials may adversely affect our results of operations and profitability.
We may have difficulty establishing adequate management and financial controls in China.
The People's Republic of China has only recently begun to adopt the management and financial reporting concepts and practices that investors in the United States are familiar with. We may have difficulty in hiring and retaining employees in China who have the experience necessary to implement the kind of management and financial controls that are expected for a United States public company. If we cannot establish such controls, we may experience difficulty in collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet U.S. standards.
We face intense competition that may prevent us from maintaining or increasing market share for our existing products and gaining market acceptance of our future products. Our competitors may develop or commercialize products before or more successfully than us.
The pharmaceutical and traditional Chinese medicine markets in China are intensely competitive, rapidly evolving and highly fragmented. Our competitors may develop products that are superior to or more affordable than ours or they may more effectively market products that compete with ours. We face direct competition from manufacturers of other traditional Chinese medicines that are similar to our products. We also face competition from manufacturers of western medicines, including multinational companies, that manufacture western medicines with similar effects and that can be used as substitutes for our products. Many of our existing and potential competitors have substantially greater financial, technical, manufacturing and other resources than we do. Our competitors' greater size in some cases provides them with a competitive advantage with respect to manufacturing costs because of their economies of scale and their ability to purchase raw materials at lower prices. Many of our competitors also have better brand name recognition, more established distribution networks and larger customer bases. In addition, many of our competitors have extensive knowledge of our target markets. As a result, they may be able to devote greater resources to the research, development, promotion and sale of their products or respond more quickly to evolving industry standards and changes in market conditions than we can. Alternative products may be developed that are more effective, work faster and are less costly than our products. Competitors may succeed in developing products earlier than us, obtaining approvals and clearances for such products more rapidly than us, or developing products that are more effective than those of our company. In addition, other forms of treatment may be competitive with our company's products. Over time, our products may become obsolete or uncompetitive. Our failure to adapt to changing market conditions and to compete successfully with existing or new competitors may materially and adversely affect our financial condition and results of operations.
10
Our success depends on collaborative partners, licensees and other third parties over whom we have limited control.
Due to the complexity of the process of developing pharmaceuticals, should we develop pharmaceutical products in addition to the Huoliyuan capsules, we may depend on arrangements with pharmaceutical institutes, corporate and academic collaborators, licensors, licensees and others for the research, development, clinical testing, technology rights, manufacturing, marketing and commercialization of our products. License agreements could obligate the parties to diligently bring potential products to market, make milestone payments and royalties that, in some instances, could be substantial, and incur the costs of filing and prosecuting patent applications. There are no assurances that we will be able to establish or maintain collaborations that are important to our business on favorable terms, or at all.
A number of risks arise from the Company's dependence on collaborative agreements with third parties. Product development and commercialization efforts could be adversely affected if any collaborative partner:
|
•
|
terminates or suspends its agreement with us;
|
|
•
|
causes delays;
|
|
•
|
fails to timely develop or manufacture in adequate quantities a substance needed in order to conduct clinical trials;
|
|
•
|
fails to adequately perform clinical trials;
|
|
•
|
determines not to develop, manufacture or commercialize a product to which it has rights; or
|
|
•
|
otherwise fails to meet its contractual obligations.
|
Our collaborative partners could pursue other technologies or develop alternative products that could compete with the products we are developing.
We may not be able to obtain the regulatory approvals or clearances that are necessary to commercialize our products.
The PRC imposes significant statutory and regulatory obligations upon the manufacture and sale of pharmaceutical and nutraceutical products. It typically has a lengthy approval process in which it examines pre-clinical and clinical data and the facilities in which the product is manufactured. Regulatory submissions must meet complex criteria to demonstrate the safety and efficacy of the ultimate products. Addressing these criteria requires considerable data collection, verification and analysis. We may spend time and money preparing regulatory submissions or applications without assurances as to whether they will be approved on a timely basis or at all.
Governmental and regulatory authorities may approve a product candidate for fewer indications or narrower circumstances than requested or may conditionally approve the performance of post-marketing studies for a product candidate. Even if a product receives regulatory approval and clearance, it may later exhibit adverse side effects that limit or prevent its widespread use or that force us to withdraw the product from the market.
11
Any marketed product and its manufacturer will continue to be subject to strict regulation after approval. Results of post-marketing programs may limit or expand the further marketing of products. Unforeseen problems with an approved product or any violation of regulations could result in restrictions on the product, including its withdrawal from the market and possible civil actions.
Manufacturing our products requires compliance with applicable good manufacturing practices regulations, which include requirements relating to quality control and quality assurance, as well as the maintenance of records and documentation. If we cannot comply with regulatory requirements, including applicable good manufacturing practices requirements, we may not be allowed to develop or market the product candidates. If we fail to comply with applicable regulatory requirements at any stage during the regulatory process, it may be subject to sanctions, including fines, product recalls or seizures, injunctions, refusal of regulatory agencies to review pending market approval applications or supplements to approve applications, total or partial suspension of production, civil penalties, withdrawals of previously approved marketing applications and criminal prosecution.
Sales of acer truncatum bunge based products are subject to seasonal fluctuations, which may cause our operating results to fluctuate from quarter to quarter. This may result in volatility in the price of our common stock.
The fourth quarter is our peak season for sales of acer truncatum bunge based products because of purchases related to the Chinese New Year holiday season that effectively lasts more than two weeks. Gift giving during the holiday season is customary. Because of these factors, we may experience quarterly fluctuations in our results of operations, which in turn may result in volatility in the price of our common stock.
We do not have product liability insurance. If we were successfully sued for product liability, we could face substantial liabilities that may exceed our resources.
We may be held liable if any product we or our suppliers develop causes injury or is found unsuitable during product testing, manufacturing, marketing, sale or use. These risks are inherent in the development of pharmaceutical and nutraceutical products. We do not have product liability insurance. If we choose to obtain product liability insurance but cannot obtain sufficient insurance coverage at an acceptable cost or otherwise protect against potential product liability claims, the commercialization of products that we develop may be prevented or inhibited. If we are sued for any injury caused by our products, our liability could exceed our total assets.
Direct-selling laws and regulations may prohibit or severely restrict our direct sales efforts and cause our revenue and profitability to decline, and regulators could adopt new regulations that harm our business.
Our direct selling system is subject to extensive laws, governmental regulations, administrative determinations, court decisions and similar constraints. These laws and regulations are generally intended to prevent fraudulent or deceptive schemes, often referred to as "pyramid" schemes, which compensate participants for recruiting additional participants irrespective of product sales, use high pressure recruiting methods and/or do not involve legitimate products.
Complying with these widely varying and sometimes inconsistent rules and regulations can be difficult and may require the devotion of significant resources on our part. There can be no assurance that we or our distributors are in compliance with all of these regulations. Our failure or our distributors' failure to comply with these regulations or new regulations could lead to the imposition of significant penalties or claims and could negatively impact our business. If we are unable to continue business in existing markets or commence operations in new markets because of these laws, our revenue and profitability may decline.
We are also subject to the risk that new laws or regulations might be implemented or that current laws or regulations might change, which could require us to change or modify the way we conduct our business in certain markets. This could be particularly detrimental to us if we have to change or modify the way we conduct business in markets that represent a significant percentage of our revenue.
12
Risks related to protection and infringement of intellectual property rights.
Our ability to compete depends, in part, on our ability to obtain and enforce intellectual property protection for our technology in China and internationally. We currently rely primarily on a combination of trade secrets, patents, copyrights, trademarks and licenses to protect our intellectual property. While the Chinese government is more supportive in recent years of the protection of intellectual property rights, if we fail to enforce our intellectual property rights, our business may suffer. We, or our suppliers, may be subject to third-party claims of infringement on intellectual property rights. These claims, if successful, may require us to redesign affected products, enter into costly settlement or license agreements, pay damage awards, or face a temporary or permanent injunction prohibiting us from marketing or selling certain of our products.
Risks related to lack of insurance.
We may be held liable if our operations or any product we or our suppliers develop causes injury or property damage to employees or others. We do not have property and casualty insurance and injury insurance. If we choose to obtain property and casualty insurance and injury insurance but cannot obtain sufficient insurance coverage at an acceptable cost or otherwise protect against potential claims, the commercialization of products that we develop may be prevented or inhibited. If we are sued for any property damage or injury caused by our operations or products, our liability could deplete our total assets.
Our future capital needs are uncertain and we may need to raise additional funds in the future.
We may require additional cash resources in the future due to:
|
Ÿ
|
changed business conditions or other future developments;
|
|
Ÿ
|
the time and expenses required to obtain regulatory clearances and approvals;
|
|
Ÿ
|
the resources we devote to developing, manufacturing, marketing and selling our products;
|
|
Ÿ
|
our ability to identify and our desire or need to pursue acquisitions or other investments; and
|
|
Ÿ
|
the extent to which our products generate market demand.
|
If we need to obtain external financing, we cannot assure you that financing will be available in the amounts or on the terms acceptable to us, if at all. Our future capital needs and other business reasons could require us to sell additional equity or debt securities or obtain credit facilities. The sale of additional equity or equity-linked securities could result in additional dilution to our then existing stockholders. The incurrence of indebtedness would result in an increase in our debt obligations and could result in operating and financing covenants that would restrict our operations.
Risks Related to Doing Business in China
If our land use rights are revoked, we would have no operational capabilities.
Under the PRC law, land is owned by the state or rural collective economic organizations. The state issues to tenants the rights to use property. Use rights can be revoked, and the tenants may be forced to vacate at any time when redevelopment of the land is in the public interest. The public interest rationale is interpreted quite broadly and the process of land appropriation may be less than transparent. If this happens, we may be forced to (i) delay the construction of facilities or (ii) curtail or cease planting on that land. We have relied on these land use rights as the cornerstone of our operations, and the loss or curtailment of such rights would have a material adverse effect on our business and results of operation.
Adverse changes in economic and political policies of the PRC government could have a material adverse effect on the overall economic growth of China, which could adversely affect our business.
All of Shandong Spring Pharmaceutical's business operations are conducted in China. Accordingly, our results of operations, financial condition and prospects are subject to a significant degree to economic, political and legal developments in China. China's economy differs from the economies of most developed countries in many respects, including with respect to:
13
|
•
|
the amount of government involvement,
|
|
•
|
level of development,
|
|
•
|
growth rate,
|
|
•
|
control of foreign exchange, and
|
|
•
|
allocation of resources.
|
While the PRC economy has experienced significant growth in the past 30 years, growth has been uneven across different regions and among various economic sectors of China. The PRC government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. Since early 2004, the PRC government has implemented certain measures to control the pace of economic growth. Such measures may cause a decrease in the level of economic activity in China, which in turn could adversely affect our results of operations and financial condition.
We rely principally on dividends paid by our PRC subsidiary to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC subsidiary to pay dividends to us could have a material adverse effect on our ability to conduct our business.
We are a holding company, and we rely principally on dividends paid by our PRC subsidiary for our cash and financing requirements, and to service any debt we may incur. If our PRC subsidiary incurs debt on its own behalf, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us.
Under PRC laws and regulations, our PRC subsidiary, as a foreign-invested enterprise in the PRC, may pay dividends only out of accumulated profits as determined in accordance with PRC accounting standards and regulations. In addition, a foreign-invested enterprise is required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund certain statutory reserve funds, until the aggregate amount of such fund reaches 50% of its registered capital. At its discretion, it may allocate a portion of its after-tax profits based on PRC accounting standards to staff welfare and bonus funds. These reserve funds and staff welfare and bonus funds are not distributable as cash dividends.
Any limitation on the ability of our PRC subsidiary to pay dividends or make other distributions to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business.
Labor laws in the PRC may adversely affect our results of operations.
On June 29, 2007, the PRC's government promulgated the Labor Contract Law of the PRC, which became effective on January 1, 2008. The Labor Contract Law imposes greater liabilities on employers and significantly affects the cost of an employer's decision to reduce its workforce. Further, the law requires certain terminations be based upon seniority and not merit. In the event that we decide to significantly change or decrease our workforce, the Labor Contract Law could adversely affect our ability to enact such changes in a manner that is most advantageous to our business or in a timely and cost-effective manner, thus materially and adversely affecting our financial condition and results of operations.
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
We are dependent on our relationship with the local government in the provinces in which we operate our business. The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those relating to taxation, environmental regulations, land use rights, property and other matters. We believe that Shandong Spring Pharmaceutical's operations in China are in material compliance with all applicable legal and regulatory requirements. However, the central or local governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
14
Future inflation in China may inhibit our ability to conduct business in China. In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. Rapid economic growth can lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the increase in the costs of supplies, it may have an adverse effect on profitability. These factors have led to the adoption by Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause the Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
Our sales and operating revenues could decline due to macro-economic and other factors outside of our control, such as changes in client confidence and declines in employment levels.
The medicine and health care products markets in China are susceptible to fluctuations in economic conditions. Our business substantially depends on the prevailing economic conditions in China. Changes in national and regional economic conditions, as well as local economic conditions where we conduct our operations and where prospective purchasers of our properties live, may result in more caution on the part of market participants and consequently fewer purchases. These economic uncertainties involve, among other things, conditions of supply and demand in local markets and changes in client confidence and income, employment levels, and government regulations. These risks and uncertainties could periodically have an adverse effect on consumer demand for and the pricing of our products, which could cause our operating revenues to decline. A reduction in our revenues could in turn negatively affect the market price of our securities.
Failure to comply with the individual foreign exchange rules relating to overseas direct investment or the engagement in the issuance or trading of securities overseas by our PRC resident stockholders may subject such stockholders to fines or other liabilities.
Other than Notice 37, our ability to conduct foreign exchange activities in the PRC may be subject to the interpretation and enforcement of the Implementation Rules of the Administrative Measures for Individual Foreign Exchange promulgated by SAFE in January 2007 (as amended and supplemented, the "Individual Foreign Exchange Rules"). Under the Individual Foreign Exchange Rules, any PRC individual seeking to make a direct investment overseas or engage in the issuance or trading of negotiable securities or derivatives overseas must make the appropriate registrations in accordance with SAFE provisions. PRC individuals who fail to make such registrations may be subject to warnings, fines or other liabilities.
We may not be fully informed of the identities of all our beneficial owners who are PRC residents. For example, because the investment in or trading of our shares will happen in an overseas public or secondary market where shares are often held with brokers in brokerage accounts, it is unlikely that we will know the identity of all of our beneficial owners who are PRC residents. Furthermore, we have no control over any of our future beneficial owners and we cannot assure you that such PRC residents will be able to complete the necessary approval and registration procedures required by the Individual Foreign Exchange Rules.
It is uncertain how the Individual Foreign Exchange Rules will be interpreted or enforced and whether such interpretation or enforcement will affect our ability to conduct foreign exchange transactions. Because of this uncertainty, we cannot be sure whether the failure by any of our PRC resident stockholders to make the required registration will subject our PRC subsidiary to fines or legal sanctions on its operations, delay or restriction on repatriation of proceeds of this offering into the PRC, restriction on remittance of dividends or other punitive actions that would have a material adverse effect on our business, results of operations and financial condition.
15
Because our funds are held in banks in the PRC that do not provide insurance, the failure of any bank in which we deposit our funds may affect our ability to continue to operate.
Banks and other financial institutions in the PRC do not provide insurance for funds held on deposit. As a result, in the event of a bank failure in the PRC, we may not have access to funds on deposit. Depending upon the amount of money we maintain in a bank in the PRC that fails, our inability to have access to our cash may impair our operations, and, if we are not able to access funds to pay our suppliers, employees and other creditors, we may be unable to continue to operate.
Under the enterprise income tax law, we may be classified as a "resident enterprise" of China. Such classification may result in unfavorable tax consequences to US and our non-PRC stockholders.
China passed a New Enterprise Income Tax Law, or the New EIT Law, which became effective on January 1, 2008. Under the New EIT Law, an enterprise established outside of China with de facto management bodies within China is considered a resident enterprise, meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. The implementing rules of the New EIT Law define de facto management as "substantial and overall management and control over the production and operations, personnel, accounting, and properties" of the enterprise. In addition, a circular issued by the State Administration of Taxation on April 22, 2009 clarified that dividends and other income paid by such resident enterprises will be considered to be the PRC's source income and subject to the PRC's withholding tax. This recent circular also subjects such resident enterprises to various reporting requirements with the PRC's tax authorities.
Although substantially all of our management is currently located in the PRC, it remains unclear whether the PRC's tax authorities would require or permit our overseas registered entities to be treated as PRC resident enterprises. We do not currently consider our company to be a PRC resident enterprise. However, if the PRC's tax authorities determine that we are a resident enterprise for the PRC's enterprise income tax purposes, a number of unfavorable PRC tax consequences may follow. First, we may be subject to the enterprise income tax at a rate of 25% on our worldwide taxable income as well as the PRC's enterprise income tax reporting obligations. This would also mean that income such as interest on offering proceeds and non-China source income would be subject to the PRC's enterprise income tax at a rate of 25%. Second, although under the New EIT Law and its implementing rules, dividends paid to us from our PRC subsidiary would qualify as tax-exempt income, we cannot guarantee that such dividends will not be subject to a 10% withholding tax, as the PRC authorities responsible for enforcing the withholding tax have not yet issued guidance with respect to the processing of outbound remittances to entities that are treated as resident enterprises for the PRC's enterprise income tax purposes. Finally, dividends paid to stockholders with respect to their shares of our common stock or any gains realized from transfer of such shares may generally be subject to the PRC's withholding taxes on such dividends or gains at a rate of 10% if the stockholders are deemed to be non-resident enterprises or at a rate of 20% if the stockholders are deemed to be non-resident individuals.
Our PRC subsidiary has taken the position that it is compliant with the taxation, environmental, employment and social security rules of China, and if that position turns out to be wrong, it may face penalties imposed by the PRC government.
While we believe our PRC subsidiary has been in compliance with PRC taxation, environmental, employment and social security rules during its operations in China, we have not obtained letters from the PRC government authorities confirming such compliance. If any PRC government authority takes the position that there is non-compliance with the taxation, environmental protection, employment and/or social security rules by our PRC subsidiary, it may be exposed to penalties from PRC government authorities, in which case the operation of our PRC subsidiary may be adversely affected.
We may be adversely affected by complexity, uncertainties and changes in PRC regulation of pharmaceutical business and companies, including limitations on our abilities to own key assets.
The PRC government regulates the pharmaceutical industry including foreign ownership of, and the licensing and permit requirements pertaining to, companies in the pharmaceutical and nutraceutical industry. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations.
16
The interpretation and application of existing PRC laws, regulations and policies and possible new laws, regulations or policies have created substantial uncertainties regarding the legality of existing and future foreign investments in, and the businesses and activities of, pharmaceutical businesses in China, including our business.
Any deterioration of political relations between the United States and the PRC could impair our financing activities and your investment in us.
The relationship between the United States and the PRC is subject to fluctuation and periodic tension. Changes in political conditions in the PRC and changes in the state of Sino-U.S. relations are difficult to predict and could adversely affect our financing activities. Such a change could lead to a decline in our profitability. Any weakening of relations between the United States and the PRC could have an adverse effect on our efforts to raise capital to expand our present business activities and your investment in us.
Price control may affect both our revenues and net income.
The laws of the PRC provide for the government to fix and adjust prices. To the extent that we are subject to price control, our revenue, gross profit, gross margin and net income will be affected since the revenue we derive from our sales will be limited and, unless there is also price control on the products that we purchase from our suppliers, we may face no limitation on our costs. Further, if price controls affect both our revenue and our costs, our ability to be profitable and the extent of our profitability will be effectively subject to determination by the applicable regulatory authorities in the PRC.
We may have limited legal recourse under Chinese law if disputes arise under contracts with third parties.
Almost all of our agreements with our employees and third parties, including our suppliers and customers, are governed by the laws of the PRC. The legal system in the PRC is a civil law system based on written statutes. Unlike common law systems, such as we have in the United States, it is a system in which decided legal cases have little precedential value. The government of the PRC has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the PRC, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance or seek an injunction under Chinese law are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. The occurrence of any such events could have a material adverse effect on our business, financial condition and results of operations.
Uncertainties with respect to the PRC legal system could adversely affect us.
Our operations in China are governed by PRC laws and regulations. We are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to wholly foreign-owned enterprises. The PRC legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value.
Since 1979, PRC legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their nonbinding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until sometime after the violation. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
17
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing original actions in China based on United States or other foreign laws against us or our management.
All of our assets are located outside the United States and all of our current operations are conducted in China. Moreover, all but one of our directors and officers are nationals or residents of China. All or a substantial portion of the assets of these persons are located outside the United States. As a result, it may be difficult for our stockholders to effect service of process within the United States upon these persons. In addition, there is uncertainty as to whether the courts of China would recognize or enforce judgments of U.S. courts obtained against us or such officers and/or directors or assets predicated upon the civil liability provisions of the securities laws of the United States or any state thereof, or be competent to hear original actions brought in China against us or such persons predicated upon the securities laws of the United States or any state thereof.
Fluctuation in the value of RMB may have a material adverse effect our financial results as reported in US dollars.
The value of RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. All of our financial assets, revenues and costs are denominated in RMB. Any significant fluctuation in value of RMB may materially and adversely affect our cash flows, revenues, earnings and financial position, and the value of the payments to us by customers. For example, an appreciation of RMB against the U.S. dollar would make any new RMB denominated investments or expenditures costlier, to the extent that we might need to convert U.S. dollars into RMB for such purposes. An appreciation of RMB against the U.S. dollar would also result in foreign currency translation losses for financial reporting purposes when we translate our RMB denominated financial assets into USD, as USD is our reporting currency.
Restrictions on currency exchange may limit our ability to utilize our revenues effectively.
Our revenues and operating expenses are denominated in RMB. The PRC government imposes controls on the convertibility of the RMB into foreign currencies and, in certain cases, the remittance of currency out of China. Pursuant to the Foreign Currency Administration Rules, promulgated on January 29, 1996 and amended on January 14, 1997, and various regulations issued by SAFE and other relevant PRC government authorities, RMB is freely convertible only to the extent of current account items, such as trade-related receipts and payments, interest and dividends. Capital account items, such as direct equity investments, loans and repatriation of investments, require the prior approval from SAFE or its local branch for conversion of RMB into a foreign currency such as U.S. dollars, and remittance of the foreign currency outside the PRC. Shortages in the availability of foreign currency may restrict the ability of our PRC subsidiary to remit sufficient foreign currency to pay dividends or other payments to us, Our PRC subsidiary may purchase foreign exchange for settlement of "current account transactions," including payment of dividends to us should we decide to pay dividends to our stockholders, without the approval of SAFE. However, approval from the SAFE or its local branch is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses, such as the repayment of loans denominated in foreign currencies.
Risks Related to Our Offering and Ownership of Our Common stock
The market price of our common stock may be volatile or may decline regardless of our operating performance, and you may not be able to resell your shares at or above the offering price.
The offering price for our common stock will be determined through negotiations between the underwriter and our Company and may vary from the market price of our common stock following our offering. If you purchase our common stock in our offering, you may not be able to resell those shares at or above the offering price. We cannot assure you that the offering price of our common stock, or the market price following our offering, will equal or exceed prices at which our common stock was traded before the offering. The market price of our common stock may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
18
|
·
|
actual or anticipated fluctuations in our revenue and other operating results;
|
|
·
|
the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections;
|
|
·
|
actions of securities analysts who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors;
|
|
·
|
announcements by us or our competitors of significant products or features, technical innovations, acquisitions, strategic partnerships, joint ventures, or capital commitments;
|
|
·
|
price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole;
|
|
·
|
lawsuits threatened or filed against us; and
|
|
·
|
other events or factors, including those resulting from war or incidents of terrorism, or responses to these events.
|
In addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. Stock prices of many companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies. In the past, stockholders have filed securities class action litigation following periods of market volatility. If we were to become involved in securities litigation, it could subject us to substantial costs, divert resources and the attention of management from our business, and adversely affect our business.
Although we intend to apply to list our common stock on the Nasdaq Capital Market, an active trading market may not develop for our securities, and you may not be able to sell your common stock at or above the offering price per share. In addition, we might not be able to maintain the listing of our common stock on the NASDAQ Capital Market.
Our common stock is quoted on the OTCQB under the symbol "CYIG". There is a limited trading market for our common stock and at times there is no trading in our common stock. We intend to apply to list our common stock on The Nasdaq Capital Market in connection with this offering. We will not consummate this offering if our application is not approved. However, we cannot predict the extent to which investor interest in our company will lead to the development of an active trading market in our common stock or how liquid that market might become. If such a market does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at the time you wish to sell them, at a price that is attractive to you, or at all. Also, there can be no assurance that we will be able to maintain the listing standards of that exchange, which includes requirements that we maintain our stockholders' equity, total value of shares held by unaffiliated stockholders, and market capitalization above certain specified levels. If we fail to conform to the Nasdaq listing requirements on an ongoing basis, our common stock might cease to trade on The Nasdaq Capital Market, and may move to the OTCQB or the "pink sheets" maintained by Pink OTC Markets, Inc. The OTC Bulletin Board and the "pink sheets" are generally considered to be markets that are less efficient, and to provide less liquidity in the shares, than The Nasdaq Capital Market. If we cease to be listed on The Nasdaq Capital Market, holders of our common stock may find it more difficult to dispose of, or to obtain accurate quotations as to the market value of, our common stock, and the market value of our common stock would likely decline.
We have broad discretion in the use of the net proceeds from our offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled "Use of Proceeds," and you will not have the opportunity as part of your investment decision to assess whether the net proceeds will be used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment. We intend to use the net proceeds from this offering to fund: (i) the expansion of manufacturing facilities for acer truncaturm bunge seed oil, (ii) the purchase of equipment, and (iii) general working capital to meet the needs of our continued development.
19
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering, or the amounts that we will actually spend on the uses set forth above.
The failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
We do not anticipate paying any cash dividends in the near future.
We presently do not anticipate that we will pay any dividends on any of our capital stock in the foreseeable future. The payment of dividends, if any, would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any dividends is within the discretion of our Board of Directors. We presently intend to retain all earnings, if any, to implement our business plan; accordingly, we do not anticipate the declaration of any dividends in the foreseeable future. Therefore, if you purchase shares in this offering, realization of a gain on your investment will likely depend on the appreciation of the price of our common stock, which may never occur.
Our principal stockholders and management own a significant percentage of our common stock and will be able to exert significant control over matters subject to stockholder approval.
Our executive officers, directors, 5% stockholders and their affiliates beneficially own approximately 38.19% of our common stock and, upon the closing of this offering, that same group will hold approximately [●]% of our outstanding common stock (assuming no exercise of the underwriters' over-allotment option), based upon the number of shares of our common stock outstanding as of July 13, 2018. Therefore, even after this offering, these stockholders will have the ability to influence us through this ownership position. These stockholders may be able to determine or significantly influence all matters requiring stockholder approval. Including the election of directors, the amendment of our organizational documents, or the approval of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.
Future sales of our common stock, or the perception that future sales may occur, may cause the market price of our common stock to decline, even if our business is doing well.
The market price of our shares could decline as a result of sales of substantial amounts of our shares in the public market, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of our common stock. An aggregate of 29,839,168 shares will be outstanding before the consummation of this offering and [●] shares will be outstanding immediately after this offering. All of the shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act. Of the current outstanding common stocks, 9,534,696 shares of common stocks are tradable without restriction. The remaining shares will be "restricted securities" as defined in Rule 144. These shares may be sold without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act. Additional restricted shares may be issued under our 2018 Stock Incentive Plan (the "Plan"), which has a total of 3,000,000 shares reserved for issuance. Awards under the Plan may also result in additional shares of common stock available for trading.
In connection with this offering, we, our officers and directors and the holders of 5% or more of our currently outstanding shares of common stock have agreed, subject to certain exceptions, not to sell or transfer any shares of common stock for six months after the date of this prospectus without the consent of the underwriter. However, the underwriter may release these shares from any restrictions at any time. We cannot predict what effect, if any, market sales of shares held by any stockholder or the availability of shares for future sale will have on the market price of our common stock.
20
You will experience immediate and substantial dilution.
The offering price of our shares is substantially higher than the pro forma net tangible book value per share of our common stock. Upon the completion of this offering, if you purchase shares in this offering, you will incur immediate dilution of approximately $[●], or approximately [●]%, in the pro forma net tangible book value per share from the price per share that you pay for the shares. Accordingly, if you purchase shares in this offering, you will incur immediate and substantial dilution of your investment. See "Dilution" on page 27.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price may decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
We may issue more shares of common stock in the future, which could result in additional dilution to existing stockholders.
Our certificate of incorporation authorizes the issuance of up to a total of 100,000,000 shares of common stock and up to a total of 5,000,000 shares of preferred stock. Because we may need to raise additional capital, we may in the future sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of equity or equity-linked securities, and any additional shares issued in connection with acquisitions, if any, may result in further dilution to investors. Such issuances may not require the approval of our stockholders unless required pursuant to the rules of The Nasdaq Capital Market.
The rights of the holders of our common stock may be impaired by the potential issuance of preferred stock.
Our board of directors has the right to create one or more new series of preferred stock. As a result, the Board of Directors may, without stockholder approval, issue preferred stock with voting, dividend, conversion, liquidation or other rights that may adversely affect the voting power and equity interest of the holders of our common stock. Although we have no present intention to issue any additional shares of preferred stock or to create any new series of preferred stock, we may issue such shares in the future.
Our management has determined that our disclosure controls and procedures are not effective and we have identified material weaknesses in our internal control over financial reporting. If we fail to maintain the adequacy of our internal controls, our ability to provide accurate financial statements and comply with the requirements of the Sarbanes-Oxley Act of 2002 could be impaired, which could cause our stock price to decrease substantially.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and the Sarbanes-Oxley Act and, after the closing of this offering, we will be subject to the rules and regulations of Nasdaq. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal controls over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with accounting principles generally accepted in the U.S., or GAAP.
21
In connection with the preparation of our financial statements for the years ended March 31, 2018 and 2017, our management concluded that our internal control over financial reporting was not effective and we identified several material weaknesses. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. In addition, as of March 31, 2018 and 2017, our management concluded that our disclosure controls and procedures were not effective due to the material weaknesses in our internal control over financial reporting.
We have committed limited personnel and resources to the development of the external reporting and compliance obligations that are required for a public company. Recently, we have taken measures to address and improve our financial reporting and compliance capabilities, and we are in the process of instituting changes to improve our obligations as a public company. We plan to obtain additional financial and accounting resources to support and enhance our ability to meet these requirements. We will need to continue to improve our financial and managerial controls, reporting systems and procedures, and documentation thereof. If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements. If that were to happen, the market price of our stock could decline substantially and we could be subject to sanctions or investigations by Nasdaq, the Securities and Exchange Commission, or the SEC, or other regulatory authorities.
22
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements about us and our industry that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations or financial condition, business strategy and plans, and objectives of management for future operations, are forward-looking statements. In some cases, you can identify forward-looking statements because they contain words such as "anticipate," "believe," "contemplate," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "will" or "would" or the negative of these words or other similar terms or expressions.
We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to known and unknown risks, uncertainties and assumptions, including risks described in "Risk Factors" and elsewhere in this prospectus, regarding, among other things:
|
·
|
the size and growth potential of the markets for our products, and our ability to serve those markets;
|
|
·
|
the rate and degree of market acceptance of our products;
|
|
·
|
ability to expand our sales organization to address effectively existing and new markets that we intend to target;
|
|
·
|
impact from future regulatory, judicial, and legislative changes or developments in the PRC and other countries;
|
|
·
|
ability to compete effectively in a competitive industry;
|
|
·
|
ability to continue to use our current direct sales channel;
|
|
·
|
the success of competing products that are or may become available;
|
|
·
|
the performance of our third-party distributors and suppliers (including Shandong Yongchuntang);
|
|
·
|
our ability to attract and retain key personnel;
|
|
·
|
the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
|
|
·
|
our ability to obtain funding for our operations;
|
|
·
|
our costs of goods sold, the supply of acer truncatum bunge seed oil and the rate at which the acer truncatum bunge trees mature; and
|
|
·
|
our use of the proceeds from this offering.
|
You should not rely on forward-looking statements as predictions of future events. We have based the forward-looking statements contained in this prospectus primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations and prospects. The outcome of the events described in these forward-looking statements is subject to risks, uncertainties and other factors described in "Risk Factors" and elsewhere in this prospectus. Moreover, we operate in a very competitive and rapidly changing environment. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this prospectus. The results, events and circumstances reflected in the forward-looking statements may not be achieved or occur, and actual results, events or circumstances could differ materially from those described in the forward-looking statements.
23
In addition, statements that "we believe" and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based on information available to us as of the date of this prospectus. And while we believe that information provides a reasonable basis for these statements, that information may be limited or incomplete. Our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all relevant information. These statements are inherently uncertain, and investors are cautioned not to unduly rely on these statements.
The forward-looking statements made in this prospectus relate only to events as of the date on which the statements are made. We undertake no obligation to update any forward-looking statements made in this prospectus to reflect events or circumstances after the date of this prospectus or to reflect new information or the occurrence of unanticipated events, except as required by law. We may not actually achieve the plans, intentions, or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments.
USE OF PROCEEDS
We estimate that our net proceeds from the sale of the common stock that we are offering will be approximately $[●] (or approximately $[●] if the underwriter exercises in full its option to purchase additional common stock) assuming a public offering price of $[●] per share, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase (or decrease) in the assumed offering price of $[●] per share, would increase (or decrease) the net proceeds to us from this offering by $[●], assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting estimated underwriting discounts and commissions.
We intend to use the net proceeds to us from this offering to fund: (i) the expansion of manufacturing facilities for acer truncatum bunge seed oil, (ii) the purchase of equipment, and, and (iii) general working capital to meet the needs of our continued development.
Our management will have broad discretion in the application of the net proceeds we receive from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds. We may not use these proceeds in a manner desired by our stockholders. Although we have no present intention of doing so, future events may require us to reallocate the offering proceeds.
We intend to invest the net proceeds to us from the offering that are not used as described above in short-term, investment-grade, interest-bearing instruments.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock is quoted on the OTCQB under the symbol "CYIG". The OTCQB is a quotation service that displays real-time quotes, last-sale prices, and volume information in over-the-counter ("OTC") equity securities. An OTC Markets equity security generally is any equity that is not listed or traded on a national securities exchange.
24
Price Range of Common Stock
The following table shows, for the periods indicated, the high and low bid prices per share of our common stock as reported by the OTC Markets' quotation service. These bid prices represent prices quoted by broker-dealers on the OTC Markets quotation service. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commissions, and may not represent actual transactions.
|
|
Year Ended
|
Year Ended
|
Year Ending
March 31, 2019
|
|||||||||||||||||||||
|
|
March 31, 2017
|
March 31, 2018
|
(through July 13, 2018)
|
|||||||||||||||||||||
|
|
||||||||||||||||||||||||
|
|
High
|
Low
|
High
|
Low
|
High
|
Low
|
||||||||||||||||||
|
First Quarter
|
$
|
0.55
|
$
|
0.20
|
$
|
0.65
|
$
|
0.04
|
$
|
0.90
|
$
|
0.51
|
||||||||||||
|
Second Quarter
|
$
|
0.20
|
$
|
0.15
|
$
|
0.67
|
$
|
0.37
|
||||||||||||||||
|
Third Quarter
|
$
|
0.24
|
$
|
0.12
|
$
|
0.44
|
$
|
0.25
|
||||||||||||||||
|
Fourth Quarter
|
$
|
0.70
|
$
|
0.22
|
$
|
0.90
|
$
|
0.50
|
||||||||||||||||
On July 13, 2018, the last reported sales prices of our common stock on the OTCQB was $0.82 per share.
Holders
As of July 13, 2018, there were 769 holders of record of our common stock. Because shares of our common stock are held by depositaries, brokers and other nominees, the number of beneficial holders of our shares is substantially larger than the number of stockholders of record.
Transfer Agent and Registrar
The transfer agent and registrar for the Company's common stock is Interwest Transfer Co., Inc. 1981 E Murray Holladay Desalt Lake City, UT 84117.The telephone number is: (801) 272-9294.
Securities Authorized for Issuance under Equity Compensation Plans
In order to compensate our officers, directors, employees and/or consultants, on April 21, 2018, our Board of Directors approved the 2018 Stock Incentive Plan (the "Plan"). The Plan has a total of 3,000,000 shares reserved for issuance.
Under the Plan, an eligible person in our service may acquire a proprietary interest in our Company in the form of shares or an option to purchase shares of our common stock.
There have been no issuances under the Plan.
We have never declared or paid a cash dividend. Any future decisions regarding dividends will be made by our Board of Directors. We currently intend to retain and use any future earnings for the development and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Our Board of Directors has complete discretion on whether to pay dividends. Even if our Board of Directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the Board of Directors may deem relevant.
25
The following table sets forth our cash and cash equivalents as well as capitalization as of March 31, 2018:
|
•
|
on an actual basis;
|
|
•
|
on a pro forma basis to give further effect to the issuance and sale by us of [●] shares of common stock in this offering at the assumed public offering price of $[●] per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
|
You should read this table together with "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations," as well as our consolidated financial statements and related notes appearing elsewhere in this prospectus.
|
|
March 31,
2018
|
Pro forma
(1)
|
||||||
|
Cash and cash equivalents
|
$
|
25,353,360
|
$
|
|||||
|
Stockholders' Equity
|
||||||||
|
Preferred stock, par value $500 per share; 45 shares authorized, issued and outstanding at March 31, 2018 and 2017.
|
22,500
|
|||||||
|
Common stock, par value $0.001 per share; 100,000,000 shares authorized; 29,789,168 shares issued and outstanding at March 31, 2018.
|
29,789
|
|||||||
|
Additional paid-in capital
|
4,322,838
|
|||||||
|
Statutory reserve
|
1,828,504
|
|||||||
|
Retained earnings
|
94,447,937
|
|||||||
|
Accumulated other comprehensive income (loss)
|
4,455,017
|
|||||||
|
Total stockholders' equity attributable to the Company
|
105,106,585
|
|||||||
|
Noncontrolling interest
|
3,048,705
|
|||||||
|
Total stockholders' equity
|
108,155,290
|
|||||||
|
Total capitalization
|
108,155,290
|
|||||||
|
(1)
|
The number of shares of our common stock outstanding is based on 29,839,168 shares outstanding as of July 13, 2018, which excludes:
|
|
·
|
Shares of our common stock reserved for future issuance under our 2018 Stock Incentive Plan.
|
|
·
|
500,000 restricted shares of common stock issuable to a consultant should our common stock be listed on The Nasdaq Stock Market.
|
|
·
|
400,000 restricted shares of common stock issuable upon the exercise of a warrant at an exercise prices of $2.00 per share, which warrant is exercisable six months after our common stock is listed on The Nasdaq Stock Market. For a discussion on the terms of the outstanding warrant, see "Description of Capital Stock—Warrants".
|
26
DILUTION
The as adjusted net tangible book value of our common stock as of [●], was $[●], or approximately $[●] per share based upon [●] shares of common stock outstanding on such date. As adjusted net tangible book value per share represents the amount of our total tangible assets reduced by the amount of our total liabilities, divided by the total number of shares of common stock outstanding after giving effect to the sale of [●] shares of the common stock we are offering based upon an assumed public offering price of $[●] per share, the closing price of our common stock on the OTCQB on [●], and after deducting underwriting discounts and commissions and estimated offering expenses of approximately $[●] million.
If you participate in this offering, your interest will be diluted to the extent of the difference between the offering price per share and the as adjusted net tangible book value per share of our common stock immediately after completion after this offering. This represents an immediate increase in as adjusted net tangible book value of $[●] per share to our existing stockholders and an immediate dilution of $[●] per share to investors participating in this offering.
The following table illustrates this dilution on a per share basis to new investors:
|
|
Post-offering (1)
|
|||
|
Assumed public offering price per share
|
$
|
|||
|
As adjusted net tangible book value per share as of [●] before giving effect to this offering
|
$
|
|||
|
Increase in as adjusted net tangible book value per share attributed to new investors purchasing shares of common stock from us in this offering
|
$
|
|||
|
As adjusted net tangible book value per share after giving effect to this offering
|
$
|
|||
|
Dilution in as adjusted net tangible book value per share to new investors in this offering
|
$
|
|||
|
(1)
|
Assumes gross proceeds from offering of [●] common stock.
|
If the underwriter exercises their over-allotment option in full, the adjusted net tangible book value will increase to $[●] per share, representing an immediate dilution of $[●] per share to new investors, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us. Investors exercising their warrants may experience additional dilution.
The number of shares of our common stock outstanding is based on 29,839,168 shares outstanding as of July 13, 2018, which excludes:
|
·
|
Shares of our common stock reserved for future issuance under our 2018 Stock Incentive Plan.
|
|
·
|
400,000 restricted shares of common stock issuable upon the exercise of a warrant at an exercise prices of $2.00 per share, which warrant is exercisable six months after our common stock is listed on The Nasdaq Stock Market.
|
|
·
|
500,000 restricted shares of common stock issuable to a consultant should our common stock be listed on The Nasdaq Stock Market.
|
the underwriter warrants to purchase that number of shares of our common stock equal to 7% of the aggregate number of the shares of common stock underlying the stocks sold in the offering (or [●] shares, assuming the over-allotment option is fully exercised).
SELECTED CONSOLIDATED FINANCIAL DATA
We derived the following selected consolidated statements of operations data for the years ended March 31, 2016, 2017, and 2018 and the selected consolidated balance sheet data as of March 31, 2017 and 2018 from audited consolidated financial statements appearing elsewhere in this prospectus. The selected financial data set forth below should be read together with the financial statements and the related notes to those statements, as well as the sections of this prospectus titled "Management's Discussion and Analysis of Financial Condition and Results of Operations."
27
|
For the Year Ended
|
||||||||||||
|
March 31,
|
||||||||||||
|
2016
|
2017
|
2018
|
||||||||||
|
(in thousands, except per share data)
|
||||||||||||
|
Consolidated Statements of Operations Data:
|
||||||||||||
|
Net sales
|
$
|
47,827
|
$
|
56,463
|
$
|
64,943
|
||||||
|
Cost of sales
|
26,554
|
33,284
|
39,604
|
|||||||||
|
Gross profit
|
21,273
|
23,179
|
25,339
|
|||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
724
|
810
|
492
|
|||||||||
|
Sales and marketing
|
3,760
|
3,934
|
4,985
|
|||||||||
|
General and administrative
|
4,447
|
4,248
|
4,719
|
|||||||||
|
Impairment of assets
|
1,115
|
986
|
332
|
|||||||||
|
Total operating expenses
|
10,046
|
9,978
|
10,528
|
|||||||||
|
Operating income
|
11,227
|
13,201
|
14,811
|
|||||||||
|
Total other income
|
31
|
55
|
767
|
|||||||||
|
Income before income taxes
|
11,258
|
13,256
|
15,578
|
|||||||||
|
Provision for income taxes
|
2,841
|
3,201
|
3,839
|
|||||||||
|
Net income
|
$
|
8,417
|
$
|
10,055
|
$
|
11,739
|
||||||
|
Net income attributable to common shareholders—basic and diluted
|
$
|
8,417
|
$
|
10,055
|
$
|
11,739
|
||||||
|
Net income per share attributable to common shareholders—basic and diluted
|
$
|
0.28
|
$
|
0.34
|
$
|
0.38
|
||||||
|
Weighted average shares of common stock outstanding—basic and diluted
|
|
29,710
|
|
29,764
|
|
29,789
|
||||||
|
Foreign currency translation adjustment
|
$ |
(3,915
|
)
|
$ |
(5,229
|
)
|
$ |
9,115
|
||||
|
Comprehensive income
|
$
|
4,502
|
$
|
4,826
|
$
|
20,854
|
||||||
|
As of March 31,
|
||||||||
|
2017
|
2018
|
|||||||
|
(in thousands)
|
||||||||
|
Consolidated Balance Sheet Data:
|
||||||||
|
Cash and cash equivalents
|
$
|
10,309
|
$
|
25,353
|
||||
|
Working capital
|
15,492
|
28,083
|
||||||
|
Total assets
|
90,287
|
110,138
|
||||||
|
Total liabilities
|
2986
|
1,983
|
||||||
|
Total shareholders' equity
|
$
|
87,301
|
$
|
108,155
|
||||
28
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS
AND RESULTS OF OPERATIONS
Overview
We were incorporated in the State of Florida in January 1989, and reincorporated in the State of Delaware on April 4, 2007. We operate principally through its wholly-owned subsidiary, Landway Nano Bio-Tech, Inc. ("Landway Nano"), incorporated in Delaware, which, in turn, owns 97% of Shandong Spring Pharmaceutical Co., Ltd. ("Shandong Spring Pharmaceutical"), incorporated in the People's Republic of China (the "PRC"). We, through Shandong Spring Pharmaceutical, are engaged in the business of (i) distributing health care supplement products manufactured by Shandong Yongchuntang, (ii) developing, manufacturing, and selling Huoliyuan capsules, a prescription medicine, and (iii) developing the acer truncatum bunge planting bases and selling acer truncatum seed oil in the PRC. Acer truncatum bunge plants are a species of maple tree.
The Huoliyuan capsule, our proprietary product, is a China Food and Drug Administration ("CFDA") approved prescription TCM that has a wide range of therapeutic benefits. It is the only TCM of its kind made in slow-release capsule form for improved absorption rate and therapeutic effects.
Since July 2015, the Company has also produced acer truncatum bunge seed oil and sold the product to customers through an Internet direct sales system operated by the Company pursuant to an agreement with Shandong Yongchuntang. The acer truncatum bunge seed oil is extracted from acer truncatum pods that are purchased from third party vendors. The Company's self-grown acer truncatum pods will not be ready to be used for production until approximately the fall of 2018. The Company currently has a 5,880 mu (approximately 968.65 acres) acer truncatum bunge plantation base coupled with modern production facilities. We believe it is the only company in China able to achieve industrial-scale production and vertically integrated capability for acer truncatum bunge seed oil products.
On March 18, 2017, we entered into an Acquisition Agreement on Acer Truncatum Industrial Project (the "Agreement") with Shandong Yongchuntang. Pursuant to the Agreement, we agreed to transfer a 3% equity interest in Shandong Spring Pharmaceutical in exchange for tangible and intangible assets related to the Acer Truncatum Industrial Project (the "Project"), which were owned by Shandong Yongchuntang. As a result of this equity transaction, Shandong Yongchuntang has become a 3% shareholder of Shandong Spring Pharmaceutical.
Results of Operations
|
Years Ended
|
||||||||||||||||
|
March 31,
|
$ |
%
|
||||||||||||||
|
2018
|
2017
|
Change
|
Change
|
|||||||||||||
|
Sales
|
$
|
64,942,737
|
$
|
56,463,164
|
$
|
8,479,573
|
15.0
|
%
|
||||||||
|
Cost of Goods Sold
|
(39,603,995
|
)
|
(33,284,237
|
)
|
(6,319,758
|
)
|
19.0
|
%
|
||||||||
|
Gross Profit
|
25,338,742
|
23,178,927
|
2,159,815
|
9.3
|
%
|
|||||||||||
|
Operating Expenses
|
(10,528,364
|
)
|
(9,978,320
|
)
|
(550,044
|
)
|
5.5
|
%
|
||||||||
|
Operating Income
|
14,810,378
|
13,200,607
|
1,609,771
|
12.2
|
%
|
|||||||||||
|
Gain on Disposal of Acer Truncatun Bunge Plants
|
642,532
|
-
|
642,532
|
100
|
%
|
|||||||||||
|
Interest Income
|
124,410
|
54,672
|
69,738
|
127.6
|
%
|
|||||||||||
|
Income Tax Provision
|
(3,838,832
|
)
|
(3,200,625
|
)
|
(638,207
|
)
|
19.9
|
%
|
||||||||
|
Net Income
|
11,738,488
|
10,054,654
|
1,683,834
|
16.7
|
%
|
|||||||||||
|
Comprehensive Income
|
$
|
20,853,809
|
$
|
4,826,286
|
$
|
16,027,523
|
332.1
|
%
|
||||||||
The following table sets forth information from our statements of comprehensive income for the years ended March 31, 2018 and 2017, in dollars:
29
Revenue
During the year ended March 31, 2018, we realized $64,942,737 in revenue, representing an increase of 15.0%, or $8,479,573, as compared to $56,463,164 for the same period in 2017. The increase in revenue in RMB was 13.3% as compared to the year ended March 31 2017. The other 1.7% of the increase in dollar denominated revenue was attributable to the RMB appreciation during the year ended March 31, 2018, compared with the same period in 2017. The total 13.3% revenue increase in RMB was due to the increased sales of acer truncatum bunge seed oil, health care products, and Huoliyuan products.
Part of our revenues was generated by us as the distributor for the health care products manufactured by Shandong Yongchuntang, a related party. We purchase the products from Shandong Yongchuntang according to the purchase contract signed between us and Shandong Yongchuntang. Pursuant to the renewed one- year contract dated February 20, 2017, we agreed to purchase nine products from Shandong Yongchuntang at fixed prices. On February 21, 2018, we further renewed the purchase contract with Shandong Yongchuntang for a term of one year ending on February 25, 2019. Pursuant to this most recently renewed one-year contract, we continue to purchase the nine products from Shandong Yongchuntang at fixed prices without changes in any terms of the previous contract. During the year ended March 31, 2018, 40.5% of our total revenue was generated as the distributor of Shandong Yongchuntang, compared to 35.7% during the year ended March 31, 2017. For the year ended March 31, 2018, our revenue from sales of the health care products was $26,297,055, representing an increase of 30.4%, or $6,137,424, as compared to $20,159,631 for the same period in 2017. The revenue from sales of health care products measured in RMB increased by 28.5%. The other 1.9% of the increase in dollar denominated revenue was attributable to the RMB appreciation during the year ended March 31, 2018, compared with the same period in 2017. The significant increase in sales of the health care products was primarily due to the growth of our customer base and the Internet direct-sales.
The sales of the Huoliyuan capsules and tablets accounted for 39.7% of our revenue during the year ended March 31, 2018, compared to 43.8% during the year ended March 31, 2017. The sales of the Huoliyuan products in the year ended March 31, 2018 were $25,800,732, an increase of 4.3% or $1,066,779 as compared to the year ended March 31, 2017. The revenue from sales of Huoliyuan products denominated in RMB increased by 2.7%. The other 1.6% of the increase in dollar denominated revenue was attributable to the RMB appreciation during the year ended March 31, 2018, compared with the same period in 2017.
Since July 2015, we have produced acer truncatum bunge seed oil and sold the product to customers through our distributors. The acer truncatum bunge seed oil was extracted from the acer truncatum pods that were purchased from third-party vendors. Our self-grown acer truncatum pods will not be ready to be used for production until approximately the fall of 2018. During the year ended March 31, 2018, 19.8% of our total revenue was generated from the sales of acer truncatum seed oil products, compared to 20.5% during the year ended March 31, 2017. For the year ended March 31, 2018, our revenue from sales of the acer truncatum seed oil products was $12,844,950, representing an increase of 11.0%, or $1,275,370, as compared to $11,569,580 for the same period in 2017. The revenue from sales of acer truncatum seed oil products denominated in RMB increased by 9.3%. The other 1.7% of the increase in dollar denominated revenue was attributable to the RMB appreciation during the year ended March 31, 2018, compared with the same period in 2017. The increase in sales of acer truncatum seed oil products in RMB was primarily due to the continuing promotion of our acer truncatum bunge seed oil by organizing conferences to introduce the features and benefits of the product to our distributors and customers.
The following is the sales breakdown by products during the years ended March 31, 2018 and 2017:
|
For the years ended March 31,
|
||||||||||||||||
|
2018
|
2017
|
|||||||||||||||
|
Health care supplements
|
$
|
26,297,055
|
40.5
|
%
|
$
|
20,159,631
|
35.7
|
%
|
||||||||
|
Drugs (Huoliyuan products)
|
25,800,732
|
39.7
|
%
|
24,733,953
|
43.8
|
%
|
||||||||||
|
Acer truncatum seed oil
|
12,844,950
|
19.8
|
%
|
11,569,580
|
20.5
|
%
|
||||||||||
|
Total
|
$
|
64,942,737
|
100
|
%
|
$
|
56,463,164
|
100
|
%
|
||||||||
30
Cost of Goods Sold
Our costs of goods sold comprised primarily the cost of finished goods that we purchased from Shandong Yongchuntang, the raw materials we purchased from third-party vendors, and the manufacturing costs of acer truncatum bunge seed oil and the Huoliyuan capsule. The cost of manufacturing the Huoliyuan capsule was approximately 46.3% and 48.0% of the total cost of goods sold during the years ended March 31, 2018 and 2017, respectively. The cost of manufacturing acer truncatum bunge seed oil was approximately 16.8% and 18.4% of the total cost of goods sold during the years ended March 31, 2018 and 2017, respectively.
During the year ended March 31, 2018, our cost of goods sold totaled $39,603,995, representing an increase of $6,319,758, or 19.0%, as compared to $33,284,237 during the year ended March 31, 2017. There was a 17.2% increase in cost in RMB. The other 1.8% of the increase in dollar denominated revenue was attributable to the RMB appreciation during the year ended March 31, 2018, compared with the same period in 2017. The increase in cost was primarily due to the increase in sales of the health care products, the acer truncatum bunge seed oil and the Huoliyuan capsule, and the increase in the production cost of the Huoliyuan capsule.
The percentages of the cost of goods sold to total revenues slightly increased from 58.9% for fiscal year 2017 to 61.0% for the fiscal year 2018 primarily due to a slight increase in raw material and manufacturing costs for the Huoliyuan capsule.
Gross Profit
Gross profit for the year ended March 31, 2018 was $25,338,742, an increase of 9.3%, or $2,159,815, as compared to the same period for the prior year. The overall gross profit as a percentage of net revenues was approximately 39.0% for the year ended March 31, 2018, a slight decrease from 41.1% for same period of 2017. The gross profit as a percentage of net revenues for the health care products was 44.5% for the year ended March 31, 2018, the same as that of the year ended March 31, 2017. The gross profit as a percentage of net revenues for the Huoliyuan capsule was approximately 28.9% for the year ended March 31, 2018, which decreased from 35.4% for the same period of 2017. The gross profit as a percentage of net revenues for acer truncatum bunge seed oil was approximately 48.1% and 47.2% for the years ended March 31, 2018 and 2017. The lower gross profit as a percentage of net revenue for the Huoliyuan capsule during the year ended March 31, 2018 was due to the increased raw material and manufacturing costs. The slight increase of gross profit as a percentage of net revenue for the acer truncatum bunge seed oil during the year ended March 31, 2018 was primarily due to the net impact of decreased sales price offset by the decreased raw material cost.
The comparison of the gross profits for the years ended March 31, 2018 and 2017 is as follows:
|
March 31,
2018
|
March 31,
2017
|
Change in $
|
Variance
|
|||||||||||||
|
Health care supplements
|
$
|
11,697,623
|
$
|
8,979,067
|
$
|
2,718,556
|
30.3
|
%
|
||||||||
|
Drugs (Huoliyuan products)
|
7,457,973
|
8,744,707
|
(1,286,734
|
)
|
(14.7
|
)%
|
||||||||||
|
Acer truncatum seed oil
|
6,183,146
|
5,455,153
|
727,993
|
13.3
|
%
|
|||||||||||
|
Total
|
$
|
25,338,742
|
$
|
23,178,927
|
$
|
2,159,815
|
9.3
|
%
|
||||||||
Research and Development Expenses
Our R&D expenses for the year ended March 31, 2018 were $492,078, or approximately 0.8% of total revenue, a decrease of $317,407, or 39.2%, as compared to $809,485, or approximately 1.4% of total revenue, for the year ended March 31, 2017. The decrease of 39.2% in research and development expense was primarily due to reduced purchase of materials for acer truncatum bunge product research. In March 2017, we acquired a group of research equipment, which has been be used for developing an enhanced manufacturing process for production of our acer truncatum bunge seed oil.
31
Our long-term goal is to utilize advanced biological technology to refine and extract the beneficial compounds in plants that have traditionally been known to have medicinal benefits, primarily gingko and acer truncatum bunge plants. As of March 31, 2018, we had 27 staff in the R&D department.
Operating expenses
Our selling expenses consist primarily of sales commissions, advertising and promotion expenses, freight charges and related compensation. Our selling expenses for year ended March 31, 2018 were $4,984,794, or 7.7% of our total revenue for the year, representing an increase of 0.7% in the percentage of total revenue from the prior year. Our selling expenses for the year ended March 31, 2018 increased by 26.7%, or $1,050,460, as compared to the prior year. The increase in selling expenses was primarily due to the increase of shipping costs, advertising expenses, and sales commission.
Our G&A expenses for the year ended March 31, 2018 were $4,719,402, or 7.3% of our total revenue for the year, representing a slight decrease of 0.2% of total revenue from the prior year. Our G&A expenses for the year ended March 31, 2018 increased by 11.1%, or $471,307, as compared to the prior year. The increase in G&A expenses was primarily due to the increase of depreciation, amortization expenses and legal and consulting fees.
Our operating expenses for the years ended March 31, 2018 and 2017 also include loss from reduction in the capitalized cost of acer truncatum bunge planting. During the years ended March 31, 2018 and 2017, approximately 1% and 2.2% of the acer truncatum bunge plants were dead or lost due to the weather, replanting of trees, relocating of trees, and other natural or technical reasons as per management's estimate. For the years ended March 31, 2018 and 2017, impairment charges of $332,090 and $986,406, respectively, were recorded as reductions of capitalized costs which were included in operating expenses.
Gain on disposal of acer truncatum bunge plants
For the year ended March 31, 2018, a gain of $642,532 from disposal of acer truncatum bunge plants was also recognized, offsetting the operating expenses.
Income Taxes
Income tax expense increased by $638,207 during the year ended March 31, 2018, as compared to the year ended March 31, 2017, as a result of the increase in income from operations.
Net Income
As a result of above, during the year ended March 31, 2018, we realized net income of $11,738,488, representing a 16.7%, or $1,683,834, increase, compared to $10,054,654 during the year ended March 31, 2017. The increase was mainly due to higher gross profit, the gain from disposition of acer truncatum bunge plants, and higher interest income.
Comprehensive Income
Our business operates entirely in Chinese RMB, but we report our results in our SEC filings in U.S. Dollars. The conversion of our accounts from RMB to Dollars results in translation adjustments, which are reported as a middle step between net income and comprehensive income. The net income is added to the retained earnings on our balance sheet while the translation adjustment is added to a line item on our balance sheet labeled "other comprehensive income," since it is more reflective of changes in the relative values of U.S. and Chinese currencies than of the success of our business. During the year ended March 31, 2018, the effect of converting our financial results to USD was a gain of $9,115,321 to our other comprehensive income, as compared to a loss of $5,228,368 during the year ended March 31, 2017 as a result of the currency exchange rate fluctuation.
Noncontrolling interest
On March 18, 2017, 3% of the equity of Shandong Spring Pharmaceutical was transferred to Shandong Yongchuntang in exchange for tangible and intangible assets related to Acer Truncatum Industrial Project. As a result of this equity transaction, Shandong Yongchuntang has become a 3% shareholder of Shandong Spring Pharmaceutical. During the years ended March 31, 2018 and 2017, $625,614 of comprehensive income and $28,108 of comprehensive loss were attributable to Shandong Yongchuntang, respectively.
32
Liquidity and Capital Resources
Our principal sources of liquidity were generated from our operations. As of March 31, 2018, we had $28,082,938 in working capital, an increase of $12,590,517, or 81.3%, as compared to $15,492,421 in working capital as of March 31, 2017. The increase in working capital primarily resulted from our increased cash inflow from higher sales and the decreased balances in purchase deposits to vendors, inventory, and accounts receivable, and an increased balance in advance from customers offset by increased cash outflow, resulting from decreased balance in tax payable and accounts payable to related party as of March 31, 2018.
Based on our current operating plan, we believe that existing cash and cash equivalents balances, and the funds to be generated by operations will be sufficient to meet our working capital and capital requirements for our current operations for at least the next 12 months. Our operations produced positive cash flow of $17,866,899 during the year ended March 31, 2018. We had accounts receivable of $174,558 outstanding as of March 31, 2018. We expect our marketing activities to continue to help generate positive cash flow. The development of our own acer truncatum bunge planting bases and expanded manufacturing of acer truncatum bunge seed oil products have put some pressure on our cash flow. We may be required to seek additional capital and reduce certain spending as needed on an ongoing basis. There can be no assurance that any additional financing will be available on acceptable terms.
In order to fully implement our business plan, however, we will require capital contributions far in excess of our current asset value. Our budget for bringing our manufacturing facility to an operating level that assures profitability is $5 million. Our expectation, therefore, is that we will seek to access the capital markets in both the U.S. and China to obtain the funds we need. At present we have no commitment from any source for additional funds and there can be no assurance that the funds will be available on terms acceptable to us.
The following table sets forth a summary of our cash flows for the periods indicated:
|
Years ended March 31,
|
||||||||||||||||
|
2018
|
2017
|
Change in $
|
Change in %
|
|||||||||||||
|
Net cash provided by operating activities
|
$
|
17,866,898
|
$
|
9,736,074
|
8,130,824
|
83.5
|
%
|
|||||||||
|
Net cash used in investing activities
|
$
|
(4,539,071
|
)
|
$
|
(6,498,520
|
)
|
1,959,449
|
(30.2
|
)%
|
|||||||
|
Effect of exchange rate change on cash and cash equivalents
|
$
|
1,716,911
|
$
|
(568,016
|
)
|
2,284,927
|
(402.3
|
)%
|
||||||||
|
Net increase in cash and cash equivalents
|
$
|
15,044,738
|
$
|
2,669,538
|
12,375,200
|
463.6
|
%
|
|||||||||
|
Cash and cash equivalents, beginning balance
|
$
|
10,308,622
|
$
|
7,639,084
|
2,669,538
|
34.9
|
%
|
|||||||||
|
Cash and cash equivalents, ending balance
|
$
|
25,353,360
|
$
|
10,308,622
|
15,044,738
|
145.9
|
%
|
|||||||||
Operating Activities
Net cash provided by operating activities was $17,866,898 for the year ended March 31, 2018, which was an increase of 83.5%, or $8,130,824, from $9,736,074 net cash provided by operating activities for the year ended March 31, 2017. The increase was primarily due to the increase in cash inflow from sales and decreased balances in inventory, accounts receivable, and purchase deposit to vendors, offset by increase in cash outflow from accounts payable to related party and tax payable.
Investing Activities
During the year ended March 31, 2018, our net cash used by investing activities was $4,539,071, which was a decrease of 30.2%, or $1,959,449, as compared to $6,498,520 of net cash used for the year ended March 31, 2017. The decrease in net cash used by investing activities was primarily due to the proceeds received from the disposition of acer truncatum bunge plants in the amount of $2,156,510 during the year ended March 31, 2018.
33
Non-cash Investing Activities
During the year ended March 31, 2017, we transferred a 3% equity interest in Shandong Spring Pharmaceutical to Shandong Yongchuntang in exchange for a group of research equipment and a group of intangible assets related to Acer Truncatum Industrial Project. The fair value of assets acquired is approximately $2,338,000 (RMB14,701,200). Approximately $583,000 (RMB3,662,800) was allocated to research equipment acquired and approximately $1,755,000 (RMB11,038,400) was allocated to intangible assets acquired.
Financing Activities
No net cash was generated or used by financing activities over the years ended March 31, 2018 and 2017.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition or results of operations.
Critical Accounting Policies and Estimates
We have made no material changes to our critical accounting policies in connection with the preparation of financial statements for fiscal year 2018.
New Accounting Pronouncements
In March 2018, FASB issued ASU 2018-05, Income Taxes (Topic 740): Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 118. ASU 2018-05 amends SEC paragraphs in ASC 740 to reflect SEC Staff Accounting Bulletin (SAB) No.118. When the 2017 Tax Cuts and Jobs Act (the "Act") was signed into law, the SEC staff released SAB 118 for applying Topic 740 as it relates to the Act. SAB 118 outlines the approach companies may take if they determine that the necessary information is not available (in reasonable detail) to evaluate, compute, and prepare accounting entries to recognize the effect(s) of the Act by the time the financial statements are required to be filed. Companies may use this approach when the timely determination of some or all of the income tax effect(s) from the Act is incomplete by the due date of the financial statements. SAB 118 also prescribes disclosures that reporting entities must provide in these circumstances. The amendments to the Accounting Standards Codification became effective upon issuance. The Company has conducted a preliminary assessment of its income tax effects of the Act. Additional analysis of the law and the impact to the Company may be performed, if needed, and any impact will be finalized no later than the fourth quarter of 2018.
In February 2018, FASB issued ASU 2018-02, Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income. ASU 2018-02 provides entities the option to reclassify certain "stranded tax effects" resulting from the recent US tax reform from accumulated other comprehensive income ("AOCI") to retained earnings. Under the ASU, reporting entities will select an accounting policy to either reclassify all stranded tax effects caused by tax reform from AOCI to retained earnings, or continue recycling stranded effects (including those caused by tax reform) through earnings in future periods. Further, disclosure of either policy is required in all cases. The reclassification from AOCI to retained earnings is presented in the statement of shareholders equity. The ASU is effective for all entities in fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted for public business entities for which financial statements have not yet been issued, and for all other entities for which financial statements have not yet been made available for issuance. Entities have the option to record the reclassification either retrospectively to each period in which the income tax effects of tax reform are recognized, or at the beginning of the annual or interim period in which the amendments are adopted. The Company determined that the adoption of this new standard will have no material impact on its consolidated statements and related disclosures.
34
In May 10, 2017, the FASB issued ASU 2017-09, Compensation—Stock Compensation (Topic 718) - Scope of Modification. ASU 2017-09 clarifies when to account for a change to the terms or conditions of a share-based payment award as a modification. Under the new guidance, modification accounting is required only if the fair value, the vesting conditions, or the classification of the award (as equity or liability) changes as a result of the change in terms or conditions. Regardless of whether the change to the terms or conditions of the award requires modification accounting, the existing disclosure requirements and other aspects of GAAP associated with modifications, such as earnings per share, continue to apply. The guidance is effective prospectively for all companies for annual periods beginning on or after December 15, 2017. Early adoption is permitted. The Company is currently evaluating the potential impact of adopting this new standard on its consolidated statements and related disclosures.
In January 2017, FASB issued ASU 2017-01, "Business Combinations (Topic 805) - Clarifying the Definition of a Business". The Board is issuing the amendments in this ASU to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The definition of a business affects many areas of accounting including acquisitions, disposals, goodwill, and consolidation. The amendments in this ASU affect all reporting entities that must determine whether they have acquired or sold a business. Public business entities should apply the amendments in this ASU to annual periods beginning after December 15, 2017, including interim periods within those periods. However, early application of the amendments in this ASU if 1) for transactions for which the acquisition date occurs before the issuance date or effective date of the amendments, only when the transaction has not been reported in financial statements that have been issued or made available for issuance, or 2) for transactions in which a subsidiary is deconsolidated or a group of assets is derecognized that occur before the issuance date or effective date of the amendments, only when the transaction has not been reported in financials. The Company determined that its recent acquisition of the Acer Truncatum Industrial Project met the early application requirement and decided to account for the acquisition in accordance with FASB ASU 2017.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). Under this guidance, lessees will be required to recognize on the balance sheet a lease liability and a right-of-use asset for all leases, with the exception of short-term leases. The lease liability represents the lessee's obligation to make lease payments arising from a lease, and will be measured as the present value of the lease payments. The right-of-use asset represents the lessee's right to use a specified asset for the lease term, and will be measured at the lease liability amount, adjusted for lease prepayment, lease incentives received and the lessee's initial direct costs. The standard also requires a lessee to recognize a single lease cost allocated over the lease term, generally on a straight-line basis. The new guidance is effective for fiscal years beginning after December 15, 2018. ASU 2016-02 is required to be applied using the modified retrospective approach for all leases existing as of the effective date and provides for certain practical expedients. Early adoption is permitted. The Company is currently evaluating the effects that the adoption of ASU 2016-02 will have on the Company's consolidated financial statements.
Internal Control Over Financial Reporting
We had identified the following material weakness in internal control over financial reporting:
Lack of expertise in U.S accounting principles among the personnel in our Chinese headquarters. Our books are maintained and our financial statements are prepared by the personnel employed at our executive offices in Shandong Province, PRC. The current staff in our accounting department of the Chinese headquarters has limited knowledge and experience in U.S. GAAP. They need substantial training to meet the demands for a US public company, including the accounting skills and understanding necessary to fulfill the requirements of US GAAP-based reporting.
Lack of adequate staff in internal audit division, which could result in insufficient internal audit work to ensure that the Company's policies and procedures have been carried out as planned.
To remediate the first material weakness identified in internal control over financial reporting of the Company, we have done the following:
35
|
·
|
Engaged two part-time consultants who are qualified financial professionals and advise us for US accounting principles and financial reporting.
|
|
·
|
Engaged a third-party consulting firm to support our continued effort to develop and implement an effective Sarbanes-Oxley compliance program. As part of the Sarbanes-Oxley compliance program, all of our accounting policies and procedures were reviewed and formalized so that the accounting staff in our Chinese headquarters has written standards to follow in daily practice. The third-party consulting firm also provided company-wide training regarding our internal controls, with particular emphasis on our finance and accounting staff.
|
|
·
|
Required all of the accounting personnel in the accounting department to take a minimum of 24 CPE credits annually with a focus on US GAAP and financial reporting standards. We also required our Chief Financial Officer and our Financial Controller to take a minimum of 40 CPE credits annually with a focus on US GAAP and financial reporting standards.
|
Therefore, management believes that we have substantially remediated the first material weakness. In an effort to remedy the second material weaknesses in the future, however, we have commenced to take the following actions:
|
·
|
Hire at least one more staff in internal audit division so that sufficient internal audit work can be performed to ensure that our policies and procedures have been carried out as planned.
|
|
·
|
Implement an ongoing initiative and training in the Company to ensure the importance of internal controls and compliance with the established policies and procedures are fully understood throughout the organization, and plan to provide continuous US GAAP knowledge training to relevant employees involved to ensure the performance of and compliance with those procedures and policies.
|
Although we are undertaking steps to address these material weaknesses, the existence of material weaknesses is an indication that there is more than a remote likelihood that a material misstatement of our financial statements will not be prevented or detected in the current or any future period. This control deficiency could adversely affect our ability to record, process, summarize and report financial data consistent with the assertions of our management in our consolidated financial statements.
Quantitative and Qualitative Disclosures About Market Risk
Foreign Exchange Risk
While our reporting currency is the US dollar, almost all of our consolidated revenues and consolidated costs and expenses are denominated in RMB. All of our assets are denominated in RMB except for some cash and cash equivalents and accounts receivables. As a result, we are exposed to foreign exchange risk as our revenues and results of operations may be affected by fluctuations in the exchange rate between US dollar and RMB. If the RMB depreciates against the US dollar, the value of our RMB revenues, earnings and assets as expressed in our US dollar financial statements will decline. We have not entered into any hedging transactions in an effort to reduce our exposure to foreign exchange risk.
Inflation
Inflationary factors such as increases in the costs of our products and overhead costs may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of gross margin, selling and distribution, and general and administrative expenses as a percentage of net revenues if the selling prices of our products do not increase to cope with these increased costs.
36
DESCRIPTION OF BUSINESS
Overview
China YCT International Group, Inc. ("CYIG", "the Company", "we" or "us") is a diversified and vertically integrated health care company, which is engaged in developing, manufacturing and selling innovative traditional Chinese medicines, including manufacturing our medicines made primarily from panax ginseng leaves extract, developing acer truncatum bunge planting bases, processing and selling acer truncatum bunge seed oils, and distributing health care products manufactured by another company in the PRC.
We operate through our 97% owned subsidiary, Shandong Spring Pharmaceutical, a corporation organized in the People's Republic of China. Shandong Spring Pharmaceutical was originally organized as a subsidiary of Shandong Yongchuntang Group Co., Ltd. ("Shandong Yongchuntang") for the purpose of focusing on advanced technology related to the use of gingko as an aide to health. Shandong Yongchuntang later transferred ownership of Shandong Spring Pharmaceutical to its equity-holders, who transferred their ownership to our Company in 2007.
Corporate History
While we now develop, manufacture and sell our own products, including Huoliyuan capsules and seed oil from the acer truncatum bunge trees, Shandong Spring Pharmaceutical initially served solely as a distributor for Shandong Yongchuntang pursuant to a distribution agreement signed in 2006 that fixed the purchase price that would be paid by Shandong Spring Pharmaceutical for products purchased from Shandong Yongchuntang. Mr. Tinghe Yan, our founder and Chief Executive Officer, was the principal stockholder of Shandong Yongchuntang until he sold all of his shares of Shandong Yongchuntang to an unrelated party on December 16, 2009.
In January 1989, the Company was originally incorporated under the laws of the State of Florida under the name of Medical Technology & Innovations, Inc. On April 4, 2007, the Company reincorporated in the State of Delaware by merging with and into ItLinkz Group, Inc., a Delaware corporation, which was a wholly owned subsidiary of Medical Technology & Innovations, Inc. As a result of the merger, ItLinkz Group, Inc. was the surviving corporation.
Landway Nano Bio-Tech Group, Inc. was organized under the laws of the State of Delaware in 2006. In June 2007, Landway Nano Bio-Tech paid $2.58 million to acquire 100% of the stock of Shandong Spring Pharmaceutical, which was then owned by Mr. Tinghe Yan and Mr. Tingrong Yan.
On September 28, 2007, ItLinkz Group, Inc. ("ItLinkz Group") acquired the outstanding capital stock of Landway Nano Bio-Tech Group, Inc. In connection with the closing of the acquisition on September 28, 2007, ItLinkz Group issued to the stockholders of Landway Nano Bio-Tech 500 shares of Series B Preferred Stock, which were convertible into 404,933,115 shares of common stock. Tinghe Yan and Jirui Zhang, the executive officers of Shandong Spring Pharmaceutical, became members of the Board, and became, respectively, the Chief Executive Officer and Chief Financial Officer of ItLinkz Group. On November 23, 2007, ItLinkz Group, Inc. changed its name to China YCT International Group, Inc. Upon conversion of the Series B Preferred Stock, Messrs. Tinghe Yan and Jirui Zhang became the principal holders of common stock of the Company.
On March 18, 2017, we entered into an acquisition agreement with Shandong Yongchuntang pursuant to which we acquired the Acer Truncatum Industrial Project. Pursuant to the agreement, we transferred 3% of the equity of Shandong Spring Pharmaceutical, in exchange for tangible and intangible assets related to the Acer Truncatum Industrial Project in Shandong Province.
The following diagram illustrates our corporate structure as of the date of this prospectus:
37
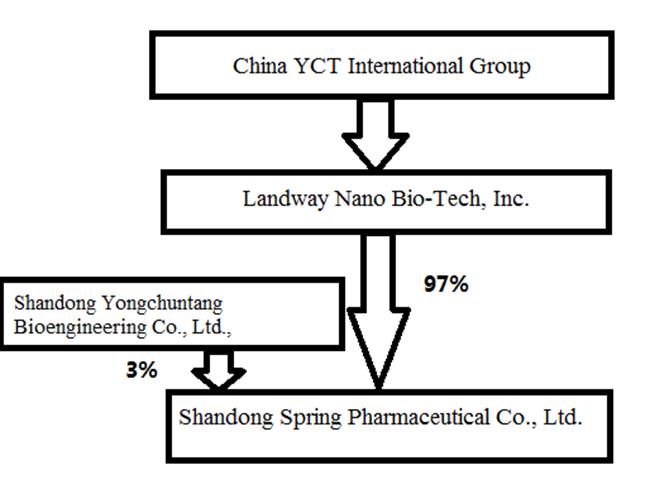
Industry Background and Market Opportunities
Acer Truncatum Bunge Market Opportunity
Acer truncatum bunge (Shantung maple, Shandong maple, or purpleblow maple) is native to the Chinese provinces of Gansu, Hebei, Henan, Jiangsu, Jilin, Liaoning, Inner Mongolia, Shaanxi, Shandong, and Shanxi, and to Korea. Traditionally, acer truncatum bunge is an important tree species for afforestation and soil and water conservation, which brings social and ecological benefits. In addition, research has demonstrated that acer truncatum bunge has great value for development and utilization as food, medicine and chemical raw materials.
Acer truncatum bunge produces nervonic acid, an extract from its seeds that is the principal extract from which acer truncatum bungee seed oil is derived. As a monounsaturated omega-9 fatty acid, nervonic acid is believed to be beneficial to memory-related brain health, anti-aging, blood lipid regulation, anti-fatigue. Omega 9 includes several types of fats. However, four of them which are the most important: oleic acid, mead acid, erucic acid and nervonic acid. Nervonic acid has been identified as important in the biosynthesis of nerve cell myelin. It is found in the sphingolipids of white matter in human brain. Nervonic acid is used in the treatment of disorders involving demyelination, such as adrenoleukodystrophy and multiple sclerosis where there is a decreased level of nervonic acid in sphingolipids.
Scientific research reveals that nervonic acid can advance the re-growth of neurons. It possesses several medical effects, such as, repairing and dredging the speedway of transferring nervonic information nerve fiber to dredge the brain route, repair the injured cranial nerve cells, and advance the re-growth of cranial nerves.
Nervonic acid can also increase the energy of brain cells, advance the multiplication and differentiation of cells, prevent the cranial nerve cells from consenescence and injury, recover the clear perceive ability, distinguishing ability, study ability and memory. Nervonic acid may be effective to prevent senile dementia.
In Europe and the United States, nervonic acid is mainly extracted from shark brains or shark oils. The extraction process and technique are difficult, and the final cost can exceed $45,359 per pound. In addition, certain species of sharks are protected by the United Nations and many countries in the world, so animal sources of nervonic acid are not as acceptable as in the past. Biosynthesis from chemicals or other intermediates are not presently available because either the yield is low or too many unhealthy by-products are produced.
38
Therefore, the most efficient principal sources of nervonic acid are plants and herbs. Malania oleifera is rich in nervonic acid. It is native to southern China in the western provinces such as Guangxi and southeastern Yunnan. Its content of nervonic acid is up to 40.92% to 50% of the lipid (usually triglyceride). However, Malania oleifera is a threatened species, and it is on the list of key wild plants for state protection. Therefore, there is no opportunity for commercial extraction form wild malaria oleifera. Cultured malania oleifera plants are experimental in some areas.
We believe the best source for producing nervonic acid at commercial scale is acer truncatum bunge seed. There are different extraction methods being used to produce nervonic acid from acer truncatum bungee seed, one of which is Supercritical Carbon Doxide. The analysis revealed that such method could yield approximately 6.22% nervonic acid. We have pioneered an extraction method that allows us to achieve similar nervonic acid yield in a more cost-effective and environmentally friendly manner.
According to the statistics provided by Herb Nutritionals Co., Ltd., the table below is a list of natural food resources of nervonic acid from both plants and animals, with nervonic acid content in mg/100g:
|
Nervonic Acid Content (mg/100g)
|
||||
|
Acer Truncatum
|
580
|
|||
|
Brassica Oil Seeds
|
69-83
|
|||
|
Sesame Seeds
|
35
|
|||
|
Macadamia Nuts
|
18
|
|||
|
Tropaeolum Speciosum
|
10
|
|||
|
Lunaria (Money Plant)
|
8
|
|||
|
King Salmon (Chinook)
|
140
|
|||
|
Sockeye Salmon
|
40
|
|||
We believe that favorable claims regarding nervonic acid from several independent studies are credible. While we rely on these independent studies for guidance, we cannot verify these claims with any degree of certainty.
We believe that demand for high-quality/low-price acer truncatum bunge seed bunge oil will increase in part due to demand from elderly Chinese and its use as a health supplement. We sell acer truncatum bunge seed oil for approximately 1,000 RMB/kg (approximately $67.41 per pound). The annual production of acer truncatum bunge oil in China was only about 12.4 tons (approximately 27337.32 pounds) in 2017.
We believe we are the leader of China's acer truncatum bunge seed oil production industry according to the market statistics that we have collected:
39
2017 Annual Production of Acer Truncatum Bunge Seed Oil in China (1 ton equals 2204.62 pounds)
|
Manufacturer
|
Acer truncatum bunge seeds used (tons)
|
Acer truncatum bunge seed oil produced(tons)
|
Percentage
|
|||||||||
|
Weifang Luyuan bio-technology Co. Ltd
|
34.86
|
2.78
|
22.5
|
%
|
||||||||
|
Liaoning Sanmark Co. Ltd
|
46.3
|
3.7
|
29.8
|
%
|
||||||||
|
Shandong Spring Pharmaceutical Co., Ltd
|
73.84
|
5.9
|
47.7
|
%
|
||||||||
|
Total
|
155
|
12.4
|
100
|
%
|
||||||||
Health Care Spending
We believe that China's increasing per capita disposable income, aging population and demand for traditional Chinese medicines and treatments should support increased demand for our Huoliyuan capsules, acer truncatum bunge seed oil products and other health care products. The increase in the per capita disposable income of Chinese urban residents and the increase in the number of elderly people in China in recent years have resulted in increasing spending on prescription and OTC medicines, including traditional Chinese medicine. According to the China Statistical Abstract 2017, the average annual per capita disposable income of Chinese urban residents increased to RMB 36,396 (approximately $5,177 ) in 2017 from RMB 21,810 (approximately $3,240) in 2011, and the average annual per capita disposable income of Chinese rural residents increased to RMB 13,432 (approximately $1,911) in 2017 from RMB 6,977 (approximately $1,036) in 2011. During the same period, the annual per capita expenditures on healthcare and medical services by Chinese urban residents increased to RMB1,777 (approximately $253) in 2017 from RMB1610.48 (approximately $239) in 2011, and the annual per capita expenditures on healthcare and medical services by Chinese rural residents increased to RMB 1,059 (approximately $151) in 2017 from RMB 697 (approximately $104 ) in 2011.
Increasing health care expenditures in China create significant opportunities for our growth. According to research conducted by CFDA Southern Medical Economic Research Institute, China's pharmaceutical market, based on both prescription and OTC medicine sales, increased to RMB1,612 trillion (approximately $229 billion) in 2017 from RMB 809.7 billion (approximately $120 billion) in 2011. China now has the world's fastest growing major healthcare market with a five-year compound annual growth rate of 17%, compared to 4% in the United States and 2% in Japan, according to data from the World Health Organization. China is the second largest healthcare market globally with total healthcare expenditures reaching $594 billion in 2015, a number projected to reach $1.1 trillion by 2020, according to 2016 Top Markets Report Pharmaceuticals by International Trade Administration. Considerable growth in China's healthcare market is anticipated as continue with per capita health spending is $420, compared to an average of over $5,800 for the world's top eight healthcare markets, according to data from IMS Market Prognosis.
We believe that China's aging population is a key contributor to the increased expenditures on medicines because elderly people on average spend more on healthcare than younger people. China's aging population is expected to increase the burden of chronic disease by 40% by the year 2030, according to a paper Toward a Healthy and Harmonious Life in China: Stemming the Rising Tide of Non-Communicable Diseases by World Bank. Over the past 20 years the number of people aged over 65 has nearly doubled reaching 150 million (10.8% of the total population) according to the Ministry of Civil Affairs. The portion of the Chinese population aged 60 and above and aged 65 and above has increased in both absolute numbers and as a percentage of the total population, and we believe that this trend is likely to continue in the foreseeable future. According to statistics from National Bureau of Statistics of China, the number of people in China aged 60 and above was approximately 231 million in 2016, or 16.7% of China's entire population, compared to approximately 153 million, or 11.6% of China's entire population in 2007. The number of people in China aged 65 and above was approximately 150 million in 2016, or 10.8% of China's entire population, compared to approximately 106 million, or 8.1% of China's entire population in 2007.
40
The following chart illustrates China's distribution of the population according to age in 2016:
Source: CIA World Factbook, last updated on January 20, 2018, https://www.cia.gov/library/publications/the-world-factbook/fields/2010.html
Traditional Chinese Medicine
Traditional Chinese medicine is a medical system developed in China about 3,000 years ago and is therefore deeply ingrained in Chinese culture. It is based on the concept that one's health depends on a constant struggle between various opposing forces (e.g.), yin and yang, or hot and cold). Excesses or imbalances in the body cause sickness or disease, with Chinese herbal medicines helping to restore balance and nurse the body back to health. Traditional Chinese medicine practitioners emphasize preventative medicine and treating diseases before they occur through healthy living and proper diets. This is especially important in China, which has increasing obesity, diabetes and smoking rates.
Historically, traditional Chinese medicine, or TCM, consisted primarily of mixtures of dried herbs and, in some cases, animal parts and minerals. These mixtures would be boiled and simmered at home to create a medicinal tea or soup. These liquid concoctions were inconvenient to prepare and take, and their dosage and quality were inconsistent due to varied methods of preparation and differences in the quality of ingredients. In recent decades, however, pharmaceutical companies in China have applied modern production technologies to extract active ingredients out of the mixtures and formulate the extracts into a variety of dosage forms such as tablets, capsules and granules, which we refer to as modernized traditional Chinese medicines. These modernized formulations offer patients convenient forms of traditional Chinese medicine and also substantially improve their quality, consistency and dosage precision.
Traditional Chinese medicine has enjoyed a resurgence championed by the Chinese government. In December 2016, China's State Council issued the "Strategic Plan on the Development of Traditional Chinese Medicine" making the development of traditional Chinese medicine a national strategy. The traditional Chinse medicine industry grew by 20% in 2017, with revenue of $130 billion, or one third of total medical industry output, according to the State Administration of Traditional Chinese Medicine.
Additionally, the Department of Forestry of Shandong Province, where we are based, has issued its plan to reform and upgrade the industry for 2016 – 2020 in Shandong (the "Plan"). The Plan specifically encourage the expansion of the wood-based oil extract industry and identifies the acer truncatum bungee trees as one of plants whose production is encouraged. The Department of Forestry of Shandong Province recognized us as one of the leaders in the province's wood-based oil extract industry. The Plan also identifies us as one of the companies in the industry that may have good potential to sell the acer truncatum bungee seed oil products internationally.
41
Our Competitive Strengths
We believe the following competitive strengths have contributed to, or will contribute to, our recent and ongoing growth:
Dominant Producer of Acer Truncatum Bunge Seed Oil
We believe that we are the largest producer of acer truncatum bunge seed oil in China, accounting for 47.7% of the market in China in 2017. We also obtained a food production license for the production of edible vegetable oil from the Food and Drug Administration of Sishui County, and expect to produce blended edible oil products that contain acer truncatum bungee seed oil as one of the major ingredients. We believe that being the dominant producer and having stronger vertically integrated production capabilities will provide us with advantages in pricing and distribution of our products and spur further growth.
Diversified Revenue and Profit Stream
We generate revenue through the sales of diversified products, including prescription medicines, acer truncatum bunge seed oil products, and nine health care products purchased from Shandong Yongchuntang. With a diversified revenue stream, which can reduce financial stress during times of economic decline by relying on more than a single product, we believe alternative revenue sources provide the diversification to create a more financially stable company as well as additional opportunities for growth.
Strong Relationship with Key Distributors
We conduct our sales primarily through our seven distributors, who collectively control a 200,000+ person direct sales force and use person-to-person marketing to promote and sell our acer truncatum bunge seed oil products and nine products purchased from Shandong Yongchuntang. Compared to health and nutrition products that are distributed through traditional market means, these personal marketing efforts are supported by various mediums, including our marketing content, websites, events and social business solutions. We believe our distribution channel is an effective vehicle to distribute our products because:
| • |
representatives can educate consumers about our products face-to-face, which we believe is more effective for differentiating our products than using traditional mass-media advertising;
|
| • |
our distribution channel allows for actual product demonstrations and trial by potential consumers;
|
| • |
our distribution channel allows the representatives to provide personal testimonials of product efficacy; and
|
| • |
as compared to other distribution methods, the representative has the opportunity to provide consumers higher levels of service and encourage repeat purchases.
|
Reputable Brands
Our acer truncatum bunge seed oil products are distributed under the recognized brand name "Bao Feng San Yi". In addition, we believe that we are the largest producer of Huoliyuan capsules and tablets in China, that our Huoliyuan capsule is the only herbal medicine of its type made in the form of a capsule, and that we are recognized by customers as a leader in the Huoliyuan market. Other peer products are produced in the form of tablets. The capsule permits the medication to be formulated for slow release. We believe that our reputable brand name will benefit our future expansion and growth efforts.
42
Raw Materials Competitive Pricing
Our goal is to be a vertically integrated acer truncatum bunge seed oil and blended edible oil producer once our own trees begin production, which is expected to occur in the fall of 2018. While to date we have produced acer truncatum bunge seed oil from raw materials purchased from third parties, we began to develop and cultivate our own trees in June 2013. We have leased 5,880 mu (approximately 2,324.77 acres) of farmland for terms of 30 years and 14 years, respectively, and have planted approximately 7 million trees at a cost of approximately $49 million through March 31, 2018. Having our own tree base will significantly reduce our cost to produce the seed oils compared to our competitors that purchase the acer truncatum bunge pods from third parties. The intended principal use of the proceeds that we will receive from this offering is to expand and further automate our manufacturing facilities for the acer truncatum bunge seed oil products.
Experienced Management
We are led by highly experienced and entrepreneurial executive officers. Mr. Tinghe Yan, our founder, Chairman and Chief Executive Officer, has over twenty years of experience in corporate management within the food and food supplements industries. Mr. Yan founded Shandong Spring Pharmaceutical in 2006. From 1998 to 2006, Mr. Yan was employed as the Chairman and General Manager of Shandong Yongchuntang, which manufactures a wide variety of food supplements and health care products and is currently the exclusive supplier of nine health care products distributed by Shandong Spring Pharmaceutical.
Our Growth Strategy
To maintain the growth of our business, we expect to rely on these key drivers:
Leverage and Expand our Existing Distributor Base Throughout China
China has been and will continue to be our largest market. Our growth strategy in China involves multiple initiatives, such as the launch our proposed edible oil products, continued investment in company-sponsored events and distributor training, and better utilization of our upper-level distributors across different geographical areas to increase our distributor base.
Expansion of manufacturing infrastructure and increased productivity for new businesses
Our production volume can be improved with additional equipment and infrastructure. We intend to use a portion of the proceeds of this offering to invest in our infrastructure to increase capacity and thereby be able to increase our production volume and expand our sales.
Vertical Integration
Our ability to initiate and increase production of the raw material for the acer truncatum bunge seed oil is expected to lower our cost of goods sold by eliminating our reliance on third party suppliers. We believe that over the next five years, the approximately seven million trees on 5,880 mu (approximately 2,324.77 acres) of land will begin to produce seeds for processing into oil beginning in the fall of 2018 until all trees are expected to mature by 2022. We also expect to increase the productivity of our acreage as we gain experience in growing, maintaining and harvesting the trees.
Direct Sales Channel
We expect to continue to expand the number of salespeople participating in our direct sales channel that is used for the sale of the acer truncatum seed oil products and the health care products that we distribute. We expect to also add additional products to this distribution channel, such as the proposed edible oil products, thereby increasing revenues.
43
Achieving a dominant position in the acer truncatum bunge seed oil products market
With years of strategic preparations and planning behind us, we are seeing tremendous growth opportunities in this business segment, as consumers have paid growing attention to personal healthcare matters associated with aging. We plan to focus our sales in this sector as our strategic priority for the next few years.
Invest in Improved and New Products
As a developer of prescription medicine with our Huoliyuan capsule, and acer truncatum bunge seed oil related products, we believe that it is vital to continue to invest in the research and development of new and innovative products. We will continue to improve and validate the efficacy of our existing product line. These types of investments should facilitate customer and distributor retention, as well as the recruitment of new distributors.
Enter new markets and further penetrate existing markets
We believe there is a significant opportunity to increase our net sales. We have a business model that is compatible across all of our markets and encourages our distributors to pursue their business in multiple markets. We believe this business model supports expansion of our distributor network into international markets and will provide a framework that facilitates our entry into new international markets. To that end, we continue to monitor business conditions in potential new markets and will selectively expand as timing and conditions are appropriate.
Mergers and Acquisitions
We closely follow and investigate potentially profitable and strategic acquisitions and combinations within our industry. We continuously seek synergistic partnerships to acquire additional technologies, products, services, operations and/or geographic capabilities that can provide long-term growth. While we currently do not have any specific mergers or acquisition targets, we plan to explore the possibility of completing a few vertical or horizontal acquisitions in the next few years to expand our presence in the industry. However, there can be no assurance that we will be able to successfully complete any such acquisitions..
Our Major Products
Huoliyuan Capsules and Tablets
We manufacture our Huoliyuan capsules and tablets according to traditional Chinese medicine concepts. The main ingredients of these Huoliyuan products are traditional Chinese herbal medicines, including Panax Ginseng leaves extracts, Radix Astragali, Radix Ophiopogonis, Schisandra Chinensis and Monkshood. We believe that our Huoliyuan capsule is the only herbal medicine of its type made in a capsule among all peer products. Other peer products are only in the form of tablets.
Our Huoliyuan products are used with respect to various medical conditions, and our Huoliyuan capsule was approved by the State Food and Drug Administration (the "SFDA") of China on June 10, 2008. The Huoliyuan capsules can be purchased only by prescription.
The therapeutic effect of Huoliyuan was tested by anindependent analysts at the Jining Institute for Drug Control in 2003. The test primarily consists of a Character Test, Identification Test, Water Index, Load Difference, Disintegration Time and Microbial Limit. The test concluded that Huoliyuan was beneficial for the human cardiovascular system and as an aid in the treatment of chronic hepatitis, diabetes, insomnia, memory loss, menopause syndrome, and other maladies. The Huoliyuan capsule is formulated for slow release and daily use.
44
We first began development of the Huoliyuan capsule in January 2007, when we purchased a patent from Beijing Boya Research Institution, Ltd. We commenced manufacture of Huoliyuan capsules in 2010. During the year ended March 31, 2018, we produced approximately 5.87 million boxes of 3 pack capsules and 3.12 million boxes of 2 pack capsules.
The Development of Acer Truncatum Bunge Planting Base
On March 22, 2011, the Chinese Ministry of Health officially approved acer truncatum bunge seed oil, along with peony seed oil, as a new food resource (No. 9 Announcement issued in 2011). Subsequently, in December 2011, the Chinese National Development and Reform Commission, jointly with the Ministry of Industry and Information Technology, issued the Twelfth Five-year Development Plan of Food Industry, whereby "the nutrition and health food manufacturing industry" was listed for the first time in the national development plan as a focused developing area.
We initiated an investment plan (the "Plan") to take advantage of the national policy and began to develop and cultivate our own planting base for acer truncatum bunge in June 2013. As of March 31, 2018, we have completed our investment of approximately $49 million in the development of the acer truncatum bunge planting base. We expect to spend about $1.5 million per year to maintain our planting base without further expansion.
The trees are expected to begin production from the fall of 2018 through 2022 and we have also completed the construction of the planting fields and related support facilities. We signed three separate lease agreements for a total of 5,880 mu (approximately 2,324.77 acres) of farmland for terms of either 30 years or 14 years, and have completed planting of approximately seven million trees. We have also developed cooperation relationships with local farmers by providing seeds and planting techniques, resulting in another 5,320 mu (approximately 2,103.36 acres) of acer truncatum bunge trees planted by local farmers.
Since July 2015, we have been purchasing acer truncatum pods from third party vendors, and have extracted the pods to produce the acer truncatum bunge seed oils. We sell the acer truncatum bunge seed oils products to customers through our distributors participating in the direct sales channel. Based on our current manufacturing technology, we expect that we will be able to extract about an average of 1 pound of acer truncatum seed oil per 10 pounds of acer truncatum bunge seeds. Until our own planting base begins production, we will continue to purchase the acer truncatum bunge pods from third parties used to manufacture our acer truncatum bunge seed oil. The following chart reflects the projected schedule of maturation of our trees.
45
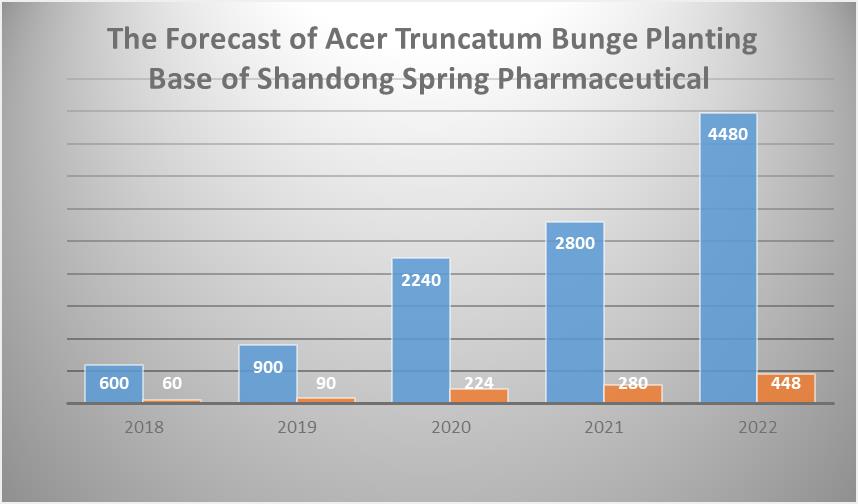
Blue: Seeds Production (tons)
Orange: Oil Production (tons)
Acer Truncatum Bunge Seed Oils and Capsules
We manufacture the "Bao Feng San Yi" brand of acer truncatum bungee seed oil by use of advanced cold-pressed extraction technology, which oil contains rich nervonic acid, oleic acid, linoleic acid, linolenic acid and other essential fatty acids. The oil is edible, clear and without impurities and the products may be purchased without a prescription.
The bottled products have two specifications, 300mg, which is for children between the ages of 7 and 18, and 500 mg, which is for middle-aged to elderly people. Long-term consumption of the 300mg product may stimulate children's nervous system and mental development and improve their memory function and their learning ability. The 500mg product may improve the nervous system and enhance the body's stress ability. It may also improve digestion, immunity, blood circulation, and memory function, and decrease the rate of brain diseases, such as Alzheimer's disease.
We also manufacture the acer truncatum bungee seed soft capsule (acer truncatum bungee seed gel-type candy) under the brand name" Bao Feng San Yi" by using acer truncatum bungee seed oil as the main raw material, and adding probiotics to promote the proliferation of fructo-oligosaccharides and lactose, and edible gelatin as a supplement. The product has a similar effect as the "Bao Feng San Yi" brand acer truncatum bungee seed oil.
High-grade Acer Truncatum Bunge Seed Blended Edible Oils (Cooking)
China's consumption of high-end edible oil is gradually increasing, the consumption of soybean oil, palm oil, rapeseed oil in the domestic market share still account for the top three. China's overall consumption of edible oil was approximately 34.67 million tons in 2015, with the average individual consumption of 24.8 kilogram.
We expect to produce blended edible oil products for use as a cooking oil that contain acer truncatum bungee seed oil as one of the major ingredients. On January 11, 2018, the Food and Drug Administration of Sishui County ratified and issued us a Food Production License for production of edible vegetable oil, which includes acer truncatum bunge seed oil, and related blended edible oil products and is valid for five years.
46
Edible oil is mainly divided into two categories: animal oil and vegetable oil. Animal oil contains more saturated fatty acids and cholesterol, which may cause hypertension, arteriosclerosis, coronary heart disease, hyperlipidemia and cerebrovascular diseases by long term consumption. Compared with animal fats and other vegetable oils, certain vegetable oils that contain more monounsaturated acid content have more nutritional value.
Our high-grade blended edible oils include extracts of acer truncatum bunge seed oils, peony seed oil and walnut oil by using advanced cold-pressed extraction, refinery and reconciliation processing technologies. We refine the oils with modern nano-activation technology and quantum-implantation, which improves the solubility, penetration and human body absorption rate. We blend the oil by using the blended ratio of 1: 1: 1.
This product will contain linoleic acid, linolenic acid and other unsaturated fatty acids. The amount of linoleic acid content in our product is twice that of peanut oil, and ten time that of olive oil. Long-term consumption of our product may have good effects, including but not limited to repairing damaged nerves, lowering lipids, softening blood vessels, lowering blood pressure and promoting blood circulation.
Other Health Care Products from Shandong Yongchuntang
Shandong Spring Pharmaceutical has served as a distributor for Shandong Yongchuntang since January 2007. Pursuant to our distribution agreement with Shandong Yongchuntang, dated as of February 21, 2018, which is subject to automatic annual renewal, we purchase nine products from Shandong Yongchuntang at fixed prices and resell them by using the direct-sales license issued to Shandong Yongchuntang. Our direct-sales sublicense agreement with Shandong Yongchuntang will expire on June 30, 2020, we are entitled the right of first refusal on the renewal of the sublicense agreement. The sublicense agreement requires us to increase the annual sales no less than 20% each year by using 2014 annual sales volume of RMB 35 million (approximately $5.28 million) as a benchmark. If we fail to reach the sales target, we are required to pay a royalty of RMB 10 million (approximately $1.6 million) to Shandong Yongchuntang. To date, we have achieved growth of sales of a minimum of 20% per year each year and not been required to pay such fee. The nine products that we purchase from Shandong Yongchuntang accounted for $26,297,055 in revenue, or 40.5% of total revenue, in the year ended March 31, 2018. Of the nine products, Healthcare Products Portfolio No. 3, Ginkgo Herbal Tea, and Magnetic mattress products, accounted for 24.4% of our total revenue in the year ended March 31, 2018. The nine products consist of the following:
|
1.
|
Healthcare Products Portfolio No. 1, which contains one bottle of each of:
|
|
(i)
|
ganoderma and rhodiola capsules (300mg / capsule× 80 / bottle)
|
|
The product is made from the extraction of two natural ingredients, ganoderma and rhodiola, and was approved by the State Food and Drug Administration as a healthcare food in April 2009 (No. G20090072). The product may enhance the body's immune system.
|
|
|
(ii)
|
spirulina green treasure capsules
|
|
This product may enhance the body's immune and digestion system, maintain body energy, anti-aging, and promote the healthy growth of infants and young children.
|
|
|
(iii)
|
zinc, calcium, vitamin tablets
|
|
The product is a supplement of calcium, iron, zinc, and vitamin C and D3. This product was approved by the State Food and Drug Administration as healthcare food (No. G20080410).
|
|
|
2.
|
Healthcare Products Portfolio No. 2, contains one bottle of each of:
|
|
ganoderma and rhodiola capsule, spirulina capsule, and ginkco green treasure protein tablets
|
|
47
|
3.
|
Healthcare Products Portfolio No. 3, contains one bottle of each of our acer truncatum bungee seed soft capsules for children (300mg per capsule), acer truncatum bungee seed soft capsules for adults (500mg per capsule), and ganoderma and rhodiola capsules.
|
|
4.
|
Healthcare Products Portfolio No. 4, contains one bottle of each of:
|
|
(i)
|
Kang Le capsules (250mg/capsule × 80 capsules/bottle)
|
|
This product is made from extractions of chitosan, astragalus, ginseng, sea buckthorn, selenium yeast powder by using delicate processing technologies, which can enhance human immune system and treat fatigue.
|
|
|
(ii)
|
Spirulina green treasure capsules and
|
|
(iii)
|
Wolfberry tablets
|
|
This product is made from extracts of wolfberry by using delicate processing technologies, which is used for anti-oxidation, anti-aging, lowering blood pressure, and lowering blood lipids.
|
|
|
5.
|
Healthcare Products Portfolio No. 5, contains one bottle of each of:
|
|
spirulina green treasure capsules, compound wolfberry tablets and compound saussurea aloe tablets
|
|
|
6.
|
Ginkgo Herbal Tea
|
|
Consumption of this product may improve circulation and pulmonary function. The flavonoid aglycone, a compound derived from the gingko plant, is contained in the product. This product is a health food approved by Ministry of Health (WeiShiJianZi 1999 No. 0529).
|
|
|
7.
|
Ginkgo Aglycone soft capsules
|
|
This product is refined with ginkgo flavonoids as the main raw material, which may assist in clearing free radicals, promoting blood rheology, improving microcirculation, inhibiting aging, preventing and treating coronary heart disease, angina pectoris, myocardial infarction, cerebral embolism, senile dementia, preventing and treatment of cardiovascular and cerebrovascular diseases.
|
|
|
8.
|
Ginkgo Skincare Product Package
|
|
Our skincare product package contains a bottle of eye cream, night cream, lotion, and essence, which have rich flavonoid aglycone and can smooth over the face, neck, and the fragile eye area.
|
|
|
9.
|
Magnetic mattress products
|
|
The mattress uses natural latex and magnetic stone powder as the main raw materials, and is implanted with special quantum chips. The magnetic mattress may stimulate and activate the activities of body cells, relieve nerve pressure, and improve sleep quality.
|
|
Patents
In March 2010, we purchased a patent from Shandong Yongchuntang for RMB 46 million (approximately $6.74 million at the time), which enabled us to manufacture and distribute medicine products for cardio cerebral vascular disease, cosmetics, and healthcare products. The patent acquired from Shandong Yongchuntang was independently assessed at a fair value of RMB 76 million (approximately $11.14 million at the time) by Beijing Beifang Yashi Asset Evaluation Company based on the current income value method. The purchase price for the patent was negotiated between our Company and Shandong Yongchuntang based on the assessed value and was purchased by us at a discount. The patent is for an aglycone type and purification method of biotransformation in herbal product manufacturing process to extracting herbal flavonoid more extensively, and it has a legal life of 16.5 years. We have not yet commercialized the patent.
48
On October 26, 2010, Shandong Spring Pharmaceutical signed an agreement to purchase two patents relating to Chinese herbal formulas from Jining Tianruitong Technology Development Limited Company for RMB 72 million (approximately $11,450,200 at the time). We received an independent assessment of the value of the patents, which substantiated the purchase price. Approval from the State Intellectual Property Office of the PRC was required for the transfer of the patent. We obtained governmental approvals for the transfer of the two patents in October 2011, which were transferred to us. The acquisition of the third patent was cancelled with the outstanding payment settled. The two patents are "Treatment to ischemic encephalopathy and its preparation method" (ZL200510045001.9) and "Chinese herbal medicine compound to treat renal insufficiency and its preparation" (ZL200710013301.8), with legal lives of approximately 14 years and 15 years, respectively. One patent was fully impaired in the year ended March 31, 2016. We have not yet commercialized the second patent.
Research and Development
Our research and product development activities include:
• Internal research, product development and quality testing;
• Joint research projects, collaborations studies;
• Identification and assessment of technologies for potential licensing arrangements; and
• Acquisition of technologies.
We are committed to developing and marketing innovative products. We have several products in development, including skin care products, nutritional supplements, and edible vegetable oil. Our goal is to utilize advanced biological technology to isolate and extract the beneficial compounds in plants that have traditionally been known to have medicinal benefits. We also focus our research on the seed selection and planting technologies for greater yield of leaves and seeds from the plants as well as the most efficient method to isolate and extract nervonic acid from seed oil.
We maintain research and product development facilities in China. We have a staff of 29 employees engaged in research and development of new technologies and resulting products. We also maintain close ties to the research staffs at Tsinghua University, China Agriculture University, Shandong Herbal Medicine University, and the Shandong Herbal Medicine Research Institute. We entered into a written R&D Cooperation Agreement with Nanjing Forestry University on August 10, 2016, which will be effective until July 31, 2019, pursuant to which we will invest in a national herbal R&D center with Nanjing Forestry University and have the right of first refusal on the transfer and use of Nanjing Forestry University's herbal related technologies. Nanjing Forest University provides assistance regarding selective breeding of the acer truncatum bunge trees and product development of acer trumcatum.
We also work to identify and assess innovative technologies developed by third parties for potential licensing or supply arrangements. Because of the nature of our distribution channel, which allows us to provide a high level of product information on a person-to-person basis, we have third parties who are interested in licensing innovative technologies to us to incorporate into our products and commercialize through our distribution channel. Licensing arrangements allow us to leverage the research activities of third parties that have resulted in demonstrated technologies, without all of the upfront costs and uncertainty associated with internal development. We have also invested in acquisitions to supplement our research capabilities and to acquire technologies.
49
During the fiscal years ended March 31, 2018 and 2017, we spent $492,078 and $809,485 on research and development. Our R&D expenses were primarily used for acquiring and testing raw material, and also the ordinary maintenance expense for our research equipment. The R&D expenses for the fiscal year ended March 31, 2018 included the payment of $267,425 for personnel costs and $221,047 for raw materials used in R&D of ginkgo biloba brain tablets. In March 2017, we acquired research equipment that will be used for developing an enhanced manufacturing process for our acer truncatum bunge seed oil.
Certifications
The manufacturing facility developed by Shandong Spring Pharmaceutical has received GMP (good manufacturing practices) certification granted by the Chinese government. GMP is the only certified manufacturing standard certificate that is authorized by the Chinese government. Only companies that pass GMP standards and obtain the certificate that is issued by Chinese government are able to manufacture medicines and related products. We have also achieved ISO9000 certification for our manufacturing processes. We received quality standard certification for acre truncatum bunge seed oil granted by the state Food and Drug Administration on February 13, 2015.
The farm operated by Shandong Spring Pharmaceutical is operated in a manner consistent with the requirements for organic certification set up by the China Organic Foods Development Center. The health products manufactured have been certified as "green" by the Chinese Ministry of Agriculture in 2006, which reflects the Company's dedication to organic agricultural methods.
Manufacturing, and Raw Material Supplies
We manufacture and package our Huoliyuan products and acer truncatum bunge seed oils products. Raw materials and packaging component, are purchased from several suppliers. Packages of the Huoliyuan products and acer truncatum bunge seed oils products, consisting of containers and packaging components, are purchased from various third-party suppliers. We design the brochures that are used by the representatives to sell our acer truncatum bunge seed oils products through our direct marketing channel.
We believe that we can continue to obtain sufficient raw materials and supplies to manufacture and produce our Huoliyuan products and acer truncatum bunge seed oils products for the foreseeable future.
Competition
We face significant competition in all of our product lines.
Acer Truncatum Bunge Seed Oil Products
While we believe that we are the largest producer of pure oil for use as a health supplement, other significant competitors include Weifang Luyuan Bio-technology Co. Ltd. and Liaoning Sanmark Co. Ltd. There are also many small producers. While we will be one of the few vertically integrated producers that also produce their own raw material once our trees begin to mature in the fall of 2018, other producers are also planting acer truncatum bunge planting bases. We also face competition from producers of other oils used as health supplements.
While we intend to enter the market for an edible oil product, Jinlongyu, Fulinmen and Luhua are the first tier brands, accounting for approximately 70% of the sales volume of small-packaged edible oil in 2017, "Shengzhou", "Jiusan"," Duoli", "Xiwang" and "Changshou Hua" are the principal second tier brands and other regional edible oil brands in the third tier. Most of the first tier brands offer several categories of edible oil, which include blended oil, rapeseed oil, rice oil, corn oil, sunflower oil, camellia oil and olive oil. Lower tier brands offer oils of lower quality.
50
Huoliyuan Capsules and Tablets
There are currently 14 pharmaceutical companies that produce Huoliyuan tablets in China. We are the only company that produces Huoliyuan capsules. We believe that we are the largest producer of Houliyuan medicine in China. During the years 2013 to 2015, the market shares of the capsule/tablet market of each of our 13 competitors ranged between 1% and 13% while our market share was 27%. The only competitors with more than a 10% market share were Jilin Yinglian Pharmaceutical Co., Ltd., and Tianjin Hezhi Pharmaceutical Co., Ltd.
We believe we achieved this success by virtue of our dosage, the advantages of selling a capsule as well as a tablet, and, efficacy and quality advantages.
Health Care Products
Our industry is very competitive and the barriers to entry are not significant. We compete with manufacturers, distributors, and retailers of nutritional products in many channels, including direct sales, specialty retail stores, wholesale stores, and the internet generally. We also compete with other public and privately owned direct sellers for distributor talent, including for example Amway, Herbalife, and Nu Skin. On both fronts, some of our competitors are significantly larger than we are, have a longer operating history, higher visibility and name recognition, and greater financial resources than we do.
Employees
Shandong Spring Pharmaceutical employed 313 individuals, each on a full-time basis, as of July 2, 2018, of which 24 employees are involved in administration; 42 are dedicated to marketing and other functions, and 29 to research and development. The remaining employees is involved in manufacturing. We believe that we have a good relationship with our employees.
Distribution Channel
Huoliyuan Capsules and Tablets
We sell our Huoliyuan products to our three major distributors, which in turn distribute the Huoliyuan products to multiple other distributors or sell the Huoliyuan products to the final point of use (e.g. hospital, pharmacy, clinic). We are required by law to sell the products through third party distributors as they are classified as a prescription medication.
Acer Truncatum Bunge Seed Oil Products, and other products from Shandong Yongchuntang
Our use of the direct sales channel enables us to build a larger customer base and increase the sales of health care products. In May 2015, we received a sublicense from Shandong Yongchuntang to sell Shandong Yongchuntang's products using the direct-sales license issued to Shandong Yongchuntang by the government. We are also permitted by Shandong Yongchuntang to sell our acer truncatum bunge seed oil products using this license.
Unlike most of our consumer packaged goods competitors, which sell their products through third-party retail establishments (e.g., drug stores and department stores), we sell our acer truncatum bunge seed oils products, and other products from Shandong Yongchuntang to the ultimate consumer through the direct-selling channel. Sales of our products are made to the ultimate consumer principally through direct selling by representatives, who are not our employees.
Our distribution channel is composed of two primary groups: our consumer group—individuals who buy the products we sell primarily for personal or family consumption; and our sales network—representatives who personally buy, use and resell products, and who also find new consumers, and recruit, train and develop new sellers. We strive to develop both our consumer group and our sales network. Our strategy for growing our consumer group is to offer high-quality, innovative products that provide demonstrable benefits. Our strategy for growing our sales network is to provide a meaningful business opportunity for those persons who demonstrate the ability to develop both a consumer group and a team of sellers, including through sales compensation and incentives.
51
Our in-house marketing staff supervises major dealers, who sub-distribute through nationwide networks, which allows us to accomplish broad geographic distribution with a marketing staff of eight people, thus keeping our overhead low. We have general dealer agreements with seven major dealers in different regions of China to control our direct sales force, covering a total of 33 provinces. The major dealers are rewarded primarily based on total sales achieved in their zones or downline team of recruited, trained and managed representatives. The primary responsibilities of the dealers are the prospecting, appointing, training and developing their downline representatives while maintaining a certain level of their own sales. Personal contacts, including recommendations from current representatives, and local market advertising constitute the primary means of obtaining new representatives.
The direct selling program gives representatives the opportunity to earn discounts on their own sales of our products, as well as commissions based on the net sales made by representatives they have recruited and trained. This program limits the number of levels on which commissions can be earned to three.
Development of the direct sales program throughout China is one part of our long-term growth strategy. Given the high rate of turnover among representatives, which is a common characteristic of direct selling, it is critical that a major dealer recruit, retain and service representatives on a continuing basis in order to maintain and grow our business.
We currently have approximately 200,000 active representatives overseen by our seven major dealers. Representatives earn by purchasing products directly from us at a discount from a published brochure price and selling them to their customers, the ultimate consumer of our products. Representatives can start their businesses for a nominal fee, or in some markets, for no fee at all. We generally have no arrangements with end users of our products beyond the representative.
A representative contacts their customers directly, selling primarily through our web-enabled systems, which highlights our products. In this sense, the representative, together with web-enabled systems, are the "store" through which our products are sold. The order is processed and the products are assembled at a distribution center and delivered to the representative through China National Post Logistic, with whom we have a strategic relationship, to deliver our products to our customers. China National Post Logistic is a subsidiary of China Post.
We employ certain web-enabled systems to increase representative support, which allow a representative to run her or his business efficiently and also allow us to improve our order-processing accuracy. Representatives can use the Internet to manage their business electronically, including order submission, order tracking, payment and communications with us.
Seasonality.
Although we are not significantly affected by seasonality, the fourth quarter is our peak season for sales of acer truncatum bunge based products because of purchases related to the Chinese New Year holiday season that effectively lasts more than two weeks. Gift giving during the holiday season is customary.
REGULATION
Our business is subject to various laws and regulations, particularly with respect to our direct selling business models and our product categories. In addition, as a United States entity operating through subsidiaries in foreign jurisdictions, we are subject to foreign exchange control that regulates the flow of funds between us and our subsidiary. If we were to sell our products overseas, we may also be subject to customs duties and other regulations of the importing country.
52
Direct Selling Regulations
Direct selling is regulated by national, provincial and local government agencies in China. These laws and regulations are generally intended to prevent fraudulent or deceptive schemes, including "pyramid" schemes, which compensate participants primarily for recruiting additional participants without significant emphasis on product sales to consumers.
The regulatory environment in China is particularly complex and continues to evolve. China's direct selling and anti-pyramiding regulations contain various restrictions, including a prohibition on the payment of multi-level compensation. The regulations are subject to discretionary interpretation by provincial and local level regulators as well as local customs and practices.
Regulators continue to act cautiously as they monitor the development of direct selling in China. As of July 2018, Shandong Yongchuntang has obtained direct selling licenses in six provinces and municipalities in China all of which we have the exclusive right to use under our supply agreement with Shandong Yongchuntang. In order to expand our direct selling model into additional provinces, Shandong Yongchuntang currently must obtain a series of approvals from the local Department of Commerce in such provinces, the Sishui Municipal Commission of Commerce (our supervisory authority), as well as the State Ministry of Commerce ("MOFCOM"), which is the national governmental authority overseeing direct selling. In the course of obtaining these approvals, the respective authorities under MOFCOM must also consult and seek opinions on our business operations from the Ministry of Public Security and the Administration for Industry and Commerce at both provincial and State levels.
China impose limits on the amount of commissions we can pay to our representatives. For example, under regulations in China, direct selling companies may pay independent direct sellers in China up to a maximum 30% of the revenue they generate through their own sales of products to consumers.
Food Regulations
The manufacture of edible oils in China requires government approval. As we expand our line of products based on acer truncatum bunge seed oil to include edible oils, on January 11, 2018, we were issued a Food Production License for production of edible vegetable oil, which includes acer truncatum bunge seed oil, and related blended edible oil products. The license was granted by the Food and Drug Administration of Sishui County and is valid for five years.
Regulations Relating to Pharmaceutical Industry
The Huoliyuan capsule is classified as a medicine in China that can only be purchased by prescription. As a result, we are subject to regulations applicable to the pharmaceutical industry. The pharmaceutical industry in China, including the traditional Chinese medicine sector, is highly regulated. The primary regulatory authority is the SFDA, including its provincial and local branches. As a developer, producer and distributor of medicinal products, we are subject to regulation and oversight by the SFDA and its provincial and local branches. The law of the PRC on the Administration of Pharmaceuticals provides the basic legal framework for the administration of the production and sale of pharmaceuticals in China and covers the manufacturing, distributing, packaging, pricing and advertising of pharmaceutical products. Its implementing regulations set forth detailed rules with respect to the administration of pharmaceuticals in China. We are also subject to other PRC laws and regulations that are applicable to business operators, manufacturers and distributors in general.
Registration and Approval of Medicine
A medicine must be registered and approved by the SFDA before it can be manufactured. The registration and approval process requires the manufacturer to submit to the SFDA a registration application containing detailed information concerning the efficacy and quality of the medicine and the manufacturing process and the production facilities the manufacturer expects to use. This process generally takes at least a few months and could be longer, depending on the nature of the medicine under review, the quality of the data provided and the workload of the SFDA. To obtain the SFDA registration and approval necessary for commencing production, the manufacturer is also required to conduct pre-clinical trials, apply to the SFDA for permission to conduct clinical trials, and, after clinical trials are completed, file clinical data with the SFDA for approval.
53
New Medicine. If a medicine is approved by the SFDA as a new medicine, the SFDA will issue a new medicine certificate to the manufacturer and impose a monitoring period of as long as five years. The length of the monitoring period is specified in the new medicine certificate. During the monitoring period, the SFDA will monitor the safety of the new medicine, and will neither accept new medicine certificate applications for an identical medicine by another pharmaceutical company, nor approve the production or import of an identical medicine by other pharmaceutical companies. For new medicines approved prior to 2002, the monitoring period could be longer than five years. As a result of these regulations, the holder of a new medicine certificate effectively has the exclusive right to manufacture the new medicine during the monitoring period. However, even though our new medicine certificate for Huoliyuan capsules has expired, as we are in the transitional period described below, we continue to have the exclusive right to produce the Huoliyuan capsules.
Provisional National Production Standard. In connection with the SFDA's approval of a new medicine, the SFDA will normally direct the manufacturer to produce the medicine according to a provisional national production standard, or a provisional standard. A provisional standard is valid for two years, during which the SFDA closely monitors the production process and quality consistency of the medicine to develop a national final production standard for the medicine, or a final standard. Three months before the expiration of the two-year period, the manufacturer is required to apply to the SFDA to convert the provisional standard to a final standard. Upon approval, the SFDA will publish the final standard for the production of this medicine. There is no statutory timeline for the SFDA to complete its review and grant approval for the conversion. In practice, the approval for conversion to a final standard is time-consuming and could take a few years. However, during the SFDA's review period, the manufacturer may continue to produce the medicine according to the provisional standard.
Transitional Period. Prior to the latter of (1) the expiration of a new medicine's monitoring period or (2) the date when the SFDA grants a final standard for a new medicine after the expiration of the provisional standard, the SFDA will not accept applications for an identical medicine nor will it approve the production of an identical medicine by other pharmaceutical companies. Accordingly, the manufacturer will continue to have an exclusive production right for the new medicine during this transitional period.
For example, although the new medicine certificates for Huoliyuan capsules have already expired, we continue to have the exclusive right to produce and sell Huoliyuan capsules until the SFDA approves the final standards for this medicine, the timing of which is uncertain.
Continuing SFDA Regulation
Pharmaceutical manufacturers in China are subject to continuing regulation by the SFDA. If an approved medicine, its labeling or its manufacturing process is significantly modified, a new pre-market approval or pre-market approval supplement will be required by the SFDA. A pharmaceutical manufacturer is subject to periodic inspection and safety monitoring by the SFDA to determine compliance with regulatory requirements. The SFDA has a variety of enforcement actions available to enforce its regulations and rules, including fines and injunctions, recall or seizure of products, the imposition of operating restrictions, partial suspension or complete shutdown of production and criminal prosecution.
Pharmaceutical Product Manufacturing
Permits and Licenses for Pharmaceutical Manufacturers. A pharmaceutical manufacturer must obtain a pharmaceutical manufacturing permit from the SFDA's relevant provincial branch. This permit is valid for five years and is renewable upon its expiration. Our current pharmaceutical manufacturing permit, issued by the SFDA, will expire on December 31, 2020. We do not currently anticipate any difficulty renewing our pharmaceutical manufacturing permit.
Good Manufacturing Practice. A pharmaceutical manufacturer must meet the Good Manufacturing Practice standards, or GMP standards, for each of its production facilities in China in respect of each form of pharmaceutical products it produces. GMP standards include staff qualifications, production premises and facilities, equipment, raw materials, environmental hygiene, production management, quality control and customer complaint administration. If a manufacturer meets the GMP standards, the SFDA will issue to the manufacturer a Good Manufacturing Practice certificate, or a GMP certificate, with a five-year validity period. However, for a newly established pharmaceutical manufacturer that meets the GMP standards, the SFDA will issue a GMP certificate with only a one-year validity period.
We have obtained a GMP certificate for all of our production facilities for the Huoliyuan capsules. On June 10, 2008, we obtained a GMP certificate for our facility for the manufacturing of medicine in the forms of tablets and capsules. The certificate was renewed in 2013 and will expire on November 27, 2018. We do not anticipate any difficulty in renewing these certificates.
54
Price Controls
The retail prices of prescription and OTC medicines that are included in the national medicine catalog are subject to price controls administered by the Price Control Office under the National Development and Reform Commission, or the NDRC, and provincial price control authorities, either in the form of fixed prices or price ceilings. The controls over the retail price of a medicine effectively set the limits for the wholesale price of that medicine. From time to time, the NDRC publishes and updates a national list of medicines that are subject to price control. Fixed prices and price ceilings on medicines are determined based on profit margins that the NDRC deems reasonable, the type and quality of the medicine, its production costs, the prices of substitute medicines and the extent of the manufacturer's compliance with the applicable GMP standards. The NDRC directly regulates the price of some of the medicines on the list, and delegates the power to provincial price control authorities to regulate the remainder on the list. For those medicines under the authority of provincial price control authorities, each provincial price control authority regulates medicines manufactured by manufacturers registered in that province. Provincial price control authorities have the discretion to authorize price adjustments based on the local conditions and the level of local economic development.
Only the manufacturer of a medicine may apply for an increase in the retail price of the medicine and it must apply either to the NDRC, if the price of the medicine is nationally regulated, or to the provincial price control authorities in the province where it is registered, if the price of the medicine is provincially regulated. For a provincially regulated medicine, when provincial price control authorities approve an application, they will file the new approved price with the NDRC for confirmation and thereafter the newly approved price will become binding and enforceable across China.
The retail prices of our Huoliyuan capsules fall under the direct regulation of the Shandong provincial price control authorities. We have discretion to determine the retail prices of the nine products we distribute for Shandong Yongchuntang as well as our acer truncatum bunge seed oil products.
Reimbursement under the National Medical Insurance Program
Accrding to the National Health and Family Planning Commission of China, as of the end of 2017, over 1.35 billion people were enrolled into the National Medical Insurance Program. The Ministry of Labor and Social Security, together with other government authorities, determines which medicines are to be included in or removed from the national medicine catalog for the National Medical Insurance Program, and under which tier a medicine should fall, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are based on a number of factors, including price and efficacy. A National Medical Insurance Program participant can be reimbursed for the full cost of a Tier 1 medicine and 80-90% of the cost of a Tier 2 medicine. Although it is designated as a national program, the implementation of the National Medical Insurance Program is delegated to various provincial governments, each of which has established its own medicine catalog. A provincial government must include all Tier 1 medicines listed in the national medicine catalog in its provincial medicine catalog, but may use its discretion based on its own selection criteria to add other medicines to, or exclude Tier 2 medicines listed in the national medicine catalog from, its provincial medicine catalog, so long as the combined numbers of the medicines added and excluded do not exceed 15% of the number of the Tier 2 medicines listed in the national catalog. In addition, provincial governments may use their discretion to upgrade a nationally classified Tier 2 medicine to Tier 1 in their provincial medicine catalogs, but may not downgrade a nationally classified Tier 1 medicine to Tier 2. Huoliyuan capsules are in the provincial medicine catalogs of 30 provinces.
The total amount of reimbursement for the cost of prescription and OTC medicines, in addition to other medical expenses, for an individual program participant in a calendar year is capped at the amount in that participant's individual account. The amount in a participant's account varies, depending upon the amount of contributions from the participant and his or her employer. Generally, on average, program participants who are from relatively wealthier eastern parts of China and relatively wealthier metropolitan centers have greater amounts in their individual accounts than those from less developed provinces.
55
Other Regulations
In addition to the regulations relating to pharmaceutical industry in China, our operating subsidiary is also subject to the regulations applicable to a foreign invested enterprise in China.
Foreign Currency Exchange
Pursuant to the Foreign Currency Administration Rules promulgated in 1996 and amended in 1997 and various regulations issued by State Administration of Foreign Exchange, or the SAFE, and other relevant PRC government authorities, the Renminbi is freely convertible only to the extent of current account items, such as trade-related receipts and payments, interest and dividends. Capital account items, such as direct equity investments, loans and repatriation of investment, require the prior approval from the SAFE or its local counterpart for conversion of Renminbi into a foreign currency, such as U.S. dollars, and remittance of the foreign currency outside the PRC.
Payments for transactions that take place within the PRC must be made in Renminbi. Unless otherwise approved, PRC companies other than foreign investment vehicles must convert foreign currency payments they receive from abroad into Renminbi. On the other hand, foreign investment vehicles may retain foreign exchange in accounts with designated foreign exchange banks, subject to a cap set by the SAFE or its local counterpart.
Foreign Exchange Registration of Offshore Investment by PRC Residents
Pursuant to SAFE's Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents to Engage in Financing and Inbound Investment Through Overseas Special Purpose Vehicles, or SAFE Circular No. 75, issued on October 21, 2005, (1) a PRC resident must register with a local branch of the SAFE before he or she establishes or controls an overseas special purpose vehicle, or SPV, for the purpose of overseas equity financing (including convertible debt financing); (2) when a PRC resident contributes the assets of or his or her equity interests in a domestic enterprise into an SPV or engages in overseas financing after contributing assets or equity interests into an SPV, such PRC resident must register his or her interest in the SPV and the change thereof with the local branch of the SAFE; and (3) when the SPV undergoes a material change outside of China, such as a change in share capital, a merger or an acquisition, the PRC resident must, within 30 days from the occurrence of the event that triggers such change, register such change with a local branch of the SAFE. PRC residents who are stockholders of SPVs established before November 1, 2005 were required to register with a local branch of the SAFE before March 31, 2006.
Under SAFE Circular No. 75, failure to comply with the registration procedures set forth above may result in penalties, including imposition of restrictions on a PRC subsidiary's foreign exchange activities and its ability to distribute dividends to the SPV.
Our principal beneficial owners who are PRC residents have registered with the local branch of the SAFE as required under SAFE Circular No. 75.
Regulation on Overseas Listing
On August 8, 2006, six PRC regulatory agencies; the Ministry of Commerce, the State-owned Assets Supervision and Administration Commission, or SASAC, the State Administration for Taxation, the State Administration for Industry and Commerce, the CSRC, and the SAFE, jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rule, which became effective on September 8, 2006. This New M&A Rule requires offshore SPVs that are controlled by PRC companies or individuals and that have been formed for the purposes of seeking a public listing on a stock exchange outside China through acquisitions of PRC domestic companies to obtain the CSRC approval prior to publicly listing their securities on a stock exchange outside China. On September 21, 2006, the CSRC published a notice on its website specifying the documents and materials required to be submitted to the CSRC when seeking the CSRC approval for their listings outside of China.
The interpretation and application of the M&A Rule remain unclear, and we cannot assure you that this offering does not require approval from the CSRC, and if it does, how long it will take us to obtain the approval. See "Risk Factors—Risks Related to Doing Business in China—The approval of the China Securities Regulatory Commission, or the CSRC, may be required in connection with this offering under a recently adopted PRC regulation."
56
Dividend Distribution
The principal regulations governing dividend distributions by wholly foreign-owned enterprises and Sino-foreign equity joint ventures include:
|
Ÿ
|
Wholly Foreign-Owned Enterprise Law (1986), as amended;
|
|
Ÿ
|
Wholly Foreign-Owned Enterprise Law Implementing Rules (1990), as amended;
|
|
Ÿ
|
Sino-foreign Equity Joint Venture Enterprise Law (1979), as amended; and
|
|
Ÿ
|
Sino-foreign Equity Joint Venture Enterprise Law Implementing Rules (1983), as amended.
|
Under these regulations, wholly foreign-owned enterprises and Sino-foreign equity joint ventures in the PRC may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. Additionally, these foreign-invested enterprises are required to set aside certain amounts of their accumulated profits each year, if any, to fund certain reserve funds. These reserves are not distributable as cash dividends.
Patents
There are three types of patents under the PRC patent law. The first type, an external design patent, refers to a new design of a product's shape, pattern or a combination of shape and pattern and the combination of a product's color and its shape and pattern, where such a design is aesthetically appealing and suitable for industrial application. The second type of patent is called an invention patent and the third type of patent is referred to as a new model or utility patent. Invention patents and utility patents are similar in that both of them relate to scientific or technological inventions. A utility patent, compared to an invention patent, requires a lower level of creativity and covers a narrower scope. In addition, an invention patent can be a new technology introduced in respect of an existing product, method or their improvements, while a utility patent is restricted to a product's shape, constitutions or a combination of these two. Invention patents are valid for 20 years, whereas utility patents and external design patents are each valid for 10 years.
LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. Litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
DESCRIPTION OF PROPERTY
Office, Manufacturing and Research Facility.
Shandong Spring Pharmaceutical operates on a property of approximately 56,894 square meters (approximately 14.06 acres) in Shandong Province to which it has a land use right. Besides housing our executive offices, the property is home to a manufacturing facility measuring 17,200 square meters (approximately 185,139.25 square feet) and a research facility measuring 3,000 square meters (approximately 32,291.73 square feet).
57
We completed a new manufacturing facility during the fiscal year ended March 31, 2012, which is mainly used to facilitate the full production of Huoliyuan capsules and the acer truncatum bunge seed oil products. Because we have installed two more packing machines, the annual production capacity of the Huoliyuan capsule manufacturing factory is 15 million boxes per year. Our factory includes a powder injection production line and cleaning and purifying equipment. The factory also includes a pin powder workshop, and a solid manufacturing workshop. Huoliyuan capsules and tablets are manufactured in a dedicated workshop, and the second workshop is used as our research facility. We have no immediate plan to build more workshops or factories for the Huoliyuan capsules. We plan to increase production capacity for the acer truncatum bunge seed oil with the proceeds of this offering.
Acer Truncatum Bunge Plantings.
Beginning June 2013, we started development of our own acer truncatum bunge planting bases. As of March 31, 2018, we had three separate lease agreements for the leasing of a total of 5,880 mu (approximately 2,324.77 acres) of farmland for terms of either 30 years or 14 years. As of March 31, 2018, we had completed planting of approximately seven million trees with production expected to begin in the fall of the calendar year 2018.
Pursuant to a Farmland Leasing Agreement with Shiqiao Village executed on June 20, 2013, as amended, we lease 1880 mu (approximately 743.29 acres) of farmland. The lease term is from July 1, 2013 to June 30, 2043. The lease payment is about RMB1,000 (approximately $159) per mu annually and payable for five years of rents in advance.
On March 1, 2014, we entered into a second Farmland Leasing Agreement with Zhongce No.4 Village for the lease of 200 mu (approximately 79.07 acres) of farmland. The lease was terminated by the local government in 2017 for building a new urban district. We received a refund and other compensation from the government of approximately $420,000 and sold our plants from the farmland for approximately $2 million. We do not believe that there is a material risk that the leases other than the one with Zhongce No.4 Village could be terminated because the parcel subject to the terminated lease is located in a relatively urban area, while our other land is located in rural areas.
On January 7, 2015, we entered into a third Farmland Leasing Agreement with Shandong Wanziyuan Tourism Development Co. to lease 2,000 mu (approximately 790.74 acres) of farmland. The lease term is from January 15, 2015 to December 31, 2029. The lease payment is RMB1,000 (approximately $159) per mu annually and is paid every five years in advance.
On July 2, 2015, we entered into a fourth Farmland Leasing Agreement with a Zhongce Shen Village for the lease of 2,000 mu (approximately 790.74 acres) farmland. The lease term is from July 2, 2015 to July 2, 2029. The lease payment is RMB1, 000 (approximately $159) per mu annually and is paid every five years in advance.
MANAGEMENT
Our directors, executive officers and key employees are listed below. The number of directors is determined by our Board of Directors. All directors hold office until the next annual meeting of the Board or until their successors have been duly elected and qualified. Officers are elected by the Board of Directors and their terms of office are, except to the extent governed by employment contract, at the discretion of the Board of Directors.
58
|
Name
|
|
Age
|
|
Position with the Company
|
|
Tinghe Yan
|
|
62
|
|
Chairman, Chief Executive Officer
|
|
Chuanmin Li
|
|
52
|
|
Chief Financial Officer
|
|
Jirui Zhang
|
|
61
|
|
Director
|
|
Dong Li
|
|
29
|
|
Independent Director
|
|
Robert J. Fanella
|
|
66
|
|
Independent Director
|
|
Wengao Zhang
|
|
72
|
|
Independent Director
|
|
Maogang Sun
|
|
47
|
|
General Manager of Shandong Spring Pharmaceutical
|
|
Xuzhong Ding
|
|
45
|
|
Chief Marketing Officer
|
|
Qiang Zhang
|
|
45
|
|
Chief Administration Officer
|
|
Zecheng Shao
|
|
44
|
|
Vice President
|
Directors and Executive Officers
Tinghe Yan. Mr. Yan has served as our Chairman and Chief Executive Officer since 2007. Mr. Yan has over twenty years of experience in corporate management within the food and food supplements industries. In 2005, he founded Shandong Spring Pharmaceuticals and has served as its Chairman since January 2006. During the eight years prior to founding Shandong Spring Pharmaceutical, Mr. Yan was employed as the Chairman and General Manager of Shandong Yongchuntang, which manufactures a wide variety of food supplements and is currently the exclusive supplier of certain products for Shandong Spring Pharmaceutical. During the period from 1988 to 1997, Mr. Yan served as Executive Vice President of Shishui Sanyin Company, and from 1985 to 1987 as Factory Director of Beijing Shishui Lianhe Preserved Fruits, both of which were multi-facility enterprises in the food industry. The Board nominated Mr. Yan to serve as a director because of his current and prior senior executive and management experience at our company and his significant industry experience, including having founded our company.
Chuanmin Li. Mr. Li has been our Chief Financial Officer since 2005 and has been involved in corporate financial management and accounting for over 29 years. Since 2005, he has been employed as Chief Financial Officer of the Company's subsidiary, Shandong Spring Pharmaceutical Co., Ltd. From 2000 to 2005, Mr. Li was employed as Chief Financial Officer of Shandong Yongchuntang. From 1998 to 2000, Mr. Li was a teacher at the Shandong Finance Institute. From 1990 to 1998, he was employed by an accounting firm in Jining City. In 1986, Mr. Li received a diploma from the Shandong Finance Institute.
Jirui Zhang. Mr. Zhang brings over twenty years of technical training to Shandong Spring Pharmaceutical, where he has served as a as Director since January 2006. During 2005, Mr. Zhang was the Manager of the International Market Department for Shandong Yongchuntang, which manufactures a wide variety of food supplements and is currently the exclusive supplier of certain products for Shandong Spring Pharmaceutical. During the 22 years prior to joining Shandong Yongchuntang, Mr. Zhang was employed as an instructor in the Shandong Chemical Engineering Vocational School. The Board nominated Mr. Zhang to serve as a director because of his significant prior management experience at Shandong Yongchuntang.
Dong Li. Mr. Li has served on our Board of Directors since April 1, 2017. As a Director, Mr. Li focuses particularly on the Company's research and development. Previously, Mr. Li successively served as a technician, the director of research and development, the technical director, and deputy general manager at Shandong Yongchuntang from November 2011 to March 2017, where he was in charge of the research, development and design of the products, network platform development, and technical service, logistics center, research and experimental center, and technical quality center. Mr. Li served as the server director at Shenzhen Sunshine Technology Co., Ltd. from September 2009 to September 2011, where he was responsible for the operation and management of the server. Mr. Li received his bachelor degree from Wuhan University of Science and Technology in Wuhan, China. The Board nominated Mr. Li to serve as a director because of his prior leadership and management experience and his significant prior experience in the area of information technology.
59
Robert J. Fanella. Mr. Fanella, a Certified Public Accountant, was appointed as an independent director, effective April 6, 2009. During Mr. Fanella's more than 36 years career specializing in corporate finance and accounting, he was responsible for audit and financial service oversight for both private and publicly traded companies. Since 2006, Mr. Fanella has been an independent financial consultant, working on various financial and operational projects for companies in industries such as electronic manufacturing, industrial plating, chemical, and health products. From April 2011 to March 2012, Mr. Fanella has served as CFO for ARCIS Resources Corporation (OTCBB: ARCS). From 2002 to 2006, Mr. Fanella was employed as CFO/Owner of Tru-Way, Inc., a metal fabrication business mainly serving the electronics manufacturing industry. The business was sold in 2006. From 1984 to 2002, Mr. Fanella was employed as CFO by MicroEnergy, Inc, a public company of which he was co-founder. MicroEnergy, Inc was a manufacturing firm designing and selling custom switch-mode power supplies to major companies in the OEM electronics market. During the 12-year period prior to founding MicroEnergy, Inc., Mr. Fanella was the CFO/Controller for two smaller businesses in the electronics manufacturing business and welding supplies distribution business, and he spent seven years at Motorola, Inc., in various capacities from financial analyst to business controller. Mr. Fanella currently serves on the Board of Directors and also is Audit Committee Chairman for American Nano Silicon Technologies, Inc. (OTCBB: ANNO). Mr. Fanella was awarded a Bachelor of Science Degree in Finance by Northern Illinois University in 1972. He was awarded a Masters of Business Administration Degree in Finance with a Marketing concentration from the University of Chicago in 1979. In 1975, Mr. Fanella is registered as a certified public accountant in Illinois. Mr. Fenella was selected to serve on our Board of Directors and chair our Audit Committee for his extensive financial, analytical and audit experience.
Wengao Zhang. Professor Zhang was appointed as an independent director of China YCT International Group on April 6, 2009. Professor Zhang has over 30 years of experience in the pharmaceutical, Chinese traditional medicine and diagnostic industries. Since 1978, Professor Zhang has been a full time professor of Shandong University of Traditional Chinese Medicine, specializing in clinical treatment via both Chinese and western methods. From 1985 to 1998, Professor Zhang was a dean of the research and development department of Shandong University of Traditional Chinese Medicine. Professor Zhang has received various awards within the clinical medicine field, including awards for combining western clinical treatment with traditional Chinese medicine methods, and the 20th Geneva Invention Silver Award. Professor Zhang has published more than 200 papers concerning Chinese traditional medicine in clinical treatment. Among his other engagements, Mr. Zhang is an associate commissioner of the International Chinese Medicine Association and director of the International Chinese Medicine Association Cardiovascular Committee. In 1968, Mr. Zhang received his bachelor degree majoring in Pharmacy from Shandong University of Traditional Chinese Medicine. Mr. Zhang brings valuable expertise of pharmaceutical, Chinese traditional medicine and diagnostic to the Board of Directors.
Key Employees
Maogang Sun. Mr. Sun has served as our general manager, deputy general manager, director of pharmaceutical production, quality management, marketing, for Shandong Spring Pharmaceutical Co., Ltd. since 2006. Mr. Sun has been engaged in drug manufacturing for 20 years. From 1995 to 2000, he worked in the Sishui pharmaceutical factory in Shandong province. From 2001 to 2007, he worked in the department of pharmaceutical preparation in a hospital for the Chinese People's Liberation Army. Mr. Sun graduated from the Shandong Province County Peoples College, where he majored in Chinese Traditional Medicine and engineering.
Qiang Zhang. Mr. Zhang has served for Shandong Spring Pharmaceutical Co., Ltd., as its Chief Administrative Officer and Head of Human Resources since 2009. He has been involved with Human resource management for over 10 years. From October 2003 to December 2008, he was employed by Shandong Huajin Group, Inc. as the Head of Administration. Mr. Zhang graduated from Shandong Economies College and is pursuing his Senior HR Manager Certificate.
Xuzhong Ding. Mr. Ding has been serving for Shandong Spring Pharmaceutical Co., Ltd., as its Chief Marketing Officer since 2008. He joined us in 2003 and was involved in our marketing development since then. Mr. Ding has over 20 years of experience in marketing, since he graduated from Shandong University in 1991, majoring in marketing.
60
Zecheng Shao. Mr. Shao has been involved with capital market for over 10 years. Since 2007 he has been employed as vice president of the Company's subsidiary, Shandong Spring Pharmaceutical Co., Ltd. From 1997 to 2007, Mr. Shao was employed as Minister of Korean Daewoo Group. During that period, he successfully managed, designed and programmed 2 ERP projects and cooperated with the other departments of the company in the past years. All the projects were released on schedule, with high quality that helped the company's business growth. From 1994 to 1997, Mr. Shao was a computer engineer at the Shandong Huajin Group. In 1994, Mr. Shao received a diploma from the Shandong Teachers' University. In 2000, he received the Super Development Engineer Certificate for PoweBuilder in Sybase Center.
Involvement in Certain Legal Proceedings
None of our directors, executive officers, or control persons has been involved in any of the following events during the past ten years:
|
●
|
been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
|
●
|
had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time;
|
|
●
|
been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, by any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity;
|
|
●
|
been found by a court of competent jurisdiction in a civil action or by the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
|
|
●
|
been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
|
|
●
|
been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
Role of the Board of Directors in Risk Oversight
The Board of Directors is responsible for assessing the risks facing our Company and considers risk in every business decision and as part of our business strategy. The Board of Directors recognizes that it is neither possible nor prudent to eliminate all risk, and that strategic and appropriate risk-taking is essential for us to compete in our industry and in the global market and to achieve our growth and profitability objectives. Effective risk oversight, therefore, is an important priority of the Board of Directors.
61
While the Board of Directors oversees our risk management, management is responsible for day-to-day risk management processes. Our Board of Directors expects management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies that are adopted by the Board of Directors. The Board of Directors expects to review and adjust our risk management strategies at regular intervals following the completion of the offering, or as needed.
Director Independence
We are not listed on a national securities exchange; however, we have elected to use the definition of independence under the Nasdaq listing requirements in determining the independence of our directors and nominees for director.
Under the listing requirements and rules of The Nasdaq Stock Market LLC, or Nasdaq, independent directors must comprise a majority of our board of directors as a listed company within one year of the listing date. Our Board of Directors has undertaken a review of the independence of each director, which included a review of each director and director nominee's responses to questionnaires inquiring about any relationships with us. This review was designed to identify and evaluate any transactions or relationships between a director, or director nominee or any member of his or her immediate family and us, or members of our senior management or other members of our Board, and all relevant facts and circumstances regarding any such transactions or relationships. Based on its review, our Board of Directors has determined that Dong Li, Robert J. Fanella, and Wengao Zhang are independent directors, as defined by Rule 5605(a)(2) of the Nasdaq Listing Rules.
To the extent required by the trading market on which our shares are listed, we will ensure that the overall composition of our Board complies with the Sarbanes-Oxley Act, and the rules thereunder, and the listing requirements of the trading market, including the requirement that one member of the Board qualifies as a "financial expert."
Board Leadership Structure
Mr. Tinghe Yan holds the positions of Chief Executive Officer and Chairman of the Board of the Company. The board believes that Mr. Yan's services as both Chief Executive Officer and Chairman of the Board is in best interest and the best interest of our shareholders. Mr. Yan possesses detailed and in-depth knowledge of the issues, opportunities and challenges facing our company in our business and is thus best positioned to develop agendas that ensure that the Board's time and attention are focused on the most critical matters relating to our business. His combined role enables decisive leadership, ensures clear accountability, and enhances our ability to communicate our message and strategy clearly and consistently to our shareholders, employees and customers.
Our Board of Directors has not designated a lead director. Given the limited number of directors comprising the board, the independent directors call and plan their executive sessions collaboratively and, between meetings of the Board of Directors, communicate with management and one another directly. Under these circumstances, the directors believe designating a lead director to take on responsibility for functions in which they all currently participate might detract from rather than enhance performance of their responsibilities as directors.
Nominating, Compensation and Audit Committees
We have three standing committees of the Board, each of which is described below.
The Audit Committee consists of Robert J. Fanella, Wengao Zhang, and Dong Li. Mr. Fanella serves as the chairman of the Audit Committee. The Board has determined that each of the members of the Audit Committee satisfies the independence requirements of the NASDAQ Stock Market. The Audit Committee oversees our accounting and financial reporting processes and procedures, reviews the scope and procedures of the internal audit function, appoints our independent registered public accounting firm and is responsible for the oversight of its work and the review of the results of its independent audits.
62
The Board of Directors has determined that Robert J. Fanella, who serves as Chairman of the Audit Committee, is an audit committee financial expert by reason of his experience in corporate finance. Mr. Fanella is an independent director, within the definition of that term applicable to issuers listed on the NASDAQ Stock Market.
The Compensation Committee consists of Robert J. Fanella, Wengao Zhang, and Dong Li. Mr. Zhang serves as chairman of the Compensation Committee. The Board has determined that each of the members of the Compensation Committee satisfies the independence requirements of the NASDAQ Stock Market. The Compensation Committee oversees the Company's policies regarding compensation and benefits, evaluates the performance of the Company's executive officers, reviews and approves the compensation of the Company's executive officers, and sets the compensation for members of the Board of Directors.
The Nominating and Corporate Governance Committee consists of Robert J. Fanella, Wengao Zhang, and Dong Li. The Board has determined that each of the members of the Nominating and Corporate Governance Committee satisfies the independence requirements of the NASDAQ Stock Market. The Nominating and Corporate Governance Committee makes recommendations to the Board regarding nominees to be submitted to our stockholders for election at each annual meeting of stockholders, selects candidates for consideration by the full Board to fill any vacancies on the Board, and oversees all of our corporate governance matters.
Code of Ethics
The Board of Directors adopted a code of ethics applicable to the Company's executive officers in 2009 to ensure that our business is conducted in a consistently legal and ethical manner. Our policies and procedures cover all major areas of professional conduct, including employee policies, conflicts of interest, protection of confidential information, and compliance with applicable laws and regulations. The Code of Ethics is available at our website at www.yctgroup.com. The reference to our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus. We intend to disclose future amendments to certain provisions of our code of conduct, or waivers of these provisions, on our website or in public filings.
The following is a summary of the key points of the Code of Ethics we adopted:
|
●
|
Honest and ethical conduct, including ethical handling of actual or apparent conflicts of interest between personal and professional relationships;
|
|
●
|
Full, fair, accurate, timely, and understandable disclosure reports and documents that a small business issuer files with, or submits to, the Commission and in other public communications made by our Company;
|
|
●
|
Full compliance with applicable government laws, rules and regulations;
|
|
●
|
The prompt internal reporting of violations of the Code to an appropriate person or persons identified in the code; and
|
|
●
|
Accountability for adherence to the Code.
|
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation Committee will or was at any time been an officer or employee of the Company. None of our executive officers serve or in the past fiscal year has served as a member of the Board of Directors or Compensation Committee of any other entity that has one or more executive officers serving as a member of our Board of Directors or Compensation Committee.
63
EXECUTIVE COMPENSATION
SUMMARY COMPENSATION TABLE
The following table sets forth the compensation paid by us for the fiscal years ended March 31, 2017, and March 31, 2018 to our principal executive officers. This information includes the dollar value of base salaries, bonus awards and number of stock options granted, and certain other compensation, if any. The compensation discussed addresses all compensation awarded to, earned by, or paid to our named executive officers. There were no executive officers whose total salary and bonus for the fiscal year ended March 31, 2018 exceeded $100,000.
|
Name and Principal
|
Salary
|
Bonus
|
Stock Awards
|
Option Awards
|
All Other
Compensation
|
Total
|
|||||||||||||||||||
|
Position
|
Year
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Tinghe Yan (1)
|
2018
|
45,280
|
45,280
|
||||||||||||||||||||||
|
Chairman, CEO
|
2017
|
44,585
|
-
|
-
|
-
|
-
|
44,585
|
||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Chuanmin Li (2)
|
2018
|
30,187
|
30,187
|
||||||||||||||||||||||
|
CFO
|
2017
|
29,723
|
-
|
-
|
-
|
-
|
29,723
|
||||||||||||||||||
Benefit Plans
We have no retirement, pension, or profit sharing programs for the benefit of directors, officers or other employees. However, our officers and directors may recommend adoption of one or more such programs in the future. We do not sponsor any qualified or non-qualified pension benefit plans, nor do we maintain any non-qualified defined contribution or deferred compensation plans.
2018 Stock Incentive Plan
In June 2018, the Board of Directors adopted a stock incentive plan named China YCT International Group, Inc. 2018 Stock Incentive Plan (the "Plan").
The Plan is intended to benefit our stockholders by providing a means to attract, retain and reward individuals who can and do contribute to the longer-term financial success of our company. Further, the recipients of stock-based awards under the Plan should identify their success with that of the company's stockholders and therefore will be encouraged to increase their proprietary interest in our company. Our Compensation Committee (the "Committee") administers the Plan.
The Plan provides for the granting of up to 3,000,000 restricted shares, non-qualified stock options, incentive stock options (within the meaning of Section 422 of the Code), stock appreciation rights ("SARs"), stock award and stock unit awards, performance shares and other cash or share-based awards. In the event of any merger, reorganization, recapitalization, stock split, stock dividend, or other change in corporate structure that affects our common stock, an adjustment may be made to the (a) maximum number of shares available for grants under the plan and/or kind of shares that may be delivered under the plan, (b) the individual award limits under the plan and (c) number, kind and/or price of shares subject to outstanding awards granted under the plan, by the Committee or the Board of Directors, to prevent dilution or enlargement of rights. Shares of stock covered by an award under the plan that is cancelled, expired, forfeited or settled in cash will again be available for issuance in connection with future grants of awards under the Plan.
64
The Committee or Board of Directors has broad authority to administer the plan, including the authority to determine when and to whom awards will be made, determine the type and size of awards, determine the terms and conditions of awards, construe and interpret the plan and award agreements, establish rates and resolutions for the plan's administration, and amend outstanding awards. Generally, the plan is open to directors, employees and consultants who are selected by the Committee or Board of Directors.
The Plan may be amended, suspended or terminated at any time by our Board of Directors, provided that suspension or termination shall not of itself impair any outstanding award granted under the plan or the applicable participant's rights regarding such award.
We had not made any awards under the Plan. In the future, we may adopt and establish an equity incentive plan pursuant to which awards may be granted if the Committee determines that it is in the best interests of our stockholders and our company to do so.
Director and Officer Liability Insurance
We intend to purchase director and officer liability insurance that provides financial protection for our directors and officers in the event that they are sued in connection with the performance of their services and also provides employment practices liability coverage, which insures for harassment and discrimination suits.
Employment Agreements
On December 10, 2013, Shandong Spring Pharmaceutical entered into an agreement with Mr. Tinghe Yan to serve as the Chief Executive Officer. Pursuant to the terms of the agreement, Mr. Yan's current annual base salary is RMB 300,000 (approximately $45,280). We also reimburse Mr. Yan for all reasonable out-of-pocket expenses incurred in the performance of his duties. The term of his employment ends on December 31, 2018. The agreement can be assigned and transferred upon a change of control of our Company.
On June 15, 2013, Shandong Spring Pharmaceutical entered into an agreement with Mr. Chuanmin Li to serve as the Chief Financial Officer. Pursuant to the terms of the agreement, Mr. Li's current annual base salary is RMB 200,000 (approximately $30,187). We also reimburse Mr. Li for all reasonable out-of-pocket expenses incurred in the performance of his duties. The term of his employment ended on June 28, 2018. We expect to extend the employment agreement with Mr. Li.
Directors Compensation
The following table sets forth the compensation paid by us for the fiscal years ended March 31, 2016, March 31, 2017, and March 31, 2018 for our directors except Mr. Tinghe Yan, who does not receive any additional compensation for his services as a director of the Company. This information includes the dollar value of base salaries, bonus awards and number of stock options granted, and certain other compensation, if any.
|
Name and Principal
|
|
Salary
|
Bonus
|
Stock Awards
|
Option Awards
|
All Other
Compensation
|
Total
|
||||||||||||||||||
|
Position
|
Year
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
||||||||||||||||||
|
|
|
|
|||||||||||||||||||||||
|
Robert J. Fanella
|
2018
|
27,500
|
27,500
|
||||||||||||||||||||||
|
Director
|
2017
|
17,500
|
-
|
10,000
|
-
|
-
|
27,500
|
||||||||||||||||||
|
2016
|
17,500
|
-
|
10,000
|
-
|
-
|
27,500
|
|||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Jirui Zhang
|
2018
|
38,035
|
38,035
|
||||||||||||||||||||||
|
Director
|
2017
|
38,035
|
-
|
-
|
-
|
-
|
38,035
|
||||||||||||||||||
|
2016
|
38,035
|
-
|
-
|
-
|
-
|
38,035
|
|||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Wengao Zhang
|
2018
|
10,000
|
10,000
|
||||||||||||||||||||||
|
Director
|
2017
|
10,000
|
-
|
-
|
-
|
-
|
10,000
|
||||||||||||||||||
|
2016
|
10,000
|
-
|
-
|
-
|
-
|
10,000
|
|||||||||||||||||||
|
|
|
||||||||||||||||||||||||
|
Dong Li
|
2018
|
38,035
|
38,035
|
||||||||||||||||||||||
|
Director
|
2017
|
-
|
-
|
-
|
-
|
-
|
-
|
||||||||||||||||||
|
2016
|
-
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||||||
Our directors are compensated by cash retainers on an annual basis.
65
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information known to us with respect to the beneficial ownership of our 29,839,168 outstanding shares of common stock as of July 13, 2018 regarding the following:
|
·
|
each stockholder known by us to own beneficially more than 5% of our common stock;
|
|
·
|
each of our officers;
|
|
·
|
each of our directors; and
|
|
·
|
all directors and executive officers as a group.
|
Except as otherwise indicated, we believe that the beneficial owners of the common stock listed below have sole voting power and investment power with respect to their shares, subject to community property laws where applicable. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission.
|
Name and Address
of Beneficial Owner (1)
|
Amount and Nature
of Beneficial Ownership (2)
|
Percentage
of Class
|
||||||
|
Tinghe Yan
|
9,653,689
|
32.35
|
%
|
|||||
|
Jirui Zhang
|
1,427,782
|
4.78
|
%
|
|||||
|
Robert J. Fanella
|
300,595
|
1.01
|
%
|
|||||
|
Wengao Zhang
|
12,500
|
*
|
||||||
|
Dong Li
|
0
|
0
|
||||||
|
Chuanmin Li
|
0
|
0
|
||||||
|
All officers and directors as a group (6 persons)
|
11,394,566
|
38.19
|
%
|
|||||
|
Except as otherwise noted, each stockholder's address is c/o Shandong Spring Pharmaceutical Co., Ltd., Economic Development Zone, Gucheng Road, Sishui County, Shandong Province, P.R. China.
|
|
|
(2)
|
Except as otherwise noted, all shares are owned of record and beneficially.
|
* Indicate less than 0.01%
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Except as follows, since March 31, 2017, there has not been, nor is there currently proposed, any transaction or series of similar transactions to which we are or will be a party:
• in which the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years; and
• in which any director, executive officer, or other stockholder of more than 5% of our Common Stock or any member of their immediate family had or will have a direct or indirect material interest.
66
On March 18, 2017, we entered into an Acquisition Agreement with respect to the Acer Truncatum Industrial Project with Shandong Yongchuntang. Pursuant to the Agreement, we issued three percent of the equity of Shandong Spring Pharmaceutical in exchange for tangible and intangible assets related to the Acer Truncatum Industrial Project. Shandong Yongchuntang also received the right to nominate one director to our Board of Directors. It designated Mr. Li Dong to serve on the Company's Board of Directors on April 1, 2017. In addition, for the year ended March 31, 2018, we purchased RMB 92.8 million (approximately $14 million) in health care products from Shandong Yongchuntang,
The security deposit to related party of $1,590,305 represents the deposit paid to Shandong Yongchuntang on January 4, 2017 for using the direct-sales license issued to Shandong Yongchuntang. The amount is non-interest bearing and not secured. Shandong Yongchuntang owns 3% of the equity of Shandong Spring Pharmaceutical.
On March 31, 2018, purchase deposit to related party of $1,412,864 pertains to a purchase deposit paid in respect of the purchase of healthcare products from Shandong Yongchuntang.
On March 31, 2017, accounts payable balance of $706,048 pertains to a payable in respect of the purchase of healthcare products from Shandong Yongchuntang.
Review, Approval, and Ratification of Transactions with Related Parties
Effective upon the completion of this offering, our Board of Directors will adopt a policy regarding the review and approval of transactions with directors, officers and holders of more than 5% of our voting securities. In evaluating related party transactions, the Board of Directors expects to establish a committee to review the proposed matters and make recommendations to the Board of Directors on the course of action, and in a case where a director is the related party, such individual abstains from voting to approve the transaction. We intend to put into place a related party transactions policy which will require, among other items, that such transactions must be approved by our Audit Committee or another independent body of our Board of Directors.
DESCRIPTION OF CAPITAL STOCK
General
The following description of our capital stock and certain provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to the certificate of incorporation, certificate of amendment of certificate of incorporation and bylaws that have been filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part.
67
Authorized Capital Stock
Our authorized share capital consists of 100,000,000 shares of common stock, par value $0.001 per share, 4,999,000 shares of preferred stock, par value $0.001 per share 45 shares of 12% preferred stock, par value $500 per share, and 1,000 shares of Series B convertible preferred stock, par value $0.001 per share. As of July 13, 2018, 29,839,168 shares of our common stock and 45 shares of our 12% Preferred Stock were outstanding.
Common Stock
Each share of our common stock entitles its holder to one vote in the election of each director and on all other matters voted on generally by our stockholders, other than any matter that (1) solely relates to the terms of any outstanding series of preferred stock or the number of shares of that series and (2) does not affect the number of authorized shares of preferred stock or the powers, privileges and rights pertaining to the common stock. No share of our common stock affords any cumulative voting rights. This means that the holders of a majority of the voting power of the shares voting for the election of directors can elect all directors to be elected if they choose to do so.
Holders of our common stock will be entitled to dividends in such amounts and at such times as our Board of Directors in its discretion may declare out of funds legally available for the payment of dividends. We currently intend to retain our entire available discretionary cash flow to finance the growth, development and expansion of our business and do not anticipate paying any cash dividends on the common stock in the foreseeable future. Any future dividends will be paid at the discretion of our Board of Directors after taking into account various factors, including:
|
●
|
general business conditions;
|
|
●
|
industry practice;
|
|
●
|
our financial condition and performance;
|
|
●
|
our future prospects;
|
|
●
|
our cash needs and capital investment plans;
|
|
●
|
our obligations to holders of any preferred stock we may issue;
|
|
●
|
income tax consequences; and
|
|
●
|
the restrictions Delaware and other applicable laws.
|
If we liquidate or dissolve our business, the holders of our common stock will share ratably in all of our assets that are available for distribution to our stockholders after our creditors are paid in full and the holders of all series of our outstanding preferred stock, if any, receive their liquidation preferences in full.
Our common stock has no preemptive rights and is not convertible or redeemable or entitled to the benefits of any sinking or repurchase fund.
Preferred Stock
Our amended certificate of incorporation authorizes three classes of preferred stock.
Preferred Stock. Our preferred stock, par value $0.001 per share ("Preferred Stock") may be issued in one or more series at the discretion of the Board of Directors without approval or any other action by the holders of our common stock. The Board of Directors has the authority to fix by resolution or resolutions the designations and the power, preferences and relative, participating, optional or other special rights and qualifications, limitations or restrictions thereof, including without limitation, the dividend rate, conversion or exchange rights, redemption price and liquidation preference, of any series of shares of preferred stock and to fix the number of shares constituting any such series, and to increase or decrease the number of shares of any such series. 4,999,000 shares of Preferred Stock are authorized and no shares have been issued.
We are also authorized to issue 1,000 shares of Series B Convertible Preferred Stock, par value $0.001 per share "Series B Preferred Stock").
Series B Preferred Stock.
Each share of our Series Preferred Stock, is convertible into 28,924 shares of common stock (after giving effect to the 1-for-28 reverse stock split that became effective on November 23, 2007). Holders of the Series B Preferred Stock are entitled to receive dividends pari passu with holders of our common stock based upon the number of shares of common stock that such holder is entitled to receive upon conversion of the series B preferred stock and are entitled to cast one vote at stockholder meetings for each such share issuable upon conversion. Upon liquidation, each holder is entitled to receive $0.01 per share of series B preferred stock prior to any payments to holders of our common stock and thereafter to participate in any remaining of proceeds of liquidation pari passu with holders of our common stock based upon the number of shares of common stock that such holder is entitled to receive upon conversion of the series B preferred stock. We can redeem outstanding shares of series B preferred stock on not less than 15 and not more than 60 days' notice by paying the holders $0.001 per share. There are 1,000 shares of series B preferred stock authorized and no shares of series B preferred stock outstanding.
We are also authorized to issue shares of our 12% Preferred Stock, par value, $500 per share (12% Preferred Stock").
68
12% Preferred Stock.
Each share of our 12% Preferred Stock (i) is entitled to a 12% noncumulative dividend; (ii) is entitled to a preference in liquidation up to its $500 par value per share; (iii) is subject to redemption upon fifteen (15) days' notice, at a price equal to its $500 par value plus any accrued dividend due; (iv) is entitled to one vote per share at any meeting of the stockholders or in connection with any written consent; and (v) is convertible at the option of the holder into 333 shares of our common stock. There are 45 shares of 12% Preferred Stock authorized and currently outstanding.
Warrants
We have issued warrants to purchase 400,000 shares at an exercise price of $2.00 per share, provided that the warrants will not be exercisable unless our common stock is listed on The Nasdaq Stock Market, and which warrants will be exercisable commencing six months after such listing with a term of five years. The holder of the warrants may, upon exercise, elect to receive the net number of shares of common stock determined according to the formula set forth in the warrants in lieu of making the cash payment otherwise contemplated to be made to use upon such exercise.
Underwriters' Warrants
Please see "Underwriting" for a description of warrants to be issued to the underwriters.
Anti-Takeover Provisions
The provisions of Delaware law, certificate of incorporation and our bylaws, which are summarized below, may have the effect of delaying, deferring or discouraging another person from acquiring control of our company.
Certificate of Incorporation and Bylaws
Because our stockholders do not have cumulative voting rights, stockholders holding a majority of the voting power of our shares of common stock will be able to elect all of our directors.
Under our bylaws, a special meeting of our stockholders shall be called at the request of a majority of the members of the Board of Directors or the holder(s) of at least 10% of the total voting power of all outstanding shares of stock of the Company then entitled to vote. The request shall state the purpose of the proposed meeting. After receipt of such request, written notice stating the place, time and date (which shall be not less than ten nor more than sixty days from the date of the notice) of the special meeting and the purpose for which the special meeting is called, shall be given to each stockholder entitled to vote.
Any action required to be or may be taken at any annual or special meeting of our stockholders, may be taken without a meeting, without prior notice and without a vote, by a consent in writing. The action shall be signed by the holders of a number of shares of outstanding stock that would be sufficient to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. Prompt notice of the taking of corporate action without a meeting by less than unanimous written consent shall be given to those stockholders who have not consented in writing and who, if the action had been taken at a meeting, would have been entitled to notice of the meeting if the record date for such meeting had been the date of delivery of the written consents.
69
Our executive officers, directors, 5% stockholders and their affiliates beneficially own approximately 38.19% of our common stock and, upon the closing of this offering, that same group will hold approximately [●]% of our outstanding common stock, based upon the number of shares of our common stock outstanding as of July 13, 2018. These stockholders have a sufficient percentage of our outstanding shares to possibly block a takeover.
Section 203 of the Delaware General Corporation Law
When we have a class of voting stock that is either listed on a national securities exchange or held of record by more than 2,000 stockholders, we will be subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, subject to certain exceptions.
The foregoing provisions may make it more difficult for another party to obtain control of us. They could also have the effect of discouraging others from making tender offers for our shares and may have the effect of deterring hostile takeovers or delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts.
Lock-Up Agreements
In connection with this offering, our officers, directors, and holders of 5% or more of our common stock have agreed to enter into lock-up agreements with the underwriters. See "Underwriting" for more information.
Listing
Our shares of common stock are currently quoted on the OTCQB, operated by OTC Market Group. The symbol for our common stock is "CYIG". We intend to apply to list our common stock on The NASDAQ Capital Market under the symbol "CTYY." No assurance can be given that our application will be approved. If our application is not approved, we will not consummate this offering.
On July 13, 2018, the last reported sale price of our common stock on the OTCQB was $0.82 per share.
Indemnification
Both our certificate of incorporation and bylaws provide for indemnification of our directors to the fullest extent permitted by the Delaware General Corporation Law.
Transfer Agent and Registrar
The Transfer Agent for our common stock is Interwest Transfer Co., Inc., 1981 E Murray Holladay Desalt Lake City, UT 84117. The telephone number is: (801) 272-9294.
UNDERWRITING
We have entered into an underwriting agreement dated [●] with Maxim Group LLC, as the sole book-running manager. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter and the underwriter has agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the following number of shares of common stocks:
70
|
Underwriter
|
Number of Shares of Common Stocks
|
| Maxim Group LLC |
|
The underwriter is committed to purchase all the shares of our common stock offered by us other than those covered by the option to purchase additional shares described below, if they purchase any shares. The obligations of the underwriter may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriter's obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriter of officers' certificates and legal opinions.
We have agreed to indemnify the underwriter against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriter may be required to make in respect thereof.
The underwriter is offering the common stock, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by its counsel and other conditions specified in the underwriting agreement. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Underwriter Compensation
We have agreed to pay the underwriter a cash fee equal to 7% of the aggregate gross proceeds sold in the offering and issue to the underwriter warrants to purchase that number of shares of our common stock equal to 7% of the aggregate number of securities sold in the offering. Such underwriter warrants shall have an exercise price equal to 110% of the public offering price per share and will be, exercisable commencing six months after the effective date of the registration statement with respect to this offering of which this prospectus forms a part .. Such underwriter's warrants shall expire three and a half years after the effective date of the registration statement with respect to this offering of which this prospectus forms a part and will provide for cashless exercise. Such underwriter's warrants will be subject to FINRA Rule 5110(g)(1) in that, such warrants shall not be transferable for 180 days from the effective date of the offering except as permitted by FINRA Rule 5110(g)(2). Such warrants shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person.
The underwriter proposes to offer the common stock offered by us to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriter may offer some shares of the common stocks to other securities dealers at such price less a concession of $ [●] per share. After the initial offering, the public offering price and concession to dealers may be changed.
Option to Purchase Additional Securities
We have granted to the underwriter an option exercisable not later than 45 days after the date of this prospectus to purchase up to a number of additional shares of common stock and/or warrants to purchase shares of common stock not to exceed 15% of the number of shares of common stock sold in the primary offering (excluding any shares of common stock issued upon any exercise of the underwriter's over-allotment option) at the public offering price per share of common stock set forth on the cover page to this prospectus less the underwriting discounts and commissions. The underwriter may exercise the option solely to cover over-allotments, if any, made in connection with this offering. If any additional shares of common stock are purchased pursuant to the over-allotment option, the underwriter will offer these shares of common stock on the same terms as those on which the other securities are being offered.
71
Discounts and Commissions
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriter of their over-allotment option.
|
|
Total
|
|||||||||||
|
|
Per Share
|
Without Over-Allotment
|
With Over-Allotment
|
|||||||||
|
Public offering price(1)
|
$
|
$
|
$
|
|||||||||
|
Underwriting discount (7%)(2)
|
$
|
$
|
$
|
|||||||||
|
Proceeds, before expenses, to us
|
$
|
$
|
$
|
|||||||||
———————
|
(1) We have granted a 45 day option to the underwriter to purchase additional shares of common stock (up to 15% of the number of shares of common sold in the primary offering) at the public offering price per share of common stock set forth above less the underwriting discounts and commissions, solely to cover over-allotments, if any.
|
(2) We have agreed to reimburse the underwriter their actual, out-of-pocket expenses, including the reasonable fees and disbursements of the underwriter' counsel related to the offering, up to an aggregate maximum amount of $150,000 but in any event in compliance with the provisions of FINRA Rule 5110(f)(2). In the event of a termination of the offering, the underwriter will be entitled to reimbursement of their actual out-of-pocket expenses, not to exceed $150,000. We estimate that the total expenses of the offering including all expenses to be reimbursed to the underwriter excluding the underwriter' discount, will be approximately $[●].
Lock-Up Agreements
All of our directors and executive officers, and holders of five percent (5%) or more of our outstanding securities (or securities convertible into shares of our common stock) have agreed that, for a period of 6 months after the date of this prospectus, subject to certain limited exceptions, they will not directly or indirectly, without the prior written consent of the underwriter, (1) offer, sell, agree to offer or sell, solicit offers to purchase, grant any call option or purchase any put option with respect to, pledge, encumber, assign, borrow or otherwise dispose of or transfer any shares of common stock, warrant to purchase shares of common stock or any other security of the company or any other entity that is convertible into, or exercisable or exchangeable for, shares of common stock or any other equity security of the company owned beneficially or otherwise as of the date of this prospectus, which we refer to as relevant securities , or otherwise publicly disclose the intention to do so, (2) establish or increase any "put equivalent position" or liquidate or decrease any "call equivalent position" (in each case within the meaning of Section 16 of the Exchange Act) with respect to any relevant security or otherwise enter into any swap, derivative or other transaction or arrangement that transfers to another, in whole or in part, any economic consequence of ownership of relevant securities, whether or not such transaction is to be settled by the delivery of relevant securities, other securities, cash or other consideration, or otherwise publicly disclose the intention to do so, (3) file or participate in the filing with the SEC of any registration statement or circulate or participate in the circulation of any preliminary or final prospectus or other disclosure document, in each case with respect to any proposed offering or sale of relevant securities or (4) exercise any rights to require registration with the SEC of any proposed offering or sale of relevant securities. We may issue, pursuant to an incentive compensation plan (which would reserve a maximum of fifteen percent (15%) of our outstanding shares), grant common stock or options to purchase common stock to officers, directors, employees and consultants, provided that no such shares issuable to such persons may be sold by such persons for a period of six months after the offering.
Right of First Refusal
We have agreed to grant the underwriter for the 12 month period following the closing of this offering a right of first refusal to act as lead managing underwriter and book runner or minimally as a co-lead manager and co-book runner and/or co-lead placement agent with at least 50.0% of the economics for any and all of our future public or private equity, equity-linked or debt offerings during such twelve (12) month period, or any successor to or any subsidiary, in which we utilize the services of an underwriter, placement agent or financial advisor (excluding (i) sales to employees under any compensation or stock option plan approved by our stockholders, (ii) shares issued in payment of the consideration for an acquisition and (iii) conventional banking arrangements and commercial debt financing of ours or our subsidiaries).
72
Price Stabilization, Short Positions and Penalty Bids
The underwriter may engage in over-allotment, stabilizing transactions, syndicate covering transactions, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
· Over-allotment involves sales by the underwriter of securities in excess of the number of securities the underwriter is obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriter is not greater than the number of securities that they may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriter may close out any short position by either exercising their over-allotment option (which they anticipate will occur if our stock prices are greater than the price per security in this offering) and/or purchasing securities in the open market (which they anticipate will occur if our stock prices are less than the price per security in this offering).
· Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum.
·Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriter will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may purchase securities through the over-allotment option. If the underwriter sells more securities than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering.
· Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities, or preventing or retarding a decline in the market price of those securities. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on an exchange or in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the securities. In addition, neither we nor the underwriter make any representation that the underwriter will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
In connection with this offering, the underwriter may also engage in passive market making transactions in our securities. Passive market making consists of displaying bids on a national securities exchange limited by the prices of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation M promulgated by the SEC limits the amount of net purchases that each passive market maker may make and the displayed size of each bid. Passive market making may stabilize the market price of our securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
73
Determination of Offering Price
The offering price for our common stock will be determined through negotiations with the underwriter, and the negotiated price may not be indicative of the market price of the common stock after the offering. In determining the offering price for the common stock in this offering, we will consider a number of factors including, but not limited to, the current market price of our common stock, trading prices of our common stock over a period of time, the illiquidity and volatility of our common stock, prevailing market conditions, our historical performance, our future prospects and the future prospects of our industry in general, our capital structure, estimates of our business potential and earnings prospects, the present state of our development and an assessment of our management and the consideration of the above factors in relation to market valuation of companies engaged in businesses and activities similar to ours.
Selling Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
LEGAL MATTERS
McLaughlin & Stern, LLP is acting as counsel to our company regarding U.S. securities law matters. The validity of the shares of common stock offered hereby will be passed upon for us by McLaughlin & Stern, LLP, New York, New York. Certain legal matters as to PRC law will be passed upon for us by [●]. Loeb & Loeb LLP, New York, New York has acted as counsel to the underwriter in connection with this offering.
Our financial statements as of and for the years ended March 31, 2018 and 2017, included in this prospectus, have been audited by Paritz & Company, P.A., our independent registered public accountants, as stated in their report appearing herein and are so included herein in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
We filed with the SEC a registration statement under the Securities Act for the common stock in this offering. This prospectus does not contain all of the information in the registration statement and the exhibits and schedule that were filed with the registration statement. For further information with respect to us and our common stock, we refer you to the registration statement and the exhibits that were filed with the registration statement. Statements contained in this prospectus about the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and we refer you to the full text of the contract or other document filed as an exhibit to the registration statement.
We file annual, quarterly, and current reports and other information with the SEC. Our filings with the SEC are available to the public on the SEC's website at www.sec.gov. Those filings are also available to the public on our corporate website at www.yctgroup.com. The information we file with the SEC or contained on, or linked to through, our corporate website or any other website that we may maintain is not part of this prospectus or the registration statement of which this prospectus is a part. You may also read and copy, at the SEC's prescribed rates, any document we file with the SEC, including the registration statement (and its exhibits) of which this prospectus is a part, at the SEC's Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room.
74
CHINA YCT INTERNATIONAL GROUP, INC.
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED MARCH 31, 2018 AND 2017
Table of Contents
|
Page
|
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
F-2
|
|
Consolidated Balance Sheets as of March 31, 2018 and 2017
|
F-3
|
|
Consolidated Statements of Comprehensive Income for the Years Ended March 31, 2018 and 2017
|
F-4
|
|
Consolidated Statements of Stockholders' Equity for the Years Ended March 31, 2018 and 2017
|
F-5
|
|
Consolidated Statements of Cash Flows for the Years Ended March 31, 2018 and 2017
|
F-6
|
|
Notes to Consolidated Financial Statements
|
F-7-F-23
|
F - 1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
China YCT International Group, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of China YCT International Group, Inc. ("the Company") as of March 31, 2018 and 2017, and the related consolidated statements of comprehensive income, stockholders' equity and cash flows for each of the years in the two-year period ended March 31, 2018 and related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2018 and 2017, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Paritz & Company, P.A.
We have served as the Company's auditor since 2015.
Hackensack, New Jersey
June 29, 2018
F - 2
CHINA YCT INTERNATIONAL GROUP, INC.
CONSOLIDATED BALANCE SHEETS
|
MARCH 31,
2018
|
MARCH 31,
2017
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
25,353,360
|
$
|
10,308,622
|
||||
|
Accounts receivable
|
174,558
|
1,134,967
|
||||||
|
Inventories
|
2,383,382
|
5,483,040
|
||||||
|
Purchase deposit to vendors
|
-
|
650,790
|
||||||
|
Purchase deposit to related party
|
1,412,864
|
-
|
||||||
|
Prepaid leases – current portion
|
741,583
|
900,547
|
||||||
|
Total current assets
|
30,065,747
|
18,477,966
|
||||||
|
Prepaid leases
|
641,349
|
1,265,252
|
||||||
|
Development cost of acer truncatum bunge planting
|
48,984,881
|
42,055,972
|
||||||
|
Plant, property, and equipment, net
|
16,793,413
|
14,487,135
|
||||||
|
Intangible assets, net
|
11,862,017
|
12,042,758
|
||||||
|
Deferred tax assets
|
200,387
|
508,521
|
||||||
|
Security deposit to related party
|
1,590,305
|
1,449,422
|
||||||
|
Total assets
|
$
|
110,138,099
|
$
|
90,287,026
|
||||
|
Liabilities and Stockholders' Equity
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable to related party
|
$
|
-
|
$
|
706,048
|
||||
|
Accounts payable and other accrued expenses
|
372,782
|
251,307
|
||||||
|
Advance from customers
|
445,829
|
-
|
||||||
|
Taxes payable
|
1,164,198
|
2,028,190
|
||||||
|
Total current liabilities
|
1,982,809
|
2,985,545
|
||||||
|
Stockholders' Equity
|
||||||||
|
Preferred stock, par value $0.001 per share; 5,000,000 shares authorized, zero shares issued and outstanding
|
-
|
-
|
||||||
|
12% Preferred stock, par value $500 per share; 45 shares authorized, issued and outstanding
|
22,500
|
22,500
|
||||||
|
Common stock, par value $0.001 per share; 100,000,000 shares authorized; 29,789,168 shares issued and outstanding
|
29,789
|
29,789
|
||||||
|
Additional paid-in capital
|
4,322,838
|
4,322,838
|
||||||
|
Statutory reserve
|
1,828,504
|
1,828,504
|
||||||
|
Retained earnings
|
94,447,937
|
83,061,604
|
||||||
|
Accumulated other comprehensive income (loss)
|
4,455,017
|
(4,386,845
|
)
|
|||||
|
Total stockholders' equity attributable to the Company
|
105,106,585
|
84,878,390
|
||||||
|
Noncontrolling interest
|
3,048,705
|
2,423,091
|
||||||
|
Total stockholders' equity
|
108,155,290
|
87,301,481
|
||||||
|
Total liabilities and stockholders' equity
|
$
|
110,138,099
|
$
|
90,287,026
|
||||
The accompanying notes are an integral part of these consolidated financial statements
F - 3
CHINA YCT INTERNATIONAL GROUP, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
|
YEARS ENDED
MARCH 31, |
||||||||
|
2018
|
2017
|
|||||||
|
Sales
|
$
|
64,942,737
|
$
|
56,463,164
|
||||
|
Cost of goods sold (including $14,404,967 and $11,015,268 from a related party for the years ended March 31, 2018 and 2017, respectively)
|
39,603,995
|
33,284,237
|
||||||
|
Gross profit
|
25,338,742
|
23,178,927
|
||||||
|
Operating expenses
|
||||||||
|
Selling expenses
|
4,984,794
|
3,934,334
|
||||||
|
General and administrative expenses
|
4,719,402
|
4,248,095
|
||||||
|
Research and development expenses
|
492,078
|
809,485
|
||||||
|
Impairment of assets
|
332,090
|
986,406
|
||||||
|
Total operating expenses
|
10,528,364
|
9,978,320
|
||||||
|
Income from operations
|
14,810,378
|
13,200,607
|
||||||
|
Gain on disposal of acer truncatum bunge plants
|
642,532
|
-
|
||||||
|
Interest income
|
124,410
|
54,672
|
||||||
|
Income before income tax provision
|
15,577,320
|
13,255,279
|
||||||
|
Income tax provision
|
3,838,832
|
3,200,625
|
||||||
|
Net income
|
11,738,488
|
10,054,654
|
||||||
|
Less: Net income (loss) attributable to noncontrolling interest
|
352,155
|
(23,649
|
)
|
|||||
|
Net income attributable to the Company
|
11,386,333
|
10,078,303
|
||||||
|
Other comprehensive income (loss):
|
||||||||
|
Foreign currency translation adjustment
|
9,115,321
|
(5,228,368
|
)
|
|||||
|
Comprehensive income
|
20,853,809
|
4,826,286
|
||||||
|
Less: Comprehensive income (loss) attributable to noncontrolling interest
|
625,614
|
(28,108
|
)
|
|||||
|
Comprehensive income attributable to the Company
|
$
|
20,228,195
|
$
|
4,854,394
|
||||
|
Earnings per common share
|
||||||||
|
Basic and Diluted
|
$
|
0.38
|
$
|
0.34
|
||||
|
Weighted average number of common shares outstanding
|
||||||||
|
Basic and Diluted
|
29,789,168
|
29,763,531
|
||||||
The accompanying notes are an integral part of these consolidated financial statements.
F - 4
CHINA YCT INTERNATIONAL GROUP, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
|
12% Preferred Stock
|
Common Stock
|
Additional
Paid-in
|
Statutory
|
Accumulated
Other Comprehensive Income
|
Retained
|
Total Stockholders' Equity Attributable
to the
|
Non-controlling
|
|||||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
Reserve
|
(Loss)
|
Earnings
|
Company
|
Interest
|
Total
|
||||||||||||||||||||||||||||||||||
|
Balance - March 31, 2016
|
45
|
$
|
22,500
|
29,720,690
|
$
|
29,721
|
$
|
4,648,461
|
$
|
1,828,504
|
$
|
837,064
|
$
|
72,983,301
|
$
|
80,349,551
|
$
|
-
|
$
|
80,349,551
|
||||||||||||||||||||||||
|
Net income (loss)
|
10,078,303
|
10,078,303
|
(23,649
|
)
|
10,054,654
|
|||||||||||||||||||||||||||||||||||||||
|
Issuance of common shares for services
|
68,478
|
68
|
22,041
|
22,109
|
22,109
|
|||||||||||||||||||||||||||||||||||||||
|
Transfer 3% equity of Shandong Spring to a noncontrolling shareholder
|
(448,690
|
)
|
(448,690
|
)
|
2,451,199
|
2,002,509
|
||||||||||||||||||||||||||||||||||||||
|
Stock option cost
|
101,026
|
101,026
|
101,026
|
|||||||||||||||||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
(5,223,909
|
)
|
(5,223,909
|
)
|
(4,459
|
)
|
(5,228,368
|
)
|
||||||||||||||||||||||||||||||||||||
|
Balance - March 31, 2017
|
45
|
22,500
|
29,789,168
|
29,789
|
4,322,838
|
1,828,504
|
(4,386,845
|
)
|
83,061,604
|
84,878,390
|
2,423,091
|
87,301,481
|
||||||||||||||||||||||||||||||||
|
Net income
|
11,386,333
|
11,386,333
|
352,155
|
11,738,488
|
||||||||||||||||||||||||||||||||||||||||
|
Foreign currency translation adjustment
|
8,841,862
|
8,841,862
|
273,459
|
9,115,321
|
||||||||||||||||||||||||||||||||||||||||
|
Balance - March 31, 2018
|
45
|
$
|
22,500
|
29,789,168
|
$
|
29,789
|
$
|
4,322,838
|
$
|
1,828,504
|
$
|
4,455,017
|
$
|
94,447,937
|
$
|
105,106,585
|
$
|
3,048,705
|
$
|
108,155,290
|
||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F - 5
CHINA YCT INTERNATIONAL GROUP, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
YEARS ENDED
MARCH 31, |
||||||||
|
2018
|
2017
|
|||||||
|
Cash flows from operating activities:
|
||||||||
|
Net income
|
$
|
11,738,488
|
$
|
10,054,654
|
||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||
|
Depreciation and amortization of plant, property and equipment
|
1,300,651
|
746,931
|
||||||
|
Amortization of intangible assets
|
1,282,495
|
1,098,757
|
||||||
|
Amortization of prepaid leases
|
884,950
|
923,380
|
||||||
|
Stock-based compensation
|
-
|
123,135
|
||||||
|
Deferred taxes
|
339,358
|
(319,501
|
)
|
|||||
|
Loss on disposal of property, plant and equipment
|
-
|
184,262
|
||||||
|
Gain on disposal of acer truncatum bunge plants
|
(642,532
|
)
|
-
|
|||||
|
Impairment of assets
|
332,090
|
986,406
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Purchase deposit to vendors
|
677,695
|
(667,291
|
)
|
|||||
|
Inventory
|
3,447,670
|
(3,425,678
|
)
|
|||||
|
Accounts receivable
|
1,016,216
|
(651,680
|
)
|
|||||
|
Cash received from cancellation of lease
|
57,858
|
-
|
||||||
|
Taxes payable
|
(1,007,109
|
)
|
1,369,927
|
|||||
|
Security deposit to related party
|
-
|
(1,486,171
|
)
|
|||||
|
Purchase deposit and accounts payable to related party, net
|
(2,076,172
|
)
|
663,222
|
|||||
|
Advance from customers
|
423,132
|
-
|
||||||
|
Accounts payable and other accrued expenses
|
92,108
|
135,721
|
||||||
|
Net cash provided by operating activities
|
17,866,898
|
9,736,074
|
||||||
|
Cash flows from investing activities:
|
||||||||
|
Acquisition of property, plant and equipment
|
(2,153,063
|
)
|
(2,880,051
|
)
|
||||
|
Proceeds from disposal of acer truncatum bunge plants
|
2,156,510
|
-
|
||||||
|
Development cost of acer truncatum bunge planting
|
(4,542,518
|
)
|
(3,618,469
|
)
|
||||
|
Net cash used in investing activities
|
(4,539,071
|
)
|
(6,498,520
|
)
|
||||
|
Effect of exchange rate changes on cash and cash equivalents
|
1,716,911
|
(568,016
|
)
|
|||||
|
Net increase in cash and cash equivalents
|
15,044,738
|
2,669,538
|
||||||
|
Cash and cash equivalents at beginning of year
|
10,308,622
|
7,639,084
|
||||||
|
Cash and cash equivalents at end of year
|
$
|
25,353,360
|
$
|
10,308,622
|
||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid during the years for:
|
||||||||
|
Interest
|
$
|
-
|
$
|
-
|
||||
|
Income taxes
|
$
|
4,445,597
|
$
|
2,521,061
|
||||
|
Noncash Investing Activities:
|
||||||||
|
Transfer 3% equity of Shandong Spring Pharmaceutical in exchange for equipment and intangible assets
|
$
|
-
|
$
|
2,134,537
|
||||
The accompanying notes are an integral part of these consolidated financial statements.
F - 6
CHINA YCT INTERNATIONAL GROUP, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 - ORGANIZATION AND BUSINESS
We were incorporated in the State of Florida, in the United States of America (the "USA") in January 1989, and reincorporated in the State of Delaware on April 4, 2007. We, through its 100% owned subsidiary Landway Nano Bio-Tech, Inc. ("Landway Nano"), incorporated in Delaware, own 97% of Shandong Spring Pharmaceutical Co., Ltd. ("Shandong Spring Pharmaceutical "), incorporated in the People's Republic of China ("PRC"). The Company, through its 97% owned subsidiary, Shandong Spring Pharmaceutical, is engaged in the business of (i) distributing health care supplement products manufactured by Shandong Yongchuntang, (ii) developing, manufacturing, and selling Huoliyuan capsules, a prescription medicine, and (iii) developing the acer truncatum bunge planting bases and selling acer truncatum seed oil in the PRC. Acer truncatum bunge plants are a species of maple tree.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The consolidated financial statements of the Company are prepared in accordance with accounting principles generally accepted in the United States of America ("US GAAP").
Principles of consolidation
The consolidated financial statements include the financial statements of China YCT International Group, Inc., Landway Nano and its 97% owned subsidiary, Shandong Spring Pharmaceutical. All inter-company transactions and balances are eliminated in consolidation.
Use of estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Actual results may differ from those estimates. Significant accounting estimates reflected in the Company's consolidated financial statements include: the valuation of inventory, the estimated useful lives and impairment of property, equipment, and intangible assets and the valuation of deferred tax assets.
Cash and cash equivalents
Cash and cash equivalents including cash on hand and deposits placed with banks or other financial institutions, which are unrestricted as to withdrawal and use and with an original maturity of three months or less.
Deposits in banks in the PRC are not insured by any government entity or agency, and are consequently exposed to risk of loss. The Company believes the probability of a bank failure, causing loss to the Company, is remote.
F - 7
Accounts receivable
Accounts receivable are recognized and carried at the original invoice amounts less an allowance for any uncollectible amount. The Company extends credit to our customers based on an evaluation of their financial condition and other factors. We generally do not require collateral or other security to support accounts receivable. We perform ongoing credit evaluations of our customers and maintain an allowance for potential bad debts if required.
The Company determines whether an allowance for doubtful accounts is required by evaluating specific accounts where information indicates the customers may have an inability to meet financial obligations. In these cases, we use assumptions and judgment, based on the best available facts and circumstances, to record a specific allowance for those customers against amounts due to reduce the receivable to the amount expected to be collected. These specific allowances are re-evaluated and adjusted as additional information is received. The amounts calculated are analyzed to determine the total amount of the allowance. We may also record a general allowance as necessary.
Direct write-offs are taken in the period when we have exhausted our efforts to collect overdue and unpaid receivables or otherwise evaluate other circumstances that indicate that we should abandon such efforts.
The Company has not experienced any significant difficulty in collecting its accounts receivable in the past and is not aware of any financial difficulties being experienced by its major customers. No allowance for doubtful accounts receivable was recorded in the years ended March 31, 2018 and 2017, respectively.
Inventories
Inventories are valued at the lower of cost or net realizable value with cost determined on a weighted average basis. Management compares the cost of inventory with the net realizable value and an allowance is made for writing down the inventory to its net realizable value, if lower than cost.
Property and equipment
Property and equipment are stated at cost. The cost of an asset comprises its purchase price and any directly attributable costs of bringing the asset to its present working condition and locations for its intended use. Leasehold improvements are stated at cost and amortized over the shorter of the useful life of the assets or the length of the lease in accordance to ASC 840-10-35-6. Depreciation and amortization are calculated using the straight-line method over the following useful lives:
|
Buildings
|
10-35 years
|
|
Machinery, equipment
|
3-20 years
|
|
Office equipment and automobiles
|
3-10 years
|
|
Leasehold improvements
|
5-30 years (or the lease term, if shorter)
|
Expenditures for maintenance and repairs are charged to expense as incurred. Additions, renewals and betterments are capitalized.
F - 8
Intangible Assets
Purchased intangible assets are recognized and measured at fair value upon acquisition. Separately identifiable intangible assets that have determinable lives are amortized over their estimated useful lives using the straight-line method as follows:
|
Land use right
|
50 years
|
|
Patent (non-US No. ZL200610068850.0)
|
16.5 years
|
|
Patent (non-US No. ZL200510045001.9)
|
14 years
|
|
Intangible assets for production and marketing of Yuanbaofen product.
|
10 years
|
Development costs of acer truncatum bunge planting
The Company has developed the acer truncatum bunge planting bases and completed planting of 5,880 mu (approximately 2,324.77 acres) as of March 31, 2018. The agricultural products (e.g., seeds, oil extract, etc.) derived from the planting are intended to be the supply for an integrated use including edible oil, protein, medicine and health care, tannin extract, industrial chemicals, nectar source, nervonic acid, and specialty lumber, as well as for landscaping and conservation of soil and water.
The Company accounts for the development costs of the planting in accordance to ASC 905. Pursuant to ASC 905-360-25-3, limited-life land development costs and direct and indirect development costs of orchards, groves, vineyards, and intermediate-life plants are required to be capitalized during the development period. Pursuant to ASC 905-360-35-7, costs capitalized during the development period are depreciated over the estimated useful life of the land development or that of the tree, vine, or plant. The planting is currently in the development stage with production expected in the fall of 2018; therefore, no depreciation expenses were recognized as of March 31, 2018.
Development costs primarily include land development cost incurred for land leveling, irrigation, and fertilization, the purchase costs of acer truncatum bunge trees, and acer truncatum bunge planting fee.
Revenue recognition
The Company sells two types of products: non-medical products and medical products. Medical products are sold to certified medicine distributors. Non-medical products are sold directly to its customers through its Internet sales channel. To order non-medical products, customers place orders on the Company's order system through the Internet. Customers who purchase through the Internet need to make payment when they place their orders. Goods are shipped to customers once the orders and payments were received.
The Company's revenue recognition policies are in compliance with Staff Accounting Bulletin ("SAB") 104, included in the Codification as ASC 605, Revenue Recognition. Sales revenue is recognized when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist, and collectability is reasonably assured. According to the Company's policy, customers can exchange defective products, but are not allowed to return products. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as customer deposits.
The Company adopted the new accounting standard, ASC 606, Revenue from Contracts with Customers, and all the related amendments (new revenue standard) to all contracts using the modified retrospective method beginning on March 31, 2018. The adoption will not result in an adjustment to the retained earnings as of April 1, 2018. The comparative information will not be restated and will continue to be reported under the accounting standards in effect for those periods. The adoption of the new revenue standard will have no impact on either reported sales to customers or net earnings. The Company will continue to recognize revenue from product sales as goods are shipped or delivered to the customer, as control of goods occurs at the same time.
F - 9
Impairment of long-lived assets
The Company reviews and evaluates the net carrying value of its long-lived assets at least annually, or upon the occurrence of other events or changes in circumstances that indicate that the related carrying amounts may not be recoverable. Per ASC 360-10-35-21, a long-lived asset (asset group) shall be tested for recoverability whenever events or changes in circumstances indicate that its carrying amount may not be recoverable. Per ASC 360-10-35-17, an impairment loss shall be recognized only if the carrying amount of the long-lived asset (asset group) is not recoverable and exceeds its fair value. The carrying amount of a long-lived asset (asset group) is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset (asset group).
Related Parties and Transactions
The Company identifies related parties, and accounts for, discloses related party transactions in accordance with ASC 850, "Related Party Disclosures" and other relevant ASC standards.
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. Companies are also considered to be related if they are subject to common control or common significant influence.
Transactions between related parties commonly occurring in the normal course of business are considered to be related party transactions. Transactions between related parties are also considered to be related party transactions even though they may not be given accounting recognition. While ASC does not provide accounting or measurement guidance for such transactions, it requires their disclosure nonetheless.
Commitments and Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigations, fines and penalties, and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Income taxes
The Company accounts for income tax under the asset and liability method as stipulated by Accounting Standards Codification ("ASC") 740, Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of the events that have been included in the financial statements or tax returns. Deferred Income taxes are recognized for all significant temporary differences between tax and financial statements bases of assets and liabilities. Valuation allowances are established against net deferred tax assets when it is more likely than not that some portion or all of the deferred tax asset will not be realized.
Value-added tax
Sales revenue represents the invoiced value of goods, net of a Value-Added Tax ("VAT"). All of the Company's products that are sold in the PRC are subject to a Chinese value-added tax at a rate of 17% of the gross sales price. This VAT may be offset by VAT paid by the Company on raw materials and other materials included in the cost of producing their finished product.
F - 10
Research and development
Research and development costs are related primarily to the Company's development of its intellectual property. Research and development costs are expensed as incurred. The costs of material and equipment that are acquired or constructed for research and development activities that have alternative future uses are classified as plant and equipment and depreciated over their estimated useful lives.
The research and development expense for the years ended March 31, 2018 and 2017 was $492,078 and $809,485, respectively.
Advertising costs
Advertising costs are expensed as incurred in accordance to the ASC 720-35, Advertising Costs. Pursuant to ASC 720-35-25-5, costs of communication advertising are not incurred until the item or service has been received and shall not be reported as expenses before the item or service has been received, except as discussed in paragraph 340-20-25-2.
The Company incurred advertising costs of $427,172 and $2,972 for the years ended March 31, 2018 and 2017, respectively, which are included in selling expenses on the Company's consolidated financial statements.
Shipping and handling costs
The Company accounts for mailing and handling fees in accordance with the FASB ASC 605-45 (Emerging Issues Task Force (EITF) Issue No. 00-10, Accounting for Shipping and Handling Fees and Costs). Amounts incurred by the Company for freight are included in selling expenses. For the years ended March 31, 2018 and 2017, the Company incurred $2,421,475 and $2,070,029 shipping and handling costs, respectively.
Stock Based Compensation
The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee stock-based awards, we calculate the fair value of the award on the date of grant using the Black-Scholes method for stock options and the quoted price of our common stock for unrestricted shares; the expense is recognized over the service period for awards expected to vest. Share-based payments to consultants, service providers and other non-employees are accounted for in accordance with ASC Topic 718, ASC Topic 505, "Equity Payments to Non-Employees" or other applicable authoritative guidance. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent that actual results or updated estimates differ from original estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. We consider many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Earnings per common share ("EPS")
Basic EPS is computed by dividing net income (loss) attributable to common stockholders by the weighted average number of common shares outstanding for the period. Diluted shares reflect the potential dilution that could occur if securities or other contracts to issue common stock (convertible preferred stock, forward contracts, warrants to purchase common stock, contingently issuable shares, common stock options and warrants and their equivalents using the treasury stock method) were exercised or converted into common stock.
Fair Value of Financial Instruments
The Company has adopted the provisions of ASC Topic 820, Fair Value Measurements and Disclosures, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses are carried at historical cost basis, which approximates fair values because of the short-term maturing of these instruments.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
F - 11
Level 1 – quoted prices in active markets for identical assets or liabilities.
Level 2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 – inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company has no financial assets or liabilities measured at fair value on a recurring basis.
Foreign currency translation
The accounts of the Company's Chinese subsidiary are maintained in RMB and the accounts of the U.S. companies are maintained in USD. The accounts of the Chinese subsidiary were translated into USD in accordance with Accounting Standards Codification ("ASC") Topic 830, Foreign Currency Matters. According to Topic 830, all assets and liabilities were translated at the exchange rate on the balance sheet date; stockholders' equity is translated at historical rates and statement of comprehensive income items are translated at the weighted average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with ASC Topic 220, Comprehensive Income. Gains and losses resulting from the foreign currency transactions are reflected in the statements of comprehensive income.
The following exchange rates were used to translate the amounts from RMB into United States dollars ("USD$") for the respective periods:
|
March 31,
|
March 31,
|
|||||||
|
2018
|
2017
|
|||||||
|
Year End Exchange Rate (RMB/USD)
|
6.2881
|
6.8993
|
||||||
|
Average Period Exchange Rate (RMB/USD)
|
6.6254
|
6.7287
|
||||||
Recent accounting pronouncements
In March 2018, FASB issued ASU 2018-05, Income Taxes (Topic 740): Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 118. ASU 2018-05 amends SEC paragraphs in ASC 740 to reflect SEC Staff Accounting Bulletin (SAB) No.118. When the 2017 Tax Cuts and Jobs Act (the "Act") was signed into law, the SEC staff released SAB 118 for applying Topic 740 as it relates to the Act. SAB 118 outlines the approach companies may take if they determine that the necessary information is not available (in reasonable detail) to evaluate, compute, and prepare accounting entries to recognize the effect(s) of the Act by the time the financial statements are required to be filed. Companies may use this approach when the timely determination of some or all of the income tax effect(s) from the Act is incomplete by the due date of the financial statements. SAB 118 also prescribes disclosures that reporting entities must provide in these circumstances. The amendments to the Accounting Standards Codification became effective upon issuance. The Company has conducted a preliminary assessment of its income tax effects of the Act. Additional analysis of the law and the impact to the Company may be performed, if needed, and any impact will be finalized no later than the fourth quarter of 2018.
In February 2018, FASB issued ASU 2018-02, Income Statement - Reporting Comprehensive Income (Topic 220): Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income. ASU 2018-02 provides entities the option to reclassify certain "stranded tax effects" resulting from the recent US tax reform from accumulated other comprehensive income ("AOCI") to retained earnings. Under the ASU, reporting entities will select an accounting policy to either reclassify all stranded tax effects caused by tax reform from AOCI to retained earnings, or continue recycling stranded effects (including those caused by tax reform) through earnings in future periods. Further, disclosure of either policy is required in all cases. The reclassification from AOCI to retained earnings is presented in the statement of shareholders equity. The ASU is effective for all entities in fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted for public business entities for which financial statements have not yet been issued, and for all other entities for which financial statements have not yet been made available for issuance. Entities have the option to record the reclassification either retrospectively to each period in which the income tax effects of tax reform are recognized, or at the beginning of the annual or interim period in which the amendments are adopted. The Company determined that the adoption of this new standard will have no material impact on its consolidated statements and related disclosures.
F - 12
In May 10, 2017, the FASB issued ASU 2017-09, Compensation—Stock Compensation (Topic 718) - Scope of Modification. ASU 2017-09 clarifies when to account for a change to the terms or conditions of a share-based payment award as a modification. Under the new guidance, modification accounting is required only if the fair value, the vesting conditions, or the classification of the award (as equity or liability) changes as a result of the change in terms or conditions. Regardless of whether the change to the terms or conditions of the award requires modification accounting, the existing disclosure requirements and other aspects of GAAP associated with modifications, such as earnings per share, continue to apply. The guidance is effective prospectively for all companies for annual periods beginning on or after December 15, 2017. Early adoption is permitted. The Company is currently evaluating the potential impact of adopting this new standard on its consolidated statements and related disclosures.
In January 2017, FASB issued ASU 2017-01, "Business Combinations (Topic 805) - Clarifying the Definition of a Business". The Board is issuing the amendments in this ASU to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The definition of a business affects many areas of accounting including acquisitions, disposals, goodwill, and consolidation. The amendments in this ASU affect all reporting entities that must determine whether they have acquired or sold a business. Public business entities should apply the amendments in this ASU to annual periods beginning after December 15, 2017, including interim periods within those periods. However, early application of the amendments in this ASU if 1) for transactions for which the acquisition date occurs before the issuance date or effective date of the amendments, only when the transaction has not been reported in financial statements that have been issued or made available for issuance, or 2) for transactions in which a subsidiary is deconsolidated or a group of assets is derecognized that occur before the issuance date or effective date of the amendments, only when the transaction has not been reported in financials. The Company determined that its recent acquisition of the Acer Truncatum Industrial Project met the early application requirement and decided to account for the acquisition in accordance with FASB ASU 2017-01.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). Under this guidance, lessees will be required to recognize on the balance sheet a lease liability and a right-of-use asset for all leases, with the exception of short-term leases. The lease liability represents the lessee's obligation to make lease payments arising from a lease, and will be measured as the present value of the lease payments. The right-of-use asset represents the lessee's right to use a specified asset for the lease term, and will be measured at the lease liability amount, adjusted for lease prepayment, lease incentives received and the lessee's initial direct costs. The standard also requires a lessee to recognize a single lease cost allocated over the lease term, generally on a straight-line basis. The new guidance is effective for fiscal years beginning after December 15, 2018. ASU 2016-02 is required to be applied using the modified retrospective approach for all leases existing as of the effective date and provides for certain practical expedients. Early adoption is permitted. The Company is currently evaluating the effects that the adoption of ASU 2016-02 will have on the Company's consolidated financial statements.
NOTE 3 – OPERATING LEASES
On October 1, 2011, the Company entered into an agreement with Shandong Yongchuntang Group Co., Ltd. ("Shandong Yongchuntang") for the lease of one automobile. The lease term is from October 1, 2011 to September 30, 2021. The total lease payment of RMB131,468 (approximately USD21,000) was paid in full upon signing the lease agreement and is being amortized over the life of the lease.
On June 20, 2013, the Company entered into a Farmland Leasing Agreement with Shiqiao Village for the lease of 2,000 mu (approximately 790.74 acres) farmland for the development of the acer truncatum bunge planting bases. The lease term is from July 1, 2013 to June 30, 2043. The lease payment is RMB1,000 (approximately USD159) per mu annually and is paid every five years in advance. The first lease payment was for the rents of the first five years in the amount of RMB10,000,000 (approximately USD1.6 million), which was paid on July 10, 2013. On March 8, 2017, the Company returned 120 mu (approximately 47.44 acres) of the leased farmland to the lessor because the lessor needed to use the farmland for a strategic development project that is led by the local government. Based on the March 8, 2017 agreement between the Company and the lessor, the Company's remaining prepaid lease of RMB180,000 (approximately USD28,600) for the 120 mu (approximately 47.44 acres) farmland will be used to reduce the next prepayment on the lease. The acer truncatum bunge plants on the 120 mu (approximately 47.44 acres) farmland were moved to a nearby location. As of March 31, 2018, the total leased farmland in Shiquiao Village is 1880 mu (approximately 743.29 acres).
F - 13
On March 1, 2014, the Company entered into a Farmland Leasing Agreement with Zhongce No.4 Village for the lease of 200 mu (approximately 79.07 acres) farmland for the development of the acer truncatum bunge planting bases. The lease term is from March 1, 2014 to February 28, 2044. The lease payment is RMB1,000 (approximately USD159) per mu annually and is paid every five years in advance. The first lease payment was for the rents of the first five years in the amount of RMB1,000,000 (approximately USD159,000), which was paid on March 10, 2014. On April 1, 2017, the Company entered an agreement with Zhongce No.4 Village to terminate the lease for the 200 mu (approximately 79.07 acres) farmland because the parcel of farmland was recalled by local government for building a new urban district. Based on the agreement, the Company received the refund of all the remaining prepaid lease payment of RMB 383,333 (approximately USD58,000) as well as a compensation of RMB 2,400,000 (approximately USD360,000) from the government for the early termination of the lease. (See Note 6).
On January 7, 2015, the Company entered into a Farmland Leasing Agreement with Shandong Wanziyuan Tourism Development Co. to lease 2,000 mu (approximately 790.74 acres) farmland for the development of the acer truncatum bunge planting bases. The lease term is from January 15, 2015 to December 31, 2029. The lease payment is RMB1,000 (approximately USD159) per mu annually and is paid every five years in advance. The first lease payment was for the rents of the first five years in the amount of RMB10,000,000 (approximately USD1.6 million), which was paid on January 8, 2015.
On July 2, 2015, the Company entered into a Farmland Leasing Agreement with Zhongce Shen Village for the lease of 2,000 mu (approximately 790.74 acres) farmland for the development of the acer truncatum bunge planting bases. The lease term is from July 2, 2015 to July 2, 2029. The lease payment is RMB1,000 (approximately USD159) per mu annually and is paid every five years in advance. The first lease payment was for the rents of the first five years in the amount of RMB10,000,000 (approximately USD1.6 million), which was paid on July 3, 2015.
The Company accounts for the lease agreements as operating leases in accordance to ASC 840-10-25-37, which requires the lessee to account for the lease as a capital lease, if land is the sole item of property leased and either the transfer-of-ownership criterion in paragraph 840-10-25-1(a) or the bargain-purchase-option criterion in paragraph 840-10-25-1(b) is met. Otherwise, the lessee shall account for the lease as an operating lease. Per ASC 840-2-25-1, rent is charged to expense by lessees over the lease term.
The components of prepaid lease were as follows:
|
As of March 31,
|
||||||||||||||||
|
2018
|
2017
|
|||||||||||||||
|
Current portion
|
Long-term
|
Current portion
|
Long-term
|
|||||||||||||
|
Shiqiao Village – 1880 mu
|
$
|
103,370
|
$
|
-
|
$
|
289,884
|
$
|
72,471
|
||||||||
|
Shandong Wanziyuan – 2000 mu
|
318,061
|
238,546
|
289,884
|
507,298
|
||||||||||||
|
Zhongce No. 4 Village – 200 mu
|
-
|
-
|
28,989
|
26,573
|
||||||||||||
|
Zhongce Shen Village – 2000 mu
|
318,061
|
397,576
|
289,884
|
652,240
|
||||||||||||
|
Total prepaid land lease
|
739,492
|
636,122
|
898,641
|
1,258,582
|
||||||||||||
|
Shandong Yongchuntang - Automobile
|
2,091
|
5,227
|
1,906
|
6,670
|
||||||||||||
|
Total prepaid lease
|
$
|
741,583
|
$
|
641,349
|
$
|
900,547
|
$
|
1,265,252
|
||||||||
The prepaid lease is amortized over prepaid period based on straight-line method. The lease expenses for the years ended March 31, 2018 and 2017 were $884,950 and $923,380, respectively.
F - 14
NOTE 4 - INVENTORIES
The components of inventories were as follows:
|
March 31,
|
March 31,
|
|||||||
|
2018
|
2017
|
|||||||
|
Raw materials
|
$
|
233,138
|
$
|
1,276,254
|
||||
|
Packaging materials
|
652,179
|
476,803
|
||||||
|
Work-in-process
|
686,234
|
1,373,919
|
||||||
|
Finished goods
|
811,831
|
2,356,064
|
||||||
|
Total Inventories
|
$
|
2,383,382
|
$
|
5,483,040
|
||||
No allowances for obsolete or unsalable inventories were made for the years ended March 31, 2018 and 2017.
NOTE 5 – PLANT, PROPERTY, AND EQUIPMENT, NET
The components of plant, property and equipment were as follows:
|
March 31,
|
March 31,
|
|||||||
|
2018
|
2017
|
|||||||
|
Machinery and equipment
|
$
|
3,594,861
|
$
|
2,254,813
|
||||
|
Office equipment and automobiles
|
769,589
|
717,259
|
||||||
|
Building
|
13,606,722
|
12,401,320
|
||||||
|
Leasehold improvements
|
4,240,568
|
2,803,052
|
||||||
|
Subtotal
|
22,211,740
|
18,176,444
|
||||||
|
Less: Accumulated depreciation and amortization
|
(5,418,327
|
)
|
(3,689,309
|
)
|
||||
|
Total plant, property and equipment, net
|
$
|
16,793,413
|
$
|
14,487,135
|
||||
The depreciation and amortization expense of plant, property and equipment for the years ended March 31, 2018 and 2017 was $1,300,651 and $746,931, respectively.
NOTE 6 – DEVELOPMENT OF ACER TRUNCATUM BUNGE
Development costs primarily include land development cost incurred for land leveling, irrigation, and fertilization, the purchase costs of acer truncatum bunge trees, and acer truncatum bunge planting fee.
Since July 2013, the Company has developed the acer truncatum bunge planting bases. As of March 31, 2017, the Company had completed planting of 6,080 mu (approximately 2,403.84 acres) at four leased farmlands. On April 1, 2017, the Company entered an agreement with Zhongce No.4 Village to terminate the lease for the 200 mu (approximately 79.07 acres) farmland because the parcel of farmland was recalled by the local government to build a new urban district. Based on the agreement, the Company received a refund of all the remaining prepaid lease payments of approximately $58,000 (RMB383,333) and compensation of approximately $362,000 (RMB2,400,000) from the government for the early termination of the lease. On May 1, 2017, the Company signed two contracts with third parties to sell all of the plants from the farmland for approximately $2,042,000 (RMB13,527,000). The total capitalized cost of the acer truncatum bunge planting in Zhongce No.4 Village farmland was approximately $1,514,000 (RMB10,030,710). In addition, the Company incurred approximately $247,000 (RMB1,639,260) in expenses to pack the plants for sale. The gain from the disposition of the acer truncatum bunge plants was approximately $643,000 (RMB4,257,030). As a result of the lease termination, the Company's acer truncatum bunge planting acreage was reduced to 5,880 mu (approximately 2,324.77 acres) at March 31, 2018 from 6,080 mu (approximately 2,403.84 acres) at March 31, 2017.
The Company accounts for the development costs of the planting in accordance to ASC 905. Pursuant to ASC 905-360-25-3, limited-life land development costs, direct and indirect development costs of orchards, groves, vineyards, and intermediate-life plants shall be capitalized during the development period. During the years ended March 31, 2018 and 2017, approximately 1% and 2.2% of the acer truncatum bunge plants were dead or lost due to the weather, replanting of trees, relocating of trees, and other natural or technical reasons as per management's estimation. Impairment of $332,090 and $986,406 was recorded during the years ended March 31, 2018 and 2017.
F - 15
NOTE 7 - INTANGIBLE ASSETS, NET
The intangible assets consist of the following:
|
As of
|
|||||||||
| Amortization
Period
|
March 31,
2018 |
March 31,
2017 |
|||||||
|
Land use right
|
50 years
|
$
|
1,613,842
|
$
|
1,470,875
|
||||
|
Patent (non-US No. ZL200610068850.0)
|
16.5 years
|
7,315,405
|
6,667,343
|
||||||
|
Patent (non-US No. ZL200510045001.9)
|
14 years
|
9,859,894
|
8,986,419
|
||||||
|
Intangible assets for production and marketing of Yuanbaofen product
|
10 years
|
1,755,443
|
1,599,930
|
||||||
|
Less: Accumulated amortization
|
(8,682,567
|
)
|
(6,681,809
|
)
|
|||||
|
Intangible assets, net
|
$
|
11,862,017
|
$
|
12,042,758
|
|||||
(i)
Land Use Rights:
All land in the PRC is owned by the government and cannot be sold to any individual or company. However, the government may grant a "land use right" for occupying, developing and using land. The Company records land use rights obtained as intangible assets at cost, which is amortized on the straight line method over the grant period of 50 years.
(ii)
Patents:
In March 2010, the Company purchased one patent use right (ZL200610068850.0) from Shandong Yongchuntang. The Company has exclusive right to use an aglycone type and purification method of biotransformation in the gingko product manufacturing process for a period of 20 years from the patent application date.
In October 2011, based on a purchase agreement signed with Jining Tianruitong Technology development Company, Limited on October 26, 2010, the Company acquired a patent "Treatment to ischemic encephalopathy and its preparation method" (ZL200510045001.9). The patent was recorded at cost when purchased, and is being amortized over its legal life on a straight-line basis. The patent's legal life is considered shorter than its remaining useful life.
F - 16
(iii)
Intangible assets for production and marketing of Yuanbaofen product:
On March 18, 2017, the Company entered into an acquisition agreement (the "Agreement") with Shandong Yongchuntang Group Co., Ltd. ("Shandong Yongchuntang") to acquire a group of tangible and intangible assets for producing and marketing acer truncatum products. Pursuant to the Agreement, the Company agreed to transfer 3% equity of Shandong Spring Pharmaceutical to Shandong Yongchuntang in exchange for a group of research equipment and a group of intangible assets related to Acer Truncatum Industrial Project (the "Project). The group of the intangible assets includes a patent on the refinery process of the acer truncatum seed oils, a trademark with the name of "Bao Feng San Yi", and certain related certifications. The fair value of assets acquired is approximately $2,338,000 (RMB14,701,200). Approximately $583,000 (RMB3,662,800) was allocated to research equipment acquired and approximately $1,755,000 (RMB11,038,400) was allocated to intangible assets acquired. This acquisition is considered as an asset acquisition as per ASU 2017-01, "Business Combinations (Topic 805). The excess amount of consideration paid over the fair market value of assets acquired was $448,690, which was recorded as a reduction to the Company's equity. The tangible and intangible assets acquired related to the Project are being depreciated or amortized over the useful life of 3 to 10 years on a straight-line basis.
Pursuant to FASB ASC 350-30-50-3, the Company conducted its annual test for impairment on intangible assets, related to the project as of March 31, 2018 and 2017. No impairment of intangible assets was recorded in the years ended March 31, 2018 and 2017.
The amortization expense of land use right for the years ended March 31, 2018 and 2017 was $30,634 and $30,163, respectively.
The amortization expense of patents for the years ended March 31, 2018 and 2017 was $1,085,255 and $1,068,594, respectively.
The amortization expense of intangible assets for production and marketing of the Yuanbaofen product for the years ended March 31, 2018 and 2017 was $166,606 and $0, respectively.
NOTE 8 – SECURITY DEPOSIT – RELATED PARTY
"Security deposit – related party" represents the deposit paid to Shandong Yongchuntang for using the direct-sales license issued to Shandong Yongchuntang.
The Company was permitted by Shandong Yongchuntang to sell Shandong Yongchuntang's products using the direct-sales license issued to Shandong Yongchuntang from July 1, 2015 to June 30, 2020. To ensure the appropriate use of the direct-sales license in the market, the Company provided a deposit of $1,590,305 (RMB10,000,000) to Shandong Yongchuntang as a marketing deposit based on an agreement between the Company and Shandong Yongchuntang, which was signed on January 4, 2017. If no violations of rules or regulations related to the direct-sales occur during the contract period, the security deposit will be fully returned to the Company at the end of the contract period. The amount of security deposit is non-interest bearing and is not secured.
NOTE 9 - TAXES PAYABLE
Taxes payable consists of the follows:
|
As of
|
||||||||
|
March 31,
|
March 31,
|
|||||||
|
2018
|
2017
|
|||||||
|
Corporate Income Tax - Foreign
|
$
|
519,875
|
$
|
1,382,382
|
||||
|
Value-Added Tax - Foreign
|
580,429
|
576,086
|
||||||
|
Other Tax & Fees - Foreign
|
63,894
|
69,722
|
||||||
|
Total Taxes Payable
|
$
|
1,164,198
|
$
|
2,028,190
|
||||
F - 17
NOTE 10 - INCOME TAXES
China YCT International Group, Inc. and Landway Nano were incorporated in the United States of America and are subject to United States federal taxation. No provisions for income taxes have been made, as there was no taxable income from U.S. operations for the years ended March 31, 2018 and 2017. The Company has net loss carryforward of approximately $22,000, which will expire in 2037. The Company has set up 100% valuation allowance on deferred tax assets resulting from net operating loss incurred in the U.S.
The U.S. Tax Cuts and Jobs Act (the "Act") was enacted on December 22, 2017 and introduces significant changes to U.S. income tax law. Effective in 2018, the Tax Act reduces the U.S. statutory tax rate from 35% to 21% and creates new taxes on certain foreign-sourced earnings and certain related-party payments, which are referred to as the global intangible low-taxed income tax and the base erosion tax, respectively. The Company's deferred tax assets and liabilities were remeasured to reflect the reduction in the U.S. corporate income tax rate from 35% to 21%, resulting in a deferred tax expense of $3,095 for the year ended March 31, 2018 which was offset by the change of valuation allowance as of March 31, 2018. This expense is attributable to the Company being in a net deferred tax asset position at the time of remeasurement. This amount can be seen on the rate reconciliation as an adjustment to deferred tax asset and corresponding valuation allowance.
The Company's Chinese subsidiary is governed by the Income Tax Law of the PRC concerning the privately run and foreign invested enterprises, which are generally subject to tax at a statutory rate of 25% on income reported in the statutory financial statements after appropriate tax adjustments.
Dividend payments by PRC subsidiaries are limited by certain statutory regulations in the PRC. No dividends may be paid by PRC subsidiaries without first receiving prior approval from SAFE. Dividend payments are restricted to 90% of after tax profits.
The Company had not provided deferred taxes on undistributed earnings attributable to its PRC subsidiaries as they were to be permanently reinvested. On February 22, 2008, the Ministry of Finance and State Administration of Taxation jointly issued Cai Shui 2008 Circular 1, "Circular 1." According to Article 4 of Circular 1, distributions of accumulated profits earned by foreign investment enterprises ("FIE") prior to January 1, 2008 to their foreign investors would be exempt from withholding tax ("WHT"), while distribution of the profits earned by a FIE after January 1, 2008 to its foreign investors should be subject to WHT.
Prior to the enactment of the Act, since Shandong Spring Pharmaceutical intends to reinvest its earnings to further expand its businesses in mainland China, it does not intend to declare dividends to their immediate foreign holding companies in the foreseeable future. Accordingly, the Company has not recorded any deferred taxes in relation to US tax on the cumulative amount of undistributed retained earnings since January 1, 2008. Under the "#1703. Treatment of deferred foreign income upon transition to new participation exemption system-deemed repatriation" of the Act, U.S. shareholders owning at least 10% of a foreign subsidiary generally must include income, for the subsidiary's last tax year beginning before 2018, the shareholder's pro rata share of the accumulated post-'86 historical E&P of the foreign subsidiary as of the "measurement date" to the extent such E&P has not been previously subject to U.S. tax. The measurement date is November 2, 2017, or December 31, 2017, whichever date produces a greater result. The portion of the E&P comprising cash or cash equivalents is taxed at a reduced rate of 15.5%, while any remaining E&P is taxed at a reduced rate of 8%. At the election of the U.S. shareholder, the tax liability is payable over a period of up to eight years. The payments for each of the first five years equal 8% of the net tax liability. The amount of the sixth installment is 15% of the net tax liability, increasing to 20% for the seventh installment and the remaining balance of 25% in the eighth year.
As of March 31, 2018, the Company has conducted a preliminary assessment of its income tax effects of the Act, and based on the analysis, the Company concluded that it had no one-time transition tax liability on its cumulative amount of undistributed retained earnings since January 1, 2008 as the Company had sufficient foreign tax credits available to offset the resulting incremental tax. However, further regulatory guidance related to the Act may be expected to be issued during 2018, which may result in changes to the Company's estimates. In this case, additional analysis of the Act and the impact to the Company will be performed, and any impact will be finalized no later than the fourth quarter of 2018.
F - 18
The reconciliation of income tax expense at the U.S. statutory rate of 35% for the years ended March 31, 2018 and 2017 to the Company's effective tax rate is as follows:
|
Years Ended
|
||||||||
|
March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
U.S. Statutory rate
|
$
|
5,452,062
|
$
|
4,639,348
|
||||
|
Tax rate difference between China and U.S.
|
(1,557,732
|
)
|
(1,327,739
|
)
|
||||
|
Change in valuation allowance
|
-
|
7,738
|
||||||
|
Permanent difference
|
(55,498
|
)
|
(118,722
|
)
|
||||
|
Effective tax rate
|
$
|
3,838,832
|
$
|
3,200,625
|
||||
The provisions for income taxes are summarized as follows:
|
Years Ended
|
||||||||
|
March 31,
|
||||||||
|
2018
|
2017
|
|||||||
|
Current
|
$
|
3,499,474
|
$
|
3,520,126
|
||||
|
Deferred
|
339,358
|
(319,501
|
)
|
|||||
|
Total
|
$
|
3,838,832
|
$
|
3,200,625
|
||||
The tax effects of temporary differences that give rise to the Company's net deferred tax assets as of March 31, 2018 and 2017 are as follows:
|
March 31,
|
March 31,
|
|||||||
|
2018
|
2017
|
|||||||
|
Impairment loss
|
$
|
87,476
|
$
|
240,504
|
||||
|
Revenue and cost
|
72,658
|
181,166
|
||||||
|
Depreciation
|
-
|
50,514
|
||||||
|
Others
|
40,253
|
36,337
|
||||||
|
Loss carry forward
|
7,738
|
7,738
|
||||||
|
Effect of rate change
|
(3,095
|
)
|
-
|
|||||
|
Less valuation allowance
|
(4,643
|
)
|
(7,738
|
)
|
||||
|
Total net deferred tax assets
|
$
|
200,387
|
$
|
508,521
|
||||
F - 19
NOTE 11 - STOCKHOLDERS' EQUITY
Stock issued for compensation and service
On April 19, 2016, in accordance with the Company's agreement with an independent director, the Company issued 43,478 shares of common stock to one independent director, which were valued at $10,609 based on the quoted market price at issuance date.
On March 10, 2017, in accordance with the Company's service agreement with an investor relations firm, the Company issued 25,000 shares of common stock to the firm for the services provided, which were valued at $11,500 based on the quoted market price at issuance date.
Noncontrolling interest
On March 18, 2017, the Company entered into an Acquisition Agreement on Acer Truncatum Industrial Project (the "Agreement") with Shandong Yongchuntang. Pursuant to the Agreement, the Company agreed to transfer 3% equity of Shandong Spring Pharmaceutical in exchange for tangible and intangible assets related to the Acer Truncatum Industrial Project (the "Project"), which was owned by Shandong Yongchuntang. The assets acquired include research equipment, a patent on the refinery process of the acer truncatum's seed oils, a trademark with the name of "Bao Feng San Yi", and some relevant certifications issued to Shandong Yongchuntang. (See NOTE 7).
Statutory Reserve
Subsidiaries incorporated in China are required to make appropriations to reserve funds, based on after-tax net income determined in accordance with generally accepted accounting principles of the People's Republic of China ("PRC GAAP"). Effective January 1, 2006, the Company is only required to contribute to one statutory reserve fund at 10% of net income after tax per annum, and any contributions are not to exceed 50% of the respective companies' registered capital.
The Company appropriated nil to the statutory reserve for the years ended March 31, 2018 and 2017, respectively.
Stock Option Plan
On July 23, 2015, the Company adopted a stock option plan that was approved by its Board of Directors on June 15, 2015. This plan was intended to retain and provide incentives for talented employees, officers and directors, and to align stockholder and employee interests. Under this stock option plan, the participants of the plan include the Company's directors, officers and some employees who were previously determined by the Board of Directors. On July 23, 2015, the Company signed stock option agreements with each participant and granted options to purchase a total of 2.6 million shares of Common Stock to the participants. The vesting period of the stock options was ten months from July 23, 2015, the grant date of the stock options. Immediately following the date when the stock options were vested, the participants would have five consecutive business days to exercise the stock options at an exercise price of $0.40 per share. Stock options not exercised within the five consecutive business days would expire. The Company assessed the fair value of the total granted stock options on the grant date using a Black-Scholes Stock Option Pricing Model. Significant assumptions used in calculating fair value of options are as follows:
F - 20
|
•
|
Expected volatility 92.03%;
|
|
•
|
Risk-free interest rate 0.33%;
|
|
•
|
Expected term (year) 0.85;
|
|
•
|
Exercise price $0.4.
|
The estimated fair value of the total granted stock options on the grant date was $529,100, which is being amortized over ten months. All stock options expired on May 30, 2016 and none of the vested stock options were exercised by the end of the option exercise date. The total estimated fair value of the stock options in amount of $529,100 had been fully amortized as of March 31, 2017. For the years ended March 31, 2018 and 2017, the amortization of stock-based compensation expense was $0 and $101,026, respectively.
A summary of the changes in stock options outstanding under the Company's stock option plan for the years ended March 31, 2018 and 2017 is presented below:
|
Weighted
|
||||||||
|
Average
|
||||||||
|
Grant Date
|
||||||||
|
Shares
|
Fair Value
|
|||||||
|
Options outstanding at March 31, 2016
|
2,600,000
|
$
|
529,100
|
|||||
|
Granted
|
-
|
-
|
||||||
|
Exercised
|
-
|
-
|
||||||
|
Canceled
|
-
|
-
|
||||||
|
Expired
|
2,600,000
|
529,100
|
||||||
|
Options outstanding at March 31, 2017
|
-
|
$
|
-
|
|||||
|
Granted
|
-
|
-
|
||||||
|
Exercised
|
-
|
-
|
||||||
|
Canceled
|
-
|
-
|
||||||
|
Expired
|
-
|
-
|
||||||
|
Options outstanding at March 31, 2018
|
-
|
$
|
-
|
|||||
NOTE 12 - RELATED PARTY TRANSACTIONS AND BALANCES
Balances:
(i) Security deposit to related party:
The security deposit to related party of $1,590,305 represents the deposit paid to Shandong Yongchuntang on January 4, 2017 for using the direct-sales license issued to Shandong Yongchuntang. The amount is non-interest bearing and not secured. Shandong Yongchuntang owns 3% of the equity of Shandong Spring Pharmaceutical.
(ii) Trade related balance with related party:
On March 31, 2018, purchase deposit to related party of $1,412,864 pertains to a purchase deposit paid in respect of the purchase of healthcare products from Shandong Yongchuntang.
On March 31, 2017, accounts payable balance of $706,048 pertains to a payable in respect of the purchase of healthcare products from Shandong Yongchuntang.
F - 21
Transactions:
(i) Acquisition of assets
On March 18, 2017, 3% equity of Shandong Spring Pharmaceutical was transferred to Shandong Yongchuntang in exchange for tangible and intangible assets related to Acer Truncatum Industrial Project. (See Note 7 and Note 11.)
(ii) Sales to related party
During the years ended March 31, 2018 and 2017, the Company sold acer truncatum seed oil products to Shandong Yongchuntang for $337,535 and $134,413, respectively.
(iii) Purchase from related party (See Note 13)
Contingency:
The Company is authorized by Shandong Yongchuntang to sell Shandong Yongchuntang's products using the direct-sales license issued to Shandong Yongchuntang. As a condition for using the direct-sales license, the Company needs to make a 20% sales increase each year based on 95% of sales in the year 2014. If the Company cannot meet this sales target in any year from April 1, 2017 to June 30, 2020, a security deposit of approximately $1.6 million will be used as an annual fee for using the direct-sales license. There is a risk that the Company may fail to meet the sales target and may need to pay approximately $1.6 million in the subsequent years.
NOTE 13 - MAJOR CUSTOMERS AND VENDORS
The Company sold products mostly through ten distributors during the years ended March 31, 2018 and 2017. Sales to four distributors represented 17%, 16%, 12%, and 12% of total sales for the year ended March 31, 2018. Sales to four distributors represented 18%, 13%, 12%, and 11% of total sales for the year ended March 31, 2017.
The Company sold 11 and 14 products during the years ended March 31, 2018 and 2017. Sales of two products represented 40% and 19% of total sales for the year ended March 31, 2018. Sales of three products represented 44%, 19% and 14% of total sales for the year ended March 31, 2017.
The Company purchases its products from Shandong Yongchuntang, a related party, according to the purchase contract signed between the Company and Shandong Yongchuntang. Pursuant to the renewed one year contract dated February 20, 2017, the Company agreed to purchase nine products from Shandong Yongchuntang at fixed prices. On February 21, 2018, the Company further renewed the purchase contract with Shandong Yongchuntang for a term of one year ending on February 25, 2019. Pursuant to this most recently renewed one year contract, the Company continue to purchases the nine products from Shandong Yongchuntang at fixed prices without changes in any terms of the previous contract. Total purchases from Shandong Yongchuntang represented 42% and 36% of our total purchases during the years ended March 31, 2018 and 2017, respectively. The revenue from sale of products purchased from Shandong Yongchuntang represented 41% and 36% of total revenue for the years ended March 31, 2018 and 2017, respectively. The purchases from two other vendors represented 25% and 16% of the Company's total purchases for the year ended March 31, 2018. The purchases from three other vendors represented 24%, 15% and 12% of the Company's total purchases for the year ended March 31, 2017.
F - 22
NOTE 14 – FUTURE MINIMUM LEASE PAYMENTS
As of March 31, 2018, future minimum lease payments under the operating leases pursuant to the three Farmland Leasing Agreements were as follows:
|
Zhongce
|
Total
|
|||||||||||||||
|
Shiqiao
|
Shandong
|
Shen
|
Operating
|
|||||||||||||
|
Fiscal year ended March 31,
|
Village
|
Wanziyuan
|
Village
|
Leases
|
||||||||||||
|
2019
|
$
|
1,466,261
|
$
|
-
|
$
|
-
|
$
|
1,466,261
|
||||||||
|
2020
|
-
|
1,590,305
|
-
|
1,590,305
|
||||||||||||
|
2021
|
-
|
-
|
1,590,305
|
1,590,305
|
||||||||||||
|
2022
|
-
|
-
|
-
|
-
|
||||||||||||
|
2023
|
-
|
-
|
-
|
-
|
||||||||||||
|
2024 and thereafter
|
5,979,548
|
1,272,244
|
1,272,244
|
8,524,036
|
||||||||||||
|
Total minimum lease payments
|
$
|
7,445,809
|
$
|
2,862,549
|
$
|
2,862,549
|
$
|
13,170,907
|
||||||||
The lease of Zhongce No.4 Village farmland was terminated by the lessor on April 1, 2017. Therefore, there are no more future payments for the canceled lease of Zhongce No.4 Village farmland.
NOTE 15 – SUBSEQUENT EVENTS
On April 21, 2018, the Company's Board of Directors approved a stock incentive plan that its adoption date is still subject to approval within twelve (12) months by the stockholders of the Company. The plan covers both stock option and stock award programs. The participants of the stock incentive will be determined by the Board Committee who is appointed by the Board of Directors. The stock incentive plan is intended to promote the best interests of the Company and its stockholders by (i) assisting the Company and its Affiliates in the recruitment and retention of persons with ability and initiative, (ii) providing an incentive to such persons to contribute to the growth and success of the Corporation's businesses by affording such persons equity participation in the Corporation and (iii) associating the interests of such persons with those of the Corporation and its Affiliates and stockholders. No fixed number of common stock shares has been reserved for this plan although the share reserve and grant limitation are defined in the plan. In the event that the stockholders of the Corporation shall not approve this Plan within such twelve (12) month period, this Plan shall terminate.
On June 4, 2018, the Company issued 50,000 shares of common stock to a consultant for the marketing consulting services to be provided for the period from June 1, 2018 to May 31, 2021, which were valued at $41,000 based on the quoted market price at issuance.
The Company has evaluated subsequent events that have occurred after the date of the balance sheet through the date of issuance of these consolidated financial statements and determined that no other subsequent event requires recognition or disclosure to the consolidated financial statements.
F - 23
[●] Shares of Common Stock
China YCT International Group, Inc.

PROSPECTUS
Sole Book-Running Manager
Maxim Group LLC
, 2018
i
PART II- INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance And Distribution.
Set forth below is an itemization of the total expenses, excluding placement discounts and commissions, that we expect to incur in connection with this offering. With the exception of the SEC registration fee, the FINRA filing fee and the NASDAQ Capital Market listing fee, all amounts are estimates.
|
Securities and Exchange Commission Registration Fee
|
$
|
1,148.98
|
|
|
NASDAQ Capital Market Listing Fee*
|
|||
|
FINRA Filing Fees
|
1,884.32
|
||
|
Legal Fees and Expenses*
|
[●]
|
||
|
Accounting Fees and Expenses*
|
[●]
|
||
|
Printing and Mailing Expenses*
|
[●]
|
||
|
Miscellaneous Expenses*
|
[●]
|
||
|
Total Expenses*
|
$ [●]
|
||
* To be filed by amendment
All amounts are estimates other than the SEC's registration fee. We are paying all expenses of the offering listed above.
Item 14. Indemnification of Directors and Officers
We are governed by the Delaware General Corporation Law, or DGCL. Section 145 of the DGCL provides that a corporation may indemnify any person, including an officer or director, who was or is, or is threatened to be made, a party to any threatened, pending or completed legal action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was or is an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such officer, director, employee or agent acted in good faith and in a manner such person reasonably believed to be in, or not opposed to, the corporation's best interest and, for criminal proceedings, had no reasonable cause to believe that such person's conduct was unlawful. A Delaware corporation may indemnify any person, including an officer or director, who was or is, or is threatened to be made, a party to any threatened, pending or contemplated action or suit by or in the right of such corporation, under the same conditions, except that such indemnification is limited to expenses (including attorneys' fees) actually and reasonably incurred by such person, and except that no indemnification is permitted without judicial approval if such person is adjudged to be liable to such corporation. Where an officer or director of a corporation is successful, on the merits or otherwise, in the defense of any action, suit or proceeding referred to above, or any claim, issue or matter therein, the corporation must indemnify that person against the expenses (including attorneys' fees) which such officer or director actually and reasonably incurred in connection therewith.
Reference is made to Section 102(b)(7) of the DGCL, which enables a corporation in its certificate of incorporation to eliminate or limit the personal liability of a director for violations of the director's fiduciary duty, except (i) for any breach of the director's duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL, which provides for liability of directors for unlawful payments of dividends of unlawful stock purchase or redemptions or (iv) for any transaction from which a director derived an improper personal benefit.
Consistent with Section 145 of the Delaware General Corporation Law, Article VIII of our By-laws, as amended, provide that our directors shall be indemnified by the Company to the fullest extent permitted by Delaware law. We believe that the indemnification provisions in our By-laws, as amended, are necessary to attract and retain qualified persons as directors.
The foregoing right of indemnification shall not be exclusive of other rights to which those seeking an indemnification may be entitled. The Company may maintain insurance, at its expense, to protect itself and all officers and directors against fines, liabilities, costs and expenses, whether or not the Company would have the legal power to indemnify them directly against such liability.
If this indemnification or any portion of it is invalidated on any ground by a court of competent jurisdiction, the Company nevertheless indemnifies each person described above to the fullest extent permitted by all portions of this indemnification that have not been invalidated and to the fullest extent permitted by law.
Indemnification Against Public Policy
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
ii
Item 15. Recent Sales of Unregistered Securities
None
Item 16. Exhibits.
|
1.1
|
Form of Underwriting Agreement.*
|
|
3.1
|
|
|
|
|
|
3.2
|
|
|
|
|
|
3.3
|
|
|
|
|
|
4.1
|
|
4.2
|
|
|
|
|
|
4.3
|
Form of Underwriters' Warrant (included in Exhibit 1.1).*
|
|
5.1
|
Opinion of McLaughlin & Stern, LLP *
|
|
10.1
|
|
|
|
|
|
10.2
|
|
|
|
|
|
10.3
|
|
|
|
|
|
10.4
|
|
|
|
|
|
10.5
|
|
|
|
|
|
10.6
|
|
|
|
|
|
10.7
|
|
|
|
|
|
10.8
|
|
|
|
|
|
10.9
|
|
|
|
|
|
10.10
|
|
|
|
|
|
10.11
|
|
|
|
|
|
10.12
|
iii
|
10.13
|
|
|
14.1
|
|
|
|
|
|
14.2
|
|
|
|
|
|
14.3
|
|
|
|
|
|
21.1
|
|
|
|
|
|
23.1
|
Consent of Paritz & Company, P.A.
|
|
23.2
|
Consent McLaughlin & Stern, LLP. (contained in Exhibit 5.1 hereto). *
|
|
24.1
|
Power of Attorney* (filed herewith)
|
*To be filed by amendment
iv
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
v
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(4) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(5) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering
(6) That, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(7) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
vi
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Jining on July 13, 2018.
|
|
CHINA YCT INTERNATIONAL GROUP, INC.
|
|
|
|
|
|
By: /s/ Tinghe Yan
|
|
|
Name: Tinghe Yan
|
|
|
Title: Chief Executive Officer
|
|
|
(Principal Executive Officer)
|
|
|
By: /s/ Chuanmin Li
|
|
|
Name: Chuanmin Li
|
|
|
Title: Chief Financial Officer
|
|
|
(Principal Executive Officer)
|
Know all men by these presents, that each of the undersigned directors and officers of China YCT International Group, Inc., a Delaware corporation, which is filing a Registration Statement on Form S-1 with the Securities and Exchange Commission, Washington, D.C. 20549 under the provisions of the Securities Act of 1933, hereby constitutes and appoints Tinghe Yan and Zecheng Shao, and each of them, the individual's true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for the person and in his or her name, place and stead, in any and all capacities, to sign such registration statement and any or all amendments, including post-effective amendments to the registration statement, including a prospectus or an amended prospectus therein and any registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act, and all other documents in connection therewith to be filed with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact as agents, or any of them, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
By: /s/ Tinghe Yan
|
|
Name: Tinghe Yan,
|
|
Title: Director, Chief Executive Officer
|
|
(Principal Executive Officer)
|
|
Date: July 13, 2018
|
|
|
|
By: /s/ Robert Fanella
|
|
Name: Robert J. Fanella
|
|
Title: Director
|
|
Date: July 13, 2018
|
|
|
|
By: /s/ Chuanmin Li
|
|
Name: Chuanmin Li
|
|
Title: Chief Financial Officer
|
|
(Principal Financial Officer)
|
|
Date: July 13, 2018
|
|
|
|
By: /s/ Wengao Zhang
|
|
Name: Wengao Zhang
|
|
Title: Director
|
|
Date: July 13, 2018
|
|
|
|
By: /s/ Jirui Zhang
|
|
Name: Jirui Zhang
|
|
Title: Director
|
|
Date: July 13, 2018
|
|
|
|
By: /s/ Dong Li
|
|
Name: Dong Li
|
|
Title: Director
|
|
Date: July 13, 2018
|
vii
